Note: Descriptions are shown in the official language in which they were submitted.
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
"A dried product and a drying process"
Field of the Invention
The present invention relates to a dried product, and in
particular, though not limited to a dried biological product in
- 5 which the product is dehydrated to a water content of 20% or less.
The invention is also directed towards a process for dehydrating a
product, and in particular, though not limited to a process for
dehydrating a biological product.
Backqround Art
Many processes exist for drying products, such as various foods,
fruits, vegetables, and other biological substances. For example
W089/08229 discloses a system and a method for drying granular
material, in which the granular material is subjected to a drying
agent, such as nitrogen.
It is also known to freeze-dry vegetables. Such a method is known
to cause rupture of the cells and to increase the permeability of
vegetables to water, see U.S. Patent Specification No. 4,788,072
at Column 2, Lines 15 to 20.
It is further known to dry fruit or vegetable slices by immersing
said slices into a sugar solution, see for example, European
Patent Specification No. EP-A-0,339,175. When using a sugar
solution with a high Dextrose equivalent (DE) value, for example,
a DE value of 70, low molecular weight sugars are able to
penetrate into the cells, whereby a higher sugar content exists.
Such dried products may not have a water activity lower than 0.4,
and some of the contents of the cell, such as flavour and odour
constituents, may no longer be present in the interior of the
cell.
When using a sugar solution with a low DE value, for example, a DE
value of 25, sugars are extracted from the product. This dried
product also may not have a water activity lower than 0.4.
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
Furthermore, it is considered that due to the extraction of
sugars, the taste and odour characteristics of the product may be
altered.
The sugar osmotic drying is also considered as a pre-treatment,
that is, a treatment for lowering the water content of the product
before a final drying treatment, such as frying. The water
content of the osmotically dried product may thus not be low
enough to preclude spoilage of the product without additional
measures, such as refrigeration. sterile packaging or the addition
of preservatives. Due to the treatment of the product in a liquid
sugar solution, sugar may completely fill the voids or spaces
situated between two adjacent cell membranes.
It is further known to dry grapes by spraying them before
harvesting with a composition to facilitate removal of water from
the grapes, see U.S. Patent Specification No. 5,068,988. The
dried grapes retain a sufficiently high water content so as to
produce sufficient juice for making wine. The wines obtained by
using such dried grapes have a high alcohol content.
It is also known to dry timber using a carefully regulated
temperature and humidity regime during the drying process. The
temperature and humidity regime is species- and size-specific and
is selected to ensure that the drying operation does not cause
warping of the product as a result of excessive moisture and
temperature gradients within the material. The drying process
results in a longer drying time than would result from drying
without humidity regulation. Details of such drying processes are
presented in a large number of standard works, for example, in the
1991 ASHRAE Handbook, HVAC Applications.
It is generally accepted in the known art that "Based on the
analysis of heat and mass transfer, the most efficient dehydration
systems will maintain the maximum vapour-pressure gradient and the
maximum temperature gradient between the air and the interior
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCI~/IE96/00037
parts of the product", see Introduction to Food Engineering, R.P.
Singh and D.R. Heldman, Academic Press (1993), at Page 422.
It is also generally accepted that in known drying processes the
outer layer of the product becomes essentially impermeable to
aroma compounds but still transmits some water vapour to allow
drying to continue, see for example, "Food Dehydration", G.V.
Barbosa-Canovas and M.R. Oaks eds., A.I.Ch.E. Symposium Vol 89
(1993), Page 32.
Finally, PCT Application Specification No. WO 94/13146 of one of
the co-inventors of the present invention discloses a method and
an apparatus for dehydrating biological products in which a closed
system is used so as to ensure the retention of the essential
flavour and fragrance of the natural product.
Definitions
In this specification and in the claims, the following words and
terms as used herein have the following meanings:
Water content of an undried substance, in other words, a substance
prior to being dried is given as a pe,centage of the total weight
of the undried substance.
Water content of a dried substance is given as a pe\cLntage of the
total weight of dry matter only of the dried substance excluding
all moisture.
A hvqroscoDic substance is one in which the water content tends to
equilibrate with its surroundings.
Water activitv of a hygroscopic substance is defined as the
equilibrium relative humidity of a closed and thermally insulated
system in which the substance has been placed. The measurement
thereof should take place with a minimum head space and generally
in conformity with recognised procedures for measurement of water
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
activity. So in principle, the water activity is no different
from equilibrium relative humidity except that it is expressed in
terms of a scale from 0-1, instead of on a scale of from 0-100%.
The water activity measures the degree of freedom of water,
retained in various ways, in a hygroscopic substance.
The water activity directly determines the physical, mechanical,
chemical and microbiological properties of a hygroscopic
substance, for example, interactions such as clumping, cohesion,
electrostatic charge, and so on. In the food industry, the water
activity is a highly significant factor to be considered for the
conservation of semifinished and finished products.
In particular, the water activitv threshold for deteriorative
mechanisms in a given hygroscopic food substance is defined as a
level of water activity above which significant oxidation,
enzymatic browning, microbiological organic activity and other
deteriorative processes begin to take place to the detriment of
the organoleptic and nutritional characteristics of that
substance. For example, the proliferat;on of micro-organisms is
generally considered to be suppressed at water activity levels
below about 0.65. Other deteriorative processes become
progressively less active as the water activity decreases toward a
value between 0.2 and 0.25, that is, towards a level approximately
corresponding to the monolayer water content.
Cellular structure of a substance means the structure of the cells
of the substance, and also means the general arrangement of the
cells, whereby intercellular spaces, channels or passageways are
defined between the cells, and reference to damage to the cellular
structure, in general, refers to damage caused to the cellular
structure during a dehydrating process, it being understood that
the cells and cellular structure adjacent a face of the product
may be damaged prior to the dehydrating process, as a result, for
example, of cutting the substance or product into slices or the
like.
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
Cellular inteqritY of a substance refers to the degree to which
the cellular structure of the substance is unaltered or undamaged,
and in particular, the degree to which the cell walls remain
intact for retaining the organoleptic characteristics of the
product. Reference to the maintenance of cellular integrity
during a dehydrating process is defined as the maintenance of the
integrity of the cellular structure, which the substance had prior
to being dried throughout the drying process so that at the end of
the dehydrating process the dried product can be rehydtated to
substantially its original form in substantially all respects.
Structural inteqritv of a substance refers to the degree to which
the structure of the substance is unaltered or undamaged,
including its cellular structure. Reference to ~aintenance of
structural integrity during a drying process is defined as the
maintenance of the integrity of the structure, which the substance
had prior to being dried throughout the dehydrating process so
that at the end of the dehydrating process, the drled product can
be rehydrated to substantially its original form in substantially
all respects. In other words, in a dehydrating process which
maintains the structural integrity of the substance being dried,
the dehydration process is essentially reversible. For substances
having cells, the maintenance of structural integrity means also
the maintenance of cellular integrity, in other words, keeping
cell walls substantially intact and maintaining the intercellular
spaces and passageways. The purpose of maintaining structural
integrity during a drying process is to help retain the initial
structural characteristics of the substance, and to facilitate the
mass transfer of moisture from the substance. In food and health
products, for example, the initial structural characteristics
include organoleptic and nutritional p~ope~Lies, and other
qualities typical to each product in its initial form prior to
being dried. It is assumed that only substances having a
relatively high degree of structural integrity are submitted to
the dehydrating process according to the invention, since
otherwise, they could not be rehydrated to their original form.
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
Deqradation temDerature of a substance is defined as the
temperature above which the structural integrity of the substance
and/or some of its chemical or biochemical constituents may suffer
irreversible thermal damage.
Organoleptic tests serve as a basis for a sensory analysis of the
internal and external quality of a material. The external quality
of a material is judged according to its optical and physical
properties which are perceived by the visual and tactile senses.
These properties have to do with the appearance of the material,
for example, colour, size, shape, condition (uniformity, absence
of defects and blemishes). Of particular importance for dried
products are the hue (as it was before drying) and the lack of
browning, fading or other discolouration.
The internal quality of a material is judged according to its
flavour and aroma, and texture. Flavour and aroma are caused by
chemical p~operties and perceived mainly by the senses of taste
and smell which are closely interlinked. Taste is due to the
sensations felt on the tongue, while aroma is perceived due to the
stimulation of the olfactory senses with volatile organic
compounds. For dried products, it is most desirable to retain the
taste and aroma characteristic of that material in its initial
state. The presence of foreign off-taste and off-aromas is most
undesirable.
The chemical compounds that make up the aromatic properties of
plant products need to be volatile by definition in order to be
perceived at the temperature at which the product is going to be
utilized. That is, they must be present in a gaseous or vapour
state so that the molecules can reach the nasal passages of the
persons perceiving the aromas. Generally, only a very small
number of compounds impart the main characteristic aroma of a
substance. Some volatile compounds may exist per se in the intact
tissue, while others are formed enzymatically upon the rupture of
the cells of the substance. One may measure precisely the
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96139854 PCT/IE96/00037
concentration of compounds that impart the characteristic odour of
a substance using gas chromatography equipment.
Texture is most often expressed as an overall assessment of the
feeling that food gives in the mouth. It is a combination of
sensations derived from the lips, the tongue, the walls of the
mouth, the teeth, and even the ears. It is desirable for dried
products to maintain their textural integrity, not to be too hard,
too elastic or too brittle, during biting, chewing and swallowing.
Because the organoleptic properties of a substance are not
generally equally subject to change, and are generally affected to
a different degree by different stimuli, it is appropriate to
examine as many of the organoleptic p.openLies as is practicable
in order to evaluate substance degradation in the most tho.ough
manner possible.
In food products, the amount of ascorbic acid present in the dried
product is a good indicator of the degree of degradation which the
product has undergone from its initial state. This follows from
the fact that ascorbic acid is particularly volatile, that is
vulnerable to depletion, especially at higher temperatures and
over time (after harvesting, during storage). The losses of
ascorbic acid are greater in fruits and vegetables having a higher
pH.
Monolaver water content of a hygroscopic substance is defined as
that amount of water which can be retained by the substance at
maximum binding energy. This water content is estimated by
fitting a theoretical expression such as the BET equation for
moisture sorption to the measured moisture sorption isotherm for
the substance. See for example, BET Monolayer Values in
Dehydrated Foods and Food Components, H.A. Iglesias and J.
Chirife, Lebensm-Wiss. U.-Technol. Volume 9, (1976) at pages 107-
113.
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/~E96/00037
Obiects of the Invention
It is an object of the invention to provide a dried product, and
in particular, a dried biological product, such as, for example, a
dried fruit or vegetable product which on being rehydrated
5 possesses substantially all of the properties and characteristics
of the original product prior to being dried, and in particular,
possesses substantially all of the organoleptic properties, and in
which the structural integrity of the product is maintained. It
is also an object of the invention to provide a dried product, the
water content of which is 20% or less by weight.
Additionally, it is an object of the invention to provide a
process for dehydrating a product, and in particular, a biological
product, such as, for example, a fruit, a vegetable or the like
product in which the product may be dried to a water content of
15 20% or less, and the structural integrity of the product prior to
being dried including its cellular integrity is maintained
throughout the dehydrating process.
SummarY of the Invention
According to the invention there is provided a dried product
20 having a water content lower than 20%, wherein at least 50% of the
cells not adjacent to a face of the product still have
substantially undamaged membrane, and in which the water present
in the product has a water activity not exceeding 0.7.
In one aspect of the invention the water present in the dried
25 product has a water activity not exceeding 0.65.
In another aspect of the invention the water present in the dried
product has a water activity not exceeding 0.6. In general, the
water present in the dried product has a water activity of between
0.2 and 0.5, and preferably the water activity is between 0.25 and
0.45, and ideally the water activity is between 0.3 and 0.4.
In one aspect of the invention the water content of the dried
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96~9854 PCT~E96/00037
product is less than 10%, and typically, the dried product has a
water content in the range 4% to 7%.
Preferably, substantially all of the cells of the dried product
which were not damaged prior to drying remain substantially
undamaged after drying.
In one aspect of the invention the product is a biological
product, for example, a dried fruit, or a dried vegetable.
Typically, the dried product is in the form of a slice, and in
general, the dried product is derived from a sliced product, the
thickness of the slices of the product prior to dehydration being
in the range of 1 mm to 10 mm. Ideally, the thickness of the
slice prior to dehydration lies in the range of 3 mm to 7 mm.
In one aspect of the invention the product contains compounds
which are able to be eluted at different temperatures, the weight
ratio fraction of compounds eluted at a temperature of between
150~C and 200~C to fraction of compounds eluted at a temperature
of between 40~C and 100~C being greater than 0.3. Preferably, the
weight ratio fraction of compounds eluted at a temperature of
between 150~C and 200~C to fraction of compounds eluted at a
temperature of between 40~C and 100~C is greater than 0.5.
Ideally, the weight ratio fraction of compounds eluted at a
temperature of between 150~C and 200~C to fraction of compounds
eluted at a temperature of between 40~C and 100~C is greater than
0.6.
In another aspect of the invention the product contains compounds
which are able to be eluted at different temperatures, the weight
ratio fraction of compounds eluted at a temperature of between
150~C and 200~C to fraction of compounds eluted up to 200~C is
greater than 0.25.
In one aspect of the invention the product comprises cells
SUBSTITUTE SHEET (RULE 26)
. CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
adjacent to a face of the product, said cells defining
therebetween spaces, at least 50% of the spaces not being
completely or nearly completely filled with sugar. Preferably, at
least 50% of the spaces contain a gaseous medium. Ideally, at
least 50% of the volume of the said spaces are filled at a rate of
at least 50% with the gaseous medium.
In one embodiment of the invention, the product is banana and the
dried product has prominent gas chromatographic peaks at 6.1 and
7.3 minutes retention times.
In another embodiment of the invention the product is mango and
the dried product has prominent gas chromatographic peaks at 6.1
and 12.8 minutes retention times.
In a further embodiment of the invention the product is pineapple
and the dried product has a prominent gas chromatographic peak at
6.1 minutes retention time.
In a still further embodiment of the invention the product is kiwi
and the dried product has a prominent gas chromatographic peak at
6.3 minutes retention time.
In another embodiment of the invention the product is papaya and
20 the dried product has a prominent gas chromatographic peak at 6.2
minutes retention time.
In a further embodiment of the invention the product is ginger and
the dried product has prominent gas chromatographic peaks at 6.3,
8.4 and 10.0 minutes retention times.
25 Additionally, the invention provides an improved food preparation
having as an ingredient a dried food product wherein the dried
food product has a water content lower than 20%, said dried food
product being a product in which at least 50% of the cells not
adjacent to a face of the product still have closed membrane, and
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
in which the water present in the dried food product has a water
activity lower than 0.7.
In one aspect of the invention the food composition is useful as a
sauce composition.
Additionally, the invention provides a dried product having a
water content lower than 10%, wherein at least 50% of the cells
not adjacent to a face of the product still have substantially
undamaged membrane, and in which the water present in the product
has a water activity lower than 0.4.
In one aspect of the invention the water content of the dried
product lies in the range of 4% to 7%.
In another aspect of the invention the water present in the dried
product has a water activity of between 0.15 and 0.35.
Preferably, the water present in the dried product has a water
activity of between 0.25 and 0.3.
In another aspect of the invention the product contains compounds
which are able to be eluted at different temperatures, the weight
ratio fraction of compounds eluted at a temperature of between
150~C and 200~C to fraction of compounds eluted at a temperature
of between 40~C and 100~C being greater than 0.3. Preferably, the
weight ratio fraction of compounds eluted at a temperature of
between 150~C and 200~C to fraction of compounds eluted at a
temperature of between 40~C and 100~C is greater than 0.5.
Advantageously, the weight ratio fraction of compounds eluted at a
temperature of between 150~C and 200~C to fraction of compounds
eluted at a temperature of between 40~C and 100~C is greater than
0.6.
In a further aspect of the invention the product contains
compounds which are able to be eluted at different temperatures,
SUBSTITUTESHEET(RULE26)
CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96/00037
the weight ratio fraction of compounds eluted at a temperature of
between 150~C and 200~C to fraction of compounds eluted up to
200~C is greater than 0.25.
In another aspect of the invention the product comprises cells
adjacent to a face of the product, said cells defining
therebetween spaces, at least 50% of the spaces not being
completely or nearly completely filled with sugar. Preferably, at
least 50% of said spaces contained a gaseous medium. Ideally, at
least 50% of the volume of said spaces being filled at a rate of
at least 50% with a gaseous medium.
In one aspect of the invention the product is a dried vegetable or
a dried fruit.
The product may also be any other dried food.
In one embodiment of the invention the product comprises cells
adjacent to a face of the product, said cells defining
therebetween spaces, at least 50% of said spaces containing a
gaseous medium. Preferably, substantially all the cells of the
dried product which were not damaged prior to drying remain
substantially undamaged after drying.
Additionally, the invention provides a food composition having the
improvement of containing a dried food product having a water
content of between 4% and 7%, the dried product being a product in
which at least 50% of the cells not adjacent to a face of the
product still have closed membrane, and the water present in the
dried product has a water activity lower than 0.4.
In one aspect of the invention the food composition is useful as a
sauce composition.
The dried product according to the invention has many advantages.
It has a sufficiently low water activity to ensure long term
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCI/IE96/00037
resistance to degradation at typical storage temperatures of about
20~C to 30~C. A water activity in the range of 0.3 to 0.4 ensures
long term res;stance to degradation at such storage temperatures
over a relatively long storage period. Depending on the product,
at water activity levels up to 0.7, certain products are resistant
to degradation and deterioration at typical storage temperatures
of about 20~C to 30~C over relatively long storage periods.
In the case of fruits and vegetables, the product has improved
organoleptic properties with respect to products dried by other
known processes. This it is believed is due at least in part to
the maintenance of the structural integrity, and in particular,
the cellular integrity of the product. In general, it has been
found that where the cellular integrity of at least 50% of the
cells of the product is maintained, and in particular, 50% of the
interior cells, the dried product can be rehydrated to have
substantially all of the p~oper~ies and characteristics which the
product had prior to dehydration. Needless to say, when the
cellular integrity of substantially all of the cells is
maintained, the p)ope.Lies and characteristics of the rehydrated
20 product even more closely approach those ~,ope.Lies and
characteristics of the product prior to being dehydrated. Where
structural integrity has been maintained throughout the
dehydration process, the properties and characteristics of the
rehydrated product even still more closely approach those
25 properties and characteristics of the product prior to being
dehydrated.
Additionally, the invention provides a process for dehydrating a
product by urging a gaseous drying medium into contact with the
product in a chamber, wherein the temperature and relative
humidity of the drying medium are controlled so that the rate of
water removal from the product is such as to minimise damage to
the cellular integrity of the product during the drying process.
Preferably, the temperature and relative humidity of the drying
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96/00037
medium are controlled so that the cellular integrity of at least
50% of the cells of the product not adjacent to a face of the
product is maintained during the drying process, and ideally, the
cellular integrity of substantially the entire product is
maintained during the drying process.
In one aspect of the invention the rate of water removal from the
product is such as to minimise damage to the structural integrity
of the product during the drying process. Preferably, the
structural integrity of at least 50% of the cells of the product
not adjacent to a face of the product is maintained during the
drying process. Advantageously, the structural integrity of the
product is substantially maintained during the drying process.
The dehydration process according to the invention establishes a
gentle drying regime which maintains the optimal conditions for
the mass transfer of moisture from the product being dried while
substantially maintaining structural integrity substantially the
entire product during the drying process. By closely controlling
the temperature and relative humidity of the drying medium, the
surface of the product being dried remains permeable to water,
moisture and water vapour moving from the interior of the product
into the drying medium.
Enhanced mass transfer of water from the product as a result of
the gentle drying process of the invention results in a superior
product as well as in an efficient drying process, due to the
reduction in energy consumption.
The drying regime avoids subjecting the product and material being
dried to thermal and pressure over-stresses, hence maintaining the
structural and cellular integrity of virtually the entire product
during the drying process, while maintaining optimal conditions
for heat and mass transfer.
In one embodiment of the invention the relative humidity of the
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
drying medium is controlled so that the difference between the
relative humidity of the drying medium and the equilibrium
relative humidity of the product does not exceed 70% relative
humidity. Preferably, the relative humidity of the drying medium
is controlled so that the difference between the relative humidity
of the drying medium and the equilibrium relative humidity of the
product does not exceed 60% relative humidity. Advant~geously,
the relative humidity of the drying medium is controlled so that
the difference between the relative humidity of the drying medium
and the equilibrium relative humidity of the product does not
exceed 50% relative humidity.
In another aspect of the invention the relative humidity of the
drying medium in the chamber is allowed to rise to a maximum value
which lies within the range of 30% to 70%. Preferably, the
maximum value of the relative humidity of the drying medium in the
chamber lies in the range of 50% to 70%. Advantageously, the
maximum value of the relative humidity of the drying medium in the
chamber lies in the range of 50% to 55%.
In another embodiment of the invention on the value of relative
humidity of the drying medium reaching the maximum value, the
relative humidity of the drying medium in the chamber is
maintained substantially constant at the maximum value, or is
permitted to only gradually decrease by maintaining the relative
humidity of the drying medium being delivered to the chamber
substantially constant, until the relative humidity of the drying
medium in the chamber commences to fall or commences to fall at an
increasing rate.
Preferably, the relative humidity of the drying medium in the
chamber is permitted to fall off relatively rapidly after the fall
in relative humidity of the drying medium has commenced or the
rate of fall of the relative humidity of the drying medium has
commenced to increase, until the water content of the product is
approaching the desired water content and the rate of evaporation
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
16
of water from the product becomes substantially independent of the
drying medium.
In one aspect of the invention the rate of evaporation of water
from the product becoming substantially independent of the drying
medium, the relative humidity of the drying medium is controlled
to asymptotically approach a predetermined value of relative
humidity which provides the product dried to the desired water
content.
In another aspect of the invention the predetermined value of
relative humidity is lower than the equilibrium relative humidity
of the product which corresponds to the desired water content, and
the difference between the predetermined relative humidity of the
drying medium and the equilibrium relative humidity of the product
corresponding to the desired water content is in the range of 20%
to 40% relative humidity.
In general, the difference between the predetermined value of
relative humidity and the equilibrium relative humidity of the
product corresponding to the desired water conttnt is in the range
of 25% to 35% relative humidity, and in many cases the difference
between the predetermined value of relative humidity and the
equilibrium relative humidity of the product corresponding to the
desired water content is approximately 30% relative humidity.
Depending on the product, the relative humidity of the drying
medium may be maintained substantially at the predetermined value
of relative humidity for a time period in the range of 30 minutes
to 120 minutes.
In another embodiment of the invention the drying medium is
circulated through the chamber so that the speed of the drying
medium relative to the product lies in the range of 1 M per second
to 3 M per second. Preferably the drying medium is circulated
through the chamber so that the speed of the drying medium
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
relative to the product lies in the range of 1.5 M per second to
2.5 M per second. Advantageously, the drying medium is circulated
through the chamber so that the speed of the drying medium
relative to the product lies in the range of approximately 2 M per
second.
In general, it is preferably that during the period while the
relative humidity of the drying medium in the chamber is being
maintained substantially constant or is only gradually decreasing,
the relative humidity of thé drying medium in the chamber is not
more than 50% relative humidity lower than the equilibrium
relative humidity of the product, for minimising the damage to the
cellular and structural integrity of the product. Preferably,
during the period while the relative humidity of the drying medium
in the chamber is being maintained substantially constant or is
only gradually decreasing, the relative humidity of the drying
medium in the chamber is not more than 40% relative humidity lower
than the equilibrium relative humidity of the product, and is
preferably not more than 30% relative humidity lower than the
equilibrium relative humidity of the product.
In another embodiment of the invention during the period while the
relative humidity of the drying medium in the chamber is falling
relatively rapidly, the relative humidity of the drying medium is
controlled so that the relative humidity of the drying medium in
the chamber is not more than 70% relative humidity lower than the
equilibrium relative humidity of the product, for minimising the
damage to the cellular and structural integrity of the product.
Preferably, during the period while the relative humidity of the
drying medium in the chamber is falling relatively rapidly, the
relative humidity of the drying medium is controlled so that the
relative humidity of the drying medium in the chamber is not more
than 60% relative humidity lower than the equilibrium relative
humidity of the product, and advantageously, is not more than 50%
relative humidity lower than the equilibrium relative humidity of
the product.
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
18
Ideally, the drying medium is recirculated, and preferably, the
relative humidity of the drying medium in the chamber is
controlled by the introduction of fresh drying medium into the
recirculating drying medium, and the rate at which the fresh
drying medium is introduced does not exceed 21~ by weight of the
mass flow rate of the drying medium.
In one embodiment of the invention the rate at which the fresh
drying medium is introduced does not exceed 15% by weight of the
mass flow rate of the drying medium.
In another embodiment of the invention the rate at which the fresh
drying medium is introduced does not exceed 10% by weight of the
mass flow rate of the drying medium.
In a further embodiment of the invention the rate at which the
fresh drying medium is introduced does not exceed 7~ by weight of
the mass flow rate of the drying medium.
In a still further embodiment of the invention the rate at which
the fresh drying medium is introduced does not exceed 4% by weight
of the mass flow rate of the drying medium.
In another embodiment of the invention fresh drying medium is
introduced at a substantially constant rate of not more than 7% by
weight of the mass flow rate of the drying medium during the
period while the relative humidity of the drying medium in the
chamber is being maintained substantially constant at the maximum
value of relative humidity.
In another embodiment of the invention fresh drying medium is
introduced at a substantially constant rate of not more than 5% by
weight of the mass flow rate of the drying medium during the
period while the relative humidity of the drying medium in the
chamber is being maintained substantially constant at the maximum
value of relative humidity.
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCI~/IE96/00037
19
In a further embodiment of the invention fresh drying medium is
introduced at a substantially constant rate of not more than 4% by
weight of the mass flow rate of the drying medium during the
period while the relative humidity of the drying medium in the
chamber is being maintained substantially constant at the maximum
value of relative humidity.
In a still further embodiment of the invention fresh drying medium
is introduced at a substantially constant rate of not more than 3%
by weight of the mass flow rate of the drying medium during the
period while the relative humidity of the drying medium in the
chamber is being maintained substantially constant at the maximum
value of relative humidity.
In another embodiment of the invention fresh drying medium is
introduced at a substantially constant rate of not more than 2% by
weight of the mass flow rate of the drying medium during the
period while the relative humidity of the drying medium in the
chamber is being maintained substantially constant at the maximum
value of relative humidity.
In a further embodiment of the invention fresh drying medium is
introduced at a substantially constant rate of not more than 1% by
weight of the mass flow rate of the drying medium during the
period while the relative humidity of the drying medium in the
chamber is being maintained substantially constant at the maximum
value of relative humidity.
In another embodiment of the invention during the period while the
relative humidity of the drying medium in the chamber is falling
relatively rapidly, fresh drying medium is introduced at a rate of
not more than 21% by weight of the mass flow rate of the drying
medium.
In general, fresh drying medium is introduced is increased from
the beginning of the period to the end of the period during which
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCTIIE96/00037
the relative humidity of the drying medium in the chamber is
falling relatively rapidly.
In practice, no fresh drying medium is introduced into the
recirculating drying medium until the relative humidity of the
drying medium has reached its maximum value.
In another embodiment of the invention during the period while the
relative humidity of the drying medium in the chamber is
asymptotically approaching the predetermined value of relative
humidity fresh drying medium is introduced at a rate of not more
than 5% by weight of the mass flow rate of the drying medium.
In a further embodiment of the invention fresh drying medium is
introduced through an inlet opening and exhaust drying medium is
exhausted through an outlet opening, the size of the inlet and
outlet openings being controlled as a function of the nominal
exposed surface area of the product in accordance with a
Modulation Index (MI) which is defined as:
MI = Kp x NSP (Sen ~ Sex)/(sen x Sex)
where Sen is the cross-sectional area of the inlet opening
for make-up fresh drying medium,
Sex iS the cross-sectional area of the outlet opening
for exhaust drying medium, and
NSP is the nominal exposed surface area of the
product, and
Kp is a constant whose value depends on the product
being dried and the pressure/flow characteristics of the drying
medium, and
during the period while the relative humidity of the drying medium
in the chamber is being maintained substantially constant at the
maximum value, the value of the Modulation Index lies in the range
of 1,000 to 10,000.
Preferably, during the period while the relative humidity of the
drying medium in the chamber is being maintained substantially
SUBST~TUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
21
constant at the maximum value, the value of the Modulation Index
lies in the range of 2,000 to 8,000.
Preferably, the temperature of the drying medium i5 controlled not
to rise to or above a degradation temperature which would cause
5 irreversible thermal damage to the product.
In general, the temperature of the drying medium does not exceed
70~C.
Typically, the drying medium is maintained at a temperature within
the range 40~C to 70~C, in general the drying medium is maintained
at a temperature within the range 55~C to 65~C.
In one embodiment of the invention on reaching its maximum, the
temperature of the drying medium is maintained substantially
constant thereafter.
The drying medium may be any suitable medium, h :aver, a typical
15 drying medium is air, or for example, the drying medium may be
nitrogen, and in some cases, the drying medium may be nitrogen-
enriched air.
By recirculating the drying medium, the process according to the
invention attains a relatively high degree of energy efficiency.
20 However, the energy efficiency of the process according to the
invention also results from the enhanced rate of water removal
from the product which is made possible by virtue of the fact that
the channels and passageways adjacent the faces of the product
remain undamaged during the drying process, thus, facilitating
25 efficient water removal from the interior of the product.
Accordingly, suitable control of the temperature and relative
humidity of the drying medium results not only in superior product
quality, but also in reduced drying time and energy requirements.
By controlling the temperature and relative humidity of the drying
medium in the chamber to provide a sufficiently high water vapour
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96139854 PCT/IE96/00037
density in the chamber effectively regulates the rate at which
water is liberated from the product by limiting the numerous
gradients in temperature and humidity which occur in the removal
of moisture from a hygroscopic substance. Proper regulation of
these gradients serves to maintain structural integrity and in the
case of biological material with cellular structure p(events the
formation of an impermeable surface layer which has an adverse
effect both on the dried product and also on the removal of water
from the interior of the product during the latter stages of the
drying process.
In addition, the invention provides a process for preparing a
dried product having the following characteristics,
a water content between 2% and 20%, and preferably between 2%
and 10%, and advantageously less than the level corresponding to
the water activity threshold at which deterioratlon of the dried
product commences, but not less than the lower llmit for monolayer
water content,
substantially all the cells not adjacent to an exposed face of
the product still have a relatively high degree of structural
integrity as demonstrated by a substantially undamaged cell
membrane, and
the product has a water activity lower than 0.7, preferably the
water activity is lower than 0.4, and ideally is between 0.3 and
0.4, most preferably at a value which minimizes the effects of
deteriorative mechanisms.
In general, cells of the product are not ruptured by the drying
process according to the invention. In particular, the cell
membranes of the product, in general, are not damaged. As a
result of this many organoleptic compounds and nutritional
elements situated in or inside the cell membrane are retained. It
seems even that many of the product of the invention have membrane
which is in such a state that organoleptic compounds are more
perceptible than in the fresh state, that is can be more easily
liberated in the mouth.
SUBSTITllTE SHEET (RULE 26
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
The dried product of the invention retains many of the
organoleptic compounds of the product as they were prior to
drying, including those comprising the bouquet and taste, and
advantageously contains a substantial part of the flavour
5 compounds in the lower boiling point fractions, typically lower
than 40~C, contained in the product as they were prior to drying.
According to an embodiment of the product of the invention, said
product containing compounds which are able to be eluted at
different temperatures, the weight ratio fraction of compounds
eluted at a temperature between 150~C and 200~C to fraction of
compounds eluted at a temperature between 40 and 100~C is greater
than 0.3, preferably greater than 0.5, for exa~ple between 0.6 and
2. The weight ratio fraction of compounds eluted at a temperature
between 150~C and 200~C to fraction of compounds eluted up to
200~C is greater than 0.25, preferably greater t~an 0.3, for
example 0.35-0.4.
Advantageously, a substantial proportion of the spaces or channels
between cells adjacent to a face of the product are not filled
with sugar and remain open. Preferably, a substantial p~opor~ion
20 of the spaces or channels contain a gaseous medium, such as air,
N2, mixtures of N2 + ~2' oxygen-enriched air, water vapour, or some
other. The gentle nature of the drying process of this invention
minimizes cell wall degradation whereby both sugars and flavour
constituents remain within the cells, rather than residing in
25 spaces between the cells.
The dried product of the invention may be a fruit or vegetable
food product such as pineapple, banana, papaya, mango, other
tropical fruits, watermelon, carrots, cabbage, celery, peppers,
spinach, beans, plums, apples, pears, mushrooms, grapes, oranges,
lemons, limes, and other fruits and vegetables, but can also be
herbs, spices, tobacco, blood, sperm, bacteria, meat, seafood, tea
leaves, seaweed, algae, coffee beans, cocoa beans, nuts, eggs and
other food and other biological products.
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
24
The invention relates also to a process for dehydrating other
products and substances, for example, products not containing
cells, such as chemical compounds, pharmaceutical compounds or
preparations, as well as non-biological products and substances.
In an alternative process according to the ;nvention a gaseous
drying medium is heated and urged into contact the product being
dried, the temperature of the drying medium contacting the product
to be dehydrated being lower than the temperature of degradation
of the product, the temperature of the product to be dehydrated
being increased in a controlled manner during the drying process,
the process comprising at least one step in which the temperature
of the drying medium is increased from a first temperature up to a
temperature which may be adjacent the maximum dehydration
temperature, but lower than the degradation temperature. In one
aspect of the invention the said increase of temperature to the
drying medium is such that during said step a difference of
temperature exists between the product and the drying medium, said
difference being between 0.1~C and 5~C, may be lower than 2.5~C,
and may be lower than 1.5~C.
20 In another aspect of the invention, the temperature of the drying
medium may be increased at a rate lower than 5~C/hour.
Before this step, the product may be submitted to another
treatment, such as a treatment which does not damage cells of a
product prepared according to the invention. For example, the
25 drying medium may first be maintained at a temperature of about
40~C up to the moment a temperature of about 40~C is reached
within the product, while thereafter the drying medium may be
heated at such a rate that the difference of temperature existing
between the product and the drying medium is less than about 5~C,
preferably less than 2.5~C. In general, a steady and continuous
increase in temperature of the drying medium minimizes damage to
the cell membranes so that a high percentage thereof remain intact
while the moisture within the cells migrates therethrough and is
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCl'/IE96/00037
removed by the drying medium. The gentle drying regime in which a
controlled temperature difference between the drying medium and
the product is maintained as mentioned above, results in a process
in which colour and flavour constituents are not removed, but
s water is removed from the cells.
According to an alternative embodiment of the process according to
the invention, when the water content of the product to be
dehydrated is lowered to 10%, the drying medium is heated at such
a rate that the difference of temperature existing between the
product and the drying medium is less than about 2.5~C. In this
case, the product is pre-dried at a temperature of 40~C to 50~C
until the product reaches a water content lower than 20%,
preferably lower than 10%.
In one alternative embodiment of the invention the rate of
increase in the temperature of the drying medium is such that the
temperature of the drying medium reaches the maximum dehydration
temperature approximately at the same time as the desired water
content of the product has been attained.
In another alternative embodiment of the invention the rate of
20 increase of the temperature of the drying medium is substantially
constant.
Ideally, the maximum dehydration temperature is less than the
temperature of degradation of the product.
In general, the maximum dehydration temperature does not exceed
2S 70~C. Typically, the maximum dehydration temperature lies in the
range 40~C to 50~C.
In one aspect of the invention the product is dried to a water
content lower than 20%.
In another aspect of the invention the product is dried to a water
SUBSnTUTESHEET(RULE26)
CA 022221~3 1997-11-2~
WO 96t39854 PCT/IE96/00037
content of about 10%.
Preferably, the drying medium is recycled. Preferably, during the
end of the dehydration process less than 4% by volume of fresh
drying medium is added to the drying medium. Advantageously,
towards the end of the dehydration process not more than 1% of the
humidity of the recirculated drying medium is condensed from the
drying medium as it is being recirculated.
In another embodiment of the invention the product is dried to a
water content below about 10%, and the relative humidity of the
drying medium during the period while the water content of the
product is being reduced below about 20% lies in the range between
10% and 20%.
In a further embodiment of the invention the product is dried to a
water content of less than 7~.
Further, the invention provides a dried product dried according to
the process according to the invention.
Brief Description of the Drawinas
The invention will be more clearly unde~stood from the following
descriptions of some non-limiting examples and embodiments thereof
which are given by way of example only with reference to the
accompanying drawings in which:
Fig. 1 is a schematic view of an apparatus suitable for
carrying out a dehydrating process according to the invention,
Figs. 2 to 5 are enlarged views of parts near the skin and
parts in the interior of apple slices before drying and after
drying, respectively using the process according to the
invention and known processes,
Fig. 6 shows plots of the relative humidity and the temperature
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96t39854 PCl'/lE96tO0037
of the drying medium, and the equilibrium relative humidity of
product against time during an example of the dehydrating
process according to the invention,
Fig. 7 shows plots of the relative humidity and the temperature
of the drying medium against time during another example of the
dehydrating process also according to the invention, and
Figs. 8 to 60 show gas chromatograph traces of products before
being dried and after being dried using the dehydrating process
according to the invention, and also show gas chromatograph
traces of all but one of the products after having been
rehydrated subsequent to being dried using the dehydrating
process according to the invention and other dehydrating
processes.
Detailed DescriPtion of the Invention
Before describing examples of the dehydration process according to
the invention, a drying apparatus which is suitable for carrying
out the dehydration process will first be described with reference
to Fig. 1. It will of course be appreciated that the dehydration
process is not limited to being carried out in the apparatus of
20 Fig. 1, and many other different types of apparatus may be used.
The drying apparatus of Fig. 1 comprises an enclosed insulated
drying chamber 1 having an access opening 2 which is closed by a
door 3 for accommodating trays 4 on which products to be
dehydrated are placed into the chamber 1. An inlet opening 5 is
25 provided to the chamber 1 for introducing gaseous drying medium to
the chamber 1, and an outlet opening 6 provides for removal of the
drying medium from the chamber 1. A duct 7 extends from the
outlet 6 to the inlet 5 for recirculating drying medium back to
the chamber 1. A fan 8 in the duct 7 circulates drying medium
through the duct 7 in the direction of the arrow A, and in turn
through the drying chamber 1 also in the direction of the arrow A.
A heater 9 located in the duct 7 between the fan 8 and the inlet 5
heats the drying medium as it is being returned to the drying
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCI~/IE96/00037
chamber 1. A condenser 10 which may be operated for condensing
some of the water out of the drying medium is located in the duct
7 between the fan 8 and the heater 9.
An inlet valve 11 for introducing fresh drying medium into the
duct 7 is located upstream of the fan 8. The inlet valve 11 may
communicate directly with ambient air for introducing fresh air
into the duct 7, or alternatively may introduce fresh drying
medium from a drying medium source 12, for example, nitrogen
contained in a gas bottle or other suitable container. An outlet
valve 14 for exhausting part of the recirculated drying medium
from the duct 7 is located between the condenser 10 and the heater
9.
A control means, namely, a central programmable logic controller
15 which is computer controlled controls the apparatus in response
to signals received from sensors which are described below and
which are located at appropriate locations in the apparatus. A
control link 16 activates and deactivates the fan 8 under the
control of the central controller 15. The inlet valve 11 and the
outlet valve 14 are controlled by the central controller 15
through control links 17 and 18, respectively. Control links 19
and 20 control the heater 9 and the condenser 10, respectively.
A temperature sensor 22 located at the inlet 5 to the drying
chamber 1 monitors the temperature of the drying medium being
introduced to the drying chamber 1. Signals from the temperature
sensor 22 are relayed to the controller 15 through a link 23. A
humidity sensor 25 is located in the drying chamber 1, and in this
case is provided by a psychometric device for monitoring the
relative humidity of the drying medium in the drying chamber 1.
The humidity sensor 25 is located in the drying chamber 1 away
from the outlet 6 but towards the outlet 6. A link 26 relays
signals from the humidity sensor 25 to the controller 15. A
humidity sensor 27 is located in the duct 7 downstream of but
adjacent the condenser 10 for monitoring the relative humidity of
SIJBST~TUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96139854 PCT/IE96/00037
the drying medium as it exists the condenser 10. The humidity
sensor 27 is also provided by a psychometric device. A link 28
relays signals from the humidity sensor 27 to the controller 15.
A product water loss sensor 30 which is provided by a load cell
device is located in the drying chamber 1 for monitoring the total
weight of the product being dried for determining the cut(ent
water content of the product as the dehydration process proceeds.
In the examples described below the water loss sensor 30 was not
used, rather as will be discussed below, the equilibrium relative
humidity of the product was monitored, since water content is a
function of equilibrium relative humidity. The product water loss
sensor 30 is located in the drying chamber 1 so that the trays 4
with the product thereon are supported on the product water loss
sensor 30. A link 31 relays signals from the product water loss
sensor 30 to the controller 15. During the dehydration process
the weight of the product on the trays 4 changes, whereby the loss
of water from the product can be estimated. As the dry matter
content of the product can be estimated by heating a sample of the
product at a temperature of about 100~C in a laboratory test for a
time specified in a recognised test procedure, the water content
of the product can be determined from the weight of the product
which is monitored by the water loss sensor 30 as the drying
process proceeds.
Accordingly, in response to signals received from the temperature
sensor 22, the humidity sensors 25 and 27 and the product water
loss sensor 30 the controller 15 controls the operation of the fan
8, the heater 9 and the inlet and outlet valves 11 and 14,
respectively. Should it be desired to operate the condenser 10,
the condenser 10 is also controlled by the controller 15 in
response to the signals received from these sensors.
In controlling the inlet and outlet valves, 11 and 14,
respectively, the cross-sectional area of the opening presented to
the fresh drying medium and the exhausted drying medium is
SUBSnTUTESHEET(RULE2B~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
controlled. The cross-sectional area is the area of the opening
presented to the medium at right angles to the flow of medium.
The cross-sectional area of the opening defined by the inlet valve
11 is hereinafter represented by Sen, and the cross-sectional area
of the opening defined by the outlet valve 14 is hereinafter
represented by Sex. In certain cases, the inlet and outlet valves
11 and 14l respectively, may remain closed during all or part of
the process. The combination of the pressure drop across the fan
8 together with minute holes and cracks which in generall would
naturally occur in the duct 7 and the drying chamber 1 may provide
sufficient exchange of drying medium, namely, drying air with
fresh air for efficient operation of the dehydration process in
the drying apparatus of Fig. 1, without the need to open the inlet
and outlet valves 11 and 14, respectively. The significant
ability of air to carry water vapour at elevated temperatures also
accounts for the ability to operate the apparatus to carry out a
dehydration process according to the invention with little or no
fresh air added, and little egress of moisture laden drying air
from the apparatus.
Examples of the dehydration process according to the invention for
drying fruit and vegetable products will now be described using
the apparatus of Fig. 1. However, it will be readily apparent to
those skilled in the art that the dehydration processes may be
carried out on any other suitable apparatus. In the examples of
the dehydration process, unless otherwise statedl the drying
medium is air. The dehydration processes may be carried out at
pressures lower or higher than atmospheric pressurel howeverl it
is preferable that the dehydration processes are carried out at
substantially atmospheric pressurel or at a pressure just above
atmospheric pressurel for examplel up to 1.2 x 105 Pa. In the
examples of the dehydration processes described hereinl the
pressure of the drying medium in the drying chamber 1 is
maintained slightly above atmospheric pressure.
In a preferred dehydration process according to the invention the
SUBSTIME SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96/00037
31
process proceeds sequentially through four phases, namely,
Phase 1 - a rising temperature phase,
Phase 2 - a high humidity phase,
Phase 3 - a declining humidity phase, and
- 5 Phase 4 - an asymptotic phase.
There is no sharp cut off point between the respective phases,
rather, the phases merge gradually from one phase into the next.
For ease of understanding this dehydration process according to
the invention, the four phases will now be described under
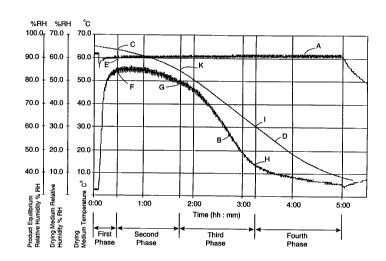
separate headings, and with reference to Fig. 6. Fig. 6
illustrates the parameters, namely, temperature and relative
humidity, of the drying medium which were monitored during a
dehydration process according to the invention in which IDARED
apples were dehydrated. Fig. 6 also illustrates the equilibrium
relative humidity of the apples during the dehydration process.
Graph A is a plot of the temperature of the drying medium against
time. Graph B is a plot of the relative humidity of the drying
medium against time. Graph D is a plot of the equilibrium
relative humidity of the apples against time. Graph A was plotted
from the signals received from the temperature sensor 22 in the
drying chamber 1. The relative humidity of the drying medium
illustrated in Graph B was obtained from the humidity sensor 25 in
the drying chamber. The equilibrium relative humidity of the
apples illustrated in Graph D was determined at half-hourly
intervals during the drying process. At each half-hourly interval
a sample of the apples was removed and its equilibrium relative
humidity was determined. The IDARED apples were sliced into
slices of approximately 3 mm thickness and loaded into the drying
chamber 1 on the two trays 4 as illustrated in Fig. 1.
Phase 1 - Risinq temPerature Phase
In Phase 1 the drying medium is circulated by the fan 8, and
exchange of drying medium with the surrounding atmosphere is
minimised. In other words, the inlet and outlet valves 11 and 14
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
are closed, and the only exchange of drying medium with the
surrounding atmosphere takes place through cracks, and
imperfections in the sealing of the duct 7 and the drying chamber
1. Heat is applied to the drying medium for raising the
temperature of the drying medium to a first set point, which
although below, is relatively close to the maximum dehydration
temperature at which the dehydration process is carried out. The
maximum dehydration temperature should never exceed, and
preferably, should be less than the degradation temperature of the
product being dried. Phase 1 essentially commences when the
product to be dried has been placed in the drying chamber 1 and
the door 3 has been closed. The temperature difference between
the drying med;um and the product should not result in damage to
the structural integrity of the product as a result of excessive
thermal stress. Typically, the product is introduced into the
drying chamber 1 when the temperature of the drying medium is
approximately 40~C. Phase 1 continues until the temperature of
the drying medium reaches the first set point, and the relative
humidity of the drying medium reaches a first set point which will
be the maximum value of relative humidity to which the drying
medium is allowed to rise. The first set points of the
temperature and the relative humidity of the drying medium depend
on the product being dried. The first set point temperature may
range from 50~C to 70~C, and the first set point of the relative
humidity of the drying medium of the drying medium may range from
30% to 70%.
In the example illustrated in Fig. 6, the first temperature set
point which is indicated by the letter E is approximately 60~C and
the first relative humidity set point which is indicated by the
letter F is approximately 55%. Thus, on the temperature of the
drying medium and the relative humidity of the drying medium
reaching 60~C and 55% respectively the first phase ends, and the
process moves into the second phase which will be described below.
During the first phase when the temperature of the drying medium
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
reaches the first set point, the heater 9 is controlled for
maintaining the temperature of the drying medium substantially at
the first set point. Additionally, during the first phase the
relative humidity of the drying medium may fluctuate, ha:~veY, it
is not permitted to exceed the first set point, and if necessary
this may be achieved by providing sufficient exchange of drying
medium with the atmosphere through the inlet and outlet valves 11
and 14, respectively. On the temperature and relative humidity of
the drying medium in the drying chamber 1 reaching the respective
first set points, the heater 9 and the inlet and outlet valves 11
and 14 are adjusted for maintaining the temperature and the
relative humidity of the drying medium substantially at the first
set points as the process moves into the second ph~se. The value
of the equilibrium relative humidity of the p(od~c~ at the end of
the first phase is indicated by the letter C on the Graph D of
Fig. 6, which is approximately 93%.
As discussed above the pressure of the drying mediu~ in the drying
chamber 1 is maintained at a pressure slightly above atmospheric
pressure, and should there be any tendency towards a build-up of
over-pressure of drying medium in the drying chamber 1 co((eclion
is made through the use of a pressure release valve (not shown).
The first phase of the drying process of the example illustrated
in Fig. 6 took approximately 30 minutes.
Phase 2 - Hiah humiditv Dhase
During the second phase, in general, the flow rate of the drying
medium is maintained constant and similar to the flow rate during
the first phase so that the speed of the drying medium over the
exposed surface of the product is maintained at a similar speed to
that during the first phase. The temperature and relative
humidity of the drying medium are maintained substantially
constant at the respective first set points. This is achieved by
maintaining the setting of the heater 9 and the inlet and outlet
valves 11 and 14, respectively, at their respective settings to
SUBSTITUTE SHEET (RULE ?~
CA 022221~3 1997-11-2~
WO 96139854 PCTIIE96/00037
which they were set at the end of the first phase. The second
phase is completed when there is insufficient moisture available
in the product to maintain the relative humidity of the drying
medium substantially constant without altering the flow rate of
the drying medium or the rate of exchange of drying medium with
the atmosphere. The end of the second phase is determined as
being the point in the process at which the relative humidity of
the drying medium commences to decrease rapidly, or if the
relative humidity of the drying medium has co ~ ?nced to decrease,
the end of the second phase is the point at which the rate at
which the relative humidity of the drying medium is decreasing
commences to significantly increase. In Graph B of Fig. 6 the end
of the second phase is indicated by the point G. At this stage,
the second phase is complete.
In general, the product being dried will contain a quantity of
free water, and the end of the second phase, in general, is
determined when this free water has been exhausted. In general,
it is at this stage that the evaporation rate of water from the
product falls below the rate of vapour removal which had been
substantially maintained during the second phase. The value of
the equilibrium relative humidity of the product at the end of the
second phase is indicated by the point K on the Graph D of Fig. 6.
During the second phase, the relative humidity of the drying
medium may not remain entirely constant. However, as can be seen
in Fig. 6 the relative humidity during the second phase remains
substantially constant for the initial part of the second phase
and gradually begins to decrease during the latter part of the
second phase. However, in such cases, as discussed above, the end
of the second phase is determined when the rate of decrease of
relative humidity of the drying medium commences to increase
significantly.
If desired, a number of set points of both temperature and
relative humidity may be set during the second phase, and when
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
there is insufficient moisture available in the product to
maintain the relative humidity of the drying medium at the last of
the set points, that is determined as being the point of
completion of the second phase.
As discussed above the relative humidity of the drying medium is
maintained substantially constant at about the maximum value of
relative humidity during the second phase, namely, the high
humidity phase. This is achieved by setting the inlet and outlet
valves 11 and 14 for controlling the exchange of drying medium
with the atmosphere at the end of the first phase. It has been
found that a relationship exists between the area through which
drying medium is exchanged with the atmosphere and the nominal
exposed surface area of the product which is exposed to the drying
medium in the drying chamber for maintaining the relative humidity
substantially constant during the second phase. This relationship
may be expressed in terms of a Modulation Index MI. The
Modulation Index is equal to a constant multiplied by the nominal
surface area of the product divided by the area S.
In the present case, assuming no leakages in the duct 7 and the
drying chamber 1 the area S through which drying medium is
exchanged with the atmosphere is equal to (Sen x SeX) / (Sen + Sex)
The Modulation Index can thus be expressed by the following
formula:
MI = Kp x NSP/S
= Kp x NSP (S~n + Sex)/sen x Sex
where
Kp is a constant whose value depends on the particular type of
product being dried and the pressure/flow characteristics of the
drying medium, and of the fan 8,
NSP is the nominal surface area of product exposed to the
drying medium.
The value of the Modulation Index during the second phase of the
dehydration process is preferably maintained within the range
SUBSTITUTE SHEET (RULE 2~\
CA 022221~3 1997-11-2~
WO 96/398!;4 PCT/lE96tO0037
1,000 < MI< 10,000
and more preferably is maintained within the range
2,000 < MI < 8,000
The purpose of establishing control over the evaporation rate is
to achieve the most rapid possible removal of water consistent
with maintaining structural integrity, and achieving optimum
retention of the initial structural p~open~ies of the product
being dried.
Instead of or as well as controlling the relative humidity of the
drying medium being returned to the drying chamber 1, by
controlling the exchange of drying medium with fresh drying
medium, the relative humidity of the drying medium may be
controlled by the condenser lO.
In the example of Fig. 6 the second phase took approximately
seventy-five minutes.
Phase 3 - Declininq humiditv Dhase
The third phase of the dehydration process commences at the end of
the second phase, in other words, when the rate at which the
relative humidity of the drying medium within the drying chamber
commences to decrease significantly, while both the temperature of
the drying medium in the drying chamber and the rate of drying
medium exchange are held substantially constant. In the third
phase the relative humidity of the drying medium in the drying
chamber is allowed to continue to decrease. I'~w ve~, the rate at
which the relative humidity is allowed to decrease is controlled
so that the rate of evaporation of water from the product does not
exceed an evaporation rate which would result in damage to the
structural integrity of the product. The rate at which the
relative humidity of the drying medium is allowed to decrease
during the third phase is controlled by regulating the exchange of
drying medium with atmosphere by the inlet and outlet valves 11
and 14, respectively. Concurrently, in some cases the temperature
SUBSTITUTE SHEET (RUBE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96/00037
of the drying medium may be varied during the third phase,
provided it does not exceed the degradation temperature of the
product being dried.
During the third phase the evaporation rate of moisture from the
product is preferably modulated in order to maintain the gradients
in temperature and relative humidity between the drying medium and
the product within limits that will maximise the rate of moisture
evaporation from the product, while at the same time avoiding
damage to the structural integrity of the product. These limits
vary from species to species, and also vary depending on the
method of preparation of the product for drying, and the final
desired characteristics of the dried product. ~ ~veY, the limits
are identifiable for each species. In general, the rate of
introduction of fresh drying medium to the recirculating drying
medium is increased as the third phase progresses.
It is important that the temperature of the drying medium should
be closely monitored during the third phase, since the evaporative
cooling effect decreases as the dehydration process continues. In
order to prEve"L undesired temperature rises during the third
phase, the heat provided to the drying medium, in general, is
reduced, and the circulating rate of the drying medium may be
reduced.
The third phase continues until the rate of water evaporation from
the product decreases virtually irrespective of the rate of drying
medium exchange with fresh drying medium. In the example of Fig.
6 the end of the third phase is identified by the point H on the
Graph B of relative humidity. The point I on the Graph D
indicates the equilibrium relative humidity of the product at the
end of the third phase.
The third phase of the example of Fig. 6 took approximately one
and a half hours.
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-25
WO 96~9854 PCTnE96/00037
38
Phase 4 - AsYmPtotic Phase
The fourth phase begins at the end of the third phase. During
the fourth phase the relative humidity of the drying medium is
allowed to asymptotically approach a predetermined value which is
below the equilibrium relative humidity of the product which
corresponds to the desired final water content of the product.
Typically, the difference between the value of predetermined
relative humidity of the drying medium and the final equilibrium
relative humidity of the product at the process temperature is in
the order of 30% relative humidity, although this value will vary
from product to product, and will also vary depending on the final
water content to which the product is to be dried. The
equilibrium relative humidity of the product during the fourth
phase is increasingly dictated by the temperature of the drying
medium within the drying chamber, and the monolayer water content
of the product, and is increasingly independtnL of the rate of
exchange of drying medium with atmosphere. The end of the fourth
phase occurs when the equilibrium relative humidity of the product
reaches the equilibrium relative humidity which corresponds to the
desired final value of water content of the product, provided the
value of water content of the product is uniform throughout the
product.
The fourth phase of the example of Fig. 6 took approximately one
and three quarter hours minutes.
There is a minimum water content which may be attained for
products during the fourth phase, the level of which depends on
factors which include the monolayer water content of the product
being dried, the relative humidity of the drying medium entering
the drying chamber 1, and the temperature of the drying medium
within the drying chamber 1.
Even though the process of the invention may yield a lower rate of
moisture removal from the product in the initial portion of the
dehydration cycle, the total drying time to reach a stated final
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
39
moisture content is in most cases significantly shorter than for
conventional forced flow warm air drying processes. It has been
found that the drying process of the invention may be completed in
a time period which may be as short as one tenth of the time it
S takes to dry product by freeze-drying processes.
Dehydration processes according to the invention whereby the
process comprises the above four phases have been carried out
using the apparatus of Fig. 1. In all cases two trays 4 carrying
respective loads of the product were introduced into the drying
chamber 1 through the access opening 2 and the door 3 was securely
closed. The fan 8 was activated to circulate the drying medium
through the drying chamber 1 at a mass flow rate sufficient to
urge the drying medium over the exposed surface of the product at
a speed of approximately 2 M per second. This speed was
maintained during all four phases of the drying processes. The
inlet valve 11 and the outlet valve 14 were closed to confine the
drying medium within the duct 7 and the drying chamber 1. The
heater 7 was activated to raise the temperature of the drying
medium to the first set point temperature. The relative humidity
20 of the drying medium in the drying chamber 1 was allowed to rise
to the first relative humidity set point. At this stage the first
phase was completed, and the second phase commenced. Immediately
upon commencement of the second phase the inlet valve 11 and
outlet valve 14 were operated so as to maintain the relative
25 humidity of the drying medium in the drying chamber 1
substantially constant at the first set point, and the heater was
controlled to maintain the temperature of the drying medium in the
drying chamber substantially constant for the duration of the
second phase. The settings of the valves 11 and 14 were
determined initially using the Modulation Index formula. Once the
inlet and outlet valves 11 and 14, respectively were set at the
beginning of the second phase, no further adjustment was
necessary, and the relative humidity of the drying medium in the
drying chamber tended to remain substantially constant.
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
The process moved from the second to the third phase when the
relative humidity of the drying medium in the drying chamber 1
commenced to decrease, or commenced to decrease more rapidly than
it had been decreasing during the latter part of the second phase.
During the third phase the inlet and outlet valves 11 and 14 were
controlled for maintaining the relative humidity gradient between
the drying medium in the drying chamber 1 and that of the product
such that the difference between the relative humidity of the
drying medium and the product did not exceed a value which would
result in damage to the structural integrity of the product as
already described. This was achieved by continuously varying the
settings of the inlet and outlet valves 11 and 14 for increasing
the rate at which fresh drying medium is added to the
recirculating drying medium.
During the fourth phase as the relative humidity of the drying
medium in the drying chamber commenced to asymptotically approach
the predetermined value of relative humidity for the drying
medium, the inlet and outlet valves 11 and 14, and the heater 9
were controlled and set for maintaining the rate at which fresh
20 drying medium was added to the recirculating drying medium and
heat was added to the drying medium, for maintaining the relative
humidity of the drying medium substantially at the predetermined
relative humidity value. The relative humidity of the drying
medium was maintained at the predetermined relative humidity value
25 for a sufficient period until the equilibrium relative humidity of
the product being dried was at a value which corresponded with the
desired final water content of the product.
Returning now to Fig. 6 which illustrates the parameters of the
drying medium during the four phase drying process in which apple
slices were dried to a final water content of approximately 5.5%
by weight. The water activity of the dried apples was 0.272. The
apples which were dried in the process illustrated in Fig. 6 were
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCI'IIE96/00037
of the IDARED variety, and had been removed from cold storage in
which they had been for approximately six months. The apples were
sliced into slices of approximately 3 mm thickness and were loaded
into the drying chamber on the two trays 4. The total nominal
surface area of the product exposed to the drying medium was 19 M2.
The loads of product on the two trays were arranged so that the
length of the loads in the direction of air flow through the
drying chamber 1, namely, in the direction of the arrow A was as
short as possible for minimising the relat;ve humidity gradient of
the drying medium across the product. The drying medium was
circulated through the drying chamber 1 at a mass flow rate
sufficient to cause the drying medium to pass over the exposed
surface area of the product at a speed of approximately 2 M per
second.
A more detailed discussion of the dried apple slices is given
below with reference to Table 1. Briefly, the tempe~atu(c of the
drying medium during the first phase of the drying process was
allowed to rise to a first temperature set point of approximately
60~C. The relative humidity of the drying medium was allowed to
rise to a first set point of approximately 55%. At the end of the
first phase the equilibrium relative humidity of the product was
approximately 93%. During the second phase of the process, the
temperature was maintained substantially constant at-the first set
point, and for the majority of the second phase the relative
humidity of the drying medium was maintained substantially
constant approximately 55%. At the end of the second phase the
relative humidity of the drying medium had fallen to approximately
50%, and the equilibrium relative humidity of the apples was
approximately 84%. During the second phase, the drying medium was
exchanged with the atmosphere and the rate at which fresh air was
added to the drying medium was approximately 2% of the mass flow
rate of the drying medium. During the second phase, the
difference between the relative humidity of the drying medium and
the equilibrium relative humidity of the product did not exceed
35% relative humidity.
SUBSTITUTE SHEET (RULE 26
CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96/00037
During the third phase of the drying process, the relative
humidity of the drying medium was allowed to drop at a rate such
that the difference between the relative humidity of the drying
medium and the equilibrium relative humidity of the apples did not
s exceed 47% relative humidity. In order to achieve this the rate
of exchange of drying medium with the atmosphere was gradually
increased during the third phase so that at the end of the third
phase fresh air was being added to the drying medium at the rate
of approximately 3.5% of the mass flow rate of drying medium.
During the third phase, the temperature of the drying medium
remained substantially constant at approximately the value of the
first set point, namely, approximately 60~C, and rose gradually
towards the end of the third phase to a temperature of
approximately 61~C. At the end of the third phase the relative
humidity of the drying medium was approximately 13%, and the
equilibrium relative humidity of the apple slices was
approximately 60%.
During the fourth phase the relative humidity of the drying medium
was allowed to drop, and to asymptotically approach a
predetermined value of 7% approximately. The relative humidity of
the drying medium was retained substantially at the predetermined
value of 7% for a period of approximately 40 minutes. This
finally yielded dried apple slices with an equilibrium relative
humidity value of approximately 38% which corresponds with the
desired final water content of approximately 5.5%, and a water
activity of approximately 0.272%. During the fourth phase, the
temperature remained substantially constant at approximately 61~C.
The fall in temperature in the Graph A at the end of the fourth
phase is as a result of the door 3 of the drying chamber 1 being
opened for the removal of the dried apples. At the end of the
fourth phase, the difference the relative humidity of the drying
medium and the equilibrium relative humidity of the product was
approximately 30% relative humidity.
Fig. 7 illustrates two graphs, namely, the Graph A of temperature,
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96139854 PCI~/IE96/00037
and the Graph B of relative humidity of the drying medium of
another example of the four phase dehydrating process according to
the invention, in which apple slices (Cultivar Jonagold) of 3 mm
thickness were dr;ed. Although the apple slices dried in this
embodiment of the invention are of a different variety to the
IDARED apples which were dried in the example of the process
according to the invention described with reference to Fig. 6, the
general cell structure and other structural characteristics of the
two types of apples are substantially similar. As can be seen
from Fig. 7 the time period for the drying process according to
this example of the invent;on was approximately 2~ hours. This
was significantly less than the approximately 5 hours taken by the
process described with reference to Fig. 6. The reason for the
shorter drying time in the process of Fig. 7 is largely accounted
for by the fact that the rate of exchange of drying medium with
fresh drying medium was approximately doubled throughout the
process. Another contributory factor to the reduced drying time
was the fact that the temperature of the drying medium was allowed
to rise gradually from 60~C to approximately 64~C during the
latter half of the third phase, and the fourth phase of the
process. Because of the increase in drying medium exchange with
fresh drying medium the relative humidity of the drying medium
only reached a maximum value of approximately 49%. Although the
maximum value of relative humidity, at 49% is less than the
maximum value of approximately 55% of the process of Fig. 6 the
lower value of maximum relative humidity was still sufficient to
avoid damage to the cellular and structural integrity of the
product, and also had the effect of accelerating the rate of water
removal from the product in both the second and third phases.
However, experiments which have been carried out indicate that any
significant reduction of the maximum value of relative humidity
much below 49% does not reduce the drying time of the process
further. In fact, the drying time at lower maximum values of
relative humidity may be either similar to that of Fig. 7, or may
increase. Most significantly at values of maximum relative
humidity much below 49%, damage to the cellular and structural
SUBSTITUTE SHEET (RULE ~\
CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96/00037
44
integrity of the product will become increasingly apparent. This,
it is believed is caused by excessive differences between the
relative humidity of the drying medium and the equilibrium
relative humidity of the product. Thus, in general, it has been
found advisable to carry out the processes according to the
invention with a maximum value of relative humidity of the drying
medium in the range of 50% to 60%, and preferably in the range of
so% to 55%.
In the example of the process described with reference to Fig. 7,
the equilibrium relative humidity of the apple slices was not
monitored. However, it is believed that the curve of the
equilibrium relative humidity of the product would substantially
follow the curve of the product equilibrium relative humidity of
Fig. 6, but modified appropriately to take account of the shorter
drying time.
Graphs of the drying medium temperature and relative humidity and
equilibrium relative humidity of the product during drying
processes on fruits and vegetables other than apples using the
four phase drying process according to the invention, although not
shown are substantially similar to those illustrated in Figs. 6
and 7. In general, the only differences are in the drying times,
and the maximum values of temperature and relative humidity, which
are dependent on the product being dried and on the treatment to
which the products are subjected to prior to drying, as well as
the final desired water content of the product. However, in
general, the maximum value of relative humidity of the drying
medium would lie within the range of 50% to 55%, and the initial
maximum value of the temperature of the drying medium, would, in
general, be in the range of 60~C to 70~C, and in the latter part
of the drying cycle, typically, towards the end of the third
phase, and during the fourth phase, the temperature may be allowed
to rise to a maximum of approximately 65~C.
In order to make a comparison between the product of the invention
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
and other dried product, which were dried by other known
techniques, the apple slices which were dried using the four phase
process described with reference to Fig. 6 were compared with
similar apple slices which had been dried by freeze-drying and
conventional forced air drying.
Table 1 sets out the results of the analysis which have been
carried out on the products dried by the three dehydration
processes as follows:
the water content of the apple product (W % by weight),
the dry content of the apple product (including flavouring
compound) (100 - W %),
the water activity (A),
ascorbic acid (vitam;n C) content % by weight,
the results of a visual inspection of the cell structure of the
product,
the fraction (F) of compounds eluted in a range of temperature
from 0 to 200~C,
the ratio (Rl) of compounds eluted between 0 to 40~C for an
amount of dried product corresponding to 1009 of dry matter, to
compounds eluted between 0 to 40~C for an amount of the initial
apple corresponding to 1009 of dry matter,
the ratio (R2) of compounds eluted between 100 and 150~C for an
amount of dried product corresponding to 100g of dry matter, to
compounds eluted between 100 to 150~C for an amount of the
initial apple corresponding to 100 of dry matter,
the ratio (R3) of compounds eluted between 150 and 200~C for an
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
46
amount of dried product corresponding to 100g of dry matter, to
compounds eluted between 150 and 200~C for an amount of the
initial apple corresponding to 100g of dry matter,
the ratio (R) of compounds other than water eluted between O to
200~C for an amount of dried product corresponding to 1009 of
dry matter, to compounds other than water eluted between O to
200~C for an amount of the initial apple corresponding to 1009
of dry matter, and
the ratio R3/Rl-
Table 1
IDARED APPLES FROM STORAGE
Fr-sh appl-s Inv ntion dri-d Freoz- dri d Alrdrying
product product not Inv ntion
Wat r contont 86 55 28 1a6
W%
(lo~w1% 15 ~45 972 81 4
Wator actlvity 1 0 0 272 Q191 O U1
ascorbic ac~d % 100 55 47 11
c lls not darna~-d not darnag-d darna~-d darn~-d
F40C(%) 13 48 18
F40-100 C ~%)940 539 03
F 100-150'C (%) 2 5 6 8 13 8
F150-200C(%) 21 345 141
F150-200~C/F4~100C 002 064 02
R, (%) 100 945 146
R2(%) lw 65ô 55
R3 (%) 100 388 66 7
R (%) 100 132 56
R3/R~ 1 4 1 46
It can be seen from Table 1 that the product dried in accordance
with the invention retains a higher level of ascorbic acid
(vitamin C) content and a higher content of low temperature eluted
compounds. These low temperature eluted compounds, in general,
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96139854 PCT/IE96/00037
are aromatic compounds. In addition as discussed below with
reference to Figs. 2 to 5, little or no cell damage was detected
in the product dried according to the invention, while cell damage
was clearly visible in the products which were freeze-dried and
forced air dried. The colour and texture of the product dried in
accordance with the dehydration process of the invention matched
more closely the colour and texture of the product prior to being
dried, than did the colour and texture of the products dried using
the other two drying processes.
Figs. 2 to 5 show on an enlarged scale the results of microscopic
analysis of the cells of the apple slices prior to drying and
after drying using the three drying processes discussed with
reference to Table 1. Figs. 2(a) and 2(b) illustrate portions of
an apple slice prior to being dried. Fig. 2(a) illustrates the
cellular structure at a skin area of the apple slice, while Fig.
2(b) illustrates the cellular structure of the flesh area of the
apple slice, in other words, an interior portion of the apple
slice. Figs. 3(a) and 3(b) illustrate portions of the apple slice
at the skin area and the flesh area, respectively, after drying by
the four phase drying process according to the invention. Figs.
3(c) and 3(d) illustrate portions of the skin area and flesh area,
respectively, of apple slices which had been dried using the four
phase process according to the invention and had subsequently been
rehydrated. Figs. 4(a) and 4(b) illustrate portions of the skin
area and flesh area, respectively, of apple slices which had been
freeze-dried. Figs. 4(c) and 4(d) illustrate portions of the skin
area and flesh area, respectively of apple slices, which had been
freeze-dried, and subsequently rehydrated. Figs. 5(a) and 5(b)
illustrate portions of the skin area and flesh area, respectively,
of apple slices which had been dried using the standard forced air
drying process. Figs. 5(c) and 5(d) illustrate portions of the
skin area and flesh area, respectively, of apple slices which had
been dried by the standard forced air drying process and
subsequently rehydrated. All the microscopic analysis on the skin
area of the apple slices were carried out at a magnification
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
48
factor of 280X. All the microscopic analysis on the flesh area of
the apple slices were carried out at a magnification factor of
140X. It should be noted that the analysis were carried out on
different slices. However, a valid comparison between the slices
s can still be made.
By comparing the sk;n area of the apple slices before and after
drying using the process according to the invention, namely, the
illustrations of Figs. 2(a) and 3(a), it can be seen that little
or no cell damage occurred to the cellular structure of the apple
slices in the skin area during the drying process accord;ng to the
invent;on. Furthermore, by comparing the skin area of the apple
slice as illustrated ;n F;g. 3(c) wh;ch had been dr;ed by the
process according to the invention and subsequently rehydrated, it
can be seen that the cellular structure after rehydration returns
substantially to its structure prior to being dried. The cell
walls have a substantially normal convex shape, and the cell
membrane of each cell in general, is in its normal posit;on
adjacent the cell wall. Moreover, a waxy layer at the sk;n
surface and colour components v;s;ble w;th;n several of the cells
adjacent the skin are substant;ally undisturbed, while significant
disturbance can be detected in the correspond;ng areas of product
dried by the a;r-flow and freeze-dry;ng processes.
The continuous lines in the Figs. 2 to 5 illustrate the walls of
the cells, and the partly broken l;nes illustrate the membrane of
each cell. In Figs. 2(a) and 2(b), only the cell walls
illustrated by the continuous line are visible. The reason for
this is that the membrane in any fresh product, or a product prior
to dehydration, in general will be adjacent the cell wall. Thus,
in the portions of the apple slices illustrated in Figs. 2(a) and
2(b), the membrane being adjacent the cell wall is not visible in
these two illustrations. Ideally if a product is to be rehydrated
to its form prior to being dried, the membrane and cell wall of
each cell should coincide.
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
While part of the membrane of some of the cells of the skin area
of the rehydrated apple slices of Fig. 3(c) have not entirely
returned to the cell wall, in general, the membrane and the cell
wall in the majority of cells do coincide.
S Similarly, much the same conclusion can be drawn from a comparison
between Figs. 2(b), 3(b) and 3(d). In Fig. 3(b), it can be seen
that little or no damage has occurred to the cellular structure of
the flesh area of the apple slice after being dried according to
the invention, and after rehydration, the flesh area of the apple
slice substantially returns to normal. In general, in the
majority of cells virtually the entire membrane returns to the
cell walls.
A comparison between Figs. 2(a) and 2(b) on the one hand, and
Figs. 4(a) to 4(d) on the other hand shows that as a result of
freeze-drying significant damage occurs to the cells both in the
skin area and the flesh area as a result of freeze-drying. Not
only are the cells damaged, but the membranes of the cells are
also dislocated during freeze drying. The extend of the damage
can clearly be seen in Figs. 4(c) and 4(d) where after rehydration
20 the cells in the skin area fail to return to normal. In Fig.
4(d), it can be seen that while some of the cell walls partly take
up the convex shape which they had prior to being freeze-dried, in
virtually all cells the membrane fails to return to normal, and
remains substantially shrivelled up within the cell. In addition,
25 the intercellular spaces are generally enlarged in the freeze
dried material as a consequence of the freezing process.
Comparisons between Figs. 2(a) and 2(b) on the one hand, and Figs.
5(a) to 5(d) on the other hand show a somewhat similar result as
that from a comparison of Figs. 2 and 4. As can be seen from Fig.
5(a) a substantial amount of damage has occ~t,ed to the cells in
the skin area of the apple slice as a result of the standard
forced air drying process. Broken cell walls are clearly visible
in several locations of the forced air dried product. This is
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96/00037
further emphasised in Fig. 5(c) after rehydration where the cells
in the skin area of the apple slice have failed to return to
normal, and furthermore, in all cases the membrane has failed to
return to the cell wall, and remains substantially shrivelled up
within the cell. In Fig. 5(b) cell damage to the flesh area of
the apple slice after the standard forced air drying process is
less evident. However, after rehydration of the flesh area the
cells in the apple slice failed to return to normal, since as can
be seen in Fig. 5(d) in virtually all cases the membrane fails to
return to the cell wall, and furthermore, the cell walls fail to
regain their normal convex shape. This failure of the cells to
reassume their original convex shape shows that the turgor of the
fresh product has been irreversibly damaged.
Dried tropical fruit materials obtained using three technologies
15 were tested. The dried tropical fruits which were dried in
accordance with the invention were dried using a four phase
dehydration process, wh;ch is substantially similar to that
described with reference to Fig. 6. The other two drying
technologies were freeze-drying and conventional air-flow drying
20 which is identical to the standard forced air drying process to
which the apple slices referred to in Table 1 were subjected. The
freeze-drying process is also identical to the freeze-drying
process to which the apple slices referred to in Table 1 were
subjected. Dried materials including banana, mango, pineapple,
25 kiwi, papaya, and ginger were prepared according to the four phase
dehydration process of the invention, air-flow dried material
prepared in ISK (Institute of Pomology and Floriculture) of
Skierniewice, Poland, and freeze-dried material produced in ISK.
The dried products were subjected to organoleptic and physical and
chemical analyses.
A sensory analysis was carried out of the slices of fresh fruits,
and dried fruits dried according to the invention, dried by
conventional air-flow drying and freeze-drying, and of rehydrated
slices of the fruits with the use of a scaling method. The method
SUBSTITUTE SHEET (RULE 2~
CA 02222153 1997-11-25
w O 96~9854 pc~rnE96looo37
for the tests - an unstructured linear scale (a segment on a
straight line, of length 100 mni, with the proper descriptions at
the edges, defining unequivocally the subject of the evaluation).
The persons performing the test marked onto the scale their
ratings, col,espondingly to the sensation experienced; next, the
results of the test were conve~ed to numeric values, assuming the
whole range of the scale to be 10 imaginary units. ~ables 2a - 2f
show the results obtained based upon averages for 10 pe~so.~s,
each of whom received samples coded in a different o-der.
Table 2a
Banana - a sumniary of average organoleptic ratings
...
2 3 ~ S ~ 7 8
r~shtruit 7~8 018 046 ~ 316 a27055 a'o
invntiondlbd in~dry403 156 037 718198 38821 400
m~bi condi~on
toilowino 1 99 4 65 7 90 1 ~ - 1 09 4 16 1 17
hydr~tion
Air-flow dried fruitin ~ dry 3~8 1 52 3 97 7 77 6 25 391 1 61 4 ~
(not inv ntion)condilion
tollowino 1 84 4 87 6 46 2 02 - 1 19 3 19 1 36
hydr ~ion
r. d in u dry 3 05 2 44 0 78 5 51 8 13 5 80 1 42 5 tS
2 0 mat ri i condi00n
foiiowinq 1 96 3 13 6 79 1 19 - 0 90 3 35 1 20
hydrdion
Ou~iity
1 i3 nan-sm ll '~i 10int nse)
2 For ion sm-ll r! ~ 10intense)
3 iiu--d or~ofbrown ~0~ ht,nobrownhue, 10brown)
2s 4 Hardn- s 0-soft, 10~rd)
5 ermbn ss O~l~ic, 10-brmie)
6 i3an n~t~s~ r i, , ' 10int nse)
7 Forei~n t~ 4! , , ' 10 intense)
8 Gen r~i; IOiowqu iity 10~ighquaiity)
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCT~E96/00037
Table 2b
Mango - a sunnary of average organoleptic ratings
i~o Gl ~ I
2 3 4 5 6 ~ 8
Fr ~htrult 73801B 046 308 373 864 027 851
Imronliondrbd in~dry 3,28 087 176 742 125 410 039 429
mat riai condition
foilowing 1 63 1~51 39 049 - 1 96 1 71 223
hydrdion
Airtiow dri d truit in ~dry 462 033 067 7B9 - 483 034 5 11
~not inv nlion) condition
bilowin~1 942 88Q951 52 - 1 8B2 00 2 04
hydrdion
r. di~ in ~ dry3 040 750 506 028 165 080 38 5 ~2
md rbi oondilion
bibwin~ Q58 489 059 0~4 - 062 342 1~
hydration
Chaiity ' ' ~ I'
1 i~ngounoll !~ 10inbn- )
2 ror ~n m~ , 10inbn~)
lS 3 iiu- d~r -otbrown ~ ht,nobrownhu-, 10brown)
4 iiardn-# ~ 10hard)
5 i~tli-n-~ D i~ic 10britti-)
6 i~bngou~o D' ;~"~lc, 10inbn~)
7 rorcign t~b r ~ 10inbn~)
20 8 G n~r iImpr ~ ion Oiowqu iity 10-hi~hqu iity)
SUBSTITUTE SHEET (RULE 2B~
CA 02222153 1997-11-25
WO 96/39854 PCI~/IE96/00037
Table 2c
Pineapple - a summary of organoleptic rating
i~ pph Cl ' ~ "
2 3 4 5 6 7 8
F~h truit 7R90 95 0~93 43 2 438 78 025 7 66
inv ntion dri d in ~ dry 641 024 291 742 227 S30 OJ3 482
m~rbl oondition
bib~ln~0~5 348 104 344 - 086 342 12D
tlydr~lon
Ahflowdri dWt h ~dry 4~ 055 335 702OE51 521 0 3 579
(not inv ntion) oondWon
biiowino 1103 460 46 4 59 - 0 43 3 16 113
hydr tion
r. - in ~ dry3 330 660 05451 9 46 5290 72 6 78
m t ri i condition
bilow~0 665 830 613 34 - 0 325 08 OR3
hydl tion
au.lity ~ -
1 ~in- ppl- ~ll r~, , ' 10int~)
2 For i~n ~ll r ! , F '~- 10int n -)
l5 3 Hu- d~r -otbrown Oiioht nobrownhu, 10brown)
4 H rdn~ ~ 10~rd)
5 i~ n-# ~b~u~c, lObrittb)
6 i~inc Pid-t~ b ~!, , ' 10int n~ )
7 F~i~nt~ ~ uhb 10int n~)
8 G-n~l impr - lon ~bwqu-lity 10hl~h qu-lity)
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCI'/IE96/00037
54
Table 2d
Kiwi - a summary of average organoleptic ratings
aw ~ '''
2 3 4 5 6 7 8
Frnhhuii 796025 505 306 342826 0~8 a39
inv ntion dri d h ndry 438 133 al2al3 326 501 105 40
rn~rbl condltion
blh~n~ 350214 a700 71 - 2 572 492 60
hydrdion
Air-flowdri dtruit inndry3~i2 0~6 5067U 376 512 107 535
~not inv nffon) oondition
~diowini~ 3S5 173 477 1~ 287 lJ3 3S9
hydr tion
r~ d ~ J in ~ dry 4300 830 354 70 83ri~350 766 50
rn~rbl oondWon
bib~ 3192 713 24QU - 2 092 89 215
hyd~on
Ou~ity ~
1 i~wi~11 ~, , 10int n~)
2 Fo~ign~m~ ! 10in~n~
15 3 i~-d~r~d~wn Oli~ht,noiorownhw, 10brown)
4 i~rdn-~ ~on, 10~rd)
5 ~ ~~ ~c, 10brlt~)
6 IGwi~b ~i F"~-, lOint n~)
7 Ford~n t~b r~ 101nt n~)
8 G-n r limF~don ~lowqu lity 10hl~hqu llty)
SUBSTITUTE SHEET (RULE 26~
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
Table 2e
Papaya - a sunniary of average organoleptic ratings
i~ ,~ ....
2 3 4 5 ~ 7 8
F-mh 1mit 5~61.271.431.88~2 7.02 1376.14
inv n~on d~d h ~dry 4311.721.86 7.17 4.08 4.74 0~7 4.i~4
rna~i oonditon
bllowino 5 ao1.39 2~01.51 , 2 49 1.~4 2 67
hydr lion
Air lbw dri-d hui~ in ~ dry3.971.08 130 6~33.62 4.89 O~B 4D1
(not inv~ion) oondition
tdo~ino 8.40 _31 1361.81 - _201~3 2.08
i~tion
r. ' ~ ~ in ~ dry 2.3S3.490.47 3.82 8.81 521 129 5~iO
malula oond~ion
folbwino 2.11 4J42.480.82 - 1.44 2 41 1.73
hydr~tion
Ou~y_ ~ ~
1. i~a ~11 ~ , , --- ~ 10~n~)
2 r~ion~m li ~ . ~ 10b~
15 3. Hu- - d~r of b~wn ~ii~h~, no i~wn im-, 10~own)
4. Hudn~ o~On, 10~rd)
5. i~-n-# 4~1~c, 10brml-)
6 i' p~ r~ ' . 10inl n~ )
~. ror ion t~ r~ 10-~
8. G~n~r~i ! . 11 4~owqu iity 10hiohqu-lily)
SUBSTITUTE SHEET (RULE 2~'
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
56
Table 2f
6inger - a summary of average organoleptic ratings
Gin~r
2 3 4 5
F~ hbult 825 1~0 280 388 712
inv nU~ondd d in~dly 850 058 a10 840 844
m~i ~ondi~on
hiiowin~ ~41 1 45 3 0~ - 4 71
hy~on
Air~bwdd dhdt In~dly 815 078 710 azo 1~80
not in~on) oond~ion
1nUow~ ~55 153 2eo - ~55
hyd~on
r ~ - in~dly a.2~i o.~io 225 954 aog
m~rbi condiUon
hiiowinl 6 21 1~ 2J8 - 453
hydrnU~on
~i ty _ k
1 GinQ~r m ii r~ Uhh, 1~int n~ )
2 r~ignun ii ~1, , , 10int~)
15 3 i~-d~gr olbrown ~Oiight,nobrownhu-, 10brown)
4 iCritt-n-~ O~c, 10brmi )
5 G n-r i imp~ion O iow qu iity 10~i~h qu iity)
Note: Bec~use the ginger is spicy, the organoleptic ratings of
ginger taste and foreign taste were not determined.
20 A numerical comparison of the leve1 of chromatographic traces for
volatile compounds produced by the above six fruits and tubers, and
maracuja (passion fruit), in the temperature ranges indicated is
shown in Table 3a. The data is given in integrated units per lOOg
of tested material and per litre of air. The results are an average
25 taken from three measurements. This analysis was performed on gas
chromatography HP equipment with an FID sensor.
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
T~ble 3a
Spoci- 0 M~rbl 10r ~na Inb~on unil-llOO o. ot m~brl~lll llb~ ol ~Ir
*~
~p ~.o ~C 40-100'C 100 1s~rG 1S~ Sum
h~or~ dry ~790 S365~S 304~8 1~ S75U7
drbd
mabrb. r hydrd d ~527 210~1 3~ 82 2~3133
Air~ow dly ~851 1~9~ 1~4612S11~4470
drbd ~ruit
~ ) r hydr~d 37~02 101~6 101302201 2Z8179
h~ dly107~1 17581S ~XK~187021~072
dd d
~ W ~hyd~S33~1 1~ 2~22Z~27U78
Mnnoo h~h 1ruit 30~09 2187~B0 ~388 11173 22l1r230
hvu~on dry 2U53 111SS10 3082~ 2~t2 1180031
drbd
rn_I l~d lXS2 486410 18351 17S08 S38021
~Jr-flow dry10092 38~620 1~81121441~748
dri d tNlt
~not~ ) rohydr~d 20~01 485008 21882 21427 49117
Fr~ dry11647 83841 18425 132~ llE42
drbd
mabri-l r hyd~d221021813584 17S17 31151 1083544
Pin- ppl- F~htruK 21~8 14~435 13~49~8410??65
hnn~on dry3S784 286~18 2WC9~B238824
drhd
rn brhl r hydral d 374S5 167733 12814 3404 Z1406
Airlbw dry 4324 77395 2411~ 3 107321
drhd 1ruit
(not r hydr bd
F~z~ dry12382 223756 48863 1875 287876
drbd
m~ri l r hydr-t~d 845643 l~i238 2312423005 2117014
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
T~ble 3~ (cont.)
Sp~ d iU~ri l 1Or t~n~ ~r~on unlt~/100 o ot m~r4Ul ii~ ot ~ir
1ruit~
up to 40 C 40100'ClOO lSO'C150200'C Sum
~ r~ dt U241 116873~7Z3 10~ 180505
im~on dry05224 1225642117337 ll~W37 lS24240
drbd
m~ r nydrabd 37984238664 ôl~!l5 27728 369231
Alr ~bw dry~1344 389W82~U0 443~8 S525~0
drhd Wt
~not r hydrd d 208~430~8S7 220~ 21~26B 37~5
inv n~on)
~ dry210B1S 12928471019~ 188052 1~42~8
dlbd
awl -~h 1ruil 2617r522458 7~ 36821 68412S
inv ntion dry 41037838027 U701 4820B 101287t
drl d
m brbl r hydr~d 20904302130 86783 8D773 490880
Alr ~bw dry 7168 13337439127 310a9 210786
dri d tNlt
irm n~on) ~d 1783711102B7 37301 18393 28170B
i~ dry65379 80Q322172824 eO241 1107736
drbd
m~brW r hydr~hd 33167380260 98544 90Q43 602914
Gin~r i-~h fmtt 8145728684S4 186263 143744 3m918
inv ntion dry 3U10ô415635 U7090 130020 7128155
dri d
m~ri l r hydrabd 222592382750 328U3 87831 2821393
Air 1bw dry35797 98106383339 74943 1175142
dri-d truit
(not r hydr hd 257141424850 160946 18482 162W72
inv ntion)
Fr~z~. dry38034 1753864203369 161739 2157006
drbd
m~rid rohydra~d 281571551262 190494 72531 1842444
iM racup Fr h 1ruit 60421 40391S2126841 29330 4255744
inv ntion dry 30273908475 83137 22088 1043973
drhd
masnrid r hydrat~d 8U3104654 36850 21001 171048
~r.flow dry29449 15570240396 27165 252702
drhd truit
~not r hydr~t~d 11751144101 37612 9248 202712
invnntion)
Fr ze dry60623 1550407138764 69129 1829923
dri d m bri
d r hydrat~d 941421325725 9180~ 38018 1550680
SUBSTITUTE SHEET (RULE 26'
CA 02222153 1997-11-25
WO 96139854 PCT/IE96/00037
59
Another numerical comparison of the level of chromatographic
traces for volatile compounds p(oduced by the same materials is
shown in Table 3b. The data in integration units per 1009 of
tested material per litre of air passed through has been
5 recalculated in terms of 1009 of dry mass of the tested material.
The results are averages for three measurements.
Table 3b
Si~ ot M~ri i br t- ang int~ration unH 1100 ~. o1 mat~riai/1 IHr ot air
~
up to 40~C40-100'C100-150'C150.200 CSum
~na Fr~h truH 1087313492957 426781 7316 403m5
inv~ion dry 7320 578477 ~l~79 1729 B2~5
dd d
mat ri i r hyd~d 3152362037302 364869 18179 273A'i86
Air~bw dry10210 176589 17B11 1324 205744
d bd 1ruH
i~n~ion)r hydr~d 227!iB310U5B4 117B50 13530 1403316
F~ dry11086 18081~ 2i~5 1~21 21~21
drbd
mat~i r hydr~d 3403121240714 180777 14202 1776005
i~an~o Fr~h fruH 16089611~76Y0735E 551 B0703 12154657
innnhon dry2B678 1197~2 39641 28011266875
dri d
mat riair hydrat d1782735539982 309008 200544 6127807
Ur flow dry - 415861 70131 1296 448060
dri d 1tuH
~not rnhydrn~d 30689871~~i63 ~1795 31S097 8075253
inv ntbn)
Fr~ dry12242 88124 20417 1397 122180
dri d
mat rbi r hydrat d31395D911560122248822442190 15391243
i'in-apple Fr h 1ruit 2040298810841808338105 7596 315904g7
inv n~ion dry 38239285230 31053 1028 355550
dri d
mnbri i r hydrat d6923213100427 236853 62i128 4032529
Air~iow dry 4548 81418 25373 1560 112893
dri-d truH
(not r hydrat d5221494240161 369343 388692 5520345
inv n~ion)
Free~ dry13086 236478 52698 1982 304244
dri d
mabri i r hydrabd1504702921801390411524409343 376t9292
SUBSTITUTE SHEET (RULE 26)
CA 022221S3 1997-11-2S
WO 96t39854 PC r/IE96looo37
T~ble 3b (cont.)
Si~oi-~ ot M~riai tor t~tino int~ration units/100 9. ot mat rial/1 litr ot air
truils
up to 40 C40-100 C100-150'C 150-200'C Sum
i~ya -~h 1ruit 456580 1195797 100342 11009818i62797
Inv n~on dry70827 1327170 127057 125849 1850503
dri d
md riai r hydrabd 7034084382680 1238057513475 8387600
Air~lbw dry97704 4171U 28709 47490 01W
dri d trult
hln~t O ) r~hydr~bd 4811457156053 510350 806802 8754150
Fr~ dry2220281361808 107408 198885 1889729
dri d
m~dai r hydr~t d77g48116700926 13627331917725 20760865
lawi Fra h truit 118287 23608~ 355488 188386 3001020
hv ntion dry45415 ~7U4 91m 52205 1098891
dri d
m~i r hydrd d 1886282428819 6970S1 648r76 3841274
AiWbw dry 7835 142D99 416BB 33133 224553
dri d truit
~not~ ) r-hydr~d1583211708139335U4 1471S8 2349282
F~ dry 6~00 838284 179223 62471 1148778
dri d
m t ri i r hydr#bd 2700033096582 802476 740576 49097.27
Ging-r i-~h truit 2780319720277 631185 487101 11114534
in~on dry3747g 67gO469 579053 ~137616 7544617
dri d
r hydr t~d10858211623169 1602E48 428442 13762839
Air~v dry38434 1053320 88477 804631261694
dri d truit
~not r hydrahd 1333707390301 834784 95757 8454212
F~ dry39385 1815219 210483 167397~464
dri d
mat rial r hydrat~i1654369114347 1119234 426153 10B25170
Mu~cuja i-~sh truit 273027 18251931 573165 132533 19230656
inv ntion dry 32863 986188 90248 23977 1133274
dfi d
mat fial r hydra~ d 46454 565304 199082 113460 924390
Air-tiow dry31067 164280 42656 28658268591
dfi-d truit
Inot r~hydr~t~d 59197 725948 189479 46590 1021214
inv ntion)
Fr z~ dry63975 1848694 146438 729511930058
dri d
m~riai r hydratod3836275402303 374101 1S8997 6319528
SuBsTlTuTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
Gas chromatographic (GC) traces illustrated in Figs. 8 to 11 were
prepared of banana slices prior to and after drying using the
three drying processes discussed w;th reference to Table 1. Gas
chromatographic traces ;llustrated in Figs. 12 to 14 were also
prepared of the banana slices after they had been subsequently
rehydrated. The gas chromatographic traces were prepared on a HP
gas chromatograph with an FlD sensor in the range of temperature
from room temperature up to 200~C. The time period of each test
for preparing the corresponding trace is plotted on the x-axis in
units of minutes. During the test period the temperature of the
sample slices were raised in a controlled manner from room
temperature to 200~C. The y-axis is a measure of the
chromatographic response measured by the gas chronatograph. The
scale of this response is in machine units, and the scale is
adjusted for each test to accommodate the most prominent peak
measured. The numbers associated with the peaks in the traces are
the retention times in minutes for the release of the respective
volatile compounds. These retention times in con~unction with the
temperature profile used for the tests make it possible to
associate each peak unambiguously with a specific molecular
volatile compound.
Fig. 8 illustrates the gas chromatographic trace for a banana
slice prior to dehydration. Fig. 9 illustrates the gas
chromatographic trace for a banana slice having been dried by the
four phase process according to the invention. Fig. 10
illustrates the gas chromatographic trace for a banana slice which
has been dried by the conventional air-flow process. Fig. 11
illustrates the gas chromatographic trace for a banana slice which
has been dried by the freeze drying process. Fig. 12 illustrates
the gas chromatic trace of a banana slice which has been
rehydrated after having been dried by the four phase process
according to the invention. Fig. 13 illustrates the gas
chromatographic ~race for a banana slice which has been rehydrated
after having been dried by the conventional air-flow drying
process. Fig. 14 illustrates the gas chromatographic trace for a
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
62
banana slice which has been rehydrated after having been dried by
the freeze drying process.
The flavour and bouquet or characteristic aroma of foods eaten in
their raw state are dominated by volatile compounds which are
eluted, namely, liberated or activated at temperatures up to 40~C,
in other words, up to normal body temperature. Accordingly, the
similarity between the elution chromatographic signature of a
fresh fruit or vegetable, in other words, a fruit or vegetable
which has not been dried, and the dried product is a strong
indication of how well the fresh fruit taste and aroma is retained
during a drying process. A comparison between the gas
chromatographic trace of Fig. 8, namely, the trace of the fresh
banana with the gas chromatographic trace of Fig. 9 of the banana
dried by the process of the invention illustrates that the banana
dried by the process according to the invention retains
substantially all of the compounds which are eluted at
temperatures of up to 40~C. Furthermore, it can be seen from the
trace of Fig. 12 that the rehydrated banana which had been dried
by the process according to the invention also retains
substantially all of the compounds which are eluted at
temperatures up to 40~C. On the other hand, a comparison of the
traces of Figs. 10 and 11 which illustrate the traces of the
banana which had been dried by the conventional air-flow drying
process and the freeze-drying process, respectively, and the trace
of Fig. 8, shows that after conventional air-flow drying and
freeze-drying a significant number of the compounds which are
eluted at temperatures of up to 40~C which were present in the
banana prior to being dried are absent in the product after
drying. Similarly, a comparison between the trace of Fig. 8, and
Figs. 13 and 14 which illustrate the banana having been rehydrated
after conventional air-flow drying and freeze drying,
respectively, shows a somewhat similar result to that of the
comparison of the traces of Fig. 8 on the one hand, and Figs. 10
and 11 on the other hand.
SUBSTITUTE SHEET (RULE 26
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
Additionally, it can be seen from the trace of Fig. 9 that the
dried banana which was dried by the process according to the
invention has prominent gas chromatographic peaks at 6.110 and
7.374 minutes retention times.
The main fraction of volat;le compounds were compounds evaporating
in the range of temperatures of 40~C to 100~C. In the fresh
fruits, this fraction contained predominantly: butanoic acid
methyl ester (RT=7.3); an unidentified compound with RT=10.2;
butanoic acid propyl ester (RT=11.9); 2-methyl butanol and/or 3-
methyl butanol (RT=14.8); ethanoic acid hexil ester (RT=16.5).
Hexanol (RT=19.3) was the main constituent of the 100~C to 150~C
fraction. In the dried banana which were dried by all three
drying processes, the main constituents of the 40~C to 100~C
fraction were: ethanol (RT=6.1); butanoic acid methyl ester and 2-
methyl butanol and/or 3-methyl butanol. In the dried banana which
was dried by the drying process of the invention the main
components of the above mentioned fraction were a compound with
RT=11.0 (probably isobutanoic acid isobutyl ester) and an
unidentified compound with RT=17.6. In the dried banana which was
dried by the freeze-drying process, in the 40~C to 100~C fraction
butanoic acid methyl ester (RT=7.3) and an unidentified compound
with RT=10.5 dominated. Following rehydration of the banana which
had been dried by all three drying processes, a drop in the level
of ethanol in the aroma was detected, the dominating compound was
still the butanoic acid methyl ester (RT=7.3). Overall, the
proportion of the low-boiling fraction (up to 40~C) rose in the
aroma of both the rehydrated banana which had been both dried by
the process according to the invention and the freeze-drying
process.
Figs. 15 to 18 illustrate gas chromatographic traces of the IDARED
apple slices which were prepared in similar fashion to those of
Figs. 8 to 11. Fig. 15 is a gas chromatographic trace of an apple
slice prior to being dried. Fig. 16 is a gas chromatographic
trace of an apple slice having been dried by the four phase
SUBSTITUTE SHEET (RULE 26!
CA 022221S3 1997-11-2S
WO 96/39854 PCT/~E96/00037
64
process according to the invention. Fig. 17 is a gas
chromatographic trace of an apple slice having been dried by the
conventional air-flow drying process, and Fig. 18 is a gas
chromatographic trace of an apple slice having been dried by the
freeze-drying process. A comparison between the four traces of
Figs. 15 to 18 indicate that as in the case of the banana slices,
the apple which was dried by the process according to the
invention retains a substantial amount of the volatile compounds
which are eluted at temperatures up to 40~C, while the apples
which were conventionally air-flow dried and freeze-dried do not
retain the same amount of such compounds. However, a significant
feature which can be seen by a comparison between the traces of
Figs. 15 and 18 is the fact that the apple which was dried by the
freeze-drying process shows the development of significant peaks
in the higher temperature range, in other words, temperature
ranges towards 200~C which, in general, tend to be associated with
the development of off tastes. Such peaks are not present in the
trace of the apple dried by the process according to the
invention.
It can also be seen from the trace of Fig. 16 that the apple dried
by the process according to the invention has prominent gas
chromatographic peaks at 6.101, 10.186 and 11.834 minutes
retention times.
Figs. 19 to 25 are gas chromatographic traces for mango slices
which correspond to the gas chromatographic traces of the banana
slices of Figs. 8 to 14. In other words, Fig. 19 is a gas
chromatographic trace of mango prior to drying, Fig. 20 is a trace
of the mango dried by the four phase process according to the
invention, Fig. 21 is a trace of the mango dried by the
conventional air-flow drying process, Fig. 22 is a trace of the
mango dried by the freeze-drying process, Fig. 23 is a trace of
the mango which was rehydrated subsequent to being dried by the
process according to the invention, Fig. 24 is a trace of the
mango having been rehydrated subsequent to being dried by the
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
conventional air-flow drying process, and Fig. 25 is a trace of
the mango having been rehydrated subsequent to being dried by the
freeze-drying process. Comparisons between the traces of Figs. 19
to 23 yield substantially similar conclusions to those drawn from
the comparisons made between Figs. 8 to 14 of the banana as
already discussed. In addition, it can be seen from Fig. 20 that
the dried mango which was dried by the process according to the
invention has prominent gas chromatographic peaks at 6.181 and
12.771.
The main fraction of volatile compounds in the ~ango are compounds
evaporating in the 40~C to 100~C range of temperatures. The
dominant compounds to be found in this fraction for the fresh
fruit are: unidentified compounds with RT-12.5, RT 12.8 (probably
butanol), RT-13.1, RT=14.2 and RT=17Ø The co~pound with RT-12.7
15 remains the main compound emitted by the dried mango which were
dried by all three drying processes. I'~we~r, in the aromas from
the dried mango which was dried by the process according to the
invention there is also a large quantity of ethanol (RT-6.81).
Following rehydration of the mango dried by the process of the
20 invention, three compounds dominate, one with RT-9.2 (probably
propanol), two others with RT=12.5 and RT-16.6. Following
rehydration of the mango which had been dried by the conventional
air-flow drying process butanol (RT=12.8) additionally dominates.
After rehydration of the mango which had been dried by the freeze-
25 drying process, the rehydrated mango becomes very aromatic, with a
large participation of a compound with RT=4.0 (ethanoic acid
methyl ester) in the fraction up to 40~C. This rehydrated mango
also displays compounds with RT=5.1 (methanol), RT~9.2 (propanol),
RT=12.3 and RT=16.5 in the 40~C to 100~C fraction, and compounds
with RT=27.6 and RT=29.9 in the 150~C to 200~C fraction.
Figs. 26 to 32 illustrate gas chromatographic traces for papayawhich also correspond to the gas chromatographic traces of Figs. 8
to 14. In other words, Fig. 26 is a trace of papaya prior to
being dried, Figs. 27 to 29 are traces of papaya ha~ing been dried
~UBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
by the process according to the invention, the conventional air-
flow drying process and the freeze-drying process, respectively,
and Figs. 30 to 32 are traces of papaya having been rehydrated
subsequent to being dried by the drying process according to the
invention, the conventional air-flow drying process and the
freeze-drying process, respectively. A comparison between the
traces of Figs. 26 to 32 yields substantially similar conclusions
as those drawn from the comparison between the traces of Figs. 8
to 14 in connection with banana. Additionally, the dried papaya
dried by the process according to the invention has prominent gas
chromatographic peaks at 6.254, 7.941 and 9.323 minutes retention
times.
Figs. 33 to 39 illustrate gas chromatographic traces for kiwi
slices which also correspond to Figs. 8 to 14. In other words,
Fig. 33 is a trace of the kiwi prior to being dried, Figs. 34 to
36 are traces of the kiwi having been dried by the process of the
invention, the conventional air-flow drying process and the
freeze-drying process, respectively, and Figs. 37 to 39 are traces
of the kiwi having been rehydrated subsequent to being dried by
the process according to the invention, the conv~ntional air-flow
drying process and the freeze-drying process, respectively. A
comparison between the traces of Figs. 33 to 39 yields
substantially similar conclusions to those drawn from the results
of the comparison of the traces of Figs. 8 to 14 for banana. In
addition, it can be seen from Fig. 34 that the dried kiwi which
was dried by the process according to the invention has prominent
gas chromatographic peaks at 6.344, 7.946, 8.649 and 9.336 minutes
retention times.
Figs. 40 to 46 illustrate gas chromatographic traces of ginger
which correspond to the traces of Figs. 8 to 14. In other words,
Fig. 40 illustrates a trace of the ginger prior to drying, Figs.
41 to 43 illustrate traces of the ginger having been dried by the
process according to the invention, the conventional air-flow
drying process and the freeze-drying process, respectively, and
SUBSTITUTE SHEET (RULE 26)
. CA 02222153 1997-11-2~
W096~9854 PCT~E96/00037
67
Figs. 44 to 46 illustrate traces of the ginger having been
rehydrated subsequent to being dried by the process according to
the invention, the conventional air-flow drying process and the
free~e-drying process, respectively. Comparisons between the
traces of Figs. 40 to 46 yield substantially similar conclusions
as those drawn from the comparison of the traces of Figs. 8 to 14
for banana. Additionally, it can be seen from Fig. 41 that the
dried ginger which had been dried by the process according to the
invention has prominent gas chromatographic peaks at 5.741, 6.356,
7.996, 8.496, 9.407, 10.006 and 11.213 minutes retention times.
Figs. 47 to 53 illustrate gas chromatographic traces of pineapple,
which correspond to the gas chromatographic traces of Figs. 8 to
14. In other words, the traces of Fig. 47 is of pineapple prior
to drying. The trace of Figs. 48 to 50 are of pineapple which has
been dried by the process according to the invention, the
conventional air-flow drying process and the freeze-drying
process, respectively, and the traces of Figs. 51 to 53 are of
pineapple which has been rehydrated, prior to having been dried by
the process according to the invention, the con~entional air-flow
drying process and the freeze-drying process, respectively. A
comparison between the traces of Figs. 48 to 53 yields similar
conclusions to those drawn from the comparison between the traces
of Figs. 8 to 14 in respect of banana, and furthermore, it can be
seen from Fig. 49 that the pineapple dried by the process
2S according to the invention has a prominent gas chromatographic
peak at 6.104 minutes retention time.
In the pineapple prior to being dried, the most intense was the
fraction up to 40~C, with the compound RT=4.9 (ethyl ethanoate)
dominating. In the 40~C to 100~C fraction the predominant
compounds were RT=7.4 (butanoic acid methyl ester), RT=8.1 (2-
methylbutanoic acid methyl ester) and RT=14.0 (hexanoic acid
methyl ester). In the dried pineapple which had been dried by the
conventional air-flow drying process, the main component was an
unidentified compound with RT=12.6, and in the dried pineapple
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/398S4 PCT/IE96/00037
68
which had been dried by the process according to the invention the
main component was ethanol (RT=6.1). The dried pineapple which
had been dried by the freeze-drying process contained mainly the
compounds with RT=12.6. Following rehydration of the dried
s product which had been dried by the conventional air-flow drying
process and the freeze-drying process, there was an increase in
the proportion of compounds belonging to the up to 40~C fraction.
In the case of the rehydrated pineapple which had previously been
conventionally air-flow dried, the main compounds were
unidentified compounds with RT=9.2 and RT=16.5. Additionally, in
the rehydrated pineapple which had been dried by the conventional
air-flow drying process there were unidentified co~pounds
belonging to the 150~C to 200~C fraction which were characterised
by RT=27.6 and RT=29.8. The main compounds in the rehydrated
material which had previously been dried by the freeze-drying
process were compounds with RT=2.8, RT=3.8 (ethanoic acid methyl
ester), RT=5.0 (ethyl ethanoate), RT=6.1 (ethanol), RT=11.2 and
RT=14.9 (3-methylbutanol).
Figs. 54 to 60 illustrate gas chromatographic traces of maracuja
slices, which correspond to the gas chromatographic traces of
Figs. 8 to 14. In other words, Fig. 54 illustrates a trace of the
maracuja prior to being dried, Figs. 55 to 57 illustrate traces of
the maracuja dried by the process according to the invention, the
conventional air-flow drying process and the freeze-drying
process, respectively, and Figs. 58 to 60 illustrate traces of the
maracuja having been rehydrated subsequent to being dried by the
process according to the invention, the conventional air-flow
drying process and the freeze-drying process, respectively. A
comparison process between the traces of Figs. 55 to 60 yields
substantially similar conclusions to those drawn from the
comparison of the traces of Figs. 8 to 14 for banana.
Additionally, it can be seen from Fig. 55 that the dried maracuja
which was dried by the process according to the invention displays
prominent gas chromatographic peaks at 6.367, 7.983, 8.682 and
9.375 minutes retention times.
SUBSTITUTE SHEET (RULE 26
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
69
In the above analysis which has been made with reference to Figs.
8 to 60 it should be noted that the various compounds which have
been identified, have been identified on the basis of the
comparison of retention times with reference times, and by relying
upon data which is available in the literature. The compound with
the retention time of 27.0 which is visible on the gas
chromatograph is an octanoic acid ethyl ester, which is used as a
reference substance. Additionally, it should be noted that the
abbreviation "RT" in the above discussion with reference to Figs.
8 to 60 indicates "retention time".
The results of the analysis of the volatile compounds using gas
chromatography give evidence of the influence of the drying
technology on the composition of the level of aromatic compounds
in the dried materials. The main fraction of volatile compounds
consists of compounds evaporating in the 40~C to 100~C range of
temperature. In all the dried materials because of the
technological processing, a fall in the level of aromatic
substances occurs. ~owever, the dried fruits and vegetables which
were dried by the process according to the invention have the
highest level of aromatic compounds for each of the species of
fruits and vegetables. Following rehydration an increase in the
content of volatile compounds occurs in the case of mango and
pineapple. This is greatest in the case of the mango and
pineapple which had been dried by the freeze drying process. An
increase in the content of volatile compounds in the rehydrated
banana also occurs, and this increase is greatest in the case of
the rehydrated banana which had previously been dried by the
process according to the invention. At the same time, in the
organoleptic rating, the highest level of foreign smell for banana
has been observed in rehydrated banana, while in the case of mango
and pineapple, the highest level of foreign smell has been found
in rehydrated mango and banana which had been previously dried by
the freeze drying process.
An assessment of hue was conducted on each fruit and tuber tested,
SUBSTITUTE SHEET (RULE 2~'
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
using the test procedure according to the Hunter Colorimeter at L*
a* b* is set out in Table 4.
Table 4
Vui-ty ot fruit iUahrial for t~stins Hu- acoordin~ to Hunbr Lab
L~ a b~
i3anuna Fresh 1ruit 80.574.07 26.39
Inv nffon drbddry 62.485.89 27.15
mabriai
~ y ~ 49.376.21 2020
Air flow drbd fruit dry 6~i.98 5.78 28.04
(not inv~on)
~ 54.035.81 18.79
r. - ~ dry 86.1 t2.95 1424
mat rial
~ _ 51.956.71 16.01
Man~o F~sh 1ruit 69.641S.83 60.30
Inv ntion dri d dry65.89 20~0 6233
mat rial
~1~ 60.74 16.88 6~i.91
Air now drbd fruit dry77.95 16.57 67.8!i
(not inv ntion)
~ 68.39 15.14 67.89
r. d ~ dry 83.8410.97 U.47
m~rlal
~y 72.4813.01 60.37
Pin appb Fr sh truit 84.521.51 28.63
Invention drled dry81.50 3.88 32.67
mat riai
. 1~ 78.57 0.55 31.78
Air-flow dri d fruit dry78.3t3 3.83 33.05
(not inv ntion)
I ~ 79.01 0.87 29.43
F ~ ~ dry 92.444.14 18.02
mat riai
~ 85.784.75 17.90
SUBSTITUTE SHEET (RULE 26~
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
Table 4 (cont.)
Variety of fruit iUateriai tor bsting Hu~ Accordin~ to Huntur l-b
L a- b-
Papaya Frosh frult 63.0 25.0 36.3
Invontion ddod dry 66.6 20.? 40.7
ma~dai
~y' - 4O.0 263 36.4
Air-flow ddod fruK dry 68.8 215 45.1
(not Invontion)
- h/~ ~ S1.9 24.1 44.0
r. d- - dry 7B.5 12.9 24.5
matndai
'Iy' ~ 69.0 16.7 36.7
Kiwi Fr~h fruit 472 -1.9 20.5
invontion driod dry Sl.0 5.9 29.7
matariai
,~ ' 47.4 3.~ 25.6
Air-fiowdd d fruit dry S7~ 3.7 27.7
~not inv ntion)
~ S52 0.7 242
r. . - dry 84.4 -1.8 19.1
mat d i
~ ' e3~ on 25.7
i~aracuja Frosh fruK 442 7J 30.7
Invuntion driod dry 44.3 132 36.4
mat riai
~, ~ 43.7 75 30.0
Air-now driod fruit dry 3D.5 8.6 132
(not invention)
. ' /' ' 345 9.5 30.9
Fn _ ~ dry 721 11.9 4B.7
rnatoriai
~,~ ' 49.7 7.1 34~i
Ginger Frosh fruK 66~ 1.3 33.4
Innntion driod dry 562 3.0 1a6
m~dai
h~ '- d 63.4 3.9 32 0
Air-flow ddnd fruit dry 69.9 1.1 19.0
- (not invention)
~ ~ 62.3 4.8 33.3
F. ' d dry 83.3 -2.0 22 0
materiai
~hJ~ ~ 67.3 3.5 34.1
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
The dry mass content of each fruit and tuber tested was evaluated
by desiccating samples to substantially total dryness. The
results are shown in Table 5. The table shows that for the
majority of fruits tested, the products dried by the four phase
process of the invention could be rehydrated to a water content
closer to the water content of the product prior to being dried,
than could product dried by the other two drying processes. The
ability of a dried product to be rehydrated to a state approaching
that of the product prior to being dried is an indication of the
retention of the properties of that product which it had prior to
being dried.
Table 5
Vari~y of tnuit ~Ihhrial for testing Ory ~ubstanc~ l%]
i3anana Fr h fruit 24 60
inv ntion dri-d m-brial dry 9276
,_'1, J 10 35
Air-11ow dri-d fruit (not inventjon) dry 94 52
~, ~ 1626
r. d ~ mahrid dry 9580
--~ ~ J~ -- Ti 15 46
iUango Fresh fruit 18 90
Inv ntion dried mahriai dry 93 16
_'1~_ 8 78
Air-llow dried fruit ~not invention) dry 93 69
'~' ' 680
r,. 'r; ~ mahriai dry 95 14
:~,' i 7.94
Pine-ppl- Fresh truit 13 00
Inv ntion driod mat-rial dry 93 58
- "Jr_ 5 41
Air-tlowdried fruit (not inv nUon) dry 9506
,_ 534
r d~isd materiai dry 9462
5 62
SUBSTITUTE SHEET (RULE 26~
CA 02222153 1997-11-25
WO 96/39854 PCT/~E96/00037
Tsble 5 (cont.)
Variety of fnuit Materiai tor te~in3 Dry ~L]
Papaya Frersh fruit 9 69
Inv-ntion dri-d materlaidry 9235
'q S4
Air flow dried truit ~not invention) dry 93 49
~ ~ 4~i3
r. - - materhi dry 949s
Iy r~ S23
Kiwi Fre~ it 16 75
Inv ntion dri d mat riaidry 9234
_~ 12 45
Air-flowdrbd truit (not inv ntion) dly 93U
~ - 11 14
r. F m-t r~i dry 98 43
~ - 122~
Uaracuja Fr sh tmit 2~13
b~ntion dri d mat rbi dry 9212
18 51
Air-flow drbd fruit (not invention) dry 94 79
~ ~ 1985
r. - mabri i dry 94 76
24 54
Gin~er Fresh ftult 2351
Irnr ntion drl d mat riai dry 94U
~ ~ 205
Air-11ow driod 1ruit (not irN ntion) dry 9314
19 28
r. d~kd mat riai dry 96 62
i 1702
Measurements of water activity of the above dried fruits and
tubers have been taken and the results are presented in Table 6.
The measurements of water activity were taken using a water
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
74
activity meter AW-THERM 40 with automatic temperature
compensation.
Table 6
Vari-ty ot Iruit ~iabriais hr 1 st W br activity
-
i3anana Inv ntion dried mabriai 0 392
Air-tlow dried truit ~not invention) 0 374
F ~ mat riai o ~3
~ani30 Invention dried materiai Q410
Air tlow drbd truit (not inwntion) 0 355
r~ ~ - material o ~7
Pineappb inv ntion dried materiai 0362
Air-11ow dried truR (not invention) 0 3~37
F ~ - r ri mat riai 0 30i~
Papaya inv ntion drbd mat riai 0 347
Air-tlow dri-d ~rult (not Invention) 0 301
r. d - mat riai 0~7
0 Kiwi Inv ntion drbd materid 0330
Air-fiow drbd truit (not inv ntion) 0294
r~ materid 0 144
iUarscuja invention dried mat riai 033~3
Air-tlow dried truit (not invention) 0 309
r. ~ materiai 0169
Gin~er Inwntion dried mat riai 0~3
Air flow dried truit (not inv ntion) 0 345
r~ r ~m-teriai 0099
Ascorbic acid content of the above dried fruits and tubers tested
is presented in Table 7.
lS The ascorbic acid presence was tested using the HPLC method,
Supelco LC - 18 column, with the solvent 1% KHP04, pH=3, the rate
of flow = 0.8 ml/min. The detection was conducted at a wavelength
of 243 nm.
SUBSTITUTE SHEET (RULE 26
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
A representative sample of the material was taken, which was
homogenized in 6% metaphosphoric acid, and then filtered. From
the solution that was obtained, phenol compounds were removed on
the C-18 column and then the solution was subjected to the HPLC
chromatography analysis. The retention time of ascorbic acid was
equal to 6.31 min. In the tested range of ascorbic acid content,
(from 0.2 to 1.5 mg of ascorbic acid in 100 ml of solution
prepared for injection) the square of the correlation of the
content of ascorbic acid in the reference solutions and the
surface areas of the peak equalled to 0.9998.
SUBSTITUTE SHEET (RULE 26
CA 02222153 1997-11-25
WO 96/39854 PCT/IE96/00037
Table 7
Vari~ty of truit ~aterial for t~sbng Ascorb;c acid contant
[mg/100g]Img/100~ dry ma~] l%]
Ban na Froshfn~it 58 236 1000
Im~ntion drbd mat~al 32 3 4 14 4
Air-flow dried fn it (not inv~ntion) 0 5 0 5 02
r. ~ i matorlal 3 0 3 2 13 5
Mango Frnsh fn R 21 3 112 7 100 0
Inwnbondri dm-t rid 1033 1109 984
Air tlow dri d fn it (not hv-nbon) 93 8 98 6 87 5
r. ~ ~ matcrlal 87 7 90 2 80 0
Pinoappl- Fr~h fn~R 32 7 251 6 100 0
Inv nt~on drbd mat-rial217 6 232 5 ~4
Air-flowdrl d fn it (notinvcnbon)1989 2102 835
r. ~ - mat riai 2392 2465 979
Papaya Frndl fn~R 44 6 460 0 100 0
Invontion drl d matcrbi3921 4137 899
Air flow dried fnJit (not invenbon) 4052 433 4 842
r. ~ - matcrlal 41? 8 435 8 94 7
IGwi Fr~h fn~R 104 4 623 1 1W 0
Inv nbon dried m~t-rial5740 6216 998
Air-tlow drl d fruit (not invcnbon) 459 9 499 1 801
r. ~ mabriai 5491 569 5 91 4
Maracuja Fr shfruit 130 587 1W0
Iml nbon dri d mat rial38 9 422 72 0
Air-flowdried fruit (not invcnbon) 25 26 44
r. ~ i mat~rial 46 4 50 5 86 0
Of the above fruits and tubers, a comparison has been made of the
content of b-carotene and carotenoids, and the level of nitrates
and nitrites for banana, mango and pineapple. The result of the
comparison are shown in Table 8. The analytical methods used were
as follows:
SUBSTITUTE SHEET (RULE 26)
CA 02222153 1997-11-25
WO 96/39854 PCI~/~E96/00037
carotenoids and b-carotene - method described by Czapski and
Saniewski, Experimentia, 39, 1373-1374, 1983;
nitrates - potentiometric method, using the ORION apparatus;
nitrites - spectrophotometry method, according to Polish
standard PN-92 A-75112.
Table 8
V rhtyof fruit8 ~fiaterbiforbsiinC t _ ~ ¦ wm d al! ¦ nn~s ¦ ni~ab~
[m~/lODo D W ]
3anana r~osh fnJit Z75 3ti3 350
<100
invention dried mabriai 255 487 330
Air-11ow drhd fmit 389 ~5 310
(not inv ntion)
r, d- . mat~i 3S3 S~7 ~70
i~an~o r~h fnJit ~30 875 105
<100
invention drhd materbi 371 734 110
Air-llvw dri~ fnJit 402 9~7 100
(not inv ntion)
fi '' m bri i ~.18 ~81 105
rin-apple F~h fn~it 326 131 1~5
<100
invention drhd mat ri i 3i~4 ô45 1~iO
Air-tlow dri d fn it ~1 558 150
(not inv ntion)
r, ~ mabriai 300 594 1~iO
Table 9 shows the results of texture tests on the slices of the
above fruits and tubers, with the exception of maracuja (passion
fruit). Each result is an average of at least 20 measurements.
The measurements of the penetration force and the Young's modulus
were performed using the structural solidity meter Instron 4303
with a sensor having a radius of 3.7 mm. To make the
measurements, the perforation of a slice of a given material was
SUBSTITUTE SHEET (RULE 26~
CA 02222153 1997-11-25
WO 96/39854 PCTtlE96/00037
placed between two plates with holes having a radius of 16.7 mm.
As can be seen from Table 9, most of the products dried by the
dehydration process according to the invention have a Young's
modulus comprised between 0.11 MPa and about 0.20 MPa.
s Table 9
Variety ottruit Mabrial fort~tina i~ Appar nt Youn~'s
Forcs l~ modulus [MPal
i3anana Fresh truit OJ1 0 031
inv ntion dri~d mabriai ~~ 0138
Air llow drled tru~t ~not ~nwntion) 1~ 0238
r ~ mabrial r.14 0 8B7
Man~o r~h tnJit 0~ 1004
invsntion dri d matsrial10~ 0175
Air-flow dri d truit (not inv ntionl 1~32 0231
r '' rma1arhi 10Q0 0311
Pinsappls F~sh trult 1J0 0013
inventiondrhdmat rial 119~ 0119
Air 11ow dd d truit ~not inv ntlon) 790 Q106
r ' t mat rbi 874 0235
Papaya F~sh truit 0 39S 0 0095
inv ntion dri d mabrial7302 02239
Air-tlow dri d tn~lt ~not Inwntion) 7171 01382
r. ~d ~ mabriai 5207 02970
iGwi F~sh truit 0 624 0 0045
Inv ntion dfisd matsriai10280 01265
Air-flow dri d truit ~not inwntion) 9 782 0 093
r. d - mabriai 11075 02534
Gin~-r hesh fruit 17130 00875
inv ntion drbd mat riai17570 102~0
Air-flow drisd truit ~not inv ntion) 20 100 0 5U8
r. ~ - materiai 9 077 0 3866
SUBSTlTUtE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96139854 PCT/IE96/00037
Evaluation of the orqanolePtic test results
Banana
The banana slices dried by the four phase process of the invention
and the conventional air-flow dried slices received similar
ratings, both higher than the ratings for the freeze-dried banana
slices, for the presence of typical banana flavour and the absence
of off-flavour. The slightly different overall rating for the
conventional air-flow dried material is the result of its greater
hardness, caused by its having been dried to a ~ater content level
of 5.5% (v. 7.2% for the material dried in accordance with the
invention). Banana slices dried in accordance with the invention
were much less brittle than the other dried pro~cLs tested. In
addition, the hue of the banana slices dried in ~ccordance with
the invention was closer than the hue of the freeze-dried banana
slices to the hue of the banana slices prior to drying.
Following rehydration, because of a comparable w~ter-absorption
capacity (cf. Table 6), the materials dried in accordance with the
invention and conventional air-flow dried material reached a
similar level of hardness. At the same time, the freeze-dried and
the conventional air-flow dried materials were characterised by a
high degree of b~. ..ess and a strongly perceptible foreign smell
and taste after rehydration. They were rated as unpalatable, with
an unnatural hue and smell. The hue ratings are confirmed in the
colour parameter measurements according to the Hunter scale (Table
4)-
ManqoThe conventional air-flow dried material received the highest
rating for mango smell with the lowest level of foreign smell,
slightly higher than the rating for material dried in accordance
with the invention. Like the freeze-dried material, the
conventional air-flow dried material was characterised by a low
degree of browness, comparable to that of the mango prior to
drying. The conventional air-flow dried material and the material
dried in accordance with the invention received similar hardness
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
. . .
wo 96/39854 PcTnEs6/00037
ratings, reflecting the nearly equal water content (6.3% and 6.8%
respectively). The product dried in accordance with the invention
had a much lower degree of brittleness.
Following rehydration, the differences between the various dried
materials became more noticeable. Thus, the material dried using
conventional air-flow drying had a higher level of foreign smell
and taste after rehydration, in addition to being harder and
lighter in colour than the material dried in accordance with the
invention. The rehydrated freeze-dried material, while retaining
a light hue comparable to that of the mango prior to drying, was
characterised by a particularly high level of foreign smell and
taste.
PineaPPle
Of the dried pineapples tested, the pineapple dried in accordance
15 with the invention was characterised by the highest organoleptic
ratings (Table 2c). In particular, it had an exceptionally strong
pineapple smell, similar to that for pineapple prior to drying,
with almost no foreign smell. In addition, it was characterised
by low brittleness.
20 Slices of rehydrated pineapple which had been dried in accordance
with the invention were characterised by a hardness close to that
of the slices of pineapple prior to drying. This feature is
important in the use of dried fruit for the production of
breakfast mixtures to which liquids, for example, milk are
25 normally added. Also in those slices of pineapple which were
rehydrated after being dried in accordance with the invention, the
most intense pineapple aroma was recorded. The highest level of
foreign smell, as in the case of mango, was noticed in the
rehydrate obtained from the freeze-dried material. These results
are in line with those acquired from the analysis of volatile
compounds as shown in Figs. 9 and 12.
SUBSTITUTE SHEET (RULE 26
CA 022221~3 1997-11-25
WO 96/39854 PCT~E96/00037
Kiwi
Kiwi slices dried in accordance with the invention had low
brittleness and a high level of kiwi flavour retention. The
freeze-dried material also had a high level of kiwi flavour
retention, but was otherwise light in colour and brittle.
After rehydration, the structure became too soft and a strong off-
flavour and odour developed in the freeze-dried and conventionally
air-flow dried materials. The kiwi dried in accordance with the
invention was superior to all others tested in colour retention
and was rated substantially better than the others in flavour and
odour after rehydration.
PaPava
The papaya slices dried by conventional air-flow drying received
generally average rankings, while after rehydration it received
low rankings. Papaya dried in accordance with the invention
displayed higher intensity of taste-flavour compounds typical for
papaya prior to drying, and the lowest presence of foreign taste
and off-flavour. The freeze-dried material received higher
rankings in general in the dry state, compared to conventional
air-flow dried material, while after rehydration the density of
compounds responsible for undesirable taste and odour
significantly increased. All papaya fruit products, both in dry
form and after rehydration, displayed fair colour with no
indication of browning. The rehydrated papaya which had been
dried in accordance with the invention received substantially
higher rankings than the papaya dried using the other processes.
Ginqer
The freeze-dried ginger slices displayed fair colour and a high
degree of brittleness. The highest level of ginger flavour with
almost no foreign smell was observed in material dried in
accordance with the invention. Relatively good quality was
displayed by the conventional air-flow dried material except for
significant browning which was reflected in the hue evaluation and
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
82
the general quality ranking. In all dried products, the density
of volatile compounds giving the typical ginger fragrance
decreased after rehydration, while the intensity of foreign odour
returned to the level typical for ginger prior to drying. The
5 rehydrated ginger which had been dried in accordance with the
invention received a slightly higher overall rating compared to
the rehydrated ginger which had been dried according to the other
techniques.
Hue
Table 4 presents the colour coordinates determined in the Hunter
L*a*b* apparatus for various materials before and after drying.
The materials dried in accordance with the invention exhibited
superior co10ur retention and colour stability after rehydration
in all cases.
Other Observations
CaPacitv for rehvdration
The highest capacity for water absorption (~ehyd)ation) was shown
by the dried papaya and ginger dried in accordance with the
invention. In the case of the kiwi, the conve..Lionally air-flow
20 dried material had a slightly higher rehydration capacity than the
material dried in accordance with the invention. The freeze-dried
material of the various fruits and tubers tested indicated the
lowest levels of rehydration capacity compared to the others.
Water activitv
25 The water activity measurements (Table 6) indicate that all the
tested dried materials were completely protected against
microbiological deterioration. Thus, these materials would not
require any additional treatment to ensure preservation, other
than packaging because of their high hygroscopicity. The
pineapple dried in accordance with the invention had the highest
water activity value, which is in line with the dry mass
measurements and the organoleptic assessments. The water activity
value for all of the tested materials dried in accordance with the
SUBSTITUTE SHEET (RULE 26)
. CA 022221~3 1997-11-2~
WO 96/39854 PCI'/IE96J00037
invention were less than 0.41, which indicates that they were
completely protected against microbiological decomposition.
Ascorbic acid content
The results of tests for the presence of ascorbic acid in the
materials before and after drying can be found in Table 7. For
the mango and the pineapple dried in accordance with the
invention, there were only insignificant losses of vitamin C
during processing. Thus, the mango dried in accordance with the
invention retained approximately 98% of the vitamin C content of
the mango prior to drying. Similarly, the pineapple dried in
accordance with the invention retained 92~. The mango and
pineapple slices dried by conventional air-flow drying exhibited
greater losses of vitamin C, 20% for the mango and 16% for the
pineapple. A comparison of the vitamin C losses in the materials
dried in accordance with the invention and freeze-dried materials
allows one to conclude that the proportion of vitamin C preserved
in the former is exceptionally high.
8ecause bananas contain a very high level of native enzymes from
the group of oxidases, drying brought about a very strong fall in
the content of ascorbic acid. The highest preservation rate of
ascorbic acid (about 15%) was found in the bananas dried in
accordance with the invention. For the freeze-dried bananas, the
preservation rate was about 14%. The conventionally air-flow
dried bananas had a vitamin C loss in the range of 98%, meaning
that there was nearly complete destruction of the vitamin C in
that material.
The kiwi dried in accordance with the invention had practically no
ascorbic acid losses. In the freeze-dried kiwi, 80% of the
vitamin C was preserved.
Presence of carotenoids and of beta-carotene
The results of the tests, given in Table 8, indicate that all
species of materials tested retain a similar high level of b-
SUBSTITUTE SHEET (RULE 26~
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
84
carotene and carotenoids. Thus, each of the drying processes
results in a small loss of these elements. Pineapple dried in
accordance with the invention demonstrated superior results in the
retention of b-carotene.
S Level of nitrates and nitrites
The level of nitrates and nitrites in the fresh and dried
materials tested is below the accepted maximum value given by
FAO/World Health Organisation and the Polish National Hygiene
Office. With respect to the presence of the above mentioned
compounds in these materials, they can be considered completely
acceptable for consumption by adults and children.
ComDosite Droducts usinq materials dried in accordance
with the invention
Materials dried in accordance with the invention can be added to
composite food preparations. For example, dried apple or banana
slices may be added to a mixture to be baked for making a cake.
Dried fruit slices as such or dried fruits in powder form may also
be advantageously used as additives to sugar syrup, jams,
marmalades, yogurt, sauces, for example, salad dressing,
20 gelatinous products, for example, a mixture of dry gelatine and
dried particles of fruits or herbs, and so on.
After drying, herbs, spices, vegetables and the like are
advantageously cut into small particles and are possibly mixed
with salts or other seasoning materials.
25 Use of the inventive Processes as a pre-treatment
Tests made on various dried materials, for example, apple slices,
have shown that the product of the invention had a higher vitamin
C content than freeze-dried apple slices. Because of this high
retention of vitamin C content, the process of the invention can
be used as a prior treatment so as to increase the concentration
of the structural components of the material being dried,
including vitamin C content, and to increase the efficiency of
SUBSTITUTE SHEET (RULE 26)
CA 022221~3 1997-11-2~
WO 96/39854 PCI~/IE96/00037
another process for which the material is to be used, without
significant degradation of those components. The process of the
invention can therefore be used, for example, for the drying of a
culture medium containing microorganisms so as to have dried
material with a higher concentration of the component sought in
the process.
Processes according to other embodiments of the invention can also
be carried out by means of the apparatus shown in Fig. 1, and this
will be readily apparent to those skilled in the art.
While the apparatus of Fig. 1 which has been described for
carrying out the dehydration process according to the invention,
provides for carrying out the dehydration processes of the
invention as batch processes, it will be readily apparent to those
skilled in the art that other suitable apparatus may be provided
so that the processes according to the invention may be carried
out as continuous processes.
It is also envisaged that it is not essential that the same drying
medium, subject to the addition of fresh drying medium be used in
the complete drying process. For example, it is envisaged that the
product may be conveyed on a production line through a number of
different drying chambers in which different drying media would be
used. However, in such cases, the temperature and relative
humidity of the drying media in general, would be regulated in the
various drying chambers so that the change in drying medium
temperature and relative humidity to which the product is
subjected in the transition from one drying chamber to another
would be gradual, and would be substantially similar to that
already described with reference to Figs. 6 and 7. Furthermore,
it is envisaged that where the product is transferred from one
drying chamber to another in a drying process, the drying medium
may be circulated a number of times through the respective drying
compartments, and then may subsequently be circulated to the next
drying chamber in sequence.
SUBSTITUTE SHEET (RULE 26
CA 022221~3 1997-11-2~
WO 96/39854 PCT/IE96/00037
It will of course be appreciated that the dehydrating processes
for dehydrating various fruits and tubers which have been
described above may be varied without departing from the scope of
the invention. The examples of the inventive drying process
S described have been given purely for the purpose of describing the
best method known for carrying out the process according to the
invention.
SUBSTITUTE SHEET (RULE 26