Note: Descriptions are shown in the official language in which they were submitted.
CA 02230019 1998-02-19
WO 97!06889 PCT/IB96/00930
- 1 -
A METHOD FOR REACTIVATING SORBENT
TO ENABLE THE REUSE THEREOF
BACKGROUND OF THE INVENTION
s This invention relates to S02 emission reduction and, more
specifically, to a method for reactivating for purposes of the reuse thereof
the sorbent, which is injected into a fossil fuel-fired combustor in order to
effectuate the capture therewith of the SOZ that is released during the
combustion of the fossil fuel within the fossil fuel-fired combustor.
io It has long been known in the prior art to employ a sorbent
embodying Ca0 to effect therewith capture of the S02 that is released
during the combustion of the fossil fuel within fossil fuel-fired combustors.
Moreover, such sorbent has proven to be particularly effective in capturing
the SOZ that is released within a circulating fluidized bed combustor during
is the combustion therewithin of fossil fuels that are solid in nature.
However, notwithstanding the fact that sorbent has proven to be
particularly effective when utilized to accomplish the capture of S02 in
circulating fluidized bed combustors, it has nevertheless been found that
sorbent consumption and the need to effect the disposal of the ash, in
2o which sorbent is contained, that is produced as a consequence of the
combustion, which occurs within the circulating fluidized bed combustor,
represent major operating costs to the operator of the circulating fluidized
bed combustor. Furthermore, it is expected that these costs will continue
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-2-
to escalate as environmental constraints become even more stringent in
the future. Thus, in summary the increased calcium-to-sulfur mole ratio
necessary to meet very high levels of suffer removal will seriously impact
the cost effectiveness of circulating fluidized bed technology unless the
s consumption of sorbent can be reduced.
In accordance with the process employed in circulating
fluidized bed technology, fossil fuel, which may take any one of a number
of different forms, e.g., coal, etc., is burned in the combustor of a
circulating fluidized bed system in the presence of a sorbent embodying
to CaO, such sorbent being injected into the combustor in order that while
the fossil fuel is being burned in the combustor such sorbent undergoes
calcination and sulfation reactions. To this end, such sorbent, while the
fossil fuel is being burned in the combustor, is operative to effect the
capture of S02. In doing so though, not all of the Ca0 in the sorbent is
is fully utilized for sulfur capture. This is attributable to the fact that in
capturing the sulfur the Ca0 in the sorbent combines with the sulfur to
form CaS04. As such, because the specific volume of the CaS04 is
greater than the specific volume of the CaO, the pore structure of the
sorbent becomes plugged with CaS04. That is, the surface of the Ca0
zo essentially becomes covered with an outer layer of CaS04, which
functions to effectively shield the unreacted free Ca0 in the interior of the
particles of sorbent from further reaction with SOz, i.e., inhibits further
capture of sulfur by the sorbent.
It has been known heretofore to reinject ash into the
2~ combustor of the circulating fluidized bed system in an effort to effect
the
reuse of the sorbent contained in the reinjected ash. This reinjection of
ash containing sorbent eventually reaches a point of diminishing returns.
Namely, the particles of sorbent eventually become sulfated and the
internal pores of the sulfur become blocked by CaS04. Accordingly,
~o further sulfur capture by the sorbent can only occur if the Ca0 in the
interior of the sorbent particles becomes exposed. Attempts, to which
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-3-
reference will be had more fully hereinafter, have been made to effect
such exposure of the CaO.
Thus, by way of summary, a substantial effort has been put
forth heretodate in the prior art in an attempt to develop new methods for
s reducing sorbent consumption, diminishing solids waste disposal and
improving solids waste utilization. In this regard, by way of exemplification
and not limitation, reference is had to U.S. Patent No. 5,342,594 entitled
"Fluidized Bed Process For SOx Removal," which issued on August 30,
1994. As taught in U.S. Patent No. 5,342,594, hot, unclean gases having
to gaseous sulphur oxide-containing impurities are mixed within a mixing
chamber with a solid process material comprising CaC03 such that the
gaseous sulphur oxide-containing impurities react with the CaC03 to form
a solid impurity reaction product thereof. Thereafter, the solid process
material and the solid impurity reaction product are separated from the
is unclean gases to provide purified gases. Whereupon, the separated solid
impurity reaction product and solid process material are returned to the
mixing chamber to be once again mixed with hot, unclean gases.
Also, further in this regard, reference is had, by way of
exemplification and not limitation, to U.S. Patent No. 5,345,883 entitled
?o "Reactivation Of Sorbent In A Fluid Bed Boiler," which issued on
September 13, 1994. In accordance with the teachings of U.S. Patent No.
5,345,883, a jet of fracturing medium of liquid water or steam is injected at
a sufficiently high pressure and in being so injected is directed so as to
impinge upon sorbent particles containing unreacted sorbent material
2s inside, whereby these sorbent particles are mechanically fractured to
expose the unreacted sorbent contained therewithin. To this end, as
taught in U.S. Patent No. 5,345,883, the fracturing medium when being so
injected is at a temperature lower than the temperature of the sorbent
particles that the fracturing medium is made to impinge upon such that the
3o fracturing of the sorbent particles is the result of thermal shock. Or, as
taught in U.S. Patent No. 5,345,883, the fracturing medium when being so
CA 02230019 2000-08-02
62898-1469
-4-
injected is directed at the sorbent particles such that the sorbent particles
are caused to be mechanically broken apart by striking a target surface or
other particles.
In addition, reference is had further in this regard, by way of
exemplification and not limitation, to U.S. Patent No. 5,344,632 entitled
"Method, For Reducing Sulfur Oxides Emissions In A Combustion
Process," which issued on September 6, 1994. As taught in U.S. Patent
No. 5,344,632, the mixture of flue gases and entrained fine particles, a
portion of which are particles of limestone, which are both unsulfated and
to have undergone chemical conversion to calcined limestone, are made to
enter a humidifying reactor at a reduced temperature, wherein the reduced
temperature results from heat having been previously extracted from the
mixture. Thereafter, water is dispersed in the form of a piuratity of fine
water particles that evaporate and humidify the mixture of flue gases and
is entrained fine particles, which in combination with the reduced
temperature of the mixture, is said to be highly conducive to the formation
of a thin ftlm of alkali solution of calcium hydroxide on the surface of the
particles of Limestone. This alkali solution is then effective in absorbing
sulfur oxides present in the mixture of flue gases and entrained fine
zo particles to form calcium sulfate and calcium sulfide precipitation.
Lastly, reference is had in this regard, by way of
exemplification and not limitation, to U.S. Patent No. 5,341,753 entitled
"Circulating Fluidized Bed Power Plant With Improved Mixing Of Sorbents
Wth Combustion Gases," which issued on August 30, '1994. In
2s accordance with the teachings of U.S. Patent No. 5,341,753, recognition is
had therein of the fact that the calcium sulfur ratio required for a desired
amount of sulfur removal is a function of how much excess particle density
in the gas stream is required in order to ensure that a sufficient number of
sulfur dioxide molecules come in contact with the calcium dioxide
3o particles. Thus, it is said to be desirable to improve the contact between
the calcium and the sulfur dioxide particles. To this end, high velocity
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-5-
steam is injected info the circulating fluidized bed boiler to improve the
mixing therewithin of the recirculating solids with the combustion gases
whereby the circulating fluidized bed boiler for burning sulfur containing
fuels is allegedly made to utilize limestone more efficiently.
s From the teachings of the aforementioned U.S. patents, it is
thus apparent that the utilization of spent ash from a circulating fiuidized
bed system can be enhanced by hydration or even simple size reduction
followed by the reinjection of the spent ash into the combustor of the
circulating fluidized bed system in order to thereby achieve additional
Io sulfation of the reinjected spent ash. Moreover, it is apparent from the
teachings of the foregoing U.S. patents that it is possible to realize even
additional sulfation through the proper selection of the location whereat
the spent ash is reinjected into the circulating fluidized bed system. In
addition to those which form the subject matter of the aforementioned U.S.
is patents, there are also known to exist in the prior art other ash
activation/injection processes. Considered collectively, all of these ash
activation/injection processes, for ease of reference, may be categorized
as follows: direct spent ash reinjection with no treatment of the spent ash;
mechanical grinding of the spent ash in order to expose unreacted Ca0
zo prior to the spent ash being reinjected; humidification of the flue gases
without any spent ash recycle; injection of the sorbent into flue gases
without any spent ash recycle; reinjection in dry form of hydrated spent
ash; reinjection in moist form of hydrated spent ash; and reinjection in
slurry form of hydrated spent ash.
2s Although generally speaking these ash activation/injection
processes have been demonstrated to be operative for their intended
purpose, there has nevertheless been evidenced in the prior art a need for
such ash activation/injection processes to be further improved. Namely,
there has been evidenced in the prior art a need for a new and improved
~o method for enhancing the capture of the S02 that is released during the
combustion of fossil fuel within a fossil fuel-fired combustor, and, in
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-6-
particular, a new and improved method for reactivating for purposes of the
reuse thereof the sorbent, which is injected into the combustor of a
circulating fluidized bed system in order to effectuate the capture therewith
of the S02 that is released during the combustion of fossil fuel within the
s combustor of the circulating fluidized bed system. '
To this end, there has been evidenced in the prior art a need
for such a new and improved method for reactivating sorbent for purposes
of the reuse thereof that is characterized in a number of respects. One
such characteristic is that such a new and improved method for
to reactivating sorbent for purposes of the reuse thereof would enable a
substantial reduction to be realized in the amount of limestone that would
otherwise be required to be utilized to achieve the same amount of SOZ
capture, i.e., enables a lower Ca/S ratio to be utilized than that which has
heretofore been required to achieve the same amount of S02 capture.
is Another such characteristic is that such a new and improved method for
reactivating sorbent for purposes of the reuse thereof would enable a
substantial reduction to be realized in the amount of spent ash that
otherwise would be required to be disposed of. A third such characteristic
is that such a new and improved method for reactivating sorbent for
2o purposes of the reuse thereof would enable a substantial reduction to be
realized in the amount of Ca present in the spent ash that eventually must
be disposed of thereby enabling the spent ash to be more readily
disposed of. A fourth such characteristic is that such a new and improved
method for reactivating sorbent for purposes of the reuse thereof would be
2s capable of implementation without requiring any process modifications to
be made in the combustion process by which fossil fuel is burned in fossil
fuel-fired combustors and from whence is released the SOz, the capture of ,
which is effected with sorbent. A fifth such characteristic is that such a
new and improved method for reactivating sorbent for purposes of the
~o reuse thereof would be capable of implementation without requiring any
significant equipment modifications to be made in the fossil fuel-fired
CA 02230019 1998-02-19
WO 97/06889 PCT/IS96/00930
_7_
combustors in which the fossil fuel is burned and from whence is released
the SO2, the capture of which is effected with sorbent. A sixth such
characteristic is that such a new and improved method for reactivating
sorbent for purposes of the reuse thereof would be capable of utilization
~ s with virtually any form of fossil fuel-fired combustor in which fossil
fuel is
burned and from whence is released the S02, the capture of which is
effected with sorbent. A seventh such characteristic is that such a new
and improved method for reactivating sorbent for purposes of the reuse
thereof would be suitable for application in new fossil fuel-fired
io combustors. An eighth such characteristic is that such a new and
improved method for reactivating sorbent for purposes of the reuse thereof
would be suitable to be retrofitted for application in existing fossil fuel-
fired
combustors.
It is, therefore, an object of the present invention to provide a
is new and improved method for reactivating for purposes of the reuse
thereof the sorbent containing unreacted CaO, which is injected into a
fossil fuel-fired combustor in order to effectuate the capture therewith of
the SOZ that is released during the combustion of the fossil fuel within the
fossil fuel-fired combustor.
o It is another object of the present invention to provide a new
and improved method that is particularly suited to being utilized for
reactivating for purposes of the reuse thereof the sorbent containing
unreacted CaO, which is injected into the combustor of a circulating
fluidized bed system in order to effectuate the capture therewith of the
2s S02 that is released during the combustion of fossil fuel within the
combustor of the circulating fluidized bed system.
It is still another object of the present invention to provide a
new and improved method for reactivating for purposes of the reuse
thereof sorbent containing unreacted CaO, which is characterized in that
~o through the use thereof a substantial reduction is enabled to be realized
in
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
_$_
the amount of sorbent that would otherwise be required to be utilized to
achieve the same amount of SOZ capture.
Another object of the present invention is to provide such a
new and improved method for reactivating for purposes of the reuse
s thereof sorbent containing unreacted CaO, which is characterized in that
through the use thereof a substantial reduction is enabled to be realized in
the amount of spent ash that otherwise would be required to be disposed
of.
A still another object of the present invention is to provide
io such a new and improved method for reactivating for purposes of the
reuse thereof sorbent containing unreacted CaO, which is characterized in
that through the use thereof a substantial reduction is enabled to be
realized in the amount of Ca present in the spent ash that eventually must
be disposed of thereby enabling the spent ash to be more readily
is disposed of.
A further object of the present invention is to provide such a
new and improved method for reactivating for purposes of the reuse
thereof sorbent containing unreacted CaO, which is characterized in that
the implementation thereof does not require any process modifications to
2o be made in the combustion process by which fossil fuel is burned in fossil
fuel-fired combustors and from whence is released the S02, the capture of
which is effected with sorbent.
A still further object of the present invention is to provide
such a new and improved method for reactivating for purposes of the
2s reuse thereof sorbent containing unreacted CaO, which is characterized in
that the implementation thereof does not require any significant equipment
modifications to be made in the fossil fuel-fired combustor in which the
fossil fuel is burned and from whence is released the S02, the capture of
which is effected with sorbent.
o Yet an object of the present invention is to provide such a
new and improved method for reactivating for purposes of the reuse
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
_g_
thereof sorbent containing unreacted CaO, which is characterized in that
the utilization thereof may be with virtually any form of fossil fuel-fired
combustor in which fossil fuel is burned and from whence is released the
S02, the capture of which is effected with sorbent.
s Yet a further object of the present invention is to provide
such a new and improved method for reactivating for purposes of the
reuse thereof sorbent containing unreacted CaO, which is characterized
by its suitability for application in new fossil fuel-fired combustors.
Yet another object of the present invention is to provide such
io a new and improved method for reactivating for purposes of the reuse
thereof sorbent containing unreacted CaO, which is characterized by its
suitability to be retrofitted for application in existing fossil fuel-fired
combustors.
is SUMMARY OF THE PRESENT INVENTION
In accordance with the present invention there is provided a
method for reactivating for purposes of the reuse thereof the sorbent
containing unreacted CaO, which is injected into a fossil fuel-fired
combustor in order to effectuate the capture therewith of the SOZ that is
ao released during the combustion of the fossil fuel within the fossil fuel-
fired
combustor. More specifically, in accord with the subject method of the
present invention for reactivating for purposes of the reuse thereof sorbent
containing unreacted CaO, such reactivation of the sorbent containing
unreacted Ca0 is effected through steam reactivation. To this end, spent
2s ash, which is derived from the combustion of fossil fuel in a fossil fuel-
fired
combustor and which has present therein particles of sorbent containing
unreacted CaO, is subjected to steam, which is at a predetermined
temperature and a predetermined partial pressure. As a consequence of
this subjection of the spent ash to steam, the unreacted Ca0 contained in
~o the sorbent particles present in the spent ash is converted to Ca(OH)2 by
virtue of the reaction of the Ca0 contained in the sorbent particles with the
CA 02230019 2000-08-02
62898-1469
steam. Moreover, because the volume of Ca(OH)2 is greater than
the volume of CaO, this increase in volume results in a
fracturing of the sorbent particles, which had previously
contained unreacted Ca0 but which now contain Ca(OH)2, such
5 that by virtue of the fracturing of the sorbent particles the
Ca(OH)2 thereof becomes exposed. After having been so exposed
to steam, in accord with the subject method of the instant
invention the spent ash now having present therein sorbent
particles containing Ca(OH)2 rather than unreacted Ca0 is then
10 reinjected into the fossil fuel-fired combustor whence the
spent ash was derived. To this end, the spent ash now having
present therein sorbent particles containing Ca(OH)2 is
reinjected into the fossil fuel-fired combustor at a location
thereof whereat the temperature is sufficiently high, i.e., in
excess of 580 degrees C., so as to cause the Ca(OH)2 contained
in the sorbent particles present in the reinjected spent ash to
convert once again to Ca0 such that by virtue of this reactiv-
ation thereof the sorbent present in the reinjected spent ash
is rendered once again operative to effectuate the capture
therewith of S02 that is released during the combustion of
fossil fuel within the fossil fuel-fired combustor.
In summary, the invention provides a method for
reactivating ash particulates containing unreacted Ca0 for
purposes of the reuse thereof in a fossil fuel-fired combustor
wherein sorbent containing Ca is injected for purposes of
effecting the capture therewith of S02 released during the
combustion of fossil fuel in the fossil fuel-fired combustor
including the step of providing a supply of ash particulates
containing unreacted Ca0 generated as a consequence of the
combustion in the fossil fuel-fired combustor of the fossil
fuel in the presence of sorbent containing CaO, the improvement
CA 02230019 2000-08-02
62898-1469
11
camprising the steps of: (a) subjecting the ash particulates
containing unreacted Ca0 to steam having a predefined
temperature and a predefined partial pressure wherein the
predefined temperature and the predefined partial pressure
jointly define a point in the region lying above the curve
representative of the minimum partial pressure of water vapor
operative to effect the rehydration of Ca0 as a function of
temperature depicted in Figure 2 in order to convert the ash
particulates containing unreacted Ca0 to ash particulates
containing Ca(OH)2 for the purposes of causing the ash
particulates containing Ca(OH)2 to fracture as a result of the
expansion thereof caused by the specific volume of Ca(OH)2
being greater than the specific volume of CaO; (b) injecting
into the fossil fuel-fired combustor the ash particulates
contain Ca(OH)2 produced from the conversion of the ash
particulates containing unreacted CaO; (c) converting the
Ca(OH)2 of the ash particulates containing Ca(OH)2 to Ca0 by
subjecting the ash particulates containing Ca(OH)2 while within
the fossil fuel-fired combustor to a temperature in excess of
580 degrees C.; and (d) effecting within the fossil fuel-fired
combustor the capture of the S02 released during the combustion
of fossil fuel in the fossil fuel-fired combustor by the
exposure of such S02 to the Ca0 produced from the conversion
within the fossil fuel-fired combustor of the Ca(OH)2 of the
ash particulates containing Ca(OH)2.
The invention also provides a method for reactivating
ash particulates containing unreacted Ca0 for purposes of the
reuse thereof in a circulating fluidized bed system including a
combustor, a cyclone separator and a heat exchanger wherein
sorbent containing Ca is injected into the combustor of the
CA 02230019 2000-08-02
62898-1469
11a
circulating fluidized bed system for purposes of effecting the
capture therewith of S02 released during the combustion of
fossil fuel in the combustor of the circulating fluidized bed
system including the step of providing a supply of ash
particulates containing unreacted Ca0 generated as a
consequence of the combustion in the combustor of the
circulating fluidized bed system of the fossil fuel in the
presence of sorbent containing CaO, the improvement comprising
the steps of: (a) subjecting the ash particulates containing
unreacted Ca0 to steam having a predefined temperature and a
predefined partial pressure wherein the predefined temperature
and the predefined partial pressure jointly define a point in
the region lying above the curve representative of the minimum
partial pressure of water vapor operative to effect the
rehydration of Ca0 as a function of temperature depicted in
Figure 2 in order to convert the ash particulates containing
unreacted Ca0 to ash particulates containing Ca(OH)2 for the
purpose of causing the ash particulates containing Ca(OH)2 to
fracture as a result of the expansion thereof caused by the
specific volume of Ca(OH)2 being greater than the specific
volume of CaO; (b) injecting into the combustor of the
circulating fluidized bed system the ash particulates
containing Ca(OH)2 produced from the conversion of the ash
particulates containing unreacted CaO; (c) converting the
Ca(OH)2 of the ash particulates containing Ca(OH)2 to Ca0 by
subjecting the ash particulates containing Ca(OH)2 while within
the combustor of the circulating fluidized bed system to a
temperature in excess of 580 degrees C.; and (d) effecting
within the combustor of the circulating fluidized bed system
the capture of the S02 released during the combustion of fossil
fuel in the combustor of the circulating fluidized bed system
CA 02230019 2000-08-02
62898-1469
llb
by the exposure of such S02 to the Ca0 produced from the
conversion within the combustor of the circulating fluidized
bed system of the Ca(OH)2 of the ash particulates containing
Ca(OH)2.
BRIEF DESCRIPTION OF THE DRAWING
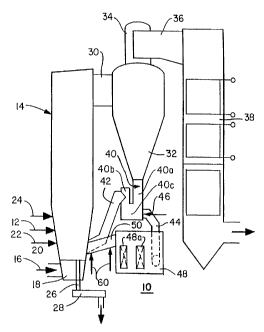
Figure 1 is a side elevational view of one form of
fossil fuel-fired combustor, i.e., a circulating fluidized bed
system, with which the method of the present invention may be
employed;
Figure 2 is a graphical depiction of the
disassociation pressure of Ca(OH)2 as a function of temperature
based on thermodynamic considerations and
Figure 3 is a graphical depiction of the conversion
of hydrated fly ash with 502.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Referring now to the drawing, and more particularly
to Figure 1 thereof, there is depicted therein a form of fossil
fuel-fired system generator with which the method of the
present invention is capable of being utilized. More
specifically, in accord with the best mode embodiment of the
invention the fossil fuel-fired combustor illustrated in Figure
1 of the drawing comprises a circulating fluidized bed system,
generally denoted in Figure 1 by the reference numeral 10.
Fossil fuel, which is most frequently in the form of coal,
along with sorbent, which is most frequently in the form of
limestone, along with sorbent, which is most frequently in the
form of limestone, are fed, as indicated by the arrow denoted
by the reference numeral 12 in Figure 1, to the combustor,
CA 02230019 2000-08-02
62898-1469
11c
denoted generally in Figure 1 by the reference numeral 14, of
the circulating fluidized bed system 10. As indicated by the
arrow denoted by the reference numeral 16 in Figure 1, primary
fluidizing air, which has been preheated, is fed in known
fashion to the air plenum chamber, denoted in Figure 1 by the
reference numeral 18, which is located in the bottom of the
combustor 14 such as to be positioned below the air
distribution plate, denoted in Figure 1 by the reference
numeral 20.
Continuing with the description of the circulating
fluidized bed system 10 depicted in Figure 1 of the drawing,
combustion supporting air, as indicated by the arrows denoted
by the reference numerals 22 and 24, respectively, is fed into
the combustor 14. Ash, which is generated as a consequence of
the combustion in the combustor 14 of the fossil fuel
introduced at 16 thereinto is removed from the combustor 14
through the pipe, denoted in Figure 1 by the reference numeral
26, and through the ash cooler, denoted in Figure 1 by the
reference numeral 28.
The bottom portion, which comprises the primary
combustion zone, of the combustor 14 in accordance with
conventional practice is normally refractory lined in order to
thereby eliminate therefrom high heat losses. On the other
hand, in accordance with conventional practice the
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-12-
upper portion of the combustor 14 normally contains evaporative waterwail
tubes in which steam is generated.
The flue gas generated as a consequence of the combustion
within the combustor 14 of the fossil fuel introduced at 16 thereinto along
with any solids, which may be entrained in the flue gas, are made to flow
from the combustor 14 through the duct, denoted in Figure 1 by the
reference numeral 30, to the cyclone separator, denoted in Figure 1 by the
reference numeral 32. In the cyclone separator 32, the entrained solids
are separated from the flue gas. After being separated in the cyclone
Io separator 32 from the flue gas, the solids drop to the bottom of the
cyclone
separator 32 whereas the flue gas now minus the previously entrained
solids flows out of the top, the tatter being denoted in Figure 1 of the
drawing by the reference numeral 34, of the cyclone separator 32. From
the top 34 of the cyclone separator 32, the flue gas now minus the
is previously entrained solids then flows through the tangential duct, denoted
in Figure 1 by the reference numeral 36, to the convective pass, denoted
in Figure 1 by the reference numeral 38, of the circulating fluidized bed
system 10, wherein in accordance with conventional practice heat
exchange surfaces are typically to be found.
'-o Referring again to Figure 1 of the drawing, the circulating
fluidized bed system 10 as illustrated therein is further provided on the
bottom of the cyclone separator 32 with a J-leg or seal pot, denoted
generally by the reference numeral 40 in Figure 1. The function of the J-
leg or seal pot 40 is to effect the recirculation of the solids, which are
~s collected in the cyclone separator 32 after the separation thereof from the
flue gas, back to the combustor 14 against the pressure present in the
combustor 14. Namely, these solids flow down on the inlet side, i.e., on
the side, denoted in Figure 1 by the reference numeral 40a, of the J-leg or
seal pot 40, up the outlet side, i.e., the side, denoted in Figure 1 by the ,
~o reference numeral 40b, of the J-leg or seal pot 40, and then back to the
combustor 14 through the duct, denoted in Figure 1 by the reference
62898-1469
CA 02230019 2000-08-02
_~3_
numeral 42. In accordance with conventional practice, the bottom portion,
denoted in Figure 1 by the reference numeral 40c, of the J-leg or seal pat
40 is normally fluidized, by virtue of the injection thereinto of air, to
permit
the solids in the J-leg or seal pot 40 to flow therethrough. As is known to
those skilled in this art, the difference in solids level between the inlet
side
40a thereof and the outlet side 40b thereof corresponds to the pressure
differential across the J-leg or seal pot 40. To this end, solids entering the
inlet side 40a of the J-leg or seal pot 40 displace the solids flowing into
the
duct 42 out of the outlet side 40b of the J-leg or seal pot 40.
to Continuing, the circulating fluidized bed system 10, in
accordance with the illustration thereof in Figure 1 of the drawing, further
embodies a solids withdrawal pipe, denoted in Figure 7 by the reference
numeral 44, and a solids flow control valve, represented by the an-ow
identified in Figure 1 by the reference numeral 46. As seen with reference
is to Figure 1 of the drawing, the solids withdrawal pipe 44 and the solids
flow control valve 46 are suitably supported in the lower portion 40c of the
J-leg or seal pot 40. The function of the solids withdrawal pipe 44 is to
feed the desired portion, as established through operation of the solids
flow control valve 46, of the hot recircuiating solids from the J-leg or seal
'o pot 40 to the extemai fluidized bed heat exchanger, denoted in Figure 1
by the reference numeral 48. In known fashion, the extemai fluidized bed
heat exchanger 48 typically consists of one or more compartments,
schematically depicted at 48a in Figure 1, with most compartments 48a
containing immersed tube bundles (not shown in the interest of
~s maintaining clarity of illustration in the drawing), which are designed to
be
operative as evaporative and/or reheat andlor superheat and/or
economizer heat transfer surface. However, some of the compartments
48a may not be provided with immersed tube bundles. The solids, which
enter the external fluidized bed heat exchanger 48, are fluidized for
~o purposes of effecting the flow thereof therethrough. During the course of
their passage through the external fluidized bed heat exchanger 48 the
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-14-
solids, as they gradually pass from one to another of the compartments
48a, transfer heat to the heat transfer surfaces housed therewithin.
Thereafter, the solids flow out of the external fluidized bed heat exchanger .
48 through the outlet pipe, denoted in Figure 1 by the reference numeral
s 50, and back to the combustor 14.
The solids, which are circulating around the circulating
fluidized bed system 10, i.e., through the combustor 14, the cyclone
separator 32 and the external fluidized bed heat exchanger 48, consist of
a mixture of unreactive fossil fuel ash and particles of sorbent, which are
io only partially reacted. To this end, the core of these particles of sorbent
comprises unreacted CaO, whereas the shell or outer layer of these
particles of sorbent consist of CaS04.
A discussion will next be had herein of SOZ capture by
sorbent in the combustor 14 of the circulating fluidized bed system 10. In
is this regard, overall S02 capture by sorbent consisting of limestone, which
is added to a circulating fluidized bed system, such as the circulating
fluidized bed system 10 illustrated in Figure 1 of the drawing, is
traditionally described by two consecutive reactions; namely, those of
endothermic caicination and exothermic sulfation. Exothermic sulfation is
~o a net reaction and is not to be interpreted as describing reaction
mechanisms on a molecular level. The calcination reaction, which is
significant above 700 degrees C., creates very porous Ca0 particles. As
the sulfation reaction proceeds, the pore structure of Ca0 particles
changes, since the molar volume of CaS04 is larger than that of Ca0 and
~s this tends to block the pores near the outer surface of the particle,
preventing further diffusion of S02 into the interior of the sorbent
particles.
Although there are cases of quite uniform sulfation across the particles
found in small particles of fly ash from circulating fluidized bed systems,
most of this type of heterogeneous reaction is commonly modeled with an
~o unreacted-core model, which makes allowance for the following: diffusion
of gaseous S02 to the surface of the solid particle, penetration and
CA 02230019 2000-08-02
62898-1469
-15-
diffusion of SOZ through the CaS04 layer to the surface of the unreacted
core, and reaction of SOZ with sorbent within the core.
Low calcium utilization, typically only 25 to 45%, leads to a
relatively large quantity of waste products for disposal. Thus, if the overall
utilization of fresh sorbent can be increased, a significant improvement of
the desulfurization process, in terms of bath economics and pollution
control, could be accomplished. As noted herein previously, among the
methods that have been found to have the potential to increase the
utilization of sorbent signincantly is hydration of spent sorbent/ash by
to steam reactivation at low temperature combined with recirculation of the
reactivated sorbent/ash back to the combustor. Enhanced utilization of
sorbent by such hydration is attributable to the difference in molar volume
of Ca0 and Ca(OH)2. To this end, when spent sorbent/ash is exposed to
steam, the strong affinity between water and unreacted Ca0 leads to
is absorption of water in the pores of the particles so that Ca0 is converted
to Ca(OH)2.
In Figure 2 of the drawing the partial pressure of water in
equilibrium with a mixture of Ca0 and Ca(OH)Z is depicted by means of
the curve denoted therein by the reference numeral 52. Thus, the curve
'0 52 effectively represents the minimum partial pressure of water vapor in
an environment to effect rehydration of Ca0 as a function of temperature.
The curve 52 was computed using free energies of formation and heat
capacities of the pure solids and gases. From a reference to Figure 2 of
the drawing, it can be seen that hydration is possible at high temperatures,
~5 i.e., at temperatures within the range of 300 degrees C. to 500 degrees C.
and high HZO partial pressures up to 1.0 and beyond. Moreover, it can be
seen from a reference to Figure 2 of the drawing that Ca(OH)2 is stable
below 450 degrees C. with a volume percent moisture of 60%. As such,
given the smaller size of water vapor molecules compared with S02,
~o penetration of H20 to previously unutilized Ca0 sites is therefore
feasible.
Furthermore, under appropriate conditions in-situ rehydration of Ca0 to
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-16-
Ca(OH)2 can be had with a corresponding volume change and
concomitantly exposure of unreacted Ca0 surface area, which would then
allow further S02 capture when the sorbent particles that have been
subjected to such in-situ rehydration of Ca0 are reinjected into a
s circulating fluidized bed system, e.g., into the combustor 14 of the '
circulating fluidized bed system 10 illustrated in Figure 1 of the drawing.
Heretofore, the effect of temperature on hydration of partially
sulfated limestone has been the subject of several studies. Such studies
have found that the conversion of Ca0 to Ca(OH)2 decreased when the
~o temperature was increased from 100 degrees C. to 300 degrees C. at a
constant moisture content. Further, it was observed in such studies that
the rate of hydration increased as the temperature was decreased.
Moreover, it was suggested that this was due to the mechanism by which
steam reaches active Ca0 sites and proposed that the diffusional
is resistance toward steam is partially caused by the increase in molar
volume resulting from the formation of Ca(OH)Z. To this end, it could be
that the decrease in both reaction rate and ultimate conversion is caused
by pore plugging, similar to that occurring during sulfation, with an
increasing temperature causing the pores of the sorbent particles to plug
'o faster.
With further reference to the aforementioned studies, it has
been found therefrom that the rate of hydration is much slower with steam
than with water. Such studies assumed that this was caused by the
transport mechanism of water through the sorbent particles rather than
~s because of pore plugging. To this end, it was suggested in such studies
that water molecules are quickly absorbed because of the surface tension
of water, which draws water into the pores of the sorbent particles, where
the water reacts with the CaO.
All the work done in the course of such studies has been
~o carried out at temperatures below 300 degrees C. In these studies, there
does not appear to have been any systematic consideration given to
CA 02230019 1998-02-19
WO 97/06889 PCT/dB96/00930
- 17-
reactivation at temperatures above 300 degrees C., perhaps partially
because decomposition of Ca(OH)2 becomes significant at 300 degrees
C., and thermodynamic as well as kinetic factors become important. In
particular, a minimum partial pressure of steam, above the decomposition
o s pressure of Ca(OH)2, is needed to effect reactivation.
Reference will next be had herein to Figure 3 of the drawing
wherein a series of curves, denoted therein by the reference numerals 54,
56 and 58, respectively, are depicted. The trends, which are shown by the
curves 54, 56 and 58, can be explained by a combination of
io thermodynamic and kinetic factors. Thermodynamic factors enter into this
discussion in two ways. First, there is the determination of whether at a
given steam partial pressure reactivation can occur at a specified
temperature in accord with curve 52 of Figure 2 of the drawing. Secondly,
thermodynamic factors have an influence on the reaction rate.
is It appears that the effect of temperature and steam partial
pressure as shown by the curves 54, 5.6 and 58 in Figure 3 of the drawing
is explainable where the rate of reactivation is limited by diffusion of steam
through pores in a surface layer of spent ash. To this end, the rate of
diffusion is proportional to two temperature dependent factors, i.e., the
2o diffusion coefficient divided by the temperature, and the driving force.
Furthermore, the driving force is equal to the difference in steam partial
pressure in the bulk gas and in the center of the sorbent particle (the
decomposition pressure). As regards the diffusion coefficient, the
diffusion coefficient increases with temperature to a power varying
zs between approximately 1.5 and 0.5 depending upon whether the diffusion
is bulk diffusion or Knudsen diffusion. However, for a given partial
pressure of steam, the driving force for diffusion decreases with
temperature because of the increase in the steam partial pressure in the
center of the sorbent particle. The result of these two counteracting trends
~o is a maximum in the reactivation rate, provided that the diffusion
coefficient varies with temperature to a power greater than unity. The
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-18-
overall trend, as illustrated by the curves 54, 56 and 58 in Figure 3 of the
drawing, shows that the reaction rate increases with temperature to a
maximum, because the diffusion coefficient increases. Moreover, at a
temperature higher than this maximum point the reaction rate falls off as
s the driving force fails, reaching zero when the bulk diffusion is equal to
the
decomposition pressure.
The spent ash, which is to be subjected to steam
reactivation in accordance with the method of the present invention may
be obtained from several locations in the circulating fiuidized bed system
io 10. Namely, the spent ash, which is to be subjected to steam reactivation
in accordance with the present invention may be obtained from the ash
cooler 28, or from the J-leg or seal pot 40, or from the external fluidized
bed heat exchanger 48. In addition, it is also contemplated in accordance
with the method of the present invention that steam reactivation of the
is spent ash may take place in-situ. That is, steam may be employed to
perform a dual function, i.e., to effect the fluidization of the solids being
recirculated from the cyclone separator 32 to the combustor 14 while at
the same time this same steam is operative to effect the steam
reactivation in-situ of the recirculated solids. To this end, as depicted by
?o the arrows denoted by the reference numeral 60 in Figure 1 of the
drawing, the steam employed for this purpose may be introduced into the
outlet pipe 50 of the circulating fluidized bed system 10. Other than when
the steam reactivation of the spent ash takes place in-situ, the spent ash
after being subjected to steam reactivation in accordance with the present
~s invention preferably is reintroduced into the combustor 14 at 12 along with
the fossil fuel that is to be burned therewithin.
Thus, in accordance with the present invention there has
been provided a new and improved method for reactivating for purposes
of the reuse thereof the sorbent containing unreacted CaO, which is
~o injected into a fossil fuel-fired combustor in order to effectuate the
capture
therewith of the S02 that is released during the combustion of the fossil
62898-1469
CA 02230019 2000-08-02
-~s-
fuel within the fossil fuel-fired cornbustor. Besides, there has been provided
in
accord with the present invention a new and improved method that is
particularly suited to being utilized far reactivating for purposes of the
reuse thereof the sorbent containing unreacted CaO, which is injected into
s the combustor of a circulating fiuidized bed system in order to effectuate
the capture therewith of the S02 that is released during the combustion of
fossil fuel within the combustor of the circulating fluidized bed system. As
well, in accordance with the present invention there has been provided a
new and improved method for reactivating for purposes of the reuse
to thereof sorbent containing unreacted CaO, which is characterized in that
through the use thereof a substantial reduction is enabled to be realized in
the amount of sorbent that would otherwise be required to be utilized to
achieve the same amount of SOZ capture. Moreover, there has been
provided in accord with the present invention a new and improved method
is for reactivating for purposes of the reuse thereof sorbent containing
unreacted CaO, which is characterized in that through the use thereof a
substantial reduction is enabled to be realized in the amount of spent ash
that otherwise would be required to be disposed of. Also, in accordance
with the present invention there has been provided a new and improved
?o method for reactivating for .purposes of the reuse thereof sorbent
containing unreacted CaO, which is characterized in that through the use
thereof a substantial reduction is enabled to be realized in the amount of
Ca present in the spent ash that eventually must be disposed of thereby
enabling the spent ash to be more readily disposed of. Further, there has
been provided in accord with the present invention a new and improved
method for reactivating far purposes of the reuse thereof sorbent
containing unreacted CaO, which is characterized in that the
implementation thereof does not require any process modifications to be
made in the combustion process by which fossil fuel is burned in fossil
3o fuel-fired combustors and from whence is released the SOz, the capture of
which is effected with sorbent. In addition, in accordance with the present
CA 02230019 1998-02-19
WO 97/06889 PCT/IB96/00930
-20-
invention there has been provided a new and improved method for
reactivating for purposes of the reuse thereof sorbent containing
unreacted CaO, which is characterized in that the implementation thereof
does not require any significant equipment modifications to be made in the
s fossil fuel-fired combustor in which the fossil fuel is burned and from -
whence is released the SO2, the capture of which is effected with sorbent.
Furthermore, there has been provided in accord with the present invention
a new and improved method for reactivating for purposes of the reuse
thereof sorbent containing unreacted CaO, which is characterized in that
io the utilization thereof may be with virtually any form of fossil fuel-fired
combustor in which fossil fuel is burned and from whence is released the
S02, the capture of which is effected with sorbent. Penultimately, in
accordance with the present invention there has been provided a new and
improved method for reactivating for purposes of the reuse thereof sorbent
is containing unreacted CaO, which is characterized by its suitability for
application in new fossil fuel-fired combustors. Finally, there has been
provided in accord with the present invention a new and improved method
for reactivating for purposes of the reuse thereof sorbent containing
unreacted CaO, which is characterized by its suitability to be retrofitted for
2o application in existing fossil fuel-fired combustors.