Note: Descriptions are shown in the official language in which they were submitted.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
TITLE OF THE INVENTION
PROCESS FOR THE PREPARATION OF A GROWTH HORMONE
SECRETAGOGUE
5 BA~KGROUND OF THE INVENTION
Growth hormone, which is secreted from the pituitary,
stimulates growth of all tissues of the body that are capable of growing.
In addition, growth hormone is known to have the following basic effects
on the metabolic processes of the body: (1) Increased rate of ~lotei
10 synthesis in all cells of the body; (2) Decreased rate of carbohydrate
tili7~tion in cells of the body; (3) Increased mobilization of free fatty
acids and use of fatty acids for energy. A deficiency in growth hormone
secretion can result in various medical disorders, such as dw~ sm.
Various ways are known to release growth hormone. For
15 example, chemicals such as arginine, L-3,4-dihydroxyphenyl~l~nine
(L-DOPA), glucagon, vasopressin, and insulin induced hypoglycernia,
as well as activities such as sleep and exercise, indirectly cause growth
hormone to be released from the pituitary by acting in some fashion on
the hypoth~l~mus perhaps either to decrease somatostatin secretion or
20 to increase the secretion of the known secretagogue growth hormone
releasing factor (GRF) or an unknown endogenous growth hormone-
releasing hormone or all of these.
In cases where increased levels of growth hormone were
desired, the problem was generally solved by providing exogenous
25 growth hormone or by ~(1mini.ctering GRF or a peptidal compound
which stimulated growth hormone production and/or release. In either
case the peptidyl nature of the compound necessitated that it be
~lmini~tered by injection. Tniti~lly the source of growth hormone was
the extraction of the pituitary glands of cadavers. This resulted in a
30 very expensive product and carried with it the risk that a disease
associated with the source of the pituitary gland could be transmitted
to the recipient of the growth hormone. Recombinant growth
hormone has become available which, while no longer carrying any
risk of disease tr~n.smi.ssion, is still a very expensive product which
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
must be given by injection or by a nasal spray. Other compounds have
been developed which stimulate the release of endogenous growth
hormone.
In particular, certain spiro compounds are disclosed in
S PCT Patent Publication WO 94/13696 and Proc. Natl. Acad. Sci.
US~, 92, 7001-7005 (July 1995) as being non-peptidal growth
hormone secretagogues. These compounds have the ability to
stimnl~te the release of natural or endogenous growth hormone and
thus may be used to treat conditions which require the stim~ tion of
growth hormone production or secretion such as in humans with a
deficiency of natural growth hormone or in ~nim~l.c used for food or
wool production where the stimulation of growth horrnone will result
in a larger, more productive ~nim~l.
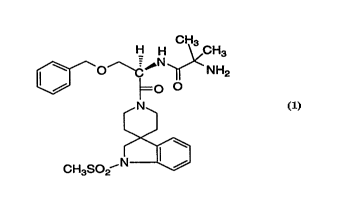
Among the preferred compounds disclosed therein is
l S spiro[3H-indole-3,4'-piperdin] - 1 '-yl)carbonyl]-2-(phenylmethyl-
oxy)ethyl]-2-amino-2-methylpropanamide which has the structure:
H H \ 3
--~--C~ ~NH2
g~ ' so2CH3
PCT Patent Publication WO 94/13696 discloses methods
for preparing this compound (see Examples 18, 19 and SS). However,
the syn~hç~i~ of the compound was accomplished by using the very
expensive amino acid coupling agent EDC ($1100/kg); the use of
numerous equivalents of trifluoroacetic acid as the catalyst for the
BOC group deprotections; extensive chromatographic purifications;
and resulted in "gllmming" of the final product.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
The advantages of the present invention include: a 6-step
high yielding non-isolation process providing material of 299 9%
purity; decreased expense through the use of DCC t$40/kg] in~te~-l of
EDC [$1100/kg]; ~1imini~hed environmental impact through the use of
5 methanesulfonic acid instead of trifluoroacetic acid as the catalyst (as
well as lesser equivalents of catalyst) in the deprotections; and ease of
isolation of the final product.
SUMMARY OF THE INVENTION
The instant invention is directed to a process for the
preparation of the compound N-[l(R)-[(1,2-dihydro-1-methanesulfonyl-
spiro[3H-indole-3,4'-piperdin]- 1 '-yl)carbonyl]-2-(phenylmethyl-
oxy)ethyl]-2-amino-2-methyl-propanamide which has the structure:
CH3
o ~N~H
CH3SO2--~3
15 and salts thereof, in particular, the methanesulfonate salt.
This compound has the ability to stimulate the release of
natural or endogenous growth horrnone and may be used to treat
conditions which require the stimulation of growth hormone production
or secretion such as in humans with a deficiency of natural growth
20 hormone or in ~nim~l~ used for food or wool production where the
~ stimulation of growth hormone will result in a larger, more productive
~nimzll .
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
l:)ESCRIPTION OF THE INVENTION
The present invention is directed to a novel process for the
preparation of the compound N-[ 1 (R)-[( 1 ,2-dihydro- 1 -methanesulfonyl-
spirot3H-indole-3,4'-piperdin]- 1 '-yl)carbonyl]-2-(phenylmethyl-
S oxy)ethyl]-2-amino-2-methyl-propanamide which has the structure:
CH3
H H \ CH3
~0--~ ~ ~< NH2
~3
CH3SO2 -
and salts thereof, in particular, the methanesulfonate salt.
The instant process provides the desired compound from
readily available inexpensive and environmentally acceptable starting
10 materials reagents and solvents. The process does not require the use any
chromatographic purifications, and it is possible to produce the fimal
product from the intermediate spiroindoline sulfonamide without
isolation of any of the intermediates.
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
The individual processes within ~e general process are
sllmm~rized as follows:
SCHEME I
H ,L L
~N~ ProtectwithAmino ~ ~ Prepare ~N~ Reductionof
P~ule~ g Group ~ Acid Chloride ~ Acid Chloride
COOH COOH COCI
L, IL Fisher Indole ,L
~N~ React with ~ N~ Synthesis rN\ Reduction
in Presence of
PhNHNH2 ~ Catalyst
CHOCH=NNHPh ~N
Mesylation ~ Removal of Amino
of Amine F,ule.;li,,g Group N
HSO2Me SO2Me
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
SCHEME I (CONT'D)
H H
,~N--L
N Ph~O~ 2 N~)=O
H ~ > Removal of Amino
~N Peptide CouplingW_N P~u~ lg Group
SO2Me S 02Me
Ph~O~NH2 ~Ç= c H3
N ~ HO CX O O H
2 H,~,, $~ Removal of Amino
~N Peptide Coupling ~ Plult:cli,)g Group
s 02M e S 02M e
H H H3C CH3 H H H3C CH3
Ph ~~~ < NH2 Ph~O~ ~~ NH3+ ~ X~
~=0 0 ~=0 0
r~""c.~i~". of Salt ~
s 02M e so2M e
(wherein L is an appropriate amino protecting group and X~
is an ~ iate counterion).
,
CA 02235511 1998-04-22
WO 97/lSS73 PCT/US96/16954
Within this general process, a first process concerns the
preparation of a compound of formula I:
H H _ L
SO2M e
I
5 wherein L is an amino protecting group, by coupling an amino acid of the
forrnula:
Ph~O~C02H
H--L
10 with a compound of the formula:
H
SO2M e
in the presence of an acid activating agent in an inert solvent in the
presence of a catalytic agent, to give the compound of formula I.
CA 0223~11 1998-04-22
WO 97/1~;573 PCT/US96/16954
Acid activating agents suitable for this process include:
DCC, EDC, ECAC and BOP, in which the preferred acid activating agent
is DCC (N,N'-dicyclohexylcarbodiimide).
Catalytic agents suitable for this process include: HOBT and
S the like in which a preferred catalytic agent is HOBT
(hydroxybenzotriazole) .
Inert solvents appropriate for this processes include:
acetonitrile; iso-propyl acetate; ethyl acetate; propionitrile; water;
chlorinated hydrocarbons such as dichloromethane, chloroforrn, carbon
tetrachloride, dichloroethane, chlorobenzene, ortho-dichlorobenzene;
benzene; toluene; xylenes; and the like; and mixtures thereof, in which
the preferred solvent is either acetonitrile or isopropyl acetate and water.
The preferred reaction temperature range is between -40 and
150~C, and the most plefelled range is between 20 and 35~C.
Suitable arnino protecting groups include: benzyl,
benzyloxymethyl, benzyloxycarbonyl (carbobenzyloxy), benzylsulfonyl,
2-bromo-ethyloxycarbonyl, t-butoxy-carbonyl, 2-chloro-benzyloxy-
carbonyl, 2-chloroethyloxycarbonyl, di-t-amyloxycarbonyl, 9-fluoroenyl-
methyloxycarbonyl, isopropoxycarbonyl, 4-1nethoxy-benzyloxycarbonyl,
4-nitrobenzyloxycarbonyl, 2-nitrophenyl-sulfonyl, ph~aloyl, 2,2,2-
trichloro-t-butyloxycarbonyl, trifluoroacetyl, triphenylmethane,
allyloxycarbonyl, and vinyloxycarbonyl groups, and the like, in which the
preferred ones include benzyloxycarbonyl (carbobenzyloxy), t-butoxy-
carbonyl groups, and in which the most preferred one is the t-butoxy-
carbonyl group.
In the interest of efficiency, it is preferred that this coupling
be conducted in situ without isolation of the compound of forrnula I
following its preparation by the aforementioned process.
CA 0223~11 1998-04-22
W O 97/15573 PCT~US96/16954
Within this general process, a second process concerns the
preparation of a compound of formula II:
Ph~ o~N H2
~N
~>
SO2M e
II
5 which comprises reacting a compound of the formula I:
H H
Ph~O~
~=0
S 02M e
I
wherein L is an amino protecting group, with an amino
deprotecting agent to give the compound of formula II.
Suitable amino protecting groups include: benzyl,
benzyloxymethyl, benzyloxycarbonyl (carbobenzyloxy), benzylsulfonyl,
2-bromo-ethyloxycarbonyl, t-butoxy-carbonyl, 2-chloro-benzyloxy-
carbonyl, 2-chloroethyloxycarbonyl, di-t-amyloxycarbonyl, 9-:fluoroenyl-
methyloxycarbonyl, isopropoxycarbonyl, 4-methoxy-benzyloxycarbonyl,
4-nitrobenzyloxycarbonyl, 2-nitrophenyl-sulfonyl, phthaloyl, 2,2,2-
trichloro-t-butyloxycarbonyl, trifluoroacetyl, triphenylmethane,
allyloxycarbonyl, and vinyloxycarbonyl groups, and the like, in which the
preferred ones include benzyloxycarbonyl (carbobenzyloxy), t-butoxy-
CA 0223~11 1998-04-22
WO 97/lSS73 PCT/US96/16954
- 10-
carbonyl groups, and in which the most preferred one is the t-butoxy-
carbonyl group.
In this process, the removal of the amino protecting group
may be accomplished by use of an appropriate catalytic agent. Removal
S of a t-butoxycarbonyl protecting group may be carried out in a solvent
such as methanol, ethanol, methylene chloride, ethyl acetate, or iso-
propyl acetate, with a strong acid. Such strong acids include
methanesulfonic acid, trifluoroacetic acid, hydrochloric acid, hydrogen
chloride gas, hydrogen bromide; hydrogen iodide; trifluoromethane-
sulfonic acid; camphorsulfonic acid; sulfuric acid; phosphoric acid; and
an arylsulfonic acid, such as benzenesulfonic acid, p-toluenesulfonic acid,
and p-chlorobenzene-sulfonic acid. Preferred catalytic agents include:
trifluoroacetic acid; methanesulfonic acid; camphorsulfonic acid;
benzenesulfonic acid, p-toluenesulfonic acid; and p-chlorobenzene-
sulfonic acid. The most preferred catalytic agent is methanesulfonic acid.
The preferred solvent is methanol or ethanol, and the most preferred
solvent is ethanol.
The preferred reaction temperature range is between -40 and
1~0~C, and the most preferred range is between 10 and 40~C.
Removal of a benzyloxycarbonyl (carbobenzyloxy) group
may be achieved by a number of methods, for exa~nple, catalytic
hydrogenation with hydrogen in the presence of a noble metal or its oxide
such as palladium on activated carbon in a protic solvent such as ethanol.
In cases where catalytic hydrogenation is contraindicated by the presence
of other potentially reactive functionality, the removal of
benzyloxycarbonyl (carbobenzyloxy) group may also be achieved by
treatment with a solution of hydrogen bromide in acetic acid, or by
treatment with a ~ixture of TFA and dimethylsulfide.
In the interest of efficiency, it is preferred that this acid-
catalyzed deprotection be conducted in situ without isolation of the
compound of formula II following its preparation by the aforementioned
process.
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
Within this general process, a third process concerns the
preparation of a compound of formula III:
H H H3C CH3
Ph ~~ b~ NH--L
SO2M e
III
S wherein L is an amino protecting group, by coupling an amino acid of the
formula:
X
HO2C H - L
wherein L is an amino protecting group, with a compound of the
formula II:
~~
N
S02M e
II
in the presence of an acid activating agent in an inert solvent in the
presence of a catalytic agent, to give the compound of formula III.
Acid activating agents suitable for this process include:
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 12-
DCC, EDC, ECAC and BOP, in which the preferred acid activating agent
is DCC (N,N'-dicyclohexylcarbodiimide).
Catalytic agents suitable for this process include: HOBT and
the like in which a preferred catalytic agent is HOBT
5 (hydroxybenzotriazole).
Inert solvents appropriate for this processes include:
acetonitrile; isopropyl acetate; ethyl acetate; propionitrile; water;
chlorinated hydrocarbons such as dichloromethane, chloroform, carbon
tetrachloride, dichloroethane, chlorobenzene, ortho-dichlorobenzene;
10 benzene; toluene; xylenes; and the like; and mixtures thereof, in which
the preferred solvent is a mixture of iso-propyl acetate and water,
~rerel~bly in a ratio of approximately 40:60 to 60:40 (by volume) and
more preferably in a ratio of approximately 50:50 (by volume).
The preferred reaction temperature range is between -40 and
150~C, and the most preferred range is between 20 and 50~C.
Suitable amino protecting groups include: benzyl,
benzyloxymethyl, benzyloxycarbonyl (carbobenzyloxy), benzylsulfonyl,
2-bromo-ethyloxycarbonyl, t-butoxy-carbonyl, 2-chloro-benzyloxy-
carbonyl, 2-chloroethyloxycarbonyl, di-t-amyloxycarbonyl, 9-fluoroenyl-
20 methyloxycarbonyl, isopropoxycarbonyl, 4-methoxy-benzyloxycarbonyl,
4-nitrobenzyloxycarbonyl, 2-nitrophenyl-sulfonyl, phthaloyl, 2,2,2-
trichloro-t-butyloxycarbonyl, trifluoroacetyl, triphenylmeth~n~,
allyloxycarbonyl, and vinyloxycarbonyl groups, and the like, in which the
preferred ones include benzyloxycarbonyl (carbobenzyloxy), t-butoxy-
25 carbonyl groups, and in which the most preferred one is the t-butoxy-
carbonyl group.
In the interest of efficiency, it is preferred that this coupling
be conducted in situ without isolation of the compound of formula III
following its preparation by the aforementioned process. Alternatively,
30 the compound of formula III may be isolated as a discrete intermediate.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 13-
, Within this general process, a fourth process concerns the
preparation of a compound of formula IV, or a ph~ ceutically
acceptable salt thereof:
H H H3C~CH3
Ph 0~ --~ N H2
~CO O
SO2M e
IV
which comprises reacting a compound of the formula III:
~ ~HN~<CNH3 L
~>
SO2M e
III
wherein L is an amino protecting group, with an amino
10 deprotecting agent to give the compound of formula IV.
Suitable amino protecting groups include: benzyl,
benzyloxymethyl, benzyloxycarbonyl (carbobenzyloxy), benzylsulfonyl,
2-bromo-ethyloxycarbonyl, t-butoxy-carbonyl, 2-chloro-benzyloxy-
carbonyl, 2-chloroethyloxycarbonyl, di-t-amyloxycarbonyl, 9-fluoroenyl-
15 methyloxycarbonyl, isopropoxycarbonyl, 4-methoxy-benzyloxycarbonyl,
4-nitrobenzyloxycarbonyl, 2-nitrophenyl-sulfonyl, phthaloyl, 2,2,2-
trichloro-t-butyloxycarbonyl, trifluoroacetyl, triphenylmethane,
CA 0223~511 1998-04-22
W O 97/15573 PCT~US96/16954
- 14 -
allyloxycarbonyl, and vinyloxycarbonyl groups, and the like, in which the
preferred ones include benzyloxycarbonyl (carbobenzyloxy), t-butoxy-
carbonyl groups, and in which the most preferred one is the t-butoxy- -
carbonyl group.
In this process, the removal of the amino protecting group
may be accomplished by use of an a~lol)liate catalytic agent. Removal
of a t-butoxycarbonyl protecting group may be carried out in a solvent
such as methanol, ethanol, methylene chloride, ethyl acetate, or iso-
propyl acetate, with a strong acid. Such strong acids include
10 methanesulfonic acid, trifluoroacetic acid, hydrochloric acid, hydrogen
chloride gas, hydrogen bromide; hydrogen iodide; trifluoromethane-
sulfonic acid; camphorsulfonic acid; sulfuric acid; phosphoric acid; and
an arylsulfonic acid, such as benzenesulfonic acid, p-toluenesulfonic acid,
and p-chlorobenzene-sulfonic acid. Preferred catalytic agents include:
15 trifluoroacetic acid; methanesulfonic acid; camphorsulfonic acid;
benzenesulfonic acid, p-toluenesulfonic acid; and p-chlorobenzene-
sulfonic acid. The most preferred catalyhc agent is methanesulfonic acid.
It is preferred that compound of formula V is isolated in the form of the
methanesulfonate salt. The preferred solvent is methanol or ethanol, and
20 the most preferred solvent is ethanol.
The preferred reaction temperature range is between -40 and
150~C, and the most preferred range is between 10 and 40~C.
Removal of a benzyloxycarbonyl (carbobenzyloxy) group
may be achieved by a number of methods, for example, catalytic
25 hydrogenation with hydrogen in the presence of a noble metal or its oxide
such as palladium on activated carbon in a protic solvent such as ethanol.
In cases where catalytic hydrogenation is contraindicated by the presence
of other potentially reactive functionality, the removal of
benzyloxycarbonyl (carbobenzyloxy) group may also be achieved by
30 treatment with a solution of hydrogen bromide in acetic acid, or by
treahnent with a rnixture of TFA and dimethylsulfide.
In the interest of efficiency, it is preferred that this acid-
catalyzed deprotection be conducted in situ without isolation of the
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 15 -
compound of formula IV following its preparation by the aforementioned
process.
Within this general process, a fifth process concerns the
preparation of a pharmaceutically acceptable salt of a compound of
5 formula IV, in particular, the methanesulfonate salt, i.e. a compound of
formula V:
H H H3C CH3
Ph 0~ --~NH2- CH3S03H
,~0 0
SO2M e
V
which comprises reacting a compound of the formula IV:
H HN ~<CNHH2
O O
SO2Me
IV
with an acid, preferably methanesulfonic acid, to give the compound of
formula V.
It is preferred that compound of forrnula V is isolated in the
15 form of the methanesulfonate salt. The preferred solvent comprises ethyl
acetate and ethanol, and the most preferrred solvent is a mixture of ethyl
~cet~te and ethanol.
CA 022355ll l998-04-22
WO 97/1 5S73 PCT/US96/16954
- 16-
In the interest of ef~lciency, it is preferred that the formation
of the salt be conducted in situ without isolation of the compound of
formula V following its preparation by the ai~orementioned process.
In a preferred embodiment of ~e present invention, ~e
individual processes within the general process are outlined as follows:
SCHEME II:
Oq~OBn Oq,OBn
~N~ BnOCOCI, ~ ~ 1.04 eq. (COC1)2~ ~ Pd/C, H2
K2CO3, H2O ~ cat. DMF ~ DIEA
COOH COOH COCI
2 3 4
Oq,OBn Oq,OBn ~OBn
Pl.~HNI 12 ~ TFA ~ NaBH4/MeOH
CHO CH=NNHPh N
~OBn ~OBn H
r N~ r N 1 ) Activated r N~
MsCI C > carbon
~ DIEA ~ 2)10% Pd/~
SO2Me SO2Me
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
SCHEME II (CONT'D)
Ph~O~NH BOC
O
PhO~CO2H r N
/ 10 NHBOC ,~ 1) MsOH, EtOH
~N HOBT ~? 2) basicworl<up
S02Me iPrOAc:H20 S02Me
lb 11
Ph~O~NH2 --~ c H3
~ H3CXCH3 0 0
HO2C NHBOC ~ ~
/ 13 ~/ 1) MsOH, EtOH
DCC l ~ 2) basic workup
--N HOBT ~--N
iPrOAc-H20
S O2M e SO2M e
12 14
H H H3C CH3 H H H3C CH3
Ph~O~ b~<NH2 Ph~o~N~<NH2- MsOH
~=00 ~=0 0
~N ~N
MsOH
SO2M e S O2M e
16
CA 0223~11 1998-04-22
WID 97/15573 PCT/US96/16954
- 18 -
In this preferred embodiment, the CBZ-Spiroindoline 1
is treated with Darco (20% by weight) prior to hydrogenation. The
hydrogenation is carried out in ethanol at 65~C over 10% Pd/C with
vigorous stirring.
A solution of lb in isopropyl acetate and water is coupled
with commercially available N-BOC-O-benzyl-D-serine in the presence
of dicyclohexylcarbodiimide (DCC) and 1-hydroxybenzotriazole (HOBt).
After filtration of the dicyclohexylurea (DCU) side product, the 2-phase
filtrate is separated and the organic layer is washed successively with lM
aqueous sodium hydroxide solution, O.5M aqueous hydrochloric acid and
finally saturated aqueous sodium hydrogen carbonate. Improved results
in this coupling are achieved when a solution of the free amino in
iPrOAc/H20 is treated with DCC, HOBT followed by addition of ~e
amino acid at ambient temperature and followed by reaction for 3-5 hrs
The batch is then concentrated in vacuo and the solvent is switched from
isopropyl acetate to ethanol. This solvent switch generally proceeds
swiftly by "feeding and bleeding" 3x batch volumes to remove isopropyl
~et~te
The BOC-group of 11 is removed by treatment with
methanesulfonic acid (MsOH) (3 eq) in ethanol at 35-40~C. Partitioning
between isopropyl acetate and aqueous lM sodium hydroxide solution
affords 12.
The coupling of 12 with N-BOC-oc-aminoisobutyric acid is
best conducted in a two-phase solvent system, isopropyl ~cet~te/water
(1:1) in the presence of DCC and HOBt (l.l eq. each). Removal of the
DCU by filtration, separation of the layers and washing the organic layer
successively with lM aqueous sodium hydroxide, 0.5M aqueous
hydrochloric acid and saturated aqueous sodium hydrogen carbonate
affords 14.
The mixture is solvent switched to ethanol for the
subsequent methanesulfonic acid cleavage of the Boc group.
Deprotection of 14 is more difficult than that of 11 and requires a
concentrated solution of ethanol/methanesulfonic acid and h~ting to 35-
40~C. After extractive workup (EtOAc-NaOH), the free amine 15 is
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 19-
isolated. The organic layer is washed well with lN NaOH to ensure
complete removal of methanesulfonic acid.
The ethyl acetate solution of the free base lS is concentrated
to low bulk in vacuo and is azeotroped dry (KF <500 mgml~l) by
S "fee.-ling and bleeding" 2x batch volumes of 90/10, ethyl acetate/ethanol
followed by 2x batch volumes of ethyl acetate. The resulting dry, slightly
hazy solution of the free base lS in ethyl acetate is treated with Darco
G-60 (25 weight %) at room temperature for about 10 hours. Removal of
the Darco by filtration with a filtration agent gives the free base lS.
Forrnation of the methanesulfonic acid salt 16 from 15 is
carried out in EtOAc with 1.1 eq of MsOH at about 50~C. The free base
lS is treated with 8 volume % of EtOH and 1 eq of H2O and heated to
55~C until complete dissolution. Cooling to ambient temperature and
stirring the resulting slurry for 4 hours gives crystalline material of 16
15 desi~n~e-1 as crystal Form II [solubility in IPA = 12 mg/mL].
The conversion of Form II to Form I is accomplished where
the salt is formed in EtOAc-EtOH as above, but instead of cooling the
initial solution of the salt (at 55~C) to ambient temperature, it is cooled to
45~C. Crystals should start appearing at that temperature and the slurry
20 should become thicker with time. The tempelatul~ is then raised to S l ~C
and the slurry is aged overnight. Complete conversion to Form I of 16
should be expected.
Preferably, the conversion of Form II to Form I is achieved
by ~ lin~ seed crystals of Form I to a solution of the free base in EtOAc-
25 EtOH at 50-55~C followed by aging. Accordingly, the free base lS may
be treated with 1.1 equivs. of methanesulfonic acid in 8% ethanol in ethyl
acetate at 50-55~C. The batch is then seeded with approximately 2% by
weight of Form I of the methanesulfonate salt 16, and then aged at 55~C
overnight. The batch is cooled to room temperature and aged for
30 approximately 2-3 hours. The product is isolated by filtration at room
temperature under a nitrogen atmosphere, dried at 35~C in vacuo and
sieved to give the methanesulfonate salt 16.
The methanesulfonic acid salt 16 may also be formed by
alternating the stepwise addition of MsOH (1.1 eq) and seed crystals of
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 20 -
Form I to a solution of the free base in EtOAc-EtOH at about 50~C,
wherein the order of addition of the MsOH and the seed is not critical.
Throughout the instant application, the following
abbreviations are used with the following meanings:
S Bu butyl
Bn benzyl
BOC, Boc t-butyloxycarbonyl
BOP Benzotriazol-l-yloxy tris(dimethyla~ino)-
phosphonium hexafluorophosphate
calc. calculated
CBZ, Cbz Benzyloxycarbonyl
DCC N,N'-Dicyclohexylcarbodiimide
DIEA Di-isopropylethyl~n~ine
DMF N,N-dimethylformamide
DMAP 4-Dimethylaminopyridine
EDC 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride
EDAC Ethyl-3-(3-dimethylamino)-propylcarbodiimide
EI-MS Electron ion-mass spectroscopy
Et ethyl
eq. equivalent(s)
FAB-MS Fast atom bombardment-mass spectroscopy
h, hr. hours
HOBT, HOBt Hydroxybenzotriazole
HPLC High pressure liquid chromatography
iPrOAc iso-Propyl acetate
KHMDS Potassium bis(trimethylsilyl)a~ide
LAH Lithium aluminum hydride
LHMDS Lithium bis(trimethylsilyl)amide
Me methyl
MF Molecular formula
MHz Megahertz
MPLC Medium pressure liquid chromatography
MsOH Methane sulfonic acid
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
NMM N-Methylmorpholine
NMR Nuclear Magnetic Resonance
Ph p~enyl
Pr propyl
prep. prepared
TFA Trifluoroacetic acid
THF Tetrahydrofuran
TLC Thin layer chromatography
TMS Tetramethylsilane
In the above structural formula and throughout the instant
specification, the following terms have the indicated me~nin~:
The phrase "peptide coupling reaction" as used herein is
intended to mean the coupling of a carboxylic acid with an amine using
an acid activating agent such as EDC, DCC, and BOP in an inert solvent
in the presence of a catalyst such as HOBT. Inert solvents a~lo~liate for
such couplings include: acetonitrile; iso-propyl acetate; ethyl acetate;
propionitrile; water; chlorinated hydrocarbons such as dichloromethane,
chloroform, carbon tetrachloride, dichloroethane, chlorobenzene, ortho-
dichlorobenzene; benzene; toluene; xylenes; and combinations thereof;
and the like.
The variable "L" and the term "amino protecting group" is
intended to indicate the presence of an appropriate protecting group for
amino, such as those described in Greene, T.W., Wuts, P.G.M. Protective
Groups in Organic Synthesis, 2nd ed., John Wiley & Sons, Inc., New
York, 1991. An ~lo~liate protecting group will be able to withstand
the reaction conditions of intermediate processes, prior to being removed
when desired. The amino protecting group is independently selected for
each process within the entire processes.
Suitable amino protecting groups include: benzyl,
benzyloxymethyl, benzyloxycarbonyl (carbobenzyloxy), benzylsulfonyl,
2-bromo-ethyloxycarbonyl, t-butoxy-carbonyl, 2-chloro-benzyloxy-
carbonyl, 2-chloroethyloxycarbonYl, di-t-amyloxycarbonyl, 9-fluoroenyl-
methyloxycarbonyl, isopropoxycarbonyl, 4-methoxy-benzyloxycarbonyl,
4-nitrobenzyloxycarbonyl, 2-nitrophenyl-sulfonyl, phthaloyl, 2,2,2-
CA 0223~511 1998-04-22
WO 97/lS573 PCTAJS96/16954
trichloro-t-butyloxycarbonyl, trifluoroacetyl, triphenylmeth~ne, and
vinyloxycarbonyl groups, and the like, in which the preferred ones
include benzyloxycarbonyl (carbobenzyloxy), t-butoxy-carbonyl groups,
and in which the most preferred one is the t-butoxy-carbonyl group.
The removal of the amino protecting group rnay be
accomplished by use of an appropriate catalytic agent. Removal of a t-
butoxycarbonyl protecting group may be carried out in a solvent such as
methanol, ethanol, methylene chloride, ethyl acetate, or iso-propyl
acetate, with a strong acid. Such strong acids include methanesulfonic
acid, trifluoroacetic acid, hydrochloric acid, hydrogen chloride gas,
hydrogen bromide; hydrogen iodide; trifluoromethane-sulfonic acid;
camphorsulfonic acid; sulfuric acid; phosphoric acid; and arylsulfonic
acids, such as benzenesulfonic acid, p-toluenesulfonic acid, and p-
chlorobenzene-sulfonic acid. Preferred catalytic agents include:
trifluoroacetic acid; methanesulfonic acid; camphorsulfonic acid;
benzenesulfonic acid, p-toluenesulfonic acid; and p-chlorobenzene-
sulfonic acid. The most preferred catalytic agent is metharlesulfonic acid.
The preferred solvent is methanol or ethanol.
Removal of a benzyloxycarbonyl (carbobenzyloxy)
protecting group may be achieved by a number of methods, for example,
catalytic hydrogenation with hydrogen in the presence of a noble metal or
its oxide such as palladium on activated carbon in a protic solvent such as
ethanol. In cases where catalytic hydrogenation is co~ indicated by the
presence of other potentially reactive functionality, the removal of
benzyloxycarbonyl (carbobenzyloxy) group may also be achieved by
treatment with a solution of hydrogen bromide in acetic acid, or by
treatment with a mixture of TFA and dimethylsulfide.
The amine compounds employed as starting materials for the
process of the present invention may be present as their acid salts, such as
the salts derived from using inorganic and organic acids. Examples of
such acids are hydrochloric, nitric, sulfuric, phosphoric, formic, acetic,
trifluoroacetic, propionic, maleic, succinic, malonic, methane sulfonic
and the like. Similarly the compounds produced by the processes of the
in~t~nt invention may be isolated in the form of their ph~ ceutically
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/169~;4
acceptable acid salts. In addition, certain compounds cont~inin~ an acidic
function such as a carboxy can be in the form of their inorganic salt in
which the counterion can be selected from sodium, potassium, lithium,
calcium, magnesium and the like, as well as from organic bases.
The preparation of compounds with the process of the
present invention may be carried out in sequential or convergent synthetic
routes. It is noted that in some cases the order of carrying out the
foregoing reaction schemes may be varied to facilitate the reaction or to
avoid unwanted reaction products. In general, the process of the present
invention is conducted in a sequential manner as presented herein.
Many of the starting materials are either commercially
available or known in the literature and others can be prepared following
literature methods described for analogous compounds. The skills
required in carrying out the reaction and purification of the resulting
reaction products are known to those in the art. Purification procedures
include cry~t~lli7~tion, normal phase or reverse phase chromatography.
The following e~amples are provided for the purpose of
further illustration only and are not intended to be limitations on the
disclosed invention.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 24 -
EXAMPLE 1
O~,OBn
CO2H
Isonipecotic acid-N-benzyl carbamate (3)
5 Materials:
Isonipecotic acid (2) T.C.I. 4.02 kg (31.1 mol)
Benzyl chloroformate (Schweitzerhall) 6.91 kg (40.5 mol)
K2CO3 10.1 kg (72.9 mol)
Water 40.2 L
Isonipecotic acid (2) and K2co3 were dissolved in 40.2 L of
water in a 100 L 4 neck flask with mechanical stirring under N2 and the
solution was cooled to 10~C. Benzyl chloroformate was added,
m~int~ininp the temperature between 9 and 14~C, and the mixture was
warmed up to 22~C after the addition was complete and aged for 58 h.
The addition was completed in 4 h at which point the pH was 9Ø After
aging for 58 h there was no change in the pH.
The reaction mixture was transferred to a 200 L extractor
and washed with 3 x 13 kg ( 15 L) of IPAC and 1 x 12 L of EtOAc. The
aqueous layer was extracted with 8 L of toluene. After the washes the
benzyl alcohol content was reduced from 3.8% to 1.4% by HPLC
analysis. HPLC analytical: Dupont Zorbax 25 cm RXC8 column with
1.5 mL/min flow and detection at 254 nm; isocratic mixture with 35%
M[eCN, 65% of 0.1% aqueous H3PO4; retention times: 3 = 6.9 min,
benzyl alcohol = 3.3 min, toluene = 17.3 min.
The aqueous phase was acidified with 37% aqueous HCl to
pH 1.8. Carbon dioxide was evolved during the addition of HCl, but gas
evolution was easily controlled. The addition of HCl took <1 h and
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
required 10 L of conc. HCl. The aqueous phase was extracted with 3 x
6.6 L of toluene. The toluene extracts were dried with 2 kg of sodium
sulfate and filtered through a pad of Solka-flocTM. The combined filtrates
weighed 17.8 kg. The crude yield of carbamate 3 was 7.89 kg (97%) (as
S obtained by evaporation of weighed aliquots of the filtrates to dryness).
The filtrates were transferred through a 10 ,u inline filter to a 100 L flask.
The extracts were concentrated at 10 mbar at <25~C to a volume of 18 L.
The final concentration of carbamate 3 was 440 g/L. The concentration
of the toluene filtrate served to azeotropically remove final traces of water
(final KF = 170mg/L). The product was 99.1 area % pure with 0.9 area
% benzyl alcohol as the only impurity.
EXAMPLE 2
O~,OBn
[~ 4
COCI
15 Isonipecotic acid chloride-N-benzyl carbamate (4)
Materials:
Isonipecotic acid N-benzyl carbamate (3) 7.89 kg (30.0 mol) in
in toluene. (MW = 263.30) 17.9 L
Oxalyl chloride (MW = 126.93) 3.94 kg (31.0 mol)
DMF (MW = 73.10) 10 mL
Toluene 12 L
To the toluene solution of benzyl carbamate 3 from the
25 preceding step was added 5 mL of DMF and 10 L of toluene. The oxalyl
chloride was added over a period of 20 min. The reaction mixture was
aged for 16 h at 18~C under a slow stream of nitrogen. HPLC analysis of
the reaction mixture showed that 1.3% of the carboxylic acid 3 still
CA 02235511 1998-04-22
WO 97/15~;73 PCT/US96/16954
- 26 -
remained unreacted. The reaction mixture was warmed to 26~C, and 5
mL of DMF were added. The mixture was aged for 2.5 h.
A 1.0 mL aliquot of the reaction mixture was quenched with
5.0 mL of tert-butylamine and analyzed after evaporation by HPLC: 25
5 cm Dupont Zorbax RXC8 column at 50~C with 1 mL/min flow and
detection at 220 nm; isocratic 42% MeCN, 58% of 0.1% aqueous H3PO4.
This method showed that <0.05% of the acid 3 remained (as judged by A)
alld showed >3 area % B (> 1 mol% (COCl)2).
Oq,OBn
N~ Oq, N H-t-Bu
0~ N H-t-Bu
CONH-t-Bu
A B
The mixture was concentrated at 10 mbar and a temperature
of 20-25~C until 5 L of solvent had been removed.
The typical HPLC profile of concentrated toluene solution
after t-BuNH2 quench described above is as follows:
l~etention time (min) Area % Identity
2.1 <0.5% carboxylic acid3
7.8 <0.5% benzyl chloride
11.0 >99% Cbz-t-butylcarboxamide A
12.1 NA toluene
12.7 <0.5% ditert-butyloxamide B
CA 0223~11 1998-04-22
WO 97/t5573 PCT/US96/16954
EXAMPLE 3
O~,OBn
~N~
J 5
CHO
Piperidine-4-carboxaldehyde-1-benzyl carbamate (5)
Materials:
Isonipecotic acid chloride N-benzyl carbamate (4) 3.38 kg (12.0 mol)
in toluene (MW = 281.74) in 5.54 kg
DIEA (KF = 18 mg/L) 1.55 kg (15.0 mol)
10% Pd/C (KF < 20 mg/g) 101 g
thioanisole (MW = 124.21, d = 1.058) 0.56 g
The DIEA and thioanisole were added to the solution of (4)
in toluene from the previous step and the catalyst was suspended in this
mixture. The mixture was immediately placed into the 5 gal autoclave
and hydrogenated at 20~C and 40 psi of H2. After 18 h the reaction had
taken up 70% the theoretical amount of hydrogen and HPLC analysis of
an aliquot that was quenched with tert-butylamine indicated that 14.2 area
% of acid chloride 2 remained. HPLC conditions same as above.
Retention time: 5 = 8.1 min.
A second charge of catalyst (101 g) and thioanisole (0.54 g)
were added as a slurry in 1375 mL toluene to the hydrogenator. After 23
h HPLC analysis of an aliquot that was quenched with tert-butyl:~mine
indicated that 1.8 area % of acid chloride 2 remained. The rnixture was
purged with nitrogen and the catalyst and precipitated DIEA-HCl were
removed by filtration through Solka-flocTM. The filter cake was washed
with 10 L of toluene. The filtrates were transferred through a 10 ,u inline
filter to a 50 L extractor and washed with 2 x 7.2 L of 1 M aqueous HCl
and 2 x 7.2 L of water. The mixture was concentrated at 10 mbar and a
temperature of 25-30~C until 5 L of residue remained.
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
- 28 -
~etention time (min) Area % Identity
2.1 <2 carboxylic acid3
6.6 <1 dimer 21
8.1 >95 aldehyde 5
The assay yield of aldehyde 3 was 94% by HPLC analysis.
EXAMPLE 4
0~, OBn
~N~
~,
~N~
CBZ-Spiroindoline (9)
Materials:
Piperidine~-carboxaldehyde-1-benzyl 1.71 kg (6.89 mol)
carbamate (5) in toluene solution in 21.4 kg
Phenylhydrazine 900 mL, 981 g (9.15 mol)
Trifluoroacetic acid (TFA) 2.20 L, 3.26 kg (28.6 mol)
NaBH4 300 g, (7.93 mol)
Toluene 34.4 kg
MeCN 7.0 L
MeOH 7.0 L
The crude aldehyde 5 solution from the previous step was
transferred through a 10 ,u inline ~llter to a 100 L reactor equipped with
25 Teflon coated copper coils for cooling or he~ting and a mech~qnic~l stirrer.
Toluene (34.4 kg) and MeCN (7 L) were added, and the resulting solution
was cooled to 0~C. Phenylhydrazine was added in portions and the
CA 0223~ll l998-04-22
WO 97/lS573 PCT~S96/16954
- 29 -
temperature was maintained at -1 to 3~C while nitrogen was continuously
bubbled through the reaction mixture.
The phenylhydrazine was added until TLC and HPLC
analysis indicated complete consumption of the aldehyde 5 and the
5 appearance of a slight excess (<5%) of phenylhydrazine. TLC
conditions: Silica, E. Merck Kieselgel G60 F254 0.25 IIlIIl; diethyl
ether/pentane (4/1); and developing agent 0.5% ceric sulfate, 14%
ammonium molybdate in 10% aqueous sulfuric acid then heat; Rf:
aldehyde 5 = 0.52, phenylhydrazone 7 = 0.61, phenylhydrazine 6 = 0.21.
10 HPLC conditions: 25 cm Dupont Zorbax RXC8 column at 30~C with 1.0
mL/min flow and detection at 254 nm; gradient schedule:
Time (min) acetonitrile:water
0 57:43
15 10 65:35
75:25
18 75:25
retention times: phenylhydrazine 6 = 4.5 min, toluene = 7.2 min,
phenylhydrazone 7 = 11.4 min.
The reaction mixture was aged for 30 min at 0-2~C, and TFA
was added m~int~ining the temperature between 2 and 7~C. The reaction
mixture was warmed to 50~C over 30 min, and m~int~ined for 17 h. The
nitrogen sparge through the reaction mixture was stopped and a slow
stream of nitrogen was m~int~in~d over the reaction mixture. During the
first hour at 5~C the color gradually darkened to a deep green, and a
relatively small amount of a white crystalline precipitate (ammonium
trifluoroacetate) formed. After 17 h HPLC analysis (same conditions as
above) indicated that the reaction mixture contained 91.6 area %
indolenine 8 and 1.5% of unreacted phenylhydrazone remained. Aging
~ 30 the mixture for longer periods of time did not increase the assay yield of
indolenine 8.
The reaction mixture was cooled to 12~C, and 7.0 L of
MeOH was added. NaBH4 was added in small (<20 g) portions
m~int~ining the temperature below 15~C. The addition took 30 min.
CA 0223~11 1998-04-22
WO 97/15S73 PCT/US96/16954
- 30-
Moderate hydrogen evolution was observed during the addition, but it
was easily controlled and there was virtually no frothing. Near the end of
the addition the color rapidly changed from green to brown and then
bright orange. A small amount (<200 mL) of a heavier phase had
S separated (presumably aqueous salts). HPLC analysis (conditions as
before) indicated that all of the indolenine 8 had been consumed (90.4
area % CBZ-indoline 9); retention times: indolenine 8 = 7.5 min, indoline
9 = 8.2 min. TLC: ethyl ether as solvent, ceric sulfate-ammonium
molybdate stain or 1% anisaldehyde stain; retention factors: indolenine 8
= 0.18, CBZ-indoline 9 = 0.33.
The color change from green to orange corresponds very
closely to reaction end point. The quantity of NaBH4 required to
complete the reaction is heavily dependent on the temperature and rate of
addition of NaBH4, but the yield and quality of the product is virtually
lS unaffected provided that the reaction is complete. The reaction mixture
was cooled to 5~C over a period of 30 min. Then 8 L of 3% aqueous
NH40H (8 L) were added to bring the pH of the aqueous phase to 7.4,
the mixture was agitated, and allowed to settle. The temperature rose to
15~C. The cloudy yellow lower aqueous phase was separated. The
organic phase was washed with 4 L of 3% aqueous NH4OH, 2 x 4 L of
water, and 2 x 4 L of brine. The weight of the organic phase after the
washings was 53.5 kg, and the assay yield was 94%.
The washed toluene solution was combined with the washed
organic phases of two other .simil~rly processed reactions. ~he total
aldehyde used in the three reactions was 5.06 kg, (20.5 mol). The total
weight of CBZ-indoline 9 assayed in the combined organic phases was
5.91 kg, (18.3 mol, 90% assay yield). The combined organic phases were
dried with 5 kg of sodium sulfate, treated with 250 g of Darco G60
carbon for 30 min, and filtered through SoLka-flocTM. The filtrates were
vacuum concentrated at 10 mbar at <25~C until the residue was near
dryness. The solvent switch was completed by slowly bleeding in 30 L of
IPAC and reconcentrating to 14 L at 200 mbar at 50-60~C. The mixture
was heated to reflux in order to obtain a clear homogeneous deep orange
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 31 -
solution. lH NMR analysis indicated that the solution contained ca. 6
mol% of residual toluene after solvent switch.
- The solution was cooled to 68~C and seeded with 4 g of
crystalline CBZ-indoline 9. The solution was allowed to gradually cool
to 26~C over 6 h and aged for 9 h at 20-26~C. The slurry was cooled to
2~C over 1 h and aged at 2~C for lh. The product was isolated by
filtration, and the filter cake was washed with 2 x 2 L of 5~C IPAC and 2
x 2 L of 5~C MTBE. The product was dried in the vacuum oven at 30~C
under a nitrogen bleed to give 4.37 kg (74%) of the title compound 9 as a
light tan crystalline powder. HPLC analysis of the product indicated 99.5
area % purity. The mother liquor (11 L) and the washes contained 1.15
kg (19%) of additional product 9 and ca 3% of Cbz-isonipecotic acid
phenylhydrazide (retention time = 4.8 min).
EXAMPLE 5
O;~OBn
~N~
~'
SO2Me
CBZ-Spiroindoline-methanesulfonamide (1)
Materials:
CBZ-Spiroindoline (9) 1.69 kg (5.23 mol)
Methanesulfonyl chloride 599 g (5.23 mol)
Et3N (KF = 151) 635 g (6.27 mol )
THF (KF = 41) 12 L
A 22 L flask was charged with the solid CBZ-spiroindoline 9
and then 11.5 L of THF and the Et3N were transferred into the flask
through a 10 ,u inline filter. The resulting homogenous solution was
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
cooled to 0~C. A 1 L dropping funnel was charged with the
methanesulfonyl chloride and 500 mL of THF. The solution of the MsCl
in THF was added to the reaction mixture m~int~ining the temperature
between 0 and 4~C. The addition took S h and was exotherrnic. A white
S precipitate, presumably triethylamrnonium hydrochloride formed during
the addition. HPLC analysis indicated that the reaction was complete at
the end of the addition (9 was undetectable).
HPLC conditions: 25 cm Dupont Zorbax RXC8 column with
1.5 mL/min flow and detection at 254 nm. Gradient Schedule:
Time (min) 0.1% aq. H~P04:MeCN
0 70:30
3 70:30
12 20:80
25 20:80
Retention times: 9 = 7.6 min, 1 = 13.6 min.
After the addition was complete the reaction mixture was
warmed to 18~C and aged for 16 h. There was no change in the
appearance of the reaction mixture, and HPLC profile between the end of
the addition and after the 16 h age. The reaction mixture was slowly
transferred over lh into a vigorously stirred solution of 30 L of water and
200 mL of 37% aqueous HCl in a 50 L flask. The temperature in the 50
L flask rose from 22 to 28~C. The product separated as a pale tan gummy
solid which changed to a granular solid. The aqueous suspension was
cooled to 22~C and aged for I h. The suspension was filtered, and the
filter cake was washed with 2 x 4 L of MeOH/water (50/50). HPLC
analysis indicated that <0.1% of the CBZ-Spiroindoline-
methanesulfonamidel was in the mother liquors.
The filter cake was washed with 4 L of MeOH/water (50/50)
to which 50 mL of 28% aqueous NH40H had been added. The filter
cake was washed with 2 x 4 L of MeOH/water (50/50), and the solid was
dried in the vacuum oven at 50~C under a nitrogen bleed to give 2.03 kg
-
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 33 -
(97%) of the title product 1 as an off-white powder. HPLC analysis of
the solids indicated 93.7 area % 1.
EXAMPLE 6
O~OBn
~N~ 8
N
Optional Procedure for Isolation of Inter~nediate
CBZ-Spiroindolenine (8)
Materials:
Piperidine-4-carboxaldehyde-1-benzyl 12.37 g (0.050 mol)
carbamate (5)
Phenylhydrazine 5.41 g (0.050 mol)
Trifluoroacetic acid (TFA) 11.56 mL,17.10 g
(0.150 mol)
Methylene chloride 500 mL
The CBZ-aldehyde 5 was dissolved in dichloromethane in a
1 L flask equipped with Teflon coated magnetic stirring bar. The
resulting solution was cooled to 0~C. Phenylhydrazine was added via a
weighed syringe over 5 min and the temperature was m~int~in~d at -1 to
3~C while nitrogen was continuously bubbled through the reaction
mixture.
TLC and HPLC analysis indicated complete consumption of
the CBZ-aldehyde 5 and the appearance of a slight excess (<2%) of
phenylhydrazine. TLC conditions: Silica, L. Merck Kieselgel G60 F254
0.25 mm; diethyl ether/pentane (4/1); and developing agent 0.5% ceric
sulfate, 14% ammonium molybdate in 10% aqueous sulfuric acid then
heat; Rf: aldehyde 5 = 0.52, phenylhydrazone 7 = 0.61, phenylhydrazine
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 34 -
6 = 0.21. HPLC conditions: 25 cm Dupont Zorbax RXC8 column at
30~C with 1.0 mL/min flow and detection at 254 nm; gradient schedule:
Time (min) acetonitrile:water
0 57:43
10 65:35
lS 75:25
18 75:25
retention times: phenylhydrazine 6 = 4.5 min, toluene = 7.2 min,
phenylhydrazone 7 = 11.4 min.
The reaction mixture was aged for 10 min at 0-2~C, and TFA
was added by syringe maintaining the temperature between 2 and 7~C.
The reaction rnixture was warmed to 35~C over 30 min, and m~int~inç~l
for 17 h. The nitrogen sparge through the reaction mixture was stopped
and a slow stream of nitrogen was maintained over the reaction mixture.
During the first hour at 35~C the color gradually darkened to a rosy pink
~en to a deep green, and a relatively small amount of a white crystalline
precipitate (ammonium trifluoroacetate) formed. After aging for 17 h
HPLC analysis (same conditions as above) indicated that the reaction
mixture contained 93 area % indolenine 8 and <0.5% of unreacted
phenylhydrazone remained. Aging the mixture for longer periods of time
did not increase the assay yield of indolenine 8.
The reaction mixture was cooled to 10~C, and a mixture cont~inin~ 60
mT . 28-30% ammonium hydroxide, 90 mL water and 150 g crushed ice
was added with good stirring. The color of the rnixture changed to a
salmon color. The organic phase was separated and washed twice with
400 mL water then 100 mL saturated aqueous NaCl. The organic phase
was dried over magnesium sulfate and filtered through a plug of 5 g of
silica. The filtrate was evaporated to give 15.g4 g (99%) of indolenine 8
as a pale orange oil.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/169~4
EXAMPLE 7
O~OBn
~,
SO2Me
Procedure for the Preparation of CBZ-Spiroindoline-methanesulfon~ le
(1) without Isolation of Inte~nediate CBZ-Spiroindoline (9)
Step 1: CBZ-Spiroindoline (9)
Materials:
Piperidine-4-carboxaldehyde- 1-benzyl 49.5 g (0.20 mol)
carbamate (5)
Phenylhydrazine (Aldrich) 23.7 g (0.22 mol)
Trifluoroacetic acid (TFA) 75.4 g (0.66 mol)
Toluene (KF < 250 mg/L) 654 mL
MeCN (KF < 250 mg/L) 13.3 mL
NaBH4 11.3 g, (0.30 mol)
Toluene 20 mL
MeOH 50 mL
A 2% (by volume) solution of MeCN in toluene was made
up using 654 mL of toluene and 13.3 mL of MeCN. In a 2 L 3 neck flask
equipped with a mechanical stirrer 617 ml of the above solution were
degassed by passing a fine stream of nitrogen through the solution for 5
~ rnin. Phenylhydrazine and TFA were added to the mixture while still
degassing.
~ 25 The CBZ-aldehyde 5 was dissolved in the rest of the solution
prepared above (50 mL) and degassed by bubbling nitrogen through the
solution while in the addition funnel. The solution in the flask was heated
-
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 36 -
to 35~C, and the aldehyde solution was slowly added to the
phenylhydrazine-TFA over 2 h. The mixture was aged at 35~C for 16h.
HPLC conditions: 25 cm Dupont Zorbax RXC8 column at
50~C with 1 mL/min flow and detection at 220 nm; isocratic 55% MeCN,
45% of 0.1% aqueous H3PO4. Typical HPLC profile after 16 h age:
Retention time (min) Area % Identity
1.6 0.1-0.5 phenylhydrazine 6
4.1 <0.1 dimer 21
4.7 <0.1 aldehyde 5
5.0 NA spiroindoline 9
6.3 NA toluene
6.9 97 spiroindolenine 8
10.3 <0.2 phenylhydrazone 7
2-3 tot. other impurities <0.2% ea.
The mixture was cooled to -10~C and MeOH was added. A
suspension of sodium borohydride in 20 mL toluene was added in small
portions (1 mL) over 30 min taking care that the temperature did not
exceed -2~C.
Area % Identity
0.1-1 phenylhydrazine 6
85-90 CBZ-spiroindoline 9
<0.1 CBZ-spiroindolenine 8
10-15 tot. other impurities (<3% ea.)
The temperature was raised to 10~C over lh, and 6%
aqueous ammonia (200 mL) was added. The mixture was agitated for 10
min, allowed to settle for another 10 min, and the lower aqueous phase
was drawn off. Acetonitrile (20 mL) and MeOH (20 mL) were added to
the orgaI~ic phase and it was washed with 150 mL of 15% brine. The
organic phase was found to contain a 92% assay yield of CBZ-
spiroindoline 9.
Step 2: CBZ-Spiroindoline-methanesulfonamide (1)
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
Matenals:
CBZ-Spiroindoline (9) (MW = 322.51) (0.184 mol)
Methanesulfonyl chloride 21.1 g (0.184 mol)
DIEA (KF = 150 mg/L) 29.7 g, 40.1 mL (0.230 mol)
THF (KF = 41 mg/L) 150 mL
The crude solution of CBZ-spiroindoline 9 solution from
Step 1 above was concentrated in a lL 3 neck flask (60-70~C, 150-200
10 Torr) until 250 g of residue remained. The THF and DIEA were added,
and the resulting homogenous solution was cooled to 0~C. A 125 mL
dropping funnel was charged with the methanesulfonyl chloride and 50
mL of THF. The solution of MsCl in THF was added over 2 h to the
reaction mixture m~int~ining the temperature between 0 and 4~C and the
15 mixture was aged for 2 h at 5-8~C. The addition was slightly exotherrnic.
A white precipitate, presumably DIEA-hydrochloride, formed during the
addition. HPLC conditions were the same as above. HPLC analysis
indicated that the reaction was complete 1 h after the end of the addition
(9 was undetectable) and the assay yield was 94% from 9. Retention
20 time: 1 = 7.8 min. Typical HPLC profile of reaction mixture after 2 h
age:
Area % Identity
<0.1 CBZ-spiroindoline 9
90-92 CBZ-sulfonamide 1
8-10 tot. other impurities (<2% ea.)
The mixture was warmed to 20~C, and 200 mL of lM
aqueous HCl was added. The mixture was warmed to 50~C, and the
aqueous phase was separated. The organic phase was washed sequentialy
with 100 mL water, 100 mL 5% aqueous sodium bicarbonate, and 100
mL water. The organic phase was transferred to a 1 L 3 neck flask
equipped for mechanical stirring and distillation. The mixture (ca 400
mL) was distilled at atmospheric pressure until 150 mL of distillate had
been collected. The head temperature reached 107~C; the pot
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
- 38 -
temperature was 110~C. The distillation was continued with continuous
addition of n-propanol at such a rate as to maintain a constant volume (ca
350 mL) in the pot. The distillation was stopped when a total of 525 mL
of n-PrOH had been added and a total of 800 rnL of distillate had been
S collected.
The temperature of both the head and pot rose from 94~C to
98~C during the solvent switch. Toluene and n-PrOH form an azeotrope
boiling at 97.2~C composed of 47.5% toluene and 52.5% n-PrOH. The
rnixture was allowed to cool gradually to 20~C over 3h and aged for 12 h.
10 The mother liquor was found to contain 2% toluene and 4 mg/mL of
sulfonamide. The solubility of the sulfonamide in various nixtures of
toluene and n-PrOH has been determined by HPLC assay:
%toluene in n-PrOH solubility of 1 in mg/mL
lS 0 2.36
3.02
4.23
7.51
10.3
The crystalline slurry was filtered and washed with 3 x 100
rnL of n-PrOH. The product was dried in a vacuum oven at 50~C with a
nitrogen bleed for 16 h to furnish 65.5g (82 % from aldehyde 5) of 6 as a
tan solid with 93.5 wt% purity.
Typical HPLC profile of solid:
Area % Identity
<0.1 CBZ-spiroindoline 9
>99 CBZ-sulfonamide 1
< 1 tot. other impurities (<0.2 Yo ea.)
For additional purification, a 40.0 g sample of the n-PrOH
crystallized sulfonamide was dissolved in 134 rnL of EtOAc at 60~C and
treated with 8.0 g of Darco G-60 carbon for 1 h at 60~C. After the
addition of 2.0 g SolkaflocTM, the slurry was filtered through a pad of 4.0
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/169~4
- 39 -
g SoL~aflocTM, and the pad was washed with 90 mLof EtOAc at 60~C.
Prior to the addition of the carbon the solution was a brown color. The
fîltration proceeded well without plugging to give a golden yellow
filtrate. The filtrate was distilled at atmospheric pressure in a 500 mL
flask (pot temperature 80-85~C) until 100 g (100 mL) of residue
remained. This solution was allowed to cool to 35~C over 3 h. Over a lh
period, 116 mL of cyclohexane was added with good agitation at 35~C.
The mixture was cooled to 20~C over 1 h and aged at 20~C for 12 h. At
35~C much of the sulfonamide has crystallized out and the mixture was
thick. Addition of cyclohexane at 20~C makes agitation difficult. After
the aging period, the supernatant was found to contain 2.5 mg l/g. The
crystalline slurry was filtered and the cake was washed with 77 mL of 2:1
cyclohexane-EtOAc and 2 x 77 mL of cyclohexane. The product was
dried in a vacuum oven at 50~C with a nitrogen bleed for 16 h to furnish
34.2 g of 1 (MW = 400.3) as a white crystalline solid (85 % recovery
from crude 1, 70 % from 5 with >99.9 wt % purity).
EXAMPLE 8
,H
~ HCI
~ ' SO2Me
20 HCl Salt of Spiroindoline-methanesulfonamide (la)
Materials:
CBZ-spiroindoline-methanesulfonamide (1) 941 g (2.35 mol)
Pearlman's catalyst 20% Pd(OH)2/C 188 g
25 THF 8 L
MeOH 7 L
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 40 -
The catalyst was suspended in 7 L of MeOH and transferred
into the S gal autoclave followed by the solution of 1 in 8 L of THF. The
mixture was hydrogenolyzed at 25~C at 80 psi of H2. After 2.5 h the
temperature was raised to 35~C over 30 min.
HPLC analysis indicated complete consumption of Cbz-
spiroindoline-methanesulfonamide. HPLC conditions: 25 cm Dupont
Zorbax RXC8 column with 1.5 mL/min flow and detection at 254 nm.
Gradient Schedule:
Time (min) 0.1% aq. H~PO4:MeCN
0 70:30
3 70:30
12 20:80
25 20:80
retention times: Spiroindoline = 7.6 min,
Cbz-spiroindoline-methanesulfon~mitle = 13.6 min.
The mixture was purged with nitrogen and the catalyst was
removed by filtration through Solka-flocTM while still warm. Ihe catalyst
was washed with 4 L of THF and 2 L of MeOH. The pale yellow filtrates
20 were concentrated to a thick oil at 10 mbar and <25~C. The solvent
switch was completed by slowly bleeding in 15 L of EtOAc and
reconcentrating to dryness. The residue solidified to a hard off-white
mass. MeOH (1.5 L) was added and the mixture was he~te-l to 70~C to
give a homogenous solution. While the solution was at 70~C, 10.5 L of
25 EtOAc at 20~C was added. The temperature fell to 40~C, and the mixture
remained homogenous.
Subsequent experiments suggested that it is more convenient
to solvent switch the MeOH-THF filtrates to MeOH, concentrate to the
desired volume, and then add the EtOAc. This avoids the solidification
30 of the residue upon concentration of the EtOAc solution.
Hydrogen chloride diluted with about an equal volume of
nitrogen was passed into the solution. The temperature rose to 60~C over
the course of 15 min, and a white precipitate of the hydrochloride salt
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 41 -
formed. Diluting the HCl with nitrogen only avoids the reaction mixture
sucking back and may not be necessary.
The mixture was cooled in an ice bath, and the hydrogen
chloride addition was continued for lh. The temperature gradually fell to
S 20~C. The suspension was aged for 2 h while the temperature was
lowered to 10~C. The crystalline product was isolated by filtration, and
the filter cake was washed with 3 L of EtOAc. It was dried in the
vacuum oven at 35~C to give 1.18 kg (86%) of the title product la as an
off-white crystalline solid of >99.5 area % purity by HPLC analysis.
HPLC conditions: 25 cm Dupont Zorbax RXC8 column with 1.5 mL/min
flow and detection at 230 nm; isocratic 35% MeCN, 65% of 0.1%
aqueous ammonium acetate. Retention time: la = 5.4 min.
EXAMPLE 9
~N~ lb
~,
SO2Me
~piroindoline-methanesulfonamide (Free base form) (lb)
A 250 mL aliquot of the filtrate from the Cbz-
hydrogenolysis cont~ining 4.67 g of lb (free base) was concentrated to ca
10 mL. The residue was dissolved in 20 mL of EtOAc and the solution
20 was reconcentrated to ca 10 mL. This was repeated once more, and 10
mL of EtOAc was added to the residue. A crystalline precipitate began to
form. MTBE (20 mL) was added in one portion. Additional crystalline
solid precipitated, but the supernatent still contained a substantial quantity
of dissolved product which did not precipitate on st~n~lin~. Hexanes (70
25 mL) were added dropwise over 2 h to the mixture with vigorous stirring.
The slow addition of the hexanes is neccessary to avoid the oiling out of
the amine.
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
- 42 -
The agitated mixture was aged for lh and filtered. The filter
cake was washed with 20 mL of l: 1 MTBE-hexanes and then with 20 mL
of hexanes. The product was dried under a stream of nitrogen to give
3.86 g (82%) of the free amine of lb as an off white crystalline solid of
S >99.5 area % purity. HPLC conditions: 25 cm Dupont Zorbax RXC8
column with 1.5 mL/min flow and detection at 230 mn; isocratic 35%
MeCN, 65% of 0.1 % aqueous ammonium acetate. Retention time: lb =
5.4 min.
EXAMPLE 10A
~1 lb
~'
SO2Me
Spiroindoline-methanesulfonamide (Free base forrn) (lb)
Materials:
CBZ-Spiroindoline-sulfonamide (1) 833.5 gr (2.08 mol)
Pd(OH)2/C (20% weight of Pd(OH)2) 124.5 (15%)
THF 6.5 L
MeOH 19.5 L
NH40H (conc) 60 mL
The hydrogenation was run three (3) times due to equipment
limitations; this procedure refers to a single run. The CBZ spiroindoline
sulfonarnidel was dissolved in THF (6.5 L, KF = 53 ,ug/,uL) and then
25 MeOH (KF=18,ug/rnL, 4L) was added followed by addition of the
catalyst and the slurry was transferred to a 5 gal autoclave. The
rem~in~1er of the MeOH (2.5 L) was used for rin~in~. The mixture was
hlo~te-l to 40~C at 50 psi for 24 hours. The catalyst loading and reaction
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 43 -
time are a function of the purity of starting material 1. This m~tçri~l was
unique requiring 2 15% catalyst and long reaction time. Purer batches of
spiroindoline required only 5% of catalyst and 4-6 hrs reaction time.
Upon completion (<0.1 A% 1 by LC) the l -~i x ~l l. e was
5 filtered thru Solka FlocTM and the carbon cake washed with MeOH (13 L)
cont~ining NH40H (0.5%, 60 mL). The combined ~lltrates (assay shows
1587 g of spiroindoline amine lb) were concentrated in vacuo and the
resulting solids were partitioned between 40 L (of toluene:THF (3:1) and
0.5N NaOH (18 L). Although the layers separated easily a heavy
10 precipitate could be seen in the aqueous layer. The aqueous suspension
was thus extracted with CH2C12 (15 L). The aqueous and organic layer
separated slowly. Prior to CH2C12 addition THF was added to the
aqueous layer along with enough NaCl to saturate the layer. However
dissolution of the product was not achieved which necessitated the use of
CH2C12.
The combined toluene, THF and CH2C12 layers were
combined and concentrated in the batch concentrator. The residue was
flushed with 7 L of CH3CN. Finally 10 L of CH3CN were added and the
solution stood overnight under N2 atmosphere.
EXAMPLE 10B
H
I
1b
~'' ' SO2Me
Spiroindoline-methanesulfonamide (Free base form) (lb)
25 Materials:
CBZ-Spiroindoline-sulfonamide (1) 3 kg (7.49 mol)
Darco G-60 600 g
Ethyl Acetate 36 L
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
-- 44 --
Absolute Ethanol 189 L
10% Pd/C 450 g
Ammonia Solution 500 ml
Solka FlocTM 2.5 kg
Isopropyl Acetate 65 L
A mixture of CBZ-spiroindoline (1) (1 kg) and Darco G-60
(200 g) in ethyl acetate (9 L) was stirred and heated at 60-65~C under a
nitrogen atmosphere for 8 hours. The Darco was removed by filtration at
60-65~C, the solid washed with hot ethyl acetate (3 L) and the ~lltrate and
washings combined. LC wt/wt assay con~lrmed negligible loss to the
Darco. The ethyl acetate solution was evaporated to dryness in vacuo
using a 20 L Buchi apparatus and then flushed with absolute ethanol (2 x
S L). This material was then slurried in absolute ethanol (8 L) warmed to
65-70DC and placed in the 20 L autoclave. The batch was rinsed in with
absolute ethanoI (1 L). A slurry of 10% Palladium on charcoal (75 g,
7.5% by weight) in absolute ethanol (750 ml) was then added to the
autoclave and rinsed in with a further portion of absolute ethanol (250
ml).
The batch was hydrogenated at 65~C with vigorous stirring
under 40 psi hydrogen pressure for 3 hours, a second portion of 10%
palladium on charcoal (75 g) was added, the batch was hydrogenated for
a fur~er 2 hours and then sealed overnight. The batch was transferred
(still hot, 60-65~C) to a 20 L Buchi apparatus and degassed in vacuo to
remove formic acid by "feeding and bleeding" absolute ethanol (18 L
total).
This procedure was repeated twice rnore and the three
batches were combined in a 10 gallon glass-lined vessel and the
combined batch was degassed again by the addition and distillation (in
vacuo) of absolute ethanol (2 x 10 L). Solka flocTM (0.5 kg) was added to
the batch and rinsed in with ethanol ( 10 L). An Estrella ~llter was loaded
with SoLkaflocTM (2 kg) as a slurry in ethanol (20 L). The resulting
mixture was warmed to 60-65~C and then transferred at this temperature
via heated filter using pump to two tared stainless-steel bins. The initial
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 45 -
vessel, the filter, the pump and the lines were rinsed with a hot (60-65~C)
mixture of aqueous ammonia (500 ml) in absolute ethanol (25 L). The
filtrate and washings were combined in the two stainless-steel bins.
The batch was then transferred to a vessel using an in-line
S filter cont~inin~: a 10 micron cartridge, and then concentrated in vacuo to
low buL~ (~15 L). The ethanol was replaced by isopropyl acetate by the
"feeding and bleeding" of 3x batch volumes of isopropyl acetate (45 L
total), while m~int~ining a batch volume of ~15 L. The solvent switch,
when complete, contained <1% residual ethanol by GC. The batch was
10 then diluted to ~33 L by the addition of isopropyl acetate (20 L), and this
solution of spiroindoline-amine lb (1.855 kg by LC analysis) in isopropyl
acetate was used for the next stage of the process.
EXAMPLE 1 1 A
~H,N--BOC
~ ~0
SO2Me
Boc-O-Benzylserine Spiroindoline (11)
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
- 46 -
Materials:
Spiroindoline-amine (lb) 1587 g (5.966 moles)
Amino acid (10) 1938 g (6.563 moles)
Ph~O~CO2H
NHBOC
DCC 1334.5 g (6.563 moles)
HOBT 884 g (6.563 moles)
CH3CN 25 L
0.5N NaOH 18 L
0.5N HCl 18 L
NaHCO3 sat. 18 L
iPrOAc 28 L
The spiroindoline-aminelb in CH3CN or iPrOAc:H2O (25
L) at arnbient temperature under N2 was treated in sequence with HOBT
(884 g; 1.1 eq) as a solid, DCC (1334.5 g, 1.1 eq) as the melt (heating in
hot water at 60~C for ca. 1 hr) and finally the amino acid 10 (1938 g) as
the solid. The mixture was stirred for 3 hr upon which time heavy
precipitation of DCU occurred and LC analysis showed ca. 0.5 A% of
amine lb rem~ining. IPAc (9 L) was added, the slurry was filtered
through Solka FlocTM and the cake was washed with IPAc (19 L). The
combined organic solution was washed in sequence with 0.5N NaOH (18
L), 0.5N HCl (18 L) and saturated NaHCO3 (18 L). A final water wash
at this point resulted in an emulsion and was thus elimin~ted.
The organic layer was concentrated in vacuo and the residue
was dissolved in MeOH or EtOH (10 L final volume). Assay yield 3026
gr (89%).
The use of alternative peptide coupling agents such as
carbonyldiimidazole or formation of mixed anhydrides, such as sec-butyl
carbonate, gave inferior yields of 11 and/or 14 with a high degree of
epimerization in the case of the forrner compound. Other peptide
coupling reagents were prohibitively expensive.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 47 -
EXAMPLE 1 lB
~ ~H,N--BOC
~ ~0
[~ 11
~ ' SO2Me
Boc-O-Benzylserine Spiroindoline (11)
Materials:
Spiroindoline-amine (lb) 1.855 kg (6.96 mol)
Isopropyl acetate 29 L
Dicyclohexylcarbodiimide (DCC) 1.58 kg (7.65 mol)
l-Hydroxybenzotriazole (HOBt) 1.03 kg (7.62 mol)
N-Boc-O-benzyl-D-Serine 2.26 kg (7.65 mol)
lM Aqueous sodium hydroxide 26 L
0.5M Aqueous hydrochloric acid 26 L
Satd. Aqueous sodium hydrogen carbonate 26 L
Absolute Ethanol 50 L
Water (20 L) was added to a stirred solution of the
spiroindoline-aminelb (1.855 kg) in isopropyl acetate (33 L) in a reaction
vessel. The following chemicals were then added sequentially at room
temperature under a nitrogen atmosphere: DCC (1.58 kg, 1.1 equivs.),
HOBt (1.03 kg, 1.1. equivs.) and finally N-Boc-O-benzyl-D-Serine (2.26
~ kg, 1.1 equivs.). The reagents were rinsed in with isopropyl acetate (7 L).
The batch was stirred at room temperature under nitrogen atmosphere for
5 hours when LC showed the ratio of product/starting material to be
99.4/0.6. The mixture was then filtered through an Estrella filter using
cloth and cardboard only and lltili7ing a pump into another vessel. The
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 48 -
sending vessel was rinsed with isopropyl acetate (22 L) and this was used
to rinse the filter, the pump and the lines into the receiving vessel. The
2-phase mixture in the vessel was stirred for 10 minutes and then allowed
to settle for 15 minutes. The lower aqueous layer was separated off and
5 the organic solution was left to stand at room temperature overnight.
The next day, the organic solution was washed with lM
aqueous sodium hydroxide solution (26 L) then O.5M aqueous
hydrochloric acid (26 L) and finally saturated aqueous sodium hydrogen
carbonate (26 L). LC analysis gave an assay yield of 3.787 kg, 93%
overall yield from 7.49 moles (3 kg) of starting CBZ-spiroindoline (1).
The batch was concentrated i7t vacuo (internal temperature = 13-15~C.
jacket temperature = 40~C, Vacuum = 29") to low bulk (~15 L) and
solvent switched to ethanol by '~feeding and blee~lin~" ethanol (50 L)
whilst m~int~ining the volume at ~15 L. GC showed <1% isopropyl
15 ~cet~ rem~ining. This solution was used for the next stage of the
process.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 49 -
EXAMPLE 12A
O ~C~ NH2
~~ I=o
~N~ 1 2
SO2Me
O-Benzylserine Spiroindoline (free base form) (12)
Materials:
Boc-O-Benzylserine Spiroindoline (11) 3026 g (5.57 moles)
Methane sulfonic acid (MsOH) 1.16 L (17.9 moles)
MeOH 10 L
iPrOAc 24 L
0.5 N NaOH 35 L
The Boc-O-benzylserine spiroindoline 11 in 10 L of MeOH
(or EtOH) was treated with neat MsOH (1.16 L) added over ca. 30-40
min, (initial temperature 16~C, final temperature 28~C). The dark red
15 solution was aged overnight under N2. The mixture was then pumped
into a 100 L extractor cont~inin~ 24 L iPrOAc and 35 L 0.5 N NaOH.
The pH of the aqueous layer was 7. NaOH (6M) was added until pH 2
10.5. As the pH increased the color changed from red to yellow. The
layers were separated and the organic layer (24 L) was shown by NMR to
contain 13 mole % of MeOH in iPrOAc [5 volume %]. LC assay 2.48 kg.
CA 02235511 1998-04-22
WO 97/lS573 PCT/US96/16954
- 50 -
EXAMPLE 12B
3~ ~ H, NH2
12
~N'sO2Me
C)-Benzylserine Spiroindoline (free base forrn) (12)
5 Materials:
Boc-O-Benzylserine Spiroindoline (11) 3.787 kg (6.96 mol)
Methanesulphonic acid 2.006 kg (20.87mol)
Isopropyl acetate 38 L
lM Aqueous sodium hydroxide 16 L
50% Aqueous sodium hydroxide 1.6 L
Methanesulphonic acid (2.006 kg, 1.355 L, ~3 equivs.) was
added to the stirred solution of Boc-O-benzylserine spiroindoline (11)
(3.787 kg) in ethanol (total volume ~ lS L) in a reaction vessel. The batch
was walmed to 35-40~C. After 7 hours, LC showed the absence of
starting material and the reaction was allowed to cool to room
temperature overnight. The next day, water (44 L) was added to ~e
batch with stiITing. The batch was cooled to ~5~, stirred for 301minutes
and then filtered through an in-line filter (loaded with a lO,u cartridge)
into a bin. The batch was then sucked back into the vessel. A water rinse
(10 L) was used to rinse the vessel and lines into the bin and this was
used to then rinse back into the vessel. Isopropyl acetate (38 L) was
added followed by a lM aqueous sodium hydroxide (16 L). The batch
was cooled to 10- 15~C, the pH of the lower aqueous layer was confirmed
as ~7 and 50% aqueous sodium hydroxide solution was added (1.6 L) (pH
>10). I~e batch was stirred at 10-15~C for 25 minutes and then allowed
CA 02235511 1998-04-22
WO 97/15573 PCT/US96tl6954
to settle for 10-15 minutes. The lower aqueous layer was separated (78.1
kg). LC assay indicated 28.4 g of 12 (0.85% of theory) contained in the
aqueous liquors. Volume of the organic solution = 51 L. LC assay
indicated 3.057 kg, 92% overall yield from 3 kg, 7.49 moles of CBZ-
5 spiroindoline sulfonamide (1). This solution was used for the next stage.
EXAMPLE 13A
H H CH3 CH3
-- ~ O O H
~N~
~N~
SO2Me
Boc-Aminoisobutyryl O-Benzylserine Spiroindoline (14)
Materials:
Spiroindoline amine (12) 2481 g (5.57 moles)
amino acid peptide (13) 1247.1 g (6.16 moles)
CH~<CH3
HO2C NH-BOC
DCC 1266.7 g (6.16 moles)
HOBT 827 g (6.16 moles)
IPAc 52 L
H2O 37L
0.5N NaOH 36 L
0.5NHC1 36 L
Sat. NaHCO3 36 L
The solution of ~he amine 12 in IPAc was diluted to a total
volume of 39 L with IPAc and 37 L of H2O was added. The biphasic
CA 0223~11 1998-04-22
W O 97/15573 PCTAUS96/16954
mixture was then treated in sequence with HOBT (827 g) as a solid, DCC
(1266.7 g) as a melt, and amino acid 13 at ambient temperature under
nitrogen. The reaction mixture was stirred for 2 h upon which time LC
analysis indicated dissappearance of the starting material 12 (<0.3 A%).
S ~e rnixture was ~lltered through Solka FlocTM and the solids were
washed with 13 L of IPAc. The material may be stored at this point as a
biphasic mixture overnight.
The mixture was transferred to a 100 L extractor, the
aqueous layer was separated and the organic layer was washed
successively with 36 L of 0.5N NaOH, 0.5N HCl and saturated NaHCO3.
Assay yield 3160 g (81% from spiroindoline + 5% for volume
measurement error). The solution was concentrated to a small volume
and was ~lushed with ethanol (2 x 4 L). If desired, the inermediate
compound 14 may be isolated by adding water to crystalize it out.
The use of alternative peptide coupling agents such as
carbonyldiimidazole or formation of mixed anhydrides, such as sec-butyl
carbonate, gave inferior yields of 14 with a high degree of epimerization.
Other peptide coupling reagents were prohibitively expensive.
EXAMPLE 13B
H H CH~3 CH3
[~0 C ' N ~N' BOC
~N~
~N~
SO2Me
Boc-Aminoisobutyryl O-Benzylserine Spiroindoline (14)
CA 0223~11 1998-04-22
WO 97/15573 ~ PCT/US96/16954
- 53 -
Materials:
Spiroindoline amine (12) 3.057 kg (6.89 mol)
Dicyclohexylcarbodiimide (DCC) 1.56 kg (7.56 mol)
l-Hydroxybenzotriazole (HOBt) 1.02 kg (7.55 mol)
Boc-2-Aminoisobutyric acid (13) 1.54 kg (7.58 mol)
Isopropyl acetate 32 L
lM Aqueous sodium hydroxide 38 L
0.5M Aqueous hydrochloric acid 38 L
Satd. aqueous sodium hydrogen carbonate 38 L
Absolute ethanol 45 L
Water (49 L) was added to the stirred solution of the
spiroindoline amine 12 (3.057 kg) in isopropyl acetate (total volume ~51
L) in a reaction vessel at room temperature under a nitrogen atmosphere.
15 The following chemicals were then added sequentially: DCC (1.56 kg,
~1.1 equivs.), HOBt (1.02 kg, ~1.1 equivs.) and finally, N-Boc-2-
aminoisobutyric acid 13 (1.54 kg, ~1.1 equivs.). The mixture was stirred
vigorously at room temperature for 2 hours when LC showed the reaction
to be complete. The mixture was filtered to to another vessel via an
20 Estrella filter using a pump. Isopropyl acetate (22 L) was used to rinse
vessel, the filter, the pump and the lines into the receiving vessel. The 2-
phase mixture was then stirred for 5 minutes and the layers were allowed
to separate. The lower aqueous layer was separated without incident
(weight of aqueous liquors = 51.1 kg). The organic solution was then
25 washed sequentially with lM aqueous sodium hydroxide (38 L), 0.5M
aqueous hydrochloric acid (38 L) and finally, saturated aqueous sodium
hydrogen carbonate (38 L) without incident.
The organic solution was then transferred using a pump via
an in-line filter (containing a 10,u cartridge) to another vessel for the
30 solvent switch to ethanol. The vessel was rinsed with isopropyl acetate
(10 L) and this was used to rinse the pump, the filter and the lines into
the receiving vessel. The filtrate and washings were combined. Total
volume = 75 L (by dipstick). LC assay gave 4.395 kg of Boc-
CA 02235511 1998-04-22
WID 97/15573 PCT/US96/16954
- 54 -
aminoisobutyryl O-benzylserine spiroindoline (14), i.e. 93% overall from
7.49 moles of starting CBZ-spiroindoline sulfonamide (1).
T~e batch was concentrated in vacuo to low bulk (~15 L)
and the isopropyl acetate switched to ethanol by "~eeding and bleeding"
5 absolute ethanol (45 L total). At the end of the solvent switch, GC
showed <1% isopropyl acetate rem~ining. This solution (25 L)
cont~ining 4.395 kg of 14 was used for the next stage. If desired, the
inermediate compound 14 may be isolated by ~ lin,e~ water to crystalize it
out.
EXAMPLE 14A
~~O~C~ N ~ NH
~N~ lS
~C SO2Me
AminoisobutyrYl O-Benzylserine Spiroindoline (15)
Materials:
Boc Spiroindoline (14) 3 l 60 g (5.03 moles)
Meth~n~sulfonic acid (MsOH) 979 mL (15.1 moles)
EtOH 6.2 L
H2O 30L
lN NaOH ll L
EtOAc 26 L
Darco 60 activated carbon 1 Kg
The Boc spiroindoline 14 was dissolved in 6.2 L of EtOH
and treated with MsOH (979 mL). The temperature rose from 20 to 30~C
and the reaction was allowed to proceed overnight. After 12 hours at
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 55 -
20~C there was still 15 A% of starting material left so the mixture was
heated to 35~C for 6 hours. Upon completion (<0.1 A% 14) the reaction
was cooled to 20~C and 30 L of H2O were added and the solution was
filtered through a glass funnel with a polypropylene filter to filter off
5 residual DCU. The mixture was transferred to a 100 L extractor and 26 L
of EtOAc were added. The aqueous layer was basified via addition of
chilled lN NaOH (11 L) and 1 L of 50% NaOH. Addition of ice was
required to keep the temperature below 14~C. Higher temperatures
resulted in significant emulsion problems.
The organic layer was distilled at 50~C at ca. 21" of Hg until
KF <1000 ,ug/mL. Lower KF's result in more efficient carbon treatments
and better recovery at the salt formation step. KF's of 160 ~g/mL were
achieved at the 700 g scale. The solution was diluted with ethyl acetate
to a total volume of 31 L (LC assay 2.40 kg). Activated carbon (Darco
15 G-60) was added and the mixture was stirred for 24 h. The mixture was
filtered through Solka FlocTM and the ~llter cake was washed with ethyl
~cet~te (16 L), assay 2.34 Kg.
EXAMPLE 14B
H H CH CH3
[~~~C' ~ NH2
SO2Me
Aminoisobutyryl O-Benzylserine Spiroindoline (15)
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 56 -
Materials:
Boc Spiroindoline (14) 4.395 kg (6.99 mol)
Methanesulfonic acid 2.017 kg (20.99 mol)
Ethyl ~eet~te 185 L
lM Aqueous sodium hydroxide 16 L
50% Aqueous sodium hydroxide 2.6 L
Darco G-60 900 g
Solka FlocTM 2.5 kg
Methanesulfonic acid (2.017 kg, 1.36 L, ~3 equivs.) was
added to the stirred solution of the Boc spiroindoline 14 (4.395 kg) in
ethanol (total volume ~25 L) in a reaction vessel at room temperature.
The batch was warmed to 35-40~C, and stirred overnight. On the next
day, the batch contained ~1.1 A% of starting material and so the reaction
was continued for a further 4 hours, then LC showed ratio of product/
starting material to be 99.6/0.4. The batch was concentrated in vacuo to
~15 L volume and then diluted with water (44 L). The batch was cooled
to 5~C, stirred for 30 minutes and then filtered through a Sparkler in-line
filter (cont~ining a 10}1 cartridge) using a pump to another vessel to
remove a small amount of residual DCU.
The vessel, the pump, the filter and the lines were rinsed
with water (10 L), and this was added to the vessel. Ethyl acetate (36 L)
was added to the vessel and the stirred mixture was cooled to 10~C. A
solution of cold (5-10~C) lM aqueous sodium hydroxide solution (16 L)
and cold (5-10~C) 50% aqueous sodium hydroxide solution (2.6 L) were
added at 10~C and the temperature rose to 14~C. The resulting mixture
was stirred for 15 minutes at <14~C and then the lower aqueous layer
separated off.
The batch was concentrated in vacuo to ~20 L volume and
then a mixture of ethyl acetate (35 L) and ethanol (5 L) was fed in while
m~int~ining the volume at ~20 L. At the end of this distillation the KF
was 9160 mgml~1. The batch was solvent switched to ethyl acetate by
"feeding and bleeding" ethyl acetate (40 L total). At the end of this
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 57 -
distillation, KF was 446 mgml~ 1. The batch was diluted with ethyl
acetate (10 L).
Darco G-60 (900 g) was added to the hazy mixture. This
was rinsed in with ethyl acetate (6 L). This mixture was stirred at room
5 temperature overnight. Next day, Solka FlocTM (0.5 kg) was added to the
stirred batch in the vessel and then Solka FlocTM (2.0 kg) was stirred in a
little ethyl acetate and loaded into an Estrella filter . The excess solvent
was pumped away through a Sparkler in-line filter Cont~ining a 10~
cartridge. The slurry was transferred from the vessel through a filter
10 using a pump and then through another filter to 2 x 40 L stainless steel
bins. Visual inspection showed the liquors to be clear and clean. The
vessel was rinsed with ethyl acetate (22 L) and this was used to rinse
through the route outlined above to the stainless steel cans. The contents
of both cans was transferred into a reaction vessel and the solution was
15 mixed thoroughly.
The batch (58 L) had a KF of 2950 mgml~l and so was
redried by concentrating i~l vacuo to 20-25 L volume. The batch was
diluted to 46 L volume (dipstick) by the addition of ethyl acetate (25 L).
The KF was 363 mgml~l. The batch was diluted to 62 L volume by the
20 addition of ethyl acetate ( 17 L) and was used for the final stage of the
process.
CA 02235511 1998-04-22
WO 97/15573 PCT/US96/16954
- 58 -
EXAMPLE l5A
H H CH CH3
~o ~ ~ NH2~ CH3SO3H
g ~'SO2Me
Spiro[3H-indole-3,4'-piperdin]- 1 '-yl)carbonyl]-2-(phenylmethyl-
oxy)ethyll-2-amino-2-methylpropanamide Methanesulfonate (16)
Materials:
Amine (15) 2340 g (4.43 moles)
Methane sulfonic acid (MsOH) 316 mL (4.88 moles)
EtOAc 60 L
EtOH 4.8 L
8% EtOH in EtOAc 20 L
The volume of the solution of 15 from the previous step was
adjusted to 60 L with ethyl acetate and EtOH (4.8 L) was added. The
MsOH (316 mL) was added in 3 L of EtOAc at 45~C. To the deep red
homogeneous solution was added 496 g of the title compound Form I
seed (10~o seed based on the weight of the free amine was employed).
The temperature rose to ca. 48~C and the reaction was aged at 52~C for
1.5 hours. Analysis indicated complete conversion to the title compound
(Form I). (At less than 10% seed longer age (> 3 hours) was required).
The slurry was allowed to cool to 20~C overnight and was filtered in a
centrifuge under N2. The cake was washed with 20 L of 8% EtOH in
EtOAc. N2 is essential during filtration because the wet crystals are very
hygroscopic. The batch was dried at 35~C under vacuum to afford 2.7Kg
(56% overall yield) of the title compound (Forrn I) (99.9 A% purity; <
0. 1% enantiomer).
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
_ 59 _
The conversion of Form II to Form I is also accomplished
where the salt is formed in EtOAc-EtOH by addition of MsOH as above
and the initial solution of the salt (at 55~C) is cooled to 45~C. Crystals
start appearing at that temperature and the slurry becomes thicker with
5 time. The temperature is then raised to 51 ~C and the slurry is aged
overnight. Complete conversion to Form I of 16 should be expected.
This procedure may also be employed to prepare seed crystals of Form I
of 16.
EXAMPLE 15B
H H CH ~cH3
[~0 C ~ NH2- CH3SO3H
SO2Me
Spiro[3H-indole-3,4'-piperdin]- l '-yl)carbonyl]-2-(phenylmethyl-
oxy)ethyll-2-amino-2-methylpropanamide Methanesulfonate (16)
Materials:
Amine (15) 3.1 kg (5.86 mol)
Methanesulfonic acid 620 g (6.45 mol)
Ethyl acetate 37 L
Absolute ethanol 8.7 L
Spiro[3H-indole-3,4'-piperdin]- l '-yl)-
20 carbonyl]-2-(phenylmethyl-oxy)ethyl]-
2-amino-2-methylpropanamide
methanesulfonate (Form I) 70 g (0.11 mol)
Absolute ethanol (6.4 L) was added to the solution of the
amine (15) (3. l kg) in ethyl acetate (total volume ~62 L) in a reacttion
vessel. The batch was warmed to 50~C and a solution of methanesulfonic
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96/16954
- 60 -
acid (620 g, 412 ml, 1.1 equivs.) in ethyl acetate (11 L) was added over
~5 minutes at 50-54~C. The batch was seeded with spiro[3H-indole-3,4'-
piperdin]- 1 '-yl)-carbonyl]-2-(phenylmethyl-oxy)ethyl]-2-arnino-2-
methylpropanamide methanesulfonate (Form I) (70 g) and the resulting
slurry was stirred and heated at 55~C under nitrogen atmosphere
overnight.
The next day, the slurry was cooled to 15-20~C, held for 2
hours and then dropped to the 50 cm polypropylene filter under nitrogen
atmosphere. The solid product was washed with a mixture of absolute
ethanol (2.3 L) in ethyl acetate (26 L). The white, solid product was dug
off and dried in an Apex oven in vacuo at 35~C for an a~ liate time
(approx. two days). The dried spiro[3H-indole-3,4'-piperdin]-1'-yl)-
carbonyl]-2-(phenylmethyl-oxy)ethyl]-2-amino-2-methylprop~n~micle
methanesulfonate (3.352 kg) was sieved using a Jackson-Crockatt sieve
to give 3.347 kg (including seed, 70 g) } yield = 3.277 kg.
Form I of N-[1(R)-[(1,2-dihydro-1-methanesulfonyl-
spiro[3H-indole-3,4'-piperdin]- 1 '-yl)carbonyl]-2-(phenylmethyl-
oxy)ethyl]-2-amino-2-methylpropanamide methanesulfonate is an
anhydrous polymorph characterized by the following properties:
a melting point of 168-171~C and solubility in isopropanol of
4.6 mg/mL.
The DSC curve for Form I of N-[l(R)-[(1,2-dihydro-1-
methanesulfonyl-spiror3H-indole-3,4'-piperdin]- 1 '-yl)carbonyl]-2-
(phenylrnethyl-oxy)ethyl]-2-amino-2-methylprop~n~mide
methanesulfonate at 10 ~C/min in an open cup under nitrogen flow
e~hibits a single endotherm, due to melting, with a peak temperature of
about 180~C and an extrapolated onset temperature (melting point) of
about 170~C with an asociated heat of approximately 53 J/g.
Form I was characterized by an X-ray powder diffraction
pattern with reflections at approximately: 6.5, 14.7, 16.9, 17.1, 17.9,
19.5, 21.1, 21.7, and 22.0~ (2 theta). Data collected using a Philips
APD3720 Automated Powder Diffraction instrument with copper Koc
radiation. Measurements were made from 2~ to 40~ (2 theta) with the
sample m~int~ined at ambient room temperature.
CA 0223~11 1998-04-22
WO 97/15573 PCT/US96116954
- 61 -
HPT C Conditions:
LC Retention times on Zorbax RX-C8 (4.6 mm x 25 cm),
~ = 210 nm, flow rate = 1.5 ml/min.
Compound 1: 60:40 CH3CN-H20 (1% H3PO4) RT = 5.0 min
Compound lb: 35:65 CH3CN-H20 (0.1 w % NH40Ac) RT = 6.2 min.
Compound 10: 60:40 CH3CN-H20 (0.1 H3PO4) RT = 2.9 min.
Compound 11: 60:40 CH3CN-H2O (0.1% H3PO4) RT = 5.4 min.
Compound 12: 40:60 CH3CN-H20 [pH 5.25 NaH2PO4 (6.9 g/L of H20)
(adjust pH with NaOH)] RT = 5.6 min
Compound 14: 60:40% CH3CN-H20 (0.1% H3PO4) RT = 4.65 min
Compound 15: 40:60% CH3cN-H2o [pH = 5.25 NaH2PO4 (6.9 g/L
of H2O)] adjust pH with NaOH)RT = 4.9 min
LC Retention times on Zorbax RX-C8 (4.6 mm x 25 cm),
= 210 nm, flow rate = 1.2 ml/min, column temperature = 48~C
Solvent A = 0.05% Phosphoric acid + 0.01% Triethylamine in water
Solvent B = Acetonitrile
Gradient system:
Time % A % B
0 rnin 95 5
35 min 10 90
38 min 95 5
40 min 95 5
Retention time (mins)
Compound 1 25.2
Compound lb 8.5
Compound 10 20.5
Compound 11 26.3
Compound 12 14.8
Compound 14 25.6
Compound 15 15.7
CA 0223~11 1998-04-22
WIO 97/15S73 PCT/US96/16954
- 62 -
While the invention has been described and illustrated with
reference to certain particular embodiments thereof, those skilled in the
art will appreciate that various adaptations, changes, modifications,
substitutions, deletions, or additions of procedures and protocols may be
S made without departing from the spirit and scope of the invention. For
example, reaction conditions other than the particular conditions as set
forth herein above may be applicable as a consequence of variations in
the reagents or methodology to prepare the compounds from the
processes of the invention indicated above. Likewise, the specific
10 reactivity of starting materials may vary according to and depending upon
the particular substituents present or the conditions of manufacture, and
such expected variations or differences in the results are contemplated in
accordance with the objects and practices of the present invention. It is
intended, therefore, that the invention be defined by the scope of the
15 claims which follow and that such claims be interpreted as broadly as is
reasonable.