Note: Descriptions are shown in the official language in which they were submitted.
CA 02210477 1997-07-15
WO 96/22117 PCTIUS96100458
ON-LINE DRUG DELIVERY SYSTEM IN ERTRACORPOREAL THERAPY
background Of The invention
This invention relates to the field of extracorporeal
treatment of fluids and particularly blood, where drugs or
other biological solutions, such as monoclonal antibody
solutions, need to be added at precisely controlled rates,
including at rates responsive to other information and/or
conditions throughout the extracorporeal treatment
circuit. More particularly, it relates to the treatment
of cells with photoactivatable compounds and radiation and
specifically, to clinically useful systems for the
extracorporeal treatment of blood cells, especially
leukocytes, with W radiation.
It is well-known that a number of human disease
states may be characterized by the overproduction of
certain types of leukocytes, including lymphocytes, in
comparison to other populations of cells which normally
comprise whole blood. Excessive or abnormal lymphocyte
populations result in numerous adverse effects to patients
including the functional impairment of bodily organs,
leukocyte mediated autoimmune diseases and leukemia
related disorders many of which often ultimately result in
fatality.
For best results in extracorporeal chemotherapy, it
is necessary to deliver drug molecules to a target
location in the blood at a desired drug concentration.
For instance, in current extracorporeal photochemotherapy
(photopheresis) the patient takes crystalline 8-
methoxypsoralen ("8-MOP") capsules orally. Two (2) hours
later, when the 8-MOP concentration in patient's blood is
at maximum level, the peripheral blood is drawn from the
. patient, anticoagulated, and pumped into a rotating
-1-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
centrifuge bowl where it is separated into three layers;
plasma, huffy coat, and packed red blood cell layers. The
plasma and huffy coat layers are separated from the bowl.
See, for example, U.S. Pat. No. 4,568,328 to King, U.S.
Patent No. 4,573,960 to Goss, and U.S. Patent No.
4,623,328 to Hartranft.
The collected huffy coat is mixed with plasma and
normal saline and recirculated through a photoactivation
chamber (photoceptor) where the blood cells in the
circulating solution are exposed to the UVA irradiation in
the presence of the photoactivatable drug, 8-MOP
molecules. The treated cells are immediately returned to
the patient. In this therapy the drug concentration in
the patient blood is one of the most important parameters.
Inter- and intra-patient variation in bioavailability
of 8-MOP is extremely high, however, and in certain
individuals, such as uremic patients, the bioavailability
of 8 MOP is near zero. It is therefore, very difficult to
deliver consistent and optimum therapy to patients.
Several methods are currently used to deliver drugs
in liquid form into an extracorporeal circuit, but, none
of them can achieve the goal satisfactorily.
Among the more common methods currently in use is to
inject a precalculated amount of liquid drug into a part
of an extracorporeal blood circuit, such as a drip chamber
or blood bag, and hand-mix it. Another common approach is
to drip the drug into the drip chamber. These methods are
not very precise, thereby making it difficult to control
the concentration of the drugs during the extracorporeal
therapy process.
'
-2-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCTlUS96100458
Anotheb rii~thod currently in use imparts a drug
solution into the blood circuit by means of a syringe
pump. In this method the drug injection rate can be ~,
precisely controlled, but is independent of the blood flow '
rate. Thus, the drug concentration in the blood circuit
or reaction chamber varies as the blood flow rate in the
extracorporeal circuit changes.
Still another commonly used method uses a peristaltic
drug delivery pump, such as heparin pump, which is slaved
to the blood pump rate. This method has limited
applications for several reasons. First, the blood supply
from the patient peripheral circuit to the extracorporeal
circuit should remain substantially constant throughout
the treatment. However, if the blood supply changes,
which happens frequently during the treatment due to the
movement of needle or other reasons, a negative pressure
can develop within the extracorporeal circuit,
endangering the patient. For this reason, most dialysis
patients need to have a fistula implanted as a blood
access. This method also requires the use of an elastic
pump chamber tubing, such as silicone or PVC, which in
many instances adsorbs drug molecules.
In the method mentioned above, no attempt is made to
achieve a good mixing of the delivered drug with the
blood. Mixing is left to the natural behavior of the
f luid f low. Because the f low is laminar in most of the
extracorporeal blood circuit, any liquid drug stream
injected into the blood stream requires a substantial
amount of time or flowing distance to achieve good mixing.
Known methods for extracorporeal therapy hold the
buffy coat portion of the patient's blood throughout all
the treatment cycles of a therapy session, and therefore
do riot return any portion of the patient's buffy coat to
-3 -
SUBSTITUTE SHEET (RULE 26)
CA 02210477 2000-10-16
the patient until the conclusion of the treatment session.
In order to minimize the time during which a patient is
without buffy coat, it is desirable to have an
extracorporeal treatment system which returns whole blood
s (including buffy coat) to the patient at the end of each
treatment cycle in the therapy session.
It is therefore an object of the present invention
to overcome the foregoing drawbacks by providing a system
for increasing the effectiveness of extracorporeal treatment
~o and for increasing patient safety thereby also raising
patient comfort level as well as meeting acceptable
regulatory standards.
It is another related object to provide a suitably
automated system which can be monitored and operated by less
15 trained personnel thereby lowering treatment costs in
accordance with recently enacted fiscal policies.
It is still another related object to provide a
system for use in the extracorporeal treatment of patients
wherein drug concentration is regulated and delivered to a
zo reaction chamber, such as used in extracorporeal
chemotherapy, to maximize the effectiveness of such therapy.
It is still another related object to provide an
extracorporeal treatment system which returns whole blood to
the patient at the end of each treatment cycle in the
2s therapy session.
It is still another related object to provide an
extracorporeal treatment system which achieves a complete
and uniform mixing of a delivered drug with blood flowing in
an extracorporeal circuit.
3o It is still another related object to provide an
extracorporeal treatment system which is able to detect
blood loss in the extracorporeal circuit.
- 4 -
CA 02210477 2000-10-16
It is still another related object to provide an
extracorporeal treatment system which is able to detect the
presence of air in the extracorporeal circuit.
It is still another related object to provide an
s extracorporeal treatment system which includes a syringe
pump and a pressure pillow sensor which prevents a drug in
the syringe from being drawn into the blood circuit when
there is negative pressure in the bloodline.
According to the above objects, from a broad
~o aspect, the present invention provides an on-line system for
extracorporeally delivering a predetermined concentration of
a medicinal solution into a patient's blood. The system
comprises means for extracting blood from the patient and
introducing the extracted blood into a blood reservoir.
15 First pumping means is provided for pumping the extracted
blood from the blood reservoir into a blood line at a first
pumping rate. The blood reservoir level is sensed by a
sensing means in response to the extracted blood remaining
in the blood reservoir to determine a sensed blood reservoir
20 level. First adjustment means is provided for adjusting the
first pumping rate in response to the sensed blood reservoir
level. Second pumping means is provided for pumping the
medicinal solution into the blood line at a second pumping
rate. Second adjustment means adjust the second pumping
z5 rate in response to the first pumping rate. Mixing means
mixes the extracted blood in the blood line with the
medicinal solution in the blood line. A treatment chamber
is provided in which the blood-medicinal solution mixture is
introduced into and exposed to radiation to form a quantity
30 of treated blood. Means is provided to return the extracted
blood to the patient after the mixing.
These and still other objects of the invention
will become apparent upon study of the accompanying drawings
and description of the invention.
- 5 -
CA 02210477 2000-10-16
Brief Description of The Drawings
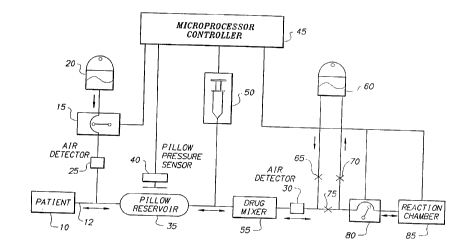
Fig. 1 is a block diagram illustrating an on-line
extracorporeal drug treatment system operating in accordance
s with a preferred embodiment of the present invention.
Fig. 2 is a flow diagram illustrating the
operation of the system of Fig. 1 in accordance with a
preferred embodiment of the present invention.
Fig. 2A is a flow diagram illustrating the
~o operation of the system of Fig. 1 in accordance with a
further preferred embodiment of the present invention.
Fig. 3 is a flow diagram illustrating the
operation of blood and syringe pumps in accordance with a
preferred embodiment of the present invention.
- 5a -
-CA 02210477 1997-07-15
WO 96!22117 PCT/US96/00458
Figs. 4 and 4A are a flow diagram illustrating the
operation of a system for controlling the irradiation
time of blood contained in a centrifuge treatment chamber
in accordance with a preferred embodiment of the present
invention.
Fig. 5 is a block diagram of a system for detecting
air in the blood circuit of an extracorporeal blood
treatment system in accordance with a preferred embodiment
of the present invention.
Fig. 6 is a side view of a drug mixer for mixing a
drug solution with a patient's blood within an
extracorporeal blood circuit in accordance with a
preferred embodiment of the present invention.
Fig. 7 is a block diagram of a system for detecting
blood loss from the blood circuit of an extracorporeal
blood treatment system in accordance with a preferred
embodiment of the present invention.
Fig. 8 is a circuit diagram of a system for
monitoring the state of irradiation lamps positioned
within a centrifuge chamber in accordance with a preferred
embodiment of the present invention.
Figs. 9 and 9A are front views of a syringe pump
according to a preferred embodiment of the present
invention.
Summary Of The Invention
The present invention is directed to a method and
apparatus for extracorporeally treating a patient's blood
on-line. Whole blood is initially collected from the
patient and mixed with a medicinal solution to form a
-6-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCTIUS96100458
whole-blood medicinal solution mixture. A quantity of
treated whole blood if formed by providing the whole-blood
medicinal solution mixture to a centrifuge chamber and
exposing the whole-blood medicinal solution to radiation.
The quantity of treated whole blood is then emptied from
. the centrifuge chamber, stored in a return storage medium,
and re-infused into the patient. The process is
successively repeated from the initial collection stage
for a plurality of cycles. In a preferred embodiment, the
1o treatment process is expedited after the first volume of
whole blood has been treated by simultaneously collecting
whole blood from the patient while at the same time
treating the volume of whole blood that was collected from
the patient in the previous cycle.
In accordance with another aspect of the present
invention, a continuous flow of blood collected from a
patient is provided into a rotating centrifuge chamber.
As the flow of blood collected from the patient is being
received by the rotating centrifuge chamber, the blood
currently contained in the rotating centrifuge chamber is
irradiated with light. As the blood is being irradiated
in the rotating centrifuge chamber, changes in the volume
of blood contained in the rotating centrifuge chamber and
the cumulative light energy applied to the blood in the
chamber are monitored over time. A remaining irradiation
time value is determined in accordance with changes in the
volume of blood currently contained in the rotating
centrifuge chamber and the cumulative light energy value.
A determination is made whether the irradiating step is
finished by comparing the remaining irradiation time value
- to a predetermined constant. If the comparison of the
remaining irradiation time value with the predetermined
constant does not indicate that the irradiating step is
finished, then new remaining irradiation time values are
successively determined until a comparison of the
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
remaining irradiation time value with the predetermined
constant indicates that the irradiating step is finished.
In accordance with a further aspect of the present
invention, an on-line system and method for
extracorporeally delivering a predetermined concentration -
of a medicinal solution into a patient's blood are
provided. Initially, blood is extracted from the patient
and provided to a blood reservoir. The extracted blood is
then pumped from the blood reservoir into a blood line at
a first pumping rate. The level of blood in the reservoir
is sensed based on the extracted blood currently remaining
in the blood reservoir to determine a sensed blood
reservoir level. The first pumping rate is adjusted in
response to the sensed blood reservoir level. A medicinal
solution is pumped into the blood line at a second pumping
rate which is adjusting in response to the first pumping
rate. The extracted blood in the blood line is mixed with
the medicinal solution in the blood line, and later
returned to the patient.
In accordance with a still further aspect of the
present invention, an improved syringe pump for use in an
on-line system for extracorporeally delivering a
predetermined concentration of a medicinal solution into
a patient s blood is provided. The syringe pump includes
a mounting block for rigidly receiving a body portion of
a syringe, the syringe being filled with a medicinal
solution and being coupled to a blood path in the on-line
system. A push block is slidably secured to the mounting
block, the push block having a plunger clip opening for
securing a top end of a plunger portion of the syringe to
the push block. Driving means, coupled to the mounting
block and the push block, are provided for driving the
push block toward the mounting block in response to a
control signal. The plunger clip opening in the push
_g_
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCTlI3S96J00458
block is shaped so as to prevent the on-line system from
drawing medicinal solution from the syringe when there is
negative pressure in the blood path.
In accordance with yet a still further aspect of the
present invention, a blood loss detector is provided for
use in connection with an on-line system for
extracorporeally treating a patient's blood that includes
a reaction chamber for processing the patient's blood, the
reaction chamber having a drain line for removing blood
from the on-line system. A first conductive tube having
a first end coupled to the drain line is provided. An
insulator block having a first channel for receiving a
second end of the first conductive tube is also provided.
The insulator block further includes a second channel for
receiving a first end of a second conductive tube. The
first and second channels are connected in the insulator
block by a hollow fluid bridge for carrying fluid from the
second end of the first conductive tube to the first end
of the second conductive tube. Sensing means are provided
for signaling the presence of an electrical connection
between the first and second conductive tubes when a
patient's blood flows through the fluid bridge, thereby
indicating a blood loss from the extracorporeal circuit.
In accordance with a still further aspect of the
present invention, an air detector is provided for use in
connection with an on-line system for extracorporeally
treating a patient's blood. First and second oscillators
are positioned on opposing sides of a blood transmission
line for transporting the patient's blood through the on
- line system. A signal transmitter is coupled to the first
oscillator, and a signal receiver is coupled to the second
oscillator. A microprocessor is coupled to the signal
transmitter and the signal receiver. The microprocessor
includes comparing means for comparing signals transmitted
_g_
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
by the signal transmitter to signals received by the
signal receiver. The microprocessor further includes air
detection means, responsive to the comparing means, for
signalling the presence of air in the blood transmission
line.
In accordance with a still further aspect of the
present invention, an improved method and apparatus for
mixing first and second fluids moving in a combined
laminar flow within a single fluid transmission line are
provided. The combined laminar flow is directed into a
fluid mixer through a mixer input port. The combined
laminar flow is then passed through a mesh material
positioned inside the fluid mixer, thereby forming a
mixture of the first and second fluids.
Detailed Description Of The Preferred Embodiment
Referring now to Fig. 1, there is shown a block
diagram of an on-line extracorporeal drug treatment system
100 operating in accordance with a preferred embodiment of
the present invention. System 100 operates in several
modes which are performed in a repetitive fashion for a
plurality of cycles during a treatment session. In the
collection mode, system 100 collects whole blood from
patient 10, adds a precisely controlled amount of
medicinal solution to the whole-blood via syringe pump 50,
and provides the whole blood medicinal solution mixture to
reaction chamber 85 for treatment. After the blood in
reaction chamber 85 has been treated, system 100 switches
to a return mode, during which treated whole blood is
pumped by blood pump 80 to return bag 60. When the
contents of reaction chamber 85 have been pumped into
return bag 60, system 100 switches to a re-infusion mode
during which treated whole blood from return bag 60 is
returned to patient 10 through gravity pressure.
-10-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCTIUS9b100458
In a f first pref erred embodiment ( shown in Fig . 2 ) ,
system 100 successively cycles through its three modes ten
times during a treatment session. In each such cycle, as
little as 150 ml of whole blood may be collected, treated
and returned (or re-infused) into patient 10. In
_ alternate embodiments of the system shown in Fig. 2, an
operator may vary both the number of treatment cycles and
the volume of whole blood that is treated in each cycle.
As shown in Fig. 2, system 100 is preferably primed with
a saline solution prior to initiation of the treatment
session.
In a second preferred embodiment (shown in Fig. 2A),
system 100 repeats its three modes ten times during a
treatment session. However, in contrast to the system of
Fig. 2, in this embodiment the treatment process is
expedited after the first volume of whole blood has been
collected, treated and emptied into the return bag 60. In
the system of Fig. 2A, after the first volume of whole
blood has been collected, treated and emptied into the
return bag 60, a second volume of blood is collected from
the patient 10, mixed with a medicinal solution and
provided to reaction chamber 85. Thereafter, the second
volume of blood then in reaction chamber 85 is treated
while at the same time the blood in return bag 60 ( from
the previous treatment cycle) is simultaneously re-infused
into patient 10. It was found that the overall treatment
time for a patient could be reduced by performing the
treatment and re-infusion steps simultaneously. In
addition, it was found that blood line 12 was less prone
to clogging when the treatment and re-infusion steps were
performed simultaneously because this reduced the amount
of time that blood line 12 was stagnant or inactive. As
was the case with the system of Fig. 2, in the system of
Fig. 2 as little as 150 ml of whole blood may be
collected, treated and re-infused into patient 10 in each
-11-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96l00458
treatment cycle. In alternate embodiments, however, an
operator may vary both the number of treatment cycles and
the volume of whole blood that is treated in each cycle.
As discussed more fully below, the flow of blood
throughout system 100 is precisely controlled throughout
the treatment session by microprocessor controller 45
which controls both the flow rate and direction of blood
in the extracorporeal circuit. Among other devices,
microprocessor controller 45 is coupled both to blood pump
80 and syringe pump 50, as well as to solenoid activated
pinch clamps 65, 70, 75. During the collection mode of
system 100, blood pump 80 pumps whole blood from pressure
pillow reservoir 35 into reaction chamber 85. As the
whole blood exits pressure pillow reservoir 35, syringe
pump 50 injects a medicinal solution into the blood
circuit at a controlled rate. In the collection mode,
microprocessor controller 45 controls the flow direction
of whole blood in the extracorporeal circuit by placing
clamps 65, 70 in a closed state, while placing clamp 75 in
an open state.
During the return mode of system 100, the direction
of blood pump 80 is reversed, and blood pump 80 pumps
treated whole blood from reaction chamber 85 to return bag
60. In the return mode, microprocessor controller 45
controls the direction of treated blood in the
extracorporeal circuit by opening clamp 70, while
maintaining clamps 65, 75 in a closed position. Finally,
during the re-infusion mode, treated blood from the return
bag 60 flows by gravity first to pressure pillow reservoir
35, and then back into the patient. In the preferred
embodiment, the same needle that was used to collect blood
from patient 10 in the collection mode is used to re-
infuse the treated blood into the patient. In the re-
infusion mode, microprocessor controller 45 controls the
-12-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22!!7 PCTII3S96100458
flow of blood in the extracorporeal circuit by opening
clamp 65, while maintaining clamps 70, 75 in a closed
position.
Against this overview of the operation of the present
invention, the details of system 100 will be described
more fully below. Referring again to Fig. 1, during the
collection mode, whole blood is extracted from patient to
through a needle and provided to system 100 through
disposable blood tubing 12. The patient's blood
preferably flows by gravity into pressure pillow reservoir
35. Prior to reaching pressure pillow reservoir 35, the
patient's blood is anticoagulated by anticoagulation pump
15, which provides heparin from bag 20 to the patient's
blood. The whole blood in pressure pillow reservoir 35 is
pumped into reaction chamber 85 by blood pump 80 at a rate
controlled by microprocessor controller 45. During the
collection mode, pressure pillow sensor 40 continuously
senses the level of blood in reservoir 35 and sends a
signal representative of the reservoir level to
microprocessor controller 45. If the blood level in
reservoir 35 falls below a predetermined minimum
threshold, the pump speed of pump 80 is reduced to zero
until such time as the blood level in reservoir 35 exceeds
the predetermined minimum level. The purpose behind
reducing the speed of pump 80 when the level of blood in
reservoir 35 is low, is to insure that the blood flow out
of patient 10 is entirely gravity driven and that blood is
never pumped out of the patient by blood pump 80. The
pillow sensor 40 could be made from many different types
of sensors such as electromechanical, optical, ultrasonic,
or piezoelectric sensors. In addition, in an alternate
embodiment, the pump speed of pump 80 could be ramped down
gradually as the blood level in the pressure pillow
reservoir decreases.
-13-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
As pump 80 pumps whole blood from pressure pillow
reservoir 35 to reaction chamber 85 during the collection
mode, a liquid drug is pumped into the blood circuit from
syringe pump 50 at a rate controlled by microprocessor
controller 45. In calculating the syringe pump rate,
microprocessor controller 45 in effect slaves the pumping
rate of syringe pump 50 to the pumping rate of blood pump
80 such that, syringe pump 50 injects drug solution into
the blood circuit only when blood pump 80 is in an ON
state. In addition, as described more fully below in
conjunction with Fig. 3, syringe pump 50 operates at an
increased pumping rate during the first cycle of a
treatment session in order to compensate for drug
absorption by the tubing and other materials forming
system 100.
Following the injection by syringe pump 50 of the
drug solution into the extracorporeal circuit, the blood
stream, combined with the delivered liquid drug, flows
into specially designed drug mixer 55 where the two
relatively unmixed streams (i.e., the whole blood stream
and the drug solution stream) are broken up and mixed to
form a whole blood drug solution mixture which is pumped
by pump 80 into reaction chamber 85. Further details of
drug mixer 55 are set forth below in conjunction with the
description of Fig. 6.
In a preferred embodiment of the present invention,
a photoactivatable agent, such as a psoralen, and still
further preferably 8-methoxypsoralen, in liquid form is
injected in the blood circuit by syringe pump 50 during
the collection mode of system 100, although other drug or
biological solutions, such as monoclonal antibody
solutions or other photoactivatable agents may also be
used. Also in a preferred embodiment, reaction chamber 85
constitutes a rotatable centrifuge that includes within
-14-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 2000-10-16
its interior a photoactivation system for irradiating the
whole blood drug solution mixture with UV light while the
centrifuge is rotating. A preferred reaction chamber 85
which includes a photoactivation system within its interior
s is illustrated and operates substantially as shown and
described in U.S. Patent No. 4,921,473.
Although in the preferred embodiment of system
100, whole-blood from a patient 10 is collected and provided
to the system for treatment and then re-infused into the
io patient, it will be understood by those skilled in the art
that blood provided from other sources, such as for example,
a donor center (not shown) may be provided to system 100 for
treatment. Where blood collected from a donor center is
provided to system 100 for treatment, the treated blood may
either be directly infused into a patient by system 100 or
collected in a container (not shown) and infused into a
patient at a later time. In addition, although in the
preferred embodiment, system 100 operates to treat whole-
blood, it will be understood by those skilled in the art
zo that blood formed from fractional components of whole-blood
may also be treated by system 100.
Referring now to Fig. 3, there is shown a
flow diagram illustrating a system 300 for operating
blood pump 80 and syringe pump 50 in accordance with
2s a preferred embodiment of the present invention. When
system 100 is operating in its collection mode, the
operation rate of blood pump 80 is set in such a way that
the pumping rate is less than the blood flow from patient 10
to pressure pillow reservoir 35. Consequently, during
so normal operation, pressure pillow reservoir 35 should remain
fully filled with the patient blood. However, if
the blood flow from the patient to pressure pillow reservoir
- 15 -
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
35 slows down for any reason, the blood volume inside
pressure pillow reservoir 35 may decrease eventually
causing pressure pillow reservoir 35 to collapse. This
undesirable situation is prevented by the pillow sensor 40
which detects the level of the blood in pressure pillow
reservoir 35 and signals this condition to microprocessor .
controller 45. If the blood level in pressure pillow
reservoir 35 decreases, microprocessor control system 45
slows down the blood pump rates of pumps 50 and 80. This
feedback mechanism helps patient 10 receive uninterrupted
and safer treatment.
The operation of the feedback system described
generally in the paragraph immediately above is
illustrated in more detail as feedback system 300 in Fig.
3. Referring still to Fig. 3, system 300 begins at step
310 by monitoring pressure pillow sensor 40 to determine
the level of blood in reservoir 35. A control signal
representative of the level of blood in the reservoir is
then provided to microprocessor controller 45 by sensor
40. In step 320, microprocessor controller 45 determines,
in response to the control signal received from sensor 40,
whether the level of blood in pressure pillow reservoir 35
has fallen below a predetermined level, thereby indicating
that pressure pillow reservoir 35 has collapsed. If a
determination is made that pressure pillow reservoir 35
has collapsed, then processing proceeds to step 330,
wherein microprocessor 45 sends control signals to pumps
50 and 80 setting both pumps to an OFF state.
Alternatively, if a determination is made at step 320 that
pressure pillow reservoir 35 has not collapsed, then
processing proceeds to step 340, wherein microprocessor 45
sends a control signal to pump 80 setting that pump to an
ON state.
-16-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCTlIJS96100458
Next, in step 350, microprocessor controller 45
determines whether system 100 is operating in the first
cycle of a treatment session. If system 100 is in the
first cycle of the treatment session, microprocessor
controller 45 sends a control signal to syringe pump 50
turning that pump to an ON state and setting its pumping
rate at a first rate (R1). Alternatively, if system 100
is not in the first cycle of the treatment session,
microprocessor controller 45 sends a control signal to
syringe pump 50 turning that pump to an ON state and
setting its pumping rate at a second rate (R2 ) which is
less than (Rl). In the preferred embodiment, an increased
pumping rate (R1) is used in the first cycle to compensate
for the drug adsorption rate of the tubing and other
materials that form system 100. After the pumping rate of
syringe pump 50 is set, processing continues with step
380, wherein microprocessor controller 45 determines
whether the volume of whole blood to be treated in the
current cycle has been provided to reaction chamber 85 by
pump 80. Microprocessor controller 45 determines whether
the cycle volume has been reached by repeatedly monitoring
the state and pumping rate of blood pump 80 during the
collection mode. If the cycle volume has not been
reached, system 300 returns to step 310 and the process
described above is repeated; otherwise, processing passes
to step 390. In step 390, microprocessor controller 45
sends control signals to pumps 50 and 80 setting both
pumps to an OFF state.
Referring now to Figs. 4, 4A, there is shown a flow
diagram illustrating the operation of an irradiation time
control system 400 for controlling the irradiation time of
blood contained in reaction chamber 85 during the
treatment mode of system 100 in accordance with a
preferred embodiment of the present invention. During the
treatment mode, a continuous flow of blood collected from
-17-
SUBSTITUTE SliEET (RULE 2G)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
patient 10 is mixed with a medicinal solution and provided
to reaction chamber 85 by blood pump 80. As mentioned
above, in the preferred embodiment, reaction chamber 85 is
a centrifuge which includes an interior photoactivation
system for irradiating the patient s blood with UV light.
In the present invention, system 100 does not wait until
the entire cycle volume of blood (described in connection
with step 350 above) is received into the centrifuge
chamber before beginning to irradiate the patient s blood
with UV light. Instead, on a continuous basis as blood is
received into chamber 85 during the treatment mode, the
blood is separated into its constituent parts by the
rotating centrifuge and then irradiated by the W lights
positioned inside the centrifuge chamber. By separating
and irradiating the patient s blood on a continuous basis
during the collection mode, the present invention
minimizes the treatment time for patient 10.
The operation of the irradiation time control system
described generally in the paragraph immediately above is
illustrated in detail as system 400 in Fig. 4. Referring
still to Fig. 4, system 400 begins at the beginning of the
treatment mode in step 405 by initializing a remaining
irradiation time value (TI) to a seed value (T,). The
remaining irradiation time value is a running value which
is repeatedly incremented and decremented, and which
represents the remaining time (in seconds) during which
the blood in chamber 85 is to be subjected to UV light
before such blood is returned and re-infused into the
patient. The seed value (T,) is set to compensate for the
fact that W light from the photoactivation system cannot
penetrate whole blood, but instead such light can only
penetrate through a buffy coat layer. Since it takes
whole blood approximately 2-3 minutes after it has been
received into a rotating centrifuge to separate into its
constituent parts, any W applied to the blood during this
-18-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCTIUS96J00458
initial 2-3 minutes is useless for purposes of treating
the blood. Therefore, in the preferred embodiment, Ts is
preferably set between 120-180 seconds. In initialization
step 405, a time marker Tp~" is set to zero, and a volume
marker VP"~,, is set to zero. By setting Tp,.~, and Vp"~" to zero,
initialization step 405 signifies that the irradiation
step begins at time zero with chamber 85 in an empty
state.
After initialization step 405, a determination is
made in step 410 regarding the current volume of blood
(V~"n) in chamber 85. Since blood is continuously being
pumping into chamber 85 during at least part of the
treatment mode, the value of V~"n will vary with time.
Microprocessor controller 45 determines V~"n bY monitoring
the state and speed of pump 80 throughout each treatment
cycle. In step 415, a change in blood volume (deltaV) is
determined by differencing V~,~ and V~" and in step 420 Vpn,,
is replaced with V~"~. In step 425, the time that has
elapsed since initiation of the irradiating step (T~urc) is
saved. In step 430, a change in elapsed time (deltaT) is
determined by differencing T~"n and Tp,.~," and in step 435 TP,.~"
is replaced with T~",~. In step 440, a cumulative light
energy value (W~"m) representing the total light energy
provided to the blood in chamber 85 during the treatment
cycle is calculated. In order to determine this
cumulative light energy value, microprocessor controller
45 monitors the state (ON/OFF) of the UV lamps in chamber
85 during the treatment cycle. In addition,
microprocessor controller 45 maintains a running record of
the age (in hours used) of each bulb in chamber 85 and
adjusts the cumulative light energy value for the fact
that the light energy emitted by each bulb decreases as
the bulb is used.
-19-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
Although in Fig. 4, the steps for determining deltaV
(steps 410, 415, 420) , deltaT (steps 425, 430, 435) and
UV~"~, (step 440) are shown in parallel, these values may
also be determined sequentially. In the preferred
embodiment of system 400, deltaV is determined 40 times
per second, while deltaT and UV~ are determined 5 times
per second. Frequent calculations of these values are
important to insure that the transition of TI to zero is
caught immediately.
In step 445, following calculation of deltaV, deltaT
and UV~"~" V~"~ is compared to a threshold (V,~h) which
represents the threshold volume of blood that activates
the deltaV and deltaT terms during the calculation of TI in
step 485 (described below). If V~"~, is greater than or
equal to V"",~b, then in step 450 first (A) and second (B)
irradiation time calculation constants are set to one. If
V~"~. is not greater than or equal to V,bn,e, then in step 455
UV~"m is compared to a threshold (UV~h) . If UV~"o, is
2 0 greater than or equal to UV,b"~,h, then in step 4 6 0 the f first
and second irradiation time calculation constants A and B
are set to zero and one, respectively; otherwise, in step
465, the first and second irradiation time calculation
constants A and B are both set to zero. A preferred
value for UV,~,~ is 300. In step 470, system 400 determines
(or confirms) whether the UV lights in chamber 85 are in
fact in an ON state. A preferred circuit for determining
the state of the UV lights in chamber 85 is described
below in conjunction with Fig. 8. If step 470 determines
that the UV lights are in fact in an ON state, then a
third irradiation time calculation constant (U) is set to
one in step 475; otherwise the third irradiation time
calculation constant (U) is set to zero.
Following the determination of the three irradiation
time calculation constants, microprocessor controller 45
-20-
SUBSTITUTE SHEET {FU~~ «)
CA 02210477 1997-07-15
WO 96/22117 PCTIUS96100458
updates the value of T, in accordance with equation (1)
below:
TI = TI + (A * C * deltaV) - (B * U * deltaT) (1)
where, C is a constant representing the number of seconds
of irradiation time to be added to the remaining
irradiation time TI as each milliliter of blood is added
into chamber 85. In step 490, TI is compared against a
zero threshold. If TI is not greater than zero, this
indicates that the irradiation step is finished;
otherwise, the process is repeated as shown in Fig. 4
until TI reaches or falls below the zero threshold.
Referring again to Fig. 1, the preferred embodiment
of system 100 includes a plurality of air detectors 25, 30
for detecting the presence of air in the extracorporeal
circuit. In the preferred embodiment, if air is detected
in system 100, an alarm is generated immediately informing
an operator that air has been detected. Referring now
to Fig. 5, there is shown a block diagram of an air
detector 25, 30. First and second oscillators 505, 510
are positioned on opposing sides of blood transmission
line 12 for transporting the patient s blood through the
on-line system. First and second crystal oscillators 505,
510 are held in place by air detector mounting block 515
which has recesses that are adapted to receive oscillators
505, 510. A signal transmitter 520 is electrically
coupled to first oscillator 505, and signal receiver 525
is electrically coupled to second oscillator 510.
Microprocessor controller 45 is coupled to signal
transmitter 520 and signal receiver 525. Microprocessor
controller 45 includes comparing means for periodically
comparing signals transmitted by signal transmitter 52o to
signals received by signal receiver 525. Since
transmitter 520 repeatedly broadcasts the same signal,
-21-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
unexpected changes in the signal received by receiver 525
indicate the presence of air in tube 12. Microprocessor
controller 45 includes air detection means, responsive to
the comparing means, for signalling the presence of air in
the blood transmission line when there is an unexpected
change in the signal received by receiver 525.
In the preferred embodiment of the present invention,
the operation of each air detector 25, 30 is periodically
l0 tested to verify that it is functioning properly. In
particular, on a periodic basis, signal transmitter 520 is
turned off and any signal received by receiver 525 is
monitored by microprocessor controller 45. If, during
this verification test, an unexpected signal is received
by receiver 525, this condition would indicate that the
air detector is not operating properly either because the
output of transmitter 520 is stuck or for some other
reason. In the preferred embodiment, if microprocessor
controller 45 determines that an air detector is not
functioning properly, an alarm is sounded signalling the
state of the system to an operator.
Although in Fig. 1, air detectors 25, 30 are
positioned adjacent to anticoagulation pump 15 and mixer
55, respectively, it will be understood by those skilled
in the art that air detectors 25, 30 may be positioned
throughout system 100. It will also be understood by
those skilled in the art that air detectors 25, 30 may be
used to sense the presence of air in fluid circuits other
than extracorporeal blood circuits.
Referring now to Fig. 6, there is shown a side view
of drug mixer 55 for mixing a drug solution with a
patient's blood within an extracorporeal blood circuit in
accordance with a preferred embodiment of the present
invention. Mixer 55 is formed of a sealed hollow chamber
-22-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCTIUS96lDU458
610 with an opening 600 for receiving unmixed fluids and
an opening 650 for outputting mixed fluids. The interior
of chamber 610 is divided into compartments 620 and 630 by
a mesh bag 640 which is secured in a circular manner along
its opening 660 to the interior of hollow chamber 610.
_ During operation of fluid mixer 55, first and second
fluids moving in a combined laminar flow within a single
fluid transmission line are provided to mixer 55 through
input port 600. The combined laminar flow is then passed
through mesh bag 640 positioned inside the fluid mixer,
thereby forming a mixture of the first and second fluids.
Mesh bag 640 achieves an efficient mixing of the first and
second fluids by disrupting the combined laminar flow of
these fluids. When mixer 55 is used to mix blood with
other solutions, mesh 640 preferably has a hole size
between 100 and 600 microns. It will be understood by
those skilled in the art that mixer 55 may be used to mix
fluids other than blood, and may be used in applications
other than extracorporeal blood circuits.
In the preferred embodiment of the present invention,
system 100 includes means for detecting blood loss from
the extracorporeal circuit. Such blood loss could occur,
for example, if a hole or crack develops in the centrifuge
treatment chamber, or if an overflow condition were to
develop inside the centrifuge chamber. When system 100
detects that blood is being lost from the extracorporeal
circuit, an alarm is triggered signalling this event to an
operator. Referring now to Fig. 7, there is shown a block
diagram of a preferred system 700 for detecting blood loss
from system 100 in accordance with a preferred embodiment
of the present invention. In the preferred embodiment,
reaction chamber 85 includes an outer housing (not shown)
for catching any leakage or overflow from the reaction
chamber. The lower-most portion of this outer housing is
connected to a drain line 710 for carrying away any blood
-23-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PCT/US96/00458
that leaks or spills into the outer housing. In system
700, a first electrically conductive tube 720 having a
first end coupled to drain line 710 is provided. An
insulator block 730 having a first hollow channel 735 for
receiving a second end of first conductive tube 710 is
also provided. Insulator block 730 is not electrically
conductive. Insulator block 730 further includes a second
hollow channel 740 for receiving a first end of a second
conductive tube 750. First and second hollow channels
735, 740 are connected in insulator block 730 by a hollow
fluid bridge 755 for carrying fluid from the second end of
first conductive tube 720 to the first end of second
electrically conductive tube 750. A comparator circuit
760 is provided for sensing the presence of an electrical
connection between first and second conductive tubes 720,
750. Since the conductivity of blood flowing through
hollow fluid bridge 755 is sufficient to form an
electrical connection between the first and second
conductive tubes 720, 750, comparator circuit 760 will
sense an electrical connection between the conductive
tubes whenever blood flows through hollow fluid bridge
755, thereby signalling a blood loss from the
extracorporeal circuit. The output of comparator circuit
760 is coupled to a latching relay circuit 770 that causes
a shutdown of blood pump 80 and the sounding of an audible
alarm whenever an electrical connection is sensed between
first and second conductive tubes 720, 750.
As discussed above in connection with Fig. 4, in step
470 of system 400, the present invention repeatedly
monitors the state (ON/OFF) of the UV irradiation lamps
inside reaction chamber 85. Referring now to Fig. 8,
there is shown a circuit diagram of a preferred system 800
for monitoring the state of an irradiation lamp 810
positioned within a centrifuge chamber in accordance with
a preferred embodiment of the present invention. In
-24-
SURD TiTUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 rcT~s96~ooa~s
system 800, a sine wave generator 820 and a resistor 830
are placed in series with irradiation lamp 810. When lamp
810 is in an ON state, a voltage is generated across
resistor 830, thereby causing a logic 0 signal at the
output of circuit 840. Alternatively, when lamp 810 is in
an OFF state, no voltage is generated across resistor 830
and the output of circuitry 840 will be a logic 1 signal.
In the preferred embodiment of the present invention,
three separate irradiation lamps are positioned within
chamber 85, and a separate system 800 is coupled to each
of these lamps so that the state of each lamp may be
separately monitored during the treatment process.
Finally, referring now to Figs. 9, 9A, there are
shown front views of a preferred embodiment of syringe
pump 50 according to the present invention. Syringe pump
50 includes a mounting block 900 for rigidly receiving a
body portion of a syringe 910. Syringe 910 is preferably
a glass syringe and is filled with a medicinal solution.
Syringe 910 is coupled (at syringe tip 920) to the
extracorporeal blood circuit. A push block 930 is
slidably secured to mounting block 900 by a pair of metal
rods 940, 950 positioned on opposing sides of the plunger
portion 960 of syringe 910. In the preferred embodiment,
push block 930 includes a plunger clip opening 970 for
securing the top end of plunger portion 960 to push block
930. The plunger clip opening 970 in push block 930 is
shaped so as to prevent the on-line system from drawing
medicinal solution from syringe 910 when there is negative
pressure in the blood path connected to tip 920. Driving
means (not shown, but positioned behind blocks 900, 930 in
Figs. 9, 9A), coupled to mounting block 900 and push block
930, are provided for driving the push block 930 toward
the mounting block 900 in response to a control signal
provided by microprocessor controller 45. This control
signal will communicate to the driving means whether
-25-
SUBSTITUTE SHEET (RULE 26)
CA 02210477 1997-07-15
WO 96/22117 PC°T/US96/00458
syringe pump 50 should be pumping at rate Rl, rate R2 or
whether pump 50 should be in an OFF state. In the
preferred embodiment, the driving means used for driving
push block 930 toward mounting block 900 is a worm drive
mechanism. In the preferred embodiment of the present
invention, syringe pump 50 includes a safety latch 980
that prevents system 100 from operating unless syringe 910
has been installed in mounting block 910. Fig. 9 shows
safety latch 980 in its open state; and Fig. 9A shows
safety latch 980.in its closed or locked state. A control
signal prevents operation of the present invention
whenever latch 980 is in an open state.
It will be understood by those skilled in the art
that syringe pump 50 may be used to precisely deliver
controlled quantities of fluids other than medicinal
solutions and in environments other than extracorporeal
blood circuits. In addition, it will also be understood
by those skilled in the art that system 100 has
applications in dynamic liquid mixing environments
including any extracorporeal blood treatments where drug
solution or any biological solutions, such as monoclonal
antibody solutions, needs to be added into the blood or
other circuit at precisely controlled rate and well mixed
before going into a treatment chamber.
The present invention may be embodied in other
specific forms without departing from the spirit or
essential attributes of the invention. Accordingly,
reference should be made to the appended claims, rather
than the foregoing specification, as indicating the scope
of the invention.
-26-
SUBSTITUTE SHEET (RULE 26)