Note: Descriptions are shown in the official language in which they were submitted.
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
Method of controlling harmful fungi
The present invention relates to a method of controlling harmful
5 fungi and to synergistic mixtures suitable for this purpose
which, in addition to an active compound IA or IB which inhibits
respiration at the cytochrome complex III, contain an active
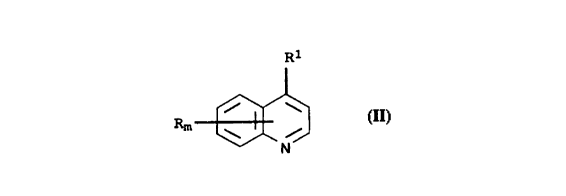
compound of the formula II
Rl
l~ ,J~ , II
where the index and the substituents have the following meanings:
m is an integer from 1 to 6, it being possible for the radicals
R to be different if m iQ greater than 1;
R is hydrogen, cyano, nitro, hydroxyl, mercapto, amino,
carboxyl, aminocarbonyl, aminothiocarbonyl, sulfo (S03H),
aminosulfonyl, halogen,
Cl-C6-alkyl, hydroxy-Cl-C6-alkyl, Cl-C6-alkoxy-
Cl-C6-alkyl, Cl-C6-alkoxy, Cl-C6-alkoxy-Cl-C6-alkoxy,
Cl-C6-alkylthio, Cl-C6-alkylamino, di-Cl-C6-alkylamino,
Cl-C6-alkylsulfonyl, Cl-C6-alkylsulfoxyl, Cl-C6-alkyl-
sulfonyloxy, Cl-C6-alkylcarbonyl, Cl-C6-alkylcarbonyloxy,
Cl-C6-alkylcarbonylamino, Cl-C6-alkoxycarbonyl,
Cl-C6-alkoxycarbonylamino, Cl-C6-alkylaminocarbonyl,
di-Cl-C6-alkylaminocarbonyl, Cl-C6-alkylaminothiocarbonyl,
di-Cl-C6-alkylaminothiocarbonyl, Cl-C6-alkylaminosulfonyl,
di-C1-C6-alkylaminosulfonyl, C2-C6-alkenyl, C2-C6-alkenyl-
oxy, C2-C6-alkenylcarbonyloxy, C2-C6-alkynyl and
C2-C6-alkynyloxy, it being possible for these groups to be
partially or completely halogenated;
C3-C6-cycloalkyl, aryl, aryl-Cl-C6-alkyl, aryl-C1-C6-alkoxy,
aryloxy, arylthio, arylcarbonyl, arylcarbonyloxy, heteroaryl,
heteroaryl-Cl-C6-alkyl, heteroaryl-Cl-C6-alkoxy,
heteroaryloxy, heteroarylthio, heteroarylcarbonyl and
heteroarylcarbonyloxy, it being possible for these groups to
be partially or completely halogenated and/or to carry one to
three of the following radicals: cyano, nitro, hydroxyl,
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
C1-C4-alkyl, Cl-C4-haloalkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy or
Cl-C4-alkylthio;
R1 is hydrogen, cyano, nitro, hydroxyl, mercapto, amino,
carboxyl, aminocarbonyl, aminothiocarbonyl, sulfo (S03H),
aminosulfonyl, halogen,
Cl-C6-alkyl, hydroxy-Cl-C6-alkyl, Cl-C6-alkoxy-
Cl-C6-alkyl, Cl-C6-alkoxy, Cl-C6-alkoxy-Cl-C6-alkoxy,
Cl-C6-alkylthio, Cl-C6-alkylamino, di-Cl-C6-alkylamino,
Cl-C6-alkylsulfonyl, Cl-C6-alkylsulfoxyl, Cl-C6-alkyl-
sulfonyloxy, C1-C6-alkylcarbonyl, Cl-C6-alkylcarbonyloxy,
Cl-C6-alkylcarbonylamino, Cl-C6-alkoxycarbonyl,
Cl-C6-alkoxycarbonylamino, Cl-C6-alkylaminocarbonyl,
di-Cl-C6-alkylaminocarbonyl, C1-C6-alkylaminothiocarbonyl,
di-Cl-C6-alkylaminothiocarbonyl, Cl-C6-alkylaminosulfonyl,
di-Cl-C6-alkylaminosulfonyl, C2-C6-alkenyl, C2-C6-alkenyl-
oxy, C2-C6-alkenylcarbonyloxy, C2-C6-alkynyl and
C2-C6-alkynyloxy, it being possible for these groups to be
partially or completely halogenated;
C3-C6-cycloalkyl, aryl, aryl-C1-C6-alkyl, aryl-C1-C6-alkoxy,
arylthio, arylcarbonyl, arylcarbonyloxy, heteroaryl,
heteroaryl-Cl-C6-alkyl, heteroaryl-Cl-C6-alkoxy,
heteroaryloxy, heteroarylthio, heteroarylcarbonyl and
heteroarylcarbonyloxy, it being possible for these groups to
be partially or completely halogenated and/or to carry one to
three of the following radicals: cyano, nitro, hydroxyl,
Cl-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4-haloalkoxy or
C1-C4-alkylthio.
It is known from the literature that active compounds which
inhibit the cytochrome bcl complex (cytochrome complex III) can be
employed as fungicides [cf. U. Brandt, U. Haase, H. Schagger,
35 G. von Jagow: Spezifitat and Wirkmechanismu~ der Strobilurine
[Specificity and mechanism of action of the strobilurins],
Dechema-Monographie Vol. 129, 27-38, VCH Verlagsgesellschaft
Weinheim, 1993; J.M. Clough: Natural Product Reports, 1993,
565-574; F. Rohl, H. Sauter: Biochem. Soc. Trans. 22, 635
40 (1993)].
Examples of such active compounds are compounds of the formula IA
or IB
CA 0221~14 1997-10-02
-
BASF Aktienges~llschaft940769 O.Z. 0050/45781
y/ C
Xn ~Q ~ X
C\ C
R" R"
IA IB
where is a double or single bond and the index and thesubstituents have the following ?~nings:
15 R' is -C[CO2CH3]=CHOCH3, -C[CO2CH3]=NOCH3, -C[CONHCH3]=NOCH3,
-C[CO2CH3]=CHCH3, -C[CO2CH3]=CHCH2CH3, -C[COCH3]=NOCH3,
-C[COCH2CH3]~NOCH3, -N(OCH3)-CO2CH3, -N(CH3)-CO2CH3,
-N(CH2CH3)-CO2CH3~
20 R" is a C-organic radical which is bonded directly or via an
oxy, mercapto, amino or alkylamino group, or
together with a group X and the ring Q or T to which they are
bonded, are an unsubst. or subst. bicyclic, partially or
completely unsaturated system which, in addition to carbon
ring members, can contain heteroatoms from the group
consisting of oxygen, sulfur and nitrogen,
RX is -OC[CO2CH3]=CHOCH3, -OC[CO2CH3]=CHCH3, -OC[CO2CH3]=CHCH2CH3,
-SC[CO2CH3]=CHOCH3, -SC[CO2CH3]=CHCH3, -SC[CO2CH3]=CHCH2CH3,
-N(CH3)C[CO2CH3]=CHOCH3, -N(CH3)C[CO2CH3]=NOCH3,
-CH2C[CO2CH3]=CHOCH3, -CH2C[CO2CH3]-NOCH3,
-CH2C[CONHCH3]=NOCH3,
35 RY i9 oxygen, sulfur, =CH- or =N-,
n is 0, 1, 2 or 3, it being possible for the radicals X to be
different if n > l;
40 X is cyano, nitro, halogen, Cl-C4-alkyl, Cl-C4-haloalkyl,
Cl-C4-alkoxy, Cl-C4-haloalkoxy, Cl-C4-alkylthio or,
in the case where n > 1, a C3-Cs-alkylene, C3-C5-alkenylene,
oxy-C2-C4-alkylene, oxy-Cl-C3-alkylenoxy, oxy-
C2-C4-alkenylene, oxy-C2-C4-alkenylenoxy or butadienediyl
group bonded to two adjacent C atoms of the phenyl ring, it
being pos~ible for these chains in turn to carry one to three
CA 0221~14 1997-10-02
~3ASF Aktiengesellschaft 940769 O.Z. 0050/45781
of the following radicals: halogen, Cl-C4-alkyl,
Cl-C4-haloalkyl, C1-C4-alkoxy, Cl-C4-haloalkoxy or
Cl-C4-alkylthio,
5 Y is =~- or -N-,
Q is phenyl, pyrrolyl, thienyl, furyl, pyrazolyl, imidazolyl,
oxazolyl, isoxazolyl, thiazolyl, thiadiazolyl, triazolyl,
pyridinyl, 2-pyridonyl, pyrimidinyl or triazinyl,
T is phenyl, oxazolyl, thiazolyl, thiadiazolyl, oxadiazolyl,
pyridinyl, pyrimidinyl or triazinyl.
Active compounds I (or IA and I~) of this type are described, for
15 example, in the following specifications:
EP-A 178 826, EP-A 203 606, EP-A 203 608, EP-A 206 523,
EP-A 212 859, EP-A 226 917, EP-A 229 974, EP-A 242 070,
EP-A 242 081, EP-A 243 012, EP-A 243 014, EP-A 251 082,
20 EP-A 253 213, EP-A 254 426, EP-A 256 667, EP-A 260 794,
EP-A 260 832, EP-A 267 734, EP-A 273 572, EP-A 274 825,
EP-A 278 595, EP-A 280 185, EP-A 291 196, EP-A 299 694,
EP-A 307 101, EP-A 307 103, EP-A 310 954, EP-A 312 221,
EP-A 312 243, EP-A 329 011, EP-A 331 966, EP-A 335 519,
25 EP-A 336 211, EP-A 337 211, EP-A 341 845, EP-A 350 691,
EP-A 354 571, EP-A 363 818, EP-A 370 629, EP-A 373 715,
EP-A 374 811, EP-A 378 308, EP-A 378 755, EP-A 379 098,
EP-A 382 375, EP-A 383 117, EP-A 384 211, EP-A 385 224,
EP-A 385 357, EP-A 386 561, EP-A 386 681, EP-A 389 901,
30 EP-A 391 451, EP-A 393 428, EP-A 393 861, EP-A 398 692,
EP-A 400 417, EP-A 402 246, EP-A 405 782, EP-A 407 873,
EP-A 409 369, EP-A 414 153, EP-A 416 746, EP-A 420 091,
EP-A 422 597, EP-A 426 460, EP-A 429 968, EP-A 430 471,
EP-A 433 233, EP-A 433 899, EP-A 439 785, EP-A 459 285,
35 EP-A 460 575, EP-A 463 488, EP-A 463 513, EP-A 464 381,
EP-A 468 684, EP-A 468 695, EP-A 468 775, EP-A 471 261,
EP-A 472 224, EP-A 472 300, EP-A 474 042, EP-A 475 158,
EP-A 477 631, EP-A 480 795, EP-A 483 851, EP-A 483 985,
EP-A 487 409, EP-A 493 711, EP-A 498 188, EP-A 498 396,
40 EP-A 499 823, EP-A 503 436, EP-A 508 901, EP-A 509 857,
EP-A 513 580, EP-A 515 901, EP-A 517 301, EP-A 528 245,
EP-A 532 022, EP-A 532 126, EP-A 532 127, EP-A 535 980,
EP-A 538 097, EP-A 544 587, EP-A 546 387, EP-A 548 650,
EP-A 564 928, EP-A 566 455, EP-A 567 828, EP-A 571 326,
45 EP-A 579 071, EP-A 579 124, EP-A 579 908, EP-A 581 095,
EP-A 582 902, EP-A 582 925, EP-A 583 806, EP-A 584 625,
EP-A 585 751, EP-A 590 610, EP-A 596 254,
CA 0221~14 1997-10-02
~ BASF Aktiengesellschaft 9gO769 O.Z. 0050/45781
WO-A 90/07,493,WO-A 92/13,830, WO-A 92/18,487, WO-A 92/18,494,
WO-A 92/21,653, WO-A 93/07,116, WO-A 93/08,180, WO-A 93/08,183,
WO-A 93/15,046, WO-A 93/16,986, WO-A 94/00,436, WO-A 94/05,626,
WO-A 94/08,948, WO-A 94/08,968, WO-A 94/10,159, WO-A 94/11,334,
5 JP-A 02~/121,970, JP-A 04/182,461, JP-A OS/201,946,
JP-A 05/201,980, JP-A 05/255,012, JP-A 05/294,948,
JP-A 06/025,133, JP-A 06/025,142, JP-A 06/056,756,
FR-A 2 670 781,
G~-A 2 210 041, GB-A 2 218 702, G~-A 2 238 308, GB-A 2 249 092,
10 GB-A 2 253 624, GB-A 2 255 092,
DE-A 39 05 911, DE Pat. Appl. 43 05 502.8,
DE Pat. Appl. 43 lO 143.7, DE Pat. Appl. 43 18 397.2, DE Pat.
Appl. 43 34 709.6, DE Pat. Appl. 44 03 446.6, DE Pat. Appl. 44 03
447.4, DE Pat. Appl. 44 03 448.2, DE Pat. Appl. 44 10 424.3, DE
15 Pat. Appl. 44 21 180.5, DE Pat. Appl. 44 21 182.1, DE Pat. Appl.
44 lS 483.6, DE Pat. Appl. 44 23 615.8, DE Pat. Appl. 44 23 612.3
and DE Pat. Appl. 44 41 674.1.
In the use of these active compounds it is seen, however, that
20 their action i8 only temporary, ie. after some time renewed
growth of the fungus occurs.
In addition, compound~ II having fungicidal action are known from
the literature [cf. Monat~hefte fur Chemie, 125, S1 f. and 723 f.
25 (1994); Farm Chemicals Handbook Vol. 79 (1993); The Agrochemicals
Handbook, 2. Ed., Royal Society of Chemistry (1987)]. The
preparation of these compounds is additionally described in
Houben-Weyl, Vol. E7a, 290-491.
30 It is an object of the present invention to eliminate the
disadvantages in the use of the compounds IA and IB.
We have found that harmful fungi can be fundamentally better
controlled if, in addition to an active compound IA or IB which
35 inhibits respiration at the cytochrome complex III, a further
active compound of the formula II is used.
The process according to the invention is probably based on the
fact that the fungus, on inhibition of respiration at the
40 cytochrome complex III, utilizes a secondary route of alternative
respiration such that complete destruction doe~ not occur. This
would mean that the active compounds of the formula II are
suitable for inhibiting alternative respiration. The combination
of the inhibition of both respiration via the cytochrome complex
45 III and alternative respiration could therefore be responsible
for the fact that the fungus is completely destroyed.
CA 0221~14 1997-10-02
~ BASF Aktiengesellschaft 940769 O.Z. 0050/45781
By means of the combination of appropriate active compounds
according to the invention, a more effective control of the
fungal pest is achieved, as by the combination of the active
compounds IA or IB and II lower application rates of the
5 individual active compounds are necessary (synergism).
Fundamentally all active compounds described in the
specifications mentioned at the outset are suitable in the
inventive method for inhibiting respiration at the cytochrome
10 complex III, where in particular the compound~ mentioned in the
examples given there are to be taken into account. Of particular
importance in this case are compounds IA and IB in which R'' is
one of the following groups:
15 unsubst. or subst. aryloxy, unsubst. or subst. hetaryloxy,
unsubst. or subst. aryloxymethylene, unsubst. or subst.
hetaryloxymethylene, un~ubst. or subst. arylethenylene, unsubst.
or subst. hetarylethenylene, or a group
20 R~R~C=NOCH2- or ~YON=CR~CRe=NOCH2 where the radicals ~~, R~, RY, R~
and RF in general and in particular have the meanings described in
the following specifications:
EP-A 370 629, EP-A 414 153, EP-A 426 460, EP-A 460 575,
25 EP-A 463 488, EP-A 472 300, EP-A 498 188, EP-A 498 396,
EP-A 515 901, EP-A 585 751,
WO-A 90/07,493, WO-A 92/13,830, WO-A 92/18,487, WO-A 92/18,494,
WO-A 93/15,046, WO-A 93/16,986, WO-A 94/08,948, WO-A 94/08,968,
JP-A 05/201,946, JP-A 05/255,012, JP-A 05/294,948,
30 JP-A 06/025,133, JP-A 06/025,142,
DE Pat. Appl. 44 03 447.., DE Pat. Appl. 44 03 448.., DE Pat.
Appl. 44 21 180.5 and DE Pat. Appl. 44 21 182.1;
particularly preferred ~unsubst. or subst. aryloxy, or unsubst.
35 or subst. hetaryloxy~' radical~ correspond in general and in
particular to the meanings described in the following
specifications:
EP-A 178 826, EP-A 242 070, EP-A 242 081, EP-A 253 213,
40 EP-A 254 426, EP-A 256 667, EP-A 260 794, EP-A 280 185,
EP-A 307 103, EP-A 341 845, EP-A 382 375, EP-A 393 861,
EP-A 398 692, EP-A 405 782, EP-A 430 471, EP-A 468 684,
EP-A 468 695, EP-A 477 631, EP-A 483 985, EP-A 498 188,
EP-A 513 580, EP-A 515 901,
45 WO-A 93J15,046, WO-A 94/10,159,
GB-A 2 253 624,
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
JP-A 04/182,461 and
DE Pat. Appl. 44 23 612.3;
particularly preferred "unsubst. or subst. aryloxymethylene, or
5 unsubst. or subst. hetaryloxymethylene" radicals correspond in
general and in particular to the meanings described in the
following specifications:
EP-A 178 826, EP-A 226 917, EP-A 253 213, EP-A 254 426,
10 EP-A 278 595, EP-A 280 185, EP-A 299 694, EP-A 335 519,
EP-A 350 691, EP-A 363 818, EP-A 373 775, EP-A 378 308,
EP-A 385 224, EP-A 386 561, EP-A 398 692, EP-A 400 417,
EP-A 407 873, EP-A 472 224, EP-A 477 631, EP-A 498 188,
EP-A 498 396, EP-A 513 580, EP-A 515 901, EP-A 579 124,
15 WO-A 93/08,180, WO-A 93/15,046, WO-A 94/00,436,
JP-A 04/182,461,
DE Appl. No. 43 05 502.., DE Appl. No. 44 10 424.. and
DE Pat. Appl. 44 15 483.6;
20 particularly preferred ~unsubst. or subst. arylethenylene or
unsubst. or subst. hetarylethenylene" radicals correspond in
general and in particular to the meanings described in the
following specifications:
25 EP-A 178 826, EP-A 203 606, EP-A 253 213, EP-A 254 426,
EP-A 280 185, EP-A 378 755, EP-A 398 692, EP-A 402 246,
EP-A 474 042, EP-A 475 158, EP-A 477 631, EP-A 487 409,
EP-A 498 188, EP-A 498 396, EP-A 513 580, EP-A 515 901,
EP-A 528 245, EP-A 544 587,
30 WO-A 93/15,046, WO-A 94/11,334,
FR-A 2 670 781 and DE Pat. Appl. 44 23 615.8;
particularly preferred active compounds of the formula IA in
which R' is -C[CO2CH3]=CHOCH3 correspond in general and in
35 particular to the compounds described in the following
specifications:
EP-A 178 826, EP-A 203 606, EP-A 226 917, EP-A 242 070,
EP-A 242 081, EP-A 256 667, EP-A 260 794, EP-A 278 595,
40 EP-A 299 694, EP-A 307 103, EP-A 335 519, EP-A 341 845,
EP-A 350 691, EP-A 370 629, EP-A 373 775, EP-A 378 308,
EP-A 378 755, EP-A 382 375, EP-A 385 224, EP-A 386 561,
EP-A 393 861, EP-A 402 246, EP-A 405 782, EP-A 407 873,
EP-A 414 153, EP-A 426 460, EP-A 430 471, EP-A 463 488,
g5 EP-A 468 695, EP-A 472 224, EP-A 474 042, EP-A 475 158,
EP-A 483 985, EP-A 487 409, EP-A 515 901, EP-A 528 245,
EP-A 544 587,
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/4~781
WO-A 90/07,493, WO-A 92/18,487, WO-A 92/18,494, WO-A 93/08,180,
WO-A 93/16,986, WO-A 94/00,463, WO-A 94/08,948, WO-A 94/08,968,
WO-A 94/10,159, WO-A 94/11,334,
FR-A 2 670 781,
5 JP-A 06-/025,133,
DE Appl. No. 44 03 447.., DE Appl. No. 44 10 424.. and DE Pat.
Appl. 44 21 180.5;
particularly preferred active compounds of the formula IA in
10 which R' is -C[CO2CH3]=NOCH3 correspond in general and in
particular to the compounds de~cribed in the following
specifications:
EP-A 253 213, EP-A 254 426, EP-A 299 694, EP-A 363 818,
15 EP-A 378 308, EP-A 385 224, EP-A 386 561, EP-A 400 417,
EP-A 407 873, EP-A 460 575, EP-A 463 488, EP-A 468 684,
EP-A 472 300, EP-A 515 901,
WO-A 94/00,436, WO-A 94/08,948, WO-A 94/10,159, WO-A 94/11,334,
JP-A 05/201,946, JP-A 05/255,012, JP-A 05/294,948,
20 DE Appl. No. 44 03 447.., DE Appl. No. 44 10 424.. and
DE Pat. Appl. 44 21 180.5;
particularly preferred active compounds of the formula IA in
which R' is -C~ CONHCH3]=NOCH3 corre~pond in general and in
25 particular to the compounds described in the following
specifications:
EP-A 398 692, EP-A 463 488, EP-A 477 631, EP-A 515 901,
EP-A 579 124, EP-A 585 751,
30 WO-A 92/13,830, WO-A 93/08,180, WO-A 94/08,948, WO-A 94/10,159,
WO-A 94/11,334,
GB-A 2 253 624,
JP-A 04/182,461, JP-A 05/201,946, JP-A 05/255,012,
JP-A 05/294,948,
35 DE Appl. No. 43 05 502.., DE Appl. No. 44 03 448.., DE Appl. No.
44 10 424.., DE Pat. Appl. No. 44 23 615.8 and DE Pat. Appl. 44
21 182.1;
particularly preferred active compounds of the formula IA in
40 which R~ is -C[CO2CH3]=CHCH3 or -C~CO2CH3]=CHCH2CH3 correspond in
general and in particular to the compounds described in the
following specifications:
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
EP-A 280 185, EP-A 463 488, EP-A 501 901, EP-A 513 580,
EP-A 515 901,
DE Appl. No. 44 03 447.., DE Appl. No. 44 10 424.., DE Pat. Appl.
No. 44 21 180.5 and DE Pat. Appl. No. 44 15 483.6;
particularly preferred active compounds of the formula IA in
which R' is -C[COCH3]=NOCH3 or -C[COCH2CH3]=NOCH3 correspond in
general and in particular to the compounds described in
EP-A 498 188;
particularly preferred active compounds of the formula IA in
which R' is -N(OCH3)CO2CH3, -N(CH3)-CO2CH3 or -N(CH2CH3)-CO2CH3
correspond in general and in particular to the compounds
described in the following specifications:
EP-A 498 396, WO-A 93/15,046, JP-A 06/025,142, JP-A 06/056,756,
DE Pat. Appl. 44 23 612.3 and DE Pat. Appl. 44 41 674.1;
particularly preferred active compounds of the formula IB in
20 which Rx is -OC[CO2CH3]=CHOCH3, -OC[CO2CH3]=CHCH3,
-OC[CO2CH3]=CHCH2CH3, -SC[CO2CH3]=CHOCH3, -SC[CO2CH3]=CHCH3,
-SC[CO2CH3]=CHCH2CH3, -N(CH3)C[CO2CH3]SCHOCH3,
-N(CH3)C[CO2CH3]=NOCH3, -CH2C[CO2CH3]=CHOCH3, -CH2C[CO2CH3]=NOCH3 or
-CH2C[CONHCH3]=NOCH3 correspond in general and in particular to
25 the compounds described in the following specifications:
EP-A 212 859, EP-A 331 966, EP-A 383 117, EP-A 384 211,
EP-A 389 901, EP-A 409 369, EP-A 464 381, EP-A 471 261,
EP-A 503 436, EP-A 546 387, EP-A 548 650, EP-A 579 908 and
30 EP-A 584 625.
Examples of particularly suitable active compounds I are compiled
in the following Tables.
35 Table l.lA
Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=CHOCH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxymethylene, where the unsubst. or subst. (het)aryl
40 group has the following meaning
CA 022l~l4 l997-l0-02
BASF Aktiengesellschaft 940769 0.Z. 0050/45781
No. unsubst. or subst. (het)aryl Reference
I.lA-l2-CH3-C6H4 EP-A 226 917
I.lA-22,5-(CH3)2-C6H3 EP-A 226 917
I.lA-32-CH3, 4-C[CH3]=NOCH3-C6H3 EP-A 386 561
I.lA-42-CH2CH2CH3, 6-CF3-pyrimidin-4-yl EP-A 407 873
I.lA-52,4-(CH3)2-C6H3 EP-A 226 917
Table l.lB
Compounds-of the formula IA in which R' is -C(CO2C~3J=CHOCH3, Q is
phenyl, n has the value 0, R is unsubst. or subst. (het)aryloxy,
lS where the unsubst. or subst. (het)aryl group has the following
~~A~i ng
No.unsubst. or sub~t. ~Het)~ry1 Roforenco
20I.lB-l C6H5 EP-A 178 826
I.lB-26-[2-CN-C6H4-O]-pyrimidin-4-yl EP-A 382 375
Table l.lC
Compounds of the formula IA in which R' is -C~CO2CH3 J=CHOCH3, Q is
phenyl, n has the value 0, R~ is unsubst. or subst.
(het)arylethenylene, where the unsubst. or subst. (het)aryl group
has the following meaning
No.unsubst. or subst. (Hot)~ryl Referonco
- I.lC-l1-(2,4-Cl2-C6H3), 5-CF3-pyrazol-4-yl EP-A 528 245
35I.lC-21-(4-Cl-C6H4)-pyrazol-4-yl EP-A 378 755
- I.lC-33-CF3-C6H4 EP-A 203 606
I.lC-43-Cl-C6H4 EP-A 203 606
I.lC-54-C6Hs-C6H4 EP-A 203 606
Table l.lD
Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=CHOCH3, n has the value 0, R" is CH20N=CR~R~, where Ra
45 and R~ have the following meanings
CA 0221~14 1997-10-02
- ~ BASF Aktiengesellschaft 940769O.Z. 0050/45781
No. Ra R~ Reference
I.lD-l CH3 4-Cl-C6H4 EP-A 370 629
I.lD-2 CH3 3-CF3-C6H4 EP-A 370 629
I.lD-3 CH3 4-OCH2CH3-pyrimidin-2-yl WO-A 92/18,487
Table l.lE
10 Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=CHOCH3, n has the value 0, R i8 CH20N=CRYCR~=NOR~,
where RY, R~ and R~ have the following meanings
15No. RY R~ R~ Roforonco
I.lE-l CH3 CH3 CH3DE Appl. No. 44 03 447.4
I.lE-2 CH3 CH3 CH2CH3DE Appl. No. 44 03 447.4
I.lE-3 CH3 C6H5 CH3DE Appl. No. 44 03 447.4
I.lE-4 CH3 C6H5 CH2CH3DE Appl. No. 44 03 447.4
I.lE-5 CH34-Cl-C6H4 CH3DE Appl . No. 44 21 180.5
I.lE-6 CH34-Cl-C6H4 CH2CH3DE Appl. No. 44 21 180.5
Table 1.2~
Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=NOCH3, n has the value 0, R" is unsubst. or subst.
30 (het)aryloxymethylene, where the unsubst. or subst. (het)aryl
group has the following e~ni ng
No. u~subst. or subat. (het)ary1 Roforonce
I.2A-1 2-CH3-C6H4 EP-A 253 213
I.2A-22,5-(CH3)2-C6H3 EP-A 400 417
I.2A-32,4-(CH3)2-C6H3 EP-A 400 417
I.2A-42,3,5-(CH3)3-C6H2 EP-A 400 417
40I.2A-52-Cl, 5-CH3-C6H3 EP-A 400 417
I.2A-62-CH3, 4-C[CH3]=NOCH3-C6H3EP-A 386 561
Table 1.2
Compounds of the formula IA in which Q is phenyl, R' is
CA 022l~l4 l997-l0-02
- BASF Aktienyesellschaft 940769 O.Z. 0050/45781
-C(CO2CH3)=NOCH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxy, where the unsubst. or subst. (het)aryl group has
the following meaning
5 No.~- unsubst. or subst. (het)arylReferencc
I.2B-1 C6H5 EP-A 253 213
I.2B-2 6-[2-CN-C6H4-O]-pyrimidin-4-ylEP-A 468 684
10 Table 1.2C
Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=NOCH3, n has the value 0, R" is CH2ON=CRaR~, where R~
and R~ have the following meanings
No. Ra RP Roferenco
I.2C-1 CH3 4-Cl-c6H4 EP-A 463 488
I.2C-2 CH3 .3-Cl-C6H4 EP-A 463 488
I.2C-3 CH3 4-CF3-C6H4 EP-A 463 488
I.2C-4 CH3 3-CF3-C6H4 EP-A 463 488
I.2C-5 CH3 4-CH3-C6H4 EP-A 463 488
25I.2C-6 CH3 4-OCH2CH3-pyrimidin-2-yl EP-A 472 300
I.2C-7 CH3 3,5-Cl2-C6H3 EP-A 463 488
Table 1.2D
Compounds of the formula IA in which Q i~ phenyl, R' is
-C(CO2CH3)=NOCH3, n has the value 0, R" is CH2ON=CRYCR~=NOR~, where
RY, R~ and R~ have the following meanings
No. RY R~ R~ Reference
I.2D-1 CH3 CH3 CH3DE Appl. No. 44 03 447.4
I.2D-2 CH3 CH3 CH2CH3DE Appl. No. 44 03 447.4
I.2D-3 CH3C6Hs CH3DE Appl. No. 44 03 447.4
40I.2D-4 CH3C6H5 CH2CH3DE Appl. No. 44 03 447.4
I.2D-5 CH34-Cl-C6H4 CH3DE Appl. No. 44 21 180.5
I.2D-6 CH34-Cl-c6H4 CH2CH3DE Appl. No. 44 21 180.5
45 Table 1.3A
Compounds of the formula IA in which Q is phenyl, R~ is
CA 022l~l4 l997-l0-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
-C(CONHCH3)=NOCH3, n has the value 0, R~ is unsubst. or subst.
(het)aryloxymethylene~ where the unsubst. or subst. (het)aryl
group has the following meaning
5 No.-~ un~ub~t. or ~ub~t. (hot)ary1Rofercnce
I.3A-12-CH3-C6H4 EP-A 477 631
I.3A-22,5-(CH3)2-C6H3 EP-A 477 631
I.3A-32,4-(CH3)2-C6H3 EP-A 477 631
I.3A-42,3,5-(CH3)3-C6H2 EP-A 477 631
I.3A-52-CH3, 4-C[CH3]=NOCH3-C6H3 EP-A 579 124
I.3A-61-t4-Cl-C6H4]-pyrazol-3-ylDE Appl. No. 43 05 502.8
15 I.3A-7 1-[2,4-Cl2-C6H3]-pyrazol-3-yl DE Appl. No. 43 05 502.8
Table 1.3B
Compounds of the formula IA in which Q is phenyl, R' is
20 -C(CONHCH3)=NOCH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxy, where the unsubst. or subst. (het)aryl group has
the following meaning
No. un~ub~t. or ~ub~t. ~hot)aryl Roferenco
I.3~-1 C6H5 EP-A 398 692
I.3B-2 6-[2-CN-C6H4-O]-pyrimidin-4-yl GB-A 2 253 624
Table 1.3C
Compounds of the formula IA in which Q is phenyl, R' i8
-C(CONHCH3)=NOCH3, n has the value 0, R" is unsubst. or subst.
(het)aryl-ethenylene, where the unsubst. or subst. (het)aryl
group has the following meaning
No. unsub~t. or subst. (het)aryl Reference
I.3C-1 1-[2,4-C12-C6H3]~ DE Appl. No. 44 23 615.8
5-CF3-pyrazol-4-yl
~able 1.3D
Compounds of the formula IA in which Q is phenyl, R' is
-C(CONHCH3)=NOCH3, n has the value 0, R" is CH20N=CRaR~, where Ra
45 and R~ have the following meanings
CA 022l~l4 l997-l0-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
14
No. Ra R~ Reference
I.3D-1 CH34-Cl-C6H4 EP-A 463 488
I.3D-2 CN33-Cl-C6H4 EP-A 463 488
r . 3D-3 CH34-CF3-C6H4 EP-A 585 751
I.3D-4 CH33-CF3-C6H4 EP-A 585 751
I.3D-5 CH34-CH3-C6H4 EP-A 463 488
lOI.3D-6 CH33,5-C12-C6H3 EP-A 463 488
I.3D-7 CH32-OCH2CH3-pyrimidin-2-ylWO-A 92/13,830
Table 1.3E
Compounds of the formula IA in which Q is phenyl, R' is
-C(CONHCH3)=NOCH3, n has the value 0, R" is CH2ON=CRYCR~=NORe,
where RY, R~ and R~ have the following meanings
20No. RY R~ RE Refor-nce
I.3E-1 CH3 CH3 CH3DE Pat. Appl. 44 21 182.1
I.3E-2 CH3 CH3 CH2CH3DE Pat. Appl. 44 21 182.1
I.3E-3 CH3C6Hs CH3DE Pat. Appl. 44 21 182.1
I.3E-4 CH3C6H5 CH2CH3DE Pat. Appl. 44 21 182.1
I.3E-5 CH34-Cl-C6H4 CH3DE Pat. Appl. 44 21 182.1
I.3E-6 CH34-Cl-C6H4 CH2CH3DE Pat. Appl. 44 21 182.1
Table 1.4A
Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=CHCH3, n has the value 0, R" is unsubst. or subst.
35 (het)aryloxymethylene, where the unsubst. or subst. (het)aryl
group has the following meaning
No. unsubst. or subst. (hot)arylReforence
I.4A-12-CH3-C6H4 EP-A 280 185
I.4A-22,5-(CH3'2-C6H3 EP-A 513 580
I.4A-32,4-(CH3)2-C6H3 EP-A 513 580
r. 4A-42,3,5-(CH3)3-C6H2 EP-A 513 580
45~.4A-52-Cl, 5-CH3-C6H3 EP-A 513 580
CA 022l~5l4 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
I.4A-6 2-CH3, 4-C[CH3]=NOCH3-C6H3 EP-A 513 580
I.4A-7 1-[4-Cl-C6H4]-pyrazol-3-yl DE Pat. Appl. 44 15 483.6
5 Table 1-.4B
Compounds of the formula IA in which Q is phenyl, R' iQ
-C(CO2CH3)=CHCH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxy, where the unsubst. or subst. (het)aryl group has
10 the following meaning
No.un~ub~t. or ~ubst. Reference
(hot)aryl
I.4B-1 C6H5 EP-A 513 580
Table 1.4C
Compounds of the formula IA in which Q is phenyl, R' is
2 -C(CO2CH3)=CHCH3, n has the value 0, R" i~ CH2ON=CRYCR~=NOR~, where
RY, R~ and Re have the following meanings
No. RY R~ RE Roforence
I.4C-1 CH3 CH3 CH3DE Pat. Appl. 44 21 180.5
I.4C-2 CH3 CH3 CH2CH3DE Pat. Appl. 44 21 180.5
I.4C-3 CH3C6Hs CH3DE Pat. Appl. 44 21 180.5
I.4C-4 CH3C6H5 CH2CH3DE Pat. Appl. 44 21 180.5
30I.4C-5 CH34-Cl-C6H4 CH3DE Pat. Appl. 44 21 180.5
I.4C-6 CH34~Cl~C6HqCH2CH3DE Pat. Appl. 44 21 180.5
Table 1.5A
Compounds of the formula IA in which Q is phenyl, R' is
~ -C(CO2CH3)-CHCH2CH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxymethylene, where the unsubst. or subst. (het)aryl
group has the following meaning
No.unsub~t. o_ ~ub~t. Reference
(hot)aryl
I.5A-12-CH3-C6H4 EP-A 513 580
I.5A-22,5-(CH3)2-C6H3 EP-A 513 580
I.5A-32,4-(CH3)2-C6H3 EP-A 513 580
I.5A-42,3,5-(CH3)3-C6H2 EP-A 513 580
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
I.5A-5 2-Cl, S-CH3-C6H3 EP-A 513 580
I.SA-6 2-CH3, 4-C[CH3]=NOCH3-C6H3 EP-A 513 580
5 Table 1-.5B
Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=CHCH2CH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxy, where the unsubst. or sub~t. (het)aryl group has
10 the following meaning
No. un~ub~t. or ~ub~t. Roforonco
~ (hot)aryl
I.5B-1 C6Hs EP-A 513 580
Table 1.5C
Compounds of the formula IA in which Q is phenyl, R' is
-C(CO2CH3)=CHCH2CH3, n has the value 0, R" is CH2ON=CRYCR~=NORE,
where RY, R~ and R~ have the following meanings
No. RY R~ RE Roforonco
I.5C-1 CH3 CH3 CH3DE Pat. Appl. 44 21 180.5
I.5C-2 CH3 CH3 CH2CH3DE Pat. Appl. 44 21 180.5
I.5C-3 CH3 C6Hs CH3DE Pat. Appl. 44 21 180.5
I.5C-4 CH3 C6H5 CH2CH3DE Pat. Appl. 44 21 180.5
30I.5C-5 CH34-Cl-C6H4 CH3DE Pat. Appl. 44 21 180.5
I.5C-6 CH34-Cl-C6H4 CH2CH3DE Pat. Appl. 44 21 180.5
Table 1.6A
Compounds of the formula IA in which Q is phenyl, R' is
- -C(COCH3)=NOCH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxymethylene, where the unsubst. or subst. (het)aryl
group has the following ~An;ng
No. unsubst. or subst. (hetj~ryl Roforonco
I.6A-12-CH3-C6H4 EP-A 498 188
I.6A-22,5-(CH3)2-C6H3 EP-A 498 188
45I-6A-32,4-(CH3)2-C6H3 EP-A 498 188
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
I.6A-4 2,3,5-(CH3)3-C6H2 EP-A 498 188
I.6A-5 2-CH3, 4-C[CH3]=NOCH3-C6H3 EP-A 498 188
5 Table 1-.6B
Compounds of the formula IA in which Q is phenyl, R' is
-C(COCH3)=NOCH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxy, where the unsubst. or subst. (het)aryl group has
10 the following meaning
No. unsubst. or sub~t. (hot)aryl Reforenco
I.6B-1 C6H5 EP-A 498 188
15 I.6B-2 6-~2-CN-C6H4-O]-pyrimidin-4-yl EP-A 498 188
Table 1.7A
Compounds of the formula IA in which Q is phenyl, R' is
20 -C(COCH2CH3)=NOCH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxymethylene, where the unsubst. or subst. (het)aryl
group has the following meaning
No. un~ubst. or aub~t. (hot)ary1 Roforenco
I.7A-12-CH3-C6H4 EP-A 498 188
I.7A-22,5-(CH3)2-C6H3 EP-A 498 188
I.7A-32,4-(CH3)2-C6H3 EP-A 498 188
30 I-7A-42,3,5-(CH3)3-C6H2 EP-A 498 188
I.7A-52-CH3, 4-C[CH3~=NOCH3-C6H3 EP-A 498 188
Table 1.7B
Compounds of the formula IA in which Q is phenyl, R' is
-C(COCH2CH3)=NOCH3, n has the value 0, R~' is unsubst. or subst.
(het)aryloxy, where the unsubst. or subst. (het)aryl group has
the following meaning
No. unsubst. or s~bst. (het)aryl Referenco
I.7B-1 C6Hs EP-A 498 188
I.7B-2 6-[2-CN-C6H4-O]-pyrimidin-4-yl EP-A 498 188
Table 1.8A
CA 022l~l4 l997-l0-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
Compounds of the formula IA in which Q is phenyl, R' is
-N(OCH3)-CO2CH3, n has the value 0, R" is unsubst. or subst.
(het)aryloxymethylene, where the unsubst. or subst. (het)aryl
group has the following meaning
No.unsub~t. or ~ub~t. (het)aryl Roference
I.8A-1 2-CH3-C6H4 WO- A 93/15,046
I.8A-2 2,5-(CH3)2-C6H3 WO-A 93/15,046
lOI.8A--32,4--(CH3)2--C6H3 WO--A 93/15,046
I.8A-42,3,5-(CH3)3-C6H2 WO - A 93/15,046
I.8A-5 2-Cl, 5-CH3-C6H3 WO - A 93/15,046
15 I.8A-62-CH3, 4-c[cH3]=NocH3-c6H3 WO- A 9 3/15,046
I.8A-72-CH3, 4-C~CH3]=NOCH2CH3-C6H3 WO-A 93/15,046
I.8A-82-CH3, 4-c[cH2cH3]=NocH3-c6H3 WO-A 93/15,046
I.8A-9 2-CH3, WO - A 93/15,046
4-C[CH2CH3]=NOCH2CH3-C6H3
I.8A-101-[4-Cl-C6H4]-pyrazol-3-yl DE Pat. Appl. 44 23
612.3
Table 1.8B
Compounds of the formula IA in which Q is phenyl, R' is
-N(OCH3)-CO2CH3, n haQ the value 0, R~ is CH2ON=CRaR~, where Ra and
R~ have the following meanings
No. R~ R~ Roforenco
I.8B-1 CH3 3,5-C12-C6H3 WO- A 9 3/15,046
With respect to their u~e in the mixtures according to the
35 invention, compounds of the formula II.1 are particularly
preferred
RC Rl
R \ ,1~ ~ ~ / II.l
where the substituents have the following meanings:
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
Ra is hydrogen or Cl-C4-alkyl;
Rb is hydrogen, halogen or Cl-C4-alkyl;
5 Rl is hydrogen;
Rc is hydrogen, nitro, sulfoxyl, halogen, Cl-C4-alkyl,
Cl-C4-alkylcarbonyl or Cl-C4-alkylcarbonyloxy;
lO Rd is hydrogen;
Re is hydrogen, nitro or halogen;
Rf i9 hydrogen, hydroxyl, carboxyl, halogen, Cl-C4-alkyl,
Cl-C4-alkoxy, C1-C4-alkylcarbonyl, Cl-C4-alkylcarbonyloxy or
C2-C4-alkenylcarbonyloxy.
Preferred compounds II.l are further those in which at least one
of the radicals Rl and Ra to Rf are hydroxyl.
Particularly preferred compounds II.1 are those in which Rf i9
hydroxyl.
Examples of particularly suitable active compounds of the formula
25 II are compiled in the following Table:
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
~ u)
_,
~ ~ O --
_~ ~ o ~ -- ~ a~
o ~1
~ k ~ ~ ~ - x - - ~
o . o
1~ o
u u ~ _~ o u u_~ c; ~ u . _I
UO O ~ a~ O ~ ~ O U
ou~ o
~ ~u~. o o ~ . ~x . . u~
~ e ~ u ~
a~ J h a~ ~ ~ a
s u u u~ ~ u ~ ~ u ~c ~
U ~ ~ ~ N ~ ~ ~ um
u~ mu
m
m mmU~mmmmmm~mmm
~~um~~U~OOOoooooo
~mmmmmmmmmU~zm H mU
~mmmmmmmmmmmmmmmm
mouomomomo~m
~mmmmmmmmmmmmmmmm
~mmmm~mmmmmmmmmmm
~., ~ ~
~mmmummmmuummmmmm
ooooooooo~
O
CA 02215514 1997-10-02
BASF l~ctiengesellschaft 940769 0. Z . 0050/45781
21
u~
o~
_,
o~
OC ~ a~
~r
,,
o
J~
~r r ~ ~ r~
H
O
O O
S
U ~ ~ I' o~
r ~ l l I
r o ~ ~ ~
~ ~ a a
m
u m m
m m .
o o o o
,-, u u
o
.
m a~ u u
oc m m m m m
~: m u u m m
p m m !r: m m
m m u u
oc m~ m m m m
~ co o~ o
o
z
CA 02215514 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
Preferably, in the preparation of the mixtures the pure active
compounds IA or IB and II are employed to which, as required,
further compounds active against pests (eg. insects, arachnids or
5 nemato~es) or harmful fungi or alternatively herbicidal or
growth-regulating active compounds or fertili2ers can be admixed.
The mixtures of the compounds IA or IB and II and the
simultaneous joint or separate use of the compounds IA or IB and
10 II are distinguished by an outstanding action against a broad
spectrum, in particular, of phytopathogenic fungi. In some cases,
they are systemically active (ie. they can be absorbed by the
treated piant when used for crop protection without loss of
action and if appropriate transported within the plant) and can
lS therefore also be employed as foliar and soil fungicides.
They are of particular importance for the control of a plurality
of fungi on various crop plants such as cotton, vegetable plants
(eg. cucumbers, beans and cucurbits), barley, grass, oats,
20 coffee, corn, fruit plants, rice, rye, soybeans, vines, wheat,
decorative plants, sugar cane and a plurality of seeds. In
particular, they are suitable for controlling the following
phytopathogenic fungi: Erysiphe graminis ~powdery mildew) on
cereals, Erysiphe cichoracearum and Sphaerotheca fuliginea on
25 cucurbits, Podosphaera leucotricha on apples, Puccinia species on
cereals, Rhizoctonia species on cotton and grass, Ustilago
species on cereals and sugar cane, venturia inaequalis (scab) on
apples, Helmintho~porium species on cereals, Septoria nodorum on
wheat, Botrytis cinerea (gray mold) on strawberries and vines,
30 Cercospora arachidicola on groundnuts, Pseudocercosporella
herpotrichoides on wheat and barley, Pyricularia oryzae on rice,
Phytophthora infestans on potatoes and tomatoes, Plasmopara
viticola on vines, Alternaria species on vegetables and fruits,
and Fusarium and Verticillium specie~.
They can additionally be used in the protection of materials (eg.
wood preservation), for example against Paecilomyces variotii.
The compounds IA or IB and II can be applied simultaneously,
40 together or separately, or successively, the sequence in the case
of separate application in general having no effect on the
control result.
The compounds IA or IB and II are customarily used in a weight
45 ratio of from 5:1 to 1:50, preferably 2:1 to 1:20, in particular
1:1 to 1:10 (I:II).
CA 0221~14 1997-10-02
~ASF Aktiengesellschaft 940769 O.Z. 0050/45781
Depending on the nature of the desired effect, the application
rates of the mixtures according to the invention are from 0.015
to 10 kg/ha, preferably 0.1 to 7 kg/ha, in particular 0.2 to
3 kg/ha.
The application rates here for the compounds IA and I~ are from
0.005 to 3 kg/ha, preferably 0.02 to 2 kg/ha, in particular 0.05
to 1 kg/ha.
10 The application rates for the compounds II are in general from
0.05 to 10 kg/ha, preferably 0.1 to 5 kg/ha, in particular 0.2 to
2 kg/ha.
In the treatment of seed, in general application rates of mixture
15 of from 0.001 to 0.1 g/kg of seed, preferably 0.002 to 0.05 g/kg
of seed, in particular 0.005 to 0.5 g/kg of seed, are used.
If harmful fungi which are pathogenic to plants are to be
controlled, the joint or separate application of the compounds IA
20 or I~ and II or of the mixtures of the compounds IA or I3 and II
is carried out by spraying or dusting the seeds, the plants or
the soils before or after the sowing of the plants or before or
after the germination of the plants.
25 The fungicidal synergistic mixtures or the compounds IA or I~ and
II according to the invention can be prepared, for example, in
the form of directly sprayable solutions, powder and suspensions
or in the form of high-percentage aqueous, oily or other
suspensions, dispersions, emulsions, oil dispersions, pastes,
30 dusting compositions, broadcasting compositions or granules and
applied by spraying, atomizing, dusting, broadcasting or
watering. The application form is dependent on the intended use;
in each case it should guarantee a dispersion of the mixture
according to the invention which is as fine and uniform as
35 posslble.
The formulations are prepared in a manner known per se, eg. by
addition of solvents and/or carriers. Inert additives such as
emulsifiers or dispersants are customarily admixed to the
40 formulations.
Suitable surface-active substances are the alkali metal, alkaline
earth metal and ammonium salts of aromatic sulfonic acids, eg.
lignosulfonic, phenolsulfonic, naphthalenesulfonic and
45 dibutylnaphthalenesulfonic acid, and also of fatty acid alkyl-
and alkylarylsulfonates, alkyl, lauryl ether and fatty alcohol
sulfates, as well as salts of sulfated hexa-, hepta- and
CA 0221~14 1997-10-02
BASF Aktiengesellschaft 940769 O.Z. 0050/45781
24
octadecanols or fatty alcohol glycol ethers, condensation
products of sulfonated naphthalene and its derivatives with
formaldehyde, condensation products of naphthalene or of the
naphthalenesulfonic acids with phenol and formaldehyde,
5 polyoxyethylene octylphenyl ethers, ethoxylated isooctyl-, octyl-
or nonylphenol, alkylphenyl or tributylphenyl polyglycol ethers,
alkylaryl polyether alcohols, isotridecyl alcohol, fatty
alcohol-ethylene oxide condensates, ethoxylated castor oil,
polyoxyethylene or polyoxypropylene alkyl ethers, lauryl alcohol
10 polyglycol ether acetate, sorbitol esters, lignin-sulfite waste
liquors or methylcellulose.
Powder, broadcasting and dusting compositions can be prepared by
mixing or joint grinding of the compounds IA or IB and II or of
15 the mixtures of the compounds IA or I3 and II with a solid
carrier .
Granules (eg. coated, impregnated or homogeneous granules) are
customarily prepared by binding the active compound or the active
20 compounds to a solid support.
Fillers or solid carriers used are, for example, mineral earths
such as silicic acids, silica gels, silicates, talc, kaolin,
limestone, lime, chalk, bole, loess, clay, dolomite, diatomaceous -
25 earth, calcium sulfate and magnesium sulfate, magnesium oxide,ground synthetic substances, and also fertilizers such as
ammonium sulfate, ammonium phosphate, ammonium nitrate, ureas and
vegetable products such as cereal flour, tree bark, wood and
nutshell meal, cellulose powder and other solid carrier~.
The formulations in general contain from 0.1 to 95% by weight,
preferably 0.5 to 90% by weight, of one of the compounds IA or IB
and II or the mixture of the compounds IA or IB and II. The
active compounds are employed here in a purity of 90~ to 100%,
35 preferably 95% to 100% (according to lH-NMR spectrum or HPLC).
The compounds IA or I~ and II or the mixtures or the
corresponding formulations are used by treating the harmful
fungi, their habitat or the materials, plants, seeds, soils,
40 surfaces or spaces to be protected from fungal attack with a
fungicidally effective amount of the mixture or of the compounds
IA or I9 and II applied separately. Treatment can be carried out
befo~e or after attack by the harmful fungi.
45 The synergistic action of the mixtures according to the invention
was shown by the following experiments:
CA 0221~14 1997-10-02
-
BASF Aktienge~ell~chaf t 940769 O. Z . 0050/45781
Laboratory experiments:
Spore germination test:
5 Suspensions of fungal spores (Botrytis cinerea, 106 spores / ml)
in microtiter plates were incubated at 18 C for 24 h after
addition of test solution. After 48 h and 72 h, the germination
was assessed under the microscope by estimation of the growth in
comparison with untreated controls.
Assessment was carried out in % in comparison with the untreated
control.
Active com- Action after 48 h Action after 72 h
pound II
8010a + Colbyc 8010a + ColbyC
[ppm~ I.2A-lb I.2A-lb
II.01 [10] 100 52 56 98 67 70
II.03 llO] 85 41 47 90 56 64
II.04 [l.Ol 42 1 23 55 4 39
II.05 [0.1] 28 2 15 33 17 24
II.06 [1.0] 61 14 34 75 44 53
II.07 [0.31 99 53 55 101 69 72
II.10 [10] 87 25 48 88 49 63
II.12 [3.3] 67 2 37 60 14 43
II.13 [0.3] 81 28 45 82 49 59
II.14 [3.3] 25 3 14 26 7 19
II.15 tl.0] 50 5 28 62 23 45
II.16 [1.0] 7 0 4 24 3 17
II.17 ~10] 85 30 47 86 50 62
II.18 [1.0] 75 25 42 81 47 58
4 0 a % action of the compound II
b % action of the mixture of II with 1 ppm of the compound I.2A-l
c % of expected value calculated according to Colby
CA 0221~14 1997-10-02