Note: Descriptions are shown in the official language in which they were submitted.
CA 02210801 2006-07-10
1
METHOD AND APPARATUS FOR THREE-DIMENSIONAL
MICROSCOPY WITH ENHANCED DEPTH RESOLUTION
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention pertains generally to three-dimensional optical
microscopy, and more particularly to a method and apparatus for three-
dimensional
optical microscopy which employs dual opposing objective lenses about a sample
to obtain a high level of depth resolution.
2. Description of the Background Art
Optical microscopy has experienced a remarkable renaissance in the medical
and biological sciences during the last decade. The increased importance of
optical
microscopy has been due to new developments in fluorescent probe technology,
and the availability of quantitative three-dimensional image data obtained
through
either computational deconvolution or scanning confocal microscopy.
Optical microscopy offers several advantages over non-optical microscopy
techniques. Use of optical microscopy allows viewing of living tissue samples
in
their natural state. Electron microscopy, in comparison, requires microscopy
samples which are dried and exposed to vacuum. Additionally, the interior of
the
sample can be viewed and mapped in three dimensions using optical microscopy,
whereas scanning electron microscopy and other scanned probe microscopies map
only the surface of the sample, and thus cannot provide information about the
sample interior. Yet another advantage of optical microscopy is that
particular
cellular components can be recognized and mapped out with great specificity by
staining with fluorescent probes. It is now possible to synthesize fluorescent
probes with specificity for nearly any given biomolecule.
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
2
The only important drawback to optical microscopy is its limited resolution,
which is related to the angle over which the objective lens can collect light,
and
ultimately from the finite wavelength of light. Thus, any technology such as
the =
present invention that significantly increases the resolution of optical
microscopy
will have importa.nt applications in cellular biology, medical imaging, and
other biotechnology fields.
Presently there are two primary approaches to three-dimensional optical
microscopy: optical sectioning microscopy, which is also known as
computational
deconvolution, and scanning confocal microscopy.
In optical sectioning microscopy, a series of images of the microscopy
sample are acquired, with the focus moved successively through sections of the
sample to obtain successive images. Each image contains in-focus information
from the parts or sections of the sample which are in the focal plane, and
blurred,
out-of-focus information from the other parts of the sample. Analysis of the
entire
data set by computer allows reconstruction of the three-dimensional structure
of the
sample. The reconstruction process employs computational algorithms and a
previously stored reference data set describing the blur caused by a single
point
source. Optical sectioning microscopy is a"widefield" microscopy in which
large
area images are recorded, typically by a charge-coupled device array (CCD)
camera. Thus, high light throughput and high data acquisition speeds are
possible
with this technique.
In confocal microscopy, a focused laser beam is used as a light source, and
light is detected by a photomultiplier tube through a pinhole which is focused
onto
the same spot in the sample as the laser. This combined focal point is then
scanned in three dimensions through the sample, and the detected intensity as
a
function of spot position is used to obtain a three-dimensional image of the
sample.
The pinhole partially suppresses out-of-focus information and improves the
resolution, but at the cost of discarding much of the light. This loss of
light
necessitates long exposure times, which makes operation slow and often causes
severe sample bleaching problems. Confocal microscopy operations are further
slowed down by the fact that the data pixels are acquired one at a time, as
opposed
W O 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCTIUS96/01324
1 3
to up to a million in parallel for the large area imaging employed in optical
sectioning microscopy.
, Both optical sectioning microscopy and confocal microscopy suffer an
important drawback in that the depth resolution or Z-direction resolution is
several
times worse than that in the transverse, or XY, plane. The limitation on Z-
direction resolution is caused by fundamental geometrical limitations which
are
discussed in detail below. The present invention provides a method and
apparatus
for optical microscopy in which the Z-resolution is not only equal to that of
the
resolution in the XY plane, but is increased to more than double the
resolution in
the XY plane obtained heretofore with optical sectioning microscopy. This
increase in Z-direction resolution is achieved by the present invention while
also
maintaining the high light throughput and data acquisition speeds available
through
optical sectioning microscopy.
There are two previously known optical microscopy methods which employ
dual opposing objective lenses. One method, which is known as 4Pi Confocal
Microscopy, is a confocal, rather than a widefield, microscopic method. 4Pi
Confocal Microscopy can generally be employed in three ways. In a first mode,
focused laser light is used to illuminate a sample from both objective lenses
and
interfere in the sample. In a second mode the emitted light is collected from
both
directions and combined onto a single pinhole detector. The third mode
involves
the combination of the first two modes simultaneously. Being a confocal
technique, however, all modes of 4Pi Confocal Microscopy have poor light
throughput and lengthy data acquisition times due to loss of light caused by
the
pinhole photodector and the slowness of the pixel-by-pixel data acquisition.
The second known optical microscopy method which employs two opposing
lenses is generally called Standing Wave Fluorescence Microscopy (SWFM). This
technique requires a light source with great temporal and spatial coherence,
typically in the form of a laser. The spatially and temporally coherent light
source
results in an interference pattern in sample space which is a sinusoidal
standing
wave (hence the name) that extends throughout the observed region of the
sample.
W 0 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
4
SWFM could in principle achieve similar Z resolution as one embodiment
of the present invention (the I3M embodiment described herein) but only by
combining several different standing wave patterns in sequence through use of
=
scanning mirrors on similar dynamic devices, or by using multiple individually
coherent but mutually incoherent light sources, such as a plurality of lasers.
The present invention provides the increased Z-direction resolution without
requiring
such moving parts, requires only a single, spatially incoherent light source
such as
an arc lamp or incandescent bulb, and does not require temporal coherence
beyond
that exhibited by any band-limited light source. The use of a simple
incoherent
light source allows free choice of wavelength of the illumination light, while
lasers
are available in only a limited selection of wavelengths. Furthermore, one
embodiment of the present invention (the ISM embodiment described herein)
achieves greater Z resolution than is possible through SWFM alone.
Thus, the present invention differs from, and has advantages compared to,
all previously known 3D microscopy techniques. Compared to any mode of
microscopy that uses a single objective lens, the present invention offers
higher Z
resolution. Compared to SWFM, the present invention uses simpler illumination
means and offers a greater selection of illumination wavelengths, and in one
of its
embodiments offers higher Z resolution. Compared to 4Pi Confocal Microscopy,
the present invention offers simpler illumination means, a greater selection
of
illumination wavelengths, greater data acquisition speed, and more efficient
use of
observed or emitted light, which can lead to less severe sample bleaching.
Thus, there is a need for a method and apparatus for three-dimensional
optical microscopy which provides greatly enhanced depth or Z-direction
resolution, which has a high light throughput, which has a high data
acquisition
speed, and which does not require use of spatially coherent light sources. The
present invention satisfies these needs, as well as others, and generally
overcomes
the deficiencies found in known optical microscopy devices and methods.
CA 02210801 2007-03-22
-5-
SUMMARY OF THE INVENTION
The present invention generally pertains to a method and apparatus for three-
dimensional optical microscopy which employs dual opposing objective lenses
about a
sample. There are three preferred embodiments of the invention which,
employing
essentially the same apparatus, allow the sample to be illuminated from one or
both
objective lenses, and to be observed and recorded through one or both
objective lenses.
Accordingly, the present invention provides a three dimensional optical
microscopy apparatus, comprising: (a) first and second spaced-apart objective
lenses; (b)
means for supporting a microscopy sample between said objective lenses; (c)
means for
beam splitting and recombining light; (d) first and second observation paths,
said first
observation path extending from said microscopy sample to said beam splitting
and
recombining means via said first objective lens, said second observation path
extending
from said microscopy sample to said beam splitting and recombining means via
said
second objective lens; (e) a plurality of means for directing light, at least
one of said light
directing means positioned along each of said first and second observation
paths to direct
observed light from said microscopy sample along said first and second
observatiions
paths to said beam splitting and recombining means; ( fl optical path length
balancing
means for adjusting the optical path length of at least one of said first and
second
observations paths so as to make said optical path lengths of said first and
second
observations paths be closely equal; and (g) imaging means for detecting and
recording
images by detecting and recording the points of the image in parallel, said
imaging
means being positioned to detect and record all or part of said observed
light, said
observed light having been combined by said beam splitting and recombining
means.
The present invention also provides a three dimensional optical microscopy
apparatus, comprising: (a) a first objective lens and a second objective lens,
said
objective lenses mounted opposite to each other; (b) means for supporting a
microscopy
sample between said objective lenses; (c) means for beam splitting light; (d)
first and
second optical paths, said first optical path extending from said beam
splitting means to
said microscopy sample via said first objective lens, said second optical path
extending
from said beam splitting means to said microscopy sample via said second
objective
lens; (e) illuminating means for producing extended, spatially incoherent
light, said
illuminating means positioned to provide illuminating light to said beam
splitting means;
CA 02210801 2007-03-22
-5 a-
(f) a plurality of means for directing light, at least one of said light
directing means
positioned along each of said first and second optical paths to direct
illuminating light
from said beam splitting means along said first and second optical paths to
said sample;
(g) optical path length balancing means for adjusting optical path lengths of
at least one
of said first and second optical paths, so as to make said optical path
lengths of said first
and second optical paths be closely equal; and (h) imaging means for detecting
and
recording images by detecting and recording the points of the image in
parallel, said
imaging means positioned to record observed light from at least one of said
objective
lenses.
The present invention also provides an apparatus for optical microscopy,
comprising: (a) means for supporting a sample; (b) means for providing
spatially
structured illuminating light to said sample, said spatially structured
illuminating light
containing lateral structure, said means for providing spatially structured
illuminating
light comprising light source means for producing light, an illuminating path
from said
light source means to said sample, and at least one mask located along said
illuminating
path; (c) optical magnification means for producing magnified images of said
sanlple
illuminated by said spatially structured illumination light; (d) imaging means
for
detecting and recording said magnified images of said sample; and (e)
processing means
for processing said recorded images from said imaging means to obtain a
reconstruction
of said sample with improved resolution including improved lateral resolution,
said
processing means arranged to cause information components from said recorded
images
to assume new positions in Fourier space.
The present invention also provides an apparatus for optical microscopy,
comprising: (a) means for supporting a sample; (b) means for providing
spatially
structured illuminating light to said sample, said spatially structured
illuminating light
containing lateral structure, said means for providing spatially structured
illuminating
light comprising means for providing at least two mutually coherent beams of
light to
said sample, said at least two mutually coherent beams of light arranged so as
to interfere
with each other at said sample; (c) optical magnification means for producing
magnified
images of said sample illuminated by said spatially structured illumination
light; (d)
imaging means for detecting and recording said magnified images of said
sample; and (e)
processing means for processing said recorded images from said imaging means
to
CA 02210801 2007-03-22
-5b-
obtain a reconstruction of said sample with improved resolution, including
improved
lateral resolution.
By way of example and not of limitation, the present invention generally
includes
first and second objective lenses which are mounted opposite to each other
about a thin
sample, with at least one of the objective lenses including translational
adjustnlent
means. Illuminating means, preferably in the form of one or more arc lamps or
other
extended spatially incoherent light source, provides illumination for the
sample. The
invention generally includes beam splitter and beam combiner means, preferably
in the
form of a beam splitter/recombiner cube, for splitting the illuminating light
into two
paths so that it may be directed to the sample through both objective lenses,
and for
combining observed or emitted light from both objective lenses for recording.
A plurality
of adjustable mirrors allow the direction of illuminating and/or observed
light to and
from the objective lenses and image recording means. The image recording means
preferably comprises a CCD camera. Means for selectively transmitting and
reflecting
light of different wavelengths, preferably in the form of one or more dichroic
mirrors, are
generally included in the invention. Optical path length adjustment means,
preferably in
the form of a translating stage with one or more suitably positioned mirrors,
allows
tuning of optical path lengths. Phase compensation means, preferably in the
form of
chromatic phase compensator plates, may be included for compensation of pliase
differences between illuminating and observed or emitted light, and/or between
different
wavelength components within the illumination light and/or within the observed
or
emitted light. Alignment means for positioning the sample relative to the
objective lenses
are provided, which preferably include a removable mirror and eyepiece. The
invention
also may employ vibration isolation supporting means such as a vibration
isolated
platform or housing.
W U 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/US96/01324
6
In a first embodiment of the present invention the two opposing objective
lenses are used to a sample simultaneously to obtain two images of the sample,
while illuminating light is generally directed to the sample from one
objective lens. The two images from the two objective lenses are combined and
brought into
coincidence on the CCD camera or other imaging means. The optical lengths of
the two optical paths from the two objective lenses are adjusted to differ by
less
than the coherence length of the light emitted from the sample, and preferably
by
significantly less than a wavelength of the observed or emitted light. The two
images will then interfere on the CCD camera to provide sample information.
The
enhanced depth or Z-resolution information provided by the present invention
stems
from the interference of these two images when they are combined coherently on
the same CCD camera with the length of the two optical paths carefully
balanced.
While the first embodiment of the present invention is generally described
herein
in the context of fluorescence microscopy, it will be readily understood by
persons
skilled in the art that this embodiment is applicable to most other modes of
optical
microscopy as well, including brightfield, darkfield, and phase contrast
microscopies. The first embodiment of the present invention is generally
called
"Image Interference Microscopy" or IZ microscopy, and for convenience and
clarity
will hereinafter be referred to as the IZM embodiment. The operation of the
IZM
embodiment of the invention, as well as the other embodiments related below,
proceeds in a manner similar to that used in standard optical sectioning
microscopy: a series of images of the sample are acquired at different focal
planes,
with the whole data set being computationally deconvolved to remove the out-of-
focus blur by using a previously measured sample of the blur caused by a point
source.
In a second embodiment of the invention, which applies primarily to
fluorescence or phosphorescence microscopy, illuminating or excitation light
from
an extended, spatially incoherent source is split by beam splitting means, and
used
to illuminate the sample from both sides simultaneously through both opposed
objective lenses. When the optical path lengths are balanced, the two
illumination
beams interfere at the focal plane of the two objectives. This narrow
interference
CA 02210801 2007-03-22
-7-
fringe causes the illumination intensity to vary with depth, Z, in a thin
slice or region of
the sample surrounding the focal plane. This spatial structure of the
illumination light
causes a corresponding modulation of the fluorescent emission from the sample,
which is
the source of the increased Z-direction resolution. In the second embodiment
the sample
is generally observed through a single objective lens. The second embodiment
of the
invention is called "Incoherent Interference Illumination" or I3 microscopy,
and for
convenience and clarity will hereinafter be referred to as the I3M embodiment
of the
invention.
In a third embodiment of the invention, the 12 M embodiment and 13M
embodiment are combined and, using essentially the same apparatus, achieve
greater Z-
direction resolution than is possible with either the 12M or 13M embodiments
alone. The
third embodiment is hereinafter referred to as the IS microscopy or 15 M
embodiment
since it involves a combination of the 12 M and 13 M embodiments. In the 15M
embodiment, the sample is observed through both lenses as in the 12 M
embodim.ent,
while the sample is illuminated from both objective lenses as in the I3M
embodiment.
The same beam splitter may be used for both the illumination light and the
observed
light, since the necessary alignment is essentially identical for both.
In a further aspect, the present invention provides a method for three-
dimensional
optical microscopy, comprising the steps of: (a) placing a sample between
first and
second opposing objective lenses; (b) focussing said objective lenses on a
section of said
sample; (c) directing observed light from said section of said sample along
first and
second paths to imaging means for detecting and recording images by detecting
and
recording the points of the image in parallel, said first and second paths
leading from
said section of said sample to said imaging means through said first and
second objective
lenses respectively, and causing said observed light from said first and
second paths to
coincide on said imaging means; (d) adjusting optical lengths of at least one
of said first
and second paths so as to make said first and second optical path lengths be
closely
equal, thereby causing said observed light from said first and second
objective lenses to
interfere on said imaging means; (e) recording said interfering observed light
on said
imaging means; (f) focussing said objective lenses on another section of said
sample; and
(g) repeating steps (c), (d), (e), and (f) until a plurality of sections of
said sample have
been observed and recorded, forming a data set of recorded images.
CA 02210801 2007-03-22
-7a-
The present invention also provides a method for three-dimensional optical
microscopy, comprising the steps of: (a) placing a sample between first and
second
opposing objective lenses; (b) focussing said first and second objective
lenses onto a
section of said sample; (c) directing illuminating light from an extended,
spatially
incoherent light source along first and second illumination paths to said
section of said
sample, said first illumination path extending from said light source to said
section of
said sample via said first objective lens, said second illumination path
extending from
said light source to said section of said sample via said second objective
lens; (d)
directing observed light from at least one of said first and second objective
lenses to
imaging means for detecting and recording images by detecting and recording
the points
of the image in parallel; (e) adjusting optical lengths of at least one of
said first and
second illumination paths, so as to make said optical lengths of said first
and second
illumination paths be closely equal, thereby causing said illuminating light
from said first
and second illumination paths to interfere in said section of said sample; (f)
recording
said observed light on said imaging means; (g) refocusing said first and
second objective
lenses onto another section of said sample; and (h) repeating steps (c), (d),
(e), (f) and. (g)
until a plurality of sections of said sample (16) have been observed and
recorded,
forming a data set of recorded images.
In a still further aspect, the present invention provides a method for three-
dimensional optical microscopy, comprising the steps of: (a) placing a sample
between
first and second opposing objective lenses; (b) focussing said first and
second objective
lenses onto a section of said sample; (c) directing illuminating light from an
extended,
spatially incoherent light source along first and second illumination paths to
said section
of said sample, said first illumination path extending from said light source
to said
section of said sample via said first objective lens, said second illumination
path
extending from said light source to said section of said sample via said
second objective
lens; (d) directing observed light from at least one of said first and second
objective
lenses to imaging means for detecting and recording images by detecting and
recorcling
the points of the image in parallel; (e) adjusting optical lengths of at least
one of said first
and second illumination paths, so as to make said optical lengths of said
first and second
illumination paths be closely equal, thereby causing said illuminating light
from said first
and second illumination paths to interfere in said section of said sample; (f)
recorcling
CA 02210801 2007-03-22
-7b-
said observed light on said imaging means; (g) refocusing said first and
second objective
lenses onto another section of said sample; and (h) repeating steps (c), (d),
(e), (f) and (g)
until a plurality of sections of said sample have been observed and recorded,
forming a
data set of recorded images.
The present invention also provides a method of optical microscopy comprising
the steps of: (a) placing a luminescent sample in a microscope containing in-
iage
detecting and recording means; (b) illuminating said sample with an
illumination pattern
that contains lateral structure; (c) recording at least one image of said
sample using said
image detecting and recording means; (d) altering said illumination pattern at
least one
time, each time recording at least one image of said sample illuminated with
said altered
illumination pattern; (e) collecting said images into a data set; and (f)
computationally
processing said data set to obtain a reconstruction of said sample with
improved
resolution, including improved lateral resolution, said step of
computationally processing
said data comprising the steps of separating a plurality of information
components,
causing said information components to assume new positions in Fourier space,
and
recombining said information components.
The invention provides a method and apparatus for three-dimensional optical
microscopy which provides greatly enhanced depth or Z-direction resolution.
The invention also provides a method and apparatus for three-dimensional
optical
microscopy which has high light throughput.
The invention also provides a method and apparatus for three-dimensional
optical
microscopy which allows high data acquisition speed.
The invention also provides a method and apparatus for three-dimensional
optical
microscopy which does not require use of a coherent light source.
Still further, the invention provides a method and apparatus for three-
dimensional
optical microscopy which does not cause unnecessary bleaching of samples.
CA 02210801 2006-07-10
8
Further features and advantages of the invention will be brought out in the
following portions of the specification, wherein the detailed description is
for the
purpose of fully disclosing preferred embodiments of the invention without
placing
limitations thereon.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be more fully understood by reference to the following
drawings which are for illustrative purposes only:
FIG. 1 is a schematic diagram of a first (IZM) embodiment of an optical
microscope in accordance with the present invention.
FIG. 2 is a schematic diagram of an optical microscope as generally used
in a second (I3M) embodiment and : a third (15 M) embodiment of the present
invention.
FIG. 3 is a schematic diagram of the I'M embodiment of an optical
microscope.
FIG. 4 is a schematic diagram of the I3M embodiment of an optical
microscope wherein shared beam splitting optics are employed for illuminating
and
detected light.
FIG. 5 is a schematic diagram of the I'M embodiment of an optical
microscope wherein separate beam splitting optics are employed for
illuminating
and detected light.
FIG. 6 shows the schematic diagram of the I'M embodiment of an optical
microscope with an additional beam splitter included adjacent to the
illumination
source and image detector.
FIG. 7 is a graphic representation of the region of support of the incoherent
optical transfer function obtained through conventional widefield optical
sectioning
microscopy, shown generally in the kYkZ plane, i.e., in the plane of Fourier
space
spanned by the kY and kZ axes, which correspond to the Y and Z axes of real
space, respectively.
FIG. 8 is a graphic representation of the region of support of the incoherent
optical transfer function obtained through the I'M embodiment of the present
invention, shown generally in the kYkZ plane.
WU 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
9
FIG. 9 is a graphic representation of the region of support of the spatial
frequency content of the illuminating light intensity used in the I3M and I5M
embodiments of the present invention, shown generally in the kYkZ plane.
FIG. 10 is a graphic representation of the region of support of the
incoherent optical transfer function obtained through the I3M embodiment of
the
present invention, shown generally in the kYkZ plane.
FIG. 11 is a graphic representation of the region of support of the
incoherent optical transfer function obtained through the ISM embodiment of
the
present invention, shown generally in the kYkZ plane.
FIG. 12 is a graphic representation of the spatial frequency components or
coherent transfer function in Fourier space of the emitted light from a
sample,
shown generally in the kYkZ plane.
FIG. 13 is a graphic representation of the autocorrelation function or
incoherent transfer function of the coherent 1:ransfer function shown in FIG.
12,
shown generally in the kYkZ plane.
FIG. 14 is a simplified representation of a conventional objective lens with
acceptance angle a.
FIG. 15 is a graphic representation of the portion of the coherent transfer
function shown in FIG. 12 which is obtained by the conventional objective
leans
shown in FIG. 14.
FIG. 16 is a graphic representation of the portion of the incoherent transfer
function shown in FIG. 13 which is obtained by the conventional objective lens
shown in FIG. 14.
FIG. 17 is a simplified representation of two opposing objective lenses in
accordance with the present invention, with each objective lens having an
acceptance angle a.
FIG. 18 is a graphic representation of the portion of the coherent transfer
function shown in FIG. 12 which is obtained by the dual objective lens
arrangement shown in FIG. 17.
W O 96124082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
FIG. 19 is a graphic representation of the portion of the incoherent transfer
function shown in FIG. 13 which is obtained by the dual objective lens
arrangement shown in FIG. 17.
FIG. 20 is a simplified diagrammatic representation of the illumination
5 arrangement generally used in a conventional microscopy system employing a
single objective lens, showing light from a single point of light source.
FIG. 21 is a simplified diagrammatic representation of the illumination
arrangement used in the I3M and ISM embodiments of the present invention,
showing light from a single point of the illumination source illuminating the
sample
10 from both directions after having been split into two beams by the beam
splitting
means.
FIG. 22 is a graphic depiction of the Fourier space representation
corresponding to illumination or excitation light amplitude from the point
source
illumination arrangement shown in FIG. 21, viewed generally in the kYkZ plane.
FIG. 23 is a graphic representation of the autocorrelation function of the
Fourier space representation shown in FIG. 22 corresponding to the intensity
of the
illumination light in the point source illumination situation shown in FIG.
21.
FIG. 24 is a graphic depiction of the union, over all points of the
illumination source, of the regions (point pairs) depicted by FIG. 22.
FIG. 25 is a graphic representation of the union, over all points of the
illumination source, of the regions (point triplets) depicted by FIG. 23.
FIG. 26 is a plan view of the IZM embodiment of the present invention.
FIG. 27 is plan view of a more compact version of the I2M embodiment
shown in FIG. 26.
FIG. 28 is a plan view of the I3M embodiment of the present invention.
FIG. 29 is a plan view of the ISM embodiment of the present invention.
FIG. 30 is a plan view of the ISM embodiment of the present invention in
which two image recording devices are employed.
FIG. 31 is a plan view of the ISM embodiment of the present invention
wherein different portions of the same image detector are employed.
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCTIUS96/01324
11
FIG. 32 is a simplified schematic representation of the present invention
incorporated into a standard inverted microscope equipped for epi-
illumination.
FIG. 33 is a simplified schematic representation of a standard inverted
microscope equipped for both epi- and trans-illumination.
FIG. 34 is a flow diagram showing the general steps of the method for
using the I2M embodiment of the present invention.
FIG. 35 is a flow diagram showing the general steps of the method for
using the I3M embodiment of the present invention.
FIG. 36 is a flow diagram showing the general steps of the method for
using the I5M embodiment of the present invention.
FIG. 37 is a graphic representation of the amplitude of two coherent beams
of light in Fourier space, shown generally in the kYkZ plane.
FIG. 38 is a graphic representation of the autocorrelation of the Fourier
space amplitude shown in FIG. 37, shown generally in the kYkZ plane.
FIG. 39 is a graphic representation of the directly observable and displaced
regions in Fourier space obtained by observation through a conventional single
lens
microscope, shown generally in the kYkZ plane.
FIG. 40 is a graphic representation of the directly observable and displaced
regions in Fourier space obtained by observation through the dual opposing
objective lens arrangement of the present invention, shown generally in the
kYkZ
plane.
FIG. 41 is a graphic representation of the light amplitude distribution in
Fourier space for the four beam standing wave microscopy technique, shown
generally in the kYkZ plane.
FIG. 42 is a graphic representation of the resulting intensity field from the
autocorrelation of the function shown in FIG. 41, shown generally in the kYkZ
plane.
FIG. 43 is a graphic representation of the light amplitude distribution in
Fourier space for the four beam standing wave microscopy technique which
occurs
if the two illuminating point sources are arranged symmetrically opposite each
other in the aperture, shown generally in the kYkZ plane.
CA 02210801 1997-07-18
WO 96/24082 PCT/US96/01324
12
FIG. 44 is a graphic representation of the resulting intensity field from the
autocorrelation of the function shown in FIG. 43, shown generally in the kYkZ
plane.
FIG. 45 is a graphic representation of the region of support for the
illumination amplitude for two mutually cohereiit light sources which are
located
at diametrically opposite points on the edge of the aperture, shown generally
in the
kYkZ plane.
FIG. 46 shows the region of support for the illumination intensity
corresponding to the function shown in to FIG. 45, shown generally in the kYkZ
plane.
FIG. 47 is a graphic representation of the directly observable and displaced
regions of Fourier space obtained using the four-beam standing wave microscopy
technique, for the illumination arrangement described by FIG. 45 and FIG. 46,
shown generally in the kYkZ plane.
FIG. 48 is a graphic representation of the region of support for the
illumination amplitude for two mutually coherent light sources wherein one
light
source is located at the center of the aperture and one light source is placed
at the
edge of the aperture, shown generally in the kYkZ plane.
FIG. 49 shows the region of support for illumination intensity
corresponding to the function shown in FIG. 48, shown generally in the kYkZ
plane.
FIG. 50 is a graphic representation of the directly observable and displaced
regions of Fourier space obtained using the four-bream standing wave
microscopy
technique, for the illumination arrangement described by FIG. 48 and FIG. 49,
shown generally in the kYkZ plane.
DESCRIPTION OF THE PREFERRED EMBODIlVIENTS
Referring more specifically to the drawings, for illustrative purposes the
method and apparatus comprising the present invention and the underlying
theory
behind the invention are generally shown in FIG. 1 through FIG. 50. It will be
=
appreciated that the apparatus of the invention may vary as to configuration
and as
to details of the parts, and that the method of the invention may vary as to
the
W O 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
- 13
steps and their sequence, without departing from the basic concepts as
disclosed
herein.
Referring first to FIG. 1, a simplified schematic diagram of a microscope
apparatus 10 in accordance with the first or I2M embodiment of the present
invention is generally shown. A first objective lens 12 and a second objective
lens
14 are mounted about a sample 16, with objective lenses 12, 14 being focused,
from opposite directions, on one and the same section or plane of sample 16.
Sample 16 is preferably thin and mounted between two cover glasses. The
observed light or images from first and second objective lenses 12, 14 is
reflected
by a plurality of mirrors 18 along paths 24, 26 respectively and directed to
beam
splitting and recombining means, preferably in the form of beam
splitter/recombiner 20. The observed light or images from objective lenses 12,
14
are brought into coincidence on image detection means 22 for image recording
by
mirrors 18 and beam splitter/recombiner 20. Preferably, image detection means
22 is a CCD camera or the like. The optical lengths of the two optical paths
24,
26 are adjusted to differ by less than the coherence length of the emitted
light.
Optical path length adjustment is carried out by suitable means (not shown)
which
are discussed below in more detail. Once optical path lengths 24, 26 are
adjusted,
the observed light or images from first and second objective lenses 12, 14
will
interfere on image detection means 22. Generally, illuminating light from
illuminating means (not shown) is directed to sample 16 through one of the
objective lenses 12 or 14 using a beam splitter, which may or may not be
dichroic,
and may or may not also serve as one of the mirrors 18a or 18d. The image from
the interfering observed light is recorded by iinage detection means 22 and
stored
by data processing means (not shown) which are interfaced with image detection
means 22.
The operation of the microscope apparatus 10 proceeds in a fashion which
is generally similar to standard optical sectioning microscopy. After the
observed
light or images of the section of sample 16 are recorded by image detection
means,
objective lenses 12, 14 are focused on another section or plane within sample
16
using sample positioning means (not shown) to obtain another image
corresponding
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
14
to the new section. A series of images of the sample are acquired at different
focal
planes, and a data set of images for the desired portions of sample 16 is
formed
from the series of images. As in optical sectioning microscopy, each image
includes in-focus information from sample 16 from the section or focal plane
in
which first and second objective lenses 12, 14 are focused, as well as out-of-
focus
or blurred information from the sections of sample 16 which are outside the
focal
plane. The entire data set is computationally processed (a process we will
generally refer to, without implying limitation, as deconvolution) to remove
the
out-of-focus blur, using a previously measured sample of the blur caused by a
point
source. Image detection means 22 is preferably interfaced to a microprocessor
or
other data processing means (not shown) to facilitate computational
deconvolution
of the data set from sample 16.
The enhanced Z-direction resolution results from essentially the same
physical process that takes place in a standard microscope with a single
objective.
Resolution in a standard single objective microscope can be regarded as
generated
by the interference between light emitted in different directions, leading to
the well
known fact that objective lenses of larger aperture, i.e. which accept light
with a
larger range of angles, and have greater resolution. The present invention
extends
this process to include light emitted in "backward," as well as "forward,"
directions by employing two opposing objective lenses.
Several arrangements of mirrors 18 may be employed for microscope
apparatus 10, and the mirror arrangement shown in FIG. 1 is one of the
simplest
preferred arrangements. It will be appreciated that some arrangements of
mirrors
18 will not work with the present invention as shown in FIG. 1 because the
images
from one of the objective lenses 12, 14 will become inverted relative to the
image
from the other lens.
The IZM embodiment of the present invention as shown in FIG. 1 is
generally applicable to fluorescence microscopy. However, it will be apparent
to
those skilled in the art that this embodiment is also applicable to
brightfield,
darkfield, phase contrast, and other modes of optical microscopy. Thus, when
the
present invention is employed for fluorescence microscopy or phosphorescence
CA 02210801 1997-08-12
L- t,L' d >#; ; ~,~
4
IpE" 06JAN1997
microscopy, the term "observed light" from the objective lenses as related
herein is
used to refer generally to emitted light from a sample, and the term
"illuminating
light" generally refers to excitation light. When non-luminescent types of
microscopy are employed, "observed light" generally refers to light reflected
by or
5 transmitted through a sample.
Referring next to FIG. 2, a simplified schematic diagram of a microscope
apparatus 28 is shown which is suitable for use in the second or I3M
embodiment and
third or ISM embodiment of the present invention, wherein like reference
numerals
denote like parts. As can be seen by referring also to FIG. 1, the I3M and ISM
10 embodiments employ generally the same apparatus as used in the IZM
embodiment,
with the primary difference being that illuminating light, preferably from a
spatially
incoherent light source 30, is directed initially to beam splitter/recombiner
20 by an
illumination focusing means generally comprising one or more lenses or mirrors
and
depicted schematically as lens 32, rather than directed initially to sample 16
as in the
15 I2M embodiment. The I3M and ISM embodiments of the present invention as
shown
in FIG. 2 are contemplated for use primarily with luminescent microscopies,
though
they can be used with other modes of microscopy. Thus, in the I3M and ISM
embodiments the term "illuminating light" will generally refer to excitation
light, and
the term "observed light" will generally refer to emitted light from a sample.
The illuminating light in apparatus 28 of FIG. 2 is split into two beams by
beam splitter/recombiner 20, and directed by mirrors 18 to first and second
objective
lenses 12, 14 along paths 24, 26 respectively. The illuminating light is
focused and
brought into coincidence on a section or plane of sample 16 by objective
lenses 12,
14. As in the 12 M embodiment, the optical lengths of two optical paths 24, 26
are
adjusted by suitable means (not shown) to be closely equal. This adjustment of
optical path lengths 24, 26 results in interference of the illuminating light
within the
focal plane of sample 16. Thus, in the I3M and ISM embodiments of the present
invention, the illuminating light is brought into interference in the focal
plane of a
section of sample 16 rather than the observed light on the detection means as
in the
12 M embodiment. However, all three
~ e erp ""~ C~`,,~'L='~
W O 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/US96/01324
16
embodiments use essentially the same apparatus, as can be seen by comparing
FIG.
1 and FIG. 2.
Referring now to FIG. 3 and FIG. 4, which show the I3M and I5M
embodiments of the present invention respectively, it can be seen that the
primary
difference between the apparatus comprising the I3M and I5M embodiments is the
position of image detection means 22. Focussing means (not shown), generally
lenses, may be used to focus the image onto image detection means 22.
Referring
more particularly to FIG. 3, an apparatus 34 in accordance with the 13M
embodiment of the present invention is generally shown, wherein image
detection
means 22 records observed light from first objective lens 12 alone. Observed
light
from first objective lens 12 reaches image detection means 22 through
selective
reflectance and transmittance means, shown here as beam spitter 36 (which may
be dichroic) which selectively transmits observed or emitted light from first
objective lens 12 to image detection means 22, while selectively reflecting
illuminating or excitation light away from image detection means 22. Beam
splitter
36 may occupy generally the same position as mirror 18a in the I'M and I5M
embodiments, or may be a separate component.
Referring more particularly to FIG. 4, an apparatus 38 in accordance with
the ISM embodiment of the present invention is generally shown, wherein image
detection means 22 is positioned adjacent to beam splitter/recombiner 20 for
recording observed light from both first and second objective lenses 12, 14.
Focussing means (not shown), such as lenses, may also be included between beam
splitter/recombiner 20 and image detection means 22 if desired. The I'M
embodiment of the present invention records interfering images on image
detection
means 22, as in the I'M embodiment, and causes illuminating light from first
and
second objective lenses to interfere within sample 16 as in the I'M
embodiment,
and thus is merely a combination of these two embodiments. The same optical
path length adjustment which causes illuminating light to coincide and
interfere
within the focal plane of a section of sample 16 results in coincidence and
interference of observed light on image detection means 22, and thus in many
W 0 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCTIUS96/01324
17
instances the same apparatus may be used for both IZM and I3M embodiments by
simply moving the light source 30.
= Several mirror and beam splitter/recombiner arrangements are contemplated
for each embodiment of the invention, witli the ISM embodiment perhaps having
the greatest possible number of configurations. As shown in FIG. 4, apparatus
38
employs a single beam splitter/recombiner 20 which both splits illuminating
light
and recombines observed light for detection by image detection means 22. The
apparatus 40 shown in FIG. 5 employs separate beam splitter/recombiners 42, 44
for splitting illuminating light and combining observed light respectively, to
achieve the same beam splitting and recombining effected by apparatus 38 in
FIG.
4. Additional mirrors 18e, 18f, and dichroic mirrors 46, 48, are required for
use
of separate beam splitters 42, 44 in FIG. 5. Shown in FIG. 6 as apparatus 50
is
yet another possible arrangement of separate beam splitter/recombiners 42, 44
in
accordance with the ISM embodiment which does not require additional mirrors
or
dichroic mirrors. Possible advantages for each of the arrangements shown in
FIG.
4 through FIG. 6 are discussed below.
The operation of the microscope apparatus shown in FIG. 2 through FIG.
6 in accordance with the 13 M and ISM embodiments of the present invention
proceeds in the same manner related above for the 12 M embodiment. After the
observed light or images of the section of sample 16 are recorded by image
detection means 22, objective lenses 12, 14 are focused on another section or
plane
within sample 16 by moving the sample using sample positioning means (not
shown) to obtain another image corresponding to the new section. A series of
images of the sample are acquired at different focal planes, and a data set of
images for the desired portions of sample 16 is formed. The entire data set is
computationally deconvolved to obtain three dimensional sample information
with
enhanced Z-direction resolution.
The present invention will be more fully understood by referring to FIG.
7 through FIG. 25, which relate generally the theory behind the present
invention
and the physical basis for the enhanced Z-direction resolution achieved by the
present invention. Graphic representations of various three dimensional
functions
W U 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/US96/01324
18
included in FIG. 7 through FIG. 25 are shown generally in the kY and kZ plane
of
Fourier space.
As related above, the reconstruction process in currently used optical
sectioning microscopy employs a reference data set which describes the blur
from
a point source. This reference blur is generally known as the "point spread
function" of the microscope and characterizes its optical properties. The
Fourier
transform of the point spread function is known generally as the "optical
transfer
function" or OTF, and describes to what extent the different spatial frequency
components of the sample information are represented in the data. For reasons
outlined below, the optical transfer function of any optical imaging system is
zero
everywhere except within a certain finite region which is generally called the
"region of support". Thus, only those spatial frequencies of the sample which
lie
within the region of support influence the sample data, and no information is
contained in the data set about any other spatial frequencies. Since the
computational reconstruction can be made more accurate the more information
about the sample is available, it is thus advantageous for a microscope to
have as
large as possible a region of support of its optical transfer function. The
size and
shape of the region of support of the optical transfer function for a three-
dimensional optical microscope is determined by the angle over which the
objective
lens can accept light. The IZM embodiment of the present invention extends the
solid angle over which light is collected, thereby increasing the resolution.
FIG. 7 shows generally, in graphic representation in the YZ plane, the
region of support of the incoherent optical transfer function obtained through
conventional optical sectioning microscopy. The Z-direction resolution
obtained
through the conventional microscopy is described generally by the equation
Zres = [1-Cos(a)]/X
where a is the angle over which the objective lens can collect light, and X is
the
wavelength in the sample medium of the observed or emitted light. In contrast,
FIG. 8 shows the corresponding region of support of the incoherent optical
transfer
function obtained with the I2M embodiment of the present invention, which
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCTIUS96/01324
- 19
provides a Z direction resolution described by the equation
Zres = 2/X
where X is the wavelength in the sample medium of observed or emitted light.
Both graphs represent three-dimensional objects, rotationally symmetric about
the
kZ axis. As can readily be seen, the present 'invention provides significantly
increased Z-direction resolution over state of the art optical sectioning
microscopy.
The introduction of the illumination method characteristic of the I'M and
I5M embodiments of the present invention changes the optical transfer
functions
shown in FIG. 7 and FIG. 8 by convolving them with the function shown in FIG.
9. FIG. 9 shows generally the region of support for the spatial frequency
content
of the illuminating or excitation light generaily used in the I3M and I5M
embodiments of the present invention. Thus, the optical transfer function of
the
I'M embodiment is given by the convolution of the functions shown in FIG. 7
and
FIG. 9, which results in the region of support shown generally in Fig. 10. The
I'M embodiment thus provides a Z-direction resolution described by the
equation
Zres = 2/Aexcitation + [1-COS( )]/Aemission
Similarly, the region of support for the optical transfer function of the I5M
embodiment, shown generally in FIG. 11, is obtained by the convolution of the
functions shown in FIG. 8 and FIG. 9. The region of support of the optical
transfer function for the 15M embodiment is described by the equation
Zres = 2/Xexcitation 4- 2/Xemission
Note that the optical transfer function for the I'M embodiment not only more
than
doubles the Z-resolution obtained with the I'M embodiment, but also fills the
"gaps" in the I'M optical transfer function regions of support shown in FIG. 8
and
FIG. 10.
In the following sections, corresponding to FIG. 12 through FIG. 25, the
physical reasons behind the shapes of the objects in FIG. 7 through FIG. 12
will
be discussed in more detail. A quasi-monochromatic light field, such as that
emitted by a fluorescent microscopy sample, has effectively a single
wavelength
Xemission and, therefore all of its spatial frequency components are confined
to a thin
shell in Fourier space, of radius R = 1/X, as is shown graphically in FIG. 12.
W O 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
The field amplitude contains information only about those spatial frequency
components of the sample emission amplitude (and phase) which fall within the
same thin shell shown in FIG. 12. This shell can be regarded as a coherent
transfer function between the sample emission and the electric field. For an
5 incoherent emitting (e.g. fluorescent) sample, however, the average
intensity of the
light field contains information about the emission intensity, and the
relevant
"incoherent" transfer function can be obtained by the autocorrelation function
of
the coherent transfer function. The autocorrelation function of the shell
shown in
FIG. 12 is nonzero within a sphere of radius R= 2/X, as shown in FIG. 13.
10 However, as shown in FIG. 14, a conventional objective lens 52 can only
access the light from a sample 54 within a certain angle of its optical
axis. The
effect is to truncate the coherent transfer function shown in FIG. 12 to a
spherical
cap-shaped function contained within a cone of half angle a as shown in FIG.
15.
The corresponding incoherent transfer function, which is the autocorrelation
15 function of the spherical cap-shaped function of FIG. 15, is shown by FIG.
16.
The incoherent transfer function thus is nonzero only within the donut-shaped
region of Fourier space shown in FIG. 16, and no information about the sample
is available from outside of this region. This donut-shaped region represents
the
fundamental resolution limits of the microscope, regardless of the optical
quality
20 of the lenses used. That this is indeed the main limitation on resolution
of three
dimensional optical microscopy is clear from the similarity of theoretical
predictions to experimental results. In microscopy employing the IZM
embodiment
of the present invention, two objective lenses 52, 56 are present, as shown in
FIG.
17, so that light within two cones of half angle a can be accessed. This
provides
a coherent transfer function consisting of two spherical caps, as shown in
FIG. 18.
The corresponding incoherent transfer, which is the autocorrelation function
of the
function depicted in FIG. 18, is shown in FIG. 19. The significantly increased
width (in the kZ direction) in FIG. 19 as compared to FIG. 16 is directly
related
to the increased Z-resolution obtained in the present invention. The graphic
representations shown in FIG. 7 through FIG. 19 are shown for an angle of 67
degrees, corresponding to the most high-resolving objective lenses currently
W O 96/24082 CA 02210801 19 9 7- 0 7-1 s pCT/US96/01324
21
available. For such lenses, the IZM embodiment will generally improve Z
direction
resolution by a factor of 3.2 over the background art.
The ability of the I3M and I'M embodiments to access information outside
the regions depicted in FIG. 16 and FIG. 19 can be understood in terms of a
distinction between on the one hand the spatial structure of the light
emission and,
on the other hand, the spatial structure of the sample itself. The above
description
of the I'M embodiment of the invention relates information one can access
about
observed or emitted light from a sample, wliich will hereinafter be referred
to as
E(r). Of more interest, however, is the sample itself or, more specifically,
in the
context of fluorescence microscopy, the distribution F(r) of fluorescent
molecular
groups (fluorophores) within the sample. E(r) is related to F(r) by
E(r) = F(r) I(r)
where I(r) is the local illumination intensity or pattern. If the illumination
is
uniform, as is typically the case in widefield microscopies, then I(r) is
constant,
so that E(r) and F(r) are identical except for an uninteresting constant scale
factor.
Thus, the limitations to E related above for the I'M embodiment apply to F
exactly
as they do to E.
If, as in the case in the I3M and I'M embodiments of the present invention,
the illumination is not uniform, the situation is different. The real space
product
in the equation E(r) = F(r) I(r) corresponds to a convolution in Fourier
space.
If I(r) has Fourier components outside of the origin (which occurs when I(r)
is
non-uniform), this convolution operation "inoves" sample information to new
positions in Fourier space. In particular, it moves some information into the
observable region of E from the normally unobservable region. This is the
essence
of the physical basis for the resolution enhancements of I3M and I'M
embodiments
of the invention. However, as related above, all three embodiments employ
essentially the same apparatus, and merely have a different physical basis for
the
Z direction resolution enhancement.
If the non-uniform illumination pattern were to remain fixed relative to the
sample as the data acquisition progressed from section to section, there would
be
no way to tell the sample fluorescence information F(r) apart from the
illumination
W O 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/1JS96/01324
22
pattern I(r). In other words, the illumination pattern would look like part of
the
sample. This would result in the acquired data remaining restricted to the
usual
(small) observable region of Fourier space shown in FIG. 7, but the
information
which belonged to other areas of the sample would get mixed in with it. This
mixed-together data would in general be difficult to separate. If, on the
other
hand, the illumination pattern stays fixed in relation to the focal plane, it
will "look
like part of the point spread function, " in which case the acquired data
stays in its
correct position in Fourier space, and instead the optical transfer function
itself
becomes extended. This latter, clearly preferable state of affairs is the case
for the
present invention as described herein.
It is possible to change the relative strength of the different parts of the
optical transfer functions by apodization, e.g., by introducing masks, in
planes
conjugate to the back focal planes of the objective lenses, into the imaging
beams,
illumination beams, or both. Use of polarizing components to restrict the
illumination light, the imaging light, or both, to a single polarization
state, may
also be employed. In the case of both, these states can be the same or
different.
Interference microscopies generally employ light sources with high temporal
and spatial coherence, and typically require use of lasers. One might ask how
it
can be possible to achieve interference with "incoherent" light. The standard
way
of analyzing this involves consideration of individual point sources of an
incoherent
light source. In a spatially "incoherent" light source, such as a thermally
glowing
light bulb filament, light "rays" emitted from different points have a
randomly
varying relative phase, that is to say they are mutually incoherent. Each
source
point by itself, however, can be considered a coherent light source, since a
point
source cannot have a phase difference relative to itself. The total effect of
the
entire light source can therefore be found by first considering each
individual
source point by itself, then calculating the light intensity caused by that
point alone
(which, since each point is coherent, will provide a bona fide interference
pattern),
followed by adding all of all these intensities. In most situations, such as
when
using a standard desk lamp, the various interference patterns cancel each
other out
and add up to a smooth intensity distribution. The particular geometry
employed
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
23
in the present invention, however, is designed so that every source point
interference pattern has a peak at the focal plane, and therefore their sum,
the total
intensity distribution, also has such a peak.
Referring to FIG. 20, the illumination arrangement generally employed in
standard microscopies is called Kohler illumination, wherein the light from
each
source point 58 of an illuminating source 60 is focused into a parallel beam
62 in
sample space and in sample plane 64 by lens 66, at an angle 0 that differs
from
source point to source point. In the I3M and ISM embodiments of the present
invention, however, the light from each source point is split by a beam
splitter and
instead corresponds to two beams in sample space, as shown in FIG. 21. In
Fourier space this corresponds to two points on the sphere of radius
Mexcitation as
is shown graphically in FIG. 22. The resulting intensity (from the particular
source point under consideration) is the autocorrelation function of these two
points
as shown by the three points in FIG. 23, which in real space corresponds to a
sinusoidal interference pattern aligned with tlie Z-axis. The total light
intensity in
the sample is the sum of the intensity contributions from all the points of
the light
source. The set of possible light beams that can be transmitted by the
objective
lenses is described in Fourier space by the double spherical cap shown in FIG.
24,
which is generally the same as was shown in FIG. 18, except that FIG. 24 shows
a radius of 1/Xexcitatioti instead of 1/Aemission as in FIG. 18. Each point on
the light
source will give rise to some particular angle 0 (as defined in FIG. 21 and
FIG.
22), and thus to a particular value of OkZ, which is given by =
2Cos(,6)/Xexcitazion=
With full aperture illumination, every such ray, and thus every such value of
0, is
generated by some point on the light source. Thus (3 takes on all values from -
to a, so OkZ takes on all values from OkZmin = 2Cos( )/Aexcitation to OkZmax =
2/Xexcitat;on. The total illumination intensity, the sum of all the
contributions from
all the points on the light source, is thus represented in Fourier space by
the union
of the regions of FIG. 23 for all values of OkZ between OkZmin and OkZma,. The
resulting region is shown in FIG. 25. This region, which can be recognized by
comparison to FIG. 9, is the region of supporl: of the Fourier transform of
the total
CA 02210801 2006-07-10
-24-
illumination light intensity in the sample. The region can be thought of as a
version of
FIG. 19 with everything outside of the kZ axis discarded.
For illustration purposes only, by way of example and not of exclusion,
FIG. 26 through FIG. 29 show possible embodiments of the present invention.
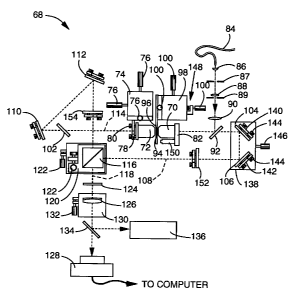
Referring
first to FIG. 26, there is shown a schematic diagram of an apparatus 68 for
use with the
12M embodiment of the invention. All components of apparatus 68 are mounted on
supporting means, such as a platform or housing (not shown), which preferably
is
vibrationally isolated. First and second objective lenses 70, 72 may include
translation
adjustment means, although generally, second objective lens 72 alone is
mounted on
translational adjustment means, shown here as translation stages 74, which can
undergo
XYZ adjustment by actuating screws 76, which are oriented in the X, Y, and Z
directions. Second objective lens 72 is mounted to translation stages 74 by
angular
adjustment means, such as a tiltable mount 78, containing actuating means such
as
adjustment screws 80. First objective lens 70 is preferably mounted directly
to support
member 82. Objective lenses 70, 72 must generally be carefully aligned in
position and
angle for interference to take place. Such precision adjustment is allowed by
adjusting
translation stage 74 positionally on the supporting means. Illuminating light
from
illuminating means, such as light from a filtered mercury arc lamp (not shown)
directed
through an optical fiber 84, is focused and directed through illumination
focusing means,
shown here as lenses 86, 88, 90 and optical beam delimiting means, shown here
as field
stop 87 and aperture stop 89, onto beam splitter 92. Optical fiber 84
preferably has a
wide core (1 mm), so as to act as a spatially incoherent light source. Beam
splitter 92
reflects illuminating light through first objective lens 70 and onto a sample
94. Sample
94 is mounted by support 96 to translational adjustment means, shown here as
translation
stage 98, which is translated by screws 100. Observed light, or, in the case
of
fluorescence microscopy, emission light from the sample, emerges through both
first and
second objective lenses 70, 72, and is selectively transmitted by beam
splitter 92 and
optional second beam splitter 102. Said beam splitters 90, 102 may be
dichroic, and will
be referred to herein as dichroic mirrors 92, 102 for simplicity. Light
transmitted by
dichroic mirror 92 traverses mirrors 104, 106 along path 108, while light
transmitted by
dichroic mirror 102 traverses mirrors 110, 112 along path 114. The light
directed along
paths 108 and 114 is directed to and combined into a single beam 118 by beam
splitting
CA 02210801 2006-07-10
-25-
and recombining means, preferably in the form of beam splitter cube 116. Beam
splitter
cube 116 is preferably mounted on a translating and tilting stage 120 which is
moved by
screws 122. The light in beam 118 passes through filter 124 to remove
illuminating light,
and finally may be focused by focusing means, shown here as achromatic lens
126, onto
image detection means 128, preferably in the form of a CCD camera or the like.
Lens
126 preferably includes a focusing stage 130 which is positionally adjusted by
screw
132. Image detection means 128 is generally interfaced to data processing
means (not
shown), wherein data sets from samples may be stored for computational
deconvolution.
For alignment purposes, the beam can be deflected by a removable mirror 134
into an
eyepiece 136 and/or other alignment aides (not shown) which may be mounted on
kinematic base plates (not shown) so they can be swapped and replaced with
precision.
The path length difference between paths 108, 114 can be fine tuned to within
the
coherence length of the observed and illuminating light with the "phasing"
translation
stage 138, to which mirrors 104, 106 are mounted by angle adjustment means,
such as
tiltable mirror mounts 140, 142, with actuating means such as screws 144.
Translating
stage 138 is positionally adjusted by screw 146, and will lengthen or shorten
path length
108 relative to path 114. Fine adjustment of the phase can be done by
precision motion
of stage 138 as well as off-line in software after the data are acquired. The
interference
pattern on image detection means 128 can be monitored using a pinhole-
apertured photo
diode (not shown) where eyepiece 136 is shown.
All optical surfaces used with the present invention, including beam
splitter/recombiner cube 116, should preferably be of high optical flatness,
preferably
X/20 or better, to preserve the relative phase of different rays. Since the Z-
direction
resolution is increased by the present invention, the sample has to be moved
with
increased precision relative to current state of the art microscopes. This is
ensured by
use of a piezoelectric actuator 148 on the sample translating stage 98, which
is
responsive to feedback control from capacitive sensor 150 which is responsive
to
feedback control from capacitive sensor 150 which
CA 02210801 1997-08-12
PCT/u596/Oi32~
~OR e JA1~
26
measures the actual sample position. Similar position sensors and actuators
may also
be employed to sense and correct the position of second objective lens 72
and/or of
phase adjusting stage 138.
Since fluorescence emission typically occurs over a fairly wide range of
wavelengths (-50 nm), and restriction of the bandwidth with narrow filters is
undesirable as light would be discarded unnecessarily, care should be taken to
ensure
that the equality of the two optical path lengths holds true (within
tolerances) for all
wavelengths in this band. A potential problem is the dispersion (dependence of
refractive index on wavelength) of optical materials. Thus, when the IzM
i o embodiment is used for fluorescence microscopy, one should assure that all
components through which the light is transmitted (i.e. the lenses, the
dichroic
mirrors, and the two halves of the beam splitter cube) are of identical
optical
thickness in the two beams or paths 108, 114, to within sufficiently tight
tolerances.
An alternative approach to dispersion problems is to include, if necessary,
compensating plates 152, 154, which can be tilted to change their effective
thickness,
or one of which consists of two thinner plates separated by index matching
fluid, so
that its total thickness can be adjusted, or one of which consists of two
wedges that
can be moved past each other so as to form a single plate of variable
thickness. The
same potential dispersion problem applies to wavelength differences within the
illuminating light in the I3M and ISM embodiments, and to the wavelength
difference
between the illuminating light and the observed light in the ISM embodiment
when
used for fluorescence. Chromatic phase compensation means such as compensating
plates 152, 154 may be used to address this problem in all three embodiments.
Referring now to FIG. 27 an alternative apparatus 156 consistent with the IZM
embodiment of the present invention is generally shown, wherein like reference
numerals denote like parts. The apparatus 156 is slightly more compact, which
is
achieved simply by replacing mirrors 104, 110 as shown in FIG. 26 with
dichroic
mirrors 158, 160, so that illuminating light can aimed directly at sample 96
through
dichroic mirror 158, which transmits the illumination or excitation light but
reflects
the emission light, instead of vice versa. Apparatus 156 is more compact, but
~ 8?W
CA 02210801 1997-08-12
~UM o s JAN 1997
26A
somewhat less symmetric: since the two dichroic mirrors 158, 160 are used at
different angles, they can no longer both be identical and at the same time
have
identical phase and spectral effects on their respective beams.
The 12 M embodiment described above in FIG. 26 and FIG. 27 can be turned
into an I3M system simply by exchanging the positions of the illuminating
AIIENKD ~KEO
W0 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
27
light from fibre optic 84 with that of image detection means 128 and focusing
lens
126. This is readily apparent by referring to FIG. 28, wherein like reference
numerals denote like parts. Thus, FIG. 28 shows an apparatus 162 wherein
illuminating or excitation light from optical fiber 84 is split by beam
splitter/recombiner 116 and directed along paths 108, 114 to first and second
objective lenses 70, 72 respectively, which then focus the illuminating light
onto
a plane of sample 96. As related above, the I3M embodiment records observed
light from only one objective lens. This is carried out by placing beam
splitter
158, which may be dichroic, between image detection means 128 and first
objective lens 70, so that emitted or observed light is selectively
transmitted
through dichroic mirror 158 to image detection means 128. Otherwise, the
apparatus 162 is generally the same as the apparatus shown in FIG. 26 and FIG.
27 for the IZM embodiment of the present invention, and is operated in
generally
the same manner, as will be more clearly described below.
Referring now to FIG. 29, an apparatus 164 consistent with the ISM
embodiment of the present invention is generally shown. As related above, the
ISM embodiment is a combination of the IZM and I3M embodiments, and thus both
records observed or emitted light from both objective lenses 70, 72 as in the
IZM
embodiment and illuminates the sample from both objective lenses 70, 72 as in
the
I3M embodiment. This is readily apparent by comparing FIG. 26 through FIG. 29.
In the apparatus 164 shown in FIG. 9, illuminating or excitation light from
optical
fiber 84 is split by beam splitter/recombiner 116 and directed along paths
108, 114
to first and second objective lenses 70, 72 respectively, where the
illuminating light
is focused on a section of sample. Observed or emitted light collected by
first and
second objective lenses 70, 72 is directed back along paths 108, 114
respectively
and combined by beam splitter/recombiner 116 and focused onto image detection
means 128. Thus, once the general apparatus for the present invention is
aligned
for operation of the IZM embodiment, it is then automatically aligned for I5M
embodiment as well, except for one detail: the relative phase of the two
illumination beams is the opposite of the ideal one, so that the illumination
intensity gets a minimum instead of a maximum at the focal plane. It is
possible
CA 02210801 1997-08-12
PCTIUs9~f 12
28
to use the apparatus 164 in this state, but it decreases the signal-to-noise
ratio. This
problem is caused by the phase shift upon reflection in the beam splitter,
which is an
unavoidable result of energy conservation. There are, however, several ways
around
the problem. For example, a separate beam-splitting loop for the illumination
light
can be employed as shown generally in FIG. 5. Such a configuration would allow
the phase of the illumination light to be adjusted independently of the phase
of the
observed light, using a second, independent optical path length adjusting
means.
Alternatively, one can make the illumination light incident on the beam
splitter cube
from the same side from which the emission light is detected, as shown
generally in
FIG. 6. This approach also requires an additional beam splitter/recombiner,
which
may be dichroic. Yet another approach is to exploit the wavelength difference
between the illuminating or excitation light and the observed or emitted
light, to
create a compensating phase difference between them by slightly offsetting the
chromatic phase compensation plates 152, 154. Such an offset however, is made
at
the expense of getting some phase variation within the excitation and emission
bands
themselves. The apparatus used in ISM embodiment of the present invention may
allow illumination light to be introduced through either side of the beam
splitter cube
116, so as to be able to acquire data at both phase conditions.
As related above, the phases of the two beam paths 108, 114 for each
embodiment shown in FIG. 26 through FIG. 29 must generally be adjusted to be
equal, which may be carried out using phase adjusting stage 138. Phase
measurements to determine the amount of adjustment required are easily done
using
test samples such as fluorescent microbeads. A more practical method for
commercial application of the present invention, however, would involve dual
detection, wherein both of the two beams of emission light that emerge from
the
beam splitter are detected and recorded, as in generally shown in FIG 30 and
FIG. 31.
In FIG. 30, image detection means 166 records light from one side of beam
splitter/recombiner 168, which is directed to image detection means 166 along
path
170. Light from the other side of beam splitter/recombiner 168 along path 172
is
3o reflected off dichroic mirror 174 to image detection means 176. In FIG. 31,
the same
"om
CA 02210801 1997-08-12
PGTIU~ ~ 6/ 01 3 2 4
28A
general effect is obtained by directing the light from beam
splitter/recombiner 168
along path 170 to one portion of image detection means 178 via mirror 180, and
directing light from beam splitter/recombiner along path 172 to another
portion
WEWO
CA 02210801 2006-07-10
-29-
of image detection means 178 via dichroic mirror 174 and truncated mirror 182.
Lenses
184, 186, 188 focus light on paths 170, 172 to separate portions of the
detector on image
detection means 178. Dual detection results in the positive side effect of
using the
emitted light even more efficiently. The two beams exiting the beam
splitter/recombiner
168 represent different combinations of the two incoming beams, differing by a
phase
shift of 180 degrees. By detecting both beams, either on separate cameras 166,
176, as
shown in FIG. 30 or on different parts of the same camera 178, as shown in
FIG. 31, and
comparing the two data sets in Fourier space, one can deduce the phase angles
of both
the emission and the illumination light paths, and thereby adjust the phase
adjusting
stage 190 and the chromatic phase compensator plates (not shown) if such are
used. This
could easily be done automatically.
One may want to acquire multiple data sets with different relative phase, of
the
imaging beams, illumination beams, or both. In particular, using the 13M or15M
embodiments of the present invention, one data set could be acquired with the
illumination phase adjusted so as to have constructive interference at the
focal plane, and
also a second data set with the opposite illumination phase, where the
illumination
intensity would then have a minimum at the focal plane. Using the difference
between
these two data sets, the interferometric information components could be
enhanced and
the background suppressed.
While the I3M and I5M embodiments of the present invention have been
described generally in the context of using Kohler illumination, several other
illumination arrangements are suitable for use with these embodiments. For
example,
critical illumination will give similar results to K6hler illumination, as
will any
intermediate arrangement.
The present invention generally requires that the first and second objective
lenses
are focused on the same point in the X, Y and Z directions. This can be done
by taking
two three-dimensional test data sets (which may be smaller than an actual data
set, for
increased speed), one data set using the first objective lens only, (e.g. by
closing a shutter
in the beam path from the second lens), and one data set with the second
objective lens
only (by similarly blocking the path from the first
WU 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCTIUS96/01324
lens during data recording). A simple cross-correlation procedure then would
determine the focus and lateral offset errors, which can be corrected by
moving
one of the objective lenses relative to the other lens. This procedure can be
done
automatically, and applies to the I2M and ISM embodiments, with or without
dual
5 detection.
Referring now to FIG. 32 and FIG. 33, commercial implementations of the
present invention as microscope 192 in FIG. 32 may look more like a typical
microscope depicted schematically in FIG. 33 than the previous schematics may
indicate. For example, as shown in FIG. 32, beam splitter/recombiner 196,
phase
10 adjusting stage 198, lens 200, filter 202, and mirrors 204 as well as other
components (not shown) can be incorporated into a structure 206 similar to
existing
commercial microscopes, with light source 208 and image detection means 210
suitably positioned for the embodiment or embodiments of invention to be used.
Referring now to FIG. 34, FIG. 35, and FIG. 36, flow charts relating the
15 method of employing each embodiment of the present invention are generally
shown. Referring first to FIG. 34, a flow diagram for the I2M embodiment of
the
present invention is generally shown. At step 212, a microscope sample is
positioned between first and second opposing objective lenses. As related
above,
the microscope sample is preferably mounted between glass cover slides. For
20 fluorescent microscopy, the sample will be suitably labeled by selected
fluorescent
probes.
At step 214, the two opposing objective lenses are focused upon a section
or plane within the sample. Focusing is preferably carried out by moving one
or
both objective lenses, or the sample, on precision translating means such as
25 translating stages.
At step 216, the light or image observed by first and second objective lenses
is directed along first and second paths to image detection means such as a
CCD
camera or the like for image recording, whereupon the observed light from the
two
paths is caused to coincide. This step is generally carried out by a plurality
of
30 mirrors that direct light along first and second paths to a beam
splitter/recombiner
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/US96/01324
31
which combines the light from the two paths and directs it to the image
detection
means.
At step 218, the optical path lengths of the first and second paths are
adjusted so that the two path lengths differ by less than the coherence
length, and
preferably much less than a wavelength, thereby causing the coinciding
observed
light on the image detection means in step 216 to interfere on the image
detection
means. Optical path length adjustment is generally carried out by a
translating
stage with mirrors mounted thereon.
At step 220, the interfering observed light or images in step 218 are
recorded by the image detection means. The image detection means is preferably
interfaced to data processing means such as a microprocessor, allowing the
recorded image to be stored.
At step 222, the first and second objective lenses are focused on another
section of the sample. This step is preferably carried out by translating the
sample
relative to the objective lenses, translating of the objective lenses, or
translation of
sample and lenses.
At step 224, steps 220 through 222, or optionally steps 218 through 222,
are repeated, until each section of the sample has been observed and recorded
as
related above. The recorded images from each section of sample form a data set
for the entire sample, which is stored by the microprocessor interfaced to the
image detection means.
At step 226 means for computational deconvolution are applied to the data
set of step 224, to produce a three dimensional image of the sample which has
enhanced Z direction resolution. The term "deconvolution" as used herein
should
be understood to mean any form of reconstruction method or algorithm. The
computational deconvolution will generally involve software which may employ a
plurality of Fourier transformation algorithms. The image data may also or
instead
be displayed after simpler processing and prior to a full computational
deconvolution, or after none at all. One reason to do this would be to display
the
data in real time. Even unprocessed, it will still confer more information
than in
conventional widefield microscopy.
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
32
An additional step (not shown), wherein the illuminating light is provided
to the sample through one of the objective lenses, may be included between
step
212 and 214. While Kohler illumination, as related above, is the preferred
illumination technique, other illumination methods generally used in the art
are also
contemplated.
Another additional step (not shown), wherein chromatic phase matching of
the observed light is carried out, may also be included prior to step 220.
Phase
matching is preferably carried out with phase compensator plates, one of which
comprises two wedges of which one can be translated past the other in order to
vary the optical thickness of the combination.
Yet another additional step (not shown) may be included prior to step 220
in which the observed light from first and second objective lenses is focused
onto
the image detection means. This focusing is preferably carried out by one or
more
translatable lenses.
Still another additional step (not shown) may be included between steps 212
and 214, wherein the sample is aligned between the first and second objective
lenses. The alignment is preferably carried out using an eyepiece which
observes
the sample by a removable mirror or mirrors.
Referring now to FIG. 35, a flow chart is shown which relates the general
steps comprising the method of the I3M embodiment of the present invention. At
step 228, a microscope sample is positioned between first and second opposing
objective lenses. As related above for the IZM embodiment, the microscope
sample
is preferably mounted between glass cover slides. Since the I3M embodiment is
primarily contemplated for use in fluorescent microscopy, the sample will
preferably be suitably labeled by selected fluorescent probes.
At step 230, excitation or other illumination light is directed through first
and second objective lenses onto a section of the microscope sample, and
focused
thereupon. This step is generally carried out by directing illuminating light
to a
beam splitter/recombiner which splits the light into first and second paths,
and then
directing the light along first and second paths by a plurality of mirrors to
first and
W 0 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
33
second objective lenses respectively. Generally, an extended, spatially
incoherent
light source is used to provide illuminating light.
At step 232, observed or emitted light from the first objective lens is
directed towards an image detection means such as a CCD camera or the like.
Generally, a dichroic mirror is used for this step, which selectively
transmits
observed light while reflecting illuminating light or vise versa.
At step 234, the illumination light directed to the sample is caused to
interfere within the section of sample. Causing the interference is generally
carried
out by adjusting the optical path lengths of first and/or second paths.
Generally,
optical path length adjustment is achieved by moving a translating stage which
includes mirrors mounted thereon.
At step 236, the observed or emitted light which was directed to the image
detection means is recorded. The image detection means is preferably
interfaced
to a microprocessor, as in the IZM embodiment of the invention, so that a
plurality
of images may be stored.
At step 238, illuminating or excitation light is directed onto another section
of sample and focused thereupon by first and second objective lenses.
At step 240, steps 236 through 238, or optionally steps 234 through 236,
are repeated until a data set comprising the recorded images of each section
of
sample has been obtained and stored.
At step 242, computational deconvolution means are applied to the data set
from step 240 to provide a three dimensional image of the sample with enhanced
Z direction resolution. As in the I2M embodiment, a phase matching step and
an alignment step, may be included, as well as a step in which observed light
is
focused onto the image detection means.
Referring now to FIG. 36, a flow chart is shown which relates the general
steps comprising the method of the I5M embodiment of the present invention. At
step 244, a microscope sample is positioned between first and second opposing
objective lenses. As related above for the I2M and I3M embodiments, the
microscope sample is preferably mounted between glass cover slides. As the ISM
W U 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/US96/01324
34
embodiment is primarily contemplated for fluorescent microscopy, the sample
will
preferably be suitably labeled by selected fluorescent probes.
At step 246, excitation or other illumination light is directed through first
and second objective lenses onto a section of the microscope sample, and
focused
thereupon. This step is generally carried out by directing illuminating light
to a
beam splitter/recombiner which splits the light into first and second paths,
and then
directing the light along first and second paths by a plurality of mirrors to
first and
second objective lenses respectively.
At step 248, the light observed or emitted by the sample is directed from
first and second objective lenses along the first and second paths to an image
detection means such as a CCD camera or the like, whereupon the observed light
from first and/or second paths is caused to coincide. The same mirrors and
beam
splitter/recombiner as was used in step 246 may be employed for directing
observed light from the sample to the image detection means. Alternatively,
separate beam splitters and additional mirrors may be used, as related above
in
FIG. 4 through FIG. 6.
At step 250, the illumination light directed to the sample is caused to
interfere within the section of sample. Causing the interference is generally
carried
out by adjusting the optical path lengths of first and second paths.
Generally,
optical path length adjustment is achieved by moving a translating stage which
includes a mirror or mirrors mounted thereon.
At step 252, the observed or emitted light which was directed to and
coincided upon the image detection means is recorded by the image detection
means. The image detection means is preferably interfaced to a microprocessor,
as in the other embodiments of the invention, so that a plurality of images
may be
stored.
At step 254, illuminating or excitation light is directed onto another section
of sample and focused thereupon by first and second objective lenses.
At step 256, steps 252 through 254, or optionally steps 250 to 254 are
repeated until a data set comprising the recorded images of each section of
sample
has been obtained and stored. One may want to acquire multiple data sets with
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCTIUS96/01324
different relative phase, of the imaging beains, illumination beams, or both.
In
particular, using the 13M or I5M embodiments of the present invention, one
data
set could be acquired with the illumination phase adjusted so as to have
constructive interference at the focal plane, and also a second data set with
the
5 opposite illumination phase, where the illuniination intensity would then
have a
minimum at the focal plane. Using the difference between these two data sets,
the
interferometric information components could be enhanced and the background
suppressed.
At step 258, computational deconvolution means are applied to the data set
10 from step 258 to provide a three dimensional image of the sample with
enhanced
Z direction resolution. As in the other embodiments, a phase matching step
and an alignment step, may be included, as well as a step in which observed
light
is focused onto the image detection means.
Comparison of FIG. 34 through FIG. 36 shows that the method comprising
15 the ISM embodiment involves a combination of steps from both the 12M and
13M
embodiments, which reflects the similarity of the apparatus used in the three
embodiments of the invention.
Computational algorithms applied at steps 226, 242 and 258 may include
the application of external constraints. Such constraints generally involve
spatial
20 confinement constraints in the deconvolution algorithm, and positivity of
the
emission intensity and of the fluorophore density when the invention is used
for
fluorescence microscopy.
The concepts related in the disclosure of the present invention may be used
in combination with existing microscopy techniques to extend the lateral or XY
25 resolution to a level which is greater than can presently be achieved. For
example,
the present invention may be used in combination with aspects of the existing
technology Standing Wave Fluorescence Microscopy, hereinafter referred to as
"SWFM", by using an "aperture synthesis" approach to SWFM.
In SWFM, two coherent beams of light are used to illuminate a sample as
30 related above. In Fourier space, the amplitude of these two beams is
nonzero only
at two points as shown in FIG. 37. The autocorrelation or intensity of the two
CA 02210801 2006-07-10
-36-
points shown in FIG. 37 is related by FIG. 38 wherein there are three points
which may
lie anywhere within the outlined regions of FIG. 38.
SWFM generally involves a standing wave aligned in the Z-direction. The
present invention as described above already incorporates all of the Z-
direction
resolution that can in principle be achieved with SWFM. However, it is
possible to
achieve increased lateral or XY resolution using a form of SWFM wherein the
direction
of the standing wave is not parallel to the Z-direction. For a certain
standing wave
direction and wavelength having wave vector kst, wave' three image stacks at
different
phases of the standing wave are acquired. Alternatively, two image stacks at
different
phases and one reference stack without any standing wave may be used. The same
reference stack could then be used for different standing wave angles,
decreasing the
total number of stacks that have to be acquired. Each of these image stacks by
itself
contains no Fourier components outside of the region of support of the optical
transfer
function or "directly observable region", but the information therein pertains
to three
different regions of sample information: the directly observable region
itself, and two
copies of the directly observable region displaced therefrom by +kst. wave and
-kst. wave
respectively, as shown in FIG. 39 and FIG. 40. FIG. 39 shows generally the
directly
observable and displaced regions as observed through a conventional single
lens
microscope, while FIG. 40 shows the directly observable and displaced regions
as
observed through the dual opposing objective lens arrangement of the present
invention
in its 12 M embodiment. The black region in FIG. 39 and FIG 40 represents the
optical
transfer function for the directly observable region, while the regions offset
by +kst. wave
and -kst. wave are shown as shaded. From the combined data set, it is possible
to separate
out these three components of sample information, and computationally move
them to
their appropriate positions in Fourier space. Repetition of this operation for
different
wave vectors kst. wave allows successive filling in of different parts of
Fourier space, until
a large region is covered. The extent of the region of Fourier space that can
be accessed
in this fashion is determined by the convolution of the directly observable
region with
the set of possible wave vectors kst, wave that can be created.
WO 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 PCT/US96/01324
37
The set of wave vectors of the light that can be sent in through the objective
lens(es) is limited by the light wavelength and the acceptance angle
(numerical
aperture) of the objective lenses exactly the same way as outgoing emission
light
as shown above in FIG. 12 through FIG. 19. Thus, the set of possible standing
wave wavevectors for a single objective lens system is shown FIG. 7, and the
set
of possible standing wave vectors for a dual objective lens system is shown by
FIG. 8, wherein X is in this context understood to denote xexcitaion. The set
of
standing wave vectors for a dual objective lens system that lacks the ability
to send
both laser beams through the same lens is described by the side lobes of FIG.
8,
where X is X ezcitation=
The procedure related above can be carried out either with conventional
single-lens detection, in which case the phrase "directly observable region"
in the
previous paragraph refers to the region shown generally in FIG. 7, or with the
dual-lens detection of the IZM embodiment of the present invention, for which
case
the same phrase refers to the region shown generally in FIG. 8. The
corresponding regions of Fourier space that can be accessed with the above
standing wave aperture synthesis procedure are shown graphically by the
unfilled
outlines in FIG. 39 and FIG. 40 for single and dual lens configurations
respectively. Note in FIG. 39 that there is a disk-shaped region near the kYkZ
plane, represented graphically by the hatched area along the kY axis, that
cannot be
accessed by the above standing wave aperture synthesis procedure in its
conventional form unless the microscope has the ability to send both laser
beams
through the same objective lens. As seen in FIG. 40, the present invention
accesses this region without requiring such an ability.
There are two further advantages to using the combination with the 12M
embodiment of the invention. First, fewer image stacks (fewer different
standing
wave vectors kt. Weoe) are required to achieve a reasonable coverage of the
accessible regions of Fourier space. Second, the Z-resolution is increased, as
can
seen by the black region in FIG. 40 being longer in the kZ direction than the
corresponding region in FIG. 39. In fact, the accessible region in Fig. 40 is
W O 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/US96/01324
38
almost the entire sphere of radius 1/Aexcitation + 1/xemissioni ~'~ch
represents all the
spatial information that can be accessed by any farfield optical means.
An even larger transfer function, similar to the one for ISM embodiment of
the present invention, can be had in the above standing wave/aperture
synthesis
procedure, so that even fewer image stacks are needed for full coverage. This
technique, which will hereinafter be referred to as four-beam standing wave
microscopy, involves substitution of two mutually coherent point light
sources, in
a plane conjugate to the back focal plane of the objective, for the extended
light
source in a setup otherwise identical to that used in the ISM embodiment as
shown
in FIG. 4 through FIG. 6 and FIG. 29. These mutually coherent point sources
could be, for example, focused laser beams or single mode optical fiber
outputs,
which in both cases may be supplied by a single laser. Because of the presence
of a beam splitter, the resulting illumination at the sample consists of four
mutually
coherent plane waves. These will interfere to form an intensity field with
structure
in both the Z and the lateral (XY) directions. As described above regarding
the
I3M and ISM embodiments, the Z structure, because it is fixed in the sample
reference frame, will simply give rise to an extension of the optical transfer
function in the Z direction. The lateral structure, as related below, consists
entirely of a sinusoidal modulation of the light intensity, so that the above
aperture
synthesis procedure can generally be directly applied.
FIG. 41 shows graphically the light amplitude distribution in Fourier space
for the four beam standing wave microscopy technique. The two point sources
give rise to one plane wave each entering through the "left" objective lens,
shown
as the two right side dots in FIG. 41. Because of the beam splitter, the two
point
sources also provide mirror image beams entering through the right objective
lens
shown as the left side dots in FIG. 41. FIG. 42 shows the resulting intensity
field
from the autocorrelation of FIG. 41. This intensity field contains 13 points
which
correspond to all 12 possible difference vectors between the four points shown
in
FIG. 41, plus a point at the origin, which can be considered as the difference
vector of any point with itself. The intensity field shown in FIG. 42 may
appear
complicated, but note that all 13 points lie either on the kZ axis or are
W O 96/24082 CA 0 2 210 8 01 19 9 7- 0 7-18 pCT/US96/01324
39
symmetrically placed on two lines parallel to it. This means that the light
intensity
is the sum of one component which is uniform in the XY plane (but which has
structure in the Z direction), and one component which is sinusoidally
modulated
in a lateral direction (and which has a different Z structure). Because of
this fact,
it is still possible to proceed with the information separation and aperture
synthesis
procedure as related above, with only slight modification due to the fact that
the
optical transfer functions for the modulated and the central information
components
are no longer identical.
There exists a special case where even the minor modification due to non-
identical optical transfer functions is unnecessary, which occurs if the two
point
sources are arranged perfectly symmetrically (placed diametrically opposite
each
other in the lateral plane). In that case, some of the points in FIG. 42
coincide,
resulting in three identical rows as shown by FIG. 43 and FIG. 44, which give
rise
to identical "central" and "modulation" transfer functions.
The effective optical transfer functions for the four beam standing wave
microscopy technique depend on the particular positions of the two light
sources
in the back focal plane. FIG. 45 through FIG. 47 and FIG. 48 through FIG. 50
describe two examples. Shown generally in FIG. 45 is the region of support for
the illumination amplitude for two mutually coherent light sources which are
located at diametrically opposite points on the edge of the aperture. FIG. 46
shows
the region of support for the illumination intensity corresponding to FIG. 45,
and
FIG. 47 shows resulting optical transfer function. The central transfer
function is
shown in black, while the additional regions of sample information which can
be
obtained through modulation are shown as shaded regions. FIG. 48 shows the
region of support for the illumination amplitude for two mutually coherent
light
sources wherein one light source is located at the center of the aperture and
one
light source is placed at the edge of the aperture. FIG. 49 shows the region
of
support for illumination intensity corresponding to FIG. 45, and FIG. 50 shows
the
resulting optical transfer function. The central transfer function is shown in
black,
while the shaded portions depict the regions of sample information available
through modulation. Clearly, only very few data stacks of the types
CA 02210801 2006-07-10
-40-
corresponding to FIG. 47 and/or FIG. 50 would be required to cover most of the
accessible region of Fourier space.
In all the aperture synthesis techniques related above, one may need to
determine
the absolute phases of the various standing waves, which are likely to be
unknown. They
can easily be deduced by successively comparing the different information
components
in the areas where they overlap, as shown generally in FIG. 40, starting from
the zero-
phase "central" component.
As an alternative to the four-beam standing wave microscopy technique, one
could use masks in the excitation light path, in planes conjugate to the image
plane, to
create lateral structure in the sample illumination. For example, in the
embodiments
depicted in FIG. 26 through FIG. 29, lateral structure could be introduced
into the
illumination light by placing a mask in the position generally occupied by
field stop 87.
Using masks to provide the lateral structure could allow aperture synthesis
methods
similar to the ones described above to be used without the need for a coherent
light
sources, which are limited by high price and limited choice of wavelengths
available.
Those skilled in the art will appreciate that, while the abovementioned
methods of
improving lateral resolution by introducing lateral structure into the
illumination light
and applying aperture-synthesis computer processing to the resulting image
data have
been described here in the context of dual-objective-lens microscopes, these
methods
apply to conventional single-objective-lens microscopes as well.
Any combination of the 12M, I3M, and ISM embodiments of the present invention,
as well as the methods for lateral resolution enhancement related herein can
be used
sequentially on the same sample. The resulting information may then be
combined by
computer into a single reconstruction. For example, one may well want to
combine data
from microscopy using the I5M embodiment with data from the standing wave
applications of the present invention described above.
Accordingly, it will be seen that the present invention provides a method and
apparatus for three dimensional optical microscopy which has greater depth or
Z direction resolution than has previously been attained for widefield
microscopy.
Although the description above contains many specificities, these should not
be
construed as limiting, but as merely providing illustrations of some of the
presently
preferred embodiments of this invention. Thus, the scope of the invention
should be
determined by the appended claims and their legal equivalents.