Note: Descriptions are shown in the official language in which they were submitted.
CA 02224551 1998-02-25
Specification:
This invention embodies a diagnostic method which detects disease antigens in
situ in the
alimentary tract, such as infection of the gastric mucosa by Helicobacter
pylori, using a
detection capsule restrained by a control string.
Helicobacter pylori can infect gastric mucosa and is implicated in diseases
such as gastritis
and gastric and duodenal ulcer. Once a diagnosis is made, the disease can
usually be cured
by using antibiotic regimens to eradicate the infection.
Current commercially available diagnosis are including invasive endoscopic
gastric biopsies
followed by urease test using urea and a pH indicator, microscopic examination
or bacterial
culture, which requires very skilled medical workers to perform, expensive
equipment and
very time consuming. Serological test measuring bacterial antigen-specific
antibody levels
is less invasive and a relatively routine clinical assay. But the presence of
the antibodies in
patients blood samples does not necessarily indicating there is a current
infection. Other
non-invasive method, such as '3C-labeled COZ breath test, in which radioactive
material is
administered, and special equipment is required to measure the results.
This invention embodies a in situ diagnostic method for alimentary diseases,
such as
Helicobacter infection. Compared to other commercially available method for
detecting
alimentary diseases, such as Helicobacter infection in the stomach, this
detection method is
very suitable for rapid in-office diagnosis and therapeutically monitoring
disease
conditions. It can also be used by patients themselves for at-home monitoring
of such
CA 02224551 1998-02-25
conditions with simple instructions. There are no radioactive or other
hazardous materials
used, no special equipment required, and the results can be easily
interpreted. That is in
addition to the rapid assay format (the whole process can be done within 20
min) and as a
result of all these, the diagnostic test embodied by this invention is very
cost effective.
f 1
CA 02224551 1998-02-25
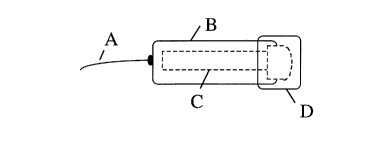
The whole assembly embodies the detection capsule is the size of a
pharmaceutical capsule.
In drawings which illustrate embodiments of the invention, Figure 1 is a plan
view of the
embodiment, Figure 2 is a top view of the Part B and C of the embodiment,
Figure 3 is a
plan view of Part C, and Figure 4 is a section of Part C.
Part A in Figure 1, is the control string which is used to hold and control
the destination of
the detection capsule. The destination of the detection capsule is simply
controlled by the
length of the string. Color paint or other markers can be used to mark the
length of the
string. A chart is provided as the reference of individuals body height
compared to the
physiological location of the specific alimentary organs, such as the stomach.
Part B in Figures 1, is attached by Part A. Part B embodies a plastic material
non-digestible
under physiological conditions of the alimentary tract. Part B' can be totally
transparent or
with a transparent window for monitoring the detection results.
Part C is inside Part B and D and held by Part B at the filter unit, C6 as
shown in Figures 1
and 2. Part C is a mini-detection device, which embodies a nitrocellulose
membrane strip,
C2 as shown in Figures 3 and 4, sitting on top of a plastic support, C7 in
Figure 4. A filter
paper C 1 is sitting on the left side of the plastic support as shown in
Figures 3 and 4. Two
or more different antibodies or other ligands can be immobilized on the
nitrocellulose
membrane strip forming invisible ligand bands and with certain distance in
between each
band. One of the ligand bands, C3 as shown in Figure 3, is used as the
positive control
band. The positive control band is composed of antibodies against the capture
ligands
conjugated with a colloidal dye. One or more additional different ligand bands
can be used
as detection ligand bands, C4, as shown in Figure 3, which is used to detect
disease
antigens (such as catalase and urease of Helicobacter) carried by the capture
ligands
conjugated with a colloidal dye. The capture ligands conjugate is pre-absorbed
in excess
into a small filter paper, CS as shown in Figure 3, that is attached to the
opposite end of the
positive control band with detection bands in between. The filter unit, C6 as
shown in
Figures 3 and 4, is buffered to neutralized the extreme conditions of the
alimentary fluid,
and with certain pore size to prevent macro-molecular and debris from entry
into the
detection device.
Part D as shown in Figure l, is the capsule cap that embodies a special
membrane for
coating the filter unit of Part C. Part D is sealed tightly with the outside
of Part B to prevent
premature contact with fluid before the detection capsule reaches the destined
location.
Upon reaching the specific location of the alimentary tract, such as the
stomach, the
membrane can be solublized because of the specific physiological condition of
that location,
CA 02224551 1998-02-25
such as low pH and the presence of pepsin in the stomach. This allows the
filter unit of
Part C to have direct contact with the alimentary fluid.
To perform a test, the detection capsule is ingested by patients with the aid
of drinking
water, while one end of the control string is being held. The detection
capsule restrained
by the control string will then destined to certain locations of the
alimentary tract, such as
the stomach. Upon reaching such location of the alimentary tract, such as the
stomach, the
detection capsule restrained by the control string stays, and one end of the
capsule coated
by special membrane becomes soluble because of the physiological conditions of
the
specific alimentary location, such as low pH and the presence of pepsin in the
stomach.
This allows the filter unit to have direct contact with the alimentary fluid,
such as gastric
juice. The alimentary fluid, such as gastric juice, filtered and buffered by
the filter unit, is
then moving towards the detection strip driven by capillary force.
The fluid first encounters the capture ligands conjugated with a colloidal
dye, such as
colloidal gold particles. Antigens present in the fluid sample bind with the
capture ligand
conjugate and travel together with free capture conjugate towards detection
ligand bands.
Antigens carned by the capture conjugate that are recognized by the detection
ligand bands
pre-immobilized on the detection strip form the colloidal dye-specific color
bands, such as
red bands if colloidal gold particles are used for the capture conjugate. The
unbound
capture conjugate continues to move towards the positive control band and
forms a colored-
band upon binding with the positive control ligands pre-immobilized on the
detection strip.
The maximal time course required for the detection is 20 min. Upon finishing
the test, the
detection capsule is then pulled out and testing results is inspected through
the transparent
Part A of the detection capsule.
The results can be easily interpreted by a layman with simple instructions. If
the disease
antigens, such as antigens from Helicobacter infection, are not present in the
patient's
alimentary fluid, i.e. gastric juice, only the positive control band is
visible. Because free
capture conjugate does not bind with the detection ligands by itself. But if
the disease
antigen is present in the in situ alimentary fluid sample, both the positive
control and
detection bands will be visible.
This invention can be used for detecting diseases, such as bacteria, viruses,
fungi and
parasites infection, as well as cancers, in the upper alimentary tract and its
peripheral
organs, including the mouth, esophagus, stomach, duodenum, gallbladder, bile
duct and
pancrease. In particular, this invention can be used for testing Helicobacter
pylori infection
in the stomach.