Note: Descriptions are shown in the official language in which they were submitted.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
NEW CRYPTOPHYCINS FROM SYNTHESIS
This invention was made in part with U.S. Government support under Grant Nos.
CA12623 and CA53001 from The National Cancer Institute, Department of Health
and
Human Services. Accordingly, the U.S. Government may have certain rights in
this
invention.
Background of the Invention
Neoplastic diseases, characterized by the proliferation of cells not subject
to the
normal control of cell growth, are a major cause of death in humans. Clinical
experience
in chemotherapy has demonstrated that new and more effective drugs are
desirable to treat
these diseases. Such experience has also demonstrated that drugs which disrupt
the
microtubule system of the cytoskeleton can be effective in inhibiting the
proliferation of
neoplastic cells.
The microtubule system of eucaryotic cells is a major component of the
cytoskeleton and is in a dynamic state of assembly and disassembly; that is,
heterodimers
of tubulin are polymerized to form microtubules, and microtubules are
depolymerized to
their constituent components. Microtubules play a key role in the regulation
of cell
architecture, metabolism, and division. The dynamic state of microtubules is
critical to
their normal function. With respect to cell division, tubulin is polymerized
into
microtubules that form the mitotic spindle. The microtubules are then
depolymerized
when the mitotic spindle's use has been fulfilled. Accordingly, agents which
disrupt the
polymerization or depolymerization of microtubules, and thereby inhibit
mitosis, comprise
some of the most effective chemotherapeutic agents in clinical use.
Such anti-mitotic agents or poisons may be classified into three groups on the
basis
of their molecular mechanism of action. The first group consists of agents,
including
colchicine and colcemid, which inhibit the formation of microtubules by
sequestering
tubulin. The second group consist of agents, including vinblastine and
vincristine, which
induce the formation of paracrystalline aggregates of tubulin. Vinblastine and
vincristine
are well known anticancer drugs: Their action of disrupting mitotic spindle
microtubules
preferentially inhibits hyperproliferative cells. The third group consists of
agents,
including taxol, which promotes the polymerization of tubulin and thus
stabilizes
microtubule structures.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-2-
However, merely having activity as an antimitotic agent does not guarantee
efficacy against a tumor cell, and certainly not a tumor cell which exhibits a
drug-
resistant phenotype. Vinca alkaloids such as vinblastine and vincristine are
effective
against neoplastic cells and tumors, yet they lack activity against some drug-
resistant
tumors and cells. One basis for a neoplastic cell displaying drug resistance
(DR) or
multiple-drug resistance (MDR) is through the over-expression of P-
glycoprotein.
Compounds which are poor substrates for transport of P-glycoprotein should be
useful in
circumventing such DR or MDR phenotypes.
Accordingly, the exhibition of the DR or MDR phenotype by many tumor cells
and the clinically proven mode of action of anti-microtubule agents against
neoplastic cells
necessitates the development of anti-microtubule agents cytotoxic to non-drug
resistant
neoplastic cells as well as cytotoxic to neoplastic cells with a drug
resistant phenotype.
Agents which have shown promise in this regard include a class of compounds
known as
cryptophycins.
With respect to methods of producing cryptophycins, no method for total
synthesis
of cryptophycins is currently known. Cryptophycin compounds are presently
produced
via isolation from blue-green alga or are semi-synthetic variations of such
naturally
produced compounds. The lack of a total synthetic method necessarily makes it
difficult
to produce stereospecific cryptophycins which can maximize activity and
increase the
stability of the compound. For example, research has shown that cryptophycins
with an
intact macrocyclic ring are more active. Accordingly, a total synthetic method
which
could produce cryptophycins with a macrocyclic ring that is more stable than
naturally
derived cryptophycins would be desirable. The present invention solves these
problems.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-3-
Disclosure of the Invention
The present invention provides novel cryptophycin compounds having the
following structure:
RiR2R3 ~R5
13 O
Ar
18 18 14
X HN Rs
R9 3
Rio Y HN O
O g
R77 R8
wherein
Ar is methyl or phenyl or any simple unsubstituted or substituted aromatic or
heteroaromatic group;
Rl is a halogen, SH, amino, monoalkylamino, dialkylamino, trialkylammonium,
alkylthio,
dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
Rl and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring, a sulfate ring or a monoalkyiphosphate ring; or
R, and R2 may be taken together to form a double bond between C18 and C19;
R3 is a lower alkyl group;
R4 and R5 are H; or
R4 and R5 may be taken together to form a double bond between C13 and C14;
R6 is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
R7, Rg, R9 and Rlo are each independently H or a lower alkyl group; and
X and Y are each independently 0, NH or alkylamino.
CA 02214565 2005-07-22
- 3a -
In a further aspect, the present invention provides a cryptophycin represented
by
the structure:
Rt R3 Rs
R2 ~
Ar ,a 0
98 48 14
X Gl HN Rs
~ c
; ::C
Rio Y HN 0
O ~
R"t ~s
wherein
Ar is a phenyl group, a phenyl group substituted with a C1-C5 alkyl group, a
phenyl group
substituted with a Cl-C5 alkoxyl group, a phenyl group substituted with a
halogen, a
naphthyl group, a furyl group, an indolyl group, a pyrrolyl group, a pyridyl
group, or a
thienyl group;
Rl is a halogen, SH, amino, monoalkylamino, dialkylamino, trialkylammonium,
alkylthio, dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
Rl and R2 taken together form an epoxide ring, an aziridine ring, an
episulfide ring, a
sulfate ring or a monoalkylphosphate ring; or
R, and R2 taken together form a double bond between C18 and C19;
R3 is a C1-C5 alkyl group;
R4 and R5 are H; or
R4 and R5 taken together form a double bond between C13 and C14;
R6 is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
R7 and Rg are each independently a CI -C5 alkyl group;
R9 and Rlo are each independently H or a C1-C5 alkyl group; and
CA 02214565 2005-07-22
-3b-
X and Y are each independently 0, NH or alkylamino, wherein the following
structures
are excluded:
H GHs
I Q ,yi
O O HN G!
4 HN O OCHg
CH3
t~)
CH3
o HN cl
HN OCH3
fl
CH3
(2)
The invention further provides a pharmaceutical composition useful for
inhibiting
the proliferation of a hyperproliferative mammalian cell comprising an
effective amount
of a compound as described in the preceding paragraph together with a
pharmaceutically
acceptable carrier.
In a further aspect, the invention provides use of a cryptophycin as described
above for the production of a medicament.
CA 02214565 2005-07-22
- 3c -
In a further aspect, the invention provides use of a cryptophycin as described
above for the treatment of a pathological condition caused by
hyperproliferating
mammalian cells.
10
20
30
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-4-
The present invention further provides total synthetic methods for producing
cryptophycins. The present invention also provides for the use of
cryptophycins in
pharmaceuticals, to inhibit the proliferation of mammalian cells and to treat
neoplasia.
Brief Description of the Drawings
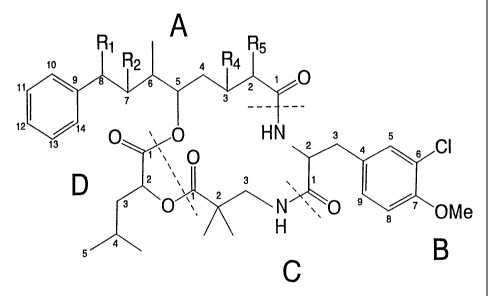
Figure 1 provides a general structure of selected cryptophycin compounds of
the
present invention and a numbering system for the hydroxy acid units A and D
and amino
acid units B and C in selected embodiments;
Figures 2A and B graphically depict the effects of cryptophycin compounds and
vinblastine on Jurkat cell proliferation and cell cycle progression. Jurkat
cells were
incubated with the indicated concentrations of cryptophycin compounds (A) or
vinblastine
(B) for 24 hours. For each sample, the number of viable cells (~) and the
mitotic index
(0) were determined as described in the Experimental Section. Values represent
the
means standard deviation (sd) for triplicate samples in one of three similar
experiments;
Figure 3 graphically depicts the reversibility of the effects of vinblastine,
cryptophycins and taxol on cell growth. SKOV3 cells were treated with 0.1nM
vinblastine (0), 0.1nM cryptophycins (M) or 1nM taxol (19) at time = 0. These
concentrations inhibited cell growth by 50% for each compound. After 24 hours
the cells
were washed and incubated in drug-free medium for the time indicated. The cell
density
was determined by sulforhodamine B (SRB) staining as described in the
Experimental
Section,. and is expressed as the mean sd absorbance at 560 run for
triplicate samples
in one of three experiments;
Figure 4 provides Isobolograms for combinational effects of vinblastine and
cryptophycins on cell proliferation. SKOV3 cells were treated with vinblastine
(0-
600pM) and/or cryptophycins (1-100pM) for 48 hours. Cell numbers were then
determined by SRB staining as described in the Experimental Section, and the
IC50s (M)
and the line of additivity (---) were determined for combinations of
vinblastine and
cryptophycin compounds. Values represent means for two experunents each
containing
triplicate samples;
Figure 5 provides a first scheme for synthesizing cryptophycins in accordance
with
the present invention;
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-5-
Figure 6 provides a scheme for producing a hydroxy acid unit A;
Figure 7 provides a scheme for producing the subunit of a cryptophycin
comprising a hydroxy acid unit A and amino acid B;
Figure 8 provides a scheme for producing the subunit of a cryptophycin
comprising an amino acid unit C and hydroxy acid D;
Figure 9 provides a first scheme for synthesizing selected cryptophycins in
accordance with the present invention;
Figure 10 provides a second scheme for synthesizing selected cryptophycins in
accordance with the present invention;
Figure 11 provides a scheme for synthesizing a subunit of a cryptophycin
comprising a hydroxy acid D;
Figure 12 provides a third scheme for synthesizing selected cryptophycins in
accordance with the present invention;
Figure 13 provides a fourth scheme for synthesizing selected cryptophycins in
accordance with the present invention; and
Figure 14 provides a fifth scheme for synthesizing selected cryptophycins in
accordance with the present invention.
4
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-6-
Detailed Description of the Invention
The present invention provides novel cryptophycin compounds having the
following structure:
Ri ~
R2 ~Rs
Ar ,3 O
,8 ,s ,4
X HN Rs
Rs s
Rio Y HN O
O s
R77 R8
wherein
Ar is methyl or phenyl or any simple unsubstituted or substituted aromatic or
heteroaromatic group;
Rl is a halogen, SH, amino, monoalkylamino, dialkylamino, trialkylammonium,
alkylthio,
dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
R, and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring, a sulfate ring or a monoalkyiphosphate ring; or
Rl and R2 may be taken together to form a double bond between C18 and C19;
R3 is a lower alkyl group;
R4andR5 are H;or
R4 and R5 may be taken together to form a double bond between C13 and C14;
R6 is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
R7, R8, R9 and Rlo are each independently H or a lower alkyl group; and
X and Y are each independently 0, NH or alkylamino.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96103246
-7-
In one aspect of the present invention, novel cryptophycin compounds are
provided
having the following structure:
R1 R3 Rs
11 10 8 s 6
4
R2 R4 R5 HN3 R7
2
R8 O
Wherein
R, is H, OH, a halogen, 0 of a ketone group, NH2, SH, a lower alkoxyl group or
a
lower alkyl group;
R2 is H, OH, 0 of a ketone group, NH2, SH, a lower alkoxyl group or a lower
alkyl
group; or
Rl and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring or a double bond between C,o and Cll; or
Rl and R4 may be taken together to form a tetrahydrofuran ring;
R3 is H or a lower alkyl group;
R4 is OH, a lower alkanoyloxy group or a lower a-hydroxy alkanoyloxy group;
R5 is H or an OH group;
R6 is H; or
R5 and R6 may be taken together to form a double bond between C5 and C6;
R7 is a benzyl, hydroxybenzyl, methoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
halomethoxybenzyl, or dihalomethoxybenzyl group;
R. is OH, a lower #-amino acid wherein Cl is bonded to N of the Q-amino acid,
or an
esterified lower 0-amino acid wherein Cl is bonded to N of the esterified
lower 0-amino
acid group;
R4 and R$ may be taken together to form a didepsipeptide group consisting of a
lower a-
amino acid bonded to a lower a-hydroxy alkanoic acid; and
R5 and RS may be taken together to form a didepsipeptide group consisting of a
lower a-
amino acid bonded to a lower a-hydroxy alkanoic acid; and
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-8-
with the following provisos:
R, is H, a lower alkyl group, or a lower alkoxyl group only if R2 is OH, 0 of
a ketone
group, NHZ, SH;
R2 is H, a lower alkyl group, or a lower alkoxyl group only if Rl is OH, 0 of
a ketone
group, NH2, SH;
when R, is OH, R. is OH, R3 is methyl, R5 and R6 are taken together to form a
double
bond between C5 and C6, R4 and R8 are taken together to form the
didepsipeptide group
with the structure X:
O O O
X= 3 4 JL a a
Rio 0 '
R9 H
wherein O, of X corresponds to R4, N8 of X corresponds to R8,. R9 is methyl,
and
Rlo is isobutyl, R7 is not 3-chloro-4-methoxybenzyl;
when R, and R2 are taken together to form an epoxide ring, R3 is methyl, R5
and R6 are
taken together to form a double bond between C5 and C6, R4 and R8 are taken
together to
form a didepsipeptide with the structure X, R9 is methyl, and Rlo is isobutyl,
R7 is not 3-
chloro-4-methoxybenzyl;
when R, and R2 are taken together to form a double bond between Cla and Cl1,
R3 is
methyl, R5 and R6 are taken together to form a double bond between C5 and C6,
R4 and
R8 are taken together to form a didepsipeptide with the structure X, R9 is
methyl, and Rio
is isobutyl, R7 is not 3-chloro-4-methoxybenzyl; and
when Rl and R2 are taken together to form an epoxide group, R3 is methyl, R5
and R6 are
taken together to form a double bond between C5 and C61 R4 is bonded to the
carboxy
terminus of leucic acid, and R8 is bonded to the nitrogen terminus of either 3-
amino-2-
methylpropionic acid or 3-amino-2-methylpropionic acid methyl ester, R7 is not
3-chloro-
4-methoxybenzyl.
The invention further provides cryptophycin compounds wherein at least one of
the
groups attached to C2, Cg, C9, C,o, and Cll has R stereochemistry. In a
further
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-9-
embodiment of the invention, at least one of the groups attached to C2, C81
C91 C,o, and
C11 has S stereochemistry.
The invention further provides cryptophycin compounds in accordance with the
above structure where the structure of the didepsipeptide that is formed when
R4 or R5 is
taken together with R. is the following structure X:
0
O
1
2 0
X= J3 4 6 8
Ri0 :OS 7 H
R9
wherein O1 of X corresponds to R4 or R5, N8 of X corresponds to R8, R9 is H or
a lower
alkyl group, and Rlo is H or a lower alkyl group.
As used herein, the following terms have the indicated meanings unless a
contrary
meaning is clearly intended from the use in context:
"lower Q-amino acid" means any Q-amino acid having three to eight carbons and
includes linear and non-linear hydrocarbon chains; for example, 3-amino-2-
methylpropionic acid.
"esterified lower (3-amino acid" means any 0-amino acid having three to eight
carbons where the hydrogen of the carboxylic acid group is substituted with a
methyl
group; for example, 3-amino-2-methylpropionic acid methyl ester.
"lower alkanoyloxy group" means an alkanoyloxy group of one to seven carbons
and includes linear and non-linear hydrocarbon chains.
"lower a-hydroxyalkanoyloxy group" means an a-hydroxyalkanoyloxy group of
two to seven carbons and includes linear and non-linear hydrocarbon chains;
for example,
2-hydroxy-4-methylvaleric acid.
"lower alkoxyl group" means any alkyl group of one to five carbons bonded to
an
oxygen atom.
"lower alkyl group" means an alkyl group of one to five carbons and includes
linear and non-linear hydrocarbon chains including, for example, methyl,
ethyl, propyl,
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-10-
isopropyl, butyl, isobutyl, tert-butyl, sec-butyl, methylated butyl groups,
pentyl, and tert-
pentyl groups.
"allylically substituted alkene" means any alkene which contains an alkyl
substitution.
"epoxide ring" means a three-membered ring whose backbone consists of two
carbons and an oxygen atom.
"aziridine ring" means a three-membered ring whose backbone consists of two
carbons and a nitrogen atom.
"episulfide ring" means a three-membered ring whose backbone consists of two
carbons and a sulfur atom.
"sulfate ring" means a five-membered ring consisting of a carbon-carbon-oxygen-
sulfur-oxygen backbone with two additional oxygen atoms connected to the
sulfur atom.
"monoalkyiphosphate ring" means a five-membered ring consisting of a carbon-
carbon-oxygen-phosphorus-oxygen backbone with two additional oxygen atoms, one
of
which bears a lower alkyl group, connected to the phosphorus atom.
"simple unsubstituted aromatic group" refers to common aromatic rings having
4n+2 pi electrons in a monocyclic conjugated system (for example, furyl,
pyrrolyl,
thienyl, pyridyl) or a bicyclic conjugated system (for example, indolyl or
naphthyl).
"simple substituted aromatic group" refers to a phenyl group substituted with
single group (e.g. a lower alkyl group or a halogen).
"heteroaromatic group" refers to aromatic rings which contain one or more non-
carbon substituents such as oxygen, nitrogen, or sulfur.
"halogen" refers to those members of the group on the periodic table
historically
known as the halogens. Methods of halogenation include, but are not limited
to, the
addition of hydrogen halides, substitution at high temperature,
photohalogenation, etc.,
and such methods are known to those of ordinary skill in the art.l=2
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-11-
One embodiment of a cryptophycin compound of the present invention is when R,
and R2 are taken together to form an epoxide group, R3 is methyl, R5 and R6
are taken
together to form a double bond between C5 and C6, R7 is 4-methoxybenzyl, and
R4 and R8
are taken together to form the didepsipeptide with the structure X where R9 is
methyl and
Rlo is isobutyl. The structure of this compound, Cryptophycin 2, is as
follows:
O
O =
li
O-_ O O HN
NO
~Ha
CRYPTOPHYCIN 2
A further embodiment of a compound of the present invention is when Rl and R2
are taken together to form a double bond between the C,o and Cll carbons, R3
is methyl,
R5 and R6 are taken together to form a double bond between C5 and C6, R7 is
4-methoxybenzyl, and R4 and R. are taken together to form the didepsipeptide
with the
structure X where R9 is methyl and R,o is isobutyl. The structure of this
cryptophycin
compound, Cryptophycin 4, is as follows:
CJi5iTO
O" 00 HN
o H ~o
OOFIs
CRYPTOPHYCIN 4
~
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-12-
A further embodiment of a compound of the present invention is when R, and R4
are taken together to form a tetrahydrofuran ring, R2 is an OH group, R3 is
methyl, R5
and R6 are taken together to form a double bond between C5 and C6, R7 is 3-
chloro-4-
methoxybenzyl, and R8 is a (2-carbomethoxypropyl)amino group. The structure of
this
compound, Cryptophycin 6, is as follows:
0
HN CI
10 1
H OCH3
CH3O 0
CRYPTOPHYCIN 6
A further embodiment of a compound of the present invention is when Rl and R4
are taken together to form a tetrahydrofuran ring, R2 and R8 are OH groups, R3
is methyl,
R5 and R6 are taken together to form a double bond between C. and C6 such that
there is
a double bond, and R7 is 3-chloro-4-methoxybenzyl. The structure of this
compound,
Cryptophycin 7, is as follows:
0
I ~ HN q
\
/ HOO I /
OCHy
CRYPTOPHYCIN 7
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-13-
A further embodiment of a compound of the present invention is when R, is a
chloro group, R2 is an OH group, R3 is methyl, RS and R6 are taken together to
form a
double bond between C5 and C6, R7 is 3-chloro-4-methoxybenzyl, and R4 and R8
are taken
together to form the didepsipeptide with the structure X where R9 is methyl
and R,o is
isobutyl. The structure of this compound, Cryptophycin 8, is as follows:
- CI
i O
OH N
O / p f ~ CI
"O O
OCHa
CRYPTOPHYCIN 8
A further embodiment of a compound of the present invention is when Rl is a
methoxy group, R2 is an OH group, R3 is methyl, R5 and R6 are taken together
to form a
double bond between C5 and C6, R7 is 3-chloro-4-methoxybenzyl, and R4 and R8
are taken
together to form the didepsipeptide with the structure X where R9 is methyl
and RIO is
isobutyl. The structure of this compound, Cryptophycin 9, is as follows:
OCH3
O
OH HN
O O CI
Ha
CRYPTOPHYCIN 9
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-14-
A further embodiment of a compound of the present invention is when R, is a
methoxy group, R2 and R4 are OH groups, R3 is methyl, R5 and R6 are taken
together to
form a double bond between C5 and C6, R7 is 3-chloro-4-methoxybenzyl, and R$
is a (2-
carboxypropyl)amino group. The structure of this compound, Cryptophycin 10, is
as
follows:
OCH3
0~ O
I OH OH
HN Cl
:C
~N O I
H OCH3
HO O
CRYPTOPHYCIN 10
A further embodiment of a compound of the present invention is when R, and R4
are taken together to form a tetrahydrofuran ring, R2 is an OH group, R3 is
methyl, R5
and R6 are taken together to form a double bond between CS and C6. R7 is 3-
chloro-4-
methoxybenzyl, and R8 is a (2-carboxypropyl)amino group. The structure of this
compound, Cryptophycin 12, is as follows:
j.jL i O
HN
NO OCH3
H
HO O
CRYPTOPHYCIN 12
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-15-
A further embodiment of a compound of the present invention is when Rl and R,
are taken together to form a double bond between the Clo and Cõ carbons, R3 is
methyl,
R4 is an OH group, P. and R6 are taken together to form a double bond between
C5 and
.
C6, R7 is 3-chloro-4-methoxybenzyl, and R8 is a (2-carboxypropyl)amino group.
The
structure of this compound, Cryptophycin 14, is as follows:
\ \ / O
8H
HN ~CI
~O N
~H ~~
HO' ~O
CRYPTOPHYCIN 14
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form an epoxide group, R3 is methyl, R5 and R6 are taken
together
to form a double bond between C5 and C6, R, is 3-chloro-4-hydroxybenzyl, and
R4 and R8
are taken together to form the didepsipeptide with the structure X where R9 is
methyl and
Rlo is isobutyl. The structure of this compound, Cryptophycin 16, is as
follows:
QLrO
O HN CI
N~c O OH
CRYPTOPHYCIN-16
Y
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-16-
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form a double bond between C,o and Cõ carbons, R3 is
methyl, R5
and R6 are taken together to form a double bond between C5 and C6, R7 is 3-
chloro-4-
hydroxybenzyl, and R4 and R8 are taken together to form the didepsipeptide
with the
structure X where R9 is methyl and R,o is isobutyl. The structure of this
compound,
Cryptophycin 17, is as follows:
O
co 00 HN a CI
~O
N~~ OH
H
CRYPTOPHYCIN-17
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form a double bond between C,o and Cõ carbons, R3 is
methyl, R5
and R6 are taken together to form a double bond between C5 and C6, R7 is 3-
chloro-4-
methoxybenzyl, and R4 and R8 are taken together to form the didepsipeptide
with the
structure X where R9 is methyl and R,o is sec-butyl. The structure of this
compound,
Cryptophycin 18, is as follows:
o
~ % o a
JOHN)(
O
~H OCHa
CRYPTOPHYCIN-18
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-17-
A further embodiment of a compound of the present invention is when R, and R,
are taken together to form a double bond between C,o and Cli carbons, R3 is
methyl, R5
and R6 are taken together to form a double bond between C5 and C6, R7 is 3-
chloro-4-
methoxybenzyl, and R4 and R8 are taken together to form the didepsipeptide
with the
structure X where R9 is methyl and R,o is isopropyl. The structure of this
compound,
Cryptophycin 19, is as follows:
I/ O 0 HN~ CI
NO ,
OCHa
CRYPTOPHYCIN-19
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form an epoxide group, R3 is methyl, P. and R6 are taken
together
to form a double bond between C5 and C6, R7 is 3-chloro-4-methoxybenzyl, and
R4 and R.
are taken together to form the didepsipeptide with the structure X where R9 is
hydrogen
and Rlo is isobutyl. The structure of this compound, Cryptophycin 21, is as
follows:
QLO
0
0 HN CI
o ~
~
oNo
H OCN'
CRYPTOPHYCIN-21
~
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-18-
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form an epoxide group, R3 is methyl, R5 and R6 are taken
together
to form a double bond between C. and C6, R7 is 3,5-dichloro-4-hydroxybenzyl,
and R4
and R8 are taken together to form the didepsipeptide with the structure X
where R9 is
methyl and R,o is isobutyl. The structure of this compound, Cryptophycin 23,
is as
follows:
~ 0
I O 00 HN
O N~O
OH
~C'
CI
CRYPTOPHYCIN-23
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form an epoxide group, R3 is methyl, R5 and R6 are taken
together
to form a double bond between C5 and C6, R7 is 4-methoxybenzyl, and R4 and R8
are
taken together to form the didepsipeptide with the structure X where R9 is
hydrogen and
R,o is isobutyl. The structure of this compound, Cryptophycin 24, is as
follows:
o
I o ~
:~Oa
~Jv \N
H OCHa
CRNPTOPHYCIN-24
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-19-
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form a double bond between Clo and C11 carbons, R3 is
methyl, R4 is
hydroxy, R6 is hydrogen, R7 is 3-chloro-4-methoxybenzyl, and R. and R8 are
taken
together to form the didepsipeptide with the structure X where R9 is methyl
and Rlo is
isobutyl. The structure of this compound, Cryptophycin 26, is as follows:
O
HO O HN
~ CI
I O HN~O 00143
O"/ Y
CIiYPT (OPHYCIN-26
A further embodiment of a compound of the present invention is when Rl and R2
are taken together to form a double bond between the Clo and Cll carbons, R3
is
hydrogen, R5 and R6 are taken together to form a double bond between C5 and
C6, R7 is
3-chloro-4-methoxybenzyl, and R4 and R. are taken together to form the
didepsipeptide
with the structure X where R9 is methyl and Rlo is isobutyl. The structure of
this
compound, Cryptophycin 28, is as follows:
O OO HN
O 25 ~H
CRYPTOPHYCIN-28
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-20-
A further embodiment of a compound of the present invention is when RI and R2
are taken together to form a double bond between the Cio and Cl1 carbons, R3
is methyl,
R5 and R6 are taken together to form a double bond between C5 and C6, R7 is 3-
chloro-4-
methoxybenzyl, and R4 and R8 are taken together to form the didepsipeptide
with the
structure X where R9 is hydrogen and Rlo is isobutyl. The structure of this
compound,
Cryptophycin 29, is as follows:
O
YO HN~p ~ G
'
O v\N
H 0CHs
CRYPTOPHYCiN-29
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form a double bond between the Clo and Cll carbons, R3
is methyl,
R5 is hydroxy, R6 is hydrogen, R7 is 3-chloro-4-methoxybenzyl, and R4 and R8
are taken
together to form the didepsipeptide with the structure X where R9 is methyl
and Rlo is
isobutyl. The structure of this compound, Cryptophycin 30, is as follows:
o
~ O OH HN
p + ~ CI
O NO OCHa
CRYPTOPHYCIN-30
.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-21-
A further embodiment of a compound of the present invention is when R, and R,
are taken together to form an epoxide group, R3 is methyl, R5 and R6 are taken
together
to form a double bond between C5 and C6, R7 is 3,5-dichloro-4-methoxybenzyl,
and R4
and R8 are taken together to form the didepsipeptide with the structure X
where R9 is
methyl and R,o is isobutyl. The structure of this compound, Cryptophycin 31,
is as
follows:
O
vH CI
O ~'-~O CI
CRYPTOPHYCIN31
A further embodiment of a compound of the present invention is when Rl and R2
are taken together to form an epoxide group, R3 is methyl, R5 is hydrogen, R6
is
hydrogen, R7 is 3-chloro-4-methoxybenzyl, and R4 and R$ are taken together to
form the
didepsipeptide with the structure X where R9 is methyl and R,o is isobutyl.
The structure
of this compound, Cryptophycin 35, is as follows:
~ o
I -
8 HN
p CI
O N~
~H OCHs
CRYPTOPHYCIN35
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-22-
A further embodiment of a compound of the present invention is when Rl and R2
are taken together to form an epoxide group, R3 is hydrogen, R5 and R6 are
taken together
to form a double bond between C5 and C6, R7 is 3-chloro-methoxybenzyl, and R4
and R.
are taken together to form the didepsipeptide with the structure X where R9 is
methyl and
R,o is isobutyl. The structure of this compound, Cryptophycin 40, is as
follows:
o
N
CI
'OV N~OOC~
H
CRYFrTOPHYCIN-40
A further embodiment of a compound of the present invention is when Rl and R.
are taken together to form a double bond between the Clo and C11 carbons, R3
is methyl,
R5 and R6 are taken together to form a double bond between C5 and C6, R7 is
3,5-
dichloro-4-hydroxybenzyl, and R4 and R8 are taken together to form the
didepsipeptide
with the structure X where R9 is methyl and Rlo is isobutyl. The structure of
this
compound, Cryptophycin 45, is as follows:
-01 C1r0
p HN
(,~
O''~0
H
CRYPTOPHYCIN-45
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-23-
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form an epoxide group, R3 is methyl, R. and R6 are taken
together
to form a double bond between C5 and C6, R7 is 3-chloro-4-methoxybenzyl, and
R4 and R.
are taken together to form the didepsipeptide with the structure X where R9 is
methyl and
R,o is propyl. The structure of this compound, Cryptophycin 49, is as follows:
O
Q HN ~,y
N' :~O
OCHg
CRYPTOPHYCIN-49
A further embodiment of a compound of the present invention is when Rl and R2
are taken together to form a double bond between the CIo and C11 carbons, R3
is methyl,
RS and R. are taken together to form a double bond between C. and C6, R7 is 3-
chloro-4-
methoxybenzyl, and R4 and R8 are taken together to form the didepsipeptide
with the
structure X where R9 is methyl and Rio is propyl. The structure of this
compound,
Cryptophycin 50, is as follows:
c o
C 0 0 HN ~
a
p N' C f /
OCH3
CRYPTOPHYCIN-50
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-24-
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form an epoxide group, R3 is methyl, R5 and R6 are taken
together
to form a double bond between C5 and C6, R7 is 3-chloro-4-methoxybenzyl, and
R4 and R8
are taken together to form the didepsipeptide with the structure X where R9 is
methyl and
Rlo is sec-butyl. The structure of this compound, Cryptophycin 54, is as
follows:
o
I ~ 0 8
0 HN CI
~
N~
OCHs
CRYPTOPHYCIN-54
Of the above compounds, Cryptophycins 2, 4, 16-19, 21, 23, 24, 26, 28-31, 40,
43, 45, 49, 50, and 54 are metabolites produced by a strain of Nostoc sp. of
blue-green
algae (cyanobacteria) which has been cultured, with these compounds
subsequently
isolated from this culture. Cryptophycins 6 and 7 are artifacts that are
produced if the
isolation procedure utilizes solvents containing methanol. Cryptophycins 8, 9,
10-12, 14,
and 35 are derivatives of these naturally-produced metabolites, having been
chemically
modified with the methods described in the Experimental Section of this
application, with
alternate methods to create the exemplified compounds, as well as the non-
exemplified
compounds, available to those of ordinary skill in the art.
The present invention provides methods of producing the above cryptophycin
compounds through the culturing of a strain of the Nostoc sp. The
morphological
characteristics of the Nostoc sp. of blue-green algae (cyanobacteria), as
provided in U.S.
Patent No. 4,946,835, are that they are filamentous and consist of vegetative
cells. In
longer filaments, heterocysts occasionally are observed in an intercalary
position; akinetes
are not observed. Reproduction is by hormogonia in addition to random trichome
breakage. The basis for an identification of a Nostoc sp. can be found in J.
Gen. Micro.,
111:1-61 (1979).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-25-
The invention further provides that a Nostoc sp. may be cultured and that
novel
cryptophycin metabolites, as well as previously disclosed cryptophycin
metabolites, may
be isolated from this culture. In a preferred embodiment of the present
invention, the
Nostoc sp. strain designated GSV 224 is the strain which is cultivated and
from which are
isolated compounds represented by the following structure:
Ri F~ R6
7
11 10 5 O 4
R2 R4 R5 HN3 R7
C
Fia O
Wherein
Rl is H, OH, a halogen, 0 of a ketone group, NH2, SH, a lower alkoxyl group or
a
lower alkyl group;
R2 is H, OH, 0 of a ketone group, NH2, SH, a lower alkoxyl group or a lower
alkyl
group; or
R, and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring or a double bond between Cio and C11; or
Rl and R4 may be taken together to form a tetrahydrofuran ring;
R3 is H or a lower alkyl group;
R4 is OH, a lower alkanoyloxy group or a lower a-hydroxy alkanoyloxy group;
R5 is H or an OH group;
R6 is H; or
R5 and R6 may be taken together to form a double bond between C5 and C6;
R7 is a benzyl, hydroxybenzyl, methoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
halomethoxybenzyl, or dihalomethoxybenzyl group;
R. is OH, a lower j6-amino acid wherein Cl is bonded to N of the (3-amino
acid, or an
esterified lower 0-amino acid wherein C, is bonded to N of the esterified
lower 0-amino
acid group;
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-26-
R4 and R8 may be taken together to form a didepsipeptide group consisting of a
lower /3-
amino acid bonded to a lower a-hydroxy alkanoic acid; or
R5 and R. may be taken together to form a didepsipeptide group consisting of a
lower /3-
amino acid bonded to a lower a-hydroxy alkanoic acid; 5 with the following
provisos:
Rl is H, a lower alkyl group, or a lower alkoxyl group only if R2 is OH, 0 of
a ketone
group, NH2, SH.
In a preferred embodiment of the invention, chemically modifying a
cryptophycin
metabolite isolated by the above method provides a distinct compound also
having this
structure. Procedures for chemically modifying cryptophycin compounds to
produce
additional compounds within the scope of the present invention are available
to those of
ordinary skill in the art. Moreover, additional procedures are described in
greater detail
in the Experimental Section of this application.
In addition to the novel cryptophycin compounds of the present invention, the
present invention provides novel methods of producing, as well as using, the
above
structure which includes the following previously disclosed cryptophycin
species,
Cryptophycins 1, 3, 5, 13 and 15. The structures of these compounds are as
follows:
H
O
O =H =
O~ O O HN CI
O N~O I /
OCH3
CRYPTOPHYCIN 1
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-27-
~ O
O O O HN CI
= p N~p + /
H
CRYPTOPHYCIN 3
H
C O
O :
OH O HN q
OH N~p /
\~~
CHsO CH
O
CRYPTOPHYCIN S
H
O Y14 HN
CI
H Np OCH~
H
HO O
CRYPTOPHYCIN 13
OH
O
- I / OH d H
O O C~
CRYPTOPHYCIN 15
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-28-
The invention provided herewith is directed to any strain of the Nostoc sp.
and
preferably to the Nostoc sp. GSV 224 strain to produce cryptophycin compounds.
To that
end, the GSV 224 strain of Nostoc sp. was deposited on October 7, 1993
pursuant to the
Budapest Treaty on the International Deposit of Microorganisms for the
Purposes of
Patent Procedure with the Patent Culture Depository of the American Type
Culture
Collection, 12301 Parklawn Drive, Rockville, Maryland 20852 U.S.A. under ATCC
Accession No. 55483. Other strains of Nostoc sp., in particular strain MB 5357
previously deposited by Merck and Co. under ATCC accession No. 53789, are
strains
contemplated to be utilized to practice the present invention.
As is the case with other organisms, the characteristics of Nostoc sp. are
subject to
variation. For example, recombinants, variants, or mutants of the specified
strains may
be obtained by treatment with various known physical and chemical mutagens,
such as
ultraviolet ray, X-rays, gamma rays, and N-methyl-N'-nitro-N-nitrosoguanidine.
All
natural and induced variants, mutants, and recombinants of the specified
strains which
retain the characteristic of producing a cryptophycin compound are intended to
be within
the scope of the claimed invention.
The cryptophycin compounds of the present invention can be prepared by
culturing
a strain of Nostoc sp. under submerged aerobic conditions in a suitable
culture medium
until substantial antibiotic activity is produced. Other culture techniques,
such as surface
growth on solidified media, can also be used to produce these compounds. The
culture
medium used to grow the specified strains can include any of one of many
nitrogen and
carbon sources and inorganic salts that are known to those of ordinary skill
in the art.
Economy in production, optimal yields, and ease of product isolation are
factors to
consider when choosing the carbon sources and nitrogen sources to be used.
Among the
nutrient inorganic salts which can be incorporated in the culture media are
the customary
soluble salts capable of yielding iron, potassium, sodium, magnesium, calcium,
ammonium, chloride, carbonate, phosphate, sulfate, nitrate, and like ions.
Essential trace elements which are necessary for the growth and development of
the organisms should also be included in the culture medium. Such trace
elements
commonly occur as impurities in other constituents of the medium in amounts
sufficient to
meet the growth requirements of the organisms. It may be desirable to add
small
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-29-
amounts (i.e. 0.2mL/L) of an antifoam agent such as polypropylene glycol (M.W.
about
2000) to large scale cultivation media if foaming becomes a problem.
For production of substantial quantities of the cryptophycin compounds,
submerged aerobic cultivation in tanks can be used. Small quantities may be
obtained by
shake-flask culture. Because of the time lag in metabolite production commonly
associated with inoculation of large tanks with the organisms, it is
preferable to use a
vegetative inoculum. The vegetative inoculum is prepared by inoculating a
small volume
of culture medium with fragments of the vegetative trichome or heterocyst-
containing
form of the organism to obtain a fresh, actively growing culture of the
organism. The
vegetative inoculum is then transferred to a larger tank. The medium used for
the
vegetative inoculum can be the same as that used for larger cultivations or
fermentation,
but other media can also be used.
The organisms may be grown at temperatures between about 20' C and 30' C and
an incident illumination intensity of about 100 to 200 mol photons ni 2Sec-1
(photosynthetically active radiation).
As is customary in aerobic submerged culture processes of this type, carbon
dioxide gas is introduced into the culture by addition to the sterile air
stream bubbled
through the culture medium. For efficient production of the cryptophycin
compounds, the
proportion of carbon dioxide should be about 1%(at 24' C and one atmosphere of
pressure).
The prior art, specifically U.S. Patent No. 4,946,835, provides methods of
cultivating Nostoc sp., the contents of which are hereby incorporated by
reference.
Cryptophycin compound production can be followed during *the cultivation by
testing samples of the broth against organisms known to be sensitive to these
antibiotics.
One useful assay organism is Candida albicans.
Following their production under submerged aerobic culture conditions,
cryptophycin compounds of the invention can be recovered from the culture and
from the
culture media by methods known to those of ordinary skill in this art.
Recovery is
generally accomplished by initially filtering the culture medium to separate
the algal cells
and then freeze-drying the separated cells. The freeze-dried alga can be
extracted with a
- suitable solvent such as ethanol, methanol, isopropanol, or dichloromethane.
The
cryptophycins can be separated by subjecting this extract, as well as the
culture media, to
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-30-
rapid chromatography on reversed-phase column. The cryptophycins can be
purified by
reversed-phase high-performance liquid chromatography (HPLC).
As will be apparent from their structures, the cryptophycin compounds have
groups which are capable of chemical modification. The genus compound of the
present
invention contemplates those cryptophycins which exhibit anti-neoplastic
activity. For
example, the derivatives exemplified in the present invention include
compounds having
the epoxide oxygen or hydroxy groups on C-7 and C-8 of unit A or the leucic
acid group
of unit B of Figure 1. Such derivatives of the novel and previously disclosed
compounds
which display the desired anti-neoplastic activity are included in the claimed
invention.
Moreover, the relationship between the structure of the cryptophycin compounds
and anti-
neoplastic activity is provided in the Experimental Section hereinbelow.
While selected cryptophycin compounds are known to be metabolites produced by
the alga of the present invention, other cryptophycin compounds, e.g.
Cryptophycins 8-
15, can be derived from the metabolites using published techniques which are
known to
those of ordinary skill in the art; for example, the syntheses disclosed in
U.S. Patent Nos.
4,868,208, 4,845,086, and 4,845,085, the contents of which are hereby
incorporated by
reference, or by utilizing other methods which are known to those of ordinary
skill in the
art. Moreover, the present invention provides methods of producing derivatives
in the
Experimental Section.
Cryptophycins are potent antitumor and antifungal depsipeptides from blue-
green
algae (cyanobacteria) belonging to the Nostocaceae. The first cryptophycin,
Cryptophycin
1, was isolated from terrestrial Nostoc sp. ATCC 53789 and found to be very
active
against fungi, especially strains of Cryptococcus (R.E. Schwartz et al., J.
Ind. Microbiol.
1990, 5:113-124). Cryptophycin 1 has also been isolated from terrestrial
Nostoc sp. GSV
224, along with twenty-four additional cryptophycin analogs as minor
constituents of the
alga, and found to be very active against subcutaneously transplanted solid
tumors in mice
(G. Trimurtulu et al., J. Am. Chem. Soc. 1994, 116:4729-4737; R. Barrow et
al., J. Am.
Chem. Soc. 1995, 117:2479-2490). Two of the analogs from Nostoc sp. GSV 224,
Cryptophycins 3 and 5, had been described previously as semi-synthetic analogs
of
Cryptophycin 1 (D.F. Sesin, U.S. Patent 4,845,085, issued July 4, 1989; D.F.
Sesin et
al., U.S. Patent 4,868,208, issued September 19, 1989). The cryptophycins
showed
significant tumor selective cytotoxicity in the Corbett assay and were equally
cytotoxic
CA 02214565 2006-04-19
-31-
against drug 'sensitive and drug resistant tumor cells. Cryptophycin 1
appeared to have
the same mode of action as vinblastine, but differed from the latter drug in
irreversibly
inhibiting microtubule assembly (C:D. =Smith et ai., Cancer Res. 1994, 54:3779-
3784).
One of the cryptophycins from Nostoc sp. GSV 224, Cryptophycin 24, has been
isolated
from a marine sponge and named arenastatin A (M. Kobayashi et al:,
Tetrahedrori l.ett.
1994, 35:7969-72; M. Kobayashi et al., Tennen Yuki Kagobutsu Toronkai Koen
Yoshishu
1994, .36st, 104-110).
Twenry-two additional cryptophycin compounds, herein designated 'Cryptophycins
2, 4, b, 7, 16-19, 21, 23; 24, 26, 28-31, 40, 43, 45, 49, 50 and 54 are
disclosed in
International Patent Application VKO/1995/017093 ftled
December 21, 1994, such compounds eitheF being metabolites isolated from a
strain of
Nostoc sp; or having been semi-synthesized from such-metabolites. Also
disclosed in
these patent applications is the -characteriiation of selected cryptophycin
compounds' as
anti-microtubule agents with clinical-type activity expressed toward a broad
spectrum of
tumors implanted in mice, including DR and MDR tumors.
The prescnt invention provides novel cryptophycin compounds having the
following structure: -
.
Ri R2 R9 q5
qr O
U 14
X O HN Ra '
Ro 3CY 'R, HN O
O
P-i Re
wherein
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-32-
Ar is methyl or phenyl or any simple unsubstituted or substituted aromatic or
heteroaromatic group;
Rl is a halogen, SH, amino, monoalkylamino, dialkylamino, trialkylammonium,
alkylthio,
dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
Rl and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring, a sulfate ring or a monoalkylphosphate ring; or
Rl and R2 may be taken together to form a double bond between Cl$ and C19;
R3 is a lower alkyl group;
R4andR5areH;or
R4 and R5 may be taken together to form a double bond between C13 and C14;
R6 is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
R7, R8, R9 and Rlo are each independently H or a lower alkyl group; and
X and Y are each independently 0, NH or alkylamino.
In a preferred embodiment of this cryptophycin, R8 of the cryptophycin is
ethyl,
propyl, isopropyl, butyl, isobutyl, pentyl or isopentyl. In another preferred
embodiment
of this cryptophycin, R7 is ethyl, propyl, isopropyl, butyl, isobutyl, pentyl
or isopentyl.
In an additional preferred embod'unent of this cryptophycin, R7 is H, R. is
methyl, R3 is
methyl; X and Y are not both O.
The present invention provides an additional preferred embodiment of this
cryptophycin wherein R3 is ethyl, propyl, isopropyl, butyl, isobutyl, pentyl
or isopentyl.
In an another preferred embodiment of this cryptophycin, R9 is methyl, ethyl,
propyl,
butyl, isobutyl, pentyl or isopentyl. In a further preferred embod'unent of
this
cryptophycin, Rlo is methyl, ethyl, propyl, butyl, isobutyl, pentyl or
isopentyl.
The invention further provides cryptophycin compounds wherein at least one of
the
groups attached to C3, C6, C,o, C16, C,7, and Cl8 has R stereochemistry. In a
further
embodiment of the invention, at least one of the groups attached to C3, C61
C,o, C162 C17,
and C18 has S stereochemistry.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-33-
One embodiment of a cryptophycin compound of the present invention is when Ar
is phenyl, R, and R2 are taken together to form a double bond between C18 and
C19, R3,
R7 and R8 are methyl, R4 and R5 are taken together to form a double bond
between C13
and C14, R6 is 3-chloro-4-methoxybenzyl, Rg is isobutyl, Rlo is hydrogen, and
X and Y
are oxygen. The structure of this compound, Cryptophycin 51, is as follows:
O
O
O HN ==~'' ci
O N O
~-----H OCH3
CRYPTOPHYCIN-51
A further embodiment of a compound of the present invention is when Ar is
phenyl, Ri and R2 are taken together to form an R,R-epoxide ring, R3, R7 and
R8 are
methyl, R4 and R5 are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, Rlo is hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 52, is as follows:
O
. I / O O
ci
O HN C
O N
OCH3
H
CRYPTOPHYCIN-52
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-34-
A further embodiment of a compound of the present invention is when Ar is
phenyl, Rl and R2 are taken together to form an S, S-epoxide ring, R3, R7 and
R8 are
methyl, R4 and R5 are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, RIo is hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 53, is as follows:
- - ,Q
I / O
O HN ci
I0NO ~
= H OCH3
CRYPTOPHYCI N-53
A further embodiment of a compound of the present invention is when Ar is
phenyl, R, is S-chloro, R2 is R-hydroxyl, R3, R7 and R8 are methyl, R4 and R5
are taken
together to form a double bond between C13 and C14, R6 is 3-chloro-4-
methoxybenzyl, R9
is isobutyl, R,o is hydrogen, and X and Y are oxygen. The structure of this
compound,
Cryptophycin 55, is as follows:
CI
1O
I/ O OH O
O HN CI
O N
= H OCH3
CRYPTOPHYCIN-55
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-35-
Another embodiment of a compound of the present invention is when Ar is
phenyl, R, and R2 are taken together to form an R,R-epoxide ring, R3, R7 and
R8 are
methyl, R4 and R5 are hydrogen, R6 is 3-chloro-4-methoxybenzyl, R9 is
isobutyl, R10 is
hydrogen, and X and Y are oxygen. The structure of this compound, Cryptophycin
57, is
as follows:
I / O O
O HN ci
O N
H OCH3
CRYPTOPHYCIN-57
Another embodiment of a compound of the present invention is when Ar is
phenyl, Rl is S-chloro, R2 is R-hydroxyl, R3, R7 and R. are methyl, R4 and R5
are
hydrogen, R6 is 3-chloro-4-methoxybenzyl, R9 is isobutyl, R,o is hydrogen, and
X and Y
are oxygen. The structure of this compound, Cryptophycin 58, is as follows:
CI
O
OOH O
O HN ci
I0c O H OCH3
CRYPTOPHYCIN-58
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-36-
Another embodiment of a compound of the present invention is when Ar is
phenyl, Rl and R. are taken together to form an R, R-episulfide ring, R3, R7
and Ra are
methyl, R,, and R5 are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, R,o is hydrogen, and X and Y are
oxygen. The
structnre of this compound, Cryptophycin 61, is as follows:
S
101~ O
O O HN CI
O HN : O aOMe
O
CRYPTOPHYCIN 61
Another embodiment of a compound of the present invention is when Ar is p-
methoxyphenyl, Rl and R2 are taken together to form a double bond between Cls
and C19,
R3 and R7 are methyl, R4 and R5 are taken together to form a double bond
between C13
and C14, Rs is 3-chloro-4-methoxybenzyl, R9 is isobutyl, R$ and Rlo are
hydrogen, and X
and Y are oxygen. The structare of this compound, Cryptophycin 81, is as
follows:
O
O O HN CN
Me0
O HN O ~ OMe
CRYPTOPHYCIN 81
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-37-
Another embodiment of a compound of the present invention is when Ar is
methyl, Ri and R2 are taken together to form a double bond between Cla and
C19, R3 and
R7 are methyl, R4 and RS are taken together to form a double bond between C13
and C14,
Rs is 3-chloro-4-methoxybenzyl, R9 is isobutyl, R8 and R,o are hydrogen, and X
and Y
are oxygen. The structure of this compound, Cryptophycin 82, is as follows:
O
O HN
O HN O OMe
O
CRYPTOPHYCIN 82
Another embodiment of a compound of the present invention is when Ar is
methyl, Rl and R2 are taken together to form an R,R-epoxide ring, R3 and R7
are methyl,
R4 and RS are taken together to form a double bond between C13 and C14, R6 is
3-chloro-
4-methoxybenzyl, R9 is isobutyl, R$ and Rlo are hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 90, is as follows:
O
O O HO O
CRYPTOPHYCIN 90
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-38-
Another embodiment of a compound of the present invention is when Ar is
methyl, Rl and R2 are taken together to form an S, S-epoxide ring, R3 and R7
are methyl,
R4 and RS are taken together to form a double bond between C13 and C14, R6 is
3-chloro-
4-methoxybenzyl, R9 is isobutyl, R8 and RIO are hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 91, is as follows:
O
cx: :xtc:
O O
CRYPTOPHYCIN 91
Another embodiment of a compound of the present invention is when Ar is
phenyl, Ri and R2 are taken together to form an R,R-aziridine ring, R3, R7 and
R8 are
methyl, R4 and R5 are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, Rg is isobutyl, Rlo is hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 97, is as follows:
NH
0
O O HN CI
.==~~\\
O HN O ~ OMe
0-01
CRYPTOPHYCIN 97
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-39-
Another embodiment of a compound of the present invention is when Ar is p-
fluorophenyl, R, and R2 are taken together to form a double bond between Cls
and C29,
R3 and R7 are methyl, R4 and Rs are taken together to form a double bond
between C13
and C14, R6 is 3-chloro-4-methoxybenzyl, R9 is isobutyl, R8 and Rlo are
hydrogen, and X
and Y are oxygen. The structure of this compound, Cryptophycin 110, is as
follows:
O
p O :Xo:
W
O
CRYPTOPHYCIN 110
Another embodiment of a compound of the present invention is when Ar is p-
tolyl,
Rl and R2 are taken together to form a double bond between C,$ and C19, R3 and
R7 are
methyl, R4 and R5 are taken together to form a double bond between C,3 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, R$ and Rio are hydrogen, and X and Y
are
oxygen. The structure of this compound, Cryptophycin 111, is as follows:
O
O O HN
. NIa
O HN O aOw
O
CRYPTOPHYCIN 111
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-40-
Another embodiment of a compound of the present invention is when Ar is 2-
thienyl, Rl and R2 are taken together to form a double bond between Cla and
C19, R3 and
R7 are methyl, R4 and Rs are taken together to form a double bond between C13
and C14,
R6 is 3-chloro-4-methoxybenzyl, R9 is isobutyl, Rs and Rlo are hydrogen, and X
and Y
are oxygen. The structure of this compound, Cryptophycin 112, is as follows:
S O
p O HN
O HN O Ow
O
CRYPTOPHYCIN 112
Another embodiment of a compound of the present invention is when Ar is p-
fluorophenyl, Rl and R2 are taken together to form an R, R-epoxide ring, R3
and R7 are
methyl, R4 and RS are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, R8 and Rlo are hydrogen, and X and Y
are
oxygen. The structure of this compound, Cryptophycin 115, is as follows:
O O
O HN \ a
O HN 0 O LA9
O
CRYPTOPHYCIN 115
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-41-
Another embodiment of a compound 'of the present invention is when Ar is p-
fluorophenyl, Ri and R2 are taken together to form an S, S-epoxide ring, R3
and R7 are
methyl, R4 and Rs are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, R$ and R,o are hydrogen, and X and Y
are
oxygen. The structure of this compound, Cryptophycin 116, is as follows:
O
O O HN
,O HN O
CRYPTOPHYCIN 116
Another embodiment of a compound of the present invention is when Ar is p-
tolyl,
Rl and R2 are taken together to form an R,R-epoxide ring, R3 and R7 are
methyl, R4 and
RS are taken together to form a double bond between C13 and C14, R6 is 3-
chloro-4-
methoxybenzyl, R9 is isobutyl, Ra and R,a are hydrogen, and X and Y are
oxygen. The
sMature of this compound, Cryptophycin 117, is as follows:
0 0
O O HN ,, aCl
Ms
.
O HN O OMe
CRYPTOPHYCIN 117
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
_42_
Another embodiment of a compound of the present invention is when Ar is p-
tolyl,
Rl and R2 are taken together to form an S, S-epoxide ring, R3 and R7 are
methyl, R4 and
RS are taken together to form a double bond between C13 and C14, R6 is 3-
chloro-4-
methoxybenzyl, R9 is isobutyl, R8 and R,o are hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 118, is as follows:
.14 O
~
Lo HN CI
Me
O HN O LL/ OMe
O
CRYPTOPHYCIN 118
Another embodiment of a compound of the present invention is when Ar is 2-
thienyl, Rl and R2 are taken together to form an R,R-epoxide ring, R3 and R7
are methyl,
R4 and R5 are taken together to form a double bond between C13 and C14, R6 is
3-chloro-
4-methoxybenzyl, R9 is isobutyl, R8 and R,o are hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 119, is as follows:
O
S 0
~ I O O HN õ~~~~ CI
I
)%0 HN O / OMe
O
CRYPTOPHYCIN 119
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-43-
Another embodiment of a compound of the present invention is when Ar is 2-
thienyl, Rl and R2 are taken together to form an S, S-epoxide ring, R3 and R7
are methyl,
R; and R5 are taken together to form a double bond between C13 and C14, R6 is
3-chloro-
4-methoxybenzyl, Rg is isobutyl, R8 and R,o are hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 120, is as follows:
0
S
~ I C p HN
I /
p HN O OMe
O
CRYPTOPHYCIN 120
Another embodiment of a compound of the present invention is when Ar is
phenyl, Rl and R2 are taken together to form a double bond between C18 and
C19, R3 and
R7 are methyl, R4 and R5 are taken together to form a double bond between C13
and C14,
R6 is 3-chloro-4-methoxybenzyl, R9 is isobutyl, R$ and Rlo are hydrogen, X is
oxygen and
Y is a nitrogen bearing a single hydrogen. The structure of this compound,
Cryptophycin
121, is as follows:
O
O O HN ~
' ~~~
NH HN O / O~
O
CRYPTOPHYCIN 121
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-44-
Another embodiment of a compound of the present invention is when Ar is
phenyl, R, and R2 are taken together to form an R,R-epoxide ring, R3 and R7
are methyl,
R4 and RS are taken together to form a double bond between C13 and C14, R6 is
3-chloro-
4-methoxybenzyl, R9 is isobutyl, R8 and Rlo are hydrogen, X is oxygen and Y is
a
nitrogen bearing a single hydrogen. The structure of this compound,
Cryptophycin 122,
is as follows:
O
O
O O HN C O
NH HN / OMe
O
CRYPTOPHYCIN 122
Another embodiment of a compound of the present invention is when Ar is
phenyl, Rl and R2 are taken together to form an S, S-epoxide ring, R3 and R7
are methyl,
R4 and R5 are taken together to form a double bond between C13 and C14, R6 is
3-chloro-
4-methoxybenzyl, R9 is isobutyl, R8 and R,o are hydrogen, X is oxygen and Y is
a
nitrogen bearing a single hydrogen. The structure of this compound,
Cryptophycin 123,
is as follows:
.111\2
O
O O HN Cl
..J NH HN :: O / OMe
O
CRYPTOPHYCIN 123
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-45-
Another embodiment of a compound of the present invention is when Ar is p-
chlorophenyl, R, and R2 are taken together to form a double bond between Cls
and C19,
R3 and R7 are methyl, R4 and R5 are taken together to form a double bond
between C13
and C14i R6 is 3-chloro-4-methoxybenzyl, R9 is isobutyl, Rs and Rio are
hydrogen, and X
and Y are oxygen. The structure of this compound, Crvptonhvcin 124. is ac
fntlows:
O
O O HN a
a
(il
O HN O
O
CRYPTOPHYCIN 124
Another embodiment of a compound of the present invention is when Ar is p-
chlorophenyl, Rl and R2 are taken together to form an R,R-epoxide ring, R3 and
R7 are
methyl, R4 and RS are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, Rs and Rlo are hydrogen, and X and Y
are
oxygen. The structure of this compound, Cryptophycin 125, is as follows:
O O
.=='~~~
p O HN a
a
O HN O ~ OMe
CRYPTOPHYCIN 125
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-46-
Another embodiment of a compound of the present invention is when Ar is p-
chiorophenyl, R, and R2 are taken together to form an S S epoxide ring, R3 and
R7 are
methyl, R4 and R5 are taken together to form a double bond between C13 and
C14, R6 is 3-
chloro-4-methoxybenzyl, R9 is isobutyl, R8 and Rlo are hydrogen, and X and Y
are
oxygen. The structure of this compound, Cryptophycin 126, is as follows:
.142 O
O O HN .~~~~~ CI
G't
O HN O / OMe
O 01
CRYPTOPIiYCIN 126
Another embodiment of a compound of the present invention is when Ar is
phenyl, RI is S-chloro, R2 is R-hydroxyl, R3 and R7 are methyl, R4 and R5 are
taken
together to form a double bond between C13 and C14, R6 is 3-chloro-4-
methoxybenzyl, R9
is isobutyl, R$ and RIO are hydrogen, X is oxygen and Y is a nitrogen bearing
a single
hydrogen. The structure of this compound, Cryptophycin 127, is as follows:
O
OH O O HN ::M 25 NH HN OMe
O
CRYPTOPHYCIN 127
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-47-
Another embodiment of a compound of the present invention is when Ar is p-
tolyl,
Rl is S-chloro, R2 is R-hydroxyl, R3 and R7 are methyl, R4 and R5 are taken
together to
form a double bond between C13 and C14, R6 is 3-chloro-4-methoxybenzyl, R9 is
isobutyl,
Ra and R,o are hydrogen, and X and Y are oxygen. The structure of this
compound,
Cryptophycin 128, is as follows:
Cf
O
q.{ p O :xc:
N 10 O Me
CRYPTOPHYCIN 128
Another embodiment of a compound of the present invention is when Ar is p-
tolyl,
Rl is R-chloro, R2 is S-hydroxyl, R3 and R7 are methyl, R4 and R5 are taken
together to
form a double bond between C13 and C14, R6 is 3-chloro-4-methoxybenzyl, R9 is
isobutyl,
R$ and Rlo are hydrogen, and X and Y are oxygen. The structure of this
compound,
Cryptophycin 130, is as follows:
cl
a H 0 O HN CI
.=
NIa
O HN O OMe
O
CRYPTOPHYCIN 130
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-48-
Another embod'unent of a compound of the present invention is when Ar is p-
tolyl,
Rl is R-chloro, R2 is R-hydroxyl, R3 and R7 are methyl, R4 and R5 are taken
together to
form a double bond between C13 and C14, R6 is 3-chloro-4-methoxybenzyl, R9 is
isobutyl,
Ra and R,o are hydrogen, and X and Y are oxygen. The structure of this
compound,
Cryptophycin 131, is as follows:
C:I
O
O!-1 O O HN a
O HN O O~
O
CRYPTOPHYCIN 131
Another embodiment of a compound of the present invention is when Ar is p-
chlorophenyl, Rl is S-chloro, R2 is R-hydroxyl, R3 and R7 are methyl, R4 and
RS are taken
together to form a double bond between C13 and C14, R6 is 3-chloro-4-
methoxybenzyl, Rg
is isobutyl, R8 and Rio are hydrogen, and X and Y are oxygen. The structure of
this
compound, Cryptophycin 132, is as follows:
q
O
OH O O HN
O HN I: O ~ OMe
O f-ly
CRYPTOPHYCIN 132
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-49-
Another embodiment of a compound of the present invention is when Ar is p-
chlorophenyl, Rl is R-chloro, R2 is S-hydroxyl, R3 and R7 are methyl, R4 and
Rs are taken
together to form a double bond between C13 and C14, R6 is 3-chloro-4-
methoxybenzyl, R9
is isobutyl, Ra and Rlo are hydrogen, and X and Y are oxygen. The structure of
this
compound, Cryptophycin 133, is as follows:
O
OH O O HN a
CI
O HN O Ow
CRYPTOPHYCIN 133
Another embodiment of a compound of the present invention is when Ar is p-
chlorophenyl, RI is R-chloro. R2 is R-hydroxyl, R3 and R7 are methyl, R4 and
R5 are
taken together to form a double bond between C13 and C14, R6 is 3-chloro-4-
methoxybenzyl, R9 is isobutyl, R8 and R,o are hydrogen, and X and Y are
oxygen. The
structure of this compound, Cryptophycin 134, is as follows:
O
CH O tzc
0 0 OMe
O
CRYPTOPHYCIN 134
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-50-
Set forth hereinbelow are additional cryptophycin compounds, their substituent
groups based upon the following structure:
R,~ ae <
O~FR12
o
~-O R* HN ~
~
~~H o + ~ ~Fb
Wherein
Rl is H or a halogen;
R2 is H, an oxygen of a ketone or OH; or
R, and R2 may be taken together to form an epoxide ring; or R, and R2 may be
taken
together to form an episulfide ring;
R3 is H, or a lower alkyl group;
R4isHorOH;
R5 is H or OH; or
R4 and R5 may be taken together to form a double bond;
R6 is H or a halogen;
With the following proviso
when R, and R2 are taken together to form an epoxide group, R4 and R5 are
taken
together to form a double bond and R6 is chlorine, R3 is not methyl.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-51-
An embodiment of a cryptophycin compound of the present invention is when Rl
is hydrogen, R2 is an oxygen of a ketone group, R3 is S-methyl, R4 and R5 are
taken
together to form a double bond and R6 is chloro. The structure of this
compound,
Cryptophycin 20 is as follows:
o
I
CI
O
HNO
OCHs
~J
O
CRYPTOPHYCIN 20
A further embodiment of a compound of the present invention is when R, is S-
bromo, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form a
double
bond and R6 is chloro. The structure of this compound, Cryptophycin 25 is as
follows:
Br
o
7,~O O HN~ a
HN O-------\OCH3
OIY
CRYPTOPHYCIN 25
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-52-
A further embodiment of a compound of the present invention is when Rl is R-
chloro, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form
a double
bond and R6 is chloro. The structure of this compound, Cryptophycin 27 is as
follows:
ci
i
-
OHrOHN
O HN~ G
o
OCH3
~J
CR1fPTOPHYCIN 27
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form a R, R-epoxide ring, R3 is S-methyl, R4 and R5 are
hydrogen
and R6 is chloro. The structure of this compound, Cryptophycin 32 is as
follows:
O
_
o HN G
O HN)~ I
O~~ ~~
CRYPTOPHYCIN 32
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-53-
A further embodiment of a compound of the present invention is when R,, R4 and
R5 are hydrogen, R2 is S-hydroxy, R3 is R-methyl and R6 is chloro. The
structure of this
compound, Cryptophycin 33 is as follows:
\ o
I
CI
/ ~~n O HN
HN)~O
C"3
OJY
CRYPTOPHYCIN 33
A further embodiment of a compound of the present invention is when Rl, R,, R4
and R5 are hydrogen, R3 is R-methyl and R6 is hydrogen. The structure of this
compound, Cryptophycin 34 is as follows:
o
_
CHr
O 20
~
O HN O \% ,
OCH3
CRYPTOPHYCIN 34
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-54-
A further embodiment of a compound of the present invention is when R, is R-
bromo, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form a
double
bond and R6 is chloro. The structure of this compound, Cryptophycin 37 is as
follows:
Br
O OH O HN CI
O HN~O \ /
H
CRYPTOPHYCIN 37
A further embodiment of a compound of the present invention is when RI and R2
are taken together to form a S, S-epoxide ring, R3 is S-methyl, R4 and RS are
taken
together to form a double bond and R6 is chloro. The structure of this
compound,
Cryptophycin 38, is as follows:
O
I _
O HN
~'~~ ~.. ! CI
O HN
OCH~
O
~J
CRYPTOPHYCIN 38
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-55-
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form a S,R-epoxide ring, R3 is S-methyl, R4 and R. are
taken
together to form a double bond and R6 is chloro. The structure of this
compound,
Cryptophycin 39, is as follows:
o O HN C~
O HN~O yi
~ J C~
0i +Y
CRYPTOPHYCIN 39
A further embodiment of a compound of the present invention is when RI and R2
are taken together to form a R, R-epoxide ring, R3 is S-methyl, R4 is S-
hydroxy, R5 is R-
hydroxy and R6 is chloro. The structure of this compound, Cryptophycin 41, is
as
follows:
OH
a
OH HN CI
O y
O HNO /
O~
~~
CRYPTOPHYCIN 41
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-56-
A further embodiment of a compound of the present invention is when R, and R2
are taken together to form a R,R-epoxide ring, R3 is S-methyl, R4 is R-
hydroxy, R5 is S-
hydroxy and R6 is chloro. The structure of this compound, Cryptophycin 42, is
as
follows: 5
OH
O
H FiN ci
O ~
~
O HNp / OCj3
O-1y
CRYPTOPHYCIN 42
A further embodiment of a compound of the present invention is when R, is
hydrogen, R2 is S-hydroxy, R3 is R-methyl, R4 and RS are taken together to
form a double
bond and R6 is chloro. The structure of this compound, Cryptophycin 48, is as
follows:
OHN
CI
HN O
~ J ~~
O
CRYPTOPHYCIN 48
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-57-
A further embodiment of a compound of the present invention is when R, is S-
chloro, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are hydrogen and R6 is
chloro. The
structure of this compound, Cryptophycin 59, is as follows:
C'OH
. \ O
-
O HN ~
O HN
Op I i
~J CH3
CRYPTOPHYCIN 59
A further embodiment of a compound of the present invention is when Rl and R2
are taken together to form a S, S-episulfide ring, R3 is S-methyl, R4 and R5
are taken
together to form a double bond and R6 is chloro. The structure of this
compound,
Cryptophycin 60, is as follows:
o
O HN ci .'~
O HN:CO
OCH3
CRYRTOPHYCIN 80
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-58-
A further embodiment of a compound of the present invention is when Rl is S-
chloro, R2 is R-hydroxy, R3 is hydrogen, R4 and R5 are taken together to form
a double
bond and Rs is chloro. The structure of this compound, Cryptophycin 63, is as
follows:
~ OH
O
O HN
a
O HNO
OCH3
CRYPTOPHYCIN 63
A further embodiment of a compound of the present invention is when Rl is R-
chloro, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are hydrogen and R6 is
chloro. The
structure of this compound, Cryptophycin 64, is as follows:
CI
OH
O HN
"~O HN)~ Tl\
O~ O ~ a
Hs
CRYPTOPHYCIN 64
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-59-
A further embodiment of a compound of the present invention is when R, is R-
chloro, R2 is S-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form
a double
bond and R6 is chloro. The structure of this compound, Cryptophycin 69, is as
follows:
= c_i
o
Cr OH O HN a
O HN~CO
C"3
CRYPTOPHYCIN 69
A further embodiment of a compound of the present invention is when R, is S-
chloro, R2 is S-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form
a double
bond and R6 is chloro. The structure of this compound, Cryptophycin 70, is as
follows:
CI
i O
CHOHN
CI
L"~O HN~O
oclis
O-Iy
CRYPTaPHYCIN 70
4
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-60-
A further embodiment of a compound of the present invention is when R, is R-
bromo, R2 is S-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form a
double
bond and R6 is chloro. The structure of this compound, Cryptophycin 71, is as
follows:
Br
O
, _ -
OH O HN
~ CI
O HN~O
O
-Y
CRYPTOPHYCIN 71
A further embodiment of a compound of the present invention is when Rl is S-
bromo, R2 is S-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form a
double
bond and R6 is chloro. The structure of this compound, Cryptophycin 72, is as
follows:
cJZ1rOHN0
~.. cl
O HN O \~..
OCH6
CRYPTOPHYCIN 72
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-61-
A further embodiment of a compound of the present invention is when RI is S-
chloro, R2 is S-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form
a double
bond and R6 is chloro. The structure of this compound, Cryptophycin 73, is as
follows:
OH
O
HN
O HNO
OCH3
O--J~
CRYPTOPHYCIN 73
A further embodiment of a compound of the present invention is when R, is S-
chloro, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form
a double
bond and R6 is hydrogen. The structure of this compound, Cryptophycin 74, is
as
follows:
ci
= OH
O
O HN
O HN' O
OCH3
O~y
CRYPTOPHYCIN 74
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-62-
A further embodiment of a compound of the present invention is when R, is S-
fluoro, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form
a double
bond and R6 is chloro. The structure of this compound, Cryptophycin 75, is as
follows:
F
O
7t'~~ HN ~
HNO
H3
CRl(PTOPHYCIN 75
A further embodiment of a compound of the present invention is when R, is R-
fluoro, R2 is R-hydroxy, R3 is S-methyl, R4 and R5 are taken together to form
a double
bond and R6 is chloro. The structure of this compound, Cryptophycin 76, is as
follows:
F
O
HN q
IY
HN
OOH3
J,J
CRYPTOPHYCIN 78
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-63-
The present invention provides methods of producing the above cryptophycin
compounds, as well as all previously known cryptophycins, through total
synthesis.
The invention further provides that novel cryptophycin metabolites, as well as
previously disclosed cryptophycin metabolites, may be synthesized using the
methods
provided in this invention.
The present invention provides a method for producing a cryptophycin
comprising
selecting an allylically substituted E alkene; rearranging the allylically
substituted E
alkene via a stereospecific Wittig rearrangement; converting this compound to
a first 8-_
amino acid or 6-hydroxy acid; coupling the first acid to a second a-amino acid
to form a
first subunit; coupling a third j6-amino acid to a fourth a-hydroxy acid or a-
amino acid to
form a second subunit; and coupling the first subunit to the second subunit to
form a
cryptophycin.
The present invention further provides a preferred embodiment of the method,
wherein the cryptophycin produced has the following structure:
RiR2R3 ~~
Ar 'g ' 13 O
18 18 14
X HN Rs
R9 3
Rio Y HN O
O
~-i R8
wherein
Ar is methyl or phenyl or any simple unsubstituted or substituted aromatic or
heteroaromatic group;
Rl is a halogen, SH, amino, monoalkylamino, dialkylamino, trialkylammonium,
alkylthio,
dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-64-
Rl and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring, a sulfate ring or a monoalkylphosphate ring; or
Rl and R2 may be taken together to form a double bond between C18 and C19;
R3 is a lower alkyl group; 5 R4andR5areH;or
R4 and R5 may be taken together to form a double bond between C13 and C14; R6
is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
R7, R8, R9 and Rlo are each independently H or a lower alkyl group; and
X and Y are each independently 0, NH or alkylamino.
In a preferred embodiment of the present invention, the method produces a
cryptophycin wherein Ar is phenyl; R3 is methyl; R6 is halomethoxybenzyl; R7
is H; R. is
methyl; R9 is isobutyl; R,o is H; X is 0; and Y is O.
In addition to the present invention providing cryptophycins with the above
structure, the present invention provides methods of producing previously
disclosed
cryptophycins and prior art cryptophycins. Cryptophycins 1, 8 and 35 were
produced by
total synthesis. Provided hereinbelow is a representation of previously
disclosed and
prior art cryptophycins produced by total synthesis:
RI R2 ~ ~
O
):X: ::ccc:H3
OIY
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-65-
wherein
R, is a halogen; R2 is OH; or
Rl and R2 may be taken together to form an epoxide ring;
R3 is H; and R4 is H; or
R3 and R4 may be taken together to form a double bond.
The present invention also provides a pharmaceutical composition useful for
inhibiting the proliferation of a hyperproliferative mammalian cell comprising
an effective
amount of a cryptophycin with the following structure:
Ri R2 R3 ~ R5
Ar 'a 13 O
,e ,q
X O HN Rs
~ 3
Rio Y HN O
O e
R7 R8
wherein
Ar is methyl or phenyl or any simple unsubstituted or substituted aromatic or
heteroaromatic group;
RI is a halogen, SH, amino, monoalkylamino, dialkylamino, trialkylammonium,
alkylthio,
dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
Rl and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring, a sulfate ring or a monoalkylphosphate ring; or
Rl and R2 may be taken together to form a double bond between C18 and C19;
R3 is a lower alkyl group;
R4andR5areH; or
R4 and R5 may be taken together to form a double bond between C13 and C14;
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-66-
R6 is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
R7, R8, R9 and R,o are each independently H or a lower alkyl group; and
X and Y are each independently 0, NH or alkylamino;
together with a pharmaceutically acceptable carrier.
In a preferred embodiment of the present invention, the pharmaceutical
composition further comprises at least one additional anti-neoplastic agent.
The present invention also provides a method for inhibiting the proliferation
of a
mammalian cell comprising contacting the mammalian cell with a cryptophycin
compound
in an amount sufficient to inhibit the proliferation of the cell, the
cryptophycin compound
having the following structure:
RiR2R3 R5
Ar 19 ' 13 O
18 1B 14
X O HN Rs
~ 3 3
Rio Y HN O
O 6
wherein
Ar is methyl or phenyl or any simple unsubstituted or substituted aromatic or
heteroaromatic group;
Rl is a halogen, SH, amino, monoalkylamino, dialkylamino, trialkylammonium,
alkylthio,
dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
Rl and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring, a sulfate ring or a monoalkylphosphate ring; or
Rl and R2 may be taken together to form a double bond between C18 and C19i
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-67-
R3 is a lower alkyl group;
R4 and R5 are H; or
R4 and R5 may be taken together to form a double bond between C13 and C14;
R6 is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
R7, R8, R9 and RIO are each independently H or a lower alkyl group; apd
X and Y are each independently 0, NH or alkylamino.
In a preferred embodiment of the present invention, this method further
comprises
contacting the cell with at least one additional anti-neoplastic agent. In a
preferred
embodiment of the present invention, the mammalian cell contacted is
hyperproliferative.
In a further preferred embodiment of the present invention, the
hyperproliferative cell is
human.
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-68-
The present invention also provides a method of inhibiting the proliferation
of a
hyperproliferative mammalian cell having a multiple drug resistant phenotype
comprising
contacting the cell with an amount of a cryptophycin compound effective to
disrupt the
dynamic state of microtubule polymerization and depolymerization to arrest
cell mitosis,
thereby inhibiting the proliferation of the cell, the cryptophycin compound
having the
following structure:
RtR2R3 ~~
Ar 1 1 13 O
Ie ie 'a
X HN Rs
R9 3
Rto Y HN O
O 6
R8
wherein
Ar is methyl or phenyl or any simple unsubstituted or substituted aromatic or
heteroaromatic group;
Rl is a halogen, SH, amino, monoalkylamino, diallcylamino, trialkylammonium,
alkylthio,
dialkylsulfonium, sulfate, or phosphate;
R2 is OH or SH; or
Rl and R2 may be taken together to form an epoxide ring, an aziridine ring, an
episulfide
ring, a sulfate ring or a monoalkylphosphate ring; or
Rl and R2 may be taken together to form a double bond between C18 and C19;
R3 is a lower alkyl group;
R4andR5are H; or
R4 and R5 may be taken together to form a double bond between C13 and C14;
R6 is a benzyl, hydroxybenzyl, alkoxybenzyl, halohydroxybenzyl,
dihalohydroxybenzyl,
T
haloalkoxybenzyl, or dihaloalkoxybenzyl group;
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-69-
R7, R8, R9 and Rlo are each independently H or a lower alkyl group; and
X and Y are each independently 0, NH or alkylamino.
In a preferred embodiment of the present invention, the method further
comprises
contacting the cell with at least one additional anti-neoplastic agent. In a
further preferred
embodiment of the present invention, the mammalian cell is human.
The present invention also provides a method of alleviating a pathological
condition caused by hyperproliferating mammalian cells comprising
administering to a
subject an effective amount of the pharmaceutical composition disclosed herein
to inhibit
proliferation of the cells. In a preferred embodiment of the present
invention, the
mammalian cells are human.
In preferred embodiment of the present invention, the method further comprises
administering to the subject at least one additional therapy directed to
alleviating the
pathological condition. In a preferred embodiment of the present invention,
the
pathological condition is characterized by the formation of neoplasms. In a
further
preferred embodiment of the present invention, the neoplasms are selected from
the group
consisting of mammory, small-cell lung, non-small-cell lung, colorectal,
leukemia,
melanoma, pancreatic adenocarcinoma, central nervous system (CNS), ovarian,
prostate,
sarcoma of soft tissue or bone, head and neck, gastric which includes
pancreatic and
esophageal, stomach, myeloma, bladder, renal, neuroendocrine which includes
thyroid
and non-Hodgkin's disease and Hodgkin's disease neoplasms.
The method for preparing the cryptophycin compounds is summarized in Scheme 1
as depicted in Figure 5. The starting material is a 3E-alkene (a) substituted
at C-2 with a
XH group in the S-configuration where X is oxygen or NH. L-Alanine and L-
lactic acid
serve as inexpensive sources of the starting material a. The key step in the
synthesis is a
stereoselective [2,3] Wittig rearrangement (D.J-S. Tsai et al., J. Org. Chem.
1984,
49:1842-1843; K. Mikami et al., Tetrahedron 1984, 25:2303-2308; N. Sayo et
al., Chem
Lett. 1984, 259-262) of the propargyl ether of a (b) to the (3R,4R)-3-(XH-
substituted)-4-
alkylhept-5(E)-en-1-yne (c) where X is an oxygen or a protected nitrogen (e.g.
t-
butyldimethylsilylamino). Compound c can then be converted to the 8-hydroxy or
amino
acid unit A precursor of the cryptophycin, methyl (5S,6R)-5-(XP-substituted)-6-
alkyl-8-
aryl-octa-2E,7E-dienoate (d) where P is a suitable protecting group, using
methods known
to those of ordinary skill in the art.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-70-
One strategy for synthesizing a cryptophycin, which is composed of a S-hydroxy
or amino acid unit A, an a-amino acid unit B, a/3-amino acid unit C, and an -
hydroxy
or amino acid unit D, is to assemble the macrocycle from two precursors
representing
moieties of the cryptophycin molecule, for example, an A-B precursor (e)
containing the
8-hydroxy or amino acid unit A and the a-amino acid unit B and a C-D precursor
(f)
containing the fl-amino acid unit C and the a-hydroxy or amino acid unit D.
In the method described herein, a cryptophycin is assembled from A-B and C-D
precursors in two steps by (1) connecting the termini of units A and D in the
A-B and C-
D precursors to form an acyclic C-D-A-B intermediate and (2) connecting the
termini of
units B and C to form the cyclic product.
In the synthesis of Cryptophycin 51 described in the Experimental Section, an
ester linkage is formed between the S-hydroxy group of unit A in the A-B
moiety and the
carboxylic acid group of unit D in the C-D fragment to form an acyclic C-D-A-B
intermediate and then an amide linkage is formed between the carboxylic acid
group of
unit B in the A-B moiety and the (3-amino group of unit C in the C-D moiety.
Compound
K is the A-B moiety precursor, Compound P is the C-D moiety precursor, and
Compound R is the acyclic C-D-A-B precursor of Cryptophycin 51. Compounds K
and
P have protecting groups on the carboxylic acid group of unit B and the )3-
amino group of
unit C to limit coupling to ester formation between units A and D in step 1.
These
protecting groups are removed from the C-D-A-B intermediate so that amide
formation
can occur between units B and C in step 2.
The synthesis of methyl (5S,6R)-5-t-butyldimethylsilyloxy-6-methyl-8-phenyl-
octa-
2E,7 E-dienoate (G), the unit A precursor of the Cryptophycin 51, is
summarized in
Scheme 2 (Figure 6). (S)-trans-3-penten-2-ol (A), the starting material, was
prepared by
enzymatic resolution of the racemic compound. Reaction of A with propargyl
chloride
and base under phase-transfer condition formed propargyl ether B in 86% yield.
Treatment of B with butyl lithium at -90 C led to alcohol C in 71 % yield. The
desired
3R,4R anti compound C was the only product formed in the Wittig rearrangement.
After
protection of the hydroxyl group of C as the tert-butyldimethylsilyl ether (or
tert-
butyldimethylsilyl ether), hydroboration of the triple bond (H.C. Brown,
Organic
Synthesis Via Boranes, Wiley, 1975) led to an aldehyde D in 73 % yield from C.
Next D
was converted into the trans a, 16-unsaturated ester E by a Horner-Emmons
reaction in
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-71-
90 % yield. Selective ozonolysis of the C6-C7 double bond in D gave aldehyde F
in 83 %
yield. Finally a Wittig reaction of F with benzyltriphenylphosphonium chloride
in the
presence of butyllithium produced G in 80 % yield. The yield of G from A was
26 %.
The coupling of the unit A precursor G with the D-3-(3-chloro-4-methoxyphenyl)
alanine unit B to produce the A-B precursor (K) is summarized in Scheme 3
(Figure 7).
Hydrolysis of the methyl ester group in G with lithium hydroxide in acetone
produced
carboxylic acid H in 95 % yield. Coupling of H with trichloroethyl ester I to
produce J
could be accomplished in 65 % yield by treating a solution of H in
N,N-dimethylformamide (DMF) with a small excess of
pentafluorophenyldiphenylphosphinate (FDPP), an equimolar quantity of the
trifluoroacetate salt of I, followed by 3 equiv of diisopropylethylamine
(DIEA) at 25 C
(S. Chen et al., Tetrahedron Lett. 1991, 32:6711-6714). Fluorodesilylation of
J then led
to K in 95 % yield.
Protected amino acid I was prepared from D-tyrosine in five steps. First,
D-tyrosine was chlorinated with sulfuryl chloride in glacial acetic acid (R.
Zeynek,
Hoppe-Seyler's Z. f. Physiol. Chemie 1926, 144:247-254). Next N-(tert-
butoxycarbonyl)-
3-(3-chloro-4-hydroxyphenyl)-D-alanine was obtained in 94% yield by treating a
suspension of the amino acid in 50% aqueous dioxane with di-tert-
butyldicarbonate in the
presence of triethylamine. The resulting product was dimethylated with
dimethyl sulfate
in the presence of potassium carbonate in refluxing acetone in 84% yield. The
methyl
ester was then saponified with sodium hydroxide in aqueous dioxane to yield N-
(tert-
butoxycarbonyl)-3-(3-chloro-4-methoxyphenyl)-D-alanine in 86 % yield. Exposure
of the
BOC-protected amino acid to trichloroethanol, pyridine and DCC in
dichloromethane led
to trichloroethyl ester I in 65 % yield. Treatment of this material with
trifluoroacetic acid
led to a quantitative yield of the trifluoroacetate salt of I.
The synthesis of (2S)-2-[3'(ten-butoxycarbonyl)amino-2',2'-
dimethylpropanoyloxy]-4-methylpentanoic acid (P), the C-D precursor, is
summarized in
Scheme 4 (Figure 8). The starting point for the unit C portion of P was the
aminoalcohol
L. Protection of the amino group in L by treatment with di-tert-
butyldicarbonate in the
presence of triethylamine (93 % yield), followed by oxidation of the primary
alcohol with
ruthenium tetroxide (P.H.J. Carlsen et al., J. Org. Chem. 1981, 46:3936-3938)
gave
carboxylic acid M(66 % yield). L-Leucic acid was converted to allyl ester N in
93 %
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-72-
yield under phase-transfer conditions, by exposing it to a mixture of allyl
bromide in
dichloromethane and aqueous sodium bicarbonate containing tetra-n-
butylammonium
chloride (S. Friedrich-Bochnitschek et al., J. Org. Chem. 1989, 54:751-756).
The
coupling reaction of M with N was carried out with 4-dimethylaminopyridine
(DMAP)
and dicyclohexylcarbodiimide (DCC) in dichloromethane to produce 0 in 75%
yield.
Cleavage of the allyl ester in 0 was carried out in THF containing morpholine
and
catalytic tetrakis(triphenylphosphine)-palladium to give P in 95% yield (P.D.
Jeffrey et
al., J. Org. Chem. 1982, 47:587-590).
Coupling of the A-B precursor (K) and the C-D precursor (P) was accomplished
as shown in Scheme 5 (Figure 9). Treatment of K and P with DCC/DMAP in
dichloromethane led to the fully protected C-D-A-B intermediate (Q) in 84%
yield.
Reductive cleavage of the trichloroethyl ester group in Q was achieved using
activated
zinc dust in acetic acid. The BOC-protecting group was then removed by
trifluoroacetic
acid to give R as the trifluoroacetate salt in 91 % overall yield from Q.
Macrolactamization of R with FDPP led to Cryptophycin 51 in 61 % yield (J.
Dudash, Jr.
et al., Synth. Commun. 1993, 23:349-356). The overall yield from S-trans-3-
penten-2-ol
(A) was 7%.
Cryptophycin 51 served as the precursor of Cryptophycin 52, the R, R-epoxide,
and Cryptophycin 53, the S, S-epoxide. In turn, Cryptophycin 52 served as the
precursor
of Cryptophycin 55, the 18R,19S-chlorohydrin, and Cryptophycin 57, the 13,14-
dihydro
analog. Cryptophycin 57 served as the precursor of Cryptophycin 58.
Cryptophycin 53
served as the precursor of Cryptophycin 61 using a method described by T.H.
Chan and
J.R. Finkenbine, J. Am. Chem. Soc. 1972, 94:2880-2882 and Cryptophycin 97
using a
method described by Y. Ittah et al., J. Org. Chem. 1978, 43:4271-4273.
For the synthesis of cryptophycins that have Ar groups that are different from
phenyl, unit A precursors of general structure d (Scheme 1, R3 = Me) can be
prepared
by a Wittig reaction of aldehyde F (Scheme 2; TBS protecting group) or S
(Scheme 6;
TBPS protecting group) with the appropriate aryltriphenylphosphonium chloride
in the
presence of butyllithium. Cryptophycin 81 was prepared from precursor d (Ar =
p-
methoxyphenyl, R3 = Me) as shown in Schemes 6 and 7 (Figures 10 and 11).
The Ar group can also be introduced into the new cryptophycin at a later step
in
the synthesis. First precursor d (Ar = R3 = Me) was converted into
Cryptophycin 82 by
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-73-
coupling the appropriate A-B (e) and C-D (f) precursors as shown in Scheme 8
(Figure
12). Selective ozonolysis of Cryptophycin 82 or periodic acid oxidation of the
corresponding epoxides Cryptophycins 90 and 91 led to an aldehyde,
Cryptophycin 108.
} A Wittig reaction of Cryptophycin 108 with the appropriate
aryltriphenylphosphonium
chloride in the presence of butyllithium gave the new cryptophycin (Scheme 9;
Figure
13). Using this procedure, Cryptophycin 110 (Ar = p-fluorophenyl),
Cryptophycin 111
(Ar = p-tolyl), Cryptophycin 112 (Ar = 2-thienyl) and Cryptophycin 124 (Ar = p-
chlorophenyl) were prepared. Cryptophycin 110 served as the precursor of the
epoxides
Cryptophycins 115 and 116. Cryptophycin 111 served as the precursor of the
epoxides
Cryptophycins 117 and 118 and the chlorohydrins Cryptophycins 128, 130 and
131.
Cryptophycin 112 served as the precursor of the epoxides Cryptophycins 119 and
120.
Cryptophycin 124 served as the precurcur of the epoxides Cryptophycins 125 and
126 and
the chlorohydrins Cryptophycins 132, 133 and 134.
Another strategy for synthesizing a cryptophycin is to assemble the macrocycle
from three precursors, for example, an A-B precursor (e) containing the 6-
hydroxy or
amino acid unit A, a precursor containing the 6-hydroxy or amino acid unit D,
and a
precursor containing the fl-amino acid unit C. In the method described herein,
a
cryptophycin is assembled from A-B, C and D precursors in three steps by (1)
connecting
the termini of units A and D in the A-B and D precursors to form an acyclic D-
A-B
intermediate, (2) connecting the termini of units D and C in the D-A-B and C
precursors
to form an acyclic C-D-A-B intermediate, and (3) connecting the termini of
units B and C
to form the cyclic product.
In the synthesis of Cryptophycin 121 described in the Experimental Section, an
ester linkage is formed between the 6-hydroxy group of unit A in the A-B
moiety and the
carboxylic acid group of unit D in the D fragment to form an acyclic D-A-B
intermediate.
An amide linkage is then formed between the carboxylic acid group of unit C
and the a-
amino group of unit D in the D-A-B fragment. Finally an amide linkage is
formed
between the carboxylic acid group of unit B in the A-B moiety and the 0-amino
group of
unit C in the C-D moiety. Compound K is the A-B moiety precursor, BOC-L-
leucineanhydride is the D unit precursor, and Compound AL is the unit C
precursor.
Compound AK is the acyclic C-D-A-B precursor of Cryptophycin 121 (Scheme 10;
Figure 14). Compounds K and BOC-L-leucineanhydride have protecting groups on
the
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-74-
carboxylic acid group of unit B and the a-amino group of unit D to limit
coupling to ester
formation between units A and D in step 1. The protecting group is removed
from the S-
amino group of unit D in the D-A-B intermediate so that amide formation can
occur
between the amino group in unit B and the carboxylic acid C in step 2. These
protecting
groups are removed from the C-D-A-B intermediate so that amide formation can
occur
between units B and C in step 2.
Novel cryptophycin compounds of the present invention have epoxide rings that
are opened by nucleophiles at different rates than the epoxide ring in
Cryptophycin 1, or
have chlorohydrin functionalities that form epoxide rings at different rates
than the
chiorohydrin functionality of Cryptophycin 8 is transformed into the epoxide
ring of
Cryptophycin 1. The epoxide ring of Cryptophycin 1 or the chlorohydrin
functionality (a
masked epoxide ring) of Cryptophycin 8 is essential for optimum in vivo
activity. If the
epoxide oxygen is eliminated (such as found in Cryptophycin 3) or the epoxide
ring
hydrolyzed to a diol (such as found in Cryptophycin 15), antitumor activity is
greatly
diminished. Cryptophycin 1 shows appreciable toxicity in animals compared with
Cryptophycin 8. This is reflected in the T/C (mostly >0%) and gross log kill
values
(mostly < 2.0) for Cryptophycin 1 compared with those for Cryptophycin 8
(mostly T/C
values of 0% and gross log kill values of >2.8). Cryptophycin 25, the
corresponding
bromohydrin analog, shows T/C and gross log kill values that are comparable
with those
for Cryptophycin 1. This striking difference in in vivo activity suggests that
the
bromohydrin Cryptophycin 25 is converted more rapidly into Cryptophycin 1 in
vivo than
Cryptophycin 8. This further suggests that the less toxic Cryptophycin 8 might
have
more time to accumulate at the tumor site before being transformed into the
active
compound Cryptophycin 1. 'The epoxide group of Cryptophycin 1 probably binds
covalently to its target receptor in the tumor cell. The novel cryptophycins
in the present
invention could potentially possess better in vivo activity than Cryptophycin
1 and
Cryptophycin 8 by exhibiting more favorable rates of epoxide formation in vivo
from the
corresponding chlorohydrin pro-drugs and covalent binding to the target
receptor in the
tumor cell.
The compounds of the present invention are more stable towards hydrolysis and
solvolysis than Cryptophycins 1 and 21. The ester bond connecting units C and
D in
Cryptophycin 1 is relatively sensitive to mild base hydrolysis, cleaving at pH
11 to a
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-75-
hydroxy acid with a half-life of 0.83 hour. The C-D ester bond in Cryptophycin
21,
which lacks the methyl group on C-2 of unit C, opens at a faster rate with a
half-life of
0.25 hour. The C-D ester bond is also sensitive to solvolysis. When methanol
is used in
the isolation scheme, considerable methanolysis of Cryptophycins 1 and 21
occurs.
Cryptophycin 21 is much more susceptible to methanolysis than Cryptophycin 1.
Cryptophycin 1 shows antitumor activity whereas Cryptophycin 21 is inactive,
probably
because the C-D ester bond of Cryptophycin 21 is hydrolyzed faster than the C-
D ester
bond of Cryptophycin 1 in vivo. Hydrolysis of the C-D ester bond may also
explain in
part the diminished in vivo activity of Cryptophycin 1 by intraperitoneal and
subcutaneous
routes of drag administration. The C-D ester bond of cryptophycins possessing
two
methyl groups on C-2 of unit C, such as the one found in Cryptophycin 52, is
stable at
pH 11.
The compounds of the present invention and the previously disclosed
cryptophycin
compounds can be therapeutically employed as anti-neoplastic agents and
thereby used in
methods to treat neoplastic diseases. As used herein, "neoplastic" pertains to
a neoplasm,
which is an abnormal growth, such growth occurring because of a proliferation
of cells
not subject to the usual limitations of growth. As used herein, "anti-
neoplastic agent" is
any compound, composition, admixture, co-mixture or blend which inhibits,
eliminates,
retards or reverses the neoplastic phenotype of a cell.
Chemotherapy, surgery, radiation therapy, therapy with biologic response
modifiers, and immunotherapy are currently used in the treatment of cancer.
Each mode
of therapy has specific indications which are known to those of ordinary skill
in the art,
and one or all may be employed in an attempt to achieve total destruction of
neoplastic
cells. Chemotherapy utilizing one or more cryptophycins is provided by the
present
invention. Moreover, combination chemotherapy, chemotherapy utilizing
cryptophycins
in combination with other neoplastic agents, is also provided by the subject
invention as
combination therapy is generally more effective than the use of single anti-
neoplastic
agents. Thus, a further aspect of the present invention provides compositions
containing
a therapeutically effective amount of at least one new cryptophycin compound
of the
present invention, including nontoxic addition salts thereof, which serve to
provide the
above-recited therapeutic benefits. Such compositions can also be provided
together with
physiologically tolerable liquid, gel or solid carriers, diluents, adjuvants
and excipients.
CA 02214565 2005-07-22
-76-
Such carriers, diluents, adjuvants and excipients may be found in the United
States
Phannacapeia Vol. XXII and National Fotmularv VolXVII, U.S. Phannacopeia
Convention, Inc., Rockville, MD (1989).
Additional modes of treatanent are provided in AHFS Drug Infor7nation,
1993 ed. by the American Hospital Formulary Service, pp. 522-660,
The present invention further provides that the pharmaceutical composition
used to
treat neoplastic disease contains at least one cryptophycin compound and at
least one
additional anti-neoplastic agent. Anti-neoplastic compounds which may be
utilized in
combination with cryptophycin include those provided in The Merck Index, 11th
ed.
Merck & Co., Inc. (1989) pp. Ther 16-17.
In a further embodiment of the invention, anti-neoplastic agents may be
antimetabolites which may include, but are not limited to, methotrexate, 5-
fluorouracil, 6-
mercaptopurine, cytosine arabinoside, hydroxyurea, and 2-chlorodeoxyadenosine.
In
another embodiment of the present invention, the anti-neoplastic agents
contemplated are
alkylating agents which may include, but are not limited to, cyclophosphamide,
melphalan, busulfan, paraplatin, chlorambucil, and nitrogen mustard. In a
further
embodiment of the subject invention, the anti-neoplastic agents are plant
alkaloids which
may include, but are not limited to, vincristine, vinblastine, taxol, and
etoposide. In a
further embodiment of the present invention, the anti-neoplastic agents
contemplated are
antibiotics which may include, but are not limited to, doxorubicin
(adriamycin),
daunorubicin, mitomycin c, and bleomycin. In a further embodiment of the
subject
inv.ention, the anti-neoplastic agents contemplated are hormones which may
include, but
are not limited to, calusterone, diomostavolone, propionate, epitiostanol,
mepitiostane,
testolactone, tamoxifen, polyestradiol phosphate, megesterol acetate,
flutamide,
nilutamide, and trilotane. In a further embodiment of the subject invention,
the anti-
neoplastic agents contemplated include enzymes which may include, but are not
limited
to, L-Asparaginase or aminoacridine derivatives which may include, but are not
limited
to, amsacrine. Additional anti-neoplastic agents include those provided in
Skeel,
Roland T., "Antineoplastic Drugs and Biologic Response Modifier:
Classification, Use
and Toxicity of Clinically Useful Agents," Handbook of Cancer Chemotherapv
(3rd ed.),
Little Brown & Co. (1991).
CA 02214565 2005-07-22
.77_
The present cryptophycin compounds and compositions can be administered to
mammals for veterinary use, such as for domestic animals, and clinical use in
humans in
a manner similar to other therapeutic agents. In general, the dosage required
for
therapeutic efficacy will vary according to the type of use and mode of
administration, as
well as the particularized requirements of individual hosts. Ordinarily,
dosages will range
from about 0.001 to 1000mg/kg, more usually 0.01 to 10mg/kg, of the host body
weight.
Alternatively, dosages within these ranges can be administered by constant
infusion over
an extended period of time, usually exceeding 24 hours, until the desired
therapeutic
benefits have been obtained. Indeed, drug dosage, as well as route of
administ.ration,
must be selected on the basis of relative effectiveness, relative toxicity,
growth
characteristics of tumor and effect of cryptophycins on cell cycle, drug
pharmacokinetics,
age, sex, physical condition of the patient, and prior treatment.
The cryptophycin compounds, with or without additional anti-neoplastic agents,
may be formulated into therapeutic compositions as natural or salt forms.
Pharmaceutically acceptable non-toxic salts include the base addition salts
(formed with
free carboxyl or other anionic groups) which may be derived from inorganic
bases such
as, for example, sodium, potassium, ammonium, calcium, or ferric hydroxides,
and such
organic bases as isopropylamine, trimethylamine, 2-ethylamino ethanol,
histidine,
procaine, and the like. Such salts may also be formed as acid addition salts
with any free
cationic groups and will generally be formed with inorganic acids such as, for
example,
hydrochloric or phosphoric acids, or organic acids such as acetic, oxalic,
tartaric,
mandelic, and the like. Additional excipients which the further invention
provides are
those available to one of ordinary skill in the art, for example, that found
in the United
States Pharnracopeia Vol. XXII and National Formularv Vol XVII, U.S.
Pharmacopeia
Convention, Inc., Rockville, MD (1989).
The suitability of particular carriers for inclusion in a given therapeutic
composition depends on the preferred route of administration. For example,
anti-
neoplastic compositions may be formulated for oral administration. Such
compositions
are typically prepared either as liquid solution or suspensions, or in solid
forms. Oral
formulations usually include such normally employed additives such as binders,
fillers,
carriers, preservatives, stabilizing agents, emulsifiers, buffers and
excipients as, for
example, pharmaceutical grades of mannitol, lactose, starch, magnesium
stearate, sodium
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-78-
saccharin, cellulose, magnesium carbonate, and the like. These compositions
take the
form of solutions, suspensions, tablets, pills, capsules, sustained release
formulations, or
powders, and typically contain 1%-95 % of active ingredient, preferably 2%-
70%.
Compositions of the present invention may also be prepared as injectable,
either as
liquid solutions, suspensions, or emulsions; solid forms suitable for solution
in, or
suspension in, liquid prior to injection may be prepared. Such injectables may
be
administered subcutaneously, intravenously, intraperitoneally,
intramuscularly,
intrathecally, or intrapleurally. The active ingredient or ingredients are
often mixed with
diluents or excipients which are physiologically tolerable and compatible with
the active
ingredient(s). Suitable diluents and excipients are, for example, water,
saline, dextrose,
glycerol, or the like, and combinations thereof. In addition, if desired, the
compositions
may contain minor amounts of auxiliary substances such as wetting or
emulsifying agents,
stabilizing or pH buffering agents.
The invention further provides methods for using cryptophycin compounds
encompassed by the genus structure to inhibit the proliferation of mammalian
cells by
contacting these cells with a cryptophycin compound in an amount sufficient to
inhibit the
proliferation of the mammalian cell. A preferred embodiment is a method to
inhibit the
proliferation of hyperproliferative mammalian cells. For purposes of this
invention,
"hyperproliferative mammalian cells" are mammalian cells which are not subject
to the
characteristic limitations of growth, e.g., programmed cell death (apoptosis).
A further
preferred embodiment is when the mammalian cell is human. The invention
further
provides contacting the mammalian cell with at least one cryptophycin compound
and at
least one additional anti-neoplastic agent. The types of anti-neoplastic
agents
contemplated are the same as those disclosed hereinabove.
The invention fureher provides methods for using cryptophycin compounds
encompassed by the genus structure to inhibit the proliferation of
hyperproliferative cells
with drug-resistant phenotypes, including those with multiple drug-resistant
phenotypes,
by contacting said cell with a cryptophycin compound in an amount sufficient
to inhibit
the proliferation of a hyperproliferative mammalian cell. A preferred
embodiment is
when the mammalian cell is human. The invention further provides contacting
the
mammalian cell with a cryptophycin compound and at least one additional anti-
neoplastic
CA 02214565 2005-07-22
-79-
agent. The types of anti-neoplastic agents contemplated are the same as those
disclosed
hereinabove.
The invention further provides a method for alleviating pathological
conditions
caused by hyperproliferating mammalian cells, for example, neoplasia, by
administering
to a subject an effective amount of a pharmaceutical composition provided
hereinabove to
inhibit the proliferation of the hyperproliferating cells. As used herein
"pathological
condition" refers to any pathology arising from the proliferation of mammalian
cells that
are not subject to the normal limitations of cell growth. Such proliferation
of cells may
be due to neoplasms, including, but not limited to the following neoplasms:
mammary,
small-cell lung, non-small-cell lung, colorectal, leukemia, melanoma, central
nervous
system (CNS), ovarian, prostate, sarcoma of soft tissue or bone, head and
neck, gastric
which includes pancreatic and esophageal, stomach, myeloma, bladder, renal,
neuroendocrine which includes thyroid and lymphoma, non-Hodgkin's and
Hodgkin's. In
a further embodiment of the invention, the neoplastic cells are human. The
present
invention further provides methods of alleviating such pathological conditions
utilizing
cryptophycin in combination with other therapies, as well as other anti-
neoplastic agents.
Such therapies and their appropriateness for different neoplasia may be found
in Cance
Principles and Practice of Oncology, 4th ed., Editors DeVita, V., Hellman, S.,
and
Rosenberg., S., Lippincott Co. (1993),
In the present disclosure, cryptophycin compounds are shown to potently
disrupt
the microtubule structure in cultured cells. In-addition, and in contrast with
the 1rRca
alkaloids, cryptophycin compounds appear to be a poor substrate for the drug-
efflux pump
P-glycoprotein. Cryptophycin 1 is the major cytotoxin in the blue-green alga
(cyanobacteria) Nosioc sp. strain designated GSV 224 and shows excellent
activity against
tumors implanted in mice. This cyclic didepsipeptide had previously been
isolated from
Nostoc sp. ATCC accession no. 53789 as an antifungal agent and its gross
structure was
previously determined. The relative and absolute stereochemistry of this
potentially
important drug has now been established using a combination of chemical and
spectral
techniques. Twenty-four additional cryptophycin compounds, Cryptophycins 2-7,
16-19,
21, 23, 24, 26, 28-31, 40, 43, 45, 49, 50 and 54 have also been isolated from
GSV 224
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-80-
and their total structures and cytotoxicities determined. Several derivatives
and
degradation products are described, both chemically and pharmacologically.
The following examples serve to illustrate certain preferred embodiments and
aspects of the present invention and are not to be construed as limiting the
scope thereof.
Experimental Section
In the experimental disclosure which follows, all weights are given in grams
(g),
milligrams (mg), micrograms ( g), nanograms (ng), picograms (pg), moles (mol)
or
millimoles (mmol), all concentrations are given as percent by volume (%),
molar (M),
millimolar (mM), micromolar ( M), nanomolar (nM), picomolar (pM), or normal
(N), all
volumes are given in liters (L), milliliters (mL) or microliters ( L), and
linear
measurements in millimeters (mm), unless otherwise indicated.
The following examples demonstrate the isolation and synthesis of cryptophycin
compounds as well as their use as therapeutic agents in accordance with the
invention.
In screening extracts of over 1000 blue-green algae (cyanobacteria) for
antitumor
activity, the lipophilic extract of Nostoc sp. GSV 224 was found to be
strongly cytotoxic,3
exhibiting minimum inhibitory concentrations (MICs) of 0.24ng/mL against KB, a
human
nasopharyngeal carcinoma cell line, and 6ng/mL against LoVo, a human
colorectal
adenocarcinoma cell line. More importantly, this extract showed significant
tumor
selective cytotoxicity in the Corbett assay.4=5 Bioassay monitored reversed-
phase
chromatography of the algal extract led to a fraction which was predominantly
Cryptophycin 1, a potent fungicide that had been isolated earlier from Nostoc
sp. ATCC
53789 by researchers at Merclc6=' and found to be very active against strains
of
Cryptococcus.
Cryptophycin 1 accounted for most of the cytotoxic activity of the crude algal
extract of Nostoc sp. GSV 224 and the pure compound showed IC50 values of 3
and
5pg/mL against KB and LoVo, respectively. In the Corbett assay Cryptophycin 1
was
found to be strongly tumor selective and equally cytotoxic against drug-
sensitive and
drug-resistant tumor cells. Immunofluorescence assays showed that Cryptophycin
1
interact with a cellular target similar to that of vinblastine, but differed
from the latter
drug in having a longer time course of action and in not forming
paracrystalline bodies.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-81-
In preliminary in vivo experiments, Cryptophycin 1 exhibited very promising
activity
against tumors implanted in mice.
Minor amounts of several other cryptophycin compounds were present in Nostoc
sp. GSV 224. Twenty-one of these could be isolated in sufficient quantities
for structure
determinations and antitumor evaluation in vitro by extraction of the alga
with 1 : 5
dichloromethane/acetonitrile and reversed-phase HPLC of the extract. .
Cryptophycins 2,
3, 4, 16, 17, 18, 19, 21, 23, 24, 26, 28, 29, 30, 31, 40, 43, 45, 49, 50 and
54
accompanied Cryptophycin 1 in the fraction eluted from a reversed-phase flash
column
with 65:35 acetonitrile/water. Cryptophycins 2, 3, 4, 5, 6, and 7 were the
only
compounds found when the alga was extracted with methanol and the reversed-
phase
chromatography was carried out with methanol/water. Cryptophycins 2, 3, 4, 5
and 6
were eluted with 3:1 methanol/water and Cryptophycin 7 was found in an
earlier, less
cytotoxic fraction eluted with 1:3 methanol/water. Acyclic Cryptophycins 5, 6
and 7
appear to be artifacts generated by decomposition of Cryptophycin 1 during the
isolation
procedure.
Cryptophycins 3 and 5 appeared to be identical with fungicidal semi-synthetic
compounds prepared from Cryptophycin 1 by researchers at Merck.8=9
Cryptophycin 3
was prepared by treating Cryptophycin 1 with a zinc-copper couple or with
diphosphorus
tetraiodide.8 Cryptophycin 5 was prepared by methanolysis of Cryptophycin 1.9
Examnle 1 Structure Determination
The determination of the structures of the new compounds, viz. Cryptophycins
2,
4, 6, 7, 8, 9, 10, 12, 14, 16, 17, 18, 19, 21, 23, 24, 26, 28, 29, 30, 31, 40,
43, 45, 49,
50 and 54, as well as those previously disclosed, were carried out in a
straightforward
manner using methodology that is well-known to those trained in the art. Mass
spectral
data were consistent with the molecular compositions. Proton and carbon NMR
data
obtained from COSY, HMQC, HMBC and NOESY spectra allowed one to assemble all
of the gross structures of these depsipeptide-type compounds. The presence of
the
various hydroxy and amino acid units in each compound were confirmed by gas
chromatographic mass spectral analysis. Total structures, including absolute
stereochemistries, were determined using a combination of chemical degradative
and
special analytical techniques on appropriate derivatives of the cryptophycin
compounds.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-82-
Example 2 Structure-Activity Relationships (SAR)
To probe the structural features in Cryptophycin 1 needed for optimal
activity, all
of the compounds described herein were evaluated for cytotoxicity against KB
(human
nasopharyngeal carcinoma), LoVo (human colon carcinoma), and SKOV3 (human
ovarian
carcinoma) cell lines. IC50 values are listed in Tables 1 and 2. Comparison of
the
cytotoxicities show that the intact macrolide ring, the epoxy and methyl
groups and the
double bond in the 7,8-epoxy-5-hydroxy-6-methyl-8-phenyl-2-octenoic acid unit
(see unit
A in Figure 1), the chloro and 0-methyl groups in the 3-(3-chloro-4-
methoxyphenyl)
alanine unit (unit B), the methyl group in the 3-amino-2-methylpropionic acid
unit (unit
C), and the isobutyl group in the leucic acid unit (unit D) of Cryptophycin 1
are needed
for optimal cytotoxicity. The potent cytotoxicity of Cryptophycin 8 is most
likely due to
the chlorohydrin functionality which acts as a masked epoxide.
The most active compounds were also evaluated for selective cytotoxicity
against
four different cell types, viz. a murine leukemia (L1210 or P388), a murine
solid tumor
(colon adenocarcinoma 38, pancreatic ductal adenocarcinoma 03, mammary
adenocarcinoma M16/M17), a human solid tumor (colon CX-1, HCT8, H116; lung
H125;
mammary MX-1, MCF-7), and a low malignancy fibroblast (LML), using the Corbett
assay4, a disk diffusion assay modeled after the one commonly used in
antifungal and
antibacterial testing. The results, shown in Table 1, indicated that
Cryptophycins 1-5 and
8 were neither solid tumor nor leukemia selective, but rather equally active
against tumor
cell lines, including drug-resistant ones such as M17. None of the compounds
showed a
zone of inhibition for any of the solid tumor cell lines that was 250 zone
units, i.e.
7.5mm, larger than the zone of inhibition for the leukemia cell line.
Cryptophycins 1-5
and 8, however, displayed markedly larger zones of inhibition (400 zone units)
for all of
the tumor cell lines compared with the zone of inhibition for the fibroblast
LML.
Diagnostically LML has been found to behave more like a normal cell than a
tumor cell
with respect to clinically-useful cytotoxic agents (see Corbett assay data for
5-
fluorouracil, etoposide and taxol in Table 1). Since the differential
cytotoxicities were
> 250 zone units, Cryptophycins 1-5 and 8 were tumor selective. These
compounds
therefore became candidates for in vivo testing.
Cryptophycin 1 is active against a broad spectrum of murine and human tumors
implanted in mice, including drug-resistant ones (Table 3). It exhibits
excellent activity
CA 02214565 1997-09-03
WO 96/40184 PCT/US96103246
-83-
against five early stage murine tumors, viz. colon adenocarcinomas #38 and
#51, taxol-
sensitive and taxol-resistant mammary #16/C/RP, and pancreatic ductal
adenocarcinoma
#03, and two early stage human tumors tested in SCID mice, viz. MX-1 breast
and H125
adenosquamous lung, showing tumor burden T/C (mean tumor burden in treated
animals/mean tumor burden untreated animals) values that are less than 10%.
T/C values that are less than 42% are considered to be active by NCI
standards;
T/C values that are less than 10% are considered to have excellent activity
and potential
clinical activity by NCI standards.4 Two of the trials showed gross (tumor
cell) log kill
values of 2Ø Gross log kill is defmed as T-03.2 Td where T is the median
tiune in
days for the tumors of the treated group to reach 750mg, C is the median time
in days for
the tumors of the control group to reach 750mg, and Td is the tumor volume
doubling
time. Gross log kill values of >2.8, 2.0-2.8, 1.3-1.9, 0.5-0.8, and <0.5 with
duration
of drug treatment of 5-20 days are scored + + + +, + + +, + +, + and -
(inactive),
respectively. An activity rating of +++ to ++++, which is indicative of
clinical
activity, is needed to effect partial or complete regression of 100-300mg size
masses of
most transplanted solid tumors of mice.
Cryptophycin 8 is also active against a broad spectrum of tumors implanted in
mice (Table 4). It has shown excellent activity against all of the tumors
tested to date,
showing tumor burden T/C values < 10%, but more importantly gross log kill
activity
ratings of +++ to ++++ and some cures.
Good in vivo activity was also seen with Cryptophycin 35 in the one trial that
has
been run to date.
Lethal toxicity observed during testing of Cryptophycins 1 and 8 was
attributed to
leucopenia which is common to all clinically used antitumor drugs.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-84-
Example 3 Culture Conditions
Nostoc sp. GSV 224 was obtained from Professor C.P. Wolk, MSU-DOE Plant
Research Laboratory, Michigan State University. Nostoc sp. ATCC 53789 was
purchased
from the American Type Culture Collection. A 1L flask culture of alga was used
to
inoculate an autoclaved 20L glass carboy containing an inorganic medium,
designated
modified BG-113, the pH of which had been adjusted to 7.0 with NaOH. Cultures
were
continuously illuminated at an incident intensity of 200 mol photons m-Zsec'
(photosynthetically active radiation) from banks of cool-white fluorescent
tubes and
aerated at a rate of 5L/min with a mixture of 0.5 % CO2 in air at a
temperature of 24 t
1' C. Typically, the culture was harvested by filtration after 21 days. The
yields of
lyophilized Nostoc sp. GSV 224 and ATCC 53789 averaged 0.61 and 0.3g/L of
culture,
respectively.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-85-
Example 4 Isolation
Method A: The lyophilized Nostoc sp. GSV224 (50g) was extracted with 2 L of 1:
5
CH2C12/CH3CN for 48 h and the extract concentrated in vacuo to give a dark
green solid.
The residue (1g; KB MIC 0.24ng/mL) was applied to an ODS-coated silica column
(55g,
7 x 5cm) and subjected to flash chromatography with 1:3 CH3CN/H20 (0.8 L), 1:1
CH3CN/H20 (0.8 L), 65:35 CH3CN/H20 (1.0 L), MeOH (0.8 L), and CH2C12 (0.5 L).
The fraction that was eluted with 65:35 CH3CN/H20 (420mg; KB MIC 14pg/mL) was
subjected to reversed-phase HPLC (Econosil C18, 10 , 25cm x 21.5mm, UV
detection at
250nm, 65:35 CH3CN/H2O, flow rate 6mL/min) to obtain Cryptophycin 1(tR 49.3
min,
220mg) and a number of impure fractions. The fraction eluted from the Econosil
C18
column at tR 28.8 min was further purified by normal phase HPLC (Econosil
silica 51t
cartridge, 250 x 4.6mm, 6:4 ethyl acetate/hexane, 3mL/min) to give
Cryptophycin 16
(3.0mg). The fraction eluted from the Econosil C18 column at tR 32.5 min was
subjected
to HPLC on the Econosil silica column using 55:45 ethyl acetate/hexane at
3mL/min to
give Cryptophycin 24 (0.8mg). The fraction eluted from the Econosil C18 column
at tR
35.5 min was subjected to HPLC twice on the Econosil silica column, first
using 1: 1
ethyl acetate/hexane at 3mL/min and second using 4 : 6 ethyl acetate
/methylene chloride
at 2.5mL/min to give Cryptophycin 23 (1.2mg) and Cryptophycin 43 (0.1mg). The
fraction eluted from the Econosil C18 column at tR 39.5 min was subjected to
HPLC on
the Econosil silica column with 1:1 ethyl acetate/hexane at 3mL/min to give
Cryptophycin
2 (6mg) and Cryptophycin 21 (14mg) and a complex mixture of cryptophycins
eluted at tR
32.5 min. This latter fraction, accumulated from 400g dry alga, was
chromatographed
successively on a semi preparative column (partisil C18, 250 x 9.4mm, l01L)
with 35:65
water/acetonitrile and a reversed phase analytical column (Econosil, 150 x
4.6mm, 5 )
with 5:4:1 water/acetonitrile/methanol at 1.3mL/min to give Cryptophycin 50
(tR 34.8,
0.4mg) and Cryptophycin 40 (tR 38.8 min, 0.3mg). The fraction eluted from the
Econosil
C18 column at tR 44.5 min was subjected to HPLC on the Econosil silica column
with 1:1
ethyl acetate/hexane at 3mL/min to give Cryptophycin 17 (0.3mg). Normal phase
HPLC
purification of the fraction eluted from the Econosil C18 column at tR 54.5 as
a shoulder
to Cryptophycin 1 yielded Cryptophycin 45 (tR 6.7 min, 0.1mg), Cryptophycin 26
(tR 8.9
min, 0.5mg), and Cryptophycin 54 (tR 19.8 min, <0.1mg) on elution with 1:1
ethyl
acetate/hexane. The fraction eluted from the Econosil C18 column as a broad
peak (tR 58
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-86-
to 70 min) was subjected to HPLC on the Econosil silica colunm with 43:57
ethyl
acetate/hexane at 2.5mL/min to give Cryptophycin 4(tR 19.6 min, 1.5mg),
Cryptophycin
31 (tR 9.4 min, 0.8mg), Cryptophycin 19 (tR 25.8min, 0.3mg), Cryptophycin 49
(tR 28
min, 0.lmg), Cryptophycin 28 (tR 29.0min, 0.5mg) and impure Cryptophycin 29
(tR 52.5
min, 2.0mg) and Cryptophycin 30 (tR 49 min, 3.0mg). Cryptophycins 29 and 30
obtained
pure after reversed phase HPLC (Econosil C18, 10 , 250 x 10mm, 3:1
methanol/water). The fraction eluted from the Econosil C18 column at tR 78.9
min was subjected to HPLC
on the Econosil silica column with to give Cryptophycin 3(tR 16.4 min, 3.0mg).
The
fraction eluted from the Econosil C18 column at tR 82.8 min was subjected to
HPLC on
the Econosil silica column with 45:55 ethyl acetate/hexane at 3mL/min to give
Cryptophycin 18 (tR 19.2, 0.8mg).
Method B: The lyophilized Nostoc sp. GSV 224 (12.23g) was extracted twice with
700mL and 400mL portions of MeOH for 12 and 5 hours (h), respectively. The
extracts
were combined and concentrated in vacuo to give 1.84g of a dark green solid
which was
partitioned between water and CH2C12. The lipophilic portion (0.65g; KB MIC
0.24ng/mL) was applied to an ODS-coated silica column (55g, 7 x 5cm) and
subjected to
flash chromatography with 1:3 MeOH/H20 (0.8L), 1:1 MeOH/H20 (0.8L), 3:1
MeOH/H2O (0.8L), MeOH (0.8L), and CH2C12 (0.5L). The fraction that was eluted
with
3:1 MeOH/H20 (22mg; KB MIC 14pg/mL), which accounted for essentially all of
the
cytotoxic activity, was subjected to reversed-phase HPLC (Econosil C18, 10 ,
250
x10mm, UV detection at 250nm, flow rate 3mL/min) using 1:5 MeOH/H20 as the
eluant
to give Cryptophycins 7(tR 7.6 min, 0.2mg), 5(tR 15.4 min, 2.3mg), 2(tR 16.0
min,
1.0mg), 1(tR 19.0 min, 12.0mg), 4(tR 26.5 min, 1.2mg), and 3(tR 30.2 min,
1.4mg).
From one of the cultures the fraction (8.lmg) that eluted from the flash
column with 1:3
MeOH/H20 showed milder cytotoxicity (KB MIC 2 g/mL). Purification on HPLC
using
2:3 MeOH/H2O as the eluant yielded Cryptophycin G (7, tR 6.0 min, 2.4mg).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-87-
Example 5 Spectral Data for Cryptophycins 1-7
The bold italicized letters in the spectral data refer to the units A-D in
Figure 1.
Cryptophycin 1
[a]D +33.8' (MeOH, c 1.83); UV X.(e) 208 (42,400), 218 (33,700), 228
(23,800), 280 (2,210); CD [0]202 +15,900, [0]206 +64,900, [0]214 +26,900,
[0]224
+46,300, [0]237 +10,500. IR (CHC13) P. 3425, 2963, 1751, 1719, 1677, 1502,
1259
cm'. EIMS m/z (rel intensity) 654/656 (20/9), 412/414 (33/12), 280/282
(31/12), 227
(80), 195/197 (92/44), 91 (100); high resolution EIMS m/z 654.2665 (calcd for
C35H43C1N208, 4.3mmu error). 'H NMR (CDC13): amino or hydroxy acid unit
S(carbon
position, multiplicity; J in Hz) 7, 8-epoxy-5-hydroxy-6-methyl-8 phenyl-2-
octenoic acid (A)
5.74 (2, dt; 15.5 and 0.9), 6.68 (3, ddd; 15.5, 9.6 and 5.2), 2.45 (4, ddd;
14.2, 11.1 and
9.6), 2.55 (4, brdd; 14.2 and 5.2), 5.16 (5, ddd; 11.1, 4.9 and 1.9), 1.80 (6,
m), 1.14
(6-Me, d; 7.1), 2.92 (7, dd; 7.5 and 2.0), 3.69 (8, d; 2.0), 7.25 (10/14, m),
7.34-7.39
(11/12/13, m); leucic acid (D) 4.83 (2, dd; 6.8 and 3.3), 1.70 (3, m), 1.36
(3, m),
1.70(4, m), 0.86 (5, d; 6.6), 0.85 (4-Me, d; 6.6); 3-amino-2-methylpropionic
acid (C)
2.71 (2, m), 1.22 (2-Me, d; 7.1), 3.30 (3, ddd; 13.4, 5.8 and 3.8), 3.48 (3,
ddd; 13.4,
6.3 and 5.8), 6.93 (3-NH, brt; 5.8); 3-chloro-4-methoxyphenylalanfne (B) 4.80
(2, ddd;
8.7, 7.3 and 5.4), 5.61 (2-NH, d; 8.7), 3.03 (3, dd; 14.4 and 7.3), 3.13 (3,
dd; 14.4 and
5.4), 7.21 (5, d; 2.1), 3.87 (7-OCH3,s), 6.83 (8, d; 8.5), 7.07 (9, dd; 8.5
and 2.1). 13C
NMR (CDC13): unit 6(carbon position) A 165.3 (1), 125.3 (2), 141.0 (3), 36.7
(4), 76.2
(5), 40.6 (6), 13.5 (6-Me), 63.0 (7), 59.0 (8), 136.7 (9), 125.6 (10/14),
128.7 (11/13),
128.5 (12); D 170.7 (1), 71.3 (2), 39.4 (3),.24.5 (4), 22.9 (5), 21.3 (4-Me);
C 175.6(1),
38.2 (2), 14.1 (2-Me), 41.1 (3); B 170.9 (1), 53.6 (2), 35.0 (3), 129.7 (4),
131.0 (5),
122.4 (6), 154.0 (7), 56.1 (7-OCH3), 112.2 (8), 128.4 (9).
Cryptophycin 2
[a]D +20.4' (MeOH, c 0.54); UV X.(e) 206 (43,800), 218 (37,500), 232
(22,900), 278 (2,410); CD [0]203 +54,100, [0]212 +16,500, [0]=5 +53,600,
[0]236 -
14,000. IR (CHC13) P. 3423, 3029, 2961, 1742, 1724, 1678, 1512, 1258 cm-'.
EIMS
m/z (rel intensity, assignment) 620 (11, M*), 431 (3), 378(8), 377 (6), 311
(11), 246
(10), 244 (8), 227 (14), 195 (17), 161 (84, CH3O-C6H4-CH=CH=CO+), 121 (79,
CH3O-
C6H4-CHZ+), 91 (100); high resolution EIMS m/z 620.3094 (calcd for C35H44N2O8,
0.3mmu error); 161.0605 (calcd for C10I1902, -0.2mmu error); 121.0658 (calcd
for
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-88-
C8H9O, -0.4mmu error). 'H NMR (CDC13): amino or hydroxy acid unit S(carbon
position, multiplicity; J in Hz) 7, 8-epoxy-5-hydroxy-6-methyl-8 phenyl-2-
octenoic acid (A)
5.71 (2, dd; 15.4 and 1.3), 6.70 (3, ddd; 15.4, 10.2 and 5.0), 2.45 (4, m),
2.55 (4, m),
5.18 (5, ddd; 11.3, 4.8 and 2.0), 1.79 (6, m), 1.14 (6-Me, d; 7.0), 2.92 (7,
dd; 7.7 and
2.0), 3.68 (8, d; 2.0), 7.24 (10/14, m), 7.34-7.39 (11/12/13, m); leucic acid
(D) 4.82 (2,
dd; 10.1 and 3.7), 1.70 (3, m), 1.33 (3, m), 1.70 (4, m), 0.86 (5, d; 6.4),
0.84 (4-Me,
d; 6.4); 3-amino-2-methylpropionic acid (C) 2.68 (2, m), 1.23 (2-Me, d; 7.3),
3.39 (3-
H2, m), 7.02 (3-NH,brt; 6.0); O-methyltyrosine (B) 4.79 (2, ddd; 8.1, 7.0 and
5.7), 5.55
(2-NH, d; 8.1), 3.07 (3, dd; 14.5 and 7.0), 3.13 (3, dd; 14.5 and 5.7), 7.10
(5/9, d;
8.6), 6.81 (6/8, d; 8.6), 3.78 (7-OCH3, s). 13C NMR (CDC13): unit S(carbon
position) A
165.1 (1), 125.1 (2), 141.1 (3), 36.7 (4), 76.0 (5), 40.7(6), 13.6 (6-Me),
63.0 (7), 59.0
(8), 136.7 (9), 125.6 (10/14), 128.7 (11/13), 128.5 (12); D 170.6(1), 71.3
(2), 39.4 (3),
24.5 (4), 21.3 (5), 22.9 (4-Me); C 176.0 (1), 38.1 (2), 14.2 (2-Me), 40.7 (3);
B 171.1
(1), 53.9 (2), 35.3 (3), 131.0 (4), 130.2 (5/9), 114.1 (6/8), 158.6 (7), 55.2
(7-OCH3).
Crxptophycin 3
[a]D +20.3 (MeOH, c 1. 13); UV X.(e) 206 (51,700), 218 (31,200), 230
(22,900), 246 (18,800), 280 (3,230); CD [0]205 +50,000, [0]212 -390, [0]218 -
47,200, [61233
-100, [8]25l +33,400, [0]271 +4,310. IR (CHC13) v,,. 3417, 2926, 1742, 1721,
1676,
1499, 1336 cm 1. EIMS m/z (rel intensity) 638/640 (2/0.7, M+), 412/414
(63/19),
280/282 (15/5), 227 (100), 195 (63), 91 (98); high resolution EIMS m/z
638.2764 (calcd
for C35H43C1N207, -0.5mmu error), 412.1516 (calcd for C20H27CIN06, 1.lmmu
error),
227.1293 (calcd for C15H17NO, 1.Ommu error). 'H NMR (CDC13): amino or hydroxy
acid unit 8(carbon position, multiplicity; J in Hz) 5-hydroxy-6-methyl-8
phenyl-2, 7-
octadienoic acid (A) 5.77 (2, d; 15.5), 6.68 (3, ddd; 15.5, 9.5 and 5.3), 2.37
(4, m),
2.54 (4, m), 5.01 (5, ddd; 11.4, 6 and 1.5), 2.56 (6, m), 1.14 (6-Me, d; 7.0),
6.01 (7,
dd; 15.8 and 8.8), 6.41 (8, d; 15.8), 7.28-7.34 (10/11/13/14, m), 7.23 (12,
m); leucic
acid (D) 4.84 (2, dd; 10.1 and 3.6), 1.62 (3, m), 1.36 (3, m), 1.62 (4, m),
0.77 (5, d;
6.5), 0.73 (4-Me, d; 6.3); 3-amino-2-methylpropionic acid (C) 2.71 (2, m),
1.22 (2-Me,
d; 7.3), 3.28 (3, dt; 13.5 and 7.0), 3.50 (3, ddd; 13.5, 4.9 and 4), 6.93 (3-
NH, brt; 6.3);
3-chloro-4-methoxyphenylalanine (B) 4.82 (2, m), 5.64 (2-NH, d; 8.8), 3.05 (3,
dd; 14.5
and 7.0), 3.13 (3, dd; 14.5 and 5.5), 7.22 (5, d; 2.2), 3.87 (7-OCH3, s), 6.84
(8, d;
8.5), 7.08 (9, dd; 8.5 and 2.2). 13C NMR (CDC13): unit 8(carbon position) A
165.4 (1),
CA 02214565 1997-09-03
WO 96/40184 PCTNS96/03246
-89-
125.2 (2), 141.4 (3), 36.5 (4), 77.1 (5), 42.3 (6), 17.3 (6-Me), 130.1(7),
130.0 (8),
136.7 (9), 126.1 (10/14), 128.6 (11/13), 128.4 (12); D 170.1 (1), 71.6 (2),
39.5 (3),
24.5 (4), 21.2 (5), 22.7 (4-Me); C 175.6 (1), 38.3 (2), 14.0 (2-Me), 41.2 (3);
B 170.9
(1), 53.5 (2), 35.1 (3), 129.8 (4), 131.0 (5), 122.4 (6), 154.0 (7), 56.1 (7-
OCH3), 112.2
(8), 127.6 (9).
Cryptophycin 4
[a]D +36.7 (MeOH, c 1.93); UV) A.(e) 206 (41,800), 228 (25,000), 240
(21,200), 248 (22,500), 280 (3,000), 290 (1,230); CD [0]205 +63,900, [0]211
+3,040,
[e1218 -71,900, [0]229 -11,700, [0]234 -130,[01u2 +47,500, [91270 +5,400. IR
(CHC13)v.
3410, 2962, 2917, 1741, 1718, 1678, 1511, 1251 cm-'. EIMS mlz (rel intensity)
604 (2,
M+), 378 (74), 246 (11), 227 (46), 161 (100), 91 (96); high resolution EIMS
m/z
604.3127 (calcd for C35H44N2O7, 2.2mmu error), 378.1910 (calcd for C20H28NO6,
0.7mmu
error), 227.1293 (calcd for C15H17NO, 1.7mmu error), 161.0605 (calcd for
C,oH902, -
0.2mmu error). 'H NMR (CDC13): amino or hydroxy acid unit 8(carbon position,
multiplicity; J in Hz) 5-hydroxy-6-methyl-8 phenyl-2, 7-octadienoic acid (A)
5.74 (2, dd;
15.3 and 1.2), 6.71 (3, ddd; 15.3, 10.3 and 5.0), 2.37 (4, m), 2.53 (4, m),
5.03 (5, ddd;
11.2, 6.4 and 2.0), 2.55 (6, m), 1.13 (6-Me, d; 6.8), 6.01 (7, dd; 15.8 and
8.8), 6.40 (8,
d; 15.8), 7.28-7.37 (10/11/13/14, m), 7.22 (12, m); leucic acid (D) 4.84 (2,
dd; 10.1 and
3.6), 1.65 (3, m), 1.34 (3, m), 1.65 (4, m), 0.75 (5, d; 6.5), 0.72 (4-Me, d;
6.3); 3-
amino-2-methylpropionic acid (C) 2.69 (2, m), 1.22 (2-Me, d; 7.5), 3.39 (3-H2,
m), 7.03
(3-NH, brt; 6.0); O-methyltyrosine (B) 4.79 (2, m), 5.61 (2-NH, d; 7.8), 3.08
(3, dd;
14.5 and 7.0), 3.13 (3, dd; 14.5 and 5.3), 7.11 (5/9, d; 8.8), 6.81 (6/8, d;
8.8), 3.78 (7-
OCH3, s). 13C NMR (CDC13): unit S(carbon position) A 165.3 (1), 125.1 (2),
141.5 (3),
36.5 (4), 77.1 (5), 42.3 (6), 17.3 (6-Me), 130.1 (7), 131.8 (8), 136.7 (9),
126.2 (10/14),
128.7 (11/13), 127.6 (12); D 170.8 (1), 71.6 (2), 39.5 (3), 24.5 (4), 21.2
(5), 22.7
(4-Me); C 175.9 (1), 38.2 (2), 14.2 (2-Me), 40.9 (3); B 171.2 (1), 53.8 (2),
35.3 (3),
131.0 (4), 130.2 (5/9), 114.1 (6/8), 158.6 (7), 55.2 (7-OCH3).
CrXptophycin 5
[a]D +36.0' (MeOH, c 0.55); UV X.(e) 206 (45,600), 218 (37,700), 280
(3,790), 286 (3,480), 325 (2,080); CD [0]203 +7,710, [9]206 +29,000, [0]210
+21,400,
[0]= +59,800, [0]234 +12,800, [0124, +13,700. IR (CHC13) v,,. 3426, 2958,
1728,
1672, 1502, 1259 cm'. EIMS m/z (rel intensity) 686/688 (0.1510.05), 655/657
(1/0.3),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-90-
654/656 (1.5/0.5), 311/313 (75/27), 195 (66), 155 (54), 121 (51), 91 (100);
high
resolution EIMS m/z 686.2983 (calcd for C36H47CIN209, -1.3mmu error). 'H NMR
(CDC13): amino or hydroxy acid unit 6(carbon position, multiplicity; J in Hz)
7, 8-epoxy-
S-hydroxy-6-methyl-8 phenyl-2-octenoic acid (A) 5.87 (2, d; 15.3), 6.72 (3,
dt; 15.3 and
6.8), 2.60 (4, m), 2.52 (4, ddd; 15.2, 7.8, and 6.8), 5.11 (5, ddd; 12.3, 7.8,
and 7.1),
1.87 (6,m), 1.12 (6-Me, d; 7.1), 2.91 (7, dd; 7.3 and 2.1), 3.70 (8, d; 2.1),
7.24 (10/14,
brd; 7.4), 7.29-7.36 (11/12/13, m); leucic acid (D) 4.09 (2, m), 2.86 (2-OH,
brd, 6.1),
1.83 (3, m), 1.42 (3, m), '1.86 (4, m), 0.90 (5, d; 6.6), 0.87 (4-Me, d; 6.8);
3-amino-2-
methylpropionic acid (C) 3.64 (1-OCH3, s), 2.60 (2, m), 1.07 (2-Me, d; 7.3),
3.27 (3,
ddd; 13.5, 8.0 and 5.5), 3.39 (3, m), 6.32 (3-NH, t; 5.4); 3-chloro-4-
methoxyphenylalanine (B) 4.59 (2, dt; 6 and 7.5), 6.30 (2-NH, d; 7.5), 2.95
(3, dd; 13.6
and 7.5), 3.0 (3, dd; 13.6 and 6.0), 7.2 (5, d; 2.1), 3.86 (7-OCH3, s), 6.84
(8, d; 8.5),
7.05 (9, dd, 8.5; 2.1). 13C NMR (CDC13): unit 6(carbon position) A 164.8 (1),
126.5
(2), 139.2 (3), 34.4 (4), 75.5 (5), 39.2 (6), 12.9 (6-Me), 63.3 (7), 58.7 (8),
136.8 (9),
125.7 (10/14), 128.6 (11/13), 128.4 (12); D 175.1 (1), 69.2 (2), 43.2 (3),
24.3 (4), 21.2
(5), 23.2 (4-Me); C 175.4 (1), 51.9 (1-OMe), 39.1 (2), 14.7 (2-Me), 41.6 (3);
B 170.6
(1), 54.6 (2), 37.4 (3), 129.5 (4), 131.0 (5), 122.4 (6), 154.1 (7), 56.1 (7-
OMe), 112.2
(8), 128.4 (9).
Cryptophycin 6
[a]D +17.1 (MeOH, c 1.1); UV X,.(e) 206 (40,000), 218 (30,100), 228
(21,400), 282 (2,430); CD [0]203 +37,700, [01210 -5,430, [0]213 -1,260, [0]221
+24,100,
[6]232 +8,480, [9]2 +13,400, [0]254 +790. IR (CHC13) v11187C 3425, 3006, 2956,
1726,
1672, 1641, 1502, 1462, 1259 cm 1. FABMS (thioglycerol) m/z, (rel intensity)
573/575
(13/6) [M-H2O]+, 217 (26), 91 (100). 'H NMR(CDC13): amino or hydroxy acid unit
S
(carbon position, multiplicity; J in Hz) 5, 7, 8-trihydroxy-6-methyl-8 phenyl-
2-octenoic acid
(A) 5.92 (2, dt; 15.0 and 1.5), 6.94 (3, dt; 15 and 7.5), 2.51 (4, m), 2.64
(4, m), 3.97
(5, ddd; 9.3, 6.5 and 4.5), 2.03 (6, m), 1.10 (6-Me, d; 6.5), 3.70 (7, dd; 9.0
and 7.5),
4.64 (8, d; 7.5), 7.33-7.39 (10/11/13/14, m), 7.28 (12, tt; 6.5 and 2.0); 3-
chloro-4-
methoxyphenylalanine (B) 4.60 (2, td; 8.0 and 6.0), 6.09 (2-NH, brd; 8.0),
2.96 (3, dd;
.30 13.8 and 8.0), 3.02 (3, dd; 13.8 and 6.0), 7.22 (5, d; 2.0), 3.86 (7-OCH3,
s), 6.84 (8, d;
8.5), 7.07 (9, dd; 8.5 and 2.0) 3-amino-2-methylpropionic acid (C) 3.63 (1-
OCH3,s),
2.58 (2, m), 1.07 (2-Me, d; 7.0), 3.24 (3, ddd; 13.8, 8 and 6.5), 3.41 (3,
ddd; 13.8, 6.5
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-91-
and 4.8), 6.21 (3-NH, brt; 6.5). 13C NMR (CDC13): unit 6(carbon position) A
165.2
(1), 125.6 (2), 141.3 (3), 36.9 (4), 82.5 (5), 46.3 (6), 14.3 (6-Me), 85.1
(7), 84.8 (8),
140.9 (9), 125.8 (10/14), 128.6 (11/13), 127.8 (12); B 170.6 (1), 54.5 (2),
37.3 (3),
129.6 (4), 131.0 (5), 122.5 (6), 154.1 (7), 56.1 (7-OCH3), 112.2 (8), 128.5
(9) C 52.0
(1-OCH3), 175.4 (1), 39.2 (2), 14.7 (2-Me), 41.6 (3).
Cryptoph cy in 7
[a]D -51.9 (MeOH, c 0.89); UV X.(e) 206 (23,400), 220 (14,900), 282 (1,670);
CD [0]202 +35,400, je1206 -1,730, 101211 -19,200, [0]220 -15,800, [0]232
+29,000, [0]263
+2,040. IR (CHC13) P. 3426, 2946, 1732, 1675, 1501, 1258 cm-1. EIMS m/z (rel
intensity) 455/457 (1/0.3, [M-2HZO]+), 105 (100), 77 (98); FABMS m/z (magic
bullet
matrix) 496/498 [M-H20+Na]+, (thioglycerol matrix) 474/476 [M-H201+H]+. 'H NMR
(CD3OD): amino or hydroxy acid unit a(carbon position, multiplicity; J in Hz)
5, 7, 8-
trihydroxy-6-methyl-8 phenyl-2-octenoic acid (A) 6.06 (2, ddd; 15.5, 1.3 and
1.0), 6.80
(3, dt; 15.5 and 7.5), 2.49 (4, m), 2.59 (4, m), 3.92 (5, ddd; 9.5, 6.3 and
4.7), 1.95 (6,
m), 1.08 (6-Me, d; 6.7), 3.59 (7, dd; 9.0 and 7.8), 4.56 (8, d; 7.8), 7.37
(10/14, brd;
7.3), 7.31 (11/13, brt; 7.3), 7.24 (12, tt; 7.3 and 1.5); 3-chloro-4-
methoxyphenylalanfne
(B) 4.52 (2, dd; 6.9 and 5.0), 2.93 (3, dd; 13.8 and 6.9), 3.15 (3, dd; 13.8
and 5.0),
7.20 (5, d; 2.2), 3.78 (7-OCH3, s), 6.88 (8, d; 8.4), 7.08 (9, dd; 8.4 and
2.2). 13C
NMR (CD3OD): unit S(carbon position) A 167.4 (1), 127.6 (2), 140.9 (3), 37.9
(4), 84.0
(5), 47.6 (6), 14.4 (6-Me), 86.0 (7), 85.8 (8), 142.9 (9), 127.1 (10/14),
129.3 (11/13),
128.5 (12); B 177.6 (1), 57.3 (2), 38.2 (3), 132.8 (4), 132.1 (5), 122.9 (6),
155.0 (7),
56.5 (7-OCH3), 113.2 (8), 130.1 (9).
Cryptophycin 16
[a]D + 41.3 (MeOH, c 5.2); UV ~, (e) 242 (4963), 280 (2430), 286 (2212); IR
(neat) v11187C 3402, 3270, 2960, 1748, 1724, 1676, 1514, 1466, 1343, 1239,
1177 cm-1;
EIMS m/z (rel intensity) 640/642 (66/27), 398/400 (47/16), 265 (55), 227 (93),
181
(100); high resolution EIMS m/z 640.25676 (calcd for C34H41C1N2O8, -1.6mmu
error).
'H NMR (CDC13): amino or hydroxyacid unit 6(carbon position, multiplicity; J
in Hz) 7,
8-epoxy-5-hydroxy-6-methyl-8-phenyl-2-octenoic acid (A) 5.74 (2, d; 16), 6.67
(3, ddd;
15.3, 9.7 and 5.5), 2.45 (4, dt; 14.3 and 10.4), 2.55 (4, brdd; 14.3 and 5.3),
5.15 (5,
ddd; 11.2, 4.8 and 1.8), 1.8 (6, m), 1.14 (6-Me, d; 7.0), 2.92 (7, dd; 7.5 and
2.0), 3.69
(8, d; 2.0), 7.24-7.26 (10/14, m), 7.33-7.39 (11/12/13, m); 3-chloro-4-
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-92-
hydroxyphenylalanine (B) 4.8 (2, m), 5.64 (2-NH, d; 8.8), 3.03 (3, dd; 14.5
and 7.0),
3.11 (3, dd; 14.4 and 5.6), 7.17 (5, d; 2.2), 5.61(7-OH, s), 6.91 (8, d; 8.3),
7.0 (9, dd;
8.3 and 2.2); 3-amino-2-methylpropionic acid (C) 2.71 (2, m), 1.22 (2-Me, d;
7.3), 3.28
(3, dt; 13.6 and 6.8), 3.49 (3, ddd; 13.6, 5 and 4.1), 6.92 (3-NH, br t; 6.1);
leucic acid
(D) 4.83 (2, dd; 10.1 and 3.3), 1.36 (3, m), 1.67-1.75 (3, m), 1.67-1.75 (4,
m), 0.85 (5,
d; 7.5), 0.86 (4-Me, d; 6.8). 13C NMR (CDC13) unit 6(carbon position) A 165.3
(1),
125.3 (2), 141.0 (3), 36.7 (4), 76.2 (5), 40.6 (6), 13.5 (6-Me), 63.0 (7),
59.0 (8), 136.8
(9), 125.6 (10/14), 128.7 (11/13), 128.6 (12); B 170.9 (1), 53.6 (2), 35.1
(3), 129.9 (4),
129.6 (5), 120.0 (6), 150.4 (7), 116.4 (8), 129.2 (9); C 175.6 (1), 38.3 (2),
14.1 (2-Me),
41.1 (3); D 170.8 (1), 71.3 (2), 39.4 (3), 24.6 (4), 21.3 (5), 22.9 (4-Me).
Cryptophvcin 17
[a]D + 27.8 (CHC13 c. 0.37); UV ~(e) 248 (14740), 268 (8100), 278 (3400),
284 (2840); IR (neat) v11187C 3412, 2958, 1750, 1723, 1668, 1504, 1463, 1290,
1177, 751
cm-'; EIMS m/z (rel intensity) 624/626 (10/3), 398/400 (95/35), 284 (100), 149
(95);
high resolution EIMS m/z 624.26161 (calcd for C34H41C1NZO7, -1.4mmu error). 'H
NMR
(CDC13): amino or hydroxyacid unit 6(carbon position, multiplicity; J in Hz) 5-
hydroxy-
6-methyl-8 phenyl-2, 7-octadienoic acid (A) 5.77 (2, d; 15.4), 6.67 (3, ddd;
15.4, 9.5, and
5.3), 2.37 (4, m), 4.99 (5, ddd; 11.2, 6.3, and 1.6), 2.54 (6, m), 1.14 (6-Me,
d; 6.7),
6.01 (7, dd; 15.7, and 8.7), 6.41 (8, d; 15.9), 7.28-7.34 (10/11/13/14, m),
7.23 (12, m);
3-chloro-4-hydroxyphenylalanine (B) 4.82 (2, m), 5.63 (2-NH, d; 8.7), 3.12 (3,
dd; 14.7,
and 5.6), 3.03 (3', dd; 14.7, and 7.1), 7.18 (5, d; 2.0), 5.47 (7-OH, br s),
6.91 (8, d;
8.3), 7.02 (9, dd; 8.3, and 2.0); 3-amino-2-methylpropionic acid (C) 2.71 (2,
m), 1.21
(2-Me, d' 6.9), 3.25 (3, m), 3.52 (3', m), 6.89 (3-NH, br t; 6.1); luecic acid
(D) 4.84
(2, dd; 9.6, and 3.1), 1.62 (3, m), 1.36 (3', m), 1.62 (4, m), 0.77 (5, d'
6.5), 0.73
(4-Me, d; 6.5); 13C NMR (CDC13) unit 6(carbon position) A 165.4 (1), 125.3
(2), 141.3
(3), 36.5 (4), 77.1 (5), 42.3 (6), 17.3 (6-Me), 130.0 (7), 129.9 (8), 136.7
(9), 126.2
(10/14), 128.6 (11/13), 127.6 (12); B 170.9 (1), 53.5 (2), 35.1 (3), 129.6
(4), 131.9 (5),
126.2 (6), 150.3 (7), 116.3 (8), 127.6 (9); C 175.9 (1), 38.4 (2), 13.9 (2-
Me), 41.3 (3);
D 170.9 (1), 71.6 (2), 39.5 (3), 24.5 (4), 21.2 (5), 22.7 (4-Me).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-93-
Cryptophvcin 18
[a]D + 54.9 (MeOH, c 0.93); UV A. (e) 250 (20518), 284 (3857); IR (neat)
P. 3411, 3271, 2966, 1746, 1728, 1668, 1505, 1463, 1258, 1178 cm-1; EIMS m/z
(rel
intensity) 638/640 (4.5/1.1), 412/414 (59/19), 280(17), 227 (100); high
resolution EIMS
m/z 638.272934 (calcd for C35H43C1N207, 2.9mmu error). 'H NMR (CDC13): amino
or
hydroxy acid unit S(carbon position, multiplicity; J in Hz) 5-hydroxy-6-methyl-
8 phenyl-
2, 7-octadienoic acid (A) 5.76 (2, d; 15.5), 6.65 (3, ddd; 15.4, 9.2 and 6.2),
2.38-2.47
(4, m), 5.08 (5,ddd; 10.6, 4.9 and 2.2), 2.58 (6, m), 1.15 (6-Me, d; 6.8),
6.07 (7, dd;
15.9 and 8.5), 6.43 (8, d; 15.9), 7.21-7.35 (10/11/12/13/14, m); 3-chloro-4-
methoxy-
phenylalanine (B) 4.83 (2, m), 3.05(3, dd; 14.5 and 7.1), 5.65 (2-NH, d; 8.7),
3.14 (3,
dd; 14.4 and 5.5), 7.21 (5, d; 2.4), 3.86 (7-OCH31 s), 6.83 (8, d; 8.3), 7.08
(9, dd; 8.3
and 2.2); 3-amino-2-methylpropionic acid (C) 2.73 (2, m), 1.23 (2-Me, d; 7.2),
3.23 (3,
dt; 13.5 and 6.8), 3.56 (3, ddd; 13.5, 5.7 and 4.0), 6.85 (3-NH, dd; 7.1 and
6.2); leucic
acid (D) 4.8 (2, d; 4.6), 1.86-1.89 (3, m), 0.94 (3-Me, d; 7.0), 1.20-1.26 (4,
m), 1.39-
1.44 (4, m), 0.77 (5, d; 7.4). 13C NMR (CDC13) unit 8(carbon position) A 165.5
(1),
125.2 (2), 141.5 (3), 36.4 (4), 77.7 (5), 41.9 (6), 17.1 (6-Me), 129.8 (7),
131.9 (8),
136.8 (9), 128.6 (10/14), 126.2 (11/13), 127.6 (12); B 170.0 (1), 53.5 (2),
35.1 (3),
129.9 (4), 131.1 (5), 122.4 (6), 153.9 (7), 56.1 (7-OCH3), 112.2 (8), 128.5
(9); C 175.3
(1), 38.6 (2), 14.0 (2-Me), 41.4 (3); D 169.5 (1), 76.6 (2), 36.2 (3), 15.5 (3-
Me), 24.2
(4), 14.0 (5).
Cryptophvcin 19
[aJD +62.6 (MeOH, c 0.67); UV (MeOH) X. (e) 204 (44900), 230 (17000),
248 (15600), 280 (2500); IR (neat) v,,m 3413, 3272, 2966, 1745, 1726, 1672,
1504,
1258, 1199, 1178, 1066, 692 cm'; EIMS m/z (rel intensity) 624/626 (3.0/1.4),
398/400
(58/21), 280/282(15/5), 227 (100), 195/197 (57/22); high resolution EIMS m/z
624.2585
(calcd for C34H41C1N2O7, 1.8mmu error). 'H-NMR (CDC13):amino or hydroxy acid
unit 6
(carbon position, multiplicity; J in Hz) 5-hydroxy-6-methyl-8 phenyl-2, 7-
octadienoic acid
(A) 5.76 (2, d; 15.2), 6.64 (3, ddd; 15.4, 9.1 and 6.2), 2.38 (4, m), 2.47 (4,
m), 5.04
(5, ddd;7.1, 5.1 and 1.8), 2.57 (6, m), 1.15 (6-Me, d; 6.9), 6.05 (7, dd; 15.8
and 8.5),
6.43 (8, d; 15.8), 7.29-7.35 (10/11/13/14, m), 7.23 (12, m); 3-chloro-4-
methoxyphenylalanine (B) 4.84 (2, m), 5.67 (2-NH, d; 8.9), 3.04(3, dd; 14.3
and 7.1),
3.14 (3, dd; 14.3 and 5.3), 7.22 (5, d; 2.0), 3.86 (7-OCH3, s), 6.83 (8, d;
8.2), 7.08 (9,
LZZ '(L/0~/6~) ~0~/TO~/66Z '(Z/S/TT) 9~ti/tl~fi/Z~t, 8L9/9L9/tL9 VlIsIIalui
Iai)
Z/Ui SWIEI fiLIT 'ZLZT '6~~T '06171 '0179T '~99T 'VZLI 'Lt~LT '096Z 'fi8Z~
781lf Ovau)
2II =(LLIZ) 06Z '(fiLIZ) Z8Z '(TLStl) OtiZ (3) "o"v, Afl '=(SS'T a'HOaW) oLt,
+ a[701 0~
~Z uta q o~ O
'(aY1i-t,) Z'iZ '(S) 8'ZZ '(b) fi'tZ '(~)
9'6~ '(Z) Z'IL '(I) S'OLT a '(~) fi't~ '(Z) t,'Z~ '(i) 9'ZLT a =(6) ~'8ZT '(8)
Z'ZTI '(aJ6i0
-L) i'99 '(L) 6'~ST '(9) tl'ZZT '(S) 6'0~I '(V) 8'6ZT '(~) 0'9~ '(Z) 6'~S '(T)
L'OLI S!(ZT)
9'8ZT '(~i/TI) L'8Zi '(ti/0i) 9'SZT '(6) L'9~i '(8) 0'69 '(L) 0'~9 '(aL1i-9)
S'~T '(9) 9'0v SZ
'(S) 6'9L '(t,) L'9~ '(~) 0'TbT '(Z) ~'SZT '(T) S'S9T V (uoilisod uoqjua) g
jiun (~IOQO)
UY1IN O~i =(t,'9 tP ~8'0 '(t,'9 =P 'S) V8'0 '(Eu 'V) L9'i '(Iu '~) i~'I '(UI
'~) L9'T
'(~'~ PUL' 0'OT 'PP 'Z) 68'ti( a) PFan a10nap '.(8=S :I iq 'HN-E) 06'9 '(ui
'~) St,'~ '(Tu '~)
IS'~ '(iu 'Z) 9S'Z( j) p?an atuoido.tdouiurn-F :(0'Z PuLl fi'8 !PP '6) LO'L
'(i7'8 '=P '8) ~8'9
'(s 'aW0-L) 98'~ '(0'Z 'P 'S) TZ'L '(9'9 Puu ~'fii 'PP '~) VI'~ '(L'L Put,
~'tii 'PP '~) 86'Z OZ
'(9'8 =P 'HN-Z) 89'9 '(Z'9 Puu 8'9 'Z'8 !PPP 'Z) tL't,( g)
auzuvpvpduayddxoyjazu-p,-o.ro1ua
-F '(Ta '~I/ZI/TI) 8~'L-~~'L '(Iu '1~T/OI) SZ'L '(8'1 =P '8) 89'~ '(0'Z Pug
S'L 'PP 'L)
Z67 '(T'L =P 'aLN-9) ViT '(Iu '9) 08'T '(S'T Pu'e T'S 'Z'II =PPP 'S) 6T'9 '(Iu
't,) 99'Z '(Tu
't) Si,'Z '(6'V Pu'c 6'6 '0'ST =PPP '~) 89'9 '07'0 '=P 'Z) ~L'S ( V) P?an
a?ounlao Jdiraud
-S-1,C~flau,i-g-dxo.rpdq-S-droda-8 'L (zH uT f'satltaildt;Inui 'suoiltsod
uoq.ma) 9 ygun ppn ST
,Cxo.rp4 .10 ounun (~IO(IO) 2IViN Hi '(jo.r.xa nun.uZ'0 'SOzNIOi'HKO J03
PatL'o) 09SZ'Of9
z/iu Sy~g uoi~niosai 142ul :(06) LZT '(001) SSI '(96) S6T '(9L) LZZ '(~~) 99Z
'(Ofi)
86E '(SI) 8Lf, '(S) Z19 '(t7/0i) Z=b9/0t9 (AliSUaJut antIEla.z) Z/u-l SWIg
'i_uia 688 '~ZOT
'9901 'VLT I '89Zi 'ZLET '60171 '=b9fiI '~OSI '~L9I 'I ~LI 'L96Z '6LZ~ '~Oti~
"wulf O'Cau)
2II '(OOTZ) 88Z '(OOVZ) 08Z '(OOL9) OtZ (3) ""X Afl '(ZL'0 a~IOHO) oZ'Ot7 +
a[al OI
IZ uta u OT O
'(aY1i-~) L'91 '(t,) 0'6I '(~)
8'6Z '(Z) 6'9L '(T) 9'69I Q'(~) S'it, '(aY1I-Z) 6'~I '(Z) L'8~ '(I) I'SLT 0
!(6) S'8ZI '(8)
Z'ZTI '(ab1I0-L) 1'99 '(L) 6'~ST '(9) t,'ZZi '(S) I'I~T '(t,) 0'0~T '(~) T'S~
'(Z) 1,'~9 '(I)
0'TLI S-(ZI) 9'LZI '(~T/IT) 9'8ZT '(t,1/01) I'9ZI '(6) 8'9~i '(8) 6'I~i '(L)
6'6ZT '(aNI S
-9) T'LI '(9) 0'Zfi '(S) L'LL '(t,) ~'9~ '(~) ~'IVT '(Z) ~'SZI '(T) S'S9T V
(uontsod uoq.rea)
9 Jiun (~IOQO) ?Iy1IN O~t '(6'9 'P 'aW-~) 96'0 '(6'9 'P 't,) t,8'0 '(Iu '~)
607 '(Z'f,
'P 'Z) ~L't, (Q) p?On auapnnosidxoJpCzf-Z '(L'9 uq 'HN-~) 08'9 '(ul '~) 69'~
'(uT '~) 6T'~
'(T'L '=P 'aNi-Z) ~Z'I '(w 'Z) SL'Z 0) P?on 9tuotdo.rdp4lazu-z-ouiutv-~ '(0'Z
Pim Z'8 '=PP
-t,6-
9tiZ~0/96Sf1/.LDd b8I0V/96 OM
~0-60-L661 S9SbiZZO Va
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-95-
(64), 215/217/219 (31/20/8), 141 (100); high resolution EIMS m/z 674.21643
(calcd. for
C34H4C12N2O8, -0.3mmu error); 1H NMR (CDC13) amino or hydroxyacid unit
b(carbon
position, multiplicity; J in Hz) 7, 8-epoxy-5-hydroxy-6-methyl-8 phenyl-2-
octenoic acid
(A) 5.77 (2, d; 15.4), 6.65 (3, ddd; 15.4, 9.3 and 6.0), 2.47 (4, dt; 14.2 and
10.2), 2.55
(4, br dd; 14.2 and 5.6), 5.13 (5, ddd; 11.0, 4.6 and 1.6), 1.81 (6, m), 1.15
(6-Me, d;
6.9), 2.93 (7, dd; 7.6 and 2.0), 3.7 (8, d; 2.0), 7.22-7.26 (10/14, m); 7.32-
7.39
(11/12/13, m); 3,5-dichloro-4-hydroxyphenylalanine (B) 4.81 (2, m), 5.69 (2-
NH, d;
8.6), 3.11 (3, dd; 14.5 and 5.6), 3.50 (3, dd; 14.3 and 7.0), 7.13 (5/9, s),
5.78 (7-OH,
s); 3-amino-2-methylpropionic acid (C) 2.73 (2, m), 1.22 (2-Me, d; 7.1), 3.19
(3, dt;
13.4 and 6.9), 3.58 (3, ddd; 13.6, 5.8 and 4.1), 6.82 (3-NH, br t; 5.9);
leucic acid (D)
4.84 (2, dd; 9.9 and 3.2), 1.38 (3, m), 1.68-1.75 (3, m), 1.68-1.75 (4, m),
0.86 (4-Me,
d; 6.7), 0.87 (5, d; 6.7). 13C NMR (CDC13) unit S(carbon position) A 165.4
(1), 125.4
(2), 140.9 (3), 36.7 (4), 76.3 (5), 40.6 (6), 13.5 (6-Me), 63.0 (7), 58.9 (8),
136.7 (9),
125.6 (10/14), 128.7 (11/13), 128.6 (12); B 170.7 (1), 53.3 (2), 35.0 (3),
130.3 (4),
129.0 (5/9), 121.0 (6/8), 146.7 (7); C 175.3 (1), 38.4 (2), 13.9 (2-Me), 41.5
(3); D
170.8 (1), 71.3 (2), 39.4 (3), 24.6 (4), 21.3 (4-Me), 22.9 (5).
Cryptophvcin 24
[a]D + 48.8 (CHC13, c 0.63); UV A,,. (e) 228 (19006), 242 (8249), 274 (2351);
IR (neat) v,,,a~ 3400, 3284, 2959, 1732, 1678, 1652, 1514, 1248, 1178 cm 1;
EIMS m/z
(rel intensity, assignment) 606 (2, M+), 364 (7), 161 (55, CH3O-C6H4-
CH=CH=CO+),
121 (100, CH3O-C6H4-CH2+), 91 (68); high resolution EIMS m/z 606.2954 (calcd
for
C34H42N2O8, -1.3mmu error); 1H NMR (CDC13) amino or hydroxy acid unit S(carbon
position, multiplicity; J in Hz) 7, 8-epoxy-5-hydroxy-6-methyl-8 phenyl-2-
octenoic acid (A)
5.70 (2, dd; 15.2 and 1.3), 6.70 (3, ddd; 15.2, 10.3 and 4.7), 2.43 (4, dt;
14.3 and
10.9), 2.56 (4, m), 5.20 (5, ddd; 11.3, 5.1 and 2.0), 1.79 (6, m), 1.14 (6-Me,
d; 7.0),
2.92 (7, dd; 7.5 and 2.0), 3.68 (8, d; 2.0), 7.23 -7.38 (10/11/12/13/14, m); 0-
methyl-
tyrosine (B) 4.73 (2, m), 5.58 (2-NH, d; 8.3), 3.03 (3, dd; 14.5 and 7.5),
3.14 (3, dd;
14.5 and 5.7), 7.11 (5/9, d; 8.6), 6.81 (6/8, d; 8.6), 3.78 (7-OMe, s); 3-
aminopropionic
acid (C) 2.55 (2-H2, m), 3.42 (3, m), 3.53(3, m), 6.97 (3-NH, br t; 5.7);
leucic acid (D)
4.89 (2, dd; 9.9 and 3.5), 1.29 (3, m), 1.62 - 1.70 (3/4, m), 0.83 (5, d;
5.9), 0.84
(4-Me, d; 6.1); 13C NMR (CDC13): unit 6(carbon position) A 165.4 (1), 125.3
(2), 141.0
(3), 36.7 (4), 75.9 (5), 40.6 (6), 13.4 (6-Me), 63.0 (7), 59.0 (8), 136.7 (9),
125.6
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-96-
(10/14), 128.7 (11/13), 128.5 (12); B 170.7 or 170.6 (1), 54.1 (2), 35.2 (3),
128.5 (4),
130.2 (5/9), 114.1 (6/8), 158.6 (7), 55.2 (7-OMe); C 172.8 (1), 32.5 (2), 34.2
(3); D
170.6 or 170.7 (1), 71.2 (2), 39.5 (3), 24.4 (4), 21.3 (5), 22.8 (4-Me).
Cryptophycin 26
[a]D + 28.2 (CHC13, c 1.31); UV A. (e) 254 (14615), 284 (2949); IR (neat)
v1187C 3299, 2960, 1732, 1644, 1504, 1258, 1209 cm 1; EIMS m/z (rel intensity)
656/658
(0.5/0.1, M+), 638/640 (1.7/1.0), 525/527 (3.7/1.8), 412/414 (10/4), 280/282
(12/11),
227 (20), 195 (48), 131 (68); high resolution EIMS m/z 656.2836 (calcd for
C35H45C1N208, 2.8mmu error), 638.2712 (calcd for C35H43C1N207, 4.7mmu error);
iH
NMR (CDC13) amino or hydroxy acid unit 6(carbon position, multiplicity; J in
Hz) 3, 5-
dihydroxy-6-methyl-8 phenyl-7-octenoic acid (A) 2.46 (2, dd; 14.8 and 7.8),
2.58 (2, dd;
14.8 and 3.0), 5.46 (3, m), 1.86 - 1.90 (4-H2, m), 3.61 (5, m), 2.37 (6, m),
1.14 (6-Me,
d; 6.8), 6.06 (7, dd; 16 and 8.7), 6.47 (8, d; 16), 7.37 (10/14, br d; 7.9),
7.32 (11/13,
br t; 7.6), 7.22 - 7.28 (12, m); 3-chloro-4-methoxyphenylalan ine (B) 4.73 (2,
br dt; 6.4
and 8.1), 6.14 (2-NH, d; 8.6), 2.84 (3, dd; 14.4 and 8), 3.18 (3, dd; 14.4 and
6.3), 7.21
(5, d; 2.2), 3.85 (7-OMe, s), 6.82 (8, d; 8.6), 7.08 (9, dd; 8.6 and 2.2); 3-
amino-2-
methylpropionic acid (C) 2.87 (2, m), 1.19 (2-Me, d; 7.0), 3.01 (3, ddd; 13.4,
10.6 and
4.9), 3.73 (3, ddd; 13.4, 8.2 and 4.7), 6.72 (3-NH, br dd; 7.3 and 5.2);
leucic acid (D)
4.95 (2, dd; 9.7 and 4.2), 1.62 - 1.72 (3, m), 1.79 - 1.84 (3, m), 1.62 - 1.72
(4, m),
0.90 (4-Me, d; 6.4), 0.95 (5, d; 6.4). 13C NMR (CDC13): unit 6(carbon
position) A
170.0 (1), 41.5 (2), 71.4 (3), 37.3 (4), 71.9 or 71.8 (5), 43.6 (6), 16.6 (6-
Me), 130.8
(7), 132.5 (8), 136.8 (9), 126.2 (10/14), 128.6 (11/13), 127.6 (12); B 170.9
(1), 53.2
(2), 34.7 (3), 130.3 (4), 131.1 (5), 122.2 (6), 153.8 (7), 56.1 (7-OMe), 112.2
(8), 128.5
(9); C 174.3 (1), 40.1 (2), 14.4 (2-Me), 42.5 (3); D 170.7 (1), 71.8 or 71.9
(2), 38.9
(3), 24.6 (4), 21.6 (4-Me), 22.9 (5).
Cryptovhcyin 28
[a]D + 65.6 (MeOH, c 0.93); UV (MeOH) X. (e) 204 (48000), 230 (19300),
248 (18700), 280 (3400); IR (neat) v. 3413, 3270, 2958, 1745, 1726, 1665,
1504,
1258, 1197, 1175, 1066, 694 cm'; EIMS m/z (rel intensity) 624/626 (3.0/1.3),
412/414
(70/24), 280/282(13/6), 213 (100), 195/197 (86/40); high resolution EIMS m/z
624.2626
(calcd for C34H41C1N2O7, -2.4mmu error); 1H NMR(CDC13) amino or hydroxy acid
unit 6 (carbon position, multiplicity; J in Hz) 5-hydroxy-8 phenyl-2, 7-
octadienoic acid (A) 5.78
'(L) 6'~ST '(9) b'ZZI '(S) 0'I~T '41) 6'6ZI '(~) 6'V~ '(Z) 8'~S '(1) 6'0Li
S'=(ZT) 9'LZT
'(~T/ii) 9'8ZT '411/01) Z'9ZI '(6) L'9~T '(8) 8'T~i '(L) i'0~i '(alN-9) ~'LI
'(9) ~'Zti 0~
'(S) i'LL '(t,) fi'9~ '(~) S'Tt~i '(Z) Z'SZT '(I) 9'S9I V (IIouisod uoqjuo) 9
ziun (~IO(IO)
2iNiN O~i '(f~'9 P 'S) ZL'0 '(1,'9 P 'aL1i-0 9L'0 (uT 'v) ~9'T '(ul '~) ~9'T
'(S'~ PUL, ~'01
'V'SI PPP '~) V~'I '(S'~ PUL' 6'6 'PP 'Z) 061, (a) P?an a?anal !(L'S !Ijq 'HN-
E) 68'9
'(Tu '~) SS'~ '(Lu '~) irb'~ '(Iu 'zH-Z) SS'Z (3) P?an O?uoido.rdouiwn-F :(Z'Z
Puu fi'8 IPP
'6) 80'L '(t'8 !P '8) ~8'9 '(s 'aL1i0-L) L8'~ '(Z'Z '=P 'S) ZZ'L '(6'S PUL,
VVT 'PP '~) bT'~ SZ
'(Z'OI Puv t,'ti =PP '~) 0'~ '(9'8 lP 'HN-Z) L9-S '(ui 'Z) 9L't, (g)
auruv1vtduaqd~Cxoujaru
-/,-o.ropqa-~ '(ui 'ZT) ZZ'L '(Iu 'fiT/~T/ii/0i) ~~'L-8Z'L '(8'ST =P '8) Tfi'9
'(8'8 PUL, 8'Si =PP 'L) 10'9 '(8'9 =P 'aY1t-9) tT'T '(Tu '9) 9S'Z '(8'1 P~
t,'9 '0'IT '=PPP 'S)
~0'9 '(ul 'fi) VS'Z '(tu 'fi) 9~'Z '(~'S Pug 1'01 '~'ST =PPP '~) 69'9 '(T'T
Pu'u ~'ST '=PP 'Z)
SL'S (V) P1an ozouazpvjao -Z'z-IiCuayd-g-p4laui-9-iCxojP4-S (zH ui
f:Slt3i1di1Inui 'uoi~isod OZ
uoq.reo) 9 1iun Pzan dxo.rPif4 .ro ouiurn (~ID(IO)2IY1IN HI :(so.uo nunus=O-p
'LOzNI014HKD
jo3 Pop,:3)L09Z'tZ9 z/ui Sb1tI3 uoi1nlosai u8q
i :(S6) 16 '(~9) i~T '(OZ/69) LSI/SST
'(91/09) L6I/961 '(001) LZZ 001,/86~ '(1'1/9'Z) 9Z9/tZ9 Vltsualut Iai) z/ru
Sym
=rTuo V69 'L901 'tLIT 'L611 '6SZT 'WST 't7L91 'V~LT 'ttLT '096Z 'ZLZ~ 'Siv~
(IL'au) UI 400~~) "Z '(OOOLT) OSZ (3) m"'X Afl '=(~T'T 3 '~IOHO) oZ'ZZ + a[70]
ST
6Zut3qoT D
' (S)
97Z S'TZ '(t,) S'tZ '(~) 9'6~ '(Z) 9'IL '(1) 6'0LI Q'(~) Z'Tb '(aL1I-Z) 0'K
'(Z)
~'S~ '(T) 9'SLI 0 '=(6) fi'8ZI '(8) ~'ZII '(aL1i0-L) T'99 '(L) 0'tSi '(9) VZZI
'(S) 0'T~T
'(fi) 8'6ZI '(~) i'S~ '(Z) 9'~S '(I) 6'0LI S!(ZT) 9'LZI '(~T/ii) 9'8ZT
'(t,1/01) T'9ZT '(6) 01
L'9~I '(8) 8'~~i '(L) I'tiZi '(9) 9"8~ '(S) S'~L '(17) 9'8~ '(~) Z'Tii '(Z)
Z'SZI '(T) ti'S9i
V (uoTltsod uoqjuo) 9 Iiun (~ID(IO) gy1iN D~, '=(~'9 !P 'S) 17L'0 '(~'9 lP
'aL1T-v) 9L'0 '(M
'b) Z9'i '(UJI '~) Z9'T '(Iu '~) OvI '(Iu 'z) Z8't, (a) Ptan g?onal :(9'S :1
iq 'HN-~) L6'9
'(8'~ Pug 6't, 'S'~T 'PPP '~) 6fi'~ '(0'L PuL' S'~i V '~) 6Z'~ '(Z'L 'P 'aY1i-
Z) IZ'I '(Iu
'Z) ZL'Z (.7) P?on a?uoidojdp41au'-z-ouizun-~ 407 Puu 9'8 =PP '6) 80'L '(9'8
=P '8) t,8'9 S
'(s 'aW0-L) L8'~ '(0'Z 'P 'S) ZZ'L '(t''S PuL' S'tiT -PP '~) t71'~ '(Z'L PUL,
S'fii -PP '~) W'~
'(S'S !P 'HN-Z) ZL'S '(tu 'Z) ZS'i, (S) auiuvpvpdirat/ddxor/iaur-/,-o.rolua-~
'=(ui 'ZI) ZZ'L '(Iu
'tT/~I/II/0T) 8~'L-LZ'L '(8'SI 'P 18) tt'9 '(1,'L PuL, 8'SI '=TP 'L) L0'9
'(L'9 =I iq 'zH-9)
~S'Z '(uI 'S) LI'S '(UI 'V) ~S'Z '(uI 'V) 0VZ '(VS PuR 6'6 '9'9I 'PPP '~)IL'9
'(9'SI 'P 'Z)
-L6-
9VZ~0/96Sfl/,LDd b8TOti/96 OM
~0-60-L661 S9SbiZZO Va
CA 02214565 1997-09-03
WO 96/40184 PCT/[JS96/03246
-98-
56.1 (7-OMe), 112.2 (8), 128.4 (9); C 172.6 (1), 32.4 (2), 34.5 (3); D 170.4
(1), 71.5
(2), 39.7 (3), 24.4 (4), 21.2 (4-Me), 22.6 (5).
Cryptophycin 30
[a]D - 12.3 (CHC13, c 1.53); UV X. (e) 254 (17200), 284 (3600); IR (neat)
v,,=
3414, 3306, 2961, 1738, 1729, 1660, 1504, 1258, 1205, 1183, 1066, 695 cni 1;
EIMS
m/z (rel intensity) 656/658 (1.0/0.3), 638/640 (3.0/1.0), 525/527 (3.8/1.3),
412/414
(10.5/3.6), 280/282(10.3/3.8), 227 (29), 195/197 (48/17), 155/157 (74/21), 131
(100);
high resolution EIMS m/z 656.2852 (calcd for C35H45C1N208, 1.3mmu error); 1H-
NMR
(CDC13) amino or hydroxy acid unit S(carbon position, multiplicity; J in Hz)
3,5-
dihydroxy-6-methyl-8-phenyl-7-octenoic acid (A) 2.25 (2, dd; 16.0 and 9.6),
2.64 (2, brd;
16.0), 3.89 (3, m), 2.51 (3-OH, d; 6.4), 1.77 (4, ddd; 14.3, 9.8 and 2.1),
1.88 (4, ddd;
14.3, 11.3 and 3.8), 4.88 (5, ddd; 11.3, 6.2 and 2.1), 2.53 (6, m), 1.10 (6-
Me, d; 6.8),
5.99 (7, dd; 15.9 and 9.0), 6.40 (8, d; 15.9), 7.28-7.33 (10/11/13/14, m),
7.23 (12, m);
3-chloro-4-methoxyphenylalanine (B) 4.60 (2, m), 6.61 (2-NH, d; 8.1), 3.09 (3,
dd; 14.2
and 5.6), 3.15 (3, dd; 14.2 and 7.3), 7.22 (5, d; 2.1), 3.86 (7-OMe, s), 6.83
(8, d; 8.3),
7.07 (9, dd; 8.3 and 2.1); 3-amino-2-methylpropionic acid (C) 2.67 (2, m),
1.21 (2-Me,
d; 7.3), 3.26 (3, ddd; 13.6, 7.3 and 6.4), 3.63 (3, ddd; 13.6, 6.2 and 3.9),
6.75 (3-NH,
br t; 6.3); leucic acid (D) 4.83 (2, dd; 9.6, 4.1), 1.42 (3, m), 1.64 (3, m),
1.64 (4, m).
0.79 (4-Me, d; 6.4), 0.76 (5, d; 6.4); 13C NMR (CDC13) unit S(carbon position)
A 171.6
(1), 42.4 (2), 66.0 (3), 41.3 (4), 76.0 (5), 42.0 (6), 17.3 (6-Me), 130.0 (7),
131.9 (8),
136.7 (9), 126.1 (10/14), 128.6 (11/13), 127.6 (12); B 170.8 (1), 54.3 (2),
35.1 (3),
130.1 (4), 131.1 (5), 122.2 (6), 153.8 (7), 56.1 (7-OMe), 112.1 (8), 128.7
(9); C 175.6
(1), 39.7 (2), 13.8 (2-Me), 41.5 (3), D 171.9 (1), 72.1 (2), 39.1 3), 24.6
(4), 21.4 (4-
Me), 22.7 (5).
Cryptophvcin 31
ja]D + 50.6 (MeOH, c 1.13); UV ~(e) 242 (3800), 284 (700); IR (neat) v.
3412, 3272, 2961, 1745, 1725, 1678, 1537, 1481, 1270, 1196, 1176, 1000, 698 cm
1;
EIMS m/z (rel intensity) 688/690/692 (1.2/1.0/0.4), 446/448/450 (7.9/6.7/3.1),
314/316/318 (17/11/3), 91 (100); high resolution EIMS m/z 688.2336 (calcd for
30 C35H42C12N208, -1.8mmu error); 'H-NMR (CDC13) amino or hydroxy acid unit
8(carbon
position, multiplicity; J in Hz) 7, 8-epoxy-S-hydroxy-6-methyl-8 phenyl-2-
octenoic acid (A)
5.78 (2, d; 15.5), 6.66 (3, ddd; 15.5, 9.4 and 6.0), 2.47 (4, ddd; 14.1, 10.8
and 9.4),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-99-
2.56 (4, m), 5.14 (5, ddd; 10.8, 4.7 and 1.7), 1.82 (6, m), 1.15 (6-Me, d;
7.1), 2.93 (7,
dd; 7.5 and 1.9), 3.70 (8, d; 1.9), 7.24-7.26 (10/14, m), 7.34-7.39 (11/12/13,
m); 3,5-
dichloro-4-methoxyphenylalanine (B) 4.83 (2, m), 5.68 (2-NH, d; 9.0), 3.0 (3,
dd; 14.4
and 7.3), 3.14 (3, dd; 14.4 and 5.6), 7.16 (5/9, s), 3.87 (7-OMe, s); 3-amino-
2-
methylpropionic acid (C) 2.74 (2, m), 1.22 (2-Me, d; 7.1), 3.20 (3, m), 3.58
(3, ddd;
13.5, 5.6 and 4.1), 6.82 (3-NH, br t; 5.6); leucic acid (D) 4.83 (2, m), 1.38
(3, m), 1.72
(3, m), 1.72 (4, m). 0.87 (4-Me, d; 6.8), 0.86 (5, d; 6.8); 13C NMR (CDC13)
unit S
(carbon position) A 165.4 (1), 125.4 (2), 141.0 (3), 36.7 (4), 76.3 (5), 40.6
(6), 13.5 (6-
Me), 63.0 (7), 58.9 (8), 136.7 (9), 125.6 (10/14), 128.7 (11/13), 128.6 (12);
B 170.8
(1), 53.3 (2), 35.2 (3), 129.3 (4), 129.6 (5/9), 134.5 (6/8), 151.2 (7), 60.6
(7-OMe); C
175.3 (1), 38.3 (2), 13.9 (2-CH3), 41.5 (3), D 170.6 (1), 71.3 (2), 39.4 (3),
24.6 (4),
22.9 (4-Me), 21.3 (5).
Cryptophycin 40
[a]D + 41.6 (CHC13, c 0.31); UV X,,. (e) 242 (4974), 266 (3911), 274 (3666),
286 (2359), 328 (511); IR (neat) P. 3415, 2959, 1748, 1723, 1667, 1505, 1463,
1289,
1176 cm-'; EIMS m/z (rel intensity) 640/642 (5/2), 280/282 (7/3), 213 (13),
195/197
(51/17), 155 (29), 141 (32), 121 (28), 91 (100), 69 (47); high resolution EIMS
m/z
640.2570 (calcd. for C34H41C1N2O8, -1.8mmu error); 1H NMR (CDC13) amino or
hydroxy
acid unit 8(carbon positions, multiplicities; J in Hz) 7, 8-epoxy-S-hydroxy-8
phenyl-2-
octenoic acid (A) 5.77 (2, d; 15.1), 6.72 (3, ddd; 15.1, 9.7 and 4.9), 2.42
(4, m), 2.58
(4, m), 5.33 (5, m), 1.89 (6, ddd; 12.9, 8.1 and 5.0), 2.13 (6, ddd; 12.9, 9.3
and 5.0),
2.98 (7, ddd; 6.7, 4.5 and 1.9), 3.64 (8, d; 1.9), 7.31-7.39 (10/11/13/14, m),
7.22 (12,
m); 3-chloro-4-methoxyphenylalanine (B) 4.83 (2, m), 5.64 (2-NH, d; 8.6), 3.03
(3, dd;
14.3 and 7.5), 3.14 (3, dd; 14.3 and 5.4), 7.21 (5, d; 2.0), 3.87 (7-OMe, s),
6.84 (8, d;
8.3), 7.08 (9, dd; 8.3 and 2.0); 3-amino-2-methylpropionic acid (C) 2.72 (2,
m), 1.23 (2-
Me, d; 7.3), 3.31 (3, dt; 13.8 and 6.9), 3.50 (3, ddd; 13.6, 5.7 and 3.9),
6.96 (3-NH, br
t; 6.0); leucic acid (D) 4.85 (2, dd; 6.7, 3.4), 1.42 (3, m), 1.72 (3, m),
1.72 (4, m),
0.86 (4-Me, d, 3.7), 0.87 (5, d, 3.7); 13C NMR (CDC13) unit S(carbon position)
A 165.3
(1), 125.2 (2), 140.9 (3), 39.0 (4), 72.0 (5), 37.3 (6), 59.0 (7), 58.7 (8),
140.9 (9),
125.6 (10/14), 128.7 (11/13), 128.5 (12); B 170.9 (1), 53.6 (2), 35.1(3),
129.8 (4),
131.0 (5), 122.5 (6), 157.0 (7), 56.1 (7-OMe), 112.3 (8), 128.4 (9); C 175.6
(1), 38.3
(2), 14.1 (2-Me), 41.1 (3); D 170.9 (1), 71.4 (2), 39.4 (3), 24.5 (4), 21.5 (4-
Me),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-100-
22.8 (5).
Cryptophycin 43
[ca]D + 20' (CHC13, c 0.2); UV X,,. (e) 250 (20512), 282 (4083), 294 (1734);
IR
(neat) P. 3400, 3272, 2927, 1727, 1660, 1516, 1455, 1242, 1175 cm'; EIMS m/z
(rel
intensity) 533 (24), 484 (3), 445 (14), 398 (9), 364 (29), 227 (59), 149 (67),
91 (100)
high resolution EIMS m/z 590.3044 (calcd for C34H41N207, -5.2mmu error); 1H
NMR
(CDC13) amino or hydroxy acid unit 8(carbon position, multiplicity; J in Hz) 5-
hydroxy-
6-methyl-8 phenyl-2, 7-octadienoic acid (A) 5.75 (2, d; 15.3), 6.69 (3, ddd;
15.3, 9.9 and
5.3), 2.37 (4, dt; 14.2 and 10.4), 2.52 (4, m), 5.01 (5, ddd; 11.2, 6.4 and
1.8), 2.55 (6,
m), 1.13 (6-Me, d; 6.9), 6.01 (7, dd; 15.8 and 8.9), 6.41 (8, d; 15.8), 7.21-
7.34
(10/11/12/13/14, m); 4-methoxyphenylalanine (B) 4.80 (2, m), 5.64 (2-NH, d;
8.4),
3.06(3, dd; 14.5 and 7.2), 3.13 (3, dd; 14.4 and 5.3), 7.06 (5/9, d; 8.4),
6.74 (6/8, d;
8.4); 3-amino-2-methylpropionic acid (C) 2.69 (2, m), 1.22 (2-Me, d; 7.3),
3.33.(3, m),
3.44 (3, dt; 14.0 and 4.7), 7.0 (3-NH, m); leucic acid (D) 4.84 (2, dd; 10.0
and 3.6),
1.60-1.67 (3, m), 1.35 (3, m), 1.60-1.67 (4, m), 0.76 (5, d; 6.4), 0.73 (4-Me,
d; 6.7);
13C NMR (CDC13) unit 6(carbon position) A 125.2 (2), 141.5 (3), 36.5 (4), 77.5
(5),
42.3 (6), 17.3 (6-Me), 130.1 (7), 131.8 (8), 136.8 (9), 126.2 (10/14), 128.6
(11/13),
127.6 (12); B 53.8 (2), 35.3 (3), 129.8 (4), 130.5 (5/9), 115.6 (6/8), 154.6
(7); C 38.3
(2), 14.1 (2-Me), 41.0 (3); D 71.6 (2), 39.6 (3), 24.5 (4), 21.2 (5), 22.9 (4-
Me). Due to
the small sample size, carbonyl carbon signals could not be seen.
Cryptophvcin 45
[a]D + 72.0 (MeOH, c 0.12); UV X. (e) 250 (25500), 284 (5300); IR (neat)
P. 3407, 3239, 2958, 1743, 1727, 1667, 1538, 1469, 1242, 1196, 1177, 694 cm 1;
EIMS m/z (rel intensity) 658/660/662 (2.1/1.4/0.3), 483 (7.6) 432/434/436
(9.5/6.4/1.8),
300/302/304 (8.0/5.5/1.2), 227 (100) 91 (87); high resolution EIMS m/z
658.2207 (calcd
for C341-I,oC12N2O7, 0.6mmu error); 'H NMR (CDC13):amino or hydroxy acid unit
8
(carbon position, multiplicity; J in Hz) 5-hydroxy-6-methyl-8 phenyl-2, 7-
octadienoic acid
(A) 5.80 (2, d; 14.7), 6.66 (3, ddd; 14.7, 8.5 and 5.5), 2.38 (4, m), 2.53 (4,
m), 4.97
(5, br dd; 10.4 and 6.2), 2.57 (6, m), 1.14 (6-Me, d; 6.7), 6.01 (7, dd; 15.9
and 8.7),
6.42 (8, d; 15.9), 7.28-7.34 (10/11/1314, m), 7.22 (12; m); 3,5-dichloro-4-
hydroxyphenyl-alanine
(B) 4.82 (2, m), 5.73 (2-NH, br d; 8.7), 3.02 (3, dd; 14.3 and
6.2), 3.10 (3, dd; 14.3 and 5.2), 7.14 (5/9, s), 5.79 (7-OH, s); 3-amino-2-
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/0324.6
-101-
methylpropionic acid (C) 2.73 (2, m), 1.21 (2-Me, d; 7.0), 3.17 (3, m), 3.60
(3, m),
6.81 (3-NH, br t; 6.7); leucic acid (D) 4.84 (2, dd;10.0 and 3.2), 1.38 (3,
ddd; 14.9,
10.2 and 3.2), 1.65 (3, m), 1.65 (4, m). 0.78 (4-Me, d; 6.5), 0.73 (5, d;
6.5); 13C NMR
(CDC13) unit 8(carbon position) A 165.5 (1), 125.4 (2), 141.2 (3), 36.4 (4),
77.6 (5),
42.3 (6), 17.3 (6-Me), 130.0 (7), 131.9 (8), 136.7 (9), 126.2 (10/14), 128.6
(11/13),
127.6 (12); B 171.0 (1), 53.2 (2), 35.0 (3), 130.4 (4), 129.1 (5/9), 121.0
(6/8), 146.7
(7); C 175.2 (1), 38.5 (2), 13.9 (2-Me), 41.6 (3), D 170.7 (1), 71.5 (2), 39.5
(3), 24.6
(4), 22.7 (4-Me), 21.2 (5).
Cryptophycin 49
[a]D +68.1 (MeOH, c 0.075); W X. (e) 246 (25500), 284 (5200); IR (neat)
v.. 3401, 3282, 2962, 1744, 1728, 1668, 1540, 1505, 1464, 1258, 1198, 1177,
1066,
694 cm''; EIMS m/z (rel intensity) 624/626 (0.8/0.3), 398/400 (43/14),
227(78), 195/197
(58/26) 91 (100); high resolution EIMS m/z 624.2650 (calcd for C34H41C1NZO7, -
4.8mmu
error); 'H NMR(CDC13):amino or hydroxy acid unit 6(carbon position,
multiplicity; J in
Hz) 5-hydroxy-6-methyl-8 phenyl-2, 7-octadienoic acid (A) 5.77 (2, d; 14.1),
6.67 (3, m),
2.38 (4, m), 2.50 (4, m), 5.01 (5, m), 2.56 (6, m), 1.13 (6-Me, d; 6.5), 6.03
(7, dd;
15.8 and 8.6), 6.42 (8, d; 15.8), 7.29-7.35 (10/11/13/14, m), 7.23 (12; m); 3-
chloro-4-
methoxyphenylalanine (B) 4.82 (2, m), 5.64 (2-NH, m), 3.06 (3, m), 3.13 (3,
m), 7.22
(5, m), 3.87 (7-OMe, s), 6.83 (8, m), 7.08 (9, m); 3-amino-2-methylpropionic
acid (C)
2.72 (2, m), 1.22 (2-Me, d; 6.7), 3.26 (3, m), 3.53 (3, m), 6.90 (3-NH, m); 2-
hydroxyvaleric acid (D) 4.81 (2, dd; 8.8 and 3.9), 1.63 (3, m), 1.68 (3, m),
1.33 (4-H2,
m). 0.74 (5, t; 7.3).
Cryptophycin 50
[a]D + 32.0 (CHC13 c. 0.44); UV A. (e) 242 (4933), 262 (3996, 274 (3719),
286 (2430), 332 (359); IR (neat) v. 3412, 3274, 2958, 1752, 1724, 1676, 1648,
1503,
1465, 1258, 1177, 1066, 753; EIMS m/z (rel intensity) 640/642 (4/2), 398/400
(11/4),
280/282 (10/3), 227 (17), 195/197 (57/18), 157 (20), 141 (31), 91 (100); high
resolution
EIMS m/z 640.2531 (calcd. for C34H41C1N2O8, 2.lmmu error); 'H NMR (CDC13)
amino
or hydroxy acid unit 8(carbon positions, multiplicities; J in Hz) 7, 8-epoxy-5-
hydroxy-6-
methyl-8-phenyl octanoic acid (A) 5.73 (2, d; 15.7), 6.67 (3, ddd; 15.7, 9.7
and 5.4),
2.45 (4, m), 2.55 (4, m), 5.13 (5, ddd; 11.2, 5.0 and 1.7), 1.78 (6, m), 1.15
(6-Me, d,
6.9), 2.91 (7, dd; 7.5 and 1.9), 3.68 (8, d; 1.7), 7.25 (10/14, m), 7.33-7.38
(11/12/13;
(S) Z' T T '(t,) 0'fiZ '(aY1i-~)
9'9I '(~) I'9~ '(Z) 9'9L '(T) t'69T a '(~) ~'It, '(aL1I-Z) 0'tiT '(Z) 9'8~
'(T) t,'SLI a '=(6)
17'8ZT '(8) Z'ZTT '(aL1T0-L) T'99 '(L) 6'~ST '(9) Z'SZT '(S) 0'T~T '(fi) 8'6ZI
'(~) 0'S~ '(Z)
S'~S '(T) 6'0LT S'(ZI) 9'8ZI '(~T/TT) 9'8ZT '(tii/OT) tl'SZT '(6) L'9~I '(8)
L'8S '(L) T'~9 SZ
(aNi-9) Z'~T '(9) 9'0j7 '(S) ~'9L '(t,) 9'9~ '(~) 0'TtiT '(Z) fi'SZT '(T)
~'S9T V (uouisod
uoq.reo) g iiun :(~10(ID) gyiN DEI :(S'L 11 'S) 08'0 '(ui 'i,) OZ'I-8T'T '(u,
'fi) Tt,'I
-9~"T '(8'9 =P 'aYN-~) Z6'0 '(Iu '~) Z8'I-8L'T '(Z'17 'P 'Z) ~L'i, (a) '=(8'9
=1 sq 'HN-~) 88'9
'(~'S '8'9 't'~T PPP '~) TS'~ '(8'9 '8'9 't,'~T =PPP '~) 9Z'~ '(~'L 'P 'aL1i-
Z) ZZ'T '(o
'z) ~L'Z (.7) =(Z'Z 't'8 PP '6) LO'L '41'8 !P '8) ~8'9 '(S 'ay1tO-L) L8'~
'(Z'Z 'P 'S) IZ'L OZ
'(S'S 'S'tT !PP '~) ~T'~ '(~'L 'S'fT !PP '~) ~0'~ '(9'8 'P 'HAI-Z) ~9'S '(u,
'Z) 18'i, (S)
=(u '~T/ZI/IT) 8~'L-0~'L '(U1 'tT/0T) SZ'L '(6'I 'P '8) 69'~ '(8'T 't'L !PP
'L) 687 '(8'9
=P 'aW-9) tT'T '(w '9) 6L'I '(L'T 'Z'fi '0'TI =PPP 'S) 9I'9 '(uI 't,) ~S'Z
'(Iu 'ti) 9V'Z '(L'S
'L'6 *SI =PPP '~) 99'9 '(ti'SI P 'Z) ~L'S (V) (zH u! f '=)4i3jIdijinui
uopTsod uoq.xto)
q ziun pian dxo.rpdzf io ouzzun :(~ID(ID) 2IY1llN HI :(ioua nuruiZ'Z
'gOzAtIOsvHSSD io3 SI
PaPa) 989Z't,99 2/zu SLNIa uoUniosai u8zq :(OOT) I6 '(0~) TVT '(SZ/St) L6T/961
'(SZ) LZZ
'(91) 08Z '(t,/ZT) ~It7/TTi7 '(S) ~67 '(OT/LI) 9S9/tS9 (ITSaaIut ant~~ia.z)
z/ur Swig
tiSuioqo~ ~
'(t,) v8I '(~)
L'Z~ '(Z) VZL '(T) VOLT a !(~) Z'T1, '(aW-Z) 1'17I '(Z) V8~ '(1) 9'9LT 3 !(6)
9'8ZT '(8) OT
~'ZTI '(aL1I0-L) T'99 '(L) 0'fiST '(9) S'ZZI '(S) 0'I~T '(t,) 8'6ZT '(~) T'S~
'(Z) 9'~S '(1)
6'0LI S'(ZT) 9'8ZT '(~i/TT) L'8ZT '(fiT/OT) S'SZI '(6) 8'9~T '(8) T'69 '(L)
Z'~9 '(aL1T-9)
9'~I '(9) 8'0t, '(S) ~'9L '(fi) 6'9~ '(~) 0'TiT '(Z) ~'SZT '(I) ~'S9i V
(suoi1isod uoqreo)
sanien g lzun (~IDQD) gyiN D~T (~'L 'T 'S) fi8'0 '(T-u 'zH-t,) ~~'i '(u '~)
S9'T '(Iu '~)
SS'I '(L'~ Put' Z'6 !PP 'Z) SL'fi (a) P?an a?ounJuaddxo.'p4-Z !(L'9 'I iq 'HN-
~) Z6'9 '(0'S S
Put' L'9 '9'~T '=PPP '~) 6V~ '(6'9 WE 9'~T 4 '~) 6Z'~ '(~'L '=P 'ayli-Z) ZZ'T
'(ra 'Z) TL'Z
0) pian o2uordo.rd1Xzflaut-Z-ouizun-~ '=(Z'Z Put, t,'8 !PP '6) LO'L '(1,'8 !P
'8) ~8'9 '(s 'ab1iO
-L) L8'~ '(6'I '=P 'S) TZ'L '(9'9 PuRV'17T !PP '~) EVE '(~'L PuLfi'17T 'PP '~)
~0'~ '(~'8
'P 'HAI-Z) I9'9 '(fi'S PuE I'L '~'8 '=PPP 'Z) 08't, (S)
auzun1nptuar/ddxoi/lauc-p-ojo/z/a-g '(Lu
-ZO I-
9tiZ~0/96Stl/.LDd b81Ob/96 OM
~0-60-L661 S9SbiZZO Va
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-103-
Example 6 Synthesis of Cr~Utophycin Derivatives
Cryptonhvcin 8
To a solution of 3.8mg of Cryptophycin 1 in 1.5mL of 2:1 1,2-
dimethoxyethane/water was added 91tL 1N HCI. The solution was allowed to stir
at room
tempierature for 4 h, neutralized with potassium carbonate, and evaporated.
The residue
was partitioned between water and CHZC12. The CHZC12-soluble material was
purified by
reversed-phase HPLC to obtain 3.3mg of pure Cryptophycin 8.
EIMS m/z (relative intensity) 690/692/694 (0.8/0.5/0.2). High resolution EIMS
m/z 690.2533 (calcd for C35H44C12N2O8, -5.8mmu error). 'H NMR (CDC13): amino
or
hydroxy acid unit S(carbon position, multiplicity; J in Hz) 8-chloro-5, 7-
dihydroxy-6-
methyl-8 phenyl-2-octenoic acid (A) 5.79 (2, d; 15.4), 6.69 (3, ddd; 15.4, 9.7
and 5.6),
2.68 (4, ddt; 14.0, 5.5 and 1.8), 2.38 (4,m), 5.11 (5, ddd; 10.8, 8.6 and
1.8), 2.51 (6,
m), 1.05 (6-Me, d; 7.0), 4.01 (7, dd; 9.6 and 1.9), 4.65 (8, d; 9.6), 7.36-
7.41
(10/11/12/13/14, m); leucic acid (D) 4.92 (2, dd; 10.1 and 3.5), 1.76 (3/4,
m), 1.45 (3,
m), 0.94 (5, d; 6.6), 0.94 (4-Me, d; 6.4); 3-amino-2-methylpropionic acid (C)
2.73(2,
m), 1.22 (2-Me, d; 7.2), 3.25 (3, ddd; 13.6, 6.8 and 6.1), 3.54 (3, ddd; 13.5,
6.1 and
3.4), 6.91(3-NH, brt; 6.1); 3-chloro-4-methoxyphenylalanine (B) 4.82 (2, ddd;
8.8, 7.2
and 5.6), 5.64 (2-NH, d; 8.8), 3.03 (3, dd; 15.4 and 7.2), 3.16 (3, dd; 15.4
and 5.6),
7.23 (5, d; 2.2), 3.88 (7-OCH3, s), 6.85 (8, d; 8.5), 7.09 (9, dd; 8.5 and
2.2).
Cryptophvcin 9
To a solution of 10mg of Cryptophycin 1 in 1mL dry methanol was added 10 L
methanolic HCl (obtained by treating 1.25g thionyl chloride with 25mL MeOH).
After
stirring for 4 h the solvent was removed in vacuo and the sample was left
under vacuum
for 12 h. Reversed-phase HPLC gave 8mg of pure Cryptophycin 9.
'H NMR (CDC13): amino or hydroxy acid unit S(carbon position, multiplicity; J
in Hz); 5, 7-dihydroxy-8-methoxy-6-methyl-8 phenyl-2-octenoic acid (A) 5.76
(2, d; 15.5),
6.67 (3, ddd; 15.5, 9.5 and 5.6), 2.34 (4, ddd; 14.1, 11.1 and 9.5), 2.62 (4,
dddd; 14.1,
5.6, 1.8 and 1.5), 5.09 (5, ddd; 11.1, 7.8 and 1.8), 2.24 (6, dqd; 7.8, 7.0
and 2.2), 1.03
(6-Me, d; 7.0), 3.71 (7, dd; 8.3 and 2.2), 4.03(8, d; 8.3), 3.20 (8-OCH3, s),
7.31-7.40
(10/11/12/13/14, m); leucic acid (D) 4.86 (2, dd; 9.8 and 3.5), 1.71 (3/4, m),
1.41 (3,
m), 0.89 (5/4-Me, d; 6.4); 3-amino-2-methylpropionic acid (C) 2.71 (2, ddq;
6.8, 3.9
and 7.2), 1.21 (2-Me, d; 7.2), 3.23 (3, ddd; 13.5, 6.8 and 6.0), 3.52 (3, ddd;
13.5, 6.0
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-104-
and 3.9), 6.90 (3-NH, brt; 6.0); 3-chloro-4-methoxyphenylalanine (B) 4.82 (2,
ddd; 8.8,
7.4 and 5.7), 5.66 (2-NH, d; 8.8), 3.02 (3, dd; 14.4, 7.4), 3.15 (3, dd; 14.4
and 5.5),
7.23 (5, d; 2.2), 3.87 (7-OCH3, s), 6.84 (8, d; 8.5), 7.08 (9, dd; 8.5 and
2.2).
Cryptoph ci~10
To a stirred solution of 7mg of Cryptophycin 9 in lmL of acetone and 0.3mL
water was added 8 L of 2N NaOH. After stirring for 4 h the solution was
neutralized to
pH 7 with 1N HCl and the solvent was removed under reduced pressure. The
residue
was subjected to reversed-phase HPLC using 7:3 MeOH/H20 to yield pure
Cryptophycin
(5mg).
10 'H NMR (CD3OD): amino or hydroxy acid unit 6(carbon position, multiplicity;
J
in Hz); 5, 7-dihydroxy-8-methoxy-6-methyl-8 phenyl-2-octenoic acid (A) 5.99
(2, dt; 15.4
and 1.3), 6.82 (3, dt; 15.4 and 7.3), 2.30 (4, m), 2.50 (4, m), 3.66 (5, td;
7.8 and 3.5),
2.05 (6, d pentet; 1.8 and 7.0), 0.96 (6-Me, d; 7.0), 4.04 (7, dd; 8.8 and
2.0), 4.01 (8,
d; 8.8), 3.12 (8-OCH3, s), 7.26-7.36 (10/11/12/13/14, m); 3-amino-2-
methylpropionic
acid (C) 2.50 (2, m), 1.02 (2-Me, d; 7.3), 3.16 (3, dd; 13.4 and 6.9), 3.82
(3, dd; 13.4
and 6.6); 3-chloro-4-methoxyphenylalanine (B) 4.57 (2, dd; 8.5 and 6.5), 2.82
(3, dd;
13.9 and 8.6), 3.03 (3, dd; 13.9 and 6.5), 7.25 (5, d; 2.2), 3.82 (7-OCH3, s),
6.96 (8, d;
8.6), 7.13 (9, dd; 8.6 and 2.2). 13C NMR (CD3OD): 8 179.5, 173.4, 168.2,
155.4,
143.7, 141.7, 131.9, 131.7, 129.8, 129.3 (2C), 129.2 (2C), 128.8, 126.2,
123.2, 113.4,
85.9, 74.5, 74.1, 56.8, 56.6, 56.3, 43.3, 41.2, 40.2, 38.8, 38.0, 15.5, 9.9.
Cryptoph cin 12
To a solution of 5mg of Cryptophycins 1, 5 or 8 in lmL of 4:1 acetone/water
was
added 15 L of 2N NaOH. After stirring at room temperature for 5 h, the
reaction
mixture was neutralized to pH 7 with 1N HCl and evaporated. The CH2C12-soluble
material was passed through a small silica-cartridge with CH2C12, 1:1
EtOAc/CH2C12, and
EtOAc. The fraction eluted with EtOAc contained pure Cryptophycin 12.
'H NMR (CD3OD): amino or hydroxy acid unit 6(carbon position, multiplicity; J
- in Hz); 5, 7, 8-trihydroxy-6-methyl-8 phenyl-2-octenoic acid (A) 6.07 (2,
ddd; 15.5, 1.3
and 1.2), 6.40 (3, dt; 15.5 and 7.3), 2.49 (4, m), 2.60 (4, m), 3.92 (5, ddd;
9.3, 6.7 and
4.5), 1.94 (6, m), 1.07 (6-Me, d; 6.6), 3.61 (7, dd; 8.9 and 7.6), 4.56 (8, d;
7.6), 7.36
(10/14, dd; 7.4 and 1.5), 7.32 (11/13, brt; 7.5), 7.25 (12, m); 3-amino-2-
methylpropionic
acid (C) 2.54 (2, ddq; 7.0, 6.6 and 7.0), 1.02 (2-Me, d; 7.0), 3.14 (3, dd;
13.5 and 7.0),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-105-
3.42 (3, dd; 13.4 and 6.6); 3-chloro-4-methoxyphenylalanine (B) 4.57 (2, dd;
8.4 and
6.7), 2.83 (3, dd; 13.8 and 8.4), 3.02 (3, dd; 13.8 and 6.6), 7.25 (5, d;
2.1), 3.82 (7-
OCH3, s), 6.95 (8, d; 8.5), 7.12 (9, dd; 8.5 and 2.1). Methylation of
Cryptophycin 12
with diazomethane gave Cryptophycin 6.
Crvptophvcin 14
To a solution of 3mg of Cryptophycin 6 in 1mL of 3:1 acetone/H20 was added
5 L of 2N NaOH. After stirring for 5 h, the reaction mixture was neutralized
to pH 7
with 1N HCl and then evaporated to dryness. The residue was subjected to
reversed-
phase HPLC to give 2.4mg of Cryptophycin 14.
'H NMR (CD3OD): amino or hydroxy acid unit S(carbon position, multiplicity; J
in Hz); 5-hydroxy-6-methyl-8 phenyl-2, 7-octadienoic acid (A) 5.98 (2, d;
15.3), 6.78 (3,
dt; 15.3 and 7.5), 2.35 (4, m), 3.64 (5, td; 7.2 and 4.8), 2.47 (6, m), 1.14
(6-Me, d;
6.9), 6.22 (7, dd; 15.9 and 8.1), 6.39 (8, d, 15.9), 7.24-7.36
(10/11/12/13/14, m); 3-
amino-2-methylpropionic acid (C) 2.35 (2, m), 1.02 (2-Me, d; 6.9), 3.18 (3,
dd; 13.2 and
6.6), 3.36 (3, dd; 13.2 and 4.5); 3-chloro-4-methoxyphenylalanine (B) 4.58 (2,
dd; 8.7
and 6.3), 2.80 (3, dd; 13.8 and 9.0), 3.05 (3, dd; 13.8 and 6.3), 7.25 (5, d;
2.1), 3.82
(7-OCH3, s), 6.95 (8, d; 8.4), 7.13(9, dd; 8.4 and 2.1).
Cryptophycin 35
A catalytic amount of Pt02 was added to a flask containing 0.5mL of CHZC12.
The air in the flask was evacuated, H2 was introduced, and the mixture was
stirred at
room temperature for 20 min. A solution of 10mg of Cryptophycin 1 in minimum
CH2C12 was added and the mixture was stirred at room temperature for 45 min.
The
catalyst was removed by filtration through celite/cotton and the solvent was
evaporated.
Reversed phase HPLC of the residue on a C18 column yielded 6.5mg of
Cryptophycin
35.
EIMS m/z (relative intensity) 656/658 (25/10), 412/414 (25/12), 280/282
(20/10),
195/197 (78/25), 141 (58), 91 (100); high resolution EIMS m/Z 656.2864 (calcd
for
C35H45C1N20g, 0.0mmu error); 'H NMR (CDC13) amino or hydroxy acid unit S
values
(carbon positions, multiplicities; J in Hz) 2,3-dihydro-7,8-epoxy-5-hydroxy-6-
methyl-8-
phenyl octanoic acid (A) 2.32 (2, ddd; 14.5, 9.2, 5.8), 2.10 (2, ddd; 14.5,
9.2, 6.2), 1.5-
1.8 (3/4 overlapping m), 5.07 (5, ddd; 12.5, 5.6, 2.0), 1.80 (6, m), 1.12 (6-
Me, d; 7.0),
2.90 (7, dd; 7.4, 1.8), 3.67 (8, d; 1.8), 7.24 (10/14, m), 7.32-7.38
(11/12/13, m); 3-
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-106-
chloro-4-methoxyphenylalanine (B) 4.71 (2, ddd; 8.7, 6.4, 6.3), 5.62 (2-NH, d;
8.7),
3.08 (2H-3, br d; 6.4), 7.19 (5, d; 2.0), 3.87 (7-OMe, s), 6.83 (8, d; 8.5),
7.07 (9, dd;
8.4, 2.0); 3-amino-2-methylpropionic acid (C) 2.72 (2, m), 1.18 (2-Me, d;
6.9), 3.12 (3,
ddd; 11.4, 10.6, 5.6), 3.70 (3, ddd), 6.76 (3-NH, br t, 6.0); leucic acid (D)
4.83 (2, dd;
9.9, 3.8), 1.39 (3, m), 1.70 (3, m), 1.72 (4, m), 0.87 (4-Me, d; 5.3), 0.86
(5, d; 5.3);
13C NMR (CDC13) unit 8 values (carbon positions) A 172.4 (1), 36.2 (2), 32.0
(3), 21.1 (4), 76.6 (5), 40.2 (6), 13.6 (6-Me), 63.3 (7), 59.2 (8), 136.8 (9),
125.6 (10/14), 128.7
(11/13), 128.6 (12); B 170.7 (1), 53.7 (2), 35.5 (3), 130.0 (4), 131.1 (5),
122.2 (6),
.153.8 (7), 56.1 (7-OMe), 112.1 (8), 128.5 (9); C 175.2 (1), 38.2 (2), 13.6 (2-
Me), 42.1
(3); D 171.9 (1), 71.7 (2), 39.6 (3), 24.5 (4), 22.9 (4-Me), 21.4 (5).
Examnie 7 Analysis of Microtubule Depolvmerizing Activity of Cryptophycin
Materials
Vinbiastine, cytochalasin B, tetramethylrhodarnine isothiocyanate
(TRITC)-phalloidin, sulforhodamine B (SRB) and antibodies against 0-tubulin
and
vimentin were obtained from the Sigma Chemical Company. Basal Medium Eagle
containing Earle's salts (BME) was from Gibco and Fetal Bovine Serum (FBS) was
purchased from Hyclone Laboratories.
Cell Lines
The Jurkat T cell leukemia line and A-10 rat aortic smooth muscle cells were
obtained from the American Type Culture Collection and were cultured in BME
containing 10% FBS and 50 g/mL gentamycin sulfate. Human ovarian carcinoma
cells
(SKOV3) and a sub-line which has been selected for resistance to vinblastine
(SKVLBI)
were a generous gift from Dr. Victor Ling of the Ontario Cancer Institute.
Both cell
lines were maintained in BME containing 10% FBS and 501Ag/mL gentamycin
sulfate.
Vinblastine was added to a fmal concentration of 1 g/mL to SKVLB1 cells 24
hours after
passage to maintain selection pressure for P-glycoprotein-overexpressing
cells.
Cell Proliferation and Cycle Arrest Assays
Cell proliferation assays were performed as described by Skehan et al.ll For
Jurkat cells, cultures were treated with the indicated drugs as described in
Skehanll and
total cell numbers were determined by counting the cells in a hemacytometer.
The
percentage of cells in mitosis was determined by staining with 0.4% Giemsa in
PBS
CA 02214565 2005-07-22
-107-
followed by three rapid washes with PBS. At least 1000 cells per treatment
were scored
for the presence of mitotic figures and the mitotic index was calculated as
the ratio of
cells with mitotic figures to the total number of cells counted.
Imrnunofluorescence Assays
A-10 cells were grown to near-confluency on glass coverslips in BME/10% FBS.
Compounds in PBS were added to the indicated final concentrations and cells
were
incubated for an additional 24 hours. For the staining of microtubules and
intermediate
filaments, the cells were fixed with cold methanol and incubated with PBS
containing
10% calf serum to block nonspecific binding sites. Cells were then incubated
at 37'C for
60 min with either monoclonal anti-0-tubulin or with monoclonal anti-vimentin
at
dilutions recommended by the manufacturer. Bound primary antibodies were
subsequently visualized by a 45-minute incubation with fluorescein-conjugated
rabbit
antimouse IgG. The coverslips were mounted on microscope slides and the
fluorescence
patterns were examined and photographed using a Zeiss Photomicroscope IIl
equipped
with epifluorescence optics for fluorescein. For staining of microfilaments,
cells were
fixed with 3% paraformaldehyde, permeabilized with 0.2% Triton X-100 and
chemically
reduced with sodium borohydride (lmg/mL). PBS containing 100nM TRITC-
phalloidin
was then added and the mixture was allowed to incubate for 45 min at 37' C.
The cells
were washed rapidly three times with PBS before the coverslips were mounted
and
inunediately photographed as described above.
Effects of cryptonhvcins and vinbiastine on Jurkat cell proliferation and cell
cycle
Dose-response curves for the effects of cryptophycin compounds and vinblastine
on cell proliferation and the percentage of cells in mitosis are indicated in
Figures 2A and
2B, respectively. Less than 3% of untreated cells displayed mitotic figures.
Both the
cryptophyein-compounds and vinblastine caused dose-dependent increases in the
percentage of cells observed in mitosis. The increase in the mitotic index was
closely
correlated with decreases in cell proliferation, i.e. the concentrations of
both cryptophycin
compounds and vinblastine that caused 50% of the cells to accumulate in
mitosis was
virtually the same as the concentration which inhibited cell proliferation by
50%. The
IC_4Os for the cryptophycin compounds and vinblastine for these effects were
0.2 and 8nM,
respectively.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-108-
Effects of cytochalasin B, vinblastine and cryptophycins on the cytoskeleton
Aortic smooth muscle (A-10) cells were grown on glass coverslips and treated
with PBS, 2 M cytochalasin B, lOOnM vinblastine or lOnM cryptophycin
compounds.
After 24 hours, microtubules and vimentin intermediate filaments were
visualized by
indirect immunofluorescence and microfilaments were stained using TRITC-
phalloidin.
The morphological effects of each drug were examined. Untreated cells
displayed
extensive microtubule networks complete with perinuclear microtubule
organizing centers.
Vimentin intermediate filaments were also evenly distributed throughout the
cytoplasm,
while bundles of microfilaments were concentrated along the major axis of the
cell.
Cytochalasin B caused complete depolymerization of microfilaments along with
the
accumulation of paracrystalline remnants. This compound did not affect the
distribution
of either microtubules or intermediate filaments. Both vinblastine and the
cryptophycin
compound caused marked depletion of microtubules. Neither compound affected
microfilament organization; however, vimentin intermediate filaments
collapsed, forming
concentric rings around the nuclei of cells treated with either vinblastine or
a
cryptophycin compound.
Effects of cryptophycins and vinblastine on taxol-stabilized microtubules
A-10 cells were treated for 3 hours with 0 or 10 M taxol before the addition
of
PBS, l00nM vinblastine or lOnM cryptophycin compound. After 24 hours,
microtubule
organization was examined by immunofluorescence as described above. Compared
with
those in control cells, microtubules in taxol-treated cells were extensively
bundled,
especially in the cell polar regions. As before, vinblastine caused complete
depolymerization of microtubules in non-pretreated cells. However,
pretreatment with
taxol prevented microtubule depolymerization in response to vinblastine.
Similarly, taxol
pretreatment completely stabilized microtubules against cryptophycin-induced
depolymerization.
Reversibilitv of microtubule depolymerization by vinblastine and cryptophvcin
A-10 cells were treated with either lOOnM vinblastine or lOnM cryptophycins
for
24 hr, resulting in complete microtubule depolymerization. The cells were then
washed
and incubated in drug- free medium for periods of 1 hour or 24 hours.
Microtubules
repolymerized rapidly after the removal of vinbiastine, showing significant
levels of
microtubules after 1 hour and complete morphological recovery by 24 hour. In
contrast,
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-109-
microtubules did not reappear in cells treated with cryptophycin compounds at
either 1
hour or 24 hours after removal of the compound.
Reversibilitv of cryptophycins-. vinblastine- and taxol-inhibition of cell
proliferation
SKOV3 cells were treated for 24 hours with previously determined IC" doses of
vinblastine, cryptophycin compounds or taxol (i.e. values determined in
experiments
summarized in Table 5). During this time the cell density increased from 0.4
to 0.5
0.05 absorbance units (Figure 3), indicating a 25% increase in cell number for
all three
treatments. Removal of the drugs resulted in rapid growth of the vinblastine-
treated cells,
such that their numbers were increased approximately 3-fold in 24 hours. In
contrast,
cells treated with cryptophycin compounds or taxol remained arrested,
increasing only
0.2- to 0.4-fold in the 24 hours following removal of the drug. The
proliferative capacity
of cryptophycins or taxol-treated cells was subsequently restored since the
cells then
doubled in the next 24 hours.
Effects of combinations of vinblastine and cryptophycins on cell proliferation
SKOV3 cells were treated with combinations of cryptophycins and vinblastine
for
48 hours. The percentages of surviving cells were then determined and the
IC50s for each
combination was calculated. The effects of these combinational treatments, as
well as
single drug treatments, are depicted as an isobologram (Figure 4). The IC50s
for
combinations of cryptophycin compounds and vinblastine fell very close to the
line of
additivity, indicating that these two drugs induce only additive inhibitions
of cell
proliferation.
Toxicitv of cryptophycins, vinblastine and taxol toward SKOV3 and SKVLB1 cells
SKVLB1 cells are resistant to natural product anticancer drugs because of
their
over expression of P-glycoprotein12. The abilities of taxol, vinbiastine and
cryptophycin
compounds to inhibit the growth of SKOV3 and SKVLBI cells are summarized in
Table 5. Taxol caused dose-dependent inhibition of the proliferation of both
cell lines
with IC50s for SKOV3 and SKVLB1 cells of 1 and 8000nM, respectively.
Vinblastine
also inhibited the growth of both cell lines, with IC50s of 0.35 and 4200nM
for SKOV3
and SKVLB1 cells, respectively. Cryptophycins demonstrated IC50s of 7 and
600pM for
SKOV3 and SKVLB1 cells, respectively. The resulting resistance factors for
SKVLB1
cells to the compounds are calculated as the IC50s for SKVLB1. IC50s for SKOV3
cells
are also indicated in Table 5.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-110-
Thus it is demonstrated that the present invention provides novel cryptophycin
compounds, as well as previously-disclosed cryptophycin compounds, which are
potent
inhibitors of cell proliferation, acting by disruption of the microtubule
network and
inhibition of mitosis. The cryptophycin compounds disrupt microtubule
organization and
thus normal cellular functions, including those of mitosis.
Classic anti-microtubule agents, such as colchicine and Vinca alkaloids,
arrest cell
division at mitosis. It seemed appropriate to compare the effect of one of
these agents on
cell proliferation with the cryptophycin compounds. For this purpose, the
Vinca alkaloid
vinblastine was selected as representative of the classic anti-microtubule
agents.
Accordingly, the effect of cryptophycin compounds and vinblastine on the
proliferation
and cell cycle progression of the Jurkat T-cell leukemia cell line was
compared. Both
compounds caused parallel dose-dependent inhibitions of cell proliferation and
accumulation of cells in mitosis.
Since antimitotic effects are commonly mediated by disruption of microtubules
in
the mitotic spindles, the effects of cryptophycin compounds on cytoskeletal
structures
were characterized by fluorescence microscopy. Immunofluorescence staining of
cells
treated with either a cryptophycin compound or vinblastine clearly
demonstrated that both
compounds caused the complete loss of microtubules. Similar studies with SKOV3
cells
demonstrate that the anti-microtubule effects of cryptophycin compounds are
not unique to
the smooth muscle cell line. Neither drug affected the levels or distribution
of
microfilament bundles, as was readily induced by cytochalasin B, indicating
that the loss
of microtubules may not be due to a non-specific mechanism, e.g. activation of
proteases
or loss of energy charge. Both vinbiastine and cryptophycin compounds also
promote
marked collapse of vimentin intermediate filaments, such that brightly
staining rings were
formed around the cell nucleus.
Removal of vinblastine from the culture medium resulted in rapid
repolymerization
of microtubules. In contrast, cells treated with cryptophycin compounds
remained
depleted of microtubules for at least 24 hours after the compound was removed
from the
cultures.
The present invention demonstrates that cryptophycin compounds circumvent
P-glycoprotein-mediated multiple drug resistance. Transport by P-glycoprotein
limits the
ability of natural product anticancer drugs to inhibit the growth of tumor
cells with
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-111-
acquired or de novo drug resistance.13-1s Vinca alkaloids, while very useful
in the initial
course of chemotherapy, are extremely good substrates for transport by P-
glycoprotein,
and so are of very limited usefulness against P-glycoprotein-mediated MDR
tumors.
Therefore, identification of agents which overcome multiple drug resistance
may, should
lead to the development of useful and novel anticancer agents. The
cryptophycin
compounds of the present invention appear to be such agents since they are
poor
substrates for P- glycoprotein-mediated transport. This fact is reflected in
the low cell
resistance factor for cryptophycin compounds compared with vinblastine, taxol
and other
natural product drugs.
Total Synthesis of Cryptophvcins
The structures of the novel synthesized compounds, viz. Cryptophycins 51, 52,
53, 55, 56, 57, 58, and 61 were confirmed in a straightforward manner using
methodology that is well-known to those trained in the art. Mass spectral data
were
consistent with the molecular compositions. Proton and carbon NMR data were
very
similar to those of cryptophycin 1 and related naturally-occurring and semi-
synthetic
analogs.
The following examples demonstrate the total synthesis of cryptophycin
compounds as well as their use as therapeutic agents in accordance with the
invention.
Example 8 Synthesis of Cryptophvcin 51
S-trans-3-Penten-2-ol (A)
A mixture of racemic trans-3-penten-2-ol (933mg, 11mmo1), trifluoroethyl
laurate
(4.14g, 15mmo1), and porcine pancreatic lipase (PPL, 2.0g) in 25mL of
anhydrous
diethyl ether was stirred for 80 hours. The PPL was then filtered off and
washed with
ether three times. The ether filtrate was evaporated and the sticky oil was
then subjected
to short-path vacuum distillation. The S-trans-3-penten-2-ol (A) was condensed
in a .
liquid nitrogen cooled trap (383mg). 'H NMR (CDC13) 8 5.57 (4-H; dq, -
15.3/6.0), 5.47
(3-H; ddd, -15.3/6.4/1.2), 4.19 (2-H; 1:4:6:4:1 pentuplet, 6.4), 2.24 (OH;
bs), 1.63 (5-
H3; d, 6.0), 1.19 (1-H3; d, 6.4). 13C NMR (CDC13) S 135.5 (3), 125.5 (4), 68.7
(2),
23.3 (5), 17.5 (1).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-112-
S-trans-2-(2-Prop nloxy)-3 pentene (B)
To a vigorously stirred mixture of S-enantiomer A (628mg, 7.3mmol),
tetrabutylammonium hydrogen sulfate (138mg, 0.41mmo1), and 40% NaOH in water
(5mL) at 0 C was added dropwise propargyl chloride (767mg, 10.3mmol, 7451LL).
Vigorous stirring was continued overnight after which time the mixture was
neutralized
by HCI at 0 C and the propargyl ether extracted into pentane. The extract was
evaporated and the propargyl ether was purified on a short silica gel column
(2% diethyl
ether/pentane) to give 778mg of propargyl ether B, [a]D -118.9 (c 2.0,
CHC13); 'H
NMR (CDC13) 6 5.70 (4-H; dq, 18.5/6.5), 5.31 (3-H; ddd, 18.5/7.2/1.4), 4.15
(1'-H; dd,
-15.6/2.1), 4.01 (1'-H; dd, -15.6/2.1), 4.01 (2-H; m), 2.38 (3'-H; t, 2.1),
1.73 (5-H; dd,
6.5/1.4), 1.25 (1H; d, 6.3).
(3R.4R)-4-Methylhept-5(E)-en-l-yn-3-ol (C)
An aliquot of butyl lithium hexane solution (2.5M, 5.1mL, 12.8mmol) was
evaporated in vacuo and the residue cooled to -90 C. A solution of propargyl
ether B
(454mg, 3.66mmol) in lOmL of THF was slowly added. After allowing the
temperature
to increase to room temperature overnight, the reaction mixture was quenched
with
NH4C1 solution. Extraction with ether three times, evaporation of the dried
extract, and
purification of the residue on a silica gel column (5 % EtOAc/hexane) gave
322mg of
alcohol C (71% yield), [a]D +32.9 (c 3.0, CHC13); IR (NaCI) v,,,a, 3306,
2968, 1455,
1379, 1029, 975 cm 1. 1H NMR (CDC13) 6 5.61 (6-H; dq, 15.3/6.3), 5.38 (5-H;
dd,
15.3/7.7), 4.13 (3-H; bs), 2.45 (1-H; d, 1.5), 2.38 (4-H; m), 2.20 (OH; bd,
3.3), 1.68
(7-H; d, 6.2), 1.09 (4-CH3; d, 6.8). 13C NMR (CDC13) S 131.5 (5), 127.9 (6),
83.5 (2),
73.6 (1), 66.2 (3), 43.4 (4), 18.1 (7), 15.7 (4-Me).
f3S.4R)-3-tert-Butvldimethylsilvloxy-4-methylhent-5E-enal lD)
To a stirred solution of alcohol C (248mg, 2mmol) and imidazole (340mg,
5mmo1) in 3mL of dry DMF was added tert-butyldimethylsilyl chloride (452mg,
3mmo1).
After stirring the mixture overnight, l OmL of 10% NaOH was added to destroy
the
excess tert-butyldimethylsilyl chloride. The product was extracted into ether
and the
extract washed successively with water, 0.5 N HCI, and water, dried and
evaporated.
Purification of the residue by chromatography on silica gel with hexane gave
457mg of
(3R,4R)-3-tert-butyldimethylsilyloxy-4-methylhept-5(E)-en-1-yne (96% yield),
'H NMR
(CDC13) 6 5.50 (6-H; dq, 15.3/6.1), 5.38 (5-H; dd, 15.3/7.5), 4.16 (3-H; dd,
5.7/1.7),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-113-
2.37 (1-H; d, 1.7), 2.35 (4-H; m), 1.68 (7-H; d, 6.1), 1.07 (4-Me; d, 6.8),
0.90 (CMe3;
s), 0.12 (SiMe; s), 0.09 (SiMe; s).
Using the same procedure the corresponding TBDPS derivative, (3R,4R)-3-tert-
butyldiphenylsilyloxy-4-methylhept-5(E)-en-1-yne, was formed in 92% yield,
[a]D +32.9
(c 3.0, CHC13). 'H NMR (CDC13) S 7.72/7.38 (2Ph-H5), 5.32 (6-H; m), 5.25 (5-H;
dd,
16.2/7.3), 4.29 (3-H; dd, 5.2/2.0), 2.38 (4-H; m), 2.33 (1-H; d, 2.0), 1.64 (7-
H; d,
5.3), 1.11 (4-Me; d, 6.9), 1.06 (CMe3). 13C NMR (CDC13) 8 136.1/ 135.9 /133.6
/129.7/129.6/127.5/127.3 (Ph), 132.4 (5), 126.1 (6), 83.3 (2), 73.5 (1), 68.0
(3), 43.6
(4), 26.9 (CM%), 19.4 (CMe3), 18.0 (7), 14.7 (4-Me).
2-Methylbutene (1.15mL 2M solution in THF, 2.3mmol) was added to 1.1mL of
BH3 THF solution (1M, 1.lmmol) at -25 C and the mixture was stirred in an ice
bath for
two hours. The temperature was then cooled to -50 C and a solution of the TBS
derivative (238mg, lmmol) in lmL of THF was added all at once. The cooling
bath was
removed and the reaction mixture was allowed to warm to and remain at room
temperature for one hour. Then 2.2 M KH2PO4/K2HPO4 solution (4. 8mL) and 30%
H202
(0.8mL) were added at 0 C. One hour later, the THF was evaporated and the
residue
was extracted into ether. The dried ether extract was evaporated and the
residue
chromatographed on silica gel (1 % EtOAc/hexane) to give 194mg of aldehyde
D(76%
yield). 'H NMR (CDC13) 8 9.78 (1-H; t, 2.3), 5.46 (6-H; dq, 15.3/6.1), 5.34 (5-
H; dd,
15.3/7.5), 4.13 (3-H; m), 2.47 (2-H; m), 2.31 (4-H; m), 1.66 (7-H; br d, 6.1),
0.99 (4-
Me; d, 6.8), 0.87 (CMe3; s), 0.07 (SiMe; s), 0.04 (SiMe; s).
The tert-butyldiphenylsilyl ether (TBDPS) derivative of the aldehyde was
formed
in 83% yield, 1H NMR (CDC13) 6 9.52 (1-H; t, 2.4), 7.69/7.40 (2Ph-HS), 5.28 (6-
H; m),
5.22 (5-H; dd, 16.2/6.2), 4.19 (3-H; m), 2.42 (2-H; m), 2.29 (4-H; m), 1.60 (7-
H; d,
5.4), 1.07 (CMe3), 1.02 (4-Me; d, 6.9). 13C NMR (CDC13) S 202.0 (1),
136.1/133.6/
133.3 /130.2/129.7/127.7/127.6 (Ph), 132.3 (5), 126.2 (6), 72.8 (3), 47.6 (2),
42.2 (4),
27.1 (CM%), 19.6 (CMe3), 18.3 (7), 14.9 (4-Me).
Methyl (5S.6R)-5-tert-Butyldimethylsilvloxy-6-methyl-7-oxonona-2E.7 E-dienoate
(E)
To a stirred solution of aldehyde D(0.74g, 2.9mmol) and trimethyl
phosphonoacetate (632mg, 3.5mmol) in 5mL of THF cooled to -78 C was added
tetramethylguanidine (435 L, 3.5mmo1). After 30 minutes the cooling bath was
removed
and the mixture was stirred for another four hours. The mixture was
neutralized with 1N
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-114-
HC1 and the product was extracted into ether. Evaporation of the dried ether
extract left
a residue which was chromatographed on silica gel (5 % EtOAc/hexane) to give
0.814g of
E (90% yield). 'H NMR (CDC13) 6 6.93 (3-H;dt, 15.6/7.8), 5.62 (2-H; dd,
15.6/1.2),
5.37 (8-H, m), 5.37 (7-H, m), 3.71 (OCH3, s), 3.61 (5-H, m), 2.29 (4-H2, m),
2.22 (6-
H, m), 1.66 (9-H3; br d, 6.1), 0.99 (6-Me; d, 6.8), 0.88 (CMe3; s), 0.03
(SiMe; s), 0.01
(SiMe; s).
The tert-butyldiphenylsilyl ether (TBDPS) derivative of the aldehyde was
formed
in 90% yield, 'H NMR (CDC13) 6 7.68/7.38 (2Ph-H5), 6.75 (3-H;dt, 15.6/7.4),
5.62 (2-
H; d, 15.6), 5.34 (8-H, m), 5.29 (7-H, m), 3.70 (5-H, m), 3.68 (OCH3, s), 2.28
(4-H2,
m), 2.20 (6-H, m), 1.62 (9-H3; d, 5.3), 1.08 (CMe3), 0.99 (6-Me; d, 6.9). 13C
NMR
(CDC13) S 166.7 (1), 146.4 (3), 136.0/134.2/133.8/129.62/129.56/127.5/127.4
(Ph),
132.5 (7), 125.8 (8), 122.6 (2), 76.2 (5), 51.3 (OCH3), 41.7 (6), 36.8 (4),
27.0 (CM~e ),
19.4 (CMe3), 18.1 (9), 14.7 (6-Me).
Methvl (5S.6R)-5-tert-Butyldimethylsilyloxv-6-methvl-7-oxohept-2(E)-enoate (F)
Ozone was passed through a solution of methyl ester E (328mg, 1.0mmo1) and
971LL of pyridine in 15mL of CH2C12 at -78 C and the progress of the
ozonolysis was
monitored by TLC analysis. After the methyl ester had been consumed, about
500mg of
zinc dust and lmL of glacial acetic acid were added. The temperature was
slowly
increased to 25 C. The mixture was filtered and the filtrate was washed
successively
with saturated CuSO4 and NaHCO3 solutions. After evaporation of the solvent,
the crude
aldehyde F (249mg, 83 %) was used in the next step without further
purification. 'H
NMR (CDC13) 8 9.96 (7-H; t, 2.3), 6.96 (3-H; dt, 15.7/7.6), 5.90 (2-H; dd,
15.7/0.7),
4.05 (5-H; m), 3.74 (OMe; s), 2.51 (6-H; m), 2.45 (4-H2; m), 1.09 (6-Me; d,
6.9), 0.88
(CMe3; s), 0.04 (SiMe; s), 0.03 (SiMe; s).
Methyl (5S.6R)-5-t-butvldimethvlsilvloxv-6-methvl-8-phenyl-octa-2E 7 E-
dienoate (G)
To a stirred solution of aldehyde F(25.(kmg, 0.08mmo1) in 1.5mL of THF at -
78 C was added 0.80mL of a cold (-78 C) mixture of benzyltriphenylphosphonium
chloride (268mg, 0.69mmol, in 6.9mL of THF) and n-butyl lithium (280 L, 2.5M
in
hexane). After 15 min, the cold bath was removed and stirring was continued
for 2 h.
The reaction was quenched with saturated ammonium chloride solution and the
THF was
evaporated. The concentrate was extracted with hexane twice and the combined
extract
was washed with brine, dried and evaporated. The residual oil, a 5:1 mixture
of the E
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-115-
and Z isomers, was dissolved in 1.5mL of benzene containing thiophenol (0.02M)
and
1,1'azobis(cyclohexanecarbonitrile) (VAZO, 0.006M) and the mixture was
refluxed for
5h. After cooling to RT, hexane (15mL) was added and the organic solution was
washed
successively with 10% NaOH and brine, dried (MgSO4), and evaporated.
Chromatography of the residue on silica gel (2% EtOAc/hexane) led to 24mg
(80%) of
G, [a]D +68.2 (c 1.5, CHC13); EIMS mlz 374 (< 1%; M+), 359 (1; M+-CH3), 317
(10;
M+-Bu), 275 (10), 243, (73), 143 (20), 115 (10), 97 (64), 89 (31), 73 (100);
HREIMS
m/z 374.2232 (C=H34O3Si, 0+4.5mmu), 359.2031 (C21H31O3Si, 0+1.1mmu), 317.1579
(C18HuO3Si, A-0.6mmu); UV (MeOH) a,1187C (e) 206 (33500), 252 (20100) nm; IR
P.
2952, 2855, 1725, 1657, 1435, 1257, 1168, 1097, 970, 836, 775 cm-1; 'H NMR 8
7.2-
7.4 (Ph-H5; m), 6.96 (3-H; ddd, 15.6/7.8/7.5), 6.37 (8-H; d, 15.9), 6.16 (7-H;
dd,
15.9/8.1), 5.84 (2-H; d, 15.6), 3.75 (5-H; ddd, 10.2/6.0/4.2), 3.72 (OMe; s),
2.44 (6-H;
m), 2.36 (4-H2; m), 1.10 (6-Me; d, 6.9), 0.91 (Si-CMe3; s), 0.06 (Si-Me; s),
0.05 (Si-
Me; s); 13C NMR S 166.8 (1), 146.4 (3), 137.6 (Ph 1'), 131.9 (8), 130.4 (7),
128.5 (Ph
3'/5'), 127.0 (Ph 4'), 126.0 (Ph 2'/6'), 122.9 (2), 75.0 (5), 51.4 (OMe), 42.8
(6), 37.6
(4), 25.9 (Si-CMe--
J, 18.1 (Si-CMe3), 16.2 (6-Me), -4.4 (Si-Me), -4.5 (Si-Me). Calcd for
C=H34O3Si: C, 70.52; H, 9.17. Found: C, 70.72; H, 9.42.
(5S,6R)-5-t-Butvldimethylsilyloxy-6-methyl-8-phenylocta-2E.7 E-dienoic acid
(H)
To a solution of ester G (159mg, 0.43mmo1) in 7mL acetone was added 5mL of
1N aq LiOH. The mixture was stirred at 25 C for 3h, diluted with 20mL of Et20,
and
acidified to .= pH 4 with iN HC1. The organic layer was separated and washed
with
20mL portions of brine and water, dried (MgSO4) and evaporated. Chromatography
of
the residual oil on silica gel with 40% EtOAc in hexane containing 0.5% AcOH
resulted
in pure acid H as a pale yellow mobile oil (145mg, 95% yield): [a]D +87.0 (c
1.4,
CHC13); EIMS m/z; 343 (1; M+-OH), 303 (5), 275 (9), 257 (4), 229 (62), 213
(16), 171
(22), 143 (37), 131 (16), 115 (23), 97 (100), 91 (44); HREIMS m/z 343.2107
(CZ1H31O2Si, A-1.3mmu), 229.1220 (C15H1702, 0+0.9mmu); UV X. (e) 206 (24500),
252 (15600) nm; IR P. 3300-2800 (br), 2956, 2856, 1697, 1651, 1419, 1256,
1097,
836, 693 cm 1; 'H NMR 8 10.4 (CO2H; bs, W1,2 =100), 7.2-7.4 (Ph-H5; m), 7.09
(3-H;
ddd, 15.6/7.6/7.6), 6.39 (8-H; d, 15.9), 6.16 (7-H; dd, 15.9/8.1), 5.85 (2-H;
d, 15.6),
3.78 (5-H; ddd, 6.0/6.0/4.2), 2.46 (6-H; m), 2.40 (4-H2; m), 1.12 (6-Me; d,
6.9), 0.92
(Si-CMe3; s), 0.07 (SiMe2; s); 13C NMR d 171.6 (1), 149.1 (3), 137.5 (Ph 1'),
131.8 (8),
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-116-
130.5 (7), 128.5 (Ph 3'/5'), 127.1 (Ph 4'), 126.1 (Ph 2'/6'), 122.7 (2), 74.9
(5), 42.9
(6), 37.6 (4), 25.8 (Si-CM~e ), 18.1 (Si-CMe3), 16.1 (6-Me), -4.4 (Si-Me), -
4.5 (Si-Me).
2.2.2-Trichloroethyl Ester of 3-(3-Chloro-4-methoxyphenyl)-D-alanine (I)
A sample of the D-chlorotyrosine BOC derivative (160mg, 0.35mmol) was
dissolved in 3mL neat trifluoroacetic acid and allowed to stand at room
temperature for
lh. Removal of the excess reagent under reduced pressure returned the desired
amine I
,as the trifluoroacetate salt (165mg, 100% yield), [ ]D +1.7 (c 5.2, CHC13);
IR v1i87C
3400-2500 (br), 1760, 1680, 1500, 1200, 1130, 1070, 805, 710 cm 1; 1H NMR S
8.07
(NH2; br m, W1/2 =45), 7.27 (5-H; s), 7.12 (9-H; d, 8.1), 6.88 (8-H; d, 8.1),
4.86/4.67
(CH2CC13; AB q, -12.0), 4.41 (2-H; bs, W l/2 = 20), 3.86 (OMe; s), 3.33 (3-H;
dd,
-14.4/3.6), 3.22 (3-H'; dd, -14.4/6.6); 13C NMR 8 167.6 (1), 155.0 (7), 130.9
(5), 128.8
(9), 125.4 (4), 123.1 (6), 112.7 (8), 93.4 (CC13), 75.3 (CH2CC13), 56.1 (OMe),
54.2 (2),
34.9 (3).
Compound J
To a stirred solution of H (25mg, 0.07mmo1) in 3mL of anhydrous DMF under
argon was added successively pentafluorodiphenylphosphinate (FDPP, 32mg,
0.08mmo1),
trifluoroacetate salt I (35mg, 0.07mmo1) and diisopropylethylamine (DIEA,
27mg,
= 36 L, 0.21mmo1, = 3 equiv). Stirring was continued at 25 C for lh and then
the
reaction mixture was extracted with 20mL of EtZO. The ether extract was washed
with
lOmL of 1N HCI, followed by lOmL of sat'd NaHCO3, 2OmL of brine and 20mL of
water, dried (MgSO4), and evaporated. The residual pale yellow oil was
subjected to
chromatography on silica gel (15% EtOAc in hexane) to give J as a colorless
oil (32mg,
65% yield): [a]D +11.8 (c 1.2, CHC13); EIMS m/z; 644/646/648/650 (7/8/6/3; M+-
Bu),
570/572/574 (46/100/21), 536/538 (18/15), 394/396 (67/29), 275 (20), 155/157
(29/9);
HREIMS m/z 644.0981 (C29H34C14NOSSi, A-2.13mmu); UV N'. (e) 204 (54900), 230
(23200), 248 (19200), 284 (3500) mn; IR vn= 3290, 2980, 2850, 1760, 1680,
1640,
1505, 1380, 1270, 1169, 990, 720 cm-'; 1H NMR unit A 8 7.2-7.4 (Ph-H5; m),
6.87 (3-
H; ddd, 15.0/7.8/7.5), 6.37 (8-H; d, 16.2), 6.18 (7-H; dd, 16.2/8.1), 5.82 (2-
H; d,
15.0), 3.75 (5-H; ddd, 9.9/6.0/4.8), 2.46 (6-H; m), 2.36 (4-H2; m), 1.11 (6-
Me; d, 6.9),
0.91 (SiCMe3; s), 0.07 (SiMe; s), 0.06 (SiMe; s); unit B S 7.19 (5-H; d, 2.1),
7.04 (9-H;
dd, 8.4/2.1), 6.85 (8-H; d, 8.4), 5.85 (NH; d, 7.8), 5.08 (2-H; ddd,
7.8/6.0/5.7),
4.81/4.74 (CH2CC13; AB q, -11.7), 3.87 (OMe; s), 3.22 (3-H; dd, -14.1/5.7),
3.12 (3-
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-117-
H'; dd, -14.1/6.0). 13C NMR unit A 6 165.1 (1), 143.0 (3), 137.6 (9), 132.0
(8), 130.4
(7), 128.5 (11/13), 127.0 (12), 126.0 (10/14), 124.7 (2), 75.0 (5), 42.6 (6),
37.6 (4),
25.9 (Si-CMe3), 18.1 (Si-CMe3), 16.5 (6-Me), -4.3 (Si-Me), -4.6 (Si-Me); unit
B 6 170.1
(1), 154.3 (7), 131.1 (5), 128.5 (4/9), 122.6 (6), 112.2 (8), 94.2 (CC13),
74.8 (CHZCCI3),
56.1 (OMe), 53.0 (2), 36.5 (3).
(1'R.5S.6R)-N-1'-(carbo-2".2".2"-trichloroethoxy)-2'-(3-chloro-4-met hoxyphen
1=~yl-5=
t-butvldimethylsilyloxv-6-methyl=8-phenyl-octa-2E .7E-dienamide (K)
To a solution of J (50mg, 0.07mmo1) in 4mL MeCN was added 400 L of 50% aq
HF and the mixture stirred for 1h at 25 C. Extraction into 30mL of EtZO,
followed by
washing the ether extract with 30mL portions of sat'd NaHCO3, brine and water,
drying
(MgSO4) and evaporation, gave alcohol K as a colorless foam (40mg, 95% yield):
[a]D -
6.1 (c 1.3, CHC13); EIMS m/z (rel intensity) 587/589/591/593 (M+, < 1%),
552/554/556 (1/2/0.5), 456/458/460/462 (1/2/1/0.2), 342/344/346 (7/8/4),
212/214
(15/5), 195/197 (6/2), 155/157 (99/34), 131 (100), 91 (77); HREIMS m/z
587.0721
(C27H29 5C14NO5, 0+7.9mmu); UV )4. 204 (56500), 230 (22100), 248 (18100), 284
(3600) nm; IR v,,m 3400, 3300, 2980, 1780, 1680, 1640, 1505, 1270, 1180, 1090,
1000,
770 cm-'. 'H NMR unit A 8 7.2-7.4 (Ph-H5; m), 6.92 (3-H; ddd, 15.3/7.8/7.5),
6.46 (8-
H; d, 15.9), 6.14 (7-H; dd, 15.9/8.4), 5.90 (2-H; d, 15.3), 3.65 (5-H; ddd
7.8/5.6/4.0),
2.39 (6-H/4-H2; bm), 1.78 (OH; bs, W1n=40 Hz), 1.14 (6-Me; d, 6.9); unit B 6
7.18 (5-
H; d, 1.8), 7.03 (9-H; dd, 8.4/1.8), 6.84 (8-H; d, 8.4), 5.97 (NH; d, 7.8),
5.06 (2-H;
ddd, 7.8/6.0/5.7), 4.79/4.72 (CH2CC13; AB q, -12.0), 3.86 (OMe; s), 3.20 (3-H;
dd, -
14.1/5.7), 3.10 (3-H'; dd, -14.1/6.0). 13C NMR unit A 6 165.3 (1), 142.6 (3),
137.0 (9),
131.7 (8), 131.0 (7), 128.5 (11/13), 127.3 (12), 126.1 (10/14), 125.0 (2),
73.8 (5), 43.2
(6), 37.2 (4), 16.8 (6-Me); unit B 6 170.2 (1), 154.2 (7), 131.0 (5), 128.4
(9), 128.3 (4),
122.5 (6), 112.2 (8), 94.2 (CC13), 74.7 (CH2CC13), 56.1 (OMe), 53.0 (2), 36.5
(3).
3-(tert-Butoxxcarbonyl)amino-2.2-dimethylpropanoic Acid (M)
To a solution of 3-amino-2,2-dimethylpropan-l-ol (L) (3.0g, 29mmo1) in 51mL of
a 10 % solution of triethylamine in MeOH was added di-tert-butyl dicarbonate
(6.7g,
31mmo1) and the mixture was stirred at 25 C for lh. After removal of solvent,
the
residue was dissolved in CH2C12 (30mL) and the solution was washed twice with
1M
KHSO4 (pH 2) and once with saturated NaCI solution, and dried (MgSO4). Removal
of
solvent in vacuo afforded 5.8g (93 % yield) of 3-(tert-butoxycarbonyl)amino-
2,2-
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-118-
dimethylpropan-l-ol as a white solid which was directly used for the next step
without
further purification (> 95 % pure by NMR analysis), mp 70.5-71.5 C; IR P.
3350,
1685, 1456 cm'1; 'H NMR 8 4.87 (NH; br s), 3.72 (OH; br s), 3.19 (1-H2; d,
5.1), 2.95
(3-H2; d, 6.0), 1.44 (CMe3; s), 0.85 (2-Me2; s); 13C NMR (CDC13) 6 157.6 (BOC
CO),
79.7 (CMe3), 68.1 (1), 47.1 (3), 36.7 (2), 28.3 (CMe3), 22.4 (2-Me2).
To a solution of alcohol 3-(tert-butoxycarbonyl)amino-2,2-dimethylpropan-l-ol
(5.3g, 25.9mmo1) and sodium periodate (16.6g, 77.7mmo1) in carbon
tetrachloride
(52mL), acetonitrile (52mL) and water (78mL) was added ruthenium trichloride
hydrate
(122mg), and the mixture was stirred at 25 C for lh. The mixture was filtered
through
Celite and a saturated solution of potassium carbonate in water (50mL) was
added. The
water layer was separated, washed with ether (20mL), acidified with HCl to pH
2 at 0 C
and extracted with CH2C12 (30mL x 3). The combined extracts were washed with
saturated NaCI solution and dried (MgSO4). Removal of solvent in vacuo yielded
a
residue that was first subjected to flash reversed-phase chromatography on a
C18 silica
(ODS 120A, 50 to 90% MeOH) and then crystallized from ether to give 3.7g (66%
yield)
of M as a white solid, mp 106-108 C; EIMS m/z (rel intensity) 217 (0.1), 161
(11), 98
(25), 88 (71), 57 (100); HREIMS m/z 217.1292 (C,oH19N04, 0+2.2mmu); IR P. 3450-
2500, 1710, 1694, 1510 cm 1; 'H NMR of major conformer S 5.03 (NH; br s), 3.26
(3-
H2; m), 1.45 (CMe3; s), 1.24 (2-Me2; s); 13C NMR (CDC13) a 183.2 (1), 156.3
(BOC
CO), 79.6 (CMe3), 49.5/47.9 (2/3), 28.4 (CM~e ), 22.9 (2-Me2).
Allyl (2S)-2-Hydroxv-4-methvlpentanoate (N)
To a solution of 2.66g of L-leucic acid (20mmo1) and 1.74g of sodium
bicarbonate
(20mmo1) in 30mL water at 0 C was added 30mL of a CH2C12 solution of 6.44g of
tetrabutylammonium chloride (20mmo1) and 1.74mL of allyl bromide (20mmo1).
After
vigorously stirring the mixture for 24h, the CHZC12 was evaporated. About 50mL
water
was added and the aqueous layer was extracted four times with EtZO. The ether
solution
was dried over anhydrous sodium sulfate and then evaporated. The residue was
passed
---- through a short Si column to give 3.21g of allyl ester N (93% yield) as a
colorless oil,
[a]D -8.4 (c 1.1, CHC13); IR P. 3464, 2957, 1732, 1203, 1140, 1087 cm 1; 1H
NMR 6
5.92 (allyl 2-H; m), 5.34 (allyl 3-Hz; dd, 17.4/1.1), 5.28 (allyl 3-HE, dd,
10.5/1.1), 4.67
(allyl 1-H2; d, 5.7), 4.23 (2-H; br s), 2.64 (OH; br s), 1.89 (4-H; m), 1.57
(3-H2; m),
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-119-
0.96 (5-H3; d, 6.5), 0.95 (4-Me; d, 6.7); 13C NMR 6 175.3 (1), 131.4 (allyl C-
2), 118.6
(3), 68.9 (2), 65.7 (allyl C-1), 43.2 (3), 24.1 (4), 23.0 (5), 21.3 (4-Me).
Allyl (2S)-2-(3'(tert-Butoxvcarbonyl)amino-2' 2'-dimeth lnropanoylox1 -4-
methvlpentanoate (0)
To a solution of 0.8g of M(3.7mmo1), 0.76g of N(4.4mmol), and 92mg DMAP
in lOmL of dry CHZC12 at 0 C was added 0.84g of DCC (4.lmmol) in CH2C12. The
mixture was stirred at 25 C for 3h and filtered. The filtrate was washed with
saturated
aqueous sodium bicarbonate, dried (Na2SO4), and evaporated in vacuo. Flash
chromatography (silica gel, 5% EtOAc/hexane) afforded 1.0g (92% yield) of pure
0 as a
colorless oil, Rf 0.68 (17:83 EtOAc/hexane), [a] D-29.4 (c 18.1, CHC13); EIMS
m/z
(rel intensity) 371 (2, M+), 242 (13), 184 (12), 126 (20), 84 (100); HREIMS
m/z
371.2317 (C19H33N06, A-0.9mmu); IR (neat) v,,.3385, 2963, 1731, 1720, 1513 cm
1; 1H
NMR (300 MHz, CDC13) unit C 8 5.39 (NH; obscured br s), 3.33 (3-H; dd, -
13.5/7.4),
3.27 (3-H'; dd, -13.5/5.9), 2.78 (2-H, m), 1.44 (CMe3; s), 1.23 (2-Me; s),
1.22 (2-Me;
s); unit D 8 5.91 (allyl 2-H; ddt, 16.6/10.3/6.0 Hz), 5.34 (allyl 3-Hz; bd,
16.6), 5.27
(allyl 3-HE; bd, 10.3), 5.08 (2-H; dd, 9.6/3.6), 4.65 (allyl 1-H2; m), 1.6-1.9
(3-HZ/4-H;
m), 0.94 (5-H3; d, 6.3), 0.94 (4-Me; d, 7.3). 13C NMR unit C 8 176.5 (1),
156.3 (BOC
CO), 79.0 (CMe3), 48.6 (3), 44.0 (2), 28.4 (CMe=), 22.2/23.0 (2-Me2); unit D 8
170.6
(1), 131.4 (allyl C-2), 119.1 (allyl C-3), 70.9 (2), 66.0 (allyl C-1), 39.5
(3), 24.8 (4),
23.0 (5), 21.5 (4-Me).
(2S)-2-[3'(tert-Butoxvcarbonvl)amino-2' 2'-dimethylpropanovloxyl-4-
methvlpentanoic
Acid
To lOmL of a solution of 180mg (0.49mmol) of 0 and 60mg (0.05mmol) of
tetrakis(triphenylphosphine)palladium in dry THF (under argon atmosphere) was
slowly
added 470 L (5.4mmol) of dry morpholine over 10 min. After stirring for 50
min,
40mL of ether was added and the solution was washed with 1N HCl (4OmL) and
then
extracted with saturated sodium bicarbonate (2 x 30mL). The aqueous extract
was
acidified with 0.5N HCl and extracted with ether (40mL). The ether extract was
washed
with water (2 x 30mL), dried (MgSO4) and evaporated in vacuo to give P as a
colorless
mobile oil (152mg, 95%); [a]D -22.2 (c 2.2, CHC13); EIMS m/z (rel intensity)
331 (1,
M+), 275 (1), 258 (4), 231 (9), 202 (36), 174 (13), 144 (31), 126 (16), 114
(14), 98
(54), 88 (50), 84 9100); HREIMS m/z 331.2004 (C16H29N06, 0-1.Ommu). 'H NMR
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-120-
(CDC13) unit C 6 5.41 (NH; dd, 5.7/5.4), 3.30 (3-H2; m), 2.68 (2-H; m), 1.43
(CMe3; br
s), 1.22 (2-Me; s), 1.21 (2-Me; s); unit D S 6.47 (1-OH; br s, W1/2 = 35),
5.09 (2-H; dd,
9.3/2.7), 1.7-1.9 (3-H2/4-H; m), 0.97 (5-H3; d, 6.0), 0.94 (4-Me; d, 6.0). 13C
NMR
(CDC13) unit C 8 176.5 (1), 156.5 (BOC CO), 79.3 (CMe3), 48.6 (3), 44.0 (2),
28.3
(CMe ), 23.0 (2-Me), 22.2 (2-Me); unit D S 175.4 (1), 70.6 (2), 39.5 (3), 24.8
(4), 23.0
(5), 21.4 (4-Me).
Compound 0
To a solution of alcohol K (80mg, 0.14mmo1), acid P (68mg, 0.21mmo1) and
DMAP (4mg) in dry CH2C12 (4mL) stirred at 0 C under an argon atmosphere was
added
DCC (44mg, 0.21mmo1) in dry CH2C12 (1mL). The mixture was stirred at 0'C for
30
minutes, during which time a white precipitate developed, and then allowed to
warm to
room temperature and stirred for a further 4 hours. The precipitate was
filtered off and
the filtrate diluted with Et10 (4OmL) and washed successively with dilute HCl
(1M,
40mL), saturated NaHCO3 (4OmL) and brine (4OmL). The ethereal layer was dried
(MgSO4) and evaporated in vacuo to give a waxy solid. Chromatography (silica,
EtOAc:hexane, 1:3) led to pure Q as a colorless, viscous oil (103mg, 84%),
[a]D -3.1 (c
2.9, CHC13); EIMS m/z 800/802/804/806 (<1, M+-C5H8O2), 415/417/419/421
(5/3/3/2),
342/344/346 (7/9/4), 286/288/290 (2/6/2), 207 (34), 178 (22), 155/157 (66/24),
131 (36),
91 (70), 70 (100); HRRiMS m/z 800.2179 (C38H4$N20835C14, 0-1.4mmu); UV (MeOH)
X. (e) 204 (51200), 230 (18500), 248 (17200), 282 (2200) nm; IR (NaCI) vjõ.
3376,
2965, 1755, 1728, 1712, 1678, 1504, 1258, 1150, 1067, 732 cm'. iH NMR (CDC13)
8
unit A: 7.28-7.33 (10-H/14-H/11-H/13-H; m), 7.22 (12-H; m), 6.78 (3-H; ddd,
15.8/6.4/6.3), 6.40 (8-H; d, 15.8), 6.01 (7-H; dd, 15.8/8.7), 5.88 (2-H; d,
15.8), 5.06
(5-H; bm, WõZ = 20 Hz), 2.62 (6-H; m), 2.53 (4-H2; bm, WI,2 = 15 Hz), 1.12 (6-
CH3; d,
6.8); unit B 7.18 (5-H; d, 2.0), 7.05 (9-H; dd, 8.5/2.0), 6.83 (8-H; d, 8.5),
6.49 (NH; d,
7.9), 5.06 (2-H; bm, Wla = 20 Hz), 4.79/4.70 (CH2CC13; AB q, -11.7), 3.85
(OCH3; s),
3.20 (3-Hb; dd, -14.1/5.8), 3.07 (3-H,; dd, -14.1/6.7); unit C 5.38 (NH; bt,
6.5), 3.27
(3-H2; d, 6.5), 1.20 (2-CH3; s), 1.15 (2-CH3'; s); unit D 4.92 (2-H; dd,
10.0/3.8), 1.72
(4-H; bm, Wõ2=20 Hz), 1.67 (3-Hb; ddd, -14.1/10.0/5.0/), 1.56 (3-Ha; ddd, -
14.1/9.1/3.8), 1.43 (CO2CMe3; s), 0.86 (4-CH3; d, 6.4), 0.82 (5-H3; d, 6.4).
13C NMR
(CDC13) S unit A 165.4 (1), 139.3 (3), 136.9 (9), 131.7 (8), 130.1 (7), 128.6
(11/13),
127.5 (12), 126.2 (10/14), 125.4 (2), 76.5 (5), 41.1 (6), 33.4 (4), 16.7 (6-
Me); unit B
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-121-
170.0 (1), 154.1 (7), 131.2 (5), 128.8 (4), 128.5 (9), 122.3 (6), 112.1 (8),
94.3
(CH CC13), 74.6 (CH2CC13), 56.1 (7-OMe), 53.2 (2), 36.6 (3); unit C 176.9 (1),
156.4
(CO2CMe3), 79.1 (CO CMe3), 48.7 (3), 44.0 (2), 22.8 (2-Me), 22.3 (2-Me'); unit
D
170.7 (1), 71.4 (2), 39.5 (3), 28.4 (CO2CMe=), 24.8 (4), 23.0 (4-Me), 21.4
(5).
Amino Acid R
To the amino acid Q (100mg, 0.11mmol) was added activated Zn dust (400mg,
excess) and AcOH (4mL). The heterogenous mixture was subject to sonication for
45
minutes, stirred for a further 90 minutes at room temperature, and then poured
onto a pad
of Celite. The organic material was washed from the Celite pad with CH2C12.
The
solvent was removed in vacuo, leaving the carboxylic acid as a colorless
amorphous solid.
Without purification the crude acid was dissolved in trifluoroacetic acid
(TFA,
5mL) and allowed to sit at room temperature for 1 hour. After this time excess
TFA was
removed in vacuo and the resulting amorphous solid was then subjected to
chromatographic purification (Sep-PakT", silica, initially CHZC12 then 10%
MeOH/CH2C12), yielding the trifluoroacetate ammonium salt of the desired
compound.
Repeated lyophilization of an aqueous solution of the salt resulted in the
free amino acid
R as a colorless amorphous solid (68mg, 91 % over two steps); IR (NaC1) P.
3300,
3200, 2965, 1693, 1606, 1504, 1441, 1259, 1201, 1146, 1066, 727 cm''. 'H NMR
(CD3OD) 8 unit A: 7.33 (10-H/14-H; d, 7.4), 7.28 (11-H/13-H; t, 7.4), 7.18-
7.23 (12-H;
m), 6.69 (3-H; ddd, 15.6/7.7/7.0), 6.43 (8-H; d, 15.8), 6.04 (7-H; dd,
15.8/8.9), 6.00
(2-H; d, 15.6), 5.01 (5-H; ddd, 9.1/6.9/3.1), 2.64 (4-Hb; bm, Wlt2 _= 30 Hz),
2.60 (6-H;
bm, WIn = 20 Hz), 2.49 (4-H8; ddd, 15.8/9.1/7.7), 1.13 (6-Me; d, 6.7); unit B
7.18-7.23
(5-H; m), 7.11 (9-H; dd, 8.3/1.6), 6.92 (8-H; d, 8.3), 4.59 (2-H; bm, W1t2 =20
Hz),
3.81 (OCH3; s), 3.14 (3-Hb; dd, -13.7/4.3), 2.96 (3-H$; m, W1,2 = 20 Hz); unit
C 2.96 (3-
H2; bm, W1/2.= 20 Hz), 1.31 (2-CH3; s), 1.25 (2-CH3'; s); unit D 4.90 (2-H;
dd, 9.6/4.0),
1.66 (4-H; bm, Wl/2= 25 Hz), 1.59 (3-Hb; ddd, -14.4/9.6/4.8), 1.53 (3-Ha; ddd,
-
14.4/9.1/4.0), 0.81 (4-Me; d, 6.5), 0.74 (5-H3; d, 6.5). 13C NMR (CD3OD) 6
unit A
167.7 (1), 140.7 (3), 138.4 (9), 133.0 (8), 131.7 (7), 129.6 (11/13), 128.5
(12), 127.3
(10/14), 127.1 (2), 78.4 (5), 43.1 (6), 35.7 (4), 17.4 (6-Me); unit B 179.8
(1), 155.2 (7),
132.3 (4), 132.1 (5), 130.1 (9), 123.0 (6), 113.4 (8), 56.6 (7-OMe), 56.6 (2),
37.8 (3),
unit C 176.8 (1), 48.2 (3), 42.2 (2), 23.3 (2-Me), 23.3 (2-Me'); unit D 172.0
(1), 73.4
(2), 40.7 (3), 26.0 (4), 23.1 (4-Me), 21.8 (5).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-122-
Cryptophvcin 51
To a stirred solution of amino acid T (75mg, 0.11mmo1) in anhydrous DMF
(2OmL) at room temperature under argon was added diisopropylethylamine (DIEA,
44mg,
60 L, 0.34mmol, --3 equiv.) followed by pentafluorodiphenylphosphinate (FDPP,
55mg,
0.14mmo1, --1.3 equiv.) in DMF (2mL). The mixture was stirred for 12 hours,
Et20
(4OmL) was added, and the ether layer was washed successively with HCI (1M,
40mL),
brine (4OmL) and H20 (40mL), dried (MgSO4) and evaporated under reduced
pressure.
The residual waxy solid was further purified by reverse phase chromatography
(ODS,
, 30% H20/MeCN, 3mL miri') to give Cryptophycin 51 as a colorless aznorphous
10 solid (45mg, 61 %), [a]D +26.4 (c 0.25, CHC13); EIMS m/z 652/654 (M+,
3/1),
632/634 (3/2), 426/428 (51/15), 227 (64), 195/197 (64/22), 155/157 (71/15),
131 (59),
91 (100); HREIMS m/z 652.2936 (C36H45N20735C1, A-2.1mmu); UV (MeOH) A. (e) 204
(52000), 228 (20400), 250 (13400), 284 (2800) nm; IR (NaCI) P. 3376, 3270,
2960,
1747, 1721, 1659, 1536, 1514, 1259, 1150, 1066, 1013, 980, 694 cm''. 'H NMR
(CDC13) 8 unit A 7.32 (10-H/14-H; dd, 8.0/1.5), 7.29 (11-H/13-H; t, 8.0), 7.24
(12-H;
bm, WõZ = 15 Hz), 6.77 (3-H; ddd, 15.2/10.8/4.3), 6.40 (8-H; d, 15.8), 6.01 (7-
H; dd,
15.8/8.8), 5.76 (2-H; dd, 15.2/1.1), 5.04 (5-H; ddd, 11.1/6.4/1.9), 2.54 (4-
Hb/6-H; bm,
W1,2 = 15 Hz), 2.37 (4-Ha; ddd, -14.3/11.1/10.8 ), 1.13 (6-Me; d, 6.8); unit B
7.20 (5-H;
d, 2.0), 7.05 (9-H; dd, 8.4/2.0), 6.84 (8-H; d, 8.4), 5.61 (NH; d, 7.8), 4.74
(2-H; ddd,
7.8/7.6/5.4), 3.87 (OMe; s), 3.11 (3-Hb; dd, -14.2/5.4), 3.06 (3-H$; dd, -
14.2/7.6); unit
C 7.24 (NH; bm, W12 =15 Hz), 3.40 (3-Hb; dd, -13.5/8.5), 3.12 (3-H8; dd, -
13.5/3.6),
1.22 (2-Me; s), 1.15 (2-Me', s); unit D 4.85 (2-H; dd, 10.2/3.6), 1.66 (3-Hb;
ddd, -
14.0/10.2/4.6), 1.61 (4-H; bm Wl,2= 20.0 Hz), 1.33 (3-H8; ddd, -14.0/9.0/3.6),
0.74 (4-
Me; d, 6.6), 0.72 (5-H3; d, 6.6). 13C NMR (CDC13) 6 unit A 165.1 (1), 142.2
(3), 136.7
(9), 131.7 (8), 130.1 (7), 128.6 (11/13), 127.5 (12), 126.1 (10/14), 124.6
(2), 77.0 (5),
42.2 (6), 36.5 (4), 17.3 (6-Me); unit B 170.3 (1), 154.1 (7), 130.9 (5), 129.5
(4), 128.3
(9), 122.5 (6), 112.3 (8), 56.1 (7-OMe), 54.2 (2), 35.3 (3); unit C 178.0 (1),
46.5 (3),
42.7 (2), 22.8 (2-Me), 22.6 (2-Me'); unit D 170.6 (1), 71.5 (2), 39.5 (3),
24.5 (4), 22.7
(4-Me), 21.2 (5).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-123-
Example 9 Synthesis of Cryptophvcin 52 and Cryptophycin 53
To a stirred solution of Cryptophycin 51 (75mg, 0.12mmol) in anhydrous
dichloromethane (7.5mL) at 0 C under argon was added a solution of m-
chloroperbenzoic
acid (mCPBA, 50mg, 0.23mmol, = 2 equiv. based on 80% active oxygen) in
dichloromethane (1mL). After 30 minutes the reaction mixture was allowed to
warm to
room temperature and stirred for a further 12 hours. The solvent was then
removed
under reduced pressure to give a 1.8:1 mixture of Cryptophycins 52 and 53 (by
NMR
analysis), respectively, as an amorphous solid. The mixture of regioisomeric
epoxides
was dissolved in minimal acetonitrile and subjected to reverse phase
chromatography
(YMC-ODS, 10 , 250mm x 22.5mm, 30% H20/MeCN, 6mL min') to separate
Cryptophycin 52 (37mg, 48 %) and Cryptophycin 53 (19mg, 25 %).
Snectral data for Cryptophvcin 52
ja]D +19.9 (c 0.5, CHC13); EIMS mlz 668/670 (4/2, M+), 445 (35), 244 (12),
227 (22), 195/197 (66/27), 184 (45), 155/157 (38/10), 91 (100); HREIMS m/z
668.2873
(C36H45N2 0 835C1, 0-0.9mmu), 445.2497 (C25H35NO6, A-3.3mmu); UV (MeOH) X. (e)
204 (35100), 218 (20900) nm; IR (NaCI) v. 3415, 3270, 2960, 1748, 1721, 1650,
1536, 1504, 1260, 1192, 1150, 1066, 1013, 800, 698 cm 1. 1H NMR (CDC13) 6 unit
A
7.33-7.38 (11-H/12-H/13-H; bm, W1/2=25 Hz), 7.24 (10-H/14-H; m, W1n=15 Hz),
6.76 (3-H; ddd, 15.1/10.8/4.3), 5.71 (2-H; dd, 15.1/1.7), 5.20 (5-H; ddd,
11.0/5.0/1.8),
3.68 (8-H; d, 1.9), 2.92 (7-H; dd, 7.5/1.9), 2.57 (4-Hb; ddd, -14.6/1.8/1.7),
2.45 (4-Ha;
ddd, -14.6/11.0/10.8), 1.78 (6-H; bm, W12=15 Hz), 1.14 (6-Me; d, 6.9); unit B
7.18
(5-H; d, 2.2), 7.04 (9-H; dd, 8.4/2.2), 6.83 (8-H; d, 8.4), 5.56 (NH; d, 7.9),
4.73 (2-H;
ddd, 7.9/7.4/5.3), 3.87 (OMe; s), 3.09 (3-Hb; dd, -14.6/5.3), 3.05 (3-Ha; dd, -
14.6/7.4);
unit C 7.20 (NH; dd, 8.6/3.2), 3.41 (3-Hb; dd, -13.4/8.6), 3.10 (3-He; dd, -
13.4/3.2),
1.22 (2-Me; s), 1.15 (2-Me'; s); unit D 4.82 (2-H; dd, 10.2/3.5), 1.73 (3-Hb;
bm,
W1,2 = 20 Hz), 1.66 (4-H; bm, W1,2 = 20 Hz), 1.31 (3-H8; ddd, -13.8/9.1/3.5),
0.84 (4-
Me; d, 6.6), 0.82 (5-H3; d, 6.6); 13C NMR (CDC13) S unit A 164.9 (1), 141.8
(3), 136.7
(9), 128.7 (11/13), 128.3 (12), 125.6 (10/14), 124.7 (2), 75.9 (5), 63.0 (7),
59.0 (8),
40.7 (6), 36.9 (4), 13.5 (6-Me), unit B 170.3 (1), 154.1 (7), 130.9 (5), 129.5
(4), 128.5
(9), 122.6 (6), 112.4 (8), 56.1 (7-OMe), 54.3 (2), 35.3 (3), unit C 178.0 (1),
46.5 (3),
42.8 (2), 22.8 (2-Me), 22.8 (2-Me'), unit D 170.5 (1), 71.2 (2), 39.3 (3),
24.6 (4), 22.7
(4-Me), 21.2 (5).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-124-
Spectral data for Cryptophycin 53
(a]D +20.8 (c 1.7, CHC13); EIMS m/z 668/670 (5/4, M ), 445 (32), 244 (15),
227 (24), 195/197 (64/21), 184 (60), 155/157 (33/9), 91 (100); HREIMS m/z
668.2853
(C36H45N20 8 35C1, Al.lmmu); UV (MeOH) X11187C (e) 204 (38700), 218 (22900)
nm; IR
(NaCI) v. 3415, 3280, 2917, 2849, 1748, 1722, 1660, 1504, 1465, 1260, 1190,
1150,
1066, 755 cm'. 'H NMR (CDC13) 6 unit A 7.29-7.36 (11-H/12-H/13-H, bm, W112=20
Hz), 7.23 (10-H/14-H; dd, 8.3/1.7), 6.77 (3-H; ddd, 15.1/10.9/4.3), 5.81 (2-H;
dd,
15.1/1.3), 5.17 (5-H; ddd, 11.2/4.9/1.8), 3.58 (8-H; d, 1.7), 2.90 (7-H; dd,
7.8/1.7),
2.67 (4-Hb; ddd, 14.7/11.2/10.9), 2.56 (4-Ha; dddd, 14.7/4.3/1.8/1.3), 1.67-
1.78 (6-H;
bm, W1,2.= 45), 1.03 (6-CH3; d, 7.1); unit B 7.21 (5-H; d, 2.1), 7.07 (9-H;
dd, 8.5/2.1),
6.84 (8-H; d, 8.4), 5.90 (2-NH; d, 7.9), 4.75 (2-H; ddd, 7.9/7.9/4.9), 3.85 (7-
OCH3; s),
3.14 (3-Hb; dd, 14.5/4.9), 3.03 (3-Ha; dd, 14.5/7.9); unit C 7.29-7.36 (3-NH;
bm,
W122 = 25), 3.43 (3-Hb; dd, 13.7/8.8), 3.10 (3-He; dd, 13.7/3.4), 1.23 (2-CH3;
s), 1.17
(2-CH3'; s); unit D 4.92 (2-H; dd, 10.3/3.2), 1.67-1.78 (3-Hb/4-H; bm, Wõ2 =
45), 1.48
(3-Ha; ddd, 13.9/8.8/3.2), 0.89 (4-CH3; d, 6.6), 0.86 (5-H3; d, 6.6). 13C NMR
(CDC13)
6 unit A 165.1 (1), 142.0 (3), 137.0 (9), 128.5 (11/13), 128.5 (12), 125.3
(10/14), 124.6
(2), 76.7 (5), 63.2 (7), 56.2 (8), 40.8 (6), 36.7 (4), 13.4 (6-Me); unit B
170.4 (1), 154.0
(7), 130.8 (5), 129.7 (4), 128.2 (9), 122.5 (6), 112.3 (8), 56.1 (7-OMe), 54.4
(2), 35.3
(3); unit C 177.9 (1), 46.4 (3), 42.7 (2), 23.0 (2-Me), 22.7 (2-Me'); unit D
170.5 (1),
71.3 (2), 39.2 (3), 24.7 (4), 22.8 (4-Me), 21.3 (5).
Example 10 Synthesis of Cryptophycin 55
To a solution of Cryptophycin 52 (6mg, 0.009mmol) in 0.6mL of 2:1 1,2-
dimethoxyethane/water was added 2 L of 12 N HCI. The solution was allowed to
stir at
room temperature for 20 h, neutralized with potassium carbonate, filtered
through a 5
filter, and evaporated. The acetonitrile-soluble material was purified by
reversed-phase
HPLC on C18 (250 x 10mm column) using 4:1 MeOH/H20 to obtain 3.0mg of
Cryptophycin 55 (48%). [a]D +42.5 (c 1.1, CHC13); EIMS m/z 704/706/708 (M+ <
1),
668/670 (1.5/0.5, M+-HCl), 445 (6), 226 (8), 195/197 (16/5), 184 (10), 155/157
(33/11),
135 (100), 91 (99), 77 (30); HREIMS m/z 668.2873 (M+-HCI, C36H45N20835C1, A-
0.8mmu); UV (MeOH) A. (e) 204 (48400), 218 (29200), 284 (1600) nm; IR (NaCI)
v,,,.
3410, 3286, 2959, 1748, 1723, 1666, 1538, 1504, 1455, 1257, 1178, 1066, 753 cm-
'.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-125-
1H NMR (CDC13) 6 unit A 7.35-7.42 (10-H/11-H/12-H/13-H/14-H; m), 6.78 (3-H;
ddd,
15.1/10.6/4.5), 5.78 (2-H; dd, 15.1/1.7), 5.16 (5-H; ddd, 11.1/8.3/2.1), 4.65
(8-H; d,
9.7), 4.01 (7-H; bd, 9.7), 2.69 (4-Hb; dddd, -14.5/4.5/2.1/1.7), 2.50 (6-H;
bm,
Wln.= 15), 2.38 (4-He; ddd, -14.5/11.1/10.6), 1.53 (7-OH, s), 1.04 (6-Me, d,
7.1); unit
B 7.21 (5-H; d, 2.2), 7.07 (9-H; dd, 8.5/2.2), 6.85 (8-H; d, 8.5), 5.57 (2-NH;
d, 7.8),
4.74 (2-H; ddd, 7.8/7.6/5.2), 3.88 (7-OCH3; s), 3.13 (3-Hb; dd, 14.5/5.2),
3.05 (3-H8;
dd, 14.5/7.6); unit C 7.21 (3-NH; m), 3.38 (3-Hb; dd, 13.5/8.3), 3.17 (3-Hg;
dd,
13.5/4.1), 1.23 (2-CH3; s), 1.17 (2-CH3'; s), unit D 4.93 (2-H; dd, 10.1/3.5),
1.78 (3-
Hb; ddd, 13.5/10.1/5.0), 1.72 (4-H; bm, W1n=20), 1.43 (3-Ha; ddd,
13.5/8.8/3.5), 0.92
(4-CH3; d, 6.6), 0.92 (5-H3, d, 6.4). 13C NMR (CDC13) b unit A 165.1 (C-1),
142.4 (C-
3), 138.4 (C-9), 129.0 (C-i1/13), 128.3 (C-12), 128.0 (C-10/14), 124.6 (C-2),
76.1 (C-
5), 74.1 (C-7), 62.0 (C-8), 38.4 (C-6), 36.5 (C-4), 8.6 (6-Me); unit B 170.3
(C-1), 154.1
(C-7), 130.9 (C-5), 129.6 (C-4), 129.2 (C-9), 122.6 (C-6), 112.3 (C-8), 56.1
(7-OMe),
54.3 (C-2), 35.3 (C-3); unit C 177.8 (C-1), 46.5 (C-3), 42.8 (C-2), 22.9 (2-
Me), 23.0
(C-2-Me'); unit D 170.3 (C-1), 71.3 (C-2), 39.7 (C-3), 24.8 (C-4), 22.7 (4-
Me), 21.6
(C-5). The corresponding diol, Cryptophycin 56 (2.8mg, 44% yield), was also
obtained.
Example 11 Synthesis of Cryptophycin 57
A small amount of Pt02 (=1mg) was added to a flask containing 0.5mL of
CH2C12. The air in the flask was evacuated, H2 was introduced, and the mixture
was
stirred at room temperature for 20 minutes. A solution containing 10.2mg of
Cryptophycin 52 (0.015mmo1) in CHZCIZ (0.3mL) was added and the mixture
stirred at
room temperature for a further 30 minutes. The catalyst was removed by
filtration
through Celite/cotton and the solvent was removed in vacuo. Reverse phase HPLC
of the
residue (ODS, 101t, 250 x 22.5mm, MeCN/H20 (3:1), 5mL min') yielded pure
Cryptophycin 57 (9.1mg, 89%). [a]D +3.4 (c=4.5, CHC13); EIMS m/z 670/672 (M+,
9/3), 447 (10), 246 (63), 229 (20), 195/197 (78/25), 184 (58), 155/157
(39/13), 128 (21),
- 91 (100), 77 (23); HREIMS m/z 670.3037 (C36H47N2O835C1, A-1.6mmu); UV (MeOH)
X. (e) 204 (31400), 218 (12000), 284 (1200) nm; 'H NMR (CDC13) S unit A: 7.30-
7.37
(11/12/13-H, bm), 7.23 (10/14-H, bdd, 7.9, 1.9), 5.03 (5-H, ddd, 9.0, 5.6,
3.4), 3.66
(8-H, d, 2.1), 2.89 (7-H, dd, 7.7, 2.1), 2.27 (2-Hb, ddd, 14.3, 8.7, 6.2),
2.04 (2-Ha,
ddd, 14.3, 8.8, 6.8), 1.64-1.75 (6-H/4-H2, bm), 1.61 (3-H2, bm, W,n = 25),
1.11 (6-CH3,
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-126-
d, 7.1), unit B: 7.19 (5-H, d, 2.1), 7.04 (9-H, dd, 8.3, 2.1), 6.83 (8-H, d,
8.3), 5.55 (2-
NH, d, 8.3), 4.65 (2-H, ddd, 8.3, 7.3, 5.3), 3.87 (7-OCH3, s), 3.16 (3-Hb, dd,
14.3,
7.3), 3.08 (3-He, dd, 14.3, 5.3), unit C. 6.91 (3-NH, dd, 6.4, 6.4), 3.41 (3-
Hb, dd, 13.5,
6.4), 3.30 (3-Ha, dd, 13.5, 6.4), 1.21 (2-CH3, s), 1.13 (2-CH3', s), unit D:
4.80 (2-H,
dd, 9.8, 4.1), 1.64-1.75 (3-Hb/4-H, bm), 1.34 (3-H8, ddd, 15.4, 10.1, 4.1),
0.86 (4-CH31
d, 6.5), 0.84 (5-H3, d, 6.5); 13C NMR (CDC13) 8 unit A: 172.6 (1), 136.9 (9),
128.7
(11/13), 128.5 (12), 125.6 (10/14), 76.8 (5), 63.4 (7), 59.2 (8), 40.2 (6),
36.2 (2), 32.2
(4), 21.4 (3), 13.6 (6-Me), unit B: 170.4 (1), 154.0 (7), 131.1 (5), 130.0
(4), 128.5 (9),
122.5 (6), 112.2 (8), 56.1 (7-OMe), 54.3 (2), 35.3 (3), unit C.- 177.6 (1),
47.0 (3), 43.1
(2), 22.5 (2-Me'), 22.4 (2-Me), unit D: 171.7 (1), 72.0 (2), 39.0 (3), 24.6
(4), 22.8 (4-
Me), 21.8 (5).
Example 12 Synthesis of Cryptophycin 58
To a stirred solution of Cryptophycin 57 (5.5mg, 0.008mmo1) in 3mL of ethanol
free chloroform at =-60 C was added TMSC1 (Used as obtained from Aldrich, =
4.5mg,
,= 5.2 L, = 0.04mmo1). The reaction mixture was stirred for 20 minutes, by
which time
tlc indicated no starting material remained. The volatile components were then
removed
under reduced pressure to leave an amorphous solid. This material was taken up
in
acetonitrile and subject to HPLC (ODS, 10 , 250 x 22.5mm, MeCN/H20 (3:1), 5mL
min') to return pure Cryptophycin 58 (5.4mg, 93%) as the major product. [a]D
+7.2
(c=2.1, CHC13); EIMS m/z 706/708/710 (M+, 27/23/8), 670/672 (M+-HCI, 14/13),
583
(54), 581 (53), 485 (23), 483 (21), 447 (34), 294 (21), 282 (39), 246 (57),
195/197
(87/27), 184 (73), 155/157 (45/10), 128 (30), 91 (95), 77 (30), 69 (100);
HREIMS mlz
706.2844 (C36H48N20835C12, 0-5.6mmu), m/z 670.3070 (M+-HCI, C36H47N20835C1, A-
4.9mmu); UV (MeOH) X. (e) 204 (331900), 218 (11800), 284 (1800) nm; 'H NMR
(CDC13) 6 unit A: 7.34-7.42 (10/11/12/13/14-H, bm), 5.01 (5-H, ddd, 9.6, 8.3,
2.5),
4.65 (8-H, d, 9.6), 4.00 (7-H, dd, 9.6, 1.9), 2.42 (6-H, ddq, 8.3, 1.9, 7.0),
2.29 (2-Hb,
ddd, 14.3, 9.4, 4.5), 2.06 (2-H., ddd, 14.3, 8.3, 7.5), 1.62-1.82 (3-H2/4-H2,
bm), 0.99
(6-CH3, d, 7.0), unit B: 7.20 (5-H, d, 2.1), 7.06 (9-H, dd, 8.3, 2.1), 6.84 (8-
H, d, 8.3),
5.62 (2-NH, d, 8.3), 4.61 (2-H, ddd, 8.3, 7.7, 5.4), 3.87 (7-OCH31 s), 3.17 (3-
Hb, dd,
14.3, 7.7), 3.11 (3-H., dd, 14.3, 5.4), unit C: 6.97 (3-NH, dd, 6.4, 6.2),
3.43 (3-Hb, dd,
13.4, 6.2), 3.31 (3-Ha, dd, 13.4, 6.4), 1.23 (2-CH3, s), 1.16 (2-CH3', s),
unit D: 4.93 (2-
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-127-
H, dd, 10.0, 4.0), 1.86 (3-Hb, ddd, 14.0, 10.0, 5.5), 1.58 (3-He, ddd, 14.0,
8.3, 4.0),
0.97 (4-CH3, d, 6.8), 0.94 (5-H3, d, 6.6); 13C NMR (CDC13) 6 unit A: 172.8
(1), 138.7
(9), 129.0 (12), 128.9 (11/13), 128.0 (10/14), 76.5 (5), 73.8 (7), 62.1 (8),
38.1 (6), 35.9
(2), 31.8 (4), 21.4 (3), 8.7 (6-Me), unit B: 170.6 (1), 153.9 (7), 131.0 (5),
130.2 (4),
128.5 (9), 122.4 (6), 112.2 (8), 56.1 (7-OMe), 54.4 (2), 35.0 (3), unit C:
177.2 (1), 47.0
(3), 43.2 (2), 22.5 (2-Me'), 22.4 (2-Me), unit D: 171.8 (1), 72.0 (2), 39.4
(3), 24.9 (4),
22.9 (4-Me), 21.7 (5).
Example 13 Synthesis of Cryptophvcin 61
To a solution of Cryptophycin 53 (5mg, 0.007mmol) in 0.5mL of dry benzene was
added triphenylphosphine sulfide (4mg, 0.014mmo1) followed by 0.65 L of
trifluoroacetic
acid as a solution in dry benzene (100 L). The solution was allowed to stir at
room
temperature for 6 h, neutralized with sodium bicarbonate, filtered and
evaporated. The
residue was partitioned between water and CH2C12. The CH2C12-soluble material
was
purified by reversed-phase HPLC on C18 using 4:1 MeCN/H20 to obtain pure
Cryptophycin 61 (1.9mg, 37 %). [a]D +28.4 (c=0.7, CHC13); EIMS m/z 684/686
(M+,
not observed), 652/654 (M+-S, 5/4), 426/428 (90/29), 294 (10), 227 (100),
195/197
(57/20), 184 (20), 155/157 (34/9), 131 (45), 129 (44), 91 (76), 77 (27);
HREIMS m/z
652.2973 (M+-S, C3J'45N20 735C1, 0-5.8mmu); UV (MeOH) X. (e) 204 (26700), 218
(11600), 284 (820) nm; IR (NaCI) v. 3410, 3271, 2958, 1749, 1724, 1670, 1503,
1463,
1258, 1176, 1066, 758 cm'. 'H NMR (CDC13) 6 unit A 7.29-7.34 (11/12/13-H; m),
7.25
(10/14-H, bd, 6.6), 6.73 (3-H, ddd, 15.2/10.6/4.5), 5.66 (2-H; dd, 15.2/1.7),
5.22 (5-H,
ddd, 11.2/4.2/2.0), 3.68 (8-H, d, 5.1), 3.01 (7-H, dd, 8.4/5.1), 2.52 (4-Hb,
dddd, -
14.4/4.5/2.0/1.7), 2.41 (4-Ha, ddd, -14.4/11.2/10.6), 1.68-1.74 (6-H, m), 1.14
(6-Me, d,
6.9); unit B 7.18 (5-H, d, 2.2), 7.04 (9-H, dd, 8.4/2.2), 6.84 (8-H, d, 8.4),
5.45 (NH, d,
7.8), 4.75 (2-H, ddd, 7.8/7.3/5.4), 3.87 (OMe, s), 3.09 (3-Hb, dd, -14.5/5.4),
3.05 (3-
Hõ dd, -14.5/7.3); unit C 7.17 (NH, dd, 8.3, 3.9), 3.39 (3-Hb, dd, -13.5/8.3),
3.14 (3-
H., dd, -13.5/3.9), 1.23 (2-Me, s), 1.16 (2-Me', s); unit D 4.86 (2-H, dd,
10.2/3.4),
1.77 (3-Hb, ddd, -14.0/10.2/4.9), 1.68-1.74 (4-H, m), 1.42 (3-H., ddd, -
14.0/8.7/3.4),
0.92 (4-Me, d, 6.6), 0.88 (5-H3, d, 6.4). 13C NMR (CDC13) 6 unit A 164.9 (1),
141.7
(3), 138.3 (9), 128.8 (11/13), 128.0 (12), 126.7 (10/14), 124.7 (2), 76.6 (5),
45.8 (7),
43.9 (8), 43.9 (6), 36.6 (4), 16.0 (6-Me), unit B 170.2 (1), 154.1 (7), 130.9
(5), 129.4
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-128-
(4), 128.3 (9), 122.8 (6), 112.4 (8), 56.1 (7-OMe), 54.2 (2), 35.3 (3), unit C
177.9 (1),
46.5 (3), 42.7 (2), 22.9 (2-Me), 22.8 (2-Me'), unit D 170.4 (1), 71.3 (2),
39.4 (3), 24.7
(4), 22.7 (4-Me), 21.4 (5).
Example 14 Synthesis of Cryptophvcin 81
Compound S
Compound S is the tert-butyidiphenylsilyl ether (TBDMS) derivative of F.
Compound T
To lOmL of a THF solution of p-methoxybenzyltriphenylphosponium chloride
(lmmol) at -78 C was added 4001AL of butyl lithium solution (lmmol, 2.5M in
hexane).
The mixture was stirred for 30 minutes and then a 2.64mL aliquot was added to
3mL of a
THF solution of aldehyde S(75.Omg, 0.24mmol) at -78 C. After 30 minutes,
cooling
was ceased, but stirring was continued for another two hours during which time
the
temperature rose slowly to 25 C. The reaction was quenched with saturated
ammonium
chloride solution and the THF was evaporated. The products were extracted with
hexane
twice and the combined organic layer was washed with brine, dried and then
concentrated. The residue was applied to a flash silica column (3 %
EtOAc/hexane) to
give 63mg of compound T and 40mg of a mixture of T and the Z isomer.
Compound T had the following properties: [a]D+110.5' (CHC13, c 0.75); IRv.
2956, 2857, 1724, 1608, 1511, 1428, 1250, 1173, 1111, 1037, 821, 703, 505 cnm-
1;
EIMS m/Z (relative intensity %) 497 (< 1, M+-OMe), 471 (31, M+-Bu), 367 (56),
294
(31), 199 (75), 135 (100); high-resolution EIMS 497.24770 (calcd for
C32H37O3Si,
A+3.5mmu, M+-OMe), 471.19859 (calcd for C29H31O4Si, A+0.6mmu, M+-Bu'). 'H
NMR 8 7.71/7.68 (SiPh2, 2'-H, 6'-H/2"-H, 6"-H; d; 6.5), 7.45/7.43 (SiPh2, 4'-
H/4"-H;
t; 7.4), 7.39/7.38 (SiPh2, 3'-H, 5'-H/3"-H, 5"-H; dd; 7.4, 6.5), 7.24 (10-H,
14-H; d;
8.7), 6.85 (11-H, 13-H; d; 8.7), 6.79 (3-H; dt; 15.7, 7.5), 6.19 (8-H, d,
16.1), 6.00 (7-
H, dd, 16.1, 8.1), 5.66 (2-H, dt, 15.7, 1.3), 3.82 (5-H, m), 3.81 (-OCH3, S),
3.69
(CO2CH3, S), 2.41 (6-H, m), 2.36 (4-H, m), 2.30 (4-H, m), 1.12 (6-CH3, d,
7.0), 1.09
(CMe3, S). 13C NMR 8 166.7 (1), 158.8 (12), 146.0 (3), 136.0 (SiPh2, 2',
6'/2", 6"),
134.1/133.7 (SiPh2, 1'/1"), 130.4 (9), 130.0 (8), 129.7/129.6 (SiPh2, 4'/4"),
129.5 (7),
127.6/127.5 (3', 5'/ 3' , 5"), 127.1 (10, 14), 122.8 (2), 113.9 (11, 13), 76.4
(5), 55.2
(OCH3), 51.3 (CO2CH3), 42.1 (6), 37.1 (4), 27.0 (CMe3), 19.5 (CMe3), 16.2 (6-
CH3).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-129-
Additional compound T was obtained from the mixture of T and the Z-isomer.
The 40mg of the mixture of E and Z isomers was dissolved in 4mL of a benzene
solution
containing thiophenol (0.02M) and ACN (0.006M). The mixture was heated under
reflux
for 5h. Work-up and purification by a short Si column give 37.2mg compound T.
Compound U
To 6mL acetone solution of compound T (76mg, 0.15mmol) was added 4.4mL of
iN LiOH in water. The clear solution was stirred overnight. Acetone was
evaporated
and the aqueous solution was acidified with 1N HCI. The product was extracted
with
EtOAc three times. The organic layer was dried and concentrated. Purification
by silica
colunm (20% EtOAc/hexane with 0.5 % AcOH) gave 62.2mg acid of compound U (81
%);
[a]D +120.8 (CHC13, c 3.1); IR P. 2960, 2858, 1695, 1650, 1511, 1427, 1250,
1111,
1036, 702 cm 1; 'H NMR 8 7.73/7.70 (SiPh2, 2'-H, 6'-H/2"-H, 6"-H, d, 7.0),
7.50
(SiPh2, 4'-H/4"-H, m), 7.44 (SiPh2, 3'-H, 5'-H/ 3"-H, 5"-H, m), 7.29 (10-H, 14-
H, d,
8.6), 6.96 (3-H; dt; 15.6, 7.8), 6.89 (11-H, 13-H, d, 8.6), 6.22 (8-H, d,
16.0), 6.03 (7-
H, dd, 16.0, 7.9), 5.70(2-H, d, 15.6), 3.88 (5-H, m), 3.83 (OCH3, S), 2.43 (6-
H, m),
2.40 (4-H, m), 1.17 (6-CH3, d, 6.9), 1.14 (CMe3, s); 13C NMR, 171.7 (1), 158.8
(12),
148.8 (3), 135.0 (SiPh2, 2', 6'/2", 6"), 133.9/133.7 (SiPh2, 1'/1"), 130.3
(9), 130.0
(8), 129.7 (SiPh2, 4'/4"), 129.4 (7), 127.6 (SiPh2, 3', 5'/3", 5"), 127.1 (10,
14), 122.5
(2), 113.9 (11, 13), 76.2 (5), 55.2 (OCH3), 42.3 (6), 37.1 (4), 27.0 (CMe=),
19.5
(CMe3), 16.0 (6-CH3).
Compound V
Compound U (59mg, 0.12mmol), the trifluoroacetate salt of compound I (57.2mg,
0.12mmol) and diisopropylethylamine (DIEA, 62 L, 0.36mmol) were dissolved in
1.5mL
of dry DMF. To this solution was added FDPP (55mg, 0.14mmo1), in 0.6mL DMF)
and
the reaction mixture was stirred for two hours. Ether was added and the
organic layer
was washed successively with iN HCI, saturated sodium bicarbonate, and brine,
respectively. Concentration and purification by chromatography (silica column,
8%
EtOAc/hexane) gave 74.2mg of compound V (72%); [a]D +53.8 (CHC13, c 1.6); IR
P.
3286, 2959, 1760, 1667, 1640, 1607, 1510, 1253, 1174, 1111, 1067, 1027, 703
cm'';
EIMS m/z (relative intensity %) 798/799/800/801/802/803/804/805
(31/14/44/17/23/10/6/3, M+-Bu), 766 (40), 694/695/696/697/698/699/700/701
(70/31/100/38/58/19/14/5), 662 (67), 622 (71), 544 (70), 518 (83); high-
resolution EIMS
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-130-
798.1443 (calcd for C4oH40C14NO6Si, A-6.4mmu, M+-Bu'). 'H NMR 6 unit A
7.69/7.65
(SiPh2, 2'-H, 6'-H/2"-H, 6"-H; d; 6.5), 7.41 (SiPh2, 4'-H/4"-H, m), 7.35
(SiPh2, 3'-H;
5'-H/3"-H, 5"-H, m), 7.24 (10-H, 14-H; d, 8.7), 6.85 (11-H, 13-H, d, 8.7),
6.65 (3-H,
.
dt, 15.3, 7.5), 6.20 (8-H, d, 16.1), 6.03 (7-H, dd, 16.1, 8.0), 5.50(2-H, d,
15.3), 3.81
(OCH3, S), 3.77 (5-H, m), 2.39 (6-H, m), 2.34 (4-H, m), 2.29 (4-H', m), 1.11
(6-Me,
d, 6.9), 1.06 (CMe3, S); unit B 7.15 (5-H, d, 1.8), 7.00 (9-H, dd, 8.4, 1.8),
6.83 (8-H,
d, 8.4), 5.65 (NH, d, 7.7), 5.01 (2-H, ddd, 7.7, 6.0, 5.5), 4.78/4.72
(CH2CC13, ABq, -
11.9), 3.86 (OMe, S), 3.15 (3-H; dd, 6.1, -14.5), 3.08 (3-H', dd, 5.8, -14.5).
13C NMR
S unit A 165.1 (1), 158.8 (12), 142.5 (3), 136.0 (SiPh2, 2', 6'/2", 6"),
134.2/133.6
(SiPh2, i'/1"), 130.4 (9), 129.9 (8), 129.7/129.6 (SiPh2, 4'/4"), 129.5 (7),
127.6/127.5
(SiPh2, 3', 5'/3", 5"), 127.1 (10, 14), 124.6 (2), 113.9 (11, 13), 76.4 (5),
55.2 (OMe),
42.1 (6), 37.2 (4), 27.0 (CM~e. ), 19.5 (CMe3), 16.6 (6-Me); unit B 170.0 (1),
154.2 (7),
131.0 (5), 128.4 (4/9), 122.5 (6), 112.1 (8), 94.2 (CC13), 74.7 (CH2CC13),
56.1 (OMe),
52.9 (2) 36.4 (3).
Compound W
To a solution of compound V (55.8mg, 0.065mmo1) in 5.7mL of acetonitrile was
added 2.OmL of 49% hydrofluoric acid at 0 C. The ice bath was removed after
five
minutes and the reaction mixture was stirred vigorously for 17 hours. The
product was
extracted into ether and the extract was washed successively with saturated
sodium
bicarbonate and brine. Concentration and normal-phase chromatography (silica
column,
25% EtOAc/hexane) gave 31.6mg of compound W(79%); [a]D -3.7 (CHC13, c 1.3);
IR
3286, 2961, 1756, 1668, 1634, 1607, 1510, 1251, 1175, 1066, 812 cm'. 1H NMR S
unit A 7.27 (10-H, 14-H, d, 8.5), 6.93 (3-H, dt, 15.4, 7.6), 6.83 (11-H, 13-H,
d, 8.5),
6.34 (8-H, d, 15.9), 5.88 (7-H, dd 15.9, 8.2), 5.86 (2-H, d, 15.4), 3.81 (OMe,
S), 3.80
(5-H, m), 2.40 (6-H, m), 2.36 (4-H, m), 1.13 (6-Me, d, 6.8); unit B 7.17 (5-H,
d, 1.9),
7.05 (9-H, dd, 8.5, 1.9), 6.83 (8-H, d, 8.5), 5.90 (NH, d, 7.7), 5.03 (2-H,
ddd, 7.8,
5.9, 5.6), 4.79/4.72 (CH2CC13, ABq, -11.9), 3.86 (OCH31 S), 3.20 (3-H, dd,
6.0, -14.3),
3.09 (3-H', dd, 5.9, -14.3). 13C NMR S unit A 165.2 (1), 159.1 (12), 142.6
(3), 131.3
(9), 129.8 (7), 128.7 (8), 127.3 (10, 14), 125.0 (2), 114.0 (11, 13), 73.8
(5), 55.3
(OMe), 43.3 (6), 37.2 (4), 16.9 (6-Me) unit B 170.1 (1), 154.2 (7), 131.0 (5),
128.5
(4/9), 122.5 (6), 112.2 (8), 94.2 (CC13), 74.7 (CH2CC13), 56.1 (OMe), 53.0
(2), 36.5 (3).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-131-
(2S.2'R)-2-f3'(tert-Butoxycarbonyl)amino-2'-methvlpropanoyl-oxyl-4-m
ethylnentanoic
acid (AC)
A solution of methyl (S)-(+)-3-hydroxy-2-methylpropanoate (X) (lOg, 85mmol) in
300mL of ca. 9M ammonia in methanol was heated to 50 C in a sealed glass flask
for
168h, flushed with nitrogen to remove excess ammonia, and then evaporated to
dryness in
vacuo. The residue was triturated with ether, leaving behind (S)-3-hydroxy-2-
methylpropanamide (5.7g, 66% yield) as a white solid, mp 85 . 5-87.5 C; [a]
D+28. 7 (c
3.5, MeOH); EIMS m/z (rel intensity) 88 (19, M-Me), 85 (35), 73 (69); HREIMS
m/z
88.0397 (C3H6NO2, 0+0.2mmu); IR vtõ. 3384, 2960, 1671, 1473 crri 1; 1H NMR 6
5.83
(NH; br s), 5.42 (NH; br s), 3.73 (3-H2; m), 2.55 (2-H; m), 1.19 (2-Me; d,
7.2); 13C
NMR S 180.7 (1), 65.4 (3), 44.0 (2), 14.5 (2-Me). Anal. Found: C, 46.45; H,
8.83.
Calcd for C4H9NO2: C, 46.59; H, 8.79.
A suspension of (S)-3-hydroxy-2-methylpropanamide (2.1g, 20mmo1) in anhydrous
THF (2OmL) was added slowly to 1M borane-THF complex (61mmo1, 61mL) cooled to
0 C. The mixture was refluxed for 6h, cooled to 0 C, carefully decomposed with
conc
HC1 (lOmL), and concentrated in vacuo. The concentrate was saturated with NaOH
(20g), extracted with CHC13 (15mL x 4), and the combined extracts were dried
(MgSO4).
After filtration and removal of solvent, distillation in vacuo yielded 1.4g
(77% yield) of
(R)-3-amino-2-methylpropan-l-ol (Y) as a colorless oil, bp 110-112 C (40mmHg);
[a]D
+8.9 (c 22.6, MeOH); IR v. 3358, 1873, 1598, 1466 cm 1; 'H NMR S 5.18 (NH2;
br
s), 3.8 (1-H2; m), 2.95 (3-H; m), 2.68 (3-H; m), 1.81 (2-H; m), 0.82 (2-Me; d,
7.2); 13C
NMR S 66.9 (1), 46.4 (3), 37.1 (2), 14.4 (2-Me).
To a solution of amino alcohol Y(2.0g, 22mmol) in 39mL of a 10 % solution of
triethylamine in MeOH was added di-tert-butyl dicarbonate (5.4g, 25mmo1) and
the
mixture was stirred at 25 C for 30 min. After removal of solvent, the residue
was
dissolved in CH2C12 and the solution was washed twice with 1M KHSO4 (pH 2) and
once
with saturated NaC1 solution, and dried (MgSO4). Removal of solvent in vacuo
afforded
4.3g (100% yield) of (R)-3-(tert-butoxycarbonyl)amino-2-methylpropan-l-ol as a
viscous
oil which was directly used for the next step without further purification (>
95 % pure by
NMR analysis); IR P. 3356, 1976, 1686, 1523, 1456 cm'1; 'H NMR 6 4.82 (NH; br
s),
. 3.54 (1-H; dd, -11.4/4.2), 3.31 (1-H/3-H; m), 3.25 (3-H; dd, -14.1/6.6),
1.77 (2-H; m),
1.44 (CMe3; s), 0.87 (2-Me; d, 6.9).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-132-
To a solution of alcohol (R)-3-(tert-butoxycarbonyl)amino-2-methylpropan-l-ol
(2.2g, 12mmo1) and sodium periodate (7.5g, 35mmol) in carbon tetrachioride
(25mL),
acetonitrile (25mL) and water (38mL) was added ruthenium trichloride hydrate
(51mg,
0.25mmo1), and the mixture was stirred at 25 C for lh. The mixture was
diluted with
CH2C12 (100mL) and then filtered through Celite. The filtrate was basified (pH
9) with 2
M K2CO3 solution and the water layer was washed with ether. The aqueous layer
was
acidified with 1M KHSO4 to pH 2 at 0 C and extracted with CH2C12 (20mL x 3).
The
combined extracts ware washed with saturated NaCI solution and dried (MgSO4).
Removal of solvent in vacuo yielded 2.Og (85% yield) of (R)-3-(tert-
butoxycarbonyl)amino-2-methylpropanoic acid (Z) as a sticky solid. Pure Z
(1.75g, 74%
yield) crystallized from ether, mp 69.5-70.5 C; [a]D -18.4 (c 2, MeOH); EIMS
m/Z (rel
intensity) 147 (60; M+-Me2C=CH2), 130 (12), 74 (29), 57 (100); HREIMS m/z
147.0517
(C5H9NO4, A+1.4mmu); IR v111eJ1 3322-2400, 2797, 1711, 1654, 1413 cm-1; 1H NMR
of
major conformer 8 5.00 (NH; br s), 3.32 (3-H; m), 3.24 (3-H'; m), 2.71 (2-H;
m), 1.44
(CMe3; s), 1.20 (2-Me; d); 13C NMR of major/minor (2:1 ratio) conformers S
180.7/179.5 (1), 156.0/157.7 (BOC CO), 79.5/81.0 (CMe3), 42.7/44.0 (3),
39.9/40.2
(2), 28.3/28.3 (CM~e ), 14.6/14.6 (2-Me). Anal. Found: C, 53.04; H, 8.62.
Calcd for
C9H17NO4: C, 53.18; H, 8.43.
To a solution of 2.66g of L-leucic acid (20mmo1) and 1.74g of sodium
bicarbonate
(20mmo1) in 30mL water at 0 C was added 30mL of a CH2C12 solution of 6.44g of
tetrabutylammonium chloride (20mmo1) and 1.74mL of allyl bromide (20mmo1).
After
vigorously stirring the mixture for 24h, the CH2C12 was evaporated. About 50mL
water
was added and the aqueous layer was extracted four times with EtzO. The ether
solution
was dried over anhydrous sodium sulfate and then evaporated. The residue was
passed
through a short Si column to give 3.21g of allyl (2S)-2-hydroxy-4-
methylpentanoate (AA)
(93% yield) as a colorless oil, [a]D -8.4 (c 1.1, CHC13); IR P. 3464, 2957,
1732,
1203, 1140, 1087 cm''; 'H NMR 8 5.92 (allyl 2-H; m), 5.34 (allyl 3-Hz; dd,
17.4/1.1),
5.28 (allyl 3-HE; dd, 10.5/1.1), 4.67 (allyl 1-H2; d, 5.7), 4.23 (2-H; br s),
2.64 (OH; br
s), 1.89 (4-H; m), 1.57 (3-H2; m), 0.96 (5-H3; d, 6.5), 0.95 (4-Me; d, 6.7);
13C NMR 8
175.3 (1), 131.4 (allyl C-2), 118.6 (3), 68.9 (2), 65.7 (allyl C-1), 43.2 (3),
24.1 (4),
23.0 (5), 21.3 (4-Me).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-133-
To a solution of 1.74g of Z(8.55mmo1), 1.34g of AA (8.Ommol), and 64mg
DMAP in 12mL of dry CH2C12 at 0 C was added dropwise 8mL of a solution of DCC
(2.47g, 12mmol) in CH2C12. The clear solution was stirred at 0 C for 30 min
and then at
23 C for 3h. The white precipitate was filtered off, the solvent was
evaporated, and the
residue was redissolved in Et20. The ether solution was washed successively
with cold
0.5N HCI, sodium bicarbonate, and brine. The dried (Na2SO4) ether layer was
evaporated and the product was purified by flash column chromatography (silica
gel) to
give 2.62g (92% yield) of pure allyl (2S,2'R)-2-[3'(tert-butoxycarbonyl)amino-
2'-methyl-
propanoyl-oxy]-4- methylpentanoate (AB) as a colorless oil, [a]D -51.3 (c
3.41, CHC13);
EIMS m/z (rel intensity) 301 (5.2), 284 (4.0), 258 (1.5), 228 (43.5), 170
(41.8), 130
(74.5), 112 (100); HREIMS m/z 301.1532 (C14H23NO6, A-0.7mmu, M-Me.2C=CH2),
284.1496 (C14H22NO5, A+0.2mmu); IR P. 3395, 2962, 1747, 1715, 1515, 1251,
1175,
1083 cm-'. 'H NMR unit C S 5.17 (NH; br s), 3.42 (3-H; m), 3.22(3-H'; m), 2.78
(2-H,
m), 1.43 (CMe3; br s), 1.21 (2-Me; d, 7.1); unit D 8 5.90 (allyl 2-H; m), 5.33
(allyl 3-
Hz; d, 16.3), 5.27 (allyl 3-HE; d, 10.3), 5.09 (2-H; dd, 9.7/3.7), 4.63 (allyl
1-H2; m),
1.80 (3-H2; m), 1.64 (4-H; m), 0.96 (5-H3; d, 6.5), 0.94 (4-Me; d, 7.3). 13C
NMR unit
C 8 174.7 (1), 156.0 (BOC CO), 79.2 (CMe3), 43.1 (3), 40.3 (2), 28.3 (CMe ),
14.5 (2-
Me); unit D 8 170.4 (1), 131.4 (allyl C-2), 119.0 (allyl C-3), 70.9 (2), 65.9
(allyl C-1),
39.6 (3), 24.7 (4), 23.0 (5), 21.5 (4-Me).
To lOmL of a solution of 282mg (0.8mmol) of AB and 91mg (0.08mmo1) of
tetrakis(triphenylphosphine)palladium in dry THF was slowly added 688 L
(8mmol) of
dry morpholine. After stirring for 40 min, the solvent was evaporated and
100mL of
CHZC12 was added. The solution was washed successively with 2N HCI and water.
The
organic layer was filtered and the filtrate was extracted twice with saturated
sodium
bicarbonate. After back-washing with CHZCl2, the aqueous layer was first
acidified to pH
3 with cold KHSO4 at 0 C and then extracted three times with ether. The dried
ether
extract was evaporated to give 250mg of (2S,2'R)-2-[3'(tert-
butoxycarbonyl)amino-2'-
methylpropanoyl-oxy]-4-methylpentanoic acid (AC) (100% yield) as a wax-like
solid, [a]
D-47.9 (c 4.7, CHC13); EIMS m/z (rel intensity) 261 (12), 244 (18), 217 (28),
198 (17),
188 (100), 160 (61); HREIMS m/z 261.1221 (C11H19NO6, A-0.8mmu, M-Me2C=CH2),
244.1221 (C11H18NO5, 0-3.6mmu); IR v,,. 3376, 2960, 1738, 1518, 1174, 786 cm-
'. 1H
NMR (CDC13+D20) unit C 6 3.49 (H-3; dd, -13.8/3.5), 3.12(3-H; dd, -13.8/8.7),
2.68
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-134-
(2-H; m), 1.43 (CMe,; br s), 1.21 (2-Me; d, 7.1); unit D b 5.12 (2-H; dd,
9.6/3.5), 1.90-
1.68 (3H/4-H; m), 0.97 (5-H; d, 6.1), 0.94 (4-Me; d, 6.0). 13C NMR unit C 6
174.6 or
174.8 (1), 156.1 (BOC CO), 79.5 (CMe3), 43.0 (3), 40.4 (2), 28.3 (CMez), 14.5
(2-Me);
unit D 6 174.6 or 174.8 (1), 70.5 (2), 39.5 (3), 24.7 (4), 23.0 (5), 21.4 (4-
Me).
Compound AD
Alcohol W (34.8mg, 0.056mmo1), compound AC (26.8mg, 0.085mmo1) and
DMAP (1.74mg) were dissolved in 2831LL of dichloromethane. To this mixture was
added 666 L of dichloromethane solution of DCC (17.5mg, 0.085mmol). The
reaction
mixture was stirred overnight. The solvent was evaporated with a stream of
nitrogen,
ether was added, and the white precipitate was filtered off. The filtrate was
washed
successively with 0.5N HC1, sat. sodium bicarbonate, and brine. Concentration
and
chromatography (silica column, 25 % EtOAc/hexane) gave 46mg of compound AD (90
%);
[a]D -11.8' (CHC13, c 2.0); IR v,,.3369, 2961, 1737, 1511, 1252, 1174, 1066,
813, 756
cm''. 'H NMR (500MHz) 8 unit A 7.25 (10-H/14-H, dt, 8.5, 2.1), 6.84 (11-H/13-
H, dt,
8.5, 2.1), 6.76 (3-H, ddd, 15.5, 6.5, 6.4), 6.34 (8-H, d, 15.6), 5.88 (2-H,
bd, 15.5),
5.86 (7-H, dd 15.6, 8.7), 5.04 (5, m), 3.80 (OMe, s), 2.56 (6-H, m), 2.52 (4-
H, m),
1.10 (6-Me, d, 6.8); unit B 7.19 (5-H, d, 2.1), 7.05 (9-H, dd, 8.5, 2.1), 6.83
(8-H, d,
8.5), 6.55 (NH, bd, 7.3), 5.04 (2-H, m), 4.78/4.70 (CH2CC13, ABq, -11.8), 3.85
(OCH3,
S), 3.19 (3-H, dd, 6.3, -13.8), 3.08 (3-H', dd, 6.8, -13.8); unit C 5.14 (NH,
bt, 6.3),
3.32 (3-H, m), 3.20 (3-H', m), 2.73 (2-H, m), 1.42 (CMe3, s), 1.18 (2-Me, d,
7.0); unit
D 4.93 (2-H, dd, 10.0, 3.7), 1.67 (3-H/4-H, m), 1.55 (3-H', m), 0.86 (5-H, d,
6.5),
0.83 (4-Me-H, d, 6.5). 13C NMR 6 unit A 165.4 (1), 159.1 (12), 139.3 (3),
131.1 (9),
129.7_(7), 128.5 (8), 127.3 (10, 14), 125.4 (2), 114.0 (11, 13), 76.5 (5),
55.3 (OMe),
41.1 (6), 33.4 (4), 16.7 (6-Me); unit B 170.5 (1), 154.1 (7), 131.2 (5), 128.9
(4), 128.5
(9), 122.4 (6), 112.1 (8), 94.3 (CC13), 74.6 (CH2CCl3), 56.1 (OMe), 53.2 (2),
36.6 (3);
unit C 175.2 (1), 156.0 (BOCCO), 79.3 (CMe3), 43.1 (3), 40.4 (2), 28.3 (CMe=),
14.4
(2-Me); unit D 170.1 (1), 71.4 (2), 39.5 (3), 24.7 (4), 22.9 (5), 21.4 (4-Me).
Cryptophycin 81
Compound AD (46mg, 0.05mmo1) was mixed with activated Zn dust (178mg, 30
excess) in 1.3mL HOAc. The mixture was subjected to ultrasonication for 45
minutes,
and then stirred for another 90 minutes. About 30mL of dichloromethane was
added.
The solid was filtered off and the filtrate was evaporated under vacuum. The
residue was
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-135- -
dissolved in 1.1mL of TFA and the solution wasstirred for one hour. TFA was
evaporated under vacuum and water was added. Lyophilization gave the free
amino acid.
The amino acid was dissolved in 4.6mL DMF. To this solution was added 26 L
DIEA
and FDPP (30mg, 0.075mmol, in 2.2mL DMF), respectively. After stirring for 6
hours,
the solvent was evaporated and EtOAc was added. The solution was washed with
0.5N
HCI, and brine respectively. Evaporation of solvent followed by
chromatographic
purification (Si, ether) produced 20.5mg of Cryptophycin 81 (61 %); [a]D +34.9
(CHC13,
c 0.45); IR v,,= 3409, 3270, 2958, 1746, 1725, 1672, 1511, 1251, 1175, 1066,
1025,
972, 816 cm'. 'H NMR (500 MHz) S unit A 7.26 (10-H/14-H; dt, 8.6, 2.5),
6.84(11-
H/13-H, dt, 8.6, 2.5), 6.68 (3-H, ddd, 15.4, 9.9, 5.6), 6.35 (8-H, d, 15.9),
5.86 (7-H,
dd, 15.9, 8.8), 5.77(2-H, dd, 15.4, 0.9), 4.99 (5-H, ddd, 11.2, 6.0, 1.7),
3.80 (OCH3,
S), 2.53 (4-H/6-H, m), 2.37 (4-H', ddd, 11.2, 9.9,-14.6), 1.12 (6-Me, d, 6.9);
unit B
7.22 (5-H, d, 2.2), 7.08 (9-H, dd, 8.4, 2.4), 6.84 (8-H, d, 8.4), 5.64 (NH, d,
8.6), 4.81
(2-H, m), 3.86 (OMe, S), 3.13 (3-H; dd, 5.6, -14.5), 3.05 (3-H', dd, 7.1, -
14.5); unit C,
6.93 (NH, bdd, 5.8, 5.6), 3.50 (3-H, ddd, 5.2, 3.9, -13.5), 3.28 (3-H', ddd,
6.9, 6.7, -
13.5), 2.71 (2-H, m), 1.22 (2-Me; d, 7.3); unit D 4.84 (2-H, dd, 10.1, 3.4),
1.67 (3-H/
4-H; m), 1.38 (3-H', m), 0.78 (5-H, d, 6.5), 0.75 (4-Me-H, d, 6.5). 13C NMR
(125
MHz) 8 unit A 165.4 (1), 159.2 (12), 141.4 (3), 131.1 (9), 129.6 (7), 128.4
(8), 127.3
(10, 14), 125.2 (2), 114.1 (11, 13), 77.5 (5), 55.3 (OMe), 42.2 (6), 36.4 (4),
17.4 (6-
Me); unit B 171.0 (1), 154.0 (7), 131.2 (5), 129.9 (4), 128.4 (9), 122.5 (6),
112.1 (8),
56.2 (OMe), 53.5 (2), 35.1 (3); unit C 175.6 (1), 41.2 (3), 38.3 (2), 14.0 (2-
Me); unit D
170.9 (1), 71.6 (2), 39.5 (3), 24.5 (4), 22.7 (5), 21.3 (4-Me).
Exar_nple 15 Synthesis of Cryptophvcin 82
Compound AE
Compound AE is the tert-butyldiphenylsilyl ether (TBDMS) derivative of E.
Compound AF
The hydrolysis of AE (150mg) to AF (125mg) was carried out in 87% yield using
the procedure described above for the hydrolysis of T to U. 1H NMR 5 7.69/7.64
(SiPh2,
2'-H, 6'-H/2"-H, 6"-H, d, 6.3), 7.41 (SiPh2, 4'-H/4"-H, m), 7.39 (SiPh2, 3'-H,
4-Me-H/
3"-H, 5"-H, m), 6.86 (3-H; dt; 15.5, 7.5), 5.62 (2-H, d, 15.5), 5.30 (7-H/8-H,
m),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-136-
3.72 (5-H, m), 2.28 (4-H, m), 2.20 (6-H, m), 1.52 (9-H, bd, 7.7), 1.08 (CMe3,
s), 1.00
(6-CH3, d, 6.7).
Compound AG
The preparation of AG (96mg) from AF (76mg, 0.18mmo1) was carried out in 5 70%
yield using the procedure described above for the preparation of V from U. 1H
NMR 8 unit A 7.67 (SiPh2, 2'-H, 6'-H/2"-H, 6"-H; m), 7.46--7.31 (SiPh2, 3'-H,
4'-H,
4-Me-H/3"-H, 4"-H, 5"-H, m), 6.62 (3-H, dt, 15.4, 7.6), 5.51/5.48 (2-H, d,
15.4),
5.33 (7/8, m), 3.70 (5-H, m), 2.22 (4-H/6-H, m), 1.61 (9, bd, 7.4), 1.06
(CMe3, S),
0.98 (6-Me, d, 6.8); unit B 7.16 (5-H, d, 1.7), 7.00 (9-H, dd, 8.5, 1.7),
6.83/6.82 (8-H,
d, 8.5), 5.68/5.66 (NH, d, 7.3), 5.03 (2-H, m), 4.78/4.73 (CH2CC13, ABq, -
11.9), 3.87
(OMe, S), 3.16 (3-H; m), 3.09 (3-H', m). 13C NMR 8 unit A 165.1 (1),
143.0/142.9 (3),
136.0 (SiPh2, 2', 6'/2 ', 6"), 134.3/133.7 (SiPh2, i'/1"), 132.5 (7), 129.6
(8), 129.6
(SiPh2, 4'/4"), 127.5 (SiPh2, 3', 4-Me/3", 5"), 125.7/125.4 (2), 76.3 (5),
41.6 (6),
36.9/36.8 (4), 27.0 (CMe=), 19.5 (CMe3), 18.1 (9), 16.4/16.3 (6-Me); unit B
170.0 (1),
154.2 (7), 131.0 (5), 128.4 (4/9), 122.5 (6), 112.1 (8), 94.2 (CC13), 74.7
(CHZCCI3),
56.1 (OMe), 52.9 (2) 36.4 (3).
Compound AH
The preparation of AH (53.4mg) from AG (84mg, 0.llmmol) was carried out in
92% yield using the procedure described above for the preparation of W from V.
1H
NMR 8 unit A 6.83 (3-H, dt, 15.4, 7.5), 5.80 (2-H, d, 15.4), 5.51 (8-H, m),
5.33 (7-H,
dd, 15.2, 8.3), 3.50 (5-H, m), 2.42 (4-H, m), 2.30 (4-H', m), 2.28 (6, m),
1.68 (9, d,
7.3), 0.99 (6-Me, d, 6.7); unit B 7.20 (5-H, d, 1.8), 7.03 (9-H, dd, 8.4,
1.8), 6.80 (8-H,
d, 8.4), 6.10 (NH, bd, 7.0), 5.07 (2-H, m), 4.80/4.70 (CH2CC13, ABq, -11.5),
3.88
(OCH3, S), 3.20 (3-H, dd, 5.5, -14.3), 3.10 (3-H', dd, 7.2, -14.3). 13C NMR S
unit A
165.4 (1), 142.8 (3), 132.2 (7), 127.7 (8), 125.7 (2), 73.6 (5), 42.8 (6),
36.9 (4), 18.1
(9), 16.8 (6-Me); unit B 170.2 (1), 154.2 (7), 131.0 (5), 128.5 (4/9), 122.5
(6), 112.2
(8), 94.2 (CC13), 74.7 (CH2CC13), 56.1 (OMe), 53.1 (2), 36.5 (3).
Cr,yptophycin 82
Compound Al (36.8mg, 86% yield) was prepared from 27.4mg (0.052mmo1) of
compound AH using the procedure described above for AD.
Compound Al (68mg, 0.083mmo1) was cyclized to Cryptophycin 82 (28.5mg)
using the procedure described above for the cyclization of AD to Cryptophycin
81; [a]D
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-137-
+19.9 (CHC13, c 2.0). 'H NMR (500 MHz) S unit A 6.65 (3-H, ddd, 15.4, 9.5,
5.6),
5.76 (2-H, d, 15.4), 5.48 (8-H, dq, 15.3, 6.5), 5.27 (7-H, ddd, 15.3, 8.4,
1.5), 4.89 (5-
H, ddd, 10.7, 5.6, 1.5), 2.38 (4-H, m), 2.33 (4-H'/6-H, m), 1.66 (9-H, dd,
6.4, 1.5),
1.00 (6-Me, d, 6.9); unit B 7.22 (5-H, d, 2.0), 7.08 (9-H, dd, 8.4, 2.0), 6.83
(8-H, d,
8.4), 5.74 (NH, m), 4.81 (2-H, ddd, 8.5, 7.3, 5.6), 3.87 (OMe, S), 3.13 (3-H;
dd, 5.6, -
14.4), 3.04 (3-H', dd, 7.3, -14.4); unit C, 6.93 (NH, bdd, 6.7, 4.7), 3.52 (3-
H, ddd,
4.7, 3.9, -13.5), 3.27 (3-H', ddd, 6.8, 6.7, -13.5), 2.72 (2-H, dqd, 7.1, 6.8,
3.9), 1.20
(2-Me; d, 7.1); unit D 4.88 (2-H, dd, 9.6, 3.4), 1.75 (3-H/ 4-H; m), 1.47 (3-
H', m),
0.94 (5-H, d, 6.2), 0.91 (4-Me-H, d, 6.4). 13C NMR (125 MHz) b unit A 165.5
(1),
141.7 (3), 131.4 (7), 127.1 (8), 125.2 (2), 77.7 (5), 41.5 (6), 36.2 (4), 17.9
(9), 17.2 (6-
Me); unit B 171.0 (1), 153.9 (7), 131.1 (5), 129.9 (4), 128.4 (9), 122.4 (6),
112.2 (8),
56.1 (OMe), 53.5 (2), 35.1 (3); unit C 175.5 (1), 41.3 (3), 38.2 (2), 14.0 (2-
Me); unit D
170.9 (1), 71.5 (2), 39.6 (3), 24.6 (4), 23.0 (5), 21.5 (4-Me).
Example 16 Synthesis of CrYptophycin 90 and Crvptophvcin 91
General Procedure for Epoxidation of S rene-tvne Cryptophycins
To a solution of the cryptophycin (about lOmg/mL) in dichloromethane was added
three equivalents of m-chloroperbenzoic acid in dichloromethane (about
lOmg/mL). The
solution was stirred at room temperature until all starting material was
consumed. The
solution was passed through a short silica column using CH2C12 and then 1:4
CH2C12: EtZ0
to give a mixture of the two epoxides. The epoxides were separated by HPLC (C-
18, 7:3
MeCN:H20).
Using this procedure, Cryptophycin 82 (5mg) was converted to 2.2mg of
Cryptophycin 90 and 1.2mg of Cryptophycin 91.
Spectral Data for Cryptoph cin 90
'H NMR (500 MHz) S unit A 6.67 (3-H, ddd, 15.4, 9.5, 5.8), 5.79 (2-H, d,
15.4), 5.09 (5-H, ddd, 10.4, 4.5, 2.6), 2.84 (8-H, qd, 5.2, 2.2), 2.57 (7-H,
dd, 7.6,
2.2), 2.46 (4-H, m), 1.82 (6-H, m), 1.32 (9-H, d, 5.2), 1.03 (6-Me, d, 6.9);
unit B 7.22
(5-H, d, 2.2), 7.08 (9-H, dd, 8.4, 2.2), 6.84 (8-H, d, 8.4), 5.74 (NH, d,
8.6), 4.81 (2-
H, ddd, 8.3, 7.4, 5.7), 3.87 (OMe, S), 3.14 (3-H; dd, 5.4, -14.5), 3.03 (3-H',
dd, 7.3, -
14.5); unit C, 6.95 (NH, bdd, 6.7, 4.8), 3.52(3-H, ddd, 4.8, 3.7, -13.4), 3.29
(3-H',
ddd, 6.7, 6.5, -13.4), 2.74 (2-H, dqd; 7.3, 6.5, 3.7), 1.24 (2-Me; d, 7.3);
unit D 4.90
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-138-
(2-H, dd, 9.9, 3.7), 1.75 (3-H, m), 1.60 (4-H; m), 1.49 (3-H', ddd, 8.7, 3.7, -
13.7),
0.95 (5-H, d, 6.5), 0.91 (4-Me-H, d, 6.7). 13C NMR (125 MHz) 6 unit A 165.4
(1),
141.1 (3), 125.3 (2), 76.3 (5), 60.0 (7), 56.8 (8), 40.2 (6), 36.5 (4), 17.5
(9), 13.3 (6-
Me); unit B 171.0 (1), 154.0 (7), 131.1 (5), 129.9 (4), 128.4 (9), 122.5 (6),
112.3 (8),
56.1 (OMe), 53.6 (2), 35.1 (3); unit C 175.5 (1), 41.2 (3), 38.3 (2), 14.1 (2-
Me); unit D
170.7 (1), 71.4 (2), 39.6 (3), 24.7 (4), 23.0 (5), 21.5 (4-Me)
Spectral Data for Cryptophycin 91
'H NMR 6 unit A 6.68 (3-H, ddd, 15.4, 9.8, 5.4), 5.78 (2-H, d, 15.4), 5.09 (5-
H,
ddd, 11.1, 3.7, 2.0), 2.75 (8-H, m), 2.62 (7-H, dd, 9.5, 2.1), 2.48 (4-H, m),
1.78 (6-H,
m), 1.32 (9-H, d, 5.2), 0.99 (6-Me, d, 7.1); unit B 7.23 (5-H, d, 1.9), 7.09
(9-H, dd,
8.4, 1.9), 6.85 (8-H, d, 8.4), 5.68 (NH, d, 8.4), 4.82 (2-H, m), 3.88 (OMe,
S), 3.15 (3-
H; dd, 5.4, -14.4), 3.04 (3-H', dd, 7.2, -14.4); unit C, 6.95 (NH, bdd, 6.0,
4.6), 3.53(3-
H, ddd, 5.0, 4.0, -13.4), 3.29 (3-H', ddd, 6.9, 6.7, -13.4), 2.75 (2-H, m),
1.23 (2-Me;
d, 6.7); unit D 4.92 (2-H, dd, 9.8, 3.4), 1.77 (3-H, m), 1.58 (4-H; m), 0.96
(5-H, d,
7.3), 0.92 (4-Me-H, d, 6.7).
Example 17 Synthesis of Cryptophycin 97
To a solution of the cyclic depsipeptide, Cryptophycin 53 (9mg, 0.013mmo1),
dissolved in
dimethyl sulfoxide (lmL), was added sodium azide (40mg) and concentrated
sulfuric acid
(4 L). The mixture was then allowed to stir at 75-85 C for 2 days. After this
time the
reaction mixture was cooled to room temperature, diluted with Et20 (15mL) and
the
organic layer washed with brine (2 x 20mL) and H20 (20mL). The ethereal
extract was
then dried (MgSO4) and the solvent removed in vacuo to return an amorphous,
colorless
solid, that was predominantly the azido-alcohol, Cryptophycin 86. Purification
was
achieved by reverse phase chromatography (ODS, 101L, 250 x 10mm, 25% H20/MeCN,
3mL min71) to return the pure azido-alcohol, Cryptophycin 86, as an amorphous
colorless
solid (7.4mg, 77%).
Spectral Data for Crvytophycin 86
[a]D -22.0 (c=3.0, CHC13); MS (EI) m/z 482/484 (highest observed ion, M+-229,
8/3), 625/627 (14/5), 259 (7), 195/197 (100/34), 184 (14), 155/157 (82/70), 91
(23), 77
(22); HRMS, obsd mlz 482.1791, C23H31N2O735C1 (A 2.9mmu); MS (FAB) m/z (magic
bullet matrix) 712/714 (M++H, 79/36), 686/688 (31/12), 232 (74), 184 (100). 'H
NMR
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-139-
(CDC13) 8 unit A: 7.37-7.42 (10/11/12/13/14-H, bm, W1,z =20), 6.77 (3-H, ddd,
15.2,
10.6, 4.4), 5.77 (2-H, dd, 15.2, 1.3), 5.45 (5-H, ddd, 11.0, 4.2, 2.0), 4.55
(8-H, d,
5.7), 3.75 (7-H, dd, 7.3, 5.7), 2.55 (4-Hb, dddd, 14.5, 4.4, 2.0, 1.3), 2.43
(4-Ha, ddd,
14.5, 11.0, 10.6), 2.34 (7-OH, s), 1.80 (6-H, ddq, 7.3, 4.2, 7.0), 0.99 (6-Me,
d, 7.0),
unit B: 7.20 (5-H, d, 2.2), 7.06 (9-H, dd, 8.4, 2.2), 6.84 (8-H, d, 8.4), 5.76
(NH, d,
7.7), 4.74 (2-H, ddd, 7.7, 7.5, 5.5), 3.87 (OCH3, s), 3.10 (3-Hb, dd, 14.5,
5.5), 3.06 (3-
Ha, dd, 14.5, 7.5), unit C. 7.22 (NH, dd, 8.4, 3.7), 3.40 (3-Hb, dd, 13.6,
8.4), 3.14 (3-
Ha, dd, 13.6, 3.7), 1.23 (2-CH3, s), 1.16 (2-CH3', s), unit D: 4.85 (2-H, dd,
9.5, 5.1),
1.71 (3-Hb, ddd, 13.6, 9.5, 5.9), 1.59 (4-H, bm, Wln = 25), 1.50 (3-H$, ddd,
13.6, 7.9,
5.1), 0.89 (4-CH3, d, 6.6), 0.85 (5-H3, d, 6.6); 13C NMR (CDC13) S unit A:
165.3 (1),
143.0 (3), 135.1 (9), 129.1 (12), 128.9 (11/13), 128.6 (10/14), 124.3 (2),
75.1 (7), 74.6
(5), 67.8 (8), 39.3 (6), 34.7 (4), 11.9 (6-Me), unit B: 170.5 (1), 154.1 (7),
130.9 (5),
129.7 (4), 128.3 (9), 122.5 (6), 112.4 (8), 56.1 (7-OMe), 54.3 (2), 35.3 (3),
unit C:
177.8 (1), 46.5 (3), 42.8 (2), 22.9 (2-Me), 22.7 (2-Me'), unit D: 170.2 (1),
71.4 (2),
39.4 (3), 24.7 (4), 22.6 (4-Me), 21.8 (5).
Cryptophycin 97
To a solution of the cyclic depsipeptide, Cryptophycin 86 (5.5mg, 0.008mmo1),
dissolved in Et20/CH2C12 (3:1, 0.5mL), was added an ethereal solution (0.5mL)
of
triphenylphosphine (3mg, 0.011mmo1). The mixture was then allowed to stir at
room
temperature for three days. After this time the solvent was removed in vacuo
and the
residue dissolved in CH2C12 and subject to HPLC purification (CN column, 10 ,
250 x
10mm, 80% EtOAc/CH2C12, 3mL min'') to return pure Cryptophycin 97 as an
amorphous
colorless solid (4.2mg, 82 %).
UV (MeOH) Xn. (e) 202 (24400), 218 (9400), 284 (2200) nm; MS (EI) m/z 667/669
(M+, 11/3), 639/641 (41/16), 442 (21), 226 (17), 195 (43), 196 (32), 197
(100), 198
(71), 199 (11), 182/184 (25/16), 155/157 (63/22), 146 (30), 91 (40), 77 (29);
HRMS,
obsd m/z 667.3061, C36H46N30735C1 (0-3.6mmu). 'H NMR (CDC13) 6 unit A 7.35 (11-
H/13-H; m, W1n=15 Hz), 7.28 (12-H, m), 7.16 (10-H/14-H; m, W1,2-:=15 Hz), 6.74
(3-
H; ddd, 15.2/9.0/6.1), 5.69 (2-H; d, 15.2), 5.21 (5-H; ddd, 9.2/4.3/4.2), 2.79
(8-H, bs),
2.51 (4-H2, m), 2.11 (7-H, bd, 6.5), 1.48 (6-H, m), 1.13 (6-Me, d, 6.9); unit
B: 7.18 (5-
H, d, 2.1), 7.04 (9-H, dd, 8.4, 2.1), 6.83 (8-H, d, 8.4), 5.53 (NH, m), 4.73
(2-H, ddd,
7.6, 5.6, 5.4), 3.87 (OMe, s), 3.09 (3-Hb, dd, 14.7, 5.4), 3.04 (3-Ha, dd,
14.7, 7.6),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-140-
unit C.- 7.20 (NH, m), 3.40 (3-Hb, dd, 13.5, 8.6), 3.11 (3-Ha, dd, 13.5, 3.3),
1.22 (2-
Me, s), 1.15 (2-Me', s), unit D: 4.84 (2-H, dd, 10.1, 3.7), 1.71 (3-Hb, m),
1.67 (4-H,
bm, W1,2 = 25), 1.35 (3-H~, m), 0.86 (4-Me, d, 6.7), 0.84 (5-H3, d, 6.5); 13C
NMR
(CDC13) 6 unit A: 165.0 (1), 142.1 (3), 139.2 (9), 128.8 (11/13), 127.5 (12),
125.4
(10/14), 124.6 (2), 76.6 (5), 42.5 (6), 42.1 (7), 40.5 (8), 36.6 (4), 14.8 (6-
Me), unit B:
170.2 (1), 154.1 (7), 130.9 (5), 129.5 (4), 128.2 (9), 122.6 (6), 112.4 (8),
56.1 (7-
OMe), 54.3 (2), 35.3 (3), unit C: 177.9 (1), 46.5 (3), 42.7 (2), 22.8 (2-Me/2-
Me'), unit
D: 170.5 (1), 71.3 (2), 39.5 (3), 24.6 (4), 22.7 (4-Me), 21.3 (5).
Example 18 Synthesis of Cryptophycins 110-112 and 124
Cryptophycin 108
To a mixture of Cryptophycin 90 and Cryptophycin 91 (27mg, 0.045mmo1) in
0.8mL of tetrahydrofuran was added 400 L of an aqueous solution of periodic
acid
(32mg, 0.14mmo1). The clear solution was stirred at room temperature for 5
hours.
Water was added and the aqueous layer was extracted twice with ethyl acetate.
The
organic layer was washed with water, dried and concentrated. The residue was
purified
by reversed-phase chromatography on an ODS column (1:1 MeCN:H20) to give a 90%
yield of Cryptophycin 108. 'H NMR (500 MHz) 6 unit A 9.64 (7-H, d, 1.9), 6.67
(3-H,
ddd, 15.3, 10.0, 5.4), 5.81 (2-H, dd, 15.3, 0.9), 5.32 (5-H, ddd, 11.0, 6.6,
2.1), 2.65
(6-H, qdd, 7.2, 6.9, 1.9), 2.53 (4-H, m), 2.44 (4-H', m), 1.17 (6-Me, d, 7.2);
unit B
7.21 (5-H, d, 2.2), 7.08 (9-H, dd, 8.4, 2.2), 6.84 (8-H, d, 8.4), 5.91 (NH, d,
8.4), 4.80
(2-H, m), 3.86 (OMe, S), 3.16 (3-H; dd, 5.4, -14.6), 3.00 (3-H', dd, 7.8, -
14.6); unit C
7.05 (NH, bdd, 6.9, 5.0), 3.47(3-H, ddd, 4.6, 4.2, -13.5), 3.32 (3-H', ddd,
6.9, 6.6, -
13.5), 2.73 (2-H, m), 1.23 (2-Me; d, 7.2); unit D 4.83 (2-H, dd, 9.5, 3.6),
1.75 (3-H,
m), 1.70 (4-H; m), 1.40 (3-H', ddd, 9.5, 3.9, -14.0), 0.93 (5-H, d, 6.6), 0.88
(4-Me-H,
d, 6.6). 13C NMR (125 MHz) 6 unit A 200.6 (7), 165.4 (1), 140.5 (3), 125.6
(2), 73.7
(5), 50.1 (6), 36.1 (4), 10.8 (6-Me); unit B 171.1 (1), 154.0 (7), 131.0 (5),
129.9 (4),
128.3 (9), 122.4 (6), 112.3 (8), 56.1 (OMe), 53.8 (2), 35.0 (3); unit C 175.6
(1), 41.0
(3), 38.1 (2), 14.1 (2-Me); unit D 170.4 (1), 71.3 (2), 39.3 (3), 24.6 (4),
22.9 (5), 21.4
(4-Me).
Cryptophycin 108 was also produced by selective ozonolysis of Cryptophycin 82
using the procedure described above for the ozonolysis of E to F.
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-141-
General Procedure for the Wittig Reaction
Butyl lithium (0.4mL, 2.5 M in hexane, lmmol) was added to a 10mL solution of
the aryltriphenylphosphonium chloride (immol) in THF at -78 C. The reaction
mixture
was stirred for 15 minutes at -78 C and then was placed in a ice-bath for one
hour.
Three equivalents of the above mixture was added to the THF solution of
Cryptophycin
108 (about 30mg/mL) at -78 C. The solution was stirred for 20 minutes before
the cold
bath was removed. When the temperature had risen to 25 C, the reaction was
quenched
with saturated ammonium chloride aqueous solution. The mixture was extracted
twice
with ethyl acetate. The organic extract was washed with water, dried and
concentrated.
The residue was purified on an ODS flash column (65:35 MeCN:H20) to give a
mixture
of E and Z isomers (contains 8% to 13 % Z isomers depending on the nature of
the aryl
group; analysis determined by NMR). The desired E isomer crystallized from
ether.
Cryptophvcin 110
The Wittig reaction led to 5. lmg of the p-fluorophenyl analog (contained
about
8% Z isomer) from 7.3mg of Cryptophycin 108 (about lmg unreacted aldehyde was
recovered). After crystallization from ether, 4.0mg of pure Cryptophycin 110
was
obtained; [a]D +42.4 (MeOH, c 2.1). 'H NMR 6 unit A 7.29 (10-H/14-H; dd, 8.6,
5.6), 6.99(11-H/13-H, dt, 8.6, 8.5), 6.68 (3-H, ddd, 15.3, 9.7, 5.6), 6.38 (8-
H, d,
15.8), 5.83 (7-H, dd, 15.8, 8.8), 5.78 (2-H, d, 15.3), 5.00 (5-H, ddd, 10.8,
7.3, 1.3),
2.53 (4-H/6-H, m), 2.36 (4-H', m), 1.13 (6-CH3, d, 6.8); unit B 7.21 (5-H, d,
1.8), 7.07
(9-H, dd, 8.4, 1.8), 6.84 (8-H, d, 8.4), 5.68 (NH, d, 8.5), 4.82 (2-H, m),
3.87 (OMe,
s), 3.14 (3-H; dd, 5.6, -14.4), 3.04 (3-H', dd, 7.2, -14.4); unit C, 6.95 (NH,
bdd, 6.8,
5.9), 3.50 (3-H, td, 4.4, -13.5), 3.28 (3-H', ddd, 6.8, 6.7, -13.5), 2.72 (2-
H, m), 1.23
(2-Me; d, 7.2); unit D 4.82 (2-H, m), 1.65 (3-H/4-H; m), 1.35 (3-H', ddd, 4.5,
3.8, -
10.9), 0.78 (5-H, d, 6.4), 0.74 (4-Me-H, d, 6.4). 13C NMR (75 MHz) S unit A
165.4
(1), 162.3 (12, d, 'Jc-F 245.8 Hz), 141.4 (3), 132.9 (9), 130.6 (7), 129.9
(8), 127.6
(10/14, d, 3Jc-F 8.0 Hz), 125.2 (2), 115.5 (11/13, d, 2Jc-F 21.5 Hz), 77.4
(5), 42.2 (6),
36.4 (4), 17.3 (6-Me); unit B 170.9 (1), 153.9 (7), 131.0 (5), 129.9 (4),
128.4 (9), 122.4
(6), 112.2 (8), 56.1 (OMe), 53.6 (2), 35.1 (3); unit C 175.6 (1), 41.1 (3),
38.3 (2), 14.0
(2-Me); unit D 170.9 (1), 71.5 (2), 39.5 (3), 24.5 (4), 22.7 (5), 21.2 (4-Me).
,
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-142-
Cryptophycin 111
The Wittig reaction led to 43mg of the p-tolyl analog (contained about 9% Z
isomer) from 55mg of Cryptophycin 108. After crystallization from ether, 34mg
of pure
Cryptophycin 111 was obtained; [a]D +44.3' (CHC13, c 0.6); EIMS m/z (relative
intensity %) 652 (2.9, M+), 497 (3.6), 412 (23.8), 242 (20), 145 (46), 105
(75); high-
resolution EIMS 652.29094 (calcd for C36H45C1N207, A-0.6mmu, M+). 'H NMR 6
unit A
7.21 (10-H/14-H; d, 8.0), 7.11 (11-H/13-H, d, 8.0), 6.68 (3-H, ddd, 15.2, 9.6,
5.4),
6.37 (8-H, d, 15.8), 5.95 (7-H, dd, 15.8, 8.6), 5.76 (2-H, d, 15.2), 4.99 (5-
H, dd, 10.4,
6.2), 2.51 (4-H/6-H, m), 2.38 (4-H', m), 2.32 (12-Me, s), 1.13 (6-CH3, d,
6.8); unit B
7.22 (5-H, d, 2.1), 7.08 (9-H, dd, 8.4, 2.1), 6.83 (8-H, d, 8.4), 5.80 (NH, d,
8.4), 4.81
(2-H, m), 3.86 (OMe, s), 3.14 (3-H; dd, 5.6, -14.4), 3.03 (3-H', dd, 7.3, -
14.4); unit C
6.98 (NH, bdd, 6.0, 5.7), 3.49 (3-H, td, 4.6, -13.4), 3.29 (3-H', ddd, 6.7,
6.6, -13.4),
2.70 (2-H, m), 1.22 (2-Me; d, 7.2); unit D 4.81 (2-H, m), 1.65 (3-H/4-H; m),
1.37 (3-
H', m), 0.78 (5-H, d, 5.8), 0.73 (4-Me-H, d, 6.4). 13C NMR (75 MHz) 8 unit A
165.5
(1), 141.5 (3), 137.4 (12), 133.9 (9), 131.7 (7), 129.3 (10/14), 129.0 (8),
126.0 (11/13),
125.1 (2), 77.4 (5), 42.2 (6), 36.4 (4), 21.1 (12-Me), 17.3 (6-Me); unit B
171.0 (1),
153.9 (7), 131.0 (5), 129.9 (4), 128.4 (9), 122.4 (6), 112.2 (8), 56.1 (OMe),
53.6 (2),
35.1 (3); unit C 175.6 (1), 41.1 (3), 38.3 (2), 14.1 (2-Me); unit D 170.9 (1),
71.6 (2),
39.5 (3), 24.5 (4), 22.7 (5), 21.2 (4-Me).
CrYptophvcin 112
The Wittig reaction led to 35mg of the 2-thienyl analog (contained about 13% Z
isomer) from 51mg of Cryptophycin 108. After crystallization from ether, 25mg
of pure
Cryptophycin 111 was obtained. 'H NMR (500 MHz) S unit A 7.12 (12-H; d, 4.9),
6.94
(11-H, dd, 4.9, 3.4), 6.90 (10-H, d, 3.4), 6.68 (3-H, ddd, 15.2, 9.5, 5.3),
6.54 (8-H, d,
15.7), 5.83 (7-H, dd, 15.7, 8.7), 5.78 (2-H, d, 15.2), 4.96 (5-H, dd, 9.5,
6.5), 2.51 (4-
H/6-H, m), 2.35 (4-H', m), 1.13 (6-CH3, d, 6.8); unit B 7.21 (5-H, d, 1.6),
7.07 (9-H,
dd, 8.4, 1.6), 6.84 (8-H, d, 8.4), 5.74 (NH, d, 7.1), 4.82 (2-H, m), 3.87
(OMe, s), 3.13
(3-H; dd, 5.5, -14.4), 3.04 (3-H', dd, 7.1, -14.4); unit C, 6.97 (NH, bt,
5.8), 3.50 (3-H,
ddd, 4.4, 4.3, -13.4), 3.28 (3-H', ddd, 6.8, 6.6, -13.4), 2.71 (2-H, m), 1.22
(2-Me; d,
7.2); unit D 4.82 (2-H, m), 1.67 (3-H/4-H; m), 1.39 (3-H', m), 0.80 (5-H, d,
6.4), 0.77
(4-Me-H, d, 6.4).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-143-
Cryptophvcin 124
The Wittig reaction led to 131mg of the p-chlorophenyl analog (contained about
10% Z isomer) from 153mg of Cryptophycin 108. After crystallization from
ether,
107mg of pure Cryptophycin 124 was obtained; [a]D +29.2 (CHC13, c 0.5); high-
resolution EIMS m/z 672.23691 (calcd for C35H42C12N2O7, 0 O.Ommu). 'H NMR (500
MHz) 8 unit A 7.26 (10-H/11-H/13-H/14-H, s), 6.68 (3-H, ddd, 15.2, 9.6, 5.4),
6.36 (8-
H, d, 15.8), 5.98 (7-H, dd, 15.8, 8.8), 5.77 (2-H, d, 15.2), 5.00 (5-H, bdd,
9.4, 6.3),
2.54 (4-H/6-H, m), 2.38 (4-H', m), 1.13 (6-CH3, d, 6.8); unit B 7.22 (5-H, d,
1.7), 7.07
(9-H, dd, 8.4, 1.7), 6.84 (8-H, d, 8.4), 5.73 (NH, bd, 7.8), 4.82 (2-H, m),
3.86 (OMe,
s), 3.13 (3-H; dd, 5.5, -14.4), 3.04 (3-H', dd, 7.2, -14.4); unit C, 6.97 (NH,
bt, 5.8),
3.49 (3-H, td, 4.2, -13.4), 3.29 (3-H', ddd, 6.7, 6.6, -13.4), 2.71 (2-H, m),
1.22 (2-Me;
d, 7.2); unit D 4.81 (2-H, m), 1.65 (3-H/4-H; m), 1.35 (3-H', m), 0.77 (5-H,
d, 7.1),
0.75 (4-Me-H, d, 7.1). 13C NMR (75 MHz) 8 unit A 165.4 (1), 141.3 (3), 135.2
(12),
133.2 (9), 130.9 (7), 130.6 (8), 128.7 (11/13), 127.3 (10/14), 125.2 (2), 77.3
(5), 42.2
(6), 36.5 (4), 17.3 (6-Me); unit B 170.9 (1), 153.9 (7), 131.0 (5), 129.8 (4),
128.4 (9),
122.4 (6), 112.2 (8), 56.1 (OMe), 53.6 (2), 35.1 (3); unit C 175.6 (1), 41.1
(3), 38.3
(2), 14.1 (2-Me); unit D 170.8 (1), 71.5 (2), 39.6 (3), 24.5 (4), 22.8 (5),
21.3 (4-Me).
Example 19 Synthesis of Cryptophycins 115-120. 125 and 126
Cryptophycins 115 and 116
Using the general procedure described above for epoxidation of styrene-type
cryptophycins, Cryptophycin 110 (3.5mg) was converted to 2.0mg of Cryptophycin
115
and lmg of Cryptophycin 116.
Spectral Data for Cryptophycin 115
[a]D +29.1 (MeOH, c 0.8); EIMS m/z (relative intensity %) 672 (1.9, M+), 412
(5.8), 245 (17), 195 (52), 155 (31), 141 (23), 135 (15), 109 (100); high-
resolution EIMS
668.2853 (calcd for C35H42C1FN208, A+3.4mmu, M+). 'H NMR (500 MHz) 6 unit A
7.22 (10-H/14-H; ddt, 8.7, 5.2, 2.0), 7.01 (11-H/13-H, ddt, 8.7, 8.5, 2.0),
6.68 (3-H,
ddd, 15.2, 9.7, 5.2), 5.74 (2-H, dd, 15.2, 0.8), 5.15 (5-H, ddd, 11.2, 5.0,
1.8), 3.67 (8-
H, d, 2.0), 2.88 (7-H, dd, 7.4, 2.0), 2.54 (4-H, dtd, 5.2, 1.8, -14.4), 2.44
(4-H', ddd,
11.2, 9.7, -14.4), 1.79 (6-H, m), 1.13 (6-CH3, d, 6.8); unit B 7.21 (5-H, d,
2.0), 7.06
(9-H, dd, 8.3, 2.0), 6.83 (8-H, d, 8.3), 5.63 (NH, d, 8.4), 4.80 (2-H, ddd,
8.4, 7.2,
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-144-
5.4), 3.87 (OMe, s), 3.14 (3-H; dd, 5.4, -14.4), 3.03 (3-H', dd, 7.2, -14.4);
unit C 6.94
(NH, bdd, 6.7, 5.0), 3.48 (3-H, ddd, 5.0, 3.7, -13.4), 3.30 (3-H', ddd, 6.8,
6.7, -13.4),
2.72 (2-H, m), 1.22 (2-Me; d, 7.4); unit D 4.83 (2-H, dd, 9.9, 3.6), 1.70 (3-
H/4-H; m),
1.35 (3-H', m), 0.87 (5-H, d, 6.5), 0.85 (4-Me-H, d, 6.5). 13C NMR (125 MHz) 8
unit
A 165.3 (1), 162.9 (12, d, 1Jc-F 245.4 Hz), 141.0 (3), 132.5 (9), 127.3
(10/14, d, 3JC-F
8.3 Hz), 125.3 (2), 115.7 (11/13, d, 2Jc-F 21.9 Hz), 76.1(5), 63.0 (7),.58.3
(8), 40.5 (6),
36.7 (4), 13.4 (6-Me); unit B 170.9 (1), 154.0 (7), 131.0 (5), 129.7 (4),
128.4 (9), 122.5
(6), 112.3 (8), 56.1 (OMe), 53.6 (2), 35.0 (3); unit C 175.6 (1), 41.1 (3),
38.2 (2), 14.1
(2-Me); unit D 170.7 (1), 71.3 (2), 39.4 (3), 24.5 (4), 22.9 (5), 21.3 (4-Me).
Cryptophycins 117 and 118
Using the general procedure described above for epoxidation of styrene-type
cryptophycins, Cryptophycin 111 (6.2mg) was converted to 3.5mg of Cryptophycin
117
and 1mg of Cryptophycin 118.
Spectral Data for Cryptophvcin 117
[a]D +25.5 (MeOH, c 1.8); EIMS m/z (relative intensity %) 668 (4.8, M+), 412
(6.2), 280 (11), 173 (9.4), 145 (15), 135 (34), 105 (100); high-resolution
EIMS m/z
668.28532 (calcd for C36H45C1N208, A+1.1mmu, M+). 'H NMR (500 MHz) S unit A
7.17/7.13 (10-H/11-H/13-H/14-H, A2B2 q, 8.0), 6.67 (3-H, ddd, 15.4, 9.8, 5.6),
5.73 (2-
H, dd, 15.4, 0.9), 5.14 (5-H, ddd, 11.2, 4.9, 2.0), 3.65 (8-H, d, 2.0), 2.91
(7-H, dd,
7.6, 2.0), 2.54 (4-H, bdd, 5.6, -14.3), 2.44 (4-H', ddd, 10.7, 9.8, -14.3),
2.35 (12-Me,
s), 1.77 (6-H, m), 1.14 (6-CH3, d, 6.9); unit B 7.21 (5-H, d, 2.2), 7.07 (9-H,
dd, 8.5,
2.2), 6.83 (8-H, d, 8.5), 5.65 (NH, d, 8.5), 4.80 (2-H, ddd, 8.3, 7.4, 5.6),
3.87 (OMe,
s), 3.13 (3-H; dd, 5.6, -14.5), 3.02 (3-H', dd, 7.4, -14.5); unit C 6.93 (NH,
bd, 6.8,
5.1), 3.48 (3-H, ddd, 5.1, 3.8, -13.2), 3.29 (3-H', ddd, 6.9, 6.8, -13.2),
2.71 (2-H, m),
1.22 (2-Me; d, 7.1); unit D 4.82 (2-H, dd, 9.8, 3.6), 1.70 (3-H/4-H; m), 1.33
(3-H', m),
0.85 (5-H, d, 6.5), 0.84 (4-Me-H, d, 6.5). 13C NMR (125 MHz) 8 unit A 165.3
(1),
141.0 (3), 138.4 (12), 133.7 (9), 129.4 (10/14), 125.6 (11/13), 125.3 (2),
76.2(5), 62.9
(7), 59.0 (8), 40.7 (6), 36.7 (4), 21.1 (12-Me), 13.6 (6-Me); unit B 170.9
(1), 154.0 (7),
131.0 (5), 129.8 (4), 128.4 (9), 122.4 (6), 112.3 (8), 56.1 (OMe), 53.6 (2),
35.1 (3);
unit C 175.5 (1), 41.1 (3), 38.3 (2), 14.1 (2-Me); unit D 170.7 (1), 71.3 (2),
39.4 (3),
24.5 (4), 22.8 (5), 21.2 (4-Me).
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-145-
Spectral Data for Cryptophvcin 118
'H NMR spectrum is similar to that of Cryptophycin 38, except that the
multiplets
at 7.30-7.38 for the phenyl protons has been replaced by a 4H multiplet at
7.14 and a 3H
singlet at 2.35 for the p-tolyl protons.
CrYptophycins 119 and 120
Using the general procedure described above for epoxidation of styrene-type
cryptophycins, Cryptophycin 112 (8mg) was converted to 1.2mg of Cryptophycin
119 and
0.5mg of Cryptophycin 120.
Suectral Data for Crvntophycin 119
'H NMR (500 MHz) S unit A 7.28 (12-H, d, 5.2), 7.11 (10-H, d, 3.3), 7.00 (11-
H, dd, 5.2, 3.3), 6.68 (3-H, ddd, 15.1, 9.9, 5.2), 5.76 (2-H, dd, 15.1, 1.3),
5.15 (5-H,
ddd, 11.2, 5.5, 1.8), 3.94 (8-H, d, 2.0), 3.10 (7-H, dd, 7.5, 2.0), 2.56 (4-H,
bdd, 5.2, -
14.3), 2.44 (4-H', ddd, 11.2, 9.9, -14.3), 1.76 (6-H, m), 1.13 (6-CH3, d,
6.8); unit B
7.21 (5-H, d, 2.3), 7.07 (9-H, dd, 8.4, 2.3), 6.83 (8-H, d, 8.4), 5.63 (NH, d,
8.4), 4.80
(2-H, ddd, 8.5, 7.4, 5.7), 3.87 (OMe, s), 3.13 (3-H; dd, 5.7, -14.5), 3.03 (3-
H', dd,
7.4, -14.5); unit C 6.95 (NH, bd, 6.5, 5.3), 3.48 (3-H, ddd, 5.3, 3.6, -13.5),
3.31 (3-H',
ddd, 6.8, 6.5, -13.5), 2.72 (2-H, m), 1.23 (2-Me; d, 7.3); unit D 4.85 (2-H,
dd, 10.3,
3.5), 1.70 (3-H/4-H; m), 1.33 (3-H', m), 0.86 (5-H, d, 6.3), 0.85 (4-Me-H, d,
6.3).
CrMtophycins 125 and 126
Using the general procedure described above for epoxidation of styrene-type
cryptophycins, Cryptophycin 124 (47mg) was converted to 26mg of Cryptophycin
125
and 12mg of Cryptophycin 126.
Spectral Data for Cryptophvcin 125
[a]D +35.6 (CHC13, c=0.9); high-resolution EIMS m/z 688.2301 (calcd for
C35H42C12N208, 0+1.7mmu). 'H NMR (500 MHz) 6 unit A 7.33 (11-H/13-H, dt, 8.5,
2.0), 7.18(10-H//14-H, dt, 8.5, 2.0), 6.67 (3-H, ddd, 15.1, 9.9, 5.2), 5.73 (2-
H, dd,
15.1, 0.9), 5.15 (5-H, ddd, 11.0, 4.7, 1.5), 3.66 (8-H, d, 1.9), 2.87 (7-H,
dd, 7.4, 1.9),
2.53 (4-H, m), 2.42 (4-H', ddd, 10.6, 10.5, -14.4), 1.78 (6-H, m), 1.12 (6-
CH3, d, 7.0);
unit B 7.20 (5-H, d, 2.1), 7.06 (9-H, dd, 8.3, 2.1), 6.83 (8-H, d, 8.3), 5.72
(NH, d,
8.1), 4.79 (2-H, ddd, 8.3, 7.9, 5.4), 3.86 (OMe, s), 3.12 (3-H; dd, 5.4, -
14.5), 3.02 (3-
H', dd, 7.4, -14.5); unit C 6.97 (NH, bdd, 6.3, 5.5), 3.46 (3-H, ddd, 4.4,
4.1, -13.7),
3.22 (3-H', ddd, 7.0, 6.3, -13.7), 2.70 (2-H, m), 1.22 (2-Me; d, 7.2); unit D
4.82 (2-H,
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-146-
dd, 9.9, 3.4), 1.70 (3-H/4-H; m), 1.33 (3-H', m), 0.87 (5-H, d, 6.5), 0.84 (4-
Me-H, d,
6.5). 13C NMR (125 MHz) 8 unit A 165.3 (1), 141.0 (3), 135.3 (12), 134.4 (9),
128.9
(11/13), 126.9 (10/14), 125.3 (2), 76.0 (5), 63.1 (7), 58.2 (8), 40.4 (6),
36.7 (4), 13.4
(6-Me); unit B 170.9 (1), 154.0 (7), 131.0 (5), 129.8 (4), 128.3 (9), 122.4
(6), 112.3 (8),
56.1 (OMe), 53.7 (2), 35.0 (3); unit C 175.6 (1), 41.0 (3), 38.2 (2), 14.1 (2-
Me); unit D
170.7 (1), 71.2 (2), 39.4 (3), 24.5 (4), 22.9 (5), 21.3 (4-Me).
Spectral Data for Cryptophycin 126
1H NMR (300 MHz) unit A 6 7.26 (11-H/13-H, d, 8.1), 7.17 (10-H//14-H, d,
8.1), 6.70 (3-H, ddd, 15.1, 9.4, 5.3), 5.81 (2-H, d, 15.1), 5.14 (5-H, bdd,
10.0, 4.5),
3.57 (8-H, bs), 2.85 (7-H, bd, 7.6), 2.66 (4-H, m), 2.59 (4-H', m), 1.77 (6-H,
m), 1.04
(6-CH3, d, 7.0); unit B 7.23 (5-H, bs), 7.08 (9-H, bd, 8.4), 6.83 (8-H, d,
8.4), 5.82
(NH, d, 6.8), 4.81 (2-H, ddd, 7.2, 6.8, 5.4), 3.86 (OMe, s), 3.14 (3-H; dd,
5.4, -14.1),
3.03 (3-H', dd, 7.4, -14.1); unit C 7.03 (NH, bt, 5.7), 3.47 (3-H, ddd, 4.0,
3.7, -13.1),
3.34 (3-H', ddd, 6.8, 6.4, -13.1), 2.72 (2-H, m), 1.24 (2-Me; d, 7.0); unit D
4.90 (2-H,
dd, 10.0, 2.5), 1.74 (3-H/4-H; m), 1.45 (3-H', m), 0.91 (5-H, d, 6.5), 0.86 (4-
Me-H, d,
6.5). 13C NMR (125 MHz) 6 unit A 165.4 (1), 141.3 (3), 135.6 (12), 134.1 (9),
128.8
(11/13), 126.8 (10/14), 125.2 (2), 76.8 (5), 63.3 (7), 55.6 (8), 40.8 (6),
36.7 (4), 13.4
(6-Me); unit B 170.9 (1), 153.9 (7), 131.0 (5), 129.8 (4), 128.4 (9), 122.3
(6), 112.2 (8),
56.1 (OMe), 53.7 (2), 35.0 (3); unit C 175.7 (1), 41.0 (3), 38.2 (2), 14.1 (2-
Me); unit D
170.8 (1), 71.4 (2), 39.3 (3), 24.6 (4), 23.1 (5), 21.3 (4-Me).
Examnle 20 Synthesis of Cryptophycins 121-123 and 127
Compound AJ
To a solution of BOC-L_-leucine monohydrate (1.245g, 5mmol) in 30mL anhydrous
CH2C12was added solid EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride)(0.53g, 2.75mmo1) under N2 with stirring at 0 C. After stirring
at 0 C for
1.5h the mixture was concentrated below 5 C and the residue was diluted with
35mL cold
- EtOAc and washed successively with two portions (lOmL) each of ice-cold
solutions of
5% aqu. KHSO4, 5% aqu. NaHCO3 and brine. The organic phase was separated,
dried
(MgSO4) at 5 C and evaporated below 5 C. The residual oil was diluted with ice-
cold
THF (5mL) and added to a solution of compound K (295mg, 0.5mmo1) in ice-cold
anhydrous THF (5mL). A few crystals of DMAP were then added to the mixture
which
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-147-
was stirred and allowed to reach 25 C overnight. 5% aqu. NaHCO3 solution
(5mL) was
added and the resulting two-phase mixture was stirred vigorously at 25 C for
2h. EtOAc
(4OmL) was added, the aqueous phase was extracted with additional EtOAc (2x
20mL)
and the combined organic layers were washed with water and brine, dried
(NaSO4),
filtered and evaporated. The residue was filtered through a plug of silica gel
with 25 %
EtOAC in hexanes to yield compound AJ as a colourless oil (385mg, 96% yield):
[a)D
13.6 (c= 0.63, CHCC13); EIMS m/z (rel. intensity) 805/803/801(M+, 1%),
568/570/572/574 (9/10/6/2), 523/525/527 (5/6/1), 418/420/422/424 (15/15/6/2),
341/343/345/347 (58/70/35/9), 307/309/311 (29/22/10), 224/227/228/229
(10/100/31/14),
208/210/211/2'12 (20/83/45/54); HREIMS m/z 800.2218 (C35H48 35C14N208, A-
5.3mmu);
'H NMR (CDC13) unit A d 7.21-7.35 (Ph-H5; m), 6.77 (3-H; ddd, 6.7/6.7/15.3),
6.4 (8-
H; d, 15.8), 6.05 (7-H; dd, 8.7/15.8), 5.9 (2-H; d, 15.3), 5.0 (5-H; m), 2.6
(6-H; m),
2.55 (4-H2; m), 1.17(6-Me; d, 6.7); unit B 8 7.22 (5-H; s), 7.05 (9-H: d,
8.1), 6.84 (8-
H; d, 8.1), 6.55 (NH; d, 7.8), 5.0 (2-H; ddd, 5.5/7.1/7.8), 4.68-4.8 (CH2CC13;
ABq,
11.9), 3.86 (OMe; s), 3.2 (3-H; dd, 5.5/14.0), 3.06 (3-H': dd, 7.1/14.0); unit
D S 4.8
(NH; d), 4.2 (2-H; m), 1.65 (4-H; m), 1.55 (3-H; m), 1.4 (CMez); s), 1.37 (3-
H'; m),
0.85 (4-Me; d, 6.5), 0.8 (5-H; d, 6.5); 13C NMR unit A 8 165.4(1), 139.3 (3),
137.0 (9),
131.5 (8), 130.5 (7), 128.5 (11/13), 127.3 (12), 126.2 (10/12), 125.8 (2),
76.2 (5), 40.8
(6), 33.4 4), 16.8 (6-Me); unit B 8 170.0 (1), 154.2 (7), 131.0 (5), 129.0
(9), 122.5 (6),
112.2 (8), 94.4 (CC13), 74.7 (CH2CC13), 56.1 (OMe), 53.3 (2), 36.5 (3), unit D
8 173.2
(1), 155.7 (BOC-CO), 80.0 (CMe3), 52.4 (2), 41.2 (3), 28.3 (CMe,), 24.8 (4),
22.8
(4-Me), 21.6 (5).
Comnound AK
Compound AJ (115mg, 0.134mmo1) was dissolved in TFA (3mL) and left at 25 C
for 1 h. The solvent was removed and the residue was evaporated repeatedly
from
CH2C12 then from toluene to leave an amorphous solid. This solid was dissolved
in
anhydrous THF (5mL) and sufficient diethylisopropylamine was added to adjust
the pH of
the solution to pH 8-9 when an aliquot of the solution was spotted onto moist
pH paper.
The solution was cooled to 0 C with stirring and a solution of compound AL in
THF
(3mL) was added. To prepare compound AL compound Z (55mg, 0.27mmol) was
dissolved in THF (3mL) and diethylisopropylamine (0.046mL, 0.27mmol) was
added.
To this mixture, after cooling to -15 C, pivaloylchloride (0.033mL, 0.27mmol)
was
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-148-
added dropwise and the solution was stirred at -15 C for 10 min and at 0 C for
20 min.
The resulting suspension was then transferred into the solution of compound AJ
in THF.
The resulting mixture was stirred at 0 C and allowed to reach 25 C overnight.
After 12
hrs at 25 C 5% aqu. NaHCO3 solution was added and the resulting mixture was
stirred
vigorously at 25 C for 1.5h. EtOAC (40mL) was added and the phases were
separated.
The aqueous phase was extracted with additional EtOAC (2x lOmL). The combined
organic extracts were washed with 5% aqu. NaHCO3 solution (2OmL), 5% aqu.
KHSO4
solution (20mL) and brine, dried (NaSO4) and evaporated. The residual oil was
chromatographed over silica gel eluting with 35% EtOAc in hexanes to yield
compound
AK as a colourless foam (98mg, 83 % yield): [a]D -8.3 (c 0.88, CHC13); 'H NMR
(CDC13) unit A 8 7.28-7.35 (Ph-H4; m), 7.22(H-12;m), 6.75 (H-3; ddd,
6.4/6.4/15.4),
6.4(H-8; d, 15.9), 6.04 (H-7; dd, 8.6/15.9), 5.95
(H-2; d, 15.4), 5.0 (H-5; m), 2.6 (H-4; m), 1.1 (6-Me; d, 6.8); unit B 8 7.22
(H-5, d,
1.5), 7.15 (NH; d, 7.6), 7.05 (H-9; dd, 1.5/8.2), 6.85 (H-8; d, 8.2), 5.0 (H-
2; m), 4.8-
4.68 (CH CCl3; ABq, 12), 3.86 (OMe; s), 3.2 (H-3; m), 3.1 (H-3'; dd,
7.2/14.1); unit C
8 5.0 (NH; m), 3.2 (H2-3; m), 2.55 (H-2; m), 1.1 (2-Me; d, 7.1); unit D S 6.12
(NH;
m), 4.4 (H-2; m), 1.65 (H-4; m), 1.55 (H-3; m), 1.4 (H-3'; m), 0.86 (4-Me; d,
6.8),
0.81 (5-H; d, 6.8); 13C NMR (CDC13) unit A 8 165.8(1), 138.9(3), 131.5(8),
130.4(7),
128.6(11/13), 127.4(12), 126.2(10/14), 125.8 (2), 76.3(5), 33.6(4), 16.6(6-
Me); unit B 6
170.2(1), 154.1(7), 136.9(4),131.2(5), 128.6(9), 122.3(6), 112.2(8), 94.4(-
CC13),
74.5(CHZCC13), 56.1(OMe), 53.4(2), 36.6(3); unit C S 175.4(1), 156.3(BOC-CO),
79.5(OCMe3), 43.6(3), 41.3(2), 15.1(2-Me); unit D 8 172.6(1), 51.3(2),
40.5(3), 24.5(4),
22.7(4-Me), 21.5(5).
Cryptophvcin 121
Compound AK (73mg, 0.082mmo1) was dissolved in AcOH (3.5mL), activated Zn
dust (400mg) was added and the resulting suspension was sonicated for 45 min.
After
stirring at 25 C for an additional 1.5h the mixture was diluted with CH2C12
(5mL) and
filtered through CeliteR. The solids were washed with additional CH2C12 (lOmL)
and the
resulting filtrate was evaporated. The residue, without further purification,
was dissolved
in TFA (3mL), kept, at 25 C for 1 h and the solvent was removed in vacuo. The
residue
was evaporated repeatedly from CH2C12, then from toluene until a colourless
solid was
obtained. This solid was dissolved in dry DMF (3mL), FDPP (43mg, 0.108mmol)
was
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-149-
added followed by a sufficient amount of diethylis6propylamine to adjust the
pH of the
solution to pH 8-9 when an aliquot was spotted onto moist pH paper. After
stirring at
25 C for 16 hrs, the mixture was diluted with ether (4OmL) and washed with 5%
aqu.
KHSO4 solution (2 x lmL), 5% aqu. NaHCO3 (15mL) and brine. After drying
(Na2SO4)
and evaporation the residue was purified by reversed-phase HPLC on C-18 silica
(EconosilR C-18, 22x 250mm) eluting with CH3CN/water 65:35 at 6mL/min. The
fraction eluting at tR 48 min was collected and evaporated to leave an
amorphous solid
(26mg, 50% yield): [a]D +46.5 (c 0.81, CHC13); EIMS m/z 637/639 (M+, 1%),
449/451 (2/1), 420 (5), 411/413 (7/5), 227/228 (9/7), 195 (10), 184 (15),
167/168/169
(40/86/29), 155/157 (100/26), 91 (85), 69 (86); HREIMS m/z 637.2864 (C35 H44
35C1 N3
06, A + 5.5mmu); 'H NMR unit A 6 7.21- 7.35 (Ph-H5; m), 6.75 (H-3; ddd,
4.2/10.8/16), 6.4 (H-8; d, 16), 6.04 (H-7; dd, 8.8/16), 5.75 (H-2; d, 16), 5.1
((H-5; m),
2.55 (H-4; m; H-6; m), 2.35 (H-4'; m); unit B 8 7.18 (H-5; d, 2), 7.05 (H-9;
dd,
2.0/8.3), 6.85 (H-8; d, 8.3), 5.7 (NH; d, 7.2), 4.7 (H-2; ddd, 4.9/7.2/7.7),
3.86 (OMe;
s), 3.1 (H-3; dd, 4.9/14.4), 3.0 (H-3'; dd,7.7/ 14.4); unit C 8 7.25 (NH; m),
3.5 (H-3;
m), 3.4 (H-3'; m), 2.55 (H-2; m), 1.2 (2-Me; d, 7.2); unit D 8 5.8 (NH; d,
7.2), 4.4 (H-
2; m), 1.55 (H-4; m), 1.38 (H2-3; dd, 7.2/7.7), 0.76 (4-Me; d, 6.6), 0.74 (H-
5; d, 6.6):
13 C NMR (CDC13) unit A 6 165.1 (1), 141.9 (3), 136.8 (9), 131.7 (8), 130.2
(7), 128.5
(11/13), 127.3 (14), 126.1 (10/12), 125.1 (2), 76.5 (5), 42.3 (6), 36.3 (4),
17.2 (6-Me);
unit B 6 171.0 (1), 154.1 (7), 130.8(5), 129.6 (4), 128.4 (9), (122.5(6),
112.4 (8), 56.2
(OMe), 54.4 (2), 35.4 (3); unit C S 175.8 (1), 41.0 (3), 38.6 (2), 14.8 (2-
Me); unit D S
173.2 (1), 51.1 (2), 40.9 (3), 24.7 (4), 23.4 (4-Me), 21.5 (5)
Cry_ptophycins 122 and 123
To a stirred solution (0 C) of Cryptophycin 121 (20mg, 0.032mmol) in anhydrous
CH2C12 (1.3mL) was added 99% mCPBA (17mg, 0.lmmol) in one portion. Anhydrous
toluene (0.7mL) was then added and the resulting mixture was stirred at 25 C
for 72 h.
The solvent was evaporated in vacuo and the residual solid was purified by
reverse-phase
HPLC on C-18 silica (Econosil R C-18, 22x 250mm) eluting with CH3CN/water
65:35 at
6mL min '. Cryptophycin 122 (9mg, 44 %) eluted at tR 37.5 min and Cryptophycin
123
(5mg, 23 % ) eluted at tR 40 min.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-150-
Cryptophycin 122
[a] D+35.0 (c 1, CHC13); EIMS m/z 653/655 (1.6/0.7, M+), 411/413 (20/5),
280/282 (39/19), 252/254 (13/8), 223/225/227 (19/10/23), 211/213 (18/6),
195/197
(51/13), 184/186 (49/11), 176/168/169(20/16/21), 155/156/157 (95/59/42),
139/141/143
(60/40/24), 135/135 (30/11), 129/131(40/29), 91(100); HREIMS m/z 653.2906 (C35
H44
35C1 N3 07, A-3.8mmu); 'H NMR (d6-acetone) unit A 8 6.65 (H-3; ddd,
38/11.0/15.0),
5.9 (H-2; dd, 1.9/15.0), 5.25 (H-5; ddd, 1.9/4.9/11.5), 3.82 (H-8; d, 2.0),
3.0 (H-7; dd,
2.0/7.7), 2.65 (H-q.; dddd, 2.0/2.0/3.8/14.5), 2.4 (H-4'; ddd, 11.0/11.5/
14.5), 1.85 (H-
6; dqd, 4.9/7.4/7.7), 1.1 (6-Me; d, 6.9); unit B 8 7.45 (NH; d, 7.9), 7.22 (H-
9; dd,
2.0/8.4), 7.0 (H-8; d, 8.4), 4.45 (H-2; ddd, 3.6/7.9/11.2), 3.84 (OMe; s), 3.2
(H-3; dd,
3.6/14.5), 2.75 (H-3'; dd, 11.2/14.5); unit C 8 7.8 (NH; d, 8.8), 3.65 (H-3;
ddd,
3.3/8.8/13.2), 3.1 (H-3'; ddd, 2.1/2.1/13.2), 2.55 (H-2; m); unit D S 7.35
(NH; d, 8.1),
4.25 (H-2; ddd, 4.8/8.1/10.8), 1.65 (H-4; m), 1.45 (H-3; ddd, 5.1/10.8/13.7),
1.35 (H-
3'; 4.8/9.0/13.7), 0.8 (4-Me/ H-5; d, 6.5); 13C NMR unit A S 165.9(1),
140.8(3),
138.6(9), 129.4(11/13), 129.2(12), 126.7(2), 126.6(10/14), 76.0(5), 63.9(7),
59.3(8),
41.2(6), 37.7(4), 13.9(6-Me); unit B S 171.9(1), 154.6(7), 132.5(4), 131.4(5),
129.0(9),
122.4(6), 113.3(8), 56.9(2), 65.4(OMe), 36.4(3); unit C 8 177.2(1), 41.3(3),
38.9(2),
15.7(2-Me); unit D 8 174.2(1), 51.7(2), 40.6(3), 25.3(4), 23.1(4-Me), 21.5(5).
Crvptophvcin 123
[a]D +25.2 (c 0.58, CHC13); 'H NMR (CDC13) unit A 8 7.21- 7.35 ((Ph-H5; m),
6.75 (H-3; ddd, 4.1/10.9/14.1), 5.85 (H-2; dd, 1.4/15.2), 5.25 (H-5; m), 3.6
(H-8; d,
2.0), 2.9 (H-7; dd, 2.0/7.8), 2.7 (H-4; m), 2.55 (H-4'; m), 1.75 (H-6; m),
1.05 (6-Me;
d, 7.1), unit B d 7.18(H-5;d,2.0), 7.05 (H-9; dd, 2.0, 8.5), 6.85 (H-8; d,
8.5), 5.95
(NH; d, 7.7), 4.7 (H-2;ddd, 4.9/7.7/8.1), 3.86 (OMe;s), 3.15 (H-3; dd,
4.9/14.5), 3.05
(H-3'; dd, 8.1/14.5); unit C 8 7.25 (NH; m), 3.55 (H-3; ddd, 4.6/8.7/13.3),
3.15 (H-3';
ddd, 3.0/3.1/13.3), 2.55 (H-2; m), 1.2 (2-Me; d, 7.3); unit D 6 6.0 (NH; d,
8.2), 4.45
(H-2; m), 1.6 ( H-4; m), 1.55 (H2-3; m), 0.88 (4-Me; d, 7.1), 0.87 (5-H; d,
7.1);
Crvntophvcin 127
Cryptophycin 122 (5mg, 0.0075mmo1) was dissolved in CHC13 (2mL) and cooled
to -40 C under N2. Trimethylsilylchloride (0.02mL, 0.157mmo1) was added
dropwise
and the resulting mixture was stirred at -40 C for lh. The solvent was
evaporated and
the residue was filtered through a plug of silica gel with 15 % EtOH in
diethylether to
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-151-
yield Cryptophycin 127 (4mg, 77% yield): [a]D +28.6 (c 1.16, CHC13); EIMS m/z
653
(0.5; M+-HCl); 411(2); 182/184 (22/26); 153/155 (68/40); 135(31);
107/108/109(55/22/31); 91/92(100/30); 79/81(45/35); HREIMS m/z 653. 2841
(C35H4435C1N3O7, 0 2.6mmu); ' H NMR (CHC13) unit A 8 7.3-7.4 (Ph-H5; m), 6.75
(H-3;
ddd, 4.2/10.9/15.0), 5.8 (H-2; dd, 1.5/15.0), 5.2 (H-5; ddd, 1.9/9.9/9.9),
4.65 (H-8; d,
9.6), 4.0 (H-7; brd, 9.6), 2.65 (H-4; m), 2.48 (H-6; m), 2.35 (H-4'; ddd,
10.9/11.2/14.5), 1.02 (6-Me; d, 7.0); unit B S 7.18 (H-5; d, 2.2), 7.05 (H-9;
dd,
2.2/8.6), 6.85 (H-8; d, 8.6), 6.05 (NH; d, 7.7), 4.65 (H-2; ddd, 4.7/7.7/8.6),
3.86
(OMe; s), 3.15 (H-3; dd, 4.7/14.6), 2.9 (H-3'; dd, 8.6/14.6); unit C 8 7.25
(NH; brdd,
4.1/5.7), 3.4 (H2-3; m), 2.55 (H-2; m), 1.15 (2-Me; d, 7.5); unit D S 6.2 (NH;
d, 8.2),
4.45(H-2; m), 1.65 (H-4; m), 1.55(H2-3; m), 0.93 (4-Me; d, 6.8), 0.92 (5-Me;
d, 6.8);
13C NMR (CDC13) unit A S 165.4(1), 142.4(3), 138.8(9), 128.8(11/13),
128.2(12), 128.0
(10/14), 124.0 (2), 75.6(5), 73.9(7), 62.1(8), 38.6(6), 36.4 (4), 8.5(6-Me);
unit B S
171.1(1), 154.0(7), 130.8(5), 129.8(4), 128.2(9), 122.4 (6), 112.4 (8), 56.1
(OMe),
54.5(2), 35.4(3); unit C b 175.8(1), 41.1(3), 38.8(2), 14.8(2-Me); unit D S
173.2(1),
51.1(2), 40.9(3), 25.0(4), 22.8(5), 21.8(4-Me).
Example 21 Synthesis of Cryptophvcins 128 and 130-134
CrYptophycins 128. 130 and 131
A crude 2:1 mixture of 5.7mg of Cryptophycins 117 and 118 was dissolved in
CHC13 (0.5mL) and treated with trimethylsilyl chloride (511L) at -60 C for 3
h.
Reversed-phase HPLC separated the resulting mixture of chlorohydrins into
Cryptophycin
128, Cryptophycin 130, and Cryptophycin 131. Further purification by normal-
phase
HPLC gave pure Cryptophycin 128 (2.1mg).
Spectral Data for Clyptophycin 128
[a]0 +51.4' (CHC13, c 0.4); IR v. 3408, 3281, 2958, 1747, 1731, 1668, 1538,
1505, 1258, 1179, 1067, 910, 733 cm-'; EIMS m/z (relative intensity %) 668
(0.3, M+-
HC1), 500 (1.2), 445 (4.2), 407 (6), 318 (12), 274 (17), 240 (33), 199 (29),
155 (31),
141 (23), 135 (15), 109 (100); high-resolution EIMS 668.2851 (calcd for
C36H45C1N208,
A+1.3mmu, M+-HCl). 'H NMR (500 MHz) 8 unit A A 7.27 (10-H/14-H, d, 8.0), 7.18
(11-H/13-H, d, 8.0), 6.69 (3-H, ddd, 15.2, 9.6, 5.4), 5.79 (2-H, dd, 15.2,
0.8), 5.10 (5-
H, ddd, 11.0, 8.4, 1.7), 4.63 (8-H, d, 9.6), 3.99 (7-H, bd, 9.6), 2.67 (4-H,
bdd, 5.4, -
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-152-
14.2), 2.49 (6-H, dqd, 7.6, 7.6, 1.7), 2.36 (4-H', td, 10.7, -14.2), 2.35 (12-
Me, s), 1.04
(6-CH3, d, 7.1); unit B 7.23 (5-H, d, 2.1), 7.09 (9-H, dd, 8.5, 2.1), 6.84 (8-
H, d, 8.5),
5.83 (NH, d, 8.6), 4.80 (2-H, ddd, 8.2, 7.5, 5.7), 3.87 (OMe, s), 3.16 (3-H;
dd, 5.6, -
14.4), 3.00 (3-H', dd, 7.5, -14.4); unit C 6.94 (NH, bt, 5.8), 3.53 (3-H, ddd,
5.4, 4.1, -
13.5), 3.24 (3-H', ddd, 6.9, 6.6, -13.5), 2.74 (2-H, m), 1.22 (2-Me; d, 7.3);
unit D 4.92
(2-H, dd, 9.9, 3.2), 1.75 (3-H/4-H; m), 1.47(3-H', m), 0.94 (5-H, d, 6.4),
0.92 (4-Me-
H,
d, 6.4). 13C NMR (125 MHz) 6 unit A 165.6 (1), 141.6 (3), 139.2 (12), 135.3
(9),
129.7 (10/14), 127.9 (11/13), 125.2 (2), 76.4 (5), 74.0 (7), 62.0 (8), 38.4
(6), 36.3 (4),
21.1 (12-Me), 8.6 (6-Me); unit B 171.1 (1), 153.9 (7), 131.0 (5), 130.0 (4),
128.4 (9),
122.4 (6), 112.2 (8), 56.1 (OMe), 53.6 (2), 35.0 (3); unit C 175.3 (1), 41.3
(3), 38.3
(2), 14.0 (2-Me); unit D 170.6 (1), 71.3 (2), 39.7 (3), 24.7 (4), 23.1 (5),
21.5 (4-Me).
Spectral Data for Cryptophvcin 130
1H NMR (300 MHz) S unit A 7.27 (10-H/14-H, d, 7.8), 7.16 (11-H/13-H, d, 7.8),
6.69 (3-H, ddd, 15.1, 9.6, 5.2), 5.75 (2-H, d, 15.1), 5.40 (5-H, ddd, 10.6,
3.0, 1.8),
5.05 (8-H, d, 5.9), 3.74 (7-H, ddd, 10.5, 5.4, 5.2), 2.59 (4-H, m), 2.55 (6-H,
m), 2.37
(4-H', m), 2.33 (12-Me, s), 1.06 (6-CH3, d, 6.9); unit B 7.21 (5-H, bs), 7.07
(9-H, bd,
8.4), 6.83 (8-H, d,,8.4), 5.77 (NH, d, 6.6), 4.80 (2-H, m), 3.87 (OMe, s),
3.12 (3-H;
dd, 5.6, -14.4), 3.02 (3-H', dd, 7.2, -14.4); unit C 7.00 (NH, bt, 6.5), 3.47
(3-H, ddd,
4.3, 4.0, -13.3), 3.24 (3-H', ddd, 6.7, 6.6, -13.3), 2.72 (2-H, m), 1.23 (2-
Me; d, 7.3);
unit D 4.80 (2-H, m), 1.70 (3-H/4-H; m), 1.42(3-H', ddd, 7.9, 4.7, -13.0),
0.91 (5-H,
d, 6.4), 0.86 (4-Me-H, d, 6.4). 13C NMR (125 MHz) unit A 165.5 (1), 142.1 (3),
138.8
(12), 135.2 (9), 129.5 (10/14), 127.5 (11/13), 124.8 (2), 77.8 (5), 74.2 (7),
67.2 (8),
39.4 (6), 34.6 (4), 21.1 (12-Me), 12.3 (6-Me); unit B 171.0 (1), 153.9 (7),
131.0 (5),
129.5 (4), 128.4 (9), 112.2 (8), 56.1 (OMe), 53.6 (2), 35.0 (3)' unit C 175.6
(1), 41.1
(3), 38.3 (2), 14.1 (2-Me); unit D 170.3 (1), 71.5 (2), 39.5 (3), 24.6 (4),
22.7 (5), 21.6
(4-Me).
Spectral Data for Cryptophycin 131
1H NMR (300 MHz) S unit A 7.18 (10-H/11-H/13-H/14-H, s), 6.64 (3-H, ddd,
15.2, 9.8, 5.4), 5.70 (2-H, d, 15.2), 5.06 (5-H, bt, 9.1), 4.86 (8-H, d, 9.7),
4.06 (7-H,
bd, 9.7), 2.54 (4-H, bdd, 5.2, -14.2), 2.37 (12-Me, s), 2.13 (4-H', m), 1.85
(6-H, m),
0.97 (6-CH3, d, 6.6); unit B 7.21 (5-H, d, 2.1), 7.07 (9-H, dd, 8.4, 2.1),
6.83 (8-H, d,
8.4), 5.66 (NH, d, 10.0), 4.79 (2-H, bq, 7.7), 3.87 (OMe, s), 3.14 (3-H; dd,
5.6, -
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-153-
14.4), 3.02 (3-H', dd, 7.2, -14.4); unit C 6.93 (NH, bt, 6.2), 3.51 (3-H, ddd,
5.0, 4.2, -
13.5), 3.27 (3-H', ddd, 6.7, 6.4, -13.5), 2.73 (2-H, m), 1.23 (2-Me; d, 7.3);
unit D 4.86
(2-H, dd, 9.7, 3.4), 1.75 (3-H/4-H; m), 1.46(3-H', m), 0.94 (5-H, d, 6.0),
0.93 (4-Me-
H, d, 6.4).
Cryptophycin 132. 133 and 134
A mixture of Cryptophycin 124 and the Z isomer (134mg, 0.199mmo1) dissolved
in dichioromethane (6mL) was allowed to react with m-chloroperbenzoic acid
(103mg,
0.598mmo1) at room temperature for 36 h. Phosphate buffer (lOmL, pH 8) was
added to
remove the chlorobenzoic acid generated during the reaction. After 30 minutes
the
aqueous layer was replaced with dimethyl sulfide (50 L) and a fresh sample of
phosphate
buffer (lOmL). Stirring was continued for 30 minutes. The organic layer was
separated,
the solvent evaporated and the residue dried under vacuum for 12 h. The crude
mixture
of predominantly Cryptophycins 125 and 126 was dissolved in CHC13 (5mL) and
treated
with excess trimethylsilyl chloride (50 L) at -60 C for 3 h. The solvent was
evaporated
and the residue purified on a reversed phase HPLC (Econosil ODS silica, 250mm
x
22mm, 35 % H2O/CH3CN, 6mL/min) to give Cryptophycin 134 (tR 52.5 min, 9mg,
6%),
partially pure Cryptophycin 133 (tR 61 min, 32mg, 22%) and crude Cryptophycin
132 (tR
67.5 min, 72mg). Further purification by normal-phase HPLC (Econosil silica,
250mm x
10mm, 56 % EtOAc/hexane, 3mL/min) gave pure Cryptophycin 132 (65mg, 45 %).
Spectral Data for Cryptophvcin 132
[a]D +60.1 (CHC13, c=1.1); EIMS m/z (relative intensity %) 688 (1.6, M+-
HCl), 412 (10), 261 (11), 195 (57), 184 (28), 165 (28), 155 (92), 135 (85);
high-
resolution EIMS m/z 688.23563 (calcd for C35H42C12N208, A-3.8mmu, M+-HCl). iH
NMR (500 MHz) 8 unit A 7.33 (10-H/11-H/13-H/14-H, s), 6.67 (3-H, ddd, 15.2,
9.9,
5.1), 5.79 (2-H, dd, 15.2, 1.1), 5.10 (5-H, ddd, 11.1, 8.1, 1.5), 4.63 (8-H,
d, 9.5), 3.98
(7-H, bd, 9.3), 2.62 (4-H, bdd, 5.1, -14.2), 2.47 (6-H, dqd, 7.4, 7.4, 1.5),
2.40 (4-H',
td, 10.8, -14.2), 2.35 (12-Me, s), 1.02 (6-CH3, d, 7.0); unit B 7.21 (5-H, d,
2.1), 7.06
(9-H, dd, 8.4, 2.1), 6.82 (8-H, d, 8.4), 6.04 (NH, d, 8.5), 4.74 (2-H, td,
8.0, 5.4), 3.85
(OMe, s), 3.13 (3-H; dd, 5.3, -14.4), 2.95 (3-H', dd, 7.8, -14.4); unit C 7.00
(NH, bt,
5.9), 3.48 (3-H, ddd, 5.0, 3.9, -13.4), 3.23 (3-H', ddd, 6.6, 6.3, -13.4),
2.71 (2-H, m),
1.21 (2-Me; d, 7.2); unit D 4.91 (2-H, dd, 9.7, 3.4), 1.75 (3-H/4-H; m),
1.45(3-H', m),
0.92 (5-H, d, 6.6), 0.91 (4-Me-H, d, 6.6). 13C NMR (125 MHz) 6 unit A 165.7
(1),
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-154-
141.6 (3), 137.3 (12), 134.8 (9), 129.4 (11/13), 129.0 (10/14), 125.2 (2),
76.4 (5), 74.0
(7), 61.4 (8), 38.4 (6), 36.2 (4), 8.7 (6-Me); unit B 171.1 (1), 153.9 (7),
131.0 (5),
130.1 (4), 128.4 (9), 122.3 (6), 112.2 (8), 56.1 (OMe), 53.8 (2), 34.9 (3);
unit C 175.3
(1), 41.0 (3), 38.1 (2), 14.1 (2-Me); unit D 170.6 (1), 71.3 (2), 39.7 (3),
24.7 (4), 23.1 5 (5), 21.5 (4-Me).
Spectral Data for Cryptophycin 134
'H NMR spectram is similar to that of Cryptophycin 27, except that two
multiplets
at 7.31 (10/14) and 7.36-7.40 (11/12/13) for the phenyl protons has been
replaced by two
doublets at 7.26 (10/14) and 7.36 (11/13) for the p-chlorophenyl protons.
Example 22 Structure-Activitv Relationships (SAR) and In Vivo Evaluation
The cytotoxicities of Cryptophycins 1, 3 and 8 and the new cryptophycins
against
the human tumor cell lines KB and LoVo are shown in Table 6. Cryptophycin 51
and
Cryptophycin 3 show comparable cytotoxicities (IC50's 3.5-5.8 nM).
Cryptophycin 52
(IC50's 43-7OpM), however, is slightly less cytotoxic than Cryptophycin
1(IC50's 9-
29pM), and Cryptophycin 55 (33-47pM) is slightly less cytotoxic than
Cryptophycin 8
(IC50's 9-19pM). The cytotoxicity IC50's for Cryptophycins 117, 122, 125, 127,
128 and
132 are comparable with those for Cryptophycins 1, 8, 52, and 55; however, the
data
does not presently admit to more meaningful comparisons.
Cryptophycin 52 is active in vivo, but requires roughly three times the total
dosage
compared with Cryptophycin 1. The in vivo activity of Cryptophycin 52 against
five
solid tumors of murine origin and three human solid tumors is summarized in
Table 7.
Similarly Cryptophycin 55 was active in vivo, but also required three times
the total
dosage compared with Cryptophycin 8. The in vivo activity of Cryptophycin 55
against
six solid tumors of murine origin and four human solid tumors is summarized in
Table 8.
The in vivo data appears to correlate with the in vitro data.
Cryptophycins 117, 125, 128 and 132 are active in vivo against a pancreatic
adenocarcinoma of murine origin (Panc 03). Cryptophycins 117 and 128 appear to
be
more potent, that is they require smaller total doses, than Cryptophycins 1
and 8,
respectively, whereas Cryptophycins 125 and 132 are less potent, that is they
require
higher total doses, than Cryptophycins 1 and 8, respectively. The data is
summarized in
Table 9.
CA 02214565 2005-07-22
-155-
T/C values that are less than 42% are considered to be active by NCI
standards;
T/C values that are less than 10% are considered to have excellent activity
and potential
clinical activity by NCI standards. Gross log kill is defined as T-C/3.2 Td
where T is the
median time in days for the tumors of the treated group to reach 750mg, C is
the median
time in days for the tumors of the control group to reach 750mg, and Td is the
tumor
volume doubling time (T.H. Corbett et al., Cytotoxic Anticancer Drugs: Models
and
Concepts for Drug Discovery and Development, pp 35-87; Kluwer: Norwell, 1992).
Gross log kill values of > 2.8, 2.0-2.8, 1.3-1.9, 0.7-1.2, and <0.7 with
duration of drug
treaunent of 5-20 days are scored + + + +, + + +, + +, + and - (inactive),
respectively. An activity rating of +++ to ++++, which is indicative of
clinical
activity, is needed to effect partial or complete regression of 100-300mg size
masses of
most transplanted solid tumors of mice. Cryptophycin 52 shows T/C values
ranging from
0 to 14% for murine tumors and 4.1 to 16 for human tumors and gross log kill
values
ranging from 1.1 to 2 for murine tumors and 0 to 3.2 for human tumors.
Cryptophycin 1
shows T/C values ranging from 0 to 27 % and log kill values ranging from < 1
to 2.
Cryptophycin 55 shows T/C values ranging from mostly 0 to 4.4 % and log kill
values
ranging from 2.1 to >4.6 (cures) for all but one trial, the Colon 26
experiment which
showed a gross log kill value of 1.2. Cryptophycin 8 shows T/C values of
mostly 0%
and log kill values > 2.8 (several cures) as shown in Table 10. Cryptophycins
117 and
125 appear to have T/C and gross log kill values that are comparable with
those of other
epoxide-type cryptophycins, viz Cryptophycins 1 and 52, whereas Cryptophycins
128 and
132 have T/C and gross log kill values that are comparable with those of other
chlorophycin-type cryptophycins, viz Cryptophycins 8 and 55.
Although the foregoing invention has been described in some detail by way of
illustration and example for purposes of clarity and understanding, it will be
apparent to
those of ordinary skill in the art in light of the teaching of this invention
that certain
changes and modifications may be made thereto without departing from the
spirit or scope
of the claims.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-156-
Table 1. Cytotoxicity data for cryptophycins and semi-synthetic analogs.
Corbett/Valeriote assay
data for 5-fluorouracil, etoposide (VP-16) and tazol are included for
comparison.
Type of Cytotozicity (Differential in Zone Units)
Corbett Valeriote KB IC50 LoVo IC50
Compound g/disk Assay' g/disk Assayb ng/mL ng/mL
1 12.5 E/T( > 400)' N 0.005 0.003
2 25 E/T( > 400) N 0.007 0.0002
3 25 E/T( > 400) N 0.3 0.5
4 20 E/T( > 400) N 1.3 0.5
2.9 E/T( > 600) N 0.02 0.02
6 250 I a100 a100
7 z750 a480
8 30 E/T( > 500) 30 N 0.0002 0.01
9 15 Not
Deter-
mined
a100 a100
12 Z100 z100
14 1.8 3
5-FU 2.5 M/T( > 400) 2.5 LL( > 400)
VP-16 5 L(350),T(530)d 5 LL(260)
tazol 0.2 M/H/T(z400)
'L= leukemia selective (e.g. ZLt2IO-7c3s and ZLUW - Zxa>-250ZU)
M= murine solid tumor selective (e.g. ZC38 - ZLI210 a250 zu)
H= human solid selective (e.g. ZH: - ZLi2io OZ0 ZU)
E= equally cytotozic towards leukemia and solid tumor cell lines (inhibition
zones
2250 zu)
T= tllmor selective (e.g. ZL1210 -ZLMLsZC38 -ZLMLs and ZE{s - ZLvt 2250 Zll
I= inactive (inhibition zones <250)
6N= non-selective towards tumor (leukemia) and normal cell (CFU-GM) lines
LL= lymphocytic leukemia selective (ZLUiQ - ZCSJ-GM i250 Zu)
ML= acute myelogenous leukemia (AML) selective (ZAõL - ZcRj.GM a250 zu).
Selective against drug-sensitive and drug-resistant cell 'lines (ZC38 - ZLML,Z
17 -
ZLmL andZEõ-ZLm}.
Selective against drug-sensitive cxll lines only.
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-157-
Table 2. In Vitro Cytotoxicity Data of Cryptophycins
Cryptophycin KBIC50 LoVoIC50 SKOV3IC50
ng/mL ng/mL ng/mL
1 0.0025 0.001 0.026
2 0.023 0.021 0.18
3 1.8 0.6 2.8
4 6 2.5 21
12 2 7.4
8 0.01 0.0022 0.15
12 18 3
12
16 0.08 0.02 0.64
17 4.7 5.9 11
18 15 4.5 23
19 9.8 5.9 41
21 0.01 0.0003 0.029
23 0.89 0.4 1.7
24 0.12 0.095 0.3
26 19 9.8 95
28 1.5 0.75 6.1
29 1 0.49 3.4
30 11 8 21
31 0.53 0.062 1.9
35 0.055 0.01 0.092
40 9.0 1.0 1.7
43 0.72 0.8 1.1
45 2.3 2.4 1.6
49 1.4 1.9 1.1
50 0.17 0.17 0.2
54 0.80 2.2 2.2
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-158-
Table 3. In Vivo Activity of Cryptophycin I
mgfkg % Body
#. of Inj. Total . . Wt.
Exp # SC :Tumor : IV Dose Lossat T%C Log Cure
Nadir Kill s
1560 Colon 38 8 10.3 Gain 6% 1.5 0/5
1694 Panc 03 8 16.0 Gain 0% 2.0 0/5
1636 Colon 51 7 28.1 -11% 7% 1.3 0/5
1720 Mam 16/C 5 13.2 -1% 5% 1.4 0/5
1733 Mam 16/Taxol 5 16.5 0% 2% 1.8 0/4
1833 M17/0 (Adr. 5 5.4 -10% 23% <1 0/5
Sens.)
1749 Panc 02 5 11.0 -5% 20% 1.1 0/5
1596 Human Sm Cell 6 7.3 0% 27% < 1 0/5
L.
DMS273
SCID
1806 MX-1 Human 8 12 -3% 3% 2.0 0/5
Breast
1823 H125 Human 8 14.4 -15% 9% 1.1 0/5
Adenosq-lung 1/5 dead
1841 LNCaP Human 6 6.5 -6% 26% <1 0/5
Prostate
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-159-
Table 4. In Vivo Activity of Cryptophycin Analogs
~o ..
#Of,
Inj. mgllt'..,Body:.,
:Exp Agent ;:SC IV g Tll:: :Wt. 'T/C ' L+vg Cure
# 7Ytmo I:ass . . I~ill s; ,
r rtt:;
Nadir
1813 Cryptophycin-2 P03 10 37 -2% 44% <1 0/5
1843 Cryptophycin-3 P03 4 28/5 -9% 54% <1 0/5
1769 Cryptophycin-5 C38 15 45 -2% >10 None 0/5
0%
1825 Cryptophycin-8 P03 11 106 -6% 4% 4.6 0/5
1885 Cryptophycin-8 Mam 7 21.3 -4.5% 6% 2.5 0/5
16/C
1887 Cryptophycin-8 C38 6 30 -2% 0% 2.8 1/5
B
1900 Cryptophycin-8 Colon 9 67.5 -1% 7% 1.8 0/5
51
1843 Cryptophycin-15 P03 5 18 -7% 83% None 0/5
1878 Cryptophycin-16 P03 9 82 -1% 89% None 0/5
1813 Cryptophycin-21 P03 9 27 -11% 61% None 0/5
(1/5
dead)
= 1843 Cryptophycin-35 P03 7 23 -2% 11 k 1.3 0/5
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-160-
Table 5. Cytotoxicities of antimitotic agents for SKOV3 and SKVLB1 cells
Cells were treated with varying concentrations of the compounds indicated
below
for 48 hours. Cell numbers were then determined as indicated in the Methods
section and
the IC50 for each compound was calculated. Values represent the mean SEM for
three
experiments.
Cell Line '
Compound SKOV3 SKVLB1 Resistance Factor
IC50 (nM)
Vinblastine 0.35 0.25 4200 1700 12,000
Taxol 1 0.4 8000 2000 8,000
Cryptophycins 0.007 f 0.002 0.60 f 0.19 86
CA 02214565 1997-09-03
WO 96/40184 PCTIUS96/03246
-161-
Tabie S. lN Vlb CyMxkfly Dat o[CYyptoplq-t3os
Sp LOVO
icso
nM nM
1 0.0092 0.0104
3 3.1-4.6 1 Si-S.8
8 0.019 0.0091
51 3.5 5.2
52 0.043 0.070
53 42 5.5
33 0.033 0.047
57 0.064 0.27
58 0 048 0.20
61 21 15
81 5.5 5.7
82 310 45
90 0.34 0.30
91 30 34
97 3.6 5.7
110 4.6 5.3
111 4.8 3.2
112 5.4 6.2
115 0ACi9 0.048
117 0.036 0.031
118 2.3 1.2
119 4.4 6.1
120 4.6 6d
122 0m1 69
1?S 0.05 0.006
127 0.176 0.075
128 0.023 2.S
132 0.082 0.037
IN VIVO ACTIVITY OF CRYPTOPHYCIN-52
SUMMARY
A =
-r
00
.. ... . : , s Log,o Tumor Day
Totei Ooie .. ~r of YNt Loss~. TIC Pertlel . '' Complete';, Turtwr Ce9. Free
Tumor
Exp # SC Tumor mglkg inJ. ot NadEr IMr 96= Regressions : Regresaiorn- KiN
Cikes Free Actlvtty
Human Tumors
2069* H116 Colon 30 4 -6.3% 4.1 215 0l5 2.4 015 84 +++
(Upstagedl
* LNCeP Prostate 48 8 -4.896 6.9 5/5 115 3.2 0l5 66 + + + + (Upstaged)
2072
* OlbiPanc 48 9 -2.396 16 - - None 0f5 68 - >
2076
N>1~',
2087* H125 Lung In)ectlons in pru9reas ~
Mouse Tumors N
2063 Mem 171Adr 24 12 -4.796 10 - - 1.6 0I5 18 ++
2065 Colon 261A 64 10 -14.0% 14 - - 1.1 015 24 +
2068* Colon 38 55 8 =2296 0 1/5 1l5 1.6 0l5 95 + + (Upategedl
2080 e Mem 161C 30 3 -1.6% 5 - - 1.2 1 f5 59 + +
2082 Mam 16/ClAdr 36 4 0% 2 - - 2 015 22 +++
2088* Penc 02 Iniecttona In progreu
~ In Progress rA
OTable 7
1 .
IN.VlVO ACTIVITY OF CRYPTOPHYCIN-55
SUMMARY
.% Body . Lopto : Turrwr Day Total Dose A~ of Wt. Loss TIC Parttal Complete.
Tumor Celt Free : Tumor
Exp X SC Tumor mg/kg inj. at Nadir fn 96 Regressions Regressions Kill, Cures
Free Activity
Human Tumors
2066 =' TSU Prostate 298.5 10 =8.1 % 0 515 515 4.0 216 d 94 + + + +
(Upstaged)
2069" H 116 Colon 315 * 7 -10.4% 0 4/5 3/5 6.3 215 d 84 ++++
& (Upstagedl 157 5 7 =7.6% 8 4/5 0/5 3.6 Ol5 d 84 ++++
2072" LNCaP Prostete 300 12 .3.1% 0 515 515 5.9 315 d 77 ++++ y
I (Upsteged) 200 5 =7.7% 0 4/5 315 6.0 0/6 d 77 ++++
N
2075'* Gbb Panc 320 8 0.0% 4.4 - - >2.0 1/5 d 68 + + + bi
2087 H125 Lung Injections In progress
rn
Mouse Tumors
2064"' Mem 161C 144 10 =7.596 0 - - 3.9 015 d 92 ++++
2065 Colon 26 260 10 =296 0 - - 1.2 015 d 24 +
2068 Colon 38 237 8 .13% 0 515 415 4.7 015 d 95 + + + + (Upstaged)
2070" Peno 03 402 6 =6.0% 0 - - > 4.5 5!5 d 84 ++++
270 6 =5.0% 0 - - >4.5 4/5 d 84 + + + +
~ { 1 180 4 Ø8% 0 -- - >4.5 4/5 d 84 ++++
2071 Mam 17/Adr 180 6 =4.7% 0 - - 2.1 0/5 d 18 +++
2082 Mam 161ClAdt 180 6 =1.7% 0 - - 2.4 015 d 22 +++ w
2088 Panc 02 injections in progress o
~ Produced 1/5 drug deaths
** Exp. In Progress Table 8
0
Evaluation of Cryptophycin Analogs Against
Early Stage Panc 03 in BDFI Male Mice
Total % Body Median Tumor Tumor
No. of Dose Drug Wt. Loss Size on day 14 T/C Free
Cage Agent Inj. IV mg/kg Deaths or Gain (day) (Range) In % Day 14
C 1 No Rx -- -- -- +6.8% (12) 830 (393-1458) -- 0/5
m 2 Crypto #117 1 7.0 3/4 -20.% (12) 0 (one mouse) 0% 1/4
3 1 6 13.5 1/4 -8.2 (13) 75 (0-256) 9% 1/4
4 1 6 6.75 0/3 -2.4 (8) 247 (80-415) 30% 0/3
M 5 Crypto #125 7 42 0/4 0% (10) 0(0-63) 0% 3/4 6 1 7 21 0/4 -1.6% (10) 101 (0-
126) 12% 1/4
tA 7 1 7 10.5 0/4 0% (8) 295 (264-467) 35% 0/4
m
tt1 8 Crypto #128 3 57 1/4 -15.2% (10) 0 (all zero) 0% 3/4
9 1 4 32.5 0/4 -5.7 %(6) 0 (all zero) 0% 4/4
7t~ Ll 1 4 16.25 0/4 0% (12) 0 (all zero) 0% 4/4
C
~ Crypto #132 6 180 0/4 -5.7% (10) 0 (all zero) 0% 4/4
1 6 90 0/4 -3.3 %(8) 0(all zero) 0% 4/4
~ 1 6 45 0/4 1.7% (10) 304 (108-505) 37% 0/4
Tumors implanted day 0; Drug treatments started day 3.
As of Day 14 all the mice appear to be in good condition and gaining weight.
There should be no more drug deaths.
Table 9
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
=165-
/N V/Vp ACTiVtTY OF CRYPTOPHYCIN-8
{R~~
t~ii Mna 03 11 106 -a 4 4.8 t1V5
7917 rana 03 12 QO .1 0 2.8 OJS
188iA Mate 16/C 7 213 -4.6 a 2.5 O/S
18878 Colen 88 a 80 +2 0 3.0 0!5
1954 Coloq 38 11 132 .8 0 >4.5 5/3
+ 4 11 s8 .6 0 >4.5 415
1lQS Co1on 2B 10 100 .6.4 0 23 OJS
2007 Ovsry-135 6 50 3.5 0 >2 215 Id 20)
8 60 O >2 113 (d 20)
18K Aiiaat 17/Adr 8 53.1 -0.8 0 3.2 O!8
! 36.9 =1.8 .0 2.6 0!5
1!!0 Mun 161C/Adr 7 iS .6 O 2.5 015
=001 rana 02 9 126 =3.5 0 >2 'Ub (d25)
1921 9 67.5 -6 18 1.8 015
1974 IN P388 L.ru1c 7 81 -1116 5096 8.8 2.3 015
1924 Colon 51 9 67.5 .6 7 2.2 015
1848 11pC-1 t=lttnan 11 50 .6.9 0 >4.5 315
4 Bsasst 11 34.2 -4.5 0 4.5 1J5
1852 K125 tkiman 8 98 +3.8 0 1.8 015
i Adeno. Sq. Isnv 8 57.6 +9.8 0 1.6 Ol5
1883 TSU Muinan 7 84.5 -3.4 0 >4 515
Praststa 7 66.5 -3.3 0 3.6. O15
1878 tCa Hwnn 8 96 +3.1 7.3 1.6 016
rrestsb 8 67.2 -F 3.Q 14.7 0.9 015
l880 Adv. itsoa 8 108 -6.7 E!5 PR's 3.8 015
iitsman H1i6 8 76 .6.7 .415 Mt's 3.5 0/5
Cdon 8 53 -39 1!s PB's 2.9 0/5
1278 Adv. Stsea 6 Q1 3.2 515 CR's 8.10 4/5
LKCsP Hunun 6 67 =1.4 5l3 CH's 7.10 4/5
Proststs 6 40.2 3.2 515 CR's 8.00 215
Llailted numbers ot tuenor prowths on wblch to carry out log WA calculation.
.wa~..
Table 10
CA 02214565 1997-09-03
WO 96/40184 PCT/US96/03246
-166-
References
1. Eglof, G., Organic Chemistry: An Advanced Treatise, Gilmar et al. (ed.),
pp. 31-46, John Wiley & Sons (1943).
2. Kemp, et al., Organic Chemistrv, Worth Publishers, Inc. (1980). 5 3.
Patterson, G. M. L. et al., J. Phycol. 27:530-536 (1991).
4. Corbett, T.H. et al., Cytotoxic Anticancer Drugs: Models and Concepts for
Drug Discovery and Development, pp 35-87, Kluwer Academic
Publishers: Norwell, 1992.
5. Valeriote, F.A. et al., Discovery and Development of Anticancer Agents,
Kiuwer Academic Publishers: Norwell, 1993; in press.
6. Schwartz, R.E. et al., J. Ind. Microbiol. 5:113-124 (1990).
7. Hirsch, C.F. et al., U.S. Patent 4,946,835, issued August 7, 1990.
8. Sesin, D.F., U.S. Patent 4,845,085, issued July 4, 1989.
9. Sesin, D.F. and Liesch, J.M., U.S. Patent 4,868,208, issued September
19, 1989.
10. Sesin, D.F., U.S. Patent 4,845,086, issued July 4, 1989.
11. Skehan, P. et al., J. Natl. Cancer Inst. 82:1107-1112 (1990).
12. Bradley, G. et al., Cancer Res. 49:2790-2796 (1989).
13. Endicott, J.A. et al., Ann. Rev. Biochem. 58:137-171 (1989).
14. Beck, W.T., Biochem. Pharm. 36:2879-2887 (1987).
15. Moscow, J.A. et al., J. Natl. Cancer Inst. 80:14-20 (1988).
16. Trimurtulu, G. et al., J. Am. Chem. Soc. 116:4729-4737 (1994).
17. Smith, C.D. et al., Cancer Res. 54:3779-3784 (1994).