Note: Descriptions are shown in the official language in which they were submitted.
CA 0221~39 1997-09-1~
Catalyst and method for catalytic off-qas cle~n;nq
in the DMT process
The invention relates to a catalyst for cleaning a
pressurized off-gas obtained after the oxidation of para-
xylene (p-X) with air in the liquid phase in the preparation
of dimethyl terephthalate (DMT), to a process for preparing
such a catalyst, and to a method of cleaning the pressurized
off-gas obtained after the oxidation of para-xylene (p-X)
where the pressurized, oxygen-containing off-gas from the
oxidation is guided first of all via a single-stage or
multistage condensation, a single-stage or multistage
absorption and subsequently, with or without the supply of
oxygen, via a catalytic afterburner which is operated under
pressure.
It is known that the current Witten DMT process
essentially comprises the steps of:
(1) oxidation of para-xylene (p-X) and methyl para-
toluate (p-TE), generally with downstream off-gas
cleaning,
(2) esterification with methanol of the reaction
products from the oxidation,
(3) separation of the resulting so-called crude ester
(DMT, crude)into a p-TE-rich fraction, which is
customarily recycled to the oxidation, a crude DMT
fraction, which generally contains more than 85~ by
weight of DMT, and a high-boiling residue fraction,
workup thereof, when required, for example by a
downstream methanolysis or thermolysis, and
subsequent recovery of the catalyst, and
o.z. 5104
23443-614
CA 0221~39 1997-09-1~
(4) purification of the crude DMT fraction, for example
by washing, recrystallization and distillation
("Terephthalsauredimethylester", Ullmann Vol. 22, 4th edition,
pp. 529-533; EP 0 464 046 B1; DE-A 40 26 733). It is also
known that terephthalic acid of appropriate quality can be
prepared from DMT, i.e. from particularly DMT-rich fractions
or ultrapure DMT, by specific hydrolysis.
A mixture of para-xylene (p-X) and methyl para-
toluate (p-TE or p-T ester) is oxidized generally with
atmospheric oxygen in the presence of a heavy metal catalyst
(DE-C 20 10 137) at a temperature from about 140 to 180~C and
at a pressure of from about 4 bar to 8 bar absolute in the
liquid phase. The oxidation stage produces a reaction mixture
which predominantly contains monomethyl terephthalate (MMT),
p-toluic acid (p-TA) and terephthalic acid (TA) dissolved or
suspended in p-TE and is esterified with methanol, at a
temperature from about 250 to 280~C and at a pressure of from
20 to 25 bar absolute. In addition, the oxidation produces an
off-gas which depending on the pressure and temperature is
substantially saturated with aliphatic and also aromatic
compounds. Thus the off-gas contains not only target products
but also reaction by-products, including the low-boiling
compounds acetaldehyde, formaldehyde and the corresponding
methyl acetals, dimethyl ether, acetic acid and formic acid
and their methyl esters. Apart from these organic constit-
uents the off-gas from the oxidation essentially comprises
atmospheric nitrogen, a residual oxygen content of from about
0.5 to about 4~ by weight, CO2 at from about 1 to 3~ by
weight, and from about 0.3 to 2.0~ by weight of CO. In the
O.Z. 5104
23443-614
CA 0221~39 1997-09-1~
Witten DMT process the off-gas is usually first of all
subjected to multistage cooling, in the course of which the
relatively high-boiling and middle-boiling target products
gradually condense out. The target products that have
remained in the off-gas, predominately methanol and p-X, are
subsequently removed in all but traces from the off-gas in a
multistage absorption procedure, the target products that have
accumulated in the absorbents being recycled to the process.
In numerous countries there are statutory
regulations requiring the removal of organic carbon compounds
and CO from off-gases and expelled air.
EP 0 544 729 Bl discloses a process for cleaning an
oxidation off-gas originating from atmospheric oxidation of
xylene and under a pressure of from 5 to 50 bar, wherein at
least the xylene is first of all substantially removed in an
off-gas scrubber under pressure by absorption with an ester,
for example methyl para-toluate (p-TE) or methyl benzoate
(BME) or an ester mixture, for example of p-TE and BME. It is
also possible for the absorption procedure to be preceded by a
condensation stage. In addition, it is intended that oxidiz-
able substances still present in the off-gas after the
absorptive cleaning should be incinerated under pressure and
that the pressurized off-gas should be utilized in an
expansion turbine for producing energy. The incinerators
required for such incineration of off-gases under pressure are
complex and expensive both to procure and to operate.
Furthermore, appropriate pressure incineration chambers are
now almost impossible to obtain on the market.
In this context EP 0 544 726 Bl mentions the
O.Z. 5104
23443-614
CA 022l~39 l997-09-l~
possibility of subjecting the oxidizable constituents in the
off-gas to catalytic incineration under pressure, where the
off-gas to be incinerated, following the absorption scrubber,
is generally in a state in which it is saturated with steam.
The VDI [German Engineers Association] guideline
3476, "Katalytische Verfahren der Abgasreinigung" [Catalytic
methods of waste-gas purification], VDI-Handbuch Reinhaltung
der Luft, Volume 6 (June 1990), describes inter alia the
removal of CO, hydrocarbons and NOx from car exhaust gases
using Pt/Rh/Pd on ceramic supports at temperatures from 300 to
950~C. Support materials employed for catalysts for off-gas
cleaning include metals in the form of shaped sheets (expanded
mesh), weaves, nets, moldings of metal oxide, such as A1203,
SiO2, TiO2, ZrO2, MgO, and also natural and synthetic
minerals, such as pumice, mullite, cordierite, steatite or
zeolites. Mention is also made here, quite generally, for the
removal of CO and vapors of organic compounds from industrial
waste gases, of noble metal catalysts or metal oxide catalysts
on ceramic supports, supported catalysts of high surface area,
or unsupported catalysts.
EP 0 664 148 Al provides a process for cleaning a
pressurized off-gas by catalytic afterburner at an operating
temperature of between 250 and 800~C and at a pressure of
between 2 and 20 bar, with the use in particular of a catalyst
comprising platinum and/or palladium on ~-aluminum oxide.
Experiments show that under the operating conditions in an
off-gas from the oxidation of para-xylene in the preparation
of DMT such a catalyst is deactivated after only a short
operating period and that the required reduction in pollutants
O. Z . 5104
23443 -614
CA 0221~39 1997-09-1
is thus no longer achieved.
A major object of the invention, therefore, is to
provide a catalyst system which satisfies the requirements for
the cleaning of the off-gas produced in the oxidation in the
course of the preparation of DMT . An additional'concern of
the present invention is to tie such an off-gas cleaning
measure into the DMT process from an economic standpoint as
well. The invention also applies to the cleaning of an off-
gas from the oxidation of other alkyl aryls, for example o-
xylene, m-xylene, toluene, and xylene and toluene derivatives.
It has now been found that a catalyst which
comprises at least one oxide of titanium and at least one
element from subgroup VIII of the Periodic Table of the
Elements in the metal or oxide form is outstandingly suitable
for the method of cleaning a pressurized off-gas originating
from the oxidation of para-xylene (p-X) with air in the liquid
phase in the preparation of dimethyl terephthalate (DMT),
since even after a comparatively long operating period no
notable deactivation was found, the required off-gas values
were achieved, and thus the catalyst is also notable for an
excellent economic service life.
The present invention therefore provides a catalyst
for cleaning a pressurized off-gas obtained after the
oxidation of para-xylene (p-X) or a mixture of para-xylene
with methyl para-toluate (p-TE) with air in the liquid phase
in the preparation of dimethyl terephthalate (DMT), wherein
the catalyst comprises:
a) at least one oxide of titanium, and
b) at least one element from subgroup VIII of the
O.Z. 5104
23443-614
CA 0221~39 1997-09-1~
Periodic Table of the Elements in the metal or oxide form.
The novel catalyst preferably contains, based in
each case on the weight of the catalyst:
the component a) in an amount of from 50 to 99~ by
weight, more preferably 80 to 99~ by weight, and
the component b) in an amount of from 0.01 to 5~ by
weight,
where the component b) is calculated in total and as
metal.
The titanium oxide or a precursor compound for
component a) preferably originates from the so-called sulfate
process, so that the novel catalyst preferably contains as
additional component c), a sulfate. The component c) is
possibly present, in particular, in an amount of from 0.1 to
10~ by weight, based on the weight of the catalyst.
The sulfate as the component c) can be present in
the novel catalyst as such or else, for example, as hydrogen
sulfate or oxide sulfate or water-containing oxide sulfate of
a corresponding metal or in the form of sulfuric acid which is
adhering to or has formed an adduct with a metal oxide
compound. The sulfate can also be present in two or more of
the abovementioned forms alongside one another.
In the novel catalyst, the component a) is
preferably present as titanium dioxide, for example as rutile.
More preferably, however, in the novel catalyst, the component
a) is predominantly in the anatase form. It is also possible,
however, for titanium oxide with an oxygen deficit or water-
containing oxide, oxide hydroxide or hydroxide or sulfate to
be present, including hydrogen sulfate, for example oxide
O.Z. 5104
23443-614
CA 0221~39 1997-09-1~
sulfate or water-containing oxide sulfate of titanium, or
titanium oxide with sulfuric acid in adduct form or adhering.
It is also possible for a proportion of the component b) to be
present in the novel catalyst in a sulfatic form.
Preferably, the sulfate c) is present in the form of
sulfate of barium, tungsten, vanadium, zirconium or a mixture
thereof.
As additional components, the novel catalyst may
optionally include an oxide of tungsten, an oxide of vanadium,
an oxide of zirconium, or a corresponding phosphate, including
a hydrogenphosphate, an oxide of silicon or a silicate.
The structure given to the novel catalyst is not
critical and, may be any practical form such as spherical,
strand-like, tubular, annular or else like a saddle or
honeycomb. The novel catalyst preferably in general has a
geometric surface area of from around 200 to 2,000 m2/m3 and
is preferably a strand extrudate, for example with a diameter
of 4 mm. The novel catalyst may also have a honeycomb
structure, especially such a structure in which the hydraulic
diameter (4a/U=dh where a = inside channel cross-sectional
area, U = circumference of the inside channel cross-sectional
area and dh = hydraulic diameter) of the individual channel
cross-sections of the flow-traversed honeycomb channels is in
the range from 1.5 mm to 6.5 mm.
The present invention also provides a process for
preparing the above-mentioned catalyst, which comprises
applying the component b) to a shaped support which is formed
essentially of the component a), individually or in a mixture
by means of a dip impregnation or spray impregnation.
O.Z. 5104
23443-614
CA 0221~39 1997-09-1~
A process for preparing a shaped support based on
titanium oxide, also referred to as a molding, is disclosed
for example, in DE-C 26 58 569. The novel catalyst is
generally prepared by using a molding based on titanium oxide
which may be obtained inter alia by the preparation and
extrusion of a moldable composition containing titanium oxide
and subsequent drying and calcining of the molding, and
preferably has a BET surface area of between 10 and 200 m2/g
and a pore value of between 0.1 and 0.6 cm3/g. To improve the
mechanical properties, the molding may be reinforced by means
of glass fibers, for example.
In general, the component b) of the novel catalyst
is applied to the support by impregnation. To this effect,
the molding is generally brought into contact with a solution
which contains the component b), preferably in the form of a
dissolved salt. In the novel process, the molding may be
impregnated using a solution prepared preferably by employing
a nitrate of the component b). In preparing the solution, it
may be necessary to adjust the pH of the solution in an
appropriate manner, for example by adding an organic or inor-
ganic acid, for example nitric acid, or an alkali, or to add a
complexing agent or stabilizer, for example for stabilizing a
noble metal sol. The impregnation can be effected by spraying
the molding one or more times with the solution or by dipping
the molding one or more times into the solution.
The impregnated molding is dried, preferably with
the ingress of air, in a temperature range of from 30 up to
650~C and is subsequently calcined, i.e. subjected to a
thermal aftertreatment.
O.Z. 5104
23443-614
CA 0221~39 1997-09-1~
When a nitrate is employed, a particular advantage
of the novel preparation process is that the component b) can
be fixed on the molding by a simple thermal treatment of the
impregnated molding in the metal and/or oxide form and without
further residues, for example halides. In this way it is also
possible to save costly and time-consuming operations which
may be necessary, for example, in the case of wash coating, or
a reduction step with hydrogen in the gas phase.
In the novel catalyst, the component b) is prefer-
ably present predominately in the region of a surface of the
catalyst molding; in other words, preference is given here to
a so-called shell impregnation. As the component b), the
novel catalyst preferably comprises at least one of platinum,
palladium and rhodium. Particular preference is given to the
novel catalyst having a platinum content in the range from
0.05 to 0.5~ by weight, especially from 0.1 to 0.2~ by weight
of Pt, based on the weight of the catalyst.
The present invention provides, furthermore, a
method of cleaning a pressurized off-gas obtained after the
oxidation of para-xylene (p-X) or a mixture of para-xylene and
methyl p-toluate (p-TE) with air in the liquid phase in the
preparation of dimethyl terephthalate (DMT), where the
pressurized, oxygen-containing off-gas from the oxidation is
led first of all via a single-stage or multistage conden-
sation, a single-stage or multistage absorption and
subsequently, with or without the supply of oxygen, via a
catalytic afterburner which is operated under pressure, using
the catalyst as defined previously.
In general, in the novel off-gas cleaning method,
O.Z. 5104
23443-614
CA 0221~39 1997-09-1
- 10 -
the target products present in the oxidation off-gas from air
oxidation of p-X (these products are essentially p-X, p-TE,
DMT, methyl benzoate (BME) and methanol) are predominately
removed from the off-gas in a condensation unit, also in an
optional downstream scrubber unit or absorption unit, and are
recycled appropriately to an earlier stage of the process.
The condensation is in general operated at a temperature in
the range from 15 to 80~C and at a pressure of from 3 to 20
bar absolute.
The pressurized gas originating from the
condensation is preferably heated in a countercurrent heat
exchanger, for example from about 25~C to around 120~C at a
pressure of from 3 to 20 bar absolute, and then is run into a
scrubber unit (i.e., absorption unit). The absorption unit
may consist of a plurality of scrubbing stages; for example,
off-gas scrubbing with BME or an ester mixture can be carried
out first of all.
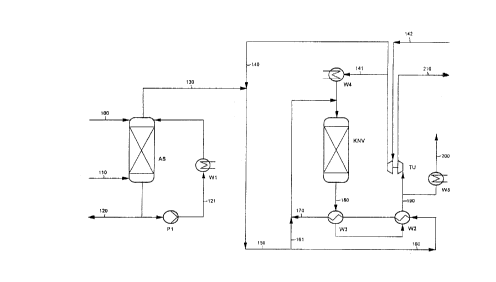
Figure 1 shows a preferred embodiment of the novel
method for cleaning a pressurized off-gas according to the
present invention.
Downstream of such an absorption unit, the off-gas
(110) may preferably be further saturated with wastewater
(100) obtained in the process, the so-called process water
generally containing organic constituents. In general this is
done using a so-called saturator (AS). The process water is
preferably circulated via a pump (P1) and a heat exchanger
(W1) which is heated with low-pressure steam, and is fed to
the top of the saturator (AS). In this procedure the off-
gas generally becomes saturated with water and with the
O.Z. 5104
23443-614
CA 0221~39 1997-09-1~
essentially volatile organic constituents present in the
original process water. In order to avoid clogging of the
saturator (AS) as a result of the accumulation of solids and
of high-boiling constituents which are generally obtained in
the bottom of the saturator, it is possible to draw off a
small amount of the wastewater (120) from the bottom section
of the saturator (AS) and to recycle it to the process at an
appropriate point, for example.
The off-gas leaving the absorption unit, preferably
the saturator (AS), normally comprises by-products obtained in
the DMT process, such as CO and also low-boiling compounds
such as acetaldehyde, formaldehyde, methyl acetate, dimethyl
ether, acetic acid and formic acid and their methyl esters.
In order to dispose of these by-products in the most
environmentally friendly way, the pressurized off-gas may then
be passed to a catalytic afterburner.
In the catalytic afterburner (KNV) the novel
catalyst is employed here. The reactor of the catalytic
afterburner is in general designed as a fixed-bed reactor and
is able to accommodate bulk catalysts and monolithic
catalysts. It is appropriate to employ honeycomb-shaped
monoliths, which are generally notable for a very low pressure
loss, which in the case of the novel off-gas cleaning method
has a positive effect in connection, in particular, with
energy recovery by an off-gas turbine.
In the novel method the catalytic afterburner is
preferably operated at a pressure of between 2 and 20 bar
absolute, particularly preferably at from 5 to 10 bar
absolute, and at a working temperature of between 160 and
O.Z. 5104
23443-614
CA 022l~39 l997-09-l~
- 12 -
650~C, particularly preferably between 200 and 550~C. With
preference, the catalytic afterburner in the novel method is
operated with a space velocity (GHSV) in the range from 1000
h-1 to 50,000 h-1, particularly preferably in the range from
5000 h-1 to 80,000 h-1 (GHSV = V(s.t.p.)/Vcat [m3 (s.t.p./
m3 ~ h], where V(s.t.p.) = volume flow under standard
conditions [m3 (s.t.p.)/h] and Vcat = catalyst volume [m3])
For the first run-up of the reactor (KNV), process
air (141) is in general first of all heated via an electri-
cally operated heat exchanger (W4) and passed via the reactor
(KNV) until the latter has reached its intended operating
temperature, which ensures the activation of the catalyst.
The reactor can then be loaded with off-gas. Suitably, the
catalytic afterburner then runs with substantial thermal
autonomy. In the case of the novel method, the off-gas
upstream of the catalyst usually contains water as steam in an
amount of from 0.04 to 2 . 8 kg/m3 (s.t.p.), in particular from
0.1 to 0.4 kg of water per3 m (s.t.p.) of off-gas.
Before it enters the catalyst region, the off-gas
stream to be cleaned (150) is preferably preheated (160,161,
170) in countercurrent with the cleaned off-gas (clean gas)
(180) coming from the reactor by means of an appropriate
arrangement of corresponding heat exchangers (W2, W3 ) .
The oxidation in the DMT process is generally set
such that the oxygen required for catalytic combustion is
already present in the off-gas before the off-gas enters the
reactor. The oxygen content in the off-gas (130) may, if
required, be raised by supplying compressed air (140). In
this way the organic compounds and CO present in the off-gas
O.Z. 5104
2 3 44 3 - 6 14
CA 022l~39 l997-09-l~
- 13 -
are generally converted almost completely to carbon dioxide
and water over the novel catalyst. The oxygen content in the
clean gas (180, 190) is preferably adjusted to a level of from
0.5 to 2 % by volume.
In order to recover the compression energy, it is
possible to depressurize the clean gas (190) by way of an off-
gas turbine (TU) to produce mechanical or electrical energy.
For energy recovery by means of an expansion turbine, use is
generally made of those clean gas streams which are under a
pressure of, preferably, more than 3 bar absolute. Since the
off-gas is then generally in accordance with requirements, it
can be led off (210) via a stack. For this purpose the
temperature may be controlled, possibly by supplying unheated
off-gas, such that the clean gas leaving the off-gas turbine
has a temperature of about 125~C.
In the case of the novel method, it is also possible
to divert at least some of the clean gas stream upstream of
the turbine, to dry it appropriately and then cool it (W5) to
a temperature lower than 40~C, and to use it again (200)
appropriately as inert gas, for example for blanketing in the
process.
In addition to the good economic catalyst service
life obtained, the particular advantages of the novel method
also consist in the simultaneous utilization of the waste
water obtained, in the incineration of all of the by-products
which are obtained in the oxidation and which pass into the
off-gas, and the minimization of the CO content in the clean
gas to a value which in many cases can no longer be detected
with measuring instruments used in everyday operation.
O. Z . 5104
23443-614
CA 0221~39 1997-09-1
- 13a -
The invention is illustrated in more detail by the
following examples:
Example~
Example 1:
- Preparing a catalyst for cleaning the off-gas from the
oxidation in the Witten DMT process -
2000 g of a commercial, TiO2-based catalyst support
in strand form (type H9050 from Huls AG prepared by a process
described in DE-C 2658 569) were charged to a rotating drum
and heated to 110~C by means of a stream of hot gas which was
guided onto the support. When the temperature reached, 50Oml
of an aqueous Pt nitrate solution where w(Pt) = 5.2 g/l were
sprayed on at 110~C over a period of about 20 minutes. In the
course of this procedure the solvent evaporated and the metal
was deposited on the support in a thin marginal zone. The
material was then removed and calcined at 450~C in a stream of
air for 4 hours.
Example 2:
- Cleaning the off-gas from the oxidation in the Witten DMT
process -
Figure 1 shows a preferred embodiment of the novelmethod of cleaning pressurized off-gas from the oxidation in
the Witten DMT process. In this connection, Table la + b sets
out material streams, their composition and the respective
operating conditions; cf. key. The amounts of off-gas and
wastewater and their compositions are typical of a DMT/PTA
plant with a capacity of 240 kt/a.
The off-gas which is obtained downstream of the
condensation and absorption unit in the DMT/PTA plant and has
o.z. 5104
23443-614
CA 0221~39 1997-09-1
- 13b -
been substantially freed from useful products enters the
bottom section of the saturator (AS) as a material stream
(110) at a rate of 70,768 kg/h and at a temperature of 120~C.
The wastewater (20,000 kg/h) (100) originating from the
DMT/PTA plant is fed in at the top section of the saturator.
The saturator is fitted either with valve trays or with
structured packing. By means of a circulation pump (P1) the
wastewater is circulated (121) via a heat exchanger (W1) which
is heated with low-pressure steam. In order to avoid
accumulation of solids in the saturator with the possibly
attendant functional disruptions to the plant, a small part of
the bottom product from the scrubber is removed (120) and
recycled to the DMT/PTA process at an appropriate point.
For the run-up of the reactor (KNV), process air
(141) is first of all passed via the electrically heated heat
exchanger (W4) into the catalytic afterburner, until a temper-
ature is reached which ensures the commencement of the
reaction.
The scrubbed off-gas (130) which is saturated with
water and with the organic constituents of the wastewater,
with a temperature of 121.5~C and a pressure of 7.1 bar, is
heated by the countercurrent heat exchangers to 250~C (150,
160, 170) and is passed into the reactor of the catalytic
afterburner.
The entry temperature of the off-gas before the
reactor can be adjusted both in the course of run-up and
during normal operation by means of the material stream (161).
In addition, atmospheric oxygen required for combustion can be
supplied by way of the material stream (140).
O.Z. 5104
23443-614
CA 022l~39 l997-09-l~
- 13C -
The reactor (KNV) is charged with the catalyst
type H 5922 from Huls AG - cf. Example 1 - and is operated
with a space velocity in the region of around 30,000
[m (s.t.p.)/m h].
Combustion is complete and requires only relatively
small excesses of oxygen.
As a result of the heat of reaction of the exother-
mic combustion process, the cleaned off-gas (clean gas) leaves
the reactor with a temperature of 401~C (180) and is guided
onto the jacket side of the heat exchangers (W2, W3) for
heating of the off-gas, in the course of which the clean gas
is cooled to a temperature of 277~C (190) and is then let down
(210) to atmospheric pressure by way of the off-gas turbine
(TU) coupled to the air compressor, and is led off into the
atmosphere by way of the stack.
In order to obtain inert gas it is possible to cool
the clean gas (190), in part under pressure, and to use it
(200) in the DMT/PTA plant for blanketing.
O . Z . 5104
23443 -614
CA 02215539 1997-09-lS
- 14 - o. z . 5104
Key to Fiqure 1, Table 1 and the mater;al streams
Figure 1 shows a preferred embodiment of the novel method of cleaning
pressurized off-gases from the oxidation in the Witten DMT process.
Material streams in Figure 1 and Tables 1a and b:
5 100: process water
110: off-gas from the condensation and absorption unit
1 2û: bottom product, recycled to the process
121: saturator circuit
130 off-gas from the saturator
10 140: - compressed air
141: compressed air for running up the reactor
142: air intake
150: off-gas, enriched with oxygen
160: flow of off~as to the neat exchangers W 2 + 3
15 161: flow of off-gas for regulating the reaction temperature
170: off-gas heated by countercurrent heat exchanger, upstream of the
reactor
180: clean gas downstream of the reactor
190: clean gas downstream of the countercurrent heat exchanger
20 200: substream of c!ean gas, cooled and under pressure, recycled to the
process as inert gas
210: residual, depressurized stream of clean gas upstream of the stack
Plant comp~nents in Figure 1:
AS : saturator
25 P1 : pump
W1 : heat exchanger, operated with steam
W2+3: c~untercurrent heat exchangers
KNV : reactor for catalytic afterburning
W4 : heat exchanger, electrically operated
30 W5 : heat exchanger
TU : expansion turbine
Key to Tables 1a and b:
In Tables 1a and b the material streams, their compositions and the
respective operating conditions are set out in relation to Figure 1.
CA 0221~39 1997-09-1~
- 15 - o . z . 5 104
Key to abbreviations:
p-X : para-xylene
p-TA : para-toluic acid
p-TE : methyl paratoluate (pT ester)
BME : methyl benzoate
HM-BME : methyl hydroxymethylbenzoate
MM-BME : methyl methoxymethylbenzoate
DMT : dimethyl terephthalate
DMT, crude = crude ester (DMT crude ester stream after the
l 0 esterification)
Crude DMT : dimethyl terephthalate fraction after the crude ester
distillation
DMT-ultrapure : ultrapure dimethyl terephthalate (highly pure DMT
intermediate or end product)
DMO : dimethylorthophthalic acid
DMI : dimethylisophthalic acid
DMP : dimethyl phthalate = isomer mixture of DMT, DMO
and DMI
MMT : monomethyl terephthalate (terephthalic acid
2 0 monomethyl ester)
TA : terephthalic acid
MTA : medium-purity terephthalic acid
PTA : high-purity terephthalic acid
PTA-p : very pure, i.e. ultrapure, terephthalic acid (contents
2 5 of MMT and p-TA together of < 50 ppm by weight)
TAS : terephthalaldehyde acid (4-CBA)
TAE: : terephthalaldehyde acid methyl ester
Formaldehyde-DMA: formaldehyde dimethyl acetal
Acetaldehyde-DMA: acetaldehyde dimethyl acetal
CA 022l5539 l997-09-l5
-- 16 --
o u~ O tq ~ ~
o - ~ O ~ al ~ Y
a) ~ ~
o
7 o ~-- ~ ~ Y
$
o U~ ~ o
$ ~ U~
, ln ~ ~ ~ y ~
O O ~ ~ ~ 0 ~ 0 a~ n 0
~ ~ o ~_ Y U~ ~ ~
O ~ ~ ~ cn a~ D ~ ~ '~
o ~ 0 V~ 0 0
~ ~ U~ ~ ~ O ~
.. , _
8 V V ~ o S
5 i o ~ S
~_ 5 ~ 3 ~ ~Q ~ ~ LL ~ e~. ~ llJ ~.1 C
23443-6 1 4
CA 02215539 1997-09-15
-- 1 7
o a~ ~ o
' ~
~ C ~ ~ O
~ (D r- ~r
o ~ a~ D C C:~
~D
o ~ ~ q' O ~n
O ~ ~ ~
~ O O
o
~n
O O
.. ~ ~ :
' ~.
n ~ - ' O O
2344 3-6 1 4
Table 1b
Malerlal slream- 100 110 120 130 140 150 1.70 180 190 210
Amounls Ikg/hl 20000 7076B s68 90200 54 90254 90254 90254 90254 90254
Amounls [m lhl 16229 7.97 16242 21648 28382 23109 106052
T. - ~ e l-Cl35 110 105 1215 110 122 250 401 277 123
Pressure Ibarl 7.1 7.1 7.1 7.1 7.5 71 7 7 6 9 1.1
Cr , - -~ r r1% by wl.l 1~h by wl.ll% by wl.ll% by wll 1% by wt IlUh by wt I seo 150 lUh by w1.1 see 180 1% by wt.l
D
W~ter 95.47 0.23 94.01 20 75 20 74 21.33 21.33 1
Melhanol 0.01 0 03 0.02 0 03 0 03 _
p-TE traces Iraces traces Iraces X
p-xyleno 0 01 0 1 ~
BME traces 0.04 0 01 0.03 0 03 O
Acellc acld2.60 0 00 4 56 0 55 o 55
Fommlc acld1.20 0 00 1.36 0 26 0 26
r. j~- 0.02 0.15 015
Ac~ I I jd~0.66
Melhyl acotato 0 01 0 02 0 02 0.02
~ Elhyl acetato Iraces Iraces Iraces Iracoa
w
C ~ 'Dorl~h by wt.ll% by wt.l1% by wl.l 1% bY ~ 11% by v~ 11~h bY wt l see 1501% by w~.l see 180 1% by wt.
Dlmethyl ether lraces Iraces Iraces traces
Methvl torm. ~ 0 05 0 01 0 02 0 02 0 02
CA 02215539 1997-09-15
-- 19 --
o ~ ~ U~ o
o
a~
ao ~ ~ U~ o
o
U7 ~ ~ ~ 0 'D ~
- _ g ~ ('~ O
O (D
~r ~
,,, n ~ ~ D ~
5 ~ r N ~ b
~o o o
-- o o
~
_ g g ,~ o
oO 9 9
,, s
. o
1- 5 '~ Z 0 8 8
23443-6 1 4