Note: Descriptions are shown in the official language in which they were submitted.
CA 0222~763 1997-12-23
PSEUDO-PLUG-FLOW REACTOR
CROSS-REFERENCE TO RELATED APPLICATION
The present application claims priority from U.S. Provisional
Application No. 60/033,831 filed December 23, 1996, and from U.S. Non-Provisional
Application No. 08/861,210 filed May 21, 1997, which applications are incorporated
herein by reference in their entireties.
10 TECHNICAL FIELD
This invention relates to devices for controlling reactions with special
emphasis to oxidations. The reactor of this invention behaves in a manner similar to the
manner that a plug-flow reactor behaves.
15 BACKGROUND OF THE INVENTION
Plug-flow reactors and processes are well known to the art. They are
usually characterized by their ability to allow a mixture of reactants to undergo a
reaction as the mixture flows from one end to the other end of the reactor without any
substantial back mixing. In order to achieve conditions which allow plug-flow, it is
20 important that the reactants are homogeneous, in either gaseous or liquid state
(including dispersions, aerosols, and homogeneous powder or dust containing gases),
and preferably in one phase. If all reactants are in the same liquid phase, which flows
from one end to the other end of the reactor, back mixing may be avoided more easily
than in the case that all reactants are in the gaseous phase, because of molecular
25 mobility reasons.
If one reactant is in a liquid state and the other in gaseous state, it
becomes extremely difficult, if not impossible to produce plug-flow conditions with the
presently known techniques.
There are many reactions, which require that one reactant is in the liquid
30 state, while the other reactant is in the gaseous state. One example is the case of
miscellaneous oxidations, wherein for example a hydrocarbon is oxidized to form an
CA 0222~763 1997-12-23
intermediate oxidation product. In such cases, the present art utilizes stirred-tank
conditions, which lack completely the advantages of plug-flow conditions. Our recent
patents and patent applications on atomization technology, although they provide a
great advance in the art of reactions in general, with special emphasis in oxidations, are
5 not characterized by plug-flow conditions.
There is a plethora of references (both patents and literature articles)
dealing with the formation of intermediate oxidation products, such as diacids, for
example, one of the most important being adipic acid. Adipic acid is used to produce
Nylon 66 fibers and resins, polyesters, polyurethanes, and miscellaneous other
1 0 compounds.
There are different processes of manufacturing adipic acid. The
conventional process involves a first step of oxidizing cyclohexane with oxygen to a
mixture of cyclohexanone and cyclohexanol (KA mixture), and then oxidation of the
KA mixture with nitric acid to adipic acid. Other processes include, among others, the
15 "Hydroperoxide Process," the "Boric Acid Process," and the "Direct Synthesis
Process," which involves direct oxidation of cyclohexane to adipic acid with oxygen in
the presence of solvents, catalysts, and initiators or promoters.
Initiators or promoters are presently being used to shorten considerably
an induction period at the beginning of the reaction. Accepted explanations, which
20 have been given regarding the role of the initiators or promoters, involve oxidation of
the catalyst, which is usually cobaltous ions to cobaltic ions.
The Direct Synthesis Process has been given attention for a long time.
However, to this date it has found little commercial success. One of the reasons is that
although it looks very simple at first glance, it is extremely complex in reality. Due to
25 this complexity, one can find strikingly conflicting results, comments, and views in
different references.
It is well known that after a reaction has taken place according to the
Direct Synthesis Process, a mixture of two liquid phases is present at ambient
temperature, along with a solid phase mainly consisting of adipic acid. The two liquid
30 phases have been called the "Polar Phase" and the "Non-Polar" phase. However, no
CA 0222~763 1997-12-23
attention has been paid so far to the importance of the two phases, except for separating
the adipic acid from the "Polar Phase" and recycling these phases to the reactor partially
or totally with or without further treatment. Further, no attention has been paid to the
behavior of catalyst, such as solubility, for example, during reaction conditions.
It is also important to note that most, if not all, studies on the Direct
Synthesis Process have been conducted in a batch mode, literally or for all practical
purposes.
There is a plethora of references dealing with oxidation of organic
compounds to produce acids, such as, for example, adipic acid, benzoic acid, phthalic
10 acid, isophthalic acid, terephthalic acid, etc.
The following references, among the plethora of others, may be
considered as representative of oxidation processes relative to the preparation of
diacids, and especially adipic acid.
U.S. Patent 5,463,119 (Kollar) discloses a process for the oxidative
15 preparation of C5-C8 aliphatic dibasic acids by
( 1 ) reacting,
(a) at least one saturated cycloaliphatic hydrocarbon having
from 5 to 8 ring carbon atoms in the liquid phase and
(b) an excess of oxygen gas or an oxygen-containing gas in
20 the presence of
(c) a solvent comprising an organic acid containing only
primary and/or secondary hydrogen atoms and
(d) at least about 0.002 mole per 1000 grams of reaction
mixture of a polyvalent heavy metal catalyst;
(2) removing the aliphatic dibasic acid; and
(3) recycling intermediates, post oxidation components, and
derivatives thereof rem~ining after removal of the aliphatic dibasic acid into the
oxidation reaction.
U.S. Patent 5,374,767 (Drinkard et al) discloses formation of
30 cyclohexyladipates in a staged reactor, e.g, a reactive distillation column. A mixture
CA 0222~763 1997-12-23
containing a major amount of benzene and a minor amount of cyclohexene is fed to the
lower portion of the reaction zone and adipic acid is fed to the upper portion of the
reaction zone, cyclohexyladipates are formed and removed from the lower portion of
the reaction zone and benzene is removed from the upper portion of the reaction zone.
The reaction zone also contains an acid catalyst.
U.S. Patent 5,321,157 (Kollar) discloses a process for the preparation of
C5- C8 aliphatic dibasic acids through oxidation of corresponding saturated
cycloaliphatic hydrocarbons by
(1) reacting, at a cycloaliphatic hydrocarbon conversion level of
10 between about 7% and about 30%,
(a) at least one saturated cycloaliphatic hydrocarbon having
from 5 to 8 ring carbon atoms in the liquid phase and
(b) an excess of oxygen gas or an oxygen cont~ining gas
mixture in the presence of
(c) less than 1.5 moles of a solvent per mole of cycloaliphatic
hydrocarbon (a), wherein said solvent comprises an organic acid containing only
primary and/or secondary hydrogen atoms and
(d) at least about 0.002 mole per 1000 grams of reaction
mixture of a polyvalent heavy metal catalyst; and
(2) isolating the C5 - C8 aliphatic dibasic acid.
U.S. Patent 5,221,800 (Park et al.) discloses a process for the
manufacture of adipic acid is disclosed. In this process, cyclohexane is oxidized in an
aliphatic monobasic acid solvent in the presence of a soluble cobalt salt wherein water
is continuously or intermittently added to the reaction system after the initiation of
25 oxidation of cyclohexane as indicated by a suitable means of detection, and wherein the
reaction is conducted at a temperature of about 50~C to about 150~C at an oxygen partial
pressure of about 50 to 420 pounds per square inch absolute.
U.S. Patent 3,987,100 (Barnette et al.) describes a process of oxidizing
cyclohexane to produce cyclohexanone and cyclohexanol, said process comprising
30 contacting a stream of liquid cyclohexane with oxygen in each of at least three
CA 0222~763 1997-12-23
successive oxidation stages by introducing into each stage a mixture of gases
comprising molecular oxygen and an inert gas.
U.S. Patent 3,957,876 (Rapoport et al.) describes a process for the
preparation of cyclohexyl hydroperoxide substantially free of other peroxides by5 oxidation of cyclohexane containing a cyclohexane soluble cobalt salt in a zoned
oxidation process in which an oxygen containing gas is fed to each zone in the
oxidation section in an amount in excess of that which will react under the conditions of
that zone.
U.S. Patent 3,932,513 (Russell) discloses the oxidation of cyclohexane
10 with molecular oxygen in a series of reaction zones, with vaporization of cyclohexane
from the last reactor effluent and parallel distribution of this cyclohexane vapor among
the series of reaction zones.
U.S. Patent 3,530,185 (Pugi) discloses a process for manufacturing
precursors of adipic acid by oxidation with an oxygen-containing inert gas which15 process is conducted in at least three successive oxidation stages by passing a stream of
liquid cyclohexane maintained at a temperature in the range of 140 ~C to 200 ~C and a
pressure in the range of 50 to 350 p.s.i.g. through each successive oxidation stage and
by introducing a mixture of gases containing oxygen in each oxidation stage in an
amount such that substantially all of the oxygen introduced into each stage is consumed
20 in that stage thereafter causing the residual inert gases to pass countercurrent into the
stream of liquid during the passage of the stream through said stages.
U.S. Patent 3,515,751 (Oberster et al.) discloses a process for the
production of epsilon-hydroxycaproic acid in which cyclohexane is oxidized by liquid
phase air oxidation in the presence of a catalytic amount of a lower aliphatic carboxylic
25 acid and a catalytic amount of a peroxide under certain reaction conditions so that most
of the oxidation products are found in a second, heavy liquid layer, and are directed to
the production of epsilon-hydroxycaproic acid.
U.S. Patent 3,361,806 (Lidov et al) discloses a process for the production
of adipic acid by the further oxidation of the products of oxidation of cyclohexane after
30 separation of cyclohexane from the oxidation mixture, and more particularly to stage
CA 0222~763 1997-12-23
wise oxidation of the cyclohexane to give high yields of adipic acid precursors and also
to provide a low enough concentration of oxygen in the vent gas so that the latter is not
a combustible mixture.
U.S. Patent 3,234,271 (Barker et al) discloses a process for the
5 production of adipic acid by the two-step oxidation of cyclohexane with oxygen. In a
preferred embodiment, mixtures comprising cyclohexanone and cyclohexanol are
oxidized. In another embodiment, the process involves the production of adipic acid
from cyclohexane by oxidation thereof, separation of cyclohexane from the oxidation
mixture and recycle thereof, and further oxidation of the other products of oxidation.
U.S. Patent 3,231,608 (Kollar) discloses a process for the preparation of
aliphatic dibasic acids from saturated cyclic hydrocarbons having from 4 to 8 cyclic
carbon atoms per molecule in the presence of a solvent which comprises an aliphatic
monobasic acid which contains only primary and secondary hydrogen atoms and a
catalyst comprising a cobalt salt of an organic acid, and in which process the molar ratio
of said solvent to said saturated cyclic hydrocarbon is between 1.5:1 and 7:1, and in
which process the molar ratio of said catalyst to said saturated cyclic hydrocarbon is at
least 5 millimoles per mole.
U.S. Patent 3,161,603 (Leyshon et al) discloses a process for recovering
the copper-vanadium catalyst from the waste liquors obtained in the manufacture of
adipic acid by the nitric acid oxidation of cyclohexanol and/or cyclohexanone.
U.S. Patent 2,565,087 (Porter et al) discloses the oxidation of
cycloaliphatic hydrocarbons in the liquid phase with a gas containing molecular oxygen
and in the presence of about 10% water to produce two phases and avoid formation of
esters.
U.S. Patent 2,557,282 (Hamblet et al) discloses production of adipic acid
and related aliphatic dibasic acids; more particularly to the production of adipic acid by
the direct oxidation of cyclohexane.
U.S. Patent 2,439,513 (Hamblet et al) discloses the production of adipic
acid and related aliphatic dibasic acids and more particularly to the production of adipic
acid by the oxidation of cyclohexane.
CA 0222~763 1997-12-23
U.S. Patent 2,223,494 (Loder et al) discloses the oxidation of cyclic
saturated hydrocarbons and more particularly to the production of cyclic alcohols and
cyclic ketones by oxidation of cyclic saturated hydrocarbons with an oxygen-containing
gas.
5U.S. Patent 2,223,493 (Loder et al) discloses the production of aliphatic
dibasic acids and more particularly to the production of aliphatic dibasic acids by
oxidation of cyclic saturated hydrocarbons with an oxygen-containing gas.
German Patent DE 44 26 132 A 1 (Kysela et al.) discloses a method for
dehydration of process acetic acid from the liquid-phase oxidation of cyclohexane with
10air, in the presence of cobalt salt as a catalyst after separation of the adipic acid by
filtration and the cyclohexane phase by phase separation, while simultaneously avoiding
cobalt salt precipitates in the dehydration column, characterized in that the acetic acid
phase to be returned to the beginning of the process is subjected to azeotropic
distillation by the use of added cyclohexane, under distillative removal of the water
15down to a residual content of less than about 0.3 to 0.7 wt %.
PCT Demand International publication WO 96/03365 (Costantini et al.)
discloses a method of recycling a cobalt-cont~ining catalyst in a reaction involving the
direct oxidation of cyclohexane into adipic acid using an oxygen containing gas. The
method is characterized in that the reaction mixture, obtained in a preceding stage
20where the cyclohexane was oxidized into adipic acid, of which at least part of the
intermediate oxidation products, such as cyclohexanol and cyclohexanone, the
carboxylic acid and water has been separated and of which at least part of the adipic
acid formed has been recovered by cryst~lli7~tion, undergoes at least one extraction
operation using at least one cosolvent or a mixture comprising a cosolvent and a25carboxylic acid. The method is also characterized by the separation of a mixture
containing at least part of the cobalt catalyst, part of the carboxylic acid and optionally
residual quantities of other compounds and a solution containing the co-solvent and at
least part of the glutaric and succinic acids formed during the oxidation reaction, and
the carboxylic acid.
CA 0222~763 1997-12-23
The following references, among others, describe processes conducted in
intermixing liquid with gaseous materials, mostly under increased surface area
conditions.
U.S. Patent No. 5,399,750 (Brun et al.) discloses methods for preparing
5 maleamic acid (aminomaleic acid) by reacting gaseous ammonia with molten maleic
anhydride under reactant contact conditions of high surface area, for example reacting
said gaseous NH3 with a thin film of said molten maleic anhydride or with said molten
maleic anhydride in a state of vigorous agitation.
U.S. Patent No. 5,396,850 (Connote et al.) discloses a method of
10 destroying organic waste in a bath of molten metal and slag contained in a vessel. The
method comprises injecting organic waste into the bath to form a primary reaction zone
in which the organic waste is thermally cracked and the products of the thermal
cracking which are not absorbed into the bath are released into the space above the
surface of the bath. The method further comprises injecting an oxygen-containing gas
15 toward the surface of the bath to form a secondary reaction zone in the space above the
surface of the bath in which the oxidizable materials in the products from the primary
reaction zone are completely oxidized and the heat released by such oxidation istransferred to the bath. In order to facilitate efficient heat transfer from the second
reaction zone to the bath, the method further comprises injecting an inert or other
20 suitable gas into the bath to cause molten metal and slag to be ejected upwardly from
the bath into the secondary reaction zone.
U.S. Patent No. 5,312,567 (Kozma et al.) discloses a complex mixing
system with stages consisting of propeller mixers of high diameter ratio, where the
blades are provided with flow modifying elements, whereby the energy proportions25 spent on dispersion of the amount of gas injected into the reactor, homogenization of
the multi-phase mixtures, suspension of solid particles, etc. and the propertiescorresponding to the rheological properties of the gas-liquid mixtures and to the special
requirements of the processes can be ensured even in extreme cases. Open channels
opposite to the direction of rotation are on the blades of the dispersing stage of the
30 propeller mixers fixed to a common shaft, where the channels are interconnected with
CA 0222~763 1997-12-23
gas inlet. The angle of incidence of a certain part of the blades of mixing stages used
for homogenization and suspension is of opposite direction and the length is shorter
and/or the angle of incidence is smaller than those of the other blades. Baffle bars are
on the trailing end of the blades on a certain part of the propeller mixers used similarly
for homogenization and suspension, and/or auxiliary blades at an angle of max. 20~ to
the blade wings are arranged above or below the trailing end of the blades.
U.S. Patent No. 5,244,603 (Davis) discloses a gas-liquid mixing system
which employs an impeller/draft tube assembly submerged in liquid. Hollow eductor
tubes affixed to the impeller drive shaft are used to flow gas from an overhead gas space
10 to the liquid in the vicinity of the assembly. The positioning and size of the eductor
tubes are such as to maximize the desired gas-liquid mixing and reaction rate.
U.S. Patent No. 5,270,019 (Melton et al.) discloses an elongated,
generally vertically extending concurrent reactor vessel for the production of
hypochlorous acid by the mixing and reaction of a liquid alkali metal hydroxide and a
15 gaseous halogen, wherein an atomizer is mounted near the top of the reactor vessel to
atomize the liquid alkali metal hydroxide into droplets in the vessel. The vessel has a
spraying and reaction zone immediately beneath the atomizer and a drying zone beneath
the spraying and reaction zone to produce a gaseous hypochlorous acid and a
substantially dry solid salt by-product.
U.S. Patent No. 5,170,727 (Nielsen) discloses a process and apparatus in
which supercritical fluids are used as viscosity reduction diluents for liquid fuels or
waste materials which are then spray atomized into a combustion chamber. The
addition of supercritical fluid to the liquid fuel and/or waste material allows viscous
petroleum fractions and other liquids such as viscous waste materials that are too
25 viscous to be atomized (or to be atomized well) to now be atomized by this invention by
achieving viscosity reduction and allowing the fuel to produce a combustible spray and
improved combustion efficiency. Moreover, the present invention also allows liquid
fuels that have suitable viscosities to be better utilized as a fuel by achieving further
viscosity reduction that improves atomization still further by reducing droplet size
30 which enhances evaporation of the fuel from the droplets.
CA 0222~763 1997-12-23
U.S. Patent No. 5,123,936 (Stone et al.) discloses a process and
apparatus for removing fine particulate matter and vapors from a process exhaust air
stream, and particularly those emitted during post-production curing or post-treatment
of foamed plastics, such as polyurethane foam, in which the exhaust air stream is passed
5 through a transfer duct into which is introduced a water spray in the form of a mist of
fine droplets in an amount which exceeds the saturation point; thereafter the exhaust air
stream is introduced into a filter chamber having a cross-sectional area that issubstantially greater than that of the transfer duct, and the exhaust air stream passes
through at least one, and preferably a plurality of high surface area filters, whereby a
10 portion of the water is removed from the exhaust air stream and collected in the filter
chamber prior to the discharge of the exhaust air stream into the environment.
U.S. Patent No. 5,061,453 (Krippl et al.) discloses an apparatus for
continuously charging a liquid reactant with a gas. The gas is dispersed in the reactant
through a hollow stirrer in a gassing tank. The quantity of gas introduced per unit time
15 is kept constant.
U.S. Patent No. 4,423,018 (Lester, Jr. et al.) discloses a process
according to which a by-product stream from the production of adipic acid from
cyclohexane, containing glutaric acid, succinic acid and adipic acid, is employed as a
buffer in lime or limestone flue gas scrubbing for the removal of sulfur dioxide from
20 combustion gases.
U.S. Patent No. 4,370,304 (Hendriks et al.) discloses methods by which
ammonium orthophosphate products are prepared by reacting ammonia and phosphoricacid together at high speed under vigorous mixing conditions by spraying the reactants
through a two-phase, dual coaxial mixer/sprayer and separately controlling the supply
25 and axial outflow rate of the phosphoric acid at 1 to 10 m/sec. and the outflow rate of
ammonia at 200 to 1000 m/sec. (N.T.P.). Thorough mixing and a homogenous productis obtained by directing the outflow spray into a coaxial cylindrical reaction chamber of
a specified size with respect to the diameter of the outermost duct of the sprayer/mixer.
The product may be granulated on a moving bed of granules and adjusted in respect of
CA 0222~763 1997-12-23
the NH3 to H3PO4 content by ch~n~;ing the concentration of the phosphoric acid and/or
supplying additional ammonia to the granulation bed.
U.S. Patent No. 4,361,965 (Goumondy et al.) discloses a device for
atomizing a reaction mixture, said device enabling the reaction mixture to be atomized
5 in a reactor with the aid of at least a first gas and an atomizing nozzle. This device
further comprises a supply of a second hot gas at the top of the atomizing device,
serving to dry the atomized mixture, a supply of a third gas and means for distributing
this third gas comprising an annular space of adjustable width and adapted to distribute
in the reactor said third gas in the form of a ring along the inner wall of the reactor, so
10 as to avoid any contact between the reaction mixture and said wall. The invention is
applicable to the atomization of a reaction mixture.
U.S. Patent No. 4,308,037 (Meissner et al.) discloses methods according
to which high temperature thermal exchange between molten liquid and a gas stream is
effected by generating in a confined flow passageway a plurality of droplets of molten
15 liquid and by passing a stream through the passageway in heat exchange relationship
with the droplets. The droplets are recovered and adjusted to a predetermined
temperature by means of thermal exchange with an external source for recycle. The
process provides for removal of undesired solid, liquid or gaseous components.
U.S. Patent No. 4,065,527 (Graber) discloses an apparatus and a method
20 for handling a gas and a liquid in a manner to cause a specific interaction between them.
The gas is placed into circulation to cause it to make a liquid circulate in a vortex
fashion to present a liquid curtain. The gas is then passed through the liquid curtain by
angled vanes to cause the interaction between the two fluids, such as the heating of the
liquid, scrubbing of the gas, adding a chemical to the liquid and the like. The vanes are
25 spaced apart and project inwardly from the inner periphery of an annular support so that
the circulating liquid readily moves into the spaces between the vanes to create the
liquid curtain. A number of embodiments of the invention are disclosed.
U.S. Patent No. 4,039,304 (Bechthold et al.) discloses methods
according to which waste gas is contacted with a solution of a salt from a pollutant of
30 the gas. This solution is obtained from another stage of the process used for cleaning or
CA 0222~763 1997-12-23
purifying the gas. The resulting mixture of gas and solution is subjected to vaporization
so as to obtain a dry gaseous substance constituted by the waste gas and the evaporated
solvent for the salt. The gaseous substance thus formed contains crystals of the salt as
well as the pollutant present in the original waste gas. The salt crystals and other solid
5 particles are removed from the gaseous substance in the form of a dry solids mixture.
The gaseous substance is subsequently mixed with an absorption fluid such as an
ammonia solution in order to wash out and redissolve any salt crystals which mayremain in the gaseous substance and in order to remove the pollutant present in the
original waste gas from the gaseous substance. The pollutant and the redissolved salt
10 crystals form a salt solution together with the absorption fluid and it is this salt solution
which is brought into contact with the waste gas. The gaseous substance is exhausted to
the atmosphere after being mixed with the absorption fluid.
U.S. Patent No. 3,928,005 (Laslo) discloses a method and apparatus for
treating gaseous pollutants such as sulfur dioxide in a gas stream which includes a wet
15 scrubber wherein a compressed gas is used to atomize the scrubbing liquid and a nozzle
and the compressed gas direct the atomized liquid countercurrent to the flow of gas to
be cleaned. The method and apparatus includes pneumatically conveying to the nozzle
a material such as a solid particulate material which reacts with or modifies the
pollutant to be removed or altered. The gas used for atomizing the scrubbing liquid is
20 also used as a transport vehicle for the solid particulate material. In the case of sulfur
oxides, the material may be pulverized limestone.
U.S. Patent No. 3,677,696 (Bryk et al.) discloses a method according to
which, the concentration of circulating sulfuric acid is adjusted to 80-98% by weight
and used to wash hot gases cont~ining mercury. The temperature of the acid is
25 m~int~ined between 70-250~C, and the solid material separating from the circulating
wash solution is recovered.
U.S. Patent No. 3,613,333 (Gardenier) discloses a process and apparatus
for removing cont:~min~nts from and pumping a gas stream comprising indirectly heat
exch~nging the gas and a liquid, introducing the liquid under conditions of elevated
30 temperature and pressure in vaporized and atomized form into the gas, mixing same
CA 0222~763 1997-12-23
thereby entrapping the cont:~min~nts, and separating clean gas from the atomized liquid
containing the cont~min~nts.
U.S. Patent No. 2,980,523 (Dille et al.) discloses a process for the
production of carbon monoxide and hydrogen from carbonaceous fuels by reaction with
5 oxygen. In one of its more specific aspects it is directed to a method of separating
carbonaceous solid entrained in the gaseous products of reaction of carbonaceous fuels
and oxygen wherein said products are contacted with a limited amount of liquid
hydrocarbon and thereafter scrubbed with water, and said carbonaceous solid is
decanted from said clarified water.
U.S. Patent No. 2,301,240 (Ba-lm~nn et al.) discloses an improved
process for removing impurities from acetylene gas which has been prepared by thermal
or electrical methods by washing with organic liquids, as for example oils or tars.
U.S. Patent No. 2,014,044 (Haswell) discloses an improved method for
treating gas and aims to provide for the conservation of the sensible heat of such gas.
U.S. Patent No. 1,121,532 (Newberry) discloses a process of recovering
alkalis from flue-gases.
None of the above references, or any other references known to the
inventors disclose, suggest or imply, singly or in combination, oxidation of cyclic
hydrocarbons to dibasic acids in multiple stages of temperature/conversion, subject to
20 the intricate and critical controls and requirements of the instant invention as described
and claimed.
Our U.S. Patents 5,580,531, 5,558,842, 5,502,245, and our co-pending
applications 08/477,195 (filed 06/07/95), 08/587,967 (filed 01/17/96), and 08/620,974
(filed 03/25/96), all of which are incorporated herein by reference, describe methods
25 and apparatuses relative to controlling reactions in atomized liquids.
Our co-pending U.S. Patent Application No. 08/812,847 of Mark W.
Dassel, Eustathios Vassiliou, David C. DeCoster, Ader M. Rostami, and Sharon M.
Aldrich, titled "Methods and Devices for Controlling the Reaction Rate of a
Hydrocarbon to an Acid by Making Phase-related Adjustments", filed on March 6,
30 1997, is also incorporated herein by reference.
CA 0222~763 1997-12-23
14
Our co-pending U.S. Patent Application No. 08/824,992 of Mark W.
Dassel, David C. DeCoster, Ader M. Rostami, Sharon M. Aldrich, and Eustathios
Vassiliou, titled "Methods and Devices for Preparing Dibasic Acids", filed on March
27, 1997, is also incorporated herein by reference.
All of the following patent applications, each of which was filed on May
21, 1997 are also incorporated herein by reference:
U.S. Patent Application No. 08/859,985 of Eustathios Vassiliou, Mark
W. Dassel, David C. DeCoster, Ader M. Rostami, and Sharon M. Aldrich, titled
"Methods and Devices for Controlling the Reaction Rate of a Hydrocarbon to an
10 Intermediate Oxidation Product by Pressure Drop Adjustments";
U.S. Patent Application No. 08/861,281 of Mark W. Dassel, Eustathios
Vassiliou, David C. DeCoster, Ader M. Rostami, and Sharon M. Aldrich, titled
"Methods and Devices for Controlling the Reaction Rate of a Hydrocarbon to an
Intermediate Oxidation Product by Monitoring Flow of Incoming and Outcoming
15 Gases";
U.S. Patent Application No. 08/861,180 of David C. DeCoster, Ader M.
Rostami, Mark W. Dassel, and Eustathios Vassiliou, titled "Methods and Devices for
Controlling the Oxidation Rate of a Hydrocarbon by Adjusting the Ratio of the
Hydrocarbon to a Rate-Modulator";
U.S. Patent Application No. 08/861,176 of Mark W. Dassel, Eustathios
Vassiliou, David C. DeCoster, and Ader M. Rostami, titled "Methods of Preparing an
Intermediate Oxidation Product from a Hydrocarbon by Utilizing an Activated
Initiator"; and
U.S. Patent Application No. 08/859,890 of Ader M. Rostami, Mark W.
25 Dassel, Eustathios Vassiliou, David C. DeCoster, titled "Methods and Devices for
Controlling the Oxidation of a Hydrocarbon to an Acid by Regulating
Temperature/Conversion Relationship in Multi-Stage Arrangements."
SUMMARY OF THE INVENTION
As aforementioned, this invention relates to reactors behaving in a
similar manner as plug-flow reactors. More particularly, the instant invention relates to
CA 0222~763 1997-12-23
a reactor comprising a reaction chamber having an elongate body, a liquid feed side, a
coalescing side, a front end, a back end, a cross section, a length, a width, and an axis,
the reactor comprising:
a first reactant feed line for introducing a first reactant to the reactor, the
first reactant being non-gaseous;
a plurality of liquid-feed inlets at the liquid feed side of the reactor, the
liquid-feed inlets being adapted to feed a liquid in a direction substantially
perpendicular to the direction of the axis, and in a manner that the liquid changes
direction from substantially perpendicular to substantially parallel to the direction of the
10 axis at the coalescing side of the reactor;
a gas feed line adapted to feed a gas containing a second reactant to the
reactor; and
reaction-inducing means for causing a reaction to take place between the
first reactant and the second reactant to form a reaction product, in at least a portion of
15 the reaction chamber. Examples of such means are temperature and/or pressure control
means, such as for example heaters, coolers, compressors, etc., preferably accompanied
by monitoring equipment, etc., all well known to the art.
It is highly preferable that at least one of the plurality of the liquid-feed
inlets may comprise an atomizer for atomizing the liquid in a form of a pattern, which is
20 preferably linear. However, at least one of the plurality of the liquid-feed inlets may
comprise an orifice adapted to form streams of liquid, and/or a spatter producing
mech~ni .~m .
The cross-section of the reaction chamber may have any shape, but it is
preferably circular, and more preferably triangular.
Also preferably, the gas feed line is located in the vicinity of the front
end of the reaction chamber and the first reactant feed line is in the vicinity of the back
end of the reaction chamber.
It is preferable that the reaction chamber further comprises restrictive
baffles forming pools at the coalescing side of said reaction chamber. The reactor may
CA 0222~763 1997-12-23
16
also preferably comprise a re-circulator for transferring liquid from the coalescing side
to predeterrnined liquid feed inlets.
The predetermined liquid feed inlets, the feed of which originates from a
given pool, may preferably be located between the pool and the front end, or above the
pool, while the gas feed line may be also located under said pool.
It is preferable that at least two of the pools have different capacities,
more preferable that pools toward the front end have higher capacities than pools
toward the back end, and even more preferable that the pools have progressively larger
capacities going from the back end toward the front end of the reaction chamber.10 Degree of inclination of the reaction chamber, different heights of baffles or different
distances between consecutive baffles are examples of ways to modify the capacities of
the pools.
The ratio of the length of the reaction chamber to the width of said
reaction chamber is preferably higher than 10. The reaction chamber may be inclined or
15 it may be substantially horizontal. Means for adjusting the degree of inclination of the
reaction chamber may also be provided. Such means may be for example hydraulic or
other types of lifters, well known to the art, which may adjust one or both ends of the
reaction chamber, thus ch~n~;ing the degree of inclination of the reaction chamber. In
addition, one or both ends of the reaction chamber may be pivoted.
The reaction chamber may be divided into different sections adapted to
operate under different conditions. The reactor may comprise additional inlets in at
least one of the different sections, for introducing matter ch~nging reaction
characteristics in the at least one of the different sections. The reaction chamber may
further comprise means for removing reaction product between at least one pool and the
25 respective liquid feed inlet. Also, by-products such as water for example, or recyclable
products, may be removed in a similar manner.
BRIEF DESCRIPTION OF THE DRAWING
The reader's understanding of this invention will be enhanced by
30 reference to the following detailed description taken in combination with the drawing
figures, wherein:
CA 0222~763 1997-12-23
FIGURES 1 A, I B, AND 1 C illustrate schematically examples of
miscellaneous general configurations of the reactors according to the present invention.
FIGURES 2A through 2E illustrate schematically examples of
miscellaneous general cross sections of the reaction chambers according to the present
invention.
FIGURE 3 illustrates schematically an inclined reactor according to a
preferred embodiment of the present invention wherein liquids are recirculated from
pools to sets of liquid feed inlets.
FIGURE 4 illustrates schematically a fragmented view of another
10 preferred embodiment of the present invention, wherein a filter is used in the re-
circulation line.
FIGURES 5 and 6 illustrate schematically examples of segments of the
reaction chamber according to a preferred embodiment of the present invention.
FIGURES 7 and 8 illustrate schematically examples of atomized liquids
15 through liquid feed lines or atomizers.
FIGURE 9 illustrates schematically a fragmented view of a reaction
chamber according to another preferred embodiment of the instant invention, wherein
the liquid feed inlets in the form of atomizers are stacked very close to each other in
order to provide high concentration of droplets in linear spray patterns.
FIGURE 10 illustrates schematically a linear spray pattern.
FIGURE 11 illustrates schematically a circular or elliptical spray pattern.
FIGURE 12 illustrates schematically a reaction chamber with a circular
cross section having triple feed inlets in order to maximize the density of liquid droplets
after the triple inlets have been stacked as illustrated in the reaction chamber of Figure
25 9.
FIGURE 13 illustrates schematically a reaction chamber having a
triangular cross section, wherein three substantially flat plates are connected together.
FIGURE 14 illustrates schematically a fragmented view of a reaction
chamber according to another preferred embodiment of the instant invention, wherein
30 the gas feed line is positioned under a pool.
CA 0222~763 l997-l2-23
18
FIGURE 15 illustrates schematically a horizontal reaction chamber
according to another preferred embodiment of the instant invention.
FIGURE 16 illustrates schematically a horizontal reaction chamber
according to another preferred embodiment of the instant invention, wherein each re-
5 circulator pump, re-circulates the liquid through a respective 3-way valve in a way that
part of the liquid is dispensed, preferably atomized, immediately above the pool under
consideration and part of the liquid is dispensed above a pool further away from the
back end and closer to the front end of the reaction chamber.
FIGURE 17 illustrates schematically a reaction chamber according to
10 another preferred embodiment of the instant invention, wherein a first section of the
reaction chamber is controlled to have a higher temperature than a second section.
FIGURE 18 illustrates schematically a reaction chamber according to
another preferred embodiment of the instant invention, wherein the reaction chamber is
separated in a back section and a front section, as well as an additional inlet for
15 introducing other desirable ingredients.
DETAILED DESCRIPTION OF THE INVENTION
As aforementioned, this invention relates to reactors behaving in a
similar manner as plug-flow reactors. The reactor of the instant invention is very useful
20 in most cases wherein one reactant is a liquid or a substance contained in a liquid and
the other reactant is a gas, or a substance contained in a gas. Examples include but are
not limited to formation of maleamic acid, formation of ammonium phosphate, etc.,
wherein one reactant is in the liquid state and the other reactant is in the gaseous state.
This invention is particularly fit for oxidations, such as for example oxidation of
25 cyclohexane to cyclohexanone and/or cyclohexanol, and/or cyclohexyhydroperoxide,
and/or adipic acid, oxidation of cyclohexanone and/or cyclohexanol to
cyclohexylhydroperoxide, and/or adipic acid, oxidation of cyclohexylhydroperoxide to
adipic acid, oxidation of toluene to benzoic acid, oxidation of o-xylene to phthalic acid,
oxidation of m-xylene to isophthalic acid, oxidation of p-xylene to terephthalic acid,
30 etc.
CA 0222~763 1997-12-23
19
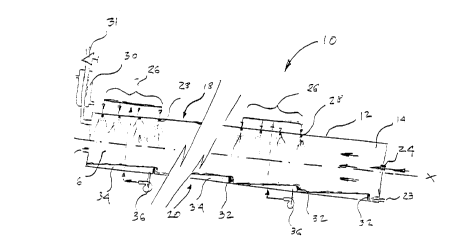
The reactor 10, as better illustrated in Figures lA to lC, comprises a
reaction chamber 12, which in turn comprises an elongated body 13, a front end 14, a
back end 16, a length L, a width W, and an axis X. The ratio of the length L to the
width W is preferably larger than 10
The general shape of the reactor l O of this invention may have any of a
plurality of configurations. In one example, illustrated in Figure lA, the general shape
of the reactor l O has the form of a coil. In another example, illustrated in Figure 1 B, the
general shape of the reactor 10 has the form of two inclined straight segments connected
together. In still another example, illustrated in Figure lC, the general shape of the
10 reactor lO has the form of a single inclined segment. The reactor lO, may also be
substantially horizontal, as will be illustrated at a later point.
The inclination of the reaction chamber 12 is preferably lower than 45
degrees and more preferably in the range of 5 to 20 degrees. The reaction chamber 12
may also be substantially horizontal.
Means for adjusting the degree of inclination (not shown) of the reaction
chamber 12 may also be provided. Such means may be for example hydraulic lifters or
other types of lifters, well known to the art, which may adjust one or both ends of the
reaction chamber, thus ch:~nging the degree of inclination of the reaction chamber. In
addition, one or both ends of the reaction chamber may be pivoted.
The cross-sections of the different segments of the reactor 10 may have
different shapes. Examples of some of the most common cross-sections are illustrated
in Figures 2A through 2E. The most preferred cross-sections, for reasons to be
explained later are a circular cross-section illustrated in Figure 2A, and a triangular
cross-section illustrated in Figure 2D.
The reaction chamber 12, as better illustrated in Figure 3, also has
preferably a liquid feed side 18, a coalescing side 20, a first reactant feed line 22, a
liquid exit port 23, a gas feed line 24, sets 26 of liquid feed inlets 28, each set 26
cont~ining one or more liquid feed inlets 28, and a condenser 30, provided with a valve
31, for condensing condensables and allowing off-gases to be moved out of the system.
30 Preferably, a demister (not shown), well known to the art, may be placed between the
CA 0222~763 1997-12-23
reaction chamber 12 and the condenser 30. It is preferable that that the reaction
chamber 12 has baffles 32, which form pools 34 in the coalescing side 20. The pools 34
are adapted to restrict liquid matter from free flowing from the back end 16 to the front
end 18 of the reaction chamber 12. In many occasions, it is preferable that at least two
5 pools have different capacities, more preferable that the pools toward the front end 14
are larger or have larger capacities than the pools toward the back end 16, and it is even
more preferable that the pools have progressively higher capacities going from the back
end 16 toward the front end 14. One of the reasons why is that the reaction rate usually
decreases as the reaction progresses, and the reaction progresses in the reaction chamber
10 12 as the liquid moves from the back end 16 to the front end 14.
The reactor 10, may also comprise a re-circulator, in the form of a pump
36 for example, which is adapted to re-circulate liquid matter from the pools 34 to
predetermined inlets 28 or sets of inlets 26. A filter 35 may be used in the re-circulator
line 37 for removing precipitated matter, such as adipic acid for example in the case of
15 oxidation of cyclohexane to adipic acid, as better shown in Figure 4. A cooler (not
shown) may be used between the pool and the filter 35 to promote precipitation and to
remove heat of reaction. Also, a heat exchanger (not shown), preferably a heater, may
be used after the filter and before the liquid feed inlets 28 to raise the temperature
adequately for the reaction to proceed, and ensure solubilization of any precipitate that
20 might form after the filter.
Filters in the re-circulation lines are preferably employed in pools
located after the reaction has progressed adequately to filter out the product of reaction,
adipic acid for example. In this particular case, at the early stages, no filters are
necessary. In order to remove the heat of reaction at the early stages and in the absence
25 of a filter, a cooler may be used in such a manner that the temperature does not decrease
to a point that reaction cannot proceed and/or to the point that reaction product, adipic
acid for example, is precipitated. In addition, it may be prudent to m~int~in the
temperature of the heat exchanger cooling fluid at a higher temperature than thetemperature of precipitation of any solids dissolved, to avoid deposition of solids on the
30 heat exchanger surfaces.
CA 0222~763 1997-12-23
The re-circulation line may also be divided in one stream of lower
temperature and one stream of higher temperature, the two streams being fed through
different atomizers in the same vicinity. As explained in more detail in our co-pending
application Serial Number 08/587,967, filed 01/17/96, which is incorporated herein by
5 reference, the heat produced by the reaction in the higher temperature droplets is
removed by evaporation of volatile matter, which matter condenses on the lower
temperature droplets.
A jacket around the reaction chamber may also be used for removing
heat of reaction by recirculating cooling liquids through the jacket.
Exemplary segments of the reaction chamber 12 are better illustrated in
Figures 5 and 6. A plurality of segments as shown in Figure 5 may be connected
together through flanges 38.
Preferably, the liquid feed inlets 28 comprise or are in the form of
atomizers. Atomized liquids have the advantage of providing an extremely high surface
15 area, as compared to bubbling or other methods of contact with a gas. The reaction in
the case of atomized liquids takes place between the gas as continuous phase and the
droplets of the atomized liquid as the discontinuous phase. Figures 7 and 8 illustrate
some examples of atomized liquids 40 through liquid feed lines or atomizers 28. As
aforementioned, other examples of liquid feed inlets are orifices which may produce
20 substantially linear streams of liquid, or spatter. Spatter is characterized by a course
spray, wherein the average diameter of the droplets is larger than about 1/16".
The liquid feed inlets 28, if they are in the form of atomizers, may be
stacked very close to each other, as better shown in Figure 9, and provide high
concentration of droplets if the spray pattern is linear. A spray pattern is linear if the
25 atomizer produces a substantially linear pattern 42 on a flat substrate facing the
atomizer 28, as better shown in Figure 10. Common shapes of patterns, in contrast with
the substantially linear pattern 42, are circular or elliptical patterns 44, better shown in
Figure 11. For the reasons stated above, the linear pattern atomizers are highlypreferable.
CA 0222~763 1997-12-23
The first reactant feed line 22 should most preferably be in the vicinity of
the back end 16 of the reaction chamber 12. It can be over or under pool 34. It can also
be part of one or more of sets 26 of inlet 28, or inlets 28, which are located toward the
back end 16.
The gas feed line 24 can preferably be in the vicinity of the front end 14.
However, it can also be in the vicinity of the back end, or in any other place or places
within the reaction chamber 12.
As aforementioned, the preferable cross-sections of the reaction chamber
12 are circular and triangular. For the same conditions, materials of construction, and
cross-sectional area, the circular cross section provides higher strength than the
triangular one. However, the triangular cross section provides higher density ofatomized droplets under the right atomization conditions, wherein the pattern of spray is
linear. When a reaction chamber with a circular cross section is used, a preferred
arrangement of the locations of liquid feed inlets 28 is illustrated in Figure 12, so that a
lS plurality of triple liquid feed inlets 28 may be stacked closely to each other, as better
illustrated in Figure 9.
An example of a reaction chamber having a triangular cross section is
illustrated in Figure 13. Three substantially flat plates 46 are connected together by the
bolts and nuts 48. Gaskets and other well known to the art features are not shown for
purposes of brevity and clarity. Two or all three plates may be integrally connected. A
tube having a triangular configuration, for example, may be used. It is preferable,
however, that at least one plate is removable for easy access, installation and
maintenance of the liquid feed inlets, the baffles, and other accessories, which are not
shown, such as for example thermocouples, pressure monitors, etc.
The operation of these embodiments, regardless of shape of cross
section, arrangement or size of baffles, location and type of liquid feed inlets 28, and
other variations, may be better explained by considering Figure 3.
Initially, first reactant, for example cyclohexane, contained in a liquid,
for example acetic acid with catalyst and initiator, is caused to flow into the slightly
30 inclined reaction chamber 12 through the first reactant feed line 22. A pool 34, closest
CA 0222~763 1997-12-23
to the first reactant feed line 22, starts being filled. After this pool 34 has been filled, it
starts overflowing and liquid starts entering the second pool, next to it. This process
repeats itself until all pools are full and overflowing, at which point the liquid is
removed from the reaction chamber 12 through the liquid exit port 23 for further5 treatment. At the same time that this process of liquid movement is taking place from
pool to pool in a direction substantially parallel to the axis X-X of the reaction chamber,
re-circulating devices, such as for example pumps 36, remove liquid from a respective
pool, and recirculate it to the reaction chamber 12 through a set 26 of liquid feed inlets
28, preferably in the form of an atomized liquid. The atomized droplets fall back to the
10 respective pools 34, and the recirculation continues. The direction of the falling
droplets is substantially perpendicular to the axis X-X of the reaction chamber 12, while
the overall movement of the liquid is from the back end to the front end of the reaction
chamber 12, parallel to the axis X-X.
Simultaneously, a second reactant, for example oxygen, mixed in a gas,
15 for example nitrogen, or just oxygen, enters the reaction chamber 12 through gas feed
line 24 and moves in a direction substantially parallel to the axis X-X of the reaction
chamber 12. During this movement of the gas, the first reactant, for example
cyclohexane, reacts with the second reactant, for example oxygen, to form a reaction
product, for example adipic acid. The movement of the gas may be co-current with the
20 general movement of the liquid or counter-current, as it will be exemplified
hereinbelow. The off-gases leave the system through valve 31 of condenser 30, while
condensable matter returns to the reaction chamber 12, after it has been condensed in
the condenser 30.
Temperatures, pressures, and other conditions regarding the reactor 10
25 depend on the particular reactants and reaction products, so that they are not specifically
described here, as also being very well known to the art.
The concentration of second reactant is higher closer to the front end 14,
and it decreases as the gas moves toward the back end 16, since second reactant is
replenished at the front end 16, and since the conversion of the first reactant to reaction
30 product has been progressed considerably to a desired degree toward the front end.
CA 0222~763 1997-12-23
24
If all other conditions are the same, the lower the feed rate of first
reactant through the first reactant feed line 22, the higher the conversion of first reactant
to reaction product of liquid in the vicinity of the front end 14, provided that the
reaction is slow enough. Of course, higher temperatures, and higher partial pressures of
S the second reactant in the reaction chamber favor higher reaction rates, and therefore,
conversions as the liquid moves from the back end 16 toward the front end 14.
This re-circulation arrangement allows the first reactant to move slowly
in a controlled manner from the back end 16 to the front end 14, while it is continually
or continuously, and controllably reacting during this movement with the second
10 reactant. Thus it performs in a manner similar to a plug flow reactor, having important
added advantages. The first and second reactants according to the instant invention can
have an independent inlet/outlet relation from any end to the opposite end, and most
importantly, the reactor of the present invention can handle heterogeneous reactants,
such as liquids with gases, in a plug flow manner, which is not possible with a
15 conventional plug flow reactor.
In the embodiment shown in Figure 4, the liquid from pool 34 passes
through a filter 35 via re-circulation line 37, wherein solid products of l-eaction, such as
for example adipic acid, terephthalic acid, etc., are removed from the liquid before it
enters the liquid feed inlets 28. A cooler (not shown) in the recirculation line 37
20 between pool 34 and filter 35 is in many occasions beneficial as precipitating the
product of reaction to a high degree. For example, in the case that the first reactant is
cyclohexane and the reaction product is adipic acid, such a cooler may be desirable for
precipitating the adipic acid before filtration. Two phases which may be formed during
cooling may be separated, if so desired, and treated separately or together before they
25 are further pumped toward the set 26 of liquid feed inlets 28. A heater (not shown) after
the filter 35 may be desirable to re-produce one phase from the two phases.
In another embodiment of the instant invention, the gas feed line 24 may
be positioned under one or more pools 34, as better illustrated in Figure 14. In this case
the gas not only comes in contact with the droplets above the pool, but it also bubbles
CA 0222~763 1997-12-23
through the content of the pool, thus speeding up the reaction. In all other respects this
embodiment is similar to the embodiments already discussed.
In still another embodiment, better shown in Figure lS, the reaction
chamber is substantially horizontal, and each pump 36 re-circulates liquid from each
5 pool to a set 26 of liquid feed inlets 28, which are preferably atomizers above the same
individual pool. The operation of this embodiment is substantially identical as the
embodiments discussed above.
In still a different embodiment of the instant invention, better illustrated
in Figure 16, each re-circulator pump 36, re-circulates the liquid through a respective 3-
lO way valve 50 in a way that part of the liquid is dispensed, preferably atomized,immediately above the pool under consideration and part of the liquid is dispensed
above a pool further away from the back end 16 and closer to the front end 14 (not
shown in Figure 16, but shown in Figure 3). A combination of flow rate of liquidentering the reaction chamber 12 through first reactant feed line 22, and settings of 3-
15 way valves 50, determines the conversion of first reactant to reaction product at thedifferent stages of the reaction chamber, all other parameters or conditions rem~ining
constant.
In still another embodiment, better illustrated in Figure 17, a first section
52 of the reaction chamber 12 is temperature controlled to have a higher temperature
20 than a second section 54 by well known to the art techniques. The details regarding the
liquid feed inlets, baffles, pools, etc., are not shown for purposes of clarity and brevity,
and also since they have already been discussed in detail above. The purpose of this
arrangement is to initiate a reaction, such as for example of cyclohexane to adipic acid,
in section 52, and then continue the reaction at a lower temperature in second section 54
25 at a lower temperature, in order to improve selectivity and yield to final reaction
product, for example adipic acid, without suffering from a long induction or initiation
period. More than 2 sections similar to sections 52 and 54 may be utilized, and be
operated at different temperatures. In other regards, the operation of this embodiment is
substantially the same as discussed above regarding the other embodiments.
CA 0222~763 1997-12-23
26
In another embodiment, better shown in Figure 18, the reaction chamber
12 is separated in a back section 56 and a front section 58. In the vicinity of the back
part 57 of the front section 56, there is located an additional inlet 60 for introducing
other materials, such as for example other reactants, solvents, catalysts, initiators, or any
5 other desirable ingredients. More additional inlets at different desirable points and
more than two section may be used depending on the particular circumstances. Thedifferent sections are adapted to attain different temperatures and operate under
different conditions, so that one type of reaction may take place in one section and a
different reaction in a different section. As in the previous case, details regarding the
10 liquid feed inlets, baffles, pools, etc., are not shown for purposes of clarity and brevity,
and also since they have already been discussed in detail above.
The operation of this embodiment will be better understood if it is
described in terms of a specific example. In this example, the purpose is to produce
adipic acid from cyclohexane. Cyclohexane containing catalyst, such as a cobalt
15 compound for example, and a small amount of initiator, such as cyclohexanone for
example, are fed to the back section 56 ofthe reaction chamber 12. The back section 56
is preferably kept at a temperature of 130 to 160~C. The mixture is atomized andrecirculated in this section as already has been described in the previous embodiments,
and it progresses from the back point 16 of the reaction chamber 12 toward the back
20 point 57 of the front section 58. Oxygen, or a gas containing oxygen enters the gas feed
line 24, which in this case is preferably located in the vicinity of the back end 16 of the
reaction chamber 12. Acetic acid is introduced into the front section 58 of the reaction
chamber 12 through additional inlet 60. The temperature of the front section 58 is
preferably maintained in a range of 90 ~C to 120 ~C. The gas entering from the gas feed
25 line 24 and moving through the reaction chamber 12 in a direction from the back end 16
toward the front end 12 does not allow vapors of acetic acid to move to the back section
56 and condense therein. The liquid which is re-circulated from pools (similar to the
ones described in the previous embodiments, but not shown for purposes of clarity) to
respective liquid feed inlets (similar to the ones described in the previous embodiments,
CA 0222~763 1997-12-23
but not shown for purposes of clarity), contains acetic acid as solvent within the front
section 58.
These conditions favor the formation of cyclohexanone and
cyclohexanol in the back section 56, while they also favor the formation of adipic acid
5 in the front section 58, from oxidation of an excess of cyclohexane which passes
unreacted through the back section 56, and from oxidation of cyclohexanone,
cyclohexanol and other adducts, which were formed in the back section 56. Thus,
according to this embodiment of the instant invention, an adequate amount of initiator
may be produced in a reaction chamber at a back section, while the final product may be
10 formed in the same reaction chamber in a front section.
The examples given above illustrate very clearly the versatility and
adaptability of the reactor of the instant invention, wherein even more than one reaction
may take place within a single reaction chamber in different sections of said reaction
chamber in a highly controlled fashion.
A major advantage of the methods and devices of the present invention is
that an outstanding balance between productivity and selectivity/yield of the desired
product may be achieved. In this respect high yields and selectivities may be obtained
without sacrificing productivity.
Another major advantage of this invention is that the conversion of first
20 reactant, for example cyclohexane, to reaction product, for example adipic acid,
increases substantially uniformly along the length of the reaction chamber from the
back end to the front end, and all the liquid reaching the front end, and exiting
therefrom, has been subjected to the same treatment gradually. In the contrary, in the
case of a stirred tank reactor, the exiting liquid contains completely unconverted first
25 reactant (which is being mixed substantially instantaneously with the rest of the liquid
as it enters the stirred tank reactor), as well as reaction product which was formed and
remained in the reactor for an excessively long time, so that the average conversion of
first reactant to reaction product reaches an average conversion in the stirred tank
reactor which is equivalent to the conversion level reached at the front end of the
30 reaction chamber of the present invention. In other words, the striking difference is that
CA 0222~763 1997-12-23
28
in the case of the reaction chamber of this invention, and for all practical purposes, the
molecules of first reactant and reaction product exiting the reaction chamber have been
subjected to substantially the same treatment, while in the case of a stirred tank reactor,
even some molecules of first reactant which just entered the reactor, exit, while other
5 molecules have been reacted at a considerably earlier time, in order to reach an average
conversion which is equivalent to the conversion level reached at the front end of the
reaction chamber of the present invention. This promotes substantially better
selectivities and yields when the reaction is conducted according to the teachings of the
present invention.
Reactions, such as oxidations for example, according to this invention,
are non-destructive oxidations, wherein the oxidation product is different than carbon
monoxide, carbon dioxide, and a mixture thereof. Of course, small amounts of these
compounds may be formed along with the oxidation product, which may be one product
or a mixture of products.
Examples include, but of course, are not limited to plepa~lion of C5-C,2
aliphatic dibasic acids from the corresponding saturated cycloaliphatic hydrocarbons,
such as for example preparation of adipic acid from cyclohexane.
Regarding adipic acid, the preparation of which is especially suited to the
methods and apparatuses of this invention, general information may be found in aplethora of U.S. Patents, among other references. These, include, but are not limited to:
U.S. Patents 2,223,493; 2,589,648; 2,285,914; 3,231,608; 3,234,271;
3,361,806; 3,390,174; 3,530,185; 3,649,685; 3,657,334; 3,957,876; 3,987,100;
4,032,569; 4,105,856; 4,158,739 (glutaric acid); 4,263,453; 4,331,608; 4,606,863;
4,902,827; 5,221,800; and 5,321,157.
Examples demonstrating the operation of the instant invention have been
given for illustration purposes only, and should not be construed as limiting the scope of
this invention in any way. In addition it should be stressed that the preferred
embodiments discussed in detail hereinabove, as well as any other embodiments
encompassed within the limits of the instant invention, may be practiced individually,
or in any combination thereof, according to common sense and/or expert opinion.
CA 0222~763 1997-12-23
29
Individual sections of the embodiments may also be practiced individually or in
combination with other individual sections of embodiments or embodiments in their
totality, according to the present invention. These combinations also lie within the
realm of the present invention. Furthermore, any attempted explanations in the
5 discussion are only speculative and are not intended to narrow the limits of this
nventlon.
All explanations given hereinabove are to be considered as speculative
and should not be construed as limiting the breadth of the claims.