Note: Descriptions are shown in the official language in which they were submitted.
CA 02211190 1997-07-23
W 096/23223 PCTrUS9Çl~
DISPOSABLE HEMOLYSIS DETECTOR
BACKGROUND OF THE lNV~NllON
The present invention relates to systems and methods
for detecting hemolysis in a patient's blood, and in
particular, to disposable systems that may be used to
detect hemolysis in non-laboratory environments. Even
more particularly, the present invention relates to
systems and methods that clinicians may use in a patient's
room for detecting hemolysis.
During the treatment of patients by extracorporeal
blood circuits during hemodialysis, hyperthermia, open-
heart surgery, immunosorbent therapy, extracorporealphotochemotherapy, and blood transfusions, there is a risk
that hemolysis, or the breaking of red blood cells, may
occur. Such breaking of red blood cells is deleterious
not only from the loss of function of those cells, but
also by the release into the blood plasma of hemoglobin
which is toxic.
At present, hemolysis is typically detected during
extracorporeal therapies by first taking a sample of a
patient's blood to a laboratory where the sample is placed
in a rotating centrifuge for separating the red blood
cells in the sample from the plasma, and '_hen comparing
the colors of the plasma before and after or during
treatment. Hemolysis testing systems which require the
use of a centrifuge and a laboratory are unsatisfactory
because it is possible that hemolysis may occur to a
significant degree during the time that the t~st i~self is
being performed.
CA 022lll90 l997-07-23
W O 96/23223 PCTrUS9C,'~31
In addition to a centrifuge, another known method for
separating out the plasma portion of a blood sample
involves the use of a microporous membrane device which
allows only non-cellular elements of a blood sample to
permeate a membrane. However, such microporous membrane
devices typically require a relatively high shear rate of
the blood sample at the surface of the membrane to prevent
the cellular elements of the blood sample from clogging or
plugging the membrane pores. Membrane plasma separators
therefore require complicated flow systems to maintain a
shear rate at the membrane surface that is both high
enough to promote a good plasma flux through the membrane
without any clogging of the membrane pores and also low
enough to prevent damage to blood cells that are separated
from the plasma by the membrane. As a result, such
systems are complex, expensive, and typically require a
large sample volume in order to detect hemolysis.
As described above, known membrane plasma separators
require high shear rates at the membrane surface to keep
blood cells away from the membrane surface and to prevent
such blood cells from clogging the membrane pores.
Without such high shear rates, the pores in these known
membrane plasma separators will immediately become clogged
by blood cells and only a very small amount of plasma will
be able to permeate through the membrane before it is
completely masked by blood cells. This small amount of
plasma is typically just enough to wet the membrane and is
insufficient for determining whether hemolysis has
occurred unless a massive hemolysis has occurred in the
sample.
Two examples of known microporo~s membrane systems
for separating the plasma portion of a blood sample from
CA 022lll90 l997-07-23
W O 96/23223 PCTrUS96/00631
its cellular elements are shown in U.S. Patent No.
~ 3,705,100 to Blatt et al. and U.S. Patent No. 4,191,182 to
Popovich et al. As described in the paragraph immediately
above, plasma separation is achieved in these systems by
creating a high shear rate at the membrane surface. In
addition, in order to further prevent clogging or plugging
of the membrane pores by the cellular elements of the
blood, these systems further include means for inducing a
transmembrane pressure across the membrane surface. In
order to generate the high shear rate and transmembrane
pressure reguired at the membrane surface, these prior art
systems incorporate special mechanisms for controlling the
blood flow velocity and pressure at the membrane surface.
It is an object of the present invention to provide
a system for detecting hemolysis in an extracorporeal
circuit which can be performed quickly by a clinician in
a patient or treatment room without the necessity of a
laboratory.
It is a further object of the present invention to
provide a system for separating a blood sample into its
plasma and cellular elements which is inexpensive, and
which does not require external instruments or mech~n;sms
for controlling the velocity and flow of blood at a
membrane surface.
It is a still further object of the present invention
to provide a system for detecting hemolysi~ which requires
only a small amount of a patient's plasma to permeate a
membrane in order to detect whether hemolysis has
occurred.
CA 02211190 1997-07-23
W 096/23223 PCTrUS~6~00~1
These and still other objects of the invention will
become apparent upon study of the accompanying drawings
and description of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a perspective view of a hemolysis detector
according to a preferred embodiment of the present
invention .
Fig. 2 is a perspective view showing the preferred
hemolysis detector of Fig. 1 in its inverted position.
Fig. 3 is a sectional view showing the preferred
hemolysis detector of Fig. 2.
Fig. 4 is a perspective view of a system for
collecting and channeling the plasma portion of a blood
sample in accordance with a preferred embodiment of the
present invention.
Fig. 5 is a top view of the system for collecting and
channeling the plasma portion of a blood sample shown in
Fig. 4.
Fig. 6 is a sectional view of the system for
collecting and channeling the plasma portion of a blood
sample shown in Fig. 5.
Fig. 7 is a diagram illustrating a preferrei method
for using the hemolysis detector of Fig. 1 to detect
hemolysis in a patient's blood sample.
CA 02211190 1997-07-23
W096/23223 PCT~S96/00631
Fig. 8 is a perspective view of a hemolysis detector
according to a further preferred embodiment of the present
invention.
Fig. 9 is a perspective view showing the preferred
hemolysis detector of Fig. 8 in its inverted position.
Fig. 10 is a sectional view showing the preferred
hemolysis detector of Fig. 8.
Fig. 11 is a diagram illustrating a preferred method
for using the hemolysis detector of Fig. 8 to detect
hemolysis in a patient's blood sample.
Fig. 12 is a perspective view of a hemolysis detector
according to a still further preferred embodiment of the
present invention.
Fig. 13 is a perspective view showing the preferred
hemolysis detector of Fig. 12 in its inverted position.
Fig. 14 is a sectional view showing the preferred
hemolysis detector of Fig. 12.
Z5 Fig. 15 is a diagram illustrating a preferred method
for using the hemolysis detector of Fig. 12 to detect
hemolysis in a patient's blood sample.
Fig. 16 is a top view showing a hemolysis detector
according to a still further preferred embodiment of the
present invention.
Fig. 17 is a sectional view of the hemolysis detector
of Fig. 16.
CA 02211190 1997-07-23
W 096/23223 PCTrUS9~'OQ~l
Fig. 18 is sectional view showing a hemolysis
detector according to a still further preferred embodiment
of the present invention.
Fig. 19 is sectional view showing a hemolysis
detector according to yet a still further preferred
embodiment of the present invention.
Fig. 20 is sectional view showing a hemolysis
detector according to yet a still further preferred
embodiment of the present invention.
SUMMARY OF THE lNv~:...lON
lS The present invention is directed to a method and
apparatus for detecting hemolysis from a sample of a
patient's blood. A sealed chamber having a fixed volume
is provided for receiving the sample of blood. The sealed
chamber has an internal pressure resulting from a presence
of a fixed quantity of air inside the chamber. A volume
of fluid that includes the sample of blood is received
into the sealed ch~rh~r. While the sample is being
received into the sealed chamber, the internal pressure of
the sealed chamber is raised to an increased internal
2S pressure by retaining the fixed quantity of air inside the
sealed chamber as the fluid is received into the chamber.
The increased internal pressure causes the plasma portion
of the blood sample in the chamber to permeate a membrane
that forms at least a portion of one side of the chamber.
A test volume of the plasma portion Gf the sample is
received by a hemolysis detection means after the test
volume of the plasma portion has permeated the membrane,
and a hemolysis condition is detected in accQrdance with
CA 02211190 1997-07-23
W096/23223 PCT~9G/OQ~1
=~ _
a hue associated with the test volume received into the
hemolysis detection means.
In accordance with a further aspect of the present
invention, a hemolysis detector for detecting hemolysis
from a sample of blood includes a sealable chamber having
means for receiving a volume of fluid that includes a
sample of blood into the chamber. Means for transforming
the sealable chamber into a sealed chamber having an
internal pressure resulting from a presence of a fixed
quantity of air inside the sealed chamber are also
provided. The means for receiving the volume of fluid
into the chamber includes means for raising the internal
pressure to an increased internal pressure as the fluid is
received into the chamber by retaining the fixed quantity
of air in the sealed chamber as the volume of fluid is
received into the chamber. The means for transforming the
sealable chamber into a sealed chamber is formed of a
membrane which is permeable to a plasma portion of the
sample in the sealed chamber when the internal pressure in
the chamber is equivalent to the increased internal
pressure. ~emolysis detection means positioned outside of
the chamber for receiving a test volume of the plasma
portion of the sample after it has permeated the membrane
is also provided. The hemolysis detection means detects
a hemolysis condition in accordance with a hue associated
with the test volume of the plasma portion of the sample.
In accordance with a still further aspect of the
present invention, a system for detecting a blood
constituent of interest from a sample of whole blood
comprises a membrane for separating a plasma portion of
the sample from a cellular portion of the sample. The
membrane has a first side for receiving the sample of
CA 02211190 1997-07-23
W096/23223 PCT~S9G~6~1
whole blood and a second side for passing only a plasma
portion of the whole blood sample. A blood barrier is
coupled to and positioned against the first side of the
membrane such that the blood barrier defines a perimeter
enclosing the first side of said membrane. An indicator
paper that is responsive to the blood constituent of
interest is also provided. The indicator paper is coupled
to the membrane and positioned against the second side of
the membrane. During operation of this aspect of the
invention, the sample of whole blood is placed inside the
perimeter and in contact with the first side of the
membrane. A determination of whether the constituent of
interest is present in the whole blood sample is then made
by observing the hue of the indicator paper.
In accordance with yet a further aspect of the
present invention, a system for detecting a blood
constituent of interest from a sample of whole blood
comprises a membrane for separating a plasma portion of
the sample from a cellular portion of the sample of blood.
The membrane has a first side for receiving the sample of
whole blood and a second side for passing only the plasma
portion of the sample of whole blood. A blood barrier is
coupled to and positioned against the first side of the
membrane such that the blood barrier defines a perimeter
enclosing the first side of the membrane. An indicator
paper is coupled to the membrane by a plasma channelling
means that is positioned against the second side of the
membrane. The indicator paper is responsive to the blood
constituent of interest. During operation of this aspect
of the present invention, the sample of whole blood is
placed inside the perimeter and in contac_ wi~h the first
side of the membrane. The plasma portion o~ the ~ample is
collected as it permeates the membrane and then channelled
CA 02211190 1997-07-23
W096/23223 PCT~S96/00631
with the channelling means to the indicator paper. A
~ determination of whether the constituent of interest is
present in the whole blood sample is then made by
observing the hue of the indicator paper.
DET~Tr~n DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now to Figs. 1-3, there are shown
perspective and sectional views of a hemolysis detector
lO0 according to a preferred embodiment of the present
invention. Hemolysis detector 100 is formed of a sealable
chamber 110 having a sample injection port 120 for
receiving a sample of blood into the sealed chamber.
Sealable ch~mh~r 110 is preferably formed of a clear rigid
plastic and has a fixed internal volume. Suitable
plastics for forming sealable chamber 110 include acrylic,
PVC, polycarbonate or polysulfone. Sample injection port
120 is preferably formed of latex rubber or other elastic
material. Sealable chamber 110 is mounted on a chamber
base 130 which is also preferably formed of a clear rigid
plastic. A microporous membrane disk 140 (not shown in
Fig. 1, but shown in Fig. 3) is provided at and forms the
bottom end of sealable chamber 110. Microporous membrane
disk 140 preferably has a pore size in the range of 0.2 to
1.2 microns, and still more preferably between 0.45 - 0.80
microns. In the preferred embodiment, membrane disk 140
is only permeable to the plasma portion of a whole blood
sample and non-permeable to the cellular portion of the
sample. A channelling means 150 is positioned immediately
below microporous membrane disk 140. ~he channelling
means 150 collects the plasma portion of a whole blood
sample as that plasma portion permeates through the
membrane disk 140 and then channels that plasma portion to
a clear capillary tube 160. Hemolysis de'ector 100 is
CA 02211190 1997-07-23
wo96n32~ PCT~S9~00~31
-- 10 --
preferably assembled by positioning the channelling means
150 within the chamber base 130, and then ultrasonically
welding sealable chamber llO to chamber base 130 with
membrane disk 140 located in-between.
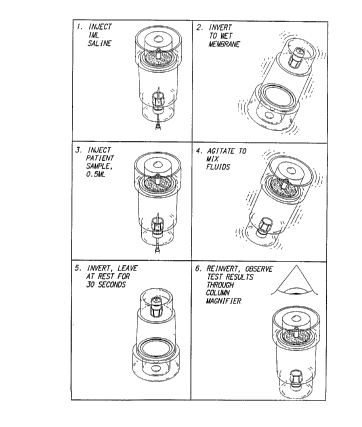
Referring now to Fig. 7, there is shown a diagram
illustrating a preferred method for using hemolysis
detector lOO to detect hemolysis in a patient's blood
sample. As shown in Fig. 7, the process begins in step 1
by injecting a wetting solution such as saline into
sealable chamber 110. The wetting solution is preferably
injected into sealable chamber llO with a syringe that has
been inserted through the sample injection port 120. In
step 2, hemolysis detector lOO is inverted thereby causing
the wetting solution injected during step 1 to wet the
membrane disk 140 positioned at the bottom of sealable
chamber 110. This wetting step causes the membrane disk
140 (which was previously dry and therefore permeable to
air) to become impervious to air, thereby transforming
sealable chamber llO into a sealed chamber having a fixed
volume of air inside. In step 3, a sample of a patient's
whole blood is injected into sealed ch~her llO through
sample injection port 120. Since sample injection port
120 is made of latex or rubber, no air escapes from sealed
chamber llO during injection of the whole blood sample
into the chamber.
In accordance with the preferred system for operating
hemolysis detector lOO, the volume of the whole blood
sample injected into sealed chamber 110 in step 3
preferably corresponds to 1-10% of the fixed internal
volume of sealed chamber 110. Since flu d is added to
sealed chamber 110 in step 3 but no air is allowed to
e~--c~p~ from the chamber, the internal pressure in sealable
CA 02211190 1997-07-23
W 096/23223 PCTrUSg~
chamber rises by approximately 8-8Omm Hg as a result of
the injection of the whole blood sample into the sealed
chamber during this step. In a still further preferred
embodiment, the volume of whole blood injected during step
3 is measured precisely such that the internal pressure in
sealable chamber 110 is raised by 28-30 mm Hg when the
whole blood sample is injected into the sealed chamber
110. The change in pressure that results from the
injection of a whole blood sample into the sealed chamber
110 can be easily determined by solving equation (1) below
for the quantitY P2:
Pl * V, = P2 * V2 ( 1 )
where, Vl represents the volume of air in sealed chamber
110 prior to the injection of the whole blood sample into
the chamber, Pl represents the pressure of the air in
sealed chamber 110 prior to the injection of the whole
blood sample into the chamber (this will typically be the
ambient air pressure), V2 represents V~ minus the volume of
the whole blood sample injected into sealed chamber llo
during step 3, and P2 represents the air pressure within
sealed chamber 110 after the whole blood sample has been
injected into the chamber.
In the preferred embodiment of detector 100, the
membrane disk 140 is itself used as the means for
transforming sealable chamber 110 into a sealed chamber
because the membrane disk 110 (which originally was in a
dry state and pervious to air) becomes imperViQus to air
when it is wetted. In alternate embodiments, other means,
such as a sealable valve or opening (not shown~ positioned
between the interior of chamber 110 and the cutside may be
used to transform chamber 110 into a sealod st~te.
CA 02211190 1997-07-23
W096l23223 PCT~S9G/0
- 12 -
Referring still to Fig. 7, in step 4 of the process,
hemolysis detector lOo is shaken to mix the whole blood
sample injected into the sealed chamber 110 during step 3
with any wetting solution remaining in the sealed chamber
110 from step 1. Next, in step 5, hemolysis detector 100
is inverted, and the plasma portion of the whole blood
sample previously injected into the chamber then permeates
through membrane disk 140. The increased internal
pressure generated by the injection of the whole blood
sample into sealed chamber 110 in step 3 functions during
step 5 as an urging force to push the plasma portion of
the whole blood sample through the membrane disk 140. In
the preferred embodiment, the increased internal pressure
generated by the injection of the whole blood sample into
sealed chamber 110 should be great enough to urge the
plasma portion of the blood sample through the membrane
disk 140, but not so great as to cause damage to the blood
sample.
In a preferred embodiment of hemolysis detector 100,
the surface area of membrane disk 140 may be on the order
of 3.14 square centimeters and, in step 5 of the process
of Fig. 7, 0.1 to 0.15 ml of plasma will permeate membrane
disk 140. Thus, in this preferred embodiment, the ratio
of plasma volume permeating the membrane to the membrane
surface area is 0.318 ml/sq. cm. of membrane surface area.
In alternate embodiments, the ratio of plasma volume to
membrane surface area may range from 0.1 to 1.0 ml/sq. cm.
of membrane surface area.
Although in the preferred embodiment, the sample
injection port 120 functions to raisG t.~e ir,ternal
pressure inside chamber 110 as the sample is njected into
the sealed chamber by retaining a fixed q~antity of air
CA 02211190 1997-07-23
W096/23223 PCT~S9~'00'~1
inside the chamber as the sample is received into the
chamber, in alternate embodiments other means, such as a
manual pressure pump (not shown) may also be used to
increase the internal pressure inside the sealed ch~h~r
110.
As the plasma portion of the whole blood sample
permeates membrane disk 140 in step 5, this plasma portion
is collected by channelling means 150 and then channeled
into clear capillary tube 160. In step 6, hemolysis
detector 100 is inverted again and the hue or tint of the
plasma in capillary tube 160 is observed either with the
naked eye or with a column magnifier (not shown). If the
hue of the plasma is amber, this indicates that the whole
blood sample was normal. Alternatively, if the hue of the
plasma is pink, this indicates that hemolysis has
occurred.
Although in the preferred embodiment of detector llO,
described immediately above, the clear capillary tube 160
may be used alone to detect whether hemolysis has occurred
in the sample simply by observing the hue or tint of
plasma in the tube, in alternate embodiments an indicator
paper such as guaiac paper (described below in conjunction
with detector 400) may be used to detect hemolysis from
the plasma after it has permeated through the membrane
disk 140.
Referring now to Figs. 4-6, there are shown
perspective and sectional views of channelling r.ec,~s 150
for collecting and channeling the plasma portion of a
blood sample in accordance with a preferred embod~m~nt of
the present invention. Channelling means 150 is formed of
a plurality of interconnected v-shaped channels 152. Each
CA 02211190 1997-07-23
W 096/23223 PCTrUS9fl0~31
- 14 -
adjacent pair of channels 152 is joined at a ridge 154.
Ridges 154 are positioned against membrane disk 140 when
hemolysis detector 100 is in its assembled state. A
collection channel 156 is coupled to each of the channels
152 and to clear capillary tube 160. During operation of
hemolysis detector 100 (and, in particular, during step 5
shown in Fig. 7), plasma permeating through membrane disk
140 flows first into channels 152 and then into collection
channel 156. Thereafter, the plasma in collection channel
156 flows by gravity into clear capillary tube 160.
Although in the preferred embodiment of channeling
means 150, channels 152 are v-shaped, in alternate
embodiments such channels may be u-shaped. In addition,
in alternate embodiments, channels 152 may be coupled to
capillary tube 160 through multiple collection channels.
Finally, in a still further alternate embodiment (not
shown), a channelling means 150 may be formed of a bowl-
Ch~r~ container with capillary tube 160 coupled to the
lower-most portion of the bowl, such that the bowl catches
plasma as it permeates through membrane disk 140 and then
channels that plasma by gravity to the capillary tube 160.
Referring now to Figs. 8-10, there are shown
perspective and sectional views of a hemolysis detector
200 according to a further preferred embodiment of the
present invention. Hemolysis detector 200 is
substantially equivalent to hemolysis detector 100,
except, as explained more fully below, hemol~sis detector
200 is shaped slightly differently and has its sample
injection port 220 located on the lengthwise portion of
sealable chamber 210. Thus, hemolysis detector 200 is
formed of a sealable chamber 210 having a sample injection
port 220 for receiving a sample of blood into the sealed
CA 02211190 1997-07-23
W O 96/23223 PCTAUS96100631
chamber. Like sealable chamber 110, sealable chamber 220
is preferably formed of a clear rigid plastic and has a
fixed internal volume. Sealable chamber 210 is mounted to
a chamber base 230 which is also preferably formed of a
5 clear rigid plastic. A microporous membrane disk 240 (not
shown in Fig. 8, but shown in Fig. 10) is provided at and
forms one end of sealable chamber 210. Microporous
membrane disk 240 is substantially equivalent to membrane
disk 140. A channelling means 250 is positioned
10 immediately adjacent to microporous membrane disk 240.
The channelling means 250 collects the plasma portion of
a whole blood sample as that plasma portion permeates
through the membrane disk 240 and then channels that
plasma portion to a clear capillary tube 260.
Referring now to Fig. 11, there is shown a diagram
illustrating a preferred method for using hemolysis
tletector 200 to detect hemolysis in a patient's blood
sample. As shown in Fig. 11, hemolysis detector 200 is
20 used in substantially the same manner as hemolysis
detector 100, except that in hemolysis detector 200 the
injection of the saline solution and whole blood sample in
steps 1 and 3 is accomplished using a sample injection
port positioned along the lengthwise portion of chamber
210.
Referring now to Figs. 12-14, there are shown
perspective and sectional views of a hemolysis detector
300 according to a still further preferred embodiment of
30 the present invention. Hemolysis detector 300 is formed
of a sealable chamber 310 having a sample injection port
320 for receiving a sample of blood ir.to the sealed
chamber. Sealable chamber 310 is preferakiy formed of a
clear rigid plastic and has a fixed internal volume. A
CA 02211190 1997-07-23
W096/23223 PCT~S96/00631
- 16 -
capillary tube cover 330 is secured to sealable chamber
310. Capillary tube cover 330 is also preferably formed
of a clear rigid plastic. A microporous membrane disk 340
(not shown in Figs. 12-13, but shown in Fig. 14) is
provided at and forms a boundary defining one wall of
sealable chamber 310. Thus, in contrast to the systems of
hemolysis detectors 100 and 200 which were formed of
sealable chambers that were cylindrical in shape,
hemolysis detector 300 is formed of a sealable chamber 310
that is essentially L-shaped. For purposes of clarity,
the internal portion of hemolysis detector 300 occupied by
sealable chamber 310 is indicated in Fig. 14 by parallel
line shading. As was the case in hemolysis detectors 100
and 200, the microporous membrane disk 340 in hemolysis
detector 300 preferably has a pore size in the range of
0.2 to 1.2 microns, and still more preferably between 0.45
- 0.80 microns, and is only permeable to the plasma
portion of a whole blood sample and non-permeable to the
cellular portion of the sample. A channelling means 350
is positioned immediately adjacent to microporous membrane
disk 340. The channelling means 350 collects the plasma
portion of a whole blood sample as that plasma portion
permeates through the membrane disk 340 and then channels
that plasma portion to a clear capillary tube 360.
Hemolysis detector 300 is preferably assembled by
positioning the channelling means 350 within the capillary
tube cover 330, and then ultrasonically welding sealable
chamber 310 to the cover 330 with membrane disk 340
located in-between.
Referring now to Fig. 15, there is shown a diagram
illustrating a preferred method for using hemolysis
detector 300 to detect hemolysis in a p~~ient's blood
sample. As shown in Fig. 15, the process beains in step
CA 02211190 1997-07-23
W 096/23223 PCT~US96/OOC~l
1 by injecting a wetting solution such as saline into
sealable chamber 310 through the sample injection port
320. In step 2, hemolysis detector 300 is tipped sideways
and agitated, thereby causing the wetting solution
injected during step 1 to wet the membrane disk 340. This
wetting step causes the membrane disk 340 (which was
previously dry and therefore permeable to air) to become
impervious to air, thereby transforming sealable chamber
310 into a sealed chamber having a fixed volume of air
inside. In step 3, a sample of a patient's whole blood is
injected into sealed chamber 310 through sample injection
port 320. Since sample injection port 320 is made of
latex or rubber, no air escapes from sealed chamber 310
during injection of the whole blood sample into the
chamber. The volume of the whole blood sample injected
into sealed chamber 310 in step 3 preferably corresponds
to 1-10% of the fixed internal volume of sealed chamber
310. As ~;sc~lcced above in connection with hemolysis
detector 100, the addition of this fluid into the sealed
chamber causes its internal pressure to rise by
a~oximately 8-80mm Hg, and preferably by 28-30 mm Hg.
Referring still to Fig. 15, in step 4 of the process,
hemolysis detector 300 is shaken to mix the whole blood
sample injected into the sealed chamber 310 during step 3
with any wetting solution remaining in the sealed ch~h~r
310 from step 1. Next, in step 5, hemolysis detector 300
is inverted, and the plasma portion of the whole blood
sample previously injected into the chamker tllen permeates
through membrane disk 340. The increased internal
pressure generated by the injection of the whole blood
sample into sealed chamber 310 in step 3 ~nd the àownward
pressure created by the weight of the whole blocd sample
itself together function during step 5 as an ur~ing force
CA 02211190 1997-07-23
W096/23223 PCT~S,~'C~31
- 18 -
to push the plasma portion of the whole blood sample in an
upward direction through the membrane disk 340. As the
plasma portion of the whole blood sample permeates
membrane disk 340 in step 5, this plasma portion is
collected by channelling means 350 and then channeled in
an upward direction into clear capillary tube 360. In
step 6, the hue or tint of the plasma in capillary tube
360 is observed either with the naked eye or with a column
magnifier (not shown). If the hue of the plasma is amber,
this indicates that the whole blood sample was normal.
Alternatively, if the hue of the plasma is pink, this
indicates that hemolysis has occurred.
Referring now to Fig. 17, there is shown a sectional
view of a hemolysis detector 400 according to a still
further preferred embodiment of the present invention.
Hemolysis detector 400 is formed of a microporous membrane
disk 410 for separating the plasma portion of a sample of
whole blood from the cellular portion of the whole blood
sample. Membrane disk 410 has a first side 420 for
receiving the sample of whole blood to be separated. In
the embodiment shown in Fig. 17, the whole blood sample is
preferably received onto membrane disk 410 by placing one
or more drops of the whole blood sample onto the first
side 420 of membrane disk 410. Microporous membrane disk
410 preferably has a pore size in the range of 0.2 to 1.2
microns, and still more preferably between 0.45 - 0.80
microns. Since membrane disk 410 is only permeable to the
plasma portion of a whole blood sample and non-permeable
to the cellular portion of the sample, cnly plasr.la from
the whole blood sample placed on first side 420 will pass
through to the second side 430 of membrane disk 410.
-
CA 02211190 1997-07-23
W O 96/23223 PCTrUS9~/00~1
Membrane disk 410 is preferably formed from of a
hydrophilic membrane such as Thermopor 800, Verapor 800,
or Supor 800 made by Gelman Science, Inc. It will be
- understood by those skilled in the art that other
microporous membranes, including hydrophobic membranes
that require a pre-wetting step, could be used to form
membrane disk 410. In addition, in the preferred
embodiment membrane disk 410 is 5 - lo mm in diameter,
although disks having larger or smaller diameters could
also be used. It will also be understood by those skilled
in the art that membranes formed in shapes other than
disks could also be used in place of membrane disk 410.
A blood barrier 440 is coupled to and positioned
lS against the first side 420 of membrane disk 410. Blood
barrier 440 is preferably circular in shape and defines a
perimeter 450 enclosing the first side 420 of membrane
disk 410. In the preferred embodiment, membrane disk 410
and blood barrier 440 are glued together, although these
elements could be secured to each other using other means
of attachment. Blood barrier 440 is preferably formed of
a molded plastic material.
Referring still to Figs. 16 and 17, hemolysis
detector 400 is further formed of an indicator paper disk
460 which is secured inside a plastic casing 470 by glue
480 or by other attachment means. Tndic~tor paper disk
460 is preferably positioned directly adjacent to and in
contact with the second side 430 cf membrane disk 410.
Indicator paper disk 460 is also preferably ccupled to
membrane disk 410 in a detachable manner so that indicator
disk 460 and membrane disk 410 can be easily separated.
Indicator paper disk ~60 is preferably formed of a porous
paper impregnated with guaiac resin such as Hemoccult
CA 02211190 1997-07-23
W O 96/23223 PCTAUS96/00631
- 20 -
paper manufactured by SmithKline Diagnostics, Inc. Such
paper displays a blue color in the presence of hemoglobin
when treated with a hydrogen peroxide solution. A
suitable hydrogen peroxide solution for use in conjunction
with this aspect of the present invention is the Hemoccult
Developer manufactured by SmithKline Diagnostics, Inc.
Indicator paper disk 460 preferably has a larger
diameter than membrane disk 410 and, in the embodiment
shown, is approximately 20mm in diameter. Since in the
preferred embodiment indicator paper disk 460 is larger
than membrane disk 410, a portion of indicator paper disk
460 will lie outside of perimeter 450. It will, however,
be understood by those skilled in the art that indicator
paper formed in shapes other than disks could also be used
in place of disk 460.
To test for the presence of free hemoglobin in a
sample of whole blood using hemolysis detector 400, one or
more drops of a whole blood sample is placed within
perimeter 450 on the first side 420 of membrane disk 410.
If membrane disk 410 is formed of a hydrophobic or
slightly hydrophilic paper, membrane disk 410 should be
~Le ueL~ed with a wetting solution such as an aqueous
solution of 5-20~ isopropyl alcohol or ethyl alcohol prior
to dropping the whole blood sample onto the membrane disk
410. Other organic solvents can also be used as a wetting
agent, so long as these solvents do not leave a residue in
membrane disk 410 that could interfere with the blood
test. The purpose of this pre-wetting step is to
hydrophilize the membrane disk. The pre-wetting step can
be accomplished by simply dropping one or more drops of
the wetting solution onto the membrane disk 410.
CA 02211190 1997-07-23
W 096/23223 PCTrUS9~10
- 21 -
After one or more drops of a whole blood sample is
placed within perimeter 450 on the first side 420 of
membrane disk 410, the plasma portion of the whole blood
- sample will wet the membrane disk 410 as well as the
indicator paper disk 460 lying beneath the membrane disk
410. If the wetting of the indicator paper disk 460
propagates beyond perimeter 450, a drop of developer
solution is applied to the wetted portion of the indicator
paper disk 460 lying outside of perimeter 450. If blue
color appears in the wetted area after approximately one
minute, this is an indication of free hemoglobin in the
plasma and hemolysis is therefore detected as being
present. Alternatively, if the wetting of the indicator
paper disk 460 does not propagate beyond perimeter 450,
membrane disk 410 should be removed from its position
above the indicator paper disk 460 and a drop of developer
solution should then be applied to the wetted portion of
the indicator paper disk 460 previously lying below
membrane disk 410. Again, if blue color appears in the
wetted area after approximately one minute, this is an
indication of free hemoglobin in the plasma and hemolysis
is therefore detected as being present.
Although in the embodiment of detector 400 described
immediately above, the blood constituent of interest is
free hemoglobin and an indicator paper impregnated with
guaiac resin is used to detect such hemoglobin, it will be
understood by those skilled in the art that detector 400
of the present invention could be applied to detect other
blood constituents in plasma by varying the type of
indicator paper used to form disk 460.
Referring now to Fig. 18, there is shown a sectional
view of a hemolysis detector 500 according to a still
CA 02211190 1997-07-23
W096/23223 PCT~S96100~1
further preferred embodiment of the present invention.
Hemolysis detector 500 is formed of chamber 510 which is
enclosed on all sides except its bottom-most side 520. At
the top of chamber 510, a sample injection port 530 is
provided for receiving a whole blood sample into chamber
510. A chamber base 540 is also provided. Both chamber
510 and chamber base 540 are preferably formed from molded
plastic. A microporous membrane disk 550 and an indicator
paper disk 560 are also provided. Membrane disk 550 is
preferably formed from of a hydrophilic membrane such as
Thermopor 800, Verapor 800, or Supor 800 made by Gelman
Science, Inc. It will be understood by those skilled in
the art that other microporous membranes, including
hydrophobic membranes can also be used. Similarly,
indicator paper disk 560 is preferably formed of a porous
paper impregnated with guaiac resin such as Hemoccult
paper manufactured by SmithKline Diagnostics, Inc. In
c:oslLrdst to the system of detector 400, membrane disk 550
and indicator paper disk 560 in detector 500 have
substantially the same diameter.
During assembly of detector 500, membrane disk 550
and indicator disk 560 are positioned between the top edge
570 of chamber base 540 and the bottom-most side 520 of
chamber 510, and the top edge 570 of chamber base 540 and
the bottom-most side 520 of chamber 510 are secured to
each other with both disks in between, preferably by
ultrasonic welding. Once detector 500 is assembled, the
preferred application of the device be~ins with the
injection of one or two milliliters of a wetting agent
(such as 15% isopropyl alcohol in water) into the chamber
510 through the sample injection port 530. The injected
wetting solution wets both the membranP disk 550 and the
indicator paper disk 560, and mos' of the injection
-
CA 02211190 1997-07-23
W 096/23223 PCTrUS~/C~
- 23
solution permeates out of the device within a minute.
Next, a predetermined volume of a whole blood sample
(preferably 1-10~ of the internal volume of chamber 510)
is injected into chamber 510 through sample injection port
530. After maintaining detector 500 in its upright
position with sample injection port 530 at the top for
approximately one minute, detector 500 is next inverted
180 degrees and one or two drops of developer solution are
applied to the indicator paper disk 560. The bottom of
chamber base 540 is preferably open or exposed to the
outside so as to allow the developer to be applied
directly onto the indicator paper. If blue color appears
in the area of the developer solution within approximately
30 seconds, this is an indication of free hemoglobin in
the plasma and hemolysis is therefore detected as being
present.
Although in the embodiment of detector 500 described
immediately above, the blood constituent of interest is
free hemoglobin and an indicator paper impregnated with
guaiac resin is used to detect such hemoglobin, it will be
understood by those skilled in the art that detector 500
of the present invention could be applied to detect other
blood constituents in plasma by varying the type of
indicator paper used to form disk 560.
Referring now to Fig. 19, there is shown a sectional
view of a hemolysis detector 600 according to yet a still
further preferred embodiment of the present invention.
Hemolysis detector 600 is formed of chamber 610 which is
enclosed on all sides except its bottom-most side 620. At
the top of ~A~h~r 610, a sample injection port 630 is
provided for receiving a whole blood sample into chamber
610. A chamber base 640 is also provided. Both chamber
CA 02211190 1997-07-23
W096/23223 PCT~S9~'C~31
- 24 -
610 and chamber base 640 are preferably formed from molded
plastic. A microporous membrane disk 650 is also
provided. Membrane disk 650 is the substantial equivalent
of membrane disk 550 described above.
During assembly of detector 600, membrane disk 650 is
positioned between the top edge 660 of chamber base 640
and the bottom-most side 620 of chamber 610, and the top
edge 660 of chamber base 640 and the bottom-most side 620
of chamber 610 are secured to each other with the membrane
disk in between, preferably by ultrasonic welding. Once
detector 600 is assembled, the preferred application of
the device begins with the injection of one or two
milliliters of a wetting agent (such as a normal saline or
15% isopropyl alcohol in water) into the chamber 610
through the sample injection port 630. The injected
wetting solution wets the membrane disk 650, and most of
the injection solution permeates out of the device within
a minute. Next, a predetermined volume of a whole blood
sample (preferably 1-10% of the internal volume of chamber
610) is injected into chamber 610 through sample injection
- port 630. After maintaining detector 600 in its upright
position with sample injection port 630 at the top for
approximately one minute, detector 600 is next inverted
180 degrees and a strip of indicator paper (such as guaiac
paper) is wetted by bringing the indicator paper (not
shown) into contact with side 670 of membrane disk 650.
The bottom of chamber base 640 is preferably open or
exposed to the outside so as to allow the indicator paper
to be brought into direct contact with the membrane disk.
One or two drops of developer solution are next applied to
the indicator paper strip. If blue color a~pears in the
area of the developer solution within apprcximately 30
seconds, this is an indication of free hemoglcbin in the
=
CA 02211190 1997-07-23
W 096/23223 PCT~US9G/O~f31
- 25 -
plasma and hemolysis is therefore detected as being
~ present.
Although in the embodiment of detector 600 described
immediately above, the blood constituent of interest is
free hemoglobin and an indicator paper impregnated with
guaiac resin is used to detect such hemoglobin, it will be
understood by those skilled in the art that detector 600
of the present invention could be applied to detect other
blood constituents in plasma by varying the type of
indicator paper used during operation of the system.
Referring now to Fig. 20, there is shown a sectional
view of a hemolysis detector 700 according to yet a still
further preferred embodiment of the present invention.
Hemolysis detector 700 is formed of chamber 710. At the
top of chamber 710, a sample injection port 720 is
provided for receiving a whole blood sample into chamber
710. A ch~hPr base 730 is also provided. Both chamber
710 and chamber base 730 are preferably formed from molded
plastic. A microporous membrane disk 7~0 is also provided
on the bottom-most side of chamber 710. Membrane disk 740
is the substantial equivalent of membrane disk 550
described above. A channellin~ means 750 is positioned
immediately below microporous membrane disk 740.
Channelling means 750 is substantially equivalent to
~h~nneling means 150, and thus collects the plasma portion
of a whole blood sample as that plasma portion permeates
through the membrane disk 740 and then channels that
plasma portion to a clear capillary tube 760. In contrast
to hemolysis detector loO wherein the bottom end of
capillary tube 160 is closed, in hemolysis detector 700
the bottom end 770 of capillary tube 760 ~ open.
CA 02211190 1997-07-23
W 096123223 PCTAUS9~0~1
- 26 -
During assembly of detector 700, membrane disk 740
and channelling means 750 are positioned between the
chamber base 730 and the chamber 710, and the chamber base
730 and the ch~rh~r 710 are secured to each other with the
membrane disk and channeling means in between, preferably
by ultrasonic welding. Once detector 700 is assembled,
the preferred application of the device begins with the
injection of one or two milliliters of a wetting agent
(such as a normal saline or 15% isopropyl alcohol in
water) into the chamber 710 through the sample injection
port 720. The injected wetting solution wets the membrane
disk 740, and most of the injection solution permeates out
of the device within a minute. Next, a predetermined
volume of a whole blood sample is injected into chamber
710 through sample injection port 720. After maintaining
detector 700 in its upright position with sample injection
port 720 at the top for approximately one minute, a strip
of indicator paper (such as guaiac paper) is wetted by
dropping plasma exiting from end 770 of tube 760 onto an
indicator paper strip such as guaiac paper (not shown).
One or two drops of developer solution are next applied to
the indicator paper strip. If blue color appears in the
area of the developer solution within approximately 30
seconds, this is an indication of free hemoglobin in the
plasma and hemolysis is therefore detected as being
present.
Although in the preferred embodiment of detector 700,
an indicator paper is applied to plasma exiting tube 760
to determine whether hemolysis is pre~ent, it w~ll be
understood by those skilled in the art that an optical
sensor system (formed of a light transmitting source 780
and a light sensor 790) may alternati~ely be used to
CA 02211190 1997-07-23
W096123223 PCT~S9G,~G31
analyze the plasma from tube 760 in order to determine
whether hemolysis has occurred.;. ~
The present invention may be embodied in other
specific forms without departing from the spirit or
essential attributes of the invention. Accordingly,
reference should be made to the appended claims, rather
than the foregoing specification, as indicating the scope
of the invention.