Note: Descriptions are shown in the official language in which they were submitted.
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
Thermallv stable l.i,~ red amines as .st~hili~rs
q
The invention relates to novel 2,2,6,6-tetramethylpiperidines which are sl ~hstitl ~ted on the
ring nitrogen and contain, in the 4-position, a s-lbstit--ent with a polymerizable double bond,
to their homopolymers and copolymers, to their use for st~hili~ing organic material against
damage by light, oxygen and/or heat, and to organic material stabilized by means of said
compounds or polymers.
A number of hindered amines of the 2,2,6,6-tetramethylpiperidine type have already been
disclosed as additives for synthetic polymers, in particular as light st~h~ ers; these include
4-suhstitlIted derivatives of 1-propargyl-2,2,6,6-t~lrd,-,e~hylpi~ueridi,le. By way of example,
the following publiG~tions may be mentioned in this respect:
US-A-4 472 547 (C.A.102:46831z); EP-A-11 051 (C.A.94:39518r); DE-A-2 805 838
(C.A.90:22820c). US-A-4 386 127 tC.A.99:89380p) also describes 1-butynyl-2,2,6,6-
tetramethyl-4-hydroxypiperidine.
The use of some compounds of the 2~6-diarylpiperidin-l-yl-sllhstitllted 2-butene type as
sf~ ers has also been disclosed (US-A-5 204 474).
A number of scienli~ic puhlic~tions describe 1-butynyl-2,2,6,6-tetramethylpiperidines whose
piperidine ring is unsllhstitllted in the 4-position, and their possihlE use as medicaments; for
e,~d.--,clE M. E. Zuhair et al., Eur. J. Med. Chem. 27, 93 (1992) (C.A.117:26405v); J. M. A.
Al-Rawi et al. in Org. Magn. Reson.19, 91 (1982) (C.A.97:155132w) and Org. Magn.Reson.15, 285 (1981) (C.A.95:60789k); B. Karlen et al., J. Med. Chem.13, 651 (1970)
(C.A.73:44857w) .
.
1,6-Bis(2,2,6,6-tetramethylpiperidin-1-yl)hexadiyne, whose piperidine rings are
unsl~hstitl,ted in the 4-position, is described in the publications US-A-3 755 586
(C.A.80:19550c); W. B. Lutz et al., J. Org. Chem. 27,1695 (1962) and US-A-3 085 093
(C.A.59:9998e) as a pharmaceutically active compound.
CA 02232~73 1998-03-20
W O 97/13752 PCT/~G/0~264
It has now been found that certain compounds of the 2,2,6,6-tetramethylpiperidine type
which contain both a triple bond in the ,~-position on the ring nitrogen atom and a
polymerizable double bond in the 4-position on the piperidine ring are surprisingly suitable
as st~h~ ers for organic ~-al~rial. They also have high thermal stability and thus allow
higher processing lempe~lures in the ~rplic~tion and consequently a higher throughput on
further processing of the products st~h~ ed in this way.
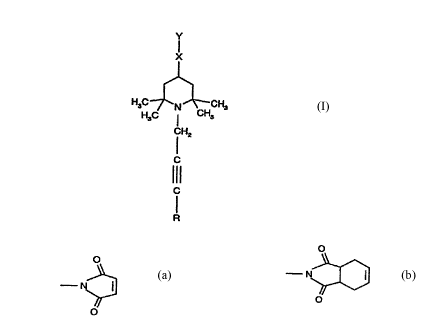
The invention therefore relates firstly to compounds of the formula I
H3C~CH3
H3C I CH3
CH2 (1)
111
R
in which
R is H, C1-C12alkyl, phenyl, (C1-C4alkyl)phenyl, phenyl-Cl-C4alkyl, halogen,
C2-C12alkynyl or C1-C12alkoxy,
X is NR', NCOR' or O, and
Y is R" or Rn-CO-, where R" is C2-C6alkenyl or C3-C6alkenyl which is interrupted by -O-,
-CO-, -NR'-, -S-, -SO-, -SO2- or phenylene, or (C1-C4alkenyl)phenyl, and
R' is H or C1-Cl2-alkyl, or
-X-Y
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
O O
is a group of the formula --N;~ or N
O O
C,-C12alkyl R and R' are, for example, methyl, ethyl, propyl, isopropyl, n-butyl, sec-butyl,
isobutyl, t-butyl, 2-ethylbutyl, n-pentyl, isopentyl, 1-methylpentyl, 1 ,3-dimethylbutyl,
n-hexyl, 1-methylhexyl, n-heptyl, isoheptyl, 1,1,3,3-tetramethylbutyl, 1-methylheptyl,
3-methylheptyl, n-octyl, 2-ethylhexyl, 1,1,3-trimethylhexyl, 1~1~3~3-L~Lrdlllelllylpentyl~ nonyl,
decyl, undecyl, 1-methylundecyl, dodecyl, or 1,1,3,3,5,5-hexamethylhexyl. Alkyl R and R'
contain, in particular, from 1 to 6, preferably from 1 to 4, carbon atoms.
C,-C4alkylphenyl R is phenyl which is mono- or polysllhstituted by alkyl. This group
contains, for example, from 1 to 3, in particular 1 or 2, alkyl suhstituents.
C,-C4alkylphenyl can be, for example, methylphenyl (tolyl), dimethylphenyl (xylyl),
trimethylphenyl (mesityl), ethylphenyl, propylphenyl or butylphenyl.
Phenyl-C,-C4alkyl R is, for example, benzyl, phenethyl, 3-phenylpropyl, a-methylbenzyl or
a,a'-dimethylbenzyl.
C2-C,2alkynyl R is, for exa." 'E, ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl,
3-butynyl, etc.. These alkynyl groups preferably have 2 to 4 carbon atoms.
C1-C12alkoxy R is, for example, methoxy, ethoxy, propoxy, butoxy, etc.. These alkoxy
groups preferably have 1 to 6, in particular 1 to 4, carbon atoms.
Halogen is F, Cl, Br or 1, prer~rdbly Cl or Br, but in particular Cl.
C2-C6alkenyl is, for example, vinyl, 1-propenyl, 2-propenyl, 1-butenyl, 2-butenyl, 3-butenyl
etc.. Preference is given to alkenyl groups having 2 to 4 carbon atoms. Preference is
generally given to alkenyl groups containing a C=C double bond in the 1,2-position.
CA 02232~73 1998-03-20
W O 97/137S2 PCT/EP96/04264
r, ~rt ~nce is given to compounds of the formula I in which
R is H, C1-caalkyl~ phenyl, (C,-C4aikyl)phenyl or phenyl-C,-C4alkyl, and
R" is C2-C4alkenyl, C3-C4alkenyl which is interrupted by -O-, -CO- or-NR'-, or
benzyl or phenyl which is substituted by C2-C4alkenyl.
Of particular il ~lere: il are compounds of the formula I in which
R is H, C,-C8alkyl or phenyl,
X is NH, N(Ct-C4alkyl) or 0, and
Y is a group of the formula CH2=CH-CO-, CH2=C(CH3)-CO-,
CH~CH2-- /CH~CO-- or CH~CH2--CO--
CH2 , CH2 CH2
or
-X-Y is the group of the formula N~ .
Of very particular il"pO~ lance are compounds of the formula I in whichR is H or methyl,
X is O or NH, and
Y is CO-CH=CH2, CO-C(CH3)=CH2 or vinylbenzyl.
The invention furthermore relates to homopolymers and copolymers obtainable by addition
polymerization of one or more compounds of the formula 1.
These novel homopolymers and copolymers preferably conform to the formula 11 c
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
14 12_
n
( l ~)m
H3C N CH3
H3C l H2
111
in which m is O or 1,
R2, R3 and R4, independently of one another, are H or C,-C10alkyl, C3-C,Oalkyl which is
interrupted by-O-, -CO-, -NR'-, -S-, -SO-, -SO2- or phenylene,
Z is a direct bond, phenylene, phenylene(C,-C4alkylene), C,-C4alkylene or
C3-C4alkylene, which is interrupted by -O-, -CO-, -NR'-, -S-, -SO-, -SO2- or phenylene,
with the proviso that the total number of carbon atoms in all four radicals R2, R3, R4 and Z
does not exceed 10, excluding carbon atoms in optional interruptions, or, if
R2 and R4 are H and m is 0, R3, Z and X together can also be the
~ o~ o
t~i
unit, and Z and R are as defined under the formula 1, and n is a number from 5 to 10,000,
and the general symbols in each recurring unit are identical or different.
-
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
Preference is given to compounds of the formula ll in which Z is a direct bond and
R2, R3 and R4, independently of one another, are H or C1-C4alkyl.
n in the compounds of the formula ll is preferably from 2 to 1,000, in particular from 2 to
100.
The invention furthermore relates to copolymers obtainable by addition copolymeri~Lion of
compounds of the formula I with at least one comonomer containing a polymeri~ble,
ethylenically unsaturated C=C double bond.
r, ~r~rence is given to copolymers in which the comonomer is one or more compounds from
the group consisting of a-olefins, styrenes, ~-methylstyrenes, acrylic acid,
methacrylic acid, acrylates, methacrylates, acrylamides, methacryla".:~'Ps, acrylonitrile, vinyl
acetate or vinyl chloride.
These copolymers pr~:rt:r~bly contain from 1 to 99 mol% of structural units of the formula ll
and from 99 to 1 mol% of structural units of the formula lll
_ IRs Hl --
--C C - (ill)
R6 H
in which
R5 is H, C1-C,0alkyl, phenyl, -COOR7, -CONH2, -GN or-CI,
R6 is H or Cl-C10alkyl and
R7 is H or C,-C10alkyl.
Preference is given to copolymers in which, in the formula lll,
Rs is C1-C2alkyl, phenyl, -COOR,, -CONH2, -C=N or-CI,
,
CA 02232573 l998-03-20
W O 97/13752 PCT~EP96/04264
R6 is H or methyl and
R7 is H or C1-C2alkyl.
~ The copolymers co",pris;"g compounds of the formula I with a comonomer conl~i"il,g a
C=C double bond pre:r~r~bly have a mean molecular weight Mn of from 500 to1,000,000,
plere,dbly from 1,000 to 100,000, in particular from 1,000 to 10,000.
Compounds of the formula I are prepared, for example, by reacting
4-(alkyl)amino- or 4-hydroxy-2,2,6,6-tetramethylpiperidines of the formula IV or V
NHR' OH
H3C ~ CH3 H3C ~ CH3
H3C I CH3 H3C I CH3
f H2 (,", f H2 ""
C C
111 111
Cl C
R R
in which R and R' are as defined above, with unsaturated, polymerizable carboxylic acids,
carboxylic acid chlorides or carboxylic acid ester derivatives in a manner known per se, or
by reacting 4-sl Ihstitl It~-l 2,2,6,6-tetramethylpiperidines of the formula Vl
CA 02232~73 l998-03-20
W O 97/137S2 PcT/~l9~lo1264
~_ (Vl)
H3C N CH3
H
in which X and Y are as defined above, with an appropriate alkynyl-p-toluenesulfonic acid
compound in a manner known per se.
The preparation is pr~rerably carried out by reacting compounds of the formula IV or V in
which R is H or CH3 and
R' is C,-C4alkyl or H with, for example, acryloyl chloride, methacryloyl chloride, methacrylic
acid, methylmethacrylic acid or vinylbenzyl chloride, or by reacting compounds of the
formula Vl in which X and Y together are CH2=CHCONH- or CH2=C(CH3)CONH- with
propargyl tosylate.
Both compounds of the formula IV and of the formula Vl can be prepared in a l"anner
known per se from 4-alkylamino-2,2,6,6-tetramethylpiperidines of the formula Vll or
compounds of the formula V or Vl can be prepared in a manner known per se from 4-
hydroxy-2,2,6,6-tetramethylpiperidine of the formula Vlll
NHR' OH
H3C ~ CH (Vl 1) H3C ~CH (Vl 11)
H3C I CH3 H3C I CH3
H H
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/04264
which are commercially available or can easily be prepared by known processes, and the
appropriate reagents mentioned above.
The reaction is preferably carried out in an inert solvent. The solvents which can be
employed are polar or nonpolar organic solvents, for example hydrocarbons, halogenated
hydrocarbons, esters, ethers, ketones, amides, nitriles, tertiary amines or sulfoxides;
examples of 5Uit~h'e solvents are toluene, hexane, cyclohexane, ligroin, petroleum ether
and other hydrocarbon mixtures, dimethylformamide, tetrahydrofuran, dioxane, chlo~urur,,,.
diethyl ether, dimethyl sulfoxide and acetonitrile; particular preference is given to
ac~lul liLI ile.
The Len"~er~lure during the reaction can be in the range from -5û to 200~C, preferably from
-25 to 1 6û~C, in particular from -15 to 1 40~C.
The temperature of the reaction mixture can be kept in the boiling range (reflux) for the
duration of the reaction. To this end, a solvent-containing reaction mixture is warmed to the
boiling point, generally under atmospheric pressure, and the evaporated solvent is
condensed with the aid of a suit~lE condenser and fed back into the reaction mixture.
The (ea~;lion can be carried out with exclusion of oxygen, for example by flushing with an
inert gas such as argon; however, oxygen does not always i"l~, rt:re, so that it may also be
poss~ lc to carry out the reaction without this measure.
When the ,eaclion is complete, work-up can be carried out by customary methods; the
mixture is eYrediently first diluted with water, for example by adding the, t:a~lion mixture to
from 1 to 4 times the volume of (ice) water; the product can subsequently be separated off
directly or extracted, expediently using, for example, ethyl acetate or toluene. In the case of
extraction, the product can be isolated in a conventional manner by removing the solvent;
~ this is Yxred Qntly carried out after drying of the organic phase. It is also possible to insert
further purification steps, for example washing with aqueous sodium bicarbonate solution,
dispersion of activated charcoal, chromatography by means of silica gel, filtration,
recry~ lion and/or distillation.
CA 02232~73 l998-03-20
W O 97/13752 PCT/~l~/o~26
-10-
The homopolymers and copolymers of compounds of the formula 1, for example of those of
the formula ll or lll, are prepared by free-radical polyme,i~dlion.
The free-radical polymerization can be carried out using various methods. These are
described, for example by S. Sandler and W. Karo in Polymer Synthesis, Vol. 1-3,Academic Press, New York 1968. Examples of conventional polymerization p,ocesses are
bulk, solvent, emulsion, suspension and preciri~tion polymerization.
The polymerization is generally i"ilidled by a conventional free-radical polyme~ lion
initiator. These include thermal irlilialur:j~ such as azo compounds, for example
azoisobutyronitrile (AIBN), pe,uxides, for example benzoyl peroxide, redox initiator systems,
such as a mixture of iron(lll) acetylacetonate, benzoin and benzoyl peroxide, and
phuluchemical free-radical ~unller:j~ such as benzoin or benzil methyl ketal.
The initiator is expe~liently added to the reaction solution in an amount of from 0.1 to
5 mol%, preferably from 0.5 to 3 mol%, based on the amount of ethylenically unsaturated
monomers.
In order to control the molecular weight, chain l~ansrer compounds can be added, for
example in amounts of from 5 to 20 mol%. Examples of such compounds are disulfides,
mercaplans, halides, dibutyl sulfide, and others.
The polyl"e,i~alio" is prer~,ably carried out in solution. The reaction le",perdlure is
generally in the range from 10 to 200~C, preferably from 30 to 1 50~C, particularly preferably
from 40 to 1 00~C.
Any solvent present must be inert under the reaction conditions. Suitable solvents include
aromatic hydrocarbons, ketones and ethers. Examples thereof are benzene, toluene,
xylene ethylbenzene, isopropylbenzene, methyl ethyl ketone, acetone, cyclohexanone,
diethyl ether, dibutyl ether, tetrahydrofuran and dioxane. Particular prt~ nce is given to
toluene and tetrahydrofuran.
-
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/04264
- 1 1 -
The polymeri~dlion is expediently carried out in the absence of oxygen, for exa",ple under
argon or under nitrogen.
The compounds of the formula I and novel homopolymers and copolymers thereof areparticularly suitable for st~hi~ ng organic n~dl~rials against thermal, oxidative or actinic
degradation.
Examples for those mdL~rials are:
1. Polymers of monoolefins and diolefins, for example polypropylene, polyisobutylene,
polybut-1-ene, poly-4-methylpent-1-ene, poly;soprel-e or polybllt~ ne, as well as polymers
of cycloolefins, for i"~ance of cyclopentene om~or~o,~ene, polyethylene (which opliollally
can be crosslinked), for example high density polyethylene (HDPE), high density and high
molecular weight polyethylene (HDPE-HMW), high density and ultrahigh " -'ec-~l~r weight
polyethylene (HDPE-UHMW), medium density polyethylene (MDPE), low density polyethy-
lene (LDPE), linear low density polyethylene (LLDPE), branched low density polyethylene
(BLDPE).
Polyolefins, i.e. the polymers of monoolefins exemplified in the preceding pardy,dph, prefe-
rably polyethylene and polypropylene, can be prepared by dirr~,enl, and especially by the
following, methods:
a) radical pol~",erisdlion (normally under high pressure and at elevated temperature).
b) catalytic poly" ,erisdlion using a catalyst that normally contains one or more than
one metal of groups IVb, Vb, Vlb or Vlll of the Periodic Table. These metals usually
have one or more than one ligand, typically oxides, halides, alcoholates, esters,
ethers, amines, alkyls, alkenyls and/or aryls that may be either p- or s-coorcJi, ,clled.
These metal complexes may be in the free form or fixed on suL,~IIdles, typically on
activated magnesium chloride, titanium(lll) chloride, alumina or silicon oxide. These
catalysts may be soluble or insolut'~ in the polymerisation medium. The catalysts
can be used by themselves in the polymerisation or further activators may be used,
typically metal alkyls, metal hydrides, metal alkyl halides, metal alkyl oxides or me-
tal alkyloxanes, said metals being elements of groups la, lla and/or Illa of the
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
-12-
Periodic Table. The activators may be modified conveniently with further ester,
ether, amine or silyl ether groups. These catalyst systems are usually termed
Phillips, Slandard Oil Indiana, Ziegler (-Natta), TNZ (DuPont), metallocene or single
site catalysts (SSC).
2. Mixtures of the polymers mentioned under 1), for example mixtures of polypropylene
with polyisobutylene, polypropylene with polyethylene (for example PP/HDPE, PP/LDPE)
and mixtures of different types of polyethylene (for example LDPE/HDPE).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl monomers,
for example ethylene/propylene copolymers, linear low density polyethylene (LLDPE) and
mixtures thereof with low density polyethylene (LDPE), propylene/but-1-ene copolymers,
propylene/isobutylene copolymers, ethylene/but-1-ene copolymers, ethylene/hexene copo-
lymers, ethylene/methylpentene copolymers, ethylene/heptene copolymers, ethylene/oc-
tene copolymers, propylene/butadiene copolymers, isobutylene/isoprene copo!ymers, ethy-
lene/alkyl acrylate copolymers, ethylene/alkyl methacrylate copolymers, ethylene/vinyl
acetate copolymers and their copolymers with carbon monoxide or ethylene/acrylic acid
copolymers and their salts (ionomers) as well as terpolymers of ethylene with propylene and
a diene such as hexadiene, dicyclopentadiene or ethylidene-norbornene; and mixtures of
such copolymers with one another and with polymers " ~e~ ~Lioned in 1 ) above, for example
polypropylene/ethylene-propylene copolymers, LDPE/ethylene-vinyl acetate copolymers
(EVA), LDPE/ethylene-acrylic acid copolymers (EAA), LLDPE/EVA, LLDPE/EAA and alter-
nating or random polyalkylene/carbon monoxide copolymers and mixtures thereof with other
polymers, for example polyamides.
4. Hydrucalbon resins (for example C5-Cg) including hydrogenated modifications thereof
(e.g. tackifiers) and mixtures of polyalkylenes and starch
5. Polystyrene, poly(p-methylstyrene), poly(a-methylstyrene).
6. Copolymers of styrene or a-methylstyrene with dienes or acrylic derivatives, for example
styrene/butadiene, styrene/acrylonitrile, styrene/alkyl methacrylate, styrene/butadiene/alkyl
acrylate, styrene/butadiene/alkyl methacrylate, styrene/rr aieic anhydride, styrene/acrylo-
nitrile/methyl acrylate; mixtures of high impact strength of styrene copolymers and another
~ == :
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
polymer, for example a polyacrylate, a diene polymer or an ethylene/propylene/diene terpo-
lymer; and block copolymers of styrene such as styrene/butadiene/styrene, styrene/iso-
prene/styrene, styrene/ethylene/butylene/styrene or styrene/ethylene/propylene/ styrene.
7. Graft copolymers of styrene or a-methylstyrene, for example styrene on polybutadiene,
styrene on polybutadiene-styrene or polybl ~t~iene-acrylonitrile copolymers; styrene and
acrylonitrile (or methacrylonitrile) on polybutadiene; styrene, acrylonitrile and methyl meth-
acrylate on polybutadiene; styrene and maleic anhydride on polybutadiene; styrene, acrylo-
nitrile and maleic anhydride or malei.~ de on polybutadiene; styrene and maleimide on poly-
butadiene; styrene and alkyl acrylates or methacrylates on polybuPdiene; styrene and
acrylonitrile on ethylene/propylene/diene terpolymers; styrene and acrylonitrile on polyalkyl
acrylates or polyalkyl methacrylates, styrene and acrylonitrile on acrylate/but~iiene copoly-
mers, as well as mixtures thereof with the copolymers listed under 6), for example the
copolymer mixtures known as ABS, MBS, ASA or AES polymers.
8. Halogen-containing polymers such as polychloroprene, chlorinated rubbers, chlorinaled
and brominated copolymer of isobutylene-isoprene (halobutyl rubber), chloril,~L~d or sulfo-
chlorinated polyethylene, copolymers of ethylene and chlorinated ethylene, epichlorohydrin
homo- and copolymers, especially polymers of halogen-containing vinyl compounds, for
example polyvinyl ch~oride, polyvinylidene chloride, polyvinyl fluoride, polyvinylidene fluo-
ride, as well as copolymers thereof such as vinyl chloride/vinylidene chloride, vinyl chlo-
ride/vinyl acetate or vinylidene chloride/vinyl acetate copolymers.
9. Polymers derived from a,b-unsaturated acids and derivatives thereof such as polyacry-
lates and polymethacrylates; polymethyl methacrylates, polyacrylamides and polyacryloni-
triles, impact-modified with butyl acrylate.
10. Copolymers of the monomers mentioned under 9) with each other or with other unsatu-
rated monomers, for example acrylonitrile/ butadiene copolymers, acrylonitrile/alkyl acrylate
copolymers, acrylonitrile/alkoxyalkyl acrylate or acrylonitrile/vinyl halide copolymers or
acrylonitrile/ alkyl methacrylate/butadiene terpolymers.
11. Polymers derived from unsaturated alcohols and amines or the acyl derivatives or
acetals thereof, for example polyvinyl alcohol, polyvinyl acetate, polyvinyl stearate, polyvinyl
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/04264
benzoate, polyvinyl maleate, polyvinyl butyral, polyallyl phLhalale or polyallyl r"ela",i,le; as
well as their copolymers with olefins mentioned in 1) above.
12. Homopolymers and copolymers of cyclic ethers such as polyalkylene glycols, polyethy-
Iene oxide, polypropylene oxide or copolymers thereof with bisglycidyl ethers.
13. Polyacetals such as polyoxymethylene and those polyoxymethylenes which contain
ethylene oxide as a comono",er; polyacetals modified with ther."opl~slic polyl"~li,anes,
acrylates or MBS.
14. Polyphenylene oxides and sulfides, and mixtures of polyphenylene oxides with styrene
polymers or polyan,ides.
15. Polyurethanes derived from hydroxyl-terminated polyethers, polyesters or polybuta-
dienes on the one hand and aliphatic or aromatic polyiso.;yanates on the other, as well as
precursors thereof.
16. Polyamides and copolyamides derived from diamines and dicarboxylic acids and/or
from a" li. ,oca,l-oxylic acids or the cor,esponding lactams, for example polyamide 4, poly-
amide 6, polyamide 6/6,6/10,6/9,6/12,4/6, 12/12, polyamide 1 1, polyamide 12, aromatic
polyamides starting from m-xylene diamine and adipic acid; polyamides prepared from
hexamethylenediamine and isophthalic or/and terephLl,alic acid and with or without an
elc.~ er as modifier, for example poly-2,4,4,-trimethylhexamethylene tert:phll,alamide or
poly-m-phenylene isophthalamide; and also block copolymers of the aforementioned poly-
amides with polyolefins, olefin copolymers, ionomers or chemically bonded or grafted
elastomers; or with polyethers, e.g. with polyethylene glycol, polypropylene glycol or poly-
tetramethylene glycol; as well as polyamides or copolyamides modified with EPDM or ABS;
and polyamides condensed during processing (RIM polyamide systems).
17. Polyureas, polyimides, pol~a~" ~e imides, polyetherimids, polye:jLt:,i"i'-, polyhydanto-
ins and polybenzimid~.,les.
18. Polyesters derived from dicarboxylic acids and diols and/or from hydroxycarboxylic
acids or the corresponding lactones, for example polyethylene terephthalate, polybutylene
-
CA 02232~73 1998-03-20
W O 97/13752 PCT/~r5/01264
-15-
terephlhalate, poly-1,4-dimethylolcyelohe,~dne LerephLl,aldl~7 and polyhydroxybenzoates, as
well as block copolyether esters derived from hydroxyl-te", li. ,aled polyethers; and also poly-
esters modified with poly~;~,bonales or MBS.
19. Polycarbonates and polyester carbonates.
20. Polysulfones, polyether sulfones and polyether ketones.
21. Cn~ssli. ,ked polymers derived from aldehydes on the one hand and phenols, ureas and
melamines on the other hand, such as phenol/~r"~aldehyde resins, urea/formaldehyde
resins and " ,ela" ,ine/~r, . ,aldehyde resins.
22. Drying and non-drying alkyd resins.
23. Unsaturated polyestcr resins derived from copolyesters of saturated and unsaturated
dicarboxylic acids with polyhydric alcohols and vinyl compounds as cluss!;nhi-lg agents, and
also halogen-cor,l~in;"g ~~lud;ric~lions thereof of low flammability.
24. Crossli.~ ''e acrylic resins derived from suhstitllted acrylates, for ~ mple epoxy acry-
lates, u, ~lhane acrylates or polyester acrylates
25. Alkyd resins, polyester resins and acrylate resins crossliliked with ",elar":ne resins,
urea resins, isocyanates, isocyanurates, polyisocyanates or epoxy resins.
26. Crosslinked epoxy resins derived from ali~ hdLic, cycloaliphatic, heterocyclic or aromatic
glycidyl compounds, e.g. products of diglycidyl ethers of bisphenol A and bisphenolF, which
are crosslinked with customary hardeners such as anhydrides or amines, with or without
accelerators.
27. Natural polymers such as cellulose, rubber, gelatin and chemically modified homolo-
gous derivatives thereof, for example cellulose acetates, cellulose propion~l~s and cellu-
lose butyrates, orthe Ics'l(l'cse ethers such as methyl cellulose; as well as rosins and their
derivatives.
_
CA 02232~73 1998-03-20
O 97113752 PCTrEP96/04264
-16-
28. Blends of the aforementioned polymers (polyblends), for ex~n,ple PP/EPDM, Poly-
amide/EPDM or ABS, PVC/EVA, PVC/ABS, PVC/MBS, PC/ABS, PBTP/ABS, PC/ASA,
PC/PBT, PVC/CPE, PVC/acrylates, POM/ther",opl~f~lic PUR, PC/thermoplastic PUR,
POM/acrylate, POM/MBS, PPO/HIPS, PPO/PA 6.6 and copolymers, PA/HDPE, PA~PP,
PA/PPO, PBT/PC/ABS or PBT/PET/PC.
The invention therefore furthermore relates to compositions co""~risi"g a) an organic
material which is sensitive to oxidative, thermal and/or actinic degradation/build-up and b) at
least one compound of the formula I and/or a homopolymer and/or copolymer thereof, and
to the use of compounds of the formula I and/or homopolymers and/or copolymers thereof
for ~i~hil;,; lg organic material against oxidative, thermal or actinic degradation/build-up.
The invention also relates to a process for st~h ~ 7ing organic material against thermal,
oxidative and/or actinic degradation/build-up which comprises adding at least one
compound of the formula I and/or a homopolymer and/or copolymer thereof to this material.
Of particular interest is the use of compounds of the formula I as 5t~hjii~er5 in synthetic
organic polymers and co"~spo"ding composilions.
The organic materials to be protecLed are preferably natural, semisynthetic or preferably
synthetic organic materials. Particular pr~f~rence is given to synthetic organic polymers or
mixtures of such polymers, in particular thermoplastic polymers, such as polyolefins,
especially polyethylene (PE) and polypropylene (PP). Other particularly pr~:fe"~d organic
materials are coating compositions. Coating compositions which can advantageously be
5t~hjii~ed in accordance with the invention are described, for example, in Ullmann's
Encyclopedia of Industrial Chemistry, 5th. Edn., Vol. A18, pp. 359-464, VCH
Verlagsgesellschaft, Weinheim 1991.
Other materials which can be stabilized by means of the novel compounds are photographic
materials. These are taken to mean, in particular, those described in Research Disclosure
1990, 31429 (pages 474-480) for photographic reproduction and other reproductionmethods.
In general, the compounds of the formula I are added to the material to be st~hili~ed in
amounts of from 0.01 to 10%, preferably from 0.01 to 5%, in particular from 0.01 to 2%,
-
-
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
based on the total weight of the '~ ed col"posilion. The novel compounds are
particularly pfert:rdbly used in amounts of from 0.0~ to 1 .~i%, especis'ly from 0.1 to 0.5%.
The incGr~uolalion into the ~I~aL~:lials can be carried out, for exd,- ,~!e, by i,.co.~.or~ling or
applying the compounds of the formula I and, if desired, further additives by the usual
ods in industry. If the ",alelials are polymers, in particular synthetic polymers, the
i"cor~.ordlion can be carried out before or during shaping or by apply;"g the dissolved or
dlspe,~ed compound to the polymer, if necessary with s~bse-lllent evdporalion of the
solvent. In the case of elastomers, these can also be SIA~ ed as lattices. Another way of
i"col~,ordli"g compounds of the formula I into polymers cG",prises adding them before,
during or immediately after poly.neri~dlion of the co..t:sponding l--onome~ or before
clossli~ .lg. The compounds of the formula I can be added as such, or allt:r"dli-/ely in
enc~ps~ t~d form (for exa...ple in waxes, oils or polymers). In the case of addition before
or during the polymeri,dlion, the compounds of the formula I can also act as regl~'~rs for
the chain length of the polymers (chain terminators).
The compounds of the formula I can also be added to the plastics to be ~ hil;~ed in the
form of a ma~terL,alcl . which co. Ildil Is these compounds, for example, in a concenlrdlion of
2.5 to 25% by weight.
The compounds of the formula I can expediently be incorporated by the r~,llo~ ;ng methods:
- as an emulsion or dispersion (for e~xdl I Ir !e to lattices or emulsion polymers),
- as a dry mix during mixing of adcJilional components or polymer mixtures,
- by direct addition into the p-ocessing equipment (for ~,cd---ple extruder, internal mixer,
etc.),
- as a solution or melt.
Novel polymer co..~posilions can be used in various forms or converted into various
products, for example as or into films, fibres, tapes, moulding co",posilions, profiles, or as
binders for paints, adhesives or adhesive cements.
CA 02232~73 1998-03-20
W O 97/137S2 PCTAEP96/04264
-18-
ln addition to the compounds of the formula I or homopolymers and/or copolymers thereof,
the novel coi"posilions can contain, as additional component (c), one or more conventional
additives, such as, for example, the following:
1. Antioxidants
1.1. Alkylated monoDhenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-tert-butyl-4,6-
dimethylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-butylphenol, 2,6-di-tert-
butyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol, 2-(a-methylcyclohexyl)-4,6-di-
methylphenol, 2,6-~1io~t~decyl-4-methylphenol, 2,4,6-tricyclohexylphenol, 2,6-di-tert-butyl-4-
methoxymethylphenol, nonylphenols which are linear or branched in the side chains, for
example, 2,6-di-nonyl-4-methylphenol, 2,4-dimethyl-6-(1'-methylundec-1'-yl)phenol, 2,4-di-
methyl-6-(1'-methylheptadec-1'-yl)phenol, 2,4-dimethyl-6-(1'-methyltridec-1'-yl)phenol and
mixtures thereof.
1.2. AlkylthiomethylDhenols, for example 2,4-dioctylthiomethyl-6-tert-butylphenol, 2,4-di-
octylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-di-dodecylthio-
methyl-4-nonylphenol .
1.3. Hydroquinones and alkylated hydroquinones, for exdr"ple 2,6-di-tert-butyl-4-methoxy-
phenol, 2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-octa-
decyloxyphenol, 2,6-di-tert-butylhydroquinone, 2,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-
butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyphenyl stearate, bis-(3,5-di-tert-butyl-4-
hydroxyphenyl? adipate.
1.4. Tocopherols, for example a-tocopherol, b-tocopherol, g-tocopherol, d-tocopherol and
mixtures thereof (\/itamin E).
1.5. Hydroxvlated thiodiDhenyl ethers, for example 2,2'-thiobis(6-tert-butyl-4-methylphenol),
2,2'-thiobis(4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol), 4,4'-thiobis(6-tert-butyl-
2-methylphenol), 4,4'-thiobis-(3,6-di-sec-amylphenol), 4,4'-bis-(2,6-dimethyl-4-hydroxyphe-
nyl) disulfide.
CA 02232573 l998-03-20
WO 97/13752 PcT/~ r f.'C1264
- 1g-
1.6. Alkylidenebisphenols. for example 2,2'-methylenebis(6-tert-butyl-4-methylphenol), 2,2'-
methylenebis(6-tert-butyl-4-ethylphenol), 2,2'-methylenebis~4-methyl-6-(a-methylcyclohexyl)-
phenol~, 2,2'-methylenebis(4-methyl-6-cyclohexylphenol), 2,2'-methylenebis(6-nonyl-4-
methylphenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), 2,2'-ethylidenebis(4,6-di-tert-
butylphenol), 2,2'-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2'-methylenebis[6-(a-
methylbenzyl)-4-nonylphenol3, 2,2'-methylenebis[6-(a,a-dimethylbenzyl)-4-nonylphenol],
4,4'-methylenebis(2,6-di-tert-butylphenol), 4,4'-methylenebis(6-tert-butyl-2-methylphenol),
1,1-bis(5-tert-butyl-4-hydroxy-2-methylphenyl)butane, 2,6-bis(3-tert-butyl-5-methyl-2-
hydroxybenzyl)-4-methylphenol, 1,1,3-tris(5-tert-butyl-4-hydroxy-2-methylphenyl)butane,
1 ,1-bis(5-tert-butyl-4-hydroxy-2-methyl-phenyl)-3-n-dodecylmerc~ptobllt~ne, ethylene ~Iycol
bis[3,3-bis(3'-tert-butyl-4'-hydroxyphenyl)butyrate], bis(3-tert-butyl-4-hydroxy-5-methyl-
phenyl)dicyclopentadiene, bis[2-(3'-tert-butyl-2'-hydroxy-5'-methylbenzyl)-6-tert-butyl-4-
methylphenyl]terephthalate, 1,1-bis-(3,5-dimethyl-2-hydroxyphenyl)butane, 2,2-bis-(3,5-di-
tert-butyl-4-hydroxyphenyl)propane, 2,2-bis-(5-tert-butyl-4-hydroxy2-methylphenyl)-4-n-
dodecylmercaptobutane, 1,1,5,5-tetra-(5-tert-butyl-4-hydroxy2-methylphenyl)pentane.
1.7. O-. N- and S-benzvl comPounds, for example 3,5,3',5'-tetra-tert-butyl-4,4'-dihydroxydi-
benzyl ether, ocl;1decyl-4-hydroxy-3,5-dimethylbenzyll,,er~,-aplu~cet~te, tridecyl-4-hydroxy-
3,6-di-tert-butylbenzyl" ,e. captoacetate, tris(3,5-di-tert-butyl-4-hydroxybenzyl)amine, bis(4-
tert-butyl-3-hydroxy-2,6-dimethylbenzyl)di~l,iuterephll)alate, bis(3,5-di-tert-butyl-4-hydroxy-
benzyl)sulfide, isooctyl-3,5di-tert-butyl-4-hydroxybenzylmercapto~cet~te.
1.8. Hvdroxvbenzylated malonates, for example dioctadecyl-2,2-bis-(3,5-di-tert-butyl-2-
hydroxybenzyl)-malonate, di-octadecyl-2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)-malonate,
di-dodecylmercaptoethyl-2,2-bis-(3,5-di-tert-butyl-4-hydroxybenzyl). . .alondLe, bis[4-(1 ,1 ,3,3-
tetramethylbutyl)phenyl]-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate.
1.9. Aromatic hydroxybenzyl compounds, for example 1,3,5-tris-(3,5-di-tert-butyl-4-hydroxy-
benzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-2,3,5,6-tetra-
methylbenzene, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)phenol.
1.1û. Triazine Compounds, for example 2,4-bis(octylmercapto)-6-(3,5-di-tert-butyl-4-
hydroxyanilino)-1,3,5-triazine, 2-octylmercaptû-4,6-bis(3,5-di-tert-butyl-4-hydroxyanilino)-
1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,3,5-triazine,
CA 02232~73 l998-03-20
W O 97/13752 PCT/~l~G~I~1264
-20-
2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris-(3,5-di-tert-butyl-4-
hydroxybenzyl)isocyanurate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-
isocyanurate, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxyphenylethyl)-1,3,5-triazine, 1,3,5-tris(3,5-
di-tert-butyl-4-hydroxyphenylpr~F ~nyl)-hexahydro-1,3,5-triazine, 1,3,5-tris(3,5-dicyclohexyl-
4-hydroxybenzyl)isocyanurate.
1.11. Benzylphosphonates, for example dimethyl-2,5-di-tert-butyl-4-hydroxybenzylphospho-
nate, diethyl-3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl3,5-di-tert-butyl-4-
hydroxybenzylphosphonate, dioctadecyl-5-tert-butyl-4-hydroxy3-methylbenzylphosphonate,
the calcium salt of the monoethyl ester of 3,5-di-tert-butyl-4-hydroxybenzylphosphonic acid.
1.12. AcylaminoPhenols. for example 4-hydroxylauranilide, 4-hydroxystearanilide, octyl N-
(3,5-di-tert-butyl-4-hydroxyphenyl)ca, bar"ate.
1.13. Esters of b-(3,5-di-tert-butvl-4-hydroxvPhenyl)propionic acid with mono- or polyhydric
alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol, 1,9-
nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethy-
lene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl) isocyanurate, N,N'-bis(hy-
droxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylol-
propane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo~2.2.2]octane.
1.14. Esters of b-(5-tert-butyl-4-hydroxv-3-methylphenvl)proPionic acid with mono- or poly-
hydric alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, oct~lec~nol, 1,6-hexane-
diol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene
glycol, diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl) isocyanurate,
N,N'-bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol,
trimethylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo~2.2.2~octane.
1.15. Esters of b-(3,5-dicyclohexyl-4-hydroxYPhenvl)Propionic acid with mono- or polyhydric
alcohois, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl~isocyanurate, N,N'-bis(hydroxyethyl)ox-
amide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-
hydroxymethyl-1 -phospha-2,6,7-trioxabicyclo~2.2.2]octane.
CA 02232~73 l998-03-20
W O 97/13752 PCT/~,'/C1264
-21-
- 1.16. Esters of 3,5-di-tert-butyl-4-hydroxyphenvl acetic acid with mono- or polyhydric alco-
hols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol,
ethylene glycol, 1 ,2-propanediol, neopentyl glycol, thiodiethylene. glycol, diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N'-bis(hydroxyethyl)-
O~dll d~, 3-thiaundecanol, 3-thiapent~dec~nol, trimethylhexanediol, trimethylolpropane, 4-
hydroxymethyl-1 -phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.17. Amides of b-(3,5-di-tert-butyl-4-hvdroxyphenyl)propionic acid e.g. N,N'-bis(3,5-di-tert--
butyl-4-hydroxyphenylprop.anyl)hexamethylenediamine, N,N'-bis(3,5-di-tert-butyl-4-hydroxy-
phenylpropionyl)trimethylenediamine, N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-
hydrazine.
1.18. Ascorbic acid (vitamin C)
1.19. Aminic antioxidants, for 6','CdlllplE: N,N'-di-isopropyl-p-phenylenedia".i.le, N,N'-di-sec-
butyl-p-phenylenediamine, N,N'-bis(1,4-dimethylpentyl)-p-phenylenediar"i,le, N,N'-bis(1-
ethyl-3-methylpentyl)-p-phenylenediamine, N,N'-bis(1-methylheptyl)-p-phenylenediamine,
N,N'-dicyclohexyl-p-phenylenediamine, N,N'-diphenyl-p-phenylenediamine, N,N'-bis(2-naph-
thyl)-p-phenylenedid~ le, N-isopropyl-N'-phenyl-p-phenylenediamine, N-(1,3-dimethylbutyl)-
N'-phenyl-p-phenylenediamine, N-(1-methylheptyl)-N'-phenyl-p-phenylenedidn,i"e, N-cyclo-
hexyl-N'-phenyl-p-phenylenediamine, 4-(p-toluenesulfamoyl)diphenylamine, N,N'-dimethyl-
N,N'-di-sec-butyl-p-phenylenediamine, diphenylamine, N-aliyldiphenylamine, 4-isopropoxy-
diphenylamine, N-phenyl-1-naphthylamine, N-(4-tert-octylphenyl)-1-naphthylamine, N-phe-
nyl-2-naphthylamine, octylated diphenylamine, for example p,p'-di-tert-octyldiphenylamine,
4-n-butylaminophenol, 4-butyrylaminophenol, 4-nonanoylamino-phenol, 4-dodecanoyl-
aminophenol, 4-octadecanoylal"i"ophenol, bis(4-methoxyphenyl)amine, 2,6-di-tert-butyl-4-
dimethylaminomethylphenol, 2,4'-diaminodiphenylmethane, 4,4'-diaminodiphenylmethane,
N,N,N',N'-tetramethyl-4,4'-diaminodiphenylmethane, 1,2-bis[(2-methylphenyl)amino]ethane,
1,2-bis(phenylamino)propane, (o-tolyl)biguanide, Bis[4-(1',3'-dimethylbutyl)phenyl]amine,
tert-octylated N-phenyl-1-naphthylamine, a mixture of mono- and dialkylated tert-butyl/tert-
octyldiphenylamines, a mixture of mono- and dialkylated nonyldiphenylamines, a mixture of
mono- and dialkylated dodecyldiphenylamines, a mixture of mono- and dialkylated isopro-
pyl/isohexyldiphenylamines, a mixture of mono- und dialkylated tert-butyldiphenylamines,
CA 02232~73 l998-03-20
W O 97/13752 PCTrEP96/04264
-22-
2,3-dihydro-3,3-dimethyl-4H-1,4-benzothiazine, phenothiazine, a mixture of mono- und dial-
kylated tert-butyl/tert-octylpher,ull ,i~i"es, a mixture of mono- und dialkylated tert-octyl-phe-
noLl,ia~ es, N-allylphenothid~il" N,N,N',N'-tetraphenyl-1,4-diaminobut-2-ene, N,N-bis-
(2,2,6,6-tetramethyl-piperid-4-yl-hexamethylenediamine, bis(2,2,6,6-tetramethylpiperid-4-
yl)seb~c~te, 2,2,6,6-tetramethylpiperidin-4-one, 2,2,6,6-tetramethylpiperidin-4-ol.
2. UV absorbers and liqht stabilisers
2.1. 2-(2'-Hydroxv~henYl)ben~ulli~ùles~ for example 2-(2'-hydroxy-5'-methylphenyl)-benzo-
triazole, 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)be-,~ul.ia~ùle, 2-(5'-tert-butyl-2'-hydroxyphe-
nyl)be, l~ul- i~ole, 2-(2'-hydroxy-5'-(1 ,1 ,3,3-tetramethylbutyl)phenyl)be, ,~ul, ia~ùle, 2-(3',5'-di-
tert-butyl-2'-hydroxyphenyl)-5-chloro-bel ,~ul, ia~ole, 2-(3'-tert-butyl- 2'-hydroxy-5'-methyl-
phenyl)-5-chloro-bel l~ulr i~ole, 2-(3'-sec-butyl-5'-tert-butyl-2'-hydroxyphenyl)ben~ul~ ule,
2-(2'-hydroxy-4'-octyloxyphenyl)ben~ul,i~ùle, 2-t3',5'-di-tert-amy,-2'-hydroxyphenyl)benzo-
triazole, 2-(3',5'-bis-(a,a-dimethylbenzyl)-2'-hydroxyphenyl)ben~ul,ia~ùle, mixture of 2-(3'-
tert-butyl-2'-hydroxy-5'-(2-octyloxycarbonylethyl)phenyl)-5-chloro-ben~ul,id~ole, 2-(3'-tert-
butyl-5'-[2-(2-ethylhexyloxy)-carbonylethyl]-2'-hydroxyphenyl)-5-chloro-ben~ulrid~ole, 2-(3'-
tert-butyl-2'-hydroxy-5'-(2-methoxyl;arbonylethyl)phenyl)-5-chloro-ben~ulrid~ole, 2-(3'-tert-
butyl-2'-hydroxy-5'-(2-methoxycarbonylethyl)phenyl)ben~ul~ia~ùle, 2-(3'-tert-butyl-2'-
hydroxy-5'-(2-octyloxycarbonylethyl)phenyl)ben ,ull ia~ole, 2-(3'-tert-butyl-5'-~2-(2-ethylhexyl-
oxy)carbonylethyl]-2'-hydroxyphenyl)ber,~ulri~,ule, 2-(3'-dodecyl-2'-hydroxy-5'-methylphe-
nyl)bel,~ulric~ule, and 2-(3'-tert-butyl-2'-hydroxy-5'-(2-isooctyloxycarlJo"ylethyl)phenylben-
~ulri~ùle~ 2,2'-methylene-bisi-4-(1,1,3,3-tetramethylbutyl)-6-ben~ul,i~ole-2-ylphenol]; the
transe:jleriric~lion product of 2-i3'-tert-butyl-5'-(2-methoxycarbonylethyl)-2'-hydroxyphenyl]-
2H-ben~ul,i~ùle with polyethylene glycol3ûO;, where R = 3'-tert-butyl-4'-hydroxy-5'-2H-ben-
~ul~i~ù1-2-ylphenyl.
2.2. 2-Hydroxybenzophenones. for example the 4-hydroxy, 4-methoxy, 4-octyloxy, 4-decyl-
oxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-dimethoxy derivatives.
2.3. Esters of substituted and unsubstituted benzoic acids, as for example 4-tertbutyl-phenyi
salicylate, phenyl salicylate, octylphenyl salicylate, dibenzoyl resorcinol, bis(4-tert-butylben-
zoyl) resorcinol, benzoyl resorcinol, 2,4-di-tertbutylphenyl 3,5-di-tert-butyl-4-hydroxybenzo-
CA 02232~73 l998-03-20
W O 97/13752 PCT/~l~/0l264
ate, hexadecyl 3,5-di-tert-butyl-4-hydroxyberl~oale, octadecyl 3,5-di-tert-butyl-4-hydroxy-
benzoate, 2-methyl-4,6-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzoate.
2.4. Acrylates. for example ethyl a-cyano-b,b-diphenylacrylate, isooctyl a-cyano-b,b-di-
phenylacrylate, methyl a-carbomethoxycil~nal"dle, methyl a-cyano-b-methyl-p-methoxy-
~i. ,na~ le, butyl a-cyano-b-methyl-p-methoxy-ci. ~nal "aLe, methyl a-car60, l I~Ll ,~x~-p-
methoxyci- ~na"~le and N-(b-ca, l.on It:Lh~xy-b-cyanovinyl)-2-methylindol;i)e.
2.5. Nickel comoounds, for ~,~d",ple nickel complexes of 2,2'-thio-bis-[4-(1,1 ,3,3-tetra-
methylbutyl)phenoq, such as the 1:1 or 1:2 complex, with or without additional ligands such
as n-butylamine, I,i~Ll,anolamine or N-cyclohexyl~i~ ',anola",i"e, nickel dibutyldiLl.iocar-
ba,ndle, nickel salts of the ",onoalkyl esters, e.g. the methyl or ethyl ester, of 4-hydroxy-3,5-
di-tert-butylbenzylphosphonic acid, nickel con,r'sx~s of keluxi",es, e.g. of 2-hydroxy-4-
methylphenyl undecylkt:luxi",e, nickel complexes of 1-phenyl-4-lauroyl-5-hydroxypyrazole,
with or without additional ligands.
2.6. Stericallv hi.,deled amines, for exdr, FIE bis(2,2,6,6-tetramethyl-4-piperidyl)seb~le,
bis(2,2,6,6-tetramethyl-4-piperidyl)succ;..dle, bis(1,2,2,6,6-pentamethyl-4-piperidyl)seba-
cate, bis(1 -octyloxy-2,2,6,6-l~lr~ Lhyl-4-piperidyl)seh~o~te, bis(1 ,2,2,6,6-pentamethyl-4-pi-
peridyl) n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate, the condensate of 1-(2-hydroxy-
ethyl)-2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, the condensate of N,N'-
bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-tert-octylamino-2,6-di-
chloro-1 ,3,5-tri~ine, tris(2,2,6,6-t~ll dm~l hyl-4-piperidyl)nitrilotri~cePte, tetrakis(2,2,6,6-
tetramethyl-4-piperidyl)-1,2,3,4-butane-tetracarboxylate, 1,1'-(1,2-ethanediyl)bis(3,3,5,5-
tetramethylpiperazinone), 4-benzoyl-2,2,6,6-tetramethylpiperidine, 4-stearyloxy-2,2,6,6-
tetramethyl,uiperidine, bis(1,2,2,6,6-pentamethylpiperidyl)-2-n-butyl-2-(2-hydroxy-3,5-di-tert-
butylbenzyl)malonate, 3-n-octyl-7,7,9,9-t~ dl l leLl lyl-1 ,3,8-LI i~spil ~[4.5]decan-2~4-dion~
bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)seb~c~te, bis(1-octyloxy-2,2,6,6-tetramethyl-
piperidyl)succinate, the condensate of N,N'-bis-(2,2,6,6-tetramethyl-4-piperidyl)hexamethy-
lenediamine and 4-morpholino-2,6-dicllloro-1,3,5-triazine, the condensate of 2-chloro-4,6-
bis(4-n-butylamino-2,2,6,6-t~rd",~ll,ylpiperidyl )-1,3,5-triazine and 1,2-bis(3-aminopropyl-
amino)ethane, the condensate of 2-chloro-4,6-di-(4-n-butylamino-1,2,2,6,6-pentamethyl-
piperidyl)-1,3,5-triazine and 1,2-bis-(3-aminopropylamino)ethane, 8-acetyl-3-dodecyl-
7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, 3-dodecyl-1-(2,2,6,6-tetramethyl-
CA 02232~73 1998-03-20
O 97113752 PCT/~i5./01264
-24-
4-piperidyl)pyrrolidin-2,5-dione, 3-dodecyl-1-(1 ,2,2,6,6-pentamethyl-4-piperidyl)pyrrolidine-
2,5-dione, a mixture of 4-hexadecyloxy- and 4-stearyloxy-2,2,6,6-tetramethylpiperidine, a
condensation product of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and
4-cyclohexylamino-2,6-dichloro-1,3,5-tri~ine, a condensation product of 1,2-bis(3-amino-
propylamino)ethane and 2,4,6-trichloro-1,3,5-triazine as well as 4-butylamino-2,2,6,6-tetra-
methylpiperidine (CAS Reg. No. [136504-96-6]); N-(2,2,6,6-tetramethyl-4-piperidyl)-n-do-
decylsuccinimid, N-(1,2,2,6,6-pentamethyl-4-piperidyl)-n-dodecylsuccinimid, 2-undecyl-
7,7,9,9-tetramethyl-1-oxa-3,8-di~a-4-oxo-spiror4,51decane, a reaction product of 7,7,9,9-
tetramethyl-2-cycloundecyl-1-oxa-3,8-di~a-4-oxospiro [4,5]decane und epichlorohydrin.
2.7. Oxamides. for example 4,4'-dioctyloxyoxanilide, 2,2'-diethoxyoxanilide, 2,2'-dioctyloxy-
5,5'-di-tert-butoxanilide, 2,2'-didodecyloxy-5,5'-di-tert-butoxanilide, 2-ethoxy-2'-ethyloxani-
lide, N,N'-bis(3-dimethyla",inopropyl)oxamide, 2-ethoxy-5-tert-butyl-2'-ethoxanilide and its
mixture with 2-ethoxy-2'-ethyl-5,4'-di-tert-butoxanilide and mixtures of ortho- and para-
methoxy-rlisubstituted oxanilides and mixtures of o- and p-ethoxy-disllhstitllted oxanilides.
2.8. 2-t2-HvdroxyPhenyi)-1 .3,5-tri~ines, for example 2,4,6-tris(2-hydroxy-4-octyloxyphenyl)-
1,3,5-tri~ine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-tri~ine, 2-
(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2,4-bis(2-hydroxy-4-propyl-
oxyphenyl)-6-(2,4-dimethylphenyl)-1,3,5-tri~ine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(4-
methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-dodecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-
1,3,5-triazine, 2-(2-hydroxy-4-tridecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine,
2-[2-hydroxy-4-(2-hydroxy-3-butyloxy-propoxy)phenyll-4,6-bis(2,4-dimethyl)-1,3,5-triazine, 2-
[2-hydroxy-4-(2-hydroxy-3-octyloxy-propyloxy)phenyl]-4,6-bis(2,4-dimethyl)-1,3,5-triazine, 2-
[4-(dodecyloxy/tridecyloxy-2-hydroxypropoxy)-2-hydroxy-phenyll-4,6-bis(2,4-dimethylphe-
nyl)-1 ,3,5-triazine, 2-[2-hydroxy-4-(2-hydroxy-3-dodecyloxy-propoxy)phenyl]-4,6-bis(2,4-di-
methylphenyl)-1,3,5-tri~ine, 2-(2-hydroxy-4-hexyioxy)phenyl-4,6-diphenyl-1,3,5-triazine, 2-
(2-hydroxy-4-methoxyphenyl)-4,6-diphenyl-1,3,5-triazine, 2,4,6-trisl2-hydroxy-4-(3-butoxy-2-
hydroxy-propoxy)phenyl]-1,3,5-tri~ine, 2-(2-hydroxyphenyl)-4-(4-methoxyphenyl)-6-phenyl-
1 ,3,5-tri~ine.
3. Metal deactivators, for example N,N'-diphenyloxamide, N-salicylal-N'-salicyloyl hydrazine,
N,N'-bis(salicyloyl) hydrazine, N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl) hydrazine
, 3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalyl dihydrazide, oxanilide, isophthaloyl
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/04264
-Z5-
dihydrazide, sebacoyl bisphenylhydr~ide, N,N'-diacetyladipoyl dihydrazide, N,N'-bis(salicyl-
oyl)oxalyl dihydrazide, N,N'-bis(salicyloyl)thiopropionyl dihydrazide.
4. PhosPhites and phosphoniles. for example triphenyl phosphite, diphenyl alkyl phosphites,
phenyl dialkyl phosplliles, tris(nonylphenyl) phosphite, trilauryl phospl~ile, trioctadecyl phos-
phite, distearyl pentaerythritol cli~.hosphiLe, tris(2,4-di-tert-butylphenyl) phosph;le, ~iiisodecyl
pentaerythritol di,~llos~llile, bis(2,4-di-tert-butylphenyl3 pentaerythritol di,uhosphite, bis(2,6-
di-tert-butyl-4-methylphenyl)-pentaerythritol di~hospl ,iLe, diisodecyloxypentaerythritol di-
phospllilt:, bis(2,4-di-tert-butyl-6-methylphenyl)pentaerythritol diphosphite, bis(2,4,6-tris(tert-
butylphenyl)pentaerythritol di~)hosphiLe, tristearyl sorbitol L~iphosphiLe, tetrakis(2,4-di-tert-
butylphenyl) 4,4'-biphenylene diphosphonite, 6-isooctyloxy-2,4,8,10-tetra-tert-butyl-12H-di-
benz[d,g~-1 ,3,2-dioAdphospl)oci, 1, 6-fluoro-2,4,8,1 0-tetra-tert-butyl-1 2-methyl-dibenz[d,g]-
1,3,2-dioxaphosphocin, bis(2,4-di-tert-butyl-6-methylphenyl)methylphosphite, bis(2,4-di-tert-
butyl-6-methylphenyl)ethyllJhosphite.
5. Hydrox~lamines. for eAdlllple, N,N-dibenzylhydroxylamine, N,N-diethylhydroxylamine,
N,N-dioctylhydroxylamine, N,N-dilaurylhydroxylamine, N,N-ditetradecylhydroxylamine, N,N-
dihexadecylhydroxylamine, N,N-dioctadecylhydroxylamine, N-hexadecyl-N-octadecyl-hydroxylamine, N-hepl;l-le~;yl-N-oct~decylhydroxylamine, N,N-dialkylhydroxylamine derived
from hydrogenated tallow amine.
6. Nitrones. for example, N-benzyl-alpha-phenyl-nitrone, N-ethyl-alpha-methyl-nitrone, N-oc-
tyl-alpha-heptyl-nitrone, N-lauryl-alpha-undecyl-nitrone, N-tetradecyl-alpha-tridecyl-nitrone,
N-hexadecyl-alpha-pentadecyl-nitrone, N-octadecyl-alpha-heplddecyl-nitrone, N-hexadecyl-
alpha-heptadecyl-nitrone, N-oc~t~-lecyl-alpha-pentadecyl-nitrone, N-heptadecyl-alpha-hep-
tadecyl-nitrone, N-oct~-~ecyl-alpha-hexadecyl-nitrone, nitrone derived from N,N-dialkyl-
hydroxylamine derived from hydrogendled tallow amine.
7. Thiosvnerqists, for example, dilauryl thiodipropionate or distearyl Ihiodi~ ,upionate.
8. Peroxide scavenqers. for example esters of b-thiodipropionic acid, for example the lauryl,
stearyl, myristyl or tridecyl esters, mercaptoben,i",idazole or the zinc salt of 2-mercapto-
benzimidazole, zinc dibutyldithiocarbamate, dioctadecyl disulfide, pentaerythritol tetrakis(b-
dodecylmercapto)propionate.
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
-26-
9. Polyamide st~ sers, for example, copper salts in cornbi"dliorl with iodides and/or phos-
phorus compounds and salts of divalent manganese.
10. Basic co- ~ ~ " ,e. ~. for example, melamine, polyvinylpy" olidone, dicyan~lia" ,;de, triallyl
cyanurate, urea derivatives, hydr~i"e derivatives, amines, poly~n ~!es, polyurethanes,
alkali metal salts and alkaline earth metal salts of higher fatty acids for e~dr"ple calcium
stearate, zinc stearate, magnesium behenate, magnesium stearate, sodium ricinoleate and
pot~sciurn palmitate, antimony pyrocatecholate or tin pyrocatecholate.
11. Nuclealillg agents. for example, inorganic sul,~L~nces such as talcum, metal oxides
such as titanium dioxide or magnesium oxide, phosphdl~s, carbonates or sulfates of, prefe-
rably, alkaline earth metals; organic compounds such as mono- or polycalbokylic acids and
the salts thereof, e.g. 4-tert-butylbenzoic acid, adipic acid, diphenylacetic acid, sodium suc-
cinate or sodium ber,~o~le; polymeric compounds such as ionic copolymers ("ionomers").
12. Fillers and le;"rurei"~ agents. for example, calcium carbonate, 5jli~ 5, glass fibres,
glass bulbs, ~-~bestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxides, car-
bon black, graphite, wood flour and flours or fibers of other natural products, synthetic fi-
bers.
13. Other additives, for example, p!-slioi~ers, lubricanL~, emulsfflers, pigments, rheology
additives, catalysts, flow-control agents, optical brighteners, flameproofing agents, anli~l~lic
agents and blowing agents.
14. Benzofuranones and indolinones, for example those disclosed in US-A-4325863, US-A-
4338244, US-A-5175312, US-A-5216052, US-A-5252643, DE-A-4316611, DE-A-4316622,
DE-A-4316876, EP-A-0589839 or EP-A-0591102 or 3-[4-(2-acetoxyethoxy)phenyl]-5,7-di-
tert-butyl-benzofuran-2-one, 5,7-di-tert-butyl-3-[4-(2-stearoyloxyethoxy)phenyl]benzofuran-
2-one, 3,3'-bis[5,7-di-tert-butyl-3-(4-[2-hydroxyethoxy]phenyl)benzofuran-2-one], 5,7-di-tert-
butyl-3-(4-ethoxyphenyl)benzofuran-2-one, 3-(4-acetoxy-3,5-dimethylphenyl)-5,7-di-tert-but-
yl-benzofuran-2-one, 3-(3,5-dimethyl-4-pivaloyloxyphenyl)-5,7-di-tert-butyl-benzofuran-2-
one.
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/04264
-27-
These additional additives are expediently employed in amounts of from 0.1 to 10% by
weight, for example from 0.2 to 5% by weight, based on the polymer to be st~hili~ed
The examples below illustrate the invention in greater detail. All parts and percentages, in
the examples, in the remainder of the description and in the claims, are by weight, unless
stated otherwise. The following abbreviations are used in the examples:
GPC: Gel permeation chromatography;
'H-NMR: Magnetic nuclear resonance of the nuclide 1H;
Mn Number average molar mass (unit g/mol);
Mw: Number average molar mass (unit g/mol);
TGA: Thermogravimetric analysis;
THF: Tetrahydrofuran.
Example 1: Propargvl tosvlate
381 9 (2 mol) of toluenesulfonyl chloride are dissolved in 400 ml of methylene chloride in a
2.5 I sulfonation flask fitted with a mechanical stirrer, thermometer and dropping funnel. A
solution of 112 9 (2 mol) of propargyl alcohol in 160 ml of water is poured in. 88 9 (2.2 mol)
of sodium hydroxide, dissolved in 132 ml of water, are added dropwise at such a rate that
the temperature of the emulsion does not exceed 25~C. The mixture is then stirred
overnight at room ter"per~ re and then diluted with 400 ml of water and 200 ml of
methylene chloride. The organic phase is separated off and washed twice with water. The
solvent is removed on a rotary evapora~ur. The residue is distilled via a Vigreux column.
Yield: 325 9 (77 %) of a liquid of boiling point 1 02~C / 0.008 mbar.
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
-28-
Microanalysis:
C~IGI ~ted Found
C 57.13 57.07
H 4.79 4.80
S 15.25 15.24
1H-NMR (CDC13):
2.45 ppm (3H, s) : CH3
2.47 - 2.53 ppm (1 H, m) : ~C-H
4.69- 4.70 ppm (2H, m) :.C-CH2-O
7.34 - 7.37 ppm '
(4H, m) : H-Ar
7.80 - 7.83 ppm
FxamPle 2: P~ epardlion of 1 -proParqyl-4-hydroxv-2,2~6,6-tetramethylpiperidine
197 g (1.25 mol) of 4-hydroxy-2,2,6,6-tetramethylpiperidine, 105 9 (0.5 mol) of the
compound from F~ample 1 and 1.81 of aceLunilr:!e are introduced into a 2.51 sulrurl~lion
flaskfitted with precision glass stirrer, ll,er,non,eL~r and condenser. The suspension is
warmed to the boil, a clear solution initially being obtained above 70~C. After half an hour, a
slight suspension is formed. The mixture is refluxed for 18 hours, cooled to room
temperature, diluted with 51 of water and extracted three times with ethyl acetate, and the
combined organic phases are dried and evaporated. The solid residue is recrystallized from
ethyl acetate.
Yield: 72.2 9 (74%) Melting Point: 151 ~C
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96rO4~64
Microanalysis:
C~ t~d Found
C 73.80 73.78
H 10.84 10.83
N 7.17 7.21
H-NMR (CDC13):
1.07 ppm and 1.26 ppm (12H, s) : CH3
1.38 - 1.45 ppm (2H, m) : CH2 (pi~.ed~' ~e)
1.54- 1.~6 ppm (1H, d) : OH
1.79- 1.84 ppm (2H, m) : CH2 (piperidine)
2.11 - 2.12 ppm (lH, t) : H-C~C
3.32 - 3.33 ppm (2H, d) : ~C-CH2-N
X~
3.91 - 3.96 ppm (1H, m)
ExamPle 3: ~\ epardlion of 1 -(but-2-ynyl)-4-hvdroxy-2,2,6,6-tetramethylPiperidine
27.2 9 (0.12 mol) of 2-butynyl p-toluenesulfonate [Lancaster Synthesis Ltd., Lancashire
LA 30Y, England], 47.7 9 (0.3 mol) of 4-hydroxy-2,2,6,6-tetramethylpiperidine and 0.6 1 of
ac~:lonil,ile are introduced into a 1 I round-bottom flask fitted with magnetic stirrer and
condenser. The mixture is warmed to reflux. After 19 hours, the solution is poured onto ice
and extracted three times with ethyl acetate. The organic phases are dried and evaporated.
The crude product is recry~ Hd from n-hexane.
Yield: 20 9 (80%) Melting point: 106~C
CA 02232573 1998-03-20
W O 97/137S2 PCT~EP96/04264
-30-
Microanalysis:
C~ICUi-~PCI Found
C74.59 74.47
H11.08 11.04
N6.69 6.80
'H-NMR (CDCI3):
1.07- 1.25 ppm (12H, s) : CH3 (piperidine)
1.16-1.19ppm (1H d) : OH
1.38 - 1.46 ppm (2H, m) : CH2 (piperidi"e)
1.78 - 1.95 ppm (5H m) : CH2 (piperidine) and CH3-C~
3.29 ppm (2H, m) : ~C-CH2-N
\ o
3.91 - 3.99 ppm (1 H m) : X
ExamDle 4: PreParation of N-(2 2.6 6-tetramethylpi~eridin-4-yl)acrylamide
469 9 (3 mol) of 4-amino-2 2 6 6-tetramethylpipe, idil ,e are dissolved in 500 ml of diethyl
ether in a 1.51 sulroncllion flask fitted with precision glass stirrer Ll,e""o",eter d,opping
funnel and condenser. 90.5 g (1 mol) of acryloyl chloride are slowly added dropwise at
-15~C. ~f~er the dropwise addition the mixture is allowed to warm to room temperature and
is poured into ice-water and the solid is separated off and recrystallized from acetonitrile.
Yield: 67 g (32%)Meltinq Point: 122~C
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
Microanaiysis:
C~lc~ ~'~ed Found
C 68.53 68.65
H 10.54 10.61
N 13.32 13.22
1H NMR (CDCb):
0.69 ppm (1H, s) : NH (piperidine)
0.91 - 0.99 ppm (2H, t) : CH2 (piperidine)
1.13 ppm and 1.27 ppm (12H, s) : CH3 (piperidine)
1.91 - 1.96 ppm (2H, m) : CH2 (pipe,idi"e)
N
4.29 - 4.40 ppm (1H, m) : ~
5.46 ppm (1 H, m) : -NH-CO-
5.61 - 5.65 ppm (1 H, m) : CO-CH=C
6.02- 6.32 ppm (2H, m) : C=CH2
Example 5: P~e~a,dlion of N-(2,2,6,6-tetramethyl"iperidi,--4-vl)methacrylamide
344 9 (2.2 mol) of 4-amino-2,2,6,6-lelr~",e:ll,ylpiperidine are dissolved in 1.51 Of methylene
chloride in a 2.51 sulfonation flask fitted with precision glass stirrer, thermometer and
d~upp;. ,g funnel. The mixture is cooled to -10~C, and 104.5 g (1 mol) of methacryloyl
chloride are added dropwise with stirring. The mixture is then stirred for a further 3 hours at
room te""~e,dlure and poured into 21 Of 0.5 N sodium hydroxide solution, the organic phase
is separdled off, washed with water and dried, and the volatile constituents are removed by
evaporation. The residue is recryst~lli7ed from n-hexane.
Yield: 87 9 (39%) Meltinq Point: 119~C
CA 02232~73 1998-03-20
O 97/13752 PCT~EP96/04264
-32-
Microanalysis:
C~lcu'-ted Found
C 69.60 69.52
H 10.78 10.79
N 12.49 12.42
1H-NMR (CDCI3):
0.6g ppm (1H, s) : NH (piperidine)
0.91 - 0.99 ppm (2H, t) : CH2 (piperidine)
1.13 ppm and 1.27 ppm (12H, s) : CH3 (piperidine)
1.91 -1.95 ppm (2H, m) : CH2 (piperidine)
1.96 ppm (3H, s) : CH3 (methacrylate)
N
4.25 - 4.36 ppm (1 H, m) : ~,~
5.31 ppm and 5.65 ppm (2H, s) : CH2 =C
5.55 pm (1H, d) : -NH-CO-
Example 6: rlel~ar~Lion of 4-acrylolyloxy-1-proParayl-2,2,6,6-tetramethylPiPeridine
290 9 (1.48 mol) of the compound prepared in Example 2, 195 9 (1.9 mol) of triethylamine
and 2.71 of diethyl ether are introduced under argon into a 51 flask with plane ground joints
fitted with pl ecision glass stirrer, thermometer and dropping funnel. This mixture is cooled to
5~C, and 161 9 (1.8 mol) of acryloyl chloride are then slowly added dropwise with stirring.
The mixture is then stirred at room temperature for about 18 hours. The reaction solution is
poured into sodium bicarbonate solution, and the organic phase is separated off. The water
phase is extracted once with ethyl acetate. The combined organic phases are dried and
evaporated. The residue is recrystallized from n-hexane.
Yield: 206.5 g (56%) Meltinq Point: 48~C
CA 02232~73 1998-03-20
W O 97/13752 PCT/~l~c~1264
-33-
Microanalysis:
C~lc~ ted Found
~ C72.25 72.40
H9.30 9.34
I r N5.62 5.64
'H-NMR (CDCI3):
1.14 ppm and 1.27 ppm (12H, s) : CH3 (piperidine)
1.54- 1.62 ppm (2H, m) : CH2 (piperidine)
1.83- 1.89 ppm (2H, m) : CH2 (piperidine)
2.11 - 2.13 ppm (lH, t) : H-C~C
3.33 - 3.34 ppm (2H, d) : N-CH2-
\ o
5.10-5.21 ppm (lH,m) : ~,<
5.78 - 5.82 ppm (1 H, d) : C0-CH=C
6.05 - 6.14 and 6.3~ - 6.41 ppm (2H, m) : C=CH2
Example 7: r~epardlion of 4-methacryloxy-1-ProPargvl-2.2,6.6-tetramethvlPiperidine
240 9 (1.25 mol) of the compound described in Example 2, 177 9 (1.75 mol) of triethylamine
and 1.7 1 of diethyl ether are introduced under argon into a 2.5 I sulfonation flask fitted with
preci~ion glass stirrer, thermometer and dropping funnel. The suspension is cooled to 5~C,
and 1 56 9 (1.5 mol) of methacryloyl chloride are then added dropwise. The mixture is
allowed to warm to room temperature, stirred for a further 20 hours and poured into
aqueous sodium bicarbonate solution, and the organic phase is separated off, dried and
evaporated. The residue is recryst~ 7ed from n-hexane.
Yield: 163 9 (50%) Melting point: 49~C
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
-34-
Microanalysis:
C~lc~ d Found
C 72.97 72.28
H 9.57 9.80
N 5.32 5.22
-NMR (CDCI3):
1.13 ppm and 1.27 ppm (12H, s) : CH3 (piperidine)
1.48- 1.68 ppm (2H, m) : CH2 (piperidine)
1.84- 1.90 ppm (2H, m) : CH2 (piperidine)
1.93 ppm (3H, s) : Clt3 (methacrylate)
2.11 - 2.13 ppm (1 H, t) : H-C-C
3.34- 3.35 ppm (2H, d) : N-CH2
\ o
5.08 - 5.18 ppm (1 H, m) : ~
H
5.53 ppm and 6.07 ppm (2H, s) : CH2=C
Example 8: P,ePar~lion of 4-acryloyloxy-1-but-2-ynyl-2.2.6.6-tetramethylpi~eridine
10.5 9 (50 mmol) of the compound prepared in Example 3, 5.6 9 (55 mmol) of triethylamine
and 65 ml of diethyl ether are introduced under argon into a 250 ml sulfonation flask fitted
with precision glass stirrer, thermometer and dropping funnel. 4.5 g (50 mmol) of acrylic acid
are added dropwise at 5~C. The mixture is then stirred at roorn temperature for a further
24 hours and washed twice with sodium bicarbonate solution, and the organic phase is
dried using sodium sulfate and evaporated. The residue is chromatographed on silica gel
60 [Merck, Darmstadt] using n-hexane/ethyl acetate = 3: 1, giving 10.3 g (78%) of a
colourless liquid.
CA 02232~73 l998-03-20
O 97/13752 PCT~EP96/04264
-35-
Microanalysis:
C~lcul~t~sd Found
C72.97 72.77
H9.57 9.63
N5.32 5.11
H NMR (CDCI3):
1.06 ppm and 1.18 ppm (12H, s) : CH3 (pi~.eridi.,e)
1.46 - 1.54 ppm (2H, t) : CH2 (pi~,eri.Jil,e)
1.69 - 1.70 ppm (3H, m) : CH3 (butyne)
1.74- 1.80 ppm (2H, m) : CH2 (piperidine)
3.21 - 3.22 ppm (2H, m) : CGC CH2 N
\ o
5.02 - 5.12 ppm (1H, m) :
5.70 - 5.74 ppm ~
5.97-6.06 ppm ~ (3H, m) : CH2=CH-CO
6.26 - 6.32 ppm J
ExamDle 9: r~epar~lion of 4-(4-vinvlbenzyloxv)-1-proPa,u~/1-2,2~6~6-tetramethylPiPeridine
30 9 (154 mmol) of the compound prepared in Example 2, 1 50 ml of toluene, 7.7 g(7.7 mmol) of polyethylene glycol 1000 and 43.1 g (770 mol) of powdered pot~ssi~hydroxide are introduced under argon into a 350 ml sulfonation flask fitted with precision
glass stirrer, thermometer and dropping funnel. This suspension is warmed to 80~C, and
25.9 g (170 mmol) of vinylbenzyl chloride [Fluka AG, Buchs] are then added dropwise. The
mixture is stirred at 80~C for a further 4 hours, then cooled to room temperature and poured
into water. The organic phase is separated off and extracted with 2N HCI solution. This
aqueous phase is washed with toluene and then rendered basic by means of 1 N sodium
hydroxide solution. The water phase is extracted with toluene, and the organic phase is
CA 02232573 1998-03-20
W O 97/13752 PCT~EP96/04264
-36-
dried using sodium sulfate and the solvent is evaporated co,~ tely. Bulb-tube distillation at
150~C/0.02 mbar gives a colourless liquid.
Yield: 23.5 g ( 51 %)
Microanalvsis:
C~ fed Found
C80.98 80.89
H9.38 9.39
N4.50 4.47
'H-NMR (CDCI3):
1.05 ppm and 1.26 ppm (12H s) : CH3 (piperidine)
1.46 - 1.54 ppm (2H t) : CH2 (piperidine)
1.86 - 1.92 ppm (2H, m) : CH2 (piperidine)
2.10-2.11 ppm (1H t) : H-C~C
3.31 - 3.32 ppm (2H, d) : C~C-CH2-N
\ o
3.64 - 3.75 ppm (1 H m) : ~<
4.52 ppm (2H s) : Ar-CH2-O-
5.19 - 5.25 ppm l
5.69 - 5.77 ppm ~ (3H, m) : CH2=CH-
6.65 - 6.75 ppm J
7.21 - 7.38 ppm (4H m) : H-Ar
CA 02232573 l998-03-20
W O 97/13752 PCT/EP96/04264
ExamPle 10: Preparation of N-(1-Proparqyl-2.2.6.6-tetramethylpiperdin-4-vl)acrvlamide
66.6 g (0.31 mol) of the compound from Example 1,131 9 (0.62 mol) of the compound
described in Example 4 and 940 ml of acetonitrile are introduced into a 21 round-bottomed
flask fitted with magnetic stirrer and condenser. The mixture is warmed to reflux, a clear
solution forming. The mixture is refluxed for 6 hours, giving a suspension again. The solid is
filtered off, and the solution is evaporated to dryness. The residue is purified on silica gel 60
[Merck] by means of ethyl acetEe and recryst~lli7~d from xylene.
Yield: 47 9 (61 %) Melting point: 125.3~C
Microanalysis:
C~lcul~ted Found
C72.54 72.39
H9.74 9.71
N11.28 11.30
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/04264
-38-
1H-NMR (CDC13):
1.13 ppm and 1.22 ppm (12H, s) : CH3 (p;~ elidine)
1.30 - 1.38 ppm (2H, t) : CH2 (piperidine)
1.79- 1.84 ppm (2H, m) : CH2 (piperidine)
2.11 - 2.12 ppm (1 H, t) : H-C~C-
3.32 - 3.33 ppm (2H, d) : C-=C-CH2-N
N
4.20 - 4.32 ppm (1 H, m) : ~
H
5.50 ppm (lH, d) :-NH-C0-
5.60 - 5.64 ppm ~
6.03 - 6.12 ppm ~ (3H, m) :CH~=CH-
6.24 - 6.31 ppm J
ExamPle 11: r~epardlion of N-(1-ProDar~lvl-2~6~6-tetramethylPiperidin-4-yl)methacrylamide
40.2 g (0.19 mol) of the compound from Example 1,85.8 9 (0.38 mol) of the compound
described in Example 5 and 600 ml of acetonitrile are introduced into a 1 I round-bottomed
flask fitted with magnetic stirrer and condenser. The solution is warmed to reflux, stirred for
6 hours, cooled to room tei"pe~dlure and separated from the solid. The solvent is
evaporated. The residue is purified on silica gel 60 [Merck] using ethyl acetate and
sl lhsequently recryst~ d from cyclohexane.
Yield: 25.4 9 (51 %)Meltinq point: 117.4~C
Microanalysis:
C~lcul~ted Found
C 73.2¢ 73.21
H 9.99 9.98
N 10.68 10.65
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
-39-
lH-NMR (CDCI3):
1.13 ppm and 1.25 ppm (12H, s) : CH3 (piperidine)
1.29-1.37 ppm (2H,t) : CH2 (piperidine)
1.80 -1.86 ppm (2H, m) : CH2 (piperidine)
1.95 ppm (3H, s) : CH3 (methacrylate)
2.11-2.12ppm (1H,t) : H-C-C-
3.32-3.33 ppm (2H, d) : C=C-CH2-N
N
4.17 - 4.28 ppm (1H, m) :
5.31 ppm and 5.64 ppm (2H, s) : CH2=
5.52 ppm (1 H, m) : -NH-C0-
ExamPle 12: P~ePardlion of Poly(4-acryloyloxv-1-ProPa,~/1-2,2,6,6-tetramethvlpiperidine)
90 g (0.36 mol) of the monomer from Example 6, 1.19 9 (7.2 mmol) of ~,o~isic017lltyronitrile
(AIBN), 3.2 g (36 mmol) of butyl ")er.;aptan and 270 9 of tetrahydrofuran (THF) are
introduced under argon into a 500 ml round-bottom flask fitted with magnetic stirrer. The
solution is freed from oxygen and polymerized overnight at 70~C under argon. The reaction
solution is precipil;.l~d in ",~ll,anol, and the solid is re-dissolved in THF and preci~ led in
methanol. The white polymer powder is dried under a high vacuum, giving 33.1 9 (37%) of
polymer.
GPC (THF): Mn = 6700
Mw = 10600
TGA (Heating rate 2~C / min., air) : 10% weight loss at 303~C
CA 02232~73 l998-03-20
W O 97/13752 PCT/EP96/04264
-40-
ExamPle13:PreParation ofpoly(4-methacryloxy-1-proparqyl-2,2.6.6-tetramethylpiPeridine)
70 g (0.27 mol) of the monomer from Example 7 are polymerized with 0.87 g (5.3 mmol) of
AIBN and 3.6 g (40 mmol) of butyl mercaptan in 210 9 of THF as described in Example 12.
Yield: 40.3 g (58%) of a white polymer powder.
GPC (THF): Mn = 6100
Mw = 9900
TGA (Heating rate 2~C / min., air) : 10% weight loss at 298~C
ExamPle 14: PreParation of Poly(4-acryloyloxy-1-but-2-ynvl-2.2.6,6-tetramethvlpi,.eri.li"e)
20 g (76 mmol) of the monomer from Example 8 are polymerized with 0.12 g (0.76 mmol) of
AIBN and 0.68 g (7.5 mmol) of butyl mercaptan in 63 9 of toluene as described inExample 1 2.
Yield: 7 g (35%) of polymer.
GPC (THF): Mn = 4400
Mw = 5600
TGA (Heating rate 2~C / min., air) : 10% weight loss at 278~C
CA 02232573 1998-03-20
W O 97/13752 PCT~EP96/04264
ExamPle 15: PreParation of Poly~N-t1-proparqvl-2~2~6~6-tetramethylpiperidin-4-yl)acrvlamide
42 g (169 mmol) of the monomer from Example 10 are polymerized with 280 mg (1.7 mmol)
of AIBN and 3.05 g (33.8 mmol) of butyl mercaptan in 120 g of THF as described in
Example 1 2.
The polymer is precil-iL~ied in n-hexane.
Yield: 32.5 g (77%) of white polymer.
GPC (THF): Mn = 1500
Mw = 41 00
TGA (Heating rate 2~C / min., air) : 10% weight loss at 31 5~C
Example 16: P~ePardLion of poly~N-(1-Proparqyl-2~2~6~6-tetramethvlpiperidin-4-yl)
methacrvlamidel
25 g (95 mmol) of the monomer from Example 1 1 are polymerized with 156 mg (0.95 mmol)
of AIBN and 1.71 9 (19 mmol) of butyl mercaptan in 75 9 of THF as described in
Example 12.
The polymer is precirit~ted in n-hexane.
Yield: 14 g (56%) of white polymer.
GPC (THF): Mn = 1500
Mw = 4000
TGA (Heating rate 2~C / min., air) : 10% weight loss at 321 ~C
,
CA 02232~73 l998-03-20
W O 97/13752 PCT/EP96/04264
Determination of the PKa of the substance
Titration in acetonitrile/chloroform = 1: 1 using 0.1 N HCI04 in dioxane. The half
neulrdli~lion point (HNP) is relative to 2-ai,.;nopyridine (= 0 m\/):
HNP (m\/) pKa
2- 0 6.86
Aminopyridine
Polymer + 75 6.45
ExamPle 1 7: PreParation of poly(4-vinvlbenzyloxy-1 -proPargvl-2,2,6.6-tetramethylpiperidine)
29 9 (97 mmol) of the monomer from Example 9, 0.64 9 (3.9 mmol) of azobisisobutyronitrile
(AIBN) and 11 6 g of toluene are introduced into a 250 ml round-bottom flask fitted with
magnetic stirrer and reflux condenser. The solution is freed from oxygen and stirred
overnight at 70~C under argon.
The viscous solution is poured into methanol. The preci~iL~Le is taken up in toluene and re-
precipit~tPd in methanol. The polymer is dried under a high vacuum.
Yield: 24 g (83%)
MALDI-MS (~aatrix_ssisted Laser Desorption/~onization-Mass Spectrometry):
Mn = 6000 Mw = 8500
TGA (Heating rate 2~C / min., air) : 10% weight loss at 316~C
CA 02232~73 l998-03-20
O 97/13752 PCT~EP96/04264
ExamPle 18: Liqht st~hi~ qtion of PolyProPylene fibres
2.5 9 of each of the novel stfih~ erS from Exal"ples 12 to 14 together with 1 9 of tris(2,4-di-
tert-butylphenyl) phosphite, 1 9 of calcium monoethyl 3,5-di-tert-butyl-4-hydroxyben~yl-
phosphonate, 1 9 of calcium stearate and 2.5 9 of TiO2 (Kronos RN 57) are mixed with
1000 9 of polypropylene powder (melt flow index 12 g/10 min, measured at 230~C/2.16 kg)
in a turbomixer. The mixtures are extruded at 200-230~C to give pellets, which are
sl Ihsec-lently converted into fibres with the aid of a pilot plant (Leonard; Sumirago/VA, Italy)
under the following conditions:
Extruder temperature: 1 90-230~C
Head temperature: 255-260~C
SLI~lchillg ratio: 1:3.5
Sl.~lchi, ,9 temperature: 1 00~C
Fibres: 12 den
The fibres produced in this way are exposed against a white background in a type 65 WR
Weather-O-Meter~ (Atlas Corp.) at a black panel temperature of 63~C in accordance with
ASTM D 2565-85. After various exposure times, the residual tensile strength of the samples
is measured. The measurements are used to cfllc,J'qte the exposure time Tso after which the
tensile strength of the samples has halved.
For comparative purposes, fibres are produced without novel st~qhi'i~er under oll,er~ise
identical conditions and are tested. The test results are shown in Table 1.
Table 1:
Exposure time for the initial tensile
strength to halve
.SPhiii7erExposure time
None 300 h
from Example 122270 h
from Example 132470 h
from Example 1431 10 h
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/04264
The fibres sl;-hil;~ed in accordallce with the invention have excellent ~lrenyLll retention.
Example 19: Stabilization of a two-coat Paint
The light sPhili7er from Example 10 is incorporated into 5-10 g of xylene and tested in a
varnish of the following co,llposilion:
SynthacrylD SC 303 l) 27.51 9
Synthacryl~ SC 370 2) 23.34 9
Maplenal~ MF 650 3) 27.29 9
Butyl acetate/butanol (37/8) 4.33 9
Isobutanol 4.87 9
Solvessoe 150 4) 2.72 9
Crystal Oil K-30 5) 8.74 9
Flow-control agent Baysil~ MA6) 1.20 9
100.00 9
1) Acrylate resin, Hoechst AG; 65% solution in xylene/butanol 26:9
2) Acrylate resin, Hoechst AG~ 75% solution in Solvessoe 1004)
3) Melamine resin, Hoechst AG; 55% solution in isobutanol
4) Manufacturer: ESSO
5) Manufacturer: Shell
6) 1% in Solvesso~ 150; manufacturer: Bayer AG
The varnish is mixed with 1 % of the stabilizer from Example 10, based on the solids content
of the varnish. The control is a varnish containing no light st~bili7er.
The varnish is diluted to spray consi~Lency with Solvesso~100 and sprayed onto a prepared
aluminium sheet (coil coat, filler, silver-metallic base coat) and baked for 30 minutes at
130~C, giving a dry film thickness of 40-50 ~Lm.
CA 02232573 1998-03-20
W ~ 97113752 PCT~EP96/04264
-45-
The samples are then weathered in a UVCON~weathering unit from Atlas Corp.
(UVB-313 Lamps) with a cycle of 4 hours' UV irradiation at 60~C and 4 hours' condensation
at 50~C.
The samples are regularly assessed for cracks.
The samples co,~Lhi.,;ny the novel .~l~h~ er have high cracking ,~sisl~nce.
E~xamPle 20: Sl~ lion of a Dhotoqraphic material
0.087 9 of the yellow coupler of the formula
CH3
(CH3)3 C--C--fH--C NH~ I H2
CH3 ~ NH-C--(CHz)3 O~L ICHH' CHzCH,
CH3
is dissolved in 2.0 ml of a solution of the novel 5t~hjli7er from Example 6 in ethyl acetate
(2.25 9/100 ml). 9.0 ml of a 2.3% aqueous gelatin solution which has been ~just~cl to a pH
of 6.5 and 1.744 g/l of the wetting agent of the formula
CA 02232~73 1998-03-20
W O 97/13752 PCT~EP96/04264
CH3cHcH2cHs
~SO3Na
CH3CHCH2CH3
are added to 1.0 ml of this solution.
2 ml of a silver bto", de emulsion having a silver content of 6.0 9/l and 1.0 ml of a 0.7%
aqueous solution of the curing agent of the formula
Cl
>=N
N /~OH
~N
Ci
are added to 6.0 ml of the resultant coupler emulsion, and the mixture is cast onto a
13 x 18 cm resin-coated paper. After a curing time of 7 days, the samples are exposed to
125 lux-s behind a silver step wedge and subsequently processed by the Kodak Ektaprint
2~ ,c r~cess.
The yellow wedges obtained are irradiated with a total of 60 kJ/cm2 behind a UV filter
(Kodak 2C) in an Atlas Weather-O-Meter~ using a 2500 W xenon lamp.
A sample without ~ hili~er is processed in the same way as standard.
The drop in colour density at the absorption maximum of the yellow dye that occurs on
irradiation is measured using a Macbeth TR 924 A densitometer.
The light sPhi~ tion effect is evident from the drop in colour density. The smaller the drop
in density, the greater the light Sphili~tion effectiveness.
The novel st~hili~er has a good light st~hi~ tiQn action.
CA 02232~73 l998-03-20
W O 97/13752 PCT~EP96/0426
Exam~le 21: SPhili7~tion of Polypropylene ta~es
1.0 9 of the novel 5t~hili'er from Example 9 together with 1 9 of tris(2,4-di-tert-butylphenyl)-
pl ~osphile, 0.5 g of pentaerythrityl tetrakis(3-[3',5'-di-tert-butyl-4'-hydroxyphenyqpropionate)
and 1 9 of calcium ~lear~L~ are mixed with 1000 9 of polypropylene powder (STATOILMF;
melt flow index 4.0 g/10 min, measured at 230~C/2.16 kg) in a turbo mixer.
The mixtures are extruded at 200-230~C to give pellets, which are converted into stretch
tapes 2.5 mm wide and 50,um thick with the aid of a pilot plant (Leonard; Sumirago/VA,
Italy) under the following condilions;
Extruder temperature: 210-230~C
Head temperature: 240-260~C
Slr~l~;hing ratio: 1 :6
SLr eL~,-hil lg temperature: 110~C
The tapes produced in this way are exposed against a white background in a type 65 WR
Weather-O-Meter~ (Atlas Corp.) at a black panel temperature of 63~C in accordance with
ASTM D 2565-85. After various exposure times, the residual tensile strength of the samples
is measured. The measurements are used to c~icul~te the exposure time T50 after which
the tensile strength of the samples has halved.
For comparative purposes, tapes are produced without novel st~hili~er under otherwise
identical conditions and are tested.
The sample 5t~hi~i~ed in accordance with the invention has excellent strength retention.