Note: Descriptions are shown in the official language in which they were submitted.
CA 02234611 1998-04-14 ..-
F b_ a . '~
'' ' FlLE, P+IW-ht-TH IS A~",~ ",-~~-
~'TT TRANSLATION
5016645J.64
1/1012 - Foreign
New phenylamidine derivatives, processes for
preparing them and their use as pharmaceutical
compositions
The invention relates to new phenylamidine derivatives,
processes for preparing them and their use as
pharmaceutical compositions. The phenylamidines
according to the invention correspond to the general
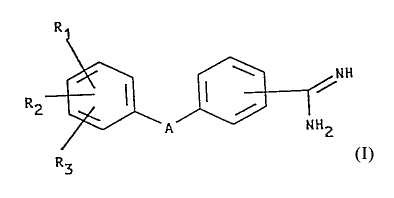
formula I
R
RZ ~ ~ ~ /NH
A NN2
R3
wherein
A denotes X1-CmH2m-X2-, in which m is an integer 2, 3,
4, 5 or 6
or
X4
X3
R7 8
and
X1 denotes O, NH or NCH3;
X2 denotes O, NH, NCH3 or /x~ /
CA 02234611 1998-04-14
' r . ~ ~ ,
- 2 -
X3 denotes X1-CnH2n in which n is the integer 1 or 2 ;
X4 denotes Cn-H2n-X1, wherein n is the integer 1 or 2 ;
Rl denotes CS_~-cycloalkyl, Arl, OArl, CH2-Ar2;
CR4RSAr3 , C ( CH3 ) 2R6
R2 denotes H, C1_6-alkyl, OH, halogen, O- (Cl_6) -alkyl;
R3 denotes H, Cl_6-alkyl;
R4 denotes Cl_4-alkyl, CF3, CH20H, COON, COO (C1_4) -alkyl;
RS denotes H, Cl_4-alkyl, CF3 and
R4 and RS may also together form a C4_6-alkylene group;
R6 denotes CH20H, COOH, COO (C1_4) -alkyl, CONR9Rlo,
CH2NR9Rlo ;
R~ denotes H, halogen, OH, C1_6-alkyl or C1_6-alkoxy;
Ra denotes H, halogen, OH, Cl_6-alkyl or C1_6-alkoxy;
R9 denotes H, Cl_6-alkyl, phenyl, phenyl- (Cl_6-alkyl) ,
CORM, COORll, CHO, CONH2, CONHRll, S02- (C1_6-alkyl) ,
S02-phenyl, wherein the phenyl ring may be mono- or
polysubstituted by halogen, CF3, C1_4-alkyl, OH,
Cl_4-alkoxy;
Rlo denotes H or Cl_6-alkyl and
R9 and Rlo together may represent a C4_6-alkylene group;
R11 denotes Cl_6-alkyl, C5_~-cycloalkyl, aryl,
heteroaryl, aralkyl or heteroaryl-(Cl_6-alkyl),
wherein the aryl or heteroaryl groups may be mono-
CA 02234611 2004-03-17
27400-185
- 3 -
or polysubstituted by Cl, F, CF3, Cl_4-alkyl, O~i or
C~_4-alkoxy; T .
Arl denotes an optionally mono- or polysubstituted aryl
group, with the exception of the unsubstituted
phenyl group and the phenyl group which is
monosubstituted by halogen, Cl_4-alkyl or
monosubstituted by C1_4-alkoxy;
Ar2 denotes an optionally mono- or polysubstituted aryl
group, with the exception of the unsubstituted
phenyl group;
Ar3 denotes an optionally mono- or polysubstituted aryl
group
with the proviso that
R1 cannot represent an unsubstituted phenyl group
bound via a C1_4-alkylene unit;
optionally in the form of the individual optical
isomers, mixtures of the individual enantiomers or
racemates and in the form of the free bases or the
corresponding acid addition salts with pharmacologically
acceptable acids.
According to one aspect of the present invention,
there is provided a compound of formula I
R~
NH
R2
R A / NH2
3
wherein
CA 02234611 2004-03-17
27400-185
- - 3a -
A is -Xl-CmH2m-Xz-, wherein m is 2, 3, 4, 5 or 6 or
/ Xaw
X3
R7 ~ ~a
wherein
X1 is O;
~X~
X2 is O or ;
X3 is -Xl-CyH2y, wherein y is 1 or 2;
X4 is -Cn-H2n-Xl-, wherein n is 1 or 2;
Rl is CS_7-cycloalkyl, CR4RSAr3, or C (CH3) ZRs:
R2 is H, C1_6-alkyl, OH, or O- (Cl_6) -alkyl;
R3 is H, or Cl_6-alkyl;
R4 is Cl_4-alkyl, or CF3;
RS is Cl_4-alkyl, or CF3; or
R4 and RS together form a C4_6-alkylene group;
R6 is CH20H, COOH, COO (Cl_4) -alkyl, CONR9RIO, or
CH2NR9Rlo ;
R7 is H;
R8 is H;
R9 is H, C1_6-alkyl, phenyl, phenyl- (C1_6-alkyl) ,
CORM, COORll, CHO, CONH2, CONHRll, S02- (C1_6-alkyl) , S02-
phenyl, wherein the phenyl ring is optionally mono- or
polysubstituted by one or more substituents wherein the
CA 02234611 2004-03-17
27400-185
- 3b -
substituents are selected from halogen, CF3, C1_4-alkyl, OH,
and C1_4-alkoxy;
Rlo is H or C1_6-alkyl; or
R9 and Rlo together form a C4_6-alkylene group;
Rll is C1_6-alkyl;
Ar3 is an optionally mono- or polysubstituted
phenyl group
optionally in the form of the single optical isomers, a
mixture of individual enantiomers or a racemate thereof, a
free base or a corresponding acid addition salt with a
pharmacologically acceptable acid.
According to another aspect of the present
invention, there is provided a compound which is represented
by the following formula
H3C CH3
O ~ O
HO / / / NH2
NH
optionally in the form a free base or an acid addition salt
with a pharmacologically acceptable acid.
Preferred compounds according to general formula I
are those wherein
A denotes X1-Cm-H2m-X2 in which m is the integer 2
Image
CA 02234611 1998-04-14
r , . ,
- 4 -
X1 is O;
X2 is /x~
i
X3 denotes -Xl-CnH2n- wherein n is the integer 1 or
2 ;
X4 denotes -CnH2n-Xl- wherein n is the integer 1 or
2 ;
Rl denotes CS_~-cycloalkyl, Arl, OArl, CH2-Ar2;
CR4RSAr3 C ( CH3 ) 2R6 ;
,
R2 denotes H, C1_6-alkyl, OH, Cl, O- (Cl_6) -alkyl;
R3 denotes H, C1_6-alkyl;
R4 denotes C1_4-alkyl , CF3 , CH20H;
R5 denotes H, C1_4-alkyl, CF3, CH20H and
R4 and RS ether may also form a C4_6-alkylene group;
tog
R6 denotes CH20H, COOH, COO (Cl_4) alkyl, CONR9Rlo
CHaNR9Rlo
;
R~ denotes H, F, C1, Br, OH, Cl_6-alkyl or C1_6-alkoxy;
R8 denotes H, F, Cl, Br, OH, C1_6-alkyl or C1_6-alkoxy;
R9 denotes H, C1_6-alkyl ;
Rlodenotes H or Cl_6-alkyl and
R9 and Rlo gether may also represent a C4_6-alkylene
to
group;
r . ~ . ,
CA 02234611 1998-04-14
- 5 -
Arl denotes an optionally mono- or polysubstituted aryl
group, with the exception of the unsubstituted phenyl
group and the phenyl group which is monosubstituted by
halogen, C1_4-alkyl and monosubstituted by C1_4-alkoxy;
Ar2 denotes an optionally mono- or polysubstituted
aryl group, with the exception of the unsubstituted
phenyl group;
Ar3 denotes an optionally mono- or polysubstituted aryl
group
with the proviso that
Rl cannot represent an unsubstituted phenyl group
bound via a Cl_4-alkylene unit;
optionally in the form of the individual optical
isomers, mixtures of the individual enantiomers or
racemates and in the form of the free bases or the
corresponding acid addition salts with pharmacologically
acceptable acids.
Particularly preferred compounds of general formula I
are those wherein
A denotes
X4
X3
R7 $
and
X1 is O;
X3 denotes Xl-CH2;
CA 02234611 1998-04-14
- 6 -
X4 denotes CH2-Xl;
Rl denotes C5_~-cycloalkyl, Arl, OArl, CH2-Ar2;
CR4RSAr3 , C ( CH3 ) 2R6 ;
RZ denotes H, OH, O- (Cl_6) -alkyl;
R3 denotes H;
R4 denotes CH3, CH20H;
RS denotes H, CH3, CH20H and
R4 and R5 together may also denote a C4_6-alkylene group;
R6 denotes CH20H, COOH, COO (Cl_4) -alkyl, CONR9Rlo,
CH2NR9Rl o ;
R~ denotes H;
R8 denotes H;
R9 denotes H, Cl_6-alkyl;
Rlo denotes H or Cl_6-alkyl and
R9 and Rlo together may also denote a C4_6-alkylene group;
Arl denotes an aryl group optionally mono- or
polysubstituted by hydroxy or by hydroxy and
C1_6-alkyl;
Ar2 denotes an aryl group optionally mono- or
polysubstituted by hydroxy or by hydroxy and
C1_6-alkyl;
Ar3 denotes an aryl group optionally mono- or
CA 02234611 1998-04-14
- 7 -
polysubstituted by hydroxy or by hydroxy and
C1_6-alkyl ~ . .
optionally in the form of the individual optical
isomers, mixtures of the individual enantiomers or
racemates and in the form of the free bases or the
corresponding acid addition salts with pharmacologically
acceptable acids.
Unless specifically stated otherwise, the general
definitions are used as follows:
C1_4-alkyl, Cl_6-alkyl and C1_8-alkyl, respectively,
generally denote a branched or unbranched hydrocarbon
group having 1 to 4 or 6 or 8 carbon atoms, which may
optionally be substituted by one or more halogen atoms,
preferably fluorine, which may be the same or different
from one another. The following hydrocarbon groups are
mentioned by way of example:
methyl, ethyl, propyl, 1-methylethyl(isopropyl), n-
butyl, 1-methylpropyl, 2-methylpropyl, 1,1-
dimethylethyl, pentyl, 1-methylbutyl, 2-methylbutyl, 3-
methylbutyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl,
2,2-dimethylpropyl, 1-ethylpropyl, hexyl, 1-
methylpentyl, 2-methylphenyl, 3-methylpentyl, 4-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-
dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl,
3,3-dimethylbutyl, 1-ethylbutyl, 2-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-1-
methylpropyl and 1-ethyl-2-methylpropyl. Unless
otherwise specified, lower alkyl groups having 1 to 4
carbon atoms such as methyl, ethyl, propyl, isopropyl,
n-butyl, 1-methylpropyl, 2-methylpropyl or 1,1-
dimethylethyl are preferred.
Aryl generally denotes an aromatic group having 6 to 10
CA 02234611 1998-04-14
_ g _
carbon atoms, also in compositions in which the aromatic
group may be substituted by oneror more lower alkyl
groups, trifluoromethyl groups, cyano groups, alkoxy
groups, nitro groups, amino groups and/or one or more
halogen atoms - which may be identical or different; the
preferred aryl group is an optionally substituted phenyl
group, the preferred substituents being halogen (such as
fluorine, chlorine or bromine) and hydroxyl.
Aralkyl generally denotes a C~_14-aryl group bound via an
alkylene chain, in which the aromatic group may be
substituted by one or more lower alkyl groups, alkoxy
groups, nitro groups, amino groups and/or one or more
halogen atoms, Whl.Ch may be identical or different.
Aralkyl groups having 1 to 6 carbon atoms in the
aliphatic part and 6 carbon atoms in the aromatic part
are preferred.
Unless otherwise stated, the preferred aralkyl groups
are benzyl, phenethyl and phenylpropyl or 2-phenyl-
isopropyl.
Alkoxy generally represents a straight-chained or
branched Cl_8-hydrocarbon group bound via an oxygen atom.
A lower alkoxy group having 1 to 3 carbon atoms is
preferred. The methoxy group is particularly preferred.
Unless otherwise stated, amino denotes an NH2 function
which may optionally be substituted by one or two
C1_s-alkyl, aryl or aralkyl groups, which may be
identical or different.
Alkylamino represents, by way of example, methylamino,
ethylamino, propylamino, 1-methylene-ethylamino,
butylamino, 1-methylpropylamino, 2-methylpropylamino or
1,1-dimethylethylamino.
CA 02234611 1998-04-14
- 9 -
Dialkylamino denotes, for example, dimethylamino,
diethylamino, dipropylamino, dibutylamino, di-(1-
methylethyl)amino, di-(1-methylpropyl)amino, di-2-
methylpropylamino, ethylmethylamino or
methylpropylamino.
Cycloalkyl generally denotes a saturated or unsaturated
cyclic hydrocarbon group having 5 to 9 carbon atoms
which may optionally be substituted by a halogen atom or
a number of halogen atoms, preferably fluorine, which
may be the same or different. Cyclic hydrocarbon groups
having 3 to 6 carbon atoms are preferred. Examples
include cyclopropyl, cyclobutyl, cyclopentyl,
cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl,
cycloheptenyl, cycloheptadienyl, cyclooctyl,
cyclooctenyl, cyclooctadienyl and cyclononinyl.
Heteroaryl, within the scope of the above definition,
generally represents a 5- to 6-membered ring which may
contain oxygen, sulphur and/or nitrogen as heteroatoms
and onto which another aromatic ring may be fused. S-
and 6-membered aromatic rings which contain an oxygen, a
sulphur and/or up to two nitrogen atoms and which are
optionally benzocondensed are preferred. _
Examples of particular heterocyclic systems include:
acridinyl, acridonyl, alkylpyridinyl, anthraquinonyl,
ascorbyl, azaazulenyl, azabenzanthracenyl,
azabenzanthrenyl, azachrysenyl, azacyclazinyl,
azaindolyl, azanaphthacenyl, azanaphthalenyl, azaprenyl,
azatriphenylenyl, azepinyl, azinoindolyl, azinopyrrolyl,
benzacridinyl, benzazapinyl, benzofuryl,
benzonaphthyridinyl, benzopyranonyl, benzopyranyl,
benzopyronyl, benzoquinolinyl, benzoquinolizinyl,
benzothiepinyl, benzothiophenyl, benzylisoquinolinyl,
bipyridinyl, butyrolactonyl, caprolactamyl, carbazolyl,
carbolinyl, catechinyl, chromenopyronyl,
CA 02234611 1998-04-14
- 10 -
chromonopyranyl, cumarinyl, cumaronyl,
decahydroquinolinyl, decahydroquinolonyl,
diazaanthracenyl, diazaphenanthrenyl, dibenzazapinyl,
dibenzofuranyl, dibenzothiphenyl, dichromylenyl,
dihydrofuranyl, dihydroisocumarinyl,
dihydroisoquinolinyl, dihydropyranyl, dihydropyridinyl,
dihydropyridonyl, dihydropyronyl, dihydrothiopyranyl,
diprylenyl, dioxanthylenyl, oenantholactamyl, flavanyl,
flavonyl, fluoranyl, fluoresceinyl, furandionyl,
furanochromanyl, furanonyl, furanoquinolinyl, furanyl,
furopyranyl, furopyronyl, heteroazulenyl,
hexahydropyrazinoisoquinolinyl, hydrofuranyl,
hydrofuranonyl, hydroindolyl, hydropyranyl,
hydropyridinyl, hydropyrrolyl, hydroquinolinyl,
hydrothiochromenyl, hydrothiophenyl, indolizidinyl,
indolizinyl, indolonyl, isatinyl, isatogenyl,
isobenzofurandionyl, isobenzfuranyl, isochromanyl,
isoflavonyl, isoindolinyl, isoindolobenzazapinyl,
isoindolyl, isoquinolinyl, isoquinuclidinyl, lactamyl,
lactonyl, maleimidyl, monoazabenzonaphthenyl,
naphthalenyl, naphthimidazopyridindionyl,
naphthindolizinedionyl, naphthodihydropyranyl,
naphthofuranyl, naphthyridinyl, oxepinyl, oxindolyl,
oxolenyl, perhydroazolopyridinyl, perhydroindolyl,
phenanthracquinonyl, phthalideisoquinolinyl,
phthalimidyl, phthalonyl, piperidinyl, piperidonyl,
prolinyl, parazinyl, pyranoazinyl, pyranoazolyl,
pyranopyrandionyl, pyranopyridinyl, pyranoquinolinyl,
pyranopyrazinyl, pyranyl, pyrazolopyridinyl,
pyridinethionyl, pyridinonaphthalenyl,
pyridinopyridinyl, pyridinyl, pyridocolinyl,
pyridoindolyl, pyridopyridinyl, pyridopyrimidinyl,
pyridopyrrolyl, pyridoquinolinyl, pyronyl, pyrrocolinyl,
pyrrolidinyl, pyrrolizidinyl, pyrrolizinyl,
pyrrolodioazinyl, pyrrolonyl, pyrrolopyrimidyl,
pyrroloquinolonyl, pyrrolyl, quinacridonyl, quinolinyl,
quinolizidinyl, quinolizinyl, quinolonyl, quinuclidinyl,
CA 02234611 1998-04-14
- 11 -
rhodaminyl, spirocumaranyl, succinimidyl, sulpholanyl,
sulpholenyl, -.tetrahydrofuranyl, tetrahydroisoquinolinyl,
tetrahydropyranyl, tetrahydropyridinyl,
tetrahydrothiapyranyl, tetrahydrothiophenyl,
tetrahydrothipyranonyl, tetrahydrothipyranyl, tetronyl,
thiaphenyl, thiachromanyl, thiadecalinyl,
thianaphthenyl, thiapyranyl, thiapyronyl,
thiazolopyridinyl, thienopyridinyl, thienopyrrolyl,
thienothiophenyl, thiepinyl, thiochromenyl,
thiocumarinyl, thiopyranyl, triazaanthracenyl,
triazinoindolyl, triazolopyridinyl, tropanyl, xanthenyl,
xanthonyl, xanthydrolyl, adeninyl, alloxanyl,
alloxazinyl, anthranilyl, azabenzanthrenyl,
azabenzonaphthenyl, azanaphthacenyl, azaphenoxazinyl,
azapurinyl, azinyl, azoloazinyl, azolyl, barbituric
acid, benzazinyl, benzimidazolethionyl,
benzimidazolonyl, benzisothiazolyl, benzisoxazolyl,
benzocinnolinyl, benzodiazocinyl, benzodioxolanyl,
benzodioxolyl, benzopyridazinyl, benzothiazepinyl,
benzothiazinyl, benzothiazolyl, benzoxazinyl,
benzoxazolinonyl, benzoxazolyl, cinnolinyl, depsidinyl,
diazaphenanthrenyl, diazepinyl, diazinyl,
dibenzoxazepinyl, dihydrobenzimidazolyl,
dihydrobenzothiazinyl, dihydrooxazolyl, _-
dihydropyridazinyl, dihydropyrimidinyl,
dihydrothiazinyl, dioxanyl, dioxenyl, dioxepinyl,
dioxinonyl, dioxolanyl, dioxolonyl, dioxopiperazinyl,
dipyrimidopyrazinyl, dithiolanyl, dithiolenyl,
dithiolyl, flavinyl, furopyrimidinyl, glycocyamidinyl,
guaninyl, hexahydropyrazinoisoquinolinyl,
hexahydropyridazinyl, hydantoinyl, hydroimidazolyl,
hydroparazinyl, hydropyrazolyl, hydropyridazinyl,
hydropyrimidinyl, imidazolinyl, imidazolyl,
imidazoquinazolinyl, imidazothiazolyl,
indazolebenzopyrazolyl, indoxazenyl, inosinyl,
isoalloxazinyl, isothiazolyl, isoxazolidinyl,
isoxazolinonyl, isoxazolinyl, isoxazolonyl, isoxazolyl,
.. E ,
CA 02234611 1998-04-14
- 12 -
lumazinyl, methylthyminyl, methyluracilyl, morpholinyl,
naphthimidazolyl, oroticyl, oxathianyl, oxathiolanyl,
oxazinonyl, oxazolidinonyl, oxazolidinyl, oxazolidonyl,
oxazolinonyl, oxazolinyl, oxazolonyl,
oxazolopyrimidinyl, oxazolyl, perhydrocinnolinyl,
perhydropyrroloazinyl, perhydropyrrolothiazinyl,
perhydrothiazinonyl, perimidinyl, phenazinyl,
phenothiazinyl, phenoxathiinyl, phenoxazinyl,
phenoxazonyl, phthalazinyl, piperazindionyl,
piperazinodionyl, polyquinoxalinyl, pteridinyl,
pterinyl, purinyl, pyrazinyl, pyrazolidinyl,
pyrazolidonyl, pyrazolinonyl, parazolinyl,
pyrazolobenzodiazepinyl, pyrazolonyl,
pyrazolopyrimidinyl, pyrazolotriazinyl, pyrazolyl,
pyridazinyl, pyridazonyl, pyridopyrazinyl,
pyridopyrimidinyl, pyrimidinethionyl, pyrimidinyl,
pyrimidionyl, pyrimidoazepinyl, pyrimidopteridinyl,
pyrrolobenzodiazepinyl, pyrrolodiazinyl,
pyrrolopyrimidinyl, quinazolidinyl, quinazolinonyl,
quinazolinyl, quinoxalinyl, sultamyl, sultinyl,
sultonyl, tetrahydrooxazolyl, tetrahydropyrazinyl,
tetrahydropyridazinyl, tetrahydroquinoxalinyl,
tetrahydrothiazolyl, thiazepinyl, thiazinyl,
thiazolidinonyl, thiazolidinyl, thiazolinonyl, _
thiazolinyl, thiazolobenzimidazolyl, thiazolyl,
thienopyrimidinyl, thiazolidinonyl, thyminyl,
triazolopyrimidinyl, uracilyl, xanthinyl, xylitolyl,
azabenzonaphththenyl, benzofuroxanyl, benzothiadiazinyl,
benzotriazepinonyl, benzotriazolyl, benzoxadiazinyl,
dioxadiazinyl, dithiadazolyl, dithiazolyl, furazanyl,
furoxanyl, hydrotriazolyl, hydroxytrizinyl, oxadiazinyl,
oxadiazolyl, oxathiazinonyl, oxatriazolyl, pentazinyl,
pentazolyl, petrazinyl, polyoxadiazolyl, sydonyl,
tetraoxanyl, tetrazepinyl, tetrazinyl, tetrazolyl,
thiadiazinyl, thiadiazolinyl, thiadiazolyl,
thiadioxazinyl, thiatriazinyl, thiatriazolyl,
thiatriazolyl, triazepinyl, triazinoindolyl, triazinyl,
CA 02234611 1998-04-14
- 13 -
triazolinedionyl, triazolinyl, triazolyl, trioxanyl,
triphenodioxazinyl, triphenodithiazinXl,
trithiadiazepinyl, trithianyl or trioxolanyl.
Particularly preferred heteroaryl groups include, for
example, thienyl, furyl, pyridyl, pyrimidyl, pyrazinyl,
pyridazinyl, quinolyl, isoquinolyl, quinazolyl,
quinoxalyl, thiazolyl, benzothiazolyl, isothiazolyl,
oxazolyl, benzoxazolyl, isoxazolyl, imidazolyl,
benzimidazolyl, pyrazolyl and indolyl.
The new compounds may be prepared using the following
conventional methods:
1. Reaction of imidoesters of formula II
R
R ,C ~ ~ /N H
2 /
OR ('Jt" SR)
R3
(II)
wherein R1 to R4, A and B are as hereinbefore
defined and R preferably denotes a C1_6-alkyl group
or benzyl (however, the person skilled in the art
may also, if desired, use derivatives of other
alcohols), and ammonia. The reaction is
conveniently carried out in an organic solvent at
temperatures between about 0°C and the boiling
temperature of the reaction mixture, preferably
between ambient temperature and about 100°C or
boiling temperature, if this is lower. Suitable
solvents are polar solvents such as methanol,
ethanol and propanol.
CA 02234611 1998-04-14
- 14 -
If the starting materials are sufficiently acid-
stable, the reaction may be carried out via the
corresponding acid imide chlorides instead of the
imido esters.
2. In order to prepare compounds of formula I wherein
A is linked via O or S to at least one of the ring
systems:
reacting
(a) a phenol or thiophenol of formula III
R
R
2
Z
R3
(III)
wherein Z denotes OH or SH and Rl, R2 and R3 are as
hereinbefore defined, with a compound of general
formula IV
/ NH
/ _
L A NHZ
(IV)
wherein A is as hereinbefore defined and L denotes
a nucleofugic leaving group, or
(b) a phenol or thiophenol of formula V
/ NH
Z NHz
(V)
CA 02234611 1998-04-14
- 15 -
wherein Z is as hereinbefore defined, with a
compound of formula VI: _ _.
R
R
2
A -L
R3
(VI)
wherein A, R1, R2, R3 and L are as hereinbefore
def fined .
The reaction is carried out in aprotic solvents
such as dimethylsulphoxide, dimethylformamide,
acetonitrile or alcohols such as methanol, ethanol
or propanol using a base (metal carbonate, metal
hydroxide, metal hydride) at temperatures between
about 0 and 140°C or the boiling temperature of the
reaction mixture.
The phenols or thiophenols may also be used in the
form of salts, e.g. the alkali metal salts. The
nucleofugic leaving group may be, for example, a
halogen such as Br or C1.
3. Reduction of an amidoxime of formula VII
R
R ~ ~ ~ /NOH
2
A NH2
R3
(VII)
wherein A and R1 to R3 are as hereinbefore defined.
CA 02234611 1998-04-14
- 16 -
Catalytic hydrogenation, particularly with Raney nickel
in a lower alcohol such as methanol, is suitable for the
step of reducing the amidoxime.
Appropriately, the amidoxime of the formula is dissolved
in methanol with the addition of the calculated quantity
of the particular acid the salt of which is desired as
an end product, and hydrogenated at ambient temperature
under slight pressure, e.g. 5 bar, until the uptake of
hydrogen has ceased.
The starting materials may be obtained from known
compounds by conventional methods.
Thus, the starting materials for process 1 may be
obtained from the corresponding nitriles by reacting
with HC1 via the step of the imide chlorides or directly
by reacting with C1_6-alcohols or benzyl alcohol, for
example, in the presence of an acid such as HCl.
Reacting the nitrites with HZS in solvents such as
pyridine or dimethylformamide in the presence of a base
such as triethylamine with subsequent alkylation or
benzylation also results in compounds of formula II.
Starting from carboxylic acid amides, which moreover
correspond to the compounds of formula II by reacting
with a trialkyloxonium salt such as triethyloxonium-
tetrafluoroborate in a solvent such as dichloromethane,
tetrahydrofuran or dioxane at temperatures of between 0
and 50°C, preferably at ambient temperature, compounds
of formula II are obtained.
In order to prepare the starting materials of general
formula VII, the corresponding amidoximes may be reacted
instead of amidines analogously to process 1 or 2, or by
analogous reaction of corresponding nitrites, from which
the starting materials of general formula VII are
CA 02234611 2004-03-17
27400-185
- 17 -
finally obtained by the addition of hydroxylamine.
It has been found that the compounds of formula I are
characterised by their ~.-~rsatility in therapeutic
applications. Particular mention should be made of
those applications in which the LTB4-receptor-
antagonistic properties play a part. These include, in
particular, arthritis, asthma, chronic obstructive lung
diseases, e.g. chronic bronchitis, psoriasis, ulcerative
colitis, gastro- or enteropathy induced by non-steroidal
antiphlogistics, cystic fibrosis, Alzheimer's disease,
shock, reperfusion damage/ischaemia, atherosclerosis and
multiple sclerosis.
The new compounds may also be used to treat illnesses or
conditions in which the passage of cells from the blood
through the vascular endothelium into the tissue is of
importance (e. g. metastasis) or diseases and conditions
in which the combination of LTB4 or another molecule
(such as 12-HETE) with the LTB4-receptor affects cell
proliferation (such as chronic myeloid leukaemia).
The new compounds may be used in conjunction with other
active substances, e.g. those used for the same_
indications, or with antiallergics, secretolytics, ~32-
adrenergics, steroids administered by inhalation,
antihistamines and/or PAF-antagonists. They may be
administered topically, orally, transdermally, nasally,
parenterally or by inhalation.
The activities may be investigated pharmacologically and
biochemically using tests such as those described in
WO 93/16036, pages 15 to 17.
The therapeutic or prophylactic dose is deper_dent not
only on the potency of the individual compounds and the
CA 02234611 1998-04-14
- 18 -
body weight of the patient but also on the nature and
gravity of the condition being treated. For oral use
the dose is between 10 and 500 mg, preferably between 20
and 250 mg. For administration by inhalation, the dose
given to the patient is between about 0.5 and 25,
preferably between about 2 and 20 mg of active
substance.
Solutions for inhalation generally contain between about
0.5 and 50 of active substance. The new compounds may
be administered in conventional preparations, e.g. as
tablets, coated tablets, capsules, lozenges, powders,
granules, solutions, emulsions, syrups, aerosols for
inhalation, ointments and suppositories.
The Examples which follow show some possible
formulations for the preparations:
Examples of formulations
1. Tablets
Composition:
Active substance according to the
invention 20 parts by weight
Stearic acid 6 parts by weight
Glucose 474 parts by weight
The ingredients are processed in the usual way to form
tablets weighing 500 mg. If desired, the content of
active substance may be increased or reduced and the
quantity of glucose reduced or increased accordingly.
CA 02234611 1998-04-14
- 19 -
2 . ~~,1_JOSltO~"i es
Composition: '
Active substance according to the
invention 1000 parts by weight
Powdered lactose 45 parts by weight
Cocoa butter 1555 parts by weight
The ingredients are processed in the usual way to form
suppositories weighing 1.7 g.
3. Powders for inhalation
Micronised powdered active substance (compound of
formula I; particle size approximately 0.5 to 7 ~Cm) are
packed into hard gelatine capsules in a quantity of
mg, optionally with the addition of micronised
lactose. The powder is inhaled from conventional
inhalers, e.g. according to DE-A 33 45 722, to which
reference is hereby made.
Example of synthesis
i ~
o ~~ ~o
OH
X
Amidoxime: X = pare-C(=NOH)NH2
2.0 g of the nitrile of the above formula (X = pare-CN)
are placed in 40 ml of ethanol, refluxed and a mixture
of 1 g of Na2C03 in 5 ml of water and 1.24 g of
hydroxylamine x HCl is added dropwise. After 5 hours'
refluxing the solvent is distilled off, the residue is
. CA 02234611 1998-04-14
- 20 -
stirred with 50 ml of water, extracted 3 x with 50 ml of
ethyl acetate and the combined organic phases are dried.
After filtering, the substance is evaporated down in
vacuo and the residue is purified by flash
chromatography (silica gel 60, CH2Cla/methanol 9:1). The
product is dissolved in ethanol, acidified with
ethanolic HC1 and precipitated as the hydrochloride
using ether. The oil obtained is crystallised with
ethyl acetate. Yield: 2.0 g of white crystals.
4- [ [3- [ [4- [1- (4-Hydroxyphenyl) -1-methylethyl] phenoxy] -
methyl]phenyl]methoxy]benzolcarboximidamide
hydrochloride (X = para-C (=NH) -NHa)
2.0 g of the amidoxime of the above formula (X = para-
C(=NOH)-NH~) are dissolved in 50 ml of methanol and
hydrogenated with 5 g of methanol-moistened Raney nickel
with the addition of 1 ml of 20o ammonium chloride
solution for 5 hours under normal pressure and at
ambient temperature. The nickel is suction filtered and
the solution is filtered through kieselguhr. After
concentration by evaporation in vacuo, the residue is
stirred with 50 ml of water. The crystals are suction
filtered and recrystallised twice from ethanol/ether.
Yield: 1.0 g of the amidine compound (the above formula,
X = para-C(=NH) -NH2 as hydrochloride, m.p. 234-236°C.
The following compounds are also obtained, inter alia,
using this procedure:
CA 02234611 1998-04-14
- 21 -
No. Compound . - - Salt form M.p. M.p.
(ocl foci
min max
2 Chloride 135 140
\ I I / o
o i
\ NHz
NH
3 Chloride 136
I
/
o \ / o I / o /
I
I / \ I \ NH2
NH
4 Fumarate 199 200
I .
/ o / o /
I NHZ
/ \ \
OH
NH
Methane- I93 198
sulphonate
I
o i °
I I I
\ HHz
OH
NH
CA 02234611 1998-04-14
- 22 -
6 Methane- 118 125
I sulphonate
o ~ o
I \ ~ I ~
/ \ \ NHZ
OCH3
NH
7 Chloride 156
'H3 \
I / o
I \ / I
/ \ \ I NNZ
NH
8 \ Chloride 218 220
I
/ o / o
I I
\ \ NHZ
HH
\ Chloride 130 132
I
o / o
I
/ \ \
NH NHZ
Chloride 117 121
\
OH
\ / O / 0
/ \ \ ~ NH2
CF3 CF3 NH
11 \ Dichloride 206
NH2 \ / I / /
\ ~ \ ( NHZ
NH
" - ~ CA 02234611 1998-04-14
- 23 -
12 ~i Chloride 165
I
N \ / I/ /
i I I
/ \ \ NH2
NH
13 ~~H3 ' Chloride 220
~2 I \
NH I \ / ( 0 / o / I
/ \ \ NHZ
NH
14 \ Chloride 172 175
I
\ / o / o /
I
/ \ \ ~ NH2
NH
OH
15 Chloride 199 275
/ \
I I _
o~/o /
\ /
I
NH2
NN
16 Chloride 152 155
/ \
I /
0 0
/
_ I
\ NH2
NH
CA 02234611 1998-04-14
- 24 -
17 Chloride 186 193
I I ..
o ~/ o
I
\ NHZ
NH
18 Chloride 162 165
I
I \ I \ o / o
/
/ / \ I NHZ
NH
20 Methane- 148 154
o ~ sulphonate
\ I \ / o /
/ /
\ I NHZ
NH
21 \ Sulphate 195
I
I \ I \ o / o
/
/ /
I NHZ
NH
21 Methane- 153 156
o~ \
sulphonate
I \ o ~ o , I
/ \ NHZ
NH
22 ~ Fumarate 215 240
\ o \
/ I _ o I / o /
/ i
NH2
' HH
CA 02234611 1998-04-14
- 25 -
23 ~ ~ Methane- 221 224
- sulphonate
\ o~
/ o~/o . \
/
\ NHZ
NH
24 Sulphate 217
o I / o
_ / i
\ ~ \ ~ NHZ
NH
25 Methane- 215 218
sulphonate
/~/o \
0
/
\ NH2
NH
26 I \ Methane- 178 181
/ o / o / sulphonate
CF3 ~ ~ ~ NH
\ \ 2
NH
27 Methane- 138 140
\
cF3 ~ sulphonate
/ o / o
\ ~ \ ~ NH2
' I
NH
CA 02234611 1998-04-14
- 26 -
28 Methane- 123 126
w \
off I sulphonate
o / o
/ /
I I
\ \ NH2
NH
29 Chloride 193 196
0 0
_ / ~ \
\ I I ~ /
\ I NH2
NH
30 Methane- 133 137
sulphonate
I I
o / o / o /
w \ I \ I NH2
/ a
NH
31 Fumarate 225
H \
I
O 0 / O
/ /
\ \ NHz
NH
32 I Sulphate 230
0
\
/ o / o /
\ I NH2
NH
33 ~ Methane- 230
/ o I / o sulphonate
/ \ I \ I NH2
N\O \ I . I IH
CA 02234611 1998-04-14
- 27 -
34 \ Methane- 230
° I / ° sulphonate
/ /
/ \ I \ I NH2
CH3, \ NH
O
35 Methane- 230 233
0
/ o~ I \ sulphonate
I / /
\
\ I NHZ
O~ I
H NH
36 Methane- 184 187
I sulphonate
o ~ o
\ i /
I I
/ \ \ I NHz
NH
37 Methane- 175 177
\
I sulphonate
\ ~ o / o /
/ \ \ I NH2
°H
NH
38 Methane- 160 167
off
\ / sulphonate
I / \ ~ ° I / ° /
\ NHZ
NH
3g \ Chloride 258 259
I
o / o
/ /
o r I
\ HH2
NH2 _ NH
CA 02234611 2004-03-17
27400-185
- 28 -
40 \ Chloride 212 213
o ~ o
H~0
\ NHz
NH
41 ~ Fumarate 219 220
\ _
NH / o I / o ~
0 \ ~ \ ~ NHz
~N-CHz
H NH
42 Fumarate 25 r
o i o
N ' \ I NHZ
p/ ~ ~ NH
43 ~ Fumarate 211 212
o I ~- o
\ -/ ~z r ~ I
NH ~ N
NH
44 \ Fumarate 258 260
p i o
i
N \ NHZ
p NH
45 Fumarate 224 226
o / o ~ /
~N-~HZ \ ~ \ ~ NH2
H
NH
CA 02234611 2004-03-17
27400-185
- 29 -
46 ~ Fumarate 224 226
CH3-SOz ~ C~..~C
NCH2 \ \ ~ NHz
H
NH
47 Fumarate 216
o i o
N ~ ~ \ ~ HH2
NH
0
Surprisingly, the compounds in the Example and in the
Table have outstanding Ki values which are largely within
the range from 0.2 to 0.7 nmol/1 (RB.LTB4/U937 cells).