Note: Descriptions are shown in the official language in which they were submitted.
CA 0222898~ 1998-02-09
New Ionically Conductive Material with
Improved Conductivity and Stability
BACKGROUND OF INVENTION
5 (a) FieldoftheInvention
This invention relates to an ionically conductive m~t~ri~l with
improved conductivity and stability. More particularly, the present
invention is concerned with sulfamide derivatives of the formula:
Rl R2 NSO2 N R3 R4
wherein Rl, R2, R3 and R4 are methyl, the rem~ining groups being ethyl
groups, or one of Rl, R2, R3 and R4 is a methoxyethyl, the rem~ining
groups being methyl or ethyl groups, which are used as aprotic solvents
15 which can be used as media for organic reactions and/or synthesis; as
ionically conductive material when a salt is added as a solute to a
sulfamide of the above formula; as solid or gel polymer electrolytes
useful for electrochemical applications, such as lithium type batteries or
so-called rocking chair or lithium ion batteries, the electrolytes being
20 obtained by adding a sulfamide of the above formula with a salt, to a
solid or liquid polymer.
(b) Description of the Prior Art:
Sulfamides (RIR2NSO2NR3R4) wherein Rl, R2, R3 and R4
represent alkyl groups and their derivatives are used in many chemical
25 and biological applications. Richey et al (Journal of Organic
Chemistry, Vol. 52, No 4, (1987) p. 479-483) report that when used as
solvents they are particularly stable toward polar organometallic
compounds as well as toward other strongly basic and nucleophilic
reagents. It has been found by Richey et al that organomagnesium
30 compounds are indefinitely stable in tetraethylsulfamide identified as
TES when Rl, R2, R3 and R4 are all ethyl groups and that the half-life of
butyllithium in TES exceeds 15 minutes while in other solvent the half-
lives are less than 5 mimltes.
Sulfamides are also used in the field of pesticides (U.S.
35 Patent No. 5,596,017) and in biological applications. A method for
preparing sulfamide compounds is described in U.S. Patent No.
5,506,355. U.S. Patent No. 3,879,528, U.S. Patent No. 5,539,102, and
CA 0222898~ 1998-02-09
U.S. Patent No. 3,923,886 describe routes and processes for producing
sulfamide compounds for those applications.
Sulfamides are also well known for their capability to
increase the flame resistance of polymers. U.S. Patent Nos. 3,888,819
and 3,915,931 describe sulfamide compositions and a process for that
particular application.
Proton-vacancy conducting polymers based on
polyethylene oxide (PEO) and sulfamide-type salts were also reported in
the literature by Bermudez and al. (Electrochimica Acta, Vol. 37, No 9,
10 (1992) pp 1603-1609) as a novel basic proton conducting polymer. The
presence of the sulfamide-type salt is a key issue of this concept for
proton conducting polymer.
In the field of lithium battery, battery performances of
PEO polymer modified with sulfonamide end groups provide better
15 conductivity than the PEO system alone. It was reported for example by
Ito et al (Solid State Ionics, 86-88, (1996), p 325-328) that PEO having
aLkyl-sulfonamide end groups such as PEOlooo-(NKso2Me)2 result in a
higher ionic conductivity as compared to PEOlooo. The authors explain
this higher conductivity by the higher dissociation constant of the
~0 termin~l sulfonamide.
The identification of new solvents having high dielectric
constants, and solvatation powers (adequate high donor (DN) and
acceptor (AN) numbers) as well as low viscosities is a paramount for
high energy lithium cell development operating at ambient temperature.
25 Mixture of solvents intended to improve overall battery performances
may also be of utility as exemplified for instance in U.S. Patent No
4,129,691. These solvents could be used in liquid lithium batteries as
well as in gel electrolyte containing polymers networks (cf. U.S. Patent
Nos. 4,792,504 and 5,501,920).
U.S. Patent Nos. 4,851,307 and 5,063,124 describe new
ionically conductive materials which comprise a salt dissolved in a
solvent or mixed therewith in variable amounts of this solvent with a
polymer having solvating groups; this solvent being a sulfamide
derivative of general formula:
Rl R2 NSO2 N R3 R4
CA 0222898~ 1998-02-09
wherein Rl, R2, R3 and 1~4 are each independently a C(1-10) aL~yl group
or a C(1-10) oxaalkyl group. A representative example of this group is
Rl = R2, = R3 = R4= ethyl, abbreviated TES, will be considered the
5 reference molecule in the description which follows.
Neither U.S. Patent No. 4,851,307 or U.S. Patent No.
5,063,124 report or claim specific chemical or electrochemical
properties or benefits by selecting specific R groups nor describe their
relative position on the two nitrogen atoms.
10 SUMMARY OF INVENTION
It is an object of the present invention to provide a new
family of sulfamides having different Ri groups and which possess
different chemical propelties, m~kin~ them useful for different
applications such as in the field of lithium batteries and other organic
15 chemistry in general.
It is another object of the present invention to provide a
family of sulfamide derivatives that can be used as aprotic solvents for
organic reactions and/or synthesis.
It is another object of the present invention to provide
20 derivatives of the present invention to provide sulfamide derivatives
which can be used to produce ionically conductive m~tçri~l~, solids or
gel polymer electrolytes or solid and liquid electrolytes.
It is another object of the present invention to provide
sulfamide compositions whose chemical and electrochemical properties
25 are especially advantageous for applications as solvent for chemical and
electrochemical reactions.
lt is another object of the present invention to provide
sulfamides whose properties are especially advantageous for
applications as solvent for chemical and electrochemical reactions, and
30 especially for liquid and/or solid and/or gel lithi~lm baKeries or rocking
chair batteries.
The present invention relates to sulfamides or llfi~ es for
use in an electrolytic composition or as aprotic solvents, said sulfarnides
having the general formula:
RIR2Nso2NR3R4
CA 0222898~ 1998-02-09
wherein one to three R substituents are methyl groups, the rem~inder
being ethyl groups, or one R is a methoxyethyl, the rem~ining R being
methyl or ethyl groups.
When Rl = R2 = R3 = methyl, R4 = ethyl, the sulfamide
has a dielectric constant of 57, and a melting point of -33~C.
When Rl = R2 = methyl, R3 = R4 = ethyl, the sulfamide
has a dielectric constant of 40.5, a melting point of -24~C and a glass
transition temperature of-122~C.
When Rl = R3 = methyl, R2 = R4 = ethyl, the sulfamide
has a dielectric constant of 44, a melting point of -56~C and a glass
transition temperature of-130~C.
When Rl = R2 = R3 methyl, R4 = methoxy-ethyl, the
sulfamide has a dielectric constant of 41.7 and a glass transition
15 temperature of-110~C.
When Rl = R2 = R3 = ethyl, R4 = methoxy-ethyl, the
sulfamide has a dielectric constant of 29, a glass transition temperature
of-118~C and a stability window ext~n(1ing from 0 to 5 V. vs. lithium.
The invention also relates to an electrolytic composition
20 which comprises at least one sulfamide composition defined above and
at least one dissociable salt in solution therein. The sulfamide may be in
a-lmixtllre with a co-solvenl, and the salt may be a lithium salt.
According to a preferred embodiment the lithinm salt is
selected from the group consisting of LiBF4, LiPF6, Li(RFSO2)2N
25 Li(RFSO2)3C where RF is fluorine or a fluorinated carbon radical having
1 - 4 carbon atoms.
The co-solvent may be chosen from the group consisting
of dimethyl ethers of ethylene glycol, diethylene glycol, triethylene
glycol, and/or ethylene carbonate, propylene carbonate, dimethyl
30 carbonate, diethyl carbonate r-butyrolactone.
In the electrolytic composition the sulfamide may be in
~l.";x~ e with an aprotic polymer, such as polyethers comprising at
least 70% ethylene oxide units, poly(methyl methacrylate),
poly(acrylonitrile), and copolymers cont~ining at least 70% vinylidene
35 fluoride units.
CA 0222898~ 1998-02-09
The sulfamide to polymer ratio is preferably less than or
equal to 20% and the sulfamide acts as a plasticizer.
The solvent to polymer ratio may be greater than or equal
to 50% and the resulting mixture then constitutes a gel.
The invention also relates to a lithium battery wherein the
electrolyte, alone or as a component of composite electrodes comprises
an electrolytic composition as defined above. In this battery the
negative electrode may contain either metallic lithium, a lithium alloy, a
carbon intercalation compound or a low voltage intercalation oxide or
10 nitride, and may contain a high voltage intercalation electrode derived
from v~n~ m, m~ng~nese, cobalt or nickel oxides, iron or m~ng~rlese
iron phosphate or pyrophosphate. The positive electrode may also
contain a polydisulfide or sulfur mixed with a conductive additive such
as high surface area carbon.
The invention also relates to a supercapacity type device
wherein the electrolyte comprises a composition as defined above,
wherein the electrodes may contain high surface area carbon.
The invention also relates to an electrochomic device
wherein the electrolyte comprises a composition as defined above, and
wherein one of the electrodes may be a thin film of tungsten trioxide.
The invention also relates to a solvent composition for use
as media co~ lg a sulfamide as defined above alone or in ~ ixll-,es
with organic solvents. Such composition may include co-solvents such
as aromatic hydrocarbons, or halocarbons, and may be used as media for
organic reactions comprising carbanion preparation, Friedel & Craft
reactions, Diels & Alder or Michael reactions, cationic or anionic
polymerizations .
It is known to the specialist in the field that:
~shortening of a alkyl groups usually results in molecules
with less conformational freedom thus increased meltin~ points,
viscosities or glass transition temperalules Tg for glass-forming
compositions. Examples are for instance the well know propylene
carbonate, turning into a glass below -40~C and ethylene carbonate
(melting point Tmp= 39~C); dimethylcarbonate, Tmp= 4~C,
diethylcarbonate, Tmp= -43~C; acetonitrile Tmp= -48~C, propionitrile
CA 0222898~ 1998-02-09
Tmp- -93~C. Similarly, tetramethyl~-llf~mi~e abbreviated TMS melts at
73~C;
~shorter alkyl groups, especially methyl have reduced
electron-releasing properties, and the donor numbers (DN) in Gl~tm~nn's
5 definition [V. G-l~n~nn Electochimica Acta 21, 661-670, (1976)] of the
corresponding molecules built from these alkyl chains are lower (i.e.
acetonitrile, DN = 14.1, butyronitrile DN = 16.6). Conversely, the
acceptor number (AN) is usually moderately affected by chain length
(ethanol: AN = 37, n-butanol AN = 36.8);
10 ~ the oxidative stability of ether groups does not extend
beyond 3.8 Volts vs. the lithium reference electrode;
~ an increase in dielectric constant is usually accompanied
by in increase in reactivity, as the dipoles bear a higher charge and/or
are closer to the outer part of the molecule, thus are more exposed to
15 reactants.
Properties varying progressively could be expected when
going from TES to TMS with sequential replacement of ethyl by methyl
groups. Surprisingly, outstanding behavior was observed on these
compounds and those cont~ining the ether linkage in methoxyethyl
20 substi~nt~. The properties were not foreseen or anticipated by one
skilled in the art, thus clearly establishing the novelty aspect of the
present invention.
The principal properties of interest as solvent for
chemical and electrochemical applications are: the liquid temperature
25 range, the dielectric constant (e), the donor (DN) and acceptor (AN)
numbers, the glass transition temperature, (Tg) alone or in ~ iX~ es,
especially with polar polymers. For electrochemical use, the property
values of the first three parameters allow for an increase in the specific
conductivity. The electrochemical stability window towards metallic
30 lithium and the potential beyond which solvent oxidation takes place is
also a very important property for battery application and must be as
wide as possible.
A good solvent for liquid lithium batteries must have a
low viscosity, a high boiling point, a low melting point and should be
35 able to dissolve a large quantity of salt (high DN and AN). For lithillm
solid polymer batteries, the solvent can be used as a plasticizer (small
CA 0222898~ 1998-02-09
amount of solvent is added to the polymer, typically less than 50%
volume). In this particular application, a good plasticizer will lower the
Tg of the polymer matrix and its crystallinity. For gel electrolytes (large
volume fraction, 50% or higher, of the solvent immobilized by a
compatible polymer matrix), all the mentioned properties are important
since ion transport mech~ni~m (specific conductivity) can be dependent
on both media (solvent and polymer).
DRAWINGS
The invention is illustrated but is not limited by the
10 following drawings in which:
FIGURE 1 is a graph showing the increase in
conductivity of NES-I-4 as compared to TES;
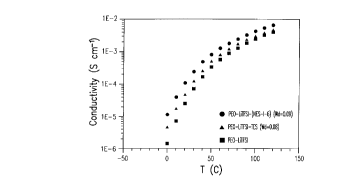
FIGURE 2 illustrates the conductivity of PEO-LiTFSI
mixture, POE-LiTFST-NES-I-6 (9% weight) mixture and PEO-LiTFSI-
15 TES (8% weight) mixture, all systems having a 35% weight LiTFSI;
FIGURE 3 is a graph comparing the electrochemical
stability of NES-II-17 and that of TES; and
FIGURE 4 is a graph comparing the capacities of cells
having NES-I-3 and NES-II-65 with cells having TES under cycling.
The invention will also be illustrated by means of the
following non limitin~ examples.
EXAMPLES
Example 1
The following table summarizes some of the properties of
25 the new sulfamides (Me = methyl, Et = ethyl, s is the conductivity in
mS/cm, e the dielectric constant, DN the donor number and AN the
acceptor number according to Glltnl~nn~s clefinition; n.m.: not
measurable; n. misc.: not miscible) and show the outst~n(lin~ behavior of
these compounds:
CA 02228985 1998-02-09
Example 1
Solvent Trn T solvent r~ mixture Max Conc. at E oxidation St biliq v~
Rl,R2,R3~i Narne~olvent (CB~ solvent and ~ DN AN ~ rnax~ (V + va Li~ (> than
~C) PEO (mSlcm) moUlCg Li/Li ) 200 l~n)
(C)
Tr ',' '' ' TES -38 -111 47 29.2 19.3 10.6 0.974 0.310 +4.3 Izood
T ' : '' ' TMS +73 none 70t misc n m n m n m n m
Me Me Me Et ES-14 ~ 0-455 ;~
Me Me,E~,Et NES-I-3 ~ .. 8~:;;i.:.. ~ ,:. :;,.i~ ~ {~" t~ 0.420 ~
Me Et,Me,Et NES-I~ ~ ~0.4 0 ~;" ~l '. ~,
Me, Me, Me, EtOMeNES-11~5 ~ ' ~it~ 0-500 ~ A~
Et,Et,Et,E:OMe NES-11-17 - ~ l:~:.:::.::::~: ~:i::.. ::.. : .::~;.:.:~.:: ~ -'i~ '~ ~"~::::: 0.794 0.446
5 Example 2
In this example, the conductivity of a solution co~
0.33 moVkg of LiTFSI in the solvent NES-I-4 (which have the highest
dielectric constant) is compared to the conductivity of the TES-LiTFSI
(TESA) system at the same concentration of salt. Conductivity data are
10 measured at di~erenl temperatures (from -20~C to 55~C) with a built in-
house bridge similar to the Beckman model RC-15 commercial bridge.
Pl~tini~e~ pl~tinllm Orion (Boston, USA) cells are used and resistance
measurements are made at 1 and 3 kHz and extrapolated to 0 kHz.
From FIGURE 1 it is noted that replacement of 3 ethyl
15 groups of TES by 3 methyl groups (NES-I-4) considerably increases the
conductivity, more than three times for all temperalu,es.
Example 3
In this example, the conductivity of PEO-LiTFSI
mixtures are illustrated. Samples are prepared as follows: 1) PEO (Mw
20 = 4 x 106) and LiTFSI are dissolved in acetonitrile; 2) the solution
obtained is coated on an inert support and then dried under partial
vacuum (40 mm Hg) for 2-3 hours at ambient temperature and 3) the
electrolyte film is dried under vacuum at 140~C for 48 hours. Samples
having plasticizer or diluent NES-I-6 or TES are prepared ~imil~rly.
25 NES-I-6 or TES are added to the mixture of PEO-LiTFSI in acetonitrile
and the same procedure as above is respected except that the final drying
step is done under 2 mm Hg during 48h at ambient temperature. NES-I-
CA 0222898~ 1998-02-09
6 or TES concentration in the final solid electrolyte film is controlled by
RMN lH
The SPE films are then disposed between two stainless
steel electrodes. Conductivity cells are introduced in an air-tight cell
5 holder that permit electrical contact. The conductivity measurements are
performed in an Instron (Burlington, Ont., Canada) temperature chamber
equipped with a con~uler-driven controller. Impedance data were
collected at intervals of 10~C over the frequency range 5Hz-13MHz
using a mode 4192A Hewlett-Packard impedance analyser.
The conductivity of the PEO-LiTFSI-(NES-I-6) ~ e is
around 2 times higher as compared to PEO-LiTFSI-TES and close to an
order of m~gnit~lde (at 0~C) higher compared to the PEO-LiTFSI system.
Example 4
In this example, the electrochemical stability of NES-II-
15 17 is compared to TES for the same concentration of LiTFSI introducedin the solution (0.37 M). A conventional three-electrode cell is used for
that study and the working electrode is a glassy carbon rod embedded in
Kel-F. Current responses in function of voltage sc~nning (scan rate = 20
mV/sec) are recorded and t~le results are pres~nte~ in Figure 3. Massive
20 solvent oxidation occurs near 5 V for NES-II-17 which is almost 700
mV more noble than for TES. NES-II-17 is therefore a better c~n~lid~te
for high voltage-energy lithium batteries.
Example 5
In this example, the effect of different sulfamides on the
25 power and cyclability of lithium polymer cells is compared. The three
cells are made of a lithium anode (35 mm thick) supported on a thin
sheet of nickel and a composite cathode whose composition by volume
is about 40% TiS2, 10% acetylene black and 50% of an ethylene oxide
copolymer. This copolymer includes about 80% ethylene oxide as
30 described in the following patents: EPO 0,013,199; U.S. 4,578,326; and
U.S. 4,758,483, to which there is added the lithillm salt LiTFSI in a ratio
of 30/1. The cathode of effective capacity near 3C/cm2 is placed on a
thin nickel collector. The thickness of the separator of the polymer
electrolyte is 15 mm and the latter is also made of an ethylene oxide
35 base polymer. The batteries with useful surface of 3.89 cm2 are
assembled by hot pressing at 80~C. The sulfamide is added to the
CA 02228985 1998-02-09
- 10-
separator and the cathode just before the hot pressing step and the
molecular sulfamide ratio introduced is 1:1 coll~aled to the amount of
LiTFSI in the cell.
5 The batteries are cycled at 25~C at constant current (both charge and
discharge) at around C/40 rate and the power performance in discharge
of these cells is obtained between cycles 7 to 15. Figure 4 show that
cells having NES-I-3 and NES-II-65 retain higher capacity for C/x rate
where x = 20 through 4 compared to TES. The cyclability at C/40 for
10 all the cells is very good and similar.