Note: Descriptions are shown in the official language in which they were submitted.
CA 02211114 2001-08-21
Description
METHOD FOR TREATING PRODUCE AND PROCESS WATER
Technical Field
This invention relates to a method for treating
contaminants in process water and treating produce to remove
debris, organic chemicals, and hard water deposits from the
produce and inhibit the formation of mold on the produce, and
more particularly, to a method of treating contaminants in
process water and cleaning, sanitizing, and descaling produce
by the use of an effective amount of a chlorine dioxide
solution.
Background Information
After harvest, fresh produce is washed before being sorted
and packed. Once clean produce has been sorted and packed,
fungus adhering to the surface of the produce may cause mold
to develop before the produce has reached the market. The
presence of fungus on even one piece of produce in a crate can
cause decay of substantially all of the produce in the crate.
For these reasons, it is desirable to reduce the occurrence of
decay in packed produce so that the overall value of the
harvested produce is increased.
If produce is treated by process water, the used process
water contains contaminants and debris. Conventionally,
process water for treating produce has been used for a short
time before being dumped due to the buildup of contaminants in
the process water.
Disclosure of the Invention
The present invention provides a method for treating
process water and an object submerged in process water when the
process water and the object include at least one contaminant
from the group of debris, soil, fungus, and organic chemicals.
The method comprises immersing the object in the process water.
Then, a chlorine dioxide solution is generated. An effective
amount of the chlorine dioxide solution is admixed with the
1
CA 02211114 1997-07-22
WO 96/25049 PCT/1JS95/02128
process water. The chlorine dioxide solution is present in the
process water in an amount sufficient to treat contaminants on
the object and in the process water. The oxidation reduction
potential of the process water is monitored. When the
oxidation reduction potential of the process water falls below
a predetermined level, the steps of generating, admixing, and
monitoring are repeated until substantially all of the
contaminants in the process water and on the objects have been
treated.
In a preferred form of the invention, the method includes
generating the chlorine dioxide solution by the reaction of a
solution comprising sodium chlorite with a solution comprising
phosphoric acid. In another form of the invention, the
chlorine dioxide solution may be generated by the reaction of
a solution comprising sodium chlorite and sodium chloride with
a solution comprising phosphoric acid. Alternatively, the
chlorine dioxide solution may be generated by the reaction of
a solution comprising sodium chlorite with a solution
comprising phosphoric acid and sodium 2-ethylhexyl sulfate.
In yet another form of the invention, the chlorine dioxide
solution may be generated by the reaction of a solution
comprising sodium chlorite and sodium chloride with a solution
comprising phosphoric acid and sodium 2-ethylhexyl sulfate.
The method may further include monitoring the pH of the
process water admixed with the chlorine dioxide solution.
Additionally, the method may include maintaining of the pH of
the process water admixed with the chlorine dioxide solution
below about 11. In a more preferred form of the invention, the
method includes maintaining the pH of the process water admixed
with the chlorine dioxide solution between about 2 and about
10.5.
The present invention also provides a method for treating
fresh produce to remove debris and inhibit the growth of
fungus. The method comprises submerging the produce in process
water. The process water comprises an effective amount of a
chlorine dioxide solution. The chlorine dioxide solution is
present in the process water in an amount sufficient to clean
2
CA 02211114 1997-07-22
WO 96125049 PC'T/US95/02128
substantially all debris from the surface of the produce and
to inhibit the growth of fungus on the produce. In a preferred
form of the invention, the effective amount of chlorine dioxide
solution in the process water is at least about 0.1 ppm. In
an even more preferred form of the invention, the effective
amount of chlorine dioxide solution in the process water is
between about 0.1 ppm and about 10 ppm. In an even more
preferred form of the invention, the effective amount of
chlorine dioxide solution in the process water is between about
0.5 ppm and about 1 ppm.
The method of the present invention may also include
providing process water comprising chlorine dioxide solution
that has a pH of less than 11. In a more preferred form of the
invention, the process water comprising the chlorine dioxide
solution has a pH of between about 2 and about 10.5. In an
even more preferred form of the invention, the pH of the
process water comprising the chlorine dioxide solution is
between about 3 and 10. It is also preferred that the produce
is submerged in the process water comprising the chlorine
dioxide solution for at least about thirty seconds.
The method may further comprise generating the chlorine
dioxide solution in the process water by the reaction of a
solution comprising sodium chlorite with a solution comprising
phosphoric acid. Alternatively, the chlorine dioxide solution
in the process water may be generated by the reaction of a
solution comprising sodium chlorite and sodium chloride with
a solution comprising phosphoric acid. In yet another form of
the invention, the chlorine dioxide solution in the process
water may be generated by the reaction of a solution comprising
sodium chlorite and sodium chloride with a solution comprising
sodium 2-ethylhexyl sulfate and phosphoric acid.
Additionally, the method may include passing the produce
under a flow of a second chlorine dioxide solution to wash
debris from the produce. The second chlorine dioxide solution
may be generated by the reaction of a solution comprising
sodium chlorite with a solution comprising phosphoric acid,
sodium 2-ethylhexyl sulfate, and either dodecylbenzenesulfonic
3
CA 02211114 1997-07-22
WO 96/25049 PCT/US95/02128
acid or sodium dodecylbenzene sulfonate. Alternatively, the
second chlorine dioxide solution may be generated by the
reaction of a solution comprising sodium chlorite and sodium
chloride with a solution comprising phosphoric acid, sodium
2-ethylhexyl sulfate, and either dodecylbenzenesulfonic acid
or sodium dodecylbenzene s,ulfonate.
Additionally, the present invention provides a method for
treating process water. The method comprises providing process
water having at least one contaminant from the following group:
debris, soil, fungus, and organic chemicals. An effective
amount of a chlorine dioxide solution is admixed with said
process water to treat the process water by oxidizing
contaminants in the process water. The method includes
providing a monitor for sensing the oxidation reduction
potential of the process water, positioning in the process
water the monitor for sensing the oxidation reduction potential
residual of the process water, and monitoring the oxidation
reduction potential residual of the process water. Additional
amounts of the chlorine dioxide solution are generated when the
oxidation reduction potential residual drops below a
predetermined level and the additional amounts of the chlorine
dioxide solution are admixed with said process water to
continue to treat contaminants in the process water.
The method may further include generating the chlorine
dioxide solution by the reaction of solution comprising sodium
chlorite with a solution comprising phosphoric acid.
Alternatively, the method may include generating the chlorine
dioxide solution by the reaction of solution comprising sodium
chlorite and sodium chloride with a solution comprising
phosphoric acid or phosphoric acid and sodium 2-ethylhexyl
sulfate. In another form of the invention, the method includes
generating the chlorine dioxide solution by the reaction of a
solution comprising sodium chlorite and sodium chloride with
a solution comprising phosphoric acid and sodium 2-ethylhexyl
sulfate.
The method may include monitoring the pH of the process
water admixed with the chlorine dioxide solution and
4
CA 02211114 2001-08-21
maintaining the pH of the process water admixed with the
chlorine dioxide solution below about 11. In a more preferred
form of the invention, the pH is maintained between about 2 and
about 10.5.
These and other advantages and features will become
apparent from the detailed description of the best mode for
carrying out the invention that follows.
Brief Description of the Drawings
In the drawings, like element designations refer to like
parts throughout the several views, and:
Fig. 1 is a schematic view of a produce cleaning assembly
line used in the method of the present invention;
Fig. 2 is a fragmentary schematic view of the chlorine
dioxide generator and controller shown in Fig. 1; and
Fig. 3 is a fragmentary schematic view of another chlorine
dioxide generator and controller shown in Fig. 1.
Best Mode for Carrying out the Invention
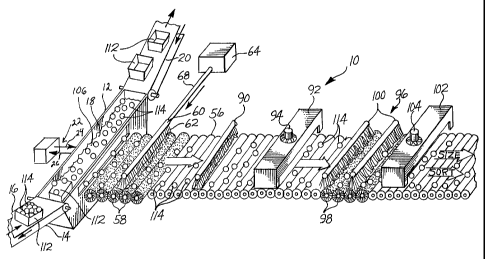
Referring to Figs. 1 and 2, a produce washing assembly line
10 is shown. The assembly line 10 includes a dump tank 12 and
a bin conveyor 14 which has an entrance side 16, a tank portion
18, and an exit side 20. A process water control loop 22
extends off of the dump tank 12. The process water control
loop 22 has an inlet 24 from the dump tank 12 into the loop 22
and an outlet 26 from the loop 22 into the dump tank 12. A
filter 28 is positioned on the inlet 24 of the control loop 22.
Process water from the dump tank 12 is pumped through the loop
22 so that the composition of the process water can be
monitored and maintained.
A process water controller 30 and a chlorine dioxide
reactor 32 are attached to the control loop 22. The process
water controller 30 includes an Oxidation Reduction Potential
(ORP) probe 34 which senses the ORP residual in the process
water passing through the control loop 22. The controller 30
also includes a flow indicator 36 which indicates to the
controller 30 that process water is flowing through the loop
5
CA 02211114 1997-07-22
WO 96/25049 PCT/US95/02128
22. When the flow indicator 36 indicates that process water
is flowing through the loop 22, the controller 30 enables the
ORP probe 34 to measure the ORP residual in the process water.
If the ORP probe 34 indicates that the ORP residual in the
process water is too low, the controller 30 activates the
chlorine dioxide reactor 32 to generate chlorine dioxide
solution.
A pH probe 38 monitors the pH of the process water.
Storage tanks 40, 42 of pH adjusting agents are attached to
pumps 44, 46 which are controlled by controller 30. Storage
tank 40 stores a pH reducing agent. Storage tank 42 stores a
pH boosting agent. The controller 30 is operable for feeding
pH adjusting agents into the process water.
The controller 30 controls a first pump 48 and a second
pump 50 which are attached to a first storage tank 52 and a
second storage tank 54, respectively. The pumps 48, 50 are
operable for pumping solutions from the storage tanks 52, 54
to the reactor 32. The controller 30 activates pumping of
proportional amounts of solutions from each of the tanks 52,
54.
An apple conveyor 56 extends into the dump tank 12. The
apple conveyor 56 is operable for moving apples out of the dump
tank 12 and through a series of stations before the apples are
sorted, sized and packed. The first station along the apple
conveyor 56 includes a plurality of rotating brushes 58 and a
spray bar 60. The spray bar 60 is operable for emitting a flow
of a second chlorine dioxide solution 62 onto apples. The
chlorine dioxide solution 62 is monitored and produced by a
chlorine dioxide generation system 64, shown in more detail in
Fig. 3.
The chlorine dioxide generation system 64 includes an inlet
66 which is operable for feeding potable water through conduit
68 past flow meter 70. The flow meter 70 is operable for
transmitting flow rate information to controller 72.
Controller 72 is operable for signalling a first pump 74 to
pump a solution from a first storage tank 76 and a second pump
78 to pump a solution from a second storage tank 80. The first
6
CA 02211114 2001-08-21
and second pumps 74, 78 are operable for pumping solutions to
reactor 82 through feed lines 84 and 86. The chlorine dioxide
solution from the reactor 82 are fed through line 88 and into
conduit 68 which feeds into the spray bar 60.
Further down the apple conveyor 56 from the spray bar 60
is a rinsing sprayer 90. The rinsing sprayer 90 is operable
for rinsing apples with potable water. The next station along
the apple conveyor 56 is a drying station 92. The drying
station 92 includes a fan 94 for blowing ambient temperature
air over apples on the conveyor 56. Following the drying
station 92 is a waxing station 96 which includes a plurality
of rotating brushes 98 and overhead spraying mechanisms 100
which are operable for spraying wax on the surface of apples.
After the waxing station 96 is a final drying station 102 which
includes a fan 104 which is operable for blowing heated air on
apples on the conveyor 56.
In a preferred form of the invention, the produce washing
assembly line 10 is operated in the following manner. The dump
tank 12 is filled with approximately 3,000 gallons of process
water 106. The process water 106 is monitored by pumping the
process water 106 through the control loop 22. As process
water 106 enters the inlet 24 of the control loop 22, the
process water 106 passes through the filter.28. The filter 28
separates particulate matter, such as leaves, twigs, and other
orchard debris, from the process water 106. In a preferred
form of the invention, the control loop 22 includes a
differential switch (not shown) installed on the filter 28 for
measuring the accumulation of particulate matter on the filter
28. When a predetermined level of particulate matter has
accumulated on the filter 28, the differential switch activates
an automatic backwash (not shown) which washes the particulate
matter out of the system through conduit 108.
The process water 106 is admixed with between about 0.1 and
about 10 ppm chlorine dioxide solution from the reactor 32.
In a more preferred form of the invention, the process water
106 is admixed with between about 0.5 and about 1.0 ppm
chlorine dioxide solution. In an even more preferred form of
7
CA 02211114 1997-07-22
WO 96/25049 PCT/US95/02128
the invention, the process water 106 is admixed with about 1.0
ppm chlorine dioxide solution.
Preferably, the process water 106 admixed with the chlorine
dioxide solution has a pH of less than 11, and in a more
preferred form of the invention, a pH of between about 2 and
about 10.5, and more commonly, between about 3 and about 10.
If the apples being cleaned in the assembly line 10 are heavily
scaled, i.e. covered in hard water deposits, the pH of the
process water 106 admixed with the chlorine dioxide solution
is maintained well below 7, and preferably at about 3. If the
apples are not scaled, but instead are covered mainly in
debris, such as orchard soil, the pH of the process water 106
admixed with the chlorine dioxide solution is maintained at
between about 7 and about 10. Accordingly, the preferred pH
of the process water 106 admixed with the chlorine dioxide
solution depends on the type of debris present on the apples
114 and in the process water 106.
The chlorine dioxide solution is generated by reacting one
part of a First Solution fed by the first pump 48 from tank 52
with between one and five parts of a Second Solution fed by the
second pump 50 from tank 54. In a preferred form of the
invention, one part of the First Solution is reacted with two
parts of the Second Solution. The First Solution comprises an
active ingredient of sodium chlorite. In a more preferred form
of the invention, the First Solution comprises 9.4% of 80%
technical sodium chlorite and the balance water. In another
form of the invention, the First Solution comprises 9.4% of 80%
technical sodium chlorite, 15% sodium chloride and the balance
water.
The Second Solution comprises phosphoric acid as an active
ingredient. It is preferable to include an anionic surfactant
and coupling agent, such as sodium 2-ethylhexyl sulfate in the
Second Solution. In a more preferred form of the invention,
the Second Solution comprises 1% sodium 2-ethylhexyl sulfate,
7.5% phosphoric acid and the balance water.
The First Solution and the Second Solution are fed into the
reactor 32 by pumps 48, 50. In the reactor 32, the First
8
CA 02211114 1997-07-22
WO 96125049 PCT/[TS95/02128
Solution and Second Solution are allowed a contact time of at
least about 15 minutes. When the controller 30 determines that
more chlorine dioxide solution is needed in the process water
106 due to the reading of the ORP probe 34, the controller 30
activates the pumps 48, 50 to feed more of the First and Second
Solution to the reactor 32. As the First and Second Solutions
enter the reactor 32, the chlorine dioxide solution in the
reactor 32 is displaced from the reactor 32 into conduit 110
which empties into the loop 22 and into the dump tank 12.
Substantial foaming of the chlorine dioxide solution is
undesirable since foam could be detrimental to the pumps 48,
50 on the control loop 22. Accordingly, it is preferred that
the First Solution and the Second Solution include surfactants
which produce little to no foam.
The controller 30 monitors and controls the composition of
the process water 106 in the following manner. The desired ORP
level is set on the controller 30. The ORP level is indicative
of a particular chlorine dioxide residual in the process water
106. The pH of the process water 106 is monitored by the pH
probe 38.
As process water 106 flows through loop 22, the pH probe
38 monitors the pH of the process water 106. If the pH in the
process water 106 is too high, the second pump 50 is activated
to pump a higher proportion of the Second Solution from storage
tank 54 to the reactor 32. Generally, the second pump 50 is
manually calibrated to pump a higher proportion of the Second
Solution for pH control, although it is foreseeable that this
calibration of the second pump 50 could be automated. The
amount of the Second Solution can be increased until the 5
parts of the Second Solution are being fed for each 1 part of
the First Solution. Generally, it is undesirable to feed the
Second Solution at a rate higher than 5 parts for each 1 part
of First Solution since proper reactions may not take place in
the reactor 32.
If a lower pH is desired, an effective amount of a pH
reducing agent may be added to the process water 106. The
controller 30 activates pump 44 to pump pH reducing agent from
9
CA 02211114 1997-07-22
WO 96/25049 PCT/US95/02128
storage tank 40 to feed through conduit 110 and into the
process water 106. In a preferred form of the invention, the
pH reducing agent comprises phosphoric acid as an active agent.
Sodium 2-ethylhexyl sulfate may be present in the pH reducing
agent to provide an increase in surface activity. In a more
preferred form of the invention, the pH reducing agent
comprises 26.25% phosphoric acid, 1.4% sodium 2-ethylhexyl
sulfate and the balance water.
If the pH of the process water 106 is too low, the
controller 30 activates pump 46 to pump pH boosting agent from
storage tank 42 into feed to the process water 106.
Preferably, the pH boosting agent comprises sodium hydroxide.
A chelant, such as sodium gluconate, may be part of the
boosting agent to aid in cleaning mineral type debris. In a
more preferred form of the invention, the pH boosting agent
comprises 42.5% sodium hydroxide, 17.5% sodium gluconate, and
the balance water.
Bins 112 of freshly-harvested apples 114 are placed on the
entrance side 16 of the bin conveyor 14. The apples 114 may
have contaminants such as debris, orchard soil, fungus, organic
chemicals such as agricultural chemicals, and hard water scale
on their surfaces. As the bins 112 move into the tank portion
18 of the conveyor 14, the bins 112 become submerged in process
water 106 in the dump tank 12. The apples 114 float out of the
bins 112 into the process water 106 in the dump tank 12. While
the apples 114 are in the dump tank 12, the apples 114 are
cleaned, descaled, and sanitized by contact with the process
water 106 which has admixed with it the chlorine dioxide
solution. As the apples 114 float toward the surface of the
dump tank 12, a water current (not shown) pushes the apples 114
toward the apple conveyor 56. Preferably, the apples 114
remain in the dump tank 12 for at least 30 seconds before being
pushed onto the conveyor 56. An estimated 75-85% of the
cleaning, descaling, and sanitizing of the apples 114 is
accomplished by the process water 106 comprising the chlorine
dioxide solution in the dump tank 12.
CA 02211114 1997-07-22
WO 96125049 PCT/US95/02128
Along with the apples 114 being cleaned, descaled and
sanitized by the process water 106 in the dump tank 12, the
bins 112 are cleaned and sanitized. As the apples 114 float
to the surface of the dump tank 12, the bins 112 continue along
the bin conveyor 14 and out the exit side 20 of the conveyor
14 for re-use.
An additional benefit of the present invention is the
treatment of the process water 106. Just as the chlorine
dioxide solution treats the apples 114 and the bins 112,
organic chemicals and contaminants in the process water 106 are
treated by the chlorine dioxide solution. Without the use of
the chlorine dioxide solution in the process water in the dump
tank, the process water became brown, had an unpleasant odor,
and was full of sludge after apples had been washed in the
system for a few days. With the addition of the chlorine
dioxide solution to the process water 106, the process water
106 remains clear and odorless, even after weeks of treating
apples in the system.
As the apples 114 proceed down the apple conveyor 56, the
apples 114 roll on top of the rotating brushes 58 and pass
underneath the spray bar 60. The spray bar 60 emits a second
chlorine dioxide solution 62 onto the apples 114. The second
chlorine dioxide solution 62 is generated by the generation
system 64 illustrated in Fig. 3. The generation system 64 is
operated by feeding potable water through the inlet 66 of
conduit 68. The flow of potable water is monitored by flow
meter 70. The readings from flow meter 70 are transmitted to
controller 72. The controller 72 signals pumps 74, 78 to pump
solutions from storage tanks 76, 80. The solutions are fed to
the reactor 82 and then into the potable water in conduit 68
to create the chlorine dioxide solution 62. Storage tank 76
stores the First Solution, as described above.. Storage tank
80 stores a Third Solution.
The controller 72 activates pumps 74, 78 to pump First
Solution and Third Solution from tanks 76, 80, respectively,
through feed lines 84, 86 and into the reactor 82., As the
solutions enter the reactor 82, previously generated product
11
CA 02211114 1997-07-22
WO 96/25049 PCT/US95/02128
is expelled from the reactor 82 into line 88 and then into
conduit 68 where it mixes with the water to form the second
chlorine dioxide solution 62. Preferably the solutions are
allowed a contact time of at least about 15 minutes in the
reactor 82.
The Third Solution comprises an anionic surfactant, such
as dodecylbenzenesulfonic acid or sodium dodecylbenzene
sulfonate, and an anionic surfactant coupling agent, such as
sodium 2-ethylhexyl sulfate. In a preferred form of the
invention, the Third Solution can be in the form of 1.6% sodium
2-ethylhexyl sulfate, 4% dodecylbenzenesulfonic acid, 7.5%
phosphoric acid, and the balance water. In another form of the
invention, the Third Solution comprises 3% sodium 2-ethylhexyl
sulfate, 7.5% dodecylbenzenesulfonic acid, 15% phosphoric acid,
and the balance water. The sodium 2-ethylhexyl sulfate
provides stabilizing properties in the second chlorine dioxide
solution 62 so that the solution 62 will feed through the spray
bar 60 without substantial clogging.
The controller 72 controls flow rates of the First Solution
and Third Solution to the reactor 82 such that the second
chlorine dioxide solution 62 has a chlorine dioxide residual
of between about 0.1 ppm and about 10 ppm. It is preferred
that the second chlorine dioxide residual in the solution 62
is between about 1 and about 5 ppm. Although, the solution 62
is generally very effective at about 1.0 ppm.
In a preferred form of the invention, the chlorine dioxide
solution 62 is generated by reacting one part of the First
Solution, as described above, with between one and five parts
of the Third Solution. In an even more preferred form of the
invention, one part of the First Solution is reacted with 5
parts of the Third Solution.
The pH of the second chlorine dioxide solution 62 is
monitored. Preferably, the pH of the solution 62 is between
about 3 and about 7. In a more preferred form of the
invention, the pH of the solution 62 is maintained at about 3.
If the pH. of the solution 62 is too high, more of the Third
Solution is fed to the reactor 82. To adjust the pH, the feed
12
CA 02211114 2001-08-21
rate of the Third Solution can be increased to 5 parts for each
1 part of the First Solution. Generally, it is undesirable to
feed the Third Solution at rates higher than 5 parts for each
one part of the First Solution, since undesired reactions may
take place in the reactor 82.
The second chlorine dioxide solution 62 emitted by the
spray bar 60 completes cleaning, descaling, and sanitizing of
the apples 114. The dodecylbenzenesulfonic acid or sodium
dodecylbenzene sulfonate in the Third Solution which is fed to
the chlorine dioxide reactor 82 produces a slight bubbling of
the solution 62 from the spray bar 60 which is helpful for
indicating to an observer that a chlorine dioxide is present
in solution 62. As the apples 114 pass over the rotating
brushes 58, the apples 114 are tumbled by the brushes 58 so
that chlorine dioxide solution 62 is emitted from the spray bar
60 onto all surfaces of the apples 114. Further, the
frictional action of the brushes 58 on the apples 114 provides
a mechanical agitation of the apples 114 which gently wipes
debris and residue from the surface of the apples 114. After
passing over the apples 114, the second chlorine dioxide
solution 62 may pass to a drain (not shown) or be added to the
process water 106 in the dump tank 12.
As the apples 114 continue on the apple conveyor 56, the
apples 114 pass under the rinsing sprayer 90 which emits
potable water onto the apples 114 to remove residual amounts
of chlorine dioxide solution 62 from the surface of the apples
114. After the rinsing sprayer 90, the apples 114 enter drying
station 92 where a fan 94 blows ambient temperature air over
the surface of the apples 114. The apples 114 are dried
sufficiently in the drying station 92 so that wax will adhere
to the surfaces of the apples 114 during the next phase of
processing.
After the apples 114 have been dried, the apples 114
continue on the apple conveyor 56 to the waxing station 96.
As the apples 114 pass through the waxing station 96, the
apples 114 are tumbled on rotating brushes 98 as an overhead
spraying mechanism 100 sprays wax onto the apples 114. The
13
CA 02211114 1997-07-22
WO 96/25049 PCT/US95/02128
rotating brushes 98 spread the wax across the surface of the
apples 114. After the waxing station 96, the apples 114 are
moved into the final drying station 102 where heated air is
blown across the surface of the apples 114 by fan 104 to
completely dry the apples 114. After the apples 114 exit the
final drying station 102, the apples 114 proceed to sorting and
sizing stations (not shown) for final packing.
This invention provides a simple and effective method for
cleaning, sanitizing, and descaling produce after harvest. The
method of the present invention would be useful on many types
of produce, such as pears, peaches, plums, apricots, oranges,
grapefruit, lemons, limes, avocados, cantaloupe, honeydew,
watermelon, zucchini, squash, carrots, potatoes, and cucumbers.
In addition, the present invention provides a simple and
effective method for cleaning and sanitizing of contaminated
water or other contaminated objects submerged in water, such
as apple bins.
In addition, the present invention provides a safe and
effective method for the use of a chlorine dioxide solution by
allowing for on site generation. According to the present
invention, small amounts of chlorine dioxide solutions,
including solutions with up to 50% active chlorine dioxide, can
be generated on site as needed. Accordingly, the present
invention provides environmental and safety benefits by
eliminating the need for the shipment, storage, and handling
of hazardous chlorine dioxide solutions.
The nature and substance of the instant invention as well
as its objects and advantages will be more clearly understood
by referring to the following specific examples.
Example I
Apples were rolled in moist orchard soil until they were
covered with soil. Then, the apples were allowed to dry. Once
dry, the apples were allowed to soak in a 5 ppm solution of
chlorine dioxide produced from a reaction of one part of First
Solution with one part of Second Solution. No direct brush
action was applied. After one minute, an apple was removed
14
CA 02211114 1997-07-22
WO 96125049 PCTlUS95/02128
from the chlorine dioxide solution. Based upon visual
inspection of the apple, approximately 95%- of the soil had been
removed from the surface of the apple. After 30 minutes,
another apple was removed from the chlorine dioxide solution.
Based upon visual inspection of the apple, approximately 980
of the soil had been removed from the surface of the apple.
r
Example 2
Three identical containers were obtained. Container #1 was
filled with a chlorine dioxide solution having a concentration
of chlorine dioxide at 5 ppm. The solution was obtained from
a reaction of one part of First Solution with one part of
Second Solution. Container #2 was filled with a chlorine
dioxide solution having a chlorine dioxide concentration of 11
ppm. The solution was obtained from a reaction of one part of
First Solution with one part of Second Solution. Container #3
was filled with deionized water. Mold was introduced into each
of the containers. A first sample was taken from each of the
three containers after five minutes. A second sample was taken
from each of the three containers after 50 minutes. All
samples were placed onto prepared mold growth media and allowed
to incubate for three days. At the end of three days, the mold
plates were observed. For the samples taken from the 5 ppm and
11 ppm chlorine dioxide solutions, the samples showed negative
for mold growth. For the samples taken from the solution which
contained no chlorine dioxide, the sample showed positive for
mold growth.
Example 3
Two identical one liter volumetric flasks were obtained.
Flask #1 was filled with deionized water and Flask #2 was
filled with an 8 ppm chlorine dioxide solution. The chlorine
dioxide solution was obtained from a reaction of one part of
First Solution with one part of Second Solution. Both flasks
were spiked with precisely the same amount of o-phenylphenol.
When tested, 7 ppm residual of o-phenylphenol was present in
Flask #1. Flask #2 showed a mild visual reaction upon addition
CA 02211114 2001-08-21
of the o-phenylphenol. When tested, 1 ppm residual of
o-phenylphenol was present in Flask #2.
Example 4
Two identical one liter volumetric flasks were obtained.
Flask #1 was filled with deionized water and Flask #2 was
filled with an 8 ppm chlorine dioxide solution. The chlorine
dioxide solution was obtained from a reaction of one part of
First Solution with one part of Second Solution. Both flasks
were spiked with precisely the same amount of
1-hydroxyethylidene-1, 1-diphosphonic acid (HEDPA). When
tested, 6 ppm residual of HEDPA was present in Flask #1. Flask
#2 showed a mild visual reaction upon addition of the HEDPA.
When tested, Flask #2 indicated that no HEDPA residual was
present.
Example 5
Two identical one liter volumetric flasks were obtained. Flask
#1 was filled with deionized water and Flask #2 was filled with
an 8 ppm chlorine dioxide solution. The chlorine dioxide
solution was obtained from a reaction of one part of First
Solution with one part of Second Solution. Both flasks were
spiked with precisely the same amount of chlorophenol red (an
organic dye). Flask #1 tested positive for approximately
8 ppm chlorophenol red. Residual was determined visually by
the presence of a reddish color. Flask #2 tested for virtually
no chlorophenol red as determined by Flask #2 being absolutely
colorless upon visual inspection.
While specific embodiments of the present invention have
been shown and described in detail to illustrate the
utilization of the inventive principles, it is to be understood
that such showing and description have been offered only by way
of example, and not by way of limitation. Protection by
Letters Patent of this invention in all its aspects are set
forth in the appended claims and is sought to the broadest
extent that the prior art allows.
16