Note: Descriptions are shown in the official language in which they were submitted.
CA 02212861 1997-0X-13
W O 96/25278 PCTrUS96/01866
ACRYLIC E M mLSIO N C O ATnNGS FO R RlneBER ARTICLES
Field ofthe Invention
The invention relates to thin walled articles formed of natural or synthetic rubber
having thereon a powder-free polymeric coating which enables or enhances mold or substrate
stripping and donning.
Back~round of the Invention
Rubber articles made from natural or synthetic rubber include surgeons gloves,
?hysicians examining gloves, workers gloves, prophylactics, catheters, balloons, tubing,
sheeting and the like. Some of such articles and in particular gloves require the ability of
donning i.e. the ability of the rubber article to be slid on and off skin (tissue) surfaces
without undue clinging or friction. Surgeons gloves require the wet donning, i.e., the ability
to be slid over wet skin surfaces while physicians e~minin~ and workers gloves require the
ability to be slid over dry skin sllrf~s Other rubber articles like catheters and tubing,
require some means to isolate the rubber from body fluids and tissue.
While this invention pertains to polymeric coatings for all rubber articles, it will focus
on gloves which are the most complex of rubber articles in terms of use and manufacture.
To achieve acceptable donning properties, the rubber surface of a glove which comes
in contact with skin or tissue has to be modified to reduce friction.
Surgeons gloves, as of today, require the donning surface to be suffioiently hydrophilic
in order to absorb moisture that may be present on the surface of skin or tissue when the
article is donned. Hydrogel co~ting~ as described for in~t~nce in U.S. Patent 3,813,695,
incorporated herein by reference, have been employed to achieve this property.
Ex~min~tion and other gloves, by contrast, do not have a hydrophilicity requirement
but still require the ability of the rubber article to be slid over skin (tissue) surfaces with
minim~l drag or friction.
Traditionally, this had been achieved by applying talc or other powdered materials,
such as modified corn starch, over the skin or tissue contacting surface. Talc can no longer
be used and other powders can cont~min~t--. the field of work. The same applies for gloves
used by workers in dust-free environments such as the manufacture of computer chips and
other electronic articles.
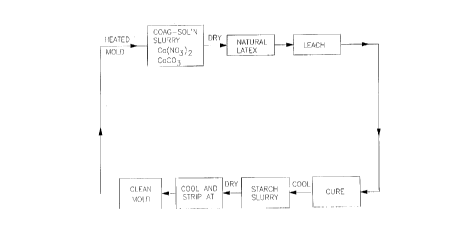
With reference to FIG. 1, the conventional way of manufacturing rubber gloves has
been to dip a mold in the shape of the article to be formed in a coagulant slurry containing
calcium nitrate and calcium carbonate. After drying, the mold is immersed in a rubber
emulsion (latex) for a time sufficient for the rubber to coagulate and form a coating of
desired thickness. The formed coagulated rubber article is oven cured, cooled, then dipped
into a starch slurry. The starch coated surface is dried to provide a powder coat. After
CA 02212861 1997-08-13
W 096/25278 PCTrUS96/01866
cooling, the rubber article is stripped from the mold. This turns the glove inside out. The
mold is cleaned and recycled.
Water leaching may and usually is employed as part of the procedure to remove rubber
impurities.
The methods and materials of glove manufacture are also described, for instance, in
U.S. Patents 3,411,982 and 3,286,011 to Kavalier et al., both incorporated herein by
reference, "Polyurethane Latexes for Coagulation Dipping," Sadowski et al., Elastomerics,
August 1979, pp. 17-20, incorporated herein by reference, and "Dipping with Natural
Rubber Latex," Pendle et al., Natural Rubber Technical Bulletin, also incorporated herein
by reference.
Halogenation, e.g. chlorination, and other chemical surface treatments have been used
to Plimin~t~ the need for a powder coat on the final product to enable dry donning. While
effective, this step is expensive and has the shortcoming of reducing shelf life of the rubber
article. It would be desirable to provide a rubber article with a powder-free donning surface
without resorting to the expensive and article deteriorating practices now in vogue. This
could substantially reduce the cost of manufacture and m~imi7e the shelf life of the rubber
article.
U.S. Patent 4,302,852 to Joung, incorporated herein by reference, proposed to
covalently bond an RTV silicone coating to the interior surface of a rubber surgeons glove
after formation of the glove. This is stated to reduce but not çlimin~tt- the need for a
donning powder.
U.S. Patent 4,304,008 also to Joung and incol~oldted herein by reference, applies a
covalently bonded silicone or urethane to the outer surface of the glove and halogenates the
inner surface. The halogenated inner surface çlimin~tes the need for a donning powder.
U.S. Patent 4,310,928, also to Joung and incol~oldted herein by reference, teaches
the deposition of a lipo compound (lipid or lipophilic substances) in place of a powder of
mineral origin in combination with a surfactant in a coagulant solution to form a uniform film
on a glove mold onto which the rubber is coagulated. The lipo compound and surfactant
enable stripping of a formed glove from its mold.
These and other proposals have not achieved commercial acceptance.
Summarv of the Invention
According to the present invention there are provided acrylic-based copolymers which
coat and firmly adhere to and may, in part, be absorbed on the surface of a rubber article
undergoing formation thereon by coagulation. The copolymers may also be deposited on a
preformed rubber article. The copolymers, as to gloves and other rubber articles, are
effective to induce the properties of mold stripping to the formed rubber article and donning
without the need for further chemical treatment. Dry or wet donning and mold stripping
properties may be achieved by depositing the same or a different acrylic based copolymer
on opposed surfaces of the formed rubber article, e.g., gloves.
-2 -
-
CA 02212861 1997-08-13
W O 96/2~278 PCTrUS96/01866
The acrylic based copolymers are preferably emulsion based copolymers of at least one
reactive low surface energy monomer, preferably a silicone oligomer, at least one alkyl
acrylate and at least one reactive hard monomer, the total of hard monomers being present
in an amount sufficient to form a non-tacky copolymer directly or by blending ofcopolymers. Preferably the copolymers exhibited at least one dominant glass transition
temperature (Tg) peak above O ~ C and typically at least one dominant glass transition
temperature peak below about O~C. One effective glass transition ~e~ eldture is generally
above about 15~C, preferably from about 15 to about 60~C. It is presently preferred that
at least the donning surface be formed by sequential polymerization of two monomer
systems; one providing the dominant low glass transition temperature peak, the other
providing the dominant high glass transition temperature peak.
The presently L,l~felled copolymers for release from a mold are copolymers
comprising copolymerizable silicone oligomers, butyl acrylate, methyl acrylate, methacrylic
acid, acrylic acid and styrene.
The presently l,lefellc~d copolymers for dry and wet donning are copolymers
comprising a silicone oligomer, styrene, butyl acrylate, methyl acrylate, acrylic acid,
trimethylpropanetriacrylate and n-isobutoxymethyl acrylamide.
The emulsion copolymers are, for efficiency of the emulsion polymeri7~tion process,
produced as high solids content emulsions. High solids are not required for product coating.
Accordingly, the emulsions may be diluted to form a solids sllcpçncion containing from about
3 to about 10% by weight preferably about 6% by weight total of the acrylic based emulsion
copolymer of a solids content typically employed for mold coating and for depositing a
coating on the surface of a formed rubber article. In the alternative the acrylic based
emulsion copolymer can be recovered from emulsion and later resuspended.
The suspension is used in combination with a water soluble multivalent metal salt
which serves as a coagulant for the rubber emulsion. The coagulant may be deposited on
the acrylic based copolymer or deposited therewith from suspension. The object is to
provide a surface concentration of coagulant salt which will enable coagulation of the latex
onto the deposited copolymer coat in a commercially acceptable time. The ~lef~lled
coagulant salt is calcium nitrate and is used in a concentration up to about 40, preferably
from about 20 to about 40 percent by weight of the suspension.
In article manufacture, in particular glove manufacture, the mold having a coagulant
and polymer coated surface is immersed into a rubber emulsion or latex from which the
rubber deposits and coagulates on the surface of the copolymer forming a coated interior
glove surface. The formed rubber article is dried, cured, then immersed into an aqueous
suspension of the same or a different acrylic based copolymer which exhibits good donning
characteristics. Dipping forms a donning polymer coat on the exterior of the rubber glove.
The formed coated glove is then stripped from the mold. This reverses the glove placing the
donning coat on the interior of the glove. As is known, water leaching may be effectively
employed for rubber purification.
-3--
CA 02212861 1997-08-13
W 096/25278 PCTrUS96/01866
In the practice of the invention the plc:f~ d mold is a contoured mold. While molds
having a textured to highly polished ceramic or porcelain surface and molds having a
fluorocarbon coating may be employed, it is pl~fellc~d to employ a mold which is sufficiently
textured to produce a dull finish in the deposited l~min~te formed by depositing the rubber
from the latex onto the copolymer coating. This is achieved by rollghening the mold surface
by blasting with sand or glass beads. The surfaces used have been measured to have a
roughness of from about 8 to 10 microns peak to valley.
It is preferred to use the same copolymer for the donning and release surfaces of the
gloves with the donning side containing corn starch and a small amount of oil, i.e., about
0.1% by weight of solids.
The lJlert;ll~d copolymers have a surface friction which requires an average force of
about 0.05 lb to less than about 0.3 lb, preferably about 0.2 to about 0.25 lb to move a sled
weighing 200 g over the copolymer coated surface of the rubber article.
Brief Dese. ;~lion of the Drawin~J~
FIG. 1 is a flow diagram of the current method of rubber glove manufacture;
FIG. 2 is a Differential Scanning Calorimetry (DSC) plot of derivative of heat flow
vs. temperature showing glass transition temperature peaks for the plcft;ll~d mold release
copolymer coating of the invention; and
FIG. 3 is a Differential Ss~nnin~ Calorimetry plot of derivative of heat flow asfunction of lel,lp~lature showing glass transition te~peld~LIre peaks for the ~l~relled donning
copolymers of the invention.
Detailed Des~ tion
According to the present invention, there are provided non-tacky, acrylic based
copolymers which adhere aggressively to the surface of rubber and other articles to provide
flexible copolymeric co~ting~ which can be stretched without separation from a rubber
surface to which they are bonded. The copolymers are formed of at least one reactive low
surface energy monomer, preferably one or more copolymerizable silicone oligomers, at least
one alkyl acrylate and at least one hard monomer. The copolymers provide powder-free
coated rubber products which exhibit excellent mold stripping and dry donning and provide
protective coatings for other articles.
By the term "rubber article" as used herein, there is meant articles formed fromnatural and/or synthetic rubbers. They are typically deposited by coagulation from a latex
onto a multivalent metal salt.
By the term "copolymerizable low surface energy monomer" there is meant monomerswhich if homopolymerized would require only a low level of force to release the
homopolymer from a surface to which it was applied. In copolymers the copolymerizable
low surface energy monomers reduce the energy to release the copolymers from a surface
whether a mold, tissue or skin.
Exemplary of copolymerizable low surface energy monomers there may be mentioned
reactive silicones, fluorocarbons, and fatty acid esters and the like, the low surface energy
-4-
CA 02212861 1997-08-13
W O 96/25278 PCTrUS96/01866
monomers having vinyl, acrylic and/or meth~crylic functionalities. Preferred arecopolymerizable silicone oligomers.
By the term "copolymerizable silicone oligomer," as used herein, there is meant
polymeric siloxanes and silicones which have acrylate, methacrylate or vinyl functionality,
including but not limited to acrylated polysiloxanes. Acrylate, methacrylate or vinyl
functionality is at least l, preferably from 2 to about 3.
Exemplary of the copolymerizable silicone oligomers there may be mentioned the
Tegro~ Silicone acrylates RC 149, 300, 450, 710, 720 and 802 and ZMR1395 manufactured
and sold by Goldschmidt Chemical Corporation of Hopewell, Virginia, which polymers are
linear dimethylpolysiloxanes with multiacrylate functionality and a molecular weight between
about 1,000 and 20,000 g/mol. They include the reaction product of dimethylpolysiloxanes
with pentaerythritoltriacrylate. There may also be mentioned silicone systems such as GE
6000, a vinyl polydimethyl siloxane and 6010 catalyst concentrate manufactured and sold by
GE Silicones division of General Electric Company. Ethoxy-substituted polysiloxanes may
lS also be used.
The copolymerizable low surface energy monomer(s) and preferably the
copolymerizable silicone oligomer are employed in a concentration of from about 0.7 to
about 20%, preferably about 1 to about 15% by weight of the monomers forming the acrylic
based copolymer. They function to enable dry and wet donning and f~rilit~tP mold release.
The balance of the monomers are SPl~Pctp-d to provide good bonding to a latex surface,
donning (wet and dry), a good tactile feel, mold release and a non-tacky copolymer having
a ~ignifi~nt glass transition te,-,p~.dture (Tg) of at least about 15~C, preferably from about
15 to about 60~C. The monomers are also selected to provide sllffi~ient elongation so that
the acrylic based copolymer coating will stretch or elongate with the rubber with minim~l
cracking, flaking or debonding. Suitable copolymers have an elongation of 100 to 500% or
more, typically from about lO0 to about 300% when self bonded to a rubber sllrf~re
Of the rubber articles which can be manufactured in accordance with the instant
invention, the articles and surgeons which have the most critical requirements are
e~min~tion and surgeons gloves. Rec~lse of their complex shape, they must be capable of
being stripped from a mold using commercially acceptable practices and yet provide, a
surface, which when stripped from the mold, has good tactile feel; that is, the ability of a
person to pick up articles with a good grip. In this regard, good tactile feel is contra to good
mold stripping.
The opposed surface of the glove must enable good (dry or wet) donning, that is the
ability to be stretched and slid over the surface of skin without excessive resistance.
The tactile and mold release surface may be glassy and smooth but is preferably rough
and dull as induced by a textured or roughened mold. The donning surface normally requires
a more course or irregular (dull) surface.
In respect of glove manufacture, copolymers of the instant invention provide good
mold stripping and/or donning properties.
-5 -
CA 02212861 1997-08-13
W 096/25278 PCTrUS96/01866
In addition to the low surface energy monomers, the following monomers and
combination thereof can be used in accordance to the invention to provide suitable polymers
having good donning and/or stripping characteristics.
One class of the monomers used in forming the copolymers are alkyl acrylate
monomers cont~ining from 1 to about 10 carbon atoms in the alkyl group, present in a total
amount of from about 30 to about 85 % by weight of the monomers, preferably from about
40 to about 85% by weight of the monomers forming the acrylic based copolymers. The
alkyl acrylate monomers that may be used include methyl acrylate, ethyl acrylate, butyl
acrylate, propyl acrylate, 2-ethylhexyl acrylate, isooctyl acrylate, isodecyl acrylate, and the
like. The presently preferred alkyl acrylate monomers are butyl acrylate and methyl acrylate.
The balance of the monomer system is comprised of hard monomers. As used herein,"hard monomers" are monomers which, if homopolymPri7P~, would have glass transition
te.nL~ ture (Tg) greater than about 25~C. Hard monomer content is from about 20 to about
60% by weight of the monomers forming the acrylic based copolymer.
Among such monomers there may be mentioned styrenic monomers such a styrene,
alpha methyl styrene and the like; alkyl mPth~rylates such as methyl methacrylate, ethyl
methacrylate, butyl methacrylate and the like; and amides such as an n-isobutoxymethyl
acrylamide and the like.
There may be present and preferably are present one or more unsaturated carboxylic
acids containing from 3 to about 5 carbon atoms, such as acrylic acid, methacrylic acid,
itaconic acid and the like. They serve to improve cohesive strength and promote adhesion
to rubber and other surfaces and are present in a concentration of from about 1 to about 6%
by weight preferably from about 2 to about 6% by weight of the emulsion copolymer.
Other vinyl unsaturated monomers which aggressively copolymerize with the principal
monomers of the invention and do not create a residual monomer cont~min~tion problem may
also be used to modify polymer pr~llies.
Such monomers include one or more vinyl esters c~ ;.hlh-~ from 2 to about 16 carbon
atoms in the alkyl group of the acid. R~-~ser.t~live vinyl esters include vinyl acetate, vinyl
butyrate, vinyl propionate, vinyl isobutyrate, vinyl valerate, vinyl versitate, and the like.
Other useful monomers there may be mentioned diesters of dicarboxylic acids and
mixtures thereof, in which each ester group of the diester independently contains from about
8 to about 16, preferably from about 8 to about 12, carbon atoms. The plefell~d diesters
are di-2-ethylhexyl maleate (dioctyl maleate), di-2-ethylhexyl fumarate and mixtures thereof.
The emulsion copolymers may be prepared in accordance with the present inventionby polymerizing the monomers to yield a polymer having a suitable average or net glass
transition temperature (Tg) above about 15~C, preferably from about 15~C to about 60~C,
and a solids content ranging from about 40 to about 70% by weight of the emulsion.
Catalysts, such as potassium persulfate, tertiary butyl hydroperoxide and the like, and Redox
catalysts such as sodium meta-bisulfite and the like, present in an amount of from about 0.15
to about 0.5 part by weight per 100 parts weight monomers with surfactant levels ranging
--6--
CA 02212861 1997-08-13
W 096/25278 PCTrUS96/01866
from about 0.5 to about 5% by weight based on weight of the monomers being L,ler~lred.
Reaction temperature generally ranges from about 65 to about 85~C.
There may be included "chain transfer agents" by which there is meant organic
compounds containing mono- or multi-merca~L~n groups, chlorinated groups, hydroxy
groups, and the like, as are known in the art. The presently plef~lled chain transfer agents
are n-dodecyl melca~ and t-dodecyl mercaptan provided in a concentr~tinn from about
0 01 to about 0.1% by weight of the monomers. In addition, internal cross linking may be
induced by the use of multifunctional acrylates and methacrylates.
While the copolymers prepared in accordance with the instant invention can be used
for a variety of rubber article applications, including gloves, catheters, tubing, protective
coverings, prophylactics, and the like, the primary focus is their use in the manufacture of
powder-free gloves and other articles.
With reference again to FIG. 1, a mold in a contoured shape of a glove is coated with
an acrylic based copolymer of this invention which exhibits good mold stripping ~lup~llies.
The copolymer coating is preferably applied from suspension in combination with a
coagulating electrolyte. The steps of copolymer coating onto a mold for transfer to a latex
rubber replaces the step of depositing a slurry of calcium carbonate on the mold surface
while depositing the copolymer on a formed article replaces coating the article with a starch
slurry. The copolymer is applied from suspension. The article is formed by deposition onto
a coagulating salt such as calcium nitrate provided on a mold, on a copolymer coating on the
mold or codeposited with the copolymer. For gloves, the copolymer coated mold providing
a coagulant salt surface, preferably codeposited with the copolymer, is emersed into a rubber
latex which coagulates onto the surface of the mold providing shape to the rubber article,
dried, cured and then immersed into a second suspension of the same or another acrylic
based copolymer which exhibits excellent donning p~ ies i.e. the ability to be slid over
a skin surface with minim~l friction and blocking. T ~rhing is employed as in the prior art
either before or after cure or prior to after providing a donning coat. The formed article is
then stripped from the mold. The mold is cleaned and recycled. Stripping turns the glove
inside out causing the donning surface to be on the inside of the glove and the mold stripping
surface to be outside of the glove.
For glove manufacture, it is presently prerellc~d to use a roughened mold as formed
by blasting with sand or glass beads to provide a rollghene~l surface which as been measured
as having a ro~lghne~ of about 8 to l0 microns peak to valley. Other textures may also be
used.
The emulsion copolymers of the instant invention may be used as such or combinedwith inert granular solids such as calcium carbonate, starch and the like provided to enh~nçe
donning. Inert solids content may range in an amount based on total solids weight of from
about 3 to about 10% by weight of solids. It is presently ~l~f~ d to employ corn starch
in an amount of from about 0.3 to about 0.7, more preferably from about 0.3 to about 0.5
part per part by weight copolymer. A suitable corn starch is 400 L-NF corn starch made by
-7 -
CA 02212861 1997-08-13
W 096/25278 PCTrUS96/01866
Roquetle Americ~, Inc., Keokuk, Iowa. The donning coat also preferably contains a small
amount, about 0.1% by weight copolymer, of an oil such as octyl isononanoate or Neobee
M-20, a polyol diester of a short cleaned fatty acid manufactured by Stepan Chemical Co.,
Northfield, Illinois.
It has been found that for good donning and stripping properties, each of the
copolymer co~tingC are preferably formed of at least a two polymers. One is a copolymer
having a low glass transition temperature, and the other a copolymer having a high glass
transition temperature. The two, in combination, provide a non-tacky copolymer having at
least one significant glass transition temperature of about 15~C or more and preferably in a
range of about lS to about 60~C.
While this may be achieved by using a blend of polymers, it is preferably achieved
by sequential or "core and shell" polymerization of at least two monomer systems, the first
forming an acrylic based copolymer having a glass transition temperature of less than about
25~C, typically from about -50 to about 25~C; the other forming an acrylic based copolymer
having a glass transition temperature of from about 25 to about 100~C, the first and second
monomer systems providing no tacky copolymer coating systems. It has been observed that
for a copolymer exhibiting good mold release, the copolymer having glass transition
temperatures below O~C occupies the bulk of the total glass transition temperatures. The
opposite may be true for a donning copolymer.
In particular, FIG. 2 is a plot of glass transition temperature of a sequential
polymPri7e~1 mixture of copolymers with a major glass transition temperature peak below 0~C
and a minor glass transition te-llpeldlllre peak above 0~C for use as a mold release copolymer
coating. While, not bound by theory in this "core and shell" approach to sequential
polymçri7~tion of two monomer systems, it is believed there is formed a continuous phase
of the low glass transition Lellll)eldtule copolymer having dispersed therein or thereon, the
high glass transition leu~peldture copolymer.
FIG. 3 is a glass transition te---~ eldture plot for a good donning copolymer coating
where relative peak intPncities of glass transition temperature are reversed.
For good mold release characteristics, it is presently ~lerelled that weight ratio of the
soft or low glass transition temperature copolymer to high glass transition temperature
copolymer be in the range of about 1:1, to about 1:3, preferably about 1 to 1.5.For good donning characteristics, the weight ratio of low to high glass transition
temperature copolymers is in the range of about 3:1, to about 1:1, preferably about 1.2 to
1.
It has been observed that if too much high Tg copolymer is present, flaking will occur.
In addition, for good dry donning, it is desirable that the copolymer form as domains or
microparticles. This provides in essence, a rough surface which is desirable for good
donning and fillers such as calcium chloride may be included in the suspension. If too much
low glass transition te---peldt~lre copolymer is employed, there is a problem of sticking or
blocking.
-8-
CA 02212861 1997-08-13
W 096/25278 PCTJUS96/01866
As indicated above, for the process of copolymer manufacture it is desirable to employ
a surfactant system present in the amount of from about 0.5 to about 5 parts by weight to 100
parts by weight monomers, preferably about 3 parts per 100 parts by weight monomers. It
is also desirable that the suspension formed from the emulsion copolymer tolerate electrolytes
S typically employed for latex coagulation in concentration typically used for latex coagulation,
i.e., in the range of about 3 to about 10% by weight of suspension. The presently preferred
surfactant system is a combination of anionic surfactants. One of the anionic surfactants has
a molecular formula C20H3707NaS and containing 20 mols of ethylene oxide and is used in
admixture with anionic surfactants which are salts of sulfated nonyl phenoxypoly(ethyleneoxy) ethanol and sodium lauryl ether sulfate. The presently preferred surfactant
system is one employing 37.4% by weight of the ammonia salt of sulfated nonylphenoxypoly
(ethyleneoxy) ethanol, 21.8% by weight of sodium dioctyl sulfosuccinate and 40.8% by
weight sodium lauryl ether sulfate. The combination of anionic surfactants enables the
formation of a stable sllspencion of the acrylic based emulsion copolymers of the invention.
The emulsion copolymers of the instant invention can be prepared to provide both a
high cohesive strength and holding power to natural and synthetic rubber surfaces as well as
the ability to stretch with the rubber surfaces and enable donning. The copolymers have an
elongation of 100 to 500%, preferably 100 to 300% when adhered to rubber and a mean
coefficient of friction from about 0.05 to about 0.3 lb typically from about 0.2 to about 0.25
lb. Copolymer coating thi~knesc is from about 10 to about 25 microns, preferably from
about 12 to 16 microns.
The inc.lllcion of multifunctional monomers such as tetramethylpropane triacrylate and
the like, which undergo cross linking reactions and chain transfer agents as part of the
monomer system results in formation of internally cross-linked emulsion polymers. This
differs from externally cross-linked polymer in that the functional groups, such as carboxyl,
hydroxyl, and/or amino groups, remain free and available for improving bonding and are
available for external cross-linking reactions such as by exposure to actinic, electron beam
radiation and/or through external cross-linking agents.
The emulsion polymers can be used as such or modified by the addition of
vinyl-addition silicone polymers present in an amount up to about 30% by weight based on
the weight of the emulsion polymer and vinyl-addition silicone system. The vinyl-addition
silicone systems used are those which comprise silicone monomers having vinyl unsaturation
which, when mixed with silicone hydride containing crosslinkers, is cured by a Group VIII
metal catalyst, preferably a platinum catalyst.
The presently preferred vinyl-addition silicone emulsions are mixtures of reactive vinyl
silicone polymers of the formulas:
_
CA 02212861 1997-08-13
W 096125278 PCTrUS96/01866
1 CH3 IH3 IH3 IH3
l.CH2 CH - Si -O- (SiO)m - (;Sio)n Si - CH = CH~
CH3 CH3 CH CH3
S CH2
where m and n are independent integers, and silicone hydride cros~linking polymers of the
formula;
H3 IH3 IH3 IH3
0 2.CH3 - Si -0- (SiO)m - (SiO)p - Si-CH3,
CH3 CH3 H CH3
where m and p are independent integers.
There may also be included conventional ingredients decigned to modify mold release
properties.
Vinyl-addition silicone systems react by thermally in~ ced addition-cure (hydrosilation)
between polydimethyl-hydrogen siloxane crosslinkPrs and reactive vinyl-functional silicone
polymers to furnish a cured polymer.
The vinyl-functional silicone polymers are polydimethyl siloxanes, where some of the
methyl groups have been substituted with vinyl groups or groups cont~ining vinylunsaturation, i.e., the reaction takes place between vinyl substituted polydimethyl siloxane
and polydimethylhydrogen siloxane.
The whole hydrosilation is catalyzed by silicone soluble complex compounds of Group
VIII transition metals, particularly platinum. In normal use of vinyl-addition silicone
systems, a small amount of inhibitor is added to prevent ~ ul~ reaction between the
silicone hydride and vinyl silicone groups following mixing of the coating components,
before deposition onto the substrate. This inhibitor is removed or made ineffectual during
the thermal curing process. Suitable silicone emulsion systems for practice of this invention
can be obtained from Dow Corning, Rhone-Poulenc and Wacker-Chemic GmbH, e.g., the
Wacker VP 1571E/1572 System.
Emulsion copolymers of the instant invention are formed to high solids content during
emulsion polymerization for efficiency of the emulsion polymerization process. They are
normally diluted to form a su~pen~ion having a less solids content for use coating onto a
mold for the rubber article to be formed or onto the formed rubber article. As is known in
the art typical solids content is in the range of about 3 to about 10% by weight of suspension.
A coagulant salt is required to cause the rubber to deposit from its emulsion (latex)
onto a surface of the polymer. The coagulant may be applied after drying of the copolymer
on the surface of the mold, a considerable savings in time and cost can be realized by
combining a coagulant with the copolymer suspension. It has surprisingly been found that
the copolymer suspensions of the instant invention can tolerate the high amount of polyvalent
-10-
CA 02212861 1997-08-13
W 096125278 PCTrUS96/01866
metal salts which serve as a coagulant if the multi-component anionic surfactant system is
employed. Examples of coagulants that can be used are water soluble salts of calcium, zinc,
aluminum and the like. Calcium nitrate is presently pl~ft;li~d. A coagulant salts, preferably
calcium nitrate, is normally provided in a concentration of up to about 40% by weight of the
suspension typically from about 20 to about 40% by weight of suspension for mold coating.
Combining the coagulant with the emulsion polymers of the instant invention elimin~t~-s a
significant step in the production of copolymer coated rubber articles. If the copolymer is
applied without coagulant, then the coagulant is applied to the surface of the copolymer after
the copolymer has been deposited and dried on the surface of the mold. This adds a step and
is, therefore, a more expensive measure.
The presently plefelled molds are smooth contoured molds having a textured, or
smooth ceramic, porcelain or a fluorocarbon surface which will accept the coating of the
copolymer or the copolymer and coagulant and release the formed rubber article at the
completion of the process.
The amount of time of immersion in the rubber emulsion determines the thickness of
the formed rubber coat. A typical thickness is from about 6 to about 10 mils.
After removal, the coagulated rubber articles may be allowed to stand or heat dried
in an oven. The formed rubber article is then leached in water. The leached rubber coating
is then cured.
After cure, the rubber coated copolymer is allowed to cool to about 60 to 80~C and
then dipped into a second acrylic based emulsion copolymer suspension preferably free of
coagulant to avoid the waste of salt by need for another le~hing step. Immersion is for
about 3 to about 6 seconds. The coating is dried at about 100 to 125~C for about 2 to 3
minutes.
The double coated article is then cooled to about 40~C and stripped from the mold.
Because stripping turns the glove inside out, the second coating becomes the donning coating
for contact with tissue or skin while the exterior or outside coating which was the stripping
coating becomes the tactile coating.
While the focus of the discussion has been directed to copolymer co~tingc for rubber
articles and in particular gloves, the copolymers have been found to have other utilitiPs, and
in particular, coatings for what is known as "soft touch" products for use in the automobile
industry. An example is to provide polymeric l~min~tes having a leather look with a feel to
match.
The copolymers of the instant invention have been successfully evaluated for use in
vacuum formable l~min~tes. The product construction would consist of a polyester film
acting as a carrier which is removed prior to vacuum forming. The copolymers of the instant
invention are deposited on the carrier at about 0.25 to about 2 mils in thickness to save as
a clear or pigmented top coat and as the surface that is felt by the user. The balance consists
of material deposited to give the appearance of leather, wood grain or the like, as the layer
of the copolymer of the instant invention adds or insures the "soft touch". There is then
-11-
CA 02212861 1997-08-13
W O 96/25278 PCTrUS96/01866
added a layer of heat activated adhesive followed by heat and pressure bonding to a 20 mil
sheet of polymeric material compatible with the injection molding plastic. The polyester
carrier is then removed and the l~min~te iS vacuum formed and insert molded to give a
contoured plastic part with a unique and desirable soft feel.
The copolymers of the instant invention could also be used as a spray coating or could
be deposited on a backing having a pressure-sensitive adhesive on the opposite side. The
copolymers provide protective and unusual "soft touch" performance ~lup~l Lies. In either
application, the polymer is believed to have unusual exterior performance properties due to
containing silicone and acrylic monomers.
EXAMPLE 1 - EMULSION COPOLYMER FOR MOLD STRIPPING.
There was formed on a parts by weight basis a first soap solution of 98.2 parts by
weight deionized water, 0.87 parts tekasodium pyrophosphate 10.9 parts by weight Aerosol~
NPES 458, a 58% solution of the ammonium salt of sulfated nonylpoly (ethyleneoxy) ethanol
manufactured by Cyanamid, 4.9 parts AerosoFU OT 75, a 75% solution of sodium dioctyl
sulfonate and 3 parts Disponil FE S77 a 32.5% solution of sodium lauryl ether sulfate
manufactured by Henkel and, a second soap solution cont~ining 82.2 parts deionized water,
0.73 parts tetrasodium pyrophosphate, 9.1 parts NPES 450, 4.1 parts OT 75 and 17.8 parts
FES 77 surfactants.
There were formed two separate monomer systems. The first monomer system
contained 39.6 parts styrene, 318 parts butyl acrylate, 50 parts methyl acrylate, 3.8 parts
methacrylic acid, 3.8 parts acrylic acid, 71.3 parts RC 726, a silicone acrylate manufactured
and sold by Go~ chmi(lt 30 parts GE silicone SL 6000-Dl and 23.5 parts GE silicone SL
6010-Dl .
The second monomer system contained 210 parts styrene, and 102.9 parts butyl
acrylate, 43.9 parts methyl acrylate, 3 parts meth~rrylic acid, 2.7 parts acrylic acid, and 56
parts GE silicone SL 6000-Dl.
There was s~aldl~ly formed a catalyst solution cont~ining 78 parts of deionized water
and 2 parts potassium persulfate, and an activator solution cont~ining 78.5 parts by weight
deionized water and 1.5 parts of sodium metabisulfite. There was formed an initial reactor
charge containing 320 parts deionized water, 0.08 parts of NPES 458, 4.5 parts of sodium
bicarbonate and 3.85 parts potassium persulfate and 0.15 parts sodium meta-bisulfate. To
the initial reactor charge there was slowly added, with agitation, the mixture of the first
monomers in the first soap solution followed by incremental addition of the activator solution
then incremental addition of the catalyst. Reactor temperature was m~int~ined between 65
and 73~C. Following completion of the reaction there was added the second soap solution
cont~ining the second charge of monomers incrementally. After completion of reaction were
treated with an ammonium and biocide, solutions water. The emulsion had a pH of 8.7 and
a solids content of 57%.
EXAMPLE 2 - EMULSION COPOLYMER FOR DRY DONNING.
A procedure of Example 1 was substantially repeated except that the monomers forthe first charge were in proportion of 95 parts styrene, 220.7 parts butyl acrylate, 38.8 parts
-12-
CA 02212861 1997-08-13
W 096125278 PCTrUS96/01866
methyl acrylate, 9.8 parts acrylic acid, 15 parts GE SL 6000-Dl, 9 parts
trimethylpropane-triacrylate and 3.3 parts n-isobutoxymethylacrylamide.
For the second monomer charge, there was employed a monomer mix of 302.3 parts
styrene, 146.2 parts butyl acrylate, 57.9 parts methyl acrylate, 17.5 parts acrylic acid, 40.4
parts GE SL 6000-Dl, 20 parts trimethylpropane triacrylate and 4.48 parts
N-isobutoxymethyl acrylamide, the incremental catalyst solution contained 70.5 parts
deionized water, and 2.5 parts of potassium persulfate. To the first soap solution contained
6.91 parts NPES 458, 3.3 parts OT 75 and 14.7 parts FES 77 and the first monomers were
added a to the initial reactor charge followed by incremental addition of the catalyst solution
and after completion of reaction, there was incrementally added second monomer system in
a soap solution containing 4.9 parts OT-75, 10.29 parts NPES 458 and 21.89 parts FES 77.
Temperature was m~int~ined at 78 to 85~C and after treatment with the ammonium biocide
solution, the emulsion had a pH of 6.6 and a total solid content of 57.6%.
EXAMPLE 3 - GLOVE MANUFACTURE.
The copolymer of Example 1 and ç~lcillm nitrate as a coagulant were coated onto a
mold for an eY~min~tinn glove. The coated mold was immersed into a latex solution and
allowed to remain in the solution until a coat of 6 to 10 mils built up on the coating. The
coating was then immersed in a solids s~-spencic~n of the copolymer of Example 2. After
drying the coating of the copolymer of Example 2, the glove was stripped from the mold.
The glove formed was fisheye free and had a shiny outside of post cure coating of the
copolymer of Example 1 and a dull side of the coating of the copolymer of Example 2. The
copolymers of Examples 1 and 2 were strongly bonded to the latex and the formed glove had
excellent dry and wet donning properties for use as an e~min~tion glove. No flaking
occurred when the glove was stretched. FIG. 3 shows the DSC profile for the copolymer
with major glass transitive temperature peaks at -21.04~C, 11.74~C + 12.45 and +28.55~C.
Surface morphology revealed a slightly irregular continuous surface with microcraters and
submicron protrusions, the microcrater rli~meters ranging from 0.1 to 1 ~L. FIG. 4 shows
the DSC plot for this polymer and the several glass transition peaks, the major ones being
at - 12.51 ~ C, 13.57~C and 29.76~ C. Morphology studies revealed a slightly irregular surface
with submicron protrusions, shallow submicron craters and agglomerated particle protrusions
and microcraters.
EXAMPLE 4 - EMULSION COPQLYMER FOR DRY DONN~G.
There was formed a surfactant solution comprising, on a parts by weight basis, 122.70
parts deionized water, 1.60 parts tetrasodium pyrophosphate (0.96 parts dry), 31.27 parts
surfactant (21.56 parts dry) .
There was separately formed a monomer mixture cont~ining on a parts by weight base
is 100 parts styrene, 405.12 parts butyl acrylate, 37.42 parts methyl acrylate, 6.5 parts
methacrylic acid, 12.30 parts acrylic acid, 6.25 parts RC 705, a silicone acrylate sold by
Goldschmidt and 0.08 parts n-dodecyl mel.;a~n. The monomer mixture was added to the
surfactant solution in a weight ratio of 4 parts monomer to 1 part surfactant solution.
CA 022l286l l997-08-l3
W 096125278 PCTrUS96/01866
There was separately formed an incremental catalyst solution of 63 parts by weight
deionized water and 2 parts by weight potassium persulfate as well as a minor amount of
surfactant.
To a stirred, nitrogen bl~nkPtecl reactor, following the charge of 200 parts by weight
deionized water, 3.08 parts by weight surfactant and 1.5 parts potassium persulfate, there
was incrementally added the monomers and surfactant solution with the catalyst solution at
a rate to enable a reaction to be carried out with a slight exotherm. Temperature was kept
at 80+2~C. There was formed an emulsion having a total solids content of about 54.3%.
The emulsion was then adjusted to a pH of about 6.5 with a biocide/ammonia neutralizing
solution.
EXAMPLE 5 - EMULSION COPOLYMER FOR DRY DONN~G.
Example 4 was subst~nti~lly repeated except that the monomer mixture contained on
a parts by weight based 219.2 parts methyl methacrylate, 219.2 parts butyl acrylate, 51.33
parts meth~rylic acid, 13 parts n-isobutylmeth~rrylate, 16.66 parts silicone acrylate (RC
lS 705), and 0.13 parts n-dodecyl mercaptan and the surfactant solution was formed of 27.73
parts Aerosol NPES 930, 2.7 parts OT-75 and 24.9 parts FES-77 and contained 0.66 parts
sodium metabisulfite and 18.81 parts surfactant in 113.3 parts deionized water.
EXAMPLE 6 - GLOVE MAl~UFACTURE.
The emulsion copolymer of Example S was evaluated as a 4.8% solids suspension
contains 4.5% soap. It was used to coat a washed eY~min~tion glove. The deposited coat
gave very good dry donning and stripping char~c-teri~ti-s No flaking occurred.
EXAMPLE 7 - DRY DONN~G COPOLYMER AND GLOVE MANUFACTURE.
Following the procedure of Example 4, there was formed a copolymer containing
46.4% by weight styrene, 38% by weight butyl alkylate, 1.3% by weight methacrylic acid,
3.3% by weight acrylic acid, 8.5% by weight silicone acrylate, and 1.9% isobutoxymethyl
acrylamide. The formed copolymer was combined with, by weight of the copolymer; of
0.6% vegetable oil and providing a good donning copolymer for glove manufacture.EXAMPLE 8 - DRY DONN~G COPOLYMERS AND GLOVE MANUFACTIJRE.
There was formed a blend of copolymers in a weight ratio of 1 :3 of the copolymer of
Example S and a copolymer obtained by emulsion polymerization of 48.2% by weightstyrene, 33.1 % by weight butyl acrylate, 6.1 % by weight methyl acrylate, 1.1 % by weight
methacrylic acid, 2.1% by weight acrylic acid, 6.3% by weight silicone acrylate, 0.9% by
weight n-isobutoxymethyl acrylamide and 2.3 % by weight trimethylpropane triacrylate. The
blend of polymers was deposited onto a mold. Calcium nitrate was the coagulant. The
procedure of Example 3 was used to form a coating of the latex on the copolymer.EXAMPLE 9 - DRY DONN~G COPOLYMER AND GLOVE MANUFACTURE.
There was formed an emulsion copolymer from a monomer mix containing 43% by
weight styrene, 36.5% by weight butyl acrylate, 8.6% by weight methyl acrylate, 1% by
weight methacrylic acid, 1.9% by weight acrylic acid, 5.2% by weight silicone acrylate,
0.8 % by weight n-isobutoxymethyl acrylamide and 3 % by weight trimethylpropane
-14-
CA 02212861 1997-08-13
W 096/25278 PCTrUS96/01866
triacrylate. A suspension of the copolymer which was coated onto medium and large glove
molds, onto which there was formed using the procedure of Example 3, a rubber coat.
EXAMPLE 10 - FRICTIONAL MEASUREMENTS.
For purposes of del~l"linillg surface friction of the copolymer coating, there were
prepared samples 1" wide, over which there was drawn at a crosshead speed of 15 inches
per minute a 200 g sled cont~ining three glass balls having a diameter of 5/8" positioned at
the corners of an equilateral triangle. A force of about 0.112 lb. of reci~t~nce was required
to draw of the sled over a powdered surface. The force was 0.07 lb for a chlorinated surface
and a biogel surface. By contrast, untreated surfaces gave a resistance of about 0.33 and
0.34 lb with a tendency to lift from the surface onto which they were applied with a
considerable tendency toward jerky movement The composition of Example 7 required a
force of about 0.22 lb. The blend of polymers of Example 8 required a force of about 0.23
lb and the composition of Example 9, when deposited from a 6% solids suspension, required
a force of about 0.22 lb. Re~i~t~nce increased to about 0.225 lb. at an 8% solids
concentration. Measurements were made under conditions of 50% humidity and a
tellll~e,dture of 23~C.
EXAMPLE 11 - EMULSION COPOLYMER FOR PROTECTIVE COATING.
A procedure of Example 1 was substantially repeated except that the monomers forthe first charge were in plo~ollion of 150 parts styrene, 237 parts butyl acrylate, 22 parts
acrylic acid, 31 parts RC 726, 9 parts meth~rylic acid triacrylate and 8.7 partsN-isobutoxymethylacrylamide .
For the second monomer charge, there was employed a monomer mix of 348 parts
styrene, 136.6 parts butyl acrylate, 9 parts methylacrylic acid, 248 parts acrylic acid, 30.8
parts RC 726, 8.7 parts N-isobutoxymethyl acrylamide, the incremPnt~l catalyst solution
contained 70.5 parts deionized water, and 2.5 parts of potassium persulfate. There was
formed by sequential emulation polymPri7~fion emulsion of the copolymers which a total
solid content of 53.3. The formed copolymers provided aggressive protective coatings for
rubber surfaces.
EXAMPLE 12 - DONN~G COATING
The aqueous solution of the emulsion copolymer of Example l was combined with
calcium carbonate present in an amount of about 3.3% by weight of the emulsion as formed
which there was added water and a minor amount of propylene glycol dicaprylate. The
mixture formed an excellent composition for providing a wet and dry donning surface in a
glove.
EXAMPLE 13
~xample 12 was repeated except that starch was substituted for calcium carbonate.
Again the coating had excellent dry and wet donning properties.