Note: Descriptions are shown in the official language in which they were submitted.
CA 02226842 1998-01-13
W 0 97/03022 PCT/AU96/OW35
HIGH YIELD PRECIPITATION PROCESS
The present invention relates to an improved precipitation process used in
the production of ~ min~ by the Bayer process.
The Bayler process is widely used to recover alumina from bauxite ores. The
5 Bayer process involves contacting bauxite with a caustic liquor at elevated
temp~ldLule to dissolve the alumina contained therein. lnsolubles, commonly called
red mud, are separated from the resulting liquor. Dissolved impurities, such as
silicates and organics, may also be removed ~om the liquor.
The dissolved alumina is recovered firom the liquor by precipitation. The
10 precipitation stage of the Bayer process involved passing a supersaturated Bayer
liquor to a series of precipitation tanks, which are generally arranged in
agglomeration and growth sections. Seed hydrate is typically added to both
sections to prom~ote precipitation of hydrate and produce particles of required size.
Precipitation trains at alumina refineries include a plurality of stages, usually in the
15 form of separate precipitation tanks, and the liquor is cooled as it moves through
each successive tank. At the end of the precipitation stage, the precipitated hydrate
particles are separated and classified. Smaller particles are generally retained as
seed particles whilst particles in the desired size range are recovered and calcined
to produce alumina. The Bayer process is widely used throughout the world and
20 is well known to those involved in the production of alumina.
As will be known to the skilled person, the dissolution of alumina in caustic
solution results in sodium alllmin~te (NaAl02) and other dissolved aluminium
values being formed as the dissolved species. Throughout this specification, this
will be referred to as alumina in solution. Moreover, the precipitation stage results
25 in the precipitation of alumina trihydrate (Al2O3-3H2O). This will be referred to
as hydrate or precipitated hydrate. The precipitated hydrate is calcined to remove
the water of hydration to form the final product alumina.
Current practice at most alumina refineries utilises Bayer liquors having high
caustic concentrations, often in the range of from 200 - 450g/~ caustic, calculated
30 as Na2CO3. Throughout this specification, all caustic concentrations will be
CA 02226842 1998-01-13
W O 97/03022 PCT/AU~5~ 5
calc~ ted as Na2CO3. At these high caustic concentrations, large amounts of
alumina are extracted into solution during digestion of the bauxite and this allows
for good recovery of alumina from the Bayer liquor. Tndee-l, alumina recovery ofup to 85g/~ A1203 can be obtained from high caustic precipitation. However, thisS does appear to represent the upper limit of alumina recovery using known
precipitation processes.
The present invention provides an improved precipitation process that has the
potential to increase alumina recovery.
The present invention provides an improved precipitation process for
10 producing hydrate from a Bayer liquor comprising precipiL~tillg hydrate from a
Bayer liquor having a high caustic concentration, diluting the Bayer liquor to reduce
the caustic concentration and precipitating further hydrate.
In another aspect, the present invention provides a process for precipiL~Ling
alumina trihydrate from a pregnant caustic liquor cont~ining dissolved aluminium15 values, the process comprising the steps of
- supplying the pregnant caustic liquor to a precipitation stage of a
Bayer plant,
- precipilaling alumina trihydrate from the pregnant liquor to form a
slurry of precipitated alumina trihydrate in caustic liquor,
- adding an aqueous stream to the slurry to thereby dilute the caustic
liquor and
- precipitating further alumina trihydrate.
The present invention is particularly suitable for the production of smelter
grade alumina.
In the present invention, a Bayer feed liquor having high caustic
concentration and a high dissolved alumina concentration is fed to a precipitation
train. After precipitation in a high caustic environment, a dilution stream is added
to reduce caustic concentration which thereby decreases alumina solubility and
promotes further precipitation. This increases yields above currently achievable30 levels.
It is preferred that the slurry obtained from precipitation in the high caustic
CA 02226842 1998-01-13
W O 97/03022 PCT/AU96/00435
e.lvirolmlent is not deslurried before dilution, which means that the dilution stream
is added to the slurry of precipitated hydrate in the caustic liquor.
~The dilution stream is preferably water or wash water which has been
produced elsewhere in the Bayer process. Wash water may have a low caustic
Sconcentration. ][t will be appreciated that any liquid stream that has a lower caustic
concentration than the Bayer liquor will be suitable for use as a dilution stream.
When the Bayer liquor is diluted, the ratio of alumina concentration to
caustic concentlration (A/C) stays substantially the same, because both the alumina
and caustic coneentrations are reduced by equal amounts. However, at the lower
10caustic concenttation resulting from the dilution, the equilibrium A/C is reduced
and thus the supersaturation of the liquor is increased. This promotes further
precipitation of hydrate.
Dilution of the liquor may occur in a single step, for example, by addition
of a la~ge amolLmt of dilution stream at a single point in the precipitation train.
15~lt~ tively, the dilution may occur as a plurality of smaller dilution steps, for
exarnple, by adding the dilution stream at two or more stages of the precipitation
train.
In a preferred embodiment, the Bayer liquor that is used as a feed stream to
the high caustic precipitation has a caustic concentration in the range of 200-350g/Q,
20calculated as Na~CO3 more preferably 240-275g/e. After completion of the dilution
(which, as explained above, may be carried out in a single step or in a plurality of
smaller steps), tlle caustic concentration is preferably reduced to from 200-250g/Q.
The alumina concentration of a Bayer liquor is usually measured by
reporting the ~'C ratio, which is:
A/C = 2 3 9IQ
25The A/C ,~t the start of the high caustic precipitation is preferably within the
range of 0.65-0.80, more preferably within the range of 0.72 to 0.75 at the
eft;ll~;d caustic concentration of 240-275g/Q.
CA 02226842 1998-01-13
W O 97/03022 PCT/AU96/00435
The high caustic precipitation preferably proceeds until the A/C ratio in the
precipitation liquor falls within the range of 0.40 to 0.60, more plefeldbly 0.45 to
0.50. Addition of the dilution liquor does not significantly alter the A/C but it does
reduce the caustic concentration. After completion of the low caustic precipitation,
S the A/C is preferably within the range of 0.25 to 0.35, more preferably 0.30 to
0.33.
As with all Bayer process precipitation processes, it is generally necessary
to seed the liquor in order to promote precipitation of hydrate. The present
invention enco~ asses all seeding strategies within its scope. A cu~ tly preferred
10 seeding strategy uses a double seeding strategy which is similar to that practiced at
many alumina refineries throughout the world. This strategy includes:
1) Washed, fine seed free of solid phase organic matter. Medium
particle size in the range of 40- 150 ~m preferably 40-lOO,um. The
fine seed charged to give 20 - 200g/e solids in feed liquor, and more
preferably about 100 - 120g/e solids in feed liquor. This seed is
preferably added to the agglomeration stage.
2) A coarse seed with a medium particle size in the range of 50 - 110
,um, more preferably about 75 - 85 ~lm. Coarse seed would be
charged to give a solids content of 200 - 700g/Q in the last
precipitator, more preferably about 400 - 500g/e solids in the last
precipitator. This seed is preferably added to the first tank of the
growth stage of the precipitative process.
The tempe~dlul~ profile used in the precipitation train may also be any
suitable profile. Suitably, the feed liquor to the precipitation has a temperature of
from 65 - 85~C, more preferably 75 - 80~C. Precipitators would be progressively
cooled to achieve 45- 55~C in the last of the precipitators. It is preferred that each
precipitator is cooled by about 1-3~C relative to the adjacent upstream precipitator,
in accordance with the disclosure in our co-pending Australian Patent Application
No. 36212/93 entitled "Improvements in Alumina Plants".
The total residence time for the precipitation process may be in the range of
about 30 to 50 hours, more preferably about 40 - 45 hours, with the high caustic
CA 02226842 1998-01-13
WO 97r03022 PCT/AU96/0043S
precipitation su;,tably having a residence time of about 15 - 20 hours.
The i,~ oved precipitation process of the present invention allows a yield
of up to 95 to l.?.Og/Q Al203 or higher. This is considerably higher than current best
practice that obtains hydrate yields of about 85g/Q Al203.
The slur~ y from the last precipitator is treated to separate the solids from the
liquor. After removing suitable quantities of the solids for see~ling, the particles of
the desired size range are calcined to form the alumina product.
The liquor recovered from the last precipitator of the precipitation train is
conventionally r eturned to the digestion step in which the liquor is contacted with
bauxite to extract alumina into solution. However, this liquor has a lower caustic
concentration due to the dilution carried out during the precipitation process.
Accordingly, it is likely to be necessary to treat this liquor to increase its caustic
concentration prior to re-using the liquor in the extraction step. Preferably, this is
achieved by evaporating off some of the water from the liquor equivalent to the
dilution added. However, any other process that increases the caustic concentration
of the liquor may also be used.
The process of the present invention adds another degree of freedom to the
precipitation phase of the Bayer process. Conventional Bayer precipitation
processes control the inlet A/C ratio, feed caustic concentration, seeding parameters
and temperature profile. The process of the present invention also allows for
control of the caustic concentration of the liquor during the precipitation process
by providing for dilution during precipitation.
The process of the present invention provides increased production and
improved efficiency. Hydrate quality may be improved by reducing soda pick-up.
Moreover, stand-alone ancillary processes that are normally uneconomic may be
attached to Bayer processes in an economic way, e.g. recovery of soda from DSP
in mud. If an evaporation plant is used to concentrate the diluted caustic liquor
'' after precipitation, power generation by high efficiency co-generation power stations
becomes possible.
Processes which recover soda from DSP in mud usually produce a dilute
caustic stream of say 10-50 gpl Na2CO3. Although this stream contains valuable
CA 02226842 1998-01-13
W O 97/03022 PCT/AU96/00435
caustic it also contains water. To reuse (recover) the caustic, the stream must be
re-introduced to the Bayer process circuit. If the stream is introduced as a dilute
stream in a Bayer process circuit having a convelllional precipitation step there is
too much water added to the circuit and as a consequence the evaporation capacity
5 of the refinery has to be increased. This requires capital and increased energy.
Such processes do not provide for economic recovery of caustic when the cost of
caustic and energy (fuel) are considered. With the improved precipitation process
of the present invention the dilute caustic stream can be introduced to the Bayer
circuit to give increased precipitation yield. It is still necessary to evaporate the
10 additional water but when the additional alumina production from increased yield
is considered the process of caustic recovery may produce favourable economics.
With regard to power generation, Bayer refineries require electrical power.
The power can be purchased from a State authority if the refinery is suitably
located but has to be generated by the refinery if located in a remote area. In such
15 a case power is usually generated by steam turbines. In a refinery which has a
requirement for low pressure steam, e.g. to run an Evaporation Plant, the power can
be generated by operating back pressure or let-down turbines, so producing low
pressure steam and power from the feed high pressure steam from the Boilerhouse
(co-generation). However, in a refinery that does not use low pressure steam the20 power is generated by condensing turbines where cooling water is used to condense
the steam. The fuel efficiency of the co-generation power station is 70-80%
whereas in the power station using condensing turbines it is 25-35%.
Soda pick-up in hydrate occurs primarily in areas of the process with high
alumina supersaturation, i.e. in front-end of the precipitation process. The
25 improved process may achieve higher yields by recovering more hydrate from the
area where low soda hydrate is produced, i.e. within latter part of the process where
supersaturations are lower. While the addition of dilution liquor increases the
supersaturation to promote precipitation the increase is not sufficient to increase
soda pick-up, so the high soda hydrate produced at the front-end gets diluted by the
30 increase of low soda hydrate.
The present process is also especially useful for modern alumina refineries.
CA 02226842 1998-01-13
W O 97/03022 PCT/AU96/00435
Such refineries typically utilise very high caustic concentration to digest the bauxite.
Although use of liquors having very higl1 caustic concentrations should enable
dissolution of large quantities of alumina to give a pregnant caustic liquor having
a large amount of dissolved alumina therein, caustic liquors having a high caustic
concentration are very aggressive, corrosive liquors that can cause severe corrosion
of the process vessels used in digestion, especially at higher temp~ld~u,es used for
boehmite digestion. As a consequence, limitations may be put on the digestion
process (in terms of either or both of residence time and digestion temperatures).
Accordingly, although the content of dissolved alumina in the pregnant liquors may
be high, the supersaturation ofthose liquors may be low. The dilution step or steps
included in the present invention act to increase the supersaturation of the liquor
and allow recovery of a larger proportion of the dissolved alumina content of the
caustic liquor.
The present invention will now be described in more detail with reference
to the following Figures. It will be appreciated that the accompanying Figures
illustrate embo~iment~ of the present invention and the Figures should not be
construed as limiting the invention. In the Figures:
FIGURE, 1 is a schematic diagram of the general flowsheet of the process
of the present invention;
FIGURE~ 2 is a schematic diagram showing a general flowsheet of a
conventional precipitation process used in the Bayer process;
FIGURE' 3 shows an expanded flowsheet of the conventional precipitation
process of FIGURE 2;
FIGURE: 4 shows an expanded flowsheet of a precipitation process
according to the present invention, and
FIGURE: 5 shows another expanded flowsheet of a precipitation process
according to the present invention.
The process of the present invention is schematically depicted in Figure 1.
As can be seen by reference to Figure 1, feed liquor is supplied to a high caustic
precipitation process which includes a double seeding skategy. The slurry resulting
from the high caustic precipitation is diluted and a low caustic precipitation then
CA 02226842 1998-01-13
W O 97t03022 PCT/AU96/0043S
occurs to precipitate further hydrate. The slurry bearing the low caustic
precipitation stages is subsequently sent to classification.
Figure 2 shows a schematic diagram of a prior art precipitation process. In
this process, feed liquor is supplied to a high caustic precipitation process which
S includes a doubled seeding strategy. The slurry levering the high caustic
precipitation process is then sent to classification.
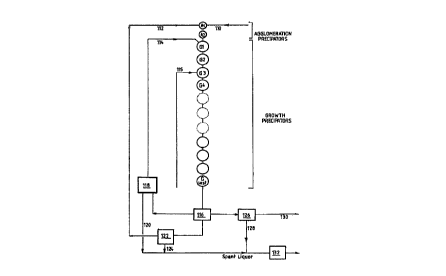
Figure 3 is an expanded flowsheet of the conventional precipitation process
shown in Figure 2. In Figure 3, the precipitation train includes two agglomeration
precipitators Al and A2 and a growth stage having a multiplicity of growth
10 precipitators Gl, G2,G Last. A Bayer liquor lO is fed to the first agglomeration
precipitator Al. Washed tertiary seed 12 is also supplied to agglomeration
precipitator Al.
After a suitable residence time in precipitator Al, the slurry of seed and
liquor (which will also include some agglomerated or precipitated hydrate) then
passes to precipitator A2 and then into the first of the growth precipitators Gl.
Deliquored secondary seed 14 is also fed to first growth precipitator Gl. The
slurry of liquor and hydrate sequentially passes throu~h the growth precipitators and
it is slowly cooled. The A/C ratio of the liquor gradually reduces but the caustic
concentration remains essentially constant.
After leaving the final growth precipitator (G Last), the slurry is classified
(16) into secondary seed, tertiary seed and product. The secondary seed is
deliquored at 18 to produce a deliquored secondary seed 1~ and spent liquor stream
20. The tertiary seed is deliquored and washed at 22 to produce tertiary seed 12and spent liquor stream 24. The product hydrate is ~vashed at 26 which produces
a wash water/spent liquor stream 28 and a washed product hydrate 30.
Figure 4 is an expanded flowsheet showing one embodiment of the
precipitation process of the present invention. This flowsheet includes
agglomeration precipitators Al and A2 and a multiplicity of growth precipitatorsGl, G2,...G Last. Pregnant liquor 110 and tertiary seed 112 are supplied to
30 agglomeration precipitator Al. The slurry passes sequentially through precipitator
A2 to precipitator Gl, wherein secondary seed 114 is added. Up to this point, the
CA 02226842 1998-01-13
W 0 97/03022 PCT/AU96/0043S
flowsheet of Figure 4 is essentially identical to the flowsheet of Figure 3.
However, at precipitator G3, a dilution stream 115 is added to the slurry. This
reduces the caustic concentration without cl-~n~ing the A/C ratio and this increases
the yield of hydrate in the overall process. The slur~ leaving the last precipitator
5 (G Last) is classified into secondary seed, tertiary seed and product hydrate. The
respective spent liquor streams 120, 124 and 128 are combined and evaporated in
an evaporation plant 132. Evaporation plant 132 is required in order to remove the
dilution water added during the precipitation process to thereby concentrate thespent liquor to ia caustic concentration suitable for use in digesting bauxite.
10Figure 5 is another embodiment of the precipitation process of the present
invention. The flowsheet of Figure S is similar to that shown in Figure 4, with the
exception that the dilution stream is added as a series of dilution streams 215a,
215b ... 215x to the respective growth precipitators Gl, G2,... G(Last-1).
In the em~bodiments shown in Figures 3, 4 and 5, like features are denoted
15by like reference numerals that differ by 100. For example, Features 10 in Figure
3 corresponds to Feature 110 in Figure 4 and Feature 210 in Figure 5.
The embodiments shown in Figures 4 and 5 respectively show addition of
the dilution stre;am in one step and addition of the dilution stream in a number of
steps. It will be appreciated that the dilution stream may be added as a single
20 stream to one precipitator, as two streams to two precipitators or as a number of
streams to a nun~ber of precipitators. All such additions fall within the scope of the
present invention. The embodiments of Figures 4 and 5 also show seed additions
to precipitators Al and Gl. Other seeding strategies may be used that vary the
number of seed additions and/or the precipitators to which the seed is added. All
25 such seeding strategies fall within the scope of the present invention.
In order to demonstrate the advantages of the present invention, preliminary
modelling of the process was carried out. In particular, the process was modelled
without dilution (Base Case-prior art), with dilution after the 7th precipitator (Case
A), with dilution after the first precipitator (Case B) and with dilution and addition
30 for further seed ~with increased residence time (Case C). The results are shown in
Table 1.
CA 02226842 1998-01-13
W O 97/03022 PCT/AU96/0043S
TABLE 1
Base Case A B C D E
Caustic (in)g/Q 250 250 250 250 275 300
Caustic (out)g/Q 254.6 205.6 205.7 205.7 220 240
Dilution No Yes Yes Yes Yes Yes
P-7 P-l +
Add'n
Seed
Add'n
Time
5 Yieldg/Q 86.7 90.3 94.2 95 .2 1 05 11 5
As can be seen from Table 1, the process in accordance with the present
invention produces significantly higher yields than the base case.
A series of experimental runs were conducted to determine the effect of the
present invention on Bayer process precipitation. The tests that were conducted
10 were bottle tests in which a pregnant caustic liquor and seed were placed in a
bottle. The caustic liquor was then cooled in accordance with one of two
tempeldLu,e profiles detailed in Tables 2 and 3 below as the Profile 1 and Profile
2, respectively.
CA 02226842 1998-01-13
W O 97/03022 PCT/AU9~C'~S
11
TABL]E 2
Temperature Profile 1
Tank Temperature (~C) Holding Time (Hours)
1 &2 78 5.5
S 3 76 4
4 74 4
72 4
6 70 4
7 68 4
8 66 4
9 64 4
61 4
11 58 4
12 55 4
Total= 45.5
-
CA 02226842 1998-01-13
W O 97/03022 PCT/A~'~5.'.~'~5
12
TABLE 3
Temperature Profile 2
TankTemperature (~C)Holding Time (Hours)
1 &2 78 5.5
3 75.2 1.95
4 72.4 1.95
69.6 1.95
6 66.8 1.95
7 64 1.95
8 61.2 1.95
9 58.4 1.95
55.6 1.95
11 52.8 1.95
12 50 1.95
Total= 25
The results for the bottle precipitation tests are given in Tables 4 to 6.
Tables 4 and 5 detail the experimental results obtained using the profile 1 in which
dilution occurred just before the commencement of the stage at 72~C. Table 6
details the results obtained using Profile 2 in which dilution took place just prior
to commencement of the stage at 72.4~C. In each of Tables 5 and 6, the liquor was
20 diluted with the aim of obtaining a caustic concentration within the range of 200-
250 g/Q following dilution. This was a calculated value and the actual caustic
concentration obtained after dilution is similar to the end liquor caustic
concentrations given in Tables 4, 5 and 6.
CA 02226842 1998-01-13
W O 97/03022 PCT/AU95J~C~S
13
TABLE 4
Single Pass Test
Parameter Start End
A/C 0.640 0.325
CS (g/Q as Na2CO3) 234 217
C/S 0.90 o.so
Oxalate (g/e as Na2C204) n/a (~1) n/a
Organics n/a (~10) n/a
Yield (g/e as Al2O3) 74
TABLE S
Single Pass Test
Pa~rameter Start End
A/C 0.769 0.334
CS (gle as Na2CO3) 362 242
15 C/S 0.91 0.91
Oxalate (gl~ as Na2C204) n/a (~1) n/a
Organics n/a (~10) n/a
Yield (g/e as Al2O3) 158
TABLE 6
25 Hour Single Pass
Par,ameter Start End
A/C 0.656 0.347
CS (g/~ as Na2CO3) 275 227
I~/S 0.91 0.90
~= 25Oxalate (gle as Na2C204) 0.72 1.1
Organics n/a (~10~ n/a
Yield (g/e as Al2O3) 85
CA 02226842 1998-01-13
W O 97/03022 PCT/AU96/00435
14
Tables 7 and 8 provide the results of similar tests to those of Tables 4 and
5, but using recycled liquor having higher organics content. The results were as follows:
TABLE 7
SAverage Values from Dilution Recycle Test
Parameter Start End
A/C 0.656 0.3 14
CS (g/Q as Na2CO3) 275 247
C/S 0.91 0.91
10Oxalate (g/Q as Na2C2O4) 0 90
Organics 8.7 8.2
Yield (g/~ as Al2O3) 94
TABLE 8
Average Values from High Organics Dilution Recycle
l SParameter Start End
A/C 0.716 0.339
CS (glQ as Na2CO3) 299 224
C/S 0.87 0.98
Oxalate (g/e as Na2C2O4) n/a n/a
20Organics 32.1 23.9
Yield (g/Q as Al2O3) 112
In order to compare the above results obtained using a process in accordance
with the present invention with conventional precipitation technology, a series of
bottle tests using Profile 1 but no dilution were conducted. The results thereof are
25 given in Table 9.
CA 02226842 1998-01-13
W O 97t03022 PCTIAU9610043S
TABLE 9
Single Pass Bottle Tests with No Dilutioll
Test 1 Test 2 Test 3
Parameter Start Finish Start Finish Start Finish
CS (gl~ 212.0 211.0 229.7 243.6 224.8 234.0
S Na~CO3)
A/C 0.659 0.37 0.668 0.341 0.692 0.374
C/S 0.875 - 0.907 - 0.792
Yield (glp 61 70 68
Al203)
In order to allow for easy comparison between the experimen~l results
obtained using a process in accordance with the present invention with the
experimental results obtained using conventional precipitation, Table 10 below
tabulates the start and finish caustic soda contents of the liquor and the yield (in g/~
as Al203) obtained from the experiments detailed in Tables 4 to 9.
TABLE 10
Comparison
l[able Caustic Concentration Yield Comment
Start Finish
4 234 217 74 Dilution
362 242 158 Dilution
6 275 227 85 Dilution
7 275 247 94 Dilution
8 299 224 112 Dilution
9(1) 212 211 61 No Dilution
9(2) 230 244 70 No Dilution
9(3) 225 234 68 No Dilution
CA 02226842 1998-01-13
W O 97/03022 PCT/AU96/00435
16
The results ~unmlal;sed in Table 10 show that the addition of water
(dilution) during the precipitation process resulted in an increased yield of alumina.
Current precipitation cilcuil~ produce a yield of alumina in the range 60-
80g/~. This is a low productivity, at approximately half the ~lllmin~ capacity of the
5liquor. The yield is ~letern ined by a number of solution and process conditions,
chiefly the pregnant liquor A/C, caustic soda concentration, and inlet/outlet
tempc.dlules. The tempcldlulc profile plays an important role in the quality of the
product, influencing such aspects as soda percentage and strength. The liquor
conditions det~rmine the equilibrium alumina concentration, and as Bayer liquors10are supersaturated, the difference between the actual and equilibrium alumina
concentration (the supersaturation) is also dependent on the liquor condition. This
difference is generally accepted as the driving force for precipitation, and is the
main influence on the yield obtained in any given process configuration.
The process of the present invention includes a dilution step in which water
15or a low strength caustic stream is added to the slurr- of liquor and hydrate. This
decreases the caustic concentration of the liquor, and although the A/C ratio of the
liquor remains unchanged, the equilibrium alumina concentration of the liquor islower at the lower caustic concentration and this increases the supersaturation of the
liquor. This results in an increase in the driving force for precipitation.
20Those skilled in the art will appreciate that the invention described herein is
susceptible to variations and modifications other than those specifically disclosed.
It is to be understood that the invention is considered to encompass all such
variations and modifications that are all within its spirit and scope.