Note: Descriptions are shown in the official language in which they were submitted.
CA 02222926 1997-12-01
WO 96/40978 PCT/US96109805
ELECTROCHEMILUMINESCENT MONITORING OF COMPOUNDS
FIELD OF THE INVENTION
The present invention is directed generally to analytical biochemistry.
More specifically, the present invention is useful for monitoring chemical
transformations of detectable compounds having a chemically-transformable
first
compound covalently Iinked to an electrochemiluminescent compound.
BACKGROUND OF THE INVENTION
An ever-expanding field of applications exists for rapid, highly specific,
sensitive, and accurate methods of detecting and quantifying chemical,
biochemical, and biological substances, including enzymes such as may be found
in
biological samples. Because the amount of a particular analyte of interest
such as
an enzyme in a typical biological sample is often quite small, analytical
biochemists
are engaged in ongoing efforts to improve assay performance characteristics
such
as sensitivity.
One approach to improving assay sensitivity has involved amplifying the
signal produced by a detectable label associated with the analyte of interest.
In
this regard, luminescent labels are of interest. Such labels are known which
can be
made to luminesce through photoluminescent, chemiluminescent, or
electrochemilu.minescent techniques. "Photoluminescence" is the process
whereby
a material luminesces subsequent to the absorption by that material of light
(alternatively termed electromagnetic radiation or emr). Fluorescence and
phosphorescence are two different types of photoluminescence.
"Chemiluminescent" processes entail the creation of the luminescent species by
a
chemical reaction. "Electrochemiluminescence" is the process whereby a species
luminesces upon the exposure of that species to electrochemical energy in an
appropriate surrounding chemical environment.
The signal in each of these three luminescent techniques is capable of very
1
CA 02222926 2007-08-07
66822-803
effective amplification (Le., high gain) through the use of known instruments
(e.g.,
a photomultiplier tube or pmt) which can respond on an individual photon by
photon basis. However, the manner in which the luminescent species is
generated
differs greatly among and between photoluminescent, chemiluminescent, and
electrochemiluminescent processes. Moreover, these mechanistic differences
account for the substantial advantages as an bioanalytical tool that
electrochemiluminescence [hereinafter, sometimes "ECL"] enjoys vis a vis
photoluminescence and chemiluminescence. Some of the advantages possible with
electrochemiluminescence include: (1) simpler, less expensive instrumentation;
(2)
stable, nonhazardous labels; and (3) increased assay performance
characteristics
such as lower detection limits, higher signal to noise ratios, and lower
background
levels.
As stated above, in the context of bioanalytical chemistry measurement
techniques, electrochemiluminescence enjoys significant advantages over both
photoluminescence and chemiluminescence. Moreover, certain applications of
ECL have been developed and reported in the literature. U.S. .Patent Numbers
5,147, 806; 5,068,808; 5,061,445; 5,296,191; 5,247,243; 5,221,605; 5,238,808,
and 5,310,687 detail
certain methods, apparatuses, chemical moieties, inventions, and associated
advantages of ECL.
United States Patent Nuinber 5,641,623
details certain aspects of ECL in connection witb beta-lactam and beta-
lactamase (neither of which is conjugated through a covalent linkage to an
electrochemiluminescent compound).
None of the above-identified documents disclose nor suggest the present
invention. Additionally, the practice of the invention offers significant
advantages
to the skilled bioanalytical chemist in comparison to the
electrochemiluminescent
techniques taught by these documents. Accordingly, the invention meets the as-
yet unmet needs of skilled workers with respect to the achievement of improved
2
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
assay performance characteristics (e.g., signal output, detection limits,
sensitivity,
etc.) for the measured species and represents a patentable advance in the
field.
SUMMARY OF THE INVENTION
The present invention is directed to compounds, processes, and kits useful
for electrochemiluminescent monitoring of compounds. A critical feature of the
invention which is common to these compounds, processes, and kits is
detectable
compounds comprising a chemically-transformable first compound covalently
linked to an electrochemiluminescent compound.
In brief, these detectable compounds and their uses represent a patentable
advance in the field of electrochemiluminescent measurements because of their
attributes. These attributes include the following:
1. They are electrochemiluminescent.;
2. They can be used to monitor chemically-transformable first compounds
covalently linked to electrochemiluminescent compounds.; and
3. The above-described monitoring can be extended to become an integral
step in performing assays for separate, nonconjugated compounds in sample
solutions (e.g., enzymes).
Applicants' present inventions are set forth immediately below in the
following nonexclusive, nonlimiting objects of the invention.
A first object of the invention is to provide electrochemiluminescent
detectable compounds comprising a chemically-transformable first compound
covalently linked to an electrochemiluminescent compound.
A second object of the invention is to provide electrochemiluminescent
processes for monitoring chemical transformations of the first compound.
Consistent with this second object, assays are provided wherein the chemical
transformation of the first compound is an integral step in performing that
assay.
A third object of the invention is to provide kits useful for practicing the
invention and for implementing the above-described first and second objects of
the
invention. Consistent with this third object, kits are provided wherein at
least one
set of solutions containing the detectable compounds is included.
3
CA 02222926 2007-08-07
66822-803
According to one aspect of the present invention,
there is provided a method of electrochemiluminescently
determining an analyte of interest in a sample comprising
exposing a composition containing said sample and an
electrochemiluminescent label to conditions suitable for
inducing electrochemiluminescence and determining emitted
luminescence, wherein said electrochemiluminescent label
contains a coordinate complex of a metal and is covalently
linked to a first compound which reacts with the
electrochemiluminescent label under said conditions to cause
the electrochemiluminescent label to electrochemiluminesce.
According to another aspect of the present
invention, there is provided a compound for determining an
analyte of interest in a sample, which comprises an
electrochemiluminescent label containing a coordinate
complex of a metal, which label is covalently linked to a
first compound which reacts with the electrochemiluminescent
label under conditions suitable for inducing
electrochemiluminescence to cause the
electrochemiluminescent label to electrochemiluminesce.
According to still another aspect of the present
invention, there is provided a compound for determining an
analyte of interest in a sample, which comprises an
electrochemiluminescent label containing a coordinate
complex of a metal, which label is covalently linked to a
catalyst substrate wherein the catalyst substrate and
corresponding catalytic product differ in ability to react
with the electrochemiluminescent label to cause the
electrochemiluminescent label to electrochemiluminesce.
According to yet another aspect of the present
invention, there is provided a system for
3a
CA 02222926 2003-06-26
78037-28
electrochemiluminescently determining an analyte of interest
in a sample, wherein the system comprises: (a) the compound
as described herein; (b) a voltage source for exposing said
compound to electrochemical energy; and (c) a detector for
detecting or measuring emitted luminescence.
According to a further aspect of the present
invention, there is provided an electrochemiluminescent
process for monitoring chemical transformations of
compounds, comprising: (a) contacting the compound for
determining an analyte of interest comprising the label and
the first compound as described herein to a sample solution
suspected of containing at least one second compound; (b)
exposing the label to electrochemical energy to cause
electrochemiluminescence; (c) measuring the luminescence
emitted by the label; and (d) monitoring the presence of any
such chemical transformations of the first compound by
comparing the measured luminescence with a predetermined
standard.
3b
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows a proposed ECL mechanism depicting reaction steps
associated with the use of TPA as a nonconjugated reductant. Figure 2 shows a
proposed ECL mechanism depicting reaction steps 5 associated with the use of
beta-lactam as a nonconjugated reductant.
Figure 3(a-c) shows a proposed ECL mechanism depicting reaction steps
associated with the use of a chemically-transformable first compound as a
conjugated reductant.
Figure 4 shows the synthesis of Ru-AMP.
Figure 5 shows the mass spectrum of the ammonium hexafluorophosphate
salt of Ru-AMP.
Figure 6 shows the proton NMR spectrum of the ammonium
hexafluorophosphate salt of Ru-AMP.
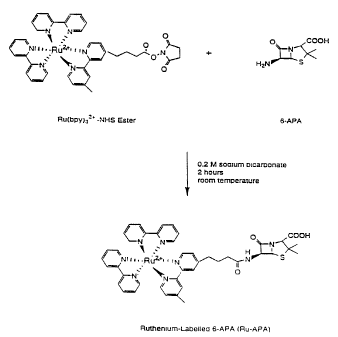
Figure 7 shows the synthesis of Ru-APA.
Figure 8 shows the structures of 5 specific beta-lactams.
Figure 9 shows the hydrolysis by NaOH or by beta-lactamase enzyme of
Ru-AMP (left side) and of Ru-APA (right side).
Figure 10 shows the comparison of measured ECL for a series of different
samples.
Figure 11 shows the comparison of measured ECL for a series of different
samples.
Figure 12 shows the effect of unhydrolyzed (closed circles) and
hydrolyzed (open circles) Ru-AMP concentration on the measured ECL.
Figure 13 shows the comparison of measured ECL for a series of different
samples.
Figure 14 shows the effect of unhydrolyzed (closed circles) and
hydrolyzed (open circles) Ru-APA concentration on the measured ECL.
Figure 15 shows the comparison of measured ECL for a series of different
samples.
Figure 16 shows a proposed ECL mechanism depicting reaction steps
4
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
associated with the NADH-promoted ECL of Ru(bpy)3+2
Figure 17 shows the synthesis of Ru-NAD.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is concerned with detectable compounds comprising
(A) a chemically-transformable first compound (B) covalently linked to (C) an
electrochemiluminescent compound. The salient features of each of these three
portions [(A), (B), and (C)] of the detectable compounds are individually
described below. Uses of the detectable compounds appear at (D) while specific
examples of the present invention appear at (E).
(A) The chemically-transformable first compounds.
The terms "chemically-transformable first compound(s)" (hereinafter
"CTFC") and "electrochemilu.minescent compound(s)" (hereinafter "EC") each
refer to the respective compound independent of certain minor variations of
that
compound. The skilled worker will understand which minor variation, if any,
applies to any particular usage of either CTFC or EC by its context. The
following explanations aid in the understanding of this context.
The term CTFC encompasses the following minor variations: (i) certain
changes in the formal redox state caused by reduction or oxidation reactions
and
certain chemical changes to the CTFC that do not destroy the covalent linkage
between it and the EC (e.g., the ejection by the CTFC of H+'); and (ii)
certain
chemical transformations (e.g., the hydrolysis of the CTFC) which alter the
measurable luminescence of the detectable compound in comparison to the
measurable luminescence before any of such chemical transformations have
occurred.
In comparing the measurable luminescence of the detectable compound
before and after such chemical transformation, several combinations are
possible
as detailed in the chart below.
measurable luminescence before measurable luminescence after
none yes (an increase from zero)
yes none (a decrease to zero)
5
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
yes yes (an increase from nonzero)
yes yes ( a decrease from nonzero)
yes yes (no change) INOPERATIVE none none INOPERATIVE
As depicted in this chart, the measurable luminescence of the detectable
compound is altered by the chemical transformation of the CTFC; i.e., the
measured luminescence before and after the chemical transformation differ from
one another. However, there must be some measurable luminescence either before
or after, or both before and after, any such chemical transformation. Thus,
the
fifth and sixth entries in the above chart do not represent compounds
encompassed
by the present invention while the first four entries do represent compounds
encompassed by the present invention.
Figure 3(a-c) shows a proposed ECL mechanism depicting reaction steps
associated with the use of a CTFC as a conjugated reductant that is covalently
linked to an EC. More particularly, the EC is exemplified by the ruthenium
(II)
tris-bipyridyl cation (hereinafter "Ru(bpy)3+2") throughout Figure 3(a-c).
Figure
3(a-c) illustrates contemplated minor variations in these two compounds (i.e.,
in a
CTFC and in an EC).
Figure 3(a) depicts the postulated ECL mechanism for a detectable
compound comprising a CTFC covalently linked to an EC. The chart below
further explains the depicted reactions.
6
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
&ymbol Definition
CTFC electrochemically unchanged CTFC (starting compound)
CTFC'+I radical, electrochemically oxidized CTFC
CTFC'(-W') radical, electrochemically neutral CTFC formed by W'
leaving CTFC+' and able to act as a high-energy reductant
in a manner similar to TPA
CTFC(-H+', -e') electrochemically neutral, nonradical CTFC formed by
CTFC'(-W') intramolecularly donating an electron (e') to
the covalently linked EC
Ru(bpy)3+2 nonexcited EC before electrochemical oxidation
Ru(bpy)3+3 nonexcited EC after electrochemical oxidation
*Ru(bpy)3+2 excited EC after being intramolecularly reduced by
the CTFC'(-H+')
Ru(bpy)3+2 nonexcited, regenerated EC formed by the emission
of light by excited EC
hv light emitted by the excited EC
Figure 3(b) shows, relative to Figure 3(a), all of the analogous reactions to
those of Figure 3(a) with the exception that the symbol YCTFC is consistently
used
to represent the resulting chemically-transformed rCTFC that is produced by
the
7
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
interaction between a CTFC and a second compound (hereinafter "SC").
Figure 3(c) shows the schematic depiction of the interaction between a
CTFC with a SC to form the resulting YCTFC.
The detectable compound depicted at Figure 3 (a), (b), (c) represents a
compound of the present invention that is able to produce measurable
luminescence both before and after the chemical transformation of the CTFC.
Thus, this compound exemplifies the third and fourth possible combinations of
measurable luminescence contained in the previously discussed chart. The
depicted reactions are consistent with postulated reaction mechanisms which
culminate in measurable luminescence both before and after the CTFC has been
chemically transformed. For compounds of the present invention falling within
the
first and second entries of the previously discussed chart (i.e., for those
compound
that only produce measurable luminescence either before or (exclusively) after
the
chemical transformation of the CTFC), only the reaction mechanisms of either
Figure 3 (a) or (exclusively) Figure 3 (b) is representative for any
particular
compound.
With regard to the minor variations of (i), both electrochemical redox
reactions and non electrochemical redox reactions are encompassed.
Additionally,
such changes are integral with and associated with the postulated
electrochemiluminescent mechanism including (a) steps leading to the formation
of
a high-energy reductant as a form of the CTFC; and (b) steps leading to the
actual
luminescence by the EC.
With regard to the minor variations of (ii), chemical transformations that
affect the intramolecular electron-donating ability of the CTFC when it acts
as a
high-energy reductant in electrochemiluminescence mechanisms are encompassed.
The hydrolysis of a CTFC/substrate by either NaOH or an enzyme is an example
of such a chemical transformation.
8
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
A critical feature of the CTFC is that it remains covalently linked to the EC
throughout all of the postulated reactions. Thus, it is understood that the
previously discussed changes in and to the CTFC specifically exclude changes
which would destroy/break the covalently linkage of the CTFC to the EC. It is
also understood that the changes in and to the CTFC alter the intramolecular
electron-donating ability of the CTFC so that the measurable luminescence
differs
before and after any such changes. The CTFC is chosen so that there is
measurable luminescence either before or (exclusively) after, or both before
and
after, any such changes.
Returning to the explanation of the term CTFC and the minor variations
encompassed therein, the scope of these variations is clear to the skilled
worker.
The CTFC must be able to participate in the ECL mechanisms that cause
the EC to luminesce. Specifically, the CTFC must function as a high-energy
reductant capable of intramolecularly providing an electron to the EC so the
EC is
reduced into an excited (i.e., emissive) state. Suitable high-energy
reductants for
forming the excited state EC often have an unpaired electron and are knows as
radicals. Figure 1 illustrates a proposed ECL mechanisms which uses TPA as a
nonconjugated high-energy reductant. This mechanisms generates the actual high-
energy reductant in situ subsequent to the initial electrochemical oxidation
(triggering) of the TPA precursor. Suitable candidates for the CTFC of the
present invention are within the knowledge of the skilled worker based on the
disclosure herein.
Applicants are not required to understand the theoretical underpinnings
which explain the observed behavior of the detectable compounds. While not
wishing to be bound by any particular scientific explanation for these
observed
properties, applicants postulate the following explanations (I) and (II).
(I.) The ability of the covalently linked CTFC to act as a high-energy
reductant
by intramolecularly donating an electron to the EC varies according to whether
that CTFC has or has not yet undergone a suitable chemical transformation.
This
variance can, depending upon the particular CTFC involved, either increase or
9
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
decrease the measured luminescence in any one of the four previously discussed
combinations. The chemical transformation in a CTFC resulting from the
interaction with a SC appears to cause this variance in intramolecular
electron-
donating ability because of structural changes in the CTFC which might effect
(i)
the route through which the reducing electron has to pass through; (ii) the
ability
of the reducing electron to begin traveling through any such route; or (iii)
stereochemical/spatial orientation considerations.
(II.) The ability of the covalently linked CTFC to act as a high-energy
reductant
by intramolecularly donating an electron to the EC is greater in comparison to
the
ability of that same (nonconjugated) CTFC to intermolecularly donate an
electron
to the EC. Correspondingly, the measured luminescence for the detectable
compounds of the present invention is greater in comparison with the measured
luminescence of electrochemiluminescent compounds where the high energy
reductant is not covalently linked to the electrochemiluminescent compound.
Although nonconjugated high energy reductants are not the subject of the
present invention; they nicely illustrate the importance of the mechanistic
differences. Certain electrochemiluminescent techniques, however, have focused
on using such nonconjugated high energy reductants. Figures 1 and 2 illustrate
proposed electrochemiluminescent mechanisms with such nonconjugated
reductants. Specifically, Figure 1 depicts electrochemiluminescent reactions
which
use tri-n-propylamine (hereinafter "TPA") as such a reductant while Figure 2
likewise depicts these reactions with beta-lactam as the reductant. The
postulated
electrochemiluminescence mechanism shown in Figure 1 using nonconjugated
TPA and Ru(bpy)3+2 has been previously reported in the literature. The
postulated
electrochemiluminescence mechanisms for beta-lactams (nonconjugated) shown in
Figare 2[as noted previously, the use of beta-lactams as nonconjugated, high
energy reductants in electrochemiluminescence techniques is the subject of a
copending and commonly-assigned United States Patent Application] and for the
conjugated high energy reductants of the present invention are derived in part
from
and are thought to be consistent with the mechanistic explanation for the TPA-
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
induced electrochemiluminescence shown in Figure 1.
Applicants theorize the following explanation as to why, for example, beta-
lactam as a nonconjugated reductant generates less electrochemiluminescence
than
beta-lactam as a conjugated reductant (i.e., as a CTFC). The nonconjugated
beta-
lactam must first diffuse through solution to become sufficiently proximate to
the
EC and then intermolecular donate an electron thereto. Moreover, during this
diffusion process, the nonconjugated beta-lactam may react with any available
species (other than the EC) because it is a very reactive, radical species. In
direct
contrast, the CTFC does not have to diffuse through the solution as a free
species.
The CTFC need only intramolecularly donate an electron to the covalently
linked
EC.
The above analysis teaches attributes of the CTFC sufficiently detailed to
enable the skilled worker to practice the present invention. To augment the
above
teachings, applicants later provide examples using particularly-identified
compounds as the CTFC. However, applicants' invention is not limited to any
specific compounds; rather applicants' invention is limited only to suitable
CTFC
as taught by the foregoing.
(B) The covalent IinkaQe
The covalent linkage comprises a linker group that covalently links one of
the chelating ligands of the EC to the CTFC. Thus, the near end of the linker
group terminates with and extends into a covalent bond between an atom of the
linker group and an atom of one of the chelating ligands of the EC while the
far
end of the linker group terminates with and extends into a covalent bond
between
an atom of the linker group and an atom of the CTFC.
This linker group must have the following attributes to ensure that
applicants' detectable compounds are operative. As detailed below, these
attributes are divided into two main categories; namely, noninterfering and
enhancing.
The noninterfering attributes are properties that the linker group must have
because otherwise their presence would interfere with the operability of the
11
CA 02222926 1997-12-01
WO 96/40978 PCTIUS96/09805
invention. Specifically, the linking group during the contemplated practice of
the
invention must not: (i) prohibit the electrochemical reactions; (ii) prohibit
the
interactions between the CTFC and the SC; (iii) prohibit the overall
electrochemiluminescence mechanism; and (iv) be itself destroyed by the
necessary reactions of the invention. For example, a linker group containing
an
electrochemically oxidizable species having a formal oxidation potential close
to
that of the central metal cation of the EC would not serve as an effective
linking
group.
The enhancing attributes of the linker group are those attributes that
specifically relate to the ability of the CTFC to intramolecularly transfer an
electron to the central metal cation of the EC. These enhancing attributes
include
the length of the linking group and the nature of the bonds within such
length.
First, the length of the intervening linker group between the CTFC and the EC
must (i) allow and permit the appropriate intramolecular electron transfer to
occur;
and (ii) not prevent any necessary reaction from occurring due to steric or
other
considerations.
The term "intramolecular" transfer of an electron from the CTFC to the
EC encompasses both transfer though bonds and through space. Such
"intramolecular" transfers, however, are limited to transfers between a
donating
compound (i.e., the CTFC) and a coffesponding receiving compound (i.e., the
EC)
which are covalently linked to each other through the linking group. The
covalent
linkage portion of the detectable compounds must allow and permit at least one
these two types of intramolecular transfer.
For intramolecular transfer through bonds, the linker group must provide
sufficient delocalized, conductive electrons (e.g., conjugated 7c-systems) to
enable
the electron to travel through the bonds of the linking group to than reach
the
central metal cation of the EC.
For intramolecular transfer through space, the linker group must enable the
CTFC to approach in relative close proximity the central metal cation of the
EC.
The linker group should be long enough and stereochemically flexible enough so
12
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
that the CTFC attached to the far end of the linker group can swing back
towards
the metal cation and then the electron can intramolecularly transfer through
the
space then separating the CTFC and the EC. An additional limitation on the
appropriate length of the linker group is that it should not be so long that
the
frequency of the described swinging around effect (which effect is thought to
be
necessary for intramolecular transfer through space) significantly decreases.
In the
case of an excessively long linker group, the amount of luminescence produced
would be lowered.
For example, a linking group that is not sufficiently long/flexible to enable
intramolecular transfer through space and contains only saturated bonds
without
any delocalized electrons (e.g., alkyl chains) would not be an effective
linker
group.
There are several advantages that the linker group imparts to the detectable
compounds as compared to the electrochemilumi.nescent compounds that are used
with nonconjugated high-energy reductants. The detectable compounds of the
present invention avoid the use of any diffusion of free species through
solutions.
Possible advantages include a more rapid generation of the exited, luminescent-
form of the EC and higher signals associated with the more effective
intramolecular transfer of applicants' present invention as compared to the
intermolecular transfer used with nonconjugated high-energy reductants.
This linkage also ensures that the ratio of the CTFC and the EC is one-to-
one. This ratio is unlike that associated with electrochemiluminescent
techniques
which use TPA nonconjugated beta-lactams as the high-energy reductant. Because
of this ratio between the two portion of the detectable compounds of the
present
invention, applicants are able to qualitatively and quantitatively monitor
chemical
transformations in the CTFC. Unlike known electrochemiluminescent techniques,
the compound monitored is simultaneously (i) covalently lin.ked to the EC and
(ii)
capable of intramolecularly donating an electron to the EC.
Suitable candidates to be tested as linker groups in the present invention are
available to those of ordinary skill in the art. In particular, Vol. 136,
Methods in
13
CA 02222926 2007-08-07
66822-803
Enzymology, K. Mosbach, Ed., pp. 3-30, Academic Press, NY (1987) discloses a
series of "spacer molecules" for immobilized active coenzymes, including NAD
and ATP. The spacer molecules of this article, which article is fully
incorporated
by reference, are examples of such suitable candidates.
The above analysis, in connection with the disclosure herein, teaches
attnbutes of the covalent linkage sufficiently detailed to enable the skilled
worker
to practice the present invention. Thus, the skilled worker can select
appropriate
candidates as linking groups and determine, by routine experimentation, those
which do and do not work. To augment the above teachings, applicants later
provide examples using specific detectable comppunds having identified linker
groups. However, applicants' invention is not limited to any such exemplified
linker group. Rather, the present invention is limited only to covalent
linkages as
taught herein to the skilled worker.
(C) The electrochemiluminescent compounds.
The third and final portion of the detectable compounds are EC. These
and their applications in certain contexts have been reported in the
literature. See,
for example, the U.S. Patents previously mentioned. The,
attnbutes and identities of such known EC are known to skilled workers and
need
not be repeated in detail here. Thus, the term electrochemiluminescent
compound
is a term of art whose metes and bounds are known to skilled workers.
Nonlimiting, nonexclusive examples of particular detectable compounds
(including
the EC portion) and their uses are later provided.
The present invention, however, is not directed to EC in and of themselves
nor is it directed to any of their known applications. The invention is
directed to a
novel and nonobvious use of EC; namely, their use in detectable compounds
comprising a CTFC covalently linked to an EC. Accordingly, the skilled worker
can practice the present invention in accordance with the disclosure herein in
combination with the existing knowledge of EC. Notwithstanding this,
applicants
provide guidelines for providing EC operative in the present invention.
The minor variations encompassed by the term CTFC discussed earlier at
14
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
(A) apply in an analogous m.anner to those for the term EC and need not be
reexamined here. Thus, changes in formal redox state of the EC due to, for
example, electrochemical oxidation and intramolecular reduction as well as
excited/nonexcited states are encompassed by the term EC and such changes
represent acceptably differing forms of the EC.
The following formula (I.) depicts suitable electrochemiluminescent
compounds for use in the present invention:
M(L')a(L2),(L3)c(L4)a(L5).(L6)f (I.); wherein
M is a central metal cation comprising ruthenium or osmium;
L' through L6 are each ligands of M, each of which may be monodentate or
polydentate, and each of which may be the same or different from each other;
a through e are each 0 or 1;
provided that the ligands of M are of such number and composition that the
compound can be induced to electrochemiluminescence; and
further provided that the total number of bonds provided by the ligands to the
central metal cation M equals the coordination number of M.
In the practice of the present invention, preferred electrochemiluminescent
compounds include those wherein the central metal cation is ruthenium Ru or
osmium Os. A particularly preferred compound is Ru(bpy)3+2
Having established (i) that electrochemiluminescent compound is a term of
art; (ii) guidelines for providing such EC; the term EC as used herein is
clear to
skilled workers. Nonetheless, applicants later amplify this teaching by
providing
nonlimiting, nonexclusive particular examples which identify the EC.
(D) Uses of the detectable compounds.
The identities, attributes, and theoretical basis of the detectable compounds
of the present invention have previously been detailed. Consequently, this
section
details the uses of such detectable compounds.
The electrochemiluminescent processes that use the detectable compounds
can be viewed as being divided into two main categories; namely, monitoring
and
assaying.
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
The detectable compounds can be used to monitor chemical
transformations in the CTFC that alter the effective intramolecular donating
ability
of that CTFC. These monitoring processes are not primarily designed to
qualitatively nor quantitatively identify the presence/amount of any
particular SC.
Rather, the monitoring processes are designed to qualitatively and/or
quantitatively
indicate the presence/extent of chemical transformations in the CTFC without
requiring identifications directed to which particular SC in the sample
solution is
responsible for any such chemical transformations.
By comparing (i) the measured luminescence of the detectable compound
after the exposure of that detectable compound to sample solutions suspected
of
containing at least one SC that is capable of interacting with the CTFC and of
effecting a chemical transformation in the CTFC with (ii)the measured
luminescence of the predetermined standard, the CTFC is effectively monitored.
More specifically, the presence and extent of such chemical transformations in
the
CTFC can be monitored. The predetermined luminescence standard of the
monitoring process is generated in the following manner.
The preparation of this calibration curve is illustrated for a detectable
compound able to produce measurable luminescence before any chemical
transformations in the CTFC. Known differing amounts of a particular
detectable
compound are (in the purposeful absence of any SC capable of interacting with
the
CTFC to cause a chemical transformation) prepared in a series of sample
solutions. Each of these sample solutions is caused to
electrochemiluminescence
upon exposure to electrochemical energy in the form of a positive voltage bias
imposed on an electrode of an electrochemiluminescent cell. The resulting
experimentally measured luminescence is recorded. The predetermined
luminescence standard for monitoring techniques comprises a calibration curve
having experimentally measured luminescence on a first axis and known amounts
of the particular detectable compound on the second axis. By comparing the
experimentally measured luminescence of a solution containing a known quantity
of the detectable compound and also containing a sample suspected of
containing
16
__ e
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
any second compounds with the corresponding luminescence value from the
calibration curve, the CTFC is effectively monitored. Changes in the CTFC
caused by interactions with any second compounds in the sample solution will
result in measurable differences (deviations) from the calibration curve.
The monitoring processes can be used to screen suspected solutions for
activity against the CTFC. Specifically, a series of sample solutions could be
monitored with the detectable compounds. A positive electrochemiluminescence
test result (i.e., a result that is either higher or lower than the
predetermined
standard) for any particular sample solution is indicative of at least one SC
in that
particular sample solution. Accordingly, that solution would then be an
appropriate candidate for further detailed investigations.
The assaying processes are extensions of the monitoring processes in that
the assaying processes are designed to specifically test for the presence
and/or
amount of a particular SC. As such, the assaying processes likewise are based
on
the chemical transformations in the CTFC which alter the effective
intramolecular
donating ability of the CTFC to the EC.
By comparing (i) the measured luminescence of the detectable compound
after the exposure of that detectable compound to a sample solution suspected
of
containing a particular SC that is capable of interacting with the CTFC and of
effecting a chemical transformation in the CTFC with (ii)the measured
luminescence of the predetermined standard, the particular SC is effectively
assayed. More specifically, the presence and amount of the particular SC can
be
assayed. The predetermined luminescence standard of the assaying process is
generated in the following manner.
Known amounts of a particular detectable compound are exposed to a
series of sample solutions each containing known differing amounts of a
particular
SC that is capable of interacting with the CTFC of the detectable compound in
accordance with the present invention. The exposure is effected under
conditions
favorable to and consistent with the desired interactions. Subsequent to such
interactions, each of these sample solutions is caused to
electrochemiluminescence
17
CA 02222926 1997-12-01
WO 96/40978 PCTIUS96/09805
and the experimentally measured luminescence is recorded. The predetermined
luminescence standard for assaying processes comprises a calibration curve
having
experimentally measured luminescence on a first axis and known amounts of the
particular SC on the second axis.
For both monitoring and assaying processes, the experimentally measured
luminescence may be either greater than or less than the luminescence for the
applicable predetermined luminescence calibration curve. In other words, the
interaction between the CTFC and the at least one second compound may either
increase or decrease the effective intramolecular electron donating ability of
that
CTFC (which would correspondingly increase or decrease the experimentally
measured luminescence).
Preferred applications of the detectable compounds are monitoring and
assaying processes when the CTFC first compound comprises a substrate and the
SC comprises an enzyme that is specific to that substrate. Particularly
preferred
substrates are beta-lactams. Such beta-lactams are useful in assaying
processes
that test for the corresponding beta-lactamase.
Another application of the detectable compounds of the present invention
takes advantages of coupled, regenerative reaction mechanism that involve the
conversion of a separate, nonconjugated substrate in solution into a separate,
nonconjugated product in solution via exposure to an appropriate enzyme and co-
mediators. The interactions between the CTFC and the enzyme-catalyzed, co-
mediated conversion of a substrate species in solution to a product species in
solution forms the theoretical underpinnings for an assay that can be specific
to the
substrate in solution, the enzyme in solution, and/or the CTFC.
Another use of the detectable compounds of the invention are in kits
specifically designed to implement the processes of the present invention.
Accordingly two types of kits are provided. The monitoring kits each comprise
a
plurality of sample standard solutions each containing known amounts of a
particular detectable compound with the purposeful absence of any SC. These
monitoring kits can be used to determine the predetermined luminescence
standard
18
CA 02222926 1997-12-01
WO 96/40978 PCT/US96109805
calibration curve. The assaying kits each comprise a plurality of sample
solutions
each containing known amounts of a particular detectable compound in addition
to
a corresponding plurality of test solutions each containing known differing
amounts of a particular SC that is capable of interacting with the detectable
compound in the described manner.
(E) Examples.
Notwithstanding the previous detailed description of the present invention,
applicants below provide specific examples solely for purposes of illustration
and
as an aid to understanding the invention. Particularly with respect to the
protection
to which the present invention is entitled to, these examples are both
nonlimiting
and nonexclusive. Accordingly, the scope of applicants' invention as set forth
in
the appended claims is to be determined in light of the teachings of the
entire
specification without incorporating in such claims the specific limitations of
any
particular example.
P.xamyle 1. Preparation of Ru(bpy)3+? labeled beta-lactam antibiotics
(a) Preparation of Ru(bpy)3+J-labeled ampicillin (Ru-AMP):
Ru(bpy)3+2-NHS ester (15.1) mg in acetonitrile (250 L) was mixed with
ampicillin (29.1 mg) in 0.2 M sodium bicarbonate, pH 8.0 (250 pL) and the
reaction was allowed to proceed at room temperature for 2 hours (Figure 4). Ru-
AMP was purified using a Waters HPLC system (Milford, MA) equipped with a
ProgelTM-TSJ CM-5PW column (7.5 cm x 7.5 mm) (Supelco, Inc., Bellefonte,
PA) using a 1.0 mL/minute, 15-minute linear gradient from 20-180 mM sodium
phosphate, pH 7Ø Substrate was quantitated spectrophotometrically by
measuring the absorbance of the ruthenium complex (the molar extinction
coefficient at 453 nm is 13,700 M-'cm'). Following formation of the ammonium
hexafluorophosphate salt, the structure and purity of Ru-AMP was confirmed by
mass spectroscopy and proton NMR (Figures 5-6).
(b) Preparation of Ru(bpy)3+'-labeled 6-aminopenicillanic acid
(hereinafter "Ru APA')
Ru(bpy)3+2-NHS ester (15 mg) (IGEN, Inc., Gaithersburg, MD) in
19
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
acetonitrile (250 L) was mixed with 6-aminopenicillanic acid (12.4 mg) in 0.2
M
sodium bicarbonate, pH 8.0 (350 pL) and the reaction was allowed to proceed at
room temperature for 2 hours (Figure 7). Ru-APA was purified with a Waters
HPLC system (Milford, MA) equipped with a ProgelTM-TSK CM-5PW column
(7.5 cm x 7.5 mm) (Supelco, Inc., Bellefonte, PA) using a 1.0 mL/minute, 20-
minute linear gradient from 20-100 mM sodium phosphate, pH 7Ø Substrate was
quantitated spectrophotometrically by measuring the absorbance of the
ruthenium
complex (the molar extinction coefEicient at 453 nm is 13,700 M''cm 1).
(G) Preparation of other Ru(bpy)3+?-labeled beta-lactams
Other beta-lactams, such as 7-aminocephalosporanic acid, that have a
primary amine in their structures can also react with Ru(bpy)3+2-NHS ester to
form
similar conjugates as described above. The reaction and purification
conditions
will be similar, potentially differing somewhat in ways solvable by one
skilled in
the art. Figure 8 shows the structure of 5 specific beta-lactams.
Example 2. ECL assay of Ru-AMP hydrolysis
Experiments were performed to compare the ECL properties of Ru-AMP
(conjugated) with Ru(bpy)3+2 and ampicillin mixtures (nonconjugated). ECL
properties were compared both before and after NaOH and enzymatic hydrolysis
(Figure 9, left side).
Ru-AMP was found to be a very good substrate of beta-lactamase.
Hydrolysis of Ru-AMP (33 pM) by beta-lactamase I from Bacillus cereus (0.3
nM) was monitored spectrophotometrically at 240 nm using a Hitachi U3200
spectrophotometer (Danbury, CT) at 25.0 C in 0.1 M sodium phosphate, pH 7Ø
Half-time (t1i2) analysis gave a k~at/Km for enzymatic hydrolysis of Ru-AMP of
3.9
x 10R min'M-'.
The ECL properties of equimolar mixtures of Ru(bpy)3+2 and ampicillin
(hydrolyzed or unhydrolyzed) were compared to the same concentration of the
Ru-AMP conjugate (hydrolyzed or unhydrolyzed). In separate experiments,
ampicillin and Ru-AMP were hydrolyzed by either 250 mM NaOH (base
hydrolysis) or 441 nM beta-lactam I from Bacillus cereus (enzyme hydrolysis).
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
For base hydrolysis, 50 pL of 5 M NaOH were added to 1.0 mL solutions of
deionized water containing either 24.85 pM Ru-AMP or a mixture of 25 M
ampicill.in and 25 pM Ru(bpy)3+2. Following 30 minute incubations, the
solutions
were neutralized with 50 L of 5 M HCI. For the unhydrolyzed counterpart
experiments, 50 L of HZO were added to solutions of either 24.85 M Ru-AMP
or a mixture containing 25 M ampicillin and 25 M Ru(bpy)3+2. Following 30
minute incubations, 50 L of 5 M NaCI was added to these solutions. The
results
shown in Figure 10 demonstrate: (1) that ampicillin hydrolysis by either NaOH
or
beta-lactamase causes an increase in the ECL of the mixtures; and (2) that the
increase in the ECL caused by the hydrolysis is dramatically greater when the
light-emitting ruthenium complex is covalently linked to ampicillin. With base
hydrolysis, ECL increased 1.5-fold when ampicillin was hydrolyzed in a mixture
of
ampicillin and Ru(bpy)3+2, while ECL increased 5.2-fold when Ru-AMP was
hydrolyzed. Similar results were obtained in enzyme hydrolysis: ECL increased
2.1-fold when ampicillin was hydrolyzed in a mixture of ampicillin and
Ru(bpy)3+2,
while ECL increased 9.8-fold upon hydrolysis of Ru-AMP. The data establishing
these conclusions is found in Figure 10 which shows the experimentally
measured
electrochemiluminescence of (from left to right):
Ru(bpy)3+2 alone;
Ru(bpy)3+2 plus unhydrolyzed ampicillin;
Ru(bpy)3+2 plus NaOH-hydrolyzed ampicillin;
unhydrolyzed Ru-ANII';
NaOH-hydrolyzed Ru-AMP;
Ru(bpy)3+2 plus unhydrolyzed ampicillin;
Ru(bpy)3+2 plus beta-lactamase-hydrolyzed ampicillin;
unhydrolyzed Ru-AMP; and
beta-lactamase-hydrolyzed Ru-AMP.
This work was confirmed in a second experiment using enzyme hydrolysis
which differed in that the incubation time with enzyme was lengthened from 30
to
60 minutes (Figure 11). Here, enzyme hydrolysis caused a 2.5-fold increase in
21
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
ECL when ampicillin and Ru(bpy)3+2 were unconjugated and an 11.1-fold increase
in ECL when the Ru-AMP conjugate was hydrolyzed. The data establishing these
conclusions is found in Figure 11 which shows the experimentally measured
lu.minescence of (from left to right):
Ru(bpy)3+2 alone;
Ru(bpy)3+2 plus unhydrolyzed ampicillin;
Ru(bpy)3+2 plus beta-lactamase-hydrolyzed ampicillin;
unhydrolyzed Ru-AMP; and
beta-lactamase-hydrolyzed Ru-AMP.
These results show that Ru(bpy)3+2-conjugation caused intramolecular
effects that dramatically increase the experimentally measured luminescence
when
the beta-lactam ring is hydrolyzed.
Figure 12 shows that low concentrations of Ru-ANII' can be detected by
hydrolysis. The lower limit of detection was found to be 50 nM (464 relative
ECL
counts for hydrolyzed Ru-AMP versus an average instrument reading of -152
relative counts for unhydrolyzed Ru-AMP). This compares favorable to the lower
limit for detection of (unconjugated) ampicillin hydrolysis which was 5000 nM.
Example 3. ECL assay of Ru-APA hydrolysis
It was thought that Ru-APA might have different ECL properties (before
and after hydrolysis) from those of Ru-AMP. The differences would be a
consequence of the structural differences between APA and AMP, especially the
difference in distance between the beta-lactam ring and the primary amino
group
used to conjugate Ru(bpy)3+2-NHS ester (Figure 9, right side). In Ru-AMP, the
beta-lactam ring is three bond lengths farther from the amino group than in Ru-
APA. Specifically, hydrolysis of Ru-APA (or other beta-lactam conjugates) may
be more or less sensitively detected by ECL than Ru-AlVIP hydrolysis.
The ECL properties of the Ru-APA conjugate were compared with those
of the mixtures of unconjugated Ru(bpy)3+2 and 6-APA. ECL properties were
compared before and after NaOH and enzymatic hydrolysis. The data was then
compared to the results of similar experiments with Ru-AMP described in
22
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
Example 2.
Ru-APA was found to be a very good substrate of beta-lactamase.
Hydrolysis of Ru-APA (23 M) by beta-lactamase I from Bacillus cereus (0.6
nM) was monitored spectrophotometrically at 240 nm using a Hitachi U3200
spectrophotometer (Danbury, CT) at 25.0 C in 0.1 M sodium phosphate, pH 7Ø
Hal.f-time (tv2) analysis gave a k~t/Km for enzymatic hydrolysis of Ru-APA of
9.8
x 10' min 1M-1. This rate indicates that the enzyme hydrolyzed Ru-APA with a 4-
fold lower efficiency than Ru-AMP, but that Ru-APA hydrolysis by beta-
lactamase is still exceptionally efficient.
The ECL properties of equimolar mixtures of Ru(bpy)3+2 and APA
(hydrolyzed or unhydrolyzed) were compared with the same concentration of the
Ru-APA conjugate (hydrolyzed or unhydrolyzed). In separate experiments, 6-
APA and Ru-APA were hydrolyzed by either 250 mM NaOH (base hydrolysis) or
347nM beta-lactamase I from Bacillus cereus (enzyme hydrolysis).
For base hydrolysis, 50 pL of 5 M NaOH were added to 1.0 mL solutions
of deionized water containing either 23.0 M Ru-APA or a mixture containing
23.0 M APA and 23.0 N.M Ru(bpy)3+2. Following 60 minute incubations, the
solutions were neutralized with 50 L of 5 M HCI. For unhydrolyzed counterpart
experiments, 50 L of H20 were added to solutions of either 23.0 pM Ru-APA or
a mixture of 23.0 M APA and 23.0 M Ru(bpy)3+2. Following 60-minute
incubations, 50 L of 5 M NaCl was added to these solutions. The results shown
in Figure 13 demonstrate: (1) that 6-APA( conjugated or nonconjugated)
hydrolysis by either NaOH or beta-lactamase causes an increase in ECL; and (2)
that the increase in ECL caused by hydrolysis is dramatically greater when the
light-emitting ruthenium complex is covalently coupled to 6-APA. With base
hydrolysis, ECL increased 1.9-fold when 6-APA (nonconjugated) in a mixture of
6-APA and Ru(bpy)3+2 was hydrolyzed, while ECL increased 13.2-fold when Ru-
APA (conjugated) was hydrolyzed. Similarly with enzyme hydrolysis, ECL
increased 1.4-fold when 6-APA (nonconjugated) in a mixture of 6-APA and
Ru(bpy)3+2 was hydrolyzed, while ECL increased 31.8-fold when Ru-APA
23
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
(conjugated) was hydrolyzed. The data establishing these conclusions is found
in
Figure 13 which shows the experimentally measured luminescence of (from left
to
right):
Ru(bpy)s+Z alone;
Ru(bpy)3+2 plus unhydrolyzed 6-APA;
Ru(bpy)3+2 plus NaOH-hydrolyzed 6-APA;
unhydrolyzed Ru-APA;
NaOH-hydrolyzed Ru-APA;
Ru(bpy)3+2 plus unhydrolyzed 6-APA;
Ru(bpy)32 plus beta-lactamase-hydrolyzed 6-APA;
unhydrolyzed Ru-APA; and
beta-lactamase-hydrolyzed APA.
This work clearly demonstrates that conjugation of the 6-APA and the
electrochemiluminescent ruthenium complex result in intramolecular effects
that
increase the electrochemiluminescence when the beta-lactam ring is hydrolyzed.
Moreover, comparison with the results described in Example 2 for the
ampicillin
conjugate show that hydrolysis of Ru-APA results in a much greater
electrocheniiluminescence signal than hydrolysis of Ru-AMP. Because the
ruthenium atom is closer to the beta-lactam ring in Ru-APA than in Ru-AMP,
these results indicate that there may be a critical effect of the distance
between the
ruthenium complex and the beta-lactam ring. Other, as-yet untested beta-lactam-
Ru(bpy)3+2 conjugates may give an even more dramatic change in the
electrochemiluminescence upon beta-lactam hydrolysis.
Figure 14 shows that the hydrolysis of very low concentrations of Ru-APA
can be detected by ECL. More specifically, Figure 14 shows the effect of
unhydrolyzed (closed circles) and hydrolyzed (open circles) Ru-APA
concentration on the experimentally measured electrochemiluminescence. The
lower limit of detection was found to be 50 nM (an instrument reading of -33
relative ECL counts for hydrolyzed Ru-APA versus an average of -648 relative
ECL counts for unhydrolyzed Ru-APA (conjugated).) This compares favorably to
24
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
the lower limit for detection of (unconjugated) APA hydrolysis which was 50 M
(in the presence of 10 M Ru(bpy)3+2)
An experiment was performed to quantitate the advantage of conjugating a
beta-lactam to the ECL label, Ru(bpy)3+2. The increase in ECL upon hydrolysis
of
10 pM Ru-APA was compared to an ECL standard curve in which various
concentrations of 6-APA (nonconjugated) were hydrolyzed in the presence of 10
pM Ru(bpy)3+2. By extrapolation of the 6-APA standard curve, the results
(Figure
15) demonstrates that the ECL change upon hydrolysis of 10 pM Ru-APA
(conjugated) is equivalent to the ECL change in the hydrolysis of 1250 M 6-
APA
(nonconjugated) in the presence of 10 pM Ru(bpy)3+2. This demonstrates that
conjugation of Ru(bpy)3+2 and 6-APA results in a 125-fold increase in the ECL
change seen during 6-APA hydrolysis. The data establishing these conclusions
is
found at Figure 15 which shows a comparison of electrochemiluminescence
effects
of Ru-APA (conjugated) to Ru(bpy)3+2 plus 6-APA (unconjugated). Triangles
represent the electrochemiluminescence of 10 pM unhydrolyzed (open triangles)
and hydrolyzed (closed triangles) Ru-APA. Circles represent the
electrochemiluminescence effects of unhydrolyzed (closed circles) and
hydrolyzed
(open circles) 6-APA (0-1000 pM) in the presence of 10 M Ru(bpy)3+z
Extrapolation in Figure 15 indicates the electrochemiluminescence change upon
hydrolysis of 10 pM Ru-APA is equivalent to the electrochemiluminescence
change upon hydrolysis of 1250 pM free 6-APA in the presence of 10 M
Ru(bpy)s+2.
Example 4. Preparation of Ru{bpy)3+~-labeled fl-nicotinamide adenine
cofactors
(a) Theory of Oxidoreductase Enzymes and Their Use in Assays
(3-Nicotinamide adenine cofactors (such as NAD+, NADH, NADP+,
NADPH) are widely used in nature by oxidoreductase enzymes as oxidants or
reductants during reduction or oxidation of metabolites. Such enzymes include
many dehydrogenases (lactate dehydrogenase, alcohol dehydrogenase, glucose
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
dehydrogenase, etc.). The oxidized forms of these cofactors (NAD+ or NADP+)
have little or no TPA-like effects in ECL. However, the reduced forms (NADH or
NADPH) behave like TPA in promoting Ru(bpy)3+2 electrochemiluminescence (1,
2). Consequently, ECL can be used to measure the enzyme-catalyzed formation
or disappearance of the reduced forms of these cofactors. Hence, substrates
(glucose, ethanol, etc.) of dehydrogenases can be detected by ECL since their
chemical transformations by the appropriate enzyme stoichiometrically results
in
oxidation or reduction of nicotinamide adenine cofactors.
Reduced nicotinamide cofactors (NADH or NADPH) are not believed to
be destroyed during the ECL reactions as are TPA and beta-lactams, but are
instead converted to their oxidized forms (NAD+ or NADP+). This means that, in
the presence of an appropriate dehydrogenase enzyme, nicotinamide adenine
cofactors can be reused such that a single cofactor molecule that is
covalently
linked to an electrochemiluminescent compound can participate in multiple ECL
reactions (Figure 16). Note also in Figure 16 that the Ru(bpy)3+2 is also
regenerated so that it is possible for a single detectable compound comprising
such
a cofactor covalently linked to an electrochemiluminescent compound can
possibly
emit multiple photons one after another.
Nicotinamide adenine cofactors have advantages over present
electrochemiluminescent techniques that use TPA. Specifically, these cofactors
(i)
can participate in regenerative ECL reaction mechanisms; (ii) can be used to
detect
and quantitate dehydrogenases and their corresponding substrates. One
disadvantage is that the ECL signal (i.e., the experimentally measured
luminescence) is less in an ECL reaction with NADH or NADPH than in an ECL
reaction with TPA. This disadvantage could be reduced or obviated by using a
conjugate of derivatives of Ru(bpy)3+2 and the nicotinamide adenine cofactor.
As
shown in the Examples above, when Ru(bpy)3+2 is conjugated to a chemically-
transformable first compound which can act as a high energy reductant and
intramolecularly donate an electron to the covalently linked
electrochemiluminescent compound (such as a beta-lactam), the ECL signal
26
CA 02222926 1997-12-01
WO 96/40978 PCTJUS96/09805
generated is much greater than when the CTFC is not conjugated with the EC.
Similarly, a Ru(bpy)3+2-nicotinamide adenine cofactor (reduced form) conjugate
will also have more ECL than a nonconjugated mixture of Ru(bpy)3+2 and the
reduced cofactor. Similarly, the difference in ECL signal between the reduced
(NADH or NADPH) and oxidized forms (NAD+ NADP+) of the cofactors will be
greater when the cofactors are covalently linked to the Ru(bpy)3+2 than when
they
are not conjugated.
Conjugates of nicotinamide adenine cofactor derivatives are known and are
enzymatically functional (3,4). One such cofactor derivative, N6-([6-
aminohexyl]carbamoylmethyl) nicotinamide adenine dinucleotide, is commercially
available (Sigma Chem. Co., St. Louis, MO). The primary amino group of this
compound can be used to couple this compound to the same Ru(bpy)3+2-NHS ester
described above (obtainable from IGEN, Inc., Gaithersburg, 1VID) by the same
or
similar method (Figure 17) (3,4). Other similar coupling methods will also
work.
The conjugate (Ru-NAD) can be purified by HPLC in a similar manner as
described for purification of Ru AMP and Ru-APA. The four references noted
above are (1) Downey, T.M. & Nieman, T.A. (1992) Anal. Chem. 64 261-268;
(2) Martin, A.F. & Nieman, T.A. (1993) Anal. Chem. Acta. 281. 475-481; (3)
Mansson, M.-O., Larsson, P.-O., & Mosbach, K. (1982) Methods Enzym. 89,
457-468; and (4) Persson, M., Mansson, M.O., Bulow, L., Mosbach, K. (1991)
Bio/Technology 9 280-284. Each of these four references is incorporated by
reference. Figure 17 shows the preparation of Ru-NAD.
The oxidized form of Ru-NAD (Ru-NAD+) can be used in enzyme assays
in an ECL instrument to detect and quantitate a dehydrogenase enzyme or a
substrate of a dehydrogenase (or some compound that gives rise to either). The
assays will be performed according to conventional protocols (duration,
temperature, pH, buffer, salt, substrate and enzyme concentrations, etc.)
except
that NAD+ normally included will be excluded and Ru-NAD+ will be used in
place.
The concentration of Ru-NAD+ may be lower or higher than the conventional
assays owing to differences in substrate specificity, solubility, cost, or
other
27
CA 02222926 1997-12-01
WO 96/40978 PCTIUS96/09805
factors. Following the incubation, the mixture will be analyzed in an ECL
instrument (IGEN, Inc., Gaithersburg, MD). No additional Ru(bpy)3+2 will be
added. Reduction of Ru-NAD+ will be recognized by an increase in ECL signal
over background and will indicate the presence of the relevant dehydrogenase
and
substrate.
Similarly, oxidation of the reduced form of Ru-NAD (Ru-NADH) can be
detected by ECL. Again, conditions, and the presence of relevant enzyme and
enzyme substrate will be considered and will be derived from known conditions
for assays involving nonconjugated NADH. NADH will be omitted from the assay
and Ru-NADH (at an appropriate concentration that may not be the conventional
concentration) will be included. Following incubation, the mixture will be
analyzed with an ECL instrument. Any decrease in ECL from the initial Ru-
NADH signal will indicate that some Ru-NADH has been oxidized and will be
evidence of the presence of the relevant enzyme or substrate.
(b) Preparation of Ruthenium-Labelled N6[6-aminohexyl-
(carbamoylmethyl)-NAD+
To a solution containing 6.6 mg N6[6-aminohexyl-(carbamoylmethyl)-
NAD+ (Li+ salt, Sigma Chem. Co., St. Louis, MO) in 0.4 mL of a 1:1 mixture of
acetonitrile and NaHCO3 (0.2 M, pH 8.6) was added an NHS ester of Ru(bpy)32+
(IGEN, Inc., Gaithersburg, MD) in 0.2 mL of a 1:1 mixture of acetonitrile and
NaHCO3 (0.2 M, pH 8.6). The reaction mixture was run overnight at room
temperature. The following morning, the reaction was stopped, the solvent
removed, and the compound was purified by size exclusion chromatography
(BioRad Bio-Gel P-2, BioRad Laboratories, Richmond, CA). Proton NMR
showed the compound to be correct, but not completely pure. The compound
(Ru-NAD) was repurified on a column of Sp-Sephadex (Pharmacia, Uppsala,
Sweden), eluting with changes of increasing concentrations of trifluoroacetic
acid
(0, 0.05, 0.2, 0.3 M). NMR showed to compound to be pure Ru-NAD.
(c) Ru-NAD as an Enzyme Cofactor
To determine whether Ru-NAD was functional as an enzyme cofactor, a
28
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
reaction involving oxidation of D-glucose-6-phosphate by glucose-6-phosphate
dehydrogenase was tested. The reaction was monitored spectrophotometrically at
340 nm. This wavelength is commonly used to observe the interconversion of
NAD+ and NADH. A mixture of 63 M Ru-NAD, 400 M glucose-6-phosphate,
and 22 nM enzyme in 55 mM Tris buffer, pH 7.8 containing 33 mM MgC12 was
incubated at 30 C in a cuvette. Continuous absorbance readings showed that
absorbance increased over approximately 40 minutes in a fashion characteristic
of
enzymatic reduction of NAD+. This indicated that Ru-NAD was indeed accepted
as a functional cofactor by glucose-6-phosphate dehydrogenase.
(d) Effect of Enzymatic Reduction on the ECL of Ru-NAD
Ru-NAD was found to be accepted as a cofactor by the dehydrogenase,
glucose-6-phosphate dehydrogenase. An experiment was performed involving
oxidation of glucose-6-phosphate by this enzyme with concurrent reduction of
Ru-
NAD. Here, ECL measurements were made to determine if; (1) the ECL-inducing
effects of NADH (but not NAD+) are also present in Ru-NADH (but not Ru-
NAD) and (2) if conjugation of Ru(bpy)32+ with NADH causes an increase in ECL
measurement sensitivity as compared to the ECL of a mixture of unconjugated
Ru(bpy)32+ and NADH. The results are shown below (all solutions contain the
substrate, glucose-6-phosphate, solutions not containing Ru-NAD contained 1.0
M Ru(bpy)3+2);
Sample ECL counts
21 M NAD+ 45,500
21 M NAD+ + enzyme 45,200
21 M NADH 47,900
21 M NADH + enzyme 40,800
21 M Ru-NAD 71,700
21 pM Ru-NAD + enzyme 132,000
These results show that addition of enzyme to Ru-NAD increases the ECL
signal. Also the results show that, at unconjugated NAD concentrations too low
for ECL effects to be seen, Ru-NAD clearly gives a large amount of ECL when
29
CA 02222926 1997-12-01
WO 96/40978 PCT/US96/09805
enzyme is added. In conclusion, Ru-NAD behaves in the same way as free
Ru(bpy)32+ plus free NAD+ in an ECL instrument (enzyme addition causes an
increase in ECL), but Ru-NAD is much more sensitively detected. T'lus
indicated
that low concentrations of dehydrogenases or their substrates can be
sensitively
detected by ECL of Ru-NAD+ reduction or Ru-NADH oxidation.
The scope of the patent protection which the present invention is entitled to
is not limited by the preceding text. Rather, the present invention is defined
by the
claims appended hereto and all embodiments falling thereunder.