Note: Descriptions are shown in the official language in which they were submitted.
CA 02228674 1998-02-04
W O 97/06233 1 PCT~US96/11409
PIGMENTED RHEOPECTIC CLEANING COMPOSITIONS
WITH THIXOTROPIC PROPERTIES
Field of the Invention
This invention relates to thickened liquid bleach-cc.. ~ compositions
useful for hard surface cle~ning, and particular to such compositions which
include bleach-stable pigments.
Back~round of the Invention
Thickened bleach compositions possess a number of advantages over
unthickened bleach compositions. The more viscous, thickened solutions a&ere to
vertical and inclined surfaces for a longer period of time as co~ aled or
disinfectant activity of the thickened compositions is more effective on the
int~nclefl areas.
To provide a thickened hypochlorite composition having an acceptable
shelf-life, the rate of decomposition of alkali metal hypochlorite as well as the
phase behaviour of the composition must be considered. As is known, alkali metalhypochlorite degradation may be illustrated by the following equation:
2 o NaOC 1 ~ NaC 1 + I/2 ~2
Many con~ lional thickening agents accelerate the degradation of the
hypochlorite and thus are problematic for use in hypochlorite compositions. Also,
~ the inclusion of conventional thickening agents and surf~ct~nt~ is difficult because
2 5 the resulting hypochlorite composition has a tendency to separate into two or more
phases, particularly at elevated temp-,ldLules. Many thickening agents are
~emselves unsta'ble in the presence of an alkali metal hypochlorite. Thus,
SUBSTITUTF SHEEl ~ULE26~
CA 02228674 1998-02-04
W O 97/06233 2 PCT~US96/11409
achieving sufficient viscosity in hypochlorite compositions by conventional agents
and additives in addition to providing a hypochlorite composition having
acceptable stability is difficult.
It is also desirable, for commercial and aesthetic reasons, to provide
5 thickened bleach-cont~ining cleaner compositions which have distinctive
coloration. The realm of bleach stable colourants is fairly small due to the
tendency of organic dyes to degrade in the presence of strong oxidizers.
U.S. Patent No. 4,474,677 (Foxlee) suggests the use of certain halogenated
copper phthalocyanine pigments for aqueous alkali metal hypochlorite
l o compositions. While this class of pigments is considered to be bleach-stable, slow
degradation of the pigment molecule releases copper which catalyzes the
degradation of hypochlorite. U.S. PatentNo. 4,271,030 (Brierley) describes a
suspension of ultramarine blue using calcium soap flocs. Use of calcium soap
flocs is not desirable due to the high concentration required, 50% by volume of the
15 composition, or from a cleaning standpoint due to a tendency to precipitate onto
hard surfaces. U.S. Patent No. 4,952,333 (Cramer) describes a bleaching and
brightening composition using polymers to suspend ultramarine blue in an
Pm~ ified polymer matrix. This composition however would not be an effective
hard surface cleaner due to its low detergency. U.S. PatentNo. 4,917,814
20 (MacIntyre) describes the use of cobalt ~lllmin~te to colour thickened hypochlorite
solutions. Cobalt pi~nentc were found to be superior to ultramarine blue for
suspension properties. While this is not disputed, it should be pointed out that,
under higher temperature conditions than employed in MacIntyre, surfactant
thickened compositions will lose viscosity and allow se-liment~tion of the pigment.
25 Consumer products require stability when exposed to these higher te~ cl~luies during distribution and storage.
~lJBST~TUTE SHEET ~RULE 26~
CA 02228674 1998-02-04
W O 97/06233 3 PCTAUS96/11409
- SummarY of the Invention
This invention provides thickened hypochlorite compositions with enhanced
rheological pr~c,lies which are capable of stably suspending inorganic pigments.The thickening system is a blend of surfactants and clay that is rheopectic at low
5 shear rates, which helps to stably suspend the pigment, but thixotropic at higher
shear rates, which allows the product to dispense easily from a container to aid in
the cleaning of hard surfaces. By definition, rheopexy and thixotropy are opposite
flow properties. Having both properties present in a single fluid is quite
advantageous.
Non-limiting examples of inorganic colourants that can be ~ltili7e(1, include
ultramarine blue, cobalt alllmin~te blue, Lil~iulll dioxide and calcium carbonate.
This invention focuses on the use of ultramarine blue which is preferred due to its
consumer appeal, low toxicity, and colour intensity col.l~aled to other pigments.
The composition behaves as a highly structured liquid and exhibits some
unique and unexpected flow properties. This occurs despite the low solids content,
less than 10%, of the formula as compared to other structured liquids, some of
which are known in the category of liquid laundry d~l~ .genl~. This characteristic
helps to solve the problem of pigment se-lim~nt~tion while still m~int~inin~ thin
fluid flow properties which help to achieve good surface coverage for products
2 o such as liquid toilet bowl cleaners. The composition also has good phase-stability
and hypochlorite-stability.
The compositions of this invention comprise:
(a) an alkali metal hypochlorite, preferably sodium hypochlorite,
(b) bentonite clay,
2 5 (c) a tertiary amine oxide having one long-chain alkyl group of from 10
to 16 carbon atoms and two lower alkyl groups,
(d) an alkali metal salt, preferably sodium chloride,
SUB~ 111 IJTE SHEET (RULE 26)
-
CA 02228674 1998-02-04
W O 97/06233 4 PCTAUS96/11409
(e) a pH stabilizer to provide a pH of l l or higher,
an alkali metal Clo- Cl6 alkyl sarcosinate,
(g) a Clo - Cl4 straight chain alkylbenzène sulphonate, and
(h) an inorganic pigment, preferably ultramarine blue.
5 The desired rheological properties and phase stability described above are achieved
through the careful blending of clay, surfactants and electrolytes. In particular, the
molar ratio of the tertiary amine oxide (c) to the alkylbenzene sulphonate (g)
should range from about 5: l to about l l: l . Useful non-pigmented compositionsanalogous to the compositions of this invention can be ~l~paled without the
10 pigment (h).
The viscosity of the composition can range from about 200 cps to about
lO00 cps. The preferred range is from about 300 - S00 cps.
Brief Description of the Drawin~s
Figures l and 2 are graphs showing the rheological properties of a preferred
embodiment of this invention.
Figures 3 and 4 are graphs showing certain rheological properties of a
for~ tion according to this insertion (Figure 3) and conl~al~ble properties of asimilar composition without the clay (Figure 4).
Detailed Disclosure
The inventive composition is a hypochlorite stable, single phase, thickened
hypochlorite bleach composition capable of adhering to vertical or inclined
surfaces longer than thinner compositions. The composition is an effective agent2 5 for stain and soil removal as well as disinfection. The high level of hypochlorite
stability and single solution phase behaviour of the composition enables the
composition to have an acceptable shelf life. The compositions include also an
~UBSTITUT~ SHEE~ ~RULE 26)
CA 02228674 1998-02-04
W O 97/06233 5 PCTrUS96/11409
organic pigment in suspension. In these pigmented compositions, the colour
stability, particularly where the pigment is ultramarine blue, is uniquely
advantageous.
Preferably the alkali metal of the alkali metal hypochlorite is selected from
5 lithillm, potassium or sodium. For purposes of cost and availability, sodium
hypochlorite is currently preferred. The alkali metal hypochlorite may have other
by-products of the m~nllf~r*lring process present without adversely affecting the
composition. The amount of alkali metal hypochlorite employed is within the
range of about O.S weight % to about 10 weight %, preferably from 1.0 weight %
o to 5.0 weight %, and more preferably from l.S weight % to 3.0 weight %.
Bentonite clay is a colloidal hydrated alllminllm silicate clay found in North
America. It consists principally of montmorillonite (A12O3.4SiO2.H2O) and
usually also contains some magnesium, iron and calcium carbonate. Bentonite
clay is preferred for use in the compositions of this invention, but other clays of
15 similar structure and/or properties may be used. The amount of Bentonite clay in
the composition should range from about 0.15 weight % to about l.S weight %,
preferably from 0.25 weight % to 1.0 weight %.
The tertiary amine oxide is of the formula:
R2
Rl_N~
R3
wherein Rl is an alkyl group cont~ining from about 10 to about 16 carbon atoms
and each of R2 and R3 is a lower alkyl group cont~ining from 1 to 3 carbon atoms.
~UBSTITUTE S!~ET (RULE 26)
CA 02228674 1998-02-04
W O 97/06233 6 PCTAJS96/11409
~1, R2 and R3 may be a straight or branched chain; Rl may contain an odd or an
even number of carbon atoms. Amine oxides of mixed chain length may be used,
which may contain a predomin~nce of one or more chain lengths. Preferably, the
tertiary amine oxide is selected from myristyldimethylamine oxide,
5 lauryldimethylamine oxide, and mixtures thereof. Most preferably employed is
myristyldimethylamine oxide. The amount of the tertiary amine oxide employed is
pLerclably in the range from about 0.5 weight % to about 2.5 weight %, more
preferably from 0.9 weight % to 1.8 weight %, and most preferably from 1.0
weight % to 1.5 weight %.
The alkali metal salt may be selected from any number of water-soluble
alkali metal salts and mixtures thereof, with the alkali metal preferably being
lithium, potassium, or sodium, and the anion ion preferably being a halide (such as
chloride, fluoride, bromide and iodide). More preferably the alkali metal salt is
selected from the group consisting of sodium chloride, lithium chloride, potassium
chloride, and mixtures thereof. For purposes of cost and availability, the alkali
metal salt most preferred is sodium chloride and may be used in varying amounts
to reduce hypochlorite degradation, limited only by the avoidance of a "salting
out" of the solution (where the surfactants become insoluble in water). When
sodium chloride is used, the preferred amount is in the range of about 0.25 weight
2 0 % to about 2.0 weight %, preferably from 0.5 weight % to 1.5 weight %.
An alkali metal hydroxide is the plefe,led pH stabilizer included in the
composition, although any pH stabilizer may be employed as long as the stabilityand viscosity of the composition are not adversely affected. Other pH stabilizers
which may be used, for example, include carbonate buffers. The alkali metal of
2 5 the preferred hydroxide may be lithium, potassium, or sodium. Sodium hydroxide
and potassium hydroxide are particularly useful pH stabilizers due to cost and
availability, with sodium hydroxide most preferred. The alkali metal hydroxide is
SUBSTITUTE SHEET (RULE 26)
CA 02228674 1998-02-04
W O 97/0~233 7 PCT~US96/11409
included in the composition in an effective amount to adjust the composition to a
pH level of at least about 11, more preferably from 12 to 13.5, and most preferably
within the range from 12 to 13.
The alkali metal alkyl sarcosinate may be represented by the formula:
CH3
R4 - C - N - CH2COO- M+
ll
0 wherein R4 is a branched or straight chain ~ 1 o-C 16 alkyl group and M is an alkali
metal cation (such as lithium, potassium or sodium). Sodium lauroyl sarcosinate is
most ~rere~.~ d. The amount of alkali metal alkyl sarcosinate that may be used
preferably ranges from about 0.10 weight % to about 0 75 weight %, more
preferably 0.12 weight % to 0.60 weight %, and most preferably from 0.15 weight
% to 0.30 weight %.
The aL~ali metal Clo to C14 straight chain alkylbenzene sulphonate is
preferably defined wherein the alkali metal is pot~eil-m, lithium, or sodium. Most
preferably employed is sodium dodecylbenzene sulphonate. Preferably the àmount
of sulphonate used is within the range of from about 0.08 weight % to about 0.8
2 o weight %, more preferably from 0.1 weight % to 0.5 weight %, and most
preferably from 0.15 weight % to 0.4 weight %.
In these pi~nen~ed compositions, the plerc.l~,d pigment is ultramarine blue
which is an inorganic silicate. Although this material is inert to hypochlorite
oxidation and does not catalyze decomposition of hypochlorite, it is insoluble and
2 5 requires suspension in the hypochlorite solution. Such suspension cannot be
achieved merely by dispensing particles of ultramarine blue in hypochlorite
solution, because the pigment has a density of 2.35 and settles out even when it is
of very fine particle size. The thickening system employed in the composition ofthis invention provides excellent suspension for ultramarine blue pigment particles.
SIJBSTITUTE SHEET ~RULE 26)
CA 02228674 1998-02-04
W O 97/06233 8 PCT~US96/11409
The amount of ultramarine blue in the composition of this invention ranges from
about 0.01 weight % to about 0.50 weight %, preferably about 0.05 weight %.
The molar ratio of the tertiary amine oxide to alkali metal alkylbenzene
sulphonate should fall within the range of from about 5 :1 to about 1 1:1.
5 Preferably, the molar ratio is from 6:1 to 10:1, and more preferably from 7:1 to
9:1.
The composition offers improved viscosity for alkali metal hypochlorite
bleaches while at the same time providing a commercially acceptable pigmented
composition with excellent colour stability. Although not wishing to be bound to10 any particular theory, it is believed that the primary interaction is between the clay
and the amine oxide components of the formula. In the preferred embo-liment of
the example set forth below, the combination of the clay, sodium chloride, and the
sodium hydroxide in solution causes the clay platelets to align in an edge-to-face
structure. Some of the amine oxide acts to stabilize the structure through both
15 ionic and steric interaction. Sulphonate and sarcosinate surf~t~nt~ combine with
the rem~inin~ amine oxide to forrn organic structures or micelles which boost
viscosity. It is further theorized that these micelles interact with the clay structure
to develop the unique rheology of the composition.
This invention provides a commercially advantageous coloured thickening
2 0 system which exhibits thixotropic properties for easy dispensing, particularly from
a spray container. Cleaning products employing this thickening system have a
sufficiently high level of quiescent viscosity to keep the inorganic pigment
particles in suspension.
The invention will be better understood by reference to the following
2 5 examples which are included for the purpose of illustration, and are not be be
construed as limit~tions.
~UBSTITUTE SHEET (RULE 26)
CA 02228674 1998-02-04
W O 97/06233 9 PCT~US96/11409
Example l
A blue-pigmented hand surface cleaner was prepared which had the
following ingredients, all percentages being by weight.
In~redient
bentonite clay (Gelwhite H) 1.00%
ultramarine blue 0.05%
sodium chloride 1.00%
sodium hydroxide 2.50%
myristyldimethylamine oxide 5.60%
sodium hypochlorite 2.50%
sodium dodecylbenzene sulphonate 0.72%
sodium lauroyl sarcosinate 1.00%
fragrance 0.065%
deionized water q.s. to 100%
The cleaner composition was p~ ed by dispensing in the main vessel ( l)
Gelwhite H, a montmorillomite clay (Southern Clay Products) in water, using a
homogenizer until the clay is fully hydrated, and adding the Ultramarine blue with
further agitation. In a separate vessel (2), sodium chloride and a 25% solution of
2 o sodium hydroxide were dissolved in water. The contents of vessel (2) were added
to vessel (l) with high agitation. The rem~ining ingredients were added, with
agitation, in the following order: Ammonyx MO, a 30% solution of
myristyldimethylamine oxide, Stepan Company; fragrance; a 16.67% solution of
sodium hypochlorite bleach; Biosoft D-40, a 40% solution of sodium
25 dodecylbenzene sulphonate, Stepan Company; and Hamposyl L-30, a 30% solution
of sodium lauroyl sarcosinate, W.R. Grace & Co~ y.
~UBSTITUTE SHEET ~RlJLE 26)
CA 02228674 1998-02-04
W O 97/06233 10 PCTAUS96/11409
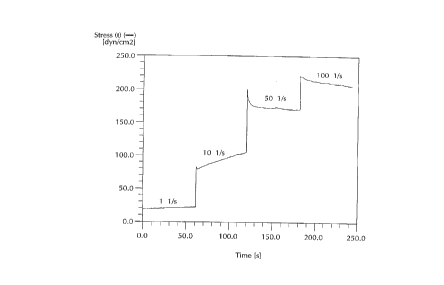
Figure 1 shows the rheology profile of this preferred embodiment. It
snmm~rizes the shear stress as a function of time at four shear rates. The formula
appears to be rheopectic at both 1 and 10 sec-l, under conditions of constant shear.
The thixotropic character is evident at 50 sec~l. Figure 2 captures the stress growth
5 behaviour at the inception of flow at the first shear rate, 1 sec~ 1. Testing was
conducted with the Rheometrics Scientific RFSII rheometer, 50 mm parallel plate,0.9 mm spacing, 316SS tooling, 25C, 0.002 - 10 gr-cm force rebalance.
An analogous formula without the clay and pigment components exhibits
dramatically different rheological properties. See Figures 3 and 4. In the
10 e~min~tion of plots of G', a measure of the elastic strength of a viscoelastic fluid,
and G' ', a measure of the mechanical energy dissipated during the deformation of
structured fluid, the formula with clay behaves as a highly structured fluid with
significant strain dependence. The analogous formula behaves as a predominzmtly
viscous fluid with no significant strain dependence. This difference indicates a15 significant interaction between the clay component and the surfactants present in
the forrn~ tion.
Examples 2-5
Following the procedure of Example 1, the following additional compositions
were prepared:
SUBSTITUTE SHEET (RULE 26)
CA 02228674 1998-02-04
W O 97/06233 11 PCT~US96/11409
v~ 8
-- o ~ o o o o o ~, ~
~1 ~. ~. ? ~ ~", ~ . V,, _ _ o o
~ -- ~ O O
o
.~ o ~ 1-- oo o o o ~ ~ --
~1 ~ ~ ' ~ ' ~ ~ "'' -- ~ ~ U~
o
o
. ~ ~ o o o o o ~ t-- --
~1 ~ ~ ~' ~ ' ~ ~ ~ a~
C'l o
-- o ~ o o o o ~ ~
~1 o. o., , u~, ~, v~ o., _ o. o
_ o ~ V~ ~ _ o o
X r
r~
O
X .
~ ~ O ~ ~
a ~? a a
O ~ ~ ~ ~ o ~ O
SUBSTITUTE SHE~T (RUI E 26)
CA 02228674 1998-02-04
W O 97/06233 ~2 PCT~US96/11409
Comparative Example
A cleaning composition containing ultramarine blue pigment, but without
bentonite clay was pr~aled, and the pigrnent-settling characteristics were
co~ ~ed with the composition of Example 1. The comparative composition was
5 ~l~al~d using the method of Example 1. The two compositions were m~int~ined
in a quiescent state for a period of six weeks at 40~C. The following table shows the
ingredients of the compositions and the relevant rheological data.
TABLE
(~omparative
In~redient Example 1 Example
bentonite clay 1.00% --
ultramarine blue 0.05% 0.025%
sodium chloride 1.00% 1.()0%
sodium hydroxide 2.50 % 2.60 %
myristyldimethylamine oxide (30%) 5.6()% 6.20 %
sodium hypochlorite 2.50 % 2.50 %
sodium dodecylbenzene sulphonate (40 % ) 0./2 % 0.~0 %
sodium lauroylsarcosinate (30%) 1.00% 1.0()%
~agrance 0.~65 % 0.0 /5%
deionized water q.s. to 1(~0% q.s. to 100%
viscosity 464 448
pigment settling none a~ter 6 settled during 3l~1
weeks week
10 These data show that, in contrast to the excellent suspension characteristics of
Example 1, in the Comparative Example, which contains only half the amount of
pigment, settles out within three weeks.
SIJBSTITUTE SHEET (RULE 26)
_