Note: Descriptions are shown in the official language in which they were submitted.
CA 02235976 1998-04-28
1
METHOD OF TREATING A METAL
The present invention relates to a method of pickling or surface treating a
metal
in a solution containing nitric acid to which hydrogen peroxide is supplied to
decrease the
formation of nitrous fumes.
At manufacturing of many metals such as steel, particularly stainless steel,
an
oxide layer forms at the surface during the annealing, and this layer must be
removed.
This is normally done by pickling which means that the steel is treated in an
acidic oxidis-
ing pickling bath to affect some dissolution of metal under the oxide layer
which then
comes loose. Pickling and surface treatment of metals is often performed in a
solution
based on nitric acid as an oxidising agent which treatment, however, involves
emissions
of nitrous fumes, mainly NO and N02. These emissions can be reduced
significantly by
adding hydrogen peroxide to the nitric acid containing solution as disclosed
in the US
patent 4938838 and 3945865 as well as in H. T. Karlsson et al, "Control of NOx
in Steel
Pickling", Environmental Progress, Vol. 3, No. 1, 1984, pp. 40-43. In pickling
of steel the
following reactions occur:
2 Fe + 6 H+ + 3 N03 ~ 2 Fe3+ + 3 N02- + 3 HZO
2 N02 + 2 H+ ~ NO + NOZ + H20
N02- + H202 ~ N03- + H20
This process generally works very well, but it has been found that in order to
decrease the emissions below a certain level far more than stochiometric
amounts of
hydrogen peroxide must be supplied. At the same time, increasing consciousness
of en-
vironme~ntal problems call for more effective reduction of nitrous fumes.
The present invention intends to solve the problem of further reducing the
emis-
sions of nitrous fumes or NOX, particularly NO and N02, without increasing the
hydrogen
peroxide consumption to unacceptable levels. According to the invention it has
surpris-
ingly been found that the reduction of NOx emissions can be improved
considerably with-
out significantly increasing the (hydrogen peroxide consumption if at least a
part of the
hydrogE:n peroxide is sprayed or flushed directly on the metal instead of
being added to
the nitric acid containing solution, either directly into a tub in which the
metal is treated or
into a circulation conduit for the nitric acid containing solution.
Thus, the present invention concerns a method of pickling or surface treating
a
metal in an aqueous solution containing nitric acid wherein hydrogen peroxide
is supplied
to decrease the formation of nitrous fumes. At least a portion of the hydrogen
peroxide is
supplied by spraying or flushing an aqueous solution thereof directly on the
metal through
one or several separate nozzles. Preferably the hydrogen peroxide is sprayed
in a way to
CA 02235976 2000-07-12
2
obtain as small droplets as possible which makes the reaction with the NOX
more effi-
cient. Although it is possible to supply substantially all the hydrogen
peroxide through the
separate nozzles, the preferred portion is from about 20 to about 80%, most
preferably
from about 40 to about 60% of the total amount of hydrogen peroxide supplied.
Without being bound to any theory it is assumed that hydrogen peroxide coming
into contact with metal ions in a pickling solution decomposes catalytically
into water and
oxygen and is thus consumed to no use. It is also assumed that the main part
of the ni-
trous fumes are generated at the surface of the metal and that the hydrogen
peroxide
therefore is most likely to contact the NOX before it comes into contact with
metal ions if it
is sprayed or flushed directly on the metal. This is supposed to be
particularly true when
nitric acid containing solution is sprayed or flushed directly on the metal in
which proc-
esses considerable amounts of nitrous fumes evolve even at very low
concentrations of
dissolved NOx.
The nitric acid solution normally contains from about 0.1 to about 4 mols/l,
pref-
erably from about 0.5 to about 3 molsll of nitric acid, and suitably also
hydrofluoric acid,
for example from about 0.01 to about 5 molsll, preferably from about 0.1 to
about 3
mols/I. The content of dissolved NOX is normally from about 0.01 to about 0.7
g/I, pref-
erably from about 0.1 to about 0.4 g/l. The invention is particularly
advantageous when
the content of dissolved NOX is below about 0.7 gll. Normally most of the
dissolved NOX
is in the form of NOZ .
According to the invention it is generally possible to maintain the emissions
of
NOX gas below about 7 g NOX per m2 treated metal and often even below about 4
g NOX
per m2 treated metal at a hydrogen peroxide consumption from about 2 to about
60 g
H20z, preferably from about 5 to about 40 g H202 per m2 treated metal.
The amount of hydrogen peroxide added can be controlled by conventional
method such a by measuring the redox potential in~the nitric acid containing
solution or
measuring the content of NOX in the exhaust gas. Preferred redox potential
control
methods are described in US 4938838 and EP 442250.
The invention is advantageous in all processes for surface treatments of
metals
such as steel, copper or brass with nitric acid containing solutions. It is
particularly advan-
tageous in pickling of steel, especially stainless steel.
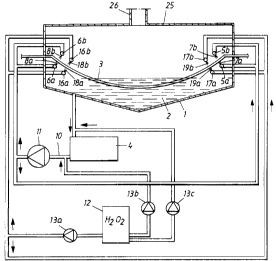
The invention will now be described in connection with the enclosed Figure
schematically showing an embodiment of a process of treating a metal.
The figure shows a tub 1 containing a surface treating or pickling bath 2 of
an
aqueous solution containing nitric acid and preferably also hydrofluoric acid
through
which a running strip 3 of a metal, preferably stainless steel, is conducted
continuously.
Nitric acid containing solution is supplied through lances 5a, 5b, 6a, 6b,
each containing
CA 02235976 1998-04-28
3
a plurality of nozzles 7a, 7b, 8a, 8b spraying the solution on each side of
the metal strip 3
so it is distributed over substantially the entire width thereof. Solution
from the bath 2 is
withdrawn to a tank 4 and is fed ~;o the lances 5a, 5b, 6a, 6b at sufficiently
high pressure
via a circulation conduit 10 and a pump 11. The process also involves supply
of an aque-
ous solution of hydrogen peroxide from at storage tank 12. A portion of the
hydrogen
peroxide is brought by a pump 13a to separate lances 16a, 16b, 17a, 17b, each
contain-
ing a plurality of nozzles 18a, 18b, 19a, 19b spraying the solution on each
side of the
metal strip 3 so it is distributed over substantially the entire width
thereof. The suitable
number of nozzles depends on the size of the metal strip 3 and on the type of
nozzles,
but normally from about 4 to about 12 nozzles per lance is sufficient. Any
conventional
nozzle can be used, for example nozzles also blowing air which prevents
clogging at in-
terruption of the hydrogen peroxide flow. The remaining part of the hydrogen
peroxide
supplied is added by pumps 13b, 13c to the nitric acid containing solution in
the tank 4
and the circulation conduit 10 at the suction side of the pump 11. The
hydrogen peroxide
from the pump 13c is preferably mixed with the solution from the bath 2 just
before it en-
ters the 'tank 4. Above the tub 1 a hood 25 containing a vent 26 is arranged.
Any nitrous
fumes farmed is evacuated through the vent 26. The supply of hydrogen peroxide
is
preferably controlled on basis of the NOx content in the gas stream in the
vent 26 or of
the redox potential in the bath 2. It is also possible to have fixed flow of
hydrogen perox-
ide added through the nozzles 1 F3a, 18b, 19a, 19b and only regulate the pumps
13b, 13c
supplying hydrogen peroxide to the tank 4 and the circulation conduit 10.
Although not shown in the Figure it is possible to treat the metal strip 3
without
immersing it into the bath 2. It is also possible to convey the metal strip 3
vertically and
spray the nitric acid containing :solution and the hydrogen peroxide on the
vertical sur-
faces.
The invention is further illustrated through the following example. If not
otherwise
stated all contents and percentages refer to wt%.
EXAMPLE: In a plant according to the Figure stainless steel was pickled in a
an
aqueous. solution of 2.9 molsll nitric acid and 2.7 molsll hydrofluoric acid.
When all the
hydrogen peroxide was added to the nitric acid containing solution in the tank
4 and the
circulation conduit a hydrogen peroxide consumption of 60-70 ml 35% aqueous
H20z per
m2 pickled steel was required to (keep a NOX concentration below 280 ppm in
the vent 26
(corresponding to 3.5 g NOX per m2 pickled steel). When the process was
operated ac-
cording to the invention and about 45% of the hydrogen peroxide supplied was
sprayed
directly on the steel surface through the separate lances 16b, 17b above the
steel strip 3,
each containing six nozzles 18b, 19b, the consumption required to keep a NOX
concen-
tration below 280 ppm in the vent 26 was only 40-45 ml 35% aqueous H202 per m2
pick-
led steel.