Note: Descriptions are shown in the official language in which they were submitted.
CA 02223846 1997-12-0~
METHOD AND APPAR~TUS FOR CONTINUOUS AMBULATORY
PERITONEAL DIALYSIS
This invention relates to a method and a~alalus for p~lr~ ling peritoneal
S dialysis, capable of use in both the hospital and home ellvilo~ lent.
BACKGROUND OF THIS rNVENTION
Generally, peritoneal dialysis is used to correct the following m.o~ica
disorders:
10 1. Acute and chronic renal failure;
2. Severe water retention;
3. Electrolyte disorders; and
4. Drug intoxication (acute poisoning).
The kidneys have the normal function of excreting metabolic waste products
15 from the body. They also regulate the amount and composition of body fluids, as
well as performing important endocrine functions, some of which are the regulation
of blood pressure, bone marrow function, and bone composition.
In kidney failure, the above functions are affected. Mild or moderate kidney
malfunction may result in abnorm~lities that can be collccled or ameliorated by
20 drugs or dietary measures. However, when kidney failure is severe, artificialkidney function becomes nPcess~ry to m~int~in life. Artificial kidney (dialysis)treatment cannot totally colllpcllsatc for the patient's own kidney. Dialysis
primarily substitutes for the lost excretory function and helps regulate fluid,
electrolyte and acid-base balance.
Two types of artificial kidney tre~tm~nt are recognized, namely hemodialysis
and peritoneal dialysis.
In hemodialysis, the blood is treated directly by using an extra-corporeal
system with an artificial membrane (kidney). Peritoneal dialysis uses the principles
of osmosis and diffusion across the peritoneal membrane to indirectly remove toxic
30 substances from the blood, and thereby correct certain electrolyte and fluid
imbalances.
CA 02223846 1997-12-0~
In result, extra-corporeal hemodialysis is used when rapid and efficient
dialysis is n-ocess~ry to preserve the life of the patient in case of severe renal failure
or drug intoxication.
Teclmi.~lly, hemodialysis is more de.~ g than peliloneal dialysis, and
this along with other mto(lic~l reasons has led to the increasing use of the relatively
simple pelilolleal operation in the event of (a) acute and chronic renal failure, (b)
severe water retention, (c) electrolyte disorders and (d) drug intoxication (acute
poisoning).
Prior to the 1970's, peritoneal dialysis treatment was a manual operation.
10 Subsequently, however, further developments were made, these falling into twocategories: (a) complete automatic fluid proportioning system, and (b) simple semi-
automatic cycler. The semi-automatic cycler system required the least amount of
technical opel~ling skill, and was based upon the principles of gravity and siphon.
This system allows the dialysis fluid to flow into the abdominal cavity under
15 gravitational action, keeps it there for a period of time, and then allows the fluid to
flow out. This cycle is continuously repeated until the end of the dialysis treatment.
The Prior Art
In order to improve efficiency, reliability and patient safety, we developed a
20 peritoneal dialysis apparatus which was patented on June 27, 1978 as U.S. patent
4,096,859. This development was embodied in a complex peritoneal dialysis
apparatus capable of being programmed to provide a predetell~ ed quantity of fresh
dialysis solution, passing this solution into the peritoneal gravity of a patient using
gravity feed, allowing the solution to remain in the peritoneal cavity for a
25 predetermined length of time, and draining spent dialysis solution from the
peritoneal cavity into a waste solution container, again by the urging of gravity.
Although this prior system functions well, certain users have found it to be
llnn.ocess~rily complex and expensive.
30 GENERAL DESCRIPTION OF THIS INVENTION
CA 02223846 1997-12-0~
Accordingly, it is an object of one aspect of this invention to provide a
simple but reliable apparatus for continuous ambulatory peritoneal dialysis (CAPD),
which is easy for the patient to program.
More particularly, this invention provides an appa.alus for CAPD,
S COlll~ lllg:
a support frame,
a weighing means secured to the support frame,
first support means ope-a~ively associated with the weighing means for
suspending from the weighing means a container of fresh dialysis solution,
second support means for suspending a container for receiving spent dialysis
solution,
a first, a second and a third conduit each having a distal end and a proximal
end,
said first conduit being a fill conduit adapted for connection at its distal endto the container of fresh dialysis solution,
said second conduit being a drain conduit adapted for connection at its distal
end to the container for receiving spent dialysis solution,
said third conduit being a patient conduit adapted for comml-nic~tion at its
distal end with a patient catheter through which dialysis solution can both enter and
leave the peritoneal cavity of the patient,
junction means where the proximal ends of the first, second and third
conduits are conn~cted together and are in co",-"~ ic~tion with each other,
clamp means for selectively permitting and halting fluid flow through each of
the first, second and third conduits, and
central processor means operatively associated with the weighing means and
receiving from the weighing means a signal representing the weight being supported
at any given time by the weighing means, the central processor means also havingdata input/output capability by which it can be programmed to carry out a three-stage cycle consisting of:
CA 02223846 1997-12-0~
a) a fill stage during which the drain conduit is closed, and during which dialysis
solution passes from the container of fresh dialysis solution through the fill
conduit, thence through the patient conduit to the pelilolleal cavity of the patient;
5 b) a dwell stage during which the dialysis solution remains in the peritoneal cavity
of the patient; and
c) a drain stage during which the dialysis solution flows from the ~Klilolleal cavity
of the patient through the patient conduit, thence through the drain conduit to the
container for receiving spent dialysis solution;
the central processor means including a real time clock allowing the central
processor means to track the time taken to fill, the dwell time and the drain time.
Further, this invention provides a process for peritoneal dialysis utilising an
appal~lus complisillg:
a support frame,
a weighing means secured to the support frame,
first support means operatively associated with the weighing means for
suspending from the weighing means a container of fresh dialysis solution,
second support means for sl-cpen~ing a container for receiving spent dialysis
solution,
a first, a second and a third conduit each having a distal end and a proximal
end,
said first conduit being a fill conduit adapted for connection at its distal endto the container of fresh dialysis solution,
said second conduit being a drain conduit adapted for connection at its distal
end to the container for receiving spent dialysis solution,
said third conduit being a patient conduit adapted for connection at its distal
end to a patient catheter through which dialysis solution can both enter and leave the
peritoneal cavity of the patient,
junction means where the proximal ends of the first, second and third
conduits are connrcte-l together and are in co,,,,,,~ ir~tion with each other,
CA 02223846 1997-12-0~
clamp means for selectively permitting and halting fluid flow through each of
the first, second and third conduits, and
central processor means operatively associated with the weighing means and
being adapted to receive from the weighing means a signal le~lesen~ g the weight5 being supported at any given time by the weighing means, the central processormeans having a real time clock and also having data input/output capability by
which it can be progr~mm~;
the said process comprising the steps:
passing a signal from the weighing means to the central processor means,
10 said signal represe"~ g the weight being supported at any given time by the
weighing means, and, in sequence,
closing the drain conduit and opening the other two conduits to allow dialysis
solution to pass from the container of fresh dialysis solution through the fill conduit,
thence through the patient conduit to the peritoneal cavity of the patient;
closing the patient conduit and allowing the dialysis solution to remain in the
patient's peritoneal cavity for a predel~ led dwell period; and
closing the fill conduit and opening the other two conduits to allow spent
dialysis solution to pass from the pe~ilolleal cavity of the patient through the patient
conduit, thence through the drain conduit to the container for receiving spent
20 dialysis solution.
GENERAL DESCRIPI ION OF THE DRAWINGS
Several embodiments of this invention are illustrated in the accompanying
drawings, in which like numerals denote like parts throughout the several views,25 and in which:
Figures 1 (a) and (b) represent the fill stage and the drain stage utilized in the
prior art;
Figure 2 shows the main components in a complete CAPD appalalus,
adapted to carry out the prior art stages illustrated in Figure 1;
Figure 3 is a sc~ ic view of one embodiment of this invention;
CA 02223846 1997-12-0~
Figure 4 is a schematic view of a further embodiment of this invention;
Figure 5 is a front view of a pro~ ."",i~g and display panel used in this
invention;
Figure 6 shows the components of a quick conllect cap which can be utilized
5 with this invention;
Figure 7 shows the use of the cover and cap illustrated in Figure 6; and
Figure 8 is a sc-h~mAtic view of a further version of this invention, similar tothat shown in Figure 4.
10 DETAILED DESCRIPTION OF THE DRAWINGS
In Figure l(a), ~se.llhlg the prior art, a container 10 of fresh dialysis
solution is suspended at a level above the peritoneal cavity of the patient 12, and a
conduit or line 14 allows the dialysate to flow by gravity into the peliloll~al cavity.
For a typical patient and a standard bag of dialysis solution, the fill stage requires an
15 average of about ten mimltes.
When the fill stage is completed, the empty bag 10 may either be
disconn~cted or simply rolled up and carried around under the clothing. After
filling, the dwell stage is initiAt~d This takes typically from four to six hours, after
which the patient is ready to drain the spent dialysis solution either into the empty
20 bag from the previous fill or into a new bag. In Figure l(b) the bag 16 collects the
spent dialysate from the peritoneal cavity of the patient, and it will be noted that the
bag 16 is located below the level of the peritoneal cavity of the patient. When the
drain stage is completed, the patient is ready to begin a new cycle. Typically, an IV
pole is often used to suspend the bags at suitable heights. This technique of
25 treatment is often referred to as single line or straight line CAPD.
CAPD systems have evolved during the past two decades into two main
types: standard ~y~Lellls and disconnect systems. The former implies the need tocarry an empty bag and tubing during dialysate dwell, while the latter techniquefully disconnects the spent bag and the tubing sets. Numerous comleclors and
30 transfer sets have been designed to simplify the procedure and reduce the rate of
cont~min~tion.
CA 02223846 1997-12-0~
While the straight line system provides the most economical form of CAPD
tre~tm~nt it also has many drawbacks. One of the most common problems is
cont~min~tion, which often results in peritonitis or infl~mm~tion of the pe.iLoll~u
If the patient, during spiking of the new solution bag, inadvellelllly touches the
5 comle.;lion site, the bacteria will flow straight into the pelilol~al cavity.
A number of dirÇ~lelll CAPD sets are preselllly in use which address the
problem of cont~min~tion. These include the Y connection type lla,lsrer sets andvariations of these including double bag ~y~l~llls wherein the solution bags andtransfer set are all m~m-f~ctllred as a single unit.
Figure 2 shows a typical Y tubing set 17 co~ Pct~d to the patient's cath~oter
40 via a llal~rel set 28. The transfer set 28, which is also known as an adapter, is a
short piece of tubing with a manual clamp 46 and suitable mating connectors at both
ends to provide properly sealed connections to the ~ l... adapter 48 of the
catheter 40 and to the connector 49 of the patient line 38 of the Y set 17. The Y set
coll,l,lises a Y junction 42 from which three separate tubes branch out. The first
tube 30, called the fill line, is conn~cted to a fresh sterile solution bag 10a at the
time of performing the exchange. The second tube 34, called the drain line, comes
attached to an empty sterile drain bag 16a. The third tube 38, called the patient
line, connects to one end of the llansr~,l set 28 as shown. Each of these three lines
has a manual clamp (explained more fully below) for controlling the flow of solution
through that path. There are available other variations of this standard configuration
which are used to save cost. For example the drain bag need not be ~tt~rh~d as part
of the sterile Y set. ~n~tead, the empty solution bag from the previous exchange can
be saved to be used as the drain bag for the next exchange, as described in U.S.A.
patent 5,053,003, October 1, 1991, Joseph E. Dadson et al.
To explain the functioning of the Y set, let us assume that the patient has
been dwelling. Once the dwell period expires, the patient takes a new Y set 17,
connects the fill line 30 to a fresh sterile solution bag 10a, and connects the
connector end 49 of the patient line 38 to the transfer set 28. At this point the three
clamps of the Y set 17 and the clamp 46 of the llall~rer set 28 are all closed. The
patient begins by ope~ g the transfer set clamp 46, the patient line clamp 47 of the
CA 02223846 1997-12-0~
Y set 17, and the drain line clamp 48 of the Y set 17. This allows the patient to
drain, i.e. the dialysis fluid and the toxic wastes flow via the catheter 40, the
tla~r~,l set 28, the patient line 38 and via the drain line 34 to the drain bag. When
the drain phase is completed, the patient closes the patient line clamp 47 and then
5 opens the fill line clamp 45 for about 10 seconds. This permits fluid to flow from
the fresh solution bag lOa through the fill line 30, through the Y junction 42, and
through the drain line 34 into the drain bag 16a. Lastly, the patient closes the drain
line clamp 48 and opens the patient line clamp 47. This allows the fresh solution to
flow from the solution bag into the peritoneal cavity via the fill line 30, the Y
10 junction 42, the patient line 38, the ll~ rel set 28 and the catheter 40. When the
fill is completed, the patient clamps the llal~rel set clamp 46. The patient then
disconnects the Y set 17 from the tl~l~r~r set 28 and discards it. At the same time
the patient caps off the open end of the lldl~Çer set with a suitable connector.This type of Y set treatment is called "Drain Before Fill~ and also "Flush
15 Before Fill". As can be seen, this technique is definitely superior to the straight line
technique in that a drain phase right after cormection to a new Y set 17 flushes out
any bacteria that might be present ~dj~cent to the transfer set connection site and
also in the catheter or the transfer set. In addition, the flushing of the fill line before
filling the solution into the peritoneal cavity might flush out any bacteria acquired
20 while connPcting the fill line to the new solution bag. The resuits obtained with the
use of Y set 17 are definitely far superior to the straight line technique and have
been proven to be so by experts all over the world. The Y set technique thelefole
has become the industry standard although slight variations of it are prevalent
depending upon the economic cir~ msldnces in the individual situations. Recently,
25 the concept of the double bag system, although more expensive, is being promoted,
wherein the sterile solution bag is preattached to the Y set and the whole set comes
sterile in one package. This elimin~tes the need to connect the fill line 30 into the
new sterile solution bag lOa.
As il~il"~ed previously, the cost of the treatment is a major obstacle
30 preventing rapid growth of the simpler CAPD. In CAPD the infusion volume is not
controlled and the entire contents of the dialysate bag are emptied into the
CA 02223846 1997-12-0~
peritoneum. Since most current CAPD sets are disconnect "Y" type and because
the patient normally needs a Ill;nil"",~ of from 2L exchanges per day, four "Y"
sets with four solution bags or al~e~ iv~ly four double bag systems are needed per
day. This number increases as the peliLoll~al cavity loses its efficiency and more
S exch~nges per day are required. The problem becomes more severe when CAPD is
done with children. Pediatric CAPD in many countries is very e~ensive because
solution bags with smaller volumes are more costly (per unit of solution) than
normal 2L bags. In addition, these special pediatric size bags are ~iffi~'l-lt to acquire
and llecessi~te keeping stock of different sizes of bags. Moreover when extremely
10 low fill volumes other than the standard pediatric bag sizes are required, special
measulillg devices like bu~les are introduced in the tubing sets making the tubing
sets extremely e~ensive and cumbersome.
Another problem with the current CAPD treatment is patient compliance.
The role that compliance plays in ~etçrmining the a~qll~ry of dialysis dosage will
15 be described later in more detail. As seen above, CAPD is done 4-6 times a day
and seven days a week. This causes a lot of mental, psychological, and physical
fatigue for the patients. Patients have to remember the exact sequence of opening
and closing the clamps every time they perform the exchange and this can be veryconfusing for some of the patients. All this fatigue eventually leads to non-
20 compliance whereby patients skip exchanges and also do not collfolm to all thesterility guidelines. The new CAPD system addresses the problems of (1) ch~c~ing
the compliance itself, and (2) reducing patient fatigue by initi~ting the CAPD steps
at the touch of a button.
Despite the increasing use of continuous ambulatory peritoneal dialysis
25 (CAPD), the ability of this form of dialysis to provide adequate treatment over
longer periods of time remains a cause of some concern. A number of patients
receiving CAPD will transfer to other forms of dialysis, particularly hemodialysis
due to the inability of CAPD to provide adequate dialysis in terms of biochemical
control or fluid removal. One of the major issues cul~elllly facing nephrologists
30 caring for patients receiving long term peritoneal dialysis (PD) is the adequacy of
the treatm~nt Dialysis adequacy has a major impact on morbidity and mortality.
CA 02223846 1997-12-0~
Additionally if patients are to receive adequate dialysis, whether the target Kt/V is
1.7, 2.0, or higher, the patient needs to pelro,lll all prescribed exchanges. This
leads to another issue of patient compliance. Compliance indicates the extent towhich the patient has carried out the task as ~ign~d
Although l~easulelllc~ of compliance with prescribed Llleld~y is
strai~,hlrc,lvvdld in hemodialysis, no such method exists for continuous ambulatory
peritoneal dialysis (CAPD). An indirect method based on clca~ kin~ti~s for
measuring compliance in CAPD patients has been described in the literature but
subsequent reports have failed to validate this method. There is only one report of
10 compliance in CAPD patients in the li~erdLure. This was ~sesse~ by inventory
checks of supplies in patients' homes over a 4-9 week period. This study revealed
noncompliance in 57% of U.S.A. CAPD patients, with these patients pelrollllillg a
mean of 73 % of prescribed exchanges. This degree of noncompliance, if verified
and m~int~in~ in the longer term, would have a major deleterious effect on
15 OU~COIIR; many patients would be ,eceiving inadequate dialysis.
Nol~colllpliance of treatment regimens in patients on dialysis may have
adverse and possibly fatal consequences. A review of compliance liL~,ldLule in end
stage renal disease (ESRD) patients in-lir~es that at least 80% of patients have some
form of noncompliance. Since peritoneal dialysis is performed at home, at issue is
20 whether or not the patient actually does all of the prescribed exchanges. Results of
the CANUSA study demonstrated the association of decreased survival with lower
Kt/V and decreased cre~tinin~ clearance. If less than the prescribed dialysis isactually delivered to a patient due to failure to pe,roll" all exchanges, then survival
could clearly be affected.
If patients are to receive adequate dialysis, whether the target Kt/V is 1.7,20,or higher, the patient needs to perform all prescribed exchanges. To illustrate the
effect of nonco,llpliance on an individual patient, one of the noncompliant patients
had a weekly Kt/V of 2.09 based on a 24-hour dialysate and urine collection. Thehome visit supply inventory revealed the patient was performing only 74% of the
30 prescribed exchanges, thus making the Kt/V of the actual dialysis delivered only
1.55.
CA 02223846 1997-12-0~
It is clear from this study that the measured-to-predicted cre~tinin~- ratio will
not predict compliance with prescribed exc~ nges. Patient compliance with
prescribed elr~h~nges in PD can be ~l~le~ d to some accuracy with supply
hlvenlolies. However, this approach of d~l~ ...in;.~ compliance would be very
S costly, requiring periodic home visits and illv~ oly ch~c~ing by hlo~lthrare
personnel, and would still not verify whether patient underwent specified Dwell
time and other pl~sclil~lion parameters which play a major role in adequacy. T_is
new invention solves the problem of çhPc~ing patient compliance by storing all the
tre~trn~t data in a semiconductor memory or PCMCIA card-type memory which
10 can be verified by the h~lthr~re personnel at any time anywhere.
Bioincompatibility of the ~;ullellLly used dialysis solutions has been suggestedas a reason for the changes of the pe.iloh~uln during long term pe~iloll~al dialysis
(PD). Studies suggest that daily exposure to cullell~ly used glucose-based PD
solutions may result in functional changes in the p~ olleulll. The decreased fluid
15 removal, due to increased fluid reabsorption, res~lting in decreased cleaMnces for
small solutes as well as increased glucose absorption, represents changes which, in
clinical use, may prevent the peritoneum from with~t~n~ling long-term PD use. The
results in(lic~te that the functional studies of the peritoneal transport characteristics
after daily infusion of dialysis fluid may be a useful model to assess the
20 biocompatibility of PD solutions. Continuous measurement and monitoring of the
dialysate flow rates and ultrafiltrations of individual exchanges will help to
determine the effect of long dwells on the functioning of the peritoneum.
Early detection of poor ultrafiltration ~ymptollls may perhaps warn the
physician of a failing peritoneum and a res~llting change in prescription may allow
25 the patient to stay on PD longer. Presently, there is no mechanism for studying
such parameters on a continuously on-going basis.
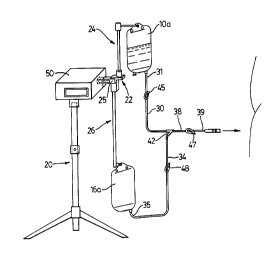
Referring now to Figure 3, there is illustrated one embodiment of an
apparatus for continuous ambulatory peritoneal dialysis, which comprises a support
frame shown generally at 20 and typically resembling an IV pole, a weighing means
30 shown generally at 22 secured to the support frame, a first support means 24 for
suspending from the weighing means 22 a container 10a of fresh dialysis solution,
CA 02223846 1997-12-0~
and a second support means 26 for suspending from the weighing means a container16a for receiving spent dialysis solution. Both the first and the second supportmeans 24 and 26 are in the form of vertical posts, which are mounted to a commonflange 25 forming part of the weighing means 22. A first conduit 30 is provided,5 having a distal end 31 adapted for connection to the container lOa of fresh dialysis
solution. A second conduit 34 has a distal end 35 adapted for connection to the
container 16a for receiving spent dialysis solution. A third conduit 38 is a patient
conduit having a distal end 39 adapted to connect with a tl~l~r~r set 28, in turn
co~n~-le~ to the patient catheter 40.
Each conduit has, opposite its distal end, a proximal end, the proximal ends
of the conduits being connPct~ together at a Yjunction 42. All three conduits are
in co"~ tion with each other at the Yjunction 42.
In the embodiment shown in Figure 3, each conduit 30, 34 and 38 has a
re~ecLive m~ml~lly operable clamp 45, 48 and 47 respectively. Each clamp is
capable of selectively pelllliuillg or halting fluid flow through its respective conduit.
A housing 50 is suspended at the top of the support 20 and contains a central
processor means operatively associated with the weighing means 22, and receivingfrom the weighing means a signal replese,l~ g the weight being supported at any
given time by the weighing means 22. The central processor means also has data
input/output capability by which it can be programmed to carry out a three-stagecycle consi~ g of:
a) a fill stage during which the drain conduit is closed, and during which dialysis
solution passes from the container of fresh dialysis solution through the fill
conduit, thence through the patient conduit to the peritoneal cavity of the patient;
b) a dwell stage during which the dialysis solution remains in the peritoneal cavity
of the patient; and
c) a drain stage during which the dialysis solution flows along the catheter from the
peritoneal cavity of the patient, thence through the transfer set 28, thence
through the patient line 38, thence through the drain line 34 to the container 16a
for receiving spent dialysis solution.
CA 02223846 1997-12-0~
The central processor means further includes a real time clock allowing the
central processor means to track the time taken to fill, the dwell time, and the drain
time.
It will also be noted that the weight tr~ncdl~cer 22 is suspended from and
supported by the housing 50.
The construction described with respect to the Figure 3 embodiment a~le
to m~int~in the simplicity and functionality of the conventional CAPD (manual).
However, the basic principle of the new system can be expanded to include other
functions and add more flexibility to the control system. For example, dirrelellt
10 kinds of tr~ncd~lcers, flow meters or other devices could be used to monitor the
amount of fluid infused or drained. Since the patient will never be filling and
draining at the same time, a single tr~nc~cer can be employed to weigh both the fill
volume and the drain volume.
The patient inputs the amount of fluid to be delivered by an electronic
15 means, for example by manipulating the "up" and "down" buttons 52 and 54,
shown to the right on the control panel 53 seen in Figure 5. A llulllelical display 56
registers the total amount as controlled by the buttons 52 and 54, and a "set" button
58 tells the central processor to use the value shown in the display at the time the set
button 58 is depressed. When the patient has input the applopiiate hlrollllation, he
20 opens the necess~ry clamps 45, 46 and 47 to allow the fill stage to begin. As the
patient fills, the amount infused or rem~ining to be infused is shown on the display
56. After a sufficient amount has been infused, the patient closes the fill line clamp
45 to prevent any more infusion of fluid. When the sufficient amount has been
delivered, the scale device could generate an electronic beep or a similar alert. The
25 patient than closes the clamp 47 to initiate the dwell stage. The electronic control
could then measure and display the passage of real time by showing either the true
clock time or the number of ",i"."es still to go for the dwell stage. The total time of
the dwell would have been previously input by the patient.
When the dwell stage is completed, the patient opens the clamps 46, 47 and
30 48 to allow the drain phase to occur. The amount of drain fluid is again measured
by the weighing means 22 and shown on the display 56. The patient then flushes
CA 02223846 1997-12-0
14
the line in the manner normally accomplished with the Y system. At this point, the
patient is ready to open the nPcess~ry clamps to allow the fill phase to begin again.
Ideally, during the fill and drain phases, the scale measures flow rates, total
fill volume, total drain volume, and ultrafiltration. Because the electronic control
S system has a real time clock, it can record all pal~lllete,s, such as the actual clock
time of the exchange, the time taken for infusion, the dwell time, and the drain time.
This allows clini~i~n~ to dete. ~ patient compliance and to study the transport
characteristics of the patient from exrll~nge to exchange. It becomes possible to
optimize the dialysis solution dosage for each patient, and to determine many other
10 criteria for presc,il~ion CAPD. The basic weighing m~cll~ni~m permits calculation
of many other treatment parameters as and when desired by reprog,i1.--,--i~-g the
control electronics in the scale. An optical system could be inserted into the drain
flow path to detect cloudiness of the fluid, thus permitting early detection of one of
the symptoms of peritonitis.
In another embodiment of this invention as shown in Figure 4, in addition to
the scale m~cll~ni~m, the Y set lines are routed through a clamping system on the
control unit which selectively ~ fll~nir~lly occludes one line at a time. This
permits the CAPD exchange to be carried out without any manual hllel ~elllion. The
principle of one form of cam-operated m~çll~nic~l occlusion can be derived from
20 U.S. patent 4,096,859, granted to us on June 27, 1978.
As already pointed out, Figure S shows a typical control panel for this
invention. Ideally, the "set" button in conjunction with the ~up" and ~down"
buttons allows the patient or the nurse-in-charge to enter a variety of l~,all"enl
variables, including the fill volume, the dwell and drain times, and the number of
25 exchanges. When the power is first turned on, the control system selects the
reference position of the occlusions cams, in which all Y set lines are closed. This
is the start position. Pressing the "start" button then initi~tes the treatment process
whereby the drain, the flush, and the fill phases of the process are autom~ti~ally
performed. The scale keeps track of the flow rates at all times. During fill, the
30 control system autom~tic~lly clamps off the fill line when the required amount of
fluid has been delivered to the patient. During drain, the control system clamps off
CA 02223846 1997-12-0~
the patient line when the drain time has elapsed and the preselected Illilli,,,,,-,~ drain
volume limit is exceeded. If suitable conditions are not met, an alarm will be
generated. Moreover, in CAPD, the patient is not asleep but rather is fully aware of
the operation taking place. Therefore safety is not an issue.
S Storage of tre~tmPnt pa~ L~,L~ and variables is provided via electronic
means such as PCMCIA memory cards or some other devices. These portable
devices can be removed and taken to the hospital for e~r~min~tion by the physicians
at regular intervals. With mini~tllrization in electronics, the whole control system
can be assembled into a very small portable unit which is completely battery
10 powered. The illustration in Figure 5 is only one example of many possible
representations. The ~setn, up", and "down" buttons could be elimin~ted by
pe~ al~llLly storing the ll~aLIIRllt variables into the memory card so that the control
panel is simplified and also the variables cannot be changed by the patient.
Currently, as ~iccllsse~ above, CAPD cost is a very important issue. As
15 more exchanges are needed to improve adequacy, the cost of the tre~tm.ont is
signifir~ntly increased. A 2L infusion is considered a norm for most adults, butrecently a 2.5L infusion is being suggested for improving the adequacy. This
becomes a very heavy burden to the hPalthr~re system of the country, especially in
the developing world where patients do not have enough money to receive even the20 basic minimllm 4 exchanges a day and thus cannot receive adequate dialysis.
Recently, in APD (automated peliLolleal dialysis), the use of 5L solution
bags is being advocated to help reduce the cost of dialysis because 5L solution bags
are proportionately much cheaper in cost than the 2L or 3L bags. The CAPD
system disclosed herein makes it possible to use the cost-saving 5L bags for CAPD.
25 A simply "Y" system in conjuncLion with a 5L bag will provide two 2.5L
exchanges. At the end of the first exchange, the patient will disconnect from the
system by using a unique quick connect cap 60 (U.S. patent 4,983,161, issued on
January 8, 1991, to Joseph E. Dadson, et al) which is prefilled with disinfectant (see
Fig. 6). The patient uses one half 62 of the quick connect cap 60 to cover the
30 transfer set connector 66, and uses the other half 64 of the quick connect cap 60 to
cover the patient connector 68 of the Y set. The quick connect cap and its
CA 02223846 1997-12-0~
16
application are shown in Figures 6 and 7 respectively. To start a new exchange the
patient will simply remove the covers from the transfer set connector 66 and thepatient connector 68 of the Y set and rejoin them. Since the two ends were covered
with comleclors filled with disinfectant, they will remain sterile.
Along the same principle, double bag ~y~lcllls can be m~n--fartllred with a
SL pre~tt~rh~ solution bag instead of convclllional 2L bags. The use of the present
invention in conjul~lion with the quick connect cap 60 will permit two 2.5L
exchanges from one double bag system and will significantly reduce the cost per
exchange. Also possible is the m~m-f~rtl-re of double Y/double bag systems or the
10 use of one Y set 70 in conjull;lion with two 5L solution bags 72 which comprise one
Y set with another Y in the fill line to connect to two solution bags (Figure 8).
With this variation the patient can even receive up to four 2.5L exchanges from one
system. This will further reduce the cost of the lledllllcnt and make it affordable for
patients around the world.
lS In dialyzing children, normally much smaller amounts of fluid are infused.
Solution bags of 250 ml, 500 ml, and lL sizes re quite common. In many cases
these special bags are relatively expensive and their availability and the keeping of
inventory becomes a problem in many places and many countries. The use of the
present CAPD system will elimin~te the requirement of special pediatric bags. For
20 example, one 2L double bag system will permit four 500 ml exchanges, or one SL
double bag system will permit five lL exchanges which will be sufficient for most
children. This will result in tremendous savings to the h~lthr~re system. DirÇclc
combinations of solution bags can be utilized to get the most Optllllum dialysis and
cost savings.
It is clearly seen from the above description that the addition of the scale
device and the occlusion mechanism tremendously improves the flexibility and
simplicity of CAPD treatment and opens a large number of new options. Some of
the advantages of the new device are:
1) Precise delivery and monitoring of the fluids in all phases of the CAPD
exchange;
CA 02223846 1997-12-0~
2) Complete automation of all CAPD steps by the touch of a single button;
3) For children, there will be no need to use special pediatric bags. With this
device, it will be possible to use standard 2L or 3L bags and do 4 or S
exch~ng,os with a single bag.
5 4) It will be possible to do CAPD with SL bags or with any other size bags. The
cost of h~lth~re is a very critical issue these days and it is especially so in
development countries. By reducing the cost of treatment or of each exchange,
they will be able to perform adequate ~Y~h~n~es and get sufficient dialysis. 5L
commercial bags are cheaper than the equivalent amount of solution in 2L bags.
One "Y~ set in conjul~lion with one SL bag and one quick connect cap will
provide 2 CAPD exchallges, or a double "Y~ system will provide all 4
exchanges. Twin bags or ultra bags or double bag systems can in future be built
in 5L solution bags.
S) The device will store all the ~reall~Rn~ parameters to determine patient
l S compliance and decide whether the patient is getting sufficient dialysis dosage.
6) The device will allow the clinicians to obtain exact patient ultrafiltration
characteristics from one exchange to another over an extended period of time.
This will aid them in determining the transport characteristics of the peritoneal
cavity, and in making many other observations which otherwise would not be
obtainable.
While several embodiments of this invention have been illustrated in the
attached drawings and described hereinabove, it will be evident to those skilled in
the art that changes and modifications may be made therein without depallhlg from
the essence of this invention, as set forth in the appended claims.