Note: Descriptions are shown in the official language in which they were submitted.
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
DESCRIPTION
IMMUNOSUPPRESSANT
Technical Field
The present invention relates to a pharmaceutical
composition, more specifically to an immunosuppressant
useful in the prevention or treatment of diseases assumed
to be associated with immunity, including autoimmune
diseases, or in the prevention of graft rejection following
organ transplantation.
Backqround Art
In diseases assumed to be associated with immune reac-
tion, including autoimmune diseases, accentuation of immunereaction or accentuation of body defence reaction based on
immune reaction may be involved in their pathology, whose
site is affected varies among diseases. Traditionally,
these diseases have been treated using steroids or certain
immunosuppressants (e.g., azathioprine, cyclophosphamide,
methotrexate, mizoribine, cyclosporine A, Tacrolimus
(FK506; THE MERCK INDEX 12th Edition, pl546, No. 9200
(1996)). All these agents have severe side effects;
immunosuppressants mentioned above, in particular, have
side effects characteristic to respective agents, as well
as leukopenia and thrombocytopenia due to bone marrow
suppression [The New England Journal of Medicine, Vol.
330(19), p. 1368 (1994)]. The same applies in the
prevention of graft rejection following organ
transplantation; to cope with this situation, such
immunosuppressants as cyclosporine A and Tacrolimus (FK506)
~ have been developed, but all have problems to be resolved,
including severe renal toxicity.
The diseases associated with immune reaction,
including autoimmune diseases are chronic and their cases
are complicated. Therefore, the most suitable agent for
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
the each condition of a patient should be chosen. But, it
is usually difficult to chose a proper agent in a chlinical
use because of several problems such as side effects due to
the increase of dose and the long-term administration,
since the treatment of the diseases associated with immune
reaction takes long time. And all these agents, which have
been used for the treatment of those diseases and for the
prevention of graft rejection following organ
transplantation, have severe side effects as mentioned
above. Therefore there is strong demand for the
development of a drug of very low prevalence of side
effects.
Against this background, the present inventors inves-
tigated in search of a new agent possessing immunosup-
pressing activity, and discovered a safe quinoline- or
quinazoline-series compound with low toxicity.
Disclosure of Invention
The present inventors found that a quinoline or
quinazoline compound or a pharmaceutically acceptable salt
thereof possesses immune cytokine production-suppressing
activity, and that it is effective in the prevention or
treatment of diseases assumed to be associated with
immunity, or in the prevention of graft rejection following
organ transplantation. The present inventors also found
unexpectedly that a combination comprising the quinoline or
qunazoline compound and the traditionally used
immunosappressant has a remarkable immunosuppressing
activity. The inventors made further investigation based
on these finding, and developed the present invention.
Accordingly, the present invention relates to:
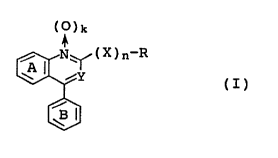
(1) an immunosuppressant containing as an active
ingredient a compound represented by the formula (I);
CA 0222691~ 1998-01-14
WO 97/09984 PCT/JP96/01693
(~)k
~ ~ (X)n-R (I)
~ 5 ~ 3
wherein Y is a nitrogen atom or C-G (G is an optionally
esterified or amidated carboxyl group, an acyl group, an
optionally protected hydroxyalkyl group, or a halogen
atom); R is an optionally substituted hydrocarbon residue
or an optionally substituted heterocyclic group; X is an
oxygen atom or an optionally oxidized sulfur atom; n is 0
or l; k is 0 or 1; G and R may bind together to form a
ring; rings A and B may each have a substituent; or a
pharmaceutically acceptable salt thereof,
(2) the immunosuppressant of the above item (1),
wherein n is 0, and wherein the optionally substituted
hydrocarbon residue represented by R is a group represented
by the formula:
-CH2-xl_zl
wherein xl is an oxygen atom, an optionally oxidized sulfur
atom, or ~(CH2)m- (m is an integer from 0 to S); Zl is a
hydrogen atom, an optionally substituted hydrocarbon
residue, an optionally substituted heterocyclic group, or
an optionally substituted amino group,
(3) the immunosuppressant of the above item (2),
wherein m is 0, provided that Xl is ~(CH2)m-,
(4) the immunosuppressant of the above item (1),
wherein the optionally substituted heterocyclic group
represented by Zl is an aromatic 5-membered heterocyclic
group containing 2 or 3 hetero atoms,
(5) the immunosuppressant of the above item (1),
wherein Y is C-G,
(6) the immunosuppressant of the above item (S),
wherein G is a Cl_6 alkyloxycarbonyl group,
,
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
(7) the immunosuppressant of the above item (l),
wherein G is an ethoxycarbonyl group,
(8) the immunosuppressant of the above item (l),
wherein ring A is substituted for by at least l alkoxy
group,
(9) the immunosuppressant of the above item (l),
wherein ring A is substituted for by 2 methoxy groups,
(10) the immunosuppressant of the above item (l),
wherein ring A is substituted for by 2 methoxy groups
respectively at the 6- and 7-positions of the quinoline
ring or quinazoline ring,
(11) the immunosuppressant of the above item (1),
wherein ring B is substituted for by at least 1 alkoxy
group,
(12) the immunosuppressant of the above item (1),
wherein ring B is substituted for by 2 identical or
different alkoxy groups,
(13) the immunosuppressant of the above item (l),
wherein k is 0,
(14) the immunosuppressant of the above item (1),
wherein the compound represented by the formula (I) is
methyl 4-(3,4-dimethoxyphenyl)-2-ethyl-6,7-dimethoxy-
quinoline-3-carboxylate;
ethyl 6-chloro-2-methyl-4-(3,4-
dimethoxyphenyl)quinoline-3-carboxylate;
6,7-dimethoxy-9-phenylfuro[3,4-b]quinoline-1(3H)-one;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(1-
methylimidazol-2-yl)thiomethyl]quinoline-3-carboxylate;
4-(3,4-dimethoxyphenyl)-2-(2-hydroxyethylthiomethyl)-
6,7-dimethoxyquinazoline;
4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(4-methyl-
1,2,4-triazol-3-yl)thiomethyl]quinazoline;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(l-
methylimidazol-2-yl)ethyl]quinoline-3-carboxylate;
methyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-3-
methoxycarbonylquinoline-2-acetate;
CA 0222691~ 1998-01-14
WO g7/(19g8~t PCT/JP96/al6g3
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-
triazol-l-ylmethyl)quinoline-3-carboxylate;
~ ethyl 4-(3-isopropoxy-4-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(3-hydroxy-4-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(4-hydroxy-3-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 7-hydroxy-6-methoxy-4-(3,4-dimethoxyphenyl)-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-
triazol-l-ylmethyl)quinoline-3-carboxylate l-oxide; or
ethyl 2-(N,N-diethylaminomethyl)-4-(3,4-
dimethoxyphenyl)-6,7-dimethoxyquinoline-3-carboxylate,
(15) the immunosuppressant of the above item (1)
possessing immune cytokine production-suppressing activity,
(16) the immunosuppressant of the above item (1) for
the prophylaxis or therapy of autoimmune diseases,
(17) the immunosuppressant of the above item (1) for
the prophylaxis or therapy of systemic lupus erythematosus,
chronic hepatitis, interstitial liver diseases, asthma,
psoriasis, ulcerative colitis, Crohn disease, regional
ileitis or multiple sclerosis,
(18) the immunosuppressant of the above item (1) for
the prophylaxis of graft rejection following organ
transplantation,
(19) a method of preventing or treating autoimmune
diseases in mammals which comprises administering to the
mannals a therapeutically effective amount of a compound
represented by the formula (I) of the above item (1) or a
pharmaceutically acceptable salt thereof,
(20) the method of the above item (19), wherein
autoimmune diseases is systemic lupus erythematosus,
chronic hepatitis, interstitial liver diseases, asthma,
psoriasis, ulcerative colitis, Crohn disease, regional
ileitis or multiple sclerosis,
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
(21) a method of preventing graft rejection following
organ transplation in mammals which comprises administering
to the mannals a therapeutically effective amount of a
compound represented by the formula (I) of the above item
S (1) or a pharmaceutically acceptable salt thereof,
(22) use of a compound represented by the formula (I)
of the above item (1) or a pharmaceutically acceptable salt
thereof for the manufacture of a medicament to be used as
an immunosuppressant,
(23) a pharmaceutical composition which comprises a
quinoline or quinazoline compound represented by the
formula (I):
(~)k
~ ~ (X)n-R (I)
wherein Y is a nitrogen atom or C-G (G is an optionally
esterified or amidated carboxyl group, an acyl group, an
optionally protected hydroxyalkyl group, or a halogen
atom); R is an optionally substituted hydrocarbon residue
or an optionally substituted heterocyclic group; x is an
oxygen atom or an optionally oxidized sulfur atom; n is 0
or l; k is 0 or 1; G and R may bind together to form a
ring; rings A and B may each have a substituent; or a
pharmaceutically acceptable salt thereof; and an
immunosuppressant except for a quinoline or quinazoline
compound mentioned above,
(24) the pharmaceutical composition of the above item
(23), wherein n is 0, and wherein the optionally
substituted hydrocarbon residue represented by R is a group
represented by the formula:
3S -CH2-xl_zl
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
wherein xl is an oxygen atom, an optionally oxidized sulfur
atom, or ~(CH2)m- (m is an integer from o to 5); Zl is a
hydrogen atom, an optionally substituted hydrocarbon
residue, an optionally substituted heterocyclic group, or
~ 5 an optionally substituted amino group,
(25) the pharmaceutical composition of the above item
(24), wherein m is 0, provided that xl is ~(CH2)m-,
(26) the pharmaceutical composition of the above item
(23), wherein the optionally substituted heterocyclic group
represented by zl is an aromatic 5-membered heterocyclic
group containing 2 or 3 hetero atoms,
(27) the pharmaceutical composition of the above item
(23), wherein Y is C-G,
(28) the pharmaceutical composition of the above item
(23), wherein G is a Cl_6 alkyloxycarbonyl group,
(29) the pharmaceutical composition of the above item
(23), wherein G is an ethoxycarbonyl group,
(30) the pharmaceutical composition of the above item
(23), wherein ring A is substituted for by at least 1
alkoxy group,
(31) the pharmaceutical composition of the above item
(23), wherein ring A is substituted for by 2 methoxy
groups,
(32) the pharmaceutical composition of the above item
(23), wherein ring A is substituted for by 2 methoxy groups
respectively at the 6- and 7-positions of the quinoline
ring or quinazoline ring,
(33) the pharmaceutical composition of the above item
(23), wherein ring B is substituted for by at least 1
alkoxy group,
(34) the pharmaceutical composition of the above item
(23), wherein ring B is substituted for by 2 identical or
different alkoxy groups,
(35) the pharmaceutical composition of the above item
(23), wherein k is 0,
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
(36) the pharmaceutical composition of the above item
(23), wherein the compound represented by the formula (I)
is
methyl 4-(3,4-dimethoxyphenyl)-2-ethyl-6,7-dimethoxy-
quinoline-3-carboxylate,
ethyl 6-chloro-2-methyl-4-(3,4-
dimethoxyphenyl)quinoline-3-carboxylate;
6,7-dimethoxy-9-phenylfuro[3,4-b]quinoline-1(3H)-one;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(1-
methylimidazol-2-yl)thiomethyl]quinoline-3-carboxylate;
4-(3,4-dimethoxyphenyl)-2-(2-hydroxyethylthiomethyl)-
6,7-dimethoxyquinazoline;
4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(4-methyl-
l,2,4-triazol-3-yl)thiomethyl]quinazoline;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(1-
methylimidazol-2-yl)ethyl]quinoline-3-carboxylate;
methyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-3-
methoxycarbonylquinoline-2-acetate;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-
triazol-l-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(3-isopropoxy-4-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-l-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(3-hydroxy-4-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(4-hydroxy-3-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 7-hydroxy-6-methoxy-4-(3,4-dimethoxyphenyl)-2-
(1,2,4-triazol-l-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-
triazol-l-ylmethyl)quinoline-3-carboxylate l-oxide; or
ethyl 2-(N,N-diethylaminomethyl)-4-(3,4-
dimethoxyphenyl)-6,7-dimethoxyquinoline-3-carboxylate,
(37) the pharmaceutical composition of the above item
(23) possessing immune cytokine production-suppressin~
activity,
CA 0222691~ 1998-01-14
wo 97~agg84 PCT/JP96/01693
(38) the pharmaceutical composition of the above item
(23) for the prophylaxis or therapy o~ autoimmune diseases,
(39) the pharmaceutical composition of the above item
(23) for the prophylaxis or therapy of systemic lupus
- 5 erythematosus, chronic hepatitis, interstitial liver
diseases, asthma, psoriasis, ulcerative colitis, Crohn
disease, regional ileitis or multiple sclerosis,
(40) the pharmaceutical composition of the above item
(23) for the prophylaxis of graft rejection following organ
transplantation,
(41) the pharmaceutical composition of the above item
(23) wherein the immunosuppressant is Tacrolimus (FK506),
cyclosporm or methotrexate,
(42) a method of preventing or treating autoimmune
diseases in mammals which comprises administering to the
mammals a therapeutically ef~ective amount of a
pharmaceutical composition of the above item (23),
(43) the method of the above item (42), wherein
autoimmune diseases is systemic lupus erythematosus,
chronic hepatitis, interstitial liver diseases, asthma,
psoriasis, ulcerative colitis, Crohn disease, regional
ileitis or multiple sclerosis,
(44) a method of preventing graft rejection following
~ organ transplantation in mammals which comprises
administering to the mammals a therapeutically effective
amount of a pharmaceutical composition of the above item
(23), and
(45) use of a pharmaceutical composition of the above
item (23) for the manufacture of a medicament to be used as
an immunosuppressant.
The immunosuppressant in the present invention generi-
cally refers to the class of immunosuppressants possessing
a quinoline or quinazoline skeleton. Their active ingredi-
ents, along with production methods, are described in de-
tail in Japanese Patent Unexamined Publication Nos.306052/1994 (EP-A-0567107), 118266/1995 (EP-A-0608870),
-
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
069890/1995 (EP-A-0634169), WO 95/24394 and 53419/1996 (EP-
A-0686630), for instance. The compounds described in these
documents all possess immunosuppressing activity and are
advantageously used for the present invention.
In the above formula (I), the optionally substituted
hydrocarbon residue represented by R is exemplified by
aliphatic hydrocarbon residues, alicyclic hydrocarbon
residues, alicyclic-aliphatic hydrocarbon residues,
aromatic carbon ring-aliphatic hydrocarbon residues and
aromatic hydrocarbon residues.
Such aliphatic hydrocarbon residues include Cl_g
saturated aliphatic hydrocarbon residues (e.g., methyl,
ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-
butyl, pentyl, isopentyl, neopentyl, tert-pentyl, hexyl,
isohexyl, heptyl, octyl) and C2_g unsaturated aliphatic
hydrocarbon residues (e.g., vinyl (ethenyl), l-propenyl, 2-
propenyl, l-butenyl, 2-butenyl, 3-butenyl, 2-methyl-1-
propenyl, l-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl,
3-methyl-2-butenyl, l-hexenyl, 3-hexenyl, 2,4-hexadienyl,
5-hexenyl, l-heptenyl, l-octenyl, ethynyl, l-propynyl, 2-
propynyl, l-butynyl, 2-butynyl, 3-butynyl, l-pentynyl, 2-
pentynyl, 3-pentynyl, 4-pentynyl, l-hexynyl, 3-hexynyl,
2,4-hexadynyl, 5-hexynyl, l-heptynyl, l-octynyl).
Such alicyclic hydrocarbon residues include C3-7
saturated alicyclic hydrocarbon residues (e.g.,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl) and C5-7 unsaturated alicyclic hydrocarbon
residues (e.g., l-cyclopentenyl, 2-cyclopentenyl, 3-
cyclopentenyl, l-cyclohexenyl, 2-cyclohexenyl, 3-
cyclohexenyl, l-cycloheptenyl, 2-cycloheptenyl, 3-
cycloheptenyl and 2,4-cycloheptadienyl).
Such alicyclic-aliphatic hydrocarbon residues include
those C4-9 ones comprising a combination of one of the
above-mentioned alicyclic hydrocarbon residues and one of
the above-mentioned aliphatic hydrocarbon residues, (e.g.,
cyclopropylmethyl, cyclopropylethyl, cyclobutylmethyl,
CA 0222691~ 1998-01-14
w~s7~asss4 PCT/~P96~01693
cyclopentylmethyl, 2-cyclopentenylmethyl, 3-
cyclopentenylmethyl, cyclohexylmethyl, 2-cyclohexenyl-
methyl, 3-cyclohexenylmethyl, cyclohexylethyl, cyclohexyl-
propyl, cycloheptylmethyl, cycloheptylethyl).
Such aromatic carbon ring-aliphatic hydrocarbon re-
sidues include C7_9 phenylalkyls (e.g., benzyl, phenethyl,
l-phenylethyl, 3-phenylpropyl, 2-phenylpropyl, 1-
phenylpropyl) and Cll_l3 naphthylalkyls (e.g., a-
naphthylethyl, ~-naphthylmethyl, ~-naphthylethyl).
Such aromatic hydrocarbon residues include phenyl,
naphthyl (e.g., ~-naphthyl, ~-naphthyl).
In the above formula (I), the optionally substituted
heterocyclic group represented by R is exemplified by (i)
5- to 7-membered heterocyclic groups containing 1 sulfur
atom, 1 nitrogen atom or 1 oxygen atom; (ii) 5- or 6-
membered heterocyclic groups containing 2 to 4 nitrogen
atoms; (iii) 5- or 6-membered heterocyclic groups
containing 1 or 2 nitrogen atoms and 1 sulfur atom or 1
oxygen atom; (iv) these heterocyclic groups may be
condensed with a 6-membered ring containing 2 or fewer
nitrogen atoms, a benzene ring, or a 5-membered ring
containing 1 sulfur atom.
Examples of such heterocyclic groups include 2-pyrid-
yl, 3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-
pyrimidinyl, 6-pyrimidinyl, 3-pyridazinyl, 4-pyridazinyl,
2-pyrazinyl, 2-pyrrolyl, 3-pyrrolyl, 2-imidazolyl, 4-imid-
azolyl, 5-imidazolyl, 3-pyrazolyl, 4-pyrazolyl, isothiaz-
olyl, isoxazolyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-
oxazolyl, 4-oxazolyl, 5-oxazolyl, 1,2,4-triazol-3-yl,
1,2,3-triazol-4-yl, tetrazol-5-yl, benzimidazol-2-yl,
indol-3-yl, lH-indazol-3-yl, lH-pyrrolo[2,3-b]pyrazin-2-yl,
lH-pyrrolo[2,3-b]pyridin-6-yl, lH-imidazo[4,5-b]pyridin-2-
yl, lH-imidazo[4,5-c]pyridin-2-yl and lH-imidazo[4,5-
b]pyrazin-2-yl.
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
In the above formula (I), the optionally substituted
heterocyclic group for R is preferably 2-imidazolyl or
1,2,4-triazol-3-yl.
In the above formula (I), the hydrocarbon residue for
R is preferably a group represented by the formula:
-CH2-Xl _ z 1
wherein xl is an oxygen atom, an optionally oxidized sulfur
atom, or ~(CH2)m- (m is an integer from 0 to 5); Zl is an
optionally substituted a hydrocarbon residue, an optionally
substituted heterocyclic group, or an optionally
substituted amino group.
The optionally oxidized sulfur atom for Xl is
exemplified by thio group, sulfinyl group and sulfonyl
group, with preference given to thio group.
Xl is pre~erably ~(CH2)m- (m is an integer from 0 to
2, preferably 0).
The optionally substituted hydrocarbon residue for zl
is exemplified by the same hydrocarbon residues as those
exemplifying the optionally substituted hydrocarbon residue
for R mentioned above.
In the above formula (I), the optionally substituted
heterocyclic group for Zl is exemplified by the same
heterocyclic groups as those exemplifying the optionally
substituted heterocyclic group for R mentioned above. Of
such heterocyclic groups, aromatic 5-membered heterocyclic
groups containing 2 or 3 hetero atoms (e.g., oxygen atom,
nitrogen atom, sulfur atom) are preferred, with greater
preference given to 2-imidazolyl or 1,2,4-triazol-3-yl.
In the above formula (I), the hydrocarbon residue and
heterocyclic group each represented by R or Z1 mentioned
above may have 1 to 3 substituents at any substitutable
positions on the chain or the ring thereof. Such
substituents include aliphatic chain hydrocarbon groups,
alicyclic hydrocarbon groups, aryl groups, aromatic
heterocyclic groups, non-aromatic heterocyclic groups,
halogen atoms, nitro groups, optionally substituted amino
=
CA 0222691~ 1998-01-14
WO 97/09984 PCT/JP96/01693
groups, optionally substituted acyl groups, optionally
substituted hydroxyl groups, optionally substituted thiol
groups, optionally esterified carboxyl groups and so on.
Aliphatic chain hydrocarbon groups mentioned as sub-
- 5 stituents for the hydrocarbon residue and heterocyclic
group each represented by R or zl include linear or
branched aliphatic hydrocarbon groups such as alkyl groups
(preferably Cl_lo alkyl), alkenyl groups (preferably C2-1o
alkenyl), and alkinyl groups (preferably C2_l0 alkinyl).
Preferable alkyl groups include methyl, ethyl, propyl,
isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl,
isopentyl, neopentyl, tert-pentyl, l-ethylpropyl, hexyl,
isohexyl, l,l-dimethylbutyl, 2,2-dimethylbutyl, 3,3-di-
methylbutyl, 2-ethylbutyl, hexyl, pentyl, octyl, nonyl and
decyl.
Preferable alkenyl groups include vinyl, allyl, iso-
propenyl, l-propenyl, 2-methyl-1-propenyl, l-butenyl, 2-
butenyl, 3-butenyl, 2-ethyl-1-butenyl, 3-methyl-2-butenyl,
l-pentenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl, 4-methyl-3-
pentenyl, l-hexenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl and 5-
hexenyl.
Preferable alkinyl groups include ethynyl, l-propynyl,
2-propynyl, l-butynyl, 2-butynyl, 3-butynyl, l-pentynyl, 2-
pentynyl, 3-pentynyl, 4-pentynyl, l-hexynyl, 2-hexynyl, 3-
hexynyl, 4-hexynyl and 5-hexynyl.
Alicyclic hydrocarbon groups mentioned as substituents
for the hydrocarbon residue and heterocyclic group each
represented by R or Zl include saturated or unsaturated C3-
8 alicyclic hydrocarbon groups such as C3-8 cycloalkyl
groups, C3-8 cycloalkenyl groups and C4-8 cycloalkadienyl
groups.
Preferable examples of the C3-8 cycloalkyl groups
include cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl, cyclooctyl, bicyclo[2.2.1]heptyl,
bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
14
bicyclo[3.2.2]nonyl, bicyclo[3.3.1]nonyl,
bicyclo[4.2.1]nonyl and bicyclo[4.3.1]decyl.
Preferable examples of the C3_g cycloalkenyl groups
include those having 5 to 7 carbon atoms, such as 2-
cyclopenten-l-yl, 3-cyclopenten-1-yl, 2-cyclohexen-1-yl and
3-cyclohexen-1-yl.
Preferable examples of the C4-8 cycloalkadienyl groups
include those having 5 to 7 carbon atoms, such as 2,4-
cyclopentadien-l-yl, 2,4-cyclohexadien-1-yl and 2,5-
cyclohexadien-l-yl.
Aryl groups mentioned as substituents for the hydro-
carbon residue and heterocyclic group each represented by R
or Zl are monocyclic or condensed polycyclic aromatic
hydrocarbon groups, preferably phenyl, naphthyl, anthryl,
phenanthryl, acenaphthylenyl and others, with greater
preference given to phenyl, l-naphthyl, 2-naphthyl and
others.
Preferable examples of aromatic heterocyclic groups
mentioned as substituents for the hydrocarbon residue and
heterocyclic group each represented by R or Zl include
aromatic monocyclic heterocyclic groups (e.g., furyl,
thienyl, pyrrolyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, imidazolyl, pyrazolyl, 1,2,3-oxadiazolyl,
1,2,4-oxadiazolyl, 1,3,4-oxadiazolyl, furazanyl, 1,2,3-
thiadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl,
1,2,3-triazolyl, 1,2,4-triazolyl, tetrazolyl, pyridyl,
pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl) and
aromatic condensed heterocyclic groups (e.g., benzofuranyl,
isobenzofuranyl, benzo[b]thienyl, indolyl, isoindolyl, lH-
indazolyl, benzimidazolyl, benzoxazolyl, 1,2-
benzisoxazolyl, benzothiazolyl, 1,2-benzisothiazolyl, lH-
benzotriazolyl, quinolyl, isoquinolyl, cinnolinyl, quina-
zolinyl, quinoxalinyl, phthalazinyl, naphthylizinyl, puri-
nyl, pteridinyl, carbazolyl, a-carbolinyl~ ~-carbolinyl, y-
carbolinyl, acridinyl, phenoxazinyl, phenothiazinyl,phenazinyl, phenoxathiinyl, thianthrenyl, phenathridinyl,
CA 0222691~ 1998-01-14
W~ 97~9984 PCT/.rP96~(~1693
phenathrolinyl, indolizinyl, pyrrolo[l,2-b]pyridazinyl,
pyrazolo[l,5-a]pyridyl, imidazo[l,2-a]pyridyl, imidazo[l,5-
a]pyridyl, imidazo[l,2-b]pyridazinyl, imidazo[l,2-a]pyrimi-
dinyl, 1,2,4-triazolo[4,3-a]pyridyl, 1,2,4-triazolo[4,3-
~ 5 b]pyridazinyl).
Preferable examples of non-aromatic heterocyclic
groups mentioned as substituents for the hydrocarbon resi-
due and heterocyclic group each represented by R or z
include oxylanyl, azetidinyl, oxetanyl, thietanyl,
pyrrolidinyl, tetrahydrofuryl, thiolanyl, piperizinyl,
tetrahydropyranyl, morpholinyl, thiomorpholinyl and piper-
azinyl.
Examples of halogens mentioned as substituents for the
hydrocarbon residue and heterocyclic group each represented
by R or Zl include fluorine, chlorine, bromine and iodine,
with preference given to fluorine and chlorine.
Examples of the optionally substituted amino groups
mentioned as substituents for the hydrocarbon residue and
heterocyclic group each represented by R or zl include (i)
amino group and (ii) substituted amino groups having 1 or 2
substituents selected from Cl_10 alkyl groups, C2_10 alkenyl
groups, C2_10 alkinyl groups, Cl_lo acyl groups, C6_l2
aromatic groups, heterocyclic groups and so on (e.g.,
methylamino, dimethylamino, ethylamino, diethylamino,
dibutylamino, diallylamino, cyclohexylamino, phenylamino,
N-methyl-N-phenylamino, acetylamino, propionylamino,
benzoylamino, nicotinoylamino).
Examples of the optionally substituted acyl groups,
mentioned as substituents for the hydrocarbon residue and
heterocyclic group each represented by R or Zl include (i)
formyl and (ii) groups resulting from binding o~ an Cl_10
alkyl group, C2_10 alkenyl group or aromatic group and a
carbonyl group (e.g., acetyl, propionyl, butyryl,
isobutyryl, valeryl, isovaleryl, pivaloyl, hexanoyl,
heptanoyl, octanoyl, cyclobutanecarbonyl, cyclopentane-
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
16
carbonyl, cyclohexanecarbonyl, cycloheptanecarbonyl,
crotonyl, 2-cyclohexenecarbonyl, benzoyl, nicotinoyl).
Examples of the optionally substituted hydroxyl groups
mentioned as substituents for the hydrocarbon residue and
heterocyclic group each represented by R or zl include (i)
hydroxyl group and (ii) substituted hydroxyl groups having
an appropriate substituent, particularly a substituent for
use as a hydroxyl group protecting group, such as alkoxy,
alkenyloxy, alkinyloxy, aralkyloxy, acyloxy and aryloxy.
Said alkoxy is preferably Cl_lo alkoxy (e.g., methoxy,
ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, sec-butoxy,
tert-butoxy, pentyloxy, isopentyloxy, neopentyloxy,
hexyloxy, heptyloxy, nonyloxy, cyclobutyloxy,
cyclopentyloxy, cyclohexyloxy).
Said alkenyloxy is exemplified by C2_10 alkenyloxy
(e.g., allyloxy, crotyloxy, 2-pentenyloxy, 3-hexenyloxy, 2-
cyclopentenylmethoxy, 2-cyclohexenylmethoxy).
Said alkinyloxy is preferably C2_l0 alkinyloxy (e.g.,
ethynyloxy, 2-propinyloxy).
Said aralkyloxy is exemplified by phenyl-Cl_4 alkyloxy
(e.g., benzyloxy, phenethyloxy).
Said acyloxy is preferably C2_4 alkanoyloxy (e.g.,
acetyloxy, propionyloxy, n-butyryloxy, isobutyryloxy).
Said aryloxy is exemplified by phenoxy and 4-
chlorophenoxy.
Examples of the optionally substituted thiol groups
mentioned as substituents for the hydrocarbon residue and
heterocyclic group each represented by R or zl include the
(i) thiol group and (ii) substituted thiol groups having an
appropriate substituent, particularly a substituent for use
as a thiol group protecting group, such as alkylthio,
alkenylthio, alkinylthio, aralkylthio, acylthio and
arylthio.
Said alkylthio is preferably Cl_lo alkylthio (e.g.,
methylthio, ethylthio, propylthio, isopropylthio,
butylthio, isobutylthio, sec-butylthio, tert-butylthio,
-
CA 0222691~ 1998-01-14
WO 97/09984 PCT/JP96/01693
pentylthio, isopentylthio, neopentylthio, hexylthio,
heptylthio, nonylthio, cyclobutylthio, cyclopentylthio,
cyclohexylthio).
Said alkenylthio is exemplified by C2_l0 alkenylthio
(e.g., allylthio, crotylthio, 2-pentenylthio, 3-
hexenylthio, 2-cyclopentenylmethylthio and 2-
cyclohexenylmethylthio).
Said alkinylthio is preferably Cz_lO alkinylthio (e.g.,
ethynylthio, 2-propinylthio).
Said aralkylthio is exemplified by phenyl-Cl_4 alkyl-
thios (e.g., benzylthio, phenethylthio).
Said acylthio is preferably an alkanoylthio having 2
to 4 carbon atoms (e.g., acetylthio, propionylthio,
butyrylthio, isobutyrylthio).
Said arylthio is exemplified by phenylthio and 4-
chlorophenylthio.
Examples of the optionally esterified carboxyl groups
mentioned as substituents for the hydrocarbon residue and
heterocyclic group each represented by R or zl, include (i)
carboxyl group, (ii) groups resulting from binding of a
carboxyl group and a C1_6 alkyl group (i.e.,
alkoxycarbonyl, e.g., methoxycarbonyl, ethoxycarbonyl,
propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl,
isobutoxycarbonyl, sec-butoxycarbonyl, tert-butoxycarbonyl,
pentyloxycarbonyl, hexyloxycarbonyl), (iii) groups
resulting from binding of a carboxyl group and a C3-6
alkenyl group (i.e., alkenyloxycarbonyl e.g.,
allyloxycarbonyl, crotyloxycarbonyl, 2-pentenyloxycarbonyl,
3-hexenyloxycarbonyl), and (iv) groups resulting from
binding of a carbonyl group and an aralkyl group (i.e.,
aralkyloxycarbonyl, e.g., benzyloxycarbonyl,
phenethyloxycarbonyl).
In the above formula (I), the substituent on the
hydrocarbon residue and heterocyclic group each represented
by R or Zl may optionally have further 1 or more,
preferably 1 to 3, substituents at any substitutable
-
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
18
positions. Examples of such substituents include Cl_lo
lower alkyl groups, C2_10 lower alkenyl groups, C2_10 lower
alkinyl groups, C3_g cycloalkyl groups, C3-8 cycloalkenyl
groups, C4-8 cycloalkadienyl groups, aryl groups, aromatic
heterocyclic groups, non-aromatic heterocyclic groups,
aralkyl groups (e.g., aryl-Cl_6-alkyl groups etc.), amino
groups, N-mono-substituted amino groups, N,N-di-substituted
amino groups, amidino groups, acyl groups, carbamoyl
groups, N-mono-substituted carbamoyl groups (e.g.,
methylcarbamoyl, ethylcarbamoyl, phenylcarbamoyl), N,N-di-
substituted carbamoyl groups (e.g., N,N-dimethylcarbamoyl,
N,N-diethylcarbamoyl, piperidinocarbamoyl,
morpholinocarbamoyl)~ sulfamoyl groups, N-mono-substituted
sulfamoyl groups (e.g., methylsulfamoyl, ethylsulfamoyl,
phenylsulfamoyl, p-toluenesulfamoyl), N,N-di-substituted
sulfamoyl groups (e.g., N,N-dimethylsulfamoyl, N-methyl-N-
phenylsulfamoyl, piperidinosulfamoyl, morpholinosulfamoyl),
carboxyl groups, Cl_10 lower alkoxycarbonyl groups (e.g.,
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
isopropoxycarbonyl, isobutoxycarbonyl, sec.-butoxycarbonyl,
tert.-butoxycarbonyl), hydroxyl groups, Cl_10 lower alkoxy
groups, C2_10 lower alkenyloxy groups, C3_7 cycloalkyloxy
groups, aralkyloxy groups, aryloxy groups, mercapto groups
Cl_lo lower alkylthio groups, aralkylthio groups, arylthio
groups, sulfo groups, cyano groups, azide groups, halogen
atoms, nitro groups and nitroso groups. These substituents
are exemplified by the same substituents as those for the
hydrocarbon residue and heterocyclic group each represented
by R or zl.
In the above formula (I), provided that R is -CH2-Xl-
zl, the optionally substituted amino group for zl, is
represented by -N(Rl)(R2) (Rl and R2, whether identical or
not, represent a hydrogen atom, an optionally substituted
hydrocarbon, or an optionally substituted heterocyclic
group; or Rl and R2 may bind together to form a ring).
CA 0222691~ 1998-01-14
WO 97/09984 PCT/JP96~01693
19
The optionally substituted hydrocarbon residue or the
optionally substituted heterocyclic group represented by Rl
- or R2 is exemplified by the same optionally substituted
hydrocarbon residues or optionally substituted heterocyclic
- 5 groups as those mentioned to exemplify the group for R
above.
Rl and R2 may bind together to form a ring. Examples
of such -N(Rl)(R2) rings include l-pyrrolidinyl, 1-
imidazolidinyl, l-pyrazolidinyl, l-piperidyl, piperazinyl,
4-morpholinyl, 4-thiomorpholinyl, homopiperazin-l-yl,
1,2,4-triazol-1-yl, 1,3,4-triazol-1-yl, pyrazol-l-yl,
imidazol-l-yl, 1,2,3-triazol-1-yl, 1,2,3-triazol-2-yl,
tetrazol-l-yl, benzimidazol-l-yl, indol-l-yl and 1~-
indazol-l-yl.
The hydrocarbon residue and heterocyclic group each
represented by Rl or R2 may have 1 to 3 substituents at any
substitutable positions on the chain or the ring thereof.
Such substituents are exemplified by the same substituents
as those for the optionally substituted hydrocarbon residue
and optionally substituted heterocyclic group each
represented by R. These substituents on the hydrocarbon
residue and heterocyclic group each represented by Rl or R2
may have 1 or more, preferably 1 to 3, substituents at any
substitutable positions. Examples of such substituents
include Cl_lo lower alkyl groups, C2_10 lower alkenyl
groups, C2_10 lower alkinyl groups, C3_7 cycloalkyl groups,
aryl groups, aromatic heterocyclic groups, non-aromatic
heterocyclic groups, aralkyl groups, amino groups, N-mono-
substituted amino groups, N,N-di-substituted amino groups,
amidino groups, acyl groups, carbamoyl groups, N-mono-
substituted carbamoyl groups, N,N-di-substituted carbamoyl
groups, sulfamoyl groups, N-mono-substituted sulfamoyl
groups, N,N-di-substituted sulfamoyl groups, carboxyl
groups, lower alkoxycarbonyl groups, hydroxyl groups, lower
alkoxy groups, lower alkenyloxy groups, cycloalkyloxy
groups, aralkyloxy groups, aryloxy groups, mercapto groups,
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
lower alkylthio groups, aralkylthio groups, arylthio
groups, sulfo group, cyano group, azide group, nitro group,
nitroso group and halogens. These substituents are
exemplified by the same substituents as those for the
optionally substituted hydrocarbon residue and the
optionally substituted heterocyclic group each represented
by R.
In the above formula (I), rings A and B may each have
substituents. Such substituents include halogen atoms,
nitro group, optionally substituted Cl_lo alkyl groups,
optionally substituted C2_l0 alkenyl groups, optionally
substituted C2_l0 alkinyl groups, optionally substituted
hydroxyl groups, optionally substituted thiol groups,
optionally substituted amino groups, optionally substituted
acyl groups (e.g., Cl_10 alkanoyl, C2_10 alkenoyl, C2_10
alkinoyl), optionally esterified carboxyl groups, and
optionally substituted aromatic ring groups.
Examples of halogens mentioned as substituents for
rings A and B include fluorine, chlorine, bromine and io-
dine, with preference given to fluorine and chlorine.
Examples of the optionally substituted Cl_lo alkylgroups, mentioned as substituents for rings A and B, may be
Cl_10 linear alkyl, C3_10 branched alkyl or C3-10 cyclic
alkyl, including methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl, neo-
pentyl, hexyl, heptyl, octyl, nonyl, decyl, cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl and cycloheptyl. These
Cl_lo alkyl gorups, C2_l0 alkenyl groups or C2_l0 alkinyl
groups, respectively may have 1 to 3 substituents
exemplified by the same substituents as those for the
optionally substituted hydrocarbon residue and the
optionally substituted heterocyclic gorup each represented
by R or zl.
Examples of the optionally substituted hydroxyl groups
mentioned as substituents for rings A and B, include (i)
hydroxyl group and (ii) substituted hydroxyl groups having
CA 0222691~ 1998-01-14
W<~ g7/~9984 PCT/JP96~al693
an appropriate substituent, particularly a substituent for
use as a hydroxyl group protecting group, such as alkoxy,
- alkenyloxy, alkinyloxy, aralkyloxy and acyloxy, as well as
aryloxy.
Said alkoxy is preferably an Cl_lo alkoxy (e.g.,
methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy,
sec-butoxy, tert-butoxy, pentyloxy, isopentyloxy,
neopentyloxy, hexyloxy, heptyloxy, nonyloxy, cyclobutyloxy,
cyclopentyloxy, cyclohexyloxy).
Said alkenyloxy is exemplified by C2_l0 alkenyloxys
(e.g., allyloxy, crotyloxy, 2-pentenyloxy, 3-hexenyloxy, 2-
cyclopentenylmethoxy, 2-cyclohexenylmethoxy).
said alkinyloxy is exemplified by C2_l0 alkinyloxys
(e.g., propinyloxy).
Said aralkyloxy is exemplified by phenyl-Cl_~ alkoxys
(e.g., benzyloxy, phenethyloxy).
Said acyloxy is preferably a C2-4 alkanoyloxy (e.g.,
acetyloxy, propionyloxy, butyryloxy, isobutyryloxy).
Said aryloxy is exemplified by phenoxy and 4-
chlorophenoxy.
Examples of the optionally substituted thiol groups
mentioned as substituents for rings A and B include (i)
thiol group and (ii) substituted thiol groups having an
appropriate substituent, particularly a substituent for use
as a thiol group protecting group, such as alkylthio,
alkenylthio, alkinylthio, aralkylthio, acylthio, arylthio
and so on.
Said alkylthio is preferably Cl_lo alkylthio (e.g.,
methylthio, ethylthio, propylthio, isopropylthio,
butylthio, isobutylthio, sec-butylthio, tert-butylthio,
pentylthio, isopentylthio, neopentylthio, hexylthio,
heptylthio, nonylthio, cyclobutylthio, cyclopentylthio,
cyclohexylthio).
Said alkenylthio is exemplified by C2_l0 alkenylthio
(e.g., allylthio, crotylthio, 2-pentenylthio, 3-
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
hexenylthio, 2-cyclopentenylmethylthio and 2-
cyclohexenylmethylthio).
Said alkinylthio is preferably C2_l0 alkinylthio (e.g.,
ethynylthio, 2-propinylthio).
Said aralkylthio is exemplified by phenyl-Cl_4 alkyl-
thios (e.g., benzylthio, phenethylthio).
Said acylthio is preferably an alkanoylthio having 2
to 4 carbon atoms (e.g., acetylthio, propionylthio,
butyrylthio, isobutyrylthio).
Said arylthio is exemplified by phenylthio and 4-
chlorophenylthio.
Examples of the optionally substituted amino groups
mentioned as substituents for rings A and B, include (i)
amino group and (ii) substituted amino groups having 1 or 2
substituents selected from Cl_lo of alkyl groups, C2_l0
alkenyl groups, C2_10 alkinyl groups, Cl_lo acyl groups, C6-
12 aromatic groups, heterocyclic groups and so on (e.g.,
methylamino, dimethylamino, ethylamino, diethylamino,
dibutylamino, diallylamino, cyclohexylamino, phenylamino,
N-methyl-N-phenylamino, acetylamino, propionylamino,
benzoylamino, nicotinoylamino).
Examples of the optionally substituted acyl groups
mentioned as substituents for rings A and B, include (i)
formyl and (ii) groups resulting from binding of a Cl_lo
alkyl group, a C2_10 alkenyl group, a C2_10 alkinyl or a C6-
12 aromatic group and a carbonyl group (e.g., acetyl,
propionyl, butyryl, isobutyryl, valeryl, isovaleryl,
pivaloyl, hexanoyl, heptanoyl, octanoyl,
cyclobutanecarbonyl, cyclopentanecarbonyl,
cyclohexanecarbonyl, cycloheptanecarbonyl, crotonyl, 2-
cyclohexenecarbonyl, benzoyl, nicotinoyl).
Examples of the optionally esterified carboxyl groups
mentioned as substituents for rings A and B, include the
(i) carboxyl group, (ii) groups resulting from binding of a
carboxyl group and a C1_6 alkyl group (i.e.,
alkoxycarbonyl, e.g., methoxycarbonyl, ethoxycarbonyl,
CA 0222691~ 1998-01-14
WO 97/09984 PCT~JP96~0~693
propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl,
isobutoxycarbonyl, sec-butoxycarbonyl, tert-butoxycarbonyl,
pentyloxycarbonyl, hexyloxycarbonyl), (iii) groups
resulting from binding of a carboxyl group and a C3-6
~ 5 alkenyl group (i.e., alkenyloxycarbonyl, e.g.,
allyloxycarbonyl, crotyloxycarbonyl, 2-pentenyloxycarbonyl,
3-hexenyloxycarbonyl), and (iv) groups resulting from
binding of a carbonyl group and an aralkyloxy group (i.e.,
aralkyloxycarbonyl, e.g., benzyloxycarbonyl and
phenethyloxycarbonyl).
Examples of the optionally substituted aromatic ring
groups mentioned as substituents for rings A and B include
C6_l4 aromatic hydrocarbon residues (e.g., phenyl,
naphthyl, anthryl) and heterocyclic aromatic residues
(e.g., pyridyl, furyl, thienyl, imidazolyl, thiazolyl).
Such substituents for rings A and B may each be pre-
sent at any substitutable position of the ring thereof; 1
to 4 identical or different substituents may be present.
Provided that substituents on ring A or B are mutually
adjoining, they may bind together to form a ring
represented by -(CH2)t- or -O-(CH2)l-O- (t is an integer
from 3 to 5; 1 is an integer from 1 to 3); such rings
include 5- to 7-membered rings formed in cooperation with
carbon atoms of the benzene ring.
Preferably, ring A is substituted for by at least 1
alkoxy group (preferably Cl_3 alkoxy gorup), more
preferably at least 1 methoxy group. Still more
preferably, ring A is substituted for by 2 identical or
different alkoxy groups (preferably Cl_3 alkoxy gorup),
more preferably by methoxy groups. Most preferably, ring A
is substituted for by 2 methoxy groups respectively at the
6- and 7-positions of the quinoline ring or quinazoline
ring.
Preferably, ring B is substituted for by at least 1
alkoxy group (preferably Cl_3 alkoxy gorup), more
preferably at least 1 methoxy or isopropoxy group. Still
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
24
more preferably, ring B is substituted for by 2 identical
or different alkoxy groups (preferably Cl_3 alkoxy gorup).
Most preferably, ring B is substituted for by a methoxy or
isopropoxy group at the 3-position and by a methoxy gorup
at the 4-position of the quinoline ring or quinazoline
ring.
The optionally oxidized sulfur atom represented by X
is exemplified by the thio group, sulfinyl group and
sulfonyl group, with preference given to the thio group.
In the above formula (I), provided that Y is C-G, the
optionally esterified carboxyl group for G include the (i)
carboxyl group, (ii) groups resulting from binding of a
carboxyl group and a Cl_6 alkyl group (i.e., alkoxycarbonyl
e.g., methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
isopropoxycarbonyl, butoxycarbonyl, isobutoxycarbonyl, sec-
butoxycarbonyl, tert-butoxycarbonyl, pentyloxycarbonyl,
hexyloxycarbonyl), (iii) groups resulting from binding of a
carboxyl group and a C3-6 alkenyl group (i.e.,
alkenyloxycarbonyl e.g., allyloxycarbonyl,
crotyloxycarbonyl, 2-pentenyloxycarbonyl and 3-
hexenyloxycarbonyl), and (iv) groups resulting from binding
of a carbonyl group and an aralkyloxy group (i.e.,
aralkyloxycarbonyl, e.g., benzyloxycarbonyl and
phenethyloxycarbonyl). The aralkyl group in said
aralkyloxycarbonyl groups is an alkyl group having an aryl
group as a substituent (arylalkyl group). Said aryl group
is exemplified by phenyl and naphthyl, each of which may
have the same substituents as those for ring A above. Said
alkyl group is preferably a lower Cl_6 alkyl group.
Preferable aralkyl groups include benzyl, phenethyl, 3-
phenylpropyl, (l-naphthyl)methyl and (2-naphthyl)methyl,
with preference given to benzyl, phenetyl and others.
In the above formula (I), provided that Y is C-G, the
amidated carboxyl group for G is represented by -
CON(Rl)(R2) (Rl and R2 have the same definitions as thosegiven above).
CA 0222691~ 1998-01-14
w<~s7~ass~4 PCT/.JP96/01693
In the above formula (I), provided that ~ is C-G, the
acyl group for G is represented by -Co-R4 (R4 is a Cl_5
alkyl group or an aryl group). The Cl-5 alkyl group for
R4, is exemplified by methyl, ethyl, isopropyl, butyl,
isobutyl, sec-butyl, tert-butyl, pentyl, isopentyl,
neopentyl, tert-pentyl and l-ethylpropyl, preferably
methyl, butyl, isobutyl and pentyl. The aryl group for R4
is a monocyclic or condensed polycyclic aromatic
hydrocarbon group, preferably examples including C6_14
monocyclic or condensed polycyclic aromatic hydrocarbon
group such as phenyl, naphthyl, anthryl and phenanthryl.
In particular, phenyl, l-naphtyl and 2-naphtyl are more
preferred.
Provided that G is a hydroxyalkyl group, the alkyl
group thereof is exemplified by the Cl_8 saturated
aliphatic hydrocarbon residues, mentioned to exemplify the
hydrocarbon residue represented by R above. Said
hydroxyalkyl group is preferably represented by -CH2OH or -
CH(oH)-R4 (R4 has the same definition as that given above),
wherein R4 is preferably methyl, ethyl, or the like.
Provided that G is a protected hydroxyalkyl group,
said protected hydroxyalkyl group is represented by -
CH2OCOR5 or -CH(oCoR5)-R4 (R4 has the same definition as
that given above; R5 represents an alkyl group, aralkyl
group or aryl group each of which may have a substituent).
The alkyl group represented by R5 is exemplified by Cl_6
alkyl groups, such as methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, sec-butyl and tert-butyl. The aralkyl
group represented by R5 is a Cl_4 alkyl group having an C6_
14 aryl group as a substituent (arylalkyl group), or the
like. Said aryl group is exemplified by phenyl and
naphthyl. Said aralkyl group is exemplified by benzyl,
phenethyl, 3-phenylpropyl, (l-naphthyl)methyl and (2-
naphthyl)methyl. The aryl group for R5 is exemplified by
phenyl and naphthyl.
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
26
Provided that G is a halogen atom, said halogen atom
is an atom of chlorine, bromine, iodine or fluorine, with
preference given to chlorine or bromine.
In the above formula (I), provided that Y is C-G, R
and G may bind together to form a 5-membered ring. Such a
structure is represented by the following formula (II) or
(III).
(~)k R3 (~)k R3
~--~ ~o @[~ ~N~Z
~1~ ~o
(II) (III)
In these formulas, R3 represents a hydrogen atom, an
optionally substituted hydrocarbon residue, or an
optionally substituted heterocyclic group; the other
symbols have the same definitions as those given above.
In the above formulas (II) and (III), the optionally
substituted hydrocarbon residue and the optionally
substituted heterocyclic group for R3 are exemplified by
the same optionally substituted hydrocarbon residues and
optionally substituted heterocyclic groups mentioned to
exemplify R and zl above.
In the above formula (I), Y is preferably C-G, with
greater preference given to a Cl_6 alkyloxycarbonyl group
for G, and greatest preference given to an ethoxycarbonyl
group for G.
n in the above formula (I) is preferably 0.
k in the above formula (I) is preferably 0.
Of the compounds represented by the above formula (I),
preference is given to those wherein Y is C-G (G is
ethoxycarbonyl), R is -CH2-Zl, zl is 1,2,4-triazol-1-yl,
CA 0222691~ 1998-01-14
WO 97/()9984 PCT/JP96/01693
the substituents for ring A are methoxy groups each present
at the 6- or 7-position of the quinoline ring, each sub-
stituent for ring B is methoxy or isopropoxy group present
at the 3- position and methoxy group present at the 4-
position thereof, n is 0, and k is 0.
Preferable examples of compounds represented by theformula (I) is
methyl 4-(3,4-dimethoxyphenyl)-2-ethyl-6,7-dimethoxy-
quinoline-3-carboxylate;
ethyl 6-chloro-2-methyl-4-(3~4-
dimethoxyphenyl)quinoline-3-carboxylate:
6,7-dimethoxy-9-phenylfuro[3,4-b]quinoline-1(3H)-one;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(1-
methylimidazol-2-yl)thiomethyl]quinoline-3-carboxylate;
4-(3,4-dimethoxyphenyl)-2-(2-hydroxyethylthiomethyl)-
6,7-dimethoxyquinazoline;
4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(4-methyl-
1,2,4-triazol-3-yl)thiomethyl]quinazoline;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-[(1-
methylimidazol-2-yl)ethyl]quinoline-3-carboxylate;
methyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-3-
methoxycarbonylquinoline-2-acetate;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-
triazol-l-ylmethyl)quinoline-3-carboxylate (hereinafter
reffered to as compound (A));
ethyl 4-(3-isopropoxy-4-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(4-hydroxy-3-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate
(hereinafter reffered to as compound (B)):
ethyl 4-(3-hydroxy-4-methoxyphenyl)-6,7-dimethoxy-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 7-hydroxy-6-methoxy-4-(3,4-dimethoxyphenyl)-2-
(1,2,4-triazol-1-ylmethyl)quinoline-3-carboxylate;
ethyl 4-(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-
triazol-l-ylmethyl)quinoline-3-carboxylate l-oxide; or
CA 0222691~ 1998-01-14
W 097/09984 PCT/JP96/01693
ethyl 2-(N,N-diethylaminomethyl)-4-(3,4-
dimethoxyphenyl)-6,7-dimethoxyquinoline-3-carboxylate.
The salt of compound (I) for the present invention is
preferably a pharmaceutically acceptable salt, exemplified
by salts with inorganic bases, salts with organic bases,
salts with inorganic acids, salts with organic acids and
salts with basic or acidic amino acids.
Preferable salts with inorganic bases include alkali
metal salts such as sodium salt and potassium salt; alka-
line earth metal salts such as calcium salt and magnesiumsalt; aluminum salt and ammonium salt.
Preferable salts with organic bases include salts with
trimethylamine, triethylamine, pyridine, picoline, ethanol-
amine, diethanolamine, triethanolamine, dicyclohexylamine
and N,N'-dibenzylethylenediamine.
Preferable salts with inorganic acids include salts
with hydrochloric acid, hydrobromic acid, nitric acid,
sulfuric acid and phosphoric acid.
Preferable salts with organic acids include salts with
formic acid, acetic acid, trifluoroacetic acid, fumaric
acid, oxalic acid, tartaric acid, maleic acid, citric acid,
succinic acid, malic acid, methanesulfonic acid, benzene-
sulfonic acid and p-toluenesulfonic acid.
Preferable salts with basic amino acids include salts
with arginine, lysine and ornithine. Preferable salts with
acidic amino acids include salts with aspartic acid and
glutamic acid. Among these salts, sodium or potassium
salts are most preferable. Compound (I) or a
pharmaceutically acceptable salt thereo~ for the present
invention may be a hydrate.
Compound (I) for the present invention can, for
example, be produced by or according to the methods
described in Japanese Patent Unexamined Publication Nos.
306052/1994 (EP-A-0567107), 118266/1995 (EP-A-0608870) and
069890/1995 (EP-A-0634169), 53419/1996 (EP-A-0686630) and
W095/24394.
CA 0222691~ 1998-01-14
WO 97/09984 PCT/JP96/01693
These dosage forms can be manufactured by the per se
known technique conventionally used in pharmaceutical
procedures.
Compound (I) for the present invention can be admin-
- 5 istered orally or non-orally to mammals, including humans,
as ~ormulated with a pharmaceutically acceptable carrier,
in the form of solid preparations such as tablets, cap-
sules, granules/ powders or suppositories, or liquid prepa-
rations such as syrups and injectable preparations.
Pharmaceutically acceptable carriers are various or-
ganic or inorganic carrier substances in common use as
pharmaceutical materials, including excipients, lubricants,
binders and disintegrating agents for solid preparations,
and solvents, dissolution aids, suspending agents, isoton-
izing agents, buffers and soothing agents for liquid
preparations. Other pharmaceutical additives such as
preservatives, antioxidants, coloring agents and sweetening
agents may be used as necessary.
Preferable excipients include lactose, sucrose, D-
mannitol, erythritol, starch, crystalline cellulose andlight silicic anhydride.
Preferable lubricants include magnesium stearate,
calcium stearate, talc and colloidal silica.
Preferable binders include crystalline cellulose,
sucrose, D-mannitol, dextrin, hydroxypropyl cellulose,
hydroxypropylmethyl cellulose and polyvinylpyrrolidone.
Preferable disintegrating agents include starch, car-
boxymethyl cellulose, carboxymethyl cellulose calcium,
crosscarmellose sodium and carboxymethyl starch sodium.
Preferable solvents include water for injection, alco-
hol, propylene glycol, macrogol, sesame oil and corn oil.
Preferable dissolution aids include polyethylene
glycol, propylene glycol, D-mannitol, benzyl benzoate,
ethanol, tris-aminomethane, cholesterol, triethanolamine,
sodium carbonate and sodium citrate.
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
Preferable suspending agents include surfactants such
as stearyltriethanolamine, sodium lauryl sulfate, lauryl-
aminopropionic acid, lecithin, benzalkonium chloride,
benzethonium chloride and monostearic glycerol; and hydro-
philic polymers such as polyvinyl alcohol, polyvinylpyr-
rolidone, carboxymethyl cellulose sodium, methyl cellulose,
hydroxymethyl cellulose, hydroxyethyl cellulose and hy-
droxypropyl cellulose.
Preferable isotonizing agents include sodium chloride,
glycerol and D-mannitol.
Preferable buffers include buffer solutions of phos-
phates, acetates, carbonates and citrates.
Preferable soothing agents include benzyl alcohol.
Preferable preservatives include p-oxybenzoic acid
esters, chlorobutanol, benzyl alcohol, phenethyl alcohol,
dehydroacetic acid and sorbic acid.
Preferable antioxidants include sulfites and ascorbic
acid.
Compound (I) for the present invention is of low tox-
icity; no deaths occurred during a l-week observation
period following oral administration of compound (A), for
instance, to female F344/Jcl rats at 6 weeks of age at a
dose of 2,000 mg/kg. As such, compound (I) for the present
invention is useful as an immunosuppressant for mammals
(e.g., humans, horses, bovines, pigs, dogs, cats).
Although the dose of compound (I) for the present in-
vention can be chosen over a wide range, depending on tar-
get disease, route of administration, and patient condition
to be treated, it can normally be chosen over the range
from 1 mg to 2,000 mg a day, preferably 10 mg to 400 mg,
for oral administration, and from 1 mg to 1,000 mg,
preferably from 1 mg to 100 mg, for non-oral
administration, per adult. These doses can be administered
in about 1 to 3 portions a day.
In the present invention, examples of the drug which
is used in combination with the quinoline or quinazoline
CA 0222691~ 1998-01-14
W O 97~984 PCT/JP96/01693
compound represented by the formula (I) or a
pharmaceutically acceptable salt thereof include
immunosuppressants ecept for a quinoline or quinazoline
compound mentioned above, for example, (i) T cell
inhibitors, (ii) cytotoxic chemicals, (iii) anti-tumor
antibiotics, (iv) anti-lymphocyte immunoglobulins, and so
on.
(i) T cell inhibitors are drugs having the property to
suppress the immune response of T cells by inhibiting the
production of both Thl and Th2 cytokines which are derived
from the T cells (e.g., Tacrolimus (FK506), cyclosporin A,
cyclosporin G), or drugs having the property to inhibit the
activity of cytokines produced by T cells (e.g.,
rapamycin). Consequently, these drugs suppress the immune
reaction.
(ii) Cytotoxic chemicals are drugs, which are also
used as anticancer drugs, having the property to act
nonspecifically on actively proliferating cells, i.e.
hematopoietic stem cells, germ cells, malignant cells or
lymphocytes (T lymphocytes and B lymphocytes), which
inhibit both responses of cellular immunity and humoral
immunity as a result of the suppression of both T
lymphocytes and B lymphocytes. Accordingly, these drugs
always have both activities of myelosuppression and
hypofertility as well as immunosuppressing activity.
Examples of the cytotoxic chemicals include ~ alkylating
agents, ~ nucleic acid metabolic antagonists, @~ folic
acid metabolic antagonists and so on. ~ The alkylating
agents are drugs having the property to suppress the immune
response by damaging T cells or B cells, mainly through the
inhibition of DNA symthesis or DNA replication. Examples
of the alkylating agents include cyclophosphamide,
ifosfamide, chlorambucil and the like. ~ The nucleic acid
metabolic antagonists, which inhibit the metabolism of
nucleic acid, are drugs having the property to suppress the
antibody production by damaging mainly T cells. Examples
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
of the nucleic acid metabolic antagonists include 6-
mercaptopurine, azathiopurine, 6-thioguanine, 5-
fluorouracil, cytosine arabinoside, mizoribine and the
like. ~3 The folic acid metabolic antagonists, which
inhibit the metabolism of folic acid, are drugs having the
property to suppress the antibody production by damaging
mainly T cells. Examples of the folic acid metabolic
antagonists include methotrexate and the like.
(iii) Anti-tumor antibiotics are drugs having the
property to inhibit DNA or RNA synthesis by intercalating
therein, as a result of damaging T cells or B cells.
Examples of the anti-tumor antibiotics include adriamycin,
daunomycin and the like.
(iv) Anti-lymphocyte immunoglobulins are drugs having
the property to suppress the immune response by damaging
mainly T cells. Examples of the anti-lymphocyte
immunoglobulins include anti-CD3 antibody, anti-CD4
antibody, anti-CD5 antibody, anti-IL-2 receptor antibody
and the like.
On the other hand, a quinoline or quinazoline compound
represented by the formula (I) of the present invention,
are the drugs having the property to suppress the immune
response of T cells by specific inhibition of the
production of Thl cytokines such as interferon-y (IFNy)
and interleukin-2 (IL-2) among the cytokines which are
derived from the T cells. Therefore, the inhibition
mechanism of the quinoline or quinazoline compound
represented by the formula (I) is different from those of
the immunosuppressants mentioned above.
In the present invention, especially preferred is the
pharmaceutical composition which comprises a quinoline or
quinazoline compound represented by the formula (I) or a
pharmaceutically acceptable salt thereof (e.g., compound A
or compound B) in combination with Tacrolimus (FK506),
methotrexate or cyclosporin A, particularlly Tacrolimus
(FR506).
CA 0222691~ 1998-01-14
WO 97/09984 PCT/JP96~1693
The pharmaceutical composition of the present
invention comprising the quinoline or quinazoline compound
represented by the formula ( I) or pharmaceutically
acceptable salt thereof in combination with an
immunosuppressant except for a quinoline or quinazoline
compound mentioned above, which is provided in accordance
with the present invention, can be respectively put to use
by mixing the respective active components either all
together or independently with a physiologically acceptable
carrier, excipient, binder, diluent, etc. and administering
the mixture or mixtures either orally or non orally as a
pharmaceutical composition. When the active components are
formulated independently, the respective formulations can
be extemporaneously admixed using a diluent or the like and
aministered or can be administered independently of each
other, either concurrently or at staggered times to the
same subject.
The dosage form for said pharmaceutical composition
includes such oral dosage forms as granules, powders,
tablets, capsules, syrups, emulsions, suspensions, etc. and
such non-oral dosage forms as injections (e.g.
subcutaneous, intravenous, intramuscular and
intraperitoneal injections), drip infusions, external
application forms (e.g. nasal spray preparations,
transdermal preparations, ointments, etc.), and
suppositories (e.g. rectal and vaginal suppositories).
These dosage forms can be manufactured by the per se
known technique conventionally used in pharmaceutical
procedures.
To manufacture an oral dosage form, an excipient (e.g.
lactose, sucrose, starch, mannitol, etc.), a dis-integrator
(e.g. calcium carbonate, carboxymethylcellulose calcium,
etc.), a binder (e.g. ~-starch, gum arabic,
carboxymethylcellulose, polyvinylpyrrolidone,
hydroxypropylcellulose, trehalose etc.), and a lubricant
(e.g. talc, magnesium stearate, polyethylene glycol 6000,
,
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
34
etc.), for instance, are added to the active component or
components and the resulting composition is compressed.
Where necessary, the compressed product is coated, by the
per se known technique, for masking the taste or for
enteric dissolution or sustained release. The coating
material that can be used includes, for instance, ethyl-
cellulose, hydroxymethylcellulose, polyoxyethyleneglycol,
cellulose acetate phthalate, hydroxypropylmethylcellulose
phthalate, and Eudragit (Rohm & Haas, Germany, methacrylic-
acrylic copolymer).
Injections can be manufactured typically by the
following procedure. The active component or components
are dissolved, suspended or emulsified in an aqueous
vehicle (e.g. distilled water, physiological saline,
Ringer's solution, etc.) or an oily vehicle (e.g. vegitable
oil such as olive oil, sesame oil, cottonseed oil, corn
oil, etc. or propylene glycol) together with a dispersant
(e.g. Tween 80 (Atlas Powder, U.S.A.), HCO 60 (Nikko
Chemicals), polyethylene glycol, carboxymethylcellulose,
sodium alginate, etc.), a preservative (e.g. methyl p-
hydroxybenzoate, propyl p-hydroxybenzoate, benzyl alcohol,
chlorobutanol, phenol, etc.), an isotonizing agent (e.g.
sodium chloride, glycerol, sorbitol, glucose, inverted
sugar, etc.) and other additives. If desired, a
solubilizer (e.g. sodium salicylate, sodium acetate, etc.),
a stabilizer (e.g. human serum albumin), a soothing agent
(e.g. benzalkonium chloride, procaine hydrochloride, etc.)
and other additives can also be added.
A dosage form for external application can be
manufactured by processing the active component or
components into a solid, semi-solid or liquid composition.
To manufacture a solid composition, for instance, the
active component or components, either as they are or in
admixture with an excipient (e.g. lactose, mannitol,
starch, microcrystalline cellulose, sucrose, etc.), a
thickener (e.g. natural gums, cellulose derivatives,
CA 0222691~ 1998-01-14
WO 97/09984 PCT~JP96/01693
acrylic polymers, etc.), etc., are processed into powders.
The liquid composition can be manufactured in substantially
the same manner as the injections mentioned above. The
semi-solid composition is preferably provided in a hydrous
or oily gel form or an ointment form. These compositions
may optionally contain a pH control agent (e.g. carbonic
acid, phosphoric acid, citric acid, hydrochloric acid,
sodium hydroxide, etc.), and a preservative (e.g. p-
hydroxybenzoic acid esters, chlorobutanol, benzalkonium
chloride, etc.), among other additives.
Suppositories can be manufactured by processing the
active component or components into an oily or aqueous
composition, whether solid, semi-solid or liquid. The
oleaginous base that can be used includes, for instance,
higher fatty acid glycerides [e.g. cacao butter, Witepsols
(Dynamite-Nobel), etc.], medium-chain fatty acids [e.g.
Migriols (Dynamite-Nobel), etc.], vegetable oils (e.g.
sesame oil, soybean oil, cotton-seed oil, etc.), etc. Ther
water-soluble base includes, for instance, polyethylene
glycols, propylene glycol, etc. The hydrophilic base
includes, for instance, natural gums, cellylose
derivatives, vinyl polymers, and acrylic polymers, etc.
The pharmaceutical composition of the present
invention is low in toxicity and can be safely used in
mammals (e.g. humans, mice, rats, rabbits, dogs, cats,
bovines, horses, swines, monkeys).
The dosage of the pharmaceutical composition of the
present invention may be appropriately determined with
reference to the dosages recommended for the respective
active components and can be selected appropriately
according to the recipient, the recipient's age and body
weight, current clinical status, administration time,
dosage form, method of administration, and combination of
the active components, among other factors. The preferred
frequency of administration is 1 to 3 times a day.
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
The proportions of the active components in the
pharmaceutical composition of the present invention can be
appropriately selected according to the recipient, the
recipient's age and body weight, current clinical status,
administration time, dosage form, method of administration,
and combination of active components, among other factors.
When, for example, the compound represented by the formula
(I) or a pharmaceutically acceptable salt thereof (e.g.
compound A or B) and Tacrolimus (FK506), which is an
example of T cell inhibiting agents, are to be administered
in combination to a human subject, Tacrolimus (FK506) is
used in a proportion of usually about 0.001 to 10 weight
parts and preferably about 0.01 to 1 weight parts relative
to 1 weight part of the compound or a salt thereof. When,
for example, the compound represented by the formula (I) or
a pharmaceutically acceptable salt thereof (e.g. compound A
or B) and cyclosporin A, which is an example of T cell
inhibiting agent, are to be administered in combination to
a human subject, cyclosporin A is used in a proportion of
usually about 0.01 to 100 weight parts and preferably about
0.1 to 10 weight parts relative to 1 weight part of the
compound or a salt thereof. When, for example, the
compound represented by the formula (I) or a
pharmaceutically acceptable salt thereof and methotrexate
which is a metabolic antagonist for a folic acid are to be
administered in combination to a human subject,
methotrexate is used in a proportion of usually about
0.0001 to 1 weight parts and preferably about 0.001 to 0.1
weight parts r relative to 1 weight part of the compound or
a pharmaceutically acceptable salt thereof.
The pharmaceutically composition of the present
invention shows a marked synergistic effect compared with
administration of either active component alone. For
example, compared with cases in which each of these active
components was administered to patients with the diseases
associated with immunity, administration of these active
CA 0222691~ 1998-01-14
W O 97109984 PCT/JP96rO1693
components in combination resulted in marked improvements
in both e~ficacy and toxicity. Thus, the pharmaceutical
composition of the present invention improves the disorder
of immune response more effectively than it is the case
with administration of each component drug alone and,
therefore, can be used advantageously for the prophylaxis
and treatment of diseases assumed to be associated with
immunity including autoimmunity, and the prevention of
graft rejection following transplantation.
Furthermore, since the pharmaceutical composition of
the present invention develops sufficient efficacy with
reduced doses as compared with the administration of any
one of the active components alone, the side effects of the
respective components (e.g. nephrotoxicity,
myelosuppression, pneumonitis, etc.) can be reduced.
The immunosuppressant of the present invention spe-
cifically suppresses the production of immune cytokines
[e.g., interleukin-2 (IL-2), interferon-y (IFN-y)], and is
useful in treating or preventing diseases assumed to be
associated with immunity, including autoimmune diseases, in
m~mm~ls, including humans. Such target diseases include
systemic lupus erythematosus, ulcerative colitis, Crohn
disease, multiple sclerosis, psoriasis, chronic hepatitis,
gallbladder cancer, breast cancer, uterine cervical cancer,
chronic lymphatic leukemia, chronic myelocytic leukemia,
large intestine cancer, colic cancer, rectal cancer,
Helicobacter pylori infection, Hodgkin's disease, insulin-
dependent diabetes mellitus, malignant melanoma, multiple
myeloma, non-Hodgkin lymphoma, non-small cell lung cancer,
ovarian cancer, digestive ulcer, prostatic cancer, septic
shock, tuberculosis, infertility, arteriosclerosis,
Behcet's disease, asthma, atopic dermatitis, nephritis,
systemic fungal infection, acute bacterial meningitis,
acute myocardial infarction, acute pancreatitis, acute
viral encephalitis, adult respiratory distress syndrome,
bacterial pneumonia, chronic pancreatitis, herpes simplex
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
38
viral infection, varicella-zoster viral infection, AIDS,
human papilloma viral infection, influenza, invasive
staphylococcal infection, peripheral vascular disease,
sepsis, interstitial liver diseases, regional ileitis and
multiple sclerosis, especially systemic lupus
erythematosus, chronic hepatitis, interstitial liver
diseases, asthma, psoriasis, ulcerative colitis, Crohn
disease, regional ileitis and multiple sclerosis. The
immunosuppressant of the present invention is also useful
in the prevention of graft rejection following organ
transplantation.
Brief Description of Drawinqs
Fig. 1 shows the effect of concomitant administration
of compound A and Tacrolimus (FK506) on body weight change
in adjuvant arthritic rats.
Fig. 2 shows the effect of concomitant administration
of compound A and methotrexate on body weight change in
adjuvant arthritic rats.
Best modes for carryinq out the invention
The immunosuppressing activity of the pharmaceutical
composition of the present invention is hereinafter de-
scribed with reference to experimental examples.
Experimental Example 1
Action on interleukin-2 and interferony production by rat
splenocytes
Interleukin-2 (IL-2) and interferon-y (IFN-y), both
cytokines produced by T-cells, show diverse actions, in-
cluding T-cell growth and macrophage activation, respec-
tively, and play key roles in immune reaction. Suppression
of production of these cytokines serves as a good index of
immunosuppressing activity.
i) Experimental materials and methods
CA 0222691~ 1998-01-14
WO 97/099~4 PCT/JP96/01693
Splenocytes were isolated from the spleen of a Lewis
rat (male, 8 weeks of age) r and suspended in RPMI-1640
f (containing penicillin, streptomycin and 2-mercaptoethanol)
supplemented with 5% fetal bovine serum), followed by cul-
~ 5 tivation of 1 x 107 splenocytes in the presence of 2 ~g/ml
concanavalin A for 24 hours. After completion of the cul-
tivation, the supernatant was collected. The IL-2 content
in the supernatant was determined with 3H-thymidine incor-
poration as an index, by 50 fold diluting the culture su-
10 pernatant, and carrying out a reaction for growing CTLL-2
cells, an IL-2 dependent cell line. The IFN-y content was
determined by a commonly known method using an ELISA kit
(GIBCO PBL, USA).
15 ii) Results
Compounds (A) and (B) both suppressed IL-2 and IFN-y
production dose dependently (Table 1).
Table 1
Concen-
Interleukin-2 Interferon-y
tration
~M cpm %Inh.ng/ml ~Inh.
Medium 39,093 + 4,755 54.8 + 2.9
25Compound (A) 132,202 + 752189~36.6 ~t 2.7** 339
1022,273 i 1,088**43~ 27.9 ~ 0.7** 49~
100446 i 78** 99% 1.1 i 0.6** 98%
Compound (B) 134,355 ~ 1,744 12~ 53.2 i 4.03%
1018,955 + 661** 52~ 36.6 ~ 1.7** 33
100248 ~ 14** 100~ 1.0 + 0.8** 98
Data are shown mean for 3 cases + standard error.
*: p < 0.05 versus drug-free control group
**: p < 0.01 versus drug-free control group
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
Experimental Example 2
Action on interleukin-2 and intlerferon-y production by
antigen-stimulated mouse T-lymphocytes
i) Experimental materials and methods
Lymphocytes were isolated from a lymph node of a
Balb/c mouse at 10 days after sensitization with ovalbumin
(OVA, 100 ~g), and suspended in RPMI-1640 (containing peni-
cillin and streptomycin) supplemented with 10~ fetal bovine
serum). Lymph node cells were subjected to alternative
cultivation in the presence of splenocytes from an X-ray-
irradiated mouse of the same line and 50 ~g/ml OVA, and in
the presence of 20 ng/ml IL-2, to yield an OVA-reactive T
cell line, from which an OVA-reactive T cell clone was es-
tablished by the limiting dilution analysis method. An
allo-reactive T-cell line was prepared by subjecting Balb/c
mouse splenocytes to alternative cultivation in the pres-
ence of splenocytes from an X-ray-irradiated A/J mouse, and
in the presence of 20 ng/ml IL-2.
To assess drug effect, subject cells were cultured
with each drug in the presence of splenocytes from an X-
ray-irradiated mouse of the same line and 50 ~g/ml OVA for
48 hours for the OVA-reactive T cells, and in the presence
of splenocytes from an X ray-irradiated A/J mouse for 24
hours for the allo-reactive T-cells, after which the IFN-y
and IL-2 contents in the supernatant were determined by
ELISA (according to the same method mentioned in
Experimental Example 1).
ii) Results
Compound (A) significantly suppressed IFN-y production
by the OVA-reactive T cell line, OVA-reactive T cell clone
and allo-reactive T cell line at concentrations exceeding 1
~M, and also significantly suppressed IL-2 production by
the allo-reactive T cell line at concentrations exceeding 1
~M (Table 2).
CA 0222691~ 1998-01-14
W ~ ~7~9~84 PC~JP96/~1693
Table 2
Cytokine Compound (A) (~M)
0.1 1 10
OVA-reactive T cell line AIFN-y 27 43* 72**
OVA-reactive T cell line BIFN-y 17 36* 98**
OVA-reactive T cell clone 5A7 IFN-y ND 35* 71**
OVA-reactive T cell clone SE3 IFN-y ND 38* 69**
Allo-reactive T cell line AIFN-y ND 28* 38*
IL-2 ND 25* 63*
Allo-reactive T cell line BIFN-y 11 22* 30**
~L-2 ND 26* 45**
Data are shown in suppression rate (%).
*: p < 0.05 versus drug-free control group
**: p < 0.01 versus drug-free control group
ND: Not done
Experimental Example 3
Action on rat adjuvant arthritis
Male Lewis rats (7 weeks of age), 6 per group, were
sensitized by intradermally injecting 0.05 ml of Freund's
complete adjuvant (0.5% liquid paraffin suspension of dead
tubercle bacillus cells) to the right hind leg paw (0 day).
Five to 6 times weekly from just before sensitization (0
day) to 30 days, Compound A (3.13 mg/kg/day or 12.5
mg/kg/day), Tacrolimus (FK506) (0.63 mg/kg/day) and
methotraxate (O.OS mg/kg/day), in suspension in 0.5% methyl
cellulose, singly or concomitantly, w.ere orally
administered. Just before sensitization (0 day) and at 14
and 18 days, the left hind leg paw volume was measured to
obtain the paw swelling suppression rate, in comparison
with non-sensitized rats (see Table 3). Body weight was
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
measured just before administration; the body weight
increase was obtained in comparison with non-sensitized
rats (see Fig. 1 and Fig. 2).
The results are shown in mean + standard error for
each group (N = 6) and compared by Student's t-test at a
significance level of < 5%.
As shown in Table 3, concomitant administrations of
Compound A and Tacrolimus (FK506), or compound A and
methotratate showed more potent action in terms of edema
suppression, in comparison with either drug administered
alone.
Particularly, with respect to the action in terms of
edema suppression, the result of concomitant administration
of compound A and Tacrolimus (FK506) at 18 days, also of
compound A and methotrexate at 18 days, showed a remarkable
effect beyond the sum effect of both agents. As is clear
from Fig. 1 and Fig. 2, concomitant administrations of
Compound A and Tacrolimus (FK506), or Compound A and
methotraxate showed more potent effect in terms of body
weight change (increase), in comparison with either drug
administration alone.
CA 0222691~ 1998-01-14
WO 97l09984 PCT/JP96/O~ 693
43
Table 3
Swelling Suppression Rate
Manner of Administration
(mg/kg)
14 days 18 days
I) Compound A 3.13 34* 31*
FK506 0.63 55** 42**
Concomitant use 98** 100**
(Compound A + FK506)
II)Compound A 12.5 40 37
Methotrexate 0.05 83** 37
Concomitant use 100** 88**
(Compound A ~ Methotrexate)
*: p c 0.05 versus drug-free control group
**: p < 0.01 versus drug-free control group
Examples
The present invention is hereinafter described in more
detail by means of, but are not limited to, the following
working examples and reference examples.
Reference Example 1
Production of N-(3,4-dimethoxyphenyl)acetamide
3,4-dimethoxyaniline (100 g) was suspended in H2O (800
ml); after an aqueous solution of NaOH (34.0 g/200 ml) and
acetic anhydride (86.6 g) were alternatively added in one-
fifth portions at under 65~C, the mixture was stirred at
55-60~C for about 20 minutes. After the reaction mixture
was cooled to about 5~C and stirred for 1 hour, the result-
ing crystal was collected by filtration and dried under
reduced pressure to yield 119 g (recovery 93.2%) of N-~3,4-
dimethoxyphenyl)acetamide as a brown crystal.
A sample for IR and lH-NMR determination was obtained
by recrystallization from ethyl acetate.
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
44
IR (cm~l, KBr): 3276, 1653, 1606, 1575, 1520, 1462
H-NMR (CDC13, 300 MHz) ~: 2.15 (3H, s), 3.84 (6H, s),
6.78 (lH, d, J=8.6 Hz), 6.88 (lH, dd, J=8.6, 2.4 Hz), 7.30
(lH, d, J=2.4 Hz), 7.55 (lH, brs)
Reference Example 2
Production of 2-acetylamino-3',4,4',5-tetramethoxybenzo-
phenone
A mixture of N-(3,4-dimethoxyphenyl)acetamide (114 g),
3,4-dimethoxybenzoic acid (117 g) and polyphosphoric acid
(1,203 g) was stirred at 95-110~C for 3 hours. After cold
water (3 1) was added, the reaction mixture was extracted
with ethyl acetate (3 1); the extract was washed with a 2 N
aqueous solution of NaOH (3 1), then with water (3 1).
After the reaction mixture was concentrated under reduced
pressure, n-hexane (1.2 1) was added to the residue, fol-
lowed by stirring at about 5~C for 30 minutes. The result-
ing crystal was collected by filtration, then dried under
reduced pressure to yield 129 g (recovery 61.3~) of 2-
acetylamino-3',4,4',5-tetramethoxybenzophenone as a yellow
crystal.
A sample for IR and lH-NMR determination was obtained
by recrystallization from ethyl acetate.
IR (cm~l, KBr): 2947, 1695, 1678, 1616, 1595
lH-NMR (CDC13, 300 MHz) ~: 2.22 (3H, s), 3.76 (3H, s),
3.94 (3H, s), 3.98 (3H, s), 4.00 (3H, s), 6.93 (lH, d,
J=7.7 Hz), 7.09 (lH, s), 7.28 (lH, dd, J=7.7, 1.9 Hz), 7.32
(lH, s), 8.38 (lH, s), 11.0 (lH, brs)
Reference Example 3
Production of 2-amino-3',4,4',5-tetramethoxybenzophenone
hydrochloride
To a solution of 2-acetylamino-3',4,4',5-tetramethoxy-
benzophenone (129 g) in isobutanol (1.49 1), concentrate
hydrochloric acid (446 ml) was added, followed by thermal
refluxing for 2 hours. After the reaction mixture was
CA 0222691~ 1998-01-14
WO g7~'flgg84 PC'r/JP96/lJ1693
cooled and stirred at about 5~C for 2 hours, the resulting
crystal was collected by filtration, then dried under re-
duced pressure to yield 117 g (recovery 92.3%) of 2-amino-
3',4,4',5-tetramethoxybenzophenone hydrochloride as a
yellowish white crystal.
A sample for IR and lH-NMR determination was obtained
by recrystallization from EtOH.
IR (cm~l, KBr): 3618, 2839, 2567, 1657, 1628, 1583
lH-NMR (DMSO-d6, 300 MHz) ~: 3.58 (3H, s), 3.80 (6H, s),
3.85 (3H, s), 3.61 (lH, brs), 6.93 (lH, s), 7.06 (lH, d,
J=8.6 Hz), 7.20-7.22 (2H, m)
Reference Example 4
36.0 g of 2-amino-3',4,4',5-tetramethoxybenzophenone
hydrochloride and 21.4 g of the ethyl ester of 4-chloro-
acetoacetic acid were thermally refluxed in 350 ml of
ethanol for 7 hours during stirring. After completion of
the reaction, 10.6 g of triethylamine was added drop by
drop at under 20~C, followed by stirring at 5~C for 1 hour.
The resulting crystal was filtered, twice washed with 50 ml
of ethanol, then dried under reduced pressure to yield 41.0
g (recovery 92~) of the ethyl ester of 2-chloromethyl-4-
(3,4-dimethoxyphenyl)-6,7-dimethoxyquinoline-3-carboxylic
acid.
Reference Example 5
Production of 4-amino-1-[4-(3,4-dimethoxyphenyl)-3-ethoxy-
carbonyl-6,7-dimethoxyquinolin-2-ylmethyl]-4H-1,2,4-tria-
zolium bromide
The ethyl ester of 2-chloromethyl-4-(3,4-dimethoxy-
phenyl)-6,7-dimethoxyquinoline-3-carboxylic acid (22.5 g,
content ratio 99.1%, 50.0 mmol), sodium bromide (5.81 g,
56.5 mmol) and 4-amino-1,2,4-triazole (5.47 g, 65.1 mmol)
were suspended in DMF (50 ml) and stirred at 65~C for 3
hours.
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
46
After ethyl acetate (100 ml) was added to the reaction
mixture, the resulting crystal was collected by filtration
and dried to yield 4-amino-1-[4-(3,4-dimethoxyphenyl)-3-
ethoxycarbonyl-6,7-dimethoxyquinolin-2-ylmethyl]-4H-1,2,4-
triazolium bromide as a white crystal (31.1 g, contentratio 83.6%, recovery 90.6~).
A sample for structural identification was purified by
silica gel column chromatography (mobile phase CH2C12:MeOH
= 5:1) and recrystallization (4.8% hydrated EtOH).
IR (cm 1, KBr): 3196, 1706, 1518, 1472
lH-NMR (DMSO-d6, 90 MHz) ~: 0.92 (3H, t, J=6.9 Hz,
CO2CH2CH3), 3.72 (3H, s, OMe), 3.77 (3H, s, OMe), 3.86 (3H,
s, OMe), 3.96 (3H, s, OMe), 3.72-4.09 (2H, m, CO2CH2), 5.94
(2H, s, CH2N), 6.93-7.31 (7H, m), 9.28 (lH, s, CH=N), 10.41
(lH, s, CH=N)
Anal. Calcd for C25H2gN5O5Br(0.73 Hp): C, 51.10; H, 5.05;
N, 11.92; Br, 13.60
Found: C, 51.10; H, 4.91; N, 11.88; Br, 13.55
mp: 183.8-184.4~C
Reference Examples 6 through 12
Reaction was carried out using the same starting quin-
oline and triazole compounds as those used in Reference
Example 5 in a 1:1.3 molar ratio and the same procedure,
but different reaction solvents and additives were used.
The results are shown in Table 4 (percent areal ratios for
HPLC peaks confirmed with the reaction mixture).
CA 02226915 1998-01-14
W O 97/09984
PCT/JP96/01693
47
~, ~i 1~ o N CO 00
a~
~ S O --
.,1 ~ C ~
~ C~ ~r N I o
~ ~ n ~:
a~
_ p~ n N 1'~
~ ~ ~ co ~ ~ ~ co a~ a~
~ ~ a ~
U~ ~
C o
3 ~ _ ~ ~ ~ N ~
o
O ~5 U o o
-- O o O ~ ~ O O
D I m I I I ~ m
O o ~ ~ ~D ~ o O O O
J3 Z Z ~ o ~
~1 a) ,,
d' a) ~ Ei ~ I~ ~ a~ o ~--I N
, ~ ~ Z
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
48
Reference Example 13
Production of ethyl ester of 4-(3,4-dimethoxyphenyl)-6,7-
dimethoxy-2-(1,2,4-triazol-1-ylmethyl)quinoline-3-car-
boxylic acid
4-amino-1-[4-(3,4-dimethoxyphenyl)-3-ethoxycarbonyl-
6,7-dimethoxyquinolin-2-ylmethyl]-4H-1,2,4-triazolium
bromide (10.31 g, content ratio 83.6~, 15.0 mmol) was sus-
pended in H2O (37.5 ml); under ice cooling conditions, con-
centrate hydrochloric acid t3.8 ml, 45 mmol) and a 5.6 M
aqueous solution of NaNO2 (4.00 ml, 22.5 mmol) were added,
followed by stirring at room temperature for 2 hours.
After a 5 N aqueous solution of NaOH (8.7 ml) wasadded to neutralize the reaction mixture, the resulting
crystal was collected by filtration and washed with water
to yield 6.66 g (recovery 92.8%) of the ethyl ester of 4-
(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-triazol-1-
ylmethyl)quinoline-3-carboxylic acid as a white crystal.
IR (cm~l, KBr): 1720, 1504, 1468, 1430
lH-NMR (CDC13, 90 MHz) ~: 0.89 (3H, t, J=7.1 Hz,
CO2CH2CH3), 3.79 (3H, s, OMe), 3.86 (3H, s, OMe), 3.96 (3H,
s, OMe), 4.04 (3H, s, OMe), 3.86-4.13 (2H, q, J=7.1 Hz,
CO2CH2), 5.72 (2H, s, CH2N), 6.86-6.95 (4H, m), 7.41 (lH,
s), 7.93 (lH, s), 8.23 (lH, s)
mp: 175.4-176.0~C
Reference Example 14
Production of ethyl ester of 4-(3,4-dimethoxyphenyl)-6,7-
dimethoxy-2-(1,2,4-triazol-1-ylmethyl)quinoline-3-car-
boxylic acid
4-amino-1-[4-(3,4-dimethoxyphenyl)-3-ethoxycarbonyl-
6,7-dimethoxyquinolin-2-ylmethyl]-4H-1,2,4-triazolium bro-
mide (503 g, content ratio 80.9~, 0.709 mol) was suspended
in H2O (5.44 1); under ice cooling conditions, concentrate
hydrochloric acid (159 g, 1.56 mol) and a 0.63 M aqueous
CA 0222691~ 1998-01-14
W O 97l09984 PCT/JP96/01693
49
solution of NaNO2 (1.46 1, 0.920 mol) were added, followed
by stirring at room temperature for 3 hours.
~ After a 5 N aqueous solution of NaOH (295 ml) was
added to neutralize the reaction mixture, the resulting
crystal was collected by filtration and washed with water
to yield 329 g (recovery 97.0~) of the ethyl ester of 4-
(3,4-dimethoxyphenyl)-6,7-dimethoxy-2-(1,2,4-triazol-1-
ylmethyl)quinoline-3-carboxylic acid as a white crystal.
Example 1
Table 5
12.5 mg 25 mg 50 mg 100 mg
Composition (per Tablet)
Tablet Tablet Tablet Tablet
(1) Compound (A) 12.5 mg 25.0 mg 50.0 mg 100.0 mg
(2) Lactose 133.13 120.63 95.63 45.63
(3) Corn starch 26.7 26.7 26.7 26.7
(4) Hydroxypropyl cel- 5 7 5 5 5
lulose
(5) Crosscarmellose so- 9 5 9 5 9 5 9 5
dium
(6) Macrogol 6000 1.9 1.9 1.9 1.9
(7) Magnesium stearate 0.57 0.57 0.57 0.57
Total 190.0 mg190.0 mg 190.0 mg 190.0 mg
In a granulating machine (Powrex Co., model FD-5S,
FV-25: Japan), 75 g of the above component (1), 798.8 g of
component (2) and 160.2 g of component (3) were granulated
with an aqueous solution of 34.2 g of component (4) as a
binder solution. After drying and size reduction, 961.4 g
of the granules were mixed with 51.3 g of component (5),
10.26 g of component (6) and 3.08 g of component (7) to
yield a powder mixture. This powder mixture was tableted
CA 0222691~ 1998-01-14
W O 97/09984 PCT/JP96/01693
using a per se known tablet machine to yield a tablet
preparation 8.0 mm in diameter and 190 mg in weight
containing 12.5 mg of component (1).
Tablets of other dose units were produced in the same
manner as above.
The tablets obtained were all found to have excellent
tablet properties, including hardness, disintegrability and
solubility.
Industrial Applicability
The immunosuppressant containing compound (I) or a
pharmaceutically acceptable salt thereof for the present
invention is safe and of low toxicity, and can be used to
prevent or treat diseases assumed to be associated with
immunity, including autoimmune diseases, and to prevent
graft rejection following organ transplantation.
And, the pharmaceutical composition comprising a
quinoline or quinazoline compound (I) or a pharmaceutically
acceptable salt thereof and an immunosuppressant except for
the quinoline or quinazoline compound mentioned above, has
a remarkable effect beyond the sum effect of both agents in
comparison with either agent administered above.
Therefore, the pharmaceutical composition can be
used to prevent or treat diseases assumed to be associated
with immunity, including autoimmune diseases, and to pre-
vent graft rejection following organ transplantation, with
very low prevalence of side effects even in chronic
administration, provided that drug combination,
administration method, dose etc. are appropriately chosen
according to symptoms.