Note: Descriptions are shown in the official language in which they were submitted.
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
1
VITAMIN D ANALOGUES
' This invention relates to a hitherto unknown class of
compounds which shows strong activity in inducing
differentiation and inhibiting undesirable proliferation of
l0 certain cells, including skin cells and cancer cells, as
well as immunomodulating and antiinflammatory effects, to
pharmaceutical preparations containing these compounds, to
dosage units of such preparations, and to their use in the
treatment and/or prophylaxis of diseases characterized by
abnormal cell differentiation and/or cell proliferation
such as e.g. psoriasis and other disturbances of
keratinization, HIV-associated dermatoses, wound healing,
cancer, including skin cancer, and of diseases of, or
imbalance in, the immune system, such as host versus graft
and graft versus host reaction and transplant rejection,
and autoimmune diseases, such as discoid and systemic lupus
erythematosus, diabetes mellitus and chronic dermatoses of
autoimmune type, e.g. scleroderma and pemphigus vulgaris,
and inflammatory diseases, such as rheumatoid arthritis and
asthma, as well as a number of other disease states includ-
ing hyperparathyroidism, particularly secondary hyperpara-
thyroidism associated with renal failure, cognitive impair-
ment or senile dementia (Alzheimers disease) and other neu-
rodegenerative diseases, hypertension, acne, alopecia, skin
atrophy, e.g. steroid induced skin atrophy, skin ageing,
including photo-ageing, and to their use for promoting
osteogenesis and treating/preventing osteoporosis and
osteomalacia.
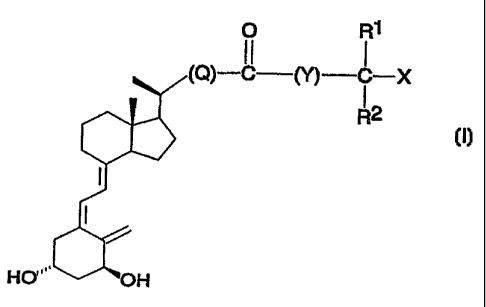
The compounds of the invention constitute a novel
class of vitamin D analogues represented by the general
formula I:
CA 02222785 1997-11-28
WO 97/20811 PCT/DK9b/00502
2
~J
10
Ha'''
in which formula X is hydrogen or hydroxy; R3 and R2 stand
for methyl or ethyl, or, when taken together with the
carbon atom bearing the group X, can form a C3-C5 carbo-
cyclic ring; Q is either a single bond or a Cl-C$
hydrocarbylene in which one of any methylene groups not
directly bonded to the carbonyl group may optionally be
replaced by an oxygen atom (or methyl byhydroxy); Y is
either a single bond or CI-C$ hydrocarbylene.
In the context of this invention, the expression
hydrocarbylene indicates the diradical obtained after
removal of 2 hydrogen atoms from a straight, branched or
cyclic, saturated or unsaturated hydrocarbon.
Examples of Q and Y (when not a single bond) include,
but are not limited to, methylene, ethylene, CH=CH, C-C,
trimethylene, CH=CHCH2, CH2CH=CH, C=CCH2, CH2-C--__C, analog-
ously derived C4- (tetramethylene) and C5-diradicals, and
additionally, for Q: O-CH2, O-CH2CH2, CH2-O-CH2CH2, CH2CH2-
O-CH2, CH(OEt) -C=CH, CH(OH) -CH2, CH(OEt) -C=C, and for Y:
phenylene (o-, m-, p-). ,
The compounds of the invention can comprise more than
one diastereoisomeric form (e.g. E or Z configurations when '
a double bond is present in the group Q or Y; R and S con-
figurations when a branching carbon is present}. The inven-
tion covers all these diastereoisomers in pure form and
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
3
also mixtures thereof. In addition, prodrugs of I in which
one or more of the hydroxy groups are masked as groups
which can be reconverted to hydroxy groups in vivo are also
within the scope of the invention.
The compounds I may be obtained in crystalline form
either directly by concentration from an organic solvent or
by crystallisation or recrystallisation from an organic
solvent or mixture of said solvent and a cosolvent which
may be organic or inorganic, such as water. The crystals
may be isolated in essentially solvent-free form or as a
solvate, such as a hydrate. The invention covers all cry-
stalline modifications and forms and also mixtures thereof.
A number of vitamin D analogues have recently been
described that show some degree of selectivity in favour of
the cell differentiation inducing/cell proliferation in-
hibiting activity in vitro as compared with the effects on
calcium metabolism in vivo (as measured in increased serum
calcium concentration and/or increased urinary calcium
excretion), which adversely limit the dosage that can safe-
ly be administered. One of the first of these to appear,
calcipotriol (INN) or calcipotriene (USAN), has been devel-
oped on the basis of this selectivity and is now recognized
worldwide as an effective and safe drug for the topical
treatment of psoriasis.
A study with another analogue (EB 1089) selected on
this basis supports the concept that systemically adminis-
tered vitamin D analogues may inhibit breast cancer cell
proliferation in vivo at sub-toxic doses (Colston, K.W. et
al., Biochem. Pharmacol. 44, 2273-2280 (1992)).
Promising immunosuppressive activities of vitamin D
analogues have been reviewed (Binderup, L., Biochem. Phar-
' macol. 43, 1885-1892 (1992)). Thus, a series of 20-epi-
vitamin D analogues has been identified as potent
" inhibitors of T-lymphocyte activation in vitro (Binderup,
L. et al, Biochem. Pharmacol. 42, 1569-1575 (1991)). Two
of these analogues, MC 1288 and KH 1060, systemically ad
ministered, have shown immunosuppressive activities in vivo
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
4
in experimental animal models. Additive or synergistic
effects were observed in combination with low-dose
cyclosporin A. KH 1060, alone or in combination with
cyclosporin A, has also been shown to prevent autoimmune
destruction of transplanted islets in diabetic NOD mice
(Bouillon, R. et al. In: Vitamin D, Proceedings of the
Ninth Workshop on Vitamin D, Orlando, Florida, Walter de
Gruyter, Berlin, 1994, pp 551-552). MC 1288 was able to
prolong survival of cardiac and small bowel grafts in rats
(Johnsson, C. et al. In: Vitamin D, Proceedings of the
Ninth Workshop on Vitamin D, Orlando, Florida, Walter de
Gruyter, Berlin, 1994, pp 549-550). However, in all these
studies, the dosages of the analogues that produced sig-
nificant immunosuppression also induced increases in serum
calcium levels. There is therefore a continuing need for
new analogues with high potency showing an acceptable com-
bination of prolonged therapeutic activity and minimum
toxic effects.
The present invention provides a hitherto undisclosed
series of 20-epicholecalciferol analogues which are
characterised by the presence of a keto function in the
side chain. Analogues of vitamin D with a keto moiety (a
carbonyl group bonded to two carbon atoms) in the side
chain are not new: For example, Kureha Chemical Industries
KK in Japanese Patent Application No. 210016/1983 disclose
the use of 23-oxo-1,25,26-trihydroxycholecalciferol as an
antitumour drug. Norman, A.W. and Mayer, E. of the Univer-
sity of California describe the synthesis of 1,25-
-dihydroxy-24-oxo-vitamin D3 and 1,23,25-trihydroxy-24-oxo-
-vitamin D3 (US 4,495,181, 1985). The Teijin Company dis-
closes 24-oxo-vitamin D3 derivatives as neoplasm inhibitors
in Japanese Patent Application No. 067,423/1985. Hamma, N. '
et al. describe fluorine derivatives of vitamin D3 and
process for producing the same (EP 250,755, 1988). McLane,
J.A. et al. disclose stable and active metabolites of 1,25-
-dihydroxy-16-ene-cholecalciferol (US 5,401,733, 1995). An
example of a 22-oxa-24-ketone with cell differentiating
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
activity is described by the Chugai Pharmaceutical Company
in Japanese Patent Application No. 8,113,559/1995. How-
ever, it should be noted that these and other prior art
side chain keto compounds that have been exemplified are
5 characterised by the presence of the natural vitamin D
configuration of the C-20 methyl group. Furthermore, these
compounds contain the keto function located at either the
second or the third atom away from C-20 (position 23 or
position 24).
The compounds of the present invention differ from
the prior art side chain keto compounds in the configur-
ation of the C-20 methyl group; this has the ,(3-projection
as shown in the conventional representation used in formula
I. In addition, the skeleton of the other C-20 substituent
(the rest of the side chain) is not restricted to being
either aliphatic or six-carbon, nor is the location of the
keto group limited to either the second or the third atom
away from C-20. These compounds have been discovered to
possess exceptionally high immunosuppressive activities
together with high tumour cell proliferation inhibiting
activities. For example, the compound of Example 4
(Compound 104) was found to be more potent than the ana-
logues MC 1288 and KH 1060 (the hitherto most potent
compound tested) tablified in the above mentioned review
(Binderup, 1992) in inhibiting allogeneic T-lymphocyte
activation (mixed lymphocyte reaction) in vitro while it
was less calcemic in vivo. Furthermore compound 104 was
found to be at least ten times more potent than its corre-
sponding 20-normal-configurated (20R) isomer in this immu-
nological test system. The same orders of activity were
observed in other in vitro immunological test systems such
as the inhibition of the proliferative response of human
lymphocytes induced by phytohemagglutinin (using the method
described in Piekoszewski, W. et al., Immunopharmacology
and Immunotoxicology, 16, 389-401 (1994)). The compounds of
Examples 1 and 2 (Compounds 101 and 102) were also found to
be over ten times more potent than their 20-isomers which
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
6
had been synthesised for comparison purposes. The compounds
were additionally highly active in the U937 cancer cell
differentiation test, also referred to in the same review
(Binderup, 1992), with the compounds of the present inven-
tion being approximately ten-fold more potent than their
20-isomers.
CA 02222785 1997-11-28
WO 97/2081 I PCT/DK96/00502
7
Scheme 1
I I ( R= CHO )
IIa (R= H)
0
(~-c-t~~-z
III IV
0
cc~-c-cv~-z
V I
CA 02222785 1997-11-28
WO 97120811 PCT/DK96/00502
S
A compound of formula I may be prepared by the general
method of Scheme 1. In this Scheme, the vitamin D nucleus
building block aldehyde II, in which the aldehyde carbon is .
positioned appropriately to become the side chain keto
carbon in the target compound I, is the starting material.
This aldehyde II is either the simple compound in which Qa
represents a single bond or a known derivative, or a new
derivative prepared by a standard sequence of reactions. In
the following, the symbol Qa in II indicates that this
linking group may either be identical to Q in the compound
I, or alternatively may be a group which can be converted
to Q at any subsequent stage in the synthesis. Furthermore,
the identity of Qa may change from intermediate to inter-
mediate along the reaction sequence. However the actual
identity will be apparent from the particular context.
Following the synthetic scheme as depicted:-
1 II is reacted with an organometallic reagent of
formula Z-(Ya)-M (wherein the metal radical M
represents optionally Li or Mg-Hal; Hal= C1,
Br, or I), derived from a side chain building
block of formula Z- (Ya) -H or Z- (Ya) -Hal, to
establish the remainder of the carbon side
chain skeleton in the intermediate III. The
side chain alcohol configuration may be R or S
or a mixture and is irrelevant to the syn-
thesis. Again, the symbol Ya is used to indi-
cate optional identity with or convertibility
to Y (see above far analogous use of Qa), and
the symbol Z bears an analogous relationship to
the group C(RI)(R2)(X). In a complementary
approach to the intermediate III, the metal
radical (-M) replaces the aldehyde (-CHO) func-
tion in starting material II which is reacted
with an aldehyde side chain building block of
formula Z-(Ya)-CHO. The precursor IIa to the
metallated derivative II (R= M) is prepared by
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
9
a standard reaction sequence from an aldehyde
of type II.
The remaining steps in the synthesis involve:-
2 Oxidation of the alcohol to the ketone;
3 Optional conversion of the group Q to Q;
a
4 Optional conversion of the group Ya to Y;
5 Optional conversion of the group Z to
C (Rl) (R2) (X) ;
6 Triplet-sensitised photoisomerisation of the
vitamin D triene (5E to 5Z); and
7 Removal of the vitamin D nucleus silyl
protective groups;
The sequence of steps 1 through 7 may be altered
(e. g. the photoisomerisation step (6) may precede the reac-
tion (step 1) with the side chain building block), and
several steps may be combined (e.g. the conditions of the
desilylation step (7) may also effect a deprotection of the
alcohol group X (step 5). Examples of conditions and
reagents for the specified reactions (i.e. for steps 2, 6,
and 7) are well known in the prior art of vitamin D ana-
logue synthesis. Alternative routes to any one of the
intermediates II through v or the compound I are available
and will be obvious to the man skilled in the art.
The present compounds are intended for use in pharma-
ceutical compositions which are useful in the treatment of
human and veterinary disorders as described above.
The amount required of a compound of formula I
(hereinafter referred to as the active ingredient) for
- therapeutic effect will, of course, vary both with the
particular compound, the route of administration and the
- mammal under treatment. The compounds of the invention can
be administered by the parenteral, intra-articular, enteral
or topical routes. They are well absorbed when given
enterally and this is the preferred route of administration
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
in the treatment of systemic disorders. In the treatment of
dermatological disorders like psoriasis or eye diseases
topical or enteral forms are preferred.
In the treatment of respiratory diseases like asthma
5 an aerosol is preferred.
While it is possible for an active ingredient to be
administered alone as the raw chemical, it is preferable to
present it as a pharmaceutical formulation. Conveniently,
the active ingredient comprises from 0.1 ppm to 0.2o by
10 weight of the formulation.
By the term "dosage unit" is meant a unitary, i.e. a
single dose which is capable of being administered to a
patient, and which may be readily handled and packed, re-
maining as a physically and chemically stable unit dose
comprising either the active material as such or a mixture
of it with solid or liquid pharmaceutical diluents or
carriers.
The formulations, both for veterinary and for human
medical use, of the present invention comprise an active
ingredient in association with a pharmaceutically accept-
able carrier therefore and optionally other therapeutic
ingredient(s). The carriers) must be "acceptable" in the
sense of being compatible with the other ingredients of the
formulations and not deleterious to the recipient thereof.
The formulations include e.g. those in a form suit-
able for oral, rectal, parenteral (including subcutaneous,
intramuscular and intravenous), intra-articular and topical
administration.
The formulations may conveniently be presented in
dosage unit form and may be prepared by any of the methods
well known in the art of pharmacy. All methods include the
step of bringing the active ingredient into association
with the carrier which constitutes one or more accessory
ingredients. In general, the formulations are prepared by
uniformly and intimately bringing the active ingredient
into association with a liquid carrier or a finely divided
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
11
solid carrier or both, and then, if necessary, shaping the
product into the desired formulation.
_ Formulations of the present invention suitable for
oral administration may be in the form of discrete units as
capsules, sachets, tablets or lozenges, each containing a
predetermined amount of the active ingredient; in the form
of a powder or granules; in the form of a solution or a
suspension in an aqueous liquid or non-aqueous liquid; or
in the form of an oil-in-water emulsion or a water-in-oil
emulsion. The active ingredient may also be administered in
the form of a bolus, electuary or paste.
A tablet may be made by compressing or moulding the
active ingredient optionally with one or more accessory
ingredients. Compressed tablets may be prepared by com-
pressing, in a suitable machine, the active ingredient in a
free-flowing form such as a powder or granules, optionally
mixed by a binder, lubricant, inert diluent, surface active
or dispersing agent. Moulded tablets may be made by mould-
ing, in a suitable machine, a mixture of the powdered
active ingredient and suitable carrier moistened with an
inert liquid diluent.
Formulations for rectal administration may be in the
form of a suppository incorporating the active ingredient
and a carrier such as cocoa butter, or in the form of an
enema.
Formulations suitable for parenteral administration
conveniently comprise a sterile oily or aqueous preparation
of the active ingredient which is preferably isotonic with
the blood of the recipient.
Formulations suitable for intra-articular administra
tion may be in the form of a sterile aqueous preparation of
' the active ingredient which may be in microcrystalline
form, for example, in the form of an aqueous micro-
crystalline suspension. Liposomal formulations or biode-
gradable polymer systems may also be used to present the
active ingredient for both intra articular and ophthalmic
administration.
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
12
Formulations suitable for topical administration,
including eye treatment, include liquid or semi-liquid
preparations such as liniments, lotions, gels, applicants,
oil-in-water or water-in-oil emulsions such as creams,
ointments or pastes; or solutions or suspensions such as
drops.
For asthma treatment inhalation of powder, self-
-propelling or spray formulations, dispensed with a spray
can, a nebulizer or an atomizer can be used. The formula-
dons, when dispensed, preferably have a particle size in
the range of 10 to 100 ~..
Such formulations are most preferably in the form of
a finely comminuted powder for pulmonary administration
from a powder inhalation device or self-propelling powder-
-dispensing formulations. In the case of self-propelling
solution and spray formulations, the effect may be achieved
either by choice of a valve having the desired spray
characteristics (i.e. being capable of producing a spray
having the desired particle size) or by incorporating the
active ingredient as a suspended powder in controlled par-
ticle size. These self-propelling formulations may be
either powder-dispensing formulations or formulations
dispensing the active ingredient as droplets of a solution
or suspension.
Self-propelling powder-dispensing formulations pref-
erably comprise dispersed particles of solid active ingre-
dients, and a liquid propellant having a boiling point
below 18°C at atmospheric pressure. Generally, the propel-
lant constitutes 45 to 99.9% w/w of the formulation whilst
the active ingredient constitutes 0.1 ppm to 0.1% w/w, of
the formulation.
In addition to the aforementioned ingredients, the
formulations of this invention may include one or more
additional ingredients such as diluents, buffers, '
flavouring agents, binders, surface active agents,
thickeners, lubricants, preservatives, e.g. methyl hydroxy-
benzoate (including anti-oxidants), emulsifying agents and
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
I3
the like. The compositions may further contain other thera-
peutically active compounds usually applied in the treat-
ment of the above mentioned pathological conditions.
The present invention further concerns a method for
treating patients suffering from one of the above patho-
logical conditions, said method consisting of administering
to a patient in need of treatment an effective amount of
one or more compounds of formula I, alone or in combination
with one or more other therapeutically active compounds
usually applied in the treatment of said pathological con-
ditions. The treatment with the present compounds and/or
with further therapeutically active compounds may be simul-
taneous or with intervals.
In the treatment of systemic disorders daily doses of
from 0.1-100 E.cg, preferably from 0.2-25 ~Cg, of a compound
of formula I are administered. In the topical treatment of
dermatological disorders, ointments,' creams or lotions
containing from 0.1-500 ~Cg/g, and preferably from 0.1-100
~.g/g, of a compound of formula I are administered. For
topical use in ophthalmology ointments, drops or gels con-
taining from 0.1-500 ~tg/g, and preferably from 0.1-100
~.g/g, of a compound of formula I are administered. The oral
compositions are formulated, preferably as tablets, cap-
sules, or drops, containing from 0.05-50 E,r.g, preferably
from 0.1-25 ~.g, of a compound of formula I, per dosage
unit.
The invention is further illustrated by the following
non-limiting Preparations and Examples:
Preparations and Examples
The exemplified compounds I are listed in Table 1,
whereas the starting materials and intermediates of general
formulae II, III, IV and V are listed in Table 2.
The following standard abbreviations are used
throughout this disclosure: Me = methyl; Et = ethyl; TBS =
t-butyldimethylsilyl; THP = tetra-hydro-4H-pyran-2-yl; THF
- tetrahydrofuran.
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
14
General:
Ether refers to diethyl ether. Tetrahydrofuran (THF)
was dried over sodium/benzophenone. Reactions were routine- -
ly run under an argon atmosphere unless otherwise noted.
In the standard work-up procedure, the organic layer was
separated, washed with saturated sodium chloride solution,
dried over anhydrous magnesium sulphate, and concentrated
in vacuo to give the product.
For fH nuclear magnetic resonance spectra (300 MHz)
chemical shift values (b) (in ppm) are quoted, unless oth-
erwise specified, for deuteriochloroform solutions relative
to internal tetramethylsilane (b - 0.00) or chloroform (b =
7.25). The value for a multiplet, either defined (doublet
(d), triplet (t), quartet (q)) or not (m) at the
approximate mid point is given unless a range is quoted (s
- ringlet, b = broad).
CA 02222785 1997-11-28
WO 97/20811 PCTlDK96/00502
Table 1: Exemplified I (Details t~ro-
Compounds are
vided for comp ounds ExampleNumber
where
an
is given; the other may pre-
compounds be
y pared using reaction
analogous sequences
from the appro priate II or IIa)
Comt~ound
Compound Example Y R1 R2 X
Q
No. No.
10
101 1 (CH2)2 single bond Me Me H
102 2 single bond (CH2)4 Me Me OH
103 3 single bond C-C-~CH 2 Et Et OH
104 4 (CH2)2 single bond Me Me OH
15 105 5 (CH2)2 single bond -(CH2 )4- OH
106 6 *CH=CH single bond Me Me H
107 *CH=CH single bond Me Me OH
108 7 O-(CH2)2 single bond Et Et H
109 8 O-(CH2)2 single bond Et Et OH
110 CH2-O-(CH2)2 single bond Me Me OH
111 *CH=CH single bond -(CH2)2- H
112 cCH(OEt)-C=CH* single bond Et Et OH
113 #CH(OH)-CH2 single bond Me Me OH
114 cCH(OEt)-C=C single bond Et Et OH
115 CH2 (CH2)2 Me Me OH
116 (CH2)3 single bond Et Et OH
117 CH2 CH2 Me Me OH
* E-Configuration of this double bond.
# R-Configuration at this carbon.
' a S-Configuration at this carbon.
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
16
N N
N
M .IJ M
W N N N N N '
M V M
x a~~ a~ ~ x a~
N U N ~ ~ W N ~ W N N U N
~ ~ ~ ~ ~
O 0 U U 0 O 0 O
_ _ _ _ U _ _ U U _ U _
U U U U U U U U U
d ~ ~ .
R
N N N
~r a~x ~rx a~~r x a~ a~ a
_ U _ U _ W
N L7~ N X31N 57 tTl~7 t51N
x ~ U x U ~ x U ~ ~ ~ ~ x
" - " _ " ~ a _ _ _
i m m
m a U ~ r
n
0
b b .
0 0 0 0 0 0 0 0
W O Ot Q? N N N N N ~ * ~ O N N N N N
OctSO v b1 N b1N tJ1tT1CnU b1l71N N N N bl
~ x ~ x ~ ~ ~ n ~ ~ x x x x
Us~ ccf -.-IU -r-tU ~ -~-tr1x -r1-~ U U U U -fi
a~s~ ~ ~ -- m -- ~ a~ cnU r~v~
*
HHHHHHH,'~','>,'~,'~,'~* ,'~~Jv'
øa H H H H H H H H H H H H -k H
O ?,
U E-~
cLS
O UJ rl N M d~ Ln l0 t~ ~0 01 O ri rl N N M d'
O O O O O O O O O ri rl rl rt c-i rl c-i
O O O O O O O O O O O O O O O O
O O O O O O O O O O O O O O O O
U~
N ~-i ~-i
id N
rl Q O
,~.t ~r-! '~ * v-1 N M di !11 l0 C~ CO a1 O ri ~ N M
((j p., .~ 'z, -k -k O O O O o O O o O ri r-i r1 c-1 v-!
H
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
17
N N N
W W
- M M M
N ~ .-. --~
~ 1J.i.1
M M M
W U N N U N U
N -- ~ f.~.~G~
-r1 .1.1 .I~ -rlJ-1r1
W w W
O O O O U U U
C
U U U
U U U U U U U
C
~t ..CZ ~ .L7..Q.~ .CZ ~ .Ct
.~
N
x a~ a~ a~ as a~ a~ a~ a~a~
a
flj U ~-I rl rl rl r-Ir-I r-! r-Ir-1
r-
~t t7~b~ b'~ ~ tJtb~
b
U
'r'~ 'r~ r~ W-~-r'~-r'~ r-~ r~'r~
r
U rn ca m U1 cn m ~n u~m
cr.
N
N
U
O --
,.Q N N N N N N s *
O
M N
C
x x '.~',x ~ ~ _
t~ N U U U U U U N N U U N
C
x x N x !Ill x
x
U ~
O O O O O O ~ U U
* *
O * H -x
* H H H * H H H H H H H H
U Ea
~ ~-I ra td
O N Lf1 l0[~(~ DD01 O O r1N M tipO1 O rI
d~ N
r-I rirlv-Ir-Ic-1N N N N N N N M M
N M
O O O O O O O O O O O O O O O
O O
O o o O o 0 0 0 0 0 0 0 0 0 0
0 0
.r.,
0
U i
rti
N ~-I ~-1
c~ N
N
r-I N O E (LS.~ c
S-1 -ri d~ tnl0l0 C~QO 0101 O ri * -k N
;~
L3~ J-1 -I rlrlri rir-irlu-1N N * * * N
,"y
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
18
N H O
x
x
o U
M '~
ro ~a .~
M O
rl ~-I -~-i
~
N U N ~ fd
w ~ ~ ~ N
a~
~
x 'Tj O N
U U Z ._
'
x
N ~ N
ro ro ro o
~ m o
. . ro ,-~
c~ ~
o ~ b a
i~ ~ ri ri ,
a ~
,
cn m ~ U
lDto d~ .~''.l~
~ o ~
x - O 't3~
~
~
x a iia ~ o
U i U U N N
~ W -~-t._
cd * cJ~-
QI * N .l,~1 l-1.4~ (d.t'', S-1ftSN J...l
x -- w H w w U -~ . a~ v o 4..,
c o 0 0 0 ~ ~ ~ ~
~ x ; ~
a U ~ ~ v
0
~
~ N ~ U o 0
'
N o o ~
~ ro ~ o ,.Q.i.~~ .c~
3 ~ O ~ ~ ~ ~ _ ~ rt
~
H
,7 H H H H ~ ~ ro-rl ~ roO C~!U U
H H H H rfO N ~-Ir-'I~ -~i
E ~ ~ ~ o Ul.l-1
U H N
N U
O
O 3
rn~ H ~ ~ ~
O =~ -~ N O ~
~ N N J-3~1 h i-1.t~, O -=-IO O O
4-a O c1J M W W ~ t~co N N ~ ~ -rl , -ri-rl
~ M M M M M M U ~i.1.~~ ~ H O ~ O
5~
, O O O O O O rl N (0 t1S U (l~t~ O ft$f0
0 0 0 0 0 0 ~ E ~--~ ~ O ~
~ v ~ ~ -
H i~tn N tn a~ M tnro ~ tntn
~ ~ b i ~ ~
O rn- ~ - 7 ro~ ~+-~~
O ~ ~ ~ O ~ O N ~ O O
~ ! r- t
-1 -a I 'tf
ca -~r~ r-trt!n'f U .u N U U
O ~ .~ ni.u ~
J~ Cl~U U1 tCjN W H E-~~'
N ~I ~-1 ~
~.' ~I
r-t Qy O ~ .t~ U
~ -r-t ~ M d m uo~ O U1 * *
pa ~..1 N N N N N O N
'Z, * * * ~ LT
f~ G1*
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
19
General Procedure 1 (Preparations OZ 02 17)
To a solution, maintained at about 5°C, of the Grig-
. nard reagent prepared from magnesium (3.3 mmol) and the
Side chain buildinct block (3 mmol) in dry THF (3 ml) was
added via a syringe the Aldehyde (ca. 1 mmol) in dry THF (5
ml). After stirring at the same temperature for 30 min, the
reaction mixture was partitioned between ether and satu-
rated ammonium chloride solution. Standard work-up gave the
oily Product.
Preparation O1: Compound 0003
(This preparation is a modification without separ-
ation of isomers of the synthesis described in Calverley,
M.J. and Binderup, E.T. Novel vitamin D analogues. Interna-
tional Patent Application WO 9,100,271-A: (1991), Prepara-
tion 32:) Szde chain building block: 6-bromo-2-methyl-
-2(trimethylsilyloxy)hexane (0.80 g); Aldehvde: Compound
0001 (0.55 g, 0.96 mmol); Without further purification, the
Product containing the Title Compound was used in the next
step.
Preparation 02: Compound 0004
fide chain building block: 2-bromopropane (0.37 g);
Aldehyde: Compound 0002 (0.60 g); Purification of the Prod-
uct by chromatography on silica gel (50 g) (eluant: 1.5~
ether in petroleum ether) gave the Title Compound. Oil; b
0.05 (12H, m), 0.54 (3H, s), 0.75 to 1.0 (9H, m), 0.85 (9H,
s), 0.9 (9H, s), 1.05 to 2.1 (20H, m), 2.3 (1H, bd), 2.55
(1H, dd). 2.87 (1H, bd), 3.31 (1H, m), 4.21 (1H, m), 4.52
(1H, m), 4.93 (1H, m), 4.98 (1H, m), 5.81 (1H, d), 6.45
( 1H, d) .
Preparation 03: Compound 0005
(This preparation is a modification without separ-
ation of isomers of the synthesis described in Bretting,
C.A.S. and Grue-Smrensen, G., International Patent Applica-
tion WO 9,319,044-A1 (1993), Preparation 13:) To a solu-
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
tion, maintained at about -70°C, of the lithio-derivative
prepared from n-butyl-lithium (1.6 M in hexanes; 3 mmol)
and the side chain building block 4-ethyl-4-(tetrahydro- ,
pyranyloxy)-hex-1-yne (0.65 g, 3.1 mmol) in dry THF (5 ml)
5 was added via a syringe Compound 0001 {0.575 g, 1 mmol) in ,
dry THF (2 ml). After stirring at the same temperature for
10 min and thereafter at 0°C for 45 min, the reaction
mixture was partitioned between ether and water. Standard
work-up gave the oily Product. Purification by chromatogra-
10 phy on silica gel (50 g) (eluant: 30% ether in petroleum
ether) gave the Title Compound as an oil.
General Procedure 2 (Preparatior~.s 04, 05)
To a solution, maintained at about 25°C, of the Tri-
15 methylsilyl ether (0.96 mmol} or the THP ether (0.22 mmol)
in THF (2 ml) was added pyridinium p-toluenesulphonate
(0.010 g, 0.04 mmol, for trimethylsilyl ether; 0.150 g, 0.6
mmol, for THP ether) in ethanol (10 ml}. After stirring at
the same temperature for 15 min {trimethylsilyl ether) or 2
20 h {THP ether), the reaction mixture was partitioned between
ethyl acetate and 5% sodium hydrogen carbonate solution.
Standard work-up gave the oily Product.
Preparation 04: Compound 0006
Trimethylsil~l ether: Compound 0003, the crude prod-
uct from Compound 0001, (0.96 mmol}; Purification of the
Product by chromatography on silica gel (50 g) (eluant: 40%
ethyl acetate in petroleum ether) gave the Title Compound.
Oil; S 0.06 {12H, m}. 0.54 (3H, s), 0.83 (3H, d), 0.86 (9H,
s), 0.89 {9H, s), 1.01 to 2.15 (24H, m), 1.2 (6H, s), 2.31
{1H, bd), 2.55 (1H, dd), 2.88 (1H, bd}, 3.85 (IH, m), 4.21
(1H, m), 4.52 (1H, m), 4.93 (1H, m), 4.98 (1H, m), 5.83 -
( 1H, d} , 6 . 45 { 1H, d) .
Preparation 05: Compound 0007
THP ether: Compound 0005 (0.170 g, 0.217 mmol); With-
out further purification, the Product Containing the Title
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96100502
21
Compound was used in the next step. 8 0.05 {12H, m), 0.55
(3H, s) , 0.85 (9H, s) , 0.88 (6H, t) , 0.89 (9H, s) , 1.03
(3H, d) , 1.15 to 2.1 (20H, m) , 2.31 -(1H, bd) , 2.37 (2H, m) ,
2.54 (1H, dd), 2.87 (1H, m), 4.21 (1H, m), 4.52 (1H, m),
4.61 (1H, m), 4.93 (1H, m), 4.98 (1H, m), 5.82 (IH, d),
6.44 (1H, d).
Preparation 06: Compound 0008
To a solution, maintained at about 25°C, of Compound
0001 (0.481 g, 0.84 mmol) in dry toluene (3 ml) was added
the side chain building block 2-propylcarbonylmethylenetri-
phenylphosphorane (0.60 g, 1.72 mmol). After stirring at
the same temperature for 10 min and thereafter at 110°C for
5 h, the reaction mixture was partially concentrated in
vacuo and diluted with ether. The solution was set aside to
crystallise and filtered. The filtrate was concentrated in
vacuo to give an oil. Purification by chromatography on
silica gel (50 g) (eluant: loo ether in petroleum ether)
gave the Title Compound. Needles {from methanol); m.p. 123-
124°C; b 0.05 (12H, m), 0.5 (3H, s}, 0.85 (9H, s), 0.89
(9H, s), 1 (3H, d), 1 to 1.82 (lOH, m), 1.09 {6H, d), 1.95
( 3H, m) , 2 . 25 ( IH, m) , 2 . 3 ( IH, bd) , 2 . 54 ( 1H, dd) , 2 . 81
(1H, m), 2.84 (IH, bd), 4.21 (1H, m), 4.52 (1H, m), 4.93
(1H, m), 4.97 {1H, m), 5.81 (1H, d), 6.1 (1H, d), 6.43 (IH,
d}, 6.77 (1H, dd).
Preparation 07: Compound 0009
To a solution, maintained at about 25°C, of Compound
0006 (0.69 g, 0.1 mmol) in dry dichloromethane (2 ml) was
added solid pyridinium chlorochromate (0.04 g, 0.19 mmol).
After stirring at the same temperature for 1 h, the reac-
tion mixture was extracted with ether. The organic layer
was separated and filtered and concentrated in vacuo to
' give an oil. Purification by chromatography on silica gel
(15 g) (eluant: 20% ethyl acetate in petroleum ether) gave
the Title Compound. Oil; b 0.06 (12H, m), 0.51 (3H, s},
0.85 (9H, s), 0.88 (9H, s), 1.02 (3H, d}, 1.1 to 2.1 (20H,
CA 02222785 1997-11-28
WO 97/20811 ~'CT/DK96/00502
22
m) , 1.2 (6H, s) , 2.31 (1H, bd) , 2.5 (4H, m) , 2.85 (1H, m) ,
4.21 (1H, m), 4.51 (1H, m), 4.93 (1H, m), 4.97 (1H, m),
5.81 (1H, d) , 6.43 (1H, d) .
General Qrocedure 3 (Preparations 08. 09. I8) .
To a solution, maintained at about 25°C, of the Alco-
ho, (0.2 to 0.5 mmol) in dry dichloromethane (10 ml) was
added portionwise 1,1,1-triacetoxy-1,1-dihydro-1,2-benz-
iodoxol-3(1H)-one (1.2 mol equiv.). After stirring at the
same temperature for I5 min, the reaction mixture was par-
titioned between ether and 5~ aqueous sodium bicarbonate
solution also containing excess sodium thiosulphate. Stan-
dard work-up gave the oily Product.
I5 Preparation 08: Compound 0010
Alcohol: Compound 0007, the crude product from
Compound 0005, (0.217 mmol); The Product was purified by
chromatography on silica gel (30 g) (eluant: 40o ether in
petroleum ether) gave the Title Compound. Oil; b 0.05 (12H,
m) , 0.54 (3H, s) , 0.85 (9H, s) , 0.88 (9H, s) , 0.92 (6H, t) ,
1.1 to 2.1 {14H, m) . 1.14 (3H, d) , 2.3 (1H, bd) , 2.52 (2H,
m), 2.56 (2H, s), 2.86 (1H, m), 4.2I (1H, m), 4.51 (1H, m),
4.93 (1H, m), 4.97 (1H, m), 5.81 {1H, d), 6.43 (1H, d).
Preparation 09: Compound OOI1
Alcohol: Compound 0004 (0.322 g, 0.5 mmol); The Prod-
uct was purified by chromatography on silica gel (30 g)
(eluant: 5~ ether in petroleum ether) gave the Title
Compound. Needles (from methanol); m.p. 95-96°C; b 0.05
(12H, m) , 0.55 (3H, s) , 0.83 (3H, d) , 0.85 (9H, s) , 0.89
(9H, s), 1.08 (6H, d), 1.2 to 2.1 (16H, m), 2.25 to 2.7
(5H, m), 2.86 {1H, bd), 4.21 (1H, m), 4.52 (1H, m), 4.93
(1H, m) , 4.98 (1H, m) , 5.81 (1H, d) , 6.45 {1H, d) .
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
23
Preparation 10: Compound 0011 (Alternative s~n-
thesis)
. To a solution, maintained at about 25°C, of Compound
0008 (1.15 g, 1.79 mmol) in toluene (50 ml) was added an
. 5 aqueous solution of sodium dithionite (5.45 g, 31 mmol)
containing sodium hydrogen carbonate {5.5 g) and methyltri-
decylammonium chloride (0.55 g). After vigorous stirring at
the same temperature for 5 min and thereafter at 80°C for 3
h, the reaction mixture was partitioned between ether and
l0 water. Standard work-up gave the oily Product. Purification
by chromatography on silica gel (30 g) (eluant: 30~ ether
in petroleum ether) gave the Title Compound. Needles {from
methanol); m.p. 95-96°C; b 0.05 (12H, m), 0.55 (3H, s),
0.83 (3H, d) , 0.85 (9H, s) , 0.89 {9H, s) , 1.08 (6H, d) , 1.2
15 to 2.1 (16H, m) , 2.25 to 2.7 (5H; m)', 2.86 (1H, bd) , 4.21
(1H, m), 4.52 (1H, m). 4.93 (1H, m), 4.98 (1H, m), 5.81
(IH, d) , 6.45 {1H, d) .
General Procedure 4 preparations 11a, 19a
20 To a solution, maintained at about 5°C, of the Ketone
(ca. 0.5 mmol) in dry dichloromethane (3 ml) was added
hexamethyldisilazane (2 molar equiv.) and then iodotri-
methylsilane {0.6 molar equiv.). After stirring at the same
temperature for 1 h and thereafter at -20°C overnight, the
25 reaction mixture was partitioned between ether and 5~
sodium hydrogen carbonate solution. Standard work-up gave
the oily Product.
reparation lla: 24-Trimethvlsilvloxv-1(S),3(R)-bis-
30 (TBS-oxy)-20~(S)-9.10-seco-cholesta-
-5 (E) , 7 (E) , 10 (19) . 24-tetraene
(Compound 0012a)
Ketone: Compound 0011 (0.395 g, 0.61 mmol); Purifica-
tion of the Product by chromatography on silica gel {30 g)
35 (eluant: 1 ~ ether in petroleum ether) gave the Title
Compound. Needles (from methanol); m.p. 69-71°C; b 0.05
(12H, m) , 0.15 (9H, s) , 0.53 {3H, s) , 0.86 {3H, d) , 0.86
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
24
(9H, s), 0.89 (9H. s), 1 to 2.08 (17H, m), 1.56 (3H, s),
1.59 (3H, s), 2.16 (1H, m), 2.3 (1H, bd), 2.55 {1H, dd),
2.86 (1H, bd), 4.21 (1H, m), 4.52 {1H, m), 4.93 (1H, m),
4.98 (1H, m) , 5.81 (1H, d) , 6.45 (1H, d) .
General Procedure 5 (Preparations llb, 19b)
To a solution, maintained at about 5°C, of the Enol
ether (ca. 0.2 mmol) in dry dichloromethane (5 ml) was
added solid m-chloroperbenzoic acid (85-°s) (1.1 molar
equiv.). After stirring at the same temperature for 15 min,
the reaction mixture was partitioned between ether and 5%
sodium hydrogen carbonate solution. Standard work-up gave
the oily Product.
Preparatior~ llb: Comgound 0012
Enol-ether: Compound 0012a (0.152 g, 0.21 mmol);
Purification of the Product by chromatography on silica gel
(15 g} (eluant: 5 % ether in petroleum ether) gave the
Title Compound. Needles (from methanol); m.p. 82-84°C; b
0.05 (12H, m), 0.15 (9H, s), 0.55 (3H, s), 0.84 (3H, d),
0.85 (9H, s) , 0.89 (9H, s) , 1.15 to 2.08 (16H, m) , 1.32
(6H, s), 2.3 (1H, bd), 2.56 (1H, dd), 2.62 (2H, m), 2.86
(1H, m), 4.21 (1H, m), 4.52 {1H, m), 4.93 (1H, m), 4.98
( 1H, m) , 5 . 81 { 1H, d) , 6 . 45 ( 1H, d) .
General Procedure 6 (Preparations 12 through 15, 20,
21, 23)
To a solution of the 5E-Vitamin D compound (ca. 0.1 t
mmol), anthracene (9-acetylanthracene in Preparations 20
and 21) (0.03 g) and triethylamine (0.1 ml) in toluene or
~d.ichloromethane (5 ml) in a Pyrex flask was irradiated with
light from a high pressure ultraviolet lamp, type TQ718Z2 '
(Hanau) at about 10°C for 30 minutes. The reaction mixture
was partially concentrated in vacuo to give the oily Prod- '
uct.
CA 02222785 1997-11-28
WO 97/20811 PCTlDK96/00502
Preparation 12 Compound 0013
5E-Vitamin D compound: Compound 0011 (0.067 g, 0.104
. mmol) (in toluene); Purification of the Product by chro-
matography on silica gel (15 g) (eluant: 5 % ether in pet-
', 5 roleum ether) gave the Title Compound. Oil; 6 0.05 (12H,
m) , 0.53 (3H, s) , 0.82 (3H, d) , 0.87 (18H, s) , 1.05 to 2.05
(16H, m), 1.08 (6H, d), 2.2 (1H, dd), 2.3 to 2.67 (4H, m),
2.81 (1H, m), 4.I8 (1H, m), 4.36 (1H, m), 4.85 (1H, m),
5 .17 (1H, m) , 6.00 (IH, d) , 6.22 (1H, d) .
Preparation 13: Compound 0014
5E-Vitamin D compound: Compound 0009 (0.080 g, 0.116
mmol) (in dichloromethane); Purification of the Product by
chromatography on silica gel (15 g) (eluant: 30 ~ ethyl
acetate in petroleum ether) gave the Title Compound. Oil; 8
0.05 (12H, m), 0.49 (3H, s), 0.87 (9H, s), 0.87 (9H, s), 1
(3H, d) , 1.19 (3H, s) , 1.19 (3H, s) , 2.2 {1H, dd) , 2.3 to
2.7 (4H, m), 2.81 (1H, m), 4.18 (1H, m), 4.36 (1H, m), 4.85
( 1H, m) , 5 . 17 ( 1H, m) , 6 ( 1H, d) , 6 . 22 ( 1H, d) .
Preparation 14: Compound 0015
5E-Vitamin D compound: Compound 0010 (0.076 g, 0.109
mmol) (in dichloromethane): Purification of the Product by
chromatography on silica gel (15 g} (eluant: 50o ether in
petroleum ether) gave the Title Compound. Oil; b 0.05 (12H,
m) , 0.53 (3H, s) , 0.87 (18H, s) , 0.92 (6H, t} , 1.14 (3H,
d) , 2.2 (1H, dd) , 2.4 (1H, dd) , 2.53' (1H, m) , 2.57 (2H, s) ,
2.81 (1H, m), 4.18 {1H, m), 4.36 (1H, m), 4.85 (1H, m),
5.17 {1H, m) , 6 (1H, d) , 6.22 (1H, d) .
Preparation 15: Compound 0016
5E-Vitamin D compound: Compound 0012 (0.062 g, 0.085
mmol) (in toluene); Purification of the Product by chroma-
tography on silica gel (15 g) (eluant: 2 o ether in pet-
roleum ether) gave the Title Compound. Oil; b 0.05 (12H,
m) , 0.15 (9H, s) , 0.53 (3H, s) , 0.84 (3H, d) , 0.87 (18H,
s) , 1.32 (3H, s) , 1.32 (3H, s) , 2.2 (1H, dd) , 2.4 (1H, m} ,
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
26
2.62 (2H, m), 2.81 (1H, m), 4.18 (1H, m), 4.36 (1H, m),
4. 85 (IH, m} , 5.17 (1H, m) , 6 (1H, d} , 6.22 (IH, d) .
Preparation 16a: (2-Cyanoethoxv-1 (S) , 3 (R) -bis (TBS-
oxy) -9. 10-20 (R) -seco-prec~na-5 (E) . -
7(E),I0(19),24-triene (Compound
0017a
To a solution, maintained at about 25°C, of 20(R)-
hydroxy-1(S),3(R)-bis-(TBS-oxy)-9,10-20(R)-seco-pregna-
-5(E),7(E),10(19),24-triene (1.3 g, 2.32 mmol} in
dichloromethane (40 ml) was added a 40% aqueous solution of
tetrabutylammonium hydroxide (20 ml, 15 mmol) followed by
acrylonitrile (4.84 g, 91 mmol). After vigorous stirring at
the same temperature overnight, the reaction mixture was
partitioned between ether and water. Standard work-up gave
the oily Product. Purification by chromatography on silica
gel (80 g) (eluant: 20o ether in petroleum ether) gave the
Title Compound; b = 0.05 (m, I2H), 0.56 (s, 3H), 0.86 (s,
9H), 0.88 (s, 9H), 1.10 (d, 3H), 1.10-2.20 (m, I3H}, 2.30
{bd, 1H}, 2.55 (dd, 1H), 2.56 (t, 2H), 2.87 (bd, iH), 3.34
(m, 1H), 3.44 (m, 1H), 3.75 (m, 1H), 4.20 (m, 1H), 4.52
(dd, 1H), 4.92 (bt, 1H), 4.98 (bs, 1H), 5.79 (d, 1H), 6.45
(d, 1H) .
Preparation 16b: Compound 0017
To a solution, maintained at about -70°C, of compound
0017a (0.9 g, 1.46 mmol) in dry ether {45 ml) was added via
a syringe diisobutylaluminium hydride (1 M in hexanes; 2
mmol). After stirring at the same temperature for 1 h, the
reaction mixture was partitioned between ether and satu-
rated ammonium chloride solution. After standard work-up,
the oily Product was purified by chromatography on silica °
gel (50 g) (eluant: 30o ether in petroleum ether) to give
the Title Compound as an oil; 8 = 0.05 (m, 12H), 0.53 (s,
3H), 0.86 (s, 9H), 0.88 (s, 9H), 1.09 (d, 3H), 1.05-2.10
(m, 13H) , 2.30 (bd, 1H) , 2.54 (dd, 1H) , 2.62 (dt, 2H) , 2.86
{bd, 1H), 3.30 (m, 1H), 3.55 (m, 1H), 3.88 (m, 1H), 4.20
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
27
(m, 1H), 4.52 (dd, 1H), 4.93 (bt, 1H), 4.98 (bt, 1H), 5.79
(d, 1H) , 6.44 (d, 1H) , 9. 78 {t, 1H) .
Preparation 17: Compound 0018 (General Procedure 1)
Side chain building block: 3-bromopentane (0.45 g);
Aldehvde: Compound 0017 (0.49 g, 0.8 mmol); Purification of
the Product by chromatography on silica gel (50 g) (eluant:
20% ether in petroleum ether) gave the Titie Compound as
separate isomers which were recombined for use in the next
step. First eluted isomer: Oil; b = 0.05 (m, 12H), 0.54 (s,
3H), 1.85 {m, 6H), 0.86 (s, 9H), 0.88 (s, 9H), l.ll {d,
3H), 1.10-2.15 (m, 20H), 2.30 (bd, 1H), 2.54 (dd, 1H), 2.86
(bd, 1H), 3.18 (bs, 1H), 3.26 (m, 1H), 3.49 (m, 1H), 3.75
(m, 2H), 4.21 (m, 1H), 4.52 (dd, 1H), 4.93 (bt, 1H), 4.98
(bs, 1H), 5.79 (d, 1H), 6.45 {d, 1H); Second eluted isomer:
Oil; b = 0.05 (m, 12H), 0.55 (s, 3H), 0.87 (m, 6H), 0.86
(s, 9H), 0.88 (s, 9H), 1.09 (d, 3H), 1.10-2.15 (m, 21H),
2.30 (bd, 1H) , 2.54 (dd, 1H) , 2.86 (bd, 1H) , 3 .29 (m, 1H) ,
3.44 (m, 1H), 3.72 (m, 2H), 4.21 (m, 1H), 4.52 (dd, 1H),
4.93 (bs, 1H), 4.98 (bs, IH), 5.79 (d, 1H), 6.45 {d, 1H).
Preparation 18: Compound 0019 (General Procedure 3)
Alcohol: Compound 0018 (0.22 g,, 0.32 mmol); The Prod-
uct was purified by chromatography on silica gel (30 g)
(eluant: 10~ ether in petroleum ether) gave the Title
Compound; 8 = 0.05 (m, 12H), 0.54 (s, 3H), 0.83 (t, 3H),
0.84 (t, 3H), 0.86 (s, 9H), 0.89 (s, 9H), 1.07 (d, 3H),
1.05-1.80 (m, 16H), 1.85-2.07 (m, 3H), 2.25-2.35 (m, 2H),
2.55 (m, 1H), 2.86 {bd, 1H), 3.29 (m, 1H), 3.46 (m, 1H),
3.79 (m, 1H), 4.21 (m, 1H), 4.51 (dd, 1H), 4.93 (m, 1H),
4. 97 (m, 1H) , 5.79 (d, 1H) , 6.45 (d, 1H) .
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
28
Preparation
19a: (3-Trimethylsilyloxy-4-ethyl-hex-3-
en~rloxy) -1 (S) , 3 (R) -bis (TBS-oxv) -
-20 (R) -9 10-seco-preana-5 (E) . 7 (E) . -
10(19)-triene (Compound 0020a)
(General Procedure 4)
Ketone: Compound
0019
(0.178
g,
0.25
mmol);
Purifica-
tion of the by chromatography on silica gel (30 g)
Product
(eluant: in petroleum ether) gave the Title
1 %
ether
Compound; 0.06 (m, 12H), 0.55 (s, 3H), 0.86 (s, 9H),
b =
0.90 (s, 9H), 0.92 (t, 3H), 0.93 (t, 3H), 1.08 (d, 3H),
1.16 (s, 9H), 1.00-2.25 (m, 17H), 2.31 (bd, 1H), 2.37 (m,
2H), 2.55 (dd, 1H), 2.86 (bd, 1H), 3.3I (m, 2H), 3.63 (m,
1H), 4.21 (m, 1H), 4.53 (m, 1H), 4.93 (m, 1H), 4.98 (m,
1H) , 5 .79 (d, 1H) 6.46 (d, 1H) .
,
Preparation 19b: Compound 0020 (General Procedure 5)
F'nol-ether: Compound 0012a (0.1 g, 0.13 mmol); Puri-
fication of the Product by chromatography on silica gel (15
g) (eluant: 5 % ether in petroleum ether) gave the Title
Compound; b = 0.06 (m, 12H), 0.17 (s, 9H), 0.55 (s, 3H},
0.77 (t, 3H), 0.78 (t, 3H), 0.86 (s, 9H), 0.89 (s, 9H),
1. 08 (d, 3H) , 1. 00-2.15 (m, 17H) , 2.29 (bd, 1H) , 2.55 (dd,
1H), 2.70-2.92 (m, 3H), 3.30 (m, 1H), 4.45 (m, 1H), 3.78
(m, 1H) , 4.21 (m, 1H) , 4.53 (m, 1H) , 4.93 (m, 1H) , 4. 98 (m,
1H), 5.79 (d, 1H), 6.45 (d, 1H}.
Preparation 20: Compound 0021 (General Procedure 6)
5E-Vitamin D compound: Compound 0019 (0.06 g, 0.87
mmol) (in toluene); Without further purification, the Prod-
uct containing the Title Compound was used in the next
step. b in agreement with structure.
Preparation 21: Compound 0022 (General Procedure 6)
~F-yitamin D compound: Compound 0020 (0.07 g, 0.09
mmol) (in toluene); Without further purification, the Pr -
uct containing the Title Compound was used in the next
step. The following signals for the Title Compound could be
CA 02222785 1997-11-28
WO 97/20811 PCTJDK96/00502
29
discerned: b = 0.06(m, 12H), 0.17 {s, 9H), 0.56 (s, 3H),
0.78 (t, 3 H) 0.79 (t, 3H) , 0.88 (s, 18H) , 1.08 3H)
, (d, ,
1.00-2.15 (m,17H), 2.2 2 (dd, 1H), 2.45 (dd, 1H), 80
a 2. (m,
3H), 3.30 (m,1H), 3.45(m, 1H), 3.79 (m, 1H), 4.19 (m,
S 1H) , 4 .38 {m,1H) 4. (m, 1H) , 5.18 (m, 1H) , 6.00{d,
, 87
IH) , 6 .25 (d,1H)
.
Preparations 22: Compound 0030
By replacing the side chain building block
cyclopentylcarbonylmethylenetriphenylphosphorane for
2-propylcarbonylmethylenetriphenylphosphorane in Prepara-
tion 06, the Title compound was prepared.
Pre aration 23: Compound 0033
I5 5E-Vitamin D compound: Compound 0008 (0.09 g, 0.14
mmol) {in dichloromethane); Purification of the Product by
chromatography on silica gel (15 g) (eluant: 30a ether in
petroleum ether) gave the Title Compound; b in agreement
with structure.
Preparation 24: Compound 0035
The compound was prepared from Compound 0001 by the
sequence: 1. CH2=CH-MgBr; 2. separation of isomers by chro-
matography; 3. EtBr, KH; 4. S02; 5. 03; 6. PPh3; 7. heat,
NaHC03; 8. Ph3PCHC02Me; 9. di-isobutylaluminium hydride;
10. L,Z,I-triacetoxy-l,l-dihydro-1,2-benziodoxol-3(1H)-
- one ) .
Preparation 25: Compound 0036
The compound was prepared from Compound 0001 by the
sequence: 1. CH3C02Et, LiN(SiMe3)2; 2. TBS-trifluoro-
methanesulphonate, 2,6-lutidine; 3. di-isobutylaluminium
hydride.
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
Preparation 26: Compound 0037
The compound was prepared from Compound 0001 by the
sequence: 1. Me3SiCzCH, n-butyl-lithium; 2. EtBr, KH; 3.
tetrabutylammonium fluoride.
5
Preparation 27: Compound 0038
To a solution, maintained at about -70°C, of the
lithio-derivative prepared from n-butyl-lithium (1.6 M in
hexanes; 1 mmol) and Compound 0037 {1 mmol) in dry THF (5
10 ml) was added via a syringe 2-ethylbutanal (1 mmol) in dry
THF (2 ml). After stirring at the same temperature for l0
min and thereafter at 0°C for 45 min, the reaction mixture
was partitioned between ether and water. Standard work-up
gave the oily Product. Purification by chromatography on
15 silica gel {50 g) (eluant: 30~ ether in petroleum ether)
gave the Title Compound as an oil.
General Procedure 7 (Examples)
To a mixture, maintained at about 25°C, of an ethyl
20 acetate solution (about 0.3 ml} of the TBS-ether (ca. 0.1
mmol} in acetonitrile (5 ml) was added 48% aqueous
hydrofluoric acid (0.5 g, 12 mmol). After stirring at the
same temperature for 1 h, the reaction mixture was parti-
tioned between ethyl acetate and 1N sodium hydroxide sol-
25 ution. After standard work-up gave the oily product was
purified by chromatography on silica gel {15 g) (eluant:
ethyl acetate) to give the Title Compound.
Example 1 : 24-Oxo-1(S), 3 (R) -dihvdroxy-20 (S) -9 . 10-
30 -seco-cholesta-5 (Z) , 7 (E) , 10 (19) -triene
(Compound 101)
TBS-ether: Compound 0013 (0.050 g, 0.078 mmol); Title
Compound: Oil; S 0.56 (3H, s) , 0.83 (3H, d) , 1.09 {6H, d) ,
1.15 to 2.1 (18H, m), 2.31 (1H, dd), 2.4 (1H, ddd), 2.5
(1H, m), 2.59 (IH, dd), 2.61 {1H, m), 2.82 (1H, dd), 4.23
(1H, m) , 4.43 (1H, m) , 5 (1H, m) , 5.32 (1H, m) , 6.02 (1H,
d) , 6.37 (1H, d) .
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
31
Example 2: 1(S),3(R)-Dihydroxy-20(R)-(6-hvdroxy-6-
-methyl-1-heptanovl)-9,10-seco-preana-
-5(Z),7(E),10(19)-triene (Compound 102)
TBS-ether: Compound 0014 (0.065 g, 0.095 mmol); Title
Compound: Oil; b 0.52 (3H, s), 1.02 (3H, d), 1.1 to 2.05
(22H, m), 1.21 (6H, s), 2.3 (1H, s), 2.37 to 2.65 (4H, m),
2.81 (1H, m), 4.22 (1H, m), 4.42 (1H, m), 4.99 (1H, m),
5.33 (1H, m) , 6.01 (1H, d) , 6.35 (1H, d) .
Example 3: 1(S),3(R)-Dihydroxv-20(R)-(5-hydroxy-5-
-ethyl-2-hept5m-1-byl)-9,10-seco-preana-
-5 (Z) , 7 (E) , 10 (19) -triene (Compound 103)
TBS-ether: Compound 0015 (0.07 g, 0.1 mmol); Title
Compound: Oil; b 0.55 (3H, s), 0.93 (6H, t), 1.1 to 2.1
(20H, m) , 1.14 (3H, d) , 2.31 (1H, dd) , 2.53 (1H, m) , 2.57
(2H, s) , 2.59 (1H, m) , 2.82 (1H, dd) , 4.23 (1H, m) , 4.42
(1H, m), 4.99 (1H, m), 5.33 (1H, m), 6.01 (1H, d), 6.36
( 1H, d) .
Example 4 : 24-Oxo-1 (S) , 3 (R) , 25-Trihydrox~r-20 (S) -
-9.10-seco-cholesta-S(Z),7(E),10(19)-tri-
ene (Compound 104)
TBS-ether: Compound 0016 (0.053 g, 0.073 mmol); Title
Compound: Oil; b 0.57 (3H, s), 0.85 (3H, d), 1.2 to 2.1
(18H, m), 1.39 (6H, s), 2.31 (1H, dd), 2.55 (3H, m), 2.83
(1H, m) , 3.83 (IH, s) , 4.23 (1H, m) ,' 4.43 (1H, m) , 5 (1H,
m) , 5.33 (1H, m) , 6.02 (1H, d) , 6.38 (1H, d) .
Example 5: 24-(1-Hydroxy-cyclopentyl)-24-oxo-
-1(S),3(R)-dihydroxy-20(S)-9,10-seco-
-chola-5 (Z) , 7 (E) , l0 (19) -triene (Compound
' 105
TBS-ether: Compound 0034 (0.05 g, 0.066 mmol); Titie
Compound: Oil; b 0.57 (3H, s), 0.85 (3H, d), 1.2 to 2.1
(26H, m), 2.31 (1H, dd), 2.55 (3H, m), 2.83 (1H, m), 3.83
(1H, s) , 4.23 (1H, m) , 4.43 (1H, m) , 5 (1H, m) , 5.33 (IH,
m) , 6.02 (1H, d) , 6.38 (1H, d) .
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
32
Example 6 : 24-Oxo-1 (S), 3 (R) -dihydroxv-20 (R) -.9, 10-
-seco-cholesta-5 (Z) , 7 (E) , 10 (19) , 22 (E) -
-tetraene (Compound 106)
_ ,
TBS-ether: Compound 0033 (0.050 g, 0.078 mmol); Title
Compound: Oil; 8 0.56 (3H, s} , I (3H, d) , 1.09 (6H, d) ,
1.I5 to 2.1 (16H, m), 2,25 (1H, m), 2.31 (1H, dd), 2.59
(1H, dd), 2.81 (1H, m), 2.82 (1H, dd}, 4.23 (1H, m), 4.43
(1H, m) , 5 (1H, m) , 5.32 (IH, m} , 6.02 (1H, d) , 6.1 (1H,
d) , 6 .37 (1H, d) , 6.77 (1H, dd) .
Example 7 : 1 (S) , 3 (R) -Dihydroxy-20 (R) - (3-oxo-4-
-ethyl-1-hexyloxy)-9,10-seco-preana-
-5 (Z) 7 (E) . 10 (19) -triene (Compound 108)
TBS-ether: Compound 0021, the crude product from
Preparation 20}; Title Compound: Oil; a 0.55 (s, 3H), 0.84
(t, 3H), 0.85 (t, 3H), 1.08 (d, 3H), 1.10-1.80 (m, 15H),
1.90-2.07 (m, 4H}, 2.25-2.35 (m, 2H), 2.60 (bd, IH), 2.75
(bq, 2H), 2.82 (bd, IH), 3.29 (m, 1H), 3.47 (m, 1H}, 3.79
(m, 1H), 4.23 (m, 1H), 4.42 (m, 1H), 5.00 (bs, 1H), 5.33
(bs, 1H}, 5.99 (d, 1H), 6.38 (d, 1H).
Example 8 : 1 (S) , 3 (R) -Dihydroxy-20 (R) - (3-oxo-4-
hvdroxy-4-ethyl-1-hexyloxy)-9,10-seco-
-preana-5 (Z) 7 (E) , 10 (19) -triene (Compound
1 9
TBS-ether: Compound 0022, the crude product from
Preparation 21); Title Compound: Oil; b 0.54 (s, 3H), 0.77
(t, 3H), 0.79 (t, 3H), I.07 (d, 3H), 1.05-2.10 (m, I9H},
2.30 (dd, 1H), 2.58 (m, 2H), 2.81 (m, 2H), 3.28 (m, 1H),
3.50 (m, 1H), 3.83 (s, 1H), 3.85 (m, 1H), 4.22 (m, IH),
4.41 (m, 1H}, 4.98 (m, 1H), 5.3I (m, 1H), 5.98 (d, 1H),
6.37 (d, 1H) .
Example 7: Capsules containina Compound 104
Compound 104 was dissolved in arachis oil to a final
concentration of 1 ~.g/ml oil. Ten parts by weight of gela-
tine, 5 parts by weight of glycerin, 0.08 parts by weight
CA 02222785 1997-11-28
WO 97/20811 PCT/DK96/00502
33
potassium sorbate, and 14 parts by weight distilled water
were mixed together with heating and formed into soft gela-
tine capsules. These were then filled each with 100 E.~.1 of
the oily solution of Compound 104.
Example 8: Dermatological Cream containin Compound
104
Compound 104 (0.05 mg) was dissolved in almond oil (1
to g). To this solution was added mineral oil (40 g) and self-
emulsifying beeswax (20 g). The mixture was heated to
liguifidation. After the addition of hot water (40 ml), the
mixture was mixed well. The resulting cream contains ap-
proximately 0.5 ~.g of compound 104 per gram of cream.