Note: Descriptions are shown in the official language in which they were submitted.
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-1-
~
10 Title: TRICYCLIC BENZAZEPINE VASOPRESSIN
ANTAGONISTS
1. Field of the Invention
This invention relates to new tricyclic non-
peptide vasopressin antagonists which are useful in
treating conditions where decreased vasopressin levels
are desired, such as in congestive heart failure, in
disease conditions with excess renal water reabsorption
and in conditions with increased vascular resistance and
coronary vasoconstriction.
2. Backaround of the Invention
Vasopressin is released from the posterior
pituitary either in response to increased plasma
osmolarity detected by brain osmoreceptors or decreased
blood volume and blood pressure sensed by low-pressure
volume receptors and arterial baroreceptors. The
hormone exerts its action through two well defined
receptor subtypes:" vascular V1 and renal epithelial V2
receptors. Vasopressin-induced antidiuresis, mediated
by renal epithelial V2 receptors, helps to maintain
normal plasma osmolarity, blood volume and blood
pressure.
Vasopressin is involved in some cases of
congestive heart failure where peripheral resistance is
increased. Vi antagonists may decrease systemic
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-2-
vascular resistance, increase cardiac output and prevent
vasopressin induced coronary vasoconstriction. Thus, in
conditions with vasopressin induce increases in total
peripheral resistance and altered local blood flow, V1-
antagonists may be therapeutic agents. Vl antagonists
may decrease blood pressure, induced hypotensive effects
and thus be therapeutically useful in treatment of some
types of hypertension.
The blockage of V2 receptors is useful in
treating diseases characterized by excess renal
reabsorption of free water. Antidiuresis is regulated
by the hypothalamic release of vasopressin (antidiuretic
hormone) which binds to specific receptors on renal
collecting tubule cells. This binding stimulates
adenylyl cyclase and promotes the cAMP-mediated
incorporation of water pores into the luminal surface of
these cells. V2 antagonists may correct the fluid
retention in congestive heart failure, liver cirrhosis,
nephritic syndrome, central nervous system injuries,
lung disease and hyponatremia.
Elevated vasopressin levels occur in
congestive heart failure which is more common in older
patients with chronic heart failure. In patients with
hyponatremic congestive heart failure and elevated
vasopressin levels, a V2 antagonist may be beneficial in
promoting free water excretion by antagonizing the
action of _antidiuretic hormone, On the basis of
biochemical and pharmacological effects of the hormone,
antagonists of vasopressin are expected to be
therapeutically useful in the treatment and/or
prevention of hypertension, cardiac insufficiency, =
coronary vasospasm, cardiac ischemia, renal vasospasm,
liver cirrhosis, congestive heart failure, nephritic
syndrome, brain edema, cerebral ischemia, cerebral
hemorrhage-stroke, thrombosis-bleeding and abnormal
states of water retention.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-3-
The following prior art references describe
peptide vasopressin antagonists: M. Manning et al.,
J. Me . Chem., 35, 382(1992); M. Manning et al., 1. Meci.
Chem., 35, 3895(1992); H. Gavras and B. Lammek,
U.S. Patent 5,070,187 (1991); M. Manning and
W.H. Sawyer, U.S. 'Patent 5, 055, 448 (1991) F.E. Ali,
U.S. Patent 4,766,108(1988); R.R. Ruffolo et al., Dua
News ~n Perspective, 4(4), 217, (May)(1991). P.D.
Williams et al., have reported on potent hexapeptide
oxytocin antagonists [7. Med. Chem., 31, 3905(1992)]
which also exhibit weak vasopressin antagonist activity
in binding to V1 and V2 receptors. Peptide vasopressin
antagonists suffer from a lack of oral activity and many
of these peptides are not selective antagonists since
they also exhibit partial agonist activity.
Non-peptide vasopressin antagonists have
recently been disclosed, Y. Yamamura et al., Science,
.252, 579 (1991) ; Y. Yamamura et al., Br. 7. Pharmacol,
105, 787(1992); Ogawa et al., (Otsuka Pharm Co., LTD.)
EP 0514667-Al; EPO 382185-A2; W09105549 and
U.S.5,258,510; WO 9404525 Yamanouchi Pharm.Co.,Ltd.,
WO 9420473; WO 9412476; WO 9414796; Fujisawa Co. Ltd.,
EP 620216-Al Ogawa et al, (Otsuka Pharm. Co.) EP 470514A
disclose carbostyril derivatives and pharmaceutical
compositions containing the same. Non-peptide oxytocin
and vasopressin antagonist have been disclosed by Merck
and Co.; M.G. Bock and P.D. Williams, EP 0533242A; M.G.
Bock et al., EP 0533244A; J.M. Erb, D.F. Verber, P.D.
Williams, EP 0533240A; K. Gilbert et al.,.EP 0533243A.
Premature birth can cause infant health
problems and mortality and a key mediator in the
mechanism of labor is the peptide hormone oxytocin. On
the basis of the pharmacological action of oxytocin,
antagonists of this hormone are useful in the prevention
of preterm labor, B.E. Evans et al., J. Med. Chem. 35,
3919 (1992) ,J. Med. Chem., 3E, 3993 (1993) and references
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-4-
therein. The compounds ofthis invention are
antagonists of the peptide hormone oxytocin and are
useful in the control of premature birth.
The present invention relates to novel
tricyclic derivatives which exhibit antagonist activity
at V1 and/or V2 receptors and exhibit in vivo
vasopressin antagonist activity. The compounds also
exhibit antagonist activity at oxytocin receptors.
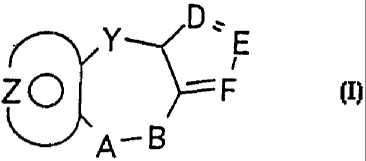
SUMMARY OF THE INVENTION
This invention relates to new compounds
selected from those of the general formula I:
"D`
Y- N ,
Z~ >=:= F
A- B
wherein Y is a moiety selected from; -(CH2)n- wherein n
is an integer from 0 to 2,
0
- CHI oweral kyI (Ci- C3) and --C
A-B is a moiety selected from
- (CH2) m i- and - N- (CH2) rrr
R3 R3
wherein m is an integer from 1 to 2 provided that when Y
is -(CH2)n- and n is 2, m may also be zero and when n is
zero, m may also be three, provided. also that when Y is
-(CH2)n- and n is 2, m may not be two;
and the moiety:
z 3o -
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-5-
represents: (1) phenyl or substituted phenyl optionally
substituted by one or two substituents selected from
, (C1-C3)lower alkyl, halogen, amino, (C1-C3)lower alkoxy
or (Cl-C3)lower alkylamino; (2) a 5-membered aromatic
(unsaturated) heterocyclic ring having one heteroatom
selected from 0, N or S; (3) a 6-membered aromatic
(unsaturated) heterocyclic ring having one nitrogen
atom; (4) a 5 or 6-membered aromatic (unsaturated)
heterocyclic ring having two nitrogen atoms; (5) a 5-
membered aromatic (unsaturated) heterocyclic ring having
one nitrogen atom together with either one oxygen or one
sulfur atom; wherein the 5 or 6-membered heterocyclic
rings are optionally substituted by (C1-C3)lower alkyl,
halogen or (Cl-C3)lower alkoxy;
the moiety:
N/ D E
~
F
is a five membered aromatic (unsaturated) nitrogen
containing heterocyclic ring wherein D, E and F are
selected from carbon and nitrogen and wherein the carbon
atoms may be optionally substituted by a substituent
selected from halogen, (C1-C3)lower alkyl, hydroxy, -
COC13, -COCF3,
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-6-
O
~1 R b
-C-O- I ower al kyl (C1-C3), - (CH2)q<
F~
. /_\ .
- (CH2)q-N~ , - (CH2)qN (CH2)q NO
-(CH2) q- 0-1 ow er al kyl (C1- C3), -(CH2) qCH,
0
7%,
-C-lower alkyl (C -C ), -CHNN CHNi s 2 N
N1 /-~
/a
- CH2- N,,,:~, N - CH2- N~ N~-(CH2) q N NR
/-~ / ~
- (CH2) q- NN
-CHO, amino, (C1-C3)lower alkoxy, (C1-C3)lower
alkylamino, CONH-lower alkyl(Cl-C3), and -CON[lower
alkyl(C1-C3)]2; q is one or two; Rb is iridependently
selected from hydrogen, -CH3 or -C2H5;
R3 is a moiety of the formula:
0
I I
- C- Ar
wherein Ar is a moiety selected from the group
consisting of
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-7-
R1 R
R6 Ri4
R N
R2
wherein R4 is selected from hydrogen, lower alkyl(Ci-
C3), -CO lower alkyl (C1-C3) ;
R1 and R2 are selected from hydrogen, (Cl-C3)lower
alkyl, (C1-C3)lower alkoxy and halogen; R5 is selected
from hydrogen, (C1-C3) lower alkyl, (Cl-C3) lower alkoxy
and halogen; R6 is selected from (a) moieties of the
formulae:
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-$-
Ra R Ra 'b
(
- NCQAr , - NCOCH2Ar , - NCCNAr',
R
- NCO- (CH2) n -cycl oal kyl
1 1
R R R
a a
I I
-N-S02 , -N-S02CH2
R2 R2
O R RO R'
,a,I II
N-P N-P
R2 [o12 R2 2
O
s - NH- C- O= I ow er al kyl (C3- C8) st rai ght or branched,
0
11
- NH- C- I ower al kyl (C3- C8) st rai ght or branched,
- NHSO2- I ow er al kyl (C3- C$) st rai ght or branched,
- NH- C- O- I ower al kenyl (C3- C$) st rai ght or branched,
0
~) .
- NH- C- I ower al kenyl (C3- C8) st rai ght or branched,
- NHSO2- I ower al kenyl (C3- C8) st rai ght or branched,
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96/01076
-9-
wherein cycloalkyl is defined as C3-C6 cycloalkyl,
cyclohexenyl or cyclopentenyl; Ra is independently
selected from hydrogen, -CH3, -C2H5,
'b
- (CH2)q - <R b - (CN)q N~
- (CH2)q- N - (CH2)q- N 0
-(CH2) q-0-lower alkyl (Cl-C3) and -CH2CH2OH, q is one or
two, and R1, R2 and Rb are as hereinbefore defined;
(b) moieties of the formula:
R1
R1 R2
/
7
-XR, - ,
I
F~ R 2 - N
wherein R7 is lower alkyl(C3-C8), lower alkenyl(C3-C8),
- (CH2) p-cycloalkyl (C3-C6) ,
R1
R
- (CH2) p ~ , - (CH2)P \ I ,
2 N
R
Ri
R
(CH2)P ~ , - ( CH2)
P
s Q
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCTIUS96/01076
-10-
wherein p is one to five and X is selected from 0, S,
NH, NCH3; wherein R1 and R2 are as hereinbefore defined;
(c) a moiety of the formula:
F~
= I
- N- COJ
wherein J is Ra, lower alkyl(C3-CO branched or
unbranched, lower alkenyl(C3-C8) branched or unbranched,
0-lower alkyl(C3-C8) branched or unbranched, -0-lower
alkenyl(C3-C8) branched or unbranched, tetrahydrofuran,
tetrahydrothiophene, the moieties:
R
R N
S O
or -CH2-K' wherein K' is (C1-C3) lower alkoxy, halogen,
tetrahydrofuran, tetrahydrothiophene or the heterocyclic
ring moiety:
-
D\
N/E
~ / =
G-F
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-11-
wherein D, E, F and G are selected from carbon or
nitrogen and wherein the carbon atoms may be optionally
. substituted with halogen, (Cl-C3)lower alkyl, hydroxy, -
CO-lower alkyl(C1-C3), CHO, (C1-C3)lower alkoxy, -C02-
5.lower alkyl(C1-C3), and Ra and Rb are as hereinbefore
defined;
(d) a moiety of the formula:
R
Ia
- N- COCHAr'
R
c
w her ei n R i s sel ect ed f r om hal ogen, (C1- C3)
l ower al kyl, -O- l ower al kyl (C1-C3), CH,
0
I I
-O-C-lower alkyl(Ci-C3), -S-lower alkyl(C1-C3),
- S- (CH2) 2 N~ ~' NH (CH2)q- (;UV~ ~
~ R,
NH(CH2)q- N~ R ~ -p.. (CH 2) 2< R b
b
F~
wherein Ra and Rb are as hereinbefore defined and Ar' is
selected from moieties of the formula:
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-12-
s Ra
R
R8
Rs
Rs w
R10
)CnN
and N Rs (
R4
wherein W' is selected from 0, S, NH, N-lower alkyl(C1-
C3), NHCO-lower alkyl(C1-C3), and NS021ower alkyl(C1-
C3)'
R8 and R9 are independently selected from hydrogen,
lower alkyl(C1-C3), -S-lower alkyl(C1-C3), halogen,
NH-lower alkyl(Cl-C3), -N-[lower alkyl(C1-C3)]2, -OCF3,
-OH, -CN, -S-CF3, -N02, -NH2, 0-lower alkyl(C1-C3),
0
I I
-aG(C,-cO
-N (Rb) (CH2) vN (Rb) 2, and CF3 wherein v is one to three
and;
R10 is selected from hydrogen, halogen and lower
alkyl (C1-C3) ; R14 is
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-13-
- O i ower al kyl (C3- C8) branched or unbranched ,
f Rio
. 'I b
R 1
- 0- CH2- / O- CI-h- ( CH2) ~ /\
- -
F~
- NH 1 ower al kyl (C3- C8) branched or unbranched ,
R1 S02N-,, R'
- NH- CH2 ( CH2) R b
NHCO
F~
I / R'
-NH-CH2-C _ -NH
I
Rb R1o
SUBSTtTUTE SHEET (RUILE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-14-
R la
~ ~ R ~
N I
-
-NCO
(CH2)n
/ =
R,
R8 N
R10
Ra R
f la
-N -N
R'
S R' 0
R
a
I ~ ~ j 20
- NCO(CH2)n - NCO(CH2)n R
N p -N
R20
wherein n is 0 or 1; Ra is hydrogen, -CH3 or -C2H5; R'
is hydrogen, (C1-C3)lower alkyl, (C1-C3)lower alkoxy and
halogen; R20 is hydrogen, halogen, (C1-C3)lower alkyl,
(C1-C3)lower alkoxy,=NH2, -NH(Cl-C3)lower alkyl, -N-
[ (C1-C3) lower alkyl] 2,
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-15-
-N - N , , - N \--/ O
-N \N- I ower al kyi (C1- C3)
- NH- (CH2)p NHI ower al kyl (C1- C) - NH- (CH2)p N[i ower al kyl (Cl- C3)]2
- NH- (CH2)P- <I) - NH- (CH2) P- N
- NH- (CH2)p NN- I ower al kyl (C1- C3)
Ra I~ Ri
- NH- (CH2) p NO , I /\
- N- COL C-
-'b
and the pharmaceutically acceptable salts thereof.
DETAILED DE RT TION OF THE INVENTION
Within the group of the compounds defined by
Formula I, certain subgroups of compounds are broadly
preferred. Broadly preferred are those compounds
wherein R3 is the moiety:
0
II
- CA r
and Ar is selected from the moieties:
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-16-
R~ R1
s
R6 R14
FN
R2 R2
Y is (CH2)n and n is one or zero;
wherein R1, R2, R4, R5, R6 and R14 are as hereinbefore
defined.
Especially preferred are compunds wherein R3
is the moiety:
0
11
-CAr and
Ar is selected from the moieties:
R R
R6 Ria
F N
R2 R2
Y is -(CH2)n and n is one and m is one;
wherein R1, R2, R4, R6 and R14 are as hereinbefore
defined.
Especially preferred are compounds wherein R3
is the moiety:
O
- CAr and
Ar is selected from the moieties:
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-17-
R R
R6 R14
1N
R2 R2
Y is -(CH2)n and n is one or zero;
R6 is
ra Ra a ~
~ ( (
- NCOAr', - NCOCH2Ar', - NCCNAr',
Ra
7 I
- X R NCO- (CH2)n- cycl oaI kyi
wherein cycloalkyl is defined as C3-C6 cycloalkyl,
cyclohexenyl or cyclopentenyl; and wherein X, Ra, Rb and
R14 are as hereinbefore defined; and Ar' is selected
from the moieties:
R8
R 8
R5
R9
R9 V1P
wherein R8, R9 and W' are as hereinbefore defined.
Also especially preferred are compounds
wherein Y in Formula I is -(CH2)n- and n is zero or one;
A-B is
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-18-
3 3
-(CH2)m N- R or R- N- (CH2) and Rl, R2, R4, -R5, R6, R7, R8, R9, R10 and R14
are as
hereinbefore defined; and m is an integer from 1-2.
The most preferred of the compounds of Formula
I are those wherein Y is -(CH2)n- and n is one; A-B is:
1 3 3 1
- CH2- N- R or R - N- CH2-
R3 is the moiety:
0
11
-CAr
Ar is selected from the moieties:
R R
Rs R 14
1 N
R2 R2
R6 is
Ra
I Ra Ra F~
I f I
- NCOAr', - NCOCH2Ar', - NCCNAr',
R a
- X- R' NCO- (CH2)n- cycl oal kyl
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-19-
(CH2)n-cycloalkyl wherein cycloalkyl is defined as C3-C6
cycloalkyl, cyclohexenyl or cyclopentenyl; wherein X,
Ra, Rb and R14 are as hereinbefore defined;
and Ar' is:
R$
R5
/
R9
wherein R5, R8 and R9 are as previously defined.
The most highly broadly preferred of the
compounds of Formula I are those wherein Y is -(CH2)n-
and n is zero or one; wherein the moiety:
z 3o
is a phenyl, substituted phenyl, thiophene, furan,
pyrrole or pyridine ring;
A-B is:
( 3 3
-(CH2) m N- R or R- N- ( CH2)
m
m is one when n is one and m is two when n is zero; D,
E, F, Rl, R2, R4, R5, R7, R8, R9, R10 are as previously
defined;
R3 is the moiety:
O.
II
- CAr
wherein Ar is selected from the moieties:
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCTIUS96/01076
-20-
R R~
R6 R1a
N
R2 R2
and R6 is selected from the group:
ra Ra rarb
- NCOAr', - NCOCH2Ar', - NCCNAr',
R
(a
- X R7 NCO- (CH2)n- cyci oal kyl
where Ar' is selected from the group:
R8 $
R R9 Ri o
R5
/ R9
R 9
W N
and R14, X, W', Ra, Rb and cycloalkyl are as previously
described.
More particularly preferred are compounds of
the formulae:
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-21-
R12 R 11 R12
"
N _ N
Zo and Zo
N CN
3 3
R R
wherein the moiety:
Zo
is selected from a phenyl, thiophene, furan, pyrrole, or
pyridine ring;
R3 is the moiety:
0
II
- CAr
wherein Ar is selected from the moieties:
R' R1
C/\ Rs Ria
N
R2 R2
R6 is
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-22-
Ra R R F
- NCOAr', - NCOCH2Ar', - NCCNAr',
R
la
- X.R~ NCO- (CH2)~- cycl oal kyl
and Ar' is selected from the moieties:
R8 R8 Rs
R5 R1o
/ Rs
9
w N
wherein X, Ra, Rb, R5, R7, R8, R9, R14, cycloalkyl and
W' are as hereinbefore described;
R11 is selected from hydrogen, halogen, (C1-C3) lower
alkyl, hydroxy,
F%
_ (CH 2) /
Q~'b
0
II
-C-lower alkyl(C1-C3),
-CHO, and (C1-C3)lower alkoxy; and R12 is selected from
hydrogen, (Cl-C3)lower alkyl, halogen and (Cl-C3) lower
alkoxy.
Also particularly preferred are compounds of
the formulae:
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-23-
R12 R11 12
- R 11
N /
t ,(CH2)m and Z~
N .N-,, s
I (CH) m R
R3
wherein m is one or two;
the moiety:
Z9
is selected from a phenyl, thiophene, furan, pyrrole or
pyridine ring;
R3 is the moiety:
0
II
- CAr
wherein Ar is selected from the moieties:
R R
R14
F N
R2 R2
R6 is
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-24-
R R rarb
- NCOAr', - NCOCH2Ar', - NCONAr',
R
' la
- X R r - NCO- (CH2),,- cycl oal kyl
(CH2)n cycloalkyl; Ar' is selected from the moieties:
R8
R a
R5
R9
R9
VV'
wherein X, Ra, Rb, R5, R6, R8, R9, R14, cycloalkyl and
W' are as hereinbefore defined;
R11 is selected from hydrogen, halogen, (Cl-C3) lower
alkyl, hydroxy,
-(CH2>q< R b
0
I I
- C- I ow er al kyl (C1 - C3),
-CHO, and (C1-C3)lower alkoxy; and
R12 is selected from hydrogen, (Cl-C3)lower alkyl,
halogen and (C1-C3)lower alkoxy.
More particularly preferred are compounds of
the formulae:
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-25-
11 R12 R11
R12 R
N N
I
and
3 N
R
I 13 1 R3 R R3
R3 is the moiety:
0
11
-CAr
wherein Ar is selected from the moieties:
R1 R1
Rs 6 R14
N ,
R2 R2
R6 is
R a R a R a ' b
I I I I
- NCOAr', - NCOCH2Ar', - NCCNAr',
R
la
- X R' - NCO- (CH2)n- cycl oal kyl
R14 is
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-26-
R la
R
~ ~
-N - (
NCC>
- ,
tCH2)n
R,
R8 N
Rio
Ra R
I (
-N -N
R'
S R'
R
a
- NCO(CH2)n - NCO(CH2)n
D-N R zo
N
R2o
wherein n is 0 or 1; Ra is hydrogen, -CH3 or -C2H5; R`
is hydrogen, (C1-C3)lower alkyl, (Cl-C3)lower alkoxy and
halogen; R20 is hydrogen, halogen, (C1-C3)lower alkyl,
(Cl-C3)lower alkoxy, NH2, -NH(C1-C3)lower alkyl, -N-
I (C1-C3) lower alkyl] 2, AL
-N N
SUBSTtTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-27-
wherein cycloalkyl is defined as C3-C6 cycloalkyl,
cyclohexenyl or cyclopentenyl; Rb is hydrogen; Ra is
independently selected from hydrogen, -CH3, -C2H5 or -
(CH2) qN (CH3) 2; Ar' is selected from the moieties:
R
R R9 R10
R Rs
I
Rs ~
w N
5
wherein q, X, Ra, Rb, R5, R7, R8, R9, R10, Rl1 and W'
are as hereinbefore described;
R12 and R13 are independently selected from hydrogen,
(Cl-C3) lower alkyl, halogen, amino, (Cl-C3) lower
alkoxy or (Cl-C3) lower alkylamino.
Also particularly preferred are compounds of
the formulae:
11 R
R12 - R R12 --
N / N
~
and d
N1-1*,(CH2)m 3
R13 3 R 13 (C 2 m R
R
wherein m is one or two;
R3 is the moiety:
0
- -CAr
wherein Ar is selected from the moieties:
SUBSTITUTE SHEET (RULE 26)
CA 02210631 2004-05-17
-28-
R'
R
R6 R1a
N
2
R R2
R6 is
R
l a Ia rarb
- NCOAr', - NCOCH2Ar', - NCCNAr',
R
Ia
- X R~ , - NCO- (CH2)n- cycl oal kyl
wherein cycloalkyl is defined as C3-C6 cycloalkyl, cyclohexenyl
or cyclopentenyl; Rb is hydrogen; Ra is independently selected
from hydrogen, -CH3, -C2H5 or -(CH2) qN (CH3) 2; and Ar' is selected
from the moieties:
R8 Ra
R5
R9
R9
w
wherein q, X, Ra, Rb, R5, R7, R8, R9, Rll, R14 and W' are as
hereinbefore defined;
R12 and R13 are independently selected from hydrogen, (C1-C3)
lower alkyl, halogen, amino, (C1-C3)lower alkoxy or (C1-C3)lower
alkylamino. Further examples of compounds according to the
present invention are N-[4-(4H-pyrazolo[5,1-
c][1,4]benzodiazepin-5(10H)yl-carbonyl)-3-chlorophenyl][1,1'-
biphenyl]-2-carboxamide; N-[4-(4H-pyrazolo[5,1-
CA 02210631 2004-05-17
- 28a -
c][1,4]benzodiazepin-5(lOHyl-carbonyl)phenyl][1,1'-biphenyl]-2-
carboxamide; N-[4-(4H-pyrazolo[5,1-c][1,4]benzodiazepin-
5(10H)yl-carbonyl)-3-chlorophenyl]-2-(dimethylamino)pyridine-3-
carboxamide; N-[5-(4H-pyrazolo[5,1-c][1,4]benzodiazepin-
5(10H)yl-carbonyl)-2-pyridinyl]-2(dimethylamino)pyridine-3-
carboxamide.
Compounds of this invention may be prepared as shown
in Scheme I by reaction of tricyclic derivatives of
Formula 3a and 3b with a substituted or unsubstituted 6-
nitropyridine-3-carbonyl chloride 4 to give the inter-
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-29-
mediates 5a, and 5,b. Reduction of the nitro group in
intermediates 5a and 5b. gives the 6-aminopyridine deri-
vatives 6a and 6b. The reduction of the nitro group in
intermediates 5a and 5b may be carried out under
catalytic reduction conditions (hydrogen-Pd/C; Pd/C-
hydrazine-ethanol) or under chemical reduction condi-
-tions (SnC12-ethanol; Zn-acetic acid TiC13) and related
reduction conditions known in the art for converting a
nitro group to an amino group. The conditions for con-
version of the nitro group to the amino group are chosen
on the basis of compatability with the preservation of
other functional groups in the molecule.
Reaction of compounds of Formula La and b
with aroyl chloride or related activated aryl carboxylic
acids in solvents such as chloroform, dichloromethane,
dioxane, tetrahydrofuran, toluene and the like in the
presence of a tertiary base such as triethylamine and
diisopropylethylamine or pyridine and the like, affords
the compunds $a and .$b, which are vasopressin antago-
nists.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCTIUS96/01076
-30-
Scheme 1 =
Y N /p\ Y N/p\E
- E 1
ZO F Z0 F
N N_(CH)m
m I (
H H
3a 3b
CI 0
' R2
R N
N02
4 y
Y- N iD E Y- N E
}=F
ZO F aN-(CH)m
m N 0
O
R' R2 R R2
N N
N02 NO 2
5a
! I .
= =
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-31-
Scheme 1 (cont'd)
Y` iD~ E Y-N~D~ E
N 1 _ 1
ZO F ZQ ~-F
O O
R~ R2 R1 R2
N N
NH2 NH2
fl
al kyl (C3- Ce)CCG AAC'~ al al kyl ken i (C - C C8C SO CI
SO
aikyl(c - C) aCOCI ~ y( 3 8) 2CI
3 8 cycl oal kyl (CH2)nCOCI R'
al kenyl (C3- C8)COCI Ar'NCOCI ~
al kenyi (C3- C8) OCOCI Z s4~a
2
(~ R
Y-N~ E Y-N~ E
Z 0 >-- F aN-(cl-6),, F
m N
O 0.
. R1 R2 R~ R 2 N N
- R6 6
R
$s~ $b
SUBSTITUTE SHEET (RUL.E 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-32--
R6= NHCCaAr' ;- NHCONAr' ;- NHCO(CH2),cycl oal ky ~
I
F~
- NHCOCH2Ar' ,- NHCOai kyi (C3-,C8), - NHCO2aI kyl (C3 C8),
- NHCOaI kenyl (C3- C$), - NHCO2aI kenyi (C3- C8),
- NHSO2ai kyl (C3- C8), - NHSO2ai kenyi (C3- C8),
R O R~
- NHSO2 q , ~ ~
NHP
2
2
2
Reaction of tricyclic derivatives of Formula
_Ea and j6h with either a carbamoyl derivative 2 or a
isocyanate derivative .1Q gives compounds (Scheme 2) of
formula 11a and 11h which are vasopressin antagonists of
Formula I wherein R6 is
- NHCCNAr '
I
F~
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-33-
Scheme 2
~ ~
~
9 CI-C-NAr'
or F~
O=C=NAr'
14
=
Y'N E Y N/p\ E
ZO F Z0 F
m N N-CH)
O O=
R' / R2 R1 / R2
N N
NHCOI Ar' NHCONA r'
iia llb
Reaction of tricyclic derivatives of Formula
La and _6h with arylacetic acids, activated as the acid
chlorides .12., anhydrides, mixed anhydrides or activated
with known activating reagents, gives compounds J_-ja and
13b (Scheme 3).
SUBSTITUTE SHEET (RU!_E 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-34-
Scheme 3
O
CI-C-CH2Ar'
Y-N~ E Y E
F
~ - N / ZO F aN-P%)m
( m N O
R~ / R2 R' ~ R2
~ N ~ N
NHCOCH2Ar' NHCOCH2Ar'
13a iat
The compounds of Formula I wherein Y, A-B, Z,
R1, R2 and R3 are as defined and the Ar moiety of R3
(-COAr) is
R2
~
R N X R7
may be prepared, as shown in Scheme 4, by reacting an
activated ester of the pyridine-3-carboxylic acid 1g
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-35-
with tricyclic derivatives 3a, and ~. The pyridine-3-
carboxylic acids 14 may be activated by preparing the
anhydride, a mixed anhydride or reacting with diethyl
cyanophosphonate, N,N-carbonyldiimidazole or related
peptide coupling reagents. Alternatively, the acid
chloride derivatives !a may be prepared from the acid
derivatives. 14 and oxalyl chloride or thionyl chloride
in an inert solvent. The solvent is removed and the
derivative reacted with 3a or 3b at 0 C to 25 C in
dichloromethane as solvent and a tertiary amine such as
triethylamine as a base. The activating reagent for the
pyridine-3-carboxylic acids 14 is chosen on the basis of
its compatibility with other substituent groups and the
reactivity of the activated derivat'ive toward the
tricyclic derivatives 3a and 3b to give the vasopressin
antagonists 16a and 16b.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-36-
Scheme 4
3a 3b
O
R
R2 N X- R'
14
O
R
CI
R N X RT
Y-N~D.:,
E Y-N.00,D~ E
_ I I
Z0 )-'F a ~F
m N N-
R1
R
O
R z
N X- R7 N X R7
16a 1rob
Alternatively, the compounds of Formula I
wherein Y, A-B, Z, R1, R2 and R3 are as defined and the Ar moiety of R3 (-
COAr) is
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-3~-
R
Rs
R2
N
wherein R6 is the moiety
-X-R7 and X is S, NH, NCH3
may be prepared as shown in Scheme 5 by first converting
tricyclic derivatives 3a and 3b into the intermediates
17 and 1 b and then reacting these intermediates with
potassium, sodium, or lithium anions (R7-X-) to give the
products 16a and 16b. The symbol M+ is a metal cation
derived from reacting a compound HXR7 with a metal
hydride (sodium or potassium hydride, for example) or
LDA, n-butyl lithium, lithium bis(trimethylsilyl)amide
and the like.
The reaction of intermediates 17 and 7 , with
the moieties R7-NH2 and R7-NHCH3 may also be carried
without first forming the corresponding anions. Thus,
heating intermediates 7. and 17b with excess R7-NH2 or
R7-NHCH3 in an inert solvent or without solvent gives
the products 1'i and 16b wherein X is NH or NCH3.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-38-
Scheme 5
3a 3b
CA O CI O
R1 R2 R
N R'
Rd Rd
Rd = CI, Br
D
Y-N E Y-.N E
*oL >---F Z Q >-F
m N N~(CH)m
R R1 R2
N R \ N
Rd Rd
Rd = CI, Br
17a ~
M+ X R7 M+ X R7
Y~ ZD~E Y- ZD~ E
N
ZO F ZC' rF
m N N-~~)m
_O O R1 R2 Ri R2
N
XR7 XR7
16a lu
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-39-
Alternatively, the intermediates 17a and 7~
may be converted to the more reactive fluoride deriva-
tives 18a and 18b as shown in Scheme _E. Reaction of the
fluoride intermediates 18a and 18b with amines NH2R7 and
CH3NHR7 gives the 6-aminonicotinoyl derivatives laa and
. .1.~~= .
SUBSTiTUTE SHEET (RUI_E 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-40-
Scheme 6
17a ~
KF KF
. = D~ iD~
Y~N~ E
QN
F
N N'(cH)m
2
#---N R~ R2
R N R
F F
18a R' ~
NH2R7 ; 4)
HN- R7
I k, R2
y C'H3
Y~D~ E Y-
N I
Z}=F Zo F
_( ~)m
N N
o
R1 R2 R~ R2
N
' R1
~ 7
XR
~ 6 19b
X=NH; X- R'_
NCH3 ; 2
R R
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-41-
As an alternative method for synthesis of
compounds of this invention as depicted in Formula I
wherein Y, A-B, D, E, F and Z are as previously
described and R3 is
R1
O
11
-CAr and Ar i s Rs
R2 /
N
is the coupling of pyridinyl carboxylic acids 2.2 with
the tricyclic derivatives 3a and 3J2 to give 2ja and 2JJ2.
The pyridine carboxylic acids are activated
for coupling by conversion to an acid chloride, bromide
or anhydride or by first reacting with an activating
reagent such as ZI,Il-dicyclocarbodiimide, diethyl
cyanophosphonate and related "peptide type" activating
reagents. The method of activating the acids 2,2 for
coupling to the tricyclic derivatives 3a and -~a is
chosen on the basis of compatibility with other
substituent groups in the molecule. The method of
choice is the conversion of the 3-pyridinyl carboxylic
acids 2_Q to the corresponding 3-pyridinylcarbonyl
chlorides. The 3-pyridinylcarbonyl chlorides 22- may be
prepared by standard procedures known in the art, such
as reaction with thionyl chloride, oxalyl chloride and
the like. The coupling reaction is carried out in
solvents such as halogenated hydrocarbons, toluene,
xylene, tetrahydrofuran, or dioxane in the presence of
pyridine or tertiary bases such as triethylamine and the
like (Scheme 7). Alternatively, the 3-pyridinyl-
carbonyl chlorides 2,2-, prepared from the carboxylic
acids 22, may be reacted with derivatives 3a and -3h in
pyridine with or without 4-(dimethylamino)pyridine.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96/01076
-42-
In general, when the 3-pyridinyl carboxylic
acids 20 are activated with "peptide type" activating
reagents, higher temperatures are required than when the
3-pyridinylcarbonyl chlorides are used.
Scheme 7
R 0 R
k \ )1_R6 - 11 ~ ~ s
CI C R
F-D N N
20 22 R
Y-N~D E Y-N E
z0 F
a F
( N ~ N ~"~ (`^~~ m
m
=p
1 / 2 R1 R2
R N R N
6 R6
21a 21b
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-43-
Scheme 8
CH2Br / D~ / p
O + HN E \E
2 F ~ --- F
N0
R15C=O NO2 C=0
I 15
R
2 5: R25=CH ; O- al ky I R15~
Pd/ C:H2 Pd/ C; H2
DkE Np'E
_
ZO ZO ~F
F N
NH2
CO R15 H
27 2
B2H4 Ar COCI
or LAH
D:
,D:
N E N E
F ZO F
Jr
Tr
0
p
Ar
The starting materials 3a and 3J2 in the foregoing
Schemes 1-7 may be prepared as follows. In accordance
with Scheme 8, alkylation of heterocycles of structural
type 24 with an alkylating moiety such as ,2,a gives
intermediates 2.a. The heterocycle 2A may contain an a-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/1JS96/01076
-44-
carboxaldehyde function or an a-carboxylic and/or ester function as shown in
Scheme 8. Where the intermediate
2.a (R15=H) contains an a-carboxaldehyde group,
hydrogenation with palladium-on-carbon gives reduction
.5 and ring closure in one step to give 2.
In derivatives 2a where R15 is an a-carboxylic
and/or an a-carboxylic ester function, the intermediate
amino acid derivative 27 is first isolated and then ring
closed. The ring closure of derivatives 27 may be
carried out by heating or by activation of the acid
function (27:R15=H) for ring closure. The cyclic
lactams 28 are conveniently reduced with diborane or
lithium aluminum hydride to give intermediates 2.
Reaction of tricyclic derivatives 2 with aroyl
chlorides (ArCOCl), where Ar is as hereinbefore defined,
gives diazepines 26.
Tricyclic derivatives of structural type 36
may be prepared as shown in Scheme 9. Formylation of U
under known conditions in the literature, such as
Vilsmeier formylation, gives intermediates 35, which on
reduction and ring closure affords tricyclics 37.
Where the ring containing the symbol Z is a
substituted or unsubstituted phenyl group, the procedure
gives 4,5-dihydropyrrolo[1,2-a]-quinoxalines ,36. These
derivatives 3-E and 37 may be reacted with aroyl
chlorides (ArCOCl) wherein Ar is as previously defined
or with a substituted or unsubstituted 6-nitropyridine-
3-carbonyl chloride or with a nitrogen protecting group,
such as benzyloxycarbonyl chloride to'give compounds 3$
and 39. The compounds ~3$ and 39, may be reacted with
chlorine, bromine or halogenating reagents such as N-
chlorosuccinimide, N-bromosuccinimide and the like to
give compounds 40 and 41 wherein R17 is a halogen atom. The derivatives 3$ and
39 may be formylated and
acetylated to give products 12 and 41 wherein R17 is a
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
= -45-
CHO or a -COCH3 group. Halogenation, formylation and
acetylation of derivative.s IL gives 1-substituted 4,5-
dihydropyrrolo[1,2-alquinoxalines. The derivatives
.3.g, .12 and _U wherein R16 is a substituted or unsubsti-
tuted 6-nitro-3-pyridinylcarbonyl group are reduced to
give the 6-amino-3-pyridinylcarbonyl derivatives A2,d and
43d which are reacted with reagents Ar'COC1, Ar'CH2COC1
or
Ar'- NCOCI
F~
wherein Ar' and Rb are as previously hereinbefore
defined, to give tricyclic diazepines ~4. and ~.
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTNS96/01076
-4 6-
Scheme 9
H 2
Z O o CH3
0 ~~ -~- Z p ~
o.-- ~
2 2
~ ~ 32
B
N
H
-
hal ogen g- i
Z ~ CN'\ ZO
~2 ~ G-O
H NO2
34
ZQ = phenyl
. ~
N / N /
ZO
H H
A r COCI 37
0
II
P hCH2OC- CI
= ~
O2N COCI
N-
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-47-
Scheme 9 (cant'dl
= R,s ArCO
N ~ 0 N /
a ~1
N R16=Ph-CF-120C Z~
~
N
R1s R R 16
R, 6= NO2 00
~ N
2
R
Reagent 5.
R R 17
halogen
N (CH3)2N=Ca-ICI N
O
N Ci_ N
R16 CF-~CCCI :FeCI 3 h16
4 0 R 16 41
17 hal ogen,CHO a) ArCO R17
R hai ogen,CHO
- COCH3 b) Ph- Ct-tzCO - COCH
1 3
R
c ) NO2 COCI
N
R2
R 1 =
~c [H] ~ R1s _ NH 2 CO 43 [H] 41 c
N
R2
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-48-
Scheme 9 (cont'd)
42 43
Reagent
A r'OOCI
A r' Cq OOCJ
A r' NCOCI
Ia
R'7 R~7
aR1 Z O 1
N N R
O j \ Rs 0 R6
N N
2
44 Rs 45 2
Ar'CCNH
Ar'CH2CCNH
Ar'-N-CCNH
RO
The compounds of this invention wherein R3 is
the moiety:
}
0
11 =
-CAr
and the Ar group is the moiety:
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/1JS96/01076
-4 9-
R
Rs
N
R2
and R6, Ra, Rb, Y, R1, R2, Z and Ar' are as previously
defined and wherein R11 is selected from the moieties:
- CH2N,,- Rb - CH2N - CH2N
"-~Fb
- CH2 N \-/ 0 CH2- N
- CH2N
~ , - CH2 NN- R4 and
N
- CH2N 0
may be synthesized as shown in Scheme 10.
The tricyclic pyrrolodiazepines 46 and 47 are
reacted with appropriate amines in the presence of
formaldehyde to give the aminomethylene derivatives 48
and 49. The reaction may be carried out with aqueous
formaldehyde or its equivalent in the presence of the
appropriate amine in a lower alkanol at room temperature
or preferably at temperatures of 50 C-100 C. The
aminomethylene derivatives 48 and Al may be converted to
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-50-
hydrochloride salts or succinic acid and maleic acid
salts as well as other pharmaceutically acceptable acid
salts.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-51-
Scheme 10
Y- N Y N
ZO ~O
N R N H2) m
~C 2 ~
R
R O / \ 6
rN R
2 N
R
46 4.7 2
CH2O
+ NH~~'
R b
HN HN
~\ r F~ ~
HN O et c .
Y-N
Y--N
ZO
Z O N 2) m
N R
R
~C C 2 m
O ~ ~ Rs O Rs
N N
4 R2 49 R2
= R11=CH2NR' , CH2N~ -CH2N
R b
CH2N% et c.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96/01076
-52-
Scheme 11
Y1N iD= E Y`N E
F Z O )--- F
Z O
m
N N~(cH) m
1 1
H H
CI O
R1 R 2
R14
Y-N'
ZQ E Y--N E
F ZO ~F
m N N-(CH) m
0 p
R1 R2 R1 ~ R2
14 14
R R
52
I I =
= y
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
- -53-
As shown in Scheme 11, reaction of tricyclic
derivatives of Formula 3a and _~h with substituted and
= unsubstituted arylcarbonyl chlorides 50, wherein R1, R2
and R14 are hereinbefore defined gives compounds 5-1- and
_~2 which are vasopressin antagonists.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-54-
Scheme 12
E --N I E
y--N y
~
zo F ZO F
. . ~
m
N \N~(CH)
~ I .
H H
3a 3b
CI
R~ R2
I\D 2
53
iD.:,~~ .1-11
Y-N E Y N. E
zo F zo F
CH_
~m
m N (
=o 0
R~ R2
R2
ND 2 ND 2
~ 54b
r
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-55-
Scheme 12 (cont' i
Y- E Y- N~D\ E
N I 1
ZO F a ~=F
( m N N_/
(c;N)m
=o 0
R1 R2 R1 R2
\ \
NH2 NH2
55a 55b
R25COCi
57
Y- N E Y-N~ E
Zo ~F a F
m N N P'O m
0 O
R1 R2 R1
R 2
. \ ~
w - I 14
R R 14
56a 56b
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-56-
Reaction of tricyclic derivatives of Formula
3a and 3b with a substituted or unsubstituted phenyl
carbonyl chloride 5 gives intermediates 54a and 54b.
The reduction of the nitro group in intermediates 54a
and 5~4 may be carried out under catalytic reduction
conditions (hydrogen-Pd/C; Pd/C-hydrazine-ethanol) or
under chemical reduction conditions (SnC12-ethanol; Zn-
acetic acid TiC13) and related reduction conditions
known in the art for converting a nitro group to an
amino group. The conditions for conversion of the nitro
group to the amino group are chosen on the basis of
compatability with the preservation of other functional.
groups in the molecule.
Reaction of compounds of Formula 55a and 55t
with acid chlorides, R25C0C1 or related activated acid
carboxylic acids in solvents such as chloroform,
dichloromethane, dioxane, tetrahydrofuran, toluene and
the like in the presence of a tertiary base such as
triethylamine and diisopropylethylamine or pyridine and
the like, affords the compounds 56a and 5&1~- which are
vasopressin antagonists.
The acid chlorides R25COC1 are those wherein
R25 is selected from the group
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-57-
S02N~
Rb
o
- ' ~ R10 (CH2)n
I
R8/R10
/ ~
R~ R
,
N S R'
O
_ (CH2 n (CH2 n R20
N
R20
Wherein n is 0 or 1; Ra is hydrogen, -CH3 or -
C2H5; R' is hydrogen, (C1-C3)lower alkyl,' (C1-C3)lower
alkoxy and halogen; R20 is hydrogen, halogen, (C1-C3)-
lower alkyl, (C1-C3)lower alkoxy, NH2, -NH(C1-C3)-lower
alkyl, -N-[(C1-C3)lower alkyl]2,
R . .
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-58-
-N _N
,
- N \-~ O
/-\
- N\~~ N- I ow er al kyl (Cl - C) - NH- (CH2)p NHi ower al kyl (C1- C) - NH-
(CH2)p N[I ower al kyl (Ci- C3)]2
- NH- (CH2)P- N - NH- (CH2)p N
-NH- (CH2)P- NN- I ower al kyl (Cl- C3) ,
Ra R R1
- NH- (CH2) p NO /\
-N- CO-
KO
Preparation of some tricyclic diazepines
useful for starting materials for the synthesis of
compounds of this invention are shown in Schemes 8 and
9. Other tricyclic diazepines are prepared by
literature procedures or by methods known in the art or
by procedures reported for the synthesis of specific
known tricyclic diazepines. These diazepine ring systems discussed below when
subjected to reaction
conditions shown in Schemes 1, 2, 3, 4, 5, 6, 7, 9 and
10 give the compounds of this invention.'
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-59-
The tricyclic diazepine ring system, 10,11-
dihydro-5H-imidazo[2,1-c][1,4]benzodiazepine,
N
N
is reported by G. Stefancich, R. Silvestri and M.
Artico, 1. Het. Chem. 30, 529(1993); ring substitution
on the same ring system is reported by G. Stefancich, M.
Artico, F. Carelli, R. Silvestri, G. deFeo, G. Mazzanti,
I. Durando, M. Palmery, IL Farmaco, E~j. Sc., 44,
429(1985).
Y / N \
X=H, - OCI-t3, - OCH2CsH5 ; Y-H,
X N
I CI, -OCH3 ; X,Y=O-CH2-Oa
H
The synthesis of 9,10-dihydro-4H-furoj2,3-e]-
pyrrolo[1,2-a][1,4]diazepin-9-one
N
N O
H
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-60-
is reported by F. Povazunec, B. Decroix and J. Morel, T. ,
Het. Chem. 2, 1507(1992) and is reduced to give the
tricyclic heterocycle 9,10-dihydro-4H-furo[2,3- t
e]pyrrolo[1,2-a][1,4]diazepine.
N
N
H
The tricyclic 5,10-dihydro-4H-pyrazolo[5,1-c][1,4]benzo-
diazepine ring system is reported by L. Cecchi and G.
Filacchioni, Chem., 2Q, 871(1983);
N
N
N
The synthesis of 9-oxo-9,10-dihydro-4H-pyrrolo[1,2-a]-
thieno[2,3-e][1,4]diazepine is reported by A. Daich and
B. Decroix, Bull. Soc. Chim. Fr 129, 360(1992);
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-61-
N
S N O
I
H
and is reduced with boron-dimethylsulfide to give 9,10-
dihydro-4H-pyrrolo[1,2-a]thieno[2,3-e][1,4]diazepine.
N
. / \
S N
H
Also reported by A. Daich and B. Decroix is 5-oxo-4,5-
dihydropyrrolo[1,2-a]thieno[3,2-e][1,4]diazepine
S
N
N
1 o
H
which is also reduced to give 4,10-dihydro-5H-pyrrolo-
[1,2-a]thieno[3,2-e][1,4]diazepine
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-62-
S
N
N
Reported by B. Decroix and J. Morel, E. Chem., 28,
81 (1991) are 5H-pyrrolo [ 1, 2-a] thierio [3, 2-e] [1, 4] diaze-
pine;
N
S
N 'I
N
N- S
S N \
N
and 4a-pyrrolo[1,2-a]thieno[2,3-e][1,4]diazepi.ne. The
10H-pyrrolo[1,2-a]thieno[3,4-a][1,4]diazepine is
reported by A. Daich, J. Morel and B. Decroix, J,
Heterocyclic Chem., 31, 41(1994). Reduction by
hydrogen-Pd/C or chemical reduction with reagents such
as sodium cyanoborohydride and acetic acid gives the
dihydro tricyclic heterocycles
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-63-
.+
S
N N
-- ~ S
N- N-
H H
S N
H
The synthesis of the tricyclic 1,5-benzodiazepine ring
system, 6,7-dihydro-5H-pyrrolo[1,2-a][1,5]benzodiaze-
pine, has been reported by F. Chimenti, S. Vomero, R.
Giuliano and M. Artico, IL Farmaco,
339(1977). Annelated 1,5-benzodiazepines containing
five membered rings have been reviewed by A. Chimirri,
R. Gitto, S. Grasso, A.M. Monforte, G. Romeo and M.
Zappala, Heterocycles, 3-E, No. 3, 604(1993), and the
ring system 6,7-dihydro-5H-pyrrolo[1,2-a][1,5]benzo-
diazepine is described.
N
N
. I .
H
The preparation of 5,6-dihydro-4H-[1,2,4]-
` triazolo[4,3-a][1,5]benzodiazepin-5-ones from 1,2-
dihydro-3H-4-dimethylamino-1,5-benzodiazepin-2-ones has
been described by M. DiBroccio, G. Roma, G. Grossi, M.
Ghia, and F. Mattioli Fur. J. Med. Chem; a, 489(1991).
Reduction of 5,6-dihydro-4H-[1,2,4]triazolo[4,3-a]-
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-64-
[1,5]benzodiazepin-5-ones with diborane or lithium
hydride gives the tricyclic 5,6-dihydro derivatives.
R21 R21
~N\N ~N
N N /
\ I \ ( .
N N
I o I
H H
R21 = H, CH3
The compounds of this invention and their
preparation can be understood further by the following
examples, but should not constitute a limitation
thereof.
Reference Examplg 1
1-(2-Nitrophenyl)-1H-pvrrole-2-carboxaldehyde
To a solution of 3.76 g of 1-(2-nitro-
phenyl)pyrrole in 20 ml of N,N-dimethylformamide at 0 C
is added dropwise with stirring 3 ml of phosphorus
oxychloride. Stirring is continued for 30 minutes and
the reaction mixture is heated at 90 C for 1 hour.
After cooling to room temperature the mixture is treated
with crushed ice and the pH adjusted to 12 with 2 N
sodium hydroxide. The resulting suspension is filtered,
washed with water and dried to give 5.81 g of the
desired product as a light yellow solid, m.p. 119 -
122 C.
Reference Example 2
4 5-Dihydro-byrrolo-i1,2-al-quinoxalin
To a solution of 1.0 g of 1-(2-nitrophenyl)-
1H-pyrrole-2-carboxaldehyde in 40 ml of ethyl alcohol
and 40 ml of ethyl acetate, under argon, is added 40 mg
of 10% Pd/C. The mixture is hydrogenated at 40 psi for
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT1US96/01076
-65-
2 hours and filtered through diatomaceous earth. The
filtrate is concentrated in vacuo to a residue which is
dissolved in ether and treated with hexanes to give
0.35 g of the desired product as a beige solid, m.p.
108 -110 C.
Reference Example 3
N-(2-Nitrobenzoyl)pyrrole-2-carboxaldehyde
To an ice bath cooled solution of 5.6 g of 2-
pyrrolecarboxaldehyde in 40 ml of tetrahydrofuran is
added 2.4 g of 60% sodium hydride in mineral oil. The
temperature elevates to 40 C. After stirring for 20
minutes a solution of 11.0 g of 2-nitrobenzoyl chloride
in 20 ml of tetrahydrofuran is added dropwise over 20
minutes. After stirring in the cold for 45 minutes, the
reaction mixture is poured into ice water and ether then
filtered. The cake is washed with additional ether.
The two phase filtrate is separated and the ether layer
dried and concentrated jn vacuo to give 10 g of a
residue as a dark syrup which is scratched with ethanol
to give crystals which are collected by filtration,
washed with ether and then dried to afford 3.2 g of
solid, m.p. 95-99 C.
Reference Example 4
10,11-Dihvdro-5H-pyrroloi2,1-clfl,4lbenzodiazepin-5-one
A mixture of 1.5 g of N-(2-nitrobenzoyl)-
pyrrole-2-carboxaldehyde in 50 ml of ethyl acetate, 2
drops of concentrated HCl and 0.3 g of 10% Pd/C is
shaken in a Parr apparatus under hydrogen pressure for
1.75 hours. The mixture is filtered, 0.4 g of 10% Pd/C
ti 30 added and the mixture shaken in a Parr apparatus under
hydrogen pressure for 2 hours. The reaction mixture is
filtered through diatomaceous earth and the filtrate
concentrated in vacuo to give 1.0 g of a yellow oil.
The residue is purified on thick layer chromatography
plates by elution with 4:1 ethyl acetate:hexane to give
107 mg of the desired product as an oily solid.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96/01076
-66-
Reference Example 5
1-12-Nitrobenzyll-2-pyrrolecarboxaldehyde
To 5.56 g of 60% sodium hydride in mineral
oil, washed three times with hexane, is added 300 ml of
N,N-dimethylformamide under argon. The reaction mixture
is cooled in an ice-bath and 13.2 g of pyrrole-2-
carboxaldehyde is added slowly. The reaction mixture
becomes a complete solution and is stirred for an
additional 10 minutes. While stirring, 30.0 g of 2-
nitrobenzyl bromide is added slowly. After complete
addition, the reaction mixture is stirred for 30
minutes, the ice bath is removed and the reaction
mixture stirred at room tempez<ature for 24 hours. The
N,N-dimethylformamide is concentrated in vacuo to give a
residue which is stirred with ice water for 1 hour. The
resulting solid is collected, air dried, then vacuum
dried to give 30.64 g of the desired product as a tan
solid, m.p. 128-132 C.
Reference Example 6
10 11-Dihydro-5H-pyrrolof2 1-clfl 4lbenzodiazepine
A mixture of 30.6 g of 1-=(2-nitrobenzyl)-2-
pyrrolecarboxaldehyde and 3.06 g of 10% Pd/C in 400 ml
of ethyl acetate and 400 ml of ethyl alcohol is hydro-
genated over 18 hours. The reaction mixture is filtered
through diatomaceous earth and the filtrate is treated
with activated carbon and filtered through diatomaceous
earth. The filtrate is concentrated in vacuo to give a
residue which is dissolved in methylene chloride con-
taining ethyl alcohol. The solution is passed through a
pad of silica gel and the pad washed with a 7:1 hexane-
ethyl acetate solution to give 16.31 g of the desired
product as solid, m.p. 145-148 C.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-67-
Reference Example 7
3-Methylbenzorblthiophene-2-acetyl chlorid-
A mixture of 2.0 g of 3-methylbenzo[b]-
thiophene-2-acetic acid and 19.4 ml of thionyl chloride
5.is heated at reflux for 1 hour. The volatiles are
evaporated in vacuo to give a residue which is con-
centrated from toluene three times and dried under
vacuum to give 2.25 g of the desired product as a
residue.
Reference Example 8
4-Chloro-2-methoxybenzoyl chloride
A solution of 2.0 g of 4-chloro-,Q-anisic acid
in 22 ml of thionyl chloride is heated at reflux for 1
hour. The volatiles are evaporated in vacuo to give a
residue which is concentrated from toluene three times
and dried under vacuum to give 2.0 g of the desired
product as a residue.
Reference Example 9
2- (Trifluoromethyl) benzoy_l chloride
A solution of 2.0 g of o-trifluoromethyl-
benzoic acid in 21 ml of thionyl chloride is heated at
reflux for 1 hour. The volatiles are evaporated in
vacuo to give a residue which is concentrated from
toluene three times and dried under vacuum to give 2.1 g
of the desired product as a residue.
Reference Example 10
2-Methylphenylacetyl chloride
A solution of 2.0 g of o-tolylacetic acid in
27 ml of thionyl chloride is heated at reflux for 1
hour. The volatiles are evaporated in vacuo to give a
residue which is concentrated from toluene three times
and dried under=vacuum to give 2.1 g of the desired
product as a light brown oil.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT1US96/01076
-68-
Reference Example1l
3-Methyl-4-n' r -b nzovl chloride
A mixture of 1.81 g of 3-methyl-4-nitrobenzoic
acid and 1.25 g of thionyl chloride in 75 ml of chloro-
form is heated at reflux under argon for 48 hours. The
volatiles are removed in vacuo to a residue which is
evaporated with toluene several times in'vacuo. The
residue is partially dissolved in methylene chloride and
filtered free of solids and the filtrate evaporated j_n
vacuo to give 1.47 g of the desired acid chloride.
Reference Example 12
1-(o-Nitrobenzyl)-imidazole-2-carboxaldehyde
A 2.0 g portion of sodium hydride (60% in oil)
is washed with pentane two times. To the residue is
added 110 ml of N,N-dimethylformamide under argon. With
stirring and external cooling, 4.80 g of 2-imidazole-
carboxaldehyde is added and the cooling bath removed.
Slight external heating results in a yellow solution.
The reaction mixture is chilled in ice and 10.8 g of 2-
nitrobenzyl bromide is added. The reaction mixture is
stirred at 0 C for 18 hours. The volatiles are removed
.jZ vacuo to a residue which is stirred with ice waterr,
filtered and the cake washed well with water and suction
dried to give 10.9 g of the desired product as a solid,
m.p. 141-144 C. MH+ 232.
Reference Example 13
10 11-Dihydro-5H-imidazof2 1-clfl 41b nzodiazepine
A 5.0 g sample of"1-(o-nitrobenzyl)-imidazole-
2-carboxaldehyde is dissolved in 150 ml of hot ethyl
alcohol, cooled to room temperature and filtered. To
the filtrate is added 0.5 g of 10% Pd/C and the mixture
hydrogenated at 48 psi for 4 hours. An additional 0.5 g
of 10% Pd/C is added and hydrogenation continued for 25 hours at 65 psi. The
mixture is filtered through
diatomaceous earth and the cake washed with ethyl
acetate. The filtrate is evaporated in vacuo to a
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-69-
residue which is dissolved in methylene chloride,
treated with activated carbon, filtered through
diatomaceous earth and hexanes added to the filtrate at
the boil to give 1.86 g of the desired product as a
.5 crystalline solid, m.p. 164-170 C.
Reference Example 14
10,11-Dihydro-5H-imidazo(2,1-clt1..41benzodiazepine
To a suspension of 4 mmol of lithium aluminum
hydride in 20 ml of anhydrous tetrahydrofuran is added a
1 mmol solution of 10,11-dihydro-11-oxo-5H-imidazo-
[2,1-c][1,4]benzodiazepine and the mixture is refluxed
for 24 hours and cooled at 0 C. To the mixture is added
dropwise 0.12 ml of water and 6 ml of 1 N sodium
hydroxide. The mixture is extracted with ethyl acetate
and the solvent removed to give the desired product as a
solid. Recrystallization from methylene chloride-hexane
gives crystals, m.p. 164-170 C.
Reference Example 15
9,10-Dihydro-4H-furof2,3-elpyrrolofl,2-alf1,41diazepine
To a suspension of 4 mmol of lithium aluminum
hydride in 25 ml of anhydrous tetrahydrofuran is added 1
mmol of 9,10-dihydro-4H-furo[2,3-e]pyrrolo[1,2-a][1,4]-
diazepin-9-one. The mixture is refluxed for 12 hours
and allowed to stand overnight. To the mixture is added
dropwise 0.12 ml of water and then 6 ml of 1 N sodium
hydroxide. The mixture is extracted with ethyl acetate
and the extract dried (Na2SO4). The volatiles are
removed in vacuo to give the desired product as a solid.
Reference Example 16
9,10-Dihydro-4H-furof2,3-elpyrrolorl,2-a1r1,41diazepine
A solution of 1 mmol of 4H-furo[2,3-e]pyrrolo-
[1,2-a][1,4]diazepine and 0.2 g of 10% Pd/C in 10 ml of
ethanol is hydrogenated for 18 hours. The reaction
mixture is filtered through diatomaceous earth and the
filtrate is evaporated in vacuo to give the desired
product as a solid.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96101076
-70-
Reference Examp le 17
9.10-Dihydro-4H-pvrrolofl 2-althienof 3-el-
f1,41diazepine
To a mixture of 7.0 g of 9-oxo-9,10-dihydro-
4H-pyrrolo[1,2-a]thieno[2,3-e][1,4]diazepin in 25 ml of
anhydrous tetrahydrofuran is added 9 ml of 10 molar
boron-dimethylsulfide in tetrahydrofuran. The mixture
is refluxed for 6 hours. The solution is cooled to room
temperature and 25 ml of methanol added dropwise. The
volatiles are removed under vacuum. To the residue is
added 100 ml of 2 N NaOH. The mixture is refluxed 5
hours and filtered. The solid is extracted with di-
chloromethane and the extract is washed with 2 N citric
acid, water and dried (Na2SO4). The solvent is removed
'.~n vacuo to give the desired product as a solid.
Reference Example 18
4,10-Dihydro-5H-pyrrolofl 2-althienof3 2-el-
f1.41diazepine
To a suspension of 7.0 g of 5-oxo-4,5-dihydro-
pyrrolo[1,2-a]thieno[3,2-e][1,4]diazepine in 25 ml of
anhydrous tetrahydrofuran is added 9 ml of 10 M borane-
dimethylsulfide in tetrahydrofuran. The mixture is
refluxed for 6 hours. The solution is cooled to room
temperature and 25 ml of methanol added dropwise. The
volatiles are removed under vacuum. To the residue is
added 100 ml of 2 N NaOH. The mixture is refluxed 5
hours and filtered. The solid is extracted with di-
chloromethane and the extract is washed with 2 N citric
acid, water and dried (Na2SO4). The solvent is removed
to give a solid.
Reference Example 19 5 6-Dihydro-4H-fl 2 4ltriazolof4 3-=alfl 51benzodiazepine
A mixture of 7.0 g of 5,6-dihydro-4H-[1,2,4]-
triazolo-[4,3-a][1,5]benzodiazepin-5-one in 25 ml of
tetrahydrofuran is added 9 ml of 10 M borane-
dimethylsulfide in tetrahydrofuran. The mixture is
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTlUS96/01076
-71-
refluxed for 6 hours, cooled to room temperature and 25
ml of methanol added dropwise. The volatiles are
removed under vacuum and to the residue is added 100 ml
of 2 N sodium hydroxide. The mixture is refluxed for 5
hours, chilled and extracted with dichloromethane. The
extract is washed with 2 N citric acid, water and dried
(Na2SO4). The solvent is removed under vacuum to give a
solid. The solid is purified by chromatography on
silica gel to give the desired product.
Reference Example 20
1-(2-Nitrophenyl)-1H-pyrrole-2-carboxaldehyde
A sample of 4.7 g of sodium hydride (60% in
oil) is washed with hexane (under argon). To the sodium
hydride is added 200 ml of dry N,N-dimethylformamide and
the mixture is chilled to 0 C. To the mixture is added
10.11 g of pyrrole-2-carboxaldehyde in small portions.
The mixture is stirred 10 minutes arid 15.0 g of 1-
fluoro-2-nitrobenzene added dropwise. After the addi-
tion, the mixture is stirred at room temperature 16
hours and the mixture concentrated (65 C) under high
vacuum. To the residue is added 400 ml of dichloro-
methane and the mixture washed with 150 ml each of H20,
brine and dried (Na2SO4). The solvent is removed in
vacuo to give a yellow solid. Crystallization from
ethyl acetate-hexane (9:1) gives 17.0 g of light yellow
crystals, m.p. 119 -122 C.
Reference Example 21
4 10-Dihydro-5H-pyrrolofl,2-althienoF3,2-el-
f1,41diazepine
To an ice cooled mixture of 2.1 g of pyrrole-
2-carboxylic acid and 2.3 g of methyl 3-aminothiophene-
2-carboxylate in 40 ml of dry dichloromethane is added 4
g of N,N-dicyclohexylcarbodiimide. The mixture is
stirred at room temperature for 3 hours-and filtered.
The filter cake is washed with dichloromethane and then
extracted twice with 60 ml of acetone. The acetone
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/1JS96/01076
-72-
extract is concentrated to dryness to give 0.8 g of
solid, m.p. 214-218 C. To a suspension of the preceding
compound (1.19 g) in 20 ml of dry tetrahydrofuran is
added 0.2 g of sodium hydride (60% in oil). After the
hydrogen evolution, the mixture is stirred and refluxed
for 4.5 hours, cooled and poured into ice-water. The
precipitated solid is filtered and the solid triturated
with petroleum ether (bp 30-60 C) to give 0.75 g of
4,10-dihydro-4,10-dioxo-5H-pyrrolo-[1,2-a]thieno[3,2-
e] [ 1, 4] diazepine as a solid, m.p. 280-290 C. The
preceding compound (0.362 g) is added to an ice-water
cooled solution of 1 M diborane in tetrahydrofuran. The
mixture is stirred at room temperature for 65 hours.
The solution is concentrated to dryness and ice-water
added to the residue. The mixture is acidified with
dilute HC1, stirred and then basified with solid NaHCO3.
The mixture is filtered to give 0.223 g of a solid
(foam) m.p. 80-85 C.
Reference Example 22
10,11-Dihydro-5H-1,2,4-triazolof3,4- 1-
l1,41benzodiazepine
A mixture of 2.2 g of 2-cyanoaniline, 2.0 g of
methyl bromoacetate and 1.3 g of potassium carbonate in
12 ml of dry N,N-dimethylformamide is heated at 150-
155 C for 40 minutes. The cooled mixture is poured into
ice-water and the mixture filtered to give 2 g of methyl
[N-(2-cyanophenyl)amino]acetate as a yellow solid, m.p.
70-78 C. The preceding compound (2.0 g) is added to a
solution of 0.5 g of sodium methoxide in 50 ml of
methanol. The mixture is shaken under an atmosphere of
hydrogen with the catalyst Raney-Ni for 19 hours. The
mixture is filtered through diatomaceous earth and the
filtrate evaporated. Water is added to the residue and
the mixture filtered to give 2,3,4,5-tetrahydro-lH-1,4-
benzodiazepin-3-one as a yellow solid, m.p. 167-170 C.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-73-
A mixture of the preceding compound (1.6 g)
and 0.84 g of phosphorus pentasulfide in 10 ml of dry
(dried over KOH) pyridine is stirred and heated at 80-
85 C for 15 minutes. The mixture is poured into water
and stirred for 30 minutes. Filtration gives 1.0 g of
1,2,4,5-tetrahydro-3H-1,4-benzodiazepin-3-thione as
yellow solid, m.p. 150-153 C.
The preceding compound (0.5 g) and 0.5 g of N-
formylhydrazine in 6 ml of dry n-butanol is refluxed for
16 hours and the solvent removed. The gummy residue is
triturated with cold water and the mixture filtered.
The solid is triturated with acetone to give 0.19 g of
yellow solid, m.p. 232-237 C.
Reference Example 23
4 5-Dihydro-6H-f1,2,41triazol.of4,3-alf1,51-
benzodiazepine
A mixture of 2,3,4,5-tetrahydro-lli-1,5-benzo-
diazepin-2-thione (0.8 g) and 0.80 g of N-formyl-
hydrazine in 8 ml of n-butanol is stirred and refluxed
for 18 hours and the solvent removed under vacuum. Ice
water is added to the residual solid and the mixture
filtered to give 0.312 g of a gray solid, m.p. 162-
165 C .
Reference Example 24
4,5-Dihydro-6H-imidazofl,2-a1f1,51benzodiazepine
A mixture of 30 g of acrylic acid, 33 g of Q-
phenylenediamine is heated on a steam bath for 1.5 hours
and the cooled black mixture triturated with ice-water.
The aqueous phase is decanted and ice and aqueous
ammonium hydroxide added to.the residue. The mixture is
=
extracted with dichloromethane and the extract concen-
trated to dryness. The residue is triturated with
carbon tetrachloride and filtered. The oily solid is
triturated with a small amount of ethanol to give 9.7 g
of a solid. Trituration of the solid with ethyl acetate
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-74-
gives 2,3,4,5-tetrahydro-lij-1,5-benzodiazepin-2-one as
an impure solid, m.p. 75-107 C.
A mixture of the preceding compound (11.3 g)
and 5.9 g of phosphorus pentasulfide in 70 ml of dry
pyridine is stirred and heated at approximately 80 C for
20 minutes. The mixture is poured into water and the
mixture stirred for 30 minutes. Filtration gives 8.6 g
of 2,3,4,5-tetrahydro-lFi-1,5-benzodiazepin-2-thione as a
solid, m.p. 154-157 C.
A mixture of the preceding compound (0.70 g),
1.0 g of aminoacetaldehyde dimethyl acetal and 15 mg of
4-methylbenzenesulfonic acid monohydrate in 6 ml of dry
n-butanol is refluxed for 4 hours and the solvent
removed under vacuum. The residue is heated (refluxed)
with 10 ml of 3 N hydrochloric acid for 55 minutes. Ice
is added to the cooled mixture and the mixture made
basic with solid NaHCO3. The mixture is extracted with
dichioromethane and the extract dried (Na2SO4). The
solvent is removed to give an orange syrup which solidi-
fied on standing. The oily solid is triturated with
acetone to give a light yellow solid (0.185 g) m.p. 119-
122 C .
Reference Example 25
1-(2-Nitrophenyl)-2-pyrroleacetic acid, ethyl ester
To a stirred mixture of 1.88 g of 1-(2-
nitrophenyl)pyrrole, 4.80 g of ethyl iodoacetate and
2.22 g of FeSO4.7H20 in 40 ml of dimethyl sulfoxide is
added dropwise 10 ml of 30% hydrogen peroxide while
keeping the reaction mixture at room temperature with a
cold water bath. The mixture is stirred at room
temperature for one day. An additional 2.4 g of ethyl
iodoacetate, 1.1 g of FeSO4.7H20 and 5 ml of 30%
hydrogen peroxide is added and the mixture stirred at
room temperature for 1 day. The mixture is diluted with
water and extracted with diethyl ether. The organic
extract is washed with water, brine and dried (Na2SO4).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96101076
-75-
The solvent is removed and the residue (2.12 g) chroma-
tographed on silica gel with ethyl acetate-hexane (1:4)
as solvent to give 0.30 g of product as a brown gum.
Reference Example 26
6,7-Dihydro-5H-pvrrolofl.2-alf1,51benzodiazepin-6-one
To a solution of 0.8 mmol of 1-(2-nitro-
phenyl)-2-pyrroleacetic acid, ethyl ester in 3 ml of
ethanol is added stannus chloride dihydrate (SnC12.2H20)
in 2 ml of concentrated hydrochloric acid (with cooling
in water bath). The mixture is stirred at room tempera-
ture for 5 hours and chilled in an ice bath. To the
mixture is added slowly saturated sodium carbonate
solution. The solid which precipitates is filtered and
the solid washed with water and then extracted with
ethyl acetate. The ethyl acetate extract is dried
(Na2SO4) and the solvent removed to give 0.16 g of solid
which is triturated with ether to give 0.11 g of product
as an off-white solid.
Reference Example 27
6,7-Dihydro-5H-pyrrolorl,2-a1r1,51benzodiazepine
To a solution of 0.070 g of 6,7-dihydro-5ii-
pyrrolo[1,2-a][1,5]benzodiazepin-6-one in 2 ml of
tetrahydrofuran is added 0.45 ml of a 2.0 M solution of
diborane-dimethylsulfide in tetrahydrofuran. The
mixture is refluxed for 3 hours, poured into water and
made basic with 2 N NaOH. The tetrahydrofuran is
removed under vacuum and the residual aqueous mixture
extracted with diethyl ether. The extract is washed
with brine, dried (Na2SO4) and the solvent removed to
give 0.065 g ofa colorless oil; one spot by thin layer
chromatography (silica gel) with ethyl acetate-hexane
(1:2) as solvent (Rf 0.81).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-76-
Reference Example?
1- f 2-Nitro-5- (ethoxycarbonyl) benzyll -pyrrole-2-
carbox ldehyde
To a stirred slurry of 2.2 g of sodium hydride
(60% in oil, washed with hexane) in tetrahydrofuran is
added at 0 C a solution of 4.5 g of pyrrole-2-carbox-
aldehyde in 25 ml of tetrahydrofuran. After the addi-
tion is complete, a solution of 15 g of ethyl 4-nitro-3-
bromomethylbenzoate in 30 ml of dry tetrahydrofuran is
slowly added under nitrogen. The reaction mixture is
stirred at 20 C for 8 hours and carefully quenched with
water. The reaction mixture is extracted with chloro-
form which is washed with water, dried with Na2SO4 and
concentrated in vacuo to give 12 g of the desired
product as a solid; mass spectrum (M+H)349.
Reference Example 29
1-f2-Nitro-4-(ethoxycarbonyl)benzyll-pyrrole-2-
carboxaldehyde
The conditions of Example 28 are used with
ethyl 3-nitro-4-bromomethylbenzoate to give 13.0 g of
the desired product as a solid; mass spectrum (M+H)349.
Reference Example 30
Ethyl 10,11-Dihydro-5H-pyrrolof2,1-clf1,41-
benzodiazepine-7-carboxylate
A solution of 10.0 g of 1-[2-nitro-5-(ethoxy-
carbonyl)benzyl]-pyrrole-2-carboxaldehyde in 150 ml of
absolute ethanol containing 1.0 g of 10% Pd/C is
hydrogenated in a Parr apparatus for 16 hours under 40
psi of hydrogen. The reaction mixture is filtered
through a pad of diatomaceous earth and the filtrate concentrated in vacuo to
a residue of 5.5 g of the
desired product as a solid; mass spectrum (M+H)255.
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-77-
Reference Example 31
Ethyl 10 11-Dihydro-5H-pvrrolor2,1-cl(1,41-
benzodiazepine-8-carboxylate
The hydrogenation conditions of ethyl 10,11-
dihydro-5H-pyrrolo[2,1-c][1,4]-benzodiazepine-7-car-
boxylate are used with 1-[2-nitro-4-(ethoxycarbonyl)-
benzyl]-pyrrole-2-carboxaldehyde to give 5.0 g of the
desired product as a solid; mass spectrum (M+H)255.
Reference Example 32
2-Methylfurane-3-carbonyl chloride
A mixture of 4.0 g of inethyl-2-methylfurane-3-
carboxylate, 30 ml of 2 N NaOH and 15 ml methanol is
refluxed for 1.5 hours. The solvent is removed under
vacuum to give a solid. The solid is extracted with
dichloromethane (discarded). The solid is dissolved in
water and the solution acidified with 2 N citric acid to
give a solid. The solid is washed with water and dried
to give crystals 1.05 g of crystals of 2-methylfuran-3-
carboxylic acid. The preceding compound (0.95 g) and 3
ml of thionyl chloride is refluxed for 1 hour. The
solvent is removed, toluene added (20 ml, three times)
and the solvent removed to give the product as an oil.
Reference Example 33
2-f2-(Tributylstannyl)-3-thienyll-1.3-dioxolane
To a stirred solution of 15.6 g (0.10 mol) of
2-(3-thienyl)-1,3-dioxolane in 100 ml of anhydrous
ether, n-butyl-lithium (1.48 N, in hexane, 74.3 ml) is
added dropwise under nitrogen at room temperature.
After being refluxed for 15 minutes, the reaction
mixture is cooled to -78 C and tri-n-butyltin chloride
(34.18 g, 0.105 mol) in 100 ml of dry tetrahydrofuran is
added dropwise. ' After the addition is complete, the
mixture is warmed to room temperature and the solvent
evaporated. To the oily residue 100 ml of hexane is
added, and the resulting precipitate (LiCl) is filtered
off. The filtrate is evaporated and the residue dis-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-78-
tilled at reduced pressure, giving 34.16 g (77%) of the
desired product.
Reference ExamplQ 34
Methyl 6-aminopyridine-3--carboxylate
Dry methanol (400 ml) is cooled in an ice bath
and HC1 gas is bubbled into the mixture for 25 minutes.
To the MeOH-HCl is added 30 g of 6-aminopyridine-3-
carboxylic acid and then the mixture is stirred and
heated at 90 C for 2 hours (all the solid dissolved).
The solvent is removed under vacuum and the residual
solid dissolved in 100 ml of water. The acidic solution
is neutralized with saturated sodium bicarbonate (solid
separated) and the mixture chilled and filtered to give
30 g of white crystals, m.p. 150 -154 C.
Reference ExamQle 35
6-f(5-fluoro-2-methylbenzoyl)aminolpyridine-3-carboxylic
acid
To a mixture of 4.5 g of methyl 6-amino-
pyridine-3-carboxylate and 5.53 ml of triethylamine in
40 ml of dichloromethane (cooled in an ice bath) is
added 6.38 g of 5-fluoro-2-methylbenzoyl chloride in 10
ml of dichloromethane. The mixture is stirred at room
temperature under argon for 18 hours and an additional
3.4 g of 5-fluoro-2-methylbenzoyl chloride added. After
stirring at room temperature for 3 hours, the mixture is
filtered to give 3.0 g of methyl 6-[[bis(5-fluoro-2-
methylbenzoyl)]amino]pyridine-3-carboxylate. The
filtrate is concentrated to dryness and the residue
triturated with hexane and ethyl acette to give an
additional 9.0 g of bis acylated compound.
A mixture of 12.0 g of methyl 6-[[bis(5-
fluoro-2-methylbenzoyl)]amino]pyridine-3-carboxylate, 60
ml of methanol-tetrahydrofuran (1:1) and 23 ml of 5 N =
NaOH is stirred at room temperature for 16 hours. The
mixture is concentrated under vacuum, diluted with 25 ml
of water, cooled and acidified with 1 N HC1. The mix-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96101076
-79-
ture is filtered and the solid washed with water to give
6.3 g of the product as a white solid.
As described for Reference Example 35, but
substituting the appropriate aroyl chloride, heteroaroyl
*chloride, cycloalkanoyl chlorides, phenylacetylchlorides
and related appropriate acid chlorides, the following 6=
[(aroylamino]pyridine-3-carboxylic acids, 6-[(hetero-
aroyl)amino)pyridine-3-carboxylic acids and related 6-
[(acylated)amino]pyridine-3-carboxylic acids are
prepared.
Reference Example 36
6-f(3-Methyl-2-thienylcarbonyl)aminolpyridine-3-
carboxylic acid
Reference Example37
6-f(2-Methyl-3-thienylcarbonyl)aminolPvridine-3-
carboxylic acid
Reference Example 38
6-f(3-Methyl-2-furanylcarbonyl)aminolpyridine-3-
carboxylic acid
Reference Example 39
6-f(2-Methyl-3-furanylcarbonyl)amino]pvtidine-3-
carboxylic acid
Reference Example 40
6-f(3-fluoro-2-methylbenzoyl)aminolpyridine-3-carboxylic
acid
Reference Example 41
6-f(2-Methylbenzoyl)aminolpyridine-3-carboxylic acid
Reference Example 42
6-f(2-chlorobenzoyl)aminolpyridine-3-carboxylic acid
Reference Example 43
6-f(2-Fluorobenzoyl)aminolpyridine-3-carboxylic acid
Reference Example 44
6-f(2-Chloro-4-fluorobenzoYl)aminoipyridine-3-carboxylic
acid
Reference Example 45
6-f(2,4-Dichlorobenzovl)aminolpyridine-3-carboxylic acid
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-80-
Reference Example 46
6-((4-Chloro-2-fluorobenzoyl)aminolT)yridine-3-carboxylic
acid
Reference Example 47
6-f(3,4,5-Trimethoxybenzoyl)aminolpyridine-3-carboxylic
aci~
Reference Example 48
6-((2,4-Difluorobenzoyl)aminolpyridine-3-carboxylic acid
Reference Example 49
6-f(2-Bromobenzoyl)aminolpyridine-3-carboxylic acid
Reference Example 50
6-r(2-Chloro-4-nitrobenzoyl)aminolpyridine-3-carboxylic
acid
Reference Ex.ample 51
6-((Tetrahydrofuranyl-2-carbonyl)aminolpyridine-3-
carboxylic acid
Reference Example 52
6-f(Tetrahydrothienyl-2-carbonyl)aminolpyridine-3-
carboxylic acid
Reference Example 53
6-f(Cyclohe xvlcarbonyl)aminolpyridine-3-carboxylic acid
Reference Example 54
6-f(cYclohex-3-enecarbonyl)aminolpyridine-3-carboxylic
acid
Reference Example 55
6-((5-Fluoro-2-methylbenzeneacetyl)aminolpyridine-3-
carboYylic acid
Reference Example 56
6=f(2-Chlorobenzeneacetyl)aminolpyridine-3-carboxylic
acid
Reference Example 57
6-i(cyclopentylcarbonyl)aminolpyridine-3-carboxylic acid
Reference Example 58
6-f(cyclohexylacetyl)aminolpyridine-3-carboxylic acid
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-81-
Reference Example 59
6-f(3-Methyl-2-thienylacetyl)aminolpyridine-3-carboxylic
' acid
Reference Example 60
6-f(2-Methyl-3-thienylacetyl)aminolpyridine-3-carboxylic
acid
Reference ExamT)le 61
6-f(3-Methyl-2-furanylacetyl)aminolpyridine-3-carboxylic
acid
Example 62
6-f(2-Methyl-3-furanylacetyl)aminolpyridine-3-carboxy ic
acid
Reference Example 63
6-f(3-Methyl-2-tetrahydrothienylacetyl)aminolpyridine-3-
carboxylic acid
Reference ExamQle64
6-f(2-Methyl-3-tetrahydrothienylacetyl)aminolpyridine-3-
carbox_ylic acid
Reference Example 65
6-f(2,5-Dichlorobenzoyl)aminolipvridine-3-carboxylic acid
Reference Example 66
6-f(3,5-Dichlorobenzoyl)aminol-pvridine-3-carboxylic acid
Reference Example, 67
6-f(2-Methyl-4-chlorobenzoyl)aminolpvridine-3-carboxylic
acid
Reference Example. 68
6-i(2,3-Dimethylbenzoyl)aminolpyridine-3-carboxylic acid
Reference Example 69
6-f(2-Methoxybenzoyl)aminolpyridine-3-carboxvlic acid
Reference Ex.ample 70
6-f(2-Trifluoromethoxybenzoyl)aminolpyridine-3-
carboxylic acid
Reference Example 71
6-f(4-Chloro-2-methoxybenzoyl)aminolpyridine-3-
carboxylic acid
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-82-
Reference Example 72
6-ff2-(Trifluoromethyl)benzoyllaminolpyridine-3-
carboxylic acid
Reference Exampla 73
6-f(2 6-Dichlorobenzovl)aminolpyridine-3-carboxylic acid
Reference Example 74
6-f(2 6-Dimethylbenzoyl.)aminolpyridine-3-carboxylic acid
Reference Example 75
6-f(2-Methylthiobenzoyl)aminolpyridine-3-carboxylic acid
Reference Example 76
6-f(4-Fluoro-2-(trifluoromethyl)benzoyl)aminolpyridine-
3-carboxylic acid
Reference Example 77
6-f(2 3-Dichlorobenzovl)aminolpyridine-3-carboxylic acid
Reference Example 78
6-f(4-Fluoro-2-methylbenzoy_l)aminolpyridine-3-carboxylic
acid
Reference Example 79
6-f(2,3,5-Trichlorobenzoyl)aminolpyridine-3-carboxylic
acid
Reference Example 80
6-f(5-Fluoro-2-chlorobenzoyl)aminoipvridine-3-carboxvlic
acid
Reference Example 81
6-f(2-Fluoro-5-(trifluoromethyl)benzoyl)aminolpyridine-
3-carboxylic acid
Reference Example 82
6-f(5-Fluoro-2-mPthylbenzoyl)aminolp-yridine-3-carbonyl
chlori,de
A mixture of 6.2 g,of 6-[(5-fluoro-2-methyl-
benzoyl)amino]pyridine-3-carboxylic acid and 23 ml of
thionyl chloride is refluxed for 1 hour. An additional
12 ml of thionyl chloride is added and the mixture
refluxed for 0.5 hour. The mixture is concentrated to
dryness under vacuum and 30 ml of toluene added to the
residue. The toluene is removed under vacuum and the
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTNS96/01076
-83-
process (add toluene and remove) is repeated to give 7.7
g of crude product as a solid.
= As described for Reference Example 82, the
following 6-(acyl)amino)pyridine-3-carbonyl chlorides
are prepared.
Reference Example 83
6-f(3-Methyl-2-thienylcarbonyl)aminolpyridine-3-carbonyl
chloride
Reference Example 84
6-((2-Methyl-3-thienylcarbonyl)aminolpyridine-3-carbonyl
chloride
Reference Example 85
6-f(3-Methyl-2-furanvlcarbony_l)aminolpyridine-3-carbonyl
chloride
Reference Example 86
6-f(2-Methyl-3-furanylcarbonyl)aminolpyridine-3-carbonyl
chloride
Reference Example 87
6-f(3-Fluoro-2-methylbenzovl)aminolpyridine-3-carbonvi
chloride
Reference Example 88
6-f(2-Methylbenzoyl)aminolp,vridine-3-carbonyl chloride
Reference Example 89
6-i(2-Chlorobenzoyl)aminolpyridine-3-carbonyl chloride,
white crystals
Reference Ex.ample 90
6-f(2-Fluorobenzovl)'aminolpyri.dine-3-carbonyl chloride
Reference Example 91
6-f(2-Chloro-4-fluorobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example 92
6-((2 4-Dichlorobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example93
6-((4-Chloro-2-fluorobenzoyl)aminolpyridine-3-carbonyl
chloride
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-84-
Reference Example 94
6-f(3.4,5-Trimethoxybenzoyl)aminolpyridine-3-carbonvl
chloride
Reference Example 95
6-r(2,4-Difluorobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example 96
6-f(2-Bromobenzovl)aminolpvridine-3-carbonyl chloride
Reference Example 97
6-f(2-Chloro-4-nitrobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example 98
6-f(Tetrahydrofuranyl-2-carbonyl)aminolpyridine-3-
carbonyl chloride
Reference Example 99
6-r(Tetrahydrothienyl-2-carbonyl)aminolpyridine-3-
carbonyl chloride
Reference Examp1e 100
6-r(Cyclohexylcarbonyl)aminolpyridine-3-carbonyl
chloride
Reference Example 101
6-f(Cyclohex-3-enecarbonyl)aminolpyridine-3-carbonyl
chloride
Reference Example 102
6-f(2-Methylbenzeneacetyl)aminolpyridine-3-carbonvl
chloride
Reference Example 103
6-r(2-Chlorobenzeneacetyl)aminolpyridine-3-carbonyl
chloride
Reference Example 104 6-((Cyclopentylcarbonyl)aminolpyridine-3-carbonyl
chloride
Reference Example 105
6-f(Cyclohexylacetyl)aminolpyridine-3--carbonyl chloride
SUBSTtTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-85-
Reference Example 106
6-f(3-Methyl-2-thienylacetyl)aminolpyridine-3-carbonvl
= chloride
Reference Example 107
6-f(2-Methyl-3-thienylacetyl)aminolpyridine-3-carbonvl
chloride
Peference Example 108
6-f(3-MethYl-2-furanylacetyl)aminolpyridine-3-carbonyl
chloride
Reference Ex.ample 109
6-f(2-Methvl-3-furanylacetyl)aminolpyridine-3-carbonyI
chloride
Reference Example 110
6-f(2-Methyl-5-fluorobenzeneacetvl)aminolpyridine-3-
carbonyl chloride
Reference Example 111
6-f(3-Methyl-2-tetrahydrothienylace-yl)aminolpyridine-3-
carbonyl chloride
Reference EYamiple 112
6-f(2-Methyl-3-tetrahydrothienYlacetyl)aminolpyridine-3-
carbonyl chloride
Reference Example 113
6-f(2,5-Dichlorobenzoyl)aminolpvridine-3-carbonyl
chloride
Reference Example 114
6-f(3,5-Dichlorobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example 115
6-f(2-Methyl-4-chlorobenzoyl)aminolpvridine-3-carbonyl
chloride
Reference Example 116
6-f(2.3-Dimethylbenzoyl)aminolpyridine-3-carbonyl
T chloride
Reference Ex.ample 117
6-f(2-MethoYybenzoyl)aminolpyridine-3-carbonyl chloride
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-86-
Reference Example 118
6-r(2-Trifluoromethoxybenzoyl)aminolpyridine-3-carbonvl
chloride
Reference Example 119
6-r(4-Chloro-2-methoxybenzoyl)aminolpvridine-3-carbonyl
chloride
Reference Example 120
6-ff2-(Trifluoromethyl)benzoyllaminolpyridine-3-carbonvl
chloride
Reference Example 121
6-f(2,6-Dichlorobenzoyl)aminolpyridine-3-carbonvl
chloride
Reference Example 122
6-f(2 6-Dimethylbenzoyl)aminolpyridine-3-carbonyi
chloride
Reference Example 123
6-((2-Methylthiobenzoyl)aminolp_yridine-3-carbonyl
chloride
Reference Example 124
6-((4-Fluoro-2-(trifluoromethyl)benzoyl)aminolpvridine-
3-carbonyl chloride
Reference Example 125
6-f(2,3-Dichlorobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example 126
6-f(4-Fluoro-2-methylbenzoyl)aminolpyridine-3-carbonvl
Chloride
Reference Example 127
6-f(2,3,5-Trichlorobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example 128
6-i(5-Fluoro-2-chlorobenzoyl)aminolpyridine-3-carbonyl
chloride
Reference Example 129
6-f(2-Fluoro-5-(trifluoromethyl)benzoyl)aminolpyridine-
3-carbonyl chloride
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-87-
Reference Example 130
1-(3-Nitro-2-pyridinyl)-1H-pyrrole-2-carboxaldehyde
A sample (3.6 g) of sodium hydride (60% in
oil). is washed with hexane under argon. To the sodium
hydride is added 100 ml of dry N,N-ciimethylformamide.
The mixture is cooled in an ice bath and 7.8 g of 1H-
pyrrole-2-carboxaldehyde is added in small portions.
After the addition the cooled mixture is stirred for 15
minutes and 13.0 g of 2-chloro-3-nitropyridine is added.
The mixture is heated at 120 C for 16 hours. The
solvent is removed under vacuum at 80 C and to the dark
residue is added 200 ml of ethyl acetate. The mixture
is filtered and to the filtrate is added 100 ml of
water. The mixture is filtered through diatomaceous
earth and then filtered through a thin pad of hydrous
magnesium silicate. The filtrate is diluted with water,
the organic layer separated, washed 2 times with 100 ml
of water and once with 100 ml of brine and then dried
(Na2SO4). The solvent is removed under vacuum to give
16 g of solid. The solid is chromatographed on a silica
gel column with hexane-ethyl acetate (2:1) as solvent to
give crystals which are recrystalizzed from ethyl
acetate-hexane (97:3) to give 8.5 g of product as
crystals, m.p. 122 -125 C.
Reference Example 131
5,6-Dihydropyridot3.2-elpyrrolofl.2-alpyrazine
To a suspension of 8.0 g of 1-(3-nitro-2-
pyridinyl)-1H-pyrrole-2-carboxaldehyde in 150 ml of
ethyl acetate is added 800 mg of 10% Pd/C. The mixture
is shaken in a Parr hydrogenator for 3 hours and then
filtered through diatomaceous earth. The filtrate is
concentrated under vacuum to give 8.5 g of solid. The
solid is purified by chromatography over silica gel with
solvent hexane-ethyl acetate (2:1) as solvent to give
2.6 g of product as white crystals, m.p. 92 -94 C and
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-88-
1.6 g of pyrido[3,2-A]pyrrolo[1,2-.~t]pyrazine as tan
needles, m.p. 88 C to 90 C.
As described for Reference Example 35, the
following bis acylated products (Table A) are prepared
and purified by silica gel chromatography. These
compounds are then hydrolysed to the acids as described
in Example 35 (Table B).
Table A
O
X O R1
CH3
F~
N N
R1 R3
R2 R4
R3
R4
Ls?;:.....
a>:> :::::::
132 CH3 H H H H 388
133 CH3 H H F H 424
134 CH3 F H H H 426
135 H OCH3 OCH3 OCH3 H 540
136 Cl H H H H 430
137 F H F H H 396
138 Br H H H H 520
139 Cl H F H H 412
140 Ph H H H H 512
142 Ci H H Br H 474
143 CH3 H H F Br
144 CH3 H H H Br 468
M+ is molecular ion found from FAB mass spectrum
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96/01076
-89-
Table B
O
w f.p ~ X O Ri
N R2
H
Rs
R4
R~f ~1 R2
~ :.:.. ...... d..::.. ~ . ....::.:....i!~............
E~;::<>:N >':: ;:::::::i' ;}:>>:::-:`<:: `<:: :::;::::::>::>:': ::
t~...
145 CH3 H H H H 256
146 CH3 H H F H 274
147 CH3 F H H H 274
148 H OCH3 OCH3 OCH3 H 332
149 Cl H H H H 276
150 F H F H H 278
151 Br H H H H. 322
152 Cl H F H H 294
153 Ph H H H H 318
154 C1 H H Br H 356
155 CH3 H H F Cl
156 CH3 H H H Br 336
M+ is molecular ion found from FAB mass spectrum.
ge r n xample 157
6-Amino-5-bromopvridin -'~- a boxyl;c acid
To a stirred solution of 6-aminonicotinic acid
(13.8 g, 0.1 mole) in glacial acetic acid (100 ml),
bromine (16 g, 5 ml, 0.1 mole) in acetic acid (20 ml) is
added slowly. The reaction mixture is stirred for 8
hours at room temperature and the acetic acid is removed
under reduced pressure. The yellow solid residue is
dissolved in water and carefully neutralized with 30%
SUBSTtTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTlUS96/01076
-90-
NH40H. The separated solid is filtered and washed with
water to give 18 g of solid; mass spectrum: 218 (M+).
Reference Example 158
Methyl 6-amino-5-bromopyridine-3-carboxyla P
6-Amino-5-bromopyridine-3-carboxylic acid (10
g, 50 mmol)* is dissolved in saturated methanolic HC1
(100 ml) and refluxed for 24 hours. The solvent,
methanol, is removed under reduced pressure and the
residue is dissolved in ice cold water. The aqueous
solution is neutralized with 0.1 N NaOH and the solid
which separates is filtered; washed well with water and
air dried to yield 10 g of product as a solid: mass
spectrum 231 (M+).
Reference Example 159
10-((6-Chloro-3-pyridinyllcarbonyll-10 11-dihydro-5H-
py_rrolof2.1-c1f1,41benzodiazepine
To a mixture of 1.84 g of 10,11-dihydro-513.-
pyrrolo[2,1-_r,][1,4]benzodiazepine and 1.52 g of tri-
ethylamine in 20 ml of dichloromethane is added a solu-
tion of 2.11 g of 6-chloronicotinyl chloride in 5 ml of
dichioromethane. The mixture is*stirred at room
temperature for 2 hours and quenched with 30 ml of 1 N
sodium hydroxide. The mixture is diluted with 20 ml of
dichloromethane and the organic layer separated. The
organic layer is washed twice with 20 ml of 1 N sodium
hydroxide, washed with brine and dried (Na2SO4). The
solvent is removed under vacuum and the residue tri-
turated with ether to give 3.22 g of white.solid; mass
spectrum (CI) 324 (M+H) .
Reference Example 160
10-ff6-f(2-dimethylaminoethYl)aminol-3-
-ovridinvllcarbonvll-10,11-dihydro-5H-pyrrolo[2 1-_l-
f1,41benzodiazepine
A mixture of 10-[[6-chloro-3-pyridinyl]-
carbonyl]-10,11-dihydro-5H-pyrrolo[2,1-_r,][1,4]benzo-
diazepine (3.2 g), K2C03 (5 g) and the 2-dimethylamino-
SUBST1TUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-91-
ethylamine (5 ml) is heated in dimethylsulfoxide (80 ml)
for 6 hours at 100 C (with stirring). The reaction
mixture is quenched with water and the solid which
separates, is filtered off and washed well with water.
Examination of the TLC (CHC13:MeOH; 3:1) showed the
products to be sufficiently pure to be used for further
reactions without purification. Yield 3.2 g, 85%, mass
spectrum (CI) 376 (M+1).
Reference Example 161
6-f(2-Methylbenzeneacetyl)aminolpyridine-3-carboxylic
acid
To a cooled (0 C) mixture of 5.0 g methyl 6-
aminopyridine-3-carboxylate, 12.6 ml of N,N-diisopropyl-
ethylamine in 40 ml of dichloromethane is added a
solution of 12.2 g of 2-methylbenzeneacetyl chloride in
10 ml of dichloromethane. The mixture is stirred under
argon at room temperature overnight. The mixture is
diluted with 200 ml of dichloromethane and 50 ml of
water and the organic layer separated. The organic
layer is washed with 50 ml each of 1 M NaHCO3, brine and
dried (Na2SO4). The solution is filtered through a thin
pad of hydrous magnesium silicate and the filtrate con-
centrated to dryness. The residue (9.0 g) is chroma-
tographed on a silica gel column with hexane-ethyl
acetate (3:1) as eluent to give 8.6 g of solid. This
solid, mainly methyl 6-[[bis(2-methylbenzeneacetyl)]-
amino]pyridine-3-carboxylate, is dissolved in 60 ml of
tetrahydrofuran-methanol (1:1) and 23 ml of 5 N NaOH
added to the solution. The mixture is stirred at room
temperature overnight and the mixture concentrated under.
vacuum. Water (25 ml) is added and the mixture is
stirred and acidified with cold 1 N HC1. The mixture is
chilled and the solid filtered and washed with water to
give 5.9 g of off-white solid.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-92-
Reference Example 162 6-f(2-Methvlbenzeneacety_1)aminolpyridine-3-carbonyi
chloride
A mixture of 4.5 g of 6-[(2-methylbenzene-
5'acetyl)amino]pyridine-3-carboxylic acid and 25 ml of
thionyl chloride is refluxed for 1 hour and then con-
centrated to dryness under vacuum. To the residue is
added 20 ml of toluene and the solvent removed under
vacuum. The addition and removal of toluene is repeated
and the residual solid dried at room temperature under
vacuum to give 5.3 g of dark brown solid.
Reference Example 163
6-f(2-Methylbenzeneacetyl)aminolp_yridinP-3-carboxylic
acid
To a chilled solution (0 C) of 5.0 g of methyl
6-aminopyridine-3-carboxylate and 12.6 ml of diiso-
propylethylamine in 40 ml of dichloromethane under argon
is added 12.2 g of 2-methylbenzeneacetyl chloride in 10
ml of dichloromethane. The mixture is stirred at room
temperature 16 hours and diluted with 200 ml of di-
chloromethane and 50 ml of water. The organic layer is
~separated and washed with 50 ml each of 1 M NaHCO3,
brine and dried (Na2SO4). The solution is filtered
through a thin pad of hydrous magnesium silicate and the
filtrate concentrated to dryness. The residue (9.0 g)
is purified by chromatography on silica gel with hexane-
ethyl acetate (3:1) as eluent to give 0.70 g of methyl
6-[[bis(2-methylbenzeneacetyl)]amino]pyridine-3-carboxy-
late and 8.6 g of a mixture of inethyl 6-[(2-methyl-
benzeneacetyl)amino]pyridine-3-carboxylate and the bis
acylated product. The above mixture (8.6 g) of mono and
bis acylated product is dissolved in 60 ml of tetra-
hydrofuran-methanol (1:1) and 23 ml of 5 N NaOH is
added. The solution is stirred at room temperature for
16 hours, concentrated under vacuum, diluted with 25 ml
of H20 and acidified with cold 1 N HC1. The precipi-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-93-
tated solid is filtered off and dried to give 5.9 g of
white solid.
Reference Example 164
6-f(2-Methylbenzeneacetyl)aminolpyridine-3-carbonyl
chloride
A mixture of 4.5 g of 6-[(2-methylbenzene-
acetyl)amino]pyridine-3-carboxylic acid and 17 ml of
thionyl chloride is heated on a steam bath for 1/2 hour.
An additional 815 ml of thionyl chloride is added and
the mixture refluxed for 0.5 hour. The volatiles are
removed under vacuum and toluene (20 ml) added (twice)
and the solvent removed under vacuum to give 5.3 g of a
dark colored solid.
Reference Example 165
2-Biphenylcarbonyl chloride
A mixture of 5.6 g of 2-biphenylcarboxylic
acid and 29 ml of thionyl chloride is heated on a steam
bath for 0.5 hour and the volatiles removed under
vacuum. Toluene (40 ml) is added (twice) and the
solvent removed under vacuum to give 6.8 g of a yellow
oil.
Reference Example 166
Methyl 6-((bis(2-biphenylcarbonyl)laminolpyridine-3-
carboxylate
To a chilled (0 C) solution of 2.64 g of
methyl 6-aminopyridine-3-carboxylate and 5.5 ml of
diisopropylethylamine in 30 ml of dichloromethane under
argon is added 6.8 g of 2-biphenylcarbonyl chloride in
10 ml of dichioromethane. The mixture is stirred at
room temperature 2 days and then diluted with 120 ml of
dichloromethane and 50 ml of water. The organic layer
is separated, washed with 50 ml each of 1 M NaHCO3 and
brine and dried (Na2SO4). The solution is filtered
through a thin pad of hydrous magnesium silicate and the
filtrate concentrated under vacuum to give a solid.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96/01076
-94-
Crystallization from ethyl acetate gives 6.2 g of white
crystals, m.p. 180-188 C. .
Reference Example 167
6-f(2-Biphenxlcarbonyl)aminolipyridine-3-carboxylic acid
To a chilled (0 C) mixture of 6.0 g of methyl
6-[[bis(2-biphenylcarbonyl)]amino]pyridine-3-carboxylate
in 40 ml of methanol and 30 ml of tetrahydrofuran is
added slowly 18 ml of 2 N NaOH. The mixture is stirred
at room temperature overnight and brought to pH 5 with
glacial acetic acid. The mixture is concentrated,
acidified to pH 2-3 with 1 N HC1 and extracted with 250
ml of ethyl acetate. The extract is washed with 50 ml
of brine, dried (Na2SO4) and the solvent removed under
vacuum. The residual white solid is triturated with 15
ml of ethyl acetate to give 3.35 g of white crystals,
m.p. 215-217 C.
Reference Example 168
6-f(2-Biphenylcarbonyl)aminolpyridine-3-carbonyl
chloride
A mixture of 1.9 g of 6-[(2-biphenylcar-
bonyl)amino]pyridine-3-carboxylic acid and 9 ml of
thionyl chloride is refluxed for 1 hour and then con-
centrated to dryness under vacuum. Toluene (15 ml) is
added (twice) to the residue and the solvent removed
under vacuum to give 2.1 g of a light brown oil.
Reference Example 169
6-f(Cyclohexylcarboriyl)aminolpvridine-3-carboxylic acid
To a chilled (0 C) solution of 5.0 g of methyl
6-aminopyridine-3-carboxylate and 1.2.6 ml of diiso-
propylethylamine in 50 ml of dichloromethane under argon
is added a solution of 9.7 ml of cyclohexylcarbonyl chloride in 10 ml of
dichloromethane. The mixture is
stirred at room temperature overnight and diluted with
200 ml of dichloromethane and 60 ml. of water. The
organic layer is separated, washed with 60 ml of brine
and dried (Na2SO4). The solution is filtered through a
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-95-
thin pad of hydrous magnesium silicate and the filtrate
concentrated under vacuum to give 12.8 g of a solid.
The above solid (12.0 g) in a mixture of 150
ml of tetrahydrofuran-methanol (1:1) is chilled (0 C)
and 62 ml of 2 N sodium hydroxide added. The mixture is
stirred at room temperature for 3 hours, neutralized
with 10 ml of glaqial aceti.c acid and concentrated under
vacuum. The mixture (containing solid) is acidified to
pH 1 with 1 N HCl and extracted with 250 ml of ethyl
acetate and twice with 100 ml of ethyl acetate. The
combined extract is washed with 100 ml of brine, dried
(Na2SO4) and concentrated to a white solid. Trituration
with hexane gives 6.5 g of product as a white solid.
Reference Example 170
5-f(6-Chloro-3-pyridinyl)carbonyll-5,10-dihydro-4 -
gvrazolof5,1-cl(1,41benzodiazepine
To a solution of 10 mmol. of 5, 10-dihydro-4H-
pyrazolo[5,1-c][1,4]benzodiazepine and 1.5 g of tri-
ethylamine in 20 ml of dichioromethane is added a
solution of 2.11 g of 6-chloropyridine-3-carbonyl
chloride in 5 ml of dichloromethane. The mixture is
stirred for 3 hours at room temperature diluted with 20
ml of dichloromethane and washed with 30 ml of 1 N NaOH.
The organic layer is washed twice with 20 ml of 1 N
NaOH, dried (Na2SO4) and the solvent removed. The
residue is triturated with ether to give 3 g of solid.
Reference Example 171
Methyl 4-(((1,1'-Biphenyll-2-carbonyl)aminol-3-
methoxybenzoatg
A mixture of 10.0 g of [1,1'-biphenyl]-2-
carboxylic acid in 75 ml of methylene chloride and 12.52
g of oxalyl chloride is stirred at room temperature for
15 hours. The volatiles are evaporated in vacuo to give
11.06 g of an oil. A 2.16 g portion of the above oil in
25 ml of methylene chloride is reacted with 1.81 g of
methyl 4-amino-3-methoxybenzoate and 1.30 g of N,N-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-96-
diisopropylethylamine by stirring at room temperature
for 18 hours. The reaction mixture is washed with
water, saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through
hydrous magnesium silicate and hexane added to the
filtrate at the boil to give 3.20 g of the desired
product as a crystalline solid, m.p. 115-117 C.
Reference Example 172
Methyl 4-f(fl.l'-Biphenyll-2-carbonyl)aminol-2-
chlorobenzoate
A solution of 2.37 g of [1,1'-biphenyl]-2-
carbonyl chloride in 10 ml of methylene chloride is
added dropwise to an ice cold solution of 1.84 g of
methyl 4-amino-2-chlorobenzoate and 1.49 g of N,N-
diisopropylethylamine in 50 ml of methylene chloride.
The reaction mixture is stirred at room temperature for
18 hours and washed with water, saturated aqueous NaHCO3
and the organic layer dried(Na2SO4). The organic layer
is passed through a pad of hydrous magnesium silicate
and hexane added at the boil to give 1.1 g of the
desired product as a crystalline solid, m.p. 132-134 C.
M+H=365
Reference Exampl~.e 173
4-((fl,l'-Biiphenyll-2-carbonvl)aminol-2-chlorobenzoic
Acid
A mixture of 3.0 g of methyl 4-[([1,1'-
biphenyl]-2-carbonyl)amino]-2-chlorobenzoate in 75 ml of
absolute ethanol and 2.0 ml of 10 N sodium hydroxide is
heated on a steam bath for 3 hours. Water is added to
obtain a solution which is extracted with methylene chloride. The aqueous
phase is acidified with acetic
acid and the resulting solid collected and dried in
vacuo at 80 C to give 0.1 g of the desired product as a
crystalline solid, m.p. 217-219 C
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-97-
Reference Example 174
4-f(fl,l'-Biphenyll-2-carbonyl)-aminol-3-methoxybenzoyl
Chloride
A solution of 2.69 g of 4-[([1,1'-biphenyl]-2-
carbonyllamino]r3-methoxy benzoic acid in 5 ml of
thionyl chloride is heated on a steam bath for 1 hour
under Argon. The volatiles are removed in_ vacuo to give
a residue which is stirred with hexane to give 2.58 g of
crystalline solid, m.p. 121-123 C. M+=361.
Reference Example 175
Methyl 4-f((1,1'-Biphenyll-2-carbonyl)aminolbenzoate
A mixture of 10.0 g of [1,1'-biphenyl]-2-
carboxylic acid in 75 ml of methylene chloride and 12.52
g of oxalyl chloride is stirred at room temperature for
18 hours. The volatiles are evaporated in vacuo to give
11.66 g of an oil. A 7.5 g portion of the above oil in
ml of methylene chloride is added dropwise to a
solution of 4.53 g of methyl 4-aminobenzoate and 4.3 g
of N,N-diisopropylethylamine in 100 ml of methylene
20 chloride at 0 C. The reaction mixture is stirred at
room temperature for 18 hours arnd washed with water, and
saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through
hydrous magnesium silicate and hexane added to the
25 filtrate at the boil to give 8.38 g of the desired
product as a crystalline solid, m.p. 163-165 C.
Reference Example 176
4-f(fl,l'-Biphenyll-2-carbonyl)aminolbenzoic Acid
A 3.15 g sample of methyl 4-[(.[1,1'-biphenyl]-
2-carbonyl)amino]benzoate is refluxed for 8 hours in 100
ml of ethyl alcohol and 2.5 ml of lON sodium hydroxide.
The cooled reaction mixture is acidified with
hydrochloric acid and the desired product collected and
dried to give 2.9 g of the desired product as a solid
m.p. 246-249 C. M+H=318.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCTIUS96/01076
-98-
Reference Example 177
4-((f1,1'-Biphenyll-2-carbonyl)aminolbenzoyl Chloride
A mixture of 1.39 g of 4-[([1,1'-biphenyl]-2-
carbonyl)amino]benzoic acid in 2.0 ml of thionyl
.5 chloride i=s heated on a steam bath for 1 hour. Cold
hexane is added and the crystalline solid collected and
dried to give 1.34 g of the desired product, m.p. 118-
120 C.
Reference Example 178
2-(Phenylmethyl)benzoyl Chloride
A mixture of 5.0 g of 2--(phenylmethyl)benzoic
acid in 5.0 ml of thionyl chloride is heated on a steam
bath for 1 hour. The volatiles are evaporated in vacuo
to give 5.74 g of the desired product as an oil. Mf=227
as methyl ester.
Reference Example 179
Llethyl 4-(f2-(Phenylmethyl)benzoyllaminolb nznatP
To 3.03 g of methyl 4-aminobenzoate and 3.12 g
of N,N-diisopropylethylamine in 75 ml of methylene
chloride is added 5.54 g of 2-(phenylmethyl)benzoyl
chloride and the reactants stirred at room temperature
for 18 hours. The reaction mixture is washed with
water, saturated aqueous NaHC03 and the organic layer
dried(Na2SO4). The organic layer is passed through
hydrous magnesium silicate two times and hexane added to
the filtrate at the boil to give 5.04 g of the desired
product.as a crystalline solid, m.p. 138-139 C.
Reference Example 180
Sodium 4-fr2-(Phenylmethyl)benzoyllaminolb nzoa P
A mixture of 4.90 g of methyl 4-[[2-(phenyi-
methyl)benzoyl]amino]benzoate in 100 ml of absolute
ethanol and 3.50 ml of 10 N sodium hydroxide is heated
on a steam bath for 3 hours. The aqueous phase is
filtered and the resulting solid collected and dried to
give 4.25 g of the desired product m.p. 340-346 C.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96101076
-99-
Reference Example 181
4-f[2-(Phenylmethyl)benzoyllaminolbenzoi Acid
: A mixture of 4.0 g sodium 4-[[2-(phenyl-
methyl)benzoyl]amino]benzoate is suspended in water and
the pH adjusted to 5 with acetic acid. The solid is
collected by filtration and dried at 80 C in vacuo to
give 3.75 g of the desired product, 246-247 C. M+=332.
Reference Example 182
4-ff2-(Phenylmethyl)benzoyllaminolbenzoyl Chloride
A mixture of 2.0 g of 4-[[2-(phenylmethyl)-
benzoyl]amino]benzoic acid in 2.0 ml of thionyl chloride
is heated on a steam bath for 1 hour. The volatiles are
evaporated in vacuo to give 1.53 g of the desired
product as an oil. M+=346 as methyl ester.
Reference Example 183
Methyl 4-fr(2-phenylme hyl)benzoyllaminol-2-chloro-
benzoate
A mixture of 5.0 g of 2-(phenylmethyl)benzoic
acid in 5.0 ml of thionyl chloride is heated on a steam
bath for 1 hour. The volatiles are evaporated ja vacuo
to give 5.70 g of an oil. A 2.85 g portion of the above
oil in 25 ml of methylene chloride is added to a
solution of 50 ml of methylene chloride containing 1.85
g of methyl 4-amino-2-chlorobenzoate and 1.65 g of N,N-
diisopropylethylamine by stirring at room temperature
for 18 hours. The reaction mixture is washed with
water, saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through
hydrous magnesium silicate two times and hexane added to
the filtrate at the boil to give 2.96 g of the desired
product as a crystalline solid, m.p. 133-135 C. M+=380.
Reference Example 184
Methyl 4-(((2-Phenylmethyl)benzoyllaminol-3-
methoxybenzoatg
A solution of 2.85 g of 2-(phenylmethyl)-
benzoyl chloride in 25 ml of methylene chloride is added
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-100-
dropwise to an ice cold solution of 1.84 g of methyl 4-
amino-3-methoxybenzoate
and 1.61 g of N,N-diisopropyl-
ethylamine in 50 ml of methylene chloride. The reaction
mixture is stirred at room temperature for 18 hours and
washed with water, saturated aqueous NaHCO3 and the
organic l-ayer dried(Na2SO4). The organic layer is
passed through a pad of hydrous magnesium silicate and
hexane added at the boil to give 2.2 g of the desired
product as a crystalline solid, m.p. 129-131 C. M+=376.
Reference Example 185
2-Chloro-4-rf(2-Phenylmethyl)benzoyllaminolbenzoic Acid
A mixture of 2.8 g of methyl 2-chloro-4-[[(2-
phenylmethyl)benzoyl]aminobenzoate in 75 ml of absolute
ethanol and 1.84 ml of 10 N sodium hydroxide is heated
on a steam bath for 3 hours. Water is added to obtain a
solution which is extracted with methylene.chloride.
The aqueous phase is acidified with acetic acid and the
resulting solid collected and dried ig vacuo at 80 C to
give 2.6 g of the desired product as a crystalline
solid, m.p. 184-187 C. M+H=366.
Reference Example 186
3-Me hoxy-4-(i(2-phenylmethyl)benzoyllaminolb nzoa P
A mixture of 2.05 g of methyl 4-[[(2-phenyl-
methyl)benzoyl]amino]-3-methoxybenzoate in 75 ml of
absolute ethanol and 1.4 ml of 10 N sodium hydroxide is
heated on a steam bath for 3 hours. Water is added to
obtain a solution which is extracted with methylene
chloride. The aqueous phase is acidified with acetic
acid and the resulting solid collected and dried in
vacup at 80 C to give 1.87 g of the desired product as a
crystalline solid, m.p. 176-178 C. M+H=362.
Reference Example 187
_ 3-MPthoxy-4-fr(2-phenylmethyl)benzoYllaminolbenzoyl
Chloride
A mixture of 1.71 g of 3-methoxy-4-[[(2-
phenylmethyl)benzoyl]amino]benzoic acid in 2.0 ml of
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-101-
thionyl chloride is heated on a steam bath under Argon
for 1 hour and hexane added. The resulting solid is
collected and dried to give 1.71 g of the desired
product as a crystalline solid, m.p. 130-135 C. M+=376
as the methyl ester.
Reference Example 188
S4'-(Trifluoromethyl)-11,1'-bxphenyll-2-carbonyl
Chloride
A mixture of 5.0 g of 4'-(trifluoromethyl)-
[1,1'-biphenyl]-2-carboxylic acid in 5.0 ml of thionyl
chloride is heated on a steam bath under Argon for 1
hour and hexane added. The resulting solid is collected
and dried to give 5.36 g of the desired product as a
colorless oil. M+=280 as methyl ester.
Reference Example 189
Methyl 2-Chloro-4-f(f4'-(trifluoromethyl)[1,1'-
biphenyllcarbonyl)aminolbenzoate
A solution of 3.13 g of [4'-(trifluoromethyl)-
[1,1'-biphenyl]-2-carbonyl chloride in 25 ml of
methylene chloride is added dropwise to an ice cold
solution of 1.84 g of methyl 4-aminobenzoate and 1.43 g
of N,N-diisopropylethylamine in 50 ml of methylene
chloride. The reaction mixture is stirred at room
temperature for 18 hours and washed with water,
saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through a
pad of hydrous magnesium silicate and hexane added at
the boil to give 3.36 g of the desired product as a
crystalline solid, m.p. 164-165 C. M+=396.
Reference Example 190
3-Methoxy-4-f(f4'-(trifluoromethyl)f1,1'-biphenyll-2-
carbonyl)aminolbenzoyl Chloride
A mixture of 2.0 g of 3-methoxy-4-[([4'-
(trifluoromethyl)[1,1'-biphenyl]-2-carbonyl)amino]-
benzoic acid in 20 ml of thionyl chloride is heated on a
steam bath under Argon for 1 hour and hexane added. The
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT1US96/01076
-102-
resulting solid is collected and dried to give 1.92 g of
the desired product as a crystalline solid, m.p. 136-
138 C.
Reference Example 191
3-Methoxy-4-f(f4'-tri luoromethyl)(1,1'-biphenyll 2
carbonyl)aminolben oic Acid
A mixture of 3.78 g of methyl 3-methoxy-4-
[([4'-trifluoromethyl)[1,1'-biphenyl]-2-carbonyl)-
amino]benzoate in 75 ml of absolute ethanol and 2.20 ml
of 10 N sodium hydroxide is heated on a steam bath for 3
hours. Water is added to obtain a solution which is
extracted with methylene chloride. The aqueous phase is
acidified with acetic acid and the resulting solid
collected and dried in vacuo at 80 C to give 3.49 g of
the desired product as a crystalline solid, m.p. 213-
215 C .
Reference Example 192
Methyl 3-Methox.y-4-f(f4'-trifluoromethyl)(1 1l-
biphenyll-2-carbonyl)aminolbenzoa
A solution of 3.56 g of [4'-(trifluoro-
methyl)[1,1'-biphenyi]-2-carbonyl chloride in 25 ml of
methylene chloride is added dropwise to an ice cold
solution of 1.81 g of methyl 4-amino-3-methoxybenzoate
and 1.62 g of N,N-diisopropylethylamine in 50 ml of
methylene chloride. The reaction mixture is stirred at
room temperature for 18 hours and washed with water,
saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through a
pad of hydrous magnesium silicate and hexane added at
the boil to give 3.9 g of the desired product as a
crystalline solid, m.p. 112-113 C.
Reference Example 193
2-Chloro-4-((f4'-(trifluoromPthyl)fl 1'-biphenyll-2-
carbonyl)aminolbenzoyl Chloride
A mixture of 1.39 g of 2-chloro-4-[([4'-
(trifluoromethyl)[1,1'-biphenyl]-2-carbonyl)amino]-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-103-
benzoic acid in 2.0 ml of thionyl chloride is heated on
a steam bath for 1 hour. The reaction mixture is
concentrated to a residue in vacuo to a residue. Cold
hexane is added to the residue and the solid collected
and dried to give 1.39 g of the desired product.
Reference Example 194
2-Chloro-4-r(f4'-(trifluoromethvl)(1,1'-biphenyll-2-
carbonyl)aminolbenzoic acid
A mixture of 3.83 g of methyl 2-chloro-4-
[([4'-(trifluoromethyl)[1,1'-biphenyl]-2-carbonyl)-
amino]benzoate in 75 ml of absolute ethanol and 2.20 ml
of 10 N sodium hydroxide is heated on a steam bath for 3
hours. Water is added to obtain a solution which is
extracted with methylene chloride. The aqueous phase is
acidified with acetic acid and the resulting solid
collected and dried in vacuo at 80 C to give 3.42 g of
the desired product as a crystalline solid, m.p. 187-
189 C.
Reference Example 195
Methyl 2-Chloro-4-f(f4'-(trifluoromethyl)f1.i'-
biphenyll-2-carbonyl)aminolbenzoate
A solution of 3.56 g of [4'-(trifluoro-
methyl)(1,1'-biphenyl]-2-carbonyl chloride in 10 ml of
methylene chloride is added dropwise to an ice cold
solution of 1.86 g of methyl 2-chloro-4-aminobenzoate
and 1.6 g of N,N-diisopropylethylamine in 50 ml of
methylene chloride. The reaction mixture is stirred at
room temperature for 18 hours and washed with water,
saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through a
pad of hydrous magnesium silicate(3X) and hexane added
to the filtrate at the boil to give 4.0 g of the desired
product as a crystalline solid, m.p. 130-132 C.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-104-
Reference Example 196
4- f( f4'- (Trifluoromethyl) f1. 1'-
biphenyl l carbonyl)aminol benzoic Acid
A mixture of 3.0 g of methyl 4-[([4'-(tri-
fluoromethyl)[1,1'-biphenyl]-2-carbonyl)amino]benzoate
in 75 ml of absolute ethanol and 2.0 ml of 10 N sodium
hydroxide is heated on a steam bath for 3 hours. Water
is added to obtain a solution which is extracted with
methylene chloride. The aqueous phase is acidified with
acetic acid and the resulting solid collected and dried
in vacuo at 80 C to give 2.93 g of the desired product
as a crystalline solid, m.p. 243-245 C. M+=385.
Reference Example 197
Methyl 6-ff3-(2-methylpyridinyl)carbonyllaminolpyridine-
3-carboxylate
To a stirred solution of 3 g of methyl 6-
aminopyridine-3-carboxylate and 4 ml of N,N-diisopro-
pylethylamine in 100 ml of methylene chloride is added
dropwise a solution of 6.4 g of 2-methylpyridine-3-
carbonyl chloride in 25 ml of methylene chloride. The
reaction mixture is stirred at room temperature for 2
hours and quenched with water. The organic layer is
washed with water, dried(MgSO4), filtered and evaporated
in vacuo to a residue which is stirred with ether and
the resulting solid collected and air dried to give 6.8
g of the desired product. M+=390.
Reference Examole 198
6-ff3-(2-methylpyridinyl)carbonyllaminolpyridine-3-
carboxylic Acid
To a solution of 6.5 g of methyl 6-[[3-(2-
methylpyridinyl)carbonyl]amino]pyridine-3-carboxylate in
100 ml of 1:1 tetrahydrofuran:methyl alcohol is added 20
ml of 5N NaOH. The reaction mixture is stirred over-
night
and evaporated in vacuo to a residue. The residue
is dissolved in water and neutralized with acetic acid.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-105-
The separated solid is filtered and air-dried to give
3.0 g of the desired product. M+=257.
t Reference Example 199
Methyl 6-f(f1,1'-biphenyll-2-carbonyl)aminol-nyridine-3-
carboxylate
To a solution of 1.5 g of methyl 6-amino-
pyridine-3-carboxylate in 100 ml of inethylene chloride
is added 3 ml of N,N-diisopropylethylamine at room
temperature. To the stirred reaction mixture is slowly
added a solution of 2.5 g of [1,1'-biphenyl]-2-carbonyl
chloride. The reaction mixture is stirred at room
temperature for 4 hours and then quenched with water.
The organic layer is washed well with water and dried
over anhydrous MgSO4, filtered and evaporated in vacuo
to a solid residue. The residue is stirred with ether,
filtered and dried to give 3.0 g of the desired
product:M+=332.
Reference Example 200
6- ( ( (1, 1'-Biphenyll-2-carbonvl) aminolPvridine-3-
carboxylic A~;id
To a stirred solution of 2.5 g of methyl 6-
[([1,11-biphenyl]-2-carbonyl)amino]-pyridine-3-car-
boxylate in 50 ml of 1:1 tetrahydrofuran:methanol is
added 10 ml of 5N sodium hydroxide and the mixture
stirred at room temperature for 16 hours. The reaction
mixture is concentrated in vacuo to a residue which is
dissolved in water and neutralized with acetic acid.
The separated colorless solid is filtered and air dried
to give 2.0 g of the desired product:M+=318.
Reference Example 201
Methyl 2-(2-Pyridinyl)benzoate
A mixture of 12 g of methyl 2-(iodomethyl)-
benzoate, 20 g of n-butyl stannane and 2 g of tetrakis-
(triphenylphosphine)palladium (0) are refluxed in
degassed toluene for 48 hours. The reaction mixture is
concentrated in vacuo to a residue which is purified by
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-106-
column chromatography on silica gel by elution with 1:1 ethyl acetate:hexane
to give 5.5 g of the desired
product as an oil. M+=213.
Reference Example 202
2-(2-Pyridinyl)benzoic Acid
A mixture of 3.0 g of methyl 2-(2-pyridinyl)=
benzoate and 600 mg of sodium hydroxide in 50 ml of 9:1
methanol:water is refluxed for 4 hours. The reaction
mixture is concentrated in vacuo and the residue
dissolved in 50 ml of cold water. The solution is
neutralized with glacial acetic acid and the resulting
product filtered, washed with water, and dried to give
2.5 g of the desired product:M+1=200.
15. Example 1
N- f 5- (5H-Pvrrolo (2, 1-c1 F1, 41 benzodia .Ppi n-10 (11 H) -
vlcarbonvl)-2-pvridinyll-5-fluoro-2-methylbenzamid_
A mixture of thionyl chloride (100 ml) and 6-
[(5-fluoro-2-methylbenzoyl)amino]pyridine-3-carboxylic
acid (2.7 g, 10 mmol) is heated to reflux for 5 hours.
At the end, excess thionyl chloride is removed and the
acid chloride is dissolved in CH2C12 (100 ml). At room
temperature, the methylene chloride solution of the 6-
[(5-fluoro-2-methylbenzoyl)amino]pyridine-3-carbonyl
chloride is added slowly. The reaction mixture is
stirred at room temperature for 2 hours and quenched
with ice cold water. The reaction mixture is washed
with 0.1 N NaOH and subsequently washed with water. The
CH2C12 layer is separated; dried (MgSO4), filtered and
concentrated. The product is purified by silica gel
column chromatography by eluting first with 10% ethyl
acetate-hexane (1 L) and then with 30% ethyl acetate-
hexane. The product is crystallized from ethyl acetate-
hexane. Yield 1.0 g, 46 ; mass spectrum (FAB), M+1 441;
M+Na: 462.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-107-
As described for Example 1, the following
compounds are prepared (Table C).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-108-
Table C
OCP
N
X
O I
N NI-I
O
R5 / Ri
R2
R4
Ra
... ;
^:.
2 CH3 H H H H H 423
3 CH3 H H H F H
4 CH3 F H H H H 441
5 H OCH3 OCH3 OCH3 H H 499
6 C1 H H H H H 443
7 F H F H H H 445
8 Br H H H H H 489
9 C1 H F H H H 461
10 Ph H H H H H
11 C1 H H Br H H
12 CH3 H H H H Br 502
13 CH3 H H F H Cl
14 C1 H H Cl H H
15 CH3 CH3 H H H H
16 Cl H H F H H
17 Cl H H CF3 H H
18 C1 H H H F H
19 Cl H H H Cl H
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-109-
. :.. ;:.: ;
';:>::':::><R;; :::>::;::::<:
20 Cl H H F H H
21 ~ H H H H H
N
22 /, H H H H H
S
23 CH3 H H H CH3 H
24 C1 H H F H ci
25 C1 H F H H ci
26 ci ci H H H H
27 ci H H ci H H
28 -OCH3 H H H H H
29 OCF3 H H H H H
30 -CF3 H H H H H
31 C1 C1 H ci H H
32 -SCH3 H H H H H
33 ci H N02 H H H
34 CH3 H H CH3 H H
35 F H H ci H H
36 ci H H NH2 H H
37 F CF3 H H H
38 -OCH3 H H ci H H
39 C1 H H -SCH3 H H
40 F H H H CF3 H
41 F H CF3 H H H
42 CF3 H F H H H
43 N02 H H H H H
44 F H H H H H
. 45 C1 H NH2 H H H
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-110-
Example 46
N-f5-(5H-Pyrrolof2 1-clfl 41benzodiazepin-10(11H)-
ylcarbonyl)-2-gvri,dinyll-2-mei-hylbenzeneacetamide
A mixture of 2.0 mmol of 10,11-dihydro-10-(6-
amino-3-pyridinylcarbonyl)-5H-pyrrolo[2,1-.a][1,4]benzo-
diazepine, 2.1 mmol of 2-methylbenzeneacetyl chloride
and 5 mmol of triethylamine in 10 ml of dichloromethane
is stirred under argon at room temperature for 16 hours.
The solvent is removed under vacuum and the residue
partitioned between 50 ml of ethyl acetate and 25 ml of
water. The organic layer is separated, washed with H20,
1 N NaHCO3, brine and dried (Na2SO4). The solvent is
removed and the residue chromatographed on silica gel
with ethyl acetate-hexane as solvent to give the product
as a solid.
As described for Example 46, the following
compounds are prepared (Table D).
Table D
N \ / _
N
O X
N NH
~=O
CH2
Ri
R5 *R2
R4
~3
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96/01076
- 111 -
...::.. ::;:....:: ......
:<:... R >:;
47 CH3 H H CH3 H H
48 CH3 H H H H Br
49 CH3 H H H H C1
50 Cl H H H H H
51 Ci H H H H Br
52 C1 H H H H Cl
53 C1 H C1 H H H
54 C1 H C1 H H Br
55 C1 H C1 H H C1
56 -OCH3 H H H H H
57 -OCH3 H H H H Br
58 -OCH3 H H H H C1
59 -OCH3 H H -OCH3 H H
60 -OCH3 H H -OCH3 H Br
61 -OCH3 H H -OCH3 H C1
62 H -OCH3 -OCH3 H H H
63 H -OCH3 -OCH3 H H Br
64 H -OCH3 -OCH3 H H C1
65 H C1 H H H H
66 H Cl H H H Br
67 H C1 H H H C1
68 H H C1 H H H
69 H H C1 H H Br
70 H H C1 H H C1
71 F H H H H H
72 F H H H H Br
73 F H H H H C1
74 H F H H H H
75 H F H H H Br
76 H F H H H C1
77 H H F H H H
78 H H F H H Br
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-112-
. ......... ... .. ... .
;:.
;:::::.;:<;:;:::. . ...:;<::
>:>>:<:::1~ ;.:<<R <:<:;>;>::::::::::
::..
;.:.
Ex
79 H H F H H Cl
80 H CH3 H H H H
81 H CH3 H H H Br
82 H CH3 H H H Cl
Example 83
10,11-Dihydro-l0-<<6-(f(2-methylphenyl)aminol-
carbonyllaminol-3-pvr.i dinyllcarbonyll-5H-pyrrolof2.l-cl-
f1.41benzodiazQpine
A mixture of 2.0 mmol of 10,11-dihydro-l0-(6-
amino-3-pyridinylcarbonyl )-5H.-pyrrolo [2, 1-_r,] [ 1, 4] benzo-
diazepine and 4.0 mmol of (2-methylphenyl)isocyanate in
12 ml of tetrahydrofuran is refluxed for 16 hours. The
solvent is removed and the residue chromatographed on
silica gel with ethyl acetate-hexane as solvent to give
the product as a solid. .
As described for Example 83, the following
compounds are prepared (Table E).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/1JS96/01076
-113-
Table E
~ N \
~ - ~
N
X
N NH
~=o
NH
Rs Ri
R4
R 2
. . ..: ...
. ....... .,
R :>:>::
84 H CH3 H H H H
85 H CH3 H H H Br
86 H CH3 H H H Cl
87 H H CH3 H H H
88 H H CH3 H H Br
89 H H CH3 H H C1
90 C1 H H H H H
91 C1 H H H H Br
92 Cl H H H H C1
{ 93 H Cl H H H H
94 H C1 H H H Br
95 H Cl H H H Cl
96 H H Cl H H H
97 H H C1 H H Br
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
=114-
õ
>`' ::;::<;
::.:.:
98 H H Cl H H C1
99 Cl Cl H H H H
100 Cl C1 H H H Br
101 ci ci H H H Cl
102 C1 H Cl H H H
103 Cl H C1 H H Br
104 C1 H C1 H H Cl
105 C1 H H H C1 H
106 C1 H H H C1 Br
107 Cl H H H Cl C1
108 H C1 Cl H H H
109 H C1 Cl H H Br
110 H C1 C1 H H Cl
111 F H F H H H
112 F H F H H Br
113 F H F H H Cl
114 F H H F H H
115 F H H F H Br
116 F H H F H C1
117 F H H H F H
118 F H H H F Br
119 F H H H F C1
Example i20
N- f 5- t f 3- f(Dimethylamino) methyl ]- f H-pyrrolof2, 1-cl -
f1,41benzodiazepin-10(11H)1-yllcarbony11-2-pyridiny11-5-
fluoro-2-methylbenzamide
A mixture of 0.44 g of kl-[5-(5$-pyrrolo-
[2, 1-r,] [1, 4]benzodiazepin-10 (11E) -ylcarbonyl) -2-
pyridinyl]-5-fluoro-2-methylbenzamide, 5 ml of a 40%
aqueous solution of dimethylamine and 5 ml of an aqueous
solution of formaldehyde in 50 ml of tetrahydrofuran-
methanol (1:1) is refluxed for 16 hours in the presence
of a drop of glacial acetic acid. The mixture is
concentrated under vacuum and the residue extracted with
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-115-
chloroform. The extract is washed with water, dried
(MgSO4) and the solvent removed. The residue is
* purified by column chromatography on silica gel with
methanol in chloroform as eluent to give 0.45 g of
solid: mass spectrum (CI) 499 (M+1.) .
The following Examples are prepared as
described for Example 120 with formaldehyde and the
appropriate amine.
Example 121
N-f5-ff3-f(Dimethylamino)methyll-f5H-pyrrolof2,1-cl-
f1,41benzodiazepin-10(11H)1-yllcarbonyll-2-pyridinyll-5-
chloro-2-methylbenzamide
Example 122
N-f5-if3-f(Dimethylamino)methyll-f5H-pvrrolof2,1-cl-
I1,41benzodiazepin-10(11H)1-yllcarbonyll-2-pyridinyll-3-
fluoro-2-methylbenzamide
Example 123
N-f5-ff3-f(Dimethylamino)methyll-f5H-pvrrolof2,l-cl-
11,41benzodiazepin-10(11H)-yllcarbonyll-2-pyridinyll-2-
chloro-4-fluorobenzamide
Example 124
N-f5-ff3-f(Dimethylamino)methyll-f5H-pyrrolof2,l-cl-
11,41benzodiazepin-10(11H)l-yllcarbonyll-2-pyridinyll-2-
chloro-5-fluorobenzamide
Example 125
N-f5-ff3-f(Dimethylamino)methyll-f5H-pyrrolof2,1-cl-
f1.41benzodiazepin-10(11H)l-yllcarbonyll-2-pyridinyll-2-
chlorobenzamide
Example 126
N-f5-ff3-f(Dimethylamino)methyll-f5H-pyrrolof2,1-c1-
f1,41benzodiazepin-10(11H)1-yllcarbonyll-2-pyridinyll-2-
fluoro-5-chlorobenzamide
Example 127
N-f5-ff3-f(Dimethylamino)methyll-f5H-pyrroloi2,1-cl-
f1,41benzodiazepin-10(11H)1-yllcarbonyll-2-pyridinyll-
2,4-dichlorobenzamide
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96122293 PCT/US96101076
-116-
Example 128
N-f5-fi3-(1-Pyrrolidinylmet_yl)-5H-pyrrolof2,1-cl-
11,41benzodiazepin-10(11H)-yllcarbonyll-2-pyridinyll-2-
chloro-4-fluorobenzamide
5. Exa le 129
N-f5-ff3-f(Dimethylamino)methyll-f5H-pyrrolof2,l-cl-'
.il 4lbenzodiazepin-10(11H)1-vllcarbonyll-2-pyridiny~
chlorobenzeneacetamide
Example 130
N-f2-(Dimethylamino)ethyll-N-f5-(5H-wrrolof2 1-c1-
f1,41benzodia7-epin-10(11H)-ylcarbonyl)-2-pyridinyll-5-
fluoro-2-methylbenzamide
To a solution of 0.75 g of 10-[[6-[2-
(dimethylamino)ethylamino]-3-pyridinyl]carbonyl]-10,11-
dihydro-5H-pyrrolo[2,1--Q][1,4]benzodiazepine and 5 ml of
diisopropylethylamine in 75 ml of dichloromethane is
added (slowly) 0.35 g of 5-fluoro-2-methylbenzoyl
chloride in 10 ml of dichloromethane. The mixture is
stirred at room temperature for 16 hours and the
solution washed well with water. The organic layer is
dried (MgSO4) and the solvent removed under vacuum. The
residue is purified by column chromatography on silica
gel with 30% methanol in chloroform as eluent to give
0.80 g of yellow solid; mass spectrum (CI), 511 (M+1).
Example 131
N-f3-(Dimethylamino)propyll-N-r5-(5H-pyrrolof2.1-cl-
T1,41benzodiazepin-10(11H)-ylcarbonyl)-2-pyridinyll-5-
fluoro-2-methylbenzamide
A solution of 6.35 g of 5-fluoro-2-methyl-
benzoyl chloride in 10 ml of dichloromethane is added to
a solution of 2 mmol of 10-[[6-[3-(dimethylamino)-
propylamino]-3-pyridinyl]carbonyl]-10,11-dihydro-5H-
pyrrolo[2,1-c][1,4]benzodiazepine and 5 ml of diiso-
propylethylamine in 75 ml of dichloromethane. The
solution is stirred 16 hours at room temperature, washed
with water, dried (MgSO4) and the solvent removed. The
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-117-
residue is purified by column chromatography over silica
gel with 30% methanol in chloroform as eluent to give
0.75 g of solid; mass spectrum (CI) 525 (M+1).
Example 132
5' N-f2-(Dimethylamino)methyll-N-5-(5H-pyrrolof2,1-c1-
11,41benzodiazepin-10(11H)-ylcarbonyl)-2-pyridinyll-5-
fluoro-3-methvlbenzamide
As described for Example 130, a solution of 2
mmol of 10-[[6-[2-(dimethylamino)methylamino]-3-pyri-
dinyl]carbonyl]-10,11-dihydro-5H-pyrrolo[2,1-_Q][1,4]-
benzodiazepine, 8 ml of diisopropylethylamine, and 2.2
mmol of 5-fluoro-2-methylbenzoyl chloride in 100 ml of.
dichloromethane is stirred at room temperature for 16
hours. The solvent is removed anci the product purified
by chromatography on silica gel to give a solid.
Example 133
N-f5-ff3-f(Dimethylamino)methyll-f5H-pyrrolof2,l-cl-
f1,41benzodiazepin-10(11H)lyllcarbonyll-2-pyridinyll-
3,4,5-trimethoxybenzamide
A mixture of 1.0 g of N-[5-(5H-pyrrolo[2,1-
_Q][1,4]benzodiazepin-10(11H)-ylcarbonyl)-2=pyridinyl]-
3,4,5-trimethoxybenzamide, 10 ml of 40% aqueous di-
methylamine, 10 ml of 35% aqueous formaldehyde in 50 ml
of tetrahydrofuran-methanol (1:1) plus 1 drop of acetic
acid is refluxed for 16 hours. The mixture is concen-
trated and the residue extracted with chloroform. The
extract is washed with water, dried (MgSO4), concen-
trated and the residue purified by column chromatography
(silica gel) with 5% methanol in chloroform as eluent.
= 30 The fractions containing product are combined to give
0.80 g of solid; mass spectrum (CI) 556 (M+1).
Example 134
N-f5-(Pyridof3,2-elpyrrolofl,2-alpyrazin-5(6H)-
ylcarbonyl)-2-pyridinyll-5-fluoro-2-methylbenzamide
To a chilled (0 C) solution of 0.343 g of 5,6-
dihydropyrido[3,2-e]pyrrolo[1,2-a]pyrazine and 1.1 ml of
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-118-
triethylamine in 5 ml of dichloromethane is added 1.17 g
of 6-(5-fluoro-2-methylbenzoyl)aminopyridine-3-carbonyl
chloride. The mixture is stirred at room temperature
for 16 hours. To the mixture is added 50 ml of di-
chioromethane and 20 ml of water. The organic layer is
separated and washed with 20 ml each of 1 M NaHCO3 and
brine. The organic layer is dried (Na2SO4) and passed
through a thin pad of hydrous magnesium silicate and the
pad washed with dichloromethane. The filtrate is con-
centrated and the residue chromatographed on silica gel
prep-plates with ethyl acetate-hexane (1:1) as eluent.
The product is crystallized from ethyl acetate to give
0.38 g of white crystals, m.p. 226-234 C.
As described for Example 134 the following
compounds are prepared (Table F).
Table F
N N
( =
N
i ~
O aJ) X R
N N NHC
F~
Ft5 R = ; ,
R2 . . : : R R~ RS x .:. ... ::
135 H CH3 H H H H
136 H CH3 H H H Br 137 H CH3 H H H Cl
138 H H CH3 H H H
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-119-
139
H H CH3 H H Br
140 H H CH3 H H C1
141 C1 H H H H H
5. 142 Cl H H H H Br
143 C1 H H H H C1
144 H C1 H H H H
145 H C1 H H H Br
146 H C1 H H H C1
147 H H Cl H H H
148 H H C1 H H Br
149 H H C1 H H C1
150 C1 C1 H H H H
151 C1 C1 H H H Br
152 Cl C1 H H H C1
153 Cl H C1 H H H
154 C1 H C1 H H Br
155 C1 H C1 H H C1
156 C1 H H H C1 H
157 C1 H H H Cl Br
158 C1 H H H Ci C1
159 H C1 C1 H H H
160 H C1 C1 H H Br
161 H C1 C1 H H Cl
162 F H F H H H
163 F H F H H Br
164 F H F H H C1
165 F H H F H H
166 F H H F H Br
167 F H H F H C1
168 F H H H F H
169 F H H H F Br
170 F H H H F C1
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-120-
Example 171
N- f5- (Pyrrolo f 1 2-al quinoxalin-5 (4H) -ylcarbonyl) -2-
pyridiny11-5-fluo o-2-methylbenzamide
~
To a chilled (0 C) solution of 0.341 g of 4,5-
dihydropyrrolo[1,2-a]quinoxaline and 1.11 ml of tri-
ethylamine in 5 ml of dichloromethane is added 1.17 g of
6-[(5-fluoro-2-methylbenzoyl)amino]pyridine-3-carbonyl
chloride. The mixture is stirred under argon at room
temperature for 16 hours. The mixture is diluted with
50 ml of dichloromethane and 20 ml of water and the
organic layer is separated. The organic layer is washed
with 20 ml each of 1 M NaHCO3 and brine and dried
(Na2SO4). The solution is filtered through a thin pad
of hydrous magnesium silicate and the pad washed with
dichloromethane. The filtrate is concentrated and the
residue purified on silica gel prep-plates with ethyl
acetate-hexane (1:1) as solvent to give a solid. The
solid is crystallized from ethyl acetate to give 0.38 g
of crystals, m.p. 190-196 C.
As described for Example 171 the following
compounds are prepared (Table G).
Table G
N
N
X R R
2
N NHC
R 3
R5 R4
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-121-
, ::. : ::. :........:::. ::: ......:. : . . : :. .
V 'p
::: = ::... ..... ....
..: ~~
: ~:::'r::i::i}}::: ':=~=~:::::::::::::j~i:: :.~:~::::::,'.::i:ii:== ::
~.ii:~i::::~:i?:~:: ::::ii:':i::i:ti:;:;'::,::i:::::;:::i:::::
~~::~:.::s::. ~:~'
172 H CH3 H H H H
173 H CH3 H H H Br
174 H CH3 H H H C1
175 H H CH3 H H H
176 H H CH3 H H Br
177 H H CH3 H H C1
178 Cl H H H H H
179 Cl H H H H Br
180 Cl H H H H Cl
181 H Cl H H H H
182 H C1 H H H Br
183 H Cl H H H Cl
184 H H Cl H H H
185 H H C1 H H Br
186 H H Cl H H C1
187 C1 Cl H H H H
188 C1 Cl H H H Br
189 Cl Cl H H H C1
190 C1 H C1 H H H
191 Cl H C1 H H Br
192 C1 H C1 H H C1
193 C1 H H H Cl H
194 C1 H H H Cl Br
195 C1 H H H Cl Cl
196 H C1 C1 H H H
197 H C1 Cl H H Br
198 H Cl Cl H H C1
199 F H F H H H
200 F H F H H Br
201 F H F H H C1
202 F H H F H H
203 F H H, F H Br
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-122-
.::, .
.::::;:>::::::~ `':::`>:::`
~o.... :.>. ~:::.,:.,:.. ~
:::::: ::
204 F H H F H C1
205 F H H H F H
206 F H H H F Br
--T-
207 F H H H F Cl
Exa le 208
N-f5-(4H-Pyrazolo[5,1-clf1,41benzod_iazepin-5(10H)-
ylcarbonyl)-2-pyridinyll-5-fluoro-2-methylbenzamide
To a chilled (0 C) solution of 0.37 g of 5,10-
dihydro-4H-pyrazolo[5,1--Q][1,4]benzodiazepine and 836
microliters of triethylamine in 5 ml of dichloromethane
is added 0.761 g of 6-[(5-fluoro-2-methylbenzoyl)-
amino]pyridine-3-carbonyl chloride. The mixture is
stirred at room temperature under argon for 5 hours. An
additional 420 microliters of triethylamine and 0.38 g
of 6-[(5-fluoro-2-methylbenzoyl)amino]pyridine-3-
carbonyl chloride is added and the mixture stirred 16
hours. The mixture is diluted with 60 ml of di-
chloromethane and washed with 25 ml each of H20, 1 M
NaHCO3, brine and dried (Na2SO4). The solution is
filtered (twice) through a thin pad of hydrous magnesium
silicate and the pad washed with dichloromethane. The
filtrate is concentrated to give a yellow glass (0.68 g)
which is crystallized from ethyl acetate to give 0.38 g
of white crystals,m.p. 250-260 C; mass spectrum (FABL).
442.4 (M+H).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-123-
Y
Table H
1
N
N
. ~ ~
N
O R' R2
N NH R3
Ft 5 R4
::<::::<:::E4::'.`';'`~~~><~:>; ~~>f~<`~<'
1~Ãi::::: :. ::..:... . ...~ ...:...:. ::......:2.:.: :,... ... : .. ...~:: ::
: :. :..:
209 H CH3 H H H H
210 H CH3 H H H Br
211 H CH3 H H H Cl
212 H H CH3 H H H
213 H H CH3 H H Br
214 H H CH3 H H. C1
215 C1 H H H H H
216 C1 H H H H Br
217 C1 H H H H C1
218 H C1 H H H H
219 H -Cl H H H Br
220 H Cl H H H C1
221 H H Cl H H H
222 H H C1 H H Br
223 H H Cl H H Cl
224 Cl Ci H H H H
225 C1 C1 H H H Br
226 Cl Cl H H H Cl
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-124-
'; ;::;
;r ;
~ ..:..... ..:...._. ..
~x. .:i~~s...,... ~. :::. . .... .:... ..... 2.: :,.. . ...,.:: . .. 3.
...:.:.., .... .... 4. ... ,. 5 .:.: ....:: ::: ..~.,.:......:::::.. :..
227 ci H ci H H H
228 ci H ci H H Br
229 ci H ci H H ci
230 ci H H H ci H
231 ci H H H ci Br
232 ci H H H C1 ci
233 H C1 ci H H H
234 H ci ci H H Br
235 H ci ci H H C1
236 F H F H H H
237 F H F H H Br
238 F H F H H ci
239 F H H F H H
240 F H H F H Br
241 F H H F H ci
242 F H H H F H
243 F H H H F Br
244 F H H H F ci
Example 245
N-f5-(4H-PXrazolof5,1-clfl.4lbenzodiazepin-5(lOH)-
ylcarbonvl)-.-pyridinyll-f1.1'-biphenyll-2-carboxamide
To a chilled (0 C) solution of 0.185 g of
5,10-dihydro-411-pyrazolo[5,1-.Q][1,4]benzodiazepine and
417 l of triethylamine in 3.5 ml of dichioromethane is
added 0.35 g of 6-(2-biphenylcarbonyl)aminopyridine-3-
carbonyl chloride in 1.5 ml of dichloromethane. The
mixture is stirred at room temperature under argon for
16 hours,, diluted with 40 ml of dichloromethane and 20
ml of water. The organic layer is separated, washed
with 20 ml of brine and dried (Na2SO4). The solution is
filtered through a thin pad of hydrous magnesium
silicate. The filtrate is concentrated to dryness under
vacuum to give 0.4 g of solid. The solid is purified on
silica gel prep-plates with ethyl acetate-hexane (3:1)
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-125-
as eluent to give 170 mg of a brown glass, m.p. 110-
150 C.
As described for Example 245, the following
derivatives are prepared (Table H).
Table H
/N
N
N R
O X R2
N NH
:: :: :;::;> _
:
.:.> :>:::::::::::::: :::
` < :::: `::<:::>
'~
:>::,;:::
246 H Cl
247 H H
s
248 H H
s
249 H H E \ 250 H H
251 Cl Cl
S
252 Cl H
S
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-12 6-
253 H Cl
S
254 H H
255 C1 H
NI
256 H C1 ~
N
257 H H /D N( q.O 2
258 H Cl 3 N( qK)
259 H H NK2
260 H H NFCF~
Example 261
1,0-ff6-f(2-Methylpropyl)aminol-3-pyridinytlcarbonyll-
10.11-dihydro-5H-pyrrolor2.1-clf1.41benzodiazepine
A mixture of 0.16 g of 10-[(6-chloro-3-
pyridinyl)carbonyl]-10,11-dihydro-5H-pyrrolo[2,1-_q]-
[1,4]benzodiazepine, 40 mg of pyridine and 2 ml of 2-
methylpropylamine is stirred and heated at 100 C in a
sealed vessel for 1 hour. To the mixture is added 0.2
ml of N,N-dimethylpropyleneurea and the mixture is
heated at 110 C for 7 hours. The volatiles are removed
under vacuum and 10 ml of 0.5 N NaOH is added to the
residue. The mixture is filtered and the solid washed
with water and then hexane. The solid is dissolved in
ethyl acetate and the solution washed with 0.5 N NaOH,
brine and dried (Na2SO4). The solution is filtered
through a thin pad of hydrous magnesium silicate and the
filtrate concentrated to dryness. The residue is tri-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-127-
turated with diisopropylether-hexane to give 0.18 g of
white solid; mass spectrum (CI) 361 (M+H).
As described for Example 261, the following
derivatives are prepared (Table I).
Table I
/D
N
N
O
)11~
N NHR
::.:...
. . ..
:
>::;>::>::>r;<>
................ ::: : :: :::>
<;{?. 2;;iii:
:.......... .:::.: .... :..: :; :::
:.::. ...:.:::.:.
` ::~~~..... :.
*262 C -CH2CH2C (CH3) 3
**263 C - Cu -0
264 C
265 C -CH2CH2C(CH3)2-
CH2CH3 .
266 C -CH2(CH2)4CH3
267 C - CH2-` )
268 C -CH2CH2CH (~CH/3) 2
269 N -CH2CH2C (CH3) 3
270 N - CH2--0
271 N -0
272 N -CH2(CH2)4CH3
*mass spectrum (CI) 389 (M+1)
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-128-
**mass spectrum (CI) 401 (M+1)
Example 273
10-ff6-((Phenylmethyl)aminol-3-pyridinyllcarbony 1-
19,11-dihydro-5H-pyrrolor2,1-clf1,41benzodiazepine
A mixture of 0.16 g of 10-[(6-chloro-.3-
pyridinyl)carbonyl]-10,11-dihydro-5H-pyrrolo[2,1--r,][1,4]
benzodiazepine, 0.5 ml of benzylamine and 0.2 ml of
N,N'-dimethylpropyleneurea is stirred and heated at
110 C for 7 hours. After cooling to room temperature,
the mixture is washed with hexane (3 times 10 ml). The
residue is dissolved in water and made alkaline with 1 N
NaOH. The suspension is washed with H20 and extracted
with ethyl acetate. The organic extract is washed with
brine, dried (Na2SO4) and filtered through a thin pad of
hydrous magnesium silicate. The filtrate is evaporated
and the residue triturated with diethyl ether-hexane to
give 0.20 g of white solid; mass spectrum (CI) 395
(M+H).
As described for Example 273, the following
derivatives are prepared (Table J).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-129-
Table J
N C
N
O
N NHR
~
;:; . ;:'::`:>:- ':: :;:,
274 C CH
2
275 C - ~~ /-\
2
276 C CI
-cH2 /\
277 c \
- CH2
S
278 C - / \
C,u '2CH2
S
279 c - CH
2
280 c -CH 2
O-N
281 N -CH
2
282 N -CH CH CH
2 2 2
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-130-
283 N CN / \ -
CH3
284 N
CH2
S
285 N C H 2 ~ \
CH2
S
286 N i I
- CH2CH2 0
287 N - Cu ~_DN
2
.' Example 288
10,11-Dihvdro-l0-ff6-(cyclohexylthio)-3-
pvridinvllcarbonyll-5H-pyrrolo-f2 1-
c1f1,41benzodiazepine
To a suspension of 35 mg of sodium hydride
(60% in oil) in 3 ml of tetrahydrofuran is added under
argon 0.10 g of cyclohexylmercaptan. A white preci-
pitate forms and after 0.5 hour at room temperature, 1
ml of N,N'-dimethylpropyleneurea is added. To the
mixture is added 0.13 g of 10-[(6-chloro-3-pyridinyl)-
carbonyl]-10,11-dihydro-5H-pyrrolo[2,1--q][1,4]benzo-
diazepine in 2 ml of tetrahydrofuran. The mixture is
stirred at room temperature for 18 hours, quenched with
water and ammonium chloride and concentrated under
vacuum. The aqueous suspension is filtered and the
solid washed with water and hexane. The solid is
purified by chromatography on silica gel prep-plates
with ethyl acetate-hexane (1:4) as eluent to give 0.13 g
of white solid; mass spectrum (CI): 404 (M+H).
As described for Example 288, the following
derivatives are prepared (Table K).
SUBST(TUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTJUS96/01076
-131-
Table K
f
D
N
N
O ~
I
.
N S- R
Ex. Na. D
289 C CF~--o
290 C _CH 20
291 C -CH2CH2C(CH3)3
292 C Cu Cu /~\
293 c -CH2CH2 ~ \
S
294 N CH27-0
295 N - CH /-\
2
296 N -CH2CH2C(CH3)3
297 N _ CH 2CN 0
= 298 N CH2CH2
S
299 N
-CH2 z- N
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-132-
300 C
N
Example 301
10,11-Dihydro-10-rf6-f(2-methylphenyl)aminol-3-
pyridinyllcarbonyl -5H-pyrrolo[2,1-c1f1,41benzodiazepine
A mixture of 0.5 g of 10-[(6-chloro-3-pyri-
dinyl)carbonyl]-10,11-dihydro-5H-pyrrolo[2,1-_r,][1,4]-
benzodiazepine and 0.36 g of Q-toluidine in 60 ml of
N,N-dimethylformamide is refluxed for 16 hours. The
mixture is poured into 200 ml of ice-water and extracted
with three 100 ml portions of chloroform. The extract
is washed with water, dried (Na2SO4) and the solvent
removed. The residue is purified by chromatography on
silica gel prep-plates with hexane-ethyl acetate (5:1)
as solvent to give 0.56 g of yellow solid: mass spectrum
(CI) 395.2 (M+H).
As described for Example 301, the following
derivatives are prepared (Table L).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT1US96101076
-133-
:
Table L
,D
N
- I _
N
N N-R
Rs, R1
~
~
R4 R2
R3
j:i:ii`~~!ii;i:i:'iiti~El;':~'~~;>i`::.~>~`;; i>
;
302 C H C1 H H H H
5 303 C H C1 H C1 H H
304 C H C1 H H F H
305 C H F H F H H
306 C H CH3 H H F H
307 C H CF3 H H H H
308 C CH3 CH3 H H H H
309 C H H H H H H
310 N H H H H H H
311 N CH3 H H H H H
312 N H CF3 H Ci H H
313 N H CH3 H H F H
314 N H F H F H H
315 N H C1 H H F H
316 N H Cl H Cl H H
317 N H Cl H H H H
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-134-
Examiple 318.
N-f4-(5H-Pyrrolof2,1-c1f1,41benzodiazepin-10(11H)-
ylcarbonyl)-2-methoxypheny11f1,1'-bipheny11-2-
carboxamide
To a solution of 0.70 g of 10,11-dihydro-5ii-
pyrrolo[2,1-_Q][1,4]benzodiazepine and 0.56 g of N,N-
diisopropylethylamine in 50.m1 of methylene chloride is
added 1.35 g of 4-[([1,1'-bipheny].]-2-carbonyl)amino]-3-
methoxybenzoyl chloride followed by stirring at room
temperature for 18 hours. The reaction mixture is
washed with water and saturated aqueous NaHCO3 and the
organic layer dried(Na2SO4). The organic layer is
passed through hydrous magnesium silicate and the
filtrate concentrated in vacuo to give a residue which
is dissolved in methylene chloride and passed through a
pad of hydrous magnesium silicate two additional times
to give upon concentration in vacuo to give 1.5 g of
amorphous solid. M+=512.
Example 319
N-f4-(5H-Pyrrolof2,1-c1f1,41benzodiazepin-10(11H)-
ylcarbonyl)-3-chlorophenyl.lfl.1'-bipheny 1-2-carboxamide
To a solution of 0.52 g of 10,11-dihydro-511-
pyrrolo[2,1-_Q][1,4]benzodiazepine and 0.39 g of N,N-
diisopropylethylamine in 25 ml of methylene chloride is
added 1.1 g of 4-[([1,1'-biphenyl]-2-carbonyl)amino]-2-
chlorobenzoyl chloride followed by stirring at room
temperature for 18 hours. The reaction mixture is
washed with water and saturated aqueous NaHCO3 and the
organic layer dried(Na2SO4). The organic layer is
passed through hydrous magnesium silicate and the
filtrate concentrated in vacuo to give a residue which
is dissolved in methylene chloride and passed through
hydrous magnesium silicate two additional times to give
upon concentration _in vacuo 1.10 g of the desired
product as a residue. M+=516,518,520.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-135-
Example 320
N-f4-(5H-Pyrroloi2,1-c1(1,41benzodiazepin-10(11H)-
ylcarbonyl)phenyll(1 1'-bipheny11-2-carboxamide
To a solution of 0.65 g of 10,11-dihydro-SH-
pyrrolo[2,1-~][.1,4]benzodiazepine and 0.52 g of N,N-
diisopropylethylamine in 25 ml of methylene chloride is
added 1.34 g of 4-[([1,1'-biphenyl]-2-carbonyl)amino]-
benzoyl chloride followed by stirring at room tempera-
ture for 18 hours. The reaction mixture is washed with
water and saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through
hydrous magnesium silicate and the filtrate concentrated
in vacuo to give a residue which is dissolved in
methylene chloride and passed through hydrous magnesium
silicate two additional times to give upon concentration
in. vacuo to give 1.02 g of the desired product as a
residue. M+=482.
Example 321
N-f4-(5H-Pyrrolof2,i-clf1,41benzodiazepine-10(11H)-
ylcarbonyl)phenyll-2-(phenylmethyl)benzamide
To a solution of 0.75 g of 10,11-dihydro-5H-
pyrrolo [2, 1-c] [ l, 4] benzodiazepine and 0.57 g of N,N-
diisopropylethylamine in 50 ml of methylene chloride is
added 1.53 g of 4-[[2-(phenylmethyl)benzoyl]amino]-
benzoyl chloride followed by stirring at room tempera-
ture for 18 hours. The reaction mixture is washed with
water and saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through
hydrous magnesium silicate and the filtrate concentrated
in vacuo to give a residue which is dissolved in
rnethylene chloride and passed through hydrous magnesium
silicate two additional times to give upon concentration
in vacuo to give 1.97 g of the desired product as an
amorphous solid.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-136-
Example 322
N-f4-(5H-Hyrrolof2,1-cl[1,41ben.zodiazepin-10(11H)-
ylcarbonyl)-3-chlorophenyll-2-(phenylmethyl)benzamide
To a solution of 0.92 g of 10,11-dihydro-5H-
pyrrolo[2,-1-a][1,4]benzodiazepine and 0.72 g of N,N-
diisopropylethylamine in 50 ml of methylene chloride is
added 2.4 g of 2-chloro-4-[[(2-phenylmethyl)benzoyl]-
amino]benzoyl chloride followed by stirring at room
temperature for 18 hours. The reaction mixture is
washed with water and saturated aqueous NaHCO3 and the
organic layer dried(Na2SO4). The organic layer is
passed through hydrous magnesium silicate and the
filtrate concentrated in vacuo to give a residue which
is dissolved in methylene chloride and passed through
hydrous magnesium silicate two additional times to give
upon concentration in vacuo 2.87 g of the desired
product as an amorphous compound.
Example 323
N-f4-(5H-Pyrrolof2,1-c1f1,41benzodiazepin-10(11H)-
yl.carbonyl)-2-methoxyphenyll-2-(phenylmethyl)benzamide
To a solution of 0.75 g of 10,11-dihydro-5,H-
pyrrolo[2,1--r,-][1,4]benzodiazepine and 0.58 g of N,N-
diisopropylethylamine in 50 ml of methylene chloride is
added 1.69 g of 3-methoxy-4-[[(2-phenylmethyl)benzoyl]-
amino]benzoyl chloride followed by stirring at room
temperature for 18 hours. The reaction mixture is
washed with water and saturated aqueous NaHC03 and the
organic layer dried(Na2SO4). The organic layer is
passed through hydrous magnesium silicate to give upon
concentration in vacuo 1.92 g of the desired product as
an amorphous solid.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-137-
Example 324
N-f4-(5H-Pyrrolof2,1-cl(1.41benzodiazepin-10(11H)-
ylcarbonyl)phenyllf4'-(trifluoromethyl)fl.l'-biphenyll-
2-carboxamide
A solution of 1.14 g of [4'-(trifluoromethyl)-
[1,1'-biphenyl]-2-carbonyl chloride in 10 ml of
methylene chloride is added dropwise to an ice cold
s-olution of 1.0 g of 10,11-dihydro-10-(4-aminobenzoyl)-
5H-pyrrolo[2,1-c][1,4]benzodiazepine and 0.52 g of N,N-
diisopropylethylamine in 25 ml of methylene chloride.
The reaction mixture is stirred at room temperature for
18 hours and washed with water, saturated aqueous NaHCO3
and the organic layer dried(Na2SO4). The organic layer
is passed through a pad of hydrous magnesium silicate
two times to give 1.70 g of the desired product as an
amphorous compound.
Example 325
N-f4-(5H-Pyrroloi2.1-clf1,41benzodiazepin-10(11H)-
ylcarbonyl)-3-methoxyphenyllf4'-(trifluoromethyl)r1.1'-
biphenyll-2-carboxamide
A solution of 1.87 g of [4'-(trifluoromethyl)-
[1,1'-biphenyl]-2-carbonyl chloride in 10 ml of
methylene chloride is added dropwise to an ice cold
solution of 0.74 g of 10,11-dihydro-10-(4-aminobenzoyl)-
51i-pyrrolo[2,1-c.][1,4]benzodiazepine and 0.56 g of N,N-
diisopropylethylamine in 50 ml of methylene chloride.
The reaction mixture is stirred at room temperature for
18 hours and washed with water, saturated aqueous NaHCO3
and the organic layer dried(Na2SO4). The organic layer
is passed through a pad of hydrous magnesium silicate
two times to give the desired product as a residue which
is crystallized from ethyl acetate to give 2.33 g of the
desired product, 211-212 C.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-138-
Example 326
N-f4-(5H-Pyrrolof2.1-clf1,41benzodiazepin-10(11H)-
ylcarbonyll-2-chlorophenyllf4'-(trifluoromethyl)f1.1'-
biphenyll-2-carboxamide
A solution of 1.35 g of 2-chloro-4-[([4'-
(trifluoromethyl)[1,1'-biphenyl]-2-carbonyl)amino]-
benzoyl chloride in 10 ml of methylene chloride is added
dropwise to an ice cold solution of 0.63 g of 10,11-
dihydro-5H-pyrrolo[2,1--q][1,4]benzodiazepine and 0.48 g
of N,N-diisopropylethylamine in 50 ml of methylene
chloride. The reaction mixture is stirred at room
temperature for 18 hours and washed with water,
saturated aqueous NaHCO3 and the organic layer
dried(Na2SO4). The organic layer is passed through a
pad of hydrous magnesium silicate two times to give 1.63
g of the desired product as a non-crystalline solid..
Example 327
N-f4-(5H-Pyrrolof2,1-c1fl,41benzodiazepin-10(1 H)-
ylcarbonyl)phenyll-2-methylpyridine-3-carboxamide
To a stirred solution of 1.0 g of 10,11-
dihydro-10-(4-aminobenzoyl)-5H-pyrrolo[2,1-_t][1,4]benzo-
diazepine and 3 ml of N,N-diisopropylethylamine in 100
ml of methylene chloride is slowly added 600 mg of 2-
methylpyridine-3-carbonyl chloride dissolved in 15 ml of
methylene chloride. The reaction mixture is stirred at
room temperature for 2 hours. The reaction mixture is
quenched with water and the organic layer washed well
with water. The organic layer is dried(MgSO4), filtered
and evaporated in vacuo to a residue which is purified
by column chromatography on silica gel by elution with
1:1 ethyl acetate:hexane to give 800 mg of the desired
product as a pale yellow residue. M+=422.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-139-
Example 328
N- ( 4- (51-i-Pyrrolo f 2 1-cl (1 41 benzodiazepin-10 (11 )-yl-
carbonyl)-3-chlorophenyll-2-methyl-pyridine-3-
carboxamide
A mixture of 1.1 g of 10,11-dihydro-l0-(4-
amino-2-chlorobenzoyl)-SH-pyrrolo[2,1--Q][1,4]benzodiaze-
pine and 3 ml of N,N-diisopropylethylamine in 100 ml of
methylene chloride is stirred while a solution of 600 mg
of 2-methylpyridine-3-carbonyl chloride in 15 ml of
methylene chloride is added slowly. The reaction
mixture is stirred at room temperature for 2 hours. The
reaction mixture is quenched with water and the organic
layer washed with water, dried(MgSO4), filtered and
evaporated in vacuo to a residue. The product is
purified by column chromatography on silica gel by
elution with 1:1 ethyl acetate:hexane to give the
desired product as a pale yellow residue. M+=456.
Example 329
N-f5-(5H-Pyrrolof2 1-clfl 4lbenzodiazepin-10(11H)-
ylcarbonyll-2-pyridinyll-2-methylpyridine-3-carboxamide
A mixture of 2.5 g of 6-[[3-(2-methyl-
pyridinyl)carbonyl]amino]pyridine-3-carboxylic acid and
ml of thionyl chloride is refluxed for 3 hours and
the mixture evaporated to dryness in vacuo to give a
25 solid. A solution of the solid in 50 ml of methylene
chloride is added to 2 g of 10,11-dihydro-5H-pyrrolo-
[2,1-c][1,4]benzodiazepine dissolved in 50 ml of
dichloromethane containing 3 ml of N,N-diisopropyl-
ethylamine at room temperature. The reaction mixture is
stirred at room temperature for 2 hours and quenched
with water; washed with water; dried(MgSO4), filtered
and evaporated in vacua to a residue. The residue is
purified by column chromatography on silica gel by
elution with 1:1 ethyl acetate:hexane to give 2.0 g of
the desired product as a solid. M+=423.
-
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-140-
Example 330
N-(5-(5H-Pyrrolof2 1-clf1,41benzodiazepin-10(11H)-
ylcarbonyll-2-pvridinyll-2-methylpyridine-3-carboxamide
Hvdrochloride
To a solution of 1.0 g of N-[5-(5H-pyrrrolo-
[2, 1--Q] [ 1, 4 ] benzodiazepin-10 (11H) -ylcarbonyl) -2-
pyridinyl]-2-methylpyridine-3-carboxamide in 50 ml of
methanol is added hydrogen chloride gas. The mixture is
stirred at room temperature for 30 minutes and the
solvent removed under vacuum. The residue is triturated
with ether to give 1.0 g of the desired product as a
solid: mass spectrum(CCl);459(M+).
Example 331
N-f4-(5H-Pyrrolof2,1-c1(1,41benzodiazepin-10(11H)-
ylcarbony l)phenyll-2-fN-methylpinerazinvll-pvridine-3-
c-arboxamide Hydrochloride
The method of Example 330 is used to prepare
the desired product as a solid: M*=543.
Examgle 332
N- f 4- (5H-PYrrolo (2 1-cl [ 1, 41 benzodiazepin-10 (11H) -
ylcarbonyl)phenvll-2-(dimethylamino)-pyridine-3-
c-arboxamide Hydrochloride
The method of Example 330 is used to prepare
the desired product as a solid: M+=487.
Example 333
N- f 4- (5H-Pyrrolo f 2 1-cl (1, 41 benzodiazepin-10 (11H) -
ylcarbonyl)phPnyll-2-chloropyridine-3-carboxamide
To a stirred solution of 6.06 g of 10,11-
dihydro-l0-(4-aminobenzoyl)-5H-pyrrolo[2,1-_r.][1,4]benzo-
diazepine and 10 ml of N,N-diisopropylethylamine is
added a solution of 4.0 g of 2-chloropyridine-3-carbonyl
chloride in 25 ml of methylene chloride. The reaction
mixture is stirred at room temperature for 1 hour. The
reaction mixture is quenched with water and the organic
layer washed well with water. The organic layer is
dried, filtered and evaporated in vacuo to a pale yellow
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-141-
product which is crystallized from 1:1 ethyl
acetate:hexane to give 7.0 g of the desired product;
M+=442.
Example 334
N-f4-(5H-Pyrrolof2.1-c1f .41b_nzodiazepin-10(11H)-
ylcarbonyl)phenyll-2-(methylamino)pyridine-3-carboxamidP
A mixture of 1 g of N-[4-(5H-pyrrolo[2,1-_Q]-
[1,4]benzodiazepin-10(11H)-ylcarbonyl)phenyl]-2-chloro-
pyridine-3-carboxamide, 1 g of K2C03 and 10 ml of a 40%
solution of monomethylamine is heated in 25 ml of
dimethylsulfoxide for 8 hours at 100 C. The reaction
mixture is poured over water and the pale yellow solid
separated. The reaction mixture is filtered and the
collected solid washed well with water. After drying
the solid is purified by column chromatography on silica
gel by elution with 9:1 ethyl acetate:methanol to give
850 mg of the desired product as a pale yellow
solid:M+=437.
Example 335
N-f4-/5H-pvrrolo[2 1-clfl 4lbenzodiaz pin-10(11H)-
ylcarbonyl)phenyll-2-((3-dimethylaminopropyl)aminnl-
pyridine-3-carboxamide
Using the conditions of Example 334 and N-[4-.(5H-
pyrrolo[2,1-_Q][1,4]benzodiazepin-10(11H)-ylcarbonyl)-
phenyl]-2-chloropyridine-3-carboxamide and 3-(dimethyl-
amino)propylamine gives 900 mg of the desired
product:M+=508.
Example 336
N- ( 4- (5u-Pyrrolo [2 1-cl (1 41 benzodiaz pin-10 (11H) -
ylcarbonyl)phenyll-2-(i-)Diperidinyl)-pyridine-3-
4
carboxamide
Using the conditions of Example 334 and 1 g of
= N-[4-(5H-pyrrolo[2,1-c][1,4]benzodiazepin-10(11H)-
ylcarbonyl)phenyl]-2-chloropyridine-3-carbox.amide and 5
ml of piperidine t_ives 700 mg of the desired
product:M+=491.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-142-
Example 337
N-f4-(5H-pyrrolof2,1-clf1,41benzodiazepin-10(11H)-
ylcarbonyl)phenyll-2-(4-methyl-l-piperazinyl)-pyridine-
3-carboxamide
Using the conditions of Example 334 and 1 g of
N- [4- (SI.i-pyrrolo [2, 1-L] [1, 4]benzodiazepin-10 (11H) -
ylcarbonyl)phenyl]-2-chloropyridine-3-carboxamide and 5
ml of N-methylpiperazine gives 1 g of the desired
product:M+=500.
Example 338
N-f4-(5H-Pyrrolo(2,1-clf1,41benzodiazepin-10(11H)-
ylcarbonyl)phenyll-2-(dimethylamino)-pyridine-3-
carboxamide
Using the conditions of Example 334 and 1 g of
N- [4- (5)=i-pyrrolo [2, 1--Q] [ 1, 4] benzodiazepin-10 (11H) -
ylcarbonyl)-phenyl]-2-chloropyridine-3-carboxamide and
10 ml of 40% N,N-dimethylamine gives 700 mg of the
desired product:M+=451.
Example 339
N-f4-(5H-Pyrrolo[2,1-clf1,41benzodiazepin-10(11H)-ylc
arbonyl)phenyll-2-(morpholino)-g,vridine-3-carboxamide
Using the conditions of Example 334 and 1 g of
N- [4- (5H-pyrrolo [2, 1-c] [ 1, 4 ] benzodiazepin-10 (11H) -
ylcarbonyl)-phenyl]-2-chloropyridine-3-carboxamide and 5
ml of morpholine gives 800 mg of the desired
product:M+=493.
Example 340
N-f5-(5H-Pyrroloi2,1-clf1,41-benzodiazepin-10(11H)-
ylcarbonyl)-2-gvridinyllfl 1'-biphnyll-2-carboxamide
A mixture of 2.0 g of 6-[([1,11-biphenyl]-2-
carbonyl)amino]pyridine-3-carboxylic acid and 20 ml of
thionyl chloride is refluxed for 3 hours. The excess
thionyl chloride is removed in vacuo to a residue which
is dissolved in 50 -;1 of methylene chloride. This
solution is adde4 ---dded dropwise to a stirred solution
of 2.0 g of 10,11-dihydro-5H-pyrrolo[2,1--r,][1,4]benzo-
SUBS": "UTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-143-
diazepine in 50 ml of methylene chloride and 5 ml of
N,N-diisopropylethylamine. The reaction mixture is
stirred at room temperature for 2 hours and quenched
with water. The organic layer is washed well with water
5* and dried over anhydrous MgSO4. The organic layer is
concentrated in vacuo to a residue which is purified by
column chromatography on silica gel by elution with 40%
ethyl acetate:hexane to give 1.2 g of a colorless
solid:M+=484.
Example 341
N-[4-(5H-Pyrrolof2 1-clfl 4lbenzodiazepin-10(11H)-
ylcarbonyl)phenyll-2-(2-pyridinyl)benzamide
A mixture of 1.94 g of N- [4- (5.I--pyrrolo-
[2,1-_r,][1,4]benzodiazepin-10(11H)-ylcarbonyl)phenyl]-2-
bromobenzamide, 2.95 g of 2-pyridyl tri-n-butyl tin and
400 mg of tetrakis(triphenylphosphine)palladium(0) is
refluxed for 24 hours in degassed toluene for 24 hours.
The reaction mixture is concentrated in vacuo to a
residue which is purified by column chromatography on
silica gel by elution with 70% ethyl acetate:hexane to
give 900 mg of the desired product as a pale yellow
solid:M+1=485.
Example 342
N-f5-(5H-Pyrrolof2,1-clf1,41benzodiazepin-10(11H)-
ylcarbonyl)-2-pyridinyll-2-(2-pyridinyl)benzamide
A mixture of 484 mg of N-[5-(5ji-pyrrolo-
[2,1-_Q][1,4]benzodiazepin-10(11H)-ylcarbonyl)-2-
pyridinyl]-2-bromobenzamide, 814 mg of 4-(N,N-di-
methyl)anilino-tri-n-butyl stannane and 100 mg of tetra-
kis(triphenylphosphine)palladium (0) is refluxed in
degassed toluene for 24 hours. The reaction mixture is
concentrated in vacuo to a residue which is purified by
column chromatography on silica gel by elution with
ethyl acetate to give 200 mg of the desired product:
M+1=528.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-144-
Example 343
10, 11-Dihydro-10- (4- (4-butyloxy) benzoyl) -5H-pyrrolo f-- 1-
c1f1,41benzodiazepine
To a solution of 92 mg of 10,11-dihydro-5H-
pyrrolo[2,1-c].[1,4]benzodiazepine in 2 ml of methylene
chloride is added 100 mg of triethylamine followed by
130 mg of 4-(n-butyloxy)benzoyl chloride. The reaction
mixture is stirred at room temperature for 24 hours and
then treated with 4 ml of 1N sodiurn hydroxide. The
mixture is extracted with 10 ml of ethyl acetate and the
extract washed with 1N sodium hydroxide and 5 ml of
brine. The organic layer is dried over anhydrous sodium
sulfate and filtered through hydrous magnesium silicate.
The filtrate is concentrate in vacuo to a residue which
'is stirred with ether-hexanes to give 160 mg of the
desired product as a white solid:mass
spectrum (CI) , 361 (MH+) .
Example 344
5,10-Dihydro-2-hydroxymethyl-5-(4-(4-butyloxY)benzoyl)-
2,0 4H-pyrazolor5,1-clfi.4lbenzodiazepine
As described for Example 343 4-(n-butyl-
oxy)benzoyl chloride is reacted with 5,10-dihydro-4H-
pyrazolo[5,1-c][1,4]benzodiazepine to give the desired
product as a solid;mass spectrum (CI ), 392 (MH+) .
Example 345
10,11-Dihydro-l0-(4-(5-pentyloxy)benzoyl)-5H-
pyrrolof2,1-clf1,41benzodiazepine
As described for Example 343 4-(n-pentyl-
oxy)benzoyl chloride is reacted with 10,11-dihydro-51i-
pyrrolo[2,l-.g][1,4]benzodiazepine to the desired product
as a solid:mass spectrum(CI),375(MH+). SUBSTyTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-145-
Example 346
N-f4-(5H-Pyrrolof2,1-c1rl,41benzodiazepin-10(11H)-
ylcarbonyl)phenyll-2-(4-chlorophenyloxy)pyridine-3-
carboxamide
The conditions of Example 325 are used with 2-
(4-chlorophenyloxy)pyridine-3-carbonyl chloride to give-
the desired product as a crystalline solid, m.p. 211-
212 C (M+Na) = 557.3.
Example 347
N-(4-(5H-Pyrrolof2,1-clf1,41benzodiazepin-10(11H)-
ylcarbonyl)phenyll-2-mathyl-2-(4-
chlorophenyloxy)proAionamide
The conditions of Example 325 are used with 2-
(4-chlorophenoxy)-2-methylpropionyl chloride to give the
desired product as a solid. M+499.
Example 348
10-f(6-(1 1-dimethylethyl)aminol-3-pyridinyl)carbonyll-
10.11-dihydro-5H-pyrrolor2,1-clf1,41benzodiazepine
Using the conditions of Example 273 and t-
butylamine gives the desired product as a beige solid.
MS (CI) : 361 (M+H) .
Example 349
10-rf6-(1-Methylethyl)amino)-3-pyridinyl)carbonyll-
10.11-dihydro-5H-pyrrolof2,1-c1f1,41benzodiazepine
Using the conditions of Example 273 and
isopropylamine gives the desired product as a white
solid. MS (CI) : 347 (M+H) .
Example 350
10-if6-(l-Indanylamino)-3-pyridinyl)carbonyll-10,11-
dihydro-5H-oyrrolof2,l-cl(1,41benzodiazepine
Using the conditions of Example 273 and 1-
aminoindan gives the desired product as a beige solid.
MS (CI) : 421 (M+H)
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-146-
Example 351
10-ff6-(2 4-pimethoxyphenylamino)-3-pyridinvllcarbonyil-
11-dihydro-5H-pyrrolof2 1-clfl 4lbenzodiaz pine
Using the conditions of Example 273 with 2,4-
5 dimethoxybenzylamine gives the desired product as a
light yellow solid. MS(CI): 455(M+H).
Example 352
10-ff6-(2-Bromophenylamino)-3-gvridinyllcarbonyll-10 11-
dihydro-5H-Qvrrolof2 1-clfl 4lbenzodiaz pine
10 Using the conditions of Example 273 and 2-
bromobenzylamine gives the desired product as an off-
white solid. MS (CI) : 474 (M+H) .
Example 353
N-f5-(5H-Pyrrolof2,1-c1f1,41benzodiazepin-10(11H)-
ylcarbonyl)-2-pyridinyll-2-methylfurane-3-carboxamide
Using the conditions of Example 1 with
Reference Example 39 to give Reference Example 86 and
stirring overnight gives the desired product as white
crystals after column chromatography on silica gel by
elution with 1:1 ethyl acetate:hexane and crystalliza-
tion from ethyl acetate, m.p. 210-212 C.
Example 354
N-i5-(5H-Pyrrolof2,1-c1G=1,41benzodiazepa.n-10(11H)-
ylcarbonyl)-2-pyridinyll-2-aminobenzamide
A room temperature solution of 1.0 g of N-[5-
(5H-pyrrolo [2, 1--(~] [ 1, 4 ] benzodiazepin-10 (11H) -
ylcarbonyl)-2-pyridinyl]-2-nitrobenzamide in 100 ml of
ethyl alcohol is hydrogenated over 200 mg of .10$ Pd/C in
a Parr apparatus under 40 psi of hydrogen for 2 hours.
The reaction mixture is filtered through diatomaceous
earth and the cake washed with additional ethyl alcohol.
The combined filtrates are concentrated in vacuo and the
residue purified by crystallization from 2:1 ethyl
acetate:hexane to give the desired product as pale
yellow crystals: M+Na 445:M+423.
SUBSTITUTE SHEET (RUL_E 26)
CA 02210631 1997-07-16
WO 96/22293 PCT1US96/01076
-147-
Binding Assay to Rat Hepatic Vl Receptors
Rat liver plasma membranes expressing the
vasopressin V1 receptor subtypes are isolated by sucrose
density gradient according to the method described by
Lesko et al, (1973). These membranes are quickly sus-
pended in 50.0 mM Tris.HC1 buffer, pH 7.4, containing
0.2% bovine serum albumin (BSA) and 0.1 mM phenylmethyl-
sulfonylfluoride (PMSF) and kept frozen at -70 C until
used in subsequent binding experiments. For binding
experiments, the following is added to the wells of a
ninety-six well format microtiter plate: 100 l of 100.0
mM Tris.HC1 buffer containing 10.0 mM MgC12, 0.2% heat
inactivated BSA and a mixture of protease inhibitors:
leupeptin, 1.0 mg %; aprotinin, 1.0 mg %; 1,10-phen-
anthroline, 2.0 mg %; trypsin inhibitor, 10.0 mg % and
0.1 mM PMSF, 20.0 l of [phenylalanyl-3,4,5,-3H] vaso-
pressin (S.A. 45.1 Ci/mmole) at 0.8 nM, and the reaction
initiated by the addition of 80 l of tissue membranes
containing 20 .g of tissue protein. The plates are kept
undisturbed on the bench top at room temperature for 120
min. to reach equilibrium. Non-specific samples are
assayed in the presence of 0.1 M of the unlabeled
antagonist phenylalanylvasopressin, added in 20.0 l
volume. For test compounds, these are solubilized in
50% dimethylsulfoxide (DMSO) and added in 20.0 .l volume
to a final incubation volume of 200 l. Upon completion
of binding, the content of each well is filtered off,
using a Brandel cell Harvester (Gaithersburg, MD). The
radioactivity trapped on the filter disk by the ligand-
~ 30 receptor complex is assessed by liquid scintillation
counting in a Packard LS Counter, with an efficiency of
65% for tritium. The data are analyzed for IC50 values
by the LUNDON-2 program for competition (LUNDON
SOFTWARE, OH).
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-148-
Bindina Assay to Rat Kidney Medullary V~ Recep or
Medullary tissues from rat kidneys are
dissected out, cut into small pieces and soaked in a
0.154 mM sodium chloride solution containing 1.0 mM EDTA
with many changes of the liquid phase, until the solu-
tion is clear of blood. The tissue is homogenized in a.
0.25 M sucrose solution containing 1.0 mM EDTA and 0.1
mM PMSF using a Potter-Elvehjem homogenizer with a
teflon pestle. The homogenate is filtered through
several layers (4 layers) of cheese cloth. The filtrate
is rehomogenized using a dounce homogenizer, with a
tight fitting pestle. The final homogenate is centri-
fuged
at 1500 x g for 15 min. The nuclear pellet is
discarded and the supernatant fluid recentrifuged at
40,000 x g for 30 min. The resulting pellet formed
contains a dark inner part with the exterior, slightly
pink. The pink outer part is suspended in a small
amount of 50.0 mM Tris.HC1 buffer, pH 7.4. The protein
content is determined by the Lowry's method (Lowry et
al, J. Biol. Chem., 1953). The membrane suspension is
stored at -70 C, in 50.0 mM Tris.HCl, containing 0.2%
inactivated BSA and 0.1 mM PMSF in aliquots of 1.0 ml
containing 10.0 mg protein per ml of suspension until
use in subsequent binding experiments.
For binding experiments, the following is
added in l volume to wells of a 96 well format of a
microtiter plate: 100.0 l of 100.0 mM Tris.HC1 buffer
containing 0.2% heat inactivated F3SA, 10.0 mM MgC12 and
a mixture of protease inhibitors: leupeptin, 1.0 mg %;
aprotinin, 1.0 mg %; 1,10-phenanthroline, 2.0 mg %;
trypsin inhibitor, 10.0 mg % and 0.1 mM PMSF, 20.0 l of
[3H] Arginine8, vasopressin (S.A. 75.0 Ci/mmole) at 0.8
nM and the reaction initiated by the addition of 80.0 l
of tissue membranes (200.0 g tissue protein). The
plates are left undisturbed on the bench top for 120
min. to reach equilibrium. Non-specific binding is
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-149-
assessed in the presence of 1.0 M of unlabeled ligand,
added in 20 l volume. For test compounds, these are
solubilized in 50% dimethylsulfoxide (DMSO) and added in
20.0 .l volume to a final incubation volume of 200 l.
Upon completion of binding, the content of each well is
filtered off, using a Brandel cell Harvester
(Gaithersburg, MD). The radioactivity trapped on the
filter disk by the ligand-receptor complex is assessed
by liquid scintillation counting in a Packard LS
Counter, with an efficiency of 65% for tritium. The
data are analyzed for IC50 values by the LUNDON-2
program for competition (LUNDON SOFTWARE, OH). The
results of this test on representative compounds of this
invention are shown in Tables 1, 2 and 3.
Radioliaand Bindina Experiments with Human Platelet
Membranes
Platelet Source: Hudson Valley Blood Services,
Westchester Medical Center, Valhalla, NY.
Platelet Membrane Preparation:
Frozen platelet rich plasma (PRP), received
from the Hudson Valley Blood Services, are thawed to
room temperature. The tubes containing the PRP are
centrifuged at 16,000 x g for 10 min. at 4 C and the
supernatant fluid discarded. The platelets resuspended
in an equal volume of 50.0 mM Tris.HC1, pH 7.5 con-
taining 120 mM NaCl and 20.0 mM EDTA. The suspension is
recentrifuged at 16,000 x g for 10 min. This washing
step is repeated one more time. The wash discarded and
the lysed pellets homogenized in low ionic strength
buffer of Tris.HC1, 5.0 mM, pH 7.5 containing 5.0 mM
EDTA. The homogenate is centrifuged at 39,000 x g for
10 min. The resulting pellet is resuspended in Tris.HC1
buffer, 70.0 mM, pH 7.5 and recentrifuged at 39,000 x g
for 10 min. The final pellet is resuspended in 50.0 mM
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-150-
Tris.HC1 buffer pH 7.4 containing 120 mM NaCl and 5.0 mM
KC1 to give 1.0-2.0 mg protein per ml of suspension.
Bindinct to Vasopressin V1 receptor subtype in Human
Platelet Membranes:
In wells of 96 well format microtiter plate,
add 100 l of 50.0 mM Tris.HC1 buffer containing 0.2%
BSA and a mixture of protease inhibitors (aprotinin,
leupeptin etc.). Then add 20 l of [3H]Ligand (Manning
or Arg8Vasopressin), to give final concentrations
ranging from 0.01 to 10.0 nM. Initiate the binding by
adding 80.0 i of platelet suspension (approx. 100 .g
protein). Mix all reagents by pipetting the mixture up
and down a few times. Non specific binding is measured
in the presence of 1.0 M of unlabeled ligand (Manning
or Arg8Vasopressin). Let the mixture stand undisturbed
at room temperature for ninety (90) min. Upon this
time, rapidly filter off the incubate under vacuum
suction over GF/B filters, using a Brandel Harvester.
Determine the radioactivity caught on the filter disks
by the addition of liquid scintillant and counting in a
liquid scintillator.
B'~ndincz to Membranes of Mouse Fibroblast Cell Line (LV-
2) Transfected with the cDNA Expressina the Human V2
Vasopressin Receptor
Membrane Pregarati.on
Flasks of 175 ml capacity, containing attached
cells grown to confluence, are cleared of culture medium'
by aspiration. The flasks containing the attached cells
are rinsed with 2x5 ml of phosphate buffered saline
(PBS) and the liquid aspirated off each time. Finally,
5 ml of an enzyme free dissociation Hank's based
solution (Specialty Media, Inc., Lafayette, NJ) is added
and the flasks are left undisturbed for 2 min. The
content of all flasks is poured into a centrifuge tube
and the cells pelleted at 300 x g for 15 min. The
Hank's based solution is aspirated off and the cells
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96101076
-151-
homogenized with a polytron at setting #6 for 10 sec in
10.0 mM Tris.HC1 buffer, pH 7.4 containing 0.25 M
sucrose and 1.0 mM EDTA. The homogenate is centrifuged
at 1500 x g for 10 min to remove ghost membranes. The
supernatant fluid is centrifuged at 100,000 x g for 60
min to pellet the receptor protein. Upon completion,
the pellet is resuspended in a small volume of 50.0 mM
Tris.HC1 buffer, pH 7.4. The protein content is
determined by the Lowry method and the receptor
membranes are suspended in 50.0 mM Tris.HC1 buffer
containing 0.1 mM phenylmethylsulfonyifluoride (PMSF)
and 0.2% bovine serum albumin (BSA) to give 2.5 mg
receptor protein per ml of suspension.
Receptor Binding
For binding experiments, the following is
added in l volume to wells of a 96 well format of a
microtiter plate: 100.0 l of 100.0 mM Tris.HC1 buffer
containing 0.2% heat inactivated BSA, 10.0 mM MgCl2 and
a mixture of protease inhibitors: leupeptin, 1.0 mg %;
aprotinin, 1.0 mg %; 1,10-phenanthroline, 2.0 mg %;
trypsin inhibitor, 10.0 mg % and 0.1 mM PMSF, 20.0 l of
[3H] Arginine8, vasopressin (S.A. 75.0 Ci/mmole) at 0.8
nM and the reaction initiated by the addition of 80.0 l
of tissue membranes (200.0 g tissue protein). The
plates are left undisturbed on the bench top for 120 min
to reach equilibrium. Non specific binding is assessed
in the presence of 1.0 .M of unlabeled ligand, added in
20 l volume. For test compounds, these are solubilized
in 50% dimethylsulfoxide (DMSO) and added in 20.0 l
volume to a final incubation volume of 200 l. Upon
= completion of binding, the content of each well is
filtered off, using a Brandel cell Harvester
(Gaithersburg, MD). The radioactivity trapped on the
filter disk by the ligand-receptor complex is assessed
by liquid scintillation counting in a Packard LS
Counter, with an efficiency of 65% for tritium. The
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-152-
data are analyzed for IC50 values by the LUNDON-2
program for competition (LUNDON SOFTWARE, OH).
Oxytocin Receptor Bindina
(a) Membrane Preparati on
Female Sprague-Dawley rats weighing approxi-
mately 200-250 g are injected intramuscularly (i.m.)
with 0.3 mg/kg of body weight of diethylstilbestrol
(DES). The rats are sacrificed 18 hours later under
pentobarbital anesthesia. The uteri are dissected out,
cleaned of fat and connective tissues and rinsed in 50
ml of normal saline. The tissue pooled from six rats is
homogenized in 50 ml of 0.01 mM Tris.HC1, containing 0.5
mM dithiothreitol and 1.0 mM EDTA, adjusted to pH 7.4,
using a polytron at setting 6 with three passes of 10
sec each. The homogenate is passed through two (2)
layers of cheesecloth and the filtrate centrifuged at
1000 x g for 10 min. The clear supernatant is removed
and recentrifuged at 165,000 x g for 30 min. The re-
sulting pellet containing the oxytocin receptors is
resuspended in 50.0 mM Tris.HC1 containing 5.0 mM MgCl2
at pH 7.4, to give a protein concentration of 2.5 mg/ml
of tissue suspension. This preparation is used in sub-
sequent binding assays with [3H]Oxytocin.
(b) Radioligand Bindina
Binding of 3,5-[3H]Oxytocin ([3H]OT) to its
receptors is done in microtiter plates using [3H]OT, at
various concentrations, in an assay buffer of 50.0 mM
Tris.HC1, pH 7.4 and containing 5.0 mM MgC12, and a
mixture of protease inhibitors: BSA, 0.1 mg; aprotinin,
1.0 mg; 1,10-phenanthroline, 2.0 mg; trypsin, 10.0 mg;
and PMSF, 0.3 mg per 100 ml of buffer solution. Non-
specific binding is determined in the presence of 1.0 uM
unlabeled OT. The binding reaction is terminated after
60 min., at 22 C, by rapid filtration through glass
fiber filters using a Brandel cell harvester (Bio-
medical Research and Development Laboratories, Inc.,
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-153-
Gaithersburg, MD). Competition experiments are con-
ducted at equilibrium using 1.0 nM [3H]OT and varying
the concentration of the displacing agents. The
concentrations of agent displacing 50% of [3H]OT at its
sites (IC50) are calculated by a computer assisted
LUNDON-2 program (LUNDON SOFTWARE INC., Ohio, USA).
The results of this assay on representative
examples are shown in Table 4.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96101076
-154-
Table 1
Bi dina Assay to Rat Hepatic Vi Receptors and Rat Kidney
Medullary V2 Receptors or *Binding to V1 Receptor ~i type in Human Platelet
and **Binding to Membranes of
Mouse Fibroblast Cell Line (LV-2) Transfected with the
cDNA Expressing the Human V_ Receptor
D
N
X
~
N NH- R
Ex. D X R V1 V2
No. IC50 ( M) IC50 ( M)
1 C H CH 0.033 0.004
*0.020 **0.005
F
5 C H OCH 3 *51% at **47% at
mH 10 M 10 M
~ 3
OCH3
4 C H CH3 F *0.044 0.001
261 C H -CH2CH (CH3) 2 65% at 32% at
1 M 1 M
208 N H CH3 0.087 0.011
CO-0 F
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-155-
Ex. D X R Vl V2
No. IC50( M) 1IC50( M)
273 C H 0.190 0.082
262 C H -CH2CH2C (CH3) 2 64% at 50% at
1 j.lM 1 p.M
263 C H -C 0.200 0.360
2
12 C Br CH3 0.210 0.024
7 C H F 32% at 58% at
1 ,M 10 M
F
6 C H Ci 0.011 0.0018
8 C H Br 0.007 0.0016
/ \
301 C H CH3 94% at 91% at
M 10 M
10 33 C H Ci 0.450 0.030
- \ NO
9 C H Ci 0.006 0.0011
**0.0009
- F
261 C H -CH2CH (CH3) 2 89% at 55% at
10 10 M
SUBSTITTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-156-
Ex. D X R V1 V2
No. IC50 ( M) IC50 ( M)
274 C H CH 3 90% at 97% at
1 M 10 M
2
_c/\
14
C H _ /\ 96% at 95% at
- 1 .M 1 M
i
11 C H C) 100% at 93% at
/ \ 1 M 1 M
Br
5 342 C H ?YN
352 C H Br 0.088 0.059
-CH2 b
348 C H -C(CH3)3 0.08 43% at
1 M
350 C H 5DO 0.015 0.034
245 N H ~ ~ 0.019 0.001
O ~
_IC ~ \
10 329 C H p CH3 0.31 0.07
C N
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTlLTS96/01076
-157-
Ex. D X R V1 V2
No. IC50 ( M) IC50 ( M)
330 C H Q CH 3 89% at 79% at
b 1 I~ 1~
-C HG
353 C H 93% at 86% at
1 M 1 M
C~ O
43 C H 0 93% at
CP 1 RM
NJ 2
351 C H `^" 73% at 56% at
- ~ 1 M 1 ~1.M
2 3
354 C H 0 NH2 29% at 86% at
11 ~ 1 }1M 1 FtM
'14 C H 0 Cl 100% at 99% at
11 1 M 1 p.M
C
CI
18 C H I 0 F 98% at 94% at
3 1 M 1 M
C
CI
.
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-158-
Table 1A
BindincL Assay to Rat Hepatic V- Receptors and Rat Kidney
Medullary V2 Receptors or *Bindincr to V] Receptor
Subtype in Human Platelet and **Bindina to Membranes of
Mouse Fibroblast Cell Line (LV-2) Transfected with the
cDNA Expressina the Human V2 Receptor
N
O
NH- R
Ex.No. X R V1 V2
IC50 (P,M) IC50 (N.M)
341 H li 0.02 0.004
C
: N 10 327 H 0 CH3 0.35 0.028
~C N
347 H li 0.18 0.42
Cr C( CH) 2 /_` c)
328 Ci 0 CH3 3.3 0.019
C / N
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-159-
Ex.No. X R V1 V2
IC50 Q~M) IC50 ( M)
324 H 0.42 0.12
C'
\
CF3
333 H 0 G 0.25 0.41
~~ bN
-C
338 H 0 N(C= 3) 2 0.037 0.0048
N
332 H NtC= O 2 0.031 0.0034
HCI
C b
337 H CH3 1.3 0.65
O N
C N
331 H CH3 87% at 43% at
M 1 M
N
N
O
_ I) N
-C / HCI
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-160-
Ex.No. X R V1 V2
IC50 (IM) IC50 ( M)
336 H 0 99% at 69% at
II -c
-N
334 H II 15% at 79% at
M 1 M
1
P-N
NH
I
CH3
339 H ~i 41% at 55% at
iM 1 M
1 }
C
P-N
N
O
346 H 0 44% at 76% at
M 10 }iM
C
PN-
O
CI
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-161-
Ex.No. X R V1 V2
IC50 ( M) IC50 ( M)
326 Cl 0 41% at 91% at
11 10 M 10 M
-C
CF3
319 C1 ~i 0.016 0.0015
-c
320 H 0.0034 0.0026
321 H 0.018 0.0051
CH2 /_\
322 ci Ii 0.67 0.011
C / \
k /_\
335 H 0 *100% at 60% at
1 pM 1 pM
-N
NH( CF{2) sN CH3) 2
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTlUS96/01076
-162-
Table 2
Bindina Assay to Rat Hepatic V1 Receptors and Rat Kidney
Medullary V2 Receptors or *Binding to Vi Receptor SuhtygP in Human Platelet
and **Bindina to Membranes of
Mouse Fibroblast Cell Line (LV-2) Transfected with the
cDNA Expressina the Human V2 Receptor
Ex. Structure Vi V2
No. IC50 ( M) IC50 ( M)
171 - 0.630 0.031
N
N
N ~
~ / ~
o n-N c
-
F
288 N 83% at 54% at
M 10 M
N 49% at
1 M
0~-' N S~
~
10 131 ~ N 66% at 82% at
10 M 1 M
N
(i ~) sN(C~~2
o N I F
CX) 6
CH3
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-163-
Ex. Structure Vi V2
No. IC50 ( M) IC50 (I--M)
130 ~ N 98% at 92% at
\ I - 10 M 10 M
N
(I H2)2N(CH3)2
O N F
CH3
134 23% at 94% at
N N
M 10 M
N
CF~
O
,
N NHCC)
F
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-164-
Table 3
Bindina Assay to Rat Hepatic Vi Receptors and Rat Kidney
Medullary V2 Receptors or *Bindina to V] eceptor
S hfiype in Human Platelet and **Bindinq to Membranes of
Mouse Fibroblast Cell Line (LV-2) Transfected with the
cDNA Expressinq the Human V2 Receptor
CH2N( CH3) 2
N
N
X
O I
N NH- R
Ex. X R Vi V2
No. IC50 ( M) IC50 ( M)
133 H OC= ~ *11% at 21% at
/ \ 10 M 10 M
_ ""' ~
OCH3
120 H CH3 0.099 0.033
F
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-165-
Table 4
Oxytocin Bindinq Assay
Ex. No. Dose ( M) % Inhibition IC50 ( M)
1 10 92 0.20
5 10 93
344 1 58 3.8
4 10 100 0.67
133 10 59
261 0.15
120 1 8
208 10 95 0.73
273 2.5 95 0.056
262 10 76 1.6
263 10 98 0.38
171 10 73 1.1
12 10 98 0.8
7 10 66
6 1 90 0.14
8 1 89 0.15
301 10 89 0.86
288 10 94 1.36
33 10 95 0.51
9 2.5 96 0.17
131 10 60
130 10 57
134 1 63
341 1 74
327 1 56
347 10 86
328 10 85 0.57
324 1 45
333 10 98 0.88
338 10 98 0.72
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-166-
Ex. No. Dose ( M) % Inhibition IC50 (41M)
332 10 98 0.83
337 1 16
331 1 13
336 10 94 1.63
334 1 5
339 10 48 8.56
346 1 0
326 1 0
352 1.25 96 0.105
348 10 95 0.71
350 10 95 0.205
240 10 98 0.61
329 10 91 0.19
330 10 93 0.99
353 10 83 2.05
43 10 99 0.92
351 1 0
354 1 7
14 10 96 0.58
18 5 97 0.31
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-167-
The compounds of the present invention can be
used in the form of salts derived from pharmaceutically
or physiologically acceptable acids or bases. These
salts include, but are not limited to, the following:
salts with inorganic acids such as hydrochloric acid,
sulfuric acid, nitric acid, phosphoric acid and, as the
case may be, such organic acids as acetic acid, oxalic
acid, succinic acid, and maleic acid. Other salts
include salts with alkali metals or alkaline earth
metals, such as sodium, potassium, calcium or magnesium
or with organic bases. The compounds can also be used
in the form of esters, carbamates and other conventional
"pro-drug" forms, which, when administered in such form,
convert to the active moiety in vivo.
When the compounds are employed for the above
utilities, they may be combined with one or more
pharmaceutically acceptable carriers, for example, sol-
vents, diluents and the like, and may be administered
orally in such forms as tablets, capsules, dispersible
powders, granules, or suspensions containing, for exam-
ple, from about 0.05 to 5% of suspending agent, syrups
containing, for example, from about 10 to 50% of sugar,
and elixirs containing, for example, from about 20 to
50%.ethanol, and the like, or parenterally in the form
of sterile injectable solutions or suspensions contain-
ing from about 0.05 to 5% suspending agent in an
isotonic medium. Such pharmaceutical preparations may
contain, for example, from about 25 to about 90% of the
active ingredient in combination with the carrier, more
usually between about 5% and 60% by weight.
The effective dosage of active ingredient
employed may vary depending on the particular compound
employed, the mode of administration and the severity of
the condition being treated. However, in general,
satisfactory results are obtained when the compounds of
the invention are administered at a daily dosage of from
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCTIUS96/01076
-168-
about 0.5 to about 500 mg/kg of animal body weight,
preferably given in divided doses two to four times a
day, or in a sustained release form. For most large
mammals the total daily dosage is from about 1 to 100
mg, preferably from about 2 to 80 mg. Dosage forms
suitable for internal use comprise from about 0.5 to
500 mg of the active compound in intimate admixture with
a solid or liquid pharmaceutically acceptable carrier.
This dosage regimen may be adjusted to provide the
optimal therapeutic response. For example, several
divided doses may be administered daily or the dose may
be proportionally reduced as indicated by the exigencies
of the therapeutic situation.
These active compounds may be administered
orally as well as by intravenous, intramuscular, or sub-
cutaneous routes. Solid carriers include starch, lac-
tose, dicalcium phosphate, microcrystalline cellulose,
sucrose and kaolin, while liquid carriers include
sterile water, polyethylene glycols, non-ionic surfac-
tants and edible oils such as corn, peanut and sesame
oils, as are appropriate to the nature of the active in-
gredient and the particular form of administration de-
sired. Adjuvants customarily employed in the prepara-
tion of pharmaceutical compositions may be advan-
tageously included, such as flavoring agents, coloring
agents, preserving agents, and antioxidants, for
example, vitamin E, ascorbic acid, BHT and BHA.
The preferred pharmaceutical compositions from
the standpoint of ease of preparation and administration
are solid compositions, particularly tablets and hard-
filled or liquid-filled capsules. Oral administration
of the compounds is preferred.
These active compounds may also be adminis-
tered parenterally or intraperitoneally. Solutions or
suspensions of these active compounds as a free base or
pharmacologically acceptable salt can be prepared in
SUBSTiTUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-169-
water suitably mixed with a surfadtant such as hydrox-
ypropylcellulose. Dispersions can also be prepared in
glycerol, liquid, polyethylene glycols and mixtures
thereof in oils. Under ordinary conditions of storage
and use, these preparations contain a preservative to
prevent the growth of microorganisms.
The pharmaceutical forms suitable for in-
jectable use include sterile aqueous solutions or dis-
persions and sterile powders for the extemporaneous
preparation of sterile injectable solutions or disper-
sions. In all cases, the form must be sterile and must
be fluid to the extent that easy syringability exits.
It must be stable under conditions of manufacture and
storage and must be preserved against the contaminating
action of microorganisms such as bacterial and fungi.
The carrier can be a solvent or dispersion medium con-
taining, for example, water, ethanol (e.g., glycerol,
propylene glycol and liquid polyethylene glycol),
suitable mixtures thereof, and vegetable oil.
The new tricyclic non-peptide vasopressin
antagonists of this invention are useful in treating
conditions where decreased vasopressin levels are
desired, such as in congestive heart failure, in disease
conditions with excess renal water reabsorption and in
conditions with increased vascular resistance and
coronary vasoconstriction.
In particular, the vasopressin antagonists of
this invention are therapeutically useful in the
treatment and/or prevention of hypertension, cardiac
insufficiency, coronary vasospasm, cardiac ischemia,
renal vasospasm, liver cirrhosis, congestive heart
failure, nephritic syndrome, brain edema, cerebral
ischemia, cerebral hemorrhage-stroke, thrombosis-
bleeding and abnormal states of water retention.
In particular, the oxytocin antagonists of
this invention are useful in the prevention of preterm
SUBSTITUTE SHEET (RULE 26)
CA 02210631 1997-07-16
WO 96/22293 PCT/US96/01076
-170-
labor and premature birth which is a significant cause
of infant health problems and infant mortality.
SUBSTITUTE SHEET (RULE 26)