Note: Descriptions are shown in the official language in which they were submitted.
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
METHODS FOR ISOLATING
POLYHYDROXYALKANOATES FROM PLANTS
Background of the Invention
The present invention is generally in the area of isolating
polyesters from plants.
Polyhydroxyalkanoates (PHAs) are a class of naturally occurring
polyesters that are synthesized by numerous organisms in response to
environmental stress. For reviews, see Byrom, D., "Miscellaneous
Biomaterials", in D. Byrom, Ed., "Biomaterials" MacMillan Publishers,
London, 1991, pp. 333-359; Hocking, P.J. and Marchessault, R.H.,
"Biopolyesters," in G.J.L. Griffin, Ed., "Chemistry and Technology of
Biodegradable Polymers", Chapman and Hall, London, 1994, pp. 48-96;
Holmes, P.A., "Biologically Produced (R)-3-hydroxyalkanoate Polymers
and Copolymers," in D.C. Bassett, Ed., "Developments in Crystalline
Polymers," Elsevier, London, Vol. 2, 1988, pp. 1-65; Lafferty et al.,
"Microbial Production of Poly-(3-hydroxybutyric acid," H.J. Rehm and G.
Reed Eds., "Biotechnology", Verlagsgesellschaft, Weinheim, Vol. 66,
1988, pp. 135-176; Muller and Seebach, Angew. Chem. Int. Ed. Engl.,
32:477-502 (1993); and Steinbuchel, A., "Polyhydroxyalkanoic Acids,"
Byrom, D., Ed., "Biomaterials", MacMillan Publishers, London, 1991,
pp. 123-213.
The PHA biopolymers can be divided into two groups according to
the length of their side chains (Figure 1). Those with short side chains
(Figure la), such as polyhydroxybutyrate (PHB), a homopolymer of R-3-
hydroxybutyric acid units, are crystalline thermoplastics, whereas PHAs
with long side chains (Figure lb) are more elastomeric. The former have
been known for about seventy years (Lemoigne and Roukhelman, Annales
des Fermentations, 5:527-536 (1925)) whereas the latter materials were
first identified in the early 1980's. De Smet et al., J. Bacteriol.,
154:870-878 (1983).
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-2-
Due to their earlier -discovery and their desirable physical
properties, the short side chain materials have been more extensively
studied. The PHA polymers, which are natural thermoplastics, can be
processed using conventional polymer technology and have industrially
useful properties, such as biodegradability in soil and marine
environments, biocompatibility, and good barrier properties. These
characteristics make these materials useful for a wide range of industrial
applications.
The PHA polymers may constitute up to 90% of the dry cell
weight of bacteria, and are found as discrete granules inside the bacterial
cells. These PHA granules accumulate in response to nutrient limitation
and serve as carbon and energy reserve materials. Distinct pathways are
used by microorganisms to produce each class of these polymers. The
pathway leading to the short side chain polyhydroxyalkanoates
(SSCPHAs) involves three enzymes, thiolase, reductase and PHB synthase
(sometimes called polymerase). Using this pathway, the homopolymer
PHB is synthesized by condensation of two molecules of acetyl-Coenzyme
A to give acetoacetyl-Coenzyme A, followed by reduction of this
intermediate to R-3-hydroxybutyryl-Coenzyme A, and subsequent
polymerization (Figure 2a). The last enzyme in this pathway, the
synthase, has a substrate specificity that can accommodate C3-C5
monomeric units including R-4-hydroxy acid and R-5-hydroxy acid units.
This biosynthetic pathway is found, for example, in the bacteria Zoogloea
ramigera and Alcaligenes eutrophus.
The biosynthetic pathway which is used to make the long side
chain polyhydroxyalkanoates (LSCPHAs) is still partly unknown,
however, it is currently thought that the monomeric hydroxyacyl units
leading to the LSCPHAs are derived by the 0-oxidation of fatty acids and
the fatty acid pathway (Figure 2b). The R-3-hydroxyacyl-coenzyme
substrates resulting from these routes then are polymerized by PHA
synthases that have substrate specificities favoring the larger monomeric
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-3-
units in the C6-C14 range Long side chain PHAs are produced, for
example, by Pseudomonads.
The biosynthesis of PHAs has been studied in a wide range of
bacteria at both the biochemical and genetic level, and has been reviewed
in Steinbuchel et al., FEMS Microbiology Reviews, 103:217-230 (1992).
Since the first PHA synthase genes were identified and characterized in
1989 (Peoples and Sinskey, J. Biol Chem., 264:15298-15303 (1989); and
U.S. Patent Nos. 5,229,279, 5,245,023, and 5,250,430 to Peoples and
Sinskey), a number of other microbial PHA polymerases have been
investigated and their DNA and amino acid sequences published.
Steinbuchel et al., FEMS Microbiology Reviews, 103:217-230 (1992).
More recently, two subunit PHA synthases from Chromatium vinosum
(Liebersgesell, M. and Steinbuchel, A., European J. Biochem., 209:135-
150 (1992); and WO 93/02194) and Thiocystis violacea (Liebersgesell,
M. and Steinbuchel, A., Appl. Microbiol. Biotechnol. 38:493-501 (1993))
have been described.
The genes encoding the enzymes responsible for the production of
SSCPHAs in, for example, Z. ramigera and A. eutrophus, have been
isolated and sequenced. Peoples and Sinskey, Prog. Biotechnol. 3:51-56
(1987); Peoples et al., J. Biol. Chem., 262:97-102 (1987); Peoples and
Sinskey (1989), J. Biol. Chem. 264:15298-15303, J. Biol. Chem.
264:15293-15297, and Molecular Microbiol. 3:349-357; Slater et al., J.
Bacteriol., 170:4431-4436 (1988); and Schubert et al., J. Bacteriol.,
170:5837-5847 (1988).
PHA producing microorganisms produce PHA to greater than 60%
total dry weight and are readily extractable by organic solvent. Lafferty
et al. ,"Microbial Production of Poly-/3-Hydroxybutyric Acid ", in H.J.
Rehm and G. Reed, Eds., "Biotechnology", Verlagsgesellschaft,
Weinheim, Vol. 66, 1988, pp. 135-176. In plants, the extraction and
recovery of PHA is significantly complicated by the presence of large
amounts of plant oil as well as lower percentages of PHA. These
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-4-
complicating factors make the successful extraction, separation and
recovery of PHAs from plants more difficult.
There is a need for the development of methods for the large scale
processing and purification of polyhydroxyalkanoates from plant biomass.
It is therefore an object of the invention to provide methods for
processing PHAs from plant biomass on a large scale. It is another object
of the invention to provide methods for isolating PHAs from transgenic
oil crop plants. It is a further object of the invention to provide methods
for processing plant biomass derived from oil seed crop plants such that
the recovery of the non-PHA products such as plant oils also is
maximized.
Summary of the Invention
Methods are provided for separating a polyhydroxyalkanoate
("PHA") from plants. In one embodiment, methods are provided for
isolating PHAs from a plant biomass derived from transgenic crop plants
which contain plant oils. The methods advantageously permit both the oil
and the PHAs to be recovered from the plant biomass. To isolate a PHA,
in one embodiment, a biomass derived from an oil crop plant is pre-
processed, for example by grinding, crushing or rolling. The oil then is
extracted from the biomass with a first solvent in which the oil is soluble
and in which the PHA is not highly soluble, to separate the oil from the
PHA. The essentially oil-free plant biomass then is extracted with a
second solvent in which the PHA is soluble, to separate the PHA from the
biomass. Alternatively, the PHA-containing biomass is treated with a
chemical or biochemical agent, such as an enzyme, to chemically
transform the PHA into a PHA derivative. The derivatized PHA then is
separated from the mixture using, for example, a physical separation
process such as distillation, extraction or chromatography.
Advantageously, using the method, plant oils, PHAs, and PHA
derivatives all can be recovered and purified on a large scale from plants
such as transgenic oil crop plants.
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-5-
Brief Description of the Drawings
Figure la is an illustration of the structure of short side chain
polyhydroxyalkanoates .
Figure lb is an illustration of the structure of long side chain
polyhydroxyalkanoates.
Figure 2a is an illustration of a biosynthetic pathway for the
synthesis of the short side chain polyhydroxyalkanoate,
polyhydroxybutyrate.
Figure 2b is an illustration of a biosynthetic pathway for the
synthesis of long side chain polyhydroxyalkanoates.
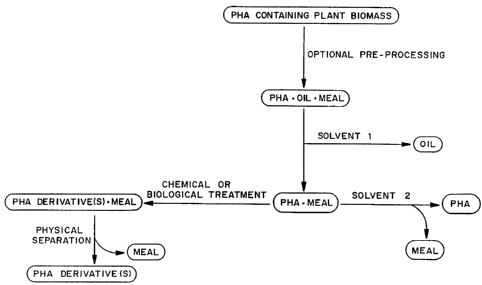
Figure 3 is flow chart illustrating one embodiment of a process for
separating polyhydroxyalkanoates from plants.
Figure 4 is a flow chart illustrating another embodiment of a
process for separating polyhydroxyalkanoates from plants.
Detailed Description of the Invention
Methods are provided for separating polyhydroxyalkanoates
("PHAs") from a plant biomass containing plant oil and meal. PHAs
which can be isolated from plant biomass include degradation or other
products of PHAs, such as monomers, dimers, oligomers, acids, esters,
amides, and lactones, which can be formed from chemical, biochemical or
physical treatment during processing of the biomass, or from processes
occurring within the plant biomass. In a preferred embodiment, the
PHAs are isolated from a biomass derived from a transgenic oil crop
plant. In addition to maximizing the recovery of PHA materials, the
recovery of commercially useful non-PHA products from the biomass also
is maximized. For example, in the case of PHA separation from the seed
of an oil-seed plant, the oil and meal also can be isolated and then used
commercially.
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-6-
I. Materials for Isolation of PHAs
A. PHA Materials which can be Isolated
PHA materials which can be isolated from plant biomass include
monomers, polymers and other products derived from PHAs including
chemically and biologically modified derivatives. The PHA materials are
defined in one embodiment as containing one or more units, for example,
to 100,000, and preferably 100-30,000 units, of the following fonnula
I:
-OCR'RZ(CR3R4)"CO-;
10 wherein n is 0 or an integer, for example, between 1-15,
and in a preferred embodiment between 1-4; and
wherein R', R2, R3, and R4 independently can be
hydrocarbon radicals including long chain hydrocarbon radicals;
halo- and hydroxy-substituted radicals; hydroxy radicals; halogen
radicals; nitrogen-substituted radicals; oxygen-substituted radicals;
and/or hydrogen atoms.
As defined herein, the formula -(CR3R ),,- is defined as including
but not limited to the following formulas:
-CR3R4- (where n= 1);
-CR3R4CR3'R4'- (where n=2); and
-CR3R4CR3'R 'CR3"R4"- (where n=3);
wherein R3, R4, R3', R4', R3", and R4" can be independently
hydrocarbon radicals including long chain hydrocarbon radicals; halo- and
hydroxy-substituted radicals; hydroxy radicals; halogen radicals; nitrogen-
substituted radicals; oxygen-substituted radicals; and/or hydrogen atoms.
Thus, formula I includes units derived from 3-hydroxyacids (n=1), 4-
hydroxyacids (n=2), 5-hydroxyacids (n=3).
These units may be the same, as in a homopolymer, or be selected
from two or more different units, as in a copolymer or terpolymer. The
polymers in one embodiment have a molecular weight above 300, for
CA 02234965 1998-04-15
WO 97/15681 PCTIUS96/16921
-7-
example between 300 and 1,000,000, and in a preferred embodiment,
betweenlO,000 and 3,000,000 Daltons. In one embodiment, PHA
homopolymers such as, for example, polyhydroxybutyrate,
polyhydroxyvalerate or polylactic acid may be isolated. Additionally,
PHA copolymers or terpolymers including at least two monomers of a
hydroxyalkanoate such as hydroxybutyrate, hydroxyvalerate,
hydroxyhexanoate, hydroxyheptanoate, hydroxyoctanoate,
hydroxynonanoate and hydroxydecanoate can be isolated. PHAs including
monomers and polymers and derivatives of 3-hydroxyacids, 4-
hydroxyacids and 5-hydroxyacids can also be isolated.
The PHA polymers also may contain or be modified to include
non-hydroxy acid units such as long chain fatty acids, amino acids,
carbohydrates, phosphorus and sulfur containing compounds, and triols,
such as glycerol. PHA products which can be isolated include derivatives
formed upon physical, chemical or biochemical treatment of the biomass
or by processes within the biomass including hydroxyacid monomers,
dimers, trimers, linear and cyclic oligomers and lactones. PHA
derivative products which can be isolated include esters, diols, unsaturated
compounds, aldehydes, acids, alcohols, lactones, cyclic and linear esters,
amides, and thioesters of polyhydroxyalkanoates or of a monomer derived
from the polyhydroxyalkanoate.
Representative PHA products which can be isolated from plant
biomass include:
esters defined by the formula: HOCR'Rz(CR3R4),,CO2R5;
amides defined by the formula: HOCR'RZ(CR3R )nCONRSR6;
thioesters defined by the formula: HOCR'RZ(CR3R4)nCOSRs;
acids defined by the formula: HOCR'RZ(CR3R4)nCO2H;
ethers defined by the formula: R6OCR'RZ(CR3R'')õCO2R5;
esters defined by the formula: R6CO2CR'R2(CR3R4),,CO2RS;
unsaturated compounds defined by the formulas:
R'R2C =CR3(CR4R5),CO2Rb; and
R'RZR3CC(R4) = C(R5)C02R 6;
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-8-
diols defined by the formula: HOCR'R2(CR3R4),,CH2OH;
lactones or macrolides, defined by the formula:
c-OCR'R2(CR3R4)"CO-]X ;
where x is an integer, for example from 1 to 10; and
ketones or aldehydes defined by the formula:
HOCR'R2(CR3R4)"CORS;
HOCR'R2(CR3R4),CHO;
R'CO(CR2R3)nCOOR4; and
R'CO(CRzR3)nCOR4;
wherein n is 0 or an integer; and
wherein R', R2, R3, R4, RS and R6 are each independently
hydrocarbon radicals including long chain hydrocarbon radicals, halo- and
hydroxy-substituted radicals, hydroxy radicals, halogen radicals, nitrogen-
substituted radicals, oxygen-substituted radicals, and hydrogen atoms; and
wherein -(CR2R3)R is defined as described above.
Commercially useful monomer PHA products such as 3-
hydroxybutyric acid or crotonic acid, or alkyl esters thereof, including
methyl-3-hydroxybutanoate, ethyl-3-hydroxybutanoate, propyl-3-
hydroxybutanoate and butyl-3-hydroxybutanoate, also can be isolated.
The PHA derived hydroxy acid monomers, in addition to the higher
molecular weight forms, are a source of valuable chemicals that can be
used commercially either with or without further modification.
As used herein, the term "PHA materials", or "PHAs" or
"polyhydroxyalkanoates" refers to monomers, polymers and other PHA-
based materials originally present in the biomass prior to processing, and
products formed during processing such as products formed from
degradation or processes occurring within the plant biomass or derivative
products formed by treatment of the biomass with chemical or biological
agents to cause a chemical transformation.
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-9-
B. Plant Sources From Which PHAs Can Be Isolated
Plant Species
PHAs and PHA products can be isolated from plant biomass
derived from plants such as soybean, cotton, coconuts, groundnuts,
rapeseed, sunflower seed, olive, palm, sesame?,ieed, linseed, castor,
safflower seed, tobacco and potato. In a preferred embodiment, the
biomass can be derived from an oil crop plant, particularly rapeseed,
sunflower seed, safflower seed, linseed and soybean. In addition to the
PHA polymers, the plant oil in the seed crop plants can be isolated and
recovered during the processing. Plant oils typically make up 10-50% of
the seed by weight. The worldwide demand for plant oil is considerable.
The methods for processing the plant biomass can be tailored based on the
properties of the particular PHA polymer or derivative being isolated, and
based on the type of plant crop and the plant components being extracted.
Production of Transgenic Plants
The use of transgenic oil crop plants offers many advantages.
Transgenic crop plants for production of PHAs can be obtained using
methods available in the art. Transgenic plant crop production can
produce PHA polymers at both a price and a scale that is competitive with
petrochemical derived plastics. Transgenic plant derived PHA polymers
or their derivatives can be processed and separated from plant biomass in
commercially useful forms. The location of the PHA in the plant crop
(e. g. , leaf, seed, stem or combinations thereof) can be varied to maximize
the yield of PHA from the plant.
The genes encoding the enzymes responsible for the production of
short side chain PHAs in, for example, Z. ramigera and A. eutrophus,
have been identified, isolated and sequenced. Peoples and Sinskey, Prog.
Biotechnol. 3:51-56 (1987); Peoples et al., J. Biol. Chem., 262:97-102
(1987); Peoples and Sinskey (1989), J. Biol. Chem. 264:15298-15303, J.
Biol. Chem. 264:15293-15297, and Molecular Microbiol. 3:349-357;
Slater et al., J. Bacteriol., 170:4431-4436 (1988); and Schubert et al., J.
Bacteriol., 170:5837-5847 (1988). In A. eutrophus, they were found to
CA 02234965 1998-04-15
-10-
form an operon, phbC phbA phbB genes, coding for the short side chain
PHA synthase, thiolase, and reductase, respectively. For the long side
chain PHAs, the synthase enzymes in the Pseudomonas organism were
found to be encoded by the pha locus. This locus includes two closely
related PHA synthase genes, phaA and phaC, as well as a depolymerase
gene which is the product of the phaB gene.
Methods which can be used for producing PHA polymers in
transgenic crop species are described in: U.S. Patent Nos. 5,245,023 and
5,250,430; WO 91/00917; WO 92/19747; WO 93/02187; WO 93/02194;
WO 94/11519; WO 94/12014; WO 94/23027; WO 95/05472; Poirier et
al., Science, 256:520-523 (1992), Poirier et al., Bio/Technol., 13:142-150
(1995) and Nawrath et al., Proc. Natl. Acad. Sci. USA, 91:12760-12764
(1994).
To form a transgenic crop species, a gene encoding a PHA
synthase is transferred from a microorganism into plant cells to obtain the
appropriate level of production of the PHA synthase enzyme. The gene
may be derived from a microorganism such as Acinetobacter, Aeromonas,
Alcaligenes, Azotobacter, Bacillus, Brevibacterium, Corynebacterium,
Chromatium, Flavobacterium, Halobacterium, Pseudomonads, Nocardia,
Rhodococcus, Thiocystis, Streptomyces, Streptococcus or Zoogloea.
Additional PHA biosynthetic genes also can be provided, for example, an
acetoacetyl-CoA reductase gene or other genes encoding enzymes required
to synthesize the substrates for the PHA synthase enzymes. The
expression in different plant tissues or organelles can be controlled using
methods known to those skilled in the art. Gasser and Fraley, Science,
244:1293-1299 (1989). PHB has been produced in genetically engineered
plant systems by standard techniques. Poirer, Y. et al., Science,
256:520-523 (1992); Poirier, Y. et al., BiolTechnol., 13:142-150 (1995);
and Nawrath, C. et al., Proc. Natl. Acad. Sci. USA, 91:12760-12764
(1994).
AMENDED SHEET
IPEA/EP
CA 02234965 1998-04-15
-11-
In a preferred embodiment, the PHA content of the plant biomass
prior to extraction is at least 1 % by weight of the dry weight of biomass,
more preferably between 5 and 95 % by weight of the dry weight of
biomass, and in another preferred embodiment between about 5 and 60%
by weight of the dry weight of biomass, most preferably between 10 and
60%. Preferably, at least 24% of the PHA is recovered in the process
separate from oil.
H. Methods For Isolation Of PHAs From Plants
A. Pre-processing of the Plant Biomass
The PHA-containing plant biomass, for example, a transgenic oil
crop plant containing a heterologous PHA synthase gene, is cultjvated and
harvested. The plant biomass may be pre-processed prior to extraction of
the PHA polymers using methods available in the art, such as agitation,
heating, cooling, pressure, vacuum, sonication, centrifugation, and/or
radiation. As used herein, the term "plant biomass" refers to plant
components including seeds, leaf, stalk and stem. Additionally, the plant
biomass can be pre-processed using any one or more combinations of
procedures including drying, dehulling, cleaning, ageing, weighing,
cracking, flaking, pressing, rolling, grinding, cooking, crushing, settling,
and/or filtering. The use of these procedures for separating oil from meal
in the processing of oil bearing plants is described in "Oil Crops of the
World," G. R6bblen et al., Eds., McGraw-Hill Publishing Company,
1989, Chapter 11. In addition, methods used for corn milling including
both dry and wet milling approaches involve separating the oil-containing
germ from the starch-containing endosperm. This can be accomplished
by centrifugation or air classification as described for example in "Corn
Chemistry and Technology", 1994 edition, Watson, S.A. and Ramstad, P.
E., eds., American Association of Cereal Chemists Inc., St. Paul,
Minnesota.
B. Extraction of Plant Biomass
The PHA monomers, polymers and derivatives can be removed
from the plant biomass using suitable means including solvent extraction
AMENDED SHEET
IPEA/EP -
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-12-
and/or washing, aqueous extraction and/or washing, crushing, temperature
treatment, enzymatic treatment, centrifugation, supercritical fluid
extraction, high and/or reduced pressure treatment, chromatography,
distillation, melting, or treatment with reagents to facilitate separation of
the PHA materials.
Methods for extracting the oil from the pre-processed material
available in the art also may be used, such as oil expeller pressing where
the oil is mechanically squeezed from the oil bearing material, and
prepressing solvent extraction where a portion of the oil is removed by
expellers and the remainder by extraction with an organic solvent, such as
a hydrocarbon, for example, hexane. Additionally, supercritical gases
including carbon dioxide and propane can be used. Other methods
include direct solvent extraction where the oil is removed directly from
conditioned seed with an organic solvent; propane refining to separate fat;
and fat splitting involving hydrolysis of fat or oil with water to produce
glycerol and fatty acid. "Oil Crops of the World," G. Robblen et al.,
Eds., McGraw-Hill Publishing Company, 1989; "Liquid Extraction, " R.
Treybal, Ed., McGraw-Hill Book, New York, 1951; and "World
Oilseeds: Chemistry, Technology, and Utilization, " D.K. Salunkhe et al.,
Eds., Van Nostrand Reinhol, New York, 1992.
Extraction of Oil from Plant Biomass
One preferred method for isolating the PHAs from a plant biomass
is illustrated in the flow chart of Figure 3. In the process, the PHA
containing plant biomass first optionally is pre-processed as described
above. The pre-processed or unprocessed PHA containing plant biomass
then is extracted in a solvent in which the oil is soluble, and in which the
PHA and the meal are not highly soluble, to remove the majority or all of
the oil from the PHA containing plant biomass. The solvent is selected
such that it is a good extractant for the oil and a poor extractant with low
solubility for the PHA and the plant meal. Extraction of the PHA-oil-
meal mixture, as illustrated in the flow chart of Figure 3, produces an oil
fraction essentially free of PHA (for example including less than about
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-13-
10% by weight of PHA) and an essentially oil free PHA-meal mixture
(including for example, less than about 10% oil by weight). The PHA-
meal mixture then is extracted with a second solvent in which the PHAs
are soluble, to obtain purified PHA materials. Alternatively, the PHA-
meal mixture can be treated chemically or enzymatically to produce PHA
derivatives which are then isolated from the meal, as illustrated in Figure
3.
The first solvent which is used to extract the oil from the plant
biomass is selected based on its ability to solubilize the oil. Preferably, a
solvent is used in which the oil is soluble and in which the PHA and plant
material is not highly soluble. Suitable solvents include hydrocarbons,
such. as propane, butane, pentane, hexane, heptane, octane, nonane and
decane. As used herein the term "solvent" includes solvents as well as
solvent mixtures, such as mixtures of hydrocarbons. Preferably, the first
solvent is chosen wherein the PHA is soluble to less than 1 %, most
preferably less than 0.1 % and the oil is soluble to more than 10% (w/v,
ambient temperature).
To isolate the PHA and oil components from the biomass, solvents
used in the extractions are selected which exploit the differences in the
physical nature and solubility characteristics of the PHA and oil
components of the biomass. The isolation steps are tailored depending on
the particular PHA, plant host or PHA/plant host combination. For
example, in the extraction of PHB and LSCPHA, different solvents or
solvent combinations are used in their extraction from PHA-containing
transgenic plant biomass based on their solubility.
In the embodiment where the PHA is separated from the PHA-
meal product by treatment with a second solvent, the second solvent
(solvent 2 in Figure 3) is selected based on its capability of being a good
extractant for the PHA and a poor extractant for the meal. Solvents
which can be used include solvents or solvent mixtures including
chloronated organic solvents such as chloroform, methylene chloride,
dichloroethane, trichloroethane, tetrachloroethane and dichloroacetate.
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-14-
For example, hydrocarbon stabilized chloroform can be used. Other
solvents which have been used to extract PHAs from microbial sources
which may be used include alkyl carbonates, such as propylene carbonate
and ethylene carbonate, trifluoroethanol, acetic anhydride,
dimethylformamide, ethylacetoacetate, triolein, toluene, dioxane,
tetrahydrofuran, diethylether, pyridine, hydroxyacids and alcohols having
more than 3 carbon atoms, as well as mixtures thereof. Lafferty et al.,
"Microbial Production of Poly-O-Hydroxybutyric Acid," in H.J. Rehm
and G. Reed, Eds.,"Biotechnology", Verlagsgesellschaft, Weinheim,
Vol. 66, 1988, pp. 135-176. In a preferred embodiment, the second
solvent is a chlorinated organic solvent or an alkyl carbonate.
Additionally, in a preferred embodiment, the first and second solvents
have boiling points between ambient temperature and 400' C, more
preferably between 30 C and 250' C.
The solvent extraction steps also can be conducted using
supercritical fluid extraction, wherein a gas is used such as ethylene,
propylene, propylene oxide, butane or carbon dioxide. In a preferred
embodiment, the gas has a boiling point between -250 C and ambient
temperature, preferably between -150 C and -20 C. The PHA also may
be extracted in a molten state.
In an alternative embodiment, as illustrated in the flow chart of
Figure 3, the PHA-meal mixture is treated with a chemical or biochemical
agent, such as an enzyme, to chemically transform the PHAs into PHA
derivatives as described in detail below. The PHA derivatives then are
separated from the plant biomass if necessary, using one or more
subsequent physical separation steps such as distillation, extraction,
centrifugation, filtration or chromatography.
Extraction of PHA and Oil.
In another embodiment, shown in the flow chart of Figure 4, the
PHA containing plant biomass optionally first is pre-processed as
described above. The pre-processed or unprocessed PHA containing plant
biomass then is solvent extracted in a solvent in which the oil and the
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-15-
PHAs are soluble, and in which the meal has low solubility, to essentially
remove the oil and the PHAs from the plant meal, such that, for example,
less than about 10% of oil and PHAs by weight remain in the plant meal.
The solvent used in this process is selected such that it is a good
extractant for the PHAs and oil, and a poor extractant for the meal. The
PHA materials in the PHA-oil product then are further separated from the
oil by a physical separation step, such as distillation, or by further
exploitation of differences in solubility between the PHA and oil.
Alternatively, the PHA-oil product may be modified by chemical
or biological treatment to provide a PHA derivative(s)-oil product as
described below (as shown in Figure 4). The PHA derivative component
of the latter may be subsequently purified by physical processing,
including distillation, solvent extraction, washing, precipitation,
centrifugation, supercritical fluid extraction, filtration, and
chromatography.
Solvents which may be used to extract the oil-PHA component
from the plant biomass include chlorinated organic solvents, for example,
chloi-oform, methylene chloride, di-, tri-, tetra-chloroethane and
dichloroacetate, alkyl carbonates such as propylene carbonate and ethylene
carbonate, trifluoroethanol, acetic anhydride, dimethylformamide,
ethylacetoacetate, triolein, acetic acid, toluene, alcohols, hydroxyacids,
dioxan, tetrahydrofuran, diethylether, and/or pyridine. The solvent also
may consist of or may include hydrocarbons such as hexane, heptane,
octane, nonane or decane or mixtures thereof. Preferred solvents are
those having boiling points between ambient temperature and 400 C,
preferably between 30 C and 250 C. Preferably, such solvents have
solubility for both PHA and oil components of at least 5% (w/v, ambient
temperature), and are chosen depending upon the structure of the PHA
defined in Figure 1. The PHA material also can be extracted in the
molten state. The choice of solvent will depend on the choice of plant
from which the biomass is derived and the solubility properties of the
PHAs, derivatives and oils being separated.
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-16-
As illustrated in the flow chart of Figure 4, the extracted PHA-oil
also can be separated by chemical modification to form a PHA derivative-
oil product, by treatment with a chemical or biological agent, such as an
enzyme which degrades the PHA material, as described in detail below.
The PHA derivative then is separated from the PHA derivative-oil
mixture using, for example, a physical process such as distillation,
extraction, centrifugation, supercritical fluid extraction, preparation
filtration, and/or chromatography.
Further refining of the essentially oil free PHA can be carried out
by standard procedures known to those skilled in the art.
III. Synthesis of PHA Derivatives
As described above, during the processing, the PHA materials in
the biomass can be derivatized by physical, chemical or enzymatic
conversion into derivatives, prior to their isolation, to facilitate the
isolation of the materials, or to produce a desired derivative product.
PHA derivatives which can be formed include acids, esters, oligomers,
cyclic oligomers, lactones, macrolides, amides, amines, thioesters, diols,
and unsaturated compounds, which can be formed using methods available
in the art. Griesbeck, A. and Seebach, D., Helv. Chim. Acta 70:1320-
1325 (1987); Plattner, D.A., Helv. Chim. Acta, 76:2004-2033 (1993);
Seebach, D. et al.,"Biological-Chemical Preparation of 3-
Hydroxycarboxylic Acids and Their Use in EPC-synthesis," W. Bartmann
and K.B. Sharpless, Eds., "Stereochemistry of Organic and Bioorganic
Transformations, " VCH, Weinheim, 1987, pp. 85-126; and Seebach, D.
et al., Chimia, 44:112-116 (1990); Org. Synth., 71:39-47 (1992); Angew.
Chem. Int. Ed. Eng., 434-435 (1992); and Helv. Chim. Acta, 77:1099-
1123 (1994). Additional methods for derivatizing esters which may be
used to form PHA polyesters are known to those skilled in the art.
Chemical agents which can be used to modify the PHA materials
in the processing of the biomass include, for example, acids, bases,
detergents, chelator, an oxidizing or reducing agent, a nucleophilic or
electrophilic reagent, metal ions, aqueous solutions or organic solutions,
CA 02234965 1998-04-15
-17-
and free radicals. Other chemical agents which can be used include
hydrogen peroxide, hypochlorite, ozone and alkyl peroxides. The
chemical transformation can be, for example, a chemical reaction such as
an esterification, transesterification, hydrolysis, saponification,
aminolysis, thiolysis, etherification, silylation, addition, elimination,
rearrangement, or condensation. The chemical agents can be used, for
example, to produce derivatives with a molecular weight less than that of
the PHA starting materials in the plant biomass. Additionally, the PHA
materials can be modified by physical treatment such as heat, cold or
agitation.
The PHA materials also can be chemically modified during
processing by treatment of biomass materials such as PHA-meal or PHA-
oil mixtures with a biological agent such as an enzyme, which for
example, degrades the biomass or the PHA material. Enzymes which can
be used include PHA depolymerases, proteases, nucleases, lipases,
hydratases, phosphorylases, cellulases and/or glycosidases. The PHA
polymers may be converted to oligomers, monomers, dimers, trimers, or
other derivatives. The PHA functionality may also be converted to non-
PHA chemical functionality.
IV. Applications
The PHAs isolated as described herein can be used in a wide
variety of different applications. In one embodiment, the isolated PHAs
can be used to form a latex. PCT WO 91 / 13207 discloses the use of
polymers or copolymers of 0-hydroxybutyrate and 0-hydroxyvalerate in
the form of a latex, i. e. , as an aqueous suspension of non-crystalline,
amorphous particles. The latex can be used, for example, to form films
or coated papers which are biodegradable and recyclable. PCT WO
96/03468 describes the use of PHA latex in architectural coatings.
Methods for forming a PHA latex from purified crystalline PHAs are
described in PCT WO 94/07940. In the method, a purified solution of
PHA in an organic solvent, which can be obtained as described herein, is
emulsified
AMENDED SHEET
tPEA/EP
CA 02234965 1998-04-15
WO 97/15681 PCT/US96116921
-18-
in an aqueous solution including a surfactant, to form an amorphous latex.
Thus, the methods disclosed herein provided purified PHAs which can be
used in a variety of industrial and biomedical applications such as the
formation of PHA latex materials.
The invention will be further understood from the following non-
limiting examples.
Example 1: Extraction of Polyhydroxybutyrate
from Plant Biomass.
The process illustrated in Figure 3 was used to isolate
polyhydroxybutyrate (PHB) from plant biomass by extraction with hexane
(solvent 1) to remove the oil followed by extraction with hydrocarbon
stabilized chloroform (solvent 2) to isolate PHB.
A sample of rapeseed (32 g) containing approximately 40 weight
% oil was admixed with 6 g of PHB powder (Aldrich) and ground using
an electric food grinder. This sample is representative of a transgenic oil
seed containing 34% by weight oil and 16% by weight of PHB. The
mixture was continuously extracted with 300 mL hexane (solvent 1) in a
soxhlet apparatus for 6 hours after which time the sample was allowed to
cool providing an organic solvent phase and a solid meal. The organic
solvent was concentrated to yield a yellow oil (11. 8 g, 31 % by weight of
the admixture). NMR analysis indicated that the oil contained no PHB.
This result indicates that PHB-free oil can be readily recovered at greater
than 90% yield from PHA containing plant. biomass. A portion of the
solid meal (7.7 g) was then further extracted with 120 mL of hydrocarbon
stabilized chloroform (Solvent 2) for 22 hours in a soxhlet apparatus.
Evaporation of the chloroform solution resulted in the formation of a
yellow/white plastic film weighing 1:15 g. A portion of the crude PHB
film (227 mg) was washed with three, one mL portions of hexane. After
air drying, the PHB film (86 mg) was off-white in color. NMR analysis
of this film indicated that it was essentially pure PHB. The recovery of
PHB film represents a 24% yield based on the original PHB content of
the admixture.
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-19-
Example 2: Extraction of a PHB Derivative from
Plant Biomass.
The process illustrated in Figure 3 was used to is olate
polyhydroxybutyrate (PHB) from plant biomass by extraction of plant
biomass with hexane (solvent 1) to remove the oil fraction followed by
chemical treatment and then physical separation of the PHB in derivative
form.
A portion of the PHB containing residual meal (2.32 g) from
Example 1 was heated at reflux for 15.5 hours with n-butanol (25 mL)
and concentrated sulfuric acid (0.33 mL). To the resultant black mixture
was added saturated sodium bicarbonate (20 mL), brine (20 mL) and ethyl
acetate (50 mL). The mixture was shaken in a separatory funnel and the
phases were separated. The organic phase was filtered through a pad of
celite, washed with brine, treated with a small amount of activated
charcoal, filtered, and concentrated to a dark oil. This material was
distilled under reduced pressure. The fraction distilling at 49-53 C and
0.25 torr was collected to yield a slightly yellow colored liquid (0.47 g).
NMR analysis of this material confirmed that it was butyl 3-
hydroxybutyrate. The amount of material recovered represents a 46%
yield of derivatized PHB based on the amount of PHB contained in the
residual meal.
Example 3: Extraction of PHAs from Rapeseed
PHA was extracted in polymer form from rapeseed using the
process of Figure 4 as follows. A sample of rapeseed (20 g) containing
approximately 40% by weight oil was admixed with small pieces of PHO,
a copolymer including approximately 94% 3-hydroxyoctanoic acid and
approximately 6% 3-hydroxyhexanoic acid (5.43 g, isolated from
Pseudomonas putida) and ground using an electric food grinder. This
sample is representative of a transgenic oil seed containing 31 % by weight
oil and 21 % by weight of PHO. The mixture was continuously extracted
with 300 mL hexane (Solvent 1) in a soxhlet apparatus for 12 hours. The
sample was allowed to cool and was filtered to provide an organic solvent
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-20-
phase and a solid meal (11-.02 g after air drying). The solvent phase was
concentrated to yield a yellow, very viscous gel-like material (12.92 g,
51 % by weight of the admixture). Upon standing, this material set into a
yellow, plastic-like solid. NMR analysis indicated that the material
contained PHO and rapeseed oil. These results indicate that PHO oil
mixture can be readily recovered at greater than 90% yield from PHA
containing plant biomass. The PHA/oil mixture obtained was suitable for
further purification.
Further purification was conducted as follows. A portion of the
yellow, plastic-like material (0.136 g, approximately 41 % wt PHO, 1.1 x
0.2 cm) was washed with 2 ml of n-propanol. After slowly swirling at
room temperature for 3 days, the supernatant was removed, and the
residual solid was washed overnight with 2 ml of methanol. The
methanol wash was combined with the propanol wash and concentrated to
yield a yellow oil (0.0876 g). After drying under vacuum, the residual
solid polymer (0.048 g) was semi-transparent and almost colorless. This
represents an 84% yield of PHO from the original rapeseed/PHO mixture.
NMR analysis of the purified polymer showed that it was PHO
(approximately 95 % purity) containing a small amount of rapeseed oil.
G.C. analysis showed a 10 fold reduction of major contaminants relative
to the yellow, plastic-like material initially isolated by hexane extraction.
Example 4: Isolation of a PHA Derivative from Plant Biomass
The yellow PHO containing plastic-like material obtained prior to
further purification, as described in Example 3, was further purified in
derivative form by chemical treatment and physical separation: A portion
of the partially purified PHO containing plastic material (2.75 g,
containing approximately 40% by weight PHO) isolated by hexane
extraction from Example 3 was dissolved in n-butanol (50 mL) with
heating. Concentrated sulfuric acid (0.7 mL) was added and the mixture
was heated at reflux for 20 hours. After cooling to room temperature,
saturated sodium carbonate (4 mL) was added to make the mixture basic
to pH paper. The reaction mixture was filtered, the phases were
CA 02234965 1998-04-15
WO 97/15681 PCT/US96/16921
-21-
separated and the organic layer washed with brine (2 x 30 mL). The
organic phase was concentrated to about 10 mL, dissolved in chloroform
(50 1nL), dried over magnesium sulfate, filtered and concentrated under
vacuum to a yellow oil (2.75 g). This material was distilled under
reduced pressure. The fraction distilling at 93-97 C and 0.45 torr was
collected to yield a clear, colorless liquid (0.41 g). The amount of
material recovered represents a 25 % yield of derivatized PHO based on
the amount of PHO contained in the plastic-like starting material. NMR
analysis of this material indicated that it is butyl 3-hydroxyoctanoate of
approximately 95% purity and that it contains a very small amount of
unsaturated material.