Note: Descriptions are shown in the official language in which they were submitted.
CA 02228886 1998-02-06
-1-
Apparatus For The Purification Of Liquids And A
ethod Of Manufacturing And Of Operating ame
FIELD OF THE INVENTION
The present invention relates to an apparatus for the purification of liquids
and a method of manufacturing and a method of operating same. More
particularly, the
present invention relates to an apparatus for the deionization of liquids, and
in particular
water, by electrodeionization and a method of manufacturing and a method of
operating the
apparatus.
BACKGROUND OF THE INVENTION
The purification of liquids is of great interest in many industries and, in
particular, pure water is used for many industrial purposes such as in the
production of
semiconductor chips, pharmaceuticals, etc. and removing ionic impurities
(deionizing;) is
one of the necessary stages of purifying water for such processes.
Recently, electrodeionization apparatus has become one of the preferred
methods of deionizing water and other liquids. The term "electrodeionization"
generally
refers to an apparatus and/or methods for removing ionic impurities from
liquids which
employ a combination of ion exchange resins, ion exchange membranes and
electrical
currents to transfer ions from the liquid to be deionized to another, "waste"
liquid.
Known electrodeionization (EDI) apparatus, such as those sold by the
assignee of the present invention, generally comprise alternating arrangements
of cation
permeable membranes and anion permeable membranes defining compartments
therebetween. In alternate ones of these compartments, there is provided ion
exchange
material and the compartments with the ion exchange material are referred to
as diluting
compartments, as ionic impurities in the fluid in these compartments are
removed. The
compartments which do not contain the ion exchange material are referred to as
concentrating compartments) as the ionic impurities removed from the diluting
CA 02228886 1998-02-06
-2-
compartments are received in the concentrating compartments.
In use, liquid to be deionized is passed through the diluting compartments
and the "waste" liquid is passed through the concentrating compartments. The
ions in the
liquid to be deionized migrate from the diluting compartments through the ion
exchange
material and ion permeable membranes to the "waste" liquid in the
concentrating
compartments under the impetus of an electrical current which is passed
through the
compartments. The waste liquid which has received the ions while flowing
through the
concentrating compartments is discarded or partially recycled and the
deionized liquid,
which has given up its ions while flowing through the diluting compartments is
recovered
as the desired deionized liquid.
While the ionic impurities removed from the first liquid are commonly ions
of mineral salts, such as NaCI, as used herein the term ionic impurities is
intended to
comprise both ions and ionizable species, such as dissolved CO2) silica,
ammonia and
ionizable organic molecules.
Various arrangements of EDI apparatus are known and used. For example,
United States Patent No. 4,632,745 to Giuffrida et al. discloses an EDI
apparatus and
method. United States Patent No. 5,1S4,809 to Oren et al. discloses a process
for
deionizing water using an EDI apparatus wherein the ion exchange material is a
mixture of
anion resin beads of substantially uniform size and cation resin beads of
substantially
uniform size. The contents of these two references are incorporated herein by
reference.
However, problems exist with known EDI apparatus. For example, they
must be manufactured with a number of diluting and concentrating compartments
which is
sufficient to achieve a desired degree of deionization for given liquids. To
date, it has been
difficult to determine the number of compartments required for a given
installation and it is
not uncommon to have an over specified EDI apparatus (i.e. - one with more
compartments
than are otherwise needed), thus unnecessarily increasing the cost of the
system, or an
CA 02228886 1998-02-06
-3-
under specified system (i.e. - one with fewer compartments than necessary to
achieve a
required degree of deionization of the first liquid) which can necessitate
remedial redesign
and/or reconstruction of the EDI apparatus or, at best, non-efficient
operation of the EDI
apparatus.
An additional problem exists in that it has been difficult to determine the
amount of electrical current applied to the EDI apparatus which is necessary
to achieve the
desired degree of deionization. In addition, changes in the makeup of the
liquid to be
deionized and/or the waste liquid can change the amount of current required to
achieve a
desired level of deionization. Thus, EDI apparatus are typically operated with
conservative
electrical current levels (i.e. - electrical currents which are higher than
are likely optimal)
resulting in increased power consumption by the EDI apparatus and
corresponding reduced
power efficiency.
Accordingly, it is desired to manufacture EDI apparatus substantially
without undue over specification and to operate EDI apparatus in a
substantially power
efficient manner.
.SUMMARY OF THE INVENTION
It is an object of the present invention to provide a novel EDI apparatus and
method of manufacturing an EDI apparatus which obviates or mitigates at least
one of the
disadvantages of the prior art. It is a further object of the present
invention to provide a
novel method of operating an EDI apparatus which monitors and controls the
electrical
current used in said EDI apparatus.
According to a first aspect of the invention, there is provided a method of
manufacturing an EDI apparatus for the deionization of a first liquid supplied
at or below a
given maximum flow rate (Q), said apparatus comprising a cathode and an anode
with a
number of diluting compartments and concentrating compartments positioned
between the
cathode and the anode and arranged in an alternating manner) said apparatus
including
CA 02228886 1998-02-06
-4-
means for passing said first liquid through said diluting compartments at or
below said
given maximum flow rate and means for passing a second liquid through said
concentrating
compartments to receive ionic impurities from said first liquid when a
electrical current is
applied between said cathode and said anode, comprising the steps of:
determining the concentration of ionic impurities in the first liquid to be
processed within said apparatus;
selecting a desired final concentration of ionic impurities for the first
liquid
after it has been processed within said apparatus;
selecting the number (n) of diluting compartments to be included in said
apparatus, said selected number of diluting compartments being at least one;
selecting the number of concentrating compartments to be included in said
apparatus, the selected number of concentrating° compartments being at
least one greater
than said selected number of diluting compartments;
selecting an electrical current (I) to be applied between said anode and
cathode, wherein the selection of said number of diluting compartments and the
selected
electrical current to be applied is such E f is greater than 1 in
1
F
Ej=
( Q) EDc~XZ~
n ;
where F is the Faraday constant to convert coulombs to moles, Dc; is the
difference in the
concentration of impurity i in the first liquid to be processed and the
selected final
concentration of impurity i, z; is the charge of impurity i and ~~c; x z; is
selected from the
larger of the summation over the cationic impurities and the anionic
impurities; and
assembling said EDI apparatus with said selected number of diluting
compartments .
Preferably, Ef is in the range of from 1 to about 50. More preferably, Ef is
in the range of from about S to about 50. More preferably, Ef is in the range
from about S
CA 02228886 1998-02-06
-$-
to about 20. More preferably, Ef is in the range of from about 8 to about 15.
According to another aspect of the present invention, there is provided a
method of operating an EDI apparatus for the deionization of a first liquid,
said apparatus
comprising a cathode and an anode with alternating diluting and concentrating
compartments positioned between the cathode and the anode) the number (n) of
diluting
compartments being at least one and the number of concentrating compartments
being at
:least one greater than the number of diluting compartments, said apparatus
including means
For passing said first liquid through said number of diluting compartments at
or below a
given maximum flow rate (Q) and means for passing a second liquid through said
number
of concentrating compartments to receive ionic impurities from said first
liquid when a
electrical current (I) is applied between said cathode and said anode,
comprising the steps
of:
determining the difference between the concentration of ionic impurities of
said first fluid prior to processing by said apparatus and a concentration of
ionic impurities
for said fluid after processing;
determining the flow rate (Q) of the first fluid through said at least one
diluting compartment;
supplying electrical current (I) between said anode and said cathode such
that Ef is at least 1 in
1
E- F
I
( Q) EOc,XZ~
n ;
where F is the Faraday constant to convert coulombs into moles, ~c; is the
difference in the
concentration of impurity i in the first liquid to be processed and the
selected final
concentration of impurity i, z; is the charge of impurity i and ~Oc; x z; is
selected from the
larger of the summation over the cationic impurities and the anionic
impurities.
CA 02228886 1998-02-06
-6-
Preferably, E f is in the range of from 1 to about 50. More preferably, Ef is
in the range of from about 5 to about 50. More preferably ) E f is in the
range from about 5
to about 20. More preferably, E f is in the range of from about 8 to about 15.
According to yet another aspect of the present invention, there is provided
an apparatus for the electrodeionization of a fluid, comprising:
a cathode;
an anode;
an alternating series of at least one diluting compartment and a number of
concentrating compartments at least one greater than the number of diluting
compartments,
each compartment defined between cation and anion permeable membranes and
positioned
between said cathode and said anode;
means to introduce said fluid to be deionized to said diluting compartments;
means to remove said fluid to be deionized from said diluting compartments
after
processing therein;
means to supply a waste fluid to said concentrating compartments and to remove
;paid waste fluid therefrom;
electrical current supply means to supply an electrical current (I) to flow
between
said cathode and anode;
means. to determine the difference in the concentration of ionic impurities in
said
fluid before deionization and after deionization within said at least one
diluting
compartment;,
means to determine the flow rate (Q) of said fluid to be deionized through
said
diluting compartment;
control means responsive to each of said concentration difference and said
determined flow rate to alter the supply of electrical current flowing between
said anode
and cathode such Ef is greater than 1 in
CA 02228886 1998-02-06
_7_
I
E- F
I
~~c~XZr
n r
where F is the Faraday constant to convert coulombs into moles, Oc; is the
difference in the
concentration of impurity i in the first liquid to be processed and the
selected final
concentration of impurity i, z; is the charge of impurity i and ~Ac; x z; is
selected from the
larger of the summation over the cationic impurities and the anionic
impurities.
S
Preferably, E f is in the range of from 1 to about 50. More preferably, E f is
in the range of from about 5 to about 50. More preferably, Ef is in the range
from about 5
to about 20. More preferably, Ef is in the range of from about 8 to about 15.
BRIEF DESCRIPTION OF THE DRAWINGS
Preferred embodiments of the present invention will now be described) by
way of example only, with reference to the attached Figures, wherein:
Figure 1 shows a block diagram representation of an EDI apparatus in
accordance with the present invention;
Figure 2 shows a chart of operating results showing the relationship of
resistivity to an E-Factor, as calculated in accordance with an embodiment of
the present
invention, for a test EDI apparatus;
Figure 3 shows a flowchart of a method of manufacturing an EDI apparatus
in accordance with an embodiment of the present invention; and
Figure 4 shows a flow chart of a method of manufacturing an EDI apparatus
in accordance: with another embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
While the following discussion only specifically discusses the deionization of
water, it will be apparent to those of skill in the art that the present
invention is not so
CA 02228886 1998-02-06
_g_
limited and it is contemplated that the present invention may be favorably
employed for any
suitable deionization process, as will occur to those of skill in the art.
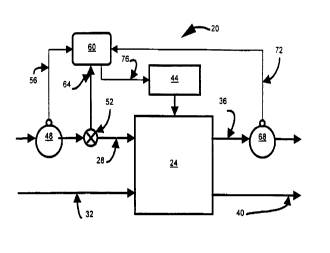
An EDI apparatus in accordance with an embodiment of the present
invention is indicated generally at 20 in Figure 1. EDI apparatus 20 includes
a known EDI
core 24 which comprises at least one diluting compartment and a number of
concentrating
compartments which is at least one greater than the number of diluting
compartments. EDI
core 24 will include an alternating series of diluting and concentrating
compartments, with
at least one concentrating compartment at each end of EDI core 24, and a
manifold or other
suitable meals to direct a supply 28 of water to be purified to the diluting
compartment or
compartments in core 24 and a manifold or other suitable means to direct a
supply 32 of
waste water to the concentrating compartments in core 24. Apparatus 20 further
includes
manifolds or other suitable means to output supply 36 of deionized water and
supply 40 of
waste water which has received ionic impurities.
The anode and cathode in apparatus 20 are connected to a suitable source 44
of do current such that, when a suitable current is applied between the anode
and cathode,
deionization of supply 28 of water occurs. As will be apparent to those of
skill in the art,
deionization comprises the removal of ionic impurities including ions such as
mineral salts
ionizable species such as COz and ammonia, and ionizable organic molecules.
As shown in the Figure, supply 28 of water to be deionized includes a
concentration measuring means 48 which is used to determine the concentration
of ionic
impurities in supply 28 of water and to produce an output signal 56, to
control device 60)
which is a nel: indication of the concentration of ionic impurities in supply
of water 28.
hor mineral salts and the like, concentration measuring means 48 can comprise
a
c:onductivity/resistivity measurement device, such as the 740 model
manufactured by
'Chorton Associates of Waltham, Mass. , USA. As will be apparent to those of
skill in the
art, the conductivity of water is directly proportional to the amount of ions
dissolved in the
water. Thus, measurements of the conductivity of water provide a reasonable
indication of
CA 02228886 1998-02-06
-9-
the amount of ions present in the water.
For ionizable organic molecules and other ionizable species, concentration
measuring means 48 can comprise a Total Organic Content (TOC) analyzer, such
as the
Model A-2000 Widerange TOC Analyzer, manufactured by Anatel of Boulder
Colorado,
or the like. It is contemplated that, in many circumstances, the concentration
of non-
mineral ions will not vary significantly and can be treated substantially as a
constant.
Thus, in many circumstances concentration measuring means 48 will comprise a
conductivity measuring device whose output will be combined with a
predetermined
constant representing the concentration of non-mineral ions to produce output
signal 56. If
the concentration of the non-mineral ions cannot be treated as a constant,
then
concentration measuring means 48 will comprise both a conductivity measuring
device and
a TOC analyzer or the like to produce output signal 56.
Apparatus 20 further includes a flow rate measuring device 52) such as a
vortex flow meter device such as the model V2-M1-A300F3000-E3, manufactured by
Universal Flow Monitors, Inc. of Hazel Park, Michigan, which provides an
output signal
64 to control device 60. It is contemplated that in some circumstances, supply
28 of water
may be provided at a constant, known, flow rate and in these circumstances
flow rate
measuring df:vice 52 may merely constitute a constant, predefined, input 64 to
control
device 60.
Control device 60 can comprise a microcontroller, such as an Allen Bradley
PLC, or any other suitable processing device as will be apparent to those of
skill in the art.
Another concentration measuring means 68 determines the concentration of
ionic impurities in supply 36 of deionized water output from apparatus 20 and
provides an
appropriate output signal 72 to control device 60. Again, concentration
measuring means
68 can comprise a conductivity/resistivity measuring device and/or a TOC
analyzer. It is
contemplated that in many circumstances it will be acceptable to assume a
constant value
CA 02228886 1998-02-06
- 10-
(for example zero) for the concentration of ionizable species in deionized
water supply 36
and in these cases, concentration measuring means 68 will comprise a
conductivitylresistivity measuring device whose output is combined with the
assumed
constant value to obtain output 72. It is also contemplated that, in other
circumstances) it
will be acceptable to assume the concentration of both ions and ionizable
species as
constants and thus concentration measuring means 68 will merely comprise a
constant value
input 72 to control device 60.
As will be described below in more detail) control means 60 examines
signals 56, 6~4 and 72, whether these signals are predefined constants,
measured values or a
mixture of both, and provides a control signal 76 to current supply 44 to
alter the current
applied between the anode and cathode of core 24 to achieve a prespecified
level of
deionization of supply 36. In addition, and as is also described below in more
detail,
control means 60 can output a signal representing the present operating
conditions of
apparatus 20, according to a predefined E-Factor.
Depending upon the design and construction of current supply 44, output
signal 76 may be sufficient to appropriately alter the current supplied to the
anode and
cathode in core 24 but in some circumstances it is contemplated that control
means 60 may
~be supplied with a signal from an ammeter (not shown) representing a measured
value of
the current bEaween the anode and cathode.
The present inventors have determined that operation of an EDI apparatus
can be controlled in accordance with a ratio, referred to by the present
inventors as an
"E-factor', or Ef, which is defined as
1
E- F
r
n ;
CA 02228886 1998-02-06
-11-
where I is the current applied between the anode and cathode of EDI core 24, F
is the
Faraday constant used to convert coulombs into moles (i.e. 96,480
coulombs/mole), Q is
the flow rate: of the supply of fluid to be deionized to EDI core 24, n is the
number of
diluting compartments, Oc; is difference in the concentration of the ionic
impurity i
between the fluid to be deionized and the deionized fluid (i.e. - the
'concentration drop'
through the .apparatus for impurity i) and z; is the corresponding unit charge
of the ionic
impurity. For example, for an Na+ or Cl- ion, the unit charge is one and for
S042, the unit
charge will be two.
More specifically, depending upon the fluid to be purified, ~~c; x z; is
calculated for anionic impurities and cationic impurities and the larger
summation is
selected for consideration in determining E f. For example, if the fluid to be
purified
contains a relatively significant amount of dissolved CO2, which will be
ionized within EDI
core 24 to HC03-, ~~c; x z; will be larger for anionic impurities and this
larger value will
be selected for use in determining E f.
It is contemplated that, in many cases, the ionic impurities in the water to
be
purified will not change greatly over time. Thus, a determination of the
whether ~Oc; x z;
is larger for cationic or anionic impurities can be performed at some initial
point in time,
from an analysis of the water to be processed, and can be confirmed, if
necessary, at
selected intervals. In other cases, the determination of the greater of the
anionic and
cationic impurities will be performed on an ongoing basis.
Ef is a dimensionless ratio and, in a preferred embodiment, Q is expressed in
litres/sec, ~c; is expressed in moles/litre and I is expressed in amperes. It
will be apparent
to those of skill in the art that other expressions of measurement may also be
employed.
It has been determined by the present inventors that an Ef of at least 1 is
required for .acceptable operation of an EDI apparatus and, more preferably,
that the E f be
in the range of from about 1 to about 50 and more preferably, that the E f be
in the range of
CA 02228886 1998-02-06
-12-
from about _'i to about 20. More preferably, that the Ef be in the range of
from about 8 to
about 15.
Accordingly, in operation of apparatus 20 control means 60 will, on an
S ongoing basis, determine the E~ for apparatus 20 and output signal 76 will
be appropriately
set such that the current supplied by current supply means 44 is varied as
necessary to
maintain the determined Ef within a predefined range for apparatus 20. For
example, it
may be desired that apparatus 20 be operated with Ef in the range of 8 to 15
and the current
supplied by current supply 44 will be increased or decreased accordingly by
control means
60 to accommodate changes in the amount of ionic impurities present in flow 28
(as
indicated by changes in the concentration measurements effected by device 48)
or changes
in the flow rate of supply 28 (as indicated by changes in signal 64 from
device 52) or both
or changes in other parameters which occur. It is also contemplated that
control means 60
can provide an output to an operator or a process control system which is
indicative of the
actual deterlr~ined E f that apparatus 20 is operating at and/or other
operating parameters
such as the concentration of ionic impurities in the deionized water supply 36
as measured
by concentration measuring device 68. These outputs can be simple numerical
values
displayed to an operator, or any other suitable display technique as will
occur to those of
skill in the act, such as graphical display, etc.
The following example is included to illustrate further the present invention
and is not intended as a representing a limitation of the present invention.
As will be seen,
this example indicates that the level of deionization of water through a
particular test EDI
;apparatus decreased, from a maximum level, when the E-Factor, Ef, was below
about 15.
n this example, an EDI core such as those sold by the assignee of the present
invention
under the model designation E-Cell were employed.
In the test, the EDI apparatus included five diluting compartments and six
concentrating compartments and was operated with a substantially constant flow
rate of
0.131 litres/sec of water to be deionized through the diluting compartments.
The water to
CA 02228886 1998-02-06
-13-
be deionized was previously deionized to remove ionic impurities and a
metering pump was
used to inject NaCI into the water to be deionized, resulting in a test range
conductivities
between about 2.7 to about 50 ~cSlcm (with minimal ionizable species present)
and the
resistivity (the inverse of conductivity) of the deionized water was measured.
The current
flow between the anode and cathode was fixed at a current of 1.0 amps and the
EDI
apparatus was operated at different rates of NaCI injection (corresponding to
different Ef's)
for approximately 20 minutes each.
A plot of the measured resistivity of the deionized water against the
corresponding E f is shown in Figure 2. As will be apparent from the plot, the
level of ions
remaining in the deionized water (as indicated by a drop in the resistivity of
the water) rose
sharply as E f fell below 6. The resistivity of the deionized water increased
to 16.3 MLl-cm
(0.61 ~,S/cm) at Ef equal to about 15.
In circumstances wherein concentration measuring device 48 and/or
concentration measuring device 68 only directly measure the ions in the fluid
and estimate
t:he ionizable species which may be present, a higher Ef can be employed than
might
otherwise be the case to ensure adequate removal of ionizable species.
Accordingly,
depending upon the concentration measuring devices and the use to which the
deionized
water will be put, acceptable results can be obtained over a wide range of
Ef's) such as
from 1 to about 50, or more preferably, from about 5 to about 50, or more
preferably from
about 5 to about 20 and most preferably from about 8 to about 15.
As will be apparent, apparatus 20 can be operated with the current supplied
by current supply means 44 being varied as necessary to maintain operation at
a preselected
l: f or within a preselected range of E f's. In this manner, over
specification of the current
supplied by current supply means 44 is substantially avoided to achieve power
efficiency in
the operation of apparatus 20.
As will also be apparent to those of skill in the art, the present invention
is
CA 02228886 1998-02-06
-14-
not limited to controlling the operation of an EDI apparatus but can also be
usefully
employed during the manufacture and design of EDI apparatus. Specifically,
given
appropriate design criteria, an EDI apparatus can be reliably designed and
manufactured
without unnecessary over specification of the apparatus.
A flowchart representing one embodiment of a manufacturing method in
accordance with the present invention is shown in Figure 3. At step 200 a
determination is
made of the concentration of the ionic impurities of the fluid to be treated
by the apparatus.
Generally, there will be some expected variation in this determined value due
to variations
in the water supplied to be deionized and the manufacturing method will
generally be
performed wiith the determined value set at the largest, normally expected
concentration
value.
At step 204, a target concentration value for the processed fluid is selected,
;according to the intended use for the processed fluid. For example, it is
generally desired
that water which is deionized for use in semiconductor fabrication processes
be deionized
to a greater level (lower concentration) than water deionized for use in
pharmaceutical
manufacturin;; processes.
At step 208 the number of diluting compartments to be included in the
apparatus is selected. Generally, the considerations here are twofold, namely
the fewer
diluting compartments of a given size included the EDI apparatus, the less
expensive the
apparatus is to manufacture and maintain but the higher the flow rate through
the diluting
compartments, of the fluid to be treated, i.e. the residency time of the fluid
to be treated in
the compartment is reduced. It is contemplated that in many circumstances the
selection of
the number of diluting compartments to be included will determined almost
entirely by the
volume of fluid to be treated in a given time (liters per minute, etc.) and
the other
parameters discussed herein will be varied as necessary to achieve the desired
range of Ef.
Once the number (n) of diluting compartments is selected, the number of
concentrating
compartments is fixed at a number at least one greater (zn+1).
CA 02228886 1998-02-06
-15-
At step 212 an electrical current is selected as a target operating current to
be supplied to the EDI apparatus.
At step 216 Ef is determined, using the values determined and/or selected in
steps 200 through 212. At step 220, if Ef is within a preselected range of
acceptable
values, such. as the above-mentioned range of 5 to 18, the method proceeds to
step 224
wherein EDI apparatus in accordance with the selected and/or determined values
is
constructed.
If at step 220, it is determined that Ef is not within the preselected range
of
acceptable values, the method revisits one or more of steps 204, 208 and 212.
It is
contemplated that, in many circumstances the target concentration of ionic
impurities of the
treated fluid is effectively prevented from being changed owing to the
requirements of the
intended use for the deionized fluid produced by the apparatus. Accordingly,
it is
contemplated that the two parameters most frequently available for
modification will be the
number of the diluting compartments and the current applied between the anode
and the
cathode. If the number of diluting compartments is varied, the number of
concentrating
compartments is changed accordingly.
Once one or more of the parameters of steps 204, 208 and 212 have been
changed, steps 216 and 220 are repeated to determine if the resulting value of
Ef is within
the preselected range of acceptable values. If Ef is now acceptable, the
method proceeds to
step 224 wherein EDI apparatus in accordance with the selected andlor
determined values
is constructed.
If E f is still outside the preselected range of acceptable values, the method
again revisits one or more of steps 204, 208 and 212. Generally, steps 204
through 220
are repeated in an iterative manner until E f is within the preselected
acceptable range of
values and th.e EDI apparatus can be manufactured at step 224.
CA 02228886 1998-02-06
-16-
In another embodiment of the method, the equation
I
E- F
I
~~c~xZr
n ;
can be re-wriitten as
Ef F Q EOclxz~
n=
I
This equation can then be solved for n, the number of diluting
compartments.
Acceptable values for n will vary in accordance with a variety of criteria,
including the costs associated with constructing the EDI apparatus with more
or fewer
diluting compartments, the cost of the operating electrical current supplied
to the EDI
.apparatus, etc.
Figure 4 shows a flowchart representing another embodiment of a~ method of
manufacturing and EDI apparatus in accordance with the present invention
wherein the
number of dilluting compartments 'n' is to be the focus consideration for the
manufacture of
the EDI apparatus. At step 300 the maximum volume of water to be treated per
unit time
~;litres/sec) is determined. At step 304, the concentration of ionic
impurities of the water to
be treated is determined and at step 308 a desired value for E f is selected
and at step 312
the desired concentration of ionic impurities for the treated fluid is
selected and at step 316
;a desired operating current is selected.
The equation given above is then solved for n at step 320 and the calculated
value for n is then rounded up to the next integer value. A determination is
made at step
CA 02228886 1998-02-06
-17-
324 as to whether the resulting value for n is acceptable. As mentioned above,
the criteria
as to whether a particular value of n is acceptable will vary in accordance
with a variety of
parameters, such as the cost of adding diluting chambers, the cost of
supplying the
electrical current, the physical size of the EDI apparatus etc. , but the
determination of
acceptable values of n will be apparent to those of skill in the art.
If at step 324 the value of n is determined as being acceptable, the EDI
apparatus is :manufactured accordingly at step 328. If at step 324 the value
of n is
determined to be unacceptable, the method revisits one or both of steps 312
and 316 to
select new values and steps 320 and 324 are performed again. If the value of n
is again not
acceptable at step 324, steps 312 through 324 are repeated in an iterative
manner until n is
acceptable and the EDI apparatus can be manufactured at step 328.
The above-described embodiments of the invention are intended to be
examples of ~.he present invention and alterations and modifications may be
effected
thereto, by those of skill in the art, without departing from the scope of the
invention
which is defined solely by the claims appended hereto.