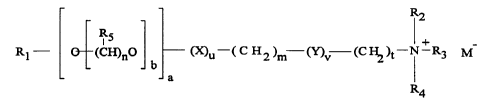Note: Descriptions are shown in the official language in which they were submitted.
CA 02226666 1998-O1-12
WO 97/03156 ' PCT/US96/11249
1
DETERGENT COMPOSITION COMPRISING CATIONIC ESTER SURFACTANT AND
PROTEASE ENZYME
Field of the invention
The present invention relates to detergent compositions adapted for use in
laundry and dish washing processes. More specifically, the present
invention relates to detergent compositions comprising a surfactant system
in combination with a proteolytic enzyme which provide unexpectedly good
detergency performance on proteinaceous soils.
Back4round of the invention
The satisfactory removal of soils/stains is a particular challenge to the
formulator of a detergent composition for use in laundry and dishwashing
machines.
Traditionally, the removal of soils/stains has been facilitated by the use of
surfactants. Of these, anionic surfactants have been found to give the most
effective cleaning performance, especially at high levels.
Additional cleaning benefits may also be seen with the use of enzymes, for
example, in the removal of proteinaceous stainslsoils such as blood, egg,
chocolate, gravy and the like.
A problem encountered with the use of enzymes as components of
detergents is that enzyme activity in the wash may be reduced by the
presence of other detergent components in the wash solution such as high
levels of anionic surfactants.
One solution to this problem would be to reduce the level of anionic
surtactant present in the detergent composition. However, whilst this would
ameliorate the problem, this is accompanied by a marked negative effect on
the overall stain/soil removal performance. To overcome this problem,
combinations of surfactants, such as anionic, nonionic and cationic
surfactants have been used.
GB-1,375,450 discloses a detergent composition which is asserted to
provide effective cleaning and soil removal performance. The composition
CA 02226666 1998-O1-12
WO 97/03156 ,; PCT/US96/11249
2
comprises an anionic surfactant, a cationic surfactant together with a
proteolytic enzyme, wherein said cationic surfactant comprises two ,
hydrophobic long chains. Specifically disclosed as suitable cationic
surfactants are alkyl quaternary ammonium species as well as the ester
compound formed from two moles of stearic acid and one mole of tri-
ethanol-methyl-ammonium chloride, e.g. N,N-di(2-stearoyloxyethyl)-N-(2-
hydroxyethyl)-N-methyl ammonium chloride. However, a problem
encountered with such cationic surfactants is their relative insolubility in
the
wash which diminishes them value in detergent compositions.
EP-B-8142 discloses liquid detergent compositions comprising a ternary
surfactant system containing anionic, nonionic and cationic surfactants.
More particularly disclosed is a liquid composition comprising 24% of an
anionic surfactant (1~4S), 18.5% of a nonionic surfactant ( C45E7) and
3.5°~
of Coconut (C12-C14) dihydroxyethylmethyl ammonium chloride together
with 0.4°~ of a Maxatase proteolylic enzyme, and wherein said
composition
is asserted to provide good soil removal performance.
EP-B-51986 discloses a granular laundry detergent composition which is
asserted to provide good grease and oil removal performance together with
clay soil detergency. The composition comprises, as a surfactant system a
specified mixture of anionic, nonionic and water-soluble C1p-C14 alkyl
trimethyl quaternary ammonium cationic surfactant, wherein said cationic
surfactant is in amount from 0.2% to 2% by weight. The composition may
additionally comprise a proteolytic enzyme.
/mother potential solution to these problems would be to use detergent
particles with different solubility rates. However, this would greatly
increase
the cost of the manufacturing process. An exemplary disclosure is given in
EP A- 0,342,043 where the anionic surtactant and the enzyme are in two
different particulate multi ingredient components. The particulate containing
the enzyme has a solubility rate superior to the surfactant particulate and
this superior solubility rate may be provided by a cationic surfactant.
Notwithstanding the advances in the art represented by the above
disclosures, there is still a need for detergent compositions which provide
CA 02226666 2000-12-14
3
effective soil/stain removal performance, which avoid degradation of the
detergent components and which are not detrimental to the environment.
The Applicant has now surprisingly found that the provision of a cationic
ester
surfactant ameliorates these problems. Not to be bound by theory, it is
believed
that such component reduces the critical micelle concentration of any anionic
surfactant present in the composition. Hence, reducing the concentration of
anionic surfactant monomers in the wash improves the enzyme performance.
Further enhanced soil removal benefits are observed where the cationic ester
surfactant contains one hydrophobic chain.
The Applicant has also found that where a detergent composition contains a
surfactant system comprising an anionic surfactant and a cationic ester
surfactant, in combination with a proteolytic enzyme enhanced stain/soil
removal is obtained, especially on proteinaceous stains.
The Applicant has found that the further addition of an amylolytic enzyme to
the
composition of the present invention enhances the overall soil/stain removal
performance.
It is therefore an object of the present invention to provide compositions
suitable for use in laundry and machine dishwashing methods producing
enhanced stain removal.
Summary of the invention
According to the present invention there is provided a detergent composition
comprising: a) from 1 % to 95% by weight of a surfactant system comprising an
anionic surfactant which includes a linear alkyl benzene sulfonate and a
cationic ester surfactant selected from the group consisting of stearoyl
choline
ester quaternary methylammonium halides, palmitoyl choline ester quaternary
methylammonium halides, myristoyl choline ester quaternary methylammonium
halides, myristoyl choline ester quaternary methylammonium halides, lauroyl
choline ester quaternary methylammonium halides, cocoyl choline ester
quaternary methylammonium halides, tallowyl choline ester quaternary
methylammonium halides, and mixtures thereof, present in a weight ratio of
said anionic surfactant to said cationic ester surfactant of 2.5:1 to 25:1; b)
from
CA 02226666 2000-12-14
4
0.0001 % to 5% by weight of a proteolytic enzyme; and c) from 0.0001 % to 5%
by weight of an amylolytic enzyme; wherein the % weight of proteolytic enzyme
in the formulation is based on an enzyme activity of 4 Knpu/g of the enzyme
particle, and wherein the weight ratio of said anionic surfactant to said
proteolytic enzyme is at least 1.5:1.
If the actual activities of these proteolytic enzymes in the detergent
composition
are different from their 4 KNPU/g standard activities, the level of
proteolytic
enzyme will be adjusted accordingly.
Non limiting examples of enzymes which can be used for the purpose of the
invention include SavinaseT"", enzyme of the Bacillus Lentus type backbone
such as MaxacalT"", OpticIeanT"", DurazymT"" and ProperaseT"", enzyme of the
Bacillus Licheniformis type backbone such as Alcalase and Maxatase and
enzyme of the Bacillus Amyloliquefaciens type backbone such as Primas. For
example, if a protease is used having an activity of 16 knpu/g, the amount of
protease will be reduced by a factor 4 to compensate for the extra activity of
the
protease.
For the purpose of the invention, proteases which are not supplied in Knpu/g
units will also be converted according to the process described below so as to
obtain uniform enzyme unit and enzyme activity, e.g 4 Knpu/g:
1-conversion of the level of proteolytic enzyme used into the level of pure
enzyme, and
2-conversion from the level of pure enzyme to a 4knpu/g Savinase particle
basis according to the following equation:
4 knpu/g = 16.5 mg pure enzyme/g of enzyme particle.
For example, according to the process described above, Alcalase of 1.25AU/g
is found to be equivalent to 4Knpu/g.
In a preferred embodiment, the cationic ester surfactant is a water-
dispersible
surfactant selected from those having the formula:
CA 02226666 1998-O1-12
WO 97/03156 ;; PCT/US96/11249
R2
RS I +
Rl - (CH)n0 (~u-( C H2 )m (~~ (C ~ )t-N-R3 M
b
a
R4
wherein R1 is a C5-C31 linear or branched alkyl, alkenyl or alkaryl chain or
M-. ~N+(R6R7Rg)(CH2)s; X and Y, independently, are selected from COO,
OCO, O, CO, OCOO, CONH, NHCO, OCONH and NHCOO wherein at least
one of X or Y is a COO, OCO, OCOO, OCONH or NHCOO group; R2, R3,
R4, R6, R7, and Rg are independently selected from alkyl, alkenyl,
hydroxyalkyl, hydroxy-alkenyl and alkaryl groups having from 1 to 4 carbon
atoms; and R5 is independently H or a C1-C3 alkyl group; wherein the
values of m, n, s and t independently lie in the range of from 0 to 8, the
value of b lies in the range from 0 to 20, and the values of a, a and v
independently, are either 0 or 1 with the proviso that at least one of a or v
must be 1; and wherein M is a counter anion.
Preferred water-dispersible cationic ester surfactants are the choline ester
surfactants.
Detailed description of the invention
An essential element of the invention is a surfactant system present in
amount from 1 °~ to 95%, preferably from 3% to 50%, more preferably 4%
to
40°~6 and most preferably from 5% to 30% by weight of the detergent
composition. Said system comprises as essential components an anionic
surfactant and a cationic ester surfactant.
The weight ratio of said anionic surfactant to said cationic ester surfactant
in
the surfactant system is from 2.5:1 to 25:1, preferably from 4:1 to 15:1, most
preferably from 5:1 to 10:1.
Anionic surfactant
Essentially any anionic surfactants useful for detersive purposes can be
included in the compositions. These can include salts (including, for
CA 02226666 1998-O1-12
WO 97/~3156 t: PCT/US96/11249
6
example, sodium, potassium, ammonium, and substituted ammonium salts
such as mono-, di- and triethanolamine salts) of the anionic sulfate,
sulfonate, carboxylate and sarcosinate surfactants and mixtures thereof.
Other anionic surfactants include the isethionates such as the acyl
isethionates, N-acyl taurates, fatty acid amides of methyl tauride, alkyl
succinates and sulfosuccinates, monoesters of sulfosuccinate (especially
saturated and unsaturated C~2-C~$ monoesters) diesters of sulfosuccinate
(especially saturated and unsaturated C6-C~4 diesters), N-acyl
sarcosinates. Resin acids and hydrogenated resin acids are also suitable,
such as rosin, hydrogenated rosin, and resin acids and hydrogenated resin
acids present in or derived from tallow oil.
Anionic sulfate surfactant
Anionic sulfate surfactants suitable for use herein include the linear and
branched primary and secondary alkyl sulfates, alkyl ethoxysulfates, fatty
oleyi glycerol sulfates, alkyl phenol ethylene oxide ether sulfates, .the C5-
C~7 acyl-N-(C~-C4 alkyl) and -N-(C~-C2 hydroxyalkyl) glucamine sulfates,
and sulfates of alkylpolysaccharides such as the sulfates of
alkylpolyglucoside (the nonionic nonsulfated compounds being described
herein).
Alkyl ethoxysulfate surfactants are preferably selected from the Cg-C~ g
alkyl sulfates which have been ethoxylated with from about 0.5 to about 20
moles of ethylene oxide per molecule. More preferably, the alkyl
ethoxysulfate surfactant is a Cg-C~ g alkyl sulfate which has been
ethoxylated with from about 0.5 to about 20, preferably from about 0.5 to
about 5, moles of ethylene oxide per molecule.
Anionic sulfonate surfactant
Anionic sulfonate surfactants suitable for use herein include the salts of C5-
C20 linear alkylbenzene sulfonates, alkyl ester sulfonates, Cg-C22 primary
or secondary alkane sulfonates, Cg-C24 olefin sulfonates, sulfonated
polycarboxylic acids, alkyl glycerol sulfonates, fatty acyl glycerol
sulfonates,
fatty oleyl glycerol sulfonates, and any mixtures thereof.
Anionic carboxvlate surfactant
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/US96/11249
7
Anionic carboxylate surfactants suitable for use herein include the alkyl
ethoxy carboxylates, the alkyl polyethoxy polycarboxylate surfactants and
the soaps ('alkyl carboxyls'), especially certain secondary soaps as
described herein.
Preferred alkyl ethoxy carboxylates for use herein include those with the
formula RO(CH2CH20)x CH2C00-M+ wherein R is a C6 to C18 alkyl group,
x ranges from O to 10, and the ethoxylate distribution is such that, on a
weight basis, the amount of 'material where x is 0 is less than about 20 %,
and the amount of material where x is greater than 7, is less than about 25
%, the average x is from about 2 to 4 when the average R is C1 g or less,
and the average x is from about 3 to 10 when the average R is greater than
C13, and M is a ration, preferably chosen from alkali metal, alkaline earth
metal, ammonium, mono-, di-, and tri-ethanol-ammonium, most preferably
from sodium, potassium, ammonium and mixtures thereof with magnesium
ions. The preferred alkyl ethoxy carboxylates are those where R is a C12 to
C1 g alkyl group.
Alkyl polyethoxy polycarboxylate surfactants suitable for use herein include
those having the formula
RO-(CHR1-CHR2-O)-R3 wherein R is a Cg to C1g alkyl group, x is from 1 to
25, R1 and R2 are selected from hydrogen, methyl acid radical, succinic
acid radical, hydroxysuccinic acid radical, and mixtures thereof, wherein at
least one R1 or R2 is a succinic acid radical or hydroxysuccinic acid radical,
and R3 is selected from hydrogen, substituted or unsubstituted hydrocarbon
having between 1 and 8 carbon atoms, and mixtures thereof.
Anionic secondary soap surfactant
Preferred soap surfactants are secondary soap surfactants which contain a
carboxyl unit connected to a secondary carbon. The secondary carbon can
be in a ring structure, e.g. as in p-octyl benzoic acid, or as in alkyl-
substituted cyclohexyl carboxylates. The secondary soap surfactants should
preferably contain no ether linkages, no ester linkages and no hydroxyl
groups. There should preferably be no nitrogen atoms in the head-group
(amphiphilic portion). The secondary soap surfactants usually contain 11-15
total carbon atoms, although slightly more (e.g., up to 16) can be tolerated,
CA 02226666 1998-O1-12
WO 97/~3156 ~; PCT/US96/11249
g
e.g. p-octyl benzoic acid.
The following general structures further illustrate some of the preferred
secondary soap surfactants:
A. A highly preferred class of secondary soaps comprises the secondary
carboxyl materials of the formula R3 CH(R4)COOM, wherein R3 is
CH3(CH2)x and R4 is CH3(CH2)y, wherein y can be O or an integer from 1
to 4, x is an integer from 4 to 10 and the sum of (x + y) is 6-10, preferably
7-
9, most preferably 8.
B. Another preferred class of secondary soaps comprises those carboxyl
compounds wherein the carboxyl substituent is on a ring hydrocarbyl unit,
i.e., secondary soaps of the formula R5-R6-COOM, wherein R5 is C~-C10,
preferably C8-C9, alkyl or alkenyl and R6 is a ring structure, such as
benzene, cyclopentane and cyclohexane. (Note: R5 can be in the ortho,
mete or para position relative to the carboxyl on the ring.)
C. Still another preferred class of secondary soaps comprises secondary
carboxyl compounds of the formula
CH3(CHR)k-(CH2)m-(CHR)n-CH(COOM)(CHR)o-(CH2)p-(CHR)q-CHg,
wherein each R is C1-C4 alkyl, wherein k, n, o, q are integers in the range
of 0-8, provided that the total number of carbon atoms (including the
carboxylate) is in the range of 10 to 18.
In each of the above formulas A, B and C, the species M can be any
suitable, especially water-solubilizing, counterion.
Especially prefen-ed secondary soap surtactants for use herein are water-
soluble members selected from the water-soluble salts of 2-methyl-1-
undecanoic acid, 2-ethyl-1-decanoic acid, 2-propyl-1-nonanoic acid, 2-butyl-
1-octanoic acid and 2-pentyl-1-heptanoic acid.
Alkali metal sarcosinate surfactant
Other suitable anionic surfactants are the alkali metal sarcosinates of
formula R-CON (R1) CH2 COOM, wherein R is a C5-C17 linear or branched
CA 02226666 1998-O1-12
WO 97/03156 ~: PCT/US96/11249
9
alkyl or alkenyl group, R1 is a C1-C4 alkyl group and M is an alkali metal
ion. Preferred examples are the myristyl and oleyl methyl sarcosinates in the
form of their sodium salts.
Cationic ester surfactant
An essential component of the surfactant system is a water dispersible
cationic ester surfactant. That is, a water dispersible compound having
surfactant properties comprising at least one ester (ie -COO-) linkage and at
least one cationically charged group.
Excluded from the cationic ester surfactant species of the invention is the
ester compound formed from two moles of stearic acid and one mole of tri-
ethanol-methyl-ammonium chloride, e.g. N,N-di(2-stearoyloxyethyl)-N-(2-
hydroxyethyl)-N-methyl ammonium chloride.
Suitable cationic ester surfactants, including choline ester surfactants, have
for example been disclosed in US Patents No.s 4228042, 4239660 and
4260529.
Preferred water dispersible cationic ester surfactants are those having the
formula:
R2
RS ~ +
Rl - (C~np b (X)u -( C H 2 )m (~~ (C H2 )t -N-R3 M
a
R4
wherein R1 is a C5-C31 linear or branched alkyl, alkenyl or alkaryl chain or
M-. N+(R6R7Rg)(CH2)s; X and Y, independently, are selected from COO,
OCO, O, CO, OCOO, CONH, NHCO, OCONH and NHCOO wherein at least
one of X or Y is a COO, OCO, OCOO, OCONH or NHCOO group; R2, R3,
Rd, R6, R7, and Rg are independently selected from alkyl, alkenyl,
hydroxyalkyl, hydroxy-alkenyi and alkaryl groups having from 1 to 4 carbon
CA 02226666 1998-O1-12
WO 97103156 i, PCT/US96/11249
to
atoms; and R5 is independently H or a C1-C3 alkyl group; wherein the
values of m, n, s and t independently lie in the range of from 0 to 8, the
value of b lies in the range from 0 to 20, and the values of a, a and v
independently, are either 0 or 1 with the proviso that at least one of a or v
must be 1; and wherein M is a counter anion.
Preferably R2,Rg and R4 are independently selected from CHg and
-CH2CH20H.
Preferably M is selected from halide, methyl sulfate, sulfate, and nitrate,
more preferably methyl sulfate, chloride, bromide or iodide.
Prefen-ed water dispersible cationic ester surfactants are the choline esters
having the formula:
O CH3
Rl-C-O-CH2CH2-N~ CH3 M-
CH3
wherein R1 is a C11-C19 linear or branched alkyl chain.
Particularly preferred choline esters of this type include the stearoyl
choline
ester quaternary methylammonium halides (R1=C17 alkyl), palmitoyl choline
ester quaternary methylammonium halides (R1=C15 alkyl), myristoyl
choline ester quaternary methylammonium halides (R1=C13 alkyl), lauroyl
choline ester methyiammonium halides (R1=C11 alkyl), cocoyl choline ester
quaternary methylammonium halides (R1=C11_C13 alkyl), tallowyl choline
ester quaternary methylammonium halides (R1=C15_C17 alkyl), and any
mixtures thereof.
Most preferred choline ester compounds among the above disclosed are
cocoyl choline ester quaternary methylammonium halides.
The particularly preferred choline esters, given above, may be prepared by
the direct esterification of a fatty acid of the desired chain length with
dimethylaminoethanol, in the . presence of an acid catalyst. The reaction
product is then quaternized with a methyl halide, forming the desired
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/US96/11249
11
cationic material. They may also be prepared by the direct esterification of a
long chain fatty acid of the desired chain length together with 2-haloethanol,
in the presence of an acid catalyst material. The reaction product is then
quaternized with trimethylamine, forming the desired cationic material.
Other suitable cationic ester surfactants have the structural formulas below,
wherein d may be from 0 to 20.
O CH3
Rl-O-~-( CH2 )~C-O-CH2CH2-N~ CH3 M
~H3 _
CH3 O O CH3
M CH3-N ~CHz-CH2-O-C-( CH2 )d C-O-CH2-CH2-~~ CH3M
CH3 ~H3
Preferably, the cationic ester surfactant is present in amount from 1 % to
20°~, preferably 4°i6 to 15% and more preferably 5% to 12% by
weight of the
surfactant system.
Proteolytic enzyme
An essential component of the detergent composition is an enzyme showing
proteolytic activity.
For the purpose of the invention, the level of proteolytic enzyme in the
formulation is based on an enzyme activity of 4 Knpu/g of the enzyme
particle.
The compositions herein will typically comprise from 0.0001 % to 5%,
preferably from 0.001 % to 4% and more preferably from 0.005% to 2%
active protease by weight of the composition.
The weight ratio of said anionic surfactant to said proteolytic enzyme is at
least 1.5:1, preferably at least 3:1 and more preferably at least 5:1.
CA 02226666 2000-12-14
12
Suitable enzymes, for the purpose of the invention, have for example been
. disclosed in US Patents 3,519,570 and 3,533,139.
Non limiting examples of proteolytic enzymes which can be used for the
purpose of the invention include Savinase, enzyme of the Bacillus Lentus
type backbone such as Maxacal, Opticlean, Durazym and Properase,
enzyme of the Bacillus Licheniformis type backbone such as Alcalase and
Maxatase and enzyme of the Bacillus Amyloliquefaciens type backbone
such as Primase.
Preferred commercially available protease enzymes include those sold
under the trademarks Al~lase, Savinase, Primase, Durazym, and
' Esperase by Novo Industries A/S (Denmark), those sold under the
trademarks Maxatase, Maxacal and Maxapem by Gist-Brocades, those sold
by Genencor International, and those sold under the trademarks Opticlean
and Optimase by Solvay Enzymes. Mixture of the herein before described
proteases may be used.
' A most preferred protease is Savinase.
The detergent composition of the invention has further been found to
produce an enhanced soil removal performance in presence of one or more
additional enzymes selected from amylase, cellulase, lipase, peroxidase,
endoglucanase enzymes and mixtures thereof, preferably amylase
enzymes.
These enzymes may be incorporated into the composition in accordance
with the invention at a level of 0.0001 °~ to 5% alive enzyme by weight
of
the composition.
Preferred amylases usable in the present invention include, for example, a-
amylases obtained from a special strain of B licheniformis, described in
more detail in GB-1,269,839 (Novo). Preferred commercially available
amylases inGude for example, those sold under the trademark Rapidase by
Gist-Brocades, and those sold under the trademark Termamyl and BAN by
Novo Industries AIS.
CA 02226666 2000-12-14
13
The cellulases usable in the present invention include both bacterial or
fungal cellulase. Preferably, they will have a pH optimum of between 5 and
9.5. Suitable cellulases are disclosed in U.S. Patent 4,435,307, which
discloses fungal cellulase produced from Humiccla insolens and Humicola
strain DSM1800 or a cellulase 212-producing fungus belonging to the genus
Aeromonas, and cellulase extracted from the hepatopancreas of a marine
mollusk (Doiabella Auricula Solander). Suitable cellulases are also
disclosed in GB-A-2.075.028; GB-A-2.095.275 and DE-OS-2.247.832.
TM TM
ENDO A, CAREZYME both from Novo Industries AIS are especially useful.
Suitable lipase enzymes for detergent usage inGude those produced
by microorganisms of the Pseudomonas group, such as Pseudomonas
stutzeri ATCC 19.154, as disclosed in GB 1,372,034. See also lipases in
Japanese Patent Application 53,20487, laid open to public inspection on
February 24, 1978. This lipase is available from Amano Pharmaceutical Co.
Ltd., Nagoya, Japan, under. the trade mark Lipase P "Amano," hereinafter
referred to as "Amano-P." Other commercial lipases include Amano-CES,
lipases ex Chromobader viscosum, e.g. Chromobacter viscosum var.
. lipolyticum NRRLB 3673, commercially available from Toyo Jozo Co.,
Tagata, Japan; and further Chromobacter viscosum lipases from U.S.
Biochemical Corp., U.S.A. and Disoynth Co., The Netherlands, lipases ex
Pseudomonas gladioli. Also suitable are cutinases (EC 3.1.1.SOj which can
be considered as a special kind of lipase, namely lipases which do not
require interfacial activation. Addition of cutinases to detergent
compositions
have been described in e.g. EP-B-0,322,429 (Genencor) and EP-A-
0,652,939 (Unilever).
rM
The LIPOLASE enzyme derived from Humicola lanuginosa and
commercially available from Novo (see also EP 341,947) is a preferred
lipase for use herein.
Peroxidase enzymes are used in combination with oxygen sources,
e.g., percarbonate, perborate, pe~sulfate, hydrogen peroxide, etc. They are
used for "solution bleaching," i.e. to prevent transfer of dyes or pigments
removed from substrates during wash operations to other substrates in the
wash solution. Peroxidase enzymes are known in the art, and include, for
example, horseradish peroxidase, ligninase, and haloperoxidase such as
chloro- and bromo-peroxidase. Peroxidase-containing detergent
compositions are disclosed, for example, in EP-A-0,424,398.
CA 02226666 1998-O1-12
WO 97/03156 ;; PCT/US96/11249
14
A wide range of enzyme materials and means for their incorporation
into synthetic detergent compositions are also disclosed in U.S. Patent
3,553,139. Enzymes are further disclosed in U.S. Patent 4,101,457 and in
U.S. Patent 4,507,219. Enzyme materials useful for liquid detergent
formulations, and their incorporation into such formulations, are disclosed in
U.S. Patent 4,261,868. Enzymes for use in detergents can be stabilized by
various techniques. Enzyme stabilisation techniques are disclosed and
exemplified in U.S. Patent 3,600,319 and EP 0 199 405. Enzyme
stabilisation systems are also described, for example, in U.S. Patent
3,519,570.
Optionally, the surfactant system may further comprises additional
surfactants which are not detrimental to the system. Such surfactants may
include nonionic, ampholytic, amphoteric, zwitterionic, and non-ester
cationic surfactants and mixtures thereof.
Additional surfactant
The additional surfactant is preferably present at a level from 0.1 % to 50%,
more preferably ftom 1 °r6 to 40%, most preferably from 5% to 30% by
weight
of the surfactant system.
A typical listing of nonionic, ampholytic, and zwitterionic classes, and
species of these surfactants, is given in U.S.P. 3,929,678 issued to Laughlin
and Heuring on December 30, 1975. Further examples are given in "Surface
Active Agents and Detergents" (Vol. I and II by Schwartz, Peny and Berch).
Nonionic surfactant
Essentially any anionic surfactants useful for detersive purposes can be
included in the compositions. Exemplary, non-limiting classes of useful
nonionic surfactants are listed below.
Nonionic Dolvhvdroxv fatty acid amide surfactant '
Polyhydroxy fatty acid amides suitable for use herein are those having the
structural formula R2CONR1Z wherein : R1 is H, C1-C4 hydrocarbyl, 2- '
hydroxy ethyl, 2-hydroxy propyl, ethoxy, propoxy group or a mixture thereof,
preferable C1-C4 alkyl, more preferably C1 or C2 alkyl, most preferably C1
alkyl (i.e., methyl); and R2 is a C5-C31 hydrocarbyl, preferably straight-
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/US96/11249
IS
chain C5-C1 g alkyl or alkenyl, more preferably straight-chain Cg-C17 alkyl
or alkenyl, most preferably straight-chain C11-C17 alkyl or alkenyl, or
mixture thereof; and Z is a polyhydroxyhydrocarbyl having a linear
hydrocarbyl chain with at least 3 hydroxyls directly connected to the chain,
or an alkoxylated derivative (preferably ethoxylated or propoxylated)
thereof. Z preferably will be derived from a reducing sugar in a reductive
amination reaction; more preferably Z is a glycityl.
Nonionic condensates of alkyl phenols
The polyethylene, polypropylene, and polybutylene oxide condensates of
alkyl phenols are suitable for use herein. In general, the polyethylene oxide
condensates are preferred. These compounds include the condensation -
products of alkyl phenols having an alkyl group containing from about 6 to
about 18 carbon atoms in either a straight chain or branched chain
configuration with the alkylene oxide.
Nonionic ethoxvlated alcohol surfactant
The alkyl ethoxylate condensation products of aliphatic alcohols with from
about 0 to about 25 moles of ethylene oxide are suitable for use herein. The
alkyl chain of the aliphatic alcohol can either be straight or branched,
primary or secondary, and generally contains from 6 to 22 carbon atoms.
Particularly preferred are the condensation products of alcohols having an
alkyl group containing from 8 to 20 carbon atoms with from about 2 to about
moles of ethylene oxide per mole of alcohol.
Nonionic ethoxvlated/propoxylated fatty alcohol surfactant
The ethoxylated Cg-C1 g fatty alcohols and Cg-C1 g mixed
ethoxylated/propoxylated fatty alcohols are suitable surfactants for use
herein, particularly where water soluble. Preferably the ethoxylated fatty
alcohols are the C1 p-C1 g ethoxylated fatty alcohols with a degree of
ethoxylation of from 3 to 50, most preferably these are the C12-C18
ethoxylated fatty alcohols with a degree of ethoxylation from 3 to 40.
Preferably the mixed ethoxylated/propoxylated fatty alcohols have an alkyl
chain length of from 10 to 18 carbon atoms, a degree of ethoxylation of from
3 to 30 and a degree of propoxyiation of from 1 to 10.
CA 02226666 1998-O1-12
WO 97/03156 ;; PCT/US96/11249
16
Nonionic EO/PO condensates with propvlene 4lvcol
The condensation products of ethylene oxide with a hydrophobic base
formed by the condensation of propylene oxide with propylene glycol are
suitable for use herein. The hydrophobic portion of these compounds
preferably has a molecular weight of from about 1500 to about 1800 and
exhibits water insolubility. Examples of compounds of this type include
certain of the commercially-available PluronicTM surfactants, marketed by
BASF.
Nonionic EO condensation products with propylene oxide/ethylene diamine
adducts
The condensation products of ethylene oxide with the product resulting from -
the reaction of propylene oxide and ethylenediamine are suitable for use
herein. The hydrophobic moiety of these products consists of the reaction
product of ethylenediamine and excess propylene oxide, and generally has
a molecular weight of from about 2500 to about 3000. Examples of this type
of nonionic surfactant include certain of the commercially available
TetronicTM compounds, marketed by BASF.
Nonionic alkylpolysaccharide surfactant
Suitable alkylpolysaccharides for use herein are disclosed in U.S. Patent
4,565,647, Llenado, issued January 21, 1986, having a hydrophobic group
containing from about 6 to about 30 carbon atoms, preferably from about 10
to about 16 carbon atoms and a polysaccharide, e.g., a polyglycoside,
hydrophilic group containing from about 1.3 to about 10, preferably from
about 1.3 to about 3, most preferably from about 1.3 to about 2.7 saccharide
units. Any reducing saccharide containing 5 or 6 carbon atoms can be
used, e.g., glucose, galactose and galactosyl moieties can be substituted for
the glucosyl moieties. (Optionally the hydrophobic group is attached at the
2-, 3-, 4-, etc. positions thus giving a glucose or galactose as opposed to a
glucoside or galactoside.) The intersaccharide bonds can be, e.g., between
the one position of the additional saccharide units and the 2-, 3-, 4-, and/or
6- positions on the preceding saccharide units.
The preferred alkylpolyglycosides have the formula
R20(CnH2n0)t(9lYcosyl)x
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/US96/11249
17
wherein R2 is selected from alkyl, alkylphenyl, hydroxyalkyl,
hydroxyalkylphenyl, and mixtures thereof in which the alkyl groups contain
from 10 to 18, preferably from 12 to 14, carbon atoms; n is 2 or 3; t is from
0
to 10, preferably 0, and X is from 1.3 to 8, preferably from 1.3 to 3, most
preferably from 1:3 to 2.7. The glycosyl is preferably derived from glucose.
Nonionic fatty acid amide surfactant
Fatty acid amide surfactants suitable for use herein are those having the
formula: R6CON(R7)2 wherein R6 is an alkyl group containing from 7 to 21,
preferably from 9 to 17 carbon atoms and each R7 is selected from
hydrogen, C1-C4 alkyl, C1-C4 hydroxyalkyl, and -(C2H40)xH, where x is in
the range of from 1 to 3.
Amahoteric surfactant
Suitable amphoteric surfactants for use herein include the amine oxide
surfactants and the alkyl amphocarboxylic acids.
A suitable example of an alkyl aphodicarboxylic acid for use herein is
Miranol(TM) C2M Conc. manufactured by Miranol, Inc., Dayton, NJ.
Amine Oxide surfactant
Amine oxides useful herein include those compounds having the formula
R3(OR4)xN0(R5)2 wherein R3 is selected from an alkyl, hydroxyalkyl,
acylamidopropoyl and alkyl phenyl group, or mixtures thereof, containing
from 8 to 26 carbon atoms, preferably 8 to 18 carbon atoms; R4 is an
alkylene or hydroxyalkylene group containing from 2 to 3 carbon atoms,
preferably 2 carbon atoms, or mixtures thereof; x is from 0 to 5, preferably
from 0 to 3; and each R5 is an alkyl or hydyroxyalkyl group containing from
1 to 3, preferably from 1 to 2 carbon atoms, or a polyethylene oxide group
containing from 1 to 3, preferable 1, ethylene oxide groups. The R5 groups
can be attached to each other, e.g., through an oxygen or nitrogen atom, to
form a ring structure.
These amine oxide surfactants in particular include C10-C18 alkyl dimethyl
amine oxides and Cg-C1 g alkoxy ethyl dihydroxyethyl amine oxides.
Examples of such materials include dimethyloctylamine oxide,
diethyldecylamine oxide, bis-(2-hydroxyethyl)dodecylamine oxide,
dimethyldodecylamine oxide, dipropyltetradecylamine oxide,
CA 02226666 1998-O1-12
WO 97/03156 t; PCT/US96/11249
I8
methylethylhexadecylamine oxide, dodecylamidopropyl dimethylamine
oxide, cetyl dimethylamine oxide, stearyl dimethylamine oxide, tallow
dimethylamine oxide and dimethyl-2-hydroxyoctadecylamine oxide.
Preferred are C10-C1 g alkyl dimethylamine oxide, and C10-1 g acylamido
alkyl dimethylamine oxide.
Zwitterionic surfactant
Zwitterionic surfactants can also be incorporated into the detergent
compositions hereof. These surfactants can be broadly described as
derivatives of secondary and tertiary amines, derivatives of heterocyclic
secondary and tertiarjr amines, or derivatives of quaternary ammonium,
quaternary phosphonium or tertiary sulfonium compounds. Betaine and
sultaine surfactants are exemplary zwitterionic surfactants for use herein.
Betaine surfactant
The betaines useful herein are those compounds having the formula
R(R')2N+R2C00- wherein R is a C6-C1 g hydrocarbyl group, preferably a
C10-C16 alkyl group or C10-16 acylamido alkyl group, each R1 is typically
C1-C3 alkyl, preferably methyl,m and R2 is a C1-C5 hydrocarbyl group,
preferably a C1-C3 alkylene group, more preferably a C1-C2 alkylene
group. Examples of suitable betaines include coconut
acyiamidopropyldimethyl betaine; hexadecyl dimethyl betaine; C12-14
acylamidopropylbetaine; Cg-14 acylamidohexyldiethyl betaine; 4[C14-16
acylmethylamidodiethylammonio]-1-carboxybutane; C 16-18
acyiamidodimethylbetaine; C12-16 acylamidopentanediethyl-betaine; [C12-
16 acyimethylamidodimethylbetaine. Preferred betaines are C12-18
dimethyl-ammonio hexanoate and the C10-1 g acylamidopropane (or
ethane) dimethyl (or diethyl) betaines. Complex betaine surfactants are also
suitable for use herein.
Sultaine surfactant
The sultaines useful herein are those compounds having the formula
(R(R1 )2N+R2S03- wherein R is a C6-C1 g hydrocarbyl group, preferably a
C10-C16 alkyl group, more preferably a C12-C13 alkyl group, each R1 is
typically C1-C3 alkyl, preferably methyl, and R2 is a C1-C6 hydrocarbyl
group, preferably a C1-C3 alkylene or, preferably, hydroxyalkylene group.
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/US96/11249
19
Ampholytic surfactant
Ampholytic surfactants can be incorporated into the detergent compositions
herein. These surfactants can be broadly described as aliphatic derivatives
of secondary or tertiary amines, or aliphatic derivatives of heterocyclic
secondary and tertiary amines in which the aliphatic radical can be straight
chain or branched.
Non-ester cationic surfactants
Non ester cationic surfactants can also be used in the detergent
compositions herein. Suitable non ester cationic surfactants include the
quaternary ammonium surfactants selected from mono Cg-Clg, preferably
Cg-C10 N-alkyl or alkenyl ammonium surfactants wherein the remaining N '
positions are substituted by methyl, hydroxyethyl or hydroxypropyl groups.
Optionally, the detergent composition of the invention may further comprises
additional components which are not detrimental to the composition. Such
components include builders, chelants, alkaline hydrogen peroxide sources,
peroxyacid bleach precursors, polymeric dispersing agents and
conventional detersive adjuncts.
Builders
Detergent builders can optionally be included in the compositions herein to
assist in controlling mineral hardness. Inorganic as well as organic builders
can be used. Builders are typically used in fabric laundering compositions
to assist in the removal of particulate soils.
The level of builder can vary widely depending upon the end use of
the composition and its desired physical form. When present, the
compositions will typically comprise at least 1 % builder. Granular
formulations typically comprise from 10% to 80%, more typically from 15%
to 50°~ by weight, of the detergent builder. Lower or higher levels of
builder, however, are not meant to be excluded.
Inorganic or phosphate-containing detergent builders include, but are
not limited to, the alkali metal, ammonium and alkanolammonium salts of
polyphosphates (exemplified by the tripolyphosphates, pyrophosphates, and
glassy polymeric meta-phosphates).
Non-phosphate builders may also be used. These can include, but are not
restricted to phytic acid, silicates, alkali metal carbonates (including
CA 02226666 1998-O1-12
WO 97/03156 , PCT/ITS961i1249
bicarbonates and sesquicarbonates), sulphates, aluminosilicates,
monomeric polycarboxylates, homo or copolymeric polycarboxylic acids or
their salts in which the polycarboxylic acid comprises at least two carboxylic
radicals separated from each other by not more than two carbon atoms.
Examples of silicate builders are the crystalline layered silicates, such as
the layered sodium silicates described in U.S. Patent 4,664,839. NaSKS-6
is the trademark for a crystalline layered silicate marketed by Hoechst
(commonly abbreviated herein as "SKS-6"). Unlike zeolite builders, the Na
SKS-6 silicate builder does not contain aluminum. NaSKS-6 has the delta-
Na2Si205 morphology form of layered silicate. It can be prepared by
methods such as those described in German DE-A-3,417,649 and DE-A-
3,742,043. SKS-6 is a highly preferred layered silicate for use herein, but _
other such layered silicates, such as those having the general formula
NaMSix02x+l.yH20 wherein M is sodium or hydrogen, x is a number from
1.9 to 4, preferably 2, and y is a number from 0 to 20, preferably 0 can be
used herein. Various other layered silicates from Hoechst include NaSKS-
5, NaSKS-7 and NaSKS-11, as the alpha, beta and gamma forms. As noted
above, the delta-Na2Si205 (NaSKS-6 form) is most preferred for use
herein. Other silicates may also be useful such as for example magnesium
silicate, which can serve as a crispening agent in granular formulations, as
a stabilising agent for oxygen bleaches, and as a component of suds control
systems.
Aluminosilicate builders are especially useful in the present
invention. Aluminosilicate builders are of great importance in most currently
marketed heavy duty granular detergent compositions, and can also be a
significant builder ingredient in liquid detergent formulations.
Aluminosilicate builders include those having the empirical formula:
Naz[(A102)z(Si02)y].xH20
wherein z and y are integers of at least 6, the molar ratio of z to y is in
the
range from 1.0 to 0.5, and x is an integer from 15 to 264.
Useful aluminosilicate ion exchange materials are commercially
available. These aluminosilicates can be crystalline or amorphous in
structure and can be naturally-occurring aluminosilicates or synthetically
derived. A method for producing aluminosilicate ion exchange materials is '
disclosed in U.S. Patent 3,985,669. Preferred synthetic crystalline
aluminosilicate ion exchange materials useful herein are available under the
designations Zeolite A, Zeolite P (B), Zeolite MAP and Zeolite X. In an
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/US96/11249
21
especially preferred embodiment, the crystalline aluminosilicate ion
exchange material has the formula:
Nal2[(A102)12(Si02)121.xH20
wherein x is from 20 to 30, especially 27. This material is known as Zeolite
A. Dehydrated zeolites (x = 0 - 10) may also be used herein. Preferably,
the aluminosilicate has a particle size of 0.1-10 microns in diameter.
Organic detergent builders suitable for the purposes of the present
invention include, but are not restricted to, a wide variety of
polycarboxylate
compounds. As used herein, "polycarboxylate" refers to compounds having
a plurality of carboxylate groups, preferably at least 3 carboxylates.
Polycarboxylate builder can generally be added to the composition in acid
form, but can also be added in the form of a .neutralised salt. When utilized
in salt form, alkali metals, such as sodium, potassium, and lithium, or
alkanolammonium salts are preferred.
Included among the polycarboxylate builders are a variety of
categories of useful materials. One important category of polycarboxylate
builders encompasses the ether polycarboxylates, including oxydisuccinate,
as disclosed in U.S. Patent 3,128,287 and U.S. Patent 3,635,830. See also
'TMSlTDS" builders of U.S. Patent 4,663,071. Suitable ether
polycarboxylates also include cyclic compounds, particularly alicyclic
compounds, such as those described in U.S. Patents 3,923,679; 3,835,163;
4,158,635; 4,120, 874 and 4,102, 903.
Other useful detergency builders include the ether
hydroxypolycarboxylates, copolymers of malefic anhydride with ethylene or
vinyl methyl ether, or acrylic acid, 1, 3, 5-trihydroxy benzene-2, 4, 6-
trisulphonic acid, and carboxymethyloxysuccinic acid, the various alkali
metal, ammonium and substituted ammonium salts of polyacetic acids such
as ethylenediamine tetraacetic acid and nitrilotriacetic acid, as well as
polycarboxylates such as mellitic acid, succinic acid, oxydisuccinic acid,
polymaleic acid, benzene 1,3,5-tricarboxylic acid, carboxymethyloxysuccinic
acid, and soluble salts thereof.
Citrate builders, e.g., citric acid and soluble salts thereof (particularly
sodium salt), are polycarboxylate builders of particular importance for heavy
duty liquid detergent formulations due to their availability from renewable
resources and their biodegradability. Citrates can also be used in granular
compositions, especially in combination with zeolite and/or layered silicate
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/L1S96/11249
22
builders. Oxydisuccinates are also especially useful in such compositions
and combinations.
Also suitable in the compositions containing the present invention
are the 3,3-dicarboxy-4-oxa-1,6-hexanedioates and the related compounds
disclosed in U.S. Patent 4,566,984. Useful succinic acid builders include '
the C5-C20 alkyl and alkenyl succinic acids and salts thereof. A particularly
preferred compound of this type is dodecenylsuccinic acid. Specific
examples of succinate builders include: laurylsuccinate, myristylsuccinate,
palmitylsuccinate, 2-dodecenylsuccinate (preferred), 2-pentadecenylsucci-
nate, and the like. Laurylsuccinates are the preferred builders of this group,
and are described in EP 0,200,263.
Other suitable polycarboxylates are disclosed in U.S. Patent _
4,144,226 and in U.S. Patent 3,308,067. See also U.S. Pat. 3,723,322.
Fatty acids, e.g., C12-C1g monocarboxylic acids, can also be
incorporated into the compositions alone, or in combination with the
aforesaid builders, especially citrate and/or the succinate builders, to ,
provide additional builder activity. Such use of fatty acids will generally
result in a diminution of sudsing, which should be taken into account by the
formulator.
Chelants
Chelating agents generally comprise from 0.1 % to 10% by weight of
the compositions herein. More preferably, if utilized, the chelating agents
will comprise from 0.1 % to 3.0% by weight of such compositions.
A chelating agent can be selected from amino carboxylate, organic
phosphonate, polyfunctionally-substituted aromatic compound, nitriloacetic
acid and mixture thereof. Without intending to be bound by theory, it is
believed that the benefit of these materials is due in part to their
exceptional
ability to remove transition metal ions such as iron and manganese ions
from washing solutions by formation of soluble chelates.
Amino carboxylates useful as optional chelating agents include
ethylenediaminetetracetates, ethylenediamine disuccinate, N-
hydroxyethyiethylenediaminetriacetates, 2-hydroxypropylene diamine .
disuccinate, nitrilotriacetates, ethylenediamine tetraproprionates,
triethylenetetraaminehexacetates, ethylene triamine pentaacetate,
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/ITS96/11249
23
diethylenetriaminepentaacetates, and ethanoldiglycines, alkali metal,
ammonium, and substituted ammonium salts therein and mixtures therein.
Preferred amino carboxylates chelants for use herein are ethylenediamine
disuccinate ("EDDS"), especially the [S,S] isomer as described in U.S.
Patent 4,704,233, ethylenediamine-N,N'-diglutamate (EDDG) and 2-
hydroxypropylene-diamine-N,N'-disuccinate (HPDDS) compounds.
A most preferred amino carboxylate chelant is ethylenediamine disuccinate.
Organic phosphonates are also suitable for use as chelating agents
in the compositions of the invention when at least low levels of total
phosphorus are permitted in detergent compositions, and include
ethylenediaminetetrakis (methylenephosphonates) available under the
trademark DEQUEST from Monsanto, diethylene triamine penta (methylene
phosphonate), ethylene diamine tri (methylene phosphonate),
hexamethylene diamine tetra (methylene phosphonate), a-hydroxy-2 phenyl
ethyl diphosphonate, methylene diphosphonate, hydroxy 1,1-hexylidene,
vinylidene 1,1 diphosphonate, i,2 dihydroxyethane 1,1 diphosphonate and
hydroxy-ethane 1,1 diphosphonate.
Preferably, these amino phosphonates do not contain alkyl or alkenyl
groups with more than 6 carbon atoms.
Preferred chelants are the diphosphonate derivatives selected from a-
hydroxy-2 phenyl ethyl diphosphonate, methylene diphosphonate, hydroxy
1,1-hexylidene, vinylidene 1,1 diphosphonate, 1,2 dihydroxyethane 1,1
diphosphonate and hydroxy-ethane 1,1 diphosphonate. A most preferred is
hydroxy-ethane 1,1 diphosphonate.
Polyfunctionally-substituted aromatic chelating agents are also useful in the
compositions herein. See U.S. Patent 3,812,044. Preferred compounds of
this type in acid form are dihydroxydisulfobenzenes such as 1,2-dihydroxy-
3,5-disulfobenzene.
Alkaline hydroaen peroxide sources
Detergent compositions of the present invention may include an inorganic
perhydrate bleach, normally in the form of the sodium salt, as the source of
alkaline hydrogen peroxide in the wash liquor. This perhydrate is normally
incorporated at a level of from 1 % to 40% by weight, more preferably from
5°~ to 35°~ by weight and most preferably from 8% to 30% by
weight of the
composition.
CA 02226666 1998-O1-12
WO 97/03156 ,, PCTlUS96/11249
24
The perhydrate may be any of the alkali metal inorganic salts such as
perborate monohydrate or tetrahydrate, percarbonate, perphosphate and
persilicate salts, but is conventionally an alkali metal perborate or
percarbonate.
Sodium percarbonate, which is the preferred perhydrate, is an addition
compound having a formula corresponding to 2Na2C~3.3H202, and is
available commercially as a crystalline solid. Most commercially available
material includes a low level of a heavy metal sequestrant such as EDTA, 1-
hydroxyethylidene 1,_ 1-diphosphonic acid (HEDP) or an amino-
phosphonate, that is incorporated during the manufacturing process. For
the purposes of the detergent composition aspect of the present invention, -
the percarbonate can be incorporated into detergent compositions without
additional protection, but preferred executions of such compositions utilise a
coated form of the material. A variety of coatings can be used including
borosilicate borate, boric acid and citrate or sodium silicate of Si02:Na20
ratio from 1.6:1 to 3.4:1, preferably 2.8:1, applied as an aqueous solution to
give a level of from 2% to 10%, (normally from 3% to 5%) of silicate solids
by weight of the percarbonate. However the most preferred coating is a
mixture of sodium carbonate and sulphate or sodium chloride.
The particle size range of the crystalline percarbonate is from 350
micrometers to 1500 micrometers with a mean of approximately 500-1000
micrometers.
Peroxvacid bleach yrecursor
Peroxyacid bleach precursors are compounds which react with hydrogen
peroxide in a perhydrolysis reaction to produce a peroxyacid. Generally
peroxyacid bleach precursors may be represented as
O
X-C-L
where t_ is a leaving group and X is essentially any functionality, such that
on perhydrolysis the structure of the peroxyacid produced is
CA 02226666 1998-O1-12
WO 97/03156 i, PCT/US96/11249
O
X-C-OOH
Peroxyacid bleach precursor compounds are preferably incorporated at a
level of from 0.1 % to 60% by weight, more preferably from 0.5% to 40% by
weight of the detergent composition.
Leaving groups
The leaving group, hereinafter L group, must be sufficiently reactive for the
perhydrolysis reaction to occur within the optimum time frame (e.g., a wash
cycle). However, if L is too reactive, this activator will be difficult to
stabilize
for use in a bleaching composition. '
Prefen-ed L groups are selected from the group consisting of:
Y R3 RsY
-O ~ , ~ Y , and -
O O
-N-C-R~ ~ -N N -N-C-CH-R4
R3 ' ~ ~ R3 Y
I
Y
R3 Y
I I
-O-C H=C-C H=C HZ -O-C H=C-C H=C H2
O O
O C H -C Y~--C
4
-p-C-R~ -N\CiNR4 ' -N\C jNR
' O O
R3 O Y
-O-C=C HR4 , and -N-S-C H-R4
R3 O
CA 02226666 1998-O1-12
WO 97/03156 ,, PCT/US96/11249
26
and mixtures thereof, wherein R1 is an alkyl, aryl, or alkaryl group
containing from 1 to 14 carbon atoms, R3 is an alkyl chain containing from 1
to 8 carbon atoms, R4 is H or R3, and Y is H or a solubilizing group. Any of ,
R1, R3 and R4 may be substituted by essentially any functional group
including, for example alkyl, hydroxy, alkoxy, halogen, amine, nitrosyl,
amide and ammonium or alkyl ammonium groups
The preferred solubilizing groups are -SO -M+, -CO -M+, -SO -M+,
3 2 4
-N+(R3)4X- and O<-N(R3)3 and most preferably -S03 M+ and -C02-M+
wherein R3 is an alkyl chain containing from 1 to 4 carbon atoms, M is a
ration which provides solubility to the bleach activator and X is an anion
which provides solubility to the bleach activator. Preferably, M is an alkali
metal, ammonium or substituted ammonium ration, with sodium and
potassium being most preferred, and X is a halide, hydroxide, methylsulfate
or acetate anion.
Suitable peroxyacid bleach precursor materials are compounds containing
one or more N- or O-aryl groups. These can be selected from a wide range
of classes that include anhydrides, esters, imides, lactams and acylated
derivatives of imidazoles and oximes. Examples of useful materials within
these classes are disclosed in GB-A-1586789. Suitable esters are disclosed
in GB A-836988, 864798, 1147871, 2143231 and EP A-0170386.
Detergent compositions containing mixtures of any of the precursors
hereinafter disclosed are also contemplated by the present invention.
Perbenzoic acid precursor
Perbenzoic acid precursor compounds provide perbenzoic acid on
perhydrolysis.
Suitable O-acylated perbenzoic acid precursor compounds include the
substituted and unsubstituted benzoyl oxybenzene sulfonates, including for
example benzoyl oxybenzene sulfonate:
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/US96/11249
27
0
~/ S03
Also suitable are the benzoylation products of sorbitol, glucose, and all
saccharides with benzoylating agents, including for example:
OAc
_Ac0 ~O
OAc
OAc
OBz
Ac = COCH3; Bz = Benzoyl
Perbenzoic acid precursor compounds of the imide type include N-benzoyl
succinimide, tetrabenzoyl ethylene diamine and the N-benzoyl substituted
ureas. Suitable imidazole type perbenzoic acid precursors include N-
benzoyl imidazole and N-benzoyl benzimidazole and other useful N-acyl
group-containing perbenzoic acid precursors include N-benzoyl pyrrolidone,
dibenzoyl taurine and benzoyl pyroglutamic acid.
Other perbenzoic acid precursors include the benzoyl diacyl peroxides, the
benzoyl tetraacyl peroxides, and the compound having the formula:
0 0
oil
d ~~COOH
Phthalic anhydride is another suitable perbenzoic acid precursor compound
herein:
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/US96/11249
28
0
Suitable N-acylated precursor compounds of the lactam class are disclosed
generally in GB-A-855735.
Suitable caprolactam bleach precursors which may be used herein are of
the formula:
0
p . C CH2 CH2
(~ ~ \
CH2
R6 C N
CH2 CH2
wherein R6 is H or an alkyl, aryl, alkoxyaryl or alkaryl group containing from
1 to 12 carbon atoms, preferably from 6 to 12 carbon atoms.
Suitable valero lactams have the formula:
0
p C CH2 CH2
R6 C N
CH2 CH2
wherein R6 is H or an alkyl, aryl, alkoxyaryl or alkaryl group containing from
1 to 12 carbon atoms, preferably from 6 to 12 carbon atoms. In highly
preferred embodiments, R6 is selected from phenyl, heptyl, octyl, nonyl,
2,4,4-trimethylpentyl, decenyl and mixtures thereof.
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/US96/11249
29
The most preferred materials are those which are normally solid at
<30°C,
particularly the phenyl derivatives, ie. benzoyl valerolactam, benzoyl
caprolactam and their substituted benzoyl analogues such as chloro, amino
alkyl, alkyl, aryl and alkyioxy derivatives.
Caprolactam and valerolactam precursor materials wherein the R6 moiety
contains at least 6, preferably from 6 to about 12, carbon atoms provide
peroxyacids on perhydrolysis of a hydrophobic character which afford
nucleophilic and body soil clean-up. Precursor compounds wherein R6
comprises from 1 to 6 -carbon atoms provide hydrophilic bleaching species
which are particularly efficient for bleaching beverage stains. Mixtures of
'hydrophobic' and 'hydrophilic' caprolactams and valero lactams, typically at
weight ratios of 1:5 to 5:1, preferably 1:1, can be used herein for mixed
stain
removal benefits.
Perbenzoic acid derivative precursors
Perbenzoic acid derivative precursors provide substituted perbenzoic acids
on perhydrolysis.
Suitable substituted perbenzoic acid derivative precursors include any of
the herein disclosed perbenzoic precursors in which the benzoyl group is
substituted by essentially any non-positively charged (ie; non-cationic)
functional group including, for example alkyl, hydroxy, alkoxy, halogen,
amine, nitrosyl and amide groups.
A prefer-ed class of substituted perbenzoic acid precursor compounds are
the amide substituted compounds of the following general formulae:
R1-C-N-R2-C-L R1-N-C-R2 C L
(5
O R O or R O O
wherein R1 is an aryl or alkaryl group with from 1 to 14 carbon atoms, R2 is
an arylene, or alkarylene group containing from 1 to 14 carbon atoms, and
R5 is H or an alkyl, aryl, or alkaryl group containing 1 to 10 carbon atoms
and L can be essentially any leaving group. R1 preferably contains from 6
CA 02226666 2000-12-14
to 12 carbon atoms. R2 preferably contains from 4 to 8 carbon atoms. R1
may be aryl, substituted aryl or alkylaryl containing branching, substitution,
or both and may be sourced from either synthetic sources or natural sources
including for example, tallow fat. Analogous structural variations are
permissible for R2. The substitution can include alkyl, aryl, halogen,
nitrogen, sulphur and other typical substituent groups or organic
compounds. R5 is preferably H or methyl. R1 and R$ should not contain
more than 18 carbon atoms in total. Amide substituted bleach activator
compounds of this type are described in EP-A-0170386.
Cationic ~eroxvacid precursors
Cationic peroxyacid precursor compounds produce cationic peroxyacids on
perhydrolysis.
Typically, cationic peroxyacid precursors are formed by substituting the
,. peroxyaad part . of a suitable peroxyacid precursor compound with a
positively charged functional group, such as an ammonium or alkyl
ammonium group, preferably an ethyl or methyl ammonium group. Cationic
peroxyacid prearrsors are typically present in the solid detergent
compositions as a salt with a suitable anion, such as a halide ion.
The peroxyacid prearrsor compound to be so cationically substituted may
be a perbenzoic acrd, or substituted derivative thereof, precursor compound
as described hereinbefore. Alternatively, the peroxyacid precursor
compound may be an alkyl percarboxylic acid precursor compound or an
amide substituted alkyl peroxyacid precursor as described hereinafter
Cationic peroxyacid precursors are described in U.S. Patents 4,904,406;
4,751,015; 4,988,451; 4,397,757; 5,269,962; 5,127,852; 5,093,022;
5,106,528; U.K 1,382,594; EP 475,512, 458,396 and 284,292; and in JP
87-318, 332.
Examples of preferred cationic peroxyacid precursors are described in
WO 95/29160, US 5686015, US 5460747, US 5578136 and US 5584888.
CA 02226666 1998-O1-12
WO 97/0316 ~; PCT/US96/11249
31
Suitable cationic peroxyacid precursors include any of the ammonium or
alkyl ammonium substituted alkyl or benzoyl oxybenzene sulfonates, N-
acyiated caprolactams, and monobenzoyltetraacetyl glucose benzoyl
peroxides.
A preferred cationically substituted benzoyl oxybenzene sulfonate is the 4-
(trimethyl ammonium) methyl derivative of benzoyl oxybenzene sulfonate:
0
l( )j ~-C( 3503
~/
A preferred cationically substituted alkyl oxybenzene sulfonate has the
formula:
O S03
N+
/ O
Preferred cationic peroxyacid precursors of the N-acylated caprolactam
class include the trialkyl ammonium methylene benzoyl caprolactams,
particularly trimethyl ammonium methylene benzoyl caprolactam:
O O
O ,N
~N
/+
Other preferred cationic peroxyacid precursors of the N-acylated
caprolactam class include the trialkyl ammonium methylene alkyl
caprolactams:
CA 02226666 1998-O1-12
WO 97/03156 ;; PCaYUS96/i1Z49
32
0 0
N
j + ~ (CH2)n
where n is from 0 to 12.
Another preferred cationic peroxyacid precursor is 2-(N,N,N-trimethyl
ammonium) ethyl sodium 4-sulphophenyl carbonate chloride.
Alkyl percarboxvlic acid bleach precursors
Alkyl percarboxylic acid bleach precursors form percarboxylic acids on
perhydrolysis. Preferred precursors of this type provide peracetic acid on
perhydrolysis.
Preferred alkyl percarboxylic precursor compounds of the imide type include
the N-,N,N1N1 tetra acetylated alkylene diamines wherein the alkylene
group contains from 1 to 6 carbon atoms, particularly those compounds in
which the alkylene group contains 1, 2 and 6 carbon atoms. Tetraacetyl
ethylene diamine (TAED) is particularly preferred.
Other preferred alkyl percarboxylic acid precursors include sodium 3,5,5-tri-
methyl hexanoyloxybenzene sulfonate (ISONOBS), sodium
nonanoyloxybenzene sulfonate (NOBS), sodium acetoxybenzene sulfonate
(ABS) and pentaacetyl glucose.
Amide substituted alkyl peroxvacid precursors
Amide substituted alkyl peroxyacid precursor compounds are also suitable,
including those of the following general formulae:
R1 ~~ ~ R2 II L R1 ~ - i -R2-C-L
O R5 O or R5 O OI
wherein R1 is an alkyl group with from 1 to 14 carbon atoms, R2 is an
alkylene group containing from 1 to 14 carbon atoms, and R5 is H or an
alkyl group containing 1 to 10 carbon atoms and L can be essentially any
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/US96/11249
33
leaving group. R1 preferably contains from 6 to 12 carbon atoms. R2
preferably contains from 4 to 8 carbon atoms. R1 may be straight chain or
branched alkyl containing branching, substitution, or both and may be
sourced from either synthetic sources or natural sources including for
example, tallow fat. Analogous structural variations are permissible for R2.
The substitution can include alkyl, halogen, nitrogen, sulphur and other
typical substituent groups or organic compounds. R' is preferably H or
methyl. R1 and R6 should not contain more than 18 carbon atoms in total.
Amide substituted bleach activator compounds of this type are described in
EP=A-0170386.
Benzoxazin orcranic peroxyacid precursors
Also suitable are precursor compounds of the benzoxazin-type, as disclosed
for example in EP-A-332,294 and EP A-482,807, particularly those having
the formula:
O
CEO
~C-R~
N
including the substituted benzoxazins of the type
O
~O
~C-R~
wherein R1 is H, alkyl, alkaryl, aryl, arylalkyl, and wherein R2, R3, R4, and
R5 may be the same or different substituents selected from H, halogen,
alkyl, alkenyl, aryl, hydroxyl, alkoxyl, amino, alkyl amino, COOR6 (wherein
R6 is H or an alkyl group) and carbonyl functions.
An especially prefer-ed precursor of the benzoxazin-type is:
CA 02226666 1998-O1-12
WO 97/03156 , PCT/LTS96/11249
34
O
II
CEO
~C
N
Bleaching agents other than oxygen bleaching agents are also known
in the art and can be utilized herein. One type of non-oxygen bleaching
agent of particular interest includes photoactivated bleaching agents such
as the sulfonated zinc andlor aluminum phthalocyanines. See U.S. Patent
4,033,718, issued July 5, 1977 to Holcombe et al. If used, detergent
compositions will typically contain from 0.025% to 1.25%, by weight, of such
bleaches, especially sulfonate zinc phthalocyanine.
If desired, the bleaching compounds can be catalyzed by means of a
manganese compound. Such compounds are well known in the art and
include, for example, the manganese-based catalysts disclosed in U.S. Pat.
5,246,621, U.S. Pat. 5,244,594; U.S. Pat. 5,194,416; U.S. Pat. 5,114,606;
and European Pat. 549,271 A1, 549,272A1, 544,440A2, and 544,490A1;
Preferred examples of these catalysts include MnlV2(u-O)3(1,4,7-trimethyl-
1,4,7-triazacyclononane)2(PF6)2, Mnlll2(u-O)1 (u-OAc)2(1,4,7-trimethyl-
1,4,7-triazacyclononane)2_(C104)2, MnlV4(u-O)6(1,4,7-
triazacyclononane)4(C104)4, MnIIIMnIV4(u-O)1 (u-OAc)2_(1,4,7-trimethyl-
1,4,7-triazacyclononane)2(CI04)3, MnIV( 1,4,7-trimethyl-1,4,7-
triazacyclononane)- (OCH3)3(PF6), and mixtures thereof. Other metal-
based bleach catalysts include those disclosed in U.S. Pat. 4,430,243 and
U.S. Pat. 5,114,611. The use of manganese with various complex ligands
to enhance bleaching is also reported in the following United States
Patents: 4,728,455; 5,284,944; 5,246,612; 5,256,779; 5,280,117;
5,274,147; 5,153,161; 5,227, 084;
As a practical matter, and not by way of limitation, the compositions
and processes herein can be adjusted to provide on the order of at least
one part per ten million of the active bleach catalyst species in the aqueous
washing liquor, and will preferably provide from 0.1 ppm to 700 ppm, more
preferably from 1 ppm to 500 ppm, of the catalyst species in the laundry '
liquor.
CA 02226666 1998-O1-12
WO 97/03156 ;, PCT/ITS96/11249
Polymeric Disoersinc~ A4ents - Polymeric dispersing agents can
advantageously be utilized at levels from 0.5% to 8%, by weight, in the
compositions herein, especially in the presence of zeolite and/or layered
silicate builders. Suitable polymeric dispersing agents include polymeric
polycarboxylates and polyethylene glycols, although others known in the art
can also be used.
Polymeric polycarboxylate materials can be prepared by polymerizing or
copolymerizing suitable unsaturated monomers, preferably in their acid
form. Unsaturated monomeric acids that can be polymerized to form
suitable polymeric polycarboxylates are selected from acrylic acid, malefic
acid (or malefic anhydride), fumaric acid, itaconic acid, aconitic acid, -
mesaconic acid, citraconic acid and methylenemalonic acid. The presence
in the polymeric polycarboxylates herein of monomeric segments,
containing no carboxylate radicals such as vinylmethyl ether, styrene,
ethylene, etc. is suitable provided that such segments do not constitute
more than 40°~ by weight.
Polymeric polycarboxylate materials can also optionally include further
monomeric units such as nonionic spacing units. For example, suitable
nonionic spacing units may include vinyl alcohol or vinyl acetate.
Particularly preferred polymeric polycarboxylates are co-polymers
derived from monomers of acrylic acid and malefic acid. The average
molecular weight of such polymers in the acid form preferably ranges from
2,000 to 10,000, more preferably from 4,000 to 7,000 and most preferably
from 4,000 to 5,000. Water-soluble salts of such acryliGmaleic acid
polymers can include, for example, the alkali metal, ammonium and
substituted ammonium salts. Soluble polymers of this type are known
materials. Use of polyacrylates of this type in detergent compositions has
been disclosed, for example, in Diehl, U.S. Patent 3,308,067, issued march
7, 1967. The ratio of acrylate to maleate segments in such copolymers will
generally range from 30:1 to 1:1, more preferably from 10:1 to 2:1. Soluble
acrylate/maleate copolymers of this type are known materials which are
described in European Patent Application No. 66915, published December
15, 1982, as well as in EP 193,360, published September 3, 1986, which
also describes such polymers comprising hydroxypropylacrylate. Of these
CA 02226666 1998-O1-12
WO 9710316 PCT/iTS96/11249
36
acrylicJmaleic-based copolymers, the water-soluble salts of copolymers of
acrylic acid and malefic acid are preferred.
Another class of polymeric polycarboxylic acid compounds suitable
for use herein are the homo-polymeric polycarboxylic acid compounds
derived from acrylic acid. The average molecular weight of such homo-
polymers in the acid form preferably ranges from 2,000 to 100,000, more
preferably from 3,000 to 75,000, most preferably from 4,000 to 65,000.
A further example of polymeric polycarboxylic compounds which may be
used herein include the maleic/acryliGvinyl alcohol terpolymers. Such
materials are also disclosed in EP 193,360, including, for example, the
45/45/10 terpolymer of acryliGmaleiclvinyl alcohol.
Another example of polymeric polycarboxylic compounds which may be
used herein include the biodegradable polyaspartic acid and polyglutamic
acid compounds.
Conventional detersive adjuncts
The compositions herein can optionally include one or more other
detergent adjunct materials or other materials for assisting or enhancing
cleaning performance, treatment of the substrate to be cleaned, or to modify
the aesthetics of the detergent composition (e.g., perfumes, colorants, dyes,
etc.). The following are illustrative examples of such adjunct materials.
Slav Soil Removal/Anti-redeposition A4ents - The compositions
according to the present invention can also optionally contain water-soluble
ethoxylated amines having clay soil removal and antiredeposition
properties. Granular detergent compositions which contain these
compounds typically contain from 0.01 % to 10.0% by weight of the water-
soluble ethoxylates amines; liquid detergent compositions typically contain
0.01 % to 5%.
The most preferred soil release and anti-redeposition agent is
ethoxylated tetraethylenepentamine. Exemplary ethoxylated amines are
further described in U.S. Patent 4,597,898, VanderMeer, issued July 1,
1986. Another group of preferred clay soil removal-antiredeposition agents
are the cationic compounds disclosed in EP 111,965. Other clay soil
CA 02226666 1998-O1-12
WO 97/03156 ~, 1'CT/US96/11249
37
removallantiredeposition agents which can be used include the ethoxylated
amine polymers disclosed in EP 111,984; the zwitterionic polymers
disclosed in EP 112,592; and the amine oxides disclosed in U.S. Patent
4,548,744. Other clay soil removal and/or anti redeposition agents known in
the art can also be utilized in the compositions herein. Another type of
preferred antiredeposition agent includes the carboxy methyl cellulose
(CMC) materials. These materials are well known in the art.
Polymeric Soil Release A4ent - Any polymeric soil release agent known to
those skilled in the art can optionally be employed in the compositions and
processes of this invention. Polymeric soil release agents are characterised
by having both hydrophilic segments, to hydrophilize the surface of
hydrophobic fibers, such as polyester and nylon, and hydrophobic
segments, to deposit upon hydrophobic fibers and remain adhered thereto
through completion of washing and rinsing cycles and, thus, serve as an
anchor for the hydrophilic segments. This can enable stains occurring
subsequent to treatment with the soil release agent to be more easily
cleaned in later washing procedures.
The polymeric soil release agents useful herein especially
include those soil release agents having: (a) one or more nonionic
hydrophile components consisting essentially of (i) polyoxyethylene
segments with a degree of polymerization of at least 2, or (ii) oxypropylene
or polyoxypropylene segments with a degree of polymerization of from 2 to
10, wherein said hydrophile segment does not encompass any
oxypropylene unit unless it is bonded to adjacent moieties at each end by
ether linkages, or (iii) a mixture of oxyalkylene units comprising oxyethylene
and from 1 to 30 oxypropylene units wherein said mixture contains a suffi-
cient amount of oxyethylene units such that the hydrophile component has
hydrophilicity great enough to increase the hydrophilicity of conventional
polyester synthetic fiber surfaces upon deposit of the soil release agent on
such surface, said hydrophile segments preferably comprising at least 25%
oxyethylene units and more preferably, especially for such components
having 20 to 30 oxypropylene units, at least 50% oxyethylene units; or (b)
one or more hydrophobe components comprising (i) Cg oxyalkylene
terephthalate segments, wherein, if said hydrophobe components also
comprise oxyethylene terephthalate, the ratio of oxyethylene
terephthalate:C3 oxyalkylene terephthalate units is 2:1 or lower, (ii) C4-Cg
,;",..
CA 02226666 2000-12-14
38
alkylene or oxy C4-Cg alkylene segments, or mixtures therein, (iii) poly
(vinyl ester) segments, preferably polyvinyl acetate), having a degree of
polymerization of at least 2, or (iv) C1-C4 alkyl ether or C4 hydroxyalkyl
ether substituents, or mixtures therein, wherein said substituents are
present in the forth of C1-C4 alkyl ether or C4 hydroxyalkyl ether cellulose
derivatives, or mixtures therein, and such cellulose derivatives are
amphiphilic, whereby they have a sufficient level of C1-C4 alkyl ether andlor
C4 hydroxyalkyl ether units to deposit upon conventional polyester synthetic
fiber surfaces and retain a sufficient level of hydroxyls, once adhered to
such conventional synthetic fiber surface, to incxease fiber surface
hydrophilicity, or a combination of (a) and (b).
Typically, the polyoxyethylene segments of (a)(i) will have a degree
of polymerization of from 200, although higher levels can be used,
preferably from 3 to 150, more preferably from 6 to 100. Suitable oxy C4-C6
alkylene hydrophobe segments include, but are not limited to, end-caps of
polymeric soil release agents such as MOgS(CH2)nOCH2CH20-, where M
is sodium and n is an integer from 4-6, as disclosed in U.S. Patent
4,72?,580, issued January 26, 1988 to Gosselink
Polymeric soil release agents useful in the present invention also
inGude cellulosic derivatives such as hydroxyether cellulosic polymers,
copolymeric blocks of ethylene terephthalate or propylene terephthalate
with polyethylene oxide or polypropylene oxide terephthalate, and the like.
Such agents are commercially available and inGude hydroxyethers of
cellulose such as METHOCE~ (Dow) and carboxy alkyl of cellulose such as
Metolose (Shin Etsu). Cellulosic soil release agents for use herein also
inducts those selected from the group consisting of C~-C4 alkyl and C4
hydroxyalkyl cellulose; see U.S. Patent 4,000,093, issued December 28,
1976 to Nicol, et al.
Soil release agents characterised by polyvinyl ester) hydrophobe
segments include graft copolymers of polyvinyl ester), e.g., C1-C6 vinyl
asters, preferably polyvinyl acetate) grafted onto polyalkylene oxide
backbones, such as polyethylene oxide backbones (see EP 0 219 048).
Commercially available soil release agents of this kind include the
SOKALAN type of material, e.g., SOKALAN HP-22, available from BASF
(West Germany).
One type of preferred soil release agent is a copolymer having
random blocks of ethylene terephthalate and polyethylene oxide (PEO)
CA 02226666 2000-12-14
39
terephthalate. The molecular weight of this polymeric soil release agent is
in the range of from 25,000 to 55,000. See U.S. Patent 3,959,230 to Hays
and U.S. Patent 3,893,929.
Another preferred polymeric soil release agent is a polyester with
repeat units of ethylene terephthalate units which contains 10-15°~ by
weight of ethylene terephthalate units together with 90-80°~ by weight
of
polyoxyethylene terephthalate units, derived from a polyoxyethylene glycol
of average molecular weight 300-5,000. Examp~es of this polymer include
the commeTM ally available material ZELCON 5126 (from Dupont) and
MILEASE T (from ICI). See also U.S. Patent 4,702,857.
Another preferred polymeric soil release agent is a sulfonated
product of a substantially linear ester oligomer comprised of an oligomeric
ester backbone of terephthaloyl and oxyalkyleneoxy repeat units and
terminal moieties covalently attached to the backbone. These soil release
agents are described fully in U.S. Patent 4,968,451. Other suitable
polymeric soil release agents include the terephthalate polyesters of U.S.
Patent 4,711,730, the anionic end-capped oligomeric esters of U.S. Patent
4,721,580 and the block polyester oligomeric compounds of U.S. Patent
4,702,857.
Preferred polymeric soil release agents also include the soil release
agents of U.S. Patent 4,877,896, which discloses anionic, especially sul-
foarolyl, end-capped terephthalate esters.
If utilized, soil release agents will generally comprise from 0.01
°~ to
10.0%, by weight, of the compositions herein, typically from 0.1 °~ to
5°~,
preferably from 0.2% to 3.0°~.
Still another preferred soil release agent is an oligomer with repeat
units of terephthaloyl units, sulfoisoterephthaloyl units, oxyethyleneoxy and
oxy-1,2-propylene units. The repeat units form the badkbone of the oligomer
and are preferably temninated with modified isethionate end-caps. A
partiarlarly preferred soil release agent of this type comprises one
sulfoisophthaloyl unit, 5 terephthaloyl units, oxyethyleneoxy and oxy-1,2-
propyleneoxy units in a ratio of from 1.7 to 1.8, and two end-cap units of
sodium 2-(2-hydroxyethoxy)-ethanesulfonate. Said soil release agent also
comprises from 0.5% to 20°~, by weight of the oligomer, of a
crystalline-
reducing stabilizer, preferably selected from xylene sutfonate, cumene
sulfonate, toluene sulfonate, and mixtures thereof.
CA 02226666 1998-O1-12
WO 97/03156 PCT/ITS96/11249
v
Dve Transfer Inhibiting Agents
The compositions according to the present invention may also include one
or more materials effective for inhibiting the transfer of dyes from one
fabric
to another during the cleaning process. Generally, such dye transfer
inhibiting agents include polyvinyl pyrrolidone polymers, polyamine N-oxide
polymers, copolymers of N-vinylpyrrolidone and N-vinylimidazole,
manganese phthalocyanine, peroxidases, and mixtures thereof. If used,
these agents typically comprise from 0.01 % to 10% by weight of the
composition, preferably from 0.01 % to 5%, and more preferably from 0.05%
to 2%.
More specifically, the polyamine N-oxide polymers preferred for use
herein contain units having the following structural formula: R Ax-P; wherein
P is a polymerizable unit to which an N-O group can be attached or the N-O
group can form part of the polymerizable unit or the N-O group can be
attached to both units; A is one of the following structures: -NC(O)-, -C(O)O-
,
-S-, -O-, -N=; x is 0 or 1; and R is aliphatic, ethoxylated aliphatics,
aromatics,
heterocyclic or alicyclic groups or any combination thereof to which the
nitrogen of the N-O group can be attached or the N-O group is part of these
groups. Preferred polyamine N-oxides are those wherein R is a heterocyclic
group such as pyridine, pyrrole, imidazole, pyrrolidine, piperidine and
derivatives thereof.
The N-O group can be represented by the following general
structures:
O O
~lhc-N-~2)y~ =N-~l~c
(R3)z
wherein R1, R2, R3 are aliphatic, aromatic, heterocyclic or alicyclic groups
or combinations thereof; x, y and z are 0 or 1; and the nitrogen of the N-O
group can be attached or form part of any of the aforementioned groups.
The amine oxide unit of the po(yamine N-oxides has a pKa <10, preferably
pKa <7, more preferred pKa <6.
Any polymer backbone can be used as long as the amine oxide
polymer formed is water-soluble and has dye transfer inhibiting properties.
Examples of suitable polymeric backbones are polyvinyls, polyalkylenes,
CA 02226666 1998-O1-12
WO 97/03156 ~; PCTlUS96/11249
41
polyesters, polyethers, polyamide, polyimides, polyacrylates and mixtures
thereof. These polymers include random or block copolymers where one
monomer type is an amine N-oxide and the other monomer type is an N-
oxide. The amine N-oxide polymers typically have a ratio of amine to the
amine N-oxide of 10:1 to 1:1,000,000. However, the number of amine oxide
groups present in the polyamine oxide polymer can be varied by appropriate
copolymerization or by an appropriate degree of N-oxidation. The polyamine
oxides can be obtained in almost any degree of polymerization. Typically,
the average molecular weight is within the range of 500 to 1,000,000; more
preferred 1,000 to 500,000; most preferred 5,000 to 100,000. This preferred
class of materials can be referred to as "PVNO".
The most preferred polyamine N-oxide useful in the compositions -
herein is poly(4-vinylpyridine-N-oxide) which as an average molecular
weight of 50,000 and an amine to amine N-oxide ratio of 1:4.
Copolymers of N-vinylpyrrolidone and N-vinylimidazole polymers
(referred to as a class as "PVPVI") are also preferred for use herein.
Preferably the PVPVI has an average molecular weight range from 5,000 to
1,000,000, more preferably from 5,000 to 200,000, and most preferably from
10,000 to 20,000. (The average molecular weight range is determined by
light scattering as described in Barth, et al., Chemical Analysis, Vol 113.
"Modem Methods of Polymer Characterization".) The PVPVI copolymers
typically have a molar ratio of N-vinylimidazole to N-vinylpyrrolidone from
1:1
to 0.2:1, more preferably from 0.8:1 to 0.3:1, most preferably from 0.6:1 to
0.4:1. These copolymers can be either linear or branched.
The present invention compositions also may employ a polyvinyl-
pyrrolidone ("PVP") having an average molecular weight of from 5,000 to
400,000, preferably from 5,000 to 200,000, and more preferably from 5,000
to 50,000. PVP's are known to persons skilled in the detergent field; see, for
example, EP-A-262,897 and EP-A-256,696. Compositions containing PVP
can also contain polyethylene glycol ("PEG") having an average molecular
weight from 500 to 100,000, preferably from 1,000 to 10,000. Preferably,
the ratio of PEG to PVP on a ppm basis delivered in wash solutions is from
2:1 to 50:1, and more preferably from 3:1 to 10:1.
The detergent compositions herein may also optionally contain from
0.005% to 5% by weight of certain types of hydrophilic optical brighteners
which also provide a dye transfer inhibition action. If used, the compositions
CA 02226666 2000-12-14
42
herein will preferably comprise from 0.01 % to 1.2% by weight of such optical
brighteners.
The hydrophilic optical brighteners useful in the present invention are
those having the structural formula:
Rt RZ
>--N H H N
N N =c
( ~ N ~~ N
H
N H N
RI S03M S03M
Rt
wherein R1 is selected from anilino, N-2-bis-hydroxyethyl and NH-2-
hydroxyethyl; R2 is selected from N-2-bis-hydroxyethyl, N-2-hydroxyethyl-N-
methylamino, morphilino, chloro and amino; and M is a salt-forming ration
such as sodium or potassium.
When in the above formula, R~. is anilino, R2 is N-2-bisfiydroxyethyl
and M is a ration such as sodium, the brightener is 4,4',-bis[(4-anitino-8-(N-
2-bis-hydroxyethyl)-s-tria~ine-2-yl)aminoj-2,2'-stilbenedisulfonic acid and
disodium salt. This particular brightener species is commercially marketed
under the trademark Tinopal-UNPA-GX by Ciba-Geigy Corporation.
Tinopal-UNPA-GX is the preferred hydrophilic optical brightener useful in
the compositions herein. .
When in the above formula, R~ is anilino, R2 is N-2-hydroxyethyl-N-
2-methylamino and M is a ration such as sodium, the brightener is 4,4'-
bis[(4-anitino-6-(N-2fiydroxyethyl-N-methylamino)-s-tria2ine-2-yl)amino~,2'-
stilbenedisulfonic acid disodium salt. This particxrlar brightener species is
commeraally marketed under the trademark Tinopal 58M-GX by Ciba-
Geifly Corporation.
VVhen in the above formula, R1 is anilino, R2 is morphilino and M is a
ration such as sodium, the brightener is 4,4'-bis[(4-anilino-6-morphilino-s-
triazine-2-yl)aminoJ2,2'-stilbenedisulfonic acid, sodium salt. This particular
brightener species is commercially marketed under the trademark Tinopal
AMS-GX by Ciba Geigy Corporation.
Other spec optical brightener species which may be used in the
present invention provide especially effective dye transfer inhibition
performance benefits when used in combination with the selected polymeric
dye Vansfer inhibiting agents hereinbefore described. The combination of
such selected polymeric materials (e.g., PVNO andlor PVPVI) with such
selected optical brighteners (e.g., Tinopal UNPA-GX, Tinopal SBM-GX
CA 02226666 2000-12-14
43
and/or Tinopal AMS-GX) provides significantly better dye transfer inhibition
in aqueous wash solutions than does either of these two detergent
composition components when used alone. Without being bound by theory,
it is believed that such brighteners work this way because they have high
affinity for fabrics in the wash solution and therefore deposit relatively
quick
on these fabrics. The extent to which brighteners deposit on fabrics in the
wash solution can be defined by a parameter called the "exhaustion
coefficient". The exhaustion coefficient is in general as the ratio of a) the
brightener material deposited on fabric to b) the initial brightener
concentration in the wash liquor. Brighteners with relatively high exhaustion
coefficients are the most suitable for inhibiting dye transfer in the context
of
the present invention.
Of course, it will be appreciated that other conventional optical
brightener types of compounds can optionally be used in the present
compositions to provide conventional fabric "brightness" benefits, rather
than a true dye transfer inhibiting effect. Such usage is conventional and
well-known to detergent formulations.
Conventional optical brighteners or other brightening or whitening
agents known in the art can be incorporated at levels typically from
0.005°~
to 5%, preferably from 0.01 °~ to 1.2°~ and most preferably from
0.05°~ to
1.2°~, by weight, into the detergent compositions herein. Commercial
optical
brighteners which may be useful in the present invention can be classified
into subgroups, which include, but are not necessarily limited to, derivatives
of stilbene, pyrazoline, coumarin, carboxylic acid, methinecyanines,
dibe~zothiophene-5,5-dioxide, azoles, 5- and 6-membered-ring
heterocyGes, and other miscellaneous agents. Examples of such
brighteners are disclosed in "'The Production and Application of Fluorescent
Brightening Agents", M. Zahradnik, Published by John wley ~ Sons, New
York (1982). Further optical brightener which may also be used in the
present invention inGude naphthalimide, benzoxazole, benzofuran,
benzimidazole and any mixtures thereof.
Spec examples of optical brighteners which are useful in the
present compositions are those identifired in U.S. Patent 4,790,856. These
brighteners include the PHORWHITE series of brighteners from Verona.
Other brighteners disclosed in this reference include: Tinopal UNPA,
Tinopal CBS and Tinopal 5BM; available from Ciba-Geigy; Artic Whit CC
CA 02226666 1998-O1-12
WO 97/03156 PCT/US9C/11249
i;
44
and Artic White CWD; the 2-(4-styryl-phenyl)-2H-naptho[1,2-d]triazoles;
4,4'-bis(1,2,3-triazol-2-yl)-stilbenes; 4,4'-bis(styryl)bisphenyls; and the
aminocoumarins. Specific examples of these brighteners include 4-methyl-
7-diethyl- amino coumarin; 1,2-bis(-benzimidazol-2-yl)ethylene; 1,3-
diphenyl-pyrazolines; 2,5-bis(benzoxazol-2-yl)thiophene; 2-styryl-naptho-
[1,2-d]oxazole; and 2-(stilbene-4-yl)-2H-naphtho[1,2-d]triazole. See also
U.S. Patent 3,646,015.
Suds Sus~pressors - Compounds for reducing or suppressing the formation
of suds can be incorporated into the compositions of the present invention.
Suds suppression can be of particular importance in the so-called "high
concentration cleaning process" and in front-loading European-style _
washing machines.
A wide variety of materials may be used as suds suppressors, and
suds suppressors are well known to those skilled in the art. See, for
example, Kirk Othmer Encyclopedia of Chemical Technology, Third Edition,
Volume 7, pages 430-447 (John Wiley & Sons, Inc., 1979). One category of
suds suppressor of particular interest encompasses monocarboxylic fatty
acid and soluble salts therein. See U.S. Patent 2,954,347. The
monocarboxylic fatty acids and salts thereof used as suds suppressor
typically have hydrocarbyl chains of 10 to 24 carbon atoms, preferably 12 to
18 carbon atoms. Suitable salts include the alkali metal salts such as
sodium, potassium, and lithium salts, and ammonium and alkanolammonium
salts.
The detergent compositions herein may also contain non-surfactant
suds suppressors. These include, for example: high molecular weight
hydrocarbons such as paraffin, fatty acid esters (e.g., fatty acid
triglycerides), fatty acid esters of monovalent alcohols, aliphatic C1 g-C40
ketones (e.g., stearone), etc. Other suds inhibitors include N-alkylated
amino triazines such as tri- to hexa-alkylmelamines or di- to tetra-
alkyidiamine chlortriazines formed as products of cyanuric chloride with two
or three moles of a primary or secondary amine containing 1 to 24 carbon
atoms, propylene oxide, and monostearyl phosphates such as monostearyl
alcohol phosphate ester and monostearyl di-alkali metal (e.g., K, Na, and Li)
phosphates and phosphate esters. The hydrocarbons such as paraffin and
haloparaffin can be utilized in liquid form. The liquid hydrocarbons will be
liquid at room temperature and atmospheric pressure, and will have a pour
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/US96/11249
point in the range of -40°C and 50°C, and a minimum boiling
point not less
than 110°C (atmospheric pressure). It is also known to utilize waxy
hydrocarbons, preferably having a melting point below 100°C. The
hydrocarbons constitute a preferred category of suds suppressor for
detergent compositions. Hydrocarbon suds suppressors are described, for
example, in U.S. Patent 4,265,779. The hydrocarbons, thus, include
aliphatic, alicyclic, aromatic, and heterocyclic saturated or unsaturated
hydrocarbons having from 12 to 70 carbon atoms. The term "paraffin," as
used in this suds suppressor discussion, is intended to include mixtures of
true paraffins and cyclic hydrocarbons.
Another preferred category of non-surfactant suds suppressors
comprises silicone suds suppressors. This category includes the use of '
polyorganosiloxane oils, such as polydimethylsiloxane, dispersions or
emulsions of polyorganosiloxane oils or resins, and combinations of
polyorganosiloxane with silica particles wherein the polyorganosiloxane is
chemisorbed or fused onto the silica. Silicone suds suppressors are well
known in the art and are, for example, disclosed in U.S. Patent 4,265,779
and EP 354016.
Other silicone suds suppressors are disclosed in U.S. Patent
3,455,839 which relates to compositions and processes for defoaming
aqueous solutions by incorporating therein small amounts of
polydimethylsiloxane fluids.
Mixtures of silicone and silanated silica are described, for instance, in
German Patent Application DOS 2,124,526. Silicone defoamers and suds
controlling agents in granular detergent compositions are disclosed in U.S.
Patent 3,933,672 and in U.S. Patent 4,652,392.
An exemplary silicone based suds suppressor for use herein is a
suds suppressing amount of a suds controlling agent consisting essentially
of:
(i) polydimethylsiloxane fluid having a viscosity of from 20 cs. to
1,500 cs. at 25°C;
(ii) from 5 to 50 parts per 100 parts by weight of (i) of siloxane resin
composed of (CH3)3Si01~2 units of Si02 units in a ratio of from
(CH3)3 Si01~2 units and to Si02 units of from 0.6:1 to 1.2:1;
and
(iii) from 1 to 20 parts per 100 parts by weight of (i) of a solid silica
gel.
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/i1S96/11249
46
In the preferred silicone suds suppressor used herein, the solvent for
a continuous phase is made up of certain polyethylene glycols or
polyethylene-polypropylene glycol copolymers or mixtures thereof
(preferred), or polypropylene glycol. The primary silicone suds suppressor is
branched/crosslinked and preferably not linear.
To illustrate this point further, typical liquid laundry detergent
compositions with controlled suds will optionally comprise from 0.001 to 1,
preferably from 0.01 to 0.7, most preferably from 0.05 to 0.5, weight % of
said silicone suds suppressor, which comprises (1 ) a nonaqueous emulsion
of a primary antifoam agent which is a mixture of (a) a polyorganosiloxane,
(b) a resinous siloxane. or a silicone resin-producing silicone compound, (c)
a finely divided filler material, and (d) a catalyst to promote the reaction
of
mixture components (a), (b) and (c), to form silanolates; (2) at least one
nonionic silicone surfactant; and (3) polyethylene glycol or a copolymer of
polyethylene-polypropylene glycol having a solubility in water at room
temperature of more than 2 weight %; and without polypropylene glycol.
Similar amounts can be used in granular compositions, gels, etc. See also
U.S. Patents 4,978,471 and 4,983,316; 5,288,431 and U.S. Patents
4,639,489 and 4,749,740, Aizawa et al at column 1, line 46 through column
4, line 35.
The silicone suds suppressor herein preferably comprises
polyethylene glycol and a copolymer of polyethylene glycol/polypropylene
glycol, all having an average molecular weight of less than 1,000, preferably
between 100 and 800. The polyethylene glycol and
polyethylene/polypropylene copolymers herein have a solubility in water at
room temperature of more than 2 weight %, preferably more than 5 weight
o~_
The preferred solvent herein is polyethylene glycol having an
average molecular weight of less than 1,000, more preferably between 100
and 800, most preferably between 200 and 400, and a copolymer of
polyethylene glycol/polypropylene glycol, preferably PPG 200/PEG 300.
Preferred is a weight ratio of between 1:1 and 1:10, most preferably
between 1:3 and 1:6, of polyethylene glycol:copolymer of polyethylene-
polypropylene glycol. ,
The preferred silicone suds suppressors used herein do not contain
polypropylene glycol, particularly of 4,000 molecular weight. They also
CA 02226666 1998-O1-12
WO 97/03156 PCT/US96/11249
47
preferably do not contain block copolymers of ethylene oxide and propylene
oxide, like PLURONIC L101.
Other suds suppressors useful herein comprise the secondary
alcohols (e.g., 2-alkyl alkanols) and mixtures of such alcohols with silicone
oils, such as the silicones disclosed in U.S. 4,798,679, 4,075,118 and EP
150,872. The secondary alcohols include the C6-C16 alkyl alcohols having
a C1-C16 chain. A preferred alcohol is 2-butyl octanol, which is available
from Condea under the trademark ISOFOL 12. Mixtures of secondary
alcohols are available under the trademark ISALCHEM 123 from Enichem.
Mixed suds suppressors typically comprise mixtures of alcohol + silicone at
a weight ratio of 1:5 to 5:1.
For any detergent compositions to be used in automatic laundry
washing machines, suds should not form to the extent that they overflow the '
washing machine. Suds suppressors, when utilized, are preferably present
in a "suds suppressing amount. By "suds suppressing amount" is meant that
the formulator of the composition can select an amount of this suds
controlling agent that will sufficiently control the suds to result in a low-
sudsing laundry detergent for use in automatic laundry washing machines.
The compositions herein will generally comprise from 0% to 5% of
suds suppressor. When utilized as suds suppressors, monocarboxylic fatty
acids, and salts therein, will be present typically in amounts up to 5%, by
weight, of the detergent composition. Preferably, from 0.5% to 3% of fatty
monocarboxylate suds suppressor is utilized. Silicone suds suppressors are
typically utilized in amounts up to 2.0%, by weight, of the detergent
composition, although higher amounts may be used. This upper limit is
practical in nature, due primarily to concern with keeping costs minimized
and effectiveness of lower amounts for effectively controlling sudsing.
Preferably from 0.01 °~ to 1 °~ of silicone suds suppressor
is used, more
preferably from 0.25°r6 to 0.5%. As used herein, these weight
percentage
values include any silica that may be utilized in combination with
polyorganosiloxane, as well as any adjunct materials that may be utilized.
Monostearyl phosphate suds suppressors are generally utilized in amounts
ranging from 0.1 % to 2%, by weight, of the composition. Hydrocarbon suds
suppressors are typically utilized in amounts ranging from 0.01 % to 5.0%,
although higher levels can be used. The alcohol suds suppressors are
typically used at 0.2°~-3% by weight of the finished compositions.
CA 02226666 1998-O1-12
WO 97/03156 PCT/IJS96/11249
48
Fabric Softeners - Various through-the-wash fabric softeners,
especially the impalpable smectite clays of U.S. Patent 4,062,647, as well -
as other softener clays known in the art, can optionally be used typically at
,
levels of from 0.5% to 10%, preferably from 0.5% to 2% by weight in the ,
present compositions to provide fabric softener benefits concurrently with
fabric cleaning. Clay softeners can be used in combination with amine and
cationic softeners as disclosed, for example, in U.S. Patent 4,375,416 and
U.S. Patent 4,291,071.
Other Ingredients - A wide variety of other functional ingredients useful in
detergent compositions can be included in the compositions herein,
including other active ingredients, carriers, hydrotropes, processing aids,
dyes or pigments, solvents for liquid formulations, solid fillers for bar
compositions. The detergent compositions herein will preferably be
formulated such that, during use in aqueous cleaning operations, the wash
water will have a pH of between 6.5 and 11, preferably between 7.5 and
10.5. Laundry products are typically at pH 9-11. Techniques for controlling
pH at recommended usage levels include the use of buffers, alkalis, acids,
etc., and are well known to those skilled in the art.
Other optional ingredients
Other optional ingredients suitable for inclusion in the compositions of the
invention include perfumes, colours and filler salts, with sodium sulfate
being a preferred filler salt.
Form of the compositions
The detergent compositions of the invention can be formulated in any
desirable form such as powders, granulates, pastes, liquids, and gets.
Liguid compositions
The detergent compositions of the present invention may be formulated as
liquid detergent compositions. Such liquid detergent compositions typically
comprise from 94% to 35% by weight, preferably from 90% to 40% by
weight, most preferably from 80% to 50% by weight of a liquid carrier, e.g.,
water, preferably a mixture of water and organic solvent.
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/I1S96/11249
49
Gel compositions
The detergent compositions of the present invention may also be in the form
of gels. Such compositions are typically formulated with polyakenyl
polyether having a molecular weight of from about 750,000 to about
4, 000, 000.
Solid compositions
The detergent compositions of the invention may also be in the form of
solids, such as powders and granules.
Preferably, the mean particle size of the components of granular
compositions in accordance with the invention should be such that no more
that 5% of particles are greater than l.4mm in diameter and not more than
5°~ of particles are less than 0.15mm in diameter.
The term mean particle size as defined herein is determined by sieving a
sample of the composition into a number of fractions (typically 5 fractions)
on a series of Tyler sieves. The weight fractions thereby obtained are
plotted against the aperture size of the sieves. The mean particle size is
taken to be the aperture size through which 50% by weight of the sample
would pass.
The bulk density of granular detergent compositions in accordance with the
present invention are particularly useful in concentrated granular detergent
compositions that are characterised by a relatively high density in
comparison with conventional laundry detergent compositions. Such high
density compositions typically have a bulk density of at least 400 g/litre,
more preferably from 650 g/litre to 1200 g/litre, most preferably from
800g/litre to 1000g/litre.
Bulk density is measured by means of a simple funnel and cup device
consisting of a conical funnel moulded rigidly on a base and provided with a
flap valve at its lower extremity to allow the contents of the funnel to be
emptied into an axially aligned cylindrical cup disposed below the funnel.
The funnel is 130 mm high and has internal diameters of 130 mm and 40
mm at its respective upper and lower extremities. It is mounted so that the
lower extremity is 140 mm above the upper surface of the base. The cup
CA 02226666 1998-O1-12
WO 97/~3156 ~, PCT/~S96/11249
has an overall height of 90 mm, an internal height of 87 mm and an internal
diameter of 84 mm. Its nominal volume is 500 ml.
To carry out a measurement, the funnel is filled with powder by hand
pouring, the flap valve is opened and powder allowed to overfill the cup.
The filled cup is removed from the frame and excess powder removed from
the cup by passing a straight edged implement eg; a knife, across its upper
edge. The filled cup is then weighed and the value obtained for the weight
of powder doubled to provide a bulk density in g/litre. Replicate
measurements are made as required.
Makin4 processes - granular compositions
In general, granular detergent compositions in accordance with the present
invention can be made via a variety of methods including dry mixing, spray
drying, agglomeration and granulation.
The invention is illustrated in the following non limiting examples, in which
all percentages are on a weight basis unless otherwise stated.
In the detergent compositions of the invention, the abbreviated component
identifications have the following meanings:
LAS : Sodium linear C12 alkyl benzene
sulphonate
TAS : Sodium tallow alcohol sulphate
C45AS : Sodium C14-C15 linear alkyl sulphate
CxyEzS : Sodium C1x-C1y branched alkyl sulphate
condensed with z moles of ethylene oxide
C45E7 : A C14-15 Predominantly linear primary alcohol
condensed with an average of 7 motes of
ethylene oxide
C25 E3 : A C12-15 branched primary alcohol condensed
with an average of 3 moles of ethylene oxide
C25E5 : A C12_15 branched primary alcohol condensed
with an average of 5 moles of ethylene oxide
QAS : R2.N'~(CH3)2(C21-140Fi) with R2 = C12 - C14
CEQ : R1 COOCH2CH2.N+(CH3)3 with R1 = C11-C13
Soap : Sodium linear alkyl carboxylate derived from an
80/20 mixture of tallow and a coconut oils.
TFAA : C1g-C1g alkyl N-methyl glucamide
TPKFA : C12-C14 topped whole cut fatty acids
CA 02226666 1998-O1-12
WO 97/03156 PCT/US96/11249
i;
SI
STPP : Anhydrous sodium tripolyphosphate
Zeolite A : Hydrated Sodium Aluminosilicate of formula
Nal2(A102Si02)12- 27H20
having a primary particle size in the range from
0.1 to 10 micrometers
NaSKS-6 : Crystalline layered silicate of formula
S -Na2Si205
Citric acid : Anhydrous citric acid
Carbonate : Anhydrous sodium carbonate with a particle
size
between 200~m and 900~.m
Bicarbonate : Anhydrous sodium bicarbonate with a particle
-
size distribution between 400~.m and 1200~,m
Silicate : Amorphous Sodium Silicate (Si02:Na20; 2.0
ratio)
Sulphate : Anhydrous sodium sulphate
Citrate : Tri-sodium citrate dihydrate of activity 86.4%
with
a particle size distribution between 425~.m and
850~,m
MA/AA : Copolymer of 1:4 maleic/acrylic acid, average
molecular weight about 70,000.
CMC : Sodium carboxymethyl cellulose
Savinase Proteolytic enzyme of activity 4KNPU/g
:
Alcalase Proteolytic enzyme of activity 3AU/g
:
Carezyme Cellulytic enzyme of activity 1000 CEVU/g
:
Termamyl Amylolytic enzyme of activity 60KNU/g
:
Lipolase Lipolytic enzyme of activity 100kLU/g
:
Endolase Endoglunase enzyme of activity 3000
: CEVU/g
all sold
by NOVO
Industries
A/S and
of activity
mentioned
above unless
otherwise
specified
PB4 : Sodium perborate tetrahydrate of nominal
formula NaB02.3H20.H202
PB1 : Anhydrous sodium perborate bleach of
nominal formula NaB02.H202
Percarbonate : Sodium Percarbonate of nominal formula
2Na2C03.3H202
NOBS : Nonanoyloxybenzene sulfonate in the form of the
sodium salt.
CA 02226666 1998-O1-12
WO 97/03156 : PCT/US96/11249
it
52
TAED : Tetraacetyl ethylene diamine
DTPMP : Diethylene triamine yenta (methyiene
phosphonate), marketed by Monsanto under the
Trade name bequest 2060
Photoactivated: Sulphonated Zinc Phthalocyanin encapsulated in
bleach dextrin soluble polymer
Brightener : Disodium 4,4'-bis(2-sulphostyryl)biphenyl
1
Brightener : Disodium 4,4'-bis(4-anilino-6-morpholino-1.3.5-
2
triazin-2-yl)amino) stilbene-2:2'-disulphonate.
HEDP : 1,1-hydroxyethane diphosphonic acid
PVNO : Polyvinylpyridine N-oxide
PVPVI : Copolymer of polyvinyfpyrolidone and
vinylimidazole -
SRP 1 : Sulfobenzoyl end capped esters with
oxyethylene oxy and terephtaloyl backbone
SRP 2 : Diethoxylated poly (1, 2 propylene terephtalate)
short block polymer
Silicone : Polydimethyldiloxane foam controller with
antifoam
Siloxane-oxyalkylene copolymer as dispersing
agent with a ratio of said foam controller to said
dispersing agent of 10:1 to 100:1.
Example 1-Comparative performance testing
The following formulations were prepared, where A is a prior art formulation
and B is according to the invention.
Components A B
b wei ht
t~S 8.0 8.0
C25E3 3.4 3.4
CEQ 0.7
Zeolite 18.10 18.10
Carbonate 22.50 22.50
Silicate 2.50 _ 2.50
Na Sul hate _ 26.11
26.11
MA/AA 0.30 .. 0.30
SU~ST1TUTE SHEET (RULE 26)
CA 02226666 1998-O1-12
WO 97/03156 ~; PCT/US96/11249
53
CMC 0.22 0.2
2
Savinase 0.85 _
0.85
Termam I 0.10 0.10
PB4 9.0 9.0
TAED 1.50 1.50
DTPMP 0.25 0.25
M SO 0.30 0.30
Photoactivated bleach15 m 15 m
Suds su ressor 0.55 0.55
Bri htener 1 0.09 0.09
Perfume 0.26 0.26
HEDP 0.22 0.22
Miscellaneous to
balance
Test arotocol - stain removal
Three white cotton sheets were prewashed in a non-biological bleach free
heavy duty detergent to remove any fabric finish employed by the textile
manufacturer. Blood stains (provided by the EMPA Institute) were then
evenly applied using a paintbrush to one sheet, egg stains to the second
sheet and chocolate stains to the third one. The stains were then left to dry
overnight. Sets of six test swatches of size 4cm x 4cm were cut from each
sheet.
The sets of stained fabric swatches were subjected to one wash cycle in an
automatic washing machine. The swatches were then assessed for removal
of the various proteinaceous stains by a four person grading panel using the
well-known four-point Scheffe scale.
In more detail, a Miele 820 automatic washing machine was employed, and
the 40oC short cycle programme selected. Water of 10o Cfark hardness ( _
1.5 mmol Ca2+/litre) was used. 100g of detergent, dispensed from a
granulette dispensing device was employed. One swatch of each fabric type
was washed along with a ballast load of 5lbs (approx 2.41Cg) of lightly soiled
sheets consisting of a 60%/40% mixture of synthetic and cotton fabrics.
SUBSTITUTE SHEET (RULE 26)
CA 02226666 1998-O1-12
WO 97/03156 ~, PC~'/~TS96/11249
54
Comparative testing - results
The above stain removal test protocol was followed in comparing the
efficiency of the two different Compositions A and B in removing
proteinaceous soils.
The results, averaged over all of the proteinaceous soils types, obtained
were as follows:
Com onents A reference B
Ratio anionic surfactant9.4 9.4
Protease
Ratio anionic : CEQ 11.4
surfactant
CEQ surfactant: 5_8
wt% of total surfactant
s stem
Stain removal 0.0 +1.Os
erformance PSU
s = significant at 95% confidence level
The stain removal obtained for Composition B is thus shown to be enhanced
over the reference Formulation A.
Example 2
The following laundry detergent compositions C, D and E according to the
present invention were prepared.
Components C D E
b wei ht
LAS 5.25 5.61 4.76
TAS 1.25 1.86 1.57
C45AS 2.24 3.89
AE3S - 0.76 1.18
C25E5 - 5.47 -
SUBSTITUTE SHEET (RULE 26)
CA 02226666 1998-O1-12
WO 97/03156 PCT/US96/11249
,:
SS
C45E7 3.25 5.0
CEQ 0.55 2.0 2.0
STPP 19.7 - -
Zeolite 19.52 19.52
S KS-6 8.21 8.21
Citric acid 2.24 2.24
Carbonate 6.10 21.44 21.44
Bicarbonate 2.0 2.0
Silicate 6.80 - -
Sul hate 39.74 - 14.3
MA/AA 0.80 1.65 1.65
CMC 0.20 0.36 0.36
Savinase 0.85 2.75 2.75
Termam I 0.09 0.13 0.13
PB4 5.0 12.67 -
TAED 0.50 3.13 -
DTPMP 0.25 0.20. 0.20
M SO 0.35 0.20 0.20
Photoactivated15ppm 27ppm 27ppm
bleach
Components C D E
b wei ht
Suds 0.48 2.4 2.4
su ressor
Bri htener 0.08 0.23 0.23
1
Perfume 0.26 0.47 0.47
HEDP 0.27 0.27
Miscellaneous
to balance
Example 3
The following detergent formulations, according to the present invention
were prepared, where formulation F is a phosphorus-containing detergent
composition, formulation G is a zeolite-containing detergent composition
and formulation H is a compact detergent composition:
SUBSTITUTE SHEET (RULE 26)
CA 02226666 1998-O1-12
WO 97/03156 ~, PCT/HTS96/11249
56
F G H
Blown Powder
STP P 24 - 24. 0
Zeolite A - 24.0 -
Sul hate 9.0 6.0 13.0
MA/AA 2.0 4.0 2.0
LAS 6.0 8.0 11.0
TAS 2.0 - -
CEQ 0.7 0.7 2.0
Silicate 7.0 3.0 3.0
CMC 1.0 1.0 0.5
Bri htener 2 0.2 0.2 0.2
Soa 1.0 1.0 1.0
DTPMP 0.4 0.4 0.2
S ra On
C45E7 2.5 2.5 2.0
C25E3 2.5 2.5 2.0
Silicone antifoam 0.3 0.3 0.3
Perfume 0.3 0.3 0.3
D additives
Carbonate 6.0 13.0 15.0
PB4 18.0 18.0 10.0
PB1 4.0 4.0 -
TAED 3.0 3.0 1.0
Photoactivated bleach 0.02% 0.02% 0.02%
Savinase 1.0 1.0 1.0
Li olase 0.4 0.4 0.4
Termam I 0.25 0.30 0.15
Sul hate 3.0 3.0 5.0
Balance (Moisture & 100.0 100.0 100.0
Miscellaneous
Density (g/litre) 630 670 670
Examale 4
The following nil bleach-containing detergent formulations I to K of
particular
use in the washing of colored clothing, according to the present invention
were prepared:
t J K
Blown Powder
Zeolite A 15.0 15.0 -
Sul hate 0.0 5.0 -
LAS 3.0 3.0 -
SUBSTITUTE SHEET (RULE 26,
CA 02226666 1998-O1-12
WO 97/03156 ;; PCT/L1S96/11249
57
CEQ 2.0 1.5 1.3
DTPMP 0.4 0.5 -
CMC 0.4 0.4 -
MA/AA 4.0 4.0 -
A lomerates
C45AS - - 11.0
LAS 6.0 5.0 -
TAS 3.0 2.0 -
Silicate 4.0 4.0 -
Zeolite A 10.0 15.0 13.0
CMC - - 0.5
MA/AA ~ _ _ 2.0
Carbonate 9.0 7.0 7.0
S ra On
Perfume 0.3 0.3 0.5
C45E7 4.0 4.0 4.0
C25E3 2.0 2.0 2.0
D additives
MA/AA - 3.0
NaSKS-6 - 12.0
Citrate 10. 0 - 8.0
Bicarbonate 7.0 3.0 5.0
Carbonate 8.0 5.0 7.0
PVPVI/PVNO 0.5 0.5 0.5
Alcalase 0.5 0.3 0.9
Li olase 0.4 0.4 0.4
Termam I 0.6 0.6 0.6
Carez me 0.6 0.6 0.6
I J K
D additives
Silicone antifoam 5.0 5.0 5.0
Sul hate - 9.0 -
Balance (Moisture and 100.0 100.0 100.
Miscellaneous 0
Densit /litre 700 700 700
Example 5
The following detergent formulations L to O, according to the present
invention were prepared:
L M N O
LAS 20.0 14.0 24.0 22.0
QAS 0.7 1.0 - 0.7
SUBSTITUTE SHEET (RULE 26)
CA 02226666 1998-O1-12
WO 97/03156 ;, PCT/US96/11249
5g
TFAA - 1.0 _ _
C25E5/C45E7 - 2.0 - 0.5
C45E3S - 2.5 - -
CEQ 2.0 1.5 1.0 1.0
STPP 30.0 18.0 30.0
_
Silicate 9.0 5.0 10.0 -
Carbonate 13.0 7.5 - 5.0 '
Bicarbonate - 7.5 - -
DTPMP 0.7 1.0 - -
SRP 1 0.3 0.2 - 0.1
MA/AA 2.0 1.5 2.0 1.0
CMC 0.8 ~ 0.4 0.4 0.2
Savinase 0.8 1.0 0.5 0.5
Termam I 0.8 0.4 - 0.25
Li olase 0.2 0.1 0.2 0.1
Carez me 5T 0.15 0.05 - - -
Photoactivated70ppm 45ppm - 10ppm
bleach m
Bri htener 0.2 0.2 0.08 0.2
1
PB1 6.0 2.0 - -
NOBS 2.0 1.0 - -
Balance 100.0 100.0 100.0 100.0
(Moisture and
Miscellaneous
Example 6
The following detergent formulations P to R, according to the present
invention were prepared:
P Q R
Blown Powder
Zeolite A 30.0 22.0 6.0
Sul hate 19.0 10.0 7.0
MA/AA 3.0 3.0 6.0
LAS 14.0 12.0 22.0
C45AS 8.0 7.0 7.0
CEQ 2.0 2.0 2.0
Silicate - 1.0 5.0
Soa - - 2.0
Bri htener 1 0.2 0.2 0.2
Carbonate 8.0 16.0 20.0
DTPMP - 0.4 0.4
S ra On
SUBSTITUTE SHEET (RULE 2~
CA 02226666 1998-O1-12
WO 97/03156 " PCT/US96/11249
59
C45E7 1.0 1.0 1.0
D additives
PVPVI/PVNO 0.5 0.5 0.5
Savinase 1.0 1.0 1.0
Li olase 0.4 0.4 - 0.4
Termam I 0.1 0.1 0.1
Carez me 0.1 0.1 0.1
NOBS - 6.1 4.5
PB1 1.0 5.0 6.0
Sul hate - 6.0 -
Balance (Moisture and 100.0 100.0 100.0
Miscellaneous
Example 7
The following high density (850 g/litre) and bleach-containing detergent
formulations S to U, according to the present invention were prepared:
S T U
Blown Powder
Zeolite A 15.0 15.0 15.0
Sul hate 0.0 5.0 0.0
LAS 3.0 3.0 3.0
QAS - 1.5 1.5
CEQ 2.0 1.5 2.0
DTPMP 0.4 0.4 0.4
CMC 0.4 0.4 0.4
MA/AA ~ 4.0 2.0 2.0
A lomerates
LAS 5.0 5.0 5.0
TAS 2.0 2.0 2.0
Silicate 3.0 3.0 4.0
Zeolite A 8.0 8.0 8.0
Carbonate 8.0 8.0 ~ 4.0
S ra On
Perfume 0.3 0.3 0.3
C45E7 2.0 2.0 2.0
C25E3 2.0 - -
D additives
Citrate 5.0 - 2.0
Bicarbonate 3.0 -
Carbonate 8.0 15.0 10.0
TAED 6.0 2.0 5.0
PB1 14.0 7.0 10.0
Polyethylene oxide of MW - 0.2
5,000,000
SUBSTITUTE S~E~ ~~ULE 26)
CA 02226666 1998-O1-12
WO 97/03156 ;, PCT/US96/11249
Bentonite - - 10.0
Savinase 1.0 1.0 1.0
Li olase 0.4 0.4 0.4
Termam I 0.6 0.6 0.6
Carez me 0.6 0.6 0.6
Silicone antifoam ranule 5.0 5.0 5.0
Sul hate 0.0 3.0 0.0
Balance (Moisture and 100.0 100.0 100.0
Miscellaneous
Example 8
The.following high density detergent formulations V and W, according to the
present invention were prepared:
V W
A lomerate
C45AS 11.0 14.0
CEQ 3 3.5
Zeolite A 15.0 6.0
Carbonate 4.0 8.0
MA/AA 4.0 2.0
CMC 0.5 0.5
DTPMP 0.4 0.4
S ra On
C25E5 5.0 5.0
Perfume 0.5 0.5
D Additives
HEDP 0.5 0.3
SKS 6 13.0 10.0
Citrate 3.0 1.0
TAED 5.0 7.0
pC 20.0 20.0
SRP 1 0.3 0.3
Savinase 1.4 1.4
Li olase 0.4 0.4
Carez me 0.6 0.6
Termam I 0.6 0.6
Silicone antifoam article 5.0 5.0
Bri htener 1 0.2 0.2
Bri htener 2 0.2 -
Balance Moisture and Miscellaneous 100 100
Densit /litre 850 850
SU~~TITUTE ~~tEE ~ RULE 26~
CA 02226666 1998-O1-12
WO 97/03156 " PCT/LTS96/11249
61
Example 9
The following liquid detergent formulations X to AE, according to the present
invention were prepared:
X Y Z AA AB AC AD AE
LAS 10.0 13.0 9.0 25.0 - - -
C25AS 4.0 1.0 2.0 10.0 - 13.0 18.0 15.0
C25E3S 1.0 - 3.0 - 2.0 2.0 4.0
C25E7 6.0 8.0 13.0 2.5 - - 4.0 4.0
TFAA - - - 4.5 - 6.0 8.0 8.0
QAS - - - 3.0 1.0 - -
CEQ 0.6 1.5 1.0 0.75 2.0 1.5 1.8 2.0
TPKFA 2.0 - 13.0 2.0 - 15 7.0 7.0
Rapesee - - 5.0 - 4.0 4.0
d fatty
acids
Citric 2.0 3.0 1.0 1.5 1.0 1.0 1.0 1.0
acid
Dodecen 12.0 10.0 15.0 - -
yl/tetra-
decenyl
succinic
acid
Oleic 4.0 2.0 1.0 - 1.0 - - -
acid
Ethanol 4.0 4.0 7.0 2.0 7.0 2.0 3.0 2.0
1,2 4.0 4.0 2.0 7.0 6.0 8.0 1 0.0 1 3.0
Propane-
diol
Mono - 5.0 - 9.0 9.0
Ethanol
Amine
Tri - - 8.0 - - -
Ethanol
Amine
NaOH up 8.0 8.0 7.6 7.7 8.0 7.5 8.0 8.2
to H
Ethoxyla-0.5 - 0.5 0.2 - 0.4 0.3
ted tetra-
ethylene
pentamin
a
SUBSTITUTE SHEET (RULE 26)
CA 02226666 1998-O1-12
W~ 97/03156 ;, PCT/US96/ll?r49
62
X Y Z AA AB AC AD AE
DTPMP 1.0 1.0 0.5 1.0 2.0 1.2 1.0 -
SRP 2 0.3 - 0.3 0.1 - - 0.2 0.1
PVNO - - - - - 0.10
Protease 0.5 0.5 0.4 0.25 - 0.5 0.3 0.6
Alcalase - 1.5 - - -
Lipolase 0.10 0.01 - 0.15 0.15
(109
KLU/
Termamyl 0.05 0.05 0.12 0.10 0.05 0.15 0.10 0.10
(300
KNU/
Carezym - 0.01 - - 0.03 0.03
a (5000
CEVU/
Endoglu- - - 0.10 - - 0.07 -
canase
Boric 0.1 0.2 2.0 1.0 1.5 2.5 2.5
acid
Na 1.0 - _ _
formate
Calcium 0.015 - 0.01 - - -
chloride
Softening - 4.0 4.0 - -
clay of
the
bentonite
t a
Balance 100.0 100.0 100.0 100.0 100.0100.0 100.0 100.0
(Moisture
and
Miscella-
neous
SUBSTITUTE SHEET (RULE 26)