Note: Descriptions are shown in the official language in which they were submitted.
CA 02225297 1997-12-19
1
TITLE OF THE INVENTION
Purification of Broken Frac Fluid
NAME ( S) OF INVENTOR ( S)
Shaun T. E. Mesher
Glenna L. Strickland
Dwight N. Loree
FIELD OF THE INVENTION
This invention relates to apparatus and method
used for the treatment of hydrocarbon streams, particularly
hydrocarbon streams that include a broken frac fluid.
BACKGROUND OF THE INVENTION
In the treatment of oil and gas wells by
fracturing, a frac fluid is applied to an underground
formation under sufficient pressure to form fractures in
the formation, and thus improve flow of oil and gas from
the formation into a well. It is desirable to retain the
frac fluid close to the well bore and for this reason the
frac fluids are made to gel when pressure is applied at
reservoir temperatures. The chemicals used to gel the frac
fluids contain considerable phosphate and metal
concentration. Upon completion of the fracturing treatment,
pressure is released, the frac fluid breaks and the broken
frac fluid is produced from the well along with reservoir
fluid.
When the well is produced, the well production
fluid is delivered to a refinery for refining into various
hydrocarbon fluids. In the refining process, the phosphates
have been found to cause contamination and plugging of the
refinery equipment. It has thus been found necessary either
to remove the phosphates from the chemicals used to gel the
frac fluid or remove them in the refinery itself. Customers
CA 02225297 1997-12-19
2
of Trysol Canada Ltd. have requested a solution to the
problem of removing phosphates from hydrocarbon streams. So
far as the applicant is aware, the producers of the
hydrocarbons have been unable to provide a satisfactory
solution.
SUMMARY OF THE INVENTION
The inventors have thus addressed the need for
removing phosphates from a hydrocarbon stream, particularly
a broken frac fluid.
Phosphates are removed from a hydrocarbon stream
by first contacting the hydrocarbon stream with a polar
material, wherein the polar material chemically reacts with
phosphates in the hydrocarbon stream and binds to the
phosphates and then separating the hydrocarbon stream from
the polar material.
Contacting the hydrocarbon stream with the polar
material preferably, particularly for large volume
applications at a refinery, may comprise passing the
hydrocarbon stream through a fixed filter bed composed of
polar material that is insoluble in water or oil, wherein
the hydrocarbon stream is separated from the polar material
upon passage through the fixed filter bed.
Contacting the hydrocarbon stream with the polar
material may also comprise, particularly for use at a well
site, mixing the hydrocarbon stream with an aqueous acid or
base solution.
Various polar materials may be used as described
in the following.
Apparatus for carrying out the process, either
with a filter bed in a pipe, or a container for mixing the
hydrocarbon stream with an acid or base is also provided.
CA 02225297 2007-06-12
2a
According to an aspect of the present invention,
there is provided a method of purifying a well production
fluid, wherein the fluid contains phosphates, the method
comprising the steps of:
contacting the well production fluid with a polar
material, wherein the polar material chemically reacts
with phosphates in the well production fluid and binds to
the phosphates; and
separating the well production fluid from the polar
material.
According to another aspect of the present
invention, there is provided a method of purifying a
hydrocarbon stream, wherein the hydrocarbon stream
contains phosphates, the method comprising the steps of:
mixing the hydrocarbon stream with an aqueous acid
or base solution, wherein the aqueous acid or base
solution chemically reacts with phosphates in the
hydrocarbon stream and binds to the phosphates; and
separating the hydrocarbon stream from the aqueous
acid or base solution.
According to a further aspect of the present
invention, there is provided an apparatus for purifying a
hydrocarbon stream, wherein the hydrocarbon stream
contains phosphates, the apparatus comprising:
a pipe; and
a filter bed in the pipe, the filter bed comprising
a polar material that is insoluble in water or oil,
wherein the polar material comprises alkaline carbonate
and chemically reacts with phosphates in the hydrocarbon
stream and binds to the phosphates.
CA 02225297 2007-06-12
2b
According to another aspect of the present
invention, there is provided an apparatus for purifying a
hydrocarbon stream, wherein the hydrocarbon stream
contains phosphates, the apparatus comprising:
a pipe; and
a plurality of filter beds in the pipe, at least one
of the filter beds comprising a polar material that is
insoluble in water or oil, wherein the polar material
comprises alkaline carbonate and chemically reacts with
phosphates in the hydrocarbon stream and binds to the
phosphates.
According to a further aspect of the present
invention, there is provided an apparatus for purifying a
hydrocarbon stream, wherein the hydrocarbon stream
contains phosphates, the apparatus comprising:
a mixer connected to receive the hydrocarbon stream,
wherein the mixer contains an acid or base which
chemically reacts with phosphates in the hydrocarbon
stream and binds to the phosphates.
CA 02225297 1997-12-19
3
These and other aspects of the invention are
described in the detailed description of the invention and
claimed in the claims that follow.
BRIEF DESCRIPTION OF THE DRAWINGS
There will now be described preferred embodiments
of the invention, with reference to the drawings, by way of
illustration only and not with the intention of limiting
the scope of the invention, in which:
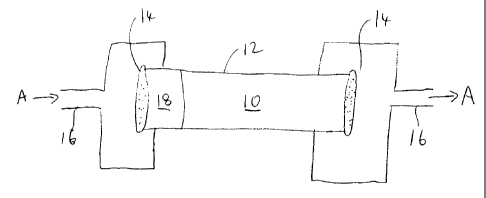
Fig. 1 shows a filter for use in treating
hydrocarbon streams according to an aspect of the
invention;
Fig. 2 shows a series of filter beds in a heat
exchanger according to an aspect of the invention;
Fig. 3 shows apparatus for aqueous treatment of
a hydrocarbon stream according to an aspect of the
invention;
Fig. 4 is a graph showing metals present in a
first untreated broken frac fluid;
Fig. 5 is a graph showing metals present in the
first broken frac fluid after treatment according to an
aspect of the invention in a continuous stirred tank
reactor;
Fig. 6 is a graph showing metals present in the
first broken frac fluid after treatment according to an
aspect of the invention in a moving burden bed reactor;
Fig. 7 is a graph showing metals present in a
second untreated broken frac fluid;
Fig. 8 is a graph showing metals present in the
second broken frac fluid after treatment according to an
aspect of the invention in a continuous stirred tank
reactor;
CA 02225297 1997-12-19
4
Fig. 9 is a graph showing metals present in the
second broken frac fluid after treatment according to an
aspect of the invention in a moving burden bed reactor;
Fig. 10 is a graph showing phosphate removal from
a continuous hydrocarbon stream passing through a first bed
of alumina and calcium carbonate; and
Fig. 11 is a graph showing phosphate removal from
a continuous hydrocarbon stream passing through a second
bed of alumina and calcium carbonate.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
This invention is particularly applicable to
purification of a hydrocarbon stream in a refinery where
production fluid from an oil reservoir is being refined.
The process may also be used elsewhere in the oil
production process, including at an oil well site before
introduction of the hydrocarbon stream into a pipeline. The
hydrocarbon stream is contacted with a polar material that
reacts with phosphates in the hydrocarbon stream and binds
to the phosphates. For example, any alkali metal salt that
is insoluble in water or oil is believed to work.
Exemplary polar materials are silica, silicates,
alumina, aluminates and alkiline carbonates, particularly
calcium carbonate. A base is preferred to an acid. Base or
acid as used herein refers to a Bronsted acid or base or
Lewis acid or base.
As shown in Fig. 1, a preferred manner of
contacting the hydrocarbon stream with a polar material
comprises passing a hydrocarbon stream A through a fixed
filter bed 10 composed of polar material that is insoluble
in water or oil. The filter bed 10 is formed within an
enlarged pipe 12, enclosed within frits 14. The frits 14
are stainless steel plates perforated with holes having a
small diameter, eg of about 2 m. The fixed filter bed 10
CA 02225297 1997-12-19
is preferably made from a packed powder form of the polar
material held in place in the tubing 16 of a refinery, such
that the hydrocarbon stream may be processed continuously.
The powder form of the polar material should have a
5 permeability that does not unduly block fluid flow through
the filter, yet should present high surface area of the
polar material to the hydrocarbon stream. Mesoporous polar
material, particularly mesoporous silica and/or calcium
carbonate is believed to provide satisfactory flow rates
and phosphate removal rates. Mesoporous means a material
having a mean hole diameter from 60 Angstroms to 150
Angstroms. The relative diameters of the pipes 16 and 12
are dependent on flow rate and desired residence time.
Greater diameter, and hence greater residence time, may be
preferred for increased removal of phosphates.
The filter bed 10 may be a single homogenous
polar material, may be formed of a mixture of one or more
polar materials, may include a filler of non-polar material
and may be formed of layered polar material. For example,
the polar material may be powdered mesoporous alumina,
which has been found to work well. A filler such as calcium
carbonate may be used in a mixture with alumina (eg 75% by
weight alumina and 25% by weight filler) to provide a flow
path for the fluid. It has been found that the phosphate
content of the hydrocarbon stream drops rapidly upon
introduction to the filter bed 10 made of alumina, with
removal of in the order of 98% of the phosphates, but the
filter bed 10 appears to reach a saturation point after
which a reduced level, eg. 50%, of phosphates is obtained.
The saturation point is believed to result from blocking of
the pores of the polar material with contaminants in the
hydrocarbon stream. This reduces the active surface area
for phosphate binding reactions to occur.
CA 02225297 2006-04-10
6
Layers of filter material may be used. For
example, an initial bed 18 of activated carbon may be used
to clean contaminants from the hydrocarbon stream and
prolong the efficacy of the filter material.
For increased time of high removal rates, several
such filter beds 10 may be used in series, such as
illustrated in Fig. 2. To further reduce the contamination
of the filter bed, removable filter bed 22 with activated
carbon may be used upstream of the other filter beds 10
to remove contaminants. The activated carbon is believed
to bind the contaminants, which then do not block the pores
of the polar material. These filter beds 10 may be housed
in a heat exchanger 24, and the process operated at elevated
temperature, for example 50 C.
Any of the filter beds 10 in a series may be made
removable. Some kinds of filter material such as alumina
may be reused by heating the filter medium to drive off
carbon material.
In a further example of an aspect of the invention,
shown in Fig. 3 schematically, an embodiment of the process
may be operated using existing equipment at a well site
configured uniquely. Acid is often used in well treatment
operations. In this embodiment, the hydrocarbon stream
B produced from the well is directed to an acid bath/mixer
30 where it is mixed with acid, such as hydrochloric acid.
A chemical reaction occurs in which the phosphate becomes
polar and dissolves in the hydrochloric acid. The mixture
of hydrocarbon stream and hydrochloric acid is then
separated in for example a hydrocyclone 32. The
hydrochloric acid may then be reused in the acid bath/mixer.
The hydrocarbon stream may then be dried conventionally
to meet pipeline requirements. A base may be used in the
acid mixer, though since use of acid is common at well
sites, use of acid may be more convenient.
CA 02225297 1997-12-19
7
EXAMPLES
In example 1, a broken frac fluid from an oil
well having total metal content of 199 ppm and total
phosphate content of 101 ppm (as shown in Fig. 4) was
treated by contact with a polar material consisting of
calcium carbonate impregnated onto silica (Si02) in a
continuous stirred tank reactor. Approximately 5 g of polar
material was stirred with 100 g of hydrocarbons. After
treatment, 0.8 ppm total phosphate remained and 1.7 ppm
total metals remained (as shown in Fig. 5).
In example 2, the same broken frac fluid as in
example 1 (Fig. 4) was treated by contact with a mixture of
about 20 g fumed silica and calcium carbonate in a moving
burden bed reactor. After treatment, 0.8 ppm total
phosphate remained and 1.7 ppm total metals remained (Fig.
6).
In example 3, a broken frac fluid from a lab
preparation which was gelled and broken in the lab, wherein
the broken frac fluid had total metal content of 636 ppm
and total phosphate content of 523 ppm (as shown in Fig. 7)
was treated by contact with the same material as in example
1 in a continuous stirred tank reactor under the same
conditions. After treatment, 8.7 ppm total phosphate
remained and 10 ppm total metals remained (as shown in Fig.
8).
In example 4, a broken frac fluid as in example
3 (Fig. 7) was treated by contact with the same polar
material as in example 2 in a moving burden bed reactor.
After treatment, 14.8 ppm total phosphate remained and 14.8
ppm total metals remained (Fig. 9).
In example 5, a hydrocarbon stream containing 65
ppm phosphate was passed at a flow rate of 0.5 mL/min
through a filter bed containing 21.9 g of polar material
consisting of 75 wt% alumina (A1203) and 25 wt% calcium
CA 02225297 1997-12-19
8
carbonate (CaCO3). Samples were taken at 30 min intervals
for a period of 6 hours. Initially, 100% phosphates were
removed, and after 5 hours efficacy of the filter began to
be degraded, with reduction in % removal.
In example 6, a hydrocarbon stream containing 65
ppm phosphate was passed at a flow rate of 0.5 mL/min
through a filter bed containing 101 g of polar material
consisting of 75 wt% alumina (A1203) and 25 wt% calcium
carbonate (CaCO3). Samples were taken at 1 hr intervals for
a period of 43 hours. Initially, 100% phosphates were
removed, and after 5 hours efficacy of the filter began to
be degraded, with reduction in % removal to 50% removal
after about 29 hours.
The mechanisms according to which the phosphates
become bound to the polar material is illustrated below for
the following cases:
I Removal of trialkyl phosphate with basic silica.
Oxygen in the silica carries out a nucleophilic attack
on the phosphorus. The resulting five member charged
phosphorus species rearranges to reform the double
oxygen bond and displace an alcohol.
II Removal of trialkyl phosphate with basic alumina. A
similar reaction occurs as with the silica.
III Removal of dialkyl phosphate with basic silica or
basic alumina.
IV Removal of monoalkyl phosphate with basic silica or
basic alumina
V Removal of trialkyl phosphate with acidic silica or
alumina. A lone pair of electrons on the
oxygen/phosphorus member attracts a proton and creates
CA 02225297 1997-12-19
9
a positive charge on the phosphorus. Nucleophilic
attack is made by the oxygen on the alumina/silica on
the phosphorus to create a five member phosphorus
species. A double bond to the oxygen is reformed,
which displaces a RO group.
VI Phosphate removal by Base Extraction. Calcium binds to
an oxygen and displaces hydrogen.
Phosphate removal by acid extraction may be
obtained when HC1 carries out the reaction shown in V, and
generates an extremely acid soluble phosphate. Silica may
also bond to the phosphate by hydrogen bonding, wherein the
hydrogen is bound to both an oxygen on the silica and an
oxygen in the phosphate.
CA 02225297 1997-12-19
- lo _
Removal of Phosphates with Basic or Acidic Silica or Alumina
Basic: I i
- Si -O-Ca+ -AI-O Ca'
Trialkyl
Phosphates
~O (:~O
RO-P-OR RO-P-OR
OR OR
I 1:
\ I- \ ,
si AI_
U= o O.
R ~P-OR RO-P-OR
OR OR
O"Si O~- AI-
I I
ROCa + O=P-OR ROCa + 0=P-OR
OR OR -
Dialkyl Phosphates
Si
O O O-1AI-
~
I ~
HO-P-OR ROCa + 0=P-OR or 0=P-OR ~
OR OH OH
Monoalkyl Phosphates
I~
O O~Si O~-AI- I ~
HO-P-OR
, = ROCa + O=P-OH or O=P-OH
OH OH OH
CA 02225297 1997-12-19
- /pA -
Phosphate Removal By Base Extaction
O O
RO-P-OH CaCO3 . RO-P-O-Ca` v I
OR OR
Any alkaline metal should accomplish this task
@ U
AL - UN2
O
Ro=p -oR
~
o2
f~I-O
pH ~
R o -` P - 02
oR
L
vA
GP,
~
+
0= P - vK
H 0
(Zo+-t p= P -OK
o
CA 02225297 1997-12-19
11
A person skilled in the art could make immaterial
modifications to the invention described in this patent
document without departing from the essence of the
invention that is intended to be covered by the scope of
the claims that follow.