Note: Descriptions are shown in the official language in which they were submitted.
CA 02217061 1997-09-30
FO/67-20399/A -1 -
TRANSDERMAL SYSTEM USING ELECTRODES AS THE INITIALISING SYSTEM
The invention relates to a transdermal system for the passive administration of a substance
according to the preamble of the independent patent claim.
Transdermal systems are generally used to administer a substance, for example a thera-
peutically active substance or mixture of substances, through the skin of a living organism
without the need for clear penetration of the outer layer of the skin - the stratum corneum -
and possibly also underlying layers of skin by mechanical means using a device such as, for
example7 an injection needle. Transdermal systems are accordingly normally classed as
being non-invasive dosage forms.
The great interest in transdermal systems stems from the fact that that dosage form has
distinct advantages over other conventional dosage forms. In the case of oral administration
undesired side effects frequently occur as a result of incompatibilities in the gastrointestinal
tract or the liver. Also, orally administered substances are frequently decomposed or so
modified in the gastrointestinal tract or the liver that the desired therapeutic effect does not
occur ("first pass" effect). Other forms of parenteral administration, such as, for example,
intravenous, subcutaneous or intramuscular injections, involve penetration of the skin or of
layers of the skin and are therefore associated by the patient with a sensation of pain. In
addition, local inflammation or infection may occur as a result of the partial damage to the
skin. Especially in the case of long-term therapy requiring regular injections of the substance
or infusions over prolonged periods - often several times a day - the patient is frequently
subjected to a high level of discomfort. This has, among other things, an adverse effect on
the co-operation of the patient in adhering to the medically necessary dosage scheme.
Since transdermal systems are not subject to those limitations they are today, especially in
the form of one typical example, that is to say the transdermal patch, numbered among the
current dosage forms that enjoy widespread usage.
Transdermal systems may be divided roughly into passive and active systems. In passive
systems the substance to be administered diffuses from a reservoir through the skin. In
active systems an additional force promotes the transport of substance through the skin.
CA 02217061 1997-09-30
Especially preferred for that purpose are electric fields, which generate a flow of current
through the skin. The administration of a therapeutically active substance through the skin
with the aid of an electric current is generally referred to as iontophoresis. It is known that
iontophoretic administration can also be used for uncharged active substances. In that
process, for example, a convection flow, which results from electroosmotic or osmotic forces,
transports the uncharged active substance through the skin.
Typically, the active iontophoretic systems used today comprise at least two electrodes, one
of which forms a contact with the reservoir containing the substance. The other electrode,
often referred to as the neutral electrode, is applied directly to the skin and serves to close
the circuit via the body. On connection to a source of electrical energy, a current then flows
through the skin and transports the substance into the body.
Iontophoretic administration, however, has a number of disadvantages. For example
undesired effects of hydrolysis or electrolysis may lead to the degradation of the
therapeutically active substance and/or to the production of new, possibly toxic, compounds.
Since, in addition, the skin constitutes an electrical resistance, which can vary substantially
according to the patient and the condition of the skin at the time in question, it is usually
necessary in iontophoretic systems for there to be provided, in addition to the source of
electrical energy, control means that monitor and, where necessary, regulate the electric
current through the skin. The production of such systems is as a result cost-intensive and
technically and structurally very complex. Also, correct application of the system can often
be carried out only by a person skilled in the art. In addition, the current flow during
iontophoretic administration of a substance may result in burns or other kinds of irritation to
the skin.
The disadvantages mentioned above do not occur in passive transdermal systems. Of
course an accompanying passive transport of substance through the skin usually occurs also
in active systems and it should therefore be stated here, for the purpose of delimitation, that
systems designated as passive are those in which the administration of the substance
through the skin is carried out purely by processes of diffusion in the physical sense and in
which no other forces are generated that significantly assist in the transdermal transport of a
substance.
CA 02217061 1997-09-30
A limitation of the passive transdermal systems customary today, however, is that the
dosage rate, that is the amount of substance administered through the skin per unit of time,
can be controlled only with great difficulty. EP-A-O 144 486 therefore proposes a passive
transdermal system in which the reservoir comprises a plurality of storage layers containing
the therapeutically active substance in different concentrations. In that system the dosage
rate can be influenced by the different concentrations, the different concentration gradients,
the number and thickness of the individual layers and the 'nature and amount of any carrier
substance that may have been added. A disadvantage of that method, however, is that the
dosage rate is, in practice, laid down during the production of the transdermal system, and
any subsequent adaptation to the individual requirements of the patient is not possible. It is
therefore necessary to produce a different system for each desired dosage rate, which is not
efficient in terms of economic mass production. Furthermore, the production of such a multi-
layered system having a precise balance of layer thicknesses and substance concentrations
is fraught with considerable technical difficl.lties.
A further problem of passive transdermal systems is that the process of diffusion by way of
the natural channels of the skin (sebaceous and sweat glands, inter- and trans-cellular
transport paths, hair follicles) proceeds very slowly. It is accordingly difficult using a passive
transdermal system to administer a substance through the stratum corneum at a dosage
rate that is high enough to achieve the desired therapeutic effect.
An aim of the present invention is therefore to prepare a transdermal system for the passive
administration pf a substance that on the one hand renders possible the administration of a
determined amount of substance within a predetermined period of time and that, on the
other hand, is a system in which the dosage rate can still be modified and controlled even
after the system's production. In addition, irritation to the skin shall be avoided with the
transdermal system and also its production on a large scale shall be simple and cost-
effective. It shall furthermore be possible for the transdermal system to be stored for a
prolonged period without there being any appreciable changes in its therapeutic activity as a
result.
CA 02217061 1997-09-30
The transdermal system for the passive administration of a substance through the skin that
achieves that aim has the features given in independent claim 1. Thus, in accordance with
the invention an electric current transports the substance from the reservoir into the transfer
means prior to the administration, which is passive per se, for example prior to the
application of the system to the skin The system is thereby initialised. By means of the
initialisation of the system it is possible to influence in a controlled manner the amount of
substance and the concentration of the substance in the transfer means. The concentration
of the substance decisive for the diffusion-dependent dosage rate is, in fact, not the
concentration at which the substance is introduced into the reservoir during the production of
the system, but the concentration produced in the transfer means by the initialisation.
A property of the transdermal system according to the invention is thus that the dosage rate
for the passive administration can still be influenced in a controlled manner after the system
has been produced. The resulting advantage is that it is possible to obtain different dosage
rates with transdermal systems that have themselves been identically produced, because
the amount and concentration of substance in the transfer means can be controlled during
the initialisation by means of the intensity of the current and the duration of the flow of
current. Such transdermal systems are consequently very well suited to economic mass
production. It is especially also possible for the concentration of the substance to be
increased during the initialisation, which means that after the initialisation the substance is
present in a higher concentration in the transfer means than it was in the reservoir before the
initialisation. In the subsequent passive administration of the substance, that increase in
concentration brings about a marked increase in the dosage rate. As a result, the substance
can be stored in the reservoir at a substantially lower concentration and its concentration not
increased to the desired value in the transfer means until prior to administration This is
especially advantageous in cases where the substance cannot be stored for a prolonged
period at the high level of concentration required for an adequately high dosage rate.
It is especially advantageous when the transfer means comprises a modification layer of
which the electrical conductivity is higher than that of the reservoir. As explained below, this
may in fact lead to the mentioned effect that as the substance migrates from the storage
layer into the modification layer, which occurs during the initialisation, its concentration is
increased .
CA 02217061 1997-09-30
Since, unlike iontophoresis systems, there is no flow of current through the skin in the
system according to the invention, there can also be no electrically induced irritations to the
skin, such as, for example, burns. This has the additional advantage that the current
intensity is not subject to any physiologically determined limitation during the initialisation of
the system according to the invention.
The transdermal system according to the invention can naturally also be used for the
administration of a plurality of substances. These may either be contained in the same
storage layer, or the reservoir may comprise a plurality of storage layers that are physically
separated from one another.
Further advantageous features and preferred arrangements of the transdermal system for
passive administration according to the invention are disclosed in the dependent claims.
In the following, the invention is explained in detail by way of example embodiments and with
reference to the drawings, which are diagrammatic and not to scale:
Fig. 1 is a section through a first example embodirnent of the transdermal system
according to the invention showing the fundamental components,
Fig. 2 shows a possible curve of the electric field strength in the transdermal system
according to the invention during the initialisation as a function of a position co-
ordinate,
Fig. 3 is a section through a further development of the first example embodiment of the
transdermal system according to the invention showing the fundamental
components, and
Fig. 4 is a section through a further example embodiment of the transdermal system
according to the invention showing the fundamental components.
CA 02217061 1997-09-30
In the following description of the preferred example embodiments with reference to the
drawings, components similar in function have been given the same reference symbols.
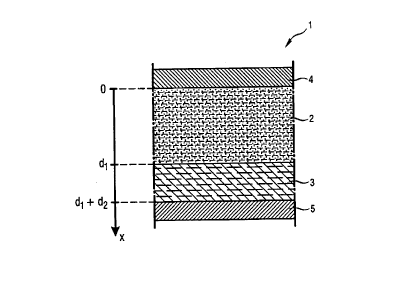
Fig. 1 is a diagrammatic representation of one example embodiment of the transdermal
system according to the invention for the passive administration of a substance through the
skin. The transdermal system 1 comprises a reservoir which, in this example embodiment,
consists of a storage layer 2 containing the therapeutically active substance that is to be
administered. The transdermal system also comprises a modification layer 3 functioning as
transfer means which, during the passive administration of the substance, is in
communication with both the storage layer 2 and the skin (not shown in Fig. 1). Also
provided in the transdermal system are a first electrode 4 and a second electrode 5 which,
before the actual administration of the substance, are so arranged that the storage layer 2
and the modification layer 3 are located between the two electrodes 4 and 5.
The storage layer 2 and the modification layer 3 are made of an electrically conductive
material so that an electric current is able to flow through those layers. The storage layer 2 is
preferably made of an ionically conductive polymer or gel, especially a hydrogel, in which the
substance to be administered is typically contained in dissolved form. The modification layer,
too, is preferably made of an ionically conductive polymer or gel, especially a hydrogel. The
two layers 2 and 3 may be made of the same material. Such polymer or gel materials per se
are state of the art and are frequently used in known active and passive transdermal
systems .
In the transdermal system according to the invention an initialisation of the system is carried
out prior to the passive administration of the substance through the skin. This means that
before the actual administration, and preferably before the system is positioned in contact
with the skin, an electric current brings the transdermal system into the desired starting state
for the administration. The use of the transdermal system according to the invention is thus
divided into two phases that are separated in time: first, the transport of substance from the
storage layer 2 into the modification layer 3 with the aid of an electric current (initialisation),
and then the passive - that is to say diffusion-induced - administration of the substance
through the skin.
CA 02217061 1997-09-30
In the preferred example embodiment of the transdermal system according to the invention
shown in Fig. 1, the electrical conductivity of the modification layer 3 is higher than that of
the storage layer 2. If the two layers 2 and 3 are made of the same material, the difference in
conductivity can be produced, for example, by means of different degrees of crosslinking of
a gel. For the purpose of improved orientation, Fig. 1 in addition shows a co-ordinate axis x
of which the origin O is located where the first electrode 4 and the storage layer 2 touch. The
storage layer 2 extends to the x co-ordinate d~, and the modification layer 3 extends from the
x co-ordinate d~ to the x co-ordinate d1+d2.
If the two electrodes 4 and 5 are connected to a source of electrical energy, for example a
battery (not shown in Fig. 1), in such a manner that the two electrodes 4 and 5 form a closed
circuit with the energy source, the storage layer 2 and the modification layer 3 then, if the
polarity is appropriate, an electric current is able to flow which transports the substance from
the storage layer 2 into the modification layer 3. In electrical terms, the modification layer 3
and the storage layer 2 form a series connection of two resistors and, since the electrical
conductivity of the modification layer 3 is higher than the electrical conductivity of the storage
layer 2, the drop in voltage across the storage layer 2 is greater than the drop in voltage
across the modification layer 3. This means, however, that the electric field strength
prevailing in the storage layer 2 is higher than that prevailing in the modification layer 3. The
corresponding curve for the electric field strength E is shown in Fig. 2 as a function of the x
co-ordinate, as defined in Fig. 1.
For the explanation that follows it is, by way of example, assumed that the electrophoretic
mobility of the substance in the storage layer 2 is substantially the same as that in the
modification layer 3. That assumption is purely for the purpose of better understanding, but
is not necessary for the invention. The transdermal system according to the invention may
alternatively be so arranged that the mobility of the substance in the storage layer 2 differs
from that in the modification layer 3.
Since the rate of migration of the substance is determined essentially by the product of the
local electric field strength and the electrophoretic mobility, it follows, based on the
assumption that the mobility of the substance in the storage layer 2 is the same as that in the
modification layer 3, that the rate of migration of the substance depends chiefly on the
CA 02217061 1997-09-30
electric field strength in each of the layers 2 and 3. For the above-described example
embodiment having the electric field strength curve E shown in Fig. 2, this means that, on
passing from the storage layer 2 of lower conductivity and higher electric field strength into
the modification layer 3 of higher conductivity and lower electric field strength, the substance
is "slowed down". The substance therefore has different rates of migration in the two layers 2
and 3. That difference in the rates of migration has the result that the concentration of the
substance in the modification layer 3, in which the rate of migration is lower, increases.
Consequently, during the initialisation of the transdermal system 1 an increase in
concentration of the substance occurs in the modification layer 3.
Such a transdermal system therefore has the advantage that the substance to be
administered is, after initialisation, available in the modification layer 3 in a concentration that
is distinctly higher than the original concentration at which the substance was contained in
the storage layer 3 before the initialisation. That increase in concentration results in a
considerably higher dosage rate in the subsequent passive administration. The increase in
concentration of the substance during the initialisation is of particular advantage in cases in
which the substance cannot be stored for prolonged periods at such a high concentration as
that required for an efficient passive administration.
As has already been mentioned above, for the transdermal system 1 according to the
invention, and especially for the increase in concentration of the substance in the
modification layer 3, it is not necessary for the electrophoretic mobility of the substance to be
the same in both layers. The only prerequisite for the increase in concentration is that the
rate of migration of the substance in the modification layer 3 is distinctly lower than its rate of
migration in the~storage layer 2. This can also be effected, for example, by the electric field
strength prevailing in the storage layer 2 being the same as that prevailing in the modification
layer 3 while the electrophoretic mobility of the substance in the two layers 2 and 3 is
different. It is naturally also possible to bring about different rates of migration of the
substance in the two layers 2 and 3 by means of different electric field strengths combined
with different electrophoretic mobilities.
It is also possible in the transdermal system 1 according to the invention to control the
concentration at which the substance is present in the modification layer 3 at the end of the
initialisation. Two parameters are available for that control: first, the intensity of the electric
CA 02217061 1997-09-30
current that flows during the initialisation, and secondly the duration of the initialisation. The
intensity of the current influences the amount of substance that migrates from the storage
layer 2 into the modification layer 3 per unit of time, and the duration of the initialisation lays
down how long the substance migrates by virtue of electric forces. By a suitable combination
of current intensity and duration it is thus possible for a determined amount of substance to
be introduced into the modification layer 3. Since the modification layer 3 preferably has the
property that in it the equalization of concentration by means of diffusion occurs rapidly by
comparison with electrically induced migration, a specifically desired concentration of the
substance can be achieved in the modification layer 3. Since the electric current only flows
during the initialisation, and therefore does not flow through the skin, there is also no
physiologically determined limitation placed on the intensity of the current.
The passive system according to the invention consequently has the great advantage that
even after its production, during which the substance is introduced at a particular
concentration into the storage layer, the concentration of the substance to be administered
can still be modified in a controlled manner and hence the system is suitable for economic
mass production.
The transdermal system 1 according to the invention may naturally also be so arranged that
at the end of the initialisation the concentration of the substance that is to be administered is
lower in the modification layer than the original concentration at which the substance was
contained in the storage layer 3 before the initialisation. This can be effected, for example,
by the electrical conductivity of the storage layer being higher than that of the modification
layer 3, with the result that the electric field strength is higher in the modification layer 3 than
in the storage layer 2. Analogously to the explanations given above, when the mobility of the
substance in the two layers 2 and 3 is substantially the same, a higher rate of migration, and
hence a reduction in concentration, results in the modification layer 3. The higher rate of
migration in the modification layer can naturally also be brought about by the electrophoretic
mobility of the substance in the storage layer 2 being different from that in the modification
layer 3 - as an alternative to or in addition to the electric field strength being different.
In the transdermal system according to the invention the substance to be administered is
therefore, at the end of the initialisation, located in the modification layer 3 in a desired
CA 022l706l l997-09-30
-10-
concentration controllable by the initialisation. The initialisation is terminated by breaking the
circuit formed by the two electrodes 4 and 5, the energy source, the storage layer 2 and the
modification layer 3. This may be effected, for example, by the second electrode 5, or
alternatively by both electrodes 4 and 5, being removed from the transdermal system 1 by
being pulled off. The transdermal system is then ready for the passive administration of the
substance. For that purpose, once the second electrode 5 has been removed the
transdermal system is fixed to the patient's skin in such a manner that the modification layer
3 is in contact with the skin. The transdermal system according to the invention is especially
preferably in the form of a patch and covered with an adhesive layer. The patch is thus
,
attached to the skin in a manner known per se, as in the case of a conventional passive
transdermal patch. Since the second electrode 5, or the two electrodes 4 and 5, is (are)
separated from the transdermal system before the administration of the substance, it (they)
may be so formed that it (they) simultaneously act(s) as a protecting means during the
storage of the system. After the transdermal system according to the invention has been
attached to the skin, the substance to be administered is able to migrate passively, that is to
say by diffusion, through the skin.
An advantageous further development of the first example embodiment of the transdermal
system according to the invention is shown in Fig. 3. In that embodiment there are provided
between the first electrode 4 and the storage layer 2, and between the modification layer 3
and the second electrode 5, intermediate layers 4a and 5a, respectively, which physically
separate the first electrode 4 from the storage layer 2 and the second electrode 5 from the
modification layer 3. The intermediate layers 4a and 5a prevent contamination of the storage
layer 2 and of the modification layer 3, respectively, since any electrolytic products that may
be formed at the electrodes 4 and 5 during the current flow are kept away from the storage
layer 2 and especially from the modification layer 3 by means of the intermediate layers 4a
and 5a. In addition, the electrolyte contained in the intermediate layers 4a and 5a helps to
close the circuit. After the initialisation of the system the intermediate layer 5a can be
removed from the transdermal system 1 by being pulled off together with the second
electrode 5. it is naturally also possible for both intermediate layers 4a and 5a to be removed
together with their respective electrodes 4 and 5 after the initialisation.
CA 02217061 1997-09-30
The transdermal system in the further development shown in Fig. 3 furthermore has a
blocking membrane 10 which is arranged between the storage layer 2 and the modification
layer 3. The blocking membrane 10 has the property that its permeability can be controlled
by the application of an electric field. Before the initialisation of the transdermal system, the
electric field between the electrodes has not yet been switched on and consequently the
blocking membrane 10 is virtually impermeable. When the electric field is switched on in
order to initialise the transdermal system, the blocking membrane 10 is as a result "opened"
and the substance to be administered is able to migrate through it. Membranes such as the
blocking membrane 10 are state of the art per se.
That further development with the blocking membrane 10 has the advantage that the
transdermal system can be stored better and for longer, since during storage the blocking
membrane 10 provides a more durable separation of the storage layer 2 and the
modification layer 3, which are, of course, phases that have different physical and chemical
properties (for example conductivity). Before the initialisation, that is to say as long as the
circuit has not been closed, the blocking membrane 10 especially prevents any significant
transport of mass, for example caused by passive diffusion, between the storage layer 2 and
the modification layer 3. On the other hand, the blocking membrane 10 presents virtually no
hindrance to the migration of the substance during the initialisation of the transdermal
system, that is to say when the membrane is in the opened state.
The administration of a plurality of therapeutically active substances is naturally also possible
using the system according to the invention. Those substances may be contained, for
example, in the~ same storage layer 2 and then during initialisation jointly migrate into the
modification layer 3.
On the other hand, it is also possible for the reservoir to comprise more than one storage
layer 2. In a further preferred example embodiment, which is shown in Fig. 4, the reservoir
comprises two storage layers 2a and 2b. Those storage layers 2a and 2b are preferably
arranged in the form of a stack and physically separated from one another, so that the
reservoir has a multi-layered structure. That example embodiment is especially
advantageous for the administration of a plurality of different substances when the different
substances cannot be stored together over a prolonged period in one storage layer at a
CA 02217061 1997-09-30
- 12-
concentration high enough for the passive administration. During the initialisation the
substances then migrate from the different storage layers 2a and 2b into the modification
layer 3 where they are brought to the desired concentration. The number of two storage
layers 2a and 2b is obviously to be understood purely as an example. The reservoir may
equally contain more than two storage layers.
When the reservoir has such a multi-layered structure, different storage layers 2a and 2b
may furthermore contain the same substance at different concentrations. Thus, during the
initialisation, a concentration profile can be produced in the assembly of the modification
layer 3 and the storage layers 2a and 2b as a whole with which it is possible, for example, to
achieve a dosage rate that is constant for longer in the subsequent passive administration.
In the multi-layered structure also, the storage layers 2a and 2b and the modification layer 3
are preferably made of an ionically conductive polymer or gel, especially a hydrogel. The
material from which the modification layer 3 is made may be the same material as that used
for the production of the storage layers 2a and 2b.
For example, storage layers 2a and 2b and the modification layer 3 may all be made of the
same polymer or gel material. The different rates of migration of the substance in the
different layers 2a, 2b and 3 can in those circumstances be achieved by means of different
degrees of crosslinking in the individual layers 2a, 2b and 3, since the conductivity and
hence the electric field strength in a particular layer during the initialisation depend on the
degree of crosslinking in that layer.
It will be understood that the further developments described for the first example
embodiment may also be applied in an equivalent manner to the second example
embodiment. For example in the second example embodiment there may also be provided in
addition, at one or at both electrodes 4 and 5, intermediate layers that keep the electrolytic
products away from the storage layers 2a and 2b and from the modification layer 3. Also, in
order to improve the shelf life of the transdermal system there may be provided between the
modification layer 3 and the adjacent storage layer 2b, and/or between adjacent storage
layers 2a and 2b, blocking membranes the permeability of which can be controlled by means
of the electric field.
CA 022l706l l997-09-30
-13-
The transdermal system according to the invention, in which an electrically driven transport
of the substance into the modification layer 3iS effected before the per se passive
administration of the substance, has the great advantage that even after the system's
production, during which the substance is introduced into the storage layer 2 at a particular
concentration, the concentration of the substance to be administered can be modified in a
controlled manner. Consequently, with such a system the dosage rate can be adapted to the
individual therapeutic requirements of a patient, making the transdermal system very flexible
in its use.