Note: Descriptions are shown in the official language in which they were submitted.
64680-1031
CA 02229786 2000-11-17
1
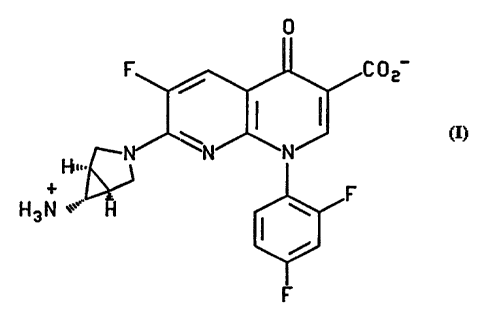
~1NITTERIONIC FORMS OF TROVAF~OXACIN
f3ackcround of the Invention
This invention relates to the naphthyridone antibiotic trovafloxacin. More
particularly, it relates to polymorphs and the pentahydrate of the
zwitterionic form of
thereof having the formula i, below, and methods for their preparation. The
invention
further relates to methods of using, and pharmaceutical compos~ions
comprising, the
compounds of the invention for treatment of bacterial infections in mammals.
The antibacterial activity of the aforementioned naphthyridone antibiotic is
described in United States Patent No. 5,164,402 [the '402 patent] and
5,229,396 issued
11/17/92 and 7/20/93, respectively.
The zwitterionic forms of trovafloxacin are useful for the administration of
the
drug as a suspension.
Summary of the Invention
According to a first embodiment of the invention there is provided a
trovafloxacin
zwitterionic crystal form having the formula
0
0 2_
H3N
which is selected from the group consisting of
a) a non hygroscopic first polymorph PI exhibiting the following
characteristic X-ray powder diffraction patiem
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
-2-
Peak 1 2 3 4 5 6 7 g
no. 8 r
2 8 () 6.9 9.8 11.3 12.0 13.9 16.1 16.6 17.1 17.4
Cu
d space 12.7 9.0 7.9 7.4 6.4 5.5 5.4 5.2 5.1
Peak 10 11 12 13 14 15 1~6 17
no.
2 8_( 19.7 22.9 23.6 24.9 25.4 25.9 27.7 29.5
)
Cu
d space 4.5 3.9 3.8 3.6 3.5 3.4 3.2 3.0
b) a hygroscopic second polymorph PII exhibiting the characteristic
X-ray powder diffraction pattern
Peak no. 1 2 3 4 5 6 7
8
2 B_( ) Cu 8.4 9.5 10.2 14.7 16.8 17.9 22.6 26.1
d space 10.6 9.3 8.7 6.0 5.3 5.0 3.9 3.4
an
c) a pentahydrate, trovafloxacin zwitterion pentahydrate, exhibiting
the characteristic X-ray powder diffraction pattern
Peak 1 2_ 3 4 5 6 7 8
9
no.
2 8_( 6.6 8.6 12.7 13.3 15.9 18.6 19.2 20.1 21.0
)
Cu
d space13.3 10.3 7.0 6.6 5.5 4.8 4.6 4.4 4.2
Peak 10 11 12 13 14 15 16 17
no.
2 8_( 22.5 22.9 23.6 24.9 25.4 25.9 27.7 29.5 ,
)
Cu
d space4.0 3.9 3.8 3.6 3.5 3.4 3.2 3.0
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
..3_
A second embodiment of the invention relates to a process for preparing a
zwitterion, of trovafioxacin, of the formula I which is selected from the
group consisting
of a non hygroscopic polymorph PI, a hygroscopic polymorph PII and a
pentahydrate
thereof, as described above, comprising
' S A. the steps of treating an aqueous suspension of a metastable form of the
compound of the formula I
1 ) with a nonpolar solvent followed by azeotropic removal of
residual water and vacuum drying to form said hygroscopic polytr~orph PII
which
exhibits the characteristic X-ray p~nrder diffraction pattern described in
claitml ;
2) with a polar solvent followed by azeotropic remove) of residual
water and vacuum drying; or
3) with water and air drying the residue at an elevated temperature,
removing the mother liquor and air drying the residue at room temperature to
constant
weight to form the pentahydrate; or
B) treating the hygroscopic second polymorph PII with a refluxing polar
solvent to form the non-hygroscopic first polymorph PI.
According to a third embodiment of the invention there is provided a process
for preparing the metastable form of the xwitterion, of trovafloxacin, of the
formula I,
by
a) treating an acid salt of trovafloxacin with a base to raise the pH of the
mixture to between 7.5 and 8.5 at an elevated temperature, removal of the
mother
liquor, washing the crystals with water and drying the crystals under vacuum
at about
35 to about 40 °C; or
b) treating a compound of the formula
a
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
-4-
o2g ,.
s r
II
AHN
F
6
wherein A is hydrogen or an amine protecting group such as t-butyloxycarbonyl,
benzyloxycarbonyl, (C,-CB)alkylcarbonyl and benzyl; and
B is hydrogen or a carboxylic acid protecting group selected from benzyl, t-
butyl
and (C,-CB) alkyl; with an amine and/or carboxylic acid deprotecting agent,
respectivel~r.
A fourth embodiment of the invention provides a method of treating bacterial
infections in a mammal which comprises administering to said mammal a
bacterial
infection treating effective amount of a compound of formula I as described
above.
According to a fifth embodiment of the invention there is provided a
composition
for treating bacterial infections in a mammal which comprises a bacterial
infection
treating effective amount of a compound of formula I and a pharmaceutically
acceptable
can-ier.
Detailed Description of the Invention
The present invention relates to a compound comprising a stable zwitterionic
form of the antibiotic trovafloxacin of the formula
w
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
-5-
0
' 5
I I
H3N
More particularly, it is related to a compound of the formula I which is
selected from
a) a non hygroscopic first polymorph PI exhibiting the characteristic
X-ray powder diffraction pattern described above;
'15 b) a hygroscopic second polymorph PII exhibiting the characteristic
X-ray powder diffraction pattern described above;
and c) a pentahydrate, trovafloxacin zwitterion pentahydrate, exhibiting
the characteristic X-ray powder diffraction pattern described above.
The invention also relates to processes for the preparation of the compounds
of the formula I as illustrated in the following schemes.
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
-6
SCHEME 1
H3N
X- .
1
0
02H
02
H3N
F
2
metastable form of zWitterion
4 3 5
- -
polymorph pII polymorph PI pentahydrate
a
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/0075fr
SCHEME 2
0
02B
s
AHN
6
0
~2
I
I
+
H3N
metastable forms of zwitterion
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
_$_
As shown in Scheme 1 a trovafloxacin salt 1, wherein X is an anion selected
from those formed from mineral acids such as hydrochloric, sulfuric, nitric
and
phosphoric; organic acids such as sulfonic acids, e. g. benzenesulfonic
(besylic), p-
toluenesulfonic (PTSA, tosylic), methanesulfonic (MSA, mesylic) and
trifluoromethanesulfonic (triflic); and carboxylic acids e.g., acetic,
proprionic, benzoic,
citric, tartaric, malefic, fumaric, succinic and malic, is converted to a
metastable
zwitterionic form 2_ by raising the pH -of a slung comprising compound y to a
pH bf
between about 7.5 and 8.5 at a temperat~s irr the-r ange of about 45 to about
55 ° C
using an aqueous basic solution. A preferred salt is the -mesylate. The bases
useful
in the practice of this aspect of the invention include inorganic bases such
as alkali or
alkaline earth hydroxides, carbonates and bicarbonates and organic bases such
as
tri(C,-CB)alkyl amines, pyridine and morpholine. A preferred aqueous base is
saturated
sodium bicarbonate. The wet product is then dried to constant weight, in
vacuo, at a
temperature from about 35 to about 40°C.
Alternatively, as shown in scheme 2, compound 2 may be prepared directly from
protected precursors 6, of the trovafloxacin salts 1, of the formula
0
OzB
II
RHN
/
F
6
wherein A is hydrogen or an amine protecting group such as t-butyloxycarbonyl,
benzyloxycarbonyl, (C,-Ce)alkylcarbonyl and benzyl; and
B is hydrogen or a carboxylic acid protecting group selected from benzyl, t-
butyl
and (C,-C6) alkyl; with an amine and/or carboxylic acid deprotecting agent,
respectively.
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00755
-9-
A preferred compound 6, wherein A is hydrogen and B is ethyl, is converted to
compound 2 by treatment with a solution of NaOH in a polar solvent at an
elevated
' temperature. A preferred solvent is methanol and the temperature is the
reflux
temperature of the solvent. The pH of the solution was then adjusted to
between about
6.5 and 8.0 with dilute HCI and saturated aqueous NaHC03 was then added to
adjust
the pH to between about 7.5 and 8.5. The product was recovered as indicated
above.
_ Metastable trovafloxacin zwitterion 2_ is converted to hygroscopic polymorph
PII,
4, by treatment with a non polar solvent such as =a hydrocarbon. A preferred
hydrocarbon is hexanes. Residual water is removed azeotropically--afld the
product
dried at about 35 to about 40°C under vacuum. ~ Solvents useful for the
azeotropic
removal of water traces include non-polar ,aliphatic hydrocarbons, such as
hexanes and
octaves, and aromatic hydrocarbons such as benzene and toluene. Preferred
solvents
are the aliphatic hydrocarbons, most preferably hexanes.
Non hygroscopic polymorph PI 3, can be prepared from compound 2 by
treatment with a polar solvent followed by azeotropic removal of water and
vacuum
drying at about 30 to about 40°C. Polar solvents useful for this
conversion include
(C~-CB)alkyl esters of (CZ CB)alkylcarboxylic acids and (C,-CB)alkanols. A
preferred
solvent is ethyl acetate.
Alternatively, compound 3 can be prepared from compound 4 by treating
compound 4 with a refluxing polar solvent , as described above. A preferred
solvent is
is ethyl acetate.
Compound 5, the pentahydrate of the compound of formula I, is prepared by
air drying the wet crystals of compound 1, at room temperature, until constant
weight
is achieved. Alternatively, compound a may be prepared from compound 4 by
treatment with water until a constant water uptake has been obtained. Compound
3
is not converted to compound 5 by exposure to water.
The antibacterial compounds of the invention, i.e., polymorph PI, polymorph
PII
and the pentahydrate (hereafter 'the active compounds') are useful in the
treatment of
animals and humans having a broad spectnrm of bacterial infections. They are
particularly useful in treating gram-positive bacterial strains.
The active compounds may be administered alone, but will generally be
administered in a mixture with a pharmaceutical carrier selected with regard
to the
intended route of administration and standard pharmaceutical practice. For
example,
they can be administered orally or in the form of tablets containing such
excipients as
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
-10-
starch or lactose, or in capsules either alone or in admixture with
excipients, or in the
form of elixirs or suspensions containing flavoring or coloring agents. In the
case of
animals, they are advantageously contained in an animal feed or drinking water
in a '
concentration of about 5 to about 5000 ppm, preferably about 25 to about 500
ppm.
They can be injected parenteraliy, for example, intramuscularly, intravenously
or
subcutaneously, For parenterai administration, they are best used in the form
of a
sterile aqueous solution which can contain other solutes, for example, enough
salt or
glucose to make the nalution isotonic. In the case of animals, the compounds
of
formula i can be administered intramuscularly or subcutaneously at
dosage~tevels of w
about 0.1 to about 50 mg/kg/day, advantageously about 0.2 to about 10
mg/kg/day
given in a single daily dose or up to 3 divided doses.
The active compounds can be administered to humans, for the treatment of
bacterial diseases by either oral or parenteral routes. They may be
administered orally
at dosage levels of about 0.1 to 500 mg/kg/day, advantageously 0.5-50
mg/kg/day
given in a single dosage or up to 3 divided dosages. For intramuscular or
intravenous
administration, dosage levels are about 0.1-200 mg/kg/day, advantageously 0.5-
50
mg/kg/day. While intramuscular administration may be a single dose or up to 3
divided
doses, intravenous administration can include a continuous drip. Variations
will
necessarily occur depending on the weight and condition of the subject being
treated
and the particular route of administration chosen as will be known to those
skilled in
the art.
The active compounds may be administered alone, but will generally be
administered in a mixture with a pharmaceutical carrier selected with regard
to the '
intended route of administration and standard pharmaceutical practice. For
example,
they can be administered orally or in the form of tablets containing such
excipients as
starch or lactose, or in capsules either alone or in admixture with
excipients, or in the
form of elixirs or suspensions containing flavoring or coloring agents. In the
case of
animals, they are advantageously contained in an animal feed or drinking water
in a
concentration of about 5 to about 5000 ppm, preferably about 25 to about 500
ppm.
They can be injected parenterally, for example, intramuscularly, intravenously
or
subcutaneously, For parenteral administration, they are best used in the form
of a
sterile aqueous solution which can contain other solutes, for example, enough
satt or
glucose to make the solution isotonic. In the case of animals, the compounds
of
formula I can be administered intramuscularly or subcutaneously at dosage
levels of
' ~ CA 02229786 2001-04-04
64680-1031
_11 _
about 0.1 to about 50 mg/kg/day, advantageously about 0.2 to about 10
mg/kg/day
given in a single daily dose or up to 3 divided doses. The active compounds
can be
administered to humans by either ore! or parenteral routes, and may be
administered
orally at dosage levels of about 0.1 to 500 mg/kg/day, advantageously 0.5-50
mg/kg/day given in a single dosage or up to 3 divided dosages. For
intramuscular or
intravenous administration, dosage levels are about 0.1-200 mg/kg/day,
advantageously
0.5-50 mg/kg/day. While intramusarlar administraation may be a single dose or
up to
3 divided doses, intravenous administration can indude a continuous drip.
Variations
will necessarily ocrxrr depending -on the weight and condition of the subject
being
treated and the particular route of administration chosen as will be known to
those
skilled in the art.
The antibacterial activity of the compounds of the invention is shown by
testing
according to the Steers replicator technique which is a standard in vitro
bacterial testing
method described by E. Steers et al., Antibiotics and Chemotherapy, 9, 307
(1959).
The following examples illustrate the methods and compounds of the present
invention. It will be understood, however, that the invention is not limited
to the specific
examples.
Example 1
Trovafloxacin zwitterion. metastable form
A. Trovafloxacin mesylate (prepared according to Example 13B of the '402
patent) (20 g) was stirred with demineralized water (100 mL). The crystal
sluny was
heated to about 50 °C and the slurry adjusted to a pH of about 8.0 by
addition of
saturated sodium bicarbonate solution. The slurry was held at about 50
°C for 30
minutes, allowed to cool to about 25 °C and stirred at this temperature
for 30 minutes.
The crystals were isolated by filtration and washed with demineralized water
(27 mL).
The wet crystals were suspended in demineralized water (100 mL) and stir-ed
for about
1 hour at about 50 °C, then cooled to about 20°C and stirred at
this temperature for
about 1 hour. The crystals were filtered from the mother liquor, washed with
demineralized water (about 27 mL) and dried to constant weight under vacuum at
about
40 °C to yield the title product which contained 2.5 % residual water
by analysis. Yeld
16 .25 g, 97 %.
B. The ethyl ester of trovafloxacin
CA 02229786 2001-04-04
64680-1031
-12-
(i 0 g) was stirred with methanol (75 mL), water (25 mL) and sodium hydroxide
pellets
(1.8 g). The resultant mixture was heated to reflux at about 72 °C to
form a solution.
The solution was cooled to about 25 °C and the pH adjusted to about
7.5, by addition
of 6N hydrochloric acid, to form a slurry. Saturated sodium bicarbonate
solution (50
mL) was added and the slung stirred for 30 minutes at about 25 °C...
The title product
was isolated and washed with water (20 ml) and dried under vacuum about 45
°'~.
Yield 7.72 g, 82.5 %.
i 0 am le
Trovafloxacin zwitterion polymorph PI Inon hvaroscopic forrn_l
Trovafloxadn mesylate, {75 g) was stir-ed with demineralised water (375 mL).
The crystal slung was heated to about 50 °C and the slung adjusted to a
pH of about
8.0 by addition of saturated sodium bicarbonate solution. The slurry was held
at about
50 °C for 30 minutes, allowed to cool to about 25 °C and stirred
at this temperature
for 30 minutes. Crystals were isolated by filtration and washed with
demineralised water
(100 mL). The wet crystals were suspended in demineralised water (375 mL) and
stirred for 1 hour at about 50 °C, then cooled to about 20 °C
and stirred at this
temperature for about 1 hour. The crystalline product was filtered from the
mother
liquor and washed with demineralised water (about 100 mL). The wet crystals
were
stirred with ethyl acetate (1125 mL) and the resultant slurry heated to reflux
and the
water azeotropically removed. The essentially anhydrous slurry was cooled to
about
° C, the crystals were isolated by filtration and dried under vacuum at
40 ° C until all
the solvent had been removed to provide the title product. Yield 60 .9 g, 94
96.
25 The product is characterized by the X-ray powder diffraction pattern
described
above.
Example 3
Trovafloxacin zwitterion hyaroscopic colymomh PII
The title product of Example 1, paragraph A, (5 g) was mixed with hexanes (150
mL) to form a slurry. The slung was heated to reflux and traces of residual
water were
removed azeotropically. After 4 hours at reflux the crystal slurry was cooled
to about
25 °C, isolated by filtration and dried to constant weight under vacuum
at about 40 °C.
Yield 4.7 g, 94 96. The title product was characterized by the X-ray powder
diffraction
pattern described above.
CA 02229786 1998-02-17
WO 97/07800 PCT/IB96/00756
-13-
F~cam~le 4
Trovafloxacin zwitterion pentahydrate
Trovafloxacin mesylate (50 g) was stirred with demineralised water (250 mL).
The crystal slung was heated to 50 °C and the slurry adjusted to a pH
of about 8.0
by addition of saturated sodium bicarbonate solution. The slung was held at
about 50
°C for 30 minutes, allowed to cool to about 25 °C and stirred at
this temperature for
30 minutes. The crystals were isolated by filtration and washed with
demineralised
water (70 mL). The wet crystals were suspended in demineralised water (250 mL)
and
stirred for 1- hour at about 50 °C, then cooled to about '20°C
and stirred at this
temperature for about 1 hour. The crystalline product was filtered from the
mother
liquor, washed with demineralised water (about 70 mL). The wet crystals were
air dried
to constatnt weight at room temperature to yield the title product which
contained 17.6
96 water by analysis. Yield 48.4 g, 84 96
The title product was characterized by the X-ray powder diffraction pattern
described above.