Note: Descriptions are shown in the official language in which they were submitted.
CA 02227934 1998-02-23
0050/461~2
~enzoyl derivatives
The present invention relates to novel benzoyl derivatives having
S herbicidal action, processes for preparing the benzoyl deriva-
tives, compositions which contain the latter and the use of these
derivatives or compositions contAining the latter for controlling
weeds .
10 ~erbicidally activ~ 2-aroylcycloheY~ne~iones are disclo~ed in the
literature, for example in EP 283261, EP 90262, EP 135191,
EP 18611~, EP 186119, EP 186120, EP 319075, WO 9005712,
WO 9404524, NO 9408988, JO3052862 and JO 3120202.
15 The herbicidal properties of the known compounds and the toler-
ability for crop plants, however, are only satisfactory to a
limited extent.
It is an ob~ect of the present invention to find novel 2-aroyl-
20 cyclohey~nediones having improved properties.
We have found that this object $8 achieved by the benzoyl deriva-
tives of the formula I
O M ~~)n
Q ~ ~X
L Y
30 where the substituent~ have the following meanings:
L and M are hydrogen, C1-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
C1-C4-alkoxy, it being possible for the~e groups to be
un~ub~tituted or sub~tituted by one to five halogen atom~
or C1-C4-alkoxy; halogen, cyano, nitro, a group
~ (A ) ~-S ( ~ ) nRl or a group -( A) m-CO-R2;
Y is a group consisting of [sic] C-O, C~N - R3~ CR7 - NR5R6,
cR7-oR8~ CR10Rl1, CR7-SR3; 1~3-~ioYAnyl or 1,3-dioxolanyl
~0 substituted by hydrogen or C1-C4-alkyl; a heteroatom
selected from the group oxygen, sulfur and nitrogen;
X consi~ting of lsic] a chain (-CR12R13-),
_CRl2Rl3_CR2lR22_) ~ (--CR12=CR13~) ~ (--CR12Rl3--CRl2--CRl3--);
~5 NR23
CA 02227934 1998-02-23
0050/46142
the bond between X and Y can be saturated or unsaturated;
A iB 0 or NR14;
5 m iB zero or one;
n is zero, one or two;
Rl is C1-C4-alkyl, Cl-C4-haloalkyl or NR14;
R2 is Cl-C4-alkyl, Cl-C4-hAloAlkyl, Cl-C4-alkoxy or NRl4;
R3 $s hydrogen, -NR9R4; Cl-C6-alkyl, Cl-C6-haloalkyl,
Cl-C6-alkoxy, Cl-C6-haloalkoxy, C2-C6-alkenyl,
C2-C6-haloAlkenyl, C2-C6-alkynyl; mono~ubstituted to
polysubstituted phenyl, it being possible for the
~ubstituents to consist of Cl-C4-slkyl, C1-C4-alkoxy,
C1-C4-haloAlkoYy~ Cl-C4-haloalkyl, halogen, cyano, nitro;
mono- to polysubstituted benzyl, it being possible for
the substituents to consist of C1-C4-alkyl, C1-C4-alkoxy,
Cl-C4-haloAlkoYy~ Cl-C4-haloalkyl, halogen, cyano, nitro;
mono- to polysubAtituted benzyloxy, it being possible for
the substituents to consist of C1-C4-alkyl, C1-C4-alkoxy,
C1-C4-haloAlkoYy~ C1-C4-haloalkyl, halogen, cyano, nitro;
R4 i9 hydrogen, Cl-C6-alkyl, Cl-C6-haloalkyl, C2-C6-alkenyl,
C2-C6-alkynyl, C-o-NRl4; monosubstituted to
polysubstituted phenyl, it being possible for the
substituents to consist of C1-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-haloAlk~xy~ C1-C4-haloalkyl, halogen, cyano, nitro;
mono- to polysubstituted benzyl, it being possible for
the substituents to conYist of C1-C4-alkyl, Cl-C4-alkoxy,
C1-C4-haloalkoxy, Cl-C4-haloalkyl, halogen, cyano, nitro;
R9 iB hydrogen, C1-C6-alkyl, Cl-C6-haloalkyl, C2-C6-alkenyl,
C2-C6-alkynyl, C=o-NR14; monosubstituted to
polysubstituted phenyl, it being possible for the
substituents to consist of C1-C4-alkyl, C1-C4-alkoxy,
Cl-C4-haloAlkoxy, C1-C4-hAloAlkyl, halogen, cyano, nitro;
mono- to polysubstituted benzyl, it being poqsible for
the substituents to consist of Cl-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-haloAlk~Yy, C1-C4-haloalkyl, halogen, cyano, nitro;
R5 and R6 independently of one another are hydrogen, Cl-C6-alkyl,
C2-C6-alkenyl, Cl-C4-haloalkyl, C2-C6-haloAlkenyl,
C1-C6-alkoxy, C1-C6-haloalkoxy; monosubstituted to
polysubstituted phenyl, it being possible for the
CA 02227934 l998-02-23
0050/46142
substituents to con~ist of Cl-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-haloalkoxy, Cl-C4-haloalkyl, halogen, cyano, nitro;
mono- to polysubstituted benzyl, it being possible for
the substituents to consi~t of Cl-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-haloalkoxy, Cl-C4-haloalkyl, halogen, cyano, nitro;
R7 is hydrogen, Cl-C6-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkyl,
Cl-C4-haloAlkoxy; substituted or unsubstituted phenyl, it
being possible for the substituents to consist of one to
three halogens, Cl-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-haloalkoxy, nitro; R7 and R21 or R7 and R23 or R7 and
Rl2 can form a bond;
R~ i8 hydrogen, Cl-C6-alkyl, Cl-C4-hAloAlkyl, substituted
phenyl, it being possible for the substituents to consist
of Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-haloalkyl, halogen, cyano, nitro; substituted
benzyl, it being possible for the substituents to consist
of Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-haloalkyl, halogen, cyano, nitro;
R10 and Rll in~epen~ently of one another are hydrogen,
C1-C6-alkyl; phenyl which is unsubstituted or substituted
by one to three halogens, Cl-C4-alkyl, C1-C4-alkoxy,
C1-C4-haloalkoxy, nitro; R10 and R12 or Rl~ and R23 or
R10 and R21 can form a bond;
Rl2 and Rl3 independently of one another are hydrogen,
Cl-C6-alkyl, Cl-C6-haloalkyl, Cl-C6-alkoxy,
Cl-C6-haloalkoxy; unsubstituted or substituted phenyl, it
being possible for the substituents to consist of
Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-haloalkyl, halogen, cyano, nitro;
35 Rl4 is Cl-C4-alkyl;
R2l is hydrogen, Cl-C6-alkyl, Cl-C6-haloalkyl, Cl-C6-alkoxy,
Cl-C6-haloalkoxy; unsubstituted or substituted phenyl,
it being possible for the substituents to consist of
Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-haloalkyl, halogen, cyano, nitro;
R22 is hydrogen, Cl-C6-alkyl, Cl-C6-haloalkyl, Cl-C6-alkoxy,
Cl-C6-haloalkoxy; unsubstituted or substituted phenyl, it
being possible for the sub~tituent~ to consist of
CA 02227934 1998-02-23
0050/46142
Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-haloalkyl, halogen, cyano, nitro;
R23 i~ hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, Cl-C6-alkoxy;
phenyl or benzyl which i~ unsubstituted or substituted by
Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-haloalkyl, halogen, cyano, nitro;
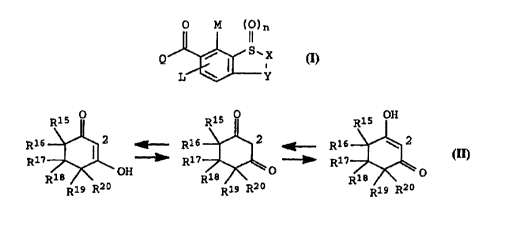
Q i~ a cyclohexane-1,3-dione ring, which is linked in the
~LO 2-position, of the formula II,
R16~2 ~. R16~2 ~ R16~2
~L5 R17 ~ ~ ~ R17 ~ ~~~~' R17 ~ o II
where
:10
Rl5, Rl6,Rl3, and R20 are hydrogen or Cl-C4-alkyl,
Rl9 i~ hydrogen, Cl-C4-alkyl or a group -CooRl4,
25 R17 i~ hydrogen, Cl-C4-alkyl, C3-C4-cycloalkyl, it being
po~sible for the~e group~, if desired, to carry one to
three of the following ~ubstituents: halogen,
Cl-C4-alkylthio or Cl-C4-alkoxy,
.10 or
Rl7 is tetrahydropyran-3-yl, tetrahydropyran-4-yl or tetrahy-
drothiopyran-3-yl,
.15 or
Rl7 and R20 together form a bond or a three to six-membered
carbocyclic ring,
40 where in the case where Y - C-O, X i8 not equal to NR23,
and agriculturally customary salts of the compound [~ic] I.
4L5
CA 02227934 1998-02-23
0050/46142
Compounds of the formula Ia-Ie are obt~ine~. by reacting compounds
of the formula II with a benzoic acid derivative of the formula
III and rearranging to give benzoyl derivative~. of the formula
Ia-Ie.
Scheme 1
R15 ~ 0 M (~)n
R~ 'X
Rlg R20
II III
~ R16~ ~ ~ ( 1~l )~ R16_ I ~ X
R ~ R2 ~ ~X R17 ~
In the above scheme 1, T in said formulae has the meaning halogen
25 or OH and L, M, X, Y and n have the ~~.nings given above.
The first step of the reaction sequence, the acylation, is car-
ried out in a generally known manner, eg. by adding an acid chlo-
ride of the formula III (T=Cl) or a carboxylic acids [sic] III
30 (T=OH) activated, for example, with DCC (dicyclocarbodiimides
[sic]) or similar agents known from the literature, eg. triphe-
nylphosphine/DEAD = diethyl azodicarboxylate, 2-pyridine tsic]
disulfide~triphenylphosphine to the solution or suspension of a
cyclohexanedione II, if appropriate in the presence of an auxil-
35 iary base. The reactants and the auxiliary base are in this casee~pe-~;iently employed in equimolar amounts. A small excess, eg.
from 1.2 to 1.5 molar equivalents, based on II, of the auxiliary
base can be advantageous under certain circumstances.
~-.0 Suitable auxiliary bases are tertiary alkylamines, pyridine or
alkali metal carbonates. The solvents used can be, eg. methylene
chloride, dioxane, diethyl ether, toluene, acetonitrile or ethyl
acetate.
~5 During the addition of the acid chloride, the reaction mixture i~
preferably cooled to from O to 10~C, then it is stirred at from 20
to 100 C, in particular 25 to 50 C, until the reaction is
CA 02227934 1998-02-23
0050/46142
complete. Working up is carried out in the customary manner, eg.
the reaction mixture is poured into water and the useful product
is extracted, eg. with methylene chloride. After drying the or-
ganic phase and removing the solvent, the crude enol ester can be
5 employed without further purification for the rearrangement.
Preparation examples for benzoyl enol esters of cyclohex-
ane-1,3-diones are found, for example, llacuna] EP-A 186 118 or
US 4,780,127.
10 The rearrangement of the enol esters to the compounds of the for-
mula Ia-Ie is expediently carried out at from 20 to 40~C in a sol-
vent and in the presence of an auxiliary base and also with the
aid of a cyano compound as a catalyst.
15 The solvents used can be, eg. acetonitrile, methylene chloride,
1,2-dichloroethane, ethyl acetate or toluene. A preferred solvent
is acetonitrile. Suitable auxiliary bases are tertiary alkyla-
mines, pyridine or alkali metal carbonates, which are preferably
employed in equimolar amounts or up to a four-fold excess, based
20 on the benzoyl enol ester. A preferred auxiliary base i8 tri-
ethylamine in twice the amount.
Suitable catalysts are potassium cyanide, acetone cyanohydrin and
trimethylsilyl cyanide, preferably in an amount of from 1 to
25 50 mol percent, based on the enol ester. Preferably, acetone cya-
nohydrin is added, eg. in the amounts from 5 to 15, in particular
10 mol percent. Examples of the cyanide-catalyzed rearrangement
of enol esters are found, for example, in EP-A 186118 or
US 4,780,127.
Working up is carried out in a manner known per se, eg. the reac-
tion mixture iB acidified with dilute mineral acids such as 5 9c
strength hydrochloric acid or sulfuric acid and extracted with an
organic solvent such as methylene chloride or ethyl acetate. For
35 purification, the extract is extracted with cold 5 to 10 %
strength alkali metal carbonate solution, the final product pass-
ing into the aqueous phase. By acidifying the aqueous solution,
the product of the formula Ia-Ie is precipitated or extracted
again with methylene chloride or ethyl acetate, dried and then
40 freed from the solvent.
The 1,3-diketones of the formula II used as a starting material
are known and can be prepared by processes known per se, such as
are described, for example, in EP-A 71707, EP-A 142741, EP-A
45 243313, uS 4,249,937 and wo 92~13821. Cyclohexanedione and
dimedone are commercially available compounds.
CA 02227934 1998-02-23
OOSO/46142
Benzoic acids of the formula III can be prepared as follows:
Benzoyl halides, for example benzoyl chlorides of the formula III
(T = Cl), are prepared in a known manner by reactin~g the benzoic
5 acids of the formula III ~T = OH) with thionyl chloride.
The benzoic acids of the formula III (T = OH) can be prepared in
a known manner by acidic or basic hydrolysis of the corresponding
esters of the formula III (T = C1-C4-alkoxy).
:LO The inteL ~ tes of the formula III are known in some cases or
can be prepared by processes known from the literature.
Scheme 2
;L50 M O M O M
J~ SN T~ ~ ~ T~
20 IV Va O VIa ~
O M O M O M
;25 ~ _ ~ ~ O~ , T
IV Vb O VIb O
O M O M O M
.. ~0 J~ SN T~J~ ~ T~
IV Vc VIC
~5
After this, for example, arylthio compounds IY as shown in scheme
2 can be reacted with substituted haloalkenyl [sic] as described
in J. Med. Chem. 1984, 27, 1516, substituted alkynylcarboxylic
acids as described in J. Org. Chem. 1980, 45, 4611 or J. Am.
4LO Chem. Soc. 1983, 105, 883, substituted haloalkylcarboxylic acids
as desribed in Chem. Ber. 1925, 58, 1612 in the presence of a
base such as alkali metal hydroxide, alkali metal hydride or
alkali metal carbonate. The resulting compounds V are cyclized to
VI under Friedel-Crafts conditions with addition of a Lewis acid
4L5 or a protic acid. Preferred Lewis acids are AlC13 or SnCl4 and
preferred protic acids are polyphoshoric [ 8iC] acid and sulfuric
acid as described in Can. J. Chem. 1981, 59, 199; Chem. Ber.
CA 02227934 1998-02-23
0050/46142
1925, 5~, 1625; Chem. Her. 1926, 5g, 1074; Phosp. and Sulf. 1984,
19, 31.
Thiochromenone acids can furthermore be prepared by, for example,
5 elimination of hydrogen halide from 3-halothiochromanone acids
or, for example, by reaction of the subfitituted thiophenol acids
with substituted a-alkylacetic ester~ in the presence of
phosphorus pentoxi~e as described in Ann. Chem. 1964, 680, 40.
~0 The arylthio compounds IV can be obtained, for example, by Sand-
meyer reaction from corresponding anilines, which for their part
are synthesized by reduction of suitable nitro compounds a~ de-
scribed in Organicum l9th Edition 1992, 552 ff.
15 In the case where, for example, X i9 equal to (-CRl2Rl3-) or
(-CRl2Rl3CR2lR22-), Y is equal to C=O and T i~ equal to
Cl-C4-alkoxy, the thiochromonone ester or dihydrobenzothiophene
ester as described in scheme 2 can be prepared by alkylation of
the arylthio compound IV with halopropionic acid or haloacetic
;~0 acid in solvent or water in the presence of one of the
abovementioned bases and cyclized to VI.
The reactantq and the base are in thi~ ca~e eYre~iently employed
in equimolar amounts. The reaction mixture is preferably stirred
;25 at 20-100 C, in particular at 20-40 C. Working-up is carried out,
for example, in ~uch a way that the reaction mixture is poured
onto water, the aqueous phase is rendered acidic using mineral
acids such a~ hydrochloric acid or sulfuric acid and the u~eful
product is filtered off with suction or extracted by extraction
.lO l~icl with methylene chloride or ethyl acetate, and the extract
is dried and freed from the solvent. The ester can be reacted
without further purification.
By stirring V in, for example, polyphosphoric acid at 40-140 C, in
35 particular at 70-100~C, or by activating the carboxylic acid by
conversion into the acid chloride and stirring with 2-6, in par-
ticular 3.5 to 4.5, mol equivalents of a Lewis acid such as AlC13
or SnCl4 in a solvent or by stirring with or in sulfuric acid, an
intermediate of the formula III is obtained by working up in a
~IO manner known per se, ie. adding ice water and filtering off the
useful product with suction or extracting the aqueous phase with
ethyl acetate or methylene chloride, drying and removing the
solvent.
~15 In the case where, for example, X is equal to an ethylene group
~-CR12=CRl3-), Y is equal to C=O and T is equal to C1-C4-alkoxy,
the thiochromenone ester can be converted by, for example,
CA 02227934 1998-02-23
0050/46142
reaction of an arylthio compound ~ith an acetylenecarboxylic acid
derivative in water or solvent at 0-140 C. Working up is carried
out in a manner known per se by adding water and dilute mineral
acid, eg. hydrochloric acid. The useful product is either fil-
5 tered off with suction or obt~i n~ by extraction with methylenechloride or ethyl acetate, then drying and removing the solvent.
The intermediates of the formula III can be further functional-
ized by reactions known from the literature, ~uch as reduction as
l0 described in Jerry March, Advanced Organic Chemistry, Fourth Ed.,
eg. p.910ff, oximation as described in Jerry March, Advanced
organic Chemi~try, Fourth Ed., eg. p. 934, 935, 1039, 1226,
405ff, conversion into imines and amines as described in Jerry
March, Advanced Organic Chemistry, Fourth Ed., ketalization,
15 alkylation, halogenation, elimination and oxidation as described
in Jerry March, Advanced Organic Chemi~try, Fourth Ed.
The acids of the 3-alkoxy-1,2-benzisothiazole-1,1-dioxides or
3-alkoxy-1,2-benzisothiazoles can be obtained starting from cor-
20 responding ~AcchArin derivatives or 1,2-benzisoth;A~oles by, for
example, reaction with PCl5, POC13 or chlorine and alcohol, if ap-
propriate in the presence of an auxiliary base ~uch as triethyl-
amine, which is described, for example, in US 4,571,429, Arch.
Pharm. 1984, 317, 807, US 4461901, VS 450916, J. Med. Chem. 1986,
25 29, 359. Saccharincarboxylic acids can be obtained by processe~
known from the literature a~ described in Ann. Chem. 427, 231,
1922, Chem. Ber. 13, 1554, 1980, Chem. Ber. 25, 1740, 1892,
German Offenlegungsschrift 3607343, German Patent Application
P 44 27 995.7.
The derivative~ of the benzo-1,4-oxathiin acids are known in some
cases, eg. from J. Org. Chem. 1968, 33, 456 or can be
synthesized, for example, by reaction [sic] from the
corre~ponding phenol derivatives as described in Chem. Comm.,
35 1975, 451, J. org. Chem. 1974, 39, 1811, J. Am. Chem. Soc. 1954,
76, 1068 or by combination of, for example, substitution
reaction~ of halogen-sub~tituted thiophenol derivativeR and
secondary reactions such as oxidation, reduction or addition a~
described in J. Het. Chem. 1983, 20, 867.
~0
The benzoic acids of the formula III can al~o be obtA~ne~ by
reacting the corresponding bromo- or iodo-sub~tituted compound of
the formula VII
~5
CA 02227934 1998-02-23
0050/46142
Scheme 3
M (~)n ~ M ~~)n
Br,I ~ y T ~ 'X
VII III
10 T i8 OH, Cl-C4-alkoxy
Y,L,M,X, have the meanings de~cribed above
with carbon monoxide and water at elevated pressure in the pres-
ence of a palladium, nickel, cobalt or rhodium transition metal
15 cataly~t and a base.
The catalysts nickel, cobalt, rhodium and in particular palladium
can be present in metallic form or in the form of customary
salts, such as in the form of halogen compounds, eg. PdC12,
20 RhCl3-H20, acetates, eg. Pd~OAc)2, cyanides etc., in the known
valency states. Metal complexes with tertiary phosphines, metal
alkylcarbonyls, metal carbonyls, eg. CO2~CO)8, Ni~CO) 4, metal
carbonyl complexes with tertiary phosphines, eg. (PPh3)2Ni(CO) 2~
or transition metal salts complexed with tertiary phosphines can
25 further be present. The last-mentioned embodiment iB preferred,
in particular, in the case of palladium as a catalyst. The nature
of the phosphine ligands here is widely variable. For example,
they can be repre~ented by the following formulae:
~ R24 R24~ ~ R26
P ~ R25 or R2s ~ P (CH2)~ - P ~
where n is the numbers 1, 2, 3 or 4 and the radicals R24 to R27
35 are low molecular weight alkyl, eg. Cl-C6-alkyl, aryl,
Cl-C4-alkylaryl, eg. benzyl, phenethyl or aryloxy. Aryl is, for
example, naphthyl, anthryl and preferably unsubstituted or
~ubstituted phenyl, where with respect to the sub~tituents
attention only has to be paid to their inertness to the
40 carboxylation reaction, otherwise they can be widely varied and
include all inert C-organic radicals such as C1-C6-alkyl r~icAls~
eg. methyl, carboxyl radicals such as COOH, COOM (M is, for
example, an alkali metal, alkaline earth metal or ammonium salt
[sicl), or C-organic radicals bonded via oxygen, such as
45 C1-C6-alkoxy radicals.
CA 02227934 1998-02-23
0050/46142
The phosphine complexes can be prepared in a manner known per se,
eg. as described in the documents mentioned at the outset. For
example, the starting materials used are customary commercially
available metal salts such as PdCl2 or Pd(OCOCH3)2 and the
5 phosphine for example P(c6Hs)3~ P(n-C4Hs)3, PCH3(C6Hs) 2r
1,2-bis(diphenylphosphino)ethane is added.
The amount of phosphine, based on the transition metal, is cus-
tomarily from O to 20, in particular 0.1 to 10, mol equivalents,
10 particularly preferably 1 to 5 mol equivalents.
The amount of transition metal is not critical. Of course, for
co~t reason~, rather a small amount, eg. from 0.1 to 10 mol%, in
particular from 1 to 5 mol%, based on the starting substance II
15 or III, is used.
For the preparation of the benzoic acids III (T = OH), the reac-
tion is carried out with carbon monoxide and at least equimolar
amounts of water, based on the starting ~ubstanceQ VI. The reac-
20 tion component water can simultaneously be used also as a sol-
vent, ie. the maximum amount is not critical.
However, depenAing on the starting substances and the catalyst~
used, it can al~o be advantageous instead of the reaction compo-
25 nent to use as the solvent another inert solvent or the base usedfor the carboxylation.
Suitable inert solvents are solvents customary for carboxylation
reaction~, such a~ hydrocarbons, eg. toluene, xylene, hex~ne~
30 pentane, cyclohexane, ethers, eg. methyl tert-butyl ether, tetra-
hydrofuran, dioxane, dimethoxyethane, substituted amides such as
dimethylformamide, persubstituted ureas ~uch as tetra-Cl-C4-alkyl-
ureas, or nitriles such as benzonitrile or acetonitrile.
35 In a preferred embodiment of the process, one of the reaction
components, in particular the base, is used in an excess 80 that
no additional solvent is necessary.
Bases suitable for the process are all inert bases which are able
40 to bind the hydrogen iodide or hydrogen bromide liberated in the
reaction. Examples which may be mentioned here are tertiary
amines such as tert-alkylamines, eg. trialkylamines such as tri-
ethylamine, cyclic amines such a~ N-methylpiperidine or N,N'-di-
methylpiperazine, pyridine, alkali metal carbonates or hydrogen
45 carbonates, or tetraalkyl-substituted urea derivatives such as
tetra-Cl-C4-alkylurea, eg. tetramethylurea.
CA 02227934 1998-02-23
0050/46142
The amount of base is not critical, customarily from 1 to 10, in
particular from 1 to 5, mol are u~ed. When the base is simulta-
neously used as a solvent, the amount is generally proportioned
such that the reaction components are dis~olved, unnecessarily
5 high excesses being avoided for practicability reasons, in order
to save costs, to be able to employ small reaction vessels and to
guarantee maximum contact of the reaction components.
During the reaction, the carbon monoxide pressure is adjusted
10 ~uch that an excess of C0, based on VI, is always present. Pre-
ferably, the carbon monoxide pressure at room temperature is from
1 to 250 bar, in particular from 5 to 150 bar of CO [sic].
The carbonylation is generally carried out continuously or batch-
15 wise at from 20 to 250 C, in particular 30 to 150 C. In the ca~e
of batchwise operation, carbon monoxide iB expediently injected
into the reaction mixture continuously to maintain a constant
pressure.
20 The arylhalogen compounds VII used as starting compounds are
known or can easily be prepared by suitable combination of known
synthe~es and by reaction sequences described above.
With respect to the inte~ed use of the benzoyl derivatives of
25 the general formula I, suitable substituents are the following
radicals:
L and M are hydrogen,
30 Cl-C6-alkyl such as methyl, ethyl, propyl, 1-methylethyl, butyl,
1-methylpropyl, 2-methylpropyl, l,1-dimethylethyl, pentyl,
1-methylbutyl, 2-methylbutyl, 3-methylbutyl, 1,1-dimethylpropyl,
1,2-dimethylpropyl, 2,2-dimethylpropyl, 1-ethylpropyl, hexyl,
1-methylpentyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl,
35 1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl,
2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl,
l-ethylbutyl, 1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl,
l-ethyl-l-methylpropyl or l-ethyl-2-methylpropyl,
40 in particular methyl, ethyl, l-methylethyl, l-methylpropyl,
2-methylpropyl, 1,1-dimethylethyl and 1,1-dimethylpropyl;
C2-C6-alkenyl such as 2-propenyl, 2-butenyl, 3-butenyl,
l-methyl-2-propenyl, 2-methyl-2-propenyl, 2-pentenyl,
45 3-pentenyl, 4-pentenyl, 3-methyl-2-butenyl, 1-methyl-2-butenyl,
2-methyl-2-butenyl, 1-methyl-3-butenyl, 2-methyl-4-butenyl,
3-methyl-3-butenyl, 1,1-dimethyl-2-propenyl, 1,2-dimethyl-
CA 02227934 1998-02-23
0050/46142
2-propenyl, 1-ethyl-2-propenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl,
5-hexenyl, 1-methyl-2-pentenyl, 2-methyl-2-pentenyl,
3-methyl-2-pentenyl, 4-methyl-2-pentenyl, 1-methyl-3-pentenyl,
2-methyl-3-pentenyl, 3-methyl-3-pentenyl, 4-methyl-3-pentenyl,
5 1-methyl-4-pentenyl, 2-methyl-4-pentenyl, 3-methyl-4-pentenyl,
4-methyl-4-pentenyl, 1,1-dimethyl-2-butenyl, l,l-dimethyl-
3-butenyl, 1,2-dimethyl-2-butenyl, 1,3-dimethyl-3-butenyl,
2,2-dimethyl-3-butenyl, 2,3-dimethyl-2-butenyl, 2,3-dimethyl-
3-butenyl, 1-ethyl-2-butenyl, 1-ethyl-3-butenyl, 2-ethyl-2-but-
10 enyl, 2-ethyl-3-butenyl, 1,1,2-trimethyl-2-propenyl, l-ethyl-
1-methyl-2-propenyl and ethyl-2-methyl-2-propenyl,
in particular 1-methyl-2-propenyl, 1-methyl-2-butenyl,
1,1-dimethyl-2-propenyl and 1,1-dimethyl-2-butenyl;
C2-C6-al~ynyl such as propargyl, 2-butynyl, 3-butenyl, 2-pentynyl,
3-pentynyl, 4-pentynyl, 1-methyl-3-butynyl, 2-methyl-3-butynyl,
1-methyl-2-butynyl, 1,1-dimethyl-2-propynyl, 1-ethyl-2-propynyl,
2-hexynyl, 3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-2-pentynyl,
20 1-methyl-3-pentynyl, 1-methyl-4-pentynyl, 3-methyl-4-pentynyl,
4-methyl-2-pentynyl, 1,1-dimethyl-2-butynyl, l,l-dimethyl-
3-butynyl, 1,2-dimethyl-3-butynyl, 2,2-dimethyl-3-butynyl,
l-ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl and
l-ethyl-l-methyl-2-propynyl;
Cl-C4-alkoxy such a~ methoxy, ethoxy, n-propoxy, l-methylethoxy,
n-butoxy, l-methylpropoxy, 2-methylpropoxy and l,1-dimethyl-
ethoxy,
30 in particular C1-C3-alkoxy such as methoxy, ethoxy or i-propoxy,
where these groups can be unsubstituted or substituted by one to
five halogen atoms such aq fluorine, chlorine, bromine and
iodine, preferably fluorine and chlorine or Cl-C4-alkoxy as men-
35 tioned above.The group ~(A)m-S(O)nRl defined above i~, for example
Cl-C4-alkylthio such as methylthio, ethylthio, n-propylthio,
40 l-methylethylthio, n-butylthio, 1-methylpropylthio, 2-methyl-
propylthio and l,l-dimethylethylthio, in particular methylthio;
Cl-C4-alkylsulfinyl such as methylsulfinyl, ethylsulfinyl,
n-propylsulfinyl, l-methylethylsulfinyl, n-butylsulfinyl,
45 l-methylpropylsulfinyl, 2-methylpropylsulfinyl and l,1-dimethyl-
ethylsulfinyl, in particular methylsulfinyl;
CA 02227934 1998-02-23
0050~46142
Cl-C4-a:1kylsulfonyl such as methylsulfonyl, ethylsulfonyl,
n-propylsulfonyl, l-methylethylsulfonyl, n-butylsulfonyl,
l-methylpropylsulfonyl, 2-methylpropylsulfonyl and l,l-dimethyl-
ethylsulfonyl, in particular methylsulfonyl;
C1-C4-alkoxysulfonyl such as methoxysulfonyl, ethoxysulfonyl,
n-propoxysulfonyl, l-methylethoxysulfonyl, n-butoxysulfonyl,
l-methylpropoxysulfonyl, 2-methylpropoxysulfonyl and
l,l-dimethylethoxysulfonyl, in particular methoxysuIfonyl;
.10
N-Cl-C4-alkylsulfamoyl such as N-methylsulfamoyl, N-ethylsul-
famoyl, N-n-propylsulfamoyl, N-l-methylethylsulfamoyl, N-n-butyl-
sulfamoyl, N-l-methylpropylsulfamoyl, N-2-methylpropylsulfamoyl
and N-l,l-dimethylethylsulfamoyl, in particular N-methyl-
.15 sulfamoyl;
N-C1-C4-alkylsulfinamoyl such as N-methylsulfinamoyl, N-ethyl-
BUl finamoyl, N-n-propyl 8ul finamoyl, N-l-methylethylsulfinamoyl,
N-n-butylsulfinamoyl, N-l-methylpropylsulfinamoyl, N-2-methyl-
.20 propylsulfinamoyl and N-l,l-dimethylethylsulfinamoyl, in
particular N-methylsulfinamoyl;
di-C1-C4-alkylsulfamoyl such as dimethylsulfamoyl, diethyl-
sulfamoyl, dipropylsulfamoyl, dibutylsulfamoyl, N-methyl-N-ethyl-
:25 sulfamoyl, N-methyl-N-propylsulfamoyl, N-methyl-N-l-methylethyl-
sulfamoyl, N-methyl-N~ dimethylethylsulfamoyl, di-l-methyl-
ethylsulfamoyl, N-ethyl-N-l-methylethylsulfamoyl and N-ethyl-
N-l,l-dimethylethylsulfamoyl; in particular dimethylsulfamoyl;
30 di-Cl-C4-alkylsulfinamoyl such as dimethylsulfinamoyl, diethyl-
sulfinamoyl, dipropylsulfinamoyl, dibutylsulfinamoyl, N-methyl-
N-ethylsulfinamoyl, N-methyl-N-propylsulfinamoyl, N-methyl-N-l-
methylethylsulfinamoyl, N-methyl-N-l,l-dimethylethylsulfinamoyl,
di-l-methylethylsulfinamoyl, N-ethyl-N-l-methylethylsulfinamoyl
35 and N-ethyl-N-l,l-dimethylethylsulfinamoyl; in particular
dimethylsulfinamoyl,
C1-C4-alkylsulfinyloxy such as methylsulfinyloxy, ethylsulfinyl-
oxy, n-propylsulfinyloxy, l-methylethylsulfinyloxy, n-butylsul-
40 finyloxy, l-methylpropylsulfinyloxy, 2-methylpropylsulfinyloxy
and l,l~dimethylethylsulfinyloxy, in particular methylsulfinyl-
oxy;
C1-C4-alkylsulfonyloxy such as methylsulfonyloxy, ethylsulfonyl-
~15 oxy, n-propylsulfonyloxy, l-methylethylsulfonyloxy, n-butyl-
sulfonyloxy, l-methylpropylsulfonyloxy, 2-methylpropylsulfonyloxy
CA 02227934 1998-02-23
0050/46142
and l,~-dimethylethylsulfonyloxy, in particular methyl-
sulfonyloxy;
Cl-C4-alkylsulfinylamino such as methyl~ulfinylamino, ethyl
5 sulfinylamino, n-propylsulfinylamino, l-methylethylsulfinylamino,
n-butylsulfinylamino, 1-methylpropylsulfinylamino, 2-methyl-
propyl~ulfinylamino and l,l-dimethylethylsulfinylamino, in
particular methylsulfinylamino;
10 Cl-C4-alkylsulfonylamino such as methylsulfonylamino, ethyl-
sulfonylamino, n-propylsulfonylamino, l-methylethylsulfonylamino,
n-butylsulfonylamino, l-methylpropylsulfonylamino, 2-methyl-
propyl~ulfonylamino and l,l-dimethylethylsulfonylamino, in
particular methylsulfonylamino;
N-Cl-C4-alkylsulfinyl-N-methylamino such as N-methylsulfinyl-
N-methylamino, N-ethylsulfinyl-N-methylamino, N-n-propylsulfinyl-
N-methylamino, N-l-methylethylsulfinyl-N-methylamino, N-n-butyl-
sulfinyl-N-methylamino, N-l-methylpropylsulfinyl-N-methylamino,
20 N-2-methylpropylsulfinyl-N-methylamino and N-l,l-dimethylethyl-
sulfinyl-N-methylamino, in particular N-methylsulfinyl-
N-methylamino;
N-Cl-C4-alkylsulfinyl-N-ethylamino such as N-methylsulfinyl-
25 N-ethylamino, N-ethylsulfinyl-N-ethylamino, N-n-propylsulfinyl-
N-ethylamino, N-l-methylethylsulfinyl-N-ethylamino, N-n-butyl-
sulfinyl-N-ethylamino, N-l-methylpropylsulfinyl-N-ethylamino,
N-2-methylpropylsulfinyl-N-ethylamino and N-l,l-dimethylethyl-
sulfinyl-N-ethylamino, in particular N-methylsulfinyl-N-ethyl-
30 amino;
N-Cl-C4-alkylsulfonyl-N-methylamino ~uch as N-methylsulfonyl-
N-methylamino, N-ethylsulfonyl-N-methylamino, N-n-propylsul-
fonyl-N-methylamino, N-l-methylethylsulfonyl-N-methylamino,
35 N-n-butylsulfonyl-N-methyl-amino, N-l-methylpropylsulfonyl-
N-methylamino, N-2-methylpropylsulfonyl-N-methylamino and
N-l,l-dimethylethyl~ulfonyl-N-methylamino, in particular
N-methylsulfonyl-N-methylamino;
40 N-Cl-C4-alkylsulfonyl-N-ethylamino such as N-methylsulfonyl-
N-ethylamino, N-ethylsulfonyl-N-ethylamino, N-n-propylsulfonyl-
N-ethylamino, N-l-methylethylsulfonyl-N-ethylamino, N-n-butyl-
sulfonyl-N-ethylamino, N-l-methylpropylsulfonyl-N-ethylamino,
N-2-methylpropylsulfonyl-N-ethylamino and N-l,l-dimethylethylsul-
45 fonyl-N-ethylamino, in particular N-methylsulfonyl-N-ethylamino;
CA 02227934 1998-02-23
0050/46142
Cl-C4-haloalkylthio such as chloromethylthio, dichloromethylthio,
trichloromethylthio, fluoromethylthio, difluoromethylthio,
trifluoromethylthio, chlorofluoromethylthio, chlorodifluoro-
methylthio, l-fluoroethylthio, 2-fluoroethylthio, 2,2-difluoro-
5 ethylthio, 2,2,2-trifluoroethylthio, 2-chloro-2,2-difluoro-
ethylthio, 2,2-dichloro-2 fluoroethylthio, 2,2,2-trichloro-
ethylthio and pentafluoroethylthio, in particular trifluoro-
methylthio.
10 The group -(A)m-C0-R2 defined above is, for example,
Cl-C4-alkylcarbonyl such as methylcarbonyl, ethylcarbonyl,
n-propylcarbonyl, l-methylethylcarbonyl, n-butylcarbonyl,
1-methylpropylcarbonyl, 2-methylpropylcarbonyl and
15 l,1-dimethylethylcarbonyl, in particular methylcarbonyl;
Cl-C4-alkoxycarbonyl such as methoxycarbonyl, ethoxycarbonyl,
n-propoxycarbonyl, 1-methylethoxycarbonyl, n-butoxycarbonyl,
1-methylpropoxycarbonyl, 2-methylpropoxycarbonyl and
20 1,1-dimethylethoxycarbonyl, in particular methoxycarbonyl;
N-Cl-C4-alkylcarbamoyl such as N-methylcarbamoyl, N-ethyl-
carbamoyl, N-n-propylcarbamoyl, N-l-methylethylcarbamoyl,
N-n-butylcarbamoyl, N-l-methylpropylcarbamoyl, N-2-methylpropyl-
25 carbamoyl and N-l,l-dimethylethylcarbamoyl, in particular
N-methylcarbamoyl;
di-Cl-C4-alkylcarbamoyl such as dimethylcarbamoyl, diethyl-
carbamoyl, dipropylcarbamoyl, dibutylcarbamoyl, N-methyl-N-
30 ethylcarbamoyl, N-methyl-N-propylcarbamoyl, N-methyl-N-l-methyl-
ethylcarbamoyl, N-methyl-N-l,l-dimethylethylcarbamoyl,
di-l-methylethylcarbamoyl, N-ethyl-N-l-methylethylcarbamoyl and
N-ethyl-N-l,l-dimethylethylcarbamoyl; in particular dimethyl-
carbamoyl;
Cl-C4-alkylcarbonyloxy such as methylcarbonyloxy, ethylcarbonyl-
oxy, n-propylcarbonyloxy, l-methylethylcarbonyloxy, n-butyl-
carbonyloxy, l-methylpropylcarbonyloxy, 2-methylpropylcarbonyloxy
and 1,1-dimethylethylcarbonyloxy, in particular methylcarb-
40 onyloxy;
C1-C4-alkylcarbonylamino such as methylcarbonylamino, ethyl-
carbonylamino, n-propylcarbonylamino, l-methylethylcarbonylamino,
n-butylcarbonylamino, l-methylpropylcarbonylamino, 2-methyl-
45 propylcarbonylamino and 1,1-dimethylethylcarbonylamino, in
particular methylcarbonylamino;
CA 02227934 l998-02-23
OOSO/~6142
N-Cl-C4--alkylcarbonyl-N-methylamino such as N-methylcarbonyl-
N-methylamino, N-ethylcarbonyl-N-methylamino, N-n-propyl-
carbonyl-N-methylamino, N-l-methylethylcarbonyl-N-methylamino,
N-n-butylcarbonyl-N-methylamino, N-1-methylpropylcarbonyl-
5 N-methylamino, N-2-methylpropylcarbonyl-N-methylamino and
N-l,l-dimethylethylcarbonyl-N-methyl-amino, in particular
N-methylcarbonyl-N-methylamino.
X i~, for example:
.10
CH2, CH(CH3), C((CH3)2), CH(c2Hs)~ C(~C2Hs)2), CH~C6Hs), CH2-CH2,
CH2-CH~CH3), CH2-C~(CH3)2), CH~CH3)-CH(CH3), CH(CH3)-C((CH3)2),
C( (CH3)2)--C( (CH3)2),
CE~2--CH~C2H5), CH2--C((C2H5)2), CH(c2Hs)-cH(c2Hs)~ CH(C2Hs)~C((C2Hs)2),
~lS C((C2HS)2)--C((C2H5)2 ), CH2--CH(C3H7), CH2--CH(iC3H7), CH2-CH~C4Hg)~
CH2-CH(iC4Hs),
CH2-CH(Br), CH2-C((Br)2), CH(Br)-CH(Br), C((Br)2)-C((Br)2),
CH2-CH(Cl), CH2-C((C1)2), CH(Cl)-C((C12), C((C1)2)-C((C1)2),
CH2~CH(C6Hs), CH(c6Hs)-cH(c6Hs)~ CH2-CH(p-NO2C6Hs)]
.20 CH=CH, C(CH3)=CH, C(CH3)=CCH3, CH=CBr, CH=CCl, CBr=CBr, CCl=CCl,
CH=C(OCH3), CH=C(C6Hs), C(C6Hs)=C(C6Hs)~ C(C2Hs)=CH,
C(C2Hs)-C(C2Hs), CH=C(C3Hs), CH=C(C4H7), CH2-CH=CH, CH(CH3)-CH=CH,
C((CH3) 2)-- CH=CH, CH2--CH--C(CH3), CH2--C(CH3)=CH, CH2--C(CH3)=C(CH3),
CH(CH3)-C(CH3)=C(CH3), C((CH3)2)-C(CH3)=C(CH3), N-H, N-CH3, N-C2Hs,
25 N-C3Ht, N-C4Hg, N-iCH3H7, N-OCH3, N-OC2Hs, N-CH2C6Hs, N-C6H
Y i8, for example:
C=O, CH-OH, CH-OCH3, CH-OC2H5, CH-OC3H7, CH-OiPr, CH-OC4Hg,
30 CH- oiBu ~ CH-OCsHll, CH-OC6H13, CH-OC6H5, C(CH3)-OCH3, C(CH3)_0C2H5,
C(CH3~_0C3H7, C(CH3)-OC4Hg, C(CH3)-OiPr, C(CH3)-OiBu, C(CH3)-OtBu,
c(cH3)--OPh, CH2, CH(CH3), C((CH3) 2)~ C--N--CH3, C--N--C2Hs, C=N--C3H7,
C=N-C4Hg, C=N-iC4Hg, C=N-tC4Hg, C=N-iPr, C=N-OCH3, C=N-OC2H5,
C-N-OC3H7, C=N-OC4Hg, CsN-oiC4Hg, C-N-OtC4Hg, C=N-OCH2CH=CH2,
.35 C=N-OCH(CH3)CH=CH2, C=N--OCH2CH=CHCH3, CSN-OCH2CHsC(CH3) 2~
C=N--OCH2CH=CHBr, C=N--OCH2CH=CHCl, C=N--OCH2CH=CHC2H5, C=N--OCH2CS--SCH,
C=N-OCH2C-CCH3, C=N-OCH2C6Hs, CH-NH(OCH3), CH-NH(OC2Hs),
CH-NH(OiPr), CH-NH(OnPr), CH-NH(OC6H5), CH-NCH3(OCH3),
CH-NCH3(0C2H5), CH-NCH3(0iPr), CH-NCH3(0nPr), CH-NCH3(0C6Hs),
40 CH-NH(CH3), CH-NH(C2Hs), CH-NH(C3H7), CH-NH(C4Hg), CH-NH(iPr),
CH-NH(iBu), CH-NH(tBu), CH-NH(C6H5), CH-N(CH3) 2~ CH-NCH3~C2H5),
CH-NCH3(C3H7), CH-NCH3(C4Hg), CH-NCH3(iPr), CH-NCH3(iBu), C=N-NH2,
C=N-NHCH3, C=N--N((CH3) 2 ), CsN-NH(c2Hs)~ C=N--NCH3(C2Hs)~
CSN-N((C2Hs)2)~ CH-SCH3, CH--SC2Hs, CH--SC3H7, CH--SC4Hg, CH--SPr,
~5 CH-SiBu, CH-SH, C(CH3)-SCH3, C(CH3)-SC2Hs, C(CH3)-SC3H7~
1,3-dioxanyl, 1,3-dioxolanyl, 5,5-dimethyl-1,3-dioxanyl
CA 02227934 1998-02-23
0050/46142
Prefer.red benzoyl derivatives are those of the formula Ia,
O M (~)n
Q ~ S~X Ia
L
10 where L i8 hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
Cl-C4-alkoxy, Cl-C4-alkylthio, Cl-C4-haloalkyl, Cl-C4-haloalkoxy,
Cl-C4-haloalkylthio, Cl-C4-alkylsulfonyl, halogen, nitro or cyano
and H is hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
Cl-C4-alkoxy, Cl-C4-alkylthio, Cl-C4-haloalkyl, Cl-C4-haloalkoxy,
15 Cl-C4-haloalkoxy, Cl-C4-haloalkylthio, Cl-C4-alkylsulfonyl,
halogen, nitro or cyano and Q, X, n and Y have the meanings given
in claim 1, where in the case where Y = C=0, X is not equal to
NR23.
20 Furthermore preferred benzoyl derivatives are those of the
formula Ib,
0 M (~)n
Q ~ 'X Ib
L
where L is Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, Cl-C4-alkoxy,
Cl-C4-haloalkyl, Cl-C4-haloalkoxy, halogen, nitro or cyano and M
30 is hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
Cl-C4-alkoxy, Cl-C4-haloalkyl, Cl-C4-haloalkoxy, halogen, nitro or
cyano and Q, X, n and Y have the meanings given in claim 1, where
in the case where Y = C=0, X is not equal to NR23.
35 Preferred benzoyl derivatives are also those of the formula I as
claimed in claim 1, where the radicals L and M are hydrogen,
methyl, methoxy, chlorine, cyano, nitro or trifluoromethyl.
Preferred benzoyl derivatives are those of the formula Ic,
o M (~)n
21 Ic
CA 02227934 l998-02-23
0050/46142
19
where L is hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
C1-C4-a:Lkoxy, Cl-C4-haloalkyl, Cl-C4-haloalkoxy, halogen, nitro or
cyano and M i~ hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
Cl-C4-a:Lkoxy, Cl-C4-haloalkyl, Cl-C4-haloalkoxy, halogen, nitro or
5 cyano and Q, n, Y and also R22, R21, R12 and Rl3 have the -Ani~gs
given in claim 1.
Likewise preferred benzoyl derivatives are those of the
formula Id,
,10
o n (11 ) R12 Id
L
where L is hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
C1-C4-alkoxy, C1-C4-haloalkyl, Cl-C4-halo~lkoxy, halogen, nitro or
cyano and M i~ hydrogen, C1-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
20 C1-C4-a:Lkoxy, C1-C4-haloalkyl, C1-C4-haloAlkoxy, halogen, nitro or
cyano and Q, n, Y and also R12 and R13 have the meanings given in
claim l.
Also preferred benzoyl derivatives are tho~e of the formula Ie,
0 M (~)n
~ 1I R12 Ie
L R13
where L is hydrogen, C1-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
Cl-C4-alkoxy, Cl-C4-haloalkyl, Cl-C4-haloalkoxy, halogen, nitro or
cyano and M is hydrogen, Cl-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
35 Cl-C4-alkoxy, Cl-C4-haloalkyl, Cl-C4-haloAl~o~y, halogen, nitro or
cyano and Q, n, Y and also Rl2 and Rl3 have the meanings given in
claim l.
Preferred benzoyl derivatives are likewise those of the formula I
40 as claimed in claim l, where n is l or 2 and Y is CR7-oR8, where
R7 and R8 have the meanings given in claim l.
~5
CA 02227934 1998-02-23
0050~46142
Table l; Compounds of the formula
OH O M ~~)n
Rl 7 ~yl>< RRl 3
Rl9 L
No. R17 R18 Rl9 n R12 R13 Y L M
1.1 H H H 0 H H C=O H H
1.2 H H H 2 H H C=O H H
1 3 H H H 0 H H CHOCH3 H H
1.4 M H H 2 H H CHOCH3 H H
1.5 H H H 0 H H CHOC2Hs H H
1.6 H H H 2 H H CHOC2Hs H H
1.7 H H H 0 H H CHOiPr H H
20 1.8 H H H 2 H H CHOiPr H H
1.9 H H H 0 H H CHOH H H
1.10 H H H 2 H H CHOH H H
1.11 H H H 0 H H C=NOCH3 H H
1 12 H H H 2 H H C=NOCH3 H H
1 13 H H H 0 H H c=Noc2Hs H H
1.14 H H H 2 H H c=Noc2Hs H H
1.15 H H H 0 H H C=NOiPr H H
1.16 H H H 2 H H C=NOiPr H H
1.17 H H H 0 H H C=NOCH2CH=CHCI H H
1.18 H H H 2 H H C=NOCH2CH=CHCI H H
1.19 H H H 0 H H c=NocH2c6Hs H H
1.20 H H H 2 H H C=NOCH2C6Hs H H
1.21 H H H 0 H H C(CH3)2 H H
1.22 11 H H 2 H H C(CH3)2 H H
1.23 ('H3 H H 0 H H C=O H H
1.24 ('H3 H H 2 H H C=O H H
1.25 ('H3 H H 0 H H CHOCH3 H H
1.26 ('H3 H H 2 H H CHOCH3 H H
1.27 CH3 H H 0 H H CHOC2Hs H H
1.28 l'H3 H H 2 H H CHOC2Hs H H
1.29 CH3 H H 0 H H CHOiPr H H
1.30 CH3 H H 2 H H CHOiPr H H
1.31 CH3 H H 0 H H CHOH H H
1.32 CH3 H H 2 H H CHOH H H
CA 02227934 1998-02-23
0050/461g2
No. Rl7 R18 R19 n R12 R13 Y L M
1.33 CH3 H H 0 H H C=NOCH3 H H
1.34 CH3 H H 2 H H C=NOCH3 H H
1.35 CH3 H H 0 H H C=NOC2Hs ~ H H
1.36 CH3 H H 2 H H C=NOC2Hs H H
1.37 CH3 H H 0 H H C=NOiPr H H
1.38 CH3 H H 2 H H C=NOiPr H H
1.39 CH3 H H 0 H H C=NOCH2CH=CHCI H H
1.40 CH3 H H 2 H H C=NOCH2CH=CHCI H H
1.41 CH3 H H 0 H H C=NOCH2C6Hs H H
1.42 CH3 H H 2 H H C=NOCH2C6Hs H H
1.43 CH3 H H 0 H H C(CH3)2 H H
1.44 CH3 H H 2 H H C(CH3)2 H H
1.45 CH3 CH3 H 0 H H C=O H H
1.46 CH3 CH3 H 2 H H C=O H H
1.47 CH3 CH3 H 0 H H CHOCH3 H H
1.48 CH3 CH3 H 2 H H CHOCH3 H H
1.49 CH3 CH3 H 0 H H CHOC2Hs H H
1.50 CH3 CH3 H 2 H H CHOC2Hs H H
1.51 CH3 CH3 H 0 H H CHOiPr H H
1.52 CH3 CH3 H 2 H H CHOiPr H H
1.53 CH3 CH3 H 0 H H C=NOCH3 H H
1.54 CH3 CH3 H 2 H H C=NOCH3 H H
1.55 CH3 CH3 H 0 H H C=NOC2Hs H H
1.56 CH3 CH3 H 2 H H C=NOC2Hs H H
1.57 CH3 CH3 H 0 H H C=NOiPr H H
1.58 CH3 CH3 H 2 H H C=NOiPr H H
1.59 CH3 CH3 H 0 H H C=NOCH2CH=CHCI H H
1.60 CH3 CH3 H 2 H H C=NOCH2CH=CHCI H H
1.61 CH3 CH3 H 0 H H C=NOCH2C6Hs H H
1.62 CH3 CH3 H 2 H H C=NOCH2C6Hs H H
1.63 CH3 CH3 H 0 H H C(CH3)2 H H
1.64 CH3 CH3 H 2 H H C(CH3)2 H H
1.65 H H H 0 H H C=O H CH3
1.66 H H H 2 H H C=O H CH3
1.67 H H H 0 H H CHOCH3 H CH3
1.68 H H H 2 H H CHOCH3 H CH3
1.69 H H H 0 H H CHOC2Hs H CH3
1.70 Hl H H 2 H H CHOC2Hs H CH3
1.71 H H H 0 H H CHOiPr H CH3
1.72 Hl H H 2 H H CHOiPr H CH3
1.73 H H H 0 H H C=NOCH3 H CH3
CA 02227934 1998 - 02 - 23
0050/~6142
No. R17 R18 Rl9 n Rl2 ~13 y L M
1.74 H H H 2 H H C=NOCH3 H CH3
1.75 H H H 0 H H C=NOC2Hs H CH3
1.76 H H H 2 H H C=NOC2Hs H CH3
1.77 H H H 0 H H C=NOiPr H CH3
1.78 H H H 2 H H C=NOiPr H CH3
1.79 H H H 0 H H C=NOCH2CH=CHCI H CH3
1.80 H H H 2 H H C=NOCH2CH=CHCI H CH3
1.81 H H H 0 H H C=NOCH2C6Hs H CH3
1.82 H H H 2 H H C=NOCH2C6Hs H CH3
1.83 H H H 0 H H C(CH3)2 H CH3
1.84 ~ H H 2 H H C(CH3~2 H CH3
1.85 CH3 H H 0 H H C=O H CH3
1.86 CH3 H H 2 H H C=O H CH3
1.87 CH3 H H 0 H H CHOCH3 H CH3
1.88 CH3 H H 2 H H CHOCH3 H CH3
1.89 CH3 H H 0 H H CHOC2Hs H CH3
1.90 CH3 H H 2 H H CHOC2Hs H CH3
1.91 CH3 H H 0 H H CHOiPr H CH3
1.92 CH3 H H 2 H H CHOiPr H CH3
1.93 CH3 H H 0 H H C=NOCH3 H CH3
1.94 CH3 H H 2 H H C=NOCH3 H CH3
1.95 CH3 H H 0 H H C=NOC2Hs H CH3
1.96 CH3 H H 2 H H C=NOC2Hs H CH3
1.97 CH3 H H 0 H H C=NOiPr H CH3
1.98 CH3 H H 2 H H C=NOiPr H CH3
1.99 CH3 H H 0 H H C=NOCH2CH=CHCI H CH3
1.100 CH3 H H 2 H H C=NOCH2CH=CHCI H CH3
1.101 CH3 H H 0 H H C=NOCH2C6Hs H CH3
1.102 CH3 H H 2 H H C=NOCH2C6H5 H CH3
1.103 CH3 H H 0 H H C(CH3~2 H CH3
1.104 CH3 H H 2 H H C(CH3)2 H CH3
1.105 CH3 CH3 H 0 H H C=O H CH3
1.106 CH3 CH3 H 2 H H C=O H CH3
1.107 CH3 CH3 H 0 H H CHOCH3 H CH3
1.108 CH3 CH3 H 2 H H CHOCH3 H CH3
1.109 CH3 CH3 H 0 H H CHOC2Hs H CH3
1.110 CH3 CH3 H 2 H H CHOC2Hs H CH3
1.111 CH3 CH3 H 0 H H CHOiPr H CH3
1.112 CH3 CH3 H 2 H H CHOiPr H CH3
1.113 CH3 CH3 H 0 H H CHOH H CH3
1.114 ICH3 CH3 H 2 H H CHOH H CH3
CA 02227934 1998 - 02 - 23
0050/46142
No. Rl7 Rl8 Rl9 n Rl2 Rl3 Y L M
1.115 CH3 CH3 H 0 H H C=NOCH3 H CH3
1.116 CH3 CH3 H 2 H H C=NOCH3 H CH3
1.117 CH3 CH3 H 0 H H C=NOC2Hs H CH3
1.118 CH3 CH3 H 2 H H C=NOC2Hs H CH3
1.119 CH3 CH3 H 0 H H C=NOiPr H CH3
1.120 CH3 CH3 H 2 H H C=NOiPr H CH3
1.121 CH3 CH3 H 0 H H C=NOCH2CH=CHCI H CH3
1.122 CH3 CH3 H 2 H H C=NOCH2CH=CHCI H CH3
1.123 C:H3 CH3 H 0 H H C=NOCH2C6Hs H CH3
1.124 CH3 CH3 H 2 H H C=NOCH2C6Hs H CH3
1.125 CH3 CH3 H 0 H H C(CH3)2 H CH3
1.126 CH3 CH3 H 2 H H C(CH3)2 H CH3
1.127 H H H 0 H H C=O H Cl
1.128 H H H 2 H H C=O H Cl
1.129 H H H 0 H H CHOCH3 H Cl
1.130 H H H 2 H H CHOCH3 H Cl
1.131 H H H 0 H H CHOC2Hs H Cl
1.132 H H H 2 H H CHOC2Hs H Cl
1.133 H H H 0 H H CHOiPr H Cl
1.134 H H H 2 H H CHOiPr H a
2 1.135 H H H 0 H H C=NOCH3 H Cl
1.136 H H H 2 H H C=NOCH3 H Cl
1.137 H H H 0 H H C=NOC2Hs H Cl
1.138 H H H 2 H H C=NOC2Hs H Cl
1.139 H H H 0 H H C=NOiPr H Cl
1.140 H H H 2 H H C=NOiPr H Cl
1.141 H H H 0 H H C=NOCH2CH=CHCI H Cl
1.142 H H H 2 H H C=NOCH2CH=CHCI H Cl
1.143 H H H 0 H H C=NOCH2C6Hs H Cl
1.144 H H H 2 H H C=NOCH2C6Hs H Cl
1.145 H H H 0 H H C(CH3)2 H Cl
1.146 H H H 2 H H C(cH3)2 H Cl
1.147 CH3 H H 0 H H C=O H Cl
1.148 CH3 H H 2 H H C=O H Cl
1.149 CH3 H H 0 H H CHOCH3 H Cl
1.150 CH3 H H 2 H H CHOCH3 H Cl
1.151 CH3 H H 0 H H CHOC2Hs H Cl
1.152 CH3 H H 2 H H CHOC2Hs H Cl
1.153 CH3 H H 0 H H CHOiPr H Cl
1.154 ClH3 H H 2 H H CHOiPr H Cl
1.155 CH3 H H 0 H H C=NOCH3 H Cl
0050/46142 CA 02227934 1998-02-23
24
No Rl7 Rl8 Rl9 n Rl2 Rl3 Y L M
1.156 CH3 H H 2 H H C=NOCH3 H Cl1.157 CH3 H H 0 H H c=Noc2Hs H Cl1.158 CH3 H H 2 H H c=Noc2Hs H Cl1.159 CH3 H H 0 H H C=NOiPr H Cl1.160 CH3 H H 2 H H C=NOiPr H Cl1.161 CH3 H H 0 H H C=NOCH2CH=CHCI H Cl1.162 CH3 H H 2 H H C=NOCH2CH=CHCI H Cl1.163 CH3 H H 0 H H c=NocH2c6Hs H Cl1.164 CH3 H H 2 H H c=NocH2c6Hs H Cl1.165 OEl3 H H 0 H H C(CH3)2 H Cl1.166 CH3 H H 2 H H C(CH3)2 H Cl~L 1.167 CH3 CH3 H 0 H H C=O H Cl
1.168 CH3 CH3 H 2 H H C=O H Cl1.169 CH3 CH3 H 0 H H CHOCH3 H Cl1.170 CH3 CH3 H 2 H H CHOCH3 H Cl1.171 CH3 CH3 H 0 H H CHOC2Hs H Cl;!0 1.172 CH3 CH3 H 2 H H CHOC2Hs H Cl
1.173 CH3 CH3 H 0 H H CHOiPr H Cl1.174 C}13 CH3 H 2 H H CHOiPr H Cl1.175 CH3 CH3 H 0 H H C=NOCH3 H Cl1.176 CH3 CH3 H 2 H H C=NOCH3 H Cl1.177 CH3 CH3 H 0 H H c=Noc2Hs H Cl1.178 CH3 CH3 H 2 H H C=NOC2Hs H Cl1.179 CH3 CH3 H 0 H H C=NOiPr H Cl1.180 CH3 CH3 H 2 H H C=NOiPr H Cl.lo 1.181 CH3 CH3 H 0 H H C=NOCH2CH=CHCI H Cl
1.182 CH3 CH3 H 2 H H C=NOCH2CH=CHCI H Cl1.183 CH3 CH3 H 0 H H c=NocH2c6Hs H Cl1.184 CH3 CH3 H 2 H H C=NOCH2C6Hs H Cl1.185 CH3 CH3 H 0 H H C(CH3k H Cl1.186 CH3 CH3 H 2 H H C(CH3)2 H Cl
~0
~5
CA 02227934 1998-02-23
0050/46142
Table 2: Compounds of the formula
OH O M (~)n
R17~ R13
Rl9 L
The radicals Rl2 of X and R7 of Y form a bond
No. Rl7 Rl8 Rl9 n R13 y L M
2.1 H H H 0 H COCH3 H H
15 2.2 H H H 2 H COCH3 H H
2.3 H H H 0 H COC2Hs H H
2.4 H H H 2 H COC2Hs H H
2.5 H H H 0 H COiPr H H
20 2.6 H H H 2 H COiPr H H
2.7 CH3 H H 0 H COCH3 H H
2.8 CH3 H H 2 H COCH3 H H
2.9 CH3 H H 0 H COC2H5 H H
2.10 CH3 H H 2 H COC2H5 H H
25 2.11 CH3 H H 0 H COiPr H H
2.12 CH3 H H 2 H COiPr H H
2.13 CH3 CH3 H 0 H COCH3 H H
2.14 CH3 CH3 H 2 H COCH3 H H
30 2.15 CH3 CH3 H 0 H COC2Hs H H
2.16 CH3 CH3 H 2 H COC2Hs H H
2.17 CH3 CH3 H 0 H COiPr H H
2.18 CH3 CH3 H 2 H COiPr H H
2.19 H H H 0 H COCH3 . H CH3
35 2.20 H H H 2 H COCH3 H CH3
2.21 H H H 0 H COC2Hs H CH3
2.22 H H H 2 H COC2Hs H CH3
2.23 H H H 0 H COiPr H CH3
40 2.24 H H H 2 H COiPr H CH3
2.25 CH3 H H 0 H COCH3 H CH3
2.26 CH3 H H 2 H COCH3 H CH3
2.27 CH3 H H 0 H COC2Hs H CH3
2.28 CH3 H H 2 H COC2Hs H CH3
45 2.29 CH3 H H 0 H COiPr H CH3
2.30 CH3 H H 2 H COiPr H CH3
CA 02227934 1998-02-23
0050/46142
No. Rl 7 Rls Rl9 rl Rl3 y L M
2.31 CE~3 CH3 H 0 H COCH3 H CH3
2.32 CH3 CH3 H 2 H COCH3 H CH3
. 2.33 C~3 CH3 H 0 H COC2Hs ~ H CH3
2.34 CE~3 CH3 H 2 H COC2Hs H CH3
2.35 C~3 CH3 H 0 H C OiPr H CH3
2.36 CH3 CH3 H 2 H C OiPr H CH3
2.37 H H H 0 H COCH3 H Cl
IO 2.38 H H H 2 H COCH3 H Cl
2.39 H H H 0 H COC2Hs H Cl
2.40 H H H 2 H COC2Hs H Cl
2.41 H H H 0 H C OiPr H Cl
2.42 H H H 2 H COiPr H Cl
2.43 CH3 H H 0 H COCH3 H Cl
2.44 CH3 H H 2 H COCH3 H Cl
2.45 CH3 H H 0 H COC2Hs H Cl
2.46 CH3 H H 2 H COC2Hs H Cl
2.47 C,H3 H H 0 H C OiPr H C1
2.48 CH3 H H 2 H C OiPr H Cl
2.49 ~,H3 CH3 H 0 H COCH3 H Cl
2.50 C'H3 CH3 H 2 H COCH3 H Cl
2.51 (~H3 CH3 H 0 H COC2Hs H Cl
2.52 CH3 CH3 H 2 H COC2Hs H Cl
2.53 (',H3 CH3 H 0 H C OiPr H Cl
2.54 CH3 CH3 H 2 H C OiPr H Cl
CA 02227934 1998-02-23
0050/46142
Table 3: Compounds of the formula
OH O M (~)n
Rl 7 ~S~< Rl 2
Rl9
No. R17 R18 R19 n R12 R13 y L M
3.1 H H H 0 H H C=O CH3 H
3.2 H H H 2 H H C=O CH3 H
3.3 H H H 0 H H CHOCH3 CH3 H
3.4 H H H 2 H H CHOCH3 CH3 H
3.5 H H H 0 H H CHOC2Hs CH3 H
3.6 H H H 2 H H CHOC2Hs CH3 H
3.7 H H H 0 H H CHOiPr CH3 H
3.8 H H H 2 H H CHOiPr CH3 H
3.9 H H H 0 H H C=NOCH3 CH3 H
3.10 H H H 2 H H C=NOCH3 CH3 H
3.11 H: H H 0 H H C=NOC2Hs CH3 H
3.12 H: H H 2 H H C=NOC2Hs CH3 H
3.13 H: H H 0 H H C=NOiPr CH3 H
3.14 H H H 2 H H C=NOiPr CH3 H
3.15 H H H 0 H H C=NOCH2CH=CHCI CH3 H
3.16 ~ H H 2 H H C=NOCH2CH=CHCI CH3 H
3.17 Hl H H 0 H H C=NOCH2C6Hs CH3 H
3.18 H H H 2 H H C=NOCH2C6Hs CH3 H
3.19 H[ H H 0 H H C(CH3)2 CH3 H
3.20 H[ H H 2 H H C(CH3)~ CH3 H
3.21 C'H3 H H 0 H H C=O CH3 H
3 5 3.22 CH3 H H 2 H H C=O CH3 H
3.23 CH3 H H 0 H H CHOCH3 CH3 H
3.24 C'H3 H H 2 H H CHOCH3 CH3 H
3.25 C'H3 H H 0 H H CHOC2Hs CH3 H
3.26 C'H3 H H 2 H H CHOCzHs CH3 H
3.27 CH3 H H 0 H H CHOiPr CH3 H
3.28 CH3 H H 2 H H CHOiPr CH3 H
3.29 C'H3 H H 0 H H C=NOCH3 CH3 H
3.30 C'H3 H H 2 H H C=NOCH3 CH3 H
3.31 C'H3 H H 0 H H C=NOC2Hs CH3 H
3.32 C'H3 H H 2 H H C=NOC2Hs CH3 H
3.33 ('H3 H H 0 H H C=NOiPr CH3 H
CA 02227934 l998-02-23
0050/4~142
28
No. Rl7 Rl8 R19 n R12 R13 y L M
3.34 CH3 H H 2 H H C=NOiPr CH3 H
3.35 CH3 H H 0 H H C=NOCH2CH=CHCI CH3 H
3.36 CH3 H H 2 H H C=NOCH2CH=CHCI CH3 H
3.37 CH3 H H 0 H H C=NOCH2C6Hs CH3 H
3.38 CH3 H H 2 H H C=NOCH2C6Hs CH3 H
3.39 C:H3 H H 0 H H C(CH3)2 CH3 H
3.40 CH3 H H 2 H H C(CH3)2 CH3 H
3.41 CH3 CH3 H 0 H H C=O CH3 H
3.42 CH3 CH3 H 2 H H C=O CH3 H
3.43 CH3 CH3 H 0 H H CHOCH3 CH3 H
3.44 CH3 CH3 H 2 H H CHOCH3 CH3 H
3.45 CH3 CH3 H 0 H H CHOC2Hs CH3 H
3.46 CH3 CH3 H 2 H H CHOC2Hs CH3 H
3.47 CH3 CH3 H 0 H H CHOiPr CH3 H
3.48 CH3 CH3 H 2 H H CHOiPr CH3 H
3.49 CH3 CH3 H 0 H H C=NOCH3 CH3 H
3.50 CH3 CH3 H 2 H H C=NOCH3 CH3 H
3.51 CH3 CH3 H 0 H H C=NOC2Hs CH3 H
3.52 CH3 CH3 H 2 H H C=NOC2Hs CH3 H
3.53 CH3 CH3 H 0 H H C=NOiPr CH3 H
3.54 CH3 CH3 H 2 H H C=NOiPr CH3 H
3.55 CH3 CH3 H 0 H H C=NOCH2CH=CHCI CH3 H
3.56 CH3 CH3 H 2 H H C=NOCH2CH=CHCI CH3 H
3.57 CH3 CH3 H 0 H H C=NOCH2C6Hs CH3 H
3.58 CH3 CH3 H 2 H H C=NOCH2C6Hs CH3 H
3.59 CH3 CH3 H 0 H H C(CH3)2 CH3 H
3.60 CH3 CH3 H 2 H H C(CH3)2 CH3 H
3.61 H H H 0 H H C=O Cl H
3.62 H H H 2 H H C=O Cl H
3.63 H H H 0 H H CHOCH3 Cl H
3.64 H H H 2 H H CHOCH3 Cl H
3.65 H H H 0 H H CHOC2Hs Cl H
3.66 H H H 2 H H CHOC2Hs Cl H
3.67 H H H 0 H H CHOiPr Cl H
40 3.68 H H H 2 H H CHOiPr Cl H
3.69 H H H 0 H H C=NOCH3 Cl H
3.70 H H H 2 H H C=NOCH3 Cl H
3.71 Hl H H 0 H H C=NOC2Hs Cl H
3.72 H H H 2 H H C=NOC2Hs Cl H
45 3.73 H H H 0 H H C=NOiPr Cl H
3.74 H H H 2 H H C=NOiPr Cl H
OOSOt46142 CA 02227934 1998-02-23
No. Rl7 Rl8 Rl9 n Rl2 Rl3 y L M
3.75 H H H 0 H H C=NOCH2CH=CHCI Cl H
3.76 H H H 2 H H C=NOCH2CH=CHCI Cl H
3.77 H H H 0 H H C=NOCH2C6Hs Cl H
3.78 H H H 2 H H C=NOCH2C6Hs Cl H
3.79 H H H 0 H H C(CH3)2 Cl H
3.80 H H H 2 H H C(CH3)2 Cl H
3.81 CH3 H H 0 H H C=O Cl H
l0 3.82 CH3 H H 2 H H C=O Cl H
3.83 CH3 H H 0 H H CHOCH3 Cl H
3.84 CH3 H H 2 H H CHOCH3 Cl H
3.85 CH3 H H 0 H H CHOC2Hs Cl H
1 5 3.86 CH3 H H 2 H H CHOC2Hs Cl H
3.87 CH:3 H H 0 H H CHOiPr Cl H
3.88 CH3 H H 2 H H CHOiPr Cl H
3.89 CH3 H H 0 H H C=NOCH3 Cl H
3.90 CH3 H H 2 H El C=NOCH3 Cl H
a'o 3.91 CH3 H H 0 H H C=NOC2H5 Cl H
3.92 CE~3 H H 2 H H C=NOC2Hs Cl H
3.93 CE~3 H H 0 H H C=NOiPr Cl H
3.94 CH3 H H 2 H H C=NOiPr Cl H
3.95 CH3 H H 0 H H C=NOCH2CH=CHCI Cl H
~'5 3.96 CEI3 H H 2 H H C=NOCH2CH=CHCI Cl H
3.97 CH3 H H 0 H H C=NOCH2C6Hs Cl H
3.98 CE[3 H H 2 H H C=NOCH2C6Hs Cl H
3.99 CEI3 H H 0 H H C(CH3)2 Cl H
3.100 CEI3 H H 2 H H C(CH3)2 Cl H
3.101 CEI3 CH3 H 0 H H C=O Cl H
3.102 CEI3 CH3 H 2 H H C=O Cl H
3.103 CEI3 CH3 H 0 H H CHOCH3 Cl H
3.104 CE13 CH3 H 2 H H CHOCH3 Cl H
3.105 CE~3 CH3 H 0 H H CHOC2Hs Cl H
3.106 CE~3 CH3 H 2 H H CHOC2Hs Cl H
3.107 CEI3 CH3 H 0 H H CHOiPr Cl H
3.108 CH3 CH3 H 2 H H CHOiPr Cl H
~o 3.109 CH3 CH3 H 0 H H C=N-OCH3 Cl H
3.110 CH3 CH3 H 2 H H C=NOCH3 Cl H
3.111 CH3 CH3 H 0 H H c=Noc2Hs Cl H
3.112 CH3 CH3 H 2 H H c=Noc2Hs Cl H
3.113 CH3 CH3 H 0 H H C=NOiPr Cl H
3.114 CH3 CH3 H 2 H H C=NOiPr Cl H
3.115 CH3 CH3 H 0 H H C=NOCH2CH=CHCI Cl H
CA 02227934 1998 - 02 - 23
0050/46142
No. R17 R18 Rl9 n R12 R13 y L M
3.116 CH3 CH3 H 2 H H C=NOCH2CH=CHCI Cl H
3.117 CH3 CH3 H 0 H H C=NOCH2C6Hs Cl H
3.118 CH3 CH3 H 2 H H C=NOCH2C6Hs Cl H
3.119 CH3 CH3 H 0 H H C(CH3)2 Cl H
3.120 CH3 CH3 H 2 H H C(CH3)2 Cl H
3.121 H H H 0 H CH3 C=O CH3 H
3.122 H H H 2 H CH3 C=O CH3 H
3.123 H H H 0 H CH3 CHOCH3 CH3 H
3.124 H H H 2 H CH3 CHOCH3 CH3 H
3.125 ~I H H 0 H CH3 CHOC2Hs CH3 H
3.126 H H H 2 H CH3 CHOC2Hs CH3 H
3.127 H H H 0 H CH3 CHOiPr CH3 H
3.128 H H H 2 H CH3 CHOiPr CH3 H
3.129 H H H 0 H CH3 C=NOCH3 CH3 H
3.130 H H H 2 H CH3 C=NOCH3 CH3 H
3.131 H H H 0 H CH3 C=NOC2Hs CH3 H
3.132 H H H 2 H CH3 C=NOC2Hs CH3 H
3.133 H H H 0 H CH3 C=NOiPr CH3 H
3.134 H H H 2 H CH3 C=NOiPr CH3 H
3.135 H H H 0 H CH3 C=NOCH2CH=CHCI CH3 H
3.136 H H H 2 H CH3 C=NOCH2CH=CHCI CH3 H
3.137 H H H 0 H CH3 c=NocH2c6Hs CH3 H
3.138 H H H 2 H CH3 c=NocH2c6Hs CH3 H
3.139 H H H 0 H CH3 C(CH3)2 CH3 H
3.140 H H H 2 H CH3 C(CH3)2 CH3 H
3.141 CH3 H H 0 H CH3 C=O CH3 H
3.142 CH3 H H 2 H CH3 C=O CH3 H
3.143 CH3 H H 0 H CH3 CHOCH3 CH3 H
3.144 CH3 H H 2 H CH3 CHOCH3 CH3 H
3.145 CH3 H H 0 H CH3 CHoc2Hs CH3 H
3.146 CH3 H H 2 H CH3 CHOC2Hs CH3 H
3.147 CH3 H H 0 H CH3 CHOiPr CH3 H
3.148 C'H3 H H 2 H CH3 CHOiPr CH3 H
3.149 CH3 H H 0 H CH3 C=NOCH3 CH3 H
3.150 CH3 H H 2 H CH3 C=NOCH3 CH3 H
3.151 CH3 H H 0 H CH3 C=NOC2Hs CH3 H
3.152 CH3 H H 2 H CH3 C=NOC2Hs CH3 H
3.153 CH3 H H 0 H CH3 C=NOiPr CH3 H
3.154 CH3 H H 2 H CH3 C=NOiPr CH3 H
3.155 CH3 H H 0 H CH3 C=NOCH2CH=CHCI CH3 H
3.156 CH3 H H 2 H CH3 C=NOCH2CH=CHCI CH3 H
CA 02227934 l998-02-23
0050/4~142
No. R:17 Rl8 Rl9 n Rl2 Rl3 y L M
3.157 CH3 H H 0 H CH3 C=NOCH2C6Hs CH3 H
3.158 CH3 H H 2 H CH3 C=NOCH2C6Hs CH3 H
3.159 CH3 H H 0 H CH3 C(CH3)2 (:H3 H
3.160 CH3 H H 2 H CH3 C(cH3)2 CH3 H
3.161 CH3 CH3 H 0 H CH3 C=O CH3 H
3.162 CH3 CH3 H 2 H CH3 C=O CH3 H
3.163 CH3 CH3 H 0 H CH3 CHOCH3 CH3 H
3.164 CH3 CH3 H 2 H CH3 CHOCH3 CH3 H
3.165 CH3 CH3 H 0 H CH3 CHOC2Hs CH3 H
3.166 CH3 CH3 H 2 H CH3 CHOC2Hs CH3 H
3.167 CH3 CH3 H 0 H CH3 CHOiPr CH3 H
3.168 CH3 CH3 H 2 H CH3 CHOiPr CH3 H
3.169 CH3 CH3 H 0 H CH3 C=NOCH3 CH3 H
3.170 CH3 CH3 H 2 H CH3 C=NOCH3 CH3 H
3.171 CH3 CH3 H 0 H CH3 C=NOC2Hs CH3 H
3.172 ClH3 CH3 H 2 H CH3 C=NOC2Hs CH3 H
3.173 CH3 CH3 H 0 H CH3 C=NOiPr CH3 H
3.174 CH3 CH3 H 2 H CH3 C=NOiPr CH3 H
3.175 CH3 CH3 H 0 H CH3 C=NOCH2CH=CHCI CH3 H
3.176 CH3 CH3 H 2 H CH3 C=NOCH2CH=CHCI CH3 H
2 3.177 CH3 CH3 H 0 H CH3 C=NOCH2C6Hs CH3 H
3.178 CH3 CH3 H 2 H CH3 c=NocHzc6Hs CH3 H
3.179 CH3 CH3 H 0 H CH3 C(CH3)2 CH3 H
3.180 C}I3 CH3 H 2 H CH3 C(CH3)2 CH3 H
3.181 H H H 0 H CH3 C=O Cl H
3.182 H H H 2 H CH3 C=O Cl H
3.183 H H H 0 H CH3 CHOCH3 Cl H
3.184 H H H 2 H CH3 CHOCH3 Cl H
3.185 H H H 0 H CH3 CHOC2Hs Cl H
3.186 H H H 2 H CH3 CHOC2Hs Cl H
3.187 H H H 0 H CH3 CHOiPr Cl H
3.188 H H H 2 H CH3 CHOiPr Cl H
3.189 H H H 0 H CH3 C=NOCH3 Cl H
3.190 H H H 2 H CH3 C=NOCH3 Cl H
3.191 H H H 0 H CH3 C=NOC2Hs Cl H
3.192 H H H 2 H CH3 c=Noc2Hs Cl H
3.193 H H H 0 H CH3 C=NOiPr Cl H
3.194 H H H 2 H CH3 C=NOiPr Cl H
3.195 H H H 0 H CH3 C=NOCHzCH=CHCI Cl H
3.196 H H H 2 H CH3 C=NOCH2CH=CHCI Cl H
3.197 H H H 0 H CH3 C=NOCH2C6Hs Cl H
CA 02227934 1998-02-23
OOSOt46142
No. Rl7 R18 R19 n R12 Rl3 y L M
3.198 H H H 2 H CH3 C=NOCH2C6Hs Cl H
3.199 H H H 0 H CH3 C(CH3)2 Cl H
3.200 H H H 2 H CH3 C(CH3)2 Cl H
3.201 C:H3 H H 0 H CH3 C=O Cl H
3.202 CH3 H H 2 H CH3 C=O Cl H
3.203 CH3 H H 0 H CH3 CHOCH3 Cl H
3.204 CH3 H H 2 H CH3 CHOCH3 Cl H
3.205 CH3 H H 0 H CH3 CHOC2Hs Cl H
3.206 CH3 H H 2 H CH3 CHOC2Hs Cl H
3.207 CH3 H H 0 H CH3 CHOiPr Cl H
3.208 CH3 H H 2 H CH3 CHOiPr Cl H
3.209 CH3 H H 0 H CH3 C=NOCH3 Cl H
3.210 CH3 H H 2 H CH3 C=NOCH3 Cl H
3.211 CH3 H H 0 H CH3 C=NOC2Hs Cl H
3.212 CH3 H H 2 H CH3 C=NOC2Hs Cl H
3.213 CH3 H H 0 H CH3 C=NOiPr Cl H
3.214 CH3 H H 2 H CH3 C=NOiPr Cl H
3.215 CH3 H H 0 H CH3 C=NOCH2CH=CHCI Cl H
3.216 CH3 H H 2 H CH3 C=NOCH2CH=CHCI Cl H
3.217 CH3 H H 0 H CH3 C=NOCH2C6Hs Cl H
3.218 CH3 H H 2 H CH3 C=NOCH2C6Hs Cl H
3.219 CH3 H H 0 H CH3 C(CH3)2 Cl H
3.220 C'H3 H H 2 H CH3 C(CH3)2 Cl H
3.221 CH3 CH3 H 0 H CH3 C=O Cl H
3.222 C'H3 CH3 H 2 H CH3 C=O Cl H
3.223 C'H3 CH3 H 0 H CH3 CHOCH3 Cl H
3.224 C'H3 CH3 H 2 H CH3 CHOCH3 Cl H
3.225 C'H3 CH3 H 0 H CH3 CHOC2Hs Cl H
3.226 C'H3 CH3 H 2 H CH3 CHOC2Hs Cl H
3.227 C'H3 CH3 H 0 H CH3 CHOiPr Cl H
3 228 C'H3 CH3 H 2 H CH3 CHOiPr Cl H
3.229 C'H3 CH3 H 0 H CH3 C=NOCH3 Cl H
3.230 C'H3 CH3 H 2 H CH3 C=NOCH3 Cl H
3.231 CH3 CH3 H 0 H CH3 c=Noc2Hs Cl H
3-232 C'H3 CH3 H 2 H CH3 C=NOC2Hs Cl H
3.233 C'H3 CH3 H 0 H CH3 C=NOiPr Cl H
3.234 C'H3 CH3 H 2 H CH3 C=NOiPr Cl H
3.235 C'H3 CH3 H 0 H CH3 C=NOCH2CH=CHCI Cl H
3.236 C'H3 CH3 H 2 H CH3 C=NOCH2CH=CHCI Cl H
3 237 C~H3 CH3 H o H CH3 C=NOCH2C6Hs Cl H
3.238 C'H3 CH3 H 2 H CH3 C=NOCH2C6Hs Cl H
CA 02227934 1998 - 02 - 23
0050/46142
No. Rl 7 Rl8 Rl9 n R12 Rl3 y L M
3.239 CH3 CH3 H 0 H CH3 C(CH3)2 Cl H
3.240 CH3 CH3 H 2 H CH3 C(CH3)2 Cl H
3.241 H H H 0 H CH3 C=O CH3 CH3
3.242 H H H 2 H CH3 C=O CH3 CH3
3.243 H H H 0 H CH3 CHOCH3 CH3 CH3
3.244 H H H 2 H CH3 CHOCH3 CH3 CH3
3.24s H H H 0 H CH3 CHOC2Hs CH3 CH3
:lO 3.246 H H H 2 H CH3 CHOC2Hs CH3 CH3
3.247 H H H 0 H CH3 CHOiPr CH3 CH3
3.248 H H H 2 H CH3 CHOiPr CH3 CH3
3.249 H H . H 0 H CH3 COCH3 CH3 CH3
3.250 H H H 2 H CH3 COCH3 CH3 CH3
3.251 H H H 0 H CH3 COC2Hs CH3 CH3
3.252 H H H 2 H CH3 COC2Hs CH3 CH3
3.253 H H H 0 H CH3 C=NOCH3 CH3 CH3
3.2s4 H H H 2 H CH3 C=NOCH3 CH3 CH3
3.255 H H H 0 H CH3 C=NOC2Hs CH3 CH3
3.256 H H H 2 H CH3 C=NOC2Hs CH3 CH3
3.2s7 H H H 0 H CH3 C=NOiPr CH3 CH3
3.2s8 H H H 2 H CH3 C=NOiPr CH3 CH3
3.2s9 H H H 0 H CH3 C=NOCH2CH=CHCI CH3 CH3
3.260 H H H 2 H CH3 C=NOCH2CH=CHCI CH3 CH3
3.261 H H H 0 H CH3 C=NOCH2C6Hs CH3 CH3
3.262 H H H 2 H CH3 C=NOCH2C6Hs CH3 CH3
3.263 H H H 0 H CH3 C(CH3)2 CH3 CH3
3.264 H H H 2 H CH3 C(CH3)2 CH3 CH3
3.265 CH3 H H 0 H CH3 C=O CH3 CH3
3.266 CH3 H H 2 H CH3 C=O CH3 CH3
3.267 C:H3 H H 0 H CH3 CHOCH3 CH3 CH3
3.268 CH3 H H 2 H CH3 CHOCH3 CH3 CH3
3.269 CH3 H H 0 H CH3 CHOC2Hs CH3 CH3
3.270 CH3 H H 2 H CH3 CHOC2Hs CH3 CH3
3.271 CH3 H H 0 H CH3 CHOiPr CH3 CH3
3.272 CH3 H H 2 H CH3 CHOiPr CH3 CH3
3.273 CH3 H H 0 H CH3 C=NOCH3 CH3 CH3
3.274 CH3 H H 2 H CH3 C=NOCH3 CH3 CH3
3.27s CH3 H H 0 H CH3 C=NOC2Hs CH3 CH3
3.276 CH3 H H 2 H CH3 C=NOC2Hs CH3 CH3
3.277 CH3 H H 0 H CH3 C=NOiPr CH3 CH3
3.278 CH3 H H 2 H CH3 C=NOiPr CH3 CH3
3.279 CH3 H H 0 H CH3 C=NOCH2CH=CHCI CH3 CH3
CA 02227934 1998 - 02 - 23
0050/46142
No. Rl7 Rl8 Rl9 n Rl2 Rl3 y L M
3.280 CH3 H H 2 H CH3 C=NOCH2CH=CHCI CH3 CH3
3.281 CH3 H H 0 H CH3 C=NOCH2C6Hs CH3 CH3
3.282 CH3 H H 2 H CH3 C=NOCH~C6Hs CH3 CH3
3.283 CH3 H H 0 H CH3 C(CH3)2 CH3 CH3
3.284 CH3 H H 2 H CH3 C(CH3)2 CH3 CH3
3.285 CH3 CH3 H 0 H CH3 C=O CH3 CH3
3.286 CH3 CH3 H 2 H CH3 C=O CH3 CH3
3.287 CH3 CH3 H 0 H CH3 CHOCH3 CH3 CH3
3.288 CH3 CH3 H 2 H CH3 CHOCH3 CH3 CH3
3.289 CH3 CH3 H 0 H CH3 CHOC2Hs CH3 CH3
3.290 CH3 CH3 H 2 H CH3 CHOC2Hs CH3 CH3
3.291 CH3 CH3 H 0 H CH3 CHOiPr CH3 CH3
3.292 CH3 CH3 H 2 H CH3 CHOiPr CH3 CH3
3.293 CH3 CH3 H 0 H CH3 C=NOCH3 CH3 CH3
3.294 CH3 CH3 H 2 H CH3 C=NOCH3 CH3 CH3
3.295 CH3 CH3 H 0 H CH3 C=NOC2Hs CH3 CH3
3.296 CH3 CH3 H 2 H CH3 C=NOC2H5 CH3 CH3
3.297 CH3 CH3 H 0 H CH3 C=NOiPr CH3 CH3
3.298 CH3 CH3 H 2 H CH3 C=NOiPr CH3 CH3
3.299 CH3 CH3 H 0 H CH3 C=NOCH2CH=CHCI CH3 CH3
3.300 CH3 CH3 H 2 H CH3 C=NOCH2CH=CHCI CH3 CH3
3.301 CH3 CH3 H 0 H CH3 C=NOCH2C6Hs CH3 CH3
3.302 CH3 CH3 H 2 H CH3 C=NOCH2C6Hs CH3 CH3
3.303 CH3 CH3 H 0 H CH3 C(CH3)2 CH3 CH3
3.304 CH3 CH3 H 2 H CH3 C(CH3)2 CH3 CH3
~5
CA 02227934 1998-02-23
/46142
N P~ ;~ a a a ~ ~ ~ 8 8 ~ ~ ~ u ~ . ~
ô = ~
0=~0
~ K
~
c o ~ ~ o ~ ~ o - l ~ o - ~ ~ o ~ ~
o~
r~
CA 02227934 1998-02-23
0050/~L6142
~ N
~ ~, ~, z~ ~ ~ ~ ~ z 3 3 ~
C O ~ ~ O _I ~ O ~ ~ O ~ ~ O _~ ~ O _~ ~ O
O~
~ ~ X ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 2 ~ ~ ~ ~
CA 02227934 l998-02-23
0050/4614~
m m m m m m
U U U U U U
~ ~N ~ N ~N ~N ~N
m ~ :~s m m m m ~ ~ m
U U ~U ~ C~ ~ U U U ~
0 1 1 1 1 ~ Z
~ ~ U ~ U ~ ~ U ~ ~ U ~
~
~ c x :: :c x 3~ x ~
c ~ ~ o - l ~ o ~ ~ o ~ ~ o
~: x ~ x x :~: x :~ x x :~ x
- 4
p~ ~ $ ~: x x ~ ~ x x x
3: x x ~ :~: x :~: ~ x :~
CA 02227934 1998-02-23
0050/46142
I Z Z Z Z Z Z I
~
~; _I ~ o _ ~ o ~ ~ o _I ~ o _~ ~ o ~ ~ o ~ ~ o
o~
~ X :r: X ~ $ X
x
CA 02227934 1998-02-23
OOSO/46142
~3'
~, 3 3 ~ ~ ~ o o o ~ a ~ 3 ~ - 8 ~ ~ ~
c - ~ ~ o - ~ ~ o - ~ ~ o - ~ ~ o - l ~ o ~ ~ o ~ ~ o
o ~ 0 ~ ~ I~ I~ X~ X ~ X ;~
CA 02227934 1998-02-23
OOSO/46142
Z ~Z ~ Z ~
$ ~ ~ ~ ~ I ~ ~ ~ ~ ~ ~ ~ $
2 ~ X ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ X
C _~ ~ o ~ ~ o _ ~ o ~ ~ o ~ ~ o ~ ~ o _ ~
x
o~ o ~ ~ ~ ~ ~ ~ ~ x o~ 8 o o 8 ;~; o~ 'o~ S ~
CA 02227934 1998-02-23
0050/4614~
u u U m u u
u = u = u u~ U m u U u
U ~ ~ U ~ ~ U ~ ~ U ~ ~ U
X $ ~ ~ ~ 1X 3: X ::
~
C ~ X ~ X X ~ X
X X ~ X ~
~: o _ ~ O _ ~o _ ~ o _
~ X ~ 1 X X
00
~ 3: X ~ ~ ~ X ~:
g O _ ~ ~ ~ v~ x o~
CA 02227934 1998-02-23
0050/~6142
-
-
c: ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o - '
o~
CA 02227934 1998-02-23
0050/46142
3 $ ~ X :C ~:
. C C :C C C X X C ~ C :~ ~ C :~ ~
a~ ~ ~ O O O ~ ~ a a a a 3 a a a
:C X ~ C C 5: C C ~ ~ C ~ ~ ~ C ~ C $
$ ~ I ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ C
C ~ ~ ~ ~ o ~ ~ o _I ~ O ~ ~ O ~ ~ 0 ~4 ~ O _
CA 02227934 l998-02-23
OOSO/~6142
3 3 ~ 3
o~ o~ ~ o~ o~
c~
~ ~ o - ~ ~ o - ~ ~ o - ~ ~ o ~ ~ o ~ ~ o ~ ~ o - ~
o~
-
o ~ ~ ~ ~ 'o x
CA 02227934 1998-02-23
0050/46142
C ~ :~ 1 C
C ~ ~ C ~ ~ ~ ~ I
U O U U
U U U = U U U U U U U U
U ~ ~ U ~ ~ U ~ U ~
C ~ C :~
:C :~ C
:C ,C C ~ ~ :C C
c ~ a ~ ~ o _ ~ o ~ ~ o
-
~: C C ~ C C C C :C ~
~ x o~ o ~
o x ~ x oo x oo o'
CA 02227934 l998-02-23
0050/~6142
~ ~ ~ Z Z Z Z Z ~ ~ Z, ~ Z ~
~
_.
C -- ~ o ~ ~ o _~ ~ o _ ~ o _ ~ o _ ~ o ~ ~ o
o~
U ~ ~ U ~ U ~ V U ~ ~ ~ U
8 o o o o ~ o o o ~, ~ ~ _.
CA 02227934 1998-02-23
0050~6142
47
V~ ~~ ~
'' X ;~ $
Z ~ ~~ ~~~ ~~ 8 ~ ~
s r s ~ ~ ~ x s s c ~ s ~ ~ s s ~ s x s s
X :r: X :~ X X S ~ S X S ~
c ~ ~ o ~ o ~ o ~ ~ o ~ o 4 ~ o ~ ~ o
o~
~ X 1~ ~ ~ ~ ~ ~ S ~ ~ ~ S S
x ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
oc ~ 3 ~ ~ ~ S ~ s: c s s 3: s $
CA 02227934 l998-02-23
0050/46142
48
X~ X~ X~ X~ X~ ~ ~ X X ::
_1 X ~ X X X X ~ X $ ~ X X ~: X :~: X X X X
0~ 0 ~C ~0 0 0 ~
O O O O O ~ Z Z Z Z Z Z Z Z Z Z Z Z ' Z ~
P~ X X X X 3: X X :C X X X X :~ X X X X ~ X X
~: X 3: X X ~ :~: ~ X ~: X :~: ~ X X X X ~ X
X ~: :C X X X X X :~: X X . X X ~ X
X ~ X X ~ X X X X X X X X X X ~ X X :~ X X
~ o ~ ~ o ~ ~ o ~ ~ o ~ C~ o ~ ~ o --4 ~ o
o~
~: X X :~ X X ~ 3: X ~: ~ X X ;~: X X X :~ X X ~ X
x
:~; X 3~ C X X :~: X ~ X X ~ X X ~ X X
~: X X X X X :~ ~ X X X :~ :C X X :~: X X X ~ X X
~o ~ ~ ~ ~ ~r ~ ~ ~ ~ ~ v~
O
CA 02227934 1998-02-23
0050/46142
_1 ~ $ ~ ~ X
m m m m m m
U O U O
~ N ~ ~ ~ ~ ~N ~N ~
m m m m m m m m m m m m
~C~ UC,) C~ U C,~ t ~
-
~ ~r X ~ X
c - ~ o ~ ~ o - ~ ~ o - ~ ~
oo
Z ~ ~ ~ ~ ~i ~ ~r
CA 02227934 1998-02-23
0050/4bl42
Z Z Z ~ Z ~Z, Z Z, =, Z, = ~
3~ 3: ~ 33 ~ ~ ~ ~ ~ ~ 3~ ~ ~ $ 3333
3 ~ 3 ~ ~ ~ ~ ~ ~ ~ X ~ 3 ~ ~ 333
X ~ ~ ~ ~ 3 ~ ~ ~ ~ 3 ~ ~ ~ ~ ~ ~ ~ 3
C o .~ ~ o _~ ~ o ~ ~ o _~ ~ o _- ~ o _ ~ o -- ~
~ 3 ~ ~ 3 ~ ~ 33 ~ 3 ~ 3 ~ X ~ 33 ~ ~ 3 X
oo '
3: 3 ~ 333 ~ 33 ~ ~ ~ 3 ~ ~ 3 ~ 333
3: 3 ~ ~ 3 ~ 33 ~ 3 ~ ~ X ~ 33
o~ o~ ~ ~ ~ r ~ ~ ~ ~ ~ x~
~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~r ~ ~ ~r
CA 02227934 1998-02-23
0050/~6142
~ V) V~
O O O a a a 8 8 8 8 8
c o - ~ ~ o ~ ~ o ~'
o~
~ ~ 8 ", ~o~ o,, ~o~, ~ ~o ~o
CA 02227934 l998-02-23
0050/46142
~ g ~ O 0~ O O O ~
O O P O Z Z _ Z Z Z Z Z Z Z Z _ Z Z Z
R o ~ ~ o ~ ~ o ~ ~ o ~ ~ O ~ ~ o ~ ~ o ~ ~
Cl~
x
o _ ~ ~ ~ U~ ~ r~ oo o~ o ~ ~ ~ 1~ 0
CA 02227934 1998-02-23
0050/46142
r 2 2 2 2 2 ~ 2 2 2
u o o U
~tC ~ ~~ ~ ~ m~U ~ U ~
l u - ' u ~
2 ~ $ ~ $ 2 2 $ 2 $ 2
2 ~ 2 2 2 ~ 2 $ 2 2 2
$ ~ ~ 2 $
I ~ 2 2 $ 2 X ~ $ 2 $
~ o ~ ~ o _I ~ o ~ ~ o ~
~ 2 2 2 2 ~ ~ 2 ~ 2 $ ~
oo
2 ~ 2 2 2 2 $ 2 $ ~ $
O ~ ~ ~ ~ ~ r~
CA 02227934 1998-02-23
0050/~6142
~ U ~ Z Z Z Z Z Z Z Z Z Z = Z Z ~ ~ Z Z;
u ~3 b ~ ~ b ~
C ~ o ~ ~ o ~ ~ o ~ ~ o _ ~ o ~ ~ o ~4 ~ o
-
X O~ O ~ 0
CA 02227934 1998-02-23
OOSO/9~6142
~ ~ ~ X ~ $ ~ V ~
V) ~ V~
V
a ~ O O ~~~
~
~ ~ ~ ~ $ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ X ~ I
C: ~ o _ ~ o _~ ~ o ~ ~ o ~ ~ o _I ~ o ~ ~ o _-
X ~ ~ rl ~ ~q
$ ~ $ ~ V ~ ~
~ V V ~ V V V V ~ V~ V V V ~ V ~ ~ ~ V~
CA 02227934 1998-02-23
/~6142
~? ~? ~? ~? ~ rj? ~ ~? ~? ~? ~ ~? ~ ~ jj? ~ rj? ~? jj? ~ ~
T T Ti ~i T T i~ Ti T T ~ ~ r x ~ ~ T ~ iT T ~i
~ ~ ~? ~?
? ~ ~j? jj? ~_ ~ o ~ ~ ~ o o o o o o
O O O ~ O O ~ Z Z Z ' Z Z Z Z Z Z ~ Z Z Z
T X T T iT T ~ T T T i iT iT ~ T T iT iT T T T
iT ~ T $ X iT ~ T ~ T T ~ ~i ~ T X Xi ~ iT X ~
iT ~ ~ T Ti T Xi T T T T X S T Ti T ~ i iT T iT
X i T X X T T X Ti iT T Ii T X Ti i Ti T T ~ T
C ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o _-
-
I X T iT iT i T X iT T Ti T X X iT X ~ X X T
jrj? ~ T ~? jj ~? T ~ ~ ~ T ~ ? Ti ~ T
~ j? Ij? T ~j? ~ T ~ X ~ ~j? ~ Ij? ~ ~ ~ ~ iT ~ ~ ~j? ~j?
~: V V V V ~ V V V iv v v iv v v iv ~ v v
~ ~ ~ ~ ~ o ~ ~ ~ ~ ~ ~ ~ ~ ~ Q o ~
~ ~ X X CO oo oo o~ ~ ~ ~ ~
CA 02227934 1998-02-23
0050~9L6142
Iq r7 ~ ~ rq Iq
m ~ m :~
~ U U U O U
u m U ~ ~N ~N ~N
C U u u u u U C' U U U ~ U
-
C ~ O ~ ~ O _ ~ O ~ ~ O
O~
~ O
o ~o ~ o ~ o ~ ~
CA 02227934 1998-02-23
0050/46142
58
_l X '~ X :C :C X ~: X :~ X :~ X
~ X ~: X ~ :; 1 ~ X
,
r X ~ X X ~ ~ X ~ X ~X 3: ::
~
:~: x ~ ~: x ~ x ~x ~ ~ x ~:
~: x x ~ x :~: x ~ x ~
~ - ~ ~ o - ~ o - l ~ o ~ ~ o ~ ~ o - ~ ~ o ~ ~ o
~ X :~: X ~ X ~ X X ~ 1 ~ X ~ X ~
CA 02227934 1998-02-23
/46142
Z ~
z z ~ 0 0 0 a ~ ~ 8 ~ ~~ ~
~ o _ ~ o ~ ~ o _I ~ O --~ ~ O ~ ~ O ~ ~ O
~ o ~ $ ~o ~ ~ ~
CA 02227934 1998-02-23
0050/46142
~ ~ 5 i ) ~ i3 i3 ~i3 ~ i3 c~
_1 S, ,T! S~ ~ ~ $ ~ X ~
3 a 3 ~
T ~ ~ ~ ' ~ J ~
~ @i ~ ~ ~ ~ t ~ o o o ~ o o o o o o o o o
5 0 ~ O O 0 O O Z z z z z z z z z z 7 z
~i
C S. S. S~ C S. :C S. ~ S.
s. x :r: ~ X 3: :~ s. ~ ~ s.
_1
S. ~ S. ~ S. ~~ S. X ~: S. ~
~ ~ ~ o _~ ~ o _ ~ o _I ~ o _. ~ o _~ ~ o _ ~ o
o~
P~ S. S. ~ S. ~ S. ~~ S. ~:
x
~ S. ~ S. :C X :~ :C S. :C S. X S. S. ~ :: S. S. ~: S. ~ :1:
oo O~ O ~ ~ r~ ~ X C~ O _~ ~ ~ ~ u~ 'D ~ 00
CA 02227934 1998-02-23
0050/4~6142
, ~ $ 5
um um um ~ um
U ~ U um u U ~
gc~ ~l 11 11 1 1 11 11 1
U ~ ~ U ~ U ~ ~ U ~ ~ U
C _~ ~ o ~ ~ o ~ ~ o --1 ~
~' :~C 3: :I 3
~ ~
CA 02227934 1998-02-23
0050/46142
62
Z = Z ~ ~ Z Z
~
~ ~ X ~ 2 $ ~ ~ ~ ~ ~ X ~ ~ ~ ~ X ~ ~ ~ ~ ~
~: o _~ ~ o ~ ~ o ~ ~ o ~ ~ o _~ ~ o --~ ~ o
oo
o ~J ~' ~ ~ ~' ~ o~ ~, ~, ~ 8 ~,o, ,,o, ,,, o ,o~, o~ ~ o~ o~ _,
CA 02227934 1998-02-23
~ 0050/46142
~ e3 c3 c3 e3 ~3 e3 e3 ~3 o e3 U ~3 e3 e3 e3 e3 e3 e3 c~
~ c c c c c c ~c c x c c x c c c c ~c c c c
c~
c~ c~ c~
L ~ ~ c $ c
~ Zl c~ ~ Z~ ~ ~, b b b ~ ~ e~ O O O $ ~o co~ 8 8 8
~ X C C ;C C C C C C C $ C ~ C C X 'lC ~C C C X
C :C C C C C C C C C C C C C C C ~C C C C C
;C :C ~C C C ~C C ~C C C C C C C C C C C C C C
:C C C C C ~C C C C C C C C C C C C C C C
o .~ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o .
o~
~: C :C C C C C ~C C C X C C C C C C $ C C C C
,~. :C C C C C $ C $ C C C C $ C ~C C C C C C
~: ~ :C C C C C C C C C C ~C C C C erC $ e~ e~ cc
~ r ~ ~ ~ ~ ~ ~o ~ ~ ~ ~r ~ ~ o
~ ~ ~? ~ ~ ~ ~ 4? ~ ~ ~ ~ ~ ~ ~? ~? ~? ~?
~r ~ ~r ~r ~r ~ ~r ~ ~ ~ ~r ~r ~ ~ ~ ~ ~ ~r ~ ~r
CA 02227934 1998-02-23
0050/~161~2
64
8 8
ll ll l
O O O O a O O
8 ~ 8 ~
c O ~~ ~ O ~ ~ O , ~ O ~ O ~ O ~ O ~ ~
2 ~ ~ ~ ~ X
V~ ~ ~ ~ ~ ~~? ~ ? ~ ~ ? V~ U~ V? ~ V? . 11?
CA 02227934 l998-02-23
0050/46142
C-) C~ c-3 c~ c~ C) c~ c.~ c~ c~ c~
3: ~C C C ~C X C C 3C ~ ~C
U U um um
m ~ m m =~ ~ m m~m
C; C~ t~ U u o u u U U U
U~ ~ U~O ~ U~ ~ U~ ~ U~
:c c c ~c ~c c ~c ~c X C ~c
:C ~C ~C ~C C ~C C3C C X C
~C ~C C ~C X C C C C ~C 3C
~ ~C C C X C ~C C C X X ~C
O _~ ~'1 0 -- N O _ N O --~
3C C ~ C ~C $ C C X C ~C
3C C C C C C ~C C X C C
Cc C3~ C~ c~Cc~ C~ Cc C3C
~ ~ ~ 3 ~ ~o ~
CA 02227934 1998-02-23
0050/~61~2
~ ~ U U U U U ~ ) U C.~ U ~,) U U U U U t~ U U
~q r7
~ U
I q U ~ U ~ Z Z Z Z ~ Z Z =~
$ ~ $ ~ I ~ $ $ $ $ $ ~ $ ~ $ ~ $
$ ~ $ :~ ~ $ ~ $ X :: $ 3: :I $ $
:C ~ $ :~: $ :C $ ~ $ ~ $ ~ $ $ ~ ::C
C5 ~ o _ ~ o ~ ~ o _ ~ o _ ~ o ~ ~ o _I ~
$ :r: $ ~: $ ~ $ $ ~: $ :~ x ~:
$ $ ~: . $ ~: $ :r: $ $ $ :~: $ $ ~ $ ~ $ ~
CA 02227934 1998-02-23
0050/46142
E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3 E3
x :~ r x r :c ~: r 3: r r r
:r Ir ~ r ~ r :~ r r
:r ~ ~ :c r ~ ~ r ~ ;: r ~ ~: x ~ r
~: r :r r r r r :~ r ~ r 5~ r r r
e o ._1 ~ O ~ ~ O ~ ~ O _. ~ O _, ~ o ~ ~ o ~ ~
:r r r ~: :r r r :1: r r r :: r :r r ~ z :c
i3 i ~ @3 j,3 ~ j,3 j3 I ~3 j,3 j;~ ~3 ~ ~ E3 E~ ~3 i3 u i.3
~ O~ o~O ~ ~ ~
CA 02227934 1998-02-23
0050/4:61~2
$ ~ ~ ~ $ ~ X ~ ~ :C
X _ ~ $ ~ O O O ~ O O ~ O
O l~ O ~ O O O ~ O O ' Z 7 Z Z Z Z ' Z
C $ $ 5: $ :C $ :C $ ~ ~: 5 $ :C $
$ ~ ~ $ ~ $ :C $ ~
$ ~: $ :: $ ~ :C ~ $ :C $ 3: $ X $ $ $ ::
$ ~ ~ X ~ 3: :~ $ ~ $ ~ ~ $
~ o .-- ~ o ~ ~ o _ ~ o --~ ~ o --~ ~ o ~ ~ o
o~
:r ~ $ ~ X $ ~ C X :~ $
:r: $ $ ~ :c
~ ~ ~ ~ o ~} ~' ~ _, ~ ~ ~ ~ ~o l- ~0 cr~ O ~ ~ ~ ~
~ 'D 'D 'O ~O 'D 'D 'O 'D 'O 'D ~O 'D ~ ~D ~O 'D. 'D 'O ~O
CA 02227934 1998-02-23
0050/4~6142
~ i5 i;~ i3 i3 i3 i3 i3 i3 i3 ¢ i3 i3 i3
~ X ,~ 5. ~ ~ ~ ~ 5 ~ 5. ~ 5. 5,
j~ ~ U U
0 1~ 0 0 ~ O ~ ~, ~ 1 1 1 1 1 1 1 1
_.
,4
X ,r ~ 5, 5. 5. X ~ ~ ~ 5 5. 5'
5' ~ 5 ~ ~ ~ ~ ~ ~ 5'
c: o .~ ~ o ~ ~ o _~ ~ o ~ ~ o
~ ~ :~ 5 ~ 5 5 5 ~ ~ ~ 5 5 ~
~ ~o 1~ O ~ ~ ~ ~ o
O ~O 'O ~O ~O ~O ~ ~O. ~ ~ ~ O.
CA 02227934 l998-02-23
0050/4~614~2
O ~ o ~ $ ~
''~ b ~ ~ b ~
~ ~ ~ ~ ~ ~ I ~ ~ ~ X ~ ~ ~ $ ~ ~ .
~
- .
c - l ~ o - ~ o - ~ ~ o ~ ~ o ~ ~ o - ~ ~ o
o~
x ;~; lo~ ~ ~ x ~
CA 02227934 1998-02-23
0050/461g2
Y
~ b b ~ b ~ o o o ~
5. ~; 5. 5. 5 5 ~ 5 ~ 5 X ~ ~ ~ 5 X 5 5 5 X 5
X 3: X X ~ X ~ 5 X X 5 5' X ~ 5. X X
X 5: X X 5 X X X ~ X X X 5 $ ~ X $ 5. ~ X X
o ~ ~ o ~ ~ o _~ ~ o _~ ~ o ~ ~ o ~ ~ o
~ ~ , 5 5 5 ~ X 5 X 5 ~ X X ~ ~ X X X 5. X ~
x ~ o ~ 0 ~ ~ ~ ~ v~ 'D
CA 02227934 1998-02-23
0050/461g2
72
Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
~1 I C ~ ~ rC 5 C rC 5 rC rC X5~ 5 rC rC C 5 rC 5 rC rC
$ r ~ C ~ 5 5 r
8 E~) 8 8 8 5 8 ~
P~ 5 5~ ~C 5 5 C 5 5 5 5 C 5 rC 5 C 5 5 rC rC ~r 5
~ 1~ 5 5 rC 5 ~C ~CC 5 I CrC 5 I C 5 C rC 5 5 X . 5
pt ~c ~rc ~C 5 X C 5 ~C rC X ~C 5 ~rC C ~ rCrC 5 5
1~ rC C $ 5C 1~I C 5CrC rC 5 rC rC 5CrCrC rC 5C rC rC rC ~ C
~ 'I O --~ ~O ~ ~1 0 ~ J O--~ ~ O_~ ~ O ~ O
O~
C~ rC ~C I C rC ~ C~C C rC rC5C rC rC rC ~C I C 5C ~ C rc ~ rC
C rc rc 5C rc rc rc rC 5C rc 5C 5C C C 5C C C C C 5C C
~Y C I C 5C C C C C C C C C 5C C C ;C 5C C C C C C
~ ~ Il~ 1~ XO X CO 00 ;~; X 00 CO 00 CO O~
CA 02227934 1998-02-23
0050/416142
73
O O O ~ O O O O O
~> U
U~ ~U U
c ~ o --~ ~ o --~ ~ o --l ~ o
o ~ 3, ~ ~~ ~~ ~ ~ o~ o o ~ g
CA 02227934 1998-02-23
0050/4~il42
~4
_, O ~ O O O O O ~ O O O O O O O ~ O O
z z z z z z z z z z z z z z ~ z z z
$ $ ~ ~ $ ~ ~X $ S $ ~ $ ~:
m m m m
u ~
l 1 l ~ C.) $, ~ Z Z z $~ $
'~ b ~ $ ~ ~ $ ~ ~
$ ~ ~ S $ $ 3: $ $ $ :C $ ~ S
$ ~~ S $ $ ~: $ $ $ ~ ~ $ ~ $
~; $ ~$ $ $ $ :~: ~ $ ~ $ ~: ~
-
~ ~ ~ ~ ~ ~ ~ 5: ~ ~ :C X $ ~ ~ ~ ~ ~ ~
C ~ o . ~ o -- ~ o ~ o -- ~ o ~ o
o~
~ ~$ ~ ~ $ ~ 1 $ :~S ~ $ ~ ~: S X
oo
s ~ $ 3~$ ~: $ X S
P~ $ ~ $ ~ S ~ ~ $ ~ :C
CA 02227934 1998-02-23
OOSO/~61~2
~ Z ~' Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
_l C :C C C C C C C C C ~C C C C C C ~ C ~ C
~ Z Z Z ~
;;,,, C ~ O O O ~
C :C ~C C C C C C C C ~C C C C C C C C C C C
:C C C ;C C C C C C ~C C C C C C C C C C C
C :C C ;C C C ~: C C C C C C C C C C $ C C C
C :C C C C C C C C C ~C I C ~C C C C C C C C
o ~ ~ o _ ~ o ~ ~ o _~ ~ o ~ ~ o _ ~ o
P~ C :IC C C C C C C rC C C l C C C C C C C C C C
OD
~ $ ~ X C C ~C C C C $ C ~C C C $ $ $ $ C C $
~ $ ~: c c ~c c $ ~c c $ $ ~c $ x $ c $ c c $ a~
CA 02227934 1998-02-23
0050/46142
~ Z 'Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
O O O ~
8 8 8 8 8 a 8 8 ~
~ o --~ ~ o --~ ~ o --~ ~ o --~ ~ o ~ ~ o ~ ~ o
~ ~ t~ ~ t~ t~ t~ ~ ~ ~ t ~ ~ ~ ~ ~ ~ ~ ~ ~ x ~
CA 02227934 1998-02-23
0050/46142
m~ m~
o m m m \ /
u' ~ ~ 3, ~ 'm ~ 'm ~ 'm ~:~
U~ U~ '.u'
C ~ ~ O ~ ~ O _ ~ O _~ ~ O
x
X X
o
CA 02227934 1998-02-23
0050~46142
O O O O ~ O O ~ O O ~ O O O
Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
u C~ m C~
m~ m cm C ~ '~ ~ ~ ~ ~ ~ '~
~ q ~ Z Xz ~ Z ~ Z
''' '~ b ~ X ~ ~
~ X X X ;~ :C X ~ X ~T~ 3 X X :~
~
~: X ~: X X x X X :: X ~ X X :: X 3: X
_.
X :~ X X ~ X X X X :: X X X :C X X
~ -- ~ o _ ~ o ~ ~ o _ ~ o _ ~ o -- ~ o
X X X ~: X ::: X ~ X :C X ~ X $ ~ X
oo
~ X X ~ X :C X ~ X X X !r $ X X :~ X X X
u ~ u ~
~ oo oo ~ X X X X ~~
CA 02227934 1998-02-23
0050/46142
~ Z .Z Z Z Z Z ~ ~ ~ ~ Z ~ Z ~ ~ Z ~ ~ O O O
~r ~ O O O
1 o _ ~ o ~ rl o ~ ~ o ~ ~ o -- ~ o ~ ~ o
o~
X
g t~ O O O ~g S O ~ ~~~ t~ t~ 1 '~ ~ t~~ t~' ~
~ X 1~0 X tl~O Y 00 X 00 X ~~ X X tlO tX~ tX~ tlO t~O tlO t~O tX~
CA 02227934 1998-02-23
0050/46142
O O O O O O O O O O O O O O O O C~ O O
Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
5~ ~ 5 ~ 5 5 5 5 ~ 5 5 5 5 5 ~ 5 ~ ~ 5
O ~ - ~ O O G O O O O o o
8 ~ ~ 8 8 ~5 8 ~
~ x ~ c x ~ x x x ~ x x x ~ ~
x ~ ~ ~ x ~ x x x x :c x
c - ~ o- ~ o ~ ~ o ~ ~ o - ~ o - ~ ~ o - ~ o
o~
:~: x x x x ~ : x ~
x x x x
u ~ u u ~
~: U ~ X X ~ U X ~ X
~ ~ ~ ~o r~ ~ o~ o _ ~ ~ ~ v~ ~ t~ X ~' ~~t ~
o 00 ~ ~ . ~ ~ 00 ~. 00 X ~ ~ 0~ 00 ~ X 00 00 04 00
CA 02227934 1998-02-23
0050/46142
81
Z ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ Z
X ~ $ x ~
m $
N m ,
u c~ u m c~
3 3 3
-
~ _ ~ o ~ ~ o _ ~ o ~ ~ o
o~
v
o ~ ~ ~ x u~
CA 02227934 1998-02-23
OOSO/~6142
82
O O O O O O O O O O O O O O O O O O
Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
U C~
u ~ z
'~ , Z~ b b b b b ~ b b b b
:~ ~ x :~ x ~ ~ ~ x x x x ~ ~ ~ x
Y X ~ X X X X X X X X X :: X ~
~ o ~ ~ o --~ ~ o --~ ~ o --~ ~ o --' ~ o
o~
DS $ X :r: X ~: X X X X X X X X :~ X X X x
r~ x x ~ x ;2: x ~ x x c~
o CO 00 00 X X CO ~ 00 00 X 00 X X X X 0~ 00 X
CA 02227934 1998-02-23
OOSO/46142
83
O ~ ZO o o o o o Z O o ~ Zo O O o o Zo o o
Q ~ ~o~
aS ~ ' ~
~ b l~ b b ~ u ~ b b b ~ ~ ~o o o ~~
¢ _ ,~ o ~ ~ o _- ~ o --~ ~ o _ ~ o ~ ~ o ~ ~ o
o x ~o x oo ~ o~ ~o x oo x oo x ~o oo oo oo oo x o~ x x
CA 02227934 1998-02-23
0050/46142
84
C ) ~ ~ ~ o o o o o o O O O G
r C 50 50, 50 C ~ ~ Z
~: $, $ $ $ 3: X $ $ $ $ $ $ $ $ $ $ $ $ $ $
t~: $ xr $ :~ $ X X :C $ $ X X X X $ $ $ 5, X :I: X
$ ,~ $ $ $ X ~ $ $ X $ 5, $ $ :~ $ $ $ $ 5 $
~: $ '~ X X X ~ ~ X $ 5' $ $ X $ ~ :r: X $ $
C ~ o -- ~ o ~ ~ o --, ~ o ~ ~ o ~ ~ o _, ~ o
O~
~: $ '~ ~ $ $ S :~ $ X X :c x X X X $ 5' $ $ X X
$ ,~ $ X ~ $ $ $ X $ X $ $ X X $ $ X $ $ X
X ,~ X X 2 X X X X X X ~ X $ X X $ ~ X X $
Ooo~oxxxxx ~~ . .O~~~
CA 02227934 1998-02-23
0050/46142
:~ ~ c~3 $ ~ ~ c~ c~ c~ c~ c~ 3: c,
_1 u ~ C3 C~ 3 CC CC C~ 3 CC ~c ~,
w~ L
Z~; Z~ Z~ Z~
X :C ~C C ~C C ~C ~C C :~ ~C C
C :C C ~C ~C C ~C ~C C ~C C 1
~C 2C ~C ~C ~C ~C ~C ~C C ~C C C
~: ~C :CC ~C C ~C ~C C ~C ~C ~C ~C
0 r~ O
O~
~C ~~C ~C ~C ~ C C C ~C ~C ~C ~C
~Y ~C ~C ~C ~C C ~C C C ~C ;C C
5 ;~IC ~C ~C ~C CIC C ~C C IC
~ r l r3 ~ 1~ ~~ ~~ 0~ ~
O C~ Cl~Cr~ C~ C~
CA 02227934 1998-02-23
0050/46142
~ $~ ~ $ ~ ~ ~ $~ S~ $~ S $ S~
S ~ 3 S ~ $ S $ S :~: $
m ~
U C~
" ~
m N
S S C~ ~ S S :~
q q q ~ Z ~
~: :c c ~ ~ c c s :~ x ~: c c c c :c :~ c
:c c :~ c c c c c ~c c :~: :c ~ c :~ c c
r~
:C :: C C C C C :~: C C C C C ~ C :r: C
:c ~ :~ C :C C C :C X C :~ ~ c
c ~
J~
:c c :: c :c c c c c c ~: c c c c :~: ~
:c c X c C C c c :r c c c x c :~ c c
~ C C C C C ~ C ~ ~C ~ C C C C C C ~
~~ c~ o ~
.7 C~' C~' CJ~ C~ CJ~ C~ CJ' C~' CJ~ C~' C~ CJ~ CJ~ CJ~ C~ C~ C7~
CA 02227934 1998-02-23
0050/46142
87
I ~ ~ ~ ~ X
U U U ~ U ~
l l l ~ o o o
c o ~ ~ o ~ ~ o - ~ ~ o ~4 ~ o - ~ ~ o - ' ~ o ~ ~
:c ~ !r ~ 3: x x ~ 3~ $ $ ~ $
$ ~ $ ~: $ :~: $ $ ~ 5: ~ $
CA 02227934 1998-02-23
0050/46142
$ $ $ ~ $ ~ ~ $
~ V ~ U'~ U ~
~ $~ $~ $~ ~ $~ ~ ~ $ ~ $ ~ $~
g ~ ~ g ~ o O O O
8 8 $ 8 ~ 8 8 ~
~ X ~ X ~ $ :C X S ~
c o ~ ~ o ~ ~ o ~ o ~ ~ o ~ ~ o ~ ~ o
c~
' CA 02227934 1998-02-23
0050~46142
~ C~
~ ~ ~ ~ ~ ~ N
~ ~ r~ ~ ~ ~ l l
u~ ~ o / ~ o o o o
:c c c c c c c c ~: c c c
~: c :c c c c $ c c c c c c c
:c c :~ ~c c c c c c c c c
:c :c ~: c c c ~c ~c c c c c
C O ~ ~ O r-4 ~ O _I ~ O ~ ~ O
p~ c :c c c c c c c c c c c c
o~
~ $ ~c c c c c c c c c c c c
~ oo oo x oo oo o~ o~ ~o~ c~' o' o' o'
CA 02227934 1998-02-23
0050/46142
... ~ ~ ~
~c m D: tl5
U C) U
U U
~ ~ ~ o ~ ~ o _ ~ o ~ ~ o _ ~ o _~ ~ o
o~
oo
~ u ~ u ~
O ~ O ~ X O~ O _I
00 O~ O O O O O O O O O O _ ~
o o~ o~ o o o o o o o o o o o o - o o~ - o o
CA 02227934 l998-02-23
0050~46142
U C C ~ C3C~ C C C ~C ~3~3C C C
~r,~ " tC C ~ C tC rE,~tC t tC 3C C C ~3 C C
~ v.
t ' 3 tC
~C C C ~ V
ff ,. a u ff ~ Z l ~ O O O v~~
~Y tC :C C C C X C C C C C C .~C C ~C C !C .C 3C C C
P:3C:C~tC C~C~tC,C~C tC ~tC ;C 3C C~tCtC C~tC~C~C C C
C C ~C ~C ~C tC ~C C C ~C ~C ~C tC C ~ C 3C3C3C3CX,C
3C:C3C3C tC 3C tC 3"3C3C3C$ tC ttC C C3C3C3C3C tC
C ~ O --~ ~ O -- ~ O ~ ~ O ~ ~ O _~ ~ O _~ ~ O
O~
~ 3C3:3CX~C3C3C3C~C3C3C3C tC tC ~tC 3C3C3C3C C3C
x
P~ C3:X3:C C C C3C C3C3C C C C C3C3C3C3C
VC3~)V3C33 C~Jv33~3~ ~33~3C3C3C~ V V
~r~ o~ r~ xo~o ~ ~ ~r~o
~ o c, o o o o o o o o ~ o o o 8 8 8 8 8 8 8
Z;~r~r~r~r~r~r~r~r~r~r~r~r~r~r~r~
CA 02227934 1998-02-23
0050/46142
Sl N N ~
8 ~ ~ ~ 8 ~ Z
~: X :~ X 3~ X ~ ~ ~ X
C ~ ~ o ~ ~ o , ~ o ~ ~ o ~ o ~ ~ o ~ ~ o
z ~ ~ ~ ~ o o o o o o o o o
CA 02227934 1998-02-23
0050/46142
93
C
g ~g ~ g ~ g
g g g ~ O g ~
~ m
3 i v ~ 3~ ~ ~N ~ ~!.. ~ ~ C.)~U~
O C~ O O O ~ ~ ~' O O O O O O O O
C CC C C ~ C C C C C C
C :C C ;C C C ~: C ~ $ . C C
~; C ~C C C $ C C C X ~C 3: $
$ ''C , C $ $ $ 3: 3: C ~:
C ~O _ O ~ ~ O ~ ~ O
~ C ~: $ C C $ C C C S $ $
g g C,) g g g g ~ g
p~ g ~ g ~C g g ~C
~ ~l ~ ~ ~ ~ O ~o ~o ~ X ~
~ o C~ o ~ ~ o o o o o o
Z ~r ~r ~r ~Ir ~r ~r ~r d~ ~r ~r ~r ~r
CA 02227934 l998-02-23
0050~46142
94
~ V V V ~ ~ V ~ V V V 1 V V ~
~ ~ m 1
~ u u ~
m ~ u u
V ~ V ~ N ~ 8 ~ v v v
o o o o . . . v v c~ z z z z z z z z z z
'U- Zv ~ ~ v ~ Z~ v v ~ v v ~ v v b v
~ ~ ~ o ~ ~ o . ~ o ~ ~ o ~ ~ o ~ o
p~ v v v v v v v v v v v v v ~ v v v v
~ v v v v v v v v v v v v c~ v v ~ v v
o ~ ~ <~ x o~ o ~ ~ ~ ~ u) ~
~ ~ o ~ ~ o o ~ o o o o o o o o
CA 02227934 1998-02-23
0050/~6142
~ ~n ~
Z Z ~ Z ~ ~ Z Z Z Z Z ~ Z ~ o
P ~ O O O ~
-
O _ ~'I O _I ~'I O --~ ~ O -- ~'1 0 _ ~'I O _I ~J O
~ C X 5 X ~
o o o o o o o o o ~o o o ~o o o o o o o o o
CA 02227934 1998-02-23
0050/46142
_~ ~ _ _ _ _ _ _ _ _ ~ _ _ _ _ ~ ~ ~ ~ ~ ~ _ _
ll ll
O C ~ ~ ~ ~ ~ ~ ~ ~ O O O O
~ ~ ~ 8 8 ~ 8 5 ~i 8 8 ~
P~ :C X ~ ~ X ~ ~ X :: X ~ X X ::
$ ~ ~ I X ~ ~ ~ ~ ~ ~ ~ X ~ X ~ ~ ~
C --~ ~ o ~ ~ o _ ~ o ~ ~ o _ ~ o _ ~ o _~ ~ o
o~
5 ~ X ~ X ~ X ~ ~ ~ ~ ~ ~ ~ X 5
X ~ ~ ~ X X ~ ~ X ~ X ~ ~ ~ ~ 5 ~ ~ X X
X :: ~ ~ X ~ ~ X ~: X ~: X 5~
CA 02227934 1998-02-23
0050/46142
U U U Cl 1~ ~N
C~ C O ';J ~ ~ ~ O O ~ O ~ ~ ~ O
C ~ O ~ C~l O _I N O --~ ~ O --I
X 3 ~ ~ $
X
~ ~ 5 X ~ ~ ~ X ~ 1
~'
CA 02227934 1998-02-23
/~6142
,_~ ~ _ _ _ _ _ _ ~, _ ~ _ ~, _ ~, _ ~ ~, _ ~~ _ _
m m
~ U
~ N v~
u u
,4
c ~ o - ~ ~ o ~ ~ o - ~ ~ o ~ ~ o ~ ~ o - ~ ~ o - ~
o~
oo
~ ~ I~ ~o 1-- x cr~ o ~ ~~ ~ ~ ~ ~ oo 0~ 0 --I
CA 02227934 1998-02-23
0050/g6142
~ ~.
Z Z Z Z; -' Z Z Z Z Z ~ ~ ~ o O O ~ 0
0 0 0 ~
_1
c:
o~
~o r oo o~ o _I ~ ~ ~ ~ ~o 1-- x o~ o --~ ~ ~ ~ ~
x x oo x x x
CA 02227934 1998-02-23
OOSO/46142
100
O U ~ ~ ~ U U ~ U U ~ U
.) U U U ~) t,) U ~ ~ C.) U U U U ~ U U
~ ~ ~ h ~ o~ o o o ~ ~ ~ o L ~ ~
~
C O ~ ~ o _~ ~ o _1 ~ O ~ ~ O ~ ~ O ~ ~ O ~ ~ O
x
x x ~ x 3 O~ O~ 3 ~ 3 0~ 3 3 ~
CA 02227934 1998-02-23
0050/46142
101
:~ c3 c3 c3 c3 c3 c~ c3 c~ c3 e3 c3
_1 ~3 t3 ~3 ~3 ~3 c3 ~3 ~ ~3 ~3 ~3
m m m m m m
V ~ V V U V
~ ~N ~ ~ ~N ~
C~ g C~ V V ~ V C~ V V V V
~ ~ O O O O O O ~ O O O ~ ~
p ~ V ~ ~ V ~ ~ V ~ ~ U ~ ~ V ~ ~ Z~
~ ~: :r $ ;~
~ o _~ ~ o -- ~ o
CO
~ c~3 ~.3 ~ c~ ~3 c~3 c~3 ~ c~3
O ~ ~ ~ ~ ~ ~O 1~ X
o '3 ~3 ~ ~ ~ ~ ~ ~ ~ ~ ~
Z ~ .~, ~ ~ ~ ~ ~ ~ ~ ~
CA 02227934 1998-02-23
0050/461.42
102
~
~ o - l ~ o - ~ ~ o - l ~ o - l ~ o - ' ~ o - l ~ o - ' ~ o
oo
o~ c~ ~ C~ O ~ ~ ~ ~ v~ ~o ~ X cr~
CA 02227934 1998-02-23
0050/~6142
103
u ~3 i3 o i3 u iu i3 i;~ lu i;~ i5 i3 i;~ i~ i3 i~3 i~ u i~i
U iU iU i5 i3 i~ u i3 i~ lu i3 i~ i5 i;~ u i~ ~u i~ i~ iu
u ~ ~ L ",
u ~ o o o ~ $
r 'r ~ r ~ 5~
~ ~ ~ O ~ ~ O _~ ~ O _. ~ O _~ ~ O ~ ~ O _ ~ O _
~ ~ r ~r ~ :C X 3~
~ ~q ~ q ~q ~ @~ q ~q ~q ~q ~ q ~q ~
CA 02227934 1998-02-23
0050/~6142
104
~ U U O U U U U U ~ C~ U U U U ~ U U U ~ U
_1 U ~ ~ U U ~ ) o V O U U U U ~ U ~ U U U
~ ~ Ua u~
~~ o o o o O O O o o ~ ~ ~ O o
X ~ ~ X !r ~ X ~
C: ~ O -- ~ O ~ ~ O _I ~ O _1 ~ O _ ~ O ~ ~ O
~ o~ x o~ ~o ~ ~
CA 02227934 1998-02-23
0050/46142
105
u m m u u~
m U U m C ~ m m u u
~ ~ ~ O _ ~ O _/ ~ O _~ ~ O _
O~
~~
CA 02227934 1998-02-23
0050/46142
106
V ~ V ~ ~ V ~ V C~ C~ V V V ~ O V V V
5~, ~ 5~ ( 5' 5' ~ u
b b ~ b ~ Z~ b ~
C r~ o _ ~ o _1 ~ O ~4 ~ O _. ~ O _, ~ O _, ~ O
c~
~ ~ ~ V V ~ V ~ V V V ~ V V ~
rr~ ~ ~ rrl rrl ~ r~ ~ rr~ r~ rrl r~ r~ rr~ ~ rr~ ~
~ ~,; ~ ~ ~ O O ~0 ~ 0~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
CA 02227934 1998-02-23
OOSO/'~6142
107
~3 ~ ~3 t3 ~ a~3 ~ 3 3 ~ ~ 3 ~ ~ T3
~3 a3 ~ t3 t3 a3 ~
~ o~ ~
"L~ 3 ~ O ~
o o o ~ 8 8 ~ 8 8 8 c~ ~ ~
c c c x c c c c c c c ~ c c x c c c
c c c ~ ~ ~ c c 3 $ c ~ 3 c c c c c c c
:c c :c :~: c :~ 3 ~ a~
:c :~: c ~ 3 ~ ~3 ~3 ~3
~ ~ o _ ~ o _- ~ o _~ ~ o ~ ~ o ~ ~ o _ ~
~ :C C C 2: C C C C C C C C C C C C C C C C C
~ ~C ~ c c ~ c :r: C C c C c c C 3: c
C C :~ C C :: 1 C C :C C C C ::
O.~ ~~
Z 'J ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
CA 02227934 1998-02-23
0050/4~6142
108
r7 r7 ~7 ~C rC ~37~ 7 ~ rC rC ~ ~C ~~C C rC ~ cc
I_1 C C ;C C C 1~ ~C C $ ~IC rC C C C C C C
27~7 C7 ~ CS7
o~ C~ OS O O ~--~ c~ ~ ~ C~) C~ ~ ~ ~ 1 1 0 0
Z~ ~ Z Z Z Z Z Z Z Z Z Z C~ C~
~ C :C C C C r~c C C C C C C C C C C
~ ~ C ~C C C C C CC C~C C C C C C C
~r~ r7 r~l C7 ~ rC7 rC7 Cg7 ~ ~ r ~ rC ~C ~ r~
r7 r7 ~7 r7 r7 r7 ~r7 ~q r7 ~ r7 r7 r7 1~l r7 r7
rr ~ ~ ~ !1~rc ~ ~!r ~ c rc
P~ ~3 c3 c~ ~3 c~ c3
c O ~ O r~ O _~ ~ O ~ O _~ ~ O _
~ c ~ c ~ c rc ~c rc ~rc r~c r~c l c c ~c ~ c rc rc r
P~ c ~ rc rc c ~ c c rc rc rc ~c IC ~c ~ c c rc ~ c
~1 rc ~ c c rc rc rc ~ c ~c rc rc rc rc rc rc
x O~ O --~ ~ ~ ~ ~ ~~ ~ ~
CA 02227934 1998-02-23
0050/~6142
109
u O m m m~
m)~m
o I Z ~ Y ~ ~ ~ Z Z, Z, Z- ~
U U ~ b ~
c ~ o ~ ~ o - ~ ~ o ~ ~ o - l ~ o - l
oo
CA 02227934 1998-02-23
0050/46;142
110
X :~: X X ~ ~ X ~ X X
;~ z z z z z z z z z z ~ z ~ z z zl zl zl ~
~ ~ o --~ ~ o --l ~ o ~ ~ o ~ ~ o --~ ~ o --l ~
:C C ~ C C 2 ~ ~ ~ C C ~ C C ~ C ~ ~ C C C
:C 3: C :: :~: ~ ~ C C ~ C C C ~ ~ $ C
C C ~: C C ~ C :~: C C
00 Cr~ O ~ ~ 00 Cr~ O --I ~ ~ ~ ~
CA 02227934 l998-02-23
0050/~6142
111
~ ~ ~ O -- ~ O O O ~ ~ O O
~ ~ l~ ~ ~ ~ ~ I ~ ~ ~ ~ ~ $ ~ ~ X ~ ~ ~ ~ ~ ~
~ i3 i~ ~ i3 j~ ~ 3 ~ X~
~ o l~ o ~ ~ o - l ~ o - ~ ~ o - l ~ o - ' ~ o ~ ~ o -
~Y X r ~ ~ x
oo
~r
r ~ j3 j~ j~3 j3 j~ jr~
o t-- '-- t-- t~ X X X X ~; X X X ~
CA 02227934 1998-02-23
0050/46142
112
2 ~ U ~ ~ ~
U U
U~
~ ~ U ~ ~ UU U U ~ U U
o O ~ O ~ O ~ Oo o o o o O o
C ~ O _ ~ O _~ ~ O _ ~ O ~ ~ O
O~
x
o O~ ~ 8 o ~ 8 g o o o o o ~ _.
CA 02227934 1998-02-23
0050/46142
113
... ,., ~ ~
u m C~ m
-
~ ~ ~ ~ ~ ~ ~ ~ ~ X ~ ~ ~ ~ ~ ~ 2 ~ ~ ~
U ~ ~ U ~ U ~ ~ ~
U ~ ~ ~ V ~ U U U U ~ ~ ~ U ~
C: -- ~ o _ ~ o _ ~ o _ ~ o ~ ~ o _~ ~ o _
x
U ~ U ~ ~ U ~
~ O ~ O 1' X O~ O
CA 02227934 1998-02-23
0050/g6142
114
3 a ~ 3 ~ 3 ~{ u ~ ~ ~ o o a ~ ~ ~
~ ~ ~ u ~ u ~ u ~ u u ~ ~ u ~
~ ~ ~ x ~ u ~ ~
c ~ o - ~ ~ o ~ ~ o - ~ ~ o - l ~ o ~ ~ o ~ o ~ ~ o
-
x
CA 02227934 1998-02-23
0050/4L6142
115
_l tS :S 't'C tC tS tC tC tC ~ tC ttC ,tr ttC tC tC S X tC tr ttC S tt
~ S S t~
~ 8 S S ~ 8 S 8 ~
C :C C C C C C C C ~ C C C C C C C C C C C ~
C :C C C C C C C C ~ I C C ~ C ~ C C C C C C
:C C
o ~ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o _ ~ o
o~
~ c :c c c c c c c c :~: c c c c c c c c c c c c
~ ~ ~c ~ C V E.~ ~ C ~
~ 't V~ x 0~ 'I ~ ~ 'O ~ X O~ O -- ~ ~ 't
Z ~r ~t ~t 't ~ ~t ~ 't 't ~ ~J 't ~ ~t 't 't ~ ~ ~ ~t ~ ~
CA 02227934 1998-02-23
0050/9,6142
116
~ ~ E~ E~ E~ E~ ~ ~ E~
C :C C :~: C :C: C 5: :C ~ C
~ ~ m m m m
m m ~ ~ ~ m m ll~ m~m m m
~ ~ ~) U ~) U C) t) C.~
E~ I E~
O ~ C ~ ~ o o o o o ~ o o o ~ o
~ ~ E~ ~ ~ , ~ ~ z
C ~ C ~: C :~ X C C
C :C C :~ C S C C ~ :~ C
~ ~ EC ~ C
--~ ~C ~ C
t3 ~ C~ _4 ~ o _I ~ o _ ~ o
:C ~ $ 2~ C C X C
~CC ~ r~ ~ ~ ~
r~ oo o~ ~ ~ ~ u,
r~ r~ r~ r~ r~ o x ;~; x
CA 02227934 1998-02-23
0050/461g2
117
Z q ~ Z ~ Z Z Z ~ Z Z Z Z Z Z Z ~ ~ Z Z Z Z
:: $ $ $ $ $ :~: $ ~ ~: $ $ $ $ ~ $ ~ $ $ :~ $ $
$ ~ 5: $ $ $ 3: $ ~ $ 3: $ $ $ ~ 5: $ $ $ s: $ $
~ _I ~ o ~ ~ o ~ ~ o _ ~ o ~4 ~ o _~ ~ o ~ ~ o ~
~ $ 3: $ $ $ ~ $ :C $ $ $ $ $ ~ $ $ $ $ $ $ $
V ~ $ ~ $ ~ $ ~ ~ $ ~ $ O ~ ~
~ ~ ~ $ ~ U ~
,~ ~o 1-- X ~ ~ o
oo CO C~ o~ g o o C~ o C O
CA 02227934 1998-02-23
0050/41614~
118
U U ~ U ~ U U ~ U U
Vl ~ ~
U U U
~~ ~ ~ o o o ~ " r
~ ~~ ~ U U I I I ~ ~ ' ~ ~-
Z i---. Z z z z ~ C U ~ ~ ~ t-
Z i ~ O O ~ 8 ~ 8 ~
~ c c ~ c c ~c c c c c c ~ c ~c c c c c c c c c
-
~Y C :C ~C ;C C ;C ~ C ~ C C C C C C C C C C C C C
~ 2 C C C C C C C C C
C ~ ~, ~ ~ o ~ ~ o , ~ o ~ o ~ ~ o ~ ~ o ~ ~ o
~ C :C C C C :: C ~ ~: C C C C C C C C C C ~C C C
C U ~ C C C C C C C
c c c c :c ~: c c c c
~ O ~ o~ X ~ ~ ~
CA 02227934 1998-02-23
0050/46142
119
o ~3 ~ 3 ~3 u c3 ~ e3 o c~ 3 ~3 c3 ~ ~3
~ ;C :C ~ :C X X X C X C C C X C C C C :C :C :I:
~ ~ ~ 5 c c ~ a a ~ u
'- o ~ ~ g ~ ~ o o o ~ 0 ~ o
~3 ~ Z ~ Z Z Z Z Z Z ~ Z Z Z Z Z Z
:C 3: ~ C :~ ~: C C C C X X C $ ~c C ~ C C
C ~: C :~ C X X C C C C ;C C C C C C C
'C 3: X C X C C C C C C C C X X C C C C C
~y jc j~ ~ jc c ~ ~ jc jc 3 ~ jc ~
C ~ ~ o _ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o
o~
~; $ ~C C C C C C :1: C C X 3: C ;C C C :~ C ~ C C
o~
C , C C ~ C C 3: X ~ :~ C C C ~: ~ C C 3: C C
C ~ X I X X C : ~ C C X C C 3: C : ~ X
q ~ $ ~
CA 02227934 1998-02-23
OOS0/4 6142
120
~: $ $ ~ $ ~ C $ $ $ ~ :C
m m m m m m
U O U O C~ U
~,~ N m~m m~m m m
U ~ U U C' u u
$ ~ $ X :~: X ~
$ $ ~ X ~ ~ ~ $ 3: :C
$ ~ ~ $
$ ~
C: _I ~ o ~ ~ o _ ~ o ~ ~ o --I
oo
$ $ $ ~ ~ :~: $ ~ :~
$ ~ ~: $ $ ~ S
CA 02227934 1998-02-23
0050/46142
12
:~ E3 t3 c-3 c-3 c3 c-3 ~ c-3 B c-3 c3 c3 c-3 c-3 c~ c~ c~ c~ c- c~ v c~
_1 C :C ~C C 3: C C ~ C C C :~: C C C C C C C C C C
CC CC ~ ~ ~ C~ U CC CC ~ C~ Z~ q Z~ Z~ Z,~ Z,~ C~ CC CC
C :C C C C C C ~ C C C C C C C C C C C C C C
~ C :C C C C C C C ;C C C C C C C C C C C C C C
a: c :c c c c c c c c c c c c c c c c c c c c c
c ~C C~ C~ CC CC CC CC CC CC CC ~ ~ ~ CC CC C~ CC CC CC CC
~ ~ C~ ~ ~ o _- ~ o _, ~ o ~ ~ o _, ~ o _4 ~ o ~ ~
PC C :C C C C C C C C C C C C C C C C C C C C C
oo
C :C C C C C C C X C C C C C C C C C C C $ C
C :C C C C C X ~ C C C C C C C I C C C C C C
~ ~n ~D 1' ~ C~ ~ t ~ ~ t a~ C~
~t'l t'~ t''J t~ ~3 t'~ t~ t'l ty ty ty ty ty ty ty . C~ ty ty
CA 02227934 1998-02-23
0050/416142
122
Ej Ej ~j u E3 u E3 E3 u Ej u E3 ~j Ej Ei --u u Ej u u Ej u
3 ~ E3 ~ ~ u~ o o o o o
o o ~ 5 8 8 8 8 ~ 8 8 8 ~
~,
~ E3 ~ E3 ~ ~ @ ~ ~ E ~
C o ~ ~ o ~ o ~ ~ o _ ~ o ~ ~ o ~ o _~ ~ o _-
.
Cr.
x
~ ~ ~ ~ ~ I ~ ~ ~ E3 ~ @ E~ ~3 ~ ~ E3 ~ ~ ~3
~ x x g~ x O~ ~ 0~8 o o o ~o o o o
CA 02227934 l998-02-23
OOSO/'~6142
123
N ~ ~m ~ '
z ~ o z o o o ~ ~ ~ o~ ~o o o o o
~ ~ ~ ~ ~ I ~ ~ ~ ~ ~ ~ X ~ X ~ ~
C ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o _
o~
oo o o _~ ~ ~ ~ ~ ~ ~ X o~ o
~ ~~ ~ ~? ~
CA 02227934 1998-02-23
0050/4~6142
24
U U O C~ U ~
m m U g
g~ m
~
C o _ ~ o _~ ~ o _ ~ o ~ ~ o _I ~ o ~
-
o~
oO
o ~ ~ ~ ~ ~ ~o ~ x o~ ~
CA 02227934 1998-02-23
0050/46142
125
~ ~ ~3 ~3 c3 u ~ 3 o ~3 ~3 ~3 ~3 ~3 ~3 ~ 3
3 ~ ~ ~z ~ 3z ~ z ~ 3, z, ~ 3 ~ ,~ o
~3 E ~3 @ ~3 ~3 ~ E~3 ~ ~ ~ o o ~1
E~ E~3 E~ E5 E~3 E~3 ~ E~ T3 ~3 E~3 ~3 ~3 ~ E~3 ~3 ~ E~3
~ ~ ~ ~ ~ o ~ ~ o ~ ~ o _, ~ o ~ ~ o ~ ~ o ~ o
~ G :r ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
X r~
~ E~ 3 ~ ~ ~ E3 ~ 'E~
CA 02t27934 1998-02-23
0050/~16142
26
~ U U
~ 8 5~
$ l~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 2 ~ ~ 2 ~ ~ 2 ~ ~ ~ 5
2 ~ 2 5 2 2 2 ~ 2 2 5 ~ 2 2 2 2 ~ ~ 2
U ~ 2 ~ ~ ~ 2 ~ 2 ~ 2
~ o ~ t'l o ~ ~'I o ~ t'~ o ~ ~ o ~ t'~ o -- ~ o ~
2 ~ ~ ~ ~ ~ ~ ~ ~ 2 2 2 ~ 2 ~ ~ ~ ~ ~ ~
5' ~ U ~ ~ ~ U ~ ~ ~ U ~ ~ ~ U
CA 02227934 1998-02-23
0050/~6142
127
U ~ ~ ~o U O ~ U U ~
m :c m ::
U U C~ U
m~' :~
~ m ~ :~ m m ~ ~
C) U U ~ U U C~ ~ U
G O O ~ ~ ~ ~ O O o O O ~ O O O
Z ~ U ~ ~ U ~ U t~
X ~ ~ ~ X
-
~ X ~ ~ ~ ~ ~ ~ ~ $ I ~ ~
C ~ o _~ ~ o _ ~ o ~ ~ o
~ ~ X oo oo CO o~
CA 02227934 1998-02-23
0050/46142
128
m ~
= a ~
.
X ~ ~ ~ ~ ~ I $ X
~
C ~ o ~ ~ o ~ ~ o _- ~ o _~ ~ o _ ~ o ~ ~ o ~
8 o ~ o o o o o o o ~~ ~ ~
CA 02227934 1998-02-23
0050/4~6142
129
~ i3 i3 i3 i3 i3 i3 i3 ~ i3 i3 i3 i3 i3 i3 i3 ~
o~ ~
z z z z Z Z zl ~ zl~ ~ ~ O O 0~ ~
-- o o ~ c~~ ~~~ i~ i3 ~3 8
i3 ~ 3 ~ 3
C ~ o _1 ~ o _. ~ o _4 ~ o _. ~ o ~ o ~ ~ o _. ~ o
x r ~ ~ r~ ~ r~
r ~ 3 i~ ~ r~ ~ r ~ ~ r ~ r~ r
r~ r.~ ~ r~
~ ~ ~ r~ r~ ~ r~ ~ r) ~ ~ ~ r~ x ~
CA 02227934 1998-02-23
0050/46142
130
5, ~ 5
5, 51 ~ 5, 5, ~ 5. ~ ~ ~ $ ~ $ ~ ~ ~ 5, ~ 5
O O
O O O O G G O O O ~ O O O O O 5, 5
~ ~ ~ 8 8 8 ~
~: X 3: rC 3 :I 3 3 5~ ~ 5~ 3 5. 5~5~ : 5. 3 5~ 5. 3~ 5
~3 ~ ~3 33 ~ ~3 X ~ 3 ~ ~3 ~3 3~ 33 ~3 ~3
3 ~ : 3 51 3: :~: 5' 5' 3 3 ~ : 5 3 5' 5, ~: 3 5
33 ~ 3 ~ 53
o _~ ~ o _~ ~ o ~ ~ o _ ~ o _- ~ o ~ ~ o
o~
:~ C X X X X 5, 5, 5, X X X 5' ~ X X 5C ~ 3 5' 5' $
~Y 3~ ~ 3 X X ~ 3C C :~ ;C 3C C :~: 3 X 3 X 5C 3 C 3 5
p~ X r 3 I X 3 C C 3 C X X C 3C X X X C 3 X ~ 5
o ~ :$ $ $ ~ $ ~$ $ $ $ ~
CA 02227934 1998-02-23
0050/~6142
131
:~ x :C $
m m m ~
U U c,~ U U
r 1 1 1 0 1 0 1 1 1 l 1 1
~ ~ ~ ~ ~ ~ 2 ~ ~ ~ ~
C ~ o _~ ~ o _I ~ o ~ ~ o
CA 02227934 1998-02-23
0050/46142
132
X ~ ~ ~ ~ I ~ ~ ~ ~ X
Z ~ Z Z Z ~ Z Z ~ Z Z ~ ~ ~ Z Z ~ Z Z
~ _ ~ o _ ~ o ~ ~ o _~ ~ o _ ~ o ~ ~ o ~ ~ o _
o~
CA 02227934 1998-02-23
OOSO/4~6142
133
~ ~ E~ E~ 1 ~
8 ~ ~ 5~~ 5~ 5
r 3 x c ~ 5
~ ~ ~ ~ ~ o - ~ o ~ o - ~ ~ o - ~ o - ~ o - ~ ~ o
~ 5 :~ 5 5 ~ 5 5 ,~r ~ X 5 ~ 5 5 5 ~ X X X 5 5 X
oo
~ ~ ~ ~ ~ ~ 5 ~ X ~ 5 X X 5 ~ X X ~ ~ 5 X !r X
v~ ~~ ~ 8 ~ o 1~ oo o~ o ~ o
O ~ o ~,, ,, ~ ~ u~ o ~
CA 02227934 1998-02-23
0050/ ~614Z
134
:~: $ ~: ~ $ $ $ $ ~ $
$ $ ~ $ $ :~ $ $ ~ $ $ ~
3 ~ ~ ~ u
o o o o ~ o o o ~ ~ o o o zo ~ 3 o~ ~o o o
$ :c $ $ $ ~ $ ~: $ $ $ $ $ :~ $ $
$~ $~
$ ~ $ 3~ $
~ ~ ~ o ~ ~ o - ~ ~ o ~ ~ o - ~ o - ~ o
$ ~ ; ~ X ~ X :C
pc :c 3: $ :~: $ $ ~ $ :~: $ ~ $ 3~
~o ~ o ~ ~ ~ ~
CA 02227934 l998-02-23
0050/4~6142
135
:~ m m m :~ m
U U U U U U
um ~, u um ~ u
o o O O o I O o q q q ~ ~ ~ Z Z
'~ u- ~u- 'U- ~ b~
c: ~ o - ~ ~ o ~ ~ o ~ ~ o ~ ~ o - ~
-
CA 02227934 1998-02-23
0050/~6142
136
rt rt rt rt r" ~ rt rt ~ r~) ~ rt rt ~ r~ rt rt ~'1 rt rt ~ rt
~ ~: X ~ X ~ X ~ C ~ X ~
~t i? ~S ~ Q Q c
z z z z z z z z ~ I I Z Z Z rt ~ t rt
~ X ~ ~ b ~ ~ ~ v ~ 5 X b b
~ ~ x ~ x :c 3 ~ ~ ~ ~ x ~
~ V ~ V ~ ~ V ~ V V ~) V ~
~ ~ X X X X X ~ X ~ C X :C X X
V U U V ~ U U ~ V V C~ V ~ U C~ V ~ ~ U
C ~ o ~ ~ o _- ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o _~ ~
~ X X :~ $ X X X $ ~ $ $ ~r: $ $ I $ X $ ~: X ~ $
oo
~ X X X X $ $ $ :~: X $ X $ $ $ $ $ X $ $ $ :1: $
~ X ~ $ ~t ct $t $t xt rt rt ~ rt rt
U ~ ) U U C.) ~_) U ~ O U U U ~ ~ U U
~ t ~ ~O 1~ X O~ O --~ ~
O Vl 1~ ~ ~t ~O t '.D ;~; ~t t ~t ~ I~ t--
CA 02227934 1998-02-23
0050/~6142
137
X~ ~ 5~ 5~
X ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 5 5 ~ ~ $ ~ 5
~ ~ 5 ~ ~ ~ ~ F ~ o o o o 0 3 0 0
o o o O O O O O O O ~ O Z Z Z Z Z Z
O O ~ 5 ~
X ~ ~ ~ ~ 5 ~ 5 ~ ~ 5 ~ 5
~ ~ ~ ~ 5
~; o ~ o _ ~ o _ ~ o _~ ~ o ~ ~ o ~ ~ o ~ ~ o -
~ ~ ~ ~ S 5 ~ ~ ~ ~ ~ X ~ 5 ~ 5 5 ~ 5 ~ 5
X ~ x~ x ~ x ~ ~
CA 02227934 1998-02-23
0050/4:6142
138
~3 ~C t~3 CC~ c,C) c,) c~C ~ c~ C~C ~ r~C
_~ X C C C C C C C ~ l C ~: C C C
~ O~
C~
~ ~, a u U~ ~ ~ ~N
O G G ~ ~ ~ . ~ ~~ ,~ ~~ ,~
C :C C X C ~ C ~ C C C C ~C C
~ ~ CC ~ C~ C~ 3 C~ C~ ~ C~
_.
C~ rC C C C C C ~ C 1 I C ~ ;C C
p ~ cc c~ ~3 cc ~ cc c~ c
c ~ c~ - ~ ~ o - ~ ~ o - ~ o - ~ o
C C ~ ~C C ~ I ~ C C ~C C
C~
cC c~3 ~ C5~ C ~ ~C ~C ~ ~C C~ c~ C~ C~
C~ C~ C~ C~ C~ C~ ~ C~ C~ C~ C~ C~ C~
C C~~ o o ~ o o o o ~
Z ~ ~t ~t ~ ~ ~ ~ ~ ~ ~ ~ t
CA 02227934 1998-02-23
0050/46142
139
m ~ m m
C~ C~ C~
U um U C~
u ~ ~ u
c: - ' ~ o - l ~ o ~ ~ o ~ ~ o - ~ ~ o ~ ~ o ~
u ~ u x ~ ~ ~
~Y ~ u ~ ~ u u ~ u ~ u
CA 02227934 1998-02-23
OOSO/4~614~2
140
~ ~ a a ,~ u ~ ~ ~
~ ~ o ~ c~ o ~ ~ o _~ ~ o ~ ~ o -- ~ o r~ o ~ ~ o
o~
CA 02227934 1998-02-23
0050/Ç~6142
141
E3 E3 E3 E3 E3 E3 e3 E3 E3 E3 c~ E3 E3 E3 E3 c~ c~ E3 ~ E3 E3
c c c c c c ~c c :~ c ~: 3 c c c 3 c c c c c
B E3 E3
v~
._ 3 ~ 3~ 3c _ - - cr5 E~ ~3 c~ ~S c~ ~ ~ C'' E ~ ~ c~
:~ O cJ c ~ c ~ ~ O O O O O O O o o o o G O
O C c O t_ C, O ~ z z z z~ z z 7 '7 '~ '7 2. 7
;~ I E3 E, c, ~ ~ c, c53' cq c~ c~ c~ q c~ c~ cll c~ 3 c~ c~
:C 3: C C C ~ C C 3: :C 3: C C $ C 3: C C C C :C
5 E ) 3 3) c3~ ~ c) Ec E~ c~ cc cc Ec ~ E~ ~3 E3 ~ E3
:r 3c 3 3 :~ c :r: 3~ 3 c 3 3c c 3 3 3c 3 3c 3 3 3
- ~r 3 33 ~ C35 E~3 C~ E~ ~ C~ E~ C~ C3 C~ C3 E5 E5 E3 ~5
~ ~ ~ o ~ ~'I o -- ~ o ~ ~ o ~ ~ o --~ ~ o --I ~'I o
~ :C 3C 3C 3C 3C 3C 3C 3C :r: 3r 3 3C C C C 3 C 3C 3C 3r C
x
~ :C 3C 3C 3C 3r 3C C $ 3 :~ 3 3C :~: 3 ~ ,r 3C 3C 3r 3C 3C
PC :r 3 ~: 3 3C 3 3 3 3 3C 3 C C 3 C 3 :r $ 3C 3: 3C
CA 02227934 1998-02-23
OOSO/4~6142
142
i5 i5 i5 u i5 i5 15 v i5 i5 i3
m :~: m m :r: m
U U ~ U C~ U
~, x ~u~ U ,~U~ ,~m m ~N m mN
~S U ~ C~ u u u u U U u u U
o O ~ ~ ~ O o o O o o O o o o O o
3 ~5 i3
C _. ~ o _. ~ o _I ~ o _.
oo
_.
CA 02227934 1998-02-23
OOSO/46142
143
_1 ~C ~ X :: X X X :~ C C X X C X X X X ;C C X X
S ~ S ~ ~ = ~
~ S :C ~C C X C ~ C C X X S X X X C X 5C ~C C C C
p~ S ~C S ~ S ~
X :C S X X X X X C C :~ C X X C X X X X C X S
S S S S S
C o .~ ~ o _~ ~ o ~ ~ o _ ~ o _~ ~ o ~ ~ o _I t'~ o
~ C :C X X C C C X ~C ~C C C X X X C X X ~C S ~C C
tlo '
~C C C C X C C C X X X ~C ~C X X C C X C S ~C C
X :C C .C X C C S C ~C ~: C C S X X S C X C X C
o ~ t~ ~; ~ 00 ~ 88 ~ ~ ~"O ~' 'D ~~ ~ ~O ~~ 'D 8 ~ ~~ ~~
CA 02227934 1998-02-23
0050/4L61g2
144
~ U ~3 U ~ ~ U U U U U U U ~ U U ~ ~ U U U U U
_l X : X X :C: X X X X X X X X ~ X X X ~: X X X X
Z ~ o o~ ~ U
o o $ $ $ ~3 ~ 8 8 ~ x
x r x x x x x x ~ c x 3 x ~ c x ~ ~ x x x
x ~ X ~ X ~ X ~ X ~ ~ ~ U ~ 3: x ~
~ ~ Ir~ o -- ~ o _ ~ o ~ ~ o ~ o -- ~ o ~ ~ o _, ~
X X 1 ~ ~ X ~ :~ X ~ ~ C ~ X
-
~ ~ ~ ~ ~ ~ ~ I ~ ~ ~ ~ ~ ~ ~ $ ~ ~ ~ ,X ~ X ~
P~ 3 X ~ X ~
~ O O O O ~0 0 ~1 ~ ~ ~ ~ ~ ~~ ~ ~ O~ ~0 ~
CA 02227934 1998-02-23
OOSO/~16142
145
_
U U U U U ~ U U U ~ U U U O U O U U ~ U
O U ~
g O O O O O O O O O O O O O O
u~ u u ~u ~ ~ u ~
-
~ ~ $ ~: X $ 3~ ~ X 3~
~ ~ ~ u ~ u ~ u ~ ~ u ~u ~ ~
c o ~ ~ o - l ~ o - ~ ~ o ~ ~ o - ~ ~ o - ~ ~ o - ~ ~
o'
oo
~ ~ u ~ u ~ u ~ u ~
~ ~ x o~ o ~ ~ ~ ~ v~ ~o 1~ x cr~ ~o ~ ~ ~ ~
CA 02227934 1998-02-23
0050/4L6142
146
U U U U U ~ U U U U U
m m m m m m
m~ m" ~ ~ C~ U ~ U U ~c ~
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ Z Z Z Z Z
r~
~ O ~ ~ O ~ ~ O _I ~ O _.
O~
co
CA 02227934 1998-02-23
0050/415142
147
u ~i ~i u u ~ ~ u ~ ~i u ~i u ai ~ u ~ u
~ 5, 5, 5' ~ 5, ~ ~ X ~ 5, 5, 5' 5~ 5' 5' 5, 5. ~ 5, ~ ~
V~ V~ Vl
$~ S z ~ S~ ~~ 5 ~ ~ s?
U 5U ~i Ji ~
51 ~ ~ ~ ~ ~ 5, 5, ~ ~ ~ 5' 5, ~ 5, 5 5
~i U ~ ''i U ~ ~ ~ 5U
~
~i 5, 3: 3 3 5, 3 3 5, ~ 5, 3 ~ 5' 5, 51 5' 5' 5' 5' 3 5, 3
3 ~ ~3 U ~ U ~33 ~ ~ 5'j ~ 5' 3
~:: ~ C~ --I ~ O ~ ~ O _~ ~ O _~ ~ O _~ ~ O ~ ~ O _1 ~
~i X ~: ~ ~ 3 ~ 5, 5, ~ 5, 5, 5' 3~ 5, 3 5. 5, 5, ~ ~ 5, 3
~i 5. 3: X 5' 5, 3 ~ 5, 3 5, 5, 5, 5, 5, 5, 3 5, 5, 3 ~ 5
U ~33 ~ ~ 5U ~i ~ U
x c~ j ~ ~ ~ ~ ~ ~ x ~ o ~ ~ ~ ~r ~ ~ r~
Ci rl r~ ~ '~ r~ ~ ~ 'r~ ~ '~ 'rD 'r~ ~ r~ r~ r~ ~ ~
Z; ~r ~r ~r ~r ~ ~r ~r ~r ~r ~r ~r ~r ~ ~r ~r ~r ~r ~r ~r ~r ~r ~r
CA 02227934 1998-02-23
0050/4~6142
14~8
3 ~ ~ ~3 u ~ ~3 ~ 3 ~3 ~3 ~ 3 ~ 3 ~3 ~3
Z 'Z Z ~ U ~ o o
b ~ b ~ ~ ~ o o ~ ~ ~~ 8 8 8 8 8 8 8 8 8 ~ ~
~: 3 ~ u ~ ~ u u~ u u u ~ ~ 3
3 3 iu u ~ 3 ~ ~ 3 ~ u
C: o .~ ~ o ~ o ~ o ~ ~ o ~ ~ o . ~ o . ~ o ~
r ~ 3 c 1 ) ~) 3 ~ ~ 3 i~ ~ u ~ i3 i3
u 1~ u ~ 3 3 ~ i3 i3 ~ 3 ~ ~
~o x x ~; Y co oo x x ~ 8 o
CA 02227934 1998-02-23
0050/J~6142
149
U ~ V V ~ V ~ V V V V V ~ U V ~ V
~ 0~ 0~ ~ ~ 0~
O O O O O O O O ~ O " ~ ~ O O o o
~ ~ V V V C~
~ ~ ~5 ~ U U ~ E~ U ~ ~ ~
C ~ o _- ~ o _. ~ o _1 ~ O ~ ~ O _~ ~ O _~
X ~ X $ ~
O oo ~ ~ oo oo XO oo X X oo X ~~ oo X X X X oo
CA 02227934 1998-02-23
5O/~6142
~50
m m m m m m
U U~ U mN ~
o o ~ o o o O o ~ Y . ~ ~ Z Z Z Z Z
~ ~ o - ~ ~ o - ' ~ o - ~ ~ o ~ ~ o - l
CA 02227934 l998-02-23
OOSO/'~6142
151
~ ~ u u ~u c~ B v o c~
ggg~g~ 888~
~ ~ 2 ~ ~ ~ ~ 2 ~ ~ ~ ~ ~ ~ ~ ~ 2 ~ 2 ~ ~ ~ ~
C: ~ o _~ ~ o _ ~ o _- ~ o ~ ~ o _- ~ o ~ ~ o ~ ~
~ ~ I ~ ~ ~ ~ ~ ~ X ~ ~ ~ X ~ ~ ~ ~ ~ ~ ~ ~ ~
r-- oo O~ O ~ X o~ O ~ ~ ~ ~t v) ~o
0 00 ~ . X ~. ~, X
CA 02227934 l998-02-23
0050~4~6142
152
~ E~ ; C ~ 5 ~ ~ ~ C
X ~ 1 rCrC I C ~5rC rC 5C~C rC $ ~-5 rr5 ~rC ~r~ rC 1 5 r~ l ~
O ~ Y Y O ~ ~ O ~ ~~ Z ~ Z Z Z
~ O C 5 ~
P~ c 'r 15 C C C 5 C 5 C XC $ C C 5 5 C 5C C C C
5~ C
; ) C ~ ~ C C C C 5C C C C 55 5 55 5 5 C C C 5C C
-
P~ CIT' X C C5 C 5C 5 C C 5C $ 5 5C ~C C ~ C 5 $ 5C
PC gI C C C CE~ 5C ~ 5C
O rl O _~~JO _ t~J O ~ ~ O ~ I O _~ ~ O ~ ~ O ~
-
P~ 5 C 5 rC CC C 5 ~ ~ C 5 $ rC 5 $ 5 C 5 rC I~C C
0~ ~ ~
~ $ ~ C 5C C C 5C $ r5 rC rC ~ $ I C 5 C 5C C 5C C
1-- ~ ~
~1 ~ r5
C $ 5 ~; $ C 5C $ C $ 1 ~ ~ $ ~r~ r5 $ r5 r5 $
x o~ o ~ X
~ X X 00 ~ X X X X X 00 CO X X 00 X 00 W X 00 CO oo o~
~Z ~ ~
CA 02227934 1998-02-23
0050/46142
153
~2 ~
C~ U
~ 3 ~ u ~ u u
o ~ g ~ ~o~o ~
~ ~ ~ ~ ~ ~ X ~ ~ ~ ~ ~ 2 ~ ~
~ ~ o _~ ~ o ~ ~ o _~ ~ o ~ ~ o
oo
o~ o ~ ~ ~ ~ ~ o
~ I' X X oo oo ~ oo X oo X X o~
O CO oo X X X X X X C~ X X X X CO
CA 02227934 1998-02-23
0050/46142
154
$~ ~ ~ $~ ~ $ ~ ~ ~ $~ $~ $~ ~
$ $ $ ~ $ :~: $ ~ $ $ ~ :: :C X $ ~ $
~: m ~ m
o t~
~ N ~N
U = U U ~ g
'~ Z~ 5 Z~ o $ ~ b $ $ ~
$ $ $ $ $ $ $ $ $ ~: $ $ :~: $ :~ $ ~
$ ~ 1 $ $ ~ I $ $ $ ~ ~ $ ~ $
$ $ $ $ $ $ ~ $ $ ~ $ 3: $ $ 3: $ ~
~: u ~ ~ u ~ o u ~ u ~ u ~ u ~ u
~ - ~ ~ o - 4 ~ o ~ ~ o - ~ ~ o - ~ o ~ ~ o - ~
$ :~: $ $ ~ $ :~: $ $ $ $ $ ~ $ x ~:
oo
:~ $ ~: $ !r $ ~: $ $ $ $ :c $ :c $ $ :~: $ ::
~Y 5 X 3: ~ $ ~ ~ 3: $ ~ r $ x $ $ $ :: $
~ ~ ~ ~o ~ x ~' 8 --' ~ ~ ~o o ~ x g o _.
O ~ ~ ~ ~ O~ o O~
CA 02227934 1998-02-23
0050/46142
155
5~,,QQ~ a a
O ~ a ~ 5 0 0
S ~ ~ O O ~ ~ ~ SO
X ~ C X ~ S S
S ~ ~ ~ ~ ~ S ~ ~ ~ ~ ~ S
C: ~ o ~ ~ o _~ ~'1 o ~ ~ o ~ ~ o _ ~ o ~ o _- ~'I o
x
~ S ~ S S ~ :~: S ~ X
CA 02227934 l998-02-23
OOSO/'~61~2
156
0~ O O O ~ a ~ ~ ~
O O O O ~ ~ ~ Z Z Z Z Z Z ~ Z Z Z Z Z Z Z
~ ~ $ $ $ 3~ $ :r: $ ~: X $ ~ ~ $ $ X X ~
P~ $ ~ $ X ~ X $ ~ X X :: X ~ X :~: $ $ $ X :~:
X X $ ~; :: $ $ X ~: X ~ :r: $ ~ $ $ X
p~ ~ $ ~ $ X X 3: U C~ X X $ ~ X ~ X $ X
C ~ ~ o _~ ~ o ~ ~ o _~ ~ o ~ ~ o--~ o _ ~ o _
$ :: $ X x x ~ :~ $ ~ X X $ :r: $ $ $ $ $ $ $
00
x ~ $ $ $ x ~ $ $ ~ ~ X x $ x $ x $ $ x
X ~ $ $ X X $ X X X :~: X X ~ ~: X :~ $ ,~ $
~ ~ ~o r~ ~ o~ o ~ ~ ~ $ ~ ~ o _ ~ ~ ~ vl
CA 02227934 1998-02-23
0050/ 46 1g2
157
U ~ ~:
m U m ~, u u~
C.~ V
~, ~ m ~ m ~ m m ~N ~ m
O ~ ~ 'T ~ O O O O O ~ O O O ~ O q
~
~ u ~ ~ u ~ ~ u
~ ~ o - ~ ~ o - ~ ~ o ~ ~ o
o~
u ~ u ~ u ~ ~ ~
o ~ ~ ~ ~ ~
O ~ ~ ~ ~ ~ ~ ~ 9 ~ ~ ~
CA 02227934 l998-02-23
0050/46142
158
Z ~ Z ~ ~ Z Z Z Z Z Z Z Z ~ Z Z Z ~ Z Z Z Z
C _' ~ o _1 ~ O _~ ~ O _ ~ O _I ~ O ~ ~ O ~ ~ O _I
oo ~ ~ ~
1-- X O~ O _ ~ ~ ~ ~ ~O l-- 00 O~ O
CA 02227934 1998-02-23
0050/~6142
159
$ ~ ~ ~ ~ ~ ~ X $ ~ 3:
a 3 3 ~ ~ ~ o o $ $ $ - a
.
c: ~ o ~ ~ o - l ~ o - ~ ~ o ~ o - ~ ~ o ~ ~ o - l ~ o
X ~ ~ ~ ~ I ~ ~ 2 ~ ~ ~ ~ ~
x o~ oo o o ~ g ~ ~ o
CA 02227934 1998-02-23
0050/46142
160
3 N
N
C~ ~ o o o o o o o o o o o o o O ~ ~ 2 ~ ~
-
C -- t' o ~ ~ o ~ ~ o _~ ~ o ~ ~1 o ~ ~ o _ ~ o
U ~ ~ ~ U $ ~ U ~ U
O U ~ U ~ U U V ~ U U C~
o ~o o o~ o o o o o - o o o o o o o - o o o o
CA 02227934 1998-02-23
0050/~6142
161
U U ~ U U U
U = U ~ U U U c~ U U
U~ ~ ~ Z Z ~ ~
~ ~ ~ o - l ~ o - ~ ~ o - ' ~ o - '
: v v ~ ~ v v v v ~ v v ~ v
$ ~ v v v v ~ v $ v $
~ ~ ~ ~ ~ l-- x o~ o ~
~ 8 o 8 o o8 o o o o o o o o
CA 02227934 1998-02-23
0050/46142
162
c ~ o - ~ o ~ ~ o - <~ o ~ ~ o - l ~ o - ~ ~ o - ~ ~
o ~ 3 ;~; ~; 3 ~~ ~ o o o o~ ~ o~ o~ o~ Y~ o~ ~g o ;~; o~ Y~
CA 02227934 1998-02-23
0050/46142
163
~~ ~ ~
O O O O O
0 0 a a a 8 ~ ~ 8 ~ 8 8 8 ~
:; o - ~ ~ o ~ o - ~ ~ o ~ ~ o ~ ~ o - ~ ~ o - l ~ o - ~
~ $ - ~ $ 1: - $ $ ~
~ ~ ~ ~ ~o ~ o ~o o o o o ~o o ~o ~o ~o ~o o o o o
O ~
CA 02227934 1998-02-23
0050/~6142
164
~3 ~3 ~3 u u ~3 ~3 u u ~3 u u u ~3 u ~3
~1 ~ c c c c ~c c tc rc c c c c rc c c ~
C U U V~ ~ ~ N ~ ~
~ ~ r~ r~c~ ~ ~ m ~ m
r -~ ~ u~
G ~ E ) 3 ~3 u
G ~J O O O O O O G O rr ~ rc ~~ ~ ~ O ~ ~
z z z ~ Z
P~C C C ~ C C ~C C C C rc c rc ~ c c c
:C C ;C rc c c 1 rc ~T c c c c c c
E~ ~c E3 ~ Ec u E~ E~ E~ E3 Ec Ec u
g~ rc ~ Er3 rc u~ c Ec u E~ ~ c c c c rc
~ ~ c~ _ ~ O ~ ~ O _, ~ O ~ ~ O
O~
P:; rCrl rC ~ C ~C rC X rC ~ C rC rC tr,c ~C ~ C ~ ~C
-
P~ ~C,1:~C C rC rC ~C ~C rC ~C t C C rC ~C rC r~c
P:: C ~ rC rC C ~ C rC rC rC X C C rC ~ C rrc rC
o~ c~ o ~ ~ ~
o_, ,' O O O O O o 8 o O O O O O ~
CA 02227934 l998-02-23
0050/~6142
165
:c X X ~ ~ X ~: ~ X X X ~ X X !T~
m m ~,
~ N ~N ~
m m m m
=~
X $ ~ ~ 3 X X ~ X :: X ~
X $ X ~ X :C X ~: X ~: X ~ :~: X ~:
-~ ~ x ~ x
c o ~ ~ o - ~ ~ o - ~ ~ o - l ~ o - l ~ o - ~
~ :~ x ~: x x ~: x x x x 3: x ~ ~ :~: x ::
x
~ :C X :1: X ~ :~ :C X X ~: X X :~: X :~: X X
r-- oo ~ O ~ ~ ~ ~ x o~ o _
. o o o o o ~
CA 02227934 1998-02-23
0050/46142
166
U ~ U C~ ~ U ~ ~ V ~ V C~
~ z ~ ~~ a~ ~ ~ O a O
~: c c c c c c c c c ~ c :~: c
C :r C :C :~: C C X 3:C :~ C ~ C :1: C C ~: C :C
C ~ C ~ C ~
~ ~ C~ --~ ~ O _~ ~ O _1 ~ O ~ ~ O ~ ~ O ~ ~ O ~ O
Cl~
o~
1--
CA 02227934 1998-02-23
0050/~6142
167
5. ~ 5. 5. ~ 5' I ~ ~ ~ ~ ~ ~ ~ 5. ~ ~ ~ ~ 5. ~
C~ V
~) ~5)
,? ~ S C- ~ o o ~ o CS o o o ~ c
~ ~ ~ 8 8 8 8 8 8 5 i_ 8 ~
X ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 5' 5' 5 5.
5, ~ 5 ~ 5 S 5. S ~ 5. 5. 5. ~ S 5 5' 5. S, 5 5'
? j~ ~ x~ ~ V? ~ jJ? S V? ~ V? V V V j~? V? V j~? V 5' V
5? ~ ~ ~? 5? ~ ~ s? ~? ~? 5? ~
~ V ~ ~ V V V i~ ~ V V V V V V V V V V V V V V
C -- 1~ o _I ~ O _~ ~ o _~ ~ o ~ ~ o _1 ~ O _1 ~ O _~
~ ~ :~ ~ ~ S ~ 5 S. ~ .~ S. 5 ~ ~ ~ ~ 5' ~ 5 ~ 5 S
CD
_.
~ ~ ~ ~ ~ 5 ~ S 5' 5 5 5. S S S S' 5 5 S. ~ 5'
1' 5~ S :~: S? ~ V~ V? V? V? V V? V? V V v? v v? V? V i~J V
CA 02227934 1998-02-23
OOSO/4L6142
168
~ c~ (3 ~3 E3 ~3 ~3 E3 ~ t3 ~3 ~3 ~3
_1 c :~ c c ~ c c :~ c
O U C,) U
~ N ~ ~ ~ ~ N ~ N ~ N
U ~ U U U U
c E~
o ~j o o ~ ~? C ~ ~ o o ~ o ~ o o o
U ~ ~ U ~ U C~ ~
r C C C C C ~ C ~: X
C C C C X C C C ~ C
-' ~ 'C C ~ C ~ ~ ~ C ~ ~ :~
.1 !r 'C ~ ~ !r ~ ~ ~ ~ :~! !r C
C ~ C~ ~~ ~ o ~~ ~ o ~ ~ o ~-,
o~
~ :: :C $ :~: C C C C C :C C :~:
oo
~Y; ~ :C C ~ C ~: C C C 3:
~ ~ C~C C~ E~ ~ CC E~; E~ E~c E; ~
t' 00 CJ~ O ~ D t'
t-- t' t' t-- t' t' t' t'
CA 02227934 1998-02-23
0050/4:6142
169
O 0 , ~ . U t~ ~ Z Z Z Z Z Z Z Z Z Z Z Z Z
b ~ b
~ 3 ~ ~ !r X ~ ~ X
c ~ o ~ ~ o - ~ ~ o - ~ o - '
o~
CA 02227934 1998-02-23
0050/~6142
170
~ Z Z Z Z Z Z Z ~
b b b ~ ~ ~ o o ~ T
E~ u ~ C U ~ ~.~ U U EC C EC EC ~ U ~ U c ~ c, ~:
~; u ~C ~ EC E ) ~ EC ~, EC ~, U E~ EU EC ~, ~, c
C ~ C~ _ ~ O -- ~ o _~ ~ o ~ r~ o ~ o -- ~ o ~ ~ o
~ C :C C C C C C :: C $ C C C C C C C ~: C :: C C
c :c c c c c ~: c c c c c c C . c ~ E~ C
u Uc u EC u ~ E~ 3 ~ uc ~ C El EC ElC ~ i3 EC
o ~ ~ ~ ~ ~ o ~ o q oo C~ ~
CA 02227934 1998-02-23
-
OOSO/46142
171
~9 ~ C o ~ Z Z Z Z ~ ~ ~ ~ o ~ ~ o ~
_.
3 ~3 ~3 ~3 ~3 ~3 ~3 ~3 ~3 ~3 ~ ~3 ~3 ~3 ~3 ~3 ~ ~3 ~3 ~3
~3 ~3 ~3 ~3 ~ ~3 o ~ 3 ~ ~3 ~3 ~3 ~ ~3 ~3 ~3 ~ ~ ~3
~ . ('. o ~ rJ o ~ ~ o , ~ o . ~ o , ~ o ~ ~ o .
Yp, ~3 ~3 ~3 ~3 ~3 x) ~ ~3 ~3 ~ ~3 ~3 ~3 ~3 x~ ~3 ~3 ~ ~3 ~3 ~3
x ~3~ 3 o~ c~) x~ 3 ~3 i~3 ~3 ~3 ~3 ~3 x~ 3
r ~ ~ r- oo ~ o ~ r ~ ~o ~ oo o~ o
r ~r ~r ~r ~Ir ~r ~Ir ~r ~r ~r ~r ~r ~r ~r Ir ~r ~r ~r ~r ~r ~Ir ~Ir
CA 02227934 1998-02-23
OOSO/g6142
72
3 U
~5.3: 3 3~ 3 3 X 3333
oc ~c m PC oc m
U U U C,) U U
U U U C U U ~ U = ~ U
~ 1c1 ~ 3
3~U~ ~ ~ ~U~U~ ~
~33: 5. 3 3 3 ~ 3X33
~33: ~ 3 3 ~ 3 333
5'
~ ~ C~ ~ ~ o ~ ~ o ~ ~ o
5~ 3~ 3 3 5~ 3 3 3 ~ 3~ 3
r~ 5 ~ U U ~ U
U C ~ ~ U ~ U ~ U U U U
CA 02227934 1998-02-23
0050/46142
173
V V V
V~ V~ V~ ~ ~ 3 ~ O C ~
~ ~ ~ b b b b b ~, @ $ @i ~ @ ~ u @ ~ Z
$ $ $ ~: $ $ 5~ C $ $ X ~: $ $ :: $ $ $ $ ~C
$ ~ $ $ ~ C $ $ $ $ $ 5: $ ~ $ $ ~ $ $ $ ~ $
U ~ U U C~ ~ U ~i U ~
~ U ~ U ~ U ~ ~ U U ~ U ~
~ -- ~ o ~ ~ o _ ~ o ~ ~ o ~ ~ o ~ ~ o ~ ~ o ~
$ $ $ $ ~ $ $ $ 3: $ $ $ ~: ~ $ $ ~ $ $ ~: $
$ $
~ i U ~ U ~$i U$ U ~ U$ ~ '$ ~ @
-' $ $ $ $
~ U ~ ~ ~ U U$ U ~ ~ U$ ~ ~ U$ ~ @ u @i ~ ~ @ @ $
CA 02227934 l998-02-23
- O~SO/46142
174
_
U U U O U
~C C ~ C 21 2C 3 ~ 1
vl v~ o~
L ~ O O
P~ C C C C ~ 2C X 2 2
p~ 2C rC 2 ~C C ~C C 2C C
2] 3C X ~C !r~ 2~ 3C
) C ~ t ) U U U U O
U ~ 3~ C C
c; ~ cl --~ ~ O ~ ~ O
O~
P~ C 2' C 2 2C ~ ;C 1 ~
~ ~.) 3 U ~
U
oo o~ o
O ~
CA 02227934 1998-02-23
. 0050/~461g2
~ X $ X X :~ :C X X X X X X
_l X X X :~ Z X X Z X X X X
8 ~ ~ ~ 8 8 ~ ~ ~ 8 8 8
~. ~ ~
IY~
~-- , ~ ~ X X X X X X X Z X Z X X
~<
X X X X X Z X X X X X X
o~
~ ~ X X X X X X X Z X X X X
.~ o
a~ ~0 c o ~ o ~ o ~ o ~ o ~ o
~C
~ ~ :: X X X X X X Z X X X r:
o o
~ oo
~ X ~ x x x x x x x z x x r x
U
", ~ ~ X X X X X X ~ ~ V
h
O ~ ~ ~ ~ ~
CA 02227934 1998-02-23
0050/416142
76
S ,S ,S 'S ,S ,S ~ U O U ~ U ~ ~ ~ U
,S ,S :~ ,S :~: ,S ,S $ ~ ,S ,S ,S 1 S ~
~ 8 ~ 8 8 Oa 8 ~ ~ 8 8 ~ 8 ~ 8 8 8 8 ~
s ,s ,s ,s ~ 'S 'S ,s ,s ~C ,s ,: ~ ,s ,s ,s 'S ,s ,s
s ,s ~ ,s ,s ~: ,s ,s ,s 'S X ,s ,s :: 'S ,s 'S X
~; ,s :S ~ 'S :C ,s $ ~ s S ,s ~ ,s s 'S ~ :S ~ ~
c o ,~ o ~ o ~ o ~ o ~ o ~ o ~ o ~ o ~ o
o~
S :r ,S ,S ,S ,S ~ ,S ,S ,S 'S :~ 'S 'S 'S ,S ,S 'S 3:
) O U ~ ~) ,S :I: X ,S ,S ~ ,S 'S :~: ,S ~
,S :r ,S 'S ,S S U U ~ ~ S ~
CA 02227934 l998-02-23
0050t46142
177
~ .~, ~ .,.
~3C:C33C3~C3C3C3C3C3C33C3C3C3C3C3C3C3C
~ ~ ~. 8 8 ~ ~ u ~ 8 8 u ~ ~ ~ 8 8 ~
~3C~:3C3C3~3CC3~3C3~33~33C33C
~3~:33C33C3C3C33C3C3C3C3C3C3C3C33CC
~3C3:~C~C3C3~C3C3~3C33C3C3C~C3C33~C
~: o ~'~ o ~ o ~ o ~ o ~ o ~ o ~ o ~ o ~ o ~
~3C3:~33C3C3C3C3C33C33C3C3C3CC3C3$
~3'~3~3?'~ 3'~
~;~3C3C33C33C3C33C3C~C~C~
3'3.;C ' ~ ~3.3'~
X ~C 3C 3C 3C 3C
v~ o ~ v~ X O~ O --
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 't ~ ~ ~ V~ ~ V~
:Z v; vi vi vi vi l~i vi v; vi vi vi vi vi ~ v; vi vi ~n vi vi
CA 02227934 l998-02-23
OOSO/~6142
78
C :C
.~ !._
;~ 8
P~ ~ r
~: c :c
c o
~Y c
P~ ~33 ~3
t' ~ ~
~ ~3 ~3
~ '~
~ U? u~
Z U~ Ui
CA 02227934 1998-02-23
0050/~6142
179
_1 v ~ u v ~ r v 5~ V ~X~ V V I
~ X ~ X :c X X X I :S X :~: X :~
~ v ~ ~ a ~ ~ 8 ~ ~
x x ~ x x x ~ x ~ ~
~ ~\ /) ~
~ ~ ~ ~ x $ ~ ~ x ~ x ~ x ~ x ~
,~0
~ ~ ~ x :~ x ~ x :r x
~,~
~ ~ Y x x s
o
~ c O ~ O - ~ O ~ ~ O , ~ O -
J
o~
~ ~; x ~ x x x x
tn
~, X ~ X X ~ X ~: X
C) ,_
~- ~ X X 2 ~ ~ ~ ~ ~ ~ X
~D
a
CA 02227934 l998-02-23
0/46142
180
~ ~ ~ a O ~ ~ ~ D
Z ~ U ~
.~.
~ o --~ ~ o --l ~ o --~ ~ o ~ ~ o --~ ~ o --l
CA 02227934 1998-02-23
0050/46142
181
_l V C~ V ~ ~ V V ~ ~ V V
U
X U ~ ~ ~ U
~ 1 1 1 ~ O ~ V ~ Z =
'~~ Z ~ V ~ ~ bvbv
~ o --' ~ o ~ ~ o --~ ~ o ~ ~ o
co
O ~ ~ v~ ~o ~ ,." $ ~ ~
CA 02227934 1998-02-23
OOSO/46~.42
182
V ~ ~ $ ~ V~
:~ ~ V~
Z ~ Z ~ Z ~ Z
U ~ ~ V ~ V V V V V
5 ~ ~ ~ ~ ~ ~ 5 5 ~ ~ 5 5 ~ $ 5
~ $ 5' ~ ~ ~ 5 X 5 ~ ~ 5 ~ $ ~ ~ ~ ~
5. ~ ~ 5 ~ ~ 5 ~ ~ 5 ~ ~ ~
5 ~ X ~ 5' ~ ~ 5 ~
C ~ ~1 0 _~ ~ O ~ ~ O ~ ~ O _~ ~ O _~ ~ O ~ ~ O ~
~ ~ 5: 5 ~ 5 ~ X 5 5 ~ ~ 5 ~ ~ ~ ~
00
X 5 ~ ~ ~ $ ~ ~ ~ $ X ~ ~ x x
x ~ ~ :: x
O ~ o~ ~. o ~
CA 02227934 l998-02-23
0050/46142
183
r~ ~ 5~ 5 ~ 5
5 r ~ C ~ ~ 3~ $ 5 5 C C 5 ~ ~ !r 1 5
0~ O O O ~ O ~
~ ~ o o o $ 3 3 ~ 8 8 ~ 8 ~ 8 8 ~
C C C C C C C C C C C C C C C 5C C C C C C
~: C :C C 5C C C 5C C X C C C 5C C C C 5C C C C C 5C
:C 5C 5C C C 5C C C C C 5C 5C ~: C 5C 5C C C C C 5C
C :C 5 C C C C C C C C ~ 5C ~: C C 5C C C C S ~
~ ,.~ c~ _ ~ o _ ~ o _, ~ o ~ ~ o _ ~ o --~ ~ o _I
O~
X C :C C C C C C 5C 5C C C 5C C C C C C C C C 5C C
:C 5C C C ~: C C C C C 5C 5C C C C X C C C C 5C
~: 5C :C C C o ~ ~ ~ C ~ C ~ 5C ~ tc 5~ ~3
O ~ t~ t' t' ~ ~ t' t' t' t' t~ '3 ~ c~ c~ c~ ~ x x ~ '~ c~~
CA 02227934 1998-02-23
0050/46142
84
U U U
O O O O G O O G O ~ ~ ~ O O o o o o
X ~ X X ~ 3~
o --l ~ o --'
o~
-
o~ o o o O O
CA 02227934 1998-02-23
0050/46142
185
O ~ U U
5 ~ 5~ ~ 5
1 1 1 1 1 ~ Z Z ~ ~ ~
o --~ ~ o ~ ~ o ~ ~ o --l ~ o --~ ~ o
o~
~ ~ $ ~ c x ~ x ~
~: ~ v v ~ v v v v v v ~ v v v v v
O ~ ~ ~ ~ _1 ~ _1 ~ ~ ~ 1-- 00 O~ ~0 ~
CA 02227934 l998-02-23
0050/4:6142
186
~q~g5q~g5qg~5c5cg~guuu5q
~5q;C5C5C5q5C5~5C5C5C5C5C5C5q5C5C5CX;C5C5C5C
5C5C
5C~ ~c 5q~
Z ~. ~ Z Z Z ~ ~ Z~ ~ Z,~ C~ 'J C,' O O
.~.
~C5C5C5C5q5C5C5C5.5C5q5~5C5C5~5C5C5C5C~C~CX
~C~:~X~CXXXXX~C~CX3XC~CX~C~C~CX
~C~:~CXX2C~CX~C~C3XX33X~C~C~C3~C~C
~C3:X~CXXXXX~C~CXX~CXXX~C~C~C~C
C ~ t I o _ ~ o ~ ~ o ~ ~ o ~ ~ o --I ~ o --4 ~ o
o.
~33:3XXXXX$3C33C~33C3CXX3C~C3CX
X 3: X X 3C 3C 3C 3C X 3C 3 X X X 3C 3C X X 3C ~C ~C ~C
g~ ~ gC ~ g gC gC g g U 3 gC ~C gC gC u~ g g 3C g~ 3C g
~ ~ ~ ~ ~ X Cr o ~ ~ ~ ~ o ~ ~ ~
CA 02227934 1998-02-23
0050/4~6142
187
X~ X~ ~ ~ V ~ X 51~ X~ ~
X ;r ~ X X ~ 'r X !r X ~ X X 5 X X ~ ~ X X X
r I ~ X ~ 5N ~ t~, tL~
~ ~ t t O O O O O G O O O
3 ~o ~ o ~ ~ c o ~ 5 ~
t~ ~ ~r X X X ~ X X X X X 5 X ~ X X X ~ X X X X
~ ~ :r X X X X X X X X X X X X X ~ X ~ X X X X
X X :~ ~ X ~ X I 5 ~ ~ X X ~ X ~ X X X X !r X X
5 ~r ~ !r ~ X 5 X ~ ~ ~ ~ ~ ~ ~ 5 5
c ~ ~ _ ~ o _ ~ o ~ ~ o ~ ~ o _~ ~ o _~ ~ o
o~
~ X :~ X X X X X X X X X X ~ X X ~ X X X 5 X
t~ X ~ U
X ~ ~ U ~ U
c~ o ~ o 1-- x o~ ~ ~ ~0
O ~ ~ ~ ~ o ~ ~o ~o
Z; ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o ~o
CA 02227934 l998-02-23
0050/46142
188
~ t~ ~ tl t~ ? tr ~ t~
E ~ 2 tr t~ tt~ t~ tr tl t~ tr tT~ t~ t
U C~
t ~ m~ ~ m~ ~m m~ m m m
O O O O O O t~\ t ~ O O O O O O O O
Z Z i! Z Z a~
C tr ~ ~ ~ tT~
r ~ ~ !r
:r X
c o .~ ~ o _~ ~ o _ ~ o _~ ~ o
-
X ~ r
~ r~ ~ ~ o
O ,~ ,~ ~ ~ ~ ~ r~
CA 02227934 1998-02-23
ooso/~a6l~2
189
U ~ U
c) u C~ m
~ u u ~ g
o o O o . ~ . u v z z z Z Z z Z Z Z Z
'~ v v ~ b ~ v v v v v
~ X :CI X ~: X ~ :~ X ~ C X ~ X X
P: X ~: X ~ X ~ I ~ X ~ X X X
X ~X ~ 3: X :~: X ~: X 3~ X :C
X :~X ~ X ~: X X :C 1 X ~ X 3: ::
C ~ ~ o , ~ o ~ ~ o ~ ~ o ~ ~ o , ~ o
~: X :r:X X 3: X x X X :~ ~ X X ~ C X X
X X ~ X :C X 5: ~ ~ X
V V U V V V V ~ V ~V V V ~ U ~ V
V ~ X~ ~ ~X ~ ~ ~X~ V
CA 02227934 1998-02-23
0050/4~6142
190
.
oo
Z Z Z Z Z Z; Z Z , ~. ;Z Z Z Z ; ~ ~ o o
U ~o o o ~ ~
~ ~ ,~ ~ ~ ~ ~ ~ X ~ ~ ~ ~ 2 ~ ~ ~ ~ ~ ~ ~ ~ ~
C _ rJ o _~ ~ o _1 ~ o _~ ~ o _.
o~
O ~ ~ ~ ~o ~ ~ ~ ~ ~ ~ ~ ~ ~ N ~
CA 02227934 1998-02-23
OOSO/4~6142
191
,~ ~3 ~3 ~3 ~ ~3 c~ ~ o (3 o ~3 ~ E3 ~ ~3 B c~ ~3 ~3 ~ ~3
~ tC ~ t~C C C tC tC ~ tC tC ~ '1 ~ 1: C tC ~ ~ ~ :C ~ 1~
c~ ~3
~ ~3
O ~ g ~ o ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ O O O o
3 E~ ~ ~ 8 8 8 ~
~ c r x c c c c c c c c c c c c c c c :~: tC tC rtC
~ ;C :C tC C C tC C C C tC C C C tC tC C tC tC tlC C tC C
O tC :C C C tC tC tC ~1 tC tC ~ ~ tC lC C C ~ $ tC tC tC tC
C :C ~ tC tC tC tC ~ ~ ~ C ~ $ tC tC ~ ~ ~ tC tC tC tC tC
C ~'1 C~ --I ~ O _4 ~ O --~ t"l O --I ~ O _~ ~ O --~ t~ O _~
O~
X C ~ C tC tC tC C ~S ;C tC ~ C tC ;C tC C ~ C ;C C tC C
1-0
I~C ;C :1: C tC tC tC X ~ ;C tC tC tC C X C ~ tC tC tC C tC X
CC tC ~ X ~C X X X 3 X X tC 5 X X C X C tC X X X X
C~ C --~ ~ ~ 'O 1' O' O --~ t'l ~ ~ ~ 'O 1-- OD CJ~
CA 02227934 1998-02-23
0050/~6142
192
3 ~3 c~ ~3 ~3 c~ ~3 ~3 ~3 ~3
C :C ~C ~ 2 3 3 3 3 ~C ~
~ U U U
~ ~,' c~ u u U ~ m u U 5~ U u
C C, CC ~
O ~3 O ~ O O O o O O O O O O
S '~ U U U
3 :C ~ ~C ~ 3 X ~C ~ ~ C
3 ~: ~ ~ 3 X ~ 3 C 3
~C ~: 3 C 3 3 ~ C 3 ~ C
-
~ 3 ~: 3 3 ~ 3 3 ~ 3 3
1: o _~ ~ o _~ ~ o_ ~ o ~
X 3 ~: 3C ~ 3 X 3 3 3 3 3C
3C 3: 3 3 3 3 ~ 3 3 ~ 3
3 3: 3C 3 3 3C 3 C 3
CA 02227934 1998-02-23
0050/~L6142
193
t~ U ~ 3 U U U V U U U U U C.) U ~i U U
tC tC tC tC ~tC tC X tr tC C tC tC tC tC tC tC tC tC tC ~C
~ C~
t~
tC ~C tC tC U ~ S t
o ~ u z z Z I I I c ~c c c
Y tC tC tC tC1 ~ C tC tC tC tC tC tC ~tC C tC tC tC tC C tC
P~ ~ C.~C ~ C CtC tC tC C tC tC ~ C . C tC tC tC ~C tC ~C tC tC
tC CtCtC tC tCC C X tC tC tC tC tC tl tC ~ C C X
~ C :1: C C C ~C ;C C C C C C C C C C C C C
C ~ O --~ ~O --~~ O ~ ~ O ~ ~ O ~ ~ O _~ ~ O
X C C C C ~ CC C C 3: C C C C ~: C C C X C
~ ~C C C C C CC C C C C C ' C T C C C C C
~ C C ~ C C ~ ~ C C C rC C C C C C C C C C
O ~ ~ ~ ~ ~ ~ ~y
CA 02227934 1998-02-23
OOSO/46142
194
i Ei Ei ~ ~.'i Ei o C~ Ei c~ ~ E'i Ei Ei Ei o Ei
~ ~ ~ a~ a ~ ~ a' ~ ~ O ~ ~
Ei ~ ~ o o o ~ ~ ~ 8 8
~ ~ o --~ ~ o ~ ~ o -- ~ o ~ ~ o --~ ~ o ~ ~ o ~
o~
-
~ $ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 2 ~ ~ ~ ~ ~ ~ ~ ~ ~
~ c ~ i E~ Ei ~ Ei
~ v~ x ~ o ~ ~ ~ ~; ~ ~ r-- oo 0~ 0 ~ ~ ~
CA 02227934 1998-02-23
0050/g6142
195
jUU~jU~ UU~ UOOU~
333~3~3~33X3333~33
3~3"3~3~ 3~ X~t'~ a 3 ~ a a
U ~ ) t' t ~t4 o o o o o o o o o o o o O O o
OC'OOO~-OZZZZZZZZZZZZZZZ
t X ~ C X ~
X~l:~X~X~3~333
~ 3 ~: 3 ~ ~ 3 ~ 3 ~ ~ 3 ~ ~ 333 ~ 3 ~ 333
t ~ 5~ ~ 5' 33333 X I 33 ~ 3 ~ ~ ~ 333
C ~ Cl _ ~ o _. ~ o _ ~ o _ ~ o _ ~ o _ ~ o ~ ~1
o~
~ I 3' 33 ~ 3333333 ~ 33 X ~ 333 X 3
o~
t~ ~ 333 ~ 333 ~ 33 ~ 3 ~ 3 ~ 3333 ~ 3
U U ~ U U ~ U U U U ~ U ~ 3
~ 8 ~ ~ ~ o U, o ,~ ~ o~ o _ ~ ~ ~ ~
~ r~ ~' ~' ~ ~~ ~~ ~~ ~ ~~ ~ ~ ~ ~
CA 02227934 1998-02-23
0050/46142
196
x x x X x x x :~ 3:
D: m m m ~ m
~ ~ ~ ~ N N ~N
"~ ~~ c"~ ~~ c ~ ~~ ~ c_~ ~~ ~~ ~ ~~ ~~ c ~ ~~ ~z "
X X ~ X ~ X X 1 ~ ~ X
$ x 3 x x ~ ~ :r:
X ~ X X X ~ X ~ X X
X :r X X X :r; X :1: X X ~
Co ~~ ~ o _I ~ o ~ ~ o ~
~:X :r X X X ~: ~ X X X X
Y X :~ X X X X X ~ X 3:
O ~ r --~ r ~ ~ ~ r.~
CA 02227934 1998-02-23
OOSO/46142
197
_~ u ~ u u B u u u u u u o u ~ u u u u u u u u
q CS ~ ~ Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
U U ~3 U ~
C C'J ~ ~ ~ O ~ ~ O _~ ~ O _I ~ O _I ~ O _1 ~ O ~ ~
CA 02227934 1998-02-23
0050/~6142
198
C C C C C C C C ~C C C C C C ~ C ,rC lr,c :C rr C C
C rC rC rC r
z ~ z ~3 ~c rc ~ ~ ~ o o o c~ 8 8 8 8 8 8
~ C :C ~ C C C C C C C C C C C C C I C C C C C
C C C C C r C C C C C ~C ~ ~C ~C ~C ~C C rc ~c ~c ~c
rC C rc C rC rc ~ rc rc ~rC $ ~rc trc rc rc rc c ~c ~c rc ~ c ~ c
:C rc rc C rC rc rc rc rc ~rc lrc rc rc ~c ~c ~c rc rc rc ~c
c O ~ l O -- ~J O ~ O -- ~ O _l ~ O ~ I O --~ ~ O
~ C ~: C C C C C C C C C C C C ~ C C C C C C, C
~ C ~ C C C C C C C C C C ~ U ~ ~ U
C E ~ C ~ g ~ C C~ C C ~ ~ C ~ , c c
~ ~ C~ ? ~ ~ ? ? ~ ~ ~ ? ~? ~ ? ~ ~ ? ~ ? ~ ~. ~?
CA 02227934 1998-02-23
0050/4~S142
199
5. ,~ ~ 5. 5. ~ 5. 5 ~ ~ 5 ~ ~ 5 $ 5. 5. ~ 5
U
C~
5 $ 5 ~ ~ ~ -~
~- O O O O O O O O O G O G O O O ~ ~ ~ ~
~ X ~ ~ 5 ~ 5 ~ ~ ~ ~ 5 ~ ~ 5555.5555
X 5 ~ ~ 55 ~ 55 ~ ~ ~ 555555.55' ~ 5
55 ~ ~ 5 X 5 ~ ~ 5555 ~ 55
5. ~ ~ 5 ~ ~ ~ ~ 5. ~ ~ ~ 55 ~ ~ $ 5
C _ ~ o _I ~ o ~ ~ o ~ ~ o ~ ~ o --' ~ o _I ~ o
o~
~ ~ 55~ ~ ~ 5555 ~ 55 ~ 5 ~ ~ 5 ~ ~ 55
X ~ 5~' ~ 5? ~ 5
~ ~ x~ oo ~ ~ ~ ~ X o
CA 02227934 1998-02-23
0050/'~6142
200
S ~ ,~ S S S ~: $ S S
$ ~ m 5: ~: m
U U U U U U
U U U U C~ ~ S ~ ~ ~
I I I o~ Z ~ ~ Z
X S S :~ ~ ~ S S S
X ~ S :~ S ~ S S S $ 3:
~: S S S S S S ~
S 1 S ~ S S ~r
C ~ ~ O _~ ~ O _~ ~ o _ ~ O
O~
S S ~ :~: S :: S S :C S
S S ~:
~: S S S
~n ~o 1-- X ~ O
O O O
CA 02227934 1998-02-23
0050/'~614Z
201
_1 u i3 Ej i3 u i;~ i5 u i~ i3 i;~ v iJ u ~3 u u
x: x c c c c c ~ x x c c x c c c c c c c 2
Z .. ~ Z ~ ~ Z Z Z = Z~ Z Z Z Z Z Z Z Z Z
u ~ b ~C b ~ u ~ X X ~ u u
~ X :C ~ C C C C C C C C C C C X C C C X X C C
X C :C X C C C C X C C X C C C C ~ C X C C C X
X :C ~ X X C X C X X X $ X X X X C ~: C X X X
X X C C C X C C C C C C C X :C C C X X C X
C . ~ o ~ ~ o ~ o ~ ~ o ~ ~ o ~ ~ o ~4 ~ o
o
~ C ~: X X X X C C X X X :r: X X c c x X X x C x
3 g u j~ C j~J j~J ~ X X C U ~ 3 u u u u
U ~ jC ~ ~ ~ X X ~ ~ X X X X X 3 ~ x ~ c
. ~ y~ O ~ 0~ ~ ~ ~ ~ ~ ~ ~ ~0 ~ ~
~ ~r ~ ~t ~ ~ ~ ~ ~ ~ ~ ~t ~ ~ ~ ~ ~ ~ ~ ~t
CA 02227934 1998-02-23
0050/~6142
202
_~ ~3 ~3 ~3 ~3 ~3 ~3 ~
ooo
3:
X :C
X ~
C: ~ C~ ~ ~ ~ o ~
P ~ E3 E3 $ E3 ~ ~
r 3 ~ ' ~ $ 2: $ $
~ U ~
o _~
O ~ ~t ~ ~ ~ ~
CA 02227934 1998-02-23
0050/46142
203
Table 7: Compounds of the formula
OH O M (~)n
R17 ~ N
Rl9 L
The rad.icals R7 and R23 form a bond.
No. :R17 R18 Rl9 n y L M
7.1 ~H H H 2 COCH3 H H
15 7.2 ]H H H 2 COC2Hs H H
7.3 CH3 H H 2 COCH3 H H
7 4 C 'H3 H H 2 COC2H5 H H
7.5 CH3 CH3 H 2 COCH3 H H
7.6 ~'H3 CH3 H 2 COC2Hs H H
7.7 IH H H 2 COCH3 H CH3
7.8 lH H H 2 COC2H5 H CH3
7.9 CH3 H H 2 COCH3 H CH3
7.10 ~-'H3 H H 2 COC2Hs H CH3
7.11 I'H3 CH3 H 2 COCH3 H CH3
7.12 ~H3 CH3 H 2 COC2Hs H CH3
7.13 1{ H H 2 COCH3 H Cl
7.14 II H H 2 COC2Hs H Cl
7.15 ('H3 H H 2 COCH3 H Cl
7.16 ('H3 H H 2 COC2H5 H Cl
7.17 ('H3 CH3 H 2 COCH3 H Cl
7.18 ~H3 CH3 H 2 COC2Hs H Cl
7.19 El H H 2 COCH3 H NO2
7.20 E~ H H 2 COC2Hs H NO2
7.21 (~H3 H H 2 COCH3 H NO2
7.22 (~H3 H H 2 COC2H5 H NO2
7.23 ('H3 CH3 H 2 COCH3 H NO2
7.24 ('H3 CH3 H 2 COC2Hs H NO2
7.25 El H H 2 COCH3 H SO2CH3
7.26 El H H 2 COC2H5 H SO2CH3
7.27 C'H3 H H 2 COCH3 H SO2CH3
7.28 C~H3 H H 2 COC2Hs H SO2CH3
7.29 C'H3 CH3 H 2 COCH3 H SO2CH3
7.30 ~'H3 CH3 H 2 COC2H5 H SO2CH3
7.31 El H H 2 COCH3 CH3 CH3
CA 02227934 l998-02-23
0050~46142
204
No. R17 R18 Rl9 n y L M
7.32 H H H 2 COC2Hs CH3 CH3
7.33 CH3 H H 2 COCH3 CH3 CH3
7.34 CH3 H H 2 COC2H5 CH3 CH3
7.3~ CH3 CH3 H 2 COCH3 CH3 CH3
7.36 CH3 CH3 H 2 COC2Hs CH3 CH3
7.37 H H H 2 COCH3 Cl Cl
7.38 H H H 2 COC2H5 Cl Cl
10 7.39 CH3 H H 2 COCH3 Cl Cl
7.40 CH3 H H 2 COC2H5 Cl Cl
7.41 CH3 CH3 H 2 COCH3 Cl Cl
7.42 CH3 CH3 H 2 COC2Hs Cl Cl
CA 02227934 1998-02-23
0050/4~6142
205
Table ,B: Compounds of the formula
OH O M (o)~
R17~N
R18 L
Rl~
No. R17 R18 R19 n y M L
8.1 H H H 2 COCH3 H CH3
8.2 H H H 2 COC2H5 H CH3
8.3 CH3 H H 2 COCH3 H CH3
8.4 CH3 H H 2 COC2H5 H CH3
8.5 CH3 CH3 H 2 COCH3 H CH3
8.6 CH3 CH3 H 2 COC2H5 H CH3
8.7 H H H 2 COCH3 H Cl
8.8 H H H 2 COC2H5 H Cl
8.9 CH3 H H 2 COCH3 H Cl
8.10 CH3 H H 2 COC2H5 H Cl
8.11 CH3 CH3 H 2 COCH3 H Cl
8.12 CH3 CH3 H 2 COC2H5 H Cl
8.13 H H H 2 COCH3 H NO2
8.14 H H H 2 COC2H5 H NO2
8.15 CH3 H H 2 COCH3 H NO2
8.16 CH3 H H 2 COC2Hs H NO2
8.17 CH3 CH3 H 2 COCH3 H NO2
8.18 CH3 CH3 H 2 COC2H5 H NO2
8.19 H H H 2 COCH3 H SO2CH3
8.20 H H H 2 COC2H5 H SO2CH3
8.21 CH3 H H 2 COCH3 H SO2CH3
8.22 CH3 H H 2 COC2H5 H SO2CH3
8.23 CH3 CH3 H 2 COCH3 H SO2CH3
8.24 CH3 CH3 H 2 COC2H5 H SO2CH~
8.25 H H H 2 COCH3 CH3 CH3
8.26 H H H 2 COC2H5 CH3 CH3
8.27 CH3 H H 2 COCH3 CH3 CH3
8.28 CH3 H H 2 COC2H5 CH3 CH3
8.29 CH3 CH3 H 2 COCH3 CH3 CH3
8.30 CH3 CH3 H 2 COC2H5 CH3 CH3
8.31 H H H 2 COCH3 Cl Cl
8.32 H H H 2 COC2H5 Cl Cl
CA 02227934 1998-02-23
0050/461 a2
206
No. Rl 7 R18 R19 n y M L
8.33 CH3 H H 2 COCH3 Cl Cl
8.34 CH3 H H 2 COC2H5 Cl Cl
8.35 CH3 CH3 H 2 COCH3 Cl Cl
8.36 CH3 CH3 H 2 COC2H5 Cl Cl
CA 02227934 1998 - 02 - 23
0050/415142
207
Table 9: Compounds of the formula
Rl9 L
No. RIs Rl6 R19 n X Y M L
9.1 ~ H3 CH3 H 2 CH2 C=O H CH3
9.2 I~H3 CH3 H 0 (cH2)2 C=O H CH3
9 3 I~H3 CH3 H 2 (CH2)2 C=O H CH3
9.4 C H3 CH3 H 2 CH2 CHOCH3 H CH3
9.5 ICH3 CH3 H 0 (cH2)2 CHOCH3 H CH3
9.6 ~ H3 CH3 H 2 (CH2k CHOCH3 H CH3
9.7 ~CH3 CH3 H 2 CH2 CHOC2Hs H CH3
9.8 CH3 CH3 H 0 (CH2)2 CHOC2Hs H CH3
9.9 lCH3 CH3 H 2 (CH2)2 CHOC2Hs H CH3
9.10 ICH3 CH3 H 2 CH2 CHOiPr H CH3
9.11 ICH3 CH3 H 0 (CH2)2 CHOiPr H CH3
9.12 ICH3 CH3 H 2 (CH2)2 CHOiPr H CH3
g.l3 I~H3 CH3 H 2 CH2 C=NOCH3 H CH3
9.14 I~H3 CH3 H 2 (CH2)2 C=NOCH3 H CH3
9.1S I~H3 CH3 H 0 (CH2k C=NOCH3 H CH3
9.16 I~H3 CH3 H 2 CH2 C=NOC2Hs H CH3
9.17 I-H3 CH3 H 2 (CH2k C=NOC2Hs H CH3
9.18 I-H3 CH3 H 0 (cH2)2 c=Noc2Hs H CH3
9.19 I~H3 CH3 H 2 CH2 C=NOiPr H CH3
9.20 I~H3 CH3 H 2 (cH2)2 C=NOiPr H CH3
9.21 CH3 CH3 H 0 (cH2)2 C=NOiPr H CH3
9.22 I~H3 CH3 H 2 CH2 C=NOCH2CH=CHCI H CH3
9.23 ~-'H3 CH3 H 2 (cH2)2 C=NOCH2CH=CHCI H CH3
9.24 I~H3 CH3 H 0 (CH2)2 C=NOCH2CH=CHCI H CH3
9.25 ~r~H3 CH3 H 2 CH2 C=NOCH2C6HS H CH3
9.26 ~ H3 CH3 H 2 (CH2)2 c=NocH2c6Hs H CH3
9.27 ~ H3 CH3 H 0 (CH2)2 C=NOCH2C6Hs H CH3
9.28 ~ H3 CH3 H 2 CH2 C(CH3k H CH3
9.29 ~ H3 CH3 H 2 (cH2)2 C(CH3k H CH3
9.30 ' H3 CH3 H 0 (CH2)2 C(CH3)2 H CH3
CA 02227934 1998-02-23
0050/g,6142
.
208
No. Rls Rl6 Rl9 n X y M L
9.31 C'H3 CH3 H 2 CH2 ~0--CH2~ H CH3
C CH2
\ o- CH2
9.32 I~H3 CH3 H 2 (cH2)2 ~0--CH2~ H CH3
C CH2
\ 0 CH2
9.33 CH3 CH3 H 0 (CH2)2 ~0--CH2~ H CH3
C CH2
\ O--CH2/
9.34 CH3 CH3 H 2 CH2 / ~--CH~< CH H CH3
\ 0--CH2 CH3
9-35 I ~H3 CH3 H 2 (cH2)2 / ~--CH~< CH H CH3
\ O--CH2 CH3
9.36 ~'H3 CH3 H 0 (cH2)2 ~ ~--CH~<CH H CH3
\ 0 - CH2 CH3
9.37 CH3 CH3 H 2 CH2 CH-NHCH3 H CH3
9.38 ~'H3 CH3 H 2 (CH2)2 CH-NHCH3 H CH3
9.39 CH3 CH3 H 0 (cH2)2 CH-NHCH3 H CH3
9.40 I~H3 CH3 H 2 CH2 CH-NHC2H5 H CH3
9.41 ~H3 CH3 H 2 (cH2)2 CH-NHC2H5 H CH3
9.42 ~H3 CH3 H 0 (cH2)2 CH-NHC2H5 H CH3
9.43 ('H3 CH3 H 2 CH2 CH-N(CH3)2 H CH3
9.44 CH3 CH3 H 2 (cH2)2 CH-N(CH3)2 H CH3
9.45 (~H3 CH3 H 0 (cH2)2 CH-N(CH3k H CH3
9.46 ~H3 CH3 H 2 CH2 CH-NHOC2H5 H CH3
9.47 ('H3 CH3 H 2 (cH2)2 CH-NHOc2Hs H CH3
9.48 ~H3 CH3 H 0 (CH2)2 CH-NHOC2Hs H CH3
9.49 ('H3 CH3 H 2 CH2 CH-N(CH3)0C2H5 H CH3
3 5 9 50 ('H3 CH3 H 2 (cH2)2 CH-N(CH3)0C2H5 H CH3
9.51 (~H3 CH3 H 0 (CH2)2 CH-N(CH3)0C2H5 H CH3
9.52 ('H3 CH3 H 2 CH2 C=N-NH2 H CH3
9.53 (,H3 CH3 H 2 (CH2)2 C=N-NH2 H CH3
9.54 ('H3 CH3 H 0 (cH2)2 C=N-NH2 H CH3
9 55 t'H3 CH3 H 2 CH2 C=N-N(CH3k H CH3
9.56 ('H3 CH3 H 2 (cH2)2 C=N-N(CH3)2 H CH3
9.57 CH3 CH3 H 0 (CH2)2 C=N-N(CH3k H CH3
9.58 ('H3 CH3 H 2 CH2 CH-N(CH3)0CH2C6H5 H CH3
9 59 ('H3 CH3 H 2 (cH2)2 CH-N(CH3)OCH2C6Hs H CH3
9.60 ('H3 CH3 H 0 (CH2)2 CH-N(CH3)0CH2C6Hs H CH3
CA 02227934 1998-02-23
0050/46142
209
No. Rl~ Rl6 Rl9 n X y M L
9.61 CH3 CH3 H 2 CH2 C(CH3)-OCH3 H CH3
9.62 CH3 CH3 H 2 (CH2)2 C(CH3)-OCH3 H CH3
9.63 CH3 CH3 H 0 (CH2)2 C(CH3)-OCH3 H CH3
9.64 CH3 CH3 H 2 CH2 O H CH3
9.6S CH3 CH3 H 2 (C~2)2 ~ H CH3
9.66 CH3 CH3 H 0 (CH2)2 ~ H CH3
9.67 CH3 CH3 Br 2 CH2 CHOCH3 H CH3
9.68 CH3 CH3 Br 2 (CH2)2 CHOCH3 H CH3
9.69 CH3 CH3 Br 0 (CH2)2 CHOCH3 H CH3
9.70 CH3 CH3 Br 2 CH2 CHOC2Hs H CH3
9.71 CH3 CH3 Br 2 (CH2)2 CHOC2Hs H CH3
9.72 CH3 CH3 Br 0 (cH2)2 CHOC2Hs H CH3
9.73 CH3 CH3 Br 2 CH2 CHOiPr H CH3
9.74 CH3 CH3 Brr 2 (cH2)2 CHOiPr H CH3
lsicl
9.75 CH3 CH3 Br 0 (cH2)2 CHOiPr H CH3
9.76 CH3 CH3 Cl 2 CH2 CHOCH3 H CH3
9.77 CH3 CH3 Cl 2 (cH2)2 CHOCH3 H CH3
9.78 CH3 CH3 Cl 0 (cH2)2 CHOCH3 H CH3
9.79 CH3 CH3 Cl 2 CHz CHOC2Hs H CH3
9.80 CH3 CH3 Cl 2 (cH2)2 CHOC2Hs H CH3
9.81 CH3 CH3 Cl 0 (CH2)2 CHOC2Hs H CH3
9.82 CH3 CH3 Cl 2 CH2 CHOiPr H CH3
9.83 CH3 CH3 Cl 2 (cH2)2 CHOiPr H CH3
9.84 CH3 CH3 Cl 0 (CH2)2 CHOiPr H CH3
CA 02227934 1998-02-23
0050/4~;142
210
Table l0: Compounds of the formula
OH O M (~)n
R17~Y3( R13
Rl9 L
No. R17 R18 R19 n R12 R13 y L M
10.1 H H H 0 H H C=O H CH3
10.2 C]H3 H H 0 H H C=O H CH3
l5 10.3 C]H3 CH3 H 0 H H C=O H CH3
10.4 H H H 2 H H C=O H CH3
10.5 C'H3 H H 2 H H C=O H CH3
10.6 CH3 CH3 H 2 H H C=O H CH3
10.7 H H H 0 H H C=O H Cl
20 10.8 CH3 H H 0 H H C=O H Cl
10.9 C:H3 CH3 H 0 H H C=O H Cl
10.10 H H H 2 H H C=O H Cl
10.11 CH3 H H 2 H H C=O H Cl
25 10.12 CH3 CH3 H 2 H H C=O H Cl
10.13 H H H 0 H H CHOCH3 H CH3
10.14 CH3 H H 0 H H CHOCH3 H CH3
10.15 CH3 CH3 H 0 H H CHOCH3 H CH3
10.16 H H H 2 H H CHOCH3 H CH3
30 10.17 CH3 H H 2 H H CHOCH3 H CH3
10.18 CH3 CH3 H 2 H H CHOCH3 H CH3
10.19 H H H 0 H H CHOCH3 H Cl
10.20 CH3 H H 0 H H CHOCH3 H Cl
10.21 C:H3 CH3 H 0 H H CHOCH3 H Cl
lo.~ H H H 2 H H CHOCH3 H Cl
10.23 CH3 H H 2 H H CHOCH3 H Cl
10.24 CH3 CH3 H 2 H H CHOCH3 H Cl
10.25 H H H 0 H CH3 C=O H CH3
40 10.26 CH3 H H 0 H CH3 C=O H CH3
10.27 CH3 CH3 H 0 H CH3 C=O H CH3
10.28 H H H 2 H CH3 C=O H CH3
10.2g CH3 H H 2 H CH3 C=O H CH3
10.30 CH3 CH3 H 2 H CH3 C=O H CH3
10.31 H H H 0 H CH3 C=O H Cl
10.32 CH3 H H 0 H CH3 C=O H Cl
CA 02227934 1998-02-23
0050/g~ilq2
211
No. Rl7 R18 Rl9 n Rl2 Rl3 Y L M
10.33 CH3 CH3 H 0 H CH3 C=O H Cl
10.34 H H H 2 H CH3 C=O H Cl
10.35 CII3 H H 2 H CH3 C=O H Cl
10.36 Cl~3 CH3 H 2 H CH3 C=O H Cl
10.37 H H H 0 H CH3 CHOCH3 H CH3
10.38 CH3 H H 0 H CH3 CHOCH3 H CH3
10.39 CH3 CH3 H 0 H CH3 CHOCH3 H CH3
10.40 H H H 2 H CH3 CHOCH3 H CH3
10.41 CH3 H H 2 H CH3 CHOCH3 H CH3
10.42 Cl~3 CH3 H 2 H CH3 CHOCH3 H CH3
10.43 H H H 0 H CH3 CHOCH3 H Cl
10.44 CH3 H H 0 H CH3 CHOCH3 H Cl
10.45 CH3 CH3 H 0 H CH3 CHOCH3 H Cl
10.46 H H H 2 H CH3 CHOCH3 H Cl
10.47 CH3 H H 2 H CH3 CHOCH3 H Cl
10.48 CH3 CH3 H 2 H CH3 CHOCH3 H Cl
10.49 H H H 0 H 4H5 C=O H CH3
10.50 C]H3 H H 0 H C6H5 C=O H CH3
10.51 C]H3 CH3 H 0 H C6H5 C=O H CH3
10.52 H H H 2 H 4H5 C=O H CH3
10.53 C]H3 H H 2 H 4H5 C=O H CH3
10.54 C]H3 CH3 H 2 H C6H5 C=O H CH3
10.55 H H H 0 H 4H5 C=O H Cl
10.56 C]H3 H H 0 H C6H5 C=O H Cl
10.57 C]H3 CH3 H 0 H C6H5 C=O H Cl
10.58 H H H 2 H 4H5 C=O H Cl
10.59 CH3 H H 2 H 4H5 C=O H Cl
10.60 CH3 CH3 H 2 H 4H5 C=O H Cl
10.61 H H H 0 H 4Hs CHOCH3 H CH3
10.62 C]H3 H H 0 H 4H5 CHOCH3 H CH3
10.63 CH3 CH3 H 0 H 4H5 CHOCH3 H CH3
10.64 H H H 2 H C6H5 CHOCH3 H CH3
10.65 CH3 H H 2 H C6H5 CHOCH3 H CH3
10.66 CH3 CH3 H 2 H 4H5 CHOCH3 H CH3
10.67 H H H 0 H 4H5 CHOCH3 H Cl
10.68 CH3 H H 0 H 4H5 CHOCH3 H Cl
10.69 C]H3 CH3 H 0 H C6H5 CHOCH3 H Cl
10.70 H H H 2 H 4H5 CHOCH3 H Cl
10.71 CH3 H H 2 H 4H5 CHOCH3 H Cl
10.72 CH3 CH3 H 2 H 4H5 CHOCH3 H Cl
10.73 H H H 0 H H CH2 H CH3
CA 02227934 1998-02-23
0050/~6142
212
No. Rl7 R18 Rl9 n Rl2 Rl3 Y L M
10.74 CH3 H H 0 H H CH2 H CH3
10.75 CH3 CH3 H 0 H H CH2 H CH3
10.76 ]H H H 2 H H CH2 H CH3
10.77 CH3 H H 2 H H CH2 H CH3
10.78 C'H3 CH3 H 2 H H CH2 H CH3
10.79 H H H 0 H H CH2 H Cl
10.80 CH3 H H 0 H H CH2 H Cl
10.81 CH3 CH3 H 0 H H CH2 H Cl
10.82 H H H 2 H H CH2 H Cl
10.83 CH3 H H 2 H H CH2 H Cl
10.84 CH3 CH3 H 2 H H CH2 H Cl
CA 02227934 1998-02-23
0050~4~jl42
213
Table 11: Compounds of the formula
OH O M (~)n
~'~S\
R17 L
No. ~'17 n X Y L M
11.1 2-Ethylthiopropyl 2 CH2 C=O H CH3
11.2 2-Ethy' ' ~p u~"l 0 (CH2k C=O H CH3
113 2-Elhy''-~ v~l 2 (CH2)2 C=O H CH3
11.4 2-Ethy"'~~; opyl 2 CH2 CHOCH3 H CH3
11.5 2-EthyllLiop.opyl O (CH2)2 CHOCH3 H CH3
11.6 2-Ell~ r.ll 2 (CH2k2 CHOCH3 H CH3
11.7 2-Ethy''~~ opyl 2 CH2 CHOC2Hs H CH3
11.8 2-Ellly 'F (~pyl 0 (CH2)2 CHOC2Hs H CH3
11.9 2-Ethylthiopropyl 2 (CH2)2 CHOC2Hs H CH3
11.10 2-Elh~llhiv~nOp.~l 2 CH2 CHOiPr H CH3
11.11 2-EIl~y~hi.~,l,,p~l 0 (CH2)2 CHOiPr H CH3
1112 2-EIh~lll.iol,.vp, I 2 (CH2k2 CHOiPr H CH3
11.13 2-Ethylthiopropyl 2 CH2 C=NOCH3 H CH3
11.14 2-Ethyl ~r JPYI 2 (cH2k C=NOCH3 H CH3
11.15 2-Ethyllhio~,upyl 0 (CH2)2 C=NOCH3 H CH3
11.16 2-Elh~' ' ' p~up~,l 2 CH2 c=Noc2Hs H CH3
11.17 2-Elh~ propyl 2 (CH2)2 C=NOC2Hs H CH3
11.18 2-Eth~'tl i~r u~"l 0 (CH2)2 C=NOC2H5 H CH3
11.19 2-Etll~ propyl 2 CH2 C=NOiPr H CH3
11.20 2-Ethylthiopropyl 2 (CH2)2 C=NOiPr H CH3
11.21 2-Ethy"' '-~ opyl 0 (CH2)2 C=NOiPr H CH3
11.22 2-Etll~ propyl 2 CH2 C=NOCH2CH=CHCI H CH3
11.23 2-Etl-y' ' '~propyl 2 (CH2)2 C=NOCH2CH=CHCI H CH3
11.24 2-Elhy' ' ~ op~l 0 (CH2)2 C=NOCH2CH=CHCI H CH3
11.25 2-EIL~ ofJ~I 2 CH2 - C=NOCH2C6Hs H CH3
11.26 2-Elhy' ' '~F v~/~l 2 (CH2k C=NOCH2C6Hs H CH3
11.27 2-Elhy' ' '~ ùpyl 0 (CH2)2 C=NOCH2C6Hs H CH3
11.28 2-Ethylt~! lplupyl 2 CH2 C(CH3k H CH3
11.29 2-Elh~ )r ùp~l 2 (CH2)2 C(CH3k H CH3
11.30 2-Ethylt~ nvpyl O (CH2)2 C(CH3)2 H CH3
11.31 2-Ethyl~ Dp.c",yl 2 CH2 CH-NHCH3 H CH3
11.32 2-Ethylthiopropyl 2 (CH2k CH-NHCH3 H CH3
CA 02227934 1998 - 02 - 23
0050/~6142
214
No. Rl7 n X Y L M
11.33 2--Elhylthiopropyl 0 (CH2)2 CH-NHCH3 H CH3
11.34 2- Ethyllbiop.up~l 2 CH2 cH-NHc2Hs H CH3
11.35 2- Ethylthiopropyl 2 (CH2)2 CH-NHC2Hs ~ H CH3
11.36 2- Ethylthiopropyl 0 (CH2k CH-NHC2Hs H CH3
11.37 2--Ethylthiopropyl 2 CH2 CH-N(CH3k H CH3
11.38 2- Ethyllhiop.ù~,yl 2 (CH2)2 CH-N(CH3)2 H CH3
11.39 2--Ethylthiopropyl 0 (CH2)2 CH-N(CH3)2 H CH3
11.40 2- EthyllLio~lvpyl 2 CH2 cH-NHoc2Hs H CH3
11.41 2--Éthylthiopropyl 2 (CH2)2 CH-NHOC2Hs H CH3
11.42 2--Ethylthiopropyl 0 (CH2)2 CH-NHOC2Hs H CH3
11.43 2--Ethyllhioylo~ I 2 CH2 CH-N(CH3)OC2Hs H CH3
11.44 2--Ethylthiopropyl 2 (CH2k CH-N(CH3)OC2Hs H CH3
11.45 2--Ethylllliop.op~l O (CH2)2 cH-N(cH3)oc2Hs H CH3
11.46 2--EII-~ t~l 2 CH2 C=N-NH2 H CH3
11.47 2--Ethylll-io~,-ut~yl 2 (CH2k C=N-NH2 H CH3
11.48 2--Ethylthiopropyl 0 (CH2)2 C=N-NH2 H CH3
11.49 2--Ethylthiopropyl 2 CH2 C=N-N(CH3k H CH3
11.50 2--Ethylthiopropyl 2 (cH2k C=N-N(CH3)2 H CH3
11.51 2--Ethylthiopropyl 0 (CH2k C=N-N(CH3k H CH3
11.52 2--Elhy' ' -~ o~,yl 2 CH2 CH-N(CH3)OCH2C6Hs H CH3
11.53 2--EII-y'' -~ of/yl 2 (CH2)2 CH-N(CH3)OCH2C6Hs H CH3
11.54 2--Ethylthiopropyl 0 (CH2)2 CH-N(CH3)OCH2C6Hs H CH3
11.55 2--Ethylthiol .u~,l 2 CH2 C(CH3)-OCH3 H CH3
11.56 2--Ethylthiopropyl 2 (CH2k C(CH3)-OCH3 H CH3
11.57 2--Ethylthiopropyl 0 (CH2)2 C(CH3)-OCH3 H CH3
11.58 2--Ethylthiop.ù~"l 2 CH2 0 H CH3
11.59 2--Ethylthiopropyl 0 (CH2k ~ H CH3
11.60 2--Ethylthiopropyl 2 (CH2k ~ H CH3
11.61 T~ tlahyd~op~_a 3-yl 2 CH2 C=O H CH3
11.62 T~ tl hy.i.upy. 3-yl 0 (CH2)2 C=O H CH3
11.63 T. t~ yJ~.~p,.ail 3-yl 2 (CH2k C=O H CH3
11.64 TetrahyJ.u~,y.an-3-yl 2 CH2 CHOCH3 H CH3
11.65 TetrahyJ.opy. 3-yl 0 (CH2)2 CHOCH3 H CH3
11.66 Tetrahyd-(Jpy.. )-3-yl 2 (CH2)2 CHOCH3 H CH3
11.67 TetrahyJ~ùpj,an 3-yl 2 CH2 CHOC2Hs H CH3
11.68 Tetrahyd.opl.~n-3-yl 0 (CH2k CHOC2Hs H CH3
11.69 Tetrahyd.o~,y.u.)-3-yl 2 (CH2)2 CHOC2Hs H CH3
11.70 TetrahyJ.upj.dn-3-yl 2 CH2 CHOiPr H CH3
11.71 Tetrahydlo~J~. 3-yl 0 (CH2)2 CHOiPr H CH3
11.72 Tetrahyd.upy.an-3-YI 2 (CH2)2 CHOiPr H CH3
11.73 Tetrahydropyran-3-yl 2 CH2 C=NOCH3 H CH3
CA 02227934 1998 - 02 - 23
0050/416142
215
No. ~tl7 n X Y L M
11.74 l'etrahyd~opy._~ 3-yl 2 (CH2)2 C=NOCH3 H CH3
11.75 l'etrahyd.oy~- 3-yl 0 (cH2)2 C=NOCH3 H CH3
11.76 l'ct. ~lyllùy~ n-3-yl 2 CH2 c=Noc2Hs H CH3
11.77 1'.. 1. h;rJIùp~._~ 3-yl 2 (CH2)2 c=Noc2Hs H CH3
11.78 l'el~ '~yl~up~l_ 3-yl 0 (CH2)2 C=NOC2Hs H CH3
11.79 l'etrah~d.op.~ .. n 3-yl 2 CH2 C=NOiPr H CH311.80 l'etl hylloy~.an 3-yl 2 (CH2)2 C=NOiPr H CH3
11.81 lètrahydropyran-3-yl 0 (cH2)2 C=NOiPr H CH3
11.82 lètlahyJ-uyyran-4-yl 2 CH2 C=O H CH3
11.83 l'etrah~ opy~an-4-yl 0 (CH2)2 C=O H CH3
11.84 l'etrahyd-uyy-àn-4-yl 2 (CH2)2 C=O H CH3
11-85 TètldhY.I~uyfran-4-YI 2 CH2 CHOCH3 H CH3
11.86 lèt, h5JIuyyl~ln-4-yl 0 (CH2)2 CHOCH3 H CH3
11.87 l'ct- hyd,opyran-4-yl 2 (CH2)2 CHOCH3 H CH3
11.88 l~tl h~Jtùy~l-- 4 yl 2 CH2 CHOC2Hs H CH3
11.89 l'etrahyJ.~)py.àn-4-yl O (CH2k CHOC2Hs H CH3
11.90 lètrahydluy~lan-4-yl 2 (cH2)2 CHOC2Hs H CH3
11.91 lètrahyJ.ùy~ 4 yl 2 CH2 CHOiPr H CH3
11.92 lètrah~J,oy~ ~ 4 yl 0 (cH2)2 CHOiPr H CH3
11.93 lètl hyd ùy~, - 4-yl 2 (CH2)2 CHOiPr H CH3
11.94 lètrahyJ,uyJl~ln-4-yl 2 CH2 C=NOCH3 H CH3
11.95 lètrahyJ,ùy~ldll-4-yl 2 (CH2)2 C=NOCH3 H CH3
11.96 lètral,yJ.uy~ldll-4-yl 0 (CH2)2 C=NOCH3 H CH3
11.97 l'ttrahyJ,of"- - ~ yl 2 CH2 C=NOC2Hs H CH3
11.98 let, ' tJIùy~làn-4-yl 2 (CH2)2 c=Noc2Hs H CH3
11.99 l'etrahy-l-uyt-_~ 4 Yl 0 (CH2)2 c=Noc2Hs H CH3
11.100 lèh hyd.uy~ 4 yl 2 CH2 C=NOiPr H CH3
11.101 ~et._~.~J.ùy~ldn-4-yl 2 (CH2)2 C=NOiPr H CH3
11.102 ~ttrahyd,oy~,.... n 4 yl 0 (CH2)2 C=NOiPr H CH3
11.103 ~e~rahyd,utl,ioyy~i- 3-yl 2 CH2 C=O H CH3
11.104 ~ettrahydrûthiûpyran-3-yl 0 (cH2)2 C=O H CH3
11.105 ~ettrah~J,uth;oy,-an-3-yl 2 (CH2)2 C=O H CH3
11.106 ~ettrahyd.. ~ ) ran-3-yl 2 CH2 CHOCH3 H CH3
11.107 T~l. hyl... "'~ n-3-yl 0 (cH2)2 CHOCH3 H CH3
11.108 ~ttr- ' ~d~ n-3-yl 2 (CH2)2 CHOCH3 H CH3
11.109 ~tt,.,hyJ.. ' ' pj/. - 3-yl 2 CH2 CHOC2Hs H CH3
11.110 'Tetl~yJ~ al ~1 - 3-yl 0 (CH2)2 CHOC2Hs H CH3
11.111 'rttl_hyl.otlliopyran-3-yl 2 (CH2)2 CHOC2Hs H CH3
11.112 Tetrahydrothiopyran-3-yl 2 CH2 CHOiPr H CH3
11.113 ~ttrahydrothiopyran-3-yl 0 (CH2)2 CHOiPr H CH3
11.114 ~etrahydrothiopyran-3-yl 2 (CH2)2 CHOiPr H CH3
CA 02227934 1998-02-23
0050/46142
216
No. ~R17 n X Y L M
11.115 r tl8b~d~11liopyran-3-yl 2 CH2 C=NOCH3 H CH3
11.116 rtt~ h~l~ulbio~ldll-3-yl 2 (CH2)2 C=NOCH3 H CH3
11.117 rel. hy~.uLhio~ 3-yl 0 (CH2)2 C=NOCH3 H CH3
11.118 rct~ h~d,othiopyran-3-yl 2 CH2 c=Noc2Hs H CH3
11.119 relrahy~LutLiopy~- 3-yl 2 (CH2)2 c=Noc2Hs H CH3
11.120 retrahyd.othiopy.. ,n-3-yl 0 (CH2)2 c=Noc2Hs H CH3
11.121 retrahydrothiopyran-3-yl 2 CH2 C=NOiPr H CH3
11.122 retrahydrothiopyran-3-y~ 2 (CH2)2 C=NOiPr H CH3
11.123 retrahydrothiopyran-3-yl 0 (CH2)2 C=NOiPr H CH3
11.124 ;L-Methylthiocyclopropyl 2 CH2 C=O H CH311.125 ]L-Methyllllio. ~_loprupyl O (CH2)2 C=O H CH3
11.126 1 Mcth~lll,io~lop,(,;~yl 2 (CH2)2 C=O H CH3
11.127 1 M_tllylll,io- ~. Iop.ol)yl 2 CH2 CHOCH3 H CH3
11.128 1 M ~Ill-io yclopropyl 0 (CH2)2 CHOCH3 H CH3
11.129 l-Methylthiocyclopropyl 2 (CH2)2 CHOCH3 H CH3
11.130 ].-M_lh~lllliocyclopropyl 2 CH2 CHOC2Hs H CH3
11.131 ] ~1: ;Ill,iocyclopropyl 0 (CH2)2 CHOC2Hs H CH3
11.132 ].-Methyllhio ~clûprûpyl 2 (CH2)2 CHOC2Hs H CH3
11.133 ~ M_tlll~llhio. yclopropyl 2 CH2 CHOiPr H CH3
11.134 ] ~: ylll-io. ~r lo~,.upyl 0 (CH2)2 CHOiPr H CH3
11.135 ~ yllhio~ ~clop~opyl 2 (CH2)2 CHOiPr H CH3
11.136 I-~ I-iocyclopropyl 2 CH2 C=NOCH3 H CH3
11.137 I-Meth~llhiocyclo~).o?yl 2 (CH2)2 C=NOCH3 H CH3
11.138 I Mclhyllllio~-~cloplop~l O (CH2)2 C=NOCH3 H CH3
11.139 I-Methylthiocyclop.(J~yl 2 CH2 c=Noc2Hs H CH3
11.140 1-~ ~:hylllliocycloprûpyl 2 (CH2)2 c=Noc2Hs H CH3
11.141 l-Methylthiocycloplopyl 0 (cH2)2 c=Noc2Hs H CH3
11.142 l-Methylthio. ~clo~,,u~yl 2 CH2 C=NOiPr H CH3
11.143 l-Methylthio~ yclop~o?yl 2 (CH2)2 C=NOiPr H CH3
11.144 l-Methylthiocyclopropyl 0 (CH2)2 C=NOiPr H CH3
11.145 (IDi~.,. lhoxy)methyl 2 CH2 C=O H CH3
11.146 (Dimethoxy)methyl 0 (cH2)2 C=O H CH3
11.147 (Dimethoxy)methyl 2 (CH2)2 C=O H CH3
11.148 (Dimcthoxy)methyl 2 CH2 CHOCH3 H CH3
11.149 (Di : ~ y)methyl 0 (CH2)2 CHOCH3 H CH3
11.150 (Dimethoxy)methyl 2 (CH2)2 CHOCH3 H CH3
11.151 (I)imethoxy)methyl 2 CH2 CHOC2Hs H CH3
11.152 (I)imethoxy)methyl 0 (CH2)2 CHOC2H5 H CH3
11.153 (Dimethoxy)methyl 2 (CH2)2 CHOC2Hs H CH3
11.154 (I)imethoxy)methyl 2 CH2 CHOiPr H CH3
11.155 (I)imethoxy)methyl 0 (CH2)2 CHOiPr H CH3
CA 02227934 1998 - 02 - 23
0050/46142
217
No. Rl7 n X Y L M
11.156 (Dimethoxy)methyl 2 (CH2)2 CHOiPr H CH3
11.157 (Dimethoxy)methyl 2 CH2 C=NOCH3 H CH3
11.158 (Dimethoxy)methyl 2 (CH2)2 C=NOCH3 ~ H CH3
11.159 (Dimethoxy)methyl 0 (CH2)2 C=NOCH3 H CH3
11.160 (Dimethoxy)methyl 2 CH2 C=NOC2Hs H CH3
11.161 (Dimethoxy)methyl 2 (CH2)2 C=NOC2Hs H CH3
11.162 (Dimethoxy)mcthyl 0 (CH2)2 C=NOC2Hs H CH3
11.163 (D- ~ y)methyl 2 CH2 C=NOiPr H CH3
11.164 (Dimcthoxy)methyl 2 (cH2)2 C=NOiPr H CH3
11.165 (Dimethoxy)methyl 0 (CH2)2 C=NOiPr H CH3
CA 02227934 1998-02-23
0050~4~;142
218
Preparation Examples
A) Preparation of the starting materials and intermediates
1. 3-1~hio-2-methylbenzoic [sic] acid
lOCI g (0.66 mol) of 3-amino-2-methylbenzoic acid are
initially introduced together with 270 g of ice and 127 ml of
concentrated hydrochloric acid. 45.7 g (0.66 mol) of sodium
nitrite in 270 ml of water are then added dropwise at 0-10~C.
In a second vessel, 84.2 g (0.79 mol) of sodium carbonate and
106 g (0.66 mol) of potassium methylxanthate are dissolved in
45CI ml of water and heated to 60-70~C. The diazonium solution
is cautiously added dropwise. The mixture is subsequently
stirred for 1 hour. 106 g (2.65 mol) of sodium hydroxide in
27CI ml of water are then added, the mixture is stirred for a
further 2 hours, the solution is rendered acidic using hydro-
ch]oric acid and the resulting precipitate is filtered off
wit:h suction. The solid is washed with water and dried.
Yield: 110 g (100 % of theory) of 3-thio-2-methylhe~oic
[sic] acid; melting point 155 C;lH-NMR (d6-DMSO): ~ lppm] -
13.0 (lH, bs), 7.7 (2H, m), 7.3 (lH, tr), 2.4 (3H, B).
2. Met;hyl 3-thio-2-methylbenzoate lsic]
llCI g (0.66 mol) of 3-thio-2-methylbenzoic [sic] acid are
dissolved in 1.6 1 of methanol which contains 5 ~i sulfuric
acid and the mixture is refluxed for 5 hours. The alcohol iq
then distilled off, the residue iB taken up in ethyl acetate,
andl the organic phase is washed with water and with sodium
carbonate, dried using sodium sulfate and concentrated.
Yield: 104 g (87 % of theory) of methyl 3-thio-2-methylben-
zoate lsic]; 1H-NMR (CDCl3):~ [ppm] = 7.6 (lH, d), 7.4 (lH,
d), 7.1 (lH, d), 3.9 (3H, 8), 3.4 (lH, B), 2.5 (3H, s).
CA 02227934 1998-02-23
0050/41il42
219
3. Met;hyl 3-thio(2'-propionic acid)-2-methylhenzoAte lsic]
O CH3
O ~ S ~ OH
70 g (0.38 mol) of methyl 3-thio-2-methylbenzoate [sic] are
dissolved in 400 ml of water and refluxed for 7 hours with
30.8 g (0.77 mol) of sodium hydroxide solution and 58.8 g
(0.45 mol) of bromopropionic acid. After cooling, the aqueous
phase is washed with methyl tert-butyl ether. The aqueous
phase is then acidifled with 2 N hydrochloric acid, the re-
sulting precipitate is filtered off with suction and washed
wit:h water, and the product is dried.
Yield: 75.5 g (78 % of theory) of methyl 3-thiopropionic
acid-2-methylhenzo~te [sicl; lH-NMR (CDCl3): ~ [ppml - 7.66
(lEI, d), 7.51 (lH, d), 7.20 (lH, tr), 3.96 (3H, s), 3.18 (2H,
tr~, 2.70 (2H, tr), 2.63 (3H, s).
4. Met:hyl 8-methylthiochroman-4-one-7-carboxylate
o CH3
11 1 8 Sl
4 cl (15.8 mmol) of methyl 3-thiopropionic acid-2-methylben-
zoate [sic] are stirred at 70~C for 15 minutes in 40 g of
polyphosphoric acid. The reaction solution is then added to
ice-water and the resulting precipitate i8 filtered off with
suc:tion. The product is washed with water and dried in a dry-
inq cabinet. As a by-product of the cyclization, methyl 8-me-
thylthiochromen-4-one-carboxylate [sicl can be formed, which
can be ~eparated off by chromatography.
Yield: 3.1 g (83 % of theory) of methyl 8-methylthiochroman-
4-c~ne-7-carboxylate; lH-NMR (CDCl3): ~ [ppm] ~ 8-00 (lH~ d),
7.30 (lH, d), 3.94 ~3H, ~), 3.15 (2H, m), 2.98 (2H, m), 2.50
(3~l, 8);
Sec:ondary component: methyl 8-methylthiochromen-4-one-
ca~boxylate lsicl: lH-NMR ~CDCl3): ~ [ppm] s 8.4 (lH, d),
7.~1 (lH, d), 7.8 (lH, d), 7.0 (lH, d), 4.0 (3H, s),
2.7 (3H, 8).
CA 02227934 1998-02-23
0050/g~6142
220
5. 8-Methylthiochroman-4-one-7-carboxylic acid
41.1 g ~0.17 mol) of methyl 8-methylthiochroman-4-one-7-car-
boxylate are hydrolyzed under reflux with 10.3 g ~0.26 mol)
of NaOH in a mixture of 400 ml of water and methanol. The
methanol is then distilled off and the residue is diluted
with water and acidifed with 2N hydrochloric acid. The useful
product precipitates out and iB filtered off with suction,
washed with water and dried.
Yield: 34.4 g ~89 % of theory) of 8-methylthiochro-
man-4-one-7-carboxylic acid; melting point: 243-246~C.
6. 8-Methyl-l,l-dioxothiochroman-4-one-7-carboxylic acid
~ ~ 23
20 g ~0.09 mol) of 8-methylthiochroman-4-one-7-carboxylic
acid are dissolved in 100 ml of acetic acid. A spatula tipful
of sodium tungstate is added. 24.9 g ~0.22 mol) of 30 S
strength hydrogen peroxide solution are then added dropwise
at 50 C. The mixture is subsequently stirred at about 20 C
for 1 hour. The reaction solution i8 then added to water, a
precipitate being formed which i~ filtered off with ~uction.
After washing the product with water, it is dried.
Yield: 18.4 g ~80 % of theory) of 8-methyl-1,1-dioxothiochro-
mam-4-one-7-carboxylic acid; melting point: 224-225 C.
35 7. Me1thyl 4-hydroxy-8-methylthiochroman-7-carboxylate
O CH3
CN
OH
30 g ~0.127 mol) of methyl 8-methylthiochroman-4-one-7-car-
boxylate are dissolved in a mixture of 120 ml of methylene
ch:Loride and 60 ml of methanol and cooled to 0-5 C. 2.4 g
(0.064 mol) of sodium borohydride are then added in portions.
The mixture is subsequently stirred at this temperature for
CA 02227934 1998-02-23
- 0050/416142
221
1 hour, and 200 ml of 2N hydrochloric acid are added to the
reaction solution. Two phases are formed. The organic phase
is separated off and dried, and the solvent is removed by
di~jtillation. The crude product is directly reacted further
wi1:hout further purification.
Yield; 27.6 g (91 ~ of theory) of methyl 4-hydroxy-8-methyl-
thiochroman-7-carboxylate.
10 8. Me1;hyl 4-ethoxy-8-methylthiochroman-7-carboxylate
13 8 g (0.058 mol) of methyl 4-hydroxy-8-methylthiochro-
man-7-carboxylate are heated at boiling point for 4 hours in
60 ml of ethanol with addition of 1 g of sulfuric acid. The
solvent is then distilled off and the residue is taken up
wi1:h water. The aqueous phase is extracted with ethyl acet-
ate. The organic phase is washed with sodium hydrogen carbon-
at~ ~olution, dried and concentrated. The product is purified
by chromatography.
Yield: 10.1 g (60 % of theory) of methyl 4-ethoxy-8-methyl-
th:iochroman-7-carboxylate; lH-NMR (CDCl3): ~ [ppm] s 7.44 ~lH,
d)" 7.13 (lH, d), 4.40 (lH, m), 3.90 (3H, s), 3.60 (2H, m),
3.:38 (1 H, dtr), 2.90 (lH, m), 2.50 (3H, s), 2.40 ~lH, m),
1.'38 (lH, m), 1.10 (3H, tr).
The reaction to give methyl 4-methoxy-8-methylthiochro-
man-4-c,ne-7-carboxylate and methyl 4-isopropoxy-8-methylthiochro-
man-4-c,ne-7-carboxylate i~ carried out analogously to the above
30 proceA-lre, in the case of methyl 4-methoxy-8-methylthiochro-
man-4-a,ne-7-carboxylate ethanol being replaced by methanol and in
the ca~e of methyl 4-isopropoxy-8-methylthiochroman-4-one-7-car-
boxylate ethanol being replaced by isopropanol.
35 9. 4-~thoxy-8-methylthiochroman-7-carboxylic acid
2.~L g of ~odium hydroxide solution [sic] are dissolved in
20 ml of water. [lacunal Methyl 4-ethoxy-8-methylthiochro-
man-4-one-7-carboxylate di~solved in 20 ml of methanol i9
adcled dropwise at about 20 C The mixture is refluxed for 2
hours. The solvent i~ then di~tilled off and the residue is
adcled to 2N hydrochloric acid. The aqueous pha~e is extracted
wit:h methylene chloride and the organic phase is dried and
concentrated .
0050/46142 CA 02227934 1998-02-23
222
Yield: 9.3 g (100 % of theory) of 4-ethoxy-8-methylthiochro-
man-7-carboxylic acid; melting point: 89-98 C.
Hydrolyl3is of the corresponding esters to 4-ethoxy-8-methyl-
S thiochroman-7-carboxylic acid and 4-isopropoxy-8-methylthiochro-
man-7-carboxylic acid proceeds in a similar manner. The same
applies to the hydroly~is of the corresponding benzo[b]thiophene
derivatives shown below.
10 10. 8-Methyl-4-ethoxy-1,1-dioxothiochroman-7-carboxylic acid
8.4 g (0.033 mol) of 4-ethoxy-8-methylthiochroman-7-carbox-
ylic acid are initially introduced in 60 ml of acetic acid. A
spatula tipful of ~odium tungstate i8 added. 7.9 g (0.07 mol)
of 30 % strength hydrogen peroxide solution are slowly added
dropwise at 50 C and the reaction mixture is subsequently
stirred for 2 hours. It i~ then poured into water and the
aqu,eou~ phase i~ extracted with ethyl acetate. The organic
phase is washed with bisulfite solution, then dried and con-
centrated.
Yield: 9.5 g (100 % of theory) of 8-methyl-4-ethoxy-
1,1-dioxothiochroman-7-carboxylic acid; melting point: 150 C.
25 11. 8-~lethylthiochroman-4-one-7-carboxylic acid 0-ethyl oxime
o CH3
H'o~2
OC2H5
0.88 g ~9 mmol) of ethylhydroxylamine is initially introduced
in 20 ml of methanol. 0.62 g (4.5 mmol) of potassium carbon-
ate iB then added, followed by 2.0 g (9 mmol) of 8-methyl-
thiLochroman-4-one-7-carboxylic acid. The reaction is stirred
at about 20~C for 10 day~. It is worked up by addition of
wa1:er and 2N hydrochloric acid, and the resulting precipitate
is filtered off with suction and dried.
Yield: 2.2 g (92 % of theory) of 8-methylthiochro-
man-4-one-7-carboxylic acid 0-ethyl oxime; melting point:
161;~C.
CA 02227934 1998-02-23
0050/46142
223
12. 8-Methyl-l,l-dioxothiochroman-4-one-7-carboxylic acid O-ethyl
oxime
3.0 g (0.011 mol) of 8-methylthiochroman-4-one-7-carboxylic
acid O-ethyl oxime are initially introduced into 30 ml of
acetic acid together with a spatula tipful of sodium tung-
state. 2.8 g (0.024 mol) of 30 % strength hydrogen peroxide
solution are added dropwise at S0 C. After stirring for
1 hDur, the reaction mixture is poured into ice-water and the
resulting precipitate is filtered off with suction. The prod-
uct is washed with water and dried.
Yield: 2.5 g (74 % of theory) of 8-methyl-1,1-dioxothiochro-
man-4-one-7-carboxylic acid O-ethyl oxime; melting point
198~C.
13. 8-Methyl-l-oxothiochroman-4-one-7-carboxylic acid
7.0 g (31.5 mmol) of 8-methylthiochroman-4-one-7-carboxylic
acid are initially introduced into 70 ml of acetic acid to-
gether with a spatula tipful of sodium tungstate. 3.6 g
(31.5 mmol) of 30 % strength hydrogen peroxide solution are
added dropwise at 50 C and the reaction solution is subse-
quently stirred for 3 hours. It is then stirred into water
and the product iB extracted with ethyl acetate. The organic
pha~e is dried and the solvent is removed. The product is
purified by chromatography.
Yield: 5.4 g (72 % of theory) of 8-methyl-1-oxothiochro-
man-4-one-7-carboxylic acid; lH-NMR ~d6-DMSO): ~ [ppm] -
8.0 (2H, m), 3.5 (3H, m), 2.8 (lH, m), 2.7 (3H, s).
14. Methyl 3-carboxymethylthio-2-methylbenzoate
O CH3 ~
oJ~ SJl~
I
CH3
12.4 g (0.068 mmol) of methyl 3-mercapto-2-methylbenzoate in
80 ml of dimethylformamide are added dropwise to 1.6 g
(0.068 mol) of ~odium hydride in 40 ml of dimethylformamide
and the mixture is stirred at about 20~C for 60 min. 8 g
(0.068 mol) of chloroacetic acid are then added and the mix-
ture is stirred at about 20~C for 4 hours.
0050/41~142 CA 02227934 1998-02-23
224
The reaction mixture is worked up by stirring it into ice-
wat:er contAini ng hydrochloric acid.
The resulting precipitate is filtered off with suction,
washed with water and dried.
Yield: 14.6 g (89 % of theory) of methyl 3-carboxy-
met:hylthio-2-methylbenzoate; lH-NMR (d6-DMSO): ~ lppm~ =
7.'i5 (lH, d), 7.45 (lH, d), 7.21 (lH, tr), 3.82 (2H, 9),
2.'i0 (3H, s).
15. Met:hyl 7-methylbenzo[b]thiophene-312Hl-one-6-carboxylate
o CH3
C~ ~ ~ 2
14.3 g (0.06 mmol) of 3-carboxymethylthio-2-methylbenzoic
aci.d are dissolved in 300 ml of methylene chloride and 13.1 g
~0.11 mmol) of thionyl chloride are added dropwise. The mix-
ture iB refluxed for 1 hour and the solvent and exce~s thio-
ny]. chloride are then distilled off. The residue i9 taken up
in 100 ml of methylene chloride and treated with 31.8 g
(0.24 mmol) of aluminum trichloride. The reaction i8 Btirred
at about 20~C for 1 hour. The mixture is then added to ice-
wat;er and the organic phase i8 separated off. After washing
ancl drying the organic phase, the Bolvent i8 removed. The
product is reacted further without purification.
Yield: 12.9 g (97 ~ of theory) of methyl 7-methylhe~70[b]-
thiophene-3[2H]-one-6-carboxylate; lH-NMR (CDCl3): ~ [ppm] =
7.65 (2H, m), 3.93 (3H, B), 3.88 (2H, s), 2.50 (3H, B).
35 16. Met:hyl 7-methyl-3-hydroxybenzo[b]thiophene-[2H]-6-carboxylate
o CH3
~ ~ 2
OH
12.8 g (0.058 mol) of methyl 7-methylbenzo[b]thiophene-
3[i!H]-one-6-carboxylate are dissolved in 120 ml of methylene
ch]oride and 60 ml of methanol and cooled to 0 C. 1.1 g
(0.029 mol) of sodium borohydride is added in portions and
the mixture i8 stirred for 3 hours. The reaction is
CA 02227934 1998-02-23
0050/415142
225
terminated by adding water. The phases are separated and the
aqueous phase is extracted with methylene chloride. The
con~ined organic phases are dried and the solvent is dis-
tiLled off. The crude product is reacted further.
Yield: 13.2 g (100 % of theory) of methyl 7-methyl-3-hydroxy-
benzo[b]-thiophene-[2H]-6-carboxylate; lH-NMR (CDC13): ~ [ppm
= ,'.6 (2H, m), 5.3 (lH, m), 3.9 ~3H, s), 3.7 (lH, m),
3.3 (lH, m), 2.4 (3H, 5).
10 17. Met;hyl 7-methyl-3-methoxybenzo[blthiophene-12Hl-6-carboxylate
2.41 g (0.059 mol) of NaH is dissolved in 50 ml of dimethyl-
foI~amide. 13.2 g of methyl 7-methyl-3-hydroxybenzo[blthi
phene-[2H]-6-carboxylate di~solved in 50 ml [lacunal are
adcled dropwise and the mixture i~ subsequently stirred at
about 20 C for 2 hours. 8.4 g (0.059 mol) of iodomethane are
then added and the mixture is ~tirred for a further 2 hours.
The reaction Bolution i8 added to ice-water and extracted
wit;h ethyl acetate. The organic phase is dried and subse-
que!ntly concentrated, and the product is purified by chroma-
toclraphy .
Yield: 3.5 g (25 % of theory) of methyl 7-methyl-3-methoxy-
benzo[b]thiophene-[2H]-6-carboxylate; lH-NMR (CDC13): 8 [ppml
- 7.60 (lH, d), 7.20 (lH, d), 5.04 (lH, m)~ 3.90 (3H, 8),3.56
2S (lH, m), 3.40 (3H, s), 3.38 (lH, m), 2.50 (3H, s).
Similarly to the hydrolysis of the thiochromanone esters de-
scribed above, the corresponding benzothiophene acids are al~o
obtained.
The compounds shown in the following tables are obtAine~ in a
similar manner:
Table 12: Intermediates
(~)n
T~lr, S~ X
~ Y
L
No. r n X Y L M Physicaldata
12.1 HO 0 (CH2k C=O H H m.p. [~C]: 226-231
12.2 HO 2 (CH2)2 C=O H H m.p. [~Cl: 217-220
12.3 HO 0 (CH2)2 C=O H CH3 m.p. [~Cl: 243-246
CA 02227934 1998-02-23
0050/46142
226
No. T n X Y L M Physical data
12.4 HO 2 (CH2k C=O H CH3 m.p. [~C]:224-225
12.5 HO 0 (CH2)2 CHOCH3 H CH3 m.p. 1~Cl: 117-118
12.6 HO 2 (CH2)2 CHOCH3 H CH3 m.p. 1~C]: 167-172
12.7 HO 0 (CH2)2 CHOC2Hs H CH3 m.p. [~Cl: 89-98
12.8 HO 2 (CH2)2 CHOC2Hs H CH3 m.p. [~Cl: lSO
12.9 HO 0 (CH2)2 CHOiPropyl H CH3 m.p. [~Cl: 138
12.10 HO 2 (CH2)2 CHOiPropyl H CH3 m.p. 1~Cl: 142
12.11 HO 0 (cH2)z C=NOC2Hs H CH3 m.p. 1~Cl: 166
12.12 HO 2 (CH2)2 C=NOC2Hs H CH3 m.p. 1~Cl: 198
12.13 HO 0 (CH2)2 C=NOCH2CH=CHCI H CH3 m.p. 1~Cl: 163
12.14 HO 2 (CH2)2 C=NOCH2CH=CHCI H CH3 m.p. 1~Cl: 174
12.15 HO 0 (CH2)2 C=NOCH2C6Hs H CH3 m.p. [~Cl: 178
12.16 HO 2 (CH2)2 CzNO-lBulyl H CH3 m.p. [~Cl:217
12.17 H3CO 0 (CH2)z C(CH3)2 H CH3 m.p. 1~Cl: 63-65
12.18 HO 2 (CH2)2 CHOCH3 H Cl m.p. [~C]: 137-139
12.19 HO 2 (CH2)2 C=NOC2Hs H Cl m.p. ~~C]:205
12.20 HO 0 (CH2) CHOCH3 H CH3 IH-NMR,300 MHz
(d6-DMSO): ~ [ppml =
13.0 (lH, s), 7.55 (lH, d), 7.25
(lH, d), 5.10 (lH, s), 3.62 (lH,
m), 3.42 (lH, m), 3.41 (3H, s),
2.42 (3H, s)
12.21 HO 2 (CH2) CHOCH3 H CH3 lH-NMR,300 MHz
(d6-DMSO): o lppm] =
13.5 (lH, bs), 8.10 (lH, d), 7.60
(lH, d), 5.18 (lH, m), 4.07 (lH,
m), 3.75 (lH, m), 3.40 (3H, s),
2.70 (3H, s)
12.22 HO 2 (CH2)2 C=O H Cl IH-NMR,300 MHz
(d6-DMSO): o [ppm] =
14.2 (lH, bs), 8.10 (lH, d), 7.98
(lH, d), 4.13 (2H, m), 3.30 (2H,
m)
12.23 HO 0 (CH2)2 C=O H Cl IH-NMR,300 MHz
(d6-DMSO); o lppm] =
13.9 (lH, bs), 8.10 (lH, d), 7.52
(lH, d), 3.41 (2H, m), 2.90 (2H,
m)
12.24 H3CO 0 (CH2)2 CHOCH3 H CH3 IH-NMR, 400 MHz (CDCI3):
~ [ppm] = 7.46, 7.13, 4.28, 3.87,
3.38, 3.30, 2.90, 2.48, 2.39, 1.91
12.2S H3CO 0 (CH2k CHOC2Hs H CH3 m.p. 1~Cl: 94-98
12.26 H3CO 0 (CH2)2 CHOiPropyl H CH3 IH-NMR, 250 MHz (CDCI3):
~ [ppm] = 7.47, 7.17, 4.48, 3.88,
3.79,3.29,2.90,2.48,2.29,1.97,
1.21
12.27 HO 1 (CH2k C=O H CH3 m.p. 1~Cl: 98 (decomp.)
12.28 H3CO 0 CH=CH C=O H CH3 m.p. l~Cl: 128-130
CA 02227934 l998-02-23
0050/46142
..
Z27
No. T n X Y L M Physicaldata
12.29 HO 0 CH=CH C=O H CH3 IH-NMR, 2s0 MHz
(d6-DMSO): ~ lppm] =
13.52,8.48,8.30,7.87,7.03,2.66
12.30 HO O (CH2)2 C=NO-tButyl H CH3 m.p. 1~Cl: 217
12.31 HO 2 (cH2)2 C=NOC2Hs H Cl m.p. [~C]: 205
12.32 HO o (CH2)2 C(CH3)~ H CH3 m.p. [~C]: 212
12.33 HO o (CH2)2 CH2 H CH3 m.p. 1~C]: i55
lo 12.34 HO O (CH2)2 cH(c6Hs) H CH3 m.p. ~~C]: 175
12.35 HO 2 (CH2)2 CH2 H CH3 m.p. [~C]: 204
12.36 H3CO O (CH2)2 CH(c6Hs) H CH3 m.p. ~~C]: 103
12.37 HO 2 (CH2)2 cH(c6Hs) H CH3 m.p. [~C]: 145
12.38 HO o (CH2)2 CHSC6Hs H CH3 m.p. [~C]: 77
12.39 HO 2 (CH2)2 CHSO2C6Hs H CH3 m.p. 1~C]: 239
12.40 HO o (CH2k C=O Cl Cl IH-NMR,250 MHz(CDCI3):
~ Ippm] = 7.69,3.31,3.01
12.41 HO 2 (CH2)2 C=O cl cl IH-NMR, 250 MHz
(d6-DMSO): ~ lppm] =
8.04,4.16,3.31
12.42 ~:13CO O (CH2)2 CHOH Cl Cl lH-NMR, 250 MHz (CDCI3):
8 lppm] = 7.50,5.20,4.92,3.36,
2.89,2.53,1.85
12.43 HO 2 (CH2)2 CHOI Cl Cl lH-NMR, 250 MHz
(d6-DMSO): ~ lppm] = 8.03,
6.96,5.08,3.87,3.62,2.54,2.37
12.44 H10 0 (CH2)2 CHOH H CH3 m.p. [~C]: 209
Table 1i!a: Inte -1i ate~
~ M (~)n
R 1
R13
No. T n Rl2 Rl3 R21 R22 y L M Physical data
12.45 H3CO 0 CH3 CH3 H H C=O H CH3 IH-NMR, 270 MHz (CDCI3):
~ lppm] = 8.02,7.50,3.92,3.09,
2.50,1.33
12.46 H31-O O H CH3 H H C=O H CH3 m.p. [~C]: 79
12.47 H3CO 0 CH3 CH3 H H CHOCH3 H CH3 IH-NMR, 270 MHz (CDC13):
~ [ppm] = 7.44,7.02,3.89,3.59,
3.33,3.28,2.50,2.48,1.21,0.88
12.48 H3CO 0 H H CH3 H C=O H CH3 m.p. [~Cl: 83
12.49 H3('0 0 H H CH3 H CHOCH3 H CH3 1H-NMR, 270 MHz (CDCI3):
~ lppml = 7.46,7.11,4.31,3.89,
3.65,3.37,2.48,2.44,1.64,1.44
~ 0050/46142 CA 02227934 1998-02-23
228
No T n R12 Rl3 R21 R22 ~ L M Physicaldata
12.50 HCI 0 H CH3 H H CHOCH3 H CH3 m.p. l~C~: 124
12.51 H0 0 CH3 CH3 H H CHOCH3 H CH3 m.p. [~C]: 168
12.52 HCI 0 H H CH3 H CHOCH3 H CH3 m.p. [~C]: }45
12.53 H0l 2 H CH3 H H CHOCH3 H CH3 m.p. l~C]: 184
(trans)
12.54 HCI 2 CH3 CH3 H H CHOCH3 H CH3 m.p. [~C]: 161
12.55 HCI 2 H H CH3 H CHOCH3 H CH3 m.p. [~C]: 182
Preparation of the final products
1. 2-(8-Methyl-1,1-dioxothiochroman-4-one-7-carbonyl)-
1,3--cyclohexanedione [sic]
~
a) 17.4 g (0.0685 mol) of 8-methyl-1,1.-dioxothiochroman-
4-one-7-carboxylic acid are disRolved in 170 ml of to-
luene and treated with one drop of dimethylformamide, and
8.96 g (0.0753 mol) of thionyl chloride are added. After
refluxing for 4 hours, the reaction mixture i9 concen-
trated. The reaction product is directly reacted further.
Yield: 18.6 g (99 ~ of theory) of 8-methyl-1,1-dioxo-
thiochroman-4-one-7-carbonyl chloride;
b) 0.62 g ~5.5 mmol) of cyclohexane-1,3-dione i8 initially
introduced into 10 ml of acetonitrile together with
0.56 g (5.5 mol) of triethylamine. 1.5 (5.5 mmol) of
8-methyl-1,1-dioxothiochroman-4-one-7-carbonyl chloride
dissolved in 20 ml of acetonitrile are then added drop-
wise.
The mixture is subsequently stirred at about 20~C for one
hour. 0.31 g (3.7 mmol) of acetone cyanohydrin and 2.8 g
(22.5 mmol) of triethylamine are then added and the mix-
ture is ~tirred for 1 hour. For working-up, the reaction
solution is stirred into 2N hydrochloric acid and the
aqueous phaRe is extracted with ethyl acetate. The or-
ganic phase i~ then extracted with Na2CO3 solution and
CA 02227934 1998-02-23
0050/461~2
229
the alkaline aqueous phase is rendered acidic with cool-
ing. The resulting precipitate is filtered off with suc-
tion, washed with water and dried.
Yield: 1.0 g (52 ~ of theory) of 2-(8-methyl-1,1-dioxo-
thiochroman-4-one-7-carbonyl)-1,3-cyclohex~ne~;one [sic];
melting point: 173-178~C.
2. 2-(B-Methyl-l,l-dioxothiochroman-4-one-7-carbonyl O-ethyl
oxime)cyclohexAne-1,3-dione [sic
~
N ~
OC2Hs
1.0 g (3.4 mmol) of 8-methyl-1,1-dioxochroman-4-one-7-carbox-
ylic acid O-ethyl oxime i8 initially introduced into 10 ml of
acetonitrile together with 0.39 g (3.5 mmol) of cyclohex-
ane-1,3-dione, 0.75 g (3.6 mmol) of dicyclocarbodiimide [ BiC]
(DCC) is added and the mixture is stirred for 2 hours. 0.1 ml
of acetone cyanohydrin and 0.51 g (5.1 mmol) of triethylamine
are then added and the reaction mixture is stirred for a
furlcher 2 hours. It iB then stirred into sodium carbonate
solution and extracted with ethyl acetate, and the organic
pha~je is discarded. The aqueous phase is rendered acidic
using hydrochloric acid and extracted again with ethyl acet-
ate" the organic phase is dried and the solvent is distilled
off The product is purified by chromatography.
Yie,Ld: 600 mg (45 % of theory) of 2-(8-methyl-1,1-dioxo-
thiochroman-4-one-7-carbonyl O-ethyl oxime)cylohex-
ane--1,3-dione lsic]; melting point: 143~C.
35 3. 2-(8-Methyl-4-ethoxy-1,1-dioxothiochroman-7-carbonyl)cyclo-
hex;lne-1~3-dione [sic]
OH O
OC2H5
a) 8.8 g (0.031 mmol) of 8-methyl-4-ethoxy-1,1-dioxothio-
chroman-7-carboxylic acid are dissolved in 50 ml of
toluene, the solution is treated with 2 drops of dime-
0050/4~il42 CA 02227934 1998-02-23
230
thylformamide and 4.4 g (0.04 mmol) of thionyl chloride
are added. After refluxing for 4 hours, the reaction mix-
ture is concentrated. The acid chloride is directly
employed further.
b) 0.56 g of cyclohexAne-1,3-dione is initially introduced
into 10 ml of methylene chloride together with 0.47 g
(6 mmol) of pyridine. 1.5 g ~5 mmol) of the acid chloride
from 3a) in 20 ml of methylene chloride are then added
dropwise and the mixture is stirred for one hour. The re-
action solution is added to water and rendered acidic
with hydrochloric acid. The aqueous phase is extracted
with ethyl acetate, the organic phase is dried and the
solvent is removed.
Yield: 1.88 g (99 % of theory) of 0-acylated product.
c) 1.3 g (3.4 mmol) of the product from 3b are dissolved in
20 ml of acetonitrile. The reaction mixture i8 then
treated with 0.19 g (2.3 mmol) of acetone cyanohydrin and
1.7 g (17.2 mmol) of triethylamine and stirred for
2 hours. It is then added to 2N hydrochloric acid and ex-
tracted with ethyl acetate. The organic phase is then
washed with sodium carbonate solution and discarded.
Finally, the alkaline aqueous phase is rendered acidic
using hydrochloric acid. The resulting precipitate is
filtered off with suction and dried.
Yield: 0.6 g (46 % of theory) of 2-(8-methyl-4-
ethoxy-l,l-dioxothiochroman-7-carbonyl)cyclohe~ne-1,3-
dione lsic]; lH-NMR (CDCl3): ~ [ppm] - 17.5 (lH, s), 7.30
(lH, d), 7.18 (lH, d), 4.47 (lH, m), 3.95-3.83 (lH, m),
3.70-3.50 ~2H, m), 3.28 (lH, m), 2.81 (2H, tr), 2.71-2.50
(2H, m), 2.64 (3H,s), 2.43 (2H, tr)~ 2.05 (2H, m), 1.24
(3H, tr).
The comE~ounds shown in the following tables are obtA~ne~ in a
6imilar manner:
CA 02227934 1998-02-23
OOSO/46142
231
E ~ oo ~E = _,--
x ~ ~ 8----~ ~ ~ 8----" E
o~ 5 U ~~--~~ E ~ ~
-- U U U U U U U
.~ _ _ ~ _ ~ _ ~ ~ ~ ~ _ _ ~ ~ ~ E
~ E E E E E E Z ~ ~ ~ E E Z
o=u~ a $ ~ ~ ~
~ ~ x ~ X
0~ 0
r~
~U
:: ~ ~ ~ ~ O O O
O
Lq X
o
CA 02227934 1998-02-23
0050/415142
232
E = __~ E E----'" oo S E ~ "",,
o ~ E E _.
8 o o~ E r~ C ~, 8 ", o~ E ~ :~ C oo , ~ x ~ ~ ~
V v ~ C V ~ , ~ ~ oV V c~ ~ '~ E E E
E E Z r- ~ ~ ~ ~_ E Z r- ~ ~ ~ ~ E E E 2
V ~ V ~ ~ ~V $V
O - C O C O O O
~ v r ~ v ~v
X ~ ~ ~ $ ~
C ~ ~ ~ ~ ~ ~ ~ ~ _1
-
oo 5 r~
C V V .
x 3: v ~ v ~ :~
z ~
CA 02227934 1998-02-23
/4~;142
233
C~ E E ~. E ~ c
a~~~
a = ~ _ a-- E q ~ = E E
rA~ OD ''~ E '~ ~ ~ r~ c~ a ~ r ct~ ~O
X r A~ 0 E E ~
~' 2E ~' :1; :C ~ 5~! ~ r? ~ ~ ~ ~ ~ c c c c ~ G ~ C ~ OD ~ :C G
~ Z ~ ~ E ~i z r~ ~1 ~ ~ E E E E E E E E E Z o ~ ~ E
g ~ ~ O O
~ a
X ~ ~ ~ ~ ~ I ~ ~ ~ ~ ~ ~
tY r~? r? r~?
CA 02227934 1998-02-23
0050/41il42
2~4
,~
~ ~ t~
,~ ~ x ô ~ ~ ~~ ~ ~ ~ ~ ~ 8 ~ ~ ~i
V U ~ ~ ~ ~ ~ E ~ ~ ~ ~ ~ V ~ ~ ~ ~ ~ ~ ~ ~
E E E E E E E _ ~ ~ E E E E E E E E E E E E E
- - -
r~ J a i~
C X 3 1 ~ $ X ~ t ~ ~
o'
~ x o~ o ~ o~ o ~
CA 02227934 1998-02-23
0050/gl6142
235
X ~
V ~ V V ~ V
E E E E E E ~ c
V V ~ ~
C V V _l ~
N 1~1
=~ a ~ ~ ~ a ~~X~ ~
0~ 0
X ~ X ~ ~ ~ ~ O ~ O'
X ~ ~ ~ O
~ '
C
O~ ~
~
~ x ~
o
~, ~ ~ ~ oo o~ 8
o ~ ~ ~ ~ ~ ~ Z _1
CA 02227934 1998-02-23
0050/416142
236
a~
~X _
~ E ;~; '' ~' P. E u~ ~"
J ~ ~ ~ J ~ ~
~ _,
,. P~
~ / ~ X
O = U~ ~ ~ / \
0=(~ 0~
o~ o~
O O
co o o ~c co ~
o o~ o ~ ~
o ~ ~ o
o - -
a~ ~
E~ Z ~ ~'~ ~
CA 02227934 1998-02-23
0050/46142
237
The compounds I and their agriculturally utilizable salts are
suitable as herbicides, both in the form of isomer mixtures and
in pure isomer form. The isomer-cont~ining herbicidal composi-
5 tions provide highly effective control of plant growth on un-
cultivated areas, in particular at high application rates. They
act against broad-leaved weeds and grass weeds in crops such as
wheat, rice, maize, soybeans and cotton without significantly
harming the crop plants. This effect occurs especially at low ap-
10 plication rates.
In consideration of the versatility of the application methods,the compounds I or compositions contAi~ing them can additionally
be employed in a further number of crop plants for the
15 elimination of undesired plants. Suitable crops, for example, are
the following:
Allium cepa, AnAnA~ comosus, Arachis hypogaea, Asparagus
officinalis, Beta vulgaris spec. altissima, Beta vulgaris spec.
20 rapa, Brassica napus var. napus, Bra-qsica napus var.
~pobra~sica, Brassica rapa var. silvestris, Camellia sinensis,
Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
sinensis, Coffea arabica (Coffea canephora, Coffea liberica),
Cucumis sativus, Cynodon dactylon, Daucus carota, Elaeis
25 guineensis, Fragaria ve~ca, Glycine max, Gossypium hirsutum
(Go~sypium arboreum, Goqsypium herbaceum, Gossypium vitifolium),
Helianthus annuus, Hevea brasiliensis, Hordeum vulgare, Humulus
lUpUlU9, Ipomoea batatas, Juglans regia, Lens culinaris, Linum
usitatissimum, Lycopersicon lycopersicum, Malus spec., Manihot
30 esculenta, Hedicago sativa, Musa spec., Nicotiana tabacum
(N. rustica), Olea europaea, Oryza sativa, Phaseolus lunatus,
Pha~eolus vulgaris, Picea abies, Pinus spec., Pisum sativum,
Prunus avium, Prunus persica, Pyrus c lnis, Ribes sylvestre,
~icinus communis, Saccharum officinarum, Secale cereale, Solanum
35 tuberosum, Sorghum bicolor (s. vulgare), Theobroma cacao,
Trifolium pratense, Triticum aestivum, Triticum durum, Vicia
faba, Vitis vinifera, Zea mays.
Moreover, the compounds I can also be u~ed in crops which are
40 tolerant to the action of herbicides as a result of breeding,
including genetic engineering~methods.
The compDunds I and the herbicidal compositions contA;n;ng them
can be applied by spraying, atomizing, dusting, scattering or
45 watering, for example in the form of directly sprayable aqueous
solution~, powders, suspensions, even high-percentage aqueous,
oily or ~Dther suspensions, or dispersions, emulsions, oil disper-
CA 02227934 1998-02-23
0050/46;142
238
sions, pastes, dusting compositions, scattering compositions or
granules. The application forms depend on the intended uses; in
each case they should if possible ensure the finest dispersion of
the active compounds according to the invention.
Suitable inert additives are mineral oil fractions of medium to
high bo;Lling point, such as kerosene or diesel oil, further coal
tar oils and oils of vegetable or animal origin, aliphatic,
cyclic and aromatic hydrocarbons, eg. paraffin, tetrahydronaph-
10 thalene, alkylated naphthalenes or their derivatives, alkylatedhenzene3 or their derivatives, methanol, ethanol, propanol, buta-
nol, cyclohexanol, cyclohexanone or strongly polar solvents, such
as N-methylpyrrolidone or water.
15 Aqueous application forms can be prepared from emulsion concen-
trates, dispersions, pastes, wettable powders or water-dispers-
ible granules by addition of water. To prepare emulsions, paste~
or oil dispersions, the substrates 1 8iCl can be homogenized in
water as such or dissolved in an oil or solvent, by means of wet-
20 ting agentR, adhesives, dispersants or emulsifiers. However, con-
centrates consisting of active sub~tance, wetting agent, adhes-
ive, di,3persant or emul~ifier and possibly solvent or oil can
also be prepared which are suitable for dilution with water.
25 Suitable surface-active substances (adjuvants) are the Alk~
metal, alkaline earth metal or ammonium salts of aromatic sul-
fonic acids, eg. lignosulfonic, phenolsulfonic, naphthalenesul-
fonic and dibutylnaphthalenesulfonic acid, and also of fatty
acids, alkyl and alkylarylsulfonates, alkyl, lauryl ether and
30 fatty aLcohol sulfates, as well as salts of sulfated hexa-,
hepta- and oct~ec~nols, and also of fatty alcohol glycol ether,
condensation products of sulfonated naphthalene and its deriva-
tives with formaldehyde, condensation products of naphthalene or
of the naphthalenesulfonic acids with phenol and formaldehyde,
35 polyoxyethylene octylphenol [sic] ethers, ethoxylated isooctyl-,
octyl- or nonylphenol, alkylphenyl or tributylphenyl polyglycol
ethers, alkyl-aryl polyether alcoholR, isotridecyl alcohol, fatty
alcohol--ethylene oxide condensates, ethoxylated castor oil, poly-
oxyethyLene alkyl ethers or polyoxypropylene alkyl ethers, lauryl
40 alcohol polyglycol ether acetate, sorbitol esters, lignin-sulfite
waste l:iquors or methylcellulose.
Powder, scattering and dusting compositions can be prepared by
i~ing or joint grinding of the active subst~nces with a solid
45 carrier.
CA 02227934 1998-02-23
0050/4~;142
239
Granules, eg. coated, impregnated and homogeneous granules, can
be prepared by binding the active compounds to ~olid carriers.
Solid carriers are mineral earths such as silicic acids, silica
gels, silicates, talc, kaolin, limestone, lime, chalk, bole,
5 loess, ,clay, dolomite, diatomaceous earth, calcium sulfate and
magnesium sulfate, magnesium oxide, ground synthetic materials,
fertilizers, such as ammonium sulfate, ammonium phosphate, ammon-
ium nitrate, ureas and vegetable products, such as grain flour,
tree bark, wood and nutshell meal, cellulose powder or other
10 solid carriers.
The concentrations of the active compounds I in the ready-to-use
preparations can be varied within wide ranges. The formulations
in general contain from 0.001 to 98% by weight, preferably from
15 0.01 to 95% by weight, of at least one active compound. The
active compounds are employed here in a purity of from 90 to
100%, preferably 95 to 100% (according to NMR spectrum).
The compounds I according to the invention can be formulated, for
20 example, as follows
I. 20 parts by weight of the compound No. 13.1 are dissolved
in a mixture which consists of 80 parts by weight of
alkylated benzene, 10 parts by weight of the addition
product of from 8 to 10 mol of ethylene oxide to 1 mol of
oleic acid N-monoethanolamide, 5 parts by weight of cal-
cium dodecylbenzenesulfonate and 5 parts by weight of the
addition product of 40 mol of ethylene oxide to 1 mol of
castor oil. By pouring out the solution and finely dis-
persing it in 100,000 parts by weight of water, an
aqueous dispersion is obtained which contains 0.02% by
weight of the active compound.
II. 20 parts ~y weight of the compound No. 13.3 are dissolved
in a mixture which consists of 40 parts by weight of
cyclohe~none, 30 parts by weight of isobutanol, 20 parts
by weight of the addition product of [lacuna] 40 mol of
isooctylphenol and 10 parts by weight of the addition
product of 40 mol of ethylene oxide to 1 mol of castor
oil. By pouring the solution into and finely dispersing
it in 100,000 parts by weight of water, an aqueous dis-
persion is obtained which contains 0.02% by weight of the
active compound.
45 III. 20 parts by weight of the active compound No. 13.8 are
dissolved in a mixture which consists of 25 parts by
weight of cyclohe~none, 65 parts ~y weight of a mineral
CA 02227934 1998-02-23
0050/4~il42
240
oil fraction of boiling point from 210 to 280~C and 10
parts by weight of the addition product of 40 mol of
ethylene oxide to 1 mol of ca~tor oil. By pouring the
solution into and finely dispersing it in 100,000 parts
by weight of water, an aqueous dispersion is obtained
which contains 0.02% by weight of the active compound.
IV. 20 parts by weight of the active compound No. 13.9 are
well mixed with 3 parts by weight of the sodium salt of
diisobutylnaphthalene-~-sulfonic acid, 17 parts by weight
of the sodium salt of a lignosulfonic acid from a sulfite
waste liquor and 60 parts by weight of powdered silica
gel and the mixture is ground in a hammer mill. By finely
dispersing the mixture in 20,000 parts by weight of
water, a spray mixture is contAineA [sic] which contains
0.1% by weight of the active compound.
V. 3 parts by weight of the active compound No. 13.5 are
mixed with 97 parts by weight of finely divided kaolin.
In this manner, a dusting composition is obtAine~ which
contains 3% by weight of the active compound.
VI. 20 parts by weight of the active compound No. 13.15 are
intimately mixed with 2 parts by weight of calcium salt
of dodecylbenzenesulfonic acid, ~ parts by weight of
fatty alcohol polyglycol ether, 2 parts by weight of
sodium salt of a phenol/urea/formaldehyde condensate and
68 parts by weight of a paraffinic mineral oil. A stable
oily dispersion is obtAine~.
VII. 1 part by weight of the active compound No. 13.16 is
dissolved in a mixture which consists of 70 parts by
weight of cycloh~none, 20 parts by weight of ethoxy-
lated isooctylphenol and 10 parts by weight of ethoxy-
lated castor oil. A stable emulsion concentrate is ob-
tA i ne~ .
VIII. 1 part by weight of the active compound No. 13.17 is dis601ved in a mixture which consists of ~0 parts by
weight of cyclohe~Ansne and 20 parts by weight of Wettol~
EM 31 (= nonionic emulsifier based on ethoxylated castor
oil). A stable emulsion concentrate is obtA j n~ .
The app:Lication of the active compounds I or of the herbicidal
45 composi1ions can be carried out pre ~ -rgence or post-emergence.
If the active compounds are less tolerable for certain crop
plants, application techniques can be used in which the herbici-
' 0050/41jl42 CA 02227934 1998-02-23
.
241
dal compositions are sprayed with the aid of the 6pray equipment
such that the leaves of the sensitive crop plants are not
affected if possible, while the active compounds reach the leaves
of undesired plants growing under them or the uncovered soil
5 surface (post-directed, lay-by).
Depending on the aim of control, time of year, target plants and
stage of growth, the application rates of active compound are
from 0.001 to 3.0, preferably from 0.01 to 1.0, kg/ha of active
10 substance (a.s.).
To widen the spectrum of action and to achieve synergistic ef-
fects, the benzoyl derivatives I can be mixed and applied
together with numerous representatives of other herbicidal or
15 growth-regulating active compound groups. For example, suitable
mixture components are 1,2,4-thiA~iazoles, 1,3,4-thiA~iAzoles,
amides, aminophosphoric acid and its derivatives, aminotriazoles,
anilides, aryloxy-/heteroaryloxyAlkAnoic acids and their deriva-
tives, benzoic acid and its derivatives, benzothiA~iAzinones,
20 2-(heteroaryl/aroyl)-1,3-cyclohexadiones, heteroaryl aryl ke-
tones, benzylisoxazoli~ino~s, meta-CF3-phenyl derivatives, carba-
mates, ,quinolinecarboxylic acid and its derivatives, chloroacet-
anilides, cycloheYAne-1,3-dione derivatives, diazines, dichloro-
propionic acid and its derivatives, dihydrobenzofuran~, dihy-
25 drofuran-3-ones, dinitroanilines, dinitrophenols, diphenyl
ether~, dipyridyls, halocarboxylic acids and their derivatives,
ureas, 3-phenyluracils, imidazoles, imidazolinones, N-phe-
nyl-3,4,5,6-tetrahydrophthalimides ~ OYA~ i A zoles, oxiranes,
phenols, aryloxy- and heteroaryloxyphenoxypropionic acid esters,
30 phenylacetic acid and its derivatives, 2-phenylpropionic acid and
its derivatives, pyrazoles, phenylpyrazoles, pyridazines, pyri-
dinecarboxylic acid and its derivatives, pyrimidyl ethers,
sulfonamides, sulfonylureas, triazines, triazinones, triazoli-
nones, ltirazolecarboxamides and uracils.
Additionally, it may be useful to apply the c- ,-unds I on their
own or in combination with other herbicides additionally mixed
with further crop protection agents, for example with agents for
control:Ling pests or phytopathogenic fungi or bacteria. Further
40 of interest is the miscibility with mineral salt solutions, which
are emp:Loyed for the elimination of nutritional and trace element
deficiencies. Nonphytotoxic oils and oil concentrates can also be
added.
0050/4~il42 CA 02227934 1998-02-23
242
Use examples
It was Ipossible to show the herbicidal action of the benzoyl de-
rivatives of the formula I by greenhouse tests:
The cultivation contA;ners used were plastic flowerpots contain-
ing loamy sand with about 3.0% humus as a substrate. The seeds of
the test plants were sown separately according to species.
lO In the case of pre-emergence treatment, the active compounds sus-
pended or emulsified in water were applied directly after sowing
by means of finely dispersing nozzles. The containers were light-
ly wate:red to promote germination and growth and then covered
with transparent plastic hoods until the plants had taken root.
15 Thi~ corering causes uniform germination of the test plants if
this has not been adversely affected by the active compounds.
For the purposes of post-emergence application, the test plants,
depen~ g on growth form, were first raised to a height growth of
20 from 3 ltO 15 cm and only then treated with the active compounds
suspe~e~ or emulsified in water. For this purpose, the test
plants were either sown directly and grown in the same contA~rs
or they were first raised separately as seedlings and trans-
planted into the test contAin~rs a few days before treatment.
The app:Lication rate for post-emergence treatment was from 0.5 to
0.25 kgJha of a.s.
The plants were kept species-specifically at from 10-25~C or 20-
30 35 C. The test period extended over 2 to 4 weeks. During this
time, the plants were tended and their reaction to the individual
treatments was assessed.
As~;essment was carried out on a scale of from 0 to 100. 100 here
35 means no emergence of the plants or complete destruction of at
least the above-ground parts and 0 means no damage or normal
course of growth.
The plants used in the greenhouse tests were made up of the fol-
40 lowing ~Ipecies:
CA 02227934 1998-02-23
0050/46il42
243
Botanical Name English Name
Chenopodium album lambs-quarters
(CHEAL) (goosefoot)
Ipomoea subspecies morning glory
5 (IPOSS)
Sinapis alba white mustard
(SINAL)
Solanum nigrum black nightshade
(SOLNI)
10Triticum aestivum summer wheat
~TRZAS)
(ZEAMX) Indian corn
Table 1'~
Herbicidal activity in the case of post-emergence application in
the greenhouse
Ex.No. 13.3
Application rate 0 5 ¦ 0.25
(kg/lha of a.~
30Te~3t plants Damage in %
TRZAS 0 0
CHEAL 95 95
SINAL 95 95
35 SOLNI 100 95
Table 1~
Herbicidal activity in the case of post-emergence application in
the greenhouse
~,~
~ 0
-OOSO/46142 CA 02227934 1998-02-23
244
EX. No. 13.13
Application rate
(kg/ha of a.s.) 0.25
Test plants Damage in %
ZEAMX 10 0
CHEAL 95 95
IPOSS 100 100
SOLNI 100 100
~5