Note: Descriptions are shown in the official language in which they were submitted.
' CA 02222517 1997-11-27
BAYER AWGESEIL.d~.SCHAFT 51368 Leverl~tsen
Knmxrnzentrale RP
Patente Komem Rt/li/SP
b" 1 ~.. ~.f a s ' ~ f 1'. ~ .~ ~..a-.. .:~ ~:. i~.d
T~RAra~~,.~-rla~v
Compositions to comt~at i6c protozoa
The present invention relates to mixtures of substituted benzimidazoles with a
polyether
antibiotic or a synthetically prepared coccidiostat as compositions to control
parasitic
protozoa and, in particular, coccidia, and fish parasites and insect
parasites.
Substituted benzimidazoles and their use as insecticides, fungicides and
herbicides have
already been disclosed (EP-OS (European Published Specifications) 87 375, 152
360,
181 826, 239 508, 260 744, 266 984, US-A 3 418 318, 3 472 865, 3 576 818,
3 728 994).
Halogenated benzimidazoles and their effect as anthelinintics, coccidiostats
and
pesticides have been disclosed (DE-OS (German Published Specification) 2 047
369,
DE-OS (German Published Specification) 4 237 617). IVfixttlres of vitro-
substituted
benzimidazoles and polyether antibiotics have been disclosed as coccidiostats
(US-A 5 331 003). Their effect is as yet unsatisfactory in all cases.
Coccidiosis is a disease caused by single-cell parasites (protozoa). It may
cause great
losses in particular in poultry rearing. In order to avoid this, the stocks
are treated
prophylactically with coccidiostats. The development of resistance to the
compositions
used results after only a relatively few years in serious problems. On the
other hand,
it is possible by using chemically completely new coccidiostats, in particular
combinations, to control even polyresistant parasite strains.
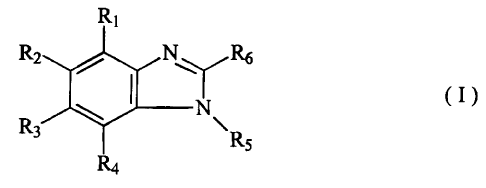
The present invention relates to mixtures of substituted benzimidazoles of the
formula
(I)
Le A 31 085
' CA 02222517 1997-11-27
R~
Rz , N ~ Rs
w I N
Rs ~ . Rs
Ra
in which
Ri, R2, R3 and Ra each, independently of one another, represent hydrogen,
halogen,
represent in each case optionally substituted alkyl, alkoxy, alkylthio,
alkylsulphinyl, allcylsulphonyl, represent optionally substituted fused-on
dioxyalkylene, but where at least one of the substituents RI, R~, R3 and Ra is
different from hydrogen and halogen,
R; represents hydrogen, represents alkyl which is substituted one or more
times,
identically or differently, by OIL CN, NHS, alkyl, cycloalkyl, alkenyl,
alkinyl,
alkoxy, halogenoalkoxy, alkylthio, halogenoalkylthio, alkenoxy, alkinoxy, -
aminocarbonyl, optionally substituted alkylcarbonyl, optionally substituted
(het-)arylcarbonyl, optionally substituted alkoxycarbonyl (AlkO-CO-), optional
1y
substituted alkoxycarbonyloxy (AIkOC00-), aminosulphonyl (SOZNH2),
optionally substituted mono- or dialkylaminosulphonyl, acylated amino
(AIkCON(R~)- or AIkOCON(R~)-), where R~ is equal to hydrogen, alkyl or
cycloalkyl, or optionally substituted alkylsulphonylamino (AlkylS02NH-), or
allLylsulphonyl-N-alkylamino (AtylS02NAllcy1-), optionally substituted aryl-
sulphonylamino (Ary1S02NH ) or arylsulphonyl-N-alkylamino (Ary1S02NAlk-),
optionally substituted dialkylamino, furthermore RS represents optionally
substituted alkoxycarbonyl, optionally substituted (het-)aryloxycarbonyl,
allylsulphonyl, alkenylsulphonyl, (het-)arylsulphonyl, or-SOZNRgRg, -CONR8R9
or -P(O)(NRBRg)z, where R8 and R9 represent H or alkyl which is optionally
substituted by one or more radicals,
R6 represents fluoroalkyl,
LeA310$~ -2-
~ CA 02222517 1997-11-27
with one or more of the following compounds:
Polyether antibiotics such as maduramycin, lasalocid, monensin, narasin,
salinomycin
or synthetic coccidiostats such as
1 (-(4-Amino-2-n-propyl-5-pyrimidinylinethyl)-2-picolinium Amprolium
chloride
1(-(4-Amino-2-n-propyl-5-pyrimidinylinethyl)-2-picolinium Amprolium +
chloride + sulfaquinoxaline sulfaquinoxaline
1 (-(4-Amino-2-n-propyl-5-pyrimidinylmethyl)-2-picolinium Amprolium +
chloride + sulfaquinoxaline + ethopabate sulfaquinoxaline + .
ethopabate
4,4-Dinitrocarbanilide + 2-hydroxy-4,6-dimethylpyrimidine Nicarba7.ln_
3,5-Dichloro-2,6-dimethyl-4-pyridinol Clopidol
3,5-Dichloro-2,6-dimethyl-4-pyridinol + methyl 7-benzyloxy- Clopidol +
6-butyl-1,4-dihydro-4-oxylquinoline-3-carboxylate methylbenzoquate
Ethyl 6-n-decyloxy-7-ethoxy-4-hydroxyquinoline-3- Decoquinate
carboxylate
9-(2-Chloro-6-fluorophenylmethyl)-9H-purin-6-amine Arprinocid
(+)-2,6-Dichloro-alpha-(4-chlorophenyl)-4--(4,5-dihydro-3,5-
Benzeneacetonitrile,
dioxo-1,2,4-iriazin-2(3H)-yl)-benzeneacetonitrile diclazuril
1-[3-Methyl-4-(4'-trifluoromethylthiophenoxy)-phenyl]-3- Toltrazuril
methyl-1,3,5-triazine-2,4,6(1H,3H,SH)-trione
4,4-Dinitrocarbanilide + 2-hydroxy-4,6-dimethylpyrimidine Robenidine
[= nicarbazin]
7-Bromo-6-chloro-febrifugin Halofuginone
3,5-Dinitro-o-toluamide Zoalene
LeA31085 -3-
' CA 02222517 1997-11-27
> >
as compositions for controlling parasitic protozoa, in particular coccidia, in
livestock.
The benzimidazoles which can be used according to the invention are known.
They are
generally defined by the formula (I). Preferred compounds of the formula (I)
are those
in which
Ri, R~, R3 and R4 each, independently of one another, represent hydrogen,
fluorine,
chlorine, bromine, iodine, represent in each case straight-chain or branched
alkyl, alkoxy, alkylthio, alkylsulphinyl, alkylsulphonyl with in each case 1
to 8
carbon atoms, represent cycloalkyl with 3 to 8 carbon atoms, represent in each
case straight-chain or branched halogenoalkyl, halogenoalkoxy,
halogenoalkylthio, halogenoalkylsulphinyl, halogenoalkylsulphonyl with in each
case 1 to 6 carbon atoms and 1 to 13 identical or different halogen atoms or
represent optionally singly or multiply, identically or differently by 1 to 10
halogens and/or straight-chain or branched alkyl with 1 to 4 carbon atoms
and/or straight-chain or branched halogenoalkyl with 1 to 4 carbon atoms and
1 to 9 halogen atoms doubly linked dioxyalkylene with: 1 to 5 carbon atoms,
but where at least one of the substituents RI, RZ, R3 and Rø is different from
hydrogen
and halogen,
RS represents hydrogen, represents alkyl with 1 to 4 carbon atoms, which is
substituted one or more times, identically or differently, by OH, CN, NH2,
alkyl
with 1 to 4 carbon atoms, cycloalkyl with 3 to 10 carbon atoms, alkenyl with
2 to 6 carbon atoms, alkinyl with 2 to 6 carbon atoms, alkyloxy with 1 to 6
carbon atoms, halogenoalkoxy with 1 to 6 carbon atoms and 1 to 5 halogens,
alhylthio with 1 to 6 carbon atoms, halogenoalkylthio with 1 to 6 carbon atoms
and 1 to 5 halogens, alkenyloxy with 2 to 6 carbon atoms, alkinyloxy with 3 to
6 carbon atoms, optionally substituted alkylcarbonyl with 1 to 6 carbon atoms,
optionally substituted phenyl- or hetarylcarbonyl, optionally substituted
alkoxycarbonyl with 1 to 6 carbon atoms, optionally substituted
alkoxycarbonyloxy with 1 to 6 carbon atoms, aminosulphonyl (NH2SO2-),
optionally substituted mono- or dialkylaminosulphonyl with 1 to 6 carbon
IxA3108~ -4-
' CA 02222517 1997-11-27
atoms, acylated amino (AIkOCON(R~)- or (AIkCON(R~~) where R~ is equal to
hydrogen or alkyl with 1 to 6 carbon atoms or cycloalkyl with 3 to 8 carbon
atoms, with 1 to 6 carbon atoms, optionally substituted alkylsulphonylamino
with 1 to 6 carbon atoms, optionally substituted alkylsulphonyl-N-alkylamino
with 1 to 6 carbon atoms, optionally substituted phenylsulphonylamino,
optionally substituted phenylsulphonyl-N-alkylamino with 1 to 6 carbon atoms,
optionally substituted dialkylamino with 1 to 8 carbon atoms, fiu-thermore RS
represents the optionally substituted radicals alkyloxycarbonyl with 1 to 6
carbon atoms, alkylsulphonyl or alkenylsulphonyl with 1 to 6 carbon atoms,
which are optionally substituted by 1 to 13 halogen atoms, optionally
substituted phenylsulphonyl, optionally substituted heteroarylsulphonyl,
phenoxycarbonyl, or -S02NR$R~, -CONR$Rg or -P(O)(NRgR9~, where Rg and R9
represent hydrogen or an alkyl with 1 to 4 carbon atoms, which are optionally
substituted by one of the radicals mentioned above for R5,
Substituents of the optionally substituted radicals which may be mentioned are
the
following substituents:
Halogen, OIL NH2, alkylamino with 1 to 6 carbon atoms, cyano, nitro, C02I~ in
each
case straight-chain or branched alkyl, alkoxy, alkylthio, alkylsulphinyl or
alkylsulphonyl with, in each case 1 to 6 carbon atoms, in each case straight-
chain or
branched halogenoalkyl, halogenoalkoxy, halogenoalkylthio,
halogenoalkylsulphinyl or
halogenoalkylsulphonyl with, in each case, 1 to 6 carbon atoms and 1 to 13
identical
or different halogen atoms, in each case straight-chain or branched
alkoxyalkyl,
alko~yallcoxy, alkanoyl, alkoxycarbonyl or alkoximinoalkyl with, in each case,
1 to 6
carbon atoms in the individual alkyl moieties, doubly linked dioxyallcylene
which is
optionally substituted 1 to 6 times, identically or differently, by halogen
and/or straight-
chain or branched alkyl with 1 to 6 carbon atoms and/or straight-chain or
branched
halogenoalkyl with 1 to 6 carbon atoms and 1 to 13 identical or different
halogen
atoms and has 1 to 6 carbon atoms, or phenyl or phenoxy which is optionally
substituted one to five times, identically or differently, by halogen and/or
straight-chain
or branched alkyl with 1 to 4 carbon atoms and 1 to 9 identical or different
halogen
atoms, each of which in turn can carry the abovementioned radicals.
GeA3108~ -5-
CA 02222517 1997-11-27
Acyl radicals of the listed radicals which may be mentioned are the radicals
alkoxycarbonyl with 1 to 6 carbon atoms, alkylcarbonyl with 1 to 6 carbon
atoms,
alkoxycarbonyloxy with 1 to 6 carbon atoms, alkylsulphonyl with 1 to 6 carbon
atoms,
benzoyl, which is optionally substituted by one of the abovementioned
radicals,
alkenylcarbonyl with 2 to 6 carbon atoms.
R6 represents 1 to 15 fluoro-Cl-C,-alkyl.
The substituents in compounds of the formula (I) particularly preferably have
the
following meaning:
RI, R2, R3 and R~ in each case, independently of one another, represent
hydrogen,
fluorine, chlorine or bromine, represent in each case straight-chain or
branched
alkyl, alkoxy, alkylthio, alkylsulphinyl, alkylsulphonyl with, in each case, 1
to
4 carbon atoms, represent cycloalkyl with 3 to 6 carbon atoms, represent in
each case straight-chain or branched halogenoalkyl, halogenoalkoxy,
halogenoalkylthio, halogenoalkylsulphinyl, halogenoalkylsulphonyl with, in
each
case, 1 to 4 carbon atoms and 1 to 9 identical or different halogen atoms, in
particular fluorine and chlorine atoms or represent optionally singly or
multiply,
identically or differently, by 1 to 6 halogens, in particular fluorine and
chlorine
atoms and/or straight-chain or branched alkyl with 1 to 4 carbon atoms and/or
straight-chain or branched halogenoallcyl with 1 to 4 carbon atoms and 1 to 6
halogen atoms doubly linked dioxyalkylene with 1 to 3 carbon atoms, but where
at least one of the substituents RI, R2, R3 and R4 is different from hydrogen
and
halogen,
RS represents hydrogen, represents alkyl with 1 to 3 carbon atoms, in
particular
represents methyl and ethyl, which is substituted by OIL CN, NHZ, alkyl, with
1 to 4 carbon atoms, in particular methyl or ethyl, alkenyl with 2 to 4 carbon
atoms, alkinyl with 2 to 4 carbon atoms, alkyloxy with 1 to 4 carbon atoms, in
particular alkoxy such as methoxy, ethoxy, propoxy, i-propo~y, halogenoalkoxy
with 1 to 4 carbon atoms and 1 to 5 halogen atoms, in particular
trifluoromethoxy, pentafluoroethoxy, fluoropropoxy, alkylthio with 1 to 4
carbon atoms, halogenoalkylthio with 1 to 4 carbon atoms and 1 to 5 halogens,
l~e A 31 085 - 6 -
. CA 02222517 1997-11-27
alkenyloxy with 2 to 4 carbon atoms, alkinyloxy with 3 to 4 carbon atoms,
optionally substituted alkylcarbonyl with 1 to 4 carbon atoms, optionally
substituted phenylcarbonyl, optionally substituted alkoxycarbonyl with 1 to 4
carbon atoms, in particular alkoxycarbonyls such as methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, i-propoxycarbonyl, t-buto~cycarbonyl,
optionally substituted alkoxycarbonyloxy with 1 to 4 carbon atoms,
aminosulphonyl (NH2S02-), optionally substituted mono- or
dialkylaminosulphonyl with 1 to 4 carbon atoms, acylated amino with 1 to 6
carbon atoms, where R~ is equal to hydrogen or alkyl with 1 to 4 carbon atoms
or cycloalkyl with 3 to 6 carbon atoms, optionally substituted
alkylsulphonylamino with 1 to 4 carbon atoms, optionally substituted
alkylsulphonyl-N-alkylamino with 1 to 4 carbon atoms, optionally substituted
phenylsulphonylamino, optionally substituted phenylsulphonyl-N-alkylamino
with 1 to 4 carbon atoms, optionally substituted dialkylamino with 1 to 4
carbon atoms, furthermore RS represents the optionally substituted radicals
alkyloxycarbonyl with 1 to 4 carbon atoms, alkylsulphonyl or alkenylsulphonyl
with 1 to 4 carbon atoms, which are optionally substituted with 1 to 9
halogens,
optionally substituted phenylsulphonyl, heteroarylsulphonyl, where heteroaryl
represents a 5 or 6-membered heterocycle which is optionally substituted by
alkyl with 1 to 4 carbon atoms or halogen or by a substituent listed under R5,
or phenoxycarbonyl, or SOZNR8R9, CONR8R9 or -P(O)(NRgRg)2, where R$ and
R9 represent hydrogen or an alkyl with 1 to 4 carbon atoms, which are
optionally substituted by one of the radicals mentioned above for R5.
Substituents of the optionally substituted radicals which may be mentioned are
the
following substituents:
Halogen, in particular fluorine or chlorine, OH, NHS, alkylamino with 1 to 4
carbon
atoms, in particular methylamino, ethylamino, dimethylamino, diethylamino, bis-
(trifluoromethylamino), cyano, nitro, C02I~ in each case straight-chain or
branched
all'yl, alkoxy, alkylthio, alkylsulphinyl or alkylsulphonyl with, in each
case, 1 to 4
carbon atoms, in particular methyl, ethyl, methoxy, ethoxy or methylmercapto,
in each
case straight-chain or branched halogenoalkyl, halogenoalkoxy,
halogenoalkylthio,
LeA3108~ -7-
' CA 02222517 1997-11-27
halogenoalkylsulphinyl or halogenoalkylsulphonyl with, in each case, 1 to 4
carbon
atoms and 1 to 9 identical or different halogen atoms, in particular
trifluoromethyl,
pentafluoroethyl, fluorochloroethyl, trichloroethyl, trifluoromethoxy,
trichloromethoxy,
trifluoromethylmercapto, in each case straight-chain or branched alkoxyalkyl,
alkoxyalkoxy, in particular methoxyethoxy, ethoxyethoxy, ethoxyethyl,
alkanoyl,
alkoxycarbonyl or alkoxyiminoalkyl with, in each case, 1 to 4 carbon atoms in
the
individual alkyl moieties, in particular acetyl, phenyl or phenoxy which is
optionally
substituted one to five times, identically or differently, by halogen and/or
straight-chain
or branched alkyl with 1 to 4 carbon atoms and 1 to 9 identical or different
halogen
atoms, in particular phenyl or phenoxy, each of which in turn can carry the
abovementioned radicals.
R6 represents 1 to 7 fluoro-Ci-C3-alkyl.
Very particularly preferred compounds of the formula (I) are those in which
the
radicals have the following meaning:
R,, Rv R3 and R4 represent hydrogen, halogen, in particular chlorine or
bromine,
represent in each case straight-chain or branched alkyl, alkoxy, alkylthio,
alhylsulphinyl, alkylsulphonyl with, in each case 1 to 4 carbon atoms, in
particular methyl, methoxy, methylthio, methylsulphinyl or methylsulphonyl,
represent in each case straight-chain or branched halogenoalkyl,
halogenoalkoxy, halogenoalkylthio, halogenoalkylsulphinyl,
halogenoalkylsulphonyl with, in each case, 1 to 4 carbon atoms and 1 to 9
identical or different fluorine or chlorine atoms, in particular for
trifluoromethyl,
trifluoromethoxy, trifluoromethylthio, trifluoromethylsulphinyl,
trifluoromethylsulphonyl, represent doubly linked dioxyalkylene which is
2~ optionally substituted optionally by fluorine and/or chlorine atoms and has
1 to
2 carbon atoms, in particular represent OCH20, OCHZCH20, OCF2CF~0,
OCF20, OCCIFCCIFO,
R, represents hydrogen, represents methyl or ethyl which are substituted by
CN, by
allyl with 1 to 4 carbon atoms, in particular methyl, alkenyl with 2 to 4
carbon
LeA3108~ -8-
' CA 02222517 1997-11-27
atoms, in particular -CH~H2 or -CH~HMe, alkinyl with 2 to 4 carbon atoms,
in particular -CCH or -CCMe, by alkoxy with 1 to 4 carbon atoms, in particular
alkoxy such as methoxy, ethoxy, propoxy, i-propox5~, by alkinyloxy with 3 to
4 carbon atoms, in particular -OCHZCCH or -OCH2CCMe, by alkylcarbonyl
with 1 to 4 carbon atoms, in particular acetyl, propionyl, i-propionyl or t-
butionyl, by optionally substituted phenylcarbonyl, in particular benzoyl, by
optionally substituted alkoxycarbonyl with 1 to 4 carbon atoms, in particular -
C02Me, -C02Et, -COZPr, -C02i-Pr or -COZtBu, by acylated amino with 1 to 6
carbon atoms, where R~ is equal to hydrogen or alkyl with 1 to 4 carbon atoms
or cycloalkyl with 3 to 6 carbon atoms, in particular N(Me)C02Me, -
N(Me)COZEt, -N(Et)C02Et, N(Et)C02Me, -N(Pr)C02Et, N(Bu)C02Me, -N(t-
Bu)C02Me, -N(Bu)C02Et, N(C~HII)C02Et, NHCOMe, -NHCOEt or . -
NHCOPr, by optionally substituted alkylsulphonylamino with 1 to 4 carbon
atoms, optionally substituted alkylsulphonyl-N alkylamino, in particular
-NMeS02Me, NEtS02Et, NMeS02Et, NEtS02Me, optionally substituted
phenylsulphonylamino, optionally substituted phenylsulphonyl-N-alkylamino
with 1 to 4 carbon atoms, in particular -NMeS02Ph, -NEtS02Ph. R5 fiirthermore
represents the optionally substituted radicals of allcyloxycarbonyl with 1 to
4
carbon atoms, alkylsulphonyl or alkenylsulphonyl with 1 to 4 carbon atoms, in
particular MeS02-, EtS02-, PrS02- or CH2~MeCHZS02-, optionally substituted
phenylsulphonyl, in particular phenylsulphonyl or 2.4.6-trimethyl-
phenylsulphonyl, optionally substituted heteroarylsulphonyl, where heteroaryl
represents a 5 or 6-membered heterocycle which is substituted by fluorine,
chlorine or bromine and/or is substituted by an alkyl with 1 to 4 carbon
atoms,
in particular methyl and/or by a substituent mentioned under R5, in particular
Me02C and contains one to three identical or different heteroatoms, in
particular nitrogen, sulphur and/or oxygen, phenoxycarbonyl, or -SOZNR8R9,
-CONRBRg or -PO(NRgR9)2, were R8 and R9 represent hydrogen or an alkyl
with 1 to 4 carbon atoms, which are optionally substituted by one of the
radicals mentioned above for R5, in particular represent -S02NMe2 or -S02NEtv
PO(NMe2~, CONMe2 or CONiPrv
R6 represents CF3, CHF2 or CZF~.
Le~A31085 -9-
CA 02222517 1997-11-27
'Ihe following substituted benzimidazoles of the general formula (I) may be
specifically
mentioned:
R~
R2 , N~ R6
c~)
Rs ~ . Rs
Ra
No. R1 R2 R3 ~ RS
1 H Br CF3 H H CF3
2 H Br CF3 H CH2CN CF3
3 H CF3 Br H CH2CN CF3
4 H Br CF3 H CH20Et CF3
5 H CF3 Br H CH20Et CF3
6 H Br CF3 H CH2OiPr CF3
7 H CF3 Br H CH20iPr CF3
8 H Br CF3 H CHZCOMe CF3
9 H CF3 Br H CH2COMe CF3
10 H Br CF3 H CH20CH(CHZF)2 CF3
11 H CF3 Br H CH20CH(CH2F~ CF3
12 H Br CF3 H CH2N(Me)COZMe CF3
13 H CF3 Br H CH2N(Me)C02Me CF3
14 H Br CF3 H CH2N(Bu)C02Et CF3
15 H CF3 Br H CH2N(Bu)C02Et CF3
16 H Br CF3 H CH2N(t-Bu)C02Et CF3
~x A 31 085 - 10 -
CA 02222517 1997-11-27
No. R1 R2 R3 R4 R5 R6
17 H CF3 Br H CH2N(t-Bu)C02Et CF3
18 H Br CF3 H CH2N(C6H1,rC02Et CF3
19 H CF3 Br H CH2N(C6H11~COZEt CF3
20 H Cl CF3 H H CF3
S 21 H Cl CF3 H CH2CN CF3
22 H CF3 Cl H CH2CN CF3
23 H Cl CF3 H CH20Et CF3
24 H CF3 Cl H CH20Et CF3
2S H Cl CF3 H CH2COMe CF3
26 H CF3 Cl H CH2COMe CF3
27 H Cl CF3 H CH(Me)COMe . CF3
28 H CF3 Cl H CH(Me)COMe CF3
29 H Cl CF3 H CH2COtBu CF3
30 H CF3 Cl H CH2COtBu CF3
1 31 H Cl CF3 H CH2COPh CF3
S
32 H CF3 Cl H CH2COPh CF3
33 H Cl CF3 H CH2CCH CF3
3-1 H CF3 Cl H CH2CCH CF3
3~ H Cl CF3 H CHZNHCOMe CF3
36 H CF3 Cl H CH2NHCOMe CF3
37 H Cl CF3 H CH2N(tBu)C02Me CF3
38 H CF3 Cl H CH2N(tBu)COZMe CF3
Ix A 31 08S - 11 -
CA 02222517 1997-11-27
No. R1 R2 R3 R4 RS R6
39 H Cl CF3 H CH2N(Me)C02Me CF3
40 H CF3 Cl H CH2N(Me)C02Me CF3
41 H CF3 CI H CH2N(Et)COZMe CF3
42 H CI CF3 H CH2N(Et)C02Me CF3
43 H CF3 Cl H CH2N(Me)S02Ph CF3
44 H Cl CF3 H CH2N(Me)S02Ph CF3
45 H CF3 Cl H CHZN(Me)S02Me CF3
46 H Cl CF3 H CH2N(Me)SOZMe CF3
47 H SOCF3 Cl H H CF3
48 H S02CF3 Cl H H CF3
49 H OCF3 CI H H CF3
50 H OCF3 Cl H CH2N(Bu)C02Et CF3
51 H CI OCF3 H CH2N(Bu)C02Et CF3
52 H OCF3 Cl H CH2N(Pr)C02Et CF3
I 53 H Cl OCF3 H CHZN(Pr)COZEt CF3
S
54 H OCF3 CI H CH2N(Me)C02Me CF3
55 H CI OCF3 H CH2N(Me)COZMe CF3
~6 H OCF3 CI H CH2CN CF3
~7 H CI OCF3 H CH2CN CF3
~ H Br OCF3 H H CF3
8
59 H Br OCF3 H CH2CN CF3
60 H OCF3 Br H CH2CN CF3
Le A 31 085 - 12 -
CA 02222517 1997-11-27
No. R1 R2 R3 ~ ~
61 H Br OCF3 H CH20Et CF3
62 H OCF3 Br H CH20Et CF3
63 H OCF3 Br H S02NMe2 CF3
64 H Br OCF3 H CH2N(Me)C02Me CF3
65 H OCF3 Br H CH2N(Me)C02Me CF3
66 H Br OCF3 H CH2N(Et)C02Et CF3
67 H OCF3 Br H CH2N(Et)C02Et CF3
68 H Br OCF3 H CH2N(Me)C02Et CF3
69 H CF30 OCH3 H H CF3
70 H CF3 OCH3 H H CF3
71 H OCF3 OCF3 H SOZNMe2 CF3
72 H CF30 OCF3 H CH20CH2CCH CF3
73 H OCF20 H H CF3
74 H OCF2CF20 H H CF3
75 H OCF2CF20 H H CHF2
76 H OCF2CF20 H H C2F5
77 H OCF2CF20 H CHZCN CF3
78 H OCF2CF20 H CHZOEt CF3
_
79 H OCFZCF20 H CHZOiPr CF3
80 H OCF2CF20 H CH2N(Me)C02Et CF3
81 H OCFZCFZO H CH2N(Me)C02Me CF3
82 H OCF2CF20 H CHZN(C6H,1)C02Et CF3
IxA31085 -13-
CA 02222517 1997-11-27
No. R, R2 R3 R4 RS R6
83 H OCFZCF20 H CH2N(C6H11)C02Et CHF2
84 H OCFHCF20 H H CF3
85 H O(CCIF)20 H H CF3
86 H O(CCIF)20 H CHZOEt CF3
87 H O(CCIF)ZO H CH2N(Me)COZEt CF3
88 H O(CCIF)20 H CH2CN CF3
89 H O(CH2)20 H CHZOEt CF3
90 Cl H S02CF3 H H CF3
91 Cl H S02CF3 H H CHF2
92 Br H CF3 H CHZCOtBu CF3
93 Br H CF3 H CH20Et CF3
94 Br H CF3 H CHZC02Bu CF3
95 Br H CF3 H CH2N(Me)C02Me CF3
96 Br H CF3 H CHZCH~H2 CF3
97 Br H CF3 H H CF3
98 Br H CF3 H CHZN(iPr)COZEt CF3
99 Br H CF3 H CH2N(C6HI,)C02Et CF3
100 Br H SOZMe H H CF3
101 Br H S02CF2 H H CF3
102 CF3 H Cl H H CF3
103 CF3 H Cl H CH2CN CF3
104 CF3 H Cl H CH2COPh CF3
Ix A 31 085 - 14 -
CA 02222517 1997-11-27
No. R1 R2 R3 R4 R5 R6
105 CF3 H Cl H CH20Et CF3
106 CF3 H Cl H CH2N(Me)COZMe CF3
107 CF3 H Cl H CH2N(Me)COZEt CF3
108 CF3 H Cl H CH2N(tBu)C02Et CF3
109 CF3 H Cl H CH2N(Bu)C02Et CF3
1I0 CF3 H Cl H CH2N(Et)C02Et CF3
111 CF3 H Cl H CH2N(C6H11)C02Et CF3
112 CF3 H Br H H CF3
113 CF3 H Br H H CHF2
114 CF3 H Br H CHZCN CF3
115 CF3 H Br H CH2N(Me)S02Me CF3
116 CF3 H Br H CH20Pr CF3
117 CF3 H Br H CH2N(Me)C02Me CF3
118 CF3 H Br H CH2N(Pr)C02Et CF3
119 CF3 H Br H CHZN(Et)C02Et CF3
120 CF3 H Br H CH2N(C6H11)C02Et CF3
121 CF3 H CF3 H H CF3
122 CF3 H CF3 H H CHF2
123 CF3 H CF3 H CH2CN CF3
124 CF3 H CF3 H CH2N(Et)C02Et CF3
125 CF3 H CF3 H CHZN(Pr)C02Et CF3
126 CF3 H CF3 H CH2N(C6HI1)C02Et CF3
LeA31085 -15-
CA 02222517 1997-11-27
No. R, R2 R3 R4 RS R6
127 Br H SCF3 H H CF3
128 Br H SCF3 H CH2N(Pr)C02Et CF3
129 CN H CF3 H H CF3
130 CN H CF3 H H CHFZ
131 CF3 OCF2- H H SOZNMe2 CF3
CHFCF3
132 H SCF3 H H CF3
.
I33 H SCF3 CH20Et CF3
134 H SOZMe H CF3
135 H S02CF3 H CF3
136 H OCF3 H H H CF3
137 Br H CF3 H CON(CH3)2 CF3
~
138 Br H CF3 H soz s ci CF3
\ /
139 Br H CF3 H SO~CH2C(CH3)CH2 CF3
140 Br H CF3 H sot S CF3
\ /
141 Br H CF3 H sot s Br CF3
\ /
Br
LeA31085 -16-
CA 02222517 1997-11-27
No. R1 R2 R3 R4 R5 R6
142 Br H CF3 H CH3 CF3
So2
O
i
-N
H3C
143 Br H CF3 H COOCH(CH3}z CF3
144 Br H CF3 H S02-CH3 CF3
145 Br H CF3 H PO(N(CH3)z)2 CF3
146 H OCFZO H soz CF3
I ~ CH3
H3COOC
147 H OCF20 H sot CF3
~
N-CH3
148 H OCF20CF20 H sot S CF3
I ~ ci
149 H OCF20CF20 H CON(C(CH3)2~ CF3
150 H OCF20CF20 H cH3 CF3
sot
. I \N
H3C ~ i
Le A 31 085 - 17 -
CA 02222517 1997-11-27
No. ( Rl ~ RZ ~ R3 R4 RS R6
151 H OCF20CF20 H CH3 CF3
sot
H3C CH3
152 H OCF20 H CH3 CF3
S Oz
I
H3C CH3
153 H OCF20CF20_ H cH3 CF3
CHZ
v
I ~N
HsC O
154 H OCF20 H S02CH2C(CH3)CH2CF3 CF3
15~ H OCF20 H sot s CF3
I / ci
156 H OCFZOCF20 H sot s CF3
I /
15 7 H OCFZO H s o 2 s CF3
I /
L,e A 31 085 - 18 -
CA 02222517 1997-11-27
No. R1 RZ R3 Rø RS R6
158 H OCF20CF20 H cH3 CF3
sot
I \N
CI N
CH3
159 H OCFZO H sot CF3
Br
S
Br
160 H OCFZCFZO H sot CF3
Br
s
Br
161 H OCF20 H CH3 CF3
sot
I ~~N
HsC O
162 H Br CF3 H SOZCH3 CF3
163 H Br CF3 H CH3 CF3
sot
I \N
,
HsC O
16-~ H Br CF3 H S02N(CH3)2 CF3
16~ H OCF2CF20 H S02CH2C(CH3)CHZ CF3
L.eA3108~ -19-
CA 02222517 1997-11-27
No. R1 R2 R3 R4 R5
166 H OCF2CF20 H S02CH3 CF3
167 H OCFZCF20 H PO-(N(CH3)2)2 CF
3
168 H Br CF3 H CO-OCH(CH3~ CF3
169 H Br CF3 H C02N(CH3)2 CF3
170 H Br CF3 H PO-(N(CH3)~ CF3
I~e A 31 085 - 20 -
CA 02222517 1997-11-27
Particularly preferred compounds from the series of substituted benzimidazoles
which
may be particularly mentioned are:
R,
R2 ~ N ~ R6
N
Rs ~ . Rs
No. ~ Rl ( R2 ~ R3_ R4 R5 R6
_4
1 H Br CF3 H H CF3
2 H Br CF3 H CHZCN CF3
3 H CF3 Br H CHZCN CF3
4 H Br CF3 H CH20Et CF3
5 H CF3 Br H CH20Et CF3
58 H Br OCF3 H H CF3
59 H Br OCF3 H CH2CN CF3
60 H OCF3 Br H CH2CN CF3
74 H OCF2-CF20 H H CF3
83 H OCF2-CF20 H CH2N(C6H11)-C02Et CF3
92 Br H CF3 H CH2COtBu CF3
9~ Br H CF3 H CH2N(Me)C02Me CF3
96 Br H CF3 H CH2CH~H2 CF3
99 Br H CF3 H CHZN(C6HI1)-COZEt CF3
102 CF3 H Cl H H CF3
LeA31 085 -21 -
CA 02222517 1997-11-27
The parasitic protazoa include:
Mastigophora (Flagellate) such as, for example Trypanosomatidae, for example
Trypanosome b. brucei, T.b. gambiense, T.b. rhodesiense, T. congolense, T.
cruzi, T.
evansi, T. equinum, T. lewisi, T. percae, T. simiae, T. vivax, Leishmania
brasiliensis,
L. donovani, L. tropics, such as, for example, Trichomonadidae, for example
Giardia
lamblia, G. cams.
Sarcomastigophora (Rhizopoda) such as Entamoebidae, for example Entamoeba
histolytica, Harlxnanellidae, for example Acanthamoeba sp., HaWnanella sp.
Apicomplexa (Sporozoa) such as Eimeridae, for example Eimeria acervulina, E.
adenoides, E. alabahmensis, E. anatis, E. anseris, E. arloingi, E. ashata, E.
auburnensis,
E. bovis, E. bnmetti, E. cams, E. chinchillae, E. clupearurn, E. columbae, E.
contorts,
E. crandalis, E. debliecki, E. disperse, E. ellipsoidales, E. falciformis, E.
faurei, E.
flavescens, E. gallopavonis, E. hagani, E. intestinalis, E. iroquoina, E.
irresidua, E.
labbeana, E. leucarti, E. magna, E. maxima, E. media, E. meleagridis, E.
meleagrimitis,
E. mitis, E. necatrix, E. ninakohlyakimovae, E. ovis, E. parva, E. pavonis, E.
perforans,
E. phasani, E. piriformis, E. praecox, E. residua, E. scabra, E. spec., E.
stiedai, E. suis,
E. tenella, E. truncate, E. truttae, E. zuernii, Globidium spec., Isospora
belli, I. cams,
I. felis, I. ohioensis, I. rivolta, I. spec., I. suis, C~stisospora spec.,
Cryptosporidium
spec. such as Toxoplasmadidae, for example Toxoplasma gondii, such as
Sarcocystidae,
for example Sarcocystis bovicanis, S. bovihominis, S. ovicanis, S. ovifelis,
S. spec., S.
suihominis such as Leucozoidae, for example Leucozytozoon simondi, such as
plasmodiidae, for example plasmodium berghei, P. falcipar<un, P. malariae, P.
ovate, P.
vivax, P. spec., such as Piroplasmea, for example Babesia argentine, B. bovis,
B. cams,
B. spec., Theileria parva, Theileria spec., such as Adeleina, for example
Hepatozoon
canis, H. spec.
The useful and breeding livestock include mammals such as, for example,
cattle,
horses. sheep, pigs, goats, camels, water. buffalo, donkeys, rabbits, fallow
deer,
LeA310$5 -23-
CA 02222517 1997-11-27
reindeer, fur-bearing livestock such as, for example, mink, chinchilla,
raccoon, birds
such as, for example, chickens, geese, turkeys, ducks, pigeons, bird species
for keeping
at home and in zoos.
Laboratory and experimental animals include mice, rats, guinea pigs, golden
hamsters,
dogs and cats.
Pet animals include dogs and cats.
Both prophylactic and therapeutic use is possible.
The active compounds are used directly or in the form of suitable
preparations,
enterally, parenterally, dermally, nasally.
Enteral use of the active compounds takes place, for example, orally in the
form of
powders, suppositories, tablets, capsules, pastes, drinks, granules, drenches,
boli,
medicated feed or drinking water. Dermal use takes place, for example, in the
form of
dipping, spraying, bathing, washing, pouring on and spotting on, and dusting.
Parenteral
use takes place, for example, in the form of injection (intramuscular,
subcutaneous,
intravenous, inh-aperitoneal) or by implants.
Suitable preparations are:
Solutions such as injection solutions, oral solutions, concentrates for oral
administration
after dilution, solutions for use on the skin or in body cavities, pour-on
formulations,
gels:
Emulsions and suspension for oral or dermal use and for injection; semisolid
preparations;
Formulations in which the active compound is incorporated in an ointent base
or in
Le A 31 085 - 24 -
CA 02222517 1997-11-27
an oil-in-water or water-in-oil emulsion base;
Solid preparations such as powders, premixes or concentrates, granules,
pellets, tablets,
boli, capsules; aerosols and inhalations, shaped articles containing active
compound.
Injection solutions are administered intravenously, intramuscularly and
subcutaneously.
Injection solutions are prepared by dissolving the active compound in a
suitable solvent
and possibly adding additives such as solubilizers, acids, bases, buffer
salts,
antioxidants, preservatives. The solutions are sterilized by filtration and
bottled.
Solvents which may be mentioned are, physiologically tolerated solvents such
as water,
alcohols such as ethanol, butanol, benzylalcohol, glycerol, hydrocarbons,
propylene
glycol, polyethylene glycol, N-methylpyrrolidone, and mixtures thereof.
The active compounds can., where appropriate, also be dissolved in
physiologically
tolerated vegetable or synthetic oils which are suitable for injection.
Solubilizers which may be mentioned are: solvents which promote dissolution of
the
active compound in the main solvent or prevent its precipitation. Examples are
polyvinylpyrrolidone, polyethoxylated castor oil, polyethoxylated sorbitan
esters.
Preservatives are: benzyl alcohol, trichlorobutanol, p-hydroxybenzoic esters,
n-butanol.
Oral solutions are used directly. Concentrates are used orally after previous
dilution to
their use concentration. Oral solutions and concentrates are prepared, as
described
above for injection solutions, it being possible to dispense with sterile
operation.
Solutions for use on the skin are spotted on, painted on, rubbed in, applied
by spraying
or jetting. or applied by dipping, bathing or washing. These solutions are
prepared, as
described above for the injection solutions.
Le A i 1 085 - 25 -
CA 02222517 1997-11-27
It may be advantageous to add thickeners during preparation. Thickeners are:
inorganic
thickeners such as bentonites, colloidal silica, aluminium monostearate,
organic
thickeners such as cellulose derivatives, polyvinyl alcohols and their
copolymers,
acrylates and metacrylates.
Gels are introduced onto the applied or painted on or into body cavities. Gels
are
prepared by adding su~cient thickeners to solutions, which have been prepared
as
described for the injection solutions, to result in a clear composition with
an ointment-
like consistency. The thickeners used are the thickeners indicated
hereinbefore.
Pour-on formulations are poured onto or sprayed onto limited areas of the
skin, in
which case the active compound either penetrates through the skin and acts
systemically or is dispersed on the surface of the body.
Pour-on formulations are prepared by dissolving, suspending or emulsifying the
active
compound in suitable skin-compatible solvents or solvent mixtiu-es. Where
appropriate,
other auxiliaries such as colourants, absorption-promoting substances,
antioxidants,
sunscreen agents, adherents are added
Solvents which may be mentioned are: water, alkanols, glycols, polyethylene
glycols,
polypropylene glycols, glycerol, aromatic alcohols such as benzyl alcohol,
phenyl
ethanol, phenoxy ethanol, esters such as ethyl acetate, butyl acetate,
benzylbenzoate,
ethers such as alkylene glycol alkyl ethers such as dipropylene glycol
monomethyl
ether, diethylene glycol monobutyl ether, ketones such as acetone, methyl
ethyl ketone,
aromatic and/or aliphatic hydrocarbons, vegetable or synthetic oils, DMF,
dimethylacetamide, N-methylpyrrolidone, 2-dimethyl-4.-oxy-methylene-1,3-
dioxolane.
Colourants are all colourants approved for use on livestock, and can be
dissolved or
suspended.
Absorption-promoting substances are, for example, DMSO, spreading oils such as
Le A 31 085 - 26 -
CA 02222517 1997-11-27
isopropyl myristate, dipropylene glycol pelargonate, silicone oils, fatty acid
esters,
triglycerides, fatty alcohols.
Antioxidants are sulphites or metabisulphites such as potassium
metabisulphite,
ascorbic acid, butylated hydroxytoluene, butylated hydroxyanisole, tocopherol.
Sunscreen agents are, for example, substances from the class of benzophenones
or
novantisolic acid.
Adherents are, for example, cellulose derivatives, starch derivatives,
polyacrylates,
natural polymers such as alginates, gelatin.
Emulsions can be used orally, dermally or as injections.
Emulsions are either of the water-in-oil type or of the oil-in-water type.
They a~ a prepared by dissolving the active compound either in the hydrophobic
or in
the hydrophilic phase and homogenizing the latter with the assistance of
suitable
emulsifiers and, where appropriate, other auxiliaries such as colourants,
absorption
promoting substances, preservatives, antioxidants, sunscreen agents, viscosity-
increasing
substances, with a solvent of the other phase.
The following may be mentioned as hydrophobic phase (oils): paraffin oils,
silicone
oils, natural vegetable oils such as sesame oil, almond oil, castor oil,
synthetic
triglycerides such as caprylic/capric acid biglyceride, triglyceride mixture
with
vegetable fatty acids of chain length C8_,2 or other specially selected
natural fatty acids,
partial glyceride mixtures of saturated or unsaturated, possibly also hydroxyl
group-
containing fatty acids, mono- and diglycerides of C8/Clo fatty acids.
Fatty acid esters such as ethyl stearate, di-n-butyryl adipate, hexyl laurate,
dipropylene
glycol pelargonate, esters of a branched fatty acid of medium chain length
with
LeA310$5 -27-
CA 02222517 1997-11-27
saturated fatty alcohols of chain length C16-C18, isopropyl myristate,
isopropyl
palmitate, caprylic%apric esters of saturated fatty alcohols of chain length
Ci2-C,s,
isopropyl stearate, oleyl oleate, decyl oleate, ethyl oleate, ethyl lactate,
waxy fatty acid
esters such as dibutyl phthalate, diisopropyl adipate, ester mixtures related
to the latter,
including fatty alcohols such as isotridecyl alcohol, 2-octyldodecanol,
cetylstearyl
alcohol, oleyl alcohol.
Fatty acids such as, for example, oleic acid and mixtures thereof.
The following may be mentioned as hydrophilic phase:
Water, alcohols such as, for example, propylene glycol, glycerol, sorbitol and
mixtures
thereof.
Emulsifiers which may be mentioned are:
nonionic surfactants, for example polyethoxylated castor oil, polyethoxylated
sorbitan
monooleate, sorbitan monostearate, glycerol monostearate, polyoxyethyl
stearate,
alkylphenol polyglycol ether;
ampholytic surfactants such as di-Na-N-lauryl-(3-iminodipropionate or
lecithin;
anionic surfactants, such as Na-lauryl sulphate, fatty alcohol ether
sulphates,
mono/dialkylpolyglycol ether orthophosphoric ester monoethanolami.ne salt;
cationic
surfactants such as cetyltrimethylammonium chloride.
Further auxiliaries which may be mentioned are:
Viscosity-increasing and emulsion-stabilizing substances such as
carbo~ymethylcellulose, methylcellulose and other cellulose and starch
derivatives,
polyacrylates, alginates, gelatin, gum arabic, polyvinylpyrrolidone, polyvinyl
alcohol,
copol~~rners of methyl vinyl ether and malefic anhydride, polyethylene
glycols, waxes,
colloidal silica or mixtures of the substances mentioned.
Le A 31 0$5 - 28 -
CA 02222517 1997-11-27
Suspensions can be used orally, dermally or as injection. They are prepared by
suspending the active compound in a liquid vehicle, where appropriate with the
addition of further auxiliaries such as wetting agents, colourants, absorption-
promoting
substances, preservatives, antioxidants, sunscreen agents.
Liquid vehicles which may be mentioned are all homogeneous solvents and
solvent
mixtures.
Wetting agents (dispersants) which may be mentioned are the surfactants
indicated
hereinbefore.
Further auxiliaries which may be mentioned are those indicated hereinbefore.
Semisolid
preparations can be administered orally or dermally. They differ from the
suspensions
and emulsions described above only by their higher viscosity.
To prepare solid preparations, the active compound is mixed with suitable
excipients,
where appropriate with the addition of auxiliaries, and converted into the
desired shape.
Excipients which may be mentioned are all physiologically tolerated solid
inert
substances. Used as such are inorganic and organic substances. Inorganic
substances
are, for example, sodium chloride, carbonates such as calcium carbonate,
bicarbonates,
aluminium oxides, silicas, aluminas, precipitated or colloidal silicon
dioxide,
phosphates.
Organic substances are, for example, sugars, cellulose, foodstuffs and
feedstuffs, such
as milk powder, animal meals, .cereals meals and coarse meals, starches.
Auxiliaries are preservatives, antioxidants, colourants, which have already
been listed
hereinbefore.
Further suitable auxiliaries are lubricants and glidants, such as, for
example,
Le A 31 085 - 29 -
CA 02222517 1997-11-27
magnesium stearate, stearic acid, talc, bentonites, disintegration-promoting
substances
such as starch or crosslinked polyvinylpyrrolidone, binders such as, for
example, starch,
gelatine or linear polyvinylpyrrolidone, and dry binders such as
microcrystalline
cellulose.
The active compounds may also be present in the preparations mixed with
synergists
or with other active compounds.
Preparations ready for use contain the active compounds in concentrations of
10 ppm
to 20 per cent by weight, preferably from 0.1 to 10 per cent by weight.
The benzimidazoles of the formula (I) are in this case present in the
following ratio by
weight to the synthetic coccidiostats or polyether antibiotics: 1 to 0.1 - 10,
preferably
ltol-10.
Preparations which are diluted before use contain the active compounds in
concentrations of 0.5 to 90 per cent by weight, preferably from 1 to 50 per
cent by
weight.
It has in general proved advantageous to administer amounts of about 0.5 to
about
50 mg, preferably 1 to 20 mg, of active compounds per kg of bodyweight per day
to
achieve effective results.
The active compounds can also be administered to the livestock together with
the feed
or drinking water.
Feedstuffs and foodstuffs contain 0.01 to 250 ppm, preferably 0.5 to 100 ppm,
of the
active compound in combination with a suitable edible material.
Such a feedstuff and foodstuff can be used both for curative purposes and for
prophylactic purposes.
LeA310$~ -30-
CA 02222517 1997-11-27
Such a feedstuff or foodstuff is prepared by mixing a concentrate or a premix
which
contains 0.5 to 30 %, preferably 1 to 20 % by weight, of an active substance
mixed
with an edible organic or inorganic vehicle, with customary feedstuffs. Edible
vehicles
are, for example, maize meal or maize and Soya bean meal or mineral salts,
which
preferably contain a small amount of an edible dust-preventing oil, for
example corn
oil or soya oil. The premix obtained in this way can then be added to the
complete
foodstuff before it is fed to the livestock.
The use for coccidiosis may be mentioned by way of example:
For the cure and prophylaxis, for example, of coccidiosis in poultry, in
particular in
chickens, ducks, geese and turkeys, 0.1 to 100 ppm, preferably 0.5 to 100 ppm,
of an
active compound are mixed with a suitable edible material, for example a
nutritious
feedstuff. If required, these amounts can be increased, especially if the
active
compound is well tolerated by the recipient. Administration via the drinking
water can
take place correspondingly.
For the treatment of single animals, for example in the case of treatment of
coccidiosis
in mammals or of toxoplasmosis, preferably amounts of active compound of 0.5
to
100 mg/kg of bodyweight are administered each day in order to achieve the
desired
results. It may, nevertheless, be necessary on occasions to deviate from the
stated
amounts, in particular as a function of the bodyweight of an experimental
animal or of
the nature of the method of administration, but also because of the animal
genus and
its individual reaction to the active compound or the type of formulation and
the time
or interval over which administration takes place. Thus, it may suffice in
certain cases
to make, do with less than the abovementioned minimum amount, whereas in other
cases the stated upper limit must be exceeded. On administration of larger
amounts, it
may be expedient to divide these into several individual administrations over
the course
of the day.
The efficacy of the mixtures according to the invention can be demonstrated,
for
LeA31085 -31-
CA 02222517 1997-11-27
example, in cage tests with the following test design, in which the livestock
are treated
with the particular individual components and with the mixtures of the
individual
components.
Cage test on coccidiosis/chicks
Male chicken chicks (for example LSL Brinkschulte/Senden) which have been
reared
coccidia-free and are 8 to 12 days old receive the compounds according to the
invention (test substances) in the concentration stated in ppm with the feed
from 3 days
before (day -3) the infection (= a.i.) to 8 (9) days after the infection (=
p.i.). Three
birds are kept in each cage. One or more such groups are used per dosage. The
infection takes place using a tube direct into the crop with about 50,000
sporulated
oocysts of Eimeria acervulina and with in each case about 20,000 sporulated
oocysts
of E. maxima and E. tenella. Highly virulent strains of these are used. The
exact
infection dose is adjusted so that, where possible, one in three untreated
experimentally
infected chicks dies from the infection. The following criteria are taken into
account for
assessing the efficacy: weight gain from start of test to end of test,
mortality rate from
the infection, macroscopic assessment of the faeces with regard to diarrhoea
and
excretion of blood on days 5 and 7 p.i. (score 0 to 6), macroscopic assessment
of the
intestinal mucosa, especially of the caeca (score 0 to 6) and excretion of
oocysts, and
the proportion (in %) of oocysts sporulating within 24 hours. The number of
oocysts
in the faeces was determined using a McMaster counting chamber (see
Engelbrecht and
coworkers "Parasitologische Arbeitsmethoden in Medizin and Veterinarmedizin,
Akademie-Verlag, Berlin (1965)). The individual findings are related to the
untreated
uninfected control groups, and a total score is calculated (cf. A. Haberkorn
(1986)
pages 263- to- 270 in Research in Avian Coccidiosis ed. L.R. McDougald, L.P:
Joyner,
P.L. Long Proceedings of the Georgia Coccidiosis Conference Nov, 18. - 20.
1985
Athens/Georgia USA).
A feed containing active compound is prepared in such a way that the required
amount
of active compound is thoroughly mixed with an animal feed which is balanced
in
Le A 31 085 - 32 -
CA 02222517 1997-11-27
nutrient terms, for example with the chick feed indicated below.
If a concentrate or a premix is to be prepared and is finally diluted to be in
the feed to
the figures mentioned in the test, in general about 1 to 30 %, preferably
about 10 to
20 % by weight of active compound are mixed with an edible organic or
inorganic
vehicle, for example maize and Soya meal or mineral salts, which contain a
small
amount of an edible anti-dusting oil, for example corn oil or soya bean oil.
The premix
obtained in this way can then be added to the complete poultry feed before
administration.
An example of a suitable composition for use of the substances according to
the
invention in poultry feed is the following.
52.00 % coarse cereal feed meal, in particular: 40 % maize, 12 % wheat
17.00 % extr. coarse soya meal
5.00 % maize gluten feed
5.00 % wheat feed meal
3.00 % fish meal
3.00 mineral mixture
%
3.00 alfalfa meal
%
2.50 vitamin premix
%
2.00 wheatgerms,
% crushed
2.00 % Soya oil
2.00 % meat and bone meal
1.50 % whey powder
1.00 % molasses
1.00 % brewers' yeast, bound to brewers' grains
2~ 100.00
Such a feed contains 18 % crude protein, 5 % crude fibre, 1 % Ca, 0.7 % P and,
per
kg, 1,200 LU. of vitamin A, 1,200 LU. of vitamin D3, 10 mg of vitamin E, 20 mg
of
Le A 31 085 - 33 -
~ CA 02222517 1997-11-27
zinc bacitracin.
Test results with combinations according to the invention are listed by way of
example
in the following tables. The synergistic activity of the combinations by
comparison
with the individual components is particularly evident from a reduction in
oocyst
excretion, but also in respect of the necropsy findings, weight gain and
better
tolerability.
In the following tables, the meanings in the "treatment" column are:
n.infcontr. = non-infected control group
inf:contr. = infected control group
ampfol comb. = amprolium combined with sulphaquinoxaline and ethopabate
1 = benzimidazole ex. No.
In the "ppm" column, the concentration of active compound used in the feed is
stated
In ppm.
In the "mortality" column, the percentage of birds which die is indicated
under %, and
the number of birds which died/birds used in the test is indicated under n.
In the "weight % of not inf. control" column, the ratio of the weight of the
treated
birds to the weight of the uninfected control group is indicated.
In the "dropping scores", "lesion score" and "oocyst control" columns,
individual data
on the effect are given.
In the "% efficay" column, the overall evaluation is scored; 0 % means no
effect,
100 % means complete effect.
Le A 31 085 - 34 -
CA 02222517 1997-11-27
Table 1
Experimental infection of chicks with Eimeria acervulina, E. maXima and E.
tenella.
Treat-ppm mortality weight drop-lesionoocyst
% in
%
of
inf.
control
ment of not ping score effi-
n ~ ~~ ac. max ten. tot.~3'
control tot.
n. 0 0 0/6 100 0 0 0 0 0 0 100
inf:
contr.
inf. 0 50 3/6 46 6 6 100 100 100 100 0
contr. 915*55* 1520*2490*
amprol.50 0 0/3 71 0,7 63 36 46 52 36
comb.
75 0 0/3 75 0 100 36 100 78 42
I 5 0 0/3 80 4-6 4 15 100 14 43 39
amprol. 0 0/3 89 0 0 1.5 2 1 1.5 82
comb. 50
+
1 +5
75 0 0/3 95 0 0.7 0.260 0.32 0.2998
+5
* = X 1 000
LeA31085 -35-
CA 02222517 1997-11-27
Table 2
Treat-ppm mortality weightdrop-lesionoocyst
% in
%
of
inf:
control
ment of ping score effi-
not
n i~ ~~ ac. max.ten. tot.~Y
control tot.
n. 0 0 0/6 100 0 0 0 0 0 0 100
inf.
contr.
inf. 0 0 0/6 61 6 100 100 100 100 0
contr. 3810*480*2980*7270*
rnonen-25 0 0/3 44 6 44 100 100 81 7
sin
50 0 0/3 88 6 13 33 19 22 43
1 1 2.5 33 t/3 83 6 6 14 38 41 31 35
~
5 0 0/3 76 6 5.7 0.6 3 3 2.1 69
10 33 I/3#100 0-2 3.5 0 0 0 0 92
monen- 0 0/3 66 6 6 13 29 24 22 32
'
sin 25
+
I 2.55
25 0 0/3 102 0-2 4.3 <0. <0.10.1 0.1 92
+ I
5
25 0 0/3 102 0-2 0.7 0 0 <0.1 <0.198
+
10
50 0 0/3 97 I 0.3 ~.1 0.1 <0.1 <0.196
+
2.5
* = X I ~~~
15 # = because of toxicity
LeA310$5 -36-
CA 02222517 1997-11-27
Table 3
Treat- ppm mortality weightdrop-lesionoocyst io
% in
%
of
inf.
control
ment of ping score effi-
not
n inf. scores ~, n~ ten. tot. ~~
control tot.
n. inf:0 0 0/6 100 0 0 0 0 0 0 100
contr.
inf: 0 83 5/6 64 6 6 100 100 100 100 0
contr. 1420220 3560*5200
robeni-16.50 0/3 95 I 5 38 9 23 23 60
dine
1 5 67 2/3 87 6 6 I1 15 11 12 40
10 33 1/3#83 6 6 0.8 0.9 0.6 0.8 71
robeni- 0 0/3 114 0 1.3 1.2 4 2 9 88
dine 16.5
+
1 +5
16.533 I/3'#96 0 4 0.010 0 <0.0 94
+ 1
10
* = x 1 000
1 ~ # = because of toxicity
Le A 31 085 - 37 -
CA 02222517 1997-11-27
Table 4
Treat- ppm mortality weightdrop-lesionoocyst
% in
~o
of
inf:
control
ment of ping score efti-
not
n i~ scorns ~. max.ten. tot.
control tot.
n. inf:0 0 0/6 100 0 0 0 0 0 0 100
contr.
inf. 0 83 5/6 64 6 6 100 100 100 100 0
contr. taso*220*3560*5200*
toltra-10 0 0/3 84 3 6 3 4 I 3 68
zuiil
15 0 0/3 106 ~ 3.3 0.6 0 0.4 0.3 88
I I ~ 67 2/3 87 6 6 11 15 11 12 40
~
10 33 I/3#83 6 6 0.8 0.9 0.6 0.8 71
toltra- 0 0/3 76 6 6. 0.7 2 0.6 1.1 72
zuril 10
+
1 +5 '
I 0 0/3 94 0 4.3 0 0 0 0 98
S
T
10
* = X 1
1 ~ # = because of poisoning
LeA3108~ -38-
CA 02222517 1997-11-27
T e5
Treat-ppm mortality weight drop-lesionoocyst
% in
%
of
inf.
control
ment of not ping score effi-
n i~ ~=~ ac. max.tea tot. ~Y
control tot.
n. 0 0 0/6 100 0 0 0 0 0 0 100
inf:
contr.
inf: 0 0 0.6 62 6 6 100 100 100 100 0
contr. 1260*150*1640*3050*
monen-25 0 0/3 72 4 5.3 86 >100>100 95 0
sin
50 0 0/3 78 6 6 35 93 38 55 30
100 0 0/3 92 0 2.7 I1 7 20 13 69
74 5 0 0/3 59 6 6 >100 40 >100 80 4.3
10 33 I/3#80 0 4.5 0.7 3 2 1.9 75
Monen-25+ 0 0/3 83 0 5.3 8 9 15 I1 58
sin 5
+
74
25 0 0/3 80 4 43 I 0 0.9 0.6 69
+
10
50 0 0/3 90 0 2.3 0.08 0 0.13 0.07 98
+
5
50 33 I/3#98 0 0.5 0.7 0 0.05 0.25 100
+ I
10
100 0 0/3 89 0 1.3 1 0.7 4 1.9 85
+5
100 0 0/3 87 0 0.7 0 0 0.02 0.01 87
+5
* X 1 000
# = because of poisoning
1,~A~108~ -39-
CA 02222517 1997-11-27
Table 6
Treat- ppm mortality weightdrop- lesionoocyst
in
%
of
inf.
control
ment % of ping score efft-
n not scores ~. may.ten. tot. ~Y
inf.
control tot.
n. inf:0 0 0/6100 0 0 0 0 0 0 100
contr.
inf: 0 0 0/659 6 6 100 100 100 100 0
contr.
2760*200*1820*4780*
halo- I 0 0/367 2 2 50 50 96 66 26
fuginone
3 0 0/372 0 0.7 5 4 19 9 70
74 5 0 0/338 6 6 84 100 45 76 4
10 33 1/348 6 6 100 44 44 63 7
halo- 1 0 0/355 0 0.7 2.5 6 13 7.2 53
+
fuginone10
74
3 0 0/376 0 0 3 0 5 2.7 85
+
5
* = x 1 000
L,e A 31 085 - 40 -
CA 02222517 1997-11-27
Table 7
Treat-ppm mortality weight drop-lesionoocyst io
% in
%
of
inf.
control
ment of not ping score eff-
inf. scores cay
control tot.
n ac. max.ten. tot.
n. 0 0 0/6 100 0 0 0 0 0 0 100
inf.
contr.
inf: 0 33 Z6 62 6 6 100 100 100 100 0
contr. 645* 115*865* 1625*
salino-15 0 0/3 85 6 6 81 87 58 75 16
mycin
30 0 0/3 100 3-5 2.3 9 0 22 10 74
40 0 0/3 112 0 0 2.5 5 1.7 3 90
60 10 0 0/3 65 6 6 59 70 53 61 5
12.5 0 0/3 86 6 6 71 100 65 79 16
15 0 0/3 85 6 6 62 100 72 78 16
20 0 0/3 84 4-6 2.3 31 87 40 53 32
salino-15 0 0/3 100 6 2.3 28 70 53 50 37
+
mycin 10
+
60
15 0 0/3 100 0 0.3 0.09 0.5 0.2 0.27100
+
12.5
30 0 0/3 98 ~ 0 0.3 0 0 0 0 100
+
10
30 0 0/3 96 0 0 0 0.2 0 0.07100
+
12.5
30 0 0/3 103 I 1 0 0 0 0 96
+
15
30 0 0/3 101 0 ~0.7 0 0 0 0 98
+
20
LeA3~ 0$~ -41-
CA 02222517 1997-11-27
Treat-ppm mortality weight drop-lesionoocyst
% in
%
of
inf.
control
ment of not pingscore effi-
inf. scores cav
control tot.
40 0 0/3 100 0 0 0.030 0.1 0.03 100
+
10
40 0 0/3 94 2 0.7 0 0 0 0 95
+
12.~
40 0 0/3 97 0 0 0 0 0 0 100
+
20
* = X 1 ~0~
LeA310$~ -42-
CA 02222517 1997-11-27
Table $
Treat-ppm mortality weight drop-lesionoocyst
ro in
%
of
inf.
control
ment of not pingscore effi-
n inf. scores ac. mat.ten. tot.
control tot.
n. 0 0 0/6 100 0 0 0 0 0 0 100
inf:
contr.
inf. 0 17 1/6 61 6 6 100 100 100 100 0
contr. 1080*220*2550*3850*
tolu~a-2.5 0 0/3 59 6 5.3 12 9 I1 II 25
zuril
59 5 0 0/3 61 6 6 91 100 99 100 0
25 33 I/3#84 I 5 0 0 0.2 0.1 79
59 2.5 0 0/3 69 6 5.7 11 9 12 1 35
+ + I
toltra-5
zuril
2.5 0 0/3 92 0 2 0 0 0 0 100
+
25
* = x 1 000
# = because of toxicity
LeA310$~ -43-
CA 02222517 1997-11-27
Table 9
Treat- ppm mortality weightdrop-lesionoocyst
ment % ping score in effi-
of %
not of
inf.
control
n inf: scores ~. ~_ ten.tot. ~y
control tot.
n. inf.0 0 0/6 100 0 0 0 0 0 0 100
contr.
inf. 0 0 0/6 54 6 6 100 100 100 100 0
contr. 1570*70* 1057*2697*
halofu-I 33 1/3 81 4-6 6 41 100 49 63 24
ginone
3 0 0/3 87 0 0 ~.1 0 <D.1<0.1 91
112 I 0 0/3 41 6 6 100 57 91 83 0
5 0 0/3 88 0-2 6 0.8 10 2.4 4.4 77
Halofu-33 1/3 88 6 6 12 100 17 43 30
ginone 1 + I
112
3+ 1 0/3 102 0 0 1 0 0.7 0.6 96
0
3 + 5 0/3 83 0 1.7 m. 0.6 0.2 0.1 89
0 I
* = x 1 000
LeA3I0$5 -44-