Note: Descriptions are shown in the official language in which they were submitted.
CA 02216371 1999-10-15
-1-
"BURST-FREE" SUSTAINED RELEASE POLY-
(LACTIDE/GLYCOLIDE) MICROSPHERES
S This invention relates to providing novel biocompatible and
biodegradable microspheres for burst-free programmable sustained released
of biologically active agents, inclusive of polypeptides, over a period of up
to 100 days in an aqueous physiological environment.
,.
i~
i
CA 02216371 1997-09-24
WO 97/26869 PCTlUS96/19440
2
I0. HAC1CGROUND OF THE INVENTION
Several publications and patents are available for sustained
release of $ctive agents from biodegradable polymers,
particularly, poly(lacti.de/glycolides) (PLGA). Prior usages of
PLGA for controlled release of polypeptides have involved the use
of molar ratios of lactide/glycalide (L/G) of 75/25 to 100/0 for
molecular weights >20,000. Further prior art preparations of PLGA
utilized fillers or additives in the inner aqueous layer to
improve the stability and encapsulation efficency and/or to
increase the viscosity of the aqueous layer, thereby modulating
polymer hydrolysis and the biologically active agent or
polypeptide release.
In addition, the prior art use of PLGA copolymers were end-
capped, i.n that the terminal carboxyl end groups were blocked. In
these end-capped co-polymers, the microcapsule preparations
exhibited a low to moderate burst release of - 10-40% of the
2o entrapped polypeptide in the first 24 hours after placement in an
aqueous physiological environment. In part, these characteristics
are due to the use of fillers in the inner aqueous phase.
Further, a 1-month release of polypeptide is known with the use
of a 75/25 co-polymer of PLGA of Mw <20,000.
Investigations in controlled release research has been
proceeding especially to obtain a 1 to 2 month delivery system ,
CA 02216371 1997-09-24
WO 97/26869 PCTIUS96/19440
' 3
for biologically active agents or polypeptides using
poly(lactide/glycolide) polymers. However, most of these systems
have one or more of the following problems: Poor encapsulation
r efficency and large 'burst release' followed by an intermediate
'no release' or 'lag phases until the polymer degrades. In
general, release from these polymers occur over a period from
about 4 weeks to about several months. In addition, in order to
achieve this release a 50/50 copolymer of Mw > 30,000 or a 75/25
copolymer of Mw > 10,000 are employed which often results in
residual polymer remaining at the site of administration long
after the release of active core.
20
CA 02216371 2001-12-21
V. SUMMARY OF THE INVENTION
This invention provides biocompati.ble and biodegradable
microspheres that have ben designed for novel., burst free,
programmable sustained release of biologically active agents,
including polypeptides over a period of up to 100 days in an
aqueous physiological environment.
Unlike currently availab:l.e :re=Lease systems, which rely on the
use of fillers/additive~~ such as gelatin, albumin, dextran, pectin,
polyvinyl pyrrolidone, polyethylene <~lycol, sugars, etc., and are
:till prone to low encapsulation efficiencies and "burst effects",
t=his invention achieves hicah e=ncap;~ul.<~ition and "bu.r~st-free" release
without the use of any additive. Ln this invention, burst-free,
programmable sustained relF~a~>e is a<<hieved through the use of a
unique blend of the ' unc:apped' and end--cad>ped forms of
holy(lac:tide/glycolide) polymer that. is preferably in the molecular
weight range of 2,000 to E>0,000 daltons.
In general, m:icrosphc=ores de;~cribed in this invention are
produced by a unique emu7_;-if icatior_ technique wherein an inner
water-in-oil (w/o) emul;~:icm is ;~t:abi Li.zed by dispersing in a
;solvent-saturated aqueous pha:>e cont:aining an emulsion stabilizer.
A ternary w/o/w emulsion i~ then foxmec~ by emulsifying the above
w/o emulsions in an external p.r~~-coo:led aqueous pha~~e containing an
o/w emulsified. For example, the
CA 02216371 2001-12-21
inner w/o emulsion may include an aqueous layer containing from -
2 to about 20% (w/w) of the active agEvnt to be entrapped and an oil
layer containing poly(=Lactide/glycolide) copolymer in
concentrations ranging from --- 5 to about. -- 50% (w/w oil phase).
5 The copolymer preferably inc_Ludes molecular weight ranging from
2,000 to about 60,000 dalto:ns, with molar composition of
lactide/glycolide from 90/1_0 to 40/60 and a blend of its uncapped
and end-capped forms in a ratio of100/0 to 1/99. Very high
encapsu:Lation efficiencies o.f about 80 to 100° are achieved
depending on polymer molecular weight and structural form.
Programmable release of active core over variable durations
between 1-100 days is acriieved by a judicious selection of process
parameters such as polymer concentration, pept:ide concentration and
the aqueous/oil phase rat.:ic->.
IS Th=is invention is part i cul.arly suitable for hicth encapsulation
efficiencies and burst-iree, continuous programm~ible release of
polypept=ides of mo.lecula:r we:ig:Eat~:=~ ranging from 1, 000 to about
250,000 daltons, and als~~ other :~iologically active agents over a
period of 1-100 days. A uniqueness of the invention is that when
using a 100/0 blend of the uncapped anti capped polymer, the final
phase of active core re:~ease is concurrent with the complete
solubilization of t;he polymer t.o innocuous components, such as
lactic <~nd glycolic acids. ~L'Izi~; is a significant advantage over
the currently available 30 day-release systems wherein a major
regulatory concern is about t~ox::icit~,T of residi_ral polymer at the
site of administration, Long
CA 02216371 1997-09-24
WO 97/26869 PCT/US96/19440
6 _
after xelease of the active core.
The microcapsules described in this invention are suitable
for administration via several routes such as parenteral
(intramuscular, subcutaneous), oral, topical, nasal, rectal and
vaginal routes.
VI. BRIEF DESCRIPTION OF DRAiPINGB '
FIG. 1 shows a comparison of drug release from a
conventional system versus a controlled release system. Peak and
1o valley levels from conventional administrations are shown, in
contrast to the steady therapeutic levels from the controlled
release administration.
FIG. 2 shows a scanning electron micrograph of PLGA
microspheres prepared by the process described in the invention
using 50/50 uncapped polymer of Mw 8-12k dalton and shows
superior sphere morphology, sphere integrity, and narrow size
distribution.
FIG. 2a shows a scanning electron micrograph of PLGA
microspheres prepared by conventional solvent evaporation method
using a 50/50 12k uncapped polymer of Mw 8-12k dalton.
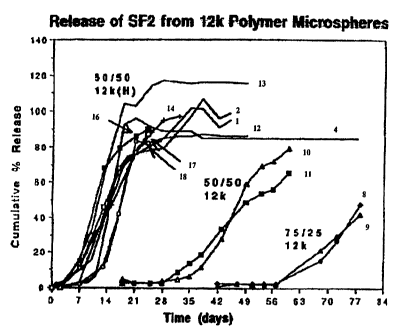
FIG. 3 shows cumulative Histatin release from PLGA
microspheres, wherein release profiles from several batches are
prepared using 50/50, uncapped polymer (of Mw 8-i2k dalton) and
wherein the process parameters are varied to modulate release
between 1 and 35 days.
FIG. 4 shows a scanning electron micrograph of solid, smooth .
CA 02216371 1997-09-24
WO 97126869 PCTlLT596/19440
7
spherical surfaces of PLGA microspheres prepared by the method of
in the invention using 50/50, end-capped polymer (of Mw 30-40k
dalton).
FIG. 5 shows cumulative Histatin release from PLGA
microspheres, wherein the release profiles are from several
batches prepared using 50/50, uncapped and end-capped polymer of
Mw 30-40k daltons, and wherein the process parameters are varied
to modulate release between 28 to 60 days.
FIG. 6 shows cumulative Histatin release from PLGA
microspheres, wherein combined release profiles from several
batches have been prepare' using 50/50, uncapped and end-capped
polymer of Mw 8-40k daltons, while varying the process parameters
to modulate release between 1 and 60 days.
FIG. 7 shows a cumulative percent release of LHRH from PLGA
micraspheres prepared using uncapped polymer of Mw 8-12 daltons.
DETAILED DESCRIPTION OF THE INVENTION
This invention relates to the design of biocompatible and
biodegradable microspheres for novel, programmable sustained
release of biologically active agents, including polypeptides
over a period of up to 100 days in an aqueous physiological
environment with little or no burst release.
Unlike currently available release systems which rely on the
use of fillers/additives such as gelatin, albumin, dextran,
pectin, polyvinyl pyrrolidone, polyethylene glycol, sugars, etc.,
and are still prone to low encapsulation efficiencies and ~~burst
effects", this invention achieves high encapsulation efficiency
CA 02216371 2001-12-21
g
and 'burst-free' release without tree use of any additive. In this
:invention, burst-free, programmable sustained release is achieved
through the use of a unique blend c;f the 'uncapped' and end-capped
forms of: poly(lactide/gl~~c=olide) Polymer.
The 'capped' form refers t:o ""poly (lactide/glycolide) with free
carboxyl end groups" whl.c:h renders the polymer more hydrophilic
compared to the routinely used end-capped form. Currently used
'end-capped' polymer hydrates between 4--12 weeks depending on the
molecular weight, resulting in <~n irutermediate 'no release' or a
'lag phase'. The uncapped po~yme.r. hydrates typically between 5 to
50 days depending on the mo:lecul<~r_ weight, thus releasing its core
continuously without. a lag pha:>e. A careful blend of the two forms
and appropriate molecular weights and L/G ratios, results in a
continuous release between 1 to 100 days. In addition, release
within this time i.s pro<~rarnmable by a judicious selection of
process parameters such as po:l.ymer concentration, peptide
concentration and the aque:ous/oi:1 phase ratio.
The coploymer i_n thi s :invention preferably includes molecular
weight ranging from 2,000 to 60,000 daltons, a lactide/glycolide
ratio of 90/10 to 40/60 and a blend of the uncapped/capped forms in
t;he rat=io of 100/0 to 1./99. 'clue molecular weight of the
polypept.ide may be in the rancfe of 1000 to 250, 000 daltons while
t: hat of other biologically active a.gent:~ may be in the range of 100
t:o 100,000 daltons.
Mic:rocapsules descrLbed :in r_hi.s invention are prepare by a
CA 02216371 1997-09-24
WO 97/26869 PCT/US96J19440
9
unique aqueous emulsification techinique which has been developed
for use with the uncapped polymer to provide superior sphere
morphology, sphere integrity and narrow size distribution. This
is accomplished by first preparing an inner water-in-oil (w/o) by
mixing the solutions of polymer in an organic solvent such as
methylene chloride and the biologically active agent in water.
This is followed by stabilization of the w/o emulsion in a
solvent-saturated aqueous solution containing an o/w emulsifier
such as polyvinyl alcohol. A ternary emulsion is then formed by
emulsifying the w/o emulsion in an external aqueous phase
containing the same emulsifier as above at concentrations ranging
from.-0.25 - 1% w/v. Microcapsules are hardened upon solvent
removal by evaporation, rinsed to remove residual emulsifier and
lyophilized. Low temperature is used both at the time of primary
Z5 emulsification (w/o emulsion formation) and during the formation
of the final w/o/w emulsion to achieve stable emulsion and
superior sphere characteristics.
In the context of the invention, a biologically active agent
is any water-soluble hormone drugs, antibiotics, antitumor
agents, antiinflammatory agents, antipyretics, analgesics,
antitussives, expectorants, sedatives, muscle relaxants,
antiepileptics, antiulcer agents, antidepressants, antiallergic
drugs, cardiotonics, antiarrhythmic drugs, vasodilators,
antihypertensives, diuretics, anticoagulants, antinarcotics, etc.
CA 02216371 1997-09-24
WO 97126869 - PCT/dTS96/19440
More precisely, applicants have discovered a
pharmaceutical composition and process with the following
itemized features:
1. A controlled release microcapsule pharmaceutical
5 formulation far burst-free, sustained, programmable release of a -
biologically active agent over a duration from 1-100 days,
comprising an active agent and a blend of uncapped and end-capped
biodegradable poly(lactide/glycolide).
2. The pharmaceutical formulation of item 1, wherein the
1o biodegradable poly(lactide/glycolide) is a blend of uncapped and
capped forms, in ratios ranging fram 100/0 to 1/99.
3. The microcapsules of items 1 or 2 wherein the copolymer
(lactide to glycolide L/G) ratio for uncapped and endcapped
polymer is 52/48 to 48/52.
4. The microcapsules of items 1 or 2 wherein the copolymer
L/G ratio for uncapped and end-capped polymer is 90/10 to 40/60.
5. The microcapsules of items 1 or 2 or 3 or 4 wherein the
molecular weight of the copolymer is between 2,000-60,000
daltons.
6. The microcapsules of items i or 2 or 3 or 4 or 5 wherein
the biologically active agent is a peptide or polypeptide.
7. The microcapsules of item 6, wherein said polypeptide is
histatin consisting of 12 amino acids and having a molecular
weight of 1563.
8. The microcapsules of items 1 or 2 or 3 or 4 or 5 or 6
characterized by the capacity to completely release histatin in
CA 02216371 1997-09-24
WO 97/26869 PCT/US96/19440
lI _
an aqueous physiological environment from 1-35 days with a 100/0
blend of uncapped and end-capped poly(lactide/glycolide) having a
L/G ratio of 48/52 to 52/48, and a molecular weight <15,000.
9. The microcapsules of items 1 or 2 or 3 or 4 or 5 or 6
characterized by the capacity to completely release histatin in
an aqueous physiological environment from 18-40 days with a 100/0
blend of uncapped and end-capped poly(lactide/glycolide) having a
L/G ratio of 48/52 to 52/48 and a molecular weight range of
28,000-40,000.
10. The microcapsules of items 1 or 2 or 3 or 4 or 5 or 6
characterized by the capacity to release up to 90% of the
histatin in an aqueous physiological environment from 28-70 days
with a 0/100 blend of uncapped and end-capped
poly(lactide/giycolide) having a L/G ratio of 48/52 to 52/48 and
a molecular weight range of 10,000-40,000 daltons.
11. The microcapsules of items i or 2 or 3 or 4 or 5 or 6
characterized by the capacity to release up to 80% of histatin in
an aqueous physiological environment from 56-100 days with a
0/100 blend of uncapped and end-capped poly(lactide/glycolide)
having a L/G ratio of 75/25 and a molecular weight of < 15,000
daltons.
12. The microcapsules of items 7 or 8 or 9 or 10 or li
having analogs of histatin with chain lengths of from 11-24 amino
acids of molecular weights from 1,500-3,000 daltons and
characterized by the following structures:
1. D S H A K R H H G Y K R K F H ~ K H H S H R G Y
CA 02216371 1997-09-24
WO 97!26869 PCT/US96/19440
12 _
2. ,K R H H G Y K R K F H ~ K H H S H R G Y R
3. K R H H G Y K R K F H E K H H S H R
R K F H E K H H S H R G Y R
5. A K R H H G Y K R K F H
6. *A K R H H G Y K R K F H
Z~ K R H H G Y K R K F -
* D-amino acid
13. The microcapsules of items 1 or 2 or 3 or 4 or 5 wherein
the biologically active agent is a polypeptide Leutinizing
hormone releasing hormone (LHRH) that is a decapeptide of
molecular weight 1182 in its acetate form, and having the
structure:
p- E H W S Y G L R P G
14. The microcapsule of items 6 or 7 or 8 or 9 or 10 or 11
or 12 or 13 having a molecular weight of from 1,000 to 250,000
daltons.
15. The microcapsules of items 6 or 7 or 8 or 9 or 10 or 11
or 12 or 13 or 14 Wherein release profiles of variable rates and
durations are achieved by blending uncapped and capped
microspheres as a cocktail in variable amounts.
16. The microcapsules of items 6 or 7 or 8 or 9 or 10 or 11
or 12 or 13 or 14 wherein release of profiles of variable rates
and duration are achieved by blending uncapped and capped polymer
in different ratios within the same microshreres.
17. The microcapsules of items 6 or 7 or 8 or 9 or 10 or 13
CA 02216371 1997-09-24
WO 97!26869 PCTIUS96/19440
13
ar 12 or 13 or 14 or 15 or 16 wherein the entrapped polypeptide
i.s any of the vaccine agents against enterotoxigenic E. coli
(ETEC) such as CFA/I,CFA/II,CS1,CS3,CS6 and CS17 and other ETEC-
related enterotoxins.
18. The microcapsules of items 6 or 7 or 8 or 9 or 10 or 11
or 12 or 13 or 14 or 15 or 16 or 17 wherein the entrapped
polypeptide consists of peptide antigens of molecular weight
range of about 800-5000 daltons for immunization against
enterotoxigenic E. coli (ETEC).
19. The microcapsules of items 1 or 2 or 3 or 4 or 5 wherein
said biologically active agents are selected from the group
consisting of water-soluyie hormone drugs, antibiotics, antitumor
agents, anti inflammatory agents, antipyretics, analgesics,
antitussives, expectorants, sedatives, muscle relaxants,
antiepileptics, antiulcer agents, antidepressants, antiallergic
drugs, cardiotonics, antiarrhythmic drugs, vasodilators,
antihypertensives, diuretics, anticoagulants, and antinarcotics,
in the molecular weight range of 100-100,000 daltons.
20. The microcapsules of items 1 or 2 or 3 or 4 or 5 or 6 or
7 or 8 wherein said biodegradable poly(lactide/glycolide) is in
an oil phase, and is present in about 1-50% (w/w).
21. The microcapsules of items 1 or 2 or 3 or 4 or 5 or 6 or
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 wherein
concentration of the active agent is in the range of O.1 to about
so% (w/w).
22. The microcapsules of items 1 or 2 or 3 or 4 or 5 or 6 or
CA 02216371 1997-09-24
WO 97/26869 PCT/US96II9440
I4
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 wherein a -
ratio of the inner aqueous to oil phases is about 1/4 to
1/40(v/v).
23. A process for preparing controlled release microcapsule
formulations characterized by burst-free, sustained, programmable
release of biologically active agents comprising: Dissolving
biodegradable poly (lactide/glycolide), in uncapped form in
methylene chloride, and dissolving a biologically active agent or
active core in Water; adding the aqueous layer~to the polymer
30 solution and emulsifying to provide an inner water-in-oil (w/o)
emulsion; stabilizing the w/o emulsion in a solvent-saturated
aqueous phase containing a oil-in-water (o/w) emulsifier; adding
said w/o emulsion to an external aqueous layer containing oil-in-
water emulsifier to form a ternary emulsion; and stirring the
resulting water-in-oil-in-water (w/o/w) emulsion for sufficient
time to remove said solvent, and rinsing hardened microcapsules
with water and lyophilizing said hardened microcapsules.
24. A process for preparing controlled release microcapsule
formulations characterized by burst-free, sustained, programmable
release of biologically active agents comprising:
dissolving biodegradable poly(lactide/glycolide) in end-
capped form in methylene chloride, and dissolving a biologically
active agent or active core in water; adding the aqueous layer to
the polymer solution and emulsifying to provide an inner water-
in-oil emulsion; stabilizing the w/o emulsion in a solvent-
saturated aqueous phase containing a oil-in-water (o/w)
CA 02216371 1997-09-24
WO 97/26869 PCT/iTS96l19440
emulsifier; adding said w/o emulsion to an external aqueous layer
containing oil-in-water emulsifier to form a ternary emulsion;
and stirring a resulting water-in-oil-water (w/o/w) emulsion for
sufficient time to remove said solvent; and rinsing hardened
5 microcapsules with water; and lyophilizing said hardened
microcapsules.
25. The process of items 23 or 24 wherein a solvent-
saturated external aqueous phase is added to emulsify the inner
w/o emulsion prior to addition of the external aqueous layer, to
10 provide microcapsules of narrow size distribution range between
0.05-500~Cm.
'2s. The process of items 23 or 24, wherein a low temperature
of about 0-4°C is provided during preparation of the inner w/o
emulsion, and a low temperature of about 4-20°C is provided
15 during preparation of the w/o/w emulsion to provide a stable
emulsion and high encapsulation efficiency.
27. The process of items wherein a 100/0 blend of uncapped
and end-capped polymer is used to provide release of the active
core in a continous and sustained manner without a lag phase.
28. The microcapsules of items 6, wherein, when the
entrapped polypeptide is active at a low pH, such as LHRH,
adrenocorticotropic hormone, epidermal growth factor, calcitonin
released polypeptide is bioactive.
29. The microcapsules of items 6 or 7 or 8 or 9 or 10 or 11,
wherein, when entrapped peptide such as histatin is inactive at a
low pH, a pH-stabilizing agent of inorganic salts are added to
CA 02216371 1997-09-24
WO 97/26869 ~ PCT/US96/19440
16
the inner aqueous phase to maintain biological activity of the
released peptide.
30. The microcapsules of items 6 or 7 or 8 or 9 or 10 or 11
wherein, when entrapped polypeptide such as histatin is inactive
at a low pH, a non-ionic surfactant such as polyoxyethylene
sorbitan fatty acid esters (Tween 80, Tween 60 and Tween 20) and
polyoxyethylene - polyoxypropylene block copolymers (Pluronics)
is added to the inner aqueous phase to maintain biological
activity of the released polypeptide.
31. The microcapsules of items 29, wherein placebo spheres
loaded with the pH-stabilizing agents are coadministered with
polypeptide-loaded spheres to maintain the solution pH around the
microcapsules and preserve the biological activity of the
released peptide in instances where the addition of pH-
stabilizing agents in the inner aqueous phase is undesirable for
the successful encapsulation of the acid pH sensitive
polypeptide.
32. The microcapsules of item 30 wherein placebo spheres
loaded with non-ionic surfactant are coadministered with
polypeptide-loaded spheres to maintain biological activity of the
released peptide where the addition of non-ionic surfactants in
the inner aqueous phase is undesirable for successful
encapsulation of the acid pH sensitive polypeptide.
33. The microcapsules of items 1 or 2 ar 3 or 4 or 5 or 6 or
8 or 9 or 10 or 11 or i2 or 13 or 14 or 15 or 16 or 17 comprising
.a blend of uncapped and capped polymer, wherein complete
CA 02216371 1997-09-24
WO 97/26869 PCT/ITS96/19440
17
solubilization of the copolymer leaves no residual polymer at the
site of administration and occurs concurrently with the complete
release of the entrapped agent.
34. A process of using microcapsules of items 1 or 2 or 3 or
4 or 5 or 6 ar 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or
16 or 17 or 18 or 19 or 20 for human administration via
parenteral routes, such as intramuscular and subcutaneous.
35. A process of using microcapsules of items 1 or 2 or 3 or
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or
16 or 17 or 18 or 19 or 20 for human administration via topical
route.
'36. A process of using microcapsules of items 1 or 2 or 3 or
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or
16 or 17 or 18 or 19 or 20 for human administration via oral
routes.
37. A process of using microcapsules of items 1 or 2 or 3 or
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or
16 or 17 or 18 or 19 or 20 for human admininstration via nasal,
transdermal, rectal, and vaginal routes.
25
CA 02216371 1997-09-24
WO 97/26869 PCT/LTS96/19440
ZS
~ons~ervatio of bioactivity of polvpeptida$
As the polymer degrades rapidly, there is a preciptitous
drop in pH accompanied by the release of soluble oligomers in the
microenvironment which may affect the biological activity of acid
pIi-sensitive peptides/proteins. In such instances, biological
activity can be maintained by the use of inorganic salts yor
buffering agents in the inner aqueous phase codissolved With the
peptide.
l0 The following unique advantages are characteristics of this
invention:
' 1. Burst-free, prolonged, sustained release of polypeptides
and other biocompatible and biodegradable microcapsules. up to 100
days in an aqueous physiological environment without the use of
1~ additives in the core.
2. Release of active core programmable for variable
durations over 1-100 days, by using a blend of uncapped and
capped polymer of different molecular weights and copolymer
ratio, and by manipulating the process parameters.
20 3. Complete release of the active core is concurrent with
complete solubilization of the carrier polymer to innocuous
components, such as lactic and glycolic acids, especially When
using a 100/0 blend of uncapped/capped polymer. This is of
tremendous significance, as most biodegradable polymers currently
25 used for 1-30 day delivery, do not degrade completely at the end
of the intended release duration, thereby causing serious concern
CA 02216371 1997-09-24
WO 97/26869 i g PCT/US96119440
of regulatory authorities on the effects of residual polymer at
the site of administration.
4. Ease of administration of the microcapsuies in various
dosage forms via several routes, such as parenterai
' S (intramuscular and sucutaneous), oral, topical, nasal, vaginal,
etc.
The hydrophilic homo-and co-polymers based on D,L-lactide
and glycolide contains hydrophilic adjusted homo-and co-polymers
with free carboxylic end groups, and is characterized by the
formula:
Paly(D,L-lsctide-co~-glycolide) 50:50
-a O-ct~-c
2
C.~3H~02)n(C2H202)~ n:m ~ I:i
Wherein Z= Molecular Weight/130; for example Z=92 for Mw
12,000 and 262 for Mw 34,000.
While the molar ratio of the lactide to glycolide may vary,
it is most preferred that the lactide to glycolide copolymer
ratio be 50:50.
Reference is now made to FIG. 1 which depicts a blood-drug
concentration versus time graph that shows conventional drug
administration using a series of dosages compared to an ideal
controlled release system. Unfortunately, many drugs have a
CA 02216371 1997-09-24
WO 97/26869 PCT/US96/I9440
therapeutic range, above which they are toxic and below which
they are ineffective. Oscillating drug levels that are commonly
observed following systemic administration causes alternating
periods of ineffectiveness and toxicity. A sustained-release
5 encapsulated biologically active agent or polypeptide
preparation, ideally, will maintain the drug in the desired
therapeutic range by means of a single dose, as depicted in the
THERAPEUTIC RANGE in FIG.1 , where the ideal case for controlled
release is shown.
10 In FIG. 2, there is shown a scanning electron micrograph of
PLGA microspheres prepared using 50/50 uncapped polymer of Mw 8-
I2k tlalton. The uncapped polymer has solid, smooth spherical
surfaces, and is suited to provide a "burst free" release system..
Table I is a summarization of the microsphere process
15 description for preparing a peptide system (Histatin peptide)
having a controlled release over the course of from 1 to 100
days.
Release profiles can be modified by a judicious blend of
uncapped and capped polymers either in separate microspheres or
20 in the same microspheres. Release from microcapsule formulations
1 through 21 listed in Table 1, occur independently of each other
and hence the cumulative release from blends of these
formulations are additive. 8y blending several formulations of
uncapped and end-capped microspheres, release curves of any
desired duration can be tailored. In addition, based on the
release characteristics of uncapped and end-capped polymers, -
CA 02216371 1997-09-24
WO 97/26869 . PCTIUS96/19440
21
blending of the two forms in a single formulation comprising
different ratios of uncapped to capped polymer, would
significantly influence the polymer hydration and hence release
of the active core thereby providing release curves of any
desirable pattern. Manipulation of polymer hydration and
degradation resulting in modulation of release of active core is
achieved by the addition of uncapped polymer to end-capped
polymer in amounts as low as 1~ up to 100.
15
25
CA 02216371 1997-09-24
WO 97/26869 PCT/US96/19440
22
~d
+~ a
b ~
a -~
.a a
wt
-.~ a
o ar
.-r N
E G
is7 O ~C rC d rIC
C
N C1 O
i~ ro i1 N N N et
~
cwt ro 3
~. ro o. ri .:, r, .
x
i
~
I
m
..
~
ar o
a ~a a
...,
o +~ ar
s
..r
.
H ro CL U m N tn ttt
i
I
a 3 w
C ~ W
O C U 3 eD m o OD
U -~ D .- e~ .-~ .-t
C
O
.,a
c
.
--a ..i x
s~
3
m
d
c
d
0 0 0 0 0
~ ~ v v v v
0 o a ~
ro
a
~
a a n , n?
. 4~rt- rw
m
0
.
U G .~ tai ref er
CA 02216371 1997-09-24
WO 97!26869 PCTlUS96/19440
23
m m ~e m m m m m m
o' 0 0 0 0 0 0 o a
., .. .~ .~ r, .~ .,
.-i :: .-i .:i ..i ri '; '; .:;
M
tti 1t7 tn itl 1i1 lI1 1lf 1l1 N
O O O t'1 O O
tW -1 e-1 rl N e~ l~ '-1 !~
Q ~' C' N N N N N N
t"7 ~'i M r-t rt ~-1 r1 ~ r1
O O O tn an O O O O
t(1 K1 Ill N N Il1 1(1 1l1 1!1
\ \ \ \ \ \ \ \ \
O O O ? N ~
O
l7 t Itf t~ N 1fl ft if
l t7 i t
O H N F!
N \O !~ O O~ H ri r1 ri
CA 02216371 1997-09-24
WO 97!26869 PCT/US96I19440
24
~ m m m m m m m
0 0 0 0 0 0
- o
. .-~ .., ~
,
'a .r ', r, .~ .-~ :; ..i
0
~
d' N ~ -1
-1
N ~ N ~f d' V'
H ~ ~ M
in ~ ~ ~ ~ o O O
\ ~. \ \
'
N
J
V' V1
r ~ ~ ~ ~' o ~-1
r .y ,. ,. "'1 H N N
.~ .~
CA 02216371 1997-09-24
WO 97/26869 PCT/LTS96/19440
c
O
iJ C
t0 O
N ..1
-.i
N
.D -.~
~0 .a
y1 ..1
mQ
ro
c~
om
a
m c
.-~
a
a .~
_
E m
E
O G!
w
41 to
'a GL
O
..,
y w
.~a
r~
O m
O.
U 41
.-r
.u
m
a~ ro
w ..i
d
0J 'a
i~
a~ ro
., E
....~
~ ~a
a a~
d
ro y
E
.-a
c sr
.~ N
w r,.i
o c
c
ro ..,,
c
o m
a.~
c
c O
ro
00
-.a
mss
m .-~
yf
~r
ro
'
o 3
3
E
O C~
c G
c
tWS
a O
O
.r -.a
~r a
~ ~
al O
s ro
ro
Ir
~.r
C~
U U
C~
s .t
.a
..~
.
r t~
C!
w w
~
~
3 m
m~
0~
O C
sa
.~
.r
v d
ro
~ ~
.~ .-c
E E
~D
G
~ ~
O .
a
m .a
ai
d 3
3 Cb
~.r
d .r
W e0
e0 ~
O O
O t~
~ ~
O
3 3
~
sr t~
~ ..
U ~
U 3
..
d ..i
C ~
d m
~
CA 02216371 1997-09-24
WO 97/26869 PCT/US96/I9440
26
While referring to Table Z in conjunction With FIG. 3, it
can be seen that the cumulative Histatin release from PLGA
microspheres from several batches prepared using 50/50 and 75/25
uncapped and end-capped, polymer modulates release between 1 to
100 days by varying the process parameters. 1-35 days by uncapped
50/50, i8-56 days by capped 50/50 and 56-100 days by capped
75/25.
In referring to FIG. 4, n view is provided through a
scanning electron micrograph of PLGA microspheres designed for a~~
one to two month release system prepared using. end-capped polymer
of Mw 30-40k daltons.
FIG. 5 depicts the cumulative Histatin release from PLGA
microspheres, in which the release profiles are from several
batches prepared using 50/50, uncapped and capped polymer., and
varying the process parameters to modulate release between 28 to
60 days.
Figure 6 represents cumulative Histatin release from PLGA
microspheres --- these combined release profiles are from several
batches prepared using 50/50 uncapped and capped polymer, and
varying the process parameters to modulate release between 1-60
days.
In the context of the invention, a biologically active agent
is any water-soluble antibiotics, antitumor agents, antipyretics
analgesics, anti-inflammatory agents, antitussives, expectorants,
sedatives, muscle relaxants, anti epileptics, antiulcer agents,
CA 02216371 1997-09-24
WO 97/26869 PCT/L1S96/19440
27 _
anti-depressants, anti-allergic drugs, cardiotonics,
antiarrhythmics drugs, vasodilators, antihypertensives,
diuretics, anticoagulants, hormone drugs, anti-narcotics, etc.
In general, "burst free" sustained release delivery of
biologically active agents from PLGA microshperes is accomplished
' in the context of this invention using of 90/10 to 40/60 molar
ratios, and ratios of uncapped polymer to end-capped polymer of
100/0 to 1/99.
In general, the approaches for designing the biologically
active agents encapsulated in the uncapped and combination
uncapped/end-capped PLGA microspheres and characteristics of
these encapsulants are briefly set forth below as follows:
1. Providing PLGA microspheres of surface morphologies using
50/50 uncapped and capped polymers of Mw - 8-40K daltons as shown
in Figs. 2 and 4.
2. Providing in vitro release of a polypeptide, Histatin
from PLGA microspheres, as shown in Figs. 3 and 5, using uncapped
and capped polymer of Mw - 8-40K daltons and molar ratios such as
50/50 and 75/25.
For example, design of a 1-12 weeek bioactive compound
release system is achieved using PLGA with the following
s~iecif ications
1. Polymer molecular weight:
-about~2-60K daltons
2. Copolymer molar ratio (L/G):
- 90/10 to 40/60
3. Polymer end groups:
- uncapped and /or end-capped
and combining judiciously within the following parameters:
4. Polymer concentration
- from 5 to 50%
5. Inner aqueous to oil phase ratio:
- 1:5 to 1:20 (v/v)
6. Peptide loads:
- from 2 to about 40% (w/w polymer)
CA 02216371 1997-09-24
WO 97/26869 . PCT/CTS96/19440
28
and by using the unique aqueous emulsification method
described in the invention.
The uniqueness and novelty of invention may generally be
summarized in a brief way as follows:
1. Use of uncapped poly(lactide/glycolide) to achieve burst-
free, continuous, sustained, programmable release of biologically
active agents over 1-100 days.
l0 2. Use of a unique aqueous emulsification system to achieve
superior microsphere characteristics such as uniform sphere
morphology and narrow size distribution.
3. Burst-free, prolonged, sustained release of polypeptides
and other biologically actice agents from biocompatible and
biodegradable microcapsules up to 100 days in an aqueous
physiological environment without the use of additives in the
inner core.
4. Release of active core programmable for variable
durations over 1-100 days by using a blend of uncapped and capped
polymer for different molecular weights and copolymer rations and
manipulating the process parameters.
5. Complete release of the active core concurrent with
complete solubilization of carrier polymer to innocuous
components such as lactic and glycolic acids, especially when
using a 100/0 blend of uncapped/capped polymer. This is of
tremendous significance as most biodegradable polymers currently
in use for 1-30 day delivery, do not degrade completely at the
end of the intended release duration causing serious concern for
regulatory authorities on the effects of residual polymer at the
site of administration.
6. Ease of administration of the microcapsules in various
dosages forms via several routes such as parenteral (intramusclar
and subcutaneous), oral, topical, nasal, vaginal, etc.
The following examples are illustrative of, but nat
limitations upon the microcapsule compositions pertaining to this
invention.
Example 1
Polylactide/glycolide (PLGA) microcapsuies are prepared by a
unique aqueous emulsification technique which has been developed
for use with the uncapped polymer to provide superior sphere
morphology, sphere integrity and narrow size distribution (See
Figures la and ib). This is accomplished by dissolving the
polymer in a chlorinated hydrocarbon solvent such as methylene
chloride and dissolving the biologically active agent in water. A
CA 02216371 1997-09-24
WO 97/2b8b9 PCTlUS96/I9440
29
w/o emulsion is then formed by mixing the solutions of polymer
and the active agent by sonication, followed by emulsion -
stabilization in a solvent - saturated aqueous solution
containing polyvinyl alcohol. A ternary emulsion is then formed
by emulsifying the w/o emulsion in an external, pre-cooled
aqueous phase containing polyvinyl alcohol (0.25 - 1% w/v).
Microcapsules are hardened upon removal of solvent by
evaporation, rinsed to remove any residual emulsifier, and then
lyophilized.
Table 1 lists the micracapsule compositions, Nos. 1-21 thus
prepared, consisting of a biologically active polypeptide,
Histatin (composed of 12 amino acids and a molecular weight of
1563) and blends of uncapped and capped polymer of ratios 100/0
to 1/99, and having a lactide/glycolide ratio of 90/10 to 40/60,
and a molecular weight range between 2000 to 60,000 daltons.
Example 2
Microcapsule compositions are prepared as described in
Example 1 wherein the copolymer L/G ratio is 48/52 to 52/48, and
the ratio of uncapped/capped polymer is 100/0. The active core is
Histatin (Mw 1563), the polymer molecular weight is < 15,000 and
the polymer concentrations vary from 7% to - 40% w/w.
Compositions 1,2,4 12-14 and 16-18 are listed in Table 1.
Release profiles of the active core from the compositions in
an aqueous physiological environment, such as phosphate-buffered
saline, pH 7.0 maintained at 37 ~ 1~C are plotted as cumulative
percentage release versus time, and presented in Figure 2.
Burst-free, variable release from 1-35 days is achieved by
varying the polymer concentration from 7 to - 40% w/w in the oil
phase.
Example 3
Microcapsule compositions are prepared as described in
Example 2, wherein the aqueous /oil ratio is varied from 1/4 to
1/20 (v/v). Compositions 1,2,4 and 12 are listed in Table 1.
Release profiles of the active core from the compositions in
an aqueous physiological environment described in Example 2 are
plotted as cumulative percentage release versus time, and
presented in Figure 2.
Burst-free, continuous release from 1-35 days, with
different onset and completion times are achieved by selecting
CA 02216371 1997-09-24
WO 97/26869 PCT/US96/19440
30 _
different w/o ratios in the inner core.
Example 4
Microcapsule compositions are prepared as described in
Example 2, wherein the polymer molecular weight is 28,000-40,000
and polymer concentrations vary from 5% to - 15% w/w.
Compositions 19-21 ase listed in Table 1.
Release profiles of the active core from the compositions in
an aqueous physiological environment are described in Example 2
are plotted as cumulative percentage release versus time and
presented in Figure 3.
Burst-free, variable release from 18-40 days is achieved by
varying the polymer concentration.
Example 5
Microcapsule compositions are prepared as described in
Example 2, wherein the ratio of uncapped/capped polymer is 1/99
and polymer concentrations vary between 5% to - 12% w/w.
Compositions 10 and 11 are listed in Table 1.
Release profiles of the active core from the compositions in
an aqueous physiological environment are described in Example 2,
and plotted as cumulative percentage release versus time and
presented in Figure 2.
Burst-free, variable release from 28-?O days is achieved by
varying the polymer concentration in the oil phase.
Examine 6
Microcapsule compositions are prepared as described in
Example 5, wherein polymer molecular weight is 28,000-40,000 and
polymer concentrations vary between 5% to - 12% w/w. Compositions
5 and 6 are listed in Table 1.
~o Release profiles of the active core from the compositions in
an aqueous physiological environment are described in Example 2
and are plotted as cumulative percentage release versus time, and
presented in Figure 3.
Burst-free, variable release from 28-70 days is achieved by
varying the polymer concentration.
Examvle ?
Microcapsule compositions are prepared as described in
Example 6, wherein the aqueous/oil ratio varies between 1/5 to
CA 02216371 1997-09-24
WO 97/26869 PCT/LTS96l19440
JZ
1/25 (v/v). Compositions 3 and 7 are listed in Table 1.
Release profiles of the active core from the compositions in
an aqueous physiological environment are described in Example 2,
and plotted as cumulative percentage release versus time, and
presented in Figure 3.
Burst-free, variable release from 28-70 days is achieved by
varying the aqueous/oil ratios.
~xamt3le 8
Microcapsule compositions are prepared as described in
Example 5, wherein the copolymer ratio is 75/25 and polymer
concentrations vary between 5% to - 25% w/w. Compositions 8 and 9
are listed in Table 1.
Release profiles of the active core from the compositions in
an aqueous physiological environment are described in Example 2,
and are plotted as cumulative percentage release versus time, and
presented in Figure 2.
Burst-free, variable release from 56->90 days is achieved by
varying the polymer concentration in the oil phase.
Example 9
Microcapsule compositions are described in Example 2,
wherein the active core is leutinizing hormone releasing hormone
(LHRH, a decapeptide of molecular weight 1182) and the polymer
concentration is -40% w/w. Release profiles of the active core
from the composition in an aqueous physiological environment is
described in Example 2, and is plotted as cumulative percentage
release versus time, and presented in Figure 4.
Burst-free, continuous and complete release is achieved
Within 35 days, similar to Histatin acetate.
~xamole 10
Microcapsule compositions are prepared as described in
Example 2, wherein an additive such as sodium salt (carbonate or
bicarbonate) is added to the inner aqueous phase at
concentrations of 1-10% w/w to maintain the biological activity
of the released polypeptide.
Burst-free, variable release from 1-28 days is achieved
similar to Examples 2 & 3, and the released polypeptide is
biologically active until 30 days, due to the presence of the
- sodium salt.
CA 02216371 1997-09-24
WO 97/26869 PCT/US96/19440
32
~xamole I1
~licrocapsule compositions are prepared as described in
Example 2, wherein an additive such as a nonionic surfactant,
polyoxyethylene/polyoxypropylene block copolymer (Pluronics F68
and F127) is added to either the inner oil or the aqueous phase
at concentrations from 10-100% w/w, to maintain the biological
activity of the released polypeptide.
15
Burst-free, continuous release from 1-35 days is achieved
similar to Examples 2 & 3, and the released polypeptide is
bioactive due to the presence of the surfactant.
Example 12
Cumulative histatin release from the microcapsule
compositions described in Examples 1 through 11 and release
profiles plotted in Figures 2 and 3 show the burst-free,
programmable peptide release for variable duration from 1-100
days. Virtually any pattern of cumulative release is achievable
over'a i0o day duration by a judicious blending of several
compositions, as shown in these figures.
30
40
50