Note: Descriptions are shown in the official language in which they were submitted.
CA 02221025 2005-03-16
30754-8
- 1 -
Flea and tick control collar containing N-
pheuylpyrazole for use on cats and dogs
The pr-esent invention relates to anexternal
'5 antiparasitic device, in particular a collar, foi pets,
in particular cats and dogs, this collar being active
against the ectoparasites of these animals, in
particular fleas and ticks.
The= invention also relates to the use of
active compounds for the manufactute of such'collars or
external devices, as=well as -to a treatment process
relating thereto.
The invention, is directed mainly towards
fleas of the genus Ctenocephalides, in particular C.
felis and C. canis, and ticks, in particular of the*
genus Rhipicephalus, especiaYly sanguineus, as well as
harvest ticks' (Trombicula automnalis),=which=are acarids
that mainly attack..hunting dogs.
Collars iritended toeliminate common
-ectoparasites from cats arid,dogs have been produced for
a long time. These collars consist of a matrix, usually
a plastic matrix, which incorpotates between' S and 40%
active substance'and* is capable of releasing it over
time. These collars thus theoretically'have the aim of
ensuring long-lasting protection.
However, despite the activity claims, out in
the field the collars do not have.the'efficacy required
to ensure the actual elimination of the-te parasites. The=
cause of this may be the low activity of the active.
substance included in the matrix. Another cause may be
the 'accelerated degradation of these active substances
under the efÃect of climatic factors; such as light,
heat and rain. Lastly, control of the release of the
active substance 'from the matrix is largely
overevaluated. Release generally proves to be difficult
and .variable, and may depend greatly on the
manufacturing conditions, which may vary from one batch
CA 02221025 2005-03-16
30754-8
- 2 -
to another,'and on the conditions of use, in particular
climatic variations and especially humidity and
temperature, etc. In addition, only a relatively small
amount of the active substance incorporated is actually
released and it proves difficult to be able to control
and optimize its release.
Another drawback of the collars encountered
in practice arises from the mode of use of this device
which may, obviously, be taken off, worn*irregularly, or
l0 even.be pulled off when the animal moves about, for
=example in undergrowth; the problem is particularly
critical for hunting dogs whose collars are removed
before a hunting 'outing even though they will be
confxonted with a flea- =and tick-ridden enviroiiment.
1s Patent applications WO-A-87/03781 and EP-A-
0,295,117 have' proposed insecticides of the N-phenyl-
pyrazole class.
These substances' are described as being
active against a very la'rge number of parasites
20 encountered in various fields, namely agriculturs,
public health and human and veterinary medicine. In the
latter field, these substances may act iri particular
against the fleas and ticks of . pets, such as cats and
dogs. These substances may be applied.in various ways,
25 namely via the oral, parenteral, percutaneous or topical
route. The latter type of administration itself covers
many possibilities, namely sprays, powders, baths,
showers, jets, greases, sha,mpoos,= creams, waxes,
preparations of skin solutiori type ' (pour-on) 'and
30 external devices such as e,arr3ngs and collars to provide
local or systemic treatment. EP-A-0,29,5,117 and EP-A-
0,500,209 propose a slow rel.ease composition which may
be in the form of a'collar or earrings for controlling
harmful insects. Such a formulation may comprise from
35 0.5 to 25% active material, from 75 to 99.5% polyvinyl
CA 02221025 2005-03-16
30754-8
- 3 -
chloride and a catalytic amount of a plasticizer,
dioctyl ptithalate.
French patent application FR-A-2,713,889
describes a pesticide composition containing an insect
growth regulator and an N-aryldiazole derivative chosen
from the derivatives 4- (2-bromo-1,1,2,2-tetrafluoro- =
ethyl)-1-(3-chloro-5-trifluoromethylpyridine-2-yl)-2-
methylimidazol=e, 5-amino-3-cyano-l-(2,6-dichloro-4-
t r i f 1ii o r o ai e t h y 1 p h e n. y 1 )- 4=
10. trifluoromethylsulphinylpyrazole and 5-amino-3 -cyano-1-
(2,6-dichloro-4-trifluoromethylphenxl)-4-
trifluoromethyltliiopyrazole. As in the prior documents
discussed above, the compositions are directed towards
a large number of insects in different fields, 'and
various types of formulations. Dog fleas and cat fleas
are targeted. Among the=formulations proposed, this
document mentions resinous preparations which giay be
made into pesticide col.lars for animals.
However, none of these dcrcuments describes
the use of pesticidal=collars comprising a compound of
the N-phenylpyrazole family to control fleas and'ticks
on pets such as cats and dogs, which make it possible to
ensure a high'level of efficacy for a long period
against these parasites. -
As for the pesticidal compounds of the prior
art, a specialist might have expected to encounter
conventional problems of release from the collars and
thus problems of activity.
Afterall, French patent application FR-A-
30. 2,713,889 proposes, does it not, the combination of such
compounds with an insect growth regulator?
The publications C. Genchi et al.,
Professione Veterinaria No. 1, supplement 1995, pages 19
to 22, J.M. Postal, Professione Veterinaria No. 1,
supplement 1995, pages 17 and 18 and A. Searle et al.,
CA 02221025 2005-03-16
30754-8
- 4 -
Australian Veterinary Practitioner, volume 25, No. 3,
1995, pages 157 and 158 propose instead to effectively
control fleas and, ticks using a sprayable solution
(spray) containing this type of active compourid.
The. Applicant has now found, surprisingly,-
that despite the fact that these. N-phenylpyrazoles
encounter the same difficulties of release as the
products of the prior art, it is however possible to
obtain collars which are entirely effective for the
elimination of cat and dog ectoparasites over a very
long period, for example from 6 to 18 months, and that,
in addition, the efficacy persists long after the collar
is taken off, namely over a period which may be equal to
or exceed 2 months, siich that it is then possible, to
' obtain a uollar which is entirely effective irrespective
, f the' conditions of use. Oneaccount of the' fact that.
the protection persists after the c llar is taken off,
.it.may be understpod that irregu3ar or voluntary use or
that resulting from loss of 'the collar does not
jeopardize the protection from which the animal should
benefit.
In addition, the Applicant has observed that
.this long-lasting efficacy was obtained with concentra-
tions of active substance= in the matrix forming the
collar which were much smaller than* those of the
standard products. It was also observed that this
efficacy was obtained within a very short time of the
collars being, put' n, in .particular an efficacy of
greater than 95% in 24 h against.fleas and gr.eater than,
90$'in 48 h against ticks.
It was observed, very surprisingly, that the
compounds.according to the invention, which are very
lipophilic and of high vapour pressure. (low volatility),
had a very high affinity for the sebum which usualZy
covers the' animal's coat (skin and hair), such that,
CA 02221025 2005-03-16
30754-8
- 5 -
when released, this compound is taken up by the s-ebum,
after which a translocation phenomenon occurs ensuring
distribution of active- substance over the animal's
entire body. In addition, and this is a noteworthy
=point, these active substances become concentrated in
the sebaceous glands which become a reservoir for them,
ensuring very long-lasting efficacy and making it
possible to compensate for the absence of the collar, by
releasing the active substance by passive diffusion.
1.0 By virtue of this phenomenon, the variations
in release of the active substance by the cbllar on
account of, for example, a variation in climatic
conditions, are compensated for by"the possibi2ita.,es of
release by the sebaceous glands.
it was ai-so observed that after a bath,
which could possibly lead to the removal of the sebum
distributed over the animal's body, the animal very
rapidly became reprotected, in the presence or absence
of the collar, owing to'the fact that secretion of new
sebum is accompanied by a rerease, by the sebaceous
glands, of the active substance that they contain.
The subject of the present invention is thus an
anti-flea and anti-tick collar for a cat or dog, to
distribute an active agent against fleas and ticks over
the cat's or dog's body, in the sebum and in the
sebaceous glands of the cat or dog, which collar is made
of a matrix in which is incorporated from 0.1 to 40% by
weight, relative to the collar, of an active agent
against fleas and ticks, this active agent comprising a
compound of formula 1- [2,, 6-C1,-4-CF,-phenylJ3,;-L'N 4- {=SO-
CF3]5-NH2 pyrazole, said collar providing more than 6
months of efficacy against fleas and more than 3 months
of efficacy against ticks.
CA 02221025 2005-03-16
30754-8
-6-
According to another embodiment of the invention there
is provided method for eliminating or preventing fleas and
ticks from cats or dogs comprising attaching to the cat or
dog a collar having a matrix into which is incorporated from
0.1 to 40% by weight, reactive to the collar, a compound
corresponding to the formula (1) below:
1- [2, 6-C1,-4-CF3-phenyl] 3-CN 4- (SO-CF3] 5-NHz pyrazole, .
wherein the collar provides more than 6 months of efficacy
against fleas and more than 3 months of efficacy against
ticks.
According to yet another embodiment of the invention
there is provided a use of a compound corresponding to
formula (I) below:
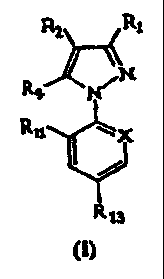
R2 R,
`N Rt N
Rij \
x
. ~ ~n =
R13
in which:
R, is CN or methyl or a halogen atom;
R2 is S(O)õR3 or 4,5-dicyanoimidazol-2-yl or haloalkyl;
R3 is alkyl or haloalkyl;
R,4 represents a hydrogen or halogen atom; or a radical
NRSR6, S(O),aRõ C(O) Rõ C(O) O-Rõ alkyl, haloalkyl or OR8 or a
radical -N=C (R9) (R),o) ;
R5 and R6 independently represent a hydrogen atom or an
alkyl, haloalkyl, C(O)alkyl, alkoxycarbonyl or S(O),CF3
radical; or R. and R. may together form a divalent alkylene
radical which may be interrupted by one or two divalent
CA 02221025 2005-03-16
30754-8
-7-
hetero atoms, such as oxygen or sulphur;
R, represents an alkyl or haloalkyl radical;
Re represents an alkyl or haloalkyl radical or a
hydrogen atom;=
R, represents an alkyl radical or a hydrogen atom;
R,,o represents a phenyl or heteroaryl group optionally
substituted with=one or more halogen atoms or groups such as
OH, -0-alkyl, -S-alkyl, cyano or a1ky1;
R11 and R12 represent, independently of each other, a
hydrogen or halogen atom, or CN or NOz;
R13 represents a halogen atom or a haloalkyl,
haloalkoxy, S(O) QCF, or SFS group;
m, n, q and r represent, independently of each other,
an integer equal to 0, 1 or 2;
X represents a trivalent nitrogen atom or a radical
C-R1z, the other three valency positions of the carbon atom
forming part of the aromatic ring;
with the proviso that when R1 is methyl, either R3 is
haloalkyl, R{ is NH2, Rll, is CF3 and X is N; or R2 is 4, 5-
dicyanoimidazol-2-yl, R4 is Cl, R11 is Cl, R13 is =CF3 and X is
=C-Cl;
for. the production of a collar intended to be attached to a
cat or dog, to distribute the substance over the'cat's or
dog's body and in the sebum and the sebaceous glands of the
pet, and which is capable of ensuring prevention and
treatment of fleas and ticks, to a high degree of efficacy
and over a period exceeding 6 months against fleas and 3
months against ticks.
Preferably, in formula (1),
Rl is CN or methyl;
R2 is S (O) R,;
R3 is alkyl or haloalkyl;
R4 represents a hydrogen or halogen atom; or a
radical NRRg, S(O),,,Rõ C(O) Rõ alkyl, haloalkyl or ORe or a
radical -N=C (R,)(Rlo) ;
CA 02221025 2005-03-16
30754-8
-7A-
R5 and R6 independently represent a hydrogen atom or an
alkyl, haloalkyl, C(O) alkyl or S (O) =CF3 radical ; or RS and R6
may together form a divalent alkylene radical which may be
interrupted by one or two divalent hetero atoms, such as
oxygen or sulphur;
R, represents an alkyl or haloalkyl radical;
R. represents an alkyl or haloalkyl radical or a
hydrogen atom;
R9 represents an alkyl radical or a hydrogen atom;
R10 represents a phenyl or heteroaryl, group optionally
substituted with one or more halogen atoms or groups such as
OH, -0-alkyl, -S-alkyl, cyano.or alkyl;
CA 02221025 2005-03-16
30754-8
- 8 -
R11 and R12 represent, independently of each
other, a hydrogen or halogen atom;
R13 represents a halogen atom or a haloalkyl,
haloalkoxy, S( )QCF3 or SFS group;
m, n, q and r represent, independently of
each other,= an integer equal to 0, 1 or 2;
X represents a trivalent nitrogen atom or a
radical C-Rlz, the other three valency positions of the
carbon atom forming part of the aromatic ring;
with the proviso that when Rl is methyl,. then
R3 is haloalkyl, R4 is NH2, Rl$ is Cl, R13 is CF3 and X is
N.
Compounds offormula (I) in which Rl is CN
will be selected anost particularly- The compounds= in
which R2 is S(o)=aR3, preferably with n = 1, R3 'preferabJLy
bea.ng CF3 or alkyl, ffor example methyl or ethyl, or
alternatively n-- 0, R, preferably being CF3, as well as
those in which X= C-R12, Ri, being a halogen atom., will
also be selected. Compounds in which R11 is a halogen
atom and those in'which R13 is haloalkyl, preferably CF31
are also preferred. In the context bf the present
invention, compounds combining two or more f. these
characteristics will advantageously be selected.
A preferred class of compounds of formula
(I) consists of compounds sudh 'that R1 is CN, R3 is halo-
alkyl, preferably CF3, or ethyl, R. is NH:2, .'R11 and= Rla
are, independently of each other, a halogen atom, and/or
R13 is haloalkyl.
The alkyl radicals in the definition of. the
compounds of formula (I) generally comprise from 1 to 6
carbon=atoms. The ring formed by the divalent alkyl.ene
radical representing RS and R6, as well as the nitrogen
atom to which R5 and R~ are attached, is generally a 5-,
6- or 7-membered ring.
A compound of formula (I) which is most
CA 02221025 2005-03-16
30754-8
- 9 -
particulerly preferred in the invention is
1- [2, 6-Cl24-CF3phenyl] 3-CN 4- [SO-CF3] 5-
M2pyrazole,
which is referred to hereinbelow as compound A.
Mention may also be made of the two=
compounds which differ from the above A by' the followi.ng
characteristics:
1- 'n = 0,R3 = CF3
2- n = 1,R3 - ethyl.
Compounds of formula (I) may be prepared
according to one or other of the processes described in
patent' applications WO-A-87%3781, 93/6089, 94/21606 or
European patent application EP-A-0,295,117, or any other
process falling within the competence of a specialist
skilled in the art of chemical synthesis. For the
chemical preparation of the products of the invention,
a persop skilled in the art is considered as having at
his disposal,, inter alia, all of the contents of
"Chemical Abstracts" and=the documents which are cited
therein.
However, low concentrations, of from 1 to
15% by weight and more particularly, especially' for
compound A, of from 1.25 to 10%, are preferred.
Under optimum conditions, compound (I) and
especially =compound A is present in the collar in a
proportion of from 2 to 6% by weight, more particularly
from 2.5 to 5% by weight.
It would obviously be possible to add to
compound A any other insecticide which might be
considered useful.
These insecticides may be present.. in the
same matrix as the compound according to the invention.
A composite collar made of at least two parts, each
including a different active substance, may also be
used.
CA 02221025 2005-03-16
30754-8
- 10 -
Within the scope of the invention, matrices
usually used to 'make collars may be used. Preferred
examples which.may be inentioned are matrices based on
PVC (polyvinyl chloride), as 'described in US-A-
3,318,769, 3,852,416 and 4,150,109 and 5,437,869, and
other vinyl polymers:
The plasticizers may be chosen iri particular
from adipates, phthalates, phosphates and citrates.
One or more plasticizers will preferably be
added to the PVC, these plasticizers being chosen in
particular from the following compounds:
- diethyl phthalate
-dioctyl sebAcate
-dioctyL adipate
.15 -diisodecyl phthalate
-acetyl tributyl citrate
-diethyl.hexy.l phthalate
-di-h-butyl phthalate
-benzyl butyl phthalate
- acetyl tributyl citrate
- tricresyl phosphate
- 2-ethylhexyl diphenyl phosphate.
= Even more preferably, a PVC matrix will be
used.in the presence of a primary remanent plasticizer
and a secondary plasticizer, in particular according to
EP-A-0,539,295 and EP-A-0,537,998.
Among the secondary plasticizers, mention
may be made of the Ãollowing products:
- acetyl triethyl citrate
- triethyl citrate
- triacetin =
- diethylene glycol monoethyl ether
- triphenyl phosphate.
A common stabilizer may'also be added thereto.
For the purposes of the present=invention, the term
CA 02221025 2005-03-16
30754-8
- 11 -
external device should be understood to refer to any
device which can be attached externally to the animal in
order to provide the same function as a collar.
By varying the concentration and/or composition of
the matrix, collars or other external devices acdording
to the =invention, which ensure effective and long-
lasting protection against fleas, may be made. Collars
or other external devices may be made with an efficacy
of greater than 6 months, in particular of greater than
dr equal - to. 12 or 18 months,even when the collar or
external device is taken off for a relatively prolonged'
period. When the collar or device is taken off, the
duration of_effective protection may range from 2 to 3
months.
By varyitng the-concentration and/or composition of
the matrix, it is possible to make collars or other
external devices according to the invention which ensure
effective and long-lasting protection against ticks.
Collars or external devices may be made with an efficacy
of greater tha-n 3 months, in particular of greater than=
or equal to 12 or 15=months, even when the collar or
external device is taken off for a relatively prolonged
period. When the collar or external device is taken off,
the duration of effective protection may range from 1 to
2 months.
It is noteworthy that this very long-lasting and
total efficacy is obtained by the compound according to
the invention alone, without addition of= another
insecticide.
The subject of the present invention is also a
method for eliminating ectoparasites, in particular
fleas and ticks, from pets such as cats and dogs, in
which method at least one collar or other external
device in accordance with the invention is attached to
the animal and the animal is afforded long-lasting,
= CA 02221025 2005-03-16
30754-8
- 12 -
effective protection against these parasi.tes, even when
the collar or other external device is taken off. The
indications of duration have been given above. Preferab-
ly, it is recommended that,=in accordance with the
method according to the invention, after it has been put
on for the first time, the collar or external devi-ce
should remain on the animal for at least 24 hours in
order for sufficient active substance to pass to the
animal and for the sebaceous glands to have been able to
store this active substance.
The object of this method is=non-therapeutic and in
particular relates to the cleaning of animal hairs and
skin by elimination'of the parasites which are present,
as well as their residues and dejections. The treated
animals thus have hair which is more pleasant to look at
and to feel.
The invention also relates to such a method for
therapeutic purposes, intended to treat and prevent
parasitoses having pathogenic consequences.
The subject of the, present invention is also the
use of a compound corresponding to formula I for the
production of a collar or other external device intended
to be attached to-a pet, in particular cats and dogs,
this compound being capable of ensuring prevention and
treating fleas and ticks to a high degree of efficacy
.and over a period exceeding 6 months against fleas and
3 months against ticks, the efficacy preferably being
maintained for several weeks if the collar or the
external device is taken off or lost or if there is a
variation in the release of the compound (I) -by the
collar or the external device. The devices as above are
concerned.
Preferably, the compound of formula I is
incorporated at a proportion of from 0.1 to 40% by
weight, preferably from 1 to 15% by weight, into a
CA 02221025 2005-03-16
30754-8
- 13 -
matrix intended to form the collar or other external
device.
Even more preferably, this compound will be
incorporated at a proportion of from 1.25 to 10% by
weight, in particular from 2 to 6% and even.more
preferably from 2.5 to 5%.
In particular, the use according to the invention
is directed towards producing collars or external
devices having, against fleas, an efficacy of greater
than 95%, or even greater than 98 or 99%.
For ticks, the desired efficacy exceeds 80% or 90%.
:Similarly, the use according to the invention,
against fleas, is directed towards the production of a
.long-lasting efficacy, longer than or equal to 12 months
l5 and even to 18 months.' "
For ticks, this duration is longer than 12 aiontris,
or even longer than 15 months.
Also preferably,, the use according to the invention
is directed towards the production of collars or other
external devices which make it possible to obtain an
efficacy maintained in the absence of= the collar or
external ,device over* a period ranging from 2 to 3
months, or more, against fleas and from 1 to 2 months,
or more, against ticks.
The present invention will now be described in
greater detail with the aid of non-limiting exau-ples
from which other*particular features and advantages of
the invention will emerge.
EXAtriPI,E 1:
Collar containing 10% AThe following two types of collar.-were prepared
(mixing and then extrusion):
Formulation 1: PVC . . . . . . . . . 50.0%
Stabilizer . . . . . . . 0.5%
. Epoxidized soybean oil .. 5.0%
CA 02221025 2005-03-16
30754-8
- 14
Diisooctyl adipate . . . 34.5%
Compound A . . . . . . 10.0%
Formulation 2: PVC. . . . . . . . . . . 50.0%
Stabilizer . . . . . . . 0.5%
Epoxidized soybean il .` 5.0%=
2-Ethylhexyl diphenyl
phosphate . . . . . . . 34.5%
Compoi,ind A . . . . . 10.0%
(% by weight).
3.0 For the tests,.9' adult dogs which had not received
any insecticide or acaricide for at least 40 days were
chosen=. The dogs were washed with an insecticide-free
shampoo and combed in order to remove any existing
parasites.
15 The dogs were divided into groups of three.
GrOLp- A :
Untreated contfols
GroLV B
Collar containing 10%'compound A formulation 1.
20 Yo w C:
Collar containing 10% compound .A. - formulation 2.
The dogs are infested with about 100 t 10
Ctenocephalides felis cat fleas (IInfed cat fleas) and.
50 t 2 Rhipicephalus sanguineus ticks (Brown dog tick).
25 The treatment follows the following general schema:
DAY
-2 Infestation with the fleas and ticks.
0 Collars are put on.
2 Fleas and ticks.are counted with a comb.
30 7:Infestation with ticks.
8 Infestation with.fleas.
9 Counting with a comb
35 Infestation with ticks.
36 Infestation with fleas.
35 37 Counting with a comb:
CA 02221025 2005-03-16
30754-8
- 15 -
63 Infestation with ticks.
64 Infestation with fleas.
65 Counting with a comb.
The same process is continued with infestation with
fleas and ticks every month for as, long as a satis-
factory efficacy is observed.
Hair samples were taken on:
D 205 and D 261 for all the dogs.
The collars were taken off on D 149 on dogs 363 and
289, on D 177 for dogs 87 and 300 and on D 2'05 for dogs
256 and 335.
The concentrations of active substance according to
the invention on the-hairs which were obtained at D 205
and D 261-are presented'in Tables 1 and,2 respectively,
in ug/g of hair.
Table 1: Concentration of active substaace according to
the inventioa on D 205.
DOG CONCENTRATION ( j.ig . g-l )
UNDER THE RIGHT LEFT 1+IIDDLE LIIMBAR,
No. COLLAR FLANR FLANK OF EACK REGION
GROUP B
363 18.63 3.11- .2.33 1.73 6.99
87 19.03 2.28 3.08 5.81 4.54
256 14.66 3.04 3.01 2.48 io.99
GROUP C
289 27.47 11.75 15.19 8.99. 30.9
300 482 . 4 34.6 69 17 : 97 36.7
335 257.4 23.86 28.86 22.8 41.2
Table Zs Conceatration of active substance according to
the invention, on hairse on D 261.
CA 02221025 2005-03-16
30754-8
- 16 -
DOG COt:CEIv'TRATIpN ( pg . g'1)
UNDER THE= RIGHT LEFT MIDDLE L=UMSAR
No. COLLAR FLANK FLANK OF BACK REGION
GROUP B
363 0.94 0.65 >0.29 >0.39 1.47
87 0.64 0.94 >0.32 >0.40 ND
256 >0.97 ND 0.81 0.78 0.69
GROUP C
289 3.16 >0.75 >0.59 >0.60 2.03
300 3.79 >0.70 2=. 04 1.. 35 - 5.52
335= 1.84 1.67 2.99 4.61 4.19
ND = Not determined.
A distributi,on of active substaace over the hair is
observed: the concentrati.ons =on the different areas are
higher with the formula'tion of Group C and are
relatively homogeneous from one area to another.
The concentrations of active substance are still
detectable 16 weeks after the collar has been taken off
and are still effecti,ve at that stage.
Tables.3, 4 and 5.make it possible to carry out a
concentration%activity correlation.
Tg.b1e 3: Concentration/activity r latioaship on D 149.
DOG CONCENTRATION (pg.g'=) ACTIVITYil)
UNDER THE BACK AND FLANKS FLEAS TICKS
No. COLLAR AV. MIN MAX
GROUP
B
363 559.5 15.6 10.7 24.8 0 1
CA 02221025 2005-03-16
30754-8
- i7 -
GROUP
C
289 871.3 48.9 30.8, 94.4 L 0 0
(1) Number of fleas and ticks present on the dog
Table 4: Concentration/activity relationship on D 205.
DOG CONCENTRATION (}ig.g`1) ACTIVITYtl)
UNDER THE BACK AND FLANKS FLEAS TICKS
No. COLLAR AV. MIN MAX
GROUP
B
363 18.6 3.54 1.73 6.99 0 12
87 19.0 3.93 2.28 5.81 0 22
256 15.0 4.88 2.48 11.0 0 15
OROUP
C
.289 27.5 16.7 8.99 30.9 0 . 12
300 482.4 26.3 15.7 36.7 0
335 257.4 29.2 22.8 41.2 0' 0
(1): Number of fleas and ticks present on the dog
Table.5=:=Concentration/activity relationahip an D 261.
DOG CONCENTRATION (ug.g-1)' ACTIVITYni
UNDER THE BACK AND FLANKS ' FLEAS TICKS
No. COLLAR AV. MIN MAX
GROUP
'30 B
363 0.94 >0.70 >0.29 1.47 0 21
87 0.64 <0.55 <LOQ 0.94 0 12
256 >0.97 <0.76 <LOQ = 0..81 1 24
GROUP35 C
289 3.16 >0.99 >0.59 2.03 0 28
300 3.79 >2.40 >0.70 5.52 0 19
335 1.84 3.37 1.67 4.61 0 3
40 (1): Number of fleas and ticks present on the dog
CA 02221025 2005-03-16
30754-8
- 18 -
AV. = average
MIN = minimum
MAX = maximum
LOQ = limit of quantification = 0.25 uglg
On D 261, the activity towards fleas is 100% on all
the dogs, except for one dog (presence of a flea):
The minimum concentrations (on the*hair) which are
effective towards fleas and ticks are-determined to be
about:
- 20'microgxams per gram of 'hair I for ticks
- 1 microgram per=gram of hair for fleas.
An activity 'of at least 5 months for ticks and of
at least 9 months. for fieas is observed with these
formulatiQns.
EX AMLS 2 _
Dogs were divided up into 6 groups of 8 dogs:
- A: Control group: collar .containing no active
substance.
- B: Reference product: comnerciaa collar agairist fleas
and ticks, containing=8% Chlorpyrifos (0,0-diethyl-0-
(3,5,6-trichloro-2-pyridyl)phosphorothioate), referred
to as ref. in the tables. -
- C: Collar containing 2.5% compound A.
- D: Collar containing 5t compound AA
- E: Collar containing 10% compound A
The collars were made with the same ingreaeats as
in formulation 2 of Example 1, with, in addition, a
pigment (titanium dioxide).
The tests follow the following schedule:
-2 Infestation with fleas and ticks
0 Collars are put on
2 Counting with a comb
7 Reinfestation with ticks
8 Reinfestatioin with fleas
CA 02221025 2005-03-16
30754-8
_ 19 -
9 Counting with a comb
35 Reinfestation with ticks
36- Reinfestation with fleas
37 Counting with a'comb
The process is continued along these lines frorn'
month to month.
Infestations and reinfestations are carried out at
a rate of:
100 t 10 fleas
50 3 ticks.
Tables 6 and 7.give the average' effectiveness
result's . '
=Tab4-s =6~z Percentag effectiveness on fleas, calculated
as a geoaae-tsic mean
.15
GROtTPS TREATED
DAY Ref. 2.5% =5% 10%
8%
CA 02221025 2005-03-16
30754-8
_ 20 _
- - - --------------- - - ---- - ---------
-5 0.0% 9.3% 0.0% 0.0%
2 63.9% 98.7% 100..0% 100.0%
9 96.3% 100.0% 100.0% 100.0%
37 96.1% 99.9% 100.0% 100.0%
65 99.1% 100.0% 100'.0% 100.0%
93 99.6% 100.0% 100.0% 100.0%
121 99.4% 100.0% 100.0% 100.10%
149 98.3% 100.0% 100.0% 100.0%
177 97.9% 100.0% 100.0% 100.0%
205 98.5% 100.0% 100.0% 100.0%
233 96.6% 100.0% 100.0% 100.0%
268 .89.6% 100.0% 10-0:0$ 100.0%
289 68.4% 100:0% 100.0% 10-0.0%
317 76..0% . 100.0% 100.0%, 100.0%'
345 79.5% 1001.0%. 100.0% 100.0%
373 - 100.0% 100.0%- 100.0%
401 - 99.4% 100:0$ 99.8%
429 - 99.2% 99.8% 99.3%
457 - 96.9% 98.8% 99.2%
485 - . 96.7% 98.2$ 98.7%
513 - 90.3% 99.0% 99.3%
541 - - 98.8% 100.0%
CA 02221025 2005-03-16
30754-8
- 21 -
Table 7 Percentage. effectiveness on ticks, calculated
as a geometric mean
GROUPS TREATED
DAY Ref. 2.5% 5% 10%
8%
-5 4.7% 19.1% 19.1%
32.0%
2 34.5% 67.0% 100.0% 98.5%
9 86.9% 96.2% 100.0% 100.0%
37 0.0% 27.2% 95.4% 95.4$
= .
65 31.7% 85.0% 100.0% 95.8%
93 31.8% 86.7% 100.096 95.7%
121 65.2% 91.3% 88.8% 92.1%
149 59.0% 93.4% 92.0% 94.5-t
177 67.2% 84.9% 95.3% 95.2%
205 62.0% 86.2% 100.0% 98.*4$
233 62.6% 89.8% 98.3% 97.9%
268 57.5% .4% 90.2*0- 99.5%
289 44.6% 92.4% '97.2% 95.1%
317 - 74.5% 85.6% 92.4%
345 - 88.1% 98.8% 98.0%
373 - 72.4% 93.9% 95.7$
401 - 63.4% 98.5% 93.6%
429 - 75.3% 83.2% 93.3%
457 - 62.4% 84.1%
86.7~
485 - - - -
513 - - - -
541 - - - -