Note: Descriptions are shown in the official language in which they were submitted.
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
ROPES OF SINGLE-WALL CARBON NANOTUBES
SUMMARY OF THE INVENTION
The invention provides a method of making single-wall carbon
nanotubes by condensing carbon vapor at appropriate conditions around the
"live
end" of a carbon nanotube, preferably a single-wall carbon nanotube. A single-
wall
carbon nanotube with a live end is formed by vaporizing carbon along with
appropriate amounts of a Group VIII transition metal or mixtures of two or
more
Group VIII transition metals, maintaining the vapor at the proper annealing
conditions and then collecting the soot and/or other material that condenses
from
the carbon/metal vapor. In one embodiment of the invention, direct laser
vaporization of a composite rod formed from a mixture of graphite and one or
more
Group VIII transition metals produced single-wall carbon nanotubes when the
transition-metal/graphite vapor was briefly maintained in a heated tube. In
another
embodiment of the invention, the composite rod was vaporized by utilizing two
different laser pulses spaced apart in time to provide a more uniform and
effective
vaporization of the composite rod.
The invention also provides a method of making ropes of single-wall
carbon nanotubes. These ropes comprise about 100 to 500 single-wall carbon
nanotubes all roughly parallel to each other arranged in a two-dimensional ("2-
D")
triangular lattice having a lattice constant of about 17 Angstroms (A). Single-
wall
carbon nanotubes in a rope have a diameter of 13.8A =L 0.3A, or about 13.8A
:I:
0.2A, and are predominant over other possible sizes of single-wall carbon
nanotubes. The invention comprises the methods of making single-wall carbon
nanotubes and ropes of single-wall carbon nanotubes disclosed herein, as well
as the
products and compositions produced by those processes.
For example, a 1:1 atom mixture of cobalt and nickel was combined
in an amount of I to 3% on an atom ratio with graphite (97 to 99 atom %
carbon)
and heated and pressed to form a composite rod. Portions of that transition-
metal/graphite composite rod were vaporized with a laser inside a tube
maintained
at a temperature of about 1000 to 1300 C. A flowing stream of argon gas was
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
2
passed through the tube and the pressure in the tube maintained at about 500
Torr. ~
Material from one end of the graphite/transition-metal composite rod was
vaporized
with a laser to form a vapor comprising carbon, cobalt and nickel. The soot
collected from that vapor produced single-wall carbon nanotubes in
concentrations
much greater than observed before. About 50% or more of all of the carbon in
the
deposits of product collected downstream of the composite rod were single-wall
carbon nanotubes present either as individual nanotubes or as ropes of
nanotubes.
Other combinations of two or more Group VIII transition metals as well as any
Group VIII transition metal used singularly will produce the single-wall
carbon
nanotubes in the method of this invention, at concentrations of 0.1 to 10 atom
%.
Preferably, one or more Group VIII transition metals selected from the group
of
ruthenium, cobalt, nickel and platinum are used.
The invention also includes an embodiment where carbon nanotubes
having a live end, preferably single-wall carbon nanotubes, are caught and
maintained in the heated portion of the tube (annealing zone). A tungsten wire
or
mesh grid may be mounted in the tube downstream of the target to catch some of
the carbon nanotubes formed from vaporization of the target comprising carbon
and
one or more Group VIII transition metals. After the carbon nanotube having a
live
end is caught, the carbon vapor supplied to the live end of the carbon
nanotube may
be supplied by: (i) continued laser vaporization of the target comprising
carbon and
one or more Group VIII transition metals; (ii) stopping laser vaporization of
the
target comprising carbon and one or more Group VIII transition metals and
starting
laser vaporization of a target comprising, consisting essentially of or
consisting of
carbon, (iii) stopping laser vaporization altogether and introducing carbon to
the
live end of the carbon nanotube from some other source. Step (iii) may be
accomplished, for example, by adding graphite particles, fullerene particles,
carbon
vapor, carbon monoxide (CO), or hydrocarbons to the argon gas flowing past the
live end of the carbon nanotube or by flowing CO or a hydrocarbon gas (without
using an inert gas) past the live end of the carbon nanotube. In this
embodiment,
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
3
after the carbon nanotubes having at least one live end are formed, the oven
temperature (annealing zone temperature) may be reduced. The temperature range
may be 400 to 1500 C., most preferably 5000 to 700 C. Other features of the
invention will be apparent from the following Description of the Several Views
of
the Drawings and Detailed Description of the Invention.
DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
Figure 1 is a diagram of an apparatus for practicing the invention.
Figure 2A is a medium-magnification transmission electron
microscope image of single-wall nanotubes.
Figure 2B is a high-magnification image of adjacent single-wall
carbon nanotubes.
Figure 2C is a high-magnification image of adjacent single-wall
carbon nanotubes.
Figure 2D is a high-magnification image of adjacent single-wall
carbon nanotubes.
Figure 2E is a high-magnification image of the cross-section of seven
adjacent single-wall carbon nanotubes.
Figure 3 is a diagram of an apparatus for practicing the invention
utilizing two different laser pulses to vaporize the composite rod target.
DETAILED DESCRIPTION OF THE INVENTION
It is known that fullerene tubes are produced in some circumstances
along with the production of fullerenes from vaporized carbon. Ebbesen et al.
(Ebbesen I), "Large-Scale Synthesis Of Carbon Nanotubes," Nature, Vol. 358, p.
220 (July 16, 1992) and Ebbesen et al., (Ebbesen II), "Carbon Nanotubes,"
Annual
Review ofMaterials Science, Vol. 24, p. 235 (1994). Such tubes are referred to
herein as carbon nanotubes. Many of the carbon nanotubes examined early on had
multiple walls, i.e., the carbon nanotubes resembled concentric cylinders
having a
small cylinder in the middle immediately surrounded by a larger cylinder that
in turn
was immediately surrounded by an even larger cylinder. Each cylinder
represented
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
4
a"wa11" of the carbon nanotube. In theory, there is no limit to the number of
walls ~
possible on a carbon nanotube, and carbon nanotubes having up to seven walls
have
been recognized in the prior art. Ebbesen II; lijima et al., "Helical
Microtubules Of =
Graphitic Carbon," Nature, Vol. 354, p. 56 (November 7, 1991).
Multi-wall carbon nanotubes have been discovered in carbon
deposits on carbon electrodes that have been used in carbon arc methods of
making
fullerenes. Ebbesen I; Ebbesen lI. It is also known that single-wall carbon
nanotubes can be made by adding a specific metal or a mixture of specific
metals to
the carbon in one or both of the carbon electrodes used in a carbon arc
apparatus
for making fullerenes. See lijima et al., "Single-Sheli Carbon Nanotubes of I
nm
Diameter," Nature, Vol. 363, p. 603 (1993); and Bethune et al., "Cobalt
Catalyzed
Growth of Carbon Nanotubes with Single Atomic Layer Walls," Nature, Vol. 363,
p. 605 (1993). The prior art recognized a method of making single-wall carbon
nanotubes using a DC arc discharge apparatus previously known to be useful in
making fullerenes described by U.S. Patent No. 5,227,038. Single-wall carbon
nanotubes were made using the DC arc discharge apparatus by simultaneously
evaporating carbon and a small percentage of Group VIII transition metal from
the
anode of the arc discharge apparatus. See Iijima et al., "Single-Shell Carbon
Nanotubes of I nm Diameter," Nature, Vol. 363, p. 603 (1993); Bethune et a1.,
"Cobalt Catalyzed Growth of Carbon Nanotubes with Single Atomic Layer Walls,"
Nature, Vol. 63, p. 605 (1993); Ajayan et al., "Growth Morphologies During
Cobalt Catalyzed Single-Shell Carbon Nanotube Synthesis," Chem. Phys. Lett.,
Vol. 215, p. 509 (1993); Zhou et al., "Single-Walled Carbon Nanotubes Growing
Radially From YCZ Particles," Appl. Phys. Lett., Vol. 65, p. 1593 (1994);
Seraphin
et al., "Single-Walled Tubes and Encapsulation of Nanocrystals Into Carbon
Clusters," Electrochent. Soc., Vol. 142, p. 290 (1995); Saito et al., "Carbon
Nanocapsuies Encaging Metals and Carbides," J. Phys. Chent. Solids, Vol. 54,
p. 1849 (1993); Saito et al., "Extrusion of Single-Wall Carbon Nanotubes Via
Formation of Small Particles Condensed Near an Evaporation Source," Chem.
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
Phys. Lett., Vol. 236, p. 419 (1995). It is also known that mixtures of such
metals
can significantly enhance the yield of single-wall carbon nanotubes in the arc
discharge apparatus. See Lambert et al., "Improving Conditions Toward
Isolating
Single-Shell Carbon Nanotubes," Chenz. Phys. Lett., Vol. 226, p. 364 (1994).
5 Single-wall carbon nanotubes of this invention are much more likely
to be free of defects than multi-wall carbon nanotubes. Defects in single-wall
carbon nanotubes are less likely than defects in multi-walled carbon nanotubes
because the latter can survive occasional defects, while the former have no
neighboring walls to compensate for defects by forming bridges between
unsaturated carbon valances. Since single-wall carbon nanotubes will have
fewer
defects, they are stronger, more conductive, and therefore more useful than
multi-
wall carbon nanotubes of similar diameter.
Carbon nanotubes, and in particular the single-wall carbon nanotubes
of this invention, are useful for making electrical connectors in micro
devices such
as integrated circuits or in semiconductor chips used in computers because of
the
electrical conductivity and small size of the carbon nanotube. The carbon
nanotubes
are useful as antennas at optical frequencies, and as probes for scanning
probe
microscopy such as are used in scanning tunneling microscopes (STM) and atomic
force microscopes (AFM). The carbon nanotubes are also useful as strengthening
agents in any composite material that may be strengthened or combined with
other
forms of carbon such as graphite or carbon black. The carbon nanotubes may be
used in place of or in conjunction with carbon black in tires for motor
vehicles. The
carbon nanotubes are useful in place of or in conjunction with graphite fibers
in any
application using graphite fibers including airplane wings and shafts for golf
clubs
and fishing rods. The carbon nanotubes may also be used in combination with
moldable polymers that can be formed into shapes, sheets or films, as is well
known
in the polymer art, to strengthen the shape, sheet or film and/or to make
electrically
conductive shapes, sheets or films. The carbon nanotubes are also useful as
CA 02231367 2006-07-26
6
supports for catalysts used in industrial and chemical processes such as
hydrogenation, reforming and cracking catalysts.
Ropes of single-wall carbon nanotubes made by this invention are
metallic, i.e., they will conduct electrical charges with a relatively low
resistance.
Ropes are useful in any application where an electrical conductor is needed,
for
example as an additive in electrically conductive points or in polymer
coatings or
as the probing tip of an STM or AFM.
In defining carbon nanotubes, it is helpful to use a recognized system
of nomenclature. In this application, the carbon nanotube nomenclature
described
by M.S. Dresselhaus, G. Dresselhaus, and P. C. Eklund, Science of Fullerne&s
and
Carbon Nanotubes, Chap. 19, especially pp. 756-760, (1996), published by
Academic Press, 525 B Street, Suite 1900, San Diego, California 92101-4495 or
6277 Sea Harbor Drive, Orlando, Florida 32877 (ISBN 0-1 2-22 1 820-5), will be
used. The dual laser pulse feature
described herein produces an abundance of (10,10) single-wall carbon
nanotubes.
The (10, 10) tubes are known as "armchair" tubes. All armchair tubes are
metallic.
Other armchair tubes are denoted as (n, n) where n is an integer from I to
infinity,
preferably I to 1000 more preferably 5 to 500. The (10,10), single-wall carbon
nanotubes have an approximate tube diameter of 13.8 A t 0.3 A or 13.8 A t 0.2
A.
The present invention provides a method for making single-wall
carbon naAptubes in which a laser beam vaporizes material from a target
comprising, consisting essentiaIly of, or consisting of a mixture of carbon
and one
or more Group VIII transition metals. The vapor from the target forms carbon
nanotubes that are predominantly single-wall carbon nanotubes, and of those,
the
(10, 10) tube is predominant. The method also produces significant amounts of
single-wall carbon nanotubes that are arranged as ropes, i.e., the single-wall
carbon
nanotubes run parallel to each other as shown by Figures 2A-2E. Again, the
(10,
10) tube is the predominant tube found in each rope. The laser vaporization
method
provides several advantages over the arc discharge method of making carbon
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
7
nanotubes: laser vaporization allows much greater control over the conditions
favoring growth of single-wall carbon nanotubes, the laser vaporization method
permits continuous operation, and the laser vaporization method produces
single-
wall carbon nanotubes in higher yield and of better quality. As described
herein, the
laser vaporization method may also be used to produce longer carbon nanotubes
and longer ropes.
Carbon nanotubes may have diameters ranging from about I
nanometer (nm) for a single-wall carbon nanotube up to 3 nm, 5 nm, 10 nm, 30
nm,
60 nm or 100 'nm for single-wall or multi-wall carbon nanotubes. The carbon
nanotubes may range in length from 50 nm up to I millimeter (mm), 1 centimeter
(cm), 3 cm, 5 cm, or greater. The yield of single-wall carbon nanotubes in the
product made by this invention is unusually high. Yields of single-wall carbon
nanotubes greater than 10 wt%, greater than 30 wt% and greater than 50 wt% of
the material vaporized are possible with this invention.
As will be described further, the one or more Group VIII transition
metals catalyze the growth in length of a carbon nanotube and/or the ropes.
The
one or more Group VIII transition metals also selectively produce single-wall
carbon nanotubes and ropes of single-wall carbon nanotubes in high yield. The
mechanism by which the growth in the carbon nanotube and/or rope is
accomplished is not completely understood. However, it appears that the
presence
of the one or more Group VIII transition metals on the end of the carbon
nanotube
facilitates the addition of carbon from the carbon vapor to the solid
structure that
forms the carbon nanotube. Applicants believe this mechanism is responsible
for the
high yield and selectivity of single-wall carbon nanotubes and/or ropes in the
product and will describe the invention utilizing this mechanism as merely an
= explanation of the results of the invention. Even if the mechanism is proved
partially or wholly incorrect, the invention which achieves these results is
still fully
= described herein.
CA 02231367 2006-07-26
8
One aspect of the invention comprises a method of making carbon
nanotubes and/or ropes of carbon nanotubes which comprises supplying carbon
vapor to the live end of a carbon nanotube while maintaining the live end of a
carbon nanotube in an anneaiing zone. Carbon can be vaporized in accordance
with
this invention by an apparatus in which a laser beam impinges on a target
comprising carbon that is maintained in a heated zone. A similar apparatus has
been
described in the literature, for example, in U.S. Patent No. 5,300,203 and in
Chai, et al., "Fullerenes with Metals Inside" J. Phys. Chem., vol. 95, no. 20,
p.
7564 (1991).
Carbon nanotubes having at least one live end are formed when the
target also comprises a Group VIII transition metal or mixtures of two or more
Group VIII transition metals. In this application, the term "live end" of a
carbon
nanotube refers to the end of the carbon nanotube on which atoms of the one or
more Group VIII transition metals are located. One or both ends of the
nanotube
may be a live end. A carbon nanotube having a live end is initially produced
in the
laser vaporization apparatus of this invention by using a laser beam to
vaporize
material from a target comprising carbon and one or more Group VIII transition
metals and then introducing the carbon/Group VIII transition metal vapor to an
annealing zone. Optionally, a second laser beam is used to assist in
vaporizing
carbon from the target. A carbon nanotube having a live end will form in the
anezesling zone and then grow in length by the catalytic addition of carbon
from the
vapor to the live end of the carbon nanotube. Additional carbon vapor is then
supplied to the live end of a carbon nanotube to increase the length of the
carbon
nanotube.
The carbon nanotube that is formed is not always a single-wall
carbon nanotube; it may be a multi-wall carbon nanotubes having two, five, ten
or
any greater number of walls (concentric carbon nanotubes). Preferably, though,
the
carbon nanotube is a single-wall carbon nanotube and this invention provides a
way
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
9
of selectively producing (10, 10) single-wall carbon nanotubes in greater and
sometimes far greater abundance than multi-wall carbon nanotubes.
The annealing zone where the live end of the carbon nanotube is
initially formed should be maintained at a temperature of 500 to 1500 C.,
more
preferably 1000 to 1400 C. and most preferably 1100 to 1300 C. In
embodiments of this invention where carbon nanotubes having live ends are
caught
and maintained in an annealing zone and grown in length by further addition of
carbon (without the necessity of adding further Group VIII transition metal
vapor),
the annealing zone may be cooler, 400 to 1500 C., preferably 400 to 1200 C.,
most preferably 500 to 700 C. The pressure in the annealing zone should be
maintained in the range of 50 to 2000 Torr., more preferably 100 to 800 Torr.
and
most preferably 300 to 600 Torr. The atmosphere in the annealing zone will
comprise carbon. Normally, the atmosphere in the annealing zone will also
comprise a gas that sweeps the carbon vapor through the annealing zone to a
collection zone. Any gas that does not prevent the formation of carbon
nanotubes
will work as the sweep gas, but preferably the sweep gas is an inert gas such
as
helium, neon, argon, krypton, xenon, radon, or mixtures of two or more of
these.
Helium and Argon are most preferred. The use of a flowing inert gas provides
the
ability to control temperature, and more importantly, provides the ability to
transport carbon to the live end of the carbon nanotube. In some embodiments
of
the invention, when other materials are being vaporized along with carbon, for
example one or more Gi-oup VIII transition metals, those compounds and vapors
of those compounds will also be present in the atmosphere of the annealing
zone.
If a pure metal is used, the resulting vapor will comprise the metal. If a
metal oxide
is used, the resulting vapor will comprise the metal and ions or molecules of
oxygen.
It is important to avoid the presence of too many materials that kill
or significantly decrease the catalytic activity of the one or more Group VIII
transition metals at the live end of the carbon nanotube. It is known that the
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
presence of too much water (H20) and/or oxygen (0 ) will kill or significantly
decrease the catalytic activity of the one or more Group VIII transition
metals.
Therefore, water and oxygen are preferably excluded from the atmosphere in the
=
annealing zone. Ordinarily, the use of a sweep gas having less than 5 wt%,
more
5 preferably Iess than 1 wt% water and oxygen will be sufficient. Most
preferably the
water and oxygen will be less than 0.1 wt%.
Preferably, the formation of the carbon nanotube having a live end
and the subsequent addition of carbon vapor to the carbon nanotube are all
accomplished in the same apparatus. Preferably, the apparatus comprises a
laser
10 that is aimed at a target comprising carbon and one or more Group VIII
transition
metals, and the target and the annealing zone are maintained at the
appropriate
temperature, for example by maintaining the annealing zone in an oven. A laser
beam may be aimed to impinge on a target comprising carbon and one or more
Group VITI transition metals where the target is mounted inside a quartz tube
that
is in turn maintained within a furnace maintained at the appropriate
temperature.
As noted above, the oven temperature is most preferably within the range of I
100
to 1300 C. The tube need not necessarily be a quartz tube; it may be made from
any material that can withstand the temperatures (1000 to 1500 C.).
Alumina or
tungsten could be used to make the tube in addition to quartz.
Improved results are obtained where a second laser is also aimed at
the target and both lasers are timed to deliver pulses of laser energy at
separate
times. For example, the first laser may deliver a pulse intense enough to
vaporize
material from the surface of the target. Typically, the pulse from the first
laser will
last about 10 nanoseconds (ns). After the first pulse has stopped, a pulse
from a
second laser hits the target or the carbon vapor or plasma created by the
first pulse
to provide more uniform and continued vaporization of material from the
surface
of the target. The second laser pulse may be the same intensity as the first
pulse,
or less intense, but the pulse from the second laser is typically more intense
than the
CA 02231367 1998-03-06
WO 97/09272 PCTIUS96/14188
11
pulse from the first laser, and typically delayed about 20 to 60 ns, more
preferably
40 to 55 ns, after the end of the first pulse.
Examples of a typical specification for the first and second lasers are
given in Examples I and 3 respectively. As a rough guide, the first laser may
vary
in wavelength from 11 to 0.1 micrometers, in energy from 0.05 to I Joule and
in
repetition frequency froni 0.01 to 1000 Hertz (Hz). The duration of the first
laser
pulse may vary from 10-13 to 10-' seconds (s). The second laser may vary in
wavelength from 1 1 to 0.1 micrometers, in energy from 0.05 to 1 Joule and in
repetition frequency from 0.01 to 1000 Hertz (Hz). The duration of the second
laser pulse may vary from 10"13 s to 10-6 s. The beginning of the second laser
pulse
should be separated froni end of the first laser pulse by about 10 to 100 ns.
If the
laser supplying the second pulse is an ultraviolet (UV) laser (an Excimer
laser for
example), the time delay can be longer, up to I to 10 milliseconds. But if the
second pulse is from a visible or infrared (IR) laser, then the adsorption is
preferably
into the electrons in the plasma created by the first pulse. In this case, the
optimum
time delay between pulses is about 20 to 60 ns, more preferably 40 to 55 ns
and
most preferably 40 to 50 ns. These ranges on the first and second lasers are
for
beams focused to a spot on the target composite rod of about 0.3 to 10 mm
diameter. The time delay between the first and second laser pulses is
accomplished
by computer control that is known in the art of utilizing pulsed lasers.
Applicants
have used a CAMAC crate from LeCroy Research Systems, 700 Chestnut Ridge
Road, Chestnut Ridge, New York 10977-6499 along with a timing pulse generator
from Kinetics Systems Corporation, 11 Maryknoll Drive, Lockport, IL 60441 and
a nanopulser from LeCroy Research Systems. Multiple first lasers and multiple
second lasers may be needed for scale up to larger targets or more powerful
lasers
may be used. The main feature of multiple lasers is that the first laser
should evenly
ablate material from the target surface into a vapor or plasma and the second
laser
should deposit enough energy into the ablated material in the vapor or plasma
plume made by the first pulse to insure that the material is vaporized into
atoms or
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
12
small molecules (less than ten carbon atoms per molecule). If the second laser
pulse
arrives too soon after the first pulse, the plasma created by the first pulse
may be so
dense that the second laser pulse is reflected by the plasma. If the second
laser pulse arrives too late after the first pulse, the plasma and/or ablated
material
created by the first laser pulse will strike the surface of the target. But if
the second
laser pulse is timed to arrive just after the plasma and/or ablated material
has been
formed, as described herein, then the plasma and/or ablated material will
absorb
energy from the second laser pulse. Also, it should be noted that the sequence
of
a first laser pulse followed by a second laser pulse will be repeated at the
same
repetition frequency as the first and second laser pulses, i.e., 0.01 to 1000
Hz.
In addition to lasers described in the Examples, other examples of
lasers useful in this invention include an XeF (365 nm wavelength) laser, an
XeCi
(308 nm wavelength) laser, a KrF (248 nm wavelength) laser or an ArF (193 nm
wavelength) laser.
Optionally but preferably a sweep gas is introduced to the tube
upstream of the target and flows past the target carrying vapor from the
target
downstream. The quartz tube should be maintained at conditions so that the
carbon
vapor and the one or more Group VIII transition metals will form carbon
nanotubes
at a point downstream of the carbon target but still within the heated portion
of the
quartz tube. Collection of the carbon nanotubes that form in the annealing
zone
may be facilitated by maintaining a cooled collector in the internal portion
of the far
downstream end of the quartz tube. For example, carbon nanotubes may be
collected on a water cooled metal structure mounted in the center of the
quartz
tube. The carbon nanotubes will collect where the conditions are appropriate,
preferably on the water cooled collector.
Any Group VIII transition metal may be used as the one or more
Group VIII transition metals in this invention. Group VIII transition metals
are iron
(Fe), cobalt (Co), nickel (Ni), ruthenium (Ru), rhodium (Rh), palladium (Pd),
osmium (Os), Iridium (Ir) and platinum (Pt). Preferably, the one or more
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
13
Group VIII transition metals are selected from the group consisting of iron,
cobalt,
ruthenium, nickel and platinum. Most preferably, mixtures of cobalt and nickel
or
mixtures of cobalt and platinum are used. The one or more Group VIII
transition
metals useful in this invention may be used as pure metal, oxides of metals,
carbides
of metals, nitrate salts of metals, or other compounds containing the Group
VIII
transition metal. Preferably, the one or more Group VIII transition metals are
used
as pure metals, oxides of metals, or nitrate salts of metals. The amount of
the one
or more Group VIII transition metals that should be combined with carbon to
facilitate production of carbon nanotubes having a live end, is from 0.1 to 10
atom
per cent, more preferably 0.5 to 5 atom per cent and most preferably 0.5 to
1.5
atom per cent. In this application, atom per cent means the percentage of
specified
atoms in relation to the total number of atoms present. For example, a I atom
%
mixture of nickel and carbon means that of the total number of atoms of nickel
plus
carbon, 1% are nickel (and the other 99% are carbon). When mixtures of two or
more Group VIII transition metals are used, each metal should be 1 to 99 atom
%
of the metal mix, preferably 10 to 90 atom % of the metal mix and most
preferably
to 80 atom % of the metal mix. When two Group VIII transition metals are
used, each metal is most preferably 30 to 70 atom % of the metal mix. When
three
Group VIII transition metals are used, each metal is most preferably 20 to
20 40 atom % of the metal mix.
The one or more Group VIII transition metals should be combined
with carbon to form a target for vaporization by a laser as described herein.
The
remainder of the target should be carbon and may include carbon in the
graphitic
form, carbon in the fullerene form, carbon in the diamond form, or carbon in
compound form such as polymers or hydrocarbons, or mixtures of two or more of
these. Most preferably, the carbon used to make the target is graphite.
Carbon is mixed with the one or more Group VIII transition metals
in the ratios specified and then, in the laser vaporization method, combined
to form
a target that comprises the carbon and the one or more Group VIII transition
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
14
metals. The target may be made by uniformly mixing carbon and the one or more
Group VIII transition metals with carbon cement at room temperature and then
placing the mixture in a mold. The mixture in the mold is then compressed and
heated to about 130 C. for about 4 or 5 hours while the epoxy resin of the
carbon
cement cures. The compression pressure used should be sufficient to compress
the
mixture of graphite, one or more Group VIII transition metals and carbon
cement
into a molded form that does not have voids so that the molded form will
maintain
structural integrity. The molded form is then carbonized by slowly heating it
to a
temperature of 810 C. for about 8 hours under an atmosphere of flowing argon.
The molded and carbonized targets are then heated to about 1200 C. under
flowing
argon for about 12 hours prior to their use as a target to generate a vapor
comprising carbon and the one or more Group VIII transition metals.
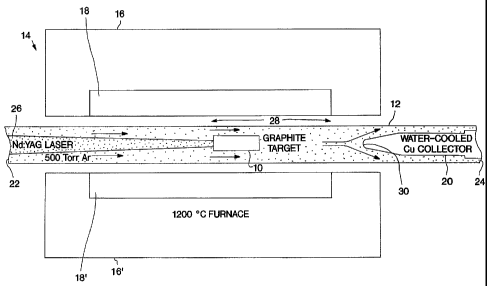
The invention may be further understood by reference to Figure 1
which is a cross-section view of laser vaporization in an oven. A target 10 is
positioned within tube 12. The target 10 will comprise carbon and may comprise
one or more Group VIII transition metals. Tube 12 is positioned in oven 14
which
comprises insulation 16 and heating element zone 18. Corresponding portions of
oven 14 are represented by insulation 16' and heating element zone 18'. Tube
12
is positioned in oven 14 so that target 10 is within heating element zone 18.
Figure 1 also shows water cooled collector 20 mounted inside
tube 12 at the downstream end 24 of tube 12. An inert gas such as argon or
helium
may be introduced to the upstream end 22 of tube 12 so that flow is from the
upstream end 22 of tube 12 to the downstream end 24. A laser beam 26 is
produced by a laser (not shown) focused on target 10. In operation, oven 14 is
heated to the desired temperature, preferably 1100 to 1300 C., usually about
1200 C. Argon is introduced to the upstream end 22 as a sweep gas. The argon
may optionally be preheated to a desired temperature, which should be about
the
same as the temperature of oven 14. Laser beam 26 strikes target 10 vaporizing
material in target 10. Vapor from target 10 is carried toward the downstream
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
end 24 by the flowing argon stream. If the target is comprised solely of
carbon, the
vapor formed will be a carbon vapor. If one or more Group VIII transition
metals
are included as part of the target, the vapor will comprise carbon and one or
more
Group VIII transition metals.
5 The heat from the oven and the flowing argon maintain a certain
zone within the inside of the tube as an annealing zone. The volume within
tube 12
in the section marked 28 in Figure 1 is the annealing zone wherein carbon
vapor
begins to condense and then actually condenses to form carbon nanotubes. The
water cooled collector 20 may be maintained at a temperature of 700 C. or
lower,
10 preferably 500 C. or lower on the surface to collect carbon nanotubes that
were
formed in the annealing zone.
In one embodiment of the invention, carbon nanotubes having a live
end can be caught or mounted on a tungsten wire in the annealing zone portion
of
tube 12. In this embodiment, it is not necessary to continue to produce a
vapor
15 having one or more Group VIII transition metals. In this case, target 10
may be
switched to a target that comprises carbon but not any Group VIII transition
metal,
and carbon will be added to the live end of the carbon nanotube.
In another embodiment of the invention, when the target comprises
one or more Group VIII transition metals, the vapor formed by laser beam 26
will
comprise carbon and the one or more Group VIII transition metals. That vapor
will
form carbon nanotubes in the annealing zone that will then be deposited on
water
cooled collector 20, preferably at tip 30 of water cooled collector 20. The
presence
of one or more Group VIII transition metals in the vapor along with carbon in
the
vapor preferentially forms carbon nanotubes instead of fullerenes, although
some
fullerenes and graphite will usually be formed as well. In the annealing zone,
carbon
from the vapor is selectively added to the live end of the carbon nanotubes
due to
the catalytic effect of the one or more Group VIII transition metals present
at the
live endofthe carbon nanotubes.
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
16
Figure 3 shows an optional embodiment of the invention that can be
used to make longer carbon nanotubes wherein a tungsten wire 32 is stretched
across the diameter of tube 12 downstream of target 10 but still within the
annealing
zone. After laser beam pulses hit the target 10 forming a carbon/
Group VIH transition metal vapor, carbon nanotubes having live ends will form
in
the vapor. Some of those carbon nanotubes will be caught on the tungsten wire
and
the live end will be aimed toward the downstream end 24 of tube 12. Additional
carbon vapor will make the carbon nanotube grow. Carbon nanotubes as long as
the annealing zone of the apparatus can be made in this embodiment. In this
embodiment, it is possible to switch to an all carbon target after initial
formation of
the carbon nanotubes having a live end, because the vapor need only contain
carbon
at that point.
Figure 3 also shows part of second laser beam 34 as it impacts on
target 10. In practice, laser beam 26 and second laser beam 34 would be aimed
at
the same surface of target 10, but they would impact that surface at different
times
as described herein.
It is also possible to stop the laser or lasers altogether. Once the
single-wall carbon nanotube having a live end is formed, the live end will
catalyze
growth of the single-wall carbon nanotube at lower temperatures and with other
carbon sources. The carbon source can be switched to fullerenes, that can be
transported to the live end by the flowing sweep gas. The carbon source can be
graphite particles carried to the live end by the sweep gas. The carbon source
can
be a hydrocarbon that is carried to the live end by a sweep gas or a
hydrocarbon gas
or mixture of hydrocarbon gasses introduced to tube 12 to flow past the live
end.
Hydrocarbons useful include methane, ethane, propane, butane, ethylene,
propylene,
benzene, toluene or any other paraffinic, olefinic, cyclic or aromatic
hydrocarbon,
or any other hydrocarbon.
The annealing zone temperature in this embodiment can be lower
than the annealing zone temperatures necessary to initially form the single-
wall
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
17
carbon nanotube having a live end. Annealing zone temperatures can be in the
range of 400 to 1500 C., preferably 400 to 1200 C., most preferably 500 to
700 C. The lower temperatures are workable because the Group VIII transition
metal(s) catalyze the addition of carbon to the nanotube at these lower
temperatures.
The invention may also be understood by reference to the following
examples.
EXAMPLE 1
The oven laser-vaporization apparatus described in Figure 1 and also
described by Haufler et al., "Carbon Arc Generation of Cbo," Mat. Res. Soc.
Symp.
Proc., Vol. 206, p. 627 (1991) and by U.S. Patent No. 5,300,203 was utilized.
An
Nd:YAG laser was used to produce a scanning laser beam controlled by a motor-
driven total reflector that was focused to a 6 to 7 mm diameter spot onto a
metal-
graphite composite target mounted in a quartz tube. The laser beam scans
across
the target's surface under computer control to maintain a smooth, uniform face
on
the target. The laser was set to deliver a 0.532 micron wavelength pulsed beam
at
300 milliJoules per pulse. The pulse rate was 10 hertz and the pulse duration
was
10 nanoseconds (ns).
The target was supported by graphite poles in a 1-inch quartz tube
initially evacuated to 10 m Torr. and then filled with 500 Torr. argon flowing
at
50 standard cubic centimeters per second (sccm). Given the diameter of the
quartz
tube, this volumetric flow results in a linear flow velocity through the
quartz tube
in the range of 0.5 to 10 cm/sec. The quartz tube was mounted in a high-
temperature furnace with a maximum temperature setting of 1200 C. The high-
temperature furnace used was a Lindberg furnace 12 inches long and was
maintained at approximately 1000 to 1200 C. for the several experiments in
Example 1. The laser vaporized material from the target and that vaporized
material was swept by the flowing argon gas from the area of the target where
it
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
18
was vaporized and subsequently deposited onto a water-cooled collector, made
from copper, that was positioned downstream just outside the furnace.
Targets were uniformly mixed composite rods made by the following
three-step procedure: (i) the paste produced from mixing high-purity metals or
metal oxides at the ratios given below with graphite powder supplied by
Carbone
of America and carbon cement supplied by Dylon at room temperature was placed
in a 0.5 inch diameter cylindrical mold, (ii) the mold containing the paste
was placed
in a hydraulic press equipped with heating plates, supplied by Carvey, and
baked at
130 C. for 4 to 5 hours under constant pressure, and (iii) the baked rod
(formed
from the cylindrical mold) was then cured at 810 C. for 8 hours under an
atmosphere of flowing argon. For each test, fresh targets were heated at 1200
C.
under flowing argon for varying lengths of time, typically 12 hours, and
subsequent
runs with the same targets proceeded after 2 additional hours heating at 1200
C.
The following metal concentrations were used in this example: cobalt
(1.0 atom per cent), copper (0.6 atom per cent), niobium (0.6 atom per cent),
nickel
(0.6 atom per cent), platinum (0.2 atom per cent), a mixture of cobalt and
nickel
(0.6 atom per cent/0.6 atom per cent respectively), a mixture of cobalt and
platinum
(0.6 atom per cent/0.2 atom per cent respectively), a mixture of cobalt and
copper
(0.6 atom per cent/0.5 atom per cent respectively), and a mixture of nickel
and
platinum (0.6 atom per cent/0.2 atom per cent respectively). The remainder of
the
mixture was primarily graphite along with small amounts of carbon cement. Each
target was vaporized with a laser beam and the soots collected from the water
cooled collector were then collected separately and processed by sonicating
the soot
for 1 hour in a solution of methanol at room temperature and pressure (other
useful
solvents include acetone, 1,2-dicholoroethane, 1-bromo, 1,2-dichIoroethane,
and
N,N-dimethylformamide). With one exception, the products collected produced a
homogeneous suspension after 30 to 60 minutes of sonication in methanol. One
sample vaporized from a mixture of cobalt, nickel and graphite was a rubbery
deposit having a small portion that did not fully disperse even after 2 hours
of
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
19
sonication in methanol. The soots were then examined using a transmission
electron microscope with a beam energy of 100 keV (Model JEOL 2010).
Rods (0.5 inch diameter) having the Group VIII transition metal or
mixture of two Group VIII transition metals described above were evaluated in
the
experimental apparatus to determine the yield and quality of single-wall
carbon
nanotubes produced. No multi-wall carbon nanotubes were observed in the
reaction products. Yields always increased with increasing oven temperature up
to
the linut of the oven used (1200 C.). At 1200 C. oven temperature, of the
single
metals utilized in the example, nickel produced the greatest yield of single-
wall
carbon nanotubes followed by cobalt. Platinum yielded a small number of single-
wall carbon nanotubes and no single-wall carbon nanotubes were observed when
carbon was combined only with copper or only with niobium. With respect to the
mixtures of two Group VIII transition metal catalysts with graphite, the
cobalt/nickel mixture and the cobalt/platinum mixtures were both approximately
equivalent and both were the best overall catalysts in terms of producing
yields of
single-wall carbon nanotubes. The yield of single-wall carbon nanotubes for
both
of these two metal mixtures were 10 to 100 times the yield observed when only
one
Group VIII transition metal was used. The mixture of nickel and platinum with
graphite also had a higher yield of single-wall carbon nanotubes than a single
metal
alone. The cobalt/copper mixture with graphite produced a small quantity of
single-
wall carbon nanotubes.
The cobalt/nickel mixture with graphite and the cobalt/platinum
mixture with graphite both produced deposits on the water cooled collector
that
resembled a sheet of rubbery material. The deposits were removed intact. The
cobalt/platinum mixture produced single-wall carbon nanotubes in a yield
estimated
at 15 weight per cent of all of the carbon vaporized from the target. The
cobalt/nickel mixture produced single-wall carbon nanotubes at yields of over
50 wt% of the amount of carbon vaporized.
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
The images shown in Figures 2A through 2E are transmission
electron micrographs of single-wall carbon nanotubes produced by vaporizing a
target comprising graphite and a mixture of cobalt and nickel (0.6 atom per
cent/0.6 atom per cent respectively) at an oven temperature of 1200 C.
Figure 2A
5 shows a medium-magnification view (where the scale bar represents 100 mm)
showing that almost everywhere, bundles of single-wall carbon nanotubes are
tangled together with other single-wall carbon nanotubes. Figure 2B is a high-
magnification image of one bundle of multiple single-wall carbon nanotubes
that are
all roughly parallel to each other. The single-wall carbon nanotubes all have
a
10 diameter of about I nm, with similar spacing between adjacent single-wall
carbon
nanotubes. The single-wall carbon nanotubes adhere to one another by van der
Waals forces.
Figure 2C shows several overlapping bundles of single-wall carbon
nanotubes, again showing the generally parallel nature of each single-wall
nanotube
15 with other single-wall carbon nanotubes in the same bundle, and showing the
overlapping and bending nature of the various bundles of single-wall carbon
nanotubes. Figure 2D shows several different bundles of single-wall carbon
nanotubes, all of which are bent at various angles or arcs. One of the bends
in the
bundles is relatively sharp, illustrating the strength and flexibility of the
bundle of
20 single-wall carbon nanotubes. Figure 2E shows a cross-sectional view of a
bundle
of 7 single-wall carbon nanotubes, each running roughly parallel to the
others. All
of the transmission electron micrographs in Figures 2A through 2E clearly
illustrate
the lack of amorphous carbon overcoating that is typically seen in carbon
nanotubes
and single-wall carbon nanotubes grown in arc-discharge methods. The images in
Figures 2A through 2E also reveal that the vast majority of the deposit
comprises
single-wall carbon nanotubes. The yield of single-wall carbon nanotubes is
estimated to be about 50% of the carbon vaporized. The remaining 50% consists
primarily of fullerenes, multi-layer fullerenes (fullerene onions) and/or
amorphous
carbon.
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
21
Figures 2A through 2E show transmission electron microscope
images of the products of the cobalt/nickel catalyzed carbon nanotube material
that
was deposited on the water cooled collector in the laser vaporization
apparatus
depicted in Figure 1. Single-wall carbon nanotubes were typically found
grouped
in bundles in which many tubes ran together roughly parallel in van der Waals
contact over most of their length. The grouping resembled an "highway"
structure
in which the bundles of single-wall carbon nanotubes randomly criss-crossed
each
other. The images shown in Figures 2A through 2E make it likely that a very
high
density of single-wall carbon nanotubes existed in the gas phase in order to
produce
so many tubes aligned as shown when landing on the cold water cooled
collector.
There also appeared to be very little other carbon available to coat the
single-wall
carbon nanotubes prior to their landing on the water cooled collector in the
alignment shown. Evidence that single-wall carbon nanotubes grow in the gas
phase, as opposed to for example on the walls of the quartz tube, was provided
in
earlier work on multi-walled carbon nanotubes using the same method. See Guo
et al., "Self-Assembly of Tubular Fullerenes," J. Phys. Chenz., Vol. 99, p.
10694
(1995) and Saito et al., "Extrusion of Single-Wall Carbon Nanotubes via
Formation
of Small Particles Condensed Near An Evaporation Source," Chem. Phys. Lett.,
Vol. 236, p. 419 (1995). The high yield of single-wall carbon nanotubes in
these
experiments is especially remarkable because the soluble fulierene yield was
found
to be about 10 weight per cent, and much of the remaining carbon in the soot
product consisted of giant fullerenes and multi-layer fullerenes.
EXAMPLE 2
In this example, a laser vaporization apparatus similar to that
described by Figure 1 was used to produce longer single-wall carbon nanotubes.
The laser vaporization apparatus was modified to include a tungsten wire
strung
across the diameter of a quartz tube mounted in an oven. The tungsten wire was
placed downstream of the target so that the wire was 1 to 3 cm downstream from
the downstream side of the target (13 to 15 cm downstream from the surface of
the
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
22
target being vaporized). Argon at 500 Torr. was passed through the quartz tube
at
a flow rate equivalent to a linear velocity in the quartz tube of about 1
cm/sec. The
oven was maintained at 1200 C. and Group VIII transition metals were combined
at I to 3 atom% with carbon to make the target.
The pulsed laser was operated as in Example I for 10 to 20 minutes.
Eventually, a tear drop shaped deposit formed on the tungsten wire, with
portions
growing to lengths of 3 to 5 mm. The deposit resembled eyelashes growing on
the
tungsten wire. Examination of the deposit revealed bundles of millions of
single-
wall carbon nanotubes.
EXAMPLE 3
Graphite rods were prepared as described in Example I using
graphite, graphite cement and 1.2 atom % of a mixture of 50 atom % cobalt
powder
and 50 atom % nickel powder. The graphite rods were pressed into shape and
then
formed into targets as described in Example I. The graphite rods were then
installed as targets in an apparatus as diagramed in Figure 3, except tungsten
wire 32 was not used. A quartz tube holding the graphite rod targets was
placed
in an oven heated to 1200 C. Argon gas which had been catalytically purified
to
remove water vapor and oxygen was passed through the quartz tube at a pressure
of about 500 Torr and a flow rate of about 50 sccm although flow rates in the
range
of about I to 500 scem (standard cubic centimeters per minute), preferably 10
to
100 sccm are also useful for a I inch diameter flow tube. The first laser was
set to
deliver a 0.532 micron wavelength pulsed beam at 250 mJ per pulse. The pulse
rate
was 10 Hz and the pulse duration was 5 to 10 ns. A second laser pulse struck
the
target 50 ns after the end of the first pulse. The second laser was set to
deliver a
1.064 micron wavelength pulsed beam at 300 mJ per pulse. The pulse rate was
10 Hz and the pulse duration was 5 to 10 ns. The first laser was focused to a
5 mm
diameter spot on the target and the second laser was focused to a 7 mm
diameter
gaussian spot having the same center point on the target as the spot from the
first
laser. About 1/10th of a second after the second laser hit the target, the
first and
CA 02231367 1998-03-06
WO 97/09272 PCT/US96/14188
23
second lasers fired again and this process was repeated until the vaporization
step
was stopped.
About 30 mg/hr of the raw product from the laser vaporization of
the target surface was collected downstream. The raw product comprised a mat
of
randotnly oriented single-wall carbon nanotubes. The raw product mat is made
up
almost entirely of carbon fibers 10-20 nm in diameter and 10 to 1000 microns
long.
About 2 mg of the raw product mat was sonicated in 5 ml methanol
for about 0.5 hour at room temperature. Transmission Electron Microscope (TEM)
analysis of the sonicated product proved that the product was comprised mostly
of
ropes of single-wall carbon nanotubes, i.e., bundles of 10 to 1000 single-wall
carbon
nanotubes aligned with each other (except for occasional branching) having a
reasonably constant rope diameter over the entire length of the rope. The
ropes
were more than 100 microns long and consisting of uniform diameter single-wall
carbon nanotubes. About 70 to 90 wt% of the product is in the form of ropes.
The
individual single-wall carbon nanotubes in the ropes all terminate within 100
nm of
each other at the end of the rope. More than 99% of the single-wall carbon
nanotubes appear to be continuous and free from carbon lattice defects over
all of
the length of each rope.
This invention includes the ropes of single-wall carbon nanotubes
described herein, particularly in the Examples. Measurements show that the
single-
wall carbon nanotubes in the ropes have a diameter of 13.8A ::h 0.2A. A (10,
10)
single-wall carbon nanotube has a calculated diameter of about 13.6A , and the
measurements on the single-wall carbon nanotubes in the ropes proves they are
predominantly the (10, 10) tube. The number of single-wall carbon nanotubes in
each rope may vary from about 5 to 5000, preferably about 10 to 1000, or 50 to
1000, and most preferably about 100 to 500. The diameters of the ropes range
from about 20 to 200A, more preferably about 50 to 200A. The (10, 10) single-
wall carbon nanotube predominates the tubes in the ropes made by this
invention.
Ropes having greater than 10%, greater than 30%, greater than 50%, greater
than
CA 02231367 2006-07-26
24
75%, and even greater than 90% (10, 10) single-wall carbon nanotubes have been
produced. Ropes having greater than 50% greater than 75% and greater than 90%
armchair (n, n) single-wall carbon nanotubes are also made by and are a part
of this
invention. The single-wall carbon nanotubes in each rope are arranged to form
a
rope having a 2-D triangular lattice having a lattice constant of about 17A.
Ropes
of 0.1 up to 10, 100 or 1,000 microns in length are made by the invention. The
resistivity of a rope made in accordance with this invention was measured to
be 0.34
to 1.0 micro ohm-meter at 27 C proving that the ropes are metallic.
The invention also produces a "felt" of the ropes described above.
The product material is collected as a tangled collection of ropes stuck
together in
a mat referred to herein as a "felt." The felt material collected from the
inventive
process has enough strength to withstand handling, and it has been measured to
be
electrically conductive. Felts of 10 mmZ, 100 mm'-, 1000 mm2 or greater, are
formed in the inventive process.
One advantage of the single-wall carbon nanotubes produced with
the laser vaporization in an oven method is their cleanliness. Typical
discharge arc-
produced single-wall carbon nanotubes are covered with a thick layer of
amorphous
carbon, perhaps limiting their usefulness compared to the clean bundles of
single-
wall carbon nanotubes produced by the laser vaporization method. Other
advantages and features of the invention are apparent from the disclosure. The
invention may also be understood by reference to Guo et al., "Catalytic Growth
Of
Single-Walled Nanotubes By Laser Vaporization," Chem. Phys. Lett., Vol. 243,
pp. 49-54 (1995) and the provisional patent applications referenced at the
beginning
of this disclosure.
The advantages achieved by the dual pulsed lasers insure that the
carbon and metal go through the optimum annealing conditions. The dual laser
pulse process achieves this by using time to separate the ablation from the
further
and full vaporization of the ablated material. These same optimum conditions
can
be achieved by using solar energy to vaporize carbon and metals. Combining any
of the Group VIII transition metals will produce the single-wall carbon
nanotubes
and ropes of this invention.