Note: Descriptions are shown in the official language in which they were submitted.
CA 02229743 1998-02-17
W O 97/11067 PCT~EP96/03954
- 1 -
Polymer-bound fluorophores as optical ion sensors
The present invention relates to functionalised rhodamines and acridines, to processes for
their preparation as well as to polyacrylates, polymethacrylates and polyurethanes which
contain these rhodamines or acridines bound covalently via their functional groups. The
invention also relates a) to an optical sensor, comprising the polymer-bound fluorophores,
counterions in the form of lipophilic salts and a ionophore, b) to a process for the optical
dele-"~inalion of ions by the fluorescence method, e.g. of cations selected from the group
consisting of metal cations and ammonium cations, or e.g. of anions selected from the
group consisting of anions of inorganic or organic acids, and also c) to the use of the optical
sensor for the determination of anions or cations, in particular in aqueous solutions.
In recent times, the optical delel");,lalion of ions has become more important, the presence
or concentration of ions being measured, for example, via the change in absorption or
fluorescence of a suitable dye. The sensors, which are also termed optrodes, usually
consist of a transparenl carrier material and an active layer. Said active layer normally
comprises a transpart:nl hy.l,oph-L ~ polymer and a lipophilic p'~-sticiser to achieve
sufficient diffusion of the ions and solubility of the active components. Active components
are a specific ionophore as sequestrant for ions, a counterion to maintain the electric
neutrality and an indicator substance which produces a measurable optical signal resulting
from a chemical or physical change of the environment.
US-A-4 645 744 describes such systems wherein the indicator substance is a neutral
compound such as a dye (p-nitrophenol) which interacts with a ionophore/metal cation
complex resulting in an optically measurable signal in the form of a change in colour. The
interaction can, for example, cause the removal of a proton from a dye resulting in a change
of the electron state. Fluorescent compounds (e.g. fluorescein) are also cited as suitable,
the fluorescence of which can be altered by the change in the electron state and can be
optically determined via fluorescence measurements.
In Chemical, Biochemical and Environmental Fiber Sensors ll, SPIE Vol. 1368, pages 165
to 174 (1990), H. He et al. describe systems using a proton carrier (nile blue) as indicator
substance, in which systems the transport of potassium into the active layer using
valinomycin as ionophore dissociates the proton carrier and one proton diffuses into the
aqueous phase. The proton carrier changes its colour from blue to red and, depending on
the chosen wavelength, it is possible to determine the decrease in fluorescence of the blue
CA 02229743 1998-02-17
W O 97/11067 PCT~P96/03954
-- 2 --
dye or the corresponding increase in fluorescence of the red dye. Owing to higher
sensitivity and selectivity, it is pr~:fer.ed to measure the fluorescence. One substantial
disadvantage of the process is the low sensitivity of the system which is due to the low
quantum yield of fluorescence of the indicator dye employed.
In Anal. Chem. Vol. 62, pages 1528-1531 and 2054-2055, Y. Kawabata describes a
membrane system for the optical determination of potassium which is based on the use of a
hydrophobic ion exchanger, namely 3,6-bis(dimethylamino)-10-dodecyl-ac.idinium b-l,,n ~ie
or3,6-bis(dimethylamino)-10-dodecyl-10-hexadecyl-acridinium bro-. :'e. A change in
fluorescence is achieved by changing the polarity of the microenvironment of the sample
because the acridinium salts diffuse via ion exchange with the potassium ion to the
i,.le.~ace of the aqueous phase.
The systems commonly known today co."p,ise in the active layer high molecularweight
hydrophobic polymers (typically PVC) in co",b:.,alion with a plasticiserto ensure f~t
response times and sufficient sensitivities. In these membrane materials, long-term stability
and repeated use is severely resl-i~;led because the pl~ticicer and other low molecular
weight components such as ionophores or fluorophores are washed out in the course of
time.
The state of the art tries to solve the long-term stability problem of the optical sensor by
introducing highly lipophilic groups into the components employed.
While the use of thick membranes also prolongs the long-term stability of the optical sensor,
response time and sensitivity are adversely affected by their use, as is described by Th.
Rosatzin in Anal. Chem.1992, 64, 2029-2035.
So far no plasticiser-free polymeric systems have become known which use an ion
exchange mechanism for the optical dete""il1alion of ions based on the determination of
the change in fluorescence of a polymer-bound fluorophore.
It has now been found that it is possible to prepare specific functionalised acridine and
rhodamine dyes and to attach them to polyacrylates or polyurethanes which, surprisingly,
meet the high demands made on them regarding sensitivity and response time without
adding a plasticiser to the system. The systems have high sensitivity while having short
response times. Said polymerically bound acridine and rhodamine dyes are lipophilic, pH-
CA 02229743 1998-02-17
W O97/11067 PCTAEP96/03954 --3--
sensitive as well as strongly basic fluorophores which are excellently suited for use in a
neutral plasticiser-free polymer membrane of low glass transition temperature Tg together
with a ionophore and a counterion for determining ions according to the ion exchange
mechanism. Their fluorescence depends strongly on the corresponding ion concenl~alions.
The fluorophores are distinguished by a high quantum yield of fluorescence, high basicity, a
great difference between the fluorescence signals of the protonated and deprolonated
form, high lipophilic properties, sufficient pholo~labilily and suitable abso.~Jlion and
emission wavelengths. It is possible to fabricale highly sensitive systems for the optical
determination of ions based on fluorescence measurements.
It is possible to substantially increase the working life and frequency because the polymers
in the membrane can be pZ~-sticiser-free hydrophobic polymers having a defined low glass
transition range and because no washing-out of the fluorophores need be feared. This is a
special advantage in particular in very thin membranes having fast response times.
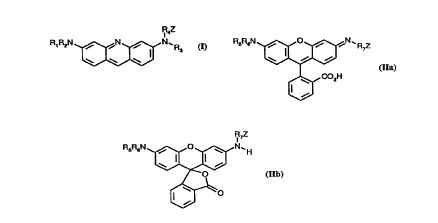
In one of its aspects, the invention relates to compounds of formula 1, lla or llb
IR"Z R5R6N~ 'R7Z
R,R2N~ (I), ~CO2H (Ila),
IR7Z
R5R6N ~N ~ H
~0
(llb), wherein
R1, R2, Rs and R6 are each independently of one another hydrogen, -SO2-(C,-C6)alkyl-
phenyl, C,-C30alkyl, C,-C30alkyl-CO- or a radical of formula ~(CnH2n~0~)m~R10;
R3 is hydrogen or-SO2-(C,-C6)alkylphenyl;
R4 and R7 are C,-C30alkylene or a radical of formula-(CnH2n-O-)m-R,O,
Z is a functional group which is selected from the group consisting of -OH, -SH, -NH2,
-COOH, -NCO, -CO~NR8YRsY, -NH-CO-CH=CH2, -NH-CO-C(CH3)=CH2;
CA 02229743 1998-02-17
W O 97/11067 PCT~EP96/03954
--4--
R~ and Rg are each independently of the other C,-C30alkylene;
R.0 is a direct bond or C,-C,2alkylene;
n is a number from 2 to 6, and m is a number from 1 to 10, with the proviso that the total
number of carbon atoms is at most 30;
and Y is -OH or -SH.
The alkyl groups can be linear or branched and p,e~erably contain 1 to 24 carbon atoms.
Linear alkyl groups are pler~r,ed. Typical examples of alkyl are methyl, ethyl and the
positional isomers of propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl,
eicosyl, heneicosyl, docosyl, tricosyl, lel,dcosyl, and tricontyl.
-SO2-(C,-Cff)Alkylphenyl is preferably toluenesulfonyl.
C2-C30Alkylene can be linear or branched and preferably contains 2 to 20, particularly
preferdbly 2 to 16, carbon atoms. Linear alkylene is prefer,ed. Typical examples are
methylene, ethylene and the posilional isomers of propylene, butylene, pentylene,
hexylene, heptylene, octylene, nonylene, decylene, undecylene, dodecylene, tridecylene,
tetradecylene, pentadecylene, hexadecylene, heptadecylene, octadecylene, nonadecylene,
eicosylene, heneicosylene, docosylene, tricosylene, tetracosylene, and tricontylene.
In the radical of formula-(CnH2n-~)m-R10, n is prefe-~bly 2 to 4, m is preferably 2 to 6, and
R10 is preferably either a direct bond or C2-C4alkylene.
Illustrative examples of C2-C30alkylene bridge groups which are interrupted by oxygen atoms
are those which are derived from ethylene oxidet propylene oxide, butylene oxide or
isobutylene oxide.
R, and R2 in the compounds of formula I are preferably each independently of the other
hydrogen or linear C,2-C24alkyl, the carbon atom number of R1 and R2 together preferably
being from 10 to 20.
R4 is preferably linear C2-C,6alkylene or a radical of formula ~(C2H4~~)m~Rlo, wherein R1o
and m have the meanings cited above.
,
CA 02229743 1998-02-17
W O 97/11067 PCT~EP96/03954
R5 and R6 in the compounds of formula 11 lla or llb are preferably each independently of the
other linear C2-C~2alkyl. R5 and R6 are particularly preferably identical and are linear C2-
C6alkyl.
R7 in the compounds of formula lla or llb is prefe,~bly linear C2-C,6alkylene.
Pr~:fened compounds are those of formula 1, lla or llb, wherein the functional group Z is
-OH -NH2 -C~NR8YRgY or-NH-C~CCH3=CH2, and R8 and R~, are each independently of
the other C2-C8alkylene. Y is preferably OH.
The compounds of formulae 1 lla and llb preferably have a pKavalue of at least 8particularly preferably of at least 10. The pKa value can be adjusted by the choice and
co",b;nalion of R1 to R7.
In another of its aspects the invention relates to a process for the preparation of the
compounds of formulae 1 lla and llb which col"plises
a) removing the phthalimide group in the compounds of formula Ic or llc
o~O ~=<CH3
R4 ~
R1R2N ~N'o--SO (IC)
O ~\
IR7 N~
R5R6N ~N'H ~
~bo
(IlC)
CA 02229743 1998-02-17
W O 97/11067 PCT~EP96103954
--6--
under acid conditions and, where ap,~,~ pl ialt3,
b) reacting the reaction products further with acrylic acid chloride or methacrylic acid
chloride in a second step or,
c) where appropriate, removing the p-toluenesulfonyl group in the reaction products of the
educts of formula Ic under acid conditions,
R" R2, R4, Rs, R6 and R7 having the mear- Igs cited above.
The processes for removing the protective groups are known per se and can be used in
analogous manner for the preparation of the compounds of formulae 1, lla and llb.
The compounds of formula Ic can be prepared in a manner known per se by stepwisealkylation using different alkylating agents or by alkylation using an alkylating agent or
acylating agent of the commercially available 3,6-diaminoacridine. Suitable alkylating
agents are, for example, dialkyl sulfates or monohalogen alkanes, preferably chloroalkanes,
bromoalkanes and iodoalkanes. Suitable acylating agents are, for example, carboxylic acid
anhydrides and, pre~erably, carboxylic acid halides, typically carboxylic acid chlorides. This
reaction can be carried out in the presence of inert polar and aprotic solvents, typically
ethers, alkylated acid amides and lactames or sulfones, and at elevated temperatures, e.g.
from 50 to 1 50~C. It is expedient to add a hydrogen halide scavenger, typical!y alkali metal
calL,onales or tertiary amines and, preferably, sl~lically hindered tertiary amines.
In one possible method of preparing the compounds of formula 1, the starting cornpound is
3,6-diaminoacridine which is reacted in a first step with p-toluenesulfonic acid chloride. The
purified reaction product is reacted first e.g. with a compound R2-Br at one nitrogen atom
and e.g. with Br-R4-phthalimide at a second nitrogen atom. Further reaction with e.g.
hydrazine hydrate gives a compound of formula 1.
The compounds of formula llc are typically obtainable by reacting phthalic acid anhydride
with 2 molar equivalents of 3-monoalkylaminophenol. Another possible method of
preparation consists in reacting 3-monoalkylaminophenol with 1 molar equivalent of 2-
hydroxy-4-dialkylamino-2'-carboxylbenzophenone. These reactions are described, inter alia,
in US-A-4 622 400. The reaction is conveniently carried out in an inert solvent, typically
hydrocarbons or ethers. Molar amounts of a condensing agent are usefully added, typically
Lewis acids, concentrated sulfuric acid, perchloric acid or phosphoric acid. The reaction
temperature is typically in the range from 50 to 250~C.
CA 02229743 1998-02-17
W O 97/11067 PCTAEP96/03954
--7--
The compounds of formula 1, lla and llb can be isolated in conventional manner by
precipitation, cryst~ s~tion or extraction and, where required, can be purified by
recryst~ilis~tion or by chr~,",alogr~phic methods. Said compounds are crystalline and dyed
e.g. a red, reddish brown or reddish violet colour.
The p-toluenesulfonyl group can be removed by known methods, such as described in J.
Chem. Soc. Chem. Com. 1973, 664.
A process for the preparation of the compounds of formula lla or llb can typically be carried
out such that 4-(2'-carboxy)benzoyl-3-hydroxy-N,N-diethylaniline is first placed in a rea~;lion
vessel and reacted with 3-methoxy-N-(3-aminopropyl)aniline. The resulting reaction product
is a compound of formula lla or llb. This compound can be further reacted by known
methods, typically with methacryloyl chloride, again resulting in a compound of formula lla
or llb.
The compounds of formula 1, lla or llb are excellently suited for covalent attachment at
polymers selected from the group consisting of polyurethanes, polyureas and polyurea-
polyurethanes.
The compounds of formula 1, lla or llb are also excellently suited for the preparation of
copolymers from olefinically unsaturated monomers, in which case Z is -NH-C~CH=CH2 or
-NH-C~C(CH3)=CH2. Some illustrative examples of olefinically unsaturated monomers are
acrylic acid, methacrylic acid, maleic acid, maleic acid anhydride, Cl-C30acrylic acid ester
and C1-C30methacrylic acid ester, Cl-C30acrylic acid amide and Cl-C30methacrylic acid
amide, or acrylic acid amide and methacrylic acid amide, vinyl ester of Cl-C20carboxylic
acids, acrylonitrile, butadiene, isoprene, chlorobutadiene, styrene, a-methylstyrene, vinyl
chloride, vinyl fluoride, vinylidene chloride and vinyl ether of Cl-C30alcohols.
The invention also relates to a copolymer which is plasticiser-free and which consists either
A) of at least one diol or at least one diamine or of mixtures of at least two of these
monomers, and of at least one diisocyanate, or
B) of at least one olefinically unsaturated monomer, which copolymer additionally contains
at least one comonomer of formula 1, lla or llb
CA 02229743 1998-02-17
WO 97111067 PCT/EP96/03954
-- 8 --
R4Z R5R6N ~N ' R7Z
R,R2N~ (I), ~CO2H (lla)
R7Z
RsR6N ~N ~H
~bo
(llb), wherein in the case of A)
Z is -OH, -SH, -NH2, -COOH, -NCO, or NR8YRgY and Y is ~H or-SH and, in the case of B)
Z is -NH-C~CH=CH2 or-NH-C~CCH3=CH2, and R" R2, Rs, R4, R5, R6, R7, R~ and Rg have
the meanings and prefer,ed meanings cited above.
The copolymer is prefer~ly formed by at least one diol or diamine and one diisocyanate.
The compounds of 1, lla or llb are preferably present in an amount of 0.01 to 10 % by
weight, more preferably of 0.1 to 5 % by weight and, most preferably, of 0.1 to 2 % by
weight, based on the amount of polymer.
Copolymer A) can be polyurethane, polyurea or polyureapolyurethane.
Polymer A) is preferably a polyurethane consisting of polyethers of C3-C6alkanediols,
polyoxyalkylenediols or hydroxy-te",linaled siloxanes and aliphatic, cyclo~ hatic,
cycloaliphatic-aliphatic, aromatic-aliphatic or aromatic C2-C20diisocyanates.
Preferred diisocyanates are those selected from the group consisting of 1 ,6-bis[isocyanate]-
hexane, 5-isocyanate-3-(isocyanatemethyl)-1,1,3-trimethylcyclohexane, 1,3-bis[5-iso-
cyanate-1,3,3-trimethylphenyl]-2,4-dioxo-1 ,3-diazetidine, 3,6-bis[9-isocyanatenonyll-4,5-
di(1-heptenyl)cyclohexene, bis[4-isocyanatecyclohexyl]methane, trans-1,4-bis[isocyanate]-
cyclohexane, 1,3-bis[isocyanatemethyl]benzene, 1,3-bis[1-isocyanate-1-methylethyl]-
benzene, 1,4-bis[2-isocyanate-ethyl]cyclohexane, 1,3-bis[isocyanatemethyl]cyclohexane,
1,4-bist1-isocyanate-1-methylethyl]benzene, bis[isocyanate]isododecylbenzene,1,4-
CA 02229743 1998-02-17
W O 97/11067 PCTAEP96/03954
_ g _
bis[isocyanate]benzene, 2,4-bis[isocyanate]toluene,2,6-bis[isocyanate]toluene, 2,4-/2,6-
bis[isocyanate]toluene, 2-ethyl-1,2,3-tris[3-isocyanate-4-methyianilinocarbonyloxy]propane,
N,N'-bis[3-isocyanate-4-methylphenyl]urea,1,4-bis[3-isocyanate-4-methylphenyl]-2,4-dioxo-
1,3-diazetidine, 1,3,5-tris[3-isocyanat~4-methylphenyl]-2,4,6-trioxohexahydro-1,3,5-triazine,
1,3-bis[3-isocyanate-4-methylphenyl]-2,4,5-trioxoin-.id~7O'idi,le, bis[2-isocyanatephenyl]-
methane, (2-isocyanatephenyl)-(4-isocyanatephenyl)methane, bis[4-isocyanatephenyl]-
methane, 2,4-bis[4-isocyanatebenzyl]-1-isocyanatebenzene, [4-isocyanate-3-(4-isocyanate-
benzyl)phenyl]-[2-isocyanate-5-(4-isocyanatebenzyl)phenyl]methane, tris[4-isocyanate-
phenyl]methane,1,5-bis[isocyanate]naphthalene, or 4,4'-bis[isocyanate]-3,3'-dimethyl-
biphenyl.
Particularly prefer-ed diisocyanates are those selected from the group consisting of 1,6-
bis[isocyanate]hexane, 5-isocyanate-3-(isocyanatemethyl)-1,1,3-trimethylcyclohexane, 2,4-
bis[isocyanate]toluene, 2,6-bis[isocyanate]toluene, 2,4-/2,6-bis[isocyanate]toluene, bis[4-
isocyanatecyclohexyl]methane or bis[4-isocyanatephenyl]methane.
Particularly suitable polyurethanes are obtained by reacting
a) 5-45 % by weight of an aromatic, cycloaliphatic or linear al;~halic diisocyanate,
b) 0-20 % by weight of a linear or branched C2-Cl2alkylenediol
c) 0-75 % by weight of a polytetrahydrofuran
d) 0-10 % by weight of a polyethylene glycol
e) 0-75 % by weight of a polypropylene glycol and
f) 15-95 % by weight of a hydroxy-terminated or hydroxypropyl-ter,llinated polydimethyl-
siloxane
g) 0.1-5 % by weight of a fluorophore of formula 1, lla or llb,
where the percentages are based on the amount of polymer and the parts by weight of the
components a) to g) give a sum of 100. A thermoplastic, randomly segmented polyurethane
is obtained.
The hydroxy-terminated or hydroxypropyl-terminated polydimethylsiloxane preferably has a
molecular weight of 900 to 4500 dalton.
The polytetrahydrofuran preferably has a molecular weight of 1000 to 4500 dalton.
Polyethylene glycol preferably has a molecular weight of 600 to 2000 dalton, andpolypropylene glycol preferably has a molecular weight of 1000 to 4000 dalton.
-
CA 02229743 1998-02-17
W O 97111067 PCT/EP96/03954
- 10-
The preparation of the polyurethanes can be carried out according to processes which are
known per se.
The compounds of formula 1, lla or llb can either be added from the start to the reaction
mixture for the preparation of polyurethane. However, it is also possible to react the
compounds of formula 1, lla or llb first with excess diisocyanate and then to mix the reaction
product with the diol or polyol components. Another possibility consisls in diluting the diol or
polyol component with a hyperstoichiometric amount of ~liisoc~nate and and .s~ ~bsec~uently
reacting the compounds of formula I with the excess diisocyanate.
The polymers obtained have essentially terminally bound fluorophors.
Another group of prefer.ed copolymers is obtained by selecting the monomers from the
compounds of the group consisting of acrylic acid, methacrylic acid, maleic acid, maleic acid
anhydride, C,-C30acrylic acid ester and C,-C3~ methacrylic acid ester, C,-C30acrylic acid
amide and C,-C30methacrylic acid amide or acrylic acid amide and methacrylic acid amide,
vinyl ester of C,-C20carboxylic acids, acrylonitrile, butadiene, isoprene, styrene, a-
methylstyrene, and vinyl ether of C,-C30alcohols and a compound of formula 1, lla or llb
Rl 4z R5R6N ~ 'R7Z
RlR2N~ R3 [~CO2H (lla)
R5R6N ~N~H
~~
(llb), wherein Rl, R2, R3, R4, Rs, R6, R7, R8 and Rg
have the meanings and prefe--ed meanings cited above and Z is -NH-CO-CH=CH2 or-NH-
CO-C(CH3)=CH2.
CA 02229743 1998-02-17
W O 97/11067 PCTAEP96/03954
- 11 -
The compounds of formula 1, lla or llb are preferably present in an amount of 0.01 to 10 %
by weight, preferably of 0.1 to 5 % by weight and, very particularly preferably, of 0.1 to 2 %
by weight, based on the entire polymer.
In another preferred embodiment of this invention, the copolymers comprise
a) 10 to 90 % by weight"~r~ferdbly 20 to 80 % by weight and, particularly prefer~l)ly, 30 to
70 % by weight, based on the polymer, of identical or different structural elements of
formula lll
Tl~ T
C C (111),
COXR,2 ~1,
b) 90 to 10 % by weight, prefer~bly 80 to 20 % by weight and, particularly preferably, 70 to
30 % by weight, based on the polymer, of identical or different structural elements of
formula IV
T3 114
C C (IV) and
R16 ~15
c) 0.1 to 5 % by weight of at least one compound of formula Va, Vb or Vc
CA 02229743 1998-02-17
W O 97/11067 PCT~EP96/03954
f c,
f f ,co
NH
R~ RsR6N ~"N--R'7
R,R2N ~ (Va), CO2H (Vb),
f co
lR7 NH
R5R6N ~N ~ H
~0
(Vc), wherein R" R2, R3, R4, Rs, R6, R,, R8 and
Rg have the meanings and prererled meaning cited above;
R10 and R11 are each independently of the other H or C1-C4alkyl, X is ~ or -NR,7-, Rl2 is C,-
C20alkyl and Rl7 is H or C1-C20alkyl;
R13 and R14 are each independently of the other H, F, Cl or C1-C4alkyl, Rl5 and Rl6 are each
independently of the other H, F, Cl, C,-C4alkyl, -COOH, -COO-C1-C5alkyl, -CONHC,-C5alkyl
or-CON~Rl7)Cl-Csalkyl~ or Rl5is H and Rl6is -CN, phenyl, chlorophenyl, C,-C,2alkoxy or C2-
Cl8acyloxy.
R1o is preferably H or methyl and R11 is preferably H. X is preferably -O-. R12 is preferably
C,-C16alkyl. Illustrative examples of R12 are methyl, ethyl, propyl, butyl, pentyl, hexyl, heptyl,
octyl, 2-ethylhexyl, nonyl, decyl, dodecyl, tetradecyl and hexadecyl.
R13 is preferably H or methyl, Rl4 is preferably H, and Rl5is preferably H. Rl6is preferably
-CN, phenyl, -COO-C1-C4alkyl, C1-C4alkoxy or C2-C6acyloxy. Typical examples of acyloxy
are acetyloxy, propionyloxy, butyroyloxy, pentanoyloxy and hexanoyloxy.
CA 02229743 1998-02-17
W O 97/11067 PCT~P96/03954
-13-
A particularly prefer,dd copolymer is that wherein the monomers forming the polymer are
selected from the group consisting of C,-C,6acrylic acid alkyl esters, C,-C,6methacrylic acid
alkyl esters, acrylamide, methacrylamide and the corresponding N-suhstihltecl derivatives,
or acrylonitrile.
The prepar~lioil of these polymers can be carried out according to known polymerisation
processes and the compounds of formula 1, lla or llb can be added e.g. at the beginning or
at a later time.
Depending on the process of preparation and the time of addition, the resulting
fluorophores are essentially terminally or randomly distributed in the polymers.
It is also poss;~'e to use alkylenetriols in minor amounts, typically 1,1,1-tris(hydroxymethyl)-
ethane in an amount of 0.1 to 5 % by weight, based on the polymer. Under these
conditions, no detec1~hlc c~ossli~king takes place yet and the resultant polyurethane
remains soluble in organic solvents.
Where the copolymer is formed from olefinically unsaturated monomers, diolefinicmonomers can also be added in minor amounts as clossl:nl~e,s.
The polymer pl efel ~bly has a glass transition temperature in the range from -150~C to
50~C, particularly preferably from -1 25~C to 40~C.
The molecular weight of the polymer is preferably from 10 000 to 250 000 dalton, more
preferably from 10 000 to 100 000 dalton and, most prefer~bly, from 10 000 to 30 000
dalton.
The dielectric constant of the polymer at 100 Hz and at room temperature is prefe~ ably from
2 to 25, particularly preferably from 5 to 15. The optical transparence is preferably in the
range from 400 to 1200 nm, particularly preferably from 400 to 900 nm.
.
The invention also relates to an optical sensor consisting of
(a) a transparent carrier which is coated on at least one side with a transparent layer of a
plasticiser-free polymer of this invention,
CA 02229743 1998-02-17
W O 97/11067 PCTAEP96/03954
-14-
(b) counterions in the form of lipophilic saits, and
(c) a ionophore which forms a complex with the ion to be determined.
The carrier can, for example, be constructed from a plastic material, typically polycarbonate
or acrylic glass, mineral materials or glass and may be in any fomm, e.g. plates, cylinders,
tubes, ribbons or filaments. Glasses are preferred.
The layer thickness on the carrier can be, for example, from 0.01 to 100 ~lm, preferably from
0.1 to 50 ~lm, more preferably from 0.1 to 30 llm and, particularly pre~e.~l~ly, from 0.1 to
1 0 ~lm.
Preferred salts containing lipophilic anions are alkali metal salts and ammonium salts with
unsl Ihstituted or sl Ihstituted tetraphenylborates.
Particularly preferred cations are Li+, Na+, K+, NH4+, and the ammonium cations of primary,
secondary and tertiary amines as well as quaternary ammonium cations containing 1 to 60
carbon atoms.
Illustrative examples of ammonium cations are methyl ammonium, ethyl ammonium, propyl
ammonium, butyl ammonium, hexyl ammonium, octyl ammonium, decyl ammonium,
dodecyl ammonium, tetradecyl ammonium, hexadecyl ammonium, octadecyl ammonium,
dimethyl ammonium, diethyl ammonium, dibutyl ammonium, butylmethyl ammonium, dioctyl
ammonium, didoceyl ammonium, dodecylmethyl ammonium, trimethyl ammonium, triethyl
ammonium, tripropyl ammonium, tributyl ammonium, trioctyl ammonium, tridodecyl ammo-
nium, dodecyldimethyl ammonium, didodecylmethyl ammonium, tetramethyl ammonium,
tetraethyl ammonium, tel-~pfopyl ammonium, tetrabutyl ammonium, tetrahexyl ammonium,
tetraoctyl ammonium, tetradecyl ammonium, tetradodecyl ammonium, dodecyltrimethyl
ammonium, octyltrimetyl ammonium, didodecyldimethyl ammonium, tridodecylmethyl ammo-
nium, tetradecyltrimethyl ammonium and octadecyltrimethyl ammonium. Quaternary ammo-
nium saits are preferred, in particular those containing 4 to 48 carbon atoms.
Borate anion is preferably unsubstituted tetraphenylborate or tetraphenylborate which is
suhstituted at the phenyl groups by one or more than one C,-C4alkyl, C,-C4alkoxy, halogen,
typically F, Cl, Br or 1, or trifluoromethyl.
Particularly ,u~erer~ed are sodium tetraphenylborate, sodium tetra(3,5-bistrifluoromethyl-
phenyl)borate, potassium tetra(4-chlorophenyl)borate, tetrabutyl ammonium tetraphenyl-
CA 02229743 1998-02-17
W O 97/11067 PCT~EP96/03954
- 15-
borate and tetradodecyl ammonium(4-chlorophenyl)borate. The salts containing lipophilic
anions serve as negative charge exchange for cations to be determined which havediffused into the active layer and which are complexed there.
The salts containing lipophilic anions can also be salts of polymers containing acid or basic
groups, typically polysulfonic acids or polycarboxylic acids.
The amount of salts containing lipophilic anions is preferably from 0.01 to 10 % by weight,
particularly preferably from 0.1 to 5 % by weight, based on the amount of polymer.
Ionophores are organic, natural or synthetic compounds which contain several, mostly
alternating, electron-rich hetero atoms, typically S, N and, preferably, O, in an open-chain or
cyclic carbon chain and which are capable of selectively complexing the ions to be
determined. The natural compounds are often macrocyclic compounds such as valinomycin
which is capable of selectively binding potassium cations. Another example is nonactin. A
large group of ionophores consists of the macrocyclic polyethers (crown ethers) which,
depending on geometry and size, are c~p~hle of complexing different metal cations. Other
examples of ionophores to be mentioned are coronandenes, cryptandenes and calixarenes.
A typical example of open-chain ionophores are the podandenes. Such ionophores are
described, inter alia, in US-A-4 645 744.
The nonionic ionophore ~ure~erdbly contains an open-chain carbon chain with several
oxygen atoms. Particuarly prefe"-3cl are (R,R)-N,N'-bis[1 1-ethoxycarbonylundecyl]-N,N',4,5-
tetramethyl-3,6-dioxaoctanediamide, N,N-dicyclohexyl-N',N'-dioctadecyl-3-oxapentane-
diamide or N,N,N',N'-tetracyclohexyl-3-oxapentane diamide.
The polymer contains the ionophore pre~erably in an amount of 0.01 to 10 % by weight,
particularly preferably in an amount of 0.1 to 5 % by weight, based on the amount of
polymer.
The optical sensor preferably contains valinomycin as potassium ionophore.
The preparation of such membranes can be carried out in a manner known per se, typically
by dissolving the composition in an organic solvent and then casting it to a film with
subsequent removal of the solvent. Once the solvent is removed, the film can be stripped
from the base, resulting in a self-supporting membrane.
CA 02229743 l998-02-l7
W O 97/11067 PCT~EP96/03954
-16-
Further possible processes for the p,~,a,alion of the membrane are those known from
coating technology, typically spinning, spraying or doctor coating processes.
Spin-coating processes are preferred.
Suitable solvents are ethers, esters, acid amides and ketones. Readily volatile solvents are
particularly suitable, prefe, dbly tetrahydrofuran.
In addition to these processes, which comprise first dissolving the cornr osi~ion, moulding it
and and then removing the solvent by evaporation, heat moulding processes are also
possible because the composition is a thermopl~stic material. Suitable heat moulding
processes are extrusion, injection moulding, compression moulding or blow moulding
processes such as those known from thermoplastic processing.
The membrane can be transparent or slighlty opaque. The membrane is preferably
transparent.
The fluorophores to be used according to this invention have very suitable ranges of
absorption and emission wavelengths which permit the use of known and inexpensiv~3 light
sources, for example halogen or xenone lamps or light-emitting diodes. Detectors s' lit~hle
for use are e.g. photodiodes. In addition, the fluorophores have high extinction coefficients
and can afford high quantum yields. The high lipophilic properties, high basicity and the
great dynamic range of the change between the fluorescence of the protonated anddeprotonated form meet in particular the high demands made on an optical determination of
ions based on the measurement of the fluorescence. Cations as well as anions can be
determined.
Suitable cations are, for example, cations of the metals of the first to fifth main group of the
periodic system of the elements, the lanthanides and actinides. Typical examples of metals
are Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, B, Al, Ga, In, Tl, Sn, Pb, Sb, Bi, Cu, Ag, Au, Zn, Cd,
Hg, Sc, Y, Ti, Zr, Hf, Cr, Mo, W, Mn, Fe, Co, Ni, Ru, Os, Rh, lr, Pt, Pd, La, Ce, Pr, Nd, Pm,
Sm, Eu, Gd, Tb, Dy, Ho, Er, Yb, Lu, Ac, Th, Pa, U, Np, Pu. Preferred cations are the alkali
metal cations and alkaline earth metal cations, in particular Li+, Na+, K+, Mg2+, Ca2+ and Sr2+,
and, more preferably, K+, Na+ and Ca++. Suitable ammonium cations are e.g. NH4+ as well
as the cations of protonated primary, secondary and tertiary amines and also quaternary
ammonium. The amines can contain 1 to 40, preferably 1 to 20 and, more preferably, 1 to
CA 02229743 l998-02-l7
W O 97/1l067 PCT/EP96/03954 -17-
12, carbon atoms. The quaternary ammonium can contain 4 to 40, preferably 4 to 20 and,
more preferably, 4 to 16, carbon atoms.
The anions to be deter~,i"ed can be derived from mineralic acids, oxygen acids and inorga-
nic complex acids. Illustrative examples are the halides and pseudohalides F, Cl-, Br~, I-, N3-,
CN-, OCN- and SCN-; anions of the inorganic oxygen acids NO2-, NO3-, co32-, PO4~, so42-,
ClOi, MnO4~ and Cl03-; anions of the inorganic complex acids Fe(CN)64~ and Co(CN)63~; the
anions of carboxylic acids, phenols; nucleotide anions such as adenosine phosphate.
The optical sensor is particularly suitable for the quantitative deler",in~lion of ions, in
particular of cations, very particularly of metal cations, typically potassium cations, in
aqueous environment, preferably using fluorescence spectrometry. The determinations can
be carried out in short time at high accuracy even at low concel,l,ations (e.g. in the ll-molar
range up to the nanomolar range) because the pH-dependent equilibria of the complexing
reactions and of the proton exchange adjust quickly and because the fluorophores are
distinguished by high fluorescence quantum yields and sensitivity. The analyses can be
typically carried out direct in body fluids (blood, urine, serum), natural waters or waste
waters, while possibly interfering cations can be specifically bound or removed beforehand.
The novel composition is particularly suitable for the determination of physiological amounts
of cations in aqueous media which, in the case of potassium, may be e.g. in the range from
about 0.5 to 10 mmol.
In addition to the plelell~d fluorescence spectroscopy, other optical methods ofmeasurement can also be used, typically surface plasmon resonance spectroscopy, ab-
sorption spectroscopy, reflection spectroscopy, interferometry or surface-enhanced Raman
or fluorescence spectroscopy.
The invention also relates to a process for the optical determination of ions in aqueous test
samples by contacting a sensor of this invention with said aqueous test sample and then
measuring the change in fluorescence of the fluorophore in the polymer layer
The process of this invention can typically be carried out such that the carrier is mounted
with the active polymer layer in an optical cell wherein the active layer is contacted with the
test sample The optical cell contains a window through which the active layer can be
CA 02229743 1998-02-17
W O 97/11067 PCTAEP96/03954
-18-
irradiated to eXcit~tion and the emitted fluorescent radiation can be measured with a
spectrofluorometer. The wavelengths are adjusted such that maximum absorption isobtained for the irradiation and maximum emission is obtained for the fluorescence
measurement. The i~lensily is measured as a function of time. The measuring system can
be such that measurement is carried out discontinuously or continuously e.g. by pumping
the measuring solution through the measuring cell. To determine unknown concentrations
of cations, the system can first be calibrated using test sa",ple;, of known concentration by
applying the concentrations as function of the intensity of the fluorescence. pH buffers are
expediently added to the test sample because, owing to the pH dependence of the
absorption spectrum of the fluorophore and consequently also of the fluorescence intensity
of the fluorophore, the sensitivity of the measurement depends on the pH of the measuring
solution. However, in another embodiment of this invention said pH dependence can also
be determined and be taken into account in the c~icul-tions~ The pH range of the test
sample can be, for example, 4 to 8, preferably 6.5 to 7.5. Suitable buffers are typically
citrate buffers and phosphate buffers. Further buffer systems are described in US-A-4
645 744, in particular also such buffers which are incorporated direct into the active layer to
avoid the addition to the test sample.
The invention also relates to the use of the optical sensor for the fluorescencespectroscopic analysis of cations or anions.
The following Examples illustrate the invention.
A) Preparation of the intermediate compounds
Ts in the structural formulae hereinbelow denotes a tosyl radical.
Example A1: Preparation of compound 101
,NH~= ~ (101)
1.21 9 of 3,6-diaminoacridine and 2.48 9 of 1,8-bisdimethylaminonaphthalene are dissolved
in 50 ml of 1,3-dimethyl-2-imidazolinon and then 2.2 9 of toluenesulfonyl chloride are
added. This mixture is heated to 80~C for 64 hours. The mixture is then poured in water
and exhausted with ethyl acetate. The organic phase is dried and concentrated by
,
CA 02229743 1998-02-17
W O 97/11067 PCT/EP96/03954
- 19-
evaporation. The product is purified by column chromatography over silica gel. Yield: 17%
red crystals.
Example A2: Preparation of compound 102
Ts
H4,C20 ~ Ts
3.0 9 of the compound 101 of Example A1 are dissolved together with 5.2 9 of 1 -bromo-
eicosane and 1.0 g of ground KOH in 45 ml of DMF and heated to 40~C for 7 hours. The
reaction mixture is poured in 500 ml of water and extracted with ethyl acetate. The organic
phase is separated, dried and concentrated by evaporation. The residue is chromato-
graphed over silica gel. Yield: 52% red resin.
Example A3: Preparation of compound 103
Ts Ts ~
H41C20 ~N~cH~cHzo~cH~H~N (103)
2.4 9 of the compound 102 of Example A2, 2.3 9 of ~N-phthalimidoethyltriethoxyethyl
bromide and 1 9 of potassium carbonate in 30 ml of DMF are heated to 70~C for 20 hours.
An additional 0.5 molar equivalent each of bromide and base are then added and the
mixture is heated to 70~C for a further 7 hours. The reaction mixure is poured in 300 ml of
2N HCI and extracted with ethyl acetate. The organic phase is separated, dried,
concentrated by evaporation and then purified over silica gel. Yield: 60%.MS(FAB): 1102.
CA 02229743 1998-02-17
W O 97/11067 PCTAEP96/03954
-20-
Example A4: Preparation of compound 104
CH3
ICH2
-CH2 'I ~ NH2
(104)
60 ml of sulfuric acid (97%) are placed in a vessel and then 9.4 9 (30 mmol) of 4-(2'-
carboxy)-benzoyl-3-hydroxy-N,N-diethylaniline are added in increments. 3.6 g (33 mmol) of
3-aminophenol are then added at room temperature and the mixture is stirred for 22 hours
at room temperature. The reaction mixture is charged to a mixture of 275 ml of water,
255 ml of 30% NaOH and 200 ml of toluene and stirred for 30 min at 70~C. After cooling to
60~C, the organic phase is separated, dried and concentrated by evaporation. The crude
product is purified by chromato~raphy. M.p.: 148-1 53~C.
Example A5: Preparation of compound 105
CH
3~ ICH2
CH2 ~NH
~0
3 (1 05)
500 mg of the compound 104 of Example A4 are dissolved in 20 ml of pyridine and the
mixture is cooled to -38~C. To this mixture are then added 492 mg (2 molar equiv.) of p-
toluenesulfonyl chloride and the solution is stirred for 1 hour at this temperature.
Subsequently, the solution is poured in 1 N of HCI and extracted with methylene chloride.
-
CA 02229743 l998-02-l7
W O 97/11067 PCTAEP96/03954
-21-
The organic phase is dried and then purified by chromatography. Yield: 60%. M.p.: 68-
73OC.1 H-NMR(DMSO-d6): 2.35 ppm (tolylme~hyl group).
Example A6: Preparation of compound 106
CH3 , CH COOCH3
~ICH2 CI H2 ;CH2~
CH2 ~ I~N~ (106)
1.5 9 of the compound 105 of Example A5,1.63g of 12-bromododecanoic acid methyl ester
and 844 mg of K2CO3 are dissolved in 25 ml of DMF and stirred for 9 hours at 65~C. The
reaction mixture is poured in 2n of HCI and extraced with ethyl acetate. The organic phase
is concentrated by evaporation and the crude product is then purified by chromatography
over silica gel. Yield: 1.02 9 pale yellow oil.1 H-NMR(CDCI3): 3.68 ppm (methyl ester).
Example A7: Preparation of compound 107
CH3
~ ICH2 Tl s O
-CH2 ~N ~ CH~
O (107)
2.5 9 of the compound 105 of Example A5 are dissolved in 25 ml of DMF and then 1.4 9 of
potassium carbonate and 3.4 9 of N-(10-bromodecyl)phthalimide are added. The mixture is
heated to 70~C for 5 hours. The mixture is then poured in 300 ml of 1 N HCI and extracted
with methylene chloride. The organic phase is dried and concentrated by evaporation.
CA 02229743 l998-02-l7
W O 97/11067 PCT~EP96/03954
-22-
Purification is carried out by coloumn chromatography over silica gel. Yield: 2.15 9 (56%)
yellowish crystals. MS (FD): 825.
Example A8: PreDaration of compound 108
CH
' ICH2 o
'CH2 ~ ~CH~fg ~
~0
(1 08)
The compound 107 of Example A7 is stirred into a mixture of 5 parts of acetic acid and
2 parts of sulfuric acid (25 ml in all) for 18 hours at room temperature. The mixture is then
poured in water and extracted with methylene chloride. The organic phase is washed with
bicarbonate solution, dried and concentrated by evaporation. Yield: 1.619 pure product.
B) Preparation of the functionalised compounds
Example B1: Preparation of compound 201
Tls Ts
H4-C20 ~[ ~N~CH~[CH2'o~CH23' ~NH2
3.7 9 of the compound 103 of Example 3 are stirred together with 1 ml of hydrazine hydrate
in 20 ml of methylene chloride and 45 ml of methanol for 21 hours at room temperature.
The resulting residue is isolated by filtration, washed thoroughly and the combined filtrates
are concentrated by evaporation. The residue is purified by chromatography over silica gel.
Yield: 41%. MS(FAB): 972.
CA 02229743 1998-02-17
WO 97/11067 PCT/EP96/03954
- 23 -
Example B2: Preparation of compound 202
H C ~N' ~ CH2 ~O~ 23~ ,NH~CHe(202)
1.2 9 of the compound 201 of Example B1 are added together with 190 mg of potassium
carbonate to 70 ml of tetrahydrofuran and the mixture is cooled to 0~C. 132 mg of meth-
acrylic acid chtoride are dissolved in 20 ml of tetrahydrofuran and this solution is slowly
added dropwise to the above suspension. After 1 hour at 0~C, the mixture is poured in
300 ml of 2N NaOH and extracted with ethyl ~cepte. The organic phase is dried and
concentrated by evaporation. The residue is purified over silica gel. Yield: 82% yellow resin.
Example B3: Preparation of compound 203
CH3
H~C~o ~NH~ ~H:~ ,CH23 HN~I~b'Hz(2o3)
980 mg of the compound 202 of Example B2 are stirred into a mixture of 5 parts of acetic
acid and 2 parts of sulfuric acid (25 ml in all) over 18 hours at room temperature. The
mixture is then poured in water and extracted with methylene chloride. The organic phase is
washed with bicarbonate solution, dried and concentrated by evaporation. The residue is
purified by chromatography over silica gel. Yield: 520 mg orange crystals. MS(FD):732. 1 H-
NMR(CDCI3): 1.95 (methacrylmethyl protons); 5.3 and 5.75 ppm (olefinic acryl protons).
CA 02229743 l998-02-l7
W O 97/11067 PCTAEP96/03954
-24-
Example B4: Preparation of compound 204
~ ICH2
'CH2 ~ N ~ CH~cH2~cH~NH2
COOH
(204)
17.49(55.5 mmol) of 4-(2'-carboxy)benzoyl-3-hydroxy-N,N-diethylaniline are added in
increments to 115 ml of sulfuric acid (95-97%) such that the temperature does not rise
above 30~C. 3-Methoxy-N-(3-aminopropyl)aniline is then slowly added. The reaction
mixture is stirred for 26 hours at room temperature and is then added, with vigorous stirring,
to a mixture of toluene and aqueous NaOH. The organic phase is separated, dried and
concentrated by evaporation. The ~qiJeolJs phase is additionally extracted with methylene
chloride and the organic phase is separated, dried and concenlr~ted by evaporation.
Example B5: Preparation of compound 205
CH
3~CI H2 ICH3
'CH2 ~5N~CH--CH2~CH--NH~CH2
~ COOH
Il I
~ (205)
500 mg of the compound 204 of Example B4 are dissolved in 40 ml of tetrahydrofuran.
After cooling the red solution to 0~C, 187 mg of K2CO3 are added and then 117 mg of
methacryloyl chloride in 10 ml of tetrahydrofuran are added dropwise. After 3 hours the
mixture is poured in water and extracted with CH2CI2. The separated organic phase is dried
_
CA 02229743 1998-02-17
W O 97tllO67 PCT~EP96/03954
-25-
and concentrated by evapo,dlion. Pu,i~icdlion by chromatography over silica gel gives
130 mg of red crystals. MS (m/z): 511 1 H-NMR(CDCI3): 5.0 and 5.55 ppm (olefinic acryl
protons).
Example B6: Preparation of compound 206
~fH2
'CH2 ~J~ ~CH
~0
(206)
1 9 of the compound 106 of Example A6 is stirred into a mixture of 5 parts of acetic acid
and 2 parts of sulfuric acid (25 ml in all) for 5 hours at 65~C. The mixture is then poured in
water and extracted with methylene chloride. The organic phase is dried and concentrated
by evaporation. The residue is purified by chromatography over silica gel. MS (m/z):584.
Example B7: Preparation of compound 207
CH3
fH2 R
CH2 ~ ~NH~I ~ ~CH2~ ,OH
~ 'CH2
~0
(207)
In in general accordance with the process described in Helv. Chim. Acta 1988, 71, 2087,
the compound 206 of Example B6 is activated at the free carboxylic acid functional group
and then reacted with diethanolamine over 72 hours at room temperature. Yield: 73%.
MS(FD): 671.
CA 02229743 l998-02-l7
W O 97/11067 PCTAEP96/03954
-26-
ExamDle B8: Preparation of compound 208
CH3
~fH2
'CH
~ (208)
1.6 g of the compound 108 of Example A8 are heated in 35 ml of 48% HBr over 21 hours to
110~C. After cooiing, the reaction mixture is poured in 2N NaOH and extracted with
methylene chloride. The organic phase is dried and concentrated by evaporation. Yield:
1.9 9 of crude product which is used direct for the next step.
Examole B9: Preparation of compound 209
CH3
fH2 R
'CH2 ~NH'~CH'~CH2'NH~cH2
~o
(209)
1.9 9 of the crude product of compound 208 of Example B8 are added to 50 ml of tetra-
hydrofuran and then 490 mg of potassium carbonate are added. To this mixture are slowly
added dropwise 245 mg of methacryloyl chloride dissolved in 20 ml of tetrahydrofuran. After
2 hours the reaction mixture is poured into 400 ml of 1 N NaOH and extracted with
methylene chloride. The organic phases are washed with 1 N Hcl, dried and concentrated by
evaporation. The crude product is purified by chromatography over silica gel. Yield: 40%.
1 H-NMR (DMSO-d6): 1.90 ppm (methacrylmethyl),5.35 and 5.68 (olefinic acryl protons).
CA 02229743 1998-02-17
WO 97/11067 PCT/EP96/03954
- 27 -
C) Preparation of the polymer-bound fluorophores
ExamPle C1: In a three-necked flask equipped with stirrer, 1. g of polytetrahydrofuran (Mn =
2000 glmol), 0.69 9 of hydroxypropyl-te"";naled polydimethylsiloxane (Mn 2740 g/mol),
0.09 9 of butanediol and 0.005 mg of diazabicycloctane are dissolved in 15 ml of anhydrous
tetrahydrofuran under inert gas conditions (N2 atmosphere). The reaction is allowed to
proceed for 3 hours at 60~C with 0.40 9 of methylene diphenyl diisocyanate.
In a second three-necked flask, also under inert gas conditions (N2 atmosphere), 0.05 9 of
methylene diphenyl diisocyanate are dissolved in 2.5 ml of tetrahydrofuran and a solution of
0.022 9 of fluorescent dye of formula 204 of Example B4 in 5 ml of tetrahydrofuran is then
added dropwise at room temperature and the reaction is allowed to proceed for 1 hour. The
resulting pink fluorescent solution is combined with the polymer solution mentioned above
and the reaction is allowed to proceed for a further 3 hours at 60~C.
The polymer is precir jt~ted by being poured in 500ml of methanol and is then isolated by
filtration and dried under vacuum at 20~C. The product obtained is dissolved once more in
20 ml of tetrahydrofuran and precirit~ted in 500 ml of methanol, giving 1.85 9 (81 % of
theory) of pink polymer.
Example C2: In a three-necked flask e~ ;ppped with stirrer, 1.0 9 of polytetrahydrofuran (Mn
= 2000 g/mol), 0.10 9 of butanediol and 0.005 mg of d~ ycloctane are dissolved in
15 ml of anhydrous tetrahydrofuran under inert gas conditions (N2 atmosphere). The
reaction is allowed to proceed for 3 hours at 60~C with 0.35 9 of methylene diphenyl
diisocyanate.
In a second three-necked flask, also under inert gas conditions (N2 atmosphere), 0.05 9 of
methylene diphenyl diisocyanate are dissolved in 2.5 ml of tetrahydrofuran and then diluted
dropwise with a solution of 0.019 9 of fluorescent dye of formula 204 of Example B4 in 5 ml
of tetrahydrofuran at room temperature, and the reaction is allowed to proceed for 1 hour.
The resulting pink fluorescent solution is combined with the polymer solution mentioned
above and the reaction is allowed to proceed for a further 3 hours at 60~C.
The polymer is then precipitated by being poured in 500ml of methanol and is then isolated
by filtration and dried under vacuum at 20~C. The product is dissolved once more in 20 ml
of tetrahydrofuran and precipitated in 500 ml of methanol, giving a pink polymer.
Exam~le C3: 4.00 9 of Tecoflex~ polyurethane, supplied by Thermedics, together with
0.05mmol/g of OH terminal groups and 0.002 9 of diazabicyclooctane are dissolved in a
mixture of 30 ml of tetrahydrofuran and 25 ml of dimethylformamide and then a solution is
added consisting of of 0.055 9 of methylene diphenyl diisocyanate and 0.067 9 of the
CA 02229743 1998-02-17
W O 97/11067 PCT~P96/03954
- 28 -
compound of formula 207 of Example B7 in 5 ml of DMF. The mixture is allowed to react for
16 hours at 60~C. Further processing is carried out in general accordance with the
procedure of Examples 1 and 2. The yield is 3.05 9 (74 % of theory) of pink polymer.
Example C4: In a vial, equipped with a three-way tap connected to vacuum and nitrogen,
50 mg of the compound 209 of Example B9 are dissolved in 0.16 g (2.9 mmol) of
acrylonitrile. To this solution are added 4.84 g (26.3 mmol) of 2-ethylhexylacrylate and 5 mg
of azoisobuty~unil,i!o (AIBN). The vial is closed and the atmosphere is exchanged three
times by a freezing/thawing cycle with nitrogen. The vial is kept in a water bath at 60~C for
2 days. The viscous contents of the vial are then dissolved in 50 ml of toluene at 50~C and
the polymer is prec;rit~ted in 1 000ml of methanol. The pale red polymer is filtered, dried,
dissolved again and then precipitated again under the same conditions. Drying is carried out
over 24 hours under high vacuum. The yield is 1.9 g (38%) and the glass transition
temperature is -58~C. The inherent viscosity of a 0.5 % solution in THF at 25~C is ~inh-2.81.
.
D) Use Examples. preparation and chard~lerisalion of the optical sensor
Example D1: Pretreated glass is used as carrier material. Round glass panes (diameter
18 mm, thickness 0.17 mm) are immersed for 1 hour into a solution of 10 vol % of dimethyl-
dodecylchlorosilane in isopropanol. The glass panes are then washed in succession with
200 ml each of isopropanol, ethanol and methanol and dried for 1 h at 1 1 0~C. The adhesion
of the me",brdne layer on the hydrophobised surface is improved.
The sensor membrane is prepared by dissolving 40 mg of the polymer described in
Example C1, 1.5 mg of valinomycin and 1.2 mg of potassium tetrakis 3,5- bis(trifluoro-
methylphenyl)borate in 1.2 ml of tetrahydrofuran. The glass carriers are mounted in the
chamber of a spin-coating apparatus (Optocoat OS 35var, Willer Company, CH-8484
Weisslingen). The chamber is rinsed with 10 ml of tetrahydrofuran and rotated for 2 min at
8000 rpm. Subsequently, 50 ~~11 of the respective coating solution are pipetted on to the
glass carrier which is then rotated for a further 10 sec. The membrane-coated glass carrier
is taken out and dried for 10 min in the air.
The coated glass carriers are mounted in an optical cell wherein the membrane is in contact
with the measuring liquid. In the optical cell the membrane can be optically excited and the
fluorescence radiation can be measured. The optical cell is placed in a spectrofluorometer
(Pekin-Elmer LS-50). The absorption and emission wavelengths are adjusted to therespective maxima of the fluorophores used in the membrane. The membrane is contacted
CA 02229743 1998-02-17
W O 97/11067 PCT~P96/03954
- 29 -
with an aqueous solution of Kcl or CaCI2 of defined concentration by pumping the solution
at a rate of 1 ml/min through the cell and deter",il-ing the change in fluorescence inlensiLy.
Rinsing with potassium ion-free buffer solutions is carried out before and after each
measurement and the fluorescence i"lensily is measured in order to determine the base
line. The fluorescence intensity (measured as change in voltage in the photodiode) at the
respective potassium concenl~lion for the fluorophore is listed below.
Potassium ion concenl,dlion in mmol Fluorescence intensity in volt
o 6.8
0.5 4.8
4.0 4.3
10.0 4.1
Example D2: The procedure of Example D1 is repeated, the membrane for the sensorbeing composed of 20 mg of the polymer of Example C2, 1.5 mg of valinomycin and 1.2 mg
of potassium tetrakis 3,5- bis(trifluoromethylphenyl)borate. The following values are
obtained:
Potassium ion concentration in mmol Fluorescence intensity in volt
0 6.3
0.5 4.9
4.0 4.7
10.0 4.6
Example D3: The procedure of Example D1 is repeated, the membrane for the sensorbeing composed of 40 mg of the polymer of Example C2, 3.0 mg of valinomycin and 2.0 mg
of potassium tetrakis 3,5- bis(trifluoromethylphenyl)borate. The following values are
obtained:
Potassium ion concentration in mmol Fluorescence intensity in volt
0 2.9
0.5 1.7
4 o 1.6
10.0 1.5
CA 02229743 1998-02-17
W O 97/11067 PCT~EP96/03954
-30-
Ex~mple D4: The procedure of Example D1 is repeated, the membrane for the sensorbeing composed of 75 mg of the polymer of Example C4, 1.5 mg of valinomycin and 1.2 mg
of potassium tetrakis 3,5-bis(trifluoromethylphenyl)borate. The following values are
obtained:
Potassium ion concentration in mmol Fluorescence intensity in volt
0 2.8
0.5 1.
4.0 1.2
10.0 1.0