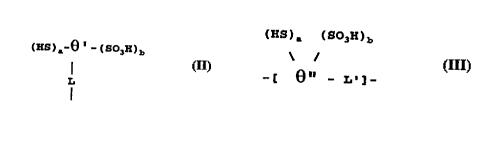Note: Descriptions are shown in the official language in which they were submitted.
CA 02230272 l998-02-24
WO 97/08122 PCT~US96/13683
ISOMERIZATION OF BlSrl IC~
This inv ntion r~latos to pr-paration o-f P~lYr~Aol Q~ more
parl ;C~ A - ly to the preparation of poly~h~~l R frvm kQtonQs or ald-hya~s
nd ~ 1A
A id catAlyzed c-~A_~tiAA~ of r~ ~ A With rla9hyd~s or kQton9 8 wa8
w~ll known Acid cataly8ts i~ acidic ion ~ J~ r~sin cataly~t~
rnd sol~hlo acia cataly~ts Sol~h~ acia catalysts can be, for - _le,
Lv_ _ chloriao, sulfuric acid, hydrochloric acid, rh.-~h~-~ c acid,
Ly~ v~ c rcid, nitric AAcid, dim9thyl ~ulfAte, sulfur ~;~Y;~ ,
4-toltt~ lf-~;c acid, boron trifluoriae, ~ lf.-~;C acia8, boron
trifluoriae A _l~ ~ and oth~r acid-acting r __ -n~ inClUaing C _ _ 'n
wh~ch w_r~ hyarolyzod by wat-r to Form rAcids; for - _le,
chlorid~, sulfonyl chloridQ and ~ho~ -
A numb~r OAf __ ~R werO kn~wn to prvmote such _n acid-catalyzQd
c '- --tion Th~s~ promotQrs ;~ ~tAAn groups that worc ~ither
rro~ or bound to a r~in Alkyl - ~-A~tan~ and bis ~,A~Lo~t~A~l~ ~9
wor~ of reportQd yromoters
It ha8 been ~l._oAr~ by Scri_bine et al , (U S Patent No
2,923,744) to produco ~;Qrh~~l A using sulfAuric acia, ~romotoa by
~_~LOA1~ fo~c ~cias or _alts or cv 9~ ;~ rulf~~-t - Qstors at
a l vel of O 1 to 5 p~ . by weight of th~ ba8e char~e, to catalyze
c~o~-~;~ of A~e-c ~ and ph~ln~ when uArQd in r of 0 1 to 5
_e ~ by woight bas-d on total char~ Sulfuric acid was usod in
~ r of 2 moles p~r ~ le of acotono
~A~ et al , (U S Pat~nt No 4,675,458) have ~ _-r-' making
9,9-bis-(4-Lyv ~y~ yl)fluor-nQ in the ~ ~- -- of sulfuric acid,
pr~fA9rAbly __ _ 8ulfuric acid, and rA ~_~L_A~ p_rticA" 1 A ly 3-
~_~Lo~ropi~;c acid, a~ a promot-r
~ io ~t al , (U S Pat-nt No 5,248,838) havo ~ lc~-~ th~ u8e
of ~A _ ~At; ~ of ~fonic acid _nd a ~A~Lan/ ~_~to-lkA~~ic
ncid for catalyzing thc c ~ ~ of rh~~~l~ with fl~oA~nv ~ ~i~h
level~ of ~ h-~-F lf~~;c acid with L~ to the foed and the
tan/ ~ ~_l ~A~; C acid, w~rQ us~d The reactions can b run in
h - 1,~ t-' Ly~ ~olvsnts
Bott-nbruch ~t al , (U S Patcnt No 4,996,373) havo ~l-_OcAF~ a
~lOC~ for proA~ g aih~ v~y~Aryl c __ ~~ from carbonyl ~ __ 'A Una
CA 02230272 1998-02-24
WO 97/08122 PCTtUS96tl3683
rhe~ol~ under hi~h pr~ssure, in the ~ of various catalysts,
including ~ulfonic Acid ro~ins CAtaly~ts c~r~t~ s sulfhydryl
func~ l;ty, for ~xampl-, ion ~Y~-h~ns~rs tl ~te~ with - o~8O
c ~ , have boen A;~l08-a for this uso
~I-yor ~t al , (U S Patent No. 4,387,251) _AVO ~ ~ v ~a~ rl~ ~
for makinSr 4,4'-a~L~a ~ ;rh~yl AlkAr~ u8ing a ~ -t;c sulfonic acids as
c~ a ~2eir~Sr aSJents ~ ~to groups wor~ ;~ lv~Aea7 within the d--finition of
R3 and w-re charactorizod as b--ins~ in~rt Fro~ta~ ot al , (U 8 Pat-nt No
5,210,328) ~a~ ID~ usin~ t_o name tys~s of 8ulfonic ncid catnlyst_ for
lO m~kin~ Cy~l~A~ aen~ h; ~?h~
;rans~n (U S Pat-nt rJo 2,468,982) has ~ ljc8~a~ vr~ParAtion of
h; ~h~r ~ uging ~hydrous hy l _ chloride in ~ A1~ with n
--~s,Lc~ Ar~ic acid, wh~Lc_ may be formed in situ by reaction of a
~~:.~ton with tho k~ton-, as C~ A~ ;n!J ag-nt
I!Cnobel ~t al, (U S Pat--nt No 4,931,594) AiP-~ thQ us-- of laryo
~mounts of sulfonic acid resin, mix ~d with ~ d 3 ~ .... ~.topro~i~r;c
acid, to cau~e the ~ ,a o_t,; ~ to occur
It has b---n ~lC_ O~ a in Briti~h Patent No 1,185,223 to uno a
mixtur-- of ~i~ l--resins, ono n~ a e~ l f~;c acid r--nin and tho othor n
r-~in c~nt-;n;n~ - ~AA~to ~rou~s, for making bisrh~
p~nAo~h et al , (U S Patent No 5,212,206) a;Pc~08~ a cataly8t,
mad- by ~o~t;n~ a sulfonatod i~n ~ o r-sin with a
d~alky~ ~tan other rofe .~c A, ~ ~trt;ve of ro~ P on
of sulfonic acid iv~ O r-sin~, ;nAl~e Wagn~r (U S
; 25 Pat-nt No 3,172,916) Mc~Att ~t al , (U S Pat-nt No 3,394,089), Fal-r
~t al , (~ S Pat nt Nos 4,455,409~ 4,294,995 and 4,396,728)~ l lch
~t al , (~ S Patont No 4,369,293)~ Bor~ ~t al , (~ S Patont No
5,302,774) ana Maki ~t al , (~ S Pat-nt No 4,423,252) Tho r~activ
cataly~ts ~enorally ;nA-~ Ld- _ ~ ~A~to-f~n~t;~nn attached to a ~ulfonic acid
~rou~ in tbe for~L of a ~ul f~ - ~ or a, oniu~L sulfonate salt
8haw (U S Pat-nt No 4,859,803) disclo80s pr-~arin~ b;~ ln from
~h nol and a kotono in tho ~ ~ of an acidic (~ulfonic acid) ion-
re d n and a ~_~Lan Tho --~AA~tan being adaed at ~art;c~ A -
~oA~rti~n~ of a s~e~Aif;~va r-actor confi~Auration to ~l_v ~ the f~ An of
cyclic di~Lers
~ i _a ~;AClAV8~d (U S Pat~nt No 4,825,010) ;q ~ 7-tion of
L -~vd~cL~ of ~ A -~-to~ of ~ -~ and k-tones, using a catalytic
' .
CA 02230272 l998-02-24
WO 97/08122 PCTAUS96/13683
amount Of acidic ~ulf~ te~ c-t;~ic-~Y~-h--~ge rosin ha~in~ sulfonic acid
~ites ;~n;C'11Y bonded to alkyl ~ Othor ~-t~ by Li (U S
Patont Nos 4,822,923 ana 5,001,281) further sug~ost the state Or tho art
of usin~ ion --~ 7~ ~ rosins to ;- - ~ by~~ ~l~cts of bisph nol synth~-
~08.
Powell et al , (U S Patent No 5,105,026) ~;~close using aciaic
iv~ ~ r~sin~ to ;- z- ~ '--;-able proaucts Or bisphenol
~y~h~in to ~ ~lo products, for _le, to R;~h-~-l A Mor~an (~ S
Patent No 3,546,165) has ~;~clc~d c~ at;~ o~ ph-nol with variou~
10 kotones, ;~ ;~g fluo OnO~ ana ;-'-1~1'----~, using hi~h l~v018 of
Lyl ochloric acid or L~ ~ chloriao, in th~ of minor ~ ~-
o~ 3 - ~_~topropionic acid The products were used for the preparation
of poly-st-r resins
S~-~olcs (U 8 Pat-nt No~ 4,467,122 ana 4,503,266) ~ lor-D
~ ~;~g crud-~ proauct, c-~t~ ~g s~pF~ from a hyarochloric acia/~inc
chlorido catalyzed ~ ~ , to remove HCl, ZnCl2 and cxcess phenol, prior
to y~ l;~ from ~;~hlorooth_ne Bee also the abstract for DE
OLS 2,948,222 (~uly 30, 1981)
~orshak et al , (SU 172,775) ~ h;~ a mixture of phonol,
ao B~PF and HCl with water, a~ter which ph~nol was l ~ ' by aist;lla~
The foll ~g refoL_~ces ~;--lc~ the v e~A -ti~~ o~ resins,
c~t-;~;~g ~lf~;c acid funct;~Al;ty, introduced oither by
copolymer~~-t~ or by sulfonation after polym-r;~-t~o~
U S Patent No 3,205,285 Turbak et al
25~ S Pat-nt No 3,366,711 MA~_O1;n; et al
U S Patont No 3,426,104 Ma3son
S Pat-nt No 4,587,304 Thaler et al
~ S Pat-nt No 4,764,557 ~;f~' -~ et al
Traya~o (U S Patent No 3,706,707) ~;~cl~- the preparation of
-~ tr _rom a polymHrizod cyclic ether and a sultone D-_n (U S Pat-nt
No 4,568,724) was o~ 1A_ interest with .~o~_L to reaction ~.vd~cLs
_rom nn EPD~ rubbor and _ nulton-
Welch (U S Pat-nt No 3,029,221) and Niwa ~t al , (~ S Pat nt No
4,912,170) ~;o-laoa ~..o no~- for modifyin~ poly~Lyl~ne rosins
It was an object o~ this invontion to provid- a ~. oer~ ~or the
~ i~ of ala-hyd 8 or k~ton-n with ~h~.- l ~, to A chi-v~ high yielas
~.
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/13683
of preforroa bis-(4-Lyd v~y~ryl) i- ~ ~ with low roaction time~ whilo
avolain~ uso of strong inor~anic acids
Furthor object~ of the invention ;~l~lAo th- dev~lopment o~
proc~ n ~or tho 8y~t~-ri~ o-f PolYrh~o' ~ charactoriz-d by hi~h yiolds
5 of high ~urity ~roducts undor roaction condition8, which w ro not
corrosivQ to vossels in which the g~ ~A ~Be~ wore conductod In addition,
~voiding the us- of sulfuric acid, ~; ~At-- tho po~ ;ty of ~ido
r-actions, ;~l~A;~ sulf~t;~ of rh~ n~
In ono a~p-ct, this invontion rolates to a ~ oc088 for thn
c~A~ t;~ of an aldohydn or kotono startinq mat-rial with a ph-nol,
un~ubstituted in at l-ast ono po~ition, cvmpri8ins r-actin~ th- ald-hyde
or kotono starting mat-rial with the phenol in a re-~t;o~ mixture in tho
pr-~OnCe of a solublo or insolublo -~tosulfonic acid _ __ ' under
condition~ suffici-nt to br~n~ about f ~;~ of a _ ~Al b;~ ic
15 moioty at oach aldohydo or k-tono moiety in tho starting mat-rial;
provided that the sol~ e ~to~ l f~; C acid _ __ ' was charactorized
by th- formula
(HS),-~-(S03H)b
whor-in ~ wa~ an alkylono, cyclc~l;ph~t;c, arylono, alkyl-noarylone,
alkyl~ - y~lo~ hatic, al~ylon-aryl, h torocyclic or alkyl~ ~'-torocyclic
r-sidue and a and b wore ;~ A~ ~ly seloctod from ;~tog ~ from 1 to
205 ana
Tho insoluble c~Los~lfo~;c acid comprisos a catalyt;c~lly-active
- - -m~ O~ ~rA -a by the f. 1~
(Hg)~-~ -(803H)b
I
L
in which ~ was an alkyl-no, cy~l~-l;~h~t~c, aryl-no, alkylonoaryleno,
~lkyl-nocy~l~l;~h~t~c, alkyl~ ~~ yl, het~ ~y~lic or alkyl~ erocyclic
rosiduos a and b w ro i ~ -'- ly seloctod from ;~e_ ~ from 1 to 205 L
wan an opti~l 1;~;~ group and - wa~ a bond, whieh eatalytieally-activo
~p-cio~ wa~ ~tt-~-' by tho bond - to an ;~ol hle organic or inorganie
~V~Ls or a eatalyticallY-activo 8pocio8 ~ a~ _e~ by the unit fc_ 1
(HS)A (8~3H)b
\
( b ) r
CA 02230272 1998-02-24
WO 97/08122 PCTAUS96/13683
whnrei~ ~ wa3 an alkyl-ne, arylene, eyrloAl;~hA~;c, alkylenearylene,
alkyl~ -_Y~-lo-l;rh~t~c, alkylon-aryl, heterocyclic or alkyl -~ torocyclic
resiaue; a and b w_ro ;n~ ly soloctod frvm ;nt~ D from 1 to 20;
L' waD an optional l; nk; ng -~roup and - wa8 a bond
ThiD invOntion furth r r-lateD to novel catalytically-activo
poly~Ly _~ rosin~, charactOriz-d by boarin~ at least one of oach of a
~Lo-funetion and a sulfonie aeia funetion on 80me indiviaual 8Ly ~ne
units of a polymer ehain
In yot An~th~r as~Oct, thiD ;nvOntion rolatOs to ~ - s~-r for
pr-~arin~ th- eatalytically-active poly~Ly ~nc resins Th-se ~~ rc-
~preferably eompri80e stOp8 of (b) 8ulf--nAt;n~- a hAloAl~yl~oly~Ly. - to
produce an ;nt-- -';Ate h vin~ ~ulfo 4unetional ~rours; (c) opt;~Ally
av~vO Li~-~ tho sulfo 4~n-ti~n-~ -~rou~ to av ~ ~ A;n~ alkali metal
~alts~ (d) th;ol~At;ng the thu8-~rodueed sulfG~LylO~o ;nl F- -~;Ato by
r aeting tho halo f n-l;~n with a r-activO th;olA~e to ~L l~c~ a
co ~--y ~A;n~ -~to ~rour or ~_o_~ ~v th roof;(O) o~ti~n~lly
hydrolyzi~g the thu~-t h; ol A~ ;n~ tc with an acid or ba~e when tho
th;~lAt~ VrouP 80 requires and (f) opt;~ lly aeidifying (if 80 r~quired)
to ~. ~_ce ~lf~n;c acid _unctional 5-vroups units
Tho ~ - rP of th inv ntion ~ormit3 u80 of vory low lovol3 of a
~in-~lO acidic a~ A ---;n~ a-~ont Tho ~-c r5 ~Ormits ~; _l;f;-d ~ vd~cL
; ~1 A~; ~n ~ ~8, rOeyelo ~1._ ~D, and/or w_8to ~ Tho
r~ aoos not ro~uire a 'rAl;7-ti~n Dtor to rOmov hydrochloric or
~ul_uric aeia ~nd aoo8 not ~roduca a wa~tO salt ~L ~ - Th acidic
25 e ~ ~;ng a~ent~ u~od in th~ ~, - rY of this i~v~ ;~n wer- r-adily
v~d from th~ L. --t;~n mixtures and can be lecvv~ and rOeycled
Tho ~ _ VJ of this invontion ro~ult~ in hi~h ~Qlt Livity toward
proforred bi~-(4-Ly~ v~y~ryl) ;~ D and v ry fast r-action rates
The ~ .~~ of thiD in~ention was partir~lA~ly useful for tho
30 ~ ~_~ ;~n of bi~(L~a_v_y~lyl) ~ , such as h;~ ~1 A and 9,9-
bi~-(4-L~-~ v_y~ yl)fluor-ne, both of which wero u~-ful in th
e~a ~tion of polyc-- h~-t~P and oth r ~ --~ ally ~ j~n;f;~nt polym r~
Tho ~ t~ cataly8ts ~;P-lo~-' herein advAn~-~-ou-ly woro
moro rOactive than hot~L ~ - ~ catalysts ~ ntly u~od Th y
35 advAn~a~ ~ly allow using lowor t' _ - ~turo8 with co O~_ ~;n~ly gr~atOr
~ ctivity for a-~ired ~roduct than was ~ ~Lly ~xp-rionc-d Groator
o ~ ;vity _ ~-r purif;c-~;~n ec q~- y to ~roduce a dosired or
-5-
CA 02230272 1998-02-24
W O 97/08122 PCTAUS96/13683
pr-sel-cted purity of product Thus~ for a ~ lly ~roducod
bisphenol like b;~ph~~l A, a hetoL~,c~s catalyst disclosed herein can
be adv_ntageously substituted in an existing c cial procQso, run with
the same or hi~hor th ~L~L at a lower t _--~ture with le88
purific-ti~ to achiove at least ~quAlly ~ure product
~ etones or aldehydQs and ~ ~l;c c _ '~ (hereinafter phenol,
rh~ phenol or ph~~~l;c startin~ material) useful in process of tho
invcntion were known in tho art and were d-scribed in the lit-rAtur0, for
~nstnnce, Jannen '982, supra, Maki ~t _1 , '252, ~u~rA, ~organ '165,
~u~ra, and Rn-bol ot al , '594, ~u~ra
Tho c~~ t;~ of this invention can be re~ by the
~quation for a L~.e8. IAt;ve c~~ ;~, that of phenol with 9-
fluorenone
z
HO OH
The pL.~P~ for making ~;~rh~~~l A can be ~ ~ y the
equation
C~3cc~ 3 2 ~ ~O ~ ~ O~
Ph~nol ~tarting mat-rials wer- adv~taL _~ly any a ~ tic L~l ~y
~which have at loast one unsubstituted ~osition, and optio~A~ly
have one or moro in~rt sub3tituent~, ~uch a~ hydrocar~yl or hAlo~n at the
ono or moro r$ng positions An inort substituent was a ~ubstituent which
ao-8 not ~nterrere ~o~ bly with thn c~ -ti~ of the ~henol and
katone or _ldehydo and which was not, itself, catalytic Pr~ferably, the
~wero unsu~st~tut-d in tho position, par_ to the LYd~G~1 group
CA 02230272 1998-02-24
WO 97/08222 PCT/U596/13683
Alkylcne (alk), alkyl, cy~lo~lirhAtic~ aryl, arylono (~r), alkyl-
~ryl~no (~llkar), arylalkylono (ar~lk), alkylcy~ls~l;phAtic and alkylene-
cyclo~li~hAtic were hyd v~rbyl function3, that was, functions c~tA;n;~g
carbon and l.~dhvg~l~ atoms ThYo alkylene functions can be straig_t-chain
or branchod-chain and ~aturatoa or ~-t~at~d, that wa~ alkylan~,
alkonyl-ne, or alkynylono Cy~lsAl;phAtic Lyd v~--~~~ rs~ includo
both satur_tod _nd ~ t~-ated cyclic ~o~iA~eo~ that was, cycloalkyl-ne
and cy~o~ ~ylone Arylon- includ-s mono- and ~olycyclic a,~ tic
r~FjA -~, ~or ~x~mplo, thoso of ~ -, bip_-nyl, biaryl, naphthyl,
10 ~h~A~ h enyl, anthracenyl or aryl ~roup8, i~ A;~ tho8e bridgod by an
alkylone grou~ Alkaryl r~o-;A~eo ;~ll-A~ alkyl, alkenyl and ~lkynyl-
xub3titut~d aromatic rings Aralkyl includos alkyl, alkonyl or alkynyl
r~niA~--o, ~ubstitutod by ono or more ~. tic ~roups
Alkyl ~roup8 ;~ A~ both 8trai~ht-chain and L~ched-ch_in ~
of methyl, ethyl, propyl, butyl, pentyl, hoxyl, hortyl, octyl, nonyl,
decyl, undocyl, dodncyl, tridocyl, totradocyl, ~ -Aocyl, h~~A~ocyl,
_~pt-docyl, ~-'-_~1 and eico_yl ~roups, A8 woll as th~ Cvl Y8~ A;~Y
unsaturated (alkenyl or alkynyl) groups, as well as hi~her ~- logue~
Pref-rably, the alkyl groups WQre of 1 to 20 carbon atoms, moro proferably
of 1 to 5 car~on atoms, mo_t rrY-ferably tho~o of 1 to 3 carbon atoms
Alkyl of 1 to 5 carbon atoms ;~ A-8 the various methyl, ethyl, propyl,
butyl and p-ntyl ;~ ~ ~
Alkyl, aryl, alk_ryl and aralkyl substituonts wor- ~uitablo
Lyllvc~rbyl substituonts on the phonol ~rct-~t
Othor in-rt ~ubstituents on thQ ~r-l~ ~, but wero not
limited to alkoxy, aryloxy or alkaryloxy, whorQin alkoxy incluaos methoxy,
othoxy, propyloxy, butoxy, pentoxy, h xoxy, heptoxy, octyloxy, nonyloxy,
decyloxy and polyoxy thylon-, ns w 11 as higher homologuos; aryloxy,
~- y~ b~ph~ y, naphthyloxy and alkaryloxy ~ '-8 alkyl, alk-nyl and
_lkylnyl-substitutod ~ -lic~
Add~tional inort substituonta in ~h-~lo includY-s halo, ~uch as
bromo, chloro or iodo
Cyano and nitro substituents may deactivato tho rh~~~lo and aldohydo
and carboxylic acid substituQnts may cause interferin~ reactions
Additional L~alv~yl ~ubstitu-nts may be suitablo in somo cas-s
Pr~f-rred substituents ;~luAs alkyl moietios c~~tA;n;~ from 1 to
~ 10 carbon atoms, more proferably, lowor alkyl ~ ieti-s, c~~1-;~;~ from 1
-7-
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/13683
to 5 carbon atoms, most prQfQrably from 1 to 3 carbon Atoms Th~ alkyl
substituents may be straiyht-chAin or branched-chain i8
r 1~-y rh -1~ ;n-~uAe~ but wer- not limit-d to, phenol,
2-cr-~ol, 3-cr-sol, 4-cr-sol, 2-chloro~h-nol, 3-chlororh~nol, 4-chloropho-
nol, 2-t-rt-butylr - -1, 2,4-dim thylrh-n~l, 2-othyl-6-methyl~h~n~l, 2-
bromophQnol, 2-fluo~v~h~ , 2-phonv~y~he~ol, 3-m tho~y~honol, 2,3,6-
trimothylr'- -1, 2,3,5,6-tetramethyl~h~nsl, 2,6-xylenol, 2,6-
A;~hl~ vL~- ~1, 3,5-diethylrh~n~l, 2-benzylrh~n~l~ 2,6-di-t~rtbutylphen~l,
2-phenylrh -1, 1-n~hthol, 2-narhlh~l Profcrred rh~n~ls ;n~lu~- ph~nol,
2- or 3-crosol, 2,6-dim~thylphenol, ~soL~lnol, ~A~h~h~l~, nnd mixtures
th-r-o~ ~o~t prefQrably, the phenol wAs un~ubstitut-d
Th- k~tones which were Advnntageously usoa includo any kotone having
A ~in~le k-tone carbonyl (C=0) group or soveral ketono carbonyl groups,
ana which were reactive und-r tho conditions used The ketoncs c~n be
3ubstituted with ~ubstituents, which were inert und-r the conditions us~d
In-rt Qub~titu~nt~ werQ as ~et forth abo~- for th~ r-active rh~nolQ
The ketv~os w~re advanta~Qously selocted from ~lirhAtic~ aromatic,
alicyclic or mixed aromatic-~l;~hat;c ketones, dik-tones or polyk-tones,
of which ac-tone, methyl ~thyl ketone, diethyl ketono, bonzil,
~c-tylac-ton~, mothyl i5v~ vyvl k~tono, methyl isobutyl kotone,
AcetoF~ -, ethyl phenyl k~tone, cy~l~h~ ~ ~, cyc~op~nt~
'~ ~, fluor-nono, ;nA~n~ns~ 3,3,5-trimethylcycl-~q- -,
~nth_Aquinon~ 4-Lyd V~yacotoP~ -, ac -rht~ -_~inon~, quinon~,
bonzoyl~-t - And A~ yl w~re ~l~r~ -~t;v~ oxampl-s
~tono~ ha~in~ halo, nitrilo or nitro substitu-nts can also bo us~d;
~or . _~-, 1,3_A; ~hl ~ro~-t~~- or hexafluoroac-tonQ
~l~h-t;c ketono~ which w~r~ us~ful starting materials ;~_l--A~, but
wer~ not l$mited to ac-tono, othyl mothyl k~tone, isobutyl methyl keton-,
1,3-A~hlo-oacetono, h-xafluoro~cot~n9 A pref-rrea ~l;ph~t;c ketono wa~
ac-tone, which c~nAen~e~ with phonol to ~ v~co 2,2-bis-(4-L~l v~y~__lyl)-
~ ly known ~s b;~p~ --1 A ~nother preferroa ~l;rh-tic
k~tone was h fl~~roaceton~, which roacts with two moles of phonol to
.n~o Z~2-bi8-(4-_y~v~yp~ yl)~h~fl~ v~v~ane (~;q~rh~n~l AF)
A pr-f-rred cla~s of l--t -8 has at least on- l.y~va~rbyl ~roup
c~nt-~n;ng an aryl group, for _~ _lo, a ph~nyl, tolyl, naphthyl, xylyl or
4-Lyd~v~y~h_~yl group
-8-
I
CA 02230272 1998-02-24
W 097/08122 PCT~US96~13~83
othor pr-~Qrrea kotonos ;n~AA those in which tho Iyd.~rbon
rA~A; CAl R co~ecLed to th- carbonyl ~rou~s of tho ketone waO in a
Cy~loAl; ~hAt; c group Ex_mplAs of ~e~;fic pr~iurrod keton~s ; n~l ~Ag 9
fluorcnon~, cy~l~h~ ~, 3,3,5-trimothylcy~o~ ~ o, ;nAAn~n9,
;~n~n_~ and Anthraguinono
~ ost ~referred ketones in~ 9-fluo e~o o, b~n-o~h~nA, A~-tAno,
_ceto~ -, 4-hy~h~y~cetorh-~ - ana 4~4~-dihyd~y ~ Most
~r-for_bly, tho ~. _ -~ of this inv ntion was u80d to make bisFhenol A by
roaction of phonol with acetone or to make 9,9-bis-(4-
l.y~.~y~henyl)fluorene (BHPF) by roaction of p_enol with 9-fl~o~an~c
The ~L._~F~ Or thi~ invention cnn ~I80 bo usod for tho c~n~Pn~-tiAn
of ~ with aldohydos; for _ _le, with fc l~hvde, acotaldehyde,
~ropi~nAl~-hyde, butyraldohyd- or hi~hor ~ -lo~1~~ of thn f 1~A RC~0,
wh-rein R w_s _lkyl of 1 to 20 c_rbon _tom~ Tho c~n~~~t;~ o- two
molo~ o_ ~hanol with ona molo of f ~ ~ydo produc-~ bin-(4-
l.yl ~y~honyl)methanA, _180 known a~ 8isphenol F
It will be ~ Lood that ~;Al~-hydos and kotoAl~~h~yos, for
= _lo, ~lyoxal, phonyl~lyoxal or yyruvic _ldohydo, c~n al80 bo used
Th~ product~ woro ~enerally ~ ~nA~ ~h~n~7 n~ th_t WaR~ R
ao having ono or moro Oin~le cArbon _toms to which wore attached nucloi of
two ~h~n~l;c moi-ti-s This singlo carbon atom ~o. o~ An to tho
c- h~n~l c_rbon of tho kotono or A ~ lo ro_ct_nt In tho c_so of
~t_rting m_tari_ls, conlA;n;n~ ~ ro th_n one aldehydo or kotone carbonyl,
the ~roduct will c~nlA;n more th_n one ~ ~Al ~;~h~n~l;c moiety For
25_le, the ~ te from acetyl acotono and phonol waO 2,2,4,4-
t-trakis-(h_dko~y~hc-yl)~ ~An~ _nd tho _ ~ ~-te from benzoylacetono was
2,2,4,4-t-trakis-(h"~l.~,y~b- yl)-4-phonylhutAno
The ~ o~lfonic acia catalyst was any ~oci~, wh-ther sol~lo
or ;n~ol~hle in tho reaction mixture, c~n~-;n;ng at loast ono thiol (8H)
~roup and at l-ast on- sulfonic _cid (S03H) ~roup, ;n~l~;n~ any ~roup
which can be ~v_.Lod to a 8--1 f~n; c acid group undor tho roaction
conaitions us-d
In tho __~ ~;c-ti~n and claims, tho solublo ~ ~a~tosulfonic acid
moi-ty wan ~ so-Lod by tho f~ 1 A
35(RS),-~-(S03H)~
~ .9_
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/13683
wh~r-in ~ was an Hlkylene, cycloaliphatic, arylene, alkylonearylen~,
alkyl-n-cycloaliphatic, alkylen-aryl, h-t-rocyclic or alkylen-hetcrocyclic
rosiauo and ~ach of "An ana b" wa~ i A~ A ~ly ~n ;~t~__L from 1 to 20
n~olublo -~ -~to~ulfonic acia," a~ usea in the spe~;fi~t;on ana
claim~, me~ns a - __ ' which hAs som~ solubility in the r-action mixture
ana which can b- ~ from the mixture, at tho ena of tho reaction, by
~xtraction, ion ~ ~, ~ ritation, absor~tion
"Inxoluble ~Losulfonic acid," ns usea in the c_~c;f;~at;~ and
claims, means a matQrial, which was ;~ol~hle in the reaction mixturo
Th~s~ material~ wore generally polym~ric or~anic resins, or catalytically-
activ ~ , bond-a to an inor~anic ~/L
Whon ~ was alkyl-ne, the alkyl-n- can be of 2 to 20 carbon atoms,
includin~ strai~ht and branched chain alkylene moietiQs, Col _L~A;n~
h-terochain moi~ties and alkylene substitut~a with inert substitu~nt~
Inort ubstituents ;~l~A-, for le, alkoxy, alkonyl, alkynyl, halo,
nitro, aryl
R~l~s l;v~ - ~Lo-l~A-~~ulfonic acias ;~l A~, but w~r~ not
limit-d to, 2-morcapto-than-sulfonic acid, 3 ~a~Lo~ v~ ~__lfA~;c ac~ a,
4 ~L~h-ut~ ul fonic ncid, 4 ~tc~e ~ ~-_lfonic acid, 3 - ~to-
2,2-dimethylprop~ -~ulfonic acid, 2,3-A;- ~toprop-~-~ul~onic acia,
c~topropane-2,3-disulfonic acid, 2-benzyl-4 ~ ~t~hut~--L.lfonic
ac~d, 5 -~c~op-~t -=_1 f~; c acia~ ~ost ~reforrea among this group of
cataly~ts worQ 3 - ~yLopropanesulfonic acid nnd 4- -~a~hut~ lfonic
~cia~ .
~ho ty~es of ~ ~to~ _lf~;c acids which w r- u---hlo w ro
_l;f~d by the followin~ _ __ '~ of r~_ la I
-10-
CA 02230272 1998-02-24
WO 97/08122 PCT/U596~13683
H8 ~ 803H Fo rmul~ I (a)
HS ~ ~S03H Formul~ I (b)
Q
SH
¦ Formula I (c)
Q ~~ S03H
whorein Q was _n inert ~ubstituent and Y was an optional hetero~7~ ,
~or Dxampl~ 0, N-Q or S Q was H, hyd~_~rbyl, halo, c~rboxy, ~ul~onyl,
~8 doscribod ~oovo ror inert substituonts on tho ~honol, koton~ or
aldohyaQ starting matorials ~ore than ono Q may opt;~Ally be prQsQnt
Th- Q substituent can be at any position on th- chain and more than on~ Q
can b- ~Le~ L As ~ot ~orth in tho gonor~l fr lA for th~ solublo
catalysts, ~ ro th~n onu 8H or s~lf~;c ~cia f~ t;~ worQ o~ti~7~11y
in th~ catalyst
C ~ of r~ 1~ I(a) are ;~ within tho genoric f~
0 H8(CH2)yCH(Q) (CH2) ~503H
wheroin y was an into~r 4rom 0 to 20, z was an ;~1 3~ from 0 to 20, Q
wa8 an optional in~rt substituont and y I z 2 1, u~ to a ~ of 40
_ ~~ 0~ r~ 1A I(b) ar~ within the sQneric formula
H8 (CH2)y~Y~ (CH2) s8~3H~
wL~ _ ~ on~ or ~ r~ inort substitu~nts, Q, can be nttach~d at any ~oint
along tho carbon chain; whorein Y was a hetoro-lQmont, for ~ , -S-;
~ach of y and z was at l~nst 1 and y -I z 2 2, up to a - of 40
P f~ ~ ~ lin~ar - ~to-l~r --~ulfonic acias woro thos~ in which tho
aistanco b_L~_ the ~ o and ~ulf~;c acid _unction~ wrre le88 than
ao 20 atoms, ;~lu~;n~ both carbon and h~t-roat ~8 C __ a~ O~ F~- 1A
I(b) can al80 havo moro than one SH and/or mor~ th~n ono sulronic acid
functi ~
C ~ of F~ 1 A I (C) are included within tho ~ormula
CA 02230272 l998-02-24
WO 97/08122 PCTAUS96/l3683
HS
I
TH ( CH2 ) y t Z SOIH
Q
wher-in y and z wor~ as abova.
r~- ~Lo8ulfonic acid pr~_ ~Gl8 can al80 be usea as catalysts, by
cv.... ...v~ ~ion to active ~Losulfonic acid catalyst8 in the roaction
mixtures. For ~ _le, a pr~cursor alkali met~l sulfonate salt can b~
noutralizeA with a min-ral acid to produce a froQ sul~onic acia.
8ulfonat~ ~ster ~ ~__ dO18 ean be hydrolyzed by treA with a stron~
baxe, for ~ _le, soaium or ~o~r; l-y~ v~ide, and thus cv~vv.Lod to a
co~ ~vnding alkali m~tal salt. A further ~ or of sulfonic acias
for the practiee of this invention, was ~ sulfonyl haliae group, which ean
roadily co..v~rLod to a co ~ 'A;~ sul~onic acid.
~ ~Losul~onie aeids can be ~ropared from co ~6~A;~g
h-lo~lkA~-~_lfonic acids by reaction with an alkali metal - ~Lide; for
~x~l~,
X-alk-803H + Na8H -~ HS-alk-803H + NaX
wh~r-in X was Cl, Br or I nnd alk was alkylono, ~enorally in accoraanco
w~th Elli8 et al., "The Preparation and P v~Al Li-s o~ a Double 8eries of
Aliphatic - ~pLans, n J Am. Chem. 80c., Volume 54 (1932), Pages 1674-
1687.
Alt~-~At;v ly, tL~- of a h~l oAl 1- - - - S ~lfonic acid with an Alkali
mHtal ~h;~-~-tat~, foll~d by hydrolysis, can be usea to preparo
to~ -r~l fonic acias .
Anoth-r route to ~Los~lf~;c acids was by co~v~ Lin~ h~l;~ to
a cv ~ thiouronium salt, which was hydrolyz~d with ~ stron~ base,
a~ ~ollow~:
X-alk-803H + ~, ~ ) HN=C-8-alk-80 H
I
NH2 HX
~ NaOH
H8-alk-803H
~-norally according to 8chramm ot al., "The 8ynt~ of
~ c~LQ-lkA~-r~lfonie Acias," J. Am. Chem. Soc., Volume 77 (1955), Pagos
40 6231-6233.
-12-
=
CA 02230272 l998-02-24
W O 97/08122 PCT~US96/13683
~yd~yAl~A~ lfonic acids can also bo c~v~Lod to tho
corro~p~n~;~ morcapto-~l -n~lfonic acid by r-action with t_iourea and
HBr/HCl to produce a thiouronium salt, which was hydrolyzod usin~ a _trong
ba~o. S~e, Frank et al., "The Preparation of - ~a~Lans 4rom Alcohols,"
J. Am. Ch m. Soc., Volumo 67 (1946), Pa~es 2103-2104.
High-r - ~to~ r-L lfonic acias can be pre2arod from high~r
olsf;n~ulfonic acids, _or examplo, oleyl sulfonic acid, by aading h~ ~J'-
r~lf;~ across tho ol~4inic bond. Alt~-~At;vely, tho olofinic bond o~ an
ol~fin;c sulfonic acid can be h~lo~ e~, for example, chlorinat~a, and
the h_l o~n moiety re~laced by a - ~-~Lo _unction, as abov~.
~ erc-pto-l~an~sulfonic acias c~n also be made from correnpnn~;n~
sultones, for example, 1,4-hut---A~ultone, in accordance with Chem. Abs.,
90:86742m (1979); R. Fischer, "Prop~ ~~ultone,~ Ind. ~n~. Chem., Volumo 56
(1964), Pa~es 41-457 or A. ~ustafa, ~Tho Chomistry of SultonQs ana
8ultams," Chemical Review~, Volume 54 (1954), Pa~es 195-223.
When -~- was arylene, th- sulfonic acid and - ~to moieti~s worQ
attached airoctly to an aromatic rin~. Reprosont~tive al. t;c
_~tosul~onic acids include 2-mercaptobon~ nulfonic acid, 3-
-~-~to~ -- -s~lfonic acid, 4 -~-~tobenz~no~lfonic acid, 2-
--~a~t~rhth-l~~sulfonic acia. Tho aromatic .s;~o8 can bo
~ub~titutod with substituent~, for oxam~le, H, alkyl, alkenyl, alkynyl,
aryl, halo, alkoxy, aryloxy (Q, ahove), which w~re inert unaer the
reaction conditions. Tho active catalysts can cAn~-;n moro than one SH
and/or moro than ono sul40nic acid function in each molecule.
Cycloaliphatic resi~leO- ;n~1-~9 thoQe o$ cyrlohoY-~o, cycloFont~no
and cyclohe~tan-; the aliphatic rin~ of indane, Tetralin or
benzocycloheptano. Ro~ C8 -tivo cyrloAl;~h-t;c - ~Losul40nic acids
include, but wero not limited to, a ~a~tocy~lo~-- -~ulfonic acid, 2-
- ~-~Locyclc~ fonic acid, 3 --c-ptocy.~l ~h~ esul40nic acid, and
3- ~Locyclo~nt~no~ulfonic acid. The cycloaliphatic rin~s can also be
~ubstitutQd with inort _ubstituents and can cnnt-;n ~ re than one S~ ~roup
and/or more than one sul40nic acid ~rou~.
R~r-~-nt-tive alkylen~cyrlo-l;rh-tic - ~Losul4onic acid
can be r-pre~ e~ by the followin~ fr 1_Q:
-13-
CA 02230272 1998-02-24
W O 97/08122 PCTAJS96/13683
CzH2~SO3H CZH2z8o3H
CzH2zSO3H
u ~ c y il 2 y e
CyH2ySH CyH2ySH
Q ~ CyH2ySH
C~cH2zSO3H
whor-in y ana z wRro i~tY~ of 0 to 20; Q wa8 an oytional inort
~ubstituent soloetad from alkyl, aryl, hAlo, ~lkoxy or aryloxy and y + z >
1. Typical c ~ includo ( --~Lomothyl)cy~ lfonic Aeid and
( --c~Lomothyl)(sulfomothyl)eyr~ Y~~.
Typienl ~lkylanoAryl morea~tosulfonie aeids ean bo a~ ~e~t~ by
tho f~
Q
HSCyH2y / ~CzH2zSO3H
Q CzH2zSO3H
~ Q ~J
CyH2y8H
¦ CyH2ySH
CzH~zSO3H
Q ~ CyH2ySH
CzH2zSO3H
-14-
-
CA 02230272 1998-02-24
WO 97~08122 PCT/[~S96/13683
whorQin x, y ~na Q woro a8 abovo ana x ~ y 2 1
A typical - __ ' of thi~ group, (mercaptomethyl)~ sulfonic
acid, can be preparea from a co 7~y~ ;~ chlorom thyl- or bromo-
mothylh ~ f~;c acid
0~ from vinylsulfonic acid can provide soluble matQrials,
c~t~;~;ng large '- ~ of -~ Lo and sl~lf~n;c acid groups This typo
of soluble c~talyst can be prepar~a from oligom~rs c~nt~;n;n~
vi~yln lfosic acid units, h~lf of which c~n b- cv.vO Loa to chlorosul f~yl
unita and ~ to ~ ~a~to units in acco~ c~ with the foll r.. n~
reaction schome
--~--CH2--CH--CH2--CH--CH2--CH--CH2--CH--]--
l l
603H S03H S03H S03H
-t-CH2-CH-CH2-CH-CH2-CH-CH2-CH-]-
l l
S03H S02Cl S03H so2cl
--[--CH2--CH--CH2--CH--CH2--CH--CH2--CE~--]--
S03H SH S03H SH
Another type of oligomeric catalysts, c~n~;n;n~ a multi~licity of
-~Lo and ~ulfon;c acia units, can be ~ ~ a from ~ ._ - ~ltono
P ~ -~ ltono WA8 propar-a as ao~cribod by G -8~9 ~t al , Chom Abs
53 2083c (1959), Helborger et al , DE 1,146,870 and Ch m Abs 59 11259
(1963) The sultono ring of the polymor can be _ -d, g-norally a8
abovO, to furni~h ~Lo~lf~;c acia ol;~ s, co~n;n~ a plurality
of 7~Lo nnd ~ f~n;c acid units
In addition, oli~ c~nt~;n;ng a plurality of ~Losulfonic
acid functions can be pr-parea from ol;~ - 5 of 4-allyl-1,4-
h~t~ lton- Tho - was preparoa ns a-scribea for 4-bonzyl-1,4-
~t-n- ,ltono, using allyl chlorido inst~ad of benzyl c~loriao 4,4-
Diallyl-1,4-but~ tono can be proparea by addition of a ~econd allyl
group
othor catnlytically activo --~a~Losulfonic acid ol;L L can be
pre~ar-d from allyl vinylsulfonat~ (C~ C020ru~u~ which was polymer-
ize~ to form a cvl ~ ~;ng sultono-c~nt~;n;n~ polymer in accordanco with
Goethal~, ~8ynlh-~is and Polymor;-~ti~n of Allyl Vinyl Sul_onato,~
-15-
-
,
CA 02230272 l998-02-24
WO 97/08122 PCTAUS96/13683
Polymer Lottors, Volume 4 (1966), Pa~s 691-693 T_o rosulting polymor,
co~t-;~;~ ~ultono groups was treated with a reacti~e th;ol~te to open tho
ultono rin~ and Droduco ~toalkyl sulfonate Folymers
The co.v~l~ions can be roprs~ d by the oquation
_
SO2 ~ SO2 ~ SO3H
~ - n - SH
~; ' 1 A~ catalytically-active ~olid ol;~ 8 can be prepared from
oligom~rs of allyl allyl~ulf~te (CH2-~rU~-~O2or~r~-~u~)~ which can bo
polymcrizod in acco d~ece with E Go-t-als et ~l , "Polymer; 7~ti~ and
Copolymor;~1;o~ of Allyl Sulfonate," J Macromol Sci - Chnm , Volume A5
(1971), Pagos 63-72 The oligomers were cv~v~lLed to mercaptosulfonic
acid functional material~ by a correspA~;n~ rin~ op~;~ reaction
The con~er~ion can be L ~ ~ by th~ equation
O o - S O2 - n - SH S03H
~ot-rocyclic re~ ad~ g~ nly ;~I ~A cyclic rAq;15 ~~~ ~g N, O, or S T_ese will g-nerally corr-spond to aromatic
, for - lo, a~ es from pyridine, th;o~' -, q~;~ol;
~idine, a~ well as the COL e~_ ~;n~ partially or fully
Alkyl; - torocyclic r~-i~--~ ot_~rwise
corro~pond to ~romatic r~ of tho ~ame configuration, a8 do alkyl
h~t~ o~y~lic l_riA~ , n~ well as cv ~-~~A;~ fully or partially
l~a~v91~~t--d , _ --n,
Pr-ferr-d nol~l ~A~tosulAfonic acids were _ ~ in which t_~
c~pLan nd ~ulfonic acid functions wer~Q ~e~aratud by A chain of 2 to lO
atoms, whother the chain or linkor arm was in an alkylone group or
i~col~o~At~d in nn a ~ -tic, cyclo~l;ph-t;c or heterocyclic ring, whether
or not thu chain includes hot_ oJ :, and ~hsth~ or not t_e -r~_~Lo
-16-
CA 02230272 1998-02-24
W 0 97~0f~122 PCT/US96/1368;~
and _ulA4Onic acid functions wor- attached directly or indirectly to the
rin~ structurAs Prcferrea 80luble cataly#ts for the practice of this
invontion werQ morca;A,tosulfonic acids in which ~ and b w~ro ;A~7p~ y
~ from 1 to 4 ~orc pr-A4~rably, a and b werc ;-- ; 'AAtly 1 or 2 Most
5 preforred were - ~_~tosulfonic acid8, contAAiniAg mercapto and sul~onic
_cid ~unction8 in a 1 1 molar ratio, that wa8 a and b wero ~ach 1, more
rticularly 3 - __~tv~Av~An~_ulfonic acid and 4 -~,A~L~but~ ulfon;c
acid
When the c~to8ulfonic acid wa8 ;n~ol~hle, the heteLv~ Y~o
10 catalyst comprises a catalyticAAlly-active -L cAie~ r-jA,r~ by r- 1
II
(H5)~-~ -(SO3H)b F~ lA II
in which ~ach of a and b was ;A~ tly an integer from 1 to 20, ~ waAA
an alkylcne, cyclo-l;;AhAtic, arylon~, alkylonoarylono, nlkylono-
cycloA~l;~hAti~A~, alkylonearyl, hetorocyclic or alkylenehcterocyclic
~i ~, L wa_ an o~tional ~ grouj, and wa8 a bond, whiCh
catalytically-active sp-ciQs was attach-d by the bond - to an ;A~ol ~e
or~A~ic or inor~anic ~o L; or a catalytically-active ~peci~
-s~ l~' by J 1A III
(HS) A ( 803H)~ (F~ 1A III)
\ /
_[
wheroin ~ was an alkyl-no, arylen-, cy~lo-l;rhAtic, alkyl-noarylono,
alky~ - y~lOAli~hAt;c~ alkylenoaryl, hoterocyclic or alkyl~h~t--ocyclic
r-siduo; a and b wero ;- ~ ' ly sol-ctea from i~1~ ~ from 1 to 20;
L' was an optional 1;~; n~ grou~ and - W_8 a bond
Catalytically-active material~ of F~ 1A~ II were generally derivQd
~rom ~olymers of ethylenic ~, whor-in the ;~ol~l- organic ~v.L
w_~ tho main chain of a rosulting ~olymor and -L- was a coval-nt bond or a
1; n~; ng group This ty~o of ~olymer will ; n~ ~e unit structure8
~ 8 F 1 by th~ ~ -~ 1 f~- 1A
-~CH2CH~-
I
(HS),-~-(SO3H)~
-17-
,
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
Pr--f--rably, th- catalytically-activ materials will i~~lude those
ha~ring from 1 to 4 of oach of -- capLo and sulfonic acid groups por
More preforably, the catalytically-acti~ro materials will ;r~ those
havinsr 1 or 2 of ~ach of ~a~.to and sulfonic acid groups Ror ~ ~ Most
5 pr--f--rably,tho catalytically-acti~r-- matorislls c~t~; ~ 1 1 ratio3 of
,Lo and sul fo; c acid function~; ~nd will correapond to tho gon--ral
formula
- lCH2CH] -
I
L
HS-~ ~ -SO3H
r - 1A y ~?olymers, made from othyl~ lly ~ t ~ted --L~ and
which can be used as carriers for the catalytically-active Fl~--cios,
15 includo, but woro not limit-d to
L ~ ---(8)
-
phenyl ~L~ _
-CH2- ~honyl ally~
-O- ~henyl phenyl ~rinyl oth-r
-COO- ~lkyl, aryl acrylic osters
-OCO- alkyl, aryl ~rinyl osters
~(C~2)r~ alk-nyl a,o3-~;olefi.. o
r ~ 4-20
-N}~- alkyl, aryl ~riny~ r
-CON~I- alkyl, aryl acryl '~
-NHCOO- alkyl, aryl vinylurothane~
- alkylphonyl ~rinyltol~ ~
- ph-nyl a-mothylsLy ~n~
-8- ph-nyl ~h nyl ~inyl et~-r
-8O2- aryl vinyl aryl ~u~ f -~
-80- aryl vinyl aryl ~u~ f~
-NSO2- aryl aryl ~ulf- ~e
The l~.l.i.~5J grou~s, -L-, accordinlJly can includo alkylone, a
co~al--nt bon~l, oxycarbonyl, carbonyloxy, oxy, uroido, amido, amino, thio
20 (~ ulfur), sulfono or sulfoxo Preferr d l;~k;~ rroups ; ~1~ io a covalont
--18-
-
CA 02230272 1998-02-24
WO 97J08122 PCT/US96~13683
bond, methylenO, sulfur or oxygen, more ~_rtie~lA~ly a eoval~nt bond
joining a phonyl ring to a carbon h,~h~e in ~ol~L~ _ - or ~oly~Ly-~o
dorivativos, o~ch e~~ A;~;~ SH An~ S03H functions in sin~lo c
units of ~oly~Ly ~o
One type of novel eatalytieAlly-acti~e poly~Lyl~ne resin~ lu~
unit struetures o~ ~8~ ~ by F~ IV
[~
B
I
CRSO3H
F~ 1 A CnH~n IV
1 1
CIIR SH
wherQin B was a bridging grou~, R _nd R1 werQ ;~ ly soloetod from
H, Alkyl or aryl, -CnH2n- wa~ str_i~ht or b~ ' chain alkylene _nd n was
an int-~er from O to 20
Tho briaging group B, can bo n~leeto~ ~rom _lkylone, generally a8
above Alkyl and aryl arQ Aof; -~ above
Yol~Ly.~ - rosins of F~ 1 A IV enn b~ m~de by tho st-ps of (_)
raacting _ hAlo-l~y~Ly~O~c ~olymer with _ lith;At~ sultone, (b) t -t;~g
a reO~ultin~ sultone-~unct;~ ' ~olymer with a reActiv~ 1h;~1Ate and
(c) acidifying th~ rosulting ;~- ';At- to ~roduc- A yolymer e~~t-;~;ng
( ~ to~ lfoAl~yl)~y~ o units
~ -~oAlkyl~Ly.~c ~olymers ;~ , but wer- not limited to
poly(chloromethyl~Ly~- ~), ~oly(L. - - hyl~Ly ~ -),
~oly(L _ ~yl~Ly e-~o)~ yoly(L ~ Ly~ ~
Ineludin~ ~ lym rs _nd eopolymers, whether m_de by ~olymer;~A~t;~~ of
h-lo-l~ylsLy.~6 --8 or hAlo-l~ylation of ~oly~Ly eno rosins
RG~ tiVQ starting materi~ls can be made by co~olymerization of
- vinylbonzyl chlorido or vinylbenzyl b ~ with styr-no Eithor startin~
~ -19-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
material can be crog~l;nke~l with divinylbenzene or Q,; ~1A CrO881;n1~;nSr
~ ~ The pol-ymers can c~ntain other ~ , for oxample, styrcne,
~-mQthyl~Ly onQ~ acrylonitrile, l~uta~;rr~ maleic anhydridc, ethylono or
propyl-no
The h-loalkylated polymer~ will adv~nta~reouQly c~nt~in ~rom 0 5
m~g/g to 10 meq/g of h~ thyl ~rroups ~alomethylated or h-loAlkylated
polymers -- lly compriso mixtureQ Or ~olymers, substitutQd in the ortho,
m ~ta- and par~-po_itions
Poly(chloromethyl~Ly-e o), con1-a;r~ ~ 2 to 5 meq/g of chlorine, wa
a preferred ntartin~ mat-rial
Tho r--action s--quonce described above can be performed ut;l;~tr~SJ a
variety of chloromethylated or Ll~ thylated ~Ly . e ~olymQrs or
co~olym~ r In particular, crorslin'~~d hAl~ ~thylatod
~Ly -/divinyl~ co~?olymers in various formQ, for Dxam;ple,
mi_ v~,, v~s or ma~v~OL~,~s boads, powdors, can be ~uncti~nal;~d to
provid the Cvl ~" Ain5J ~apto~ulfonic acid polymers
In the ca~o of all tho polymer-basod cataly~ts u1;l;~ t;~n of tho
funcl;~n~ Ly _~ ~ or othor ~olymer_ in bead form may advanta5~--ously
~; _1;fy workup ~Loced~ 8 during pr--~aration and provide for more facil--
i _~ in cataly~t A~l;cAti~n- 8eads WQrQ suitably of any size
throuSJh which Hff--ctive flow and contact was achieved Physical forms
;n~l ~;n~ ~c 1~ ~, bQads, ~xtruded ~hapes, maclo~v v~8 and mi~ v~oro~,s
con~iguration w re; '- _v~r, suitably u~ed in the ~ractice of tho
inv ntion 3n g-n~ral ller size ~?rovid 8 mor~ ~urface nr--a for
c~nt~ct, but larger ~iz-- permit- ~r--at~ r flow throuS~h a bed Opti z;ng
th----- factor~ was within tho Xill of th~ art
R--activ-- tl~iolAt6~r advAnt-~ aly ;n 1~ but wero not limit--d to,
~odium ~h~-cetat--, pot-~ri fh;Q-c tat~ 1 hioAcnt~te ana
lithium 4h;cl~-rt~to and the corres~~n~;nSr L~ _ lfjA8a Of the~o,
lithium, ~oaium or pot-o~; thi~ tato wa ~ yr--forred
Wh-n co ~,~ Dion to a -v~,to f nnlj~n was dono throuSJh a thiourea
int~ t~ the thioureas w re advanta" ~_~ly sol--ct--d from thiouroa, N-
mothylthiourea, N- ~thylthiour--a, N ~ ylthiourea In another alt~~-n-t ;ve
odium thiosulfate can be u~ed
A pr--ferrod speci--s of cntalytically-active PO1YDLY ~.e r--sin was
mad-- by r--acting poly(chloromethyl)~ly - with li~ h;~ted 1,4-
;
-20-
CA 02230272 1998-02-24
W 0 97/08~22 PCT~US96/~3683
b~l ~~ulton~ to produce an int~ ';ate sultone, ~lan~n~A by the
-L ~L~ ~1 unit formula
\~/
[ ~
CH2
I
CHS020
(CH2)2CH2
Tho re~ulting polymsr contA;~-d (~ -~Lo-~--sulfo~entyl)~L
units, that was, n in F~- 1 A IV wn~ 2 ana B wa~ -CH2
~ ost pr-~erably, this ty~e of r-8in w~8 mado from a 81ightly
cro8~1~ks~ poly~Ly~ ; th~3 rQsultin~ catalytically active matQri~l w~
~ignAt-~ as P~BSA-~ER
Anoth-r typ~ o~ cat-lyticnlly-acti~e poly~Lyl ~ rosin8 can b~3
20 ~l_p~._' by tho ~tops of
(a) alkylating a PO1YDLY1~-- with an alkenyl haliao of tho f 1
RC(Rl)3C(R~)C~2~CH(R3)X, wLc _;n nach of R, R , R and R was H, alkyl or
~ryl; m wa~ 0 to 20 ana X was F, Cl, Br or I to produc3 a hAloAl~yl
~oly~Ly -;
(b) fu~fonAI;~g thQ r-~ulting h~~sA~yl~oly~Ly ~ ~ to produce an
;nt ~ t~ ha~ing sul~o-_~n~ti~Al groups;
(c) opt;o~Ally c~v~Ling tho ~ulfo-functional groups to a 80dium
or ~ot~Q~i ~ulf~n-~- _unction;
(d) th;olAt;~ the thu~-~roduc-d ~ulfG~Ly ~ ;nt- -';At0 by
roacting tho halo function with a r-activo 1h;olAte to ~ ~c~ a
c~ __~ ~;ng ~ Lo ~unction or ~1~ ~OL thereof, and
(-) opti~-lly hydroly~ing the thu~-t~;olAted ;n0~ -';At~ with an
acid or basQ wh~n the th~lAte~ ~roup 80 requir-s; nnd
(f) opt;~n-lly aciaifying (i_ ~o r-quired) to proauco tho sulf~n;c
acid function
Thi8 ~ r~ can be l~La~ od broadly by the reaction soqu~nc~3
~ .
-21-
CA 02230272 1998-02-24
WO97/08122 PCTAUS96/13683
,~
[~ + R ( R ) C=C ( R ) CmH2mCH ( R ) X
[~ or [~
R --C--R Rl--C--CR(R ) H
H-- C ( R ) CmH2mCH ( R ) X
CmH2mCH ( R ) X
~ul f onation
/~f ~ /'
803H ~ 803H
R -C-R or R -C-CR(R ) H
2) H CmH2mCH ( R ) X
C~H~CH( R ) X
-22-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/l3683
to produc~ ;~t~ -a;~<te haloAlkyl ~ f~r~At~!d styrenQ polymsrs, of which
h~llo functionE~ were Cv~-v~l L-d to ~ Lo-functions to ~roduco th
foll r n~ typ~Js o~ products of F 1~ V
S O 3 H
HO3S R-C_Rl SO3~ R2-C-CR (R ) H
1 2 1 3
C ( R ) H CmH2mCH (R ) SH
CmX2mCH ( R ) SH
Alkonyl hnl;~ 3 o~t~ Ally c~ A;~- ~ryl and ~lkyl sub~titu~nts, A8
~fi -~ abov~ R~ tivo ~lk~nyl ~ ;cl~, u~cful for pr--p lrinSJ the
catalyt~cally-activ polym~rs, include, but worc not limitod to, ~llyl
chlorido, allyl L ~ ae, allyl iodide, methallyl chloride, m~th~llyl
Ll '~'-, crotyl chloriae, crotyl b ~A, 4-bromo-1-but-n~, 5-br~o-1-
butano, 6-bromo-1-h~xono or hiçrhor chloro or L ~ lr- -7,
tho alk--nyl ~ )3 wer ~llylic hAl;~a~~t, repre~e~tecl by the formula
RC(Rl)~C(R2)CH(R3)X Most prel~arably, th~ alkonyl h~lid~ wa~ 5-bro~-1-
pe~t--~, 11-bromo-1-ll~a-- - or ~llyl L 'd~
A ~rt;clll~-ly ~r--~~rrod product thuE~ mad-- can bH charactoriz~d by
15 tho fc_ 1 A, in thQ Ca8Q of a ~,,Lvl cL from 5-brvmo-l-p~r~
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
5~ [~SO3H
--~ SO3H
SH
or, whon th halido was allyl L ~ ~is or allyl chloride, by the fc- 1
SO3H
CHCH3 SO3H
CH~ SH
R--activo th;~ t--~ wore as ~efi -' above Mo~3t preforably, tho
25 roactiv~ thiolate was ~ alkali metal th;o~~~tate or Lyl -_lf;~~,
8y varyin~ tho choice of ~tartin~ L ~ --lkA~e (or oth~r hAlo-llrA~9)
us--d in th ~ allcylation ~tep of~ the proce ~8, tho basic ~ doscribea
bov can also bo us-d to propar- a vari-ty of catalystl~ with varying
chain l-ngth~ ~ ~t the ~O~tan nnd ~ l fo~ acid moioti-s A num}~or
30 of cat~ly~t~ with di_for nt I ~ ~ o4 ~ LOYUl f~'~; C ~-cid ~ite~,
sr upon tho d--gr--e of functi~ tion in tho alkylation and
PUlfc~tior~ ~t--ps in the ~ r~rs~ and structural r~ t;~ h;~ L~_- tho
Lan ana ~ulfonic acia xit--s, ~ 1 ';~r u~on tho choice of bromo- or
chloroalkyl~tinsJ aSrent, can ~ccordingly be mado
P ~f-- 1 ~ catalytically-activo specios of F~ V wero those
derivea from poly~Ly _ ~3, tr-atud with 5-bromo-1-p -~9, ll-bromo-l-
c~~e or an allylic halido of the _- 1 A RCH--~-u~/~U~ wher--in R wa~ R
or alkyl of 1 to 5 caroon atom~
-24-
-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96~136~3
In anoth-r aspoct, this invention relatos to novel
~ ~Loalkyl)(sulfo)phonyl~lkyl aulfonated ~oly~Ly ~ne eAt~lyst~, mado
by a procQss com~rising thQ stops of
(~) ~lkylating ~ ~ lkyl~tod ~oly ~Ly _~e with ~ haloalkylarylone
~ to produco an ;~t~ te hAlo~lkyl~oly~Ly~.ne h_ving
(h~ 1 OA 1 kyl ) phonylalkYl ntyrone unitss
(b) sulf~t;~g tho thu~-proauc~d h~loAlkylpoly~Ly ~ ~
';ate to pro~uco an ;~t- -~;at~ having sulfo-funetional groups;
(c) oP1; ~Al ly Cv~-vG' Ling tho sulfo function~l groups to
corro~p~n~;~ alkali metal salts;
(d) th;ol~t;~ tho thus-~roduced sulfG~Ly ono ;~te ~;Ate by
re~ctin~ tho halo ~unction with a r~aetive th;~l~to to p~vd~ce a
corre~p~n~;~g --~to function or ~l~C~O~ thereof and
(Q) Opl ;~nlly hydrolyzing tho thus-~h;olAtn~ ;~t~- ';~t~ with an
aci~ or b~so wh~n tho I h; ol -te~ ~roup 80 rocuir-~; and
(f) opti~lly Aci~;fying (if uo required) to ~ od~ce tho ~ulfonic
~cid f~l~ti~
This ~.e~e~ produeos polymor~ having ._--t;~ units o~ tho
~SO3H ~SO3H
HO3S (CH2)n SH
whoroin n was prof~rably an ;~t~__. from 0 to lO, moro proforably 2 or 3
A ~L ~e~ ~tivo mQmbor of this ~-rios of polym~rs, dosignatod a8
DP~SA-XE3C was mAde ~rom chlo-~ --hylstyreno polymor and 3-
25 L.. _ ~yl~ -, in accordance with the followin~ roaction ~e_~-~ce
-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
E~
Cl
alkyl at i on
Lewis acid
~ CH2 CH2 CH, Br
)~ ~ B r
-26-
-
CA 02230272 1998-02-24
W 097/OXl22 PCT~US96~13683
8~11 fonation
1) ClS03H
2) NaHCO3
NaO3S{~ [~SO3Na
~ Br
Na~S
thiolation
1) Na thioac~tat~
~ 2) aCid hYdrO1Y8i~
HO3S ~ ~ S03H
~ "~ ~SH
Tho h~lo~l~vl poly~Ly O~e ~tartin~ m t-rials can ~dvantngOou~ly be
s-l-cL~ from c_loromethylat-a poly~Ly~ -~, b ~ ~thylat--d poly~Ly ~-c~,
C_1OrO~thY1at-a PO1YDLY1O -~, iodo-t~ylat-d poly~Ly -8, gen-rally as
abov~, ~rO~Orably tho halomethyl~t~d poly~Ly . -8 Those skillOd in th-
art 5-~ ;7O that sel-ctivity dOcr~a~s with h~lo~l~yl grou~s on aryl
rin~s which ~roup~ havc ~ L-1 ectivity
T_o h~lo~l~ylary-nQ c __ ' can conv~i~~tly bo s-lOct~d ~rom
chlG~ , (chloromethyl)hq -, (chloroethyl)b~
(chlo v~ ~yl)~-~~ -, (chlG ~Lyl)~~~ -, ~8 woll ~g tho ~ ~ -A;~
~luoro, bromo and iodo analo~uos R-~ t;ve _1~ e (2-
-27-
-
CA 02230272 l998-02-24
WO 97/08122 PCT~US96/l3683
chloroothyl)b~~~r~, (2-bL~ ~thyl)h~~~, (2-iodoQthyl)~-~, 1-
chloro-3-phenyl~ ~o, 1-bromo-3-phonylpropane, and 1-iodo-3-
; phenylpropano Th~ bromo ~ ds wor~ proferr_a
Th~ alkylation wrAs convoni~ntlY carri~d out in tho ~,~ e of J
5 Fri - d~l-CrrA4t8 crAtaly~t, of which aluminum trichlorido, aluminum b ~
boron trifluor$d~, Lyd ~_, fluorido, phosphoric aeid, zinc ehlorid~,
titanium ehloride, othylrAluminum ~;rhlo id- and 3t~nnic chloride wQrQ
r-pr-r~ ;ve A prOferrQd catrAlyst was ~1 chlorido in
nitromothane or nitro~ - -, as di8closoa by A WrAr~~hawsky ot al ,
"FunctiA~-~; 7~tir~ of Poly~Lyl ~, I Alkylation with Sub8titutôd B~nzyl
Halid~ And B-nzyl ~lc~h~l C _ ~n, J Org Chem , Volume 43 (1978),
Pay--s 3151-3157
Conv~~;~~~tly, ~ft~r nlkylation, unrQactod h~lo-lkyl~ , solvOnt
and catrAlyst in th_ -~ Ytu-o w-ro l v ~ Afrom the alkylatOd poly~Ly _.o
by m~an~ within th~ art Auch a3 filtration Adv~ ly, tho -~ Yt~o
wa~ r~cyclod for roaction with ~dditional h~lo~l~yl poly~Ly~ o Th~
~lkylat~d poly,Ly ~no was optio~lly wa8hod with a solvont such as
dichloromothano and op~ ly dried
The re~ultiny- nlkylat-d ~oly"Ly ,~o was sulfonatod usin~
chloror~lf~~;c aeid, oloum or othor known sulf~~ a~onts Prior to
_ ~ion of tho halo mOiQty to thô ~ o moiety, $t wa8 eonvoni~nt to
cv~v_ L th~ gulfo moi0tios to cO~ ;ng alkali motal salts
ChlG 7f~~;c acid, ~ulfurie acid or sulfur trioxide was conv~~;~t~y
u~-d in an amount ~ff;ri~ to achi-ve a ~rodot~ or desirable
do~rO- of ~ulf~-ti~, adv~t~L l~y to avoid e~r8- y worku~, in an
mount not in lar~o ~xe-8s of thô ~uffici~nt amount whieh vari~s with oaeh
r-~in but Wa8 ~t- -' without unauo ~x~or~ -tio~ Tho adv~ r of
low~r reaetion t~ _ ~tur-s woro y _~t~r with ehloro~lf~~;e aeid
Tho th;ol~t;~g ~S ~ wore eonv~niOntly s-loetOd from tho~o
~;-cl~-~ abovO 8Odium th; ~--~tato was pro_orroa Exc-~3 soaium
L~ ~ lf;~ w~ opt~ ly usad for th;ol~t;~~ Hydrolysis was thon
~c-~r--y ~inco a thio group rathor than a ~h;o~cOtat~ wa8 formed Th~
~t 'i~te thi~lAt~' __ ' was, if n-c~ssary, acidifioa with a strong
acid to cv~v. L rulf~to salt moi~tie~ to COL e~ ~;ng sulfonic acid
moietiO~ Ad~antag-ou~ly, a minOral acid was us~d, as abovo
In an alt~ t~ '-~~; , tho ~lOC~88 co~prise8
-20-
CA 02230272 1998-02-24
WO 97/08122 PC~/US96~136X3
(~) alkylating ~ PO1YDLY OU~e resin with a hAl~ thyl
h~ lo~lkvlarylene c _ _ ' to produae an int~- -';ate h~vin~
(haloAlkyl)3;~hnnyl~1kyl ~-y _ Q unit~;
- (b) sulfonating the thus-proauced int~ te to produco an
~nt~ to having 8ul~0-functional groups;
(c) o~ ly cc VOlLing tho sulfo functions to COl O~ ;ng
alkali motal s~lts;
(a) th; ol~t;ng the thus-produced sulfG~ Ly n~ ;nl ~ te by r~-
acting the halo function with a r--active 1 h; ol AtC to produco a
10 corre81y~n~; nsJ ~ ~ ~LO function or ~ t o theroof ~Ind
(~) ol?ti~ Ally hydrolyzinS~ the t_us-th;olAte~'l ;n1-r- ~';~te with an
acid or b~se wh~n tho ~h;ol~tn~ sJrouP 80 requirod; and
op~ lly ~lciairying (if 80 roquirod) to ~ ~ l~co tho sulfonic
~cid function
Illustrntive of this reaction was tho followinSr s-quQnco
(~ "A', (~ "S~
~3 Lev~isAud ~3 1)CI503H ~SO N 1)NaSAc [~S03H
T ~ 2)Acid Hydrol 1'
D~ \~3Na \ \~
Br
ao Tho alkylatin~ 3tep was ~>orformoa as in the preco~;nçr process
opticn~lly $n a ~olvent such a~ chlo of,~ , 1,2-dichloroethan--,
~irhloromethano~ 1~2-~irhl~ .~a~a, prefer~bly ~I solvent which swells
poly~yl~o (for exam;plO, ~Lylono/divinylh~n~ - copolymOr boads) The
alkylatinq aSIont was any h-l~ -thyl h~lo~ ylarylenQ prefQrably whOrein
tho alkyl Srroup has from 0 to 10 carbon atoms Tho arylone group
prefHrably has from 6 to 14 carbon atoms
-
-29-
.,
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
It wa3 within tho skill of the art to select halomethyl
h~loAlkylarylones in which the hAlo~lk~rl and hJ~l~ thyl ~roups havQ
activiti--s E~ufficiQntly differ--nt to achiove th- d~8iroa result
R--~?r--sl~nl ~tiv~ hAl thyl hAlo~lkylarylenQ = _ _ " ;n~ lu~9
5 (2-b ~ :Lhyl)b0nzylchl0riae ana (3-~ v~yl)benzylchlorid~.
Chloromethyl h~loAlkylarlenes were conveniQntly proparea by moans within
the Dkill of tho art ~uch a8 d--Dcribea by gelva et al, Syn~ ~-ri~, l99l,
1003-1004, wher--in hAlo~lkylarylen0s were reacted with f l~ehyaQ in
acia (for - _~e, sulruric or hyl o-hloric) in the ~L--- -= of a
10 quaternary ~ ; l?ha~ tran~for catalyst Chloromethylation can also
b~ ~erformea usinSr zinc chloride ana ~araf~ 1~ hyae in accoLd-~lce with
the m~ thod doscrib0d by Daren in U S Pat0nt No 4,967,026.
Alt--rnativoly, chloromethyl ethQrs were usea to chloromQthylat0 a
h-loAll~yl arylene by methods ~ to that tau~rht by Ral y in U S
Pat--nt No 3,311,602, by Shinka, et al, J Poly Sci Polym Lott Ed
14(1), 1-3 (1976), ana by Shis~--o, et al, Chem Abstr 72:32290 (1970), or
by oth--r m ans within the skill of th0 art
In ~nother aD~oct, hydr~l Alo~_ -;nS~ aSronts~ such a8, I~Br wero adaea
to alk--nyl aryl--nos, such as, ~yl e undor radical forminSI conaitions
such a~ tauSJht by Martan in U S Patent No 4,228,106 or Plesmia in U S
Pat--nt No 3,321,536. In an ~ n of theD~ works, vinylbenzyl
chloriae w~s hydroL ~ 'nAteA by this method
The s~lf~n~ion ana th;olAtio" ste~s were as aescribea for the
prov~ OU8 p . " ~ 8 ~ .
In .~a~ tion to tho m thoas ~;~cl~o~-' abov~, a vari ty of othor
methoas w r alDo avA;~ le for ~ ;n~ the h~lo~lkyl-funct;~n-l;~
~oly"Ly _, e ro~ins which were pL ~ V16 to tho merca~tos~lf~n;c acid
polymer cataly~ts Ro~ r -t;ve a~roachQs for ~reparinsJ the
h-lo~ ylat--a poly~l_y~ nQ r--Dins include (but wore not limited to) tho
de~crib~d or ~ ~' by a) P C R eves and M S Chile~, "Pha~
Tran8fer Catalysts Anc1c,~ ' to PO1YD~-Y~nO~ " Tetrah--dron Letters (1979),
P~--s 3367-33705 b) M Tomoi, ot al , ~NOVQ1 Syn~h-s;~ of S~acer-
~ ;fl-~ Polymer S~po~L~ and Activity of PhasQ ~ fer Cataly8ts Derivea
from tho Polym~r 8up~orts," J. Poly-mer. Sci. Polymer Chem. Ed., Volume 20
(1982), Pa~oD 3015-3019; c) M. J. Farrall and ~. N. J. F~;-~-t,
-t~ and Lith; At; ~n: Two Important Ste~8 in the Functi~ ation
of Poly~Ly~ - Resins", J. Or~. Chem., Volume 41 (1976), Pa~Qs 3877-3882;
-30-
CA 02230272 l998-02-24
WO 97/08122 PCTAUS96/13683
d) S p r~ and R D Olin~er, "RQactions of Cyclic ~alonium Ions ana
Alkylene D;hAl~AQ8 with Polystyryllithium PrQ~Aration of ~Alc-1kylatod
Poly~Ly.~ Q~, J or~ Chom , Volume 45 (1980), Pa~os 2717-27195 e) M
~r_tnke, ~t al "Sorption of E' ol~ on Anion-~ o Resins Havinu
omega -~v~-lkvl or ome~_ -~yd O~y~lkyl Spacor," An~lytic~l g~i~n~s,
- Volume 4 (1988), P~on 591-594; f) ~ aauthi~r and A ~;~ - n~
"Alkylatoa SLy.~no I~ with Variable Len~th Spac-r8 I Syn~h~si~,"
J Polymer Sci PArt A Polymer Chem , Volume 28 (1990), Pag~s 1549-1568;
~) P Tundo, "Ea~y and ~--- cAl 8yn~ of Wia~ly Porous RosiAs; Vory
~ffi~;~nt Su~port~ for T ~; 1 t - - ~ ph~ ~sfor C_talysts," Sy~hA~i8
(1978), P~-~ 315-316; h) T~hik~w-~ ot al , "rl~c~8 for the Production
of SilAn~", (U S Pat-nt No 4,725,420); i) G Zhon~, ~t A1 , nSye~h~r;~
of ~l --lky1at-d Cro~rl; nk~ Poly~Ly ~na", Xinan 8hifan Daxue Xu-bao,
zir~n ~~~t~An, Volume Z (1986) Pa~s 68-70; j) ~ ~ ~llen~lobon,
"Preparation of Poly(p-( omo~a -lithiumalkyl)~Lyl -a) and Their Us~ as
Polymer MetAlAt;n~ A~Qnts", An~ow M_kromol Chem , Volum~ 31 (1973),
Pa~es 147-159; k) F Do~ ~ ot ~1 , ~8yn~h-~i~ of Sulfoalkylatod SLy e_c
Divinyl~ ~ Co~olymers", Makromol Chem , Rapid Commun , Volume 1
(1980), Pa~os 297-302
It was to bo ~d_ ~Looa that a h_loalkylat~d poly~L~_~o re~in
~repared in _ mannor ~uch as that d-scribQd in tho abovo ref- e;-er could
b~ further f n~li~nA~ ~ by thQ 8~lf~nAti~n and th;olA~;An ~ ,-- ~8
pr-viously describQd to provide a ~~Los~lf~n;c acid polymer catalyst
Ot_er ~L ~ -~iVQ roforoncos on methods for ~olymer '~f;c-ti~~
or for us-n of functional ~olymQrs ;n~ Ak-lah ot al , n~r~l;~Atinn of
Functi~n~ a~ Polymers in Or~anic Syn~~~;~n, Chom RQ~ , VO1U~Q 81
(1981), Pa~es 557-587; ~ ch~t ~t al , nFunct;~nAl;--t;~n of Cro~l;n~-A
Poly~Ly ~ Ro~ins by Ch~mical ~;f;~r1 i~n A R~viow in "Ch~mistry and
P ~_ Li-~ of Cro9~ ;nl a Polymors", 8 ~aban_, od , ~ d- c Pros~, New
York (1977), Pa~-s 59-83; and Maréchal, n~ 1 r ';f;CAt;~ of
8ynthotic Polymersn, in "C _ ehc ~iv~ Polymer Scionc~", Volumo 6, All-n,
~a., P~L Pro~, New York, Pag~s 1-47
Catalysts deri~od from poly~Ly.v~e8 will advAn~AgeJ~ly c~ntA;n from
0 2 to 5 meq of I -~tosu~f~n;c acid ~unct;~nAl;ty por ~, most ~refQrably
from 2 to 4 moq/g
It will bo una-rstooa th_t polymers, c~n1A;n;ng lar~ of
_ ~ ~a~Lo~lf~n;c acid functi~nAlity on a ~i~en carrior, p~naA~t from a
-31-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
l y l ~ chain, can be preparod by graftin~ vinylsulfonic acid,
propA~ ultone, to th~ p~ t carricr function, and c, v LLing the
gr~ft--d polym~r to matorials _avin~ .;c ~Lo/~ulfonic acid functi~ nl;ty
Catalytically-activo ~olymers in which - was an ionic bond can
~ advantagoouxly be pr~p~r~d 4rom ion-~Y~h~r~SrCI rQsin~ and r~activo
c~ t~ 5J both -- .a~ o and sulfonic acid functions
For ~ _ le, a ~trongly ba~ic ion _- -hA~ ge renin 8uch as
poly(vinylbenzyl amin~) can be reacted with a c __ ' such a~ 4 ~ c pLo-
1,2-h~lt~ ulfonic ~cid to ~roduc-- catalytically-activ-- matorial a8
cp - e~l by th~ equation
CH2NH2
1 HSCH2CH2CH ( SO3H ) CH2SO3H
+_
CH2NH3 03scH2cH ( SO3H ) CH2CH2SH
R.p r ~1 ;ve strons~ly ba~ic ion-~Y~h~-gn re~ins ;~ ~9 DOWEXTM
lX2-400, from T_-- Dow ~ r~l Campany, Amberlyst~M A-21 from Rohm nnd
Haas, DOW35XTM WGR-1, DOWEXTM WGR-2 and DOWEXTM MSA-1, from T_o Dow r~ c~
r _ y Tho WGR re~in~ were ~oly~ropyl~~ conv~ ly obtained
15 by c ~ ti~ of ~pic_lo-~ hy l-in with ~
Catnlytically-active mat. rials can also adv~r~tr;r _~ly b~ prepar-d
Erom an acidic ion - ' __ resin, for _ 1~ l~ulfonated poly~ ne by
..-cti~ with an ~ '-- - A~to~ulf~ ;c acid, for ~ _ le, a ~ o-4-
r~ ~ ~ R~lf~ ;c acid, a8 .~ e~ l~y tho ~quation
-32-
CA 02230272 l998-02-24
W O 97/08122 PCT~US96/13683
,~L~
- ~ S O3H
H~N ~ S O3H
~ SO3 H3N ~ ~ S 03H
S H
Re~ ~L ~tivo 8trongly acidic cation~ h-nge rosins ;n~lu~s DOWEX~
50X2-400, A~berlyst~ A-21, frvm Rohm and ~as, nnd DOWE~M MSC-l, from Tho
Dow ~- ~l ~ y~
In a~dition to tho u~o of ~olym rs from othyl-nically ~ t~ated
- ~, including copolym-rs, as i~oll~le D~O ~D for tho
catalytically-active ~_~c;~, the cat~lytically-active s~oc;e~ can bo
~ttachod to an inor~anic ~v L, for ex~mpl- a min-ral, ~uch as silica,
alumina, al~ ;c~t-~ or ~la~s, throu~h tho l;~;n~ ~roup -~- A
~ ~= ativo ca~- was that whoroin the ~;~;~ group was -osio- or
--O--P (=O)--O--,
mo~t pr-forably, -OSiO-
Catalytically-nctivA __-Ci~Q of F~ III will convA~;~tly be
inco ~t-d~ in tho b~k~o~ of c~ tion polymers; for ~ _le,
poly st-rs, poly ~-~, polyc~ tes, polyurothanos, polysil~
~oly --, polyother~, ~olykotonos, ~olysulfonos and polysulfoxidos Tho
dival-nt 1 i~ki ~ ~roup, -~'-, can bo seloctod from such structuros a~
~olyoxy(alk-di-yl), ~olyoxy(~r-di-yl), dioxy(alkar-di-yl), ~olyoxy(nralk-
-33-
CA 02230272 1998-02-24
W O 97/08122 PCTnJS96/13683
di-yl), polythio(alk-di-yl), polythio(aralk-di-yl), polythio(ar-di-yl),
polythio(alkar-di-yl), polythio(aralk- ai -yl ), polyamido(alk-di-yl)~
poly~mido(ar-di-yl), polyAmido-(aralk-di-yl)~ polyc~rbonyloxy(alk-di-yl)~
polyc~-honyloxy(ar-di-yl), polycarbonyloxy(alkar-di-yl), polycarbonyloxy-
(aralk-d$-yl), polycarbonyldioxy(alk-di-Yl)~ polya~rbonyldioxy(ar-di-yl)~
polycarbonyldioxy(_lkar-di-yl), polycarbonyldioxy(aralk-di-yl),
polyamino(alk-di-yl), polyamino(ar-di-yl), polyamino(alkar-di-yl),
polyamino(aralk-di-yl), PO1YCYA 1; ' ~ ( ar-di-yl), polycy~7; ~~(alkar-di-
yl), polycy~l; ~~(aralk-di-yl), polycarbonyl(alk-di-yl), poly~--h~r~vl(Ar-
di-yl), polycarbonyl(alkar-di-yl), polycarbonyl(aralk-di-yl), ~olyimido-
(alk-di-yl), polyimido(ar-di-yl), polyimido(alkar-di-yl), polyimido(aralk-
di-yl), polyur-ylone(alk-di-yl), polyuroylone(ar-di-yl), polyuroyl-
~no(ar_lk-di-yl), polyur~yl~ne alkar-di-yl), PO1YCA~ y(alk-di-
yl), polyc ~y(ar di yl), P~lyc Y(alkar di yl)~
polyc~ y(alkar-di-yl), polyc- ~ ~o~v(aralk-di-Yl), ar-di-
yl, alkaryl-di-yl, aralkyl-di-yl and A1 ~~ic-di-yl PrQA-rr-d dival-nt
l~k;~ ~roups, -D'-, includo di(cArbonyloxy)l~a ~AArbylone, siloxy,
dirA~ a~v~;_rbyl-ne~ di(oxycarbonyl)hyarv~arbylone,
dithiohy~.ocarbylQne, and Lya v~_rbylono ~roups c~~tA;n;n~ a ~ t;c rinAys
Durin~ batch ~roc~c;~v~ th~ ~tosulfonic Acid c_talyst wa~
~uitably ~ --~t in an amount r~ff;ci~t to ~nable c~ -~t;~n of th-
phonol with the kotone/ald-hyde in a ro-~AhlQ tim~ Proforably, tho
mount of ~tosul~onic acid rAn~os from O Ol o~uivalonts to 2 0
~quival-nt~ of catalyst per l OO o~uivalHnts of tho koton-/aldehydo Moro
prof-rably, tho amount of -- ~A~tC=_l f~; c acid catalyst was from 0 02 to
l O equival-nt of r~o~ l f~; C acid por ~quivalents of
ald-hyd-/k-tono Most pr-f-rably, th- roaction mixtur- will C~1A;~ from
0 03 to l O equiv_l-nt of -~to~ulf~;c acia per oquivnlonts of
ald-hyao or kotone und-r batch p .ce~rin~
Wh-n k-tono/Al~-~~~ wa_ _dd-d ovor th~ cour~- of a ._~At;~~ (for
~ _lo, a c~ ou~ l~-Atj~) tho pr-viously ~t_tod ~reforr~a
r-for to total cataly_t ana roactant~ add-d rath-r than catalyst ~ os~nt
in a reaction mixturo _t a giv~n mom nt Those ~ 1 ~d in th- art
th~t wh-n a r~act~nt w_s addoa in. ~ Ally or c~~t;~ou~]Y,
ther- w_~ oft-n a largQ ~xc-ss of catalyst Tho ratio of cataly_t to
koton-/~ld-hyde in tho r-action mixturo was advA~tA~ ly ~r~at-r than
one, conv~ ~lly on tho ordor of 20 oquiv_lents to 1 oquivalont
-34-
CA 02230272 1998-02-24
WO g7/08~22 PCT/US96/13683
Du~ to th~ high nctivity o4 tho - ~Lo8~l f~; C acia catalysts,
~oOa roaction ratos and high s~l-ctivity can bo obtained at t~ _ ~ture~
below tho melting ~oint of phenol The phonol reactant can advantageously
be ke~t in the liquid state by addition of 801vents, for examrlQ, wator,
methylene chlorid~, ~;rh- _,lm~thane Low t~ _- ~t~ ~ r~actions w-re o4ten
- ~art;cul~ly advantaaeous, bec~n- the ~roduct dirh~n~l;c _ __ a~
crystalli7e in the reaction mixture and bec~ e lower reaction
t~ _~ ~tures 4avor hi~her selectivity toward 4,4-h;~rh~~ol;c rroducts
The r~action t- _ ~turo will accordin~ly adv~t~ ly b- ~elected
10 in the ran~o ~rom 0~C to 100~C, ~re~erably from 15~C to 60~C ~ _~ ~ture
range3 can b~ chosen by routine exper; ~t;~, ~a~er~n~;~ upon thQ
k~tono/aldehyd~ and ~henol _Q-ds
Wh~n oxcess r~~~; c _ ~ wa~ us-d a8 301v nt, th~ t~ _ t~e
~or the .- a~~e-ti~ was adv~ta~ ly selected 80 that the phenol was in
the liquid 3tate In the case of high-melting ~h~ , for _le, thos~
m~lting abov~ 180~C, the us- o4 an inQrt ~olv~nt wa8 ~rQ4errQd
Di~h~nylm~than- has b-~n 4ound to bo par~;cul~-ly us-4ul ~or this ~u _o~e
Other useable inort ~olvents include, but were not limited to, the
xyl~nes, mesitylQne, the d~ . rr, ~1UG ~ -7-~~, toluene, cy~-~-Y~~,
chlorob-~7- -, halo~ ;r~tiC h_dL~.--~n and alkyl~h1h~le~--
having low melting points
I4 a sol~ent/~ t wa~ used, the amount used conv~ tly ranges
4rom 5 mL to 1 L per mole o4 ketone or aldahydQ Pre4~rably, _rom 200 mh
to 400 mL were usQd per mole o_ k-ton- or aldehyd
~he addition o4 wat-r, ~-n-rally in an amount u~ to a o 5
n~ by wei~ht ot total _-ed, was c~ red hi~hly de~irable in
~roc~rs~P _or the pr-yaration o4 b; ~h~ A, bec~ e water low~rs th~
reezing ~oint o_ ph-nol and addition o4 wAtor ~0 - ~; ts th- c~~~-ti~
to be run at low~r t~ ~tures ~ost ~referably, the amount of addQd
water was from 1 ~--c~nt by weight to 5 r~-cen~ by weight of tot~l f--d
The r-action can adv~}a~ _~ly be carri~d out by stirring th~
k-tono or aldehyde and ~ ~tos~lf~;c acid into ~ lten ph~nol in such a
way that the t~ _~ t~-e in th~ reaction ve~sel will not ri~e above 150~C
Th- molar ratio of rh~~ol;c roactant to k~tone or ald~hyde wa8
~dv~t-g_ _~ly nelect~d 50 that at least two molos of ~henol will CA~o
with the kQtone to ~roduce a c~ ~_ ';~g b;~h~~l or higher ro~~~ts
_ Therofore, molar ratios of 2 1 or higher will advantageously be sel~ctod
-35-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/l3683
It was preforred to carry out tho reactions u~inç~ lar~er ~~~r~es of
r~ ; c rez~ctant, up to a~ much ~ 50 molos of phenol per mol-- of ketone
or ald-hyao It will be ~dG ,Lood that tho oxc088 phonol acts as a
~olvent or ~ ~nt, as well as a reactant
Iower ratios of phenol to ketone/ala--hyae wQre 5Jen--rally ~r: ;~A
by An increa~- in th-- amount of by-products formed In tho preparation of
polyph~r~ol~, it has been found that molar ratios from 2 1 to 30 1 of
phonol to ald-hydo/ketone wer proforred Mor- prefQrably, the reaction
n xtur-s will cnnt-;~ from 4 1 to 25 1 molar ratios of l?h--nol to
aldehyac/ketone Mo~t ~r-forably, th-- molar ratio was from 6 1 to 25
8inco the cn~e~ tinr~ roaction was cxothormic, tho r--actant~,
inst-ad of beinsl mixea to~ether all at onco, w r~ o~1 inT~ ly ~v~ assively
mixed tosrother at a spoea ~ep~ r u~on th~ ity of tho cool;n~r
~mployed to ;r~t~ th-- temporaturo of the r--action moaium within tho
optimum limits Aftor the mixin~ of the reactants, th-y werc preferably
l--ft in contact for 80me timo in order to complot th-- c~r~--r n~t; ~ The
duration of th~ introauction of the reactants durinSr a batch p v~P8
conv--~;~r~tly varios from 15 ~u~ to 1 hour
In batch proco~ s, the -~t~ and the catalyst were ~roforably
tho v ~Lly ntirr1 d - -r~;c-~ly to a~sure b--tter mixinS~, and h~nce an
; __ov_d ~paco-time yi--ld
Wh--n tho ~-e~s of this invention was conducted batch wi~e, the
reaction time was aavanta~_ nly in tho ranSro of O l to 20 hours ~
on tho reaction conditions ;r~ 5r tho amount of the cataly8t u~ea, the
roaction t-- _ ~turo, and _ ~clfic r--actants, solvQnts and ~l ,hcL~
The ~ - ~B of thi6 inv ntion can al~o be run in a c~~ modo,
mor- pr-forably by u~e of a ~-ri~ of c~~t;~ stirr ~d tank reactor~,
tho u ~- of which ~ 8 plug flow reaction conditions It was
pr--forr--d to carry out the J?LOC-~8 of thi~ invnntion under c~~ ~u~
reaction conditions
The pro~ure in th~ roaction zone wa3 not critical, but ~r--forably
ranSI-s from O OOl to lO bar (0 1 to lO00 kPa), and moro ]?artiC~ ly from
0 5 to 3 bar (50 to 300 kPa) In many ca~os, it will be pref~rred to
carry out the r--actions under ~ t pre~ure, that was, 1 bar (lOO kPa)
DurinSr the r--action, one mole of wat~r wa8 ovolvea for oach mole of
kotono/aldohydo undergoing the c ~A~ tion with phenol It ha~ beQn
found that adaing wator to tho roaction mixture~ can be adv~ta~ _o,n for
-36-
CA 02230272 1998-02-24
WO 97/08122 PCTAUS96~13683
docrQas$ng th~ moltin~ point of ph~nol Tho w~tor ovolvod during tho
roactions n-~d not bo v_~ by distillation/ontr~; with an inert
~olvont in order to attain hi~h roaction ratQs In sQme casQs, ~ _v~r,
it will be proferrod to entrain and remove water from the reaction
mixturo, in ordor to incro~- reaction rato~
- It has bo~n found that tho solublo ~ ~Los~lf~ic acid catalystscan advantagoously bo ~ v_a from tho crude product by extraction with
water Tho aqueous Qxtracts can be c ~ trated and _c~v~L~a
- ~tosulfonic acid c~t~ly~t can b~ opti~lly recyclod to s~sc_~.L
run~ When tho ph~~lic starting matorial was phenol, a ~olutiQn o_ the
~ Losulfonic acid in phenol was conv~~~ ly l_cov~l~d and was
op~ lly recycl-d without furth-r purific~
ThQ acid c~ t ~tion can b- ~ --~ bQlow tho limits of d - tQCtio
and probably below 1 ppm by wei~ht of acid, by repe~ted extractions with
wat-r Tho _acile removal of eatalyst from the roaction mixturos wa~ a
s;~;fic-~t ~dv~nt~o ov~r tho prior ~rt, usin~ mixturos of C- '~
a~ont~ It was within th~ ~ractic~ of this invention to removQ the
Losulfonic acid by C~ti~ C0~191~ ..L extraction
The time for phasQ ~eparation durin~ extraetion of the acid catalyst
was on the order of 10 to 15 ~ t~F under batch condition~, without a
drag layor Stirring s~-od during tho ~xtraction in a mixor/settler wa~
adjusted 80 as to avoid ~ A~ f__ ; A~,
Th- ~ol~l~ - ~Losulfonic acid cataly~ts can al~o b~ __ v ~ from
reaction mixtur-s by c~L aoLion w~th a uolu~i~ of an alkali motal
Ly l~id-, c~bQ~tn or bic-~ to
In addition, the ~ol~le --c~Lo~ulfonic acid eataly~ts ean be
_ v ~ from rc-~ti~ mixtur-n by F-~;~g the r action mixturo through
column of anion-oY~h~n~o resin or amino rHsin, such as DOWEX~ WGR, from
The Dow ~ e~l C _ y
A wat-r pur~e from the p .~ will c~t~i~ phenol plus catalyst
This purge was adv~t-L_~_~ly t ~-t-d to romovo phenol by oxtraction with
mothyl isobutyl keton- boforo boing sont to a bio-pond for ~i~pos-l
For ;PO1~;A~ of B~PF from r-action mixtures, made usin~ a ~ol~o
catalyst, a phonol/wator mixtur~ was pr-fQrably distillod from the water-
wa~h~a mixturo until thQ wQight ratio of phQnol B~PF was bolow 1 5 1
Most pr-farably, phunol was 1 ~d until th~ phQnol BHPF woight ratio was
from 1 5 1 to 0 5 1 It has b~Qn found par~;c~ ly advantag-ou~ to
-37-
CA 02230272 1998-02-24
WO 97/081Z2 PCT/US96/13683
i
di~olve th- rosultin~ mat-rial in hot methylene chloride and cool the
r- ulting solution to obt~in cryst~l~;~ BEPF
Vory highly purified BHPF accorainsly can b- obtained by a process
wh-roin a ro~ultin~ crude yroduct was waBh-d with water to romove (ES),-~-
(S03H)b; the r-sultin~ ~cid-free mixture wa8 di8till~d to remove phenol and
water until tho phonol 9,9-bis-(4-1~-1 v~y~h-~.yl)fluoren- woight ratio was
le~s than 1 5; th- r-sulting mixture wa~ takon uy in hot methylone
chlorido and th- r-sulting solution was cool~d to yroduco cryst-ll;~e 9,9-
i bis-(4-hyd v~y~hQnyl)fluorene BEPF purif~ed in this way can be usQd to
m~ko ultr~hi~h quality polyc~ h~te rosins
Exc-~s phonol can also be r mov-d by boilin~ the r~action mixturo
t-'ly with wator, optionally with th~ u~e of a wateL- ~c;hl~ organic
301v~nt such au mothanol The nquoous solution was ~e~aratQd oach timo
ana th0 product, then practically yure, wa~ driod Another orfoctiv~
mothoa of r moving ~xcess phonol was by stoam dist;ll~t;~n
The r-action product solution was opt;on~lly then c ~A~t~ted by
_v~Gl~tion and ~ t~ly extractod with boil;n~ wator for the r~moval of
~xc-~8 phonol The product 80 obtain-d was opt;~nAlly thon ~_ ~dL~ll; -'
for furthor purification
BEPF can be isolat-d from tho roaction mixturos in sevoral
additional ways Th- m thod sol-ctod will dopond on the dogroo of
purific-tion d-sir-d, a~ wull as th- ~ ition of the roaction mixturo,
and th~ de~ired production rato
When -~te~ive ~urific~tion was n~-~;-ablo or i~ v~ i~te, tho
mixture, aftor b-ing t_ ~ted to remove cat~lyst, can be troated with a
volume d hot water suffici-nt to dilute th- mixture and brin~
proc~itation of BEPF Alt--n~tiv-ly, the reaction mixtur- c n be addod
to hot watHr nd the ph-nol ~ ~_ a in the form of a water/ph nol
~ t ~Q until the phenol c~nto~t- wa~ lo~ ~d suff;ciont7y to pormit
p..-lp~tation of BEPF from th- mixturo Th- B~PF solids can b- coll-ct-d
and dri-d boforQ use or can be u~od in th- form of a slurry
Whon ~ ro ~ ive purjf~c~t~ of BEPF wa8 d-~ired, the solids can
b- purifiod by pl~v~tation from a ~olvent, for ~xample, ~ ylmethano
or methylenQ chloride
Anothcr mcthod for isolatin~ BEPF compri8es adding to tho reaction
mixturo, at tho ~nd of tho reaction, a solv-nt, bo;l;n~ at a highor
t ~ than phenol, ~nd removing phenol from the phonol/BEPF/~olvont
-38-
CA 02230272 1998-02-24
WO 97/08122 PCTAUS96/13683
mixture until BHPF cry8tallizR8 or precipitate3 fr ~ the mixturo This
method can be carriod out by ~ddin~ ~;p~ ylmethanQ or triis~ ~yl~n~-
to A r~ction mixturo, from which c~talyst hoG be-n oxtractod or 1~ v_d,
prior to distill;ng the mixture Alternatively, the 801vents can be added
to the initial rcaction mixture 80 that the reaction8 wer- run in the
- p ~ -n of tho solvont ~he roAction mixturo wa~ workod u~, by
extrAction, to romove c~talyst and then by di8~ ati~n to romove 801vent
and phenol, until BHPF cryrtAll;~A~ion occurs
BHPF can also be i801ated by adding to a r-action mixtur- ~ solvent,
which boils at a higher t- _~ ~turo th~n ph-nol nnd dissolvos sufficiont
BHPF, in the ~ -e o~ phenol, that romoving phenol from the
ph-nol/BHPF/801vent mixture provide8 a ~~ ~ oll~ti~n, cool;ng of
which cau~e3 crystA~ -t;~n of BHPF 801v-nt~ ~-;n~ tho~e roqui
include ~;ph~nylmethane, diphenyl ether, do~oc-n~, ~arhfhAl~g, I~opar~
(hyaroc~on mixture commercially avA;l~h70 from Exxon Cor~~rati~n) and
trii~l~lh9n7 -,
Further purif;c~t;~n can al~o be A~ h~, aftor removing
c~talyst _rom th_ r-action mixture, by dist;ll;ng to romove phonol to n
1OVQ1 at which BHPF cry8t-71;~~r _rom tho phenol/B8PF mixture The B8PF
~Olia~ obtained can be ;~ol~to~ by c~v-~ti~nAl means and thon further
t ~-t~, for ; _lo, by ~ ~h;ng with water to remove phenol
An alt~rnative method for obt-;n;n~ hi~h purity B8PF compri8-s
removing catalyst from the r~action mixturQ, disl;ll;ng ~henol from tho
reaction mixturo to a ph-nol/BHPF lovel ~uch thAt ~;lu1;~n of the
dis~;llat;~n residue with a ~olvont ;n~l~_~ cry~t-7l;7a1i~n of the B~PF
~rom the phonol/BHPF/~olvont mixturo For ~xam~le, phenol can be r~moved
by di8t;ll0t;~n until th~ dist;ll~ ;~n rosiduo c~nt-;n~d 50 ~ , by
w i~ht o~ phRnol and 50 ~e1~o~t by w i~ht o~ BHPF Methyl-ne chloride,
trii~ ~y~ - or tolu-ne can b- -ddod to tho ro3idue and the
r-~ulting ~o~ut~~n can be cool~d to bring about cry8lA~ -t;~n of highly
purifiod B8PF
Another v ~-~ e for ;~ol-~; n~ ~uro B~PF ~olid from the roaction
compris-~ romovinu the - ~a~tosulfonic acid cataly~t, dist;ll;~ phenol
from the rosultin~ mixture, to produco a ~till r-5iduo, to which addition
of a solvent ;n~--~o~ cryst~ ti~n of BHPF For ~xample, A still
re~idue C~nt~; n; n~ 80 ~ L by wei~ht o_ ph-nol and 20 ~01_-~t by weight
-39-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
of BHPF ean b~ diluted with a solvent, for ~ _ 1Q ~; chl oromethano or
to'ln9n9, to indue~ ery~tAl1;7~tion of B}IPF.
In adaition, B~PF ean be i~olated rrom a reAetion mixture, by
r ~oving tho - ~tosulfonie aeid eataly8t, addin~ to the r~sulting
r-aetion mixturo a ~olv nt, whieh fo J an azeotrope with phenol and in
whieh B~IPF waa solubl-- in the ~ n~o of }7henol, and removin~ phonol from
tho mixturo by azootropic dist;ll~Ation Cy~1~h~"An,~l w_8 . _ 1A y of a
~301v-nt, whieh will form an azeotLv~c with ~henol ana from whieh B~PF will
proeipitate upon eool;~g the still resiaue from tho A~o_~ ~ic
10 di~tillation.
~ A ly, ~henol can bo vea $rom the reaetion mixtur-8 by
addition o~ a solvent, whieh forms an azeotrope with ~honol Aft-r
removing ph-nol by A -~Llo~ie di~t ;llAI ;A~ tho still rosidue was coolod
ana B~IPF cryst~ es out from th~ coolod mixture
In any of tho purif;e-t;on ~ ~ce~ses whieh r-gult in a cry#tAll;nq
produet, th~ eataly~t was opt-;onAlly not 1~ v_d bnfore cryst-ll;~-ti~~ of
product but rathcr oithor tolorated in the ~ od~cL or ~ ~d from the
cry~tal~, for instanco by w-~h;n~ or othor moans w~thin the skill o~ the
art, aft-r ery~tAll;~rtion
In ~ome ea~es, tho e A~ t;~~ of phonol with ~-t~--7 or aldo~d~s
ean b- run in a xolvont, for _le, methylono ehloride, from whieh th~
produet will prseipitate auring the eourso of tho reaetion, as was
do~erib-d in moro d-tail for tho ~ ~ ~tion of b;~h-no3 A
A ,~ at;ve ~olvant u~--d for ery~tA~ t-;on of BIIPF, methylen~
chloriau, can bH ~ d from tho mother liquors by bateh di~t;llAt-~n
and reeyeled baek to the ~ ; The 8till bott ~8 c~nt~;n B~IPF and
methyl--no ehloriae and ean be eooled to roeovRr additional B~IPF. B~PF
ery~tals thu~ formed were eonv~n;~nt-ly L ~_ v~ usin~ a ba~ket eentrifugo
or pres~uro filt-r and ean be roeyeled baek to a main ery~tAll;~~ Crude
mothor liquor ean al~o be reeyclod baek to the phenol ~v~l~o~nt~ s-ction
When methyleno ~hl~riA~ was used as ~olvont for crystAl~ sJ B~PF,
a common vent heaaor for coll-_Lin~ all vent8 from ~torage tanks and
~afety r-lief ffystems was ~c ~-~ Tho vont headnr systom
advAnt-~ ~ly ;n~l~A-- a flow mea~ davice in th- inlet to a carbon
aa~orption unit and a VOC analyz-r for the ~xit ~a~ The ~xit ~ ~hould
C~t-~;~ le~8 than 100 ppm of mothyleno chloride A com~lote effluont
troating ~y3tem will advantageously in~ A~ mean8 for removing or~anics
-40-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
from procoss wAters _nd me~n~ for r mov_l of ~_rtic~lAte~ from vent ~_8,
~or ~xAmpl~ a wAter venL~.- flow m t-r to scrub p~rticulAt-~ frcm the
vent he_dcr
A further advanta~o o~ the cataly8ts u8ed in the practice o~ thi~
invcntion W_8 th~t th~ catalysts c_n be used to i - 70 th~ cru
proauct mixturo, which typically c~1-;n~~7 (4-Ly~-v~y~h-nyl)(2-
hy~hv~y~honyl) ~ , tho major bi8-(4-hy~lv~y~h_~yl) = __ a~ and
c~n~on~-t-~, to p v~ce mor- of the bis-(4-Ly~ v~y~henyl) } __ '~
T - - 7'ti~n o~ A ~_ nAl ~;~h~n~l u8in~ the cat~lysta of tho
1~ invontion wa3 usoful wh~n pr_ctic-d with tho ~ r~ of th- invention or
~epar_t-ly, for example, aftor product formation usin~ catalysts within
tho skill of the _rt For in~tance, a mixture o~ p,p-
bi~(Ly~ v~y~ yl)fl~oreno ~na o,p-bis(Ly~Lv~y~h_~yl)fluorene w~
contactod with a ~ ~tos~l f~n; C acid c_taly8t, preforably of the Çormula
(HS)A-~-(8O3H)b or of formula (a)
(HS),-~ -(SOIH)b Fr 1A (A)
L
in which ~ wa~ an _lkyleno, cycloAl;phAtic, aryl-ne, alkyl-nearylen-,
alkylonecy~loA7;rhAt;c, _lkylencaryl, het-rocyclic or alkyleneheterocyclic
r-siduo; a ana b wore ;n~ __ '-~tly ~ lect-d ~rom ;n~ from 1 to 20S L
W_8 an optional 1;~; ng ~roup and - w_s a bond, which c_talytically-
active ~pe~ia~ was attached by tho bond - to _n ;n~cl~le or~anic or
inor~_nic ~vl~; ana h-atod ~fficion~ly to rosult in ~r t;~ of the
p,p (bis (4-l-y~-v~y~h_~yl) prOauct from at l-_st a portion o~ th- o,p (2-
L~l.v~y ~ yl, 4-Ly~v~y~he yl proauct Th- catalyst was a ~
(~n~ ;~y polym r) of the ~~ 1A (HS)a-~-(SO3H)b or of formula (A),
proforably tho~o pr-forroa for the roaction of an alaohyae or kotone with
a phonol a8 a-~criooa horoin, with 3 ~ ~apLv~ _- -L~lfonic _cid most
preferroa It was notod that ~uch catalyst~ r-sult in conv~~;en~ly fast
_ , 7~; ~n and less f~ ti~n o~ additional Ly-~rv~cts than do acias
~uch a5 meth_ne ~ulf~n;c acia ~ t~ e8, which will vary with the
~ boin~ ;~ ed, were suitably any t- __ ~turo at which
;~- - i7-t;~n takes place, and wer- convon;~n1ly at loast room t~ ~ture
(30~C), pre~-rably at lea~t 40~C~ ~ re pre~orably at loast 50~C The
t- ~ture was pre~-rably lower th_n that t _-~tur- at which an
-41-
.
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
i--ablo amount of additional by-~roduct or polym~r would form,
conv~ ntly 1O88 than 100~C, more pr~ferably leRs than 85~C, most
pr--f~r bly 1Q83 than 75~C Sovonty e'~_ _85 C~r~tisTraae was a conveni ~nt and
~r--f--rr~d t~ _ -- tur~ It was Eound that incr~asinsr tho t~ _ ~ ~turo
within the rang~ speeds isomerization I ik~wi~e, in~ thQ
C~ ' ation of cat~lyst in~ R i r -I 7atir~ Catalyst was
pr~ orably ~, o~ ~L in any amount surficient to result in ir - 7~
I--~; waEI no~d~d at highor t~ ratures to obtain a ~ lo rat~ of
~ ~1. 7~ TI, Conv~ tly~ tho catalyst was l?-Q~ in tho same ~ R
ana prof~rr~a rnng~s as for proparation of a bisphenol by the ~ r~g
aisclosQa herein The ~r--ssure was not critical, but was al~o
conveniently within the ~reforrQa rang-s for the ~roc~ss of }~roparing
b:8F~ -18 by th~ p ~c~r~ hor~in ~I!imo for the ;R a -t;r- was
proforably that in which, undQr the conditions, rQsultg in ;r :7-1 ;~n
15 of at l-ast a ~ortion, ~re prof~rably a pr--dat~ or aosir d fraction
of th-- o,p by-product b--inçr i~omorizQd to the dQsircd ~,~ yroduct ~ore
pr-forably th~ ratio of o,~ to l?~P product was le88 than 0 12, ~st
pr-fQrably 1-88 than O 1, ~ven morn prof-rably 1O88 than 0 075 Th- time
to achi--ve this rosult varios with ;r~ --.7~t;~ conaitions but was
20 conv~ y 1--U8 th~n a day, mor~ ~rof-rably 1~88 than 12 hours, most
~r~f--rably 1--~8 than 8 hours, for cvl~v~Ldion of half of the o,p ploa~;L to
l?~F product,
Th~ -~ ~Lo~ulfonic acid cataly~st ~ of this invontion woro
c~ ably 10~5 corrosiv~ to 81 irl 88 st--Ql than th~ mixed catalysts
25 u~d proviou~ly Corrosion rat-s for st-;r~ s sto~l, below 0 00254
cm/yoar, havo be~n measur--d Th~ reaction mixturos wor~ boli~vod to b
sub~ ;Ally frOQ of halido ions, whor--in "subs1-r~ti-lly fr~o" moans 1~88
than 5000 ypm of chloride ions
ThQ ~ -8 of this inv~ntion wa~ advAn~ ly carri-d out undor
30 conditions ~uch that tho c r~ of chloride was below 5000 p~m,
pr--l~--rably b-low lO00 p~m, ~fft ~r-f~rably b~low lOO ppm
It wns b ~ vQd that th~ low corrosion rate was r~lated to tho
--9 of minoral acids, such as L~ochloric acid or sulfuric acid, ~rom
th~ r--action mixtur--s The oc~ o of corrosion in roActions u~zing
35 minoral acias has b~on notod by Kn--b--l et al , '594 and Fal~r '995, supra
The insoluble catalysts of this invention can b-- filt--roa from tho
roaction mixtur--s, washea with a mixturs of k~ton~/aldehydo ana ph~nol,
--'12-
CA 02230272 1998-02-24
W O 97/08122 PCTAUS96/13683
and recyclod to ~u~ run~ ~lt~r~t;voly, the insoluble c_talysts
wRro usea in fixod beds ~nd tho c~ ti~ of ~J Q with
~ldohyde/kotone w_s done in C~t~ uQ up_low, cross~low or ~
f~Qh; ~n, When fixod-bea catalytic rQnctor~ woro u~od, th- catAlytically-
~ctivo r-~ins ro~ain in tho rosin beds and need not be 1 -v_d
_ Furthor _ o~ of thi_ invention will be det~- ~~d by tho
r~_ct~nts u~ed, the catalyst sQl-ctea, tho ailuont, if Any, ~na the
roactor ~m~loy d
For _lo, when usin~ _ solublo c_talyst for the co~ tio~ of
phonol with 9-fluo Onv~e, without a ~;lu~t, other than xcoss ph-nol or
in the ~r-sence of ~ diluent which does not cauno prOcipitation 0~
vd~L, it will ~on-rAlly b- proforr~d to uso A hi~h r_tio of ~henol to
~luo ~nvne to ~o selectivity to the desired ~;~ph~l product
A p_rti~ulA ly preferred proc-ss was on~ wher~in tho ~ lar rAtio o~
15 phenol fluo ~ o e wa5 from 4 1 to 25 1; tho r~action t~ _- ~tur- was from
25~C to 50~c; tho catalyst w~s ~t~ ~ fonic _cid or
-~pLoku~ _lf~;c _cid, u~od in an _mount from 5 to 10 molar ~ cc L
with e~e L to fluGLO~on~; the ~ .-PFS was carriea out undor ~ ' ~t
pressur- or undor vacuum to r~move wator of roAction _nd incrOasO th~
reaction rato; no cosolvont was usea; the catalyst was 1~ v_d from the
product by ~xtrAction with w_ter using a w_sh colu~n or by b_tch
extrAction; the wat~r ~xtr_cts thus obtA;~d w rO c -- t.~ted ana
r-cycled to th- procoss; the proauct was ;~o~At~d by removing excess
~honol to a woi~ht ratio from 1 5 1 to 0 5 1 of ~henol BEPF _nd the
product was procipit_ted with ~ ~hl oromethano
When an ~ol~le catalyst was u~ed, a part;c~ ly ~reforred
y ,-~8 was ono whor-in thc molar rntio of phenol fl~G, ~ was from 4 1
to 25 1; tho c~--ti~ w_s carri-d out at a t- _ ~ture from 40~C to 60~
C; no cosol~ent was u~ed; the catalyst was PNBSA; the c ~ ;~ was
carriea out in a c~ plu~ flow roactor; the roaction was carried
out at ~ pr-ssure or und-r ~o -~ s~u~o to r move water of
r-action and in~ the reaction rate; the product was ;~ol~tod by
rOmovin~ excess phenol to a woight rntio from 1 5 1 to 0 5 1 of
phenol B~PF nnd the product was ~ __ pitatea with ~;~hloromethane
The ~ .-SE8 for making B~PF can also be carried out at molar ratios
of ph-nol flu~ _ one from 7 1 to 5 1 in the ~e=- -~ of 0 05 to 0 15
~quivalont of ~PSA or ~BSA por molo of fluorOnon~, whor-in methyl-no
-43-
CA 02230272 1998-02-24
WO 97/08122 PCTAUS96/13683
chloriao was added to tho roaction mixture a~ter CO-lv_~ion of at loa~t 20
p~ ~L Or flu~uv o has OC~ul .od; ~t;~ tho resulting mixturo under
~~ ~ea prossurQ to r ~ ove an azootropo of mothyl~ne chlorido and wator;
and cool;~g the mixtur- At tho ena of the c~~Ao~-ti~~ reaction to caus-
5 precipitation of BHPF
Th- c~ ti~~ of phunol with fluol ~ can further be carried
out u~inD a f-oa c~~t~i~;~ from 5 1 to 3 1 molar ratio of
ph-nol rluo O - - and from 0 05 to 0 15 equival-nt of MPSA or MBSA per
molO of flu~ _ ~c, ~;l~ d with from 10 porcent by woi~ht to 30 pe~L
by woight of mothylone chlorido Cryst~lli~ BHPF can bo collected from
tho coolod r-action mixture
In addition, BHPF can be preparOd from a reaction mixturo,
c~t~;~;n~ from 18 1 to 12 1 molar ratios of phenol fluor.luono and 0 025
to 0 075 ~quivalont of MPSA or MBSA por molo of fluo Ono~O at a
t~ ~turo from 50~C to
80~C, whoroin tha mixture at tho ~nd of thn roaction was ~;luto~ with 10 to
ao volumes of wat-r to ~xtract ~ ~Losulfonic acid catalyst, tho thus-
washod mixturo was distillod to a phonol BHPF woight ratio from 1 5 1 to
1 1 And cool-d to bring cryst~ t;~n of BHPF Tho cryst~ll;~A BHPF was
~ 20 ~ v~ by ~ilt-~t;o~ and washed with mothylon- chlorido and thon with
wat-r
A proc-ss in which th- product was procipitatHd in th- roaction
mixturo was pro~Orr-d _or the ~reparation of k;~ 1 A, morO
par~ ly a ~ c-e~s whcrein th- ph-nol ncotono f-od c~~;~ea from 6 1
to 15 1 molar ratio~ of phonol ~eto~; th- ~ ~ ti~~ was carriod out
at a t~ _ ~t~ e From 25~C to 35~C~ tho roaction mixture c~~~ up to 5
c_~L by woi~ht of water to low r tho LL~ ~oint of phonol; the
cataly~t wa8 3 ~L~ ~ lfonic or 4 ~0Abnt~ ulfonic acid in
an amount from 0 05 to 0 50 equival-nt per molo of acotone in th-
~-~t~~- ~-- -1 fo-d; tho r-action was carried out undor ~ ~t prossuro;
ana th- cry~t~ll;~e bisph~ol A proauc-d by the ~ =~rs was lO v~d by
filtration or contrifu~t;~
Furth-r proces~in~ can include , ~h;~ tho bi~FhA~~l A with water to
partially r movo solublO catalyst, nnd romovin~ additional solublo
cataly~t by tlO~ ~ with an anion o '~ r-sin It was beliovod that
a proforr-d r-actor configuration for thi~ procoss was a ~erios of
-44
=_ _ __ _
CA 02230272 1998-02-24
WO 97108122 PCT/US96/13683
c~~t;nus~ stirrod tnnk roActors, 80 as to ~1~ mate plug flow reaction
conditions
Other process vari~t;o~ l A~, but wcro not limitod to
(a) ~ro~_ratiou of bis~henol A in n-at Dhenol~ using ~ solu le
cat~ly~t, with ~r-cipitation of bi~h~~ol A in the r-action mixturo ~nd
_ (b) preparation of b;~Fh~n~l A in ~henol with a _ _l~Y-formin~
cosolvent and soluble catalyst, with ~rocipitation of b; ~Fh-~ol A in the
roaction mixturo
~oro p_rticularly, it was preferred to s~lect a cAt~lyst whoroin at
l-~st 99 ~c c~nL o~ the bis~hon~l A thAt cryst~ es durin~ the r~action
was 4,4~ ~h~~l A 8uch c~talysts ;~lu~ sol~hle o~Lo8ulfonic
acidY in which A and b were ench ;~A7~l~A~ 1y ~nt~_~L4 from 1 to 4
Pr-ferr-d conditions include reaction t- _c ~turos from 0~C to 50~C, moro
pr-f-rably from 20~C to 40~C
R-pr~ ;ve _ _~Y-formin~ solvents for bi~h~ol A ;n~ll~s
di-thyl et~or, ncetone, ot_anol, p ~ol, ~;~Y~n~ acetic ncid,
~ ~n;trile, methylene chloride or c_rbon to~ hloride The _ 1-Y-
f~~ '~ solvont~ - _1~Y ~rofor~l;Ally with tho 4,4-A;ph~n~l;c isomer 8e
that tho ro~ultin~ compl-x ~as solubility ~~ Lies, di_ferin~ from that
of tho ~ _ _1~YAd 2,4-~;~h~~~l;c _ _- ' ana can bo roadily ~opAratod
therofrom
Thoso ~roc~~e~ can be run undor varyin~ ~rossuro and t~ _- ~ture
cona~tions, n~ woll n~ roactant, co~olvent and catAlyst c~ ation~, as
can bo ~t~ ~d by routino ~xper; at;~
In ono ~-ct, a ~ st preferrea ~ ~-~~ of this invention was that
- _'~ tho kotone was 9-fl 'OL. -, tha ~henol was unsubstitutQd and the
~L wA8 9,9-bi~-(4-LyalvAy~h_~yl)fluorone; the molar ratio of ph0nol
to fluv_~v o was from 8 1 to 25 1; the roaction mixture c~~t~ from
0 05 to 0 20 equival-nt of ~ osulfonic acid ~or ~ le o~ fluoreno; the
- _~to~ f~~;c acid _ __ ' was 3 J ~t-o~ v~l~~~_lf~;c acid or 4-
-~Lo~lt -_ 1f ~; C acid nnd the ~rocess was carried out at a tem-
from 45~C to 60~C
An equally ~referr-d ~Lcc~rs was that wherein the ketone was
ac~tOno, tho ~henol was unsubstituted and the ~roduct was 2,2-bis-(4-
35 L~d~VAY~ - ~ yl)~vp~n; the molar ratio of phonol to acetone was from 6 1
to 15 1; th~ r-action mixture ro~t~ from 0 10 to 0 50 equivalont of
_ ~-q~LO~ fQ~; C acid ~or molo of acotone; the - ca~tos~l fQ~; C acid
45-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
__ ~ was 3- --~pL~ ~osulfonic acid or 4 ca~tohut~ fonic
acid and tho procnss was carri-d out at a t~ ~ture from 15~C to 60~C
Without ~urther ol~boration, it wa8 believea that one skilloa in the
art can, u~in~ th~ ';~ descri~tion, ~t;l;~e the pres-nt invention to
the fullest extent The following preferred spocific _a; ~~ ' p wero,
therofore, to be construod as merely illu8trative and not limitative of
tho ~ r of the ~;~~1O8~ ~ in any way what~oevor
In the followin~ ~ _lo~, the t~ _ ~turos were set forth
.co~ L~d in ~ Cel~iu~ Unless otherwise indicatod, all parts and
0 ~GlC ~l r~eP wore by weight
Roactor Desi~n 1 A 500-mL roactor ~repared from PFA Teflon~
(matorial from DuPont) was fitted with a ~ _~le port, water
c~e~oer topped with nitro~en inlet, --~;cAl stirrQr, drain ~ort, and
u _~;~ port ~--t;~ was provided with an infrared hoat lnmp and tho
t- _ ~ture was controlled with an ~1~_L o~ic t~-- ter/t- _--~L~
controll-r
Reactor Design 2 A cap~ea 4-dram ~lass vial with a ma~netic
~tirrer Hoating was rogulat-a by P1A~; ~ tho vial in a t- _- ~L~
controlloa Al ~ block h-ator
Reactor Design 3 A 100-m~ jacketea glass reactor wa8 fitted with a
1 h- -tor port, ma~notic stirror, nitro~en inlet, ana ~ _1;~ port
~q was provia-d nnd the t _-l~turo was controlloa by circ~ ;~g
glycol sol~lti~ o~ the a~ iate t- _~ ~turo throu~h the j~kete~ fla~k
u8ing a Ne~lAb ~odel RTE-220 circ~l~t;~g bath
P---ct~-- De~ign 4 A 1 5 L, 2 L, or 3 L jacketoa srlass roactor
fitt~a with n ~ - ~r/n~ 5J ~ort, nitroSr,n inlet, and ~ ~~; r-
~tirrer Ps~t;rg was ~rovidod and th ~ tA _ - ~ture was controlled by
circ~ t;r~g SJlycol loluti~ of tho A~ iate t~ _- aturo, through tho
jackotea flask u3inq I No~lab Modol RTl!-220 circulating bath
Analytical M-thoa 1 A Vari ~n HPLC Syst m (Modol 9O10 ~olvent
deliv--ry ~y8t-m, Model 9095 ~t~- _ l~r, ~odel 9065 Polychrom dioaQ array
dotector) intorf Iced with A Varian 8tar workstation wa3 u~sod for ~naly~i
Aroa ~ - naly~i~ was re~ortad at 282 nm raL c~, v~ ,ion was
~et -~ by an ~t e~l standard method usinSr calibrated c~ _ ~tion
35 curv-~ for each major -_ _ Analytical HP~C _ ler were yre~arQd by
caro~ul quantitativo dilution of roaction ~amplo~ (rang 400-500 times
ul;" ) Column Wat--rs Nova-Pak C-18 (60 An~strom, 4 micron, 3 9 X
-46-
CA 02230272 1998-02-24
WO 97J08l22 PCT~US96/13683
150 mm) CL~ to~ _~Ly conditions ~low rat~ 1 0 mL/min, ~olv~nt
~radiont (solvont A = w~t~r, ~olv-nt B ~ acotonitril~) 0 n~t~R 65
~_ ~nL A/35 ~c ~e t B~ 9 ~-t9~: 60 ~ c_~L A/40 ~Ql.- B, 18 nllt '8:
55 ~Q ~c~L A/45 Q8lc~L B, 24 n~l1~ 45 ~ c~t A/ 55 ~nL B, 48
nl~tQ- 5 ~ClC ~ A/ 95 ~e ~l~t B, 52 n--l o~: method Dnd (10 ~t~
equilibration b~for~ ~nd n-fter run~)
Analytical ~thod 2 A Hcwlett-Packard HPBC system (Moa~l lO 84B
~olv-nt d~livory sy_t m, Mod~l 79850B ~C t~~ ) was usod ror analysis
Ar~a ~q ~nt analy~i~ was r-port-d at 254 Dm~ r~ ~nt c~v~L~ion wa~
dot ~n~ by ~n _~t~ l standard m thod usin~ calibrated co~nl-ation
curvos for oach major _ - ~ Analytical HPLC 8 ~ w re ~roparDd by
caroful quantitativ~ dilution of r-action n~mples (r~ng- 400-500 times
dilution) Column Wators Nov~-P~k C-18 (60 An~strom, 4 micron, 3 9 X
150 m~) Chromato~raphy conditions ~low rat~ 1 0 mL/min, solv nt
~radient (~olvênt A 8 water, 801vent B ~ ac-tonitril~) 0 n-~ : 65
L A/35 ~ ~L B, 9 n~t~ 60 ~CI ~t A/40 ~~ L B~ 18 ~n--t~P:
55 pOrc-nt, A/45 F- - B, 24 n~t~~ 45 ~_l~nt A/ 55 ~ t B, 36
~"tqE: 25 L ~ ' A/ 75 ~-~CQ~t B, 38 n~-~e~: 65 ~Q'- , A/35 ~LC
B, 38 nnte~ method ond NOTE This m~thod ~ive~ a - llor ~
(~ Jy on~-half tho area) ~or th- 2,4-B~PF and th- two thr-o
adauct B~PF po~ks r~l~tiv- to th- 4,4- B~PF peak than oither th~a 1 or
3 usin~ th- diodo array detector
Analytical N-thod 3 A Varian HPBC ~ystem (Modol 9010 Eolvent
delivery ~yst m, Nodel 9095 ~"t-_ _ lor, Mod~l 9065 Polychrom diodo array
dotoctor) ; n~~ C~C - d with a Varian 8tar workstation was u8-d for analysi8
Ar-a A ~_~L analysis was r~ortod at 282 rm re~L cv~v~l~ion was
dot- ~~ by an ini ~ - n~l standard method u~in~ a 801~t;~n of 0 0508
w i~ht ~--c_~t acet~L~ 9 in 60/40 (w~i~ht/wei~ht ~_,~ )
h-nol/wator for ~ro~arin~ the r _lcr Analytical ~PLC ~amples w r-
~reparod by careful quantitativn ~;luti~n of reaction _~ Column
Wat-rs Nova-Pak C-18 (60 A~L, , 4 micron, 3 9 X 150 mm)
CL -to~ ~Ly conditions flow rat- 1 0 mr/min, ~olv nt ~radi~nt
(solvent A 5 wat-r, solvent B ~ methanol) 0 n--~ 55 ~Cl ~-t A/45
~c~ B, 20 ~n~lt~8 15 ~ L A/85 ~c, B, 25 ~n~t~L: 10 ~c,~- t
A/90 ~ B, 30 n--t~: 55 ~ercont A/ 45 ~ ~ B, 35 n~t-r
mothod ona (10 n~t-n ~quilibration befor- and after runE)
-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
Analytical mothod 4 Tho ~xp-r; t-l sotup of Mothod 1 was u~ed
Th~ cL_~ to~ ~Ly conditions w~rH flow rato 1 mL/min, solvent gradiont
(solv nt A - wat-r, solvent B = mothanol) 0 ~ t~ 55 ~crc~nL A/45
pcl_cnt B, 20 minutos 15 percont A/85 pe - B, 25 ~teB 10 ~
A/90 ~CL~ - B Analyni~ - T~ l ~tandard method using 0 0508 pGLC
acet~r ~ in 60 percent mothanol/wat-r Av-ra~e r-lative standard
d~v~ations ran~cs from 1 to 2 ~c , ~o~ u~on p-Ak analyzed
Analytical Method 5 The r~action mixturo was ~ 7t~ with
ac-tonitrilo to a c~ ration of 0 01-0 1 ~c - by w ight of
- _ r and tho dilutod sample was analyz~d by HPBC on a Wat-rs NovaPak
C18 column (10 16 cm x 0 635 cm inner ~;~ -tor~ connect-d to a Varian~
9100 7JV det-ctor, s~t at 280 nm The column t~ _- ~L~ o was 30~C, tho
prcssur- wac 140 (14,000 kPa) at 0 ~t~, tho absor~tion full scale for
tho d-t-ctor wa~ 2 0, th- i~_ ~tor att~tj~ wa~ 3 and tho chart spood
15 WaB 0 5 cm/ ~ to~ ~he auto sampler inj-cts ao microlitors of sampl~
onto tho column ~v~ry 36 ~to~ Reservoir A c~~t~ o wat-r
and ~ v~ir B ~P~C ~rado ac-tonitrilo Th- followin~ protocol was usod
Time Flow RatQ %B
(min) (mL/min)
0 1 0 40
8 1 0 40
1 0 60
26 1 0 99
1 0 40
T~o poa~ ar-a ~on-rat-a by ~ach _ _ in th~ ~amplo was u~d
with its known ~ iactor, and th- dilution ratio, to c-lc~lat- tho
c~ ~ations of ~ach _ _ ~ in thc ~amplo ~o~uli~
F1UG ~ - (A1driCh 98 perc-nt), about 0 5 ~ - fluorono and
mothyl-fluor-nos
Acotone (Bakor lh~~~t, driod ovor ~ uiov-~)
D~p~ ylmcthano (Penta T~t_r~ , 99+ porcont distilloa grado)
Phenol (Dow ~' 'C~l 99t ~_ .onL), about 100 ppm ~2~ ~ 10O ppm
impuriti-s
8oaium 3 7_~Lop o~ fonato ~ource A 90 ~_ ~L purity
(Aldrich)
~ourc-- B 90 ~._ ~urity (Raschig Corp )
3 ~_~;"~Lopropr~ fonic acid (MPSA):
-48-
CA 02230272 l998-02-24
WO 97/08122 PCT/US96~13683
8Ource A: Prepared from 90 porcent Aldrich sodium
3 - ~ptoprop_nosulfonata ~y roaction with H~l or L ~-~ in
ion _~ column
urce B: Preparod from 90 percent R~schi~ Corp. sodium 3-
~ oprop~nesulfonato
_ 8Odium 2-mercaptoelh~ lfonate: 98 ~ ~L ~Aldrich)
4 ~~- ~t~b~t~ fonic ncid (MBSA): prepared from
1,4-~utAn~ultone (Aldrich) by r-action with NaSH, Ba(SH)2 or an alkali
metal ~h;oAcotate in _ccord_nce with R. Fi-cher, su~ra, A. Mu~tafa, supra,
or Chom. Abs., 90:86742m (1979).
2-Benzyl-4 - ~ hutA~sulfonic acid: prepared from
1,4-~utAn~-ultone (Aldrich) _nd benzyl bL~ A9 in accordanco with M. s.
Smith et al., "Lithium ~1 Hy~rido_~l- ~ydrido Roduction o~
8ultono~ J. Org. Ch~m., Voluma 46 (1981), Pa~e3 101-106 or T. Dur~t et
al., "M~t~llA1ion of 5- ~nd 6~ ring 8ultone8,~ C_n. J. Chem.,
Volumo 47 (1969), Pa~08 1230-1233.
2,3-D~ ~ C~PLV~L~L -aulfonic acid: ~ro~ared from ~odium
2,3-A; - ~ L ~ lf~-te (Aldrich, 95 percent) by noutrAli-~ti~~
with HCl or ~ ~- with an acid io~ hAnye re8in, for ~~ _le, DOWEX~
MSC-1, from The Dow ~- '~-1 C _ _~.
a, 2-Bi~( ~tom~thyl)-1,3-~L~~~A;~--lfonic acid: proparod from
2,2-bi~(b ~ thyl)-1,3-p ~ ;ol (Aldrich, 98 ~rc ) as follows:
A mixtur- of 2,2-bi8-(b ~ -thyl)-1,3-prop-n~A;aulfonic acid (200.0
g, 0.764 mol, 1.00 ~quivalent) and ~odium sulfito (211.7 g, 1.68 mol, 2.20
oquivalHnts) in 500 mL of ~ wat-r wa~ All_ ~ to react undor
reflux (108~C) for 28 hours. At this time, additional 80dium sulfito
(105.9 g, 0.840 mol, 1.10 oquivalont) was addod and the mixture was
~1 1, ..e~ to roact for 3 adaitional days under roflux. At this point, the
mixture consi~ts of a clear solut;~n and a c~naiAerA~lo amount of solids.
Tho mixture was coolea to room tA _~-t~-. and ~aturatoa with
g~ 3~ dh~n chloride. An ~xoth-rm to 43~C wa8 ~b-~ v_~. The mixturc
and yollow in color during t~o oarly sta~ 8 of ~Cl
adaition. A3 the mixturo ~ aturatod with HCl, a vol ~n~u~ whit~
precipitate was formed. The ~olu~;~n was cooled to room t _~-~t~.a and
filt-rea to r move ~olid Yalts, which woro ~rimar~ly 30dium chloride and
~odium L '~. Water wa~ ~ v~d from tho filtrato to provide 2,2-bis-
-49-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
(Lyd_v~y thyl)-1,3-QLv~A~A~;~ulfonic acia (190.7 g) as a hi~hly viscous
amber oil (~lass)
AlternAtiv~ly, the rc_ction mixture can be work_d up by dilution
w~th 200 m~ of ~thanol or methanol, after which the 801ia was v ~ by
filtration 801v_nt was ~ v_a from the filtrate on a rotary ~v-~o~-tor,
to ~roduce a white solid CA'~ J' mainly A;-C~; 2,2-bis-
(1,,".1 v~y ~ hyl)-1,3_pro,AA~ ; UlfA---te. C~~ ted hydrochloric acid
c_n be added to the ~olid product to give the soluble aisulfonic acid,
plu~ insoluble sodium chloride and soaium b ~-
~-Xyl-no (400 m~) was rddod to the 2,2-bis-(l~a~v~y thyl)-1,3-
prop-~ ulfonic acia _nd the resulting two-phase mixture was heatea
under rHflux (135~C to 150~C pot t~ _J _ture) to remove wat~r, ~roduced by
the d hyd~-tion, in the form of an A-A_L-v~a in a Dean-8tnrk tra~ A ter
8 bours' ~-~t;--g under roflux, thc mixturc wa~ All~ _~ to cool to room
t~ _- ~ture and the u~per xylene phase was d~-cA~to~ from the lowcr viscous
o_~L pha~e Wat-r (300 mL) was added to the coolod, lower ~ha~e
c~ 2,2-bi~-(lydlv~y thyl)-1,3-~ ~ u~fA~;c acid bis-sultone
to ~Lvd~_C~ a large ma~s of whit~ solid The wh~te solid (bis--~ultone) wa~
_ ~. J by filt-At;~~, --lurry washed ~ ively with water and with
methanol rnd driea in a vacuum oven
To a ~c3~ti~~ of ~odium ~;r--~~~-~e (9.6 ~ 114 mmol, 2.6
~~uival--nt~) in 30 mI of watar was alowly added th; ol~cetic acia (7.5 2"
96 mmol, 2 2 equivalont~) The r-sulting olution of soaium th;ola~tate
wr~ added to a nolution of 2,2-bis-(Lyl v~y thyl)-1,3-~ o~ ~~';sulfonic
acid biu-~ultone (10 g, 43 8 mmol, 1 00 equival-nt) in 280 g of
acetonitril- After all th~ 1 h~ olA~tate was add d, the resulting mixturo
wa~ ' to stana ov~r~;g~t at ~ ~t t- _e -ture 801vent was
lV _~ r u~in~ a rotnry _v-~vL-tor to ~ivo 19.6 ~ of ring-opened bis-
(th;oac~tatc) adduct as r tan, flaky ~olid
Th- th;-~A--tato adduct (18 2 ~) was hydrolyz-d by stirring ov~ ht
~t ~-~t t- _ tur~ in a nitrogon-~aturat-d mixture o_ 10 ~c~nt
~od~um l.~d_v~ do (20 g) and 100 g of wat-r The mixture was A~-i~;fiod to
3 w~th 10 parc-nt a~_~ ~ Ly_lvchloric acid sclutiA~ 801v-nt was
_~ v-' from the r-sulting mixtur- in a fumc hood, using r rotary
~v~v -tor The r-siduc was di_solved in 50 mL of water and ~aturated
with Ly~_ _ chloride ga~- The resulting solid salt was removed by
filtration and tha filtrate was c~n-e~rat~' usin~ a rotary ev_~ol-tor to
-50-
CA 02230272 1998-02-24
WO 97/08l22 PCTAJS96/13683
g$ve 2,2-bis-(mercaptomethyl)-1,3-p v~ao aisulfonic acid as a viscous
dark-colored oil
Altnr~A1;vuly, tho ~h;o~c~tato adduct can bo hydrolyzed by stirrin~
with c ~ ~t~d hydrochloric acid, removing the 801id s~lt ~roauct by
filtra~ion and rQmOving water from the filtrate u~ing ~A rotary ~v_~G _tor
~1~;V ' ATIONS
Dm = ~~- ~tors
uv ~ ultraviolet
r~m ~ revolutions per minute
mmol ~
HPLC ~ high p~o~u~e liquid chromato~ y
BH~P ~ 9~9-bis-(~-~y~ vAy~henyl)~luor~no - 4,4-inom~r ~ BF
MPSA 3 ~ A ~ ~ 1 ~lfonic A cid
~BSA ~ 4 ~A~t~u~ ulroAic acid
FN = Fn = 9-~luv ~-~vn2
2,4-isomer ~ 9-(2-hV1~VAYL-- yl)~9~(4~1~yd v~y~honyl)fluo~ono
DPM - di~henyl '-h
BPA ~ 2~2-bis-(4-Lyd~y~honyl)~v~aae - bisphonol A
n/d 8 not d~t~- ~
~ = about or a~lV~ te
ESAMPliE 1
CUN-- ~4A~ION OP 9_FL~ '~ W~TH PHENOL (3-MERCAPTOPROPANES~LFONIC ACID)
9-Fl~v ~ ~e (20 0 ~, 0 111 ~ 1, 1 0 ~quivalont) and ~ lten phenol
(156 7 g, 1 66 ~ 1, 15 0 oquival-nts) woro addod to a 500 m~ PFA Toflon~,
mat-rial ~rom DuPont, ~-~tor (reactor dosign 1)
Tho r-action mixture was hoated to 65~C with stirring at 300 to350
r~m undor a ~ad of nitrogon 3 ~ c-Itv~ _ ~ s~lfo~;c ncid (0 864 g,
5 53 m~ol, 0 0498 ~quivalent~) was added ~lowly over ~ ~ t-~y 1
minut- to tho roaction mixtur- at 65~C ~he mixtur- turns dark
yell~ o qg. upon adding the catalyst and ~radually lados to a 1; ~ht~
y llow color a tho r-action progr-~sos A sli~ht oxoth rm to 66~C wa~
obr~ The ~xot_-rm porsists for 10 ~ee~ boforo tho mixturo cool~ to
the roaction t- _ ~L~ ~ of 65~C Tho roaction was monitorod th~v~yLv L
tho r-nction yoriod by co~ Ling ~ _ 105 ana an~Alyzing by HP~C
(Analytical method 1)
Th- 9-fluoronone was found to be completoly c~~ -~ within 120
with a ~ vd~oL ~ ition, ~rt- ~-~ by quantitativo HPLC, of 98
-51-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
~oL~L of 9,9-bis-(4-l.yl.v~y~h_l.yl)-41uorene~ Tho proauct was furthor
~nalyzed by a ~ tion of HP_C and W (282 nm) and CA~I A;~d
% by aroa ~roduct
96 9 9~9-bi~-(4-Lyd v~y~honyl)fluorone (BHPF)
2 4 9-(2-~y~Lv~y~henyl)-9-(4-l~y~lv~y~h-~yl)
fluorene (2,4-isomer)
0 7 adduct cA~t~i~;~ two fluoron~ unit~ and
t_r~o rh~Alic units (two thr-o adduet)
3XAMP_E 2
C~~~ION OF 3 ~ ~PT~ss~N~sJLFONIC ACIDS FROM T~EIR SODIUM SA_TS IN
THE REACTION MIXTURE
A The ~vcel~ o o-f _lo 1 was r-~o~te~ nxee~t t_at t_o
eataly~t wau ~repared in ,BitU 4rAm 90 ~Gl.- ~ sodium
3 --_~tv~ v~-~-s lfonate (0 854 ~s, 4 79 mmol, 0 0431 equivnlsnts) and 95
to 98 ~ ulfuric acid (0 48 ~, 4 9 mmol, 0 044 equival-nts) and tho
r-action was eonduetod at 85~C
T_o 9-fluo - wa~ eomplotely eA~- -- b ~ _ 60 and 120 ~t~r,
~iv~n~ n final i~omor di~tr;hut;~, dot1 ~d as in _le 1, ofi
% by aroa ~roduet
95 3 4,4-isomer (BRPF)
3 6 2,4-i~omor
1 1 two-thr-e adduet
B T_e ~locod~. of Exam~l- 2A was _-~te~ exe-~t that 98
~ ~t sodium 2 - ~to~h~ l4Onato (0 779 ~s, 4 75 mmol, 0 0427
~quivalont~) and 95 to 98 ~-- sulfuric aeid (0 48 ~, 4 9 mmol, 0 044
~~uival-nt~) w re u~-d ns cataly~t~ Tho roaction was cv~l~L-d at 85~C
The 9-fl~vl~ - wa~ eAmpl-tely CA~ -' within 60 '~t-r, ~iving a
~roduet $~omor di~tr;~ltiA~, as doseribod in _lo 2A, of
% by nroa ~ vl~cL
91 7 4,4-isomer
6 6 2,4-isAm~r
1 7 two t_r~- aaduct
Th-~e ~x~oriment~ domonstrnto that 2 ~ca~Loothan--~ulfA~c acid,
~-norat-d in tho r-aetion mixture, was an ~ffeetive CA~ i~ a~-nt for
tho ~ vc-
~
-
-52-
CA 02230272 1998-02-24
WO 97~08t22 PCTAUS96/13683
EXAMPLE 3
CC~ - - A~ION usINa sULFURIc ACID AND 3-MERcA~ OP~OPIv lC ACID (COMPARATIVE
EXAMP~E)
9-Fluorenone (20 0 g, 0 111 mol, 1 0 ~quival-nt) and molt~n ph~nol
(156 7 g, 1 66 mol, 15 0 equivalent) w ro ~ad-a to tho r-actor (r~actor
- a-si~n 1) Th~ roaction mixture was hented to 65~C with stirring a ~ad of nitrogen 3 ~ topro~ionic acid (0 588 ~, 5 54 mmol, 0 0499
~~uivalont) was adaea to the re~ction mixturo at 65~C, foll_ ~ by tho slow
addition (over 1 minut~) of c ~ ~tod (95-98 ~ ~) sulfuric acid
(0 551 0, 5 62 mmol, 0 0506 equi~alent) to the reaction mixture at 65~C
Tho mixtur turn~ ~urpli~h Ol~h9~ upon addin~ the sul~uric acid ana
~raaually faa-s to a y6~ O~ O color within 5 to 10 ~ A slight
~xoth-rm to 66 to 67~c, w~v obs-rvoa
The ~xotherm ~orsists ror 15 n~ts~ before the reaction mixture
cool~ to tho re~cti on tomporature of 65~C The reaction was monitorOa
throu~h~t th~ r-action ~rioa by collocting _ 1~8 and analyzing by
~PLC Tho 9-~luo _~v~o was ~ound to be complet-ly ~o s~ ~ b-L~_- 240
ana 420 ~ut-~ ~P~C analysis (analytical method 3) ~ives product
di8tr;~ution:
a 0% by aroA proauct
93 0 9,9-bis-(4-1.~ ~y~h_~yl)fluorene
5 5 2,4-isomer
1 5 two thr-~ ~aauct
Thi~ ~xampl- shows that tho ~rior art proc-ss wa8 ~lower than the
~ ~ve~8 of _ 1'~ 1 or 2 and that the r-sultin~ ~ o~L c~t~in~ le
of th- 4,4-isomer, th~n producod by tho ~roce~s o~ r _~-~ 1 or 2
EXA~PLF 4
~FFECT OF ADDED WATER IN F~ P ~IONS USING MPSA (P~ENOL AS
SO~VENT)
A 9-Fluv ~ o~n (138 1 ~, 0 770 mol, 1 00 ~quival-nt) ana molt-n
~henol (1500 ~, 15 9 mol, 20 8 ~quivalent) was adaod to tho roactor
(rcactor de d ~n 4, a E) . Tho roaction mixturo was heated to 45~C with
~tirrin~ under a pad of nitro~en 3 r~to~ vL~ ~~ul~onic acid (8 28 g,
53 0 mmol, 0 0692 oquivalent) was addod slowly over ~lv~imately 1 minuto
to th- roaction mixtur- at 45~C The roaction was monitorea tl ~v~l ~u~ the
r-action ~eriod by collectin~ _ _l Q n ana analyzin~ by ~PLC Tho
9-fluo ~ ~ was found to be 22 ~ t c~~~ ~ within 9 ~l es, 52
-53-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
p-~cOnt conrum~d within 30 minutes, 76 ~LC - nt con~umod within 1 hour, 92
p~ ~L con~um~d within 1 75 hours, and 100 ~Lc~nL conuumod within 3 5
hourA ~PLC analy_is (analytical m thod 3) ~ivo~ the followin~ relativ~
ar-a ~~ ~ent analysis for tho reaction ~ vvu~Ls at 100 ~el -
conversion
% by arQA Froduct
96 9 9,9-bis(4-Lyv ~y~he~yl) fluorenO
2 4 2,4-isomor
0 6 two thro~ adduct
B 9-Fluo e..... v o (6 44 ~, 0 0358 mol, 1 00 oquivalont), molton
ph-nol (70 0 ~, 0 744 mol, 20 8 _quival-nt), and ~AiA~;~-d watOr (1 93 ~,
0 107 mol, 3 00 oquivalQnt) wa~ addod to tho rOactor (ro~ctor aosi~n 3)
Th- rOaction mixturQ was hOatod to 45~C with ~tirrin~ und~r a pad of
n~troAvon 3 ~- ~tv~ vv~sulfonic acid (0 385 ~, a 47 m~ol, 0 0690
~quival-nt) was advdOd Alowly ov-r ~ - t~ly 1 minut~ to tho r~action
mixtur- at 45~C Tho rQaction was monitor-vd tl v~ t tho reaction pcriod
by coll-_Lin~ Aam~ and analyzin~ by ~PLC Tho 9-fl~oLe~v~ was found
to bo 4 ~91~ L c~n -' w~thin 9 ~t-~, 13 ~rcc~t ~r~ ~ within 1
hour, 29 ~el - cOA -~ within 3 5 hours, and 94 ~e c_nL c~ -d within
20 5 hours RPLC analysi~ (analytical mothod 3) Ayiv~s the followin~
rolativQ aroa ~ ~_~t analysi~ for th- reaction ~roaucts (fl~oL~ . arOa
not ;n~ a) at 94 porc-nt cv~OL~ion
% by aroa product
96 5 B~PF (4,4-i~omor)
2 9 2,4-isomor
0 6 two thr-o adduct
Tho~ ~xp~rimont~ ~how that hi~h-r roaction ratos and lowor ~ -
~of ~ L,-~lvd~ct~ wer- obtain d, in thD A~-- - -~ of additional
wat-r
EXAMPLE 5
CONDENSATION OF FLUORENONE WIT~ P~ENOL VSING OT~ER ~_ ~r-~ AGENTS
A 4-NERCA~~ ~-C~T~ONIC ACID
9-Fl~v . ~ (82 9 ~, 0 460 mol, 1 00 Oquivalont) and molten ~honol
(900 ~, 9 56 mol, 20 8 oquivalent) was add~d to th~ r-actor (_~ctor
d~ n 4, 2 L) Th~ roaction mixtur- was heatOd to 45~C with ~tirrin~
undDr a ~ad of nitro~-n 4-~--c~Lo~ltr~-F~lfonic acid (5 41 ~, 31 8
mmol, 0 0692 equivalent) wa~ added ~lowly over ~ t-ly 1 minut~ to
-5~-
CA 02230272 l998-02-24
W O g7~8~22 PCT~US96/13683
the r-action mixturo at 45~C. Tho roaction was monitorod th.~ tho
roaction ~Oriod by colloctin~ sam~l~s and analyzing by HPLC. The
9-flu~ ~l-e was found to be 17 ~8 ~lL c~- -1 within 5.5 ~t~, 58
~e c~..L consumed within 30 n~lt~, 83 ~elc~ L C~n -d within 1 hour, 95
5 ~e~ t e~n -~ within 1.75 hour~, and 100 ~ nt consumod within 3.5
~ hour~. ~PLC nnalysi~ (~nalytical mothod 3) givo~ the following relativo
ar~a ~o ._ t analysis for the reaction produets at 100 ~ercenL
eo~v.l~ion:
% by ~roa product
10 97.0 B~PF
2.5 2,4-isomer
0.5 two:thr~o ~dduct
B.2,2-BIS-(NERCA~, )-1,3-PROPA~I~-u~FONIC ACID
To A 4-dram vial (r~aetor design 2) was ~dded ~ mixture of
15 fluG ~ .. ...Q (0.40 g, 2.22 ~ l, 1.00 equivalent) and phenol (2.10 g, 22.3
mmol, 10.0 ~quival~nt). Tho ca~-d vial wa~ ~lacod into tho ~~~t;~
control block re~ulatod at 63~C and ~tirring bogan.
2,2-8i8(~ ~Lomethyl)-1,3-~ a;~ c aeid (0.029 g, 0.098 mmol,
0.044 equivalent) was add-d in one ~ortion to tho vial which was thon
tightly cappoa. Tho r~t;~ was monitorod th.~Vl ~ the roaction poriod
by colloct$ng ~ and analyzing by ~PLC. The 9-fluo~ - wa8 found
to be 25 ~ ~L e~ ~' in 1.5 hour~. ~P1C analysis (analytical method
2) giv-s the foll~ ~g relativO arOa ~ 9'. ce~t analysis for tho roaction
~roducts (fluo enGne aroa not included) at 25 ~l. c~vO-dion:
% by area product
95.7 B~PF
3.4 2,4-i~omer
0.9 two:thr-e adduct
C. 2,3-DIMERCA~OPROrANES~LFONIC ACID
To a 4-dr~m vial (reactor do~ign 2) was added a mixtura of
fl~v ~ - (0.40 g, 2.22 mmol, 1.00 ~quivalent) and ~henol (2.10 g, 22.3
mmol, 10.0 oquivalent) The ca~od vial was placed into tho ~-~t;ng
control block rogulated at 63~C and ~tirring was begun.
2,3-n; ~ o~,~ fonic acid (0.021 ~, 0.011 mmol, 0.050 oquivalent)
w~ ~ddod in one ~ortion to tho vial which was then tightly cay~od. Tho
r-action was monitorod throu~hout the reaction ~eriod by collecting
-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
~am~les and analyzing by HPLC (analytieal method 2) Tho 9-~1uv.a~v o was
found to be 5 ~Ple~L e~- -' in 1 5 hours
D 3-~ERCAPTOPROPIONIC ACID AND MF~ CVLFONIC ACID
(CONPARATIVE EXANPLE)
To a 4-dram vial (reaetor ausi~n 2) was adaea a mixture o~
~1~v~Onvno (0 460 g, 2 55 ol, 1 00 cquiv~lont) and phOenol (5 00 5, 53 1
mmol, 20 8 equivalent) The eapped vial was plaeed into the hsAt;~g
eontrol bloek regulatod at 55~C and stirring was begun
3 ~~-~a~topro~;A~;c aeid (0 0217 9, 0 204 mmol, 0 080 e~uivalont) and
me~h-~-r~l~onic ncid (0 0197 g, 0 205 mmol, 0 080 ~quivalent) were added
in ono portion to the vial whieh was then tightly ea~oa The reaetion
was monitored throu~hout the reaetion perioa by eollecting sam~les ana
analyzin~ by HPLC The 9-~1~o ~nvn~ wa~ ~ound to be 32 ~CL_a~L e~ -~
within 30 ~t ~, 51 p~L_e..t C~ - ' within 1 hour, and 71 ~
e~ -' w~thin 2 hours HPLC analynis (analytieal method 3) gives the
~ollowing r-lative aroa ~el_Qnt analysis ~or the reaetion produet~
(fluv ~ ~A aroa not ;~ ) at 71 ~ l cv v~ ~ion
% by ar-a ~ vd~
93 7 BHPF
5 5 2,4-isomer
0 8 two threo adduet
E 3-MERCAE O~ROrIONIC ACID AND ~T~Y~-CVtFANIC ACID (CONPARATIVE
EXANPLE)
To a 4-dram vial (r-aetor d-sign 2) wa~ added a mixture o~
~luv.~v..c (0 460 g, 2 55 mmol, 1 00 e~uivalent) and ph-nol (5 00 ~, 53 1
mmol, 20 8 ~quival--nt) T_Q eapl?ed vial was ~laced into th~ heatin~
eontrol block ~ ated at 55~C ~nd stirring was ~egun
3-Nereaptopropionie aeia (0 0217 ~, 0 204 mmol, 0 080 equivAl-nt) and
methyl~ fl e aeid (Aldr$eh 98 ~c~co~t) ~0 0227 g, 0 204 mmol, 0 080
~quival-nt) wero adaod in on~ portion to the vial whieh wa~ thon ti~htly
eappod Tho r-action was monitored tL_v ~~ thc reaetion poriod by
eolloetin~ ~ an~ analyzin~ by HPLC The 9-~l~ol~v~c wa~ ~ound to
b- 13 ~elCent Cv~_~ - ' within 1 hour, and 21 pereont co~ ' within 2
hour~ HPLC analy~is (analytieal method 3) giv-s the ~ollowing rolative
arca p_.ccn~ analysis for the reaetion ~roduet~ (fl~o-_ ~ aroa not
e~) at 21 ~ ~cnt eonversion
-56-
CA 02230272 l998-02-24
W O 97/08122 PCT~US96/l3683
% by ar~a product
95.4 BHPF
4.6 2,4-isomor
n/d two:three adduct
F. SUB~ ON OF r~usr~uNlc ACIDS POR M~CT~T~ONIC ACID
(COMPARAT~VE EXAMP~ES)
The r-~ction conaitions doscriboa in r _lo 5D wero repr~t~
~ubstituting oach o~ the ~ollowin~ acids (each at 8 ~ 1 ~_-~ ) for
- h~ -l~onic acid in the reaction: sulfamic acid ~Aldrich 98 ~c_co L),
methylph~rh~;c acid (Aldrich 98 ~e ~nL), and phonylFh~rhA~;c acid
(Aldrich 98 yO~ ). In oach ca~H, v-ry little cvrv~L~ion o~ the
fluo.~one was ob8erved in compari8cn with the use of me~h-~~~lfonic
acid.
The~o . _l~n ~- -Lrato th~t mixturos o~ a - ~yLO _ __ ' and
an acid woro infcrior to 3 ~~ c~ytOy~vya~e sul~fonic or 4-
-c~pt~but-n~ulfonic acid for catalyzinc the c~ ti~ o~ phenol with
~1uorenon-.
EXAMPLE 6
~FF~CT OF WATER ~ ~ATION IN FT~)u~ ~IONS USING MPSA WITH
ao ~Ir~NY ~ T~ AS A CO-SOLVENT
A. 9-Fluv.onv~e (3.65 ~, 0.0200 mol, 1.00 oqu~valent), ~ lton
phonol (39.6 ~, 0.420 mol, 20.8 equival-nt), d~io~;~-' wntor (0.055 g,
3.06 mmol, 0.151 oquivalent) and diphenylmethane ~32.83 g) was added to
the r-actor ( -~tor desi~n 2). The reaction mixture was heated to 53~C
with stirrin~ undor a pad of nitro~en. 3 r!-~_aytv~ vL -3Ul fonic acid
(0.170 g, 1.10 mmol, 0.0537 ~quivalont) was addod ~lowly over
a~. -: tely 1 minuto to th- reaction mixture at 53~C. The roaction was
monitor-d th~v~yh~ the roaction period by collocting ~ and
analyzin~ by HPLC. The 9-fluv-e v o was found to be 49 ~_~ent r~ -~
30 within 2 hours and 77 ~ ~t c~ b~ ~ within 4.5 hours. HP~C analysis
(analytical method 3) gives the foll~_~ ~g r-lative area ye~c_~L analy~is
for the reaction products (fluG ~ - - area not ;~l~rd) at 77
~v,.v~.sion:
~ by area product
3596.1 BHPF
3.4 2,4-i~omor
0.5 two:throe aaauct
-57-
CA 02230272 1998-02-24
WO 97/08122 PCTnJS96/13683
B. 9-Fl~v~Onv~e (3 65 g, 0 020 mol, 1 0 equivalent), molten
~henol (39 6 ~, 0 420 mol, 20 8 equival-nt), ~ei~;7s~ watHr (0 362 g,
20 1 mmol, 0 994 oquivalont) and diphenylmothano (32 83 ~) was addod to
tho roactor (roactor dQsign 2) Th~ r-action mixturo was hOat~d to 53~C
with ~tirrin~ undor a ~ad of nitrog~Yn 3-Mercaptv~L~a~Y~lfonic acid
(0 158 g, 1 00 mmol, 0 0500 ~quivalQnt) was added ~Ylowly over
~ Y; oly 1 minuto to the , -~1;~ mixtur- at 53~C The rOaction was
monitorOd th.v ~l~t thc rOaction ~-riod by collectin~ ~ _le8 and
analyzing by HP~C. The 9-fluo.Ononn was found to be 25 ~Yl_ont cv~ -'
10 within 2 hours, 45 ~ c~ ' within 4 5 hours, and 57 ~YL
c~~ -' with$n 6 hours HPLC analy~is (analytical method 3) ~iVQB thoY
foll~_ in~ r-lativo ar~a ~L_ent analysis for the re~ction products
(fl~o .~v~e area not ;~lur~d) at 57 ~L- ~ C~vo YYion:
% by aroa product
96 3 BHPF
3 7 2,4-isomer
n/d two thro- adduct
C 9-Fl~o. - ~ (3 65 ~, 0 0200 mol, 1 00 equivalent), molton
ph-nol (39 6 g, 0 420 mol, 20 8 equivalent), ~ ' water (1 09 g, 60 7
20 mmol, 3 00 equival-nt) and diphenylmethan- (32 83 ~) wa~ add-d to the
r-actor (roactor design 2) Th- reaction mixtur- was hoatod to 53~C with
~tirring undOr a ~ad o~ nitro~en 3 ~tv~. _ -~ulfonic acid (0 158
g, 1 00 mmol, 0 0500 equival-nt) was addod ~lowly over ~-v- tely 1
minuto to tho r-action mixtur- at 53~C Th- r-~c~;o~ was ~onitor~d
tL~v~lo~t the roacti ~ period by collecting samples and nnalyzing by
HPLC. Th- 9-fluo.Onv~o was found to bo 11 ~L-- ~ C~~ - ' within 2
hours, 20 _~c consumed within 4 5 hours, and 23 ~eL. ' C~
within 6 hour~ HP~C analy~is (analytical m thod 3) ~iv 8 th following
rolativo ar~a ~- analy~i~ for tho r-action ~vd~Ls (~1~G~ V O ar-a
not i~l7~-') at 23 ~- ~ Cv~vOL~ion
% by area ~roduct
95 9 BHPF
4 1 2,4-isom r
n/d two throo adduct
35 Th-so ~ show that addition of lar~e ~ 8 of wat-r to the
roaction mixturos retards the co~ i~ r-action
-58-
CA 02230272 1998-02-24
W O 97/08122 PCTAUS96/13683
3XAMPLE 7
UEMOVAD OF WATER 'JNDER VACUUN WITH AND ~1.~. DIP~wrl~M~ o~ A8 A
CO-SO_VE.~T
A. 9-FluoL~one (127.7 g, 0.709 mol, 1.00 equivalont) and ~ lt-n
~honol (996.1 ~, 10.58 ~ 1, 14.9 equivalont) was ~dded to the roactor
(r-actor aesi9n 4, 3 L). The reaction mixture wa8 heated to 45~C with
8tirring unaer a pad of nitrogen. 3 -_~topro~r~ lfonic acid (5.53 ~,
35.4 mmol, 0.0500 oquivalont) wa8 addod slowly ov~r ~ -: t-ly 1 minute
to tho r~.action mixtur~ at 45 ~C. Tho roaction wa~ monitor-d tL~u~louL
the reaction yeriod by collectin~ ~ _la~ and analyzin~ by HPLC. Th~7
9- luor-none wAs found to bo 60 ~a c~nL consum d within 1 hour, 88 ~_ ~o~L
c~7~ _a within 2 hours, Ana 95 pc _~t r~n- ' within 2.5 hours. HPLC
~n~ly~is (analytic~1 mothod 2) ~ivos the fo~ relative area ~8YC_.~t
analysis for the reaction products at 100 ~e7c~L c~v~ ~ion:
% by ar-a ~roduct
98.0 BHPF
1.4 2,4-i~omer
0.7 two:three adauct
B. 9-FluG _~G~C (127.7 ~, 0.709 ~ 1, 1.00 ~quiv~lent) and molton
~h-nol (996.4 ~, 10.59 ~ 1, 14.9 ~quiv~l-nt) was add~d to tho r-actor
(using r~actor d-sign four (4), throo (3) L roactor with a Doan-Stark
water Je~--At;~7~ trap a d vacuum inl~7t attach-d in lie-l of tho nitro~-n
inlet). The re_ction mixtur~ was h~at~d to 45~C with ~tirring.
3 ~ ~Lo7~roF~ fonic acid (5.53 ~, 35.4 mmol, 0.0500 ~quival-nt) was
ada-d 810wly ovRr ~ ~ ely 1 minute to the reaction mixtur- at 45~C.
Tho L~-~7'i~7~ mixturo was ~11. .._~ to stir for 15 ~es at ~ --ic
~8~ - e, then vacuum wa~ ap~lied to th- re.nctor. From this point on, the
roaction wa~ conduct-d undor 1.- ce~'~ ~ro8sure conditions (<5 mn Hg) with
water/~honol di8t; 11 A~ coll- Led in the Dean-Stark tra~. Th~ roaction
was monitor-d th~u~ tho roaction ~oriod ~y roll~_Ling ~ _les and
anAlyZin~ Jy HPLC. Th- 9-~1~.~ - was found to ~o 68 ~e~e~t C~77 ~7
within 1 hour, 98 ~e~. - c~- -' within 2 hour~, and 100 _~rc
c~ ' within 2.5 hours. HPLC analysis (analytical methoa 2) ~iV08 th-
ollowing r-lativ~7 ar-a pCl__~t analy~is for the roaction product~ at 100
~9 c L convors~on:
-59-
CA 02230272 l998-02-24
W O 97/08122 PCTnJS96/l3683
% by area proauct
98 1 8~PF
1 3 2,4-isomer
0 6 two thr~ adduct
C 9-Fl~.~ ~e (191 5 ~, 1 063 mol, 1 00 oquivalont), molten
phenol (1500 g, 15 9 mol, 15 0 equivalent) and diphenylmothano (156 7 g)
w ro added to th~ r-actor (r-actor do~i~n 4, 3 L with a Doan-8tark wator
n-paration tra~ and vacuum inlot attachoa in liou of the n~tro~en inlot)
Tho r-action mixtur- was hoated to 45~C with stirring
3 ~ ~Lv~ v~-~r-~ lfonic acid (8 27 ~, 53 0 mmol, 0 0499 oquivalent) wa~
ad~oa ~lowly ovor ~ to~y 1 minut- to tho reaction mixturo at 45~C
The roaction mixture was All~ ..Oa to stir for 15 ~t~- at - ~3p'~~ - C
pr-~ure, then vacuum was ~Fl;o~ to the roactor From thi~ point on, tho
r-action was conauctod undor roduced ~r~s3uro conditions (<5 mm ~) with
wator/phonol dist~ t~ coll-_L-d in tho D-an-8tark trnp The reaction
wa-- monitorQd th ~l~L the r~action porioa by collocting ~m~les ana
an~ly~ing by ~PLC The 9-fluol~ono was founa to bo 20 po__enL c~ -~
within 15 ~t~, 80 _- ce~L c~~ i within 2 hours, 98 ~_~_~L c~~ d
within 3 5 hours, and 100 ~ _e L ____L ' within 6 hours ~P~C analy~is
(analytical method 2) givos the foll~ r~lativo area ~ nL ~naly8is
for the reaction products at 100 ~_~L c~ v.L~ion
% by ar~a product
98 3 8~PF
1 2 2,4-isomor
0 5 two throe adduct
Tho~- r-Mult~ ~how that r movnl of wat-r from th~ roaction mixturQs,
c~1~;~;~g ai~honylmQthano solvQnt, was ~----~9ce~ y. Th-so r-sults show
that remo~ing watar ~rom th- r~action mixtures accel-ratod th- rat- of the
L ~1-~;~~ reaction, but was not -c ~c-~y for ~ood roaction ratos and
c~v~ ~ions
~XAMP~E 8
REACTION OF PE~NO~ WIT~ AC~TONE TO rnvuuv~ BI8P~ENOB A USING MP8A CATALY8T
A To a 4-dram vial (roactor design 2) was rdded a mixture of
acetone (0 11 g, 1 8 mmol, 1 0 equivalont) and phenol (2 40 ~, 25 5 mmol,
14 0 ~quivalent) The cappod vial was ~lacod into the ~~~~ control
block r~gulated at 62~C and stirring was bogun 3 ~ ~tv~l~ eL~lfonic
-60-
CA 02230272 1998-02-24
WO 97/08122 PCTAJS96/13683
acid (0 021 g, 0 13 mmol, 0 070 oquivalent) was added in one ~ortion to
tho v$al which wa~ th~n tightly cap~od The r-action was monitorod
throu~hout th- roaction ~eriod by colloctin~ ~mplos ~nd ~nalyz$ng by
~PLC Tho ~cotono wa8 round to bo ~ ~ly 70 ~ ~t co~
within 2 hours ~PLC analysis (analytical mothod 2) giv~s a rolativo ~roa
~r ~61-~ ratio of 97 0 3 0 for the do~irod r-action ~roduct 2,2-bi~-
(4-l~dA~y~h-nyl)~_ ~ - (4,4-b$~ ol A) r~l~tivo to tho i- -ric
im~urity 2-(2-lyd o~he~yl)-2-(4-L~ll~y~h-~yl)-~ v~c (2,4-bis~henol A)
at 70 p~ t conv~sion~
B To a 4-dram vial (r0actor dosi~n 2) w~s addod A mixturo o~
acoton- (O 11 ~, 1 8 mmol, l O ~~uiv~lont) and ~henol (2 40 ~, 25 5 ~ol,
14 0 oquiv~lont) The capped vial was pl~cod into the ~~~t;n~ control
block regulated at 25~C and stirrin~ was be~un 3 -- ~t~ ~A~ f~;c
acid (0 074 ~, 0 47 mmol, 0 25 oyuivrAl-nt) was addod in onu ~ortion to th-
vi~l wh$ch w~s th n ti~htly c~od Tho r~action was monitored tL~v~l ut
tho roaction p-riod by collectin~ P and ~nalyzin~ by ~PLC Tho
~cotono was found to be ~y~. - t~ly 70 ~c. _~L c__~ within 2 hours
Durin~ tho latQr st~s of re~ction, th~ ro~ction product bo~ins to
crystAAl1;~o ~rom th~ ro~ction mixture ~PLC an~lysis (analytical methoa
2) giv-s a r-lative aroa ~c ~t ratio of 98 9 1 1 for tho d-sir-d
r~action ~roduct 2,2-bi~-(4-hy~L~y-~h_~yl)~ ~ne (4,4-b;~F- -1 A)
relrAtiv to tho i~om~ric impurity
2-(2-hyd~v~y~honyl)-2-(4-h~a~v~y~h-nyl)propan- (2,4-b;q~l A) for the
bulk rcaction ~o~ut;~
8eparat$on of tho cry~ ~.~d~ ~ from tho roaction mixturo
fol1 _~ by rinsin~ of the crystrAl3 with wat-r to removo ~urfaco
impuritie~ providon 4,4-b$sp~ -1 A ~roduct c~A;~;n~ 1 - ~8 than 500 parts
per ll;~ of tho 2,4-b; ~h~~~l A impurity
C (Com~ar_tiv _le) To a 4-drnm vi_l (ro_ctor dosign 2) wa3
addod a mixture of acetone (0 11 ~, 1 8 mmol, 1 0 equival~nt) and phHnol
(2 40 g, 2 5 5 mmol, 14 0 ~quivalent) The ca~p-d vial wa3 ~lac-d into
th- h--t;ng control block regulatod _t 62~C and stirring wa~ ~egun
3 - ~a~topropionic acid (0 014 g, 0 13 ~ l, 0 070 equiv_lont) and
me~h-~ lfonic acid (0 013 g, 0 13 mmol, 0 070 oquivalont) w ro added in
one portion to the vial which was then tightly ca~-d The r-~ction wns
monitorod thro~gh~t tho roaction p~riod by colloctin~ sam~l~s ~nd
an~lyzing by ~PLC Th~ _cotono was found to ~o ~. - -7y 70 ~orcent
-61-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
consumea within 2 hours HPIC analy~is (analytical method 2) gives a
r--lative aroa ~-- c~, L ratio of 96 2 3 8 for t_e desirod reaction product
2~2-bi3-~4-LYv-~ yL yl)~ v~o (4,4-bisphenol A) rolativo to th
~- c im~?urity 2-(2-lyl v,,y~"henyl)-2-(4-lyl,v y-phenyl)~;lv~o
5 (2,4-~ rh~ A) at 70 ~ o. ~lt cv v L~iOn
Thosn nxpnriments show that MPSA s~ive8 a ~roduct, with a hi~h r 4,4-
i~omor ratio than ~rior art cataly~ts
EX~MPL~! 9
PREPARATION OF THE POIYMER- su~rOR.~LI MERCAPTOS~lLFONIC ACID CATAI,YST
10 (PMB8A)
A PREPARATION OF 8~LTONE ~
1,4-Rut--~e~~lton (3 00 S~, 22 0 mmol, 1 00 oquivalent) was ndded to
dry THF (150 ml,) under a nitrogen r~ ~~ '~. The E~olution was cooloa to
-78~C us$ng a dry ico/acotono bath n-Butyllithium (1 6 molar in h~ ~ E,
13 8 mI, 1 00 oquival--nt) was added slowly dropwiso to tho -78~C ~J~lut;~
via An ada$tion funnel over c ~ ~ t~ly 40 r~t~~ with vi~orous
~tirring Th h- _ - ~ r~ action mixturo wa~ Al1_ _d to stir ~or an
add$tional 10 to 15 r~t-r at -78~C Poly(vinylbonzylchloride) (3 3 çr,
~ t-ly 1 0 equivalont of chloromothyl groups, 60/40 mixture of 3-
20 and 4-;- ~, Alar$ch ~ cAl Co ) in dry THF (10 ml) was _daea over
~ -~ t--ly 2 ~rn~tep to the r--action mixturo at -78~C Tho roaction
mixtur-- was All~ _~ to slowly w_rm to room t-- _= ~ture in th~ cool;~J bath
ov-r ~ ly 3 hours A whit ~ preci3;~itato forms in the roaction
mixtur-- auring tho r--action ~?--rioa ana ~ as a ~olid as the mixture
--rh_~ room t~ _- ~turo Wator (100 mL) was aadod to tho roaction
mixturo and the white (;~ol~ l~) solid was ,~d by filtration under
vacuum The solid w_~ 81~ y-W "h'~d w$th water, thon w$th ~mall volum 8
of methanol and mothyl-ne chloria-- and driod in a vacuum ov--n, providing
4 77 S~ of a whit ~ 801ia 8ultono-~unctional polymor
B CONVERSION OF THE SlJITONE-FVNt; _ '~ POLYMER TO POIYMER-
~u~~O~.cl MERCAPTOS~FONIC ACID (PMBSA)
The sultono-functional ~olymor from above (4 00 ~, ~ tely
15 9 mmol ~ulton--) was added to THF (125 mL) Pot-~; th; o-cotato (2 20
g, 19 0 mmol, 1 20 ~quivalont) was add d as a solid to th- slurry of the
3;~olysultone in THF On-- arop of 50 ~c~ nt tetrabutyl~ ; chloriae
was~ aad--d to the rnpidly stirroa slurry Th-- t~ _ ~turo rose to 26~C over
~--v ral r 1--r~th--n slowly l v~ed to 20~C Two additional dro~s of 50
-62-
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/l3683
v- _~nL t~trabutyl~ i chlorid- were added and tha 801ution wa~ warmea
to 40~C for 15 nl~tsA Water (100 mL) was slowly adaed over 1 hour to tho
r-action mixturo at 40~C Sub~t~Ati-7 uolid ;A~ in tho mixturo at all
st~gos o~ the r-action Tho wat-r~T~F r-action mixture was allowed to
r-act 15 hour8 at 40~C The solvent was L. ._d by rotary A~V~o ation ~na
the reDultant oOlid was ~round to a fino ~owdOr T~F (125 mA) wa~ again
addoa to tho Aolid, forming ~ ~lurry AdaitionrAl vot. ~ i thi o~Cetate
(a 20 ~, 19 0 mmol, 1 20 ~quivalent) was added, r-sultin~ in an ~xoth-rm
to 26~C Sev ral dropt7 of 50 porcent tQtrabutyl~ ; chlorid~ w ro
added and the r-action mixtur- was h-at-d to 40~C for 15 hour~ The
~olv nt w~s _~ v~ by rotary ~vO~oLa~ion Th- tan ~701ia was slurriea in
a 2 1 (by volume) m xture of tolue~e/eth~T~Al C~ ted ky~vcLloric
~cid (50 mL) was added and the mixture wa3 ~tirr~d at room t- _el~turO
overnight Most of tho ~Cl wrAA~ ~ v~d by ~7~ar~in~ th- mixture with
nitro~-n, th-n tho ~olventA~ w r- ~ a by rotn V~AVV ~tion The li~ht
tan ~olid waO 8 lurry ~ d ~ t iv~ly with 10 ~L ~t hydrochloric acid
and with wat-r Dryin~ ov~ ht in a vacuum oven (60~C/full vacuum)
yrovid-~ 4 18 g of tho ~olymor-~o Lod - ~Lonulfonic acid ~8 a lig~t
tan ~olid
C PREPARATION OF A GEL PMB8A r~TYST (PMBSA-M~R)
A catalyst was pre~ar~d rAs abov , startin~ with ~-rr;f;Al'~ r-Oin
(200 to 400, 2 ~LOo ~ cro8~Al;A-l d, ~ol), trOatOd with hut~A~rultone The
~L ~d~_ L W~A8 jd~At~f;~a ~_ PMDSA-M~R
D PREPARATION OF CATALYST FROM BROMOM~nT~n MACROE~R-uB-'
POLYSTYRA~NE (PMBSA-XEBR)
A catrAlyst wrAs ~rO~roa A8 ~bovo, ~tArtin~ with bA~ Lhylatod
AA~berlit ~ XE-305 ma~lvvv v~8 resin (4 ~_ ~ L cro~;Al d, 20 to 50 me8h,
3 7 meq Br/g)
E PREPARATION OF CATAhYST FROM C~TO~- ~ ~Y ~ MACR-urvROu~
PO~ ~ ~ (PMDSA--XA~CAL)
A cataly8t was ~rw~ared as above, 8tarting with chlG~ ~hylatea
A~bwrlit- macAoVG~vAs r~sin (4 ~rc croP~ d, 20 to 50 mosh, 4 3
~ lw ~ C l / g )
F PREPAR~TION OF CATALYST FROM h~nnI~A~D~ RESlN AND 1,3-
PROP~C~TTONA- (PMPSA-MER)
Catalyst was ~re~arQd abovo, by troatin~ Mwrr;f;~ reOin (2
~rc~nt cro8s ~ 200 to 400 mwsh, 4 3 m~q C1~Y) with li ~t~ 1,3
-63-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
~~~r~ltono, which can be preparod in acco~d~ce with T Durst et al ,
"A n-w route to 5- and 6- ~- ~d ring sulton-s", Can J Chem , Volume 48
(1970), Pagos 845-851
EXA~P~E 10
EVA~UATION OF A MERCAPTOSUBFONIC ACID PO~Y~ER (PMBSA) IN THE REACTION OF
PHENOL WITH F~UORENONE
A To a 4-dr~m vial ~~uip~od with a ~t;-ri~ bar (roactor dQsign
2) wns aadod 4 33 ~ of a 20 8 1 molo ratio mixturo o phonol to fl~vl.~v~o
and 0 26 g [(6 pe~ L by weight of the roactant soluti~n)] of the
~Losulfonic acid polymer (PMBSA) preparod as in _le 9B Tho
r~action mixtur~ consistod of a ~ _ - ~ liquid ph~so plu~ n 3-p-rato
hetolv~ polymer catalyst phase Tho mixtur~ was hoat-d to 36~C for 3
hours To in. -~c the rate of rnaction, tho t _-~ture was increased to
50~C for 18 hours Monitoring of the r-action by HPLC show~ svmo rcaction
15 at 36~C and 100 ~Cl~ ' Cv v~-~ion aftQr 18 hours at 50~C HPLC analysis
(analytical method 2) ~ives tho foll_ ng relntive area ~_-c~nL analysis
for the products aft~r 18 hours of r-action (100 ~ ._~L cv v~ldion)
by aroa vroauct
98 86 BHPF
0 98 2,4-isvmer
0 16 two throo adduct
B(Comparative _l~) DOWEX 50WX4 (a cro~ d ~ulfonatod
poly,Ly ~ne r-nin, from The Dow ~ c-l C _ y) promoted with
2,2-a$methyl~h~7oli~in~ (25 ~ ~L of the r~sin 8~lf~n;c acid
cquival-nt~) was wa~hed on a ~lass _iltcr frit with phenol at 55~C to
remove wnt-r The resin wns th-n wa~hod with a mixturo consistin~ of a
20 8 1 molo ratio mixture of phenol to fl~v-bnv~ at 55~C to ~;opl~- the
original phenol wa~h To a 4-dram vial equippod with a stirring bar
(r-actor d~sign 2) wa3 addod 2 13 ~ of a 20 8 1 ~ lo ratio mixturo of
30 phenol to fl~v - ~nd 0 74 ~ (35 ~OL.~L by w-ight of the roactant
~ol~ ~n) of th- promot-d DOWEX 50WX4, from The Dow r c-l C _ y~
catalyst activat-d a~ dQscrib-d above The wuight of catalyst us-d was
d~t~ aft~r the reaction by _ v~ly of tho r-sin from th~ reaction
mixture by filtration, w-nh;~g th~ r-sin with tol-- ~ and h~xane, nnd
drying to a constant weight in a vacuum oven at 50~C for 6 hour~
Tho r-action mixtur- consi~tod of a h- ~ ~_ o liquid phas~ plus a
~-p- ~t~ hot~~ _ o polymor catalyst pha~e Tho mixture was h at~d to
-64-
CA 02230272 l998-02-24
W O 97~08122 PCT~US96/13683
50~C for 18 hour~ Monitorin~ of th- r-Action by HPLC showoa ~ - tely
17 ~-rcont cv~v~ -ion aft~r 4 hours and 73 ~elcenL ~v~ve~ion after 18
hour~ at 50~C HPLC analysis (analytical method 2) and g~ve the foll~w ~g
relati~ ar~a ~e.c~nt analysis ~or the ~roductn aftor 18 hours of roAction
5 (73 ~vr A~t c~v_-Oion)
% by ~r~ ~v~L
91 32 BHPF
6 78 2,4-isom r
1 90 two throo ndauct
10 C (Comparntive r _lo) To a 4-dr~m ~ial ~quipped with a
Otirrin~ bar (reactor design 2) was added 2 16 ~rams of a 20 8 1 molo
ratio mixture of ~henol to fl~ - and 0 34 ~ (16 ~_~_L ~Y w~ight of
the r-actant 80l~7~) o~ dry Amb~rlyOt 15 (a cro~7sl;~l~ ~ r7ulfonat-d
~O1YDLY~- - reDin fr ~ Ro~7m and Haas C _ _~) Thc r-action mixture
consiHtod of a ~ o~g liquid phaDe ~1UD ~ s-~arato h-t~
~olymer cataly~t ~h~o The mixtur- W~8 ho~t-a to 50~C for 18 hours
Monitoring o~ the r-action by HPLC oh0w d ~-~ tely 24 percent
~v~v.~ion aÇt-r 4 hours and 64 ~e~- ' co~v~ion after 18 hours at 50~C
HP~C analy8is (analytical m thod 2)and ~ave tha fo~ g relativo ~roA
20 ~2- ~ an~lysis for the ~roaucts ~ftor 18 hours of roaction (64 ~-rcent
c~.,v~ Oion)
% by ar-a ~.vd~cL
95 82 8HPF
3 93 2,4-isomer
250 25 two thr-e adduct
The~o _ ' 'q showea that u8e of the catalysts ~i~c~~' h-roin
gavo higher c~ v_.~ions of fl~oL~ - nna highor 4,4~2,4-i~om r ratios
than tho prior art catalystO
EXAMPr~E 11
~c~v~Y AND RECYC~ING OF S08ID ~TY-CT (PM8SA)
A ~Tyc~ ~C~v~
The . ~t;o~ mixturo from Example 10A wa~7 cooled to 40~C and the mixtur-
wa~ centrifu~-d The u~-r liquid layer was ~ Y - o~ and Additional warm
(40 to 45~C) 20 8 1 ~ lo ratio ph nol/fl~vl~o~ ol-~tiA~ (~ f ~ly 3
to 4 tim 8 tho catalyst volume) was ndd~d ThD mixtur- was stirred,
c-ntrifu~ed, and the warm d liquid layer was ~ This wash
~-vcel~ . was ~ t~d for a total of thr-e wa~hes, thon the required
-65-
,
CA 02230272 l998-02-24
W O 97/08122 PCT~US96/13683
amount of phenol/fluor~none roactant mixture was added and tho r-action
b-gan
B FIRST RECYCrE
To tho 4-dram vial c~t~ ~ the ~ _~Losulfonic acid polymnr
~: .G~Od (a~ d-scribQd above) from Exam~le llA was add-d 4 33 grams of a
20 8 1 molo ratio mixture of phsnol to fl~ol~n~l.e Tho mixturo was heatod
to 50~C for 4 hours ~onitorin~ of the roaction by HPLC showed
_~ ~xlmatoly 90 ~o ~..L cv~V~ldion aftQr 4 hours at 50~C HPLC analysis
(analytical mothod 2) and gav- the followin~ r-lative area ~-
~
analysi~ for the products aft~r 4 hours of rQaction (90 ~ ~tconv-rsion)
% by area product
98 77 BHPF
1 09 2,4-is ~er
0 14 two thrQo adduct
C8ECOND RECYCLE
To th- 4-dram vial c~;~i~ thQ ~tosulfonic acid ~olymor
_avv~ (a8 d-scriboa above) from tho fir~t r~cycl- was added 4 00 grams
of a 20 8 1 molo ratio mixture of ph-nol to fluol~nG~o ThQ mixtur~ was
hoated to 50~C for 18 hours ~onitorin~ of tho roaction by HPLC shows
~ RJy 83 p_ _~nL c~v~L~ion aftor 4 hours and 100 ~c
c~v.r~ion aftor 18 hour~ at 50~C HPLC analysis (analytical mothod 2)
giv-s the fol 1 ~..in~ relativQ ar~a ~ l_~L analysis for th- products aftQr
18 hour of r-action (100 ~cLa~L co~v_~dion)
% by ar-a product
98 79 BHPF
1 10 2,4-isomQr
0 11 two throQ adduct
D THI D RECYCLE
To the 4-dram vial c~t~ th~ ~ _~to~ul f~; C acid ~olymor
_~C~v~l_~ (as ~--~r;~o~ abovQ) from tho socond rQcyclQ addod 2 00 ~ram~ of
a 20 8 1 mol~ rntio miXturQ of phQnol to fluoL~ - Tho mixturo was
h-at-d to 40~C for 18 hours ~onitorin~ of thc reaction by HPLC shows
35 a~ ~; t~ly 90 porc~nt c~vO ~ion aft-r 4 5 hours and 100 ~_ ~n~
co v~l~ion after 18 hours at 40~C ~PBC analysis (analytical method 2)
-66-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
SJives the following relativo Aroa percont e~ne~lysis for t_o ~?roductls ~fter
18 hours of roaction (100 ~eLc~ t c~ v~ liion)
% o~ area product
99 08 BHPF
0 92 Z,4~ omor
- * two throo e~dduct
* not detectable
ThH8e ~~periments showed that tho ce~talyst con b- rocycled
rersAt~ 7ly without loss of e~ctivity
~!XAMPI E 12
COMPOSITE EXP~RTM~AT. 1)~ r A'l'ION OF PIT~ rT!T~e FOR TEIE C'~ A~ION OF
PHENoL WITH FT (3-MPSA, DI~ n~L~Y~ -M~A-T~A~)
E~porimonts wer~ run in ~tirrod i801 h- 1 batch r~Actor8 (reaCtor
~o~ignQ 2 or 3) to dot~ 'n9 the effect of t __ ~ture, molar ratios of
rH_ctsnts and amount of MPSA on reaction rat 8 and }~roduct distr;hu~ n,
Re~ults woro ~_own in Te~bl~ I
GrArh;c-l e~nalysis of the rosult8 in Table I ahows that f, t;-~n of
2,4-B~IPF wa8 relatod to the reaction t _ ~ aturo As th-- t~ _ e turo
incroas--d, the 2,4/4,4 rntio in~ In contrAst, t_o ~henol/Fn ~lo
ratio _as littlo e~f~ct on t_e 2,4/4,4 re~tio T_~ yi--ld of 2 3 Aaduct
incr-~e3 --ko~ly as the ratio of ph~nol/Fn decreases _rom 15 1 to 2 5 1
and the r-action t~ eL-u-~g was i c, - ~' ~rom 25~C to 85~C
GrA~h;cAl ~ne~ly_is o~ r~sults for runD e~t 18 molo rercont ol~ D~PSA~
in torm~ o_ initial roeaction re~tos (B}~PF ~108/L~hr) ~howod a me~rkod rate
25 incr--as~e in goinS~ ~rom 25~C to 85~C
Inc ~-~;nsJ the c ~n~nt~ ation o~ MPSA catalyst also s~ivo8 th~
~~ect--d i~ in the reaction rato Tho phonol ~1 -o~ ~ o ratio al80
al~~ects tho roeaction rat-- It we~s Bolioved that hiSrher ratio# o~ ~henol
to ~1~c, - were 7~rsfic;Al for ccn~nQ~t;~na, run in a solvent, such a8
30 ~i r~ , lmnthane
-67-
.~
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
TABBE I
P~ENOL + Fn ~ > B~PF
Cataly~t MPSA, 10% Fn in DPM
~ alytical Metho~ 2 - W Dot~ctor
Run TQmp.Nol~ Molo% 2,4/4,4 2:3/4,4 Conv. In$t$al
# (~C)RatioNPSA Ar-a Aroa (%) Rat-~f
1 558.6212.8 0.0261 0.0198 99 0.16
2 252.467.9 14 0.0002
3 85 2.46 7.9 0.0389 0.114 62 0.047
4 2514.757.9 12 0.028
8514.757.9 0.0299 0.0098 99 0.53
6 252.4617.7 5 0.001
7 852.4617.7 0.0334 0.102 67 0.59
8 2514.75 17.7 0.0202 0.0074 99 0.041
9 851-.7517.7 0.0317 0.0106 99 0.88
258.6212.8 0.0239 0.0179 99 0.0042
11 858.6212.8 0.0354 0.0244 99 0.73
12 552.4612.8 0.0252 0.0673 57 0.035
135514.75 12.8 0.0237 0.0076 99 0.17
14 558.627.9 0.0264 0.0177 99 0.12
558.G217.7 0.0291 0.0248 99 0.42
16 558.6212.8 0.0285 0.0185 99 0.15
17 6314.7512.8 0.026 0.00911 99
186314.75 12.8 0.0259 0.0088 99
19 6314.7512.8 0.026 0.0088 99
6314.7512.8 0.0266 0.0084 99 0.29
21~ 6314.7512.8 0.0272 0.0096 99
22 6314.7512.8 0.0265 0.0098 98
236314.75 12.8 0.0269 0.0092 98
24 5514.7517.7 0.0255 0.0084 98 0.36
S52.4617.7 - - 24 0.14
26 639.9 5 0.0258 0.015 98 0.078
27 a514.7512.8 0.0178 0.0067 98 0.013
Fool~t-- to Tabl- I:
BF ~ BXPF (4,4-$~omor)
2s3 - t~o:thr~o adduct
~BXPF mol-~/L hr.
~ r-cyclo
-68-
CA 02230272 1998-02-24
W 097/08122 PCTAUS96/13683
TABLE II
Pu~OL ~ Fn -----> BHPF
Catalyst MPSA, Various % Pn in Solvents
Analytical Nethod 2 - UV DetHctor
Run ~emp Solvent % Fn* Mol~ MO1Q% 2,4/4,4 2z3/4,4 i~ %
_ Ratio MPSA Ar-a Area hr Conv
1 65 DPM 21 10 18 0 029 0 017 2 98
2 55 DPM 55 15 6 5 0 012 0 008 4 100
10 3 33 DPM 38 4 30 11 5 0 013 0 005 2 5 95
4 45 DPM 55 20 8 14 6 0 016 0 006 2 100
DPM 55 15 5 0 0 013 0 005 6 100
lA 27 DPM/MC 29 21 4 6 0 010 0 005 19 5 100
2A 35 ~ 55 21 8 0 015 0 004 6 5 86
15 3A 35 ~lr - - 13 21 25 6 0 018 0 005 3 25 99
4A 35 2,4,6T~Ph 14 2114 6 0 014 0 002 3 38
*I~ nolvent
DPM ~ d~phenyl t~-~
DPM/MC ~ diphonyl- -~-~o ~ methylene ~h- ~r~
e o ~ methyl l~ ~o
r - ~ chlo~
2,4,6T~tPh ~ Z,4,6-~rimothylPhe~l
Th- result~ in Table I showed that th~ 2,4/4,4 ratio ~tayg const~nt
cv~v~ dion i c ~ the 2 3/4,4 ratio in~
Th~ nmount of MPSA cataly8t w re r~lated to th- ~mount of 2,4-
i- ~ iC proauct formed ~i~h 2,4/4,4 ratios at hi~h ~v -~ tl~tions o~
MP8A wer~ prob~bly r-lat~a to a ~hift toward nn acia-catalyzed roaction to
~roauc- relntiv~ly hi~h-r ~ r of 2,4-isomer
~XAMPLE 13
B OF SOLVENTS ON rk-v~u~- DIS.~l~u 1~_ AND CONVERSIONS
Ex~orimentN wer- run in ~tirr_d tank batch r-actors to d-t~ -
wheth-r u~e of a ~olvent was aav~ . Results of thosQ experiments
w ro shown in Tabl- II The use of a ~olvont do-s not a~p-ar to b~
adv-~1r~ Comparison of a run usin~ 10 ~e ~ent DPM with neat run, at
th~ Aam~ ~P8A ~ ation, showed that re-~~ rates wer- hiqh~r for
th n-at run, although tho DPM run u~es 2 5 tim-s mor- catalyst/Fn
~i~h-r 2,4/4,4 and 2 3-aaduct ratios for th r~ti~ in DPM wa~
anothor A; ~- ~v~n~e It was th-r-fore preferred to run the CAn~ t j
in oxc-ss phonol a8 solvent
-69-
-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/l3683
EXaMPI.E 14
~rrr~C~S OF TEMPERATURE, MPSA CUNCr.~ AATION AND PHENOL/FLu~ RATIOS ON
Pk~J~uuc. DI~.,~I~u.l~w:i (EXCESS P~}ENOL AS SOLV~NT)
R--actions wore done in iso1 hr- 1 stirrod tank rQactors as d--scribed
5 above. Results were };~reso~to;l in Table III. Tb~- rosults domonstratQ
that $--~ Q;rl~ the amount o~ catalyst incro~s~d tho 2,4/4,4 isomer ratio.
IncrozL~ing tho r--action ton~oratur-- or decroa~ing tho phenol/Fn mole ratio
l-ad~ to hi~hor ~ -~ of 2:3 adduct in tho product mixturo.
EXAMPLE 15
10 R~cuv~nY AND RECYCLING OF MPSA FROM THE R3lACTION ~m~S
Runs ol~ lOO mI. to 1.5 L (roactor d--signs 3 and 4) were dono to
~let~ whether MPSA can bo ~xtracted from th- n--at BRPF reaction
~olution with wat~r and rccyclod to 8~ t rum-. Tb-- efi~oct o~
~tirrer rpm on tho timo requirod ~or hroakin5J a rosulting ~ w~re
15 ~l_o investi5rat-~d.
Phenol wa_ wni5Jhed and charSrod into the reaction v ~88el. Fluo ..no..a
was .~ ;STb-' and charged to tho roaction v-ssol, followod by a . iS,' -
~quantity ol~ MP6A cataly~t.
-70-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
TAB~E lII _
PH ENOL f Fn - - - - - > B~PF
Cat~ly~t NPSA, Neat Reaction~ - no ~olvent
~ Analytioal Nethod 2 - UV Dotector
5 Run TOEmp Nole Mole % Time 2,4/4,4 2 3/4,4% % MPSA
~ (~C) Ratio MPSA ~hr. ) Aro~ Al~ Co~. ~
1 65 10 5 2 2 0 017 o oll 99 0,0470
2 35 2 8 3 0 013 0 003 98 0 0382
3 s5 21 8 1 5 0 016 0 004 100 0 0382
4 63 lo 2 7 5 0 014 0 008 97 0 0187
5 63 1513 1 0 017 0 005 - 0 0846
6 28 lo s 20 o 011 O OOB 92 o 0470
7 45 lo 5 5 0 012 0 007 91 0 0470
81 3621 8 5 0 013 0 005 62
9 36 213 9 7 25 O OlZ 0 005 95 o ols
lo2 3621 4 6 5 0 012 0 007 96 0 0202
11 35 21159 1 83 0 028 0 011 loo 0 6932
12~ 4515 5 2.5 0.013 0.006 99 0 0330
13 45 15 5 3.5 0.014 0.007 100 0. 0330
20 14~ 5521 3 2 0.0~4 0.006 99 0.0146
153 35 21 18 mpa 20 0 034 0.011 100 0.0580
5.2 msa
~acuum used to romove water aurin~ reaction
~ ~ les~L
~ c.. l ~ 8i - ve~ u~ed to remove wat-r whilo roaction wa~ taking placo
mor- catalynt was added
2 Fn ~ddod c~ ~u~ly over 43 min
3 ~SA (mot~ - 14onic acid) ana NPA (I- ~Lopropionic ~cid) u~ea
in~t-ad o4 NPSA
-71-
~r
CA 02230272 1998-02-24
WO 97/08122 PCT~US96/13683
Tho c -- t~tions of matorial~ in tho r-sulting mixturos were
followea by HPLC (Analytical Mothod 4)
The following mixtures wor- u~od
Neat Runs DPM Runs
Chom wt% th-ory Chcm wt% th-ory
Phonol 82 35 Phonol 47
Fn0 00 Fn 0 00
NP8A0 50 MP8A 0 2l
H2O 0 84 H~O 0 48
lO BHPF16 31 BHPF 9 3
DPM 43
To tho roactor Wa8 ada-d 200 mL of the mixture _nd 200 mL of watcr
The ro3ulting mixturo was stirrod for lO ~n~te~ Th~ ~ha~08 woro A
to n-parato and tho ~oparation time not-d A ~amplo (lO mL) of the
or~anic pha~o wa~ ~ vod for analysi3 by HPLC nnd I C (ion
_L~ to~ ~hy) Tho a~uoous phaso W~8 rot~inod ~or analysis
Tho oxtraction of tho _ ;n;ng or~anic layor wa8 re~-t~, usin~ an
~qual volumo of wat-r (l90 m~) At tho ~na of the oxtraction and
~o~aration, lO mL of tho oryanic layor was ret-;ns~
Tho ~ ;~; ng 180 mL of or~anic layer wa3 oxtract-d with 180 mL of
wat-r A lO-mL ~amplo of tho or~anic layor was rotain~d, as beforu
The aquoous oxtract was distilloa und-r vacuum to ~ivo a g~lu~; ~n Of
yhonol, MPSA ana ~mall ~ r of BHPF Acid titration nna I C analysis
~n~;c-t~ that ~ll of tho NPSA was ~_v_ ~ from tho mixtures Rosults
of ~_~ ~~ -t;ve oxtractions wero ~iv n in Table IV
-72-
CA 02230272 1998-02-24
W O 97~08t22 PCr~US96/l3683
T~1Q IV
~xtraction Data for Neat 21 1 (Ph~nol/Fn) Run
(500 rpm Stirrin~ Rate)
- ~xtraction 1 2 3
5 Pha~e Or~ Aq Or~ Aq Org Aq
g u~nd 217 200 251 4 190 216 2 180
end 262 2 154 9 226 9 214 5 196 4 199 8
% C _ in Phase
Phonol 62 8 6 71 57 6 6 63 59 2 6 39
10 Fn ~ ~~ 0 ~ 0 ~ ~~ ~ ~ ~ ~~
MPSA 0 08 0 55 0 030 0 12 0 0100 0 039
~2~ 23 7 92 7 28 0 93 3 25 1 93 6
~PF 13 4 0 0098 14 4 15 8 0 0073
(150 rpm Stirrin~ R~tu)
~xtr~ction 1 2 3
Ph~o Or~. Ag. Or~ A~. Org Aq
g us~a 217 200 258 190 226 4 180
g on~ 268 6 148 4 237 Z 200 5 195 36 200 6
% C _ - in Phase
phenol 60 1 6 37 61 6 7 17 61 1 6 82
~n o 00 0 0 0 00 0 0 o 00
MPSA 0 10 0 53 0 0270 0 10 -0 005 0 03
P-l H2O 27 1 93 1 24 2 92 7 23 5 93 2
25 P-2 B~PF 12 7 0 0088 14 3 - 15 4
~ 4th and 5th extraction~ oqu~l volumos of organic and water
lay-rs MPSA in orgnnic 0 0030 (4th), 0 0005 (5th), in wat~r 0 0143 (4th),
0 0025 (5th)
~XAMPLE 16
~ ~IOW 8TUDY IN T~E r~ OF MPSA
Aeaction~ wero done in stirr-d batch isot - l reactor~ (r-actor
a~ n 2) To the .-~t~ was char~od a mixtur- of 83 2 ~- c-nt by woi~ht
of ph-nol, 0 09 ~ c by wei~ht of fl~o~ ~, 13 2 ~e.cant by wei~ht o~
B~PF, c~-;~i~g 0 92 ~_.co t by wei~ht of 2,4-isomer and 0 68 ~alcenL by
~ei~ht Or 2 3 adduct Various ~ ~ of MPSA wore ch~r~oa to the
r~nctor Tho resulting mixturos were ~tirred and heatoa Th-
~ tions in the reactor at various times w~re det~ -~ by analytical
methoa 4
~ itions of other reaction mixturos were given in Table V
R~ults woro giv~n in Table VI
-73-
CA 02230272 l998-02-24
WO 97/08122 PCTAUS96/13683
Thea~ r-sult~ ~howod th~t ~-!~;~ mixturos, in thQ pr~nc~ of MPSA,
brin~s about ~ - ;7~iA~ Or th~ r-action mixtur~s toward high~r
c~ r~ -ations of 9,9-bis-(4-hyd~o~y~he~yl)-fluor~nQ. Tho c~ ation
o~ hi~h~r adaucts also i~ 8 a8 a r~ult o~ prol~ng~
TABLE V
COMPOSITIONS FOR ISOMERIZATION ST~DY
T-mp mole/~ mol~L g Rx g
_un ~C MPSA Ph~nol Mix PSA
10 1 70 0.642 9.250 5.42 0.5710
2 55 0.340 9.705 5.42 0.2878
4 55 0.920 8.835 5.42 0.8540
6 55 0.642 9.250 5.42 0.5710
8 70 0.340 9.705 5.42 0.2878
15 9 70 0.920 8.835 5.42 0.8540
lS 55 0.180 9.940 5.42 0.1520
CA 02230272 1998-02-24
W O 97~08l22 PCTnUS96/l3683
TABLE VI
ISOMERIZATION ~uvI~S
% in R~action ~ixtur- % Total+ Tot~l*
Time Ph~nol Fn BHPF 2:4 A 2:3* 2nd add.*
Run 1 0 75.27 0.08 11.94 1.74 0.62 0.00 89.6 99.2
8#1 4 76.86 0.00 13.09 1.61 0.26 0.018 91.8 101.4
S#2 22.5 74.64 0.00 14.05 1.01 0.17 0.15 90.0 99.5
- 8#3 52 76.18 0.00 14.30 0.88 0.14 0.23 91.71 01.3
Run 2 0 79.00 0.09 12.66 1.84 0.65 0.00 94.2 99.3
8#1 4 81.9 0.0 13.1 1.9 0.5 0.00 97.4 102.5
B#2 21 76.71 0.00 13.21 1.56 0.29 0.0242 91.76 96.8
S#3 29 80.41 0.00 14.07 1.53 0.23 0.0169 96.25 101.3
15 8#4 94 80.96 0.00 14.18 0.89 0.14 0.07 92.17 101.2
8#5 101 77.22 0.00 14.62 0.94 0.21 0.10 92.99 98.0
Run 4 0 71.88 0.08 11.52 1.66 0.59 0.00 85.7 99.3
8#1 2 67.53 0.06 12.25 1.68 0.40 0.00 81.92 95.5
20 8#2 4.5 69.3 0.0 12.2 1.5 0.3 0.013 83.3 96.9
8#3 24.5 74.49 0.07 13.18 0.95 0.15 0.04 88.88 102.5
~#4 88 67.54 0.00 13.95 0.70 0.11 0.22 82.29 95.9
Run 6 0 76.02 0.08 12.06 1.75 0.62 0.00 90.5 100.1
25 8#1 2 73.72 0.00 13.01 1.83 0.51 0.00 89.0 98.6
8#2 4.5 75.4 0.0 13.2 1.8 0.4 0.0 90.8 100.3
8#3 24.5 78.39 0.00 13.36 1.17 0.18 0.02 93.13 102.7
8#4 88 74.06 0.00 14.99 0.76 0.10 0.12 89.90 99.4
30 Run 8 0 84.03 0.09 13.33 1.94 0.69 0.00 100.1 105.1
8#1 7 78.92 0.00 14.00 1.69 0.29 0.00 94.90 99.0
S#2 23 76.1 0.00 14.6 1.2 0.2 0.1 92.2 97.2
8#3 52 79.09 0.00 14.92 1.02 0.15 0.12 95.30 100.3
35 Run 9 0 72.59 0.08 11.52 1.68 0.59 0.00 86.5 100.1
8#1 3 68.42 0.00 12.90 1.31 0.20 0.03 82.87 96.5
~#2 7 70.5 0.0 13.2 1.0 0.2 0.1 84.8 98.4
8#3 22.5 70.86 0.00 12.99 0.76 0.16 0.19 84.95 98.6
40 Run 15 0 80.93 0.09 12.97 1.87 0.66 0.00 96.5 99.1
8#1 2 83.36 0.00 13.83 2.01 0.66 0.00 99.8 102.6
* -~t~
~ AI~ c~t~lyst
EXaMPL~ 17
ruKI~l~ATION OF 9,9-BIS-(4-HY~v~xY~n~ ~)F~ORENONE
A. PK~ lrl ATION FRO~ M~.~T~T.T~M~ ~T.~RTnT~
A ~ynthotic re~etion mixture (105.5 ~: 63 ~e ~ ' by woi~ht, 66.5
50 of phonol~ 20 Acrc~nt by wQi~ht, 21.1 ~ of 4,4-BRPF ~nd 17 ~ ~L by
w i~ht, 18 ~ of water) wa8 ~laeed in a 500-mL round-bottom th~
flask equi~-d w$th a ~--t;~ mantla/Variac, 1'- -tor, ~tirrin~ bar and
di~t~ t;A~ arm. A ',~1- o-Wateh" wa~ u~ed to control t~ _~~turQ of
the liquid in the ~la~k. A ~o~arate 1 hr t~r wa~ plaead in the
d$s~;ll~t;~~ towor to monitor t~ t~a in thc va~or phaca.
-75-
CA 02230272 l998-02-24
W 0 97/08122 PCTAJS96/l3683
The mixturo was stirred and heatOd at ~ ' ant prO_8ur- up to a
t~ _- atur- of 160~C, during which time dist;ll A~; on of phonol ~nd wat-r
occurred Analysis of the rOaction mixture ;nra;~-t-~ the phonol BHPF mass
ratio wao 1 1 The rOaction mixturo, whilo still hot, wAs 810wly addod to
176 g of BHPF-saturatOd methylene chlorid- and the resulting mixturo wa~
~lowly swirlod to produco a h~ a #olution, cl0ar and yOllow in
color Tho mixtur- wa8 allowOd to cool to room t _- ~tur- which causos
cryst-ll; 7~; A~ to occur
The rod-lik- crystals ~.~~ - in the ma~ma were analyz~d by
microscopo prior to filtr_tion A~L-- t~ly 80 p~ -O L o4 th~ crystals
vl-wod wore longer than 100 microns nd havo a diamet-r bcL~-n 20 and 50
micron~
Tho crystal magma was filterOd on A medium porosity gla~s frit, using
a vncuum ~ ~ ~d by wat-r jet The ~iltor cako was al;o~l-f t ~hoa
15 with 79 g of BHPP-satur_t-d methylOnO chlorid- and then 72 g o~ hot (90~C)
w_ter A~t-r drying _t 65~C in air ov~-n;~h~, 12 9 g of white ~Lvd~vL was
~C_v~L~ a, T~ol~A~tqd yiola wa~ 61 ~- ~ and HPLC purity was 99 8
-~L
B ~lrTr~S WITH S0DI W nIc~ ~ SOL~TION
Synthotic roaction mixturO (105 5 g as in Exam~le 17A) was
with 100 mL o~ a 2 ~- .~L by woight a~ ~_ ~ soln~;~~ o~ sodium
~ic--~An-te Tho mixturo was agitated and th-n the or~nnic and aA~uoou~
lay rs w r- A~ to ~-parat- in a separAtory funnel The organic layor
wa8 dr_wn o4f This proc-s~ was p-r~ormed a total o4 4 timos Washod
25 r-action mixturo (85 2 g 58 _-~.~t, 58 g of ~h-nol; 17 4 _ ~a~L, 17 g
of 4,4-BHPF and 25 pCl _~L, 21 g o4 w~t-r) w_s placod in the ap~aratus,
d-~crib-a in ~ _~o 17A
Th- mlxtur~ wan stirr-d and hoat-d at a prossuro of 80 to 100 mm Hg,
u~ to a tr _~ ~tur- o4 160~C, duriny which tim~ distillation o~ ~h-nol and
wat-r ocv~ ~d BHPF-~aturat-d phenol (100 ~) was then adaOd to tho
r-act~on mlxture and th- t~ _ aturO of the mixtur~ was controllod -t 65~C
Cry~tAll;~-t; An b-gan within 1 hour The slurry was ~tirrod o~ rniuht,
aft-r wh~ch tho rod-liko crystals ~l_- 1' in tho magma woro nnalysod by
miv~ b-fore filtration A~prA~; ~ly 30 p~lc~t of th- crystals
35 ~i-wed ha~e a length grOater than 100 micron8 and a diam~tor b_L ~ 10
nd 30 microns
-76-
CA 02230272 l998-02-24
W O 97~0g122 PCTAUS96/13683
Th~ crystAl magma was ~iltor-d on ~ m~dium poro~ity ~lass ~rit usi~g
vacuum producod by a wat~r jot Tho brown ~ilter cake was
~nhsd with 200 mL of room-t~ _~ aturQ water and th~n stir-
wa~hed with tho samQ amount of watQr Tho brown/boiu~ cryst~ls worc th~n
wn~hod with sHpF-~turated m~thylon~ chloridQ and th~n with ethylene
~ o id- Aft~r drying at 65~C in air overnight, 7 g of brown ~ vl~L
wero ~a~ The ;~ t~d yield was 47 ~v~a~- and ~P~C ~urity was
99 7 ~- __.L
C DISTILLATION OF PHENOL, CRYS~TTT~ION FROM TOLUENE
10 Synthotic r-action mixture (149 ~ 17 5 _~ c~ by weight, 24 g o~
4,4-BHPF; 95 g of phonol and 30 g of water) w r~ charg d to tbo r~actor,
d-scrib~ in ~xampl~ 17A Tho mixturo w~s 3tirr-d and hoated at a
~ro~ur- o~ 80 to 100 mm H~ up to a t~ tur~ o~ 160~C, durin~ which timo
dist~ ti~ of phenol and wator oc~ d until the ~henol B~PF m 88 ratio
wa~ ~ to L~L - tely 1 1 Th~rQ wa~ no incr-aso in adduct
~.. ~.1 LCLtion~
Tho rosulting mixtur- was added whilo 8till hot, to 121 g of BHPF-
~aL~ cted tolu~ - The rosulting ~ solution was ~ a d to cool
to room t~ _- ~L~ro, during which cryst~ll;7-ti~ oc_~ ~d Tho r-sulting
ao rOa-lik. cry~t~ls p ~ ~ in the magma w re analyz~d by mi~ o~co~u prior
to ~iltration A~ - ~ly 20 ~e~~_ L of the cry8tals vi-wed had a
length ~ -t~r than 100 microns and a ~;~ er bet ~ 10 and 50 micron~
Th~ cry~tal magma was ~iltorod on a m~dium ~orosity glass rrit uning
a vacuum ~ l~-d by a water j-t Tha pink ~iltor cnke was treat-d
25 5; ' 1 ~- ly to other ~ Aft-r drying at 65~C in air ov~-~ig~f, 18~5
of pink product wor- L~_~. ~d J~-~at~d yield was 77 ~ _~t and HPLC
purity wns 98 1 ~_.~nL
D DISTILLATION OF PHENO~; cRysT~TrTy~TIoN FROM ~U~T.~.
~'Ur.~)}2TnTC
30 Ph-nol and fluo.. r - w ro - ' ~' in tho ~-es__~n of 3-
~ a~L~,~ 1 f~; c acid (~PSA) to producH a reaction mixture which,
aft-r ~i~g to remove th- acid cat_ly8t, co~tA;~d 20 w i~ht ~9 of
4,4-BHPF, 64 wei~ht ~- ce~L of ph-nol and 16 weight ~ ~ t wnter Tho
r-action mixtur- Wa8 di~tillod under wat-r jet vacuum (a~ - tsd 80 mm
H~) up to a t- _- ature of 160~C to yi-ld a residu-, c~tA;~;~g
~- -l t~ly 50 wei~ht ~ ~. of phenol and 50 weight ~-rcont of 4,4-
B~PF, that is, a 1 1 ~h~nol 4,4-B~PF ma~s ratio
-n-
f
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/13683
The 1 1 mixtur- was cooled to 120~C and thOn pourod into 176 ~ of
room t~ _- ,ture mQthyl-nQ chlorid-, that had been proviously saturated
with 4,4-B8PF This re8ults in a cl- r ~ ~ solution at re~lux,
wh~ch wa~ ~lle~' to cool to room t~ _~ atur~ ThQ cryst~ ed mixture
wa3 ~ilt-rOd undor vacuum at room to _-~at~ ~ The filtor cake was
~;~pl~c ~ wa~hQd with 79 g of BHPF-8aturated methyl-ne chloride (no
~t$rring oi tho filter cake durin~ the wa~h) and thQn sti~ d with 72
g of hot water (90~C) ThQ reaultin~ whit- filtor cakO w~s driod in air at
60~C to provide a yi-la of 61 woi~ht ~B' -_~L Or the 4,4-B8PF orig;~lly
d-t-ctod in the r-action mixture
~ REMOVA~ OF P8ENO~-WATER AZEOTROPE
A~p v- t~ly 380 g of a r-action mixtur- si~;l~ to thnt Of _le
17D w r ~lowly dripyQa into 4 B of wator at a t~ ature of 84~C at a
prQ~ur- of 300 mm Hg This dilution in water wns ~c~ _~~;e~3 by tho
r moval or phenol in the form of a wat~r/ph-nol ~-,L ~ ThQ wator-
insoluble mat-rial~ th n procipitatQ from the liquid phase as a white
powd-r ~p - t~ly 64 ~ of "cruae" BHPF was obtainod in this mannOr
The "crud~" BHPF CA~ ~' all th impuritio8 ori~;~71y ro~t-;--' in thQ
r-action mixtur- The ~roduct wa8 filterO-d, washed with bA~ 1 ;n~ wat~r and
20 dri-a in air at 60~C to provide a .ec~ ~y of 96 wQi~ht ~7. ~ of the 4,4-
BHPF originnlly aot-ct-d in the reaction mixtur~
F CRYS~TTT7'~ION FROM TT~TT~ROEY~P~r'~-~NE
A reaction mixturo (55 8 ~ 63 1 ~e ~_ t by w~i~ht, 35 g of ~henol;
14 ~_ c_nL by woi~ht of 4,4-B~PF and 23 ~e ~o~t by w i~ht, 12 8 ~ of
wat-r) was cLa.~_' to a 250-mL round bott_m flask, otherwi~e fitted out as
in _3~ 17A Trii~ (TIPB, 106 6 ~) was add-d to the
mixtur- in thQ flask, a~ a renult of which thOe mixture ~e~ ~tOs into two
~h~-~, of which tho yollow reaction mixture was thc lowOr
The mixture wa~ ~tirrOd and heated under vacuum, ~ by wator
~et (ca 80 mm) After rOmoval of
wat-r at 50 to 88~C, the mixtur- appQar~d ~ ~e - ~ Th mixture was
~tirroa and ~ to cool Solias ap~ear when the t _J~r~L~ e r-ached
70~C Tho m$xture was ~11_ d to cool to roo~ t- _~ ,turQ ana filteroa on
a gla~n frit undOr vacuum ThQ white sol$ds on the frit w ro washod with
TIPB Th~ f$1t-r cako was left ov~r~;g~t und-r vacuum (water j-t) whilo
air was ~ullea throu~h thD filt~r cakO
-78-
CA 02230272 l998-02-24
Wo 97/08122 PC~nJS96J13683
Analysis of the r~sultin~ mother liguor showed that littl~ of tho
ph-nol in the ~eod was ~. v.~ ~8 ~ ro~ult o~ dis~ ~. The 4ilter
c~k~ c~~ 4.2 g of whito, uoarly free-flowin~ product (54 p~ L
~c_v~ry, 98.8 ~ c~t purity by ~PLC).
O. DISTILLA~ION TO REMOVE PHENOL; CRYS~TTT~IoN FROM M~VT.~.
,~ ~UT.I~T~ TnT~
A roaction mixturo (98.8 ~: 61 ~ ~L by wei~ht, 60.3 g of ~hnnol;
19.4 pc by weight, 19.2 g o~
4,4-~PF and 19.6 ~8~C~L by woi~ht, 19-4 g of wator) W~8 char~ed to an
~p~aratus, do3cribQd iA Examplo 17F. A colloction flask was attached to
tho di~t~la~j~n arm and a~ e_L~ to a vacuum source (a wator jet). Tho
t _~ aturo ~ot point was adjustea to 100~C and ~-t;~ bo~n.
-79-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
~omp (~C) Observations/Actions
50_55 ho;l;~ g ana aigt;ll A1;~ occur
100 ~light b 1~bl;r5r
~ot point raisoa to 120~C
105 di~t;~l~ti~ restarts
115 ; ~ o; 5~; f irAr~t aist~11Atj~
~et point raisoa to 140~C
120 visJorous bQ;l;~g, l$ttl- ais~; 1 At~
va~or t~oraturo risin~
121 vigorous ho;l;rçr, diE~t;ll A~ starting,
vapor t~ _ = ature 115~C
122 vapor t- _- tur-- 118~C
}~re ~k vacuum and romovo sam;plo 34 Si of dist;ll Ate
~ll~cLod, mixturc in pot _as phenol:B~PF ma~s
ratio of ca 2 1
r~c~ c L vacuum sourc ~ nd, . -~ h~At;r~5r
124 vapor t~ raturo was 119~C, dist;ll~ti~
~t Irts
125 vapor t~ _ ~turn was 119~C
bre~k vacuum ~na ~le mixturo
13.9 g o~ di8t~llAte coll - cL - d
~--t point 120~C at al ~-~ ic pros~ure
c_ - L vacuum and ~ hoating
~honol B~IPF 1 6 1 mass ratio in pot
129 vn~or t~ _- .tur- 121~C
~li8t; 11 A~ starts
131 vapor t~ _- ~ture 123~C
stop ol?oration, r~movo ~am~le
42.4 sr remain in ~ot; mixture in ~ot _a8
~_enol:B}IPF ratio ca 1.12:1s 6.7 ~ o~ di8~ te
Q~l l ~_ ~ea
-80-
CA 02230272 1998-02-24
WO 97/08122 PCT~US96~13683
Tha pot r~siauQ at 110~C wAs add-d to 172 g of fro~h d ~l~dc
mothylonQ chlorido in ~ bottlo Tho addition was done ~lowly in order to
avoid _ c-q~ive flA~hing or bo;l ;n~ of th- methylene c_lorido The
r-8ultin~ mixtur- ~ ro or 1O88 separatod into two layQrs, o_ which the
upper layer W~8 richer in the ~honol BHPF _ _ The mixturo wa8
- ~wirl-d and b-came h ~ e~8 Th- bottle wAs seal-d and placed in a ~_n
of cold wat-r (~ t-ly 10~C)
Tim Ob~or~ations/Actions
(hr min )
lO0 08 ~ _onc.~s yollow solution
0 18 ~ e~u8 yellow ~olutiAn
0 56 yollow oolu~i~n, ~osP;~ly small crystal~
1 18 samc
2 00 ~ame
152 24 cryst~ll;~A~ti~n ~n~o;n5, quit- ~ fQw crystAl8
17 48 solid yellow cry~t-ll;nA mass breaks u~
~nsily
filter und-r vacuum t_rou~h glas~ frit to
obtain nl;gh~ly yollow crystals; ___~ ~L
158 8g of yollow mother liquors
Tho cryst~ bottl- was rinsod with 29 7 ~ of fre8h methylenQ
chlorido (not all ~olids ais801ve), the rRsulting mixture being us-d to
A~O~1A- ' wash the filtor cakG, which ; ~ slightly in color
The ~iltor caka was slurry wa~hod with 49 4 g of _rosh methyl-ne
chlorid~ nnd the r-~ulting ~lurry wns ~iltorod und-r vAcuum Th- color of
tho filter cako was ~n~A~
The ~iltor cak~ w~8 ~i~l A- wa~hod with 33 ~ o_ cold water,
without a chango in tho color o_ the cako Tho _iltor cako was slurry-
wash~d with 40 g o~ ~oil;ng wator, without a chango in tho color of the
cake
Th- cako wa8 dr~-d in air undor vacuum _or ~ - tely 2 hours,
tran~forrod to a watch glass and driod in an oven ov~T~;ght at 65~C The
cako w~8 sl;gh~ly yellow
Tho mass b-l An- ~ for the ~ =-~8 was
-81-
CA 02230272 1998-02-24
WO 97/08l22 PCTnJS96/l3683
of BHPF
19.2in ~nitial mixtur-
8.lin mother liquors at ~nd o~ oporation
9.1;~ t~' ~roauct
1.7in wash
0.3~ co~t~ ~or
H. CRYS~TTT7-~IoN FROM Dl~n~Y.M~
1. A mixture of 28 ~ of DPM, 17.5 g of ~h-nol, 5.4 ~ of BHPF
(98:2 4,4- to 2,4- isomers, by HPLC) nna 12 mg of MPSA was washed w~th
water. The rosulting layors wer- s-paratod ~nd the wator layor wac
. y~d. Phenol was distilled from the or~nic layer to ~ive a mixture
e~t-;~;~g 21.8 ~ of DPN, 3.8 ~ of ~henol and 5.4 ~ of BHPF. Tho mixture
wa~ to bo cool~d to room t~ _- ~L~ a to ~ive an off-white precipitate~
whieh wax filt-roa and wash-d with DPM. The washed cake was dri~d in an
oven at 60~C to giVQ 6.1 ~ of mat-rial, c~i~;~;~ 70 pe _~L by woi~ht of
BHPF and 30 ~ . - by w i~ht of phonol. The mixtura wAs strippod at 140~C
under nitro~en at < 80 mm Hg to ~ive 4.2 g of white 301id. The solid, by
~PLC analy~is (analytical method 5), c~~t-; ~~ 99.6 ~ c_nL by ar~a Or the
4,4-i~om r ana 0.04 ~ by area of 2,4-isomor.
2. A r-action mixture from 15:1 phonol:fl~v ~nv~G~ c~t~ ~g
34.5 ~ c~nt by wei~ht of phenol and 10.5 ~9 ~nt by wei~ht of BHPF, wa3
tod with 55 ~2'~ ' by woi~ht of DPM. Phonol (80 ~e~ -- of that
$niti~11y ~l~- , t~ ~turo 105~C, 4.5 mm H~) was 1- v~d by
di~t~ t;~ to yive, after cryst~ll;=-tio~ from DPM, white BHPF meltin~
at 221 to 222~C. Th- 1~ _v_l~ of BHPF was 78 ~el -~t. Th- material
cA~t~ 99.6 ~ . of the 4,4-i~omor by HPLC.
I. CRY8~TTT7~ION FROM NEAT PEENOL
P~onol was distillud from the r-action mixturo8 to ~ v~c~ mixturos,
eA~t~;~;ng l-~8 than 50:50 phenol:BHPF by wei~ht. The r-~ultin~ materials
can bo wanhoa with mothyleno chloride. Th- products were ;~ist-nt in
color and e~t-;~ ~mall cry~tals, usually of the size of 10 to 70 micron~.
J. rn~- ~l-.ATION OF BHPF BY ADDl.lO.. TO WATER
Addition o~ r-action mixturo~ to koili~ wator or stoam z- ~d 30me
phonol a8 a ph-nol-wator A-H_LL~o. Tho r-~ulting ~roduct retained most
of th~ : - ~ i n ~ ~ and adducts ana co~ ris-d vory ~mall cryst~
-82-
CA 02230272 1998-02-24
wo 97/a8122 PCTAUS96/13683
th~ ~izc of the order of lO to 20 microns. The purity wa~ 97 to
98 ~e ._
Analytical data (HP~C) was given in T ble VII for S _le8, ~ ed
~ in accG d~.co with the practice of thQ invention and for - - cially-
av~;l~hl~ material~.
K. REMOVAL AND k~-uv~nr OF PHENoL FROM BHPF REACTION ~n~
Excoss phenol was 1. v~d from a reaction mixture to a l:l ratio of
phenol:BHPF usin~ a fA~l;n~ film ~v~y~l~tor. This was ac~ at 120~
C/120 mm H~. At thi3 t~ _ ~tur-, B~PF ~ol~ ty in ~honol w~ 45
~ ~QnL.
After removin~ phenol, the BHPF-~henol mixture was ke~t at 90~C and
stirrQa prior to adaition of methylene chlorido or other cryg~ll; ;ng
~olvent.
B~PF was cryst~ at room t~ ture under a nitrogen -y~d. A
batch cry~t~ w~ cool-d to 5 to 10~C for several hour~ auring which
BHPF cryst~ sOlia BHPF was separat~a from the rosultin~ slurry
u~ing a batch yr~s~ure filt~r or ba~k~t ~ilter. Opti~-lly~ a pressuro
~iltor can bc uuod. Nethylene chloride or other solvQnt can be recycl-d
to the p .~8.
BHPF crystals wer~ dried undor vacuum.
B~PF from 8losg (R; ~gh , ~1~ T_Q xample ~v~lu~t~1 was a
~ry solia, from lot number 9307-03.
BHPF from Isonova (Austria): The sample ev~ tr1 was ~eqi~~t~
"Isonova lO/93".
BHPF from Rut~r~-Nea~e (Stat~ Coll~_-, Pennsylvania): The dry
~olid was from lot numbor 9306099.
BHPF from I~ovolta (~ , Austria): The shmple, u~od a~ standard
_or th- comparative ~tudie~, was roc~ived in 1988 ana was ~-sig~-t-~
"Isovolta 1988."
Analy~is of the r-sults in Tablo VII s_ows that 4,4-BHPF, ~urifiod
by ai3t;1~tivQ removal of phenol:water azeotrope and ~xtraction with
m~t_yl~n~ c_loride, produces high ~urity BHPF.
-83-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
TABLE VII
SamplQLot No 4,4-BHPF 2,4-BHPF 2 3 Other Total
Sourc- BHPF Impur
Aaa.
810x~9307-03 100 -0 0 0 0
I~onov_ 10/93100 0 0 0 00
~Cl~Ex 17D 99 94 0 06 0 06
WaterEx 17E97 591 87 0 40 0 14 2 41
ppt'd
I~ovolta 1988 100 0 0 0
Rutg-r~ 9306009999 28 0 58 0 14 0 72
Noa~-
DPNEx 17H 99 6
EXANP~E 18
~RR~SI~M ~ ~l~
A REACTION MIXTURES FOR BHPF PROCESS
Corrosion t-~ts werQ ~erformed using A LC~ ~-- ' atiVe r-action
mlxturo for tho c~~~tion of ph-nol with ~l OL~e usin~ various
cataly~t~ The t-st~ were done usin~ motal ~ 3 81 cm in len~th,
10 1 59 cm in w$dt_, 0 32 cm thick, and havin~ a 0 64 cm hol- cont~rod in ono
~nd Tho _~ wHre ~ol~At~-~ from ~ach other and the ';~ rack
u d n~ polytQI A~ ~ro~thylene ~h~Ul~r ~ Tho ~ wer~
~ d to both the liquid and vapor pha~e~ of ~ach t-st c-ll The
contA~t~ o~ tho c~118 w-ro ~tirred c~ o~ly and wer- ~A;~ at the
nel-ct~a t~ _- ~tur~ u~ing YSI tffmy-rature controll-rs and C'' ~OT.~
~-a~;n~ -lor Th~ t-st~ w-re run under a nitro~en ~ad T_e chloride
co71 ~t- o_ the t-st mixtur-~ was ~500 ~pm Th~ tost~ woro run at 65~C _or
13 day~ (312 hour~) C _-~itions te~ted and rosults are ~ in
Tabl~ VIII
Rosults in Tabl- VIII d~mon~tratQ t_at tho r-action mixtur~s usea
w re c~;do ~bly l-ns corrosive than co v~~ Ally u~o~ roaction
m$xtur-~
-84-
CA 02230272 l998-02-24
W 0 97/08122 PCTAUS96n3683
TAB~E VIII
Cn~OSIn~ i~ ~lN~ FOR BHPF REACTION ~l~ ~K~S
A R~actor Mixture 90 2 ~orcent of Phenol, 8 3 ~orc~nt of Flu~ ~ ~o,
1 5 ~L~ - nL of MPSA (by woight)
Corrosion Rate (mpy)*
M-tal~iquid Vapor R~
316L ~8** nil*** < 0 1 uniform corrosion
904L ~8nil 0 1 ~;f~~ eorrosion
2205 8Bnil < 0 1 unifonm corro~ion
10 254 8M0 8~ nil nil ,,~;c corro~ion
B Water Fxtraction Mixturo 8 0 ~lccnL of Phenol, 91 ~eY~ L of Water
ana 1 0 porc~nt o~ MPSA (by weight)
Corro8ion Rate (mpy)
M-talLi~uid Vapor R _k~
15 316L ~80 1 0 1 ~;f~ corrosion
904L ~8< O 1 < O 1 uniform corrosion
2205 ~< O 1 ~ O 1 uniform corro~ion
254 SMO ~ < 0 1 < 0 1 uniform corrosion
C Rocyclo C~-~trat~ 29 87 ~c~ of Ph-nol, 69 23 ~cL~nL of Water
20 ana 0 9 ~ c~nL of ~PSA (by wei~ht)
Corrosion Rato (mpy)
MotalLiquia Vayor R~ _k~
316L ~8 0.1 0.1 uniform corro~ion
904L ~8< 0 1 < 0 1 ~;' corrosion
25 T~ol 625< 0 1 < 0 1 ~;f~ corrosion
~a~telloy C-27 0 1 < 0 1 uniform corro8ion
HastQlloy G-30 < 0 1 < 0 1 ~;f:_ corro~ion
* mpy 3 mils per year; 1 mpy = O 00254 cm/yr
** 5~ = stainlo~s st-el
*** nil ~ < O 01 mpy
-85-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
B REACTION rll~ UK~S FOR BI~r~NuL A PROCESS
A mixtur~ c~nt~in;ng 94 35 porcont by woight of phenol, 4 15 ~eL~llt
by woi~ht of acetone and 1 50 perc-nt of MPSA was ~valuatod as in Exam~l~
18A
Th- fo~low ng r-sults WorQ ~t-in~d
Corrosion Rato
~etal Li~uid Vapor
304L ~ ~a~s ~ass pasg =
316L ~ p~8~ pa~s< 0 00254 cm/y ar
904L 88 pasD paDs
a205 ns pa~s pas~
825 N-i p~88 pas8
Tho corrosion rat~s in both the liquid and vapor pha~es was < 0 00254
cm/y-ar Tho corro~ion was uniform Tho rat- of corro~ion was bolow that
for cv~v~8i~n-l reaction mixturos for making b;~rh~n~l A
EXAMPLE 19
A PR~PA~ION OF BI~r '~ A ~SING PMBSA
Ri ~ A was proparod from 14 1 ~henol acoton~ (molo ratio) at 50~
C, cQ~t~in;~ th~ i~;iic~t~ ~ r of nolid cataly~t Th proauct
distrih~ti~ was dct-- ~n~ by analytical mothod 2
The PMB8A cataly8t of Examplo 9B, at a l~vol of 6 ~e~c~L by weight,
givos 75 L ~ ' Cv~v~ iOn aftor 5 hours Tho ~roduct c~"~t~ 99 0 1 0
of 4,4 2,4~ (ar-a ~- c ~)
Tho PMBSA wa~ ~_vv~lJ~ and rousea in a ~ocond cyclo The c~lv~LDion
after 4 hourD was 60 ~ c_~L Tho proauct c~nt-;n-~ 99 1 0 9 of 4,4 2,4-
; n ~ (~r~
DOWEX~ 50WX4, from Tho Dow C' c~l C _ y, (35 ,~ c by woight
a~ dry ma~), promot-d with 25 ~ by woight of 2,2-
dim~thylth;~-o~ , was u~-d in a ~ oxp-rim nt The c~v_~dion
aft-r 4 hour~ was 43 ~_~c and tho yroauct c~nt~in-~ 98 0 2 0 (ar ar-a
) of 4,4 2 4-ir ~ D .
Th-so oxp-rim nt~ showod that a polymer-~ ~o Led catalyst of this
inv~ntion gavo high-r c~v~L~ions and ~ higher yi-ld of 4,4-isomer than a
~L~ r- -~ivo prior art catalyst
B REACTION ~SING P~BSA IN A DOWNF~OW CN LNUUUS REACTOR
Tho r-actor co~ri~oa a vertical tubo~ The bottom ~art of tho tub~
wa~ filloa with glass b~ad~, on top of which was ~rovid-~ ~ bod Or PMBSA
-86-
.
CA 02230272 l998-02-24
Wo 97~08122 PCT~U596/13683
catAlyst ro~in Tho L~ ~ of the tube was fillod with ~lass b-ads
The tub~ wa~ fitted with ~ prQ~surs ~au~e, a pr-~sure ro~ulator, h--t;~
mean8 ~Yt~ to the catalyst bod and iood moan~ at th~ bottom of tho
- t~ 7~ roactor for intro~u~;~ tho ph~nol and flUV ~~v~.O reaetants The
~Ooa was pr-pared in a e~~ , provided wlth a nitro~en stream and
_ hoated - e_n~lly by a fluid A valve was ;nt~ -~;ate th~ food
pr-~aration e~~~ r and a ~ump for introduc;~ the f~od into tho bottom
of tho r~actor A roliof ~alvo wa~ ~lacea ~L~ the ~ump and the
roactor
Tho f-ed wa8 i~L~od~ced into the r-actor at a ~ _~-t~ r~to and
pa8~8 u~waraly t_rou~h tho lower bod of ~lan8 boads, w~ich functioned as
a pro-hoator, throu~h tho catalyst bod and the upper bed of ~la88 beads,
wher-u~on the product wa8 . vel from the to~ part of the roactor for
analysis or further ~roce~sin~
~vp~-; ~ usin~ 21 1 ~h-nol fluo _~v~e and P~BSA catalyst gave tho
following ro~ults a~ a function of flow rate and reaetion t~ t~ e
49 C 69~C
Conv r~ion (%) 80 lOO
BHPF in ~honol (%) 14 16
20 Productiv~ity (~ B~PF/g eat/h) 0 57 0 44
Seleetivity (% 4,4-BHPF) 98 97
Flow rate (~ foed/g eat_) 4 39 2 71
Tho~o ox~orimont~ ~howed that lower roaetion t~ ~ture~ favored
proaucti~ity and selectivity toward 4,4-BHPF, ~- _ ;od by aecreased
cv~v~,~io~
C CONVERSION OF ACETONE AS A FUNCTION OF REACTION TE~PERAT~R~
Phonol-aeetono mixture~ (6 ~el~nL by weight acetone) were c~ v~,Led
to b;~ 1 A, using MPSA a8 catalyst in batch reactors The following
rosult~ w~r- obtain-d (T blo IX)
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
TAs LE IX
Acetone Convornion
25~C~ 35~C~ 55~C~ 65~C 75~C
Time (min)
0 0 0.10 0.22 0.25 0.26
0.40 0.48 0.49
0.04 0.42
0.59 0.62 0.67
48 0.65 0.69 0.77
0.06 0.56 0.69 0.75
72 0.82
go 0,09 0.79 0.85 0.89
120 0.13 0.72 0.83 0.89 0.94
180 0.20 0.80 0.95 0.96
240 0.26 0.97
300 0.98
360 0.32 0.99
* mixture c~ d 2 2% by woi~ht of MPSA and 2-3% by
woight of wat--r
mixturo cA~t~ 1 3% by weight Or MPSA and no added
wat--r
D REMOVAL OF MP8A CATAI YST USING ION-s!Y~ RESIN;
PURIFICATION OF CRYS~Ts T~ BIS~ ~ A
C~ A~r~--t;on of phonol with ac ton (4 ~-~c_~L hy wei5~ht),
cA~ g 2 by w--ight of wator, usinsJ 2 5 ~ "t by weight of MPSA, wa~
don-- at 35~C in plu~ ~low moaQ, with a 3-hour r~ui~ timo
Cry~t~ t;r- of l; ~ph~ A oc~ _d in the roactor The crystal~3 w ro
~-ol~t~d ~y filtration and ro~iA~ ~c tone in the moth--r liquor~ wnE~
r-cycl-d to the ~ vv at 50~C TEIE ~thor liquors wore dried at 50~C (20
mm ~Ig? and the cyclo was ~ etv~~t~ after addition o~ m~ko-up f--od A~out
90 1, c of tho ~Icotone was cv~ v, I ed to ~;F~F~ -1 A por pa-- ~
Catalyst wa~ v~d ~rom tho product by first moltin5r the crystals
nd ~ ~7';"sr tho r-~ulting oil with water, and thon ~xtracting the or~anic
layor with wat--r, which ~ r tho acid c~ ation below lOO ppm ft~r
thro oquili~rium ~tagos The ~ ~ cataly~t was ~ v_~ usinlJ Ln
~nion ~ a~ ed (<50 p~nn, the limit of dotection)
-88-
CA 02230272 1998-02-24
W O 97JO8'122 PCT/US96~136~3
Bi8~henol A, i~ool~t~ by a sin~lo crystA~ tion st~p, w~8 of
higher purity th~n ~roduct~, ~n~rally ~~tA;n~d u~ing two
crystAll;~tions. R; o~h~l A ;~l~ted. by a sin~le cry#tAl1;7AI;~,
~ c~~t-;~d a ' of 1200 p~m of 2,4-bisrh~~~l. The proc~ss of this
invention thorefore ~ ;f;~n thQ ~o~ti~~ of high purity of ~;~F~: -1
_ A, ~l~~~t~ ~At~ by oily hi~her ~ te~.
EXA~PLE 20
SOT-ID CATALYST PREPARED FROM POLY~Yn~ BY ~T~-yR~mIoN WITH A~YL RT~MTn~
SULFONATION AND T~T~-~IOU
~. Poly~L~,_ ~ (Amborlite~ XE 305) wa~ alkylated with allyl
b ~ Ag in the ~l~P~ ~e of trifluo ~ ~~;c acid in 1,2-
aichlol~ ~ano at 50~C, gQner~lly in aCcv~a~c~ w~th Tomoi et al., "A
Novel One-~ot Sy~h~ of 8~cQr- modifi~d Polymer Su~ort~ and Phase-
tr~nsfer C~talytic Activity of ph~.h....; Salts Bouna to the PolymQr
15 8~ Lsn, RQactive Polymer~, Volume 3 (1985), ~a~Q8 341-349, to produce a
matcrial having 2-bromo-1-methylethyl chains. This material was
sul~AT-~, ~e~-rA~ly ~t th~ ortho-~osition with .~e_L to the 8ide
chain, by t~_~ with chloro8~lfo~;c acid. The r~8ultin~ ~ulfonyl
chloride wa8 ~o~v.~Led to a sodium ~alt by reaction w~th sodium
ao hiCA~ho~At~. The material wa~ v~rLod to a c~--9~ -A;~ thiol by
r-action with ~odium ~h;o~ei~te nnd c~v~L-d to a co ~ A;~ acid by
acidic hydrolysis. ~at-ri~ls Vl~a ~ co. e~d to 28 and 48 ~ nL of
~lkyl _~Lan functi~l;ty (XEMSA).
B. Tho thus-pr-paroa ~olymer~ (XEMSA) w~re u~d nt a l-vel of 6
~l-.~L by waight ~or roaction ~_~.._ phonol and fl~v~e~one (20.8:1 mole
ratio) at 50~C. Product ~ _-8ition wa8 detr- ~d by nnalytical methoa 3.
Tho polymer co~;~;~ 28 ~e~c~L o~ alkyl ~ ~vt~n funct;o~l;ty
givos 75 v~ L c~v_~ion nft-r 5 hours. Th- product aistrihnti~ was
96.8:3.2 of 4,4:2,4-; - ~ (nr-a v~
The v~od~cL c~~ 48 ~-~c ~t o~ alkyl --~Lan runct;~Al;ty
givos 15 ~ ~v_ ~ion ~rt~r 2 hours. The product aistr;huti~ wa~
96.8:3.2 of 4,4:2,4-;~ (ar-a ~_l~nL).
-89-
CA 02230272 l998-02-24
WO 97/08122 PCTAJS96/13683
EXAMPLE 21
I~ SITU MET~OD FOR PREPARING THE POLYMER-xu~PO~ MERCAPTOSULFONIC ACID
CATALYST (PMBSA CLASS)
A SULTONE ~T.~VT.~ ~ION
S To a mixtur- of 1,4-b~ ultono (30 0 ~, 220 0 mmol, 1 00
~quival-nt) ana ~oly(vinylbon~ylchlorido) (PVBC, 33 6 g, ~ - t-ly 220
mmol, 1 00 oquival-nt) of chlG-~ hyl group8) was addod via a ~ 1 A dry
t-traLy~ ~f~L~ (600 mL) una~r a nitrogen a~ a Tho mixture w~s
~tirred at room t~ _ ~ture until a h ~ n 801ution was obtainod
Tho ~oluti ~n was thon coolod to -78~C u~in~ a dry ic-/nc~tonQ bath n-
Butyllithium (2 5 molar in h -~, 88 1 mL, 1 00 equivalont) was aaa-d
nlowly dro-ywin- to tho -78~C ~olut7~ of 1,4-but -nultono and
~oly(vinylb-nzylchloridc) via an addition funnel ov-r ~yy~-;~ trly 2 5
hour3 with vigorous stirrin~ A whito 801ia bo~ins to procipitato from
tho roaction miXturQ as the n-butyllithium addition was bogun, w;th
procl~itation c~t;~;~g throu~hout th- n-butyll~th~um add~tion y~riod
By the ond o~ tho ada~tion, a largo amount of whito solia has formod
within the r-action ~';
Th~ r~action mixturo (~lurry) was A ll ~' to 810wly warm to room
t~ _- ~turo in tho cool;ng bath (ovor ~yy - to~y 3 to 4 hours) ana was
~ to stir at room t~ ~turo overnight The white ~-~ ~7itatQ
which has form d in the roaction mixtur- auring th- n-butyllithium
~ddltion yoriod ~ ol~hle a~ th- mixturo ~c~-- room t~ _- ~turo
Th- whito (;~-ollhl~) 801id was ~Oa by vacuum filtration The yolymor
can b- washQd with wat-r or wator can be aadoa to thQ THF/polymer slurry
yrior to ~iltration Ada~tion of wat-r somotim 8 r-~ults in in~.~a~;~g tho
tim r-quirod for filtration Tho 801ia was 81~.-y-~ ~h~d with THF, then
with mothanol and ~inally with methylon~ chloriao (c-~ni~g uomo
_ 11;~g) The solia wan dri~d ov~rn;~ht in a vacuum ovon to ~rovide 53 4
g of a whit- solia sultono-runctional yolymor
B T~T~T ~ION
To th~ ~ulton~-_unctional polymer from abovo (110 0 ~ from two
h~t~-~-, ~y~. - --ly 0 440 mol of sulton-) was addQd nitrogon-
~aturntod THF (500 mL) In a ~e~- ~t- r-actor, a 801~1;~ of lithium
1h;~-~atat- wa~ -yr-yar-a by the dro~7wi~0 aaaition of th;ol~tic acid
(49 8 g, 0 650 mol) to a slurry of lithium c~-ho~e (24 2 g, 0 330 mol)
~n water (100 mL, nitro~on saturatoa) The lithium th;oAc-tat~ solution
-90-
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/13683
wa~ added 810wly via ~nn~ to th~ polymer/THF slurry at such ~ r~to th~t
th~ t~ _- Aturo docE not risc abov~ 35~C. Th~ yolym~r ~wells substAnf;~lly
durirA~ tho lithium ~h;o~etate adaition. After the lithium th;o~etate
ition wa8 complete, an additional 350 mIJ of water (nitro~on saturatoa)
was addod. The polymor sw~ to a volum~ of ~ t~ly 1 L. The
mixturo wa~ ho~toa to 50~c, nnd was a~low_~ to roact ove ni~ht~ The ~el-
liko ~olymer wa~ thon filtorea usin~ a coar~e ~lass-fritted funnel. The
polymer w~s washed with wat-r, thon with moth~nol, th~n with mothylono
chloride, and ~inally with ~dditional wator. The polymer slurry ~ometimes
filtor~ vory slowly durin~ thQ ~iltration process. In this case, the
W - ~h~ st~ps ro~uired ~ OA: t~ly two days.
A~tcr th~ ~-A~h;nA~ step~, cA~A~n1~atQd hydrochloric acid (300 mL,
~ -t t-~y 37 ~c _~t by woi~ht in wat-r) was addod to the yolymer.
Tho A~olym~r ~hrinks in volume ana tho HAC1 ~olution was easily - .ed by
_iltration. More cc~n~ ~t~' HC1 (300 mL) was addod to th~ filttr~d
~olid, and the mixturc wa8 ~11 _ .. d to st~nd ~t room t~ _ ~ture over 2
d~y~. Tho ~olym~r w~s th~n washod -~I-n~ively with dilut~ HCl solution,
foll ~-' by A~ -n~ivo water washos. The polymor was thon washod with
mQthanol ~na ~inally with ~ ~rom~thane. Dryin~ ov--~;g~t in a vacuum
oven (60~C/full vacuum) provided thc polym~r-~A~ALod - c~pLosul~onic
acid. The product was iA - ~t; fi ~' a8 PMBSA-SU.
C. CONVERSION OF ~P~SST- RED PO~Y~.~ ~ RAT~SIN A~O
MERCAPTOSULFONIC ACID POLYNER
A c -l~ially-availablo (Fluka ~h~ ) Cro~ n~A
25 chloromethylat-d Merri~ield~ ro~in (2 p c~lAL divinyl~ , 200 to 400
me~h, ~~ y 4.3 mmol Cl/~ , 51.2 ~, 1.0 equivalent) was react-d
with 1,4-h~ ltone (1.05 ~quivalonts) and n-butyllithium (1.0 oquiva-
lont) according to the ~ e d-scrib-d above to ~rovido a sulton--
~unctional ~olymer (70.0 g). S~ e_~-nt thiolA~;~n of the sultone ~olymor
in a m~nner gi '1-- to that describod above ~rovided the c~ ~ i ng
~t~_ lfon~c acid ~olymer (79.0 g dry mAss). Thi~ matQri_l wa~
~ i f; ~ ~ a~ PMBSA-N~R.
In this reaction ~-quonce, the lithium I h; ~A~etate reaaent was
formod in s~tu by slowly addin~ solid lithium c--h~n-t~ to a mixture of
the ~ultone ~olymer and ~h;olAcetic acid in a 3:2 volume ratio of
nitro~on-~aturated T~F/water.
-91 -
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
EXANPLE 22
PREPARATION OF POLYMER-~u~v_~ ~ MERCAPTOSULFONIC ACID CATAY~yST (X~SA
C~SS )
A ~TY~YT~ION OF POL~-Y~l~ ~
To Amberlite~ XE-305 (75 0 ~, ~- -; t-ly 0 720 mol of ~Ly ~ -
r-p-at units, 1 00 oquivalont) was aaaea 600 mL of 1,2-dichlG~
(PDC) The polymer was A~ a to swell in the Lolvent overni~ht 5-
Bromo-1-F - ~ (75 3 g, 0 702 oquivalont) and PDC (125 mL) were nda-a to
n aadition 4unnel The reactor c~~ (polymer slurry and 5-bromo-1-
~e~1~ - solution) wor~ ~vacuatoa ana back-4illed with nitrogon sRvoral
timos Trifluoromet~-n~rulfonic acid (20 0 ~, 0 133 mol, 0 19 equivalent)
wa~ aaaed to tho polymer/PDC slurry The ~lurry solution turnea a dark
~mber color The ~olymer slurry wa~ hoatod to 45 to 50~C, and ~low,
d ~ ~ addition of th~ 5-bromo-1-~e~1-n~ sslut;~n was begun The 5-
bromo-l-~ ~ ~ solution w_s addoa slowly over ~1 - tely 3 aays to tho
~tirred ~olymer slurry at 50~C A~t-r tho 5-bromo-1-~ -- addition was
com~l~to, the roaction was All~ !e~ to ~tir an aaditional 1 aay at 50~C
The polymor slurry was vory dark-colorea throughsut the addition ~oriod
Tho polymor slurry (very dark rea-brown) was cooloa to room
t~ _- ~ture and filt-rea The boads worQ wa~hed ~Yt-~Qiv-ly with
~hl~romothnn- (~till aark color-d b-ads) and th-n w~r~ washed
~Y~-n~ively with wator to remove most of the color The bead8 were then
wa~hea w$th the fsll- ng ~Hri-s of ~olvents methanol, acotone,
~ichl~ , -tl -, ac-tone, and, _inally, mothnnol After drying in a
vacuum oven at 60~C ovo-~;ghl, 96 46 g of noarly white Ll. - lkylatod
~olymer bead~ w r- obtain-d The mas~ u~tako cor~ -' to a d gre- o4
funct;~nAl;~-t~n (DF) of ~ - ~Iy 0 20
B 8a~FONATION
To tho driod b ~ -l~yl_ted ~olymcr beads ~reyar-d abovo
(a~ ~ ly 0 720 ~ 1 of ~Ly ~ ~ ro~oat unit~) was add-d 650 m~ of
~;~hlo ~ -h~n_ una~r a nitro~-n A F_ ~ ~r~ . Th~ ~olymer nlurry was
cool~d to 0~C using an ico wat-r bath Chloro~ulfonic acia (258 2 g, a 22
mol, 3 08 oquivalonts) wan aaded slowly dro~wi~e to the ~olymer slurry at
0~C ov-r 2 hour~ 40 n ~P The ~olym r beaas turn c___~ -colorod during
th- chlo ~ fonic acid addition A~tor th~ adaition was com~leto, the
roaction mixturo wa~ Al 1 _ ..ud to ~lowly warm to room t~ _- ~tur~ within th~
wat-r bath Tho volume of the swollen ~olymer was a~L~ - tely 500 to
-92-
CA 02230272 1998-02-24
W O 97/Q8122 PCTAUS96/136X3
600 mL within the roactor Aftor w- ~g to room tompQr~tur~, thQ polymor
~lurry wa~ ~llowod to st~nd ov~r~ht without stirring Th- liquia layer
was th-n ~d from tho ~olymer u8in~ a _mall-boro ran~ The b-ads
w r~ th~n wa8hed sevQral times with dichloromethane (Tho liquid layor
and ~;~hloromothano w~shos w r~ ~lowly ~nd c~rQfully q~l~h~d in a
~parat~ ves~Ql using ico ) The polymer bQads worQ then carofully
tran3forred to a fritted-gla88 funnol, and the polymer beads were qu~
by 810w, carorul addition or ic- wat-r
Artor w-~h;~ tho boad~ ~Yt- ~ively with w~ter, oXC-s~ solia sodium
bic- h~-t- wa_ ~lowly nddod to a s~a~e~ai~~ of tho polymer bead8 in
w_tor ThQ mixture wa8 Alle,, d to 8t_nd oV~rn;ght at room t~ _ ~turo
Tho ~olymer/soaium bi~- ~-to miYturo Wa3 thon h~atod to 50~C for 2 hour~
Tho polymQr ~1 rry was all_r_d to stand at roam t- _~ ~ture for 6 days
Tho ~olymor was light-colored and ~ re hi~hly ~_llo~ at this point Th-
15 81urry wa8 heat-d to 50~C and All_ ~~~ to react ovDrnight, givin~ a pH 4
_oluti~ of ov~n mor~ ~wollon polymor (~r~mat-ly 600 to 700 mL
volumo) Addition of a small amount of sodium bica~hA~-te ~ive8 a ~X 7
~olution
C THIO~ATION
To th~ aqu-ous ~olymor boad ~lurry from above was added sodium
bic--h~-t- (60 5 g, 0 720 mol) The mixturo was e~Ac~ats~ and back-
f~ d with nitrogen three times Th;ola~etic acid (41 1 ~, 0 540 mol)
wa8 addQa slowly dropwiso over 1 hours 10 ~t9P to the ~olymor ~lurry at
room t- _ ~turo Tho mixture wa8 810wly warmed to 80~C over s-veral hours
25 and all~ ' to react at 80~C for 3 aays After rooli~g to 40~C, the
_ _ -~t ~olution was , _d u8ing a ~mall-bore c-~ The ~olymor
was wa~hed ~-veral timos with wator, givin~ ~l;gh~y off-whito color-d
~olymor b-ads C ~~~ ~ted Lyd ~hloric acid (250 mL) was addod to the
~olymcr and tho slurry was heated to 50~C for 3 hours Aftor r~ol;~g to
roam t- _ ~ture, the hyd,o.Lloric acid ~olution was ~ v ' u~ing A
~mall-boro ~a~ . Tho polymor bQnds wore th-n washQd sev-ral timos with
Lyd ~-Lloric acid and the beads were transforr-d to a fritt-d-
glan~ runnel The boads were again washod ~ t~'ly w~th ~;lute~
Lyd~ll~ic acia followed by ~Yt-~;vo w-~h~ngR with water, giving
35 ~ligh1 ly off-white wat-r-swollcn boaa~ (Th- wat-r-~ 1l~ volume of the
polymor beads was ,~l - t~ly 900 m~ ) The beads were wa8hed with
methanol (mot_anol-s7_11 ~n volume a~r ~ ~ly 600 mL) and finally with
-93-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
methane After dryin~ in a vacuum oven at 60~C ov~ night, the
dark-color~d b-ads 'ad a dry volume of ~y~ ~ ~tsly 200 mL Thi8 ~roduct
va~ r7.~n7~; fi-d ag Xl~qSA-5C
D PREPA'~ATION OF CATALYST FROM POLY~ YKILN~ AND 11-BROMO-1-
UN~ (X3MSA-llC)
Catalyst was ~re~arQd as abovo, starting with ma~ ~OLO~8
poly~Ly ~ (An~berliterM XE-305) ald 11-bromo-1-~n~ec
3XAMPLE 23
EVALUATION OF POTVM~T'TC lqERCAPTOS'J~FONIC ACID ~'~TYSTS
A 3VAL'JATION OF THE MERCAPTOSULFONIC ACID POLYMER ~X3MSA-SC) IN
THE REACTION OF PHENOL WITH FL'JOR'-~NONE
To a 4-dram vial ~qui~ed with a ~tirrin~ bar wa~ add-d 4 32 g o~ a
20 8 1 ~ lar ratio mixturo of ~henol to fluo ~ - and 0 26 g (6 ~c.~t
by wei~ht of tho r-actant solution) o~ the merca~tosulfonic acid ~olymer
(XEMSA-5C) pr-par-d as describod in _le 22A-C Tho r-action mixtur~
consi~t~ of ~ ~ ~_~co~s liquid ~_axs ~lus a se~rnte hste .~ n
~oly~nor cataly~t p_ase Th~ mixture wa8 heated to 50~C for 5 hours Tho
r-action was monitored th ~ the reaction ~-riod by colloctin~
~ample~ and analyzin~ by HPLC Thc 9-fluo _n~ was found to be 36
~ - c_~L c~ -' wit'-~in 2 hours and 76 ~- c ~v = -' within 5 hours
HPLC analysis (analytical m~thod 3) gives the following relative ar-a
p~ . analy~is for the products after 5 hours of r-action (76 ~?~
c~v.l-ion) 9~9-bi~-(4-Lyd ~Ay~7- yl)fluorcno (97 45 ar-a ~c~e~L) 9-
(2-Lyl ~Ay~7-_~yl)-9-(4-Lydh~Ay~'--~yl)fluor-ne (2 17 area ~~-~enL) adduct
c~nt-~n;ng two fluor-ne unit~ and three ~',- -l;c un~ts (0 39 ar-a
p~L)
B 3VAL'JATION OF THF M3RCAPTOS'JLFONIC ACID POBYMER (XEMSA) IN THE
REACTION OF PH3NOL WITH FLul ~
To a 4-dram vial ~quipped w$th a stirring bar was add d 4 32 g of a
30 20 8 1 molar ratio mixture of phenol to fluo ~ ~ and 0 26 g (6 ~a~ce~L
by w-ight o~ the roactant #olut$on) of the mercapto~ulfonic acid polymer
(X~MSA, daçlr--e of functiA"~ at;,r "J,~- ~ t--ly 0 28 from
L.~ rylation E~t~p) prepar~d as d-scribod in Examplo 20 The r~ action
mixture consi~ted o~' a ~ liquid pha3- pIu8 a soparate
35 hote ~ polymer catalyst l?ha~-- Th-- mixture was h--at--d to 50~C l~or 5
hour~ Tll- r--action was ~nitored throu~hout tho r--action p--riod by
c~~l-cL$n~ and analyzinçr by HPLC The 9-fl~o., v o w~s ~ound to
-94-
CA 02230272 l998-02-24
WO 97/08122 PCT,'US96~13683
bo 44 p_ .-~' c~ -' within 2 hours ana 75 ~ ~ 0 consum d within 5
hours HPLC an~lysis (an~lytical mcthod 3) ~ives tho following relative
area ~e.c~t analysi8 for the ~roduct8 after 5 hours of reaction (75
~ c_ L cv.v~.sion) 9,9-bis-(4-l.y~ v~y~hc yl)fluorene (96 10 ar-a
~_l. ) 9-(2-Lyd v~y~h-nyl)-9-(4-Lyd v~y~honyl)fluor-ne (3 52 nrea
~- _Gn~): adauct C~tA;~i~ two fluorene unit8 and throe r~ -l;c units
(0 38 area ~e.~ )
C EVALUATION OF MERCAPTOS~BFONIC ACID POEY~ER (PMBSA-MER) IN THE
REACTION OF PHENOL W~TH FLUI
To a 4-dram vial e~uippea with a ntirring bar wa8 added 4 32 ~ of a
20 8 1 molar ratio of ~henol fluoL~o~ and 0 26 g (6 ~Ll_~nL by wei~ht of
tho roaction nolution) of PMBSA-MER o~ Ex~myle 21C Tho r~nction mixturo
consists of a h~ liquid pha8e ~lus a s~pA~ato heto o~ no~
polymeric cataly8t ~hA8e The mixture was heated at 50~C for 2 hour~ The
re~ct;o~ was monitor-d by collectin~ _- _l~n, which werQ anAly~-d by HPLC
Tho 9-~luor~none wan 99 5 ~_ _~L ~v = ' within 2 hours The product
after 2 hours cA~A;~s~ 96 83 aroa ~L~ ~ of 9,9-bi8-(4-
L~11V~YL~- yl)fluorene, 2 44 ar~a ~o __~L of 9-(2-Ly~Lv~y~h-nyl)-9-(4-
Ly~v~y~he~yl)-luor-no and 0 72 aroa ~e ~ of An adduct c~ A;~;~g two
~luoreno units and throo ~ ;c units by HPLC (analytical mothod 3)
D EV~T~n~I~ OF MERCAPTOSULPONIC ACID POLYMER (PMBSA-SU) FOR T~E
REACTION OF PHENoL WITL F
To a 4-dram vi~l oqui~pod with a ~tirring har was ~dded 4 32 ~ of
20 8 1 ~ lar ratio mixturo o_ yh-nol _l~o envnn ~nd 0 26 ~ (6 ~ by
weight of the r-A~t~t sQlutio~) of the polymer o~ Exam~le 21B (PMBSA-SU)
The reaction mixture consint-d o_ a ~ ~ liquid ~ha~o ~lus a
~o~ar~t~ h-t~ n ~olymor catalyst ~haso Th~ mixturo was h~at~d to
50~C for 5 hours Tho ~v~ 8 of tho roaction wns fol~ by HPLC At
the ~nd of 2 hours, 67 ~--c~nL of the fluG - was ____ d, and 85
~_~ce~t at the ~nd of 5 hour~ At the ~nd of 5 hours, th~ reaction
mixtur- c~t-; -~ 97 09 ar~a ~ _~t of 9~9-bi~-(4-hydlv~y_~ yl)fluor-n~,
2 25 aroa ~ercent of 9-(2-hyd v~y~honyl)-9-(4-Lyd v~y~henyl)fluoren~ and
0 66 area ~_o~t of an adduct c~t~;~;~ two fluorono units nnd throe
~-~o~;c units by HPLC analy~is
R-sults for tho evaluation of various ~olymer ~ osulfonic acid~
for ~ ~ -;~g ~h~nol with fluol~on~ were given in Table X
i
J -95-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/l3683
TABLE X
CONVERSION OF FLIJ~ - (%) USING 6% BY WEIGRT OF POT~YM~T~C CATALYSTS AT
50~C
Reaction tim~ (h)
Rosin 0 2 4 5 7 18
PMBSA-MER ~ 99~5
PNBSA-~;U (PVBC) 0 67 85
P~BSA (PVBC~ 0 66
XEMSA-5C O 36 76
(AT~ erlyt oTM XE--305)
~CEMSA O 49 75 82
(Ambe~rlyteTM XE-305)
DOWEX* 50 (DMT promotod) ~ 17 73
*Tr~ k of The Dow ~ C
5 *AmborlytoTM Tr~ k of Rohm and Haas
EXAMPIE 24
EVALUATION OF THE MERCAPTOSIJ~FONIC ACID ptlT-YUlP!T~ C IN THE REACTTON OF PHENOCB
WITH ACETONE
To a 4-dram vial equipy--d with a stirrinSr bar was addod 4 33 51 of a
14 1 molar ratio mixturo of phenol to acotono and 0 26 g (6 ~L ~,O'rLI. by
woight of the r--actant nolution) o~ the ~", v~,liato ;~os.lfonic acid
polymor Tho reaction mixturo typically cons~ista of a ~ ~ liquid
pha~- plu~ a ~oparato hQtc vg~ 2 polymor catalyst pha-o The mixtur
15 W~l~ hoat--d and Illow d to roact at 50~C The r-~rt;~ wa~ monitored
tl~ t tho reaction poriod by collectinsr _ l~r9 and analyzing by
HPLC HPLC anAlysis (analytical mothod 3) ~hows tho followinsJ acetono
cG~v_l, ion dat~ (bas~d u~on th~ ~auAntity of 1~;8p~- ~1 A produced)
for the difforent cataly~ts aro givon in Tablo XI
-96-
CA 02230272 l998-02-24
WO 97/08l22 PCTnUS96/13683
TABLE XI
% ACETONE CONVERSION
(BASED UPON BISPWENOL A pT~nurT~n)
Roaction time (h)
Resin 0 01 02 252 54 05 0
DOWEX* 50WX4, 25% DMT ~romoted 0 18 44
PMBSA (from uncroY~ PVBC) 0 35 60 75
X~MSA-5C (DF - 0 20) 0 38 82
XEMSA (DF - 0 28) 0 23 32
PMBSA-SU 0 2 9
PNBSA-MER (2% cros~-l;~T ~, 200- 0 66 88
400 mo~h)
*Tr~d _ k of The Dow ~- c~l C _ Y
DMT wa~ 2,2-dimothyl~h;~7ol;A;~s
EXAMPLE 25
A PREPARATION OF B~PF; ~TUVT.~ ~UT.~T~Tn~ AZEOTROPE TO REMOVE
WATER; rn~lrl ATION FROM MFTT~T-~ ~UT.~RTnT~; 5 75 1 MOLAR RATIO OF P~ENOL
TO 9-FL~ORENONE; COEK~clrI~ATION WITH ~T'~T~WT~ ~Y
To a roactor (i~oth~ 1 ~tirrod batch roactor; reactor design 4) wa~
charg-d 75 0 g (0 80 mole) of phonol and 24 98 g (0 1386 mole) of 9-
fluo ~none The mixture was hoatQd to 40~C and catalyst (0 973 g, 0 0062
mole, 3 - ~tv~ ~ ~lfonic acid) was char~ed to tho r-actor U-~t;~
was c~t;~-' The cour~e of tho reaction was fo~ d by HPLC
(analytical mothoa 3) At 30 ~ c~ v.~ion, methylene chlorid~ (15
g) was ndded to t_o ,~ ;~ mixtur- so a8 to k-Q~ thQ mixtur- stirrod,
nnd to U8 - A wator/mothyleno c_lorido ~-~ t_.~ D ( 180 mm ~g, T 5 37~C) to
r mov~ wator of roaction The reaction mixtur- was cool-d at t_e end of
th~ reaction (n arly complate fl~o-en~ e ~__s _t~on, >99 ~- _ant of
theoratical wat-r) to increasQ th- amount of cryst~ll; - ~ pitate Tho
of the run is givon in Tablo XII
Th- r-action m~xture (about 97 ~e c t sQlecti~ity to 4,4-B~PF, little
unr-act-d fluo. -) was ~plit into two parts Tho first fraction (53 3
~) wa~ filt-rad The filter cako wa~ washQd with methylone chlorid~ (49
~) nnd thon with hot wator (55 g) The ~c_v~ y wa~ 6 4 g (first crop)
-97-
_
CA 02230272 1998-02-24
W 097/081Z2 PCT~US96/l3683
~na 0 6 g (socona crop) of white crystals, Co~l~b~ ~ g to 99 ~olc6~t
purity 4,4-BHPF (33 pc ~L ec~v~ y)
To tho ~A - of tho mixturo (70 3 g) was addod 61 ~ of
t-trachloroothylene Tho cry~tals were ~ v~a by filtration and the
filt~r cak~ was wash-a with 50 g o~ tetrachloroethylone ana th-n with 115
of hot water Th- c~eloa product weighod 11 5 g (43 p- ,
~c_v_~v, 99 p~-~t a8 4,4-B~PF, white solia)
The~e r-sults showea that use of tctr~chloroethylene, in c ';~-~i~
with methylene chlorido, ~ives higher ~ A~ V~ y of 99 ~cnL pure 4,4-
10 B~PF)
TABBE XI~
T Tomp mm Hg Ob~ervations, Actions
h min ~C
OsO 37 760 Add MPSA; turns orango, thon dark green
brown
0:20 36 760 Color light L ~ ;nh
Os40 36 760 S~mplo taken
lsl5 36 760 Seod crystal add-a
2slO 36 760 Sampl- taken
2s28 36 760 Methylone chlorido (15 g) and ~ead
cry~tal addod; no cry~t~ i~; temp
rai~ea to 45~C
2s30 37 -180 Sample tak-n; seed crystal not
di~solving
3 10 45 -180 ~eater ~et at 40~C
3s30 40 -180 80ed cry~tals ada-a
3s50 40 -180 Mixturo hazy
4s30 40 -180 Mixturo hazy
5;10 40 -180 S~mplo tak-n
6s43 40 760 Vncuum turned off, stirr-r #p-ea
increasod
9s30 40 760 Sam~lo takon; methylono chlorido (about
20 g) adaea to mixture; stirrer speod
inc ~ ; hoater turnod off
-98-
__
CA 02230272 l998-02-24
WO 97/08122 PCT~US96~23683
B USE OF MFTR~T~ c~T~RTnT AZEOTROPE TO ~rTCTT'T~T' REACTION;
5 75;1 ~OLAR RATIO OF PHENOL TO 9-FLuu~
To a reactor (reactor desiqn 5) was chargod, 75 0 g (0 80 molu) of
phenol, 24 98 g (0 1386 mol~) of 9-fl~o ~Jr - and 15 0 g o~ mothylene
chloride The mixturo was hoated to 40~C Cataly8t (3-
~Lopropan-sulfonic acid, 0 757 ~, 0 0049 mole) was charg-a to the
roactor and the mixture was stirr-d The r~action wa~ fo~ a by ~PLC
(an~lytical mothod 3)
At the ena of the reaction, methylene chloride wa8 added to ~ nce a
mixture of 30 30 about 30 pe ~L by weight of methylen~
chloriae BHPF phonol and the mixtur- was h-atod to dissolve the
cryst~ll;~s material Aaditional methylono chloride was added to the
~olution and the 80~uti~ was cooled to ~romote cry8t~ t;~
Tho pro~rosg of the reaction was shown in Tabl~ XIII
At the ena of th- roactio~, half of th- resulting mixturo was filt-rod
and the filtar cake wa~ wash-d with 64 ~ of methylene chloride The first
crop Or B~PF wei~hs 7 0 ~ (dried ov~-n;~ht at 40~C) and was white with vory
littlo pink coloration A ~econd cro~ of crystals w~s collocted nnd
wa3hea w~th 28 ~ of methyl-no chlorido Weight, 4 1 g (dried ove-n; gh~
at 40~C, xlightly pink), 99 ~c purity after a further 7, nh;n~ with
wat-r
Tho ~ ;~A~r of tho reaction mixture was filt~red ~nd tho filter cake
wa5 washed with mothylone chlorid- and hot water and dri-d ovor~; ~h1 at 40~
C The ec~ly was 14 ~ (51 ~_L~_~L overall ~.c v~ y)~ 99 ~er~-- L ~urity
by ~PLC
_99_
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
TABL~ XIII
Tim-A-Temp mm ~g Ob#ervations, ~ction~
h min ~C
0 0 45 760 Add MPSA, turn~ dark
0 09 45 760 Add 20 ~ Or methylene chloride;
color wa8 dar~ L ;~h
0 30 45 760 T.; gh1 A brown; roducA pr_ssurQ to
~bout 180 mm
1 00 45 -180 Soed crystal added; no
cryst~ll;7-tion obsorved
2 00 45 -180 8-ed crystal adaod; no
crys~ tion ob~Lv~d
3 00 45 -180 S~mple t~kon; seed cryst~l ~ddodt
cryst~l not di~#ol~ing
3 45 45-~ -180 ~oater sot nt 40~C; crystal#
f L '"I
4 00 40 -180 Mor_ cryst~ls rorming
6 30 40 8~mp1_ t~kon; solid cry#t~
mass; stirrin~ inof~ectivo;
yellowi~h color; ~cuum turn_d o~f
19s0040 760 8~mplo t~kon; ~bout 50 to 70% o~
BE~PF cry8t~ 5 33% by w ig_t of
methylene chlorid~ ~dded;
t- _- ~turo increased to 58~C
bout 58 760 _ _- ~t~o lowOred to ~40~C;
20 30 mothylone chloride Added to 80%
mak_ up by w-i~ht o r mixture
24 760 Cooled to room tA _ t
-1 00-
CA 02230272 l998-02-24
W ~ 97/~8122 PCTAUS96~l3683
C REACTION VSING Me~YTT~Ne r~T~RTn~ AS SOLV_NT 3 5 1 ~OLAR RATIO
OF PH_NOL TO FLu~ ~
Phonol (30 0 ~, 0 32 molo), 9-fluo ~v~o (16 41 ~, 0 0911 ~ lo) and
- 15 0 g of mothylo~- chlorido woro ch~r~od to a rcactor (roactor d~sign 4)
The mixtur- was stirred and h-at~d at 40~C and 1 122 g (0 0072 mole) of
MPSA was adaea over about 1 minute T~ ;~ was c~ at 41~C ror tho
duration of tho roaction Th- fo~ nu ~~~v~tion~ woro mado duriny the
run
Timo (h) Tomp (~C) Obs~rv~tions, Actions
0 41 ~PSA addod; turns orange to brown to
dark brown within 30 ~ec
0 18 41 purplo color
0 5 41 sampl~ takon
1 5 41 sample taken
1 67 41 orang~ mixturo so-dQd with crystal~
3 41 ~rmplo tak-n; honter ofr; left ov~-~;~ht
4 41 hoat~r off
6 room samplQ tak-n; about 80% co~v~ dion; ~ re
2 3 aaauct than 2,4 adduct
T_e cryst~l;~ solid was 1~ v~d by filtration and tho filtor cako was
washed with mct_ylone chlorid~ A socond crop of crystals was I.- v~l~d
from the ~ thor liquors The yield was 0 86 g (first cro~), 8 66 g
(second crop), 3 5 g (third crop), ovor~ll 13 g (41 ~P ~ ), >99 p~rcont
purity by ~P~C (methoa 3)
C P~ENOL FLu~ MOLAR RATIO OF 15 1; MPSA ~TYST; PH_NOL
R~MOVED BY DISTILLATION; cRys~T~T~T7T~n FRO~ Me~UYn~ ~T.OpTne
To a mixtur- of phonol and fl~v~ 15 1 molar ratio), hoatod to 65~
C, was adaod 0 0498 ~quivalent o~
3 ~ ~-~tv~..~ fonic acid (with rQspect to fl~v~o) Tho
rosultin~ mixturo was heat-d at 65~C for 2 hours, after which the reaction
mixtur- was wa~hod with water (14 timos th- volumo of tho mixture) to
r movo MPSA The wa~hoa r-action mixtur- was distillod to a ph-nol BHPF
weight ratio of 1 1 and cool-d to brin~ about cryst~ t;~ of BHPF
Cry~t~ll;~ matorial was ~ va~ by filtration, washea with mcthylono
chloriae, wa~hea with wato~ and driod to give BHPF (99 8 ~e c~ t by weight
of 4,4-isomer)
-101-
,
CA 02230272 l998-02-24
W097/08l22 PCT~US96/l3683
EXAMPLE 26
C~ TION OF PHENOL ArYD ACETONE TO PRODVCE 2,2-BIS-(4-
TrYn~ Y y ~ ) PROPArYE
A FEED C-A-M~TMTMG 6 ~c _~ ~ BY WEIGHT OF ACETONE, P~VS WATER,
sATnRrT~ CATA~YSTS BATCH REACTION
The reaetion was carri~d out in a 2-L jacketed barfl-d resin pot,
~quippod with a eA~ er and nitro~en pur~e IsothA 1 tomp~ratur~
eontrol wa~ providoa by a fluid material, circulat-d throu~h the reactor
jack~t Stirrin~ wa~ provided by a T-;gh~;~ Labmastor~ TS2510 stirrer,
~quipped with an A-310 ; ~ r.
To the r-actor was charged 1200 g o~ fecd, cA~t~;~;n~ 90 0 ~orc~nt by
weight of ~h-nol, 6 0 Aera~nt by w~ight of acetone, 1 8 ~e~ ~ut by wei~ht
of wat~r ana 2 2 ~e e~nL by wei~ht of MPSA The mixturc was hcat~d at 35~
C At the nnd of 2 hours ~--t;~, cryst~ 7~tiA~ occurs in th~ r-action
mixtur~ Tho r-Action wa~ cA~t;~ 1 hour mor~, at the ~nd of which 80
pc _~t of tho ~cctonc was reactod (HPLC) The reaction mixtur- was
~ v.~ from the r-actor and rilterod The w~i~ht of ~ vel~a crystals
W~8 17 pOlC~t, consisting of a 1 1 adduct of BPA phonol (molar ratio)
; The cry~t-~ Adduct was w~3hcd with phenol Tho washod adduct
20 cont~; -~ 57 7 ~? ~ by wei~ht of 4,4-bispAh-~Al, 160 ppm of 2,4-
-A;~p~ -1, 200 ppm of tri~- ~1, 2270 p~m of other traee bisL~ -l;c
impuriti~s and 1170 ~pm of MPSA, tho ~lA~e b-ing phenol The mother
liquor cA~tA;~-~ 8 44 ~ _~L by wei~ht of 4,4-bi~ph-nol, 0 26 percant by
; wcight of 2,4-~;~F~ ~, 0 13 ~--c~,L by wei~ht of tr; n~h~Al 0. 62 ~ _~L
by woight of othor bi~L~ -l;c impuritio~, 0 81 ~e c~nt by wei~ht of
nc-tono, 2 95 ~ - by woi~ht of w~tor and 2 78 ~e e~ t by weight o~
MP8A, the 'A~ A~- being phonol
B R~ACTION VSING RECYC~ED MOTHER ~IQ~ORS
Th~ ~ thor liquor from (A) wa~ charged to a rotary ev~ol-tor with
30 ~ko-up ph nol (181 g) Th~ ~v~ ~tor was h-at-d at 50~C for 30 ~~"t~P,
i ~t th- nd o~ which tho conv~r8ion of aceton~ wa~ 90 ~IC ~ Pro~sure
wa~ reduced to 10 mm ~g absolute for 30 ~t~~, at the end o~ which the
mixture cA~ -' 1 4 ~_lCw~L by w ight of wat-r Tho ac~ton- c~A~te~t was
bolo~ the d-tr L i A~ limit
T_o dri-d mother liquor was ~t ~d to tha ~ctor, alon~ with make-up
phonol, ac-tone, wat-r and MPSA to give a mixtur- cA~lA;~;ng 92 0 perc~nt
by wwight of ph~nol, 4 0 percwnt by wwight of acwtone, 1 8 ~a~ce..L by
;
-102-
CA 02230272 l998-02-24
W O 97~08l22 PCTAUS96/13683
weight of water ana 2 2 ~ c by wei~ht of MPSA Tho tot~l mass
CvL -,b~ ~-.~n to (A) minus th~ woight of ~ _l~a ~ v~d Tho mixture was
~tirrea ~na heatea at 35~C for 3 houra Cryst-~ i~n of BPA wa8
obser~ed after thc initial 30 n~t~ of hAat;n~ At the end of 3 hours
--t;-g, acotone cv v_L~ion was 80 ~e c~ t Tho ra~ction mixtur_ was
proc-rsed ~ in (A) Recycling of the mothor liquors was o~Q~ted for 12
cycles Rosults ar_ gi~en in Tabl~8 XIV and XV
Th-sA ~xp~riment8 ~- -L ~tQ that catalyat and unreactea m~t-ri~ls c~n
b- _Cvv_LOd ~na r-cycled without --lv_l~ely aff~ctin~ tho proc~ss nna that
r-sult~ for suc~siv~ runs w ro ~on~rally consi8tent and ~reaictabl_
C REACTION USING 10 ~c ~_~t BY WEIGHT OF ACETONE IN THE FEED WITH 3
~a~. BY WEIGHT OF WATER
An experim~nt wa8 don~ ~3 in (A), using 1200 U o-f foea, c~-~ ~ 85 5
~ by woight of ph-nol, 10 0 ~eL- ' by wei~ht of acetone, 3 0
~c ~-~L by w igAt of water and 2 2 ~c c~t by weight of MPSA The
reaction was dono ~t 25~C Cry~t-ll;~~ BPA wa3 visiblo a_t-r 13 hours at
this tA _- ~ture At th ~nd 04 24 hour~, the co v~ ~ion of ac~tone wns
40 ~
Cry~t-ll;ns ~roduct, l~ from the r-action mixture by filtr-tion,
conDtitut-s 15 ~ c_~L o~ the mixture Th- crystals, a 1 1 adduct of
BPA ~h-nol, wer- w~h-a with phonol Tho wash_a crynt~ls c~-1-;- 51 8
. by we~ght of the 4,4-isomer, 60 ppm of 2,4-inomer, <20 ppm of
tri~ -1, 690 ppm of other trace ~;~rh--~ln nnd 840 ~m of MPSA, the
~ sr bein~ ph-nol The moth~r liquors cn-~ 6 73 ~_ ~ent by
25 w_i~ht of 4,4-i~omer, 0 15 ~ ~enL by wei~ht of 2,4-i~omer, 0 08 ~
by w_i~ht of tr;~hAn~l, 0 76 ~e~ nL by wei~ht of othor biDL~--olR, 5 71
~ ~ L by weight of ~c-tono, 4 97 ~ ~L by wei~ht o_ w~ter nna 2 66
_-- by weight 04 ~PSA, th~ _ ; n~r b-in~ phonol
-103-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
TABLE XIV
1-~ O~L-.LV~ OF WASHED BI~r~1~.J~ A CRYSTALS
CYC1- CrY8t~1 4,4- 2,4- Tri8- OthOr MPSA
NO. % i80mer i80mOr phonol bi~- PPm
Wt. % PPm ~Pm phonol
PPm
1 17 57.7 160 200 2270 1170
2 22 60.4 420 n/aA 1730 1940
3 17 59.7 460 270 2380 670
4 18 60.7 560 260 2270 1440
26 57.9 870 410 1840 1070
6 18 59.3 790 330 2050 2110
7 18 59.3 790 390 2150 740
8 19 59.6 770 280 2100 750
9 22 59.4 840 390 2000 860
31 62.5 920 380 2210 1060
11 21 64.0 710 350 2020 1410
12 23 63.0 770 280 1900 1500
AVg. 22'5 61+2 800'70 350~50 2100 1100
Cycl~ lOO ~300
7-12
* n/a = nOt dOtOCt-a
-104-
CA 02230272 l998-02-24
WO 97/08122 PCTtUS96tl3683
TABLE XV
MOTXER LIQUOR C~ ~OSl~ lVW~
Cycle 4,4- 2,4- Tris- Other Acetone Water MPSA
No Isom~r Isom~r ~henol bisphen wt % wt % wt X
wt % wt % wt % -olics
wt %
1 8 44 0 26 0 13 0 62 0 81 2 95 2 78
2 7 53 0 48 0 23 0 47 0 63 2 94 2 89
3 8 30 0 55 0 24 0 38 0 79 2 94 2 75
4 8 42 0 66 0 29 0 41 0 70 2 63 2 87
8 92 0 78 0 43 0 42 0 86 2 81 2 73
6 8 22 0 86 0 44 0 46 0 73 3 06 2 84
7 7 92 0 87 0 42 0 46 0 66 3 09 2 83
8 8 03 0 91 0 44 0 49 0 58 3 07 2 84
9 8 09 0 84 0 36 0 45 0 72 3 0a 2 44
6 86 1 03 0 51 0 46 0 25 2 64 2 92
11 7 59 0 78 0 33 0 44 0 51 3 01 2 69
12 7 87 0 84 0 39 0 42 0 27 3 17 3 14
Avg 7,7to,5 0 88 0 41 0 45 0 5~0 2 3 0'0 2 2 8
Cyclos t~0 09 ~0 06 '0 02 l0 2
7-12
r le 27
Pr-~aration of a Ro~ Os~Lative [( - ~Loalkyl)(Sulfo)Phenylalkyl]
8ulfonated Poly~Ly ~e Catalyst (Desi~natQd DPMSA-~ER3C)
A Alkylation
A 15 00 g samplQ o~ 200 to 400 mesh chloromethylatQd poly~LylOne/2
~ ~e.t aiviny~ copolymor beadQd (~l~- tely 4 3 mmol Cl/g
resin, ~l~- te~y 64 5 mmolO Cl) known in the art as a Morrif~
re3in (av~ ~le from Fluka C~emi~ AG) was adaOa to a round bottom glass
fla~k (r~actor) unaor a ~ad of plant nitrogan with a soaium l.y~lan
-crubber attachea (to ~ra~ evolved BCl) (3-B ~ ~ ~yl)~~~-_ - (102 7 g,
78 4 mL, 8 0 oquival-nts) was adaea to the dry resin beaas Dri-d (over 3
A~L l~ ~ siQv 8) nit ~ e (50 mL) wa8 added, ana the beaas
were ~lowly 8tirred at room t~ aturQ to allow for swQlling of tho
b-aas The rOactor wa~ cooled to 0~C in an ic~ water ~ath A 20 mL
~am~lQ of 1 0 M ~1 ' chlorid in nit Q~ ~ avail~lo from Aldrich
~ 'c~l Co was 81Owly aaa~a via ~yringQ to the cold ~olymer slurry with
ra~ia ~tirring ov~r ~l~- t~y 10 '~l~t~l . Tho miXturQ turns dark rod
a~ ~oon ~8 th~ ~l ' chlor~de 801~;~ was adaed ana oxotherms to
~ly 4~C within tho first 15 ~t-~ of r~action with XCl ~ein~
-105-
.,
CA 02230272 1998-02-24
WO 97/0812Z PCT~US96/13683
ovolvQd ~rom th ~olution Aft-r the addition of the AlCl3/nitroh~7~e
~olution wa~ compl-te, the mixture Wa# slowly stirred at 0~C for 2 to 3
hour~, thon was ~ d to room t _- ~ture and slowly stirrea overnight
Th- mixtur- wa~ slowly ~our-d onto ice to qu-nch th- aluminum chlorid-
Thon the boad~ were sQparated using a gla~s-rritt-d funnel with vacuum
~iltration The bead~ were s~_lA~ y washod with wator, ac-tone,
~; rh~ ~romethano, methanol, ailute a~u-ous hydrochloric acid, wat-r and
mothanol, th-n wQrQ driod ov~r~;~ht in a vacuum oven at 70~C (dry mass
23 52 ~)
B ~ulfonation
Thn polymor boaa~ from _lo 27A (23 30 g, est; tP~ 161 3 mmole
of phonyl ~roups) wore add-d to a ~lass reactor with adaition funnel and
NaOH acrubbor attnched Di~hlorom thane (100 mL) was addod to tho ~lask
and tho beads w-r- Al 1~ d to ~well (rapid ll;~g wa~ ~h-Y- v~) . Tho
~lurry was cool-d to 0~C in an ic- wat-r bath Chloro~u~ f~; c acid (37 6
~, 21 4 mL, 320 mmolo, ~r ~ tely 2 0 ~~uivalents per ~quival-nt ~henyl
groups) was slowly add-d dropwi~e over ~1 ~ tely 2 hours to the
polym r ~lurry at 0~C The mixtur- wa3 allowed to slowly warm to room
t _--~turo ove-n~ ght in tho water bath Th- mixture was slowly poured
onto ico to quench tho ~xc-~s c_lorosulfonic acid, then the b-ads wor-
~-parat-d using a ~lass-fritt-d funnel with vacuum filtration Th- beads
wor- thon wa#hod A~ iv-ly with wat-r Wator was add-d to make a
lurry, then ~olid sodium bic--h~~~te was slowly aaded in small portions
ovor ~1 - e3y 2 hour~ until no more b '~h~ wa~ f'-~ v_d (all active
acid 3~t~ noutraliz-d) The mixture was A~l_A ~ to stand 3 d_ys in the
aqueou~ ~odium bi~ t- ~olu~ omo -dditional boad _ ~l;~g was
~ v_d ovor thi~ time period) Th- b-ads were wash-d with w~ter and
trannf-rrod to a ~la~ reactor with 100 mL of water The bead~ wero thon
h-atoa to 70-80~C over 2 hours to en~ure hydrolysis of any re~
~ulronyl chlorido ~roups
C. ~h~ol~
Tho aqu-ous polym r ~lurry from _le 27B was cooled to room
t- _- ~ture 80dium h,l~ h~-t - was ~lowly added until the 81urry was
neutral (no h ~hhl ;n~ ob~orvea) ~ th-n additional sodium bic~ to (27 1
35 y, 323 mmol) was added to the aquoous b-ad slurry ~h;ol~etic acid (24 6
g, 23 1 mL, 323 mmol) was added to an aadition fuDnel Tho reactor was
ev~ At-~ and r-filled with nitro~en ~everal tim 8 to ~; ~e th air
-106-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96~13683
c~ntnnt The ~h;ol~cetic acid was 81Owly addea over ~ - t~ly 15 to
In~ltes to tho a~ueous b~aa slurry with r~id stirrin~ The Addition
rat~ wa~ adjustoa to control tho offervcscont ~vol~ti~n of carbon Ai QYi~
which was formod in the noutr~ t;An ~lOC~-8 After the Ih;ol~cetic
acid addition was complete, tho mixture was h~ated to 70~C and W~8 ~
to roact overnight with n; 1 stirrin~ Tho mixturo was then coolod to
room t- _ ~turo ana tho boads woro collocted by filtration u8ing a
frittoa-glA~8 funnel The beads were wa8hed ~Yt~ Q;v ly with wator, then
with ~; ~hl ~romethane, and thon w~hed a~ain with wat-r The boads w re
transr-rr-d back to tho glass roactor, th-n c~n~ent atQd (12 molarl
Iyalochloric acid (100 m~) was add-d Th~ mixture wa8 heat-d with mild
stirring to 50~C for 4 to 5 hour8, thon was coolea to room t~ _- tur-
D~;~n;~-~ wat-r (100 mB) w~s addoa -nd tho boads woro a~ain collectod by
filtration usin~ ~ ~ritted-~lass funnel Tho boads were wa8hod with
water, thon were washed ~Y~n~ively (~1 - t~ly 500 mL) with dilutQ
(~L-~- tely 3 molar) a~ueous h~1~.Lloric acid The beads woro thon
washed a~ain with A~; ~n;~~ wator and finally were wa3hed with methanol to
A; ~~1~ tho watQr ~nd shrink the polymer beads The boads w re dri-d
ov~-n;ghl in a vacuum oven at 70 ~C (dry mass 34 17 g) Tho final ~olymQr
catalyst was d-signatod as DPMSA-~ER3C
D Pre~aration of Thr-o-Carbon DPMSA Polymor From Morr;f;~
Ro3in B-ads
Anothor ~ ~to8ulfonic acid polymor was pro~arod usin~ tho
~ oced~l~ of ~top8 A to C of _le 27, exce~t u d n~ a chloromethylated
25 ~olyDLy ~_o r-sin (2 ~o ~_nL divinylh~n~- -, 200 to 400 mesh,
- tvly 4,3 mmol Cl/g r-~in), a M-rrifield~ r-sin c - ~ially
AV~ ~ from Fluka Ch~mi- AO as the ~olymoric ~o L and (3-
L~ - in tho alkylation Dtop of the roaction The resultin~
~olymer was ; A~~t ~f;oa as DPMSA-MER3C
E Proparation of Threo-Carbon DP~SA Polymer From Chlo ~ -hylatea
a~l-R~ in B0aas
Anoth-r - ~to~ulf~n;c acid polymor was preparaa usin~ thQ
_o-~ ~ of Dt-p~ A to C of _1Q 27, oxcopt usin~ a chloromethylated
1 5 ~el~ol~L cros81;n~A poly~Ly~no gol-rnsin (-30t70 mesh, a~l~imatoly
4 3 mmol Cl/~ ro~in) a~ tho ~olymoric D~ L ~nd (3-L _ ~yl) ~- - -
in tho al~ylation sto~ of tho roaction Thi3 ~olymor wa3 ; ~nt; fied as
DPMSA-1 5X3C
-107-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
F Proparation of Two-Carbon DP~SA Polymer From Chlorom thylated
Gel-Rosin B-ads
Another - ~tosulfonic acid ~olymer was preparOd using the
~ : ~ O of ste~ A to C of Example Z7, excopt usin~ a chloromethylat-a
1 5 ~o1~nL cro~sli~1 ' poly~Ly1enO gel-resin (-30170 mesh, ~ - tsly
4 3 mmol Cl/~ rosin) as the polymoric 8~ L and (2-L ~ _thyl) ~ ~9
~n the alkylation ~to~ of tho reaction This polym r was id~t;fi~d as
DPMSA-l 5X2C
~ PrOparation of thr-e Carbon DPMSA from 6 ~CLr ' Cro~
MaO~ ~L-~8 ROsin
Anothor ~tosulfonic acid polymer was uDin~ the ~ oc-~ e of
st-p~ A to C of Exam~le 27, except using a chloromothylatod 6 ~ ~
crsrqli~l-~ ma~ O~O~U8 ~O1YDLY e~o resin (~ v~ mately 30 to 70 mOsh,
_~ t~y 4 3 mmol Cl/g r-sin) as the polymHric s~1L and (3-
L~ ~yl)~7-~~ in tho alkylation ste~ of the r-action This polymer
wa~ i~-~t~fied as DP~SA-6/42-3C
H Pre~rntion of three Car~on DPMSA from 6 5 ~oLco~t Cror81; ~k~a
Gol Rosin
Another ~tor lf~;c acid pol-ymOr was usin~ the ~ --~ O of
ao ~t-~s A to C of _lo 27, exc-~t usin~ a chloromethylated 6 5 k~olt
cro~sli~l ' uniform particl- size poly~Ly1_~o ~ol-r-sin (380 micron,
~v~ -; t-ly 4 3 mmol Cl/g r-8in) aB the ~olymeric ~o L and
~3-b1~ _ ~yl)~ - - in the alkylation ~top of the roact~on Thi~
~olymor wa~ i~t~fi~a a8 DPMSA-6 5X3C
EXAMPLE 28
~VA~UATION OF CATA~YSTS IN CONTINUOUS ~0~
A fix-d bed downflow reactor, ha~in~ a volume of lO-mL, waD co~DL ~Led
~rom a ~ rtical tub-, 4ill-d with cataly~t ~t-r~l to tho catnlyst bed
was a pr~t ar--a, packed with çrla~s wool~ A y ~qll;~ ~
i~ a ~ ~ e re5~ulator, r--li~f valve, ~ump and hoater for th f----a
The f--ed was heat-a by ~--t;r~ luid, circ~lA~ ~d through th~ feOa pot, ana
wa~ k-pt under a nitrogen paa
Th-- f--od wa8 ~h-nol (99 9 ~"~ c .t) and fl G ~ .o~e (about 99 ~-r. ~ ) in
a 21 1 molar ratio
Tho h--t;r~lJ fluid, h~At;rtr ta~e and rOactor were turnoa on The
s--l--ctea catalyst was slurriod in ~honol at about 45~C Catalyst-phenol
mixturo was pi5~etted into the reactor, at the bottom of which a ~lug of
-108-
CA 02230272 1998-02-24
WO 97108122 PCT~US96~13683
~lass wool/~lass beads was ~laced to ~ .vOnt catalyst from loa~in~ th~
r-actor. The ~honol:flu~L~ ~ wa~ ~daod to th~ r~d ~ot ~t 55~C. Th~
~ro~uro w~ najustoa to ~bout O.34 b~r~.
Phonol: f luoronono ~ood wa~ introduced into the rcactor and th~
c _-Lition of tho ~fflu~nt from the reactor was fsll_ _a by HP1C.
-1 09-
CA 02230272 l998-02-24
WO 97/08122 PCTAJS96/13683
Tho rollowing results wore obtained:
T~ble XVI
PMBSA-~er c~t~ly.t (~x~mple 9C) ~t 50~C:
proauctivity 1.4 g B~PF/~ cat h
con~ersion time (h) % fl~vl~no~ cv~v~ ~ion
0.33 99.7
28 lOO.0
53 lO0.0
69 99.7
89 99.9
113 100.0
137 99.8
144 99.6
162 99.6
158-1Octivity 98% 4,4-B~PF
PNBSA-XEBr ( _le 9D) ~t 69~C:
yroductivity 0.6 g B~PF/g cAt h
cv v~.~ion time (h) % fl~o~vne cvnv~l~ion
2 99.8
lOO.O
39 lOO.0
63 98.9
79 99.0
103 95.5
127 87.1
164 83.4
8010ctivity 98% 4,4-BhPF
-110-
CA 02230272 1998-02-24
WO 97/08122 PCT~US96/13683
PNBSA-XECl (Ex~mplO 9E) ~ 56~C:
productivity 1.48 ~ BHPF/~ cat h
cv v~ ~ion tim~ (h) % ~l~v Onv~n cv,.v~6ion
2 99.2
27 99.1
63 99.3
87 98.7
111 98.9
135 98.6
159 98.3
164 98.0
x-l-ctivity 98~ 4~4-B~PF
XENSA-llC (~xampl~ 22D) at 60~C:
cv.~voL~ion timO (h) % rl~O ~nv~u convorsion
1 99.2
17 94~4
41 92.2
91.0
DP~SA-MER3C (~x~mpl~ 27D) ~t 50~C:
8O1-ctivity 98% p~p-B~PF
~roductivity 4 1~ B~PF/ lb cat~lyst/hour ~ 4 k~/k~/h
co..v_L~ion tim~ (h) % fluo~O~v..o cv~VO ~ion
99.9
24 99.9
39 99.9
48 100.0
66.5 100.0
75.5 98.9
99.5 99.1
103 98.6
104 98.7
-111-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
XEMSA-5C (Exampl~ 22) at 55~C:
~oloctivity 98% ~,p-B~PF
proauctivity 0.43 LB B~PF/LB catalyst/hour =0.43 k~/k~/h
c~........................ v~L~ion time (h) ~c ~~--t fl~o~o-~ convor#ion
4 99.7
a2 100.O
46 100.0
100.0
77 99.7
94 99,7
118 99.7
142 99.5
144 99.4
~68 99.3
192 99.0
216 99.0
240 98.6
264 98.6
DPMSA-XE3C ~Examplo 27A-C) at 56~C:
8010ctivity 98 ~cl~L 4,4-B~PF
c~.. v.. l6ion time (h) % fl~ o no~ c~.. ~ ion
98.0
48 95.1
72 94.7
96 92.3
123 87.3
144 86.1
170 83.8
240 77.1
Th-~o ro~ults ~how that P~BSA, XE~SA, nn~ DP~SA cat~lyst~ of th~
invontion can ~xh~bit ~ta~ility ovor tim~, thus, have very u8-~ul
li r--time~ .
~J
-112-
CA 02230272 1998-02-24
WO 97/08122 PCT~US96~l3683
EXAMPLE 29
Cv lNUUU~ PROCES~ FOR ~A~ING BHPF VSING NERCAPTOPROP~N~e~q~ONIC ACID
CATALYST
~he r-actor cvn~ri8c~ a three-staged con~;n~u~ reactor (i~oi hr- 1
porr-ctly ~tirred ty~o) Th~ roaction W~8 run at 46~C at ~ 21 1 mol~r
ratio o~ phonol fluoronone (98 ~o~c , Aldrich), tho amount of NPSA bein~
0 05 to 0 07 ~quival-nt of MPSA mole of fluo.enone The _ _-sition of
tho product~ was fo~ J~ by HPLC
Tho roaction w~s c~ntinu~d for 228 hour8, At tho end of which
~luo ~ ono cv~v. ~ion wa~ 99 95 E ~c~ t (reactor 2) or 99 9 ~ercent
(rcactor 1) n~d 8electivity to 4,4-BHPF wa~ 98 32 ~orco L ( _~ct~~ 2) or
95 2 ~ = ~ (reactor 1)
~XA~PLE 30
~VAhVATION OF CATA YST OF EXAMPBE 22D (XEMSA-5C) IN Cu_ l..JUU~ PROCESS FOR
~AKING BISPHENOL A
~he reactor compriso~ a vortic~l u~low column of 8~;nl-P8 ~toel
tubing, ~ack-a with re~in atop A ~croen ana ~la8~ beads The col = was
~oated by a water jacket Thc ~Lv~ FP of the roaction was follc ~ as
abov~ by RPLC
Tho followin~ ros~lts wuro o~t~;n~d
3-
CA 02230272 l998-02-24
WO 97/08122 PCT/US96/13683
Tabl-- XVII
Te~t 25% ~ ~ -tcd XEMSA-5C
*_~ 50Wx4 Example 22D
Ac$d capacity
dry meq/g 4 0 3 5
w t meq/mh 0 84 1 17
Swell t-3t
p_enol/w~t-r vol 0 55 0 72
Reaction t~st~
30 min r-s t$me
4% acetono, 65~C
4,4 % by wt 12 7 14 0
c~v~.~ion 0 83 >0 95
~d~cLivity 8 2 9 0 ~ 0 2
2,4/4,4 0 28 0 024 ~ 0 001
6~ ac-tone, 65~C
4,4 % Dy wt -15 19 1 ~ 0 6
~o,.v.. ~ion -0 7 0 92
proauctivity -10 12 2 ~ 0 4
2,4/4,4 -0 27 0 025 ~ 0 003
4% ~et~-, 55~C
4,4 10 7 13 0 ~0 1
cv~v_l~ion 0 67 0 92 ~ 0 01
productivity 7 2 8 3 ~ 0 06
2,4/4,4 0 21 0 019 ' 0 001
A.~_ ~ . k of Tho Dow :' c-~ Campnny
~XAMPhE 31
PREPAR~TlON OF BI8P~ENOL F
Phenol nnd f- 1 a.~hyao wor- roacted to p ~ ~c~ '~;~F' -I F S; ~_
r-~ult~ w re obtninod
T_e ~c-';ng o ~ can be . ~-tev with ~ ucce~s Dy
~U~D~titUt$n~ t_e generically or ~pocif;c~ 7 1 y aescribed reactants and/or
operatin~ cond$tions o~ this invention for t_ose u~ed in the p.~~La;~
=- ~_p,
-114-
CA 02230272 l998-02-24
W O 97~08122 PCT~US96/13683
From the fo~_ ;n~ aQscri~tion, on~ skill~d in th~ ~rt can e~8ily
a~certain tho ~ ch~rA-te~;~tics Or this inv~ntion ~nd without
a~Artin~ from th~ ~pirit and scope thereof, can make variou~ changes and
';ficAtions of the invention to ada~t it to various usage~ and
conditions
EXA~1~ 32
Alt-rn~t~ Pro~aration of a Re2r-s-ntAtive [(L'_~c~Loalkyl)(Sulfo)-
Phenylalkyl] Sulfonated Poly~Lylene Catalyst (DQ~isnated DPMSAA-0 25-
1 5X2C)
A Preparntion of (2-Bromo~thyl)bonzyl Chloride
(Al) In an ~Y~e~~ion of the ~ OC6-1u e doscribod by Selva, M; Trotta, F ;
and Tundo, P Sy~h-ri~, 1991, 1003-1004, (2-L ~thyl)benzylchlorid~ wa8
pr~arod by th~ f ollowing ~ ~--~ a
C~- -~~tad sulruric acid (132 m~) was slowly added to ice cold
15 ~ n;z~ wat-r (66 m~) in a 1 L tL ~e n~cked glA88 reactor fitt-d with ~
~~hAn;c-l ~tirrer, roflux co~ , and t~ _--~L~ ~rob- Tho r-~ctor
c~tnlA;~;n~ tho ~ul~uric acid 80l~ ~ w~s cool~d in an ice bath, then (a-
b ~ ~thyl)}-- - (92 5 g, 0 50 mol) was addod foll_l_l by 50 ~
tetr_butyl~ ; chloride in w_tor (10 ~ of nolution), parA8 l~hyde
20 (20 0~, 0 666 mol, 1 33 ~quiv_lents), and finally ~odium chloride (80 0 ~,
1 37 mol, 2 74 aquivalonts) The slurry was stirrea at ~ ~ ~ely 1000
rpm (v ry vigorous) and heated to 80~C for 2 25 hours ThQ reaction was
1-88 than 50 ~el~el~L _ _let~ as dot- -' by gas ~L~ to~-ap_ic
analysis Adaitional parAf- l~-~yd- (20 0 ~, 0 666 mol, 1 33
25 oquivalents) was nadoa and tho mixtur- was stirrea (600-700 rpm) an
adaitional 3 5 hours at 80~C ThD roaction was ~r ~ l y 50 p~ ~ ~
complQte as aet- ~ ~A by gas ch~ og_a~_ic analysis T_e mixtur- was
A11~J~ to cool ana was tran~ferr-d to a s-~aratory funnel Th- organic
ph~o was se~arnt~d and sav d for further r~action
(A2) Alt--~Atively, (2-B _thyl)h~ - (92 5 ~, 0 50 mol),
c -~ l ~tea (12 ~ lar) hydrochloric acia (125 mL, 1 5 molo HCl, 3
oquivale~ts HCl), ana ~ar~f~ lA-hyde (22 5 g, 0 75 mol, 1 5 aquivalents)
w r- aaded to a 1 ~ three-neckQd glass reactor fittad with n '- ;c-l
~tirrer, addition funnel, and t~ ~ ~ a ~robo C -- l~t-d ~ulfuric
35 acia (111 m~, ~ ~ tely 205 g, ~ ~ t~ly 4 oquival-nts of sulfuric
acia) was ndded to the nddition funnel A nmall ~ortion (~t ~ tely
~ 10-15 mL) of t_e sulfuric acid was adaod to the reaction mixture from tho
_ -115-
,
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
ad~lition Cunn--l and the slurry WA8 h~atod to 80~C with stirrin~ at
a~ ~tely 1000 rpm Aft_r th~ r~action reaches 80~C, the s ;r~;ng
c~ ~_rl ratod sulCuric acia wau adaod dro~wis~ ovor 3 hours After an
addition 1 hour r action time, the r-action wa3 loss than 50 ~
5 com~l--to as deto ~ by gas cLL~ to~ aphic analysis The mixturo w~s
~llowod to cool ~nd was transferroa to a 8 paratory f-unnol Th-- or~anic
phas~ wa~ ~e~r~ltoa and savod Cor furthor roaction
Tho - ~;r9~ proaucts from r _ le 32, parts Al and A2 r~~t~ Sr
unr-actea (2-bromoethyl)b~~ ~ and c~loromothylatoa (2-b~ ~Lhyl)~
lO product (mixture of i ~ 8 ) wore furth-r chloromethylatoa according to
tho p~,c- ' ~ doscribQd in part A1 The reaction was pel r~ -d usinsr
~ufficient time and ~ ~ ~L~ (~ar~C l~z~hyd~, soaium chlorid~, sulfuric
acia) charg--s to compl~tely ~ th-- (2-Ll~ _thyl)~ - starting
material, ÇrivinÇr a mixtur- of (2-L. ~~thyl)ben~yl chloride;~ b alonçr
15 with highor-ho;l;nçr Ly -~ .~1~ cts Th- mixture of (2-b ~ -=thyl)benzyl
chloride ~ wa~ i~o~ Ate' by oil-pum;p vacuum bulb-to-bulb Xu~lrohr
di~ t; ~ (up to 140-145~C) Tho ;~ol~t-i ~ro~uct wa~ a wat--r-whit~
oil whJch acl;~f;~ upon st~;"SJ at room t~ _--c.L-~_. Gau
cLL~ tog--aphy analysis ~hows that unreacted (2-b~ a hyl)~ - - ana tho
20 hi~her boi~ g Ly-~.v~l.Lcts w~r-- --r~ lly absont ~Crom th~ aistillod (2-
L ~ ~I thyl)b-nzyl chlorido; -~ .,d~1
E~ Alkylation
gLy ...~/divinyl~ ~ - co-yolymor rosin boads (lO OO sr, -30170 me3h,
1 5 ~ t a~vinyl~ L -; t-ly 96 0 mmolo ~Ly _no r peat units)
25 woro add--d to ~ round bottom 5JlaE~ flask (ronctor) under a pad of nitro~on
with a soaium Ly~llv8id ~crubb--r attnched (to trap ovolvod ~ICl) A
~o~ of (2-L~. ~thyl)benzyl cl~1ori~~ (mixturo of a ic rin~
-- ~, ~, .' ' .-t~ly par~) (lO O sr, 0 238 oquival--nts oasoa upon ~ Ly . -
_ ~vt~uSr units) in
30 l,2-q;~h1o~ - (25 mL) was added to tho dry ro~in b-aas Tho b-aas
w r-- ~11 9d to ~woll for ~ t-ly 5 to lO Tnlt-~, then aaditional
l,2~ hlo~ ~Lh-r - (35 ml~) wa~ addod to the ~woll--n beads Anhydrous
tin(IV) chlorido (2 5 mI, .~p - -oly 5 57 Çr~ .L - t-ly 21 4 mmol)
was Ellowly addod via ~yrin~ to tho polymor ~~lurry at room t~ turo
35 ov--r ~p - t ~ly lO 1 ~ with rapid stirrin~ Tho mixtur-- turnea
light yellow whcn tho tin(IV) chlorid-- was add--d Tha mixturo wns slowly
w~rmoa (in 5~C inc ~ -~) to 40~C ovor ~ tely 30 r~t~E~ and was
-116-
CA 02230272 1998-02-24
W O 97~08122 PCTAUS96/13683
allow a to react at 40~C for 1 hour At thi~ time the light oran~e
mixture was slowly warmQd (in 5~C incr_ ~) to 60~C over a~~ t-ly 1
hour 30 ~ t~ ana was Al 1 Oz - a to r~act ov-rni~ht at 60~c with slow
xtirrin~ Aftor ovorni~ht r-~ction, th- mixturo w~8 cOOlOa to ro ~
tomp-rature ~nd was qu-~hsd by slowly addin~ moth_nol to the w~ stirred
polymer slurry The beads were s-~arat~d from the solution usin~ a glass-
4rittQd ~ = el with vacuum filtration Th- b~aa8 woro ~ ly washod
(throo portions ~ach) with dichloromethano, wat-r, totraL~a ~L~ ~u, and
methanol, then the boads wHro driod oV~rni~ht in _ vacuum ov n at 80~C
(dry mass 13 89 g) hooL tical mass yi~ld ~ 14 49 ~ Alkyl~tion yiold
(by ma~s uptA~o) ~ 87 porc-nt A~r~Y; to d-~r-o of ~olymor
funct;~Al~r-tion (by mass yiold) - 0 21
C Sulf~ A t; t'~
The ~olymer b~ads from Exam~lo 32B (13 89 ~, Qst; -~1 116 mmolo of
~honyl groups) wor- addoa to a gla~ roactor with addition f = el and NaOH
~crubbor attachod Di~hloromethane (75 mL) was addQd to the flask and the
b-ads w r~ All_ _~ to swoll (ra~id swollin~ was obs~ v ') Th slurry was
cOOl.a to ~ - t~l y 3 to 5 ~c in an ico wat~r bath Chlorosulfonic
acid (11 7 mL, a~ - ~ly 20 5 ~, ~ ~ t~ly 176 mmol, t'~l''-~ 91y
1 5 equival-nts por ~quival~nt o~ ~henyl ~rou~s) was slowly addQd dro~wi~e
over ,~ t~y 30 '~tv~ to the cold ~olymer slurry with stirring
The mixturo was A71e.., ~ to r-act at a~l-- t~ly 3 to 5~C in an ic- wator
bath for 1 hour The mixturo was 1 ~d from th- ic~ bath and All d to
wnrm to room t~ ~turQ ovor 2 hours 45 ~t-- At this tim , thQ
~olymor slurry wa~ again coolod to 3-5~C in an ico wat-r bath, and wat~r
wa~ slowly addod with ra~id stirrin~ to quench th- ~XCQ88 chlorouulfo~;c
ncid Tho beads w rQ ~ ~r~Ated using a glass-~rittod f = ~1 with vacuum
filtration Th- b-ads w ro th n wash-d ~Y~ iVQly with wator Watar wa~
ada~d to mako a slurry, thon solid sodium bicA-~Ate was slowly nddod in
~mall portion~ with stirring until no ~ r~ ~1;~ was -b~ v~d (all
activ acid sitos w~re n-utraliz~d) Tho b-ads WQr~ wash-d with wator nd
transf-rrOd back to the ~la~s reactor with 100 mL of water Tho boAd~
wore thon hontod to 60 to 70~C ov r 2 hours to onsur- hydrolysis of any
l_ri~u-l ~ulfonyl chlorid- groups
D qh~lAti~n
Tho nquoous ~olymer 31urry from Exam~l- 32C (est; t-' ~ - t ly
20 mmol- Br) was coolod to room t~ ~ ~ 0 Sodium bicA h~nAt- was
-117-
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/13683
~lowly Add~d until tho ~lurry was neutral (no ~Uhhl in~ observed), then
additional sodium bi~A b~nAte (5 30 g, 63 0 mmol, a~rv~imatoly 3
~quivalQnts relative to est; te~ b~ c~nt~t in boads) was added to
th- aquoou~ b-Ad ~lurry Th;olA~otic acia (4 5 mL, ~ ~_ ~ly 4 8 g,
~ ~ ~ t~ly 63 mmol, ~ m_tely 3 equivalents relative to Qst~- t~
L ~ c~nte~t in be_ds) wAs added to an addition ~unnel The r actor
wa~ evA~uAt~ and r~f;71r~ with nitro~en soveral times to ~; '-e the air
c~nt~nt . Th- th;olA~tic acid was slowly added over ~ - t~ly 10-15
~ to~ to the aqueous bead slurry at room t~ _- ~turo with ra~id
~tirrin~ Tha th;olAcetic acid addition rate was adjusted to control the
~f4- v_~c- evolution o4 carbon ~;o~i~e which wAs 40rmed in tho
n~v~ Al;~At;o~ ~ _~8 A4ter tho th;ol~A~etic Acia ~ddition was complete,
tho mixtur- was heat-d to 70~C and was allowed to react ov~- n~ ght with
~ tirrin~ The mixturo was thon cooled to room t' _~ ~ ~ and the
b-ad~ woro colloctod by filtration u~in~ a frittea-~la~s funnel Tho
b-aa~ w re washed o~ ;v-ly with water, then with dichloromoth~ne
; (o~tional), and th-n wa~hod again with water The beaas wore transf-rrod
back to the gla~ reactor, then c~~ ~ated (12 molar) L~ o~Lloric acid
(100 m~) wa~ addcd Th~ mixture was hoated with mild stirring to 50~C ror
20 2-3 hours, thon wa cooled to room t~ _ tU e De; ~n; ~ water (100 mL)
wa~ add-d and the beads were a~ain collocted by filtration u~ing n
4ritt-d-~la~s funnel The beads wore wa~hnd with water, thon w re wash-d
-~t-~ivoly (a~ ~; t~ly 500 mL) with dilute (~ - t~ly 3 mol_r)
aqu-ou~ Lya ocLloric ncid Tho beads wero then washed again with
~-i on; ~ed water and wer- tran~f-rred to a ~tora~e bottlo without any
add$tional drying The final ~olymer catalyst has a titrated w-t- ~_t
(water ~wollen) acid ca~acity o4 0 ôO ll;r_~ival-nt/mL cataly~t The
~inal ~olymer cataly~t wa~ i~n-t-' as DPNSAA-0 25-1 5X2C
E Pr-paration o~ DPMSAA Ro~in with 0 71 Equival~nts Alkylation
Anothor c~to~ ~n;c acid yolymer was ~reparea from 1 5 ~ t
cro~l;nl ~ ~Ly ~ -/divinyl~ -~~~o co-polymer beadc (-30~70 mesh) using
tho ~ ~-~ ~ doscrib-d in ~teps B-D of Ex g le 32, oxc-pt using 0 71
equivalontY of (2-L ~ -~thyl)b~nzyl chlorid- in the ~lkylation r6~c~i~n
Chloro~ulronic ~cid ~2 0 ~quivalont- r-lntive to th- c-lc~lAte~ total
~quival-nts of ~honyl ~roups in the polymer) was used in tho 8Ulfon~tion
r-action ~h;ol~c tic acid and sodium hic~ te (3 0 oquival-nt~ of
~~ch r-aWont rolative to the C'1C~ ~tF~ ~mount o~ LL~ ~9 in the polymor)
-118-
=
CA 02230272 1998-02-24
W O 97~8~22 PCTAUS96/13683
worc usod in tho 1 h; ol~ti~n ro~ction Tho ~ass yiold oAf polyA~or oA~tainod
from the alkylation r-action co 1~ to a 83 ~e1C~AA~ yield in the
alkylation reaction and a degroe of functi~nAl;ty of 0 59 The final
polymor cataly~t Ahas a titr~toa water-wot (water _,_ll~n) acid cn~acity of
5' 0 94 ll;~A~uival-nt/A~ catalyst TA~AO final polymer catalyst was
~A~i~n~ted a8 DPNSAA-0 75-1 5X2C
F Preparation of DPMSAA Resin with O 43 Equivalonts Alkylation
AnotAhor --~to~lf~n;c acia polyAmor was ~repared from 1 5 A~
cro~sl;n~ ' ~Lyl~no/divinyl~en~en~ co-polymer bead8 (-30170 mo8h) usin~
10 the ~locFAd~e de8cribed in 8teps B-D of _le 32, except usin~ 0 43
oquival-nts of (2-bl ~~thyl)~onzyl chlorido in tho ~lkylation roaction
Ghloro~ulA'onic acia (2 0 equivnlonts rolativo to tho calcul~te~ total
equivalent8 of phenyl ~roup8 in the ~olymer) wa8 in the ~nlf~n~ti~n
r-action ~hiol~cetic acid ana 80dium h;C~ _h~n~te (3 0 ~quivalont~ or
o~ch ~ nt rolativo to tho ~1C ~t~~ ~mount OAC AD ~ n_ in the polymor)
wero usoa in tho th;ola~;~n roaction The mas~ yield of Apolymer obtained
from tho alkylation reaction C0~ -d to a 81 ~Ol ' yield in the
alkylation r-action ana a aegroo of f~n~t;~l;ty o~ 0 35 Tho fin~l
polymor catalyst h~s a titr~tod w~tor-wot (w~tor swollon) acia C~pACity OA'
20 0 . 85 11 i r _~ivalont/mL cataly~t Tho final polymer catalyst was
~~iq"~teA a3 DPMSAA-0 45-1 5X2C
C Preparation of DPMSA~ with 0 095 Equivalent~ Alkylation
Another - ~ o~ulf~n;c ~cid ~olymer was pre~aroa A'rom 1 5 A~o~AAL
croAA~l~n~,~ ~y,~ /divinyl~ co polyA~or b~ad8 ( 30~70 m~shA) u8in~
25 the ~ occ~ o d-seribed in steps B-D o_ _lo 32, exeept usin~ 0 095
~quivalents of ( 2 -AJ~ thyl)honzyl chloride in tho alkylation roaction
Chloro~ f~n;c ac$d (1 5 oquiv~lonts r-lativo to the ~lc~ t~ total
~quival-nts of phenyl groups in tho polymer) was u~ed in the sulf~n~t;~n
r-action Th;o~aartic acid and ~odium hie-~h~n-te (3 0 equival-nts of
~aeh relntivo to tho ~lc~ t~~ ~mount of L~ - in tho polymQr)
w ro u~od in the thiolation reaction Tho mass yield of ~olymor oht-;n~~
from the alkylation reaction corrospondn to a 99 ~elc~nt yield in tho
alkylation r-action and a d-gr~o of func~ion~l;ty o~ 0 094 The _inal
polymer cataly~t has a titratod wator-wot (wator ~ ~n) acid ca~acity of
~5 0 80 'll;~_~ivalont/mD catalyst The final polymor catalyst was
te' ns DPMSAA-0 10-1 5X2C
-119-
CA 02230272 1998-02-24
WO 97/08122 PCT/US96/13683
H Preparation of DPMSAA with 0 42 Equival-nts of (2-
B ~ -ethyl)b-nzylchlorido Alkylation and 0 30 Equivaltants of Bonzyl
Chlorid- Alkylation
Another ~ ~aptoGulfonic acid ~olymer was prepared from 1 5 pez
cro~ tyrene/divinyl~~~~ - co-~olym~r beads (-30170 mosh) usin~
the p .-- doscribed in ste~s B-D of Example 32, ~xcept using 0 42
~quival-nt~ of (2-L ~ hyl)benzyl chloride and 0 30 ~aquivalents of
b-nzyl chloride in the alkylation -ctio~ Chlorosulfonic acid (2 0
~quivalont~ r-lative to tho c_lculAted total equivalonts of phenyl grou~s
in th~ polymer) was used in the sulf~~Ati~ reaction ~h; ~A~t; C acid
and soaium b~cA h~-te (3 0 eguival-nts of ~ach r-a~ent relativ~ to the
calc~lat~d amount of L ~9 in the ~olymer) wore used in tho t h;olA,~ j~
re_ction Tho final polymor catalyst has a titratod wator-wet (water
~woll-n) acid ca~acity of 0 94 milliequival-nt/mL catalyst Tho fin_l
15 polymer catalyst was A~~;g~-t6d as DPMSAA-0 45/0 30-1 5X2C
I Preparation o~ DP~SAA Rcsin having 0 423 Equival-nts
Alkylation
Anothor - ~ptosulfonic acid polymer was preparod from 1 5 peice~L
cro~rl;~l- d ~Ly~ /diviny~ co-polymor bonds (-30l70 mesh) usin~
20 th~ p ~_~ ~ of st-ps B-D of _le 32, ~axcept usin~ 0 423 ~quival~nt8
of (2-Ll -_thyl)benzyl chloridt3 in tho alkylation e~ti~ and air-ctly
carrying tho polymer slurry ~ht-;~A from the alkylation r-action dirtctly
on to tho ~ul~Ati~ reaction without any qu~h;~ olA~ti~, or
w-~hi~ st-ps aft-r tho nlkylntion reaction Chlorosulfonic acid (1 25
~quival-nts r-lative to tho total equival-ntD of ph-nyl oroups p oso~L in
all r-actants) was addea directly to the polymer ~lurry aft-r the
alkyl_tion reaction waQ _ _l to Worku~ and ~ ~ent jPolAti~ of tho
product afttar ~Ulf~Ati~ w_s as in Example 32, except that more ~ ive
~ ~h~ wa~ r-quir~a to remova soluble roaction L~-~ ~cts from the
polym r ~lurry ~h;olA-etic acid and ~odium hic--h~-to (3 0 ~quival~nts
of each rJ - r-lat$ve to the ~sti ~-d amount of L,~ --
~ in the polymor) w rQ u~ed in the thiolation r-action The ~inal
polymor cataly8t ha~ a titrated w-t- -w~t (water ~ ) acid cnp~city of
0 78 'l~ valent/mL cataly~t The ~inal polymer catalyst was
35 ~~~i5F~Atr l as DJ?~SAA--0.45NW--1.5X2C.
-120-
CA 02230272 1998-02-24
W O g7108122 PCT~US96/13683
J Preparation of DP~SAA Rosin with 0 238 Equivalonts Alkylation
Anoth~r -~o~Lo~ul~onic ~cid polym r was ~r-~arod from 1 5 ~arcolt
cro~sl;~-~ ~Ly__nD/diviny~ co-~olymer boads (-30+70 me8h) using a
variation of tho ~oc~du~O do~cribed in Exam~l- 32 The alkylation and
~ul~Ation r-actions were ~orForm d in onQ ste~ ~t;li~;~ chlorosulfonic
acid a3 th~ alkylation c~t~lyst and ~ulfonation
8~y..~e/divinyl~7~~~ co-polymer rosin b-ads (10 00 g, -30+70 mosh,
1 5 ~ercont divinyl~ tely 96 0 m~ol ~Ly ~n~ re~at units)
woro add-d to a round bottom glass flask (ro~ctor) undor a pad of ~lant
nitro~An with ~ ~odium L~a~ido scrubber attached (to trap evolved HCl)
~ olution of (2-~ ~ -~thyl)benzyl chloride (mixtur~ o~ a~ tic ring
ir_ ~ 8, ~L~ a~ tely ~ar~) (5 32 g, 0 238 ~quivalonts banod u~on styreno
ro~-at unit~) in
1, 2-a; chl oroothano (25 m~) wa~ added to tho dry re~in bead8 The b_ads
woro All~ el to swell for ~L~ ely 5 to 10 ~OA-, th~n adaitional
1, 2~ hl oroeeh~s (35 mL) was adaod to tho ~ _11~ boads Tho slurry wa~
cool-d 2 to 3~C in an ico bath, thon chloronulf~;c acid (la 0 mL,
~ ely 21 0 g, 1 5 _quivalent8 ba8ed u~on total equival-nt~ of
phonyl group8 in the mixtur_) was ada-d slowly aro~wiso ov~r ~1 - t-ly
20 1 hour 45 '~'lteP. Tho mixturO was All~ to ~tir an additional 1 hour
at 3 to 4~C, th~n was ~ v_d to room t- _a ~turo and Al 1 ~ d to react an
additional 1 5 hours The mixture w~8 then cooloa in an ice wator bath
and wat-r wa~ ~lowly adaea to quench tho _xcAss chlorosulfonic acid
Thereart-r tho bAaas worO iPol~At-~ according to th- ~ O d~3cribod in
25 ~art C 04 _ le 32 T-; k-W; -Q~ tho th;o~Ati~~ r-action was a8 in ~art D
of _lo 32 u8ing th;o~Af~ic acid and aodium h;c~_~At - (3.0
~quivalonts O r each rea~-nt rAlative to tho o~l; -t~A~ amount of
Ll ' - in tho ~olymer) The final ~olym~Ar catalyst has a titrat-d wator-
wet (wat-r - ~) acid ca~acity of 0 94 ~ ~ivalont/m~ catalyst
30 The ~inal polymor catalyst was ~--i~At-d as DPMSAA-2S-0 25-1 5X2C
X Pro~aration of DP~SAA Resin Havin~ 6 5 ~o~ce~L Cro~sl;~king
Anothor -7~Losulfonic acid ~olymor was ~re~ar~d from 6 5
cro~ -ed ~Ly,~ -/divinylh-n~- ~ co-~olymer b~aas (380 micron ~;f-
particle si~ 8~ ) using the ~roD~ ~ d-scribed in sto~s B to D of
35 Example 32, oxco~t using 0 427 Oquivalents of (2-b~ ~thyl)benzyl
chloride in tho alkylation roaction
-121-
-
CA 02230272 1998-02-24
W O 97/08122 PCT~US96/l3683
Chlorosulfonic acid (1 5 oquivalont~ relativo to tho calc~late~
total oguival-nts of ph-nyl groups in tho polymor) wa~ usea in the
~ulfonation roaction ~h;o7~etic acid nnd ~odium bir--h~--te (3 0
oquivalonts of oach r~agent rnlative to tho c~lc~lAted amount of b ~e
in tho polymor) W~rQ used in the ~h;ol~t;on roaction Tho m 88 yield of
polymor obtainea from tho alkylation r-action oo~ ' to a 57 porcont v
yi-ld in tho alkylation roaction and a de~roo of func~ ty of 0 24
Tho final polymor catalyst han a titratod wator-wet (water _r 11~) acid
c~pacity of 1 75 milli-quiv~lont/mL catalyst Tho 4inal polymor catalyst
10 was ~-r;~-te~ as DP~8AA-0 45-6 5X2C
L Pr~paration of DPMSAA Rosin with 0 25 Equival~nts Alkylation and
Havin~ 1 8 ~e _~L Croggl;~k;~g
Another ~ _~Losulfonic acia polymor wa~ pr-par-d from 1 8 p~ c_~L
cro~Pl ;~k~a ~Ly _ne/divinyll- - ~ copolymor boaas (-25~40 mosh) using tho
15 ~loc~a~ ~ d-~cribod in ~top~ B through D of r _le 32, oxcopt usin~ 0 25
equival-nt~ o~ (2-L~ - 'hyl)b-nzyl chloride in tho alkylation reaction
Chlorosulfon$c ~cid (1 5 oquivalont8 relativo to the calc~late~
total ~quiv~l-nts of phenyl ~roup3 in tho polymer) was usod in the
nulfA~t;~n r-action ~h;olA~etic acid and ~odium hic~ ho~t~ (3 0
~quivalont~ of ~ach ~ relativ- to tho c~lc~ t-1 amount of L '~o
in tho polymor) were usod in tho th;Alatio~ r-action The ma~s yiola of
yolymor ~t~ from the alkylation reaction co~ to an 86 pOI~t
y~-la in tho alkylation r-action and a dogroo of func~ ;ty of 0 22
The final polymor cataly~t had a titratoa water-wet (wator swollen) acid
capacity of 0 85 ~ ~i~alont/m~ cataly~t The final polymor cataly~t
wa~ d n~g~-t-~ a~ DP~8AA-0 25-1 8X2C
M Prer~a~t~ of DPMSAA Rosin with 0 10 Equivalonts Alkylation and
~avin~ 1 8 ~VI_ ~ CrOrr~i~k;~
~tl- - _~tosulfonic acia polymer wa8 proparod from 1 8 po,.- -
30 ~ rl~ sLy,. -/diviny1ho~- ~ co-polymer b-ad~ (-25~40 me~h) usin~
tho ~loce~ doacrib-d in st-ps B to D of _~e 32, ~xcopt using 0 10
oquival-nt~ of (2-L, -~thyl)bonzyl ehlorid- in tho alkylation r-action
Chloros~lfo~;c acid (1 5 oquivalonts relative to tho r-lc~1at~d total
~quival-nts of phonyl groups in the polymor) wa~ u~od in the sulf~-ti~~
r-action ~h;ol~otic acid and nodium bic- h~-t6 (3 0 equival-nt~ of
~nch r-a~ont rolative to th- c-1c~lated amount of b, ~~ in thH polymer)
w re un-d in tho 1 h;o~t;o~ r~aetion The final ~olymer catalyst ha~ a
-122-
CA 02230272 l998-02-24
W ~ 97~08122 PCT~US96~13683
titrated water-wet (water 8wollen) acid capacity of 0 81
millioquivalent/m~ catalyst The fin~l polymor catalyst was dAoi7~tPd a~
DPMSAA-0 10-1 8X2C
N Proparation o~ 1 5 ~ ~L Cro8nl;~e~ DP~SAA Resin with 0 10
Equivalent8 Alkylation and U8ing Sodium ~yd~ u~ in the ~,hiol~
Roaction
Anothor -~to~ulronic acid ~olymor was pr~parcd from 1 5 ~L~_-L
cro~ Ly .~o/diviny7~-~-~ ~ co-polymer boads (-30~70 mesh) usin~ a
variation o~ the ~LOCedu~ ~ described in 8to~s B to D of Exa~ple 32, ~xce~t
using 0 10 Qquivalonts of (2-L ~ ~thyl)b~nzyl chlorid~ in th~ alkylation
roaction and ~odium Lyd G~ulfido in th~ th; ol at;~ r-action
Chloron~f~;c acid (1 5 e~uivalent~ relative to the c-lc~ tefl total
e~uivalent8 of phenyl grou~8 in the ~olymor) wa~ u~od in tho uulf~~tio~
ruaction 80dium Lyd ~ulfi~s (6 4 ~quival~nt~ rolativo to th~ c~lc~lat~
amount of L ~ ~9 in the ~olymor) wa~ usod in th- 1h~ol~ti~n reaction
The ma8s yield of polymer obtain-d from the alkylation rcaction
COrrQ~F~Q to a dogr~e of func~ ;ty of 0 10 Tho final polym~r
catalyst ha~ ~ titrat~d w~tor-wot (wator ~, ll~) acid capacity oÇ 0 82
millioquival-nt/m~ catalyst The final ~olymer catalyst was deni9n~te~ as
DPMSAA-AT-0 10-1 5X2C
0 Preyaration of DP~SAA Rosin with 0 25 Equivalunts Alkylation and
Having 4 ~ ' Cro~ol;~;~g
Anothor --c~p~osul_onic acid polymer wa8 propar-d from 4 pL~ ~
cros~ 8tyrone/divinylh-~~ ~ co-~olymer b-ads (360 micron uniform
~art$clo si2e ~hc ~) u~ing tho ~ _~~ e describ~d in ~te~ B-D of
_~e 32, ~xcopt using 0 25 oquivalonts of (2-L ~ ~~thyl)ben2yl ~h~ori~e
in the alkylation reaction ChlorosulfoniC acid (1 5 ~quivalents
r-lative to the ~lc~ ted total ~quivalent~ of phonyl grou~s in the
~olymer) was u~od in tho ~ul~n~ reaction ~h;o~a atic acid ana
~oaium bic-~h~n~te (3 0 ~quivalonts of oach rea~ent relativo to tho
r~l c~ t~ amount o~ bL. ~ n~ in th- ~olym r) w ro u~ea in th ~h; ol~t; ~n
roaction Tho mass yiOla of polymnr oht~ rom the alkylation roaction
o~- _ _ '~ to a 73 ~CL~_ ~ yiela in tho alkylation r-~ction and a d-groo
of ~uncti~n~l;ty o~ 0 18 Thc final ~olymor catalyst wa~ dQsignated as
35 DP~SAA-0 25-4X2C
P Pro~aration o~ DP~SAA Rosin with 0 10 Equival-nts Alkylation and
Having 4 ~ - Cro~l;~;~
-123-
CA 02230272 l998-02-24
W O 97/08122 PCT~US96/13683
Anothor -~Lo~ l fA~; C acid ~olymor was prc~arcd from 4 ~n c~nL
crsr~li~k~d -Ly, --/divinyl~ --ns co-polymer beads (360 micron uniform
particl- 8ize sphoro~) usin~ th~ ~C~d~Lo doscribod in ste~s B-D of
Exampl~ 3Z, oxcOpt usin~ 0 10 oqui~alents of (2-Ll ~~thyl)benzyl chloridQ
in the alkylation r~action C_lorosulfonic acid (1 5 equival-nts rolativQ
to the calculatod total Qquivalents of phenyl groups in t_o polymer) was
u~d in the ~ulfo~-t; A~ rOaction. ~h;ol~etic acid and sodium hic-~hA~Ate
(3 0 ~quival-nts Of ~ach L~-_ t rolativo to tho calculAttd amount of
b~ in the polymer) worO u~od in the fh;ol~;~ roaction The ma~s
yiold of polymQr oht~ from the alkylation r-actio~ GG~ ~ P_ '~ to an
74 ~_ cel~L yiold in tho alkylation roaction and a do~re~ of funct~ A~l i ty
Of O 074 The final polymQr catalyst was d~riD~ted as DP~SAA-0 10-4X2C
_~ 33
Evaluation of Catalysts in a CA~t;~l~~u~ Proces~
A t_ro--stagQ up-flow r-actor wa~ constructed from t_roQ vertical
~t-~ s st-ol tubos with ~-m~l;~g ports bLt~_- oach soction Each
rOactor ~ta~- was wator jacketod for t- _--~tura control wit_ all
co~ocLing lines heat-tracod to ~ OvonL reactor lino ~lu~ing Lik-wi~e
tb~ 2L roactor f - od tank was jack-ted 80 that ~ e control Of the
r-actor ~-oa can be obtained From the f-ed tank, foQd flows t_rou~h an
n70_L lcally hoat-tracod ~oction of tubin~ for control of feed input
t-mp-ratur-
Each ctor s-ction was yacked wit_ 10-20 mL of wat-r-wet catalyst
Tho reactor foOd consi~ts of a solution of 4 weight ~ ~ L acetonO
in ph-nol Thu ac-tono ~_Onol mixturO was ~ ~ -ly mot-red into t_~
t- _~ ~ture controll-d r0actor system at a ~~fi--d c '-~Atio~ of flow
rat- (1 0 mL/min to 2 0 mL/min) ana roactor t~ _ ~turo (55~C to 65~C)
U~on ~tart-up of ~ach new lo~;n~ of cataly~t, the foOd ~assos t_rou~h tho
catalyst for at loast 12 hours boforo moa~ Y were ~ ' to r~movo
water from th~ catalynt Product __~ition of the roactor effluont from
~ach of tho thr-e stages was analyzod by ~PLC while gns ~L~ tG~ ~Ly was
u~-d to analyze for acotono and wator The r-sults A~t~;--' from tost~ of
th- catalyKts at various timos of ..~cti~ (r-actor ~ ~e tim 8) w~ro
~rovia-d in Tablo XIX Productivity was ~xpr-ssod in terms of ~ounds of
~i,r_~ -1 A ~l~ ~er hour ~or cubic foot of water-~wollon catalyst
charged into th~ r-actor (NOTE Unl-ss ot~ -w 80 not-d, all of the
catalyst r-~ults w~re obt~inod at 55DC )
-124-
Table XIX
Image Image Image