Note: Descriptions are shown in the official language in which they were submitted.
CA 02215777 1997-09-18
"PROCESS FOR SHUT-DOWN OF A MEMBRANE OPERATION"
FIELD
This invention relates to a procedure for operating' a continuous membrane
separation
process wherein membrane degradation may be prevented during membrane shut-
down,
particularly during sudden or unexpected shut-down of the membrane separation
zone, and most
s specifically the invention relates to a method for safe membrane shutdown
within a process for
the removal of carbon dioxide from light hydrocarbon gases.
BACKGROUND
io Permeable membrane processes and systems are known in the art and have been
employed or considered for a wide variety of gas and liquid separations. In
such operations, a
feed stream is brought into contact with the surface of a membrane, and the
more readily
permeable component of the feed stream is recovered as a permeate stream, with
the less-readily
permeable component being withdrawn from the membrane system as a non-permeate
stream.
i s Membrane separation modules are maintained at operating conditions which
result in a non-
permeate side pressure at which the feed gas is introduced and the non-
permeate stream is
withdrawn, and a permeate side pressure at which the permeate stream is
withdrawn. The
pressure on the non-permeate side of the membrane is higher than the pressure
on the permeate
side, and the pressure differential between the non-permeate and the permeate
sides of the
a o membrane generally determines the degree. of separation attained by the
.membrane,separation. _ ~ .
Membranes are widely used to separate permeable components from gaseous feed
streams. Examples of such process applications may include removal of acid
gases from natural
gas streams, removal of water vapor from air and light hydrocarbon streams,
and removal of
25 hydrogen from heavier hydrocarbon streams. Membranes are also employed in
gas processing
applications to remove permeable components from a process gas stream. Natural
gas as
CA 02215777 1997-09-18
2
the natural gas.by bulk methods employing membrane systems,
The inherent simplicity of such fluid separation operations constitutes an
incentive in the
art to expand the use of membrane systems in practical commercial operations.
The selectivity
s and permeability characteristics of such membrane systems must be compatible
with the overall
production requirements of a given application. It is also necessary that the
membranes exhibit
acceptable stability and do not suffer undue degradation of their performance
properties in the
course of practical commercial operations.
to Membranes for gas processing typically operate in a continuous manner,
wherein a feed
gas stream is introduced to the membrane gas separation module on a non-
permeate side of a
membrane. The feed gas is introduced at separation conditions which include a
separation
pressure and temperature which retains the components of the feed gas stream
in the vapor phase,
well above the dew point of the gas stream, or the temperature and pressure
condition at which
i s condensation of one of the components might occur. However, if the flow of
the feed gas stream
is interrupted, or the feed pressure is suddenly reduced, the residual
material within the
membrane separation zone could reach its dew point and condensation would
result. The feed
gas stream fed to the gas separation membrane may contain a substantial amount
of moisture and
may cause corrosion and condensation in instrumentation, piping, pneumatic
tools, ventilators
a o and other equipment associated with the gas separation membrane. In
certain instances, it may
also lead to inferior performance of the gas separation membrane and/or other
equipment such as
adsorption traps. In anticipation of a reasonable amount of condensation,
membrane systems are
often oversized to compensate for the loss of membrane surface over the useful
life of the
membrane. For high volume gas treating application, this over design of
membrane capacity can
25 be very costly.
For gas drying applications, methods has been disclosed for the employing
sweep gases to
remove moisture from the membrane before it condenses. For example, in air
separation
applications which constitute a highly desirable field of use for permeable
membranes, oxygen is
3 o typically the more readily permeable component of the feed air for
particular membranes and is
withdrawn as the permeate gas. In such embodiments, nitrogen is the less-
readily permeable
c
CA 02215777 1997-09-18
3
component and is recovered as non-permeate gas. Liquid water is generally
removed from feed
air upstream of the membrane by conventional means such as knockout drums. Any
water vapor
present in the feed air will permeate the membrane resulting in a dry non-
permeate gas. In air
separation applications, the performance characteristics of the membranes are
sensitive to the
s presence of certain contaminants in the feed air stream. Exposure to such
contaminants may
result in a significant reduction in the permeability of the membrane in use.
Fortunately, most
contaminants commonly present in ambient air, such as light hydrocarbons, H20,
and COZ, have
been found to result in, at most, a modest decrease in membrane permeability.
The presence of
even relatively low concentrations, e.g., less than 1 ppm by volume as C,o, of
heavy hydrocarbon
io oil vapors, such as might enter the feed air stream from an oil lubricated
air compressor, can
result in rapid and extensive loss of membrane permeability.
In response to such an undesirable decrease in membrane permeability, it is
presently
common membrane practice to size the active membrane surface area with a
safety factor
i5 sufficiently large to compensate for the anticipated permeability loss from
all sources. Initially
the membrane system is significantly oversized for the desired product flow,
and the feed gas
compressor is typically operated in a turndown mode. As permeability
degradation proceeds,
either the operating temperature or pressure, or both, are increased to
compensate for the
decrease in permeability. In some instances, it is necessary or desirable to
by-pass some of the
a o modules in the membrane system initially so as to reduce excess membrane
area employed when
the membranes exhibit their full permeability capability and subsequently to
bring such by-passed
modules on stream as degradation of the initially employed modules progresses.
In addition to a
. significant capital cost penalty associated with the provision of extra
membrane surface area, such
a membrane system must operate over a significant portion of its operating
life under off design
a s conditions and that the control strategy for such a membrane system is
more complex than for a
system operating closer xo its optimum design conditions. . .. . . . . . . .
As an alternative to such over design of membrane systems to compensate for
degradation
in use, attempts have been made to restore lost performance, but such efforts
were initially
3 o unsuccessful in developing an economically feasible means for restoring
the permeability of
degraded membranes. Restoring any portion of the degraded membranes would
require
CA 02215777 1997-09-18
4
interruption of the gas treating operation, displacing large quantities of
gas. Neither over design
of the membrane system nor interruption of gas product operations for membrane
restoration
treatment, or a combination of these approaches is an entirely satisfactory
means for overcoming
permeability degradation in practical commercial air or other gas separation
operations. Further
s improvement in the response to the problem of membrane degradation is highly
desirable in the
membrane art.
US-A~,881,953 discloses an approach to the problem of preventing premature
loss of
membrane capacity by passing the feed gas mixture through a bed of adsorbent
material, such as
i o activated carbon to adsorb contaminants such as heavier hydrocarbon
contaminants without the
removal of lighter hydrocarbons. It requires that a means for removing
moisture from the feed
gas be provided because high moisture levels generally limit the ability of
activated carbon
adsorbents to retain their adsorptive capacity for heavy hydrocarbons.
i5 US-A-5,030,251 relates to the operation of a membrane separator which
removes water
vapor from a moist air feed to produce a drier air product. When such a
membrane operation is
stopped, some residual water vapor remains in the membrane separator and when
the feed flow is
resumed , the residual water vapor flows out with the non-permeate stream.
This results in a less
dry product produced during restarts than during the steady-state operation of
the membrane
a o separator. To correct this problem, a portion of the non-permeate product
is saved in a storage
tank and supplied to the membrane separation at a time when the feed is not
being supplied to the
separator to purge the residual water vapor between cycles. When the _ feed
cycle is off, the air
pressure of the non-permeate side of the separator reduces to atmospheric
pressure. Because the
pressure in the storage tank is greater than atmospheric, some of the stored
non-permeate bleeds
a s back to form the purge stream.
US-A-5,383,956, relates to processes and apparati for starting up and shutting
down
membrane gas separation systems treating a wet gas feed gas stream. The
process employs a
membrane dryer module and a gas separation membrane module in various start up
sequences
3 o and shut-down sequences for drying and separating the feed gas stream. In
the shut-down of
such a process comprising at least one gas separation module and at least one
membrane dryer,
t
CA 02215777 1997-09-18
the flow of the feed gas is stopped to both membrane modules, and the modules
are depressurized
by removing pressurized gas from the non-permeate sides of the modules. The
pressurized gas is
allowed to permeate through the respective membrane modules to the permeate
sides, followed
by purging both the permeate and non-permeate sides of the membrane modules
with a dry gas
s stream.
When a natural gas stream is processed in a membrane separation zone, the
presence of
heavy hydrocarbons, such as C6 plus hydrocarbons, and particularly C,o plus
hydrocarbons under
certain conditions such as reduction of temperature and pressure, or a change
in composition can
to result in the loss of membrane capacity and often permanent damage to the
membrane. Processes
are sought to prevent such damage to the membrane separation unit.
It is an object of the invention, therefore, to provide an improved shut-down
process for a
membrane system which process overcomes the problem of degradation of
permeability during
i s hydrocarbon gas production operations such as in natural gas production.
It is another object of the invention to provide a membrane system and shut-
down process
obviating the need for significant over design or for premature replacement of
degraded
membrane modules and minimizing the need for the interruption of gas producing
operations for
a o the treatment of membrane modules for restoration of the permeability
characteristics thereof.
SUN)~VIARY
2 5 The invention provides a process for the safe shut-down of a membrane
separation system
which minimizes: the risk of damage to the membrane. . It .was discovered that
membrane
processing streams, comprising less-readily permeable, condensible components
such as C6+
hydrocarbons in an environment in which condensing of such hydrocarbons may
occur, can be
subject to catastrophic failure when such hydrocarbon condensation is followed
by conventional
3 o shut-down procedures such as depressurization of the non-permeate side.
Depressurization of the
membrane upon shut-down was found to be desirable only when it occurred
simultaneously with
CA 02215777 1997-09-18
6
or after purging of the non-permeate zone of the membrane unit. By the present
invention, it was
found that condensation followed by drying, preferrably without change in the
relative pressures
of the permeate and non-permeate sides of the membrane, avoided degradation
and surprisingly
maintained or even improved selectivity.
In a broad aspect of the present invention is a process for the safe shut-down
of a
membrane separation zone used for the removal of a readily permeable component
from a feed
gas mixture comprising the readily permeable component, a non-permeable
component, and a
less-readily permeable, condensible component. The process comprises
intermittently passing the
i o feed gas mixture at separation conditions to a membrane separation zone to
provide a non-
permeate stream and a permeate stream. The membrane separation zone has a non-
permeate side
and a permeate side. The non-permeate stream is withdrawn from the non-
permeate side of the
membrane separation zone and a permeate stream at a permeate pressure is
withdrawn from the
permeate side of the membrane separation zone. When the feed gas mixture is
not passed to the
i5 membrane separation zone, a purge stream reduced in the less-readily
permeable, condensible
component is passed at a pressure greater than the permeate pressure used in
the separation step
to the non-permeate side of the membrane separation zone to remove at least a
portion of a
residual gas remaining in the non-permeate side of the membrane separation
zone before the
residual gas condenses in the membrane separation zone. Thereafter, the shut-
down operation is
a o completed by depressurizing the separation zone.
In a specific embodiment of the invention, the invention relates to a process
used
intermittently for the removal of carbon dioxide from a hydrocarbon gas .feed
stream to produce a
sales gas stream. The hydrocarbon gas feed mixture comprises C, to C6
hydrocarbons, carbon
2 5 dioxide and C6+ hydrocarbons. The C6+ hydrocarbons are less-readily
permeable and
- . condensible, - The hydrocarbon gas feed mixture is passed to a membrane.
separation zone having..
a non-permeate zone and a permeate zone at separation conditions to provide a
non-permeate
stream withdrawn from the non-permeate zone and a permeate stream at a
permeate pressure
withdrawn from the permeate zone. The non-permeate stream comprises C6+
hydrocarbons and a
3 o reduced amount of carbon dioxide relative to the hydrocarbon gas feed
mixture. The permeate
stream is enriched in carbon dioxide relative to the hydrocarbon gas feed
mixture. Intermittently,
E
CA 02215777 1997-09-18
7
when the hydrocarbon feed mixture is not passed to the membrane separation
zone, a purge
stream reduced in C6+ hydrocarbons relative to the C6+ hydrocarbons in the non-
permeate stream
is intermittently passed at a pressure greater than the permeate pressure to
the non-permeate zone
to prevent condensation of C6+ hydrocarbons in the non-permeate stream
remaining in the non-
permeate zone.
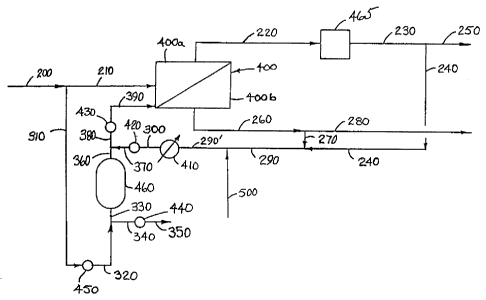
BRIEF DESCRIPTION OF THE DRAWINGS
to
Fig. 1 is a schematic flow diagram of the separation process that is subjected
to the shut-
down process of the present invention.
DETAILED DESCRIPTION
The invention is useful for the rejection of carbon dioxide from a hydrocarbon
or natural
gas stream in a membrane separation zone to provide a purified natural gas
stream which can be
subsequently processed in a natural gas process plant for the further removal
of heavy
hydrocarbons such as C6+ hydrocarbon to provide a sales gas stream or purified
natural gas
2 o stream. The gas feed stream may be passed to a membrane separation unit to
provide a permeate
gas stream comprising carbon dioxide and a non-permeate gas comprising light
hydrocarbons.
Preferably, the natural gas feed stream comprises heavy hydrocarbons in an
amount greater than
500 ppm and more preferably the gas feed stream comprises 500 ppm to 2 mol-%
heavy
hydrocarbons.
. . '),he term "enriched" as utilized herein. is meant to refer to . the
.concentration. of . a. , .
component of a product stream in relation to the concentration of that
component in the feed
stream. For example, the permeate stream from the membrane separation zone
will be enriched
in the readily permeable component relative to the concentration of the
readily permeable
3 o component in the feed. The term "membrane separation zone" means one or
more device having
at least one membrane useful for permeating or separating readily permeable
components from a
CA 02215777 1997-09-18
8
feed gas mixture. The term "dew point" means temperature at a given pressure
wherein vapor
such as hydrocarbon or water begins to condense.
Permeable membranes capable of separating at least one selected component from
a fluid
s mixture, either gas or liquids, are considered in the art as convenient,
potentially highly
advantageous means for achieving desirable fluid separation and/or
concentration. Membranes
suitable for the present invention include composite membranes such as those
membranes
disclosed in US-A-4,822,382.
to The term "intermittently" refers to a continuous process which has periods
of
intermission, particularly unplanned or sudden intermissions or interruptions
in the normal feed
flow. During the period of interruption the membrane must be safely shut-down
or damage to
the membrane may occur. The present invention provides a process for the safe
shut-down of a
membrane that is particularly useful for such unplanned, or intermittent shut-
downs wherein the
1 s conditions identified herein which result in damage to the membrane are
mitigated.
Upon shut-down, condensation in a membrane may result from a phenomena known
as
"retrograde condensation. " Retrograde condensation refers to condensation
which occurs in a
manner which is opposite to expected behavior. For example, in a membrane when
a mixture of
a o gases is present, condensation may occur when the pressure is reduced.
According to the present
invention, a feedstream comprising a mixture of a permeable, non-condensible
component and a
less-readily permeable, condensible component is passed to a membrane unit.
The membrane
comprises a non-permeate zone from which a non-permeate stream is withdrawn
and a permeate
zone from which a permeate stream is withdrawn. When the membrane is subject
to a sudden
a s interruption and the termination of the flow of the feedstream to the
membrane, there is a
. . ~ , potential for . condensation , on the membrane. Although .the flow .
of the f~edstream has stopped, . . .
..
the permeation of the permeable, non-condensible component continues. This
continuation of the
permeation process often results in a decrease in pressure in the non-permeate
zone of the
membrane. Combined with a Joule-Thompson cooling effect, both temperature and
pressure on
3 o the non-permeate side are reduced. As the pressure in the non-permeate
zone decreases, the
partial pressure of the less-readily permeable, condensible component
increases. Unless some
change is introduced to the non-permeate zone, the partial pressure of the
less-readily permeable,
t
CA 02215777 1997-09-18
9
condensable component may reach a point at which liquid will form and
condensation will take
place. The present invention acts to mitigate this retrograde condensation by
purging the non-
permeate zone of the membrane unit with a purge stream which maintains the
partial pressure of
the less-readily condensable component in the non-permeate zone above the dew
point, or the
s point at which condensation will occur. For example, in a process for the
treating of a natural
gas stream, the invention comprises passing - at an operating pressure - a
mixture of permeable,
non-condensable light hydrocarbons such as C; to C6 hydrocarbons and carbon
dioxide and less-
readily permeable, condensable components such as C6+ or C,o+ hydrocarbons to
a membrane
unit having a non-permeate zone and a permeate zone. A non-permeate stream
reduced in carbon
to dioxide is withdrawn from the non-permeate zone at a pressure essentially
equal to the operating
pressure, and a permeate stream enriched in carbon dioxide relative to the
feedstream is
withdrawn from the permeate zone at a low pressure. Suddenly terminating the
feedstream flow
to the membrane and the blocking of the non-permeate and permeate flows
results in the decrease
in pressure of the non-permeate zone as carbon dioxide continues to permeate
through the
15 membrane. The removal of carbon dioxide from the non-permeate zone by
permeation increases
the partial pressure of the C6+ or C,o+ less-readily permeable, condensable
component and, if not
prevented, may result in condensation and damage to the membrane. According to
the present
invention, upon interruption of the feedstream flow, the non-permeate zone is
purged with a
purge stream such that the partial pressure of the less-readily permeable,
condensable component
a o is maintained above the point of condensation. This requires that the
purge gas stream comprise
less of the less-readily permeable, condensable component than the amount of
less-readily
permeable, condensable component in the non-permeate zone. Preferably, the
purge gas stream
reduced in the less-readily permeable, condensable component comprises less
than 90 mol-% of
the less-readily permeable, condensable component in the non-permeate stream,
and more
25 preferably, the purge gas stream comprises less than 10 mol-% of the less-
readily permeable,
condensable component in the non-permeate stream, .and most preferably, the ~
purge..gas stream : ..
comprises less than 0.1 mol-% of the less-readily permeable, condensable
component in the .non-
permeate stream.
3 o Membrane operation is measured by the selectivity of the membrane and the
permeability
of a component through the membrane. These parameters depend upon the partial
pressure
driving forces between the non-permeate and permeate zones for each component.
In the
CA 02215777 1997-09-18
1~
separation of two components in a membrane unit when the molar flow ratio of
permeate flow to
feed flow is low, the selectivity ("S") is the product of the ratio of the
mole fractions of the
components in the feedstream and the inverse ratio of the partial pressure
driving force between
the non-permeate and permeate zones, wherein the partial pressure driving
force for a given
component of a mixture is the difference between the partial pressure of the
component in the
feed less the partial pressure of the component in the permeate. As stated in
equation form:
S = x1*(PPtz - PPPZ)~x2*(PPr~ - PPP)
i o wherein x, and x2 are the feed mole fractions of components 1 and 2; pp~,
and pp~ are the partial
pressures of the components 1 and 2 in the feedstream; and ppP, and ppp2 are
the partial pressure
of the components 1 and 2 in the permeate stream. Permeability ("PM") for a
low molar flow
ratio of permeate flow to feed flow, expressed in terms of each component, is
the flow of the
component through the membrane divided by the product of the surface area of
the membrane
i 5 and the partial pressure driving force for that component between the
feedstream and the
permeate partial pressures. In equation form, the permeability of component 1
is expressed as
follows:
PM = Fp * xfl ~ A * (P,,*xfi - Pi*xp,)
wherein FP is the permeate flow rate from the membrane zone; A is the surface
area of the
membrane; P,, and P, are the total pressures of the feedstream and the
permeate stream; and xp,
and x~, are the mole fractions of component '1 at the permeate end and
feedstream end of the
membrane.
. In one embodiment, the purge gas comprises. the sales. gas. from the gas
processing plant
downstream of the membrane separation zone. In another embodiment, the purge
gas comprises
an adsorber effluent stream derived from the adsorption of the heavier
hydrocarbons from a
portion of the feed gas stream. According to the invention, a portion of the
feed stream is passed
3 o to an adsorption bed containing a selective adsorbent for the adsorption
of heavy hydrocarbons to
produce the adsorber effluent essentially free of hydrocarbons. When the feed
to the membrane
is resumed, the adsorption bed may be regenerated in the conventional manner
with a heated
c
CA 02215777 1997-09-18
11
regeneration gas stream. Although any suitable gas stream maybe.employed for
such purpose, it
is preferred that the regeneration gas stream be selected from the group
consisting of nitrogen, a
fuel gas stream, portions of the sales gas, permeate gas, and non-permeate gas
streams, and
mixtures thereof. Permeate gas is more preferred because it is essentially
free of heavy
hydrocarbons such as C6+ or C,o+ hydrocarbons. The spent regenerant gas can be
employed for
fuel use.
The adsorption zone of the present invention relates to conventional thermal
swing
processing in which each bed of an adsorption zone undergoes, on a cyclic
basis, adsorption at an
to adsorption temperature wherein the more readily adsorbable components) in
the feed stream are
selectively adsorbed to provide an adsorption effluent stream enriched in the
less-readily
adsorbable components, regeneration at a desorption temperature that is higher
than the
adsorption temperature which is conducted by passing a purge gas at an
elevated temperature,
i.e., equal to or higher than the desired desorption temperature through the
bed, and cooling the
is bed to the adsorption temperature by passing a purge gas therethrough. Such
process steps are
disclosed, for example, in above-cited US-A-4,484,933.
It is to be understood that the adsorption zone of the present invention
contains an
adsorption bed containing adsorbent suitable for adsorbing the particular
components to be
2 o adsorbed therein. It is to be also understood that the term
"countercurrent" denotes that the
direction of gas flow through the adsorption bed, is countercurrent with
respect to the direction of
feed stream flow. Similarly, the term "cocurrent" denotes flow in the same
direction as the feed
stream flow. The term "enriched" is intended to be with reference to the feed
stream
composition unless otherwise noted.
Itwill also . be understood that the.. invention can he carried out using . a
suitable adsorbent . .
material in the adsorption bed having a selectivity for various components of
a feed stream over
other such components, thereby providing a less-readily adsorbable component
and a more
readily adsorbable component. In the present invention, the more readily
adsorbable components
3 o are heavy hydrocarbons such as C6+ hydrocarbons and water and the less-
readily adsorbable
components are C, - C6 hydrocarbons. Suitable adsorbents known in the art and
commercially
available include crystalline molecular sieves, activated carbons, activated
clays, silica gels,
CA 02215777 2005-02-10
12
activated alu~ninas and mixxures thereof. The .crystalline molecular sieves
include zeolitic.
molecular sieves.
Zeolitic molecular sieves in the calcined form may be represented by the
general
s formula;
Mez~~O : A12O3 : XSIOz
where Me is a ration, x has a value from 2 to infinity, and n is the ration
valence. Typical well-
o known zeolites which may be used include: chabazite - also referred to as
zeolite D,
clinoptilolite, EMC-2, zeolite L, ZSM-5, ZSM-11, ZSM-18, ZSM-57, EU-1,
offretite, faujasite,
ferrierite, mordenite, zeolite A, ZK-5, zeolite rho, zeolite Beta, boggsite,
and silicalite. The
adsorbent of the present invention will be selected from these zeolite
adsorbents and mixtures
thereof. Detailed descriptions of some of the above identified zeolites may be
found in D. W.
s Breck, ZEOLITE MOLECULAR SIEVES, John Wiley and Sons, New York, 1974,
It is often desirable when using crystalline molecular sieves that the
molecular sieve be
agglomerated with a binder in order to ensure that the adsorbent will have
suitable particle size.
o Although there are a variety of synthetic and naturally occurring binder
materials available such
as metal oxides, clays, silicas, aluminas, silica-aluminas, silica-zirconias,
silica-thorias, silica-
berylias, silica-dtanias, silica-alumina-thorias, silica-alumina-zirconias,
mixtures of these and the
like, silica binders are preferred. Silica is preferred because it may be
employed to agglomerate
the molecular sieve without substantially altering the adsorptive properties
of the zeolite. The
choice of a suitable binder and methods employed to agglomerate the molecular
sieves are
generally known ~tQ those. skilled in the art and need .not be further
described. herein.
The adsorption process operates most efficiently when the adsorption
temperature, the
temperature at which the adsorption step takes place, is preferably in the
range of 5 °C to 80 °C.
The desorption temperature, the temperature at which the desorption effluent
is recovered, is
preferably in the range of 120 °C to 315 °C.
CA 02215777 1997-09-18
13
Experimental results presented hereinbelow show that when .a membrane
separation unit
processing a gas mixture containing a condensible component experiences a
sudden loss of feed
flow and during the loss of feed flow the non-permeate zone of the separation
unit is purged with
a purge gas which comprises less condensible component than in the non-
permeate zone, damage
s to the membrane may be successfully prevented and possibly even improved.
When
condensation is followed by or results from depressurization or when
condensation occurs by
permitting the permeate zone pressure to rise, damage to the membrane results.
The combination of a separate adsorption zone to provide purge gas for the
membrane
i o during feedstream outages and the use of the permeate stream during normal
membrane operation
to regenerate the adsorption zone results in a novel approach to maintaining
membrane capacity
and preventing damage to membrane systems in large commercial processing
plants.
1 s DETAILED DESCRIPTION OF THE DRAWING
In the drawing, the process of the present invention is illustrated by means
of a simplified
flow diagram in which such details as pumps, instrumentation, heat-exchange
and heat-recovery
circuits, compressors, and similar hardware have been deleted as being non-
essential to an
a o understanding of the techniques involved. The use of such miscellaneous
equipment is well
within the purview of one skilled in the art.
With reference now to Fig. 1, a hydrocarbon gas feed stream 200 - at a
separation
pressure ranging from 700 kPa (100 psia) to 10.5 MPa (1500 psia) and a
separation temperature
a s ranging from 25-60 °C - is intermittently passed to a membrane
separation zone 400 via lines
200 and 210. The hydrocarbon gas feed stream comprises C,-C6hydrocarbons,.
carbon dioxide,
. and heavy hydrocarbons such as C6+ or C,o+ hydrocarbons. The membrane
separation zone 400
comprises a non-permeate zone 400a and a permeate zone 400b. A non-permeate
stream
comprising heavy hydrocarbons and having a reduced amount of carbon dioxide
relative to the
3 o feed stream is withdrawn from a non-permeate zone 400a at a non-permeate
pressure essentially
equal to the separation pressure via line 220 and a permeate stream enriched
in carbon dioxide is
withdrawn from the permeate zone at a permeate pressure ranging from 100 kPa
(15 psia) to
CA 02215777 2005-02-10
14
1050 .k.Pa (150 psia) vxa line. 260 and line 280.. The non-permeate._$tream
.220 is passed ,to a
separation zone 465 wherein the hydrocarbons such as C6+ hydrocarbons are
removed to produce
a sales gas stream in line 230 which is withdrawn as a sales gas production
line 250.
s The process is operated in an intermittent manner such that when the feed
stream is
unavailable, the membrane separation zone 400 is shut-down. By the process of
this invention,
when the feed stream 200 flow is interrupted, a portion of the feed stream 200
is passed via line
310, valve 450, and lines 320 and 330 to adsorption zone 460. Adsorption zone
460 contains an
adsorbent selected from the group consisting of activated carbon, silica gel,
alumina molecular
~ o sieves, and combinations thereof to selectively remove at least a portion
of the C6+ hydrocarbon
from the gas feed stream and produce a purge gas stream in line 360 that
comprises less than the
non-permeate stream C6+ hydrocarbons. When the feed stream is not passed to
the membrane
separation zone, the purge gas stream is passed to the non-permeate zone via
lines 360, 380,
valve 430 and line 390 to sweep any residual non-permeate gas comprising
condeasible C6+
s hydrocarbons remaining in the non-permeate zone 400a prior to reducing the
pressure in the non-
permeate zone.
The adsorption zone is thus available during intermittent feed stream outages
to provide a
purge gas stream. Preferably, the purge gas stream comprises a portion of the
non-permeate
o stream depleted in the less-readily permeable, condensible component, and
more preferably, the
purge gas comprises a portion of the feed gas mixture depleted in the less-
readily permeable,
condensible component relative to the non-permeate stream. When this purge
stream is
employed to sweep the residual, non-permeate' gas from the non-permeate zone,
the condensation
of heavy hydrocarbons is prevented and damage to the membrane separation zone
is thus
s prevented. During those periods when the feed stream is passed to the
membrane separation
. zone, the, adsorption_zone is regenerated by.any one.of the following
operations: .t~ second purge
gas stream such as a portion of the sales gas in line 240; a fuel gas stream
in line 500; or a
portion of the permeate stream in line 270; is passed via lines 240, 290, and
290' to heater 410
which heats the second purge gas stream to a regeneration temperature ranging
from 30 °C to
0 300 °C to provide a heated regeneration gas stream which is passed
via line 300, valve 420, line
370 and line 360 to the adsorption zone 460. Thus the adsorption zone may be
regenerated with a
second purge gas stream selected from the group consisting of a portion of the
sales gas, a fuel gas
CA 02215777 1997-09-18
stream, a portion of the permeate stream, nitrogen, a portion of .the non-
permeate stream and
mixtures thereof. It is preferred that the permeate stream be employed as the
regenerant during
the normal operation of the membrane unit. The permeate stream is essentially
free of heavy
hydrocarbons and always available for this regeneration without requiring
special storage. A
5 spent regeneration gas is recovered from the adsorption zone and passed via
line 330, line 340,
valve 440, and line 350 where it is withdrawn from the system. The spent
regeneration gas 350
may be used to provide fuel gas within the separation zone 465.
i o EXAMPLES
The following examples of membranes operating in a potentially condensing
environment
were based on the performance of membrane micromodules, each containing 2800
mm2
composite membrane surface having a separation layer comprised of at least one
poly
i5 (tetramethyl) bisphenol A phthalate as disclosed in US-A-4.,822,382. The
micromodules were
pressurized to a separation pressure of 3.6 MPa (515 psia) with a prepared
feed stream without
the withdrawal of a non-permeate stream. The condensible-loaded gas feed
stream was prepared
by sparging a hydrogen gas stream of 99.9 % purity through a liquid
hydrocarbon mixture of 10
vol-% benzene and 90 vol-% hexane to saturate the hydrogen gas stream with the
hydrocarbon
2 o mixture at a temperature of 24 °C (75 °F). As part of each
test, the selectivity of the membrane
was measured before and after the exposure to the saturated hydrogen gas
stream by pressurizing
the membrane micromodule with a gas mixture of 10 vol-% carbon dioxide and 90
vol-
methane. For these examples, the molar ratio of the permeate flow to the feed
flow was less than
3%.
v CONTROL EXAMPLE I .
CONDENSATION FOLLOWED BY DEPRESSURIZATION
3 o A hydrogen gas stream was sparged as described herein above and passed to
a membrane
micromodule for 6 hours at a pressure of 3.6 MPa and a temperature of 24
°C measured at the
sparger while the temperature of the micromodule was maintained at 37
°C (100 °F) without a
CA 02215777 1997-09-18
16
non-permeate flow from the membrane module. The micromodule was then
depressurized to
atmospheric pressure. Following depressurization, a gas mixture containing 10
vol-% carbon
dioxide and 90 vol-% methane was passed to the membrane micromodule to
repressurize the
micromodule to 3.6 MPa (515 psia) at a temperature of 49 °C (120
°F) and the selectivity of the
membrane was determined. The results are shown in Table 1. Prior to the test,
the four samples
tested had selectivities of 6 and permeabilities of 4. As shown in column O,
immediately
following condensation and depressurization, the average permeability reached
a value of 46 and
the selectivity was reduced to 1. Clearly, the effect of depressurization
following condensation
produced severe damage to the membrane. Furthermore, even after the
micromembrane samples
to were dried with the C02/CH4 gas mixture for several days and at a
temperature of 37 °C (100 °F)
and a pressure of 3.6 MPa (515 psia) the COZ/CH4 selectivity and COZ
permeability did not
return to original levels as shown at 4, 11, and 18 days from the point of
condensation and
depressurization. Thus, depressurization after condensation appeared to
catastrophically damage
the membrane in a manner which could not be recovered even by drying the
membrane module.
TABLE 1
CONDENSATION FOLLOWED BY DEPRESSURIZATION
BEFORE DAYS AFTER CONDENSATION
CONDENSATION
0 4 11 18
COZ PERMEABILITY 4 46 6 5 5
COZ/CIIa SELECTIVITY 6 . 1 2 2 2
IN'VEN'TION EXAMPLE II
NO DEPRESSURIZATION FOLLOWING CONDENSATION
The membrane micromodule was supplied with the sparged hydrogen gas of the
Control
Example I at 3.6 MPa and 37 °C for 6 hours without withdrawing a non-
permeate stream. At
i
CA 02215777 1997-09-18
17
the conclusion of the 6 hour .period, a non-permeate stream was withdrawn at a
rate equal to the
feed rate for a period of 30 minutes while still introducing the sparged
hydrogen gas. At the end
of the 30 minute period and without depressurizing, the COz/CH4 feed was re-
introduced and the
permeability and selectivity measured. The results are shown in Table 2 at
time periods before
s and at 3 and 4 days following condensation. The results show that the
membrane properties were
maintained and surprisingly were even slightly improved when, following
exposure to a
condensing hydrocarbon, the membrane was dried with a purge gas essentially
free of
condensible components while maintaining the differential pressure between the
non-permeate
and permeate sides of the membrane.
io
TABLE 2
is NO DEPRESSURIZATION FOLLOWING CONDENSATION
BEFORE DAY 3 DAY 4
COz PERMEABILITY 4 6 3.5
COZCIi4 SELECTIVITY 6 7.5 9
a o CONTROL EXAMPLE II
CONDENSATION FOLLOWED BY RAISING PERMEATE PRESSURE
In this Example, the membrane micromodule was sparged with the hydrocarbon
saturated
. . 2 5 . hydrogen gas of Example I for ~ 6 hours at. the conditions of
Invention Example I. ' The permeate
flow from the membrane module was blocked and the permeate side of the
membrane was raised
to the feed pressure for a period of 10 minutes. The introduction of the
sparged hydrogen gas
was replaced by the COZ/CH4 feed gas flow of Example I and the permeate and
non-permeate
flows were established at an operating pressure of 3.6 MPa and a temperature
of (49 °C) 120 °F.
3 o The measured permeability and selectivity are shown in Table 3 at 1 day
following
condensation. Following condensation and allowing the permeate pressure to
rise to the feed
CA 02215777 1997-09-18
18
pressure resulted in the loss of membrane selectivity.
TABLE 3
PERMEATE BLOCKED WITH NO DEPRESSURIZATION
BEFORE AFTER 1 DAY
COZ PERMEABILITY 5 13
COZ/CH4 SELECTIVITY 7 1.5
i