Note: Descriptions are shown in the official language in which they were submitted.
CA 0223l66l l998-04-07
0050~46271
Oxyaminooxime ethers, preparation thereof and intermediates
therefor and compositions comprising them for controlling
harmful fungi and anima:L pests
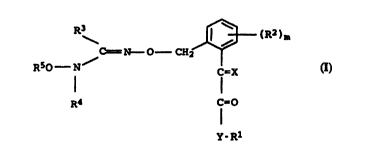
The present invention relates to oxyaminooxime ethers of the
formula I
C = N----O--CH2 l ~ (R2~m (I)
RsO - N / C=X
R4 C=O
Y-Rl
where the variables have the following meanings:
X is NOCH3, CHOCH3 or CHCH3;
Y is O or NZ, Z being hydrogen or C1-C4-alkyl;
Rl is hydrogen or C1-C4-alkyl;
R2 is cyano, nitro, ha:Logen, Cl-C4-alkyl, trifluoromethyl or
C1-C4-alkoxy;
m is 0, 1 or 2, it be:ing possible for the radicals R2 to be
different if m is 2;
R3 is hydrogen, alkyl, haloalkyl or cycloalkyl;
R4 is hydrogen or unsubstituted or substituted alkyl, alkenyl,
alkynyl, cycloalkyl,. cycloalkenyl, heterocyclyl, aryl or
heteroaryl;
R5 is hydrogen, alkylsulfonyl or unsubstituted or substituted
alkyl, alkenyl, alkynyl or cycloalkyl,
35 and their salts.
The invention additionally relates to processes and intermediates
for preparing these compounds and to compositions comprising them
for controlling animal pests and harmful fungi.
Pheny]Lacetic acid derivatives having fungicidal and insecticidal
action are disclosed in the following publications:
WO-A 93/16986, WO-A 94/14761 and WO-A 94/14322. With respect to
their action, however, the active compounds disclosed therein are
45 not a]ways satisfactory.
CA 02231661 1998-04-07
0050./4627 1
It is an object of the present invention to provide novel
compounds of this type having improved action against harmful
fungi and pests.
5 We have found that this object is achieved by the oxyaminooxime
ethers I defined at the outset. We have also found processes and
intermediates for their preparation, as well as compositions
compr-ising them for controlling animal pests and harmful fungi
and t:heir use for this purpose.
The compounds I are obtainable in various ways by processes known
per se from the literature.
In principle, it is insignificant in the synthesis of the
15 compounds I whether the group -C(X)-CO-YRl or the group
-CH2oN=C(R3)-N(R4)-oR5 is synthesized first.
The synthesis of the group -C(X)-CO-YRl is disclosed, for example,
in the following publications: EP-A 422 597, EP-A 463 488,
20 EP-A 370 629, EP-A 460 575, EP-A 472 300, W0-A 90/07493,
W0-A 92/13830, W0-A 92/18487, DE-A 44 20 416.
l.l [n the synthesis of the group -CH2oN=C(R3)-N(R4)-oR5, a
procedure is in general used in which a benzyl derivative of
the formula II is reacted with a hydroxyimine of the
:Eormula III.
~ ~(R2)m
C = NOH + Ll _ CH2
R50 - N C=X
R4 C=O
III Y-R
~ ,~(R2)m
C = ~ O--CH2 T
R50 --N ~ C=X
R4 C=O
Y-R
CA 02231661 1998-04-07
0050/4627 1
L1 in the formula II is a nucleophilically replaceable
leaving group, eg. halogen or sulfonate groups [sic],
preferably chlorine, bromine, iodine, mesylate, tosylate or
triflate.
The reaction is carried out in a manner known per se in an
inert organic solvent in the presence of a base, eg. sodium
hydride, potassium hydroxide, potassium carbonate or
triethylamine according to the methods described in
Houben-Weyl, 4th Edition, Vol. E 14b, p. 370 et seq. and
Vol. 10/1, p. 1189 et seq.
1.2 The hydroxyimine III needed is obtained, for example, by
reaction of a thioamide VII with hydroxylamine or its salt
(preferably: hydroxylammonium halide).
R3 R3
C = S H2NOH C = NOH
20R50 - N R50 - N
R4 VII R4 III
The reaction is carried out in a -nner known per se (cf. J.
Heterocycl. Chem. 17, 819 (1980); Houben-Weyl, 4th Edition,
Volume VIII, page 694 et seq.) in an inert organic solvent.
A few thioamides VII are known (cf. Acta Cienc. Indica, Chem.
11, 124 (1985); J. Org. Chem. 51, 5198 (1986); J. Org. Chem.
52, 2927 (1987); Tetrahedron Lett. 24, 1851 (1983)), The
thioamides VIIa are novel. They can be prepared in analoqy to
the known methods.
R3~
C = S
R5~o - N / VIIa
I
R4
1.3 A further possibility for preparing the hydroxyimines III
consists in reacting a hydroxylamine derivative of the
formula VIII with a hydroximic acid derivative of the formula
IX in a manner known per se in an inert organic solvent, if
appropriate using a base (see Chem.-Ztg. 100, 236 (1976);
Chem. Ber. 108, 2764 (1975); J. Org. Chem. 41, 2985 (1976)).
CA 02231661 1998-04-07
0050/4627 1
$1.50-- N --H
+ L2--C= NOH
R4
VIII 3 IX
R
C = NOH
R50--N
I
R4
III
L2 is a displaceable group such as amino, halogen,
alkylsulfonyl or Cl C4-alkoxy, preferably amino, chlorine,
bromine, methylsulfonyl, methoxy or ethoxy.
The compounds VIII and IX are known or can be prepared by
miethods known per se (cf. J. Org. Chem. 47, 1171 (1982);
Tetrahedron 48, 6335 (1992); J. Heterocycl. Chem. 12, 1063
( 1975); Am. Chem. J. 38, 257 ~1907); Tetrahedron 40, 2985
(1984); Helv. Chim. Acta 69, 1137 (1986)).
2. Alternatively, the compounds I can also be obtained by
reacting the benzyl derivative II first with the
hydroxyaminohydroxyimino derivative IV to give a
corresponding benzyloxime of the formula V, V then being
reacted with a compound VI to give I.
CA 02231661 1998-04-07
OO50~' 4627
R3 ~
C= NOH Ll--CH ~ ~(R2)m
HO--N
I C=X
R4 C =O
IV ¦ II
Y-R
~¢~ ( R2 ) m
C= N--O--CH2
HO - N C=X
R4 C=O V
Y-R
,, ~ ( R2 )m
C = N- O - CH2 r
R5-L3 R50 - N ~ C=X
VI l l
R4 C=O
I
Y-R
IL3 = nucleophilically replaceable leaving group such as
halogen, eg. chlorine, bromine, sulfonate eg. mesylate,
t:rifate [sic], tesylate [sic])
l'he reaction is carried out in a manner known per se in an
inert organic solvent in the presence of a base, eg.
potassium carbonate, potassium hydroxide, sodium hydride,
pyridine or triethylamine according to the methods described
in Houben-Weyl, 4th Edition, Vol. 10/1, p. 1189 et seq.;
ibid., Vol. E 14b, p. 307 et seq., p. 370 et seq. and p. 385
et seq.; ibid., Vol. 10/4, p. 55 et seq., p. 180 et seq. and
E). 217 et seq.; ibid., Vol. E 5, p. 780 et seq. and Farmaco.
E~d. Sci. 39, 1060 (1984). The synthesis of the compounds IV
has already been described above.
CA 02231661 1998-04-07
0050/ 4627 1
3. A further possibili1y for preparing the compounds I is the
reaction of the ben~yl derivative II with N-hydroxyphthal-
imide, subsequent hydrazinolysis to the benzylhydroxylamine
IIa and further reaction of IIa with a thioamide VII.
[ ~ ~ N-OH + Ll - CH
C=O II
I
Y-R
0
~ N - o - CH2 ~ (R2)m
ll C=X
O ¦ H2NNH2
C=O
I
y=
~ ~ (R2)m R3
H2N--O--CH2 -f c= s
C=X +R50 - N
C=O IIa
I R4
Y-Rl VII
The reaction is carried out in a manner known per se in an
inert organic solvent according to the methods described in
EP-A 463 488 or DE-~ 42 28 867 or according to the conversion
VII III (see above).
4. Correspondingly, the compounds I can also be obtained by
reacting the benzylhydroxylamine IIa first with the hydroxy-
thioamide VIIa to give the corresponding benzyloxyimino
derivative of the formula V, V then being reacted with the
compound VI as described above to give I.
CA 02231661 1998-04-07
0050/4627 1
~ ( R2 ) m
C= S + H2N--O--CH2
HO - N C=X
C=0 IIa
R4
VIIa Y-Rl
~R3 ~ (R2)m
C = N- 0 - CH2
HO - N C=X
l l
R4 C=0 V
I
Y-R
R5-L3
VI ~ ~ (R2)m
C = N ~ 0 - CH2 r
RsO - N C=X
R4 C=0
Y-Rl
5. Alternatively, the compounds I can also be prepared by
reacting a hydroxylamine derivative of the formula VIII with
a hydroximic acid derivative of the formula X in a manner
known per se in an inert organic solvent (cf. Collect. Czech.
Chem. Commun. 48, 596 (1983)).
CA 02231661 1998-04-07
0050/ 4627 1
R3 ~(R2 )m
R50 - N - H + C = N- O - CH2
L4 C=X
R4
C=O X
VIII
Y-R
J~(R2)m
C= ~ O--CH2 '~
RsO - N / C=X
R4 C=0
I
Y-R
L4 iS a displaceable group such as amino, halogen, sulfonyl
or C1-C4-alkoxy, preferably amino, chlorine, bromine,
methylsulfonyl, methoxy or ethoxy.
The compounds X are known (cf. WO-A 94/14761, EP-A 463 488,
WO-A 92/18487) or can be prepared by methods known per se.
6. In addition, the compounds I are obtained by converting a
compound III according to the methods described in
EP-A 493 711 with a lactone XI first into the corresponding
benzoic acid XII and converting XII via the corresponding
halides XIII into the cyanocarboxylic acids XIV, which are
converted by way of the Pinner reaction (Angew. Chem. 94, 1
(1982)) into the a-ketoesters XV and, if appropriate, reacted
further to give the a-ketoamides XVI (cf. EP-A 348 766,
DE-A 37 05 389, EP-A 178 826, DE-A 36 23 921, Houben-Weyl,
4th Edition, Vol. E5, p. 941 et seq.).
CA 02231661 1998-04-07
0050~46271
R3
C = NOH o ~ (R2)m
R50--N ll
S l O
R4 XI
III
~ J~--(R2)m
C = N - O - CH2 ~
R50 - N ~ C=O XII
lSR4 OH
C== N--O--CH2/~
20R50 - N ~ C=O XIII
R4 Hal
R3 ~ (R2)m
C=~O--CH2 ~r
30R50 - N ~ C=O XIV
R4 CN
C = N - O - CH2 ~ (R2)m Rl~H
R50 - N C=O XV
R4 C02R
CA 02231661 1998-04-07
0050/46271
~ f~(R2)m
C = N--O--CH2~' ~
R50 - N ~ C=O XVI
R4 CON(Z)R
(Z = hydrogen or C1-C4-alkyl)
10 The a-ketoesters XV and the a-ketoamides XIV can be converted
into the compounds I according to customary processes (cf.
EP-A 178 826, EP-A 348 766, DE-A 36 23 921, DE-A 37 05 389 and
the literature cited at the outset).
15 Compounds I in which Rl is hydrogen are obtained according to this
process by hydrolysis of the esters XV and subsequent reaction to
give I.
The compounds I in which Y is NZ can also be obtained from the
20 esters (Y=O) by reaction with the appropriate amines HN(Z)Rl.
The compounds II are disclosed in EP-A 513 580, EP-A 477 631,
EP-A 463 488, EP-A 251 082, EP-A 400 417 and/or EP-A 585 751 or
can be prepared by the methods described there.
The compounds I can be present in the form of their salts, the
nature of the salt generally not mattering. The preparation of
the salts of compounds of the formula I is carried out in a
manner known per se.
The compounds I can be obtAine~ during preparation, on account of
their C=C and C=N double bonds, as E/Z isomer mixtures, which can
be Reparated, for example, into the individual compounds in a
customary manner by crystallization or chromatography.
If isomer mixtures are formed during synthesis, in general,
however, a separation of the isomers is not absolutely necessary,
as the individual isomers can be partially converted into one
another during preparation for application or during application
40 (eg. under the action of light, acid or base). Corresponding
conversions can also take place after application, for example
during the treatment of plants, in the treated plant or in the
harmful fungus or animal pest to be controlled.
CA 02231661 1998-04-07
0050~4627 1
With regard to the C=X double bond, with respect to their
activity, the E isomers of the compounds I are preferred
(configuration based on the -OCH3 or the -CH3 group in relation to
the -COYRl group).
With regard to the -C(R3)=NoCH2- double bond, with respect to
their activity, the cis-isomers of the compounds I are preferred
(configuration based on the radical R3 in relation to the -OCH2-
group).
In the definitions of the compounds I given at the outset,
collective terms were used which are generally representative of
the following groups:
15 halogen: fluorine, chlorine, bromine and iodine;
alkyl: straight-chain or branched alkyl groups having 1 to 4, 1
to 6 or 1 to 10 carbon atoms, eg. Cl-C6-alkyl such as methyl,
ethyl, propyl, 1-methylethyl, butyl, 1-methylpropyl,
20 2-methylpropyl, 1,1-dimethylethyl, pentyl, 1-methylbutyl,
2-methylbutyl, 3-methylbutyl, 2,2-dimethylpropyl, 1-ethylpropyl,
hexyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl, 1-methylpentyl,
2-methylpentyl, 3-methylpentyl, 4-methylpentyl,
1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl,
25 2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl,
1-ethylbutyl, 2-ethylbutyl, 1,1,2-trimethylpropyl,
1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl and
1-ethyl-2-methylpropyl;
30 alkylamino: an amino group which carries a straight-chain or
branched alkyl group having 1 to 6 carbon atoms as mentioned
above;
dialkylamino: an amino group which carries two straight-chain or
35 branched alkyl groups which are independent of one another, each
having 1 to 6 carbon atoms as mentioned above;
alkylcarbonyl: straight-chain or branched alkyl groups having 1
to 10 carbon atoms, which are bonded to the structure via a
40 carbonyl group (-C0-);
alkylsulfonyl: straight-chain or branched alkyl groups having 1
to 6 or 1 to 10 carbon atoms, which are bonded to the structure
via a sulfonyl group (-S02- );
CA 02231661 1998-04-07
OOSO/4627 1
alkylsulfoxyl: straight-chain or branched alkyl groups having 1
to 6 carbon atoms, which are bonded to the structure via a
sulfoxyl group (-S(=0)-);
5 alkylaminoc~rbonyl: alkylamino groups having 1 to 6 carbon atoms
as mentioned above, which are bonded to the structure via a
carbonyl group (-C0-);
dialkylaminoc~rbonyl: dialkylamino groups each having 1 to 6
10 carbon atoms per alkyl radical as mentioned above, which are
bonded to the structure via a carbonyl group (-C0-);
alkylaminothio~rbonyl: alkylamino groups having 1 to 6 carbon
atoms as mentioned above, which are bonded to the structure via a
15 thiocarbonyl group (-CS-);
dialkylaminothior~rbonyl: dialkylamino groups each having 1 to 6
carbon atoms per alkyl radical as mentioned above, which are
bonded to the structure via a thiocarbonyl group (-CS-);
haloalkyl: straight-chain or branched alkyl groups having 1 to 6
carbon atoms, it being possible in these groups for the hydrogen
atoms to be partially or completely replaced by halogen atoms as
mentioned above, eg. Cl-C2-haloalkyl such as chloromethyl,
25 dichloromethyl, trichloromethyl, fluoromethyl, difluoromethyl,
trifluoromethyl, chlorofluoromethyl, dichlorofluoromethyl,
chlorodifluoromethyl, 1-fluoroethyl, 2-fluoroethyl,
2,2-difluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2-fluoroethyl,
2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-fluoroethyl,
30 2,2,2-trichloroethyl and pentafluoroethyl;
alkoxy: straight-chain or branched alkyl groups having 1 to 4 or
1 to 6 carbon atoms as mentioned above, which are bonded to the
structure via an oxygen atom (-0-), eg. Cl-C6-alkoxy such as
35 methoxy, ethoxy, propoxy, l-methylethoxy, butoxy, l-methyl-
propoxy, 2-methylpropoxy, l,l-dimethylethoxy, pentoxy, l-methyl-
butoxy, 2-methylbutoxy, 3-methylbutoxy, 2,2-dimethylpropoxy,
1-ethylpropoxy, hexoxy, 1,1-dimethylpropoxy, 1,2-dimethylpropoxy,
1-methylpentoxy, 2-methylpentoxy, 3-methylpentoxy, 4-methyl-
40 pentoxy, 1,1-dimethylbutoxy, 1,2-dimethylbutoxy, 1,3-dimethyl-
butoxy, 2,2-dimethylbutoxy, 2,3-dimethylbutoxy, 3,3-dimethyl-
butoxy, l-ethylbutoxy, 2-ethylbutoxy, 1,1,2-trimethylpropoxy,
1,2,2-trimethylpropoxy, 1-ethyl-1-methylpropoxy and
1-ethyl-2-methylpropoxy;
CA 02231661 1998-04-07
0050/4627 1
alkoxycarbonyl: straight-chain or branched alkyl groups having 1
to 6 carbon atoms, which are bonded to the structure via an
oxycarbonyl group (-OC(=0)-);
5 haloalkoxy: straight-chain or branched alkyl groups having 1 to
6 carbon atoms, it being possible for the hydrogen atoms in these
groups to be partially or completely replaced by halogen atoms
such as mentioned above, and these groups being bonded to the
structure via an oxygen atom;
alkylthio: straight-chain or branched alkyl groups having 1 to 4
or 1 to 6 carbon atoms as mentioned above, which are bonded to
the structure via a sulfur atom (-S-), eg. C1-C6-alkylthio such as
methy.lthio, ethylthio, propylthio, 1-methylethylthio, butylthio,
15 l-methylpropylthio, 2-methylpropylthio, 1,1-dimethylethylthio,
pentylthio, 1-methylbutylthio, 2-methylbutylthio, 3-methylbutyl-
thio, 2,2-dimethylpropylthio, l-ethylpropylthio, hexylthio,
l,l-dimethylpropylthio, 1,2-dimethylpropylthio, 1-methylpentyl-
thio, 2-methylpentylthio, 3-methylpentylthio, 4-methylpentylthio~
20 l,1-d.imethylbutylthio, 1,2-dimethylbutylthio, 1,3-dimethylbutyl-
thio, 2,2-dimethylbutylthio, 2,3-dimethylbutylthio, 3,3-dimethyl-
butylthio, 1-ethylbutylthio, 2-ethylbutylthio, 1,1,2-trimethyl-
propylthio, 1,2,2-trimethylpropylthio, 1-ethyl-1-methylpropylthio
and 1-ethyl-2-methylpropylthio;
cycloAl~yl: monocyclic alkyl groups having 3 to 6 carbon ring
members, eg. cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl;
alkenyl: straight-chain or branched alkenyl groups having 2 to 6
30 or 2 to 10 carbon atoms and a double bond in any desired
position, eg. C2-C6-alkenyl such as ethenyl, 1-propenyl,
2-propenyl, 1-methylethenyl, 1-butenyl, 2-butenyl, 3-butenyl,
1-methyl-1-propenyl, 2-methyl-1-propenyl, 1-methyl-2-propenyl,
2-methyl-2-propenyl, 1-pentenyl, 2-pentenyl, 3-pentenyl,
35 4-pentenyl, 1-methyl-1-butenyl, 2-methyl-1-butenyl,
3-methyl-1-butenyl, 1-methyl-2-butenyl, 2-methyl-2-butenyl,
3-methyl-2-butenyl, 1-methyl-3-butenyl, 2-methyl-3-butenyl,
3-methyl-3-butenyl, 1,1-dimethyl-2-propenyl,
1,2-dimethyl-1-propenyl, 1,2-dimethyl-2-propenyl,
40 l-ethyl-l-propenyl, 1-ethyl-2-propenyl, 1-hexenyl, 2-hexenyl,
3-hexenyl, 4-hexenyl, 5-hexenyl, l-methyl-1-pentenyl,
2-methyl-1-pentenyl, 3-methyl-1-pentenyl, 4-methyl-1-pentenyl,
l-methyl-2-pentenyl, 2-methyl-2-pentenyl, 3-methyl-2-pentenyl,
4-methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-3-pentenyl,
45 3-methyl-3-pentenyl, 4-methyl-3-pentenyl, 1-methyl-4-pentenyl,
2-methyl-4-pentenyl, 3-methyl-4-pentenyl, 4-methyl-4-pentenyl,
1,1-dimethyl-2-butenyl, 1,1-dimethyl-3-butenyl,
CA 02231661 1998-04-07
0050/46271
1,2-dimethyl-1-butenyl, 1,2-dimethyl-2-butenyl,
1,2-dimethyl-3-butenyl, 1,3-dimethyl-1-butenyl,
1,3-dimethyl-2-butenyl, 1,3-dimethyl-3-butenyl,
2,2-dimethyl-3-butenyl, 2,3-dimethyl-1-butenyl,
5 2,3-dimethyl-2-butenyl, 2,3-dimethyl-3-butenyl,
3,3-dimethyl-1-butenyl, 3,3-dimethyl-2-butenyl,
l-ethyl-l-butenyl, l-ethyl-2-butenyl, 1-ethyl-3-butenyl,
2-ethyl-1-butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl,
1,1,2-trimethyl-2-propenyl, 1-ethyl-1-methyl-2-propenyl,
10 1-ethyl-2-methyl-1-propenyl and 1-ethyl-2-methyl-2-propenyl;
alkenyloxy: straight-chain or branched alkenyl groups having 2 to
6 carbon atoms and a double bond in any desired position, which
are bonded to the structure via an oxygen atom (-0-);
alkenylthio or alkenylamino: straight-chain or branched alkenyl
groups having 2 to 6 carbon atoms and a double bond in any
desired position, which (alkenylthio) are bonded to the structure
via a sulfur atom or (alkenylamino) a nitrogen atom.
alkenylcarbonyl: straight-chain or branched alkenyl groups having
2 to 10 carbon atoms and a double bond in any desired position,
which are bonded to the structure via a carbonyl group (-C0-);
25 alkynyl: straight-chain or branched alkynyl groups having 2 to
10 carbon atoms and a triple bond in any desired position, eg.
C2-C6-alkynyl such as ethynyl, 2-propynyl, 2-butynyl, 3-butynyl,
l-methyl-2-propynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl,
l-methyl-2-butynyl, 1-methyl-3-butynyl, 2-methyl-3-butynyl,
30 1,1-dimethyl-2-propynyl, 1-ethyl-2-propynyl, 2-hexynyl,
3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-2-pentynyl,
l-methyl-3-pentynyl, 1-methyl-4-pentynyl, 2-methyl-3-pentynyl,
2-methyl-4-pentynyl, 3-methyl-4-pentynyl, 4-methyl-2-pentynyl,
1,1-dimethyl-2-butynyl, 1,1-dimethyl-3-butynyl, 1,2-dimethyl-
35 3-butynyl, 2,2-dimethyl-3-butynyl, 1-ethyl-2-butynyl, 1-ethyl-
3-butynyl, 2-ethyl-3-butynyl and 1-ethyl-1-methyl-2-propynyl;
alkynyloxy or alkynylthio and alkynylamino: straight-chain or
branched alkynyl groups having 2 to 6 carbon atoms and a triple
40 bond in any desired position, which (alkynyloxy) are bonded to
the structure via an oxygen atom or (alkynylthio) via a sulfur
atom or (alkynylamino) via a nitrogen atom;
alkynylcarbonyl: straight-chain or branched alkynyl groups having
45 3 to 10 carbon atoms and a triple bond in any desired position,
which are bonded to the structure via a carbonyl group (-C0-);
CA 02231661 1998-04-07
0050/ 4627 1
cyclo~l~enyl or cyclo~ nyloxy cyclo~lkenylthio and cyclo-
alkenylr ino: monocyclic alkenyl groups having 4 to 6 carbon ring
'~rs, which are bonded to the structure directly or (cyclo-
alkenyloxy) via an oxygen atom or (cycloalkenylthio~ a sulfur
5 atom or cycloalkenylamino) via a nitrogen atom, eg. cyclobutenyl,
cyclopentenyl and cyclohexenyl.
cyclo~l~o~yl ~sicl or cycloalkylthio and cycloalkylr ino:
monocyclic alkyl groups having 3 to 6 carbon ring members, which
10 are bonded to the structure (cycloalkoxy) via an oxygen atom or
(cycloalkylthio) a sulfur atom or (cycloalkylamino) via a
nitrogen atom, eg. cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl;
15 heterocyclyl or heterocyclyloxy. heterocyclylthio and hetero-
cyclylamino: three- to six-membered, saturated or partially
unsaturated mono- or polycyclic heterocycles which contain one to
three heteroatoms selected from a group consisting of oxygen,
nitrogen and sulfur, and which are bonded to the structure
20 directly or (heterocyclyloxy) via an oxygen atom or (hetero-
cyclylthio) via a sulfur atom or (heterocyclylamino) via a
nitrogen atom, such as 2-tetrahydrofuranyl, oxiranyl, 3-tetra-
hydrofuranyl, 2-tetrahydrothienyl, 3-tetrahydrothienyl,
2-pyrrolidinyl, 3-pyrrolidinyl, 3-isoxazolidinyl,
2s 4-isoxazolidinyl, 5-isoxazolidinyl, 3-isothiazolidinyl,
4-isothiazolidinyl, 5-isothiazolidinyl, 3-pyrazolidinyl,
4-pyrazolidinyl, 5-pyrazolidinyl, 2-oxazolidinyl, 4-oxazolidinyl,
5-oxazolidinyl, 2-thiazolidinyl, 4-thiazolidinyl, 5-thia-
zolidinyl, 2-imidazolidinyl, 4-imidazolidinyl, 1,2,4-oxa-
30 diazolidin-3-yl, 1,2,4-oYA~i~7olidin-5-yl, 1,2,4-thia-
diazolidin-3-yl, 1,2,4-th;~ zolidin-5-yl, 1,2,4-tria-
zolidin-3-yl, 1,3,4-oYA~i~zolidin-2-yl, 1,3,4-thia-
diazolidin-2-yl, 1,3,4-triazolidin-2-yl, 2,3-dihydrofur-2-yl,
2,3-dihydrofur-3-yl, 2,3-dihydrofur-4-yl, 2,3-dihydrofur-5-yl,
35 2,5-dihydrofur-2-yl, 2,5-dihydrofur-3-yl, 2,3-dihydrothien-2-yl,
2,3-dihydrothien-3-yl, 2,3-dihydrothien-4-yl,
2,3-dihydrothien-5-yl, 2,5-dihydrothien-2-yl, 2,5-dihydro-
thien-3-yl, 2,3-dihydl G~L1 ol-2-yl, 2,3-dihydropyrrol-3-yl,
2,3-dihydropyrrol-4-yl, 2,3-dihydropyrrol-5-yl, 2,5-dihydro-
40 pyrrol-2-yl, 2,5-dihydropyrrol-3-yl, 2,3-dihydroisoxazol-3-yl,
2,3-dihydroisoxazol-4-yl, 2,3-dihydroisoxazol-5-yl, 4,5-dihydro-
isoxazol-3-yl, 4,5-dihydroisoxazol-4-yl, 4,5-dihydro-
isoxazol-5-yl, 2,5-dihydroisoxazol-3-yl, 2,5-dihydro-
isoxazol-4-yl, 2,5-dihydrooxazol-5-yl,
45 2,3-dihydroisothiazol-3-yl, 2,3-dihydroisothiazol-4-yl,
2,3-dihydroisothiazol-5-yl, 4,5-dihydroisothiazol-3-yl,
4,5-dihydroisothiazol-4-yl, 4,5-dihydroisothiazol-5-yl,
CA 02231661 1998-04-07
(~050/ 4627 1
2,5-dihydroisothiazol-3-yl, 2,5-dihydroisothiazol-4-yl,
2,5-dihydroisothiazol-5-yl, 2,3-dihydropyrazol-3-yl, 2,3-dihydro-
pyrazol-4-yl, 2,3-dihydropyrazol-5-yl, 4,5-dihydropyrazol-3-yl,
4,5-dihydropyrazol-4-yl, 4,5-dihydropyrazol-5-yl, 2,5-dihydro-
5 pyrazol-3-yl, 2,5-dihydropyrazol-4-yl, 2,5-dihydropyrazol-5-yl,
2,3-dihydrooxazol-2-yl, 2,3-dihydrooxazol-4-yl, 2,3-dihydro-
oxazol-5-yl, 4,5-dihydrooxazol-2-yl, 4,5-dihydrooxazol-4-yl,
4,5-dihydrooxazol-5-yl, 2,5-dihydrooxazol-2-yl, 2,5-dihydro-
oxazol-4-yl, 2,5-dihydrooxazol-5-yl, 2,3-dihydrothiazol-2-yl,
10 2,3-dihydrothiazol-4-yl, 2,3-dihydrothiazol-5-yl, 4,5-dihydro-
thiazol-2-yl, 4,5-dihydrothiazol-4-yl, 4,5-dihydrothiazol-5-yl,
2,5-dihydrothiazol-2-yl, 2,5-dihydrothiazol-4-yl, 2,5-dihydro-
thiazol-5-yl, 2,3-dihydroimidazol-2-yl, 2~3-dihydroimidazol-4
2,3-dihydroimidazol-5-yl, 4,5-dihydroimidazol-2-yl, 4,5-dihydro-
15 imidazol-4-yl, 4,5-dihydroimidazol-5-yl, 2,5-dihydroimida_
zol-2-yl, 2,5-dihydroimidazol-4-yl, 2,5-dihydroimidazol-5_yl,
2-morpholinyl, 3-morpholinyl, 2-piperidinyl, 3-piperidinyl,
4-piperidinyl, 3-tetrahydropyridazinyl, 4-tetrahydropyridazinyl,
2-tetrahydropyrimidinyl, 4-tetrahydropyrimidinyl, 5-tetrahydro-
20 pyrimidinyl, 2-tetrahydropyrazinyl, 1,3,5-tetrahydrotriazin-2-yl,
1,2,4-tetrahydrotriazin-3-yl, 1,3-dihydrooxazin-2-yl,
1,3-dithian-2-yl, 2-tetrahydropyranyl, 1,3-dioxolan-2-yl,
3,4,5,6-tetrahydropyridin-2-yl, 4H-1,3-thiazin-2-yl,
4H-3,1-benzothiazin-2-yl, 1,1-dioxo-2,3,4,5-tetrahydrothien-2-yl,
25 2H-1,4-benzothiazin-3-yl, 2H-1,4-benzoxazin-3-yl and
1,3-dihydrooxazin-2-yl;
aryl or aryloxy, arylthio arylcarbonyl and arylsulfonyl:
aromatic mono- or polycyclic hydrocarbon radicals which are
30 bonded to the structure directly or (aryloxy) via an oxygen atom
~-O-) or (arylthio) a sulfur atom (-S-), (arylcarbonyl) via a
carbonyl group (-C0-) or (arylsulfonyl) via a sulfonyl group
(-SO2-), eg. phenyl, naphthyl and phenanthrenyl or phenoxy,
naphthyloxy and phenanthrenyloxy and the corresponding carbonyl
35 and sulfonyl radicals;
aryl: ino: aromatic mono- or polycyclic hydrocarbon radicals, as
mentioned above, which are bonded to the structure via a nitrogen
atom.
heteroaryl or heteroaryloxy, heteroarylthio, heteroarylcarbonyl
and hetarylsulfonyl: aromatic mono- or polycyclic radicals which,
beside carbon ring members, can additionally contain one to four
nitrogen atoms or one to three nitrogen atoms and an oxygen or a
45 sulfur atom or an oxygen or a sulfur atom and which are bonded to
the structure directly or (heteroaryloxy) via an oxygen atom
(-o-) or (heteroarylthio) a sulfur atom (-s-)~ (heteroaryl-
CA 02231661 1998-04-07
0050/46~71
carbonyl) via a carbonyl group (-CO-) or (heteroarylsulfonyl) via
a sulfonyl group (-S02-), eg.
- 5-membered heteroaryl, containing one to three nitrogen
atoms: 5-membered ring heteroaryl groups which, beside carbon
atoms, can contain one to three nitrogen atoms as ring
members, eg. 2-pyrrolyl, 3-pyrrolyl, 3-pyrazolyl,
4-pyrazolyl, 5-pyrazolyl, 2-imidazolyl, 4-imidazolyl,
1,2,4-triazol-3-yl and 1,3,4-triazol-2-yl;
- 5 - bered heteroaryl, containing one to four nitrogen atoms
or one to three nitrogen atoms and a sulfur or oxygen atom or
an oxygen or a sulfur atom: 5-membered ring heteroaryl groups
which, beside carbon atoms, can contain one to four nitrogen
atoms or one to three nitrogen atoms and a sulfur or oxygen
atom or an oxygen or sulfur atom as ring members, eg.
2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 2-pyrrolyl,
3-pyrrolyl, 3-isoxazolyl, 4-isoxazolyl, 5-isoxazolyl,
3-isothiazolyl, 4-isothiazolyl, 5-isothiazolyl, 3-pyrazolyl,
4-pyrazolyl, 5-pyrazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl,
2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-imidazolyl,
4-imidazolyl, 1,2,4-oxadiazol-3-yl, 1,2,4-oxadiazol-S-yl,
1,2,4-thiadiazol-3-yl, 1,2,4-thiadiazol-5-yl, 1,2,4-triazol-
3-yl, 1,3,4-oxadiazol-2-yl, 1,3,4-thiadiazol-2-yl and
2S 1,3,4-triazol-2-yl;
- benzo-fused 5-membered heteroaryl, cont~ining one to three
nitrogen atoms or a nitrogen atom and/or an oxygen or sulfur
atom: 5-membered ring heteroaryl groups which, beside carbon
atoms, can contain one to four nitrogen atoms or one to three
nitrogen atoms and a sulfur or oxygen atom or an oxygen or a
sulfur atom as ring members, and in which two adjacent carbon
ring members or a nitrogen and an adjacent carbon ring member
can be bridged by a buta-1,3-diene-1,4-diyl group;
- 5-membered heteroaryl bonded via nitrogen, cont~ining one to
four nitrogen atoms, or benzo-fused 5-membered heteroaryl
bonded via nitrogen, containing one to three nitrogen atoms:
5-membered ring heteroaryl groups which, beside carbon atoms,
can contain one to four nitrogen atoms or one to three
nitrogen atoms as ring members, and in which two adjacent
carbon ring members or a nitrogen and an adjacent carbon ring
member can be bridged by a buta-1,3-diene-1,4-diyl group,
these rings being bonded to the structure via one of the
45 nitrogen ring ~ ers;
CA 02231661 1998-04-07
0050/4627 1
- 6-membered heteroaryl containing one to three or one to four
nitrogen atoms: 6 hered ring heteroaryl groups which,
beside carbon atoms, can contain one to three or one to four
nitrogen atoms as ring members, eg. 2-pyridinyl, 3-pyridinyl,
4-pyridinyl, 3-pyridazinyl, 4-pyridazinyl, 2-pyrimidinyl,
4-pyrimidinyl, 5-pyrimidinyl, 2-pyrazinyl,
1,3,5-triazin-2-yl, 1,2,4-triazin-3-yl and
1,2,4,5-tetrazin-3-yl;
lO - benzo-fused 6 ~ered heteroaryl containing one to four
nitrogen atoms: 6-membered ring heteroaryl groups in which
two adjacent carbon ring members can be bridged by a
buta-1,3-diene-1,4-diyl group, eg. quinoline, isoquinoline,
quinazoline and quinoxaline,
- pyrido[3,2-dlthiazol-2-yl;
or the corresponding oxy, thio, carbonyl or sulfonyl groups;
20 hetary~ Q: aromatic mono- or polycyclic radicals which, beside
carbon ring members, can additionally contain one to four
nitrogen atoms or one to three nitrogen atoms and an oxygen or a
sulfur atom and which are bonded to the structure via a nitrogen
atom.
The statement Npartially or completely halogenated" is inten~A
to express that in the groups characterized in this way the
hydrogen atoms can be partially or completely replaced by
identical or different halogen atoms, as mentioned above.
The statement "unsubstituted or substitutedn is inten~eA to
express that in the groups characterized in this way the hydrogen
atoms can be partially or completely replaced by identical or
different groups of, for example, the type which are mentioned
35 under the collective terms set out above.
With respect to their biological action, compounds of the
formula I are preferred in which the variables have the following
meanings:
X iS NOCH3, CHOCH3 or CHCH3;
Y is 0 or NZ, Z being hydrogen or C1-C4-alkyl;
45 R1 is hydrogen or Cl-C4-alkyl;
CA 02231661 1998-04-07
0050/ 4627 1
R2 is cyano, nitro, halogen, Cl-C4-alkyl, trifluoromethyl or
C1-C4-alkoxy;
m is 0, 1 or 2, it being possible for the radicals R2 to be
different if m is 2;
R3 is hydrogen, C1-C4-alkyl, Cl-C4-haloalkyl or cyclopropyl;
R4 is hydrogen;
C1-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl, it being possible
for the three last-mentioned radicals to be partially or
completely halogenated and/or to carry one to three
substituents, in each case selected from the group: cyano,
nitro, hydroxyl, mercapto, amino, carboxyl, aminocarbonyl,
aminothiocarbonyl, Cl-C6-alkylaminocarbonyl, di-C1-C6-alkyl-
aminocarbonyl, C1-C6-alkylaminothiocarbonyl, di-C1-C6-alkyl-
aminothiocarbonyl, C1-C6-alkylsulfonyl, C1-C6-alkylsulfoxyl,
Cl-C6-alkoxy, Cl-C6-haloalkoxy, Cl-C6-alkoxycarbonyl,
Cl-C6-alkylthio, Cl-C6-alkylamino, di-Cl-C6-alkylamino,
C2-C6-alkenyloxy, C3-C6-cycloalkyl, C3-C6-cycloalkyloxy,
heterocyclyl, heterocyclyloxy, aryl, aryloxy, aryl-C1-C4-
alkoxy, arylthio, aryl-Cl-C4-alkylthio, heteroaryl, hetero-
aryloxy, heteroaryl-C1-C4-alkoxy, heteroarylthio or hetero-
aryl-Cl-C4-alkylthio, it being possible for the cyclic groups
of the fourteen last-mentioned radicals for their part to be
partially or completely halogenated and/or to carry one to
three substituents, in each case selected from the group:
cyano, nitro, hydroxyl, mercapto, amino, carboxyl, amino-
carbonyl, aminothiocarbonyl, Cl-C6-alkyl, Cl-C6-haloalkyl,
Cl-C6-alkylcarbonyl, Cl-C6-alkylsulfonyl, Cl-C6-alkylsulfoxyl,
C3-C6-cycloalkyl, Cl-C6-alkoxy, Cl-C6-haloalkoxy, Cl-C6-alkoxy-
carbonyl, Cl-C6-alkylthio, Cl-C6-alkylamino, di-Cl-C6-alkyl-
amino, Cl-C6-alkylaminocarbonyl, di-C1-C6-alkylaminocarbonyl,
Cl-C6-alkylaminothiocarbonyl, di-Cl-C6-alkylaminothiocarbonyl,
C2-C6-alkenyl, C2-C6-alkenyloxy, benzyl, benzyloxy, aryl,
aryloxy, arylthio, heteroaryl, heteroaryloxy, heteroarylthio,
C3-C6-alkynyloxy, Cl-C4-alkylenedioxy, which can be
halogenated, or C(=NoR6)-An-R7, A being oxygen, sulfur or
nitrogen and the nitrogen carrying hydrogen or Cl-C6-alkyl, n
being equal to 0 or 1, R6 being hydrogen or C1-C6-alkyl and R7
being hydrogen or Cl-C6-alkyl;
C3-C6-cycloalkyl, C4-C6-cycloalkenyl, heterocyclyl, it being
possible for the three last-mentioned radicals to be
partially or completely halogenated and/or to carry one to
three substituents, in each case selected from the group:
CA 02231661 1998-04-07
0050/ 4627 1
cyano, Cl-C6-alkyl, Cl-C6-haloalkyl, C2-C6-alkenyl,
Cl-C6-alkoxy and C1-C6-alkylthio;
aryl or heteroaryl, it being possible for these two radicals
to be partially or completely halogenated and/or to carry one
to three substituents, in each case selected from the group:
cyano, nitro, hydroxyl, mercapto, amino, carboxyl, amino-
carbonyl, aminothiocarbonyl, Cl-C6-alkyl, Cl-C6-haloalkyl,
Cl-C6-alkylcarbonyl, Cl-C6-alkylsulfonyl, Cl-C6-alkylsulfoxyl,
C3-C6-cycloalkyl, Cl-C6-alkoxy, Cl-C6-haloalkoxy, Cl-C6-alkoxy-
carbonyl, Cl-C6-alkylthio, Cl-C6-alkylamino, di-Cl-C6-alkyl-
amino, C1-C6-alkylaminocarbonyl, di-Cl-C6-alkylaminocarbonyl,
Cl-C6-alkylaminothiocarbonyl, di-Cl-C6-alkylaminothiocarbonyl,
C2-C6-alkenyl, C2-C6-alkenyloxy, C3-C6-alkynyloxy,
C1-C4-alkylenedioxy, which can be halogenated, benzyl, benzyl-
oxy, aryl, aryloxy, arylthio, heteroaryl, heteroaryloxy,
heteroarylthio, it being possible for the cyclic groups of
the eight last-mentioned radicals for their part to be
partially or completely halogenated and/or to carry one to
three substituents, in each case selected from the group:
cyano, nitro, hydroxyl, C1-C6-alkyl, C1-C6-haloalkyl,
Cl-C6-alkylcarbonyl, C3-C6-cycloalkyl, Cl-C6-alkoxy,
Cl-C6-haloalkoxy, Cl-C6-alkoxycarbonyl, Cl-C6-alkylthio,
Cl-C6-alkylamino, di-Cl-C6-alkylamino, C2-C6-alkenyl,
C2-C6-alkenyloxy, C3-C6-alkynyloxy and C1-C4-alkylenedioxy,
which can be halogenated; and C(=NOR6 ) -An-R7,
A being oxygen, sulfur or nitrogen and the nitrogen carrying
hydrogen or C1-C6-alkyl, n being equal to 0 or 1, R6 being
hydrogen or C1-C6-alkyl and R7 being hydrogen or C1-C6-alkyl;
R5 is hydrogen, C1-C6-alkylsulfonyl,
Cl-C6-alkyl, C3-C6-alkenyl, C3-C6-alkynyl, it being possible
for these radicals to be partially or completely halogenated
and/or to carry one to three substituents, in each case
selected from the group: cyano, nitro, hydroxyl, mercapto,
amino, carboxyl, aminocarbonyl, aminothiocarbonyl,
Cl-C6-alkylaminocarbonyl, di-Cl-C6-alkylaminocarbonyl,
Cl-C6-alkylaminothiocarbonyl, di-Cl-C6-alkylaminothiocarbonyl,
Cl-C6-alkylsulfonyl, Cl-C6-alkylsulfoxyl, Cl-C6-alkoxy,
Cl-C6-haloalkoxy, Cl-C6-alkoxycarbonyl, Cl-C6-alkylthio,
Cl-C6-alkylamino, di-Cl-C6-alkylamino, C2-C6-alkenyloxy,
C3-C6-cycloalkyl, C3-C6-cycloalkyloxy, heterocyclyl, hetero-
cyclyloxy, aryl, aryloxy, arylthio, heteroaryl, heteroaryloxy
and heteroarylthio, it being possible for the cyclic groups
of the 10 last-mentioned radicals for their part to be
partially or completely halogenated and/or to carry one to
three of the following substituents, in each case selected
CA 02231661 1998-04-07
0050/46;!7 1
from the group: cyano, nitro, hydroxyl, mercapto, amino,
carboxyl, aminocarbonyl, aminothiocarbonyl, Cl-C6-alkyl,
Cl-C6-haloalkyl, Cl-C6-alkylcarbonyl, Cl-C6-alkylsulfonyl,
Cl-C6-alkylsulfoxyl, C3-C6-cycloalkyl, Cl-C6-alkoxy,
Cl-C6-haloalkoxy, Cl-C6-alkoxycarbonyl, C1-C6-alkylthio,
Cl-C6-alkylamino, di-Cl-C6-alkylamino, Cl-C6-alkylamino-
carbonyl, di-C1-C6-alkylaminocarbonyl, Cl-C6-alkylaminothio-
carbonyl, di-Cl-C6-alkylaminothiocarbonyl, C2-C6-alkenyl,
C2-C6-alkenyloxy, benzyl, benzyloxy, aryl, aryloxy, arylthio,
heteroaryl, heteroaryloxy, heteroarylthio and
C3-C6-alkynyloxy,
C3-C6-cycloalkyl, which can be partially or completely
halogenated and/or can carry one to three substituents, in
each case selected from the group: cyano, Cl-C6-alkyl,
Cl-C6-haloalkyl, C2-C6-alkenyl, Cl-C6-alkoxy and
Cl-C6-alkylthio;
and their salts.
Furthermore, compounds of the formula I are preferred in which m
is 0.
Equally preferred compounds of the formula I are those in which Rl
25 is methyl.
In addition, compounds I are preferred in which R3 is hydrogen,
cyclopropyl, methyl, ethyl, 1-methylethyl or trifluoromethyl.
30 Particularly preferred compounds I are those in which R3 is
methyl.
In addition, compounds I are preferred in which R3 is
trifluoromethyl.
In addition, compounds I are preferred in which R4 is
unsubstituted or substituted C1-C6-alkyl, C2-C6-alkenyl or
C2 -C6-alkynyl .
40 Additionally, compounds I are preferred in which R4 is
unsubstituted or substituted heterocyclyl or unsubstituted or
substituted C4-C6-cycloalkenyl.
Additionally, compounds I are preferred in which R4 is
45 unsubstituted or substituted C3-C6-cycloalkyl.
CA 02231661 1998-04-07
UU3U~ StO~ I 1
In addition, compounds I are preferred in which R4 is
unsubstituted or substituted aryl or hetaryl.
In addition, compounds I are preferred in which R4 is unsubst. or
5 subst. pyridyl, pyrimidyl, pyrazinyl, pyridazinyl or triazinyl.
In addition, compounds I are preferred in which R4 is unsubst. or
subst. furyl, thienyl or pyrrolyl.
10 In addition, compounds I are preferred in which R4 is unsubst. or
subst. oxazolyl, thiazolyl, isoxazolyl, isothiazolyl, pyrazolyl
or imidazolyl.
In addition, c~ _unds I are preferred in which R4 is unsubst. or
15 subst. oxdiazolyl [sic], thiadiazolyl or triazolyl.
Additionally, compounds I are preferred in which R4 is phenyl
which is unsubstituted or carries one or two of the following
groups: nitro, cyano, hydroxyl, amino, aminocarbonyl, amino-
20 thiocarbonyl, halogen, Cl-C4-alkyl, Cl-C4-haloalkyl, C3-C6-cyclo-
alkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy, Cl-C4-alkylamino,
Cl-C4-alkylcarbonyl, di-Cl-C4-alkylamino, Cl-C4-alkylsulfonyl,
Cl-C4-alkoxycarbOnyl, Cl-C4-alkylaminocarbonyl, di-Cl-C4-alkyl-
aminocarbonyl, C1-C4-alkylthio, C2-C6-alkenyl, C2-C6-alkenyloxy,
25 C3-C6-alkynyloxy and Cl-C4-alkylenedioxy, which can be
halogenated.
In addition, compounds I are preferred in which R5 is Cl-C6-alkyl.
30 Additionally, compounds I are preferred in which Rs is methyl or
ethyl.
In addition, compounds I are preferred in which R5 is hydrogen.
35 Additionally, compounds I are preferred in which R5 is
C3-C6-alkenyl.
Additionally, compounds I are preferred in which Rs is
C3-C6-alkynyl .
In addition, compounds of the formula I are preferred in which X
is NOCH3.
In addition, compounds of the formula I are preferred in which X
45 is CHOCH3.
CA 02231661 1998-04-07
.)050/ 4627 1
In addition, compounds of the formula I are preferred in which X
is CHCH3.
In addition, compounds of the formula I are preferred in which Y
5 is 0.
Additionally, compounds of the formula I are preferred in which Y
is NH.
10 In particular, with respect to their use, the compounds I
compiled in the following tables are preferred.
The following tables (1 to 596) are based on the formulae I.l,
I.2, I.3 and I.4, the double bonds marked by "E having the E
15 configuration:
R3 ~
C=~O--CH2~ (I.l)
R50--N~ E E C=CH-OCH3
R4 C=O
OCH3
C= N--O--CH2$1 ( I . 2 )
R50--N~ E E 7=CH-CH3
R4 C=O
OCH3
C = N--O - CH2 ~ (I.3)
R50--N ~ E E C=N-OCH3
R4 C=O
OCH3
CA 02231661 1998-04-07
0050/46271
24
C = N- O - CH2 ~ (I.4)
R50 - N ~ - E C=N-OCH3
R4 C=O
NH-CH3
Table 1
Compounds of the formula I.l., where
R3 = hydrogen;
15 RS = hydrogen;
R4 = in each case one line of Table A.
Table 2
Compounds of the formula I.2, where
20 R3 = hydrogen;
R5 = hydrogen;
R4 = in each case one line of Table A.
Table 3
25 Compounds of the formula I.3, where
R3 = hydrogen;
R5 = hydrogen;
R4 = in each case one line of Table A.
30 Table 4
Compounds of the formula I.4, where
R3 = hydrogen;
R5 = hydrogen;
R4 = in each case one line of Table A.
Table 5
C~ unds of the formula I.1., where
R3 = hydrogen;
R5 = methyl;
40 R4 = in each case one line of Table A.
Table 6
Compounds of the formula I.2, where
R3 = hydrogen;
45 R5 = methyl;
R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/ 4627
Table 7
Compounds of the formula I.3, where
R3 = hydrogen;
R5 = methyl;
5 R4 = in each case one line of Table A.
Table 8
Compounds of the formula I.4, where
R3 = hydrogen;
10 R5 = methyl;
R4 = in each case one line of Table A.
Table 9
Compounds of the formula I.l, where
15 R3 = hydrogen;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 10
20 Compounds of the formula I.2, where
R3 = hydrogen;
R5 = ethyl;
R4 = in each case one line of Table A.
25 Table 11
Compounds of the formula I.3, where
R3 = hydrogen;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 12
Compounds of the formula I.4, where
R3 = hydrogen;
R5 = ethyl;
35 R4 = in each case one line of Table A.
Table 13
Compounds of the formula I.l, where
R3 = hydrogen;
40 R5 = n-propyl;
R4 = in each case one line of Table A.
Table 14
Compounds of the formula I.2, where
45 R3 = hydrogen;
R5 = n-propyl;
CA 02231661 1998-04-07
0050/ 4627 1
R4 = in each case one line of Table A.
Table 15
Compounds of the formula I.3, where
5 R3 = hydrogen;
R5 = n-propyl;
R4 = in each case one line of Table A.
Table 16
10 Compounds of the formula I.4, where
R3 = hydrogen;
R5 = n-propyl;
R4 = in each case one line of Table A.
15 Table 17
Compounds of the formula I.l, where
R3 = hydrogen;
Rs = isopropyl;
R4 = in each case one line of Table A.
Table 18
Compounds of the formula I.2, where
R3 = hydrogen;
R5 = isopropyl;
25 R4 = in each case one line of Table A.
Table 19
Compounds of the formula I.3, where
R3 = hydrogen;
30 R5 = isopropyl;
R4 = in each case one line of Table A.
Table 20
Compounds of the formula I.4, where
35 R3 = hydrogen;
R5 = isopropyl;
R4 = in each case one line of Table A.
Table 21
40 Compounds of the formula I.1, where
R3 = hydrogen;
Rs = cyclopropyl;
R4 = in each case one line of Table A.
45 Table 22
Compounds of the formula I.2, where
R3 = hydrogen;
CA 02231661 1998-04-07
0050/46271
R5 = cyc lopropyl;
R4 = in each case one line of Table A.
Table 23
5 Compounds of the formula I.3, where
R3 = hydrogen;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
10 Table 24
Compounds of the formula I.4, where
R3 = hydrogen;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
Table 25
Compounds of the formula I.l, where
R3 = hydrogen;
R5 = n-butyl;
20 R4 = in each case one line of Table A.
Table 26
Compounds of the formula I.2, where
R3 = hydrogen;
25 R5 = n-butyl;
R4 = in each case one line of Table A.
Table 27
Compounds of the formula I.3, where
30 R3 = hydrogen;
R5 = n-butyl;
R4 = in each case one line of Table A.
Table 28
35 Compounds of the formula I.4, where
R3 = hydrogen;
R5 = n-butyl;
R4 = in each case one line of Table A.
40 Table 29
Compounds of the formula I.l, where
R3 = hydrogen;
R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 30
Compounds of the formula I.2, where
CA 02231661 1998-04-07
UU~/ ~o~ ~
28
R3 = hydrogen;
R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of ~able A.
S Table 31
Compounds of the formula I.3, where
R3 = hydrogen;
R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 32
Compounds of the formula I.4, where
R3 = hydrogen;
R5 = 2-methoxyeth-1-yl;
15 R4 = in each case one line of Table A.
Table 33
Compounds of the formula I.1, where
R3 = hydrogen;
20 RS = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 34
Compounds of the formula I.2, where
25 R3 = hydrogen;
R5 - prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 35
30 Compounds of the formula I.3, where
R3 = hydrogen;
Rs = prop-2-en-1-yl;
R4 = in each case one line of Table A.
35 Table 36
Compounds of the formula I.4, where
R3 = hydrogen;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 37
Compounds of the formula I.1, where
R3 = hydrogen;
R5 = E-but-2-en-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/4627 1
Table 38
Compounds of the formula I.2, where
R3 = hydrogen;
R5 = E-but-2-en-1-yl;
5 R4 = in each case one line of Table A.
Table 39
Compounds of the formula I.3, where
R3 = hydrogen;
10 R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 40
Compounds of the formula I.4, where
15 R3 = hydrogen;
Rs = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 41
20 Compounds of the formula I.1, where
R3 = hydrogen;
R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
25 Table 42
Compounds of the formula I.2, where
R3 = hydrogen;
R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 43
Compounds of the formula I.3, where
R3 = hydrogen;
R5 = Z-but-2-en-1-yl;
35 R4 = in each case one line of Table A.
Table 44
Compounds of the formula I.4, where
R3 = hydrogen;
40 Rs = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 45
Compounds of the formula I.l, where
45 R3 = hydrogen;
R5 = E-3-chloroprop-2-en-1-yl;
CA 02231661 1998-04-07
0050/4627 1
R4 = in each case one line of Table A.
Table 46
Compounds of the formula I.2, where
5 R3 = hydrogen;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 47
10 Compounds of the formula I.3, where
R3 = hydrogen;
Rs = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
15 Table 48
Compounds of the formula I.4, where
R3 = hydrogen;
Rs = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 49
Compounds of the formula I.l, where
R3 = hydrogen;
Rs = Z-3-chloroprop-2-en-1-yl;
25 R4 = in each case one line of Table A.
Table 50
Compounds of the formula I.2, where
R3 = hydrogen;
30 R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 51
Compounds of the formula I.3, where
35 R3 = hydrogen;
Rs = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 52
40 Compounds of the formula I.4, where
R3 = hydrogen;
R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
45 Table 53
Compounds of the formula I.1, where
R3 = hydrogen;
CA 02231661 1998-04-07
0050/4627 1
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 54
5 Compounds of the formula I.2, where
R3 = hydrogen;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
10 Table 55
Compounds of the formula I.3, where
R3 = hydrogen;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 56
Compounds of the formula I.4, where
R3 = hydrogen;
R5 = prop-2-yn-1-yl;
20 R4 = in each case one line of Table A.
Table 57
Compounds of the formula I.1, where
R3 = cyclopropyl;
25 R5 = hydrogen;
R4 = in each case one line of Table A.
Table 58
Compounds of the formula I.2, where
30 R3 = cyclopropyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
Table 59
35 Compounds of the formula I.3, where
R3 = cyclopropyl;
RS = hydrogen;
R4 = in each case one line of Table A.
40 Table 60
Compounds of the formula I.4, where
R3 = cyclopropyl;
Rs = hydrogen;
R4 = in each case one line of Table A.
Table 61
Compounds of the formula I.l, where
CA 02231661 1998-04-07
0050/ 4627 1
R3 = cyclopropyl;
R5 = methyl;
R4 = in each case one line of Table A.
5 Table 62
Compounds of the formula I.2, where
R3 = cyclopropyl;
R5 = methyl;
R4 = in each case one line of Table A.
Table 63
Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = methyl;
15 R4 = in each case one line of Table A.
Table 64
Compounds of the formula I.4, where
R3 = cyclopropyl;
20 R5 = methyl;
R4 = in each case one line of Table A.
Table 65
Compounds of the formula I.1, where
25 R3 = cyclopropyl;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 66
30 Compounds of the formula I.2, where
R3 = cyclopropyl;
Rs = ethyl;
R4 = in each case one line of Table A.
35 Table 67
Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 68
Compounds of the formula I.4, where
R3 = cyclopropyl;
R5 = ethyl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
OOSO/4627
Table 69
Compounds of the formula I.l, where
R3 = cyclopropyl;
R5 = n-propyl;
S R4 = in each case one line of Table A.
Table 70
Compounds of the formula I.2, where
R3 = cyclopropyl;
10 R5 = n-propyl;
R4 = in each case one line of Table A.
Table 71
Compounds of the formula I.3, where
lS R3 = cyclopropyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
Table 72
20 Compounds of the formula I.4, where
R3 = cyclopropyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
25 Table 73
Compounds of the formula I.l, where
R3 = cyclopropyl;
R5 = isopropyl;
R4 = in each case one line of Table A.
Table 74
Compounds of the formula I.2, where
R3 = cyclopropyl;
R5 = isopropyl;
35 R4 = in each case one line of Table A.
Table 75
Compounds of the formula I.3, where
R3 = cyclopropyl;
40 Rs = isopropyl;
R4 = in each case one line of Table A.
Table 76
Compounds of the formula I.4, where
45 R3 = cyclopropyl;
R5 = isopropyl;
.,
CA 02231661 1998-04-07
, ' 0050/4627 1
34
R4 = in each case one line of Table A.
Table 77
Compounds of the formula I.1, where
5 R3 = cyclopropyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
Table 78
10 Compounds of the formula I.2, where
R3 = cyclopropyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
15 Table 79
Compounds of the formula I.3, where
R3 = cyclopropyl;
Rs = cyclopropyl;
R4 = in each case one line of Table A.
Table 80
Compounds of the formula I.4, where
R3 = cyclopropyl;
R5 = cyclopropyl;
25 R4 = in each case one line of Table A.
Table 81
Compounds of the formula I.l, where
R3 = cyclopropyl;
30 R5 = n-butyl;
R4 = in each case one line of Table A.
Table 82
Compounds of the formula I.2, where
35 R3 = cyclopropyl;
R5 = n-butyl;
R4 = in each case one line of Table A.
Table 83
40 Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = n-butyl;
R4 = in each case one line of Table A.
45 Table 84
Compounds of the formula I.4, where
R3 = cyclopropyl;
CA 02231661 1998-04-07
0051~/ 46~7 1
R5 = n-butyl;
R4 = in each case one line o:f Table A.
Table 85
5 Compounds of the formula I.1, where
R3 = cyclopropyl;
R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
10 Table 86
Compounds of the formula I.2, where
R3 = cyclopropyl;
RS = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 87
Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = 2-methoxyeth-1-yl;
20 R4 = in each case one line of Table A.
Table 88
Compounds of the formula I.4, where
R3 = cyclopropyl;
25 Rs = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 89
Compounds of the formula I.1, where
30 R3 = cyclopropyl;
RS = prop-2-en-l-yl;
R4 = in each case one line of Table A.
Table 90
35 Compounds of the formula I.2, where
R3 = cyclopropyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
40 Table 91
Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 92
Compounds of the formula I.4, where
CA 02231661 1998-04-07
0050/ 4627 1
R3 = cyclopropyl;
Rs = prop-2-en-1-yl;
R4 = in each case one line of Table A.
5 Table 93
Compounds of the formula I.l, where
R3 = cyclopropyl;
R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 94
Compounds of the formula I.2, where
R3 = cyclopropyl;
R5 = E-but-2-en-1-yl;
15 R4 = in each case one line of Table A.
Table 95
Compounds of the formula I.3 t where
R3 = cyclopropyl;
20 R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 96
Compounds of the formula I.4, where
25 R3 = cyclopropyl;
R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 97
30 Compounds of the formula I.l, where
R3 = cyclopropyl;
RS = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
35 Table 98
Compounds of the formula I.2, where
R3 = cyclopropyl;
R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 99
Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = Z-but-2-en-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
~050/46~71
Table 100
Compounds of the formula I.4, where
R3 = cyclopropyl;
R5 = Z-but-2-en-1-yl;
5 R4 = in each case one line of Table A.
Table 101
Compounds of the formula I.1, where
R3 = cyclopropyl;
10 R5 = E-3-chloroprop-2-en-1-y.l;
R4 = in each case one line of Table A.
Table 102
Compounds of the formula I.2, where
15 R3 = cyclopropyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 103
20 Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
25 Table 104
Compounds of the formula I.4, where
R3 = cyclopropyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 105
Compounds of the formula I.1, where
R3 = cyclopropyl;
R5 = Z-3-chloroprop-2-en-l-yl;
35 R4 = in each case one line of Table A.
Table 106
Compounds of the formula I.2, where
R3 = cyclopropyl;
40 R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 107
Compounds of the formula I.3, where
45 R3 = cyclopropyl;
R5 = Z-3-chloroprop-2-en-1-yl;
CA 02231661 1998-04-07
0050/46271
R4 = in each case one line of Table A.
Table 108
Compounds of the formula I.4, where
S R3 = cyclopropyl;
Rs = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 109
lO Compounds of the formula I.l, where
R3 = cyclopropyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
15 Table 110
Compounds of the formula I.2, where
R3 = cyclopropyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 111
Compounds of the formula I.3, where
R3 = cyclopropyl;
R5 = prop-2-yn-1-yl;
25 R4 = in each case one line of Table A.
Table 112
Compounds of the formula I.4, where
R3 = cyclopropyl;
30 R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 113
Compounds of the formula I.l~ where
35 R3 = ethyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
Table 114
40 Compounds of the formula I.2, where
R3 = ethyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
45 Table 115
Compounds of the formula I.3~ where
R3 = ethyl;
CA 02231661 1998-04-07
0050/4627 1
R5 = hydrogen
R4 = in each case one line of Table A.
Table 116
5 Compounds of the formula I.4, where
R3 = ethyl;
Rs = hydrogen;
R4 = in each case one line of Table A.
10 Table 117
Compounds of the formula I.l, where
R3 = ethyl;
R5 = methyl;
R4 = in each case one line of Table A.
Table 118
Compounds of the formula I.2, where
R3 = ethyl;
R5 = methyl;
20 R4 = in each case one line of Table A.
Table 119
Compounds of the formula I.3, where
R3 = ethyl;
25 R5 = methyl;
R4 = in each case one line of Table A.
Table 120
Compounds of the formula I.4, where
30 R3 = ethyl;
Rs = methyl;
R4 = in each case one line of Table A.
Table 121
35 Compounds of the formula I.1, where
R3 = ethyl;
R5 = ethyl;
R4 = in each case one line of Table A.
40 Table 122
Compounds of the formula I.2, where
R3 = ethyl;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 123
Compounds of the formula I.3, where
CA 02231661 1998-04-07
OOSO/46i!7 1
R3 = ethyl;
R5 = ethyl;
R4 = in each case one line of Table A.
5 Table 124
Compounds of the formula I.4, where
R3 = ethyl;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 125
Compounds of the formula I.l, where
R3 = ethyl;
R5 = n-propyl;
15 R4 = in each case one line of Table A.
Table 126
Compounds of the formula I.2, where
R3 = ethyl;
20 R5 = n-propyl;
R4 = in each case one line of Table A.
Table 127
Compounds of the formula I.3, where
25 R3 = ethyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
Table 128
30 Compounds of the formula I.4, where
R3 = ethyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
35 Table 129
Compounds of the formula I.1, where
R3 = ethyl;
R5 = isopropyl;
R4 = in each case one line of Table A.
Table 130
Compounds of the formula I.2, where
R3 = ethyl;
R5 = isopropyl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/4627
Table 131
Compounds of the formula I.3, where
R3 = ethyl;
R5 = isopropyl;
5 R4 = in each case one line of Table A.
Table 132
Compounds of the formula I.4, where
R3 = ethyl;
lO R5 = isopropyl;
R4 = in each case one line of Table A.
Table 133
Compounds of the formula I.1, where
15 R3 = ethyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
Table 134
20 Compounds of the formula I.2, where
R3 = ethyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
25 Table 135
Compounds of the formula I.3, where
R3 = ethyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
Table 136
Compounds of the formula I.4, where
R3 = ethyl;
R5 = cyclopropyl;
35 R4 = in each case one line of Table A.
Table 137
Compounds of the formula I.l, where
R3 = ethyl;
40 R5 = n-butyl;
R4 = in each case one line of Table A.
Table 138
Compounds of the formula I.2, where
45 R3 = ethyl;
R5 = n-butyl;
CA 02231661 1998-04-07
0050~ 4627 1
R4 = in each case one line of Table A.
Table 139
Compounds of the formula I.3, where
5 R3 = ethyl;
R5 = n-butyl;
R4 = in each case one line of Table A.
Table 140
lO Compounds of the formula I.4, where
R3 = ethyl;
R5 = n-butyl;
R4 = in each case one line of Table A.
15 Table 141
Compounds of the formula I.1, where
R3 = ethyl;
Rs = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 142
Compounds of the formula I.2, where
R3 = ethyl;
R5 = 2-methoxyeth-1-yl;
25 R4 = in each case one line of Table A.
Table 143
Compounds of the formula I.3, where
R3 = ethyl;
30 R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 144
Compounds of the formula I.4, where
35 R3 = ethyl;
R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 145
40 Compounds of the formula I.l, where
R3 = ethyl;
RS = prop-2-en-1-yl;
R4 = in each case one line of Table A.
45 Table 146
Compounds of the formula I.2, where
R3 = ethyl;
CA 02231661 1998-04-07
OOSO/ 4~Z7 1
43
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 147
5 Compounds of the formula I.3, where
R3 = ethyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
10 Table 148
Compounds of the formula I.4, where
R3 = ethyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 149
Compounds of the formula I.l, where
R3 = ethyl;
Rs = E-but-2-en-1-yl;
20 R4 = in each case one line of Table A.
Table 150
Compounds of the formula I.2, where
R3 = ethyl;
25 Rs = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 151
Compounds of the formula I.3, where
30 R3 = ethyl;
R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 152
35 Compounds of the formula I.4, where
R3 = ethyl;
R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
40 Table 153
Compounds of the formula I.l, where
R3 = ethyl;
R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 154
Compounds of the formula I.2, where
CA 02231661 1998-04-07
0050/46271
R3 = ethyl;
R5 = Z-but-2-en-l-yl;
R4 = in each case one line of Table A.
5 Table 155
Compounds of the formula I.3, where
R3 = ethyl;
R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 156
Compounds of the formula I.4, where
R3 = ethyl;
R5 = Z-but-2-en-1-yl;
15 R4 = in each case one line of Table A.
Table 157
Compounds of the formula I.l, where
R3 = ethyl;
20 R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 158
Compounds of the formula I.2, where
25 R3 = ethyl;
Rs = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 159
30 Compounds of the formula I.3 t where
R3 = ethyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
35 Table 160
Compounds of the formula I.4, where
R3 = ethyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 161
Compounds of the formula I.l, where
R3 = ethyl;
R5 = Z- 3-chloroprop-2-en-1-yl;
45 R4 = in each case one line of Table A.
CA 0223l66l l998-04-07
0050/46Z71
Table 162
Compounds of the formula I.2, where
R3 = ethyl;
R5 = Z-3-chloroprop-2-en-1-y:l;
5 R4 = in each case one line of Table A.
Table 163
Compounds of the formula I.3, where
R3 = ethyl;
10 R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 164
Compounds of the formula I.4, where
15 R3 = ethyl;
R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 165
20 Compounds of the formula I.l, where
R3 = ethyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
25 Table 166
Compounds of the formula I.2, where
R3 = ethyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 167
Compounds of the formula I.3, where
R3 = ethyl;
R5 = prop-2-yn-1-yl;
35 R4 = in each case one line of Table A.
Table 168
Compounds of the formula I.4, where
R3 = ethyl;
40 R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 169
Compounds of the formula I.l, where
45 R3 = trifluoromethyl;
R5 = hydrogen;
CA 02231661 1998-04-07
005U/ 4t~7 1
46
R4 = in each case one line of Table A.
Table 170
Compounds of the formula I.2, where
5 R3 = trifluoromethyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
Table 171
10 Compounds of the formula I.:3, where
R3 = trifluoromethyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
15 Table 172
Compounds of the formula I.4, where
R3 = trifluoromethyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
Table 173
Compounds of the formula I.l, where
R3 = trifluoromethyl;
R5 = methyl;
25 R4 = in each case one line of Table A.
Table 174
Compounds of the formula I.2, where
R3 = trifluoromethyl;
30 R5 = methyl;
R4 = in each case one line of Table A.
Table 175
Compounds of the formula I.3, where
35 R3 = trifluoromethyl;
R5 = methyl;
R4 = in each case one line of Table A.
Table 176
40 Compounds of the formula I.4, where
R3 = trifluoromethyl;
R5 = methyl;
R4 = in each case one line of Table A.
45 Table 177
Compounds of the formula I.l, where
R3 = trifluoromethyl;
CA 02231661 1998-04-07
UU:~U/ 4~
47
R5 = ethyl;
R4 = in each case one line of Table A.
Table 178
5 Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = ethyl;
R4 = in each case one line of Table A.
10 Table 179
Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 180
Compounds of the formula I.4, where
R3 = trifluoromethyl;
R5 = ethyl;
20 R4 = in each case one line of Table A.
Table 181
Compounds of the formula I.1, where
R3 = trifluoromethyl;
25 R5 = n-propyl;
R4 = in each case one line of Table A.
Table 182
Compounds of the formula I.2, where
30 R3 = trifluoromethyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
Table 183
35 Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
40 Table 184
Compounds of the formula I.4, where
R3 = trifluoromethyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
Table 185
Compounds of the formula I.l, where
CA 02231661 1998-04-07
0~51~/ 4~7 1
48
R3 = trifluoromethyl;
R5 = isopropyl;
R4 = in each case one line of Table A.
S Table 186
Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = isopropyl;
R4 = in each case one line of Table A.
Table 187
Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = isopropyl;
15 R4 = in each case one line of Table A.
Table 188
Compounds of the formula I.4, where
R3 = trifluoromethyl;
20 R5 = isopropyl;
R4 = in each case one line of Table A.
Table 189
Compounds of the formula I.l, where
25 R3 = trifluoromethyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
Table 190
30 Compounds of the formula I.2, where
R3 = trifluoromethyl;
Rs = cyclopropyl;
R4 = in each case one line of Table A.
35 Table 191
Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
Table 192
Compounds of the formula I.4, where
R3 = trifluoromethyl;
R5 = cyclopropyl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/46271
49
Table 193
Compounds of the formula I.1, where
R3 = trifluoromethyl;
R5 = n-butyl;
5 R4 = in each case one line of Table A.
Table 194
Compounds of the formula I.2, where
R3 = trifluoromethyl;
lO RS = n-butyl;
R4 = in each case one line of Table A.
Table 195
Compounds of the formula I.3, where
15 R3 = trifluoromethyl;
R5 = n-butyl;
R4 = in each case one line of Table A.
Table 196
20 Compounds of the formula I.4, where
R3 = trifluoromethyl;
R5 = n-butyl;
R4 = in each case one line of Table A.
25 Table 197
Compounds of the formula I.1, where
R3 = trifluoromethyl;
R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 198
Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = 2-methoxyeth-1-yl;
35 R4 = in each case one line of Table A.
Table 199
Compounds of the formula I.3, where
R3 = trifluoromethyl;
40 R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 200
Compounds of the formula I.4, where
45 R3 = trifluoromethyl;
R5 = 2-methoxyeth-1-yl;
CA 0223l66l l998-04-07
UU~U/4~
R4 = in each case one line of Table A.
Table 201
Compounds of the formula I.1, where
5 R3 = trifluoromethyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 202
10 Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
15 Table 203
Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 204
Compounds of the formula I.4, where
R3 = trifluoromethyl;
R5 = prop-2-en-1-yl;
25 R4 = in each case one line of Table A.
Table 205
Compounds of the formula I.1, where
R3 = trifluoromethyl;
30 R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 206
Compounds of the formula I.2, where
35 R3 = trifluoromethyl;
R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 207
40 Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
45 Table 208
Compounds of the formula I.4, where
R3 = trifluoromethyl;
CA 02231661 1998-04-07
~U:~U/ 4t~7 1
Rs = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 209
5 Compounds of the formula I.1, where
R3 = trifluoromethyl;
Rs = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
10 Table 210
Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 211
Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = Z-but-2-en-1-yl;
20 R4 = in each case one line of Table A.
Table 212
Compounds of the formula I.4, where
R3 = trifluoromethyl;
25 R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 213
Compounds of the formula I.l, where
30 R3 = trifluoromethyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 214
35 Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
40 Table 215
Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 216
Compounds of the formula I.4, where
CA 02231661 1998-04-07
0050/46271
R3 = trifluoromethyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
5 Table 217
Compounds of the formula I.l, where
R3 = trifluoromethyl;
R5 = Z-3-chloroprop-2-en-1-y:L;
R4 = in each case one line of Table A.
Table 218
Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = Z-3-chloroprop-2-en-1-y:L;
15 R4 = in each case one line of Table A.
Table 219
Compounds of the formula I.3, where
R3 = trifluoromethyl;
20 R5 = Z-3-chloroprop-2-en-1-y:L;
R4 = in each case one line of Table A.
Table 220
Compounds of the formula I.4, where
25 R3 = trifluoromethyl;
R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 221
30 Compounds of the formula I.l, where
R3 = trifluoromethyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
35 Table 222
Compounds of the formula I.2, where
R3 = trifluoromethyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 223
Compounds of the formula I.3, where
R3 = trifluoromethyl;
R5 = prop-2-yn-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
OO50/ 4627 1
53
Table 224
Compounds of the formula I.4, where
R3 = trifluoromethyl;
S R5 = prop-2-yn-1-yl;
R4 = in each case one line o:E Table A.
Table 225
Compounds of the formula I.1, where
10 R3 = methyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
Table 226
15 Compounds of the formula I.2, where
R3 = methyl;
R5 = hydrogen;
R4 = in each case one line of Table A.
20 Table 227
Compounds of the formula I.3, where
R3 = methyl;
R5 = hydrogen;
R4 = in each case one line o:E Table A.
Table 228
Compounds of the formula I.4, where
R3 = methyl;
R5 = hydrogen;
30 R4 = in each case one line of Table A.
Table 229
Compounds of the formula I.1, where
R3 = methyl;
35 R5 = methyl;
R4 = in each case one line of Table A.
Table 230
Compounds of the formula I.2, where
40 R3 = methyl;
R5 = methyl;
R4 = in each case one line of Table A.
Table 231
45 Compounds of the formula I.3, where
R3 = methyl;
R5 = methyl;
CA 02231661 1998-04-07
OOSO/4627 1
R4 = in each case one line of Table A.
Table 232
Compounds of the formula I.4, where
S R3 = methyl;
R5 = methyl;
R4 = in each case one line of Table A.
Table 233
10 Compounds of the formula I.l, where
R3 = methyl;
R5 = ethyl;
R4 = in each case one line of Table A.
15 Table 234
Compounds of the formula I.2, where
R3 = methyl;
R5 = ethyl;
R4 = in each case one line of Table A.
Table 235
Compounds of the formula I.3, where
R3 = methyl;
R5 = ethyl;
25 R4 = in each case one line of Table A.
Table 236
Compounds of the formula I.4, where
R3 = methyl;
30 R5 = ethyl;
R4 = in each case one line of Table A.
Table 237
Compounds of the formula I.1, where
35 R3 = methyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
Table 238
40 Compounds of the formula I.2, where
R3 = methyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
45 Table 239
Compounds of the formula I.3, where
R3 = methyl;
CA 02231661 1998-04-07
OOSO/4627 1
R5 = n-propyl;
R4 = in each case one line of Table A.
Table 240
5 Compounds of the formula I.4, where
R3 = methyl;
R5 = n-propyl;
R4 = in each case one line of Table A.
10 Table 241
Compounds of the formula I.1, where
R3 = methyl;
Rs = isopropyl;
R4 = in each case one line of Table A.
Table 242
Compounds of the formula I.2, where
R3 = methyl;
R5 = isopropyl;
20 R4 = in each case one line of Table A.
Table 243
Compounds of the formula I.3, where
R3 = methyl;
25 RS = isopropyl;
R4 = in each case one line of Table A.
Table 244
Compounds of the formula I.4, where
30 R3 = methyl;
R5 = isopropyl;
R4 = in each case one line of Table A.
Table 245
35 Compounds of the formula I.1, where
R3 = methyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
40 Table 246
Compounds of the formula I.2, where
R3 = methyl;
R5 = cyclopropyl;
R4 = in each case one line oi- Table A.
Table 247
Compounds of the formula I.3, where
CA 02231661 1998-04-07
0050/4627 1
56
R3 = methyl;
R5 = cyclopropyl;
R4 = in each case one line of Table A.
5 Table 248
Compounds of the formula I.4, where
R3 = methyl;
Rs = cyclopropyl;
R4 = in each case one line o:f Table A.
Table 249
Compounds of the formula I.1, where
R3 = methyl;
R5 = n-butyl;
15 R4 = in each case one line of Table A.
Table 250
Compounds of the formula I.2, where
R3 = methyl;
20 R5 = n-butyl;
R4 = in each case one line of Table A.
Table 251
Compounds of the formula I.3, where
25 R3 = methyl;
R5 = n-butyl;
R4 = in each case one line of Table A.
Table 252
30 Compounds of the formula I.4, where
R3 = methyl;
Rs = n-butyl;
R4 = in each case one line of Table A.
35 Table 253
Compounds of the formula I.1, where
R3 = methyl;
Rs = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 254
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-methoxyeth-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
OOSO/4627
Table 255
Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-methoxyeth-1-yl;
5 R4 = in each case one line of Table A.
Table 256
Compounds of the formula I.4, where
R3 = methyl;
10 R5 = 2-methoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 257
Compounds of the formula I.l, where
15 R3 = methyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of. Table A.
Table 258
20 Compounds of the formula I.2, where
R3 = methyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
25 Table 259
Compounds of the formula I.3 r where
R3 = methyl;
R5 = prop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 260
Compounds of the formula I.4, where
R3 = methyl;
R5 = prop-2-en-1-yl;
35 R4 = in each case one line of Table A.
Table 261
Compounds of the formula I.l, where
R3 = methyl;
40 R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 262
Compounds of the formula I.2, where
45 R3 = methyl;
R5 = E-but-2-en-1-yl;
CA 02231661 1998-04-07
0050/4627 1
R4 = in each case one line of Table A.
Table 263
Compounds of the formula I.3, where
5 R3 = methyl;
Rs = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 264
10 Compounds of the formula I.4, where
R3 = methyl;
R5 = E-but-2-en-1-yl;
R4 = in each case one line of Table A.
15 Table 265
Compounds of the formula I.l, where
R3 = methyl;
R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 266
Compounds of the formula I.2, where
R3 = methyl;
Rs = Z-but-2-en-1-yl;
25 R4 = in each case one line of Table A.
Table 267
Compounds of the formula I.3, where
R3 = methyl;
30 R5 = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 268
Compounds of the formula I.4, where
35 R3 = methyl;
Rs = Z-but-2-en-1-yl;
R4 = in each case one line of Table A.
Table 269
40 Compounds of the formula I.1, where
R3 = methyl;
Rs = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
45 Table 270
Compounds of the formula I.2, where
R3 = methyl;
CA 02231661 1998-04-07
0050/4627 1
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 271
5 Compounds of the formula I.3, where
R3 = methyl;
R5 = E-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
10 Table 272
Compounds of the formula I.4, where
R3 = methyl;
R5 = E-3-chloroprop-2-en-1-y.l;
R4 = in each case one line of Table A.
Table 273
Compounds of the formula I.1, where
R3 = methyl;
R5 = Z-3-chloroprop-2-en-1-yl;
20 R4 = in each case one line of Table A.
Table 274
Compounds of the formula I.2, where
R3 = methyl;
25 R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 275
Compounds of the formula I.3, where
30 R3 = methyl;
R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 276
35 Compounds of the formula I.4, where
R3 = methyl;
R5 = Z-3-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
40 Table 277
Compounds of the formula I.1, where
R3 = methyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 278
Compounds of the formula I.2, where
CA 02231661 1998-04-07
OOSO/4627 1
R3 = methyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
5 Table 279
Compounds of the formula I.3, where
R3 = methyl;
R5 = prop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 280
Compounds of the formula I.4, where
R3 = methyl;
R5 = prop-2-yn-1-yl;
15 R4 = in each case one line of Table A.
Table 281
Compounds of the formula I.1, where
R3 = methyl;
20 R5 = 2-butyl;
R4 = in each case one line of Table A.
Table 282
Compounds of the formula I.2, where
25 R3 = methyl;
R5 = 2-butyl;
R4 = in each case one line of Table A.
Table 283
30 Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-butyl;
R4 = in each case one line of Table A.
35 Table 284
Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-butyl;
R4 = in each case one line of Table A.
Table 285
Compounds of the formula I.l, where
R3 = methyl;
R5 = 2-methylprop-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/4627
Table 286
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-methylprop-l-yl;
5 R4 = in each case one line of Table A.
Table 287
Compounds of the formula I.3,. where
R3 = methyl;
10 R5 = 2-methylprop-1-yl;
R4 = in each case one line of Table A.
Table 288
Compounds of the formula I.4, where
15 R3 = methyl;
R5 = 2-methylprop-1-yl;
R4 = in each case one line of Table A.
Table 289
20 Compounds of the formula I.l r where
R3 = methyl;
R5 = l,l-dimethyleth-l-yl;
R4 = in each case one line of Table A.
25 Table 290
Compounds of the formula I.2, where
R3 = methyl;
R5 = l,l-dimethyleth-l-yl;
R4 = in each case one line of Table A.
Table 291
Compounds of the formula I.3, where
R3 = methyl;
R5 = 1,1-dimethyleth-1-yl;
35 R4 = in each case one line of Table A.
Table 292
Compounds of the formula I.4, where
R3 = methyl;
40 R5 = l,l-dimethyleth-l-yl;
R4 = in each case one line of Table A.
Table 293
Compounds of the formula I.l, where
45 R3 = methyl;
R5 = l-pentyl;
CA 02231661 1998-04-07
0050/46271
R4 = in each case one line of Table A.
Table 294
Compounds of the formula I.2, where
5 R3 = methyl;
Rs = 1-pentyl;
R4 = in each case one line of Table A.
Table 295
10 Compounds of the formula I.3, where
R3 = methyl;
R5 = 1-pentyl;
R4 = in each case one line of Table A.
15 Table 296
Compounds of the formula I.4, where
R3 = methyl;
R5 = 1-pentyl;
R4 = in each case one line of Table A.
Table 297
Compounds of the formula I.1, where
R3 = methyl;
R5 = 3-methylbut-1-yl;
25 R4 = in each case one line of Table A.
Table 298
Compounds of the formula I.2, where
R3 = methyl;
30 R5 = 3-methylbut-1-yl;
R4 = in each case one line of Table A.
Table 299
Compounds of the formula I.3, where
35 R3 = methyl;
R5 = 3-methylbut-1-yl;
R4 = in each case one line of Table A.
Table 300
40 Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-methylbut-1-yl;
R4 = in each case one line of Table A.
45 Table 301
Compounds of the formula I.1, where
R3 = methyl;
CA 02231661 1998-04-07
0050/46271
63
R5 = 2,2-dimethylprop-1-yl;
R4 = in each case one line of Table A.
Table 302
5 Compounds of the formula I.2, where
R3 = methyl;
R5 = 2,2-dimethylprop-1-yl;
R4 = in each case one line of Table A.
10 Table 303
Compounds of the formula I.3, where
R3 = methyl;
R5 = 2,2-dimethylprop-1-yl;
R4 = in each case one line of Table A.
Table 304
Compounds of the formula I.4, where
R3 = methyl;
R5 = 2,2-dimethylprop-1-yl;
20 R4 = in each case one line of Table A.
Table 305
Compounds of the formula I.1, where
R3 = methyl;
25 R5 = 2-methylbut-1-yl;
R4 = in each case one line of Table A.
Table 306
Compounds of the formula I.2, where
30 R3 = methyl;
R5 = 2-methylbut-1-yl;
R4 = in each case one line of Table A.
Table 307
35 Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-methylbut-1-yl;
R4 = in each case one line of Table A.
40 Table 308
Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-methylbut-1-yl;
R4 = in each case one line of Table A.
Table 309
Compounds of the formula I.1, where
CA 0223l66l l998-04-07
0050/46271
64
R3 = methyl;
R5 = l-methylbut-1-yl;
R4 = in each case one line of Table A.
5 Table 310
Compounds of the formula I.2, where
R3 = methyl;
R5 = l-methylbut-l-yl;
R4 = in each case one line of Table A.
Table 311
Compounds of the formula I.3, where
R3 = methyl;
RS = 1-methylbut-1-yl;
15 R4 = in each case one line of Table A.
Table 312
Compounds of the formula I.4, where
R3 = methyl;
20 R5 = 1-methylbut-1-yl;
R4 = in each case one line of Table A.
Table 313
Compounds of the formula I.1, where
25 R3 = methyl;
R5 = 3-pentyl;
R4 = in each case one line of Table A.
Table 314
30 Compounds of the formula I.2, where
R3 = methyl;
R5 = 3-pentyl;
R4 = in each case one line of Table A.
35 Table 315
Compounds of the formula I.3, where
R3 = methyl;
R5 = 3-pentyl;
R4 = in each case one line of Table A.
Table 316
Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-pentyl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/4627
Table 317
Compounds of the formula I.1, where
R3 = methyl;
Rs = 3-methylbut-2-yl;
5 R4 = in each case one line of Table A.
Table 318
Compounds of the formula I.2, where
R3 = methyl;
10 R5 = 3-methylbut-2-yl;
R4 = in each case one line of Table A.
Table 319
Compounds of the formula I.3, where
lS R3 = methyl;
R5 = 3-methylbut-2-yl;
R4 = in each case one line of Table A.
Table 320
20 Compounds of the formula I.4, where
R3 = methyl;
Rs = 3-methylbut-2-yl;
R4 = in each case one line of Table A.
25 Table 321
Compounds of the formula I.1, where
R3 = methyl;
R5 = 2-methylbut-2-yl;
R4 = in each case one line of Table A.
Table 322
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-methylbut-2-yl;
35 R4 = in each case one line of Table A.
Table 323
Compounds of the formula I.3, where
R3 = methyl;
40 R5 = 2-methylbut-2-yl;
R4 = in each case one line of Table A.
Table 324
Compounds of the formula I.4, where
45 R3 = methyl;
R5 = 2-methylbut-2-yl;
CA 02231661 1998-04-07
0050/4627 1
R4 = in each case one line of Table A.
Table 325
Compounds of the formula I.1, where
5 R3 = methyl;
R5 = l-hexyl;
R4 = in each case one line of Table A.
Table 326
10 Compounds of the formula I.2, where
R3 = methyl;
Rs = l-hexyl;
R4 = in each case one line of Table A.
15 Table 327
Compounds of the formula I.3, where
R3 = methyl;
R5 = 1-hexyl;
R4 = in each case one line of Table A.
Table 328
Compounds of the formula I.4, where
R3 = methyl;
R5 = 1-hexyl;
25 R4 = in each case one line of Table A.
Table 329
Compounds of the formula I.l, where
R3 = methyl;
30 Rs = 3,3-dimethylbut-1-yl;
R4 = in each case one line of Table A.
Table 330
Compounds of the formula I.2, where
35 R3 = methyl;
R5 = 3,3-dimethylbut-1-yl;
R4 = in each case one line of Table A.
Table 331
40 Compounds of the formula I.3, where
R3 = methyl;
R5 = 3,3-dimethylbut-1-yl;
R4 = in each case one line of Table A.
45 Table 332
Compounds of the formula I.4, where
R3 = methyl;
CA 02231661 1998-04-07
0050/4627 1
R5 = 3,3-dimethylbut-1-yl;
R4 = in each case one line of Table A.
Table 333
5 Compounds of the formula I.1, where
R3 = methyl;
R5 = 2-ethylbut-1-yl;
R4 = in each case one line of Table A.
10 Table 334
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-ethylbut-1-yl;
R4 = in each case one line of Table A.
Table 335
Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-ethylbut-1-yl;
20 R4 = in each case one line of Table A.
Table 336
Compounds of the formula I.4, where
R3 = methyl;
25 R5 = 2-ethylbut-1-yl;
R4 = in each case one line of Table A.
Table 337
Compounds of the formula I.1, where
30 R3 = methyl;
R5 = l-ethylbut-1-yl;
R4 = in each case one line of Table A.
Table 338
35 Compounds of the formula I.2, where
R3 = methyl;
R5 = 1-ethylbut-1-yl;
R4 = in each case one line of Table A.
40 Table 339
Compounds of the formula I.3, where
R3 = methyl;
R5 = l-ethylbut-1-yl;
R4 = in each case one line of Table A.
Table 340
Compounds of the formula I.4, where
CA 02231661 1998-04-07
50/ 4627 1
68
R3 = methyl;
R5 = l-ethylbut-l-yl;
R4 = in each case one line of Table A.
5 Table 341
Compounds of the formula I.l r where
R3 = methyl;
R5 = 4-methylpent-1-yl;
R4 = in each case one line of Table A.
Table 342
Compounds of the formula I.2, where
R3 = methyl;
R5 = 4-methylpent-1-yl;
15 R4 = in each case one line of Table A.
Table 343
Compounds of the formula I.3, where
R3 = methyl;
20 R5 = 4-methylpent-1-yl;
R4 = in each case one line of Table A.
Table 344
Compounds of the formula I.4 t where
25 R3 = methyl;
R5 = 4-methylpent-1-yl;
R4 = in each case one line of Table A.
Table 345
30 Compounds of the formula I.l,. where
R3 = methyl;
R5 = cyclopropylmethyl;
R4 = in each case one line of Table A.
35 Table 346
Compounds of the formula I.2, where
R3 = methyl;
RS = cyclopropylmethyl;
R4 = in each case one line of Table A.
Table 347
Compounds of the formula I.3, where
R3 = methyl;
R5 = cyclopropylmethyl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050J4627 1
69
Table 348
Compounds of the formula I.4, where
R3 = methyl;
R5 = cyclopropylmethyl;
5 R4 = in each case one line of Table A.
Table 349
Compounds of the formula I.l, where
R3 = methyl;
10 Rs = cyclopentylmethyl;
R4 = in each case one line of Table A.
Table 350
Compounds of the formula I.2, where
15 R3 = methyl;
RS = cyclopentylmethyl;
R4 = in each case one line of Table A.
Table 351
20 Compounds of the formula I.3, where
R3 = methyl;
Rs = cyclopentylmethyl;
R4 = in each case one line of Table A.
25 Table 352
Compounds of the formula I.4, where
R3 = methyl;
R5 = cyclopentylmethyl;
R4 = in each case one line of Table A.
Table 353
Compounds of the formula I.l/ where
R3 = methyl;
Rs = 2-cyclopropyleth-1-yl;
35 R4 = in each case one line of Table A.
Table 354
Compounds of the formula I.2, where
R3 = methyl;
40 Rs = 2-cyclopropyleth-1-yl;
R4 = in each case one line of Table A.
Table 355
Compounds of the formula I.3, where
45 R3 = methyl;
R5 = 2-cyclopropyleth-1-yl;
CA 02231661 1998-04-07
OOSO/4627~
R4 = in each case one line of Table A.
Table 356
Compounds of the formula I.4, where
5 R3 = methyl;
R5 = 2-cyclopropyleth-1-yl;
R4 = in each case one line of Table A.
Table 357
10 Compounds of the formula I.l, where
R3 = methyl;
Rs = 2-cyclopentyleth-1-yl;
R4 = in each case one line of Table A.
15 Table 358
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-cyclopentyleth-1-yl;
R4 = in each case one line of Table A.
Table 359
Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-cyclopentyleth-1-yl;
25 R4 = in each case one line of Table A.
Table 360
Compounds of the formula I.4, where
R3 = methyl;
30 R5 = 2-cyclopentyleth-1-yl;
R4 = in each case one line of Table A.
Table 361
Compounds of the formula I.1, where
35 R3 = methyl;
R5 = 2-cyclohexyleth-1-yl;
R4 = in each case one line of Table A.
Table 362
40 Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-cyclohexyleth-1-yl;
R4 = in each case one line of Table A.
45 Table 363
Compounds of the formula I.3 r where
R3 = methyl;
CA 02231661 1998-04-07
0050/4627 1
R5 = 2-cyclohexyleth-1-yl;
R4 = in each case one line of Table A.
Table 364
5 Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-cyclohexyleth-1-yl;
R4 = in each case one line of Table A.
10 Table 365
Compounds of the formula I.1, where
R3 = methyl;
R5 = fluoromethyl;
R4 = in each case one line of Table A.
Table 366
Compounds of the formula I.2, where
R3 = methyl;
R5 = fluoromethyl;
20 R4 = in each case one line of Table A.
Table 367
Compounds of the formula I.3, where
R3 = methyl;
25 R5 = fluoromethyl;
R4 = in each case one line of Table A.
Table 368
Compounds of the formula I.4, where
30 R3 = methyl;
Rs = fluoromethyl;
R4 = in each case one line of Table A.
Table 369
35 Compounds of the formula I.l, where
R3 = methyl;
Rs = difluoromethyl;
R4 = in each case one line of Table A.
40 Table 370
Compounds of the formula I.2, where
R3 = methyl;
R5 = difluoromethyl;
R4 = in each case one line of Table A.
Table 371
Compounds of the formula I.3, where
CA 02231661 1998-04-07
0050/4627 1
R3 = methyl;
R5 = difluoromethyl;
R4 = in each case one line of Table A.
5 Table 372
Compounds of the formula I.4, where
R3 = methyl;
R5 = difluoromethyl;
R4 = in each case one line of Table A.
Table 373
Compounds of the formula I.1, where
R3 = methyl;
R5 = 2-fluoroeth-1-yl;
15 R4 = in each case one line of Table A.
Table 374
Compounds of the formula I.2, where
R3 = methyl;
20 R5 = 2-fluoroeth-1-yl;
R4 = in each case one line of Table A.
Table 375
Compounds of the formula I.3, where
25 R3 = methyl;
R5 = 2-fluoroeth-1-yl;
R4 = in each case one line of Table A.
Table 376
30 Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-fluoroeth-1-yl;
R4 = in each case one line of Table A.
35 Table 377
Compounds of the formula I.l, where
R3 = methyl;
R5 = 3-fluoroprop-1-yl;
R4 = in each case one line of Table A.
Table 378
Compounds of the formula I.2, where
R3 = methyl;
Rs = 3-fluoroprop-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/46271
Table 379
Compounds of the formula I.3, where
R3 = methyl;
R5 = 3-fluoroprop-1-yl;
5 R4 = in each case one line of Table A.
Table 380
Compounds of the formula I.4, where
R3 = methyl;
10 R5 = 3-fluoroprop-1-yl;
R4 = in each case one line of Table A.
Table 381
Compounds of the formula I.1, where
15 R3 = methyl;
R5 = 2,2-difluoroeth-1-yl;
R4 = in each case one line of Table A.
Table 382
20 Compounds of the formula I.2, where
R3 = methyl;
R5 = 2,2-difluoroeth-1-yl;
R4 = in each case one line of Table A.
25 Table 383
Compounds of the formula I.3, where
R3 = methyl;
RS = 2,2-difluoroeth-1-yl;
R4 = in each case one line of Table A.
Table 384
Compounds of the formula I.4 r where
R3 = methyl;
R5 = 2,2-difluoroeth-1-yl;
35 R4 = in each case one line of Table A.
Table 385
Compounds of the formula I.l, where
R3 = methyl;
40 R5 = 2,2,2-trifluoroeth-1-yl;
R4 = in each case one line of Table A.
Table 386
Compounds of the formula I.2, where
45 R3 = methyl;
R5 = 2,2,2-trifluoroeth-1-yl;
CA 02231661 1998-04-07
0050/4627 1
74
R4 = in each case one line of Table A.
Table 387
Compounds of the formula I.3, where
5 R3 = methyl;
Rs = 2,2,2-trifluoroeth-1-yl;
R4 = in each case one line of Table A.
Table 388
10 Compounds of the formula I.4~ where
R3 = methyl;
R5 = 2,2,2-trifluoroeth-1-yl;
R4 = in each case one line of Table A.
lS Table 389
Compounds of the formula I.l, where
R3 = methyl;
R5 = 2-bromoeth-1-yl;
R4 = in each case one line of Table A.
Table 390
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-bromoeth-1-yl;
25 R4 = in each case one line of Table A.
Table 391
Compounds of the formula I.3, where
R3 = methyl;
30 R5 = 2-bromoeth-1-yl;
R4 = in each case one line of Table A.
Table 392
Compounds of the formula I.4, where
35 R3 = methyl;
R5 = 2-bromoeth-1-yl;
R4 = in each case one line of Table A.
Table 393
40 Compounds of the formula I.1, where
R3 = methyl;
R5 = 3-bromoprop-1-yl;
R4 = in each case one line of Table A.
45 Table 394
Compounds of the formula I.2, where
R3 = methyl;
CA 02231661 1998-04-07
0050/46271
R5 = 3-bromoprop-1-yl;
R4 = in each case one line of Table A.
Table 395
5 Compounds of the formula I.3, where
R3 = methyl;
R5 = 3-bromoprop-1-yl;
R4 = in each case one line of Table A.
10 Table 396
Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-bromoprop-1-yl;
R4 = in each case one line of Table A.
Table 397
Compounds of the formula I.l, where
R3 = methyl;
R5 = 4-bromobut-1-yl;
20 R4 = in each case one line of Table A.
Table 398
Compounds of the formula I.2~ where
R3 = methyl;
25 R5 = 4-bromobut-1-yl;
R4 = in each case one line of Table A.
Table 399
Compounds of the formula I.3, where
30 R3 = methyl;
R5 = 4-bromobut-1-yl;
R4 = in each case one line of Table A.
Table 400
35 Compounds of the formula I.4, where
R3 = methyl;
R5 = 4-bromobut-1-yl;
R4 = in each case one line of Table A.
40 Table 401
Compounds of the formula I.1, where
R3 = methyl;
R5 = 2-iodoeth-1-yl;
R4 = in each case one line of Table A.
Table 402
Compounds of the formula I.2, where
CA 0223l66l l998-04-07
0050~4~271
R3 = methyl;
R5 = 2-iodoeth-1-yl;
R4 = in each case one line of Table A.
S Table 403
Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-iodoeth-1-yl;
R4 = in each case one line of Table A.
Table 404
Compounds of the formula I.4, where
R3 = methyl;
Rs = 2-iodoeth-1-yl;
15 R4 = in each case one line of Table A.
Table 405
Compounds of the formula I.l, where
R3 = methyl;
20 RS = 2-chloroeth-1-yl;
R4 = in each case one line of Table A.
Table 406
Compounds of the formula I.2, where
25 R3 = methyl;
R5 = 2-chloroeth-1-yl;
R4 = in each case one line of Table A.
Table 407
30 Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-chloroeth-1-yl;
R4 = in each case one line of Table A.
35 Table 408
Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-chloroeth-1-yl;
R4 = in each case one line of Table A.
Table 409
Compounds of the formula I.l, where
R3 = methyl;
R5 = 3-chloroprop-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
~050/46271
Table 410
Compounds of the formula I.2, where
R3 = methyl;
R5 = 3-chloroprop-1-yl;
5 R4 = in each case one line of Table A.
Table 411
Compounds of the formula I.3, where
R3 = methyl;
10 R5 = 3-chloroprop-1-yl;
R4 = in each case one line of Table A.
Table 412
Compounds of the formula I.4, where
15 R3 = methyl;
R5 = 3-chloroprop-1-yl;
R4 = in each case one line of Table A.
Table 413
20 Compounds of the formula I.1, where
R3 = methyl;
R5 = 4-chlorobut-1-yl;
R4 = in each case one line of Table A.
25 Table 414
Compounds of the formula I.2, where
R3 = methyl;
R5 = 4-chlorobut-1-yl;
R4 = in each case one line of Table A.
Table 415
Compounds of the formula I.3, where
R3 = methyl;
R5 = 4-chlorobut-1-yl;
35 R4 = in each case one line of Table A.
Table 416
Compounds of the formula I.4, where
R3 = methyl;
40 R5 = 4-chlorobut-1-yl;
R4 = in each case one line of Table A.
Table 417
Compounds of the formula I.l~ where
45 R3 = methyl;
R5 = cyanomethyl;
CA 02231661 1998-04-07
UUSO/4~71
R4 = in each case one line of Table A.
Table 418
Compounds of the formula I.2, where
5 R3 = methyl;
R5 = cyanomethyl;
R4 = in each case one line of. Table A.
Table 419
10 Compounds of the formula I.3, where
R3 = methyl;
R5 = cyanomethyl;
R4 = in each case one line of Table A.
15 Table 420
Compounds of the formula I.4 t where
R3 = methyl;
R5 = cyanomethyl;
R4 = in each case one line of Table A.
Table 421
Compounds of the formula I.ll where
R3 = methyl;
R5 = 2-cyanoeth-1-yl;
25 R4 = in each case one line of Table A.
Table 422
Compounds of the formula I.2 r where
R3 = methyl;
30 R5 = 2-cyanoeth-1-yl;
R4 = in each case one line of Table A.
Table 423
Compounds of the formula I.3 r where
35 R3 = methyl;
RS = 2-cyanoeth-1-yl;
R4 = in each case one line of Table A.
Table 424
40 Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-cyanoeth-1-yl;
R4 = in each case one line of Table A.
45 Table 425
Compounds of the formula I.1, where
R3 = methyl;
CA 02231661 1998-04-07
0050/4627 1
R5 = 3-cyanoprop-1-yl;
R4 = in each case one line of Table A.
Table 426
5 Compounds of the formula I.2, where
R3 = methyl;
R5 = 3-cyanoprop-l-yl;
R4 = in each case one line of Table A.
10 Table 427
Compounds of the formula I.3, where
R3 = methyl;
R5 = 3-cyanoprop-1-yl;
R4 = in each case one line of Table A.
Table 428
Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-cyanoprop-1-yl;
20 R4 = in each case one line of Table A.
Table 429
Compounds of the formula I.1, where
R3 = methyl;
25 R5 = 4-cyanobut-1-yl;
R4 = in each case one line of Table A.
Table 430
Compounds of the formula I.2, where
30 R3 = methyl;
R5 = 4-cyanobut-1-yl;
R4 = in each case one line of Table A.
Table 431
35 Compounds of the formula I.3, where
R3 = methyl;
R5 = 4-cyanobut-1-yl;
R4 = in each case one line of Table A.
40 Table 432
Compounds of the formula I.4, where
R3 = methyl;
R5 = 4-cyanobut-1-yl;
R4 = in each case one line of Table A.
Table 433
Compounds of the formula I.1, where
CA 02231661 1998-04-07
0050/46271
R3 = methyl;
R5 = 2-ethoxyeth-1-yl;
R4 = in each case one line of Table A.
5 Table 434
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-ethoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 435
Compounds of the formula I.3, where
R3 = methyl;
Rs = 2-ethoxyeth-1-yl;
15 R4 = in each case one line of Table A.
Table 436
Compounds of the formula I.4, where
R3 = methyl;
20 R5 = 2-ethoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 437
Compounds of the formula I.1, where
25 R3 = methyl;
R5 = 2-isopropoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 438
30 Compounds of the formula I.2, where
R3 = methyl;
RS = 2-isopropoxyeth-1-yl;
R4 = in each case one line of Table A.
35 Table 439
Compounds of the formula I.3, where
R3 = methyl;
RS = 2-isopropoxyeth-1-yl;
R4 = in each case one line of Table A.
Table 440
Compounds of the formula I.4, where
R3 = methyl;
RS = 2-isopropoxyeth-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/ 4t~7 1
Table 441
Compounds of the formula I.l, where
R3 = methyl;
R5 = 3-methoxyprop-1-yl;
5 R4 = in each case one line of Table A.
Table 442
Compounds of the formula I.2, where
R3 = methyl;
lO R5 = 3-methoxyprop-1-yl;
R4 = in each case one line of Table A.
Table 443
Compounds of the formula I.3, where
15 R3 = methyl;
R5 = 3-methoxyprop-1-yl;
R4 = in each case one line of Table A.
Table 444
20 Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-methoxyprop-1-yl;
R4 = in each case one line of Table A.
25 Table 445
Compounds of the formula I.l, where
R3 = methyl;
R5 = 3-ethoxyprop-1-yl;
R4 = in each case one line of Table A.
Table 446
Compounds of the formula I.2,. where
R3 = methyl;
R5 = 3-ethoxyprop-1-yl;
35 R4 = in each case one line of Table A.
Table 447
Compounds of the formula I.3, where
R3 = methyl;
40 Rs = 3-ethoxyprop-1-yl;
R4 = in each case one line of Table A.
Table 448
Compounds of the formula I.4, where
45 R3 = methyl;
RS = 3-ethoxyprop-1-yl;
CA 02231661 1998-04-07
0050/46271
R4 = in each case one line of Table A.
Table 449
Compounds of the formula I.l, where
5 R3 = methyl;
R5 = 3-isopropoxyprop-1-yl;
R4 = in each case one line of Table A.
Table 450
10 Compounds of the formula I.2, where
R3 = methyl;
R5 = 3-isopropoxyprop-1-yl;
R4 = in each case one line of Table A.
15 Table 451
Compounds of the formula I.3, where
R3 = methyl;
R5 = 3-isopropoxyprop-1-yl;
R4 = in each case one line of Table A.
Table 452
Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-isopropoxyprop-1-yl;
25 R4 = in each case one line of Table A.
Table 453
Compounds of the formula I.l, where
R3 = methyl;
30 R5 = 4-methoxybut-1-yl;
R4 = in each case one line of Table A.
Table 454
Compounds of the formula I.2, where
35 R3 = methyl;
R5 = 4-methoxybut-1-yl;
R4 = in each case one line of Table A.
Table 455
40 Compounds of the formula I.3, where
R3 = methyl;
R5 = 4-methoxybut-1-yl;
R4 = in each case one line of Table A.
45 Table 456
Compounds of the formula I.4, where
R3 = methyl;
CA 02231661 1998-04-07
0050/4627 1
R5 = 4-methoxybut-1-yl;
R4 = in each case one line of Table A.
Table 457
5 Compounds of the formula I.1, where
R3 = methyl;
R5 = 4-ethoxybut-1-yl;
R4 = in each case one line of Table A.
10 Table 458
Compounds of the formula I.2, where
R3 = methyl;
R5 = 4-ethoxybut-1-yl;
R4 = in each case one line of Table A.
Table 459
Compounds of the formula I.3, where
R3 = methyl;
R5 = 4-etho~ybu~-1-yl;
20 R4 = in each case one line of Table A.
Table 460
Compounds of the formula I.4, where
R3 = methyl;
25 R5 = 4-ethoxybut-1-yl;
R4 = in each case one line of Table A.
Table 461
Compounds of the formula I.1, where
30 R3 = methyl;
R5 = 4-isopLo~okybut-1-yl;
R4 = in each case one line of Table A.
Table 462
35 Compounds of the formula I.2, where
R3 = methyl;
R5 = 4-isopropoxybut-1-yl;
R4 = in each case one line of Table A.
40 Table 463
Compounds of the formula I.3, where
R3 = methyl;
R5 = 4-isopropoxybut-1-yl;
R4 = in each case one line of Table A.
Table 464
Compounds of the formula I.4, where
CA 02231661 1998-04-07
OOSO/4627 1
84
R3 = methyl;
R5 = 4-isopropoxybut-1-yl;
R4 = in each case one line of Table A.
5 Table 465
Compounds of the formula I.l, where
R3 = methyl;
R5 = 3-methylbut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 466
Compounds of the formula I.2, where
R3 = methyl;
R5 = 3-methylbut-2-en-1-yl;
15 R4 = in each case one line of Table A.
Table 467
Compounds of the formula I.3, where
R3 = methyl;
20 R5 = 3-methylbut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 468
Compounds of the formula I.4, where
25 R3 = methyl;
R5 = 3-methylbut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 469
30 Compounds of the formula I.l, where
R3 = methyl;
Rs = 2-methylprop-2-en-1-yl;
R4 = in each case one line of Table A.
35 Table 470
Compounds of the formula I.2l where
R3 = methyl;
R5 = 2-methylprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 471
Compounds of the formula I.3~ where
R3 = methyl;
R5 = 2-methylprop-2-en-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/46271
Table 472
Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-methylprop-2-en-1-yl;
5 R4 = in each case one line of Table A.
Table 473
Compounds of the formula I.l, where
R3 = methyl;
10 R5 = but-3-en-1-yl;
R4 = in each case one line of Table A.
Table 474
Compounds of the formula I.2, where
15 R3 = methyl;
R5 = but-3-en-1-yl;
R4 = in each case one line of Table A.
Table 475
20 Compounds of the formula I.3, where
R3 = methyl;
R5 = but-3-en-1-yl;
R4 = in each case one line of Table A.
25 Table 476
Compounds of the formula I.4, where
R3 = methyl;
R5 = but-3-en--1-yl;
R4 = in each case one line of Table A.
Table 477
Compounds of the formula I.l, where
R3 = methyl;
R5 = 2-chloroprop-2-en-1-yl;
35 R4 = in each case one line of Table A.
Table 478
Compounds of the formula I.2, where
R3 = methyl;
40 R5 = 2-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 479
Compounds of the formula I.3, where
45 R3 = methyl;
R5 = 2-chloroprop-2-en-1-yl;
CA 02231661 1998-04-07
OOSO/4627 1
R4 = in each case one line of Table A.
Table 480
Compounds of the formula I.4, where
5 R3 = methyl;
R5 = 2-chloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 481
10 Compounds of the formula I.1, where
R3 = methyl;
R5 = 3,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
15 Table 482
Compounds of the formula I.2, where
R3 = methyl;
R5 = 3,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 483
Compounds of the formula I.3, where
R3 = methyl;
R5 = 3,3-dichloroprop-2-en-1-yl;
25 R4 = in each case one line of Table A.
Table 484
Compounds of the formula I.4, where
R3 = methyl;
30 R5 = 3,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 485
Compounds of the formula I.l, where
35 R3 = methyl;
R5 = 2,3,3-trichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 486
40 Compounds of the formula I.2~ where
R3 = methyl;
R5 = 2,3,3-trichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
45 Table 487
Compounds of the formula I.3, where
R3 = methyl;
CA 0223l66l l998-04-07
. 0050/46271
R5 = 2,3,3-trichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 488
5 Compounds of the formula I.4, where
R3 = methyl;
R5 = 2,3,3-trichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
10 Table 489
Compounds of the formula I.1, where
R3 = methyl;
R5 = Z-2,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 490
Compounds of the formula I.2, where
R3 = methyl;
R5 = Z-2,3-dichloroprop-2-en-1-yl;
20 R4 = in each case one line of Table A.
Table 491
Compounds of the formula I.3, where
R3 = methyl;
25 R5 = Z-2,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 492
Compounds of the formula I.4 t where
30 R3 = methyl;
R5 = Z-2,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 493
35 Compounds of the formula I.l, where
R3 = methyl;
R5 = E-2,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
40 Table 494
Compounds of the formula I.2, where
R3 = methyl;
R5 = E-2,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 495
Compounds of the formula I.3, where
CA 02231661 1998-04-07
0050/46271
88
R3 = methyl;
R5 = E-2,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
5 Table 496
Compounds of the formula I.4, where
R3 = methyl;
RS = E-2,3-dichloroprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 497
Compounds of the formula I.l, where
R3 = methyl;
R5 = Z-3-bromoprop-2-en-1-yl;
15 R4 = in each case one line of Table A.
Table 498
Compounds of the formula I.2, where
R3 = methyl;
20 Rs = Z-3-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 499
Compounds of the formula I.3, where
25 R3 = methyl;
R5 = Z-3-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 500
30 Compounds of the formula I.4, where
R3 = methyl;
R5 = Z-3-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
35 Table 501
Compounds of the formula I.l, where
R3 = methyl;
Rs = E-3-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 502
Compounds of the formula I.2, where
R3 = methyl;
R5 = E-3-bromoprop-2-en-l-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
- 0050/46271
89
Table 503
Compounds of the formula I.3, where
R3 = methyl;
R5 = E-3-bromoprop-2-en-1-yl;
5 R4 = in each case one line of Table A.
Table 504
Compounds of the formula I.4, where
R3 = methyl;
10 R5 = E-3-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 505
Compounds of the formula I.l, where
15 R3 = methyl;
R5 = 2-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 506
20 Compounds of the formula I.2, where
R3 = methyl;
R5 = 2-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
25 Table 507
Compounds of the formula I.3, where
R3 = methyl;
R5 = 2-bromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 508
Compounds of the formula I.4, where
R3 = methyl;
R5 = 2-bromoprop-2-en-1-yl;
35 R4 = in each case one line of Table A.
Table 509
Compounds of the formula I.1, where
R3 = methyl;
40 R5 = 3,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 510
Compounds of the formula I.2, where
45 R3 = methyl;
R5 = 3,3-dibromoprop-2-en-1-yl;
CA 02231661 1998-04-07
0050/4627 1
R4 = in each case one line of Table A.
Table 511
Compounds of the formula I.3, where
S R3 = methyl;
R5 = 3,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 512
10 Compounds of the formula I.4, where
R3 = methyl;
Rs = 3,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
15 Table 513
Compounds of the formula I.1, where
R3 = methyl;
R5 = 2,3,3-tribromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 514
Compounds of the formula I.2, where
R3 = methyl;
R5 = 2,3,3-tribromoprop-2-en-1-yl;
25 R4 = in each case one line of Table A.
Table 515
Compounds of the formula I.3, where
R3 = methyl;
30 R5 = 2,3,3-tribromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 516
Compounds of the formula I.4, where
35 R3 = methyl;
RS = 2,3,3-tribromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 517
40 Compounds of the formula I.1, where
R3 = methyl;
Rs = Z-2,3-dibromoprop-2-en-l-yl;
R4 = in each case one line of Table A.
45 Table 518
Compounds of the formula I.2, where
R3 = methyl;
CA 02231661 1998-04-07
0050/4627 1
R5 = Z-2,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 519
5 Compounds of the formula I.3, where
R3 = methyl;
R5 = Z-2,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
10 Table 520
Compounds of the formula I.4, where
R3 = methyl;
R5 = Z-2,3-dibromoprop-2-en-l-yl;
R4 = in each case one line of Table A.
Table 521
Compounds of the formula I.l, where
R3 = methyl;
Rs = E-2,3-dibromoprop-2-en-1-yl;
20 R4 = in each case one line of Table A.
Table 522
Compounds of the formula I.2, where
R3 = methyl;
25 R5 = E-2,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 523
Compounds of the formula I.3 r where
30 R3 = methyl;
R5 = E-2,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
Table 524
35 Compounds of the formula I.4, where
R3 = methyl;
R5 = E-2,3-dibromoprop-2-en-1-yl;
R4 = in each case one line of Table A.
40 Table 525
Compounds of the formula I.1, where
R3 = methyl;
Rs = E-2-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 526
Compounds of the formula I.2, where
CA 02231661 1998-04-07
0050/46271
R3 = methyl;
R5 = E-2-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
5 Table 527
Compounds of the formula I.3, where
R3 = methyl;
R5 = E-2-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 528
Compounds of the formula I.4, where
R3 = methyl;
R5 = E-2-chlorobut-2-en-1-yl;
15 R4 = in each case one line of Table A.
Table 529
Compounds of the formula I.l, where
R3 = methyl;
20 R5 = Z-2-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 530
Compounds of the formula I.2, where
25 R3 = methyl;
Rs = Z-2-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 531
30 Compounds of the formula I.3, where
R3 = methyl;
Rs = Z-2-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
35 Table 532
Compounds of the formula I.4, where
R3 = methyl;
R5 = Z-2-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 533
Compounds of the formula I.l, where
R3 = methyl;
R5 = E-3-chlorobut-2-en-1-yl;
~ 45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
OOS0/46271
Table 534
Compounds of the formula I.2, where
R3 = methyl;
R5 = E-3-chlorobut-2-en-1-yl;
5 R4 = in each case one line of Table A.
Table 535
Compounds of the formula I.3, where
R3 = methyl;
10 R5 = E-3-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 536
Compounds of the formula I.4, where
15 R3 = methyl;
R5 = E-3-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 537
20 Compounds of the formula I.l, where
R3 = methyl;
R5 = Z-3-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
25 Table 538
Compounds of the formula I.2, where
R3 = methyl;
R5 = Z-3-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 539
Compounds of the formula I.3, where
R3 = methyl;
R5 = Z-3-chlorobut-2-en-1-yl;
35 R4 = in each case one line of Table A.
Table 540
Compounds of the formula I.4, where
R3 = methyl;
40 R5 = Z-3-chlorobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 541
Compounds of the formula I.l, where
45 R3 = methyl;
R5 = E-2-bromobut-2-en-1-yl;
CA 02231661 1998-04-07
0050/46271
94
R4 = in each case one line of Table A.
Table 542
Compounds of the formula I.2, where
5 R3 = methyl;
R5 = E-2-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 543
10 Compounds of the formula I.3, where
R3 = methyl;
R5 = E-2-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
15 Table 544
Compounds of the formula I.4, where
R3 = methyl;
R5 = E-2-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 545
Compounds of the formula I.1, where
R3 = methyl;
R5 = Z-2-bromobut-2-en-1-yl;
25 R4 = in each case one line of Table A.
Table 546
Compounds of the formula I.2, where
R3 = methyl;
30 R5 = Z-2-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 547
Compounds of the formula I.3, where
35 R3 = methyl;
R5 = Z-2-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 548
40 Compounds of the formula I.4, where
R3 = methyl;
R5 = Z-2-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
45 Table 549
Compounds of the formula I.1, where
R3 = methyl;
CA 02231661 1998-04-07
0050/4627 1
RS = E-3-bromobut-2-en-l-yl;
R4 = in each case one line of Table A.
Table 550
5 Compounds of the formula I.2 r where
R3 = methyl;
R5 = E-3-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
10 Table 551
Compounds of the formula I.3, where
R3 = methyl;
R5 = E-3-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 552
Compounds of the formula I.4, where
R3 = methyl;
R5 = E-3-bromobut-2-en-1-yl;
20 R4 = in each case one line of Table A.
Table 553
Compounds of the formula I.1, where
R3 = methyl;
25 R5 = Z-3-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 554
Compounds of the formula I.2, where
30 R3 = methyl;
R5 = Z-3-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
Table 555
35 Compounds of the formula I.3, where
R3 = methyl;
R5 = Z-3-bromobut-2-en-1-yl;
R4 = in each case one line of Table A.
40 Table 556
Compounds of the formula I.4 r where
R3 = methyl;
R5 = Z-3-bromobut-2-en-l-yl;
R4 = in each case one line of Table A.
Table 557
Compounds of the formula I.1 r where
CA 02231661 1998-04-07
OOS0/46271
96
R3 = methyl;
R5 = 3-chloroprop-2-yn-1-yl;
R4 = in each case one line of Table A.
S Table 5S8
Compounds of the formula I.2, where
R3 = methyl;
R5 = 3-chloroprop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 559
Compounds of the formula I.3, where
R3 = methyl;
R5 = 3-chloroprop-2-yn-1-yl;
15 R4 = in each case one line of Table A.
Table 560
Compounds of the formula I.4, where
R3 = methyl;
20 R5 = 3-chloroprop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 561
Compounds of the formula I.l, where
25 R3 = methyl;
R5 = 3-bromoprop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 562
30 Compounds of the formula I.2, where
R3 = methyl;
RS = 3-bromoprop-2-yn-1-yl;
R4 = in each case one line of Table A.
35 Table 563
Compounds of the formula I.3, where
R3 = methyl;
R5 = 3-bromoprop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 564
Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-bromoprop-2-yn-1-yl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050/46271
Table 565
Compounds of the formula I.l, where
R3 = methyl;
Rs = 3-iodoprop-2-yn-1-yl;
5 R4 = in each case one line of Table A.
Table 566
Compounds of the formula I.2, where
R3 = methyl;
10 R5 = 3-iodoprop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 567
Compounds of the formula I.3, where
lS R3 = methyl;
R5 = 3-iodoprop-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 568
20 Compounds of the formula I.4, where
R3 = methyl;
R5 = 3-iodoprop-2-yn-1-yl;
R4 = in each case one line of Table A.
25 Table 569
Compounds of the formula I.l, where
R3 = methyl;
RS = but-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 570
Compounds of the formula I.2, where
R3 = methyl;
R5 = but-2-yn-1-yl;
35 R4 = in each case one line of Table A.
Table 571
Compounds of the formula I.3, where
R3 = methyl;
40 R5 = but-2-yn-1-yl;
R4 = in each case one line of Table A.
Table 572
Compounds of the formula I.4, where
45 R3 = methyl;
R5 = but-2-yn-1-yl;
CA 02231661 1998-04-07
0050/4627 1
R4 = in each case one line of Table A.
Table 573
Compounds of the formula I.l, where
5 R3 = methyl;
R5 = but-3-yn-1-yl;
R4 = in each case one line of Table A.
Table 574
10 Compounds of the formula I.2 r where
R3 = methyl;
R5 = but-3-yn-1-yl;
R4 = in each case one line of Table A.
15 Table 575
Compounds of the formula I.3, where
R3 = methyl;
R5 = but-3-yn-1-yl;
R4 = in each case one line of Table A.
Table 576
Compounds of the formula I.4, where
R3 = methyl;
R5 = but-3-yn-1-yl;
25 R4 = in each case one line of Table A.
Table 577
Compounds of the formula I.l, where
R3 = methyl;
30 R5 = but-3-yn-2-yl;
R4 = in each case one line of Table A.
Table 578
Compounds of the formula I.2, where
35 R3 = methyl;
R5 = but-3-yn-2-yl;
R4 = in each case one line of Table A.
Table 579
40 Compounds of the formula I.3, where
R3 = methyl;
R5 = but-3-yn-2-yl;
R4 = in each case one line of Table A.
45 Table 580
Compounds of the formula I.4, where
R3 = methyl;
CA 02231661 1998-04-07
OOSO~ 4627 1
Rs = but-3-yn-2-yl;
R4 = in each case one line of Table A.
Table 581
5 Compounds of the formula I.l, where
R3 = methyl;
R5 = pent-3-yn-1-yl;
R4 = in each case one line of Table A.
10 Table 582
Compounds of the formula I.2, where
R3 = methyl;
R5 = pent-3-yn-1-yl;
R4 = in each case one line of Table A.
Table 583
Compounds of the formula I.3, where
R3 = methyl;
RS = pent-3-yn-1-yl;
20 R4 = in each case one line of Table A.
Table 584
Compounds of the formula I.4, where
R3 = methyl;
25 R5 = pent-3-yn-1-yl;
R4 = in each case one line of Table A.
Table 585
Compounds of the formula I.1, where
30 R3 = methyl;
R5 = pent-4-yn-1-yl;
R4 = in each case one line of Table A.
Table 586
35 Compounds of the formula I.2, where
R3 = methyl;
R5 = pent-4-yn-1-yl;
R4 = in each case one line of Table A.
40 Table 587
Compounds of the formula I.3, where
R3 = methyl;
R5 = pent-4-yn-1-yl;
R4 = in each case one line of Table A.
Table 588
Compounds of the formula I.4, where
CA 02231661 1998-04-07
OOSO/46271
100
R3 = methyl;
R5 = pent-4-yn-1-yl;
R4 = in each case one line of Table A.
5 Table 589
Compounds of the formula I.l, where
R3 = methyl;
R5 = pent-3-yn-2-yl;
R4 = in each case one line of Table A.
Table S90
Compounds of the formula I.2, where
R3 = methyl;
R5 = pent-3-yn-2-yl;
15 R4 = in each case one line of Table A.
Table S91
Compounds of the formula I.3l where
R3 = methyl;
20 R5 = pent-3-yn-2-yl;
R4 = in each case one line of Table A.
Table 592
Compounds of the formula I.4, where
25 R3 = methyl;
R5 = pent-3-yn-2-yl;
R4 = in each case one line of Table A.
Table 593
30 Compounds of the formula I.l, where
R3 = methyl;
R5 = cyclohexylmethyl;
R4 = in each case one line of Table A.
35 Table 594
Compounds of the formula I.2, where
R3 = methyl;
R5 = cyclohexylmethyl;
R4 = in each case one line of Table A.
Table S9S
Compounds of the formula I.3, where
R3 = methyl;
R5 = cyclohexylmethyl;
45 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050,/4627 1
101
Table 596
Compounds of the formula I.4, where
R3 = methyl;
Rs = cyclohexylmethyl;
5 R4 = in each case one line of Table A.
CA 02231661 1998-04-07
0050,/46271
102
Table A
No. R4
1 H
2 CH3
3 C2Hs
4 n-C3H7
10 5 i-C3H7
6 cyclopropyl
7 n-C4Hg
8 s-C4Hg
15 9 i-C4Hg
t-C4Hg
11 n-C5H
12 i-C5H
20 13 neo-CsHll
14 cyclopentyl
n-C6H13
16 cyclohexyl
17 cyclobutyl
18 CH2CH2Cl
19 (CH2)4Cl
CH2CN
21 CH2CH2CN
30 22 (CH2)3cN
23 (CH2)4CN
24 (CH2)6cN
cyclohexylmethyl
35 26 2-cyclohexyleth-1-yl
27 cyclopropylmethyl
28 2-cyclopropyleth-1-yl
29 2-methoxyeth-1-yl
40 30 2-ethoxyeth-1-yl
31 2-isopropoxyeth-1-yl
32 3-methoxyprop-1-yl
33 3-ethoxyprop-1-yl
34 3-isopropoxyprop-1-yl
4-methoxybut-1-yl
36 4-isopropoxybut-1-yl
CA 02231661 1998-04-07
0050~'4627 1
103
No. R4
37 prop-2-en-1-yl
5 38 but-2-en-1-yl
39 3-methylbut-2-en-1-yl
2-vinyloxyeth-1-yl
41 allyloxyeth-1-yl
42 2-trifluoromethoxyeth-1-yl
10 43 3-trifluoromethoxyprop-1-yl
44 4-difluoromethoxybut-1-yl
hydroxycarbonylmethyl
46 methoxycarbonylmethyl
15 47 aminocarbonylmethyl
48 N-methylaminocarbonylmethyl
49 N,N-dimethylaminocArbonylmethyl
2-hydroxycarbonyleth-1-yl
20 51 2-methoxycarbonyleth-1-yl
52 2-aminocarbonyleth-1-yl
53 2-N-methylaminocarbonyleth-1-yl
54 2-dimethylaminocarbonyleth-1-yl
2-aminoeth-1-yl
25 56 2-aminoprop-1-yl
57 4 -Ar' i nobut- 1--yl
58 3-dimethylaminoprop-1-yl
59 4-aminothiocarbonylbut-1-yl
30 60 E-3-chloLo~Lo~-2-en-1-yl
61 Z-3-chloroprop-2-en-1-yl
62 prop-2-yn-1-yl
63 but-2-yn-1-yl
35 64 but-3-yn-1-yl
3-chloroprop-2-yn-1-yl
66 6-aminocarbonylhex-1-yl
67 3-aminothiocarbonylprop-1-yl
68 2-aminothiocarbonyleth-1-yl
69 aminothiocarbonylmethyl
4-(N,N-dimethylamino)but-1-yl
71 2-(methylthio)eth-1-yl
72 2-(methylsulfonyl)eth-1-yl
45 73 4-(methylthio)prop-1-yl
74 4-(methylsulfonyl)prop-1-yl
CA 02231661 1998-04-07
0050 /4627 1
104
No. R4
benyzl
76 2-F-C6H4-CH2
77 3-F-C6H4-CH2
78 4-F-C6H4-CH2
79 2,3-F2-C6H3-CH2
2,4-F2-C6H3-CH2
81 2,5-F2-C6H3-CH2
82 2,6-F2-C6H3-CH2
83 3,4-F2-C6H3-CH2
84 3,5-F2-C6H3-CH2
2-Cl-C6H4-CH2
86 3-Cl-C6H4-CH2
87 4-Cl-C6H4-CH2
88 2,3-C12-C6H3-CH2
89 2,4-C12-C6H3-CH2
go 2,5-C12-C6H3-CH2
91 2,6-C12-C6H3-CH2
92 3,4-C12-C6H3-CH2
93 3,5-C12-C6H3-CH2
94 2,3,4-C13-C6H2-CH2
2,3,5-C13-C6H2-CH2
96 2,3,6-C13-C6H2-CH2
97 2,4,5-C13-C6H2-CH2
98 2,4,6-C13-C6H2-CH2
99 3,4,5-C13-C6H2-CH2
100 2-Br-C6H4-CH2
101 3-Br-C6H4-CH2
102 4-Br-C6H4-CH2
103 2,3-Br2-C6H3-CH2
104 2,4-Br2-C6H3-CH2
105 2,5-Br2-C6H3-CH2
106 2,6-Br2-C6H3-CH2
107 3,4-Br2-C6H3-CH2
108 3,5-Br2-C6H3-CH2
109 2-F, 3-cl-c6H3-cH2
110 2-F, 4-Cl-C6H3-CH2
111 2-F, 5-Cl-C6H3-CH2
112 2-F, 3-Br-C6H3-CH2
CA 02231661 1998-04-07
0050/46271
105
No. R4
113 2-F, 4-Br-c6H3-cH2
114 2-F, 5-Br-c6H3-cH2
115 2-Cl, 3-Br-c6H3-cH2
116 2-Cl, 4-Br-c6H3-cH2
117 2-Cl, 5-Br-c6H3-cH2
118 3-F, 4-cl-c6H3-cH2
10 119 3-F, S-Cl-C6H3-CH2
120 3-F, 6-Cl-C6H3-CH2
121 3-F, 4-Br-c6H3-cH2
122 3-F, 5-Br-c6H3-cH2
15 123 3-F, 6-Br-C6H3-CH2
124 3-Cl, 4-Br-c6H3-cH2
125 3-Cl, 5-Br-c6H3-cH2
126 3-Cl, 6-Br-c6H3-cH2
20 127 4-F, 5-cl-c6H3-cH2
128 4-F, 6-Cl-C6H3-CH2
129 4-F, 5-Br-C6H3-CH2
130 4-F, 6-Br-C6H3-CH2
131 4-Cl, 5-Br-c6H3-cH2
132 5-F, 6-Cl-C6H3-CH2
133 5-F, 6-Br-C6H3-CH2
134 5-Cl, 6-Br-c6H3-cH2
135 3-Br, 4-Cl, 5-Br-c6H2-cH2
30 136 2-CN-C6H4-CH2
137 3-CN-C6H4-CH2
138 4-CN-C6H4-CH2
139 2-N02-C6H4-CH2
35 140 3-N02-C6H4-CH2
141 4-No2-c6H4-cH2
142 2-CH3-C6H4-CH2
143 3-CH3-C6H4-CH2
144 4-CH3-C6H4-CH2
145 2,3-(CH3)2-C6H3-CH2
146 2,4-(CH3)2-C6H3-CH2
147 2,5-(CH3)2-C6H3-CH2
148 2,6-(CH3)2-C6H3-CH2
45 149 3,4-(CH3~2-C6H3-CH2
150 3~5-(cH3)2-c6H3-cH2
CA 02231661 1998-04-07
0050~4627 1
106
No. R4
151 2-C2Hs-C6H4-CH2
152 3-C2H5-C6H4-CH2
153 4-C2H5-C6H4-CH2
154 2-i-C3H7-C6H4-CH2
155 3-i-C3H7-C6H4-CH2
156 4-i-C3H7-C6H4-CH2
10 157 2-cyclohexyl-C6H4-CH2
158 3-cyclohexyl-C6H4-CH2
159 4-cyclohexyl-C6H4-CH2
160 2-vinyl-C6H4-CH2
15 161 3-vinyl-C6H4-CH2
162 4-vinyl-C6H4-CH2
163 2-allyl-C6H4-CH2
164 3-allyl-C6H4-CH2
20 165 4-allyl-C6H4-CH2
166 2-C6H5-C6H4-CH2
167 3-C6Hs-C6H4-cH2
168 4-C6H5-C6H4-CH2
169 3-CH3, 5-t-c4Hs-c6H3-cH2
170 2-OH-C6H4-CH2
171 3-OH-C6H4-CH2
172 4-OH-C6H4-CH2
173 2-OCH3-C6H4-CH2
30 174 3-OCH3-C6H4-CH2
175 4-OCH3-C6H4-CH2
176 2,3-(OCH3)2-C6H3-CH2
177 2,4-(OCH3)2-C6H3-CH2
35 178 2,5-(OCH3)2-C6H3-CH2
179 3,4-(OCH3)2-C6H3-CH2
180 3,5-(OCH3)2-C6H3-CH2
181 3,4,5-(OCH3)3-C6H2-CH2
182 2-OC2H5-C6H4-CH2
183 3-OC2Hs-C6H4-CH2
184 4-OC2H5-C6H4-CH2
185 2-O-(n-C3H7)-C6H4-CH2
186 3-O-(n-C3H7)-C6H4-CH2
45 187 4-o-(n-c3H7)-c6H4-cH2
188 2-o-(i-c3H7)-c6H4-cH2
CA 02231661 1998-04-07
0050/4627 1
107
No. R4
189 3-o-(i-C3H7)-C6H4-CH2
190 4-O-(i-C3H7)-C6H4-CH2
191 4-O-(n-C4Hg)-C6H4-CH2
192 3-O-(t-C4Hg)-C6H4-CH2
193 4-O-(n-C6H13)-C6H4-CH2
194 2-O-allyl-C6H4-CH2
10 195 3-o-allyl-C6H4-CH2
196 4-o-allyl-C6H4-CH2
197 2-CF3-C6H4-CH2
198 3-CF3-C6H4-CH2
15 199 4-CF3-C6H4-CH2
200 2-acetyl-C6H4-CH2
201 3-acetyl-C6H4-CH2
202 4-acetyl-C6H4-CH2
20 203 2-methoxycarbonYl-c6H4-cH2
204 3-methoxycarbonyl-C6H4-CH2
205 4-methoxycarbonYl-C6H4-cH2
206 2-aminocarbonYl-c6H4-cH2
207 3-aminocarbonyl-C6H4-CH2
208 4-aminocarbonyl-C6H4-CH2
209 2-dimethylaminoCarbonyl-c6H4-cH2
210 3-dimethylaminoCarbonyl-c6H4-cH2
211 4-dimethylaminocarbonyl-C6H4-CH2
30 212 2-(N-methylaminoCarbonyl)-c6H4-cH2
213 3-(N-methylaminoCarbonyl)-c6H4-cH2
214 4-(N-methylaminoCarbonyl)-c6H4-cH2
215 2-H2N-C6H4-CH2
35 216 3-H2N-C6H4-CH2
217 4-H2N-C6H4-CH2
218 2-aminothiocarbonyl-C6H4-CH2
219 3-aminothiocarbonyl-C6H4-CH2
220 4-aminothiocarbonyl-C6H4-CH2
221 2-methoxyiminomethyl-C6H4-CH2
222 3-methoxyiminomethyl-C6H4-CH2
223 4-methoxyiminomethyl-C6H4-CH2
224 3,4-methylenedioXy-c6H3-cH2
45 225 3,4-difluoromethylenedioxy-C6H3-CH2
226 2~3-methylenedioxy-c6H3-cH2
CA 02231661 1998-04-07
0050~4627 1
108
No. R4
227 2-(l'-methoxyiminoeth-l'-yl)-C6H4-CH2
5 228 3-(l~-methoxyiminoeth-l~-yl)-c6H4-cH2
229 4-(l~-methoxyiminoeth-l~-yl)-c6H4-cH2
230 2-SCH3-C6H4-CH2
231 3-SCH3-C6H4-CH2
232 4-SCH3-C6H4-CH2
10 233 2-S02CH3-C6H4-CH2
234 3-S02CH3-C6H4-CH2
235 4-S02CH3-C6H4-CH2
236 2-OCF3-C6H4-CH2
15 237 3-OCF3-C6H4-CH2
238 4-OCF3-C6H4-CH2
239 2-OCHF2-C6H4-CH2
240 3-OCHF2-C6H4-CH2
20 241 4-OCHF2-C6H4-CH2
242 3-CF3, 4-OCF3-C6H3-CH2
243 l-naphthyl-CH2
244 2-naphthyl-CH2
245 2-phenoxyeth-1-yl
246 2-(2'-chlorophenoxy)eth-1-yl
247 2-(3'-chlorophenoxy)eth-1-yl
248 2-(4'-chlorophenoxy)eth-1-yl
249 2-(3',5'-dichlorophenoxy)eth-1-yl
30 250 2-(2'-cyanophe~oxy)eth-1-yl
251 2-(3'-cyanophenoxy)eth-1-yl
252 2-(4'-cyanophenoxy)eth-1-yl
253 2-(2'-methylphenoxy)eth-1-yl
35 254 2-(3'-methylphenoxy)eth-1-yl
255 2-(4'-methylphenoxy)eth-1-yl
256 2-(3'-t-butylphenoxy)eth-1-yl
257 2-(4'-t-butylphenoxy)eth-1-yl
258 2-(2'-nitrophenoxy)eth-1-yl
259 2-(3'-nitrophenoxy)eth-1-yl
260 2-(4'-nitrophenoxy)eth-1-yl
261 2-(2'-methoxyphenoxy)eth-1-yl
262 2-(3'-methoxyphenoxy)eth-1-yl
263 2-(4'-methoxyphenoxy)eth-1-yl
264 2-(2'-trifluoromethylphenoxy)eth-1-yl
CA 02231661 1998-04-07
0050/4627 1
109
No. R4
265 2-(3'-trifluoromethylphenoxy)eth-1-yl
266 2-(4'-trifluoromethylphenoxy)eth-1-yl
267 2-(2'-acetylphenoxy)eth-1-yl
268 2-(3'-acetylphenoxy)eth-1-yl
269 2-(4'-acetylphenoxy)eth-1-yl
270 2-(2'-methoxycarbonyl)eth-1-yl
10 271 2-(3'-methoxycarbonyl)eth-1-yl
272 2-(4'-methoxycarbonyl)eth-1-yl
273 2-(2'-dimethylaminocarbonyl)eth-1-yl
274 2-(3'-dimethylaminocarbonyl)eth-1-yl
15 275 2-(4'-dimethylaminocarbonyl)eth-1-yl
276 2-(2'-aminothiocarbonyl)eth-1-yl
277 2-(3'-aminothiocarbonyl)eth-1-yl
278 2-(4'-aminothiocarbonyl)eth-1-yl
20 279 2-(2'-methylsulfonyl)eth-1-yl
280 2-(3'-methylsulfonyl)eth-1-yl
281 2-(4'-methylsulfonyl)eth-1-yl
282 3-phenoxyprop-1-yl
283 3-(2'-chlorophenoxy)prop-1-yl
284 3-(3'-chlorophenoxy)prop-1-yl
285 3-(4'-chlorophenoxy)prop-1-yl
286 3-(3',5' dichlorophenoxy)prop-l-yl [sic]
287 3-(2'-cyanophenoxy)prop-1-yl
30 288 3-(3'-cyanophenoxy)prop-1-yl
289 3-(4'-cyanophenoxy)prop-1-yl
290 3-(2'-methylphenoxy)prop-1-yl
291 3-(3'-methylphenoxy)prop-1-yl
35 292 3-(4'-methylphenoxy)prop-1-yl
293 3-(2'-methoxyphenoxy)prop-1-yl
294 3-(3'-methoxyphenoxy)prop-1-yl
295 3-(4'-methoxyphenoxy)prop-1-yl
296 3-(2'-trifluoromethylphenoxy)prop-1-yl
297 3-(3'-trifluoromethylphenoxy)prop-1-yl
298 3-(4'-trifluoromethylphenoxy)prop-1-yl
299 4-phenoxybut-1-yl
300 2-phenyleth-1-yl
45 301 2-(2'-chlorophenyl)eth-1-yl
302 2-(3'-chlorophenyl)eth-1-yl
CA 02231661 1998-04-07
0050/'4627 1
110
No. R4
303 2-(4'-chlorophenyl)eth-1-yl
304 2-(3',5'-dichlorophenyl)eth-1-yl
305 2-(2'-cyanophenyl)eth-1-yl
306 2-(3'-cyanophenyl)eth-1-yl
307 2-(4'-cyanophenyl)eth-1-yl
308 2-(2'-methylphenyl)eth-l-yl
10 309 2-(3'-methylphenyl)eth-1-yl
310 2-(4'-methylphenyl)eth-1-yl
311 2-(2'-methoxyphenyl)eth-1-yl
312 2-(3'-methoxyphenyl)eth-1-yl
15 313 2-(4'-methoxyphenyl)eth-1-yl
314 2-(2'-trifluoromethylphenyl)eth-1-yl
315 2-(3'-trifluoromethylphenyl)eth-1-yl
316 2-(4'-trifluoromethylphenyl)eth-1-yl
20 317 3-phenylprop-1-yl
318 3-(2'-chlorophenyl)prop-1-yl
319 3-(3'-chlorophenyl)prop-1-yl
320 3-(4'-chlorophenyl)prop-l-yl
321 3-(2'-cyanophenyl)prop-1-yl
322 3-(3'-cyanophenyl)prop-1-yl
323 3-(4'-cyanophenyl)prop-l-yl
324 3-(2'-trifluoromethylphenyl)prop-1-yl
325 4-phenylbut-1-yl
30 326 4-(4'-chlorophenyl)but-1-yl
327 6-(4'-chlorophenyl)hex-1-yl
328 2-pyridylmethyl
329 3-pyridylmethyl
35 330 4-pyridylmethyl
331 4-chloropyridin-2-ylmethyl
332 5-chloropyridin-2-ylmethyl
333 6-chloropyridin-2-ylmethyl
40 334 5-chloropyridin-3-ylmethyl
335 6-chloropyridin-3-ylmethyl
336 2-chloropyridin-4-ylmethyl
337 2-pyrimidinylmethyl
338 4-chloropyrimidin-2-ylmethyl
339 5-chloropyrimidin-2-ylmethyl
340 2-chloropyrimidin-4-ylmethyl
CA 02231661 1998-04-07
0050/4627 1
111
No. R4
341 6-chloropyrimidin-4-ylmethyl
342 2-chloropyrimidin-5-ylmethyl
343 4-pyridazinylmethyl
344 2-pyrazinylmethyl
345 5-chloropyrazin-2-ylmethyl
346 6-chloropyrazin-2-ylmethyl
10 347 3-pyridazinylmethyl
348 6-chloropyridazin-3-ylmethyl
349 1,3,5-triazinylmethyl
350 2-furylmethyl
15 351 3-furylmethyl
352 4-bromofur-2-ylmethyl
353 5-chlorofur-2-ylmethyl
354 2-thienylmethyl
20 355 3-thienylmethyl
356 5-methylthien-3-ylmethyl
357 5-chlorothien-2-ylmethyl
358 2-chlorothien-4-ylmethyl
359 2-pyrrolylmethyl
360 3-pyrrolylmethyl
361 2-oxazolylmethyl
362 4-methyloxazol-2-ylmethyl
363 5-methyloxazol-2-ylmethyl
30 364 4-chlorooxazol-2-ylmethyl
365 5-chlorooxazol-2-ylmethyl
366 4-oxazolylmethyl
367 2-methyloxazol-4-ylmethyl
35 368 5-methyloxazol-4-ylmethyl
369 2-chlorooxazol-4-ylmethyl
370 5-chlorooxazol-4-ylmethyl
371 5-oxazolylmethyl
40 372 2-methyloxazol-5-ylmethyl
373 4-methyloxazol-5-ylmethyl
374 2-chlorooxazol-5-ylmethyl
375 4-chlorooxazol-5-ylmethyl
376 2-thiazolylmethyl
45 377 4-methylthiazol-2-ylmethyl
378 5-methylthiazol-2-ylmethyl
CA 0223l66l l998-04-07
0050/4627 1
112
No. R4
379 4-chlorothiazol-2-ylmethyl
5 380 5-chlorothiazol-2-ylmethyl
381 4-thiazolylmethyl
382 2-methylthiazol-4-ylmethyl
383 5-methylthiazol-4-ylmethyl
384 2-chlorothiazol-4-ylmethyl
lO 385 5-chlorothiazol-4-ylmethyl
386 5-thiazolylmethyl
387 2-methylthiazol-5-ylmethyl
388 4-methylthiazol-5-ylmethyl
15 389 2-chlorothiazol-5-ylmethyl
390 4-chlorothiazol-5-ylmethyl
391 3-isoxazolylmethyl
392 4-methylisoxazol-3-ylmethyl
20 393 5-methylisoxazol-3-ylmethyl
394 4-chloroisoxazol-3-ylmethyl
395 5-chloroisoxazol-3-ylmethyl
396 4-isoxazolylmethyl
397 3-methylisoxazol-4-ylmethyl
398 5-methylisoxazol-4-ylmethyl
399 3-chloroisoxazol-4-ylmethyl
400 5-chloroisoxazol-4-ylmethyl
401 5-isoxazolylmethyl
30 402 3-methylisoxazol-5-ylmethyl
403 4-methylisoxazol-5-ylmethyl
404 3-chloroisoxazol-5-ylmethyl
405 4-chloroisoxazol-5-ylmethyl
35 406 3-isothiazolylmethyl
407 4-methylisothiazol-3-ylmethyl
408 5-methylisothiazol-3-ylmethyl
409 4-chloroisothiazol-3-ylmethyl
410 5-chloroisothiazol-3-ylmethyl
411 4-isothiazolylmethyl
412 3-methylisothiazol-4-ylmethyl
413 5-methylisothiazol-4-ylmethyl
414 3-chloroisothiazol-4-ylmethyl
45 415 5-chloroisothiazol-4-ylmethyl
416 5-isothiazolylmethyl
CA 02231661 1998-04-07
0050~ 4627 1
113
No. R4
417 3-methylisothiazol-5-ylmethyl
5 418 4-methylisothiazol-5-ylmethyl
419 3-chloroisothiazol-5-ylmethyl
420 4-chloroisothiazol-5-ylmethyl
421 4-imidazolylmethyl
422 1-phenylpyrazol-3-ylmethyl
10 423 1-methylimidazol-4-ylmethyl
424 1-phenyl-1,2,4-triazol-3-ylmethyl
425 1,2,4-oxadiazol-3-ylmethyl
426 5-chloro-1,2,4-oxadiazol-3-ylmethyl
15 427 5-methyl-1,2,4 -OYA~ i A 701- 3-ylmethyl
428 5-trifluoromethyl-1,2,4-oxadiazol-3-ylmethyl
429 1,3,4-oYadiazol-2-ylmethyl
430 5-chloro-1,3,4-oxadiazol-2-ylmethyl
20 431 5-methyl-1,3,4_oYA~;Azol-2-ylmethyl
432 5-methoxy-1,3,4_oYA~iAzol-2-ylmethyl
433 1,2,4-thiadiazol-3-ylmethyl
434 5-chloro-1,2,4-th;a~iAzol-3-ylmethyl
435 5-methyl-1,2,4-thi A~i Azol-3-ylmethyl
436 1,3,4-thi A~ i A zol-2-ylmethyl
437 5-chloro-1,3,4-t h i ~Ad i A zol-2-ylmethyl
438 5-methyl-1,3,4-thi A~i Azol-2-ylmethyl
439 5-cyano-1,3,4-~hi A~ i A zol-2-ylmethyl
30 440 2-(2'-pyridinyloxy)eth-1-yl
441 2-(3'-pyridinyloxy)eth-1-yl
442 2-(4'-pyridinyloxy)eth-1-yl
443 2-(2'-pyrimidinyloxy)eth-1-yl
35 444 2-(4'-pyrimidinyloxy)eth-1-yl
445 2-(5'-pyrimidinyloxy)eth-1-yl
446 2-(2'-pyrazinyloxy)eth-1-yl
447 2-(2'-pyridazinyloxy)eth-1-yl
448 2-(3'-pyridazinyloxy)eth-1-yl
449 2-(l~3~5~-triazinyloxy)eth-l-yl
450 2-(5'-methylisoxazol-3'-yloxy)eth-1-yl
451 2-(5'-chloroisoxazol-3~-yloxy)eth-1-yl
452 2-(2'-methoxythiazol-4~-yloxy)eth-1-yl
45 453 2-(4~-chlorooxazol-2~-yloxy)eth-l-yl
454 2-(1'-phenyl-l'H-1',2',4'-triazol-3'-yloxy)eth-1-yl
CA 02231661 1998-04-07
0050/ 4627 1
114
No. R4
455 2-(1'-phenylpyrazol-3'-yloxy)eth-1-yl
5 456 C6H5
457 2-F-C6H4
458 3-F-C6H4
459 4-F-C6H4
460 2,3-F2-C6H3
10 461 2,4-F2-C6H3
462 2,5-F2-C6H3
463 2,6-F2-C6H3
464 3,4-F2-C6H3
15 465 3,5-F2-C6H3
466 2-Cl-C6H4
467 3-Cl-C6H4
468 4-Cl-C6H4
20 469 2,3-C1 ff 6H3
470 2,4-C12-C6H3
471 2,5-C12-C6H3
472 2,6-C12-C6H3
473 3,4-C12-C6H3
474 3,5-C12-C6H3
475 2,3,4-C13-C6H2
476 2,3,5-C13-C6H2
477 2,3,6-C13-C6H2
30 478 2,4,5-C13-C6H2
479 2,4,6-C1 ff 6H2
480 3,4,5-C13-c6H2
481 2-Br-C6H4
35 482 3-Br-C6H4
483 4-Br-C6H4
484 2,3-Br2-C6H3
485 2,4-Br2-C6H3
486 2,5-Br2-C6H3
487 2,6-Br2-C6H3
488 3,4-Br2-C6H3
489 3,5-Br2-C6H3
490 2-F, 3-Cl-C6H3
45 491 2-F, 4-Cl-C6H3
492 2-F, 5-Cl-C6H3
CA 02231661 1998-04-07
0050/46271
115
No. R4
493 2 -F, 3 -Br-c6H3
5 494 2 -F, 4 -Br-c6H3
495 2 - F, 5-Br - C6H3
496 2-Cl, 3-Br-C6H3
497 2-Cl, 4-Br-C6H3
498 2 -Cl, 5-Br-C6H3
10 499 3-F, 4-Cl-C6H3
S00 3-F, 5-Cl-C6H3
501 3-F, 6-Cl - C6H3
502 3-F, 4-Br-C6H3
15 503 3-F, 5-Br-C6H3
504 3-F, 6-Br-C6H3
505 3-Cl, 4-Br-C6H3
506 3-Cl, 5-Br-C6H3
20 507 3-Cl, 6-Br-C6H3
508 4-F, 5-cl-c6H3
509 4-F, 6-Cl-C6H3
S 10 4 -F, 5-Br-C6H3
S l l 4-F, 6-Br-C6H3
512 4-Cl, 5-Br-C6H3
513 5-F, 6-Cl-C6H3
514 5-F, 6-Br-C6H3
515 5-Cl, 6-Br-C6H3
30 516 3-Br, 4-Cl, 5-Br-C6H2
517 2-CN-C6H4
518 3-CN-C6H4
519 4-CN-C6H4
35 520 2 -N02 -C6H4
521 3 -N02 -C6H4
522 4 -N02 -C6H4
523 2 -CH3 -C6H4
40 524 3-CH3-C6H4
525 4 -CH3 -C 6 H4
526 2,3 - (CH3)2 - C6H3
527 2,4-(CH3)2-C6H3
528 2,5- ( CH3) 2-C6H3
529 2,6 - ( CH3) 2 - C6H3
530 3,4-(CH3)2 - C6H3
CA 02231661 1998-04-07
0050~ 4627 1
116
No. R4
531 3,5-(CH3)2--C6H3
532 2-C2Hs-C6H4
533 3 -C2Hs-C6H4
534 4-C2Hs-C6H4
535 2-i-C3H7-C6H4
536 3-i-C3H7-C6H4
537 4- i-C3H7-C6H4
538 3-tert-C4Hg-C6H4
539 4-tert-C4Hg-C6H4
540 2 -vinyl -C 6 H4
541 3-vinyl-C6H4
542 4 -vinyl -C6 H4
543 2 -a 1 lyl -C6 H4
544 3 -allyl-C6H4
545 4-allyl-C6H4
546 2 -C6Hs-C6H4
547 3 -C6H5-C6H4
548 4-C6Hs-C6H4
549 3-CH3, 5-tert-C4Hg-C6H3
550 2-OH-C6H4
551 3 -OH-C6H4
552 4-oH-c6H4
553 2-OCH3-C6H4
554 3-OCH3-C6H4
555 4 -OCH3-C6H4
556 2,3- (OCH3) 2-C6H3
557 2,4- (OCH3) 2-C6H3
558 2,5-(OCH3)2-C6H3
559 3,4-(OCH3)2-C6H3
560 3~5--(ocH3)2--C6H3
561 3,4,5--(OCH3)3-C6H2
562 2--Oc2Hs -C6H4
563 3-OC2Hs-C6H4
564 4-OC2Hs-C6H4
565 2 -O- ( n-C3H7) -C6H4
566 3-0- ( n-C3H7) -C6H4
567 4-O-(n-C3H7)-C6H4
568 2-0- ( i-C3H7) -C6H4
CA 02231661 1998-04-07
0050~4627 1
117
No. R4
569 ~ 3-O-(i-C3H7)-C6H4
570 4-o-(i-C3H7)-C6H4
571 4-O-(n-C4Hg)-C6H4
572 3-O-(t-C4Hs)-C6H4
573 4_o-(t-C4H9)-c6H4
574 2-O-allyl-C6H4
10 575 3-O-allyl-C6H4
576 4-O-allyl-C6H4
577 2-CF3-C6H4
578 3-CF3-C6H4
15 579 4-CF3-C6H4
580 2-acetyl-C6H4
581 3-acetyl-C6H4
582 4-acetyl-C6H4
20 583 2-methoxycarbonyl-C6H4
584 3-methoxycarbonyl-C6H4
585 4-methoxycarbonyl-C6H4
586 2-aminocarbonyl-C6H4
587 3-aminocarbonyl-C6H4
588 4-aminocarbonyl-C6H4
589 2-dimethylaminocarbonyl-C6H4
590 3-dimethylaminOcarbonyl-c6H4
591 4-dimethylaminOcarbonyl-c6H4
30 592 2-(N-methylaminocarbonyl)-C6H4
593 3-(N-methylaminocarbonyl)-c6H4
594 4-(N-methylaminocarbonyl)-c6H4
595 2-H2N-C6H4
35 596 3-H2N-C6H4
597 4-H2N-C6H4
598 2-aminothiocarbonyl-C6H4
599 3-aminothiocarbonyl-C6H4
600 4-aminothiocarbonyl-C6H4
601 2-methoxyiminomethyl-C6H4
602 3-methoxyiminomethyl-C6H4
603 4-methoxyi i n- -thyl-C6H4
604 3,4-methylenedioxy-C6H3
45 605 3,4-difluoromethylenedioxy-C6H3
606 2,3-methylenedioxy-C6H3
CA 0223l66l l998-04-07
0050/4627 1
118
No. R4
607 2-(1'-methoxyiminoeth-1'-yl)-C6H4
608 3-(1'-methoxyiminoeth-1'-yl)-C6H4
609 4-(1'-methoxyiminoeth-1'-yl)-C6H4
610 2-SCH3-C6H4
611 3-SCH3-C6H4
612 4-SCH3-C6H4
10 613 2-S02CH3-C6H4
614 3-S02CH3-C6H4
615 4-S02CH3-C6H4
616 2-OCF3-C6H4
15 617 3-OCF3-C6H4
618 4-OCF3-C6H4
619 2-OCHF2-C6H4
620 3-OCHF2-C6H4
20 621 4-OCHF2-C6H4
622 3-CF3, 4-OCF3-C6H3
623 2-NHCH3-C6H4
624 3-NHCH3-C6H4
625 4-NHCH3-C6H4
626 2-N(CH3)2-C6H4
627 3-N(CH3)2-C6H4
628 4-N(CH3)2-C6H4
629 2-ethoxycarbonyl-C6H4
30 630 3-ethoxycarbonyl-C6H4
631 4-ethoxycarbonyl-C6H4
632 2-CH2CH2F-C6H4
633 3-CH2CH2F-C6H4
35 634 4-cH2cH2F-c6H4
635 2-CH2CF3-C6H4
636 3-CH2CF3-C6H4
637 4-CH2CF3-C6H4
40 638 2-CF2CHF2-C6H4
639 3-CF2CHF2-C6H4
640 4-CF2CHF2-C6H4
641 2-CHF2-C6H4
642 3-CHF2-C6H4
45 643 4-CHF2-C6H4
644 2-(l'-oxo-n-prop-1-yl)-C6H4
CA 02231661 1998-04-07
0050/4627 1
119
No. R4
645 3-(l'-oxo-n-prop-l-yl)-C6H4
5 646 4-(l'-oxo-n-prop-l-yl)-C6H4
647 2-(l~-oxoisoprop-l-yl)-C6H4
648 3-(l'-oxoisoprop-l-yl)-C6H4
649 4-(l'-oxoisoprop-1-yl)-C6H4
650 3-cyclopropyl-C6H4
10 651 4-cyclopropyl-C6H4
652 4-cyclohexyl-C6H4
653 1-naphthyl
654 2-naphthyl
15 655 2-pyridyl
656 3-pyridyl
657 4-pyridyl
658 S-CH3-pyridin-2-yl
20 659 5-Cl-pyridin-2-yl
660 6-C1-pyridin-2-yl
661 3,5-Cl2-pyridin-2-yl
662 6-OCH3-pyridin-2-yl
663 6-CH3-pyridin-2-yl
664 6-Cl-pyridin-3-yl
665 6-CH3-pyridin-3-yl
666 6-OCH3-pyridin-3-yl
667 2-pyrimidinyl
30 668 4-OCH3-pyrimidin-2-yl
669 4-OCzHs-pyrimidin-2-yl
670 4-Cl-pyrimidin-2-yl
671 4-CH3-pyrimidin-2-yl
35 672 5-CH3-pyrimidin-2-yl
673 5-Cl-pyrimidin-2-yl
674 5-OCH3-pyrimidin-2-yl
675 5-OC2Hs-pyrimidin-2-yl
40 676 4-pyrimidinyl
677 2-Cl-pyrimidin-4-yl
678 2-OCH3-pyrimidin-4-yl
679 2-CH3-pyrimidin-4-yl
680 6-Cl-pyrimidin-4-yl
45 681 6-CH3-pyrimidin-4-yl
682 6-OCH3-pyrimidin-4-yl
CA 02231661 1998-04-07
0050/4627 1
120
No. R4
683 5-pyrimidinyl
684 2-CH3-pyrimidin-5-yl
685 2-Cl-pyrimidin-5-yl
686 2-OCH3-pyrimidin-5-yl
687 2-OC2Hs-pyrimidin-5-yl
688 2-furyl
10 689 4-C2Hs-fur-2-yl
690 4-CH3-fur-2-yl
691 4-Cl-fur-2-yl
692 4-CN-fur-2-yl
15 693 5-CH3-fur-2-yl
694 5-Cl-fur-2-yl
695 5-CN-fur-2-yl
696 3-furyl
20 697 5-CH3-fur-3-yl
698 5-Cl-fur-3-yl
699 5-CN-fur-3-yl
700 2-thienyl
701 4-CH3-thien-2-yl
702 4-Cl-thien-2-yl
703 4-CN-thien-2-yl
704 5-CH3-thien-2-yl
705 5-Cl-thien-2-yl
30 706 5-CN-thien-2-yl
707 3-thienyl
708 5-CH3-thien-3-yl
709 5-Cl-thien-3-yl
35 710 5-CN-thien-3-yl
711 4-CH3-thien-3-yl
712 5-F-thien-3-yl
713 2-oxazolyl
714 4-CH3-oxazol-2-yl
715 4-Cl-oxazol-2-yl
716 4-CN-oxazol-2-yl
717 5-CH3-oxazol-2-yl
718 5-Cl-oxazol-2-yl
45 719 5-CN-oxazol-2-yl
720 4-oxazolyl
CA 02231661 1998-04-07
0050/4627
121
No. R4
721 2-CH3-oxazol-4-yl
722 2-Cl-oxazol-4-yl
723 2-CN-oxazol-4-yl
724 5-oxazolyl
725 2-CH3-oxazol-5-yl
726 2-Cl-oxazol-5-yl
10 727 2-CN-oxazol-5-yl
728 3-isoxazolyl
729 5-CH3-isoxazol-3-yl
730 5-Cl-isoxazol-3-yl
15 731 5-CN-isoxazol-3-yl
732 5-isoxxazolyl [sic]
733 3-CH3-isoxazol-5-yl
734 3-Cl-isoxazol-5-yl
20 735 3-CN-isoxazol-5-yl
736 2-thiazolyl
737 4-CH3-thiazol-2-yl
738 4-Cl-thiazol-2-yl
739 4-CN-thiazol-2-yl
740 5-CH3-thiazol-2-yl
741 5-Cl-thiazol-2-yl
742 5-CN-thiazol-2-yl
743 4-thiazolyl
30 744 2-CH3-thiazol-4-yl
745 2-Cl-thiazol-4-yl
746 2-CN-thiazol-4-yl
747 2-SCH3-thiazol-4-yl
35 748 5-thiazolyl
749 2-CH3-thiazol-5-yl
750 2-Cl-thiazol-5-yl
751 2-CN-thiazol-5-yl
752 3-isothiazolyl
753 5-CH3-isothiazol-3-yl
754 5-Cl-isothiazol-3-yl
755 5-CN-isothiazol-3-yl
756 5-isothiazolyl
45 757 3-CH3-isothiazol-5-yl
758 3-Cl-isothiazol-5-yl
CA 02231661 1998-04-07
0050/4627 1
122
No. R4
759 3-CN-isothiazol-5-yl
760 2-imidazolyl
761 4-CH3-imidazol-2-yl
762 4-Cl-imidazol-2-yl
763 4-CN-imidazol-2-yl
764 l-CH3-imidazol-2-yl
10 765 l-CH3, 4-Cl-imidazol-2-yl
766 1,4-(CH3)2-imidazol-2-yl
767 l-CH3, 5-Cl-imidazol-2-yl
768 1,5-(CH3)2-imidazol-2-yl
15 769 4-imidazolyl
770 2-CH3-imidazol-4-yl
771 2-Cl-imidazol-4-yl
772 1-CH3-imidazol-4-yl
20 773 1,2-(CH3)2-imidazol-4-yl
774 1-CH3, 2-Cl-imidazol-4-yl
775 1-CH3-imidazol-5-yl
776 1-CH3, 3-Cl-imidazol-5-yl
777 1,2-~CH3)2-imidazol-S-yl
778 3-pyrazolyl
779 5-CH3-pyrazol-3-yl
780 5-Cl-pyrazol-3-yl
781 5-CN-pyrazol-3-yl
30 782 1-CH3-pyrazol-3-yl
783 1-CH3, 4-Cl-pyrazol-3-yl
784 1-CH3, 5-Cl-pyrazol-3-yl
785 1,5-(CH3)2-pyrazol-3-yl
35 786 1-CH3-pyrazol-5-yl
787 l-CH3, 3-Cl-pyrazol-5-yl
788 1,3-(CH3)2-pyrazol-5-yl
789 4-pyrazolyl
790 3-Cl-pyrazol-4-yl
791 3-CH3-pyrazol-4-yl
792 l-CH3-pyrazol-4-yl
793 1-CH3, 3-Cl-pyrazol-4-yl
794 1,3-(CH3)2-pyrazol-4-yl
45 795 1,3,4-oxadiazol-5-yl
796 2-CH3-1,3,4-oxadiazol-5-yl
CA 0223l66l l998-04-07
0050/4627 1
123
No. R4
797 2-Cl-1,3,4-oxadiazol-5-yl
5 798 2-CF3-1,3,4-oxadiazol-5-yl
799 2-i-C3H7-1,3,4-oxadiazol-5-yl
800 2-OCH3-1,3,4 - O~A~ i A zol-5-yl
801 1,2,4-oxadiazol-3-yl
802 5-CH3-1,2,4-oxadiazol-3-yl
803 5-i-C3H7-1,2,4-o~A~;Azol-3-yl
804 S-Cl-1,2,4-oxadiazol-3-yl
805 S-CF3-1,2,4-oxadiazol-3-yl
806 1,2,4-triazol-3-yl
15 807 1-CH3-1,2,4-triazol-3-yl
808 3-fluoropyridin-2-yl
809 3-chloropyridin-2-yl
810 3-bromopyridin-2-yl
20 811 3-methylpyridin-2-yl
812 3-trifluoromethylpyridin-2-yl
813 3-methoxypyridin-2-yl
814 4-fluoropyridin-2-yl
815 4-chloropyridin-2-yl
816 4-bromopyridin-2-yl
817 4-methylpyridin-2-yl
818 4-trifluoromethylpyridin-2-yl
819 4-methoxypyridin-2-yl
30 820 S-fluoropyridin-2-yl
821 S-bromopyridin-2-yl
822 6-trifluoromethylpyridin-2-yl
823 2-fluoropyridin-3-yl
35 824 2-chloropyridin-3-yl
825 2-bromopyridin-3-yl
826 2-methylpyridin-3-yl
827 2-trifluoromethylpyridin-3-yl
40 828 3-methoxypyridin-3-yl
829 4-fluoropyridin-3-yl
830 4-chloropyridin-3-yl
831 4-bromopyridin-3-yl
832 4-methylpyridin-3-yl
45 833 4-trifluoromethylpyridin-3-yl
834 4-methoxypyridin-3-yl
CA 0223l66l l998-04-07
0050/4627 1
124
No. R4
835 5-fluoropyridin-3-yl
836 5-chloropyridin-3-yl
837 5-bromopyridin-3-yl
838 5-methylpyridin-3-yl
839 5-trifluoromethylpyridin-3-yl
840 5-methoxypyridin-3-yl
10 841 6-fluoropyridin-3-yl
842 6-bromopyridin-3-yl
843 6-trifluoromethylpyridin-3-yl
844 2-fluoropyridin-4-yl
15 845 2-chloropyridin-4-yl
846 2-bromopyridin-4-yl
847 2-methylpyridin-4-yl
848 2-trifluoromethylpyridin-4-yl
20 849 2-methoxypyridin-4-yl
850 3-fluoropyridin-4-yl
851 3-chloropyridin-4-yl
852 3-bromopyridin-4-yl
853 3-methylpyridin-4-yl
854 3-trifluoromethylpyridin-4-yl
855 3-methoxypyridin-4-yl
856 4-fluoropyrimidin-2-yl
857 4-bromopyrimidin-2-yl
30 858 4-trifluoromethylpyrimidin-2-yl
859 5-fluoropyrimidin-2-yl
860 5-bromopyrimidin-2-yl
861 5-trifluoromethylpyrimidin-2-yl
35 862 2-fluoropyrimidin-4-yl
863 2-bromopyrimidin-4-yl
864 2-trifluoromethylpyrimidin-4-yl
865 2-trifluoromethoxypyrimidin-4-yl
866 5-fluoropyrimidin-4-yl
867 5-chloropyrimidin-4-yl
868 5-bromopyrimidin-4-yl
869 5-methoxypyrimidin-4-yl
870 5-trifluoromethylpyrimidin-4-yl
871 5-methoxypyrimidin-4-yl
872 6-fluoropyrimidin-4-yl
CA 0223l66l l998-04-07
0050/4627 1
125
No. R4
8736-bromopyrimidin-4-yl
874 6-trifluoromethylpyrimidin-4-yl
875 2-fluoropyrimidin-5-yl
876 2-br.- ~p~.imidin-5-yl
877 2-trifluoromethylpyrimidin-5-yl
878 4-fluoropyrimidin-5-yl
10 879 4-chloropyrimidin-5-yl
880 4-bromopyrimidin-5-yl
881 4-methylpyrimidin-5-yl
882 4-trifluoromethylpyrimidin-5-yl
883 3-fluoro-5-trifluoromethylpyridin-2-yl
884 3,6-dichloro-5-trifluoromethylpyridin-2-yl
885 5,6-dichloro-3-trifluoromethylpyridin-2-yl
886 5-chloro-3-trifluoromethylpyridin-2-yl
20 887 3-chloro-5-trifluoromethylpyridin-2-yl
888 6-chloro-4-cyanopyridin-2-yl
889 3-cyano-5-nitropyridin-2-yl
890 2-chloro-6-fluoropyridin-4-yl
891 6-chloro-4-fluoropyridin-2-yl
892 4,6-difluoropyridin-2-yl
893 3,5-dichloro-6-fluoropyridin-2-yl
894 6-methoxy-3-nitropyridin-2-yl
895 4-cyano-6-fluoropyridin-2-yl
30 896 6-chloro-5-cyanopyridin-2-yl
897 6-chloro-3-cyanopyridin-2-yl
898 4-cyano-3,5,6-trifluoropyridin-2-yl
899 6-chloro-5-nitropyridin-2-yl
35 900 6-chloro-3-nitropyridin-2-yl
901 5-cyano-6-fluoropyridin-2-yl
902 3-cyano-6-fluoropyridin-2-yl
903 4,6-dicyanopyridin-2-yl
904 5-trichloromethylpyridin-2-yl
905 5-cyanopyridin-2-yl
906 5-bromo-4-trifluoromethylpyridin-2-yl
907 3-nitro-5-trifluoromethylpyridin-2-yl
908 5-aminopyridin-2-yl
45 909 2,3,5,6-tetrafluoropyridin-4-yl
910 S-nitropyridin-2-yl
CA 02231661 1998-04-07
0050/4627 1
126
No. R4
911 4-methyl-5-nitroypridin-2-yl [sic]
5 912 5-difluoromethylpyridin-2-yl
913 5-fluoromethylpyridin-2-yl
914 4,6-difluoropyrimidin-2-yl
915 2,6-difluoropyrimidin-4-yl
916 2-chloro-6-trichloromethylpyrimidin-4-yl
10 917 2,6-dichloropyrimidin-4-yl
918 5-methoxycarbonylpyridin-2-yl
919 5-chloro-6-fluoropyridin-2-yl
920 5-chloro-6-hydroxypyridin-2-yl
921 5-chloro-6-methoxypyridin-2-yl
922 5-chloro-6-cyanopyridin-2-yl
923 5,6-dichloropyridin-2-yl
924 6-bromo-5-chloropyridin-2-yl
20 925 5-bromo-6-fluoropyridin-2-yl
926 5-bromo-6-chloropyridin-2-yl
927 5-bromo-6-cyanopyridin-2-yl
928 S-bromo-6-hydroxypyridin-2-yl
929 5-bromo-6-methoxypyridin-2-yl
930 5,6-dibL~ ~p~idin-2-yl
931 4-cyanopyridin-2-yl
932 6-cyanopyridin-2-yl
933 4-chloro-6-methylpyrimidin-2-yl
30 934 2-chloro-6-fluoropyridin-4-yl
935 5-bromo-4-trifluoromethylpyridin-2-yl
936 4,5-dichloropyridin-2-yl
937 4,5-dibromopyridin-2-yl
35 938 5,6-dichloropyridin-2-yl
939 4,6-dichloropyridin-2-yl
940 4,6-dibromopyridin-2-yl
941 5,6-dibromopyridin-2-yl
40 942 4-bromo-5-chloropyridin-2-yl
943 6-bromo-5-chloropyridin-2-yl
944 5-bromo-4-chloropyridin-2-yl
945 5-bromo-4-chloropyridin-2-yl
946 6-bromo-4-chloropyridin-2-yl
45 947 4-bromo-6-chloropyridin-2-yl
948 6-chloro-4-methoxypyridin-2-yl
CA 0223l66l l998-04-07
0050/4627 1
127
No. R4
949 6-bromo-4-methoxypyridin-2-yl
950 6-chloroquinazolin-2-yl
9Sl quinazolin-2-yl
952 4-cyanopyridin-2-yl
953 6-cyanopyridin-2-yl
954 5-hydroxymethylpyridin-2-yl
10 955 6-chloro-4-trifluoromethylpyridin-2-yl
956 6-chloro-4-trifluoromethylpyridin-2-yl
957 6-chloro-4-methylpyridin-2-yl
958 2,5-dichloro-6-cyanopyridin-2-yl
15 959 2,5-dichloro-6-carboxypyridin-2-yl
960 2,5-dichloro-6-methoxycarbonylpyridin-2-yl
961 6-trifluoromethylpyridin-2-yl
962 6-methoxycarbonylpyridin-2-yl
20 963 6-carboxypyridin-2-yl
964 4-phenoxypyridin-2-yl
965 5-phenoxypyridin-2-yl
966 6-phenoxypyridin-2-yl
967 4-phenoxypyrimidin-4-yl
968 4-~4-methylphenoxy)pyrimidin-4-yl
969 4-phenoxypyrimidin-2-yl
970 4-(2-fluorophenoxy)pyrimidin-2-yl
971 4-pheno~y~-yLimidin-6-yl
30 972 4-(4-chlorophenoxy)pyrimidin-6-yl
973 4-(2-pyridyloxy)pyrimidin-6-yl
974 4-(6-chloro-2-pyridyloxy)pyrimidin-6-yl
975 4-(3-pyridyloxy)pyrimidin-6-yl
976 4-(2-methyl-3-pyridyloxy)pyrimidin-6-yl
977 4-(4-pyridyloxy)pyrimidin-6-yl
978 5-bromo-2-thienyl
979 5-nitro-2-thienyl
980 2-chloro-3-thienyl
981 2-bromo-3-thienyl
982 l-methyl-3-pyrrolyl
983 1-methyl-2-pyrrolyl
984 1-benzofuran-2-yl
45 985 l-benzofuran-3-yl
986 1-benzothiophen-2-yl
CA 02231661 1998-04-07
0050~46271
128
No. R4
987 1-benzothiophen-3-yl
5 988 3-pyrrolyl
989 2-pyrrolyl
990 3-indolyl
991 2-indolyl
992 1-methyl-3-indolyl
lO 993 1-methyl-2-indolyl
994 isoxazol-4-yl
99S isothiazol-4-yl
996 1,2-benzisoxazol-3-yl
15 997 1,2-benzisothiazol-3-yl
998 1-methylindazol-3-yl
999 benzoxazol-2-yl
1000 5-chlorobenzoxazol-2-yl
20 1001 6-fluorobenzoxazol-2-yl
1002 benzthiazol-2-yl
1003 5-fluorobenzthiazol-2-yl
1004 6-fluorobenzthiazol-2-yl
1005 pyrido[3,2-d]thiazol-2-yl
1006 (6-chloropyrido)[3,2-dlthiazol-2-yl
1007 1-methyl-1,2,3-triazol-5-yl
1008 1-methyl-1,2,3-triazol-4-yl
1009 1-methyl-1,2,4-triazol-S-yl
30 1010 1-methyl-1,2,3,4-tetrazol-5-yl
1011 2-methyl-1,2,3,4-tetrazol-5-yl
1012 5-trifluoromethyl-1,3,4-thiA~i~zol-2-yl
1013 6-chlorobenzoxazol-2-yl
1014 5-fluorobenzoxazol-2-yl
1015 S-nitrothiazol-2-yl
1016 l-CH(CH3)2-pyrrol-3-y
1017 1-C(CH3)3-pyrrol-3-yl
1018 1-cyclopropylpyrrol-3-yl
1019 1-C6H5-pyrro1-3-yl
1020 1-(2-CH3-C6H4)pyrrol-3-yl
1021 l-(3-cH3-c6H4)pyrrol-3-yl
1022 1-(4-CH3-C6H4)pyrrol-3-yl
1023 1-(3-OCH3-C6H4)pyrrol-3-yl
1024 1-(4-OCH3-C6H4)pyrrol-3-yl
1025 1-(4-NO2-C6H4)pyrrol-3-yl
CA 02231661 1998-04-07
0050/46271
129
No. R4
1026 1-(3-NO2-C6Hq)pyrrol-3-yl
1027 1-(4-CN-C6H4)pyrrol-3-yl
1028 1-(3-CN-C6H4)pyrrol-3-yl
1029 1-(3-CF3-C6H4)pyrrol-3-yl
1030 l-(4-cF3-c6H4)pyrrol-3-yl
1031 1-(4-CtCH3)3-C6H4)pyrrol-3-yl
10 1032 1-(2-Cl-C6H4)pyrrol-3-yl
1033 1-(3-Cl-C6H4)pyrrol-3-yl
1034 1-(4-Cl-C6H4)pyrrol-3-yl
1035 1-(2-Br-C6H4)pyrrol-3-yl
1036 1-(3-~r-C6H4)pyrrol-3-yl
1037 1-(4-~r-C6H4)pyrrol-3-yl
1038 1-(2-F-C6H4)pyrrol-3-yl
1039 1-(3-F-C6H4)pyrrol-3-yl
1040 1-(4-F-C6H4)pyrrol-3-yl
20 1041 1-(2,4-C12-C6H3)pyrrol-3-yl
1042 1-(2,5-C12-C6H3)pyrrol-3-yl
1043 1-(2,6-C12-C6H3)pyrrol-3-yl
1044 1-(3,4-C12-C6H3)pyrrol-3-yl
1045 1-(2,4-F2-C6H3)pyrrol-3-yl
1046 1-(2,5-F2-C6H3)pyrrol-3-yl
1047 1-(2,6-F2-C6H3)pyrrol-3-yl
1048 1-(3,4-F2-C6H3)pyrrol-3-yl
1049 1-(2-Cl, 5-OCH3-C6H3)pyrrol-3-yl
30 1050 1-(2-Cl, 5-CH3-C6H3)pyrrol-3-yl
1051 1-(5-Cl, 2-OCH3-C6H3)pyrrol-3-yl
1052 1-(5-Cl, 2-CH3-C6H3)pyrrol-3-yl
1053 1-[2,5-(CH3)2-C6H3]pyrrol-3-yl
1054 1-CH(CH3)2-pyrrol-2-yl
1055 1-C(CH3)3 PYLL~1_2_Y1
1056 1-cyclopropylpyrrol-2-yl
1057 1-C6H5-pyrrol-2-yl
1058 1-(2-CH3-C6H4)pyrrol-2-yl
40 1059 l-(3-cH3-c6H4)pyrrol-2
1060 l-(4-cH3-c6H4)pyrrol-2-yl
1061 1-(3-OCH3-C6H4)pyrrol-2-yl
1062 1-(4-OCH ff 6H4)pyrrol-2-yl
1063 l-(4-No2-c6H4)pyrrol-2
45 1064 1-(3-NO2-C6H4)pyrrol-2-yl
1065 1-(4-CN-C6H4)pyrrol-2-yl
CA 02231661 1998-04-07
0050/4627 1
130
No. R4
1066 1-(3-CN-C6H4)pyrrol-2-yl
1067 1-(3-CF3-C6H4)pyrrol-2-yl
1068 1-(4-CF3-C6H4)pyrrol-2-yl
1069 1-(4-C(CH3)3-C6H4)pyrrol-2-yl
1070 1-(2-Cl-C6H4)pyrrol-2-yl
1071 1-(3-Cl-C6H4)pyrrol-2-yl
10 1072 1-(4-Cl-C6H4)pyrrol-2-yl
1073 1-(2-Br-C6H4)pyrrol-2-yl
1074 1-(3-Br-C6H4)pyrrol-2-yl
1075 1-(4-Br-C6H4)pyrrol-2-yl
1076 1-(2-F-C6H4)pyrrol-2-yl
1077 1-(3-F-C6H4)pyrrol-2-yl
1078 1-(4-F-C6H4)pyrrol-2-yl
1079 1-(2,4-C12-C6H3)pyrrol-2-yl
1080 1-(2,5-C12-C6H3)pyrrol-2-yl
20 1o8l 1-(2,6-C12-C6H3)pyrrol-2-yl
1082 1-(3,4-C12-C6H3)pyrrol-2-yl
1083 1-(2,4-F2-C6H3)pyrrol-2-yl
1084 1-(2,5-F2-C6H3)pyrrol-2-yl
1085 1-(2,6-F2-C6H3)pyrrol-2-yl
25 1086 1-(3,4-F2-C6H3)pyrrol-2-yl
1087 1-(2-Cl, 5-OCH3-C6H3)pyrrol-2-yl
1088 1-(2-Cl, 5-CH3-C6H3)pyrrol-2-yl
1089 1-(5-Cl, 2-OCH3-C6H3)pyrrol-2-yl
30 1090 1-(5-Cl, 2-CH3-C6H3)pyrrol-2-yl
1091 1-l2,5-(CH3)2-C6H3~pyrrol-2-yl
1092 5-CH(CH3)2-furan-2-yl
1093 5-C(CH3)3-furan-2-yl
1094 5-cyclopropylfuran-2-yl
35 1095 5-C6H5-furan-2-yl
1096 5-(2-CH3-C6H4)furan-2-yl
1097 5-(3-CH3-C6H4)furan-2-yl
1098 5-(4-CH3-C6H4)furan-2-yl
40 1099 5-(3-OCH3-C6H4)furan-2-yl
1100 5-(4-OCH3-C6H4)furan-2-yl
1101 5-(4-NO2-C6H4)furan-2-yl
1102 5-(3-No2-c6H4)furan-2
1103 5-(4-CN-C6H4)furan-2-yl
45 1104 5-(3-cN-c6H4)furan-2-yl
1105 5-(3-CF3-C6H4)furan-2-yl
CA 0223l66l l998-04-07
0050/46271
131
No. R4
1106 5-~4-CF3-C6H4)furan-2-yl
1107 5-~4-C~CH3)3-C6H4)furan-2-yl
1108 5-~4-C6H5-C6H4)furan-2-yl
1109 5-~2-Cl-C6H4)furan-2-yl
1110 5-~3-Cl-C6H4)furan-2-yl
1111 5-~4-Cl-C6H4)furan-2-yl
10 1112 5-~2-Br-C6H4)furan-2-yl
1113 5-~3-Br-C6H4)furan-2-yl
1114 5-~4-Br-C6H4)furan-2-yl
1115 5-~2-F-C6H4)furan-2-yl
1116 5-~3-F-C6H4)furan-2-yl
1117 5-~4-F-C6H4)furan-2-yl
1118 5-~2,4-Cl2-C6H3)furan-2-yl
1119 5-~2,5-Cl2-C6H3)furan-2-yl
1120 5-~2,6-Cl2-C6H3)furan-2-yl
20 1121 5-~3,4-Cl2-C6H3)furan-2-yl
1122 5-~2,4-F2-C6H3)furan-2-yl
1123 5-~2,5-F2-C6H3)furan-2-yl
1124 5-~2,6-F2-C6H3)furan-2-yl
1125 5-~3,4-F2-C6H3)furan-2-yl
1126 5-~2-Cl, 5-OCH3-C6H3)furan-2-yl
1127 5-~2-Cl, 5-CH3-C6H3)furan-2-yl
1128 5-(5-Cl, 2-OCH3-C6H3)furan-2-yl
1129 5-(5-Cl, 2-CH3-C6H3)furan-2-yl
30 1130 5-[2,5-(CH3)2-C6H3lfuran-2-yl
1131 4-CH~CH3)2-furan-2-yl
1132 4-C(CH3)3-furan-2-yl
1133 4-cyclopropylfuran-2-yl
1134 4-4 H5-furan-2-yl
35 1135 4-(2-CH3-C6H4)furan-2-yl
1136 4-~3-CH3-C6H4)furan-2-yl
1137 4-~4-CH3-C6H4)furan-2-yl
1138 4-~3-OCH3-C6H4)furan-2-yl
40 1139 4-~4-OCH3-C6H4)furan-2-yl
1140 4-~4-N02-C6H4)furan-2-yl
1141 4-~3-N02-C6H4)furan-2-yl
1142 4-~4-CN-C6H4)furan-2-yl
1143 4-~3-CN-C6H4)furan-2-yl
45 1144 4-~3-CF3-C6H4)furan-2-yl
1145 4-~4-CF3-C6H4)furan-2-yl
CA 02231661 1998-04-07
0050/46271
132
No. R4
1146 4-(4-C(CH3)3-C6H4)furan-2-yl
1147 4-(Z-Cl-C6H4)furan-2-yl
1148 4-(3-Cl-C6H4)furan-2-yl
1149 4-(4-Cl-C6H4)furan-2-yl
1150 4-(2-Br-C6H4)furan-2-yl
llSl 4-(3-Br-C6H4)furan-2-yl
10 1152 4-(4-Br-C6H4)furan-2-yl
1153 4-(2-F-C6H4)furan-2-yl
1154 4-(3-F-C6H4)furan-2-yl
1155 4-(4-F-C6H4)furan-2-yl
1156 4-(2,4-C12-C6H3)furan-2-yl
1157 4-(2,5-C12-C6H3)furan-2-yl
1158 4-(2~6-cl2-c6H3)furan-2-yl
1159 4-(3~4-C12-C6H3)furan-2-yl
1160 4-(2,4-F2-C6H3)furan-2-yl
20 1161 4-(2,5-F2-C6H3)furan-2-yl
1162 4-(2,6-F2-C6H3)furan-2-yl
1163 4-(3~4-F2-c6H3)furan-2-yl
1164 4-(2-Cl, 5-OCH3-C6H3)furan-2-yl
1165 4-(2-Cl, 5-CH3-C6H3)furan-2-yl
25 1166 4-(5-Cl, 2-OCH3-C6H3)furan-2-yl
1167 4-(5-Cl, 2-CH3-C6H3)furan-2-yl
1168 4-[2,5-(CH3)2-C6H3]furan-2-yl
1169 5-CH(CH3)2-thien-2-yl
30 1170 5-C(CH3)3-thien-2-yl
1171 5-cyclopropylthien-2-yl
1172 5-C6H5-thien-2-yl
1173 5-(2-CH3-C6H4)thien-2-yl
1174 5-(3-CH3-C6H4)thien-2-yl
35 1175 5-(4-CH3-C6H4)thien-2-yl
1176 5-(3-OCH3-C6H4)thien-2-yl
1177 5-(4-OCH3-C6H4)thien-2-yl
1178 5-(4-NO2-C6H4)thien-2-yl
40 1179 5-(3-No2-c6H4)thien-2
1180 5-(4-CN-C6H4)thien-2-yl
1181 5-(3-cN-c6H4)thien-2-yl
1182 5-(3-CF3-C6H4)thien-2-yl
1183 5-(4-CF3-C6H4)thien-2-yl
45 1184 5-(4-C(CH3)3-C6H4)thien-2-yl
1185 5-(2-Cl-C6H4)thien-2-yl
CA 02231661 1998-04-07
0050/4627 ~
133
No. R4
1186 5-(3-Cl-C6H4)thien-2-yl
1187 5-(4-Cl-C6H4)thien-2-yl
1188 5-(2-Br-C6H4)thien-2-yl
1189 5-(3-Br-C6H4)thien-2-yl
1190 5-(4-Br-C6H4)thien-2-yl
1191 5-(2-F-C6H4)thien-2-yl
10 1192 5-(3-F-C6H4)thien-2-yl
1193 5-(4-F-C6H4)thien-2-yl
1194 5-(2,4-C12-C6H3)thien-2-yl
ll9S 5-(2,5-C12-C6H3)thien-2-yl
1196 5-(2,6-C12-C6H3)thien-2-yl
1197 5-~3,4-C12-C6H3)thien-2-yl
1198 5-(2,4-F2-C6H3)thien-2-yl
1199 5-(2,5-F2-C6H3)thien-2-yl
1200 5-(2,6-F2-C6H3)thien-2-yl
20 12ol 5-(3,4-F2-C6H3)thien-2-yl
1202 5-(2-Cl, 5-OCH3-C6H3)thien-2-yl
1203 5-(2-Cl, 5-CH3-C6H3)thien-2-yl
1204 5-(5-Cl, 2-OCH3-C6H3)thien-2-yl
1205 5-(5-Cl, 2-CH3-C6H3)thien-2-yl
25 1206 5-[2,5-(CH3)2-C6H3]thien-2-yl
1207 4-CH(CH3)2-thien-2-yl
1208 4-C(CH3~3-thien-2-yl
1209 4-cyclopropylthien-2-yl
30 1210 4-C6H5-thien-2-yl
1211 4-(2-CH3-C6H4)thien-2-yl
1212 4-(3-CH3-C6H4)thien-2-yl
1213 4-(4-CH3-C6H4)thien-2-yl
1214 4-(3-OCH3-C6H4)thien-2-yl
35 1215 4-(4-OCH3-C6Hq)thien-2-yl
1216 4-(4-NO2-C6H4)thien-2-yl
1217 4-(3-NO2-C6H4)thien-2-yl
1218 4-(4-CN-C6H4)thien-2-yl
40 1219 4-(3-CN-C6H4)thien-2-yl
1220 4-(3-CF3-C6H4)thien-2-yl
1221 4-(4-cF3-c6H4)thien-2-yl
1222 4-(4-C(CH3)3-C6H4)thien-2-yl
1223 4-(2-Cl-C6H4)thien-2-yl
45 1224 4-(3-Cl-C6H4)thien-2-yl
1225 4-(4-Cl-C6H4)thien-2-yl
CA 02231661 1998-04-07
OOSO/46271
134
No. R4
1226 4-(2-Br-C6H4)thien-2-yl
1227 4-(3-Br-C6H4)thien-2-yl
1228 4-(4-Br-C6H4)thien-2-yl
1229 4-(2-F-C6H4)thien-2-yl
1230 4-(3-F-c6H4)thien-2
1231 4-(4-F-C6H4)thien-2-yl
10 1232 4-(2,4-C12-C6H3)thien-2-yl
1233 4-(2,5-C12-C6H3)thien-2-yl
1234 4-(2,6-C12-C6H3)thien-2-yl
1235 4-(3,4-Clz-C6H3)thien-2-yl
1236 4-(2,4-F2-C6H3)thien-2-yl
1237 4-(2,5-F2-C6H3)thien-2-yl
1238 4-(2,6-F2-C6H3)thien-2-yl
1239 4-(3~4-F ff 6H3)thien-2-yl
1240 4-(2-Cl, 5-OCH3-C6H3)thien-2-yl
20 1241 4-(2-Cl, 5-CH3-C6H3)thien-2-yl
1242 4-(5-Cl, 2-OCH3-C6H3)thien-2-yl
1243 4-(5-Cl, 2-CH3-C6H3)thien-2-yl
1244 4-[2,5-(CH3)2-C6H3]thien-2-yl
1245 2-CH3-thien-4-yl
25 1246 2-CH(CH3)2-thien-4-yl
1247 2-C(CH3)3-thien-4-yl
1248 2-cyclopropylthien-4-yl
1249 2-C6H5-thien-4-yl
30 1250 2-(2-cH3-c6H4)thien-4-yl
1251 2-~3-CH3-C6H4)thien-4-yl
1252 2-~4-CH3-C6H4)thien-4-yl
1253 2-~3-OCH3-C6H4)thien-4-yl
1254 2-(4-ocH3-c6H~)thien-4
35 1255 2-(4-NO2-C6H4)thien-4-yl
1256 2-(3-NO2-C6H4)thien-4-yl
1257 2-(4-cN-c6H4)thien-4
1258 2-(3-cN-c6H4)thien-4-yl
40 1259 2-(3-CF3-C6H4)thien-4-yl
1260 2-(4-cF3-c6H4)thien-4
1261 2-(4-c(cH3)3-c6H4)thien-4
1262 2-(2-Cl-C6H 4 ) thien-4-yl
1263 2-(3-Cl-C6H4)thien-4-yl
45 1264 2-(4-cl-c6H4)thien-4
1265 2-(2-Br-C6H4)thien-4-yl
CA 0223l66l l998-04-07
0050/4627 1
135
No. R4
1266 2-(3-Br-C6H4)thien-4-yl
1267 2-(4-Br-C6H4)thien-4-yl
1268 2-(2-F-C6H4)thien-4-yl
1269 2-(3-F-C6H4)thien-4-yl
1270 2-(4-F-C6H4)thien-4-yl
1271 2-(2,4-Cl2-C6H3)thien-4-yl
10 1272 2-(2,5-Cl2-C6H3)thien-4-yl
1273 2-(2,6-Cl2-C6H3)thien-4-yl
1274 2-(3,4-Cl2-C6H3)thien-4-yl
1275 2-(2,4-F2-C6H3)thien-4-yl
1276 2-~2,5-F2-C6H3)thien-4-yl
1277 2-~2,6-F2-C6H3)thien-4-yl
1278 2-~3,4-F2-C6H3)thien-4-yl
1279 2-~2-Cl, 5-OCH3-C6H3)thien-4-yl
1280 2-~2-Cl, 5-CH3-C6H3)thien-4-yl
20 128l 2-~5-Cl, 2-OCH3-4 H3)thien-4-yl
1282 2-~5-Cl, 2-CH3-C6H3)thien-4-yl
1283 2-[2,5-~CH3)2-C6H3]thien-4-yl
1284 1-CH~CH3)2-pyrazol-4-yl
1285 1-C(CH3)3-pyrazol-4-yl
25 1286 1-cyclopropylpyrazol-4-yl
1287 1-C6Hs-pyrazol-4-yl
1288 1-~2-CH3-C6H4)pyrazol-4-yl
1289 1-~3-CH3-C6H4)pyrazol-4-yl
30 1290 1-~4-CH3-C6H4)pyrazol-4-yl
1291 1-~3-OCH3-C6H4)pyrazol-4-yl
1292 1-~4-OCH3-C6H4)pyrazol-4-yl
1293 1-~4-NO2-C6H4)pyrazol-4-yl
1294 1-~3-NO2-C6H4)pyrazol-4-yl
35 1295 1-~4-CN-C6H4)pyrazol-4-yl
1296 1-~3-CN-C6H4)pyrazol-4-yl
1297 1-~3-CF3-C6H4)pyrazol-4-yl
1298 1-~4-CF3-C6H4)pyrazol-4-yl
40 1299 1-~4-C~CH3)3-C6H4)pyrazol-4-yl
1300 1-~2-Cl-C6H4)pyrazol-4-yl
1301 1-~3-Cl-C6H4)pyrazol-4-yl
1302 1-~4-Cl-C6H4)pyrazol-4-yl
1303 l-~2-Br-C6H4)pyrazol-4-yl
45 1304 1-(3-Br-C6H4)pyrazol-4-yl
1305 l-(4-Br-C6H4)pyrazol-4-yl
CA 02231661 1998-04-07
0050/4627~
136
No. R4
1306 1-(2-F-C6H4)pyrazol-4-yl
1307 1-(3-F-C6H4)pyrazol-4-yl
1308 1-(4-F-C6H4)pyrazo1-4-yl
1309 1-(2,4-C12-C6H3)pyrazol-4-yl
1310 1-(2,5-C12-C6H3)pyrazol-4-yl
1311 1-(2,6-C12-C6H3)pyrazol-4-yl
10 1312 1-(3,4-C12-C6H3)pyrazol-4-yl
1313 1-(2,4-F2-C6H3)pyrazol-4-yl
1314 1-(2,5-F2-C6H3)pyrazol-4-yl
1315 1-(2,6-F2-C6H3)pyrazol-4-yl
1316 1-(3,4-F2-C6H3)pyrazol-4-yl
1317 1-(2-Cl, 5-OCH3-C6H3)pyrazol-4-yl
1318 1-(2-Cl, 5-CH3-C6H3)pyrazol-4-yl
1319 1-(5-Cl, 2-OCH3-C6H3)pyrazol-4-yl
1320 1-(5-Cl, 2-CH3-C6H3)pyrazol-4-yl
20 1321 1-[2,5-(CH3)2-C6H3]pyrazol-4-yl
1322 1-CH(CH3)2-pyrazol-3-yl
1323 1-C(CH3)3-pyrazol-3-yl
1324 1-cyclopropylpyrazol-3-yl
1325 1-C6Hs-pyrazol-3-yl
25 1326 1-(2-CH3-C6H4)pyrazol-3-yl
1327 1-(3-CH3-C6H4)pyrazol-3-yl
1328 1-(4-CH3-C6H4)pyrazol-3-yl
1329 1-(3-OCH3-C6H4)pyrazol-3-yl
30 1330 1-(4-OCH3-C6H4)pyrazol-3-yl
1331 1-(4-N02-C6H4)pyrazol-3-yl
1332 1-(3-N02-C6H4)pyrazol-3-yl
1333 1-(4-CN-C6H4)pyrazol-3-yl
1334 1-(3-CN-C6H4)pyrazol-3-yl
35 1335 1-(3-CF3-C6H4)pyrazol-3-yl
1336 l-(4-CF3-C6H4)pyrazol-3-yl
1337 1-(4-C(CH3)3-C6H4)pyrazol-3-yl
1338 1-(2-Cl-C6H4)pyrazol-3-yl
40 1339 1-(3-Cl-C6H4)pyrazol-3-yl
1340 l-(4-cl-c6H4)pyrazol-3
1341 1-(2-Br-C6H4)pyrazol-3-yl
1342 1-(3-Br-C6H4)pyrazol-3-yl
1343 1-(4-Br-C6H4)pyrazol-3-yl
45 1344 1-(2-F-C6H4)pyrazol-3-yl
1345 1-(3-F-C6H4)pyrazol-3-yl
CA 02231661 1998-04-07
OOS0/46271
137
No. R4
1346 1-~4-F-C6H4)pyrazol-3-yl
1347 1-(2,4-C12-C6H3)pyrazol-3-yl
1348 1-(2,5-Cl2-C6H3)pyrazol-3-yl
1349 1-(2,6-C12-C6H3)pyrazol-3-yl
1350 1-(3,4-C12-C6H3)pyrazol-3-yl
1351 1-(2,4-F2-C6H3)pyrazol-3-yl
1352 1-(2,5-F2-C6H3)pyrazol-3-yl
1353 1-(2,6-F2-C6H3)pyrazol-3-yl
1354 1-(3,4-F2-C6H3)pyrazol-3-yl
1355 1-(2-Cl, S-OCH3-C6H3)pyrazol-3-yl
1356 1-(2-Cl, 5-CH3-C6H3)pyrazol-3-yl
1357 1-(5-Cl, 2-OCH3-C6H3)pyrazol-3-yl
1358 1-(5-Cl, 2-CH3-C6H3)pyrazol-3-yl
1359 1-[2,5-(CH3)2-C6H3lpyrazol-3-yl
1360 3-CH(CH3)2-isoxazol-5-yl
1361 3-C(CH3)3-isoxazol-5-yl
1362 3-cyclopropylisoxazol-5-yl
1363 3-C6Hs-isoxazol-5-yl
1364 3-(2-CH3-C6H4)isoxazol-5-yl
1365 3-(3-cH3-c6H4)isoxazol-5-yl
1366 3-(4-CH3-C6H4)isoxazol-5-yl
1367 3-(3-OCH3-C6H4)isoxazol-5-yl
1368 3-(4-OCH3-C6H4)isoxazol-5-yl
1369 3-(4-NO2-C6H4)isoxazol-5-yl
30 1370 3-(3-NO2-C6H4)isoxazol-5-yl
1371 3-(4-CN-C6H4)isoxazol-5-yl
1372 3-(3-CN-C6H4)isoxazol-5-yl
1373 3-(3-CF3-C6H4)isoxazol-5-yl
1374 3-(4-CF3-C6H4)isoxazol-5-yl
35 1375 3-(4-C(CH3)3-C6H4)isoxazol-5-yl
1376 3-(2-Cl-C6H4)isoxazol-5-yl
1377 3-(3-Cl-C6H4)isoxazol-5-yl
1378 3-(4-Cl-C6H4)isoxazol-5-yl
40 1379 3-(2-Br-C6H4)isoxazol-5-yl
1380 3-(3-Br-C6H4)isoxazol-5-yl
1381 3-(4-~r-C6H4)isoxazol-5-yl
1382 3-(2-F-C6H4)isoxazol-5-yl
1383 3-(3-F-C6H4)isoxazol-5-yl
45 1384 3-(4-F-C6H4)isoxazol-5-yl
1385 3-(2,4-Cl2-C6H3)isoxazol-5-yl
CA 02231661 1998-04-07
0050/46271
138
No. R4
1386 3-(2,5-Cl2-C6H3)isoxazol-5-yl
1387 3-(2,6-C12-C6H3)isoxazol-5-yl
1388 3-(3,4-C12-C6H3)isoxazol-5-yl
1389 3-(2,4-F2-C6H3)isoxazol-5-yl
1390 3-(2,5-F2-C6H3)isoxazol-5-yl
1391 3-(2,6-F2-C6H3)isoxazol-5-yl
10 1392 3-(3,4-F2-C6H3)isoxazol-5-yl
1393 3-(2-Cl, 5-OCH3-C6H3)isoxazol-5-yl
1394 3-(2-Cl, 5-CH3-C6H3)isoxazol-5-yl
1395 3-(5-Cl, 2-OCH3-C6H3)isoxazol-5-yl
1396 3-(5-Cl, 2-CH3-C6H3)isoxazol-5-yl
1397 3-[2,5-(CH3)2-C6H3]isoxazol-5-yl
1398 5-CH(CH3)2-isoxazol-3-yl
1399 5-C(CH3)3-isoxazol-3-yl
1400 5-cyclopropylisoxazol-3-yl
20 1401 5-C6H5-isoxazol-3-yl
1402 5-(2-CH3-C6H4)isoxazol-3-yl
1403 5-(3-CH3-C6H4)isoxazol-3-yl
1404 5-(4-CH3-C6H4)isoxazol-3-yl
1405 5-(3-OCH3-C6H4)isoxazol-3-yl
25 1406 5-(4-OCH3-C6H4)isoxazol-3-yl
1407 5-(4-NO2-C6H4)isoxazol-3-yl
1408 5-(3-NO2-C6H4)isoxazol-3-yl
1409 5-(4-CN-C6H4)isoxazol-3-yl
30 1410 5-(3-CN-C6H4)isoxazol-3-yl
1411 5-(3-CF3-C6H4)isoxazol-3-yl
1412 5-(4-CF3-C6H4)isoxazol-3-yl
1413 5-(4-C(CH3)3-C6H4)isoxazol-3-yl
1414 5-(2-Cl-C6H4)isoxazol-3-yl
35 1415 5-(3-Cl-C6H4)isoxazol-3-yl
1416 5-(4-Cl-C6H4)isoxazol-3-yl
1417 5-(2-Br-C6H4)isoxazol-3-yl
1418 5-(3-Br-C6H4)isoxazol-3-yl
40 1419 5-(4-Br-C6H4)isoxazol-3-yl
1420 5-(2-F-C6H4)isoxazol-3-yl
1421 5-(3-F-C6H4)isoxazol-3-yl
1422 5-(4-F-C6H4)isoxazol-3-yl
1423 5-(2,4-Cl2-C6H3)isoxazol-3-yl
45 1424 5-(2,5-C12-C6H3)isoxazol-3-yl
1425 5-(2,6-C12-C6H3)isoxazol-3-yl
CA 02231661 1998-04-07
0050/4627 1
139
No. R4
1426 5-(3,4-C12-C6H3)isoxazol-3-yl
1427 5-(2,4-F2-C6H3)isoxazol-3-yl
1428 5-(2,5-F2-C6H3)isoxazol-3-yl
1429 5-(2,6-F2-C6H3)isoxazol-3-yl
1430 5-(3,4-F2-C6H3)isoxazol-3-yl
1431 5-(2-Cl, 5-OCH3-C6H3)isoxazol-3-yl
10 1432 5-(2-Cl, 5-CH3-C6H3)isoxazol-3-yl
1433 5-(5-Cl, 2-OCH3-C6H3)isoxazol-3-yl
1434 S-(S-Cl, 2-CH3-C6H3)isoxazol-3-yl
1435 5-[2,5-(CH3)2-C6H3]isoxazol-3-yl
1436 3-CH(CH3)2-isothiazol-S-yl
1437 3-C(CH3)3-isothiazol-5-yl
1438 3-cyclopropylisothiazol-S-yl
1439 3-C6H5-isothiazol-S-yl
1440 3-(2-CH3-C6H4)isothiazol-S-yl
20 1441 3-(3-CH3-C6H4)isothiazol-S-yl
1442 3-(4-CH3-C6H4)isothiazol-S-yl
1443 3-(3-OCH3-C6H4)isothiazol-S-yl
1444 3-(4-OCH3-C6H4)isothiazol-S-yl
1445 3-(4-N02-C6H4)isothiazol-S-yl
1446 3-(3-N02-C6H4)isothiazol-S-yl
1447 3-(4-CN-C6H4)isothiazol-S-yl
1448 3-(3-CN-C6H4)isothiazol-5-yl
1449 3-(3-CF3-C6H4)isothiazol-S-yl
30 1450 3-(4-CF3-C6H4)isothiazol-S-yl
1451 3-(4-C(CH3)3-C6H4)isothiazol-S-yl
1452 3-(2-Cl-C6H4)isothiazol-S-yl
1453 3-(3-Cl-C6H4)isothiazol-S-yl
1454 3-(4-Cl-C6H4)isothiazol-S-yl
35 1455 3-(2-Br-C6H4)isothiazol-S-yl
1456 3-(3-Br-C6H4)isothiazol-S-yl
1457 3-(4-Br-C6H4)isothiazol-5-yl
1458 3-(2-F-C6H4)isothiazol-5-yl
40 1459 3-(3-F-C6H4)isothiazol-5-yl
1460 3-(4-F-C6H4)isothiazol-5-yl
1461 3-(2,4-C12-C6H3)isothiazol-5-yl
1462 3-(2,5-C12-C6H3)isothiazol-5-yl
1463 3-(2~6-Cl2-C6H3)isothiazol-5-yl
45 1464 3-(3,4-C12-C6H3)isothiazol-5-yl
1465 3-(2,4-F2-C6H3)isothiazol-5-yl
CA 02231661 1998-04-07
0050/46271
140
No. R4
1466 3-(2,5-F2-C6H3)isothiazol-5-yl
1467 3-(2,6-F2-C6H3)isothiazol-5-yl
1468 3-(3,4-F2-C6H3)isothiazol-5-yl
1469 3-~2-Cl, 5-OCH3-C6H3)isothiazol-5-yl
1470 3-(2-Cl, 5-CH3-C6H3)isothiazol-5-yl
1471 3-(5-Cl, 2-OCH3-C6H3)isothiazol-5-yl
10 1472 3-(5-Cl, 2-CH3-C6H3)isothiazol-5-yl
1473 3-[2,5-(CH3)2-C6H3]isothiazol-5-yl
1474 2-CH(CH3)2-oxazol-4-yl
1475 2-C(CH3)3-oxazol-4-yl
1476 2-cyclopropyloxazol-4-yl
1477 2-C6H5-oxazol-4-yl
1478 2-(2-CH3-C6H4)oxazol-4-yl
1479 2-(3-CH3-C6H4)oxazo1-4-yl
1480 2-(4-CH3-C6H4)oxazol-4-yl
20 148l 2-(3-OCH3-C6H4)oxazol-4-yl
1482 2-(4-OCH3-C6H4)oxazol-4-yl
1483 2-(4-N02-C6H4)oxazol-4-yl
1484 2-(3-N02-C6H4)oxazol-4-yl
1485 2-(4-CN-C6H4)oxazol-4-yl
25 1486 2-(3-CN-C6H4)oxazol-4-yl
1487 2-(3-CF3-C6H4)oxazol-4-yl
1488 2-(4-CF3-C6H4)oxazol-4-yl
1489 2-(4-C(CH3)3-C6H4)0xazol-4-yl
30 1490 2-(2-Cl-C6H4)oxazol-4-yl
1491 2-(3-Cl-C6H4)oxazol-4-yl
1492 2-(4-Cl-C6H4)oxazol-4-yl
1493 2-(2-Br-C6H4)oxazol-4-yl
1494 2-(3-Br-C6H4)oxazol-4-yl
35 1495 2-(4-Br-C6H4)oxazol-4-yl
1496 2-(2-F-C6H4)oxazol-4-yl
1497 2-(3-F-C6H4)oxazol-4-yl
1498 2-(4-F-C6H4)oxazol-4-yl
40 1499 2-(2,4-Cl2-C6H3)oxazol-4-yl
1500 2-(2,5-C12-C6H3)oxazol-4-yl
1501 2-(2,6-C12-C6H3)oxazol-4-yl
1502 2-(3,4-Cl2-C6H3)oxazol-4-yl
1503 2-(2,4-F2-C6H3)oxazol-4-yl
45 1504 2-(2,5-F2-C6H3)oxazol-4-yl
1505 2-(2,6-F2-C6H3)oxazol-4-yl
CA 02231661 1998-04-07
0050/46271
141
No. R4
1506 2-(3,4-F2-C6H3)0xazol-4-yl
lS07 2-(2-Cl, S-OCH3-C6H3)oxazol-4-yl
lS08 2-(2-Cl, S-CH3-C6H3)0xazol-4-yl
1509 2-(5-Cl, 2-OCH3-C6H3)0xazol-4-yl
1510 2-(5-Cl, 2-CH3-C6H3)oxazol-4-yl
lSll 2-[2,5-(CH3)2-C6H3]oxazol-4-yl
10 1512 2-CH(CH3)2-thiazol-4-yl
1513 2-C(CH3)3-thiazol-4-yl
1514 2-cyclopropylthiazol-4-yl
1515 2-C6H5-thiazol-4-yl
1516 2-(2-CH3-C6H4)thiazol-4-yl
1517 2-(3-CH3-C6H4)thiazol-4-yl
1518 2-(4-cH3-c6H4)thiazol-4-yl
lS19 2-(3-OCH3-C6H4)thiazol-4-yl
lS20 2-(4-OCH3-C6H4)thiazol-4-yl
20 lS21 2-(4-N02-C6H4)thiazol-4-yl
lS22 2-(3-N02-C6H4)thiazol-4-yl
1523 2-(4-CN-C6H4)thiazol-4-yl
1524 2-(3-CN-C6H4)thiazol-4-yl
1525 2-(3-CF3-C6H4)thiazol-4-yl
25 1526 2-(4-CF3-C6H4)thiazol-4-yl
1527 2-(4-C(CH3)3-C6H4)thiazol-4-yl
lS28 2-(2-Cl-C6H4)thiazol-4-yl
lS29 2-(3-Cl-C6H4)thiazol-4-yl
30 lS30 2-(4-Cl-C6H4)thiazol-4-yl
lS31 2-(2-Br-C6H4)thiazol-4-yl
lS32 2-(3-Br-C6H4)thiazol-4-yl
lS33 2-(4-Br-C6H4)thiazol-4-yl
lS34 2-(2-F-C6H4)thiazol-4-yl
35 lS3S 2-(3-F-C6H4)thiazol-4-yl
lS36 2-(4-F-C6H4)thiazol-4-yl
lS37 2-(2,4-C12-C6H3)thiazol-4-yl
lS38 2-(2,S-C12-C6H3)thiazol-4-yl
40 lS39 2-(2,6-C12-C6H3)thiazol-4-yl
lS40 2-(3~4-cl2-c6H3)thiazol-4
lS41 2-(2,4-F2-C6H3)thiazol-4-yl
lS42 2-(2~5-F2-c6H3)thiazol-4
1543 2-(2,6-F2-C6H3)thiazol-4-yl
45 1544 2-(3,4-F2-C6H3)thiazol-4-yl
lS4S 2-(2-Cl, 5-OCH3-C6H3)thiazol-4-yl
CA 02231661 1998-04-07
0050/46271
142
No. R4
1546 2-(2-Cl, 5-CH3-C6H3)thiazol-4-yl
1547 2-(5-Cl, 2-OCH3-C6H3)thiazol-4-yl
1548 2-(5-Cl, 2-CH3-C6H3)thiazol-4-yl
1549 2-[2,5-(CH3)2-C6H3]thiazol-4-yl
1550 l~3-(cH3)2-l~2~4-triazol-5-yl
1551 1-CH(CH3)2-1,2,4-triazol-3-yl
1552 1-C(CH3)3-1,2,4-triazol-3-yl
1553 1-cyclopropyl-1,2,4-triazol-3-yl
1554 1-C6H5-1,2,4-triazol-3-yl
1555 1-(2-CH3-C6H4)-1,2,4-triazol-3-yl
1556 l-(3-cH3-c6H4)-l~2~4-triazol-3-yl
1557 1-(4-CH3-C6H4)-1,2,4-triazol-3-yl
1558 1-(3-OCH3-C6H4)-1,2,4-triazol-3-yl
1559 1-(4-OCH3-C6H4)-1,2,4-triazol-3-yl
1560 1-(4-N02-C6H4)-1,2,4-triazol-3-yl
156l 1-(3-N02-C6H4)-1,2,4-triazol-3-yl
1562 1-(4-CN-C6H4)-1,2,4-triazol-3-yl
1563 1-(3-CN-C6H4)-1,2,4-triazol-3-yl
1564 1-(3-CF3-C6H4)-1,2,4-triazol-3-yl
1565 1-(4-CF3-C6H4)-1,2,4-triazol-3-yl
1566 1-(4-C(CH3)3-C6H4)-1,2,4-triazol-3-yl
1567 1-(4-C6H5-C6H4)-1,2,4-triazol-3-yl
1568 1-(2-Cl-C6H4)-1,2,4-triazol-3-yl
1569 l-(3-cl-c6H4)-l~2~4-triazol-3-yl
1570 l-(4-Cl-C6H4)-1,2,4-triazol-3-yl
1571 1-(2-Br-C6H4)-1,2,4-triazol-3-yl
1572 1-(3-Br-C6H4)-1,2,4-triazol-3-yl
1573 1-(4-Br-C6H4)-1,2,4-triazol-3-yl
1574 1-(2-F-C6H4)-1,2,4-triazol-3-yl
35 1575 1-(3-F-C6H4)-1,2,4-triazol-3-yl
1576 1-(4-F-C6H4)-1,2,4-triazol-3-yl
1577 1-(2,4-C12-C6H3)-1,2,4-triazol-3-yl
1578 1-(2,5-C12-C6H3)-1,2,4-triazol-3-yl
40 1579 l-(2~6-cl2-c6H3)-l~2~4-triazol-3
1580 l-(3~4-cl2-c6H3)-l~2~4-triazol-3
1581 1-(2,4-F2-C6H3)-1,2,4-triazol-3-yl
1582 1-(2,5-F2-C6H3)-1,2,4-triazol-3-yl
1583 l-(2,6-F2-C6H3)-1,2,4-triazol-3-yl
45 1584 1-(3,4-F2-C6H3)-1,2,4-triazol-3-yl
1585 1-(2-Cl, 5-OCH3-C6H3)-1,2,4-triazol-3-yl
CA 02231661 1998-04-07
0050/46271
143
No. R4
1586 1-(2-Cl, 5-CH3-C6H3)-1,2,4-triazol-3-yl
1587 1-(5-Cl, 2-OCH3-C6H3)-1,2,4-triazol-3-yl
1588 1-(5-Cl, 2-CH3-C6H3)-1,2,4-triazol-3-yl
1589 l-[2~5-(cH3)2-c6H3]-l~2~4-triazol-3
1590 5-C(CH3)3-1,3,4-oxadiazol-2-yl
1591 5-cyclopropyl-1,3,4-oxadiazol-2-yl
1592 5-C6Hs-1,3,4 ox~A.~iAzol-2-yl
1593 5-(2-CH3-C6H4)-1,3,4-oxadiazol-2-yl
1594 5-(3-CH3-C6H4)-1,3,4-oxadiazol-2-yl
1595 5-(4-CH3C6H4)-1,3,4-oxadiazol-2-yl
1596 5-(3-OCH3C6H4)-1,3,4- OyA~ i A zol - 2-yl
1597 5-(4-OCH3C6H4)-1,3,4- OYA~ i A zol-2-yl
1598 5-(4-NO2-C6H4)-1,3,4-oxadiazol-2-yl
1599 5-(3-NO2-C6H4)-1,3,4-oxadiazol-2-yl
1600 5-(4-CN-C6H4)-1,3,4- OY~A,~ i A 7O1-2-yl
20 1601 5-(3-CN-C6H4)-1,3,4- OyA~ i A 701 - 2-yl
1602 5-(3-CF3C6H4)-1,3,4 - OXA~ i A 7O1-2-yl
1603 5-(4 CF3C6H4)-1,3~4 OyA~ i ,A zol-2-yl
1604 5-(4-C(CH3)3C6H4)-1,3,4-oxadiazol-2-yl
1605 5-(2-Cl-C6H4)-1,3,4-oxadiazol-2-yl
1606 5-(3-Cl-C6H4)-1,3,4-oYA~iAzol-2-yl
1607 5-(4-Cl-C6H4)-1,3,4-oxadiazol-2-yl
1608 5-(2-Br-C6H4)-1,3,4-oYAAiAzol-2-yl
1609 5-(3-Br-C6H4)-1,3,4-oY~i A 7.01-2-yl
30 1610 5-(4-Br-C6H4)-1,3,4-oxadiazol-2-yl
1611 5-(2-F-C6H4)-1,3,4-oYA~iazol-2-yl
1612 5-(3-F-C6H4)-1,3,4-oY~ zol-2-yl
1613 5-(4-F-C6H4)-1,3,4-oY~iAzol-2-yl
1614 5-(2,4-C12-C6H3)-1,3,4- OyA~ i A 7O1-2-yl
35 1615 5-(2,5-Cl2-C6H3)-1,3,4-oY~iA701-2-yl
1616 5-(2,6-C12-C6H3)-1,3,4-oxadiazol-2-yl
1617 5-(3,4-C12-C6H3)-1,3,4-oxadiazol-2-yl
1618 5-(2,4-F2-C6H3)-1,3,4-oxadiazol-2-yl
40 1619 5-(2,5-F2-C6H3)-1,3,4-oxadiazol-2-yl
1620 5-(2,6-F2-C6H3)-1,3,4-oxadiazol-2-yl
1621 5-(3,4-F2-C6H3)-1,3,4-oxadiazol-2-yl
1622 5-(2-Cl, 5-OCH3C6H3)-1,3,4-oxadiazol-2-yl
1623 5-(2-Cl, 5-CH3C6H3)-1,3,4-oxadiazol-2-yl
45 1624 5-(5-Cl, 2-OCH3C6H3)-1,3,4-oxadiazol-2-yl
1625 5-(5-Cl, 2-CH3C6H3)-1,3,4-oxadiazol-2-yl
CA 0223l66l l998-04-07
0050/46271
144
No. R4
1626 5-[2,5-(CH3)2-C6H3]-1,3,4-oxadiazol-2-yl
1627 5-C(CH3)3-1,2,4-oxadiazol-3-yl
1628 5-cyclopropyl-1,2,4-oxadiazol-3-yl
1629 5-C6Hs-1,2,4-oxadiazol-3-yl
1630 5-(2-CH3C6H4)-1,2,4-oxadiazol-3-yl
1631 5-(3-CH3C6H4)-1,2,4-oxa~iazol-3-yl
1632 5-(4-CH3C6H4)-1,2,4-oxadiazol-3-yl
1633 5-(3-OCH3C6H4)-1,2,4-oxadiazol-3-yl
1634 5-(4-OCH3C6H4)-1,2,4-oxadiazol-3-yl
1635 5-(4-NO2-C6H4)-1,2,4-oxadiazol-3-yl
1636 5-(3-NO2-C6H4)-1,2,4-oxadiazol-3-yl
1637 5-(4-CN-C6H4)-1,2,4-oxadiazol-3-yl
1638 5-(3-CN-C6H4)-1,2,4-oYA~i~zol-3-yl
1639 5-(3-CF3-C6H4)-1,2,4-oxadiazol-3-yl
1640 5-(4-CF3-C6H4)-1,2,4-oY~A~iA7.ol-3-yl
164l 5-(4-C(CH3)3C6H4)-1,2,4-oxadiazol-3-yl
1642 5-(2-Cl-C6H4)-1,2,4-oxA~i~zol-3-yl
1643 5-(3-Cl-C6H4)-1,2,4-oxadiazol-3-yl
1644 5-(4-Cl-C6H4)-1,2,4-oxa~iazol-3-yl
1645 5-(2-Br-C6H4)-1,2,4-oxadiazol-3-yl
1646 5-(3-Br-C6H4)-1,2,4-oxA~ia7ol-3-yl
1647 5-(4-Br-C6H4)-1,2,4-oxadiazol-3-yl
1648 5-(2-F-C6H4)-1,2,4-oxadiazol-3-yl
1649 5-(3-F-C6H4)-1,2,4-oxadiazol-3-yl
1650 5-(4-F-C6H4)-1,2,4-oxadiazol-3-yl
1651 5-(2,4-Cl2-C6H3)-1,2,4- oXA~i A701-3-yl
1652 5-(2,5-Cl2-C6H3)-1,2,4-oxadiazol-3-yl
1653 5-(2,6-Cl2-C6H3)-1,2,4-oxadiazol-3-yl
1654 5-(3,4-Cl2-C6H3)-1,2,4-oxadiazol-3-yl
1655 5-(2,4-F2-C6H3)-1,2,4-oYa~i~Azol-3-yl
1656 5-(2,5-F2-C6H3)-1,2,4-oxadiazol-3-yl
1657 5-(2,6-F2-C6H3)-1,2,4-ox~-a7.01-3-yl
1658 5-(3~4-F2-c6H3)-l~2~4-oxadiazol-3-yl
1659 5-(2-Cl, 5-OCH3C6H3)-1,2,4-oxadiazol-3-yl
1660 5-(2-Cl, 5-CH3C6H3)-1,2,4-oxadiazol-3-yl
1661 5-(5-Cl, 2-OCH3C6H3)-1,2,4-oxadiazol-3-yl
1662 5-(5-Cl, 2-CH3C6H3)-1,2,4-oxadiazol-3-yl
1663 5-[2,5-(CH3)2-C6H3]-1,2,4-oxadiazol-3-yl
1664 3-CH3-1,2,4-oxadiazol-5-yl
1665 3-CH(CH3)2-1,2,4-oxadiazol-5-yl
CA 02231661 1998-04-07
0050/46271
145
No. R4
1666 3-C~CH3)3-1,2,4-oxadiazol-5-yl
1667 3-cyclopropyl-1,2,4-oxadiazol-5-yl
1668 3-C6Hs-1,2,4-oxadiazol-5-yl
1669 3-(2-CH3C6Hq)-1,2,4-oxadiazol-5-yl
1670 3-(3-CH3C6H4)-1,2,4-oxadiazol-5-yl
1671 3-(4-CH3C6H4)-1,2,4-oxadiazol-5-yl
10 1672 3-(3-OCH3C6H4)-1,2,4-oxadiazol-5-yl
1673 3-(4-OCH3C6H4)-1,2,4-oxadiazol-5-yl
1674 3-(4-NO2-C6H4)-1,2,4-oxadiazol-5-yl
1675 3-(3-NO2-C6H4)-1,2,4-oxadiazol-5-yl
1676 3-(4-CN-C6H4)-1,2,4-oxadiazol-5-yl
1677 3-(3-CN-C6H4)-1,2,4- OyA~; A701 - 5-yl
1678 3-(3-CF3-C6H4)-1,2,4-oYA~;A7ol-5-yl
1679 3-(4-CF3-C6H4)-1,2,4-oxadiazol-5-yl
1680 3-(4-c(cH3)3c6H4)-l~2~4-oyA~iAzol-5
20 1681 3-(2-Cl-C6H4)-1,2,4-oYA.~;A7ol-5-yl
1682 3-(3-Cl-C6H4)-1,2,4-oxadiazol-5-yl
1683 3-(4-Cl-C6H4)-1,2,4-oxadiazol-5-yl
1684 3-(2-Br-C6H4)-1,2,4-oxadiazol-5-yl
1685 3-(3-Br-C6H4)-1,2,4-sY~;Azol-5-yl
1686 3-(4-Br-C6H4)-1,2,4- OYA~; A 701 - 5 - yl
1687 3-(2-F-C6H4)-1,2,4-oYAAiA7ol-s-yl
1688 3-(3-F-C6H4)-1,2,4-oYA~;A.7ol-5-yl
1689 3-(4-F-C6H4)-1,2,4-oYa~;P701-5-yl
30 1690 3-(2,4-Cl2-C6H3)-1,2,4-oYA~;A701-5-yl
1691 3-(2,5-Cl2-C6H3)-1,2,4-oY~;A701-5-yl
1692 3-(2,6-C12-C6H3)-1,2,4-oYA~;Azol-5-yl
1693 3-(3,4-C12-C6H3)-1,2,4-oY~A~iAzol-5-yl
1694 3-(2,4-F2-C6H3)-1,2,4-oxadiazol-5-yl
1695 3-(2,5-F2-C6H3)-1,2,4-oYA~;A7ol-5-yl
1696 3-(2,6-F2-C6H3)-1,2,4-oYA~;A7ol-5-yl
1697 3-(3,4-F2-C6H3)-1,2,4-oxadiazol-5-yl
1698 3-(2-Cl, 5-OCH3C6H3)-1,2,4-oxadiazol-5-yl
40 1699 3-(2-Cl, 5-CH3-C6H3)-1,2,4-oxadiazol-5-yl
1700 3-(5-Cl, 2-OCH3-C6H3)-1,2,4-oxadiazol-5-yl
1701 3-(5-Cl, 2-CH3-C6H3)-1,2,4-oxadiazol-5-yl
1702 3-[2,5-(CH3)2-C6H3]-1,2,4-oxadiazol-5-yl
1703 5-CH3-1,2,4-thiadiazol-3-yl
45 1704 5-cH(cH3)2-l~2~4-thiadiazol-3
1705 5-C(CH3)31,2,4-thiadiazol-3-yl
CA 0223l66l l998-04-07
O05O/46271
146
No. R4
1706 S-cyclopropyl-1,2,4-thiadiazol-3-yl
1707 5-C6H5-1,2,4-thiadiazol-3-yl
1708 5-(2-CH3-C6H4)-1,2,4-~h;A~i~zol-3-yl
1709 5-(3-CH3-C6H4)-1,2,4-thiadiazol-3-yl
1710 5-(4-CH3-C6H4)-1,2,4-thiadiazol-3-yl
1711 5-(3-OCH3-C6H4)-1,2,4-thiadiazol-3-yl
10 1712 5-(4-OCH3-C6H4)-1,2,4-thiadiazol-3-yl
1713 5-(4-NO2-C6H4)-1,2,4-thiadiazol-3-yl
1714 5-(3-NO2-C6H4)-1,2,4-thiadiazol-3-yl
1715 5-(4-CN-C6H4)-1,2,4-thiadiazol-3-yl
1716 5-(3-CN-C6H4)-1,2,4-thiadiazol-3-yl
1717 5-(3-CF3-C6H4)-1,2,4-thiadiazol-3-yl
1718 5-(4-CF3-C6H4)-1,2,4-thiadiazol-3-yl
1719 5-(4-C(CH3)3-C6H4)-1,2,4-thiA~;azol-3-yl
1720 5-(2-Cl-C6H4)-1,2,4-thiadiazol-3-yl
20 1721 5-(3-Cl-C6H4)-1,2,4-thiadiazol-3-yl
1722 5-(4-Cl-C6H4)-1,2,4-thiadiazol-3-yl
1723 5-(2-Br-C6H4)-1,2,4-thiadiazol-3-yl
1724 5-(3-Br-C6H4)-1,2,4-thiA~;Azol-3-yl
1725 5-(4-Br-C6H4)-1,2,4-thiadiazol-3-yl
25 1726 5-(2-F-C6H4)-1,2,4-thiadiazol-3-yl
1727 5-(3-F-C6H4)-1,2,4-~hi ,A~i ,AZOl - 3 - yl
1728 5-(4-F-C6H4)-1,2,4-thiadiazol-3-yl
1729 5-(2,4-Cl2-C6H3)-1,2,4-~hi-AdiAzol-3
30 1730 5-(2,5-Cl2-C6H3)-1,2,4-~hi A~ i A z.ol-3-yl
1731 5-(2,6-Cl2-C6H3)-1,2,4-thi A~ i A zol-3-yl
1732 5-(3,4-Cl2-C6H3)-1,2,4-thiadiazol-3-yl
1733 5-(2,4-F2-C6H3)-1,2,4-thiadiazol-3-yl
1734 5-(2,5-F2-C6H3)-1,2,4-thiadiazol-3-yl
35 1735 5-(2,6-F2-C6H3)-1,2,4-thiadiazol-3-yl
1736 5-(3,4-F2-C6H3)-1,2,4-thiadiazol-3-yl
1737 5-(2-Cl, 5-OCH3-C6H3)-1,2,4-thiadiazol-3-yl
1738 5-(2-Cl, 5-CH3-C6H3)-1,2,4-thiadiazol-3-yl
40 1739 5-(5-Cl, 2-OCH3-C6H3)-1,2,4-thiadiazol-3-yl
1740 5-(5-Cl, 2-CH3-C6H3)-1,2,4-thiadiazol-3-yl
1741 5-[2,5-(CH3)2-C6H3]-1,2,4-thiadiazol-3-yl
1742 5-CH3-1,3,4-thiadiazol-2-yl
1743 5-CH(CH3) 2- 1, 3,4-thiadiazol-2-yl
45 1744 5-C(CH3)3-1,3,4-thiadiazol-2-yl
1745 5-cyclopropyl-1,3,4-thiadiazol-2-yl
CA 02231661 1998-04-07
0050/46271
147
No. R4
1746 5-C6H5-1,3,4-th;~ 701-2-yl
1747 5-(2-CH3-C6H4)-1,3,4-thiadiazol-2-yl
1748 5-(3-CH3-C6H4)-1,3,4-thiadiazol-2-yl
1749 5-(4-CH3-C6H4)-1,3,4-thiadiazol-2-yl
1750 5-(3-OCH3-C6H4)-1,3,4-thiadiazol-2-yl
1751 5-(4-OCH3-C6H4)-1,3,4-thiadiazol-2-yl
10 1752 5-(4-N02-C6H4)-1,3,4-thiadiazol-2-yl
1753 5-(3-NO2-C6H4)-1,3,4-thiadiazol-2-yl
1754 5-(4-CN-C6H4)-1,3,4-thiadiazol-2-yl
1755 5-(3-CN-C6H4)-1,3,4-thiadiazol-2-yl
1756 5-(3-CF3-C6H4)-1,3,4-thiadiazol-2-yl
1757 5-(4-CF3-C6H4)-1,3,4-thiadiazol-2-yl
1758 5-(4-C(CH3)3-C6H4)-1,3,4-thiadiazol-2-yl
1759 5-(2-Cl-C6H4)-1,3,4-thiadiazol-2-yl
1760 5-(3-Cl-C6H4)-1,3,4-thi A~i A zol-2-yl
20 1761 5-(4-Cl-C6H4)-1,3,4-~hi~iA7ol-2-yl
1762 5-(2-Br-C6H4)-1,3,4-thiadiazol-2-yl
1763 5-(3-Br-C6H4)-1,3,4-thiadiazol-2-yl
1764 5-(4-Br-C6H4)-1,3,4-thiadiazol-2-yl
1765 5-(2-F-C6H4)-1,3,4-thiadiazol-2-yl
25 1766 5-(3-F-C6H4)-1,3,4-thiAAi~zol-2-yl
1767 5-(4-F-C6H4)-1,3,4-thiadiazol-2-yl
1768 5-(2,4-Cl2-C6H3)-1,3,4-thi A~i ~zol-2-yl
1769 5-(2~5-Cl2-C6H3)-1~3~4-thi A~ i A 7.ol-2-yl
30 1770 5-(2,6-Cl2-C6H3)-1,3,4-thiadiazol-2-yl
1771 5-(3,4-C12-C6H3)-1,3,4-thiadiazol-2-yl
1772 5-(2,4-F2-C6H3)-1,3,4-thi~iAZol-2-yl
1773 5-(2,5-F2-C6H3)-1,3,4-thiadiazol-2-yl
1774 5-(2,6-F2-C6H3)-1,3,4-thia~iA7ol-2-yl
35 1775 5-(3,4-F2-C6H3)-1,3,4-thiadiazol-2-yl
1776 5-(2-Cl, 5-OCH3-C6H3)-1,3,4-thiadiazol-2-yl
1777 5-(2-Cl, 5-CH3-C6H3)-1,3,4-thiadiazol-2-yl
1778 5-(5-Cl, 2-OCH3-C6H3)-1,3,4-thiadiazol-2-yl
40 1779 5-(5-Cl, 2-CH3-C6H3)-1,3,4-thiadiazol-2-yl
1780 5-[2,5-(CH3)2-C6H3l-1,3,4-thiadiazol-2-yl
1781 1-CH(CH3)2-imidazol-4-yl
1782 1-C(CH3)3-imidazol-4-yl
1783 1-cyclopropylimidazol-4-yl
45 1784 1-C6H5-imidazol-4-yl
1785 1-(2-CH3-C6H4)imidazol-4-yl
CA 02231661 1998-04-07
0050/46271
148
No. R4
1786 1-(3-CH3-C6H4)imidazol-4-yl
1787 1-~4-CH3-C6H4)imidazol-4-yl
1788 1-(3-OCH3-C6H4)imidazol-4-yl
1789 1-(4-OCH3-C6H4)imidazol-4-yl
1790 1-(4-N02-C6H4)imidazol-4-yl
1791 1-(3-N02-C6H4)imidazol-4-yl
10 1792 1-(4-CN-C6H4)imidazol-4-yl
1793 1-(3-CN-C6H4)imidazol-4-yl
1794 1-(3-CF3-C6H4)imidazol-4-yl
1795 1-(4-CF3-C6H4)imidazol-4-yl
1796 1-(4-C(CH3)3C6H4)imidazol-4-yl
1797 1-(2-Cl-C6H4)imidazol-4-yl
1798 1-(3-Cl-C6H4)imidazol-4-yl
1799 1-(4-Cl-C6H4)imidazol-4-yl
1800 1-(2-Br-C6H4)imidazol-4-yl
20 1801 1-(3-Br-C6H4)imidazol-4-yl
1802 1-(4-~r-C6H4)imidazol-4-yl
1803 1-(2-F-C6H4)imidazol-4-yl
1804 1-(3-F-C6H4)imidazol-4-yl
1805 1-(4-F-C6H4)imidazol-4-yl
1806 1-(2,4-C12-C6H3)imidazol-4-yl
1807 1-(2,5-C12-C6H3)imidazol-4-yl
1808 1-(2,6-C12-C6H3)imidazol-4-yl
1809 1-(3,4-C12-C6H3)imidazol-4-yl
30 1810 1-(2,4-F2-C6H3)imidazol-4-yl
1811 1-(2,5-F2-C6H3)imidazol-4-yl
1812 1-(2,6-F2-C6H3)imidazol-4-yl
1813 1-(3,4-F2-C6H3)imidazol-4-yl
1814 1-(2-Cl, 5-OCH3-C6H3)imidazol-4-yl
35 1815 1-(2-Cl, 5-CH3-C6H3)imidazol-4-yl
1816 1-(5-Cl, 2-OCH3-C6H3)imidazol-4-yl
1817 1-(5-Cl, 2-CH3-C6H3)imidazol-4-yl
1818 1-[2,5-(CH3)2-C6H3]imidazol-4-yl
CA 02231661 1998-04-07
0050/4627 1
149
The compounds IIIa are novel
R3~
C = N - OH IIIa
R5 O - N /
I
R4'
the variables having the following meanings:
R3' is hydrogen, alkyl, haloalkyl or cycloalkyl
R4' is hydrogen or unsubstituted or substituted alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, heterocyclyl, aryl or
heteroaryl;
20 R5' is alkylsulfonyl or unsubstituted or substituted alkyl,
alkenyl, alkynyl or cycloalkyl.
Synthesis is carried out according to the processes described on
pp. 3/4, or [lacuna] can be prepared in analogy to processes
25 known per se.
The compounds VIIa
R3n
C = S VIIa
RSnO - N ~
I
R4
are also novel, the variables having the following meanings:
R3" is alkyl, haloalkyl or cycloalkyl;
R4" is unsubstituted or substituted alkyl, alkenyl, alkynyl,
cycloalkyl, cycloalkenyl, heterocyclyl, aryl or heteroaryl;
R5" is alkylsulfonyl or unsubstituted or substituted alkyl,
alkenyl, alkynyl or cycloalkyl
CA 02231661 1998-04-07
0050/4627 1
150
and it not being possible for R3 , R4 and R5 simultaneously to be
methyl
Synthesis is carried out in analogy to known processes (cf. Acta
5 Cienc. Indica. Chem 11, 124 (1985); J. Org. Chem. 51, 5198
(1986); J. Org. Chem. 52, 2927 (1987); Tetrahedron Lett. 24, 1851
(1983).)
Also novel are the compounds XII, XIII, XIV, XI [sic], XVI and
10 XVII
C = N- O - CH2~ ~ (R2)m (XII)
R50 - N ~ C=O
R4 OH
C=N - O - CH2~(R2 )m (XIII)
RsO - N / C=O
R4 Hal
(Hal = halogen)
R3 ~ (R2)m (XIV)
C= ~ O--CH2
R50 - N / C=O
R4 CN
CA 02231661 1998-04-07
0050/4627
151
C= N--O--CH 2 J3 ( R2 ) m ( XV )
5R50--N / C=0
R4 C02R
C= N--O--CH2/¢~ ( R2 ) m ( XVI )
15R50--N ~ C=0
R4 C=0
N ( Z ) _R
(Z = H or Cl-C4-alkyl)
C=N--O--CH2/~3 (R2 )m ( XVII )
R50--N CH2
C=O
R4
Y-Rl
35 the variables in each case having the same meaning as the
corresponding radicals of compounds of the formula I. The
synthesis of these compounds is described on pages 2 to 10 of
this application.
40 The compounds I are suitable as fungicides.
The compounds I are distinguished by an outstanding activity
against a broad spectrum of phytopathogenic fungi, in particular
from the class of Ascomycetes and Basidiomycetes. They are
45 systemically active in some cases and can be employed as foliar
and soil fungicides.
CA 0223l66l l998-04-07
0050/4627 1
152
They are of particular importance for the control of a
multiplicity of fungi on various crop plants such as wheat, rye,
barley, oats, rice, corn, grass, cotton, soybeans, coffee, sugar
cane, grapes, fruit and decorative plants and vegetable plants
5 such as cucumbers, beans and cucurbits, and on the seeds of these
plants.
They are specifically suitable for the control of the following
plant diseases: Erysiphe gr~ ;n;s (powdery mildew) in cereals,
10 Erysiphe cichoracearum and Sphaerotheca fuliginea on cucurbits,
Podosphaera leucotricha on apples, Uncinula necator on vines,
Puccinia species on cereals, Rhizoctonia species on cotton, rice
and grass, Ustilago species on cereals and sugar cane, Venturia
inaequalis (scab) on apples, Helminthosporium species on cereals,
15 Septoria nodorum on wheat, Botrytis cinerea (gray mold) on
strawberries, vines, vegetables and decorative plants, Cercospora
arachidicola on groundnuts, Pseudocercosporella herpotrichoides
on wheat, barley, Pyricularia oryzae on rice, Phytophthora
infestans on potatoes and tomatoes, Fusarium and Verticillium
20 species on various plants, Plasmopara viticola on vines,
Pseudoperonospora species in hops and cucumbers, Alternaria
species on vegetables and fruit.
The compounds I are applied by treating the fungi or the plants,
25 seeds, materials or the soil to be protected from fungal attack
with a fungicidally effective amount of the active compounds.
They are applied before or after the infection of the materials,
plants or seeds by the fungi.
30 Using formulation auxiliaries known per se, they can be converted
into the customary formulations (compositions), such as
solutions, emulsions, suspensions, dusts, powders, pastes and
granules. The application form depends on the particular intended
use; in each case it should guarantee a fine and uniform
35 distribution of the compounds I. The formulations are prepared in
a known manner, eg. by extending the active compound with
solvents and/or carriers, if desired using emulsifiers and
dispersants, it being possible in the case of water as a diluent
also to use other organic solvents as auxiliary solvents.
40 Suitable auxiliaries for this purpose are essentially: solvents
such as aromatics (eg. xylene), chlorinated aromatics (eg.
chlorobenzenes), paraffins (eg. petroleum fractions), alcohols
(eg. methanol, butanol), ketones (eg. cyclohexanone), amines ~eg.
ethanolamine, dimethylformamide) and water; carriers such as
45 ground natural minerals (eg. kaolins, argillaceous earths, talc,
chalk) and ground synthetic minerals (eg. highly disperse silica,
silicates); emulsifiers such as nonionic and anionic emulsifi¢rs
CA 02231661 1998-04-07
0050/4627 1
153
(eg. polyoxyethylene fatty alcohol ethers, alkylsulfonates and
arylsulfonates) and dispersants such as lignin-sulfite waste
liquors and methylcellulose.
5 The fungicidal compositions in general contain from 0.1 to 95,
preferably from 0.5 to 90, % by weight of active compound.
Depending on the type of effect desired, the application rates
are from 0.01 to 2.0 kg of active compound per ha.
In seed treatment, amounts of active compound of from 0.001 to
0.1 g, preferably 0.01 to 0.05 g, per kilogram of seed are in
general needed.
lS The compositions according to the invention can also be present
in the application form as fungicides together with other active
compounds, the [sic] eg. with herbicides, insecticides, growth
regulators, fungicides or alternatively with fertilizers.
20 On mixing with fungicides, in many cases an increase in the
fungicidal spectrum of action is obtained here.
The following list of fungicides with which the compounds
according to the invention can be applied together is inten~e~ to
25 illustrate the combination possibilities, but not restrict them:
sulfur, dithiocarbamates and their derivatives, such as ferric
dimethyldithiocarbamate, zinc dimethyldithiocarbamate, zinc
ethylenebisdithiocarbamate, manganese ethylenebisdithiocarbamate,
30 manganese zinc ethylene~i~minebisdithiocarbamate~ tetramethyl-
thiuram disulfides [sic], ammonia complex of zinc N,N-ethylene-
bisdithiocarbamate, ammonia complex of zinc N,N'-propylenebisdi-
thiocarbamate, zinc N,N'-propylenebisdithiocarbamate, N,N'-poly-
propylenebis(thiocarbamoyl) disulfide;
nitro derivatives, such as dinitro(l-methylheptyl)phenyl croto-
nate, 2-sec-butyl-4,6-dinitrophenyl 3,3-dimethylacrylate, 2-sec-
butyl-4,6-dinitrophenyl isopropyl carbonate, diisopropyl
5-nitroisophthalate;
heterocyclic substances, such as 2-heptadecyl-2-imidazoline
acetate, 2,4-dichloro-6-(o-chloroanilino)-s-triazine, O,O-diethyl
phthalimidophosphonothioate, 5-amino-1-[bis(dimethylamino)phos-
phinyl]-3-phenyl-1,2,4-triazole, 2,3-dicyano-1,4-dithioanthra-
45 quinone, 2-thio-1,3-dithiolo[4,5-b]quinoxaline, methyl l-(butyl-
carbamoyl)-2-benzimidazolecarbamate, 2-methoxycarbonylamino-
benzimidazole, 2-(fur-2-yl)benzimidazole, 2-(thiazol-4-yl)benz-
CA 0223l66l l998-04-07
0050/4627 1
154
imidazole, N - ( 1, 1, 2, 2 - tetrachloroethylthio)tetrahydrophthalimide,
N - trichloromethylthiotetrahydrophthalimide, N-trichloromethyl-
thiophthalimide;
5 N-dichlorofluoromethylthio- N', N'-dimethyl-N-phenylsulfamide,
5-ethoxy-3-trichloromethyl-1,2,4-thiadiazole, 2-thiocyanato-
methylthiobenzothiazole, 1,4-dichloro-2,5-dimethoxybenzene,
4-(2-chlorophenylhydrazono)-3-methyl-5-isoxazolone, pyridine-
2-thio-1-oxide [sic], 8-hydroxyquinoline or its copper salt,
10 2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiin,
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiin-4,4-dioxide,
2-methyl-5,6-dihydro-4H-pyran-3-carboxanilide, 2-methyl-
furan-3-carboxanilide, 2,5-dimethylfuran-3-carboxanilide,
2,4,5-trimethylfuran-3-carboxanilide, N-cyclohexyl-2,5-dimethyl-
15 furan-3-carbox~ e~ N-cyclohexyl-N-methoxy-2,5-dimethyl-
furan-3-carboxamide, 2-methylbenzanilide, 2-iodobenzanilide,
2,2,2-trichloro-1-(4-morpholinyl)ethylformamide, piperazine-
1,4-diylbis(l-(2,2,2-trichloroethyl)formamide lsic], 1-(3,4-di-
chloroanilino)-l-formylamino-2,2,2-trichloroethane, 2,6-dimethyl-
20 N-tridecylmorpholine or its salts, 2,6-dimethyl-N-cyclododecyl-
morpholine or its salts, N-[3-(p-tert-butylphenyl)-2-methyl-
propyl]-cis-2,6-dimethylmorpholine, N-[3-(p-tert-butylphenyl)-
2-methylpropyl]piperidine~ 1-[2-(2,4-dichlorophenyl)-4-ethyl-
1,3-dioxolan-2-ylethyl]-lH-1,2,4-triazole, 1-[2-(2,4-dichloro-
25 phenyl)-4-n-propyl-1,3-dioxolan-2-ylethyl]-lH-1,2,4-triazole,
N-(n-propyl)-N-(2,4,6-trichlorophenoxyethyl)- N ' - imidazolylurea,
1-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-triazol-1-yl)-
2-butanone, 1-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-tria-
zol-l-yl)-2-butanol, a-(2-chlorophenyl)-a-(4-chlorophenyl)-
30 5-pyrimidinemethanol, 5-butyl-2-dimethylamino-4-hydroxy-6-methyl-
pyrimidine, bis(p-chlorophenyl)-3-pyridinemethanol,
1,2-bis(3-ethoxycarbonyl-2-thioureido)benzene, 1,2-bis(3-methoxy-
carbonyl-2-thioureido)benzene;
35 and various fungicides, such as dodecylguanidine acetate,
3-[3-(3,5-dimethyl-2-oxycyclohexyl)-2-hydroxyethyl]glutarimide,
hexachlorobenzene, DL-methyl- N - ( 2,6-dimethylphenyl)- N - 2 - furoyl
alaninate, DL- N - ( 2,6-dimethylphenyl)-N-(2'-methoxyacetyl)alanine
methyl ester, N - ( 2,6-dimethylphenyl)- N - chloroacetyl-D,L-2-amino-
40 butyrolactone, DL-N-(2,6-dimethylphenyl)-N-(phenylacetyl)alanine
methyl ester, 5-methyl-5-vinyl-3-(3,5-dichlorophenyl)-
2,4-dioxo-1,3-oxazolidine, 3-[3,5-dichlorophenyl(-5-methyl-
5-methoxymethyl]-1,3-oxazolidine-2,4-dione [sic], 3-(3,5-di-
chlorophenyl)-l-isopropylcarbamoylhydantoin, N - ( 3,5-dichloro-
45 phenyl)-1,2-dimethylcyclopropane-1,2-dicarboximide, 2-cyano-
[ N - ethylaminocarbonyl-2-methoximino]acetamide, 1-[2-(2,4-di-
chlorophenyl)pentyl]-lH-1,2,4-triazole, 2, 4-difluoro-
CA 02231661 1998-04-07
U U3~
155
a-(lH-1,2,4-triazolyl-1-methyl)benzhydryl alcohol, N-(3-chloro-
2,6-dinitro-4-trifluoromethylphenyl)-5-trifluoromethyl-3-~hloro-
2-aminopyridine, 1-((bis(4-fluorophenyl)methylsilyl)methyl)-
lH-1,2,4-triazole;
strobilurins such as methyl E-methoximino[a-(o-tolyl-
oxy)-o-tolyl]acetate, methyl E-2-{2-[6-(2-cyanophenoxy)pyrimi-
din-4-yloxy]phenyl}-3-methoxyacrylate, methyl E-methoximino-
[a-(2-phenoxyphenyl)]acetamide, methyl E-methoximino-[a-(2,5-di-
10 methylphenoxy)-o-tolyl]acetamide;
anilinopyrimidines such as N-(4,6-dimethylpyrimidin-2-yl)aniline,
N-[4-methyl-6-(1-propynyl)pyrimidin-2-yl]aniline,
N-(4-methyl-6-cyclopropylpyrimidin-2-yl)aniline;
phenylpyrroles such as 4-(2,2-difluoro-1,3-benzodioxol-4-yl)-
pyrrole-3-carbonitrile;
cinn~m~-des such as 3-(4-chlorophenyl)-3-(3,4-dimethoxyphenyl)-
20 acrylic acid morpholide, (2RS,3SR)-1-[3-(2-chlorophenyl)-2-
[4-fluorophenyl)oxiran-2-ylmethyl]-lH-1,2,4-triazole.
The compounds of the formula I are additionally suitable for
controlling pests of the insects, arachnids and nematodes
25 classes. They can be employed as pesticides in crop protection
and in the hygiene, stored material protection and veterinary
sectors.
The harmful insects include: from the order of the butterflies
30 (Lepidoptera), for example, Agrotis ypsilon, Agrotis segetum,
Alabama argillacea, Anticarsia gemmatalis, Argyresthia
! conjugella, Autographa gamma, Bupalus piniarius, Cacoecia
murinana, Capua reticulana, Cheimatobia brumata, Choristoneura
fumiferana, Choristoneura occidentalis, Cirphis unipuncta, Cydia
35 pomonella, Dendrolimus pini, Diaphania nitidalis, Diatraea
grandiosella, Earias insulana, Elasmopalpus lignosellus,
Eupoecilia ambiguella, Evetria bouliana, Feltia subterranea,
Galleria mellonella, Grapholitha funebrana, Grapholitha molesta,
Heliothis armigera, Heliothis virescens, Heliothis zea, Hellula
40 undalis, Hibernia defoliaria, Hyphantria cunea, Hyponomeuta
malinellus, Keiferia lycopersicella, Lambdina fiscellaria,
Laphygma exigua, Leucoptera coffeella, Leucoptera scitella,
Lithocolletis blancardella, Lobesia botrana, Loxostege
sticticalis, Lymantria dispar, Lymantria monacha, Lyonetia
45 clerkella, Malacosoma neustria, Mamestra brassicae, Orgyia
pseudotsugata, Ostrinia nubilalis, Panolis flammea, Pectinophora
gossypiella, Peridroma saucia, Phalera bucephala, Phthorimaea
CA 02231661 1998-04-07
0050/4627 1
156
operculella, Phyllocnistis citrella, Pieris brassicae, Plathypena
scabra, Plutella xylostella, Pseudoplusia includens, Rhyacionia
frustrana, Scrobipalpula absoluta, Sitotroga cerealella,
Sparganothis pilleriana, Spodoptera frugiperda, Spodoptera
5 littoralis, Spodoptera litura, Thaumatopoea pityocampa, Tortrix
viridana, Trichoplusia ni, Zeiraphera canadensis;
from the order of the beetles (Coleoptera), for example, Agrilus
sinuatus, Agriotes lineatus, Agriotes obscurus, Amphimallus
10 solstitialis, Anisandrus dispar, Anthonomus grandis, Anthonomus
pomorum, Atomaria linearis, Blastophagus piniperda, Blitophaga
undata, Bruchus rufimanus, Bruchus pisorum, Bruchus lentis,
Byctiscus betulae, Cassida nebulosa, Cerotoma trifurcata,
Ceuthorrhynchus assimilis, Ceuthorrhynchus napi, Chaetocnema
15 tibialis, Conoderus vespertinus, Crioceris asparagi, Diabrotica
longicornis, Diabrotica 12-punctata, Diabrotica virgifera,
Epilachna varivestis, Epitrix hirtipennis, Eutinobothrus
brasiliensis, Hylobius abietis, Hypera brunneipennis, Hypera
posti~a, Ips typographus, Lema bilineata, Lema melanopus,
20 Leptinotarsa decemlineata, Limonius californicus, Lissorhoptrus
oryzophilus, Melanotus communis, Meligethes aeneus, Melolontha
hippocastani, Melolontha melolontha, Oulema oryzae,
Ortiorrhynchus [sic] sulcatus, Otiorrhynchus ovatus, Phaedon
cochleariae, Phyllotreta chrysocephala, Phyllophaga sp.,
25 Phyllopertha horticola, Phyllotreta nemorum, Phyllotreta
striolata, Popillia japonica, Sitona lineatus, Sitophilus
granaria;
from the order of the dipterans (Diptera), for example, Aedes
30 aegypti, Aedes vexans, Anastrepha ludens, Anopheles maculipen~is,
Ceratitis capitata, Chrysomya bezziana, Chrysomya hominivorax,
Chrysomya macellaria, Contarinia sorghicola, Cordylobia
anthropophaga, Culex pipiens, Dacus cucurbitae, Dacus oleae,
Dasineura brassicae, Fannia canicularis, Gasterophilus
35 intestinalis, Glossina morsitans, Haematobia irritans,
Haplodiplosis equestris, Hylemyia platura, Hypoderma lineata,
Liriomyza sativae, Liriomyza trifolii, Lucilia caprina [sicl,
Lucilia cuprina, Lucilia sericata, Lycoria pectoralis, Mayetiola
destructor, Musca domestica, Muscina stabulans, Oestrus ovis,
40 Oscinella frit, Pegomya hysocyami, Phorbia antiqua, Phorbia
brassicae, Phorbia coarctata, Rhagoletis cerasi, Rhagoletis
pomonella, Tabanus bovinus, Tipula oleracea, Tipula paludosa;
CA 0223l66l l998-04-07
0050/46271
157
from the order of the thrips (Thysanoptera), for example,
Frankliniella fusca, Frankliniella occidentalis, Frankliniella
tritici, Scirtothrips citri, Thrips oryzae, Thrips palmi, Thrips
tabaci;
from the order of the hymenopterans (Hymenoptera), for example,
Athalia rosae, Atta cephalotes, Atta sexdens, Atta texana,
Hoplocampa minuta, Hoplocampa testudinea, Monomorium pharaonis,
Solenopsis geminata, Solenopsis invicta;
from the order of the bugs (Heteroptera), for example, Acroster-
num hilare, Blissus leucopterus, Cyrtopeltis notatus, Dysdercus
cingulatus, Dysdercus intermedius, Eurygaster integriceps,
Euschistus impictiventris, Leptoglossus phyllopus, Lygus
15 lineolaris, Lygus pratensis, Nezara viridula, Piesma quadrata,
Solubea insularis, Thyanta perditor;
from the order of the plant-sucking insects (Homoptera), for
example, Acyrthosiphon ono~rychis, Adelges laricis, Aphidula
20 nasturtii, Aphis fabae, Aphis pomi, Aphis sambuci, Brachycaudus
cardui, Brevicoryne brassicae, Cerosipha gossypii, Dreyfusia
nordmann;An~e, Dreyfusia piceae, Dysaphis radicola,
Dysaulacorthum pseudosolani, Empoasca fabae, Macrosiphum avenae,
Macrosiphum euphorbiae, Macrosiphon rosae, Megoura viciae,
25 Metopolophium dirhodum, Myzodes persicae, Myzus cerasi,
Nilaparvata lugens, Pemphigus bursarius, Perkinsiella
saccharicida, Phorodon humuli, Psylla mali, Psylla piri,
Rhopalomyzus ascalonicus, Rhopalosiphum maidis, Sappaphis mala,
Sappaphis mali, Schizaphis graminum, Schizoneura lanuginosa,
30 Trialeurodes vaporariorum, Viteus vitifolii;
from the order of the termites (Isoptera), for example,
Calotermes flavicollis, Leucotermes flavipes, Reticulitermes
lucifugus, Termes natalensis;
from the order of the orthopterans (Orthoptera), for example,
Acheta domestica, Blatta orientalis, Blattella germanica,
Forficula auricularia, Gryllotalpa gryllotalpa, Locusta migrato-
ria, Melanoplus bivittatus, Melanoplus femur-rubrum, Melanoplus
40 mexicanus, Melanoplus sanguinipes, Melanoplus spretus, Nomadacris
septemfasciata, Periplaneta americana, Schistocerca americana,
Schistocerca peregrina, Stauronotus maroccanus, Tachycines
asynamorus;
45 from the order of the Arachnoidea, for example, spiders (Acarina)
such as Amblyomma americanum, Amblyomma variegatum, Argas persi-
cus, Boophilus annulatus, Boophilus decoloratus, Boophilus micro-
CA 02231661 1998-04-07
0050/4627 1
158
plus, Brevipalpus phoenicis, Bryobia praetiosa, Dermacentor sil-
varum, Eotetranychus carpini, Eriophyes sheldoni, Hyalomma trun-
catum, Ixodes ricinus, Ixodes rubicundus, Ornithodorus moubata,
Otobius megnini, Paratetranychus pilosus, Dermanyssus gallinae,
5 Phyllocoptruta oleivora, Polyphagotarsonemus latus, Psoroptes
ovis, Rhipicephalus appendiculatus, Rhipicephalus evertsi, Sar-
coptes scabiei, Tetranychus cinnabarinus, Tetranychus kanzawai,
Tetranychus pacificus, Tetranychus telarius, Tetranychus urticae;
10 from the class of the nematodes, for example, root gall
nematodes, eg. Meloidogyne hapla, Meloidogyne incognita,
Meloidogyne javanica, cyst-forming nematodes, eg. Globodera
rostochiensis, Heterodera avenae, Heterodera glycines, Heterodera
schachtii, Heterodera trifolii, stem and leaf nematodes, eg.
15 Belonolaimus longicaudatus, Ditylenchus destructor, Ditylenchus
dipsaci, Heliocotylenchus multicinctus, Longidorus elongatus,
Radopholus similis, Rotylenchus robustus, Trichodorus primitivus,
Tylenchorhynchus claytoni, Tylenchorhynchus dubius, Pratylenchus
neglectus, Pratylenchus penetrans, Pratylenchus curvitatus,
20 Pratylenchus goodeyi.
The compounds I can be applied as such, in the form of their
formulations (compositions) obtained using formulation
auxiliaries known per se or the application forms prepared
25 therefrom, eg. in the form of directly sprayable solutions,
powders, suspensions or dispersions, emulsions, oil dispersions,
pastes, dust compositions, scattering compositions or granules,
by spraying, atomizing, dusting, scattering or watering. The
application forms depend entirely on the intended uses; in each
30 case they should if possible guarantee the finest dispersion of
the active compounds according to the invention.
The active compound concentrations in the ready-to-use
preparations can be varied within relatively wide ranges.
In general, they are from 0.0001 to 10%, preferably from 0.01 to
1% .
The active compounds can also be used with good effect in the
40 ultra-low volume method (ULV), it being possible to apply
formulations containing more than 95% by weight of active
compound or even the active compound without additives.
Under outdoor conditions, the application rate of active compound
45 for controlling pests is from 0.1 to 2.0, preferably 0.2 to
1.0 kg/ha.
CA 02231661 1998-04-07
0050/4627 1
159
For the preparation of directly sprayable solutions, emulsions,
pastes or oil dispersions, mineral oil fractions of medium to
high boiling point are suitable, such as kerosene or diesel oil,
furthermore coal tar oils and oils of vegetable or ~nim~l origin,
5 aliphatic, cyclic and aromatic hydrocarbons, eg. benzene,
toluene, xylene, paraffin, tetrahydronaphthalene, alkylated
naphthalenes or their derivatives, methanol, ethanol, propanol,
butanol, chloroform, carbon tetrachloride, cyclohexanol,
cyclohexanone, chlorobenzene, isophorone, strongly polar
10 solvents, eg. dimethylformamide, dimethyl sulfoxide,
N-methylpyrrolidone and water.
Aqueous application forms can be prepared from emulsion
concentrates, pastes or wettable powders (oil dispersions) by
15 addition of water. To prepare emulsions, pastes or oil
dispersions, the substances can be homogenized in water as such
or dissolved in an oil or solvent, by means of wetting agents,
adherents, dispersants or emulsifiers. However, concentrates
consisting of active substance, wetting agent, adherent,
20 dispersant or emulsifier and possibly solvent or oil can also be
prepared which are suitable for dilution with water.
Suitable surface-active substances are the alkali metal, alkaline
earth metal and ammonium salts of lignosulfonic acid, naphtha-
25 lenesulfonic acid, phenolsulfonic acid and dibutylnaphthalene-
sulfonic acid, alkylarylsulfonates, alkylsulfates, alkyl-
sulfonates, fatty alcohol sulfates and fatty acids and also their
alkali metal and alkaline earth metal salts, salts of sulfated
fatty alcohol glycol ethers, condensation products of sulfonated
30 naphthalene and naphthalene derivatives with formaldehyde, con-
densation products of naphthalene or of naphthalenesulfonic acid
with phenol and formaldehyde, polyoxyethylene octylphenol [sic]
ether, ethoxylated isooctylphenol, octylphenol or nonylphenol,
alkylphenol [sic] or tributylphenyl polyglycol ether, alkylaryl
35 polyether alcohols, isotridecyl alcohol, fatty alcohol ethylene
oxide condensates, ethoxylated castor oil, polyoxyethylene alkyl
ethers, ethoxylated polyoxypropylene, lauryl alcohol polyglycol
ether acetate, sorbitol esters, lignosulfite waste liquors or
methylcellulose.
Powder, scattering and dusting compositions can be prepared by
mixing or joint grinding of the active substances with a solid
carrier.
45 The formulations in general contain from 0.01 to 95% by weight,
preferably from 0.1 to 90% by weight, of at least one active
compound. The active compounds are employed here in a purity of
CA 02231661 1998-04-07
0050/4627 1
160
from 90% to 100%, preferably 95% to 100% (according to NMR
spectrum).
Examples of formulations are:
I. 5 parts by weight of a compound I according to the
invention are intimately mixed with 95 parts by weight of
finely divided kaolin. In this way a dusting composition is
obtained which contains 5% by weight of the active
compound.
II. 30 parts by weight of a compound I according to the
invention are intimately mixed with a mixture of 92 parts
by weight of powdered silica gel and 8 parts by weight of
paraffin oil which has been sprayed onto the surface of
this silica gel. In this manner a preparation of the active
compound having good adherence is obtained (active compound
content 23% by weight).
20 III. 10 parts by weight of a compound I according to the
invention are dissolved in a mixture which consists of
90 parts by weight of xylene, 6 parts by weight of the
addition product of from 8 to 10 mol of ethylene oxide to
1 mol of oleic acid N-monoethanolamide, 2 parts by weight
of calcium dodecylbenzenesulfonate and 2 parts by weight of
the addition product of 40 mol of ethylene oxide to 1 mol
of castor oil (active compound content 9% by weight).
IV. 20 parts by weight of a compound I according to the
invention are dissolved in a mixture which consists of
60 parts by weight of cycloheY~none, 30 parts by weight of
isobutanol, 5 parts by weight of the addition product of
7 mol of ethylene oxide to 1 mol of isooctylphenol and
5 parts by weight of the addition product of 40 mol of
ethylene oxide to 1 mol of castor oil (active compound
content 16% by weight).
V. 80 parts by weight of a compound I according to the
invention are well mixed with 3 parts by weight of sodium
diisobutylnaphthalene-alpha-sulfonic acid, 10 parts by
weight of the sodium salt of a lignosulfonic acid from a
sulfite waste liquor and 7 parts by weight of powdered
silica gel and the mixture is ground in a hammer mill
(active compound content 80% by weight).
CA 02231661 1998-04-07
0050/4627
161
VI. 90 parts by weight of a compound I according to the
invention are mixed with 10 parts by weight of
N-methyl-a-pyrrolidone and a solution is obtained which is
suitable for use in the form of very small drops (active
compound content 90% by weight).
VII. 20 parts by weight of a compound I according to the
invention are dissolved in a mixture which consists of
40 parts by weight of cyclohexanone, 30 parts by weight of
isobutanol, 20 parts by weight of the addition product of
7 mol of ethylene oxide to 1 mol of isooctylphenol and
10 parts by weight of the addition product of 40 mol of
ethylene oxide to 1 mol of castor oil. By pouring the
solution into and finely dispersing it in 100,000 parts by
weight of water, an aqueous dispersion is obtained which
contains 0.02% by weight of the active compound.
VIII. 20 parts by weight of a compound I according to the
invention are well mixed with 3 parts by weight of sodium
diisobutylnaphthalene-a-sulfonic acid, 17 parts by weight
of the sodium salt of a lignosulfonic acid from a sulfite
waste liquor and 60 parts by weight of powdered silica gel
and the mixture is ground in a hammer mill. By finely
dispersing the mixture in 20,000 parts by weight of water,
a spray mixture is obtained which contains 0.1% by weight
of the active compound.
Granules, eg. coated, impregnated and homogeneous granules, can
be prepared by binding the active compounds to solid carriers.
30 Solid carriers are, for example, mineral earths, such as silica
gel, silicic acids, silica gels [sic], silicates, talc, kaolin,
attapulgous clay, limestone, lime, chalk, bole, loess, clay,
dolomite, diatomaceous earth, calcium and magnesium sulfate,
magnesium oxide, ground synthetic materials, fertilizers, such as
35 eg. ammonium sulfate, ammonium phosphate, ammonium nitrate, ureas
and vegetable products, such as grain flour, tree bark, wood and
nutshell meal, cellulose powder and other solid carriers.
Oils of various types, herbicides, fungicides, other pesticides
40 and bactericides can be added to the active compounds, if desired
only immediately before application (tank mix). These agents can
be admixed with the agents according to the invention in a weight
ratio from 1:10 to 10:1.
CA 0223l66l l998-04-07
0050/4627 1
162
Synthesis Example
The chemical shift (in ppm) of the 1H-NMR spectra was measured
against tetramethylsilane: br = broad; s = singlet, m =
5 multiplet.
Preparation of (E,E)-2-methoxyimino-2-[2'-[(1"-methyl-
l~-N-methoxy-N-methylamino)iminooxymethyl]phenyl]acetic acid
N-methylamide
~ ~ N J ~
I / N ~I ~ \ N / \
O
6.3 [lacuna] (61 mmol~ of N-methoxy-N-methylacetamide were
initially introduced into 100 ml of xylene, treated with 24.7 g
(61 mmol) of 2,4-bis(4-methoxyphenyl~ 3-dithia-2~4-diphos-
20 phetane-2,4-disulfide (Lawesson's reagent) and stirred at 100~C
for 1 hour. The reaction product was purified by column
chromatography. 5.9 g of N-methoxy-N-methylthioacetamide were
obtained as an orange oil.
25 1H-NMR (CDCl3): 2.63 (s, 3H); 3.7 (s, 3H); 3.8 (s, 3H).
1.9 g (23 mmol) of sodium acetate and 5.4 g (23 mmol) of
E-2-methoximino-2-[(2'-aminooxymethyl)phenyl]acetic acid
monomethylamide (cf. also EP-A 585 751) were added to a solution
30 of 2.7 g (23 mmol) of N-methoxy-N-methylthioacetamide in 50 ml of
ethanol and the reaction mixture was stirred at 70~C for 16 hours
and concentrated. The product was purified by column
chromatography on silica gel (methyl tert-butyl ether/n-h~Ane).
1.9 g of the title compound were obt~;ne~ as a colorless oil.
H-NMR (CDCl3): 1.94 (s, 3H); 2.81 (s, 3H); 2.87 (d, 3H); 3.57
(s, 3H); 3.92 (s, 3H); 4.85 (s, 2H); 6.69 (s, br, lH); 7.14-7.43
(m, 4H).
CA 02231661 1998-04-07
0050/4627 1
163
Use Examples
1. Example of the action against harmful fungi
5 It was possible to show the fungicidal action of the compounds of
the formula I by the following tests:
The active compounds were prepared as a 20% strength emulsion in
a mixture of 70% by weight of cyclohe~none, 20% by weight of
10 Nekanil~ LN ~Lutensol~ AP6, wetting agent having emulsifier and
dispersant action based on ethoxylated alkylphenols) and 10% by
weight of Emulphor~ EL (Emulan~ EL, emulsifier based on
ethoxylated fatty alcohols) and diluted with water according to
the concentration desired. Assessment was carried out visually.
Activity against wheat mildew
Leaves of wheat seedlings of the variety Fruhgold grown in pots
were sprayed with aqueous spray mixture and dusted with oidia
20 (spores) of wheat mildew (Erysiphe graminis var. tritici)
24 hours after the spray coating had dried on. The test plants
were then placed in a greenhouse at from 20 to 22~C and 75 to 80%
relative atomspheric humidity. After 7 days, the extent of mildew
development was determined.
The compound according to the invention, the comparison agent A
disclosed in WO-A 93/16986 (Table 1, No. H-2) and the comparison
agent B disclosed in WO-A 94/14761 (Table 8, No. 1) were employed
according to the methods described above.
~N N ~ Co.. lpli~onagentA
Comparison agentB
~\ S ~ N/ \~ (WO-A 94114761, Table 8, No. 1)
~ / NH ~ ~ / O
CA 02231661 1998-04-07
,
0050~46271
164
% attack on the leaves after application
of an aqueous active compound preparation
containing 63 ppm of active compound
Active compound 0
5 according to
synthesis example
Comparison agent A 60
Comparison agent B 40
Untreated 70
The results of the abovementioned tests clearly show the
advantages of the compound according to the invention.
2. Examples of the action against animal pests
It was possible to show the action of the compounds of the
general formula I against animal pests by the following tests
The active compounds were prepared
a) as a 0.1% strength solution in acetone or
b) as a 10% strength emulsion in a mixture of 70% by weight of
cyclohexanone, 20% by weight of Nekanil~ LN (Lutensol~ AP6,
wetting agent having emulsifing and dispersant action based
on ethoxylated alkyl phenols) and 10% by weight of Emulphor~
EL ~Emulan~ EL, emulsifier based on ethoxylated fatty
alcohols)
and diluted with acetone in the case of a) or with water in the
30 case of b) according to the concentration desired.
After conclusion of the tests, the lowest concentration in each
case was determined at which the compounds still produced an
80 - 100% inhibition or mortality in comparison with the
35 untreated control tests (activity threshold or minimum
concentration).