Note: Descriptions are shown in the official language in which they were submitted.
CA 02214120 1997-08-28
W O96/267Sl PCT~US96/02176
T~OW FRICTION COATING FOR C~RTRIDGE SEAL CAP
BACKGROUND OF THE I~V~N-1 ION
Field of the Invention
This invention relates to a low friction coating for a
coupling system for transferring liquid medicaments from one flow
conduit to another and, more particularly, to a low friction
coating for a cartridge seal cap used in conjunction with
disposable liquid medicament-containing cartridge-needle units.
Re~orted ~evelo~ments
Disposable medicament-containing cartridge needle units are
well known in the art and in widespread commerclal use. Such
cartridges conventionally feature a cartridge barrel, formed of
glass or plastic having a distal end and a proximal end. The
proximal end of the cartridge is closed with a slideable plunger,
while the distal end has a neck portion which terminates in an
opening closed by a diaphragm or septum secured to the cartridge
by a crimped-on metal collar, usually aluminum collar. The neck
portion of the distal end is adapted to receive and engage a
needle-hub-needle-needle guard assembly. The needle-hub portion
snaps onto the diaphragm in a mating relationship. When the
needle hub is snapped on the cartridge, the proximal end of the
needle cannula pierces the diaphragm thereby providing
communication between said needle cannula and the liquid
medicament contained in the cartridge barrel. Such a needle-hub-
needle-needle guard assembly is shown, for example, in U.S. Patent
No. 5,358,491.
one of the major concerns relating to the delivery of liquid
pharmaceutical products via injection to patients is the
generation and presence of particulate foreign matter which may
contaminate such products. In order to eliminate macroscopic and
microscopic particulates, elaborate measures have been taken to
--1--
CA 02214120 1997-08-28
W O96/26751 PCT~US96/02176
remove them, such as filtration of the product and special washing
and drying of the closure-system components. These steps help
assure that the products meet the requirements and guidelines of
the pharmaceutical industry, such as compendia guidelines, when
the products reach the point of use. However, at the point of use
new particulate matter is frequently generated by the practitioner
when the septum is penetrated by the needle of the injection
assembly. During such penetration a combination of elastic and
plastic deformation of the target area increases the septum
contact surface with the needle as the needle is pressed into the
septum.. Typically, untreated elastomer septums offer a high
resistance against the exterior surface of the needle as the
needle is being inserted into the penetration area. Most
frequently, when septum fragments are generated, they are the
result of the elastomeric portion of the septum being abraded off
the upper surface of the septum as it conforms to the shape of the
needle. The fragments then either enter into the needle cannula
or the interior of the cartridge as the needle drags the fragments
during penetration.
The most common solution to the problem of particulate matter
generation has been the application of silicone lubricant to the
septum to reduce the frictional drag between the septum and the
needle. While silicone does reduce generation of particulate
matter, it also increases the ris~ of product contamination from
its own composition.
Another approach proposed in the prior art to reduce the
tendency of the stopper to generate particulate matter is to coat
the elastomeric core of the septum with a thermoplastic film on
the fluid contacting side thereof.
Still another approach was proposed in U.S. Patent No.
5,219,083 to eliminate or greatly reduce particle generation from
the septum during needle penetration, by applying an inert,
abrasion -resistant coating to the proximal surface of the
stopper, i.e., to the surface of the septurn which has no contact
CA 02214120 1997-08-28
W 096J26751 PCTAUS96/02176
with the liquid pharmaceutical contained in the cartridge.
While these approaches greatly reduce the generation and
presence of particulate matter originating from the septum during
the penetration process, we have observed the presence of
particulates originating from the crimped-on metal collar holding
the septum in place in the cartridge. Such particulates are
generated during the process of making the collar and the crimping
process. Similarly to the result of generation of particulate
matter originating from the septum, the particulate matter
originating from the metal collar may get into the needle cannula
causing clogging or delivery of a liquid pharmaceutical
contaminated with metallic particles into the patient.
One of the objects of the present invention is to eliminate
or at least greatly reduce the tendency of this occurrence.
Another object of the present invention relates to the
reduction of activating force needed to activate the needle-hub-
needle-needle guard assembly when the cartridge content is to be
injected into the patient. Conventionally, the cartridge,
containing the liquid rnedicament is packaged separately from the
needle-hub-needle guard assembly. Prior to injection the assembly
is snapped on the cartridge, the proximal end of the needle
cannula penetrating the septum, and the needle guard removed to
render the device ready for injection. During the process, it is
desirable that the snap-on step is easy, not requiring excessive
force, as well as when the injection is completed and the needle-
hub-needle-needle guard assembly is removed from the cartridge,
the force of removal is not excessive to the average practitioner.
We have found that both the snap-on and the removal steps tended
to be cumbersome for some practitioners. As a result of intensive
studies directed to solving this problem, as well as solving the
problem of the presence of particulate matter originating from the
metal cap holding the septum in place, we have found that a
coating during the manufacture of the metal cap or after the cap
is crimped on the septum and the cartridge, greatly reduces the
--3--
CA 02214120 1997-08-28
W O 96/26751 PCTrUS96/02176
tendency, or completely eliminates the necessity of applying ~~
excessive force by the practitioner to activate and/or deactivate
the cartridge containing the li~uid medicament.
CA 02214120 1997-08-28
W 096~26~5~ PCT~US96/02176
SU~MARY OF THE lNV~NlION
In accordance with the present invention, there is provided a
low friction coating for a coupling system for transferring liquid
medicaments from one flow conduit to another, and more
particularly, to a low friction coating for a cartridge seal cap
used in conjunction with disposable, liguid medicament-containing
cartridge-needle units to eliminate or at least greatly reduce,
the presence of particulate materials originating from the
coupling system and to reduce the force of activation and
deactivation of the device.
In particular, the invention provides an improvement in a
cartridge-needle assembly wherein said cartridge comprises:
(a) a cartridge barrel having a distal end and a proximal endi
a plunger axially and reciprocally slideable in the cartridge
barrel;
a septum of a resilient material sealing the distal end of the
cartridge barrel; and
a cartridge seal cap having an aperturee located at its center
for allowing a needle to penetrate the septum, said cartridge
seal cap securely holding said septum in place;
(b) a needle assembly unit comprising:
a hub axially holding a needle, said needle having a distal
end and a proximal end, said hub comprising a sleeve
~ constructed to be snapped on the distal end of said cartridge
while said proximal end of said needle penetrates the septum
in the cartridge; and
(c) a sheath covering the distal end of the needle constructed to
be snapped on the hub for protection against injury by the
needle to the user;
wherein said improvement comprises:
a coating of low friction material covering the cartridge seal
cap to prevent particulate material originating from the
cartridge seal to enter into the needle and to reduce the
frictional force needed to activate and deactivate the
cartridge.
The coating of the metallic cartridge seal cap may be done
--5--
CA 02214120 1997-08-28
W O96/267Sl PCTrUS96/02176
during the manufacturing of the cap or after the cap is crimped on
the cartridge. Such coating may be: a polyolefin, such as
polypropylene and polymethylpentene; a polyvinyl, such as
polystyrene, polyvinyl acetate (PVA), polyvinyl chloride (PVC),
polyvinylidene chloride (PVDC), a copolymer of polyvinylchloride
(PVC) and polyvinylidene chloride (PVDC), polyvinyl fluoride, and
polyvinylidene fluoride; an ether, such as polymethylene oxide,
polyphenylene oxide and polyphenylene sulphone; an ester, such as
polyethylene terephthalate (PET), polycarbonate and copolyester;
an ester, such as polycaprolactam (Nylon 6), polyhexamethylene
adipamide (Nylon 66) and polyundecanoamide (Nylon 11).
We have found polytetrafluoroethylene (TEFLON) to provide one
of the best low friction coating materials. The coating thickness
will be in the range of about 0.002 to about 1.0 mm, and
preferably about 0.02 to 0.5 mm. The coating may be applied or
bonded to the metallic cap material in any suitable manner known
in the art, such as, but not limited to, by the use of adhesives,
solvents, spray applications, radio waves, infrared, microwaves,
ultrasonics and heat.
If the coating of the seal cap is done after the crimping
operation, the coating may also cover the central portion of the
septum which is exposed by the aperture in the seal cap.
CA 02214120 1997-08-28
W O 96126751 PCTAUS96/Otl76
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings illustrate an embodiment of the
present invention in which:
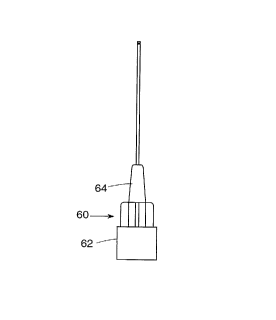
FIG. 1 is a plan view of a needle assembly unit showing a hub
axially holding the needle;
FIG. 2 iS a plan view of a cartridge;
FIG. 3 iS a plan view of a sheath to cover the needle shown in
FIG. l;
FIG. 4 iS plan view of a plunger for use in the cartridge shown
in FIG. 2;
FIG. 5 iS a plan view of a fully assembled cartridge, plunger and
hub-needle assembly without the sheath;
FIG. 6 is an enlarged, fragmentary, longitudinal cross section of
the distal end of the cartridge having the septum in
place, the crimped-on seal cap and a portion of the hub
and needle prior to the needle piercing the septum; and
FIG. 7 iS an enlarged, fragmentary, longitudinal cross section of
the distal end of the cartridge having the septum in
place, the crimped-on seal cap and a portion of the hub
and needle after the needle has pierced the septum.
CA 02214120 1997-08-28
W O96/26751 PCTrUS96/02176
DRTAIT.~D D~SCRIPTION OF THE INV~:~ ION
This invention is useful in conjunction with a wide variety
of cartridges activatable by a snap-on needle unit.
The invention will now be described with reference to the
drawings which illustrate a preferred embodiment of the invention.
Shown in FIG. 2, cartridge 10 comprises: a cartridge barrel
20, formed of glass and plastic, having a distal end 26 and a
proximal end 40. Distal end 26 has a neck portion 28 which
terminates in an opening (not shown), closed by a seal or
diaphragm cap 30 and a septum or diaphragm 36. Seal cap 30
contains an annular groove 32 to receive a snap-on hypodermic
needle assembly 60 shown in FIG. 1. Hypodermic needle assembly 60
comprises a snap-on portion 62 which is to engage seal cap 30 in a
mating relationship, and a conical portion 64 which is to receive
and engage proximal end 74 of sheath 70. When the hypodermic
needle assembly 60 is snapped on the cartridge, the proximal end
of needle 66 pierces the septum 36, thereby providing
communication between said needle and liquid medicament 24
contained in cartridge barrel 20. The proximal end 40 of
cartridge 10 is open for receipt of a plunger or piston 50, shown
in FIG. 4, which has a forward, liquid-interfacing surface 52 and
a rearwardly extending threaded portion 56 for interconnection
with a plunger rod (not shown) when the cartridge is readied for
use.
FIG. 6 shows an enlarged, fragmentary, longitudinal cross
section of the distal end of cartridge barrel 20, having septum 36
covering the distal opening of the barrel, the crimped-on seal cap
30 and snap-on portion 62 of the hypodermic needle assembly. As
shown, at this stage of the process, the proximal end of needle 66
has not pierced septum 36.
FIG. 7 shows the same parts and spacial configuration
thereof, except the proximal end of needle 66 has now pierced
septum 36.
-8-
CA 02214120 1997-08-28
W O96126751 PCTrUS96/02176
As shown, crimped-on seal cap 30 includes aperture 38 in its
center portion to expose underlying septum 36 to allow piercing of
same by the proximal end of needle 66. Crimped-on seal cap 30 is
coated with a low friction polymeric coating 80. The coating may
cover the exterior surface of the seal cap or it may cover it both
on its exterior and interior surfaces. When the coating is
applied after the seal cap is crimped on the cartridge, the
coating may also cover the center, exposed surface of the septum.
Referring now to materials of construction, such materials
are available from commercial sources. The septum can be made of
compliant, rubbery materials capable of resealing itself after
being pierced. Preferred materials possess a Shore A durometer
hardness of from about 50 to about 70. The seal cap, which holds
the septum in place after it is crimped on, is made of any
suitable metallic materials, preferably aluminum. The needle
unit, except the needle which is of stainless steel, is fabricated
of plastic. Suitable plastics include polypropylene, polystyrene,
nylons, acetals, polyethylene and polyester.
Referring now to the use of the device, the health
professional receives the device in a form wherein the cartridge
is pre-filled with the liquid medicament and is sealed at both
ends: the distal end is sealed by the septum and seal cap, while
the proximal end is sealed by the plunger. The plunger rod is not
attached to the plunger and neither is the needle unit snapped-on
the distal end of the cartridge. In readying the device for use,
the cartridge-needle unit is activated by causing the proximal end
of the needle to pierce the septum and the cartridge is moved
forward in the hub to reach the snapped position. The snap-on
step is convenient and easy since the smooth coated surface of the
3 seal cap exerts little frictional force on the hub of the needle
unit. The plunger rod is screwed onto the post of the plunger.
Lastly, the sheath covering the exposed portion of the needle is
removed. Thereafter, the distal end of the needle is inserted
into the injection site and the liquid medication is delivered to
_g _
-
CA 02214120 1997-08-28
W O96126751 PCTrUS96/02176
the patient by applying an axially directed force to the plunger
rod.
Cartridge activation force was determined using TEFLON-coated
and non-coated seal caps crimped onto cartridges. Instron 4502
equipment was used to determine the maximum load expressed in
pounds to activate the needle into the cartridges of 40-samples of
each of the coated and uncoated samples. The average forces and
standard deviations were as follows:
Sam~les Averaae Standard Deviation
TEFLON-coated 5.585 0.800
Non-coated 10.561 1.534
The present invention has been described in connection with
the preferred embodiment show in the drawings, it is to be noted,
however, that various changes and modifications are apparent to
those skilled in the art.
-10 -