Note: Descriptions are shown in the official language in which they were submitted.
CA 02222281 1997-11-25
NSC-D844/PCT
- 1 -
DESCRIPTION
LOW-ALLOY HEAT-TREATED PEARLITIC STEEL RAIL EXCELLENT
~1~T WEAR RESISTANCE AND WELDABILITY AND PROCESS FOR
PRODUCING THE SAME
Field of the Invention
The present invention relates to a pearlitic steel
rail having the significantly improved wear resistance and
weldability (welding construction, properties of the
welded joints) that rails for heavy haul railways are
required to have, and a process for producing the same.
The present invention particularly relates to a rail in
which a difference in hardness between the welded joint of
the rail and the base rail can be controlled in a given
range so that a wear dent caused by the local wear of the
rail top surface in the welded joint can be prevented,
without impairing the weldability of the rail, and a
process for producing the rail.
Background of the Invention
Train speeds and a train loading are to be increased
as means for making railroad transportation highly
efficient. Such efficient railroad transportation
signifies that the conditions of using the rail will
become severe, and a further improvement in rail materials
is required. Specifically, rails laid overseas in curved
sections in rails for heavy haul railways have shown a
drastic increase in wear, and the wear has produced
concern about the wear life of the rails. However, as a
result of the recent progress in highly strengthening heat
treatment techniques, high strength (high hardness) rails,
as described below, in which an eutectoid carbon steel is
used and which have a fine pearlite structure have been
developed. Consequently, the life of rails in the curved
sections in rails for heavy haul railways has been greatly
improved.
CA 02222281 1997-11-25
- 2 -
(1) A heat treated rail for an ultraheavy loading
having a sorbite structure or fine pearlite structure in
the head portion (Japanese Examined Patent Publication
(Kokoku)) No. 54-25490); and
(2) A process for producing a high strength rail
which has a strength of at least 130 kgf/mm2, including the
step of subjecting a rail head portion which has been
finish rolled or repeated to accelerated cooling from
austenite region temperatures to temperatures from 850 to
500°C at a rate of 1 to 4°C/sec (Japanese Examined Patent
Publication (Kokoku)) No. 63-23244).
The features of these rails, that the rails are high
strength rails, result from their eutectoid carbon steel,
and the rails are intended to have a wear resistance by
reducing the lamellar spacing in the pearlite structure.
On the other hand, rail joints have been welded for
the purpose of preventing failure and reducing the cost of
maintenance and control. Consequently, actual rails have
been used as.long rails. However, it is known that, in a
welded joint which has been repeated to the austenite
region, the cooling rate of the welded joint subsequent to
welding is slow compared with that of the heat treated
head portion during production of the rail, and that, as a
result, the hardness of the joint after welding is lowered
to form a softened portion. The softened portion tends to
suffer local wear when in contact with train wheels,
resulting in a wear dent on the rail top surface.
Consequently, serious problems have been caused by the
production of noise and the generation of vibration due to
the passage of trains, and the problems include
deterioration of the railroads.
The following method has been used as the
countermeasure:
the welded joint of a rail is subjected to accelerated
cooling heat treatment immediately after welding or after
repeating so that the hardness is improved to about the
same degree as the rail base steel. However, there arises
CA 02222281 1997-11-25
- 3 -
a new problem, due to the method, in that the welding
operation time becomes long and the operation efficiency
is decreased. Accordingly, the welded joint, of a rail is
prevented from forming a softened portion in an as-welded
state as described below. As a result, it has become
possible to improve not only the wear life but also the
weldability (welding construction).
(3) A process for producing a low alloy heat treated
rail having an improved wear resistance and an improved
weldability (welding construction, properties of welded
joint) by adding alloying elements such as Cr, Nb, etc.
(Japanese Examined Patent Publication (Kokoku)) No. 59-
19173)
However, in order to carry out still more highly
efficient railroad transportation, on rails for heavy haul
railways, in recent years, heavy loading freight has
increased. Consequently, even when the rails developed as
described above are used, ensuring the wear resistance and
perfect prevention of the wear dent of the head top
surface of the rails caused by local wear in the welded
joints cannot be achieved due to an increase in the
contact pressure of the wheels. As a result, a decrease
in life, production of noise and generation of vibration
in the welded joints and deterioration of railroads have
become serious problems again.
From such a background, development of a wear-
resistant rail as wear-resistant as the current high
strength rail which is prepared from an eutectoid carbon
steel, capable of preventing local wear in the as-welded
joints, and excellent in weldability have come to be
required.
In order to improve the wear resistance of the
conventional rail steel having a pearlite structure with a
carbon content corresponding to a eutectoid carbon steel,
a method of improving the hardness by reducing the
lamellar spacings in the pearlite structure has been
employed.
CA 02222281 1997-11-25
- 4 -
However, the current rail steel having the pearlite
structure with a carbon content corresponding to the
eutectoid carbon steel shows an upper limit Vickers
hardness of 420. lnThen the heat treatment cooling rate or
the addition amounts of alloying elements are increased
for the purpose of improving the hardness, a bainite or
martensite structure is formed in the pearlite structure
to cause a problem that the wear resistance and toughness
of the rail are lowered.
Furthermore, a method of using a material having a
metal structure showing a higher wear resistance than the
pearlite structure as a rail steel may be recognized as
another method for overcoming the problem. However, a
material which costs less and which is more excellent in
wear resistance than the fine pearlite structure in the
rolling wear conditions such as those of rails and wheels
has not been found at present.
The pearlite structure having a carbon component
corresponding to a eutectoid carbon steel, of the
conventional rail steel, has a lamellar structure of a
ferrite phase having a low hardness and a harder cementite
phase. As a result of analyzing the wear mechanism of the
pearlite structure, the present inventors have confirmed
that the wear resistance is ensured in the following
manner: a soft ferrite phase is squeezed out at first by
wheels passing the rails; and then a hard cementite phase
alone is laminated directly under rolling surfaces.
The present inventors then have experimentally
discovered that the wear resistance can be greatly
improved by increasing the ratio of a harder cementite in
the pearlite, which improves the hardness of the pearlite
to effect the wear resistance and which increases the
carbon content to ensure the wear resistance so that the
cementite phase density directly under a rolling surface
is increased.
Fig. 1 shows an experimental comparison of the
relationship between a carbon content and a wear amount.
CA 02222281 1997-11-25
- 5 -
The wear amount decreases as the carbon content increases
when the hardness of the steel materials is the same. It
has thus been confirmed that the use of a high carbon
steel (hyper-eutectoid steel) greatly improves the wear
resistance compared with that of conventional eutectoid
steels (carbon content of 0.7 to 0.8~).
Paying attention to the influence of the carbon
content on the pearlite transformation characteristics,
the present inventors have invented a heat treatment
method for stably forming a pearlite structure in high
carbon steel materials. Fig. 2 shows the relationship
between the carbon content and the pearlite transformation
characteristics with reference to a continuous cooling
transformation diagram (CCT diagram). It has been
confirmed that when the carbon content is increased, the
pearlite transformation nose shifts to a shorter time side
compared with conventional eutectoid steels (carbon
content of 0.7 to 0.8~) so that pearlite transformation
easily takes~place in a high cooling rate range.
That is, the heat treatment method to be noted of the
rail steel having a high carbon content (hyper-eutectoid
steel) is as follows: the present inventors have
discovered that even when the accelerated cooling rate of
the heat treatment is increased further compared with
conventional eutectoid steels, abnormal structures such as
martensite are not formed, and that the pearlite structure
is stably formed so that the rail steel may attain a high
strength.
Furthermore, it has been discovered that formation of
proeutectoid cementite detrimental to the ductility and
toughness, a disadvantage in a high carbon steel (hyper-
eutectoid steel), may be prevented by the accelerated
cooling of the heat treatment for highly strengthening the
steel, and that the wear resistance may be improved
without impairing the ductility and toughness by producing
a high carbon content steel.
CA 02222281 1997-11-25
- 6 -
Summary of the Invention __
In addition to the former invention, the present
inventors have investigated a method for preventing a wear
dent caused by the local wear of the head top surface of a
welded joint without impairing the weldability (welding
construction). In order to prevent the local wear of the
rail welded joint, the difference in hardness between the
welded joint having been reheated to the austenite region
and the base steel must be made as small as possible.
First, the present inventors have experimentally
investigated the influence of addition elements on the
hardness of the welded joint of a high carbon steel
(hyper-eutectoid steel). Consequently, the present
inventors have confirmed that the addition amount of Cr
and that of Si influence the hardness of the welded joint
of the high carbon steel (hyper eutectoid steel) though Si
is not so effective as Cr, and discovered that controlling
the addition amounts can prevent a decrease in the
hardness of the welded joint.
The present inventors, therefore, have used high
carbon steels (hyper-eutectoid steels) in which the
addition amount of Cr, an element most effective in
preventing a decrease in the hardness of the welded joint,
is varied, and experimentally analyzed the relationship
between the addition amount of Cr and the hardness of the
as-welded (without heat treatment) rail welded joint. As
a result, the hardness of the welded joint has been
improved when the addition amount of Cr exceeds 0.50, and
a hardness comparable to that of the base rail has been
ensured.
Furthermore, since the carbon content of the hyper-
eutectoid steel is high, Mn, Cr, etc. are segregated in
addition to C in the column portion of the rail, and a
martensite structure detrimental to the toughness of the
rail tends to form in the segregation portion. The
present inventors, therefore, have examined a method for
restricting the addition amounts of-Mn and Cr to given
CA 02222281 1997-11-25
ranges to diminish the segregation, and preventing a
decrease in the hardness of the welded joint.
Consequently, they have discovered that in high carbon
steels (hyper-eutectoid steels), controlling the addition
amount of Si which substantially does not segregate, in
the rail column portion, results in prevention of a
decrease in the hardness of the welded joint, in the same
manner as Cr.
Accordingly, the present inventors have
experimentally analyzed the relationship between the
addition amount of Si and the hardness of an as-welded
joint of the rail (without heat treatment) for the purpose
of preventing segregation using high carbon steels (hyper-
eutectoid steels) in which the addition amount of Si has
been varied while the addition amounts of Mn and Cr have
been restricted to given ranges. As a result, when the
addition amount of Si exceeds 0.40, the hardness of the
welded joint has been improved even with the addition
amounts of Mn and Cr being in small ranges, and a hardness
which is comparable to the base rail has been ensured.
From the experimental results, the present inventors
have found the following method effective as a method for
making the difference in hardness between the welded joint
- having been reheated to the austenite region and the base
steel as small as possible: in variety of rail base steels
produced under heat treatment conditions within the claims
mentioned above, the addition amounts of the alloying
elements Cr, Mn and Si principally influence the hardness,
and control of the addition amount of Mn in addition to
the control of the addition amounts of Cr and Si which are
effective in ensuring the hardness of the welded joints is
effective in controlling the difference in hardness
between the base steels and the respective welded joints.
The present inventors, therefore, have investigated the
relationship between the difference in hardness between
the rail base steel and the welded joint and the addition
amounts of Si, Cr and Mn. The contribution of Cr to the
CA 02222281 1997-11-25
_ g _
hardness is defined as 1, and the results are arranged to
derive the contribution of the three elements thereto.
Consequently, when the addition amounts of Si, Mn and Cr
are the same, the contribution of the elements are
confirmed to be as follows: Si: 1/4, Mn: 1/2. That is, in
order to allow the difference in hardness between the
welded joint and the base rail to fall into a given range
so that a collapse caused by the local wear of the head
top surface of the welded joint is prevented without
impairing the weldability (welding construction), it has
been found that the total sum Cr (wt.~), Si (wt.~)/4 and
Mn (wt.~)/2 must be in a certain range.
An object of the present invention is to provide a
rail having significantly improved wear resistance and
weldability (welding construction, properties of the
welded joints), which rails for heavy haul railways are
required to have, by the above investigation.
The present invention achieves the object mentioned
above, and the subject matter of the present invention is
a pearlitic steel rail excellent in wear
resistance and weldability obtained as described below:
the head portion of a hot rolled steel rail
having a high temperature thermal energy or steel rail
heated to high temperature for the purpose of heat
treatment which steel rail comprises, in terms of weight,
more than 0.85 to 1.20 of C, 0.10 to 1.00 of
Si, 0.20 to 1_50 of Mn, more than 0.50 to 1.00 of Cr, or
more than 0.85 to 1.20 of C, 0.40 to 1.00 of
Si, 0.20 to less than 0.40 of Mn, 0.35 to 0.50 of Cr,
the content sum Si/4 + Mn/2 + Cr being 0.8 to 1.8~ in
terms of weight,
and further comprises, in terms of weight, one or at least
two elements selected from the group consisting of
0.01 to 0.20 of Mo, 0.02 to 0.30 of V, 0.002
to 0.050 of I~'b, 0.10 to 2.00 of Co and 0.0005 to 0.005
of B,
and the balance Fe and unavoidable impurities,
CA 02222281 1997-11-25
g
is subjected to accelerated cooling and
controlled cooling by any of the following methods: (1)
the head portion is subjected to accelerated cooling from
an austenite region temperature at a cooling rate of 1 to
10°C/sec, and the accelerated cooling is stopped at the
time when the steel rail temperature reaches from 700 to
500°C; (2) the head portion is subjected to accelerated
cooling from an austenite region temperature at a cooling
rate of more than 10 to 30°C/sec, and the accelerated
cooling is stopped at the time when the pearlite
transformation of the steel rail proceeds in an amount of
70~ of the entire transformation; and (3) the head portion
is subjected to accelerated cooling from an austenite
region. temperature to a temperature from 750 to 600°C at a
cooling rate of more than 10 to 30°C/sec, and is
consecutively subjected to controlled cooling at a cooling
rate of 1 to less than 10°C/sec in a temperature region
from 750 to 600°C to 550 to 450°C;
the steel rail having a pearlite structure to
the depth of at least 20 mm from the head portion corner
and the head top surface as a starting point, the pearlite
structure having a Vickers hardness of at least 320, and
the difference in Vickers hardness between the base steel
and the welded joint of the steel rail being up to 30, and
a process for producing the same.
Brief Description of the Drawings
Fig. 1 is a graph showing the relationship between a
carbon content and a wear amount.
Fig. 2 is a graph showing the relationship between a
carbon content and pearlite transformation
characteristics.
Fig. 3 is a view showing the names of the cross-
sectional surface positions of a rail head portion, and
the reference numerals 1 and 2 designate a head top
portion and a head portion corner, respectively.
Fig. 4 is a schematic view of a Nishihara wear
CA 02222281 1997-11-25
- 10 -
tester, and the reference numerals 3, 4 and 5 designate a
rail test piece, a counterpart material and a cooling
nozzle, respectively.
Fig. 5 is a graph showing the relationship between a
hardness and a wear amount in Example 1.
Fig. 6 is a graph showing the hardness distribution
of a head portion in a welded joint in Example 1.
Fig. 7 is a graph showing the relationship between a
hardness and a wear amount in Example 2_
Fig. 8 is a graph showing the hardness distribution
of a head portion in a welded joint in Example 2.
Fig. 9 is a graph showing the relationship between a
hardness and a wear amount in Example 3.
Fig. 10 is a graph showing the hardness distribution
of a head portion in a welded joint in Example 3.
Fig. 11 is a graph showing another relationship
between a hardness and a wear amount in Example 3.
Fig. 12 is a graph showing another hardness
distribution of a head portion in a welded joint in
Example 2.
Best Mode for Carrying Out the Invention
When compared with conventional rail steels, the rail
steel of the present invention has a high carbon content,
shows a decreased wear amount at the same hardness, and
has a greatly improved wear resistance. Moreover, a
pearlite structure excellent in the wear resistance can be
stably formed without forming martensite, bainite and
proeutectoid cementite detrimental to the ductility,
toughness and wear resistance of the rail by making the
chemical composition fall into an appropriate range and
selecting appropriate heat treatment conditions.
Furthermore, a decrease in the hardness on the weld
line caused by decarburization is improved, and abnormal
structures such as martensite are not formed in the welded
joint (portion reheated to the austenite region). The
difference in Vickers hardness between the welded joint
CA 02222281 2001-04-02
- 11 -
and the base steel is up to 30, and as a result partial
wear such as a local wear dent caused by the wear of the
head top surface of the welded joint in an as-welded state
(without heat treatment) can be prevented.
According to the present invention, rails excellent
in wear resistance and weldability (welding construction,
properties of welded joints) may be provided to rails for
heavy load.
The present invention will be explained in detail.
Reasons for restricting the range of the chemical
composition, that of the pearlite structure and the
hardness will be explained in detail.
(1) On Chemical Composition
First, reasons for restricting the chemical
composition of the rail in the present invention as
described above will be explained.
C is an element which is effective in promoting
pearlite transformation and ensuring wear resistance. C
is added to the conventional rail steel in an amount of
0.60 to 0.85. However, when the C amount is up to 0.85,
the cementite phase density in the pearlite structure for
improving the wear resistance cannot be ensured, and
moreover intergranular ferrite which is to become the
starting point of a fatigue failure within the rail head
portion tends to form. Furthermore, when the C amount
exceeds 1.20, much proeutectoid cementite is formed in
the rail head portion after heat treatment to lower the
ductility and toughness considerably. The C amount is,
therefore, restricted to more than 0.85 but less than
1.20.
Si is an element which improves the hardness
(strength) of a rail base steel and the welded joint
reheated to the austenite region, by solid-solution
hardening the ferrite phase in a pearlite structure. Si
is also an element which is concentrated at boundaries
between ferrite and cementite in the pearlite structure,
CA 02222281 1997-11-25
- 12 -
and the Si concentrated zone inhibits spheroidization of
cementite in a heat-affected zone repeated to temperatures
up to the austenite region temperature during welding. As
a result, Si suppresses a decrease in the hardness of the
heat-affected zone, that is, Si increases the temper
softening resistance of the steel. v~lhen the Si amount is
less than 0.10, such effects cannot be expected.
Moreover, when the Si amount exceeds 1.00, many surface
defects are formed during hot rolling. Moreover the rail
is embrittled, and the weldability is lowered. The Si
amrn mt is. therefore, restricted to 0.10 to 1.00. In
addition, in the chemical composition system of a rail in
which the amounts of Mn and Cr are restricted to certain
values to decrease segregation in the rail column portion,
it is necessary that the Si amount be restricted to at
least 0.40 to ensure the hardness of the rail base steel
and the welded joints.
Mn is an element which contributes to the high
hardness (strength) of the rail steel by lowering the
pearlite transformation temperature and increasing the
hardenability, and moreover which inhibits the formation
of proeutectoid cementite. when the Mn amount is less
than 0.20, the effect is not significant, and
proeutectoid cementite tends to form in the rail head
portion after heat treatment. Furthermore, when the Mn
amount exceeds 1.50, a martensite structure detrimental
to the toughness of the rail tends to form. Accordingly,
the Mn amount is restricted to 0.20 to 1.50~.~ In
addition, in order to diminish the segregation of the rail
column portion and inhibit the formation of a martensite
structure detrimental to the toughness of the rail, the Mn
amount must be from 0.20 to less than 0.40.
Cr is an element which raises the equilibrium
transformation point of pearlite and as a result makes the
pearlite structure fine. Cr thus makes the rail base
steel have a high hardness (strength), improves the
hardness of the welded joint repeated to the austenite
CA 02222281 1997-11-25
- 13 -
temperature region, and makes a difference in hardness
between the rail base steel and the welded joint small.
Moreover, Cr is an element which forms Cr carbides, and
thus strengthens the cementite in the pearlite structure.
As a result, Cr not only improves the wear resistance but
also inhibits the softening of cementite in the heat
affected zone having been reheated to temperatures up to
the austenite region temperature during welding. Although
the rail base steel may be highly strengthened when the Cr
amount is less than 0.50, the hardness of the welded
joint cannot be ensured satisfactorily, and a difference
in hardness between the rail base steel and the welded
joint becomes significant. As a result, a wear dent is
formed in the welded joint due to local wear.
Furthermore, when Cr is added in an amount exceeding
1.00, namely in an excessive amount, a bainite structure
and a martensite structure are formed to lower the wear
resistance and toughness of the rail. Accordingly, the
addition amount of Cr is restricted to 0.50 to 1.00. In
addition, in order to diminish segregation of the rail
column portion and inhibit formation of a martensite
structure detrimental to the toughness of the rail, the
addition amount of Cr can be restricted to 0.20 to less
than 0.40 by adding Si in a large amount.
Furthermore, for the purpose of improving the
strength, ductility and toughness of the rail which is to
be produced with the chemical composition as mentioned
above, one or at least two elements selected from the
following elements are optionally added:
Mo: 0.01 to 0.20, V: 0.02 to 0.30$, Nb: 0.002 to
0.050, Co: 0.10 to 2.00 and B: 0.0005 to 0.005.
Next, the reasons for defining the chemical
composition as mentioned above will be explained.
Mo is similar to Cr in that it raises the equilibrium
transformation point of pearlite and as a result makes the
pearlite structure fine to contribute to highly
strengthening the rail steel and improve the wear
CA 02222281 1997-11-25
- 14 -
resistance. When the addition amount is less than 0.01,
the effect is not significant. G~Then Mo is added in an
amount exceeding 0.20, namely in an excessive amount, Mo
lowers the transformation rate of pearlite, and as a
result tends to form a martensite structure detrimental to
the toughness. Accordingly, the addition amount of Mo is
restricted to 0.01 to 0.20.
V is a constituent effective in increasing the
strength by precipitation hardening with V carbonitrides
formed in the course of cooling during hot rolling, making
austenite grains fine by the action of inhibiting the
grain growth during heat treatment by heating the steel to
high temperatures, and consequently improving the
strength, ductility and toughness required of the rail.
However, the effect cannot be sufficiently expected when
the addition amount is less than 0.02. Further effect
cannot be expected when V is added in an amount exceeding
0.30. The addition amount of V is, therefore, restricted
to 0 . 02 to 0 ~. 3 0~ .
Nb is similar to V in that it is an element effective
in making austenite grains fine by forming Nb
carbonitrides. Nb exerts an influence on inhibiting
austenite grain growth at higher temperatures close to
1,200°C than V, and improves the ductility and toughness
of the rail. The effect cannot be expected when the
addition amount is less than 0.002. Moreover, even when
lib is added in an amount exceeding 0.050, namely in an
excessive amount, a further effect cannot be expected.
Accordingly, the addition amount of Nb is restricted to
0.002 to 0.050.
Co is an element which improves the strength by
increasing the transformation energy of pearlite to make
the pearlite structure fine. However, the effect cannot
be expected when the addition amount is less than 0.10.
When Co is added in an amount exceeding 2.OO~S, namely in
an excessive amount, the effect is saturated.
Accordingly, the addition amount of Co is restricted to
CA 02222281 1997-11-25
- 15 -
0.10 to 2.00.
B is an element which has an effect on inhibiting the
formation of proeutectoid cementite from former austenite
grain boundaries, and which is effective in stably forming
a pearlite structure. However, when the addition amount.
is less than 0.0005, the effect is weak. Tn~hen B is added
in an amount exceeding 0.0050, coarse boron carbides are
formed to deteriorate the ductility and toughness of the
rail. Accordingly, the addition amount is restricted to
0.0005 to 0.0050.
Furthermore, reasons for restricting the total
content sum Si/4 + Mn/2 + Cr to 0.8 to 1.8~ in terms of ~
by weight will be explained. When the total sum Si/4 +
Mn/2 + Cr is less than 0.8~ in terms of ~ by weight, the
welded joint hardness of the rail greatly lowers, compared
with the base-steel, subsequent to welding such as
resistance flash butt welding, and the difference in
hardness between the welded joint and the base steel
increases, resulting in that the difference in Vickers
hardness of up to 30, a condition under which local wear
of the rail at the head top surface of the welded joint
can be prevented, cannot be satisfied. Moreover, when the
total sum Si/4 + Mn/2 + Cr in terms of ~ by weight exceeds
1.8~, the welded joint hardness of the rail significantly
increases compared with the base steel, and the difference
in Vickers hardness of up to 30, a condition under which
local wear of the rail at the head top surface of the
welded joint can be prevented, cannot be satisfied. In
addition to the unsatisfactoriness, abnormal structures
such as martensite are formed in the welded joint, and the
toughness and fatigue strength of the rail welded joint
greatly lowers. Accordingly, the total content sum Si/4 +
Mn/2 + Cr is restricted to 0.8 to 1.8~. In addition, in
order to prevent segregation in the rail column portion,
in a constituent system in which the addition amounts of
Mn and Cr are reduced and Si is added in a large amount to
prevent the segregation of the rail column portion,
CA 02222281 1997-11-25
- 16 -
abnormal structures such as martensite are not formed in
the welded joint even when Mn, Cr and Si are added to the
upper limits, and the difference in Vickers hardness
between the welded joint and the base steel does not
exceed 30. Accordingly, the total content sum Si/4 + Mn/2
+ Cr is restricted to up to 0.95.
The rail steel having such a chemical composition as
described above is prepared in a conventional melting
furnace such as a converter or electric furnace. The
resultant molten steel is subjected to ingot making and
blooming, or continuous casting, and the resultant steel
product is then hot rolled to give a rail. The hot rolled
rail at high temperature having thermal energy or a rail
heated to high temperature for the purpose of heat
treatment is subjected to accelerated cooling in the head
portion to improve the hardness of the pearlite structure
of the rail head portion.
(2) Hardness of Pearlite structure and Its Range
First, .reasons for restricting the Vickers hardness
of the pearlite structure to at least 320 will be
explained. When the Vickers hardness is less than 320,
the following problems arise: ensuring the wear
resistance, which rails for heavy haul railways are
required to have, in the present constituent system
becomes difficult; moreover, a metal flow is formed as due
to a heavy contact between the rail and wheels in the rail
gauge corner (G.C.) in a sharply curved section, and
consequently surface defects such as a head check or
flaking is formed. Accordingly, the Vickers hardness of
the pearlite structure is restricted to at least 320.
Furthermore, reasons for restricting the range of the
pearlite structure having a Vickers hardness of at least
320 to the depth of 20 mm from the head portion corner and
the head top surface as a starting point will be
explained. When the depth is less than 20 mm, the depth
is small as a wear-resistant region which the rail head
portion is required to have, and an effect of sufficiently
CA 02222281 1997-11-25
- 17 -
improving the rail life cannot be obtained as the wear of
the rail proceeds. Moreover, when the range of the
pearlite structure mentionecL above is to the depth of at
least 30 mm from the head portion corner and the head top
surface as a starting point, the effect of improving the
rail life is further improved. Accordingly, the range is
desirable.
Fig. 3 shows the names of the cross-sectional surface
positions of the rail head portion in the present
invention excellent in wear resistance and weldability.
In the rail head portion, the reference numerals 1 and 2
designate a head top portion and head portion corners,
respectively. One of the head portion corners 2 is a
gauge corner (G. C.) to be m~iinly contacted with wheels.
Next, reasons for restricting the difference in
Vickers hardness to up to 3U between the rail base steel
and the welded joint will be explained. When the
difference in Vickers hardness between the welded joint
and the base steel exceeds 30, partial wear such as a wear
dent is formed on the head trop surface of the rail welded
joint_ As a result, a noise is produced and vibrations
are generated when a train passes, and the deteri-oration
of the railroad track markedly proceeds. Accordingly, the
difference in Vickers hardness between the welded joint
and the base steel is restr:~cted to up to 30. In
addition, the difference in hardness is restricted to a
difference of the head port:i.on hardness distribution
between the rail welded joint reheated to the austenite
region and the base steel. The difference in hardness
does not signify a difference of the hardness between the
heat-affected zone formed around the welded joint or a
hardness-lowered region formed by decarburization on the
welded line, and the base steel. Moreover, the difference
in hardness is principally ;gin absolute value of a decrease
in the hardness of the welded joint from that of the base
steel. The hardness of the welded joint sometimes becomes
high to some degree compared with the base steel,
CA 02222281 1997-11-25
a
- 18 -
depending on the constituent. system and welding
conditions. However, since the high hardness to such a
degree does not much influence the properties of the
welded joint in the present invention, the difference in
hardness is made when the hardness of the welded joint is
low compared with that of the base steel, or when the
hardness of the welded joint is high compared therewith.
(3) On production conditions
Reasons for restricting' each of the cooling
conditions, as mentioned above during the rail production,
in claims 4 to 6 will be explained in detail.
In claim 4, the steel rail is subjected to
accelerated cooling from an austenite region temperature
at a cooling rate of 1 to 10°C/sec, and the cooling is
stopped when the steel rail temperature reaches from 700
to 500°C. Reasons for restr_Lcting the cooling conditions
will be explained. In addition, the cooling conditions
are heat treatment production conditions in which air or a
mixture containing air mainly and mist is used as a
cooling medium.
First, in the step of subjecting the steel rail to
accelerated cooling from an austenite region temperature
to 700 to 500°C at a cooling rate of 1 to 10°C/sec,
reasons for restricting the <~ccelerated cooling stop
temperature and the accelerated cooling rate as mentioned
above will be explained.
When the accelerated co~~ling is stopped at a
temperature exceeding 700°C, pearlite transformation
starts immediately after the accelerated cooling, and a
large amount of a pearlite si:ructure having a low hardness
is formed. As a result, the Vickers hardness of the rail
head portion becomes less th~.n 320, and a necessary wear
resistance cannot be ensured.. Accordingly, the
accelerated cooling stop temperature. is restricted to up
to 700°C. Moreover, when accelerated cooling is conducted
to a temperature less than 500°C, sufficient recuperation
cannot be expected from the interior of the rail after
CA 02222281 1997-11-25
v
- 19 -
accelerated cooling, and a martensite structure
detrimental to the toughness and wear resistance of the
rail is formed in the segregation portion. Accordingly,
the accelerated cooling stop temperature is restricted to
be at least 500°C.
Reasons for restricting the accelerated cooling rate
to from 1 to 10°C/sec will be explained.
When the accelerated cooling rate becomes less than
1°C/sec, pearlite transformation starts in a high
temperature region in the course of the accelerated
cooling, and a pearlite structure having a low hardness is
formed in a large amount. Consequently, the rail head
portion has a Vickers hardness of less than 320, and a
necessary wear resistance cannot be ensured. Moreover,
proeutectoid cementite detrimental to the toughness and
ductility of the rail is formed in a large amount.
Accordingly, the accelerated cooling rate is restricted to
at least 1°C/sec. Furthermore, when air or a medium
containing air, mainly, mist, etc. which media have the
least cost and have stabilized properties in the heat
treatment production, is used as a cooling medium, a
cooling rate exceeding 10°C/sec cannot be ensured stably.
Accordingly, the accelerated cooling rate is restricted to
- from 1 to 10°C/sec.
In addition, the accelerated cooling rate is defined
as an average cooling rate from the start of cooling to
the completion thereof. A temporary temperature rise may
sometimes be caused by the heat generation of pearlite
transformation or natural recuperation from the interior
of the rail in the course of the accelerated, cooling.
However, when the average cooling rate from start to
completion of the accelerated cooling is within the range
as mentioned above, no significant influence is exerted on
the properties of the pearlitic steel rail of the present
invention. The accelerated cooling conditions for the
rail of the invention, therefore, include a decrease in
the cooling rate resulting from temporary temperature rise
CA 02222281 1997-11-25
- 20 -
in the course of cooling.
A given cooling rate of 1 to 10°C/sec may be obtained
with air or a cooling medium containing air, mainly mist
and the like, or a combination of air and the cooling
medium.
Accordingly, in order to produce a rail having a
pearlite structure with a Vickers hardness of at least 320
and excellent in wear resistance and weldability, the head
portion of a steel rail is subjected to accelerated
cooling from an austenite region temperature at a cooling
rate of 1 to 10°C/sec with air, or a cooling medium
containing air mainly, mist, etc., and the accelerated
cooling is stopped at the time when the steel rail
temperature reaches from 700 to 500°C so that in the rail
head, formation of a pearlite structure having a low
hardness is prevented and abnormal structures such as a
proeutectoid cementite structure and a martensite
structure detrimental to the ductility, toughness and wear
resistance are not formed. As a result, a pearlite
structure having a high hardness can be stably formed.
Although a pearlite structure is desirable as the
metal structure of the rail, a trace amount of
proeutectoid cementite is sometimes formed therein
depending on the constituent system, the accelerated
cooling rate and the segregation state of the steel
material. However, even when a trace amount of
proeutectoid cementite is formed therein, no significant
influence is exerted on the ductility, toughness, wear
resistance and strength by the proeutectoid cementite.
The structure of the pearlitic steel rail of the invention
may, therefore, include a proeutectoid cementite structure
to a low degree.
Reasons for determining the following cooling
conditions in claim 5 will be explained: the head portion
of a steel rail is subjected to accelerated cooling from
an austenite temperature region at a cooling rate of more
than 10 to 30°C/sec; and the accelerated cooling is
CA 02222281 1997-11-25
- 21 -
stopped at the time when the pearlite transformation of
the steel rail proceeds in an amount of 70~ of the entire
transformation. In addition, the present cooling
conditions are heat treatment production conditions in
which a cooling medium mainly containing water such as
mist or water spray, is used.
As shown in Fig. 2 mentioned above, when the
accelerated cooling rate is up to 10°C/sec, a cooling
curve invariably passes the pearlite nose, and the
pearlite transformation is completed during continuous
cooling in most cases_ However, when the accelerated
cooling rate exceeds 10°C/sec, the cooling curve is found
to pass the pearlite transformation nose only when steels
containing at least a certain amount of C are used.
Furthermore, when the accelerated cooling rate exceeds
10°C/sec, continuation of cooling to temperatures as low
as up to 300°C results in the formation of a large amount
of a martensite structure in the pearlite structure.
Consequently, adverse effects are exerted on the wear
resistance and toughness of the rail.
However, when the steel rail is cooled in such a high
cooling rate range, the supercooling degree becomes
significant during pearlite transformation. lnThen the
pearlite transformation proceeds in a certain amount in
the course of cooling, heat generation of pearlite
transformation and natural recuperation from the interior
of the rail head portion are caused by stopping the
accelerated cooling in a certain temperature region in the
course of transformation, and a state similar to
isothermal transformation temporarily results.
Consequently, pearlite transformation can be completed in
the entire rail head portion.
Detailed experiments have been conducted, and it has
been confirmed that in order to complete the pearlite
transformation in the rail head portion by utilizing the
heat of pearlite transformation and the natural
recuperation from the interior of the rail head portion
CA 02222281 1997-11-25
- 22 -
after the accelerated cooling, the minimum necessary
amount of pearlite transformation is at least 70~ of the
entire transformation.
The concept of the above-mentioned production process
is shown on a continuous cooling transformation diagram
(CCT diagram) by taking a steel containing 1.0~ of C in
Fig. 2 as an example. In the example, the steel is
subjected to accelerated cooling (more than 10 to
30°C/sec) from the austenite region, and the accelerated
cooling is stopped when the amount of pearlite
transformation becomes at least 75~ of the entire
transformation. The cooling rate becomes up to 10°C/sec
after stopping the accelerated cooling due to the heat
generation of pearlite transformation and the natural
recuperation of the rail itself. The pearlite
transformation can thus be stably accomplished.
First, reasons for restricting the accelerated
cooling rate to more than 10 to 30°C/sec will be
' explained.
Ti~hen the steel rail is cooled with a cooling medium
other than air mainly containing water such as mist and
spray water at an accelerated cooling rate of up to
10°C/sec, the cooling stability becomes very poor in such
a low cooling rate region due to the very high cooling
ability, and the cooling control becomes very difficult.
Moreover, the hardness varies in the unstabilized cooling
portion, and stably adjusting the Vickers hardness of the
rail head portion to at least 320 becomes difficult.
Accordingly, the accelerated cooling rate is restricted to
more than 10°C/sec. Furthermore, as shown in the
continuous cooling transformation diagram (CCT diagram) in
Fig. 2, when the accelerated cooling rate exceeds
30°C/sec, the cooling curve does not cross the pearlite
transformation nose sufficiently during the accelerated
3.5 cooling, and a martensite structure detrimental to the
toughness and wear resistance of the rail is formed.
Moreover, even when the cooling curve crosses the pearlite
CA 02222281 1997-11-25
- 23 -
transformation nose to some degree during the accelerated
cooling, a pearlite transformation corresponding to at
least 70~ of the entire transformation cannot be expected
and, as a result, the pearlite transformation is not
completed in the whole rail head portion. A martensite
structure detrimental to the toughness and wear resistance
of the rail is subsequently formed. Accordingly, the
accelerated cooling rate is restricted to more than 10 to
30°C/sec.
In addition, the accelerated cooling rate is defined
as an average cooling rate from the start of cooling to
the completion thereof. A temporary temperature rise may
sometimes be caused by the heat generation of pearlite
transformation or natural recuperation from the interior
of the rail in the course of the accelerated cooling.
However, when the average cooling rate from start to
completion of the accelerated cooling is within the range
as mentioned above, no significant influence is exerted on
the properties of the pearlitic steel rail of the present
invention. The accelerated cooling conditions for the
rail of the invention include a decrease in. the cooling
rate resulting from the temporary temperature rise in the
course of cooling.
Furthermore, methods for obtaining a predetermined
cooling rate in the range of more than 10 to 30°C/sec are
as follows: injection cooling with a mixture of water and
air or a combination of these; the rail head portion or
the entire rail is immersed in oil, hot water, a mixture
of polymer and water or a salt bath.
Next, reasons for stopping the accelerated cooling at
the time when 70~ of the entire pearlite transformation
proceeds will be explained.
If up to 70~ of the entire pearlite transformation
proceeds when the accelerated cooling is finished, the
amount of heat generation caused by pearlite
transformation subsequent to stopping cooling is small,
and as a result the pearlite transformation cannot be
CA 02222281 1997-11-25
- 24 -
completed in the entire rail head portion. Consequently,
a large amount of martensite is formed within the rail
head portion. Moreover, when micro-segregation portions
exist within the rail head portion, the portions are
further cooled without the transformation, resulting in
the formation of island-like portions here and there each
having a martensite structure. The toughness and wear
resistance of the rail are thus lowered considerably. The
progress of the pearlite transformation at the time when
the accelerated cooling is stopped is, therefore,
restricted to at least 70~.
The progress of the pearlite transformation can be
estimated from the temperature change of the rail head
portion during the accelerated cooling. When the pearlite
transformation starts, a distinct heat generation region
resulting from the transformation is observed. According
to detailed experiments, the state of the rail immediately
before finishing the temperature rise in the heat
generation region corresponds to the temperature at which
70~ of the entire pearlite transformation is completed.
In addition, as a simple method for controlling the
transformation amount, controlling the amount mainly by a
cooling time during accelerated cooling is most desirable.
Accordingly, in order to produce a rail having a
pearlite structure with a Vickers hardness of at least 320
and excellent in wear resistance and weldability, the rail
head portion is subjected to accelerated cooling at a
cooling rate of more than 10 and up to 30°C/sec with a
cooling medium other than air mainly containing water such
as mist and spray water, and the accelerated cooling is
stopped at the time when the pearlite transformation of
the steel rail proceeds in an amount of 70~ of the entire
transformation. As a result, a pearlite structure having
a high hardness can be stably formed.
In addition, although the metal structure of the rail
is desirably a pearlite structure, a trace amount of
proeutectoid cementite may sometimes be formed therein
CA 02222281 1997-11-25
- 25 -
depending on the constituent system, the cooling rate and
the segregation state of the steel material. However,
even when a trace amount of proeutectoid cementite is
formed in the pearlite structure, the cementite exerts no
significant effect on the ductility, toughness and wear
resistance of the rail. Accordingly, the pearlitic steel
rail of the present invention may contain a proeutectoid
cementite structure, to some extent, in the structure.
Reasons for restricting the cooling conditions in
claim 6 as follows will be explained: the head portion of
a steel rail is subjected to accelerated cooling from an
austenite region temperature to a temperature from 750 to
600°C at a cooling rate of more than 10 to 30°C/sec, and
consecutively to controlled cooling at a cooling rate of 1
to less than 10°C/sec in a temperature region from 750 to
600°C to 550 to 450°C. In addition, the cooling
conditions are heat treatment production conditions in
cases where water such as mist and spray water is mainly
used in the initial cooling, and a cooling medium
containing air, or air mainly and mist is used in the
subsequent cooling.
As shown in Fig. 2, the cooling curve invariably
passes the pearlite nose when the accelerated cooling rate
is up to 10°C/sec, and most of the pearlite transformation
is finished during continuous cooling. V~lhen the
accelerated cooling rate exceeds 10°C/sec, the cooling
curve is found to pass the pearlite transformation nose
only for steels containing carbon at least in a certain
amount. Furthermore, when the accelerated cooling rate
exceeds 10°C/sec, continuation of the cooling to a low
temperature region of up to 300°C results in the formation
of a large amount of a martensite structure in the
pearlite structure. The resultant martensite structure
exerts adverse effects on the wear resistance and
toughness of the rail.
However, in cooling at an accelerated cooling rate
exceeding 10°C/sec, the pearlite transformation can be
CA 02222281 1997-11-25
- 26 -
completed in the entire rail head portion by stopping the
accelerated cooling in a temperature region where a
pearlite structure having a high hardness is stably
formed, and subjecting to cooling so that heat generation
of the pearlite transformation can be subsequently
controlled and natural recuperation can occur from within
the rail head portion.
A steel containing 1.0~ of C shown in Fig. 2 is taken
as an example, and the concept of the above production
process is shown on the continuous cooling transformation
diagram (CCT diagram). In the example, the steel is
subjected to accelerated cooling from the austenite region
at a rate of more than 10 to 30°C/sec, and the pearlite
transformation can be stably completed by further
controlling the subsequent heat generation of pearlite
transformation and natural recuperation from within the
rail head portion (1-10°C/sec).
First, in the method for subjecting the steel to
accelerated cooling from the austenite region temperature
to 750 to 600°C at a cooling rate of more than 10 to not
more than 30°C/sec, reasons for restricting the
accelerated cooling stop temperature and the accelerated
cooling rate as mentioned above will be explained.
Tnlhen the accelerated cooling is stopped at
temperatures exceeding 750°C, proeutectoid cementite is
farmed in the high temperature region in the course of the
subsequent controlled cooling, and the ductility and
toughness of the rail is considerably lowered.
Accordingly, the accelerated cooling stop temperature is
restricted to up to 750°C. TnThen the accelerated cooling
is conducted to temperatures less than 600°C, the pearlite
transformation is not completed during the subsequent
controlled cooling. As a result, abnormal structures such
as bainite and martensite, which are detrimental to the
toughness and wear resistance of the rail, tend to be
formed. Accordingly, the accelerated cooling stop
temperature is restricted to at least 600°C.
CA 02222281 1997-11-25
- 27 -
Reasons for restricting the accelerated cooling rate
to from more than 10 to 30°C/sec will be explained.
ln~h.en the steel rail is cooled with a cooling medium
other than air mainly containing water such as mist and
spray water, at an accelerated cooling rate of up to
10°C/sec, the cooling stability becomes very poor in such
a low cooling rate region due to the very high cooling .
ability, and the cooling control becomes very difficult.
Moreover, the hardness varies in the unstabilized cooling
portion, and stably adjusting the Vickers hardness of the
rail head portion to at least 320 becomes difficult.
Accordingly, the accelerated cooling rate is restricted to
more than 10°C/sec. Moreover, when the accelerated
cooling' rate exceeds 30°C/sec, the pearlite transformation
is not completed during controlled cooling subsequent to
the accelerated cooling, and abnormal structures such as
bainite and martensite detrimental to the toughness and
wear resistance of the rail tend to be formed.
Accordingly,~the accelerated cooling rate is restricted to
from more than 10 to 30°C/sec.
In addition, the accelerated cooling rate is defined
as an average cooling rate from the start of cooling to
the completion thereof. A temporary temperature rise may
sometimes be caused by the heat generation of pearlite
transformation or natural recuperation from the interior
of the rail in the course of the accelerated cooling.
However, when the average cooling rate from start to
completion of the accelerated cooling is within the range
as mentioned above, no significant influence is exerted on
the properties of the pearlitic steel rail of the present
invention. The accelerated coo~.ing conditions for the
rail of the invention, therefore, include a decrease in
the cooling rate resulting from the temporary temperature
rise in the course of cooling.
Furthermore, methods for obtaining a predetermined
cooling rate in the range of more than 10 to 30°C/sec are
as follows: injection cooling with a mixture of water and
CA 02222281 1997-11-25
- 28 -
air or a combination of these; the rail head portion or
the entire rail is immersed in oil, hot water, a mixture
of polymer and water or salt bath.
Next, in the method of controlled cooling at a
cooling rate of 1 to 10°C/sec from temperatures of 750 to
600°C to temperatures of 550 to 450°C, reasons for
restricting the controlled cooling stop temperature and.
the controlled cooling rate as mentioned above will be
explained_
When the controlled cooling is stopped at
temperatures exceeding 550°C, a large amount of a pearlite
structure having a low hardness is formed immediately
after the controlled cooling. Consequently, the rail head
portion has a Vickers hardness of less than 320, and the
wear resistance of the head portion cannot be ensured.
Accordingly, the controlled cooling stop temperature is
restricted to up to 550°C. Moreover, when the controlled
cooling is conducted at temperatures of less than 450°C,
sufficient natural recuperation from the interior of the
rail cannot be expected after the accelerated cooling, and
a martensite structure detrimental to the toughness of the
rail is formed in the segregation portion, etc.
Accordingly, the controlled cooling stop temperature is
restricted to at least 450°C.
Reasons for restricting the controlled cooling rate
to from 1 to 10°C/sec will be explained. When the
controlled cooling rate becomes less than 1°C/sec, a large
amount of a pearlite structure having a low hardness is
formed in a high temperature region in the course of
controlled cooling. As a result, the Vickers hardness of
the rail head portion becomes less than 320, and a
necessary wear resistance of the head portion cannot be
ensured. Accordingly, the controlled cooling rate is
restricted to at least 1°C/sec. Moreover, when the
controlled cooling is conducted at a cooling rate of at
least 10°C/sec, the pearlite transformation is not
completed in the course of cooling, and abnormal
CA 02222281 1997-11-25
- 29 -
structures such as bainite and martensite detrimental to
the toughness and wear resistance of the rail are formed
in the course of controlled cooling and subsequent
cooling. Accordingly, the controlled cooling rate is
restricted to from 1 to less than 10°C/sec.
In addition, the controlled cooling rate is defined
as an average cooling rate from the start of cooling to.
the completion thereof. A temporary temperature rise may
sometimes be caused by the heat generation of pearlite
transformation or natural recuperation from the interior
of the rail in the course of the controlled cooling.
However, when the average cooling rate from start to
completion of the controlled cooling is within the range
as mentioned above, no significant influence is exerted on
the properties of the pearlitic steel rail of the present
invention. The controlled cooling conditions for the rail
of the invention, therefore, include a decrease in the
cooling rate resulting from the temporary temperature rise
in the course of cooling.
A predetermined cooling rate in the range of 1 to
10°C/sec may be obtained with a cooling medium of air or
one containing mainly air and mist, etc. or a combination
of these media.
Accordingly, in order to produce a rail having a
pearlite structure with a Vickers hardness of at least 320
and excellent in wear resistance and weldability, the rail
head portion is subjected to accelerated cooling at a
cooling rate of more than 10 to 30°C/sec, and subsequently
subjected to controlled cooling at a cooling rate of 1 to
less than 10°C/sec from temperatures of 750 to 600°C to
temperatures of 550 to 450°C, using a cooling medium other
than air mainly containing water such as mist and spray
water. The pearlite structure can thus be stably formed
in the rail head portion.
Although the metal structure of the rail is desirably
a pearlite structure, a trace amount of proeutectoid
cementite is sometimes formed in the pearlite structure in
CA 02222281 1997-11-25
- 30 -
the rail head portion and the column portion, depending on
the constituent system, the cooling rate and the
segregation state of the steel material. However, even
when a trace amount of proeutectoid cementite is formed in
the pearlite structure, no significant influence is
exerted on the ductility, toughness, wear resistance and
strength of the rail. The structure of the pearlitic
steel rail may, therefore, contain a proeutectoid
cementite structure to some degree.
Examples
Next, the present invention will be explained with
reference to examples.
Example 1
The present example is one within the scope of claims
1 to 3.
Tables 1 and 2 show the chemical compositions, the
base steel hardness and microstructures, and the wear
amounts after repeating 700,000 times in the Nisihara type
wear tester shown in Fig. 4 under forced conditions, of
the rail steels of the present invention and comparative
rail steels.
Table 1
Rail ReferenceChemical
composition
(wt.~)
~_~___~________
numeral __________________________
C
Si
Nhi
Cr
Mo,V,Nb,Co,B
Si/4+Mn/2+Cr
R.S.I.*1 0.86 0.11 1.48 0.51 Co: 0.15 1.28
R.S.I.*2 0.91 0.45 0.41 0.55 - 0.87
R.S.I.*3 0.91 0.32 0.37 0.98 - 1.25
R.S.I.*4 0.95 0.81 0.22 0.52 V: 0.06 0.83
R.S.I.*5 0.96 0.84 0.21 0.94 V: 0.05 1.26
R.S.I.*6 1.01 0.61 1.05 0.82 Mo: 0.02 1.50
R.S.I.*7 1.05 0.28 0.42 0.75 B. 0.0019 1.03
R.S.I.*$ 1.11 0.98 0.52 0.51 Nb: 0.02 1.02
R.S.I.*9 1.19 0.25 0.34 0.74 - 0.97
CA 02222281 1997-11-25
- 31 -
Table 1 (Continued)
Rail ReferenceHardnessMicro- Wear Hardness Difference
numeralof head structureamount of head in
portion of head portion hardness
in base portion (g/700,000in between
rail welded base
(Hv) times) joint rail and
(Hv) welded joint
(pHv)
R.S.I.*1 386 Pearlite 0.99 405 19
R.S.I.*2 395 Pearlite 0.91 374 21
R.S.I.*3 410 Pearlite 0.85 406 4 -
R.S.I.*4 402 Pearlite 0.81 378 24
R.S.I.*5 412 Pearlite 0.74 411 1
R.S.I.*6 431 Pearlite 0.49 440 9
R.S.I.*7 385 Pearlite 0.74 402 17
R.S.I.*8 410 Pearlite 0.43 408 2
R.S.I.*9 -- L -401-_P~lite I 0.42 411 -.' - 10
[ ~
Table 2 (Continued from Table 1)
Rail ReferenceChemical
numeralcomposition
(wt.$)
_____________________________________________________________
C Si
Nhz
Cr
Mo,V,Nb,Co,B
Si/4+Mn/2+Cr
C.R.S.*10 0.76 0.55 1.08 0.28 - 0.96
C.R.S.*11 Q;81 0.62 1.31 - V: 0.06 0.81
C.R.S.*12 0.77 0.85 0.81 0.58 V: 0.04 1.20
C.R.S.*13 08181 0.81 1.24 - - 0.82
C.R.S.*14 1.01 1.25 0.61 0.65 - 1.26
C.R.S.*15 0.90 0.74 0.42 1.30 - 1.70
C.R.S.*16 ~ 1.36 0.41 0.50 0.74 - 1.09
C.R.S.*17 0.90 0.61 0.56 0.21 - 064
C.R.S.*18 0.90 0.90 1.05 - 2-Q303
Table 2 (Continued)
Rail ReferenceHardnessMicro- Wear Hardness Difference
- numeral of headstructure amount of head in
portionof portion hardness
in basehead portion(g/700,000in between
rail welded base
(Hv) times) joint rail and
(Hv) welded joint
C.R.S.*10 381 Pearlite 1.22 - -
C.R.S.*11 389 Pearlite 1.15 - -
C.R.S.*12 401 Pearlite 1.06 - -
C.R.S.*13 394 Pearlite 1.12 - -
C.R.S.*14 surface
defects
roduced
Burin
rollin
C.R.S.*15 506 Pearlite+ - - -
~7~3...G~
C.R.S.*16 452 Pearlite+ - - -
~roeutectoid
r-amPnti
tP
C.R.S.*17 396 Pearlite 0.91 352
C.R.S.*18 421 Pearlite 0.75 461 ~Q
martensite
formed
Note: R.S.I. = Rail Steel of Invention, C.R.S. = Comparative Rail Steel
The balance of the chemical composition is unavoidable impurities
and Fe.
1 0 #: Ref. N. = Reference Numeral
CA 02222281 1997-11-25
- 32 -
Further, Tables 1 and 2 clearly show a difference in
hardness between the flash butt welded joint and the base
steel of any of the rail steels of the present invention
and the comparative rail steels. In addition, the
hardness of the base steel and that of the flash butt
welded joint of any of the rail steels shown in Tables 1
and 2 are average values of a head portion, and are
neither the maximum values nor the minimum values.
Furthermore, Fig. 5 shows the relationship between a
hardness and a wear amount of the rail steels of the
present invention and the comparative rail steels
(eutectoid carbon steels: reference numerals of 10 to 13)
listed in Tables 1 and 2 to compare the wear test results.
Fig. 6 shows instances of the hardness distributions of
the head portions of the welded joints of the rail steels
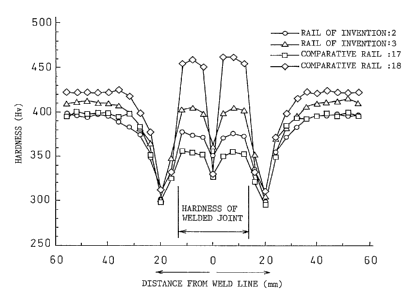
of the present invention (reference numerals: 2, 3) and
the comparative rail steels (reference numerals: 17, 18)
shown in the examples in Tables 1 and 2. In addition, the
rails used in the examples are as described below.
* Rails of the present invention (9 pieces) with
reference numerals of 1 to 9:
the steel rails being heat treated rails each
having a chemical composition as mentioned above and a
pearlite structure to the depth of at least 20 mm from the
rail head portion surface as a starting point, the
pearlite structure having a Vickers hardness of at least
320, and the head portion having been subjected to
accelerated cooling.
* Comparative rails (9 pieces):
comparative rails (4 pieces) with reference
numerals of 10 to 13: prepared from eutectoid carbon
steels having chemical compositions outside the scope of
the claims of the present invention, and
comparative rails (5 pieces) with reference
numerals of 14 to 18: prepared from hyper-eutectoid carbon
steels having chemical compositions outside the scope of
the claims of the present invention
CA 02222281 1997-11-25
1
- 33 -
The wear test conditions are as follows:
tester: the Nisihara type wear tester,
shape of a test piece: a disc-like test piece
(outside diameter: 30 mm, thickness: 8 mm),
test load: 686 N,
slip ratio: 20~,
counter material: pearlitic steel (Vickers
hardness of 390),
atmosphere: in air,
cooling: forced cooling with compressed air
(flow rate: 100 N1/min), and
number of repeats: 700,000.
Flash butt welding conditions are as follows:
welder: K-355 (manufactured in Soviet Union),
capacity: 150 KVA,
secondary current: 20,000 A (max.),
clamping force: 125 ton (max.), and
upsetting amount: 10 mm.
Example 2
The present example is one within the scope of claims
2 to 3.
Tables 3 and 4 show the chemical compositions, the
base steel hardness and microstructures, and the wear
amounts after repeating 700,000 times in the Nisihara type
wear tester shown in Fig. 4 in Example 1 under forced
cooling conditions, of the rail steels of the present
invention and comparative rail steels.
~Pahl P
Rail ReferenceChemical
composition
(wt.$)
______~_______________~______
numeral ______________~_____~__
C
Si
Mn
Cr
Mo,V,Nb,Co,B
Si/4+I~/2+Cr
R.S.I.*19 0.85 0.81 0.38 0.47 0.86
R.S.I.*20 0.90 0.45 0.39 0.49 V: 0.04 0.80
R.S.I.*21 0.90 0.98 0.39 0.48 V: 0.04 0.92
R.S.I.*22 0.95 0.98 0.21 0.48 - 0.83
R.S.I.*23 0.95 0.98 0.39 0.37 - 0.81
R.S.I.*24 1.00 0.85 0.35 0.41 Mo: 0.01 0.80
R.S.I.*25 1.04 0.78 0.39 0.43 Co: 0.21 0.82
R.S.I.*26 1.10 0.65 0.35 0.48 B: 0.0014 0.82
R.S.I.*27 1.20 0.95 0.22 0.49 Nb: 0.03 0.84
CA 02222281 1997-11-25
- 34 -
rrahle 3 (Continued)
Rail ReferenceHardnessMicro- Wear Hardness Difference
numeral of head structureamount of head in
portion of head portion hardness
in base portion (g/700,000in between
rail welded base
(Hv) times) joint rail and
(Hv) welded joint
(pHv)
R.S.I.*19 384 Pearlite 0.99 369 15
R.S.I.*20 391 Pearlite 0.95 370 21
R.S.I.*21 392 Pearlite 0.94 383 9
R.S.I.*22 384 Pearlite 0.93 375 9
R.S.I.*23 380 Pearlite 0.95 370 10
R.S.I.*24 395 Pearlite 0.75 380 15
R.S.I.*25 386 Pearlite 0.72 382 4
R.S.I.*26 395 Pearlite 0.53 385 10
R.S.I.*27 401 Pearlite 0.38 395 6
~TahlP a (Continued from Table 3)
Rail ReferenceChemical
composition
(wt.~)
~_____v-~~__e___~___-______,________
numeral ____-____________
C Si
Nhz
Cr
Mo,V,Nb,Co,B
Si/4+I&~/2+Cr
C.R.S.*28 0.77 0.50 1-00 0.25 - 0-88
C.R.S.*29 08282 0.55 - V: 0.05 0.81
C.R.S.*30 0.79 0.85 ~ ~ - 1-1616
C.R.S.*31 0.$0 0.80 1.22 - V: 0.04 0.81
C.R.S.*32 1.01 ~ 0.39 0.49 - 1-0303
C.R.S.*33 1.02 0.51 Q.84 0-8686-
C.R.S.*34 ~ 0.85 0.35 0.44 - 0.83
C.R.S.*35 0.90 Q 0.38 0.35 - 0-64
39
C.R.S.*36 0.90 0.95 0.12 0.48 - 0.78
Table
4
(Continued)
Rail ReferenceHardnessMicro- Wear Hardness Difference
numeral of headstructure amount of head in
portionof portion hardness
in basehead portion(g/700,000in between
rail welded base
(Hv) times) joint rail and
(Hv) welded joint
(pHv)
C.R.S.*28 379 Pearlite 1.22 - -
C.R.S.*29 388 Pearlite 1.15 - -
C.R.S.*30 403 Pearlite 1.06 - -
C.R.S.*31 395 Pearlite 1.12 - -
C.R.S.*32 surface
defects
produced
durin
rollin
C.R.S.*33 422 Pearlite 0.58 martensite
formed
in a
trace
amount
(3~)
in
segregation
in column
onion
C.R.S.*34 452 Pearlite+ - - -
groeutectoid
~ementite
C.R.S.*35 401 Pearlite 0.91 356 4~
C.R.S.*36 398 Pearlite 0.75 364 .~4
Note: *. R.S.I. = Rail Steel of Invention, * C.R.S. = Comparative Rail
Steel
The balance of the chemical composition is unavoidable impurities
and Fe .
#: Ref. N. = Reference Numeral
CA 02222281 1997-11-25
r
- 35 -
Further, Tables 3 and 4 clearly show a difference in
hardness between the flash butt welded joint and the base
steel of any of the rail steels of the present invention
and the comparative rail steels. In addition, the
hardness of the base steel and that of the flash butt
welded joint of any of the rail steels shown in Tables 3
and 4 are average values of a head portion, and are _ .
neither the maximum values nor the minimum values.
Furthermore, Fig. 7 shows the relationship between a
hardness and a wear amount of the rail steels of the
present invention and the comparative rail steels
(eutectoid carbon steels: reference numerals of 28 to 31)
listed in Tables 3 and 4 to compare the wear test results.
Fig. 8 shows instances of the hardness distributions of
the head portions of the welded joints of the rail steel
of the present invention (reference numeral: 21) and the
comparative rail steel (reference numeral: 35) shown in
the examples in Tables 3 and 4.
In addition, the rails used in the examples are as
described below.
* Rails of the present invention (9 pieces) with
reference numerals of 19 to 27:
the steel rails being heat treated rails each
having a chemical composition as mentioned above and a
pearlite structure to the depth of at least 20 mm from the
rail head portion surface as a starting point, the
pearlite structure having a Vickers hardness of at least
320, and the head portion having been subjected to
accelerated cooling.
* Comparative rails (9 pieces)
comparative rails (4 pieces) with reference
numerals of 28 to 31: prepared from eutectoid carbon
steels having chemical compositions outside the scope of
the claims of the present invention, and
comparative rails (5 pieces) with reference
numerals of 32 to 36: prepared from hyper-eutectoid carbon
steels having chemical compositions outside the scope of
CA 02222281 1997-11-25
y
- 36 -
the claims of the present invention.
The wear test conditions and flash butt welding
conditions are the same as in Example 1.
Example 3
The present example is one within the scope of claims
4 to 6.
Tables 5 to 10 and Tables 11 to 16 show the chemical
compositions, the heat treatment conditions (heat
treatment temperature ranges, cooling rates and pearlite
formation ratios), the base steel hardness and
microstructures, and the wear amounts after repeating
700,000 times in the Nisihara type wear tester shown in
Fig. 4 in Example 1 under forced cooling conditions, of
the rail steels of the present invention and comparative
rail steels. Further, Tables 5 to 8, and Tables 11 to 14
clearly show the hardness in the flash butt welded joints
of the rail steels of the present invention and
comparative rail steels and the differences in hardness
between the flash butt welded joints and the base steels.
Table 5
Rail R.F. Chemical Acceler-Cooling
composition
(wt.~)
N. ____~____-_____________
#
__ ______~___~______rated rate
cooling
range
in
head
C Si I~ Cr Mo,V,Nb,Si/4+ portion(C
Co,B Mn/2+Cr(C) /sec)
R.S.I.*37 0.86 0.12 1.48 0.52 Co: 0.161.29 745-5892
R.S.I.*38 0.86 0.12 1.48 0.52 Co: 0.161.29 721 11
(start)
R.S.I.*39 0.86 0.12 1.48 0.52 Co: 0.161.29 781-62511
R.S.I.*40 0.90 0.44 0.43 0.52 - 0.85 752-5444
R.S.I.*41 0.90 0.44 0.43 0.52 - 0.85 781 13
(start)
R.S.I.*42 0.90 0.44 0.43 0.52 - 0.85 756-60013
R.S.I.*43 0.92 0.34 0.35 0.98 - 1.24 731-5783
R.S.I.*44 0.92 0.34 0.35 0.98 - 1.24 721 12
(start)
R.S.I.*45 0.92 0.34 0.35 0.98 - 1.24 761-63112
CA 02222281 1997-11-25
- 37 -
Table 5 (Continued)
Rail Ref.Other Hard- Micro- Wear HardnessDifference
heat
N.# treatmentness structureamount of headin hardness
of
conditionshead of head portionbetween
portionportion in weldedbase
rail
in joint and welded
base
rail (g/700,000 joint
(Hv) times) (Hv) (pHV)
R.S.I.*37 - 386 Pearlite 0.99 401 15
R.S.I.*38 Pearlite 406 Pearlite 0.91 399 7
formation
ratio
70~
R.S.I.*39 Controlled400 Pearlite 0.94 405 5
cooling
range
625-471C
Cooling
rate
3C/sec
R.S.I.*40 - 394 Pearlite 0.92 384 10
R.S.I.*41 Pearlite 403 Pearlite 0.89 382 21
formation
ratio
84~
R.S.I.*42 Controlled408 Pearlite 0.82 380 28
cooling
range
600-501C
Cooling
rate
4C/sec
R.S.I.*43 401 Pearlite 0.89 410 9
R.S.I.*44 Pearlite 408 Pearlite 0.81 408 0
formation
ratio
74~
R.S.I.*45 Controlled409 Pearlite 0.80 411 2
cooling
range
631-540C
Cooling
rate
2C/sec
CA 02222281 1997-11-25
- 38 -
Table 6 (Continued from Table 5)
Rail Ref.Chemical Acceler-Cooling
D1 composition
# (wt.$)
___________~__________
. ___~_~_~-__~___~__ rated rate
cooling
range
in
head
C portion(C
Si
Mn
Cr
Mo,V,Nb,
Si/4+
Co,B (C) /sec)
Mn/2+Cr
R.S.I.*46 0.960.80 0.25 0.53 V: 0.050.86 752-5554
R.S.I.*47 0.960.80 0.25 0.53 V: 0.050.86 764 17
(start)
R.S.I.*48 0.960.80 0.25 0.53 V: 0.050.86 785-64118
R.S.I.*49 0.960.76 0.21 0.95 V: 0.041.25 720-5923
R.S.I.*50 0.960.76 0.21 0.95 V: 0.041.25 728 15
(start)
R.S.I.*51 0.960.76 0.21 0.95 V: 0.041.25 761-62214
R.S.I.*52 1.010.62 1.04 0.80 Mo: 1.48 704-5041
0.01
R.S.I.*53 1.010.62 1.04 0.80 Mo: 1.48 815 16
0.01
(start)
~R.S.I.*54 1.010.62 1.04 0.80 Mo: 1.48 780-64217
~ ) X X X ~ 0.01
~
CA 02222281 1997-11-25
- 39 -
Table 6 (Continued)
Rail Ref.Other Hard- Micro- Wear HardnessDifference
heat
N.# treatmentness structureamount of head in hardness
of
conditionshead of head portion between
in
portionportion welded base rail
in base joint and welded
rail (g/700,000 joint
(Hv) times) (Hv)
R.S.I.*46 - 397 Pearlite0.82 381 16
R.S.I.*47 Pearlite 404 Pearlite0.78 381 23
formation
ratio
92$
R.S.I.*48 Controlled409 Pearlite0.77 380 29
cooling
range
641-451C
Cooling
rate
3C/sec
R.S.I.*49 - 408 Pearlite0.76 414 6
R.S.I.*50 Pearlite 412 Pearlite0.75 410 2
formation
ratio
79$
R.S.I.*51 Controlled416 Pearlite0.70 412 4
cooling
range
622-490C
Cooling
rate
2C/sec
R.S.I.*52 - 410 Pearlite0.65 432 22
R.S.I.*53 Pearlite 421 Pearlite0.61 430 9
formation
ratio
72~
R.S.I.*54 Controlled432 Pearlite0.51 429 3
cooling
range
642-548C
Cooling
rate
1C/sec
CA 02222281 1997-11-25
- 40 -
Table 7 (Continued from Table 6)
Rail Ref.Chemical Acceler-Cooling
composition
(wt.~)
N ___________________~______
#
. ____ ated rate
___~_e~
cooling
range
in
head
C portion(C
Si
Mn
Cr
Mo,V,N'b,
Si/4+
Co,B (C) /sec)
I~/2+Cr
R.S.I.*55 1.040.22 0.40 0.71 B:0.00140.97 789-5623
R.S.I.*56 1.040.22 0.40 0.71 B:0.00140.97 776 19
(start)
R.S.I.*57 1.040.22 0.40 0.71 B:0.00140.97 815-69118
R.S.I.*58 1.100.97 0.52 0.51 bri~:0.031.01 851-6256
R.S.I.*59 1.100.97 0.52 0.51 I~:0.031.01 842 20
(start)
R.S.I.*60 1.100.97 0.52 0.51 Nb:0.031.01 790-62523
R.S.I.*61 1.180.21 0.35 0.78 - 1.01 880-7009
R.S.I.*62 1.180.21 0.35 0.78 - 1.01 821 29
(start)
~ R. 63 1.180 0.35 0.78 - ~ 1. O1 840-75028
S. ~ ~ .21 ~ / ~ ~
I. ~
* /
CA 02222281 1997-11-25
- 41 -
Table 7 (Continued)
Rail Ref.Other heatHard- Micro- Wear HardnessDifference
N.# treatment ness structureamount of head in hardness
of
conditionshead of head portion between
in
portionportion welded base rail
in joint and welded
base
rail (g/700,000 joint
(Hv) times) (Hv)
R.S.I.*55 - 400 Pearlite0.59 392 8
R.S.I.*56 Pearlite 409 Pearlite0.54 395 14
formation
ratio
94$
R.S.I.*57 Controlled418 Pearlite0.51 394 24
cooling
range
691-532C
Cooling
rate
5C/sec
R.S.I.*58 - 401 Pearlite0.56 402 1
R.S.I.*59 Pearlite 405 Pearlite0.54 400 5
formation
ratio
84~
R.S.I.*60 Controlled418 Pearlite0.40 404 14
cooling
range
625-531C
Cooling
rate
4C/sec
R.S.I.*61 383 Pearlite0.56 405 22
R.S.I.*62 Pearlite 401 Pearlite0.39 406 5
formation
ratio
71~
R.S.I.*63 Controlled410 Pearlite0.32 406 4
cooling
range
750-549C
Cooling
rate
10C/sec
Note: *: R.S.I. = Rail Steel of Invention, * C.R.S. = Comparative Rail
Steel
The balance of the chemical composition is unavoidable impurities
and Fe.
#: Ref. N. = Reference Numeral
CA 02222281 1997-11-25
- 42 -
Table 8 (Continued from Table 7)
Rail Ref. Chemical Acceler-Cooling
composition
(wt.$)
N.# ____________________________________________
ated rate
cooling
range
in
head
C portion (C
Si
Mn
Cr
Mo,V,Nb,
Si/4+
CO,B (C) /sec)
Mn/2+Cr
C.R.S.*64 Q.75 0.55 1.040-2828- 0.94 - -
C.R.S.*65 0-82820.52 1.33- V: 0.040.80 - -
C.R.S.*66 0.78 0.84 0.800.54V: 0.041.15 - -
C.R.S.*67 0-82820.80 1.20- - 0.80 - -
C.R.S.*68 1.01 1.24 0.660.62- 1.26 - -
C.R.S.*69 0.90 0.71 0.431.32- 1.71 752-555 4
C.R.S.*70 1-43430.40 0.500.77- 1.12 845 12
(start)
C.R.S.*71 0.91 0.61 0.500.24- 0-6464 780-574 5
C.R.S.*72 0.91 0.61 1-60601-1010- 2.05 765-625 14
Table 8 (Continued)
Rail Ref.Other heatHard- Micro- Wear HardnessDifference
N.# treatment ness structure amount of headin
of of
conditionshead head portion portionhardness
portion in between
in welded base
base rail
rail joint and welded
(g/700,000 joint
(Hv) times) (Hv) (~v)
C.R.S.*64 - 384 Pearlite 1.21 - -
C.R.S.*65 - 387 Pearlite 1.16 - -
C.R.S.*66 - 397 Pearlite 1.08 - -
C.R.S.*67 - 390 Pearlite 1.14 - -
C.R.S.*68 - surface
defects
formed
during
rolling
C.R.S.*69 - 364 Pearlite - - -
+
1-,a i
n i i-a
C.R.S.*70 Pearlite 478 Pearlite - - -
+
formation proeutectoid
ratio cementite
72~
C.R.S.*71 - 398 Pearlite 0.89 352
C.R.S.*72 Controlled421 Pearlite 0.74 478
cooling martens-
range ite
625-536C formed
Cooling
rate
1C/sec
CA 02222281 1997-11-25
- 43 -
Table 9 (Continued from Table 8)
Rail Ref.Chemical Acceler-Cooling
composition
(wt.~)
# ___~___-___________~ ated rate
__________
N. ________
cooling
range
in
head
C portion (C
Si
Mn
Cr
Mo,V,Nb,
Si/4+
Co,B (C) /sec)
Mn/2+Cr
C.R.S.*73 0.910.40 0.440.61 - 0.93 751-506 $-11
control-
lable
C.R.S.*74 0.910.40 0.440.61 - 0.93 781 $-13
(start) un-
control-
lable
C.R.S.*75 0.910.40 0.440.61 - 0.93 778-621
C.R.S.*76 0.950.91 0.220.84 V: 0.051_18 751-~ 3
C.R.S.*77 0.950.91 0.220.84 V: 0.051.18 752
(start)
C.R.S.*78 0.950.91 0.220.84 V: 0.051.18 791-684 7-15
control-
lable
C.R.S.*79 1.000.54 1.000.74 Mo: 1.38 864-~, 5
0.02
C.R.S.*80 1.000.54 1.000.74 Mo: 1.38 724 18
0.02
(start)
C.R.S.*81 1.000.54 1.000.74 Mo: 1.38 780-631 15
0.02
CA 02222281 1997-11-25
- 44 -
Table 9 (Continued)
Rail Ref.Other heatHard- Micro- Wear HardnessDifference
N.# treatment ness structureamount of headin
of
conditionshead of head portionhardness
portionportion in between
in welded base
base rail
rail joint and welded
(g/700,000 joint
(Hv) times) (HV)
C.R.S.*73 - 454 Pearlite - - -
+
martPnaitP
C.R.S.*74 Pearlite 308 Pearlite - - -
formation hard-
ratio ness
75$ in
un-
stably
cooled
portion
C.R.S.*75 Controlled542 Pearlite - - -
+
cooling martensite
range
621-522C
Cooling
rate
8C/sec
C.R.S.*76 - 471 Pearlite - - -
+
martensite
C.R.S.*77 Pearlite 564 Pearlite - - -
+
formation rn~rtensite
ratio
75$
C.R.S.*78 Controlled,, Pearlite - - -
cooling hard-
range ness
684-506C in
un-
Cooling stably
rate
8C/sec cooled
portion
C.R.S.*79 - ~ Pearlite - - -
C.R.S.*80 Pearlite 474 Pearlite - - -
+
formation martensite
ratio
42$
C.R.S.*81 Controlled461 Pearlite - - -
+
cooling ma_rr_en~ir_e
range
631-481C
Cooling
rate
12C/sec
CA 02222281 1997-11-25
- 45 -
Table 10 (Continued from Tah7P 91
Rail Ref. Chemical Acceler-Cooling
N _____composition
# (wt.~)
_-_~_________________
. _______ ______-ated rate
cooling
range
in
head
C Si Mn Cr Si/4+ portion(C
Mo,V,Nb,
Co,B I~/2+Cr(C) /sec)
C.R.S.*82 1.18 0.310.380.86 - 1.13 864-506Q~
C.R.S.*83 1.18 0.310.380.86 - 1.13 824 29
(start)
C.R.S.*84 1.18 0.310.380.86 - 1.13 820-72427
Table 10 (Continued)
Rail Ref.Other heatHard- Micro- Wear HardnessDifference
N.# treatment ness structure amount of headin
of of
conditionshead head portion portionhardness
portion in between
in welded base
base rail
rail joint and welded
(g/700,000 joint
(Hv) times) (Hv) (~V)
C.R.S.*82 - 441 Pearlite - - -
+
proeutectoid
ramantito
C.R.S.*83 Pearlite 541 Pearlite - - -
+
formation martensite
ratio
C.R.S.*84 Controlled442 Pearlite - - -
+
cooling ~roeutectoid
range cementite
724-514C
Cooling
rate
0. 5C/sec
Note: *: R.S.I. = Rail Steel of Invention, * C.R.S. = Comparative Rail
Steel
The balance of the chemical composition is unavoidable impurities
and Fe.
#: Ref. N. = Reference Numeral
CA 02222281 1997-11-25
- 46 -
Table 11
Rail Ref.Chemical Acceler-Cooling
composition
(wt.$)
N.# _____________________~______~__~__________
ated rate
cooling
range
in
head
C portion(C
Si
N~
Cr
Mo,V,Nb,
Si/4+
Co,B (C) /sec)
I~/2+Cr
R.S.I.*85 0.86 0.82 0.37 0.48 0.87 792-5623
R.S.I.*86 0.86 0.82 0.37 0.48 0.87 761 12
(start)
R.S.I.*87 0.86 0.82 0.37 0.48 0.87 776-62212
R.S.I.*88 0.92 0.48 0.37 0.49 V: 0.060.80 781-5215
R.S.I.*89 0.92 0.48 0.37 0.49 V: 0.060.80 794 14
(start)
R.S.I.*90 0.92 0.48 0.37 0.49 V: 0.060.80 754-60213
R.S.I.*91 0.92 0.99 0.36 0.47 V: 0.040.90 762-5325
R.S.I.*92 0.92 0.99 0.36 0.47 V: 0.040.90 761 15
(start)
R.S.I.*93 0.92 0.99 0.36 0.47 V: 0.040.90 721-64014
CA 02222281 1997-11-25
g7 _
Table 11 (Continued)
Rail Ref.Other heatHard- Micro- Wear HardnessDifference
N.# treatment ness structureamount of head in hardness
of
conditionshead of head portion between
portionportion in weldedbase
rail
in joint and welded
base
rail (g/700,000 joint
(Hv) times) (Hv)
R.S.I.*85 - 382 Pearlite0.99 372 10
R.S.I.*86 Pearlite 389 Pearlite0.96 374 15
formation
ratio
71~
R.S.I.*87 Controlled394 Pearlite0.95 375 19
cooling
range
622-481C
Cooling
rate
3C/sec
R.S.I.*88 - 391 Pearlite0.94 371 20
R.S.I.*89 Pearlite 400 Pearlite0.91 372 28
formation
ratio
86~
R.S.I.*90 Controlled401 Pearlite0.90 372 29
cooling
range
602-504C
Cooling
rate
4C/sec
R.S.I.*91 - 394 Pearlite0.93 380 14
R.S.I.*92 Pearlite 405 Pearlite0.87 382 23
formation
ratio
72$
R.S.I.*93 Controlled404 Pearlite0.88 384 20
cooling
range
640-547C
Cooling
rate
2C/sec
CA 02222281 1997-11-25
- 48 -
Table 12 (Continued from Table 111
Rail Ref.Chemical Acceler-Cooling
N composition e
# (wt.~)
_________________________~
_
. _ at rate
___~_______
cooling
range
in
head
C portion (C
Si
Mn
Cr
Mo,V,Nb,
Si/4+
Co,B (C) /sec)
Mn/2+Cr
R.S.I.*94 0.96 0.900.210.49- 0.82 748-549
R.S.I.*95 0.95 0.980.210.48- 0.83 781 17
(start)
R.S.I.*96 0.95 0.980.210.48- 0.83 785-661 19
R.S.I.*97 0.96 0.910.390.39- 0.81 741-562 5
R.S.I.*98 0.96 0.910.390.39- 0.81 796 16
(start)
R.S.I.*99 0.96 0.910.390.39- 0.81 771-611 17
R.S.I.*100 1:01 0.840.360.42Mo: 0.020.81 741-502 2
R.S.I.*101 1.01 0.840.360.42Mo: 0.020.81 832 17
(start)
R.S.I.*102 1.01 0.840.360.42Mo: 0.020.81 774-621 17
CA 02222281 1997-11-25
- 49 -
Table 12 (Continued)
Rail Ref.Other heatHard- Micro- Wear HardnessDifference
N.# treatment ness structureamount of head in hardness
of
conditionshead of head portion between
portionportion in weldedbase
rail
in joint and welded
base
rail (g/700,000 joint
(Hv) times) (Hv)
R.S.I.*94 - 398 Pearlite0.81 375 24
R.S.I.*95 Pearlite 403 Pearlite0.75 376 27
formation
ratio
90~
R.S.I.*96 Controlled400 Pearlite0.76 377 23
cooling
range
661-454C
Cooling
rate
3C/sec
R.S.I.*97 - 394 Pearlite0.78 374 20
R.S.I.*98 Pearlite 402 Pearlite0.75 374 28
formation
ratio
94$
R.S.I.*99 Controlled405 Pearlite0.73 376 29
cooling
range
611-514C
Cooling
rate
2C/sec
R.S.I.*100 - 384 Pearlite0.78 382 2
R.S.I.*101 Pearlite 406 Pearlite0.69 384 22
formation
ratio
89~
R.S.I.*102 Controlled396 Pearlite0.71 384 12
cooling
range
621-524C
Cooling
rate
1C/sec
CA 02222281 1997-11-25
- 50 -
Table 13 (Continued from Table 12)
Rail Ref.Chemical Acceler-Cooling
coQnposition
(wt.~)
N.# ______________________~_____--_____~ ated
rate
_
cooling
range
in
head
C portion(C
Si
Mn
Cr
Mo,V,Nb,
Si/4+
Co,B (C) /sec)
Mn/2+Cr
R.S.I.*103 1.050.77 0.39 0.44Co: 0.83 769-5604
0.14
R.S.I.*104 1.050.77 0.39 0.44Co: 0.83 801 18
0.14
(start)
R.S.I.*105 1.050.77 0.39 0.44Co: 0.83 835-66219
0.14
R.S.I.*106 1.090.68 0.36 0.49B:0.00220.84 834-6026
R.S.I.*107 1.090.68 0.36 0.49B:0.00220.84 832 21
(start)
R.S.I.*108 1.090.68 0.36 0.49B:0.00220.84 821-64222
R.S.I.*109 1.190.94 0.21 0.49Ice: 0.83 842-69510
0.03
R.S.I.*110 1.190.94 0.21 0.49Ice: 0.83 844 28
0.03
(start)
R.S.I.*111 1.190.94 0.21 0.49ice: 0.83 860-74129
0.03
CA 02222281 1997-11-25
- 51 -
Table 13 (Continued)
Rail Ref.Other Hard- Micro- Wear HardnessDifference
heat
N.# treatmentness structu.,reamount of head in hardness
of
conditionshead of head portion between
portionportion in weldedbase
rail
in joint and welded
base
rail (g/700,000 joint
(Hv) times) (Hv) (
R.S.I.*103 - 384 Pearlite0.70 385 1
R.S.I.*104 Pearlite 404 Pearlite0.58 386 18 -
formation
ratio
94~
R.S.I.*105 Controlled412 Pearlite0.52 388 24
cooling
range
662-540C
Cooling
rate
4C/sec
R.S.I.*106 - 406 Pearlite0.52 391 15
R.S.I.*107 Pearlite 410 Pearlite0.49 394 16
formation
ratio
89~
R.S.I.*108 Controlled420 Pearlite0.41 394 26
cooling
range
624-544C
Cooling
rate
~
5C/sec
R.S.I.*109 - 381 Pearlite0.57 396 15
R.S.I.*110 Pearlite 411 Pearlite0.32 397 14
formation
ratio
74~
R.S.I.*111 Controlled418 Pearlite0.21 398 20
cooling
- range
741-501C
Cooling
rate
10C/sec
Note: *: R.S.I. = Rail Steel of Invention, * C.R.S. = Comparative Rail
Steel
The balance of the chemical composition is unavoidable impurities
and Fe.
#: Ref. N. = Reference Numeral
CA 02222281 1997-11-25
- 52 -
Table 14 f~''nni-; n"o.a ~f,..l..,., m.~t-,~ .~ ~ -~ v
Rail Ref. Chani.cal Acceler-Cooling
N composition
# (wt.~)
____-_____-__________________
. _______________ ated rate
cooling
range
C in (C
Si head
Mn portion /sec)
Cr (C)
Mo,V,Nb,
Si/4+
Co,B
Mn/2+Cr
C.R.S.*112 Q.780.54 0.98 0.30 - 0.93 - -
C.R.S.*113 Q-81810.56 1.39 - V: 0.040.84 - -
C.R.S.*114 Q.800.81 0.89 0.51 - 1.16 - - .
C.R.S.*115 Q81810.79 1.30 - V: 0.060.85 - -
C.R.S.*116 1.001.44 0.23 0.44 - 0.92 - -
C.R.S.*117 1.020.42 0.88 0-6565- 1.20 745-544 6
C.R.S.*118 1-28280.88 0.38 0.40 - 0.81 764-561 2
C.R.S.*119 0.910.91 0.38 0.29 - 0.71 801 12
(start)
C.R.S.*120 0.910.38 0.38 0.48 - 0.77 741-640 13
Table 14 (Continued)
Rail Ref. Other Hard- Micro- Wear HardnessDifference
heat
N.# treatmentness structureamount of headin
of of
conditionshead head portion portionhardness
portion in between
in base welded base
rail
rail joint and welded
(g/700,000 joint
(Hv) times) (Hv) (~v)
C.R.S.*112 - 394 Pearlite 1.10 - -
C.R.S.*113 - 382 Pearlite 1.17 - -
C.R.S.*114 - 402 Pearlite 1.05 - -
C.R.S.*115 - 386 Pearlite 1.16 -
C.R.S.*116 - surface rolling
defects
produced
during
C.R.S.*117 - 404 Pearlite 0.72 trace
amount
(4~)
of
martensite
formed
in
segregation
in
column
ortion
C.R.S.*118 - 441 Pearlite - - _
+
~roeutectoid
em n i
C.R.S.*119 Pearlite 396 Pearlite 0.92 360
formation
ratio
74$
C.R.S.*120 Controlled408 Pearlite 0.82 364
cooling
range
640-544C
Cooling
rate
3C/sec
CA 02222281 1997-11-25
- 53 -
Table 15 (Continued from Table 14)
Rail Ref.Chemical Acceler-Cooling
composition
(wt.~)
N.# ________________________________-_-___-_____
ated rate
cooling
range
in
head
C portion(C
Si
Mn
Cr
Mo,V,Nb,
Si/4+
Co,B (C) /sec)
I~/2+Cr
C.R.S.*121 0.91 0.80 0.34 0.49 V: 0.86 774-5048-~,
0.04
control-
lable
C.R.S.*122 0.91 0.80 0.34 0.49 V: 0.86 741 ~-13
0.04
(start)
control-
lable
C.R.S.*123 0.91 0.80 0.34 0.49 V: 0.86 764-6423~
0.04
C.R.S.*124 0.95 0.96 0.24 0.45 - 0.81 764-~$$5
C.R.S.*125 0.95 0.96 0.24 0.45 - 0.81 761 ~2
(start)
C.R.S.*126 0.95 0.96 0.24 0.45 - 0.81 761-62114-$
control-
lable
C.R.S.*127 0.99 0.98 0.39 0.38 - 0.82 824-~ 6
C.R.S.*128 0.99 0.98 0.39 0.38 - 0.82 764 16
(start)
C.R.S.*129 0.99 0.98 0.39 0.38 - 0.82 774-60315
CA 02222281 1997-11-25
- 54 -
Table 15 (Continued)
Rail Ref.Other Hard- Micro- Wear HardnessDifference
heat
N.# treatmentness structureamount of in
of head
conditionshead of head portionhardness
portionportion in between
in base weldedbase rail
rail joint and welded
(g/700,000 joint
(HV) times) (Hv) (~Fiv)
C.R.S.*121 - 462 Pearlite- _ _
+
spa-r
n i
C.R.S.*122 Pearlite ,~ Pearlite- _ _
formation
ratio ness
72$ in un-
stably
cooled
ortion
C.R.S.*123 Controlled574 Pearlite- _ _
+
C0011ng ~-r i
range
642-464C
Cooling
rate
7C/sec
C.R.S.*124 - 486 Pearlite- _ _
+
mar n
i
C.R.S.*125 Pearlite 446 Pearlite- _
+
formation r
ma -
ncira
ratio
79~
C.R.S.*126 Controlled~ Pearlite- - _
cooling y~.d_
age ness
' 621-641C in un-
Cooling stably
rate
6C/sec cooled
ortion
C.R.S.*127 - ~, Pearlite- _ _
C.R.S.*128 Pearlite 513 Pearlite- _ _
+
formation ~,--r_e,_,_si
to
ratio
C.R.S.*129 Controlled498 Pearlite- _ _
+
cooling mo-r
;
range
603-472C
Cooling
rate
14C/sec
CA 02222281 1997-11-25
- 55 -
Table 16 (Continued from Tah~P
Rail Ref.Chemical Acceler-Cooling
composition
(wt.~)
N.# ___________~ ated rate
______-~______~_~-_____
cooling
range
in
head
- C portion (C
Si
Mn
Cr
Mo,V,Nb,
Si/4+
Co,B (C) /sec)
I~/2+Cr
C.R.S.*130 1.18 0.90 0.360.40 - 0.81 841-541 Q_,~
C.R.S.*131 1.18 0.90 0.360.40 - 0.81 836 29
(start)
C.R.S.*132 1.18 0.90 0.360.40 - 0.81 84I-741 28
Table 16 (Continued)
Rail Ref.Other Hard- Micro- Wear HardnessDifference
heat
N.# treatmentness structure amount of headin
conditionsof of
head head portion portionhardness
portion in bety~e~
in base welded base
rail
rail joint and welded
(g/700,000 joint
(Hv) times) (Hv) (~v)
C.R.S.*130 - 456 Pearlite - - -
+
nroeutectoid
t-aman_t
i to
C.R.S.*131 Pearlite 472 Pearlite - _ _
+
formation marte~ite
ratio
C.R.S.*132 Controlled466 Pearlite - - _
+
cooling proeutectoid
range cementite
741-522C
Cooling
rate
Q.4C/sec
Note: *: R.S.I. = Rail Steel of Invention, * C.R.S. = Comparative Rail
- Steel
The balance of the chemical composition is unavoidable impurities
and Fe.
#: Ref. N. = Reference Numeral
In addition, the hardness of the base steel and that
of the flash butt welded joint of any of the rail steels
shown in Tables 5 to 8 and Tables 11 to 14 are average
values of the head portions, and are neither the maximum
values nor the minimum ones.
Furthermore, Fig. 9 shows the relationship between a
hardness and a wear amount of the rail steels of the
present invention and the comparative rail steels
(eutectoid carbon steels: reference numerals of 64 to 67)
listed in Tables 5 to 10 to compare the wear test results.
Fig. 10 shows instances of the hardness distributions of
the head portions of the welded joints of the rail steels
CA 02222281 1997-11-25
- 56 -
of the present invention (reference numerals: 41, 44) and
the comparative rail steels (reference numerals: 71, 72)
shown in the examples in Tables 5 to 10.
Still furthermore, Fig. 11 shows the relationship
between a hardness and a wear amount of the rail steels of
the present invention and the comparative rail steels
(eutectoid carbon steels: reference numerals of 112 to _
115) listed in Tables 11 to 16 to compare the wear test
results. Fig. 12 shows instances of the hardness
distributions of the head portions of the welded joints of
the rail steels of the present invention (reference
numeral: 91) and the comparative rail steel (reference
numeral: 120) shown in the examples in Tables 11 to 16.
In addition, the rails used in the examples are as
described below.
(Examples in Tables 5 to 10)
* Rails of the present invention (27 pieces) with
reference numerals of 37 to 63:
the steel rails being heat treated rails each
having a chemical composition as mentioned above and a
pearlite structure to the depth of at least 20 mm from the
rail head portion surface as a starting point, the
pearlite structure having a Vickers hardness of at least
320, and the head portion having been subjected to
accelerated cooling.
* Comparative rails (21 pieces):
comparative rails (4 pieces) with reference
numerals of 64 to 67: prepared from eutectoid carbon
steels having chemical compositions outside the scope of
the claims of the present invention,
comparative rails (5 pieces) with reference
numerals of 68 to 72: prepared from hyper-eutectoid carbon
steels having chemical compositions outside the scope of
the claims of the present invention, and
comparative rails (12 pieces) with reference
numerals of 73 to 84: prepared under heat treatment
conditions outside the scope of the claims of the present
CA 02222281 1997-11-25
a
- 57 -
invention.
(Examples in Tables 11 to 16)
* Rails of the present invention (8 pieces) with
reference numerals of 85 to 111:
the steel rails being heat treated rails each having
a.chemical composition as mentioned above and a pearlite
structure to the depth of at least 20 mm from the rail
head portion surface as a starting point, the pearlite
structure having a Vickers hardness of at least 320, and
the head portion having been subjected to accelerated
cooling.
Comparative rails (21 pieces):
comparative rails (4 pieces) with reference
numerals of 112 to 115: prepared from eutectoid carbon
steels having chemical compositions outside the scope of
the claims of the present invention,
comparative rails (5 pieces) with reference
numerals of 116 to 120: prepared from hyper-eutectoid
carbon steels having chemical compositions outside the
scope of the claims of the present invention, and
comparative rails (12 pieces) with reference
numerals of 121 to 132: prepared under heat treatment
conditions outside the scope of the claims of the present
invention.
As shown in Figs. 5, 7, 9 and 11, any of the rail
steels of the present invention shows a decreased wear
amount compared with any of the corresponding comparative
steels having the same hardness as a result of making the
carbon content high compared therewith, and consequently a
significantly improved wear resistance even when the rail
steel of the invention has the same hardness as the
conventional steel_ Moreover, a pearlite structure
excellent in wear resistance can be stably formed without
forming martensite, bainite and proeutectoid cementite
detrimental to the toughness, wear resistance and
ductility by allowing the chemical composition fall into
an appropriate range and selecting appropriate heat
CA 02222281 1997-11-25
- 58 -
treatment conditions as shown in Tables 1 to 4.
As shown in Figs. 6 and 10, a.decrease in the
hardness of the welded joint taking place at the time when
the addition amount of Cr is up to 0.50 (comparative
rails with reference numerals 17, 71) or formation of
abnormal structures such as martensite taking place at the
time when the addition amount of Cr is at least 1.00
(comparative rails with reference numerals 18, 72) can be
prevented by adding Cr in an amount of more than 0.50 to
1.00, and the difference in hardness between the rail
base steel and the welded joint can be made not more than
30_ Partial wear such as a local wear dent caused by the
wear of the head top surface of the welded joint in an as-
welded state (without heat treatment) can thus be
prevented.
As shown in Figs. 8 and 12, a decrease in the
hardness of the welded joint taking place at the time when
the addition amount of Si is less than 0.40 (comparative
rails with reference numerals 35, 120) can be prevented by
adding Si in an amount of 0.40 to 1.00, and the
difference in hardness between the rail base steel and the
welded joint can be made not more than 30. Partial wear
such as a local wear dent caused by the wear of the head
top surface of the welded joint in an as-welded state
(without heat treatment) can thus be prevented.
Industrial Applicability
As shown in Figs. 5, 7, 9 and 11, any of the rail
steels of the present invention shows a decreased wear
amount compared with any of the corresponding comparative
steels having the same hardness as a result of making the
carbon content high compared therewith, and consequently a
significantly improved wear resistance. Moreover, a
pearlite structure excellent in wear resistance can be
stably formed in a rail without forming martensite,
bainite and proeutectoid cementite detrimental to the
ductility, toughness and wear resistance by allowing the
CA 02222281 1997-11-25
- 59 -
chemical composition fall into an appropriate range and
selecting appropriate heat treatment conditions as shown
in Tables 11 to 16.
Moreover, the present invention has the following
advantages as shown in Figs. 6, 8, 10 and 12: a decrease
in the hardness on the weld line caused by decarburization
is improved; no abnormal structures such as martensite are
formed in a welded joint (portion having been reheated to
the austenite region); and a difference in Vickers
hardness between the base steel and the welded joint is up
to 30, and partial wear such as a local wear dent caused
by the wear of the head top surface of the welded joint in
an as-welded state (without heat treatment) can be
prevented.
According to the present invention as described
above, a rail excellent in wear resistance and weldability
(welding construction, properties of the welded joints)
can be provided to heavy load railroads.