Note: Descriptions are shown in the official language in which they were submitted.
CA 02210780 1997-07-17
Mixtures Of Organosilanepolysulphanes And A Process
For The Production Of Rubber Compounds Containing
These Mixtures
This invention relates to mixtures of organosilane-
polysulphanes having an elevated proportion of disulphanes,
and to a process for the production of rubber compounds
containing these mixtures. In the following description,
the numerical references refer to documents listed at the
end of the disclosure.
Due to increasing environmental awareness, economies in
fuel consumption and a reduction in pollutant emissions are
today becoming a greater priority [1, 2]. The challenge to
tyre manufacturers is to develop tyres which are
distinguished by very low rolling resistance, combined with
excellent wet skid resistance and good abrasion resistance.
Proposals have been made in numerous publications and
patents with regard to reducing tyre rolling resistance and
thus fuel consumption. Proposals which can be mentioned
include reducing the carbon black content in the compound
and using special carbon blacks (U. S. Patent 4,866,131,
U.S. Patent 4,894,420). However, none of these proposed
solutions has resulted in a satisfactory balance between
the desired low rolling resistance and other important tyre
1
CA 02210780 1997-07-17
characteristics such as wet skid resistance and abrasion
resistance.
Only the use of highly active silica fillers in combination
with the organosilane bis(triethoxysilyl-
propyl)tetrasulphane (TESPT) when largely replacing the
carbon black with silica in the rubber compound, allows
production of a tyre having rolling resistance
substantially reduced in comparison with standard tyres,
while simultaneously retaining or improving the other two
above-stated tyre characteristics [3, 4, 5, 6].
At the 1986 ACS meeting in New York, S. Wolff [7] presented
a paper showing that it is possible to reduce rolling
resistance in comparison with a carbon black filled
standard compound while retaining wet skid resistance, by
using silica in combination with TESPT both in a passenger
vehicle tyre tread based on an emulsion styrene/butadiene
rubber (E-SBR) and in a transport vehicle tyre tread based
on natural rubber.
This system was further optimised with regard to all three
characteristics by using specific styrene/butadiene
polymers produced using a solution polymerisation process
(E.P.O. 447,066), sometimes blended with other polymers, in
particular polybutadiene, and additionally using novel
2
CA 02210780 1997-07-17
grades of silica (U. S. Patent 5,227,425) and polymer blends
specifically tailored to this application
(E.P.O. 620,250), sometimes with three to four different
starting polymers [8, 9].
It is stated in all the publications and patents that, in
order to achieve a lower rolling resistance while retaining
or improving wet skid resistance and abrasion resistance,
it is necessary to replace a large proportion of or the
entire content of the normally used carbon black filler
with a highly active silica [7, 9]. However, this
replacement achieves the desired objective only if the
organosilane bis(triethoxysilylpropyl)tetrasulphane (TESTP)
is used as a "coupling" agent between the silica and the
polymer.
It is now known [10, 11] that the properties which may be
achieved by using organosilanes in rubber compounds are
dependent upon two independent reactions. Firstly, during
production of the compound (preferably during the first
compounding stage) a reaction occurs at elevated
temperature between the silanol groups of the silica and
the trialkoxysilyl groups of the silane with elimination of
alcohol (hydrophobing or modification reaction). A
complete reaction is of decisive significance to subsequent
properties. Like all chemical reactions, this reaction
3
CA 02210780 1997-07-17
proceeds faster at elevated temperatures [12], such that a
rubber compounder, desiring short compounding times, would
like to use the highest possible compounding temperature.
The use of such high compounding temperatures is, however,
limited by the fact that the second, so-called rubber-
reactive group of TESPT consists of a group which is on
average a tetrasulphane group having a significant
proportion of longer sulphane chains (S5 - S8) [11].
This rubber-reactive group is generally considered to give
rise to a so-called filler/rubber bond, which determines
the technical rubber properties of the finished article
(for example tyres). This reaction, which is desired
during vulcanisation, is influenced by the thermal lability
of the tetrasulphane group and higher sulphane units.
Practical experience has, however, shown that the reaction
causes serious problems if it occurs during production of
the unvulcanised compound, during which only the reaction
between the filler and the silane should normally occur.
If sulphur is eliminated from the long-chain sulphane
units, it is incorporated into the polymer chain. This
then brings about "scorching", stiffening the sheeted
compound which may render the unvulcanised compound
unprocessable. Scorching may be measured by determining
the viscosity of the compound.
4
CA 02210780 1997-07-17
E.P.O. 732,362, which is not a prior publication, describes
the use of organosilanedisulphides in rubber compounds.
However, these sulphur compounds must be very pure or have
a disulphide content of at least 80%.
An object of this invention is to provide mixtures of
organosilanepolysulphanes which do not give rise to the
scorching at elevated temperatures which may occur during
production of unvulcanised rubber compounds, i.e.
10' vulcanisable rubber compounds, which still do not yet
contain the sulphur and accelerators) necessary for
vulcanisation.
The present invention provides mixtures of
organosilanepolysulphanes of the general formula
(RO) 3Si (CH2) x S-SZ - S (CH2) X Si (OR) 3 (I)
in which:
20 R means alkyl, linear or branched, having 1-8 C atoms, in
particular 1-3 C atoms;
x is an integer from 1-8~ and
z is 0 to 6:
wherein the proportion of organosilanepolysulphanes in
which z = 0 is less than 80% (by weight) and the proportion
CA 02210780 1997-07-17
of organosilanepolysulphanes in which z is an integer from
2 to 6 does not exceed 20 wt.% in the mixtures.
The latter should be stated as a content of <_ 20% (by
weight) and is a characterising feature. Polysulphane
fractions where z = 7 or 8 are not generally found in the
mixtures according to the invention. Exceptionally, they
are present at contents of < 1%, for example as impurities,
which have no effect on the use of the mixtures according
to the invention.
Preferrably, the sum of the proportions of
organosilanepolysulphanes in which z = 0 and z = 1 amounts
to _> 80% (by weight), providing that the proportion of
compounds in which z = 0 remains below 80%.
The sum of the constituents must, of course, always be
100%, if necessary taking account compounds where z =
7 or 8.
Particularly suitable mixtures are those in which the
proportions of the organosilanepolysulphanes assume the
following values:
6
CA 02210780 1997-07-17
z = 0 approx. 58 to < 800
z = 1 > 0 to approx. 32s, wherein the sum of these
compounds is >_ 80%, and
z = 2 to 6 <_ 200, in particular < approx. 11%.
The mixtures according to the invention are used in the
production of vulcanisable rubber compounds, in particular
for tyres. The polymers used in these compounds are
ZO natural and synthetic elastomers, whether oil-extended or
not, as individual polymers or blended with other rubbers,
such as for example natural rubbers, butadiene rubbers,
isoprene rubbers, butadiene/styrene rubbers, but in
particular SBR, produced using the solution polymerisation
process or the emulsion process. The term mixture should
be taken to mean that it is possible on the one hand to
produce compounds from a pure polysulphane of the formula
(I) where x = 0 and other polysulphanes, in which x ~ 0,
but which comply with the above specifications.
On the other hand, it is, however, also possible by means
of a suitable production process to obtain the mixtures
according to the invention directly or by adding other
polysulphanes.
7
CA 02210780 1997-07-17
The mixtures according to the invention are primarily used
in tyre tread compounds having an elevated silica content,
such as are described, for example, in E.P.O. 447,066 and
E.P.O. 620,250.
Rubber compounds produced using the mixtures according to
the invention are generally vulcanised with sulphur and/or
sulphur donors and accelerators (vulcanisation
auxiliaries), wherein the quantity of sulphur is generally
between 0.1 and 4 phr.
In addition to the polymers and the additives conventional
in practice, such as activators, anti-oxidants, processing
auxiliaries, the rubber compounds optionally contain carbon
black and also natural, light-coloured fillers, but in any
case highly active silica fillers in quantities of 10 to
200 parts, in particular 25 to 80 parts, relative to 100
parts of the polymer. These fillers are characterised in
that they have BET surface areas of 1 to 700 m2/g, in
particular of 100 to 250 m2/g, and also a DBP value of 150
to 300 ml/100 g.
Suitable presentations are not only powders, but also low-
dusting forms such as pellets and microbeads. The quantity
of the mixtures according to the invention is between 0.5
and 30 parts, relative to 100 parts of filler. In
s
CA 02210780 1997-07-17
preferred applications, such as for example tyre tread
compounds having an elevated silica content, in which
silicas of 100 to 250 mz/g are generally used, the mixtures
according to the invention are used in quantities of
between 4 and 10 parts, relative to 100 parts of the
filler.
The mixtures according to the invention may be introduced
into the compound in situ, or in an improved manner,
previously mixed with carbon black. Premodification of the
silica used as filler, as is described, for example, in DE
196 09619.7, is also possible.
Particular attention must be paid to the process for the
production of highly silica-filled compounds in combination
with organosilanes. A suitable process is described in
E.P.O. 447,066, wherein, due to the use of TESPT, it is,
however, necessary in that process not to allow the
temperature during compounding to exceed 160°C, so as not
to initiate the above-mentioned scorching. However, when
using the compounds according to the invention,
temperatures of 160 to 200°C, in particular of 175 to
190°C, are possible without causing this disruptive effect
to occur. The compounder may thus select higher
temperatures and so accelerate the reaction between the
silica and silane, i.e. reduce compounding time and/or the
9
CA 02210780 1997-07-17
number of compounding stages. Compounding conditions are
thus largely freely selectable.
The mixtures according to the invention may be used in
virtually any rubber articles. They are particularly
suitable for use in highly silica-filled compounds
(containing > 40 parts of Si02, relative to 100 parts of
rubber), in particular tyre tread compounds, in which
elevated quantities of silane must generally be used in
order to achieve the required properties.
By another aspect the present invention also provides a
process for the production of the stated rubber compounds.
The process for the production of rubber compounds
vulcanised with sulphur and/or sulphur donors and
accelerators) and containing one or more natural or
synthetic rubbers, light-coloured oxide (silicate) fillers
(optionally together with carbon black and further
conventional constituents) is characterised in that the
rubber component(s), the mixtures according to the
invention, the silicate filler and the optionally present
carbon black (optionally together with a plasticiser, anti-
oxidants and activators) are kneaded for 3 to 15 minutes in
a single stage or in multiple stages in a kneading
CA 02210780 1997-07-17
apparatus (optionally a Banbury* internal mixer) at a
temperature of 160 to 200°C, in particular at 175 to 190°,
then, either in the Banbury* internal mixer or in a roll
mill, vulcanisation auxiliary is added at 60 to 120°C,
preferably at 80 to 110°C, compounding is continued for a
further 2 to 10 minutes in the stated temperature range and
the finished rubber compound is then rolled out into sheets
or strips.
The present invention thus also relates to the use of
organosilanepolysulphane mixtures, the sulphane chain
distribution of which is selected in such a manner that
even at temperatures of 160 to 200°C (in particular of 175
to 190°C) there is no discernible scorching of the
unvulcanised compound.
In practice, this scorching may be evaluated from the
properties of the unvulcanised sheet, which becomes
increasingly rough and crumbly as scorching occurs and may
often become unprocessable on the roll mill. In the
laboratory, scorching may be identified by measuring the
viscosity of the compound and by determining the minimum
torque value of the unvulcanised compound in a rheometer
test. A guide value for any increase in viscosity in
* Tradename
11
CA 0221078012004-02-16
r a
comparison with a formulation compounded at a lower
temperature (= more reliable processing behaviour} of more
than 5 (in particular of more than 10) Mooney units may be
set as an indication of scorching of the compound.
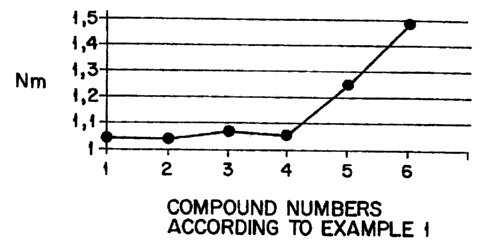
Brief Description of the Drawinas
FIG. 1 graphically illustrates the scorching behavior of
the unvulcanized compounds of Example 1. The rheometer data
is obtained at 165°C.
FIGS. 2-5 graphically illustrate a comparison in vulcanized
properties between a control (Si 69) and a product of the
invention (Si 266 mod) as used in the specific examples.
FIG. 2. shows that Si 266 mod exhibits distinctly improved
scorching behavior at all compounding temperatures.
FIG. 3 shows that Si 266 mod exhibits a distinctly more
favorable vulcanization time in comparison with Si 69.
FIG. 4 shows that even at elevated compounding
temperatures, Si 266 mod exhibits no scorching and thus has
distinctly better processing behavior.
FIG. 5 shows that Si 266 mod exhibits distinct advantages
in injection speed.
Reference now will be made to the following specific
examples illustrating the practice and compounds of the
invention, but it should be understood that they are
included herein for exemplification and are not to be
construed as limiting the scope of the inventive concept.
12
CA 02210780 1997-07-17
In the examples the following test standards were used:
3000 modulus MPa DIN 53 504
Shore A hardness - DIN 53 505
DIN abrasion mm3 DIN 53 516
MTS DIN 53 513
Mooney viscosity DIN 53 523/53 524
The following chemicals were used in the practical
examples:
Si 69* bis(triethoxysilylpropyl)-
tetrasulphane (Degussa AG).
Buna* VSL 5020 1 HM styrene/butadiene rubber, produced
using the solution polymerisation
process (Bayer AG).
Buna* CB 11S polybutadiene rubber (Bayer AG).
Naftolen* ZD aromatic plasticiser (Chemetal).
Vulkanox* 4020 discolouring anti-oxidant based on
phenylenediamine (Bayer AG) (6PPD).
Protector* G 35 ozone protection wax (Fuller).
Vulkacit* D diphenylguanidine (Bayer AG).
Vulkacit*~ CZ benzothiazyl-2-cyclohexylsulphenamide
(Bayer AG) .
Ultrasil* VN 3 GR precipitated silica having a BET
surface area of 175 m2/g (Degussa
AG ) .
Si 266* bis(triethoxysilylpropyl)disulphane.
Si 266 mod* in Example 3: sulphane chain
distribution: 57.7 S2, 31.4 S3;
8.3$ S4: 2.3~ S5; 0.2~ S6.
* Tradenames
13
CA 02210780 1997-07-17
Example 1: Determination of sulphane chain
distribution of various organosilane-
polysulphane mixtures
HPLC determination for the following -:comp.os.it:ions
Mixture Weight Graph
distribution oint
of
sulphane
chains
'7o SI266~S2 Sa S4 Ss Ss ST Ss Ss S1o n0.
Si69
1 00/0 99.7 0.3 0.0 0.0 0.0 0.0 0.0 0.0 0.0 1
90/10 83.5 15.3 1.1 0.0 0:0 0:0 0.0 0.0 0.0 2
80/20 70.2 25.1 4.1 0.6 0:0 0.0 0.0 0.0 0.0 3
70/30 57.7 31.4 8.3 2.3 0.2 0.0 0.0 0.0 0:0 4
60/40 55.7 22.7 10.2 6.0 3.1 1.3 0.6 0.4 0.1 5
0/100 16.2 29.9 24.0 15.2 7.9 3.8 2.1 0.6 0.2 6
14
CA 02210780 1997-07-17
Example 2: Determination of scorching using rheometer
curve at 180°C
All the mixtures from Example 1 were incorporated into a
rubber compound according to Example 3 at approx. 140°C
using the compound formulation for stages 1 and 2, i.e.
without the vulcanising system. The minimum torque value
of these unvulcanised compounds was then determined in
the rheometer at 180°C. The increase in the torque value
may be considered to be an indication of scorching
behaviour (c.f. Chart 1).
CA 02210780 1997-07-17
Example 3: Comparison of unvulcanised compound and
rheometer data between Si 69 and
disulphane mixture in a passenger vehicle
tread compound
Influence of output temperature.
Formulation 1 2
Buna VSL 5025 1 HM 96 96
Buna CB 11 S 30 30
Uttrasil VN 3 Gr. 80 80
N 330 6.5 6.5
Si 69 6.5
Si 266 mod - 6.5
Zn0 RS 3 3
Stearic acid 2 2
Naftolen ZD 10 10
Vulkanox 4020 1.5 1.5
Protector G35 1 1
Vulkacit CZ 1.7 1.7
Vulkacit D 2 2
Sul hur 1.4 2.1
(Si 266 mod: 57.7 S2, 31.4 S3, 8.3~ S4, 2.3~ S5,
0.2~ S6)
16
CA 02210780 1997-07-17
Compounding instructions
Stage 1
Rotor speed: 70 rpm
Flow: 80°C
Com aundin time
0-1' Buna VSL 50251 HM Buna CB 11 S
1-2' '/ silica,'/ Si-69 or Si 266 mod., N330,
ZnO, stearic
acid oil
2-3' '/2 silica '/2 Si 69 or Si 266 mod. N330,
6PPD wax
3' ~ clean shaft
3-3.5' mixin and dischar a
Output temperature:
135-145C
Intermediate stora
e: 24 hIRT
Stage 2
Rotor speed: 60 rpm
Flow: 8fl°C
Com oundin time
0-2' batch from sta a 1
2' dischar a
Output temperature:
135-150C
Intermediate stora
e: 24 h/RT
Stage 3
Rotor speed: 30 rpm
Flow: 50°C
Com oundin time
0-1.5' batch from stage 2,
accelerator sul hur
1.5' dischar a
Out ut tem erature:
<110C
m
CA 02210780 1997-07-17
Compounding instructions
Stage 1
Rotor speed: 95 rpm
Flow: 80°C
Com oundin time
0-1' Buna VSL 5025 1 HM, Buna CB 11 S
1-2' '/Z silica,'/ Si 69 or Si 266 mod., N330,
ZnO, stearic
acid oil
2-3' '/ silica '/ Si 69 or Si 266_ mod. N330,
6PPD, wax
3' clean shaft
3-3.5' mixin and dischar a
Output temperature:
155-165C
Intermediate stora
e: 24 h/RT
Stage 2
Rotor speed: 60 rpm
Flow: 80°C
Com oundin time
0-2' batch from sta a 1
2' dischar a
Output temperature:
135-145C
Intermediate stora
e: 24 h/RT
Stage 3 .
Rotor speed: 30 rpm
Flow: 50°C
Com oundin time ~ --
0-1.5' batch from stage 2,
accelerator sul hur
1.5' dischar a
Out ut tem erature:
<110C
is
CA 02210780 1997-07-17
Compounding instructions
Stage 1
Rotor speed: 115 rpm
Flow: 95°C
Com oundin time
0-1' Buna VSL 5025 1 HM, Buna CB 11 S
1-2' '/ silica,'/2 Si 69 or Si 266 mod., N330,
ZnO, stearic
acid, oil
2-3' '/ silica,'/ Si 69 or Si 266 mod., N330,
6PPD, wax
3' clean shaft
3-3.5' ~ mixin and dischar a
Output temperature:
175-185C
Intermediate stora
e: 24 h/RT
Stage 2
Rotor speed: 60 rpm
Flow: 80°C
Com oundin time
_
0 2' batch from sta a 1
2' dischar a
Output temperature:
135-145C
Intermediate stora
e: 24 h/RT
Stage 3
Rotor speed: 30 rpm
Flow : 50°C
Com oundin time
0-1.5' batch from stage 2,
accelerator sul hur
1.5' dischar a
Out ut tem erature:
<110C
19
CA 02210780 1997-07-17
Vulcanisate data: I65°C/t9s$
Si 69 Si 266 mod (output
(output temperature temperature 180C)
140C)
300% modules MPa 10.4 11.8
DIN abrasion mm3 64 61
tan8 0C 0.411 0.473
tan8 60C 0:155 0.160
Shore A hardness 73 70
Thanks to the elevated output temperature of 1$0°C
possible with Si 266 mod without any risk of scorching,
the vulcanisate data obtained in this manner may be
compared with those of Si 69 at an output temperature of
14 0°C .
Si 266 mod is distinguished by particularly good tan 8
values at 0°C, which may result in improved wet skidding
characteristics of the tyre.
CA 02210780 2004-02-16
List Of Document References In Disclosure:
[1] Auto 9I/92, Verband der Automobilindustri~e e.V.,
Frankfurt.
[2] ADAC Motorwelt 11791, 5fl (I991) .
[3] EP 0 501 227, US 5,227,425.
[4] G. Agostini, J. Berg, Th. Materne: New. Compound
- Technology, October 1994, Akroh, Ohio, USA.
[5] S. Wolff, U. Gorl, M.J..Wang, W. Wolff: Silica based
on tread compounds - .background & performance, paper
presented at TYRE TECH '93, October 1993, Basel,.
Switzerland.
[C] Ph. Cocriet, L.B. Barriquand: Precipitated silica in
tire tread, paper presented at ACS Meeting of the
Rubber Division, October 1995, Cleveland, Ohio, USA.
[7] S. Wolff: The influence of fi3lers on rolling
resistance, presented at the 129th meeting of the
Rubber Division, American Chemical Society, April 8-
11, 1986, New York. ~ .
[8] G.W. Marwede, U.G. Eisele, A.J.M. Summer: paper,
presented to the ACS Meeting of the Rubber Division,
October 1995, Cleveland, Ohio, USA.
[9] U. LeMaitre:~ .Tire rolling resistance,. AFCEP/DKG
Meeting,' 1993, Mulhouse, France.
[10] S.~Wolff: The role:of rubber-to-silica bonds in
reinforcement, presented at the First Franco-German
Rubber Symposium, November l4-16, 1985, Obernai,
France.
[il] S. Wolff: Silanes in tire compounding after ten
' years - review, Third Annual Meeting & Conference on
Tire Science & Technology, The Tire Society, March
28-29, 1984,.Akron, Ohio, USA.
[121 U. Gorl, A. Hunsche: Advanced .investigations into-
the silica/silane reaction system, paper presented
at ACS Meeting, Rubber Division, October 1996,
Louisville, Kentucky, ~ USA. - -
[13] Houben-Weyl: Production of disulphides, Methoden der
organischen Chemie, 1955.
21