Note: Descriptions are shown in the official language in which they were submitted.
CA 02234537 l998-04-09
WO 97/13454 PCT/US95/13505
AN INTEGRATE~:D MOVE;MENT ANALYZING SYSTEM
TECHNICAL FIELD
The invention pertains to the general field of
electro-diagnostic equipment and more particularly to
an integrated mo~ement ~n~lyzing system that combine~
electromyography with range of motion and funationAl
c~pacity mea~urements. to provide a non-invasive And
non-loading method for analyzin~ myofacial injuries and
repetitive stress injuries.
BACKGROUND ART
Myof'acial injuries repreYent the second largest
medical problem today, with back pain alone accounting
for the largest medical visits. Carpal tunnel syndrome
" (CTS), repetitive stres6 injuries ~RSI) account for the
most d~ys lost and ~re predicted to become the mo~t
costly health problem of our time. With the
implement~tion OI- the American's with disability (ADA)
law worker's compensation claims such as CTS c~ln now
CA 02234~37 l998-04-09
W O 97/13454 PCT~US95/1350~
sue in the federal court system allowing for the
initiation of suits in excess of 10 million dollars.
These claim~ could d~mage the economy and force
employers to go outside of the United States.
A recent study in the New England Journal of
Medicine indicates that over 58% of asymptomatic low
b~ck pain patients who underwent an MRI ~ound evidenae
of disc pathology. How reliable is an MRI - it Appears
to have no correlation to pain, impairment and may not
be alinically si~nificAnt.
A recent study revealed that over 45 peroent of
individuals who have undergone CT~ release surgery were
no better two years p~st the surgic~l intervention
bec~use they were misdiagnosed. The individuals
probably had cervical pathology th~t c~n refer pain and
mimic the symptoms of carpal tunnel, ulnar neuopathy,
cubital tunnel, tendonititis, DeQuarian'a syndrome
i.e. J repetitive stress injuries. The problem is that
until the development of the instant invention, there
was no way to ~scertain if the problem Wa8 proxim~l
tcervical or distal, CTS).
In the past, many doctors have prescribed a
pro~alati~ work reatriction limitinc the amount an
individu~l c~n lift. More often than not, the lifting
restriction is too general and too limiting which
prohibits the individual to return back to their usual
or any job. For example, a typical work restriction o~
no li~ting over 50 pounds is highly restrictive.
Doctors impose this restriction because they have no
means of evaluating the muscle and disc pathology
during movement.
The inventive integrated movement analyzer (IMA) i8
a portable, non-loading electronic instrument that
simultaneously monitors muscle activity with
~ilver-silver chloride standard ECG electrodes,
cervical, thoracic and lumbar flexion~ exten~ion, right
CA 02234537 1998-04-09
W O 971134S4 PCT~US95/13SO5
rotation, left rotation, right l~teral movement, ~nd
left lateral movement ~s well as monitorin~ the
extremitie~. The IMA also simultaneously combines a
non-loading lo~d cell ~nd strain gau~e that with a
computer ~nd software correlates the weight li~ted by
pullin~ on the strain gage. The EMG, R~nge of Motion
a~ func-tion~l c~acity evaluation) are all
conducted at the same time.
The IMA is portable and can be battery operated to
allow the patient to be monitored anywhere including at
the work sight, at home and per~ormin~ any activity
e~en their job, no m~tter wh~t or where it i8. The IMA
also complies with the new ADA law, and includes a
special device that allows for heart rate in the filter
system. This is important because when heart rate i8
~ound in the paraspinal mu8cles over the EMG, in the
upper trapezius and in the low back, the amplitude o~
the ECG aativity that overlaps the EMG correlates to
disc pathology or spinal chan~es on an MRI. Sinae the
IMA monitors active range of motion, it takes the MRI
one step ~urther and can help determine if the
monitored ~ilment, can in fact, be treated with
conservative methoda that do not in~olve surgery.
CA 02234~37 l998-04-09
W O 97/13454 PCT/US95/13505
A search of the prior art did not di~close any
patenta that read directly on the claims of the instant
invention. However, the following U.S. patents were
considered related:
PATENT NO. INVENTOR ISSUED
5,042,505 Mayer et al 27 August 1991
4,688,581 Mos8 25 August 1987
4,667,513 Konno 26 May 1987
The 5,042,505 Mayer, et al patent discloses an
10 electronic device ~or measuring rel~ti~e angular
positional displacement and angular range of motion for
body segments and articulating joints of the human
skeleton. The device has a hand-held inter~ace unit
which i~ placed against the body segment or joint to be
teated. Mounted within the houaing of the interface
unit i8 a sha~t with a pendulum at one end and an
optical encoder at the other. As the body segment
rotates or the joint articulates, the pendulum swings
in the direction of ~ravity, causing the shaft to
rotate. The optical encoder generates an electric~l
signal represent~tive of the amount of rotation of the
sha~t. The generated ~ignal is fed to a microprocessor
which proces~es the information and can produce on a
display the change in ~n~ular position relative to
initial an~ular position or the angular ran~e of motion
of the body segment or articulating joint.
The 4,688,581 Mos5 patent discloses an apparatus
and a method for non-invasive in vivo determination o~
muscle fiber composition. The method includes the steps
of electrically stimulating a chosen muscle;
determining the stimulation current; mea~uring the
electrical potenti~l of the muscle; the contraation
time; and the force produced by the contraction; and by
intercorrelating the data by multiple regression,
determining the type, percentage and size of muscle
CA 02234~37 1998-04-09
PCT/US9S/1350S
W O 97/13454
fibers within the muscle ~timulated Apparatus ~or
determining the muscle compo~ition include~ a muscle
stimul~tor of controlled voltage; electr~myogram
equipment; and a force transducer providing a tension
curve as well as force measurements.
The 4,667,513 Konno patent discloses an apparatus
and ~ method for e9timating the degree o~ the ~atigue
and p~in of muscles. The apparatus composes subjects
of different weights on the same basi~ by deriving the
variation in the muscular strength such as the dor~al
muscular strength, shoulder muscular strength, the
grasping power, and the like. An analogous electric
signal integrated the muscular output on one hand, and
provides an integrated value of the electromyogrammatic
~mplitude by processin~ the voltage induced ~rom the
muscle to be tested through an electromyogrAm amplitude
~nd a waveform processor. The ratio between these
integrAted values, after correctin~ the ratio with ~
weight/muscular strength coefficient is digitally
displ~yed.
For baakground purposes and a8 indicative o~ the
art to which the invention relates, reference may be
made to the following remainin~ patents ~ound in the
search: -
PATENT NO. INVENTO~ ISSUED
5,056,530 Butler et al 15 October 1991
5,050,618 Larsen 24 September 1991
5,038,795 Roush, et al 13 August 1991
5,012,820 Meyer 7 May 1991
4,886,0?3 Dillon et al 12 December 1989
4,845,987 Kenneth 11 July 1989
4,834,057 McLeod, Jr. 30 May 1989
4,805,636 Barry et al 21 ~ebruary 1989
6,742,832 Kauffmann et al 10 May 1988
CA 02234537 l998-04-09
W O 97/13454 PCT~US95/13505
DISCLOSURE OF THE INVENTION
The integrated movement analyzing system aomblnes
eleatromyographY with ran~e oi motion and iunctional
aapacity measurements to provide doctors and other
clinical practitioner8 with a method for accurately
analYzing myo~aci~l injuries. The ~ystem in its basic
form i 8 comprised of an integrated movement analyzer
(IMA~ that functions in combination with a surface
eleatromyography ~SFMG) cable having a set of non-
invasive S~MG electrodes that attach to a patient, arange-of-motion arm ~ROMA), and a ~unctional capaoity
sensor (FCS). The IMA i8 connected to a computer that
produces data representative of the patient's problems
being analyzed.
The IMA is a portable, non-loading electronic
instrument th~t incorporate~ a surface electromyo~raphy
eection th~t receives and proce~ses the ~ignal~
produced by the set of SEMG electrode~; a r~nKe o~
motion section th~t processes the signals from the
ROMA; and ~ functional capacity section that processes
the signals from the FCS. The aignals from all the
section~ ~re routed to ~n analo~-to-digital converter
~ADC) that further processes the signals before they
are applied to the computer. The IMA has the
capability to sample up to 32 channels of the SEMG
cable, six ch~nnel~ o~ the ROMA signals and one channel
of the FCS. All the signals are simultaneously
measured at sampling speeds of up to 10 KHz for testing
time frames.
The ROMA is a non-load bearing electro-mech~nical
deviae that includes three articulated sections. When
performing back protocol testing~ the ROMA is attached
~rom the patient's ~houlder to the patient's lower back
by use of a shoulder harness and waist belt. When
CA 02234~37 1998-04-09
WO 97/13454 PCT/US95/13505
performin~ cervical testing, the ROMA is attached from
the p~tient's head to the upper b-~ak by use o~ a
cervical cap and the shoulder h~!Lrness. The FCS
produces a signal that i5 representative of a pulling
force exerted by the p~stient. The FCS ia aomprised of
a strain gauge mounted on a plate on which the patient
standa. Attached to the stain gauge is a pull cable
having attached to its upper end a h.lndle griP. When
the grip is pulled by the patient, the stain g~luge
measures the patient's pulling force which is analogous
to the patient's lifting power. The IMA also includes a
lead failure detection section having a circuit that
causes ~ specific LED to illuminate when a
corresponding specific lead failure has occurred from a
SEMG electrode.
The simultaneous monitoring of the muscle groups
allowed by the system measures muscle tone, muscle
spasms, muscle activity and response, as well as muscle
recovery and fatigue. Thia is clccomplished f'or each
muscle group monitored while several muscles are being
monitored ~Lt the ~clme time ~bove and below the are~ of
complaint. This allows the analyst with the system, to
outline a specific therapy program for the problem and
traces the referred pain problem. With the site
specific treatment protocol, physical therapy i8
reduced to 50-60 percent less sessions, decre,lses
costs, treatment time clnd directs the specific type of
treatment like electrical stimulation, ultra sound
massage or nerve block to a specific location. Thus,
medical costs related to treatment and use of
medication are greatly reduced.
In view of the above disclosure, it is the primary
object of the invention to provide doctors and other
diagnostic personnel with a system that simultaneous
utilizes ~3urface electromyogr~phy in combination with
range of motion and functional capacity testing to
CA 02234~37 1998-04-09
W O 97/134S4 PCT~US95/13505
monitor any mu~cle ~roups in the human body.
In addition to the primary objeat, is is al80 an
object of the invention to provide a system that:
o mea~ures compliance without the patient'~
cooper~tion. ~ec~u~e the r~n~e o~ motion, FCS
are aombined with ~peci~ic EMG readings, the
system can tell i~ the patient could not
complete the range of motion or the lifting
ta~k. This is very important to the insuranae
industry to reduce and defer fraudulent worker's
compensation and personal injury claims and
reduce long term disability.
o includes a speci~ic protocol ~or aarpal tunnel
syndrome (CTS) that monitors the testin~ and
r~nge o~ motion readings for all cervical and
upper extremity muscle groups. This interactive
protocol with the system allows doctors to look
at the rel~tionship between muscle groups and to
diagnose if the problem i~ cervic~l, CTS or
cubital tunnel. The system al~o allows doctors
to determine if it i8 a repetitive stres~
injury.
o is bene~icial to ~ports in that it can tell an
~thlete what muscle groups to work out with what
procedure and for how long before the muscle
fatigues; thus, it maximizes the work-out period
without causing injury.
o is beneficial for pre-employment screenin~ to
have a "finger print" of muYcle activity if
there is a subsequent injury and with ADA law8
to determine how the work site needs to be
altered to comply with the law .
o aan diagno~e soft tissue injury.
o can tell i~ di~c pathology i~ present ~nd if it
is clinically significant,
o can provide site-specific treatment protocols,
CA 02234537 1998-04-09
W O 97/13454 PCT~US95/13505
o can eliminate the need for most aarpnl tunnel
and cubital tunnel surgeries.
~hese and other objects and advantages o~ the
present invention will become apparent ~rom the
subsequent detailed description of the preferred
embodiment and the appended claims taken in conjunction
with the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
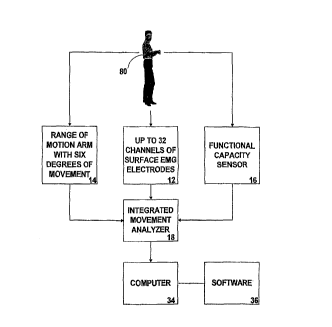
FIGURE 1 is a block dia~ram of the overall of the
integrated movement analyzing system.
FIGURE 2 i8 a block diagram showing the interface
between the integrated movement analyzer~ the computer
and a patient having the range of motion arm attached
to a patient by means of a cervic~l cap and shoulder
harness,
FIGURE 3 is a perspective view of the range of
motion arm.
FIGURE 4 is a perspective view of a patient that
has attached a range of motion arm between a cervical
cap and ~ shoulder harness, and a shoulder harness and
a waist belt.
FIGURE 5 is a perspective view o~ the shoulder
harness.
FIGURE 6 is a pergpective view of the waist belt.
FIGURE 7 i8 ~ perspective view of a cervical CAp.
FIGURE 8 is a perspective view of a typical
functional capacity sensor.
FIGURE 9 is an overall block diagram of the
integrated movement analyzer.
FIGURE 10 i9 a block diagram of the surface
electromyography section.
CA 02234537 l99X-04-09
W O 97/13454 PCT/US95/13505
/~
FIGURE 11 is a bloak diagram of the lead failure
detection section.
FIGURE lZ i8 a block diagram of the range of motion
section.
FIGURE 13 is a block diagram of the ~unctional
capaci ty ~ensing YeCt ion.
FIGURE 14 i~ a block diagram of the IMA power
5Upp ly .
FIGURE 15 i9 a schematic diagram of the
instrumentation amplifier aircuit and the lead-~ail
input circuit.
FIGURE 16A-16K are computer flow diagrams Or the
patient's data collection ~oftware program.
FIGURE 17A and FIGURE 17B are computer flow
di~rams of the patient'~ data plotting software
program.
CA 02234~37 l998-04-09
W O 97/13454 PCTrUS95/13S05
THE BEST MODE FOR CARRYING OUT THE INVENTION
The best mode ~or ~arrying out the invention i8
presented in terms of a preferred embodiment that
utilizes surface electromyography in combination with
range of motion and functional cApacity testing to
monitor any muscle ~roup in the human body.
The preferred embodiment of the integrated movement
analyzing system 10 as shown in FIGURES 1-14, i8
comprised of the following 5even major elements: a
surface electromyography (SEMG) cable assembly lZ~ a
range of motion arm (ROMA) 14, that operates in
combination with a shoulder harnesa 40, a waist belt
42, and a cervical cap 44; a functional capacity sensor
(FCS) 16, an integrated movement analyzer 18, and a
computer 34 that operates with software 36.
The overall integrated movement analyzing system 10
i9 shown in FIGURES 1 and 2. As shown in the ~igures,
the integrated movement analyzer (IMA) 18 is the focal
point of the system 10 and receives inputs ~rom the
surfaae electromyography (SEMG) cable assembly 12, the
range of motion arm (ROMA) 14 and the runctional
capacity aensor (FCS) 16; all of which are connected to
a human patient 80. The output of the IMA 18 is
provided to the computer 36 which produces comparative
analytical data which is primarily in the form of
graphic plots.
The surface electromyograph (SEMG) cable a~sembly
12 a~ shown in FIGURE 2) consists of a plurality of
paired SEMG leads having a first end 12A and a second
end 12B. The first end terminate~ at a multipin cable
connector that preferably consists of a male twist-lock
connector 12C that is sized to be attached to a mating
female connector 18A located at the IMA 18. The two
leads of the second end 12B e~ch terminate with a
CA 02234~37 1998-04-09
W O 97/13454 PCTrUS95/13505
finger-operated spring electrode attachment clip lZD.
In FIGURE 2, only three paired SEMG lead~ are shown ~or
illustrative purposes; in the aatual aable de~i~n, the
paired leads aan number ~rom 8 to 32.
The SEMG eleatrode pads 13, whi¢h pre~erab~y
consist~ of stand~rd silver-silver chloride electrodes,
include two clip attachment protrusion~ that interface
with two skin contact points. To the protrusions are
relea~ably attached the electrode attachment clips 12D
0 ~8 shown in FIGURE 2. The two skin aontact points are
adapted to be attached to selected areas of a human
patient 80 a~ al~o shown in FIGURE 2. The electrodes
produae a differential analog signal that i6
representative of the resistance between the two skin
contact points of the patient.
The cable assembly 12 is manufactured from light
weight materials to prevent or at least minimize the
dislodgment o~ the clips 12D attached to the SEMG
electrode pads 13 and is manufactured in selectable
lengths that range from 4 to 40 feet. The cable wiring
consist of individual, shielded coax wires that are
twisted in pairs for each channel. To eliminate ground
loop~, each wire shield terminate~ at an
instrumentation amplifier circuit 20A which is the
input circuit of the inte~r~ted movement analyzer 18
which is described infra. The cable assembly al80
includes a single, non-coax wire 12C that is used a8 a
signal ground for setting the ground reference ~rom the
patient 80 to the integrated movement analyzer 18.
The range of motion arm (ROMA) 14 as shown in
FIGURE 3, includes electrical circuit means and
mechanical means for producing range of motion analog
si~nals representative of the angular distance produced
from selected area5 o~ t~e patient 80. The mechanical
means is encompassed in a non-load bearing device that
includes ~n upper knuckle 16A having an attachment pin
CA 02234537 l998-04-09
WO 97/13454 PCT/USgSl1350S
14E, a middle junction 14B and a lower knuckle 14C al80
having an attachment pin 14E. Power to the ROMA 14 ia
~upplied through cable 14D.
The upper knuckle is de3igned to rotate in three
directions to measure up and down, side to side, and
rotarY movement3 of the patient' 8 shoulders ~or back
mea~urements or the top o~ the patient' 8 head l~or
aervical movements in the X, Y and Z planes. The
middle junction rotates in an angular motion to measure
the angular distance in the X-plane, and the lower
knuckle rotates in two directions to mea~ure the
angular distanae in the Y-plane as well as the rotation
in the Z-plane.
The ROMA 14, when performing upper back protocol
testing, is attached as shown in FIGURE 4, ~rom the
patient's upper back to the patient's lower back, by
means of a shoulder harness 40 as shown in FIGURE 5.
When performing lower back protocol testing, the ROMA
is attached between the shoulder harness 40 and a
waist belt 4Z as shown in FIGURES 4 and 6. For cervical
testing, the ROMA 14 a8 also shown in FIGURE 4, ie
attached from the top of the patient' 8 he~d to the
patient'a upper back, by means of a cervical cap 44 a~
shown in FIGURE 7 and the shoulder harness 40 a8 shown
in FIGURE 6.
The ROMA 14 is ~hown attached in two places in
FIGURE 4. However, in actual teating, the ROMA 14 is
attached to either the cervical cap 44 and the shoulder
harnes~3 40, or from the shoulder harness 40 to the
waist belt 42.
The shoulder harness 40 as shown in FIGURE: 5,is
typically comprised of a right shoulder support 40A, a
le~t shoulder support 40B, a horizontal baclc strap 40E
and a ROMA attachment structure 40G.
The right and left shoulder 9uPports 40A,40B each
have an upper section 40C and a lower section 40D. The
CA 02234537 1998-04-09
W O 97/13454 PCT~US95/13505
lower sections are looped under the upper arms and are
adju~tably attached to the upper section by an
attachment means 40F that allows the harness 40 to be
adjusted to fit the anatomy o~ the patient 80
undergoing the te8tin~ a8 shown in FIGURE 4. The
preferred attachment means con~ists o~ a complimentary
hook and loop ~stener 40F a5 shown in FIGUR~ 5. The
horizontal back strap 40E i9 integrally attached to the
inward edges of the back of the ri~ht and le~t shoulder
supports 40A,40B across the upper back Or the patient
protruding outward from the center o~ the back strap
40E is the ROMA attachment structure 40G. This
structure includes a pin cavity 40H that is sized to
accept an att~chment pin 14E located on the ROMA 14.
The waist belt 42 a8 shown in FIGURE ~, consists o~
an attachment means 42A that allows the belt to be
adjustablY adjusted acro~s the waist o~ the patient 80
undergoing testing as shown in FIGUR~ 4. The pre~erred
belt attachment me~ns compri~es a hook and loop
~astener 42A as shown in FIGURE 6. Protruding outward
~rom the back of the belt 42 is a ROMA attachment
structure 40B. This structure includes a pin cavity
40C that is sized to accept an attachment pin 14E
located on the ROMA 14.
The cervical cap 44 as shown in FIGUR~ 7 consists
basically of a head band 44A having a means ~or being
adjusted to ~it the head of the patient undergoing
testing as shown in FIGURE 4. Across the head band 44A
is ~ttached a head support 44C that includes a means
~or bein~ adjustably attached to the patient's head.
The pre~erred adjustment me~ns i~ a complimentary hook
and loop fastener 44B. On the center top of the head
b~nd 44C is a ROMA ~ttachment structure 44D a8 ~hown in
FIGURE 7. Thi~t structure al80 includes a pin cavity
44E that i~ sized to accept ~n attachment pin l~F
located on the ROMA 14. As also shown in FIGURE 7, the
CA 02234~37 1998-04-09
WO 97/13454 PCT/US95/13505
aervical cap 44 maY also include an adjust~lble skull
mount 64F that h~s a movable ahin ~upport 44G and
resilient head cu9hions 44G located on the inside o~
the head band 44A.
The ROMA's 14 electrical circuit means i~ compriaed
o~ a set of potentiometers. The upper knuckle has
three potentiometers, the middle junction has one
potentiometer, and the lower knuckles has two
potentiometers. The potentiometers provide 8iX
channels of range of motion analog signals. The range
of motion analog ~ nAls are in the form of voltaE~e
levels ranging from O to 5-volts d-c; where O-volts is
representative of O-degrees of angular di~placement and
5-volts d-c is representative of 270-degrees of angular
displacement. The analog signal~ are applied to the
lMA 18 through connector 14E of the cable 14D which
attache~3 to IMA connector 18B as described in~ra.
The functional capacity sensor (FCS) as shown in
FIGURES 2 and 8, includes electrical circuit mean~ and
mechanic~l means for producing a differential analog
d-c ~3i~nal representative of a pulling force exerted by
the patient.
The mechanical means for a preferred embodiment a~3
shown in FIGURE 8~ is comprised of a strain gauge 50
mounted on a flat metal plate 52 on which the patient
stands. Attached to the metal is a pull cable 54
having a ~irst end 54A that is attached to the metal
plate 52 and a second end that 54B has attached a
two-handed ~rip 54C. When the grip is pulled by the
patient 80, the strain gauge 50 measures the pulling
force o~ the patient which is analogous to the lifting
power o~ the patient.
The li~ting ~orce is measured by a range of d-c
voltage levels that are repre3entative of the ~orce
exerted upon the FCS l~ by the patient 80. The d-c
voltage range from O to 5-volts~ where O-volts is
CA 02234537 l998-04-09
W O 97/13454 PCT~US95/13505
representative of zero lbs and 5-~olts d-o i8
representative of the specific calibration of the
sensor bridge, The resulting differential analog d-c
signals are applied through connector 52 Or c~ble 56 to
IMA connector 18C to as described infra.
The integrated movement analyzer (IMA) 18 as shown
in FIGURES 2 c~nd 9~ is a self contained unit, th~t i8
comprised of a sur~aae electromyography section 20
having circuit mean8 for receiving and proces~ing the
di~ferential analog signals ~rom the SEMG cable
assemblY 12; a lead failure detection section 2Z having
circuit means for receiving and processing the
differential analoe signals from the SEMG cable
~sembly 12; a range o~ motion section Z4 having
circuit means for receiving and processing the analog
signal from the ran~e of motion arm 14; a functional
capacity sensing section 26 having circuit meana ~or
receiving ~nd processin~ the an~lo~ si~nals Prom the
functional capacity sensor (FCS) 16 and an isolated
power supply section 28 having circuit means ~or
supplying the power required to operate the IMA
circuits. The circuit me~ns of the IMA 18 ~llows the
sampling of uP to 3Z channels of the SEMG analog
signals; ~ix channels of the motion arm analog signals
and one ahannel of the functional capacity sensor
analog aignal, where ~ll the ~ignal~ ~re ~imult~neou~ly
measured at sampling speeds of up to 10 KHz at any
testing time frame.
The surface electromyography (SEMG) section 20
circuit me~ns for receiving and processing the
differential An~log ~ignals from the set of SEMG
electrodes lZB, as shown in FIGURE 10, comprises: an
instrumentation amplifier circuit ZOA havin~ means for
detecting the resistance between the contact points o~
each SEMG electrodes 1ZA, which corresponds to the
patient's skin re9i9tance) and converting this
CA 02234~37 l998-04-09
W O 97/13454 PCTrUS95/13505
resistanoe to a representative analog voltage. The
analog ~oltage i8 then applied to a voltage-to-aurrent
circuit 20B having circuit means for converting the
analog voltage to a linear aurrent drive signal.
Following the circuit ZOB as shown in FIGURE 10 i8 an
optical isolation circuit 20C that isolates the patien~
80 from the system 10. The circuit 20C aonsists of an
optiaally isolated ampli~ier having airauit mQans for
converting the linear aurrent drive signal to a voltage
representative o~ the di~erential analog signal ~rom
the SEMG electrode~ 12A. The final circuit comprising
the ~EMG seation 20 i8 a filtering airauit 20D that i8
comprised o~:
(1) a 10 Hz high-pas~ filter that eliminates any
d-c component of the output signal from the
optical isolation circuit 20C,
(2) a notch ~ilter that eliminates 60 Hz are
appliaable harmonics noise inherently
generated in the air, and
(3) a low-pass ~ilter that eliminates ~requenaies
above 2.5 KHz. The output signal o~ the
~iltering airauit 20D, represents the
resistance detected at the SEMG electrode 12B
aonnected to the patient 80.
The instrumentation amplifier circuit means 20A as
described ~bove~ is ~urther comprised a8 shown in
FIGURE 15 o~ a ~EMG input circuit ZOA1, a low pa88
~ilter ZOA2 and a voltage ampli~ying circuit 20A3.
CA 02234~37 1998-04-09
W O 97/13454 PCT/US95/13505
1~'
The SEMG input cirauit 20~1 is aomPrised o~ an
instrument~tion ~mpli~ier U1 h~ving a positive and
ne~ative input and an output. The input i8 connected
across a pair of current limiting resistors R1 and R2
respectively with each resistor having an input ~ide
and an output side. To the resistor's input side i8
applied respectively, the positive and negative input
signals from the SEMG electrodes. The resistor's
output side i9 connected ~cross a capacitor Cl and u
network of ~our diode8 CR1-CR4. The capacitor provides
stability between the two inputs of the instrumentation
ampli~ier U1 by filtering high common mode noise and
the four diodes prevent static charge or over voltage
from dama~ing the instrumentation amplifier. Any
di~ference in voltage potential between the positive
and negative inputs o~ the instrumentation ampli~ier U1
i8 equal to the difference in potential between the two
leads o~ the SEMG electrodes and i5 al80 the output o~
the instrùmentation ampli~ier.
The low pass ~ilter 20A2 is comprised o~ a coupling
capacitor C2 having an input side and an output side.
The input side i8 applied to the output o~ the
instrumentation amplifier U1 and the output ~ide is
connected to the input of a 10 KHz low pass ~ilter that
~ilters all ~requencie~ above 10 KHz. The filter
consist of a series resistor R4 that is connected
~cro~s a re~istor R5 and a capacitor C3 that i~
connected to circuit ground.
The volta~e amplifying circuit 20A3 is comprised of
a voltage amplifier U2 having a positive and negative
input ~nd an output. Connected to the positive input of
the amplifier U2 is the output from the low pass ~ilter
20AZ. The voltage amplifier U2 has a gain of at least
30. The gain is produced by a pair of voltage-dividing
feedback resistor~ R3 and R6 that have their junction
connected to the negative input of the voltage
CA 02234~37 l998-04-09
W O 97/13454 PCT~US95/13505
~4
amplifier U2.
The differential analog signals from the set of
SEMG eleatrode 12B are algo applied to a lead failure
detection section 22 as shown in FIGURE 11. The
section Z2 compri~e8 a lead-~ail detection circuit Z2A
having means for detecting when the input from the SEMG
electrodes lZB cross over a threshold differential
voltage level of 2.5 volts d-c. This voltage level
indicates that at least one of two electrode leads lZB
has failed. When such a failure ocaurs, the lead-fail
detection circuit 22A produces an output digital signal
that is applied to a lead-fail optically coupled
circuit 22B. This circuit is comprised of an optical
coupler that converts the digital input signal from the
lead-fail detection circuit 22A to an isolated optical
signal optic then back to a digital signal. The
digital signal i9 appl ied to a ~et of lead-fail
indicators 22C that consi~t of light emitting diodes
(LED's) that are located on the front panel of the
integrated movement analyzer ~8 shown in FIGURE 2. The
signal that drive8 the LED's i8 also ~ensed by An
analog to digital converter and is monitored by the
computer ~oftware program to allow the particular L~D's
corresponding to the failed lead, to illuminate uo thAt
connection action c~n be t~ken to fix the problem.
The lead-fail detection circuit 2Z a8 described
above, is further comprised, a~ also shown in FIGURE
15, of a lead-fail input circuit Z2A1 and a comparator
circuit Z2A2.
The lead-fail input circuit 22A1 is comprised of a
pair of voltage amplifiers U3 and U4 each having a
positive and negative input and an output. To the
positive inputs i8 ~pplied the positive and negative
input si~nals respectively from the SEMG eleatrodes.
The amplifiers U3 and U4 are configured a8 voltage
~ollowers to assure that the lead-fail detection
CA 02234537 l998-04-09
W O 97/13454 PCT~US95/13505
circuit 22A doe~ not interfere with the sur~aae
electromyography section ZO.
The comparator circuit 2ZA2 i9 compriaed o~ a Pair
o~ amplifier~ U5 and U6 eaoh having ~ po~itive and
negative input and an output. To the po~itive inputs
are applied the outputs from the voltage ampli~iers U3
~nd U4 re~pectively through input re~i~tora R9 ~nd R10
respeatively. Both the amplifiers operate with a
poaitive feedb~ck path that is applied through
resistors R11 and R12 respectively. The comparator
circuit further include~ a bias circuit. Thi~ circuit
sets a bias level at the negative inputs o~ the
amplifiers U5 and U6, by means of a pair of resistor~
R13 and R14 and capacitor C4, where resistor R13 is
connected to a positive voltage and capacitor C4 and
re~i~tor R14 are connected to circuit ground. The bias
circuit ~ssure~ that i~ the positive inputs o~ the
ampli~iers U5 ~nd U6 drop below a ~peci~ied thre~hold
level~ the output of either ampli~ier will ch~n~e to a
zero output. This zero output is applied to an OR
lo~ic circuit con~istin~ o~ diodes CR5 and CR6 whi~h
al~o drops to zero to produce the digital si~nal that
i~ ~pplied to the lead-fail optically coupled circuit
22B as ~hown in FIGURE 11.
The range of motion ~ection 24 circuit me~n~ as
shown in FIGURE 12, for receiving ~nd processing the
range o~ motion an~10~ ~ign~ls produced by the range o~
motion arm 14 compri~es a voltage ~ollower bu~ering
and low-pas~ filterin~ circuit 24A having means ~or:
~1) providing a d-c excitation voltage and an
isolated ground th~t is applied acro~s each o~
the potentiometers in the range o~ motion arm
(ROMA),
(2~ retaining the integrity of the potentiometer
wiper voltage by eliminating any ~-c component
above 50 Hz.
CA 02234~37 l99X-04-09
W O 97/13454 PCT~US9S/13505
From the cirauit 24A i9 produced a proaessed analog
signal that is applied to a voltage-to-current cirauit
that converts the analog voltages repre~entative o~
angular di~tanae to a linear current drive signal. In
turn, the drive signal i8 then applied to an i~olation
aircuit aonsisting o~ an analog optically i~olated
ampli~ier. The amplifier converts the signal to an
analog voltage represent~tive of the angular
di~placement of the ROMA potentiometers.
The funational aapacity sen~in~ section 26 aircuit
means as shown in FIGURE 13 for receiving and
proce~sing the di~ferential analog signals supplied by
the ~unational a~p~aity 9ensor (FCS~ 16 is aomprised of
an instrumentation ampli~ier airauit and sensor bridge
driver voltage 26A having means ~or:
(1) providing a d-a exaitation voltage and an
isolated ground ~or a sensor bridge
exaitation,
(2) reaeiving a di~ferential signal from thé
sensor bridge, whereby the di~erenae in
resistanae i~ sensed to provide a
representative d-c voltage signal.
Following the sensor bridge is a voltage to current
airauit Z6B whiah is applied and aonverts the
representative d-a voltage signal to a linear aurrent
drive signal. The drive 8 ignal i3 then ~pplied to an
optic~l i801ation airauit 26C that isolate~ the patient
80 ~rom the system 10. The airauit Z6C aonsists of an
optically isolated amplifier having cirauit means for
aonverting the drive si~nal signal to a d-a voltage
repre~entative o~ the forae exerted upon the FCS. The
airauit Z6C aan be aalibrated for variable outputs in a
typical calibration, the d-a voltage ranges from O to
5-volts, where O-volts i~ representative o~ zero lbs.
and 5-volts d-c i9 repre9entative of the specifia
calibration of the sensor bridge.
CA 02234~37 l998-04-09
W O 97/13454 PCT~US95/1350S
The final electronics circuit described is the
power supply circuit 28 shown in FIC~URE 14. The input
to the power supply i8 derived i~rom the utility
lZ0-volts a-c power which i8 applied to a bridge
rectifier and d-c ~ilter circuit where the a-c utility
power i8 rectified and filtered to produae a d-c
voltage output. The ayatem can Cl18O be designed to
operate with an internal battery that is ~elected to
produce the required d-c voltage level to operate the
8ytem lO.
The d-c voltaae i~ applied ~ set o~ d-c power
regulation circuits 28B that produce: ~5 volts d-c, +12
volt~ d-c, -12 volts d-c and -5 volts d-c. These
vo1tages are applied:
15~l) directly to the sYstem lO circuits that are
not optiaally isolated, and
(2) to a set of three isolated d-c to d-c
converting circuits 28C that convert the
non-isolated d-c volt~ges to isolated d-a
20voltages, and
(3) to an isolated i5 volts d-c and ~12 volts d-c
volta~e regulator circuit 28D which ~urther
regulate and produce the d-c regulated
voltages required for the optically isolated
25circuits.
From the respective SEMG, ROMA and FCS sections a8
shown in FIGURE 9, the respective output signals ~re
applied to an analog-to-digital converter ~ADC) ~or
~urther processing. The ADC in the preferred embodiment
30i9 a 16 bit, 16 channel device that also includes 8
lines of digital I/0. However, multiple assemblies can
be connected to provide up to 3Z channels.
The processed signal~ from the ADC 30 are
terminated at an output connector such as an IEEE 488
35interface. From the interface connector, the signals
Are routed through a cable assembly 3Z and applied to a
CA 02234~37 l998-04-09
W O 97/13454 PCT~US95/13~0~
oomputer 32 shown in FIGURE 2. The aomputer 34
operates with a software program 36 as shown in the
computer flow diagram included as FI~URES 16A-16 to
produce the comparative analytical data representative
of the patient'8 problem being analyzed. The aoftware
program 36 whiah is proteated under registered and
pending copyright regi8trations consists of a p~tient' B
data collection progr~m ~nd ~ patient '8 data plotting
program.
The patient's data collection program which i8
shown in the computer flow diagram~ of FIGURES 16A-16K
allows the seleatiOn of the following options:
a) cervical/carPal tunnel syndrome (CTS) protocol
testing,
b) extremities protocol testing,
c) mid and lower back protocol testing,
d) technical information covering lead setup and
muscle groups,
e) lead-fail integrity chec~, and
f) return to main screen option seleation.
The data collection software program:
a) interfaces with a parallel interface connector,
b) selects the voltage level that each channel will
respond to,
c) initializes the samplin~ frequency rate of the
AD~,
d) ~elects the appropriate testing protocol,
e) samples each cable lead during the test to
detect if a lead failure has occurred,
f) prompts the system 10 user as to the location of
a lead failure,
g) starts the integrated movement analyzer when the
testing should begin,
h) prompts the technician as to the muscle groups
that the individual leads should be connected to
for a given protocol,
CA 02234537 l998-04-09
WO 97/13454 PCT/US95/13505
o2 S~
i) prompts the technician as to the activitie~3 that
the patient should be per~orming durin~ the tes~t
cycle,
j) saves the data on h~rd dri~e at the completion
o:e a teat,
k) converts the patient '9 data from binary data to
computer graphic~ nd
l) time and date stamps each file a8 dat~ is taken,
The patient' 5 data plotting program which i~ ~hown
in the computer flow di~gr~m8 o~ FIGURES 17A and 17B
c~llows the plottin~ of up to ~orty cho.nnelE~ of the
patient's data for use on a final report.
The d~ta plotting softw~re program:
a) generates computer plots from 1 to 40 channels
of data,
b) plots range of motion data and correlates this
d~Lt~ to angul~r displaaement,
c) plots functional capaaity data and correlaten
this data to maximum force applied to the
function~l aapacity ~ensor by the patient,
d) ~ets the testing time for the test being
performed, and
e) produces plots which include patient
information, loaation of test, the test
performed ~nd the muscle groups.
OPERATIONAL PROCE;DURE
~ The integrated movement analyzing system is
operated by application of the following steps:
~) connect the IMA 18 to a source of electrical
power,
b) connect the computer 34 to the IMA 18,
c) connect the SEMG cable assembly 12 to the IMA
~nd test the integrity of the SEMG cable
assembly by means of the computer,
d) connect the ROMA 14 to the IMA,
CA 02234537 l998-04-09
WO 97/13454 PCT/US95/13505
e) prepare a patient by cleansing the area o~ the
patient's body encompassing a muscle group
pertaining to the test protocol that i8 to be
analyzed,
f) att~ch to ec~ch designated lead o~ the SEMG cclble
assembly 14, a SEMG electrode pad 13,
6~ mount s3aid ROMA to patient,
h) attach the electrode pad~ around the area o~ the
~elected mu~cle groups, ~8 follow~,
i) for te~3ting of repetitive stress injuries (RSI)
protocol, attach the SEMG electrode pad~ to the
following mu~cle group~3 bilaterally:
~1) external sternocleidomastoid (SCM),
(2) scalene,
(3) paraspinal cervical,
(4) upper tr~pezii,
(5) deltoid,
(6) bicep,
(7) tricep and
(8) wrist,
J) for testing the cervical region, attach the SEMG
eleatrode pads to the ~ollowing muscle groups
bilaterally:
(1) external sternocleidomastoid (SCM),
(Z) Ycalene,
(3) paracervical, and
(4) upper trapezii,
k) for testing the middle back region, attach the
SEMG electrode pads to the following muscle
groups bilaterally:
(1) middle tr~lpezii,
(Z) lower trapezii,
(3) par~aspinal Yet 1,and
(4) para~pinal Yet Z,
~5
CA 02234537 l998-04-09
W O 97/134S4 PCT/US9S/13505
~ 6
1) 170r testing the lower baak region, attach the
SEMG eleatrode pads to the following musale
groups bilaterally:
(l) paraspinal set 1,
(2) paraapinal aet 2,
(3) quadratus lumborum and
~4) gluteal,
m) ~70r testing the lower extremity region, attach
the ~MG electrode pads to the 170110wing muscle
groups:
(1~ anterior thigh,
(2) po~terior thigh,
(3) anterior a~lf, ~nd
(4) posterior cal~
n) in~truct the patient to perform a aeries Or
mo~ement~ while maintaining either a sitting or
Ftandin~ poaition aa followa:
(l) for the RSI test, instruct the patient to
perl70rm the ~ctions pertaining to the
cer~ical and extremity re~ions,
(2) for the cervical region teat, inutruct the
patient to per~orm the actions pertaining
to the cervical region7
(3) ~70r the extremities region, instruct the
patient to perf70rm the actions pertaining
to the lower extremity region, and
(4) 170r the middle and lower back region,
instruct the patient to per~orm the actions
pertaining to the middle and lower back
region.
When the operation procedure includea the use of a
functional capacity ~ensor (FCS) 16, the following
additional ~tepa are required.
a) ~ttach the FCS 16 to the IMA,
CA 02234537 l998-04-09
W O 97/13454 PCTrUS95/13505
~ 7
b) instruct patient to:
(1) stand on the flat metal plate 52,
(Z) pull on the pull cable 54 and
(3) allow the computer 34 to display and record
the pulling force exerted by the patient.
While the invention has been described in complete
detail and pictorially shown in the aacompanying
drawings it is not to be limited to such details, since
many changes and modification5 may be made to the
invention without departing from the spirit and the
scope thereo~. For example, in FIGURF 6, the
ampli~iers U1-U6 are shown for explanatory purposes as
individual di~creet components. In the actual
implementation of the circuit, ampli~iers U1-U6 are
packaged in a single integrated circuit. Hence, it i8
described to cover any and all modifications and forms
which may come within the language and scope of the
claims.