Note: Descriptions are shown in the official language in which they were submitted.
CA 02215110 1997-09-11
,° ~ ~,~ ~,'~
Description
ZINC AND ZINC-ALLOY HOT-DIP-COATED STEEL SHEET HAVING
DECREASED BARE SPOTS AND EXCELLENT COATING ADHESION AND A
METHOD FOR MANUFACTURING THE SAME
Technical Field
The present invention relates to a zinc and zinc-
alloy hot-dip-coated steel sheet having a decreased number
of bare spots and excellent coating adhesion, and a method
for manufacturing the same.
Backqround Art
Zinc and zinc-alloy hot-dip-coated steel sheets are
mainly used for automobile bodies because of low cost and
excellent corrosion resistance, and in addition to the
corrosion resistance due to coating, coating adhesion
- during press working is required for applying the steel
sheets to automobile bodies. When coating adhesion
deteriorates, coated layers peel as a powder or blocks,
which phenomenon sometimes causes galling in press forming
or deteriorates corrosion resistance of the portions from
which the coated layer peels; and also, peeled fragments
disadvantageously inflict the steel sheet.
As a conventional technique for improving coating
adhesion, Japanese Patent Laid-Open No. 61-276961
2
CA 02215110 2001-O1-15
73461-79
discloses a technique in which alloying Fe with Zn at a
high temperature ranging from 700 to 850°C is performed
after zinc hot-dip-coating. However, alloying at a high
temperature leads to not only higher costs but also
increased expenses for equipment such as rolls.
Additionally, in Japanese Patent Laid-Open No. 3-
232926, steel containsiat least one of Zr, La, Ce, Y, and
Ca, and the cooling rate from recrystallization annealing
to coating is set to not less than 50°C/sec. The cost is
10' raised due to the addition of Zr or the like to steel and
productivity deteriorates because the sheet-feeding rate
has to be lowered due to the cooling capacity.
Furthermore, in Japanese Patent Laid-Open No. 2-
163356, the O, A1, and N contents in steel are set to not
more than 0.0045 wt~, (25 x N wt$) to 0.15 wt~, and not
more than 0.0030, respectively. Moreover, restrictions
on the Ti, Si, and P contents, and Si (wt~) + p (wt~) >- Ti
(wt~) must be satisfied according to Japanese Patent Laid-
Open No. 6-81101. Anyway, the desired steel-sheet
20 properties such as strength and drawing cannot be always
achieved by such content restrictions, and there is a
possibility that coating adhesion will deteriorate because
of deviations from a predetermined composition range.
In Japanese Patent Laid-Open No. 4-333552, coating
adhesion is improved by carrying out Ni pre-plating before
galvanizing. However, in general, a continuous
3
CA 02215110 1997-09-11
galvanizing line (hereinafter referred to as "CGL") does
not have such equipment, and a large investment is
required for improving equipment or the like.
Meanwhile, automobile bodies are required to be
lighter because of recent regulations for exhaust gas.
Thinning the steel sheets is a method for lightening the
automobile bodies. According to this method, it is
necessary for ensuring safety to increase steel-sheet
strength corresponding to the decreased thickness. Thus,
high tensile-strength steel sheets have been developed for
strengthening the steel sheets by increasing the steel
contents of elements such as Si, Mn, and P. Since steel
sheets for automobiles are subjected to press forming,
excellent material characteristics with a high r-value
(high Lankford value) are required, and in particular, the
addition of these elements is essential for high-tensile
- strength steel sheets.
In the case of zinc hot-dip-coating such steel
sheets, recrystallization annealing at a high temperature
ranging from approximately 700 to 900°C is necessary to
attain excellent material characteristics. In the CGL,
recrystallization annealing is generally carried out under
a nitrogen atmosphere in the presence of hydrogen
(hereinafter referred to as reduction annealing), and
although this atmosphere is a reducing atmosphere for Fe,
4
CA 02215110 2001-O1-15
73461-79
it is an oxidizing atmosphere for some elements such as Si, Mn,
and P. Thus, elements such as Si, Mn, and P (referred to as
readily oxidizable elements) which are more oxidizable than Fe
externally diffuse during reduction annealing and bond to
oxygen on the surface of steel sheets to form oxides (called as
"surface segregated layer"). Since these oxides significantly
impede wettability between molten zinc and the steel sheets,
so-called bare spots, i.e., defects occurring when zinc does
not adhere to the steel sheets, are seen.
For overcoming such problems, Japanese Patent
Examined Publication (Kokoku) No. 61-9386 published on March
22, 1986 (corresponding to Japanese Patent Laid-Open (Kokai)
No. 57-76176; Inventors, S. Higuchi et al; Applicant Nippon
Steel Corporation) prc>poses a method of pre-plating the surface
of steel sheets with Ni before the zinc hot-dip-coating
process. However, according to this method, when steel
contains at least Si and one more element among 0.2 to 2.0 wt%
of Si, 0.5 to 2.0 wto of Mn, and 0.1 to 20 wt% of Cr, Ni
plating of not less than 10 g/mz is necessary, resulting in an
increased cost. In addition, although such a large quantity of
Ni plating improves the wettability between the zinc hot-dip
coating and the steel sheet, disadvantageously, defects caused
by Si and Ni on the coated surface frequently appear during the
alloying process.
Furthermore, for example, Japanese Patent Laid-Open
No. 57-70268 proposes a method of pre-plating the surface of
steel sheets with Fe before the zinc hot-dip-coating
5
CA 02215110 1997-09-11
process. According to this method, bare spots in Si-
containing steel are preventable by pre-plating, however,
not less than 5 g/mZ of Fe plating is required, which fact
is extremely uneconomical.
Additionally, other methods are disclosed in Japanese
Patent Laid-Open Nos. 55-122865 and 4-254531. In these
methods, steel sheets are oxidized beforehand to form a Fe
oxide film on their surface and then subjected to
reduction annealing. However, according to these methods,
alloy elements such as Si are segregated on the surface to
form an oxide film because of excess reducing during
reduction annealing, causing a problem of inferior
coating. For preventing such excess reducing, a large
amount of Fe oxide is necessary. However, if the amount
of Fe oxide film is exceedingly large, the Fe oxide film
peels due to rolling or the like, thus on the contrary, a
surface segregated layer is produced and results in
impeded coating or adverse effects on operation because
the fragments of the peeled Fe oxide-film are scattered
inside a furnace.
In addition, concerning known proposals for the steel
composition and hot-rolling conditions for zinc hot-dip-
coating the high-tensile steel sheets, Japanese Patent
Laid-Open No. 6-158172 discloses a method in which a steel
containing Si<_0.2 and Mn<_1.5 by wt~ is wound at a
temperature not less than 650°C followed by acid washing,
6
CA 02215110 2001-O1-15
73461-79
cold-rolling, annealing,, and zinc hot-dip-coating; and Japanese
Patent Laid-Open No. 6-1'79943 discloses a method in which a
steel containing 0.10 to 1.5 wto of Si and 1.00 to 3.5 wt% of
Mn is wound at a temperature ranging from 500°C to 680°C,
both
inclusive, followed by acid washing, cold-rolling, annealing,
and zinc hot-dip-coating.
Although these methods give specified processing
conditions, such as the steel composition and hot-rolling
conditions, for a serie~~ of manufacturing steps, they cannot
suppress the surface segregated layer formed during reduction
annealing or improve bare spots or coating adhesion.
Disclosure of the Invention
As a result of. detailed experiments, the inventors of
the present invention have found that bare spots and coating
adhesion are remarkably improved by providing oxides of readily
oxidizable elements just: under a coated layer of a zinc and
zinc-alloy hot-dip-coated steel sheet. Although the expression
"a zinc and zinc-alloy hot-dip-coated steel sheet" is employed
in this specification, "a zinc or zinc-alloy hot-dip-coated
steel sheet" would be more accurate.
In one aspect, the present invention provides a zinc
or zinc-alloy hot-dip-coated steel sheet having oxides of
readily oxidizable elements just under a coated layer, i.e.
inside of and near a surf=ace of a steel sheet substrate.
Moreover, in t:he zinc or zinc-alloy hot-dip-coated
steel sheet, the oxygen concentration is preferably not less
than 1 ppm, more preferably, 2 to 200 ppm, and further more
preferably, 3 to 100 ppm, in a region from
7
CA 02215110 2001-O1-15
73461-79
the surface layer of a steel-sheet substrate just under
the coated layer to 3 E.cm deep in the sheet-thickness
direction.
In addition, such hot-dip-coated steel sheets are
preferably further subjected to heat-alloying after zinc
hot-dip-coating, and excellent alloyed zinc and zinc-alloy
hot-dip-coated steel sheets are thereby obtained. Also in
the alloyed zinc and z_i.nc-alloy hot-dip-coated steel
sheets, the oxygen concentration is preferably not less
than 1 ppm, more preferably, 2 to 200 ppm, and further
more preferably, 3 to :L00 ppm, in a region of from the
surface layer of a steel-sheet substrate just under the
coated layer to 3 um dE~ep in the sheet-thickness
direction.
Furthermore, each of the zinc and zinc-alloy hot-dip-
coated steel sheets and alloyed zinc and zinc-alloy hot-
dip-coated steel sheets preferably contains at least
one element selected from the group consisting of Si, Mn,
and P as a steel component in the following ranges:
0.001 <_ Si <_ 3.0 wt~,
0.05 <_ Mn _< 2.0 Wt~, and
0.005 <_ P _< 0.2 Wt~,
Additionally, the present invention provides a method
for producing the above-mentioned zinc and zinc-alloy hot-
dip-coated steel sheet; or the alloyed zinc-alloy hot-dip-
coated steel sheets both of which show a decreased number
8
CA 02215110 2001-O1-15
73461-79
of bare spots and excellent coating adhesion. In other
words, the present invention provides a method having:
a step A for forming oxides just under the scale,
which oxides are formed from elements more oxidizable than
iron, by setting a temperature of a steel strip to not
less than 600°C and setting the mean slow-cooling rate up
to 540°C to not more than (CT - 540)°'9 . 40 (°C/min)
during coiling the steel strip hot-rolled; and
a step B for zinc and zinc-alloy hot-dip-coating the
steel strip. Accordincr to this method, the step B follows
the step A, and other steps may be also employed between
the steps A and B. In general, steps of pickling,
degreasing, cold roll.illg, annealing,. and the like may
appropriately be used as such intermediate steps.
In addition, according to a method of the present
invention, the oxides formed in the step A preferably
remain after a pre-treatment step carried out after the
step A until treatment conducted in an annealing furnace
immediately before the step B.
Furthermore, according to these~methods, a slab
subjected to hot rolling preferably contains at least one
element selected from t:he group consisting of Si, Mn, and
P as a steel component. in the following ranges:
0.001 <_ Si <_ 3.0 Wt~
0.05 <_ Mn <_ 2.0 Wt~
0.005 <_ P <_ 0.2 Wt~
9
CA 02215110 1997-09-11
Moreover, according to any of the above-mentioned
methods, an alloyed zinc and zinc-alloy hot-dip-coated
steel sheet can be produced by employing heat-alloying
treatment after the step B.
Next, oxides of readily oxidizable elements
positioned just under a coated layer will be explained.
These oxides of readily oxidizable elements are
formed during hot-rolling, in particular, the oxides are
grown when the temperature (hereinafter referred to as
"CT") during coiling is high and the cooling rate after
coiling is low.
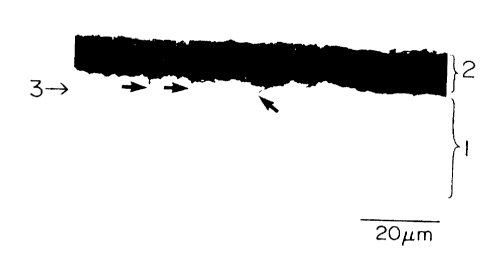
The oxides formed during hot-rolling are observed
just under the scale, as is shown in figure 6. Meanwhile,
in a conventional hot-rolled sheet, no oxide is observed
just under the scale, as is shown in figure 7. The oxides
observed during hot-rolling are analyzed by using an
' electron probe microanalyzer (hereinafter referred to as
"EPMA") and the results are shown in figure 1. Since Mn,
P, A1, and 0 show peaks, it is understood that oxides of
these elements are formed. Steel sheets shown in figures
6 and 1 contain 0.1 wt~ of Mn, 0.006 wt~ of P, and 0.03
wto of A1, and they do not contain a particularly large
amount of Mn, P, or A1.
The oxides positioned just under a coated layer of a
zinc hot-dip-coated steel sheet or an alloyed zinc hot-
CA 02215110 1997-09-11
dip-coated steel sheet of the present invention are
produced such that oxides formed just under the scale
during the hot-rolling process remain even after post-
treatment steps such as pickling and coating.
The mechanism of producing oxides just under the
scale is as follows: oxygen in a scale layer essentially
consisting of iron oxide which has been formed during hot-
rolling internally diffuses into steel during or after the
coiling process, and then, forms an oxide of a readily
oxidizable element in the steel. Therefore, oxides are
produced even when only a trace amount of readily
oxidizable elements is contained in the steel.
Although oxides of elements more oxidizable than iron
exist just under the zinc and zinc-alloy hot-dip-coating
according to the present invention, an oxide of an element
less oxidizable than iron oxide or iron may also be
contained. In addition, such an oxide is preferably
' formed in grain boundaries of a hot-rolled steel sheet.
As a result of studies and investigations conducted
on various types of steel sheets, the inventors of the
present invention have found oxides of Si-0, Mn-0, A1-0,
P-0, and Fe-Si-0 in the steel sheets.
Figure 2 shows the result of elemental analysis of a
conventional steel sheet and figure 3 shows that of an
unannealed cold-rolled steel sheet wherein oxides were
observed, which analysis was carried out in a region of
11
CA 02215110 1997-09-11
from the surface of each steel sheet to approximately 10
~m in the depth direction by glow-discharge spectroscopy
(hereinafter referred to as "GDS"). The peaks of Mn, A1,
P, and 0 observed at the depth of approximately 0.3 to 4
~m from the surface layer correspond to the oxides.
Figure 4 shows the result of elemental analysis of a
conventional steel sheet and figure 5 shows that of an
annealed cold-rolled steel sheet wherein oxides were
observed, which analysis was carried out by GDS in a
region of from the surface of each steel sheet to
approximately 10 um in the depth direction. A large
amount of surface segregated substances generated by
reduction annealing is observed in the conventional steel
sheet of figure 4, meanwhile the generation of surface
segregation products is suppressed and hardly observed in
the steel sheet with oxides produced during hot-rolling.
Next, oxides of the present invention which exist in
a surface layer of a steel sheet (surface layer of a
steel-sheet substrate)'just under a coated layer can
optical-microscopically be observed by etching the steel
sheet with a 1~ nital solution for several to several
dozen of seconds.
Figure 8 (photograph) and figure 9 (photograph) show
a conventional alloyed zinc hot-dip-coated steel sheet not
containing oxide and an alloyed zinc hot-dip-coated steel
sheet containing oxides incorporated in the present
12
CA 02215110 1997-09-11
invention, respectively. Figures 8 and 9 are cross-
sectional optical micrographs of alloyed zinc hot-dip-
coated steel sheets taken at a magnification of x1,000.
Black ribbon-like materials observed just under the coated
layer are oxides (shown by arrows).
In addition, the formation of oxides can also be
confirmed by analyzing oxygen contained in steel.
Concerning technique, oxygen in steel is analyzed in the
total sheet-thickness direction using a hot-rolled steel
sheet whose scale layer has been removed by pickling after
coiling, a steel sheet obtained by dissolving only a
coated layer of a zinc and zinc-alloy hot-dip-coated steel
sheet, an unannealed cold-rolled steel sheet, or an
annealed steel sheet, and the resulting values are
compared with those of steel sheets obtained by grinding
the surface layer in which oxides are formed. The steel
sheets in which oxides are formed have larger oxygen
values analyzed in the total sheet-thickness direction as
compared with those of',the ground sheets.
Next, the mechanism of improving bare spots and
coating adhesion by forming oxides just under a coated
layer will be investigated.
First, concerning improvement in bare spots, it was
found that surface segregation of readily oxidizable
elements is suppressed during reduction annealing in the
13
CA 02215110 1997-09-11
CGL when oxides are produced just under the scale by
internal oxygen diffusion during or after coiling, as is
above-mentioned.
This phenomenon is assumed to be due to following:
the amount of readily oxidizable elements in the surface
layer decreases because the readily oxidizable elements
already precipitate as oxides during or after coiling; the
formed oxides impede transfer (external diffusion) of the
readily oxidizable elements from bulk steel to the steel
sheet surface; and oxidation-reduction occurs inside the
steel sheet, in other words, a Fe-containing oxide
produced during or after coiling changes to an oxide of
readily oxidizable element during reduction annealing.
Therefore, the surface segregated substances of the
readily oxidizable elements, which substances impede
wettability between molten zinc and the steel sheet,
extremely decrease, thereby remarkably improving bare
spots.
Next, coating adhesion will be explained.
It has been known that coating peels mainly due to
compressive stress during press forming.
Since a steel sheet having oxides just under a coated
layer, i. e. a steel sheet of the present invention, has
spaces between oxide crystals, zinc more readily
penetrates into the steel sheet as compared with
14
CA 02215110 1997-09-11
conventional steel sheets not containing oxides. As a
result, the interface between the coated layer and the
steel sheet is significantly roughened so that the coated
layer can tightly adhere to the steel sheet. As a result,
a zinc hot-dip-coated steel sheet and an alloyed zinc hot-
dip-coated steel sheet both incorporated in the present
invention acquire excellent coating adhesion during press
forming.
Figures 10 and 11'show the observation results
obtained from a steel sheet using a SEM, a coated layer of
which steel sheet has been forcibly dissolved to the iron
potential according to a galvanostatic process (4% methyl
salicylate, 1% salicylic acid, and 10% potassium
iodide/methanol solution; 5 mA/cmZ) so as to expose. the
steel sheet. It is understood that the interface between
the coated layer and the steel sheet is apparently more
roughened as compared with the conventional steel sheet
- not containing oxides.
In addition, the technique disclosed by the present
invention exhibits more excellent effects when a steel
sheet contains at least one component selected from the
group consisting of Si, Mn, and P as a steel component in
the following ranges:
0.001 <_ Si <_ 3.0 Wt%
0.05 <_ Mn _< 2.0 Wt%
CA 02215110 1997-09-11
0.005 <_ P <_ 0.2 Wt%
Problems such as bare spots and decreased coating adhesion
hardly occur in steel sheets not containing the above
elements, thus the lower limits for these elements are
preferably 0.001 wt% for Si, 0.05 wt% for Mn, and 0.005
wt% for P. Meanwhile, the upper limit for each element is
determined considering the preferable ranges for both the
maximum effect for strengthening and cost.
Furthermore, the technique disclosed by the present
invention exhibits sufficient effects on both bare spots
and coating adhesion when even a small amount of oxides is
observed by an optical microscope in a cross-section of a
zinc and zinc-alloy hot-dip-coated steel sheet etched by
1% nital.
In addition, according to an oxygen analysis of
steel, sufficient effects are shown, particularly on bare
spots and coating adhesion when the value of the following
formula is not less than 1 ppm:
(oxygen in a steel sheet whose coating has been
removed by a hydrochloric acid~antimony method) - (oxygen
in a steel sheet whose coating has been removed by a
hydrochloric acid~antimony method and whose surface layer
is then ground to remove 3 um thereof)
Next, a technique for manufacturing the above-
16
CA 02215110 1997-09-11
described coated steel sheets will be disclosed. It is
required that the temperature for coiling after hot-
rolling be high and cooling after the coiling be slow, and
a detailed explanation will be given below.
The temperature for coiling after hot-rolling must be
600°C or more to produce oxides and the cooling rate up to
540°C after coiling must be not more than the following:
(CT - 540)°'9 . 40 (°C/min)
Oxides are not formed at not more than 540°C even when
slow-cooling is further carried out.
In addition, although pickling and/or grinding is
generally carried out to remove the scale before coating,
and sometimes, equipment for electrolytic degreasing or
pickling is also provided for the CGL inlet side, the
oxides produced in the surface layer of a steel sheet
during or after coiling in the hot-rolling process must
remain after the above treatment.
- Zinc and zinc-alloy hot-dip-coating of the present
invention is a general term for molten zinc containing
zinc and may include not only zinc hot-dip-coating but
also galfan and galvalume, in both of which Si is
contained in zinc. Moreover, Pb, Mg, Mn, etc. may be
further contained. Therefore, conditions for a zinc bath
are not particularly restricted.
Other conditions for the coated layer are not
particularly limited, however, considering corrosion
17
CA 02215110 1997-09-11
resistance and the like, the preferred amount of zinc and
zinc-alloy coating is approximately 25 to 90 g/mZ and the
preferred iron content in a coated layer in an alloyed
zinc hot-dip-coated steel sheet is 8 to 13 wt~.
Furthermore, both hot-rolled steel sheets and cold-
rolled steel sheets can be used as a material for coating.
Brief Description of Drawings
[Figure 1] An EPMA analysis chart of oxides observed just
under the scale during!hot-rolling.
[Figure 2] A graph showing the result of elemental
analysis of a conventional unannealed cold-rolled steel
sheet, which analysis was carried out by GDS from the
surface to approximately 10 um in the depth direction.
[Figure 3] A graph showing the result of elemental
analysis of an unannealed cold-rolled steel sheet of the
present invention, which analysis was carried out by GDS
from the surface to approximately 10 ~m in the depth
direction.
[Figure 4] A graph showing the result of elemental
analysis of a conventional annealed cold-rolled steel
sheet, which analysis was carried out by GDS from the
surface to approximately 10 um in the depth direction.
[Figure 5] A graph showing the result of elemental
analysis of an annealed cold-rolled steel sheet of the
present invention, which analysis was carried out by GDS
18
CA 02215110 1997-09-11
from the surface to approximately 10 ~m in the depth
direction.
[Figure 6] A cross-sectional optical micrograph, taken at
a magnification of x1,000, showing oxides positioned just
under the scale of a hot-rolled sheet of an example.
[Figure 7] A cross-sectional optical micrograph, taken at
a magnification of x1,000, showing oxides positioned just
under the scale of a conventional hot-rolled sheet.
[Figure 8] A cross-sectional optical micrograph, taken at
a magnification of x1,000, showing an example alloyed zinc
hot-dip-coated steel sheet containing oxides.
[Figure 9] A cross-sectional optical micrograph, taken at
a magnification of x1,000, showing a conventional alloyed
zinc hot-dip-coated steel sheet not containing oxides.
[Figure 10] A SEM photograph, taken at a magnification of
x1,500, showing an example steel sheet whose coated layer
has been dissolved.
[Figure 11] A SEM photograph, taken at a magnification of
x1,500, showing a conventional steel sheet whose coated
layer has been dissolved.
[Reference Numerals]
1, untreated steel-sheet portion; 2, scale; 3, oxides; 4,
a coated layer; and 5, oxides.
Best Mode for Carrying Out the Invention
19
CA 02215110 1997-09-11
An example of the present invention will be shown
below:
Each sample shown in Table 1 was melted by a
converter and formed into a slab by continuous casting.
Each of the resulting slabs was hot-rolled to 1.2 to 3.5
mm thick at a slab-heating temperature of 1150 to 1200°C,
and with a finishing temperature of 900 to 920°C, and a
coiling temperature and a cooling rate which are shown in
Table 2. After that, the resulting sheets were pickled
for 5 to 15 seconds at'80°C in an aqueous 5~ HC1 solution
to remove scale layers, and then, divided into two groups
one of which was directly subjected to the CGL and the
other was cold-rolled into 0.7 mm thick. Furthermore, in
the CGL inlet side, the following methods were also used
in combination as a pre-treatment for removing the surface
layer of a steel sheet, if required.
Electrolytic degreasing: electrolysis at 60°C in an
aqueous 3~ NaOH solution for approximately 10 seconds.
Pickling: pickling at 60°C in an aqueous 5~ HCl
solution for approximately 3 seconds.
Brushing roll: a'brushing roll with abrasive grains.
In the CGL, both the hot-rolled sheet and the cold-
rolled sheet were zinc hot-dip-coated at 470°C after
annealing at 800 to 850°C. In addition, alloyed zinc hot-
dip-coated steel sheets were obtained by successively
subjecting the annealed sheets to an alloying process
CA 02215110 1997-09-11
conducted at 480 to 530°C for 15 to 30 seconds.
o Evaluation method for oxide
Observation method for oxides in hot-rolled sheets
A cross section of each hot-rolled sheet with the
scale was ground and, without being etched, subjected to
optical-microscopic observation so as to measure the depth
of oxide invasion. The preferred magnification of the
optical microscopy was 1,000.
Quantitative evaluation of oxides in hot-rolled sheets
The following value was obtained:
(oxygen in steel analyzed in the total sheet-
thickness direction of a hot-rolled sheet whose scale has
been removed by pickling) - (oxygen in steel of a steel
sheet which has been ground to reach the depth of oxide
invention in the sheet-thickness direction after removing
the scale)
- Quantitative evaluation of oxides in coated sheets
Each of the coated sheets was immersed in the
solutions shown below until the end of the dissolving
reaction of coating and then the concentration of the
oxide-derived oxygen in a region of from the surface of
the steel sheet to 3 ~m in the sheet-thickness direction
was calculated according to the following formula:
(oxygen in a steel sheet whose coating was peeled by
a hydrochloric acid~antimony method) - (oxygen in a steel
21
CA 02215110 1997-09-11
sheet whose coating was peeled by a hydrochloric
acid~antimony method and whose surface layer was then
ground to remove 3 ~m thereof)
1% nital solution 1 vol% HN03-ethanol solution
hydrochloric acid~antimony method Sb203 (20 g) + 35%
HCl (1 1)
o Evaluation method for bare-spots
Each of the coated sheets was evaluated by
macroscopic observation.
Bare spots not observed: rank 1
a few were observed: rank 2
a small number were observed:
rank 3
observed: rank 4
o Test for coating-adhesion evaluation
Each of the coated sheets was subjected to a Dupont
impact test using a 1/2-inch punch and occurrence of
peeling was confirmed by macroscopic observation.
Peeling was notobserved: o
Peeling was observed: x
Each alloyed zinc alloy hot-dip-coated steel sheet
was bent to 90°, bent back, and then the compressed side
of the steel sheet was peeled by a tape so as to measure
22
CA 02215110 1997-09-11
the peeled amount of zinc by fluorescent X ray.
Count number of:
less than 500: rank 1
( good
not less than 500 to less than 1,000: rank 2
not less than 1,000 to less than 2,000: rank 3
not less than 2,000 to less than 3,000: rank 4
not less than 3,000 . rank 5
Table 3 shows the results of the zinc hot-dip-coated
steel sheet and Table 4 shows those of the alloyed zinc
hot-dip-coated steel sheets.
Table 1 Sample-Steel Composition
Symbol C S i M n P
wt~ wt~ wt~ wt~
A 0.105 0.010 0.08 0.008
B 0.070 0.10 0.10 0.01
C 0.070 0.50 2.0 0.07
D 0.010 1..50 0.10 0.05
E 0.003 0.003 0.05 0.005
F 0.003 0.01 0.20 0.01
G 0.003 0.30 0.50 0.04
H 0.003 0.05 1.95 0.20
23
CA 02215110 1997-09-11
Table 2 Coiling conditions, depth of oxide invasion into
hot-rolled sheet, and oxide amount in hot-rolled sheet
SampleCT Mean cooling Depth of Oxide amount Sample
steel C rate oxide in steel
to invasion hot-rolled No.
540C C/min into hot- sheet
rolled sheetppm
wm
A 540 1.0 0 0 1
A 600 1.0 1 1 2
A 600 1.5 0 <1 3
A 700 2.0 7 5 4
B 650 1.5 8 8 5
C 650 1.5 ' 6 7 6
D 580 1.0 0 0 7
D 620 1.2 <1 <1 8
E 650 1.2 5 5 9
E 650 1.6 <1 1 10
E 650 1.8 0 <1 11
F 650 1.0 10 11 12
G 650 1.0 12 15 13
H 600 1.8 0 0 14
H 650 1.0 12 18 15
24
CA 02215110 1997-09-11
%
~~
a N x O o o '~..0 0 o x o O o x o o x o
x
I
o d
i-I
G7
x
G
%
_ H
rn
O N .-a..-n.-wt -r .~N .~ .-n.-n..-nc'1.~ .~~? .a
%
N
H
G
O %
b ~ .d O .-n~ ~1O <'1r~N O ~ u1~a O ~ ,~O
a
O L
k p'
p
U
i~
.-1a
b
0
(C N
8 O O O O O O O O O O O O O O O O O
00 ~ .Y u1~t~w ~ I~ ~D~D u1 a' ~t1.T W ~t1u1u1 u1
G 00
U
U ',~
w
N
,~ a
'~ U
a
% .
,
D
O L1
~ G .~ .-n.-v,~p ~ - W .':~l1c~1 N N N N
~N it1 1
~ , . .-~ O
m
H ~ O O O Q ~ I O ~1 O ~ O O -. O O O O
~ ~ p
U %
!J
N
~
H
~
.,~
r-i
v
.J
G
H
P.
W G G
'G N m O m N O m m m d m m N N m N N
~
y G G ~O G G ~O G G G G G G G G G G G
-I ~ O O O
O
O O O O O O O O O O O O
U ~ 'O 27+~ T7b im C 27 T1 b 'O'O 'O b O 'O 'O
G G
a o b 2f
U x ~ ~ b b b b b b o ~ ro b b v b b ~ b
H N N N a1 N N N H N N N N N N H N
N D D D D ~ D D N ~ D D D D D N
TJ ~ ~ % H H H H H H H % H H H H H H % H
p alm N N N N N ~ N N N N a7 d p N
N '~ O % % % % % % % O % % % % % % O %
(~ b ~ p p l~f.1 ~1 J7.d p p p f1 p p .a
I
.- L O O O O O O O V O O O O O O 1~ O
x o 0 0
O C G p
~ N
"~
G
.~ N
N
b N
.-i
1~
~
.
Q %
df
P. ~ N N ~?~T vT h O I~ N O~~ ~ N
~ G i ,. .H.-1 .-i
.~
.-1
H H I
H
j ~
O
~
z
M C D D .-rN M ',7 ~~ f1~O D fv 00T '> O '~'
..v N W t"1 J V1
c0 .i a .,a .,~ ~ .-n
d
a ~ a d m v a d d v a v v v a '~
a~ m v ~ ,~ .-~d ,~ .-r.-,d ~ .a.~ d d d v d
a m ~a m % a
,~ ,~ .a ~ r,,
,.-i H n.a. a.H w w w H n. n.o. H r' ~'~ ,-~
H p n. ~ t3 ~ a. 9 s ~ o, a. w.n,
a ca ~a a
m m 9 6
. ~a a 6 a m ~
N 8 % ~ X k % ~ ~ a G 9 ~ ~08 N
m 6 B ~ 6
k
7 S y~ S > X k Y X c ~
E C S C .
X O O W W W O fstW W O W W W O w W U W
w a N N N m N
v
v v v
CA 02215110 1997-09-11
G Y
w a
,~
a
W f N c'1 N N
N ~ ~T ~ -n N N .-1 N .H .r .T V~ .~ N
~
U7 3 a
a
' x
1 ~ ~ ~ .-v--n-n .iN -v ~ ~ .~ .-n-ar1 M c~1 .--n-I
~ a
m
oa
N
a
C
b a v
U L
t0 N
H
r, -f
O -m'1 ~OIt1h O V y O ~t1N O O It1
-r 'r .
O a G
U r
0
Cdr
Y
,b
a y In M w ..ui o eo ...In In w .-I
,o o, o N O O
O O ~ ~ O ~ 0 ~ O O a ~ ~
~ ~ ~ ~ ~
O G
a
U
N a "
"~GN O 0 0 0 0 0 0 0 0 0 0 0 0 0 0 O 0 0
A
_ W t ~T h h ~'1~O v0 ~YJ'~T v0 ~OC ~O vT t~WO
Y
H 00
0..
O
.a
C N
r~ ri
b
v6 .1
'~ a
(n N
O C
~ O U1 N N '~ '-;O O N O ~ ~ O N N
~"IN
~ O O O O O ~ O O ~ ~~<'1 O u1~ N ~' O O
N N
..a y
mes
a
U
i~ N
~ G'
C7 N N N N
21 G N N N N O d O N O O O N d G1 N N N N
a
-i G G G d O C.O W O C O C G G 4: Y.7 C. G
' O O O O O O O O O O O O o
O ~
U 'G 'Ct7 T7L~ b Lm Ci a t~tm 0 T7'O 'C 'C 'b 'O
G G C G G
Y b b b
~
x ~ v b b b b ~ ro v b b v v b v b v
O N m m m s. m d
D, G a v v d m d N d v m m v D D ~ N D 7
' al D ~ ~ ~ ~ D D 'J~ D H m 1 FI
n
L N H H a H a al t-nH LIiJ H H N p - N
r N
N ~ p N N N m N p N N m m 4) N N N O N N
~ O UlN N W t9O ~l N N Ol % 01N N 0 f~
'7
O ~ a .D.a O f~ .Aa~ .D .~.O.a p p p . Y . O
- O O O O O O O O O O O O O O
~I
.
(~ O G G G
b0 N
L7 .-1
.a d
rI N
I N
rl
O M M ~ ~
.r N ~ p p p n o0 ~ ~ ,- . .r ~ -n
, -,n .
m .a
C
~ ~
rl
O N
O
U 2
N
G D ~ -vN ~"1~T u1D ~D h CO~ O~ ~ ~ D ',Wt'1~ M-v
.r N r1 ~
a
cC .1 " .rl rl a rl
of
to .a ~ U1N N N m W N N N a CI d y Y 1~ y N
N N N a l a a a N a a a N a N N
C4 a N 1 cC i
-1 N a N
.-I r r r r r r r r r r ' r "~'~ r r ~ rl
1-I H O.O P P. C1i~ P. CLP.H L4 a A' N 4r Q' f1
8 f1 P 6 LL H P CL
~ 8 d c6
H H
P. N H H H H H 9 of H 9 cCc6 td ~ ~ t GL
uC a' N N cCcE c6H ~ N O G4 N
H A. A O N N
X at
N H o X X X X X . X X X H X ~ k H H k X
d X W W W W H W W W X W X X
X X O O
m N
O U W O O w W W w
W O m N N U U
U U
CA 02215110 1997-09-11
Industrial availability
The technique disclosed by the present invention
relates to a zinc hot-dip-coated steel sheet and an
alloyed zinc hot-dip-coated steel sheet showing a
decreased number of bare spots and excellent coating
adhesion, and are appropriately used mainly for steel
sheets of automobile bodies.
' 27