Note: Descriptions are shown in the official language in which they were submitted.
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
1 6-T)I~'UR~;ll'l U l l~ I ) ISOC~RO~A'~S FOR TRF.Q.TMF.~T OF
MT~.RAT~n~ ~n~AnAC~n~.~
R~-cRcTRo7uN~ OF T'~F I'iWF.~TION
51. Field of the Tnv~ntinn
The invention relates to isochroman-alkyl-piperazinyVpiperidinyl-aryl
compounds useful for the treatment of hR~ h~R, especially migraine and cluster
h~P~ rhps, as analgesics, and also useful as antipsychotics and for the tre~tmPnt. of
other CNS and/or cardiovascular disorders.
10 2. ne~V~ n of thR Related ~rt
Chromans (also known as l-benzo~yL~s, where the oxygen atom is ~tt~rhe~l
to the aromatic ring) and isochromans (also known as 2-benzopyrans, where the
oxygen atom is not ~tt~rhe~l to the aromatic ring) are known in the art, as are aryl-
piperazines (or 4-alylpi,uel;dines). Chromans and aryl piperazines linked together
1~ with an alkyl chain are also known. European Patent 300,908 ~ closes (1-
benzopyran)]-alkyl-(~i~eldzhlyl or aminopiperidine)-aryls useful as antivsrrythmics
and anti-fibrillatory agents. The co~..puu. ds of this invention require -a'lkyl-
piperazinyl (or piperidinyl)-aryl at carbon 1 of a 2-benzu~y~d~l ring and also require
substitution at the 6-position of the isochroman which are useful for the tre~tnnPnt
20 of vascular (migraine and cluster) hP~ 'hPg and CNS and cardiovascular disorders.
Various isochromans, thini~orhromans~ ben7n~RpinRc, and benzothiRpinRs
with hyvllu~y, alkoxy, or o-methylenR-lim~y snhstitnt;on on their aromatic rings, and
linked to aryl pi~elaz..~es(pipPri-linPs) by alkyl chains are known. These cu~ oullds
are ~i.qrloserl as being useful as anii~yvhotics and hypotensives. The compounds of
25 this invention do not permit oxygen sllhs~'itlltinn on the aromatic ring of the
isochroman, thioisochroman, bPn7~rRpin, or benzothiepin ring system for their
usefulness in CNS and cardiovascular disorders.
Another group of isochromans, thlnj~o~h~ v~ans, bens~ rRpinR~s and
benzothiepines with hydroxy, aLkûxy, or o-methylPnR~ n~y fimrtil)nAlity ~tt~rh~ to
30 their aromatic rings, and linked to aryl piperazines(pipRri~linPs) by alkyl chains are
known, useful as antipsychotics and hy~ùte~sives. The COlllpC ullds of this invention
" do not permit oxygen sllhs~'it~lti-.n on the aromatic ring of the isochroman,
thi~ orhroman~ bPn7mrPpin, or benzothiepin ring system for their usefulness in CNS
t and cardiovascular disorders.
US Patent 4,179,510 and the many diVi~ion~lR thereof rli~rlosRs isochroman-
alkyl-~i~eL~ yl (or aminopiperidinyl)-aryls l~u,ui~hlg oxygen as a sllhstihlRnt on
-1-
CA 02225282 l997-l2-l9
W O 97/02259 PCT~US96/08681
the isoclllo,l,an aromatic ring. These compounds are ~liRrlnsed as being useful as
hypotensive and ~ iy~otic agents.
Al8o~ rl~)secl are isochroman~ othiorhroman-, 2-b~n7n~r~pin-, and -2-
benzothiepin-alkyloxyet~n-lR as being useful for prep~rinF the above compounds.
5 More sperific~lly 7~8--limPthn~ybPr7~xPpiop~ are rliRclose(l as are 1-[(6,7-
~imPthnYyisochroman)alkyl]-4-(aryl)piperazines. Further ~iRrloserl are 2-
b~n7n~rPpinq-alkyl-piperazine(aminopiperidine)-aryls, 2-benzothiPpinR and 2-
ben7m~pin~s all ~ uilillg an oxygen atom as a substituent on the aromatic ring
and useful for the same pu~oses.
Dutch Patent 8,001,981 ~ closqs 1-(2-chlorophenyl)-4-[2-(1,3,4,5-tetrahydro-
7,8 rlimethr~xy-2-bPn7n~ppin-l-yl)ethyl]piperazine useful as an antipsychotic agent.
Intern~;nn~l Patent pllhlir~tinn WO 92/18089 ~ rlospR isoch~ an-alkyl-
piperazinyl (aminopiperidinyl)-aryls, with the requirement that oxygen be present
on the aromatic ring of the isochroman which are useful in spn~it;7inF cells against
lb multi-drug rq~ n~ç.
Intern~tinn~l Patent pllhlir~tion WO 88/08424 ~liRrl~se~s isochromans-alkyl-
piperazinyl (or aminopiperidinyl)-aryls, with the e:~luil~ ent that oxygen be present
on the aromatic ring of the isochroman, useful in the tre~t nPnt of head injury,spinal trauma, and stroke.
Intern~ffon~l Patent pllhlir~tion WO 90/15056 and US Patent 5,140,040
~liRclnse isochromans, tetralins, and dihydro~n~phth~lpnes sllh~ l with various
alkyl amines for the tre~t nPnt of gl~llrcm~, depression, hy~e~ LQnRion, congestive
heart failure and vascular spastic ronrlitionR
US Patent 4,994,486 ~liRrlose~ isochroman-alkyl-~nnines for treating
psychoses, p~rkin~on's ~ e~e, and addictive behavior.
J~psinPse Patent 61083180 ~3iRrloses isochroman-alkyl-(alkyl)~minps as
antiulcer agents.
European Patent 404,197 fliRrloses isochl~..an-alkyl-piperazine-alkyl-keto
(alcohol)-aryls with bronrho~ trr and ~nti~llPrgy activity.
J~r~nP~e Patent 51125287 (J 52083846) ~ rloses isochroman-alkyl-
~minPs(piperazine) with antideples~iv~, analgesic, diuretic, ~ntiinfl~mmP~t,Qry, and
anti-~ m~ activity.
German Patent DE 2,624,693 and Great Britain Patent GB 1552004 (liRrloses
isochroman-alkyl-amines including aryl piperazines as AnAlgP~ir~, hypotensives, ,~
Anti~leprçs~Ant~, diuretics, AntiinflAmmA~orips) muscle rPlAYAnt~, and vARorlilAt~rs.
The compounds differ from the cvl~puu~ds of this invention in that oxygen
-2-
CA 02225282 1997-12-19
WO 97/022S9 PCT/1,7S96/08681
sl7hEtit~l~ion is required on the isochroman aromatic ring.
J~p~n~ce Patent 571~9713 tliR~ isochroman- and tetralin-(no alkyl
spacer)-piperazine-sryls as ~nti~ srgics. The ~v ~ouuds of this invention require at
least one carbon as a linker.
US Patents 3,549,656 and 3,467,675 and Belgium Patent 678,035 ~ ç
phthsll~n-, igochroman-, and isochromen-alkylene-amines for the tre~tm~nt of
depresslon.
Eu~/pesll Patent 458,387 and US Patent 5,137,911 ~ flose isochroman-
alkylene-piperazine-aLkylene-aryls useful as blood pl~telet a~ Lion inhihitors, as
intr~ r calcium antagonists, and for treating cardiac dy~hyLllmiss, angina
pectoris, stroke, and lllyucallial infarction.
(~Tarms~n Patent DE 3,409,612 ~1;R~ S~ rliml~th~Yyisochroman- and
bQn~ pin~-alkyl-amino-alkyls for prophylaxis of coronary heart disease or
hypertencion
Jslrs~nP~e Patent 6 1083180 .~ losç~3 isochroman-alkyl-amines useful for
treating ulcers. ~ ea~ Patent 457,686 ~iR~lnse~ phth~l~n and indane alkyl
aminopir~7- i-linyl ureas or carb~m~t~ for the tre~tm~nt of stress, pain, and
schizophrenia.
J. Med. Chem., 25(1), 75-81 (1982) t~ losçR 6~7-(limpthr~yyisochroman-alk
piperazinyl-aryl type compounds which have hy~o~ sive activity.
US P~l~llt~ 5,032,598 and 5,215,989 generically enromr~ the isochromans
and tetralins of the present invention if the variable snhstitll~nt~ are ~ppl~,~l;ately
chosen.
Tnt*rn~ti~ n~l pllhli(~ti~n No. W0 88/08424 a7ld US Patent 5,120,843 ~ lose
25 a diaLkoxyisocl-l~ ~an C0t~ iF a sllh~l;l .led pyridiyl~iperazil-ylethyl side chain.
However, the compounds of the present invention do not permit alkoxy snh~tit~ n
Intern~t;~n~l pllhli~t;~n No. W0 95/18118 (PCT/US94/13284) ~ s~
various isochromans intlutlinF 6-(snh~L~ le~ mino (6-NRR) and 6-
(snh~l;l ~l~~1)amide (6-CO-NRR) isochromans which are useful in treating hllm~n~who have a central nervous system disorders including psychosis, paraphrenia,
psychotic depression, mania, schizophrenia, schizophreniform disorders. These
c~ poullds are also useful in the tre~tm~nt of vascular he~ he~s~ particularly
migraine h~ he,s Other central nervous system disorders which can be treated
with these c~, LpouLIds include anxiety, drug ~ ff~n, convulsive disorders,
~ecLl~ disorders, personality disorders, ~t~ntion deficit disorders in children and
adults, post tr~llm~ti~ stress syndrome and dysthynua. WO 95/18118 ~ 1OBQ~
-3 -
CA 02225282 l997-l2-l9
W O 97/02259 PCT~US96/08681
racemic 1-(4-mP~ yphenyl)-4-[2-(6-~minfJf A . l.o~ylisochroman-l-yl)-eLllyl~i~erazine
(F~AMPLE 138) and 1-(4-methoxyphenyl)-4-[2-(6-methyl~minoc ~ . I.c,llylisochroman-
1-yl)-eLhyl~i~erazine (EXAMPLE 139).
SU~lvlAR.Y OF I~Vh:l~TION
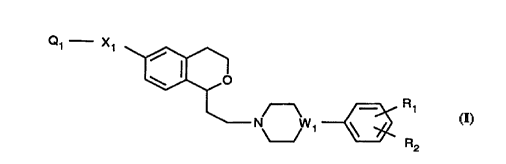
Di~lo~e~l are 1,6-~ hs~itnte~l isochromans offormula (I)
Q~--X~ ~
/~ Rl (I)
N Wl ~ R2
where:
(I) W1 iB a nitrogen (-N-) or carbon (-CH-) atom;
(II) Xl is:
(A) -(CH2)n1- where n1 is 0 thru 3,
(B) -CH=CH-;
(III) Rl is:
(A) -H,
(B) -F, -Cl, -Br, -I,
(C) Cl-C8 alkyl,
(D) C2-C8 alkenyl co~ i..i..g 1 thru 3 double bonds (=),
(E) C2-C8 alkynyl c~nt~ininF 1 or 2 triple bonds (=),
(F) C3-C8 cycloaL~yl,
(G) -Cl-C3 alkyl-C3-C8 cycloalkyl,
(H) -N02,
(I) -C~N,
(J)-CF3,
(K~ -O-Rl l where Rl l is:
(1) -H,
(2) Cl-C8 alkyl,
(3) C2-C8 aLkenyl f;o~t~i..i..F 1 thru 3 double bonds (=),
(4) C2-C8 aLkynyl c-~nt~ininF 1 or 2 triple bonds (=),
(5) C3-C8 cycloalkyl,
-4-
CA 02225282 l997-l2-l9
W O 971022~9 PCTnUS96/08681
(6) -Cl-C3 alkyl-C3-C8 cycloalkyl,
(7) -CF3,
(8) -S02-CF3,
(9) -(CH2)n2-~ where n2 i8 0 thru 4 and where -~ is optionally
5 substituted with one or two:
(a) -F, -Cl, -Br, -I,
(b) -C_N,
(c) -CF3,
(d) Cl-C3 alkyl,
(e) -O-R1 ~A where R1 LA is -H, Cl-C6 alkyl, -CF3 or
-CH2-~,
(f~ NRl lARl lB where the R1 lA and R1 1B are the
same or di~ere~lt and where R1-1B is -H, C1-C6 alkyl, -CF3 or -CH2-~, and where
R1-LA is as defined above,
(g) -co-NRl-lARl-~-B where Rl ~A and Rl ~B are as
defined above,
(h) -S02-NRl lARl B where R1 lA and R1-1B are as
defined above,
(i) -NRl lA-SO2-Rl lB where R1-1A and R1-1B are as
20 defined above,
(j) -N02,
(k) -O-SO2-CF3,
(L) -N(R1 1)2 where the R1 1 can be the same or different and are as
defined above,
(M) -CO-N(Rl l)2 where the Rl 1 are the same or dif~erent and are as
defined above,
(N) -SO2-R1 3 where R1 3 is:
(1) -H,
(2) -CF3,
(3) C1-C8 alkyl,
(4) C2-C8 aLkenyl cont~inin~ 1 thru 3 double bonds (=),
(5) C2-C8 alkynyl c.~..t~i..;..F 1 or 2 triple bonds (-),
(6) C3-C8 cycloalkyl,
p (7) -Cl-C3 alkyl-C3-C8 cycloalkyl,
(8) -(CH2)n2-~ where n2 is as defined above and ~ is optionally
gllh~l: l ., l~l with one or two:
-5 -
CA 0222~282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
(a) -F, -Cl, -Br, -I,
(b) -C-N,
(C)-CF3,
(d) C1-C3 alkyl,
(e) -O-Rl-3A where Rl3Ai8 -H, C1-C6 alkyl, -CF3 or
-CH2-~
(f-) -NRl 3ARl-3B where the R1-3A and R1-3B are the
same or different and where R1 3B is -H, C1 C6 alkyl, -CF3 or -CH2-~, and where
R1-3A is as defined above,
(g) -CO-NRl 3ARl 3B where R1 3A and R13B are as
defined above,
(h) -SO2-NR1 3ARl 3B where R1-3A and Rl 3B are as
defined above,
(i) -NRl 3A-SO2-R1 3B where R1-3A and R1-3B are as
defined above,
(j) -N02,
(k) -O-SO2-CF3,
(9) -O-Rl 3A where R1 3A is as defined above,
(10) -NR1-3AR1-3B where R1-3A and R1-3B are as defined above,
(O) -NRl l-S02-Rl 3 where Rl l and Rl 3 may be the same or dirre~ t
and are as defined above,
(P) -(CH2)n2~p where n2 is as defined above and where -~ is optionally
s~lh;~ l e-l with one or two:
( 1) -F, -Cl, -Br, -I,
(2)-C--N,
(3) -CF3,
(4) Cl-C6 alkyl,
(5) -O-R1 1 where Rl l is as defined above,
(6) -N(Rl l)2 where the Rl ls are the same or dif~erent and are
as defined above,
(7) -CO-N(Rl l)2 where the Rl ls are the saIne or different and
are as defined above,
(8) -S02-N(Rl l)2 where the Rl ls are the same or dirrel~lt and
are as defined above,
(9) -NR1 1-SO2-Rl l where the Rl ls are the same or di~ere~t
and are as defined above,
-6-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
(10) -N02,
(11) -O-SO2-CF3;
(Q) -CO-R1 1 where R1 1 i8 as defined above,
(R) -C~-~-Q1 2 where Q12 is defined below;
. (IV) R2 is defined the saIne as Rl, R2 can be the saIne or different than Rl;
(V) Ql is:
(A) -CO-NQ1 1Q12 where Q11 is
(1) -H,
(2) Cl-C8 aLkyl,
(3) C2-C8 aL~enyl cont~ining 1 thru 3 double bonds (=),
(4) C2-C8 alkynyl cont~ininF 1 or 2 triple bonds (a),
(5) C3-C8 cycloalkyl,
(6) -C1-C3 alkyl-C3-C8 cycloalkyl,
(7) -CF3,
(8)-SO2-CF3,
(9) -(CH2)n7-~ where n7 is 0 thru 4 and where -~ is optionally
8llh~L;~ l with one or two:
(a) -F, -Cl, -Br, -I,
(b) -C=N,
(c)-CF3,
(d) C1-C3 alkyl,
(e) -~-Q1-~A where Q1 1A is -H, C1-C6 alkyl, -CF3 or
-CH2-~
(f~ NQl-lAQl-lB where the Q1-1A and Q1-1B are the
25 same or cli~e~ t and where Q1-1B is -H, C1-C6 alkyl, -CF3 or -CH2-~, and where
Q1-1A i8 as defined above,
(g) CO-NQ1LAQ1 LB where Q1-~A and Q1-1B are as
defined above,
(h) -S02-NQl lAQl B where Q1-~A and Q1- LB are a8
30 defined above,
(i) -NQl-lA-so2-Ql-lB where Q1-1A and Q1-1B are a8
defined above,
(j) -NO2,
(k) -O-SO2-CF3, and where Q1-2 i8:
(1)-H,
(2) Cl-C8 alkyl,
-7 -
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08481
(3) C2-C8 alkenyl CJ--f~ g 1 thru 3 double bonds (=),
(4) C2-C8 aL~ynyl co..1~ F 1 or 2 triple bonds (=),
(5) C3-C8 cycloalkyl,
(6) -Cl-C3 aLkyl-C3-C8 cycloalkyl,
(7)-CF3,
(8) -(CH2)n2-~ where n2 is as defined above and -~ i6 optionally
substituted with one or two:
(a) -F, -Cl, -Br, -I,
(b) -C~N,
(c)-CF3,
(d) Cl-C6 alkyl,
(e) -O-Ql 2A where Q1-2A is:
(i) -H,
(ii) Cl-C6 alkyl,
(iii)-CF3,
(iV) -(CH2)~,
(9) ~(cH2)n9-Ql-2B(cH2)nlo-Ql-2c where ng and n10 are the
same or di~elent and are 0 thru 4, where Q1-2B is -O- or -NQl-2D-~ where Q1-2D is
(a) -H,
(b) Cl-C8 alkyl,
(c) C2-C8 alkenyl c~ t~ F 1 thru 3 double bonds,
(d) C2-C8 alkynyl cont~ininF 1 or 2 triple bonds,
(e) C3-C8 cycloalkyl,
(f~ -Cl-C3 alkyl-C3-C8 cycloalkyl,
(g)-CF3,
(h) -(CH2)nll <p where nll is 0 thru 4 and -~ is optionally
gllh~ ..4~d with one or two:
(i) -F, -Cl, -Br, -I,
(ii) -CaN,
(iii)-CF3,
(iv) Cl-C3 alkyl,
(V) -O-Q1-2E where Q1-2E i8 -H, C1-C6 alkyl, -CF3
or -CH2-~,
(vi) -NQl-2EQl-2F where the Q1-2E and Q1 2F are
35 the same or d~el~nt and where Q1-2F i8 -H, C1 C6 alkyl, -CF3 or C 2
where Q1-2E is as defined above,
-8-
CA 02225282 1997-12-19
WO 97/022S9 PCT/US96/08681
(vii) -CO-NQ1 2EQ12F where Q1-2E and Q12F
are as defined above,
(viii) -S02-NQl 2EQl ZF where Q1-2E and Q1-2F
are as defined above,
(ix) -NQl 2E-SO2-Ql 2F where Q1-2E and Q12F
are as defined above,
(X) -N02,
(xi) -O-SO2-CF3, and where Q1-2C is defined the
Bame as Q1-2D and the Q12C and Q1-2D can be the same or di~elellt, and
where Ql l and Ql 2 are taken together with the ~tt~rhetl nitrogen atom to
form a 5 or 6 member ring which can include one ~ ition~l nitrogen or oxygen
atom;
(B) -S~2-NQl lQl 2 where Q1-1 and Q1-2 are as defined above,
(C) -CO-O-Q1 3 where Q13 is:
(1)-H,
(2) -CF3,
(3) Cl-C8 aLkyl,
(4) C2-C8 alkenyl c.~...ts.i-);..g 1 thru 3 double bonds (=),
(5) C2-C8 alkynyl cont~inin~ 1 or 2 triple bonds (_),
(6) C3-C8 cycloaL~yl,
(7) -Cl-C3 alkyl-C3-C8 cycloalkyl,
(8) -(CH2)n7-~ where n7 is as defined above and -~ is optionally
snhs~ le~ with one or two:
(a) -F, -Cl, -Br, -I,
(b)-C=N,
(C) -CF3,
(d) Cl-C3 alkyl,
(e) -O-Ql 3A where Q1-3A is -H, C1-C6 alkyl, -CF3 or
-CH2-~
(f~ -NQl-3AQl 3B where the Q1-3A and Q1-3B are the
same or di~TeLc:llt and where Q1-3B is -H, C1-C6 alkyl, -CF3 or -CH2~, and where
Q1-3A is as defined above,
(g) CO NQl-3AQl 3s where Q1-3A and Q1-3B are as
defined above,
(h) -so2-NQl-3AQl-3B where Q1-3A and Q1-3B are as
defined above,
CA 0222~282 1997-12-19
W O 97/02259 . PCTrUS96/08681
(i) -NQ1 3A-SO2-Ql 3B where Q1-3A and Q1-3B are ag
defined above,
(j) -NO2,
(k) -0-$02-CF3,
(D) -CO-Ql 3 where Q1-3 is as defined above,
(E)-CO-i~nifl~o~
(F) -NQl lQl 2 where Q1-1 and Q1-2 are as defined above,
(F ) -NQl l-C~-Ql 2 where Q1-1 and Q1-2 are as defined above,
(G) -C(Ql 3)=N-O-Ql 4 where Q14 is defined the same as Q13 and Q1
3 is as defined above, the Q1-3 and Q1-4 can be the same or different,
(H) -SO2-Ql 3 where Q13 is as defined above,
(I) -N(Ql l)-S~2-Ql 3 where Q1-1 and Q1-3 is as defined above,
(J) 6-f~ ole optionally sllh~;t~-tP-1 with one Q15 where Ql 5 is:
(1) -H,
(2)-F,-Cl,-Br,-I,
(3) Cl-C8 aL~cyl,
(4) C2-C8 alkenyl c.~ F 1 thru 3 double bonds (=),
(5) C2-C8 alkynyl ~ol~t~i..;..~ 1 or 2 triple bonds (_),
(6) C3-C8 cycloalkyl,
(7) -Cl-C3 aL~cyl-C3-C8 cycloalkyl,
(8) -NO2,
(9) -C-N,
(10)-CF3,
(11) -O-Ql 6A where Q1-5A is
(a)-H,
(b) Cl-C8 alkyl,
(c) C2-C8 alkenyl c~r.t~i..i..e 1 thru 3 double bonds,
(d) C2-C8 aLkynyl c~ 1 or 2 triple bonds,
(e) C3-C8 cycloalkyl,
(f~ -Cl-C3 alkyl-C3-C8 cycloaLkyl,
(g) -CF3,
(h) -SO2-CF3,
(i) -(CH2)n7~ where n7 is 0 thru 4,
(12) -NQl-5AQ1 5D where Q1-5A is as defined above, Q1 5D is:
36 (a)-H,
(b) Cl-C8 alkyl,
-10-
CA 02225282 1997-12-19
WO 97/022S9 PCTIUS96/08681
(c) C2-C8 alkenyl cont~ining 1 thru 3 double bonds,
(d) C2-C8 aLkynyl cont~inin~ 1 or 2 triple bonds (_),
(e) C3-C8 cycloalkyl,
Cl-C3 alkyl-C3-C8 cycloalkyl,
. (g)-CF3,
s (h) ~(CH2)n7~~ where n7 is as defined above,
(13) -CO-NQ1 5AQl 5D where Q1-5A and Q1-5D are as defined
above,
(14) -SO2-Q1 6K where Q1-5K is:
(a)-H,
(b) -CF3,
(c) Cl-C8 alkyl,
(d) C2-C8 alkenyl cont~ininF 1 thru 3 double bonds (=),
(e) C2-C8 alkynyl cont~inin~ 1 or 2 triple bonds (_),
(f~ C3-C8 cycloaLkyl,
(g) -Cl-C3 alkyl-C3-C8 cycloaLkyl,
(h) ~(CH2)n7~P where n7 is as defined above,
(15) -NQl-5A-so2-Ql-sK where Q1-6A and Q1-6K may be the
same or di~Tele;llt and are as defined above,
(16) -(CH2)n7~p where n7 is as defined above and where -~ is
optionally sl-h~itllt~cl with one or two:
(a) -F, -Cl, -Br, -I,
(b) -C_N,
(c) -CF3,
(d) Cl-C6 alkyl,
(e) -~-Q1 5A where Q1-6A is as defined above,
(f~ -NQl-5AQl-5D where Q1-5A and Q1-5D are as defined
above,
g) Q1-6AQ1-6D where Q1-6A and Q1 5D are as
defined above,
(h) -so2-NQl-6AQl-6D where Q16A and Q1-6D are as
defined above,
(i) -NQ1 5A-SO2-Q1 5D where Q1-5A and Q1-5D are a8
defined above,
(J)-N02,
(k) -O-SO2-CF3;
-11-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
(K) 3 nY~ 701e optionally 81lhE~ with one Ql5 where Ql-5 iB as
defined above,
(L) triazole optionally sllh~ with one or two Ql-5 which may be
the same or di~ele~t, where Ql5 is as defined above,
(M) 5 thi~ ol~ optionally sllhEL:I .Le~ with one Ql 5, where Ql6 i6
as defined above,
(N) 3 thi~ 7O1e optionally sllhstitll~rl with one Ql-5~ where Ql5is
as defined above,
(O) 2-oxazole optionally sllhEL:I ..4.1 with one or two Ql5 which may
10 be the same or difrelent, where Ql5is as defined above,
(P) 2-t~ ole optionally substituted with one or two Ql5 which may
be the same or di~erellt, where Ql5is as defined above,
(Q) 2-imi~ ol~ optionally sllh~ c1 with one, two or three Ql-5
which may be the same or diCr~.e.lt, where Ql5is as defined above,
16 (R) 1-imi~ole optionally sllh~ .l~d with one, two or three Ql-5
which may be the same or dil~ , where Ql5is as defined sbove,
(S) tetrazole optionally sllh ~ 1 e.l with one Ql5~ where Ql5is as
defined above,
(T) cyclobllt~n~1irne optionally sllhsL;~ 1 with one Q11 and one Ql5
where Ql-l and Ql-5 are as defined above,
(U) l-pyrimi~linyl optionally sllh~ ~ :1 .1 e-l with one Ql5~ where Ql5 is
as defined above,
(V) 2-pyridinyl optionally Sllhhil 1 .IDd with one Ql-5~ where Ql5 is as
defined above,
(~1V) 3-pyridinyl optionally Sllhhl ;1 ~ with one Ql5~ where Ql5is as
defined above,
(X) 4-pyridinyl optionally sllhsl:l .led with one Ql5~ where Ql5is as
defined above,
(Y) -Zl-CO-Z2-Ql 2 where Q12 is as defined above and Zl is
-O- or
-NQ1 1- where Ql l is as defined above, where Z2 is
-O- or
-NQ11- where Ql l is as defined above,
with the proviso that when Xl is -(CH2)nl-, where nl is 0 and Q1 is:
-CO-NQl lQl-2~
-12-
CA 02225282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
-S02-NQl lQl-2 or
-NQl-lQl 2
-NQl l-CO-Ql 2 then Ql-l and Ql-2 cannot both be spl~octe
from:
-H,
-Cl-C6 alkyl,
-C3-C7 cycloaLkyl,
-Cl-C3 alkyl-(C3-C7) cycloalkyl and pharmaceutically
acceptable salts thereof.
Also ~ lo,se-l are aromatic bicyclic amines of the formula (ABA)
Ql--X, ~
~ R1 (ABA)
N~ ~W1~ R2
20 where:
(I) Wl is a nitrogen (-N-) or carbon (-CH-) atom;
(II) Xl is -(CH2)nl-, and nl is 0,
(III) Ql is
~ (A) -CO-NQl lQl 2 where Ql-l i8
( 1) -H,
(2) Cl-C8 alkyl,
(3) C2-C8 aLkenyl cont~ininF 1 thru 3 double bonds (=),
(4) C2-C8 aLkynyl c~nt~inin~ 1 or 2 triple bonds (_),
(5) -(CH2)n7~ where n7 is 0 thru 4 and where -~ is optionally
30 sl~hstit~ l with one or two:
(a) -F, -Cl, -Br, -I,
(b) -C-N,
(c) -CF3,
(d) Cl-C3 aL~yl,
(e) -~-Q1-1A where Q1-1A is -H, C1-C6 a~yl, -CF3 or
-CH2-~
-13-
CA 0222~282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
(f~ NQ1-~AQ1 lB where the Q1-1A and Q1-1B are the.
same or different and where Q1-1B is -H, C1-C6 alkyl, -CF3 or -CH2-~, and where
Q1-1A i8 as defined above,
(g) Co-NQl-lAQl-lB where Q1 1A and Q1-~B are as
6 defined above,
(h) -S~2-NQ1 ~AQl B where Q1-~A and Q1 1B are as
defined above,
(i) -NQl-lA-so2-Ql-lB where Q1-~ and Q1-1B are as
defined above,
(j) -N02,
(k) -O-SO2-CF3, and where Q12 is:
(6) C1-Cg aLkyl,
(7) C2-C8 alkenyl co~ i..F 1 thru 3 double bonds (=),
(8) C2-C8 alkynyl contsininF 1 or 2 triple bonds (-),
(9) -(CH2)n2-~ where n2 is as defined above and -~ is optionally
8llhs~ eA with one or two:
(a) -F, -Cl, -Br, -I,
(b) -C_N,
(c) -CF3,
(d) Cl-C6 alkyl,
(e) -O-Q1 2A where Q1-2A is:
(i) -H,
(ii) Cl-C6 aL~yl,
(iii) -CF3,
(iv)-(CH2)-~,
(B) -S~2-NQ1 lQ12 where Q11 and Q12 are as defined above,
(C) -NQ1 lQ12 where Q11 and Q12 are as defined above,
(D) -NQ1 1-CO-Ql 2 where Q1-1 and Q1-2 are as defined above,
(III) R1 is:
(A)-H,
(B) -F, -Cl, -Br, -I,
(C) C1-C8 alkyl,
(D) -C-N,
(E) -CF3,
(F) -O-R1 1 where R1 1 i8:
( 1) -H,
-1~
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
(O C1-C8 alkyl,
(3) -CF3,
(4) -S02-CF3,
(5) -(CH2)n2-~ where n2 is O thru 4
(G) -N(R1 1)2 where the R1 1 can be the saIne or different and are as
defined above,
(H) -CO-N(R1 1)2 where the R1 1 are the same or different and are as
defined above,
(I) -S02-R1 3 where R1 3 is:
(1)-CF3,
(2) C1-C8 alkyl,
(3) -O-Rl-3A where R1-3A is as defined above,
(4) -NRl-3ARl-3B where R1 3A and R1 3g are as defined above,
(J) -CO-R1 1 where R1 1 is as defined above;
(IV) R2 is defined the same as Rl, R2 can be the same or different than Rl;
and pharmaceutically acceptable salts thereof.
Further (~icrlosetl are the aromatic bicyclic amines of EXAMPES 1, 2, 11, 12,
14, 24, 40, 72, 84, 86 and 88.
T)T~,T,~TT ~1) n~CRTRTION OF T~P~ TION
The invention conAictC of novel colllpou~ds, 1,6-~ lh~ l isochroman (I)
and a small group of aromatic bicyclic amines (ABA) which are previously
generically rliRrlose-l in Intern~tion~l pllhlir~tion WO 95/18118 (PCT/US94/13284)
with a unique spectrum of activity, highly active against vascular hP~ rhPs
P~peri~lly migraine and cluster hp~ rhp~s~ The processes used to produce the novel
25 compounds of the rl~im~ invention are known to those skilled in the art. By
starting with the ap~rop~;ate starting m~teri~l~ and org~ni7in~ the process steps in
a particular order (using protective groups where nece~ ) the novel compounds ofthe invention are produced. The process of each step of the invention is known to
those skilled in the art. One skilled in the art given the rhPmir~l structure of any of
30 the 1,6-disllh~ f~ l isochroman (I) or aromatic bicyclic amines (ABA), could
readily prepare the compounds from known cc~ll,uou~ds by mPtho~ known to those
skilled in the art even without the ~;RC11~;On and EXAMPLEs below.
CHART A describes the construction of the 6-bromoi~orhroman (VI), which is
a useful intermP~i~tfe for many of the 1,6-disub~iluLed isochroman (I) and aromatic
35 bicyclic amines (ABA). RP~rti~m of 3-bromophan~th~nnl (II) with ethyl 3,3-
diethu~yulu~ionate in the presence of l~ ... tetr~rhl- ridP in nitromPth~nP or
-15-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
dichloromPt~ne gives the isochroman ester (III). Standard hydrolysis using lithium
hydroxide in THF-water provides the acid (IV), which can be coupled to a variety of
sllhstit~lt~cl arylpiperazines or 4-aLyl,ui~t ~;dines to give the amides (V). The
~Lyl~i,uelazine moiety carries the Rl and R2 sllhstitmpnt~ It is pl~relL~d that the
5 desired R1 and R2 sllhstit~lent~ be on the aryl group prior to the production of the
amide (V). The starting Rl and R2 aryl gTOUpS are known to those skilled in the art
or can be readily prepared by known mPth~ from known cu,lluoullds. Many of the
arylpiperazine moiPt;A are commPrcially available or known in the (-hPmic, l
lilelatu~.:. Those that are not commPrcially available or known can readily be
10 prepared as illustrated in CHARTS Q and R. These amides are reduced using
borane to provide the brAmniRo~hromans (VI).
CHART B describes the cullvt~ ion of the 6-bromriRochroman (VI) into the
cu~ onding 6-amide and 6-ester Qn~logR Conversion of the aryl bromide to the
primary amide is Qccomrli~hp~rl via metal-halogen PY~hQnEe uging t-butyl lithillm
15 and qllPn~.hing the reslllting aryl anion with trimPthylsilylisocyanate, see J. Med.
Chem., 36, 2208 (1993). The aryl anion can also be treated with gaseous carbon
Yi~le, followed by treQtmpnt with oxalyl chloride in DMF and subsequent reactionwith amines to provide the amides (IX) dile~ ~ly. Alternatively, the 6-
brom~iRochroman (VI), can be reacted with carbon mt~n~y~ p in the presence of
20 pQllQrlillm (II) Q~etQt~, 1,3-l~iR-3irhPnylph--?3Fhinoplùpalle, diisu,ulu,uylamine, and
h~ -yl~liRilQ~Qnp in solvents such as DMF to give the amide (VII). Other
pQllQ~illm catlysts, such as in situ pl~aled pQllQrlillm(O) with organorhosphinPR,
or pre-prepared palladium(O) pho~hine catalysts can be llt;li~.e~i The amide (VII)
can be cu~lvt:~led into either snhsl;~ lRcl amides (IX) or esters (X) via the bis-BOC
25 del;v~live (VIII) using the pl~ edul~ described in J. Org Chem., 56, 5482 (1991).
The 6-broml~iRo~hroman (VI) can be cûn~ ,ed to N-methyl sllh ~ -I~cl amides (IX)dil~ lly by using either methylamine or N-methylrnl...Qmi-le in place of
hPYslm~hyl-liQilQ7.QQnP in the pQllQ~lillm mP~liQt~tl reaction ~l~scrihe~1 above (see
~AMPLES 5 and 6). ~lt~rnQtively~ other pQtt~rn~ of N-EnhE~;tllt;on can be
30 obtained by using other primary or secc n~Qry amines in place of
hPYQmethyl(li~ilQ~QnP~ in the pQllQrlillm-mPrliQt~l reaction rl~rihecl above.
CHART C describes the enzymatic resollltinn of racemic (II). MiYing (II) with
an enzyme such as the lipase derived from Psezldomonas cepacia in aqueous buffer(plerel~2d pH 5-8) results in selective hydrolysis of the (-)-ester to give t-h-e (-)-acid
35 (XI). It is pl. r. .L~d to carry out this reaction at room temp- .dlu~e (20-35~) using 5-
20% by weight of the enzyme. The reaction is mnni~red by known means of
-16-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
removing an aliquot, acidifying, and ~Y~ by HPLC. When the reaction is
complete, the products (XI), the (-)-acid, and (XII), the (+)-ester are ~ ~Ovèl'ed and
separated by acid/base ~I-L~cLv~ techniques well-Lnown by those sLilled in the art.
These optically-active cu..ll~.,ullds can be used when appropriate in any of the5 illustrated Charts to prepare optically pure versions of the described compounds.
The undesired ~nAnt;om~r, (+)-ethyl (isochroman-1-yl)Acet~te (XII), .~cvverad
from the Pseudomonas cepacia m~rliAt~1 kinetic resolllt;~n of enAntiomars~ can be
e~ Livèly recycled back to the racemic ~ Lula for subsequent further trAAtnnant
with the Pseudomonas lipase. This ile~r~l,ive process optimizes the overall yield of
10 the desired (-)-isochroman-l-yl-acetic acid (XI). Suitable bases for this rArAmi7Atinn
are those with pKa's greater than 11, preferably greater than 12. Operable basesinclude alkali metal amide bases, aLali metal Alk-Yitl~,s, and alkali metal
carbonat,es which can all induce this rA~emi7~t;on It is pl~arel~d that the base be
alkali metal amide bases or alkali metal ~lkn~i~lç5; it is more preferred that the base
15 be the alkali metal ~lk~ , such as sodillm or pot~ lm t-butoxide or eth~
At the completion of the rAcemi~Ati~ r, the reaction is ql]~nrhe-l with a proton donor.
Virtually any proton donor is operable, for PY~mpl~ even water will quench the
re~rt;~n However, oper~t;~.n~lly water is not p~f~ d. Usually the proton donor
is an acid. Most comm~n proton donors a~ydl.,cllloric acid, Amm~nillm chloride)
20 used to quench enolate anions can be used for this qll~nrhing, however, for ease of
wu~Lup and pllrifirAt;on, acetic acid or trifluoroacetic acid is pr~ ad.
CHART D ~1e~rrihe~ the preparation of amides and esters which are linLed to
the isochroman nllrl~n~ by a one-carbon methylene spacer (I, Xl = -CH2-).
Tr~At~n~nt of the aryl bromide (VI) with ~rim~t~ylsilylacetylene in the presence of
25 p~ lm (II) Aret~t~, copper(I) iodide, and triethylamine provides the acetylenic
isochroman (XIII). R~ction of the acetylenic isochroman (XIII) with a dialkylborane
such as dicyclohexylborane followed by an oxidative work-up using basic h~dL~I~ell
peroxide gives the carboxylic acid (XIV, Ql-3 = H), from which the generalized esters
(XIV) or amides (XV) can be derived by st~n~Ard techniques known to those skilled
30 in the art.
CHART E rl~srrihe~ the preparation of amides and esters which are linked to
~, the isochroman nucleus by a two-carbon spacer which can be either 8d~u~dted (I, Xl
= -CH2CH2-) or lmAAI,~..ated (I, Xl = -CH=CH-). Tr~Atm~nt of the bromide (VI) with
an acrylate ester in the presence of a palladium catalyst, preferably pAllA~ m (II)
35 Aret~, along with 1,3 hiR-liph~nylrho~rhinopropane and diisopr~ylamine in an
organic solvent such as dimetllylru~...Ami~e gives (XVI). Hydrogenation of (XVI) by
-17-
CA 02225282 1997-12-19
W O 97102259 PCTrUS96/08681
s~n-lArd techniques known to those skilled in the art provides the saturated species
(XVII). Similarly, treAtTn~ont of the bromide (VI) with an acrylamide in the presence
of a palladium catalyst, preferably palladium(II) ~ret~te, along with 1,3-
hi~tiirhçnylrhl sphinopropane and dii~;u~urùpylamine in an organic solvent such as
dime~lylro~ mi~ gives ~VIII). IIyd~v~ Ati~n of ~rVIII by standard techniques
known to those skilled in the art provides the saturated species (XIX).
CHART F describes the preparation of amides and esters which are linked to
the isochroman nucleus by a three-carbon methylene spacer (I, Xl = -CH2CH2CH2-).Caubu~ylic acid (X, Ql 3 = H) is treated with two equivalents of propyl lithil~m to
10 provide the buLylvl~henone (X~). ~çAffng to reflux a sol~lt;on of the bu~yLvl)henon~
(X~) in morpholine with an equivalent of ~lem~nt~l sulfur and morpholine for 10 -
20 hours gives the thiolArt~m (XXI, see Org. ReArti~n.~, Vol III, Chapter 2, pp 83,
1946, John Wiley & Sons, New York.) Hydrolysis of the thi- lArt~m (XXI) with
aqueous hy-l~vchloric acid using techniques known to those skilled in the art gives
the c~l,u,.ylic acid (XXII, Q13 = H), from which the esters (XXII) and the amides
(X~II) can be readily obtained using ~.cevu~:s well-known to those skilled in the
art.
CHART G describes the preparation of isochromans bearing a 6-acyl
8llh~itllent such as an acid, an ester, a ketone, or an oxime. Metal-halogen
20 ~Yrh~nFe of the arylbromide (VI) generates an aryl-lithillm reagent which can be
qll~nrh~ with carbon dioxide to give call,u~ylic acids (X, Q13 = H). PAllA~illm-mP~iiAte~ carbonylation of the aryl brom-ide (IV) in the presence of an alcohol
generates the correspon-ling esters (X) via con-1iti-~n~ well-~ocllm~nt~(l in the
literature. Similarly, pAllA~lillm-m~liAt~l cross-coupling of (VI) with enol-ethers
gives rise to k~t~nes (~IV) following st~n-l~rd acidic hydrolysis of the enol-ether
interm~ At~. Alternatively, treAtTn~nt of the carboxylic acid (X, Ql 3 = H) with two
equivalents of an alkyl lithium l~a~ generates the corre.sponrling ketone (XXIV).
ContlQn~Ati-T- of the ketone (XXIV) with either hydlv~ylamine or any readily-
available O-sllh~ l 9~1 hydroxylamine using toluene as the solvent and a Dean-
30 Stark apparatus for water removal provides the desired oximes (~V).
C~IART H describes the preparation of the slllfon~mitles (XXVII) and theglllfnnes (~X). TreAtm~nt of the aryl bromide (VI) with t-butyl lithillm results in
metal-halogen PYrhAnge~ and the r~.slllting aryl lithillm can be qll~nrh~cl with sulfur
dioxide to afford the litl~imn salt (XXVI). This salt is then treated with phosphorûus
35 p~nts~r.hlnri-l-q and the resllltin~ sulfonyl chloride is mixed with the appru~;ate
amine to generate the cc.lle ~,uonding slllf -nAmi-3e (XXVII). ~ltsrnAtively~ aryl
-18-
CA 02225282 1997-12-19
W O 97/OZ2S9 PCT~US96/08681
bromide (VI) is cu~lvtLLed to the aryl lit~ m species as ri~R~rihe~ above a~d
qn~n~heri with the ~p~lùp~;ate ~liRlllfir-ie to give the sulfide (X2~Vlll). This 8ulfide is
then rYirii~etl using st~n-~rd procedures and rYiri~ntR such as m-chloroperbenzoic
acid to give the sulfone (X~X).
CHART I ~ Rrrihe~ the preparation of the sulfones (~IV) in which the
sulfone moiety is linked to the isochroman nllrlr~llR with a methylene tether of 1, 2,
or 3 carbon atoms. In CHARTs I thru N, the extra carbon atom present (which
becomes part of the Xl linker) in the fimr~t;nn~lit;er illustrated nr~cr~ t,e~3 the "n"
to be = 0-2, which cU~-s~on~iR to an nl in the 1,6-disnhsl;l ~.I,Pr isochromans (I). The
calbo~ylic acids (~) can be reduced to the primary- ~lr.nhnl~ (X~) using well-
known techniques and reagents such as lithitlm ~hlminllm hydride or borane. The
nh~ (XXXI) can be cv~ led to the cvl~e~l on~-inE bromides (X~II) using well-
known techniques and reagents such as phr s~horous tribromide or carbon
tetrabromide and triphenylrhosFhine. The bromides (~II) can be used to alkylate
16 thiols using techniques known to those 8killed in the art to provide the ~ulfides
x x x l l l ). The sulfides ~x xx 111 ) can be ~Y~ e(l to the sulfones (X2~V) by using
st~n-i~rd oxidative techniques and reagents such as oY.. ;.. tetroxide and N-
methylmorpholine N-oxide.
CHART J describes the preparation of the 8ll1frnzlmirlr~13(2 ~ Vll) in which
20 the slllfon~mi(le moiety is linked to the isochroman m~ with a methylene tether
of 1, 2, or 3 carbon atoms. The bromides (X~II can be treated with sodium sulfite
in ~ -X;--F 10% aqueous sodium hydroxide solnti~n to provided the snlfon~t~ salts
(XXXV). The slllfon~t~ salts are CVI1~L led to the suLfonyl chlorides (2~X2S.Vl) using
phosphnrous prntS~rh1orirlr~ and phosphorous oYych1nric7e. Tr~ tm~nt of (X2~Vl)
25 with amines (NQl lQl 2) gives the slllfnn~mirl~s (2~Vll).
CHART K ~l~s~rihe~ the preparation of sllh~ ;mitl~o1~ and triazoles
which are linked to the isochroman nucleus with a methylene tether of l, 2 or 3
carbon atoms. In CHART K, when the "X" in the ~nhstitll~nt is nitrogen the
811h~tih1~nt is a tri~o1e and when the "X" is a carbon atom the s11hst;h1F-nt is an
30 imi~l~701e. These cv-llpou~lds are obtained by alkylating the appIv~;ate imirl~o]e
or triazole with the bromides (X~II). The imirl~7ole~ and trisl~ol~!; are either~, commercially available or can be prepared as described in the ~hemic~l lil,eLa~u~e
using techniques known to those skilled in the art. In this fashion are obtained the
compounds (2~X2~Vlll).
3~ CHART L c~e~t~rih~s the preparation of the oY~ 7o1~s (XL) which are linked
to the isochroman nucleus with a methylene tether of l, 2 or 3 carbon atoms. The
-19-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
requisite oxime amides are prepared from the cc,l~ ling nitriles using
hydlu~ylamine hydr)~loride and sodium meW in mPth~nol according to the
plocedu~a ~icrlose~l in J. Med. Chem., 36, 1529 (1993). The nitriles are either
cnmm~rcially available or can be readily pla~,al~ d as degcribed in the ~h~mics~l
5 li~l~Lule: using techniques known to those skilled in the art. The oxime amides are
treated with either sodium hydride or sodium metal and then further treated withthe ester (xxxlx) according to the p~ocellula ~liR~lnse(l in J. Med. Chem., 36, 1529
(1993) to give the heteru~clic products (XL).
CHART M describes the preparation of mono-(XLII) or di-snh,-L:l ~.~d
10 tetrazoles (XLIII) which are linked to the isochroman nucleus with a methylene
tether of 1, 2 or 3 carbon atoms. The bromides (X~II) are col-vt,l L~d to the
col.~s~u~ding nitriles (XLI) via a cyanide ~liRpl~cPmpnt reaction known to thoseskilled in the art. These nitriles are then coll-vt:l L~d to the mono-snh~
tetrazoles (XLII) by the action of sodium azide in a solvent such as N-methyl-2-
15 pyrr~ linonP, accùl.li--g to the ~l~JCedu~ iR~lo~e-l in J. Med. Chem., 38, 1799 (1995).
The mono-snh L:l .l~d tetrazoles are co~lvt:lL~d to the di-sl-hs~itlltRcl tetrazoles
(XLIII) by ~tsntl~rd aIkylation re~ff~nR (R-X"~r~~o~.il ;1P" triethylamine).
CHART N de~rrihP~ the plaparaLion of the isomeric triazoles (XLIII) and
(XLIV) which are linked to the isochroman nllrlPll~ with a methylene tether of 1, 2
20 or 3 carbon atoms. The nitriles (XLI) can be COnvt~ Led to the imi~ln~~~ars (XLII) by
the action of e~h~nr~ hylllvchloric acid according to the ploced,,le ~lic~loRe~1 in J.
Med. Chem., 38, 1799 (1995). Following this same pl~cedula, l1~AI I~P~t of (XLII)
with alkyl hydrazines (either commPrcially available or prepared by means known in
the lilel.llula) in a solvent such as ethAnnl and subsequent treAtTn~nt with formic
25 acid gives a ~il-Lura of (XLIII) and (XLIV). This nli~L,ul2 can be separated into its
components by st~n~rd labolal,uly techniques such as chrom~tl~graphy or
cry8t~11i7A~;~>n .
CHART O describes the preparation of sllhsL;~ l triA7olp-R and nYA~i~7~ R
from primary call,ux~ Ps (VII) using mPt~o~R known to those skilled in the art,
30 see for PYAmplP~ J. Org Chem., 44, 4160-4164 (1979). When "X" in (0-2) is nitrogen,
the product is a triazole. When "X" in (0-2) is oYygen, the product is a nY~ 7ole
Tre~t~nPnt of Amides (VII) with dimethylamide acetals in non-polar, high boilingsolvents such as toluene at 50-100~ generates the int,çrmP~iAte (0-1). This
int-qrmf~iAte iS then reacted with either hydrazine, 1-snh~ e~ hydrazines,
35 hy~lluAylAminP, or N-sllh~ y~lru~ylAminPR under acidic con~litirlnR (usually
acetic acid) at room temperature (20-25~) to give the in~lisAte~ products (0-2).
-20-
CA 02225282 1997-12-19
W O 97/022S9 PCTnUS96/08681
CHART P describes the preparation of mono-sllhs' 1..~ oAazole d~l;v~Livt:s
(P-2) from the co~l~s~onding propargylic amides (~-1) using m~thntlR known to those
skilled in the art, see for ~Y~mrle, J. Med. Chem., 36, 1529 (1993). Tre~t~n~nt of (P-
1) with mercuric acetate in ~ xirl~ acetic acid generates the illu8trated C~ 7~le~S
(P-2).
CHART Q tli~rlnses the synthesis of piperszine (Q-3) in which Rl is an
electron withdrawing substituent ortho or para to the aniline nitrogen of t,he
piperazine.
Amine (Q-1) and aryl halide (Q-2) where a fluorine or bromine atom is ortho or para
to the electron wit,hdrawing sl]hstitll~nt are heated without solvent or in a polar
solvent such as water, DMF, dimethyl~ret~mi~e, or other such solvents with a base
(eit,her excess (Q-l) or diisopropylethylamine, pot~Qqinm carbonate or the like) at
elevated temperature (60-200~) to give piperazine (Q-3).
CHART R ~liqrl~ses the synthesis of piperazines (R-3). Nitro aryl (R-l) is
reduced to aniline (R-2) using hydlu~n and a catalyst such as palladium on carbon,
Raney nickel, stannous rhlorirl~o or the like. Alternatively, (R-2) can be purchased
cQmm~rcially. Aniline (R-2) is then heated (about 80 to aboutl65~) with bis(2-
h~lnethyl)amine hy.llochloride with or without added base in solvents such as THF,
tolll~n~, ethylene glycol, or chlorobenzene to give piperazine (R-3).
CHART S illustrates an the preparation of an important int,Prm~ te useful
for the preparation of compounds cl~im~l in this patent. The hydloAy amide (S-3) is
conveniently prepared from the hydluAy bromide (S-l; see CHART T) either directly
via a p~ lillm m~ te~l ~mitl~t;nn reaction (i~l~nti~l to that illustrated in CHART
B) or via the intermediacy of an ester (S-2). This ester is readily syntl ~qi7~d from
(S-1) via a p~ illm-based call,ullylation reaction known to those skilled in the art
as similar to those already clesrrihe~l The conversion of (S-2) to the amide (S-3) is
~rcompli.qhed by treating (S-2) with an ~l~nhnlir sollltif)n (typically m~th~nol)
co~ g the appropriate amine reagent in a mslnn~r similar to that ~ q-rrihe~l in
J. Org. Chem., 52, 2033-2036 (1987). This reaction can be carried at at room
temperature (20-25~) or preferably at 50-100~.
CHART T illustrates two i. . .~o. L~t alternative approaches to the compounds
r,k~im~rl in this patent. Reduction of the previously described acid (IV) using
st~nrl~rd re~lllring contlit;onq and reagents (p}ef~l,ed is borane) gives the primary
alcohol (S-1). This cum~oulld is then cunvt:l~,ed into the 11YI11UAY a-mide (S-3) as
~3~qrrihed in CHART S. This hydluAy amide is cc,~Led into an alkylating agent
(T-2, typical X is 8 mesylate or a bromide) by s~sn~ rd rh~mir~l transform~ti~nq
-21-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96108681
and is used to alkylate an appropriate 4-arylpiperazine or 4-~ "lpiperidine to provide
the final compounds (IX). AltArn~tively, the hydlu~y bromide (S-1) is collve~ led into
an alkylating agent (T-l, typical X is a mesylate or a bromide) by stS~ntl~rd ~hPmi~
transform~tionc and is used to alkylate an 2l~p.up~.ate 4-arylpiperazine or 4-
5 alyl~ui~ue-;dine to provide the bromides (VI). These bromides are then converted into
final compounds (IX) as previously illustrated.
CHART U tliR~loBes the Cullvè~diOn of chiral bromo acid (U-1) which is (XI) in
CHART C to the amide alcohol (U-5) which is (S-3) in CHART T. The bromo acid
(U-1) i8 alkylated to the bromo ester (U-2) using mPthorlc known to those skilled in
10 the art. An example is tre~tm~nt of the bromo acid (U-l) with 1,1'-
carbonykliimitl~7Qle in a solvent such as THF to form an activated ester, followed by
an alcohol to form the bromo ester (U-2). The bromo ester (U-2) is then treated
under the con~iti-n~ tliRCllcced for CHART V for the con~vel~ion of (V-1) to (V-2), to
give the amide ester (U-3). Hydrolysis of the amide ester (U-3) with aqueous base,
15 taking care not to also hydrolyze the amide group of (U-3), followed by aqueous acid
tre~t~n~nt gives acid amide (U~). The acid amide (U4) is then treated with
re~ inE agents such as borane or borane-methyl sulfide in THF as solvent to givethe amide alcohol (U-5).
CHART V ~1;RC1C~8ÇC a method for the co~lvè~;jiOn of ester (V-1) to ~ mi~le (V-
20 4). Ester (V-1) is prepared from bromo isochroman (T-1) and piperazine (Q-3),CHART Q, by m~thr~lc rliccl-cce.1 for CHART T. Ester (V-1) is Cu~ Led to the
amide ester (V-2) using p~ illm (II) ~etst,e, a co-catalyst such as
bis(diphenylphnsphino)propane, diisopluAuylethylamine, carbon mnnn~i~e and methyl
amine as ~li-cc~cce~l with regard to CHART B. Solvents for the cûllve~i~ion may be
25 chosen from DMF, dimethyl~cet~mi~P, N-methyl~u,...~mi-le and ~cetonitrile with
dimethyl~et~mi~le and N-melhylrul...~mirle p~c:fe-.~d when methyl ~mine gas is
used. Flefe~led tempe.c.~u~es are 50 to 120~. The amide ester (V-2) is further
co~ve~ed to the cu.Lr~l,ontling amide acid (V-3) using aqueous base followed by acid
neutr~li7~t;~n to give (V-3) or a salt thereo~ When the ester is the tert-butyl ester,
trifluoroacetic acid or hyLuchlnric acid in solvents such as ether or ethyl acetate are
used to convert the a_ide ester (V-2) to the corresponding amide acid (V-3). Theamide acid (V-3) t_en is treated with a c- nC~Pn~inF agent and an amine to provide
the Cu~e~On~inF ~ mi-le (V-4) using mPt~o-lc known to those skilled in the art
such as (~iRcll~c~cef~ with regrd to CHART W.
CHART W ~liR~loReR a synthesis of h yd~u~ic acid de-iv~tives (W-7) and (W-
8). These compounds are also prepared by the prùcesses of CHARTS A and B. The
-22-
CA 02225282 1997-12-19
W O 97/022S9 PCTÇUS96/08681
alcohol group of the ester alcohol (S-2), Chart S, i8pL~te~ d with a suitable
~/le.,Lillg group such as a dih~lLo~yL~ulyl group, which is stable to basic conllition
to give tetrahyd~ yLdLlyl ether (~7V-2). The ester group of the ether (W-2) is then
hydrolyzed with aqueous base and then ~ ifif~cl carefully (BO as not to remove the
5 protecting group) to give carboxylic acid (W-3). The carboxylic acid (W-3) is then
treated with a con-lAn~ing agent such as carbonyl~iiTnitl~ole,
diethylcyanorhosphnn~te, dicyclohexylcarbo~iimi-1e, or other suitable con~l~Ancing
agents (see, for AY~mrlA, Major Methods of Peptide Bond ForTn~t;~ n, Volume One of
The Peptides: Analysis, Srth~iR, Biology, E. Gross and d. MpiAnhofA~rl eds.,
10 ~r~lPmi~ Press) in solvents such as dichlornmPth~nP or DMF and a base such astriethylamine in the presence of an amine such as an O-alkyl, N-alkylhydL~.~.ylamine
(itself prepared by the method of Sulsky et al., Tet. Lett. 30, 31-34 (1989) to give
hydl.~ te ether (W 1). The hydl..Y,~ Ate ether (W~) is then deprotected using
m~t~ such as those found in Protective Groups in Organic Synthesis by l'heodora
15 W. Greene and pllhliRhPtl by John Wiley and Sons to give the hy~ t~ alcohol
(W-5). The hydroxyl group of hydL.~x~ te alcohol (W-5) is then COL1V~ d to a
leaving group by one of the many mPthorlR known to those skilled in the art, such as
forming a mesylate, tosylate, or chloride, bromide, or iodide, to give the hy~ x~ t,P
(W-6); the hydl.~ t~ (W-6) i6 then coupled to an amine such a6 piperazine (Q-3)
20 of CEIART Q or piperazine (R-3) of CHART R or comm~rcially available amines to
give hy~ t~ anune (W-7). The hylll..-S~...~te amine (W-7) can be further
cvLlv~lLed to hy~ ~ic acid amine (W-8) when alkyl-1 is a protecting group such
as benzyl by palladium on carbon or other such mPthorlR known to those skilled in
the art.
CHART X .li~ sçs the synthesis of the carbamate (X-6). The phenoVaniline
(X-l) is reacted with alkyl diethc.~y~L~,~L;onate in a similar m~nn~r as the
transformation of the 3-bromophenPtl ~nol (II) to the corresponding isochroman ester
(III) of CHART A, to give the phenol/aniline ester (X-2). The phenol/aniline ester (X-
2) is hydrolyzed to the phenol/aniline acid (X-3) by aqueous base followed by aqueous
acid. The phenol/aniline acid (X-3) is then con-~nRe~ with piperazines (Q-3) of
CHART Q or (R-3) of CHART R or comm~rcially available amines to give the
phenol/aniline amide (X-4) using m~thf~ such as those ~liRcl~RRed in CHART W.
The phenol/aniline amide (X-4) is then reduced to the phenol/aniline amine (X-5)with redll~in~ agents such as borane or borane-methyl sulfide in solvents such as
T~. The phenol/aniline amine (X-5) is then reacted with 1,8-
hjcyclo[5.4.0]undec-7-ene (DBU) or godium hydride or other such bases and an
-23-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
isocyanate in dichloromptll~ne or T~ as the solvent to give the carb~m~tp/urea (X-
6).
CHART Y ~ ç~ the synthesis of racemic (Y-5) starting with the phenol
(Y-1). The phenol (Y-l) is reacted with chlùlv~uropion~ hyde diethyl acetal in the
presence of a Lewis acid such as boron trifll-ori-lP etherate or I :l .. i.. tetr~ohl-)ri~
in solvents such as dichlorom~th~nP or nitrnmPth~nP, to give the chloro phenol (Y-2).
The phenol of chloro phenol (Y-2) is then CUllVel Led to a leaving group using
trifuorom~thAnesl-lfoni-~ anhydride or N-phe~ylL.;~luornmeth~nPclllfonimi~ in the
presence of a base such as triethylamine and optionally adding a catalyst such as 4-
10 dimethylaminopyridine and in a solvent such as dichloromPth~nP~ to give the triflate
(Y-3). The triflate (Y-3) is then be co~ve, Led to the amide chloride (Y4) usingps3ll~ m (II) ~et~e~ a co-catalyst, diiso~u~ylethylamine, carbon mnn~Yi-le and
methyl amine as ~liccllcce-l with regard to CHART W. Solvents for the cullveLdion
include D~, dimethyl~ et~mi~ N-meLhylrul ...Ami-le, and ~-etonitril~ with
15 ~imethylPcet~mi-lP and N-meLllyl~....~micle uiefellèd when methyl Pmine gas is
used. P~èré~èd tempo,dLules are about 50 to about 120~. The amide chloride (Y-4)is then stirred at 60 to 110~ in the presence of the piperazine (Q-3) or (R-3) or
comm~rcial ~minP~, a base such as triethylamine or dii&uu. uuylethylamine, and asolvent such as ethylene glycol, T~, DMF or ~ ~t~ to give the amide amine
20 (Y-6).
CHART Z ~P~rihes the ~l~al~tion of a nnmh~r of aniline-based de-;v~tivès
(Z-2), (Z-3), (Z4), (Z-5), (Z-6) and (Z-7). These cc,luuuuuds arise from si~nrl~rd
derivations of the aniline (Z-1), itself prè~a~ed from the bromide (VI) via metal-
halogen PY~h~nFe (typically using either n-butyllithillm or t-butyllithillm) followed
25 ~c~ n of diphenylrho~Fhoryl azide (usually in THF at -78~) and subsequent
red~lct;~.n with bis(2-mpt~yyetho~y-y)plllminllm hydride. This cu~ .dion of (VI) to
(Z-1) clûsely follows known chPmi~try, see Tetrahedron Letters, 25, 429432 (1984)
and J. Am. Chem. S~oc., 94, 6203-6205 (1972). The reA- ti~nR conve.Li~lg (Z-1) into
the dé~;vdLivës (Z-2)-(Z-7) are standard trAnRformAt~ , known to those skilled in
30 the art, and involve acylations (typically with acid ~-hlori~l~R or anhydrides),
mesylations, and stsn~Ard lactam re-lll. tionR as presviously d~R~rihe~
CHART AA illustrates the preparation of one-carbon hom--logAted
isochroman-6~arl,u~---ifl-ss (AA-5). The sequence involves metal-halogen PY~hAnge
of the bromide (VI) using alkyllit~illm reagênts (typically t-butyl lithillm) followed
35 by qn~n~hin~ of the r~slllffng anion with D~ to give the aldehyde (AA-1). This
aldehyde i8 reduced using st~n~rd reA~ntR (such as sodium borohydride in THF),
-24-
CA 02225282 1997-12-19
W O 97102259 PCTnUS96/08681
and the resulting alcohol (AA-2) iBConv~l led to the nitrile (A~-3) by activation with
m~th~ne~ f ~nyl chloride and fii~pl~ m~nt of the r. sllltinF mesylate with cyanide
anion. Hydrolysis of the nitrile i6 carried out by treating a solution of (AA-3) in
D~ with 30% h~d. v~.. peroxide in the presence of potassium carbonate and
5 stirring the reaction llli~llUle at room t~lllpeld~ul~ (20-25~) for 20 hrs. The resulting
amide (AA-4) i6 convt,~Led into snh~i;l .(etl a_ides (AA-5) as previously described.
CHART BB illustrates a generalized procedule: for the preparation of tethered
amines such as (BB-2) by reclllrti~n of the corresponding a-m-ides (BB-1) ntili7in~
~t~n~1~rd amide reduction con lition~ as previously des-~riheri (typically either
10 employing borane or lithium alulllillu~l hydride in THF).
CHART CC illustrates that fi~nrt;~n~l groups on the arylpiperazine portion of
these molec~llPs (ie, Rl and R2) can be transformed into other fi~n~t;~n~l groups.
Depicted is a standard hy~llù~t~nolytic debenzylation of the an aryl-ether (CC-1) to
provide the cul~e~ollding phenol (CC-2). Conversion of the phenol (CC-2) into the
15 corl~s~onding trifluor m~th~n~st~lfon~t,e (CC-3) by standard m~thofl~ is illU~Lldtive
of typical derivations of phen~ such as (CC-2). Conversion of the triflate (CC-8)
into numerous dt:Livt ~,ivt:s can be ~ccomrli~hf~l by p~ ]m-mf~ t~r~ collplin~
For ~Y~m~]~, coupling (CC-3) with enol-ethers provides ketone-sllh~ Pr1 aryl
d~.;v~Liv~s. These r~rt;-n~ are typically carried out in DMF or ~etonitrile using
20 p~ lillm(II) ~et~te~ 1,3-bis(diphenylphosphino)propane, and triethylamine at
elevated tempe.d~ult,s (50-120~).
CHART DD illustrates an ~ltern~t;ve preparation of isochroman-6 tri~oles
(DD-4) and isochroman-6-o~ 70les (DD-6). The primary alcohol of the previously-
described amide (DD-1) is be l lute~ Led by Et~n~rd techniques, preferably as the
25 benzyl ether (P = -CH2-phenyl). This material is be reacted with amide acetals as
described in CHART O and the resulting interm~ t;e (DD-2) is treated with
hydlr zi~e, sllh~L~ l hy.lldzi~le, hydlu~yl amine, or N-snh~;t~ltR~l hy ;ll~u~yl ~mines
as tl~srrihe~l in CHART O to generate the tri~ol~s (DD-3) or n~ 701ec (DD-5).
The plole- ti~lg group "P" is rellluve d using eton-l~rd contlition~ (typically
30 hydlù~ olysis using a tr~n~i~;on metal catalyst such as p~ linm or platinum) and
the re~lllting alcohol can be activated (usually as a snlfor ~te ester or halide) and
reacted with the d~,ulu~llate aryl piperazine as described previously in CHART T.
For the 1,6-~ llh~L;l ~L~c~ isochlulllans (I) it is pr~Çellad that nl is 0 or 1; it is
more plarell~ d that nl is 0. It is ,ul~fell~d that Rl is -O-Rl l, -CF3, -CO-N(Rl l)2,
35 -CO-Rl l and it is ~l~Ç~l.ad that Rll is Cl-C3 alkyl. It is pl~:r~ d that R2 is H.
It is plerell~d that Q1 is selected from the group con~ ;ng of -CO-NQ1 1Q12~ -S~2-
-25-
CA 02225282 1997-12-19
W 097/022S9 PCT~US96/08681
NQ1 1Q1 2 and -NQ1 1Ql 2; it i8 more prefellad that Q1 iB -CO-NQl lQl 2.
For the aromatic bicyclic amines (ABA) it i8 ~ul~r~ d that Wl iB nitrogen
(-N-) and it is plefellad that one of Rl or R2 i8 -H. It iB pr~r~llad that Q1 i8 (A)
-CO-NQl lQl 2 and that Q11 is -H and that Q12 i8 -CH3 (C1 alkyl).
The 1,6--liRl1hb~ I,ecl isochroman (I) and aromatic bicyclic amines (ABA)
contain an asymmetric center and therefore produce two Pn~ntir~mPrs one "S" which
is (-) and the other "R" which is (+). In some cases both en~ntiomp-rs (+) and (-) are
useful in the same way as the optically impure (racemic, i) .~ I,ula. Hence, they
may be utilized in the racemic form without se~u~clLi-~g them. However, if it isdesired to utilize one of the çn~ntiomP,rs, the optically impure ~ ,Ul~: or
intermP~ tç can be resolved by means known to those skilled in the art. It is
preferable to resolve the racemic intermP~ t~ (II) using the lipase mPthod described
in C~IART C, alternatively rhPmir~l mPtho~ known to those skilled in the art canbe used, see for PY~mplP, Optical RPsollltion Procedures for ChPmir~l Compounds,Vol 1, ~minPs and Rel~t~d Co l,uuuuds, Paul Newman, Optical Resolllti~n Informa-tion Center, M~nh~ttDn College, Riverdale, NY, 10471, 1978. The optically impurei~Lula can also be separated using chrrm~ . ~hic techniques on chiral
b~Dtir~n~ry phages, see Chrom~t~graphic ~ln~nt;r~sep~ration~ 2nd edition, John Wiley
& Sons, NY, 1992. These optically pure cu~,uuullds are then used in the same wayas the racemic llli~Lu~t:. When used in this patent applir~t;on the term 1,6-
~;R11h~ 1 isochroman (I) aromatic bicyclic amines (ABA) refers to and inrhl~lPR
both çn~nt;omPrs as well as optically impure forms thereof, the most c~lmmon of
which is a racemic lllL~Lule7 (~, dl).
Some 1,6-disnh~L;l~le(l isochroman (I) and aromatic bicyclic amines (ABA)
contain two asymmetric centers and therefore four stereoiRomPrs (SS, RR, SR, RS)exist producing two diastereomeric pairs of ~n~ntiomprs~ one SS,RR and the otherSR,RS. The diastereomeric pairs of çn~nti~mprs can be readily separated by meansknown to those skilled in the art. When used in this patent applir,~tion the term
1,6-~1;R11h~ isochroman(I) and aromatic bicyclic amines (ABA) inrln(la~ all four~nslnt;~mf~rs as well as optically impure forms thereof, the most commr~n of which is
a racemic ~xLul~
The 1,6--liR~hbL:I ~L~l isochroman (I) and aromatic bicyclic amines (ABA) are
slminPS, and as such form acid ~lrlit;- n salts when reacted with acids of s--ffiriPnt
strength. Pharm~rellt;r,~lly acceptable salts include salts of both inorganic and
organic acids. The ph~rm~r,eutically acceptable salt8 are s~mPt;m~qs but not always
pLef~ . . ad over the corresponding free amines since they produce compounds which
-26-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
are more water soluble and more crysts11ine The ~.~f~ d pharm~relltir~lly accep-table salts include salts of the following acids mPt~ neclllfonic, h~.hucllloric,
hydrobromic, sulfuric, phosphoric~ nitric, benzoic, citric, tartaric, fumaric, maleic,
CH3-(CH2)~,-COOH where n is O thru 4, HOOC-(CH2)n-COOH where n is as defined
5 above.
The 1,6~ 11hstituted isochroman (I) and aromatic bicyclic amines (ABA) of
this invention posses selective pharm,.~A,o1ngirA1 plop~lies and are useful in treating
hllm, n~ with vascular heArl~Arh~s, particularly migraine and duster he~ rhp~ The
1,6-~i~11h~ .1 isochroman (I) and aromatic bicyclic ,.~nines (ABA) are also useful
10 as analgesic agents.
In clinical practice the 1,6-~ 11h~L;~ .l isochroman (I) and aromatic bicyclic
amines (ABA) of the present invention will normally be ~-lmini~tP~red orally, nasally,
rectally, vaginally or by injection in the form of pharm, re11t;As~1 compo~it;~An~
c.~l1f ~i.,i..g the active ingredient either as a free base or as a pharmaceutically
acceptable acid ,.~1~fition salt in, RRo~iAtion with one or more phar~n, relltict~lly
acceptable carriers. It is p~er~ d that the 1,6-~ qllh~L;~uled isochroman (I) and
aromatic bicyclic amines (ABA) be ~ lmini~t~ ed either orally or nasally.
For the~ap~uLical treatment of migraine or cluster hePtlArhPs and for
trÇ~tnA~nt of pain as analgesic agents the suitable daily do~es of the 1,6-
rfiRllh~lil uf~,e~f isochroman (I) are aromatic bicyclic a_ines (ABA) are from about
0.005 to about 60 mg/kg for oral or nasal app.1i~tiAn, preferably from about 0.1 to
about 30 mgtkg, and from about 0.05 to about 10 mg/kg for parenteral applirAtiAn,
preferably from about 0.03 to about 3 mg/kg. The use and 7~rlmini~tration to a
patient to be treated in the clinic would be readily apparent to a person of ordinary
skill in the art.
The exact dosage and frequency of ,~lminir~ration ~epen-lR on the particular
1,6-~iC11h~ a~ isochroman (I) or aromatic bicyclic amine (ABA) used, the
particular con~liti~A,n being treated, the severity of the c ~n~liti~An being treated, the
age, weight, general physical cor.-lit;cn of the particular p~t;AA~t~ other mrtliC~ti9Tl
the individual may be taking as is well known to those skilled in the art and can be
more ac.;u~a~ely dete~ ed by measuring the blood level or Aon~r~ Lion of the 1,6-
hs~ isochroman (I) and/or aromatic bicyclic amine (ABA) in the patient's
blood and/or the patient's response to the particular cAnrlitiAn being treated.
nP~TNITION~: ~) CONVh~l~TION~
The ~finiffonR and _~p1An, tionR below are for the ter-m-s as used throughout
this entire clocllm~nt inr111rlinF both the ~perifirc~;An and the claims.
-27-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
I. CONV~ TION,q FOR FOR~UT ~ A~T) T)F.~I~ITIONS OF V~RTART.
The rhPmir~l form~ R repl-~A~ g various compounds or mohpc~ r
fr~gmPntA in the sperifir~ti~ n and clPims may contain variable snhstit~llpntA in
f3tl~1it;~n to expressly defined structural feaLur~s. These variable sllhs~itslpntA are
i~1PntifiPrl by a letter or a letter followed by a nllmpric~l subscript, for PY~mIlle~ "Zl"
or "Ri" where "i" is an integer. These variable sllhstit~lp-ntA are either monovalent or
bivalent, that is, they r~pl. ~e,1t a group ~t~rhP-l to the formula by one or two
rhPmir~l bonds. For example, a group Zl would represent a bivalent variable if
~t~rhPcl to the formula CH3-C(=Zl)H. Groups Ri and Rj would leplesent monoval-
ent variable sllhstitllpntA if ~tt~ ?tl to the form~ CH3-CH2-C(R~ )-H. When
rhPmir~l formnl~A are drawn in a linear f~hil n, such as those above, variable sub-
stitslent~ contoinpcl in parPnt~e~es are bonded to the atom immP~liAt~ly to the left of
the variable ~llhstit~lPnt enrlosed in parPnthPAiR. When two or more c~nAe. .~i ;v~
variable sllhs~;hlentR are enrlose-l in parenth~oses~ each of the con~e~ ;ve variable
sllhstitllp-ntA is bonded to the immP~ t~ply ~ edi~g atom to the left which is not
Pnrl~se-l in parPnthPseR Thus, in the forsnula above, both Ri and Rj are bonded to
the prece~ing carbon atom.
Ch~mic~l formnl~A or portions thereof drawn in a linear fashion l~pl~e t
atoms in a linear chain. The symbol "-" in general ~ e~l~ a bond between two
atoms in the chain. Thus CH3-O-CH2-CH(Ri)-CH3 ~ s~ a 2-sllh~ ule~l-1-
metho,.y~Lvpane co ,puu~d. In a siilar f~Rhion~ the symbol "=" ~ep~esellts a double
bond, e.g., CH2=C(Ri)-O-CH3, and the symbol "_" 1ePL~:Se~t~ a triple bond, e.g.,HC=C-CH(Ri)-CH2-CH3. Carbonyl groups are ~. ples~tP~ in either one of two
ways: -CO- or -C(=O)-, with the former being piefe,~ed for Aimplirity.
~hPmirs~l formlllsi~ of cyclic (ring) cu~,~uul ds or m~lPcnl~r fr~gmPntR can be
Lepr~se..tPd in a linear f~hiorl Thus, the c v~llpoulld 4-chloro-2-mel~1yl~yl;dine can
be ~ sf~..t4~l in linear fashion by N =C(CH3)-CH=CCl-CH=C H with the
cv11ve llLion that the atoms m~rk.orl with an ~riRk (*) are bonded to each otherrP~nlt;nE~ in the form~t;on of a ring. Li_ewise, the cyclic ml~lpclll~r frSIgmPnt 4
30 (ethyl)-l-~ipt.~i11yl can be le~ te~l by -N -(CH2)2-N(C2H5)-CH2-C H2.
A rigid cyclic (ring) structure for any co...~uu lds herein define8 an orient~t;~n
with respect to the plane of the ring for ~nhS~;t~lpnt~ ~tt~rh~l to each carbon atom of
the rigid cyclic co ,~uuulld. For saLulalad cvlll,uuullds which have two snh~;t,llPntR
~h~d to a carbon atom which is part of a cyclic system, -C(Xl)(X2)- the two sub-35 st;tnPnt,R may be in either an axial or equatorial position relative to the ring and
may change between axial/eqll~ri~l However, the po~it;on of the two snhs~;t~lentR
-28-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
relative to the ring and each other remains fixed. While either sllhE~ihl~nt at times
may lie in the plane of the ring (eqllAtwriAl) rather than above or below the plane
(axial), one substituent is always above the other. In rhemirs~l structural formulas
depicting such compounds, a sl~hs+it,~lçnt (X1) which is "below" another snhs+it~pnt
5 (X2) will be i-lentifiç~l as being in the alpha (a) configuration and is i(lentifipd by a
broken, dashed or dotted line ~tt~rhmpnt to the carbon atom, i.e., by the symbol"- - -" or "...". The corresponding sllhstitllPnt Att~~hP~l "above" (X2) the other (X1) is
i~ent;fiP~ as being in the beta (13) configuration and is in~lirslte~l by an unbroken line
~t+~ l m~nt to the carbon atom.
When a variable sllhst;t~lPnt is bivalent, the valences may be taken together
or separately or both in the rlPfinitio~ of the v~ri~hle For PY~m~ a variable RiAtt~'h~Pd to a carbon atom as -C(=Ri)- might be bivalent and be defined as oxo or
keto (thus forming a carbonyl group (-CO-) or as two separately ~tt~rhPCl monovalent
variable sllhstihlPnt~ a-Rij and J3-Ri k. When a bivalent v~ri~hlç, Ri, is defined to
15 consist of two monovalent variable sllhE~;hlPnt~, the convention used to define the
bivalent variable i8 of the form "a-Rij:~-Ri k" or some variant thereof. In such a
case both a-Ri j and B-Ri k are ~tt ~hP~l to the carbon atom to give -C(a-Rij)(13-R~ k)-
. For PYAmrle, when the bivalent variable R6, -C(=R6)- is defined to consist of two
monovalent variable sllhA~;tllPntc~ the two monovalent variable sllhE~itllpnt~ are a-
20 R6 1:B-R6 2~ .... a-R6 g:~3-R6 1o, etc, giving -C(a-R6 1)(~3-R6 2)-, ~--- -C(a R6 9)( 6-10
etc. Likewise, for the bivalent variable R11, -C(=R11)-, two monovalent variableEllhEt;t~lPnt~ are a-Rll l:J3-Rll 2. For a ring sllhstit~lPnt for which separate a and B
~riPnt~tion.A~ do not exist (e.g. due to the presence of a carbon carbon double bond in
the ring), and for a sllhst;tmPnt bonded to a carbon atom which is not part of a ring
25 the above co~v-:n~ion is still used, but the a and ~3 dPA~ignAtir~nr. are omittP-l
Just as a bivalent variable may be defined as two separate monovalent
variable ~llhA~it,~lPntA~, two separate monovalent variable sllhst;t~pntr~ may be defined
to be taken together to form a bivalent vAriAhlP For PY~mrl-p~ in the formula
-C1(Ri)H-C2(Rj)H- (Cl and C2 define arbitrarily a first and second carbon atom,
30 respectively) Ri and RJ may be defined to be taken together to form (1) a second
bond between Cl and C2 or (2) a bivalent group such as oxa (-O-) and the fnr nnl~
thereby describes an epoxide. When Ri and Rj are taken together to form a more
comrhPY entity, such as the group -X-Y-, then the oriPnt~tion of the entity is such
that Cl in the above fi~rmlll~ is bonded to X and C2 is bonded to Y. Thus, by
35 convention the dçRigl~Ation "... Ri and R,j are taken together to form -CH2-CH2-O-
CO- ..." means a lactone in which the carbonyl is bonded to C2. However, when
-29-
CA 02225282 l997-l2-l9
W O 97/02259 PCTnUS96/08681
cleRign~3t,P~cl 1'... R~ and R~ are taken together to form -CO-O-CH2-CH2-the ~unv~Lion
means a lactone in which the ca,l"~llyl is bonded to Cl.
The carbon atom content of variable 8llhstihl~nt.~ i8in~ tR~ in one of two
ways. The first mPthod uses a prefix to the entire name of the variable such as "C1-
5 C4", where both "1" and "4" are integers le~l~PAellt:rlg the minimnm and m~x;.......number of carbon atoms in the variable. The prefix is separated from the variable
by a space. For PY~mrle, "C1-C4 aLkyl" l~resellts alkyl of 1 through 4 carbon
atoms, (inrlllrling isomeric forms thereof unless an express intlit~t;on to the contrary
is given). Whenever this single prefix is given, the prefix in~ tes the entire carbon
10 atom content of the variable being flPfinP~ Thus C2-C4 alkoxycarbonyl flPR~rihes a
group CH3-(CH2)n-O-CO- where n is zero, one or two. By the second method the
carbon atom content of only each portion of the tlefinitinn is in~ te~l separately by
Pn~-loAing the "Ci-Cj" deAign~ti-n in parRntl~eseA and placing it imme~izi~ely (no
ing space) before the portion of the rl~finition being d~finA-l By this
15 optional collvenLion (C1-C3)aL~u~,carl,u~yl has the same mR~ning as C2-C4 alkoxy-
carbonyl because the "C1-C3" refers only to the carbon atom content of the alkoxy
group. Similarly while both C2-C6 alkoxyalkyl and (C1-C3)aLkoYy(C1-C3)alkyl define
aL~coxyalkyl groups cont~ininF from 2 to 6 carbon atoms, the two ~lRfiniti.~n~ differ
since the former flRfinition allows either the alkoxy or alkyl portion alone to contain
20 4 or 5 carbon atoms while the latter ~lPfiniti~n limits either of these groups to 3
carbon atoms.
When the claims contain a fairly complPY (cyclic) substituent, at the end of
the phrase n~min~PAign~tin~ that particular sllhstit~lRnt will be a notation in
(parRnthRses) which will cu..e ~,uulld to the same name~le~ign~ti~n in one of the
25 CHARTS which will also set forth the ~ hRmi~l structural formula of that particular
Sllhstit-uPnt
!qt~tpmpnt Ahr~ut N~mRn~ tllre
Several methods exist for the r~slmin~ of the compounds of this invention,
~iffPrin~ prinl ir~lly in the use of the term "isochroman" or "3,4-dihydro-lH-2-
30 benzopy~dnll for the bicyclic group within the compound. For PY~mple, one name forthe compound of ~Y~mrle 6 is (S)-(-)-1-[2-[4-[4-(~minoc~.lJollyl)phenyl]-1-piperazinyl]ethyl]-N-methylisochru"lan-6-c~1,..~ P or (s)-(-)-l-(bRn~mi(l~p4-yl)4
[2-(6-methylAminoc~rbonylisochroman-l-yl)eLhyl~ui~uelazil~e. Another name is (S)-l-
[2-[4-[4-(~mino~A~bù~yl)phenyl]-l-pi,u~ zillyl]ethyl]-3,4-dihydro-N-methyl-lH-2-
35 benZu~yl~11-6-Ch~l~u,c~ R.
-30-
CA 02225282 1997-12-19
W O 97/022S9 PCT/U',S.r~6~1
TT n~,~q~lTIONS
Als t~e~e~d~use8 are in degrees Centigrade.
TLC refers to thin-layer chrom~t~graphy.
HPLC refers to high plef~2~uS~ liquid chrom~tc,~.dphy.
THF refers to tetral-ydl~rulz~.
DMF refers to dimethylrul...~mi~l~
DMSO refers to dimethylgnlf Yi-l~
LDA refers to lithium diisopropylamide.
p-TSA refers to p-toln~nesulfonic acid monohydLd~e.
TEA refers to triethylamine.
BOC refers to 1,1-dimethylethoxy carbonyl or tert-blslu,.y~iarbonyl -CO-O-
C(CHS)3.
DMAP refers to dimethylaminopyridine, (CH3)2N-pyridin-l-yl.
TFA refers to trifluorscetic acid, CF3-COOH.
Saline refers to an aqueous ~alusa~d sûdium chloride sohlt;~n
Chr~m~. dphy (column and flash chro~ . d~hy) refers to
pnrifi~ti~m/geparation of cul-lpouslds é~ è6~ed as (~u~os 1" eluent). It is understood
that the appropriate fractions are pooled and c~n~~..t-dted to give the desired
co uuu~ d(s).
IR refers to inrlased spe~lsusc~llJy.
CMR refers to C-13 ms~gn~t;~ re~on~n-e ~,ue~,Lsv~cv~uy~ fh~mirsll shifts are
se:~olled in ppm (8) downfield from TMS.
NMR refers to nuclear (proton) m~gn~ti~ resrn~n-e spe~L-)scopy, t~.h~mi~l
shifts are reported in ppm (o) downfield from tetramethylsilane.
~p refers to phenyl (C6H5).
[a]D refers to the angle of rotation of plane polarized light (specific optical
rotation) at 25~ with the sodium D line (589A).
MS refers to mass ~ue.;~l~"5.etry ~lessed as m/e or mass/charge unit.
[M + sHl+ refers to the ,uo~i~ive ion of a parent plus a hydrogen atom. EI refers to
electron impact. CI refers to th~mir~l ioni7slti~n FAB refers to fast atom
bombardment.
HRMS refers to high re~ohltion mass spectrometry.
Ether refers to diethyl ether.
Pharmaceutically acceptable refers to those ,uS~per(~ies and/or sllhstsncP~
which are acceptable to the patient from a pharm~coltgi~l/t~ ol~ gi~l point of'
view and to the m~nllf~tnrinF ph~rm~euticalrh~mi~+ from a phygica~h~mi~
-31-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
point of view re~ Lg c~ l,o~il,ion, form~ t;on, stability, patient accep~a lce and
bioav~ hility.
When solvent pairs are used, the ratios of solvents used are volume/volume
(v/v).
phslrm~eutically acceptable anion salts include mesylate, ~hlnride~ sulfate,
phosphflte nitrate, citrate, CH3-tCH2)nl-COO-1 where nl is 0 thru 4, -looc-
(CH2)n1-COO~1 where n is as defined above, -lOOC-CH=CH-COO-1, ~-coo-1,
F~ANrPT,P'..C~
Without further elaboration, it i8 believed that one skilled in the art can,
10 using the prece~ g description, practice the present invention to its fullest extent.
The following ~t~ mpl~s (l~R~rihe how to prepare the various compounds
and/or perform the various p,oce~es of the invention and are to be construed as
merely illus~ldl~ive, and not limit~ffonR of the prece.li--g disclosure in any way
whal,soevt:r. Those skilled in the art will p~ tly recogni~e applopl;ate variations
15 from the p~OCédllleS both as to rçn~ and as to reaction cnn~itionR and
techniques.
PREPARATION 1 4~Piperazin-1-yl)bPn7s-mi~
A ll~i~l~ue of 4-fluorobçn7~mi-1~ (2.45 g, 17.6 mmol), piperazine (7.56 g, 87.9
mmol) and water (10 mT ) i8 stirred at 100~ for 24 hr. APcer cooling, the solid is
20 coll~ctç~l and washed with water and toluene and then dried under reduced ~ L_Juè
to give the title c~lllpc.ulld, mp = 240-248~; MS (M/Z) at 205; NMR (DMSO-d6) 2.59,
2.80, 3.14, 6.90, 7.02, 7.72 and 7.73 o.
PREPARATION 2 N-[4-(Trifluoromethyl)phenyl]piperazine
A 100 mT- flask e.lui~ped with spinbar was charged with 4-
25 bromobenzotrifluoride (19.70 g, 0.088 mol) and ~i~elf~zille (37.71 g, 0.438 mol). There~r~nt~ were heated to 130~. After 48 hours, the ~ulè cnntoin~ nifi~nt
amounts of p~ ~ and is cooled to 20-25~. During the cooling, the reaction
,Ule iS diluted with sodium hydroxide (3N, 200 mL) r~s--lting in s--ltlit;ons~l
allwull~ of ~re :l,;'Dl~ and i8 extracted twice with ethyl acetate (200 mT-). If the
30 reaction ~Lulè is allowed to cool to 20-25~ before adding aqueous base, the
reaction becom~s a solid mass m~king further maniplll~t;onR ~lifficlllt~ The comhine
organics are washed once with saline (300 mT-), dried over m~f~nPcium sulfate,
filtered, and cor.c~ a~ed to give the procuct. Recryst~lli7~t;rln~ from h~Ynn~ gives
the title ~,v~uu~ld, mp 87-89~ (lit. 86-88~), Rf = 0.20 (mPth~n-~/dichior mf~t~n~,
35 7193).
EXA~LE 1 (S)-(-)- 1-[2-[4-(4-M~h ~~Yyphenyl)- 1-pipc. ~illyUethyl]-
-32-
CA 02225282 l997-l2-l9
W O 97/02259 P~1/~ J~08681
isochroman-6-c~l,...~1...i-le (S)-(VII) also known as (S)-(~ (4-
M~thnY~yphenyl),4 [2-(6-~minoc~rbonylisochroman-1-yl)-
~Lhylui~uerazine
Step 1: Ethyl (6-bromoiRochroman-1-yl)~cet~te (III)
A lllixLu~e of 3-bromoph.?n~qthyl alcohol (II, 14.8 g) in dichlorompths~ne (37
mL) under argon is cooled to 0~ with an ice bath. Ethyl 3,3-diethu~y,ulupionate
(90%, 19.1 mL) is added via syringe. A titanium tetrachloride soll~tion (1 M in
methylene rhl~ritle, 236 mL) is added via a canula to an sl~3i*on funnel and added
semi-dropwise to the reaction ~ lu~ over one hr. The reaction is then l~lu~ d for
10 18 hr, after which time it is poured into a ~ Luie of aqueous hydrochloric acid (lN)
and saline (1/2) and extracted with methylene chloride. The organic phases are
~omhin~-l, dried over sodium sulfate, filtered, and con-~r.l-~ted. The con~ntrate is
purified by chrom~t~graphy (silica gel; ethyl acetateth~Y~ne 10/90) to give ethyl (6-
bromoiRorhroman-1-yl)~cetP~ts (III); Rf = 0.40 (ethyl acetate/h~Y~n~, 25/76); IR (neat)
15 1736, 1483, 1374, 1288, 1280, 1183, 1163, 1110, 1050, 1037 cm~1; NMR (300 MHz,
CDCl3) 7.30, 7.28, 6.92, 5.17, 4.20, 4.10, 3.79, 3.01-2.91, 2.87-2.65 and 1.28 ~; C~
(75 MH[z, CDCl3) 170.7, 136.0, 135.5, 131.6, 129.1, 126.0, 120.2, 62.6, 60.5, 41.3, 28.3
and 13.9 o; HRMS Calcd for Cl3H15O3Br = 298.0205, found = 298.0204.
Step 2: (R)-(+)-Ethyl (6-bromoiRochroman-1-yl)~retst~ (XII) and (S)-(-)-(6-
20 bromoiRûrhrûman-1-yl)acetic acid (XI).
Ethyl (6-brom- iRorhroman-l-yl)~ret~ (III, Step 1, 29.49 g), Amano P-30
lipase (15 g, lot #LPSAR01520, act = 32,600 u/g), and pH 7 buffer (590 mL) are
combined. The reaction is stirred vigorously and the hydrolysis is followed by HPLC
as follows. A 100 uL aliquot iB added to an opticlear vial c..~.t .i..i..g hydlvd~loric
25 acid (one drop). Ethyl acetate (1.5 mT.) is then added to the vial and the collt,~..t~
are mixed well. The reRlllt;nF mil~L~t: iB filtered through celite and assayed by
HPLC (llBon-l~r~k C18 3.9 mm x 30 cm reverse-phase colllmn, 10% ~cetonitrile/90%rhr 3rh~t~ buffer (4 L water, 5.22 g sodium dillyd~u~n pho,~E~h~t~ (hyLdte), 0.76 mT
phosrhoric acid) gradient to 85/15 over 15 min, then isocratic at that ratio, 2
30 mL/min, ~i~tectQr at 215 nm), (XI) Rt = 10.5 min., (XII) Rt = 13.5 min. When the
reaction reached the 50% collv~iion point, it iB filtered and the filtrate iB rinsed
succes~ively with water aqueous hyd.ucllloric acid (lN) and ethyl ~cet~te, several
times. The filtrates are comhinecl and extracted (two times) with ethyl ~-et~te. The
cc~mhin~(l organic extracts are washed with an equal volume of sa~u~a(ed aqueous35 sodium carbonate (3 x), dried over sodium sulfate, filtered and conc~ ated to give
Pn~nti~ln~rically enriched (XII). The saLul~Led aqueous sodium carbonate washes
-33-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
are ~ ified with con~ e..l ~ated hydlochloric acid and extracted three times with
methylene chloride, dried over sodium sulfate, filtered and con~entrated to give (XI).
The acid (XI) was assayed for en~nt;nmPric purity as follows. A ~xLu e of the acid
(XI, 15 mg) in THF (0.5 mL) is reduced with borane-T~ cnmrl~Y (lM in THF, 0.12
5 mL) at -5~ to 20-25~ over 18 hrs. The reaction is cooled to 0~, qllpn~hpcl with
mPth~nol (0.1 mL), then warmed to 20-25~ and hyd.ocllloric acid (lN, 0.4 mL) areadded via pipette. The reaction is then heated to reflux for 10 min., at which point
the volatiles are removed under reduced pressure and the residue is partitinned
between ethyl acetate and sa~uldted aqueous sodium carbonate. The organic phases10 are separated, dried over ma~nA~illm, filtered, and con~r-..t- dted. The residue is
weighed and diluted to a conr~t-,1Lion of 10 mg/mT with a sol~t;nn of isopropanol in
hexane (7~o). The llli~l.Ule iS assayed on a Chiracel OJ column using
hexane/isopropanol 90/10, 15 min, 1 L/min), then 80/20 for an s~ lit;onç~l 10 m-in (2
mL/min), wavelength 254 nm; (S)-alcohol Rt = 12.46 min, (R)-alcohol Rt = 10.46 min.
16 The ester (XII) could be analyzed in a similar way following hydrolysis (vide infra).
If nPe~P~, the en~nt;omPrically enriched ester could be re-subjected to another cycle
of the enzymatic hydrolysis if intlir~te~1 by the HPLC analysis. In this mnnnPr (R)-
(+)-ethyl (6-bromnicocl~v~an-l-yl)~et~t~ (XII) is obtained, (96% ee); Rf = 0.40 (ethyl
acetate~PY~nP, 25/75); [a]D +72~ (c = 0.383, ethsmnl); IR (neat) 1736, 1483, 1374,
20 1288, 1280, 1183, 1163, 1110, 1050, 1037 cm~l; NMR (300 MEIz, CDC13) 7.30, 7.28,
6.92, 5.17, 4.20, 4.10, 3.79, 3.01-2.91, 2.87-2.66 and 1.28; CMR (75 MHz, CDCl3)170.7, 136.0, 135.5, 131.6, 129.1, 126.0, 120.2, 62.6, 60.5, 41.3, 28.3, 13.9; HR~IS
Calcd for C13H1503Br = 298.0205, found = 298.0206. Also, (S)-(-)-(6-
bromoi~o~hroman-1-yl)acetic acid (XI) is i~ol~t,e-l, (99% ee); mp = 160-161~; Rf =
25 origin (ethyl ~et~t~/hPY~ne, 25M5); ta]D = - 90~ (c = 1, eth~nol); IR (neat) 1711,
1482, 1428, 14406, 1330, 1297, 1111, 1101, 1003, 971 cm~l; NMR (300 ~Iz, CDC13)
7.30, 6.92, 5.17, 4.184.11, 3.86-3.78 and 3.04-2.69; CMR (75 MHz, CDC13) 175.7,
136.2, 135.1, 132.0, 129.6, 126.2, 120.8, 72.4, 63.1, 45.5, 41.1 and 28.5 ~.
Step 3: (S)-(-)-1-[2-(6-Brnmoi~o~hroman-1-yl)acetyl]4-(4-mPthnYyphenyl)-
30 piperazine (S) (V)
(S)-(-)-(6-bromni~o~ ~an-1-yl)acetic acid (XI, Step 2, 3 g), N-(4-
methoxyphenyl)piperazine (2.34 g) and dichloromPth~nP (20 mL) are comhinP~ and
cooled to 0~. Diethylcyanophnsph~tD (2.0 mT-) and triethyla_ine (1.7 mL) are added
. eL~e~ Lively via syringe. The ice bath is allowed to expire and the ll~ is stirred
35 at 20-25~ for 18 hours. The reaction ....xLu~a is conc.q~ ated under reduced ple~ e
to give crude material which is chrom~t,o~;. aphed (silica gel; ethyl acetate/h~YSInl~,
-34-
CA 02225282 1997-12-19
W O 97/022S9 PCT/U~ 6~1
60-80/40-20) to give (S)-(-)-1-[2-(6-bromoisochroman-1-yl)acetyl]-4~4-methoxyphenyl)-
~i~t,la~i"e (S)-(V) (which after r~ y~ liPsticn from ethyl acetate~hexane), mp =122-123~; Rf = 0.26 (ethyl acetate/h~Y~ne, 70/30); [a]D = - 86~(c = 0.99, ethanol); IR
(mull) 1639, 1512, 1446, 1439, 1249, 1214, 1112, 1030, 1028, 820 c_-1; NMR (300
5 MHz, CDCl3) 7.32-7.26, 7.00, 6.88, 5.27, 4.16-4.07, 3.89, 3.80-3.60, 3.77, 3.05, 2.97-
2.90, 2.76 and 2.65 o; CMR (75 MHz, CDCl3) 168.9, 154.3, 145.2, 136.5, 136.2, 131.7,
129.3, 126.4, 120.3, 118.8, 114.4, 73.4, 63.4, 65.5, 51.3, 60.7, 46.1, 41.9, 39.9 and 28.7
o.
Step 4: (S)-(-)-1-[2-(6-BromoiAo~ ul~an-l-yl)ethyl]-4-(4-mpthr~yyphenyl)
10 piperazine (S)-(VI)
(S)-(-)-1-[2-(6-Bromoisochroman-l-yl)acetyl]-4-(4-mPthnYyphenyl)-
piperazine ((S)-(V), Step 3, 3.7 g) in THF (84 mL) iB cooled to 0~ and a u~il~ of
borane-THF com~ Y (lM in THF, 25 mL) is added via syringe. The ice bath is
removed and the llli~LUle iS heated to reflux for 18 hrs. The reaction is cooled to 0~
15 and 810wly qllPnrhP~ with aqueous hyll~ocl~loric acid (lN, 100 mL), and renu-~ed for
an S3cl~it;~n-91 1.5 hrs. The llliXl,U~ iB cooled to 20-25~ and the solvents are removed
under reduced p~ u~, and the aqueous residue is diluted with saline and b~RifiPdto pH 14 with aqueous sodium hydroYide. The ~ is eYtracted with
dichloromP~qne and the comhinP~l organic phases are dried over sodium sulfate,
20 filtered, and cnnCe~ a~ed. The conr,~.t al~ is purified by le~yYl~ pstinn from
ethyl ~cetatR~hexane to give (S)-(-)-1-[2-(6-bromoiRof~ u,l,an-1-yl)ethyl]-4-(4-mP~hm~yphenyl)-piperazine (S)-(VI), mp = 85-86~; Rf = 0.23 (ethyl acetate); ta]D -
48~ (c = 0.73, eth:~nol); IR (neat) 1518, 1479, 1266, 1250, 1155, 1140, 1112, 1103,
1041, 818 cm~1; 1H NMR (300 MHz, CDCl3) 7.29, 7.27, 6.97, 6.85, 4.78, 4.14-4.07,25 3.76-3.69, 3.76, 3.10, 2.95, 2.70-2.50, 2.13 and 2.02 ~; CMR (75 MHz, CDCl3) 153.6,
145.5, 136.8, 136.0, 131.4, 129.0, 126.3, 119.7, 117.9, 114.2, 74.1, 62.5, 55.3, 54.4,
53.3, 50.4, 32.9 and 28.6 o; HR~IS Calcd for C22H27N2o2Brl = 430-1256~ found =
430.1270.
Step 5: (S)-(-)-1-[2-[4-(4-MPt~o~ryphenyl)-1-~il,elazi~,yl]ethyl]-isochroman-6-
30 ~ le (S)-(VII)
(S)-(-)-1-[2-(6-Brnmnico-~.hroman-l-yl)ethyU-4-(4-methoxyphenyl)-piperazine
((S)-(VI), Step 4, 364 mg), p~ m(II) acetate (98%, 9.7 mg) and 1,3-bis-
diphenylph~srhinopropane (97%, 22 mg). A carbon monn~ e ~tmosph~re is
e~tohli~he~ in the vial, then into the reaction vessel are introduced via syringe DMF
35 (2 mL), 1,1,1,3,3,3-hPY~mP~hyl~ nP (98%, 1.25 mL) and diiso~u~ylethylamine
(0.29 mL). The ~u~e is heated to 100~ for 18 hr. After cooling to 20-25~, the
-35-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
reaction 8epard~Gd into two phases. The reaction ~u~Ga is poured into aqueous
llyd~v~ loric acid (lN) and washed two times with ether. The acidic solllt;-~n is
k~ifi ,~1 with aqueous sodium hydroxide and extracted three times wit~ ethyl
~r,et~t~ The ethyl acetate phases are comhinPd and con-c~ d~ed. The is purified
5 by chromS-1"J~-dphy (silica gel; mPt~nnl/dichloromPth~np~ 5/95) to give (S)-(-)-1-[2-[4-
4 mPtho,Yyphenyl)-l-piperazinyl]ethyl]-isochroman-6-ca~ x~ ;de (S)-(VII), mp =
186-187~; Rf = 0.27 (m~th~n- l/ethyl ~retstç, 10/90); [a]D - 53~ (c = 0.92, methylene
chloride/mPth~n.~l (1/1)); IR (mull) 3366, 3198, 1628, 1642, 1602, 1514, 1437, 1245,
1109 and 815 cm~l; NM, R (300 MHz,CDCl3) 7.61-7.58, 7.18, 6.85, 5.90, 4.86, 4.18-
10 4.11, 3.80-3.72, 3.76, 3.10, 2.99, 2.73, 2.66-2.49, 2.15 and 2.04 o; CMR (75 MHz,
CDCl3) 168.7, 153.5, 145.4, 142.0, 134.3, 131.0, 127.9, 124.8, 124.6, 117.8, 114.1,
74.2, 62.6, 55.2, 54.3, 53.2, 50.3, 32.8, 28.7 and 27.2 o; HRMS Calcd for
C23H29N3O3 = 395.2209, found = 395.2227.
T~'XAMPLE 2 (R)-(+)-1-t2-[4-(4-MPthnYyphenyl)-1-piperazinyl]ethyl]-
isochro lan-6-ca~ J.. irlP (R)-(VII) also known as (R)-(+)-1~4-
MPth-n~yphenyl)-4-[2-(6-, min.~,cArbonylisochroman-l-yl)-
G-Ll.yl~i~erazine
Step 1: (R)-(+)-6-(Bromni~orhroman-l-yl)acetic acid (R)-IV.
A mixlu~Ga of lit~illm hydroxide (3M, 150 mT-) is added to (R)-(+)-ethyl (6-
20 brom~i~o~l..v lan-1-yl)nr~ ~ (XII, TiXAMPLE 1 Step 2, 13.3 g) in THF (150 mL)and the ~ L~a is stirred at 20-25~ for 18 hours. The volatile 60lvents are removed
under reduced pr~ a~ and the residue is ~rirlifiP~ with aqueous llydlo~loric acid
to pH = 1. The resulting ~ix~ is extracted wit~ methylene r~hl~~ri~la and the
comhinP~ organics are dried over sodium sulfate, filtered and cnnc~ dted. The
25 cQn~ dte is purified by ~-G",.~ lli7~t;~n from dichloromP~l ~nP/hexane to give (R)-
(+)-6-(brom~i~orhroman-1-yl)acetic acid (R)-IV, mp = 160-161~; Rf = origin (ethyl
.~etst~/hPY~nP~ 25/75); [a]D = +83~(c = 0. 99, etl~nnl); IR (neat) 1711, 1482, 1428,
14406, 1330, 1297, 1111, 1101, 1003 and 971 cm~l; NMR (300 MHz, CDCl3) 7.30,
6.92, 5.17, 4.18-4.11, 3.86-3.78 and 3.04-2.69 o; CMR (75 I~Iz, CDCl3) 175.7, 136.2,
30 135.1, 132.0, 129.6, 126.2, 120.8, 72.4, 63.1, 45.5, 41.1 and 28.5 o.
Step 2: (R)-(+)-1-[2-(6-Bromni~orhroman-1-yl)acetyl]-4-(4-mPth.~Yyphenyl)-
~i~e.~zille (R)-(V).
Following the general plwedu~a of li'XAlVIPLE 1, Step 3 and m~king non-
critical v~ri~ t;~n~ but starting with (R)-(+)-6-(brom.~i~Gf,1.1 v~an-1-yl)acetic acid
35 ((~XA1VIPLE 2, Step 1, (R)-(IV)), a crude product is obtained which is first purified
by silica gel chrom~t~.d~hy using a gradient of 60-80% ethyl acetate/hPY~nQ, and
-36-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96108681
the resulting solid is then re~ L.~dl4lli7ed (ethyl f~cetDt~hexane) to give (R)-(+)-1-[2-(6-
brom~i~orhroman-l-yl)acetyl]-4-(4-methoxyphenyl)-piperazine (R)-(V), mp = 122-
123~; Rf = 0.26 (ethyl acetate/hPY~nP~ 70/30); [a]D = +89~(c = 1.00, e~llS~nol); IR
(mull) 1639, 1512, 1446, 1439, 1249, lZ14, 1112, 1030, 1028 and 820 cm~l; NMR
5 (300 MHz, CDC18) 7.32-7.26, 7.0, 6.88, 5.27, 4.16-4.07, 3.89, 3.80-3.60, 3.77, 3.05,
2.97-2.90, 2.76 and 2.65 o; CMR (75 MHz, CDCl3) 168.9, 154.3, 145.2, 136.5, 136.2,
131.7, 129.3, 126.4, 120.3, 118.8, 114.4, 73.4, 63.4, 55.5, 51.3, 50.7, 46.1, 41.9, 39.9
and 28.7 o.
Step 3: (R)-(+)-1-[2-(6-Brqmoi~orhroman-l-yl)ethyl]-4-(4-methoxyphenyl)-
10 piperazine (R)-(VI)
Following the general ~l~,cedul~ of lixAMpLE 1, Step 4 and making non-
critical v~ri~tion~ but starting with (R)-(+)-1-[2-(6-br~mniRorhroman-1-yl)acetyl]-4-(4-
methoxyphenyl)-piperazine (R)-(V) ((EXAMPLE 2, Step 2 ), (R)-(+)-1-[2-(6-
brom~ orhroman-1-yl)ethyl]-4-(4-mPthnYyphenyl)-piperazine (R)-(VI) is obtained,
15 mp = 86-86~; Rf = 0.23 (ethyl acetate); [a]D = l 47~(c = 0.94, et~nl)l); IR (neat)
1518, 1479, 1266, 1250, 1155, 1140, 1112, 1103, 1041 and 818 cm~l; NMR (300
MHz,CDCl3) 7.29, 7.27, 6.97, 6.85, 4.78, 4.14-4.07, 3.76-3.69, 3.76, 3.10, 2.95, 2.70-
2.50, 2.13 and, 2.02 8; CMR (76 MHz, CDCl3) 153.5, 145.5, 136.8, 136.0, 131.4,
129.0, 126.3, 119.7, 117.9, 114.2, 74.1, 62.5, 55.3, 54.4, 53.3, 50.4, 32.9 and 28.6;
20 HRMS Calcd for C22H27N2O2Br1 = 430.1256, found = 430.1274.
Step 4: (R)-(+)-1-[2-[4-(4-M~th~.~ryphenyl)-1-piperazinyl]ethyl]-isochroman-6-
ca~ (R)-(VII)
Following the general p~ cedu~e of F~AMpLE 1, Step 5 and making non-
critical variations but starting with (R)-(+)-1-[2-(6-brom- i~orhroman-1-yl)ethyl]-4-(4-
25 methoxyphenyl)-piperazine (R)-(VI) (~XAMPLE 2, Step 3), a crude m~t~ri~l is
obtained which is purified by flash chrom:~to~.a~hy (silica gel, 50 g; m~th~n~l/eth
~ret~to~ 5/95) to give (R)-(~)-1-[2-[4-(4-m~h- ~ryphenyl)-1-piperazinyl]ethyl]-
isochroman-6-ca~ icle (R)-(VII), mp = 187-187.5~; Rf = 0.27 (m~~h~nol/ethyl
~-~et~t~, 10/90); [a]D = +52~ (c = 0.92, methylene rhk)ri~/m ~thnnol~ 1/1); IR (mull)
30 3366, 3198, 1628, 1642, 1602, 1514, 1437, 1245, 1109 and 815 cm~l; NMR (300 ~Iz,
CDCl3) 7.61-7.58, 7.18, 6.85, 5.90, 4.86, 4.18-4.11, 3.80-3.72, 3.76, 3.10, 2.99, 2.73,
2.66-2.49, 2.15 and 2.04 ~; CMR (75 MHz, CDCl3) 168.7, 153.5, 145.4, 142.0, 134.3,
131.0, 127.9, 124.8, 124.6, 117.8, 114.1, 74.2, 62.6, 55.2, 54.3, 53.2, 50.3, 32.8, 28.7
and 27.2 ~; HRMS Calcd for C23H29N3O3 = 395.2209, found = 395.2208.~5 hxAlvlpLE 3 (S)-(-)-1-[2-[4-(4-M~thrlYyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-carbr.~r~nni-1e (S)-(LX) also known as (S)-(-)-1-(4-
-37-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
Mpthnyyphenyl)-4-[2-(6-methyls~minor~rbonylisochroman-l-yl)
~Lhylpi,ut:L ~zi~e
Step 1: (s)-(-)-N~N-Di-t-butylo~c~l,ul~yl-l-t2-[4-(4-mpthnyyphenyl)
piperazinyl]ethyl]-isochroman-6-carbnY~mi~P (S)-(VIII)
6 A ll~ib~Lu~ of (S)-(-)-1-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]-
isochroman-6-c~b~Y~1r~ p (S)-(VII), EXAMPLE 1, Step 5, 0.71 g), di-tert-butyl
dicarbonate (0.86 g) and 4-dimethylaminopyridine (0.02 g) in dichloromPth~ne (20mL) is stirred at 20-25~ under argon. After 72 hours, the reaction is concentrated
under reduced lJLe~Ule and the product i8 purified by column chrom~t~graphy
10 (silica gel, using a gradient eluant starting vwith 25% ethyl acetate in hexane and
prc~ g to 100% ethyl acetate) to give (S)-(-)-N,N-di-t-butylo~y~;a,Lonyl-1-[2-[4-(4-
methoxyphenyl)-1-piperazinyl]ethyl]-isochroman-6-carbnY~mi-1P (S)-(VIII), [a]D = -
32~ (c = 0.7201, et~ ~nol); HR~MS Calcd for C33H45N307 = 595.3257, found =
595.3282.
Step 2: (S)-(-)-1-[2-[4-(4-MPthnYyphenyl)-l-,ui~u~,~~iuyl]ethyl]-N-methyl-
isochroman-6-cdlL.n~ P. (S)-(IX)
A ~ule of (S)-(-)-N,N-di-t-butyll".y~,~l,onyl-1-[2-[4~4-mPt~lnYyphenyl)-1-
piperazinyl]ethyl]-isochroman-6-c~L~ ...icle (S)-(VIII) (1.09 g) in dichlorompth~np
(18 mL) in a sç~l~hlP tube is cooled under nitrogen using ~ret~ne and carbon
20 ~inYi-lP~ Into the cold tube is then cor rlPn~PCl methylamine (excess; typically 50
equivalent6), after which the tube is sealed and allowed to warm to room
te ~peL~tule. After stirring for 16 hr at room te~upelcll,ule, the CC~ t~ of the tube
are cnn~ a~ed under reduced pressure and the rP~ tinE crude product is purified
by column chrom~;- aphy (silica gel; using mpth~nnl/dichlornmpth~na 5/95) to give
25 (S)-(-)-1-[2-[4-(4-mPthn~ryphenyl)-1-piperazinyl]ethyl]-N-methyl-isochroman-6-
c~1,--~ P (S)-(IX), [a]D = -51~ (c = 0.9953, mpth~nol/dichlorompth~np~ 1/1); Anal.
Calcd for C24H31N3O3: C, 70.39; H, 7.63; N, 10.26 - found: C, 70.16; H, 7.84; N, 10.27.
~AMPLE 4 (R)-(+)-1-[2-t4-(4-MPthnYyphenyl)-1-pi~a~ lyUethyll-N-methyl-
isochroman-6-c~L.. ~.. itle (R)-(IX) also known as (R)-(+)-1-(4-
M~nyyphenyl) 4-[2-(6-methyls~mins~nrbonylisochroman-1-yl)-
~lhyl~i~uerazine
Step 1: (R)-(+)-N,N-Di-t-butylox~cdlbollyl-1-[2-[4-(4-mPthc)lryphenyl)-1-
piperazinyl]ethyl]-isochroman-6-c~l,u~J....i(l~P (R)-(VIII)
Following the general pl~cedu~e of F:~AMpLE 3, Step 1 and m~king non-
critical variations, but starting with (R)-(+)-1-[2-[4-(4-methoxyphenyl)-1-
-38-
CA 02225282 1997-12-19
W O 97/022S9 PCTnUS96/08681
pipt~dzi~yl]ethyU-isochroman-6-c~b~ ((R)-(VII), ~:~AlVlPLE 2, Step 4, 0.60 g)
gave (R)-(+)-N,N-di-t-butylu~y~-l.o.lyl-1-t2-t4-(4-methoxyphenyl)-1-
pi~e~dzi~yl]ethyl]-isochroman-6-ca~L--~r.-i-1e (R)-(VIII), which was used directly for
the neYt step.
6 Step 2: (R)~+)-1-[2-[4-(4-M~hnYyphenyl)-1-piperazinyl]ethyl]-N-methyl-
isochroman-6-carbnY~mi-le (R)-(~)
Following the general p~ ~du~e of ~AMPLE 3, Step 2 and making non-
critical v~ri~tinnR~ but starting with (R)-(+)-N,N-di-t-butylu~ l,onyl-1-[2-[4-(4-
methoYyphenyl)-l-piperazinyl]ethyl]-isochroman-6 cA.b..Y~ le (R)-(VIII) gave (R)-
10 (+)-1-[2-[4-(4-m~thnYyphenyl)-l-pipe,laz...~l]ethyl]-N-methyl-isochroman-6-
c~l,u~ e (R)-(IX), ta]D = +48~ (c = 0.9745, m~th~nol/dichlorom~th~n~ 1/1);
HR~IS Calcd for C24H3lN303 = 409.2365, found = 409.2391.
EXAMPLE 5 (S)-(-)- 1-t2-t4-(4-Trifluoromethylphenyl)- 1-pipt:~ a~ yl]ethyl]-N-
methyl-isochroman-6-ca~l,..~ ....i(le (S)-(IX) also known as (S)-(-)-
1-(4-l~uoromethylphenyl)~-[2~6-
methyl~minoc~ . ~u~lylisochroman-1-yl)-ethylpi~erazine
Step 1: (S)-(-)-1-[2-(6-Bromnicorhroman-1-yl)acetyl]4~4-
trifluoromethylphenyl)-piperazine (S) (V)
(S)-(-)-(6-Bromr~i~orhroman-l-yl)acetic acid (FXAMPLE 1, XI, 642 mg, 2.0
20 mmol), 10 mL dichlorom~th~n~, 1-(4-trifluoromethyl)~i~elazi,le (607 mg, 3.3 mmol)
and diethylcyanophosrhnn~ (0.33 mL, 2.2 mmol) are comhin~-l cooled to 0~ and
treated with triethylamine (0.42 mL, 3.0 mmol) with no visible change followed by
w~i~g to 20-25~. After 16 hours, the reaction ~i~Lule is cnn~Pnl ~d~ed. The
conck..t dte ig purified by LC on 58 g (230-400) silica gel eluting with ethyl
25 acetate/hexane (40/60) to give (S)-(-)-1-[2-(6-bromoiRochroman-1-yl)acetyl]-4-(4-
trifluoromethylphenyl)-piperazine (S)-(V), Rf = 0.25 (ethyl acetate/hPY~nP~ 40/60).
Step 2: (S)-(-)-1-[2-(6-BromniQorhroman-1-yl)ethyl]-4~4-
trifluoromethylphenyl)-piperazine (S)-(VI).
(S)-(-)-1-[2-(6-BromoiRor.hroman-l-yl)acetyl]-4-(~ I ;n~ v~ethylphenyl)-
30 piperazine (S)-(V) (Step 1, 876 mg, 1.8 mmol) and 18.0 mL freshly ~liP~;llP-1tetrahy-l~vruldll are comhinA-l and cooled to 0~. The ~ e is drop-wise treated
with a lM solllt;on of borane in tetrahydluruLd~l (5.4 mT, 5.4 m mol) with fo~ming.
The reaction is warmed to 20-25~ for 16 hrs. At this time, the reaction is treated
with 1M hyd~v~hlnrir acid (6.0 mL), fitted with a reflux con-lAnRAr, and heated to
35 reflux for 1 hr. The reaction is cooled to 20-25~ with the volatiles le,Luuved under
reduced p~e~ ~e- The resllltinF aqueous residue is diluted with water (30 mL),
-39-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
adjusted to pH ~ 10, and extracted twice with ethyl acetate (30 mL). The comhinP(l
organic ~ ct~ are washed once with saline (30 mL), dried over m~gnPcillm sulfate,
filtered, and concPntrated. The concent~ate is purified by LC on 43 g (230-400) silica
gel eluting with ethyl acetate/hexane (40/60) to give (s)-(-)-l-[2-(6-brnnnni~ochroman
5 1-yl~ethyl]-4-(4-trifluoromethylphenyl)-piperazine (S)-(VI), mp = 104-105~; Rf = 0.30
(ethyl ~ret~t~/h~Y~nP, 40/60).
Step 3: (S)-(-)-1-[2-[4~4-Trifluoromethylphenyl)-l-piperazinyl]ethyl]-N-
methyl-isochroman-6-cP~ le (S)-(IX)
(S)-(-)-1-[2-(6-Bromoi~o~hroman-l-yl)ethyl]-4-(4-trifluoromethylphenyl)-
10 piperazine (S)-(VI) (Step 2, 703 mg, 1.5 mmol), palladium (II) acetate (17 mg, .075
mmol), 1,3-bis(diphenylrho~phino)propane (37 mg, .09 mmol), 3.0 mT.
dimt~(hylr..~ mi~l~, diiso~uropylethylamine (0.52 mL, 3.0 mmol), and N-
m~ ylrc~ mi~le (1.8 mL, 30 mmol) are comhinP-i and purged six times with carbon
mnnnYi-lP/house vacuum followed by hP~t;ng to 120~. After 16 hours, the l~ Ule i8
15 cooled to 20-25~, treated with 25 mL lM hyl.ochloric acid, snd stirred for 10 min.
This acidic sol~lt;~ n is adjusted to pH 12 and t~ _Led three times with ethyl
acetate (20 mL). The comhinPtl organic 6l~l,r..~ are washed once with saline (30
mL), dried over m~.P~ .. sulfate, filtered, and cor.ce..l-a~ed. The con-~..l a~e is
purified by LC on 33 g (230-400) silica gel eluting with ~cetonp/hexane (40/60) to
20 give (S)~-)-1-[2-[4~4-trifluoromethylphenyl)-1-piperazinyl]ethyl]-N-methyl-
isochlu~an-6-cs~ le (S)-(IX), mp = 169-170~; Rf z 0.30 (~-~etor P/hP~ne~ 40/60).
AMpLE 6 (S)-(-)-1-[2-[4-[4-(AminocA-I~ullyl)phenyl]-1-pi,u~ yl]ethyl]-N-
methylisochroman-6 c~1,~ p (R)-(IX) also known as (S)-(-)-
l-(BPn~mi-le4-yl)-4-[2-(6-methyl~minoc~ . Lullylisochroman-l-
yl)-~l~y~ le
Step 1. (S)4)-2~6-Brnmni~o- hroman-2-yl)ethyl alcohol
(S)~-)-2-(6-Brnmnico~hroman-1-yl)acetic acid (XI) (~XAlVlPLE 1, step 2, 16.27
g, 60 mmol) and 100 mT- tetrahyd~uru~ are co~nhinptl This ~Lul~: is treated
with a lOM solllt;~n of borane methyl sulfide (18.0 mL, 0.18 mol) while m~int~ining
30 20-25~ with a water bath. After 1 hr, the reaction ~u~a is cooled to 0~ and
slowly ql~PnrhP~l with 160 mL mPth~nol Note: An in~lnrt;on period of ~ppl..~i...~tPly
1-2 minllte~ is noticed before a rapid and sudden generation of hyd~ . The
lu~a i8 warmed to 20-25~ and the volatiles are ~ uv~d under reduced pre~/iul~.
The rç?3ll1t;ng solid is diluted with lM sodium hydroxide (150 mT ) and extracted
35 three times with ethyl acetate (100 mL). The cnmhined organic ~ are washed
once with saline (100 mT ), dried over m~nPRillim sulfate, filtered, and concç~ ted
-40-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTnUS96/08681
to give a solid. This m~t,~ari~l is ~ y~ 7p~l from ethyl acetate/hexane to give (S)-
(-)-2-(6-bromniAochroman-2-yl)ethyl alcohol (S-1), mp 95-96~; Rf= 0.28
~n-p~ 30/70).
Step 2: (S)-(-)-6-Bromo-l-(bromoethyl)isochroman
(S)-(-)-2-(6-Bronnnicorhroman-2-yl)ethyl alcohol (S-1) (Step 1, 14.0 g, 54 mmol)and 91 ~ dichlorompt~np are cnmhina-l The resnltin~ d iS treated with 25
m~ tetrahyLurur~ll. The suspension is treated with carbon tetrabromide (22.6 g, 68
mlnol)~ cooled to 0~, and portion-wise treated with triphenyl rhnsphina (21.4 g, 82
mmol). The re~llltin~ e is warmed to 20-2~~ for three hours followed by
conre--t-a~ion under reduced ~laF~ to give a solid. The triphenyl phnsFhin~ oxide
is removed by l~v~ ti~n from ethyl acetate/hexane with the mother liquor
giving a solid. This m~t~ri~l is absorbed on 70 g silica gel and purified by LC on
700 g (230-400) silica gel eluting with ethyl acetate/hexane (5/95) to give (S)-(-)-6-
bromo-l-(bromoethyl)isochroman (IV), Rf = 0.47 (10% ~e~nP/hexane).
Step 3: ($)-(-)-4-[4-[2-(6-BromniAorhroman-1-yl)ethyl]-1-
pi~elazi~yl]h~qn7:~mi~
A J~ e of (S)-(-)-6-bromo-1-(bromoet~yl)isochroman (IV) (Step 2, 17.22 g,
53.8 m~nol), 14.36 g (67.0 m mol) of 4-(piperazin-l-yl?bPn7~micle (PREPARATION 1,
10.43 g (80.7 mmol) of dii~u~upylethylamine, and 125 mL of ethylene glycol is
20 heated at 85-90~ ov~rniE~ht After cooling, water (300 mT-) is added and the rPAllltinE
solid is cnll~cte~l by filtration. The cake is washed three times with water (for a
total of about 200 mL) and then with toluene (for a total of about 200 mL). The
filter cake is then dried under reduced ~l~s2iu~e. After drying, the crude product is
slurried in mP~h~n~)l/dichlorompth~ne and silica gel is added to adsorb the ~L~d.
25 After removal of the solvents, the silica gel slurry is poured onto the top of a silica
gel column equilibrated with dichloromP~nP~mPth~nol (95/5). T~ ltinn is begun
using dichloromp~np/m~tl~nnl (95/5) and then changed to
dichloromPth~n~/m~t~nol (92/8) to elute (S)-(-)-4-[4-[2-(6-bromniAochroman-1-
yl)ethyl]-l-pipe~ yl]hPn~mitle (VI), obtained as a solid after pooling of the
30 appropriate fractions and cnnrr ~ ion..
Step 4: (S)-(-)-1-[2-[4-[4-(~minncAILollyl)phenyl]-1-piperazinyl]ethyl]-N-
methylisochromanyl-6-c~boY ~ " .;-~ P
(S)~-)-4-[4-[2-(6-BromniRorhroman-l-yl)ethyl]-l-piperazinyl]h~n~mide (VI)
(Step 3, 3.34g, 7.52 mmol) is slurried in 55 mL of dry D~ and ~l~Pg~R~e~l using
35 house vacuum (rPle~Aing to argon). The slurry is transferred to a 3-necked round
bottom flask (using an ~ iition531 10 mL of DMF to rinse the flask) c~ illillg
-41-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96108681
p~ m acetate (0.084 g, 0.376 mmol) and 1~3-bis(diphenylrhosphino)propane
(0.232 g, 0.564 mmol) and the flask is placed in an oil bath. Diisrvpl o~ylethylamine
(2.6 mL, 15.3 mmol) is added and the ~ u~ is again lightly clPg~e~1 and releasedto argon. Carbon mnnnYifl~ is blown onto the surface of the ~f~Lul~ as the
temperature of the bath is raised to 60~. As the tel--pe.r l u,~ increased, the llli~l,U~
became homogeneous and the tip of the needle is then placed slightly below the
surface of the snlllt;on After bubbling carbon monnyi~l~ into the solution for several
minlltes, methyl amine gas also is bubbled into the sol~t;on Carbon mnnoYi~l~ and
methyl amine ~ ition were cont;nll~-l for 6 hr or until the starting material iscnn~llm~cl, after which the ~ u.a is cooled and DMF removed under reduced
plevv.ua. The residue is applied to a silica gel column andl eluted with
mf~th~nol/dichloromPth~ne (8/92) until the less polar i~u,,~ies were eluted. Theeluent is then switched to m~tl~nnl /dichlorom~h~n~ (10/90) and (S)-(-)-1-[2-[4-[4-
(~minor~rbonyl)phenyl]-l-piperazinyl]ethyl]-N-methylisochromanyl-6-carbnY~mide
(IX )is obtained after pooling and conr.?.,t. d~ion of the appropriate fractions and
crystslli7~tinn from m~t1~nolldichlor-7m~th~n~, mp 231.5-232.5~.
ANlpLE 7 1-[2-[4-(4-Methylphenyl)-1-piperazinyl]ethyl-isochroman-6-
Step 1: 6-Brom-i~orl .~ an-1-yl-acetic acid (IV)
Following the general p~uce~lu a of h~ANlpLE 2, Step 1 and making non-
critical variations but using racemic ethyl 6-bromni~or~ ~an-1-yl-acetate (III), 6-
bromni~orhroman-1-yl-acetic acid (IV) is obtained, mp 160-161~; NMR (300 MHz,
CDCl3) 7.30, 6.92, 5.17, 4.18-4.11, 3.86-3.78 and 3.04-2.69 ~.
Step 2: 1-[2-(6-Bromoi~orhroman-1-yl)acetyl]-4-(4-methylphenyl)-
piperazine (V)
Following the general procedure of EXAMPLE 1, Step 3 and m~king non-
critical v~ri~t;r~nC but using racemic 6-bromoi~orhroman-1-yl-acetic acid (IV) and 4-
methylphw~yl~i~el~zine, 1-[2-(6-bromni~orhroman-1-yl)acetyl]-4-(4-methylphenyl)-piperazine (V) is obtained which after flash chrom~to~.d~hy (silica gel 80 g; ethyl
acetate/h~Y~ne, 50/50), Rf = 0.20 (ethyl ~ret~t~/h~Y~n~ 50/~0); IR (neat) 1642, 1515,
1481, 1462, 1443, 1234, 1208, 1107, 1031 and 813 cm~l; NMR (300 MHz,CDC13)
7.30, 7.09, 7.01, 6.84, 5.26, 4.13-4.07, 3.95-3.87, 3.82-3.60, 3.12, 3.05-2.89, 2.77, 2.65
and 2.28 o; C~ (75 MHz, CDCl3) 168.2, 148.3, 136.5, 136.8, 131.7, 129.6, 129.2,
126.3, 121.2, 116.8, 73.3, 63.3, 60.2, 48.7, 45.9, 41.7, 39.8, 26.6 and 20.3 o.
Step 3: 1-[2-(6-Bromnicorhroman-1-yl)-ethyl]-4~4-methylphenyl)-
piperazine (VI)
-4~ 2-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
Following the general pl'~)Cedu~e of FxAlvlpLE 1, Step 4 and mPking non-
critical v~ ri5~t;~nc but using 1-[2~6-br. nn niRorh roman-1-yl)acetyl]-4~4-methylphenyl)-
azille (V), l-[Z-(6-brom~iRocl-rolL~an-l-yl)-ethyl]-4-(4-methylphenyl)piper~ine
(VI) is obtained, Rf = 0.21 (ethyl acetate/hPY~n~, 50/50); IR (neat) 2941, 2925, 2818,
5 1515, 1481, 1379, 1239, 1143, 1111 and 813 cm~l; N~ (300 ~Iz,CDCl3) 7.32-7.26,7.07, 6.97, 6.84, 4.78, 4.14-3.07, 3.78-3.69, 3.16, 2.94, 2.7-2.48, 2.26, 2.15-1.90 o;
CMR (75 ~Iz, CDCl3) 149.0, 136.9, 136.1, 131.4, 129.4, 129.0, 128.9, 126.3, 119.8,
116.1, 74.1, 62.6, 54.4, 53.2, 49.5, 33.0, 28.6 and 20.2 ~.
Step 4: 1-[2-t4-(4-Methylphenyl)-1-~i~el~hlyl]ethyl-isGchLu ,an-6-
c~l,.. ~ le (IX)
Following the general pl~cedu~e of FxAMpLE 1, Step 5 and m~kin~ ncn-
critical variations but using 1-[2-(6-br m~.iRorhroman-1-yl)-ethyl]-4~4-methylphenyl)-
pi~e~ e (VI), l-t2-[4-(4-methylphenyl)-~ e.~ yl]ethylisûchroman-6-
Ca1b~ (VII) is obtained, Rf = 0.2 (mPth~nAl/ethyl ~et~tA~ 10/90); IR (mull)15 3373, 3180, 1647, 1623, 1571, 1520, 1406, 1242, 1111 and 817 cm~l; NMR (300
MHz,CDCl3) 7.59, 7.18, 7.07, 6.84, 6.05, 4.86, 4.13, 3.77, 3.17, 3.00, 2.76-2.45, 2.26,
2.14 ard 2.02 ~; HRMS C~ t~ l for C23H29N302 = 379.2260, found = 379.2269.
FxAMPLE 8 1-[2-[4~4-Chlûrophenyl)-1-piperazinyl]ethyl-isochrûman-6-
c~L.-~...;(le (VII)
Step 1: 1-[2-(6-Brom~.iRorhroman-1-yl)acetyl]-4~4-chlorophenyl)piperazine (V)
Following the general procedure of EXAMPLE 1, Step 3 and m~king non-
critical v~ri~tinnR but using racemic 6-bromnico,~ an-1-yl-acetic acid (IV) and 4-
chlorophe~lyl~i~e,~i~e, 1-[2-(6-brnmniRo~ hroman-l-yl)acetyl]-4~4-methylphenyl)-piperazine (V) is oht~inP~l Rf = 0.20 (ethyl ~ptstplhpy~ne~ 50/50); IR (mull) 1642,
26 1594, 1496, 1482, 1443, 1275, 1232, 1107, 1030 and 821 cm~l; N~ (300
MHz,CDCl3) 7.32-7.21, 7.01, 6.84, 6.26, 4.11, 3.94, 3.79-3.60, 3.14, 3.09-2.89, 2.77
and 2.65 o; CMR (75 ~Iz, CDCl3) 178.1, 148.5, 136.0, 131.8, 129.1, 128.8, 125.8,125.0, 120.0, 117.5, 73.0, 63.2, 49.7, 49.2, 55.0, 41.9, 39.6 and 28.5 ~.
Step 2: 1-[2-(6-BrnmniRo~ ol an-1-yl)-ethyU-4~4-
chlorophenyl)~ zine (VI)
Following the general plucedu~a of ~AMpLE 1, Step 4 and m~king non-
critical v~ri~t;on~ but using 1-[2~6-brnmniRo~l-loman-1-yl)acetyl]-4{4-chlorophenyl)-
piperazine (V), 1-t2-(6-brnmniRûcl .- u an-1-yl)-ethyl]-4-(4-chlorophenyl)piperazine (VI)
is obtained, mp = 94-96~; Rf = 0.22 (ethyl acetate/hPY~nP, 50/50); IR (mull) 1500,
1483, 1448, 1248, 1242, 1152, 1144, 1113, 1102 and 815 cm~l; N~ (300
~Iz,CDCl3) 7.32-7.26, 7.19, 6.97, 6.83, 4.78, 4.14-4.07, 3.78-3.69, 3.16, 3.00-2.90,
-43-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
2.7-2.48 and 2.15-1.90 ~; CMR (75 MHz, CDCl3) 149.6, 137.1, 136.0, 131.4, 129.1,128.7, 126.3, 124.6, 120.0, 116.9, 74.0, 62.6, 54.3, 53.3, 53.0, 48.9, 33.0 and 28.6 ~.
Step 3: 1-[2-[4~4-Chlorophenyl)-1-piperazinyl]ethylisocl~vll.an-6-
C~-~YJ~ P- (IX)
Followingthe general ploce~ of ~xAlvrpLE 1, Step 5 and mnkin~ non-
critical v~ri~tionc but using 1-[2-(6-bromni~ochroman-1-yl)-ethyl]-4-(4-chlorophenyl)-
piperazine (VI), 1-[2-[4~4-chlorophenyl)- 1-piperazinyl]ethylisocln ulllan-6-
~&I,o~ P (VII) is obtained, mp = 169-171~; Rf = 0.22 (mpth~nol/ethyl ~cet~te,
10/90); IR (mull) 3365, 1649, 1661, 1623, 1500, 1403, 1241, 1112, 1096 and 821 cm~l;
N~ (300 ~Iz,CDCl3) 7.59, 7.18, 6.84, 6.05, 4.88, 4.15, 3.77, 3.17, 3.00, 2.76-2.45,
2.14 and 2.02 o.
~XAMPLE 9 1-[2-[4~4-Phenylmethyloxyphenyl)-1-piperazinyUethyl]-
isoch.v~an-6-~ ~b~-Y~ 1P (VII)
Step 1: 1-(4-Phenylmethyloxyphenyl)4-[2-(6-bromni~o~ .,.. an-1-
yl)acetyl],ui,u~ e (V)
Following the general pl~celule of F~ANlpLE 1, Step 3 and m~king non-
critical v~rint;~n~ but using racemic 6-bromni~orhroman-l-yl-acetic acid (IV) and 4-
phenylmethylu,-y~ui,ue.~zine, 1-(4-phenylmethyloxyphenyl)-4-[2-(6-brnmni~orhroman-
1-yl)acetyll,uip~i.az e (V) is obtained, Rf = 0.47 (ethyl acetate); IR (mull) 1510, 1481,
1463, 1453, 1445, 1239, 1231, 1101 and 1027 cm~1; N~ (300 MHz, CDC13) 7.43-
7.25, 7.00, 5.27, 5.02, 4.23-4.06, 3.93-3.87, 3.80-3.59, 3.06, 2.98-2.89, 2.76 and 2.65 o;
CMR (75 MHz, CDCl3) 168.9, 153.4, 145.3, 137.1, 136.4, 136.2, 131.6, 129.3, 128.4,
127.8, 127.3, 126.4, 120.2, 118.6, 115.5, 73.3, 70.3, 63.4, 51.1, 50.5, 46.0, 41.8, 39.8
and 28.7 ~. Step 2: 1~4-Phenylmethyloxyphenyl)-4-[2~6-brcmni~ofl-r~. an-1-
yl)-ethyl],ui,u~.~.zine (VI)
Following the general ,uloced of F~Al\/IPLE 1, Step 4 and m~king non-
cAtical v~ri~t;on~ but using 1~4-phenylmethyloxyphenyl)~-[2-(6-brnmni#orhroman-
l-yl)acetyl]~il,e~dzine (V), 1-(4-phenylmethyloxyphenyl)-4-t2-(6-bromni~ochroman-1-
yl)-ethyl]piperazine (VI) is obtaned, mp = 87-90~; Rf = 0.43 (ethyl acetate); IR (neat)
1578, 1517, 1452, 1258, 1153, 1113, 1054, 1049, 818 and 737 cm~l; N~ (300
MHz,CDCl3) 7.43-7.26 (m, 7H, aromatic H's), 6.97 (d, lH, J=8.2 Hz, aromatic H),
6.90 (8 with broad base, 4H, aromatic H's), 5.01 (8, 2H, PhC-H2), 4.77 (m of d, lH,
J=5.5 Hz, PhC-H), 4.14-4.07 (m, lH), 3.74 (d of t, lH, Ja=3.9 Hz, Jb=9.4 Hz), 3.11 (t,
4H, J=4.9 Hz, four of pip-H), 2.95 (m, lH), 2.7-2.54 (several m's, 7H), 2.11 (m, lH,
pipCH-H), 2.02 (m, lH, pipCH-H) ~; C~ (75 MHz, CDCl3) 153.0, 145.1, 137.4,
137.1, 136.3, 131.6, 128.5, 127.8, 126.5, 120.0, 118.0, 115.6, 74.3, 70.5, 62.8, 54.6,
-44-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTnUS96/08681
53.5, 50.5, 33.1 and 28.9 o.
Step 3: 1-[2-[4-(4-Phenylmethyloxyphenyl)-1-piperazinyl]ethyl]-
isochroman-6-c~1,~ "~ide (VII)
Following the general ~lucedu-- of F~xAMpLE 1, Step 5 and making non-
critical variations but using 1-(4-phenylmethyloYyphenyl)4-[2-(6-bromniRorhroman-
1-yl)-ethyl]piperazine (VI), 1-[2-[4-(4-phenylmethyloYyphenyl)-l-piperazinyl]ethyl]-
i~ocllru.llan-6 c~.l,o~ le (VII) is obtained, IR (mull) 3368, 3178, 1647, 1623, 1611,
1570, 1515, 1334, 124.6 and 1111 cm~l; NMR (300 MHz, CDCl3) 7.58 (m,2H,
aromatic H'_), 7.43-7.30 (m, 5H, aromatic H's), 7.15 (d, lH, J=8.4 Hz, aromatic H),
6.90 (B, 4H, aromatic H's), 6.20-5.80 (two broad ~inE~ tq~ C(O)N-H2), 5.0 (s, 2H, PhC-
H2-O), 4.87 (m of d, lH, J=5.8 Hz), 4.184.10 (m, lH), 3.81-3.73 (m, lH), 3.10 (t, 4H,
J=4.8 Hz, four of pip-H), 3.0 (m, lH), 2.75 (m, lH), 2.650-2.54 (m, 6H), 2.15 (m, lH),
2.05 (m, lH) o; CMR (75 MHz, CDCl3) 169.0, 152.9, 145.8, 142.3, 137.3, 134.5,
131.3, 128.5, 128.1, 127.8, 127.4, 125.0, 124.9, 118.0, 115.5, 74.5, 70.4, 62.8, 54.6,
53.4, 50.4, 33.1 and 29.0 ~.
EXAMPLE 10 1-[2-[4-(4-Bulu~y~henyl)-l-piperazinyl]ethyl]isocl~ an-6-
Step 1: 1-[2-(6-BrnrnoiRo-~hroman-1-yl)acetyl]-4-(4-buL~,~y~henyl)-
piperazine (V)
Following the general pl~- edul~ of F~xAMpLE 1, Step 3 and making non-
critical v~ri~ti~nR but using racemic 6-bromni~o~l~t~ù ~an-1-yl-acetic acid (IV) and 4-
buLu~y~he,~yl~ e~ 1-[2-(6-bromniRor,l . . ~ an-l-yl)acetyl]-4-(4-bul~,~yl3henyl)-
zille (V) is obtained, Rf = 0.24 (ethyl acetate/h~Y~n~, 50/50); IR (mull) 1640,
1513, 1482, 1441, 1422, 1245, 1232, 1103, 1031, 829 cm~l; NMR (300 MHz, CDCl3)
7.29, 7.00, 6.90, 5.26, 4.13-4.07, 3.92, 3.93-3.87,3.80-3.60, 3.06, 2.98-2.89, 2.76, 2.65,
1.74, 1.48, 0.96 o; CMR (75 MHz, CDCl3) 168.8, 153.8, 145.0, 136.4, 136.2, 131.6,
129.3, 126.3, 120.2, 118.7, 115.1, 73.3, 67.9, 63.3, 51.2, 50.7, 46.1, 41.8, 39.8, 31.3,
28.7, 19.1, 17.3 and 13.7 ~.
Step 2: 1-[2-(6-Bromni~o~ ...an-l-yl)-ethyl]-4-(4-buLw~ylJhenyl)-
~i~e~ e (VI)
Following the general p~u~ellul~ of ~xANlpLE 1, Step 4 and making non-
critical variations but using 1-[2-(6-bro7n~ o~ u..,an-1-yl)acetyl]-4-(4-bulu,.y~henyl)-
piperazine (V), 1-[2-(6-bro7noiRo-~hroman-1-yl)ethyl]4~4-bul~,.y~henyl)piperazine (VI)
is obtained, Rf = 0.43 (ethyl acetate); IR (neat) 2957, 2931, 28872, 1511, 1481, 1261,
35 1243, 1233, 1112, 1057 cm~l; NMR (300 MHz,CDC13) 7.32 (d, 2H, J=8.3 Hz, aromatic
H's), 7.06 (d, lH, J=8.3 Hz, aromatic H), 6.85 (q, 4H, J=9.7 Hz, aromatic H's), 4.80
45-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
(m of d, lH, J=6.1 Hz), 4.134.08 (m, lH), 3.98-3.89 (m, 3H), 3.71 (d of t, lH, Ja-3.7
Hz, Jb=13.7 Hz), 3.28 (broad 8, 4H), 3.10-2.72 (broad m, 7H), 2.65 (m of d, lH,
J=16.5 Hz), 2.40 (m, lH), 2.22 (m, lH), 1.74 (quintet, 2H, J=6.6 Hz), 1.46 (sextet,
2H, J=7.3 Hz), 1.25 (t, 2H, J=7.0 Hz), 0.96 (t, 3H, J=7.3 Hz) o; HRMS C~ te~l for
5 C25H33N2~2Brl = 473.1804, found = 473.1796.
Step 3: 1-[2-[4-(4-Bulu~y~henyl)-l-pipe,~zi~lyl]ethyl]isochroman-6-
carb-.Y~mide (VII)
Following the general plocedu~e: of lixAl\lrpLE 1, Step 5 and mslking non-
critic~l variations but using 1-[2-(6-br~m-i~orl-l~u~an-l-yl)-ethyl]-4~4-l~ulu..y~henyl)-
10 piperazine (VI), l-t2-[4~4-bul~ y~henyl)-1-piperazinyl]ethyl]i~ocllro~an-6-
C~b--Y~ e (VII) is obtained, Rf = 0.18 (mPtl~nollethyl 2qcet~te~ 10/90); IR (mull)
3364, 2820, 1647, 1624, 1570, 1517, 1413, 1260, 1245, 1111 cm~l; NMR (300 MHz,
CDCl3) 7.60 (m,2H, aromatic H's), 7.18 (d, lH, J=8.5 Hz, aromatic H), 6.86 (q, 4H,
J=9.2 Hz, aromatic H's), 6.20-5.80 (two broad cingl~t~, C(O)N-H2), 4.85 (m of d, lH,
15 J=18 Hz), 4.15 (m, lH), 3.90 (t, 2H, J=6.5 Hz, -O-C-H2-CH2CH2Me), 3.77 (m, lH),
3.10 (t, 4H, J=4.8 Hz, four of pip-H), 2.96 (m, lH), 2.80-2.50 (m, 6H), 2.15 (m, lH),
2.05 (m, lH), 1.74 (quintet, 2H, J=6.8 Hz, -OCH2C-H2-CH2Me), 1.48 (quintet, 2H,
J=7.5 Hz, -OCH2C-H2C-H2-Me), 0.96 (t, 3H, J=7.3 Hz, -OCH2CH2CH2C-H3) o; C~
(75 MHz, CDCl3) 169.1, 160.8, 153.4, 145.6, 142.4, 134.6, 131.3, 130.3, 128.2, 127.6,
20 126.1, 125.0, 118.1, 115.1, 74.5, 68.1, 62.9, 54.7, 53.5, 50.6, 41.4, 33.2, 31.5, 29.0,
19.3 and 13.9 o; HRMS C~ tetl for C26H35N303 = 437.2678, found = 437.2678.
EXA~LE 11 1-[2-[4~4-Diethylaminophenyl)- l-pi~ yl]ethyl]isocl~l ~,~an-
6-c~ 7e (VII)
Step 1: 1-[2-(6-Br~moi~o~ o~an-l-yl)acetyl]-4-(4-diethylaminophenyl)-
pi~elazi le (V)
Following the general prucedu~cs of EXAMPLE 1, Step 3 and m~king non-
critical v~ri~ n~hut using racemic 6-brl nl i~o~h~an-l-yl-acetic acid (IV) and 4-
diethylaminophe--ylt)i~ -zine, 1-[2-(6-bromri~oCl .~ ~an-1-yl)acetyl]-4-(4-
diethylaminophenyl)piperazine (V) is obtained, Rf = 0.21 (Sl-~eton~ Y~ne~ 30/70); IR
30 (mull) 1633, 1518, 1482, 1446, 1423, 1261, 1232, 1196, 1109, 809 cm~l; lH N~ (300
MHz, CDCl3) 7.32-7.26 (m, 2H, aromatic H's), 7.0 (d, lH, J=8.2 Hz, aromatic H),
6.88 (d, 2H, J=9.0 Hz, aromatic H's), 6.68 (d, 2H, Js9.0 Hz, aromatic H's), 5.27 (m of
d, lH, J=5.9 Hz, ArC-H), 4.16-4.07 (m, lH), 3.89 (m, lH), 3.76 (m, 2H), 3.64 (m, 2H),
3.28 (q, 4H, J=7.1 Hz, two of PhNC-H2), 2.98 (m, 5H), 2.76 (d of d, lH, Ja=3.7 Hz,
35 Jb=14.9 Hz), 2.65 (m of d, lH, J=16.4 Hz) 1.12 (t, 6H, J=7.0 Hz, two of NCH2C-H3)
o; C~ (7~ MHz, CDCl3) 168.8, 143.2, 141.6, 136.4, 136.2, 131.6, 129.2, 126.3,
-46-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
120.2, 119.2, 113.7, 73.3, 63.3, 51.6, 51.0, 46.1, 44.6, 41.9, 39.8, 33.1, 28.6 and 12.4 o.
Step 2: 1-[2-(6-Brom~ o~ o~an-1-yl)-ethyl]-4-(4-diethylaminophenyl)-
piperazine (VI)
Following the general plwe-lt~ . e of EXAMPLE 1, Step 4 and making non-
critical v~ri~ti~nc but using 1-[2-(6-bromni~orhroman-1-yl)acetyl]-4~4-
diethylsminophenyl)~ t~lc z e (V), 1-[2-(6-brontniRorhroman-1-yl)-ethyl]-4~4-
diethylaminophenyl)piperazine (VI) is obtained, Rf = 0.26 (ethyl acetate); IR (neat)
2931, 2965" 2814, 1516, 1374, 1262, 1232, 1144, 1109, 818 cm~l; NMR (300
MHz,CDCl3) 7.3-7.26 (m, 2H, aromatic H'~), 6.98 (d, lH, J=8.2 Hz, aromatic H), 6.88
(d, 2H, Jz9.0 Hz, aromatic H's), 6.68 (d, 2H, J=9.0 Hz, aromatic H's), 4.77 (m of d,
lH, J=5.9 Hz, ArC-H), 4.14-4.07 (m, lH), 3.74 (d of t, lH, Ja=3.9 Hz, Jb=9.3 Hz),
3.26 (q, 4H, J=7.1 Hz, two of PhNC-H2), 3.08 (t, 4H, J=4.8 Hz, four of pip-H), 2.95
(m, lH), 2.70-2.53 (m, 7H), 2.11 (m, lH, pipCH-H), 2.00 (m, lH, pipCH-H), 1.11 (t,
6H, J=7.0 Hz, two of NCH2C-H3) ~; HRMS Cstlcl~k~te~l for C25H34N301BrloO.152
C4H8O2 = 472.1964, found = 472.1956.
Step 3: 1-[2-[4~4-Diethylaminophenyl)-1-pi~ 6zi. yl]ethyU-isoclllc,~an-
6 ~ b~ e (VII)
Following the general pl'l)Ccdule of l~xANlpLE 1, Step 5 and mAking non-
critical vAriAffon~ but using 1-[2-(6-bromni~o~ c, an-1-yl)-ethyU-4~4-
diethyl~minoFhpnyl)piperazine (VI), 1-[2-t4-(4-diethylaminophenyl)-1-
yl]ethyl]isochrvl..an-6 c~b~ lP (VII) is obtained, Rf = 0.25
mPt~Annl/ethyl fl~etste~ 10/90); NMR (300 MHz,CDC13) 7.59, 7.18, 6.87, 6.68, 6.1,
5.7, 4.87, 4.13, 3.78, 3.26, 3.06, 2.78-2.57, 2.17, 2.05 and 1.11 o.~5 FxAMpLE 12 1-[2-t4-(3-Tl;~uo~ .,ethylphenyl)-1-
piperazinyl]ethyl]isochroman-6-c~l...Y,1...i-1P (VII)
Step 1: 1-t2-(6-Br mnico~l~l~an-l-yl)acetyl]-4~3
trifluoromethylphenyl)-~i~e~zi,le (V)
Following the general ~ lule of ~XAMPLE 1, Step 3 and m~king non-
30 critical v~ri~t;~nR but using racemic 6-br~mniRorh~oman-1-yl-acetic acid (IV) and 3-
uololue(llyl~he~yl~i~e~azille~ 1-[2-(6-bromniRGcl-~o Ian-l-yl)acetyl]-4~3-
uolv~elllyll~henyl)~ rnzi~e (V) is obtianed, Rf = 0.30 (ethyl acetate/hPY~nP~
40/60); IR aiq.) 1643, 1610, 1592, 1496, 1482, 1448, 1374, 1351, 1320, 1309, 1282,
1233, 1164, 1121, 1076 cm~l; NMR (300 MHz, CDCl3) 7.34 (m, 3H, aromatic), 7.08
35 (m, 4H, aromatic), 5.25 (brdd, lH, J=5.6 Hz, mP~inP), 4.11 (m, lH, OCH2a), 3.9i
m, lH, O=C-N-CH2a), 3.80-3.65 (m, 4H, ~=C-N-CH2bcd~ ~CH2b)~ 3-23 (m~ 4H~ Ph-
-47-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
NCH28), 2.95 (m, 2H, Ph-CH2a & N-CO-CH2a), 2.78 (dd, lH, J=14.8 Hz & J=3.7 Hz,
N-CO-CH2b), 2.66 (bd, lH, J=16.4 Hz, Ph-CH2b) ~; CMR (75 MHz, CDCl3) 169.2,
151.1, 136.4, 136.3, 131.8, 131.4, 129.7, 129.5, 126.5, 126.0, 122.4, 120.5, 119.2,
116.6, 112.7, 73.6, 63.6, 49.2, 48.8, 45.8, 41.6, 40.0 and 28.8 o; MS tEI, m/z) = 482.
Step 2: 1-[2-(6-Brnm. i~o~ u~an-l-yl)-ethyl]4~3
trifluoromethylphenyl)-piperazine (VI)
Following the general plùce-lul- of ~xANlpLE 1, Step 4 and m~kin~ non-
criticsl variations but using 1-t2-(6-bromoisoclnu 1an-1-yl)acetyl]-4{3-
trifluoromethylphenyl)piperazine (V), the product i8 obtained. This mr t~ri, l i8
purified by LC (silica gel, 230-400, 142 g; ethyl retstQ'hQY~nQ, 30/70) to give 1-t2~6-
brom.-Ji~orhroman-1-yl)-ethyl]-4-(3-Li~uolomethylphenyl)piperazine (VI), Rf = 0.40
(ethyl retstQ/h~QY- nQ~, 40/60); IR (liq.) 2824, 1610, 1496, 1481, 1449, 1357, 1319,
1293, 1239, 1164, 1123, 1076, 993, 949 and 695 cm~l; NMR (300 MHz, CDCl3) 7.32
(m, 3H, aromatic), 7.07 (m, 3H, aromatic), 6.97 (d, lH, J=8.2 Hz, aromatic), 4.79
(brdd, lH, J=5.7 Hz, m~Qtl~inQ), 4.11 (m, lH, OCH2a), 3.73 (m, lH, OCH2b), 3.23 (t,
4H, J=5.0 Hz, Ph-NCH2~), 2.93 (m, lH, Ph-CH2a), 2.58 (m, 7H, Ph-NC(H2)-CH2~-
NCH2 & Ph-CH2b), 2.10 (m, lH, C(H)-CH2a), 2.00 (m, lH, C(H)-CH2b) ~; C~ (75
MHz, CDCl3) 151.4, 137.0, 136.3, 131.7, 131.4 (qrt, JCF=36 Hz), 129.5, 129.3, 126.5,
126.2, 120.0, 118.6, 115.7 (d, JCF=4 Hz), 112.1 (d, JCF=4 Hz), 74.2, 62.9, 54.5, 53.2,
48.7, 33.2, 28.9 o; MS (EI, m/z) = 468.
Step 3: 1-[2-[4~3-Tri~uo.v lelhyl~henyl)-1-
~i~eL ~zillyl]ethyl]isodll u~sn-6 c~l,-. - ~ . . . ;~ (VII)
Following the general pluc6dul. of F~AMPLE 1, Step 5 snd m~kinF non-
criticsl vnri~ t;- n~ but using 1-[2-(6-brom.-i~orhroman-1-yl)-ethyl]-4~3-
trifluoromethylphenyl)~i~eL6z~e (VI) the product is ol~tsinP~l This material is
purified by LC (silics gel, 230-400, 120 g; ~cet--nP/hPY nP~ 50/50) to give 1-[2-[4~3-
trifluoromethylphenyl)-l-pipe.Azillyl]ethyl]isochromsn-6-c~l,..- ~...i~e (VII). This
m.~t,eri,li9le~ 11i7~ from ethyl r-~-t--tJlhexane to give 1-[2-[4~3-
trifluoromethylphenyl)-1-pi~.Azillyllethyl]isochromsn-6 c~l,~ (VII), mp =
30 129-131~; Rf = 0.22 ( ~-~st-nP/hPY-n.q, 50/50); IR (mull) 3383, 1647, 1618, 1606, 1567,
1407, 1359, 1322, 1312, 1287, 1161, 1139, 1115, 1098, 952 cm~l; N~ (300 MHz,
CDCl3) 7.60, 7.33, 7.18, 7.07, 6.00, 4.88, 4.14, 3.77, 3.24, 3.01, 2.78-2.48, 2.01 ~;
CMR (75 MHz, CDCl3) 169.1, 151.4, 142.4, 134.6, 131.5 (qrt, JCF=32 Hz), 131.4,
129.5, 128.2, 125.1, 125.0, 122.5, 118.6, 115.8 (d, JCF=4 Hz), 112.1 (d, JCF=4 Hz),
35 74.4, 63.0, 54.5, 53.2, 48.7, 33.2, 29.1 ~; HRMS (EI) r~lc~ t-~ for C23H26F3N3O2 =
433.1977, found = 433.1979.
-48-
CA 02225282 l997-l2-l9
W O 971022S9 P~~ 08681
~XA~rPLE 13 1-[2-t4~4-Methyl8u~u~yl~he~yl)~ e~ yl]ethyU-
isochroman e c~L~
Step 1: 1-[2-(6-Bromni~orhroman-1-yl)acetyl]-4{4-
methylsulfonylphenyl)-piperazine (V)
Following the general procedure of EXAMPLE 1, Step 3 and m~king non-
critical v~ri~t~ but using racemic 6-brnmnicorhroman-1-yl-acetic acid (IV) and 4-
methylsulfonylphe~lyl~ zine the product is obtained. This ms~t~ l is purified byLC (silica gel, 230-400, 150 g; ethyl acetate) to give 1-[2-(6-br~mnicorhroman-l-
yl)acetyl]-4-(4-methylsulfonylphenyl)piperazine (V), Rf= 0.30 (ethyl acetate); IR
(mull) 1639, 1593, 1508, 1481, 1405, 1295, 1239, 1146, 1105, 1096, 1027, 1000, 958,
825, 779 cm~l; NMR (300 MHz, CDCl3) 7.77 (d, 2H, J=8.9 Hz, aromatic), 7.28 (m,
2H, aromatic), 7.00 (d, lH, J=8.2 Hz, aromatic), 6.91 (d, 2H, J=9.0 Hz, aromatic),
5.24 (brdd, lH, J=5.7 Hz, m~t~in~), 4.10 (m, lH, OCH2a ), 3.94 (m, lH, O=CN-
CH2a), 3.78-3.60 (m, 4H, OCH2b, O=C-N-CH2bCd)~ 3-38 (m~ 4H~ Ph-N-CH28), 3-00-
2.88 (m, 5H, Ph-CH2a, CH3, N-C=O-CH2a), 2.78 (dd, lH, J=14.7 Hz & 3.6 Hz, N-
C=O-CH2b), 2.65 (brdd, lH, J=16.4 Hz, Ph-CH2b) o; CMR (75 MHz, CDCl3) 169.3,
153.9, 136.3, 131.8, 129.5, 129.2, 126.4, 120.5, 114.1, 73.7, 63.6, 47.5, 47.2, 45.5, 44.9,
41-3, 40-0 and 28-8 ~; HRMS (EI) ç~lr,~ tecl for C22H25BrN204S = 492.0719, found- 492.0714.
Step 2: 1-[2-(6-BrrmniAo~l~.v an-l-yl)-l-ethyl]~{4-
methyl_ulfonylphenyl)~i~e~ e (VI)
Following the general pl~cedu~ of F~AMpLE 1, Step 4 and making non-
critical v~ri~t;~n~ but u_ing 1-[2-(6-brQmniAo-~] . . vlllan-l-yl)acetyl]-4{4-
methylsulfonylphenyl)piperazine (V), 1-[2-(6-brnmni~o~l . . vl,.an-l-yl)-l-ethyl]-4-(4-
25 methylsulru-lyl~henyl)piperazine (VI) i8 obtained, Rf = 0.26 (ethyl acetate); IR (mull)
3586, 1592, 1507, 1481, 1424, 1405, 1296, 1249, 1145, 1109, 1095, 1004, 956, 822,
779 cm~l; N~ (300 MHz, CDCl3) 7.75 (d, 2H, J=9.0 Hz, aromatic), 7.27 (m, 2H,
aromatic), 6.95 (d, lH, J=8.3 Hz, aromatic), 6.90 (d, 2H, J=9.0 Hz, aromatic), 4.78
(brdd, lH, J=5.7 Hz, m~t~in~), 4.10 (m, lH, OCH2a), 3.74 (m, lH, OCH2b), 3.35 (t,
30 4H, J=6.0 Hz, Ph-NCH2s), 3.00 (8, 3H, CH3), 2.94 (m, lH, Ph-CH2a), 2.64 (m, 7H,
Ph-NC(H2)-CH28-NCH2 & Ph-CH2b), 2.09 (m, lH, C(H)-CH2a), 2.01 (m, lH, C(H)-
CH2b) ~; CMR (75 MHz, CDCl3) 154.3, 136.9, 136.4, 131.7, 129.3, 129.1, 128.6,
126.4, 120.1, 113.8, 74.1, 62.9, 54.4, 52.9, 47.3, 45.0, 33.1 and 28.9 8; HRMS (EI)
- r~lr~ t~ for C22H27BrN2O3S = 480.0906, found = 480.0903.
Step 3: 1-[2-[4{4-Methylsulfonylphenyl)-1-
pi~ yl]ethyl]isochroman-6 c~L
~9
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
Following the general plucc~ a of ~xAlvrpLE 1, Step 5 and mAking
non-critical vAriAt;~nR but using 1-[2-(6-bromrliRorhroman-1-yl)-1-ethyl]-4{4-
methylsulfonylphenyl)ui,u~ L~ille (VI) the product is obtained. This mntPriAl ispurified by LC (silica gel, 230-400 mesh, 75 g; mPthAnolldichlo~ hAnp~ 6/95) to
5 give 1-t2-t4~4-methylsul~u~yl~uhenyl)-1-piperazinyl]ethyl]isocl~ ,an-6 C~bUY~ P
(VII), mp = 217-219~; Rf = 0.17 (mPt~An~l/dichlorompthAnp~ 5/95); IR (mull) 3433,
1668, 1619, 1587, 1568, 1507, 1288, 1140, 1115, 1105, 1095, 1023, 998, 812, 780
cm~l; NMR (300 MHz, DMSO-d6) 7.90 (brds, lH, NH), 7.66 (m, 4H, aromatic), 7.28
(d, 2H, J=8.0 Hz, aromatic & NH), 7.06 (d, 2H, J=9.1 Hz, aromatic), 4.79 (brdd, lH,
10 J=6.5 Hz, m~inP), 4.08 (m, lH, OCH2a), 3.67 (m, lH, OCH2b), 3.32 (t, 4H, J=4.0
Hz, Ph-N-CH2~), 3.08 (8, 3H, CH3), 2.88 (m, lH, Ph-CH2a), 2.71 (dm, lH, J=16.4 Hz,
Ph-CH2b), 2.53 (m, 5H, Ph-NC(H2)-CH28-NCH2a ), 2.37 (m, lH, NCH2b), 2.13 (m,
lH, C(H)-CH2a), 1.98 (m, lH, C(H)-CH2b) o; C~ (75 MHz, DMSO-d6) 168.2, 154.3,
141.8, 134.1, 132.6, 129.0, 128.9, 128.5, 125.6, 125.2, 114.0, 74.1, 62.7, 54.6, 53.0,
15 47.2, 44.7, 32.9 and 29.0 ~; HRMS (FAB) cAlrlllAt~ for C23H29N304S+Hl =
444.1957, found = 444.1959.
LE 14 (S)~-)-1-[2-t4~4-Trifluoromethylphenyl)-l-~ yUethyl]-
isûcl~, I~-6-~ P (S)-(VII)
Following the general P1~J~dI11C of ~XAMPLE 1, Step 5 and making
20 non-critical vAriAt;~)n~ but using (S)-(-)-l-t2-(6-brc~mnicorhroman-1-yl)-1-ethyl]-4~4-
trifluoromethylphenyl),ui~ e (VI, EXA~LE 5, Step 2, 13.15 g, 28.0 mmol) the
product is oht~inP~- This mAt~riAl is purified by LC (~ilica gel, 230400 me~h, 780 g;
mPthAnnl/dichloromPthAnP~ 5/95) to give a crude product which is 1~ L.~ P~ from
mPthAnol/ethylAret~ to give (S)~-)-1-[2-[4~1 ~riflnoromethylphenyl)-l-
25 piperazinyl]ethyl]isochroman-6-ca-l,..-~...;-le (S)-(VII), mp = 166-168~; Rf = 0.20
(mPthAnnl/dichloromPthAnP~ 5/95); [a]D = -50~(c = 0.8533, m~lhAnrl); IR (mull) 3365,
3203, 1654, 1619, 1337, 1317, 1243, 1164, 1149, 1138, 1122, 1114, 1107, 1074, 825
cm~l; NMR (300 MHz, CDCl3) 7.57 (m, 2H, _romatic), 7.42 (d, 2H, J=8.7 Hz,
aromatic), 7.14 (d, lH, J=7.8 Hz, romatic), 6.87 (d, 2H, J=8.7 Hz, aromatic), 4.83
30 (brdd, lH, J=5.8 Hz, m~tllin~), 4.10 (m, lH, OCH2a), 3.73 (m, lH, OCH2b), 3.25 (t,
4H, J=4.9 Hz, Ph-N-CH28), 2.97 (m, lH, Ph-CH2a), 2.68-2.45 (m, 7H, Ph-NC(H2)-
CH2 -NCH2s, Ph-CH2b), 2.13 (m, lH, C(H)~CH2a)~ 2-02 (m~ lH~ C( ) 2b
(75 ~OEIz, CDCl3) 169.6, 153.2, 142.1, 134.5, 131.3, 128.2, 126.6, 126.3 (d, JCF=4 Hz),
125.0 (d, JCF=4 Hz), 122.9, 120.5 (qrt, JCF=33 Hz), 114.5, 74.4, 63.0, 54.5, 53.0, 47.8,
35 32.8 and 29.0 ~; HRMS (EI) cAlclllAt~ for C23H26F3N302 = 433.1977,
433.1978.
-50-
CA 0222~282 1997-12-19
W O 97/0225g PCT~US96tO8681
EXA~LE 15 1-[2-[4-(4-Ethoxyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-c~lJ~ r.~ide (IX)
Step 1: 1-[2-(6-Bromnicorhroman-l-yl)acetyl]-4-(4-ethoxyphenyl)-
piperazine (V)
Following the general procedure of ~AMPLE 1, Step 3 and making non-
critical vanations but using racemic 6-bromoisochroman-1-ylacetic acid (IV) and 4-
ethoxyphenylpiperazine, 1-[2-(6-bromoisochroman-1-yl)acetyl]-4-(4-ethoxyphenyl)-piperazine (V) is obtained, Rf = 0.46 (ethyl acetate/heY~n~, 70/30); IR (neat) 1641,
1511, 1480, 1463, 1443, 1278, 1243, 1231, 1108, 1049 cm~l; NMR (300 MHz, CDCl3)
7.28 (m, 2H, aromatic H's), 7.0 (d, lH, J=8.2 Hz, aromatic H), 6.86 (d, 4H, J=8.2 Hz,
aromatic H's), 6.25 (m of d, lH, J=6.5 Hz, PhC-H), 4.11 (m, lH), 3.99 (q, 2H, J=7.0
Hz, Proc-H2), 3.89 (m, lH), 3.80-3.59 (m, 4H), 3.05 (t, 4H, J=5.0 Hz), 3.03-2.89 (m'6,
2H), 2.77 (d of d, lH, Ja=3.6 Hz, Jb=14.9 Hz), 2.66 (m of d, lH, J=16.4 Hz), 1.39 (t,
3H, J=7.0 Hz, PhOCH2C-H3) o; CMR (75 MHz, CDCl3) 168.9, 153.6, 145.0, 136.4,
136.2, 131.6, 129.3, 126.4, 120.2, 118.7, 115.1, 73.3, 63.7, 63.55, 63.47, 63.4, 51.2,
50.7, 46.0, 41.8, 39.8, 28.7, 14.8 ~; HRMS ~~lr~ t~d for C23H27N203Br = 458.1205,
found = 458.1217.
Step 2: 1-[2-(6-BromniRorhroman-1-yl)-ethyl]-4-(4-ethoxyphenyl)-
piperazine (VI)
Following the general proc6dw.2 of EXAMPLE 1, Step 4 and m~king non-
critical variations but using l-t2-(6-bromni.corhroman-l-yl)acetyl]-4-(4-ethoxyphenyl)-
piperazine (V), 1-[2-(6-bromoi.corhroman-1-yl)ethyl]-4-(4-ethoxyphenyl)piperazine (VI)
is obtained, Rf = 0.56 (ethyl acetate/n-h~Y~n~, 70/30); IR (neat) 2850, 2810, 1512,
1482, 1251, 1231, 1153, 1108, 1048, 826 cm~1; NMR (300 MHz,CDC13) 7.29 (d, lH,
J=8.3 Hz, aromatic H), 7.27 (8, lH, aromatic H), 6.97 (d, lH, J=8.3 Hz, aromatic H),
6.85 (q, 4H, J=9.7 Hz, aromatic H's), 4.78 (m of d, lH, J=6.1 Hz), 4.14-4.07 (m, lH),
3.97 (q, 2H, J=7.0 Hz, PhOC-H2), 3.76-3.69 (m, lH), 3.10 (t, 4H, J=4.9 Hz, four of
pip-H), 2.95 (m, lH), 2.70-2.50 (m's, 7H, four pip-H, two PhCH-H, and NCH-H),
2.13 (m, lH, PhCHCH-H), 2.02 (m, lH, PhCHCH-H), 1.38 (t, 3H, J=6.9 Hz,
PhOCH2C-H3) o; CMR (75 MHz, CDCl3) 153.1, 145.6, 137.1, 136.3, 131.6, 129.3,
126.5, 1120.0, 118.1, 115.2, 74.3, 62.8, 55.6, 53.5, 50.6, 33.2, 28.9, 15.0 ~.
- Step 3: 1-[2-[4-(4-Ethoxyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-ca l,.-~...i~l~ (~)
Following the general plucedwe of EXAMPLE 5, Step 3 and m~king non-
35 critical v~ri~ti~ nR but using 1-[2-(6-brom- i~o~hroman-l-yl)ethyl]-4-(4-ethoxyphenyl)-
piperazine (VI), 1-[2-[4~4-ethoxyphenyl)-1-piperazinyl]ethyl]-N-methylisochroman-6-
-51-
CA 0222~282 1997-12-19
W O 97/02259 PCTrUS96tO8681
Cd~ r..i'1e(IX)i8 obtained, mp = 148-149~; Rf = 0.22 (m~thAnol/methylene
chloride, 5/95); IR (mull) 3334, 1633, 1536, 1515, 1310, 1245, 1237, 1146, 1108, 1050
cm~l; NMR (300 MHz, CDCl3) 7.54 (s, 2H, aromatic H's), 7.15 (d, lH, J=8.5 Hz,
aromatic H), 6.85 (d of d, 4H, Ja=9.2 Hz, Jb=19.2 Hz, four aromatic H's), 6.15 (broad
5 d, lH, C(O)N-H), 4.85 (m of d, lH, J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H),
3.97 (q, 2H, J=7 Hz, PhOC-H2), 3.77 (m, lH, PhCH2CH-H), 3.10 (t, 4H, J=4.8 Hz,
four of pip-H), 3.00 (d, 3H, J=4.9 Hz, C(O)NHC-H3), 3.00 (m, lH, NCH-H), 2.76-2.45
(several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m, lH, PhCHCH-H),
2.02 (m, lH, PhCHCH-H), 1.37 (t, 3H, J=7.0 Hz, PhOCH2C-H3) ~; CMR (75 MHz,
10 CDCl3) 168.0, 153.1, 145.6, 141.6, 134.5, 132.7, 127.7, 126.3, 125.0, 124.4, 118.1,
115.2, 74.5, 63.8, 62.9, 54.7, 53.5, 50.6, 33.2, 29.1, 26.8 and 15.0 ~.
EXAMPLE 16 1-[2-[4-(4-Propoxyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-carbnYAmi~le (IX)
Step 1: 1-[2-(6-Bromoi~o~hroman-l-yl)acetyl]-4-(4-p,v,uu,.y,uhenyl)-
piperazine (V)
Following the general procedure of F~AlvrpLE 1, Step 3 and mAking non-
critical vAriA~ n~ but using racemic 6-bromoiRo~hroman-l-yl-acetic acid (IV) and p-
pro~uo,~y,uhe-lyl,ui,ueLazine, 1-[2-(6-bromni~o~.hroman-l-yl)acetyl]-4~4-~pc.~y~henyl)
piperazine (V) is obtained, Rf = 0.50 (ethyl acetate/h~Y~n~, 70/30); IR (neat) 1641,
1511, 1481, 1464, 1443, 1278, 1242, 1230, 1108, 825 cm~l; NMR (300 MHz, CDCl3)
7.28 (m, 2H, aromatic H's), 7.0 (d, lH, J=8.2 Hz, aromatic H), 6.87 (d, 4H, J=8.2 Hz,
aromatic H's), 5.25 (m of d, lH, J=6.5 Hz, PhC-H), 4.11 (m, lH), 3.87 (t, 2H, J=7.0
Hz, Proc-H2), 3.86 (m, lH), 3.80-3.59 (m, 4H), 3.06 (t, 4H, J=5.0 Hz), 3.03-2.89 (m's,
2H), 2.77 (d of d, lH, Ja=3.6 Hz, Jb=14.9 Hz), 2.66 (m of d, lH, J=16.4 Hz), 1.78
(sextet, 2H, J=7.0 Hz, PhOCH2C-H2), 1.02 (t, 3H, J=7.0 Hz, PhOCH2CH2C-H3) ~;
CMR (75 MHz, CDCl3) 168.9, 153.6, 145.0, 136.4, 136.2, 131.6, 129.3, 126.4, 118.7,
115.1, 69.8, 63.4, 41.8, 39.8, 28.7, 22.5 and 10.4 o; HRMS cA~ lAte~l for
C24H29N2O3Br = 472.1362, found = 472.1356.
Step 2: 1-[2-(6-Brom ni ~orh roman- l-yl)-ethyl]-4-(4-p, vpo~y~henyl)-
piperazine (VI)
Following the general procedu~ of F~AlupLE 1, Step 4 and mAkinF non-
critical variations but using 1-[2-(6-brom- i~ochroman-1-yl)acetyl]-4-(4-
p~pu~y~henyl)-piperazine (V), 1-[2-(6-bromni~o~hroman-1-yl)-ethyl]-4~4-
pro~u~y~henyl)~i~elazi~le (VI) is obtained, Rf = 0.52 (ethyl acetate/h~YAn~ 70/30); IR
(mull) 1516, 1448, 1261, 1244, 1196, 1131, 1117, 1103, 100~, 985 cm~l; NMR (300
MHz, CDCl3) 7.29 (d, lH, J=8.3 Hz, aromatic H), 7.27 (s, lH, aromatic H), 6.97 (d,
-52-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
lH, J=8.3 Hz, aromatic H), 6.85 (q, 4H, J=9.7 Hz, aromatic H's), 4.78 (m of d, lH,
J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H), 3.87 (t, 2H, J=6.6 Hz, PhOC-H2), 3.77(m, lH, PhCH2CH-H), 3.10 (t, 4H, J=4.8 Hz, four of pip-H), 3.00 (m, lH, NCH-H),
2.76-2.45 (several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m, lH,
5 PhCHCH-H), 2.02 (m, lH, PhCHCH-H), 1.77 (sextet, 2H, J=6.9 Hz, PhOCH2C-H2),
1.01 (t, 8H, J=7.6 Hz, PhOCH2CH2C-H3) ~; CMR (75 MHz, CDCl3) 163.3, 14~.4,
136.8, 136.2, 131.5, 129.2, 126.4, 119.9, 118.1, 115.0, 69.8, 62.7, 54.5, 53.3, 50.4, 32.9,
28 7 22 6 10 4 o; HRMS ~lclfl~t~cl for C24H31N202Brl = 458.1561,
458.1569.
Step 3: 1-[2-[4-(4-Propoxyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochlv~an-6-carbnYS-mi~le (IX)
Following the general procedure of EXAMPLE 5, Step 3 and m~kinF non-
critical v~ri~t;onC but using 1-[2-(6-brom-iRochromarl-l-yl)-ethyl]-4~4-
propox rphenyl)-~ Ldzi~e (VI) the product i8 obtained. Rery~t~ tion from hot
15 ethyl acetate and hexane gives 1-[2-[4-(4-propol~ylJhenyl)-l-piperazinyl]ethyl]-N
methylisochroman-6-carbnY~mi~l~ (IX), Rf= 0.20 (m~th~n-~]/methylene chloride,
5/95); IR (mull) 3296, 1635, 1569, 1559, 1553, 1512, 1289, 1251, 1242, 1109 cm~l;
NMR (300 MHz, CDCl3) 7.54 (s, 2H, aromatic H's), 7.15 (d, lH, J=8.5 Hz, aromaticH), 6.85 (d of d, 4H, Ja=9.2 Hz, Jb=19.2 Hz, four aromatic H's), 6.15 (broad d, lH,
20 C(O)N-H), 4.85 (m of d, lH, J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H), 3.86 (t,
2H, J=6.6 Hz, PhOC-H2), 3.77 (m, lH, PhCH2CH-H), 3.10 (t, 4H, J=4.8 Hz, four of
pip-H), 3.00 (d, 3H, J=4.9 Hz, C(O)NHC-H3), 3.00 (m, lH, NCH-H), 2.76-2.45
(several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m, lH, PhCHCH-H),
2.02 (m, lH, PhCHCH-H), 1.77 (sextet, 2H, J=6.9 Hz, PhOCH2C-H2),1.01 (t, 3H,
25 Js7.4 Hz, PhOCH2CH2C-H3) o; CMR (7B MHz, CDCl3) 167.9, 153.2, 145.4, 141.5,
134.3, 132.5, 127.5, 124.9, 124.3, 118.0, 115.0, 69.8, 62.8, 54.5, 53.4, 50.5, 33.0, 30.5,
28.9, 26.7, 22.5 and 10.4 o.
EXAMPLE 17 (S)-(-)-1-[2-[4-(4-Tri~uornrnPthr~Yyphenyl)-l-piperazinyl]ethyl]-N-
methylisochroman-6-ca~ Y~...i~e (lX)
Step 1: (S)-(-)-1-[2-(6-Bromni~o-hroman-l-yl)acetyl]-4~4-
trifluorom~th-.xyphenyl)piperazine (S) (V)
Following the general plvCedule of EXAMPLE 1, Step 3 and making non-
critical v~ri~tionc but using 4-triflllorm~t~Yyphellyl~i~eldzine, (S)-(-)-1-[2-(6-
bromri~o-~hroman-l-yl)acetyl]-4-(4-trifluor m~th~yyphenyl)piperazine (S)-(V) is
obtained, [a]D = -70~ (c = 0.68, eth~nol); Rf = 0.52 (ethyl ~etot~ Y~ne 70/30); IR
(neat) 1641, 1511, 1482, 1465, 1445, 1266, 1232, 1211, 1160, 1108 cm~l; NMR (300
-53-
CA 0222~282 1997-12-19
W O 97/022S9 PCTrUS96/08681
MHz, CDCl3) 7.28 (m, 2H, aromatic H's), 7.14 (d, 2H, J=8.9 Hz, aromatic H's), 7.0 (d,
lH, J=8.2 Hz, aromatic H), 6.87 (d, 2H, J=8.9 Hz, aromatic H's), 5.25 (m of d, lH,
J=6.5 Hz, PhC-H), 4.11 (m, lH), 3.92 (m, lH), 3.75 (m, 4H), 3.16 (t, 4H, J=5.0 Hz),
3.03-2.89 (m's, 2H), 2.77 (d of d, lH, Ja=3.6 Hz, Jh=14.9 Hz), 2.66 (m of d, lH,5 J=16.4 Hz) 8; CMR (75 MHz, CDCl3) 168.9, 149.8, 145.0, 136.9, 136.4, 131.6, 129.3,
126.3, 122.0, 120.7, 117.1, 73.4, 63.4, 49.8, 48.2, 45.8, 42.0, 39.8, 28.7 8; HRMS
c~ t~d for C22H22N2F3~3Br = 498.0766, found = 498.0764.
Step 2: (S)-(-)-l-t2-(6-BromniRo~hroman-l-yl)-ethyl]-4-(4-
trifluoromPthnYyphenyl)-piperazine (S)-(VI)
Following the general pi'ocedul~ of ~AlVlPLE 1, Step 4 and making non-
critic~l v~t;nn~ but using (S)-(-)-1-[2-(6-bromoi~ochroman-1-yl)acetyl]4-(4-
trifluoromPthnYyphenyl)piperazine (S)-(V), (S)-(-)-1-[2-(6-bromni~o~hroman-1-yl)-
ethyl]4-(4-trifluoromPthn~ryphenyl)piperazine (S)-(VI) is obtained, Rf = 0.54 (ethyl
acetate/hPY~ne, 70/30); [a]D = -42~ (c = 1.1, eth~nol; IR (neat) 2825, 1513, 1281,
1263, 1240, 1204, 1184, 1157, 1112, 1106 cm~l; NMR (300 MHz, CDCl3) 7.29 (d, lH,J=8.3 Hz, aromatic H), 7.27 (s, lH, aromatic H), 7.11 (d, 2H, J=9 Hz, aromatic H's),
6.97 (d, lH, J=8.2 Hz, aromatic H), 6.87 (q, 2H, J=9.1 Hz, two aromatic H's), 4.78
(m of d, lH, J=6.0 Hz, PhC-H), 4.11 (m, lH, PhCH2CH-H), 3.75 (m, lH, PhCH2CH-
H), 3.18 (t, 4H, J=4.8 Hz, four of pip-H), 3.00 (m, lH, NCH-H), 2.76-2.45 (several
m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m, lH, PhCHCH-H~, 2.02 (m,
lH, PhCHCH-H) ~; CMR (75 MHz, CDCl3) 150.0, 136.5, 135.5, 131.5, 129.2, 126.3,
121.9, 120.1, 116.4, 74.1, 62.7, 54.9, 53.1, 49.1, 33.0 and 28.7 8; HRMS c~ l for
C22H24N2O2F3Brl (+1) = 486.0954, found = 486.0956.
Step 3: (S)~-)-1-[2-[4~4-TrifluoromPthr.Yyphenyl)-l-piperazinyl]ethyl]-N-
methylisochroman-6-c~ Y~ le (S)-(IX)
Following the general p~vcedu~e of EXAMPLE 5, Step 3 and m~kin~ non-
critical variations but using (S)-(-)-1-[2-(6-bromni~o~hlv an-1-yl)-ethyl]-4-(4-trifluoromPthnYyphenyl)piperazine (S) (VI) the product is obtained. Recrysts~ tinn
from hot ethyl acetate and hexane gives (S)~-)-1-[2-[4~4-trifluorom~thmryphenyl)-1-
piperazinyl]ethyl]-N-met~ylisodl-v lan-6-carbr~Y~mi-lP (S)-(IX), Rf = 0.20
mPthslnol/methylene ~hlnric~e, 5/95); IR (mull) 1636, 1614, 1572, 1551, 1513, 1450,
1270, 1238, 1157, 1107 cm~l; NMR (300 MHz, CDCl3) 7.54 (s, 2H, aromatic H's),
7.15 (d, lH, J=8.6 Hz, aromatic H), 7.11 (d, 2H, d=8.9 Hz, two aromatic H's), 6.87 (q
, 2H, J=8.9 Hz, two aromatic H's), 6.19 (broad d, lH, C(O)N-H), 4.86 (m of d, lH,
J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H), 3.77 (m, lH, PhCH2CH-H), 3.18 (t,
4H, J=4.8 Hz, four of pip-H), 3.00 (d, 3H, J=4.9 Hz, C(O)NHC-H3), 3.00 (m, lH,
-54-
CA 0222~282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
NCH-H), 2.76-2.45 (several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m,lH, PhCHCE-H), 2.02 (m, lH, PhCHCH-H) o; CMR (75 MHz, CDCl3) 167.8, 149.8,
144.7, 141.3, 134.3, 132.6, 127.5, 124.8, 124.3, 121.8, 116.5, 86.2, 74.3, 62.8, 54.54,
53.4, 48.9, 32.9, 28.9 and 26.7 S; HRMS ç~lr~ t~rl for C24HN303F3 = 463.2083,
found = 463.2086.
~AlVlPLE 18 (S)-(-)-1-[2-[4-(4-Ethylphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-ca~ 1e (S)-(IX)
Step 1: (S)-(-)-1-[2-(6-Broml~iRo-~hroman-l-yl)acetyl]-4-(4-ethylphenyl)-
piperazine (S)-(V)
Following the general procedure of ~AlVrPLE 1, Step 3 and making non-
critical variations but using 4-ethylphenylpiperazine, (S)-(-)-1-[2-(6-
BromoiRorhrom~n-l-yl)acetyl]-4-(4-ethylphenyl)piperazine (S)-(V) is obtained, Rf =
0.70 (ethyl ~ret~t~/hPY~n~, 70/30); [~]D = -81~(c = 0.7, ethanol); IR (neat) 1640, 1614,
1516, 1482, 1462, 1444, 1428, 1232, 1108 and 826 cm~l; NMR (300 MHz, CDCl3)
15 7.27 (m, 2H, aromatic H's), 7.11 (d, 2H, J=8.4 Hz, aromatic H's), 7.0 (d, lH, J=8.2
Hz, aromatic H), 6.86 (d, 2H, J=8.2 Hz, aromatic H's), 5.25 (m of d, lH, J=6.5 Hz,
PhC-H), 4.08 (m, lH), 3.89 (m, lH), 3.80-3.59 (m, 4H), 3.11 (t, 4H, J=5.0 Hz), 3.03-
2.89 (1~118, 2H), 2.77 (d of d, lH, Ja=3.6 Hz, Jb=14.9 Hz), 2.66 (m, lH), 2.57 (q, 2H,
J=7.6 Hz, PhC-H2), 1.20 (t, 3H, J=7.6 Hz, PhCH2C-H3) o; CMR (75 MHz, CDCl3)
20 169.0, 149.0, 136.5, 136.3, 131.8, 129.4, 128.6, 126.5, 120.4, 116.9, 73.5, 63.5, 50.3,
49.8, 46.1, 41.9, 39.9, 28.8, 27.9 and 15.7 o.
Step 2: ($)~-)-1-[2-(6-BromniRorhroman-l-yl)-ethyl]-4~4-ethylphenyl)-
piperazine (S)-(VI)
Following the general procedure of EXAMF~LE 1, Step 4 and making non-
25 critical variations but using (S)-(-)-1-[2-(6-brom~iRorhroman-l-yl)acetyl34-(4-
ethylphenyl)-piperazine (S)-(V), (S)~-)-1-[2-(6-BromoiRorhroman-l-yl)-ethyl]-4-(4-
ethylphenyl)-piperazine (S)-(VI) i~ obtained, Rf = 0.61 (ethyl acetate/h.~Yzln~, 30/70);
IR (mull) 2960, 2929, 2819, 1516, 1481, 1379, 1237, 1143, 1111 and 822 cm~l; NMR(300 MHz, CDCl3) 7.29 (d, lH, J=8.3 Hz, aromatic H), 7.27 (8, lH, aromatic H), 7.09
30 (d, 2H, J=8.6 Hz, aromatic H's), 6.98 (d, lH, J=8.2 Hz, aromatic H), 6.87 (d, 2H,
J=8.6 Hz, aromatic H's), 4.77 (m of d, lH, J=6.0 Hz, PhC-H), 4.13 (m, lH,
PhCH2CH-H), 3.75 (m, lH, PhCH2CH-H), 3.17 (t, 4H, J=4.8 Hz, four of pip-H), 3.00(m, lH, NCH-H), 2.70-2.53 (several m's, 9H, four pip-H, two PhCH-H, PhC-H2, and
NCH-H), 2.14 (m, lH, PhCHCH-H), 2.02 (m, lH, PhCHCH-H), 1.20 (t, 3H, J=7 6
35 Hz, PhCH2C-H3) o; CMR (75 MHz, CDCl3) 149.4, 137.1, 136.3, 135.6, 131.6, 129.3,
128.4, 126.5, 120.0, 116.3, 74.3, 62.8, 54.7, 53.5, 49.6, 33.2, 30.3, 28.9, 27.9 and 15.7
-55-
CA 0222~282 1997-12-19
W O 97/022S9 PCTrUS96/08681
o; HR~S calculated for C23H29N201Brl = 430.1444, found = 430.1443.
Step 3: (S)-(-)-1-[2-[4-(4-Ethylphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-carb- YAm~ (S)-(lX)
Following the general pl~cedu~,a of EXAMPLE 5, Step 3 and mPking non-
critical variations but using (S)-(-)-1-[2-(6-bromr iRocl-roll,an-l-yl)-ethyl]-4-(4-
ethylphenyl)-piperazine (S)-(VI) the desired product is obtained. Recryst~ 7Ation
from hot ethyl acetate and hexane gives (S)-(-)-1-[2-[4-(4-ethylphenyl)-1-
pipelazhlyl]ethyl]-N-methyl-isochroman-6-ca~L.~Y~ 1e (S)-(IX), mp = 138-140~; Rf =
0.28 (m~t~An~l/methylene chloride, 5/95); [a]D = -50~ (c = 0.93, m~t~Annl/methylene
chloride, 50/50); IR (mull) 3321, 1635, 1614, 1539, 1518, 1405, 1312, 1238, 1107 and
822 cm~l; NMR (300 MHz, CDCl3) 7.B4 (s, 2H, aromatic H's), 7.15 (d, lH, J=8.6 Hz,
aromatic H), 7.09 (d, 2H, J=8.5 Hz, 2 aromatic H's), 6.86 (d, 2H, J=8.5 Hz, 2
aromatic H's), 6.14 (broad d, lH, C(O)N-H), 4.86 (m of d, lH, J-6.0 Hz, PhC-H), 4.13
(m, lH, PhCH2CH-H), 3.77 (m, lH, PhCH2CH-H), 3.17 (t, 4H, J=4.8 Hz, four of pip-H), 3.00 (d, 3H, J=4.9 Hz, C(O)NHC-H3), 3.00 (m, lH, NCH-H), 2.76-2.45 (several
m's, 9H, four pip-H, two PhCH-H, PhC-H2, and NCH-H), 2.14 (m, lH, PhCHCH-H),
2.02 (m, lH, PhCHCH-H), 1.20 (t, 3H, J=7.6 Hz, PhCH2C-H3) o; CMR (75 MHz,
CDC13) 168.0, 149.3, 141.6, 135.7, 134.5, 132.7, 128.4, 127.7, 125.0, 124.4, 116.3,
62.9, 54.7, 53.4, 49.6, 33.1, 29.1, 27.9, 26.8 and 15.7 ~; HR~IS rA~ At,e(l for
C25H33N3~3 = 407-2573, found = 407.2581.
EXAMPLE 19 (S)-(-)-1-[2-[4-(4-EthoYyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-c~ . L. .~ le (S)-(IX)
Step 1: (S)-(-)-1-[2-(6-BromoiRo~ ,an-l-yl)acetyl]-4-(4-ethoxyphenyl)-
piperazine (S)-(V)
Following the general p-~ ce~lu-~e of EXA~LE 1, Step 3 and mAkinF non-
critical variations but using 4-ethoxyphenylpiperazine, (S)-(-)-1-[2-(6-
Brom~iRorhroman-l-yl)acetyl]4-(4-ethoxyphenyl)piperazine (S)-(V) is obtained, Rf =
0.60 (ethyl AcetAt~ YAne, 70/30); [a]D = -78~ (c = 0.82, et~An~l); IR (neat) 1627,
1515, 1478, 1441, 1429, 1250, 1230, 1102, 1031 and 821 cm~l; NMR (300 MHz,
CDC13) 7.28 (m, 2H, aromatic H's), 7.0 (d, lH, J=8.2 Hz, aromatic H), 6.86 (d, 4H,
J=8.2 Hz, aromatic H's), 5.25 (m of d, lH, J=6.5 Hz, PhC-H), 4.11 (m, lH), 3.99 (q,
2H, J=7.0 Hz, PhOC-H2), 3.89 (m, lH), 3.80-3.59 (m, 4H), 3.05 (t, 4H, J=5.0 Hz),3.03-2.89 (m's, 2H), 2.77 (d of d, lH, Ja=3.6 Hz, Jb=14.9 Hz), 2.66 (m of d, lH,J=16.4 Hz), 1.39 (t, 3H, J=7.0 Hz, PhCH2C-H3) ~; CMR (75 MHz, CDC13) 168.9,
153.5, 145.2, 136.3, 136.2, 131.6, 129.3, 126.4, 118.7, 115.1, 73.3, 63.7, 63.4, 51.2,
50.7, 46.1, 41.9, 39.8, 28.7 and 14.8 ~; HRMS ÇA~ lAt~d for C23H27N2O3Br =
-56-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
458.1205, found = 458.1203.
Step 2: (S)-(-)-1-[2-(6-BromniRochroman-l-yl)-ethyl]-4-(4-ethoxyphenyl)-
piperazine (S)-(VI)
Following the gener~l plvce~ e of EXAMPLE 1, Step 4 and making non-
5 critical variations but using (S)-(-)-l-[Z-(6-br moi~orhroman-1-yl)acetyl]-4-(4-
ethoxyphenyl)piperazine (S)-(V) gives (S)-(-)- 1-[2-(6-brom ni cof~h roman 1-yl)-ethyl]-4-
(4-ethoxyphenyl)-piperazine (S)-(VI), mp = 8~-87~; Rf = 0.28 (ethyl acetate/n-h~Y~n~,
30/70); [a]D = -46~ (c = 0.60, ethanol); IR (neat) 1516, 1476, 1261, 1246, 1196, 1130,
1117, 1104, 1060 and 932 cm~l; N~ (300 MHz,CDCl3) 7.29 (d, lH, J=8.3 Hz,
10 aromatic H), 7.27 (s, lH, aromatic H), 6.97 (d, lH, J=8.3 Hz, aromatic H), 6.85 (q,
4H, J=9.7 Hz, aromatic H's), 4.78 (m of d, lH, J=6.1 Hz), 4.144.07 (m, lH), 3.97 (q,
2H, J=7.0 Hz, PhOC-H2), 3.76-3.69 (m, lH), 3.10 (t, 4H, J=4.9 Hz, four of pip-H),
2.95 (m, lH), 2.70-2.50 (m's, 7H, four pip-H, two PhCH-H, a~d NCH-H), 2.13 (~n,
lH, PhCHCH-H), 2.02 (m, lH, PhCHCH-H), 1.38 (t, 3H, J=6.9 Hz, PhOCH2C-H3) o;
15 CMR (75 MHz, CDCl3) 153.1, 145.6, 137.1, 136.3, 131.6, 129.3, 126.5, 1120.0, 118.1,
115.2, 74.3, 62.8, 55.6, 53.5, 50.6, 33.2, 28.9 and 15.0 o.
Step 3: (S)-(-)-1-[2-t4~4-Ethoxyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-ca~ (S)-(IX)
Following the gener~l procedure of FXAMPLE 5, Step 3 and making non-
20 critical v~ri~ti~ n~ but using (S)-(-)-1-[2-(6-brnmni~o~ ,.an-1-yl)-ethyl]-4~4-
ethoxyphenyl)piperazine (S)-(VI) gives (S)-(-)-1-[2-[4~4-ethoxyphenyl)-1-
piperazinyl]ethyU-N-methyl-isochroman-6-calL..~ 1P (S)-(IX). Recrygt~ t~n
from hot ethyl acetate and hexane gives purified product, mp = 156-157~; Rf = 0.20
(m~t~ls3nol/methylene ~.hlnri~e, 5/95); [a]D = -48~ (c = 0.94, m~t~l~nol/methylene
25 chloride, 50/50); IR (mull) 3334, 1633, 1536, 1515, 1310, 1245, 1237, 1146, 1108 and
1050 cm~l; NMR (300 MHz, CDCl3) 7.54 (s, 2H, aromatic H's), 7.15 (d, lH, J=8.5 Hz,
aromatic H), 6.85 (d of d, 4H, Ja=9.2 Hz, Jb=19.2 Hz, four aromatic H's), 6.15 (broad
d, lH, C(O)N-H), 4.85 (m of d, lH, J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H),
3.97 (q, 2H, J=7 Hz, PhOC-H2), 3.77 (m, lH, PhCH2CH-H), 3.10 (t, 4H, J=4.8 Hz,
30 four of pip-H), 3.00 (d, 3H, J=4.9 Hz, C(O)NHC-H3), 3.00 (m, lH, NCH-H), 2.76-2.45
(several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m, lH, PhCHCH-H),
2.02 (m, lH, PhCHCH-H), 1.37 (t, 3H, J=7.0 Hz, PhOCH2C-H3) 8; CMR (75 MHz,
CDCl3) 168.0, 153.1, 145.6, 141.6, 134.5, 132.7, 127.7, 126.3, 125.0, 124.4, 118.1,
115.2, 74.5, 63.8, 62.9, 54.7, 53.5, 50.6, 33.2, 29.1, 26.8 and 15.0 ~; HRMS r~lclllz3t~e~
35 for C25H33N3~3 = 4~-3 ~ found = 423.26l8
~XANIPLE 20 (S)~-)-1-[2-[4-(4-Phenylmethyloxyphenyl)-l-pi~ ziLlyl]ethyl]-N
-57-
CA 0222F7282 l997-l2-l9
W O 97/02259 PCTnUS96/08681
methylisochrvL.lan-6-ca~ Y ~3 l . . i d P (S)-(IX)
Step 1: (S)-(-)-1-[2-(6-Brom~i7~o~hroman-1-yl)acetyl]-4~4-
phenylmethyloxyphenyl)piperazine (S)-(V)
Following the general pl'OCedU~e of EXAMPLE 1, Step 3 and making non-
critical v~ri~t;on~ but using 4-phenylmethyloxyphe~yl~ .dzine (3.38 g, 12.6 mmol),
the product is obtained. This m~tPri~l is purified by HPLC on a single silica gel
cartridge eluting with ethyl~et~tA/hexane (70/30) to give (S)-(-)-1-[2-(6-
bromni,~or,hroman-l-yl)acetyl]-4-(4-phenylmethyloxyphenyl)piperazine (S)-(V), Rf =
0.30 (ethyl ~ret~t~/h~Y~n~, 50/50); [a]D = -34~ (c = 0.50, m~tl~nol); NMR (300 MHz,
CDCl3) 7.35 (m, 7H, aromatic), 6.96 (d, lH, J=8.2 Hz, aromatic), 6.90 (m, 4H,
aromatic), 5.26 (brdd, lH, J=5.4 Hz, mPthinP), 5.02 (s, 2H, Ph-CH2-0), 4.09 (m, lH,
OCH2a ), 3.89 (m, lH, O=C-N-CH2a), 3.81-3.64 (m, 4H, OCH2b, O=C-N-CH2bCd),
3.05 (m, 4H, Ph-N-CH28), 3.00-2.89 (m, 2H, Ph-CH2a & N-C=O-CH2a), 2.76 (dd, lH,
J=14.9 Hz & 3.6 Hz, N-C=O-CH2b), 2.66 (brdd, lH, J=16.4 Hz, Ph-CH2b) ~; CMR
(75 MHz, CDCl3) 169.0, 153.6, 145.5, 137.3, 136.6, 136.3, 131.8, 129.4, 128.6, 127.9,
127.5, 126.5, 120.4, 118.8, 115.7, 76.7, 73.5, 70.5, 63.5, 51.3, 50.7, 46.2, 42.0, 40.0
and 28.8 ~.
Step 2: (S)~-)-1-[2-(6-Brom-.ico~hroman-l-yl)-ethyl]-4~4-
phenylmethyloxyphenyl)piperazine (S)-(VI)
Following the general p~ocedu~e of EXAMPLE 1, Step 4 and m~king non-
critical v~ri~tionc but using (S)~-)-1-[2~6-bromoicorh.v,~.an-l-yl)acetyl]-4~4-
phenylmethyloxyphenyl)piperazine (V, 5.96 g, 11.4 mmol) gives (S)-(-)-1-[2~6-
brom~ ochl~an-1-yl)-ethyl]-4-(4-phenylmethyloxyphenyl)-~ .d~hle (S)-(VI), Rf =
0.40 (ethyl ~cet~tP/hPY~n~ 50/50); [a]D = -63~ (c = 0.925, mPt~l~nol); NMR (300 MHz,
CDCl3) 7.27 (m, 7H, aromatic), 6.91 (d, lH, J=8.3 Hz, aromatic), 6.83 (8, 4H,
aromatic), 4.94 (8, 2H, Ph-CH2-O), 4.73 (brdd, lH, J=5.7 Hz, mPthinP), 4.03 (m, lH,
OCH2a), 3.67 (m, lH, OCH2b), 3.05 (t, 4H, J=4.8 Hz, Ph-NCH2s), 2.88 (m, lH, Ph-
CH2a), 2.60 (m, 7H, Ph-NC(H2)-CH2S-NCH2 & Ph-CH2b), 2.05 (m, lH, C(H)-CH2a),
1.97 (m, lH, C(H)-CH2b) ~; CMR (75 MHz, CDCl3) 153.1, 145.8, 137.4, 136.9, 136.3,
131.7, 129.3, 128.5, 127.9, 127.5, 126.5, 120.1, 118.2, 115.6, 70.5, 62.9, 54.6, 53.4,
50.4, 32.9 and 28.9 ~.
Step 3: (S)-(-)-1-[2-[4~4-Phenylmethyloxyphenyl)-1-piperazinyl]ethyl]-N-
methyl-isochroman-6-ca,l,~Y ~ P, (S)-(IX)
Following the general pl~ cedu~e of EXAMPLE 5, Step 3 and making non-
critical v~ri~ti~nc but using (S)-(-)-1-[2-(6-bromoicorhroman-1-yl)~thyl]-4-(4-
phenylmethyloxyphenyl)piperazine (S)-(VI, 5.08 g, 11.4 mmol) gives the product.
-58-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~r~/~ e 6~1
Thi8 m~tf~ri~l is purified by LC (silica gel, 230-400 mesh, 270 g; ethyl acetate) and
le. ~y~ from ethyl acetate to give (S)~ 1-[2-[4-(4-phenylmethyloxyphenyl)-1-
piperazinyl]ethyl]-N-methyl-isochroman-6-call,..x,1...ide (S)-(IX), mp = 164-167~; Rf =
0.40 (m~th~nnl/ethyl~retst~, 5/95); [a]D = -40~ (c = 0.9323, m~+ls~nol); IR (mull)
5 3302, 1639, 1544, 1515, 1498, 1314, 1291, 1272, 1252, 1153, 1138, 1111, 818, 735
and 695 cm~l; NMR (300 MHz, CDCl3) 7.53 (m, 2H, aromatic), 7.54 (m, 2H,
aromatic), 7.43-7.23 (m, 5H, aromatic), 7.14 (d, lH, J=8.6 Hz, aromatic), 6.89 (s, 4H,
aromatic), 6.19 (brdm, lH, NH), 5.01 (s, 2H, Ph0-CH2), 4.86 (brdd, lH, J=6.9 Hz,m~hinf~,), 4.14 (m, lH, OCH2a), 3.76 (m, lH, OCH2b), 3.10 (t, 4H, J=4.8 Hz, Ph-N-
10 CH28), 3.00 (d, 4H, J=4.8 Hz, N-CH3 & Ph-CH2a), 2.76-2.49 (m, 7H, Ph-NC(H2)-
CH28-NCH25, Ph-CH2b), 2.14 (m, lH, C(H)-CH2a), 2.04 (m, lH, C(H)-CH2b) ~; CMR
(75 MHz, CDCl3) 168.0, 153.0, 145.9, 141.6, 137.4, 134.5, 132.7, 128.5, 127.9, 127.7,
127.5, 125.0, 124.4, 118.0, 115.6, 74.5, 70.5, 63.0, 54.7, 53.5, 50.5, 33.2, 29.1 and 26.9
~; MS (EI, m/z) = 485; HRMS (EI) c~ t~l for C30H35N303 = 485-2678, found =
15 486.2675.
EXAMPLE 21 (R)~+)-1-[2-[4-(4-Ethoxyphenyl)-l-piperazinyl]ethyl]-N-methyl-
i~ocl~l~ ,an-6-carbnY~mi-le (R)-(IX)
Step 1: (R)~+)-1-[2-(6-Brqmni~orhroman-l-yl)acetyl]4-(4-ethoxyphenyl)-
piperazine (R-V)
Following the general p.~ce~ of EXAMPLE 2, Step 2 and m~kinF non-
critical v~ri~ti~ n~ but using 4-ethoxyphel~yl~ c~dzine gives (R)-(+)-1-[2-(6-
bromoico. ~ an-l-yl)acetyl]-4-(4-ethoYyphenyl)piperazine (R-V), Rf = 0.60 (ethylacetate/h~Y~n~, 70/30); [a]D = +76~ (c = 0.71, eth~nol); IR (neat) 1626, 1515, 1478,
1442, 1249, 1246, 1230, 1102, 1030 and 821 cm~l; NMR (300 MHz, CDC13) 7.28 (m,
2H, aromatic H's), 7.0 (d, lH, Jz8.2 Hz, aromatic H), 6.86 (d, 4H, J=8.2 Hz, aromatic
H's), 5.25 (m of d, lH, J=6.5 Hz, PhC-H), 4.11 (m, lH), 3.99 (q, 2H, J=7.0 Hz, PhOC-
H2), 3.89 (m, lH), 3.80-3.59 (m, 4H), 3.05 (t, 4H, J=5.0 Hz), 3.03-2.89 (m's, 2H), 2.77
(d of d, lH, Ja=3.6 Hz, Jb=14.9 Hz), 2.66 (m of d, lH, J=16.4 Hz), 1.39 (t, 3H, J=7.0
Hz, PhCH2C-H3) ~; CMR (75 MHz, CDCl3) 168.9, 153.6, 145.0, 136.4, 136.2, 131.6,
129.3, 126.4, 120.2, 118.7, 115.1, 73.3, 63.7, 63.55, 63.47, 63.4, 51.2, 50.7, 46.0, 41.8,
39.8, 28.7 and 14.8 o; HRMS calculated for C23H27N2O3Br (on Br 81 ion) =
460.1185, found = 460.1179.
Step 2: (R)-(+)-1-[2-(6-Bromni~orhroman-l-yl)-ethyl]-4-(4-ethoxyphenyl)-
pi~ e (R-VI)
Following the general pL.,ce.lu~e of F~XAlvlPLE 2, Step 3 and m~kinF non-
critical v~ri~t;~n~ but using (R)-(+)-1-[2-(6-brnmoi~orhroman-1-yl)acetyl]-4-(4-
-59-
CA 0222~282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
ethoxyphenyl)piperazine (R-V) gives (R)-(+)- 1-[2-(6-br~m oi ~o~h roman- 1-yl)-ethyl]-4-
(4-ethoxyphenyl)piperazine (R-VI), Rf = 0.25 (ethyl ~-et~t~/n-h~Y~ne, 30/70); [a]D =
+43~ (c = 0.73, eth~nol); IR (neat) 2820, 1511, 1478, 1446, 1250, 1225, 1116, 1108,
1047 and 825 cm~l; NMR (300 MHz,CDCl3) 7.29 (d, lH, J=8.3 Hz, aromatic H), 7.27
(s, lH, aromatic H), 6.97 (d, lH, J=8.3 Hz, aromatic H), 6.85 (q, 4H, J=9.7 Hz,
aromatic H's), 4.78 (m of d, lH, J=6.1 Hz), 4.14-4.07 (m, lH), 3.97 (q, 2H, J=7.0 Hz,
PhOC-H2), 3.76-3.69 (m, lH), 3.10 (t, 4H, J=4.9 Hz, four of pip-H), 2.95 (m, lH),
2.70-2.50 (m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.13 (m, lH, PhCHCH-
H), 2.02 (m, lH, PhCHCH-H), 1.38 (t, 3H, J=6.9 Hz, PhOCH2C-H3) ~; CMR (75
10 MHz, CDCl3) 153.1, 145.6, 137.1, 136.3, 131.6, 129.3, 126.5, 1120.0, 118.1, 115.2,
74.3, 62.8, 55.6, 53.5, 50.6, 33.2, 28.9 and 15.0 o; HRMS cAl~lllAtecl for
C23H29N2O2Brl = 444.1413, found = 444.1413.
Step 3: (R)-(+)-1-[2-[4-(4-Etho~gphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-carbnY~mi-le (R-IX)
15Following the general procedure of EXAMPLE 5, Step 3 and making non-
critical variations but using (R)-(+)-1-[2-(6-bromoi~o~hroman-1-yl)-ethyl]-4-(4-ethoxyphenyl)piperazine (R-VI) gives (R)-(+)-1-[2-[4-(4-ethoxyphenyl)-1-
piperazinyl]ethyl]-N-methylisochroman-6-call,.,Y;1..,il1e (R-IX), Rf = 0.20
(mPtl~n~l/methylene chloride, 5/95); [a]D = +49~ (c = 0.93, m~ nol/methylene
20 rhlnritlç, 50/50); IR (mull) 3334, 1633, 1536, 1515, 1310, 1245, 1237, 1146, 1108 and
1050 cm~l; NMR (300 MHz, CDCl3) 7.54 (s, 2H, aromatic H's), 7.15 (d, lH, J=8.5 Hz,
aromatic H), 6.85 (d of d, 4H, Ja=9.2 Hz, Jb=19.2 Hz, four aromatic H's), 6.15 (broad
d, lH, C(O)N-H), 4.85 (m of d, lH, J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H),
3.97 (q, 2H, J=7 Hz, PhOC-H2), 3.77 (m, lH, PhCH2CH-H), 3.10 (t, 4H, J=4.8 Hz,
25 four of pip-H), 3.00 (d, 3H, J=4.9 Hz, C(O)NHC-H3), 3.00 (m, lH, NCH-H), 2.76-2.45
(several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m, lH, PhCHCH-H),
2.02 (m, lH, PhCHCH-H), 1.37 (t, 3H, J=7.0 Hz, PhOCH2C-H3~ ~; CMR (75 MHz,
CDCl3) 168.0, 153.1,145.6, 141.6, 134.5, 132.7, 127.7,126.3,125.0, 124.4,118.1,
115.2, 74.5, 63.8, 62.9, 54.7, 53.5, 50.6, 33.2, 29.1, 26.8 and 15.0 ~; HRMS c~ te~l
30 for C25H33N3~3 = 4~3 ~5~ found = 423.2516.
EXAMPLE 22 1-[2-[4-(3-Trifluoromethylphenyl)-l-piperazinyl]ethyl]-N-
methylisochroman-6-calL..~J'...;~ (IX)
Step 1: 1-[2-[4-(3-Tri~uoromethylphenyl)-l-piperazinyl]ethyl]-N,N-di-t-
butylo~y~ ~I.ollylisochlulllan-6-carbn~r~mi-le (VIII)
35Following the general procedure of F~AlvlpLE 3, Step 1 and making
non-critical v~ri~t~ but using 1-[2-[4-(3-trifluoromethylphenyl)-1-
-60-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
pip~L~zi~yl]ethyl]-isochroman-6-c~l,--Y~ gives crude product. This mslt~ l is
purified by LC (silica gel, 230-400 mesh, 60 g; ~e+ona/haY~na, 25/75) to give 1-[2-[4-
(3-trifluoromethylphenyl)-1-piperazinyl]ethyl]-N,N-di-t-butyloxycarbonylisochroman-
6-ca~ x~lllitle (VIII), Rf = 0.37 (~etonalhaY~na, 25/75).
Step 2: 1-[2-[4-(3-Trifluoromethylphenyl)-1-piperazinyl]ethyl]-N-methyl-
isochroman-6-carb~ Y~mi~la (~)
Following the general ploceduse of F'XAl\/IPLE 3, Step 2 and making
non-critical v~ri~tior ~ but using l-[a-[4-(3 t~fll~oromethylphenyl)-1-
pi~la~ yl]ethyl]-N~N-di-t-butylu~y~ ùllylisûclllulllan-6-c~Ll~x~ (VIII, 1.13 g,
10 1.8 mmol) gives crude product. This m~t~ri~ll i8 purified by LC (silica gel, 230~00
mesh, 66 g; ~- etor~PY~n~ 50/50) to give 1-[2-[4-(3-trifluoromethylphenyl)-1-
piperazinyl]ethyl]-N-methylisochroman-6 carL-.Y~ (IX) which is rec..~ lli7~c
from ethyl~et~t~/h-oY~ne, mp = 142-143~; Rf = 0.33 (~cetonplh~yslnf~ 50/50); IR
(mull) 3307, 1637, 1612, 1606, 1558, 1443, 1311, 1290, 1247, 1151, 1136, 1122, 1109,
16 1099 and 951 cm~l; NMR (300 MHz, CDCl3) 7.55 (m, 2H, arv ,a~ic), 7.33 (t, lH,
J=8.0 Hz, aromatic), 7.12 (d, lH, J=8.6 Hz, aromatic), 7.06 (m, 4H, aromatic), 6.14
(brdm, lH, NH), 4.87 (brdd, lH, J=5.9 Hz, mat~lina)~ 4.14 (m, lH, OCH2a), 3.76 (m,
lH, OCH2b), 3.24 (t, 4H, J=5.0 Hz, Ph-N-CH28), 3.00 (d, 4E, J=4.9 Hz, N-CH3 & Ph-
CH2a), 2.76-2.48 (m, 7H, Ph-NC(H2)-CH2B-NCH2s, Ph-CH2b), 2.15 (m, lH, C(H)-
CH2a), 2.05 (m, lH, C(H)-CH2b) ~; CMR (75 MHz, CDCl3) 168.0, 151.4, 141.6, 134.5,
132.7, 131.4 (qrt, JCF=32 Hz), 129.5, 127.7, 125.0, 124.4, 118.6, 115.7 (d, JCF=4 Hz),
112.1 (d, JCF=4 Hz), 74.4, 63.0, 54.6, 53.2, 48.7, 33.2, 29.1 and 26.9 o; HR~qS (EI)
tA~ for C24H28F3N3~2 = 447.2133, found = 447.2132.
EXAMPLE 23 1-[2-[4-(4-Met~ylsulfonylphenyl)-l-piperazinyl]ethyl]N-
methylisochroman-6-carb~ mi~l.s (IX)
Step 1: 1-[2-[4-(4-Methylsulfonylphenyl)-1-piperazinyl]ethyl]-N,N-di-t-
butylo~ &bullyLsochroman-6-c~l~ le (VIII)
Following the general p,~,cedu~ of EXAMPLE 3, Step 1 and making
non-critical v~ri~ nc but using 1-[2-[4-(4-methylsulru..yl~henyl)-1-
30 piperazinyl]ethyl]-isochroman-6-c~hl,,.~;-le (825 mg, 1.9 mmol) gives crude
product. This m~ l is purified by LC (silica gel, 230-400 mesh, 58 g;
~etonP/haY~na, 45/55) to give 1-[2-~4-(4-methylsulfonylphenyl)-1-piperazinyl]ethyl]-
N,N-di-t-butylo~ycall,onylisochroman-6-ca~ e (VIII), Rf = 0.20
(~eton~ Y~n.q, 40/60).
Step 2: 1-[2-[4-(4-Methylsulfonylphenyl)-1-~i~e~ yl]ethyl]-N-methyl-
isochroman-6-carboY~mi~e (IX)
-61-
CA 0222~282 1997-12-19
W O 97/022S9 PCTrUS96/08681
Following the general procedure of EXAMPLE 3, Step 2 and making
non-critical v~ri~t;onA but using 1-[2-[4-(4-methylsulfonylphenyl)-1-
piperazinyl]ethyl]-N,N-di-t-butylu~ ~bu-lylisochroman-6-carbnY~mi-le (VIII, 660
mg, 1.0 mmol) gives crude product. This material is purified by LC (silica gel,
5 230-400 mesh, 71 g; mPtl~nnl/dichlorompth~np~ 5/95) to give 1-[2-[4~4-
methylsulfonylphenyl)-l-piperazinyl]ethyl]-N-methylisochroman-6-carb- Y~mide (IX),
Rf = 0.30 (mPth~n~)l/dichloromPth~nP~ 5/95); IR (mull) 1645, 1612, 1593, 1571, 1545,
1508, 1496, 1409, 1296, 1249, 1145, 1106, 1095, 957 and 780 cm~l; NMR (300 MHz,
CDCl3) 7.73 (d, 2H, J=8.9 Hz, aromatic), 7.55 (m, 2H, aromatic), 7.15 (d, lH, J=7.9
10 Hz, aromatic), 6.91 (d, lH, J=8.9 Hz, aromatic), 6.14 (brdd, lH, J=4.4 Hz, NH), 4.86
(brdd, lH, J=6.2 Hz, m~t~lin~), 4.12 (m, lH, OCH2a), 3.75 (m, lH, OCH2b), 3.35 (t,
4H, J=4.9 Hz, Ph-N-CH28), 2.99 (brds, 7H, N-CH3, OCH3, Ph-CH2a), 2.75-2.46 (m,
7H, Ph-NC(H2)-CH2S-NCH2s, Ph-CH2b), 2.14 (m, lH, C(H)-CH2a), 2.02 (m, lH,
C(H)-CH2b) o; CMR (75 MHz, CDCl3) 167.9, 154.3, 141.4,134.5, 132.7, 129.1, 128.6,
15 127.7, 124.9, 124.5, 113.8, 74.3, 63.0, 54.5, 52.9, 47.3, 45.0, 33.1, 29.1 and 26.8 ~;
HRMS (EI) ~~lr~ t-od for C24H31N304S = 457.2035, found = 457.2032; KF. Water =
0.87%; Melt Solvate = 0.53% ethyl acetate and 0.34% hPYstnP.
~XAlvl PLE 24 1-[2-[4~4-M~th- Yyphenyl)-l-,ui,ue..lzillyl]ethyl]isocl,lu,.lan-6-
Step 1: 1-[2~6-Br ml~iAo~hroman-l-yl)acetyl]-4-(4-methoxyphenyl)-
piperazine (V)
Following the general plucedulc: of F~ANlpLE 1, Step 3 and m~king non-
critical v~ri~t;on~ but using racemic 6-br moi~o- hroman-1-yl-acetic acid (IV) and 4-
mPthl ~ryphenyl~i,u~azine gives 1-[2-(6-bromn.i~ochroman-1-yl)acetyl]~-(4-
methoxyphenyl)piperazine (V), Rf = 0.26 (ethyl ~ret~t~p/hpy~np~ 70/30); IR (mull)
1639, 1512, 1446, 1439, 1249, 1214, 1112, 1030, 1028 and 820 cm~l; NMR (300 MHz,CDCl3) 7.32-7.26 (m, 2H, aromatic H's), 7.0 (d, lH, J=8.2 Hz, aromtic H), 6.88 (d,
4H, aromatic H's), 5.27 (m of d, lH, J=5.9 Hz, ArC-H), 4.164.07 (m, lH), 3.89 (m,
lH), 3.80-3.60 (m, 4H), 3.77 (s, 3H, -OC-H3), 3.05 (m, 4H, four of pip-H), 2.97-2.90
(m, 2H), 2.76 (d of d, lH, Ja=3.7 Hz, Jb=14.9 Hz), 2.65 (m of d, lH, J=16.4 Hz) ~;
CMR (75 MHz, CDCl3) 168.9, 154.3, 145.2, 136.5, 136.2, 131.7, 129.3, 126.4, 120.3,
118.8, 114.4, 73.4, 63.4, 55.5, 51.3, 50.7, 46.1, 41.9, 39.9, 28.7 o.
Step 2: 1-[2-(6-Brom~ o~hroman-l-yl)ethyl]-4-(4-methoxy-phenyl)
piperazine (VI)
The general P~Ced~LLe of ~A~ PLE 1, Step 4 and m~king non-critical
v~ri~tion~ but using 1-[2-(6-brom- i~ocl .l u ~an-1-yl)acetyl]-4-(4-methoxyphenyl)-
-62-
CA 02225282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
piperazine (V) gives 1-[2-(6-bromoisochroman-1-yl)ethyl]~-(4-met~oxyphenyl)-
piperazine (VI), Rf = 0.23 (ethyl acetate); IR (neat) 1518, 1479, 1266, 1250, 1155,
1140, 1112, 1103, 1041 and 818 cm~l; NMR (300 MHz,CDC13) 7.29 (d, lH, J=8.3 Hz,
aromatic H), 7.27 (s, lH, aromatic H), 6.97 (d, lH, J=8.3 Hz, aromatic H), 6.85 (q,
5 4H, J=9.7 Hz, aromatic H's), 4.78 (m of d, lH, J=6.1 Hz), 4.14-4.07 (m, lH), 3.76-
3.69 (m, lH), 3.76 (s, 3H, -OC-H3), 3.10 (t, 4H, J=4.9 Hz, four of pip-H), 2.95 (m,
lH), 2.70-2.50 (m's, 7H), 2.13 (m, lH, pipCH-H), 2.02 (m, lH, pipCH-H) o; CMR (75
MHz, CDCl8) 163.5, 146.5, 136.8, 136.0, 131.4, 129.0, 126.3, 119.7, 117.9, 114.2,
74.1, 62.5, 55.3, 54.4, 53.3, 50.4, 32.9 and 28.6 o.
Step 3: 1-[2-[4-(4-MPthnYyphenyl)-l-piperazinyl]ethyU-isochroman-6-
A dry 100 mL round bottom flask is charged with THF (18 mL) and cooled to
-78~ with a dry ice/A~etone bath. t-Butyllit~illm in h~Y~n~ (1.7M, 5.4 ml~ 9.2
mmol) is added at once. After stirring for 5 minutes, a llliX~U~ 1-[2-(6-
15 bromoi~orhroman-1-yl)ethyl]-4-(4-mPtl~oYyphenyl)piperazine (VI) in THF (20 m L) is
added via canula. After stirring for 15 minllte~ at -78~, trimethylsilyl isocyanate
(0.88 mL, 6.55 mmol) and ~inY~ne (3.52 mL) are added via srying r~e~ liv~ly. After
15 _in. the cooling bath is removed and the reaction is stirred at 20-25~ for 1.5 hrs.
The reaction lllixLu-~a is qll~nr~hetl with sa~urdted aqueous Ammonillm ~hlnriclç, the
20 volatiles are removed under reduced pressure, and the residue is b~cifiPrl with
aqueous sodium hydroxide. The crude basic SOlllt;On iB extracted with methylene
chloride. The organic extracts are comhinP~, dried with sodium sulfate, filtered and
con~entrated. The crude mAteriAl is purified by flash chromAt,o~.dphy (silica gel, 25
g; using a gradient of 0-10% mpt~nol/ethyl acetate to give 1-[2-[4-(4-
25 mpt~nxyphenyl)-l-pipel~zill~l]ethyl]-isochroman-6-carhnyAmi~e (VII), mp = 180-182~;
Rf = 0.27 (m~t~Anol/ethyl Acet~te~ 10/90); IR (mull) 3366, 3198, 1628, 1642, 1602,
1514, 1437, 1245, 1109 and 815 cm~l; NMR (300 MHz,CDCl3) 7.61-7.58 (m, 2H,
aromatic H's), 7.18, (d, lH, d=8.6 Hz, aromatic H), 6.85 (q, 4H, J=9.2 Hz, aromatic
H's), 5.90 (very broad d, 2H, C(O)N-H2), 4.86 (m of d, lH, J=5.8 Hz, PhC-H), 4.18-
30 4.11 (m, lH), 3.80-3.72 (m, lH), 3.76 (s, 3H, PhOC-H3), 3.10 (t, 4H, J=4.8 Hz, four of
pip-H), 2.99 (m, lH), 2.73 (m of d, lH, J=16.4 Hz), 2.66-2.49 (m's, 6H), 2.15 (m, lH,
pipCH-H), 2.04 (m, lH, pipCH-H) o; CMR (75 MHz, CDCl3) 168.7, 153.5, 145.4,
142.0, 134.3, 131.0, 127.9, 124.8, 124.6, 117.8, 114.1, 74.2, 62.6, 55.2, 54.3, 53.2, 50.3,
32.8, 28.7 and 27.2 ~; HRMS cAl~lllAte~l for C23H29N303 = 395.2209, found:
35 395.2219.
F'XAMPLE 25 1-[2-[4-(4-Mot~nYyphenyl)-l-piperazinyl]ethyl]-N-propyl-
-63-
CA 02225282 1997-12-lg
W O 97/022S9 PCT~US96/08681
isochroman-6-csrboY~mi-le (IX)
Step 1: 1-[2-[4-(4-MPt~ ~Yyphenyl)-1-piperazinyl]ethyl]-N,N-di-t-
butylo,.ycsLl,onylisochroman-6-caLI,..Y~ e (VIII)
Follovving the genersl P1UCedULe of EX~MPLE 3, Step 1 and m~king non-
5 critical v~ri~ti~nR but starting with 1-[2-[4-(4-methnYyphenyl)-1-piperazinyl]ethyl]-
isochroman-6-c~Lb~-Y~ irle (VII, Ti~AMPLE 24), 1-[2-t4-(4-mPthnYyphenyl)-1-
piperazinyl]ethyl]-N,N-di-t-butykJ~yc~l,ollylisochroman-6-cslL...Y~niclP. (VIII) is
obtained.
Step 2: 1-[2-[4-(4-M~t~oYyphenyl)-1-piperazinyl]ethyl]-N-propyl-
isochroman-6-caLL~.Ys.. icle (IX)
An oven-dried 100 mT- lCC~V~l~ flask eq~upped with spinbsr is chsrged with
1-[2-[4-(4-mPt~oyyphenyl)-l-piperazinyl]ethyl]-N~N-di-t-butyl~ y~Lonylisochroman-
6-cs l,..Y~...icle (VIII, 566 mg, 0.95 mmol) and 20 mL dichlorompth~na~ This ~;.cLuL~:
is trested with propylsmine (0.78 mL, 9.5 mmol). After 16 hr, the volatiles are
15 removed under reduced Pre~ULe to the crude product. This m~t~ris~l is purified by
LC (silica gel, 230-400 mesh, 30 g; mpt~n~l/ethyl~cet~t~ 5/95) to give 1-[2-[4-(4-
methoxyphenyl)-l-piperazinyl]ethyU-N-propylisochroman-6-caLI,~ le (IX), mp =
147-149~; Rf = 0.37 (mPtl~nnl/ethyl~et~t~e~ 5/95); IR (mull) 3302, 2815, 1639, 1639,
1515, 1320, 1310, 1293, 1278, 1247, 1153, 1112, 1107, 1041 and 824 cm~l; NMR (300
20 MHz, CDCl3) 7.54 (m, 2H, aromatic), 7.16 (d, lH, J=8.6 Hz, aromatic), 6.87 (m, 4H,
aromatic), 6.16 (brdt, lH, NH), 4.87 (brdd, lH, J=6.0 Hz, m~t~ine)~ 4.14 (m, lH,OCH2a), 3.76 (m, 4H, OCH3& OCH2b), 3.42 (qrt, 2H, J=6.3 Hz, N(H)-CH2), 3.10 (t,
4H, J=4.8 Hz, Ph-N-CH28), 3.00 (m, lH, Ph-CH2a), 2.77-2.53 (m, 7H, Ph-NC(H2)-
CH2B-NCH2s, Ph-CH2b), 2.17 (m, lH, C(H)-CH2a), 2.05 (m, lH, C(H)-CH2b), 1.63
25 (sxt, 2H, J=7.4 Hz, C(H3)-CH2), 0.98 (t, 3H, J=7.4 Hz, CH3-C(H2) ~; CMR (75 MHz,
CDCl3) 167.1, 153.6, 145.5, 141.3, 134.3, 132.7, 127.4, 124.8, 124.2, 117.9, 114.2,
74.3, 62.8, 55.4, 54.5, 53.3, 50.4, 41.5, 33.0, 28.9, 22.8 and 11.3 ~; MS (EI, m/z) =
437.
EX~MPLE 26 1-[2-[4-(4-MPt~ll Yyphenyl)-1-~ yl]ethyl]-N-allyl-
isochroman-6-caLL.. ~.nicie (IX)
Following the general procedure of ~AMPLE 25, Step 2 and m~king non-
critical v~t~n~ but using allylamine (0.69 mL, 9.1 mmol) give crude product.
This material is purified by LC (silica gel, 230-400 mesh, 30 g; m~~nol/ethyl
acetate 3/97) to give product which is 1~ L~ ed from ethyl acetate/hPY~nP-, mp =
35 146-148~; Rf = 0.34 (me~nr)llet,hyl~et~tP, 5/95); IR (mull) 3295, 2814, 1640, 15~6,
1515, 1494, 1443, 1310, 1281, 1246, 1148, 1107, 1037, 923 and 823 cm~l; NMR (300
-64-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
MHz, CDCl3) 7.57, 7.17, 6.85, 6.20, 5.92, 5.26, 5.19, 4.87, 4.08, 3.76, 3.11, 3.01, 2.78-
2.50, 2.18 and 2.05 o; CMR (75 MHz, CDCl3) 167.1, 153.8, 145.7, 141.8, 134.6, 134.2,
132.5, 127.8, 125.1, 124.5, 118.2, 116.8, 114.4, 74.6" 63.0, 55.6, 54.7, 53.5, 50.7, 42.5,
33.2 and 29.1 o; MS (EI, m/z) = 435; HRMS (EI) cSIlc~ tP~l for C26H33N3O3 =
435.2522, found = 435.2516.
VrPLE 27 1-[2-[4-(4-Methoxy-phenyl)-l-piperazinyl]ethyl]-N-eth
isochroman-6-ca~Ln~ 1P (IX)
Following the general procedure of EXAMPLE 25, Step 2 and making non-
critical variations but using ethylamine (approx 2 mL, cr~n~lPnce~ at 0~) gives crude
product. This m~t~ri~l is purified by LC (slicia gel, 230-400 mesth, 30 g;
mPt~l~nol/ethyl ~-~et~te, 5/95) to give product. This material is LL;Lurdled with ethyl
acetate~hexane to give 1-[2-[4-(4-mP~hn~yphenyl)-l-piperazinyl]ethyl]-N-ethyl-
isochroman-6-calL~ e (IX), mp = 127-129~; Rf = 0.30 (mPt~l~nol/ethyl ~cehte,
5/95); IR (mull) 3308, 2815, 1640, 1540, 1514, 1442, 1359, 1312, 1298, 1283, 1246,
1149, 1112, 1037 and 826 cm~l; NMR (300 MHz, CDCl3) 7.55 (m, 2H, aromatic), 7.16(d, lH, J=8.6 Hz, aromatic), 6.87 (m, 4H, aromatic), 6.12 (brdt, lH, NH), 4.87 (brdd,
lH, J=8.1 Hz, mPthinP), 4.15 (m, lH, OCH2a), 3.77 (m, 4H, OCH3& OCH2b), 3.49
(qt, 2H, J=7.2 Hz, N(H)-CH2), 3.11 (t, 4H, J=4.8 Hz, Ph-N-CH2s), 3.00 (m, lH, Ph-
CH2 ) 2.77-2.50 (m, 7H, Ph-Nc(H2)-cH2s-NcH28~ Ph-CH2b)~ 2-15 (m~ lH~ C(H)
CH2a), 2.05 (m, lH, C(H)-CH2b), 1.25 (t, 3H, J=7.2 Hz, CH3-C(H2) ~; CMR (75 MHz,CDCl3) 166.9, 153.4, 145.4, 141.2, 134.1, 132.5, 127.3, 124.7, 124.1, 117.8, 114.1,
74.2, 62.6, 55.2, 54.3, 53.2, 50.3, 34.6, 32.9, 28.7 and 14.6 o; MS (EI, m/z) = 423.
EXA~LE 28 1-[2-[4~4-MPthnYyphenyl)-l-piperazinyl]ethyl]-N-pr~pal~yl-
isochroman-6-c~Ln ~ P. (IX)
Following the general procedure of EXAMPLE 25, Step 2 and m~king non-
critical variations but using plu~al~ylamine (1.6 mL, 23.0 mmol) gives crude
product. This m~ri~l is purified by LC on 75 g (230-400) silica gel eluting withethyl acetate to give the product which is 1G~Y~I~11;7eCI from ethyl acetate/hexane to
give l-t2-[4~4-mPt~ typhenyl)-l-piperazinyl]ethyl]-N-propar~yl-isochroman-6-
30 c~.L-cii-le (IX), mp = 162-164~; Rf = 0.40 (mpt~l~n~l/ethyl ~.~etste 5/95); IR (mull)
3287, 1643, 1636, 1611, 1535, 1515, 1495, 1443, 1303, 1283, 1246, 1147, 1107, 1033
and 822 cm~l; NMR (300 MHz, CDCl3) 7.57 (m, 2H, aromatic), 7.17 (d, lH, J=8.6
Hz, aromatic), 6.85 (m, 4H, aromatic), 6.35 (brdt, lH, NH), 4.88 (brdd, lH, J=8.0 Hz,
mPt~inP), 4.25 (dd, 2H, J=5.2 Hz & J=2.5 Hz, N(H)-CH2), 4.12 (m, lH, OCH2a ), 3.76
(m, 4H, OCH3& OCH2b), 3.10 (t, 4H, J=4.8 Hz, Ph-N-CH28), 3.01 (m, lH, Ph-CH2a),
2.78-2.50 (m, 7H, Ph-NC(H2)-CH2s-NCH2S, Ph-CH2b), 2.29 (t, lH, J=2.5 Hz, alkyne),
-65-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
2.18 (m, lH, C(H)-CH2a), 2.05 (m, lH, C(H)-CH2b) 8; CMR (75 MHz, CDC13) 166.7,
153.6, 145.5, 142.0, 134.4, 131.6, 127.6, 124.9, 124.4, 118.0, 114.2, 79.3, 74.3, 71.8,
62.7, 55.4, 54.5, 53.3, 50.4, 33.0, 29.6 and 28.8 8; MS (EI, m/z) = 433; HRMS (EI)
t~-l for C26H31N303 = 433.2365, found = 433.2367.
F~AMPLE 29 1-[2-[4-(4-MPthnyyphenyl)-l-pipe~ yl]ethyl]-N-(4
m~thnYyphenylmethyl)-isochroman-6-carboy~m~
Following the general procedure of ~xAMpLE 25, Step 2 and making non-
critical variations but using 4-m~thnYyphenylmethylamine (1.2 mL, 9.2 mmol) gives
crude product. The crude is purified by flash chrom~graphy on 67 g silica gel
using m~th~nol/ethyl acetate (10/90) as the eluent to give 1-[2-[4-(4-methoxyphenyl)-
1-piperazinyUethyl]-N-(4-methoxyph~llyLllet~lyl)isochroman-6-c~L~ P (IX), mp
5 162-163~; Rf = 0.40 (mPth~n~l/ethyl ~etstç, 10/90); [a]D = -40~ (c = 0.98, eth~n-)l);
IR (mull) 3306, 1642, 1540, 1515, 1313, 1251, 1244, 1235, 1110, 1036 cm~l; NMR
(300 MHz, CDCl3) 7.57 (m, 2H, aromatic H's), 7.28 (d, 2H, J=8.7 Hz, aromatic H),7.16 (d, 2H, J=8.7 Hz, aromatic H), 6.92-6.82 (m's, 6H,aromatic H's), 6.27 (m, lH,
C(O)N-H), 4.86 (m of d, lH, J=5.8 Hz, PhC-H), 4.18-4.11 (m, lH), 3.80 (s, 3H, PhOC-
H3), 3.80-3.72 (m, lH), 3.76 (8, 3H, PhOC-H3), 3.10 (t, 4H, J=4.8 Hz, four of pip-H),
2.99 (m, lH), 2.73 (m of d, lH, J=16.4 Hz), 2.66-2.49 (m's, 6H), 2.15 (m, lH, pipCH-
H), 2.04 (m, lH, pipCH-H) 8; CMR (75 MHz, CDC13) 166.9, 145.7, 141.7, 134.5,
132.4, 130.2, 129.3, 127.7, 125.0, 124.5, 118.2, 114.4, 114.2, 74.5, 62.9, 55.5, 55.3,
54.6, 53.5, 50.6, 43.6, 33.1, 29.0 8; HRMS calculated for C31H37N304 = 515.2784,found = 515.2806.
EXAMPLE 30 1-[2-[4~4-MPthnyyphenyl)-l-piperazinyl]ethyl]-N-phenylmeth
isochroman-6 c~ Y~ e (IX)
An oven dried 15 mL micro vial, equipped with a claisen c. n~en~r~ water
cooled condenR~r, and hose adapter, is charged with 1-(2-(6-br~mni~o~hl~lllan-1-yl)-
ethyl)-4~mPt~nYyphenyl)-pipelazi~e (VI, 646 mg, 1.5 mmol), p~ lm (II) acetate
(98%, 17.2 mg, 0.075 mmol) and 1,3-bis-diphenylphosFhinnpropane (97~o, 38.3 mg,
0.09 mmol). Carbon mnnnYi~e ~tmosphpre is PqtshliRh~l in the vial. To the
react;ion vessel is introduced via syringe D~ (3.75 mT.),phenyl~t~ yl amine (1.15
mT~ 10.5 mmol) and diisu~v~ylethylamine (0.52 mL, 3 mmol). The ll~i~ is
heated to 100~ over 10 hours. After cooling to 20-25~, it sepala~ed into two phases.
The reaction lllixLula is poured into ethyl ~cetote. The llli~ is washed one time
with aqueous sodium hydroxide (lN). The organic layer is then con~ dted under
reduced ple~ to remove excess solvents and r~ct~ntc to give a crude product
which is purified by flash chrom~t~graphy on 100 g silica gel using ethyl acetate as
-66-
CA 02225282 l997-l2-l9
W O 97t02259 PCT~US96/08681
the eluent to give 1-C2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]-N-
phenylmethylisochroman-6-carbn~mif~f~ (IX), mp = 153.0-153.5~; Rf= 0.25
mf~th~n-~l/ethyl ~cePte, 10/90); IR (mull) 3263, 2819, 1640, 1543, 1513, 1245, 1234,
1112, 1039 and 826 cm-1; NMR (300 MHz,CDCl3) 7.68 (d, 2H, J=6.9 Hz, aromatic
6 H's), 7.36-7.28 (m's, ~H, aromatic H's), 7.16, (d, lH, J=8.7 Hz, aromatic H), 6.86 (q,
4H, J=9.2 Hz, aromatic H's), 6.42 (t, lH, C(O)N-H), 4.86 (m of d, lH, J=5.8 Hz, PhC-
H), 4.64 (d, 2H, J=5.7 Hz, PhC-H2), 4.17-4.10 (m, lH), 3.80-3.72 (m, lH), 3.76 (6, 3H,
PhOC-H3), 3.10 (t, 4H, J=4.8 Hz, four of pip-H), 2.99, 2.73 (m of d, lH, J=16.4 Hz),
2.66-2.49 (m's, 6H), 2.15 (m, lH, pipCH-H), 2.04 (m, lH, pipCH-H) ~; CMR (75 ~Iz,
10 CDCl3) 189.7, 167.1, 153.8, 145.7, 141.8, 138.2, 134.5, 132.4, 128.8, 127.9, 127.8,
127.6, 125.1, 124.6, 74.5, 62.9, 55.6, 54.6, 53.5, 50.6, 44.1, 33.1, Z9.0 o; HRMS
c~lr~ t~d for C30H35N3O3 = 485.2678, found = 485.2664.
EXA~LE 31 1-[2-[4-(4-Mf~hnYyphenyl)-1-piperazinyl]ethyl]-N-butyl-
isochroman-6-ca~l,.-x~...iflf~ (IX)
Following the general procedure of F~AMT'LE 30 and m~kin~ non-critical
variations but using n-butylamine (1.04 nnT.~ 10.5 mmol) gives crude product which
is purified by flash chrom~ .dphy on 100 g silica gel using ethyl acetate as theeluent to give product which is rG~ ql~ t;nn from hot ethyl acetate to give the
title ~u~ uu~d~ mp = 158.5-159.5~; Rf = 0.28 (ethyl acetate); IR (mull) 3301, 2816,
20 1637, 1537, 1515, 1444, 1308, 1244, 1111 and 823 cm~l; NMR (300 MHz,CDC13)
5.53, 7.16, 6.87, 6.10, 4.85, 3.80-3.67, 3.76, 3.45, 3.11, 3.10, 2.73, 2.66-2.49, 2.15,
2.04, 1.59, 1.43, 0.96 o; CMR (75 MHz, CDCl3) 167.3, 153.8, 145.7, 141.5, 134.5,132.9, 127.6, 125.0, 124.4, 118.2, 114.4, 74.5, 63.0, 55.6, 54.7, 53.5, 50.6, 40.0, 39.8,
33.2, 31.8, 29.1, 20.2, 13.8 ~.~5 li'XAMPLE 32 1-[2-[4-(4-Mf~t~ fnryphenyl)-l-piperazinyl]ethyl]-N-[(R)-a
methylphenylmethyl]-isochroman-6-cOlL. .~ 1 e (IX)
Following the general procedure of EXAMPLE 30 and m~kinF non-critical
v~ri~t;~n~ but using (R)-(+)-a-methylphenylmethylamine (98~o, 0.90 mL, 7 mmol)
gives crude product which is purified by flash chrom~tography on 100 g silica gel
30 using a gradient of 0 4% mf~th~nnl in ethyl ~- etqt~. The product is lG"~ i7f,.l
from hot methylene rhlnri.l~ethyl acetate/~exane to give 1-[2-[4-(4-mf~ f~yphenyl)-
1-pipwdziuyl]ethyl]-N-[(R)-a-methylphenylmethyl]isochroman-6-ca~ .x,~ le (IX) asa ~ lule of dia!i~eLGomers, mp = 140.5-141.0~; Rf = 0.28 (ethyl acetate); [~]D +25~ (c
0.94, eth~nol); IR (mull) 3310, 1636, 1630, 1514, 1495, 1275, 1148, 1110, 700 cm~1;
35 NMR (300 MHz,CDCl3) 7.58 (d, 2H, J=6.9 Hz, aromatic H's), 7.41-7.28 (m's, 5H,aromatic H's), 7.16, (d, lH, J=8.7 Hz, aromatic H), 6.85 (q, 4H, J=9.2 Hz, aromatic
-67-
CA 0222~282 1997-12-19
W O 97/02259 PCT~US96/08681
H's), 6.29 (d, lH, J=7.8 Hz, C(O)N-H), 5.34 (quintet, lH, J=7.2 Hz, PhC-H), 4.86 (m
of d, lH, J=5.8 Hz, PhC-H), 4.17-4.10 (m, lH), 3.80-3.72 (m, lH), 3.76 (B, 3H, PhOC-
H3), 3.10 (t, 4H, J=4.8 Hz, four of pip-H), 2.99 (m, lH), 2.73 (m of d, lH, J=16.4 Hz),
2.66-2.49 (m's, 6H), 2.15 (m, lH, pipCH-H), 2.04 (m, lH, pipCH-H), 1.61 (d, 3H,
5 J=6!9 Hz, PhC(H)C-H3) ~; CMR (75 MHz, CDCl3) 166.2, 153.7, 145.7, 143.0, 141.7,
134.5, 132.6, 128.7, 127.6, 127.4, 126.2, 125.0, 124.5, 118.1, 114.4, 74.5, 62.9, 55.5,
54.6, 53.4, 50.5, 49.1, 33.1, 29.0, 21.6 o.
EXAMPLE 33 1-[2-[4-(4-MPt~n~ryphenyl)-l-piperazinyl]ethyl]-N-[(S)-a-
methylphenylmethyl]isochroman-6-cd~ . . ,itle (IX)
Following the general prucedu~ of EXAMPLE 30 and m~kinE non-critical
v~ri~tinnR but using (S)-(-)-a-methylphenylmethylamine (98%, 0.90 mL, 7 mmol)
give~ crude product which is purified by flash chrom~t~graphy on 100 g silica gel
using a gradient of 0-7% m~th~nol in ethyl ~et~te~ The product is l'eC~y~ i7.eclfrom hot methylene chloride/ethyl acetate/hexane to give 1-[2-[4-(4-methoxyphenyl)-
15 1-pi~ ~yl]ethyl]-N-[(S)-a-methylphenylmethyl]isochroman-6-c~ubf.~ le (IX) as
a ll~ixLu~ of diastereomers, mp = 141.0-141.5~; Rf = 0.28 (ethyl acetate); [a]D -24~ (c
0.98, et~nol); IR (mull) 3310, 1636, 1530, 1514, 1495, 1275, 1148, 1110, 700 cm~l;
NMR (300 MHz,CDCl3) 7.58 (d, 2H, J=6.9 Hz, aromatic H's), 7.41-7.28 (m's, 5H,
aromatic H's), 7.16, (d, lH, J=8.7 Hz, aromatic H), 6.85 (q, 4H, J=9.2 Hz, aromatic
20 H's), 6.29 (d, lH, J=7.8 Hz, C(O)N-H), 5.34 (quintet, lH, J=7.2 Hz, PhC-H), 4.86 (m
of d, lH, J=5.8 Hz, PhC-H), 4.174.10 (m, lH), 3.80-3.72 (m, lH), 3.76 (s, 3H, PhOC-
H3), 3.10 (t, 4H, J=4.8 Hz, four of pip-H), 2.99 (m, lH), 2.73 (m of d, lH, J=16.4 Hz),
2.66-2.49 (m's, 6H), 2.15 (m, lH, pipCH-H), 2.04 (m, lH, pipCH-H), 1.61 (d, 3H,
J=6.9 Hz, PhC(H)C-H3) ~; CMR (75 MHz, CDCl3) 166.2, 153.7, 145.7, 143.0, 141.7,
25 134.5, 132.6, 128.7, 127.6, 127.4, 126.2, 125.0, 124.5, 118.1, 114.4, 74.5, 62.9, 55.5,
54.6, 53.4, 50.5, 49.1, 33.1, 29.0, 21.6 ~.
P'XANIPLE 34 1-[2-[4-(4-M.othnYyphenyl)-l-pipe~ lyl]ethyl]-N-phenyl-
isochroman-6 ca.L.--~....icle (IX)
Following the general procedure of P~AMPLE 30 and m~king non-critical
30 v~rint;~ n~ but using aniline (0.64 mL, 7 mmol) gives crude product which is purified
by LC on 29 g (230~00) silica gel eluting with ethyl acetate/hexane (75/25) to give 1-
[2-[4-(4-m~t~ nyyphenyl)-l-piperazinyl]ethyl]-N-phenylisochroman-6-c~~ ;de
(IX), Rf = 0.25 (ethyl ~et~t~ Y~ne, 75/25); IR (mull) 2817, 1652, 1599, 1531, 1513,
1442, 1320, 1298, 1246, 1145, 1112, 1038, 823, 754, 693 cm~l; NMR (300 MHz,
35 CDCl3) 7.87 (s, lH, NH), 7.63 (m, 4H, aromatic), 7.37 (t, 2H, J=7.7 Hz, aromatic~,
7.21 (d, lH, J=7.9 Hz, aromatic), 7.18 (t, lH, J=6.3 Hz, aromatic), 6.85 (m, 4H,
-68-
CA 02225282 l997-l2-l9
W 097/022S9 PCTnUS96/0~i81
aromatic), 4.86 (brdd, lH, J=6.0 Hz, mPt~linQ), 4.15 (m, lH, OCH2a), 3.76 (m, 4H,
OCH3& OCH2b), 3.11 (t, 4H, J=4.9 Hz, Ph-N-~H28), 3.00 (m, lH, Ph-CH2a), 2.70-
2.50 (m, 7H, Ph-NC(H2)-CH2S-NCH28, Ph-CH2b), 2.15 (m, lH, C(H)-CH2a), 2.05 (m,
lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3) 165.6, 153.8, 145.7, 142.2, 137.9, 134.8,
5 133.0, 129.1, 127.8, 125.3, 124.6, 124.5, 120.2, 118.2, 114.4, 74.5, 62.9, 55.6, 54.7,
53.5, 50.6, 33.2, 29.1 o.
EXAMPLE 35 1-[2-[4-(4-MPt~ yphenyl)-l-piperazinyl]ethyl]-N-phenylmethyl-
N-methyl-isochroman-6-carb- Yslmi-1e (IX)
Following the general procedure of EXAMPLE 30 and mAking non-critical
10 variations but using phenylmethylmethylamine (1.4 mL, 10.5 mmol) give~ crude
product which is purified by LC on 77 g (230-400) silica gel eluting with
~eton~/hexane (40/60) and gradually increa~ing to ~ceton~hexane (60/40) to give
product. Thi8 material is disolved in ether and treated with gaseous hy-lLochloric
acid resulting in the formation of a solid. Freebase Rf = 0.30 (At~etonP/hPYAnp715 40/60); bi~q salt IR (mull) 3423, 2352, 2192, 1627, 1513, 1495, 1400, 1331, 1310, 1294,
1259, 1193, 1106, 1073, 1028 cm~l; NMR of freebase (300 MHz, CDC13) 7.26 (m, 8H,aromatic), 6.84 (m, 4H, aromatic), 4.84 (bs, lH, mPthinQ), 4.75 (bs, lH, Ph-CH2a-N),
4.54 (m, lH, Ph-CH2b-N), 4.12 (m, lH, OCH2a), 3.76 (m, 4H, OCH3& OCH2b), 3.08
(t, 4H, J=4.7 Hz, Ph-N-CH28), 3.02-2.88 (m, 4H, NCH3 & Ph-CH2a), 2.64 (m, 7H, Ph-
20 NC(H2)-CH2s-NCH2~, Ph-CH2b), 2.15 (m, lH, C(H)-CH2a), 2.05 (m, lH, C(H)-CH2b) ~; HRMS (EI) ~ ~lclllAt,Qd for C31H37N303 = 499.2835, found = 499.2842.
Ti'XAlVlPLE 36 1-[2-[4-(4-M~thr.Yyphenyl)-l-pipe~dziuyuethyl]-N~N-dimeth
isochru~an-6 c~b.~Y~ le (IX)
A flame-dried 50 mL flask equipped with spinbar and ~ltliti~n funnel is
25 charged with freshly ~iFt;ll~ tetrahydivru~ (6 mL), cooled to -78~, and treated
with a 1.7 M solllti~ n of tert-butyllithillm (3.0 mL, 5.0 mmol). The r~slllting ~i~u~e
is treated drop-wise over 10 min. with a solllt;~n of 1-(2-(6-br moi~o~ hroman-1-yl)-
ethyl)-4-(4-mPthmryphenyl)piperazine (VI, 1.08 g, 2.5 mmol) and 7 mL
tetrahyd~uru~dn (507 mg, 3.3 mmol). The aryl lithillm is stirred 10 min and is
30 treated with carbon dioxide (bone dry). After an ~ 1itinn 10 min, the aryl
ca~l,u,.ylate is warmed to 20-25~ with the gas ~lflititm 8ll~pend-p~ The l~ U~: is
treated with dimel~lyl~u~ miclP (2 drops) followed by oxalyl chloride (0.33 mT., 3.76
mmol) with copious gas evolution and a rls~ lg in color. After 45 min, the
~L2~Lu~ts is treated with dimethylamine gas. After 20 min, the amine ~ lition iS35 sllcpen-l~d and the reaction ~ u~e i8 diluted with 40 mT 5M sodium hydroxide and
extracted twice with ethyl acetate (40 mL). The comhin~d organics are washed once
-69-
CA 0222~282 l997-l2-l9
W O 97/02259 PCT~US96/08681
with saline (30 mL), dried over m~gnPRi~lm sulfate, filtered, and con~ dted to give
product which is purified by LC on 63 g (230-400) 8 ica gel eluting with
~cetonP/hexane (50/50) to give 1-[2-[4-(4-mPth--xyphenyl)-l-pipt.a,iinyl]ethyl]-N,N-
dimethylisochroman-6-carb~Y~mi-le (IX), mp = 94-96~; Rf = 0.31 (~etonR/hPY~nR,
5 50/50); IR (mull) 2808, 2792, 1624, 1610, 1513, 1488, 1444, 1414, 1275, 1253, 1231,
1152, 1109, 1036, 833 cm~l; NMR (300 MHz, CDC13) 7.19 (m, 2H, aromatic), 7.12 (d,
lH, J=7.9 Hz, aromatic), 6.85 (m, 4H, aromatic), 4.85 (brdd, lH, J=6.0 Hz, mPthinR),
4.13 (m, lH, OCH2a), 3.76 (m, 4H, OCH3& OCH2b), 3.13-3.00 (m, llH, NCH3s, Ph-
NCH2s, Ph-CH2a), 2.65 (m, 7H, Ph-NC(H2)-CH28-NCH2 & Ph-CH2b), 2.17 (m, lH,
10 C(H)-CH2a), 2.05 (m, lH, C(H)-CH2b) ~; CMR (75 MHz, CDCl3) 171.2, 153.5, 145.5,
139.4, 134.2, 134.0, 127.5, 124.6, 124.4, 117.9, 114.2, 74.3, 62.7, 55.3, 54.5, 53.3, 50.4,
39.4, 35.1, 33.0, 28.8 ~; HRMS (EI) c~lculated for C25H33N3O3 = 4~3 ~5~ found =
4~ 0.
EXAMPLE 37 1-[2-[4-(4-MPth-.Yyphenyl)-l-piperazinyl]ethyl]-N-methyl-
isochroman-6-carb~Y~mi-le (IX)
Following the general pl~cedura of EXAMPLE 36 and m~king non-critical
v~ i~t;~nR but using methylamine gas and kPeping amount of other rPngent~ the
same gives 1-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]-N-methylisoch~ an-6-
carb-~Y~mide (IX) which is re~;.y~ 7~ from ethyl acetate to give the title
20 compound, mp = 174-176~; Rf = 0.40 (mPt~n~l/dichloromp~ nR~ 5/95); IR (mull)
3270, 1639, 1543, 1515, 1418, 1319, 1260, 1248, 1235, 1152, 1141, 1112, 1037, 832
and 820 cm~l; NMR (300 MHz, CDCl3) 7.53 (m, 2H, aromstic), 7.16 (d, lH, J=8.6
Hz, aromatic), 6.87 (m, 4H, aromatic), 6.19 (brdm, lH, NH), 4.86 (brdd, lH, J=5.9
Hz, mPthinP), 4.14 (m, lH, OCH2a), 3.76 (m, 4H, OCH3& OCH2b), 3.10 (t, 4H, J=4.825 Hz, Ph-N-CH28), 3.00 (d, 4H, N-CH3 & Ph-CH2a), 2.77-2.49 (m, 7H, Ph-NC(H2)-
CH28-NCH28, Ph-CH2b), 2.14 (m, lH, C(H)-CH2a), 2.05 (m, lH, C(H)-CH2b) o; CMR
(75 MHz, CDCl3) 168.0, 153.8, 145.7, 141.6, 134.5, 132.7, 127.7, 125.0, 124.4, 118.2,
114.4, 74.5, 62.9, 55.6, 54.6, 53.5, 50.6, 33.1, 29.1, 26.8 o; HRMS (EI) c~ tpfl for
C24H3lN3O3 = 409.2365, found = 409.2366.
30 ~XAlVrPLE 38 1-t2-[4-(4-Chlorophenyl)-l-pip~ yl]ethyl]-N-methyl-
isoclllu~an-6-carbnYS-mi-lR (IX)
Following the general pl'OCedu~ of EXAMPLE 36 and mQking non-critical
variations but using 1-t2-(6-brom oi corh roman- 1-yl)-ethyl]4~4-chlorophenyl)-
piperazine (VI, EXAMPLE 8, Step 2, 188 mg, 0.43 mmol) and methylamine gives
35 product which is purified by LC on 10 g (230-400) silica gel eluting with 40%~cetonP~hexane tû give 1-[2-[4-(4-chlorophenyl)-1-piperazinyl]ethyl]-N-methyl-
-70-
CA 02225282 1997-12-19
W 097/02259 PCTrUS96/08681
isochroman-6-carb--~rRmi-le (IX), mp = 158-160~; Rf = 0.21 (40% R-eton~/hexane); IR
(mull) 3319, 3263, 1639, 1613, 1597, 1571, 1545, 1497, 1334, 1314, 1239, 1150, 1139,
1109, 816 cm~l; N~ (300 ~Iz, CDCl3) 7.54 (brd~, 2H, aromatic), 7.16 (m, 3H,
aromatic), 6.84 (d, 2H, J=9.0 Hz, aromatic), 6.21 (brdm, lH, NH), 4.86 (brdd, lH,
J=6.0 Hz, mPthine), 4.12 (m, lH, OCH2a), 3.77 (m, lH, OCH2b), 3.17 (t, 4H, J=4.8Hz, Ph-N-CH28), 3.00 (d, 4H, J=4.9 Hz, N-CH3 & Ph-CH2a), 2.77-2.45 (m, 7H, Ph-
NC(H2)-CH2s-NCH28, Ph-CH2b), 2.18 (m, lH, C(H)-CEI2a), 2.05 (m, lH, C(H)-CH2b)
~; CMR (75 MHz, CDCl3) 167.7, 149.7, 141.3, 134.2, 132.4, 128.7, 127.4, 124.7,
124.2, 124.1, 116.9, 74.2, 62.7, 54.3, 53.0, 48.9, 32.9, 28.8, 26.6 o; HRMS (EI)~Rlc~ t~d for C23H28ClN3O2 = 413.1870, found = 413.1867.
~xAlvrPLE 39 1-[2-[4-(4-Chlorophenyl)-1-piperazinyl]ethyl]-N,N-dimethyl-
isochroman-6-carb- YRmi-1~ (IX)
Following the general procedure of EXAMPLE 36 and mRking non-critical
vRriRfion~ but using 1-[2-(6-brom-i~o~ an-1-yl)-ethyl]-4-(4-chlorophenyl)-
piperazine (VI, EXAMPLE 8, Step 2, 188 mg, 0.43 mmol) and dimethylamine gave
50 mg (27~o) of 1-[2-[4~4-chlorophenyl)-1-piperazinyl]ethyl]-N,N-
dimethylisochroman-6-caLl,~ e (IX) as product. This mRtPr~Rl is co~lvtlled to
the bis hyd~chloride salt with gaseous hyd~cllloric acid and ~e~ lli7e~1~ mp =
119-122~; Rf = 0.41 (40% ~cet~n~/hexane); bis salt IR (mull) 3411, 2507, 2421, 2336,
1628, 1570, 1496, 1397, 1334, 1286, 1263, 1170, 1110, 1095, 1057 cm~l; free baseNMR (300 MHz, CDCl3) 7.20 (m, 4H, aromatic), 7.12 (d, lH, J=7.9 Hz, aromatic),
6.83 (d, 2H, J=9.1 Hz, aromatic), 4.85 (brdd, lH, J=6.0 Hz, m~hine)~ 4.13 (m, lH,
OCH2a), 3.76 (m, lH, OCH2b), 3.18 (t, 4H, J=4.9 Hz, Ph-NCH2~), 3.10-3.00 (m, 7H,NCH3s & Ph-CH2a), 2.60 (m, 7H, Ph-NC(H2)-CH2E,-NCH2 & Ph-CH2b), 2.14 (m, lH,
- 25 C(H)-CH2a), 2.02 (m, lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3) 171.3, 149.7, 139.3,
134.3, 134.1, 128.8, 127.5, 124.7, 124.5, 124.3, 117.0, 74.3, 62.8, 54.4, 53.0, 48.9, 39.4,
35-2, 32-9, 28-8 o; HRMS (EI) calculated for C24H30ClN302 = 427.2026, found =
427.2020.
F'XAMPLE 40 1-t2-[4-Phenylpipelazillyl]ethyl]isochroman-6-carbmrRmi(le (VII)
Step 1: 1-[2-(6-BromniRochroman-1-yl)acetyl]-4-phe"yl~ .dzine (V)
Following the general procedure of EXAMPLE 1, Step 3 and making non-
critical vAriRt;~n~ but using racemic 6-bromr~i~orhroman-l-yl-acetic acid (IV,
F'XAlVlPLE 7, Step 1) and N-phenylpiperazine gave 1-[2-(6-br m~ orhroman-1-
yl)acetyl]-4-ph~yl~i~e~ e (V), Rf = 0.20 (40% ethyl acetate/hexane); IR (mull)
1641, 1599, 1495, 1482, 1442, 1406, 1329, 1278, 1232, 1171, 1156, 1107, 1027, 760,
694 cm~l; NMR (300 MHz, CDCl3) 7.29 (m, 4H, aromatic), 7.00 (d, lH, J=8.2 Hz,
-71-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
aromatic), 6.91 (m, 3H, aromatic), 5.26 (brdd, lH,J=5.8 Hz,m~thin~),4.11(m,1H,
OCH2a),3.91 (dt, lH,J=13.6 Hz & J=5.1 Hz,O=C-N-CH2a), 3.72 (m, 4H,O=C-N-
CH2bcd,OCH2b),3.18 (t, 4H,J=4.9 Hz,Ph-NCH2s),2.95(m,2H,Ph-CH2a & N-CO-
CH2a),2.76(dd,lH,J=14.8 Hz & J=3.7 Hz,N-CO-CH2b),2.65 ~d, lH,J=16.4 Hz,
Ph-CH2b)~;C~IR(75~DHz,CDCl3)168.8,150.7,136.2,136.1,131.5,129.2,129.0,
126.2,12~.2,120.1,116.3,73.2,63.3,49.5,49.0,45.8,41.6,39.7,28.5~;HR~S(EI)
c~lr~llA~d for C21H23BrN202 = 414.0943, found = 414.0937.
Step 2: 1-[2-(6-BromoiRorhroman-l-yl)-ethyl]-4-phenylpiperazine~)
Following the general ~rocedul~ of Ti~xAMpLE 1, Step 4 and m~kinF non-
10 critical v~ri~tinnR but using 1-[2-(6-bromniRorhroman-1-yl)acetyl]-4-phenylpiperazine
(V) gives 1-[2~6-bromoiRorhroman-1-yl)-ethyl]-4-phellyl~i~eL~i..e (VI) which is
disolved in ether (30 mL) and treated with gaseous hyd~ocllloric acid. This crude
salt is le~ ~y~ d from ethyl acetate/h~Y~nP, mp =241-242~;Rf=0.25(40% ethyl
acetate~exane); bis salt IR (mull) 2532,2510,2482,2348,2205,1596,1494,142~,
1407,1112,1100,980,884,764,694cm~l; freebase N~R(300 MHz,CDCl3)7.26 (m,
4H, aromatic), 6.94 (m, 3H, aromatic), 6.85 (t, lH,J=7.3 Hz, aromatic), 4.78 (brdd,
lH,J=5.8 Hz, m~thin~), 4.11 (m, lH,OCH2a),3.74 (m, lH,OCH2b),3.20 (t, 4H,
J=4.9 Hz,Ph-NCH2s),2.93 (m, lH,Ph-CH2a),2.65 (m, 7H,Ph-NC(H2)-CH2B-NCH2
& Ph-CH2b),2.09 (m, lH,C(H)-CH2a),2.00 (m, lH, C(H)-CH2b) o; CMR (75 MHz,
CDC13)151.6,137.8,136.3,131.6,129.3,129.1,126.5,120.0,119.6,116.0,74.3,62.8,
54.6,53.4,49.1,33.2,28.8~;MS (EI, m/z) = 400.
Step 3: 1-[2-[4-phellyl~i~el~inyl]ethyl]-isochroman-6-carbn~m
(VII).
A flame-dried 10 ml flask e.lui~ped with spinbar and ~ ;0T~ funnel is
charged with freshly ~ t;ll~d tetrahyd.uru~An(2 mL), cooled to -78~, and treatedwith a solll*-n of tert-butyllithinm (1.7 M,1.3 mL, 2.3 mmol). The resllltin~
ll~Luie is stirred 5 min and drop-treated with a solnti~n of 1-[2-(6-
bromniRorhroman-l-yl)-ethyl]4-phe~ erazine(VI,431 mg, 1.1 mmol) in 6 mT-
tetrahy-l,uru-an. The aryl lit~ lm is stirred 10 min and is added via ç~nnlll~ to a
flame-dried 25 mL flask e~uipped with spinbar contS~ininF freshly ~
+rim~thylsilylisocyanate(0.22 mL, 1.6 mmol) and 2 mT tetrahydluru-~l also cooledto -78~. The rPRlllting ~ Lule is warmed to 20-25~ for 2 hours, diluted with 25 mL
saLu ated ~mmnnillm chloride, the volatiles removed under reduced pl'.,S~iU' e,
adjusted topH = 13, and extracted twice with ethyl acetate (35 mT ). The comhin~organic e~L~cLs are washed once with saline(25 mT.), dried over m~gn~Rillm suffate,
filtered, and con-e~ aLed. This m~t~ri~l is purified by LC on 27 g (230~00) silica
-72-
CA 02225282 1997-12-19
W O 97/022S9 PCT/U~,.5.'E~'Yl
gel eluting with 5% m~th~nollethyl acetate to give 1-[2-[4-
phenyl~i~elazinyl]ethyl]isocl.lulllan-6-car~uY~ e (VII), IR (mull) 3350, 3189, 3067,
1663, 1600, 1570, 1503, 1496, 1427, 1336, 1238, 1143, 1110, 760, 692 cm~l; NMR
(300 MHz, CDCl3) 7.599 (m, 2H, aromatic), 7.26 (t, 2H, J=8.2 Hz, aromatic), 7.18 (d,
5 lH, J=8.5 Hz, aromatic), 6.93 (d, 2H, J=7.9 Hz, aromatic), 6.85 (t, lH, J=7.2 Hz,
aromatic), 6.10 (brds, lH, NH), 5.75 (brds, lH, NH), 4.88 (brdd, lH, J=5.9 Hz,
m~ in~), 4.15 (m, lH, OCH2a), 3.77 (m, lH, OCH2b), 3.21 (t, 4H, J=4.9 Hz, Ph-N-
CH28), 3.02 (m, lH, Ph-CH2a), 2.79-2.50 (m, 7H, Ph-NC(H2)-CH2~-NCH2~" Ph-
CH2b), 2.16 (m, lH, C(H)-CH2a), 2.05 (m, lH, C(H)-CH2b) ~; CMR (75 MHz, CDC13)
10 169.0, 151.3, 142.4, 134.6, 131.3, 129.1, 128.2, 125.1, 124.9, 119.7, 116.0, 74.5, 62.9,
54.6, 53.4, 49.1, 33.1, 29.0 ~; HRMS (EI) c~lr~ te~l for C22H27N302 = 365.2103,
~ound = 365.2108.
EXAMPLE 41 1-[2-[4-(3,4-Dichlorophenyl)-l-piperazinyl]ethyl]isochroman-6- ca l~n~J....i-le (VII)
Step 1: 1-[2-(6-BromniQorhroman-l-yl)acetyl]-4-(3,4-dichlorophenyl)-
piperazine (V)
Following the general procedure of EXAMPLE 1, Step 3 and m~kinF non-
critical variations but using racemic 6-bromf.iQorhroman-l-yl-acetic acid (IV,
EXAMPLE 7, Step 1) and 3,4-dichlorophenyl~i~e~ e gives 1-[2~6-
brnmniRorhroman-l-yl)acetyl]-4-(3~4-dichlorophenyl)piperazine (V), IR (mull) 1640,
1592, 1482, 1448, 1406, 1275, 1234, 1206, 1140, 1107 cm~l; NMR (300 MHz, CDCl3)
7.32-7.26 (m, 3H, aromatic H's), 7.01 (d, lH, J=8.2 Hz, aromatic H), 6.96 (d, lH,
J=2.8 Hz, aromatic H), 6.74 (d of d, lH, Ja=2.8 Hz, Jb=8.9 Hz, aromatic H), 5.24 (m
of d, lH, J=9.6 Hz), 4.11 (m, lHj, 3.94 (m, lH), 3.79-3.60 (m's, 4H), 3.16 (m, 4H),
3.09-2.89 (m, 2H), 2.77 (d of d, lH, Ja=3.6 Hz, Jb=14.8 Hz), 2.65 (d, lH, J=10.3 Hz)
o; CMR (75 MHz, CDCl3) 168.0, 150.1, 136.2, 132.8, 131.7, 130.5, 129.4, 126.4,
123.0, 120.8, 117.7, 115.7, 73.5, 63.5, 48.5, 47.9, 45.2, 41.5, 39.9, 28.7 o;
Step 2: 1-[2-(6-Bromoisochroman-l-yl)-ethyl]-4-(3,4-dichlorophenyl)-
piperazine (VI)
Following the general pl~,ceduLc of ~AlvlpLE 1, Step 4 and making non-
critical variations but using 1-[2-(6-bromni#orhroman-1-yl)acetyl]4-(3,4-
dichlorophenyl)-piperazine (V) gives l-t2-(6-bromni#orhroman-1-yl)-ethyl]-4-(3,4-
dichlorophenyl)-piperazine (VI), which after flash chrom~tr.~ .hy on 100 g of silica
gel using a gradient of 40% to 50% ethyl acetate in h~Y~n~, Rf = 0.25 (ethyl
acetate/h~Y~n~-, 25/75); IR (neat) 2825, 1593, 1483, 1467, 1455, 1449, 1380, 1239,
1142, 1111 cm~l; NMR (300 MHz,CDCl3) 7.32-7.24 (m, 2H, aromatic H's), 6.98 (m,
-73-
CA 0222~282 l997-l2-l9
W O 97/02259 PCT~US96/08681
2H, aromatic H), 6.73 (d of d, lH, Ja=2.9 Hz, Jb=8.9 Hz, aromatic H's), 4.78 (m of d,
lH, J=5.8 Hz), 4.12 (m, lH), 3.73 (m, lH), 3.16 (t, 4H, J=5.0 Hz), 3.00-2.90 (m, lH),
2.7-2.48 (m, 7H), 2.12 (m, lH), 2.02 (m, lH) o; CMR (75 MHz, CDCl3) 150.2, 137.1,
136.0, 132.2, 131.4, 130.1, 129.0, 126.2, 121.8, 120.0, 116.9, 114.9, 73.9, 62.6, 54.2,
5 52.8, 48.4, 32.9, 28.6 ~; HRMS c~ t~l for C21H23N201BrlCl2 = 468.0371, found = 418.0363.
Step 3: 1-[2-[4-(3~4-Dichlorophenyl)-l-pip~azillyl]ethyl]isochroman-6
carbn~mi-l~ (VII)
Following the general procedure of h~AlvrpLE 40, Step 3 and m~king non-
10 critical variations, but using 1-[2-(6-bromni~orl,~ ,lan-1-yl)-ethyl]-4-(3,4-dichlorophenyl)piperazine (VI) gives 1-[2-[4-(3,4-dichlorophenyl)-1-piperazinyl]ethyl]-
isochroman-6-ca.LuY~ le (VII), Rf = 0.13 (ethyl acetate); NMR (300 MHz, CDCl3)
7.59 (m, 2H, aromatic H's), 7.24 (2 m, 3H, aromatic H's), 6.94 and 5.74 (two d, lH,
aromatic H) 5.90 (broad d, 2H, PhC(O)N-H2), 4.87 (m of d, lH, J=6.0 Hz, PhC-H),
15 4.15 (m, lH, PhCH2CH-H), 3.77 (m, lH, PhCH2CH-H), 3.18 (t, 4H, J=4.8 Hz, four of
pip-H), 3.00 (m, lH, NCH-H), 2.76-2.45 (several m's, 7H, four pip-H, two PhCH-H,and NCH-H), 2.15 (m, lH, PhCHCH-H), 2.03 (m, lH, PhCHCH-H) ~; CMR (75
MHz, CDCl3) 168.3, 152.0, 142.7, 135.2, 133.0, 130.7, 129.0, 125.0, 117.5, 115.0, 74.8,
63.0, 54.5, 53.0, 48.6, 33.0, 28.8 ~; HRMS c~ t~-l for C22H26N3F102 = 433.1324,
20 found = 433.1325.
~XAMPLE 42 1-[2-[4-(4-Fluorophenyl)- l-piperazinyl]ethyl]isochroman-6-
CA - l.~
Step 1: 1-[2-(6-Bromoi~orhroman-l-yl)acetyl]-4-(4-
fluorophenyl)piperazine (V)
Following the general ~l~cedul~ of P:~AlVlPLE 1, Step 3 and m~kinE~ non-
critical variations but using racemic 6-brnmni~ochroman-l-yl-acetic acid (IV,
EXAMPLE 7, Step 1) and 4-fluorophellyl~ w~ille gives 1-[2-(6-brnmniRochroman-
l-yl)acetyl]4-(4-fluorophenyl)piperazine (V), IR (neat) 1641, 1510, 1482, 1464, 1444,
1278, 1232, 1107, 827, 817 cm~l; N~ (300 MHz, CDC13) 7.32-7.26 (m, 2H, aromatic
H's), 6.99 (m, 3H, aromatic H's), 6.92-6.86 (m, 2H, aromatic H's), 5.26 (m of d, lH,
J=9.6 Hz), 4.11 (m, lH), 3.94 (m, lH), 3.79-3.60 (m'~, 4H), 3.08 (m, 4H), 2.99-2.89 (m,
2H), 2.77 (d of d, lH, Ja=3.6 Hz, Jb=14.8 Hz), 2.65 (d, lH, J=10.3 Hz) o; CMR (75
MHz, CDCl3) 168.0, 147.2, 136.2, 131.7, 129.2, 126.2, 120.2, 118.3, 118.2, 115.6,
115.3, 73.2, 63.3, 50.6, 50.1, 45.2, 41.5, 39.7, 28.5 ~; HRMS c~ t"~l for
C21H22N2O2FBr = 432.0843, found = 432.0849.
Step 2: 1-[2-(6-Brnmni~o~-hroman-l-yl)ethyl]-4~4-
-74-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
fluorophenyl)piperazine (VI)
Following the general pl~ocedu~ of EXAMPLE 1, Step 4 and making non-
critical variations but using 1-[2-(6-bromni~orhroman-1-yl)acetyU-4-(4-fluorophenyl)-
piperazine (V) gives 1-[2-(6-brom oi .corh roman- 1-yl)ethyl]-4~4-fluorophenyl)piperazine
5 (VI) which after flash chrom~tography on 100 g of silica gel using a gradient of 40%
to 50% ethyl acetate in h~Y~n~, Rf = 0.26 (25% ethyl acetate in hexane); IR (neat)
2952, 2820, 1510, 1481, 1456, 1379, 1235, 1144, 1109, 817 cm~1; NMR (300
MHz,CDC13) 7.31-7.26 (m, 2H, aromatic H's), 6.98-6.86 (m, 5H, aromatic H's), 4.78
(m of d, lH, J=5.8 Hz), 4.14-4.07 (m, lH), 3.78-3.69 (m, lH), 3.16 (t, 4H, J=5.0 Hz),
10 3.00-2.90 (m, lH), 2.7-2.48 (m, 7H), 2.12 (m, lH), 2.02 (m, lH) o; CMR (76 MHz,
CDCl3) 158.3, 156.2, 148.2, 137.3, 136.9, 131.7, 129.3, 126.5, 117.8, 117.7, 115.6,
115.4, 74.3, 62.8, 54.6, 53.4, 50.2, 33.2, 28.9 o; HRMS calculated for
C21H24N2OlBrlFl = 418.1056, found = 418.1057.
Step 3: 1-[2-[4-(4-Fluorophenyl)-l-piperazinyl]ethyl]i~ochroman-6-
16 carb~ Y~micle (VII)
Following the general procedure of F:~AlvlpLE 40, Step 3 and making non-
critical variations but using 1-[2-(6-br~m-i~orhroman-1-yl)ethyl]-4-(4-fluorophenyl)-
piperazine (VI) gives 1-[2-[4-(4-fluorophenyl)-1-piperazinyl]ethyl]isochroman-6-c~b~ ...i(le (VII), Rf = 0.09 (ethyl acetate); NMR (300 MHz, CDCl3) 7.59 (m, 2H,aromatic H's), 7.17 (m, lH, aromatic H), 6.87 (m, 4H, aromatic H's), 6.21 (broad s,
2H, PhC(O)N-H2), 4.87 (m of d, lH, J=6.0 Hz, PhC-H), 4.15 (m, lH, PhCH2CH-H),
3.77 (m, lH, PhCH2CH-H), 3.13 (t, 4H, J=4.8 Hz, four of pip-H), 3.00 (m, lH, NCH-
H), 2.76-2.45 (several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.16 (m, lH,PhCHCH-H), 2.03 (m, lH, PhCHCH-H) o; CMR (76 MHz, CDC13) 169.1, 158.3,
155.5, 147.5, 142.0, 134.4, 131.2, 128.0, 124.9, 124.8, 117.6, 117.5, 115.4, 115.1, 74.3,
62.7, 54.4, 53.1, 499.9, 32.8, 28.8 o; HRMS cAlrlllAt,e(1 for C22H26N3F102 =
383.2009, found = 383.2010.
EXAMPLE 43 1-[2-[4-(3-Ethoxyphenyl)-l-piperazinyl]ethyuisochroman-6
c~l,.~ 1e (VII)
Step 1: 1-[2-(6-Brom-iRorhroman-1-yl)acetyl]-4-(3-
ethoxyphenyl)piperazine (V)
Following the general plwedL~,2 of EXAMPLE 1, Step 3 and mAking non-
critical variations but using racemic 6-brom-~i~o-l . . vlllan-l-yl-acetic acid (IV,
F'XAlVlPLE 7, Step 1) and 3-ethoxyph~llyl~ ,e~azine give 1-t2-(6-bromoi~orhromanyl)acetyl]4~3-ethoxyphenyl)piperazine (V) which after flssh chromAt~graphy on 200
g of silica gel u~ing 25% ethyl acetate in h~Y~nf-, Rf = 0.28 (50% ethyl acetate in
-75-
CA 0222~282 1997-12-19
W O 97/02259 PCT~US96/08681
hexane); IR (neat) 1641, 1501, 1480, 1445, 1241, 1225, 1108, 1040, 1031, 748 cm~l;
NMR (300 MHz,CDCl3) 7.32-7.26 (m, 2H, aromatic H's), 7.03-6.98 (m, 2H, aromatic
H's), 6.94-6.86 (m, 3H, aromatic H's), 5.28 (m of d, lH, J=7.4 Hz), 4.16-4.05 (m, 4H),
3.98-3.91 (m, lH), 3.83-3.65 (m, 4H), 3.08-2.91 (m, 6H), 2.80-2.64 (m, 2H), 1.46 (t,
5 3H, J=6.9 Hz, -CH3) 8; CMR (75 MHz, CDCl3) 178.5, 152.0, 140.0, 157.0, 156.5,
131.7, 129.4, 126.5, 123.3, 121.0, 120.0, 118.4, 112.6, 73.4, 63.6, 63.5, 60.0, 51.0, 50.5,
46.4, 42.2, 40.0, 28.8, 14.9 o; HRMS calculated for C23H27N2O3Br1 = 458.1205,
found = 458.1215.
Step 2: 1-t2-(6-Bromoisochroman-l-yl)ethyl]-4-(3-
ethoxyphenyl)piperazine (VI)
Following the general procedure of EXAMPLE 1, Step 4 and making non-
critical variations but using 1-[2-(6-brom.ni~orhroman-1-yl)acetyl]-4-(3-ethoxyphenyl)-
piperazine (V) gives 1-[2-(6-bromoi~orhroman-1-yl)ethyl]4-(3-
ethoxyphenyl)piperazine (VI) which after flash chrom~o~.~phy on 100 g of silica gel
using a gradient of 40% to 50% ethyl acetate in hpy~ne~ Rf = 0.30 (50% ethyl acetate
in hexane); IR (neat) 2816, 1501, 1480, 1448, 1240, 1143, 1124, 1046, 1110, 748 cm~
1; NMR (300 MHz,CDCl3) 7.31-7.26 (m, 2H, aromatic H's), 7.00-6.90 (m, 4H,
aromatic H's), 6.85-6.83 (m, lH, aromatic H), 4.78 (m of d, J=5.9 Hz, lH), 4.14-4.03
(m, 3H), 3.78-3.70 (m, lH), 3.13 (broad s, 4H), 3.00-2.90 (m, lH), 2.69-2.52 (m, 7H),
2.13 (m, lH), 1.99 (m, lH), 1.45 (t, 3H, J=7.0 Hz) ~; CMR (75 MHz, CDCl3) 151.37,
141.19, 137.0, 136.1, 131.5, 129.1, 126.4, 122.5, 120.8, 119.8, 117.9, 112.2, 74.2, 63.4,
62.6, 54.6, 53.5, 50.4, 33.0, 28.7, 14.8 o; HRMS C~ tr~l for C23H29N202Brl e
444.1413, found = 444.1400.
Step 3: 1-[2-[4-(3-Ethoxyphenyl)-l-piperazinyl]ethyl]isochroman-6-
carboY~micle (VII)
Following the general procedure of ~AMPLE 36 and m~king non-critical
variations, but using 1-[2-(6-brom~ orhroman-l-yl)ethyl]-4-(3-ethoxy-phenyl)-
piperazine (VI) gives the product which is col~vt:,ied into the hydLuchloride salt
using ethereal hyd~û~loric acid to give 1-[2-[4-(3-ethoxyphenyl)-1-piperazinyl]ethyl]-
isochroman-6-c~lJ~ P (VII), mp = 208-210~; Rf = 0.14 (10% mpt~nol in ethyl
acetate); IR (mull) 2417, 2365, 1611, 1520, 1489, 1476, 1448, 1260, 1121, 152 cm~l;
NMR (300 MHz, CDCl3) 7.60 (m, 2H, aromatic H's), 7.18 (d, lH, J=8.5 Hz, aromaticH), 6.94 (m, 3H,aromatic H's), 6.84 (m of d, lH, J=8.2 Hz, aromatic H), 6.00 (broad
d, 2H, PhC(O)N-H2), 4.86 (m of d, lH, J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H),4.06 (q, 2H, 6.9 Hz, Proc-H2), 3.77 (m, lH, PhCH2CH-H), 3.13 (broad s, 4H, four of
pip-H), 3.00 (m, lH, NCH-H), 2.76-2.45 (several m's, 7H, four pip-H, two PhCH-H,
-76-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
and NCH-H), 2.16 (m, lH, PhCHCH-H), 2.02 (m, lH, PhCHCH-H), 1.45 (t, 3H,
J=7.0 Hz, PhOCH2C-H3) ~; CMR (75 MHz, CDCl3) 151.2, 142.0, 141.2, 134.1, 131.3,
147.6, 125.0, 124.9, 121.2, 117.9, 112.2, 74.1, 63.0, 62.8, 54.8, 53.3, 50.3, 3Z.5, 31.5,
27.2, 22.2, 14.5 o; HR~IS c~ tP~ for C24H3lN303 = 409.2365, found = 409.2364.
EXAMPLE 44 (S)-(-)-1-[2-[4-(4-MPth.~.~yphenyl)-1-piperidinyl]ethyl]-N-
methylisochroman-6-carbnY~Tni~P. (S)-(IX)
Step 1: (S)-(-)-1-[2-(6-Bromoiqo~hroman-1-yl)acetyl]-4~4-
methoxyphenyl)-piperidine (S)-(V)
Following the general procedure of EXAMPLE 1, Step 3 and m~kinF non-
critical variations but using 4-methoxyphenylpiperidine (421 mg, 2.2 mmol) givescrude produce which i6 purified by LC on 53 g (230-400) silica gel eluting with 40%
ethyl ~etst~Jhexane to give (S)-(-)-1-[2-(6-br~ moiqo~hroman-l-yl)acetyl]-4-(4-
methoxy-phenyl)piperidine (S)-(V), Rf = 0.26 (505~ ethyl ~cet~te~hexane); [a]D -86 (c
0.4975, mPt~l~n~l); IR (liq.) 2933, 1638, 1612, 1513, 1481, 1463, 1446, 1283, 1268,
1247, 1179, 1106, 1036, 1005, 830 cm~l; NMR (300 MHz, CDCl3) 7.28 (m, 2H,
aromatic), 7.11 (dd, 2H, J=8.6 Hz & J=3.4 Hz, aromatic), 7.03 (m, lH, aromatic),6.85 (d, 2H, J=8.6 Hz, aromatic), 5.29 (brds, lH, O-CH), 4.85 (m, lH, Ph-CH) , 4.10
(m, 2H, OCH2a & O=C-N-CH2a), 3.79 (m, 4H, OCH3 & OCH2b), 3.15-2.66 (m, 7H,
~=C-N-cH2bcd~ Ph-CH2a~ N-CO-CH28, Ph-CH2b)~ 1.90-1.50 (m, 4 , 2s
o; CMR (75 MHz, CDCl3) 168.9, 168.8, 158.2, 137.5, 136.8, 136.3, 131.7, 129.4,
127.6, 126.6, 126.3, 120.3, 114.0, 73.5, 63.4, 55.3, 46.8, 42.8, 41.9, 40.2, 40.0, 34.2,
33.2, 33.0, 28.9 o.
Step 2: (S)-(-)-l-t2-(6-Br. moiqo-~hroman-l-yl)ethyl]-4-(4-methoxyphenyl)-
pirPritline (S)-(VI)
Following the general procedure of EXAMPLE 1, Step 4 and m~king non-
critical variations but using (S)~-)-1-[2-(6-bromoiqo~hr" ,an-1-yl)acetyl]-4-(4-mPth~Yyphenyl)pir~ri~lin~ (S)-(V) gives prodict which is purified by LC on 47 g (230-
400) silica gel eluting with 75% ethyl acetate/hexane to give (S)~-)-1-[2-(6-
brom~iqochroman-l-yl)ethyl]-4~4-mPtl-o~ry~henyl)pireri~lin-p (S)-(VI), Rf = 0.28 (75~o
ethyl acetate/hexane); [a]D = -46 (c = 0.6677, mptl~nnl); IR (liq.) 2932, 2847, 2832,
2805, 1513, 1481, 1466, 1378, 1274, 1247, 1179, 1127, 1109, 1039, 828 cm~1; NMR
(300 MHz, CDCl3) 7.29 (m, 2H, aromatic), 7.14 (d, 2H, J=8.6 Hz, aromatic), 6.97 (d,
lH, J=8.2 Hz, aromatic), 6.83 (d, 2H, J=8.7 Hz, aromatic), 4.77 (brdd, lH, J=5.7 Hz,
m~thinP), 4.10 (m, lH, OCH28), 3.78 (s, 3H, OCH3), 3.71 (m, lH, OCH2b), 3.04 (m,2H, NCH2ab), 2.95 (m, lH, Ph-CH2a), 2.70-2.40 (m, 4H, Ph-CH, NCH2Cd, Ph-CH2b),
2.04 (m, 4H, O-C(H)-CH28, NCH2ef), 1.79 (m, 4H, Ph-C(H)-CH28) o; CMR (75 MHz,
-77-
CA 0222~282 1997-12-19
W O 97/022S9 PCTrus96/08681
CDCl3) 158.2, 140.3, 137.5, 136.4, 136.2, 131.7, 129.5, 127.7, 126.6, 120.2, 113.9,
74.2, 63.1, 55.3, 54.8, 54.5, 53.9, 41.1, 32.5, 32.0, 28.8) o; HRMS (EI) ~ t~--l for
C23H28BrNO2 = 429.1304, found = 429.1286.
Step 3: (S)-(-)-1-[2-t4-(4-M~t~o~yphenyl)-1-piperidinyl]ethyl]-N-methyl-
isochroman-6-carbnY~mi-l~ (S)-(IX) r
Following the general procedure of ~.~AMPLE 5, Step 3 and making non-
critical variations but using (S)-(-)-1-[2-(6-br m~i~orhroman-l-yl)ethyl]-4~4-
methoxyphenyl)piperidine (S)-(VI, 445 mg, 1.03 mmol) gives product which is
purified by LC on 24 g (230-400) silica gel eluting with 75% ~eton~lhexane to give
(S)-(-)-1-t-2-[4-(4-methoxyphenyl)-1-piperidinyl]ethyl]-N-methylisochroman-6-
carb-Y~mi-le (S)-(IX), Rf = 0.36 (75% ~cetone/hexane); IR (liq.) 3291, 2934, 1636,
1612, 1571, 1551, 1514, 1496, 1467, 1315, 1293, 1247, 1179, 1109, 1036 cm~l; NMR(300 MHz, CDCl3) 7.54 (m, 2H, aromatic), 7.116(m, 3H, aromatic), 6.84 (d, 2H, J=8.7
Hz, aromatic), 6.20 brdm, lH, NH), 4.85 (m, lH, O-CH), 4.13 (m, lH, OCH2a ), 3.78
(m, 4H, OCH3 & OCH2b), 3.00 (m, 6H, Ph-CH2a, NCH3, Ph-CH2b, Ph-CH), 2.77-2.40
(m, 4H, N-CH2S), 2.10-1.94 (m, 4H, N-CH2S & O-C(H)-CH28), 1.80 (m, 4H, Ph-C(H)-
CH2S) o; HRMS (EI) cAl~ t,ed for C25H32N203 = 408.2413, found = 408.2414.
h~AMPLE 45 (S)-(-)-1-[2-[4-(4-l~uoromethylphenyl)-1-pi~e.~ l]ethyl]-
N,N-dimethylisochroman-6-c~l,..~ (S)-(IX)
Following the general ~ cedu-~ of ~!~AMPLE 6, Step 4 and making
non-critical v~ri~t;~n~ but using (S)-(-)-1-[2-(6-bromoiRo-hroman-l-yl)-l-ethyl]4-(4-
trifluoromethylphenyl)piperazine (S)-(VI, EXAMPLE 5, Step 2, 21.17 g, 45.1 mmol)gives product which is purified by LC on 780 g (230-400) silica gel eluting with 3%-
5% m~t~ noVdichlorom~tl~n~ to give (S)-(-)-1-[2-[4-(4-trifluoromethylphenyl)-1-
piperazinyl]ethyl]-N,N-dimethylisochroman-6-cd.L,n~ l.q (S)-(IX), mp = 149-151~;Rf = 0.34 (5% m~th~nnl/dichlorom~+~n~); [a]D = -46~ (c = 0.988, m~t~nol); IR
(mull) 1627, 1617, 1527, 1414, 1337, 1315, 1294, 1241, 1160, 1152, 1143, 1135, 1107,
1072, 824 cm~l; NMR (300 MHz, CDCl3) 7.46 (d, 2H, J=8.7 Hz, aromatic), 7.20 (m,
2H, aromatic), 7.11 (d, lH, J=7.9 Hz, aromatic), 6.90 (d, 2H, J=8.7 Hz, aromatic),
4.86 (brdd, lH, J=5.9 Hz, m~thine), 4.12 (m, lH, OCH2a), 3.75 (m, lH, OCH2b), 3.28
(t, 4H, J=5.0 Hz, Ph-N-CH28), 3.09 (brds, 3H, NCH3), 2.99 (brds, 4H, NCH3 & Ph-
CH2a), 2.74-2.48 (m, 7H, Ph-NC(H2)-CH2S-NCH2,,~, Ph-CH2b), 2.12 (m, lX, C(H)- ~
CH2a), 2.04 (m, lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3) 171.4, 153.3, 139.5, 134.5,
134.3, 127.7, 126.6, 126.3 (d, JCF=4 Hz), 126.2, 124.8, 124.6, 120.4 (qrt, JCF=33 Hz),
114.4, 74.4, 63.0, 54.6, 53.1, 48.0, 39.6, 35.4, 33.2, 29.0 o; MS (EI, m/z) = 461.
EXAMPLE 46 1-(4-M~t~ln~yphenyl)-4-[2-[6-(5-methyloY~ole-2-yl)isochroman-1-
-78-
CA 02225282 1997-12-l9
.
W O 97/022S9 PCT~US96/08681
yl)ethyl]piperazine (P-2)
An oven-dried 25 mL flask equipped with spinbar and reflux conclpn~çris
charged with 1-[2-[4-(4-mPth- ~yphenyl)-l-piperazinyl]ethyl-N-propargylisochroman-
6-carbnYAmi~le (IX, EXAMPLE 28, 433 mg, 1.0 mmol) and mercuric acetate (19 mg,
5 0.06 mmol). The ~l~Lula is diluted with 12 mL acetic acid and heated to reflux.
After 3 hr, the reaction is cooled to 20-25~, volatiles removed under reduced
p~_~.u.~, residue diluted with 35 mL lM sodium hydroxide, and extracted twice
with ethyl acetate (30 mL). The comhine-l organic extracts are washed once with
saline (20 mL), dried over mAenP~ium sulfate, filtered and oonrPntrated. This
10 material is comhin~l with the crude mAteriAl from an i~l~nti~Al 0.25 mmol scale
reaction and purified by LC on 41 g (230-400) silica gel eluting with 25%
A~etone/hexane to give 1-(4-m~th~yyphenyl)-4-[2-t6-(5-methylrlyA~ol~--2-
yl)isochr~lllan-1-yl)ethyl]piperazine (P-2) which is l~e~ YI ~lli7ecl from ethylacetate/hPYAnP mp = 129-130~; Rf = 0.40 (50% A-et~.ne/hexane)~~5 EXAMPLE 47 1-[2-(6-Aminoi~ochroman-1-yl)-ethyl]-4-(4-methoxyphenyl)-
piperazine (Z-1)
A 10 mL oven dried two neck round bottom under argon Atmn~ph~re is
charged with a sollltion of 1-[2-(6-br~moi~o~hroman-1-yl)-ethyl]4-(4-
methoxyphenyl)piperazine (VI, 406 mg, 0.94 mmol) in THF (2 mL). The ll~ula is
20 cooled to -78~ and t-butyl lithium (1.7 M in pentane, 1.081 mL, 1.83 mmol) is added
dropwise. After stirring at -78~ for 15 min, the aryl lithillm is added dropwise via a
canula to a sollltinn of diphenylrh-sph-rylazid (98%, 0.188 mT, 0.85 mmol) in THF
(9 mL) at -78~. The reaction l~ Lu~a is mAintAinptl at -78~ for two hours then
warmed to -20~ over 40 min, and then recooled to -78~. Sodium bis(2-
25 methoxyethoxy)All~minum hydride (3.4 M in tolllen~, 1.11 mL, 3.77 mmol) is addedslowly via syringe. As the reaction is warmed to 0~, e~.~ascence of nitrogen is
obsel ~,~d. The reaction lllib~l~U~e: iS stirred at 0~ for two hours and then at 20-25~ for
30 min. After cooling to 0~, the reaction is qll~nr~hecl very slowly with water. After
e~ vesc~llce sllhcitle~l, the crude is warmed to 20-25~, and filtered on a glass frit,
30 alternatively washing with water and ethyl acetate until no more product is
ob&t~ d by TLC in the filtrate. The comhinPcl filtrates were transferred to a
separatory funnel, salted out with sopdium ~hl--ri~e, shaken and the layers wereseparated. The organic layer is washed one time with 1% aqueous sodium hydroxideand one time with saline, dried with sodium sulfate, filtered and cor.~P~ ted. After
35 two flash chr~m~ al)hies on 20 g silica gel using 5~O mptl~Anol in methylene
chloride as the eluent, 1-[2-(6-Aminoi~o-~hroman-l-yl)-ethyl]-4-(4-
-79-
CA 0222~282 l997-l2-l9
W O 97/02259 PCT~US96/08681
methoxyphenyl)piperazine (Z-l) is obtained, Rf = 0.18 (5% m~~hAn- l in methylenechloride); IR (neat) 2951, 2828, 1625, 1511, 1456, 1262, 1244, 1104, 1037, 824 cm~l;
NMR (300 MHz, CDC13) 6.85 (m, 5H, aromatic HIB), 6.53 (d of d, lH, Ja=2.4 Hz,
Jb=8.2 Hz, aromatic H), 6.43 (d, lH, J=2.2 Hz, aromatic H), 4.75 (m of d, lH, J=5.8
Hz, PhC-H), 4.09 (m, lH, PhCH2CH-H), 3.76 (~, 3H, OC-H3), 3.71 (m, lH,
PhCH2CH-H), 3.67 (broad s, 2H, N-H2), 3.11 (t, 4H, J=4.9 Hz, four pip-H), 2.89 (m,
lH, PhCH-H), 2.60 (m's, 7H, PhCH-H, NC-H2 and four pip-H), 2.10 (m, lH,
PhCHCH-H), 2.00 (m, lH, PhCHCH-H) o; CMR (75 MHz, CDCl3) 153.8, 145.7,
144.6, 134.9, 129.6, 128.2, 125.7, 120.2, 118.2, 115.6, 114.9, 114.4, 113.6, 74.6, 63.2,
55.6, 54.9, 53.5, 50.6, 33.3, 29.2 o; HRMS calculated for C22H29N3O2 = 367.2260,found = 367.2255.
EXAMPLE 48 (S)-(-)-1-[2-(6-~minoiRo~hroman-l-yl)-ethyl]4-(4-
methoxyphenyl)piperazine (S)-(Z-l)
Following the general plocedula of EXAMPLE 47 and mAking non-critical
variations but using (S)-(-)-1-[2-(6-brom~liRochroman-l-yl)-ethyl]-4-(4-
methoxyphenyl)~ elazi~e (S)-(VI) gives (S)-(-)-1-[2-(6-Amin--iRo~hroman-l-yl)-ethyl]-
4 (4_mPth~xyphenyl)piperazine (S)-(Z-l), Rf = 0.18 (5% m~thAnol in methylene
~hlr~rille); [a]D = -53~ (c = 1.04, ethAnol); IR (neat) 2951, 2828, 2819, 1625, 1511,
1262, 1244, 1104, 1037, 824 cm~l; NMR (300 MHz, CDCl3) 6.85 (m, 6H, aromatic
H's), 6.53 (d of d, lH, Ja=2.4 Hz, Jb=8.2 Hz, aromatic H), 6.43 (d, lH, J=2.2 Hz,
aromatic H), 4.75 (m of d, lH, J=5.8 Hz, PhC-H), 4.09 (m, lH, PhCH2CH-H), 3.76 (s,
3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.57 (broad s, 2H, N-H2), 3.11 (t, 4H, J=4.9
Hz, four pip-H), 2.89 (m, lH, PhCH-H), 2.60 (m's, 7H, PhCH-H, NC-H2 and four pip-
H), 2.10 (m, lH, PhCHCH-H), 2.00 (m, lH, PhCHCH-H) o; CMR (75 MHz, CDCl3)
153.8, 145.7, 144.6, 134.9, 129.6, 128.2, 125.7, 120.2, 118.2, 115.5, 114.9, 114.4,
113.6, 74.6, 63.2, 55.6, 54.9, 53.5, 50.6, 33.3, 29.2 ~; HRMS cAl-~lllAt,e~l forC22H29N3O2 = 367.2260, found = 367.2258.
EXAMPLE 49 (S)-(-)-N-[Isochroman-1-[2-[4-(4-methnxyphenyl)piperazin-1-
yl]ethyU-6-yl]formAmicle (S)~Z-2)
Acetic anhydride (0.32 mL, 3.43 mmol) is cooled to 0~. Acetic formic
anhydride is generated by the dropwise Atl~iti~n of 98% formic acid (0.20 mL, 5.2
mmol) to the acetic anhydride. The llli~ Ul~ iS heated to 55~ for 2 hours and then
cooled to 0~. THF (1 mL) is added via syringe, followed by a solllt;nn of (S)-(-)-1-[2-
(6-AminoiRo-hroman-l-yl)-ethyl]-4-(4-m~h-.xyphenyl)piperazine (S)-(Z-l, 600 mg,
1.63 mmol) in THF (2 mL). The reaction i8 warmed to 20-25~ and stirred for 3
hours. The reaction is cQnc~t-dted and purified by flash chrtmAt~ hy to give
-80-
CA 02225282 1997-12-19
WO 97/OZ259 PCT/US96/08681
(s)-~-)-N-[i6oclllul~an-l-[2-[4-(4-methoxypheny~ a~ -yl]ethyl]-6-yl]form~mi~la
(S)-(Z-2), Rf = 0.20 (5% mPth~n--l in methylene ~hl~ri~e); IR (mull) 1692, 1616, 1539,
1~12,1306,1292,1266,1245,1107,825 cm-l; ~nMnR (300 ~DHZ, CDC13) 8.65 (d, ~2 H
(rûtomer), J=11.4 Hz, NC(O)-H), 8.36 (d, 1/2 H (rotomer), J=1.7 Hz, NC(O)-H), 7.91
(broad d, 1/2 H (rotomer), J=11.4 Hz, N-H), 7.43 (broad B, lJ2 H (rûtomer), N-H),
7.24 (m, lH, aromatic H), 7.07 (d of d, lH, Ja=8.4 Hz, Jb=10.9 Hz, aromatic H), 6.84
(q and m, 5H, J=9.2 Hz, aromatic H's), 4.80 (m of d, lH, J=5.8 Hz, PhC-H), 4.10 (m,
lH, PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.11 (t,4H,
J=4.9 Hz, four pip-H), 2.95 (m, lH, PhCH-H), 2.60 (m's, 7H, PhCH-H, NC-H2 and
10 four pip-H), 2.18 (m, lH, PhCHCH-H), 2.06 (m, lH, PhCHCH-H) o; HRMS
~ ~k,~ t~l for C23H29N303 = 395.2209, found = 395.2210.
EXAMPLE 50 (S)-(-)-N-[Isochroman-1-[2-[4-(4-methoxyphenyl)piperazin-1-
yl]ethyl]-6-yl]~et~mi-le (S)-(Z-2)
A 25 mT round bottom flask is charged with (s)-(-)-l-[2-(6-~min~ico~hroman
15 1-yl)-ethyl]-4~4-m~th.~Yyphenyl)piperazine (S)~Z-1, 200 mg, 0.54 mmol) and 4-dimethylaminopyridine (6.7 mg, 0.054 mmol). Methylene ~~hl~lritla (7 mL) is added
via syringe and the reaction vessel is cooled to 0~. Triethylamine (0.114 mL, 0.82
m mol) and acetyl chloride (0.042 mL, 0.60 mmol) are then added r~ e~livl ly viasyringe. The ice bath is removed after 15 min. and the reaction is stirred at 20-25~
20 for 1.5 hours. The reaction is then partitionpd b~Lween 0.5 M aqueous sodium
hydroYide and methylene ~hl-)ricle The layers were s~palaLed and the aqueous
portion is ~ ed one more time with methylene rhlor~ The organics were
c~mhina-l, dried with sodium sulfate, filtered and con~F ~l d~ed. The con-antrate is
chr~m~to~-aphed on 17 g silica gel using 5% mpth~n~l in methylene ~hlori~la as the
25 eluent to give (S)-(-)-N-[isochroman-1-[2-[4-(4-mPth--Qyphenyl)piperazin-1-yl]ethyl]-6-
yl]~et~mi-le (S)-(Z-2), Rf = 0.18 (5% mpth~nnl in methylene chloride); [a]D -44~(c
0.93, 50% eth~nol in methylene chlnri~le); IR (mull) 1667, 1615, 1599, 1546, 1512,
1421, 1333, 1312, 1247, 1036 cm~1; NMR (300 MHz, CDC13) 7.35, 7.21, 7.15, 7.04,
6.80, 4.80, 4.10, 3.76, 3.71, 3.11, 2.95, 2.60, 2.16, 2.10, 2.02 o; CMR (75 MHz, CDCl3)
30 168.3, 153.8, 145.7, 136.0, 134.9, 134.1, 125.3, 120.1, 118.2, 118.0, 114.5, 74.5, 63.1,
55.6, 54.8, 53.5, 50.6, 33.2, 29.2, 24.6 o; HRMS c~lc~ tP~l for C24H31N303 =
409.2365, found = 409.2358.
FXAlVlPLE 51 (S)-(-)-N-[Isochroman-1-[2-[4~4-mathl Yyphenyl)piperazin-1-
yUethyl]-6-yl]ban7~mi(1P (S)-(Z-2)
Following the general procedure of EXAMPLE 50 and m~king non-critica~
v~ri~t;on~ but using benzoyl chl~ri-l~ gives (S)-(-)-N-[isochroman-1-[2-[4~4-
-81-
CA 0222~282 1997-12-19
W O 97/02259 PCTrUS96/08681
methoxyphenyl)piperazin-1-yl]ethyl]-6-yl]ben~mi-le (S)-(Z-2), Rf = 0.30 (5%
mf~th~nol in methylene chloride); [a]D = 40~ (c = L0, 50% ethanol in methylene
~hl~rk~e); IR (mull) 3282, 1651, 1516, 1505, 1339, 1312, 1280, 1244, 1108, 694 cm~l;
NMR (300 MHz, CDCl3) 7.86 (d, 2H, J=6.8 Hz, aromatic H's), 7.80 (broad s, lH,
5 PhN-H), 7.50 (m, 4H, aromatic H's), 7.36 (d, lH, J=8.3 Hz, aromatic H), 7.10 (d, lH,
J=8.3 Hz, aromatic H), 6.86 (q, 4H, J=9.2 Hz, aromatic H's), 4.84 (m of d, lH, J=5.8
Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.76 (m, lH,
PhCH2CH-H), 3.12 (t, 4H, J=4.6 Hz, four pip-H), 2.99 (m, lH, PhCH-H), 2.68 (m's,7H, PhCH-H, NC-H2 and four pip-H), 2.10 (m, lH, PhCHCH-H), 2.02 (m, lH,
10 PhCHCH-H) o; HR~IS c~ t~ for C29H33N303 = 471.2522, found = 471.2525.
~XANI PLE 52 (S)-(-)-N-tIsochroman-1-[2-[4-(4-m.oth-1~ryphenyl)piperazin-l-
yl]ethyl]-6-yl]propion~mi-le (S)-(Z-2)
Following the general procedure of ~AlvlpLE 50 and m~kin~ non-critical
variations but using propionyl chloride (S)-(-)-N-[isochroman-1-[2-[4-(4-
15 methoxyphenyl)piperazin-l-yl]ethyU-6-yl]propior1~mide (S)-(Z-2) is obtained, Rf =
0.22 (5% m~th~nol in methylene ~hlnr--le); [a]D = -44~ (c = 0.97, 50% eth~nnl inmethylene chloritle); IR (mull) 3306, 1659, 1590, 1515, 1421, 1245, 1214, 1110, 1036,
821 cm~l; NMR (300 MHz, CDCl3) 7.76 (s, lH, aromatic H), 7.36 (broad s, lH, PhN-H), 7.24 (d, lH, J=8.3 Hz, aromatic H), 7.00 (d, lH, J=8.3 Hz, aromatic H), 6.80 (q,
20 4H, J=9.2 Hz, aromatic H's), 4.77 (m of d, lH, J=5.8 Hz, PhC-H), 4.06 (m, lH,PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.07 (t, 4H, J=4.9
Hz, four pip-H), 2.85 (m, lH, PhCH-H), 2.60 (m's, 7H, PhCH-H, NC-H2 and four pip-
H), 2.33 (q, 2H, J=7.5 Hz, PhNHC(O)C-H2), 2.14 (m, lH, PhCHCH-H), 2.00 (m, lH,
PhCHCH-H), 1.19 (t, 3H, J=7.5 Hz, PhNHC(O)CH2C-H3) o; CMR (75 MHz, CDC13)
25 172.5, 153.7, 145.8, 136.3, 134.7, 133.8, 125.2, 120.2, 118.1, 114.5, 74.4, 63.0, 55.6,
54.8, 53.5, 50.6, 33.3, 30.6, 29.2 and 9.8 ~.
~XANIPLE 53 (S)~-)-N-[Isochroman-1-[2-[4-(4-m~th- xyphenyl)piperazin-l-
yl]ethyl]-6-yl]acrylamide (S)-(Z-2)
Following the general procedul~ of EXAMPLE 50 and m~king non-critical
30 v~ri~t;-~n~ but using acryl chloride gives (S)-(-)-N-[isocl..~....an-1-[2-[4~4-
m~-Yyphenyl)piperazin-l-yl]ethyl]-6-yl]acrylamide (S)-(Z-2), Rf = 0.22 (5%
m~t~nol in methylene chloride); [a]D = -40~ (c = 0.79, 50% eth~nol in methylene
chloride); IR (mull) 3266, 1661, 1592, 1536, 1512, 1422, 1244, 1218, 1109, 822 cm~l;
NMR (300 MHz, CDCl3) 7.46 (broad s, lH, PhN-H), 7.26 (s, lH, aromatic H), 7.06 (d,
35 lH, J=8.4 Hz, aromatic H), 6.85 (q, 4H, J=9.2 Hz, aromatic H's), 6.43 (d of d, lH,
Ja=1.3 Hz, Jb=16.8 Hz, one acryl-H), 6.23 (m, lH, one acryl-H), 5.76 (d of d, lH,
-82-
CA 0222~282 1997-12-19
W O 97/022S9 PCT/u~r~l~8~l
Ja=1.3 Hz, Jb=10 Hz, one acryl-H), 4.81 (m of d, lH, J=5.8 Hz, PhC-H), 4.11 (m, lH,
PhCH2CH-H), 3.76 (8, 3H, OC-H3), 3.75 (m, lH, PhCH2CH-H), 3.11 (t, 4H, J=4.9
Hz, four pip-H), 2.96 (m, lH, PhCH-H), 2.60 (m's, 7H, PhCH-H, NC-H2 and four pip-
H), 2.14 (m, lH, PhCHCH-H), 2.03 (m, lH, PhCHCH-H) ~; CMR (75 MHz, CDC13)
5 163.5, 153.8, 145.8, 135.9, 134.9, 134.4, 131.1, 127.9, 125.4, 120.2, 118.2, 118.0,
114.4, 74.5, 63.0, 55.6, 54.8, 53.5, 50.6, 33.2, 29.2 ~; HRMS calculated for
C*25H31N3O3 = 421.2365, found = 421.2358.
EXAMPLE 54 (S)-(-)-N-[Isochroman-1-[2-[4-(4-m~t~-xyphenyl)piperazin-1-
yl]ethyl]-6-yl]isob uly~ d~l~ide(s)-(z-2)
Following the general procedure of EXAMPLE 50 and m~kin~ non-critical
variations but using i-butyryl chloride gives (S)-(-)-N-[Isochroman-1-[2-[4-(4-
mPtl ~Yyphenyl)~i~. ,dzi~-l-yl]ethyl]-6-yl]isol)uLyl~l.ide (S)-(Z-2), Rf = 0.27 (5%
mf~t~nnl in methylene chloride); [a]D = -42~ (c = 0.94, 50% et~nol in methylene
chloride); IR (mull) 3289, 1660, 1589, 1524, 1515, 1451, 1422, 1243, 1109, 822 cm~l;
15 NMR (300 MHz, CDCl3) 7.43 (s, lH, aromatic H), 7.23 (d, lH, J=8.4 Hz, aromatic
H), 7.13 (broad s, lH, PhN-H), 7.04 (d, lH, J=8.3 Hz, aromatic H), 6.84 (q, 4H,
J=9.2 Hz, aromatic H's), 4.80 (m of d, lH, J=5.8 Hz, PhC-H), 4.10 (m, lH,
PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.10 (t, 4H, J=4.9
Hz, four pip-H), 2.96 (m, lH, PhCH-H), 2.60 (m's, 8H, PhCH-H, NC-H2,
20 PhNHC(O)C-H) and four pip-H), 2.14 (m, lH, PhCHCH-H), 2.00 (m, lH, PhCHCH-
H), 1.24 (d, 6H, J=6.9 Hz, two of PhNHC(O)CHMeC-H3) ~; CMR (75 MHz, CDC13)
175.2, 153.8, 145.8, 136.2, 134.9, 133.9, 125.3, 120.0, 118.2, 117.8, 114.4, 74.5, 63, 1,
60.8, 55.6, 54.8, 53.5, 50.6, 36.7, 33.2, 29.3, 19.6 ~; HRMS ~s~lc~ for
C26H35N3O3 = 437.2678, found = 437.2680.~5 EXA~LE 55 (S)-(-)-1-[2-(6-Ethyl~minoiRochroman-l-yl)-ethyl]-4-(4-
mPt~loYyphenyl)piperazine (S)-(Z-4)
Following the general procedure of EXAMPLE 5, Step 2, and m~king non-
critical v~ri~t;onR but using (S)-(-)-N-[isochroman-1-[2-[4-(4-
mPt~Yyphenyl)piperazin-l-yl]ethyl]-6-yl]F~ret~mi-lP~ (S)-(Z-2, F~AlvlpLE 50) as the
30 substrate gives (S)-(-)-1-[2-(6-ethyl~min-iAochroman-l-yl)-ethyl]-4-(4-
mPt~l~Yyphenyl)piperazine (S)-(Z4), Rf = 0.32 (5% mpt~nol in methylene chloride);
[a]D = -46~ (c = 0.54, 50% et~l~nol in methylene chloride); IR (neat) 2953, 2825,
2819, 1616, 1512, 1268, 1244, 1147, 11104, 824 cm~l; NMR (300 MHz, CDCl3) 6.86
(m, 5H, aromatic H's), 6.47 (d of d, lH, Ja=2.4 Hz, Jb=8.2 Hz, aromatic H), 6.34 (d,
35 lH, J=2.2 Hz, aromatic H), 4.76 (m of d, lH, J=5.8 Hz, PhC-H), 4.09 (m, lH,
PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.13 (t and q
-83-
CA 0222~282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
overlapping, 6H, four pip-H, and PhNC-H2), 2.91 (m, lH, PhCH-H), 2.62 ~m's, 7H,
PhCH-H, NC-H2 and four pip-H), 2.10 (m, lH, PhCHCH-H), 2.00 (m, lH, PhCHCH-
H), 1.25 (t, 3H, J=7.1 Hz, PhNHCH2C-H3) o; CMR (75 MHz, CDCl3) 153.8, 146.8,
145.8, 134.8, 126.8, 125.6, 118.2, 114.4, 112.2, 111.6, 74.6, 63.4, 55.6, 54.9, 53.5, 50.5,
5 38.6, 33.3, 29.5, 15.0 o; HRMS ç~ ted for C24H33N302 = 395.2573, found:
395.2573.
~:~AMPLE 56 (S)-(-)-1-(4-M~th- ~yphenyl)-4-[2-(6-propyl~min-iRochroman-1-
yl)-ethyl]piperazine (S)-(Z-4)
Following the general procedure of EXAMPLE 5, Step 2, and making non-
10 critical variations but using (S)-(-)-N-[isochroman-1-[2-[4-(4-
methoxyphenyl)piperazin-1-yl]ethyl]-6-yl]propion~midP (S)-(Z-2, EXAMPLE MDE45)
as the substrate gives of (S)-(-)-1-(4-mPth~ryphenyl)-4-[2-(6-propyl~minsi~orhroman
l-yl)-ethyl]piperazine (S)-(Z-4), Rf = 0.52 (50% ~cetonP in hexane); [a]D = 41~ (c =
0.69, 50% et~ ~n-l in methylene chloride); IR (neat) 2812, 2804, 1614, 1514, 1271,
15 1254, 1247, 1105, 1034, 830cm~l; NMR (300 MHz, CDC13) 6.86 (m, 5H, aromatic
H'B), 6.47 (d of d, lH, Ja=2.4 Hz, Jb=8.2 Hz, aromatic H), 6.34 (d, lH, J=2.2 Hz,
aromatic H), 4.76 (m of d, lH, J=5.8 Hz, PhC-H), 4.09 (m, lH, PhCH2CH-H), 3.76 (s,
3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.64 (broad s, lH, PhN-H), 3.09 (t and q
overlapping, 6H, four pip-H, and PhNC-H2), 2.91 (m, lH, PhCH-H), 2.62 (m's, 7H,
20 PhCH-H, NC-H2 and four pip-H), 2.10 (m, lH, PhCHCH-H), 2.00, 1.63, 1.25 o;
HRMS ~ tPtl for C25H35N302 = 409.2729, found = 409.2722.
T1~XAMPLE 57 (S)-(-)-1-(4-MPth-.Yyphenyl)-4-[2-(6-methyl~minni~orhroman-1-
yl)-ethyl]piperazine (S)-(Z-4)
Acetic anhydride (0.32 mL, 3.43 mmol) is cooled to 0~. Acetic formic
25 anhydride is generated by the dropwise ~ tior~ of 98~o formic acid (0.20 mL, 5.2
mmol) to the acetic anhydride. The ~i~ e is heated to 55~ for 2 hours and then
cooled to -15~ with an ethylene glycol/carbon dioxide bath. THF (10 mL) is addedvia syringe, followed by a ssllltion of (S)-(-)-1-[2-(6-~minoico-~hroman-1-yl)-ethyl]-4-(4-
methoxyphenyl)piperazine (S)-(Z-1, EXA~LE 48, 1.99 g, 5.41 mmol) in THF (10
30 mL). The reaction stirred for 2 hours at -15~. The reaction warmed to 20-25~ and
volatiles removed under reduced ~ S~ leaving a yellow oil. A solllti~ n of the
crude in THF (30 mL) is charged into a 250 mL round bottom equipped with a reflux
cc~nrlPn~Pr. The ~ ure is cooled to 0~ and borane methyl sulfide comrlP~ (lOM,
1.73 mL, 17.3 mmol) is added slowly via syringe. The ice bath is removed when
35 e~ sc~ .lce sllhRide~l The ~i~Lu~: is then heated to gentle reflux for 3 hours, ~then
at 20-25~ for three days. The reaction is cooled to 0~ and mPth~nol (30 mT)iS added
-84-
CA 02225282 l997-l2-l9
W O 97/02259 PCTnUS96/08681
dropwise (e~ esce~ce) then stirred for 1 hour at 20-25~, followed by reflux for 2
hours. After cooling to 20-25~, the volatiles are removed under reduced l,.es~
and the aqueous residue is b~cifiP~ with aqueous sodium hydroxide and extracted 80
mL ethyl acetate (three times). The organic extracts are comhin~ dried with
sodium sulfate, filtered and con~enl-d~d to give a crude product. The crude
ms~tAris3li6 purified by flash chrrlm~tography using 25% Areton~ in hexane as the
eluent to give (s)-(~ -(4-m~t7lclxyphenyl)-4-[2-(6-methyl~minoi~o~hroman-l-yl)-
ethyl]piperazine (S)-(Z-4), IR (neat) 2933, 2831, 2817, 1616, 1513, 1275, 1246, 1107,
1038, 826 cm~l; NMR (300 MHz, CDCl3) 6.86 (m, 6H, aromatic H's), 6.47 (d of d, lH,
Ja=2.4 Hz, Jb=8.2 Hz, aromatic H), 6.35 (d, lH, J=2.2 Hz, aromatic H), 4.76 (m of d,
lH, J=5.8 Hz, PhC-H), 4.09 (m, lH, PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.71 (m, lH,PhCH2CH-H), 3.58 (broad s, lH, N-H), 3.11 (t, 4H, four pip-H), 2.92 (m, lH, PhCH-
H), 2.82 (s, 3H, NHC-H3), 2.62 (m's, 7H, PhCH-H, NC-H2 and four pip-H), 2.10 (m,lH, PhCHCH-H), 2.00 (m, lH, PhCHCH-H) 8; CMR (75 MHz, CDCl3) 153.8, 147.7,
145.8, 134.8, 126.9, 125.5, 118.2, 114.4, 111.8, 111.3, 74.64, 63.3, 55.6, 54.9, 53.5,
53.4, 50.6, 33.4, 30.9, 29.5 8; HRMS calculated for C23H31N302: 381.2416. Found:381.2415.
~AMpLE 58 ($)-(-)-1-(4-MPthnYyphenyl)-4-[2-(6-dimethyl~min~icorhroman-1-
yl)-ethyl]piperazine (S)-(Z-7)
From the preparation described in ~xAlvlpLE 57 is also i~nl~te(l (S)-(-)-1-(4-
m~tho~yphenyl)4-[2-(6-dimethyl~minoiRo-hroman-l-yl)ethyl]~i~e~ e (S)-(Z-7), I2f
= 0.22 (~- et~nP/h~y~n~ 25/75); NMR (300 MHz, CDCl3) 6.99-6.81 (m's, 5H, aromatic
H's), 6.62 (d of d, lH, Ja=2.4 Hz, Jb=8.2 Hz, aromatic H), 6.46 (d, lH, J=2.2 Hz,
aromatic H), 4.78 (m of d, lH, J=5.8 Hz, PhC-H), 4.10 (m, lH, PhCH2CH-H), 3.76 (6,
3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.11 (q, 4H, four pip-H), 2.95 (m, lH,
PhCH-H), 2.92 (s, 6H, two of NC-H3), 2.62 (m's, 7H, PhCH-H, NC-H2 and four pip-
H), 2.10 (m, lH, PhCHCH-H), 2.00 (m, lH, PhCHCH-H) 8; CMR (75 MHz, CDCl3)
153.8, 149.2, 145.8, 134.6, 126.3, 125.4, 118.1, 114.4, 112.5, 112.1, 111.4, 74.6, 63.4,
55.6, 55.0, 53.5, 50.6, 46.8, 40.7, 33.4, 29.7 8.
F~xAMpLE 59 (S)-(-)-l-t2-(6-Ethylmethyl~minoi~o~hroman-l-yl)-ethyl]4~4-
m~t~rlYyphenyl)piperazine (S)-(Z-7)
From the process described in EXAMPLE 57 is also i~ol~te
(S)-(-)-1-[2-(6-ethylmethyl~minoi~o~hroman-1-yl)-ethyl]-4~4-
mPth~ ~yphenyl)piperazine (S)-(Z-7), Rf = 0.22 (~-~eton~/hPY~n~, 25/75); [~]D = -54~(c
= 0.83, et~ ~nrl/methylene ~hlnri-le 50/50); IR (mull) 2815, 1611, 1515, 1256, 1245,
1236, 1107, 1095, 1037 and 824 cm~l; NMR (300 MHz, CDCl3) 6.97-6.81, 6.59, 6.42,
-85-
CA 0222~282 1997-12-19
W O 97/02259 PCT~US96/08681
4.77, 4.10, 3.76, 3.71, 3.37, 3.11, 2.95, 2.88, 2.62, 2.10, 2.00 and 1.11 o; CMR (75
MHz, CDCl3) 153.8, 147.6, 145.8, 134.6, 125.7, 125.5, 118.1, 114.4, 112.1, 111.0, 74.7,
63.5, 55.6, 55.0, 53.5, 50.6, 46.8, 37.5, 33.5, 29.7 and 11.3 o.
EXAMPLE 60 (S)-(-)-N-[Isochroman-1-[2-[4-(4-mPth- xyphenyl)~ Ldzi
yl]ethyl]-6-yl]-N-methyl~et~mi~e (S)-(Z-5)
Following the general procedure of EXAMPLE 50 and m~king non-critical
variations but using (S)-(-)-1-(4-mPth~ yphenyl)-4-[2-(6-methyl~min~ orhroman-1-yl)ethyl]piperazine (S)-(Z-4), the title compound is obtained, Rf = 0.26
(mPth~nf l/methylene chloride 5/95); [a]D = -38~(c = 0.69, ethnn~l/methylene chloride
(50/50)); IR (mull) 1669, 1661, 1513, 1448, 1445, 1275, 1248, 1109, 1036 and 826 cm~
l; NMR (300 MHz, CDCl3) 7.13, 6.98, 6.86, 4.83, 4.13, 3.76, 3.76, 3.23, 3.10, 2.97,
2.61, 2.14, 2.02 and 1.87 o; CMR (75 MHz, CDCl3) 170.6, 153.8, 145.7, 142.7, 137.8,
135.7, 127.2, 126.1, 124.8, 118.2, 114.4, 74.5, 62.8, 61.2, 55.6, 54.8, 53.5, 50.7, 37.2,
33.3, 29.0 and 22.5 ~.
EXAMPLE 61 (S)-(-)-N-[Isochl~an-1-t2-[4-(4-mPth- ~yphenyl)piperazin-1-
yl]ethyl]-6-yl]-N-methylisG'Gul,y~de (S)-(Z-5)
Following the general pl~cedu~ of ~xAMpLE 54 and m~kinF non-critical
variation but using (S)-(-)-1-(4-mPthnYyphenyl)-4-[2-(6-methyl~minoi~o~ u...an-1-yl)-
ethyl]piperazine (S)-(Z-4) gives (S)-(-)-N-[isochroman-1-[2-[4-(4-
20 methoxyphenyl)piperazin-1-yl]ethyl]-6-yl]-N-methylisobuLy~ ide (S)-(Z-5), Rf = 0.33
(5% mPt~nol in methylene l~hlr~ricle); [a]D = -34~ (c = 0.80, 50% eth~nnl in
methylene ~hlnri~le); IR (mull) 2962, 1658, 1512, 1468, 1457, 1386, 1245, 1109, 1038,
825 cm~l; NMR (300 MHz, CDCl3) 7.13 (d, lH, J=8.2 Hz, aromatic H), 6.98 (d, lH,
J=8.2 Hz, aromatic H), 6.86 (m and q, 5H, J=9.2 Hz, aromatic H's), 4.83 (m of d, lH,
25 J=5.8 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.76 (m, lH,
PhCH2CH-H), 3.22 (s, 3H, NC-H3), 3.10 (t, 4H, J=4.6 Hz, four pip-H), 2.97 (m, lH,
PhCH-H), 2.61 (m's, 8H, NC(O)CMe2-H, PhCH-H, NC-H2 and four pip-H), 2.14 (m,
lH, PhCHCH-H), 2.02 (m, lH, PhCHCH-H), 1.03 (d, 6H, J=6.5 Hz, two of
NC(O)CHC-H3) o; CMR (75 MHz, CDCl3) 177.4, 153.8, 145.7, 142.4, 137.8, 135.7,
30 127.4, 126.1, 125.0, 118.2, 114.5, 74.5, 62.8, 55.6, 54.8, 53.5, 50.7, 37.5, 33.3, 31.0,
29.0, 19.8 o; HRMS c~ qte~l for C27H37N303 = 451.2835, found = 451.2827.
EXAMPLE 62 (S)-(-)-N-[Isochroman-1-[2-[4~4-methoxyphenyl)piperazin-1-
yl]ethyl]-6-yl]-m~th~ne~lllf n~mitle (S)-(Z-3)
(S)-(-)-1-[2-(6-~minoi~orhroman-1-yl)-ethyl]-4-(4-m~thr.Yyphenyl)-pi~ zi~e
35 (S)-( Z-1) (200 mg, 0.54 mmol) and 4-dimethylaminopyridine (6.7 mg, 0.054 mmol)
are mixed. Pyridine (2 mL) is added via syringe, the ~lu~e is cooled to 0~, and
-86-
CA 02225282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
mf~thslne sulfonyl chloride (.045 mL, 0.60 mmol) is introduced. The ice bath is
removed after 15 min. and the reaction i8 stirred at 20-25~ for 1.5 hours. The
reaction is diluted with water and extracted two times with ethyl ~cet~te. The
organics were combined, washed one time with an saturated aqueous copper sulfate5 ~0ll7t;~n then with water, dried over m~E~n~illm sulfate, filtered and co~cPntrated.
The concentrate is chr m~tographed on 25 g silica gel using 50% acetone in hexane
as the eluent to give (S)-(-)-N-[isochroman-1-[2-[4-(4-mPt~Yyphenyl)piperazin-1-yl]ethyl]-6-yl]-metll~nPslllfor ~mi~1e (S)-(Z;-3), Rf = 0.21 (5% mPt~ ~nol in methylene
chloride); [a]D = -43~ (c = 0.89, 50% ethanol in methylene rhl~ri~); IR (mull) 1512,
1339, 1319, 1295, 1244, 1152, 1106, 1037, 973, 826 cm~l; NMR (300 MHz, CDCl3)
7.06 (m's, 3H, aromatic H's), 6.87 (q, 4H, J=9.0 Hz, aromatic H's), 4.80 (m of d, lH,
J=5.8 Hz, PhC-H), 4.11 (m, lH, PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.71 (m, lH,
PhCH2CH-H), 3.10 (t, 4H, J=4.6 Hz, four pip-H), 3.0 (s, 3H, NHSO2C-H3), 2.95 (m,lH, PhCH-H), 2.60 (m's, 7H, PhCH-H, NC-H2 and four pip-H), 2.11 (m, lH,
PhCHCH-H), 2.01 (m, lH, PhCHCH-H) o; CMR (75 MHz, CDC13) 153.8, 145.7,
135.8, 135.6, 134.8, 126.1, 121.2, 119.0, 118.2, 114.5, 74.4, 62.9, 55.6, 54.8, 53.5, 50.6,
39.4, 33.2, 29.1 O; HRMS c~ for c23H3lN3o4sl = 445-2035~ found =
445.2031.
EXAMPLE 63 (S)-(-)-6-Amino-1-[2-[4-(4-methoxyphenyl)piperazin-1-
yl]ethyl]isochroman, methyl urea (S)-(X-6)
(S)~-)-1-[2-(6-~min- i~ochroman-1-yl)-ethyl]-4-(4-mPt~- xyphenyl)piperazine (S)-(Z-l, 376 mg, 1.0 mmol) is added to ~et~nitril~ (4 mL). Methyl isocyanate (0.091mL, 1.53 mmol) is added slowly via syringe. ~lition~l ~cet~nitrilp (7 mL) is added
and the reaction is stirred for 3 hours at 20-25~. The pre~ipit~te is filtered and
25 rinsed successively with ethyl acetate and hexane to give crude product which is
purified by flash chrom~tography using 5% mPtl~nol in methylene ~hlf~ri~le to give
(S)-(-)-6-amino-l-t2-[4-(4-methoxyphenyl)piperazin-1-yUethyl]isochroman, methyl
urea (S)-(X-6), Rf = 0.07 (5% mPtl~n-.l in methylene chloride); [a]D = -43~ (c = 0.75,
50~o et~l~nol in methylene chloride); IR (mull) 3312, 1645, 1614, 1597, 1567, 1512,
30 1421, 1310, 1244, 1109 cm~l; NMR (300 MHz, CDCl3) 7.11 (s, lH, aromatic H), 7.02
(m, 2H, aromatic H's), 6.84 (q and m, 5H, J=9.2 Hz, aromatic H's), 5.09 (m of d, lH,
J=5.5 Hz, C(O)NMe-H), 4.77 (m of d, lH, J=5.8 Hz, PhC-H), 4.07 (m, lH, PhCH2CH-
H), 3.76 (8, 3H, OC-H3), 3.71 (m, lH, PhCH2CH-H), 3.09 (t, 4H, J=4.9 Hz, four pip-
H), 2.90 (m, lH, PhCH-H), 2.79 (d, 3H, J=4.7 Hz, C(O)NHC-H3), 2.60 (m's, 7H,
35 PhCH-~I, NC-H2 and four pip-H), 2.10 (m, lH, PhCHCH-H), 2.02 (m, lH, PhCHCH-
H) o; CMR (75 MHz, CDCl3) 156.8, 153.8, 145.7, 136.8, 135.0, 133.5, 125.5, 121.2,
-87-
CA 0222~282 l997-l2-l9
W O 97t02259 PCTrUS96/08681
119.1, 118.2, 114.5, 74.5, 63.1, 55.6, 54.8, 53.5, 50.6, 33.2, 29.2, 27.0 o; HRMS
ç~lr~ t*-i for C24H32N403 = 424.2474, found = 42473.
EXAMPLE 64 (S)-(-)-6-Amino- 1-[2-[4-(4-methoxyphenyl)~i ~uel azi
yl]ethyl]isochroman, t-butylcarbamate (S)-(X-6)
(S)-(-)-1-[2-(6-Aminoi~qochroman-1-yl)-ethyl]-4-(4-methn~yphenyl)piperazine (S)-(Z-1, 200 mg, 0.54 mmol) and sodil~m h~Y~mPthyl~ n~ (200 mg, 1.09 mmol) are
mixed. THF (2 mT ) i8 added via syringe and the reaction i8 stirred for 15 min. Di-t-
butyl pyloc~l,onate (108 mg, 0.50 mmol) is added as a sollltion in 1~ (2 mL). The
ll~ixl,u~è is 6tirred at 20-25~ for 20 hours. The reaction is poured into water (40 mL).
The volatiles are removed under reduced p.e8~ule, and the aqueous residue is
extracted with ethyl acetate (2 x 50 mL). The O~ ';CS were comhin~l, dried with
sodium sulfate, filtered and con-~nl ~ted. The conc~..t-a~ is chrom~tographed on30 g silica gel using 5% m~t~nol in methylene chloride as the eluent to give (S)-(-)-
6-amino- 1-[2-[4-(4-m ~tll nYyphenyl)piperazin- l-yl]ethyl]isochroman, t-butylcarbamate
15 (S)-(X-6), Rf = 0.38 (5% m~tl~nol in methylene chloride); [a]D = -39~ (c = 0.65, 50%
ethanol in methylene chloride); IR (mull) 1694, 1522, 1515, 1423, 1367, 1286, 1243,
1167, 1109, 1058 cm~l; NMR (300 MHz, CDCl3) 7.24 (8, lH, aromatic H), 7.02 (m,
2H, aromatic H's), 6.86 (q, 4H, J=9.0 Hz, aromatic H's), 6.44 (broad s, lH, N-H), 4.79
(m of d, lH, J=5.8 Hz, PhC-H), 4.10 (m, lH, PhCH2CH-H), 3.76 (s, 3H, OC-H3), 3.71
20 (m, lH, PhCH2CH-H), 3.10 (t, 4H, J=4.6 Hz, four pip-H), 2.94 (m, lH, PhCH-H),2.60 (m's, 7H, PhCH-H, NC-H2 and four pip-H), 2.11 (m, lH, PhCHCH-H), 2.01 (m,
lH, PhCHCH-H), 1.52 (8, 9H, three of CC-H3) ~; CMR (75 MHz, CDCl3) 153.8,
152.8, 145.8, 136.5, 134.8, 132.8, 125.3, 118.6, 118.2, 116.8, 114.4, 80.6, 63.1, 61.1,
55.6, 54.8, 53.5, 50.6, 33.3, 29.3, 28.4 o.~5 EXAMPLE 65 1-(4-M~tl~. Yyphenyl)-4-t2-(6-methyl~minomPtl~ylisochroman-1-
yl)ethyl]piperazine (BB-2)
Following the general procedure of li'XAMPLE 1, Step 4 and m~king non-
critical variations but using 1-[2-[4-(4-m~t~lnYyphenyl)-1-pipe.~.zi~yl]ethyl]-N-
methyl-isochroman-6-cdll,u~ (IX, ~AMPLE 37) gives crude product. This
30 m~t~ri~l is purified by LC on 13 g (230400) silica gel eluting with 5% 3M Amm~)ni~
in m~t~nol/dichlorom~ne to give 1-(4-m~t~lnxyphenyl)-4-[2-(6-
methyl~minh..,~l~lylisochroman-1-yl)ethyUpiperazine (BB-2), mp = 74-76~; Rf = 0.36
(5% 3M NH3in m~ot~l~nn]/dichlornm~t~l~n~); IR (mull) 2788, 1512, 1291, 1276, 1253,
1232, 1180, 1151, 1132, 1107, 1051, 1035, 1012, 927, 831; NMR (300 MHz, CDCl3) J
7.08 (m, 3H, aromatic), 6.85 (m, 4H, aromatic), 4.85 (brdd, lH, J=6.0 Hz, m~t~in~),
4.15 (m, lH, OCH2a), 3.77 (m, 4H, OCH3& OCH2b), 3.71 (s, 2H, Ph-CH2-N), 3.11 (t,
-88-
CA 02225282 1997-12-19
W O 97/02259 PCTAUS96/08681
4H, J=4.9 Hz, Ph-N-CH2s), 2.97 (m, lH, Ph-CH2a), 2.72-2.50 (m, 7H, Ph-NC(H2)-
CH2B-NCH28, Ph-CH2b), 2.46 (s, 3H, NCH3), Z.14 (m, lH, C(H)-CH2a), 2.04 (m, lH,
C(H)-CH2b) ~; CMR (75 MHz, CDCl3) 153.6, 145.6, 137.9, 136.7, 133.9, 128.5, 126.0,
124.7, 118.0, 114.3, 74.5, 63.0, B5.6, 55.4, 54.7, 50.5, 35.9, 33.2, 29.0 o; HRMS (EI)
rs3~ tetl for C24H33N3O2 = 395-2573~ fou
F'XAMPLE 66 1-(4-Mf~hnYyphenyl)-4-[2-(6-dimethyl~minom~thylisochroman-1-
yl)ethyl]piperazine (BB-2)
Following the general procedure of Ti~AMPLE 1, Step 4 and making non-
critical v~ri~t;o~ but using 1-[2-t4-(4-methoxyphenyl)-l-piperazinyl]etlhyl]-N~N-
dimethylisochroman-6-c~b.~ e (IX, EXAMPLE 36) gives crude product which is
purified by LC on 13 g (230-400) 6ilica gel eluting with 100~o dichlc.L~ n~ and
gradually increasing polarity to 5% 3M ~mmoni~ in mPth~nnl/dichloromPth~nP to gie
1-(4-mPthnYyphenyl)-4-[2-(6-dimethyl~minnm~t.hylisochroman-1-yl)ethyl]piperazine(BB-2), mp = 95-98~; Rf = 0.33 (5~o 3M slmmnni&~ in mpt~nol/dichlorom~t~l~n~); IR
(mull) 2809, 2791, 2770, 2762, 1512, 1442, 1277, 1253, 1232, 1179, 1150, 1107, 1045,
1037, 832 cm~l; NMR (300 MHz, CDCl3) 7.07 (m, 3H, aromatic), 6.87 (m, 4H,
aromatic), 4.83 (brdd, lH, J=6.4 Hz, m~hine)~ 4.11 (m, lH, OCH2a), 3.76 (m, 4H,
OCH3& OCH2b), 3.40 (s, 2H, Ph-CH2-N), 3.11 (t, 4H, J=4.9 Hz, Ph-NCH2s), 2.97 (m,lH, Ph-CH2a), 2.65 (m, 7H, Ph-NC(H2)-CH28-NCH2 & Ph-CH2b), 2.26 (8, 6H,
NCH3~,), 2.18 (m, lH, C(H)-CH2a), 2.05 (m, lH, C(H)-CH2b) o; CMR (75 MHz,
CDCl3) 153.8, 145.8, 137.0, 136.6, 133.9, 129.5, 127.1, 124.6, 118.2, 114.4, 74.6, 64.0,
63.2, 55.6, 54.9, 53.5, 50.6, 45.3, 33.3, 29.1 o; HRMS (EI) cf~ qt~Cl for C25H35N3O2
= 409.2729, found = 409.2733.
EXAMPLE 67 1-[2-[4-(4-M~tlt nYyphenyl)- 1-piperazinyl]ethyl]-isochroman-6-
carboxylic acid, ethyl ester (X)
An oven-dried 10 mT- flask e.lui~ped with spinbar, reflux con-lPn~Pr, and 3-
way adapter is charged with 1-[2-(6-bromoi~o~ an-1-yl)ethyl]4~4-
methoYyphenyl)-piperazine (VI, Ti~xAMpLE 24, Step 2, 431 mg, 1.0 mmol), p~ m
II acetate (11 mg, 0.05 mmol), 1,3-bis(diphenylrho~phino)propane (25 mg, 0.06
30 mmol), 2.5 mL dimelhylruL~mitlP, diisop~ ,ylethylamine (0.35 mL, 2.0 mmol), and
elhs~nol (1.2 mL, 20 mmol). The r~slllt;ng ~xLu~: is purged 8iX timeB with carbon
mnnoYi~P/under reduced ~ tUL~ followed by he~tinF to 100~. After 18 hours, the
~ixLul~ is cooled to 20-25~, conc~ ated under high V~ u~, diluted with 20 mL
lM sodium hydroxide, and e~L~c~oLt d twice with ethyl acetate (20 mL). The
~nmhin~ organics are washed once with saline (20 mL), dried over m~n~ lm
sulfate, filtered, and conrenl-~ted to give product. This m~teri~l is purified by LC
-89-
CA 0222~282 1997-12-19
W O 97/022S9 PCTrUS96/08681
on 22 g (230400) silica gel eluting with 30% ~cetonP/hexane to give 1-[2-[4~4-
methoxyphenyl)-l-piperazinyl]ethyl]-isochroman-6-carboxylic acid, ethyl ester (X),
mp = 117-119~; Rf = 0.45 (35% AretonP~hexane); IR (mull) 1712, 1513, 1422, 1286,1260, 1246, 1187, 1145, 1140, 1108, 1053, 1037, 1023, 818, 767 cm~1; NMR (300
5 MHz, CDCl3) 7.84 (d, lH, J=8.2 Hz, _romatic), 7.80 (8, lH, _romatic), 7.17 (d, lH,
J=8.1 Hz, aromatic), 6.85 (m, 4H, aromatic), 4.85 (brdd, lH, J=6.0 Hz, mPthin~), 4.36
(qrt, 2H, J=7.1 Hz, CO2CH2), 4.15 (m, lH, OCH2a), 3.76 (m, 4H, OCH3& OCH2b),
3.10 (t, 4H, J=4.7 Hz, Ph-N-CH2S), 3.00 (m, lH, Ph-CH2a), 2.79-2.50 (m, 7H, Ph-
NC(H2)-CH26-NCH2s, Ph-CH2b), 2.15 (m, lH, C(H)-CH2a), 2.06 (m, lH, C(H)-CH2b),
10 1.39 (t, 3H, J=7.1 Hz, C(H2)-CH3 o; CMR (75 MHz, CDCl3) 166.2, 153.5, 145.4,
142.9, 133.9, 129.8, 128.2, 126.9, 124.5, 117.8, 114.1, 74.3, 62.6, 60.6, 55.2, 54.3, 53.2,
50.3, 32.8, 28.7, 14.0 o.
Ti~Al\/rPLE 68 6-Acetyl-1-[2-[4~4-mPt~n ~yphenyl)-1-piperazinyl]ethyl]-
isochroman, hydrochloride salt (X~V)
An oven-dAed 10 mL flask equipped with spinbar _nd reflux cnntl~Pn~qri6
charged with 1-[2-(6-bromni~ocl~l olllan-1-yl)ethyl]4-(4-mPt~nYyphenyl)-pi~eL~lzi~e
(VI, EXAMPLE 24, Step 2, 431 mg, 1.0 mmol), pAllA~ lm II acetate (11 mg, 0.05
mmol), 1,3-bis(diphenylrhosphinn)propane (25 mg, 0.06 mmol), tl7Allinm II acetate
(290 mg, 1.1 mmol), 3.0 mL dimeLhylrul...Ami-lP, triethylamine (0.28 mL, 2.0 mmol),
and vinyl butylether (0.65 mT-, 5.0 mmol). The ~Lule iB heated to 100~ and after20 hours, the ~Lule is cooled to 20-25~, treated with hyLucllloric acid (lM, 6 mL)
and is stirred for 1 hour. The ~ Lu~e is con~ ted under high v~( ~LU~, diluted
with 20 mL 5M sodillm hydroxide, and extracted twice with ethyl acetate (20 mL).The comhinP~ organics are washed once with sAline (20 mT-), dried over mAgnp~iumsulfate, filtered and cnnl~,e~.t~dLed. This mAteriAl is purified by LC on 27 g (230400)
silica gel eluting with 25% AretonP~PY~np~ This mAt~riAl is dissolved in a ~Luleof ethyl ~ret~t~mPt~l~nnl and is treated with gaseous hydluchloric acid resulting in
the formation of a solid that is re~1Y~ C1 from ethyl acetate/mP~l~Annl to give 6-
acetyl-1-t2-[4-(4-mP~l~nYyphenyl)-l-piperazinyl]ethyl]-isochroman~ hy~loride salt
(XXIV), mp = 195-197~; Rf = 0.15 (30% AcetonP/hexane); bis salt IR (mull) 2560,
2516, 2487, 2462, 1675, 1511, 1~, 1425, 1359, 1290, 1265, 1245, 1113, 1035, 837
cm~l; freebase NMR (300 MHz, CDC13) 7.78 (d, lH, J=8.1 Hz, aromatic), 7.72 (s, lH, r
aromatic), 7.20 (d, lH, J=8.1 Hz, aromatic), 6.87 (m, 4H, aromatic), 4.89 (brdd, lH,
J=6.0 Hz, mPt~inP), 4.15 (m, lH, OCH2a), 3.76 (m, 4H, OCH3& OCH2b), 3.11 (t, 4H,J=4.8 Hz, Ph-N-CH28), 3.00 (m, lH, Ph-CH2a), 2.80-2.55 (m, 10H, Ph-NC(H2)-CH28-
NCH2~, Ph-CH2b, COCH3), 2.15 (m, lH, C(H)-CH2a), 2.06 (m, lH, C(H)-CH2b) o;
-90-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
CMR (75 MHz, CDCl3) 197.8, 153.7, 145.6, 143.5, 135.2, 134.4, 128.9, 126.1, 125.0,
118.1, 114.3, 74.5, 62.9, 55.5, 54.6, 53.4, 50.5, 33.0, 29.0, 26.5 o; HR~IS (EI) tP-l for C24H30N2O3 = 394.2256, found = 394-2262-
EXAMPLE 69 6-Formyl-1-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]-
isochroman (AA-1)
1-[2-(6-Bromoi~orhroman-l-yl)ethyl]-4-(4-methoxyphenyl)piperazine (VI,
FXAl\/rPLE 24, Step 2, 2.80 g, 6.5 mmol) and freshly t~ tillP-l tetrahy~L~ru~dn (16
mL) are mixed followed by cooling to -78~. The ~ is treated with a 1.7 M
soll7tion of tert-butyllithillm (7.7 mT, 13.0 mmol). After 15 min, the aryl lithiillm is
10 treated with dimeLllylru~ mi-l~ (1.0 mL, 13 mmol). The reaction i8 warmed to 20-
25~ over 1.5 hours then is diluted with 75 mL water and extracted twice with ethyl
acetate (75 mL). The comhin~(l organics are washed once with saline (50 mL), dried
over mAEnP~ m sulfate, filtered, and conce.ll ~al~d to give crude product. Thi~
material iB purified by LC on 160 g (230-400) silica gel eluting with 30~o
15 ~ceton~/hexane to give 6-formyl-1-[2-[4-(4-mPth~Yyphenyl)-1-piperazinyl]ethyl]-
isochroman (AA-1), Rf = 0.28 (30% ~etonP/hexane); IR (liq.) 2949, 2819, 1698, 1608,
1512, 1464, 1456, 1291, 1285, 1244, 1143, 1124, 1110, 1038, 824 cm~l; N~ (300
~Iz, CDCl3) 9.97 (s, lH, CHO), 7.69 ( d, lH, J=8.0 Hz, aromatic), 7.60 (8, lH,
aromatic), 7.28 (d, lH, J=7.7 Hz, aromatic), 6.85 (m, 4H, aromatic), 4.90 (brdd, lH,
20 J=6.0 Hz, m~thinP~), 4.16 (m, lH, OCH2a), 3.76 (m, 4H, OCH3& OCH2b), 3.10 (t, 5H,
J=5.0 Hz, Ph-N-CH2s & Ph-CH28), 2.83-2.50 (m, 7H, Ph-NC(H2)-CH2E,-NCH2E~, Ph-
CH2b), 2.16 (m, lH, C(H)-CH2a), 2.07 (m, lH, C(H)-CH2b) ~; CMR (75 MHz, CDCl3)
192.0, 153.8, 145.7, 145.2, 135.0, 134.7, 130.4, 127.4, 125.6, 118.2, 114.4, 74.6, 62.8,
55.6, 54.6, 53.5, 50.6, 33.1, 29.0 o; HRMS (EI) c~ tecl for C23H28N203 =
25 380.2100, found = 380.2098.
F~AMlpLE 70 2-[Isochroman-1-[2-[4-(4-methoxyphenyl)~ a~i~-1-yl]ethyl]-6-
yl]~-et~mi~P (AA-4)
Step 1: 1-[2-(6-Hyd.ul~y..~ethylisochroman-1-yl)-ethyl]-4-(4-
mPtl~mryphenyl)piperazine (AA-2)
6-Formyl-1-[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethylisochroman (AA-1,
EXA~LE 71, 2.51 g, 6.6 mmol) and 25 mL mpth~nol are mixed followed by cooling
to 0~. The ~ Lu~ is treated with a single portion of sodium borohydride (500 mg,13.2 mmol). The reaction is gradually warmed to 20-25~ over 2 hours and is diluted
with 75 mL water and extracted twice with ethyl acetate (75 mL). The comhinç-1
organics are washed once with saline (50 mL), dried over m~gnP~illm sulfate,
filtered and conc~ t-a~ed. This m~t~ is purified by LC on 130 g (230-400) silica
-91-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
gel eluting with mPtl-~no]/dichloromPth~nP (5/95) to give 1-[2-(6-
hy~l~u~ylllethylisûchroman-l-yl)ethyl]-4-(4-methûxyphenyl)piperazine (AA-2), Rf =
0.15 (35% ~retonP~hexane); IR (mull) 1513, 1445, 1428, 1292, 1279, 1248, 1186, 1152,
1140, 1107, 1057, 1034, 1011, 928, 826 cm~l; NMR (300 MHz, CDCl3) 7.17 ( d, lH,
5 J=8.0 Hz, aromatic), 7.09 (m, 2H, arcmatic), 6.86 (m, 4H, aromatic), 4.82 (brdd, lH,
J=6.3 Hz, mPthinP), 4.64 (8, 2H, Ph-CH2-O), 4.12 (m, lH, OCH2a), 3.76 (m, 4H,
OCH3& OCH2b), 3.10 (t, 4H, J=4.8 Hz, Ph-N-CH2S) 2.97 (m, lH, Ph-CH2a), 2.72-
2.50 (m, 7H, Ph-NC(H2)-CH2S-NCH2s, Ph-CH2b), 2.10 (m, 2H, C(H)-CH2S) ~; MS
(EI, m/z) = 382.
Step 2: 1-[2-(6-Cy~nl mpt~ylisochroman-l-yl)ethyl]-4~4
mPtho~yphenyl)-piperazine (AA-3)
1-[2-(6-IIyL u~ylllethylisochroman- 1-yl)-ethyl]-4-(4-methoxyphenyl)piperazine
(AA-2, 2.33 g, 6.1 mmol), 61 mL dichloromPth~ne and triethylamine (1.3 mL, 9.1
mmol) are mixed followed by cooling to 0~. The ~ Lu~e i8 treated with
mPth~np~ fonylrhl~ e (0.52 mmol, 6.7 mmol). The reaction i8 warmed to 20-25~
over 1.5 hours and con- e~ at~d under reduced pL~ . The crude mesylate i8
diluted with 31 mT.dimethyl8lllfr~ P and treated with sodium cyanide (896 mg,
18.3 m~ol). This ,~ is heated to 60~. After 2 hours, the volatiles are removed
under high V~ Ulll with the resulting residue diluted with 100 mL water and
~ ct~d twice with ethyl acetate (75 mL). The c- mhinP~1 organics are washed oncewith saline (75 mT,), dried over m~gnP~illm sulfate, filtered and conre~ dted. This
m~t~risll is purified by LC on 88 g (230400) silica gel eluting with 35%
~ret~nP/hexane to give 1-[2-(6-cy~nnmPthylisochroman-1-yl)ethyl]-4~4-
methoxyphenyl)piperazine (AA-3), mp = 118-119~; Rf = 0.36 (35% ~qcetone/hexane);IR (mull) 2810, 2790, 1512, 1444, 1275, 1253, 1232, 1182, 1151, 1111, 1107, 1058,
1051, 1031 and 831 cm~1; NMR (300 MHz, CDCl3) 7.10 (m, 3H, aromatic), 6.87 (m,
4H, aromatic), 4.82 (brdd, lH, J=6.3 Hz, mpthine)~ 4.14 (m, lH, OCH2a), 3.76 (m,4H, OCH3& OCH2b), 3.74 (s, 2H, NC-CH2), 3.11 (t, 4H, J=4.8 Hz, Ph-N-CH2S) 2.98
(m, lH, Ph-CH2a), 2.73-2.50 (m, 7H, Ph-NC(H2)-CH28-NCH2s, Ph-CH2b), 2.18 (m,
lH, C(H)-CH2a), 2.04 (m, lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3) 153.8, 145.8,
138.1, 135.1, 128.4, 127.9, 125.8, 125.6, 118.2, 117.9, 114.3, 74.462.9, 55.6, 54.7, 53.5,
50.6, 33.3, 29.0, 23.2 o; MS (EI, m/z) = 391.
Step 3: 2-[Isochroman-1-[2-[4-(4-methoxyphenyl)piperazin-1-yl]ethyl]-6-
yl]ncc~ P (AA-4)
1-[2-(6-Cy~n~mPthylisûchroman-1-yl)ethyl]-4-(4-methoxyphenyl)piperazine
(AA-3, 785 mg, 2.0 mmol), 5.0 mL dime~lylru~ mi-le and pot~ lm carbonate (39
-92-
CA 02225282 1997-12-19
W O 97/022S9 PCT/U~r~ 6~1
mg, 0.28 mmol) are mixed. The l~Lu~e is treated with a 30% 6Olllt;~m of Lyd~o~n
peroxide (0.24 mL, 2.3 mmol). After 20 hours, the reaction is diluted with 100 mL
dichloromPt~nP and washed once with water (20 mL), once with sali~e (20 mL),
dried over m~gnP~ lm sulfate, filtered and con~ ed. This mslt~ris~l is
5 ~ lli7gd from ethyl acetate/hexane to give 2-[isochroman-1-[2-[4-(4-
mPt~ ryphenyl)piperazin-l-yl]ethyl]-6-yl]slcet~mitle (AA-4), mp = 159-161~; Rf = 0.15
(6% mPt~n~l/dichloromPtl~n~); IR (mull) 3381, 3208, 2791, 1658, 1633, 1613, 1444,
1293, 1275, 1255, 1231, 1150, 1108, 1032, 833 cm~l; NMR (300 MHz, CDCl3) 7.09 (
8, 2H, aromatic), 7.02 (s, lH, aromatic), 6.86 (m, 4H, aromatic), 5.59 (brds, lH, NH),
10 5.43 (brds, lH, NH), 4.81 (brdd, lH, J=6.0 Hz, mPthine)~ 4.12 (m, lH, OCH2a), 3.76
(m, 4H, OCH3& OCH2b), 3.53 (8, 2H, O=C-CH2), 3.10 (t, 4H, J=4.9 Hz, Ph-N-CH28)
2.96 (m, lH, Ph-CH2a), Z.72-2.56 (m, 7H, Ph-NC(H2)-CH29-NCH28, Ph-CH2b), 2.13
(m, lH, C(H)-CH2a), 2.03 (m, lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3) 173.4,
153.8, 145.7, 137.4, 134.7, 132.8, 129.8, 127.2, 125.4, 118.1, 114.4, 74.B, 55.6, 54.8,
15 53.5, 50.6, 42.8, 33.2, 29.0 o; MS (EI, m/z) = 409.
EXAMPLE 71 2-tIsochroman- 1-[2 -[4-(4-methoxyphenyl)piperazin- 1-yl]ethyl]-6-yl]-N-methykl~etqmi-lP (AA-5)
Following the general procedure of EXAMPLE 3, Step 1 and making
non-critical v~ri~t;~n~ but using 2-[isochroman-1-[2-[4~4-mPt~-~Yyphenyl)piperazin-l-
20 yl]ethyl]-6-yl]~ce~ le (AA4, 446 mg, 1.09 mmol) crude product is obtained. This
mslt,çri~l is purified by LC on 40 g (230400) silica gel eluting with 50%
ethyl ~ ~et o t~/hexane to give 2-[isochroman- 1-[2-[4~4-m Pt~ ~.Yyphenyl)~ipe~ dZi~- 1-
yl]ethyl]-6-yl]-N,N-di-t-butylu~y.,~bù~yl~cetqmi-lP, Rf = 0.45 (60%
ethyl~- etqtP/hexane). This m~t~ri~l is reacted with methylamine according to the
25 general ,uluce~ of EXAMPLE 3, Step 2 and m~kinF non-critical v~ri~ti~n~ to give
the desired product which is purified by LC on 13 g (230-400) silica gel eluting with
60% ~- etonP/hexane to give 2-[isochroman-1-[2-[4-(4-mPt~l-.Yyphenyl)piperazin-l-
yl]ethyl]-6-yl]-N-methylAcet~...i-lP (AA-5), mp = 147-148~; Rf = 0.20 (50%
~-eton~/hexane); IR (mull) 3309, 1652, 1550, 1515, 1442, 1426, 1412, 1354, 1251,30 1228, 1153, 1147, 1114, 1036, 826 cm~l; NMR (300 MHz, CDCl3) 7.09 ( m, 2H,
aromatic), 7.01 (s, lH, aromatic), 6.87 (m, 4H, aromatic), 5.45 (brds, lH, NH), 4.83
(brdd, lH, J=6.0 Hz, mPtl~ine), 4.15 (m, lH, OCH2a), 3.77 (m, 4H, OCH3& OCH2b),
3.53 (s, 2H, O=C-CH2), 3.12 (t, 4H, J=4.8 Hz, Ph-N-CH2s) 2.95 (m, lH, Ph-CH2a),
2.77 (d, 3H, J=4.9 Hz, NCH3), 2.72-2.56 (m, 7H, Ph-NC(H2)-CH2S-NCH2s, Ph-CH2b),
35 2.15 (m, lH, C(H)-CH28), 2.04 (m, lH, C(H)-CH2b) o; C~R (75 MHz, CDCl3) 17f.6,
153.8, 145.7, 137.3, 134.7, 132.9, 130.0, 127.3, 125.4, 118.2, 114.4, 74.5, 63.0, 55.6,
-93-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
54.9, 53.6, 50.6, 43.3, 33.3, 29.0, 26.5 ~; MS (EI, m/z) = 423.
EXAMPLE 72 1-[2-[4-(4-Hyd~vAy~henyl)-1-piperazinyl]ethyl]-isochroman-6-
c&~L..~ lP (CC-2)
1-[2-[4-(4-PhenylmethyloAyphenyl)-l-piperazinyl]ethyl]isochroman-6-
carbl~Y~mi-1e (CC-1, EXAMPLE 9, 0.42 mmol, 200 mg), p~ m on carbon (10%, 20 rmg), eth~n~ l (5 mL) and methylene chloride (2 mL) are çomhin~-l After four daysthe starting material is consumed. The reaction llli~Lu~è is filtered on a bed of celite
and rinsed several times ~ltern~tively with ethanol, mpth~nol~ methylene chloride
and ethyl ~et~t~ The hltrates are comhinel:3 and conre~" ~aLed. The crude ms~t,erisil
10 is le~;~y~L~ s~l from hot etl~n~l with some mPth~nol to give 1-[2-[4-(4-
hyvfuAy~uhenyl)-l-piperazinyuethyuisochroman-6-c~L~ (CC-2), IR (mull)
3300, 3255, 3206, 1672, 1615, 1513, 1444, 1426, 1366, 1256 cm~l; NMR (300 ~Iz,
DMSO) 7.85 (d, lH, J=8.2 Hz, aromatic H), 7.82 (s, lH, aromatic H), 7.43 (d, lH,J=8.2 Hz, aromatic H), 6.96 (d, 2H, J=8.8 Hz, aromatic H's), 6.81 (d, 2H, J=8.8 Hz,
15 aromatic HIB), 5.00 (broad d, lH), 4.18 (m, 2H), 3.85 (m, lH), 3.68 (m, 4H), 3.46-3.0
(several broad m, 6H), 2.85 (broad d, 2H, J=16 Hz) 2.37 (broad m, lH) ~; HRMS
~k~ t,ecl for C22H27N303 = 382.2131, found = 382.2136.
liXAlVlPLE 73 ' (S)~-)-1-[2-[4~4-HydlvAy~henyl)-1-~i,ue~ yl]ethyl]-N-methyl-
isochrvl..an-6-carbnY~mi~P (S)~CC-2)
A Parr flask is charged with (S)~-)-1-[2-[4-(4-phenylmethylûAyphenyl)-1-
piperazinyl]ethyl]-N-methylisochroman-6 ca~L..~ (S)~CC-l, EXAMPLE 20) 50
mL mPth~n~l, 25 mL tetral,yv~c,ru~,..., and 10% p~ rlillm on carbon (200 mg). The
resulting black suspension is placed under 40 psi hyv~u~ll and ~h~kPn After 60
hours, the ~res~ e had fallen to 27 psi hyd~o~ and the reaction llliALule is
25 filtered through celite and con~ ted. The con-~Pntrae is le~y~ 7e~1 from
mPths~nr~l/ethyl~cet~tJ~ to give (S)-(-)-1-[2-[4-(4-LyLvAy,uhenyl)-1-piperazinyl]ethyl]-N-
methylisochromsn-6-c~ u~ e (S)-(CC-2), mp = 154-162~; Rf = 0.11 (5~o
mPth~nollethyl~et~tç); [a]D = -53~ (c = 0.9681, mpth~nr~l); IR (mull) 3350, 3200,
3174, 2811, 1642, 1573, 1542, 1517, 1300, 1271, 1248, 1243, 1232, 1104, 825, cm~1;
30 NMR (300 MHz, DMSO-d6) o 8.81 (s, lH, OH), 8.37 (brdd, lH, J=4.6 Hz, NH), 7.61
(m, 3H, aromatic), 7.27 (d, lH, J=8.1 Hz, aromatic), 6.76 (d, 2H, J=8.9 Hz, aromatic),
6.63 (d, 2H, J=8.9 Hz, aromatic), 4.78 (brdd, lH, J=6.0 Hz, mpthinp)~ 4.03 (m, lH,
OCH2a), 3.66 (m, lH, OCH2b), 2.93 (m, 4H, Ph-N-CH2B), 2.76 (d, 4H, J=4.5 Hz, N-
CH3 & Ph-CH28), 2.49 (m, 6H, Ph-NC(H2)-CH28-NCH2~), 2.35 (m, lH, Ph-CH2b),
35 2.15 (m, lH, C(H)-CH2a), 1.85 (m, lH, C(H)-CH2b) o; CMR (75 MHz, DMSO-d6)
166.4, 150.8, 144.2, 141.2, 133.7, 132.3, 127.5, 124.8, 124.6, 117.6, 115.4, 73.6, 62.2,
-94-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
54.2, 53.1, 50.0, 32.5, 28.5, 26.2 o; HRMS (EI) calculated for C23H29N303 =
395.2209, found = 395.2212; KF. Water = 4.28~a
EXAMPLE 74 (S)-(-)-1-[2-[4-(4-TrifluoromPth~nesulfonyloxyphenyl)-1-
piperazinyl]ethyl]-N-methyl-isochro.llan-6-call,..Y~ (S)-
(Cc-3)
(S)-(-)-1-[2-[4-(4-lly~lo~yl~henyl)-1-piperazinyl]ethyl]-N-methyl-isochroman-6-
c~l~ (S)-(CC-2, 959 mg, 2.4 mmol), 24 mL dichloromPth~n~, and N-
phenyl I ~ ;n ~.oromethane-slllfonimi~e (910 mg, 2.5 mmol) are mixed. The ~l~ib~Lu~e is
cooled to 0~ and treated with triethylamine (0.51 mL, 3.6 mmol) with no visible
10 change o-,-,u..;~g. After 16 hours, the llli~u~e is diluted with 75 mL lM sodium
hydroxide and extracted twice with dichloromPth~ne (75 mL). The comhine~
organics are washed once with saline (50 mT.)~ dried over m~n~inm sulfate,
filtered and conc~ ted. This m~t~ri~l is purified by LC on 88 g (230-400) silica
gel eluting with 5% mPth~nol/dichloroTn~th~ne to give (S)-(-)-1-[2-[4-(4-
~5 trifluorom~h~ne~ulfonyloxyphenyl)-l-piperazinyl]ethyl]-N-methylisocll~vlllan-6-
le (S)-(CC-3), Rf = 0.34 (5~0 mPth~nol/dichloromPth~n~); [a]D = -39~ (c =
0.9447, m~+hs~nol); IR (mull) 1641, 1571, 1548, 1505, 1417, 1310, 1297, 1240, 1210,
1209, 1141, 1110, 885, 826, 609 cm~1; N~ (300 MHz, CDC13) 7.55 (m, 2H,
aromatic), 7.14 (m, 3H, aromatic), 6.87 (d, 2H, J=9.4 Hz, aromatic), 6.18 (brdd, lH,
20 J=4.6 Hz, NH), 4.85 (brdd, lH, J=5.8 Hz, m.othin~), 4.13 (m, lH, OCH2a), 3.75 (m,
lH, OCH2b), 3.22 (t, 4H, J=4.9 Hz, Ph-N-CH2S), 3.00 (d, 4H, J=4.9 Hz, N-CH3 & Ph-
CH2a), 2.75-2.49 (m, 7H, Ph-NC(H2)-CH2s-NCH28 & Ph-CH2b), 2.15 (m, lH, C(H)-
CH2a), 2.04 (m, lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3) 168.0, 150.9, 142.1, 141.5,
134.5, 132.7, 127.7, 125.0, 124.5,121.9, 116.3, 74.4, 63.0, 54.5, 53.2, 48.7, 33.2, 29.1,
25 26.9; HRMS (FAB) calculated for C24H28F3N3O5S+H1 = 528.1780, found =
528.1791.
EXAMPLE 75 (S)-(-)-1-[2-[4-(4-Acetylphenyl)-1-piperazinyl]ethyl]-N-
methylisochroman-6-ca~ ", i~le (S)-(IX)
($)-(-)-1-[2-[4~4-l~uoromPth~nP~lllfonyloxyphenyl)-l-piperazinyl]ethyl]-N-
30 1~eLhylisochroman-6-carb~ mi~le (S)-(CC-3, 527 mg, 1.0 mmol), p~ illm II acetate
(11 mg, .05 mmol), 1,3-bis(diphenylphosE!hinn)propane (25 mg, .06 mmol), 3.5 mL
dim~lhylr.,....~mide, triethylamine (0.28 mL, 2.0 mmol), and bu~ylvi~.ylether (0.65
mL, 5.0 mmol) are comhinPtl The resulting ~ e is heated to 50~. After 16
hours, the reaction is cooled to 20-25~, treated with 8 mT lM hydlochloric acid, and
35 i8 stirred for 1 hour. This acidic ~ is conr~ntrated under reduced p~ uue~
diluted with 15 mL lM sodium hydroxide, and extracted twice with dichlornmpth~n~
-95-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
(25 mL). The comhinPtl organics are washed once with saline (15 mL), dried over
mslgnr~illm sulfate, filtered, and csn~ dted. This m~t~ri~l is purified by LC
36 g (230-400) silica gel eluting with 50% ~reton~/hexane to give (S)-(-)-1-[2-[4-(4-
scetylphenyl)-l-pipelazin~l]ethyl]-N-methylisochroman-6-ca l,..~...itl~ (S)-(CC-4)
5 which upon . cc, ~ lli7~tion from ethyl acetate/hPY~ne, mp = 156-157~; Rf = 0.20
50% ~retone~hexane); [a]D = ~1~ (c = 0.8481, mpth~nnl); IR (mull) 3331, 1662, 1598,
1570, 1550, 1519, 1427, 1415, 1311, 1284, 1239, 1196, 1150, 1107, 609 cm~l; NMR
(300 MHz, CDCl3) 7.85 (d, 2H, J=8.9 Hz, aromatic), 7.55 (m, 2H, aromatic), 7.14 (d,
lH, J=8.5 Hz, aromatic), 6.84 (d, 2H, J=8.9 Hz, aromatic), 6.24 (brdd, lH, J=4.6 Hz,
10 NH), 4.86 (brdd, lH, J=5.8 Hz, m~hin~), 4.12 (m, lH, OCH2a), 3.76 (m, lH,
OCH2b), 3.35 (t, 4H, J=5.0 Hz, Ph-N-CH2~,), 3.00 (d, 4H, J=4.9 Hz, N-CH3 & Ph-
CH2a), 2.75-2.53 (m, 7H, Ph-NC(H2)-CH28-NCH28 & Ph-CH2b), 2.50 (s, 3H, O=C-
CH3), 2.16 (m, lH, C(H)-CH2a), 2.02 (m, lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3)
196.6, 168.0, 154.2, 141.5, 134.5, 132.7, 130.4, 127.7, 127.6, 125.0, 124.5, 113.4, 74.4,
63.0, 54.5, 53.0, 47.3, 33.2, 29.1, 26.9, 26.1 ~; HRMS (EI) r~lr,~ t~l for C25H31N303
= 421.2365, found = 421.2365.
EXAMPLE 76 (S)-(-)-3-[Isochroman-1-[2-[4~4-mrth~.Yyphenyl)piperazin-l-
yl]ethyl]-6-yl]-N,N-dimethylacrlyamide (S)-(XVIII)
(S)-(-)-1-[2-(6-Brsmsi~orhroman-l-yl)-ethyl]-4~4-m~thnyyphenyl)-~ui~ut~zi~e
20 (S) (VI) (431.4 mg, 1.0 mmol), p~ lim (II) acetate (98%, 11.4 mg, 0.05 mmol) and
1,3-bis-diphenylrho~phinopropane (97%, 24.7 mg, 0.06 mmol) are comhin~ Argon
sltTn9~3rh~re is er~hli~h~l To the reaction vessel is introduced via syringe D~
(4.1 mL), clilllelllylacrylamide (0.72 mL, 7.0 mmol), and diisu,ulu~ylethylamine (0.35
mL, 2.0 mmol). The l~Lule is heated to 100~ over 18 hours. After cooling to 20-
25 25~, the reaction is diluted with aqueous sodium hydroxide and extracted threetimes with ethyl ~re~t~ The organics are cnmhin~-l and con~ d~ed. Re~i-lnsll
D~ is le wvGd under high vacuum. The crude m~tJ~ri~l is purified by flash
chrom~i,o~-d~hy on 80 g silica gel using 5~o m~th~nsl in methylene rhlsrirl~ as the
eluent to give a solid which is Ic~;~y~ from hot ethyl acetate/hexane to give
30 (S)-(-)-3-[isoch~ llan-1-[2-[4-(4-m~nYyphenyl)piperazin-l-yl]ethyl]-6-yl]-N~Ndimethyla.;lly~,ide (S)-(XVIII), mp = 120-121~; Rf = 0.30 (5% m~t~nnl in
methylene chloride); NMR (300 MHz,CDCl3) 7.62 (d, lH, J=15.4 Hz, aromatic H),
7.34 (d, lH, J=8.0 Hz, aromatic H), 7.10, (d, lH, J=8.0 Hz, aromatic H), 6.85 (d of d
and m, 4H and lH respectively, Ja=9.1Hz, Jb=21.4 Hz, aromatic H's), 4.83 (m of d,
35 lH, J=6.0 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H), 3.75 (m, lH, PhCH2CH-H), 3.75
(s, 3H, PhOC-H3), 3.17 (s, 3H, NMeC-H3), 3.10 (t and s, 4H and 3H respectively,
-96-
CA 02225282 1997-12-19
W O 97/OZ2S9 PCTrUS96/08681
J=4.8 Hz, four of pip-H and one of NMeC-H3), 2.94 (m, lH, NCH-H), 2.76-2.45
(several m's, 7H, four pip-H, two PhCH-H, and NCH-H), 2.14 (m, lH, PhCHCH-H),
2.02 (m, lH, PhCHCH-H) ~; CM~ (76 MHz, CDCl3) 166.7, 153.8, 146.7, 142.0, 139.8,134.5, 133.6, 128.3, 126.6, 126.2, 118.1, 117.1, 114.4, 74.6, 63.0, 56.6, 64.8, 53.5, 50.6,
5 37.4, 35.9, 33.2 and 29.1 8.
PLE 77 (S)-(-)-1-(4-M.~tllnYyphenyl)4-[2-[6-(1,2,4-triazol-3-yl)-
isochroman-l-yl]ethyl]piperazine (S)-(0-2)
Step 1: (S)-(-)-1-[2-[4-(4-M~tl nYvphenyl)-l-piperazinyl]ethyU-N-
dimethyl~minnmf~yleneisochroman-6-carbnY~mid~ (S)-(O-l)
(S)-(-)-1-[2-[4-(4-m~thnYyphenyl)-l-piperazinyl]ethyl]isochroman-6-
ca b..~ le (S)-(VII, 395.6 mg, 1 mmol) and N,N-~ ,e~lylL~ mid~im~t~ylacetal
(94~o, 0.34 mL, 2.4 mmol) and toluene (1 mL) are comhin~-l The reaction ~lu~e isheated to 90~ for 1.5 hours. After cooling to 20-26~, the volatiles are l~ ..ovt~d under
reduced ple~u~. pllrifi~tinn of the crude material by flash chr~m~tography on 90g silica gel using 5% m~thnnol in methylene l~hlori~l~ as the eluent gives (S)~-)-1-[2-
[4~4-m~tl~nyyphenyl)-l-piperazinyl]ethyl]-N-dimethyl~minl~mptl~yl~n~iRot~hroman-6
call~uY~---i(le (S)-(O-l), mp = 134-135.5~; Rf = 0.28 (5~o m~t~nnl in methylene
chloride); [a]D = -47~ (c = 0.96, 50% methylene chloride in eth~nnl); IR (mull) 1647,
1608, 1693, 1612, 1446, 1417, 1329, 1269, 1247, 1108 cm~l; NMR (300 MHz, CDCl3)
8.63 (s, lH, NMe2C-H), 8.07 (d, lH, J=8.1 Hz, aromatic H), 8.02 (s, lH, aromatic H),
7.14 (d, lH, J=8.0 Hz, aromatic H), 6.85 (d of d, 4H, Ja=9.2 Hz, Jb=21.6 Hz,
aromatic H's), 4.88 (m of d, lH, J=5.1 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H), 3.76(m, lH, PhCH2CH-H), 3.75 (s, 3H, OC-H3), 3.21 (8, 3H, NMeC-H3), 3.18 (s, 3H,
NMeC-H3), 3.10 (t, 4H, J=4.9 Hz, four pip-H), 2.98 (m, lH, PhCH-H), 2.76 (m of d,
lH, J=16 Hz, PhCH-H), 2.60 (m's, 6H, NC-H2 and fûur pip-H), 2.15 (m, lH,
PhCHCH-H), 2.05 (m, lH, PhCHCH-H) ~; CMR (75 MHz, CDCl3) 177.6, 160.8,
153.7, 145.8, 142.0, 134.9, 133.7, 130.3, 127.6, 124.5, 118.1, 114.4, 74.8, 63.1, 55.6,
54.7, 53.5, 50.6, 41.4, 35.3, 33.2 and 29.2 o.
Step 2: (S)-(-)-1-(4-M~thnYyphenyl)4-[2-[6-(l~2~1 t~ ol-3-
yl)isochroman-l-yl]ethyl]piperazine (S)-(0-2)
(S)-(-)-1-[2-[4-(4-M~thnYyphenyl)-l-piperazinyl]ethyl]-N-
dimethyl~minom~t~ylen~i~o~hroman-6-carbny~m~ (S)-(O-l, 208 mg, 0.46 mmol)
and glacial acetic acid (1 mT ) are cnmhin.o-l under argon ~tmn~rh~re. IIydls.7i~e
monohyd~a~ (0.045 mL, 0.92 mmol) is added dropwise via syringe with vigorous
35 8tirring It i~ stirred at 20-25~ for 24 hours. The reaction ~I u~a is diluted with
water and partitir~n~l between saturated aqueous sodium bicarbonate and
-97-
CA 0222~282 1997-12-19
W O 97/02259 PCTrUS96/08681
methylene ~hlm itle T}~e organics are comhin~, dried with sodium sulfate, filtered
and cQnc~ ed. The con~ entrate is purified by flash chrom~graphy on 6 g silica
gel using 5% m~oth~n~l in methylene chloride as the eluent to give (S)-(-)-1-(4-methoxyphenyl)-4-[2-[6-(1,2,4-triazol-3-yl)isochroman-1-yl]ethyl]piperazine (S)-(0-2),
5 mp = 195.5-196~; Rf = 0.11(5% m~t~nol in methylene chloride); NMR (300 MHz,
CDCl3) 8.18 (s, lH, triazoleC-H), 7.81 (d, lH, J=7.7 Hz, aromatic H), 7.8 (s, lH,
aromatic H), 7.15 (d, lH, J=8.0 Hz, aromatic H), 6.85 (d of d, 4H, Ja=9.2 Hz, Jb=21.6
Hz, aromatic H's), 4.87 (m of d, lH, J=5.1 Hz, PhC-H), 4.13 (m, lH, PhCH2CH-H),
3.76 (m, lH, PhCH2CH-H), 3.75 (s, 3H, OC-H3), 3.12 (t, 4H, J=4.9 Hz, four pip-H),
10 2.98 (m, lH, PhCH-H), 2.76-2.59 (several m's, 7H, PhCH-H, NC-H2 and four pip-H),
2.15 (m, lH, PhCHCH-H), 2.05 (m, lH, PhCHCH-H) ~; CMR (75 MHz, CDCl3)
159.4, 153.9, 146.9, 145.6, 139.8, 134.7, 127.3, 127.0, 125.3, 124.2, 118.2, 114.5, 63.1,
55.6, 54.7, 53.4, 50.6, 33.0, 29.0 ~.
~.~AMPLE 78 (S)-(-)-1-(4-M~thn~yphenyl)-4-[2-[6-(2-methyl-1,2,4-triazol-3-yl)-
isochroman-l-yl]ethyl]piperazine (S)-(0-2)
Following the general proce.lure of EXAMPLE 77, Step 2 and mslking non-
critical variations, but using methyl hydl~zi~le gives (S)~-)-1-(4-methoxyphenyl)-4-t2-
[6-(2-methyl-1,2,4-triazol-3-yl)isochroman-1-yl]ethyl]piperazine (S)-(0-2), Rf = 0.17
(5% mf.tl~nt)l in methylene chloride); NMR (300 MHz, CDC13) 8.04, 7.47, 7.46, 7.24,
6.85, 4.89, 4.16, 3.99, 3.80, 3.75, 3.10, 2.98, 2.77, 2.67-2.59, 2.18, 2.07 ~; CMR (75
MHz, CDC13) 154.4, 153.8, 160.7, 145.7, 140.4, 134.9, 129.3, 126.2, 125.9, 125.3,
118.1, 114.4, 74.5, 62.9, 55.6, 54.7, 53.5, 50.6, 37.0, 33.2, 29.0 o.
EXAMPLE 79 (S)-(-)-1-(4-M~t~r Yyphenyl)-4-[2-[6-(2-phenylmethyl-1,2,4-triazol-
3-yl)isochroman-1-yl]ethyl]piperazine (S)-(0-2)
Following the general ~l~ce~ e of EXAMPLE 77, Step 2 and mslking non-
cAtical variations but u~ing phenylmethyl hydrazine gives (S)-(-)-1~4-
methoYyphenyl)-4-[2-[6-(2-phenylmethyl-1,2,4-triazol-3-yl)isochroman-1-
yl]ethyl]piperazine (S)-(0-3), Rf = 0.28 (5% m~t~ nol in methylene chloride); NMR
(300 MHz, CDC13) 8.01 (s, lH, triazoleC-H), 7.37-7.30 (m's, 5H, aromatic H's), 7.19-
7.15 (m's, 3H, aromatic H's), 6.86 (d of d, 4H, Ja=9.2 Hz, Jb=21.6 Hz, aromatic H's),
5.43 (s, 2H, PhC-H2), 4.87 (m of d, lH, J=5.1 Hz, PhC-H), 4.14 (m, lH, PhCH2CH-
H), 3.79 (m, lH, PhCH2CH-H), 3.78 (s, 3H, OC-H3), 3.10 (t, 4H, J=4.9 Hz, four pip-
H), 2.97 (m, lH, PhCH-H), 2.73-2.52 (several m's, 7H, PhCH-H, NC-H2 and four pip-
H), 2.16 (m, lH, PhCHCH-H), 2.05 (m, lH, PhCHCH-H) o; CMR (75 MHz, CDC13)
156.0, 153.8, 151.3, 145.7, 140.5, 135.9, 134.9, 129.4, 129.0, 128.1, 126.9, 126.2,
12~.8, 125.3, 118.2, 114.4, 74.4, 62.9, 55.6, 54.7, 53.~, 52.8, 50.6, 33.2, 29.0 o.
-98-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrU$96/08681
EXAMPLE 80 (S)-(-)-1-(4-MPt~lYyphenyl)-4-[2-[6-(1,2,4-QY~ ol-5-yl)
isochroman-1-yl]ethyl]piperazine (S)-(0-2)
Hydroxylamine h~ vchloride (83.4 mg, 1.2 mmol) in a ""Ai.U~, of 6N
aqueous sodium hydroxide (0.24 mT, 1.2 mmol), 70~o acetic acid (1.2 mL) and (S)-(-)-
5 1-[2-[4-(4-methoAyphenyl)-1-piperazinyl]ethyl]-N-
dimet~ylAminomp+~ylpnpi~o~hroman-6-c~~ y~ p (S)-(O-l, EXAMPLE 77, Step 1,
450.6 mg, 1.0 mmol) are added at once. The mixture is stirred at 20-25~ for a total
of 70 minutes. The reaction is diluted with water and the pH is raised to 8 withsaluldted aqueous sodium bicarbonate. The aqueous llliXlUl~ iS extracted twice with
10 methylene chloride. The organics are comhinP~, dried with sodium sulfate, filtered
and con~ t ~ ~ed. This mA+~riAl (Rf = 0.18 (5% mPthAn~l in methylene chloride)) is
dissolved in a ~iA~Ul~ of anhydrous acetic acid (2 mL) and anhydrous p rli~YAn~ (2
mL). Argon ~tm~sphPre is Pst~hli~hP~l and the reaction is heated to 90~ for two
hours. After cooling to 20-26~, the reaction is diluted with water and the pH is15 raised to 8 with sa~ d aqueous sodium bicarbonate. The aqueous ~Lule is
~A~ ,Led twice with methylene ~hlo~lP The organics were comhinprl, dried with
sodium sulfate, filtered and cc~n~qntrated. Pllrifir~ti~ n of the ccmcçntrate by flash
chrnmAtc~.dl,hy on 50 g silica gel using 4% m~ n~l in methylene chloride as the
eluent gives (S)-(-)-1-(4-methoxyphenyl)-4-[2-t6-(1,2,4-oY~ ol~5-yl)isocl~lvlllan-1-
20 YUethYl]~iPelc zine (S)-(0-2), mp = 126-127~; Rf = 0.36 (5% mpth~nol in methylene
chloride); NMR (300 MHz, CDCl3) 8.47 (s, lH, rys~ c-H)~ 7.95 (d, lH, J=8.1
Hz, aromatic H), 7.92 (s, lH, aromatic H), 7.28 (d, lH, J=8.1 Hz, aromatic H), 6.86
(d of d, 4H, Ja=9.1 Hz, Jh=21.2 Hz, aromatic H's), 4.90 (m of d, lH, J=5.1 Hz,
PhC-H), 4.17 (m, lH, PhCH2CH-H), 3.81 (m, lH, PhCH2CH-H), 3.76 (s, 3H, OC-H3),
25 3.11 (t, 4H, J=4.9 Hz, four pip-H), 3.03 (m, lH, PhCH-H), 2.80 (m of d, lH, J=16.4
Hz, PhCH-H), 2.66-2.51 (several m's, 6H, NC-H2 and four pip-H), 2.18 (m, lH,
PhCHCH-H), 2.07 (m, lH, PhCHCH-H) 8; CMR (75 MHz, CDC13) 175.3, 157.8,
153.8, 145.7, 143.6, 135.3, 128.7, 125.8, 125.7, 122.0, 118.2, 114.4, 74.5, 62.8, 55.6,
54.6, 53.5, 50.6, 33.1, 29.0 8.
30 ~AMpLE 81 (S)-(-)-N-Methyl-1-[2-[4~4-propionylphenyl)-1-
pipel~z~yl]ethyl]isochroman-6-c~uL..Y~ (S)-(IX)
Step 1: (S)-(-)-6-Bromo-1-(2-hyd~vAy~ yl)isochroman (S)-(S-1)
(S)-(-)-(6-Brnmoi~o~hroman-1-yl)acetic acid (S)-(XI, ~AMPLE 1, Step 2, 16.27
g, 60 mmol) and 100 mL tetrahy-l~vru~ are comhin~-i This ~ is treated
35 with a 10M 8- lnti~n of borane methyl sulfide (18.0 mL, 0.18 mol) while ms~int~i~iin
20-25~ with a water bath. After 1 hour, the ~ is cooled to 0~ and 810wly
99
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
qllRn~hR~l with 160 mL mP~nnl Note: An in~ln~ n period of app~ tP~ly 1-2
...i..~l~ is noticed before a rapid and sudden generation of hyd-v~.-. The ~1~Al~U~e
i8 warmed to 20-25~ and volatiles removed under reduced ~,~e. -.~e. The re~slllt;
ll~A~u~e iB diluted with lM sodium hydroxide (150 mL) and extracted three times
5 with ethyl acetate (100 mL). The comhinR~l organics are washed once with saline
(100 mL), dried over m~FnRRium sulfate, filtered and conre~,l-dted. This m~t~ri~l is
Y~ Pfl from ethyl ~cPtstP/hexane to give (S)-(-)-6-bromo-1-(2-hy-LvAyt~ yl)-
isochrom~n (S)-(S-l), mp = 95-96~; Rf = 0.28 (30% ~- etonR/hexane); [a]D = -107~ (c =
0.4069, m~t~nol); IR (mull) 3237, 3022, 1482, 1422, 1326, 1277, 1114, 1053, 1026,
10 972, 905, 894, 880, 816, 788, cm~l; NMR (300 MHz, CDCl3) 7.28 (m, 2H, aromatic),
6.92 (d, lH, J=8.2 Hz, aromatic), 4.92 (brdd, lH, J=6.8 Hz, m.othinR), 4.15 (m, lH,
OCH2a), 3.81 (t, 2H, J=5.5 Hz, HO-CH2), 3.74 (m, lH, OCH2b), 3.00 (m, lH, Ph-
CH2a), 2.66 (dt, lH, J=16.4 Hz & J=3.1 Hz, Ph-CH2b), 2.45 (brds, lH, HO), 2.18 (m,
lH, C(H)-CH2a), 2.02 (m, lH, C(H)-CH2b) o; CMR (75 MHz, CDCl3) 136.5, 136.1,
15 131.8, 129.4, 126.3, 120.2, 75.9, 63.4, 60.8, 37.5, 28.8 o; MS (EI, m/z) = 256.
Step 2: (S)-(-)-1-(2-Hyd~uAy~lllyl)isoclllu an-6 c~l,u~ylic acid, methyl
ester (S)-(S-2)
(S)~-)-6-Bromo-1-(2-l.ydlvAyt lhyl)isochroman (S)~S-l, 5.14 g, 20.0 mmol),
ps~lls~ lm II acetate (225 mg, 1.0 mmol), 1~3-bis(diphenylrhocFhino)propane (49520 mg, 1.2 mmol), 40.0 mT- dimell.ylru~ mitle~ diisu~ ylethylamine (10.5 mL, 60.0
mmol), and mP~ nol (16 mL, 0.40 mol mmol) are comhinp~l The rR~lllt;n~ A~ e
is purged six times with carbon mon~n~ p~reduced ples~ followed by hP~tinF to
76~ quickly. The reaction ~ AI~U~e: is stirred for 19 hours. At this time, the ll~Alu~
is cooled to 20-25~, diluted with 200 mL water, and extracted twice with
25 dichlorom~'~ne (200 mT ). The comhinR-l organics are washed once with water (100
mL), once with saline (100 mL), dried over m~nP~inm sulfate, filtered and
cor.~ ted. This m~ri~l is purified by LC on 300 g (230-400) silica gel eluting
with 50% ethyl ~-et~te/hexane to give (S)-(-)-1-(2-l~yd~uAyt~l~-yl)isochroman-6-C~lJ~AY1iC acid, methyl ester (S)-(S-2), mp = 56-58~; Rf = 0.23 (60~ ethyl
30 acetate/hexane); [a]D = -114~ (c = 0.8773, mPt~l~nol); IR (mull) 3407, 3336, 1718,
1434, 1418, 1296, 1274, 1261, 1250, 1195, 1112, 1055, 1022, 997, 754 cm~l; NMR
(300 MHz, CDCl3) 7.79 (m, 2H, aromatic), 7.11 (d, lH, J=8.0 Hz, aromatic), 4.97
(brdd, lH, J=6.8 Hz, mPtl inR), 4.15 (m, lH, OCH2a), 3.88 (8, 3H, CH3), 3.82 (t, 2H,
J=5.5 Hz, HO-CH2), 3.75 (m, lH, OCH2b), 3.01 (m, lH, Ph-CH2a), 2.71 (dt, 2H,
35 J=16.8 Hz & J=3.3 Hz, Ph-CH2b & HO), 2.21 (m, lH, C(H)-CH2a), 2.03 (m, lH,
C(H)-CH2b) ~; CMR (75 MHz, CDCl3) 166.9, 142.8, 134.1, 130.3, 128.3, 127.4, 124.8,
-100-
CA 02225282 1997-12-19
WO 97/022S9 PCT/US96/0868l
75.8, 63.4, 60.6, 52.1, 37.6, 28.9 ~.
Step 3: (s)~ -(2-IIy~lru~y~lyl)-N-methyli~ûchroman-6-carbc~y~m
(S)-(S-3)
(S)-(-)-1-(2-HyLu~y~:Ulyl)isochroman-6-c~l~u~ylic acid, methyl ester (S)-(S-2,
5 473 mg, 2.0 mmol) and 8.0 mT~ 6M methylamine in mPth~nol are comhin~l The
reaction vessel is sealed with a telon 6c~ ,ap and the ~Lula is heated to 75~.After 20 hûurs, the resction ~ixLu~e is cûoled to 20-25~, cont~ ed under reducedples~ula and ~ ated with hexane to give (S)-(-)-1-(2-h~,Lu.~y~ yl)-N-methyl-
isochroman-6-c~Lu~ le (S)-(S-3), mp = 99-101~; Rf = 0.20 (5'ro
m~th~nnl/dichlûrom~th~nP); [a]D = -119~ (c = 0.8674, mPth~nnl); IR (mull) 3350,
3274, 1648, 1614, 1572, 1564, 1422, 1336, 1320, 1156, 1107, lû78, 1058, 1045, 718,
cm~l; NMR (300 MHz, CDCl3) 7.53 (m, 2H, aromatic), 7.08 (d, lH, J=8.7 Hz,
aromatic), 6.32 (brds, lH, NH), 4.97 (brdd, lH, J=6.7 Hz, m~lllinP), 4.16 (m, lH,
OCH2a), 3.82 (t, 2H, J=5.4 Hz, HO-CH2), 3.74 (m, lH, OCH2b), 3.02 (d, 4H, J=4.9
15 Hz, NCH3 & Ph-CH2a), 2.71 (dt, 2H, J=16.4 Hz & J=3.2 Hz, Ph-CH2b & HO), 2.21
(m, lH, C(H)-CH2a), 2.03 (m, lH, C(H)-CH2b) ~.
Step 4: (S)-(-)-1-(2-M~t~ ~ne~ f nylc.,.yt~ yl)-N-methyli~ûcllLv~ n-6-
carbnY~mi~l~ (S)-(T-2)
(S)-(-)-1-(2-Hyv~u~.y~:Lhyl)-N-methylisocLlu ,an-6 c~L~ P (S)~S-3, 383
20 mg, 1.6 mmol), 16 mL dichloromPt~nP and triethylamine (0.34 mL, 2.4 mmol) areco~hinP-l followed by cooling to 0~. The ~Lu~. is treated with mP~nP~l1lfonyl
chloride (0.15 mL, 1.95 mmol). After 15 min, the reaction is diluted 10 mL
dichlor~mP~ ~ne and washed once with 16 mL water, once with 15 mL saline, dried
over m~FnP~illm sulfate, filtered and conre-.l-dLed to give (S)~-)-1-(2-
25 mp~h~np~uLfonylc~y~ yl)-N-methyl-isochroman-6-c~~ 1e (S)-(T-2), Rf = 0.35(60% ~cetonP/hexane); NMR (300 MHz, CDCl3) 7.53 (m, 2H, aromatic), 7.11 (d, lH,
J=7.9 Hz, aromatic), 6.23 (brds, lH, NH), 4.90 (brdd, lH, J=7.4 Hz, mP~hine)~ 4.46
(m, lH, OCH2a), 4.34 (m, lH, MsO-CH2a), 4.12 (m, lH, MsO-CH2b), 3.76 (m, lH,
OCH2b), 3.00 (m, 7H, NCH3, S-CH3, Ph-CH2a), 2.73 (dt, lH, J=16.1 Hz & J=3.2 Hz,
30 Ph-CH2b), 2.42 (m, lH, C(H)-CH2a), 2.14 (m, lH, C(H)-CH2b) o.
Step 5: (S)-(-)-N-Methyl-1-[2-[4-(4-propionylphenyl)-1-pipe~ lyl]ethyl]-
isochroman-6-carboY~micle (S)-(IX)
A ~i~ e of (S)-(-)-1-(2-m~th~nP,alllfonylu,.y~Ulyl)-N-metlhyl-isochroman-6-
carb~ Y~mi-l~ (S)~T-2, 509 mg, 1.5 mmol), 4'-piperazinopropiophPnonP (393 mg, 1.8
35 mmol), and potsQaillm carbonate (622 mg, 4.5 mmol) in ~etonitrile (7.5 mL) isheated to 50~ overnight, then l~:nu~ed for an n~l~lit;~n~l 5 hours. The reaction
-101-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
~I ~ue is then cooled to 20-25~ and conce~lt~ d~ed to a residue, which is part;t;nned
between water and dichloromPt~nR. The aqueous layer is extracted twice more
with dichloromPt~nR and the cnmhinRfl organic layers are washed once with water,once with saline, dried over m~gnPRillm sulfate, filtered and coll~~l~t d~d. This
5 m~t,~rizll i8 purified by LC on 36 g (230~00) silica gel eluting with 60%
~cetomP/hPY~ne This m~teri~l is re"ly~ li7erl from mpth~nol/ethyl acetate to give
(S)-(-)-N-methyl-1-[2-[4-(4-propionylphenyl)-1-piperazinyl]ethyl]-i~odl~ ulllan-6-
carbnY~mic~e (S)-(IX), mp = 160-161~; Rf = 0.20 (60% ~cetor~P/hexane); IR (mull)3274, 1669, 1640, 1607, 1543, 1522, 1415, 1407, 1315, 1233, 1200, 1156, 1142, 1111,
10 798 cm~1; NMR (300 MHz, CDCl3) 7.87 (d, 2H, J=9.0 Hz, aromatic), 7.55 (m, 2H,aromatic), 7.14 (d, lH, J=8.6 Hz, aromatic), 6.85 (d, 2H, J=9.0 Hz, aromatic), 6.24
(brdd, lH, J=4.6 Hz, NH), 4.86 (brdd, lH, J=5.7 Hz, m~thinR), 4.13 (m, lH, OCH2a),
3.76 (m, lH, OCH2b), 3.34 (t, 4H, J=5.0 Hz, Ph-N-CH28), 3.00 (d, 4H, J=4.9 Hz, N-
CH3 & Ph-CH2a), 2.90 (qrt, 2H, J=7.4 Hz, O=C-CH2), 2.75-2.46 (m, 7H, Ph-NC(H2)-
15 CH28-NCH28 & Ph-CH2b), 2.14 (m, lH, C(H)-CH2a)~ 2-02 (m~ 1H~ C(H)-CH2b), 1-19(t, 3H, J=7.4 Hz, C(H2)-CH3) o; CMR (75 MHz, CDCl3) 199.3, 168.0, 154.1, 141.5,
134.5, 132.7, 130.0, 127.7, 127.3, 125.0, 124.5, 113.5, 74.4, 63.0, 54.5, 53.0, 47.4, 33.2,
31.2, 29.1, 26.8, 8.9 o; MS (EI, m/z) 435.
~AMPLE 82 (S)~-)-1-[2-[4-(4-Trifluo~o~ce,~yl~henyl)-1-pi~tLdzillyl]ethyl]-N- elllylisocllrc lan-6-c~L~ ~r~irle (S)-(IX)
Following the gener~l p~uce-lu~ of ~!~AMPLE 81, Step 5 and mslking
non-critical v~ri~ti~n~ but using 4'-piperazinotrifllloromethylrhRn-nR (504 mg, 1.95
mmol) gives crude product. This material is purified by LC on 59 g (230-400) silica
gel eluting with 50~ etonR/hexane to give (S)-(-)-1-[2-[4-(~ ' rifln~7roacetylphenyl)
25 piperazinyUethyl]-N-methylisochroman-6-carbnY~mi~le (S)-(IX), Rf = 0.20 (60%
etonP/hexane).
~XAMPLE 83 1-[1-[2-[4-(4-MPt~nyyphenyl)-l-piperazinyl]ethyl]-isochroman-6
yl]calLollyl]pyrrolidine (IX)
Following the general p~ucedu~ of F~xAMpLE 30 and ms-king non-critical
30 v~ri~ti~nc but using pyrrolidine (1.26 mL, 15.0 mmol) gives crude product. The
crude is purified by flash chrom~t~Eraphy on 100 g silica gel using a gradient of 5-
10~ mPt~l~nol in ethyl acetate as the eluent to give purified product.
Recrys~ t;~n from methylene chloride and hexane gives 1-[1-[2-[4~4-
mP~l~nYyphenyl)-1-pi~.dzillyl]ethyl]-i~ûchro an-6-yl]c~L,o~ yl]pyrrolidine (IX), mp =
35 156.0-156.5~; Rf = 0.35 (10~o mPt~nnl in ethyl acetate); IR (mull) 1615, 1609, 1563,
1514, 1441, 1254, 1234, 1154, 1106, 825 cm~l; N~ (300 M~z,CDCl3) 7.32 (d, lH,
-102-
CA 02225282 1997-12-19
W O 97/02259 PCTnUS96/08681
J=8.0 Hz, aromatic H), 7.29 (8, lH, aromatic H), 7.12 (d, lH, J=7.9 Hz, aromatic H),
6.85 (q, 4H, J=9.2 Hz, aromatic H's), 4.85 (m of d, lH, J=5.8 Hz, PhC-H), 4.17-4.10
(m, lH), 3.80-3.72 (m, lH), 3.76 (s, 3H, PhOC-H3), 3.64 (t, 2H, J=6.7 Hz, C(O)NC-
H2), 3.11 (t, 2H, J=6.6 Hz, C(O)NC-H2), 3.11 (t, 4H, J=4.8 Hz, four of pip-H), 2.99
5 (m, lH), 2.73 (m of d, lH, J=16.4 Hz), 2.66-2.49 (m's, 6H), 2.15 (m, lH, pipCH-H),
2.04 (m, lH, pipCH-H), 1.99-1.85 (two slightly overlapping ~luint~L~, 4H, J=7.0 Hz,
two of C(O)NCH2C-H2) o; CMR (75 MHz, CDC13) 169.4, 153.6, 145.6, 139.7, 135.2,
134.0,127.7, 124.7, 124.4, 118.0, 114.3, 74.4, 62.9, 55.4, 54.6, 53.4, 50.5, 49.5, 46.1,
33.0, 28.9, 26.3, 24.3 o.
10 li'XAMPLE 84 (+/-)-1-[2-(4-Phenyl-1-pir~ri-linyl)ethyl]isochroman-6-
c~
Step 1. (+/-)-2-(6-BromniRorhroman-1-yl)acetic acid
A ~LI,uL2 of ethyl (+/-)-2-(6-bromoicorhroman-1-yl)~ret~ts (III, EXAMPLE 1,
step 1; 0.77 g, 2.58 mmol), sodium hydroxide (2N, 1.9 mT,) and etl~nol (5 mL) is15 stirred for 75 min, at which time eth~nol is removed under reduced ~ie~
Several millilit~rs of water are added to the residue, followed by hyd~cllloric acid
(4N) sl~fflrir-nt to bring the pH of the ~Lu.~ to about 2. The llPi~Lul~ is ~-Ll..~ led
with ether and the organic layers are dried with m~E~n~cinm sulfate and
~.~..r_..t-dted to give (+/-)-2-(6-brnmniRorhroman-l-yl)acetic acid (IV), NMR (CDC13)
20 2.69 - 2.97, 3.83, 4.16, 5.18, 6.94 and 7.32 o.
Step 2. (+/-)-2-(6-Brrmri~orhroman-1-yl)ethyl alcohol
To (+/-)-2-(6-bromni~orh-~.".an-1-yl)acetic acid (IV, Step 1; 0.82 g, 3.0 mmol) in
dry THF (20 mT-) is added borane-methyl sll1fide (0.86 g, 9.1 mmol). After stirring
for 2.5 hr, m~~h~nnl is added and the ~Lu~e is cor..~e..l-d~ed under reduced
25 ~}e8~ule. M~t~nnl is again added and the ~i~L~ cnnre~lt c~ed twice more. The
residue is then partitionP-l between dichlorrmrth~n~ and aqueous sodium
bicarbonate and the organic layers are dried with sodium sulfate and con~ -aLed
to give (+/-)-2-(6-bromni~o~ .vll,an-1-yl)ethyl alcohol (S-1), N~ (CDCl3) 2.0, 2.2,
2.64, 2.69, 3.02, 3.70-3.79, 3.82-3.86, 4.15, 4.92, 7.28 S.
Step 3. (+/-)-1-[2-(6-Lr.~.. ~iRocl.-~an-1-yl)ethyl]-4-phe~.ylpi~e.;dine
l~/r~~h~nrRlllfi~nyl rhlnri~lr~ (0.22 mT., 2.84 mmol) is added to an ice water-
cooled ~Lus~ of (+/-)-2-(6-bromoiRorhroman-1-yl)ethyl alcohol (S-1, step 2; 0.599 g,
2.33 mmol), 4-dimethylaminopyridine (0.016 g, 0.131 mmol), diisopl~pylethylamine(0.49 mL, 2.81 _mol) and dry T~D? (7.5 mL). The ice water bath is removed and the
35 ~ is allowed to warm to 20-25~. When the mesylation is cnmp1~t~ (by TL~),
ethylene gycol (2.4 mL), diisopl~ylethylamine (1.0 mL, 5.7 mmol) and 4-
-103-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
phenylluipel;dine (0.452 g, 2.80 mmol) are added and the l~ Ule is heated
overnight at 80~. After cooling, the ~ e is poured into water and eYtracted withdichloromPt7~flnP The comhinP~7 organic layers are dried over sodium sulfate andcnnc~.lt-dted under reduced pre~ule. The residue is chromf7t~graphed (silica gel;
m~lhflnt~l/dichloromPthf7ne, 2/98) to give (+/-)-1-[2-(6-bromoi~o-hroman-1-yl)ethyl]-4-
phenylpiperidine (VI), NMR (CDCl3) 1.85, 1.99-2.17, 2.46-2.60, 2.65-2.71, 2.95, 3.09,
3.74, 4.10, 4.77, 6.99 and 7.17-7.32 o.
Step 4. (+/-)-1-[2-(4-Phenyl-1-piperidinyl)ethyl]isochroman-6-carbnYz7midP
A lllil~l,Ule of (+/-)-1[2-(6-bromoi~o-hroman-1-yl)ethyl]4-phe.lyl,ui~el;dine (VI,
step 1; 0.422 g, 1.05 mmol), DMF (2.7 mL), 1~ 3~3~3-hr~YflmPt7lylrliRilfl7flne
(Aldrich; 1.6 mL, 7.58 mmol), diisoplouylethylamine (0.38 mL, 2.18 mmol),
pfllk7~7il7m (II) acetate (0.012 g, 0.053 mmol) and 1,3-bis(diphenylrhosrhino)propane
(0.026 g, 0.064 mmol) is ~lPgflRRef7. siY. times under reduced p~ ule and released to
carbon monoYi~r each time. The lr~l,ulo is heated at 90~ overnight and then the
cooled lllixl,ul~ is poured into hydlocllloric acid (lN, 11 mL) and eYtracted with
ether. The pH of the aqueous layer i8 adjusted to 12 using aqueous sodium
hydroxide. The aqueous layer then is ~,~Ll~.cLed three times with ethyl acetate and
the com70ine~7 organic layers are washed with saline, dried over mngnP~ m sulfate
and conre .ll a~ed under reduced pi~ Ule. The residue is chromf7~graphed (si icagel; mPt~flnnl/dichloromPtl~f7nP~mmnnil7m hydroYide, 2/98/0.5) to give (+/-)-1-[2-(4-
phenyl-1-piperidinyl)ethyl]isochroman-6-carboYf7mi-7P (VII), NMR (CDCl3) 1.83, 1.99-
2.22, 2.51-2.62, 2.74-2.79, 2.97-3.08, 3.78, 4.14, 4.87, 5.63, 6.05, 7.19-7.33 and 7.59 o.
EXAMPLE 85 N-Methyl-1-[2-(4-phenyl-1-piperidinyl)ethyl]isochroman-6-
c&L~Y~r~ e~ ma eic acid sa t
Step 1. (+/-)-N-Bis(tert-butylu~y~;allJollyl)-l-[2~4-phen
piperidinyl)ethyl]isochroman 6 car7QnYflmit7e
Following the general procedure of l~xAlvl PLE 3, step 1, and mslking non-
critica. variations, (+/-)-1-[2-(4-phenylpiperdin-l-yl)ethyl]isochroman-6-c. .l,-.x~ 7,P
(VII, EXAMPLE 84, 0.231 g, 0.634 mmol), 4-dimethylaminopyridine (0.0098 g,
0.0802 mmol) and di-tert-butyldicarbonate (0.312 g, 1.43 mmol) give (+/-)-N-bis(tert-
butylu~yc~l onyl)-1-[2-(4-phenyl-1-piperdinyl)ethyl]isoclllu an-6-carbnY~mi-le (VIII)
after chrom~t~;- ~hy (silica gel; mPt~l~nol/dichloromPth~np~ 2/98), NMR (CDCl3)
1.39, 1.84, 2.00-2.20, 2.44-2.63, 2.71-2.81, 2.94-3.15, 3.78, 4.14, 4.89, 7.20-7.30 and
7.60-7.65 ~.
Step 2. N-Methyl-1-[2-(4-phenyl-1-piperidinyl)ethyl]isochroman-6-
carbnY~mi-le, maleic acid salt
-104-
-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
Methyl amine gas is cc~nclenRe~l into a glass high pres:ju~: reaction vessel
cooled at -78~ (under an argon ~t~mnsphPre) and co~ ing a LL~LuLa of ( I /-)-N-
bis(tert-butylu"~ . aL l,onyl)- 1-[2-(4-phenyl- 1-piperdinyl)ethyl]isochroman-6-caLl,.~Y~1...irle (VIII, step 1, 0.2818 g, 0.499 mmol) and dichloromPth~nP (4 mL). After
5 several mLs of methylamine are concl~n~ecl into liquid, the vessel is sealed and the
lure is allowed to warm to 20-25~, with stirring. After stirring overnight, the
vessel is recooled to -78~ and the seal broken. After again warming to 20-25~, the
L,L~uLa is conce..t- ~ted under reduced pressure and the rPSllltinF residue is
chromatographed (silica gel; mPth~nl~l/dichlornmPth~nP, 3/97 to 5/95) to give N-
10 methyl-1-[2-(4-phenyl-1-piperidinyl)ethyl~isochroman-6-carbr.Y~mide (IX).
N-Methyl-1-[2-(4-phenyl-1-piperidinyl)ethyl]isochroman-6-caLlJu~ lP. is
treated with maleic acid (0.0360 g, 0.310 mmol) in dichlornmPth~nPlmpth~nol to give
N-methyl-1-[2-(4-phenyl-1-piperidinyl)ethyl]isochroman-6-carb.~Y~mi-le, maleic acid
salt (B-l[X), NMR (CDCl3) 1.84, 2.05-2.20, 2.52-2.66, 2.72-2.78, 3.01, 3.41, 3.49, 3.78,
15 4.13, 4.35, 6.12, 7.16-7.32 and 7.53 ~.
EXAMPLE 86 (+/-)-1-[2-[4-(2,4-Dichlorophenyl)-1-
piperazinyl]ethyl]isochroman-6-c~.l,.~ e
Step 1. 1-(2,4-Dichlorophenyl)piperazine
A lnixlul~ of 1,3-dichloro-4-fluorobenzene (Q-2) (4.21 g, 25.5 mmol),
20 piperazine (Q-1, 11.0 g, 128 mmol) and dimethyl~et~mide (15 mL) i8 heated at 165~
for 6.8 ]hr, at which time the ~PLL~U a is cooled and partitionp~l between
dichlorc~mPt~nP and aqueous sodium bicarbonate. The org~nic layers are dried
with sodium sulfate and conre--l-d~ed under high vacullm to give 1-(2,4-
dichlorophenyl)piperazine (Q-3), which was sllffl~ipntly pure to use in step 2 without
25 further purific~tion
$tep 2. (+/-)-1-[2-(6-Bromni~orhroman-1-yl)ethyl]4-(2,4-
dichlorophenyl)piperazine
]Following the general pl~cedula of EXAMPLE 84, step 3 and m~king non-
critical variations, (+/-)-2-(6-bromni~ochlu lan-l-yl)ethyl alcohol (S-1) (EXAMPLE 84,
30 step 2; 0.60 g, 2.31 mmol), 4-dimethylaminopyridine (0.018 g, 0.147 mmol),
diisu~ur~"uylethylamine (0.49 mL, 2.81 mmol), mpth~npsulfonyl chlorillP (0.22 mL,
2.84 mmol) and dry THF (7.5 mL) are collv~lad to the mesylate (T-1). The mesylate
is treated with diisop.v~uylethylamine (1.0 mL, 5.7 mmol), 1-(2,4-
dichlorophenyl)piperazine (Q-3, step 1; 0.65 g, 2.82 mmol) and ethylene glycol to
35 give, after chrnm~to~ uhy (silica gel; mpth~novdichloromptl~np~ 2/98) (+/-)-1-[2-(6-
bromoi~o~hroman-1-yl)ethyl]-4-(2,4-dichlorophenyl)piperazine (VI), NMR (CDCl3)
-105-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
2.01, 2.10, 2.65-2.71, 2.95, 3.05, 3.74, 4.11, 4.78, 6.96, 7.18 and 7.26-7.36 ~.Step 3. (+/-)-1-[2-[4-(2,4-Dichlorophenyl)-1-
piperazinyl]ethyl]isochroman-6-ca~
Following the general procedure of EXAMPLE 84, step 4, and making non-
5 critical variations, (+/-)-1-[2-(6-brf~mni~ochroman-1-yl)ethyl]-4-(2,4-
dichlorophenyl)piperazine (VI, step 1; 0.373 g, 0.794 mmol), gives 0.095 g of (+/-)-1-
[2-[4-(2,4-dichlorophenyl)-1-piperazinyl]ethyl]isochroman-6-c~1.... ,1...i ~ (VII) after
chrom~;. dphy (silica gel; mf~t~n- l/dichlorom-~thAne, 2/98), NMR (CDCl3) 2.05,
2.15, 2.50-2.80, 3.05, 3.78, 4.15, 4.87, 5.62, 6.04, 6.96, 7.19, 7.35 and 7.60 o.~0 F~Al\/IPLE 87 (+/-)-N-Methyl-1-[2-[4-(2,4-dichlorophenyl)-1-
piperazinyl]ethyl]isochroman-6-call,n Y ~ . ";~
Step 1. (+/-)-N-Bis(tert-butyloAy~i~bonyl)-l-[2-[4-(2~4-dichlorophenyl)
piperazinyl]ethyl]isochroman-6-carbf~ Y~mide
Following the general proce-lule of EXAMPLE 3, step 1, and mAkinF non-
16 critical vAriAtiorl~ (+/-)-1-[2-[4-(2,4-dichlorophenyl)-1-pipel~zillyl]ethyl]isochroman-6-
c~b.~ (VI, EXAMPLE 86, step 2; 0.0854 g, 0.198 mmol), 4-
dimethyl~minopyridine (0.0046 g, 0.0377 _mol) and di-tert-butyl dicarbonate
(0.0982 g, 0.450 mmol) give, after chrnmAIo~.~phy (silica gel;
ml~t~nl~l/dichlorom~t~Ane, 2/98), (+/-)-N-bis(tert-butyluAy~ nyl)-1-[2-[4-(2,4-
20 dichlorophenyl)-1-piperazinyl]ethyl]isochroman-6-c~ rifle (VIII), NMR (CDC13)1.40, 2.05, 2.17, 2.51-2.79, 2.95-3.05, 3.78, 4.15, 4.89, 6.96, 7.19, 7.35 and 7.60-7.66
o.
Step 2. (+/-)-N-Methyl-1-[2-[4-(2,4-dichlorophenyl)-1-
piperazinyl]ethyl]igochlvluan 6 carbnY~mi~lA
Following the general p~ucedul c, of EXAMPLE 85, step 2, and mAkinE non-
critical vAriAt;r~n~ (+/-)-N-bis(tert-butyloxycarbonyl)-1-[2-[4-(2,4-dichlorophenyl)-1-
pi~e~ yl]ethyl]isochroman-6-c~l,nY,1...i-lA (VIII, step 1; 0.104 g, 0.164 mmol) gives
after chrom~l,o~phy (silica gel; mPt~lAnol/dichlorompt)lAnA~ 1.5/98.5 to 3/97 to 5/95)
a solid. This m~teri~l was cryst~lli7Ad from ~ret~nitrilPlheAane to give (+/-)-N-
methyl-1-[2-[4-(2,4-dichlorophenyl)-1-piperazinyl]ethyl]isochroman-6-c ~ 1,. .Y ~ . . .i~F!
(IX), MS (m/z) = 447; IR (mineral oil; most intense peaks) 1637, 1478, 1572, 1558,
1450, 3289 and 1107 cm~1; NMR (CDCl3) 2.05, 2.16, 2.52-2.78, 3.03, 3.77, 4.13, 4.86,
6.10, 6.96, 7.17, 7.36 and 7.54 o.
~AMPLE 88 1-[2-[4-(3-Chloro-4-methoxyphenyl)-1- .,
piperazinyl]ethyl]isochroman-6 carbnY~mi-1e
Step 1. 1~3-Chloro-4-m~t~nYyphenyl)piperazine
-106-
CA 02225282 l997-l2-l9
W O 97/02259 PCT~US96/08681
A ~llixLu~: of 3-chloro-p-~ni~i-linP (R-2, 0.633 g, 4.00 mmol), bis(2-
chlc~l~el~s~ mine slydsocllloride (0.860 4.80 mlnol~, pot~CRillm carbonate (1.11 g,
8.00 mmol) and diméthyl~-~ehmi~lP (6 mL) is stirred at 100~ for 18 hr and then
cooled. The ~ u~e ispartitinnpcl between dichloromPth~mP, water and aqueous
5 sodium bicarbonate and the organic layers are dried with sodium sulfate and
concPntrated. The residue is chrom~t~u~c.phed (silica gel;
mPths~nnl/dichloromPtl-~ne, 8/92) to give 1-(3-chloro4-mpthnyyphenyl)piperazine (R-
3), N~ (CDCl3) 3.05, 3.86, 6.80, 6.87 and 6.99 o.
Step 2. (+/-)-1-[2-(6-Br)mni~orhroman-l-yl)ethyl]4-(3-chloro4-
methoxyphenyl)piperazine
Following the general ~locedl~e of ~AMPLE 84, step 3, (+/-)-2-(6-
bromni~orhroman-l-yl)ethyl alcohol (S-l, EXAMPLE 84, step 3, 0.450 g, 1.75 mmol),
4-dimethylaminopyridine (0.012g, 0.0990 mmol), diiso~ropylethylamine (0.32 mL,
1.84 mmol), mPt~nesulfonyl chloride (0.14 mL, 1.81 mmol) and d-y TED~ (5.6 mL)
are eullv~sled to the mesySate. ~ it.icm~l portions of diisoproplyethylamine (0.18
mL, 1.03 mmol) and m~tl~nP~ fr,nyl rhlori~l~ (0.08 mL, 1.03 m~nol) are added to
complete the form~tion of the me~ylate. To the mesylate then is added
dii~u~ropylethylamine (0.65 mL, 4.26 msnol), 1-(3-chloro4-mPthnYyphenyl)piperazine
(step 1; 0.398 g, 1.75 mmol) and ethylene glycol (1.8 mL). The s~Lu~e is stirred at
80~ for 3 hr and then overs~ight at 20-25~, after which the sllixl,us~ again is heated
for 4 hr at 80~. A~ition~l 1-(3-chloro4-mPthnYyphenyl)piperazine (0.0443 g, 0.195
mmol) is added and the mi~ul~: is heated ~nn~lPr 3 hr. After cooling, the slliXl~Ule
is parfftioned be~w~sl dichlorc mPth~nP and aqueous sodium bicarbonate and the
organic layers are dried with sodium sulfate and conre~ d~ d. The residue is
chrom~to~-aphed (silica gel; mPth~nol/dichlornmpth~ne~ 2/98) to give (+/-)-1-t2-(6-
bromni~o~hroman-l-yl)ethyl]-4-(3-chloro-4-methoxyphenyl)piperazine (VI), NMR
(CDCl3) 2.01, 2.10, 2.52-2.71, 2.96, 3.10, 3.75, 3.85, 4.10, 4.78, 6.77-6.87, 6.98 and
7.29 o.
Step 3. (+/-)-l-t2-t4-(3-Chsoro-4-mPthn~s7phenyl)-l-
pipesdzssl~l]etlhyl]isochroman-6-carbny~midp
Following the general pr)celluse, of ~AMPLE 84, step 4, and m~king non-
crstical v~ri~tinnR (+/-)-1-[2-(6-brnmoi~o~h-uslsan-1-yl)ethyl]-4-(3-chloro-4-
metho~yphenyl)piperazine (VI, Step 2; 0.420 g, 0.902 mmol), DMF (2.3 mL),
3~3~3-hPy~mp-thyl~iRil~np (1.4 mL, 6.64 m_ol), diisu~su,uylethylamine (0.34
mL, 1.95 mmol), p~ m (II) acetate (0.0110 g, 0.049 mmol) and 1,3-
bis(diphenylph~Rphino)propane (0.024 g, 0.0575 ~nol) give (+/-)-l-t2-[4-(3-chloro-4
-107-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
methoxyphenyl)- 1-piperazinyl]ethyl]isochru...an-6-ca~ . x ~ l P (VII) . After
chrom~ phy (silica gel; mPtl~nnl/dichlornmpth~np~ 2/98), NMR (CDCl3) 2.06,
2.15, 2.53-2.64, 2.74-2.79, 3.02, 3.11, 3.77, 3.84, 4.14, 4.87, 5.60, 6.04, 6.77-6.87, 6.98,
7.18 and 7.60 8.
T'~x~ pLE 89 (+/-)-1-[2-[4-(3-Chloro-4-methn~yphenyl)-1-piperzinyl]ethyl]-N-
methylisochroman-6-caL 1, ~ ~ X ~ ~ ~ ~ i d e
Step 1. (+/-)-N-Bis(tert-butyloxycarbonyl)-1-[2-[4-(3-chloro-4-
methoxyphenyl)-l-piperazinyl]ethyl]isochroman-6-carbn~r~mi~p
Following the general p.ucedu~ of EXAMPLE 3, step 1, and making non-
10 critical variations, (+/-)-1-t2-[4-(3-chloro-4-mPthn~yphenyl)-1-
piperazinyl]ethyl]isochroman-6-carbnY~mi~e (VII, EXAMPLE 88, step 3; 0.153 g,
0.355 mmol), 4-dimethylaminopyridine (0.0086 g, 0.0704 mmol) and di-tert-
butyldicarbonate (0.186 g, 0.853 mmol) give, after chrom~to~.aphy (silica gel;
mf~th~noVdichlorom~th~ne, 2/98), (+/-)-N-bis(tert-butyk,Ay. arbu..yl)-1-[2-[4-(3-chloro-
15 4-mPthn~ryphenyl)-1-piperazinyl]ethyl]isocllrol..an-6 c~l.oY~...i~P (VIII). NMR
(CDCl3) 1.39, 2.03, 2.14, 2.48-2.64, 2.73-2.80, 3.00, 3.11, 3.78, 3.85, 4.14, 4.88, 6.80-
6.88, 6.98, 7.20 and 7.60-7.65 8.
Step 2. (+/-)-N-Methyl-1-[2-[4-(3-chloro-4-mPthnyyphenyl)
piperzinyl]ethyl]isochroman-6-call....,....i~le
Following the general prùce-lu e of Ti~AMpLE 85, step 2, and m~king non-
critical variations, (+/-)-N-bis(tert-butyluAycal~ullyl)-1-[2-[4-(3-chloro-4-
methoxyphenyl)-l-pip~zi--yl]ethyl]isochroman-6-ca~ le (VIII, step 1; 0.183 g,
0.290 mmol) gives 0.118 g of product after chrom~tography (silica gel;
mPth~nol/dichloromPth~ne, 2/98). The product is cry~t~ p~l from ethyl
acetate/mPth~nnl/hexane and then from ethyl acetate/dichloromPth~ne to give (+/-)-1-
[2-[4-(3-chloro4-mPthn~ryphenyl)-l-piperzinyl]ethyl]-N-methylisGchro...anyl-6-
c~bnxJ~ P (IX), MS (m/z) 443; IR (mineral oil, most intense peaks) 1508, 1642,
3266, 1112, 1548, 1274 and 949 cm~1; NMR (CDCl3) 2.09, 2.21, 2.58-2.77, 3.01, 3.15,
3.76, 3.85, 4.15, 4.88, 6.12, 6.77-6.88, 6.98, 7.17 and, 7.54 8.~0 T~'XAlVIPLE 90 N-(2-IIydluAyt~ yl)-1-[2-4~4-mptllnl~yphenyl)
piperazinyl]ethyl]isochroman-6 c~l,..x~...i-le
Step 1. (+/-)-1-2-(6-Bromni~ochroman-1-yl)acetyl-4~4-
m~thnYyphenyl)piperazine
A ~LuAluLe of (+/-)-2-(6-bromoisochroman-1-yl)acetic acid (IV, T~AMpLE 84,
35 step 1; 4.66 g, 0.0172 mol), dichloromPth~nP (18 mL), DMF (18 mT ), diethyl
cyanophnsphon~ (3.4 mL, 0.022 mol), 1-(4-mPthnxyphenyl)piperazine hydrochloride
-108-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTnUS96/08681
(R-3) (Aldrich; 4.78 g, O.OZ1 mol) and triethylamine (6.5 mL, 0.047 mol) is stirred at
20-25~ for 2.5 hours. Satulated sodium bicarbonate sodium bicarbonate (100 mL) is
added to the mixture and the l-~Lu~is allowed to stir for 20 min, at which time it
is extracted with dichloromPt~Ane The comhimP~l organic layers are dried over
ms~f~npRillnn sulfate and cnn.~ ted under reduced ~ e. The residue is
washed twice with hexane (discard) and the residue again is conl~ntrated under
reduced pressure. The residue is chromAt~ graphed (silica gel; ethyl acetate/hPYAnP~
50/50) to give (+/-)-1-2-(6-bromni~o~ .u.l.an-l-yl)acetyl4-(4-
methûxyphenyl)piperazine (V), NMR (CDCl3) 2.63-2.69, 2.74-2.80, 2.90-3.08, 3.59-
1û 3.96,4.11,5.26,6.87,7.01,7.31~.
Step 2. (+/-)-1-[2-(6-Bromoisochroman-1-yl)ethyl]-4-
methoxyphenylpiperazine
Following the general pl~Ce~ e of EXA~!LPLE 1, step 4, but using bor_ne-
methyl sulfide (14 mL) in place of borane-THF, (+/-)-1-2-(6-bromni~o. l..u.~an-1-
yl)acetyl-4-(4-mPtl~oYyphenyl)piperazine(V, step 1; 5.83 g,0.013 mol) gives 4.33 g of
(+/-)-1-[2-(6-bromni~orhroman-l-yl)ethyl]-4-methoYyphe~lylpi~elazine (VI), N~
(CDCl3) 2.00, 2.12, 2.49-2.71, 2.95, 3.10, 3.75, 4.11, 4.78, 6.87, 6.97 and 7.29 o.
Step 3. (+/-)-l-t2-t4-(4-MPt~nyyphenyl)-l-piperazinyl]ethyl]i8ochr
6-carboYAmi~P
Following the general ~.u~edul~ of F~AMPLE 84, step 4, (+/-)-1-t2-(6-
bromni.qGrh.u ~an-1-yl)ethyl]4-(methoxyphenyl)piperazine (VI, step 2; 4.29 g, 9.95
mmol) gives (+/-)-1-t2-[4-(4-methoxyphenyl)-1-pi~dzi~yl]ethyl]isochroman-6-
carboYAmi-l~ (VII), NMR (CDCl3) 2.06, 2.16, 2.54-2.79, 2.99, 3.11, 3.77, 4.15, 4.87,
5.64, 6.06, 6.87, 7.19 and 7.59 o.
Step 4. (+/-)-N-Bis(tert-butylu,Ly~bu~lyl)-l-[2-t4-(methoxyphenyl)
piperazinyl]ethyl]isochroman 6 car~oYAmi(l~
Follovring the general plocc:du~e of F'XAl\/IPLE 3, step 1, (+/-)-1-[2-[4-(4-
methoxyphenyl)-1-piperazinyl]ethyl]isochroman-6-carbc.~r~mi(le (VII, step 3; 1.97 g,
4.97 mmLol), 4-dimethylaminopyridine (0.0816 g, 0.668 mmol) and di-tert-butyl-
30 dicarbonate (2.56 g, 0.0117 mol) gives (+/-)-N-bis(tert-butylu~.y. ~bu~.yl)-1-[2-[4-
mPt~nYyphenyl)-1-piperazinyl]ethyl]isochroman-6-c~b.-~A..-i-le (VIII), NMR (CDC13)
1.39, 2.03, 2.15, 2.53-2.78, 2.99, 3.11, 3.77, 4.13, 4.88, 6.85, 7.21 and 7.63 o.
Step 5. N-(2-hy-l~u.sy~Lllyl)-1-[2-4-(4-methoxyphenyl)-1-
piperazinyl]ethyl]isoclllulllanyl-6-ca~L.~ le
A ~ule of (+/-)-N-bis(tert-butylu,.. ~bù,lyl)-1-[2-t4-(mPtl~oYyphenyl)-1-
piperazinyl]ethyl]isochroman-6-carbnY~mi-le (VIII, step 4; 0.216 g, 0.362 mmol),
-109-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
dichlorometh~ne (7 mT ) and eth~nol~min~ (0.2 ~, 3.31 Inol) is stirred overnightat 20-25~. The llliXLUlG i6 then partit;on~-l between water and dichlorom~th~n~
The comhin~l organic layers are dried over sodium sulfate and co~ dted under
reduced 1;JIG~ U1G. The residue is chrom~t~graphed (silica gel;
5 m~ths~nol/dichlorom~ths3ne (4/96) to give product which is cry~t~ 7ecl from
dichlorom~th~n~hexane/ethyl acetate to give 0.090 g of N-(2-hydroxyethyl)-1-[2-4~4-
methoxyphenyl)-l-piperazinyl]ethyl]isochromanyl-6-carb--Y~mi-1~ (IX), MS (m/z) 439;
IR (mineral oil) (most intense peaks) 1514, 1631, 1554, 1031, 3293, 1249 and 1613
cm~1; NMR (CDCl3) 2.05, 2.15, 2.51-2.78, 3.01, 3.11, 3.63, 3.77, 3.85, 4.13, 4.86, 6.59,
10 6.87, 7.17 and 7.57 ~.
EXAMPLE 91 1-[2-[4-(4-M~oth ~Yyphenyl)- l-piperazinyl]ethyl]-N-
(phenylmPthnyy)isochroman-6-cal1~ e
A llliXl~UlG of (+/-)-N-bis(tert-butylu~y~Lùnlyl)-l-[2-[4-(4-m~thnyyphenyl)-l-
piperazinyl]ethyl]isochroman-6-carl,.-~ le (VIII, EXA ret7, step 4; 0.206 g, 0.345
15 mmol), THF (7 mT.)~ O-benzylhyd-u~ylamine h ydlochloride (0.0645 g, 0.4041 mmol)
and diiso~rv,uylethylamine (0.12 mL, 0.689 mmol) i8 are heated at reflux for 7 hours.
The llli~ then is stirred at 20-25~ for two days, at which time s~ ition~l O
benzyllly.l~u,~ylamine hydlo~ ri-l~ (0.323 g, 2.03 mmol) and diiso~.u~ylethylamine
(0.35 mT-, 2.01 Inol) are added. After stirring overnight at 85-90~, THF is removed
20 by cc,~-r~ Lil-g under reduced plG~UlG and the residue is parfftion~-l between
dichlorom~th~ne and water. The cc-mhine-l organic layers are dried over sodium
sulfate and co..c~ ed under reduced ~llGI~ to give crude m~t~ri~l This
ms~t,çri~l is chr~ ms~c~;.dphed (silica gel; mf~thsln-71/dichlorom~oth~n~ 4/96) to give the
product which is cryst~ etl from h~Y~n~ethyl ~cetste/dichlorom~th~neim~th~nol to25 give the title compound, MS (m/z) 501; NMR (CDCl3) 2.03, 2.12, 2.48-2.75, 2.98,
3.10, 3.77, 4.11, 4.84, 5.05, 6.86, 7.14, 7.41 and 8.43 ~.
EXAMPLE 92 (+/-)-1-[1-[2-[4-(4-M~thnYyphenyl)-1-
piperazinyUethyl]isochroman-6-yl]-4-mel.llyl,ui~erazine
A ~ ule of (+/-)-N-bis(tert-butylc.~y. &.I.ùnyl)-1-[2-[4-(4-methoxyphenyl)-1-
30 ~i,uelazillyl]ethyl]isochroman-6-c.~L~ (VIII, EXA ret7, step 4; 0.211 g, 0.355
mmol), THF (11 mT-), diisoproplyethylamine (0.6 mL, 3.44 mmol) and 1-
methylpiperazine (0.4 mL, 3.6 mmol) is stirred over a weekend at 20-25~, after
which THF is removed under reduced ples~ and the residue is partitioned
between dichlorom~th~n~ and water. The comhin~ organic layers are dried over
35 ms~gn~ lm sulfate and con~e..l-dted under reduced p~ le to give crude product.
The crude product is chrom~twgraphed (silica gel; mpth~nol/dichlorom~th~ne~ 2/98 to
-110-
CA 02225282 1997-12-19
W 097/022S9 PCT/U~,5.'~ 6~1
4/96). The p~u1ucl i8~ ~-t~ from ethyl acetate and hexane to give the title
compound, a portion is le~ i7e~1 from ethyl o-~eto~e, MS (mJz) 478; N~
(CDCl3) 2.09, 2.32, 2.48-2.75, 2.99, 3.11, 3.47, 3.76, 4.13, 4.86, 6.86 and 7.18 ~.
- T~AlVlPLE 93 (+/-)-N-Hydlu~y-1-[2-[4-(4-mPthn~ryphenyl)-1-pipG~ yl]ethyl]-
N-methylisochroman-6-ca~ or.,ide
A ~ Llu. e of (+/-)-N-bis(tert-butyl~ yc~ ullyl)-l-[2-[4-(4-methoxyphenyl)-l-
piperazinyl]ethyl]isocLluluan-6-ca~ P (VIII, EXA ret7, step 4; 0.205 g, 0.344
mmol), dichloromPtll-one (7 mL), N-methylhy~llu~ylo-mine hy~llucl~loride (0.271 g,
3.25 mmol) and dii6ùp.~0~ylethylamine (0.60 mL, 3.44 mmol) are stirred overnight at
20-25~. The ~ U~G then is parfftionP~ between dichlorompthone and water. The
comhinP-l organic layers are dried over sodium sulfate and conc.~ ated under
reduced ~rG-~u G. The residue i8chromAtographed (silica gel;
mPth~nol/dichlorompt~l~np) 2/98 to 4/96) to give the desired product which is upon
cryst~lli7~t;~n from hexane/mPthAn~-l/dichlor ~mp~An~/ethyl acetate gives the title
cu~ ou~d, MS (Jz) 425; NMR (CDC13) 2.05, 2.17, 2.54-2.77, 3.00, 3.11, 3.42, 3.77,
4.13,4.85,6.87,7.17and7.33~.
T~Al\/IPLE 94 (S)-(-)-1-[2-[4-[4-(ter~Butylu~,~l,onyl)phenyl]-1-
piperazinyl]ethyl]-N-methylisochroman-6-c~ ,.,i-la (V-2)
8tep 1. 4-FluorobPn7oic acid, ter~butyl ester
To a ~olllt;~n of 4-fluorobenzoic acid (18.7 g, 0.133 mol) in DMF (140 mT-) is
added 1,1-ca~l,oll~kliimirlAl7ol-p (21.6 g, 0.134 mol). The lllib~ is heated for 1 hour
at 40~, at which time t-butanol (26 mL, 0.272 mol) and DBU (20.5 mL, 0.137 mol)
are added. After stirring overnight at 40~, the cooled ~lu~e is poured into ethyl
ether (1300 mL) and washed with hydrochloric acid (10%, 250 mL), followed by
water (250 mT) and then pot~ - carbonate (10%, 250 mT-). The ether layer is
dried over sodium sulfate and concenhated under redllced ple~_u~e. The residue is
chromA~ . aphed (silica gel; ethyl acetate/hPY~na, 10/90) to give 4-fluorobenzoic acid,
tert-butyl ester (Q-2); MS (m/z) 196; N~ (CDC13) 1.59, 7.07 and 7.99 ~.
Step 2. 4~ri~ az~-1-yl)benzoic acid, tert-butyl ester
A m-~Llu-~ of 4-fluorobenzoic acid, tert-butyl ester (Q-2) (step 1; 20.5 g, 0.105
mol), piperazine (52.8 g, 0.613 mol) and dimethylAcet-mi~e (121 mL) is heated at150-155~ for 160 ",;"l,lPs After CoolinF~ the solid i8 removed by filtration andwashed with hPY~ne The comhinPd filtrates are conrentrated under high vacuum
and the residue is partit;~na~l between dichlor mPthAne and water. The comhinPc~organic layers are dried over sodium sulfate and concent~ated under reduced
pIeooul~ The slightly solvent-wet solids are ~lllrrie~ in heYane and the solid is
-111-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTnUS96/08681
collecte-l and washed with h~Y~n~. The solids are dried at 20-25~ under reduced
pressure to give the t-butyl 4-(piperazin-1-yl)bPn7o~te A-1-lition~1 product i8
obtained by conr9. .t ~ alion of the filtrate and chrom~t~Fraphy of the resulting residue
(silica gel; m~th~n-~l/dichlorom~th~n~/~mmnnillm hydroxide, 3.5/96.5/0.5 to 719310.5)
5 to give 4-(pipe~ -1-yl)benzoic acid, t-butyl ester (Q-3); MS (m/z) 262; NMR
(CDCl3) 1.57, 3.01, 3.25, 6.84 and 7.87 o.
Step 3. (S)-(-)-4-[4-[2-(6-brom- iRorhroman-1-yl)ethyl]-1-
piperazinyl]benzoic acid, tert-butyl ester (V-1)
Following the general procedure of EXAMPLE 84, step 3, and m~king non-
10 critical variations, (S)-(-)-2-(6-br?moiRochroman-1-yl)ethyl alcohol (S-1) (EXAMPLE
6, step 1; 4.95 g, 0.0193 mol) and 4-(piperazin-1-yl)benzoic acid, tert-butyl ester (Q-3)
(step 2; 5.42 g, 0.0206 mol) gives the title culll,uuu~d, MS (m/z) 500; NMR (CDC13)
1.57, 2.02, 2.12, 2.50-2.70, 2.95, 3.32, 3.73, 4.10, 4.79, 6.85, 6.97, 7.29 and 7.86 o.
Step 4. (S)-(-)-1-[2-[4-[4-(tert-ButyL~y~ lJullyl)phenyl]-1-
~ zi~lyl]ethyl]-N-methyli~ocllru~an-6-c~ul,u
A l~lura of (S)-(-)-4-[4-[2-(6-brom- iRo-hroman-1-yl]ethyl]-1-
piperazinyl]h~n7Oi~ acid, tert-butyl ester (V-1) (step 3; 2.63 g, 5.25 mmol) in
dimethyl.lc~L ~ ide (45 mL) which has been tleg~RRe~l and released to argon is added
to pQll~ lm (II) acetate (0.0616 g, 0.274 mmol), 1,3-bis(diphenylrh-)sphino)propane
20 (0.164 g, 0.396 mmol) and diisop~o~uylethylamine (1.8 mL, 0.0103 mol). The ll~i~lule
is ~l~g~Re-l a second time, rele~RinF to argon. The llliXl~Ula is heated at 60-65~ while
carbon monoY~ is bubbled into the ll~ u a. After several minllt~R methylamine isalso bubbled into the n~i~ula. After he~ting for 6.5 hours at 60-65~, the )~i~lu~a is
stored overnight in the refrigerator. The lllLb~ula then i8 filtered through
25 ~ tom~eous earth and ~ ition~l portions of p~ illm (II) acetate (0.065 g, 0.290
mmol), 1,3-bis(diphenylphosphinn)propane (0.162 g, 0.392 mmol) and
dusopropylethylamine (1.8 mL, 0.0103 mol) are added to the filtrate, which is heated
at 60~ for 4 hr with the ~ it;on of carbon m~noy~ and methylamine gases, after
which the ll~ Lula is cooled and co- c~ a~ed under reduced ~ . The residue
30 is partiti~n~ between dichlorom?~n~ and water. The comhin.o~l organic layers are
washed with water and saline and dried over sodium sulfate and conce~ Led under
reduced plah~u~:. The residue is chrom~ dphed (silica gel;
mA~h~nA,l/dichloromethane, 2/98 to 4/96). Impure fractions are comhine~l and
rechrom~t4~.d~hed (silica gel; m~th~nnl/dichlor~A~m~h~n~s~ 2/98) to give the title
35 cvlll~vulld, MS (mlz) 479; NMR (CDCl3) 1.56, 2.03, 2.16, 2.48-2.79, 3.01, 3.32, 3.76,
4.14, 4.86, 6.08, 6.84, 7.15, 7.53 and 7.86 ~.
-112-
CA 02225282 1997-12-19
W O 97/02259 PCT/u~5l~s~l
FXA MPLE 95 (+/~ -[2-[4-(4-MPt~nYyphenyl)-l-piperazinyl]ethyl]isochroman-
6-ol, methyl carbamate ester (X-6)
Step 1. (+/-)-2-(6-IIyd~u~yisochroman-1-yl)acetic acid, ethyl ester
To an ice-cooled ~xture of 3-hydlv~yl~hPnethyl alcohol (X-1) (2.9 g, 21 mmol)
5 and ethyl 3,3-diethu~y~-vpionate (4.75 g, 25 mmol) in nitrom~t~np (5 mL) i8 added
boron trifluoride etherate (3.44 mL). After the ~ liti~n is comrl~tP (about 5 min)
the reaction is stirred for an A,l~litinn~l 60 min. The ...i,~u,.3 is then part;tionPcl
between dichloromPth~nP and aqueous ~mm~nillm rhlorirlP The organic layers are
dried vwith sodium sulfate and con(e..l.dled. The residue is chroTn~tographed (silica
10 gel; ethyl acetate/hPY~nP~ 10/90 to 30/70) to give (+/-)-2-(6-h~d~u~yisochroman-1-
yl)acetic acid, ethyl ester (X-2), NMR (CDCl3) 1.28, 2.6-3.0, 3.79, 4.09, 4.21, 5.07,
5.19, 6.60, 6.67 and 6.91 o.
Step 2. (+/-)-2-(6-Hydroxyisochroman-l-yl)acetic acid
To (6-hyd~v~yisochroman-1-yl)acetic acid, ethyl ester (X-2) (step 1; 2.38 g,
15 10.1 mmol) in eth~nol (10-15 mT-) is added sodium hydroxide (2N, 10 ~). The
...i,.l.~a is stirred overnight and eth~nol is then removed under reduced pl~S.i~e.
The rPRllltinE aqueous ~ is then diluted with several mLs of saline and
ifiPd with llyd~u~l-loric acid (3N), and the ~ Le is ~ ..o~d with ethyl ether
and conc~ d~ed. To remove rPm~ininE starting material, the residue is part;ti~n
20 between aqueous sodium bicarbonate and dichlorompt~ ~ne. The organic phase isremoved and the aqueous layer is ~ lifiP-l with con~e~ d~ed llyd~u~llloric acid and
then extracted with ether. The ether layer is dried over m~EnQ~inm sulfate and
conc~l dted. The conrPntrate is crystolli~P~l from THF/hPy~np~dichlorompth~n-p to
give (+/-)-2-(6-hyd.u~yisoch.v~l~an-1-yl)acetic acid (X-3), NMR (CDCl3) 2.1-3.0, 3.81,
25 4.15, 5.2, 6.59, 6.68 and 6.90 o.
Step 3. (+/-)-1-2-(6-Hydroxyisochroman-1-yl)acetyl-4-(4-
mPt~l~Yyphenyl)piperazine
To a l~ e of (+/-)-2-(6-hyd~u~yisochlulllan-1-yl)acetic acid (X-3) (step 2;
0.361 g, 1.73 mm~ol), 1-(4-methoxyphenyl)piperazine dilly.Lu~hl-ri-lQ (0.458 g, 1.73
30 mmol), dichloromPt~ne (5 m~ ), and DMF (0.5 mT~) iS added triethylamine (0.80 mL,
5.72 mmol) and then diethyl cyanophosphon~t,P (0.29 mL, 1.91 mmol). After stirring
for 50 min, water is added and the ~-~xLu~a is stirred for 1 hour. The ~xLu~a isthen extracted with dichlor mPth~ne and the organic layers are st,~alaLed, cnmhinpd
and washed with aqueous sodium bicarbonate. The organic layers are dried with
35 sodium sulfate and con~-h~ Led. The residue is cryst~ ed from ethyl acetate-
dichloromPthAnp-mpt~nol to give (+/-)-1-2-(6-hy~l~u~yisochroman-1-yl)acetyl4-(4-
-113-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
methoxyphenyl)piperazine (X-4), NMR (DMSO) 2.57-2.63, 2.73-2.90, 2.97, 3.64, 3.69,
3.95, 5.03, 6.51, 6.57, 6.8-7.0 and 9.27 ~.
Step 4. (+/-)-1-[2-t4-(4-Mathn~yphenyl)-l-piperazinyl]ethyl]i6Ochroman-
6-ol
A ~i"~u. e of (+/-)-1-2-(6-hydroxynsochroman-1-yl)acetyl-4-(4-
methoxyphenyl)piperazine (X-4) (step 3; 0.375 g, 0.98 mmol), borane methylsulfide
(0.28 _L, 2.9 mmol), and TE~ (15 mL) is stirred overnight at 20-25~ and then at 80
for 2 hr. After cooling, the n~Al,u.e is treated with mpth~nol and co~cantrated under
reduced ~U~c~S~Ule. The ~ tion of m.oth~nol and con-enl-dLion is repeated twice
more and then the residue is stirred for 3 hr in hydrochloric acid/~cetone (4N, lJ9, 5
ml). The ~-~et~na is then removed under reduced p~e~uLe and the residue is
part~tiona-l between dichlorom~t~nP and aqueous sodium bicarbonate. The organic
layers are dried with sodium bicarbonate and con--e..t~dted. The residue is
cryst~lli7e~ from dichlorom~th~n~/hexane to give (+/-)-1-[2-[4-(4-methoxyphenyl)-1-
piperazinyl]ethyl]isochroman-6-ol (X-5), NMR (CDC13) 2.05, 2.21, 2.5-2.7, 2.94, 3.13,
3.70, 3.76, 4.10, 4.82, 6.41, 6.57 and 6.80-6.91 o.
Step 5. (+/-)-1-[2-[4-(4-M~thnYyphenyl)-l-piperazinyl]ethyl]isochroman-
6-ol, methyl carbamate ester
To a ~lliA~ule of (+/-)-1-[2-[4-(4-methoxyphenyl)-1-
piperazinyl]ethyl]isoch.u .an-6-ol (X-5) (step 4; 0.064 g, 0.17 mmol) and DBU (0.032
g, 0.21 mmol) in dichlorom~th~na (2 mL) is added methylisocyanate (0.031 mL; 0.52
_mol). After sPr~ing for 1.5 hr, the ~ Lu~e is partioned between dichlorompth~n~and dilute sodium hydroxide. The organic layers are dried with sodium sulfate and
cnnr.qnt~ ated. The residue is chrom~t~ hed (silica gel;
m~th~nol/dichlorom~ np~ 2/98) to give (+/-)-1-[2-t4-(4-methoxyphenyl)-1-
piperazinyl]ethyl]isochroman-6-ol, methyl carbamate ester (X-6) which after
cryst~lli7~t;~n from ether/h.oY~ne. MS (m/z) at 425; N~ (CDC13) 2.04, 2.12, 2.65,
2.90, 3.11, 3.75, 3.77, 4.11, 4.83, 4.98, 6.82-6.95 and 7.08 o.
EXAMPLE 96 (+/-)-1-[2-[4-[4-(~minnc~- Lollyl)phenyl]-l-pipelazi~lyl]ethyl]-N-
methylisochromanyl-6-carbnY~mi~a
Step 1. (+/-)-1-(2-Chlo~ueLllyl)isochroman-6-ol
To a ~lu~e of 3-hyd~uAylJh~n~thyl alcohol (Y-l) (0.074 g, 0.537 mmol) and
chlu~u~ o~)inn~ hyde diethyl acetal (0.107 g, 0.64 mmol) in nitromath~na (0.5 mL)
is added boron trifluoride-etherate (0.007 mL, 0.054 mmol). After stirring for 100
min, water is added and the llliAllu~c: iS partit.ionarl between dichloromath~na, water
and saline. The organic layers are dried with sodium sulfate and con~ntrated and
-114-
CA 02225282 1997-12-19
WO 97/022S9 PCT/US96/08681
the residue chrom~ .a,uhed (silica gel; ethyl acetate~hPY~ne, 10/90) to give (+/~
(2-chlùioeLllyl)isochroman-6-ol (Y-2) c~).,t~i..i..~ a small amount of (+/-)-1-(2-
ethu~yel~lyl)isochroman-6-ol as an i~ U~;~y. This m~tf~ is used without further
purifir~tinn in step 2, NMR (CDCl3) 2.15 - 2.38, 2.62, 2.68, 2.91, 3.6-3.8, 4.10, 4.89,
6 5.08, 6.59, 6.68 and 6.95 o.
Step 2. (+/-)-1-(2-Chlu~v~l~lyl)isochroman-6-ol,
trifluoromPth~n~clllfon~te ester
To a "';~I~U1e of (+/-)-1-(2-chl(JroeLllyl)isochroman-6-ol (Y-2, Step 1; 0.079 g,
0.371 mmol), triethylamine (0.0413 g, 0.408 mmol), 4-dimethylaminopyridine (0.0009
10 g, 0.0074 mmol) and dichlornmPt~np (1 ~) cooled at -78~ is added
trifuoromPth~nP~ulfonic acid anhydride (0.069 g, 0.408 mmol). The cooling bath is
then removed and the ~ lure is allowed to warm slowly to 20-25~. After stirring
for a total of 60 min, the ~u~e is par~;tinn~ b~Lwe ~ dichlornm~ ne and
aqueous ~mmnninm chloride. The organic layers are dried with sodium sulfate,
15 cnn-e..l-dted, and the residue chromAt4~.aphed (silica gel; ethyl acetate/hPYslnP
10/90) to give (+/-)-1-(2-chloloetLyl)isocl~l~an-6-ol, trifluorom~th~n~elllfon~t~ ester
(Y-3), NMR (CDCl3) 2.24, 2.31, 2.73, 2.78, 3.00, 3.67, 3.80, 4.12, 4.94 and 7.05-7.18
.
Step 3. (+/-)-1-(2-Chloroethyl)-N-methylisochroman-6-carbnY~mitl~
A ~ u~e of (+/-)-1-(2-chlOloelllyl)isochroman-6-ol, trifluorol.. ~Lh~nP~lllf~n~tP
ester (Y-3, Step 2; 0.291 g, 0.844 mmol) in D~ (1.5 mT ) is de-gassed under reduced
,ule~u~e for five minllte~, after which p~ linm (II) acetate (0.019 g, 0.084 mmol)
and 1,3-bis(diphenylrhn~rhinn)propane (0.52, 0.127 mmol) i8 added. Carbon
mnnnYi~le gas i8 bubbled in and diisoplo,uylethylamine (0.29 mL, 1.69 mmol) is
added. Methylamine gas is then bubbled in and the bath temperature is raised to
50~. The s~ lit;~)n of methylamine gas and carbon monnYi~ gas is cnntinll~i for 1
~, at which time an ~ ition~l p~ linm acetate (0.010 g) and 1,3-
bis(diphenylrho~phino)propane (0.025 g) are added. A~er an slrl~itin~l 4 hr the
~Lu~ is allowed to cool and then is partitionçd belwe~ ,l ether, aqueous
~mmonillm chloride, saline-~mmnnillm ~hlori~.q, and saline. The organic phases are
separated, dried over m~gn~illm sulfate and cnn~ç~ ted. Chrom~tography of the
residue (silica gel; m~l-~nol/dichlorom~t~l~n~, 2/98), gives (+/-)-1-(2-chloroethyl)-N-
methylisochroman-6-c~L~ (Y-4), NMR (CDCl3) 2.20, 2.33, 2.73, 2.79, 2.97,
3.01, 3.65, 4.12, 4.95, 6.09, 7.14, 7.53 and 7.56 ~.
Step 4. (+/-)-1-[2-[4-[4-(~minoc~rbonyl)phenyl]-1-piperazinyl]ethyl]-N-
methylisochromanyl-6-c~l,..~ ,.... i-
-115-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
A lllLl~llUL~ of (+/-)-1-(2-chloroethyl)-N-lllal~lyLsochroman-6 c~ 1e (Y~,
step 3; 0.0937 g, 0.369 mmol), 4-(piperazin-l-yl)hPn7~mi~e (Q-3, PREPARATION 1,
0.114 g, 0.554 mmol), diisoplvpylethylamine (0.0716 g, 0.554 mmol), sodium iodide
(0.007 g) and ethylene glycol (2 mL) is heated at 100~ for 6.5 hr, after which an
5 ~iti~nAl 0.056 g of 4-(piperazin-l-yl)bPn7~mi-1Q is added. After stirring for an
s3rl~itinnAl 24 hr at 100~, the ll~LI,u.a is allowed to cool (with stirring for the
rPm~inçr of the weekend). Water is then added and the l~lL~Lu~ is extracted withdichloromPth~ne The organic extracts are con~-..l ated and comhinecl with the
gummy residue (from which the aqueous layer has been flPr~nt~-l). The comhinP(l
10 crude product is chrom~tographed (silica gel; mpth~n~vdichlorompth~ne~ 6/94) to
give (+/-)-1-[2-[4-[4-(~minoc~rbonyl)phenyl]-1-piperazinyl]ethyl]-N-
methylisochromanyl-6-carb~Y~mi-le (Y-5), NMR (CDC13) 2.05, 2.18, 2.5-2.8, 3.02,
3.32, 3.77, 4.13, 4.88, 4.8-6.0, 6.12, 6.89, 7.16, 7.54 and 7.72 o.
~XAMPLE 97 (R)-(+)-1-[2-[4-[4-(AminocA . bullyl)phenyl]-1-piperazinyl]ethyl]-N-
methylisochromanyl-6-carbnY~mi~P
Separation of (+/-)-1-[2-[4-[4-(~minoc~rbonyl)phenyl]-1-piperazinyl]ethyl]-N-
methylisocl.lu~anyl-6-caLl,n~ lP (Y-5, ~AlvlpLE 96) into its plu8 and minus
~n~nt;,lmf-rs is achieved by preparative chroms~ . d,uhy on a chiral phase pre-
packed column using as solvent ethyl alcohoVisopLu~yl alcohoVtriethylamine in a
ratio of 4/1/0.08 (V/V) and using ~iPtection at 295 nM. Peak 1, (S)-(-)-1-[2-[4-[4-
(~minocslrbonyl)phenyl]-l-piperazinyl]ethyl]-N-methylisochromanyl-6-caLL
(F~AMPLE 6), eluted first, followed by Peak 2, R)-(+)-1-[2-[4-[4-
(~mino~,~ ~ IJunyl)phenyl]-l-piperazinyl]ethyl]-N-methylisochromanyl-6-carb~y~mirle
(Y-5), MS (m/z) 422.
~XAlVIPLE 98 (S)-(-)-1-[2-[4~4-Cyanophenyl)-1-piperazinyl]ethyl]-N-
methylisochroman-6-carb--Yslmi.lP
Step 1. 1-(4-Cyanophenyl)piperazine
A ~ure of 4-fluorobPn7~mifle (Q-2, 0.700 g, 5.78 mmol), piperazine (2.49 g,
28.9 mmol), and water (5 mL) is heated at 100~ for 85 min and then allowed to cool.
Water (5-10 mL) i8 added and the lllil~LUla is partiti~npcl b~waell ethyl ~et~t~aqueous sodium bicarbonate, and saline-aqueous sodium bicarbonate. The organic
layers are dried over m~FnPRil1m sulfate and con--r~ dted to give 1-(4-
cyanophenyl)piperazine (Q-3), NMR (CDCl3) 1.77, 3.02, 3.28, 6.85 and 7.49 o.
Step 2. (S)-(-)-2-(6-Br~moico~hroman-1-yl)ethyl alcohol
Borane-methyl sulfide (3.1 mL, 33.2 mmol) i8 added to a l~u~a of (S)-(-j-2-
(6-brom~icochroman-l-yl)acetic acid (XI, EXAMPLE 1, step 2; 3.0 g, 11 mmol) and
-116-
CA 02225282 l997-l2-l9
W O 97/02259 PCT~US96/08681
l~ (40 mL). GaR evolution and a moderate exotherm ensue. After stirring for 2.5
hr, mPth~nol is slowly added to quench excess borane-methyl sulfide. The nuxl ule
i8 then con. ~..l ated under reduced plesrlu.~ and m~th~nol is added to the residue.
The Illil~l,Ule again is conce..l-dted and m~th~ncll again added. After a final
5 cc...r~ -dtion from m~th~nnl, the residue is part~tion~(l between dichlorom~thsm~,
aqueous hydlocllloric acid, and aqueous sodium bicarbonate. The organic layers are
dried over sodium sulfate and conce--t ated. The residue is chromAtographed (silica
gel; m~th~nol/dichlorl m~th~n~, 4/96) to give (S)-(-)-2-(6-bromniRorhroman-1-yl)ethyl
alcohol (S-1), NMR (CDC13) 2.03, 2.20, 2.60-2.70, 3.02, 3.70-3.85, 4.16, 4.93, 6.93 and
10 7.29 ~.
Step 3. (S)-(-)-6-Bromo-1-(2-bromoethyl)isochroman
To a llli~l,Ul13 of triphenylrhr~sphine (9.60 g, 36.6 mmol), carbon tetrabromide(6.06 g, 18.3 mmol), and dichlorom~th~n.o (25 mT, cooled to about 20~ to control the
exotherm that occurs as the reagents are mixed) is added over 10 min (S)-(-)-2~6-
15 bromoisochroman-1-yl)ethyl alcohol (S-l, step 2; 2.35 g, 9.1 mmol) in
dichlorometh~ne (25 mL). The cooling bath is L~llWVt:d and the l~ ULe is stirredfor 40 min, at which time hexane is added dropwise until no more cloudiness
appear6. The ~U~t is allowed to stand overnight in the refrigerator and then thesolids are removed by filtration. The solids are washed with ether and the comhin~-l
20 filtrates are con~ e~lt- àLed and the residue is chrom~tc.~. aphed (silica gel; ethyl
slret~t~/heYs~n~o~ 10/90) to give (S)-(-)-6-bromo-1-(2-bromoethyl)isoclll.. an (T-1),
N~ (CDC13) 2.22 - 2.46, 2.66, 2.71, 2.94, 3.51, 3.62, 4.09, 4.84, 6.94 and 7.29 o.
Step 4. (S)-(-)-4-[4-[2-(6-BromniRorhroman-1-yl)ethyl]-1-
piperazinyl]ben7onitrilf.
A IlliX~,Ule of (S)-(-)-6-bromo-1-(2-bromoethyl)isochroman (T-1, step 3, 1.53 g,4.79 mmol), 1-(4-cyanophenyl)piperazine (Q-3, step 1, 0.987 g, 5.27 mmol),
diisoplol~ylethylamine (0.681 g, 5.27 mmol), and ethylene glycol (5 mL) is heated at
95~ for 4 hr and then at 20-25~ overnight The IlliXl,Ul~ is parfitinn~l between
dichlorom~th~ne and water and the organic layers are dried over sodium sulfate and
conr~ l ~ I - àted. The residue is chrom~t~ . aphed (silica gel;
mF~th~n- l/dichlornm~th~n~ 2/98) to give (S)-(-)-4-[4-[2-(6-brnm- iRorhroman-l-
yl)ethyl]-l-pipl .a~hlyl]b~n7onitrile (VI), N~ (CDCl3) 2.00, 2.12, 2.48-2.72, 2.95,
3.33, 3.73, 4.10, 4.80, 6.85, 6.96, 7.38 and 7.49 o.
Step 5. (S)-(-)-1-[2-[4-(4-Cyanophenyl)-1-pipel~i..yl]ethyl]-N-
3B methylisochroman-6-c&~L.. ,~
Following the general pl~CedU~ of ~XAMPLE 6, step 4, and m~king non-
-117-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
critical variations, (S)-(-)-4-[4-[2-(6-brom~iRochroman-l-yl)ethyl]-1-
piperazinyl]bP-n~o~itril~ (VI, step 4, 1.61 g) is cvllvs~ d to (S)-(-)-1-[2-[4~4-
cyanophenyl)-l-piperazinyl]ethyl]-N-methylisochroman-6-call,~.,c.1...itle (IX), MS (m/z)
at 404; IR (mineral oil, most intense peaks) 1603, 1635, 2210, 1517 and 1553 cm~l;
5 NMR (CDCl3) 2.05, 2.18, 2.48-2.78, 3.00, 3.01, 3.34, 3.76, 4.12, 4.88, 6.08, 6.85, 7.15,
7.49, 7.53 and 7.54 o.
EXAMPLE 99 (S)-(-)-1-[2-[4-[4-(~min~Arbonyl)phenyl]-1-piperazinyl]ethyl]-N-
methyl-N-(phenyl m Pth ~ ry)isochroman-6-cal 1, . . x ~ . . . i d e
Step 1. (S)-(-)-1-[2-[(TetrPhy.lLv~yl~l-2-yl)oxy3ethyl]isochroman-6-
c~LoAylic acid, methyl ester
A l~ixLu-e of (S)-(-)-1-(hydlvAyel~lyl)isoclllulll_n-6-c~LvAylic acid, methyl
ester (S-2, EXA~LE 81, step 2, 1.36 g, 5.76 mmol), dichloromPt~AnP (10 mL), p-
toluene sulfonic acid monohydrate (0.0142, 0.0747 mmol) _nd 3,4-dihydro-2H-pyran(1.6 mL, 0.0175 mol) is stirred at 20-25~ for 45 minutes. The ~ALu~è then i6
partit;~.ned between aqueous sodium bicarbonate and dichloromethAne. The
comhinP~l organic layers are dried over ~odium sulfate and con-~entrated under
reduced ple~ . The residue is chromAt4~- aphed (silica gel; ethyl acetate/hPYAnP,
5/95 to 15t85) to give (S)-(-)-1-[2-[(tetrahyd.opyl~-2-yl)oxy]ethyl]isochroman-6-
c&sbuAylic acid, methyl ester (W-2), NMR (CDCl3) 1.53, 1.69-1.80, 2.04, 2.23, 2.73-
2.79, 2.98, 3.51-4.13, 4.60, 4.92, 7.18, 7.80 and 7.83 o.
Step 2. (S)~-)-1-[2-[(Tetrahyd,~"uy.dll-2-yl)oxy]ethyl]isochroman-6-
c~LuAylic acid
A ~Lb~U~ e of (S)-(-)- 1-[2-[(tetrahyvl o~yl ~-2-yl)oAy]ethyl]isochroman-6-
c~buAylic acid, methyl ester (~7V-2, step 1, 1.55 g, 4.85 mmol), et~Anol (12 mL),
sodium hydroxide (2N, 3.6 mL, 7.2 mmol) and water (1 mL) is stirred for 6.5 hours
at 20-25~, at which time the ll iA~u~è is stored in the refrigerator overnight. The
lliALule is then stirred an Arlflit;onAl 2.5 hours at 20-25~ and then is concPntrated
under reduced plee. ule. Water (6 mL) i8 added and the re~lllt;ng ~ is cooled
in an icetwater bath. The pH of the ~lliALule is adjusted to pH 5 using hydlu~loric
acid (4N) and the r~lllffnE slurry is extracted with dichloromPt~An~. The cnmhinpd
organic layers are dried over mAgnPRillm sulfate and co~ce.ll-dted under reducede~ e to give (S)-(-)-1-[2-[(tetrahydlv,uy.dll-2-yl)oYy]ethyl]isochroman-6-carboYylic
acid (W-3), MS (m/z) = 306; N~ (CDCl3) 1.55, 1.70-1.84, 2.08, 2.26, 2.77-2.82, 3.01,
3.54, 3.66-4.17, 4.63, 4.96, 7.23 and 7.88 o.
Step 3. (S)-(-)-N-Methyl-N-(phenylmet~l- Yy)-1-[2-[(tetrahydr~,~yla,l-2-
yl)oAy]ethyllisocl;llolllan-6-c&lL.~ le
-118-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
1,1'-Carbonyl-liimi-1~7.olP (0.064 g, 0.39 mmol) is added to (S)-(-)-1-[2-
[(tetrallyd~u~y.~l-2-yl)oxy]ethyl]isochroman-6-ca~Ly~ylic acid (W-3, step 2; 0.109 g,
0.356 mmol) and THF (2 mL). The llli~l,U~e iS stirred at 20-26~ for 2 hr and then N-
methyl, O-benzylhydru~ylamine (Tetrahedron Letters, 30, 31-34 (1989), 0.054 g, 0.39
5 mmol) is added and the ~i~u~e: is stirred overnight at 60~. The ..--~lure then is
corfentrated and the residue is partitinnP-l between dichlornmPth~ne, water and
aqueous sodium bicarbonate. The organic layers are dried over sodium sulfate andconrP~n~rated. The residue is chrom~t~graphed (silica gel;
meth~nnl/dichlorQmPtl~nP 2/98) to give (s)-(-)-N-methyl-N-(phenylmpthnyy)-l-[2
10 t(tetrallyd.v,uy~an-2-yl)oxy]ethyl]isochroman-6-carbQY~mide (W4), NMR (CDCl3)1.50 - 1.65, 1.70-1.90, 2.07, 2.28, 2.67, 2.72, 2.92, 3.36, 3.53, 3.66-3.95, 4.02, 4.11,
4.62, 4.69, 4.92, 7.07-7.14, 7.30, 7.36 and 7.45 ~.
Step 4. (S)-(-)-1-(2-IIy-l~u2~.y~ yl)-N-methyl-N-
phenylmpthnyyisochroman-6-carbnys~mitlp
16 (S)-(-)-N-methyl-N-(phenylmPthnYy)-1-[2-[(tetrallyd,u~yldn-2-
yl)oxy]ethyl]isochroman-6-ca bn~1r..i~P (W-4, step 3; 0.131 g, 0.308 mmol) is stirred
at 20-25~ in a ~-~u~e of acetic acid/THF/water (4/2/1, 5 mL) for 2 hr and then at
60~ for 4 hr, at which time it is stored in the refrigerator overnight. The solvents
are then removed and the reslllting l~u~e is part tionpcl between dichlornmPth~nP
20 and water. The organic layers are dried with sodium sulfate and conf~..t-d~ed and
the residue is chrom~ ;- dphed (silica gel; mPthAnnl/dichlorompth~np~ 2/98) to give
crude product. NMR of this material in~ AtP~ the presence of tetrahyLopy,dr.yl
(THP)-like protons, so mPth~nol (2 mL) and p-tolllPnf~ulfonic acid hy.l~d~e (0.006 g)
are added and the l~ u~e is stirred overnight. The solvent is then removed under25 reduced pressure and the re~idue is partitinned between dichlorompth~ne and
aqueous sodium bicarbonate. The organic layers are dried over sodium sulfate andconcentrated to give (S)-(-)-1-(2-hyd~ ye~}lyl)-N-methyl-N-
phenylmPthnYyisochroman-6-c~l,..x~ 1P (W-5), NMR (CDCl3) 2.07, 2.25, 2.64, 2.70,
3.02, 3.38, 3.77, 3.86, 4.19, 5.01, 7.06, 7.08, 7.31, 7.37 and 7.46 o.
Step 5. (S)-(-)-1-[2-t4-[4-(~minncA-l,u.. yl)phenyl]-1-pipeld~ yl]ethyl]-N-
methyl-N-(phenylmPth-.Yy)isochroman 6 carbmf~mir~e
To an ice-cooled l~ LUL~ of (S)-(-)-1-(2-hydlu,~y~ yl)-N-methyl-N-
phenylmPtl n-xyisochroman-6-carbny~mirle (W-5, step 4; 0.099 g, 0.290 mmol),
diiso~Ouylethylamine (0.049 g, 0.377 mmol) and 4-dimethylaminopyridine (0.0018 g,
."
35 0.014 mmol) in dichlorr~mPthAnP (1.5 mL) is added mpthslnpsulfonyl ~-hlori~lP (0.0~3
g, 0.377 mmol) in dichloromPt~ne (0.5 mL). The llli2~u~ is stirred for 1.5 hr and
-119-
CA 0222~282 1997-12-19
W O 97/02259 PCT~US96/08681
then part;tionPr7. between dichloromPthslne and aqueous sodium bicarbonate. The
organic layers are dried with sodium sulfate and co7lrPntrated to give a mesylate (W-
6). To the mesylate is added 4-(piperazin-l-yl)bçn7slmi~7,e (Q-3, PREPARATION 1,0.071 g, 0.348 mmol), diisu,u~v~uylethylamine (0.075 mL, 0.580 mmol), and ethylene
glycol (0.3 mL). A smal amount of dichloromeths7ne is used to wash down the sides
of the flask. The mixture is heated at 85~ for 2.5 hr and then cooled. Water is
added and the ~ uLa is allowed to stand in the refrigerator overnight. The
supç7~s7t~nt is then rlecS7ntefl and the rPmslining residue is chromsltographed (silica
gel; methslnol/dichloromPthslne, 4/96 to 6/94) to give the title compound, NMR
(CDCl4) 2.08, 2.20, 2.5-2.75, 2.95, 3.34, 3.37, 3.76, 4.13, 4.69, 4.89, 5.7, 6.89, 7.10,
7.29, 7.47 and 7.72 o.
EXAMPLE 100 (S)-(-)-1-[2-[4-[4-(~mino~All,onyl)phenyl]-1-piperazinyl]ethyl]-N-
hyll~ v2~y-N-methylisochroman-6-carbo~rslmirle
A ~ Lula of (S)-(-)-1-[2-[4-[4-(s7minoc~l1,ollyl)phenyl]-1-piperazinyl]ethyl]-N-methyl-N-(phenylmPthn~y)isochroman-6-ca~ sl~ 7~e (W-7, ~XAlVIPLE 99, step 5,
0.067 g, 0.13 mmol), pslllslr7itlm on charcoal (10~, 0.0068 g) and mPths7nnl (3 mT ) is
stirred under ap~ ; sltPly one slt nnsphPre of hyvlu~;~n gas for one hour, at which
time ethyl acetate (1 mL) is added to aid in dissolving the starting material.
Stirring is continued under a llyd~ùgell sltmnsphpre~ and after 8 hr an sldr7iffnnSll
pslllsln7illm on charcoal (10%, 0.0068 g) is added. When the starting material is
consumed (about 28 hr) the psllls7~7il7m on charcoa. is filtered off and the filtrate i8
concf~ntrated. The residue is chromslt~graphed (si ica gel;
mPthslnnl/dichloromPt)lslnP, 8/92 to 15/85) to give (S)-(-)-1-[2-[4-[4-
(slminof slrbonyl)phenyl]-l-piperazinyl]ethyl]-N-llydLu~y-N-methylisochroman-6-
carbn~S7mide (W-8), NMR (DMSO) 1.89, 2.10, 2.45, 2.65-2.70, 2.85, 3.21, 3.66, 4.02,
4.77, 6.90, 6.98, 7.22, 7.36, 7.41, 7.68, 7.72 and 9.94 o.
EXAMPLE 101 (+/-)-1-[2-[4-[4-(Aminosu fonyl)phenyl]-1-piperazinyl]ethyl]-N-
methy isochroman-6-carbn~rslmide
Step 1. 4-(Piperazin-1-yl)ben7~nPslllfsnslmir7.e
A llliXl.Ul~, of 4-fluorobPn7enP~lllfsnslmide (Q-2, 6.95 g) and piperazine (17.1 g)
in water (30 mL) is heated at 100~ overnight. The solid is then collpcterl~ washed
with water and tolllPne, and dried under reduced pla~iu~a to give 4-(piperazin-1-
yl)bP-n7pneslllfonslmi~lp (Q-3), MS (m/z) = 241; IR (mineral oil, most intense peaks)
1160, 822, 1332, 608, 1593 and 1137 cm~l; NMR (DMSO) 2.81, 3.17, 2.3, 7.01, 7.07and 7.61 o.
Step 2. (+/-)-1-[2-[4-[4~Aminosulfonyl)phenyl]-1-piperazinyl]ethyll-N-
-120-
CA 02225282 1997-12-19
W O 97102259 PCTrUS96/08681
methylisochroman-6-caL 1, ~ ~ x i1 r. . i /1~
Following the general procedure of EXAMPLE 96 ~nd m~kin~ non-critical
variations, (+/-)-1-(2-Chloroethyl)-N-methylisochroman-6-carbnYslmi~la (Y-4
EXAMPLE 96, step 3, 0.024 g, 0.095 m-mol) and 4-(piperaziny-1-
5 yl)ben7~n~sll1f~n~mi~a (Q-3, Step 1) gives (+/-)-1-[2-[4-[4-(~minnslllfonyl)phenyl]-1-
piperazinyl]ethyl]-N-methylisochroman-6-carb~Y~mi~ (Y-5), NMR (CDCl3) 2.05,
2.19, 2.5-2.8, 3.01, 3.34, 3.42, 3.79, 4.16, 4.89, 6.41, 6.91, 7.18, 7.54, 7.56 and 7.76 ~.
Following the general procedure of EXAMPLE 94 (CHART V) and making
non-critical variations and using the rç~rt~nt~ corresponding to the products, the
10 compounds of EXAMPLES 102-104 are obtained:
EXAMPLE 102 (S)-(-)-N-Methyl-1-[2-[4-[4-(methylaminocarbonyl)phenyl]-1-
piperazinyl]ethyl]isochroman-6-carbnx~micla
EXAMPLE 103 (S)-(-)-N-Methyl-1-[2-[4-[4-(dimethyl~minoc~rbonyl)phenyl]-1-
piperazinyl]ethyl]isochroman 6 carbnYslmi.l.O
EXA ~ LE 104 (S)-(-)-N-Methyl-1-[2-[4-[4-(n-propyl~minoç~rbonyl)phenyl]-1-
piperazinyl]ethyl]isochroman-6-caLI,.,x~...i~1a
Following the general plocedu. e of EXAMPLE 100 and m~kin~ non-critical
variations and using the re~t~nt~ corresponding to the products, the culllpou~lds of
~AMpLEs 105-108 are obtained:
EXAMPLE 105 (S)-(-)-N-hyLv~y-N-methyl-1-[2-[4-[4-(trifluoromethyl)phenyl]-1-
pipelazillyl]ethyl]isochroman 6 carbn~ s~mi~la
EXAMPLE 106 (S)-(-)-1-[2-t4-(4-Chlorophenyl)-1-piperazinyl]ethyl]-N-hyd~u~y-
N-methylisochroman-6-c~uL~ 1e
EXAMPLE 107 (S)-(-)-1-t2-t4-(4-Cyanophenyl)-l-piperazinyl]ethyl]-N-hydroxy-N-
methylisochroman-6-carbnY~mi-le
EX~PLE 108 (S)-(-)-N-Hydroxy-N-methyl-l-t2-t4-[4-(methylcarbonyl)phenyl]-
1-piperazinyl]ethyl]isochroman-6-carbn~ ~micle
Following the procedul~ of CHART DD and making non-critical vs~ tion~:
known to those skilled in t,he art the compounds of EXAMPLES109 thru 120 are
obtained.
EX~PLE 109 (S)-4-[4-[2-t6-(1,2,4-Triazol-3-yl)-isochroman-1-yl]ethyl]-1-
piperazinyl]ban7 slmitla
li~xAlvlpLE 110 (S)-4-[4-[2-[6-(2-Methyl-1,2,4-triazol-3-yl)-isochroman-1-yl]ethyl]-
l-pipeL~zirlyl]ban7~mi~a
EXA ~ LE 111 (S)-4-[4-[2-[6-(1,2,4-OY~ 7Ol-5-yl)-isochroman-l-yl]ethyl]-l-
piperazinyl]ben7 ~mi~le
-121-
CA 0222~282 l997-l2-l9
W O 97/02259 PCT~US96/08681
lvrpLE 112 (S)-1-[2-[6-(1,2,4-Triazol-3-yl)isochroman-1-yl]ethyl~-4-[4-
trifluoromethylphenyl] -piperazine
EXAMPLE 113 (S)-1-[2-[6-(2-Methyl-1,2,4-triazol-3-yl)-isochroman-1-yl]ethyl]-4-
[4-trifluoromethylphenyl]piperazine
lil~AMpLE 114 (S)-1-[2-[6-(1,2,4-OY~ 7ol-5-yl)isochroman-1-yl]ethyl]-4-[4-
trifluoromethylphenyl] -piperazine
EXA~LE 115 (S)-1-[4-Acetylphenyl]-4-[2-[6-(1,2,4-triazol-3-yl)isochroman-1- yl]ethyl]piperazine
EX~MPLE 116 (S)-1-[4-Acetylphenyl]-4-[2-[6-(2-methyl-1,2,4-triazol-3-
yl)isochroman-1-yl]ethyl]piperazine
EXAMPLE 117 (S)-1-[4-Acetylphenyl]-4-[2-[6-(1,2,4-~Y~ 7ol-5-yl)isochroman-l- yl]ethyl] -piperazine
~XANIPLE 118 3-[1-[2-[4-(4-Aminocarbonylphenyl)piperazin-1-
yl]ethyl]isochroman-6-yl] -N,N-dimethylacrylamide
EXAMPLE 119 3-[1-t2-[4-(4-Trifluoromethylphenyl)piperazin-1-
yl]ethyl]isochroman-6-yl]-N,N-dimethylacrylamide
~AMPLE 120 3-[1-[2-[4-(4-Acetylphenyl)piperazin-l-yl]-ethyl]isochroman-6-yl]-
N,N-dimethylacrylamide
-122-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96108681
Fo~ru~,~s OF TFr~ rPT.~ #)
- R c
, ~ H2N ~ H N~ ~
~N N~OCH3 N~N~OCH3
(-)-endl ILio, I ~er (+)-ena"~io" ,er
E-1 O E-2
H ~O
H3C~N~C --N N~OCH3
15H ~O (+)-ena'~tiu,"el ~/
~N N~OCH3
(-)-ena, lliGI "er
E-3 ~N J~
~ IN' CH3 ~N~N~COI--NH2
25 H ~O S-(-) ena,lLio",er
~NAN~CF3
(-)-en~nLio",er\
E-5 O
30 H N--C--~ H2N,C [~
~--~N N~3CH3 N~N~CI
E-7
-123-
CA 02225282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
H N~ C ~
0 ~0
H2N~ ~ ~N N~O ~CH3
E-10
10 H2N~ ~--N~N~O--CH
N N~ CH2--CH3
CH2--CH3
15 o E-11 R
I l H2N~ ~
H2N ~CH~-NA~ CH3
O H ~
H2N' =l N N~CH2--CH3
E-15
~N N~CF3
(-)-enar,Li~l"er \_/ \=/
E-14
~l
3 ' IN~ ~
36 E 16\--J ~ ÇH3
-124-
CA 02225282 1997-12-19
W O 971022S9 PCTrUS96/08681
H3C~ N,C ~ ~7 ~ ~ \CF CH3
~N~ ~
H3C~N~Q~ (-)-ena"li~",el ~/ ~ ~~
N N~O
E-l9 \--/ \=/ ~CH2--CH3
~N~ ~C~ ~N~
--N~N~O E-22
E-21 ~CH2--CH3
1O,
H2N' ~
H3C~N~C~ N N~OCH3
N N~ CH3
-125-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
R ~--N~ ~
S H ~ ~N~N~OCH3
N N~OCH3
E-25 \--/
3 H ~ H ~N N~OCH3
16 NN4~OCH3
E-27 --/o \=/
~NH ~
o N N~OCH3
CH30~N--C ~ E-30 \J \=/
N N ~OCH3
E-29
CH3 ~l
~J'''l~NH~ ~
H3C~--NH~C~ N N~OCH3
N N~OCH3
-126-
CA 02225282 l997-l2-l9
W O 97/02259 PCTrUS96/08681
CH~ R W E-34 \J ~OOH3
N N~OCH3
E-33 \J
R H3C
~~ ~ ~N~N~OCH3
N N~OCH3
~ 2 HCI E-35 \--J o
~N ~ ~
'Nb~ ~ ~N~N~CI
26 N N~OCH3
E-37 ~ N~ N~
O H2N~ J~~ E 40
H3C ~ ~
N N~CI
~ 2 HCI E-39 \--/
-127-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
O O
ll ll
H N'C~l Cl~O
~N N~CI ~N N~F
E-41 ~/ E-42 ~/
H ~
NH2-COl ICH3 N3~3OCH3
~O O (-)-en~"li~r"el E-44
~N N~
~ 2 HCI Cl I / \=/
~ .52 CH2CI E-43
~.78 H20 H3C
~0
20R N -
H3C ~ ~N N~OCH3
N N~CF3
(-)-enar~ "el E-4s
H2N~ H2N~[~ ~3,OCH3
N N~OCH3 E-48
E-47
-128-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
o H3C' --N~[~ ~N~O~CH3
H' 'NH~,~N~ 3 E-50
o
H3C~C~N ~NJ~O'CH3
~ N~J
15 ~ N ~ ~ ~CHs
N~J
E-51 o
H3C C~
o ~ H ~ ~N~O~CH3
2 ~ 'N~[~,N~ E-54
E-53
H3C~ ~ ~N~ ~CH3
H3C~NH~ ~ 3 E-56
35E-55
-129-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
H3 IC
H C'N~--N ~[~O~CH
S H3C~ ~, ~ o E-sa
N~J C
10 H3C, E 57 H C' ~r~ ~N~o~CH3
/ ~N~ E-60
E-59 '-~
H3C~C_ ~ ~N~J O~CH3
H3C H3C~3~N~ CH3
E-61
H3C o
H3C~ ¦ l l
H3C' ~O~ 'NH~N~O'CH3
R N~J
H3C'NH' 'NH~N~J E-34
E-63
36
-130-
CA 02225282 l997-l2-l9
W O 971022S9 PCTrUS96/08681
H3C~
H'N/--~ N N~OCH3
N N~OCH3
E-65 \--/
H3C' ~
H C~O~C~ E-56 OCH3
NA ~OCH3
E-67 \--/
R H2N~
H~C~ E-70 --/ ~OCH3
N N~OCH3
E-69
H N~C~
H3C--N ~ '~N~N~OH
E 71 \--/ ~OCH3
-131-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
Il ,
O H =IO
H3C~N~C 1 ~N N~O-- --CF3
H ~o (~)~ena''liolller E-74 ~/ \=J' ~
(-)-ena,,liu,"er N~N~OH
o
HaC~N,C~'IN~ ~ ~ ~ ~O'CH3
(-)-ena,lliel"er ~N~c~oH3
H3C~
N--N
HN--N N~ ~~~
'CH3 E 78
E-77 ~ ~O~
N~ J~ ~CH3
E-7s N ~J
-132-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
O H ~O
H9C~N,C~ (-)-3nanlio",er ~ ~C/~CF3
N N~C//
(~)~ena''Liolller E 81--/ ~CH3
~
~ NH2--
~/N~C~ ~N~
16 N N4~OCH3 E-84
E-83 \--/ \=/
R
H ~ NH2--C~N~N~CI
N~ CH--COOH
CH--COOH
E-85
NH2--C~
H ~O ~N N~OCH3
~-~ ~NN~ E-88 Cl
E-87Cl
3~;
-133-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
o HO~H ~,0
3 ~N~ ~ ~N N~OCH3
~N N~OcH3E-9o
E-89 O Cl
H C--N~N,C~
f~ 'N~ ~, E-92 ~N~N~OCH3
E-91 1 \~ ~OCH3
ao 1~ OH3 ~N N~Q--O--C--CH3
OH ~O S-(-)-el~ar,Liu,,,er CH3
~N N~OCH3
E-93
N CH~mic E ~/ ~~--NH2
racemic N~N~30CH3
E-95
-134-
CA 02225282 1997-12-19
W O 97/022S9 PCTAUS96/08681
H~N~
CH3 ~N N~C--NH2 E-98
R-(+)-enanlior"er E97 ~/
CH~O
[~3~O~N~C~ S ( ) en~ al N~N~3C--NH2
O ~N N~CONH2
CH3 ~
racemic N N~S~2NH2
E-101
-135-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
C~AR.T A
Br~ OH (II)
Br ~ (III)
CO2C2H5
Br ~
C02H
A /=~ Rl
~ HN\W1~ R2
~~
2~ ~N~ ~W1~
Br ~
R1 (VI)
N\W1 ~ R2
-136-
CA 02225282 l997-l2-l9
W 097/022S9 PCTrUS96/08681
C~AR.T B
Br ~
N A W1 ~ ~2 \ ~VI)
H2N~
A ~ R~
N'JW' ~ R2 (VII)
o o
1~ (C~3),CO ~ ~ ~
(CH3)3CO O N~ W~ R1 ~VIII)
o
Q11
al2 ~ R1
N W, ~ R2 (IX)
26
O
Q13 - ~
N\--W1~ R2 (X)
-137-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
CT~AF~T C
6 ~ O (II)
CO2C2H5
Br ~O t ~
CO H ~
2 (XII) CO2C2H5
26
-138-
CA 02225282 1997-12-19
WO 97/OZ259 PCT/US96/08681
~AR.T n
'' 5 ~ (VI)
~ ~ RR~
(CH3)3 Si
~
~o (XIII)
~ /--\ /=~ R,
N~ Wl ~ R2
- ~-~ (XIV)
N/--\ ~ RR~
26 Q1
(XV)
Q1-2 ~
N~W1 ~ RR,2
-139-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
C~R.T ~.
Br
/ ~ R2
Q,~ - O ~ ~
~0
N W~ ~ (XVI)
~ ~ R~ ~VII)
~NJ~/~
1-2 N W,~ RR~ ~II)
26
N W,~ R
-140-
CA 02225282 1997-12-19
WO 97/02259 PCTIUS96/08681
C~AR.T F
Q.-3--~~
~--N/--\W ~ R2
~
N~ W~ ~
(X~)
o~
~N ~
N W,~ R,
R2
a, 3--~~
2~; N/--\W,~ (XXII)
IQ,,
~N~
N/--\W,~ ~2 (X~II)
-141-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
C~AR.T G
~0 1
~ N Wl ~ F'~ \ (X)
ll
Q13--O~
R~
N\--W1~ R2 (XXIV)
o
Ql'
~ R
N~W, ~ R2
~V)
Q1~
C
N~W,~ R,
-142-
CA 02225282 1997-12-19
WO 97/02259 PCT/US96/08681
C~AR.T ~
~,~
N~ ~ R~
'10 / 11~
NAW ~ R2 (XgVI)
Ql
16 N-- 5~
N W,~ RR2 (XXVII)
Ql-3--S~[~
I N W,~ RR~ (X2SVlll)
11~
N~Wl ~ R~
-143-
CA 02225282 l997-l2-l9
W O 97/02259 PCT~US96/08681
C~AR.T I
HOOC-- (CH2)n ~ -
~ R2
10HOCH2 (CH2)n ~,~
~ N W~ ~ R ! (X~)
15BrcH2(cH2)n ~
N A w~ XXII)
Q1 3SCH2 (CH2)n ~
A /=3~ R, (XXXI I 1)
N'JW' ~ R2
Q~ 3SCH2 (CH2)n ~
0 ~~
~N W~ R, (~X2~V)
-144-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
C~AR.T J
6SIcH2(cH2)n ~
N W~ R2 (XXXlI)
o
10NaO--S--CH2(CH2)n ~
/--\ /=~ 1
N W~ ~ R2
1~l
15Cl- SCH2(CH2)n ~
NWl~ (2~Vl)
o
Q1-.
N-- SCH2 (CH2)n ~
Ql2 ~ ~~
~ ~ R~ (X~Vll)
NW~ ~ R2
-145-
CA 02225282 1997-12-19
W O 97/022S9 PCTrUS96/08681
C~ART K
BrCH2 (CH2)n ~
~ ~ RR, ~I)
Ql~
0 N~NH
\~ X X is-CH~or-N~
Ql-s
N ~ N - CH2~C~2~n ~ D
lB \--/ ~ RR2 (X~Vlll)
-146-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
~,~AR.T T,
C2H5O (CH2)n ~
b,~o (XXXIX)
A /~ 1
N W, ~ R2
OH
N
~ ' Q16--C9 N
Q1-5 NH2
Q1~ N
\~ (CH2)
~--N~W,
.~
-147-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
C~AR.T ~
BrCH2 (CH2)n ~
N W, ~ ~X~II)
0 N _ CCH2(CH2)n ~Z,
N~W, ~ RR~(XLI)
~ CH2 (CH2ln ~
N~Wl ~ RR2(XLII)
O , ~ ~ A R
N~W,~ R2 (XLIII)
-148-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
C~AR.T N
N - CCH (CH2)n ~
N~W,~ ll2 (XLI)
NH
CCH2(CH2)n
, R, (XLII)
N ~W1 ~, R2
~~
~ \>_ CH2 (CH2)n
N~W, ~ R, (XLIII)
/ Q1-5 +
~ ~>--CH2 (CH2~n ~
2~; N W, ~ R2
-149-
CA 02225282 l997-l2-l9
W O 97/02259 PCT~US96/08681
C~AR.T O
H2NJ~ (VII)
~ N~ ~W
,~-5 0
3 ~ ,N NJ~
~N/--\ ~~R2 (0-1)
Q
N--N
Q~ 5 N~
~N w,~ (0-2)
N-0
--~ N'~C~
~N W, ~ 1 (0-3)
\--/ R2
-150-
CA 02225282 l997-l2-l9
W O 97/02259 PCTnUS96/08681
CE~A RT P
Q1s H ~ (P-l)
~ 1 ~~R
Q1-5
~
N W, ~ (P-2)
-151-
CA 02225282 1997-12-19
W O 97/022S9 PCTnUS96/08681
C~AR.T ~
H--N N--H (Q )
+
Br/F~EWG orBr/F~R2 (Q-2)
EWG
It--N~N~EWG ortl--N~N~
-152-
CA 02225282 1997-12-19
WO 97/022S9 PCT/US96/08681
C~RT R
R1 (R-l)
O2N~
~R2
I
~ R1
H2N~R2 (R-2)
I
HN N~
R2 (R-3)
-153-
CA 02225282 l997-l2-l9
W O 97/022S9 PCT~US96/08681
C~R.T S
(S-l)
OH
~ (S-2)
OH
Q1-1
Q1 ~ ~ (S-3)
OH
-154-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
C~AR.T T
COOH
Br~
~NJ~ (S-l) OH Br~C~
Q~-2 ~~ (S-3) ~ (X~ Br, OMs)
OH
N~ Br~
(Vl)
(X~ Br, OMs) ~
Ql 1 ~
N~
N Wl~
--/ R2
(IX)
~,
-155-
CA 02225282 1997-12-19
PCTnUS96108681
W O 97/022S9
C~A~T U
Br~ (U-l)
COOH
l~o (U-2)
COO-alkyl
Q~lO (U-3)
~1, COO-alkyl
Q~lO (Ul)
J~ COOH
Q1-l'N ~'~ (U-5)
OH
-166-
CA 02225282 1997-12-19
PCT/US96/08681
WO 97/02259
C~ART V
- 5~N~W~3 C--O-- (C~-G3)31kyl
0~N~N~W~ G--O-- (C~-C3)alhyl(V-2)
15H~N~N~W~OH
O
CH3~,N~W,~N--R
2~; R
-157-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
C~AR.T W
alkyl-O~
~ ~ (W-l)
O ~ ~OH
alkyl -O~
o ~ ~ O o (W-2)
HO~, ~
O ~ O O
~ ~ ~ (W-3)
alkyl-
alkyl ~o
o ~ ~ ~ ~ (W 4)
~~~ ~
~ O H (W-5)
o
alkyl-1 1 ~ (W-6)
~O'N ~ ~ W ~ (W-7)
~ N ~
HO~N ~ ~ w, (W-8)
-158-
CA 02225282 l997-l2-l9
W O 97/02259 PCT~US96/08681
C~AR.T ~
HZ, ~,~OH
W (X-l)
~~
J~ CO2alkyl (X-2)
0 HZ, ~
(X-3)
CO2H
HZ~ ~
N~J(X-4)
HZ~ R2
N~J
Q-2 Z2 ~ Z1 ~ ~W, (X-6)
-159-
CA 02225282 1997-12-19
W O 97/02259 PCTrUS96/08681
C~ART ~
~0
~ 1 ~A, (Vl) ~r
H2N~
NW1 ~R1 (Z-1 )
R2
H ~ \
Q~o2s ~C~
~ 1~ 1 N W, ~R,
(Z-2)
(Z-3)
o,, --N~W,~~ CH2~N~W,~
(Z-5)
,Q-2
~2 1 ~ Ql2--C~H2
ICH2 ~3Ço ICH2 ~0
30~N W1~R, Q--t ~NAW ~R,
(Z-6) (Z-7)
-160-
CA 02225282 1997-12-19
W O 97/022S9 PCT~US96/08681
C~AR~
Br ~
~ N W ~ ' (Vi)
H ~
~N W ~R, (AA-l)
\-- R2
HO--~
~N W,~ (A~-2)
N~C~
N W, ~ (AA-3)
H2N ~
N W, ~ (AA~)
Q, ~ (AA-S)
N W ~R'
-161-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
C~AR.T RR
Q1 1 ¦¦
Q--2 ~ (BB-l)
N Wl ~ R2
~ 10
Q1-1
16 N - CH2 - Xl ~ (BB-2)
Q12 ~ O
~ 1~ RR
-162-
CA 02225282 l997-l2-l9
W O 97/022S9 PCTrUS96/08681
C~AR.T CC
Q1- Il
~N~N w,~ (CC-l)
\ o~
Ph
O
Q1 -1 ,~
16 Q- 2 N W~ R~ (CC-2)
Q~ 1ll
1 ~0
Q--2 ~N W,~OSo2cF3 (CC-3)
Q1-1 11
~ IN~ ~ (lX)
l-2 N W~ R,
\--/ R2
-
-163-
CA 02225282 1997-12-19
W O 97/02259 PCT~US96/08681
C~ART DD
o
H2~ ~"
I
oP
0 M~2N NJ~ o (DD-2)
OP
N--N N--O
15 Q15 N~ ~o (DD-3) Q1~ N ~[~o (DD-5)
~P
N--O
N--N //
Q1 ei N~ (DD 4) ~1-6 N~ (DD~)
[--N~ W1~ N~JW1
-164-