Note: Descriptions are shown in the official language in which they were submitted.
CA 02209883 1997-07-09
Pa 96/9 Stent Mini Skirt - Translation of Patent Application
The invention relates to an endoprosthesis for the internal support of
hollow organs in living creatures, comprising a flexible tubular mesh
structure with open ends and an elastic tubular covering, whereby the
mesh structure is composed of circumferentially extending mesh rows,
consisting of lined up spaces enclosed with mesh material.
Open ends of endoprostheses as described in the introduction often bear a
risk of injury of the hollow organ into which such an endoprosthesis has to
be implanted. Depending on the design they comprise for example pointed
wire ends when the mesh structure is braided from wires or sharp edges
when the mesh structure is punched or etched out of a sheet metal tube.
For this reason the tissue of the stented hollow organ can be traumatically
injured due to movements of the endoprosthesis, e.g. when repositioning
or removing the endoprosthesis, or due to movements of the hollow
organ, e.g. by the peristaltic for instance of the foodpipe.
From EP 0 621 015 A1 an endoprosthesis is known which has a
cylindrical wall formed of a wire-netting and a covering layer of elastic
material extending over a part of the length of the endoprosthesis. Since
the covering layer terminates with the open end of the endoprosthesis it
does not provide sufficient protection from pointed or sharp edged
terminating parts of an open end of the cylindrical wall. The covering layer
can be penetrated or slitted as the endoprosthesis is radially condensed for
introduction into a hollow organ to be stented or as the implanted
endoprosthesis is axially compressed due to peristaltic movements of the
hollow organ; so that injuring end parts are set free. In this state an
atraumatic displacement of the endoprosthesis within the hollow organ
can not longer be achieved.
The problem of the invention is to develop an endoprosthesis as described
in the introduction in such a way that a hollow organ which is internally
supported by it is protected from injuries by pointed or
1
CA 02209883 2005-08-23
75490-36
sharp-edged terminating parts at a front end of the mesh
structure. Moreover, a possibility shall be provided to
reposition an endoprosthesis of the kind named at the
beginning within a hollow organ or to remove it completely
out of the hollow organ without causing damage to the hollow
organ.
According to the invention the problem is solved by an
endoprosthesis for the internal support of hollow organs in
living creatures, comprising a flexible tubular mesh
structure and a tubular covering, whereby the mesh structure
is composed of circumferentially extending closed mesh rows
bounding lined up spaces enclosed with mesh material and
open ends formed by open mesh rows bounding spaces not
entirely enclosed with mesh material, characterized in that
in the region of said open end of the mesh structure the
covering comprises an embedding portion enclosing at least
partially the mesh structure, and an adjacent projecting
portion protruding beyond said open end. When in the region
of an open end of a mesh structure the elastic tubular
covering comprises an embedding portion, enclosing at least
partially the mesh structure, and a projecting portion,
following next to the embedding portion and projecting over
the front end, injuring pointed wire ends and sharp edges at
the front end of an endoprosthesis are covered by a
sufficiently long projecting material buffer, which is
secured against loss by the embedding portion being
connected with the mesh structure. Specifically when
implanting an endoprosthesis according to the invention into
a hollow organ which performs peristaltic movements in form
of axial pushes against an open end of the endoprosthesis,
e.g. the foodpipe, the partly turned back projecting portion
serves as an additional protective layer. Besides that the
covering comprising the embedding and projecting portion
2
CA 02209883 2005-08-23
75490-36
reduces the foreign body feeling of a patient into hers or
his foodpipe such an endoprosthesis has been introduced,
said feeling being enforced by uncovered wire ends touching
the inside wall of the foodpipe. As a particular advantage
it turns out that the projecting portion of the covering is
a target which can relatively well be caught by gripping
devices in order to be able to adjust the position of the
endoprosthesis for example in proximal direction. At the
same time a possibility is created for a complete removal of
the endoprosthesis out of the hollow organ.
In accordance with one aspect of the present invention there
is provided endoprosthesis for the internal support of
hollow organs in living creatures, comprising a flexible
tubular mesh structure and a tubular covering, whereby the
mesh structure is composed of circumferentially extending
closed mesh rows bounding lined up spaces enclosed with mesh
material and open ends formed by open mesh rows bounding
spaces not entirely enclosed with mesh material,
characterized in that in the region of said open end of the
mesh structure the covering comprises an embedding portion
enclosing at least partially the mesh structure, and an
adjacent projecting portion protruding beyond said open end.
Further advantages of an endoprosthesis according to the
invention can be seen in preferred embodiments described
subsequently with reference to the drawings where in
FIG. 1 an open end of an endoprosthesis shown in
perspective view of a quarter cut along the longitudinal
axis, in
2a
CA 02209883 1997-07-09
FIG. 2 an open end of a first embodiment shown in schematic side
elevation, and in
FIG. 3 a magnified detail of the open end of a second embodiment
is illustrated.
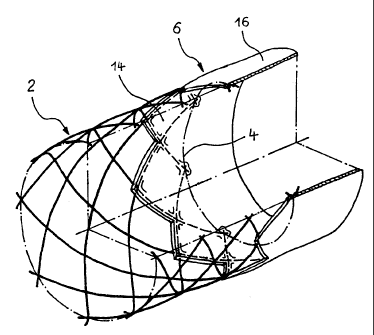
According to FIG. 1 the mesh structure 2 of an endoprosthesis according
to the invention comprises at an open end 4 a tubular covering 6,
consisting of an embedding portion 14 partially enclosing the mesh
structure 2 and of a projecting portion 16 following next to the embedding
portion 14 and projecting over the open end 4.
FIG. 2 schematically shows a portion of a mesh structure 2 which is
composed of circumferentially extending mesh rows 8 which consist of
lined up spaces 12 enclosed with mesh material 10. For clarification a
mesh row 8 is indicated with dotted spaces 12. The embedding portion 14
of the covering 6 which is indicated by a longitudinal hatch encloses the
mesh structure 2 starting from the open end 4 completely over the length
LE of at least the width B of a mesh row 8. This guarantees that the
covering 6 is interlockingly connected with the mesh structure 2 and as a
consequence the covering 6 cannot be removed from the mesh structure 2
and got lost by movements of the endoprosthesis in a hollow organ. The
length of the projecting portion 16 is in this case of the same order of
magnitude as the width B of a mesh row 8. Thus, on one hand the
projecting portion 16 provides for a sufficiently strong protection layer
between pointed wire ends and the inside wall of the hollow organ and on
the other hand enough target area in order to clamp a gripping device to it.
In an advantageous embodiment as shown in FIG. 3 the embedding
portion 14 encloses starting from the open end 4 mesh rows 8', squared
indicated, consisting of spaces 12' which are not entirely enclosed vvith
mesh material 10, whereby the spaces 12 of the first mesh row 8,
consisting of spaces 12 entirely enclosed with mesh material 10, is no
more enclosed by the embedding portion 14. In addition to the safe
3
CA 02209883 1997-07-09
anchoring of the covering 6 the freezing effect of the covering 6 to the
mesh structure 2 is reduced and therefore flexibility and compressibility of
the endoprosthesis in the region of an open end 4 is restricted only
slightly.
According to a preferred embodiment of the invention the covering 6
comprises canals, not shown in the figures, tightly enclosing the mesh
material 10 and the mesh material 10 being movable along the canals.
This enables the covering 6 to follow the deformations of the mesh
structure 2 caused by movements of the endoprosthesis as a consequence
of axial shortening and bending or radial compression and expansion. By
movement of the embedding portion 14 along the mesh material 10 it is
possible for the elastic covering 6 to take the state of minimal tension and
to reduce therefore the risk of tear or puncture of the covering 6.
In a mesh structure 2 of braided wires 10 the covering 6 comprises an
average wall thickness of about half of the diameter of the wires 10. Such
a wall thickness has proven effective with respect to the tear strength as
well as to the amount of material needed for the covering 6. The
covering 6 at the proximal open end 4 of braided mesh structures 2 has
turned out to be an advantageous protection against breaking up the
braiding which becomes effective for example when hooking with a
gripping tool into the braiding distally of the embedding portion 14 and
pulling the endoprosthesis in proximal direction.
The covering 6 of an endoprosthesis according to the invention is
preferably made of a polyurethane elastomer. Due to its biocompatibility
the polyurethane elastomer which is distributed on the trademark
ChronoFIexTM by the company PolyMedica Industries can be taken as are
example.
4
CA 02209883 1997-07-09
List of Reference Signs
2 mesh structure
4 open end
6 covering
8 mesh row (entirely enclosed spaces)
8' mesh row (not entirely enclosed spaces)
mesh material
12 space (entirely enclosed by mesh material)
12' space (not entirely enclosed by mesh material)
14 embedding portion
16 projecting portion
B width of a mesh row 8
LE length of embedding portion 14
L~ length of projecting portion 16
5