Note: Descriptions are shown in the official language in which they were submitted.
:s CA 02235680 2001-04-20
a
- 1 -
Technical Field
The present invention relates to the use of a specified surfactant in
combination with a specific alcohol to improve the activity of the hygiene
;__ agent and to a method of treating surfaces with the said composition.
1_5 Background to the Invention
Hard-surface cleaning compositions generally comprise one
or more surfactants, and, optionally, one or more hygiene
agents and/or solvents.
Typically, the surfactants used in such cleaning
compositions are selected-from anionic, nonionic,
amphoteric and cationic surfactants. This large group of
surfactants exhibit a broad range of properties. Nonionics
are very commonly used due to their effectiveness on fatty
soils and the ease with which their foaming can be
controlled. The precise function of nonionic surfactants
in cleaning compositions depends on the
hydrophile/lipophile balance or 'HLB' of the surfactant
chosen. In general terms, nonionic surfactants with a
relatively low HLB values (%-°) are regarded as wetting
agents and are used in combination with solvents where the
solvent performs the cleaning function, such as in glass
cleaners. Nonionic surfactants with relatively high HLB
values (13-15) are regarded as detergents and themselves
remove so,~l from surfaces.
. r''
- p,~dtE«D~.~ ~; v~ ~
CA 02235680 1998-04-23
c3s9g cc) we
(Amended 17 November 1997)
- 2 -
Nonionic surfactants are reported as showing low biocidal
activity, whereas certain anionic, cationic and amphoteric
surfactants show biocidal activity under specific
conditions of, for example, pH and concentration. However,
the biocidal activity of surfactants is, with a few notable
exceptions, low and it is commonplace to add a separate
hygiene agent to compositions.
Typical hygiene agents include strong acids, alkali's,
phenolics and oxidants such as peracids and hypohalites.
These oxidants, of which a typical example is hypochlorite,
are generally highly reactive species which exhibit this
reactivity alone or in effective formulations in terms of
one or more of, short shelf life, toxic, corrosive and
irritant properties. In general, these reactive components
are required at relatively high levels in formulations-.
Other less chemically reactive hygiene agents, such as
2,4,4'-trichloro-2'-hydroxy Biphenyl ether (available in
the marketplace as =RGASAN [RTM]), are effective at
relatively low concentrations but are more expensive than
simpler species and may be specific as regards their
spectrum of activity. Many organic acids, including
benzoic, salicylic and sorbic are known as preservatives in
cosmetics and some food products. but these preservatives
generally show lower biocidal activity than the
above-mentioned chemically reactive hygiene agents when
used at the same level.
In hard surface cleaning it is often necessary to disinfect
a surface. A 'disinfectant' can be understood to be a
hygiene agent which shows a 100,000 fold or better
reduction in the number of viable micro-organisms in a
specified culture when used at a level of around 0.5 wt~.
This is generally known as a 'log 5 kill'. Many of the
weaker hygiene agents do not achieve this level of
~'..',:.'' _i
CA 02235680 1998-04-23
C3698 (C) WO -_~~, - _ - ..
(Amended 17 November 1997)
- 3 -
bacterial kill, especially when present in formulations at
relatively low levels.
Some surfactants have been found to potentiate the effects
of certain hygiene agents. DE 3619375 (Henkel) discloses
that alkyl polyglycoside (APG) surfactants show a synergy
with alcohols and organic acids as regards hygiene. The
examples of this reference disclose compositions which
comprise APG and organic acids. These compositions are
used at strongly acidic pH, generally below pH 3.
Alcohols, such as ethanol and isopropanol (IPA), are well-
known components of cleaning compositions. Generally,
these are present as solvents at low levels in compositions
of near neutral pH. For example: GB 1076920 (1966) relates
to glass cleaners which comprise ~0.25~ alkyl phenol
polyoxyalkylene ether and 3.5-4~ ethanol; GB 1403919 (1972)
relates to vehicle washing compositions which comprise 3-4%
nonionic surfactant (an EO/PO system is used in the
examples) and 4~ isopropanol; US 4414128 (1981) relates to
hard surface cleaners with 0.5-3~ Dobanol 91-8 (HLB 13.8 by
calculation) and 1-2~ ethanol; GB 2103642 (1981) relates to
a spectacle-glass cleaning composition which comprises 1~
ethoxylated nonionic surfactant and loo ethanol: the
preferred pH of which is 6-8.
Often these systems are free of ethoxylated alcohol
surfactants, e.g. GB 2167083 (1984) relates to a hard
surface cleaner comprising 4~ IPA and GB 2173508 (1986)
relates to hard surface cleaners which comprise 1~ of 3,5-
dimethyl-1-hexyn-3-of and 0.450 primary alkyl sulphate as
anionic surfactant with 2-4~ IPA.
It is also known to use solvents at higher levels in 'spot
removers'. Typical examples of such formulations can be
found in GB 0962436 (1961) which relates to a solvent-based
~N1END~~ J~'c''~
CA 02235680 1998-04-23
C3698 (C) WO' ~ , - ,.
(Amended 17 November 1997)
- 4 -
spot remover of which the examples comprise 8-35~
alkoxylated components (which do not include ethoxylated
alcohols) and 20-44% alcohols.
When alcohols are present at very high levels (i.e. ethanol
at 70owt is used in 'surgical spirit') they are also known
to have disinfectant properties. However, such high
alcohol concentrations are seldom used outside of
specialist applications, due to the extreme flammability of
the compositions and the health risks associated with
consumption.
i
Thus, where alcohols are present at lower levels, as
solvent, it is commonplace to add additional hygiene agents
if antimicrobial action is desired. US 4420484-(Sterling
Drug: 1981) relates to such an antimicrobial composition.
Compositions are disclosed which comprise --12o nonionic
surfactants and 3% iso propyl alcohol. The compositions,
which have a pH around 5.5, do not appear to contain
alcohol ethoxylate, the surfactant being either betaine or
amine oxide. The antimicrobial properties of these
composition arise from the known antimicrobials, such are
chlorhexidine, which are incorporated in the composition.
EP 0478445 (Peters: 1990) discloses a composition for the
disinfection of surgical instruments. Examples of a
preferred embodiment in the cited specification relate to
compositions containing 7~ C13/8E0 ethoxy alcohol nonionic
(the HLB is around 12.8 by calculation) and 12~
ethanol/isopropanol at a pH of 8.5. The compositions cited
comprise 13~ of didecyl dimethyl ammonium chloride, which
is a known biocide and is identified as such in the
specification, while the nonionic and the alcohol are
optional.
', ... . .
1'.t.~', ~~rw~-'~'
.. .~ ;i'. v'...
CA 02235680 1998-04-23
C3698 (C) wG . ,.- . . - -
(Amended 17 November 1997) - -~ -
- 4a -
Fp 0536820 (Colgate Palmolive: 1991) discloses an acidic
composition (pH 2-4) which contains nonionic surfactants
(HLB 7-10 and 11) and anti-microbial disinfectants. It is
mentioned in the cited patent that minor amounts of
isopropyl alcohol (up to 2~) can be added to improve the
anti-microbial effects (page 5, line 48ff).
FP 0028038 (P&G: 1979) relates to compositions which
contain nonionics of HLB 10-13 and the examples contain
11.5% nonionic(HLB 12) and 1-2~ ethanol. The compositions
have a pH of 8-13. Germicides are mentioned as optional
'- ingredients in the compositions disclosed.
There is a need to provide anti-microbial, preferably
disinfecting, compositions which do not require the , _
presence of either expensive or reactive hygiene agents
such as hypochlorite or peroxides, but which show a wide
spectrum antibacterial effect and are formulated with
relatively simple and available materials such as solvents.
It is also considered important that high levels of
alcohols or other solvents should be avoided due to health
and fire risks.
~rcs~H-.-'~~~~'r-' JYL~".rL'.~~.
i:
CA 02235680 2001-04-20
Brief Description of the Invention
We have now determined that a marked synergy is exhibited
between selected ethoxylated alcohol surfactants and
S certain alcohols at alkaline pH's which lie outside of the
normal 'physiological' range in bacteria. This synergy
enables much lower levels of alcohols than had previously
been employed to have a significant antimicrobial effect.
Accordingly the present invention provides for the use of
1-30owt of a C.-CS linear or branched alcohol as a biocidal
activity improving additive in a cleaning composition of
pH>8 which comprises 0.01-30owt on product of an
ethoxylated nonionic surfactant other than an alkyl phenol
derivative, with an HLB of 10-1.4.
CA 02235680 2001-04-20
.. r c .. , .. . ..
.. . r. ~ '
- 6 -
As discussed above, ethOxylated alcohol surfactants of HLB
10-14 are inhibitors of bacterial growth but are only
weakly biocidal at typical formulation pH's. In the
presence of the specified alcohols, and above the normal
physiological pH range; a synergy is maintained and
exploited to give a product which is both an effective
cleaner and biocidal. Effective cleaning and biocidal
activity are desirable in a cleaning composition for
hygiene purposes as it is important to both to ensure a
~- high kill of bacteria and removal soil so as to retard
reinfection and regrowth of bacterial populations. In the
present invention, the important. features of effective
microbial kill and improved soil. removal are both attained
with a relatively simple and hence cost-effective
formulation.
Without wishing to be limited by any theory of operation,
it is believed that the presence of the both the selected
alcohol and the selected class of surfactant in the
membrane of bacteria results in the formation of 'pores' in
the cytoplasmic membrane of the bacteria through which the
contents of the cell may be exchanged with the ambient.
This has been confirmed by studies with the fluorophore
Propidium iodide, which is not taken up by cells with
intact membranes. It is believed that bacteria cannot
recover from this leakage under the pH conditions specified
and consequently rapid cell death occurs.
Detailed Description of the Invention
In order that the invention may be further understood it
will be described hereafter with reference to preferred
features and materials.
PNtENp~~ SN~ET
CA 02235680 2001-04-20
' . . , ~ ' . ~.
Nonionics
Nonionic, ethoxylated surfactants are present in the
compositions of the invention. These surfactants are
believed to engage in a synergistic interaction with both
the alcohol, to improve cleaning and aid the removal of
soil subsequently deposited and with the antimicrobial so
as to improve the disinfecting qualities of the
composition.
Suitable nonionic detergent active compounds can be broadly
t_ described as compounds produced by the condensation of
ethylene oxide groups, which are hydrophilic in nature,
with an organic hydrophobic compound. The length of the
hydrophilic or polyoxyethylene radical which is condensed
with any particular hydrophobic group can be readily
adjusted to yield a water-soluble compound having the
desired degree of balance between hydrophilic and
hydrophobic elements (HLB).
Particular examples include the condensation product of
aliphatic alcohols having from 8 to 22 carbon atoms in
either straight or branched chain configuration with
~- ethylene oxide, such as a coconut oil ethylene oxide
condensate having from 3 to 10 moles of ethylene oxide per
mole of coconut alcohol.
The amount of nonionic detergent active to be employed in
the composition of the invention will generally be from 0.1
to 30owt, preferably from 1 to 20owt, and most preferably
from 3 to l0owt for non-concentrated products.
Concentrated products will genE=rally have 10-20owt nonionic
surfactant present, whereas dilute products suitable for
spraying will have 0.1-5owt nonionic surfactant present.
ANiEND~D SN~E~
CA 02235680 2001-04-20
. ' ~. _ :~
_ g _
Typically, the ethoxylated nonionic surfactant is an
ethoxylated alcohol having a chain length of Cg-Cla and 4-10
ethoxy groups per molecule. It is however essential that
the HLB of the nonionic should fall in the range 10-14.
HLB can be calculated as a function of the chain length of
the molecule and its degree of ethoxylation. According to
Griffin (W. C. Griffin J. Soc. Cosmetic Chemists [5, 249,
19540 the HLB of fatty alcohol ethylene oxide adducts is
given as one fifth of the weight percent of oxyethylene
content in the adduct.
It is believed that the critical micellar concentration
(CMC) of the ethoxylated nonionic surfactants should be
below 10-'-5 moles/litre, preferably in the range 10-3~5 to
10-5 moles/litre. Quite surprisingly, we have found that
for alcohol ethoxylate nonionic surfactants acting under
optimum conditions the minimum inhibitory concentration
(MIC) for these surfactants appears to be equal to the CMC.
IMBENTIN 91-3S OFA~ (TM, ex. Kolb AG) a C9_11 alcohol with,
on average, five moles of ethoxylation had been found to be
a suitable nonionic surfactant in compositions according to
the invention. This material has a calculated HLB of 11.6
and is believed to have a CMC of 1.6x10-" moles/litre.
2S DOBANOL 91-8 (TM) a C~-Cil alcohol with on average 8 moles
of ethoxylation has also bee.~. found to be a suitable
material. This material has a ca1_culated HLB of 13.8 and
is believed to have a CMC of 4.3}:10-' moles/litre.
Alcohols
Preferably, the alcohol is selected from the group
comprising propan-2-ol, propanol, ethanol and mixtures
thereof.
.: ,' ._
Ft~fa~U~~~~ ~::~:.~.~
CA 02235680 1998-04-23
C3698 LC) WG ~ . ~ : ~ ~ - ._
< (Amended 17 November 1997)
- 9 -
10
Preferred levels of alcohol are 1-25~wt on product. At
these concentrations alcohols taken alone show little '
potential as disinfectants. Particularly preferred levels
of alcohol range from 5-150.
Preferably, the weight ratio of the alkoxylated nonionic
surfactant to the alcohol such that the alcohol is present
in weight excess over the alkoxylated nonionic surfactant.
Preferably, the compositions of the invention further
comprise a buffer to maintain the pH of the composition in
the desired pH range upon dilution by a factor of 2-5; ,
The alkaline products according to the invention have a
preferred pH of 8-11, more preferably 9.5-11. Below pH 9.5,
compositions show reduced bacterial kill, whilst above pH
11, compositions are considered unacceptably hazardous for
many uses. Sodium bicarbonate/carbonate buffers are
suitable to maintain pH in the preferred range.
~ ~~4-~~ ~,..r~' W'V~' n
CA 02235680 1998-04-23
.. , ~ ~.
C3698 (C) WO -_. - ,. .- _ . ;. _ _
, (Amended 17 November 1997) - ~-
- 10 -
Minors and Optional Components
The composition according to the invention can contain
other minor, unessential ingredients which aid in their
cleaning performance and maintain the physical and the
chemical stability of the product.
For example, the composition can contain detergent
builders. In general, the builder, when employed,
preferably will form from 0.1 to 25~ by weight of the
composition.
i _
Optionally, the composition can include one or more
amphoteric surfactants, preferably betaines, or other
surfactants such as amine-oxide and alkyl-amino-glycinates.
Betaines are preferred for reasons of cost, low toxicity
(especially as compared to amine oxides) and wide
availability.
Typically betaines in compositions according to the
invention are the amido-alkyl betaines, particularly the
amido-propyl betaines, preferably having an aliphatic alkyl
radical of from 8 to 18 carbon atoms and preferably having
a straight chain. These betaines are preferred as they are
.:.
believed to comprise relatively low levels of nitrosamine
precursors although other betaines, such as alkyl betaines.
can be used in the compositions of the invention.
Typical levels of amphoteric range from 0.01 to 8~, with
levels of 1-5wt~, particularly around 2~ being preferred
for normal compositions and up to four times the
~~4~~~i ~w ~iWr
LSt L w-1~S
- CA 02235680 1998-04-23
C3698 (C) WO - ' . .-. . . .
(Amended 17 November 1997) ~ '
- 11 -
concentration being present in so called, concentrated
products. As with the nonionic surfactant, lower levels of
around 0.05-1~ will be employed in sprayable products and
higher levels of, typically, around 4gwt in concentrates.
Metal ion sequestrants, including ethylene-diamine-tetra-
acetates, amino-poly-phosphonates (such as those in the
DEQUESTp range) and phosphates and a wide variety of other
poly-functional organic acids and salts, can also be
employed. It is believed that the hygiene performance of
_ the composition is improved by the presence of a metal ion
sequesterant.
The solutions do not generally require additional
hydrotropes to be added as the alcohols provide a ,
hydrotroping function. Suitable additional hydrotropes
include, alkali metal toluene sulphonates, urea, alkali
metal xylene and cumene sulphonates, polyglycols, >20E0
ethoxylated alcohols and glycols. Preferred amongst these
hydrotropes are the sulphonates, particularly the cumene,
xylene and toluene sulphonates. Typical levels of
additional hydrotrope range from 0-5o for the sulphonates.
Hydrotropes are not always required for dilute, sprayable
products, but may be required if lower EO or longer alkyl
ethoxylates are used or the cloud point needs to be raised
~~->;~1~.:~ r-' ..
j ;;~;;...:.i _.
CA 02235680 2001-04-20
- 12 -
considerably. The cumene sulphonate is the most preferred
hydrotrope.
Compositions according to the invention can also contain,
in addition to the ingredients already mentioned, various
other optional ingredients such as, further solvents,
colourants, optical brighteners, soil suspending agents,
detersive enzymes, compatible bleaching agents, gel-control
agents, freeze-thaw stabilisers, further bactericides,
1_0 perfumes and opacifiers.
_. The most preferred formulations according to the present
invention, excluding minors, comprise:
a) 0.1-20owt on product of an ethoxylated alcohol having
a chain length of CQ-C,a, 4-10 ethoxy groups per
molecule and HLB of 10-14,
b) 1-30owt of an alcohol is selected from the group
comprising propan-2-ol, propanol, ethanol and mixtures
thereof, wherein the alcohol (b) is present in weight excess over
the ethoxylated alcohol (a), and,
c) at least one buffering agent to maintain the pH either
in the range 9.5-11.
It is envisaged that such a composition would be packaged
in a container adapted to deliver the composition in the
form of a spray and accordingly a pre~erred method
according to the present invention co~«prises the step of
treating a surface with a composition as disclosed he=ein
by means of a spray.
In order that the present invention can be further
understood it will be illustrated herein after by reference
I,,
CA 02235680 2001-04-20
C ,. ' ,
- 13 -
to the following, non-limiting examples.
Examples
The following examples are suspension tests against a range
Of microbes at a range of pH's. T:he following bacterial
strains were used in these suspension tests.
Pseudomonas aeruginosa: ATCC 15442
w Staphylococcus aureus: NCTC 6538
Escherichia coli: ATCC 11229
.,~;~._;, .
CA 02235680 2001-04-20
- 14 -
Example 1: aga vr~t L. coli .
Bacterial suspension was prepared as follows. Incubate
bacterial culture (tryptone Soya broth) for 24 hours. Spin
down (SO ml tubes, Mistral 1000 centrifuge, 4100rpm, 10
min). Resuspend pellets in peptone water (20m1) and mix
using a vortex miner nor 15-;0 seconds. Adjust cell density
with sterile peptone water to required inoculum s~~ze using
~ENO~~ SH~~ C
CA 02235680 2001-04-20
- 15 -
a calibrated densitometer. Cell suspension is stored at 20
°C (+/- 1°C> and used within 2 hours
All tests were carried out using t:he microplate method for
a 5 minute contact time at 20°C, in a 96 well (8x12)
microplate with inoculation (30u1 of microbe suspension) of
the formulations as listed in Table 1 (270u1), to give a
total volume of 300u1. The inoculum was estimated to
contain ca.l0g cfu/ml. Inoculations were performed
simultaneously using a multi-pipette (mixing thoroughly by
charging and discharging the pipette 4 times) and the
;_ reaction mixture left for a Smin contact time.
Alkaline formulations (pH 10.5) were buffered with a mixture of
sodium carbonate (1.14 w/v percent decahydrate) and sodium
hydrogen carbonate (0.08 w/v percent).
After reaction 30u1 of each reaction mixture was
transferred into 270u1 of a quench solution using a
multipipette and mixed as previously indicated. The
composition of quench solution was as follows:
f.-
Lecithin 0.30
Sodium thiosulphate 0.50
L-histidine 0.10
Tween 80 3.Oo
pH 7 buffer(see below) lOml
Distilled water to 1 litre
(Prepare pH 7 buffer by dissolving 34gm potassium
dihydrogen phosphate in SOOmI distilled water and sterilize
(autoclave, 121°C, 15 min))
~iMENDED S!~.tE ~
CA 02235680 2001-04-20
- 16 -
After 5mins(+/-lmin), 30u1 of the quenched product was
serially diluted into 270u1 peptone solution using a
multipipette, and mixed as previously indicated.
Total viable count was determined using the Miles-Misra
spot plate method, plating out 10u7_ (in triplicate) onto
tryptone Soya agar and incubating for 24 hours at 30°C.
Results were recorded as mean count: from 3 spots on agar,
calculating Log Reduction for each formulation. as Log
Reduction - log (initial count) - log (final count)
Table 1: Synergy with Propan-2-of under Alkaline Conditions
Dobanol 91-8 Propan-2-of pH Mean Log Standard
(wlv percent)(wlv percent) (reduction) Deviation
of E-coli
0.7 0 1 0.5 0.9 0.3
0 g.p 10.5 0.3 0.1
0.7 8.0 1 0.5 3.2 0.5 I
Y
,-
Table 1 shows that under alkaline conditions a significant increase in the
biocidal activity of the composition is achieved when both the alcohol and the
alcohol ethoxylate are present.
EXample 2: further examples
35 Tables 2a and 2b b~~ ow s:no.,~ further exampl es, using t-.~o
nonicni c m~rfactants and tr.ao microbes, for wrhich the
results -,-sere obtai::ed using the method as described above
in e~:ampl~. 1 . Tn each case that the mater ial ;.~as present ,
i;C ~TT.r~ ~ ~~W.
~~.,.~ i ~;' .
CA 02235680 2001-04-20
- 17 -
the formulations contained 10o iso-propyl alcohol, 0.70
non-ionic surfactant, and were prepared at pH 11.0
Table 2a: Synergy with Propan-2-of against S.au~'eus
Nonionic Iso-propyl 7~og (reduction)
surfactant alcohol against S. aureus
0 - ,--- 0 0
010 0
Imbentin 91-35 OFA 0 0.3
Dobanol 91-5 0 0.3
Dobanol 91-8 0 0.1
Imbentin 91-35 OFA 10 1.~
Dobanol 91-5 10 2.1
Dobanol 91-8 10 1.1
Table 2b: Synergy with Propan-2-of against E.COIi.
Nonionic Iso-propyl hog (reduction)
surfactant alcohol
_ 0 0 1.2
0 10 5.1
Imbentin 91-35 OFA 0 S.l
Dobanol 91-5 0
Dobanol 91-8 0 4.8
Imbentin 91-35 OFA 10 >6
Dobanol 91-S 10 >6
Dobanol 91-8 10 >6
CA 02235680 2001-04-20
- 18 -
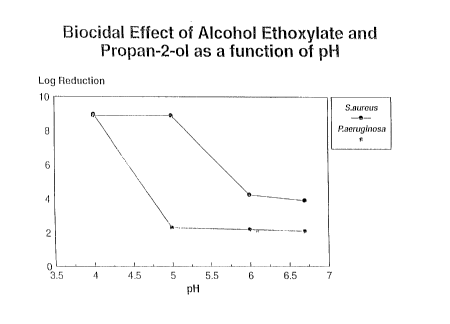
From tables 2a and 2b , it can be seen that, at pH 11,
synergy was seen when both the nonionic and the alcohol
were present.
e_
~sl~~'~~ ~c~..
jjyt~a-