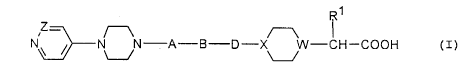Note: Descriptions are shown in the official language in which they were submitted.
CA 02212227 1997-08-0
BOEHRINGER MANNHEIM GMBH
4145/00/WO
New pyridyl- and pyridazinyl-piperazine derivatives,
processe~ for the production thereof and pharmaceutical
agents containing these compounds
It is known that compounds which carry a basic and an
acidic group are capable of inhibiting the aggregation
of blood platelets when the basic and acidic group in
the compounds are at a very specific distance from one
another (Drugs of the Future 19 (8), 757 (1994).
Compounds with an anti-aggregatory action on blood
platelets are described in the patent specifications
WO 93/14077, EP-A 0 537 980, EP-A 0 542 363, WO 94/22834
and WO 94/22835.
The present invention concerns new pyridyl- and
pyridazinyl-piperazine derivatives, processes for the
production thereof as well as pharmaceutical agents
containing these substances.
It was now found that pyridine and pyridazine
derivatives which additionally carry a carboxylic acid
group effectively inhibit the aggregation of blood
platelets and can thus be used to treat diseases that
are due to thrombo-embolic events such as stroke,
myocardial infarction or arterial occlusive diseases as
well as inflammations, osteoporosis or tumour diseases.
The present invention concerns compounds of the general
formula I
CA 02212227 1997-08-0~
N ~ N N - A - B --D X W - CH -COOH (I)
in which
Rl denotes hydrogen, lower alkyl, lower alkenyl,
cycloalkyl, cycloalkenyl, an optionally substituted
monocyclic or bicyclic aryl, an optionally
substituted hetaryl, an optionally substituted
arylalkyl or one of the groups
-oR2, -NR3R4
W denotes nitrogen or ,CR5,
X, Z independently of one another denote nitrogen or
~CH and in the case that W denotes a nitrogen
atom, X must be the ~CH group,
A denotes a valency dash or a carbonyl group,
B denotes a valency dash or a C1-C6 alkylene chain
optionally substituted once or several times by
lower alkyl or an oR2 group,
D denotes a valency dash and, in the case that X is a
nitrogen atom, can also be a carbonyl group in
which case A, B and D may not simultaneously denote
a valency dash,
R2 denotes hydrogen, lower alkyl or arylalkyl,
CA 02212227 1997-08-0
-- 3
R3,R4 independently of one another denote hydrogen or
lower alkyl or together with the nitrogen atom to
which they are bound form a five to six-membered
heterocyclic ring,
R5 denotes hydrogen or a group -oR2,
as well as pharmacologically acceptable salts thereof.
In all cases lower alkyl should represent a straight-
chained or branched Cl-C6 alkyl group such as e.g.
methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
pentyl or hexyl in particular methyl, ethyl, propyl,
isobutyl and pentyl.
Lower alkenyl denotes unsaturated residues with 3-6
carbon atoms such as allyl, but-2-enyl, hexa-2,4-dienyl
but above all allyl.
Cycloalkyl denotes an optionally substituted 3-7-
membered ring such as a cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl or cycloheptyl ring in
particular a cyclopropyl, cyclopentyl and cyclohexyl
ring. These cycloalkyl residues can be substituted once
or twice by a C1-C6 alkyl group preferably a methyl,
ethyl or isopropyl group as well as by hydroxy, methoxy,
benzyloxy, amino, methylamino, dimethylamino or
benzylamino groups or by chlorine or bromine.
Cycloalkenyl denotes an optionally substituted cyclo-
pentenyl, cyclohexenyl or cycloheptenyl ring. These
rings can be substituted once or twice by a Cl-C6 alkyl
group preferably a methyl, ethyl or isopropyl group as
CA 02212227 1997-08-0
-- 4
well as by chlorine, bromine or hydroxy, methoxy,
benzyloxy, amino, methylamino, dimethylamino or
benzylamino groups.
If the residues R3 and R4 form a heterocyclic ring
together with the nitrogen atom to which they are bound
then this is a saturated or unsaturated 5-6-membered
ring such as a pyrrolidine, piperidine, morpholine
pyrroline, piperidine or morpholine ring.
The carbocyclic and heterocyclic rings can be optionally
substituted once or twice by Cl-C6 alkyl groups,
preferably a methyl, ethyl or isopropyl groups as well
as by chlorine, bromine or hydroxy, methoxy, benzyloxy,
amino, methylamino, dimethylamino or benzylamino groups.
Aryl usually denotes a phenyl residue which is
optionally substituted once or several times. Hetaryl
usually denotes a pyridine, pyridazine, pyrrole,
thiophene, furan or imidazole ring which is substituted
once or several times.
Bicyclic aryl usually denotes an indane or naphthalene
residue which is optionally substituted once or several
times, preferably a naphthalene residue. Aryl, bicyclic
aryl and hetaryl residues can be optionally substituted
once or several times by Cl-C6 alkyl groups, preferably
a methyl, ethyl or isopropyl group as well as by
chlorine, bromine, fluorine or hydroxy, alkoxy such as
e.g. methoxy, benzyloxy, acetyloxy, carboxy, ethoxy-
carbonyl, aminocarbonyl, methylaminocarbonyl, dimethyl-
aminocarbonyl, cyano, amino, methylamino, dimethylamino,
benzylamino, acetylamino, benzoylamino and amidine
groups.
CA 02212227 1997-08-0
-- 5
Arylalkyl usually denotes an unsubstituted or once or
several-fold substituted benzyl, phenethyl, phenyl-
propyl, phenylbutyl or phenylpentyl residue, preferably
a benzyl, phenethyl or phenylpentyl residue. C1-C6 alkyl
residues, preferably a methyl, ethyl or isopropyl group
as well as chlorine, bromine, fluorine or hydroxy,
methoxy, benzyloxy, acetyloxy, carboxy, ethoxycarbonyl,
aminocarbonyl, methylaminocarbonyl, dimethylamino-
carbonyl, cyano, amino, methylamino, dimethylamino,
benzylamino, acetylamino, benzoylamino and amidino
groups come into consideration as substituents.
Compounds of the general formula I contain at least one
asymmetric carbon atom and therefore optically active
compounds of the general formula I are also a subject
matter of the present application. Furthermore
conformation isomers of compounds of the general formula
I which may occur are also a subject matter of the
present application.
Preferred compounds are compounds of formula I in which
the group A-B-D represents a group (CH2)1-3 or
CO-(CH2)1-3 and Z, X, W and Rl have the stated meaning.
Compounds of formula I are additionally preferred in
which the ring -X__~W represents a 1,4-cyclohexylidenyl
or a 1,4-piperidinyl ring and Z, A-B-D and R1 have the
meaning stated above.
Compounds of formula I are especially preferred in which
A-B-D represents ethylene or carbonylethylene group and
the ring -X W- represents a 1,4-cyclohexylidenyl ring
and Z and R1 have the stated meaning.
CA 02212227 1997-08-0
-- 6 --
Compounds of the general formula I are produced
according to well-known processes by hydrolyzing an
ester of the general formula II
R1
' ~ N~ N - A - B - D - X W - CH ~ (II)
in which R1, A, B, D, W, X, and Z have the meanings
stated above and R6 denotes a methyl, ethyl, tert.-butyl
or benzyl residue.
Compounds of the general formula II are new and are
prepared according to well-known processes and
preferably by the following:
a) in the case that W denotes nitrogen, a compound
of the general formula III
N ~ N N A B D - X N - H (III)
in which A, B, D and Z have the meanings stated
above is reacted with a compound of the general
formula IV
CA 02212227 1997-08-0~
R--CH2--4
0 ~6 (IV)
in which Rl and R6 have the meanings stated above
and L denotes a leaving group such as Hal or
o-So2_R7 in which Hal can be chloride, bromide or
iodide and R7 should be methyl, phenyl, p-methyl-
phenyl or p-nitrophenyl,
b) in the case that W denotes a group CR5 and R5
denotes a group oR2, a ketone of the general
formula V
,Z~ ~ ~
N ~ N\__/N A B - D X ~ ~ tV)
in which A, B, D, X and Z have the meanings stated
above is reacted with a carboxylic acid ester of
the general formula VI
R1_tH~ 6 (VI)
in which Rl and R6 have the meanings stated above
and the hydroxyl group of the 2-hydroxy ester which
CA 02212227 1997-08-0~
forms in this process of the general formula VII
N ~ \__/N A ~ - D - X ~ CH ~ (VII)
in which R1, R6, X, Z, A, B and D have the meanings
stated above is alkylated if desired with an
alkylating agent of the general formula VIII
R2 _ L (VIII)
in which R2 and L have the meanings stated above or
c) in the case that W denotes a CH group, a compound
of the general formula VI is alkylated with a
compound of the general formula IX
N~N~N A--B--D - X~L ~IX)
in which A, B, D, X, Z and L have the meanings
stated above or
d) the olefinic double bond of a compound of the
general formula X
CA 02212227 1997-08-0~
i
N ~ N A N - A B D ~ - ~ ~~ (X)
in which A, B, D, Rl, R6, X, and Z have the
meanings stated above is catalytically
- hydrogenated.
e) in the case that A and D each denote a carbonyl
group, a dicarboxylic acid derivative of the
general formula XI
Q ~ B ~ y (XI)
in which B has the meaning stated above and Y and Q
independently of each other denote hydrogen, the
group oR2 in which R2 has the meanings stated above
or denotes a halogen such as chlorine or bromine is
reacted successively with an amine of the general
formula XII
, ==~ A (XII)
N~N~ N H
in which Z has the meaning stated above and wi~th an
amine of the general formula XIII
CA 02212227 1997-08-0~
-- 10 --
R5 R1
H N~CH ~ 6 (XIII)
in which R1, R5 and R6 have the meanings stated
above.
Compounds of the general formula III are new and-are
produced according to well-known processes and
preferably by
reacting a compound of the general formula XII with a
compound of the general formula XIV
L A B ~ N P
(XIV)
in which A, B and L have the meanings stated above and P
denotes a protecting group for amines such as acetyl,
tert.-butyloxycarbonyl, benzyl or benzyloxycarbonyl and
subsequently the protecting group P is removed from the
product that is formed.
Compounds of the general formula IV are produced in such
a way that in the case that L = Hal, a compound of the
general formula VI is halogenated according to processes
known in the literature, or in the case that L in
formula IV denotes an o-so2-R7 group, the hydroxyl group
of a compound of the general formula XV
CA 02212227 1997-08-0~
¦H o
R1 CH ~ (xV)
O R6
in which R1 and R6 have the meanings stated above is
converted into the corresponding sulfonic acid ester.
Compounds of the general formula V are new and are
usually obtained by cleaving the ketal group of a
compound of the general formula XVI
N ~ N ~ N - A - B D X ~ 3 CH2)f
in which A, B, D, X, and Z have the meanings stated
above and f = 2,3.
Compounds of the general formula VIII can be obtained
commercially in the case that L = Hal; in the case that
L denotes an o-So2-R7 group, the hydroxyl group of
commercially available alcohols of the general formula
XVII
R2 OH (XVII)
in which R2 has the meanings stated above is converted
into the cDrresponding sulfonic acid ester.
CA 02212227 1997-08-0
- 12 -
Compounds of the general formula IX are new and if L
denotes an o-so2-R7 group they are produced by
converting the hydroxyl group of a compound of the
general formula XVIII,
N ~ N\__/N A B D x3 (XVIII)
in which A, B, D, X, and Z have the meanings stated
above, into the corresponding sulfonic acid ester; in
the case that L = Hal, the hydroxyl group of a compound
of the general formula XVIII is nucleophilically
substituted by halogen according to processes known from
the literature.
Compounds of the general formula X are new and are
produced in a well-known manner by subjecting a ketone
of the general formula V to a Wittig reaction with a
phosphorane of the general formula XIX,
O R6
in which R1 and R6 have the meanings stated above and Ar
denotes an aryl within the sense of the definition for
aryl given above, or the ketone of formula V is
subjecte~ to a Horner-Emmons reaction with a phosphono-
acetic acid ester of the generaI formula XX
CA 02212227 1997-08-0~
O
R6 o \ )~o ~6
RQo/ \\O
in which R1 and R6 have the meanings stated above.
Compounds of the general formula XI are commercially
available.
Compounds of the general formula XII are prepared by
reacting 1-benzylpiperazine with 4-chloropyridine or
4-chloropyrazine.
Compounds of the general formula XIII are produced in
such a way that
a) in the case that R5 denotes hydrogen, the double
bond of a compound of the general formula XXI
H N ~ I ~
~ : O _R6 (XXI)
in which Rl and R6 have the meanings stated above
is catalytically hydrogenated and
CA 02212227 1997-08-0
- 14 -
b) in the case that R5 denotes the group oR2, a
compound of the general formula VI is reacted with
4-piperidone and the hydroxyl group of the 2-
hydroxy ester of the general formula XXII that
forms
OH R1
H- N~L I H~O
0 ~6 (XXII)
in which R1 and R6 have the meanings stated above
is alkylated with an alkylation agent of the
general formula VIII.
Compounds of the general formula XIV are produced
according to well-known processes in such a way that
a) in the case that A denotes a valency dash, the
hydroxyl group of an alcohol of the general
formula XXIII
HO B~N P (XXIII)
in which B and P have the meanings stated above is
appropriately halogenated or sulfonated and
b) in the case that A denotes a carbonyl group, a
carboxylic acid of the general formula XXIV
HOOC B{~ (XXIV)
CA 02212227 1997-08-0~
in which B and P have the meanings stated above is
converted into the corresponding carboxylic acid
halogenide.
Compounds of the general formula XV can be obtained
according to literature methods by oxidizing the
corresponding compounds of the general formula VI.
Compounds of the general formula XVI are prepared in
such a way that a compound of the general formula XII is
reacted with a compound of the general formula XXV
L - A B -D - X ~ 3 CH2)f (XXV)
in which L, A, B, D, X and f have the meanings stated
above.
Alcohols of the general formula XVII are commercially
available.
Compounds of the general formula XVIII are prepared by
reducing the carbonyl group of a compound of the general
formula V.
Some of the compounds of the general formula XIX are
commercially available (Aldrich Chemie GmbH and Co. KG)
and are obtained in special cases according to known
processes by reacting a 2-halogen-carboxylic acid
derivative of the general formula IV with a
triarylphosphine of the general formula XXVI
CA 022l2227 l997-08-0
- 16 -
AR3P (XXVI)
in which Ar has the meanings stated above.
Some of the compounds of the general formula XX are
commercially available (Aldrich Chemie GmbH and Co. KG)
and in special cases are obtained according to known
processes by the Arbuzov reaction between a 2-halogen-
carboxylic acid derivative of formula IV and a
trialkylphosphite of the general formula XXVII
(oR6)3p (XXVII)
in which R6 has the meanings stated above.
Compounds of the general formula XXI are prepared in
such a way that a 4-piperidone of the general formula
XXVIII
O ~ N P (XXVIII)
in which P has the meanings stated above is reacted with
a compound of formula XIX or of formula XX and the
protecting group P is removed from the product that
forms.
Compounds of the general formula XXIII are obtained
by reducing the carboxyl group of a compound of
formula XXIV.
Some of the compounds of the general formula XXIV are
commercially available or are described in the
CA 02212227 1997-08-0~
literature (Ishihara, Chem. Pharm. Bull. 41, 529 (1993);
Merck and. Co. EP 478362).
Compounds of the general formula XXV are prepared
according to known processes in such a way that
a) in the case that X = CH and A denotes a valency
dash, the hydroxyl group of an alcohol of the
general formula XXIX
HO--B~< 3CH2)f
in which B and f have the meanings stated above is
converted into a halogen or a sulfonic acid ester,
b) in the case that X = CH and A denotes a carbonyl
group, a carboxylic acid of the general formula XXX
HOOC - B ~ CH2)f
in which B and f have the meanings stated above is
- converted into the corresponding carboxylic acid
halogenide and
c) in the case that X = N, a piperidone derivative of
the general formula XXXI
CA 02212227 1997-08-0~
-- 18 --
H - N ~ 3 CH2)f (XXXI)
in which f has the meanings stated above is reacted
with a compound of the general formula XXXII
L-A-B-D-L ( XXXI I )
in which L, A, B and D have the meanings stated
above.
Triarylphosphines of formula XXVI, trialkylphosphites of
formula XXVII and 4-piperidones of formula XXVIII are
commercially available.
Compounds of the general formula XXIX are obtained
by reducing the carbonyl group of a compound of
formula XXX.
Compounds of the general formula XXX are obtained by
hydrolyzing an ester of the general formula XXXIII
R _ o ~o 3 (XXXIII)
in which R6, B and f have the meanings stated above.
Compounds of the general formula XXXI are commercially
CA 02212227 1997-08-0~
-- 19 --
available.
Compounds of the general formula XXXII are commercially
available in the case that L = Hal; in the case that L
denotes a sulfonic acid residue, commercially available
1-omegadiols are sulfonated appropriately; in the case
that A or D denote a carbonyl group and L = Hal, omega-
halogen-carboxylic acids which are commercially
available are converted into the respective carboxylic
acid halogenide and in the case that A and D denote a
carbonyl group these are compounds of the general
formula XI.
Some of the compounds of the general formula XXXIII can
be obtained commercially and are in special cases
obtained by catalytic hydrogenation of the double bond
of a compound of the general formula XXXIV
O O
~ B ~ ' ~ CH2)f
R6 0 o (XXXIV)
in which B, R6 and f have the meanings stated above.
Compounds of the general formula XXXIV can be obtained
according to well-known processes by reacting a 1,4-
cyclohexanedione derivative of the general formula XXXV
- o~ 3 2f (XXXV)
CA 02212227 1997-08-0
- 20 -
in which f has the meanings stated above with a
phosphorane of the general formula XXXVI
(XXXVI)
B- PAr3
. RQo
in which R6, B and Ar have the meanings stated above.
Compounds of the general formula XXXVI are obtained in
such a way that a triarylphosphine of formula XXVI is
reacted with a commercially available omega-halogen-
carboxylic acid ester.
An ester of the general formula II or of formula XXXIII
is hydrolyzed to the corresponding carboxylic acid of
the general formula I or XXX according to the usual
methods by treating a carboxylic acid ester of the
general formula II in water or in a mixture of water,
tetrahydrofuran, dioxane, methanol or ethanol,
preferably in a mixture of water/tetrahydrofuran, with a
hydroxide such as sodium, potassium or lithium
hydroxide, preferably sodium or lithium hydroxide, or
with an acid such as hydrochloric acid, sulphuric acid
or trifluoroacetic acid, preferably trifluoroacetic
acid, and at temperatures between room temperature and
80~C, preferably at room temperature.
Reaction of a compound of the general formula III with a
compound of formula IV or a compound of formula VII with
a compound of formula VIII or a compound of formula VI
with a compound of formula IX or a compound of formula
XXII with a compound of formula VIII or a compound of
formula XII with a compound of formula XIV or a compound
of formula XII with a compound of formula XXV or a
CA 02212227 1997-08-0
- 21 -
compound of formula XXXI with a compound of formula
XXXII or reaction of 4-chloropyridine or 4-chloro-
pyridazine with 1-benzylpiperazine is usually carried
out in an aprotic solvent such as toluene,
tetrahydrofuran, diethyl ether or dimethylformamide,
preferably dimethylformamide or tetrahydrofuran using a
base such as potassium hydride, sodium hydride,
potassium carbonate or sodium bicarbonate, preferably
sodium hydride or potassium carbonate, and at
temperatures between room temperatures and 180~C,
preferably at 120~C.
A ketone of the general formula V is reacted with an
ester of the general formula VI, or 4-piperidone is
reacted with an ester of formula VI under the conditions
of an aldol reaction in a solvent such as methanol,
ethanol, toluene, tetrahydrofuran, diethyl ether or
dimethylformamide, preferably tetrahydrofuran or
dimethylformamide, using a base such as sodium or
potassium methylate or sodium or potassium ethylate,
sodium hydride, potassium hydride, lithium diisopropyl-
amide, potassium hexamethyl disilazide, preferably
sodium hydride or lithium diisopropylamide and at
temperatures between -78~C and 90~C, however, preferably
between -78~C and room temperature.
The catalytic hydrogenation of the olefinic double bond
of a compound of the general formula X or of formula XXI
or of formula XXXIV is carried out analogously to
processes known from the literature (A. Nose, Chem.
Pharm. Bull. 38, 2097 (1990); Tamura M. Bull. Chem. Soc.
Jpn. 53, 561 (1980); Liu H.-J., Synth. Commun. 15, 965
(1985); Chido N., J. Chem. Soc. Chem. Commun. 9g4
(1990); Buchi G., J. Amer. Chem. Soc. 89, 6745 (1967);
Ernst I., Coll. Czech, Chem. Comm. 24, 3341 (1959);
CA 022l2227 l997-08-0
- 22 -
Johnson W. S., J. Amer. Chem. Soc. 79, 1995 (1957);
Muchowski J. M., Can. J. Chem. 47, 857 (1969)).
The protecting group P can if necessary be removed from
compounds which carry the protecting group P and which
are described in or encompassed by this patent
specification by treating a compound carrying the
protecting group P with aqueous mineral acids or bases
such as hydrochloric acid, sulfuric acid or
trifluoroacetic acid or sodium hydroxide solution or
potassium hydroxide solution or subjecting it to a
catalytic hydrogenation such as e.g. with palladium/
carbon/hydrogen.
A compound of the general formula VI or of formula XVIII
or of formula XXIII or of formula XXIV or of formula
XXIX or of formula XXX is halogenated by reacting it
with molecular halogen (chlorine, bromine, iodine),
preferably bromine, without a solvent or in an inert
solvent such as methylene chloride, chloroform or carbon
tetrachloride, preferably carbon tetrachloride, and with
addition of red phosphorus, phosphorus trichloride or
phosphorus tribromide and at a temperature between room
temperature and 100~C, preferably at 90~C (K. Stoh,
Chem. Pharm. Bull. 34, 2078 (1986); H. J. Ziegler,
Synthesis 1969, 39)). In addition, compounds of the
general formula VI can be halogenated by metalation with
a lithium amide such as lithium diisopropylamide in an
aprotic solvent such as tetrahydrofuran and at a low
temperature, preferably at -78~C, and subsequently
reacting the compounds of the general formula XVI which
are metalized in the ~ position with bromine, iodine,
carbon tetrachloride or carbon tetrabromide (M. Hesse,
Helv. Chem. Acta 72, 847 (1989) R. T. Arnold, J. Org.
Chem. 43, 3687 (1978)) or with N-chlorosuccinimide or N-
CA 022l2227 l997-08-0
- 23 -
bromosuccinimide (W. Oppolzer, Tetrahedron Lett. 26,
5037 (1985).
The hydroxyl group of a compound of the general formula
XV, XVII, XVIII, XXIII or XXIX is converted into a
sulfonic acid ester according to the usual methods such
as by condensation with a sulfonyl chloride such as
methanesulfonyl chloride, benzenesulfonyl chloride,
p-toluenesulfonyl chloride or p-nitrobenzenesulfonyl
chloride, preferably methanesulfonyl chloride or
p-toluenesulfonyl chloride, in an inert solvent such as
methylene chloride, tetrahydrofuran or diethyl ether,
preferably methylene chloride, using an auxiliary base
such as trimethylamine or triethylamine or pyridine,
preferably triethylamine, and at a temperature between
0~C and room temperature.
Ketal cleavage of a ketal of the general formula XVI is
carried out according to standard procedures in organic
chemistry (ORGANIKUM; VEB "Deutscher Verlag der
Wissenschaften", Berlin 1977, page 486, 490).
The Wittig reaction between a ketone of the general
formula V and a phosphorane of the general formula XIX,
or a ketone of formula XXVIII and a phosphorane of the
formula XIX, or a ketone of formula XXXV and a
phosphorane of formula XXXVI is carried out according to
known methods by reflux heating the reactants in an
aprotic solvent such as benzene, toluene or xylol,
preferably toluene.
The Horner-Emmons reaction between a ketone of the
general formula V and a phosphonoacetic acid ester of
the general formula XX, or a ketone of formula XXVIII
CA 02212227 1997-08-0
- 24 -
and a phosphonoacetic acid ester of the formula XX is
usually carried out in a solvent such as dimethyl-
formamide, tetrahydrofuran, diethyl ether or
1,4-dioxane, preferably dimethylformamide or
tetrahydrofuran, using a base such as sodium hydride,
butyllithium, lithium diisopropylamide or sodium
hexamethyl disilazide, preferably sodium hydride or
lithium diisopropylamide, and at a temperature between
-78~C and 100~C preferably, however, at -78~C or room
temperature.
Oxidation of a compound of the general formula VI to
form a compound of the general formula XV is usually
carried out in a solvent such as tetrahydrofuran by
addition of a base such as lithium diisopropylamide or
lithium-N-isopropyl-N-cyclohexylamide using an oxidizing
agent such as an oxaziridine derivative, molybdenum
peroxide or atmospheric oxygen and at temperatures
between -78OC and room temperature, preferably at 50~C
(C. Tamm, Tetrahedron Lett. 26, 203 (1985); F. A. Davis
J. Org. Chem. 51, 2402 (1986); C. Wintoai Synth. Commun.
18, 2141 (1988)).
The reaction of a compound of the general formula IV
with a triarylphosphine of the general formula XXVI, or
of a compound of formula XXXVI with a triarylphosphine
of formula XXVI is carried out analogously to methods
known from the literature (Buddras J., Angew. Chem. 80,
535 (1968); Bestmann H. J. Angew. Chem. 77, 620, 651
(1965); Wittig G. Ber. Deutsch. Chem. Ges. 88, 1654
(1955)).
A compound of the general formula IV is usually reacted
with a compound of the general formula XXVII without a
CA 02212227 1997-08-0
- 25 -
solvent at temperatures between room temperature and
150~C, preferably at 130~C, with a reaction time between
30 min and 30 hours, preferably 18 hours.
A carboxylic acid derivative of the general formula XXIV
or XXX is usually reduced to an alcohol of formula XXIII
or XXIX in a solvent such as tetrahydrofuran or diethyl
ether using a reducing agent such as lithium aluminium
hydride and at a reaction temperature between 0~C and
the reflux temperature of the solvent used, preferably
at 40~C.
Acylation of an amine of the general formula XII or of
formula XIII or of formula XXXI with a carboxylic acid
derivative of formula XI or of formula XXV or of formula
XXXII or of formula XIV is usually carried out in a
solvent such as methylene chloride, dimethylformamide or
pyridine, preferably methylene chloride or pyridine,
with addition of an auxiliary base such as triethylamine
or 4-dimethylamino-pyridine and at a temperature between
-10~C and 50~C, but preferably at room temperature.
A ketone of the general formula V is usually reduced to
an alcohol of formula XVIII in a solvent such as
methanol, ethanol, tetrahydrofuran or diethyl ether,
preferably methanol, using a reducing agent such as
sodium borohydride, lithium borohydride or lithium
aluminium hydride, preferably sodium borohydride, and at
a temperature between -10~C and +30~C, preferably at
room temperature.
Alkali salts, ammonium salts, trifluoroacetates or
hydrochlorides are used above all as pharmacologically
acceptable salts which are usually produced for example
CA 02212227 1997-08-0
- 26 -
by titrating the compounds with inorganic or organic
bases or acids such as e.g. sodium or potassium
bicarbonate, sodium hydroxide solution, potassium
hydroxide solution, aqueous ammonia or amines such as
trimethylamine or triethylamine, trifluoroacetic acid or
hydrochloric acid. The salts are usually purified by
precipitation from water/acetone.
The new substances of formula I and salts thereof
according to the invention can be administered enterally
or parenterally in a liquid or solid form. In this
connection all the usual forms of administration come
into consideration such as tablets, capsules, dragées,
syrups, solutions, suspensions etc.. Water is preferably
used as the injection medium which contains the usual
additives for injection solutions such as stabilizing
agents, solubilizers and buffers.
Such additives are for example tartrate and citrate
buffer, ethanol, complexing agents (such as ethylene-
diaminetetraacetic acid and non-toxic salts thereof),
high molecular polymers (such as liquid polyethylene
oxide) in order to regulate viscosity. Liquid carriers
for injection solutions have to be sterile and are
preferably dispensed into ampoules. Solid carriers are
e.g. starch, lactose, mannitol, methylcellulose, talcum,
highly dispersed silicic acids, higher molecular fatty
acids (such as stearic acid), gelatine, agar-agar,
calcium phosphate, magnesium stearate, animal and
vegetable fats, solid high molecular polymers (such as
polyethylene glycols); preparations that are suitable
for oral application can optionally contain flavourings
and sweeteners.
CA 02212227 1997-08-0
- 27 -
The dose can depend on various factors such as manner of
administration, species, age and/or individual state of
health. The doses to be administered daily are about 10-
1000 mg/human, preferably 100-500 mg/human and can be
taken once or several times.
Within the sense of the present invention the following
pyridine and pyridazine derivatives are preferred in
addition to the compounds mentioned in the examples and
compounds derived by combination of all meanings of
substituents mentioned in the claims:
1. [4-(4-Pyridin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-acetic acid
2. {4-[2-(4-Pyridin-4-yl-piperazin-1-yl)-ethyl~-
cyclohexyl}-acetic acid
3. {4-[3-(4-Pyridin-4-yl-piperazin-1-yl)-propyl]-
cyclohexyl)-acetic acid
4. {4-[4-(4-Pyridin-4-yl-piperazin-1-yl)-butyl]-
cyclohexyl}-acetic acid
5. {4-[6-(4-(Pyridin-4-yl-piperazin-1-yl)-hexyl]-
cyclohexyl}-acetic acid
6. [4-(4-Pyridin-4-yl-piperazin-1-carbonyl)-
cyclohexyl]-acetic acid
7. {4-[2-Oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-
cyclohexyl}-acetic acid
CA 02212227 1997-08-0
- 28 -
8. {4-[3-Oxo-3-(4-pyridin-4-yl-piperazin-1-yl)-
propyl~-cyclohexyl}-acetic acid
9. {4-[4-Oxo-4-(4-pyridin-4-yl-piperazin-1-yl)-butyl]-
cyclohexyl}-acetic acid
10. {4-[6-Oxo-6-(4-pyridin-4-yl-piperazin-1-yl)-hexyl]-
cyclohexyl}-acetic acid
11. {1-[2-Oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-
acetyl]-piperidin-4-yl}-acetic acid
12. {1-[4-Oxo-4-(4-pyridin-4-yl-piperazin-1-yl)-
butyryl]-piperidin-4-yl}-acetic acid
13. {1-[S-Oxo-5-(4-pyridin-4-yl-piperazin-1-yl)-
pentanoyl]-piperidin-4-yl}-acetic acid
14. {1-[6-Oxo-6-(4-pyridin-4-yl-piperazin-1-yl)-
hexanoyl]-piperidin-4-yl}-acetic acid
15. {1-[8-Oxo-8-(4-pyridin-4-yl-piperazin-1-yl)-
octanoyl]-piperidin-4-yl}-acetic acid
16. {1-[3-(4-Pyridin-4-yl-piperazin-1-yl)-propionyl]-
piperidin-4-yl}-acetic acid
17. {1-[4-(4-Pyridin-4-yl-piperazin-1-yl)-butyryl]-
piperidin)-4-yl}-acetic acid
18. {1-[5-(4-Pyridin-4-yl-piperazin-1-yl)-pentanoyl]-
piperidin-4-yl}-acetic acid
CA 02212227 1997-08-0
- 29 -
19. {1-[2-(4-Pyridin-4-yl-piperazin-1-yl]-acetyl]-
piperidin-4-yl}-acetic acid
20. [4-(4-pyridin-4-yl-piperazin-1-carbonyl)-piperidin-
1-yl]-acetic acid
21. {4-[2-Oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-
piperidin-1-yl}-acetic acid
22. {4-[3-Oxo-3-(4-pyridin-4-yl-piperazin-1-yl)-
propyl]-piperidin-1-yl}-acetic acid
23. {4-[5-Oxo-5-(4-pyridin-4-yl-piperazin-1-yl)-
pentyl]-piperidin-1-yl}-acetic acid
24. {4-[7-Oxo-7-(4-pyridin-4-yl-piperazin-1-yl)-
heptyl)-piperidin-1-yl}-acetic acid
25. [1-Hydroxy-4-(4-pyridin-4-yl-piperazin-1-carbonyl)-
cyclohexyl]-acetic acid
26. [1-Hydroxy-4-(4-pyridin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-acetic acid
27. {1-Hydroxy-4-[2-(4-pyridin-4-yl-piperazin-1-yl)-
ethyl]-cyclohexyl}-acetic acid
28. {1-Hydroxy-4-[3-(4-pyridin-4-yl-piperazin-1-yl)-
propyl]-cyclohexyl}-acetic acid
29. {1-Hydroxy-4-[5-(4-pyridin-4-yl-piperazin-1-yl)-
pentyl]-cyclohexyl}-acetic acid
CA 02212227 1997-08-0
- 30 -
30. {1-Hydroxy-4-[6-(4-pyridin-4-yl-piperazin-1-yl)-
hexyl]-cyclohexyl}-acetic acid
31. {1-Hydroxy-4-[2-oxo-2-(4-pyridin-4-yl-piperazin-1-
yl)-ethyl]-cyclohexyl}-acetic acid
32. {1-Hydroxy-4-[3-oxo-3-(4-pyridin-4-yl-piperazin-1-
yl)-propyl]-cyclohexyl}-acetic acid
33. {1-Hydroxy-4-[4-oxo-4-(4-pyridin-4-yl-piperazin-1-
yl)-butyl]-cyclohexyl}-acetic acid
34. {1-Hydroxy-4-[6-oxo-6-(4-pyridin-4-yl-piperazin-1-
yl)-hexyl]-cyclohexyl}-acetic acid
35. {1-Hydroxy-4-[7-oxo-7-(4-pyridin-4-yl-piperazin-1-
yl)-heptyl]-cyclohexyl}-acetic acid
36. {4-Hydroxy-1-[2-oxo-2-(4-pyridin-4-yl-piperazin-1-
yl)-acetyl]-piperidin-4-yl}-acetic acid
37. 4-Hydroxy-1-[4-oxo-4-(4-pyridin-4-yl-piperazin-1-
yl)-butyryl]-piperidin-4-yl}-acetic acid
38. {1-[2,3-Dihydroxy-4-oxo-4-(4-pyridin-4-yl-
piperazin-1-yl)-butyryl]-4-hydroxy-piperidin-4-yl}-
acetic acid
39. {1-[2,3-Dihydroxy-4-oxo-4-(4-pyridin-4-yl-
piperazin-1-yl)-butyryl]-piperidin-4-yl}-acetic
acid
CA 02212227 1997-08-0~
40. {4-Hydroxy-1-[7-oxo-7-(4-pyridin-4-yl-piperazin-1-
yl)-heptanoyl]-piperidin-4-yl}-acetic acid
41. Hydroxy-[4-(4-pyridin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-acetic acid
42. Phenyl-[4-(4-pyridin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-acetic acid
43. Amino-[4-(4-pyridin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-acetic acid
44. Butane-1-sulfonylamino)-[4-(4-pyridin-4-yl-
piperazin-1-ylmethyl)-cyclohexyl]-acetic acid
45. (Butane-1-sulfonylamino)-[1-hydroxy-4-(4-pyridin-4-
yl-piperazin-1-ylmethyl)-cyclohexyl]-acetic acid
46. [1-Methoxy-4-(4-pyridin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-acetic acid
47. (Butane-1-sulfonylamino)-{1-hydroxy-4-[2-(4-
pyridin-4-yl-piperazin-1-yl)-ethyl]-cyclohexyl]}-
acetic acid
48. (Butane-1-sulfonylamino)-{4-[2-(4-pyridin-4-yl-
piperazin-1-yl)-ethyl]-cyclohexyl}-acetic acid
49. 2-[4-(4-Pyridin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-propionic acid
CA 02212227 1997-08-0
- 32 -
50. (Butane-1-sulfonylamino-)-{1-hydroxy-4-[2-oxo-2-(4-
pyridin-4-yl-piperazin-1-yl)-ethyl]-cyclohexyl}-
acetic acid
51. (Butan-1-sulfonylamino-{1-hydroxy-4-[4-oxo-4-(4-
pyridin-4-yl-piperazin-1-yl)-butyl]-cyclohexyl}-
acetic acid
52. [4-(4-Pyridazin-4-yl-piperazin-1-ylmethyl)-
cyclohexyl]-acetic acid
53. [4-(4-pyridazin-4-yl-piperazin-1-carbonyl)-
cyclohexyl]-acetic acid
54. {1-[6-(4-Pyridazin-4-yl-piperazin-1-yl)-hexyl]-
piperidin-4-yl}-acetic acid
55. {4-[2-Oxo-2-(4-pyridazin-4-yl-piperazin-1-yl)-
ethyl]-cyclohexyl}-acetic acid
56. {4-[3-Oxo-3-(4-pyridazin-4-yl-piperazin-1-yl)-
propyl]-cyclohexyl}-acetic acid
57. {1-[2-Oxo-2-(4-pyridazin-4-yl-piperazin-1-yl)-
acetyl]-piperidin-4-yl}-acetic acid
58. {1-[4-Oxo-4-(4-pyridazin-4-yl-piperazin-1-yl)-
butyryl]-piperidin-4-yl}-acetic acid
59. {1-[6-Oxo-6-(4-pyridazin-4-yl-piperazin-1-yl)-
hexanoyl]-piperidin-4-yl}-acetic acid
CA 02212227 1997-08-0
- 33 -
The following examples show several process variants
which can be used to synthesize the compounds according
to the invention. However, they should not limit the
subject matter of the invention. The structure of
compounds was established by 1H and optionally by 13C-
NMR spectroscopy as well as by mass spectrometry. The
purity of the substances was determined by means of C,
H, N, and thin layer chromatography.
Example
{4-[2-Oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-
cyclohexyl}-acetic acid
la) 76 g (4-hydroxy-cyclohexyl-)acetic acid ethyl ester
produced by catalytic hydrogenation from 4-hydroxy-
phenyl acetic acid ethyl ester (Raney-Ni/H2tl60~C/
200 bar/72 htethanol) is dissolved in 130 ml
methylene chloride and admixed with 20.6 g Dess-
Martin periodinan. The reaction solution is
subsequently stirred for 30 h at room temperature.
Afterwards the precipitated precipitate is
filtered, the methylene chloride solution is washed
successively with saturated sodium thiosulfate
solution and water and dried over sodium sulfate.
After removing the solvent 16.5 g (4-oxo-
cyclohexyl)-acetic acid ethyl ester is obtained as
a colourless oil. 1H-NMR (DMSO-d6):~ = 4.05 ppm (q,
2H); 2.48-2.25 (m, 8 lines, 3H); 2.15 (m, 3H); 1.92
(m, 2H); 1.90 (sextet, 2H), 1.15 (t, 3H).
lb) A solution of 3.6 g (4-oxo-cyclohexyl)-acetic acid
ethyl ester and 8 g (tert.-butoxycarbonyl-
methylene)-triphenylphosphorane (Aldrich GmbH and
CA 02212227 1997-08-0
- 34 -
Co. KG) in 100 ml toluene is heated for 40 hours
under reflux. Subsequently the toluene is removed
in a vacuum and the product is purified by column
chromatography (ethyl acetate/isohexane 1/10). The
(4-ethoxycarbonylmethyl-cyclohexylidene)-acetic
acid tert.-butyl ester (2.7 g) obtained in this way
is dissolved in 40 ml tetrahydrofuran, admixed with
300 mg 10 percent palladium/carbon and hydrogenated
for 20 hours at normal pressure and room
temperature. Afterwards the catalyst is removed by
filtration, the tetrahydrofuran is removed on a
rotary evaporator and the residue (2.8 g) is taken
up in 5 ml methylene chloride.
After addition of 5 ml trifluoroacetic acid the
reaction solution is stirred for 2 h at room
temperature and evaporated to dryness. 2.7 g (4-
ethoxycarbonylmethyl-cyclohexyl)-acetic acid is
obtained in this way. FAB: 228, 1H-NMR (CDCl3):~ =
7.70 ppm (broad s, lH); 4.15 (q, 2H); 2.20 (d, 2H);
2.17 (d, 2H); 1.75 (m, 5H); 1.60 (m, lH); 1.40 (m,
lH); 1.25 (t, 3H); 1.05 (m, 3H).
lc) A solution of 690 mg (4-ethoxycarbonylmethyl-
cyclohexyl)-acetic acid, 490 mg 1-(4-pyridyl)-
piperazine and 680 mg dicyclohexylcarbodiimide in
10 ml dimethylformamide is stirred for 48 h at room
temperature. Subsequently the dimethylformamide is
removed on a rotary evaporator, the residue is
taken up in 10 ml water and the aqueous mixture is
extracted three times with 10 ml methylene chloride
each time. After drying the combined organic phases
over sodium sulfate and removing the solvent, the
product is purified by means of preparative HPLC
(RP-18; methanol/buffer [pH 6.8] = 7/3). 770 mg {4-
CA 02212227 1997-08-0~
[2-oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-
cyclohexyl}-acetic acid ethyl ester is obtained.
m/e = 373; lH-NMR (DMSO-d6): ~ = 8.18 ppm (d, 2H);
6.82 (d, 2H); 4.05 (q, 2H); 3.60 (broad s, 4H);
3.42 (broad s, 4H); 2.31 (dd, 2H); 2.15 (dd, 2H);
1.55 (m, 5H); 1.45 (m, lH); 1.40 (m, lH); 1.15 (t,
3H); 0.95 (broad t, 3H).
ld) A solution of 730 mg of the ethyl ester prepared in
lc) in 6 ml methanol is stirred for 3 h at 50~C and
2 ml 1 N sodium hydroxide solution. Subsequently
the methanol is removed in a vacuum, the residue is
taken up in 10 ml water and the product is purified
by means of ion chromatography (DOWEX 50 H-form,
eluant: aqueous ammonia). 300 mg of the title
compound is obtained in this way as a white powder.
FAB: 345; 1H-NMR (CDCl3):~ = 8.20 ppm (d, 2H); 3.60
(broad s, 4H); 3.35 (broad s, 4H); 2.30 (dd, 2H);
2.10 (dd, 2H); 1.90 (m, lH); 1.65 (m, 4H); 1.55 (m,
lH); 1.40 (m, lH); 0.95 (m, 3H).
Example 2
~1-Hydroxy-4- r 2-oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-
ethyl]-cyclohexyl~-acetic acid
2a) 15.3 g of the ketone prepared in la) is dissolved
in 200 ml toluene and admixed with 6 ml ethylene
glycol and 30 mg p-toluene sulfonic acid. The
reaction solution is then heated for 4 h at 140~C
on a water separator, subsequently cooled and
washed with 50 ml saturated sodium carbonate
solution. Then the aqueous phase is extracted twice
with 30 ml methylene chloride each time, the
CA 02212227 1997-08-0
- 36 -
combined organic phases are dried over sodium
sulfate and the solvent is evaporated on a rotary
evaporator. The ketal which remains (19.3 g) is
taken up in 100 ml tetrahydrofuran and the solution
is admixed with 100 ml 1 N sodium hydroxide
solution. Then the reaction solution is heated for
24 h at 50~C, the tetrahydrofuran is withdrawn in a
vacuum, the aqueous solution is acidified with 1 N
hydrochloric acid and extracted three times with
50 ml methylene chloride each time. After drying
the combined extracts over sodium sulfate and
removing the solvent, 13 g (1,4-dioxa-spiro-
[4,5]dec-8-yl)acetic acid is obtained. FAB: 200.
2b) 11.3 g 2-(1,4-dioxa-spiro[4,5]dec-8-yl)-1-(4-
pyridin-4-yl-piperazin-1-yl)-ethanone is obtained
analogously to lc) from 13 g (1,4-dioxa-spiro[4,5]-
dec-8-yl)-acetic acid, 10.6 g 1-(4-pyridyl)-
piperazine and 15.6 g N-(3-dimethylaminopropyl)-N'-
ethyl-carbodiimide hydrochloride. 1H-NMR (CDCl3):
= 8.25 ppm (d, 2H); 6.58 (d, 2H); 3.38 (s, 4H);
3.70 (t, 2H); 3.58 (t, 2H); 3.30 (q, 4H); 2.25 (d,
2H); 1.85 (m, lH); 1.70 (m, 4H); 1.52 (sextet, 2H);
1.25 (m, 2H).
2c) A solution of 1.5 g of the product produced in 2b)
is stirred for 30 min at room temperature in 10 ml
tetrahydrofuran and 5 ml 6 N hydrochloric acid.
Subsequently the tetrahydrofuran is removed in a
vacuum and the hydrochloric acid solution is
adjusted to pH 9 with concentrated ammonia and
extracted three times with 5 ml methylene chloride
each time. After drying the combined organic phases
over sodium sulfate and removing the solvent, the
residue is purified by means of column
CA 02212227 1997-08-0
- 37 -
chromatography (mobile solvent: ethyl acetate /
methanolic ammonia = g/l). 870 mg 4-[2-oxo-2-(4-
pyridin-4-yl-piperazin-1-yl)-ethyl]-cyclohexanone
is obtained in this way. 1H-NMR (CDC13): ~ = 8.35
ppm (d, 2H); 6.65 (d, 2H); 3.85 (broad t, 2H); 3.62
(broad t, 2H); 3.38 (broad q, 4H); 2.40 (m, 7H);
2.25 (m, 2H); 1.45 (m, 2H).
2d) 1.5 ml n-butyllithium (1.6 M in hexane) is added
dropwise to a solution of 0.34 ml diisopropylamine
in 5 ml tetrahydrofuran stirred at -15~C under
nitrogen, the solution is allowed to stir for a
further 30 min at -15~C, subsequently cooled to
-78~C and a solution of 0.27 ml acetic acid tert.-
butyl ester in 2 ml tetrahydrofuran is added
dropwise. The reaction mixture is now stirred for a
further hour at -78~C and then admixed dropwise
with a solution of 600 mg of the cyclohexanone
derivative produced in 2c) in 2 ml tetrahydrofuran
and 2 ml 1,3-dimethyl-3,4,5,6-tetrahydro-2(lH)-
pyrimidione (DMPU). Afterwards the reaction mixture
is stirred for a further 30 min at -78~C,
subsequently heated to room temperature within one
hour and finally stirred for a further three hours
at room temperature. After the addition of 5 ml
saturated ammonium chloride solution, the reaction
mixture is extracted three times with 5 ml
methylene chloride each time, the combined organic
phases are dried over sodium sulfate, the solvent
is removed on a rotary evaporator and the crude
product is purified by means of preparative HPLC
(Select B, 12 ~; methanol/buffer (pH 7.5) = 65/35).
In this way the two isomers are obtained {1-
hydroxy-4-[2-oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-
ethyl]-cyclohexyl}-acetic acid tert.-butyl ester
CA 02212227 1997-08-0
- 38 -
(cis/trans isomers).
FAB: 417;
cis-isomer: 340 mg; lH-NMR (CDC13): ~ = 8.25 ppm
(d, 2H); 6.60 (d, 2H); 3.92 (broad s, lH; OH); 3.75
(m, 2H); 3.58 (m, 2H); 3.30 (m, 4H); 2.41 (s, 2H);
2.25 (d, 2H); 1.88 (m, lH); 1.75 (broad t, 4H);
1.50 (t with fine resolution, 2H); 1.40 (s, 9H);
1.08 (broad q, 2H).
trans-isomer: 100 mg; lH-NMR (CDC13): ~ = 8.25 ppm
(d, 2H); 6.60 (d, 2H); 3.72 (m, 2H); 3.60 (m, 2H);
3.31 (m, 4H); 2.28 (s, 2H); 2.21 (d, 2H); 1.80 (m,
lH); 1.70 (broad d, 2H); 1.55 (m, 2H); 1.40 (s,
9H); 1.34 (m, 4H).
2e) A solution of 300 mg cis-isomer in 1 ml
trifluoroacetic acid is stirred for 1 hour at room
temperature, it is subsequently evaporated and
dried in a high vacuum. In this way 300 mg cis-{l-
hydroxy-4-[2-oxo-2-(4-pyridin-4-yl-piperazin-1-yl)-
ethyl]-cyclohexyl}-acetic acid trifluoroacetate is
obtained as a white powder. m/e: 361.
lH-NMR (DMSO-d6): ~ = 8.29 ppm (d, 2H); 7.20 (d,
2H); 3.65 (broad t, 8H); 2.40 (s, 2H); 2.31 (d,
2H); 1.88-1.60 (m, 5H); 1.35 (broad t, 2H); 1.10
(m, 2H); 19F-NMR (DMSO-d6): ~ = -73.30 ppm (s).
2f) 50 mg trans-{1-hydroxy-4-[2-oxo-2-(4-pyridin-4-yl-
piperazin-l-yl)-ethyl]-cyclohexyl}-acetic acid-
trifluoroacetate is obtained analogously to 2e)
from 80 mg trans-isomer. m/e: 361. lH-NMR (DMSO-
d6): ~ = 8.25 ppm (d, 2H); 7.15 (d, 2H); 3.65 (m,
8H); 2.29 (s, 2H); 2.22 (d, 2H); 1.60 (m, 3H); 1.40
CA 02212227 1997-08-0
- 39 -
(m, 6H). 19F-NMR (DMS0-d6): ~ = -73.30 ppm (s).
Example 3
~1-Hydroxy-4- r 2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-
cyclohexyl~-acetic acid
3a) A solution of 12 g 1,4-dioxaspiro[4,5]decan-4-one
and 26 g (ethoxycarbonyl-methylene)-triphenyl-
phosphorane in 100 ml toluene is heated for 24
hours at 100~C. Subsequently the toluene is removed
in a vacuum, the residue is admixed with 10 ml of a
mixture of ethyl acetate/isohexane = 1/5, the
precipitated triphenylphosphinoxide is removed by
filtration, the filtrate is concentrated in a
vacuum and the product is purified by column
chromatography. 11.4 g (1,4-dioxa-spiro[4,5]-dec-8-
ylidene)-acetic acid ethyl ester is obtained as a
colourless oil.
m/e: 226
3b) The product (11 g) produced in 3a) is dissolved in
100 ml tetrahydrofuran and the solution is admixed
with 1.5 g 10 % palladium/carbon. It is then
hydrogenated at room temperature and normal
pressure until the uptake of hydrogen is completed,
it is filtered, evaporated to dryness and the
residue is taken up in 50 ml tetrahydrofuran. The
solution obtained in this way is added dropwise to
a mixture of 1.2 g lithium aluminium hydride in
50 ml tetrahydrofuran in such a way that the
reaction temperature does not exceed 50~C.
Afterwards the reaction mixture is stirred for a
further 2 h at room temperature and subsequently
CA 02212227 1997-08-0
- 40 -
excess lithium aluminium hydride is carefully
decomposed with water. After processing the
reaction mixture in the usual way, 7.3 g 2-(1,4-
dioxa-spiro[4,5]dec-8-yl)-ethanol is obtained as a
colourless oil. mte: 186. lH-NMR (CDCl3 + CD3COOD):
= 3.88 ppm (s, 4H); 3.60 (t, 2H); 1.65 (m, 4H);
1.55-1.48 (t overlapped by m, 5H); 1.20 (m, 2H).
3c) 1 g of the alcohol prepared in 3b) is dissolved in
20 ml diethyl ether and admixed with 1.4 ml tri-
ethylamine. A solution of 0.47 ml methanesulfonyl
chloride in 5 ml diethyl ether is added dropwise to
this solution at room temperature. Afterwards the
reaction solution is stirred for a further 30 min,
it is subsequently washed successively with 10 ml
saturated sodium bicarbonate solution and 10 ml
water and the organic phase is dried over sodium
sulfate. After removing the solvent, the residue is
dissolved in 5 ml dimethylformamide. 1 g 1-(4-
pyridyl)-piperazine and 0.8 g potassium carbonate
is added to this solution, the reaction mixture is
heated for 3 h at 50~C and then the dimethyl-
formamide is removed in a vacuum. The residue is
taken up in 5 ml saturated ammonium chloride
solution and extracted three times with 5 ml
methylene chloride each time. After drying the
combined extracts over sodium sulfate and removing
the solvent, the crude product is purified by
column chromatography (prep. HPLC: Select B, 12 ~,
methanoltbuffer (pH 7.5) = 7/3). 0.6 g 1-[2-(1,4-
dioxa-spiro[4,5]-dec-8-yl)-ethyl]-4-pyridin-4-yl-1-
piperazine is obtained in this way as a light grey
powder. mte: 331. lH-NMR (CDCl3): ~ = 8.20 ppm (d,
2H); 6.58 (d, 2H); 3.90 (s, 4H); 3.28 (dd, 4H);
2.48 (t, 4H); 2.35 (t, 2H); 1.67 (broad d, 4H);
CA 02212227 1997-08-0
- 41 -
1.45 (q, 4H); 1.25 (m, 3H).
3d) 0.8 g 4-[2-(4-pyridin-4-yl-piperazin-1-yl)-ethyl]-
cyclohexanone is obtained analogously to 2c) from
1.1 g of the product prepared in 3c). m/e: 287. lH-
NMR (CDCl3): ~ = 8.20 ppm (d, 2H); 6.59 (d, 2H);
3.28 (t, 4H); 2.50 (t, 4H); 2.40 (t, 2H); 2.30 (m,
4H); 2.0 (m, 2H); 1.75 (m, lH); 1.51-1.26 (m, 4H).
3e) Two stereoisomers {1-hydroxy-4-[2-(4-pyridin-4-yl-
piperazin-yl)-ethyl]-cyclohexyl}-acetic acid tert.-
butyl ester (cisttrans-isomers) are obtained
analogously to 2d) from 580 mg of the product from
3d), 0.27 ml tert.-butyl acetate, 1.5 ml n-butyl-
lithium (1.6 M in hexane) and 0.34 ml diisopropyl-
amine.
FAB: 403.
cis-isomer: 190 mg; 1H-NMR (CDCl3): ~ = 8.18 ppm
(d, 2H); 6.60 (d, 2H); 3.30 (t, 4H); 2.75 (broad s,
lH; OH); 2.50 (t, 4H); 2.35 (dd, 2H); 2.25 (s, 2H);
1.70 (d, 2H); 1.55-1.35 (s overlapped by m, 9H +
4H); 1.22 (m, 5H).
trans-isomer: 130 mg; lH-NMR (CDCl3): ~ = 8.15 ppm
(d, 2H); 6.59 (d, 2H); 3.30 (t, 4H); 2.50 (t, 4H);
2.40 (s, 2H); 2.35 (dd, 2H); 2.25 (broad s, lH;
OH); 1.68 (m, 4H); 1.55-1.30 (s covered by m, 9H +
6H); 1.0 (m, lH).
3f) 170 mg cis-{1-hydroxy-4-[2-(4-pyridin-4-yl-
piperazin-1-yl)-ethyl]-cyclohexyl}-acetic acid
trifluoroacetate is obtained analogously to 2e)
from 170 mg cis-isomer.
FAB: 347; m.p.: 130~C
CA 02212227 1997-08-0
- 42 -
100 mg trans-{1-hydroxy-4-[2-(4-pyridin-4-yl-
piperazin-l-yl)-ethyl-cyclohexyl}-acetic acid
trifluoroacetate is correspondingly obtained from
110 mg trans-isomer.
FAB: 347; m.p.: 207~C.
Example 4
~4-[2-(4-Pyridin-4-yl-piperazin-1-yl)-ethyl]-
cyclohexyl}-acetic acid
4a) 5.2 g 4-(2-Hydroxy-ethyl)-cyclohexanone is obtained
analogously to process 2c) from 9.8 g of product
3b). 1H-NMR (DMSO-d6): ~ = 4.41 ppm (t, lH; OH);
3.48 (q, 2H); 2.36 (3 xd, 2H); 2.18 (d with fine
resolution, 2H); 1.95 (dt, 2H); 1.85 (m, lH); 1.40
(q, 2H); 1.30 (m, 2H).
4b) A solution of 5.2 g 4-(2-hydroxy-ethyl)-
cyclohexanone, 6.8 g imidazole and 6 g tert.-butyl-
dimethylsilyl chloride in 50 ml dimethylformamide
is stirred for 2 h at room temperature. Afterwards
the solution is concentrated in a vacuum, the
residue is taken up in 10 ml water and the aqueous
solution is extracted three times with 10 ml
diethyl ether each time. After drying the combined
organic phases over sodium sulfate and removing the
solvent, the crude product is purified by column
chromatography (silica gel, ethyl acetate/isohexane
= 1/8). 7 g 4-[2-(tert.-butyl-dimethyl-silanyl-
oxy)-ethyl]-cyclohexanone is obtained in this way
as a colourless oil. 1H-NMR (CDCl3): ~ = 3.65 ppm
(t, 2H); 2.35 (m, 4H); 2.05 (m, 2H); 1.85 (m, lH);
1.50 (q, 2H); 1.38 (m, 2H); 0.85 (s, 9H); 0.00 (s,
CA 02212227 1997-08-0
- 43 -
6H).
4c) 8.1 g {4-[2-(tert.-butyl-dimethyl-silanyloxy-
ethyl]-cyclohexylidene~-acetic acid ethyl ester is
obtained analogously to 3a) from 7 g of product 4b)
and 9.2 g ethoxycarbonyl-methylene-triphenyl-
phosphorane. 1H-NMR (CDCl3): ~ = 5.52 ppm (s, lH);
4.10 (q, 2H); 3.70 (broad d, lH); 3.60 (t, 2H);
2.30-2.05 (m, 2H); 1.85 (m, 3H); 1.60 (m, lH); 1.40
(q, 2H); 1.20 (t, 3H); 1.06 (m, 2H); 0.85 (s, 9H);
0.00 (s, 6H).
4d) The product 4c) (8 g) is dissolved in 70 ml of a
tetrahydrofuran/methanol = 1/1 mixture and the
solution is admixed with 0.9 g 10 % palladium/
carbon. It is hydrogenated for 4 h at normal
pressure and room temperature, the catalyst is
subsequently removed by filtration, the filtrate is
evaporated to dryness, the residue is taken up in
70 ml of a 1 M hydrofluoric acid/acetonitrile
mixture and the reaction solution is allowed to
stir for 12 h at room temperature. Afterwards the
solvent is evaporated in a vacuum, the residue is
admixed with 10 ml saturated sodium bicarbonate
solution and the aqueous solution obtained in this
way is extracted three times with 10 ml methylene
chloride each time. After drying the combined
organic phases over sodium sulfate and
chromatographing the residue on silica gel
(methylene chloride/methanol = 9/1), 2.4 g [4-(2-
hydroxy-ethyl)-cyclohexyl]-acetic acid ethyl ester
is obtained as a light yellow oil. lH-NMR (DMSO-
d6): ~ = 4.35 ppm (broad s, lH; OH); 4.05 (q, 2H);
3.40 (broad s, 2H); 2.15 (sextet, 2H); 1.90 (m,
lH); 1.78-1.25 (m, 8H); 1.16 (t, 3H); 0.90 (m, 3H).
CA 02212227 1997-08-0
- 44 -
4e) In analogy to process 3c) 2.4 g mesylate is
obtained from 2.4 g of product 4d), 0.93 methane
sulfonyl chloride and 2.2 ml triethylamine, which
yielded 420 mg {4-[2-pyridin-4-yl-piperazin-1-yl)-
ethyl]-cyclohexyl}-acetic acid ethyl ester when
reacted with 1.4 g 1-(4-pyridyl)-piperazine in the
presence of 1.2 g potassium carbonate. FAB: 359;
lH-NMR (CDC13): ~ = 8.20 (d, 2H); 6.55 (d, 2H);
4.08 (q, 2H); 3.25 (t, 4H); 2.50 (t, 4H); 2.35 (m,
2H); 2.0 (d, 2H); 1.68 (m, 5H); 1.46 (m, 2H); 1.35
(m, 2H); 1.19 (t, 3H); 1.13 (m, lH); 0.90 (t, 2H).
4f) A solution of 170 mg of product 4e) in 4 ml
tetrahydrofuran and 0.5 ml water is admixed with
0.57 ml 1 N sodium hydroxide solution. The reaction
mixture obtained in this way is heated for 4 h at
50~C and subsequently the tetrahydrofuran is
removed in a vacuum. The residue is then taken up
in 5 ml water and the product is purified by means
of ion chromatography (Dowex 50 H-Form, eluant:
concentrated ammonia). In this way 70 mg of the
title compound is obtained as a light grey powder.
mte= 331.
CA 02212227 1997-08-0
Test report
A~ay
Microtitre plates are coated overnight with 2 ~g/ml
isolated activated GpIIb/IIIa receptor. After removing
the unbound receptor by several washing steps, the
surface of the plates is blocked with 1 % casein and
washed again. The test substance is added in the
required concentrations and the plates are incubated for
10 minutes while shaking in a linear shaker. The natural
ligand of the gpIIb/IIIa receptor, fibrinogen, is added.
After 1 hour of incubation the unbound fibrinogen is
removed by several washing steps and bound fibrinogen is
detected by a peroxidase-conjugated anti-fibrinogen
monoclonal antibody by measuring the O.D. at 405 nm in
an ELISA-reader. Inhibition of fibrinogen-GpIIb/IIIa
interaction results in a low O.D. An ICso is calculated
in relation to a concentration-effect curve.
Literature:
The GpIIb/IIIa-Fibrinogen-ELISA is a modification of the
assays described in the following literature:
Nachman, R. L. & Leung, L. L. K. (1982): Complex
formation of platelet membrane glycoproteins IIb and
IIIa with fibrinogen. J. Clin. Invest. 69:263-269.
Wright, P. S. et al. (1993): An echistatin C-terminal
peptide activated GpIIbIIIa binding to fibrinogen,
fibronectin, vitronectin and collagen type I and type
IV. Biochem. J. 293:263-276.
CA 02212227 1997-08-0
- 46 -
Pharmacological Data:
Example IC50 (~mol/l)
2 cis-isomer 0.30
3 trans-isomer 0.006
3 cis-isomer 0.30
4 0.10
Comparative experiments:
The compound cis-1-hydroxy-4-[4-(4-pyridyl)-piperazin-1-
yl]-acetic acid was prepared as a reference substance
which is included in the patent W0 94/22835 as example
No. 102. This compound has an IC50 value of 2.50 ~mol/l
in the above assay!