Note: Descriptions are shown in the official language in which they were submitted.
CA 02218376 1997-10-16
W~ 96/34645 PCT/US96/06335
-1-
Background of the Invention
Catheter For Administering' A Liquid Aft
Technical Field
This invention generally relates to catheters for use
in enabling the visualization of the anatomy and more
specifically to catheters that are particularly adapted
for the field of endoscopic retrograde
cholangiopancreatography.
Background Art
The use of catheter-like devices for administering
therapeutic, diagnostic and vaso-occlusive agents at
predetermined target sites in a patient is well known.
Initially catheters of this type, particularly for use in
the field of endoscopic retrograde
cholangiopancreatography (ERCP) procedures, were
constructed from Teflon~ and included a single lumen sized
to accommodate a wire guide and to act as a liquid agent
transfer channel. As the ERCP catheters typically were
also adapted to be inserted over guidewires or through
working channels of endoscopes, they were typically
shipped with a stylet wire in the lumen that would stiffen
the catheter to prevent kinking or bending. The stylet
had to be removed prior to any use of the catheter with a
guidewire or after the catheter was inserted through an
endoscopic device.
The presence of a guidewire in the lumen may restrict
the transfer of liquid agents, such as radiographic
contrast agents, through the lumen past the guidewire.~
Consequently after a physician inserted the ERCP catheter,
any guidewire would be removed to facilitate the
administration of a radiopaque contrast agent to determine
the location of various obstructions.
Often times it became necessary to reposition the
catheter. This required the reinsertion of the guidewire
through the lumen to enable catheter relocation. Then it
was necessary to remove the guidewire again. The need to
maintain sterile conditions further complicated this
procedure especially as to the guidewire while it was
CA 02218376 1997-10-16
WO 96/34645 PCT/US96/06335
-2-
removed from the catheter. Given the nature of the
contrast agents, it was also found that in some cases the
contrast agent, guidewire and lumen in combination can
become stiff thereby reducing catheter flexibility. In
some situations it was even possible for the guidewire to
stick in the catheter thereby requiring the removal of
both the catheter and the guidewire. Single lumen
catheters also were characterized by back flow whereby the
contrast agent could squirt back out the proximal end of
the catheter and onto the administering medical
professional.
More recently there have been introduced dual lumen
ERCP catheters in which one of the lumens is adapted for
receiving a guidewire or stylet and the other is adapted
for transferring the contrast agent. The transfer lumen
has typically either had a circular cross-section or
crescent-shaped cross-section, the latter being disclosed,
for example, in United States Letters Patent No. 5,397,302
to Weaver. In the Weaver patent a catheter formed of
2o polyurethane or nylon has a durometer of about 60D and a
hydrophilic coating thereby to provide lubricity, kink
resistance and suppleness. The catheter tube has
substantially cylindrical side walls, a proximal end for
connection to a source of contrast medium and a distal end
for entry into the common biliary duct of a patient. The
tube contains a first crescent-shaped liquid agent
transfer lumen for transporting contrast media from the
source of the contrast medium to the biliary duct. A
second circular lumen facilitates the insertion of the
catheter over a guidewire.
Although dual-lumen catheters of the prior art
eliminate many of the handling problems posed by single
lumen catheters, in terms of removing and reinserting a
stylet or guidewire, several undesirable characteristics
still exist. For example, dual lumen catheters for ERCP
procedures are limited to a maximum overall diameter. The
addition of a second lumen of sufficient size to provide
adequate transfer rates through the catheter of that
CA 02218376 1997-10-16
WU 96/34645 PCT/LTS96/06335
-3-
maximum size can weaken the catheter wall and subject the
catheter to being burst while a contrast agent is being
administered. Further, even if some minimum wall
thickness is maintained to prevent bursting, a catheter
being inserted through an endoscope without a guidewire is
' subject to kinking and bending under the axial pressure
required to move the catheter relative to the endoscope.
Kinking and bending have a real potential for damage to
the catheter. Consequently physicians oftentimes will
become quite deliberate and even tentative in advancing a
catheter as the transfer force increases in order to avoid
kinking or bending with the subsequent requirement that
the catheter be withdrawn and destroyed. Consequently
even dual lumen catheters typically include a stiffening
stylet for use with endoscopic devices. Still further,
these constraints on the size of the second lumen can, for
reasonable pressures exerted on the liquid agent, limit
flow rate through the lumen to an unacceptably low value.
Disclosure of Invention
Therefore it is an object of this invention to
provide a catheter for the administration of a liquid
agent at a target site that is characterized by improved
flow through the catheter.
Another object of this invention is to provide a
catheter for the administration of a liquid agent at a
target site that is characterized by improved flow through
the catheter and by separation of the paths for the liquid
agent and a guidewire.
Still another object of this invention is to provide
a catheter for the administration of a liquid agent at a
target site that can be led through an endoscopic device
without the need for a stiffening stylet.
Yet another object of this invention is to provide a
catheter for the administration of a liquid agent at a
'target site that can be led through an endoscopic device
without buckling or kinking.
Still yet another object of this invention is to
provide a catheter adapted for use in endoscopic
CA 02218376 1997-10-16
WO 96/34645 PCT/US96/06335
-4-
retrograde cholangiopancreatography procedures that
includes separate lumens for a guidewire and for the
transfer of a contrast agent from a proximal site to a
distal site.
In accordance with one aspect of this invention a
catheter for the administration of a liquid agent at a
target site within a patient comprises a catheter tube, a
handle and a conduit. The catheter tube includes first,
second and third lumens that extend through the catheter
tube and exit through a proximal end and a distal end that
is adapted for transit to a target site. The handle has
first and second entry ports and a catheter port, each
port extending between the exterior of the handle and a
central volume. The proximal end of the catheter tube is
affixed in the catheter port such liquid agent
administered under pressure through the second port
transfers through the second and third lumens in parallel
to the distal end for ejection at the target site. The
conduit extends through the central volume and has one end
terminating at the first lumen at the proximal end of the
catheter tube and the other end terminating in the first
entry port thereby to prevent the transfer of liquid agent
from the central volume into the first lumen.
In accordance with another aspect of this invention,
an ERCP cannula for administering a contrast agent in the
biliary tree comprises a catheter tube, a handle, a thin-
walled tube and an outer seal. The catheter tube has a
guidewire lumen and first and second transfer lumens
formed between a proximal end and a distal end that is
adapted for location in the biliary tree. The first and
second transfer lumens have a combined area in cross-
section of approximately 25% of the area in cross-section
of the guidewire lumen. The handle includes a first entry
port and a catheter port spaced along a first axis, a
second entry port extending along a second axis that is
transverse to the first axis and a central volume
coextensive with portions of the first and second axes.
The thin-walled tube extends along the first axis through
CA 02218376 1997-10-16
WO 96/34645 PCT/LTS96/06335
-5-
the central volume. One end of the tube is sealed to the
first entry port, and the other end of the tube is sealed
against the catheter tube in the guidewire lumen. The
outer seal is formed about portions of the exterior of the
handle at the catheter port and portions of the exterior
of the catheter extending from the catheter tube.
Brief Description of the Drawings
The appended claims particularly point out and
distinctly claim the subject matter of this invention.
The various objects, advantages and novel features of this
invention will be more fully apparent from a reading of
the following detailed description in conjunction with the
accompanying drawings in which like reference numerals
refer to like parts, and in which:
FIG. 1 is a plan view of a catheter constructed in
accordance with this invention;
FIG. 2 is a view, in cross-section, of a portion of
the catheter shown in FIG 1;
FIG. 3 is a view, in cross-section, taken along lines
3-3 in FIG 2; and
FIG. 4 is an enlarged view, in cross-section, taken
along lines 4-4 in FIG. 1.
Best Mode for Carrying Out the Invention
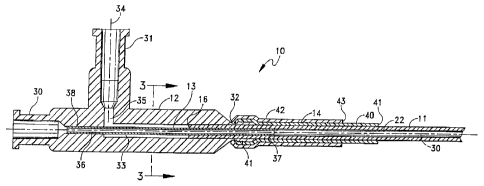
As shown particularly in FIGS. 1 and 2, a catheter 10
for the administration of a liquid agent at a target site
within a patient includes a catheter tube 11, a handle 12,
a conduit 13, a seal 14 and an end cap 15. The catheter
tube 11 extends between a proximal end 16 and a distal end
17. A section 18 of the catheter tube 11 adjacent the
distal end 17 has a reduced diameter that terminates in a
tapered tip portion 20. The reduced diameter section 18
carries a series of visual and radiographic markers 21.
The reduction of the catheter tube diameter, the taper of
the distal tip 20 and utilization of such radiographic
markers 21 is well known in the art.
FIG. 3 depicts the construction of the catheter tube
in accordance with this invention looking at the proximal
end 16 while FIG. 4 depicts a cross-section through the
CA 02218376 1997-10-16
WO 96/34645 PCT/TTS96l06335
-6-
tapered tip 20. The tube 11 can be formed of TFE or other
known biocompatible materials useful in catheter
construction. Typically the catheter tube 11 is extruded
Y
according to known methods to produce three lumens that
exit through the proximal end 16 and the distal end 17. A
first lumen 22 accommodates a guidewire. A second lumen
23 and third lumen 24 act a liquid transfer conduits for
conveying a contrast or other liquid agent from the handle
12 in FIG. 1 to the distal end 17.
For ERCP cannula applications, the catheter tube 11
may have a diameter of about 0.10". In such an embodiment
the first lumen has a diameter at the proximal end of
about 0.039" to accommodate a standard 0.035" guidewire.
Each of the lumens 23 and 24 has a diameter of about
0.020". Approximately 75% of a solid cylinder having the
diameter of the catheter 11 remains after the extrusion of
the three lumens 22 through 24.
Referring to FIG. 4, the material remaining after
forming the first lumen 22 and the second and third lumens
23 and 24 defines an outer ring 25 denoted generally by a
dashed line and three interconnected, generally radially
extending ribs designated by reference numerals 26, 27 and
28. The ribs 26 through 28 and the outer ring 25 form a
column structure that resists kinking and bending when
axial thrust is applied to the catheter tube 11 such as
thrust along the axis 30 shown in FIGS. 1 and 2. It has
been found that a catheter 10 with this catheter tube .
structure can be led through the working channel of an
endoscope without kinking or bending and without the
addition of a stylet. Thus a stylet can be eliminated to
simplify the use of and reduce the cost of the catheter
10.
As is known, after the extrusion of the tube 11
forming the lumens 22 through 24, the reduced diameter
portion 18 and tapered tip portion 20 are formed by
drawing the corresponding portions of the catheter tube 11
through a reducing die with mandrels located in each
lumen. Consequently any reduction in the overall diameter
CA 02218376 1997-10-16
WO 96/34645 PCT/US96/06335
_7_
need not significantly reduce the size of any lumens, so
the lumen 22, for example, still accommodates a standard
guidewire. Thus, in accordance with this construction,
' each of the lumens 22 through 24 exits through the distal
end 17 of the catheter device 11 and provides a passage 22
' for the guidewire and two passages 23 and 24 for the
administration of a contrast agent.
Referring now to FIGS. 1 and 2, the handle 12
includes a first entry port 30, a second entry port 31, a
catheter port 32 and an internal central volume or chamber
33. Both the first and second entry ports form
Conventional portions of Leur lock fittings. Referring
specifically to FIG. 2, the central volume 33 has a
generally cylindrical shape lying along the extension of
the catheter axis 30. The first entry port 30 and the
catheter port 32 are coaxial with the axis 30. A portion
of the catheter tube 11 adjacent the proximal end 16 lies
in the catheter port 32 with the outer surface of the
catheter tube 17 being sealed to the portions of the
handle 12 forming the catheter port 32.
Still referring to FIGS. 2 and 3, there is also
included in the handle 12 a conduit 36 in the form of a
thin-walled stainless steel tube, or "hypotube". one end
portion 37 of the conduit 36 extends into the first lumen
22 and produces a seal between the outer surface of the
conduit 36 and the inner surface of the catheter tube 11
forming the lumen 22. The other end portion 38 of the
tube 36 is sealed to the handle 12 at the entry port 30.
The volume of the chamber 33 is selected so that the total
cross-sectional area of the central volume 33 minus the
area of the tube 36 is at least as great as the combined
areas of the lumens 22 and 24.
The second entry port 31 lies along an axis 34 that
extends at right angles to the axis 33. The port 31
provides access to the central volume through a transverse
passage 35.
As will now be apparent, the tube 36, that is coaxial
with the axis 30, provides an isolated path for a
CA 02218376 1997-10-16
WO 96134645 PCT/US96/06335
_g_
guidewire that extends from the first port 30 through the
tube 36 and the first lumen 22. As contrast agent is
administered through the second entry port 34 under
pressure, it transfers through the passage 35 into the
central volume 33 around the tube 36. Given the seal
between the tube 36 and the catheter tube 11, this
material can not enter the lumen 22, but instead flows
only through the lumens 23 and 24 to be ejected at the
distal end 17 as shown in FIG. 1.
When a physician uses the catheter 10 in a procedure
requiring a guidewire, a physician first positions the
guidewire in the patient and then threads the distal tip
17 over the proximal end of the guidewire such that the
guidewire travels through the lumen 22 and the distal tip
20 moves to a target site. The radiographic markers 21
provide a means for determining the position of the distal
tip 17 during the location process.
Without removing the guidewire, the physician then
can attach a syringe or other device to the second entry
port 31 and force a contrast agent through the passage 35,
the central volume 33 and the lumens 23 and 24 in parallel
to be discharged where the lumens 23 and 24 exit the
distal end 17. The seals formed between the tube 36 and
the handle 12 and between the tube 36 and the catheter
tube 11 around the guidewire 22 assure isolation of the
guidewire lumen 22. Thus if it is necessary to relocate
the distal tip 20, there is no need to remove the
guidewire.
When the guidewire is in place, the free volume
around the guidewire 22 in the lumen 22 is sufficiently
small that any transfer of contrast agent back through the
lumen 22 as it is discharged from the distal end 17 is
blocked. When the catheter 10 shown in FIG. 1 is used
with an endoscopic device and without a guidewire, the
lumen 22 constitutes a path of low flow resistance to the
contrast agent as it exits the distal end 17 through the
lumens 23 and 24. In such procedures, the end cap 15 can
be attached to the first entry port 30 effectively sealing
CA 02218376 1997-10-16
WO 96/34645 PCT/US96/06335
_g_
the lumen 22. The end cap 15 is a typical Luer lock end
cap as known in the art. Should any contrast agent begin
to enter the lumen 22 at the distal end 17, any air in the
lumen 22 will compress and eventually reach an equilibrium
pressure whereby further displacement along the lumen 22
will not occur. Thus, the use of the end cap 15 overcomes
the prior art problem of having contrast agent flowing
back through the lumen 22 onto the personnel administering
the fluid.
Referring again to FIGS. 1 and 2, one particular
embodiment of the seal 14 comprises a two-part structure
with each part. being formed of a heat shrinkable material
such as polyolefin. A first seal 40 is formed onto a
circumferential band 41 formed integrally with the handle
12 at the catheter port 32 to improve a mechanical
i
gr
p
with the seal 40. The remaining portion of the seal 40
extends distally along the outer surface of the catheter
tube 11 for some predetermined distance to an end 41. A
- second heat shrinkable tube 42 can be applied over the
first tube 40 to terminate at an end 43 such that a small
portion of the tube 40 is exposed distally of the end 43.
In a preferred embodiment, the two tubes are formed of
different colors so the exposed portion of the tube 40
provides a marking function for facilitating measurements
during an ERCP procedure.
Therefore in accordance with the various aspects of
this invention, there has been disclosed a catheter for
the administration of liquid agents at a target site in a
patient, particularly contrast agents for use in ERCP
procedures, that includes a three-lumen catheter tube
a
,
handle and a thin-walled tube that is sealed in the
proximal end of a guidewire lumen in the catheter tube.
The proximal end of the catheter tube and the thin-walled
tube extending from the catheter tube are inserted into
a
catheter port of a handle with the other end of the thin-
walled tube being sealed to the handle at a first entry
port thereby to provide passage for a standard guidewire
through the first entry port the tube and the first lumen.
CA 02218376 1997-10-16
WO 96/34645 PCT/US96/06335
-10-
A second entry port also communicates with the central
chamber to provide a path for contrast agent through the
central volume and second and third lumens to the distal
end through the catheter tube. The combination of the
thin-walled tube and the central volume isolate the
guidewire lumen from the liquid transfer lumens thereby to
prevent the transfer of contrast agent into the guidewire
lumen so that a guidewire operates freely even during
repeated operations.
This structure eliminates the steps of removing a
guidewire during successive operations because there is a
separate path for the contrast agent. When the catheter
is used in endoscopic devices, its cross-section enables
the catheter to be inserted through the endoscope working
channel without a stylet.
This invention has been described in terms of a
particular embodiment in which the various components of
the catheter have specific configurations and dimensions
and are composed of particular materials. It will be
apparent that this invention could be embodied in
alternative structures and having different dimensions and
formed of different materials. For example, the thin-
walled tube that isolates the guidewire lumen from the
remaining lumens is shown along a straight axis, this axis
might be formed within an angular offset if desired.
Therefore, it is the intent of the appended claims to
cover all such variations and modifications as come within
the true spirit and scope of this invention.