Note: Descriptions are shown in the official language in which they were submitted.
CA 02224715 1997-12-16
W O 97/02285 PCTAEP96/029S2
MACROLIDES
The present invention relates to a novel class of macrolides having valuable
ph7...... ~rA~ and r_lated activity. For con-_nience colllpou"ds of this novel
macrolide class are refi,.l~d to herein collectively as "S~nelifrPhrin~".
The first of the Sangli~s were icol~cl from actin--mycete fe ".~ .l t;or
broths. These are the Sanglifehrins A through D of forrn~ P-
~,~ . r".A~ 0
Me ~ 3~,0 OH ~O ~ ,o, ~ ~12 ~
4 ~~ 6,l1 ~ N ~ s5Me56
Me3 5 58 ~ 3Me57
44Sanglifehrin A 61 62
OH
OH OH Me
~0
Me ~ O OH I ~ R ~ I
O~ ~ Me O ~ N ~ Me
Me Sanglifehrin B
OH
CA 02224715 1997-12-16
wo s7/02285 r~lih~s/02ss2
Me-o~Me
OH o
HO ~
Me ~ ~O OH 1O Y 1~l ~ N O
Me O ~ N ~ Me
Sanglifehrin C
OH
Me-o ~Me
OH O
Me Me Me ~
Me ~ ~ o MeN ~ O
~N ~ O ~ N
Me
Sangllfehr1n D
OH
As can be seen the macrocyclic ring of S~nglirf k. ;,~c A to D is of entirely novel
structure cha~ tc ;~d in that i) positions 1 to 6 co,ll~,ise a 3~1~Ayl,i~,;cl~7inyl
carboxylic acid residue, ii) positions 7 to 9 an aromatic a-arnino acid residue, and iii)
positions 10 to 12 an ~lirh~tic a-arnino acid residue. The rPn-~in~Pr of the macrocyclic
ring is comrricPd by an hydroxy carboxylic acid residue, providing, in the case of
~S~r~gl;f~h-;nc A to D a further 11 carbon atoms in the pl Ullaly Illa~ ;y~;liC ring.
In âccoldance with conventional ylacL;ce in macrolide cl. ..;cl,y the atorns of
the Sanglifehrin primany mac .c~yclic ring are llum~,~d as in~lir~- ~ above for
Sanglifehrin A, st~ting with the carbon atom of the c~l~nyl group of the macrocylic
lactone linkage as position 1.
CA 02224715 1997-12-16
WO g7/02285~ ~,1i~35/029S2
Sanglifi k~ C A to D are also cha~1 by the prescnce of a novel bicyclic
spiro system attached at the 23 poCitinn of the ~ ic ring via a hydocarbyl linker
group.
s ~ ;r~ h~ c A to D may be ~ Ld to e.~t~ _ ch- .,,;~1 marir~ inn to
obtain yet further macrolides of the Sangl;r~k~ dass. Such manirll~tionc includeclea~..g_ of the --r ~clic ring, in particular at the lactone oxy-group, cleavage of the
linker group between the macrolide and spiro ring ~ ms, and manipulation, e.g.
protection~ derivatisation or other çl f ~ ;rAl ~l.odirlcation of ;"~ ih. -t groupings; for
exarnple, as h~ ,hl~fte, ~çs~ ;kA Furthcr means of .~,~;r,r ~ion will be apparent to
those skilled in the art.
In acco..l~ce with the i~ lion it has been found that San~;fih.;..c, in
particular those in which the spiro ring system is present, as in the case of S~n~);r k. ;.~c
A to D, have a chl~ .;cl;r and entirely novel profile in terrns of their biological
activity. In particular they have been found to exhibit the following c~....hi~ ~ion of
activihes:
- cyclophilin binding activity;
- immllno~up~l~,ssi./e activity;
- inhibition of both B-cell and T-cell proliferation
- they do not, ho~ _l, have FK binding protein binding activity or c~k ;.. ;i~
inhibiting activity.
The Sanglifehrins can accol lh~gly be seen as providing an çYriti~ and novel
class of ;~ -osu~.lJ~ssa~ll and ~ntiinn~.. -~ol~ cc-.~ lc In particular the
I;r k.;i-c have an achivity profile that differs from that of pl~io~sly known
osu~ ssant and A.~ti;l.fl;..lllll /t~y co.-.l o~ 1s such as ~ lo~..;,-c and
macrolides, e.g. of the raparnycin and FK 506 class, indicating that the S~gl;r~h.;~c
have a dirr~ ;"t mode of achon than such previous colllpoullds. Thus the Sangl;f~k- ;I~C
provide a novel category of drug ~ s~ r~ both in terms of s~ Ule, and activity which
may be ~ntiripA-~d to m~teriAlly extend the bounds of ;-.. ~ ~o~ lessi-_ and/or
~ntiinfl~ ol~ therapy; for example, to avoid or reduce undesirable side effects of
CA 02224715 1997-12-16
W O g7/02285 r~l/hl~5102gS2
pl.,~iOU~ no~ ,l~c~ivc and nn~ .na-.. ~O~ therapies and/or to illl~)lU~_ or
extend such therapy to new disease areas or new patient c 'Pg~ 5
s~ngl;r h. ;i~c, e.g. in which the .llacl~lide ring is in ring-opened form, in which
the 26 and 27 ~ nc in the hydrocarbyl linkcr l~t~.~n the lll&lvLdlc and spiro ring
systems are both hyd~v"y ~ub~ cd, or in which the spiro residue ~tt~~hP~ to the
lll&lu~;yclic ring has been cleaved or tmncated, gPnPrally lack some or all of the
combination of San~;r~-h~ ;n cha~ I ;r ;~LviL~s. For example, San~,1ir h. ;nc inwhich the spiro residue is cleaved typically posscss cy~lophilin binding activity but do
not possess significant ;.. - ~ .o~ activity. As will be apparent to those skilled
in the art, ho~ r, such co...~ ...Ac provide valuable co...l~lu--.t~ e-...~Ai~tes or key
building blocks for the ple~&alion of further novel S~ngl;r~ k~ c~ and hence further
extend the the.~ ic potential of the Sangl;r h- ;-- class.
In that its pl.,sel.ce appears material to the biological activity, e.g. of the
~nglifehrinc A to D, the bicyclic spiro system too may be viewed as providing a
Sll~ lUlalC(:IlllpOne-~l with key biological ci~ifit~nce, useful as a sl,uclul~l co...pQn~n~
for further derivatisation or moAifi~ ion both in relation to the prod~cti~ n of further
Sanglifehrins or for applicdlion in the de.i~ n or ~ ;f.~AI;on of other drugsub~lances; for eY~mp'e to modify the activity of other ;.. ~.o~uppl~ssi~e drug
s~ s of the macrolide class.
As intlic~tp~l~ the Sanglifehrins IG~l~sent a novel class of macrolide colll~unds
of entirely novel and wholly cl~ t~, ;cl ;r S~ lule.
Accordingly in a first aspect the in~e.ltio.l provides:
a macrolide in which
i) pocitionc 2 to 6 inclusive of the Illacl~n;y~lic ring are provided by a
pj~rid~7inyl call,o~ylic acid residue; and/or
ii) positions 7 to 9 inclusive of the Illa.,lu~;yclic ring are provided by an aromatic
a-amino acid residue; andlor
CA 02224715 1997-12-16
W 0 97/02285 rCTn~6/029S2
iii) positiQnc 10 to 12 inclusive of the ~lo-,y..lic ring are prwided by an
aliphatic a-amino acid residue,
in free or protected form, or a salt thereof.
Suitably the macrolides of the invention co~n~ two, çsreri~lly all three of
the characteristic structural features i), ii) and iii).
The pire~id~7inyl ca lwAylic acid residue is suitably a 1,2 pire~ 7in-3-
carboxy-l-yl residue of which the carboxy moiety oc r-'S the l-posi~ion~ and the 1-
nitrogen atom the 6-position of the ll~&lu~;yclic ring, e.g. a residue of formula I
O H
--C j~N--
3 s
wherein the assigned nu,..~ replesent the position of the atoms of the residue in the
macrocyclic ring. This residue may be ring ~ub~liluled or u~ Jbslil~lçd Suitably it is
unsubstituted.
The a-amino moiety of the aromatic a-amino cid residue suitably oc~up:es the
9-position of the .,lacl~yclic ring. Suitably the aromatic a-amino ~id is a
phenyl~l~nine, especially 3-OH-phenyl~l~nine. residue in free or ~1~ ctr,d form.
The a-amino moiety of the ~lirh~ir a-amino cid residue suitably occ.r:es the
12-position of the ...ac.~yclic ring. Suitably the aliphatic a-amino cid residue is a
valine residue in free or protected form.
The rem~inder of the .llaclu~;yclic ring suitably CG~ ;ces a hydroxy
carboxylic acid residue, the oxy-moiety of which comrlçtçs the l..a~f~;yclic lactone
linkage and the carbonyl moiety of which forms an amide linkage with the a-aminogroup at position 12 of of the l~l&l~yclic ring. The said hydroxy carboxylic acid
residue suitably has a chain length of from 6 to 20, more suitably 11 carbon atoms. It
CA 02224715 1997-12-16
WO 97/0228s PcTlEr96lo29S2
rnay be ~lb~ ed or ~ ub~ t.,d and/or contain one or more ulls~tulated link~s
in particular c~ml~ ive double bonds along its length. More suitably the lc ~.~inA~r of
the ll~,~yclic ring co...r.;ces a 1l-oxyç~d~."rl-ll-yl, espe~ lly 11-oxy-6,8-
entlecn~lienoyl-ll-yl, residue optionally ~ ed e.g. in the 2, 3, 4 and/or 5
position. More suitably the said hydoxy carboxylic acid residue is a residue of formula
II.
I 1 R2 O ~ CH2 ) 2
,CHCH2--CH=CH--CH=CH--CH--CI--CH CH--CO-
f CH3
II
wherem
R~ and R2 are both H or ~epr~sent an extra bond;
R3 is H;
R4 is -CO-CH3 or -CH(OH)-CH3 or
R3 and R4 together ,epresenL a structure of formula m
,CH3
--,C-- I II
OCH3
in free or protected form, or salt thereof.
Preferred macrolides in accordance with the invention are accor~ingly those
comprising a macrocylic ring of formula IV
A
IV
X Y Z
wherein X, Y and Z are residues i), ii) and iii) as defined above and A is a hydroxy
carboxylic acid residue as defined above in free or protected form, or salt thereof; in
particular a l.la.,,u~;~rclic ring of formula V
CA 02224715 1997-12-16
W O 97~2285 rCTn~K~29S2
CO 'A)
3 ~ N,H ~ g lo 11
4 N - CO fH - NH-CO - CH - NH
HO ~ CH2 ~ CH
in free or protected form, or salt thereof
Generally in the SqnE;IiLk~ , as in .Sqn~l;r h.;..c A to D, the ma~ .,lic ring
is substituted at the carbon atom ~dj~e--~ the oxy moiety of the lactone bridge.Typically this substit~lent co...~ cs a 2-oxy-2'-aza-3'-oxo-spi obicycloh~YP~-3-yl
residue, e.g. of the formula VI
Me
s bJ~--
6 a O2 VI
Me~N - R5
5' ~ O
Et
wherein
-a-b- is -(Me)C=CH- or -(Me)CH-CH(OH)- and
R5 is H or Me
(wL~,.c;n Me and Et re~.~,sent methyl and ethyl ~espee~ ,ly)
in free or protected form, or salt thereof linked to the macrolide ring via a linker
cGIIlpli~ing a linear scque~-ce of from 6 to 11, typically 9, carbon atoms b~h.~,e,ll the
spiro residue and the macrolide ring.
The linker group may be ~l~bs~ cd or ~ uL.sl;n~llod and/or contain one or
more unsalulated linkages in particular cl~m~ tive double bonds along its length.
Suitably the linker group may be methyl ~Lsl;lutud. e.g. by t~vo methyl groups.
Suitably the linker group may be further ~ub~l;lu~e~ by hydroxy, e.g. by 3 hydroxy
CA 02224715 1997-12-16
WOg~102285 Pcr/Ers6~02ss2
s~bs~ ,e~ andlor rnay be ethylenically unsaturated, e.g. contain two carbon-carbon
double bonds. More suitably the linker group c~ ..;ccs a l-methyl-7-methyl-
nonanoyl-9-yl, espe~ 1y a 1-methyl-7-methyl-1-none,..u~ l-9-yl or a 1-methyl-7-
methyl-1,3-nonadienoyl-9-yl, residue optionally su~ d~ e.g. in the 3, 4, and/or 8
pc cition E~cft,~ably the linker group is a group of formula VII
VII
1 6 ~7 Cl H3
c--CH2 CH(OH)--CH(CH3)--(CH2)2 CH--CH--CH C--d
wherem
c lep~senls linkage to the spiro residue;
d .~,pl~cse.lt~ linkage to the .~ xyclic ring and
R6 and R7 are each OH or togethcr lc~ ,sc~lt an ~ ~lition~l bond,
in free or p.ute.,lcd form.
The linker group will generally be attached to the rnacrocyclic ring at the
carbon atom immptli~tely ~ cent to the lactone oxy group, i.e. when the the
macrocyclic ring co,.lplises an ll-oxy-en~ec~nQyl-ll-yl residue, at the 11 position of
thereof.
Accordingly the invention provides colllpounds of forrnula VIII
S--L~l vm
wherein
Sl~lcs~"ltS a spiro bicyclo residue as previously defined
L l~lcSc~lt~i a linker group as previously defin~d and
M lclJlcse.l~ a macrolide ring as previously defin~d
in free or protected forrn, or salt thereof.
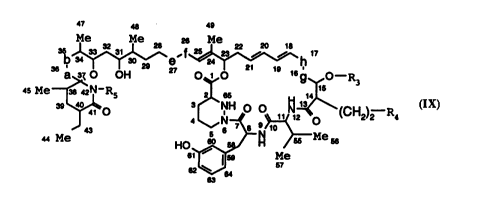
Particular colllpoullds of the invention are those of forrnula lX
CA 02224715 1997-12-16
WO g7/0228S rCTlErg6/02gS2
47 48 43
3b~27 '~h ~7
a~ o OH o~,o 169 o--R3
45 Me ~ 842N-R5 3 21 66~ 1~
4 ~N~ 2~o (CH2)2--R4
44 Me Ho~H /~ 66
62~ s7
wLe;elll
-a-b- is as defined above;
-e-f- is -CH(OH)-CH(OH)- or -CH=CH-;
-g-h- is as defined above for-a-b-, and
R3, R4 and R5 are as defined above,
in free or p,ot~:led forrn or salt thereof
The co~l.pounds of fonn~ I to IX contain ~.. ~t~;c carbon atoms and thus
may exist in a number of ep;...r-;~ forrns. All of the pcs~ C~ as well as
diastereoiso,..elic ~ lulcs thereof are enro-.-r~csed in the invention. However,comyounds of formulae VIII and lX in which the macrolide ring is in ring-closed form
and which are of app,~yliate ~ oc1-r .~ y typically possess ~livilies which are
characterisic of S~ng1ifio~rinc~ as hereinbefore referred to. ~illlCl~ which possess
sanglifehrin char~rtencti-~ activities are ylcfell~,d. In general, e.g. for p h~ ",~r~ ;r~1 use
in acco.dallce with the invention, e~i".cl~ which possess s~n~,]ir~h.;-- cha,~
activities in pure or ~ul~s~ l1y pure form (i.e. free or ~l,sl~ 1y free of epimers
which lack san~ li.h~ cha, -- tcl;cI;c activities), e.g. I~Ill~ g at least 90%, e.g. at
least 95% of active epimer (i.e. co..~ ;c;~g less than l0%, e.g. Iess than 5% of inactive
epimer) will be p~cr._l~cd.
Preferably the 3~hL ~~ 7inyl carboxylic acid residue i) at positions 1 to
6 of the macrocyclic ring has the following COl,r -- ~ Qn
CA 02224715 1997-12-16
W 0 97102285 r~ r./029S2
O H
--C _~N~N6
Preferably the aromatic amino acid ii) at po~i~ionc 7 to 9 of the ~I~;yclic
ring has the L configuration, e.g. is of confi~ration
O
~ NH
HO ~
~cf~,lably the ~lirh~ti- amino acid iii) at positions 10 to 12 of the ll~clocyclic
ring has the L configuration, e.g. is of configuration
~NH~
1 1 12
When the rçrn~in-ler of the lllac~yclic ring cQrnrri~es a residue of formula Il,
it plcfclably has the configuration
CH3 ~CH2)2
O OH ~O O
R3
or
CH3 SCH2)2
R3
When R3 and R4 together lcp~sent
CA 02224715 1997-12-16
W 0 97/02285 P~l/~r,~JOtgS2
,CH3
--,C--
OCH3
it pl~r~ bly has the configuration
CH3~ ,~ OCH3
~C
Preferably the 2-oxy-2'-aza-3'-oxo-s~ bic~clnh~Y~n-3-yl residue has the
configuration
Me
b
a~,~O
Mel~f N--R5
~~
wherein when -a-b- is -(Me)CH-CH(OH)-, it preferably has the configuration
HO~
CH3
When the linker is of formula VII, it is ~>lcfc.ably of confi~ration
CH3 R, CH3
c ~--~d
OH R6
When R6 and R~ are each OH, the c~nfig-~ration at positions 26 and 27 is
preferably either 26(S), 27(S) or 26(R), 27(R). When R6 and R7 tog~!h~r lep~ese.ll an
additional bond, the configuration at positions 26 and 27 is pl~,fc.~bly
CA 022247l5 l997-l2-l6
wo g7~0228~ r~ r,.~i/02952
27
Colllpounds of the invention of formula IX plcfe,àbly have the following
conformation
47 ~ 49
b~g O--R3
45 Me ~ 2N-R5 ~ 65 1 ~
44 M~O ~o~(cH2)2--R;
wherein when -a-b- is -(Me)CH-CH(OH)-, it preferably has the configuration:
HO~
CH3
when -e-f- is -CH(OH)-CH(OH)-, it preferably has the (S),(S) configuration or
the (R),(R) configuration;
when -g-h- is -(Me)CH-CH(OH)-, it preferably has the configuration:
~ OH
when -g-h- is -(Me)C=CH-, it plcfelably has the configuration:
\~H
,~
CH3
and when R3 and R4 are fused together they are plcfel~lbly of configuration
CA 02224715 1997-12-16
WO 97/0228~ PCT/EP96/02952
CH3~ ~" OCH3
~C
Preferably SangliÇ~,h"ns A to C have the following confi~l-rations
OH OH Me
Me Me Mle
Me ~
e O ~ N ~ ~ Me
Me
Sanglifehrin A
OH
OH OH Me
~=o
Me ~ ~o OH ~ ~ O ~ ~ o
~Me
Me Sanglifehrin B
OH
Me-o ~ Me
HO Me Me Me
~ } Me ~ ~
Me ~ OH ~ ~~
Sanglifehrin C OH
CA 02224715 1997-12-16
W 0 97/02285 PCT~EP96/02952
Me~O,,~Me
OH O ~
Me Me Me ~,I~,J
1~--~ o Me ~O
Me~ O OH I Y
Me O~N~Me
Sang 1 i f ehrin D
OH
The c~...po~ lc of the invention may be in free or protected forr4 e.g. in
protected forrns as desrribe~ in "~O~ Groups in Organic S~ thcsis" by T. W.
Greene and P. G. M. Wuts, 2nd FAitiQ~, 1991, John Wiley & Sons Inc., New York. In
particular OH groups may be in p.~,t~;t~ form, e.g. in the form of silyl ethers (for
inct~nr,e as desc~;be~l in pages 68-86 of Greene and Wuts ibid.), esters (see e.g. pages
87-103 of Greene and Wuts ibid) and ca~ t~,s (see e.g. pages 104 111 of Greene and
Wuts ibid). Such protected forms also include int~m~lly protected forms; for PY~ e
in the case of m~rolides of formula IX, wherein -g-h- is -CH(CH3)-CH(OH)-, the
protected fonn wherein the 14 to 17 positions of the I~ ,cyclic ring c~ .. ;ce a residue
of formula X:
CA 02224715 1997-12-16
WO g7~2285 rCT~Erg6~29S2
CH3
~ ~CH2)2
16 I X
- CH-CH-CH - CH -
17 1 1S 14
CH3
e.g. of configuration
O o
CH3 14
Also for example, 1,3 diols present in Sanglifehrins may be plute~led as
ap~l.)pliate ring structures, e.g. as desc~ibed on pages 118-142 of Greene and Wuts
ibid.
Compounds of the invention also exist in salt form. Examples of suitable
ph~...~re,JIically S~cept~hle salts for use in accordance with the invention include
acid and base addition salts as ap~,lo~liate having regard to the particular substihlen
present in the compound.
As previously int1ic~ the ll.acr~yclic ring of the col.l~oullds of the
invention can be cleaved, in particular at the lactone oxy group, to provide co --~ow-ds
wherein the ~ll~clo.;yclic ring is in ring-open form. Generally cleavage of the lactone
oxy group plvc~ds by hydrolysis (solvolysis), e.g. to provide co --pounds of formula
XI:
R6~X--Y--~A~H Xl
e.g. of forrnula XII
CA 02224715 1997-12-16
W 0 97~2285 ~ 5~2gS2
R60
CO XII
N~H
N - CO-ICH- NH - CO - 1 - NH - A OH
HO_~ CH2
l l CH3 CH3
e.g. a colll~uild of formula IX'
4~ 4~ 49
3b~ 26~,~h
36 a~~ OH 27 o OH21 19 169~~--R3
45 Me~3B 42N-R5 R 0~~ 65 14J~
39~ 6 3~,NH o o~ (CH2)2 R4
44 Me HO~/ H Me56
63
wherein X, Y, Z, A, R3, R4 and R5 are as defined above and R6 is H or C,~
alkyl, e.g. methyl.
Such ring-opened forms provide interm~ te means for m~lifi~-~ion of the
basic Sanglifehrin macrocyclic ring system and are also part of the present invention.
Accordingly in a futher aspect the present invention provides:
- a macrolide as hereinbefore defined in ring-opened form. said ring-opened
macrocycle being in free or protected forrn, or salt thereof;
- a compound R60 - X - Y - Z - A - OH as defined above, in free or protected
form, or salt thereof;
CA 022247l5 l997-l2-l6
WO 97/02285 r~ ,5~29S2
- a CG.~ ~ O - X - Y - Z- A' - CH(OH) - L - S wL~.~;n - A' - CH(OH) -
is a h~ Ay C~I~AY1iC acid residue, e.g. a residue of formula II, as defined above,
and the other symbols are as defined above, in free or protected form, or a salt thereof;
a compound of formula XII'
R60~
CO XII '
,NH
N CO--ICH--NH--CO--CH--NH--A '--C, H L S
HO_~ CH2
W CH3 CH3
in free or protected form, or salt thereof;
- a compound of formula IX"
47 48 49
b~27 ~h 17
4s Me~42N~Rs R O~~ 6s ~s
6 3~--NH o O ~ '~(CH2)2 R4
44 Me IX" ~HN Me56
63
in free or protected form or a salt thereof.
The invention also includes colllpoullds in which the 2-oxy-2'-aza-3'-oxo-
3'yl-spirobicyclo hexane ring system is in ring-opened form, e.g. a colnrol-n~l of
formula XII
CA 02224715 1997-12-16
WO 97/02285 PCT~EPg6/02gS2
Me
b~L--M
a o x
Me~><OH
O NH2
wherein a, b, L and M are as defined above, in free or protected form, or a saltthereof.
The ring-opened compounds of the invention are p~ bly of conformation as
the plefc.l~d conformations id~ntifi~d above for ring-closed ccill~ ndS. The ring-
opened spiro bicyclo ring system of co.,.pounds of formula ~1 is p ~,fe,d~ly of
conformation:
Me
b~L--M
a~O
- Me~ OH
O NH2
wherein when -a-b- is -(Me)CH-CH(OH)-, it pr~fcl~-bly has the configuration:
Ho~
CH3
Macrolides in acco..l~cc with the invention having a spiro bicyclo residue
.-hed to the macrocyclic ring may also be ~ubj~;lcd to cleavage of the inlc~ ing
18
CA 02224715 1997-12-16
WO 97102285 PCTlEP96/02gS2
linker group, e.g. in relation to foImula ~, in particular at the linkage ~t~ ,nresidues 26 and 27 to yield sep~tc novel spiro bicyclo col.l~ds and further
macrolides. As also previously in~lir~d these colll~oullds too are useful as
i~t~ f~ fS, the spiro bicyclic moiety of the S~ngl;r~ h,;.~c in particular having an
integral filnrtion~l role in the biological activity of the Sq~gliff h. ;.~c as a class.
Accordingly the present invention provides:
- A 2-oxy-2'-aza-3'-oxo-3'yl-spirobicyclohexane, in free or protected fo~n, or
a salt thereof, in particular a colllpo~llld of formula VI'
Me
b,~,R7
a o VI'
Me~N - R5
~0
Et
whelc;n R~. is H, an optionally protected OH group, a l~.;~ e functional
group, or a -CH2-CH(OH)-CH(CH3)-CH2-CH2-CHO group or the delta lactol
equivalent thereof, in frce or protected form or salt thereof.
Preferably the compound of formula VI' has the following configuration
Me
bJ~
a~.~O
Mel~ f N--R5
O
Et
wherein when -a-b- is -(Me)CH-CH(OH)-, it plcfe.ably has the configuration:
19
CA 02224715 1997-12-16
W 0 97/02285 rCT~W~/029S2
HO~
The invention also inrl~l"es a ring-open 2-oxy-2'-aza-3'-oxo-3'yl-
syilvbi~ oh~y~n~ in free or protected form, or a salt thereof, in particular a
cGlllyoLlnd of formula XII'
Me
b~R,
ay O XII
Me~ OH
O NH2
wherein a, b and R~ are as previously definç~1 in free or protected form, or a
salt thereof. The ring-opened spiro bicyclo ring system of colllpoullds of formula XII'
is preferably of conformation:
Me
b~R7
a~,O
Me~l~ OH
O NH2
wherein when -a-b- is -(Me)CH-CH(OH)-, it plcfelably has the configuration:
HO~
CH3
CA 02224715 1997-12-16
WO 97/02285 r~ /029S2
The invention also provides a _acrolide of formula xm
CH3
XIII H,CO~M
wherein M is a m~~oli~e ring as defined above, in particular a macrolide of
formual XIV
~ Me
H~h
~q~~ 9~0--R3
XIV ~NH o o N~(cH2)2 R4
Ho~JH~Me
, preferably of conformation
~ Me
H~h
~q~~ 9~ ~--R3
~NH o o NH--6)~ (CH2)2--R4
HoJ H~Me
wlle~e;n when -g-h- is -(Me)CH-CH(OH)-, it preferably has the configuration:
~ OH
CH3
and when -g-h- is -(Me)C=CH-, it preferably has the confi~ation:
21
CA 0222471S 1997-12-16
WO 97/02285 PCT/EP961029S2
~H
J~ .
CH3
and when R3 and R4 are fused together they are plef~,...bly of configllration
CH3~ ~ OCH3
~C
in free or protected or ring-opened form, or a salt thereof.
In a further aspect the invention in~ludes the macrolides and c~ll~ou.lds of theinvention, in particular those which are natural products in substq-nti-q-lly purified form,
e.g. at least 90%, preferably at least 95%, espe~iqlly at least 98% pure form.
In addition to the folcgo~g the present invention also provides a process for
the p~d~lcLion of any compound of the invention as hereinbefore defineA. which
process comprises:
i). for the production of any one of Sanglifehrins A, B, C or D, cultivating a
Sanglifehrin A, B, C or D producing ~ ..o...~ e strain in a culture .I~fd;~
and isolating the desired Sanglifehrin A, B, C or D from the obtained culture
broth;
ii). for the production of Sanglifc,h.iils C and D ~ubjecliilg Sq~lif~hnnc A andB to cyclisation at positions 15 and 16;
iii). for the production of Sanglifehrins A and B subjecting Sanglifehrins C
and D to ring opening of the lactol ring at posiliollslS and 16;
iv). for the production of a macrolide of formula IX or ~', wh~.c;l~ -g-h- is -
C(CH3)=CH-, dehydrating a coll,pound of formula IX or ~' whe.c;n -g-h- is -
CH(CH3)-CH(OH)- or a protected form thereof;
CA 02224715 1997-12-16
wos7/022ss rcrlErs6/02ss2
v). for the production of a macrolide of formula IX or IX' ~Lc.~l R4 is -
CH(OH)-CH3, L~hu~l~ating a CO~ uu ~d of formula IX or IX' ~L~.,m R4 is -
C(O)-CH3;
vi). for the pro~luc~ion of a macrolide of formula IX or IX' ~.l~.~;n the 14 to
16 pocitio~c of the macrolide ring CQ...l-. ;.ce a residue of formula X
CH3
O ' CH2 ) 2
o
16 1 X
--CH--CH--CH--CH--
17 1 15 14
CH3
causing a co~l,poulld of formula IX or IX' to u"dergo internal protection at
positions 15 and 17;
vii). for the production of a macrolide of formula IX or IX', causing a
compound of formula IX or IX' ~.h~ ;n the 14 to 16 pocitionC of the
macrolide ring comprise a residue of formula X
CH3
O : CH2 ) 2
16 I X
--CH--CH--CH--CH--
17 1 15 14
CH3
to undergo reversal of internal protection at positions 15 and 17;
viii). for the production of a macrolide of formula IX or IX', in which R5 is
methyl, subjecting a macrolide of forrnula IX or IX', in which R5 is H to
methylation;
CA 02224715 1997-12-16
W O 97102285 ~ ,5/029S2
ix). for the production of a macrolide of forrnula IX or IX', in which R4 is in
O-protected form, ~ubjecliug a rnacrolide of formula IX or IX', in which R4 is
in O-u~ ot~,cl~,d forrn to O-protP~ion;
x). for the l~ludu-:~ion of a macrolide of formula IX or IX', in which R4 is in O-
unprotected form, subjecting a macrolide of formula IX or IX', in which R4 is
in O-protected forrn to O-deplot~ ;on;
xi) for the production of a macrolide of formula IX or IX', which comprices
an O-~lule~,led hy~u~heny~ nine residue at pocitionc 7 to 10 of the
macrocyclic ring, ~ub~e~;Ling a rnacrolide of formula ~ or IX', in which
comprises an O-unprotected hy~uAyp}i~ nine residue at positions 7 to 10
of the macrocyclic ring to O-protection;
xii) for the production of a macrolide of formula IX or IX', which comprices
an O-unprotected hydroxyphenyl~l~nine residue at positions 7 to 10 of the
macrocyclic ring, s.~bjecling a macrolide of formula IX or IX' which
comprises an O-protected hydroxyphenyl~l~nine residue at positions 7 to 10 of
the macrocyclic ring to O-de~ul~;Lion;
xiii) for the production of a macrolide of formula IX or IX', in which -e-f- is -
CH(OH)-CH(OH)-, ~u~je~ling a macrolide of formula IX or IX' in which -e-f-
is -CH=CH- to oxidative hydrolysis;
xiv) for the production of a compound of formula V' or a compound of
formula XII, subjecling a colllpolulld of formula IX or IX' to cleavage of the
linker group bet~.een the spiro bicyclo group and the maclo.:yclic ring.
xv) for the production of a cGlll~ulld of formula R60 - X - Y - Z - A - OH or
of formula R60 - X - Y - Z - A' - CH(OH) - L - S, ~ubj~Liilg a ~l~l~ycle of
formula IV or the macrocyclic ring of a compound of formula vm to ring-
opening at the lactone bridge thereof;
24
CA 02224715 1997-12-16
W 0 97102285 r~ ,51~029S2
xvi). for the p~duc~ion of a macrolide of formula IX or XII in ring-closed
form, ~ubjecting a c~l,l~u,.d of formula R60 - X - Y - Z - A - OH or of
formula R60 - X - Y - Z - A' - CH(OH) - L - S to closure of the ll~&~l~yclic
rmg.
xvii) for the production of a coll.~,ou..d of formula X~ or XII', subjecting a
colll~ou.ld of formula IX or VI' to ring-ope ;ng within the spiro bicyclic ring
system, and
xix) for the productior~ of a col,.~u..d of formula IX or VI', ~ul~;ecth~g a
co.l.pound of formula XII or XII' to ring-closure within the spiro bicyclic rin
system
~ locesses of the invention may be pe.rolll-ed, e.g. as described in the
examples As will be appreciated the ~,.ocesses defined above rnay be applied in any
app,op.iate seque~re or combination to obtain other macrolides in free, I,lot~ .ted rig-
open and ring-closed forrn as hereinbefore described
The macrolides of the invention, e.g Sanglifehrins A to D, are, or are derived
from, natural compounds typically obtained from ...r . ~.~ of the family
Streptomycet~reae.
Microorg~nicrnc capable of producing macrolides as he~i"befole defined not
previously been identifi~d
Accordingly in a yet further aspect the present invention ~lo~ides:
- a macrolide producing i~c~ cetc strain ~he,~ the macrolide is a
macrolide in which
i) positions 2 to 6 inclusive of the l..ac,c.~;yclic ring are provided by a
piperid~7inyl carboxylic acid residue; and/or
CA 02224715 1997-12-16
WO 97/0228s PcT/Ers6/029s2
ii) po~citionc 7 to 9 inclusive of the ~ lic ring are provided by an aromatic
a-amino acid residue; andlor
iii) positions 10 to 12 inclu~ of the lllaclu~;yclic ring are provided by an
qliphqtir o~-amino acid residue,
in particular
- a Sqnglifohrin A, B, C or D producing n l;..~....~cct~, strain.
Suitably the a~ G~ ete strain is of the family Sl~ toll-y~t~ e, more
suitably of the genus SL,cplolll.yces, in particular the strain Sll~i~tol..~es sp. A92-
308110 as hereinafter described, or is derived th~,lcfl~,lll, e.g. inrll-fling ~ ;.nlc,
variants, fusants, r,col..hi-- ~ntc or m~ifiPd forms thereof.
Suitably the strains of the invention are in the form of biologically pure
isolates.
For example Sll~p~l,lyces sp. A92-308110 may be mllt~ or m~ified into
dirr~ t forllLc by conventional te~hni-lues, e.g. by UV radiation or by tlCdtlllC~lt with a
chPmir~l mllt~ge~ such as N-methyl-N'-nitro nitrosoguanidine. RP~ h~ .l clonesmay be obtained by protoplast fusion. All such mlltq~ntc or l~o...h;,.~-~tc or m~ifiP~
forms, capable of producing s~glif~ s, in~lurling mlltqntc and l~cclllbi~ lt~ capable
of producing increased yields of, sqnglif~hrinc are in~h~de~ within the scope of the
present invention.
In a particular e.llbor~ -.l of the invention Sa.,~lif.,lhills A, B, C, and D,
ong~l others, are isolated from the novel S~ nolll~s sp. A92-308110. Sqmples of
S~ lol"yces sp. A92-3081 lO were de~ositcd with the Del-l~lle Sqrnml~lng von
Mi~oolg.~ ....~n und 7~l1hlltllren GmbH, Masch~ r Weg lb, D-38124
Bla~llsellv~eig, Germany on the 3 May 1995 under the terms of the Bud~st Treaty and
have been qcci~P,d deposidon number DSM 9954. S~mp'es of SI1G~)IO1~ ;eS SP. A92-308110 may also be obtailled from Sandoz Ltd. CH4002 Basel, S~
26
CA 02224715 1997-12-16
WO g7/02285 rCT/Erg6/02952
Nodce is hereby given that access to samples of DSM 9985 is lirnited in
accc".lallce with the provisions of Rule 28 (4) and (5) EPC.
The i~o~;On of S ~gl;r h.;~c A, B, C, and D from cultures of St~ to.ll~s sp.
A92-308110 is ~ec~ in Example 2.
Sl,eptc~l"yces sp. strain A92-308110 belongs to the genus Sl,c~)lul~ cs
acco~ing to the ~c~ in ~C.E,~ Manual (Volume 4, 1989, Williams and
WiL~cins, Roltimore) and The Prokaryotcs (1992 Srrin~er Verlag, New York). The cell
walls contain LL,tliorninopimp-lir acid. The fatty acids are iso- and anteiso-~,~,cl,ed,
straight and ~ t t~d The sugar ~t,~ is non .1;~ ,. The ~e~tdLi./e
mycelium does not break down into r.~6,..~ The aerial mycelium forms long chainsof spores.
According to the lef,.~,nce books cited above, the strain de-sign-o-t~l A92-308110
is a new Streptomyces. A92-308110 grows on various organic and illu16~hliC media and
in most cases forms aerial mycelium. The primary slll~L.dte ~ ~lhilll grows as hyph~
and is generally beige to greyish-brown. The color of the ~rial mycelium belongs to the
grey series, number 4, and this l,ly~liu,,, forms lûng chains of spores which belong to
the type spira b.
The ability of St,~,p~lll~s ~. A92-308110 to grow on usual bi~ l media,
its carbon utili7~tion, and its phy~iologi~ ch~ s are pl~;se.lted in the following
tables.
Table 1 . Growth on various birl~ 91 media
Culture .. ~di~ll Culture chal.t~ ;cs
yeast extract/ growth: good
malt agar sul,~Ll~e Ill,~liulll: brownish
aerial mycelium: grayish-brown
soluble pi~n~.nt none
CA 0222471S 1997-12-16
WO 97/02285 rCTnEP96/02952
oalmeal agar growth: good
subs~ate lll~ ll; dark brown
aerial mycelium: greyish-brown
soluble pi~m-nt- ~u~. IliSh
glucose-~ala~le growth: moderate
substrate mycelium: bl~Jw,Lish
aerial ",~.icliu",; grcyish-brown
soluble pi~n~nt- none
Lol~; c salts/ growth: moderate
starch agar subst~ate ",~eliu",: grey
aerial mycelium: greyish-brown
soluble pigJ~ I~r~
Sucrose/ growth: very poor
nitrate agar s~ l.,t~ mycelium: whitish
aerial mycelium: poor, greyish brown
soluble pigrn~nt- none
Glyceroll growth: moderate
as~J~aginc agar substrate mycelium: b~llish
aerial mycelium: greish-brown
solublepigrn~nt none
Nutrient agar growth: moderate
substrate mycelium: beigc
aer al mycelium: none
soluble pigrn~nt brown
Table 2: carbon utilization
CA 02224715 1997-12-16
WO 97/02285 PCT/EPg6/02g52
moderate or good: gll~rose~ Luulose, al~k; lose, ~cylose, .. ~ ~o.~
poor .1. .. -o~ sucrose, ,~rril~ose~ cellulose, salicin
ne d~ m-inositol
Table 3: PhYsiolo~ical characteristics
nitrate reduction: positive
starch hydrolysis: moderate on ino.2;~,c salts-starch agar,
negative on oatmeal agar.
tyrosine deg, ~ inn ne~;~.e
milk peptonic~tion positive
mrl~nin formation: positive
growth te."~.~ .es: 18-37~C.
Ve~y poor growth at 13~C.
No growth at 45~C.
pH-range: rich growth at pH 5 and 7, good gro vth at pH 9
NaCI recict~nre: up to 6%, but reduced growth already at a 2%
CQl-r~ dtion.
Macrolides of the invention including S~lglif I ;i-c A B, C and D may be
produced by cultivating SL,ept~""~ces sp. A92-308110 or a mutant, ~~col. h; lant or
m~lifird folm thereof on an ap~"~,p,iate culture ...~ Y~mplr 1 desr~ihs. by way
of illustration only of the invention a p,oce~lu.~e for the cultivation of Sl,ci~tc ",~ces sp
A92-308110.
Thus in further aspects the invention inrlllrirs
29
CA 02224715 1997-12-16
WO g7/02285 ~ /02gS2
a)a bic'~rqlly pure isolate of stTain SLc~Jtùm~ces ~2. A92-308110 (DSM
9954) or a mutant, ~...h;~ 1 or ",~1;rr~ form thereof which is
capable ~f yl~~J~ e a macrolide of the invention, and
b) a process for the ylud~ ;on of a macrolide of the invention, which
cornrricf~s cultivating strain S~pto"~ ;es sp. A92-308110 (DSM 9954)
or a mutant"ecc-..k;..~-l or ,~ul;rlpd form thereof in an app,upfi~t~
culture ...~ .... and optionally ~u~ g the sar.gl;f~ k. ;--
Macrolides in accordance with the invention, e.g. colllyoullds of formula ~;for example, Sanglifehrins A, B, C and D, and their rhqrrn~~eutirqlly ~CC~Jt; '~le salts,
hereinafter genrricqlly "agents of the invention", exhibit sqn~lifrhrin ch~,-~t~ l;r
activities, i.e. the following cornhinqtiQn of activities:
- have cyclophilin binding activity;
- have i.. ~ros~ ylc;~si~/e activity;
- inhibit proliferation of both B-cells and T-cells;
- but do not have FK binding protein binding activity, and
- do not inhibit c-q-lcin~ in activity.
These activities and assays to ~ete ...inf these activities are described
hereinafter in greater detail. Biological activity of macrolides of the invention, e.g. of
forrnula IX, e.g of Sanglireti,ins A to D, may be demonstrated in s~,da~ in vitro and ~n
vivo test methods, e.g. as follows.
1. Primary Humoral ~mmllne l~r~ e to Sheep Red Blood Cells (MD,
Mishell-Dutton)
Mouse spleen cells (OF l, female, 8-10 weeks, 1 x 107) are co-cultured with sheep
erythrocytes (SRBC, 3 x 107) for 3 days in 1 ml final volume in 24 well plates.
Lymphocytes are harvested, washed and plated at a density of 1 x 106 cells onto
soft-agar with fresh antigen (SRBC). Co~ e .~f nt (guinea pig serum) is added after a
60-90 minute inrub~lion period and ;n~ bA~;O~ is co.~ for a further 60 minu~
after which the test is evaluated by co----ti.-g (lllic,uscope) the pl~ques During the 3 day
CA 0222471S 1997-12-16
W 0 97/02285 r~ gr~/02952
ion, the ly"l~ho~;~s are 3~ ;1;7~ to the antigen (SRBC). When incubated with
antigen again, B~ Jhoc~tes se~rcte specific antibody which binds to the antigen in the
vicinity of t_e sec.etuly l~ hG.;y~. ~tlitinn of complement causcs Iysis of the
antibody-coated e ~ tcs yidding a plaque. Each plaque l~,~C~ a single
antibody-~,~u~ .g cell.
Tnhihition of plaque forrn ~ion is indicative of pk~ ;r~l utility. C'~ pu....~c
of the i"~ tion, e.g. S~n~;fi h. ;nc A to D, are active in this assay at a ~ n~-.l.ation of
about..?... toabout....?....nM.
Ref~l.,nces.
R.I. Mishell & R.W. Dutton (1966) T....~ l;on of normal mouse spleen cell
hnCions in vitro. Science 153: 1004 1006
R.I. Mishell & R.W. Dutton (1967) T.. ;~,ation of dissociated spleen oell
cultures from normal mice. J.Exp.Med. 126:423-442
2. Proliferative Response Of L~u,l)ho~s to Allogenic Stim~ tion
Two-way MLR (Murine Mixed Lymphocyte Reaction):
Spleen cells (2 x 105) from Balblc mice (female, 8-10 weeks) are co-inrub-~d for 4
days with 2 x 105 spleen cells from CBA mice (female, 8-10 weeks). The ~llog~ni~ cells
induce a proliferative ~.,.c~nce in the r~spon~er spleen cell population which is
measured by l~ or inco,~o,dtion into the DNA. Macrolides of the
invention, e.g. co..~l~o~ .fls of formula IX and their ph~ y ~- pt~ salts,
e.g. Sanglif.,h,i"s A, B, C and D, have ICsos in the range from about 30 up to about 2û0
nM as colll~.,d with an ICso of about 20 nM for cyrlQsrrin A when tested in thisassay.
P~eference:
CA 02224715 1997-12-16
WO 97/02285 PCI/EP96/029S2
T. Meo (1979) The MLR in the mouse. In: "Tmmlln~D~ M~thods", L. L~kovi~, and
B. Pernis, Eds., ~~ Içmir Press, N.Y. pp. 227- 239
3. LPS- stimulated murine B-cells
Spleen cells (2 x 105) from CBA mice are incubated for 48 hours with 50 ~u~/ml
LPS plus test co,ll~ul,d. F~ulif~.ation is measured by l-~e~l~ pl~ul2,or ~lcol~olalion
into DNA. Macrolides of the invention, e.g. CCilll~ UII~S of formula ~f and their
ph~....7.~ 711y ur~fp~ le salts, e.g. S~lglifi~hrinc A, B, C and D, inhibit B-cell
proliferation and have Icsos in the range from about40 up to about 100 ~M.
References:
Greaves, M. and J. Janossy, 1972, F.li~it~tinn of sele~ T and B Iymphocyte ~ onse
by cell surface binding ligands, Tr~ncpl ~nt Rev., 11: 87
Janossy, G. and M. F. Greaves, 1971, Ly~ hoc~t~ activation, L 12fspol-~e of T and B
lymphocytes to phytomitogçnc, Clin. Exp. Tmml~nol. 9: 483498
4. Cytotoxic and cytostatic activity in vitro usin~ the THPl cell line
Cytotoxicity is ~tel.llined using the human monocytic cell line THPl (5 x 104
cellslwell) which are ;-u-,JI~ d in the ~ ence of IFN~ (100 U/ml)and LPS (5 ~ m~)
plus test colllpound (up to 10 ~M) for 24 to 72 h at 37~C. Living cells are ,~ ;r.çd
using the col~ read-out MTT which ulea~ul~s ...;I.x~ ri~l dehy~u~nase
enzymatic activity in living cells (Moscm~r 1983). Macrolides of the i~v~,n~ion, e.g.
~Illpounds of formula lX and their ph7 ~.7-,~ ly ~ e salts, e.g. S~nglifehrinc
A, B, C and D, have IC50s of about 1000 5000 nM after 24 h il.uub~iQn in this assay.
Reference:
32
CA 02224715 1997-12-16
WO 97/02285 PCT/EP96/029S2
I~Q.ccmq~l T. J. (1983), Rapid C~ ''h;~A' assay for cellular growth and survival:
aMli~ ion to prolife A-~inn and cytoto~cic assays, J. Imm. M~ Ih~lc, 65, 55-63.
5. TNF release by human p ~ ;ph~al blood l-~- n~ cells
Monor~ Ale~r cells are ~,cpa~d from the ~..;l.h..~l blood of healthy vollmt~rs
using Ficoll-Hypaque density separation according to the method of Hansell et al.
(1991). Cells (105 cellslwcll in 20û ~1 RP~ 10% FCS by volumc) are incubatcd ~vith
serial ~lil--tionc of the test co...~ c for 30 min at 37~C prior to the ~1rlitinn of
stim-lhlc. In~.r~,luil y (100 U/ml) and LPS (5~g/ml) arc uscd as stimuli to induce
Tumour Necrosis Factor (lNE) a rclease by ~ ".l blood ...f ~ k~r cells. After 3
h ;nA~l,d~ion, the cells are cPntrifugP~d (120û rpm for 10 min) and the s ~ are
collP~AtP~l The amount of TNF prcsent in the ccll ~u~ .~Atants is d~e....i~-~l using a
c~.. r-.cially available enzyme-linked ;~ n~nosû~ t assay kit. Macrolides of the
invention, e.g. colllpou.lds of formula lX and their ph~,..~ ly --r~pl-~lA salts,
e.g. Sanglir~hlills A, B, C and D, have IC50 in the range from about 200 nm to about
1000 nm when tested in this assay.
6. Cyclophilin Binding Assay
A suitable cyclophilin binding assay is the co...l~ti~ , ELISA test described byQu~PcniA- Is in Eur. J. ~.. lll--ol. 1987 17 1359-1365. In this test, the CQ~ n~l tO be
tested is added during the inA.~I.Ation of e~;lophilin (human ,~G-..b;.lant cyclophilin A)
with coated BSA-cyclosporin A and the cQn~ . ~ion lcquued to give a 50% inhihi~ion
of the control reaction without CQ-ul~ri~ol is then calculated (ICso). An Al~b... ~ assay
is the co~ ;l;ve binding test describecd by Schn~ r et aL in Biorh ~ l.y (1994), 33,
8218-8224, which involves ~lition of test COlll~U. d during the ;..~ ion of
biotinylated cyclophilin (human l~colllbir,~hlt cyclophilin A) with coated BSA-
cyclosporin A. The arnount of biotinylated cyclophilin bound in the PICS~IICe and
~hs~nre of a test co~ i is ~t.~ ;nf~ by il,.~ n with sllcpta~idin~ hr'ed
AncAlin~- pho5~.hA~;~ce Macrolides of the invcntion, e.g. co--l~u--ds of forrnula lX, e.g.
CA 0222471S 1997-12-16
W 097r02285 1~ 5/029S2
;r k.;.. A, B, C and B, have ICs~s in the range from about 10 to about 100 nM,
compared with an Icso of about 80 nM for ~ lo~ . A when tested in these assays.
Further in vitro assays which may be used to de-~n .cl.dte the biological activity of
Sangl;Lh.;~.c are IL,2 ~ gene assays and ConA-stimuhted spleen cell assays
(indicative of effect on T~ell a~:liv~Lio~
olides of the invention, e.g. cc....pol~ ~1c of formula lX, e.g. San~l;f k.;.. A, B,
C and B, do not have FK binding protein binding activity and do not inhibit
activity when tested in standard tests for these activities.
7. T ~ iced Graft-versus-Host (GvH) E2~ction in the rat
[Ford et aL, TRANSPL. PROC. 10 (1979) 258].
Spleen cells (1 x 107) from 6 week old female WistarlFurth (WF) rats are injected
P4UCIY on day O into the left hind-paw of female (F344 x WF)FI rats weighing
about 100g. Animals are treated for 4 cc...~ , days and the popliteal lymph nodes
are removed and weighed on day 7. The dirr~.e"ce in weight ~ h.~n the two lyrnphnodes is taken as the p~dlll~,t,l for ev~ ting the reaction.
Inhibition of GvH reaction in the above test is indicative of ph~ r~ utility.
Macrolides of the invention, e.g. cQ...l ou..ds of forrn~ IX and their pha,...q~..l;r~lly
~ccep1~ble salts, e.g. S~ngJ;f. h . ;..c A, B, C and D, are able to inhibit the GvH reaction
by up to about 30% when ~ d at a dose of about 1 mg~g s.c..
8. DTH in~uced by SRBC-T~ cells
Fifty microliters of a 1:1 (v/v) rnixture of a TH (sheep red blood cell primed) cell
clone (2 x 106) and a 10 % sheep red blood cell (SRBC) ~ ~ n~iol~ are injected s.c. into
the right hind footpad of female C57 BIJ6 mice (6-12 weeks old). 50 ~1 of the SRBC
cell s ~cncioll (diluted 1:1 v/v with PBS) is injerte~ s.c. into the left hind footpad (to
34
CA 02224715 1997-12-16
W O 97102285 1~ /029S2
~u,~ non specific in~;l~e in footpad swelling due to the ;..j~l;m~ l,l~lu,~,). Right
and left hind footpad th ~- ~.rCc is ~cid 24 hours later.
The percent in,_l~ in 1~ rl~ C~ of t_e dght footpad over the left footpad (z) isc~lr~ L..~cs of right footpad = x; ~ c~ of left footpad = y; % specific
ir,.,,~se = z
z=((x-y)/y). 100
M~rolides of the invention, e.g. colllpounds of formula ~ and their ph~ lir~lly
~rcep~ salts, e.g. S~nglifrhrinc A, B, C and D, reduce swelling of the DTH mouse by
up to about 50% at doses of the order of 5 mg~kg s.c..
References:
A.T.J. Bianchi, H. Hooijkaas, R. Brenner, R. Tees, AA. Nordin & M.H. Schreia (1981)
Clones of helper T-cells mediate antigen spe~ific, H-2 ...~ DTH. Nature
290:62-63
P. Herrrnann, M.H. Schreier, J.-F. Borel & C. Feurer (1988) Mast cell de~ inn as a
major event in the effector phase of delayed-type lly~A ~n~ y in~luc~d by clonedhelper T cells. In~. Archs Allergy appl. ~nunun. 86: 102- 105
9. Rat/Mouse Heart Allotr~nc~l~nt~tiol-
The in vivo efficacy of macrolides of the invention is ~ sc,d in rat and mouse
heart allotr~ncpl~nt~tiQn using Alzet o~ ;r ...;..;l.u..ls for s.c. a(~ t.~tion. In
mouse heart allol- ~cplAnt~tion (BALB/c to C3H), macrolides of the invention, e.g.
compounds of formula IX and their ~.k~ r~.~l ;r~lly ~ ~r~ salts, e.g. San~; r~ k. ;nc
A, B, C and D, prolong grah survival at doses of the order of 30 mg/kg/day. In rat heart
allotr~ncpl~nt~~ion (DA to Lewis) ~ with SUllO~ doses of cyrlnsp- rin A in
co...hin~lion with Macrolides of the invention, e.g. c~...po~ lc of formula IX and their
CA 02224715 1997-12-16
W O 97102285 P~ 6~29S2
pha . ~ul;r~lly r- rt-~lr salts, e.g. San~l;f-k.;.-c A, B, C and D, prolonged graft
survival as e~e-mrlifiP~ in the table below.
C~clospo.i" A Sanglife~i.l A Graft survival
(mg/kg) (mg/~g) (days)
- 12,12,12,13,13,14
29, 30, 45, 48, >51, >46
Control (Placebo)Control (Placebo) Control (Placebo)
Agents of the invention are useful as ph~rn ~eutir~lc, e.g. as in...... o~u~l"Gs~i~_ as
well as an anti-infl~ ....~A~ agents.
They are, in particular, useful for the pl~ ion of acute andlor chronic organ or tissue
allo- and xenot~n~ nt rejecti~n~ e.g. for the t~ rnP.nt of recipients of heart, lung,
combined heart-lung, liver, kidney, panc..,àlic, skin or comeal transplants. They are also
inAir~tP,d for the p~ htion of graft-versus-host dise~, such as following bone rnarrow
transplants.
Agents of the invention are also useful for the t~ t of anl~.;...... ~nf disease and of
infl~mm~tory conditions, in particular infl~ conditions with an aetiology
inrluAing an autollllll~unr co--lpone-lt such as arthritis (for Py~mpl~ oid arthritis,
arthritis chronica progrediente and arthritis defoll..ans) and ,I,. u",; -;r AiCP~CeS Specific
auto-immnne Aicç~cçs for which the agents of the invention may be employed include
autoimm~.nP h~pm~tological disord~ (innluAing e.g. h~molytic anaemia, aplastic
~n~Pmi~ pure red cell ~ mi~ and idiopathic ~u..~boc~rtopcnia), systemic lupus
eryll.. -.. ~lo~c, pol~. hor,d ilis, scl.,.udo-~-a. Wegener gr~n~ csiC~ de .. ~t.. ~o~ilis,
chronic active hPp~~itic my~cthPni~ gravis, psc.. ;~cis, Steven-Johnson syndrome,
idiopathic sprue, ~ltoi.. r infl~.~.. ,~o~y bowel disease (innlllAing e.g. ulc~
colitis and Crohn's disease) enA~rinp ophth~l...oF.~11.y, Graves disease, sarcoidocic,
mnltirle sclerosis, primary biliary cirrhocic~ juvenile Ai~bPtes (.' ~ s mrllihlc t~pe I),
uveitis (anterior and posterior), keratoconju..ctivitis sicca and vernal and/or allergic
k.,.atoconjuncti.~itis, int~ lung fibrosis, psoriatic arthritis, glomer~ .n~.lJ}i. ;l;c (with
36
CA 0222471S 1997-12-16
WO g7/02285 r~ 02ss2
and without nep~.~Lic S~ u..e, e.g. ;..~lu~ e i~ h;r, nCphlOtiC s~ uule or
minim~l change ~el)~û~ ) and asthm~
For these and ûther uses agents of the i~ ioL~ may be al1...;nict~ d on their ûwn or
toe~thPr with other ;~ o~ ,l~s~t ûr antiinflam~natory agents, innl~ ne
~,lo~yulills, l~Ull~;ills, FK 506, and steroids.
For the above ;-~ n.C the appropriate dosage will, of course, vary ~CpC'If~;Q~, and the
agent of the invention chosen, for example, on the subject to be treated, the mûde of
~."inic~ ion and the nature and severity of the con-lition being treated. However, in
general, s~ticf~tory results in animals are obta ned at daily ~osa~,c of from about 0.01
to 10 mg/kg/day p.o.. In larger ,,.~."",~lc, for PY~mrl~P humans, an indicated daily
dosage is in the range of from about 0.5 to about 500 mg of san~l;f~ k~ ;n ~timinict~Pred
orally once or, more suitably, in divided dosages two to four times/day.
In organ transpl~nt~tion in hllrn~nc, oral doses of 0.1 to 100, preferably 0.3 to 30, more
preferably 0.5 to 10, mg/kg of a cc~lllpound of agent of the invention. When an agent of
the invention is given along with Other;-~ nQ~ lcsS~lt~ (e.g. with coficosteroids or
with co,l,pounds of the cyclQs~ or ~ C~I class as part of a double, triple or
quadruple drug therapy) lower doses (e.g. 0.1 mg/~cg/day i.v.; 3 mg/kg/day oral initially)
may be used. In particular agents of the invention may be given with other non-steroidal
immnl-o~p~,ss<s, e.g. with cyclosporin A, l~a~ r or FK 506, with a view to thepartial or complete repl~rc ..~ .1 of steroids.
Agents of the invention may be ~ l.,,;ni~ ,d by any conve ~I;on~l route, in particular
enterally, e.g. orally, for el~mr'e in the form of sollltionc for .I.;,.~ g tablets or
carsnlPs or ~..uc.rte.~lly, for exarnple in the form of injectible solllti~nc or ~.."~ nc
Normally for systemic atlminictration oral dosage forms are p ~,f,.-.,d, ~hholl~h for some
conrlitionc, for ex~mrle for p.c~l~,"tion of rPjection of liver transplants, an intravenously
inje~ble form is desudble. t'omro!lntlC may also be r l.~ d topically or dermally,
e.g. in the form of a dermal cream or gel or like preparation or, for the pu~l)oses of
application to the eye, in the form of an occular cr~am, gel or eye drop p.~- ~ion.
CA 02224715 1997-12-16
W 0 97/02285 r~ ,.S/029S2
Sl~itiqhl~ unit dosage forms for oral ~ 1. t;~n CC~ ;~ e.g. from 0.5 to 100 mg of
the U~ .fi per dosage.
In accol.l~oe with the fol~go.ng the present invcntion also provides in a further series
Of Pr.~ho~];~,.. .~t~
A. A method of çffP~~-ting i~ o~sjion in a subject in need of such llc~-mrnt
which method ~...1.. ;~s administering to said subject an erf~ amount of an
agent of the in~lention.
B. A m,~-thod
1) for the pl~ tion of acute andlor c ronic organ allo- or ~PnO~
rejectinn for example for t_e treatment of recipients of organ transplants of
any of the particular types listed above; or
2) for the ~lc~ ntion of graft-versus-host disease, for exqmrlr in lr ~r -ntc of bone
marrow ~ , or
3) for the ~ nt of ~1~;.. - .~ disease or for the L~ .. ~1 of any such disease
or cor ~lition listed above; or
4) for the tle~ r-~l of asthma
in a subject in need of such llrA~m~nl~ which method co...l..;ces ~ tl ;n~ to
said subject an effecti~e amount of an agent of the invention.
C. An agent of the invention for use as a ph~ --I;r~l. e.g. for use as an
immllno~V~ ssant or for use in the ll~ ,t~". nt of any disease or cQntlition as set
forth under B above.
D. A ph ... ~ l co~ o~ilion co...l.. ;cing an agent of the invention in ACSo.~~ inn
with a pharm~~M~ti~~qlly ~ 1A diluent or carrier.
CA 02224715 1997-12-16
W O 97/02285 1~ 5/02952
E. The use of an agent of the invcntion.for the preparation of a medicament for use as
an ;.. - --r~ ;,sant or for use in the ~ of any disease or cQn~liti~n as
set forth under B above.
In addition rn~rolides of the il,~ tion which possess ~ ophi1in binding
~tivity, may be useful as lcage.ll~ in ~~icr1 ~r~ nl ;.. ~ .r~csqvys for cyclGs~lms and
other cyclorhi1in binding cc~ ls, for example in the assay pl~hllC d<~;h~ in
our copçn~ling patent ~ fiQn WO 95/07468. This patent application relates to an
assay procedure for d~ t~. - ...;ni~-g the conr~..1.dtion of an ;~ ~hilin-binding
~h~ArciLI;ç-q-l e.g. Ciclosporin, in blood; the pl~dulci c~...l..;~;..g adding a binding
CQ,.,I~ilor that ~licrl~es the ~.hr~ -lirql from ;~ ns~ ci~sant~ ophilin
cornpleYss in the blood; adding a l~JIor that binds to the ph~ ,"'--~,I;rq1 but not
ci~ifirqntly to the binding c4l.~ ;l0l, separating the lcceptol-ph~ )fi~ complexfrom the sample; and detl ....;ni~g the amount of the ph~ ",~ 1 Ss-~gliLh.;--c may
be used as the binding co---l~t;ln- in such assays; for i~ r~, to ~ p1~ cyclosporins
from cyclophilins, thereby releqcing the cyclosporin for q..~ ;on, e.g. by a
monoclonal antibody which is specific for the cyclosporin
The invention is further ~çsçribed by way of illustration only in the following
FY.~mplç which refers to the ~~c....p~ .jing Figures:
in which Figure 1 shows the Mass SpCCIlulll of the ~lll~oulld of Sqnglif~hrin B;Figure 2 shows the Mass ~ lll of the co~ of SAng1;r~k. ;-. A;
Figure 3 shows the Mass s~L~llll of the colll~u,.d of c~ .gl;r.,hlill D;
Figure 4 shows the Mass Sp~LIulll of the CO~1~U .~ of S~n~;r. k. ;n C;
Figure 5 shows the IR S~:tl~llll of the cc.. . ~ l of San~l;fi k. ;. . B;
Figure 6 shows the ~ S~:tlulll of the ~ n~l of San~l;r~k~ ;.- A;
Figure 7 shows the IR ~LI.llll of the colll~uund of S~agl;f~h. ;n D;
Pigure 8 shows the IR Sp~tlulll of the colll~ound of S~ l~lir. k. ;.. C;
Figure 9 shows the NMR Sp~:llUlll of the colll~,uu.ld of formula S~ir~h. ;n A;
Figure lO shows the N~ ~llulll of the co...~ of SA~glifi k. ;.- D.
Figure l l shows the N~ S~:tlulll of the colllpoLnd of S~n~lif~hrinR~ and
39
CA 02224715 1997-12-16
WO g7102285 rCT/EP96102gS2
Figure 12 shows the NMR ~:l,~ of the coLu~ulld of ~s-1~,1if~ h. ;i- C.
CA 02224715 1997-12-16
O 97/02285 P~l/rl,~0 ~ 2
EXA~LES
Culture conditions
SIlG~tulll~cGs sp. A 92-308110 may be cultured at suitable temperatures on various
culture media using al~luyliate ~ ;e~t~ and mineral substances, using aerobic ori... ~;OI~ culture procedures. The f~ ;on media typically c~ S inc a utilisable
source of carbon, sources of ~ ug_n and mineral salts inr~ i~ trace e~ , aLI of
which can be added in the form of wcll defined pl~lu~:~ or as complex l~ ul~S, for
as are found in biological products of various origins.
Fl~mpl~ 1 desr ;kc the original con~ u~ under which co~ ils of formula I
were obtained. I,~ u~cd yields rnay be obtained by opt;~ nn of the culture
conditions (aeration, t~ C.dlu,c, pH, quality and yu~ltily of the carbon and nillu~.l
sources, quantity of the mineral salts and of the trace ek".~ ) and by contTolling the
fe.".enldtion conditions in bio~
ExamPle lCulture of stMin A 92-308110.
a. Agar starting culture
Agar slant cultures of the strain A 92-308110 are grown for 10 to 14 days at 27~C on the
following agar medium:
Glucose lO.Og
Soluble starch 20.0g
Yeast extract S.Og
(Gistex, Gist Brocades)
NZ-Amine,Type A (Sheffield) S.Og
C~ um c~l,onate l.Og
Agar (Bacto) lS.Og
Dc~lin~" alised water to lOOOml
41
CA 02224715 1997-12-16
WOg7/0228S PCrtEP96/02gS2
The .. ~.1;.. is ~ to pH 6.6-6.8 with NaOH/H2SO4, then st~ili~A for 20 min. at
121~C.
The cultures can be stored at -25~-70~C. A S~ f nc;~n in glycerol-peptone can be stored
under liquid nil,ogell.
b Preculture
Spores and mycelium of 10 starting cultures are ~u~lf l~-led in 100 ml of a 0.9% salt
solution. Two 2 Liter-r.l~ ,. flasks each CQnl;~;n;~g 1 liter of preculture ,..,.1;.,...
are inoclll~t~d each with 50 ml of this ~sp~l.C;c~n The co,~ ion of the preculture
m~Ail-m is as follows:
(~lueose techn 7.50g
Glycerin 7.50g
Yeast extract (BBL) 1.35g
Malt extract liquid (Wander) 7.50g
Starch soluble 7.50g
NZ-Amine,Type A (Sheffield) 2.50g
Soya protein 2.50g
L(-) Asparagine l.OOg
CaCO3 O.O50g
NaCI 0.050g
KH2PO4 0.250g
K2HPO4 0.500g
MgSO4.7H20 O. lOOg
Trace el~rn~ont solution A lml
Agar (Bacto) lg
Dc.~ eldlised water to lOOOml
The ".~ ,... is adjusted to pH 6.8-7.2 with NaOH/H2SO4 and st~-ilicçd for 20rn at
121~C.
42
CA 02224715 1997-12-16
WO 97hl22~AS PCTlEpy6lo2gs2
The co...l o~ on of the trace cl~ .. n~ snllltifm A is as follows:
FeS04.7H20 5.0g
ZnS04.7H20 4.0g
MnCI2.4H20 2.0g
CuS04.5H20 0.2g
CoCl2.6H20 2.0g
H3B03 0. lg
KI 0.05g
H2S04 (95%) lml
Dc.llin~,lalised water to 1000 ml
The precultures are f~ - n~ ~ d for 24 hr. at 27~C on a rotary shaker at 200 rpm with an
eccentricity of 50mm.
c First intermediate culture
Two 75 Liter bioreactors cont~ining each 50 liters of preculture ."~ 1;..." are inQc~ ted
each with I liter of the preculture and fe ... ~-~ed for 96 hr. at 27~C. The f~.",f~.~t~. is
rotated at 150 rpm. Air is introduced at a rate of 0.5 liter per minute per liter ...~.1;.....
d Second h~cl...f~ te culture
Two 750 liter fe.~ nt~iQr' vessels each co~ i..g 500 liters of the ~ ".~.1;.....
are each inoc~ .e~ with 50 liter of the first j"t~ ",~ A culture. The second
h~te~ A;~tfe cultures are ;..r~lb~l~ for 70 hr at 27~C. The fermenters are rotated at 100
rpm and air into~uced at a rate of 0.8 liter per minute per liter ...f.1;.~ ..
43
CA 02224715 1997-12-16
W 0 97/0228S rCTn~6/02952
e. Main culture
Two 5'000 Liter biolca~lol~ each u~ g 3'000 liters of the main ...~ .. are
inoculated l~ ely with 250 and 300 liters of the second int~ Ai~~~ cultures. Themain cultures are incubated during 96 hr at 24~C. The biorcactors are rotated at 45 Ipm
and air introduced at a rate of 0.5 liter per minute per liter ...~ 1;....,
The c~ on of the main culture ...~.1;...., is as follows:
GlucQse techn 20g
Malt extract liquid (Wander) 2g
Yeast extract (Bacto) 2g
Soytone (Bacto) 2g
KH2PO4 0.2g
K2HPO4 0.4g
Mgso4~7H2o 0.2g
NaCI 0.05g
CaCk.6H2O 0.05g
Trace ele .. r nt solution B lml
Agar (Bacto) lg
rl~ ,.;n-,~alisedwatertol000ml.
The pH is adjusted to 6.3 with KOH/HCI. The ~ .1;n~" is st~riliced for 20 min at 121~C.
The co...l os;l;on of the trace ele-..~n~ sollltion B is the following:
FeSO4.7H20 5.0g
ZnSO4.7H20 4.0g
Mncl2.4H2o 2.0g
CuSO4.5H20 0.2g
(NH4)6 Mo7024 0.2g
44
CA 02224715 1997-12-16
W O 97/02285 1~1/r~ J029S2
CoCl2.6H20 l.Og
H3BO3 0. lg
KJ O.O5g
H2SO4 (95%) lml
iced water to 1000 ml
An op~im;ce~ culture ".~.1;.. for the main culture is as follows:
Soybean meal 20.0g
Glycerol 40.0g
MES O.lM
nemin~ised water to 1000 ml at pH 6.8
CA 02224715 1997-12-16
WO 97~2285 PCT~Erg6~2952
ExamDle 2 - Isolation of ~;~n~lifehlins A. B. C and D from StreDtom~vces sP. A92308110
The first i~ol~inn and cl~ -- t~ t;on of the 4 new CBA active metabolites was done
from two 3000 I tank r~.ll.c~ltations by activity guided rl ~I;mr~';nn and HPLC and
thinlayer chlu~ o~la~Jhic analysis. CBA (cy~lophilin binding assay) as described above
was used to test for biol~r~l activity.
The two 3000 liter fe-". ~1 t;nn~ are pr~xessed s~ ately. 1500 liter from each
fe.~rnt~iûn is stirred with 2000 l ethyl acetate in 4000 liter stainlPss steel vessel for 20
hûurs. The separation of the organic phase is done with a Wçstf~ Sep~alor typ SA-
20. The ethyl ~etate extr~ts are washed twice with 80 liters of water and e~,a~l.~t~,d to
dryness under reduced pl~Ul~i to give 1.64 and 2 kg eYtr1çtc~ The two crude extr~ts
are dPfattPd by a three step extraction with 40 liter ",.lh a-)l/water 9:1 and 40 liter of
hexane. Ev~ Lion to dryness under reduced pl~ U~, gives 1.34 kg extr~t.
The def~ttP-d extr~t is c}~ o~a~hed in two portions (670 g) on a column of 10 kgSeph~lPY H in III..IhAnOl Solution E~h portion is dissolved in 3.3 liters of mPthAnol
when added to the column After collection of the first 15 liters eluate as fr~tion 1 the
chromatography is co~tinued by collP~ting 2 liter fr~tions.
The most ~tive fr~tions were 2,3 and 4 and are the.elbl~; col..hil-~A to give 146 g.
This sample is further cll~ o~ ~l.h~d on 1 kg Silir~lgel Merck 0.04 0063 mrn with
methyl-tertiary-butyl-ether (M'I~E), MTBE/5 % mrth~ns~l and MTBE / 10 %
mPth~nol. Fr~tions of 2 liters are CQllP~ctçA Fractions 5 to 9 are the most ~tive ones
and are combined to give a sample of 43.8 g. This sample is further sepalated on a
column of 1 kg Silir~gel (Merck) 0.0~0.063 mm with a gl~.die,lt of hexane/a~etone 7:3
to ac~onp~ From this c~lllâtO~hy fraction 6 (7.0 g) is further sepal~ ted on a column
of 3 kg Lich,~l~ RP 18 (Merck) 40-63 um with mPth~nol/water 94:6 (fr~tion 4-7
2.16 g), then on a colurnn of 100 g Silicagel H with methyl~nPrhkridp and 3 %
mPth~nol (733 mg), a column of 3 kg Licl~o~l~p RP18 with l... lh~nol/water 9:1 (621
mg) and then on 100 g Liclhoyl~ RP18 with ~reto..;l.;lP/water 1:1 to yield 324 mg of
46
CA 02224715 1997-12-16
W 0 97~2285 rCTnEr96~29S2
pure Sqn~;r k-;-. A (mp 142-145~ C (~lol~ho~), (a)D25--67.30 (c=1}988,
Fractions 5 and 7 from the hex~c/acelùmc column are c~...k;..r~l (7.1 g) and further
purified on a column of 3 kg Licluul.l~ RP18 4~63 ~n with methanol/water 9:1 (769
mg), on a column of 100 g ~iliragt !l H with MTBE 3 % ~ ol (309 mg) and finally
on 100 g ~ r:lgt'l H with lll~,lh~ r~l-lnri~e and 3 % methanûl to yield 90 mg pure
San~l;fuh. ;.. B (mp. 117-121~ C (~ollJhous), (a)D25=-52.80 (c=1-128 in ...- Ih~
Fractions 9 and 10 (2.147 g) of the chromatograrnm with ..,~lh~nol/water 94:6 on 3 kg
Licl~o~lep RP18 are further pulified on 100 g Silicagel H with lll~ yl~.n~l.lnri~ 5 %
m~ thqnol (800 mg) and finally on 3 kg Lichlupl~ RP18 with ... I~ .ol/water 9:1 to
give 480 mg of S~-glir~h.;.- C (mp. 165-170~ C, (a)D25=-35.60 (c~736 in ~ !h~nol).
Fractions 11 and 12 (835 mg) of the ch~ alogramm with m~.th~nol/water 94:6 on 3 kg
Licl.,up.ep RP18 are purified on 100 g Silicagel H with MTBE/5 % m~th~-~ol to give
140 mg of Sanglifehrin D (mp. 137-142~ C, a,llull,huu~).
Sanglifehrin A, B, C and D were then char~rteri~ by UV, IR, Mass and N~
S~;lruSCOpy. The results obtained are given in Table 4 below and in the ~~rc....pP~,jillg
figures.
47
CA 02224715 1997-12-16
WO 97102285 PCT/EP96/02952
Table 4
S~n~lifi-k.;~ A
molecular formula: C60HglN5OI3 (1090.4)
W (MeOH): 275 (1962), 242 (54500), 197 (75755)
H+: 275 (1635), 242 (51884),
OH-: 292 (1973), 242 (60495)
IR-spectra: Figure 6
Mass-spectra: FAB 1096[MH+Ii]+: Figure 2
NMR spectra: Figure 9
Sanglifehrin B
m~'ecnl~r formula: C60H89NsOI2 (1072.4)
UV (MeOH): 273 (4395), 242 (50600), 197 (78577)
IR-spectra: Figure S
Mass-spec~a: FAB 1098[MH+Li]+: Figure 1
NMR spec~a: Figure 11
Sanglifehrin C
molecular formula: C6~Hg3N5O~3 (1104.4)
W (MeOH): 275 (1876)), 242 (51557), 197 (72643)
H+: 275 (1391), 242 (50120)
OH-: 292 (1832), 242 (57960)
IR-spectra: Figure 8
Mass-spectra: FAB 1110[MH+Li]+: Figure 4
NMR spectra: Figure 12
48
CA 02224715 1997-12-16
PCT~02gS2
WO g7/02285
SDr~1; f~h~ D
~ D-~fonn~a: C~IHglN5012 (1086.4)
W (MeOH~: 273 (3194),242 (47584),197 (73766)
H~: 273 (3237),242 (46389)
OHr: 285 (2600),242 (52907)
IlR-spec~: Figuue 7
Mass-spec~a: FAU31092[MH+Li]~: Figure 3
N~DR spec~a: Figure 10
49
CA 02224715 1997-12-16
WO g7/02285 rCT/EP96/029S2
47 ~ 49
--~H
H~
Sangl i f ehrin A 57
ExamPle 3 - Transformation of ,C~nvljfehrjn A into ~CAnvlifehrjn C
To a stirred, cooled (0~C) solution of 20 mg (18.3 llmol) of Sanglir.,h,in A in 0.5 mL
of methanol is added one crystal of par~tohlçnesulfonic acid monoh~yd,dte. The
res~lltine yellow solution is stirred for one hour and the reaction is quçn~h~d with
saturated aqueous sodium bic~l~onate solution. The res~ ine Illi~lUlG iS extracted
twice with ethyl acetate. The organic solution is washed twice with saturated brine,
dried over anhydrous sodium sulfate, filtered and co~ ..,t~,d under reduced
pressure. The residue is purified by column ch,oll,dtography on silica gel (95:5methyl-tert.-butyl eth~ h~nol) to yield S~nglifehrin C as a white amorphous
powder. The latter consisted of a 4:1 mixture of Sanglifehrin C and its C53 epimer,
Sanglifehrin C having the (S) configuration as ~lepictçd below (R = Me).
4:1 mixture of ~ tereG, llera
HO ~ OH
O OH O~, O ~' ~o ~ R
~o ~NH o o
HOJ~
n ~ively, this tran~Ç ~ ion can be carried out by using other protic acids (suchas pynrlinillm paratolueneslllfonate~ h~dlu-,hloric acid or sulfuric acid) or Lewis acids
~0
CA 02224715 1997-12-16
W O g7/02285 r~ r5~29S2
(such as zinc rhlnri~e, ~ ;--.-- blul~ide or çhlnri~e, ~ iSoplopoAide or
borûn trifluoride) in ...~lh~nl Use of other ~lcQholir solvents or cosolvents like
ethqnol, isopropanol, butanol, allyl ~lrohol ~,u~a,E~l ~lcQhnl~ benzyl alcohol lead in
the same rnanner to analogues where R in the structure above is l~s~~ ,ly ethyl,isoplupyl, butyl, allyl, prop~;~l, benzyl.
In the same manner as described above, ~q~gli~h.;.. B can be transÇ~ d into
Sanglifehrin D.
ExamPle 4 - Transformation of ~n~lifehrin C ;nto ~ Jl;rehrin A
A solution of 550 mg (0.50 rnmol) of sangliÇuhlin C in 5 rnL of 4:1 THF-water istreated with 0.5 rnL of 2N ~queol~s sulfuric acid and stirred for 1.5 h. The reaction is
quenchPd with s~ul~t,d aqueous sodium bic~l~nate and the resulting llliAlUl~; isextracted twice with ethyl acetate. The organic solution is washed with saturated
aqueous sodium bic~l,onate solution and twice with saturated brine, dried over
anhydrous sodium sulfate, filtered and co~e~ dted under reduced pl~S~ . The
residue is purified by column chç~lllalography on silica gel (90:10 methyl-tert.-butyl
ether:lnPth-q-nol) to yield sqngl;f~k. ;n A as a white amo~phous powder.
Other inorganic or organic acids can be used in a ~"P~ -." contqining water and
optionally an organic cosolvent. Suitable acids include hydrochloric acid,
paratoluenPs--lfonic ~id or other sulfonic ~ids, pyriAinillm par9tohlPnP~slllfonate~
acetic ~id, trifluoluaceLic acid, forrnic ~id. S~itqkle organic cosolvents are
acetonitrile, dhn~ lr.J....qmi~e, dimethylsulfoxide"lioYqnP
These reactions are accc pr- ~ by the formation of varying ~mollntC of the
compound of formula XV, ~epen~linp among others on the reaction time (for a better
procedure leading to the compound of formula XV see Example 5 below).
Analogously, sqnglifehrin D can be ll~lsÇol",ed into sanglifehrin B.
ExamPle 5 - Trhl.~r~ ...ation of .~..Jl;fehr;n A into the comPound of formula XV
CA 0222471S 1997-12-16
WO 97/0228S PCT/EP96/029S2
To a stirred, cooled (0~C) soh~tion of 50 mg (46 ~mmol) of s~n~l;r~ k ;i- A in 1.9 mL
of ~ to..;l.;le is added 0.1 mL of l~c.g~" fln~ri-le - pyridine. The rçslllting yellow
soll~tion is stirrcd for 1 hour and thc rcaction is qu~ n~'h~ with saturated ~4u~uS
sodium bica L--~te . The res~lting ll~ih~lulc is c~l. led twice with ethyl acetate. The
organic solution is washed twicc with saturatcd brine, dried over anh~li.uus sodium
sulfate, filtered and co~ e .I dtcd under l~,duced p.~ u.e. The rcsidue is purified by
column cl~lllatography on silica gel (95:5 methyl-tcrt.-butyl eth~.lll~lhanol) to yield
the cc. ~ of formula XV as a whitc &llUl~oUs powdcr.
HO ~ ~ 1 I _ ~ rO
~; O OH O~ O ~ ~7
~~ CN~ ~ O
HO ~H
xv ~
In an analogous manner s~nglifçhrin B can be ll~sÇ~,...,ed into the compound of
formula XVI. These subs~n~çc exist as a single epimer at C53, but the absolute
co~fignration has not been unambiguously d.,tu~lined.
J J~ ~0
1~ O OH O O
11 ~ NH r
HO~ )
XVI
Formula XV: MS rnlz 1078 [M+Li]' (rel. il~te.~ily 100); ~H NMR (DMSO) (only
characteristic signals listed) ~ 0.40 (3H, d, H-S0), 1.20 (3H, s, H-54), 1.69 (3H, s, H-
CA 02224715 1997-12-16
WO g7/02285 1~11r1~6~2952
49),4.20(1H,t,H-15),4.58(1H,dd,H-17),5.19(1H,dd,H-18),5.28(1H,dd,H-23),
5.62(1H,m,H-21),5.67(1H,m,H-27),5.99(1H,d,H-25),6.03(1H,dd,H-19),6.14
(lH, dd, H-20), 6.22 (lH, dd, H-26).
ExamPle 6 - Transformation of the comPound of formula XV into .cnn~lifehrin A
To a stirred solution of 54 mg (50 ~mol) of the co...~ of formula XV in 0.5 mL
of 4: 1 THF-water is added 50 IIL of 2N aqueous sulfuric acid. The rer~1tir~ SOIIltiQn
is stirred at ~llbient tc~ dtule for 12 hours and the reaction is qu~ hrd with
saturated aqueous sodium bic~l~oliate solution. This I~U~tUlC is cA~l~Led twice with
ethyl acetate. The cG...hin~d organic solution is washed with saturated aqueous
sodium bic~l,onate solution and brine, dried over anhydluus sodium s~-lf~~P~ filtered
and concen~ ed under educed ples~ul~. Colllmn ch~l..~t~, ~h~ of the residue on
silica gel gel (90: 10 methyl-tert.-butyl elhc~ ..-- -!I.P~-O1) yields san~lif. L.l~ A as a white
amorphous solid.
Analogously the compound of formual XVI can be ~ sÇul,lled into s ~ngli r~ ~. ;.. B.
The procedures described in examples 3 to 6 can be used as selectivc intramolecular
protection-deprotection sequences. Thus, by the reaction ~lesnrih~ed in eY~mple 5, the
hydroxyl at position 15 can be selectively protçcte~l which allows the selec~-~_manipulation of the rern~ining free hydroxyls. The procedure in ex~ 5 allows forthe selective protection of both the hydroxyls in the 15 and 17 positionc Both
procedures can also be used as an intr~mok ~ protectirn of the C53 ketone. The
hydroxyls and the ketone can be l. ~nel~t~d by the re~ctionc described in 4 and 6.
Sanglifehrins C and D, as well as the co~ ounds of forTnl~ XV and XVI are
therefore hll~l~ c"..P.~i~tes for the ~e~ dtion of further sanglifehrins.
CA 02224715 1997-12-16
W 0 97/02285 rCT~6/02952
ExamPle 7 ~.,ar..tion of 16-D~h~.lr., 17-D~h~d~..A~-~nvlifehrin A (Formula
XVII)
HO_~, J
'~ NH ~ ~, OH
~o ~NH o o
XVIIHO~
A solution of 54 mg (50 llmol) of the colll;)ou-,d of formula XV and a crystal of
paratolue~s~llfonic ~id monohydrate in 1 mL of 4~ reton;~ ç-water is heated to
80~C for 1.5 hours. The re~tion is q-,cn~l-rA by the ~ itinn of saturated aqueous
sodium bic~bonate solution The resulti~ l~ lul~ is e~ tud twice with ethyl
acetate. The organic layer is washed with saturated ~queo~ sodium bica,l,onate and
brine, dried over anhydrous sodium sulfate, filtered and co~ehl-ated. The residue is
purified by column clho..~tography on silica gel (90:10 methyl-tert.-butyl
ether:mçth~nol), followed by reverse phase chlOn~o~la~)lly (RP18, 50:50
acetonitrile-water to açetonitrile over 45 ...;...ll~s) to yield the pure title co~,i)uulld as a
white amorphous solid.
MS mlz 1078 [M+Li]+ (rel. intensity 100); ~H NMR (DMSO) (only ch&~ct~ ;ctic
signals listed) ~ 1.58 (3H, s, H-50), 1.71 (3H, s, H~9), 2.08 (3H, s, H-54), 4.03 (2H,
d, H-15 and C31-OH), S.57 (2H, m, H-21 and C35-OH), 5.72 (lH, dt, H-27), 5.96
(lH, d, C15-OH), 6.03 (lH, d, H-25), 6.09-6.28 (4H, m, H-18, H-l9, H-20 and H-26),
6.37 ( lH, d, H- 17).
CA 02224715 1997-12-16
WO 97/02285 rCTn~6/02g52
ExamPle 8 - PreDaration of 42~N-methvl ~ _li~f~hr~.~ A (Fonnula 2~VIII) HO_ I J~ J~, ~OH
~ O OH O O ~ OH
~ r N-Me ~ I
~0 CNNH~o o~
XVIII HO~H
To a stirred, cooled (-15~C) soludon of 109 mg (0.1 mmol) of san~l;rlk.;~ A and 67
~L (0.3 mmol) of 2,6-di-tert.-butylpyridine in 1 mL of methylene chloride is added
16.5 ~L of methyl triflate. The llu~lulc is allowed to wann to room tc,lll~.atul~ and
stirring is co..li...~ed for six hours, after which the reacdon is ~ en~ d by ~rliti~n of
saturated ayue~,us sodium bica.lonate soludon. The res~ ing ll~iAIUl~ is e~ll~t~,d
twice with ethyl acetate. The organic layer is washed with brine, dried over anhydrous
sodium sulfate, filtered and concer,t,aled. The residue is purified by two successh,~
c}~omatographies on silica gel (90:10 methyl-tert.-butyl ethel ... ~ ol then 9S:5
methyl-tert.-butyl eth~,, .... Ih~nol) to yield the pure title cG,ll~ou,ld as a white
amorphous solid.
MS m/z 1110 [M+Li]' (rel. inle.,si~ 100); IH NMR (DMSO) (only ch~--t~
signals listed) o 1.70 (3H, s, H49), 2.06 (3H, s, H-54), 3.53 (3H, s, 42 N-Me), 3.98
(lH, d, C31-OH), 4.50 (lH, d, H-65), 4.77 (lH, d, C17-OH), 5.43 (lH, d, C15-OH),5.49(1H,d,C35-OH),7.50(1H,d,H-12),8.11 (lH,d,H-9),9.22(1H,s,C61-OH).
CA 02224715 1997-12-16
WO g7/02285 rCT/EP96/02gS2
ExamPle 9 - PreParation of S3 Dihvdro ~ l;f~h~ in A (Formuh XIX)
HO ~ ~ ~ OH
~, O OH o~,O ~ OH
~~ CN ~) - ~H
XIx HO~,~J
To a stirred, cooled (0~C) sollltion of 54 mg (50 ~ol) of sanglir~h.in A in 0.5 mL of
m~.th~nol iS added 2.8 mg (75 ~lmol) of sodium bo.u},~ide. Stirring is continl~ed for
one hour and saluldt~,d ~1ueouc sodium bic&l,on,it~, is added. The ~ tUlC is e~tracted
twice with ethyl acetate. The organic solution is washed with brine, dried over
anhy~uu~ sodium sulfate, filtered and conçe~ ated. The . The residue is purified by
cl,~ l,atography on silica gel (95:5 methyl-tert.-butyl c~ h~nol followed by90:10 methyl-tert.-butyl ell,~ ol) to yield the pure title co",~und as a white
amorphous solid. The icol-~ product co,,csl,onds to a ca. 1:1 mixture of
diactereoisomers at C-53)
MS m/z 1098 [M+Li]+ (rel. intensity 63), 1104 [M+2Li-H]+ (rel. intensity 100); IH
NMR (DMSO) (only cl~ re.;,~lic signals listed) ~ 0.62 (3H, d, H-50), 1.02 (3H, d,
H-54), 3.55 and 3.59 (lH, 2m, H-53).
56
CA 02224715 1997-12-16
WO g7r02285 r~ 2gS2
Example 10 - PreParation of ~3- Tosvll.~d~ ~or.e, ..-lifehrin A (formula ~X)
HO ~, ~ ~ ~ ~OH
O OH O O ~ OH
NH
~N~)
XX HO~0~ o~o
A mixture of 55 mg (50 ~umol) of ~n~lifehrin A and 23 mg (125 lumol) oftosylhydrazide in 0.5 mL of methylene chlon~e is stirred at room temperature for six
hours. The solvent is ,~;llloved and the residue is p-- ifi~d by chromatography on silica
gel (90: 10 methyl-tert.-butyl ethe..,..- ~h~nol) to yield the title co.-l~oll"d as a white
amolphous powder.
MS m/z 1264 [M+Li]~ (rel. intensity 100); ~H NMR (DMSO) (only ch&act~islic
signals listed) o 1.70 (3H, s, H-49), 1.77 (3H, s, H-54), 2.37 (3H, s, -NSO2C6H4CH3),
6.51 (lH, s, H-60), 6.59 (2H, 2d, H-62 and H-64), 7.06 (lH, dd, H-63), 7.35 (2H, d,
tosyl meta protons), 7.73 (2H, d, tosyl para protons).
ExamPle 11 - ~r~"arat;on of 26S.27S-Dihvdroxv-c~n~lifehrin A (Formula XXI)
and 26R~27R-D:h~JruA~ lirehrin A (Formula xxm
HOJ~ ~ ~ OH
~,0 OH OH ~~,,O ~I~,OH
NH
~O ~ ,NH~
XXI HO~ H
CA 02224715 1997-12-16
W O 97/02285 P~ 9~1/02952
OH I
HO ~ ~ OH
~, O OH OH O~,O ~ OH
,.... ' NH
XXII HO~HN
W
To a stirred, cooled (0~C) solution of 49S mg (1.5 mrnol) of pOtAC~;I.... ferricyanide,
207 mg (1.5 mmol) of potassium c~l,onale, 19.5 mg (0.02S mmol) of (DHQ)2PHAL,
65 ~L (0.005 mmol) of 0.08 M O~ .. tetroxide in t-butanol and 95 mg (1 mmol) of
methyl sulror~ le in 2.5 mL of t-butanol and S mL of water is added a solutiQn of
545 mg (0.5 mmol) of sanglifellrin A in 2.5 mL of t-butanol. The resllltin~ b;l)h~'c
u,e is allowed to warm to room te.l,~.dlu.c and stirred for three hours. Then 1.08
g (8.6 mmol) of sodium sulfite is added, followed by ethyl acetate and water, and the
mixture is vigorously stirred for 15 ~ tl S, The layers are se~aldtt,d and the aqueous
layer is e~l,~led twice with ethyl acetate. The coll.bined organic layer is washed with
sdlul.~t~d ~ queous sodium bic~l~nate and brine, dried over anl~ sodium sulfate,filtered and con~e-~ t~d. The residue is p~l~ifiecl by reverse phase chromatography
(RP18, 30:70 acetor.it ile-water to ~~eto~i~rile over 60 ...;~.u~.,s) yielding the 26S,27S-
diol as an ~-,oll,hous powder.
The co"~s~,onding 26R,27R-diol is ob~h,ed by the above p,ocedu,~, but using
(DHQD)2PHAL instead of (DHQ)2PHAL.
26S,27S-diol: MS m/z 1130 rM+Li]+ (rel. h~tel~sily 100); lH N~ (DMSO) (only
charact~ tir signals listed) o 1.64 (3H, s, H49), 2.06 (3H, s, H-54), 3.20 (lH, broad
m, H-27), 3.45 (lH, broad m, H-31), 3.94 (3H, m, H-17, H-26 and C31-OH), 4.30
(lH, d, C27-OH), 4.57 (lH, d, C26-OH), 5.20 (lH, t, H-23), 5.33 (lH, d, H-25), 5.57
(3H, m, H-18, H-21 and C35-OH), 6.03 (lH, dd, H-l9), 6.14 (lH, dd, H-20).
58
CA 02224715 1997-12-16
WO g7~2285 1~ 6,~29S2
26R,27R-diol: MS m/z 1130 rM+Li]+ (rel. illte~ily 100); IH N~ (DMSO) (only
charact~"islic signals listed) ~ 1.64 (3H, s, H-49), 2.06 (3H, s, H-54), 3.16 (lH, broad
m, H-27), 3.48 (lH, broad m, H-31), 3.94 (3H, m, H-17, H-26 and C31-OH), 4.30
(lH, d, C27-OH), 4.57 (lH, d, C26-OH), 5.20 (lH, dd, H-23), 5.35 (lH, d, H-25),
5.57 (3H, m, H-18, H-21 and C35-OH), 6.03 (lH, dd, H-l9), 6.14 (lH, dd, H-20).
ExamPle 12 - Cleav~oe of the diol in 26S.27S-Dih~,.lru.~y-c~n~lifehrin A
To a solution of 90 mg (79 ~mol) of the 26S,27S diol in 0.9 mL of 2: 1 THF-water is
added 33.7 mg (157 llmol) of sodium periodate. Stirring is col.l;n~ed for one hour and
saturated aqueous sodium bicall,onate is added. The ~uAtule is extracted twice with
ethyl acetate. The organic solution is washed with brine, dried over dnliy~u~ sodium
sulfate, filtered and col-~e ~I.dted. Purification of the residue on silica gel (95:5
methyl-tert.-butylethc. ~.~rlhsnol) yields the co~l~ul.ds of formula X~ (foam) and
XXIV (powder).
~W~
J~o o J
~ f NH ~ XXIII
~o OH
1~~ ~ _~OH
0~,0 ~,OH
XXIV ~ ,NH o o 1;
HO~C~H
formula X~lI: MS m/z 366 [M+H-H2O] ' (rel. iute~ Sily 100); IH N~ (DMSO) (2: 1
mixture of OH~ OH~ C-~ at the anomeric center) (only ch&~h- ;cl;c signals
59
CA 02224715 1997-12-16
W O 97/0228S 1~ 1,5~29S2
listcd) ~ 3.54 and 4.08 (lH, 2m, H-31), 3.57 (lH, broad r4 H-35), 3.66 (lH, m, H-33),
4.38 (0.67H, ddd, H-27,,), 4.95 (0.33H, broad m, H-27eq), 5.40 (0.33H, d, C27-OHeq),
5.59 (0.33H, d, C35-OH), 5.61 (0.67H, d, C35-OH), 5.96 (0.67H, d, C27-OH~,~), 7.89
(0.67H, s, NH-42), 7.91 (0.33H, s, NH-42).
formula X~V: MS mlz 745 [M+Li]~; IH NMR (DMSO) (only ch~ signals
listed) o 0.64 (3H, d, H-50), 0.81 (6H, d, H-56 and H-57), 2.06 (3H, s, H-54), 2.17
(4H, s, H-14 and H49), 3.80 (lH, broad m, H-15), 3.94 (lH, dd, H-17), 5.33 (lH,
broad d, H-23), 5.62 (2H, m, H-18 and H-21), 6.89 (lH, d, H-25), 6.10 (lH, dd, H-
19), 6.18 (lH, dd, H-20), 10.0 (lH, d, H-26).
ExamPle 13 - AcetYlation of S~n~lifehrin A to ~ve 61-O-Acet~l-.canvlifehrin A
(Formula XXV)
HO ~ ~H
~C ~ ~H ~~~ ~' ~H
-~ NH
~ ~ ~N~ ) O
XXV ~~O~ H
To a stirred, cooled (0~C) solution of 54 mg (50 ~Lmol) of sA l~lif~hrin A and 50 IIL of
pyridine in 0.5 mL of methylene chloride is added 5.2 ~IL (55 ~nol) of acetic
anhydride. The reaction is kept at 0~C for one hour, then allowed to warm to room
te~ all~re and stirring is cor~tinl-ed for twelve hours. Satulated aqueous sodium
bic~l,onate is added and the res~lltine rnixture is extracted with ethyl acetate. The
organic layer is dried over anhydrous sodium sulfate, filtered and con~çn~atred. The
residue is purified by reverse phase chlulllàlO~Ia~hy (RP18, 40:60 ~r~lo~ -waterto acetonitrile over 45 ...~ Gs) yielding the title colllpoLI~d as an &llol~holls powder.
MS m/z 1132 [M+H]+ (rel. intc~ y 100); lH NMR (DMSO) (only chal~
signals listed) o 1.68 (3H, s, H-49), 2.06 (3H, s, H-54), 2.25 (3H, s, CH3CO2), 4.04
CA 0222471~ 1997-12-16
W O 97/0228S 1~ 5/02952
(lH, d, C31-OH), 4.67 (lH, d, C2-NH), 4.76 (lH, d, C17-OH), 5.42 (2H, m, H-8 andC15-OH), 5.57 (3H, m, H-18, H-21 and C35-OH), 6.85 (1H, s, H-60), 6.98 (1H, d, H-
62),7.06(1H,d,H-64),7.31 (lH,dd,H-63),7.51 (lH,d,H-12),7.89(1H,s,H-42),
8.23 (lH, d, H-9).
61